- 1National Regional Traditional Chinese Medicine (Lung Disease) Diagnosis and Treatment Center, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, China
- 2The First Clinical College of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan, China
- 3Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases Co-Constructed by Henan Province & Education Ministry of P.R. Zhengzhou, Zhengzhou, Henan, China
- 4Henan Key Laboratory of Chinese Medicine for Respiratory Disease, Zhengzhou, Henan, China
Objectives: Reduning (RDN) injection is a traditional Chinese medicine (TCM) extract injection commonly used as an adjunct therapy for severe pneumonia (SP) in clinical practice in China; however, further validation is required. This study aims to systematically assess the efficacy and safety of RDN injection in treating SP.
Methods: The study was conducted using the data from CNKI, WanFang, VIP, SinoMed, PubMed, Embase, Cochrane Library, and Web of Science databases from their inception to 6 April 2024. We collected randomized controlled trials on the treatment of SP using RDN injection, and used Review Manager (version 5.3) for meta analysis. The primary outcomes was clinical efficacy. We used the Cochrane Risk of Bias Assessment Tool 2 (RoB 2) to assess the risk of bias for each study.
Results: A total of 1,897 patients with SP from 22 studies were included. The meta-analysis results showed that the combination of RDN injection and conventional treatment or antibiotic treatment for patients with SP was superior to traditional and antibiotic treatments alone in terms of clinical efficacy [risk ratio = 1.25%; 95% confidence interval (CI) (1.20, 1.30); p < 0.00001], fever reduction time [mean difference (MD) = −1.55 days; 95% CI (−1.91, −1.18); p < 0.00001], cough disappearance time [MD = −1.97 days; 95% CI (−2.63, −1.31); p < 0.00001], lung rales disappearance time [MD = −2.39 days; 95% CI (−3.09, −1.70); p < 0.00001], chest X-ray improvement time [MD = −2.73 days; 95% CI (−2.96, −2.49); p < 0.00001], and hospitalization time [MD = −3.11 days; 95% CI (−3.80, −2.41); p < 0.00001]. Moreover, no significant improvement was observed in the Acute Physiology and Chronic Health Evaluation II (APACHE II) scale compared to the control group [MD = −2.45 points; 95% CI (−6.07, 1.17); p = 0.19]. Lastly, not all studies reported any serious adverse events; however, some studies did report adverse reactions.
Conclusion: The administration of RDN injection as an adjunct therapy for SP can enhance clinical efficacy, reduce fever duration, accelerate cough resolution, shorten the time for lung rales to resolve, accelerate chest X-ray improvement, and decrease hospitalization duration. Further well-designed and standardized large sample clinical studies are needed for validation.
Systematic review registration: PROSPERO, identifier CRD42024540365.
1 Introduction
Pneumonia is a common lung infection disease with infectivity, which disrupts the normal lives of patients (Liu et al., 2018). Pneumonia worsens and progresses to severe pneumonia (SP), causing obstruction of gas exchange in patients, leading to cerebral edema, hypovolemia, shock, and even other life-threatening conditions (Li et al., 2015). SP typically manifests systemically. Patients may experience high fever, severe chills, sweating, coughing up purulent or bloody sputum, fatigue, headache, muscle pain, and other symptoms (Ching and Pedersen, 2025). SP is a critical respiratory disease characterized by high mortality (Ramirez et al., 2011), multiple complications (Lee et al., 2016), a mortality rate of up to 30%–50% (Restrepo et al., 2010), and an increased burden on medical economics (Guan et al., 2025). Proactively investigating effective intervention strategies is crucial for reducing disease duration and mortality rates (Dinh et al., 2021).
The current guidelines indicate that SP is typically treated with antibiotics, mechanical ventilation, and corticosteroids. However, the use of glucocorticoids in SP is a conditional recommendation with low-quality evidence (Martin-Loeches et al., 2023), and prescribing excessive antibiotics may cause antibiotic resistance, potentially increasing mortality rates (Musher and Thorner, 2014; Aliberti et al., 2019). Studies have indicated that the use of corticosteroids to treat SP may have adverse reactions (Wu et al., 2023; See et al., 2024). Therefore, the prevention and treatment of SP remain a public health issue worthy of attention (Torres et al., 2019). The frequency of reports regarding the treatment of SP with Chinese herbal injections (CHIs) is rising, and their efficacy has been validated (Niu et al., 2022; Xiao et al., 2022). CHIs can proficiently address the medication challenges faced by patients who are comatose or have swallowing difficulties. Reduning (RDN) injection was approved as a traditional Chinese medicine in 2005 (Z20050217). RDN injection is effective in treating inflammatory diseases, with research indicating that Bcl-2, eNOS, PTGS2, PPARA, and MMPs play a crucial role in modulating the inflammatory processes associated with RDN injection compounds and metabolites (Xie et al., 2020). Recently, RDN injection has been widely used as an adjuvant therapy for SP in China, with confirmed efficacy of RDN injection in treating pneumonia (Cao et al., 2020). However, there is still a lack of systematic reviews on the use of RDN injection for SP. This study investigated randomized controlled trials (RCTs) of RDN injection as an adjunctive therapy for SP, systematically assessing its clinical efficacy and safety in treating SP, and providing guidance for the clinical management of SP.
2 Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines (Page et al., 2021), and the protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024540365).
2.1 Eligibility criteria
The inclusion criteria were: (1) All studies must be RCTs without language restrictions; (2) participants must be diagnosed with SP according to any internationally accepted guidelines, aged over 18 years, irrespective of gender or ethnicity; and (3) the experimental group received either combined or non-combined conventional treatment with RDN Injection, while the control group received either conventional treatment or a placebo.
2.2 Exclusion criteria
The exclusion criteria were: (1) Duplicate publications; (2) Integration of literature on other diseases; (3) Animal experimental studies; and (4) Inaccessibility of original literature.
2.3 Search strategy
We searched across the China National Knowledge Infrastructure (CNKI), WanFang, the Chinese Scientific Journal Database (VIP), SinoMed, PubMed, Embase, the Cochrane Library, and Web of Science databases, with no language restrictions, from the inception of the databases until 6 April 2024. The search terms were: “Reduning” or “Reduning injection” and “Severe pneumonia” or “Serious pneumonia” and “random” or “randomized controlled trial” or “controlled trial” (Supplementary Table S1).
2.4 Research selection
Two researchers (YW and BCX) independently searched, screened, and extracted data according to the inclusion and exclusion criteria. Initially, repetitive and unrelated literature was excluded based on the title and abstract; pertinent literature, along with its references, was then investigated. The full text of qualified literature was read individually. If a disagreement arose between the two researchers, a third researcher (PZ) was consulted to reach a consensus.
2.5 Data extraction
The two researchers independently extracted data, including the lead author, publication date, country, research type, research duration, diagnostic criteria, sample size, age, sex, outcome indicators, and additional information, using a pre-designed form according to the PRISMA 2020 guidelines (Page et al., 2021), and cross-checked them. When two researchers encountered disagreements, a third researcher (PZ) was tasked with reaching a consensus.
