- 1School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Orthopaedics, Chinese PLA General Hospital, Beijing, China
Introduction: Ulcerative colitis (UC) is a chronic, nonspecific inflammatory bowel disease with limited therapeutic options. Baicalein, a phenolic flavonoid extracted from Scutellaria baicalensis, has been traditionally used in Chinese medicine for its potent anti-inflammatory, anti-tumor, and antiviral properties. This plant, known as Huang-Qin, is indigenous to East Asia and has been widely used to treat various conditions such as fever, respiratory diseases, and inflammation.
Aim of the Study: This study aimed to establish a C. elegans model of UC induced by dextran sodium sulfate (DSS) and to investigate the protective effects of baicalein on intestinal injury.
Materials and Methods: DSS was used to induce acute intestinal injury in C. elegans. N2 and mutant strains (daf-2 and daf-16) were exposed to DSS at concentrations of 5% (w/v), which identified as optimal for inducing intestinal inflammation. The effects of 25 μM, 50 μM, and 100 μM of baicalein on intestinal barrier function, oxidative stress markers, and relevant gene expression were evaluated, including genes related to epithelial barrier integrity (clc-2, mtm-6, etc.), oxidative stress, and the IIS and p38 MAPK pathways.
Results: Baicalein significantly improved physiological condition and intestinal permeability in worm treated with 5% DSS. It restored the expression of epithelial barrier genes and reduced oxidative stress, as indicated by decreased ROS, enhancing SOD activity, daf-16 nuclear translocation etc. Baicalein’s protective effects were associated with the activation of the p38 MAPK and IIS pathways. In daf-2 and daf-16 mutant strains, baicalein demonstrated partial dependence on the IIS pathway for its protective effects.
Conclusion: This study established a DSS-induced UC model in C. elegans and demonstrated that baicalein exerts protective effects on intestinal barrier integrity and oxidative stress, through the IIS and MAPK pathways. These findings support the use of C. elegans as a model for UC research and provide valuable insights into baicalein’s therapeutic potential for inflammatory bowel diseases.
1 Introduction
Ulcerative colitis (UC), a chronic inflammatory bowel disease (IBD), presents significant challenges in treatment due to its complex and persistent nature (Kornbluth et al., 2010). UC is characterized by typical clinical symptoms such as bloody diarrhea, and approximately one-third of UC patients experience extraintestinal manifestations. These can include hepatic complications, hematological disorders, and even colorectal cancer, contributing to reduced life expectancy (Kornbluth et al., 2010; Voelker, 2024). Over the past decades, a notable rise in UC incidence has been observed in emerging economies, particularly in Asia (da Silva et al., 2014; Burisch et al., 2013; Ng et al., 2017).
Current therapeutic strategies for UC patients range from aminosalicylic acid derivatives, glucocorticoids, immunomodulators, to biologics. However, these treatment methods come with side effects that may affect multiple organs, including the digestive, skeletal muscle, and cardiovascular systems (Burri et al., 2020; Loftus et al., 2004; Wise, 2016; Klotz et al., 2015). Notably, prolonged use of infliximab monotherapy can result in a “loss of response” over time, with unrelieved symptoms and the emergence of negative serum antibodies. Although combining immunomodulatory agents can alleviate this issue, it imposes a significant financial burden (Rutgeerts et al., 2005; Hoffmann et al., 2004). This situation has prompted medical researchers to explore alternative treatments that are both effective and safe for UC patients.
Two commonly used models for studying UC involve chemical agents like dextran sodium sulfate (DSS) or 2,4,6-trinitrobenzene sulfonic acid (TNBS) administered to mice via gavage, a process requiring an extended experimental timeline. Another approach involves inducing UC phenotypes through genetic knockouts such as IL-2, Mdr1a, or TCR in mice. However, using gene knockout mouse models is more expensive. C. elegans, a frequently used model organism in disease research, offers several advantages due to its intestinal structural and functional similarities to humans. It allows for a shorter model induction period and high biological reproducibility. At the 99th Dahlem Conference on Infection, Inflammation, and Chronic Inflammatory Disorders in 2010, C. elegans was proposed as a potential animal model for studying host immunity and microbial pathogenic mechanisms. This suggests that C. elegans could be a valuable tool for studying UC. To our knowledge, this is the first time that DSS has been used to induce inflammatory bowel disease in worms (Irazoqui and Ausubel, 2010).
Baicalein, a flavonoid and an active component of several traditional Chinese medicinal herbs, including Scutellaria baicalensis (Huang Qin in Chinese), has significant medicinal value according to its anti-inflammatory, anti-tumor, and antiviral properties. Baicalein’s antioxidant effects are believed to be central to its therapeutic action (Chen et al., 2006; Gupta et al., 2022). In traditional Chinese medicine (TCM), Scutellaria baicalensis is used for clearing heat and dampness, purging fire, and detoxifying, making it suitable for treating UC of the damp-heat type. As one of its primary active components, baicalein provides theoretical support for its clinical application in treating UC within TCM. Studies have demonstrated that baicalein can mitigate the severity of DSS-induced UC in mouse and rat models by reducing oxidative stress and enhancing intestinal epithelial barrier function (Li Y. et al., 2022; Liang et al., 2019; Liu C. et al., 2020). Furthermore, research by Li et al. showed that 10, 20 and 40 mg/kg baicalein significantly improved the survival rate of fruit flies with DSS-induced colitis, restored reduced intestinal length, and decreased intestinal permeability (Li et al., 2024) Additionally, baicalein has been demonstrated to have significant effects in treating diseases such as UC, Alzheimer’s disease (AD), and myocardial injury caused by oxidative stress (Jadhav and Kulkarni, 2023; Cui et al., 2015; Liu B. et al., 2020). Numerous studies have shown that UC’s pathogenesis and progression are closely associated with signaling pathways such as MAPK, Nrf2/HO-1, NF-κB, and PI3K-AKT. These pathways play critical roles in regulating oxidative stress, immune reactions, and intestinal homeostasis. Their activation or inhibition can significantly influence UC development and severity, making them important therapeutic targets. Understanding the complex regulatory mechanisms of these pathways may provide insights into novel UC treatments. In C. elegans, key pathways related to oxidative stress and pathogen defense include the p38 MAPK pathway and the insulin/IGF-1 signaling (IIS) pathway, which are evolutionarily conserved in both nematodes and mammals. This study aims to investigate the potential alterations in these pathways in a DSS-induced C. elegans model of UC (Nakagawa et al., 2016; Kumar et al., 2022).
Based on these findings, we aim to establish a UC model in C. elegans using DSS. Our objective is to investigate the effects of baicalein on intestinal barrier function, oxidative stress, and transcription factor expression. This study will explore the possible mechanisms underlying baicalein’s therapeutic efficacy for UC.
2 Materials and methods
2.1 Strain acquisition and cultivation of C. elegans
The wild-type N2, daf-2, and daf-16 strains were obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota, United States. These strains were maintained on Nematode Growth Medium (NGM) seeded with Escherichia coli strain OP50 and incubated at an optimal temperature of 20°C.
2.2 Establishment and modulation of the ulcerative colitis model in C. elegans
The nematodes were synchronized and cultured at 20°C for 72 h to reach the L4 larval stage. Harvested larvae were washed in M9 buffer into EP tubes, followed by thorough rinsing, repeated more than four times, to ensure sterility.
For UC model induction, dextran sodium sulfate (DSS, molecular weight ∼5,000 Da; BioDee, Beijing, China) was added to K buffer. Nematodes were exposed to 500 μL of DSS solutions at concentrations of 1%, 2%, 5%, 10%, and 30% (w/v) respectively, while the control group received an equivalent volume of K buffer. All groups were maintained at 20°C for 24 h to allow UC symptoms to develop. Nematodes designated for intervention were then transferred onto fresh NGM plates containing 50 μM 5-fluorouracil (5-FU), either with or without baicalein (CAS 491-67-8; purity≥98%; Yuanye, Shanghai, China). These plates were incubated at 20°C for 48 h to assess baicalein’s protective effects. Baicalein was prepared at final concentrations of 25, 50, and 100 μM, dissolved in dimethyl sulfoxide (DMSO), with a standardized DMSO concentration of 0.1% (v/v) across all treatments.
2.3 Determination of the median lethal dose (LD50) of DSS on nematodes
Caenorhabditis elegans were cultured in 96-well plates in K solution containing varying concentrations of DSS: 0%, 1%, 2%, 5%, 10%, and 30%. The plates were incubated at 20°C for 24 h to assess DSS’s toxic effects. Nematodes were considered dead if they did not respond to gentle stimulation with a platinum wire. Each concentration group consisted of 120 nematodes, and the LD50 was calculated to determine the median lethal dose. This experiment was conducted in triplicate to ensure statistical reliability.
2.4 Assessment of body length and width
The body length and width of C. elegans were used as indicators of growth status (Han et al., 2022). After treatment, nematodes were transferred to microscope slides and anesthetized with 50 μL of a 10 mM levamisole solution. Dimensions were photographed and measured under somatoscopic microscopy (Chen et al., 2023). Head swing frequency, a behavioral indicator, was quantified by observing nematodes at 30-s intervals on M9 buffer-soaked slides. Each complete side-to-side head movement was recorded as a single event (Zhang JY. et al., 2024; Ye et al., 2021). Following head swing assessment, body bending frequency was recorded, defined as the number of bends per minute on an OP50-free NGM plate, tracking the posterior pharyngeal bulb along the y-axis (Han et al., 2022; Li et al., 2016; Zhang and Chen, 2023). 31 nematodes per group were evaluated, with each test repeated three times to ensure accuracy and reproducibility.
2.5 Lifespan assessment
The nematodes were maintained and treated as described above. To prevent interference from progeny, the treated nematodes were transferred to NGM plates supplemented with 50 μM 5-FU, a mitotic inhibitor (Macklin, Shanghai, China). This transfer marked the start of the experiment, denoted as day 0. For the first 5 days, nematodes were transferred daily to fresh 5-FU-NGM plates to prevent offspring proliferation, which could skew lifespan assessment. 37 nematodes per group were monitored daily. Death was determined by a lack of response to gentle prodding with a platinum wire and cessation of pharyngeal pumping. This experiment was conducted in triplicate to ensure reliability.
2.6 Assessment of intestinal permeability
Previous studies have shown that the epithelial cells of C. elegans share structural and functional similarities with mammalian cells, making them a suitable model for studying microbial dysbiosis, intestinal inflammation, and intestinal disease pathogenesis (Kim et al., 2022). Erioglaucine disodium salt (Sigma-Aldrich, United States), a recognized marker of intestinal barrier integrity (Qu et al., 2018; Gelino et al., 2016), was used to assess intestinal permeability. After 48 h of baicalein treatment, nematodes were washed three times with M9 buffer. They were then immersed in a 2.5% (w/v) staining solution at 20°C for 3 h. Subsequently, 50 μL of a 10 mM levamisole solution was added to microscope slides for observation under a super-resolution microscope (Leica, Wetzlar, Germany) with ×10 and ×40 objectives (Wu et al., 2023; Zhu et al., 2019).
Data were analyzed using the method by Ma Xuan et al., modified to account for potential erioglaucine leakage from the intestine into the body cavity due to intestinal injury (Ma, 2022). The erioglaucine blue damage coefficient was calculated as the ratio of the erioglaucine-stained area to total nematode area. The relative damage coefficient was determined by dividing the experimental group’s damage coefficient by that of the control group. The data, in TIFF format, were analyzed using ImageJ software (version 1.51; National Institutes of Health, United States) to calculate the percentage of positive blue signal area. Each group was measured in triplicate and 31 nematodes were examined per treatment group (Wu et al., 2023; Yu et al., 2020a).
2.7 Quantification of lipofuscin and reactive oxygen species (ROS) fluorescence
Lipofuscin, a pigment associated with aging, accumulates within nematodes over time (Yu et al., 2021; Qi et al., 2021). Nematodes were maintained and processed as previously described. After collection, they were washed with M9 buffer and incubated with 10 μM DCFH-DA (Nanjing Jiancheng Bioengineering Institute, China) probe for 2 h to assess ROS levels. Following incubation, nematodes were washed extensively to remove any residual probe and then placed on clean slides. For observation, 50 μL of a 10 mM levamisole solution was applied. ROS fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
To assess lipofuscin autofluorescence, nematodes treated with DSS or baicalein were similarly washed to minimize background fluorescence from bacterial debris. Fluorescence intensity was observed under blue excitation light (405–488 nm). 31 nematodes per group were evaluated and analyzed in triplicate using ImageJ software (Oh et al., 2022; Duangjan and Curran, 2022).
2.8 Assessment of superoxide dismutase (SOD) and malondialdehyde (MDA) levels
To evaluate oxidative stress, levels of SOD and MDA in the treated nematodes were measured. Approximately 1,000 nematodes were washed with M9 buffer, homogenized, and the supernatant was collected. Protein concentration was determined using the bicinchoninic acid (BCA) method. SOD and MDA levels were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, China) following the manufacturer’s protocols (Ji et al., 2022; Pei et al., 2022).
2.9 Nuclear localization assay of DAF-16
To assess the effects of low-, medium-, and high-dose baicalein on DAF-16GFP expression in Caenorhabditis elegans strain TJ356 exposed to 5% dextran sodium sulfate (DSS), treated worms were collected and washed repeatedly with M9 buffer, followed by immobilization on glass slides using 10 mM levamisole. GFP fluorescence was captured under consistent exposure settings (excitation wavelength: 485 nm, emission wavelength: 535 nm) using a fluorescence microscope. Fluorescence intensity was subsequently quantified using ImageJ software.
