- 1College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Zhejiang-Hong Kong Joint Laboratory of Liver and Spleen Simultaneous Treatment in Traditional Chinese Medicine, College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Shanghai Hope-tec Biotechnology Inc., Shanghai, China
- 4Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Background: Gymnadenia conopsea (L.) R. Br., a medicinally significant orchid used for millennia in China, is systematically reviewed regarding its botany, resources, ethnomedicinal applications, phytochemistry, pharmacology, and propagation strategies to advance therapeutic utilization and conservation.
Methods: Using keywords such as “G. conopsea,” “phytochemistry,” “propagation and breeding,” “bioactive compounds,” “immunomodulatory effects,” and “neuroprotective potential,” we systematically searched literature related to G. conopsea plants from databases including Web of Science, SciFinder, PubMed, ACS Publications, CNKI, Wanfang Data, Google Scholar, and Baidu Scholar.
Results: A total of 1,074 papers were retrieved and 133 full-text articles were ultimately selected and comprehensively reviewed. Up to now, over 203 metabolites have been identified in the tubers of G. conopsea, including benzyl ester glucosides, stilbenoids, phenanthrenes, phenolic derivatives, alkaloids and polysaccharides. Pharmacological studies validate its multi-target therapeutic potential across tonification, anti-fatigue interventions, oxidative stress mitigation, antiviral defense, and management of gastric ulcers and silicosis. Despite extensive research on the pharmacological properties of crude extracts, the relationship between specific bioactive compounds and their corresponding pharmacological activities, particularly in vivo, remains poorly understood. Critically, overexploitation and habitat degradation have led to its classification as an endangered species. Current propagation efforts face significant challenges, including low natural germination rates, and dependence on specific habitats and obligate mycorrhizal fungi, precluding the development of efficient large-scale cultivation and seedling production systems.
Conclusion: Marked progress has been made in characterizing small-molecule metabolites of G. conopsea, yet comprehensive structural elucidation of polysaccharides remains incomplete. Additionally, research must be intensified on synergistic interactions of bioactive constituents, molecular targets, mechanisms of action, and in vivo metabolic pathways to facilitate development of a quality standard system. For propagation, wild-simulated cultivation should be adopted for resource conservation, while optimizing symbiotic germination techniques is critical to overcome propagation bottlenecks, ultimately enabling sustainable utilization.
Highlights
• This review contributes to updating the botany, traditional uses, resource status, phytochemistry, and pharmacology of Gymnadenia conopsea.
• The article further elaborates on the methodologies and challenges associated with the propagation and breeding of G. conopsea.
1 Introduction
Gymnadenia conopsea (L.) R. Br. commonly known as the palmate orchid, Tibetan notoginseng, Wangla, or Buddha’s hand orchid, is a species of the genus Gymnadenia R. Br. within the Orchidaceae family, characterized as a perennial botanical drug. Among the 27 species within the genus (Bateman et al., 2021a), five are endemic to China: G. conopsea (L.) R.Br., G.orchidis Lindl., G.crassinervis Finet, G. bicornis Tang and K. Y. Lang and G.emeiensis K.Y. Lang, predominantly found in the southwestern region of China (Xue, 2023). The morphology of G. conopsea is distinctive, with a plant height that can reach up to 60 cm, predominantly featuring pink flowers, although some individuals may exhibit pinkish-white blossoms. G. orchidis is relatively shorter, reaching a maximum height of approximately 35 cm, with flowers that are primarily purplish or pink, and occasionally white. Gymnadenia crassinervis can grow to a height of 55 cm, with flowers mainly pink and some slightly tinged with white. G. bicornis has a height range of 50–70 cm and presents flowers of a pale yellowish-green color, which are smaller in comparison to other species. Lastly, G. emeiensis has a height range of 30–50 cm and is notable for its white flowers.
