- 1Department of Pharmacy, The Second Affiliated Hospital, University of South China, Hengyang, Hunan, China
- 2Hengyang Medical School, University of South China, Hengyang, Hunan, China
- 3Hunan Provincial Key Clinical Laboratory of Basic and Clinical Pharmacological Research of Gastrointestinal Cancer, the Second Affiliated Hospital, University of South China, Hengyang, Hunan, China
- 4Department of rehabilitation medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 5Wuxi School of Medicine, Jiangnan University, Wuxi, Jiangsu, China
- 6Chongqing Medical University, Chongqing, China
Background: The introduction of multi-targeted tyrosine kinase inhibitors (MTKIs) such as axitinib, lenvatinib, sorafenib, and sunitinib has greatly broadened the available treatment options for Renal Cell Carcinoma (RCC). The study aims to compare the nature of the adverse reactions associated with these four MTKIs to identify which medication poses the least risk for personalized patient management, thus enabling more accurate clinical drug oversight.
Methods: Employing a retrospective descriptive analysis methodology, this research concentrated on four commercially available MTKIs. Reports pertaining to these medications were sourced from the WHO-VigiAccess database. The data gathering process involved collecting comprehensive information on various parameters, such as age demographics, gender, and the geographical distribution of patients associated with the ADR reports. Furthermore, the study explored disease systems and symptoms that were documented alongside the adverse reactions, as outlined in the annual ADR reports produced by the WHO. To assess the relationship between these four MTKIs and the linked AEs, both the Proportional Reporting Ratio (PRR) and the Reported Odds Ratio (ROR) were utilized.
Results: At the time of the search, a total of 123,818 AEs associated with the four MTKIs had been documented in the VigiAccess database. The common ADRs for these four MTKIs include diarrhoea, fatigue, death, hypertension, nausea, asthenia, weight decreased, and vomiting. Gastrointestinal disorders and general disorders and administration site conditions emerged as the SOCs with the highest number of adverse signals, both ranking first in terms of frequency. The elevated ROR (1.08) and PRR (1.06) values associated with gastrointestinal disorders in patients treated with sorafenib suggest a higher incidence of such adverse events compared to those observed with axitinib, lenvatinib, and sunitinib.
Conclusion: Recent comparative observational research suggests that the ADR reports submitted to the WHO and the FDA for these medications highlight both common and specific ADRs. It is essential for clinical practitioners to develop personalized treatment strategies that consider the adverse effects linked to different medications, alongside the unique circumstances of their patients, thus encouraging the responsible use of these MTKIs.
Introduction
Renal Cell Carcinoma (RCC) stands out as the most prevalent malignant tumor affecting the kidneys, representing over 90% of all renal cancers. This type of cancer arises from the epithelial cells found within the renal tubules and showcases considerable heterogeneity, alongside a notable resistance to standard chemotherapy and radiotherapy treatments (Hsieh et al., 2017). The underlying mechanisms of RCC are complex, with the overproduction of Vascular Endothelial Growth Factor (VEGF) and Vascular Endothelial Growth Factor Receptor (VEGFR) playing a pivotal role in the processes of tumor angiogenesis and development. Remarkably, around 25% of patients diagnosed with RCC present with either locally advanced or metastatic disease, and between 20% and 40% of individuals with localized primary tumors will ultimately experience the spread of metastases. Given the frequently asymptomatic progression and the unfavorable outlook linked to advanced or metastatic RCC, the range of treatment options remains quite limited. Among the various subtypes of RCC, Clear Cell Renal Cell Carcinoma (ccRCC) is the most common, representing approximately 70%–80% of all RCC instances. ccRCC is distinguished by significant vascularization, and its development is closely linked to hereditary von Hippel-Lindau (VHL) disease. In ccRCC specifically, the loss of function of the VHL tumor suppressor gene leads to the excessive buildup of Hypoxia-Inducible Factor (HIF) (Sato et al., 2013). Under normal physiological circumstances, HIF serves as a transcription factor that enhances the expression of multiple pro-angiogenic factors, such as VEGF and Platelet-Derived Growth Factor (PDGF) (Chen et al., 2023). As a result, in most ccRCC cases, dysregulation of signaling pathways caused by mutations in the VHL gene facilitates angiogenesis, as well as the survival, proliferation, and differentiation of cancerous cells. The introduction of Multi-Targeted Tyrosine Kinase Inhibitors (MTKIs) has brought about a significant change in the treatment approach for RCC. MTKIs function by targeting an array of protein kinases, such as the VEGFR, PDGFR, and the Stem Cell Factor Receptor (c-KIT) (Faivre et al., 2007). Presently, commonly utilized MTKIs include axitinib, lenvatinib, sorafenib, and sunitinib, all of which have demonstrated considerable clinical effectiveness in the management of advanced or metastatic RCC. By inhibiting the activities of VEGFR, PDGFR, and other associated kinases, these agents promote their antitumor effects, hindering tumor angiogenesis and growth while triggering apoptosis (Bahadoram et al., 2022). As a result, they offer novel therapeutic avenues for patients facing advanced RCC. Despite the thoroughness of pre-marketing clinical trials, the safety of these medications remains partially undefined based on data from pre-authorization studies, as these trials are performed in controlled conditions that differ from everyday practice (Gagliardi et al., 2022). Four MTKIs have been available on the market for a considerable period, catering to a wide range of patients and serving various purposes. Consequently, it is crucial and informative to perform safety research by utilizing extensive real-world data. Therefore, a more thorough characterization of the ADRs associated with these MTKIs is necessary, utilizing spontaneous reports gathered from pharmacovigilance databases. It is important to highlight that comparative studies addressing the similarities and differences in ADRs induced by these medications are notably lacking. Since 2015, the data stored in VigiBase has been accessible to the public through VigiAccess (Watson et al., 2018; Habarugira and Figueras, 2021). The VigiAccess database facilitates searches using the trade names of drugs, while also identifying the active ingredients and presenting the corresponding results of ADR reports. This research primarily focuses on four MTKIs: axitinib, lenvatinib, sorafenib, and sunitinib. Clinicians often need to tailor treatment options based on the potential risk of AEs for each patient. To assess the occurrence of adverse reactions associated with these MTKIs, we conducted a descriptive study that analyzed spontaneously reported adverse reactions in the VigiAccess database and compared the rates of adverse reactions linked to these four MTKIs. Furthermore, we employed the Proportional Reporting Ratio (PRR) and the Reported Odds Ratio (ROR) to evaluate the relationship between these four MTKIs and the associated AEs.
