- Department of Anorectal Surgery, Shanghai Municipal Hospital of Traditional Chinese Medicine, Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Indigo naturalis (IN) has been extensively used in prescriptions of traditional Chinese medicine to treat inflammatory bowel disease (IBD), particularly ulcerative colitis (UC). However, there is a lack of quantitative, evidence-based assessments from preclinical trials.
Aims: Quantitative statistical evidence regarding the efficacy of IN in animal models of IBD remains insufficient. This study performed a meta-analysis to evaluate the therapeutic effects of IN in experiments with IBD.
Methods: Relevant animal studies were identified from PubMed, Web of Science, Embase, China National Knowledge Infrastructure, SinoMed, and Wanfang databases. Two researchers independently conducted literature screening and risk-of-bias assessments using the CAMARADES 10-point quality checklist. Meta-analysis was performed using Review Manager 5.4, focusing on the histopathological index as the primary outcome measure.
Results: Of the 15 eligible studies included, over half had low risks of bias in more than five items. Compared to controls, the histopathological index significantly improved after IN treatment (n = 151/137; SMD = −2.69 [-3.36, −2.02]; p < 0.00001). Subgroup analysis showed that a high dose of IN (>600 mg/kg; 4 studies, n = 31/22; SMD = −3.55 [-5.72, −1.39]; p < 0.001) was most effective in reducing the histopathological index. The IN group showed a significantly lower final disease activity index (DAI) score (n = 121/89; WMD = −1.69 [-2.18, −1.20]; p < 0.00001), greater percentage body weight recovery (n = 77/63; WMD = 9.99 [6.50, 13.49]; p < 0.00001), and longer colon lengths (n = 65/51; WMD = 0.95 [0.67, 1.24]; p < 0.00001) compared to controls. Additionally, IN treatment reduced IL-1β, IL-6, IL-8, and TNF-α expression while increasing IL-10 levels. These findings suggest that IN ameliorates inflammation by balancing innate and adaptive immunity, modulating the AhR/CYP1A1 signaling pathway, and altering gut microbiota structure.
Conclusion: IN demonstrated significant therapeutic efficacy in preclinical models of IBD, particularly at dosages exceeding 600 mg/kg. It protected colonic mucosal integrity and exerted beneficial effects through multiple molecular pathways.
Introduction
Inflammatory bowel disease (IBD) is a chronic disorder that affects the lower gastrointestinal tract and includes Crohn’s disease (CD) and ulcerative colitis (UC), with multiple unclear pathogenic factors (Gordon et al., 2023). Common symptoms of IBD patients include diarrhea, abdominal pain, weight loss, vomiting, and rectal bleeding (Prentice et al., 2022). The precise pathogenesis of IBD remains elusive. Extensive epidemiological studies have identified genetic predisposition, dysregulated immune responses, and environmental factors as contributors to IBD (Singh and Bernstein, 2022). In recent decades, IBD has become increasingly prevalent in developing regions such as Asia (including Asian immigrants in Western countries) and South America (Aniwan et al., 2022), making it a global public health concern. In Western countries, although IBD incidence has stabilized, its prevalence remains high at over 0.3%. Moreover, persistent IBD may lead to hyperplasia and neoplastic growth, posing additional public health challenges (Ng et al., 2017).
Current treatments for UC mainly include 5-aminosalicylic acid (5-ASA) steroids, immunosuppressants (6-methylmercaptopurine and azathioprine), and biological agents (Jeong et al., 2019). Treatment strategies for CD are similar but typically require longer immunosuppressive therapy (Dolinger et al., 2024). However, these treatments have drawbacks, including dependence, intolerance, loss of efficacy, drug resistance, and opportunistic infections, that present significant clinical challenges (Nakase, 2020). Consequently, satisfactory remission rates are not consistently achieved in clinical practice. Complementary and alternative therapies are therefore necessary for patients, clinicians, and researchers.
Indigo naturalis (IN) is an indigo-blue powder derived from the stems and leaves of the Acanthaceae plant Baphicacanthus cusia (Nees) Bremek., Polygonaceae plant Polygonum tinctorium Ait., or Cruciferous plant Isatis indigotica Fort. Its earliest medical documentation in China dates to the Theory of Medicinal Properties from the Tang Dynasty (627 A.D.). IN has been used for centuries to treat fever, hemoptysis, pediatric convulsions, oral ulcers, and sore throats (Yang et al., 2020). Its primary components are indirubin, indigo, and tryptanthrin (Naganuma, 2019). These components possess anticancer, anti-angiogenic, anti-inflammatory, and antimicrobial properties (Sun et al., 2021). IN has a long-standing application in treating inflammatory diseases, including psoriasis and IBD (Kakdiya et al., 2024; Zhang et al., 2022; Naganuma et al., 2018; Uchiyama et al., 2020; Yue et al., 2023). Clinical studies have indicated promising results of IN for UC treatment (Matsuno et al., 2022; Kudo et al., 2022; Saiki et al., 2021). However, only two randomized controlled trials have compared IN with a placebo. Further studies are required to clarify the efficacy and safety of IN (Kakdiya et al., 2024). Existing quantitative evidence is insufficient to draw definitive conclusions (Kim et al., 2024; Hu et al., 2024). Therefore, this study aimed to address these gaps by quantitatively analyzing previous research data, providing objective evidence to support the use of IN in IBD management.
Materials and methods
Literature search
Relevant animal studies were searched in PubMed, Web of Science, Embase, China National Knowledge Infrastructure, SinoMed, and Wanfang databases from inception to May 2025 without language restrictions. The following MeSH terms were used: “inflammatory bowel disease,” “IBD,” “colitis, ulcerative,” “ulcerative colitis,” “colitis,” “Crohn disease,” “Crohns disease,” “Crohn’s disease,” “Crohn’s enteritis,” “Indigo naturalis,” “Qing Dai,” “IN,” “Qing-dai,” and “indigo pulverata levis.” Abstracts and full texts of eligible studies were reviewed independently by two reviewers (Jie Hu and Li Huang). Any discrepancies were resolved through discussion with a third reviewer (Yan Chen).
Eligibility criteria
Participants
There were no restrictions regarding animal strain, sex, or age. Animal models of IBD induced by dextran sodium sulfate (DSS), trinitrobenzene sulfonic acid (TNBS), oxazolone (OXZ), or ovalbumin-induced allergic enteritis were included. The intervention duration and frequency were without restrictions. Models involving radiation-induced colitis or interleukin-10 (IL-10)−/− spontaneous chronic colitis were excluded.
Interventions and comparisons
Animals in the treatment groups received IN without restrictions on dosage form, dose, administration route, or duration. Control groups received no treatment or vehicle administration. Studies without controls were excluded.