The genus inhabits montane grasslands and semi-open woodlands (Brandrud et al., 2019), spanning temperate Eurasia to central India, including China, Japan, and the Himalayas (Bateman, 2021b; Anghelescu et al., 2023). In China, G. conopsea primarily occurs in Tibet, Qinghai, and Sichuan (Cha et al., 2024). Furthermore, G. orchidis is distributed in Qinghai, Shaanxi, Hubei, Gansu, etc.; G. bicornis, indigenous to Tibet, constitutes a distinctive species within the region; G. crassinervis, which is endemic to China, is located in Tibet, Yadong, Sichuan, and Yunnan. G. emeiensis primarily harvested in Mount Emei, Sichuan Province, originates in shrubbery-grassland habitats and boasts medicinal properties akin to that of G. conopsea (Xue, 2023). With the exception of G. emeiensis, the tubers of the other three species are frequently employed as substitutes for G. conopsea in Tibetan medicine. In this traditional medical system, G. conopsea is prescribed for conditions such as renal insufficiency, impotence, chronic pain, and urinary disorders due to its reputed yang-tonifying and essence-replenishing properties (Peng K. Z. et al., 2021). Modern studies validate its neuroprotective (anti-Alzheimer’s) (Luo, 2021), anti-oxidant properties (Yu L. et al., 2024), anti-fatigue (Liu et al., 2022), immunomodulatory (Yu, 2024b), and nootropic (Guo et al., 2022) activities. Over 200 metabolites, including glucosides, phenanthrenes, aromatic compounds, alkaloids, polysaccharides, lignans, flavones, triterpenoids, and steroids, have been isolated and characterized from the tubers of G. conopsea. Despite its therapeutic potential, the artificial cultivation on a large scale has not yet been achieved for this typical orchidaceae plant due to underdeveloped seeds, the challenges of natural reproduction, and its stringent habitat requirements. In recent years, the burgeoning market demand has spurred a proliferation of disorganized harvesting, leading to a drastic decline in G. conopsea resources, and the species is now teetering on the brink of extinction. Currently, recognized as a valuable medicinal substance, G. conopsea has been classified as an endangered species in both the “China Red List of Species” and the “IUCN Red List of Endangered Species”. Additionally, it has been designated as a second-class rare and endangered medicinal plant under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (Cheng et al., 2024a; Yu Y. P. et al., 2024).
This treatise will delineate the advancements in the study of G. conopsea, encompassing its botanical characteristics, traditional applications, phytochemical composition, pharmacological properties, and breeding. It aims to furnish a comprehensive reference for the rational and sustainable management, utilization, and conservation of G. conopsea resources in future endeavors.
2 Materials and methods
A comprehensive literature search was conducted across major scientific databases, including Web of Science, SciFinder, PubMed, ACS Publications, CNKI, Wanfang Data, Google Scholar, and Baidu Scholar, to identify studies pertaining to the phytochemical constituents, biological activities, ethnomedicinal applications, propagation, and breeding of G. conopsea (L.) R. Br. The search employed keywords such as “G. conopsea,” “phytochemistry,” “propagation and breeding,” “traditional uses,” “bioactive compounds,” “antioxidant activity,” “immunomodulatory effects,” and “neuroprotective potential,” utilizing Boolean operators (AND, OR) to optimize the search strategy. The scope was limited to literature published up to the end of 2024. Inclusion criteria encompassed peer-reviewed English articles specifically investigating G. conopsea’s phytochemistry, pharmacological properties, propagation, and breeding; relevant Chinese dissertations were also included to ensure broad representativeness. Non-peer-reviewed sources and studies unrelated to its medicinal or biological significance were excluded.
Systematic data extraction was performed on the selected studies, focusing on identified phytochemical constituents—including benzyl glucoside esters, stilbenoids, phenanthrenes, phenolic derivatives, alkaloids, polysaccharides, lignans, flavonoids, triterpenoids, and steroids—and their associated bioactivities, such as tonic effects, anti-fatigue interventions, mitigation of oxidative stress, antiviral defense, and therapeutic applications for gastric ulcers and silicosis. Notably, the isolation and characterization of dozens of novel compounds significantly expanded the known phytochemical profile of this species beyond previous literature (Shang et al., 2017; Meng et al., 2023). All compound structures presented in this review were meticulously drawn using ChemDraw Ultra 8.0 (PerkinElmer Inc., Waltham, MA, United States), facilitating the understanding of pharmacological activities and structure-activity relationships and promoting the future establishment of standardized quality control for G. conopsea.