Materials and methods
Drug sample
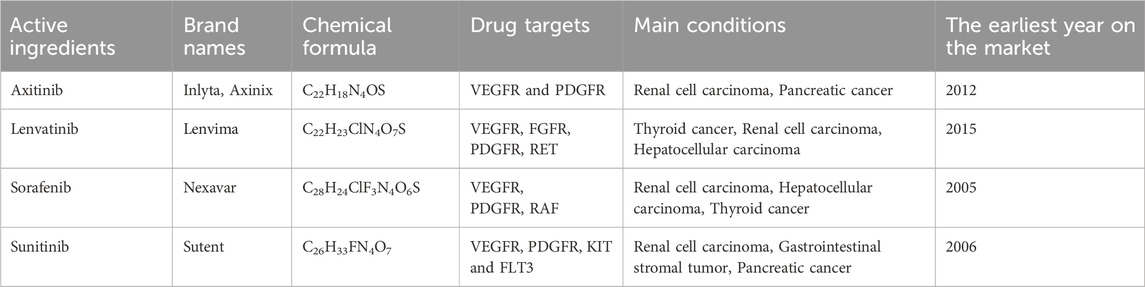
Table 1 presents the four MTKIs that we have studied and are currently available for clinical use. Axitinib is a potent and selective second-generation VEGFR inhibitor that targets VEGFR-1, VEGFR-2, and VEGFR-3, while exhibiting a weaker inhibitory effect on PDGFR. It is primarily utilized as a second-line treatment for advanced RCC (Schmidt et al., 2018). In the AXIS Phase III clinical trial, axitinib significantly extended the progression-free survival (PFS) of patients, achieving a median PFS of 6.7 months. Lenvatinib targets multiple receptor tyrosine kinases, including VEGFR, FGFR, and PDGFR, thereby inhibiting tumor angiogenesis and cell proliferation by blocking these targets. It is widely employed in the treatment of RCC, thyroid cancer, and hepatocellular carcinoma (Romero, 2019). In 2015, lenvatinib received FDA approval for the second-line treatment of advanced RCC. Based on Phase II clinical trial data, the median PFS for the lenvatinib combination therapy group reached 14.6 months, significantly surpassing that of the monotherapy group (Motzer et al., 2015). Sorafenib is the first MTKI approved for advanced RCC, targeting VEGFR, PDGFR, and RAF kinase. In 2005, sorafenib received FDA approval based on the results of the Phase III TARGET trial, which demonstrated that the median PFS for the sorafenib group was 5.5 months, significantly longer than the 2.8 months observed in the placebo group, with a disease control rate of 84%. Although subsequent drugs have surpassed sorafenib in certain metrics, it remains a crucial option for the treatment of advanced renal cancer (Wilhelm et al., 2006). Sunitinib is an oral MTKI primarily targeting VEGFR, PDGFR, KIT, and FLT3. In 2006, sunitinib received FDA approval for the first-line treatment of advanced RCC. Key Phase III trials indicated that the median PFS for the sunitinib group was 11 months, significantly better than the 5 months reported for the interferon-α group. Due to its inhibitory effect on KIT, sunitinib is also approved for the second-line treatment of gastrointestinal stromal tumors (Moran et al., 2019).
Data sources
The WHO-VigiAccess database was searched on 17 February 2025, to gather all documented AEs that occurred following the introduction of four MTKIs. The access URL is https://www.vigiaccess.org. All pharmaceutical agents under study were identified using their generic names. Data collection spanned different age ranges, genders, years of reporting, and geographic regions, as detailed by WHO-VigiAccess. Descriptive statistics were computed utilizing Excel 2021.
WHO-VigiAccess serves as an open-access portal to the PIDM database, facilitating the retrieval of safety reports concerning medicinal products provided by the UMC. The evaluation was based on system organ class (SOC) and preferred terms (PTs) as defined by the Medical Dictionary for Regulatory Activities (MedDRA). As a result, records for each MTKI were gathered, and all distinct AEs noted at the MedDRA SOC and PT levels were pinpointed to outline the range of toxicities. The reporting terms employed in MedDRA were compiled from various dictionaries, including the WHO Adverse Reaction Terminology (WHO-ART) and others (Sultana et al., 2020). In total, 27 items were classified by SOC. This research concentrated on the PTs, which represent the extent of publicly available information in the VigiBase database via WHO-VigiAccess. To assess the outcomes of the identified safety signals, we organized them using outcome codes, culminating in three essential categories: death, hospitalization, and major events, which encompass life-threatening occurrences, disabilities, and congenital anomalies.
Disproportionality analysis
In order to evaluate the possible association between axitinib, lenvatinib, sorafenib, and sunitinib with AEs under gastrointestinal disorders, we used two methods for disproportionate analysis: ROR and PRR. ROR is mainly used to measure the imbalanced probability of reporting AEs for specific drugs compared with other drugs.
The calculation formula was:
(a) refers to the quantity of reports for particular drugs and particular AEs, (b) represents the quantity of reports for specific drugs and other AEs, (c) refers to the number of reports on other drugs and specific AEs, (d) represents the number of reports on other drugs and other AEs. PRR refers to the proportion of spontaneous reports of a specific drug associated with a specific adverse outcome divided by the corresponding proportion of other drugs. The calculation formula was:
Both ROR and PRR require that at least 5 cases (a ≥ 5) of particular drug and AEs to consider the calculated results valid. If ROR ≥2 and the lower limit of the 95% confidence interval (CI) ≥ 1, the signal is considered disproportionate, indicating that there may be a safety problem. These criteria ensure that the observed disproportion is not due to random variation (Montastruc et al., 2011). In our analysis, we systematically evaluate the ratio of ADRs reports of using four MTKIs in gastrointestinal disorders. The analysis results help to provide guidance for the correct use of drugs.
Statistical analysis
A retrospective quantitative approach was adopted for this study. Descriptive analysis was conducted using Excel to evaluate the characteristics of individuals who experienced adverse reactions to the four MTKIs. The rate of ADR reporting for each MTKI was determined by dividing the number of ADR symptoms associated with that specific drug by the total number of ADR reports. The common ADRs linked to each medication were identified as those symptoms corresponding to the top 20 ADR report rates. The reported ADR symptoms for each drug were calculated, followed by a descriptive comparative analysis. Frequencies and percentages were utilized to classify the descriptive variables.