Outcome measures
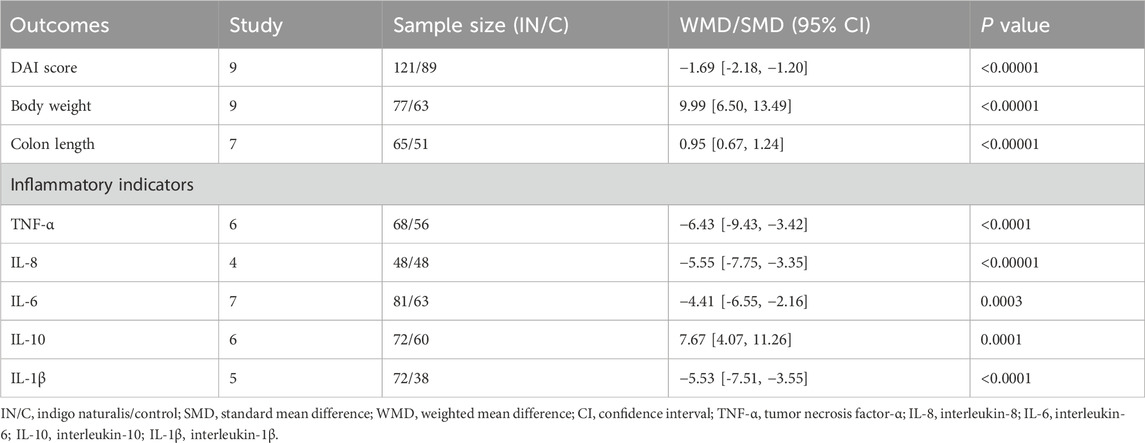
The primary outcome was the histological index, assessed by evaluating epithelial hyperplasia, loss of enterocytes, infiltration of lamina propria granulocytes, and monocyte infiltration using hematoxylin–eosin (HE) staining of colon tissue sections. Secondary outcomes included the disease activity index (DAI), final percentage change in body weight, and inflammatory cytokine levels (IL-1β, IL-10, IL-6, IL-8, and TNF-α).
Study types
Animal studies evaluating the therapeutic effects of IN against IBD in rats and mice were included. In vitro studies and clinical case reports were excluded.
Literature selection and data extraction
Two reviewers independently extracted data, which included first author, publication year, animal strain, weight and sex, number of animals, IBD induction method, IN administration details (dose, route, and timing), and outcome measures. Graphical data were extracted using GetData Graph Digitizer 2.24. Any disagreements were resolved by discussion with a third reviewer (Yan Chen).
Assessment of risk of bias
Study quality was evaluated using the 10-point quality checklist from the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) (Bahor et al., 2021). Each criterion was rated as “yes,” “no,” or “unclear,” representing low, high, or unclear risk of bias, respectively. Two researchers (Li Huang and Xiutian Guo) independently completed the assessment. Discrepancies were resolved by consultation with a third reviewer (Yan Chen).
Statistical analysis
Meta-analysis was conducted using RevMan 5.4 software. Outcomes measured in the same units were reported using mean difference (MD), and those in different units were presented as standardized mean difference (SMD). Heterogeneity was assessed using the Cochrane I2 statistic, where I2 > 50% indicated significant heterogeneity. A fixed-effects model was used when data consistency was acceptable; otherwise, a random-effects model was employed. Sensitivity analysis of primary outcome HI was then conducted to exclude one or more studies one-by-one to infer the reliability of the results.
Subgroup analyses were performed based on animal species, IBD modeling methods, IN administration timing, dose, and route. If ten studies or more were in the meta-analysis, funnel plots were used to test the potential risk of publication bias.
Results
Basic information of included studies
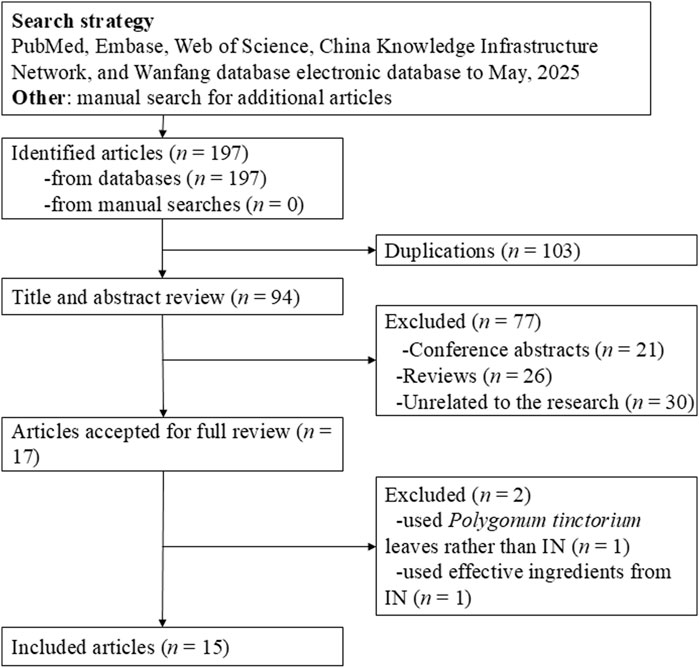
Figure 1 presents the literature search and screening process. The initial search yielded 197 articles. After removing 103 duplicates, we further excluded 21 conference abstracts, 26 reviews, and 30 irrelevant records. Full-text review was conducted on 17 articles, of which 15 were ultimately included. Among the excluded articles, one utilized Polygonum tinctorium leaves instead of IN (Asari et al., 2022) and another used active ingredients extracted from IN (Xie et al., 2023). Included studies were written in English (n = 11) and Chinese (n = 4) (Hao et al., 2013; Liu et al., 2014; Adachi et al., 2017; Kawai et al., 2017; Wang et al., 2017; Liang et al., 2019; Xiao et al., 2019; Ozawa et al., 2020; Sun et al., 2020; Qiu, 2018; Yang et al., 2021; Fan et al., 2023; Yang et al., 2023; Zhang et al., 2023; Gu et al., 2024).
Characteristics of included studies
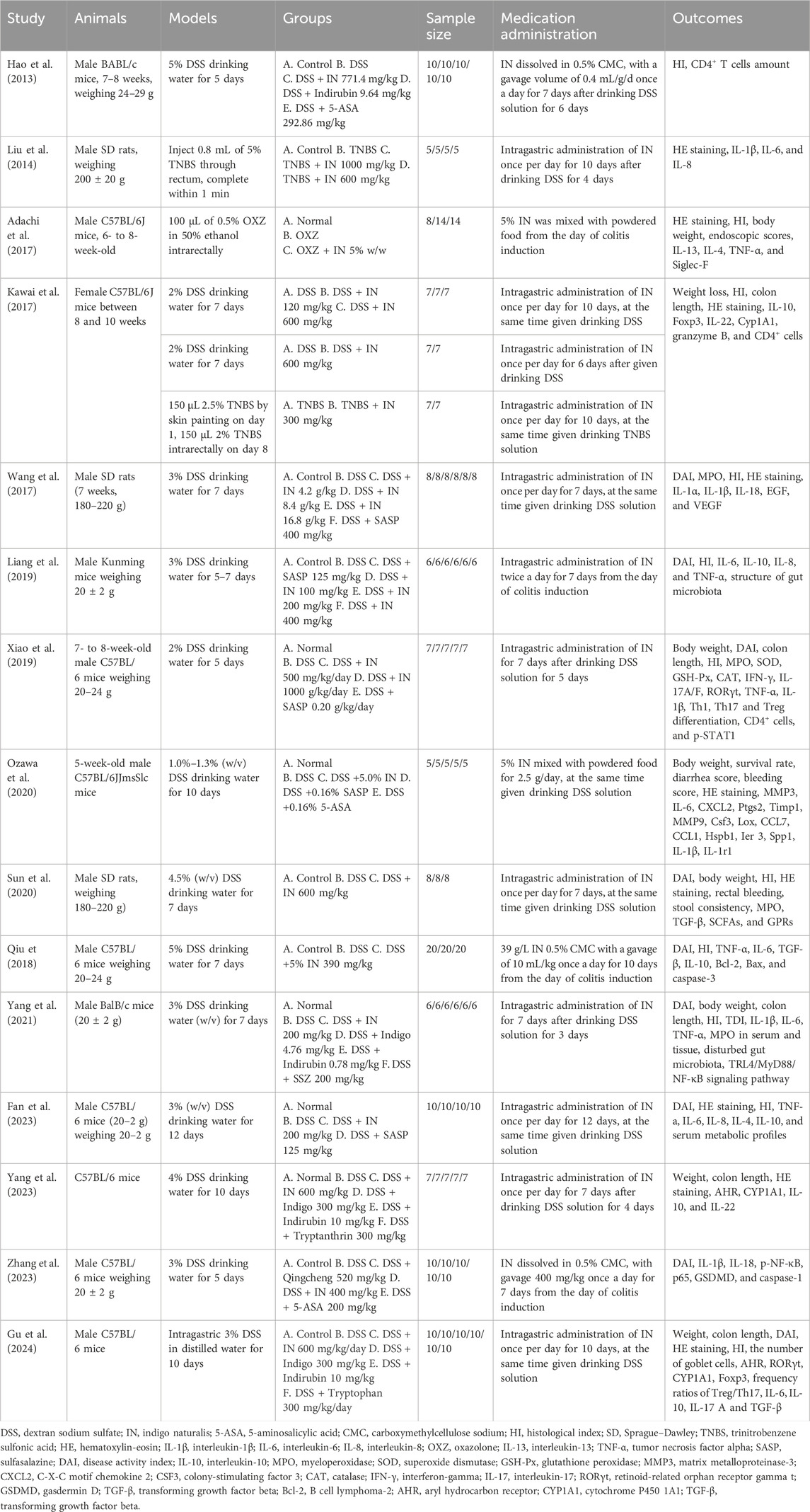
Table 1 summarizes the characteristics of the included studies. Two studies employed rats, while the remainder utilized mice (Liu et al., 2014; Wang et al., 2017). For modeling methods, 13 studies used oral DSS, two used TNBS (Liu et al., 2014; Kawai et al., 2017), and one employed OXZ (Adachi et al., 2017). Modeling duration ranged from 5 to 12 days, except for two studies using a single-time induction method (Liu et al., 2014; Adachi et al., 2017). Sample sizes ranged from 14 to 60 animals. The dosage of IN ranged from 100 mg/kg to 1,680 mg/kg. Most studies applied intragastric administration, except two that mixed IN into powdered food (Adachi et al., 2017; Ozawa et al., 2020).