As previously described (Feng et al., 2021), the nuclear localization of DAF-16GFP was classified into three distinct patterns: cytosolic (diffuse cytoplasmic fluorescence), intermediate (partial nuclear accumulation), and nuclear (clear nuclear enrichment). Each treatment group included 20 worms, and experiments were performed in triplicate to ensure statistical rigor.
2.10 Quantitative real-time polymerase chain reaction (qRT-PCR)
To assess gene expression, qRT-PCR was conducted on the N2 strain and daf-2 and daf-16 mutant nematodes exposed to 5% DSS, with and without 50 μM baicalein treatment. Total RNA was extracted and purified using the RNA Easy Fast Tissue/Cell Kit (Tiangen, Beijing, China), following the manufacturer’s protocol. RNA purity and concentration were determined by measuring the 260/280 nm absorbance ratio, with acceptable values ranging from 1.8 to 2.2, using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). cDNA synthesis was performed using the First-strand Synthesis Mast Mix kit (Lablead, Beijing, China). qRT-PCR was subsequently conducted using the 2× Realab Green PCR Fast mixture kit on a QuantStudio6 Flex system (Thermo, Beijing, China). Primers, with sequences and functions detailed in Supplementary Table S1, were synthesized by Sangon Biotech (Shanghai, China). Expression of the act-1 gene was used as an internal control. A minimum of 1,000 C. elegans were used per group, and all experiments were performed in triplicate to ensure reproducibility (Fan et al., 2023; Dilberger et al., 2019).
2.11 Statistical analyses
The numerical data obtained were subjected to statistical analysis. Comparisons between two groups were conducted using Student’s t-test, while one-way ANOVA or non-parametric tests were applied as appropriate using SPSS version 27.0.1 (International Business Machines Corporation, American). In all analyses, statistical significance was defined at p < 0.05. Data visualization was performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, United States).
3 Results
3.1 Optimal modeling of ulcerative colitis in C. elegans with 5% DSS
Under normal conditions, the erioglaucine dye is selectively taken up by the intestine of C. elegans, showing minimal leakage outside the intestinal tract when the barrier is intact. However, a slight seepage of erioglaucine from the pharynx was observed in nematodes treated with 2% DSS compared to untreated controls. More pronounced leakage, indicated by larger areas stained with erioglaucine, was observed in nematodes exposed to 5%, 10%, and 30% DSS concentrations, with significant differences in relative damage coefficients (Figures 1a,b). Notably, DSS concentrations of 10% and higher resulted in a mortality rate over 74%.
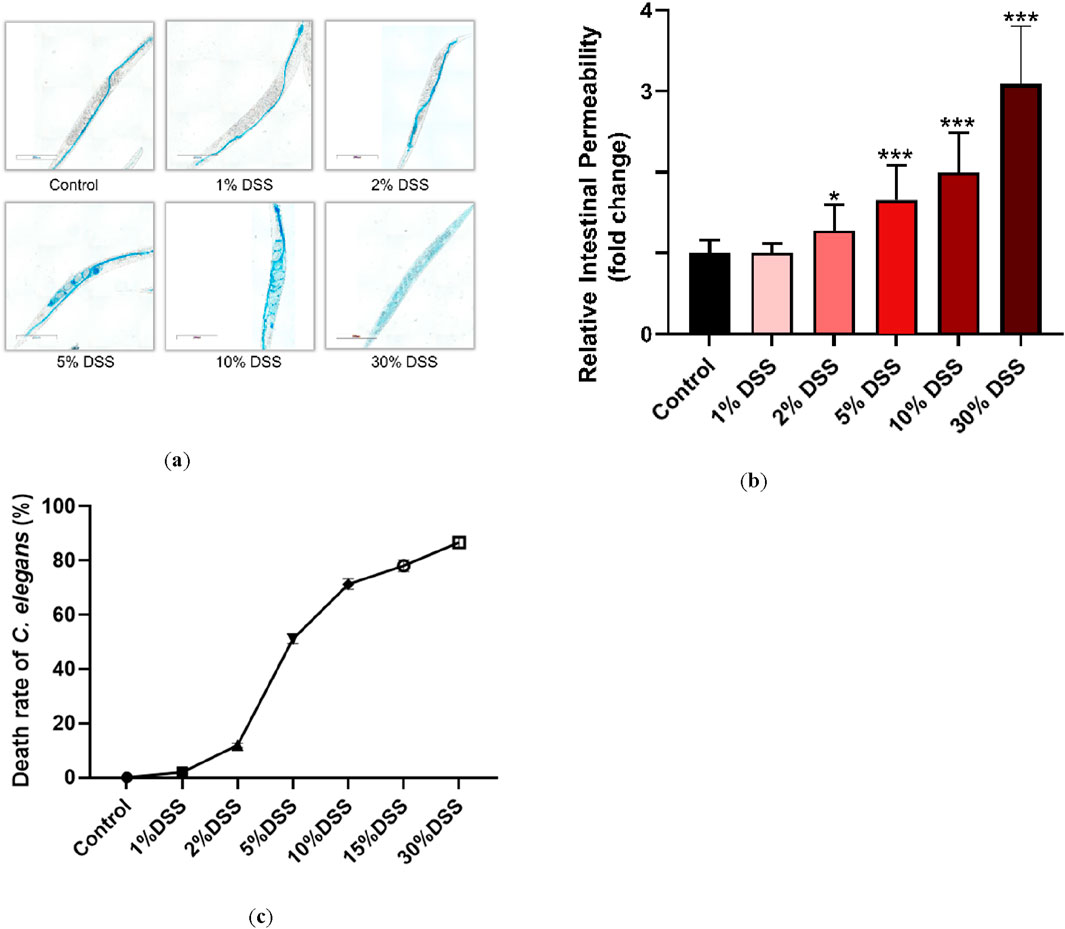
Figure 1. Effects of different DSS concentrations on C. elegans. Fourth-stage (L4) N2 C. elegans were exposed to 1%, 2%, 5%, 10%, and 30% DSS solutions for 24 h. Intestinal permeability was assessed following a rinse with M9 buffer (total magnification: ×100 and scale bar: 200 μm) (a). (b): Erioglaucine dye coverage on the nematodes was quantified to normalize the data. (c): LD50 values for each DSS concentration on C. elegans were determined. Statistical significance is denoted by '*' for comparison to the control group, where *p < 0.05 and ***p < 0.001 indicate levels of significance.
To balance experimental feasibility with concentrations commonly reported in the literature, the 5% DSS concentration was selected for further studies. This concentration corresponds to the half-lethal dose (LD50) and effectively induces intestinal barrier damage, making it a suitable choice for modeling UC in C. elegans (Figure 1c) (Prakash and Janadri, 2023; Low et al., 2013; Kim et al., 2023; Munyaka et al., 2016).
3.2 Baicalein ameliorates the growth and development of DSS-Treated C. elegans
The beneficial physiological impact of baicalein (Figure 2a) on DSS-exposed nematodes was evaluated using three metrics: lifespan, body length, and body width. Our findings showed that treatment with 5% DSS markedly decreased the average lifespan of N2 nematodes by 27.3% compared to control group, reflecting a stressed physiological state (Figure 2b). However, a 25.1% increase in lifespan was observed after the 48-h treatment with 50 μM baicalein (Supplementary Table S2).
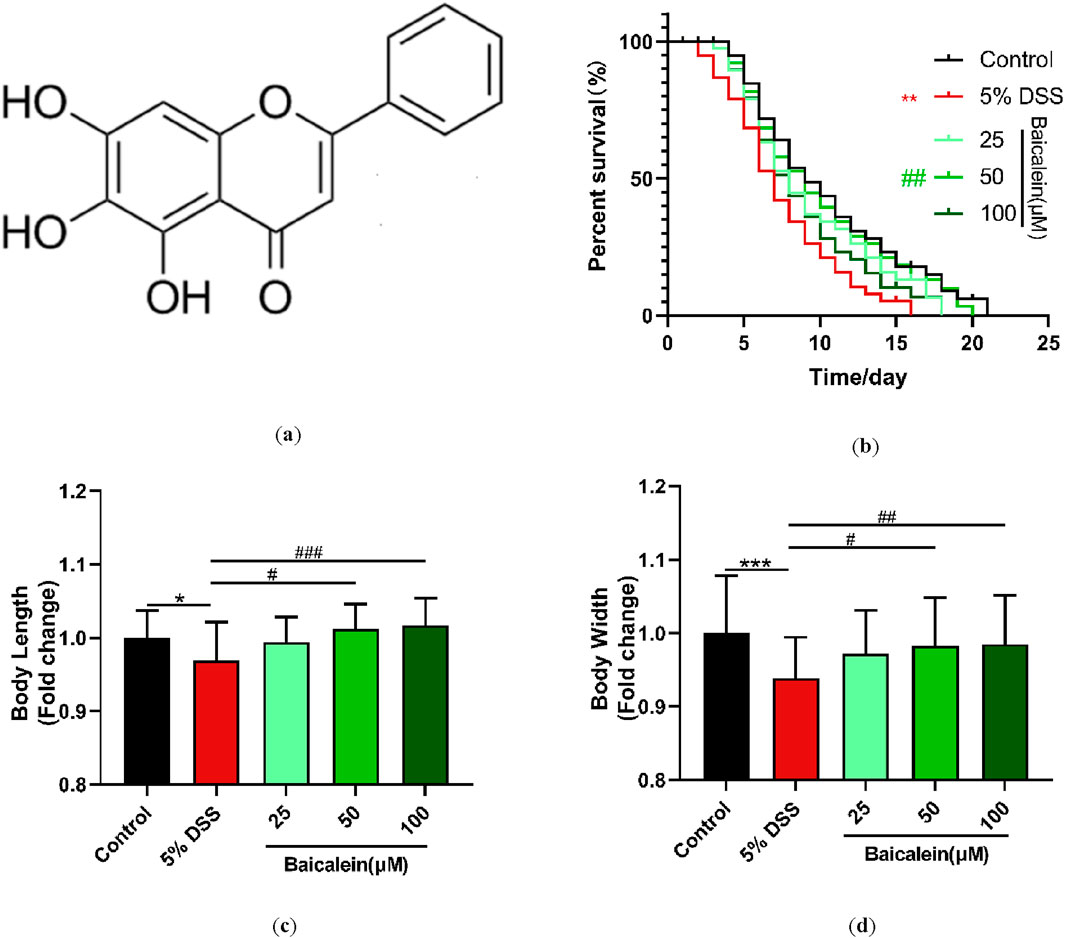
Figure 2. Baicalein intervention promotes growth and development in nematodes following DSS treatment. (a) Molecular structure of baicalein. L4 stage N2 C. elegans were treated with 5% DSS followed by 0.1% DMSO or varying concentrations of baicalein (25, 50, and 100 μM). Longevity (b), body length (c), and body width (d) were assessed, with data normalized accordingly. Statistical significance is denoted by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with *p < 0.05, ***p < 0.001, #p < 0.05, ##p < 0.01, and ###p < 0.001 indicating significance levels.
Regarding body dimensions, the mean body length and width of the control nematodes were 657.85 ± 2.46 μm and 47.01 ± 0.37 μm, respectively, while the 5% DSS-treated group measured 637.48 ± 3.44 μm in length and 44.14 ± 0.26 μm in width (Supplementary Table S3). Intervention with both 50 μM and 100 μM baicalein significantly counteracted these detrimental effects, with no significant difference observed between the two concentrations (p > 0.05) (Figures 2c,d).
These results indicate that 5% DSS exposure negatively affects the growth and developmental parameters of C. elegans. Furthermore, moderate to high concentrations of baicalein (50 and 100 μM) demonstrate a significant restorative effect on growth impairment of C. elegans induced by DSS. Previous studies have indicated that C. elegans models are reliable for testing UC therapeutic agents, particularly given their conserved genetic pathways related to stress and immunity, which parallel mammalian systems.
3.3 Baicalein enhances locomotion in DSS-Treated C. elegans
Assessment of movement behavior, including head thrashing and body bending, is critical for evaluating the locomotive capacity of C. elegans, as these locomotion behavior in C. elegans is often used as a measure of neuromuscular function and overall vitality (Liu H. et al., 2022). Our results demonstrated that 50 and 100 μM baicalein significantly improved impaired movement behaviors impaired by 5% DSS exposure, specifically increasing the frequency of body bends per minute and head thrashes per 30 s (p < 0.001). Interestingly, a 25 μM concentration of baicalein improved head thrashing (p < 0.05) without significantly affecting body bending (p > 0.05) (Figures 3a,b). These findings indicate that baicalein effectively enhances the physiological state of N2 nematodes following DSS-induced stress.