2.6 Outcomes
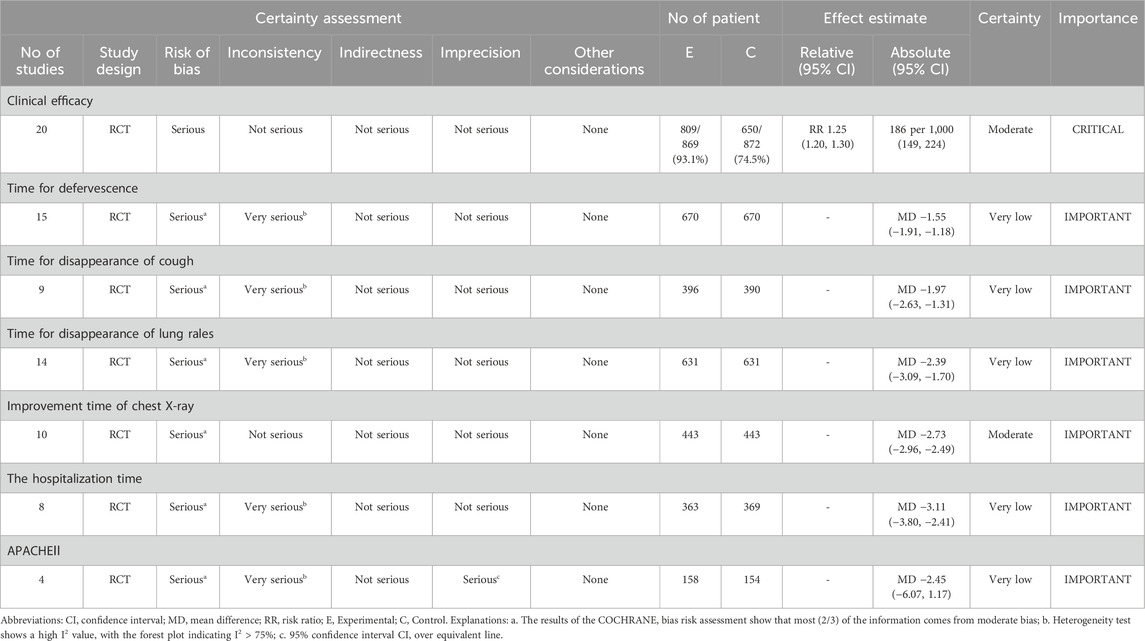
The primary outcome measure was clinical efficacy. The secondary outcome measures were duration until defervescence, to cough resolution, time to disappearance of lung rales, time to improvement in chest X-ray, length of hospitalization, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and adverse reactions.
2.7 Risk-of-bias assessment
This study employed the Cochrane Risk of Bias Assessment Tool 2 (RoB 2) (Sterne et al., 2019) to evaluate the included literature, focusing on the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results.
2.8 Statistical analysis
A meta-analysis was conducted using Review Manager (version 5.3). Risk ratio was used for ranked variables, and MD was used for continuous variables. This study utilized 95% CI to evaluate all outcome measures. I2 < 50% indicated low heterogeneity, and thus, a fixed-effects model was used; I2 ≥ 50% indicated high heterogeneity, and a random-effects model was used. When I2 was non-zero and the meta-analysis encompassed four or more studies, a subgroup analysis was performed based on RDN dosage or treatment duration. A sensitivity analysis was performed to sequentially exclude individual studies. A funnel plot was used to analyze for any publication bias. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (Gonzalez-Padilla and Dahm, 2021) was used to evaluate the quality of outcome measures. The quality of evidence was categorized into four levels: high, moderate, low, and very low.
3 Results
3.1 Search results
A total of 150 articles were retrieved, and 67 duplicate articles were excluded. Thirty-four irrelevant articles were excluded after reading the titles and abstracts. After analyzing the full text, 27 articles were excluded, including 20 studies focused on children, 3 articles on animal experiments, 2 articles that integrated on other diseases, and 2 articles for which the original text was inaccessible. Finally, we included 22 articles. The literature screening process is shown in Figure 1.
3.2 Characteristics of studies
A total of 22 studies RCTs were included (Sun et al., 2012; Yin et al., 2013; Liu and Liu, 2014; Li, 2014; Lin, 2015; Zhang et al., 2016; Nie, 2017; Zhou, 2017; Ding, 2017; Fang et al., 2018; Chen, 2018; Zhu et al., 2019; Tan et al., 2019; Yan et al., 2019; Shan, 2020; Wang et al., 2020; Bai and Xu, 2021; Pan, 2022; Guo and Huo, 2022; Li and Shao, 2022; Xing and Lin, 2022; Wang SG. et al., 2023). All studies were conducted in China, encompassing 1897 patients with SP, with 950 in the experimental group and 947 in the control group. The minimum sample size was 60 and the maximum sample size was 140. The control group received treatment methods that include conventional treatment, antibiotics, a combination of conventional treatment and antibiotics, a combination of conventional treatment and protease inhibitors, and a combination of conventional treatment and bronchoalveolar lavage. The treatment methods received by the experimental group include RDN injection combined with conventional treatment, RDN injection