- 1The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, China
- 2School of Medical Technology and Information Engineering, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Hospital of Azha Town, Naqu, Xizang, China
Acute pancreatitis (AP) is a severe inflammatory disorder of the pancreas, characterized by high morbidity and mortality rates. Despite significant advancements in understanding the pathophysiological mechanisms of AP, current treatment options still face considerable limitations. Recent studies have underscored the therapeutic potential of quercetin, a natural flavonoid, due to its potent antioxidant, anti-inflammatory, and immunomodulatory properties, positioning it as a promising therapeutic candidate for AP. This review explores the effects of quercetin on AP, highlighting its antioxidant activities, its role in immune modulation, and its protective effects on pancreatic tissue. Furthermore, it examines quercetin’s multi-target mechanisms and its advantages over conventional therapies, such as N-acetylcysteine and corticosteroids. Although preliminary studies suggest that quercetin can alleviate inflammation and oxidative stress in AP, clinical evidence remains limited. One of the main challenges for quercetin’s clinical application is its low bioavailability. Future research should focus on strategies to enhance its bioavailability and on conducting large-scale randomized controlled trials to more comprehensively assess its efficacy and safety in the treatment of AP.
1 Introduction
AP is a severe inflammatory condition of the pancreas, characterized by the rapid onset of inflammation, primarily driven by the premature activation of digestive enzymes within the pancreas. This condition can vary from mild, self-limiting episodes to severe forms that may progress to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), ultimately leading to high morbidity and mortality rates (Windsor and McClave, 2023; Zhang Haoyu et al., 2024). AP can lead to severe systemic complications, including acute lung injury (ALI) and intestinal damage, both of which are life-threatening. These complications arise from inflammatory crosstalk between the pancreas, lungs, and intestines (Zhang Zhihang et al., 2024; Swetha et al., 2025; Shen et al., 2025). Figure 1 illustrates this intricate interaction, known as the ‘pancreas-intestine-lung’ pathway, which encompasses intestinal barrier disruption, bacterial translocation, and the widespread activation of inflammatory cascades. This pathway visually demonstrates how inflammation in the pancreas can trigger systemic complications and affect multiple organs. The pathophysiology of AP is strongly influenced by oxidative stress, which results from an imbalance between free radicals and antioxidant defenses, leading to cellular damage. These mechanisms drive the progression from localized pancreatic injury to widespread systemic inflammation and MODS (Zhang Chuan et al., 2024; Li et al., 2021). Although therapeutic strategies such as fluid resuscitation, nutritional support, and pain management are available, their effectiveness remains limited, and no specific pharmacological intervention exists that can halt or reverse disease progression (Tenner et al., 2024),.
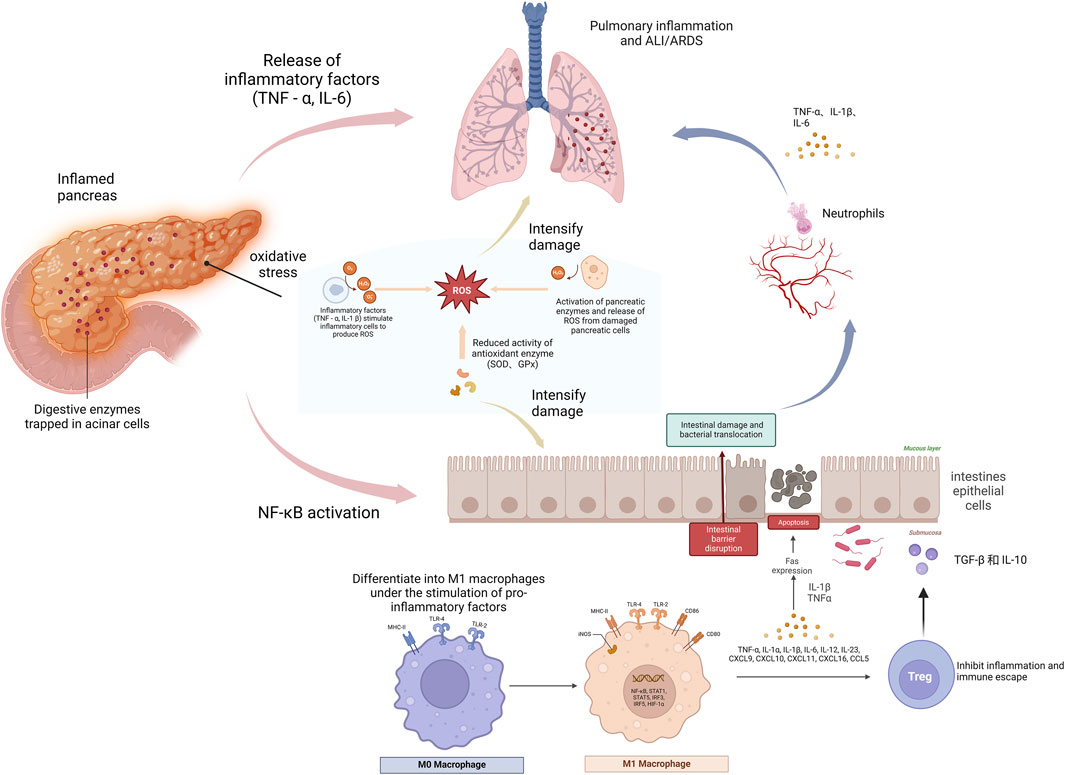
Figure 1. The role of pancreatic intestinal lung pathway in acute pancreatitis. This figure illustrates the progression of acute pancreatitis (AP), beginning with localized inflammation in the pancreas. Digestive enzymes become trapped in acinar cells, leading to oxidative stress, which subsequently triggers the release of inflammatory molecules such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), exacerbating tissue damage. As inflammation spreads, bacteria and endotoxins are transported from the pancreas into the bloodstream and lungs, further amplifying the damage and potentially leading to complications such as acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Moreover, inflammatory mediators stimulate macrophages to differentiate into the pro-inflammatory M1 phenotype, which exacerbates the inflammatory response. The figure underscores the interconnectedness of the pancreas, intestines, and lungs in the pathogenesis of AP, illustrating how the systemic inflammatory response unfolds.
Quercetin, a naturally occurring flavonoid found in various fruits and vegetables, has attracted considerable attention as a potential therapeutic agent for AP due to its potent antioxidant, anti-inflammatory, and immunomodulatory properties (Li Ke et al., 2024). Structurally, quercetin belongs to the flavonol class of flavonoids, distinguished by hydroxyl groups (-OH) attached to the aromatic rings. These hydroxyl groups are crucial to quercetin’s ability to scavenge free radicals and chelate metal ions, both of which are key mechanisms in mitigating oxidative stress (Ungurianu et al., 2024; Li X. et al., 2024). Through these actions, quercetin neutralizes reactive oxygen species (ROS) and chelates metal ions (Kashyap et al., 2019; Chiang et al., 2023), which significantly reduce oxidative damage, a key pathological factor in AP. By scavenging ROS, quercetin prevents cellular damage and tissue inflammation, thereby helping to attenuate disease progression. In addition to its antioxidant effects, quercetin also exhibits significant anti-inflammatory properties (Beken et al., 2020; Goyal and Agrawal, 2022). It achieves this by inhibiting key inflammatory mediators such as nuclear factor-kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β), all of which play central roles in the inflammatory pathways driving the pathogenesis of AP (Wang et al., 2023; Nagy-Pénzes et al., 2022). Beyond its anti-inflammatory effects, quercetin also regulates immune cell functions. It inhibits NF-κB, reducing the expression of pro-inflammatory cytokines, while modulating TNF-α and IL-1β to help reduce systemic inflammation (Sendler et al., 2020). Additionally, quercetin influences immune cell polarization by promoting the anti-inflammatory M2 phenotype in macrophages and modulating the balance between pro-inflammatory Th17 cells and regulatory T cells (Tregs). These combined actions contribute significantly to reducing the inflammatory burden associated with AP, thereby preventing the development of complications such as ALI and intestinal damage (Ge et al., 2020). Thus, quercetin shows promise not only as a potential adjunctive treatment to reduce the severity of AP but also as a strategy to prevent complications commonly associated with the disease (Liu D. et al., 2022). However, while the preclinical evidence is compelling, further research is needed to investigate quercetin’s bioavailability, long-term effects, and optimal dosing protocols (Li et al., 2022). Such research will be critical in determining the clinical applicability of quercetin as a therapeutic agent for AP (Soofiyani et al., 2021; Tang et al., 2020).
This review aims to provide a comprehensive overview of the therapeutic potential of quercetin in the context of AP. By summarizing current research, we seek to elucidate the mechanisms through which quercetin modulates oxidative stress and inflammation in AP, while also discussing the challenges and opportunities in translating these findings into clinical practice. Additionally, we will identify gaps in the existing knowledge and propose future directions for research in this promising field.