Assessment of risk of bias
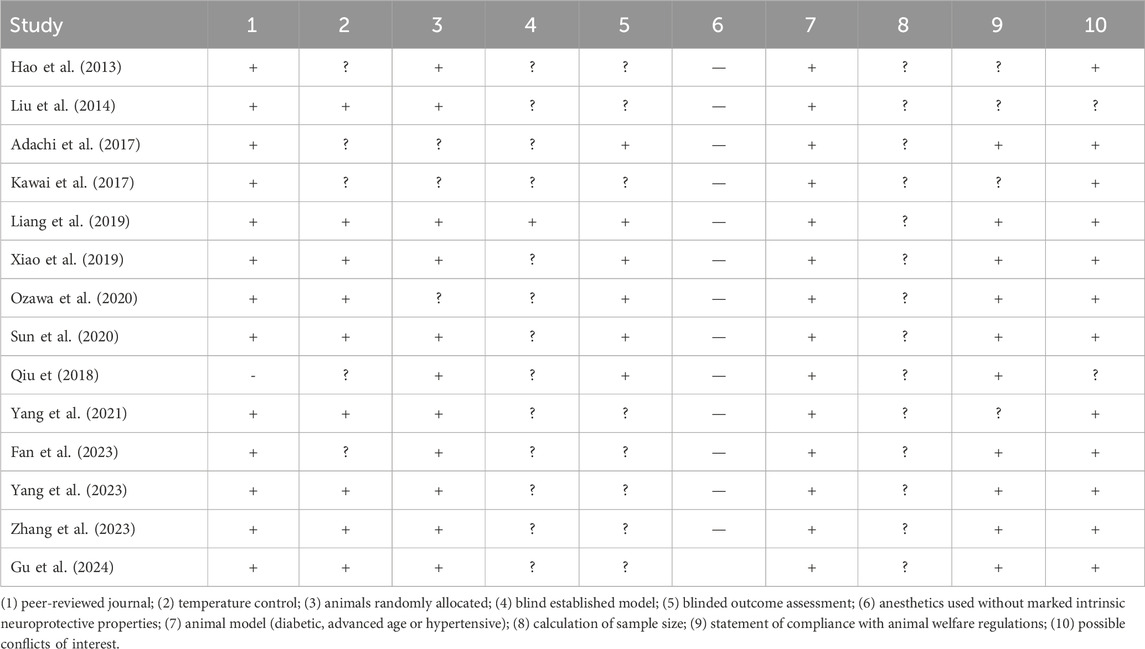
Table 2 provides details on risk-of-bias assessments. The overall quality of the included studies was acceptable. Over half had a low risk of bias in five or more items. All studies except one (Qiu, 2018) underwent peer review. Four studies (Hao et al., 2013; Liu et al., 2014; Kawai et al., 2017; Yang et al., 2021) lacked statements of confirming ethical approval for animal use or compliance with animal welfare regulations and details of the experimental implementation. Nine studies reported temperature control during animal housing, and 11 described animal randomization. All studies except one (Liang et al., 2019), failed to report blinding during model establishment. Only four studies adopted blinding when evaluating outcomes. Twelve studies did not declare conflicts of interest. Additionally, no study reported the method for sample size calculation.
Therapeutic effect of in IBD treatment
Histopathological indicators
Ten studies reported significantly reduced histopathological indices in the IN group (n = 151/137, SMD = −2.69 [-3.36, −2.02], p < 0.00001; Figure 2A), indicating protective effects on intestinal tissue. The effect was superior compared to the 3-ASA group (p < 0.05; Figure 2B). Although the heterogeneity in the included study could not be ignored, sensitivity analysis indicated that excluding individual studies does not affect the reliability of the results.
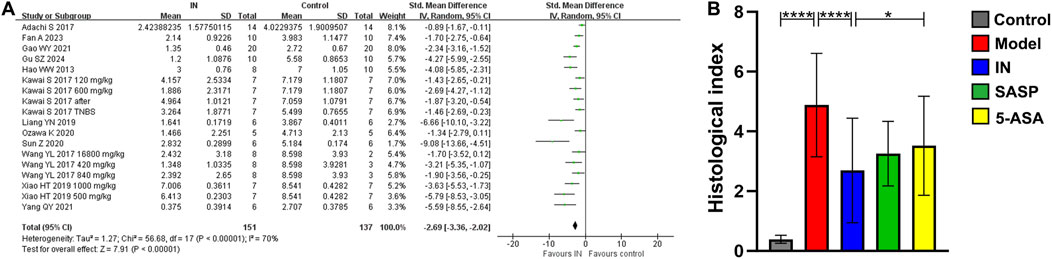
Figure 2. The meta-analysis result of histopathological index. (A). The forest plot of histopathological index related to IN treatment compared with control; (B). The bar chart of histopathological index of each group from meta-analysis.