Results
Description of the studied cases
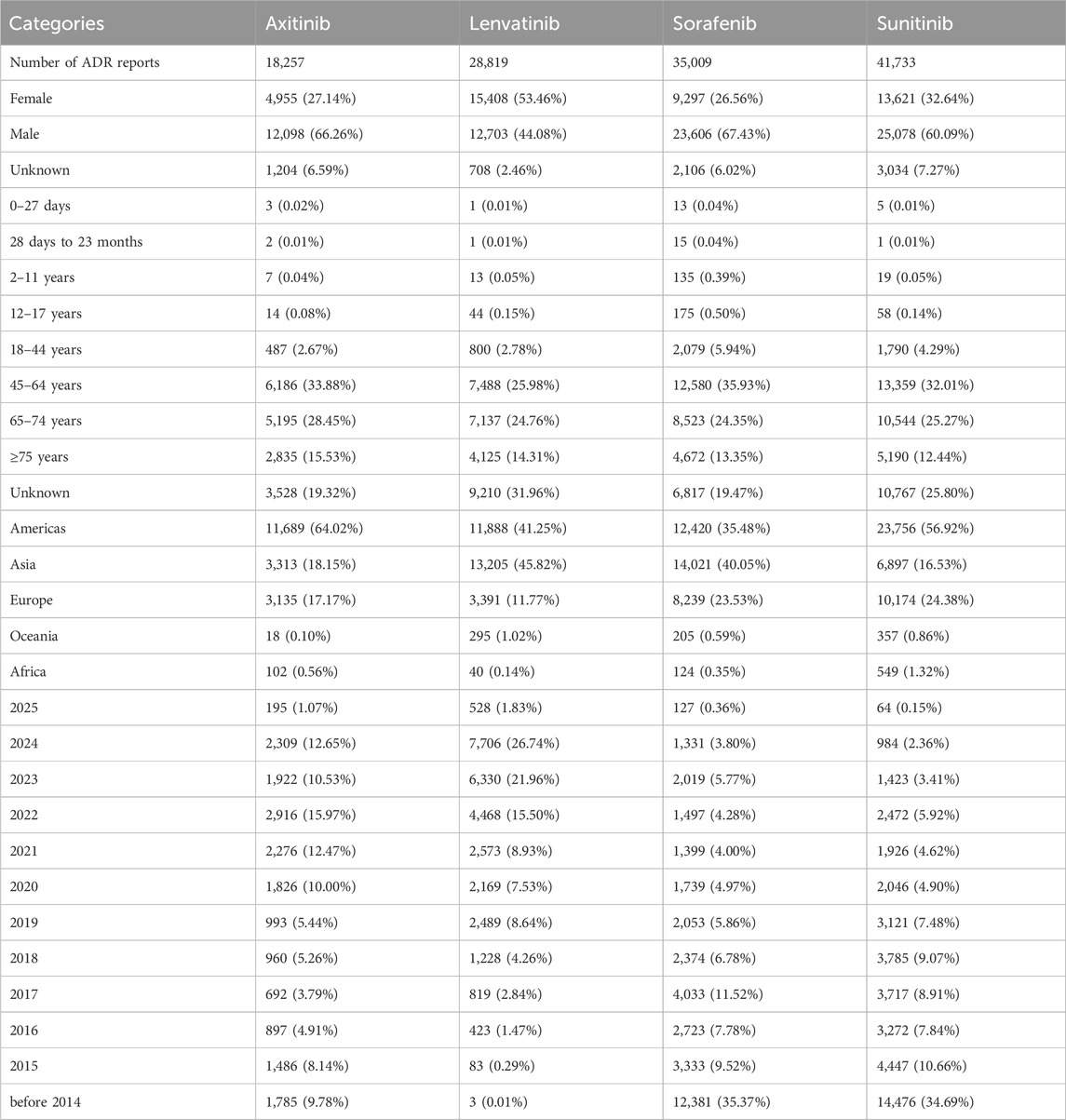
The initial documentation regarding adverse reactions to axitinib, lenvatinib, sorafenib, and sunitinib was first noted in the WHO-VigiAccess database during the years 2003, 2004, 2008, and 2013, respectively. By 2025, the WHO had gathered a cumulative total of 18,257, 28,819, 35,009, and 41,733 reports of ADRs linked to these four MTKIs, summing up to an overall total of 123,818 reports. Within these 123,818 ADR reports associated with the four MTKIs, as illustrated in Table 2, there were 7,052 cases in which the sex of the subjects was not specified. Importantly, the amount of ADR reports from males (73,485) significantly surpassed that from females (43,281), resulting in a male-to-female ratio of 1.70:1, highlighting a notable difference. When excluding reports that did not include age information, the age group most frequently reporting incidents was predominantly individuals aged 45–64 years. Additionally, most AEs were noted from the Americas, constituting 48.26% of the overall total. More information about the reporting years for each medication analyzed can be found in Table 2.
Distribution of 20 SOCs of four MTKIs
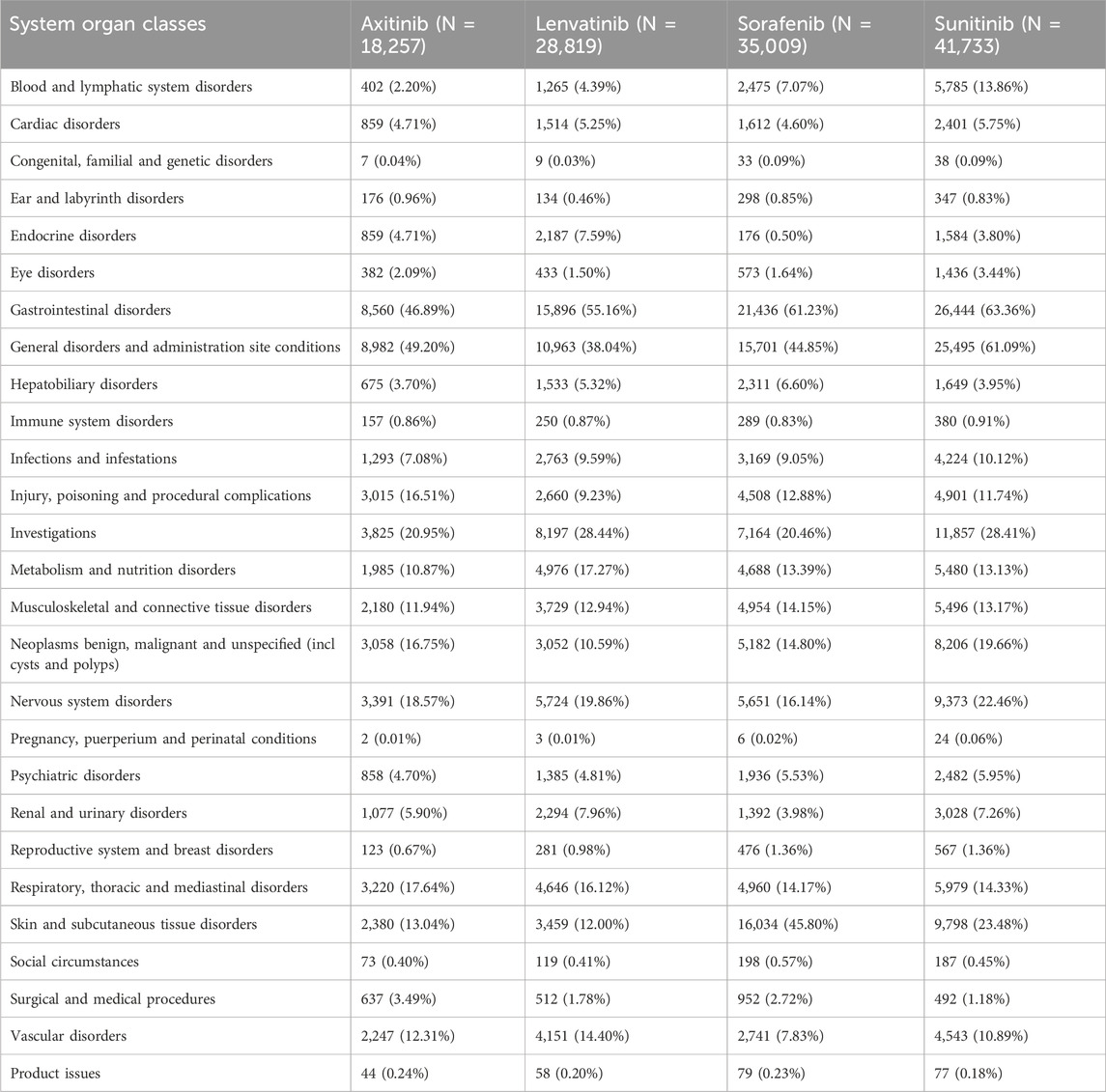
Table 3 presents the reporting frequencies of 27 SOCs associated with four MTKIs. Axitinib exhibited elevated rates of adverse reactions in the categories of respiratory, thoracic, and mediastinal disorders, as well as in injury, poisoning, and procedural complications, when compared to the other three agents. Lenvatinib demonstrated higher adverse reaction rates in endocrine disorders, metabolic and nutritional disorders, renal and urinary disorders, and vascular disorders. Sorafenib showed increased rates of adverse reactions in hepatobiliary disorders and musculoskeletal and connective tissue disorders. Notably, the incidence of skin and subcutaneous tissue disorders was significantly higher for sorafenib than for the other agents. Sunitinib exhibited elevated adverse reaction rates across multiple SOC categories, including blood and lymphatic system disorders, gastrointestinal disorders, general disorders and administration site conditions, benign, malignant, and unspecified neoplasms (including cysts and polyps), nervous system disorders, and psychiatric disorders. Furthermore, the rates of ADRs exceeding 10% in the SOC were 11 for axitinib, 10 for lenvatinib, 10 for sorafenib, and 13 for sunitinib.