2 Oxidative inflammatory and immune mechanisms underlying acute pancreatitis
2.1 Role of oxidative stress in acute pancreatitis
Oxidative stress is pivotal in the pathogenesis of AP, primarily through the induction of cellular damage and the amplification of the inflammatory response (Chen et al., 2024; Sheibani et al., 2024). The excessive generation of ROS (ROS) in AP is a critical driver of lipid peroxidation, which in turn compromises the integrity of cellular membranes and organelles (Wang et al., 2025; Li Jin-Wei et al., 2024). As lipid peroxidation escalates, it triggers cell necrosis and stimulates the release of pro-inflammatory cytokines, such as TNF-α and interleukin-6 (IL-6), thereby intensifying the inflammatory response (Li Jin-Wei et al., 2024).Furthermore, the depletion of endogenous antioxidants, including superoxide dismutase (SOD) and glutathione (GSH), exacerbates this pathological process. SOD, an essential antioxidant enzyme, facilitates the dismutation of superoxide radicals (O2−) into hydrogen peroxide (H2O2), which is subsequently decomposed by enzymes such as catalase (CAT) and GSH peroxidase, thus mitigating the deleterious effects of ROS. In the context of AP, the depletion of SOD impairs the organism’s capacity to neutralize ROS, thereby accelerating oxidative damage and exacerbating pancreatic tissue injury. Typically, these antioxidants neutralize ROS; however, their insufficient levels in AP result in ROS accumulation, thereby exacerbating oxidative damage. Cai et al. (2024) demonstrated that excessive ROS production directly causes pancreatic tissue damage and exacerbates the pathological process in animal models of AP. Their study highlights the critical role of ROS in AP, showing that ROS accumulation leads to cellular damage and amplifies the inflammatory response by activating the NF-κB pathway and inhibiting the p62-Keap1-Nrf2 pathway. Furthermore, oxidative stress induced by excessive ROS not only contributes to cellular injury but also initiates lipid peroxidation, a process that plays a key role in the inflammatory response during AP. Recent studies (Kong et al., 2021; Yang et al., 2023) have elucidated that lipid peroxidation not only compromises membrane integrity but also triggers the release of damage-associated molecular patterns (DAMPs), which serve as potent endogenous signals for immune activation. These DAMPs promote immune cell infiltration and intensify both local pancreatic and systemic inflammation. In a similar vein, Niu et al. (2024) demonstrated that Emodin confers protection against severe AP-associated lung injury by activating the Nrf2/HO-1/GPX4 signaling pathway and inhibiting ferroptosis, underscoring the pivotal role of oxidative stress in mediating not only pancreatic injury but also dysfunction in distant organs. Furthermore, Shen et al. (2025) reported that hyperlipidemia exacerbates the severity of AP through the overproduction of ROS and activation of the p38 MAPK pathway, further highlighting the pathological intersection between oxidative stress and metabolic dysregulation.
These findings collectively illustrate that oxidative stress is not merely a downstream consequence of inflammation, but rather a central pathological driver that orchestrates both local pancreatic injury and systemic complications in AP. Notably, oxidative stress and inflammation appear to be tightly interconnected, forming a self-perpetuating vicious cycle in which ROS promotes the expression of pro-inflammatory cytokines, while inflammatory mediators further enhance ROS production. This bidirectional feedback loop suggests that oxidative stress may serve as a therapeutic “hub” capable of influencing multiple downstream pathological events. Thus, oxidative stress is not just a contributing factor but a central axis in the pathophysiology of AP. Its role in driving lipid peroxidation, immune cell recruitment, and systemic inflammation makes it a compelling target for intervention. However, translating these insights into effective clinical strategies requires a nuanced understanding of the redox–inflammation interface and a shift from generalized antioxidant therapy toward more personalized, mechanism-based approaches.
2.2 Immune-regulatory mechanisms in acute pancreatitis
2.2.1 Inflammatory pathways in acute pancreatitis
AP triggers an inflammatory response through the activation of multiple signaling pathways, primarily driven by the release of key cytokines such as TNF-α, IL-6, and IL-1β (Qiu et al., 2025; Wu et al., 2021) These cytokines initiate a cascade of inflammatory events, leading to pancreatic tissue damage and dysfunction (Sheng M. et al., 2021; Jian et al., 2024). Among the various signaling pathways involved, the NF-κB and mitogen-activated protein kinase (MAPK) pathways play particularly critical roles in amplifying inflammation and exacerbating tissue injury. The MAPK pathway, which includes ERK (Extracellular Signal-Regulated Kinase), JNK(c-Jun N-terminal Kinase), and p38 subfamilies, is a key regulator of cellular responses to stress and inflammatory stimuli. Upon activation by cytokines or oxidative stress, MAPKs modulate the transcription of pro-inflammatory genes, thereby contributing to the sustained inflammatory milieu observed in AP (Shen Y. et al., 2022). demonstrated that NF-κB serves as a pivotal transcription factor that is activated in response to cytokines or ROS. Once activated, NF-κB upregulates the expression of multiple pro-inflammatory genes, thereby intensifying immune responses and worsening pancreatic injury. This finding underscores the essential role of NF-κB in the pathophysiology of AP. In parallel, Huang et al. (2023) highlighted that the MAPK pathway, which includes p38, ERK, and JNK, contributes to inflammation by activating pro-inflammatory signaling molecules, thereby further promoting cytokine release and cellular damage. Collectively, these studies emphasize the central roles of the NF-κB and MAPK pathways in the inflammatory processes of AP and suggest that targeting these pathways may represent an effective therapeutic approach. Building on this understanding, additional research has explored potential therapeutic interventions aimed at suppressing these pathwaysIn summary, these studies not only elucidate the complexity of inflammatory signaling pathways in AP but also underscore the critical importance of targeting multiple pathways for effective therapeutic intervention. Notably, quercetin’s ability to modulate key inflammatory pathways further reinforces its potential as a promising therapeutic agent for AP.
2.2.2 Macrophage polarization and pyroptosis in acute pancreatitis
Macrophages are classified into two main types based on their polarization: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages (Wang and Mei, 2023; KhaterCA et al., 2024). M1 macrophages are key drivers of inflammation, characterized by the release of cytokines such as TNF-α and IL-6, which amplify the inflammatory cascade and contribute to tissue damage. In contrast, M2 macrophages secrete anti-inflammatory cytokines, including IL-10, and play a crucial role in tissue repair and the resolution of inflammation (He et al., 2025; Sahana et al., 2022). The dynamic balance between M1 and M2 macrophages is critical in determining the extent of inflammation in AP. Ryu et al. (Liu et al., 2024) examined the role of macrophages in pancreatic diseases, with a focus on how macrophage polarization impacts these conditions. Their research demonstrated that macrophages are central to the pathogenesis of pancreatic diseases, with M1 macrophage polarization being linked to exacerbated inflammation in pancreatitis. Moreover, Liu et al. (2024) used ELAV1 gene knockout mice to develop an AP model, revealing that ELAV1 upregulates TRAF6 expression, which enhances TLR signaling and promotes M1 macrophage polarization, thereby worsening the course of AP. In addition to macrophage polarization, macrophage pyroptosis also plays a pivotal role in AP. Bansod et al. (2020), and Peng et al. (2024) showed that the cGAS-STING pathway, through activation of IRF7/IRF3, promotes pyroptosis in macrophages, which aggravates lung injury caused by AP. This finding underscores the important role of macrophage pyroptosis in amplifying the inflammatory response. Furthermore, Sheng Bo et al. (2021) investigated the impact of exosomal miR-181a-5p derived from bone marrow-derived mesenchymal stem cells (BMSCs) on macrophage polarization. They found that upregulation of miR-181a-5p promoted M2 macrophage polarization, leading to a significant reduction in pancreatic damage and inflammation, thereby suggesting that M2 macrophages play a crucial protective role in resolving inflammation. In conclusion, macrophage polarization and pyroptosis play critical roles in the progression of AP. Specifically, the heightened polarization of M1 macrophages and the potential protective effects of M2 macrophages in inflammation resolution and tissue repair underscore the importance of modulating these processes. Targeting macrophage polarization and pyroptosis presents promising therapeutic opportunities for AP and may contribute to the development of more targeted, immune-based treatments.
2.2.3 T Cell subset alterations in acute pancreatitis
In AP, T cell dysregulation often exacerbates disease progression, with Th17 cells playing a pivotal role in this process. Boodhoo et al. (2022) examined the involvement of T cells in AP and identified Th17 cells as critical mediators in its pathogenesis. Through the secretion of pro-inflammatory cytokines su as IL-17, Th17 cells promote the release of additional inflammatory mediators, thereby contributing to pancreatic tissue damage. This suggests tha excessive activation of Th17 cells significantly amplifies both inflammation and tissue injury in AP. Additionally, a reduction in Treg cell funcion is recognized as a key factor driving immune dysregulation in AP. The depletion of Treg cells results in impaired immune regulation, exacerating the inflammatory response and further aggravating tissue damage. Lethen et al. (2024) demonstrated that the Th17/Treg imbalance i AP patients correlates with disease severity, indicating that dysregulation of these T cell subsets may be a critical determinant of poor clinicaoutcomes. Beyond alterations in T cell subsets, T cell receptor (TCR) rearrangement also plays an integral role in the immune response during A. Wang et al. (Ryu and Lee, 2024) highlighted that changes in the TCR repertoire of peripheral blood T cells are significantly associated witthe progression of pancreatic disease, suggesting that TCR repertoire alterations are an inherent part of the immune response in AP. In conclusin, the functional perturbations of T cells, particularly the Th17/Treg imbalance, are central to the initiation and perpetuation of inflammatio in AP. Modulating T cell subset ratios and restoring the Th17/Treg balance may offer a promising therapeutic strategy for AP. Furthermore, alteratons in the TCR repertoire could serve as novel biomarkers for immune monitoring and disease prognosis. Thus, a comprehensive understandig of the mechanisms underlying T cell changes in AP is essential for the development of innovative immune-targeted therapies.