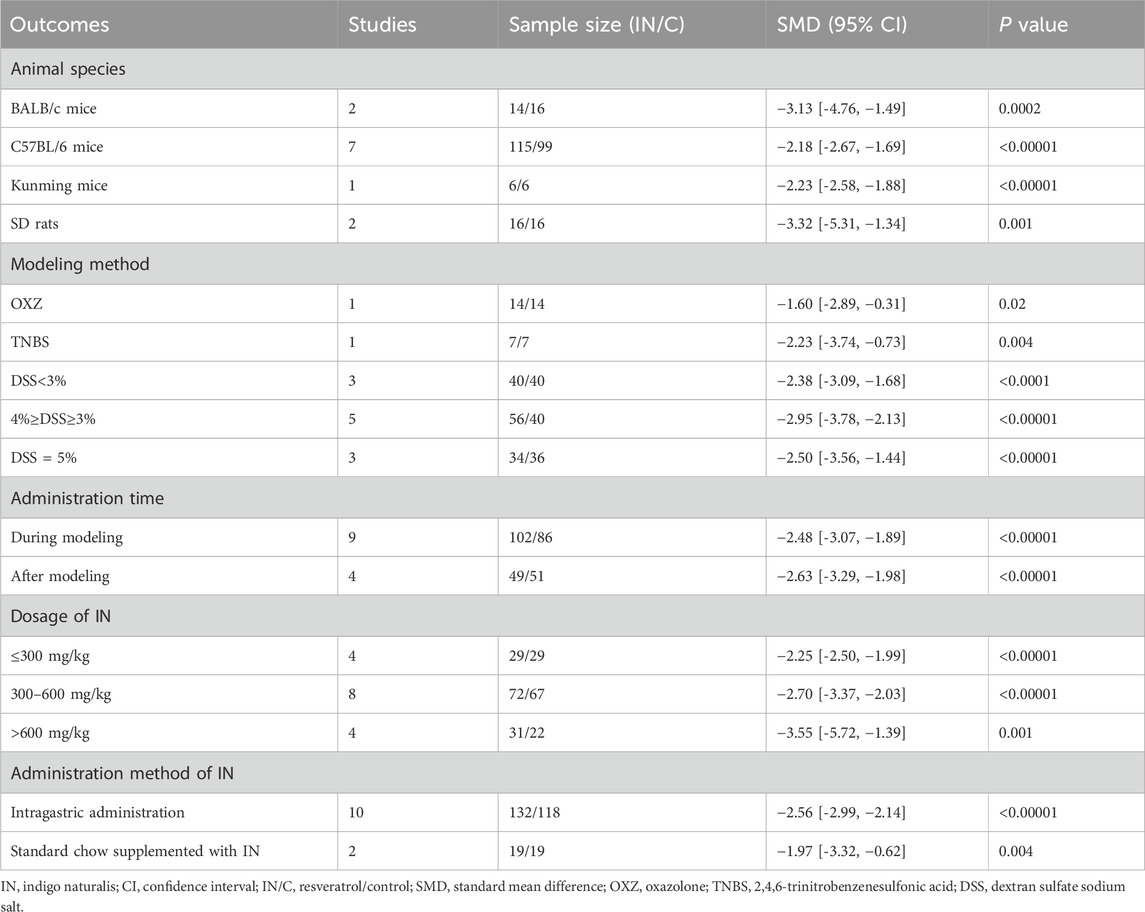
Subgroup analysis indicated that a higher IN dosage (>600 mg/kg, 4 studies, n = 31/22, SMD = −3.55 [-5.72, −1.39], p < 0.001; Table 3) produced the most significant reduction in histopathological indices compared with lower dosages (≤300 mg/kg, 4 studies, n = 29/29, SMD = −2.25 [-2.50, −1.99], p < 0.00001; 300–600 mg/kg, 8 studies, n = 72/67, SMD = −2.70 [-3.37, −2.03], p < 0.00001). No definitive conclusions were drawn regarding the influence of animal species, modeling methods, administration timing, or route of IN administration.
Final DAI score, body weight change, and colon length
Among the 15 studies, nine used final DAI to assess the therapeutic efficacy of IN. The IN group showed significantly lower final DAI scores compared to controls (n = 121/89, WMD = −1.69 [-2.18, −1.20], p < 0.00001; Figure 2).
Nine studies assessed the final percentage change in body weight. The IN group demonstrated significantly improved body weight recovery compared to the model group (n = 77/63, WMD = 9.99 [6.50, 13.49], p < 0.00001; Table 4). Additionally, colon length was significantly greater in the IN group (n = 65/51, WMD = 0.95 [0.67, 1.24], p < 0.00001; Table 4).
Effects of in on inflammatory indicators
Levels of IL-1β (five studies, n = 72/38, SMD = −5.53 [-7.51, −3.55], p < 0.0001), TNF-α (six studies, n = 68/56, SMD = −6.43 [-9.43, −3.42], p < 0.0001), IL-6 (seven studies, n = 81/63, SMD = −4.41 [-6.55, −2.16], p = 0.0003), and IL-8 (four studies, n = 48/48, SMD = −5.55 [-7.75, −3.35], p < 0.00001) significantly decreased following IN treatment. Conversely, IL-10 levels increased significantly (six studies, n = 72/60, SMD = 7.67 [4.07, 11.26], p = 0.0001). Detailed data are shown in Table 5.
Publication bias
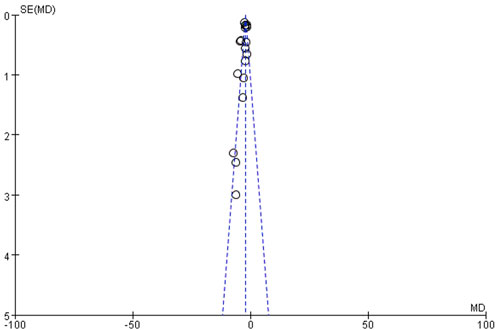
Funnel plot analysis of histopathological indices demonstrated significant asymmetry, suggesting potential publication bias (Figure 3).
Discussion
Main results
This study is the first quantitative meta-analysis to assess the efficacy of IN for IBD treatment using animal models. The overall quality of the 15 included studies was acceptable. The results indicated that IN effectively reduced IBD severity, preserved colon length, and maintained body weight by inhibiting intestinal inflammation. High-dose IN (>600 mg/kg) was more effective than low (≤300 mg/kg) and medium (300–600 mg/kg) doses in reducing the HI.