Disproportionality analysis based on gastrointestinal disorders
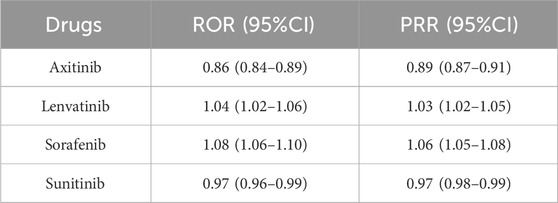
By observing and comparing the SOC distribution of four MTKIs, we found that these MTKIs exhibited the highest reported rates of adverse reactions under the categories of gastrointestinal disorders. To further compare these four medications, we conducted a disproportionate analysis using the ROR and PRR methods. Table 4 presents the results of this analysis, indicating the following ROR values for the four drugs: axitinib: 0.86 (0.84–0.89), lenvatinib: 1.04 (1.02–1.06), sorafenib: 1.08 (1.06–1.10), and sunitinib: 0.97 (0.96–0.99). Additionally, the PRR values for the four drugs were as follows: axitinib: 0.89 (0.87–0.91), lenvatinib: 1.03 (1.02–1.05), sorafenib: 1.06 (1.05–1.08), and sunitinib: 0.97 (0.98–0.99). These findings suggest that among the four MTKIs, axitinib had the lowest reported proportion of gastrointestinal disorders, whereas sorafenib exhibited a slightly higher reported proportion in comparison to the other drugs.
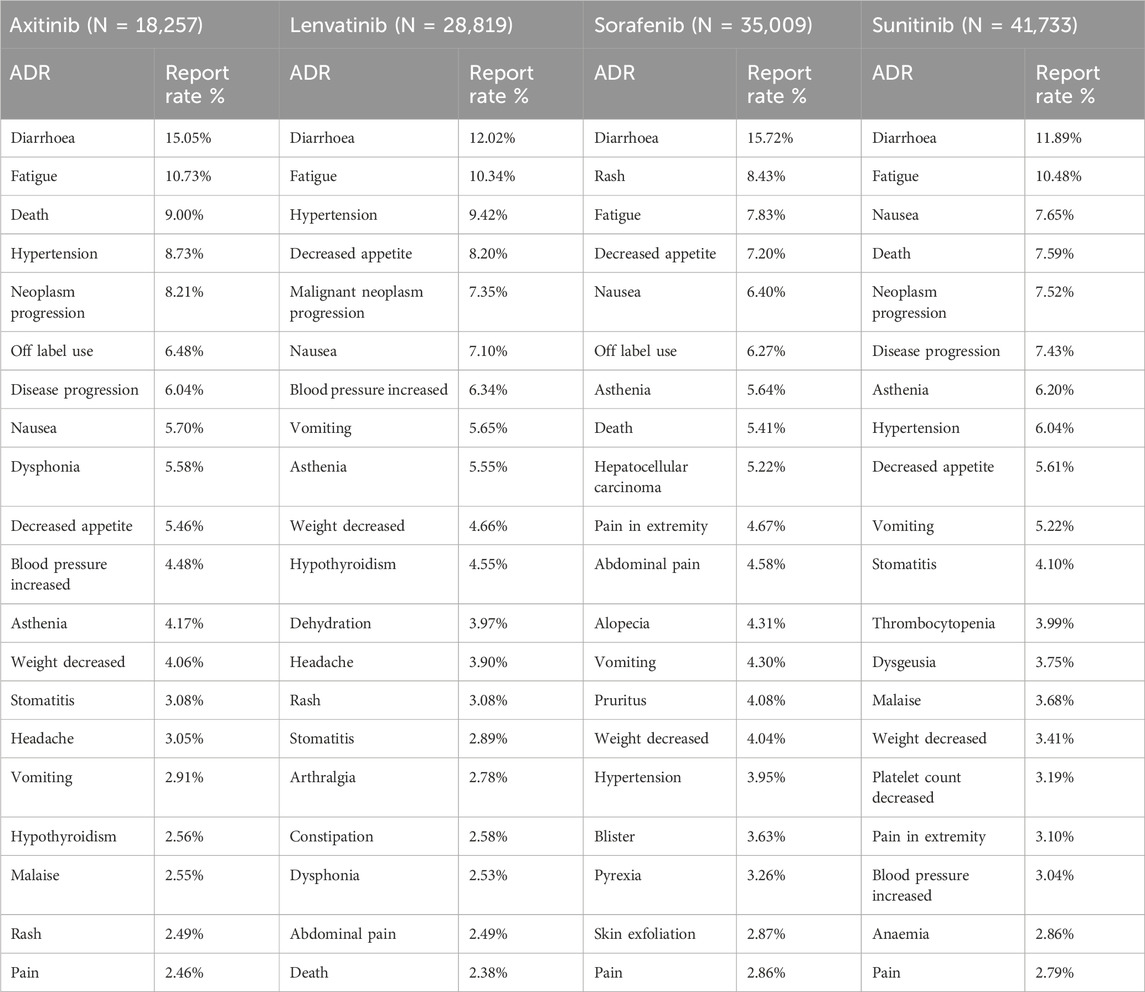
Most common ADRs of four MTKIs
Table 5 presents the 20 most frequently reported ADRs associated with the four MTKIs. The manifestations listed are preferred terms categorized within the SOC. Common ADRs for the four MTKIs include diarrhea, fatigue, death, hypertension, nausea, asthenia, weight loss, and vomiting. Among axitinib and sunitinib, death and disease progression are among the most frequently reported ADRs. Additionally, dysphonia and hypothyroidism warrant particular attention for both axitinib and lenvatinib. Furthermore, lenvatinib is associated with specific ADRs, including dehydration, arthralgia, and constipation. The reporting rates of rash and hepatocellular carcinoma for sorafenib are significantly higher than those for the other drugs. Sunitinib exhibits more pronounced hematological toxicity, primarily manifesting as thrombocytopenia, and may also lead to dysgeusia.
Serious AEs of four MTKIs
Through WHO-VigiAccess, we can identify significant AEs associated with four MTKIs, including life-threatening occurrences, disabilities, and congenital malformations. The proportions of serious adverse reactions reported for axitinib, lenvatinib, sorafenib, and sunitinib were 9.74%, 3.02%, 6.63%, and 7.95%, respectively (Figure 1).
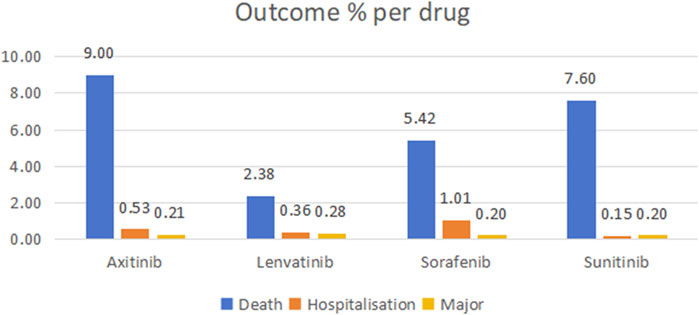
Figure 1. Outcomes for serious adverse events associated with four TIKs at the level of preferred terms (major events comprising life-threatening incidents, disabilities, and congenital anomalies).