with antibiotics, RDN injection with conventional treatment and antibiotics, RDN injection with conventional treatment and immunosuppressants, RDN injection with conventional treatment and a protease inhibitor, and RDN injection combined with conventional treatment and bronchoalveolar lavage. All patients receiving treatment with RDN injection received an intravenous injection of RDN once daily, with injection doses of 0.6 mL/kg, 20 mL, and 30 mL. The duration of treatment ranged from 3 to 14 days. The treatment duration for 13 studies exceeded 7 days, while the treatment duration for 9 studies was 7 days or less. The basic characteristics included in the study are shown in Table 1.
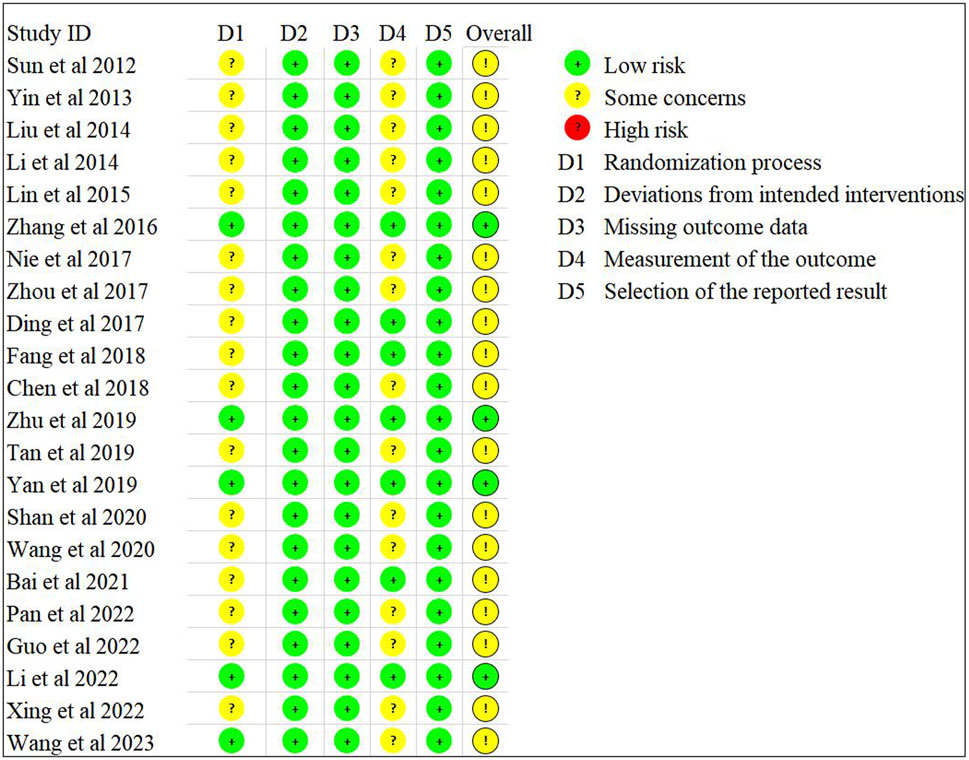
3.3 Risk-of-bias assessment
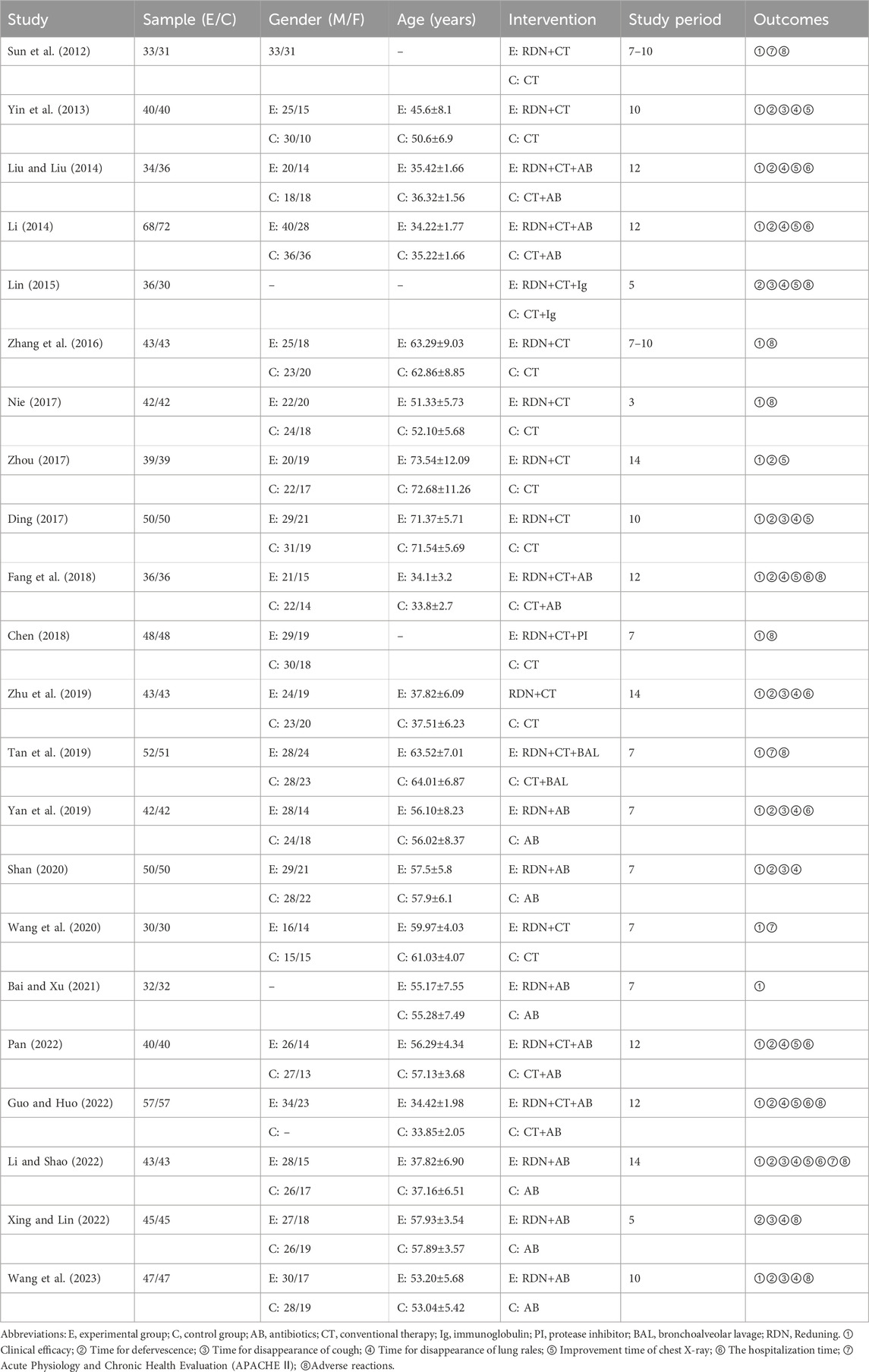
Sixteen studies (Sun et al., 2012; Yin et al., 2013; Zhang et al., 2016; Nie, 2017; Zhou, 2017; Ding, 2017; Fang et al., 2018; Chen, 2018; Zhu et al., 2019; Yan et al., 2019; Shan, 2020; Wang et al., 2020; Bai and Xu, 2021; Pan, 2022; Li and Shao, 2022; Wang SG. et al., 2023) employed a random grouping method, of which only seven studies (Zhang et al., 2016; Zhou, 2017; Chen, 2018; Zhu et al., 2019; Yan et al., 2019; Li and Shao, 2022; Wang SG. et al., 2023) utilized a random number table method, while nine studies (Sun et al., 2012; Yin et al., 2013; Nie, 2017; Ding, 2017; Fang et al., 2018; Shan, 2020; Wang et al., 2020; Bai and Xu, 2021; Pan, 2022) only mentioned “random” without detailing the specific randomization method. The 22 studies neither specified whether allocation concealment and blinding were implemented nor presented evidence of selective reporting or other possible sources of bias. The risk bias assessment is illustrated in Figures 2, 3.
3.4 Clinical efficacy
Twenty studies (n = 1,741) were included to report clinical efficacy. The meta-analysis revealed p = 0.99 and I2 = 0%, indicating low heterogeneity and the application of a fixed-effects model. It also indicated that the clinical efficacy rate of the experimental group is significantly higher than the control group (risk ratio = 1.25%; 95% CI [1.20, 1.30]; p < 0.00001) (Figure 4).
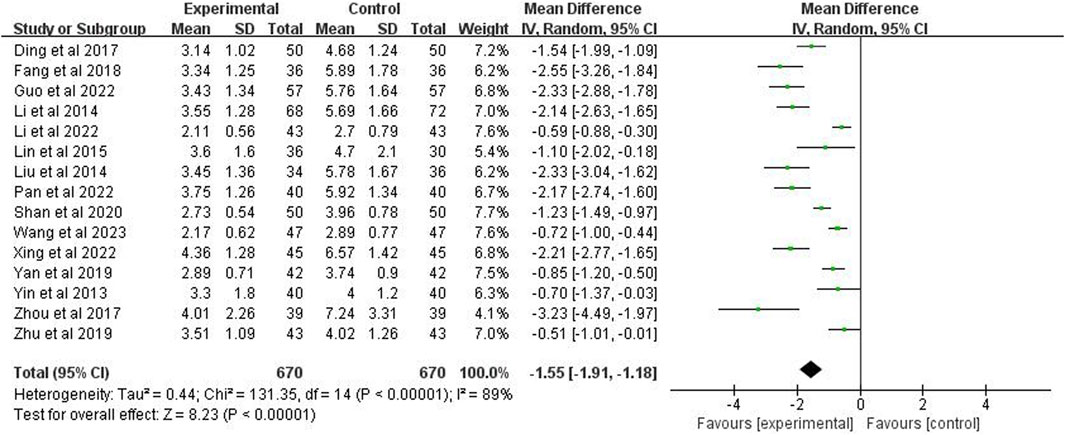
3.5 Time for defervescence
Fifteen studies (n = 1,340) were included to report the time to defervescence. The analysis demonstrated a p < 0.00001 and I2 = 89%, indicating high heterogeneity, using a random-effects model. It further indicated that the fever reduction time in the experimental group was significantly shorter than the control group (MD = −1.55 days; 95% CI [−1.91, −1.18]; p < 0.00001) (Figure 5).
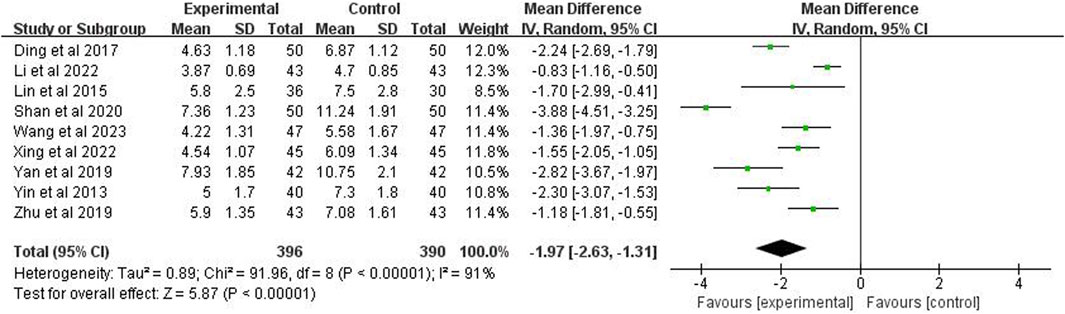
3.6 Time for cough disappearance
Nine studies (n = 786) reported the time for the disappearance of cough. The meta-analysis showed, using a random effects model, p < 0.00001 and I2 = 91%, indicating high heterogeneity. The analysis also demonstrated that the cough disappearance time in the experimental group was significantly shorter than the control group (MD = −1.97 days; 95% CI [−2.63, −1.31]; p < 0.00001) (Figure 6).