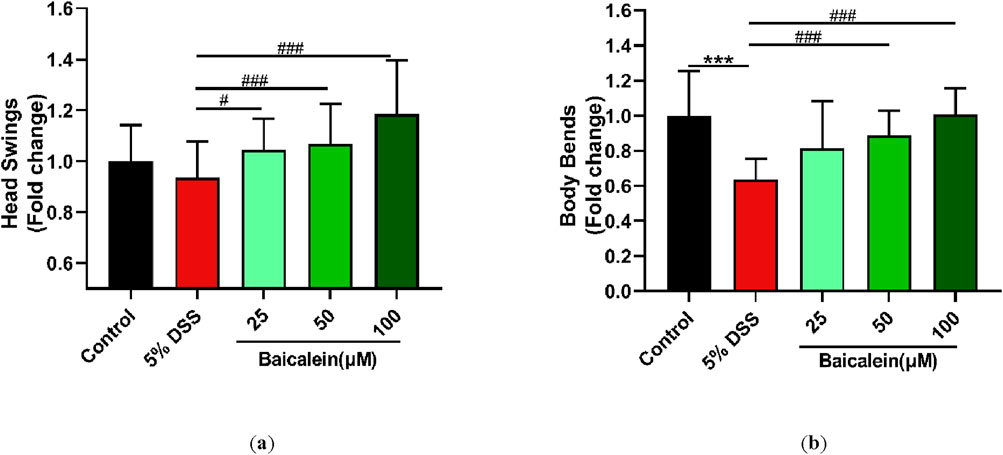
Figure 3. Baicalein ameliorates movement impairments in nematodes following DSS treatment. L4 N2 C. elegans exposed to 5% DSS were treated with 0.1% DMSO or baicalein (25, 50, and 100 μM) respectively. Locomotive abilities were evaluated by measuring head thrashes per 30 s (a) and body bends per minute (b), with normalized data for comparison. Statistical significance is denoted by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with *p < 0.05, ***p < 0.001, #p < 0.05, ##p < 0.01, and ###p < 0.001 indicating levels of significance.
3.4 Baicalein reverses intestinal permeability and gene expression in DSS-Treated nematodes
Maintaining intestinal barrier integrity is a crucial factor in UC pathology. In our study, 5% DSS treatment led to a significant infiltration of erioglaucine dye throughout the bodies of N2 nematodes, indicating a substantial increase in intestinal permeability. In contrast, treatment with various concentrations of baicalein resulted in no noticeable dye accumulation outside the intestine (Figures 4a,b). Moreover, qRT-PCR analysis revealed that treatment with 50 μM baicalein effectively restored expression levels of genes associated with intestinal barrier function, such as clc-2, egl-8, act-5, par-6, and mtm-6, which had been upregulated following DSS exposure (Figure 4c). Collectively, these findings demonstrate that while DSS treatment severely disrupts intestinal barrier function in N2 nematodes, subsequent baicalein intervention significantly alleviates this damage.
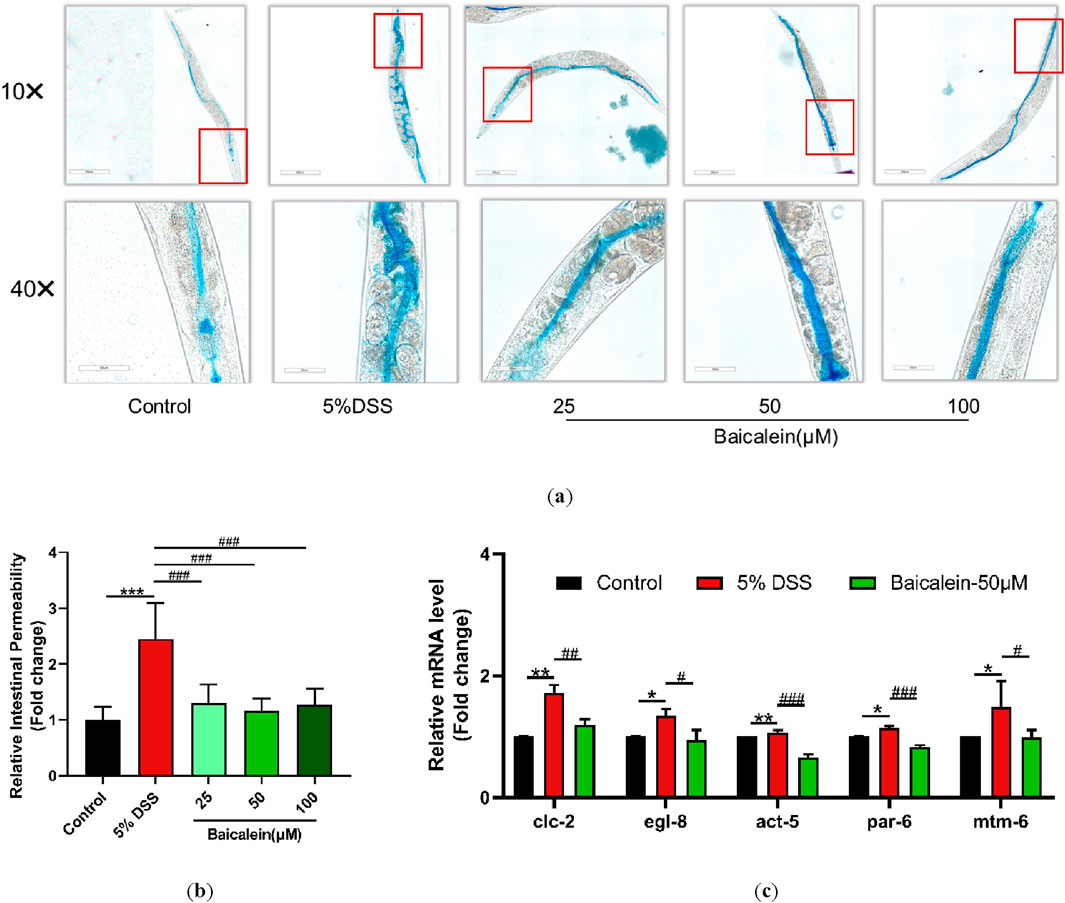
Figure 4. Baicalein mitigates intestinal permeability in DSS-treated C. elegans. The effects of baicalein treatment on C. elegans exposed to 5% DSS were assessed by observing L4 stage nematodes treated with either 0.1% DMSO or varying concentrations of baicalein (25, 50, and 100 μM). (a) Intestinal permeability was observed with total magnification ×100 (scale bar: 200 μm) and ×400 (scale bar: 50 μm) magnifications. Quantification of the observed effects is shown in (b). (c) qRT-PCR analysis was used to assess expression levels of genes involved in intestinal barrier integrity, with data normalized for comparison. Statistical significance is indicated by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with notations denoting significance levels: *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.5 Baicalein reduces lipofuscin and ROS accumulation in DSS-Treated nematodes
Fluorescence microscopy with ImageJ quantification revealed that exposure to 5% DSS increased lipofuscin fluorescence intensity by 48% compared to untreated controls, an effect that was significantly counteracted by baicalein treatment at concentrations of 25, 50, and 100 μM (Figures 5a,c). Additionally, we assessed baicalein’s impact on ROS production in DSS-treated nematodes. Fluorescence analysis of approximately 30 nematodes per group revealed intensified green fluorescence primarily around the intestines of nematodes treated with DSS alone. DSS exposure resulted in a 64.42% increase in ROS levels, which baicalein intervention reduced by 29.77% and 31.19% at concentrations of 50 and 100 μM, respectively (Figure 5b).
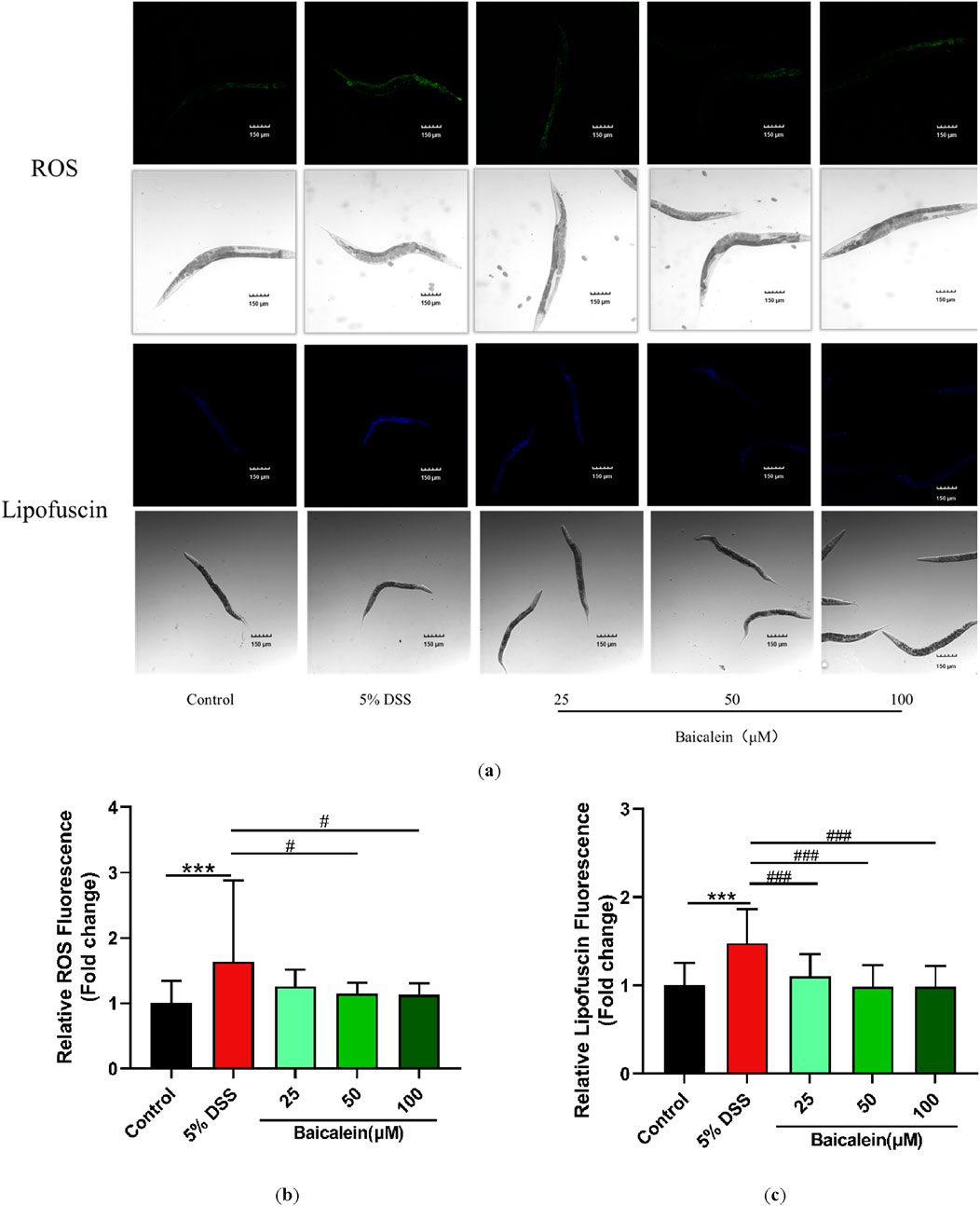
Figure 5. Baicalein reduces oxidative stress in DSS-exposed C. elegans. The L4 stage of N2 C. elegans exposed to 5% DSS were treated with either 0.1% DMSO or baicalein (25, 50, and 100 μm) to assess oxidative stress. Lipofuscin and ROS levels were observed under fluorescence microscopy with total magnification ×100 (scale bar: 150 μm) (a). Fluorescence intensities were quantified for ROS (b) and lipofuscin (c) with data normalized for comparison. Statistical significance is denoted by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with significance indicated as ***p < 0.001, #p < 0.05, ###p < 0.001.
3.6 Baicalein modulates MDA and SOD levels in DSS-Treated nematodes
Further investigations focused on measuring MDA and SOD levels in cell lysates from 5% DSS treated nematodes. MDA, a marker of lipid peroxidation, is an indicator of oxidative stress (Liu Y. et al., 2022; Xiao et al., 2021; Zhang X. et al., 2024). Exposure to 5% DSS resulted in a notable 6-fold increase in MDA levels compared to the control group incubated in K solution for 24 h (p < 0.05). Although low-dose of baicalein (25 μM) exhibited a tendency to reduce MDA, more pronounced reductions were observed with medium and high doses of baicalein (50 and 100 μM, respectively) (p < 0.05) (Figure 6a).
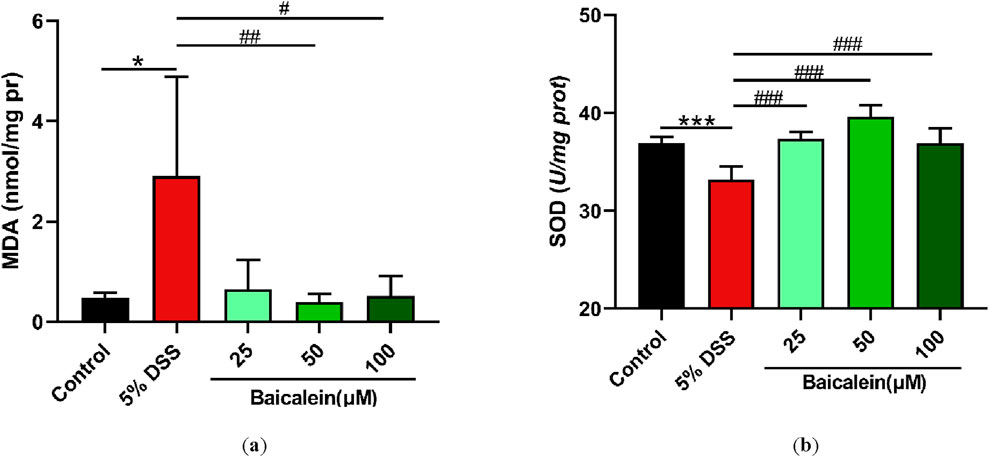
Figure 6. Baicalein alleviates oxidative stress in DSS-treated C. elegans by modulating oxidative and antioxidant markers. The L4 stage of N2 C. elegans exposed to 5% DSS were treated with 0.1% DMSO or baicalein (25, 50, and 100 μM). Levels of MDA (a) and SOD (b) were measured. Statistical significance is denoted by '*' for comparisons with the control group and '#' for 5% DSS treatment group comparisons, with notations indicating significance: *p < 0.05, ***p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001.