The same and different points of common ADRs of four MTKIs
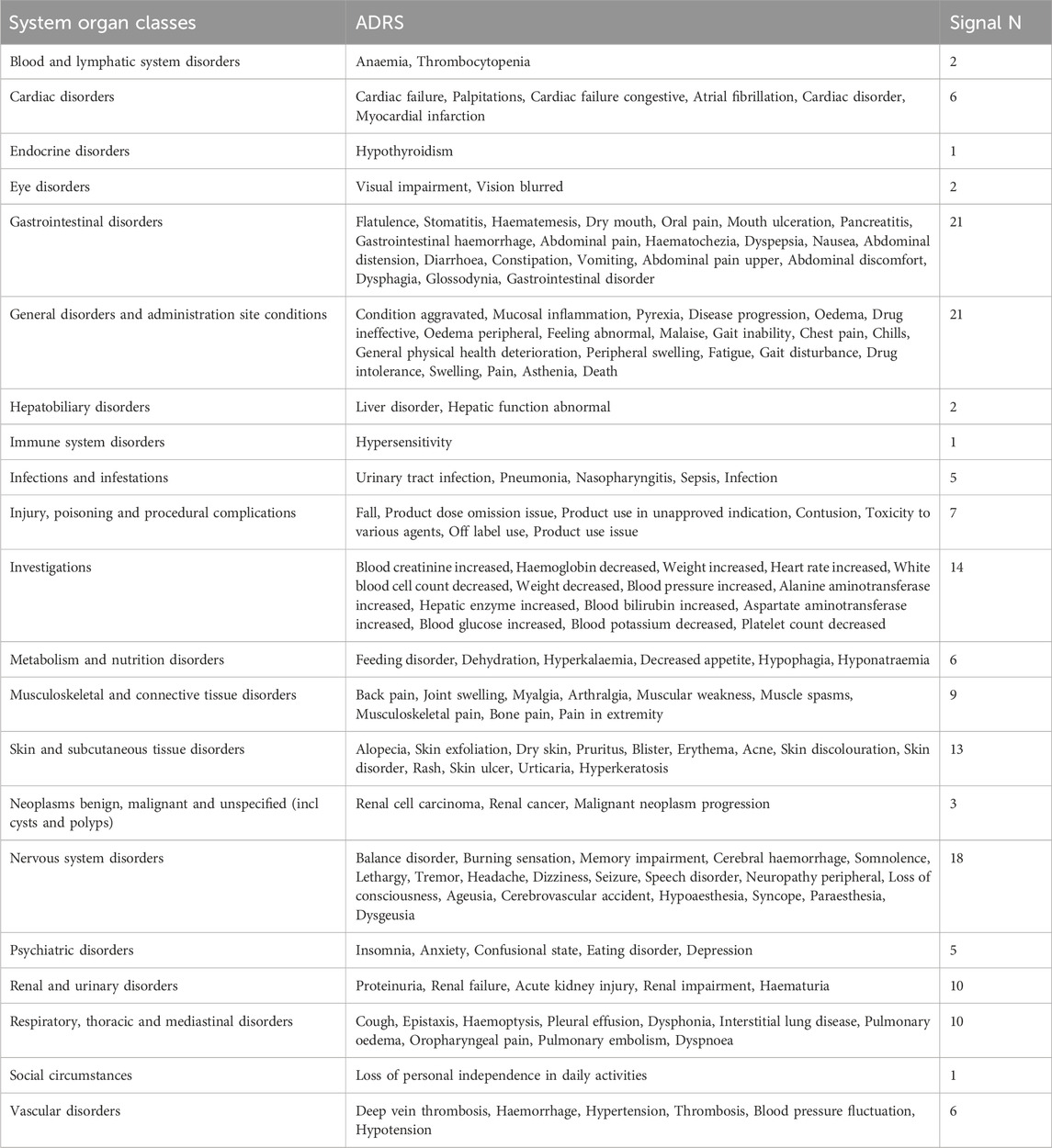
By examining the top 20 ADRs associated with each MTKI within the SOCs, a cumulative total of 163 identical signals was identified across the four MTKIs. All overlapping signals are detailed in Table 6. Gastrointestinal disorders and general disorders and administration site conditions emerged as the SOCs with the highest number of adverse signals, both ranking first in terms of frequency. For gastrointestinal disorders, the five most frequently reported reactions were flatulence, stomatitis, haematemesis, dry mouth, and oral pain. Meanwhile, for general disorders and administration site conditions, the top five reactions included condition aggravated, mucosal inflammation, pyrexia, disease progression, and oedema.
Notably, when comparing the top 20 ADRs for each MTKI drug in the SOCs, each MTKI exhibited distinct PTs of ADRs in the following categories: general disorders and administration site conditions, investigations, and vascular disorders (Table 7). The number of unique symptoms reported for axitinib, lenvatinib, sorafenib, and sunitinib was 24, 25, 35, and 22, respectively.
Discussion
In the treatment of RCC, axitinib, lenvatinib, sorafenib, and sunitinib have demonstrated significant clinical benefits. However, these MTKIs are also associated with a range of ADRs that can often be dose-limiting. By targeting not only the VEGFR but also other receptors such as the PDGFR and FGFR, these agents may induce hypertension, fatigue, gastrointestinal disturbances, and cardiovascular toxicities. Such ADRs can significantly affect patients’ quality of life, treatment adherence, and overall therapeutic outcomes (Shu et al., 2022; Zhang et al., 2024). Therefore, a systematic evaluation of their safety profiles is essential to optimize patient care and to address the ongoing challenge of rationally selecting the most appropriate MTKIs for RCC in clinical practice.
The Spontaneous Reporting System (SRS) serves an essential role in pharmacovigilance, facilitating the assessment of the safety of suspected AEs due to inherent limitations associated with clinical trials. Such limitations encompass stringent trial design, strict enrollment criteria, relatively small sample sizes, and short follow-up durations. Furthermore, data derived from clinical trials may not accurately represent real-world contexts, where variations in patient demographics and comorbidities can be significant. The SRS is crucial for detecting safety signals. Research related to the safety signals of numerous medications primarily relies on three major databases: the EudraVigilance Data Analysis System (EVDAS), the FDA Adverse Event Reporting System (FAERS), and WHO-VigiBase® (Vogel et al., 2020). In 2015, the WHO launched WHO-VigiAccess, a platform that grants public access to the data compiled in VigiBase®, which is the WHO’s comprehensive repository of documented potential adverse effects linked to medicinal products. By analyzing information from the WHO-VigiAccess database, one can reveal previously unidentified connections between medications and AEs, as well as validate certain established clinical correlations (Yamoah et al., 2022). This research intends to assess the post-market AEs associated with four MTKIs using the WHO-VigiAccess database.
According to data from WHO-VigiAccess, 48.26% of AEs related to these four MTKIs were reported from the Americas, with only 815 report of AEs originating from Africa. Prior research has highlighted a significant issue with the low reporting rates of AEs in both Africa and Oceania (Alawadhi et al., 2012; Gidudu et al., 2020). The incidence of RCC is higher in regions with elevated income levels, likely due to improved access to medical resources and the increased prevalence of imaging diagnostics. In South Africa, the limited understanding of biopharmaceuticals among healthcare workers, coupled with high costs and complex procurement procedures, further exacerbates the barriers to the utilization of these medications (Hajjaj-Hassouni et al., 2012; Martelli et al., 2017; Kvamme et al., 2020). The African region has been noted for having the lowest incidence of reported AEs, which could be linked to insufficient social mobilization, restricted access to AE reporting mechanisms, and low levels of information system coverage. The number of ADR reports from men (73,485) significantly exceeded that of women (43,281), yielding a male-to-female ratio of 1.70:1. When excluding reports lacking information on age, the demographic groups with the highest rates of reported incidents were primarily those aged 45–64 years. This is consistent with epidemiological findings that RCC incidence is approximately twice as high in men compared to women, likely due to differences in sex hormones, gender-specific tumor microenvironments, and lifestyle factors. RCC is predominantly diagnosed in individuals aged 50–80 years (Capitanio et al., 2019). When analyzing the adverse reactions associated with these four MTKIs across different age groups, the lack of age data for 30,322 cases (24.49%) will inevitably impact the accuracy of our conclusions.