3 Therapeutic potential of quercetin in acute pancreatitis
3.1 Qurcetin’s role in attenuating oxidative stress
Quercein, a natural flavonoid compound, exhibits significant antioxidant properties, effectively inhibiting ROS and lipid peroxidation, while promotingthe synthesis of intracellular GSH (GSH). Zhou et al. (2025); Hossein et al. (2024) observed in their study that quercetin significanty inhibits ROS production, demonstrating preventive effects on endothelial dysfunction in chronic kidney disease. Hossein et al. (2024) urther confirmed in cell experiments that quercetin treatment of oxidative stress-induced endothelial cells significantly reduced ROS levels, increased SH levels, and decreased cell apoptosis, highlighting quercetin’s prominent role in antioxidant defense. Additionally, several studies suggest tht quercetin can directly scavenge free radicals and enhance the activity of antioxidant enzymes in pancreatic tissues, thereby significantly reducing oidative stress. Faiza et al. (2024) found that in a cerulein-induced AP model, quercetin significantly reduced ROS productionand enhanced antioxidant enzyme activities, including SOD, CAT, and GSH peroxidase (GSH-Px). These findings further confirm quercetin’ potential in improving oxidative stress balance. Not only does quercetin reduce oxidative damage by scavenging free radicals, but it also upregulate the expression of endogenous antioxidant enzymes, helping restore oxidative balance in damaged pancreatic cells. Qi et al. (2022) repoted that quercetin upregulates the Nrf2 pathway, enhancing the cell’s antioxidant defense mechanisms. The Nrf2 pathway is crucial in the cellular resonse to oxidative stress, and by promoting Nrf2 activation, quercetin increases GSH synthesis, thereby improving the redox balance in pancreatic issues. This mechanism effectively mitigates oxidative damage in AP, further supporting the potential of quercetin as an antioxidant therapy, asdepicted in Figure 2. In conclusion, quercetin exhibits multifaceted potential in reducing oxidative stress by scavenging free radical pathways, and activating the Nrf2 pathway. These findings confirm its protective effect on pancreatic tissues and highlight its significance in systemic pathological responses, underscoring its promising therapeutic potential and providing a solid foundation for clinical application.
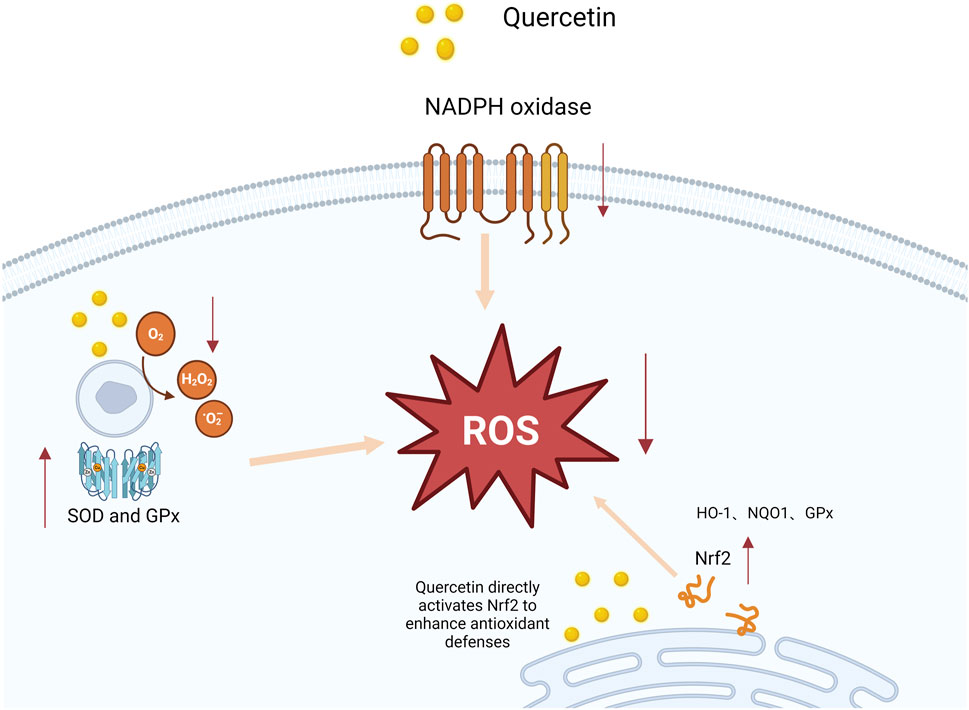
Figure 2. Mechanism of quercetin-induced Nrf2 activation and enhanced antioxidant defenses. This figure illustrates how quercetin interacts with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, resulting in an increase in reactive oxygen species (ROS). The elevated ROS levels activate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, which subsequently promotes the expression of antioxidant genes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx). As a result, the antioxidant response is potentiated, reducing oxidative stress and protecting cells from damage.
3.2 The immunoregulatory effects of quercetin
3.2.1 Quercetin’s suppression of inflammatory signaling
Quercetin has been extensively studied for its anti-inflammatory properties, particularly in the context of AP, where it alleviates the inflammatory response by inhibiting the NF-κB and MAPK signaling pathways. For example, Rezabakhsh et al. (2019) found that quercetin significantly reduced pancreatic edema and necrosis in animal models of pancreatitis by inhibiting the activation of both NF-κB and TLR4 pathways. Similarly, Liu Y. et al. (2022) demonstrated that quercetin significantly suppressed the activation of the NF-κB and MAPK pathways in AP by modulating miR-216b, targeting MAP2K6 and NEAT1, thereby further highlighting its potential therapeutic role in mitigating this condition. These findings are further corroborated by subsequent research, which continues to support quercetin’s efficacy in managing inflammation in AP. For example, Li H. et al. (2024) reported that quercetin regulates the TLR-mediated MAPK and Akt signaling pathways in hypercholesterolemic rats, leading to a significant reduction in inflammation. This result extends the anti-inflammatory effects of quercetin, suggesting its potential efficacy not only in pancreatitis but also in other inflammatory diseases involving these pathways. Additionally, Wang et al. (2022) further confirmed that quercetin alleviates kidney injury in pregnant mice with acute necrotizing pancreatitis by inhibiting the p38 MAPK pathway. The discovery highlights the crucial role of the MAPK pathway in driving inflammation and demonstrates that quercetin effectively mitigates pancreatic and renal damage by targeting this mechanism.These findings demonstrate the substantial therapeutic potential of quercetin in mitigating inflammatory responses through multi-pathway inhibition. A visual overview of the key inflammatory signaling pathways, such as NF-κB and MAPK, which are modulated by quercetin, is provided in Figure 3. Collectively, these studies support the clinical application of quercetin as a multi-target therapeutic agent, indicating its potential to mitigate AP and related inflammatory damage through the inhibition of the NF-κB and MAPK signaling pathways.
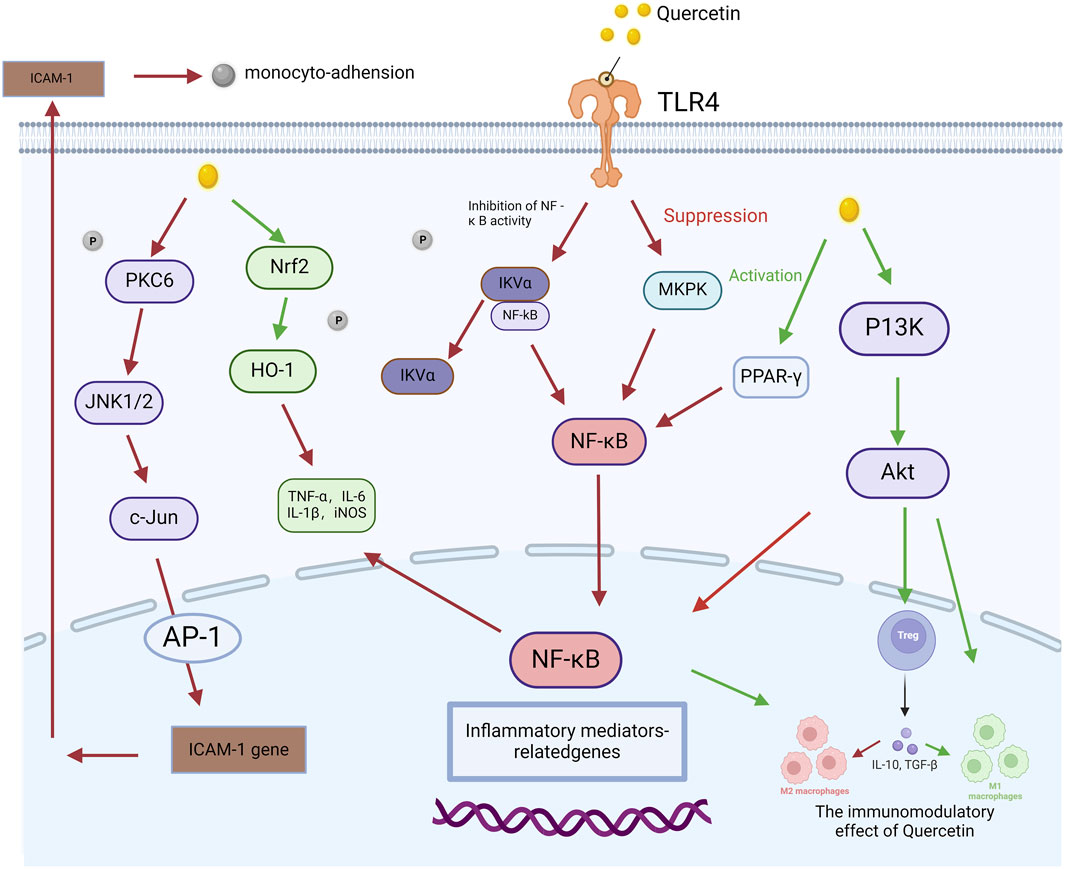
Figure 3. Quercetin-mediated signaling pathway in the regulation of acute pancreatitis. This figure illustrates how quercetin modulates the molecular pathways involved in AP. It begins by activating Toll-like receptor 4 (TLR4), initiating a cascade of signaling events involving molecules such as protein kinase C delta (PKCδ), c-Jun N-terminal kinase 1/2 (JNK1/2), and c-Jun. These molecules subsequently interact with nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1), influencing the production of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and inducible nitric oxide synthase (iNOS). Quercetin also attenuates nuclear factor-kappa B (NF-κB) activation by inhibiting inhibitor of kappa B-alpha (IκBα), leading to reduced expression of inflammation-related genes. Additionally, quercetin activates peroxisome proliferator-activated receptor gamma (PPAR-γ), which mediates anti-inflammatory effects. This figure highlights the key molecular processes through which quercetin mitigates inflammation in AP.