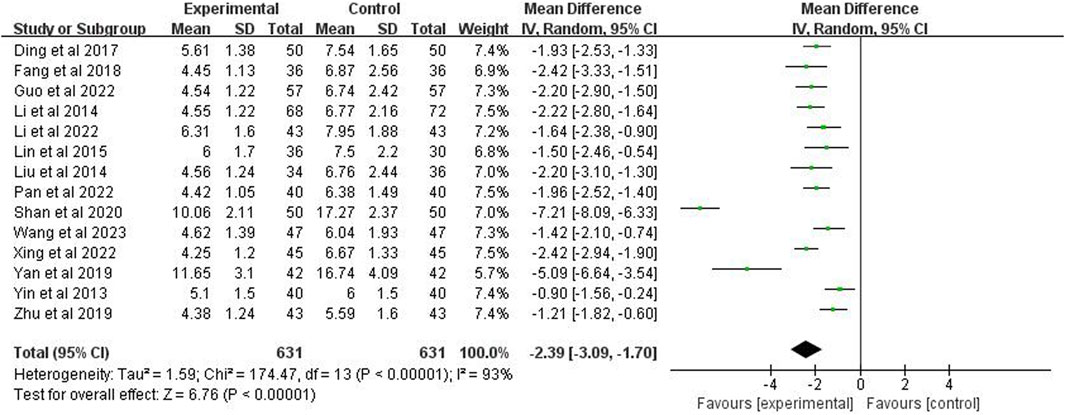
3.7 Time for disappearance of lung rales
The time to disappearance of lung rales was included in 14 studies (n = 1,262). Through a random effects model, the meta-analysis demonstrated a p < 0.00001 and I2 = 93%, indicating high heterogeneity. The analysis further showed that the disappearance time of lung rales in the experimental group was significantly shorter than the control group (MD = −2.39 days; 95% CI [−3.09, −1.70]; p < 0.00001) (Figure 7).
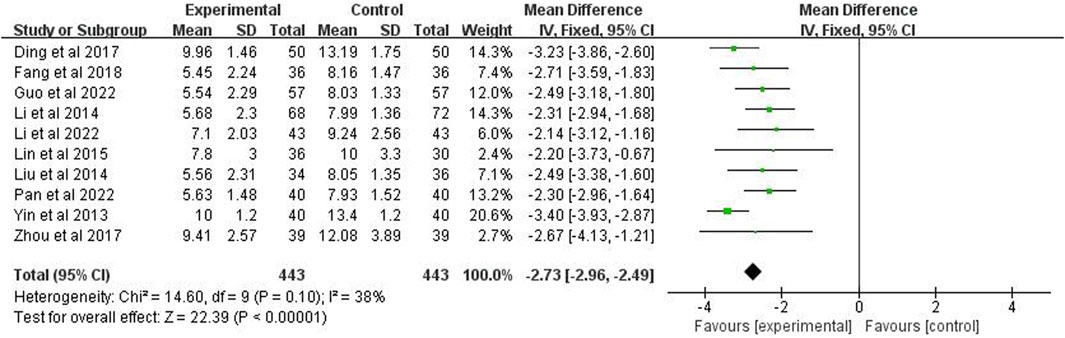
3.8 Time for improvement time of chest X-ray
The improvement time of chest X-ray was reported in studies (n = 886). The analysis showed p = 0.10 and I2 = 38% after applying a fixed effects model, indicating low heterogeneity. It also showed that the improvement time of chest X-rays in the experimental group was significantly shorter than the control group (MD = −2.73 days; 95% CI [−2.96, −2.49]; p < 0.00001) (Figure 8).
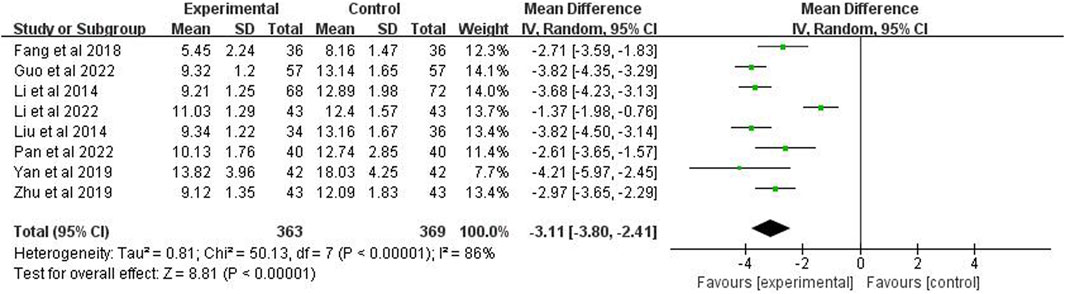
3.9 The hospitalization time
Eight studies (n = 732) reported the hospitalization time. After using the random effects model, the meta-analysis showed p < 0.00001 and I2 = 86%, indicating high heterogeneity. It also showed that the hospitalization time of the experimental group was significantly shorter than the control group (MD = −3.11 days; 95% CI [−3.80, −2.41]; p < 0.00001) (Figure 9).
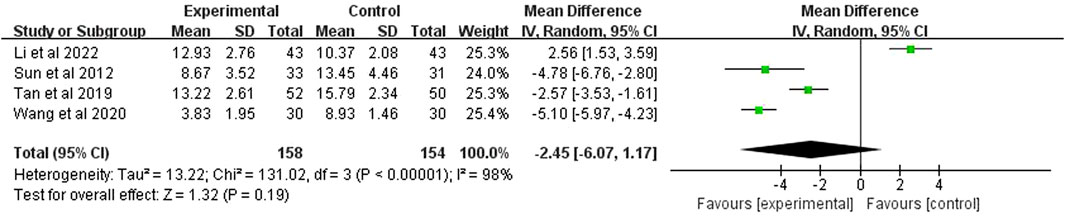
3.10 APACHE Ⅱ
APACHE II score was reported in 4 studies (n = 312). The analysis, after using a random effects model, showed p < 0.00001 and I2 = 98%, indicating high heterogeneity. It also demonstrated that the APACHE II score of the experimental group was significantly higher than the control group (MD = −2.45 points; 95% CI [−6.07, 1.17]; p = 0.19) (Figure 10).
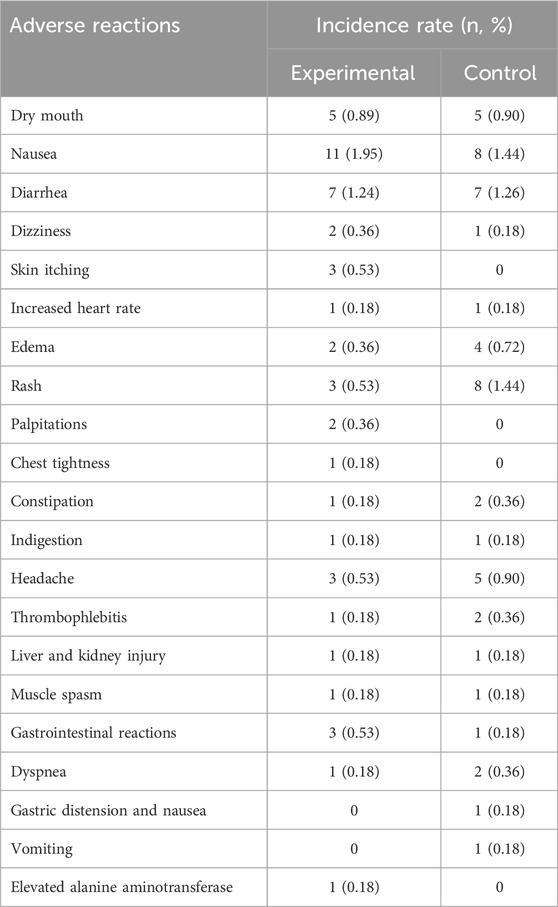
3.11 Adverse reactions
Adverse reactions were documented in 13 studies, with 2 studies indicating no adverse reactions, while 11 studies (Sun et al., 2012; Lin, 2015; Zhang et al., 2016; Nie, 2017; Zhou, 2017; Fang et al., 2018; Chen, 2018; Tan et al., 2019; Yan et al., 2019; Guo and Huo, 2022; Li and Shao, 2022; Xing and Lin, 2022; Wang SG. et al., 2023) reported reactions such as nausea, diarrhea, rash, headache, and dizziness. The remaining nine studies also reported no adverse reactions. The incidence of adverse events in the experimental group was 8.88% (50/563), whereas in the control group it was 9.21% (51/554) (Table 2). Additional evidence is necessary to establish the safety of RDN injection in the treatment of SP.