An AE reporting rate of ≥1% is typically regarded as common (Chen et al., 2019). The serious AEs associated with the four MTKIs—axitinib, lenvatinib, sorafenib, and sunitinib—include life-threatening events, disabilities, and congenital malformations. The mortality rates associated with these drugs are 9% for axitinib, 2.38% for lenvatinib, 5.42% for sorafenib, and 7.60% for sunitinib. Furthermore, sorafenib has a hospitalization rate of 1.01%. The most frequently reported ADRs for all four MTKIs include diarrhea, fatigue, death, hypertension, nausea, asthenia, weight loss, and vomiting. Notably, these four MTKIs exhibited the highest reported rates of adverse reactions within the gastrointestinal disorders category. An analysis of the ROR and PRR indicated that axitinib had the lowest reported proportion of gastrointestinal disorders, whereas sorafenib had a slightly higher reported proportion compared to the other drugs.
The adverse reaction most frequently encountered with the four MTKIs is diarrhea, which can significantly diminish treatment effectiveness and patient adherence, negatively impacting long-term outcomes for cancer patients, and in extreme cases, may even pose a threat to life (Keefe and Anthony, 2008). Diarrhea generally arises early in the treatment timeline, particularly during the initial month. The intensity of this condition is closely tied to the medication type and dosage. MTKIs can disrupt the blood supply to the intestinal lining by blocking the VEGFR, resulting in ischemia and hypoxia of the intestinal mucosa, which may trigger diarrhea. Additionally, patients on MTKIs therapy often develop submucosal fat accumulation in the gastrointestinal region, potentially linked to intestinal lymphangiectasia, which can exacerbate malabsorption and diarrhea (Liu et al., 2024). Managing diarrhea primarily depends on empirical symptomatic treatments, and educating patients is pivotal. It is vital for healthcare providers to discuss the possible side effects of MTKIs therapy with patients before starting treatment and to evaluate whether diarrhea is caused by MTKIs therapy during treatment. For those experiencing diarrhea, it is usually necessary to reduce or pause MTKIs therapy, and hospitalization may be considered if required. After diarrhea subsides, decisions regarding the resumption of treatment and dosage adjustments should be made based on the patient’s clinical situation. Regarding therapeutic options, probiotics and fecal microbiota transplantation might be utilized to adjust the gut microbiota and ease diarrhea (Ianiro et al., 2020). It is crucial to distinguish MTKI-induced diarrhea from infectious diarrhea and chemotherapy-related diarrhea, managing each case individually according to the severity and related complications (Benson et al., 2004).
MTKIs have the potential to cause hypertension in the management of RCC. This is likely due to the suppression of nitric oxide and prostacyclin synthesis that occurs when MTKIs inhibit VEGFR, resulting in the contraction of vascular smooth muscle. Moreover, another possible reason for hypertension associated with MTKIs is capillary rarefaction. This condition involves decreased vascular density, which heightens vascular resistance and, in turn, raises blood pressure (Hasinoff and Patel, 2010). It is essential for hypertensive patients, especially older adults with elevated baseline blood pressure, to establish effective blood pressure management before starting MTKI treatment. Patients who experience hypertension during therapy should follow standard protocols for hypertension management. Should blood pressure rise to alarmingly high levels, it is recommended to modify the MTKI dosage or pause the treatment. Studies suggest that the likelihood of hypertension may correlate with the dose of MTKIs, and hypertension itself could act as an important biomarker related to the effectiveness of the treatment (Ravaud and Sire, 2009). For instance, research utilizing real-world data from Japan demonstrated that patients with hypertension receiving MTKIs for RCC experienced enhanced overall survival (OS) and PFS over a 24-week period (Akaza et al., 2015). Furthermore, hypertension that develops during therapy is acknowledged as a standalone biomarker for the efficacy of sunitinib (Donskov et al., 2015). While axitinib and lenvatinib tend to show a greater frequency of hypertension compared to sunitinib, they typically pose a lower risk of cardiovascular issues. A thorough cardiovascular risk evaluation should be carried out before beginning MTKIs therapy, alongside consistent monitoring of blood pressure and potential cardiotoxic effects during the initial treatment phase (Bæk Møller et al., 2019).
Hypothyroidism might be linked to the suppression of the VEGFR, leading to the deterioration of capillary networks within the thyroid and a subsequent decrease in blood flow to thyroid cells as a result of the blockade of VEGFR (Liao et al., 2021). In addition, it can negatively influence thyroid function by lowering iodine absorption and inhibiting the activity of thyroid peroxidase. The onset of hypothyroidism usually takes time to manifest and may continue even after treatment has stopped (Wu and Huang, 2020). Consequently, during the early phases of treatment, it is advisable to closely monitor thyroid function and to inform patients about associated symptoms to allow for quick detection and treatment of thyroid-related issues. The clinical studies have verified that hypothyroidism among patients with RCC undergoing therapies like sunitinib or sorafenib acts as a favorable indicator of treatment success (Schmidinger et al., 2011; Baldazzi et al., 2012). Moreover, the emergence of skin rash is seen as a sign of increased effectiveness, with its side effects thought to relate to cross-activity among different kinases (Liu et al., 2013; Massey et al., 2015). Research has indicated that thoughtfully adjusting the medication dosage can substantially reduce adverse reactions while preserving therapeutic effectiveness. MTKIs hinder angiogenesis and disrupt the Wnt/β-catenin signaling pathway, affecting the differentiation of oral mucosal epithelial cells, preventing the regeneration of taste bud cells, and impairing nerve repair, which may result in conditions like stomatitiss, dry mouth, and dysgeusia (Boers-Doets et al., 2012; Epstein and Barasch, 2010; Naik et al., 2009). The inhibition of VEGF, recognized as a neurotrophic factor, could disrupt the conduction of taste nerves, while the broad inhibitory actions of these agents might further compromise olfactory capabilities (Carmeliet et al., 2013). Sunitinib has shown a notable association with dysgeusia, although the direct causal links remain ambiguous in current research.
These MTKIs are transported to the liver and metabolized by CYP3A4. So, avoid combining them with drugs that affect CYP3A4 activity. These drugs may alter the plasma concentrations of MTKIs, impacting efficacy or increasing toxicity (Bæk Møller et al., 2019; Wang et al., 2016). Also, a comprehensive, multidisciplinary approach can better manage MTKI - related drug ADRs, enhancing patients’' treatment experience and prognosis. Implementing regular multidisciplinary meetings to share patient information and discuss complex cases, utilizing a shared electronic health records system for real-time access to the latest patient data, jointly developing and carrying out patient education programs to ensure patients fully understand their treatment and potential ADRs, and collaborating on research projects to assess the effectiveness of management strategies and promote improvements in clinical practice.