3.2.2 Modulation of macrophage polarization by quercetin
Quercetin has been shown to effectively modulate macrophage polarization by shifting the balance from the M1 to the M2 phenotype, thereby fostering an anti-inflammatory microenvironment. This modulation is achieved through its regulation of inflammatory signaling pathways and its ability to enhance the expression of M2 macrophage markers, such as CD206 and arginase-1, while simultaneously suppressing M1-associated cytokines, including TNF-α and IL-6 (Wang et al., 2022); Gao et al., 2024. For instance, (Guo et al., 2020) demonstrated that quercetin treatment significantly reduced the population of M1 macrophages and enhanced M2 macrophage activity in a cerulein-induced AP model, resulting in reduced tissue inflammation and accelerated healing. Additionally, recent studies have highlighted quercetin’sability to target key molecular pathways involved in macrophage polarization. Wang et al. (2021) reported that quercetin, as a major component of Xiang-lian Pill, activates the peroxisome proliferator-activated receptor gamma (PPAR-γ) pathway, tipping the balance of STAT1/PPARγ, and promoting the alternative activation of macrophages to favor M2 polarization. This study highlights quercetin’s role in modulating macrophage activity and reducing inflammation in related inflammatory conditions. Similarly, Sheng B. et al. (2021) found that quercetin inhibits NF-κB activation, reducing the pro-inflammatory cytokine production associated with M1 macrophages while promoting an anti-inflammatory milieu. By enhancing the M2 phenotype and reducing M1-driven inflammation, quercetin creates a favorable environment for resolving inflammation and facilitating tissue repair. These findings strongly support the therapeutic potential of quercetin as an immunomodulatory agent in AP. Future research should explore the translational implications of quercetin in clinical settings, particularly its integration into multi-target therapies for managing inflammatory and immune dysregulation in AP.
3.2.3 Modulation of T Cell subset balance in immune responses by quercetin
Currently, there is no direct evidence in the literature indicating that quercetin can treat AP by modulating the balance of T cell subsets (Bhaskar and Helen, 2016; Zhang et al., 2021). However, existing studies suggest that the anti-inflammatory mechanisms of quercetin are closely linked to the regulation of T cell subsets (Zhou et al., 2023; Lin et al., 2023). In a study by Hai-feng et al. (2023) and Yang et al. (2018) investigating collagen-induced arthritis, it was found that quercetin exerts its anti-inflammatory effects by restoring the balance between Th17 and Treg cells, thereby promoting an increase in Treg cell proportion. Furthermore, Chen et al. (2021) and Wei et al. (2017) emphasized that quercetin regulates the Th17/Treg balance through the Tim-3 and TLR4-MyD88-NF-κB signaling pathways, thus contributing to its anti-inflammatory and hepatoprotective effects. Given that advanced stages of AP are often associated with a heightened risk of progression to pancreatic cancer, some studies (Shen M. et al., 2022)suggest that quercetin may also influence the JAK/STAT1 signaling pathway, promoting the proliferation of γδ T cells. This modulation plays a synergistic role in the elimination of breast cancer cells and the induction of cell apoptosis. Additionally, quercetin enhances the immune response, reducing the evasion of tumor cells from immune surveillance. (Shen M. et al., 2022) demonstrated, through a co-culture system of breast cancer cells and T cells treated with quercetin, that quercetin significantly increased the proportion of γδ T cells, enhancing T cell immune responses, and inhibiting both the proliferation and migration of breast cancer cells. These findings underscore quercetin’s significant immune-modulatory effects, particularly its potential to regulate the balance between Th17 and Treg cells (Zhou et al., 2024); Yu et al., 2024. In the context of AP, such mechanisms may help mitigate inflammation and improve disease outcomes by restoring the balance between pro-inflammatory Th17 cells and anti-inflammatory Treg cells. Further investigation into quercetin’s role in modulating immune responses in AP could provide novel therapeutic strategies for managing inflammation in this condition.
3.3 Advantages and challenges of quercetin in clinical applications
3.3.1 Comparison of quercetin with conventional therapies for acute pancreatitis
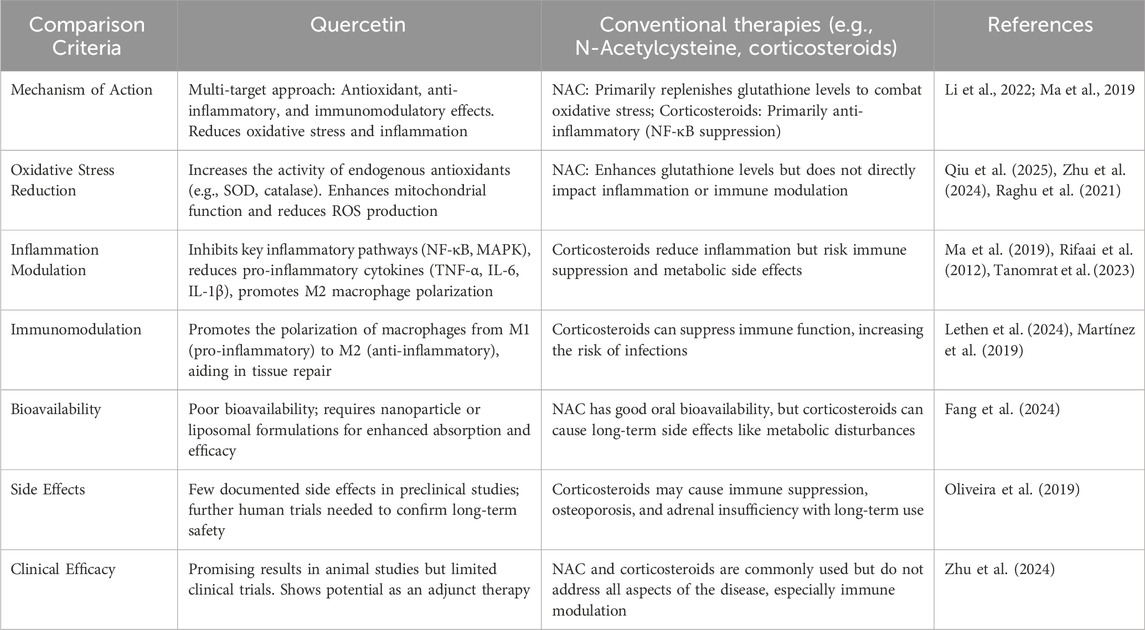
Conventional therapies for AP, such as N-acetylcysteine (NAC) and corticosteroids, primarily target specific disease components but fail to offer a comprehensive solution for the multifactorial pathogenesis of the disease (Qiu et al., 2021). NAC alleviates oxidative stress by replenishing GSH levels and neutralizing ROS. However, NAC’s efficacy is limited in its inability to address the inflammatory response and immune dysregulation, which are critical in the progression of AP (Fang et al., 2024; Minati et al., 2021). For example, a study by Ma et al. (2019) on NAC in patients with non-alcoholic fatty liver disease (NAFLD) showed that while NAC helped reduce oxidative stress markers, it failed to significantly improve inflammatory cytokine levels or liver function in the long term, highlighting NAC’s gap in managing inflammation. This finding highlights a critical consideration regarding the limited therapeutic scope of NAC, as it may alleviate oxidative damage but does not comprehensively address the immune inflammatory cascade responsible for systemic complications in AP. This emphasizes the necessity for the development of therapeutic strategies that can concurrently target oxidative stress and inflammation in a synergistic manner.Corticosteroids, on the other hand, are widely used as potent anti-inflammatory agents in treating AP and other inflammatory conditions. However, their long-term use is restricted due to a range of adverse effects, such as immune suppression, increased infection risk, and metabolic disturbances like hyperglycemia.For instance, in rheumatoid arthritis (RA) patients, long-term corticosteroid use has been associated with increased rates of infections and osteoporosis, as well as delayed wound healing, which severely hampers recovery (Rifaai et al., 2012). A critical issue with corticosteroids in the context of AP is their narrow therapeutic window, as they reduce inflammation but their immune-suppressive effects may actually hinder tissue repair and host defense, particularly in a setting where infection risk is high. This paradoxical outcome challenges the assumption that corticosteroids can be universally effective in inflammatory diseases, urging caution in their clinical application.