IN inhibited inflammation via regulating T cell differentiation
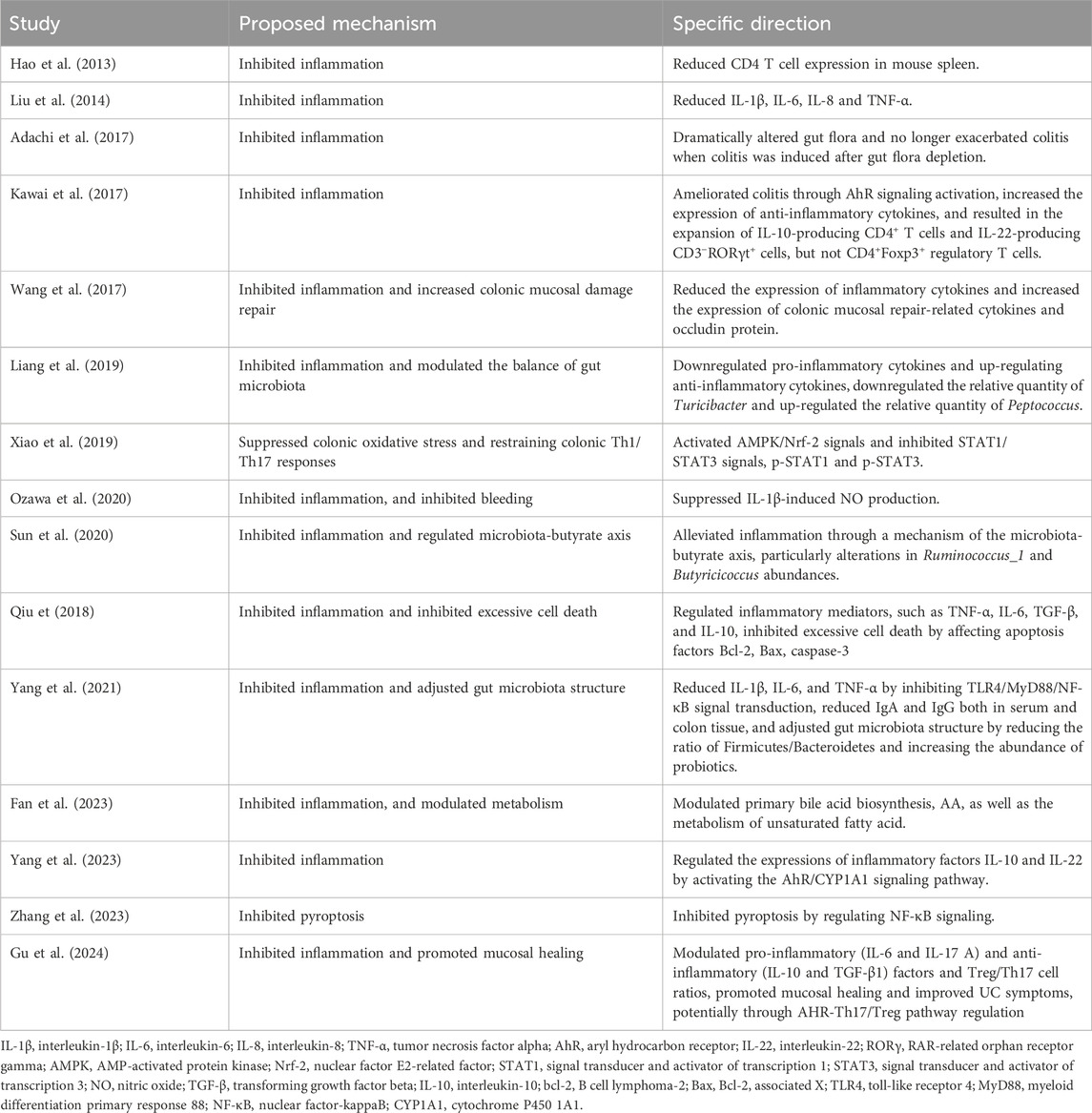
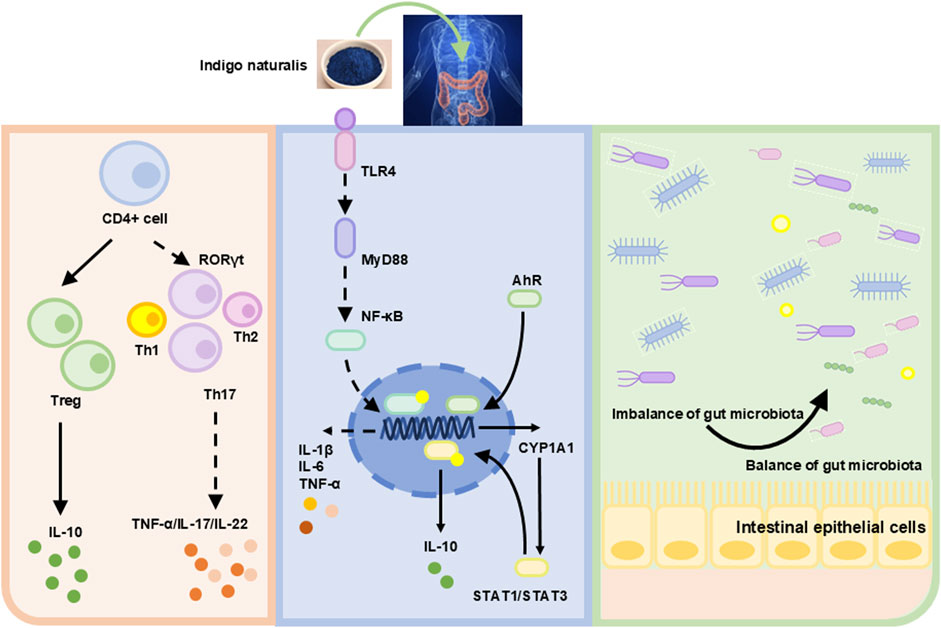
Cytokines mediate activated T-cell differentiation (Luckheeram et al., 2012). The pro-inflammatory cytokines IL-12 and IL-4 induce T cell differentiation into Th1 and Th2 cells, respectively (Ruterbusch et al., 2020). Retinoic acid receptor-related orphan receptor γ (RORγ) activates Th17 cells. Other factors, such as IL-16, also influence cell differentiation (Wu and Wan, 2020). The transcription factor forkhead box P3 (FOXP3) in regulatory T cells (Tregs) is activated by TGF-β. Elevated levels of IL-10 and TGF-β promote anti-inflammatory responses (Yue et al., 2024). RORγt, a transcription factor in innate lymphoid cells and Th17 cells, is upregulated by Tregs, specifically inhibiting type-17 immune reactions in colonic mucosa (Ren and Li, 2017). This regulatory function of Tregs exerts anti-inflammatory effects, contributing to IBD remission. Research showed that IN promoted the expansion of IL-10-producing CD4+ T cells and IL-22-inducing CD3−RORγt+ cells but did not significantly influence CD4+Foxp3+ Tregs (Kawai et al., 2017) (Figure 4).
IN inhibited inflammation through regulating the AhR/CYP1A1/STAT pathway and TLR4/Myd88 signaling pathway
The immune system consists of innate and adaptive immunity. Innate immune cells include dendritic cells, macrophages, and neutrophils, which recognize microbial patterns or products (Bruner et al., 2023). Adaptive immune cells, including B and T cells, recognize specific antigens. A balanced interaction between innate and adaptive immunity prevents excessive immune responses to commensal microbiota (Kelsen and Sullivan, 2017). Disruption of this balance leads to intestinal inflammation.
Studies have shown that microbe-associated molecular patterns (MAMPs) interacted with toll-like receptors (TLRs) or other mucosal pattern recognition receptors (PRRs) in innate immunity, potentially alleviating IBD symptoms through probiotic-associated effects (Esmaealzadeh et al., 2024). TLR4 is a primary sensor for lipopolysaccharide (LPS) derived from Gram-negative bacteria. Research indicated that TLR4 overexpression aggravated inflammation and intestinal injury in DSS-induced colitis models. Conversely, TLR4 knockout provided protection (Chen et al., 2019; Fukata et al., 2009). Thus, TLR4 signaling may significantly influence intestinal damage and repair. IN inhibits innate immunity by regulating the TLR4/MyD88/NF-κB signaling pathway (Yang et al., 2021) (Figure 4).
The aryl hydrocarbon receptor (AhR) consists of 805 amino acids. Upon activation, AhR dissociates from a protein complex and binds as a dimer to the AhR nuclear translocation protein in the nucleus, promoting downstream gene transcription (Marafini et al., 2024). Research has demonstrated reduced AhR mRNA expression in patients with UC (Monteleone et al., 2011). Animal experiments indicated that colon inflammation in UC mice worsened after treatment with AhR antagonists. AhR knockout UC mice displayed varying degrees of colon damage, including crypt deformation, epithelial cell necrosis, oedema, and accumulation of leukocytes and neutrophils (Arsenescu et al., 2011). This suggests that upregulation of AhR expression could mitigate colonic inflammation. Activated AhR can upregulate cytochrome P450 1A1 (CYP1A1) expression. Activation of the AHR/CYP1A1 signaling pathway enhances IL-10 expression and secretion, consequently increasing IL-10 receptor (IL-10R1) expression in intestinal epithelial cells (Lanis et al., 2017). IL-10 binds to IL-10R1, inducing phosphorylation of the signal transducer and activator of transcription 3 (STAT3). Phosphorylated STAT3 promotes intestinal mucosal healing by increasing the transcription of anti-apoptotic and proliferation-related genes. Clinical studies revealed lower IL-10 expression in the colonic mucosa of moderate to severe UC patients compared to mild UC or normal controls (Wittmann Dayagi et al., 2021). Additionally, IL-10 knockout mice spontaneously develop enteritis (Geng et al., 2021). This review indicated that IN alleviates colitis by activating AhR signaling and inhibiting STAT1/STAT3 signals and IL-10 (Kawai et al., 2017; Xiao et al., 2019; Yang et al., 2023).