Additionally, we evaluated the changes in SOD activity, a critical antioxidant enzyme. The 5% DSS treatment caused a significant 10.14% decrease in SOD activity in N2 nematodes, whereas baicalein treatment at 25, 50, and 100 μM increased SOD activity by 12.5%, 19.45%, and 11.14%, respectively (Figure 6b). Although baicalein at 50 μM exhibited the highest SOD activity restoration, the difference between 50 μM and 100 μM groups did not reach statistical significance (p > 0.05), suggesting a plateau or concentration-dependent divergence in its regulatory effects on SOD.
3.7 Baicalein modulates p38 MAPK pathway gene expression in DSS-Treated nematodes
The MAPK pathway, crucial in UC progression, was assessed in the DSS-induced UC model. Results showed that 5% DSS treatment upregulated genes within the p38-MAPK pathway, including sek-1 (MAPKK), nsy-1 (MAPKKK), and pmk-1 (MAPK) (Figure 7). Activation of this pathway typically increases expression of Nrf-2/Skn-1, which DSS did not alter significantly, though baicalein increased it at the concentration of 50 μM. Additionally, akt-1 expression decreased by 53.20% following 50 μM baicalein treatment. These data support baicalein’s role in ameliorating DSS-induced intestinal barrier damage and oxidative stress, especially at 50 μM.
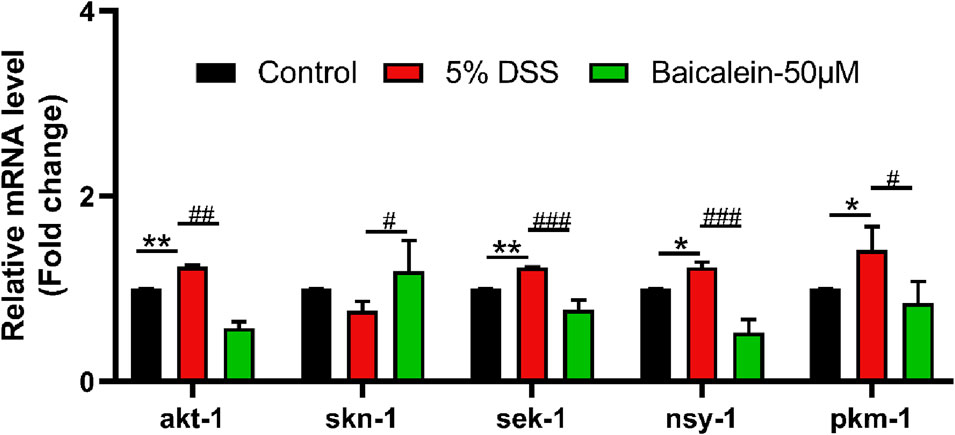
Figure 7. Baicalein modulates gene expression within the p38 MAPK pathway in DSS-exposed C. elegans. The L4 stage of N2 C. elegans subjected to 5% DSS were treated with 0.1% DMSO or baicalein (50 μM). mRNA levels of p38 MAPK pathway genes were measured in nematode homogenates. Statistical significance is indicated by '*' for comparisons with the control group and '#' for DSS model group comparisons, with significance denoted as *p < 0.05, **p < 0.01, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.8 Baicalein reverses oxidative stress-related gene expression in DSS-Treated nematodes
The IIS pathway is a critical regulator of oxidative stress in C. elegans (Li H. et al., 2021; Zaarur et al., 2019). Oxidative stress plays a central role in UC pathogenesis (Elmaksoud et al., 2021; Nikkhah-Bodaghi et al., 2019; Elkholy et al., 2023). Our results (Figure 8) demonstrated that 5% DSS treatment significantly upregulated daf-2 gene expression (analogous to mammalian insulin/IGF-1), while concurrently downregulating its downstream target DAF-16/FOXO, a forkhead box O transcription factor. This effect was reversed by 50 μM baicalein treatment. Furthermore, mRNA levels of antioxidant stress-related genes sod-3 and gst-4 (downstream of DAF-16/FOXO) were reduced by 58.02% and 40.52%, respectively, following DSS exposure, with subsequent increases of 63.03% and 67.91%, respectively, after intervention with 50 μM baicalein. In addition, baicalein reduced the DSS-induced upregulation of hif-1 mRNA, though no significant changes were observed in sod-2 and hsf-1 gene expression.
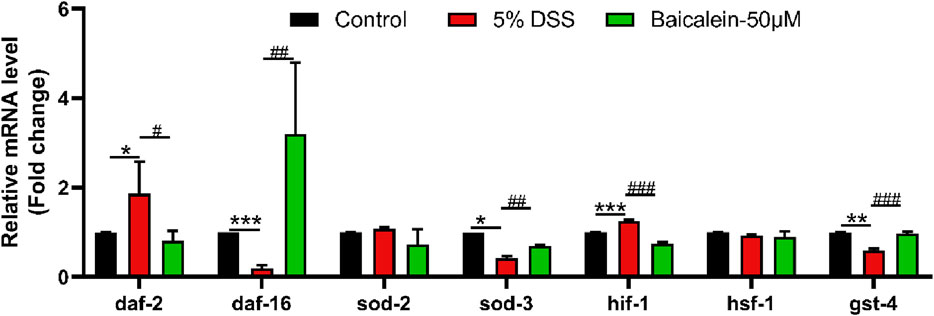
Figure 8. Baicalein alleviates oxidative stress in DSS-treated C. elegans by modulating oxidative stress-related mRNA. The L4 stage of N2 C. elegans exposed to 5% DSS were treated with 0.1% DMSO or baicalein 50 μM). Levels of oxidative stress-related mRNA was measured. Statistical significance is denoted by '*' for comparisons with the control group and '#' for 5% DSS treatment group comparisons, with notations indicating significance: *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.9 Baicalein restores the nuclear translocation of DSS-Treated nematodes
Compared to the control group, the nuclear translocation of DAF-16 in TJ356 nematodes after 5% DSS exposure was significantly reduced by 29.38% (Figures 9a,b). The addition of baicalein significantly restored the nuclear localization of DAF-16 under DSS stress, especially at 50 μM.
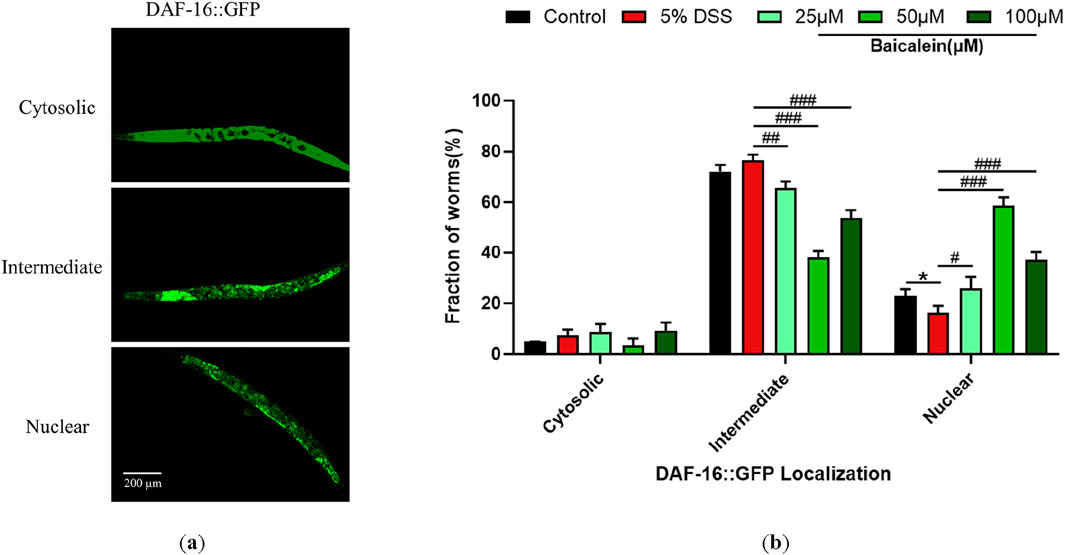
Figure 9. Baicalein influences subcellular localization of DAF-16::GFP. The L4 stage of the DAF-16GFP strains exposed to 5% DSS were treated with 0.1% DMSO or baicalein (25, 50, and 100 μM). (a) Fluorescence microscopy images depicting DAF-16GFP nuclear translocation events in TJ356 strain nematodes with total magnification ×100 (scale bar: 200 μm) (b) Quantitative analysis of nuclear translocation frequency for DAF-16GFP fusion protein. Statistical significance is indicated by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with notations denoting significance levels: *p < 0.05, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.10 Baicalein’s mechanism in DSS-Treated nematodes involves IIS pathway modulation
To elucidate the mechanism underlying daf-2 and daf-16 gene induction and intestinal barrier response following baicalein treatment in DSS-exposed C. elegans, intestinal permeability and gene expression were examined in mutant strains of nematodes. Treatment of daf-2 mutants with 5% DSS increased intestinal permeability and the expression of par-6, clc-2, and act-5, though mtm-6 remained unchanged. Baicalein (50 μM) also did not alter mtm-6 or act-5 expression (Figure 10). In daf-16 mutants, both DSS and baicalein treatments produced comparable effects on permeability, with no significant changes in par-6, mtm-6, or act-5 mRNA levels (Figure 11).
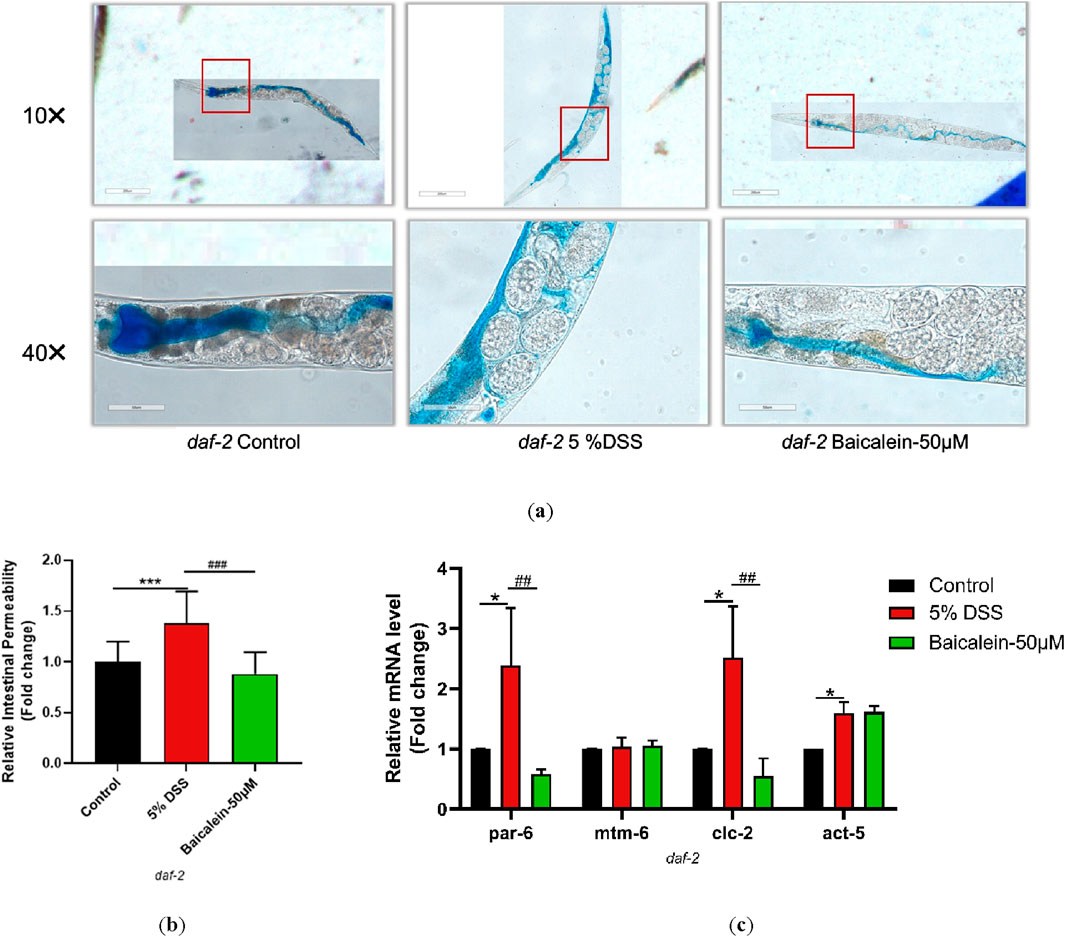
Figure 10. Baicalein Enhances Intestinal Barrier in DSS-Treated daf-2 Mutant. The L4 stage of the daf-2 mutant strains exposed to 5% DSS were treated with 0.1% DMSO or baicalein (50 μM). (a) Intestinal permeability was observed with total magnification ×100 (scale bar: 200 μm) and ×400 (scale bar: 50 μm) magnifications. Quantification of the observed effects is shown in (b). (c) qRT-PCR analysis was used to assess expression levels of genes involved in intestinal barrier integrity, with data normalized for comparison. Statistical significance is indicated by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with notations denoting significance levels: *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001.