3.12 Subgroup analyses
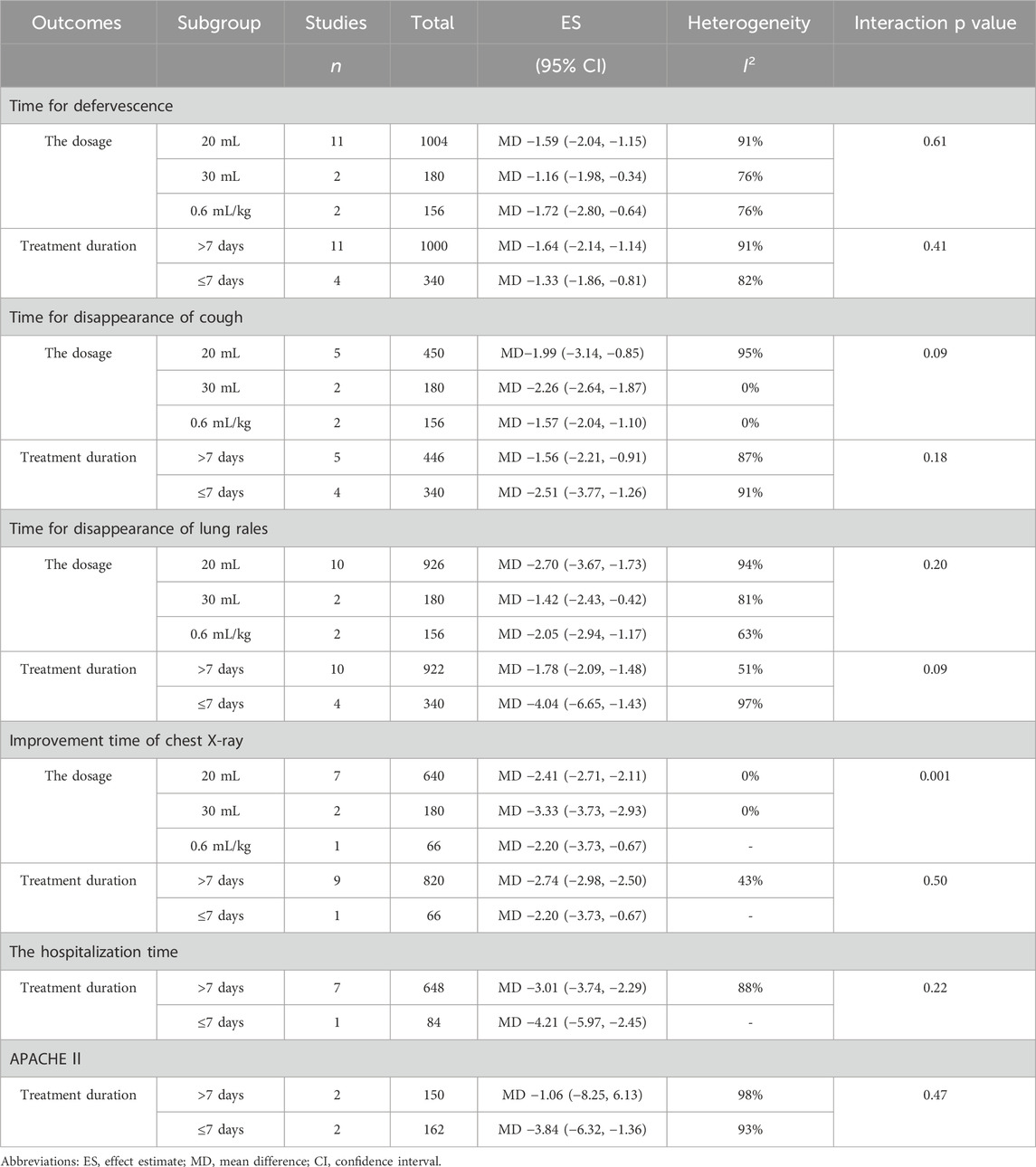
The subgroup analysis indicated that the duration of improvement in chest X-ray following treatment of SP with 30 mL of RDN Injection was superior to that observed with 20 mL and 0.6 mL/kg (MD −3.33 vs. −2.41 vs. −2.20, interaction p = 0.001). The analysis results for other subgroups showed that the p-value for the interaction was greater than 0.05, indicating no subgroup differences (Table 3).
3.13 Sensitivity analysis
An individual exclusion method was employed for the sensitivity analysis, and the results revealed no significant changes, indicating the stability of the findings.
3.14 Publication bias
A plotted a funnel plot of the main indicators of clinical efficacy was generated, and the results showed poor symmetry, with the studies distributed on both sides of the vertical line. Additionally, Begg and Egger tests were conducted, revealing a p-value of 0, signifying publication bias potentially associated with the subpar quality of the literature (Figure 11).
3.15 Quality of evidence
The GRADE evaluation indicated that the evidence for clinical efficacy and the time to improvement in chest X-ray quality was classified as moderate. In contrast, other outcome measures were deemed to be of very low quality (Table 4).
4 Discussion
SP is a significant respiratory ailment characterized by complex and challenging etiologies, affecting people of all ages globally. Patients with SP admitted to the ICU are associated with high mortality rates (Espinoza et al., 2019). Antibiotic treatment is the foundation for treating SP, should be initiated as early as possible (Garnacho-Montero et al., 2018). Due the long-term use of antibiotics, the medical costs associated with treating SP are relatively high (Welte, 2016). CHIs have certain advantages during the treatment of SP. Guidelines (Wang M. et al., 2023) advocate for the administration of Tanreqing, RDN, Xuebijing, Shenfu, and Shenmai injections for the treatment of SP in adults. In recent years, CHIs have been widely used in clinical practice (Deng and Chen, 2021; Jiang et al., 2024; Geng, 2024), with the literature continually updated. Numerous studies have shown that RDN injection can treat respiratory system diseases (Ma et al., 2021; Wang Z. et al., 2023; Wang et al., 2022). Despite clinical studies on the treatment of SP with RDN Injection, its efficacy and safety remain ambiguous. Therefore, this study sought to identify RCTs regarding the treatment of SP with RDN injection, aiming to guide the clinical management of SP.
The pathogen of SP can induce a high inflammatory response. RDN injection can address SP for the following reasons: The primary constituents of RDN Injection include Gardenia jasminoides, Lonicera japonica, and Artemisia annua, all of which possess anti-inflammatory, antibacterial, antiviral, and additional properties (Xu et al., 2009). Studies have shown that their active ingredients mainly include iridoids, lignans, coumarins, sesquiterpenes, flavonoids, caffeoylquinic acid and phenolic acids (Liu et al., 2015). The main iridoid in Gardenia and chlorogenic acid in Lonicera japonica can inhibit the macrophage response associated with inflammatory diseases, reduce the release of inflammatory factors, and inhibit infections (Cao et al., 2021). A study investigated the antipyretic and anti-inflammatory properties of RDN injection by screening its active ingredients using a mouse endotoxin shock model, resulting in the identification of two novel terpenoid compounds: designated geniposide A and identified as (1R,7R,8S,10R)-7,8,11-trihydroxy-4-guaiacin-3-one (Li et al., 2020). Research has demonstrated that RDN effectively regulates the metabolic disorders induced by endogenous components in febrile rats treated with dry yeast (Gao et al., 2020). Its antipyretic effect is mainly related to the regulation of amino acids, lipids, and energy metabolism. Network pharmacology studies have shown that RDN can regulate various biological processes and treat inflammation at the systemic level (Xie et al., 2020). These mechanisms confirm the authenticity of treating SP with RDN injection. Currently, there is a gradual increase in the focus on RDN injection.
RCTs were searched for the treatment of SP with RDN injection, and data from 1,897 patients with SP were studied. Meta-analysis results showed that RDN injection can improve clinical efficacy, reduce fever duration, shorten cough resolution time, decrease lung rales resolution time, enhance chest X-ray improvement, and decrease hospitalization time in patients with SP. There was no statistically significant difference between the two groups in terms of improving APACHE II scores, which may be related to the small number of included studies and their relatively small sample sizes. We recommend adjusting the dosage of RDN injection according to the patient’s condition, generally 20–30 mL, continuously used for 7–14 days. Although this meta-analysis provides valuable insights into the efficacy and safety of RDN injection in clinical practice, the included studies were conducted solely in China, which may limit the external validity of our results. Cultural and genetic differences may influence the safety and efficacy of RDN injection. Future research should conduct trials in different populations to evaluate the effectiveness and safety of RDN injection for various ethnic and cultural backgrounds.