IN modulated gut microbiota structure
Maintaining intestinal homeostasis requires the close interplay of immune, environmental, and genetic factors, as well as commensal microbiota. An imbalance in gut microbiota, especially involving pathogenic bacteria, disrupts intestinal mucosal immunity and causes mucosal damage. This increases intestinal permeability, allowing microbial invasion and ultimately triggering dysregulated mucosal immune responses (Franzosa et al., 2019). Previous studies have revealed altered gut microbiota composition in UC patients, characterized by reduced microbial diversity and decreased Firmicutes abundance (Vestergaard et al., 2024). Gut metabolites, derived from diet and microbiota-produced compounds, affect human metabolism. Short-chain fatty acids (SCFAs), including propionate, butyrate, and acetate, are produced by gut bacteria during dietary fiber decomposition. SCFAs regulate histone deacetylase activity, gene expression, cell proliferation, and immune responses. Butyrate specifically protects against colitis by promoting Treg cell differentiation and enhancing macrophage antibacterial activity. Studies reported reduced stool butyrate levels and decreased butyrate-producing bacteria in IBD patients (Franzosa et al., 2019). IN significantly altered gut microbiota, and microbiota depletion exacerbated colitis severity following induction (Adachi et al., 2017). IN downregulated the abundance of Turicibacter and upregulated Peptococcus. Another study demonstrated that IN decreased the Firmicutes/Bacteroidetes ratio and enhanced probiotic abundance, reshaping the gut microbiota composition (Yang et al., 2021; Liang et al., 2019). Furthermore, IN attenuated inflammation via the microbiota-butyrate axis, particularly by increasing the abundance of Ruminococcus 1 and Butyricicoccus. Additionally, IN modulated primary bile acid biosynthesis, arachidonic acid (AA) metabolism, and unsaturated fatty acid metabolism (Fan et al., 2023; Sun et al., 2020).
Dose of IN for IBD
This study found that IN doses exceeding 600 mg/kg demonstrated significant therapeutic efficacy in treating IBD, particularly in reducing HI. Among the included studies, four explored the relationship between dosage and efficacy. Two studies administered IN at 600 mg/kg and 1,000 mg/kg, finding that 1,000 mg/kg/day was superior in increasing rat body weight, improving DAI, and reducing serum IL-1β, IL-6, and IL-8 levels (Liu et al., 2014; Xiao et al., 2019). Another study compared doses of 600 mg/kg and 120 mg/kg, reporting better outcomes at 600 mg/kg regarding weight loss and colon length (Kawai et al., 2017). However, another study testing doses of 100, 200, and 400 mg/kg reported that 200 mg/kg IN exhibited better therapeutic efficacy than SASP (Liang et al., 2019). Based on our meta-analysis, the efficacy of doses above 600 mg/kg is generally confirmed, although definitive data remain limited. Additionally, the safety of a dosage of 1,000 mg/kg should be carefully considered. Future experiments should systematically determine the optimal dosage of IN before proceeding with further pharmacological evaluations.
Research progress on the active ingredients of in IBD
These two effective monomers are believed to inhibit the expression of pro-inflammatory factors such as IL-6 and IL-1 β through the TRL4/MyD88/NF - κ B signaling pathway and upregulate the expression of AhR/CYP1A1 pathway, promoting the expression of anti-inflammatory factors such as IL-10 (Yang et al., 2021; Yang et al., 2023). However, there are currently no reports on the specific targets of these active ingredients in treating IBD. Then a network pharmacology prediction on the treatment of UC with IN indicated that the top five targets were TNF, PTGS2, JUN, NOS3, and PPARγ, and the highly associated pathways in the prevention and treatment of UC by QD include the IL-17 signaling pathway, T-cell receptor signaling pathway, VEGF signaling pathway, and Th1 and Th2 cell differentiation. Further research can be conducted in these areas.
Strengths and limitations
This study provides evidence that supports the efficacy of IN for treating IBD in animal models and summarizes possible underlying mechanisms. Nevertheless, several limitations exist. First, the included studies were relatively few, and unpublished or recently published studies may have been overlooked, limiting the conclusions regarding the upper dosage limit of IN treatment. Additionally, current animal models primarily replicate general colitis. Due to differences in the pathological characteristics between CD and UC, these models may not fully reflect specific disease features. Thus, while IN can effectively ameliorate intestinal inflammation, its precise therapeutic role in specific disease subtypes remains uncertain. Although the study did not explicitly identify adverse reactions, it should be noted that the limited sample size and single model led to limitations in the conclusion.
Conclusion
IN significantly reduced disease severity in animal models of IBD by suppressing inflammation through balancing innate and adaptive immunity, regulating the AhR/CYP1A1 signaling pathway, and modulating gut microbiota composition. Subsequent research should first clarify the optimal dosage and administration method of IN for treating IBD. Based on this, the main active ingredients of IN should be identified through mass spectrometry, and then the specific mechanism of action of IN’s active ingredients in treating IBD should be clarified through RNA sequencing, protein chips, or enzyme digestion methods.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
JH: Conceptualization, Writing – original draft, Writing – review and editing. MZ: Investigation, Writing – original draft, Writing – review and editing. LH: Writing – original draft, Writing – review and editing. XG: Writing – original draft. PM: Writing – original draft. PL: Writing – original draft. YW: Writing – original draft. YC: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the National Natural Science Foundation of China (82405390; 82305230), Science and Technology Commission of Shanghai Municipality Science and Technology Program (23Y11920800), and the Traditional Chinese Medicine Science and Technology Development Project of Shanghai Medical Innovation & Development Foundation (WL-HBQN 2022012K). Training Program for High-caliber Talents of Clinical Research at Affiliated Hospitals of SHUTCM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IN, indigo naturalis; IBD, bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; 5-ASA, 5-aminosalicylic acid; DSS, dextran sodium sulfate; TNBS, trinitrobenzene sulfonic acid; OXZ, oxazolone; HE, hematoxylin–eosin; DAI, Disease Activity Index; IL, including interleukin; TNF-α, tumor necrosis factor-α; CAMARADES, Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies; MD, mean difference; MAMPs, microbe-associated molecular patterns; TLRs, toll-like receptors; RORγ, receptor-related orphan receptor γ; FOXP3, transcription factor forkhead box P3; AhR, aryl hydrocarbon receptor; CYP1A1, cytochrome P450 1A1; IL-10R1, IL-10 receptor; STAT3, signal transducer and activator of transcription 3; SCFAs, short-chain fatty acids.