In contrast, quercetin, a flavonoid with potent antioxidant and anti-inflammatory properties, offers a broader therapeutic approach by simultaneously addressing oxidative stress and inflammation. Quercetin enhances the activity of endogenous antioxidants like SOD and GSH-Px, providing robust protection against oxidative damage and preserving cellular integrity (Huang et al., 2023; Sahana et al., 2022). For instance, Minati et al. (2021) demonstrated that quercetin offers protective effects on pancreatic tissue by attenuating inflammatory responses and preserving immune homeostasis, as observed in experimental diabetes models.In human clinical trials for other diseases, quercetin has demonstrated remarkable potential. For example, a study by Zhu et al. (2024) on quercetin supplementation in patients with type 2 diabetes found that quercetin not only reduced oxidative stress but also significantly decreased inflammatory markers like C-reactive protein (CRP) and TNF-α, while improving insulin sensitivity. This suggests that quercetin’s multi-targeted action may offer more comprehensive benefits compared to NAC and corticosteroids, especially in diseases where both oxidative stress and inflammation contribute to the pathological process. However, while these findings are promising, the lack of large-scale clinical trials specifically investigating quercetin in AP remains a gap in the literature, and its therapeutic potential must be tested in rigorous clinical settings.Additionally, quercetin’s immunomodulatory effects, particularly its ability to promote M2 macrophage polarization, enhance the resolution of inflammation and facilitate tissue repair—key processes in the recovery from AP. In preclinical studies, quercetin has been shown to promote M2 macrophage polarization, which plays a critical role in tissue repair and the resolution of inflammation. For instance, in experimental colitis, quercetin has been shown to shift macrophages from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, thereby aiding in tissue regeneration and reducing inflammation (Oliveira et al., 2019). This contrasts with the inflammatory exacerbation typically observed with prolonged use of corticosteroids, which can impair immune cell function and delay healing. Quercetin’s multi-targeted action thus offers distinct advantages over traditional therapies by simultaneously addressing oxidative stress, inflammation, and immune modulation, while minimizing side effects As shown in Table 1.
While quercetin’s ability to target both biochemical pathways and immune responses positions it as a potentially safer and more comprehensive alternative to conventional treatments, it is important to note that current evidence largely stems from preclinical studies and smaller clinical trials. The full clinical validation of quercetin’s efficacy in treating AP is still needed. Emerging clinical data also suggest that quercetin may work synergistically with existing therapies, enhancing their therapeutic outcomes without exacerbating side effects. In this sense, quercetin represents not only a potential adjunct but also a novel therapeutic avenue, warranting further investigation in clinical trials.
3.3.2 The limitations of quercetin in the treatment of pancreatitis
Although quercetin demonstrates significant therapeutic potential in managing AP, its clinical application is hindered by several challenges. A primary limitation is its poor bioavailability, which arises from its low solubility and rapid metabolism in the liver (Barbulescu et al., 2023). This limitation restricts its therapeutic efficacy, particularly in severe inflammatory conditions like AP. While pharmacokinetic modifications such as nanoparticles, liposomal carriers, and co-administration with bioenhancers like piperine have shown promise in enhancing quercetin’s absorption, tissue targeting, and circulation time, the long-term clinical validation of these strategies remains limited (Ansari et al., 2022). Preclinical studies suggest that these delivery methods could optimize quercetin’s bioavailability, but human studies are sparse and often limited to small sample sizes. For instance, while a study by Kumar et al. (2022) on liposomal quercetin demonstrated improved bioavailability in cancer models, its translation into diseases like AP, with its complex pathophysiology and high systemic inflammatory load, requires more robust clinical testing.Further complicating matters, quercetin’s bioavailability issue is often addressed with adjunct therapies, such as piperine, which enhances quercetin absorption by inhibiting liver enzymes involved in its metabolism. However, the safety profile of this combination, particularly in patients with pre-existing liver conditions or drug interactions, remains underexplored. A critical question arises: while bioenhancers may improve quercetin’s efficacy, do they also increase the risk of toxicity, especially when quercetin is co-administered with other potent drugs like corticosteroids or NAC? This concern underscores the need for careful dose adjustment and patient monitoring during quercetin-based therapies.
Moreover, despite promising preclinical data, the lack of large-scale clinical trials hampers our ability to establish optimal dosages, long-term safety, and the full range of potential side effects. While quercetin has demonstrated anti-inflammatory and antioxidant effects in animal models and in vitro studies, its therapeutic application in human pancreatitis remains speculative. For instance, studies on quercetin’s use in type 2 diabetes and cardiovascular diseases suggest efficacy in reducing oxidative stress and inflammatory markers (Kumar et al., 2022); however, these findings are not easily transferable to the highly complex inflammatory environment of AP. This raises a pivotal issue: while quercetin’s potential as a synergistic agent in combination with therapies like NAC or corticosteroids holds promise, its clinical application in pancreatitis is still in its infancy. It is crucial to conduct larger randomized controlled trials to evaluate quercetin’s long-term benefits and risks in the context of AP, particularly in high-risk populations such as those with comorbid conditions.
To enhance treatment outcomes, combination therapies that pair quercetin with agents like NAC or corticosteroids may effectively address both oxidative stress and inflammation, improving therapeutic efficacy while minimizing adverse effects (Eity et al., 2024). However, caution is warranted when using such combinations, as NAC itself has limitations in treating the immune-inflammatory response, and corticosteroids carry the risk of immunosuppression. More research is needed to determine whether quercetin’s ability to modulate immune pathways can counterbalance these potential drawbacks in combination therapies.
4 Future directions
Quercetin holds significant promise as a multi-target therapeutic in AP, thanks to its antioxidant, anti-inflammatory, and immune-modulatory properties (Sun et al., 2025). However, its clinical application is limited by poor bioavailability, which affects its efficacy, particularly in severe conditions like AP. Nanoparticles and liposomal formulations have shown potential to improve its bioavailability, but these approaches need extensive validation to assess long-term safety and clinical relevance. While preclinical models show positive results, there is a critical gap in data regarding optimal dosing, side effects, and drug interactions with agents like NAC and corticosteroids (Dhanya, 2022); Xing et al., 2024. This warrants caution, as combining quercetin with these therapies could either enhance or undermine their therapeutic effects.Beyond AP, quercetin’s fibrosis-reducing and tissue-healing effects suggest that it may also hold promise for treating chronic pancreatitis and other pancreatic disorders (Georgiou et al., 2023). However, the lack of robust clinical trials in chronic conditions and comorbid patients highlights the need for more targeted research. Importantly, quercetin’s dual action on inflammation and fibrosis may offer advantages over existing treatments but also raises concerns about the balance between its anti-inflammatory and potential immunosuppressive effects.Future studies should focus not only on optimizing formulations but also on exploring quercetin’s long-term safety in real-world conditions, especially in combination therapies. Addressing these clinical gaps will be key to unlocking quercetin’s full potential as a therapeutic agent for acute and chronic pancreatic diseases.
In summary, quercetin’s ability to target multiple pathological mechanisms, including oxidative stress, inflammation, and immune dysregulation, makes it a promising candidate for the treatment of AP. Its unique multi-target profile offers distinct advantages over single-target therapies. Although current preclinical data are promising, further clinical validation is essential to establish its efficacy and safety. With continued research and clinical trials, quercetin has the potential to transform the management of pancreatitis and related inflammatory disorders, advancing from the preclinical stage to widespread therapeutic use.
Author contributions
ZY: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation. GL: Data curation, Methodology, Supervision, Writing – review and editing, Writing – original draft. MM: Investigation, Methodology, Writing – review and editing, Conceptualization, Data curation, Software, Supervision. XY: Conceptualization, Data curation, Methodology, Supervision, Writing – review and editing, Formal Analysis, Project administration, Validation. JC: Conceptualization, Investigation, Project administration, Writing – review and editing, Software, Validation. YH: Conceptualization, Writing – review and editing, Funding acquisition, Project administration, Resources, Validation, Visualization. JX: Conceptualization, Writing – review and editing, Data curation, Investigation, Methodology, Software, Supervision. LH: Conceptualization, Investigation, Project administration, Writing – review and editing, Data curation, Formal Analysis, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study design, data collection, data analysis, manuscript preparation, and publication decisions of this work were supported by the 551 Medical Forum New Talent Program, the Zhejiang Province Traditional Chinese Medicine Project for Young and Middle-Aged Renowned Clinical Practitioners, the Zhejiang Province Traditional Chinese Medicine Science and Technology Project (No. 2024ZR015 by YH, No. 2025ZR123 by SC, and No. 2022ZA056 by SQ), the Zhejiang Province Medical and Health Science and Technology Project (No. 2024KY1201 by YH), and the Zhejiang Provincial Natural Science Foundation of China (No. QN25H270030 by YH).
Acknowledgments
Figures in our manuscript was created using BioRender online tools (https://www.biorender.com/). We would like to express our gratitude for these invaluable resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. During the preparation of this work, the authors used ChatGPT-4.0 for language optimization. After using the tool, the authors reviewed, revised, and edited the content, taking full responsibility for the final version of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AP, Acute Pancreatitis; ALI, Acute Lung Injury; ARDS, Acute Respiratory Distress Syndrome; BMSC, Bone marrow-derived mesenchymal stem cell; SIRS, Systemic Inflammatory Response Syndrome; MODS, Multi-Organ Dysfunction Syndrome; ROS, Reactive Oxygen Species; NF-κB, Nuclear Factor-kappa B; MAPK, Mitogen-Activated Protein Kinase; TNF-α, Tumor Necrosis Factor-alpha; IL-6, Interleukin-6; IL-1β, Interleukin-1 beta; SOD, Superoxide Dismutase; CAT, Catalase; GSH-Px, Glutathione Peroxidase; GSH, Glutathione; TLR, Toll-Like Receptor; PPAR-γ, Peroxisome Proliferator-Activated Receptor Gamma; STAT1, Signal Transducer and Activator of Transcription 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; NAC, N-Acetylcysteine; HMGB1, High Mobility Group Box 1; ERK, Extracellular Signal-Regulated Kinase; JNK, c-Jun N-terminal Kinase; DAMPs, Damage-Associated Molecular Patterns.