The WHO-VigiAccess database, which operates on a voluntary basis for AE reporting, presents several challenges that hinder its ability to deliver a complete and thorough count of AEs. The database may not contain all the necessary information regarding reported incidents, which underscores the importance of enhancing the transparency of reporting practices. By improving the clarity and accessibility of the data provided to the public, stakeholders can engage in more effective screening for potential connections between pharmaceuticals and adverse reactions. This would also help prevent misguidance that could arise from incomplete or unclear information. The reliance on a spontaneous reporting system carries significant inherent limitations, primarily due to various biases that can affect the reporting process. These include notoriety bias, wherein more well-known drugs may receive disproportionate attention, selection bias, which can skew the data towards certain demographics, and under-reporting, which typically results in significant gaps in data collection (Faillie, 2019).
Conclusion
Our research indicates that the adverse reaction reports submitted to the WHO and the FDA for these drugs highlight both common and specific adverse reactions. Clinicians must develop personalized treatment strategies that consider the adverse reactions associated with different drugs, as well as the unique circumstances of each patient, thereby encouraging the responsible use of these MTKIs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
LJL: Writing – original draft, Writing – review and editing. JB: Writing – original draft, Writing – review and editing. XW: Writing – original draft, Writing – review and editing. XZ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Hunan Natural Science Foundation Youth Foundation (2022JJ40388).
Acknowledgments
We sincerely appreciate the significant contributions made by all the authors towards this study, their invaluable efforts have been instrumental in its success. LJL serves as both the first author and the last corresponding author of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akaza, H., Naito, S., Ueno, N., Aoki, K., Houzawa, H., Pitman, L. S., et al. (2015). Real-world use of sunitinib in Japanese patients with advanced renal cell carcinoma: efficacy, safety and biomarker analyses in 1689 consecutive patients. Jpn. J. Clin. Oncol. 45 (6), 576–583. doi:10.1093/jjco/hyv045
Alawadhi, A., Alawneh, K., and Alzahrani, Z. A. (2012). The effect of neutralizing antibodies on the sustainable efficacy of biologic therapies: what’s in it for African and Middle Eastern rheumatologists. Clin. Rheumatol. 31 (9), 1281–1287. doi:10.1007/s10067-012-2040-2
Bahadoram, S., Davoodi, M., Hassanzadeh, S., Bahadoram, M., Barahman, M., and Mafakher, L. (2022). Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 39 (3), 2022-vol3.
Bæk Møller, N., Budolfsen, C., Grimm, D., Krüger, M., Infanger, M., Wehland, M., et al. (2019). Drug-induced hypertension caused by multikinase inhibitors (sorafenib, sunitinib, lenvatinib and axitinib) in renal cell carcinoma treatment. Int. J. Mol. Sci. 20 (19), 4712. doi:10.3390/ijms20194712
Baldazzi, V., Tassi, R., Lapini, A., Santomaggio, C., Carini, M., and Mazzanti, R. (2012). The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: a prospective single-center study. Urol. Oncol. 30 (5), 704–710. doi:10.1016/j.urolonc.2010.07.015
Benson, A. B., Ajani, J. A., Catalano, R. B., Engelking, C., Kornblau, S. M., Martenson, J. A., et al. (2004). Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 22 (14), 2918–2926. doi:10.1200/JCO.2004.04.132
Boers-Doets, C. B., Epstein, J. B., Raber-Durlacher, J. E., Ouwerkerk, J., Logan, R. M., Brakenhoff, J. A., et al. (2012). Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: a structured literature review. Oncol. 17 (1), 135–144. doi:10.1634/theoncologist.2011-0111
Capitanio, U., Bensalah, K., Bex, A., Boorjian, S. A., Bray, F., Coleman, J., et al. (2019). Epidemiology of renal cell carcinoma. Eur. Urol. 75 (1), 74–84. doi:10.1016/j.eururo.2018.08.036
Carmeliet, P., Ruiz de Almodovar, C., and Carmen, R. D. A. (2013). VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell. Mol. Life Sci. 70 (10), 1763–1778. doi:10.1007/s00018-013-1283-7
Chen, C., Borrego, M. E., Roberts, M. H., and Raisch, D. W. (2019). Comparison of post-marketing surveillance approaches regarding infections related to tumor necrosis factor inhibitors (TNFi’s) used in treatment of autoimmune diseases. Expert Opin. Drug Saf. 18 (8), 733–744. doi:10.1080/14740338.2019.1630063
Chen, Y. W., Wang, L., Panian, J., Dhanji, S., Derweesh, I., Rose, B., et al. (2023). Treatment landscape of renal cell carcinoma. Curr. Treat. Options Oncol. 24 (12), 1889–1916. doi:10.1007/s11864-023-01161-5
Donskov, F., Michaelson, M. D., Puzanov, I., Davis, M. P., Bjarnason, G. A., Motzer, R. J., et al. (2015). Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br. J. Cancer 113 (11), 1571–1580. doi:10.1038/bjc.2015.368
Epstein, J. B., and Barasch, A. (2010). Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol. 46 (2), 77–81. doi:10.1016/j.oraloncology.2009.11.008
Faillie, J. L. (2019). Case-non-case studies: principle, methods, bias and interpretation. Therapie 74 (2), 225–232. doi:10.1016/j.therap.2019.01.006
Faivre, S., Demetri, G., Sargent, W., and Raymond, E. (2007). Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 6 (9), 734–745. doi:10.1038/nrd2380
Gagliardi, A., Iaquinta, F. S., Grembiale, R. D., De Sarro, C., Fabiano, A., Fraija, D., et al. (2022). Real-world safety profile of biologics used in rheumatology: a six-year observational pharmacovigilance study in the calabria region. Pharmaceutics 14 (11), 2328. doi:10.3390/pharmaceutics14112328
Gidudu, J. F., Shaum, A., Dodoo, A., Bosomprah, S., Bonsu, G., Amponsa-Achiano, K., et al. (2020). Barriers to healthcare workers reporting adverse events following immunization in four regions of Ghana. Vaccine 38 (5), 1009–1014. doi:10.1016/j.vaccine.2019.11.050
Habarugira, J. M. V., and Figueras, A. (2021). Pharmacovigilance network as an additional tool for the surveillance of antimicrobial resistance. Pharmacoepidemiol. Drug Saf. 30 (8), 1123–1131. doi:10.1002/pds.5249
Hajjaj-Hassouni, N., Al-Badi, M., Al-Heresh, A., Al-Emadi, S., El Bawendi, A., El Garf, A., et al. (2012). The practical value of biologics registries in Africa and Middle East: challenges and opportunities. Clin. Rheumatol. 31 (3), 407–416. doi:10.1007/s10067-011-1918-8
Hasinoff, B. B., and Patel, D. (2010). Mechanisms of myocyte cytotoxicity induced by the multikinase inhibitor sorafenib. Cardiovasc. Toxicol. 10 (1), 1–8. doi:10.1007/s12012-009-9056-0