Significant progress has been achieved in the isolation and characterization of G. conopsea metabolites, particularly small-molecule compounds. While further isolation and identification of trace novel structures contribute to enriching natural product libraries, they offer minimal insight into elucidating the traditional pharmacological significance of this medicinal orchid. Consequently, diverging from existing reviews (Shang et al., 2017; Meng et al., 2023), this study critically addresses research limitations and emphasizes the following imperatives: Standardized characterization of bioactive constituents with established traditional pharmacological relevance; Investigation of synergistic interactions among high-abundance efficacy components with confirmed bioactivity; Application of advanced methodologies to elucidate primary molecular targets and mechanisms of action for recognized active metabolites; collectively enabling comprehensive development of a quality standard system for G. conopsea. Critically, severe propagation constraints necessitate systematic research on wild resource domestication and artificial cultivation. This review examines key challenges and proposes: (1) Control of nematode infestations; (2) Implementation of semi-wild cultivation; (3) Supplementation with beneficial mycorrhizal fungi and recreation of conducive soil microenvironments to conserve germplasm resources; (4) Optimization of symbiotic germination technology to resolve seedling production bottlenecks. Therefore, this study synthesizes current knowledge on G. conopsea’s chemical composition, pharmacological activities, and resource status, identifies persistent challenges, and proposes strategic research directions to advance therapeutic applications and species conservation.
3 Study selection
A systematic search was conducted across six major scientific databases, yielding a total of 1,074 records. The screening process followed the PRISMA flowchart framework adapted from Page et al. (Page et al., 2021) with modifications (Figure 1). The databases included Web of Science (n = 166), PubMed (n = 69), Google Scholar (n = 263), ACS Publications (n = 17), CNKI (n = 256), Wanfang Data (n = 104), and Baidu Scholar (n = 199). After removing duplicates using NoteExpress and conducting manual screening, 503 articles were retained for preliminary evaluation. Though title and abstract screening 318 records were excluded. The remaining 185 articles underwent full-text assessment. Among these, 36 articles were excluded due to being outside the research scope, 16 were related to prescription studies, 18 focused on other Gymnadenia species, and 2 had unavailable full-text versions. Following this rigorous selection process, 113 studies met all predefined inclusion criteria and were included in the qualitative synthesis.
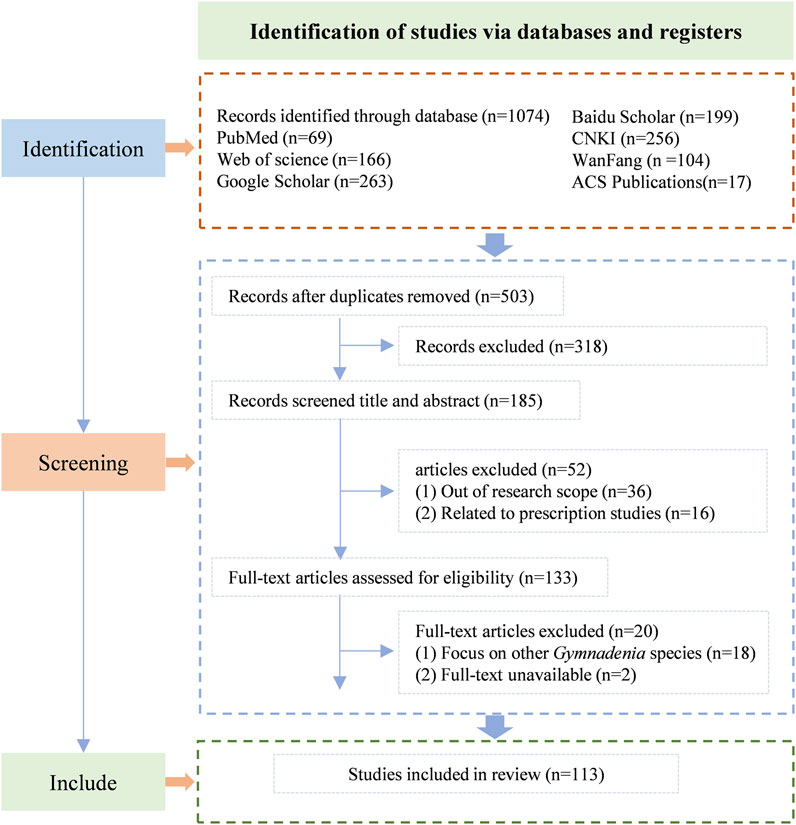
Figure 1. Flow diagram for the process of included studies identification (Page et al., 2021).