Regarding safety, although the adverse reactions are mild and self-resolving, this study cannot conclude that the use of RDN injection does not increase patient adverse reactions. Though no serious adverse events were reported in the included studies, the lack of detailed adverse event reporting in some studies is a notable limitation, which can lead to an underestimation of the true incidence of adverse events associated with RDN injection. We recommend that future trials on RDN injection adopt standardized protocols for collecting and reporting adverse events. Recommendations entail the implementation of standardized adverse event forms, precise definitions of adverse events, and uniform follow-up procedures to monitor and document such occurrences.
This research suggests that certain studies provide incomplete descriptions of randomization and blinding methods, which may lead to multifaceted biases that affect the results of this study. For instance, if randomization was not properly conducted, there might be systematic differences between the intervention and control groups at baseline, which could affect the observed treatment effects. Similarly, the absence of blinding may result in biased evaluations of outcomes, either by altering patient behavior or compromising the precision of outcome measurements. Inadequate allocation concealment may lead to outcome assessors being biased in their detection or reporting of results if they are aware of the treatment allocation (Jadad et al., 1996), which can result in overestimation or underestimation of the true therapeutic effect. Future research should aim to address these issues to provide more reliable evidence.
We have examined differences in patient characteristics, such as disease severity and comorbidities, across the included investigations. However, due to insufficient information, it is not possible to ascertain whether these are heterogeneous sources. Differences in antibiotic use may lead to high heterogeneity. There were discrepancies in the diagnostic criteria, which may contribute to heterogeneity in the results. Despite the efforts to investigate their potential sources, some unexplained heterogeneity remains in this meta-analysis. The high heterogeneity suggests that the effectiveness of interventions may vary among different populations or environments. This indicates that the findings from this study should be applied with caution in clinical practice. Clinicians must evaluate the distinct attributes of their patient demographic and the contextual factors of their practice when determining the appropriateness of the intervention. Future research should aim to identify and address the sources of heterogeneity to provide more reliable and generalizable evidence. Moreover, a substantial publication bias (p = 0) was identified in the analysis of the clinical efficacy of the primary indicator, suggesting that studies yielding negative or inconclusive results are unlikely to be disseminated. Therefore, there is a potential risk of overestimating the positive effects of treatment. Hence, more comprehensive and rigorous research is needed in the future to address this issue.
The quality of evidence of several studies included in this article is low. Biases and methodological limitations in the primary studies may result in an inaccurate estimation of the actual treatment effects. Clinicians should consider the limitations of evidence when making treatment decisions. They may need to rely on additional sources of information, such as expert opinion and clinical experience, to guide their practice. It is also important to monitor the emerging evidence from higher-quality studies to inform future clinical guidelines. It is recommended that future RCTs include the following features: strict randomization techniques and blinding of participants and outcome assessors to treatment allocation. This will help minimize the risk of selection and performance bias. Future trials should detect clinically significant differences, which requires careful planning and calculation of sample size based on expected effect size and variability. Consistent and standardized outcome measures should be used in all studies to facilitate the comparison and aggregation of results, including the use of validated tools to evaluate primary and secondary outcomes. Detailed adverse event reports and long-term follow-up should also be provided. By combining these features, future RCTs will provide better quality evidence, offering more reliable information for clinical practice and future research.
5 Limitations
This study has certain limitations: (1) The number of included studies is limited, the sample size is diminutive, and the overall quality of the literature is substandard; (2) some studies had incomplete descriptions of randomization methods; (3) all studies did not mention blinding, which may result in some bias in the results; (4) the heterogeneity cannot be determined through subgroup analysis; and (5) the included studies did not mention any long-term efficacy indicators, such as survival rate and mortality rate, and the treatment time was relatively short, making long term efficacy unclear.
6 Conclusion
In conclusion, the meta-analysis results indicate that the use of RDN injection as an adjuvant therapy for SP can enhance clinical efficacy, reduce fever duration, shorten cough resolution time, decrease lung rale resolution time, improve chest X-ray results, and decrease hospitalization time. Future validation necessitates the execution of more meticulously designed and standardized large-scale clinical studies.
Author contributions
YW: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft. BX: Data curation, Investigation, Methodology, Software, Supervision, Writing – review and editing. PZ: Data curation, Investigation, Methodology, Software, Supervision, Writing – review and editing. SL: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review and editing. YX: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0506700, 2023ZD0506702, 2023ZD0506705), the National Key Research and development Program (No.2023YFC3502601), Henan Province Scientific Research Project - Double First-Class Traditional Chinese medicine (HSRP-DFCTCM-2023-3-16, DFCTCM-2023-4-05), Special Project of Traditional Chinese Medicine Research of Henan Province (2023ZY2055), Special Project of Traditional Chinese Medicine Research of Henan Province (2023ZYZD03), the Henan Province Medical Science and Technology Program (NO.LHGJ20220586), and the “Three 100s” Initiative Program of Henan Provincial Academy of Medical Sciences (HNCRD202435).
Acknowledgments
We want to thank all the doctors and students who assisted us in completing our research. We sincerely thank Kushal Gunturu for reviewing and polishing the language of this article. His professional advice greatly improved the language quality of the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1591136/full#supplementary-material
References
Aliberti, S., Cook, G. S., Babu, B. L., Reyes, L. F., H Rodriguez, A., Sanz, F., et al. (2019). International prevalence and risk factors evaluation for drug-resistant Streptococcus pneumoniae pneumonia. J. Infect. 79 (4), 300–311. doi:10.1016/j.jinf.2019.07.004
Bai, L. H., and Xu, X. W. (2021). Observation of the effect of reduning injection as an adjuvant treatment for severe pneumonia. Med. Front. 11 (5), 30–31. doi:10.3760/cma.j.issn.1007-1245.2018.15.031
Cao, C., Zhen, Z., Kuang, S., and Xu, T. (2020). Reduning injection combined with western medicine for pneumonia: a protocol for systematic review and meta-analysis. Med. Baltim. 99 (43), e22757. doi:10.1097/MD.0000000000022757
Cao, Y. G., Ren, Y. J., Liu, Y. L., Wang, M. N., Chen, X., et al. (2021). Iridoid glycosides and lignans from the fruits of Gardenia jasminoides Eills. Phytochemistry 190, 112893. doi:10.1016/j.phytochem.2021.112893
Chen, Z. G. (2018). Clinical efficacy and safety analysis of the combination of Reduning and high-dose Ulastatin in the treatment of elderly patients with severe pneumonia. Int. Med. Health Guid News 24 (15), 2328–2331.
Ching, P. R., and Pedersen, L. L. (2025). Severe pneumonia. Med. Clin. North Am. 109 (3), 705–720. doi:10.1016/j.mcna.2024.12.011
Deng, Z., and Chen, W. (2021). The effect of Xuebijing injection combined with antibiotics in the treatment of severe pneumonia. Chin. J. Clin. Ration. Drug Use 14 (8), 71–73. doi:10.15887/j.cnki.13-1389/r.2021.08.027
Ding, F. (2017). The effect of Reduning on lung function and immune function in elderly patients with severe pneumonia. Chin. J. Pract. Med. 44 (23), 119–122. doi:10.3760/cma.j.issn.1674-4756.2017.23.041
Dinh, A., Duran, C., Ropers, J., Bouchand, F., Davido, B., Deconinck, L., et al. (2021). Factors associated with treatment failure in moderately severe community-acquired pneumonia: a secondary analysis of a randomized clinical trial. JAMA Netw. Open 4 (10), e2129566. doi:10.1001/jamanetworkopen.2021.29566
Espinoza, R., Silva, JRLE, Bergmann, A., de Oliveira Melo, U., Calil, F. E., Santos, R. C., et al. (2019). Factors associated with mortality in severe community-acquired pneumonia: a multicenter cohort study. J. Crit. Care 50, 82–86. doi:10.1016/j.jcrc.2018.11.024
Fang, C., Chen, Z. L., and Chen, J. F. (2018). Analysis of the efficacy of reduning injection combined with ceftriaxone sodium in the treatment of acute severe pneumonia. Chin. Arch. Tradit. Chin. Med. 36 (6), 1468–1470. doi:10.13193/j.issn.1673-7717.2018.06.048
Gao, X., Huang, C., Geng, T., Chen, X., Wang, J., Liu, J., et al. (2020). Serum and urine metabolomics based on UPLC-Q-TOF/MS reveals the antipyretic mechanism of Reduning injection in a rat model. J. Ethnopharmacol. 250, 112429. doi:10.1016/j.jep.2019.112429
Garnacho-Montero, J., Barrero-García, I., Gómez-Prieto, M. G., and Martín-Loeches, I. (2018). Severe community-acquired pneumonia: current management and future therapeutic alternatives. Expert Rev. Anti Infect. Ther. 16 (9), 667–677. doi:10.1080/14787210.2018.1512403
Geng, Q. (2024). Clinical study on the combination of Shenmai injection and Xuebijing injection as adjunctive treatment for severe pneumonia. J. Pract. Chin. Med. 40 (5), 881–884.