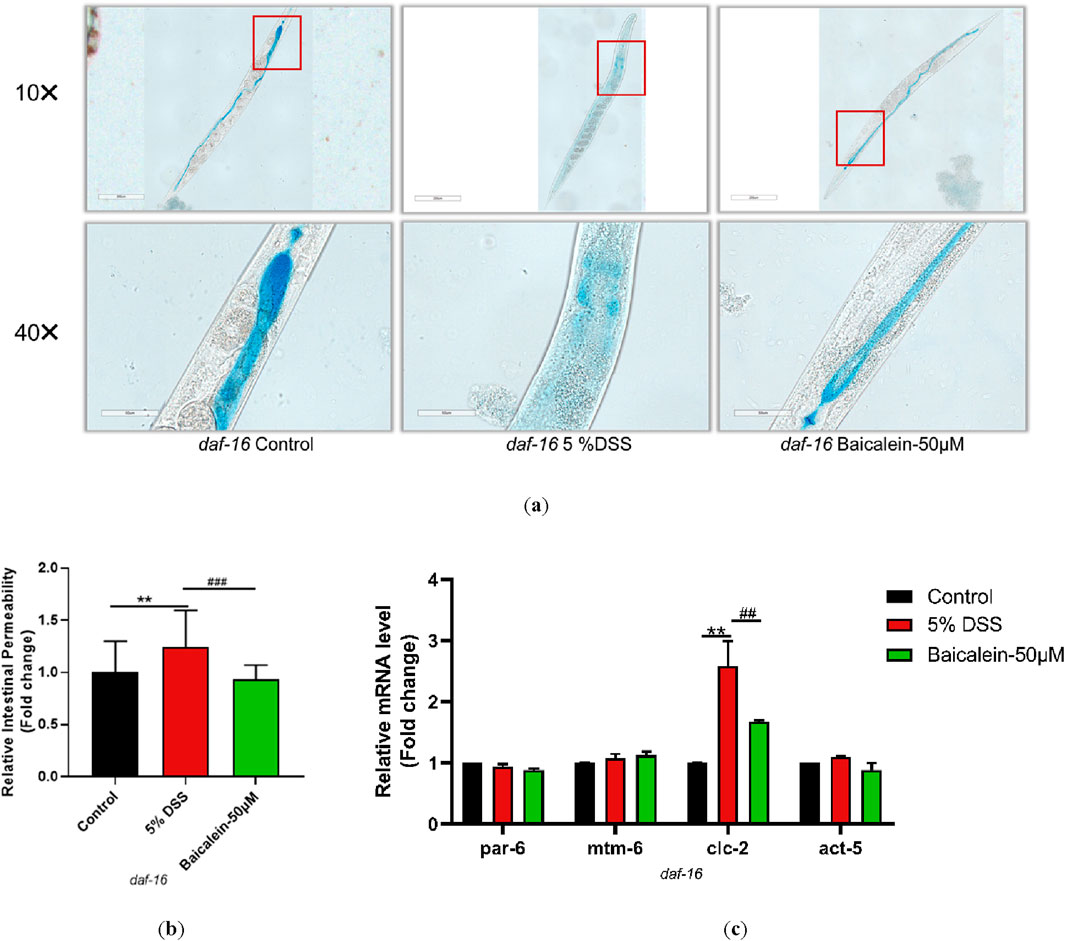
Figure 11. Baicalein Enhances Intestinal Barrier in DSS-Treated daf-16 Mutant Nematodes. The L4 stage of the daf-16 mutant strains exposed to 5% DSS were treated with 0.1% DMSO or baicalein (50 μM). (a) Intestinal permeability was observed with total magnification ×100 (scale bar: 200 μm) and ×400 (scale bar: 50 μm) magnifications. Quantification of the observed effects is shown in (b). (c) qRT-PCR analysis was used to assess expression levels of genes involved in intestinal barrier integrity, with data normalized for comparison. Statistical significance is indicated by '*' for comparisons with the control group and '#' for comparisons with the 5% DSS treatment group, with notations denoting significance levels: *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001.
4 Discussion
UC, with a projected global prevalence of approximately 5 million cases by 2023, poses significant challenges to both physical and mental health (Le Berre et al., 2023). Despite ongoing research, the precise etiology of UC remains elusive, with multiple factors including genetic predisposition, environmental triggers, immune dysregulation, disruptions to the intestinal barrier, and alterations in the gut microbiome being implicated (Wangchuk et al., 2024; Saez et al., 2023). One of the key pathological features of UC is damage to the intestinal epithelial barrier, often occurring during early disease flares. The current study underscores the potential of baicalein in mitigating UC symptoms using a C. elegans model induced by 5% DSS. The DSS model successfully mimics critical aspects of UC pathology, such as impaired barrier function and oxidative stress, thus providing a valuable platform for investigating baicalein’s therapeutic mechanisms.
Our findings suggest that baicalein exerts its protective effects through multiple pathways, particularly the IIS (insulin/IGF-1) and MAPK (mitogen-activated protein kinase) pathways. These pathways are crucial for regulating stress responses, immune function, and maintaining intestinal homeostasis. In C. elegans, the concentrations used in this study were chosen to approximate these ranges, taking into account the differences in body size, metabolism, and uptake mechanisms between nematodes and higher organisms. This approach ensures that the concentrations tested are biologically relevant and comparable to those shown to exert therapeutic effects in other models. Moreover, baicalein demonstrated a favorable safety profile, as evidenced by the normal lifespan, body dimensions, and movement behaviors of C. elegans treated with 5% DSS. Previous studies have also supported the safety of baicalein in various pharmacological contexts, Susannah Havermann et al., Saswat Kumar Mohanty et al. have demonstrated that intervention with 25 μM, 50 μM, and 100 μM baicalin significantly enhances the lifespan and stress resistance of C. elegans (Havermann et al., 2016).
Importantly, baicalein was observed to enhance intestinal barrier integrity, as indicated by reduced intestinal permeability and the upregulation of genes critical for barrier function. Increased intestinal permeability is a well-recognized hallmark of IBD, with studies indicating that patients with elevated permeability are more prone to disease relapse (Arnott et al., 2000). In the DSS-induced colitis model, mucosal permeability was found to increase prior to the onset of inflammation, underscoring the pivotal role of barrier dysfunction in UC progression (Kitajima et al., 1999). The methylene erioglaucine-stained C. elegans model employed in this study offered a more accessible and comprehensive method for evaluating intestinal barrier function across the full intestinal tract, as compared to traditional permeability assays in rodent models.
Baicalein’s ability to restore key intestinal barrier function genes, such as clc-2 and egl-8, further supports its role in strengthening intestinal integrity. The clc-2 gene, which shares homology with the claudin family in humans, plays a crucial role in cellular adhesion and signaling (Simske and Hardin, 2011), while egl-8 is associated with the activation of the PMK-1/p38 MAPK pathway, which is integral to intestinal immunity (Yang et al., 2024; Miller et al., 1999). Notably, baicalein treatment resulted in decreased egl-8 and increased daf-16 expression, consistent with its role in modulating insulin-like signaling pathways and oxidative stress response (Mack et al., 2022). Furthermore, genes like act-5, which maintains the structural stability of microvilli, and par-6, involved in cell polarity, were transiently upregulated in response to DSS exposure, contributing to the observed barrier protection (Cheng et al., 2023; MacQuee et al., 2005; Yamanaka et al., 2001; Totong et al., 2007). Also, our study observed transient upregulation of mtm-6 and act-5 following DSS exposure, which contributes to barrier function enhancement and protection against toxicants (Yu et al., 2020b; Ren et al., 2018; Zhou et al., 2024; Wu et al., 2024). While baicalein’s restoration of intestinal permeability in daf-2 mutants—without altering mtm-6 expression—reveals additional complexity. In WT nematodes, mtm-6 induction likely depends on DAF-16 activation downstream of IIS, aligning with its role in membrane trafficking (Liu Y. et al., 2022). However, in daf-2 mutants, baicalein rescued permeability despite static mtm-6 mRNA levels, suggesting compensatory mechanisms such as: post-translational activation of MTM-6’s lipid phosphatase activity, which restores phosphatidylinositol-3-phosphate (PI3P) metabolic homeostasis. This promotes Wnt secretion, and ameliorates intestinal permeability (Li W. J. et al., 2021; Liu P. et al., 2020; Silhankova et al., 2010); or (2) DAF-16-independent pathways (PI3K/AKT) reinforcing junctions via par-6/clc-2. For instance, AKT-mediated phosphorylation of PAR-6 or its binding partners (e.g., CDC-42) could stabilize apical junctions independently of DAF-16. It may regulate CDC-42 GTPase activity, promoting PAR-6/PKC-3 complex assembly for maintaining junctional continuity during cell division and elongation (Sallee et al., 2021; Wymann and Pirola, 1998). This phenotypic-genetic decoupling highlights baicalein’s capacity to engage parallel networks—spanning IIS-dependent transcription and IIS-independent post-transcriptional regulation—to restore homeostasis. Such multi-target efficacy emphasizes the therapeutic advantage of flavonoids in complex diseases like UC.
When the intestinal barrier is impaired, neutrophils clear pathogens through phagocytosis, degranulation, ROS production, and neutrophil extracellular traps (NETs) release. However, cytokines and ROS released can disrupt intestinal epithelial tight junctions, activating pathways like p38-MAPK (Elkholy et al., 2023; Althagafy et al., 2023), PI3K/AKT (Zhang J. et al., 2022; Ma et al., 2023) and NF-κB, which in turn amplify oxidative stress and recruit more immune cells to inflamed tissue (McCracken and Allen, 2014; Nakase et al., 2022; Bhattacharyya et al., 2014; Zhu et al., 2024). While C. elegans lacks certain inflammatory factors, its simple gene sequences make it suitable for initial screenings and pathway analyses (Kaletta and Hengartner, 2006).
Oxidative stress is a critical factor in UC pathogenesis, and our results show that baicalein significantly reduced ROS and MDA levels, both of which are markers of oxidative damage. Additionally, baicalein increased the activity of SOD, a key antioxidant enzyme, and reduced lipofuscin accumulation, suggesting that it not only protects against oxidative damage but also promotes healthier aging in C. elegans under DSS-induced stress conditions (Saul et al., 2021). However, the non-linear dose dependency of SOD activity—peaking at 50 μM but slightly declining at 100 μM despite oxidative damage reversal—suggests a multifactorial mechanism. At 25 μM, baicalein may primarily scavenge ROS directly through its phenolic hydroxyl groups, with minimal impact on SOD induction (Zuo et al., 2018). 50 μM baicalein likely activate transcriptional regulators like daf-16 or skn-1, upregulating SOD and other antioxidant enzymes (CAT) (Ayoub et al., 2024). At 100 μM, the plateauing or slight reduction in SOD activity—while maintaining significant efficacy over DSS controls—could reflect feedback inhibition (KEAP1-mediated Nrf2 degradation) or a shift toward non-enzymatic antioxidant mechanisms, which aligns with the hormetic responses typical of flavonoids (Wang et al., 2020). Critically, the absence of toxicity at 100 μM (supported by functional recovery of oxidative markers) underscores baicalein’s therapeutic window. Future studies should delineate the concentration-dependent crosstalk among these mechanisms to optimize dosing strategies that maximize antioxidative benefits without exceeding hormetic thresholds.
Baicalein’s therapeutic potential in UC is underpinned by its ability to modulate oxidative homeostasis and stress-responsive signaling networks. By targeting MAPK and PI3K/AKT pathways, baicalein restores intestinal redox balance, a critical factor in UC pathogenesis (Li J. et al., 2022; Tang et al., 2023). Our findings suggest its protective effects involves the IIS pathway, where it regulates DAF-16/FOXO transcription factors—key regulators of aging, immunity, and stress adaptation—downstream of DAF-2/IGF-1 (Accili and Arden, 2004). This FOXO homolog accumulates in the nucleus, where it directly binds to conserved DNA motifs (DBE/DIR elements) to coordinate stress response genes (sod-3, gst-4). Furthermore, in this study, 50 μM baicalein exhibited strong nuclear translocation ability. Mechanistically, local signaling networks, such as the Wnt/β-catenin pathway, may impose spatial constraints on DAF-16 activation by modulating its nuclear-cytoplasmic shuttling or transcriptional accessibility. Notably, high concentrations of baicalein (100 μM) could disrupt this regulatory balance, potentially through interference with Wnt/β-catenin-mediated intestinal homeostasis or feedback inhibition of IIS signaling (Mohanty and Suchiang, 2023). This hypothesis aligns with previous findings that gut-specific suppression of DAF-2 extends lifespan via enhanced DAF-16 nuclear localization, while excessive pharmacological intervention may override physiological signaling thresholds, leading to pathway desensitization or compensatory metabolic reprogramming. Further studies combining tissue-specific gene knockdown and pathway activity assays are warranted to dissect the interplay between IIS, Wnt signaling, and flavonoid-mediated lifespan modulation in intestinal epithelia (Zhang Y. P. et al., 2022). This mechanism aligns with the conserved role of FOXO homologs in mammals, which are essential for Treg cell differentiation and suppression of IFN-γ-driven inflammation in DSS colitis (Ohkura and Sakaguchi, 2010). Furthermore, the interplay between daf-16 and hif-1, another stress-responsive gene, underscores the evolutionary conservation of these pathways in maintaining barrier integrity under oxidative duress (Danese et al., 2022; Brown et al., 2020; Yin et al., 2022). Notably, HIF-1α (the mammalian counterpart of hif-1) cooperates with FOXO3 to enhance antioxidant defenses and epithelial repair, suggesting baicalein may exploit this synergy to mitigate UC progression.