References
Ansari, P., Choudhury, S. T., Seidel, V., Rahman, A. B., Aziz, M. A., Richi, A. E., et al. (2022). Therapeutic potential of quercetin in the management of type-2 diabetes mellitus. Life (Basel) 12 (8), 1146. doi:10.3390/life12081146
Bansod, S., Doijad, N., and Godugu, C. (2020). Berberine attenuates severity of chronic pancreatitis and fibrosis via AMPK-mediated inhibition of TGF-β1/Smad signaling and M2 polarization. Toxicol. Appl. Pharmacol. 403, 115162. doi:10.1016/j.taap.2020.115162
Barbulescu, A., Sjölander, A., Delcoigne, B., Askling, J., and Frisell, T. (2023). Glucocorticoid exposure and the risk of serious infections in rheumatoid arthritis: a marginal structural model application. Rheumatol. Oxf. 62 (10), 3391–3399. doi:10.1093/rheumatology/kead083
Beken, B., Serttas, R., Yazicioglu, M., Turkekul, K., and Erdogan, S. (2020). Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 33 (2), 69–79. doi:10.1089/ped.2019.1137
Bhaskar, S., and Helen, A. (2016). Quercetin modulates toll-like receptor-mediated protein kinase signaling pathways in oxLDL-challenged human PBMCs and regulates TLR-activated atherosclerotic inflammation in hypercholesterolemic rats. Mol. and Cell. Biochem. 423 (1), 53–65. doi:10.1007/s11010-016-2824-9
Boodhoo, K., de Swardt, D., Smith, C., and van de Vyver, M. (2022). Ex vivo tolerization and M2 polarization of macrophages dampens both pro- and anti-inflammatory cytokine production in response to diabetic wound fluid stimulation. Biochimie 196, 143–152. doi:10.1016/j.biochi.2021.12.009
Cai, Y., Yang, F., and Huang, X. (2024). Oxidative stress and acute pancreatitis (Review). Biomed. Rep. 21 (2), 124. doi:10.3892/br.2024.1812
Chen, J., Hamm, L. L., Bundy, J. D., Kumbala, D. R., Bodana, S., Chandra, S., et al. (2020). Combination treatment with sodium nitrite and isoquercetin on endothelial dysfunction among patients with ckd: a randomized phase 2 pilot trial. Clin. J. Am. Soc. Nephrol. CJASN 15 (11), 1566–1575. doi:10.2215/CJN.02020220
Chen, L., Wang, Z., Zhang, Y., Zhu, Q., Lu, G., Dong, X., et al. (2024). Pharmacological inhibition of phosphoglycerate kinase 1 reduces OxiDative stress and restores impaired autophagy in experimental acute pancreatitis. Inflammation. doi:10.1007/s10753-024-02173-5
Chen, S., Zhu, J., Sun, L.-Q., Liu, S., Zhang, T., Jin, Y., et al. (2021). LincRNA-EPS alleviates severe acute pancreatitis by suppressing HMGB1-triggered inflammation in pancreatic macrophages. Immunology 163 (2), 201–219. doi:10.1111/imm.13313
Chiang, M. C., Tsai, T. Y., and Wang, C. J. (2023). The potential benefits of quercetin for brain Health: a review of anti-inflammatory and neuroprotective mechanisms. Int. J. Mol. Sci. 24 (7), 6328. doi:10.3390/ijms24076328
Dhanya, R. (2022). Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 146, 112560. doi:10.1016/j.biopha.2021.112560
Eity, T. A., Bhuia, M. S., Chowdhury, R., Ahmmed, S., Salehin, S., Akter, R., et al. (2024). Therapeutic efficacy of quercetin and its nanoformulation both the mono- or combination therapies in the management of cancer: an update with molecular mechanisms. J. Trop. Med. 2024, 5594462. doi:10.1155/2024/5594462
Faiza, I. A., Toumi, A., Boudriga, S., Alanazi, T. Y. A., Alshamari, A. K., HamdenCA, K., et al. (2024). Antiobesity and antidiabetes effects of Cyperus rotundus rhizomes presenting protein tyrosine phosphatase, dipeptidyl peptidase 4, metabolic enzymes, stress oxidant and inflammation inhibitory potential. Heliyon 10 (5), e27598. doi:10.1016/j.heliyon.2024.e27598
Fang, L., Gao, D., Wang, T., Zhao, H., Zhang, Y., and Wang, S. (2024). From nature to clinic: quercetin's role in breast cancer immunomodulation. Front. Immunol. 15, 1483459. doi:10.3389/fimmu.2024.1483459
Feng, Y. L., Lu, L. P., and Zhai, G. Y. (2020). Research progress on antitumor activity of quercetin derivatives. Zhongguo Zhong Yao Za Zhi. 45 (15), 3565–3574. doi:10.19540/j.cnki.cjcmm.20200428.602
Gao, F., Zhu, F., Shuai, B., Wu, M., Wei, C., Yuan, Y., et al. (2024). Quercetin ameliorates ulcerative colitis by restoring the balance of M2/M1 and repairing the intestinal barrier via downregulating cGAS‒STING pathway. Front. Pharmacol. 15, 1351538. doi:10.3389/fphar.2024.1351538
Ge, P., Luo, Y., Okoye, C. S., Chen, H., Liu, J., Zhang, G., et al. (2020). Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: a troublesome trio for acute pancreatitis. Biomed. Pharmacother. 132, 110770. doi:10.1016/j.biopha.2020.110770
Georgiou, N., Kakava, M. G., Routsi, E. A., Petsas, E., Stavridis, N., Freris, C., et al. (2023). Quercetin: a potential polydynamic drug. Molecules 28 (24), 8141. doi:10.3390/molecules28248141
Goyal, A., and Agrawal, N. (2022). Quercetin: a potential candidate for the treatment of arthritis. Curr. Mol. Med. 22 (4), 325–335. doi:10.2174/1566524021666210315125330
Guo, J., Li, Z., Tang, D., and Zhang, J. (2020). Th17/Treg imbalance in patients with severe acute pancreatitis: attenuated by high-volume hemofiltration treatment. Med. Baltim. 99 (31), e21491. doi:10.1097/MD.0000000000021491
Hai-feng, Z., Chao, Y., Jun-yi, Li, Yu-yao, He, Huang, Y., Ren-jie, Q., et al. (2023). Quercetin serves as the major component of Xiang-lian Pill to ameliorate ulcerative colitis via tipping the balance of STAT1/PPARγ and dictating the alternative activation of macrophage. J. Ethnopharmacol. 313, 116557. doi:10.1016/j.jep.2023.116557
He, Y., Hong, Q., Chen, S., Zhou, J., and Qiu, S. (2025). Reprogramming tumor-associated macrophages in gastric cancer: a pathway to enhanced immunotherapy. Front. Immunol. 16, 1558091. doi:10.3389/fimmu.2025.1558091
Hossein, A., Firouzeh, G., Karimi, Z., and Gholamreza, D. (2024). Quercetin prevents kidney against diabetes mellitus (type 1) in rats by inhibiting TGF-β/apelin gene expression. Mol. Biol. Rep. 51 (1), 677. doi:10.1007/s11033-024-09617-z
Huang, L., Zeng, Y., Duan, L., Zhuang, Q., Zhou, Y., Wang, L., et al. (2023). Optimal timing of free total rhubarb anthraquinones on immune regulation in rats with severe acute pancreatitis. J. Ethnopharmacol. 308, 116266. doi:10.1016/j.jep.2023.116266
Jian, C., Wang, M., Qian, Y., Song, X., Wang, L., Li, L., et al. (2024). A TLR4-targeting bioactive peptide hydrogel to regulate immune-microenvironment for diabetic wound repair. Adv. Healthc. Mater 13 (19), e2400391. doi:10.1002/adhm.202400391
Kashyap, D., Garg, V. K., Tuli, H. S., Yerer, M. B., Sak, K., Sharma, A. K., et al. (2019). Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules 9 (5), 174. doi:10.3390/biom9050174
KhaterCA, S. I., Abd El-EmamCA, M. M., Hussein, A., Mostafa, M., Tarek, K. C. A., Soliman, R. H. M., et al. (2024). Lipid nanoparticles of quercetin (QU-Lip) alleviated pancreatic microenvironment in diabetic male rats: the interplay between oxidative stress – unfolded protein response (UPR) – autophagy, and their regulatory miRNA. Life Sci. 344, 122546. doi:10.1016/j.lfs.2024.122546
Kong, L., Deng, J., Zhou, X., Cai, B., Zhang, B., Chen, X., et al. (2021). Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 12 (10), 928. doi:10.1038/s41419-021-04227-0
Kumar, M., Kumar, D., Kumar, S., Kumar, A., and Mandal, U. K. (2022). A recent review on bio-availability enhancement of poorly water-soluble drugs by using bioenhancer and nanoparticulate drug delivery system. Curr. Pharm. Des. 28 (39), 3212–3224. doi:10.2174/1381612829666221021152354
Lethen, I., Lechner-Grimm, K., Gabel, M., Knauss, A., Atreya, R., Neurath, M. F., et al. (2024). Tofacitinib affects M1-like and M2-like polarization and tissue factor expression in macrophages of healthy donors and IBD patients. Inflamm. Bowel Dis. 30 (7), 1151–1163. doi:10.1093/ibd/izad290
Li, H., Du, R., Xiang, A., Liu, Y., Guan, M., and He, H. (2024d). Bone marrow mesenchymal stem cell-derived exosomal miR-181a-5p promotes M2 macrophage polarization to alleviate acute pancreatitis through ZEB2-mediated RACK1 ubiquitination. FASEB J. 38 (23), e70042. doi:10.1096/fj.202400803RR
Li, J.-W., Mao, Y.-M., Chen, S.-L., Ye, R., Fei, Y. R., et al. (2024c). The interplay between metal ions and immune cells in glioma: pathways to immune escape. Discov. Oncol. 15 (1), 348. doi:10.1007/s12672-024-01229-0
Li, Ke, Liu, J., and Zhai, G. (2024a). Research progress on quercetin derivatives and their biological activities. Chin. Pharm. J. 59 (15), 1366–1374.