Gonzalez-Padilla, D. A., and Dahm, P. (2021). Evidence-based urology: understanding GRADE methodology. Eur. Urol. Focus 7 (6), 1230–1233. doi:10.1016/j.euf.2021.09.014
Guan, S. N., Xie, K., and Ji, W. S. (2025). Network meta analysis of the efficacy and safety of seven traditional Chinese medicine injections combined with conventional treatment for severe pneumonia. Chin. J. Pharmacol. Chin. 41 (01), 116–123. doi:10.13412/j.cnki.zyyl.2025.01.003
Guo, X. C., and Huo, X. P. (2022). Analysis of the efficacy of reduning injection combined with ceftriaxone sodium in the treatment of acute severe pneumonia. Spec. Health (8), 88–90.
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Jiang, Y. H., Ni, S. H., Zhang, X. J., Huang, J., Liu, H. H., Xu, L., et al. (2024). Meta analysis and sequential trial analysis of Tanreqing injection in the treatment of severe pneumonia in elderly patients. Chin. J. Chin. Mater Med. 49 (4), 1091–1101. doi:10.19540/j.cnki.cjcmm.20231025.501
Lee, J. S., Giesler, D. L., Gellad, W. F., and Fine, M. J. (2016). Antibiotic therapy for adults hospitalized with community-acquired pneumonia: a systematic review. JAMA 315 (6), 593–602. doi:10.1001/jama.2016.0115
Li, H. B., Yang, B., Ge, W., Cao, Z. Y., Cao, L., Yu, Y., et al. (2020). Two new terpenoids from reduning injection. Chin. Herb. Med. 12 (2), 183–187. doi:10.1016/j.chmed.2019.11.006
Li, J. N., Li, X. Z., and Guo, X. (2015). The effect of early Shenfu injection on procalcitonin and brain natriuretic peptide in elderly patients with severe pneumonia. Tianjin J. Tradit. Chin. Med. 34 (2), 82–85.
Li, M., and Shao, H. Z. (2022). Clinical study on the treatment of severe pneumonia with reduning injection combined with piperacillin sodium and Tazobactam sodium. Mod. Drug Clin. 37 (12), 2790–2794.
Li, X. M. (2014). Analysis of the therapeutic effect of the combination therapy of Reduning Injection and Ceftriaxone Sodium on 140 cases of acute severe pneumonia. Fam. Ther. 10 (5), 102.
Lin, C. L. (2015). Observation on the effect of combination therapy of Reduning and high-dose immunoglobulin in the treatment of acute severe viral pneumonia. Chin. J. Rural. Med. 22 (10), 55–5658. doi:10.19542/j.cnki.1006-5180.2015.10.028
Liu, J., Sun, K., Zheng, C., Chen, X., Zhang, W., Wang, Z., et al. (2015). Pathway as a pharmacological target for herbal medicines: an investigation from reduning injection. PLoS One 10 (4), e0123109. doi:10.1371/journal.pone.0123109
Liu, J., Wu, X. L., and Wang, Y. Y. (2018). Analysis of the therapeutic effect of integrated traditional Chinese and Western medicine on severe pneumonia in the elderly. Chin. Arch. Tradit. Chin. Med. 36 (6), 1458–1461. doi:10.13193/j.issn.1673-7717.2018.06.045
Liu, J. H., and Liu, Y. M. (2014). Clinical observation of the treatment of acute severe pneumonia with reduning injection combined with ceftriaxone. J. Emerg. Tradit. Chin. Med. 23 (3), 503–504.
Ma, Q., Xie, Y., Wang, Z., Lei, B., Chen, R., Liu, B., et al. (2021). Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2. J. Ethnopharmacol. 279, 114367. doi:10.1016/j.jep.2021.114367
Martin-Loeches, I., Torres, A., Nagavci, B., Aliberti, S., Antonelli, M., Bassetti, M., et al. (2023). ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur. Respir. J. 61 (4), 2200735. doi:10.1183/13993003.00735-2022
Musher, D. M., and Thorner, A. R. (2014). Community-acquired pneumonia. N. Engl. J. Med. 371 (17), 1619–1628. doi:10.1056/NEJMra1312885
Nie, H. Y. (2017). Reduning injection as adjuvant treatment for 42 cases of severe pneumonia. J. Chin. Med. Lit. Electron 4 (52), 10262+10264. doi:10.16281/j.cnki.jocml.2017.52.094
Niu, L., Xiao, L., Zhang, X., Liu, X., Liu, X., Huang, X., et al. (2022). Comparative efficacy of Chinese herbal injections for treating severe pneumonia: a systematic review and Bayesian network meta-analysis of randomized controlled trials. Front. Pharmacol. 12, 743486. doi:10.3389/fphar.2021.743486
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pan, J. (2022). The therapeutic effect of ceftriaxone sodium combined with Reduning injection on acute severe pneumonia. Chin. Sci. J. Database Med. Health (12), 125–128.
Ramirez, P., Ferrer, M., Marti, V., Reyes, S., Martínez, R., Menéndez, R., et al. (2011). Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit. Care Med. 39 (10), 2211–2217. doi:10.1097/CCM.0b013e3182257445
Restrepo, M. I., Mortensen, E. M., Rello, J., Brody, J., and Anzueto, A. (2010). Late Admission to the ICU in Patients with community-acquired pneumonia is associated with higher mortality. Chest 137 (3), 552–557. doi:10.1378/chest.09-1547
See, X. Y., Wang, T. H., Chang, Y. C., Lo, J., Liu, W., Choo, C. Y. W., et al. (2024). Impact of different corticosteroids on severe community-acquired pneumonia: a systematic review and meta-analysis. BMJ Open Respir. Res. 11 (1), e002141. doi:10.1136/bmjresp-2023-002141
Shan, W. (2020). Clinical observation of the treatment of severe pneumonia with reduning injection combined with Biapenem. J. Prac. Tradit. Chin. Med. 36 (12), 1593–1594.
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sun, G. X., Zu, Y. N., and Li, G. Y. (2012). Clinical study on the adjuvant treatment of severe pneumonia with reduning injection. Prac. Pharm. Clin. Rem. 15 (7), 439–440. doi:10.14053/j.cnki.ppcr.2012.07.010
Tan, Z. P., Chen, G. S., and Huang, X. (2019). The effect of Reduning injection combined with fiber-reinforced bronchoalveolar lavage and sputum aspiration on blood T lymphocyte subsets and prognosis in patients with severe pneumonia. Guangzhou J. Tradit. Chin. Med. 36 (10), 1520–1526. doi:10.13359/j.cnki.gzxbtcm.2019.10.006
Torres, A., Chalmers, J. D., Dela Cruz, C. S., Dominedò, C., Kollef, M., Martin-Loeches, I., et al. (2019). Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 45 (2), 159–171. doi:10.1007/s00134-019-05519-y
Wang, M., Liu, H., Chen, Y., Yu, J., Lin, J., Sun, Z., et al. (2023b). Guideline on treating community-acquired pneumonia with Chinese patent medicines. Pharmacol. Res. 196, 106919. doi:10.1016/j.phrs.2023.106919
Wang, S. G., Ji, F., and Li, P. (2023a). Clinical study on the treatment of severe pneumonia with the combination of Reduning injection and imipenem cilastatin. Mod. Drug Clin. 38 (12), 3066–3070.