Although baicalein modulates daf-16, it likely acts through additional complementary pathways, as the activity of daf-16 is contingent on the phosphorylation of akt-1, a serine/threonine kinase downstream of PI3K. Phosphorylation of akt-1, which has been linked to chronic intestinal inflammation in IBD patients, suggests that baicalein’s protective effects might involve a more complex regulatory network, potentially modulating multiple signaling cascades (Zhang J. et al., 2022; Walldorf et al., 2021). Moreover PMK-1 may enhance DAF-16’s transcriptional activity by suppressing AKT activity, creating a feedback loop that amplifies stress resistance (Keshet et al., 2017).
Notably, sod-2 (mitochondrial SOD) and hsf-1 remained unaffected by baicalein in our model, despite their central roles in oxidative stress responses. It's likely that the expression of SOD-2 (mitochondrial superoxide dismutase) is typically regulated by redox-sensitive pathways (SIRT1), rather than Nrf2 or DAF-16 (Xiong et al., 2021). In addition, the competitive interaction between the HSF-1 and DAF-16 regulatory networks may prevent them from being simultaneously activated by the same stimulus, leading to the lack of induction of HSF-1 (Zhi et al., 2022). This complexity underscores that baicalein’s impact on intestinal inflammation and oxidative stress may not be fully explained by daf-2 and daf-16 alone.
This study adds to the growing body of evidence supporting the therapeutic potential of flavonoids, such as baicalein, in managing IBD. Flavonoids are known to enhance intestinal barrier function and modulate inflammatory responses through their effects on transcription factors and kinases such as PI3K/AKT and MAPK (Schwanke et al., 2013; Sahu et al., 2016). Importantly, while baicalein’s modulation of the DAF-2/IGF-1 and DAF-16/FOXO pathways plays a role, it is only one aspect of its broader mechanism of action. The involvement of the SKN-1/Nrf2 signaling cascade, activated by p38 MAPK in response to oxidative stress, adds another layer of complexity. This pathway is integral to innate immunity and longevity in C. elegans, suggesting that baicalein may enhance the organism’s ability to resist both oxidative and inflammatory damage through multiple molecular avenues (Dilberger et al., 2019; Hu et al., 2017; Tang and Pang, 2016; Liu F. et al., 2022). Thus, while the IIS and MAPK pathways are important targets, baicalein’s effects appear to be multifaceted, engaging a broader range of protective mechanisms.
While this study provides valuable insights into the protective mechanisms of baicalein, there are some limitations (Figure 12). We primarily focused on the effects of baicalein on intestinal barrier integrity and did not extensively examine downstream oxidative stress-related genes in epithelial cells. In mammals, DSS-induced colitis is characterized by chronic inflammation driven by adaptive immune responses (T-cell infiltration, cytokine cascades) and microbiome interactions (Zhang et al., 2025). In contrast, C. elegans lacks adaptive immunity and a microbiota, rendering DSS effects primarily acute, involving innate immune pathways (e.g., IIS, p38 MAPK), oxidative stress, and direct epithelial damage. And acute DSS exposure in worms mirrors early epithelial injury phases, whereas mammalian models replicate chronic inflammation with crypt hyperplasia and fibrosis. These differences highlight the utility of C. elegans for dissecting evolutionarily conserved pathways of epithelial stress and innate immunity, albeit with limited translatability to chronic or adaptive immune-mediated inflammation. To address species-specific limitations and strengthen pathological relevance, we propose the following refinements in subsequent studies: Dual-damage challenge: Co-exposure to DSS and pathogenic bacteria (e.g., OP50) to activate C. elegans p38 MAPK signaling and quantify antimicrobial peptide expression (abf-2, nlp-29) via qRT-PCR. Tissue-specific immune monitoring: Utilize transgenic reporters (e.g., irg-1pGFP) to dynamically assess intestinal immune activation in vivo. Future studies should leverage complementary models to bridge acute mechanistic insights with complex pathophysiology. It should aim to explore these pathways in greater detail, as well as validate the findings in mammalian models to further substantiate baicalein’s therapeutic potential in UC.
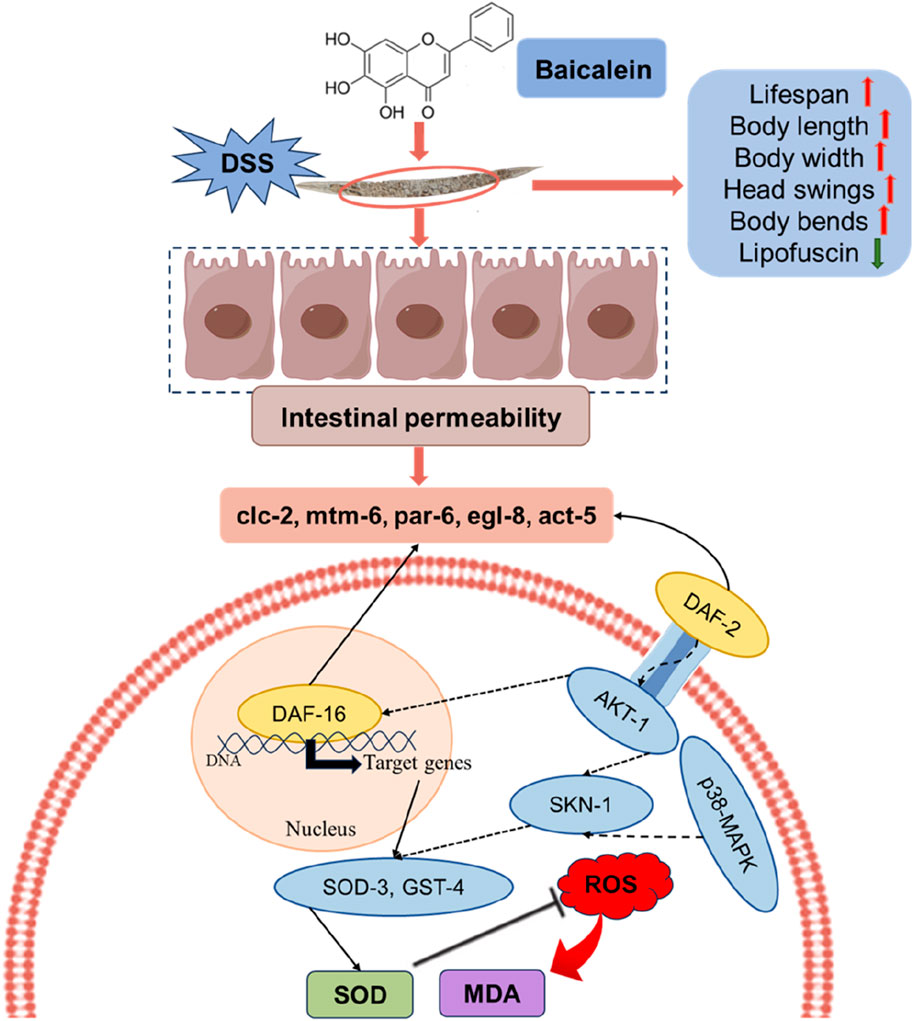
Figure 12. The proposed mechanisms by which baicalein mitigates the effects of DSS-induced damage in C. elegans. Upon DSS treatment, nematodes experience increased ROS and MDA levels, leading to oxidative stress and compromised intestinal permeability. Baicalein intervention appears to counteract these effects by enhancing several physiological parameters, including lifespan, body length, body width, head swings, and body bends, while decreasing lipofuscin accumulation, a marker of aging. In terms of oxidative damage, baicalein treatment enhances SOD activity, leading to reduced ROS levels, which subsequently lowers MDA formation, an indicator of lipid peroxidation. Additionally, baicalein improves intestinal permeability by modulating the expression of gene associated with barrier function. Mechanistically, the insulin/IGF-1 signaling pathway (IIS) and the MAPK pathway are involved in the regulation of baicalin oxidative stress and antioxidant capacity. This multi-pathway approach underscores baicalein’s potential as a therapeutic compound in enhancing health span and reducing oxidative stress under DSS-induced conditions.
5 Conclusion
The findings of this study demonstrate that baicalein confers protective effects against ulcerative colitis (UC) in a DSS-induced C. elegans model, primarily through enhancement of intestinal barrier integrity and mitigation of oxidative stress. Mechanistically, these benefits are associated with modulation of the p38/MAPK and insulin/IGF-1 signaling (IIS) pathways, underscoring baicalein’s dual role in attenuating inflammatory and oxidative damage. Furthermore, this work validates the utility of the C. elegans DSS model as a robust preclinical tool for rapid screening of UC therapeutics. The evidence presented here positions baicalein as a promising candidate for further investigation in higher-order models, with emphasis on its translational potential in targeting pathway-specific dysregulation characteristic of UC pathogenesis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
WW: Conceptualization, Data curation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. XF: Validation, Writing – review and editing. JX: Investigation, Writing – review and editing. WL: Writing – review and editing, Visualization. SH: Writing – review and editing, Validation. YW: Writing – review and editing. YX: Writing – review and editing. JH: Writing – review and editing, Investigation. KL: Writing – review and editing, Formal Analysis. CZ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was conducted under the High-Level Talent Training Program of the School of Life Sciences at Beijing University of Chinese Medicine. The project identification number is 90011451310015.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1592244/full#supplementary-material
References
Accili, D., and Arden, K. C. (2004). FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117 (4), 421–426. doi:10.1016/s0092-8674(04)00452-0
Althagafy, H. S., Ali, F. E. M., Hassanein, E. H. M., Mohammedsaleh, Z. M., Kotb El-Sayed, M. I., Atwa, A. M., et al. (2023). Canagliflozin ameliorates ulcerative colitis via regulation of TLR4/MAPK/NF-κB and Nrf2/PPAR-γ/SIRT1 signaling pathways. Eur. J. Pharmacol. 960, 176166. doi:10.1016/j.ejphar.2023.176166
Arnott, I. D., Kingstone, K., and Ghosh, S. (2000). Abnormal intestinal permeability predicts relapse in inactive crohn disease. Scand. J. Gastroenterol. 35 (11), 1163–1169. doi:10.1080/003655200750056637
Ayoub, I. M., Eldahshan, O. A., Roxo, M., Zhang, S., Wink, M., and Singab, A. N. B. (2024). Stress resistance, antiaging, and neuroprotective activities of baicalein 5,6-dimethyl ether and Alnus rugosa extract in Caenorhabditis elegans model. Arch. Pharm. Weinh. 357 (12), e2400464. doi:10.1002/ardp.202400464
Bhattacharyya, A., Chattopadhyay, R., Mitra, S., and Crowe, S. E. (2014). Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94 (2), 329–354. doi:10.1152/physrev.00040.2012
Brown, E., Rowan, C., Strowitzki, M. J., Fagundes, R. R., Faber, K. N., Güntsch, A., et al. (2020). Mucosal inflammation downregulates PHD1 expression promoting a barrier-protective HIF-1α response in ulcerative colitis patients. Faseb J. 34 (3), 3732–3742. doi:10.1096/fj.201902103R
Burisch, J., Jess, T., Martinato, M., and Lakatos, P. L.ECCO -EpiCom (2013). The burden of inflammatory bowel disease in Europe. J. Crohn's Colitis 7 (4), 322–337. doi:10.1016/j.crohns.2013.01.010
Burri, E., Maillard, M. H., Schoepfer, A. M., Seibold, F., Van Assche, G., Rivière, P., et al. (2020). Treatment algorithm for mild and moderate-to-severe ulcerative colitis: an update. Digestion 101 (Suppl. 1), 2–15. doi:10.1159/000504092
Chen, K., Shi, L., Ren, Z., and Weng, W. (2023). Antioxidant characteristics of hydrolysate from low-value sea cucumber: in vitro and in vivo activities of Caenorhabditis elegans. Food Chem. X 19, 100836. doi:10.1016/j.fochx.2023.100836
Chen, Y. C., Chow, J. M., Lin, C. W., Wu, C. Y., and Shen, S. C. (2006). Baicalein inhibition of oxidative-stress-induced apoptosis via modulation of ERKs activation and induction of HO-1 gene expression in rat glioma cells C6. Toxicol. Appl. Pharmacol. 216 (2), 263–273. doi:10.1016/j.taap.2006.05.008
Cheng, X., Zhang, P., Zhao, H., Zheng, H., Zheng, K., Zhang, H., et al. (2023). Proteotoxic stress disrupts epithelial integrity by inducing MTOR sequestration and autophagy overactivation. Autophagy 19 (1), 241–255. doi:10.1080/15548627.2022.2071381
Cui, G., Luk, S. C., Li, R. A., Chan, K. K. K., Lei, S. W., Wang, L., et al. (2015). Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1 pathway. J. Cardiovasc Pharmacol. 65 (1), 39–46. doi:10.1097/FJC.0000000000000161