References
Adachi, S., Hoshi, N., Inoue, J., Yasutomi, E., Otsuka, T., Dhakhwa, R., et al. (2017). Indigo naturalis ameliorates oxazolone-induced dermatitis but aggravates colitis by changing the composition of gut microflora. Int. Arch. Allergy. Immunol. 173, 23–33. doi:10.1159/000471923
Aniwan, S., Santiago, P., Loftus, E. V., and Park, S. H. (2022). The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United. Eur. Gastroenterol. J. 10, 1063–1076. doi:10.1002/ueg2.12350
Arsenescu, R., Arsenescu, V., Zhong, J., Nasser, M., Melinte, R., Dingle, R. W., et al. (2011). Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm. Bowel. Dis. 17, 1149–1162. doi:10.1002/ibd.21463
Asari, T., Kikuchi, H., Kawaguchi, S., Sakuraba, H., Yoshida, S., Akemoto, Y., et al. (2022). Polygonum tinctorium leaves suppress sodium dextran sulfate-induced colitis through interleukin-10-related pathway. Biochem. Biophys. Rep. 30, 101272. doi:10.1016/j.bbrep.2022.101272
Bahor, Z., Liao, J., Currie, G., Ayder, C., Macleod, M., McCann, S. K., et al. (2021). Development and uptake of an online systematic review platform: the early years of the CAMARADES systematic review facility (SyRF). BMJ. Open. Sci. 5, e100103. doi:10.1136/bmjos-2020-100103
Bruner, L. P., White, A. M., and Proksell, S. (2023). Inflammatory bowel disease. Prim. Care 50, 411–427. doi:10.1016/j.pop.2023.03.009
Chen, X., Liu, G., Yuan, Y., Wu, G., Wang, S., and Yuan, L. (2019). NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell. death. Dis. 10, 906. doi:10.1038/s41419-019-2157-1
Dolinger, M., Torres, J., and Vermeire, S. (2024). Crohn's disease. Lancet 403, 1177–1191. doi:10.1016/S0140-6736(23)02586-2
Esmaealzadeh, N., Ram, M., Abdolghaffari, A., Marques, A. M., and Bahramsoltani, R. (2024). Toll-like receptors in inflammatory bowel disease: a review of the role of phytochemicals. Phytomedicine 123, 155178. doi:10.1016/j.phymed.2023.155178
Fan, A., Hou, B. L., Tang, Z., Wang, T., Zhang, D., Liang, Y., et al. (2023). Liquid chromatography-tandem mass spectrometry-based metabolomics analysis of indigo naturalis treatment of ulcerative colitis in mice. J. Med. Food. 26, 877–889. doi:10.1089/jmf.2023.K.0132
Franzosa, E. A., Sirota-Madi, A., Avila-Pacheco, J., Fornelos, N., Haiser, H. J., Reinker, S., et al. (2019). Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4, 293–305. doi:10.1038/s41564-018-0306-4
Fukata, M., Hernandez, Y., Conduah, D., Cohen, J., Chen, A., Breglio, K., et al. (2009). Innate immune signaling by toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Dis 15, 997–1006. doi:10.1002/ibd.20880
Geng, Y., Yue, Y., Guan, Q., Ren, Y., Guo, L., Fan, Y., et al. (2021). Cereal vinegar sediment alleviates spontaneous ulcerative colitis in Il-10 deficient mice. Mol. Nutr. Food. Res. 65, e2001227. doi:10.1002/mnfr.202001227
Gordon, H., Biancone, L., Fiorino, G., Katsanos, K. H., Kopylov, U., Al Sulais, E., et al. (2023). ECCO guidelines on inflammatory bowel disease and malignancies. J. Crohns. Colitis. 17, 827–854. doi:10.1093/ecco-jcc/jjac187
Gu, S., Xue, Y., Liu, X., Tang, Y., Wang, D., Wu, D., et al. (2024). Clinical, pharmacology and in vivo studies of QingDai (Indigo naturalis) promotes mucosal healing and symptom improvement in ulcerative colitis by regulating the AHR-Th17/Treg pathway. J. Inflamm. 21, 44. doi:10.1186/s12950-024-00413-x
Hao, W. W., Wen, H. Z., Li, J., and Li, N. N. (2013). The effects of indigo naturalis and indigo carmine on CD4+T cells in the spleen of mice with ulcerative colitis. Zhejiang. J. Trad. Chin. Med. 48, 402–403.
Hu, Y., Chen, L. L., Ye, Z., Li, L. Z., Qian, H. Z., Wu, M. Q., et al. (2024). Indigo naturalis as a potential drug in the treatment of ulcerative colitis: a comprehensive review of current evidence. Pharm. Biol. 62, 818–832. doi:10.1080/13880209.2024.2415652
Jeong, D. Y., Kim, S., Son, M. J., Son, C. Y., Kim, J. Y., Kronbichler, A., et al. (2019). Induction and maintenance treatment of inflammatory bowel disease: a comprehensive review. Autoimmun. Rev. 18, 439–454. doi:10.1016/j.autrev.2019.03.002
Kakdiya, R., Jha, D. K., Choudhury, A., Jena, A., and Sharma, V. (2024). Indigo naturalis (qing dai) for inflammatory bowel disease: a systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 48, 102250. doi:10.1016/j.clinre.2023.102250
Kawai, S., Iijima, H., Shinzaki, S., Hiyama, S., Yamaguchi, T., Araki, M., et al. (2017). Indigo naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J. Gastroenterol. 52, 904–919. doi:10.1007/s00535-016-1292-z
Kelsen, J. R., and Sullivan, K. E. (2017). Inflammatory bowel disease in primary immunodeficiencies. Curr. Allergy. Asthma. Rep. 17, 57. doi:10.1007/s11882-017-0724-z
Kim, H., Jeong, S., Kim, S. W., Kim, H. J., Kim, D. Y., Yook, T. H., et al. (2024). Indigo naturalis in inflammatory bowel disease: mechanisms of action and insights from clinical trials. J. Pharmacopuncture 27, 59–69. doi:10.3831/KPI.2024.27.2.59
Kudo, T., Jimbo, K., Shimizu, H., Iwama, I., Ishige, T., Mizuochi, T., et al. (2022). Qing-dai for pediatric ulcerative colitis multicenter survey and systematic review. Pediatr. Int. 64 (1), e15113. doi:10.1111/ped.15113
Lanis, J. M., Alexeev, E. E., Curtis, V. F., Kitzenberg, D. A., Kao, D. J., Battista, K. D., et al. (2017). Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Immunol 10, 1133–1144. doi:10.1038/mi.2016.133
Liang, Y. N., Yu, J. G., Zhang, D. B., Zhang, Z., Ren, L. L., Li, L. H., et al. (2019). Indigo naturalis ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Molecules 24, 4086. doi:10.3390/molecules24224086
Liu, L. J., Wang, Z. B., Wang, Y. L., Xu, S. Q., We, S. B., and Li, J. X. (2014). In vitro and in vivo studies on the anti-inflammatory effects of indigo naturalis on experimental colitis. J. Beijing. Univ. Trad. Chin. Med. 37, 691–695.
Luckheeram, R. V., Zhou, R., Verma, A. D., and Xia, B. (2012). CD4+T cells: differentiation and functions. Clin. Dev. Immunol. 2012, 925135. doi:10.1155/2012/925135
Marafini, I., Monteleone, I., Laudisi, F., and Monteleone, G. (2024). Aryl hydrocarbon receptor signalling in the control of gut inflammation. Int. J. Mol. Sci. 25, 4527. doi:10.3390/ijms25084527
Matsuno, Y., Torisu, T., Umeno, J., Shibata, H., Hirano, A., Fuyuno, Y., et al. (2022). One-year clinical efficacy and safety of indigo naturalis for active ulcerative colitis: a real-world prospective study. Intestinal Res. 20, 260–268. doi:10.5217/ir.2021.00124
Monteleone, I., Rizzo, A., Sarra, M., Sica, G., Sileri, P., Biancone, L., et al. (2011). Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141, 237–248.e1. doi:10.1053/j.gastro.2011.04.007
Naganuma, M. (2019). Treatment with indigo naturalis for inflammatory bowel disease and other immune diseases. Immunol. Med. 42, 16–21. doi:10.1080/25785826.2019.1599158
Naganuma, M., Sugimoto, S., Mitsuyama, K., Kobayashi, T., Yoshimura, N., Ohi, H., et al. (2018). Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154, 935–947. doi:10.1053/j.gastro.2017.11.024
Nakase, H. (2020). Treatment of inflammatory bowel disease from the immunological perspective. Immunol. Med. 43, 79–86. doi:10.1080/25785826.2020.1751934
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778. doi:10.1016/S0140-6736(17)32448-0
Ozawa, K., Mori, D., Hatanaka, A., Sawano, T., Nakatani, J., Ikeya, Y., et al. (2020). Comparison of the anti-colitis activities of qing dai/indigo naturalis constituents in mice. J. Pharmacol. Sci. 142, 148–156. doi:10.1016/j.jphs.2020.01.003
Prentice, R., Wright, E. K., Flanagan, E., Prideaux, L., Goldberg, R., and Bell, S. J. (2022). Preconception, antenatal and postpartum management of inflammatory bowel disease. Aust. J. Gen. Pract. 51, 747–753. doi:10.31128/AJGP-04-22-6400
Qiu, G. Y. (2018). “Experimental study on the treatment of DSS induced ulcerative colitis in mice with indigo naturalis,”. Liaoning: Liaoning University of Traditional Chinese Medicine. Master’s thesis.