Hsieh, J. J., Purdue, M. P., Signoretti, S., Swanton, C., Albiges, L., Schmidinger, M., et al. (2017). Renal cell carcinoma. Nat. Rev. Dis. Prim. 3, 17009. doi:10.1038/nrdp.2017.9
Ianiro, G., Rossi, E., Thomas, A. M., Schinzari, G., Masucci, L., Quaranta, G., et al. (2020). Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat. Commun. 11 (1), 4333. doi:10.1038/s41467-020-18127-y
Keefe, D., and Anthony, L. (2008). Tyrosine kinase inhibitors and gut toxicity: a new era in supportive care. Curr. Opin. Support. Palliat. Care 2 (1), 19–21. doi:10.1097/SPC.0b013e3282f5273f
Kvamme, M. K., Lie, E., Uhlig, T., Moger, T. A., Kvien, T. K., and Kristiansen, I. S. (2020). Cost-effectiveness of TNF inhibitors vs synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a Markov model study based on two longitudinal observational studies. Rheumatol. Oxf. Engl. 59 (4), 917. doi:10.1093/rheumatology/kez609
Liao, X., Liu, Z., and Song, H. (2021). Thyroid dysfunction related to vascular endothelial growth factor receptor tyrosine kinase inhibitors: a real-world study based on FAERS. J. Clin. Pharm. Ther. 46 (5), 1418–1425. doi:10.1111/jcpt.13472
Liu, H. B., Wu, Y., Lv, T. F., Yao, Y. W., Xiao, Y. Y., Yuan, D. M., et al. (2013). Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PloS One 8 (1), e55128. doi:10.1371/journal.pone.0055128
Liu, J., Yan, S., Du, J., Teng, L., Yang, R., Xu, P., et al. (2024). Mechanism and treatment of diarrhea associated with tyrosine kinase inhibitors. Heliyon 10 (6), e27531. doi:10.1016/j.heliyon.2024.e27531
Martelli, L., Olivera, P., Roblin, X., Attar, A., and Peyrin-Biroulet, L. (2017). Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J. Gastroenterol. 52 (1), 19–25. doi:10.1007/s00535-016-1266-1
Massey, P. R., Okman, J. S., Wilkerson, J., and Cowen, E. W. (2015). Tyrosine kinase inhibitors directed against the vascular endothelial growth factor receptor (VEGFR) have distinct cutaneous toxicity profiles: a meta-analysis and review of the literature. Support. Care Cancer 23 (6), 1827–1835. doi:10.1007/s00520-014-2520-9
Montastruc, J. L., Sommet, A., Bagheri, H., and Lapeyre-Mestre, M. (2011). Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br. J. Clin. Pharmacol. 72 (6), 905–908. doi:10.1111/j.1365-2125.2011.04037.x
Moran, M., Nickens, D., Adcock, K., Bennetts, M., Desscan, A., Charnley, N., et al. (2019). Sunitinib for metastatic renal cell carcinoma: a systematic review and meta-analysis of real-world and clinical trials data. Target. Oncol. 14 (4), 405–416. doi:10.1007/s11523-019-00653-5
Motzer, R. J., Hutson, T. E., Glen, H., Michaelson, M. D., Molina, A., Eisen, T., et al. (2015). Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 16 (15), 1473–1482. doi:10.1016/S1470-2045(15)00290-9
Naik, S., Dothager, R. S., Marasa, J., Lewis, C. L., and Piwnica-Worms, D. (2009). Vascular endothelial growth factor receptor-1 is synthetic lethal to aberrant {beta}-Catenin activation in colon cancer. Clin. Cancer Res. 15 (24), 7529–7537. doi:10.1158/1078-0432.CCR-09-0336
Ravaud, A., and Sire, M. (2009). Arterial hypertension and clinical benefit of sunitinib, sorafenib and bevacizumab in first and second-line treatment of metastatic renal cell cancer. Ann. Oncol. 20 (5), 966–967. doi:10.1093/annonc/mdp201
Romero, D. (2019). Axitinib-ICIs boost the RCC armamentarium. Nat. Rev. Clin. Oncol. 16 (4), 207. doi:10.1038/s41571-019-0193-5
Sato, Y., Yoshizato, T., Shiraishi, Y., Maekawa, S., Okuno, Y., Kamura, T., et al. (2013). Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 45 (8), 860–867. doi:10.1038/ng.2699
Schmidinger, M., Vogl, U. M., Bojic, M., Lamm, W., Heinzl, H., Haitel, A., et al. (2011). Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer 117 (3), 534–544. doi:10.1002/cncr.25422
Schmidt, D., Rodat, T., Heintze, L., Weber, J., Horbert, R., Girreser, U., et al. (2018). Axitinib: a photoswitchable approved tyrosine kinase inhibitor. ChemMedChem 13 (22), 2415–2426. doi:10.1002/cmdc.201800531
Shu, Y., Ding, Y., Dai, B., and Zhang, Q. (2022). A real-world pharmacovigilance study of axitinib: data mining of the public version of FDA adverse event reporting system. Expert Opin. Drug Saf. 21 (4), 563–572. doi:10.1080/14740338.2022.2016696
Sultana, J., Scondotto, G., Cutroneo, P. M., Morgante, F., and Trifirò, G. (2020). Intravitreal anti-VEGF drugs and signals of dementia and Parkinson-like events: analysis of the VigiBase database of spontaneous reports. Front. Pharmacol. 11, 315. doi:10.3389/fphar.2020.00315
Vogel, U., van Stekelenborg, J., Dreyfus, B., Garg, A., Habib, M., Hosain, R., et al. (2020). Investigating overlap in signals from EVDAS, FAERS, and VigiBase(®). Drug Saf. 43 (4), 351–362. doi:10.1007/s40264-019-00899-y
Wang, X., Zhang, X., Huang, X., Li, Y., Wu, M., and Liu, J. (2016). The drug-drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4. Xenobiotica 46 (7), 651–658. doi:10.3109/00498254.2015.1109160
Watson, S., Chandler, R. E., Taavola, H., Härmark, L., Grundmark, B., Zekarias, A., et al. (2018). Safety concerns reported by patients identified in a collaborative signal detection workshop using VigiBase: results and reflections from lareb and uppsala monitoring centre. Drug Saf. 41 (2), 203–212. doi:10.1007/s40264-017-0594-2
Wilhelm, S., Carter, C., Lynch, M., Lowinger, T., Dumas, J., Smith, R. A., et al. (2006). Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 5 (10), 835–844. doi:10.1038/nrd2130
Wu, J., and Huang, H. (2020). Acquired hypothyroidism in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Drug Des. Dev. Ther. 14, 3977–3982. doi:10.2147/DDDT.S270210
Yamoah, P., Mensah, K. B., Attakorah, J., Padayachee, N., Oosthuizen, F., and Bangalee, V. (2022). Adverse events following immunization associated with coronavirus disease 2019 (COVID-19) vaccines: a descriptive analysis from VigiAccess. Hum. Vaccin. Immunother. 18 (6), 2109365. doi:10.1080/21645515.2022.2109365
Keywords: MTKIs, renal cell carcinoma, adverse drug reactions, WHO-Vigiaccess, ROR
Citation: Li L, Bai J, Wen X and Zeng X (2025) Adverse reactions of four multi-targeted tyrosine kinase inhibitors: a descriptive analysis of the WHO-VigiAccess database. Front. Pharmacol. 16:1585862. doi: 10.3389/fphar.2025.1585862
Received: 01 March 2025; Accepted: 04 April 2025;
Published: 22 April 2025.
Edited by:
Lei Yin, Shanghai Jiaotong University School of Medicine, ChinaReviewed by:
Prabhakar Mujagond, Southern Medical University, ChinaYi Heng Li, Longgang Central Hospital, China
Copyright © 2025 Li, Bai, Wen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefan Zeng, enhmY3FtdUAxNjMuY29t; Lijun Li, MTUyMDA1MDg2MzJAMTYzLmNvbQ==
 Lijun Li
Lijun Li Jiayu Bai
Jiayu Bai Xuelong Wen5
Xuelong Wen5 Xuefan Zeng
Xuefan Zeng