Danese, S., Levesque, B. G., Feagan, B. G., Jucov, A., Bhandari, B. R., Pai, R. K., et al. (2022). Randomised clinical trial: a phase 1b study of GB004, an oral HIF-1α stabiliser, for treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 55 (4), 401–411. doi:10.1111/apt.16753
da Silva, B. C., Lyra, A. C., Rocha, R., and Santana, G. O. (2014). Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J. Gastroenterol. 20 (28), 9458–9467. doi:10.3748/wjg.v20.i28.9458
Dilberger, B., Baumanns, S., Schmitt, F., Schmiedl, T., Hardt, M., Wenzel, U., et al. (2019). Mitochondrial oxidative stress impairs energy metabolism and reduces stress resistance and longevity of C. elegans. Oxid. Med. Cell Longev. 2019, 6840540. doi:10.1155/2019/6840540
Duangjan, C., and Curran, S. P. (2022). Oolonghomobisflavans from Camellia sinensis increase Caenorhabditis elegans lifespan and healthspan. GeroScience 44 (1), 533–545. doi:10.1007/s11357-021-00462-7
Elkholy, S. E., Maher, S. A., Abd el-hamid, N. R., Elsayed, H. A., Hassan, W. A., Abdelmaogood, A. K., et al. (2023). The immunomodulatory effects of probiotics and azithromycin in dextran sodium sulfate-induced ulcerative colitis in rats via TLR4-NF-κB and p38-MAPK pathway. Biomed. and Pharmacother. 165, 115005. doi:10.1016/j.biopha.2023.115005
Elmaksoud, H. A. A., Motawea, M. H., Desoky, A. A., Elharrif, M. G., and Ibrahimi, A. (2021). Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 142, 112073. doi:10.1016/j.biopha.2021.112073
Fan, S., Yan, Y., Xia, Y., Zhou, Z., Luo, L., Zhu, M., et al. (2023). Pregnane X receptor agonist nomilin extends lifespan and healthspan in preclinical models through detoxification functions. Nat. Commun. 14 (1), 3368. doi:10.1038/s41467-023-39118-9
Feng, S., Zhang, C., Chen, T., Zhou, L., Huang, Y., Yuan, M., et al. (2021). Oleuropein enhances stress resistance and extends lifespan via Insulin/IGF-1 and SKN-1/Nrf2 signaling pathway in Caenorhabditis elegans. Antioxidants (Basel). 10 (11), 1697. doi:10.3390/antiox10111697
Gelino, S., Chang, J. T., Kumsta, C., She, X., Davis, A., Nguyen, C., et al. (2016). Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet. 12 (7), e1006135. doi:10.1371/journal.pgen.1006135
Gupta, S., Buttar, H. S., Kaur, G., and Tuli, H. S. (2022). Baicalein: promising therapeutic applications with special reference to published patents. Pharm. Pat. Anal. 11 (1), 23–32. doi:10.4155/ppa-2021-0027
Han, G. C., Jing, H. M., Zhang, W. J., Zhang, N., Li, Z. N., Zhang, G. Y., et al. (2022). Effects of lanthanum nitrate on behavioral disorder, neuronal damage and gene expression in different developmental stages of Caenorhabditis elegans. Toxicology 465, 153012. doi:10.1016/j.tox.2021.153012
Havermann, S., Humpf, H. U., and Wätjen, W. (2016). Baicalein modulates stress-resistance and life span in C. elegans via SKN-1 but not DAF-16. Fitoterapia 113, 123–127. doi:10.1016/j.fitote.2016.06.018
Hoffmann, J. C., Zeitz, M., Bischoff, S. C., Brambs, H. J., Bruch, H. P., Buhr, H. J., et al. (2004). Diagnosis and therapy of ulcerative colitis: results of an evidence based consensus conference by the German society of digestive and metabolic diseases and the competence network on inflammatory bowel disease. Z Gastroenterol. 42 (9), 979–983. doi:10.1055/s-2004-813510
Hu, Q., D'Amora, D. R., MacNeil, L. T., Walhout, A. J. M., and Kubiseski, T. J. (2017). The oxidative stress response in Caenorhabditis elegans requires the GATA transcription factor ELT-3 and SKN-1/Nrf2. Genetics 206 (4), 1909–1922. doi:10.1534/genetics.116.198788
Irazoqui, J. E., and Ausubel, F. M. (2010). 99th dahlem conference on infection, inflammation and chronic inflammatory disorders: Caenorhabditis elegans as a model to study tissues involved in host immunity and microbial pathogenesis. Clin. Exp. Immunol. 160 (1), 48–57. doi:10.1111/j.1365-2249.2010.04122.x
Jadhav, R., and Kulkarni, Y. A. (2023). The combination of baicalein and memantine reduces oxidative stress and protects against β-amyloid-Induced Alzheimer's disease in rat model. Antioxidants (Basel) 12 (3), 707. doi:10.3390/antiox12030707
Ji, P., Li, H., Jin, Y., Peng, Y., Zhao, L., and Wang, X. (2022). C. elegans as an in vivo model system for the phenotypic drug discovery for treating paraquat poisoning. PeerJ 10, e12866. doi:10.7717/peerj.12866
Kaletta, T., and Hengartner, M. O. (2006). Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 5 (5), 387–398. doi:10.1038/nrd2031
Keshet, A., Mertenskötter, A., Winter, S. A., Brinkmann, V., Dölling, R., and Paul, R. J. (2017). PMK-1 p38 MAPK promotes cadmium stress resistance, the expression of SKN-1/Nrf and DAF-16 target genes, and protein biosynthesis in Caenorhabditis elegans. Mol. Genet. Genomics 292 (6), 1341–1361. doi:10.1007/s00438-017-1351-z
Kim, K.-Y., Son, J. D., Hwang, S.-J., Lee, J. K., Park, J. Y., Park, K. I., et al. (2023). Fermented glutinous rice extract mitigates DSS-induced ulcerative colitis by alleviating intestinal barrier function and improving gut microbiota and inflammation. Antioxidants (Basel) 12 (2), 336. doi:10.3390/antiox12020336
Kim, M. R., Cho, S. Y., Lee, H. J., Kim, J. Y., Nguyen, U. T. T., Ha, N. M., et al. (2022). Schisandrin C improves leaky gut conditions in intestinal cell monolayer, organoid, and nematode models by increasing tight junction protein expression. Phytomedicine 103, 154209. doi:10.1016/j.phymed.2022.154209
Kitajima, S., Takuma, S., and Morimoto, M. (1999). Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp. Anim. 48 (3), 137–143. doi:10.1538/expanim.48.137
Klotz, C., Barret, M., Dhooge, M., Oudjit, A., Chaussade, S., Coriat, R., et al. (2015). Rectocolite hémorragique: conduite diagnostique et prise en charge thérapeutique. La Presse Médicale 44 (2), 144–149. doi:10.1016/j.lpm.2014.06.025
Kornbluth, A., and Sachar, D. B.Practice Parameters Committee of the American College of Gastroenterology (2010). Ulcerative colitis practice guidelines in adults: american college of gastroenterology, practice parameters committee. Am. J. Gastroenterol. 105 (3), 501–524. doi:10.1038/ajg.2009.727
Kumar, A., Joishy, T., Das, S., Kalita, M. C., Mukherjee, A. K., and Khan, M. R. (2022). A potential probiotic Lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants (Basel) 11 (2), 268. doi:10.3390/antiox11020268
Le Berre, C., Honap, S., and Peyrin-Biroulet, L. (2023). Ulcerative colitis. Lancet 402 (10401), 571–584. doi:10.1016/S0140-6736(23)00966-2
Li, B., Xiu, M., He, L., Zhou, S., Yi, S., Wang, X., et al. (2024). Protective effect of san huang pill and its bioactive compounds against ulcerative colitis in drosophila via modulation of JAK/STAT, apoptosis, toll, and Nrf2/Keap1 pathways. J. Ethnopharmacol. 322, 117578. doi:10.1016/j.jep.2023.117578
Li, H., Yu, X., Meng, F., Zhao, Z., Guan, S., and Wang, L. (2021). Ferulic acid supplementation increases lifespan and stress resistance via Insulin/IGF-1 signaling pathway in C. elegans. Int. J. Mol. Sci. 22 (8), 4279. doi:10.3390/ijms22084279
Li, J., Li, D., Yang, Y., Xu, T., Li, P., and He, D. (2016). Acrylamide induces locomotor defects and degeneration of dopamine neurons in Caenorhabditis elegans. J. Appl. Toxicol. 36 (1), 60–67. doi:10.1002/jat.3144
Li, J., Yang, Y., Wang, H., Ma, D., Chu, L., Zhang, Y., et al. (2022). Baicalein ameliorates myocardial ischemia through reduction of oxidative stress, inflammation and apoptosis via TLR4/MyD88/MAPK(S)/NF-κB pathway and regulation of Ca(2+) homeostasis by L-type Ca(2+) channels. Front. Pharmacol. 13, 842723. doi:10.3389/fphar.2022.842723
Li, W. J., Wang, C. W., Tao, L., Yan, Y. H., Zhang, M. J., Liu, Z. X., et al. (2021). Insulin signaling regulates longevity through protein phosphorylation in Caenorhabditis elegans. Nat. Commun. 12 (1), 4568. doi:10.1038/s41467-021-24816-z
Li, Y. Y., Wang, X. J., Su, Y. L., Wang, Q., Huang, S. W., Pan, Z. F., et al. (2022). Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 43 (6), 1495–1507. doi:10.1038/s41401-021-00781-7
Liang, S., Deng, X., Lei, L., Zheng, Y., Ai, J., Chen, L., et al. (2019). The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front. Pharmacol. 10, 1466. doi:10.3389/fphar.2019.01466
Liu, B., Piao, X., Niu, W., Zhang, Q., Wu, T., Cui, T., et al. (2020). Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-Dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front. Pharmacol. 11, 1036. doi:10.3389/fphar.2020.01036
Liu, C., Li, Y., Chen, Y., Huang, S., Wang, X., Luo, S., et al. (2020). Baicalein restores the balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediat. Inflamm. 2020, 5918587. doi:10.1155/2020/5918587
Liu F., F., Wang, H., Zhu, X., Jiang, N., Pan, F., Song, C., et al. (2022). Sanguinarine promotes healthspan and innate immunity through a conserved mechanism of ROS-Mediated PMK-1/SKN-1 activation. iScience 25 (3), 103874. doi:10.1016/j.isci.2022.103874
Liu, H., Wang, Y., Zhang, W., Sun, W., Ji, X., Zhang, S., et al. (2022). Lentinan extends lifespan and increases oxidative stress resistance through DAF-16 and SKN-1 pathways in Caenorhabditis elegans. Int. J. Biol. Macromol. 202, 286–295. doi:10.1016/j.ijbiomac.2022.01.071
Liu P., P., Shao, H., Kong, Y., and Wang, D. (2020). Effect of graphene oxide exposure on intestinal wnt signaling in nematode Caenorhabditis elegans. J. Environ. Sci. (China) 88, 200–208. doi:10.1016/j.jes.2019.09.002
Liu Y., Y., Zhang, W., Wang, Y., Liu, H., Zhang, S., Ji, X., et al. (2022). Oxidative stress, intestinal damage, and cell apoptosis: toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 286 (Pt 3), 131830. doi:10.1016/j.chemosphere.2021.131830
Loftus, E. V., Kane, S. V., and Bjorkman, D. (2004). Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 19 (2), 179–189. doi:10.1111/j.0269-2813.2004.01827.x
Low, D., Nguyen, D. D., and Mizoguchi, E. (2013). Animal models of ulcerative colitis and their application in drug research. Drug Des. Devel. Ther. 7, 1341–1357. doi:10.2147/DDDT.S40107
Ma, X. (2022). Research on toxicant-induced intestinal injury screening technology based on Caenorhabditis elegans. Master's thesis. Wuhan, China: Huazhong University of Science and Technology.