Ren, J., and Li, B. (2017). The functional stability of FOXP3 and RORγt in treg and Th17 and their therapeutic applications. Adv. Protein. Chem. Struct. Biol. 107, 155–189. doi:10.1016/bs.apcsb.2016.10.002
Ruterbusch, M., Pruner, K. B., Shehata, L., and Pepper, M. (2020). In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu. Rev. Immunol. 38, 705–725. doi:10.1146/annurev-immunol-103019-085803
Saiki, J. P., Andreasson, J. O., Grimes, K. V., Frumkin, L. R., Sanjines, E., Davidson, M. G., et al. (2021). Treatment-refractory ulcerative colitis responsive to indigo naturalis. BMJ open Gastroenterol. 8 (1), e000813. doi:10.1136/bmjgast-2021-000813
Singh, N., and Bernstein, C. N. (2022). Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 10, 1047–1053. doi:10.1002/ueg2.12319
Sun, Q., Leng, J., Tang, L., Wang, L., and Fu, C. (2021). A comprehensive review of the chemistry, pharmacokinetics, pharmacology, clinical applications, adverse events, and quality control of indigo naturalis. Front. Pharmacol. 12, 664022. doi:10.3389/fphar.2021.664022
Sun, Z., Li, J., Dai, Y., Wang, W., Shi, R., Wang, Z., et al. (2020). Indigo naturalis alleviates dextran sulfate sodium-induced colitis in rats via altering gut microbiota. Front. Microbiol. 11, 731. doi:10.3389/fmicb.2020.00731
Uchiyama, K., Takami, S., Suzuki, H., Umeki, K., Mochizuki, S., Kakinoki, N., et al. (2020). Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: an investigator-initiated multicenter double-blind clinical trial. PLoS. One. 15, e0241337. doi:10.1371/journal.pone.0241337
Vestergaard, M. V., Allin, K. H., Eriksen, C., Zakerska-Banaszak, O., Arasaradnam, R. P., Alam, M. T., et al. (2024). Gut microbiota signatures in inflammatory bowel disease. United. Eur. Gastroenterol. J. 12, 22–33. doi:10.1002/ueg2.12485
Wang, Y., Liu, L., Guo, Y., Mao, T., Shi, R., and Li, J. (2017). Effects of indigo naturalis on colonic mucosal injuries and inflammation in rats with dextran sodium sulphate-induced ulcerative colitis. Exp. Ther. Med. 14, 1327–1336. doi:10.3892/etm.2017.4701
Wittmann Dayagi, T., Werner, L., Pinsker, M., Salamon, N., Barschak, I., Weiss, B., et al. (2021). Mucosal IL-10 and IL-10 receptor expression patterns in paediatric patients with ulcerative colitis. Int. J. Exp. Pathol. 102, 4–10. doi:10.1111/iep.12382
Wu, B., and Wan, Y. (2020). Molecular control of pathogenic Th17 cells in autoimmune diseases. Int. Immunopharmacol. 80, 106187. doi:10.1016/j.intimp.2020.106187
Xiao, H. T., Peng, J., Wen, B., Hu, D. D., Hu, X. P., Shen, X. C., et al. (2019). Indigo naturalis suppresses colonic oxidative stress and Th1/Th17 responses of DSS-induced colitis in mice. Oxid. Med. Cell. Longev. 2019, 9480945. doi:10.1155/2019/9480945
Xie, J., Tian, S., Liu, J., Huang, S., Yang, M., Yang, X., et al. (2023). Combination therapy with indigo and indirubin for ulcerative colitis via reinforcing intestinal barrier function. Oxid. Med. Cell. Longev. 2023, 2894695. doi:10.1155/2023/2894695
Yang, G., Gu, S. Z., Xue, Y., Liu, X. W., and Dou, D. B. (2023). Discussion on the mechanism of indigo naturalis and its main components in relieving ulcerative colitis based on aryl hydrocarbon receptor. Chin. J. Inf. TCM. 30, 126–131.
Yang, Q. Y., Ma, L. L., Zhang, C., Lin, J. Z., Han, L., He, Y. N., et al. (2021). Exploring the mechanism of indigo naturalis in the treatment of ulcerative colitis based on TLR4/MyD88/NF-κB signaling pathway and gut microbiota. Front. Pharmacol. 12, 674416. doi:10.3389/fphar.2021.674416
Yang, Q. Y., Zhang, T., He, Y. N., Huang, S. J., Deng, X., Han, L., et al. (2020). From natural dye to herbal medicine: a systematic review of chemical constituents, pharmacological effects and clinical applications of indigo naturalis. Chin. Med. 15, 127. doi:10.1186/s13020-020-00406-x
Yue, L. I., Shuting, W., Runyuan, Z., Dongmei, F., Dike, Z., Fengbin, L., et al. (2023). Efficacy of active ingredients in qingdai on ulcerative colitis: a network pharmacology-based evaluation. J. Tradit. Chin. Med. 43, 124–133. doi:10.19852/j.cnki.jtcm.2023.01.011
Yue, Y., Ren, Y., Lu, C., Li, P., and Zhang, G. (2024). Epigenetic regulation of human FOXP3+ tregs: from homeostasis maintenance to pathogen defense. Front. Immunol. 15, 1444533. doi:10.3389/fimmu.2024.1444533
Zhang, E. X., Hao, W. W., Wang, Z. H., Yuan, Z. W., Zheng, Q. W., and Wu, Y. (2023). Mechanism of action of qingdai and its active ingredient indigofera in regulating cellular pyroptosis in mice with ulcerative colitis through NF-κB signaling. Chin. J. Trad. Chin. Med. 38, 4705–4710.
Keywords: indigo naturalis, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, histopathological index
Citation: Hu J, Zhou M, Huang L, Guo X, Mei P, Li P, Wang Y and Chen Y (2025) Indigo naturalis for inflammatory bowel disease: evidence from animal studies and molecular mechanisms. Front. Pharmacol. 16:1588233. doi: 10.3389/fphar.2025.1588233
Received: 12 March 2025; Accepted: 16 June 2025;
Published: 18 July 2025.
Edited by:
Cheorl-Ho Kim, Sungkyunkwan University, Republic of KoreaReviewed by:
Hemanga Hazarika, Girijananda Chowdhury University, IndiaLiming Mao, Nantong University, China
Victor Serrano-Fernandez, University of Castilla La Mancha, Spain
Copyright © 2025 Hu, Zhou, Huang, Guo, Mei, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, NDk4MzU3NjMyQHFxLmNvbQ==
†These authors have contributed equally to this work, three of them were the first authors.
 Jie Hu†
Jie Hu† Mengen Zhou
Mengen Zhou Pingping Mei
Pingping Mei Peng Li
Peng Li Yiting Wang
Yiting Wang Yan Chen
Yan Chen