Li, X., Huang, Y., He, Y., and Ye, A. (2025). Wogonoside alleviates the proliferation and promotes the apoptosis in liver cancer cells by regulating PI3K/Akt signaling pathway. Discov. Oncol. 16 (1), 244. doi:10.1007/s12672-025-01995-5
Li, X., Wang, T., Zhou, Q., Li, F., Liu, T., Zhang, K., et al. (2024b). Isorhamnetin alleviates mitochondrial injury in severe acute pancreatitis via modulation of KDM5B/HtrA2 signaling pathway. Int. J. Mol. Sci. 25 (7), 3784. doi:10.3390/ijms25073784
Li, Y., Li, G., Suo, L., and Zhang, J. (2021). Recent advances in studies of molecular hydrogen in the treatment of pancreatitis. Life Sci. 264, 118641. doi:10.1016/j.lfs.2020.118641
Li, Z., Deng, H., Guo, X., Yan, S., Lu, C., Zhao, Z., et al. (2022). Effective dose/duration of natural flavonoid quercetin for treatment of diabetic nephropathy: a systematic review and meta-analysis of rodent data. Phytomedicine 105, 154348. doi:10.1016/j.phymed.2022.154348
Lin, Li, Jiang, W., Yu, B., Liang, H., Mao, S., Hu, X., et al. (2023). Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-κB signaling pathway. Biomed. and Pharmacother. 168, 115653. doi:10.1016/j.biopha.2023.115653
Liu, D., Wen, L., Wang, Z., Hai, Y., Yang, D., Zhang, Y., et al. (2022a). The mechanism of lung and intestinal injury in acute pancreatitis: a review. Front. Med. (Lausanne) 9, 904078. doi:10.3389/fmed.2022.904078
Liu, H., Xu, X., Li, J., Liu, Z., Xiong, Y., Yue, M., et al. (2024). Overexpression of Plakophilin2 mitigates capillary leak syndrome in severe acute pancreatitis by activating the p38/MAPK signaling pathway. J. Inflamm. Res. 17, 4129–4149. doi:10.2147/JIR.S459449
Liu, Y., Li, M., Mei, C., Cui, H., Wang, Q., Li, D., et al. (2022b). Thioredoxin-interacting protein deficiency protects against severe acute pancreatitis by suppressing apoptosis signal-regulating kinase 1. Cell Death Dis. 13 (10), 914. doi:10.1038/s41419-022-05355-x
Ma, Z., Song, G., Liu, D., Qian, D., Wang, Y., Zhou, J., et al. (2019). N-Acetylcysteine enhances the therapeutic efficacy of bone marrow-derived mesenchymal stem cell transplantation in rats with severe acute pancreatitis. Pancreatology 19 (2), 258–265. doi:10.1016/j.pan.2019.01.004
Martínez, G., Mijares, M. R., and De Sanctis, J. B. (2019). Effects of flavonoids and its derivatives on immune cell responses. Recent Pat. Inflamm. Allergy Drug Discov. 13 (2), 84–104. doi:10.2174/1872213X13666190426164124
Minati, M. A., Libert, M., Dahou, H., Jacquemin, P., and Assi, M. (2021). N-acetylcysteine reduces the pro-oxidant and inflammatory responses during pancreatitis and pancreas tumorigenesis. Antioxidants (Basel) 10 (7), 1107. doi:10.3390/antiox10071107
Nagy-Pénzes, M., Hajnády, Z., Regdon, Z., Demény, M. Á., Kovács, K., El-Hamoly, T., et al. (2022). Tricetin reduces inflammation and acinar cell injury in cerulein-induced acute pancreatitis: the role of oxidative stress-induced DNA damage signaling. Biomedicines 10 (6), 1371. doi:10.3390/biomedicines10061371
Niu, X., Sun, W., Tang, X., Chen, J., Zheng, H., Yang, G., et al. (2024). Bufalin alleviates inflammatory response and oxidative stress in experimental severe acute pancreatitis through activating Keap1-Nrf2/HO-1 and inhibiting NF-κB pathways. Int. Immunopharmacol. 142 (Pt A), 113113. doi:10.1016/j.intimp.2024.113113
Oliveira, C. P., Cotrim, H. P., Stefano, J. T., Siqueira, A. C. G., Salgado, A. L. A., and Parise, E. R. (2019). N-acetylcysteine and/or ursodeoxycholic acid associated with metformin in non-alcoholic steatohepatitis: an open-label multicenter randomized controlled trial. Arq. Gastroenterol. 56 (2), 184–190. doi:10.1590/S0004-2803.201900000-36
Peng, Y., Yang, Y., Li, Y., Shi, T., Xu, N., Liu, R., et al. (2024). Mitochondrial (mt)DNA-cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling promotes pyroptosis of macrophages via interferon regulatory factor (IRF)7/IRF3 activation to aggravate lung injury during severe acute pancreatitis. Cell Mol. Biol. Lett. 29 (1), 61. doi:10.1186/s11658-024-00575-9
Qi, W., Qi, W., Xiong, D., and Long, M. (2022). Quercetin: its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules 27 (19), 6545. doi:10.3390/molecules27196545
Qiu, D., Yan, X., Xiao, X., Zhang, G., Wang, Y., Cao, J., et al. (2021). To explore immune synergistic function of Quercetin in inhibiting breast cancer cells. Cancer Cell Int. 21 (1), 632. doi:10.1186/s12935-021-02345-5
Qiu, M., Huang, Y., Zhou, X., Yu, J., Li, J., Wang, W., et al. (2025). Hyperlipidemia exacerbates acute pancreatitis via interactions between P38MAPK and oxidative stress. Cell Signal 125, 111504. doi:10.1016/j.cellsig.2024.111504
Raghu, G., Berk, M., Campochiaro, P. A., Jaeschke, H., Marenzi, G., Richeldi, L., et al. (2021). The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr. Neuropharmacol. 19 (8), 1202–1224. doi:10.2174/1570159X19666201230144109
Rezabakhsh, A., Rahbarghazi, R., Malekinejad, H., Fathi, F., Montaseri, A., and Garjani, A. (2019). Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine Int. J. phytotherapy Phytopharm. 56, 183–193. doi:10.1016/j.phymed.2018.11.008
Rifaai, R. A., Fathy, N., Saber, E. A., and Ahmed, R. (2012). Effect of quercetin on the endocrine pancreas of the experimentally induced diabetes in male albino rats: a histological and immunohistochemical study. J. Diabetes and Metabolism 3 (3), 182. doi:10.4172/2155-6156.1000182
Ryu, S., and Lee, E. K. (2024). The pivotal role of macrophages in the pathogenesis of pancreatic diseases. Int. J. Mol. Sci. 25 (11), 5765. doi:10.3390/ijms25115765
Sahana, G. R., Nagella, P., Joseph, B. V., Alessa, F. M., and Al-Mssallem, M. Q. (2022). Flavonoids as potential anti-inflammatory molecules: a review. Molecules 27 (9), 2901. doi:10.3390/molecules27092901
Sendler, M., van den Brandt, C., Glaubitz, J., Wilden, A., Golchert, J., Weiss, F. U., et al. (2020). NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology 158 (1), 253–269.e14. doi:10.1053/j.gastro.2019.09.040
Sheibani, M., Hosseinzadeh, A., Fatemi, I., Naeini, A. J., and Mehrzadi, S. (2024). Practical application of melatonin for pancreas disorders: protective roles against inflammation, malignancy, and dysfunctions. Pharmacol. Rep. 77, 315–332. doi:10.1007/s43440-024-00683-5
Shen, G., Wen, H., Li, H., Zhang, X., Lan, B., Dong, X., et al. (2025). Emodin protects against severe acute pancreatitis-associated acute lung injury by activating Nrf2/HO-1/GPX4 signal and inhibiting ferroptosis in vivo and in vitro. BMC Gastroenterol. 25 (1), 57. doi:10.1186/s12876-025-03660-1
Shen, M., Lin, B., Qian, F., Zhao, L., Xi, Y., and Qian, Y. (2022b). Taxifolin ameliorates sepsis-induced lung capillary leak through inhibiting the JAK/STAT3 pathway. Allergol. Immunopathol. Madr. 50 (2), 7–15. doi:10.15586/aei.v50i2.550
Shen, Y., Yang, H., Wu, D., Yang, H., and Hong, D. (2022a). NLRP3 inflammasome inhibitor MCC950 can reduce the damage of pancreatic and intestinal barrier function in mice with acute pancreatitis. Acta Cir. Bras. 37 (7), e370706. doi:10.1590/acb370706
Sheng, Bo, Zhao, L., Zang, X., Zhen, J., Liu, Y., Bian, W., et al. (2021b). Quercetin inhibits caerulein-induced acute pancreatitis through regulating miR-216b by targeting MAP2K6 and NEAT1. INFLAMMOPHARMACOLOGY 29 (2), 549–559. doi:10.1007/s10787-020-00767-7
Sheng, B., Zhao, L., Zang, X., Zhen, J., Liu, Y., Bian, W., et al. (2021c). Quercetin inhibits caerulein-induced acute pancreatitis through regulating miR-216b by targeting MAP2K6 and NEAT1. Inflammopharmacology 29 (2), 549–559. doi:10.1007/s10787-020-00767-7