Ma, Y., Lang, X., Yang, Q., Han, Y., Kang, X., Long, R., et al. (2023). Paeoniflorin promotes intestinal stem cell-mediated epithelial regeneration and repair via PI3K-AKT-mTOR signalling in ulcerative colitis. Int. Immunopharmacol. 119, 110247. doi:10.1016/j.intimp.2023.110247
Mack, H. I. D., Buck, L. G., Skalet, S., Kremer, J., Li, H., and Mack, E. K. M. (2022). Further extension of lifespan by Unc-43/CaMKII and Egl-8/PLCβ mutations in germline-deficient Caenorhabditis elegans. Cells 11 (22), 3527. doi:10.3390/cells11223527
MacQueen, A. J., Baggett, J. J., Perumov, N., Bauer, R. A., Januszewski, T., Schriefer, L., et al. (2005). ACT-5 is an essential Caenorhabditis elegans actin required for intestinal microvilli formation. Mol. Biol. Cell 16 (7), 3247–3259. doi:10.1091/mbc.e04-12-1061
McCracken, J. M., and Allen, L.-A. H. (2014). Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 7, 15–23. doi:10.4137/JCD.S11038
Miller, K. G., Emerson, M. D., and Rand, J. B. (1999). Goalpha and diacylglycerol kinase negatively regulate the gqalpha pathway in C. elegans. Neuron 24 (2), 323–333. doi:10.1016/s0896-6273(00)80847-8
Mohanty, S. K., and Suchiang, K. (2023). Baicalein mitigates oxidative stress and enhances lifespan through modulation of wnt ligands and GATA factor: ELT-3 in Caenorhabditis elegans. Life Sci. 329, 121946. doi:10.1016/j.lfs.2023.121946
Munyaka, P. M., Rabbi, M. F., Khafipour, E., and Ghia, J. E. (2016). Acute dextran sulfate sodium (DSS)-Induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 56 (9), 986–998. doi:10.1002/jobm.201500726
Nakagawa, H., Shiozaki, T., Kobatake, E., Hosoya, T., Moriya, T., Sakai, F., et al. (2016). Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 15 (2), 227–236. doi:10.1111/acel.12431
Nakase, H., Sato, N., Mizuno, N., and Ikawa, Y. (2022). The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 21 (3), 103017. doi:10.1016/j.autrev.2021.103017
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 (10114), 2769–2778. doi:10.1016/S0140-6736(17)32448-0
Nikkhah-Bodaghi, M., Maleki, I., Agah, S., and Hekmatdoost, A. (2019). Zingiber officinale and oxidative stress in patients with ulcerative colitis: a randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 43, 1–6. doi:10.1016/j.ctim.2018.12.021
Oh, M., Yeom, J., Schraermeyer, U., Julien-Schraermeyer, S., and Lim, Y. H. (2022). Remofuscin induces xenobiotic detoxification via a lysosome-to-nucleus signaling pathway to extend the Caenorhabditis elegans lifespan. Sci. Rep. 12 (1), 7161. doi:10.1038/s41598-022-11325-2
Ohkura, N., and Sakaguchi, S. (2010). Foxo1 and Foxo3 help Foxp3. Immunity 33 (6), 835–837. doi:10.1016/j.immuni.2010.12.004
Pei, Y. H., Yan, N. N., Zhang, H. F., Zhang, S. T., Tang, Z. Z., Huang, Y., et al. (2022). Physicochemical characterization of a fern polysaccharide from Alsophila spinulosa leaf and its anti-aging activity in Caenorhabditis elegans. Chem. Biodivers. 19 (10), e202200156. doi:10.1002/cbdv.202200156
Prakash, T., and Janadri, S. (2023). Anti-inflammatory effect of wedelolactone on DSS induced colitis in rats: IL-6/STAT3 signaling pathway. J. Ayurveda Integr. Med. 14 (2), 100544. doi:10.1016/j.jaim.2022.100544
Qi, Z., Ji, H., Le, M., Li, H., Wieland, A., Bauer, S., et al. (2021). Sulforaphane promotes C. elegans longevity and healthspan via DAF-16/DAF-2 insulin/IGF-1 signaling. Aging (Albany NY) 13 (2), 1649–1670. doi:10.18632/aging.202512
Qu, M., Xu, K., Li, Y., Wong, G., and Wang, D. (2018). Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci. Total Environ. 643, 119–126. doi:10.1016/j.scitotenv.2018.06.173
Ren, M., Zhao, L., Ding, X., Krasteva, N., Rui, Q., and Wang, D. (2018). Developmental basis for intestinal barrier against the toxicity of graphene oxide. Part Fibre Toxicol. 15 (1), 26. doi:10.1186/s12989-018-0262-4
Rutgeerts, P., Sandborn, W. J., Feagan, B. G., Reinisch, W., Olson, A., Johanns, J., et al. (2005). Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353 (23), 2462–2476. doi:10.1056/NEJMoa050516
Saez, A., Herrero-Fernandez, B., Gomez-Bris, R., Sánchez-Martinez, H., and Gonzalez-Granado, J. M. (2023). Pathophysiology of inflammatory bowel disease: innate immune system. Int. J. Mol. Sci. 24 (2), 1526. doi:10.3390/ijms24021526
Sahu, B. D., Kumar, J. M., and Sistla, R. (2016). Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: relevance of NF-κB signaling. J. Nutr. Biochem. 28, 171–182. doi:10.1016/j.jnutbio.2015.10.004
Sallee, M. D., Pickett, M. A., and Feldman, J. L. (2021). Apical PAR complex proteins protect against programmed epithelial assaults to create a continuous and functional intestinal lumen. Elife 10, e64437. doi:10.7554/eLife.64437
Saul, N., Möller, S., Cirulli, F., Berry, A., Luyten, W., and Fuellen, G. (2021). Health and longevity studies in C. elegans: the “healthy worm database” reveals strengths, weaknesses and gaps of test compound-based studies. Biogerontology 22 (2), 215–236. doi:10.1007/s10522-021-09913-2
Schwanke, R. C., Marcon, R., Meotti, F. C., Bento, A. F., Dutra, R. C., Pizzollatti, M. G., et al. (2013). Oral administration of the flavonoid myricitrin prevents dextran sulfate sodium-induced experimental colitis in mice through modulation of PI3K/Akt signaling pathway. Mol. Nutr. Food Res. 57 (11), 1938–1949. doi:10.1002/mnfr.201300134
Silhankova, M., Port, F., Harterink, M., Basler, K., and Korswagen, H. C. (2010). Wnt signalling requires MTM-6 and MTM-9 myotubularin lipid-phosphatase function in wnt-producing cells. EMBO J. 29 (24), 4094–4105. doi:10.1038/emboj.2010.278
Simske, J. S., and Hardin, J. (2011). Claudin family proteins in Caenorhabditis elegans. Methods Mol. Biol. 762, 147–169. doi:10.1007/978-1-61779-185-7_11
Tang, H., and Pang, S. (2016). Proline catabolism modulates innate immunity in Caenorhabditis elegans. Cell Rep. 17 (11), 2837–2844. doi:10.1016/j.celrep.2016.11.038
Tang, J., Yan, B., Tang, Y., Zhou, X., Ji, Z., and Xu, F. (2023). Baicalein ameliorates oxidative stress and brain injury after intracerebral hemorrhage by activating the Nrf2/ARE pathway via miR-106a-5p/PHLPP2 axis. Int. J. Neurosci. 133 (12), 1380–1393. doi:10.1080/00207454.2022.2080676
Totong, R., Achilleos, A., and Nance, J. (2007). PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development 134 (7), 1259–1268. doi:10.1242/dev.02833
Walldorf, J., Porzner, M., Neumann, M., Joodi, G., Niess, J. H., von Boyen, G., et al. (2021). The selective 5-HT1A agonist SR57746A protects intestinal epithelial cells and enteric glia cells and promotes mucosal recovery in experimental colitis. Inflamm. Bowel Dis. 28 (3), 423–433. doi:10.1093/ibd/izab191
Wang, L., Pu, Z., Li, M., Wang, K., Deng, L., and Chen, W. (2020). Antioxidative and antiapoptosis: neuroprotective effects of dauricine in alzheimer's disease models. Life Sci. 243, 117237. doi:10.1016/j.lfs.2019.117237
Wangchuk, P., Yeshi, K., and Loukas, A. (2024). Ulcerative colitis: clinical biomarkers, therapeutic targets, and emerging treatments. Trends Pharmacol. Sci. 45 (10), 892–903. doi:10.1016/j.tips.2024.08.003
Wise, J. (2016). Glucocorticoids May increase risk of serious blood infection. BMJ 353, i3230. doi:10.1136/bmj.i3230
Wu, K., Zhao, X., Xiao, X., Chen, M., Wu, L., Jiang, C., et al. (2023). BuShen HuoXue decoction improves fertility through intestinal hsp-16.2-mediated heat-shock signaling pathway in Caenorhabditis elegans. Front. Pharmacol. 14, 1210701. doi:10.3389/fphar.2023.1210701
Wu, Z., Zhang, J., Wu, Y., Chen, M., Hu, H., Gao, X., et al. (2024). Gelsenicine disrupted the intestinal barrier of Caenorhabditis elegans. Chem. Biol. Interact. 395, 111036. doi:10.1016/j.cbi.2024.111036
Wymann, M. P., and Pirola, L. (1998). Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta 1436 (1-2), 127–150. doi:10.1016/s0005-2760(98)00139-8
Xiao, X., Zhou, Y., Tan, C., Bai, J., Zhu, Y., Zhang, J., et al. (2021). Barley β-glucan resist oxidative stress of Caenorhabditis elegans via daf-2/daf-16 pathway. Int. J. Biol. Macromol. 193 (Pt B), 1021–1031. doi:10.1016/j.ijbiomac.2021.11.067
Xiong, L., Deng, N., Zheng, B., Li, T., and Liu, R. H. (2021). HSF-1 and SIR-2.1 linked insulin-like signaling is involved in goji berry (lycium spp.) extracts promoting lifespan extension of Caenorhabditis elegans. Food Funct. 12 (17), 7851–7866. doi:10.1039/d0fo03300f
Yamanaka, T., Horikoshi, Y., Suzuki, A., Sugiyama, Y., Kitamura, K., Maniwa, R., et al. (2001). PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes cells. 6 (8), 721–731. doi:10.1046/j.1365-2443.2001.00453.x
Yang, C., Yao, L., Chen, D., Chen, C., Li, W., Tong, H., et al. (2024). Endosomal catabolism of phosphatidylinositol 4,5-bisphosphate is fundamental in building resilience against pathogens. Protein Cell 16 (3), 161–187. doi:10.1093/procel/pwae041
Ye, S., Shang, L., Xie, X., Cao, Y., and Chen, C. (2021). Optimization of in vitro culture conditions for production of Cordyceps bassiana spores (ascomycetes) and the effect of spore extracts on the health of Caenorhabditis elegans. Int. J. Med. Mushrooms 23 (6), 45–55. doi:10.1615/IntJMedMushrooms.2021038683
Yin, J., Ren, Y., Yang, K., Wang, W., Wang, T., Xiao, W., et al. (2022). The role of hypoxia-inducible factor 1-alpha in inflammatory bowel disease. Cell Biol. Int. 46 (1), 46–51. doi:10.1002/cbin.11712
Yu, X., Li, H., Lin, D., Guo, W., Xu, Z., Wang, L., et al. (2021). Ginsenoside prolongs the lifespan of C. elegans via lipid metabolism and activating the stress response signaling pathway. Int. J. Mol. Sci. 22 (18), 9668. doi:10.3390/ijms22189668
Yu, Y., Chen, H., Hua, X., Dang, Y., Han, Y., Yu, Z., et al. (2020a). Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci. Total Environ. 726, 138679. doi:10.1016/j.scitotenv.2020.138679
Yu, Y., Hua, X., Chen, H., Wang, Y., Li, Z., Han, Y., et al. (2020b). Toxicity of lindane induced by oxidative stress and intestinal damage in Caenorhabditis elegans. Environ. Pollut. 264, 114731. doi:10.1016/j.envpol.2020.114731
Zaarur, N., Desevin, K., Mackenzie, J., Lord, A., Grishok, A., and Kandror, K. V. (2019). ATGL-1 mediates the effect of dietary restriction and the insulin/IGF-1 signaling pathway on longevity in C. elegans. Mol. Metab. 27, 75–82. doi:10.1016/j.molmet.2019.07.001
Zhang, H., and Chen, W. (2023). Automated recognition and analysis of body bending behavior in C. elegans. BMC Bioinforma. 24 (1), 175. doi:10.1186/s12859-023-05307-y
Zhang, J., Cen, L., Zhang, X., Tang, C., Chen, Y., Zhang, Y., et al. (2022). MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biol. 56, 102469. doi:10.1016/j.redox.2022.102469
Zhang, J. Y., Liu, M. T., Liu, Y. H., Deng, H., Bai, J., Xie, J. H., et al. (2024). Barley protein LFBEP-C1 from Lactiplantibacillus plantarum dy-1 fermented barley extracts by inhibiting lipid accumulation in a Caenorhabditis elegans model. Biomed. Environ. Sci. 37 (4), 377–386. doi:10.3967/bes2024.042
Zhang, X., Chen, Q., Chen, L., and Ma, Z. (2024). Anti-aging in Caenorhabditis elegans of polysaccharides from Polygonatum cyrtonema hua. Molecules 29 (6), 1276. doi:10.3390/molecules29061276
Zhang, Y., Ji, W., Qin, H., Chen, Z., Zhou, Y., Zhou, Z., et al. (2025). Astragalus polysaccharides alleviate DSS-Induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 349 (Pt A), 122829. doi:10.1016/j.carbpol.2024.122829
Zhang Y. P., Y. P., Zhang, W. H., Zhang, P., Li, Q., Sun, Y., Wang, J. W., et al. (2022). Intestine-specific removal of DAF-2 nearly doubles lifespan in Caenorhabditis elegans with little fitness cost. Nat. Commun. 13 (1), 6339. doi:10.1038/s41467-022-33850-4
Zhi, D., Yang, W., Yue, J., Xu, S., Ma, W., Zhao, C., et al. (2022). HSF-1 mediated combined ginsenosides ameliorating alzheimer's disease like symptoms in caernorhabditis elegans. Nutr. Neurosci. 25 (10), 2136–2148. doi:10.1080/1028415X.2021.1949791
Zhou, Y., Chen, H., Zhong, W., and Tao, Y. J. (2024). Collagen and actin network mediate antiviral immunity against orsay virus in C. elegans intestinal cells. PLoS Pathog. 20 (1), e1011366. doi:10.1371/journal.ppat.1011366
Zhu, A., Ji, Z., Zhao, J., Zhang, W., Sun, Y., Zhang, T., et al. (2019). Effect of euphorbia factor L1 on intestinal barrier impairment and defecation dysfunction in Caenorhabditis elegans. Phytomedicine 65, 153102. doi:10.1016/j.phymed.2019.153102
Zhu, Y., Huang, X., Deng, Z., Bai, T., Gao, B., Xu, C., et al. (2024). Orally biomimetic metal-phenolic nanozyme with quadruple safeguards for intestinal homeostasis to ameliorate ulcerative colitis. J. Nanobiotechnology 22 (1), 545. doi:10.1186/s12951-024-02802-z
Keywords: C. elegans, ulcerative colitis, dextran sodium sulfate, baicalein, IIS pathway
Citation: Wang W, Fu X, Xu J, Lv W, Han S, Wang Y, Xia Y, Han J, Li K and Zhang C (2025) Baicalein from Scutellaria baicalensis mitigates oxidative stress through the IIS pathway in a C. elegans model of ulcerative colitis. Front. Pharmacol. 16:1592244. doi: 10.3389/fphar.2025.1592244
Received: 12 March 2025; Accepted: 13 June 2025;
Published: 26 June 2025.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilCopyright © 2025 Wang, Fu, Xu, Lv, Han, Wang, Xia, Han, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenggang Zhang, anhzQGJ1Y20uZWR1LmNu
 Wei Wang
Wei Wang Xiaoyu Fu
Xiaoyu Fu Jianing Xu
Jianing Xu Weiguang Lv
Weiguang Lv Shengnan Han1
Shengnan Han1 Chenggang Zhang
Chenggang Zhang