Wang, Y. Y., Zhou, D. Y., and Wang, L. H. (2020). Clinical study on the treatment of lung infection with heat toxin accumulation syndrome in intensive care unit with Reduning Injection. Anhui J. Tradit. Chin. Med. 39 (5), 12–16. doi:10.3969/j.issn.2095-7246.2020.05.004
Wang, Z., Guo, Z., Wang, X., Liao, H., Chen, F., Liu, Y., et al. (2023c). Reduning alleviates sepsis-induced acute lung injury by reducing apoptosis of pulmonary microvascular endothelial cells. Front. Immunol. 14, 1196350. doi:10.3389/fimmu.2023.1196350
Wang, Z., Liu, Y., Chen, F., Liao, H., Wang, X., Guo, Z., et al. (2022). Feasibility and mechanism analysis of Reduning in the prevention of sepsis-induced pulmonary fibrosis. Front. Pharmacol. 13, 1079511. doi:10.3389/fphar.2022.1079511
Welte, T. (2016). Severe pneumonia in the intensive care unit. Med. Klin. Intensivmed. Notfmed 111 (4), 279–289. doi:10.1007/s00063-016-0165-9
Wu, J. Y., Tsai, Y. W., Hsu, W. H., Liu, T. H., Huang, P. Y., Chuang, M. H., et al. (2023). Efficacy and safety of adjunctive corticosteroids in the treatment of severe community-acquired pneumonia: a systematic review and meta-analysis of randomized controlled trials. Crit. Care 27 (1), 274. doi:10.1186/s13054-023-04561-z
Xiao, L., Niu, L., Zhang, X., Liu, X., Liu, X., Sun, C., et al. (2022). Comparative efficacy of Chinese herbal injections for treating severe pneumonia: a protocol for systematic review and Bayesian network meta-analysis of randomized controlled trials. PLoS One 17 (5), e0262776. doi:10.1371/journal.pone.0262776
Xie, F., Xie, M., Yang, Y., Zhang, M., Xu, X., Liu, N., et al. (2020). Assessing the anti-inflammatory mechanism of reduning injection by network pharmacology. Biomed. Res. Int. 2020, 6134098. doi:10.1155/2020/6134098
Xing, W. C., and Lin, C. (2022). Clinical efficacy of traditional Chinese medicine combined with antibiotics in the treatment of acute severe pneumonia. Inner Mongolia J. Tradit. Chin. Med. 41 (6), 84–85. doi:10.16040/j.cnki.cn15-1101.2022.06.043
Xu, H. M., Wang, Y., and Liu, N. F. (2009). Safety of an injection with a mixture of extracts from Herba Artemisiae annuae, Fructus Gardeniae and Flos Lonicerae. Pharm. World Sci. 31, 458–463. doi:10.1007/s11096-009-9297-9
Yan, Y., Fan, F. J., and Zhao, J. (2019). Clinical study on the treatment of severe pneumonia with reduning injection combined with Biapenem. Mod. Drug Clin. 34 (12), 3598–3602. doi:10.7501/j.issn.1674-5515.2019.12.021
Yin, Q. W., Zhao, L., and Liu, B. (2013). Observation of the clinical efficacy of reduning injection combined with antibiotics in the treatment of severe pneumonia. Prac. J. Med. Pharm. 30 (8), 689–690. doi:10.14172/j.cnki.issn1671-4008.2013.08.038
Zhang, Z. L., Zhang, Y. Q., and Yuan, X. M. (2016). Study on the changes and correlation of plasma soluble myeloid cell trigger receptor 1 and interleukin-10 levels in patients with severe pneumonia treated with Reduning. Liaoning J. Tradit. Chin. Med. 43 (8), 1681–1683. doi:10.13192/j.issn.1000-1719.2016.08.041
Zhou, J. (2017). Clinical observation on the treatment of severe pneumonia in elderly patients with Reduning injection. Mod. Diagn Treat. 28 (6), 1013–1014.
Keywords: reduning injection, severe pneumonia, meta-analysis, systematic review, randomized controlled trial
Citation: Wang Y, Xu B, Zhang P, Li S and Xie Y (2025) Efficacy and safety of reduning injection for severe pneumonia: a systematic review and meta-analysis. Front. Pharmacol. 16:1591136. doi: 10.3389/fphar.2025.1591136
Received: 10 March 2025; Accepted: 23 July 2025;
Published: 08 August 2025.
Edited by:
Paolo Montuschi, Catholic University of the Sacred Heart, ItalyReviewed by:
Shihua Shi, ETH Zürich, SwitzerlandMarcos Pains, Federal District School of Public Health, Brazil
Copyright © 2025 Wang, Xu, Zhang, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Xie, eGlleWFuZ2huQDE2My5jb20=; Suyun Li, bGlzdXl1bjIwMDBAMTI2LmNvbQ==
†ORCID: Yan Wang, orcid.org/0009-0005-6779-6925; Baichuan Xu, orcid.org/0009-0003-3943-560X; Peng Zhang, orcid.org/0009-0009-4444-5371; Suyun Li, orcid.org/0000-0003-1634-7992; Yang Xie, orcid.org/0000-0002-6444-6090
 Yan Wang
Yan Wang Baichuan Xu
Baichuan Xu Peng Zhang
Peng Zhang Suyun Li
Suyun Li Yang Xie
Yang Xie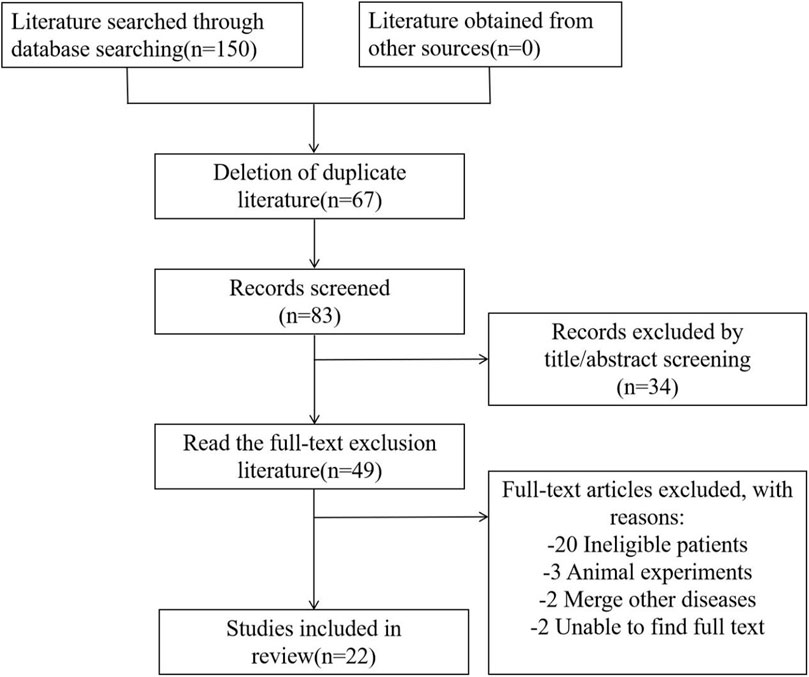
![Forest plot showing a meta-analysis of multiple studies comparing experimental and control groups. The plot lists study names, events, totals, and weights. Risk ratios with 95% confidence intervals are plotted, showing a summary effect size of 1.25 [1.20, 1.30], favoring the experimental group. Total events: experimental 809, control 650. Heterogeneity is low with I²=0%.](https://www.frontiersin.org/files/Articles/1591136/fphar-16-1591136-HTML-r1/image_m/fphar-16-1591136-g004.jpg)
![Funnel plot displaying the standard error of the log risk ratio (SE(log[RR])) against the risk ratio (RR). Data points are scattered around a central vertical line with an inverted funnel shape formed by dashed lines, indicating potential publication bias.](https://www.frontiersin.org/files/Articles/1591136/fphar-16-1591136-HTML-r1/image_m/fphar-16-1591136-g011.jpg)