Sheng, M., Lin, Y., Xu, D., Tian, Y., Zhan, Y., Li, C., et al. (2021a). CD47-Mediated hedgehog/SMO/GLI1 signaling promotes mesenchymal stem cell immunomodulation in mouse liver inflammation. Hepatology 74 (3), 1560–1577. doi:10.1002/hep.31831
Soofiyani, S. R., Hosseini, K., Forouhandeh, H., Ghasemnejad, T., Tarhriz, V., Asgharian, P., et al. (2021). Quercetin as a novel therapeutic approach for lymphoma. Oxid. Med. Cell Longev. 2021, 3157867. doi:10.1155/2021/3157867
Sun, G., Wu, Y., Li, J., Yang, M., Xu, H., Li, Y., et al. (2025). Quercetin liposomes conjugated with hyaluronidase: an efficient drug delivery system to block pancreatic cancer. J. Control Release 22, 113642. doi:10.1016/j.jconrel.2025.113642
Swetha, K., Indumathi, M. C., Kishan, R., Siddappa, S., Chen, C. H., and Marathe, G. K. (2025). Selenium mitigates caerulein and LPS-induced severe acute pancreatitis by inhibiting MAPK, NF-κB, and STAT3 signaling via the Nrf2/HO-1 pathway. Biol. Trace Elem. Res. doi:10.1007/s12011-025-04531-2
Tang, S. M., Deng, X. T., Zhou, J., Li, Q. P., Ge, X. X., and Miao, L. (2020). Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 121, 109604. doi:10.1016/j.biopha.2019.109604
Tanomrat, R., Naktubtim, C., Aimvijarn, P., and Suwannalert, P. (2023). N-acetylcysteine improves the inhibitory effect of Quercetin-rich onion extract on HT-29 and HCT-116 colorectal cancer migration and invasion through iNOS suppression. Int. J. Med. Sci. 20 (9), 1123–1134. doi:10.7150/ijms.86573
Tenner, S., Vege, S. S., Sheth, S. G., Sauer, B., Yang, A., Conwell, D. L., et al. (2024). American College of gastroenterology guidelines: management of acute pancreatitis. Am. J. Gastroenterology 119 (3), 419–437. doi:10.14309/ajg.0000000000002645
Ungurianu, A., Zanfirescu, A., and Margină, D. (2024). Exploring the therapeutic potential of quercetin: a focus on its sirtuin-mediated benefits. Phytotherapy Res. PTR 38 (5), 2361–2387. doi:10.1002/ptr.8168
Wang, F. J., and Mei, X. (2023). Expression level of serum miR-374a-5p in patients with acute pancreatitis and its effect on viability, apoptosis, and inflammatory factors of pancreatic acinar cells induced by cerulein. Kaohsiung J. Med. Sci. 39 (6), 616–623. doi:10.1002/kjm2.12666
Wang, G. Y., Shang, D., Zhang, G. X., Song, H. Y., Jiang, N., Liu, H. H., et al. (2022). Qingyi decoction attenuates intestinal epithelial cell injury via the calcineurin/nuclear factor of activated T-cells pathway. World J. Gastroenterol. 28 (29), 3825–3837. doi:10.3748/wjg.v28.i29.3825
Wang, H., Yuan, Y., Lu, C., Zhou, S., Zhang, Y., Zhao, J., et al. (2021). Analysis of T-cell receptor repertoire in peripheral blood of patients with pancreatic cancer and other pancreatic diseases. J. Cell Mol. Med. 25 (8), 3991–4000. doi:10.1111/jcmm.16358
Wang, Y., Chen, W., Wang, J., Song, C., Zhang, L., and Zhang, X. (2025). The effects of moderate to high static magnetic fields on pancreatic damage. J. Magn. Reson Imaging. doi:10.1002/jmri.29704
Wang, Y., Wan, R., Peng, W., Zhao, X., Bai, W., and Hu, C. (2023). Quercetin alleviates ferroptosis accompanied by reducing M1 macrophage polarization during neutrophilic airway inflammation. Eur. J. Pharmacol. 938, 175407. doi:10.1016/j.ejphar.2022.175407
Wei, C. B., Tao, K., Jiang, R., Zhou, L. D., Zhang, Q. H., and Yuan, C. S. (2017). Quercetin protects mouse liver against triptolide-induced hepatic injury by restoring Th17/Treg balance through Tim-3 and TLR4-MyD88-NF-κB pathway. Int. Immunopharmacol. 53, 73–82. doi:10.1016/j.intimp.2017.09.026
Windsor, J. A., and McClave, S. A. (2023). Intestinal dysfunction and failure in acute pancreatitis. Intestinal Failure, 923–934.
Wu, Z., Lu, G., Zhang, L., Ke, L., Yuan, C., Ma, N., et al. (2021). Protectin D1 decreases pancreatitis severity in mice by inhibiting neutrophil extracellular trap formation. Int. Immunopharmacol. 94, 107486. doi:10.1016/j.intimp.2021.107486
Xing, D., Du, Y., Dai, K., Lang, S., Bai, Y., and Liu, G. (2024). Polysaccharide-based injectable hydrogel loaded with quercetin promotes scarless healing of burn wounds by reducing inflammation. Biomacromolecules 25 (11), 7529–7542. doi:10.1021/acs.biomac.4c01276
Yang, L., Ye, F., Liu, J., Klionsky, D. J., Tang, D., and Kang, R. (2023). Extracellular SQSTM1 exacerbates acute pancreatitis by activating autophagy-dependent ferroptosis. Autophagy 19 (6), 1733–1744. doi:10.1080/15548627.2022.2152209
Yang, Y., Zhang, X., Xu, M., Wu, X., Zhao, F., and Zhao, C. (2018). Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 54, 153–162. doi:10.1016/j.intimp.2017.11.013
Yu, T., Xie, Y., Wang, Z., Li, J., Shen, Y., Yuan, J., et al. (2024). Quercetin ameliorates celiac-related intestinal inflammation caused by wheat gluten through modulating oxidative stress, Th1/Th2/Treg balance, and intestinal microflora structure. Food Funct. 15 (18), 9343–9356. doi:10.1039/d4fo03025g
Zhang, C., Xie, M., Wang, Y., and Han, T. (2024c). Tussilagone inhibits inflammation and oxidative stress to alleviate acute pancreatitis. SIGNA VITAE 20 (1), 133–139. doi:10.22514/sv.2024.009
Zhang, H., Wang, Z., Li, J., Jia, Y., and LiCA, F. (2024a). Timing, initiation and function: an in-depth exploration of the interaction network among neutrophil extracellular traps related genes in acute pancreatitis. Int. Immunopharmacol. 141, 112923. doi:10.1016/j.intimp.2024.112923
Zhang, J., Mei, F., Zhao, L., Zuo, T., Hong, Y., Li, M., et al. (2021). Inhibition of the p38 MAPK pathway attenuates renal injury in pregnant rats with acute necrotizing pancreatitis. Immunol. Res. 69 (3), 295–306. doi:10.1007/s12026-021-09195-3
Zhang, Z., Luo, Y., Zhuang, X., Gao, H., Qi, Y., and ChenCA, H. (2024b). Emodin alleviates lung injury via the miR-217-5p/Sirt1 axis in rats with severe acute pancreatitis. J. Pharmacol. Sci. 156 (3), 188–197. doi:10.1016/j.jphs.2024.08.007
Zhou, F., Guo, Y. X., Gao, R., Ji, X. Y., Tang, Y. X., Wang, L. B., et al. (2024). Quercetin regulates dendritic cell activation by targeting STAT4 in the treatment of experimental autoimmune encephalomyelitis. Toxicol. Appl. Pharmacol. 488, 116980. doi:10.1016/j.taap.2024.116980
Zhou, L., Yu, J., Wang, S., Ma, Y., Liu, X., Zhang, Xa, et al. (2023). Tectoridin alleviates caerulein-induced severe acute pancreatitis by targeting ERK2 to promote macrophage M2 polarization. Archives Biochem. biophysics 752, 109873. doi:10.1016/j.abb.2023.109873
Zhou, W., Wang, X., Yan, B., and Sun, Y. (2025). Embryonic lethal abnormal visual-like protein 1 aggravates caerulein-induced AR42J cell injury and macrophage M1 polarization to accelerate acute pancreatitis by upregulating TRAF6. J. Interferon Cytokine Res. 45 (1), 20–28. doi:10.1089/jir.2024.0149
Keywords: quercetin, acute pancreatitis, antioxidant, anti-inflammatory, immunomodulatory, bioavailability
Citation: Jiang Z, Lhamo G, Ma M, Ye X, Chen J, He Y, Xu J and Huang L (2025) Quercetin as a therapeutic agent for acute pancreatitis: a comprehensive review of antioxidant, anti-inflammatory, and immunomodulatory mechanisms. Front. Pharmacol. 16:1587314. doi: 10.3389/fphar.2025.1587314
Received: 04 March 2025; Accepted: 21 April 2025;
Published: 28 April 2025.
Edited by:
Ramzi Mothana, King Saud University, Saudi ArabiaReviewed by:
Jovana Rajkovic, University of Belgrade, SerbiaSidharth Mehan, Indo-Soviet Friendship College of Pharmacy, India
Copyright © 2025 Jiang, Lhamo, Ma, Ye, Chen, He, Xu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liquan Huang, aHVhbmdsaXF1YW4wMDFAMTYzLmNvbQ==; Jian Xu, MjAwNjEwMzZAemNtdS5lZHUuY24=; Yibo He, MjAxNzM2MjZAemNtdS5lZHUuY24=; Jin Chen, Y2hlbmppbjA0MjVAemNtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Zeyi Jiang
Zeyi Jiang Gamar Lhamo3†
Gamar Lhamo3† Yibo He
Yibo He Jian Xu
Jian Xu