4 Botanical characteristics and conservation status
G. conopsea, a perennial terrestrial and aromatic orchidaceous botanical drug, is commonly encountered within high-altitude forests, grasslands, shrublands, and inundated meadow regions at altitudes ranging from 0 to 4,700 m (Gao et al., 2020). It displays a marked preference for habitats with ample sunlight and flourishes predominantly on calcareous or alkaline substrates that are nutrient-poor (spanning from oligotrophic to mesotrophic conditions) (Meekers et al., 2012; Anghelescu et al., 2024). The natural population size of G. conopsea is small. It is mostly distributed sporadically or in sporadic patches in local areas of some counties in the Qinghai-Tibet Plateau (Yang et al., 2018a; Chen et al., 2023). Its abundance is inextricably linked to altitude, manifesting the unique characteristics of the plateau monsoon climate (Chen J. Y. et al., 2022; Yu Y. P. et al., 2024). Despite the existence of approximately 69 synonyms for this taxon, only G. conopsea (L.) R. Br. is widely recognized and accepted. This species typically attains a height of 20–60 cm and possesses slightly flattened roots. The lower section of the plant is usually divided into 3 to 5 digitate lobes, resembling a palm, and measures approximately 5 cm in length and 4 cm in diameter (Long et al., 2019). The stem is erect, cylindrical, and slender, adorned with 4 longitudinal leaves or oblique, and is rooted at the base with 2-3 brown, cylindrical sheaths, while the upper part bears 4-5 leaves and terminates in 1 to several bracteolate leaflets. The bracts are green, frequently tinged with purple at the margins, and are lanceolate with acuminate apices. The leaf blades are green, linear-lanceolate, narrowly oblong, or ribbon-like in morphology, with the lower leaves being erect to slightly spreading, measuring 5.5–15 cm in length and 1-2 (−2.5) cm in width, characterized by an acuminate or slightly obtuse apex, entire margins, a keel-like midrib, and a base that narrows into a clasping sheath. The racemes are densely flowered, cylindrical, and measure 5.5–15 cm in length, exhibiting a coloration that progresses from pale pink to lavender (rarely white or bright magenta). They reach full bloom in July-August, emitting an intense fragrance. The bracts are lanceolate, erect, and the apex is prolonged into a caudate shape, usually exceeding the length of the flower and ovary. The flowers are fragrant, predominantly pink, though occasionally pale pink to whitish-pink. The ovary is fusiform, with a slightly recurved apex, measuring approximately 8 mm in length including the peduncle. The pollen mass is ovoid in shape, characterized by a delicate pedicel and a mucilaginous disc. The mucilaginous disc assumes a linear-lanceolate morphology. The median sepal is broadly elliptical, measuring 3.5–5 mm in length and 3–4 mm in breadth. The apex is faintly saccate and marked by the presence of three veins. The lateral sepals are obliquely ovate, reflexed, and slightly exceed or are almost equivalent in length to the median sepal. The margins are incurved, and the apex terminates in an acute point, also traversed by three veins. The petals are vertically oriented, obliquely ovate-triangular in shape, corresponding in length to the median sepal and nearly matching the breadth of the lateral sepals. The margins are finely dentate, culminating in an acute apex. It is broadly cuneate-obovate and has three veins. The labellum projects anteriorly and exhibits a broadly obovate shape, with a length of 4–5 mm (Shang et al., 2017). Capsule, trigonal long cylindrical, sessile, ranging from 0.6 to 1.4 cm in length. The seeds are light brown, very numerous and small (Yang, 2018b). Each capsule contains approximately 8,000 to 10,000 seeds. Individual seeds measure approximately 450 μm in length. Scanning electron microscopy reveals that mature G. conopsea seeds exhibit a fusiform shape with surface ornamentation. The central portion of the seed houses the embryo, which is approximately 200 μm wide. Additionally, one end of the seed features an aperture measuring about 80 μm in width (Gao et al., 2019).
Under natural conditions, the asexual reproduction coefficient of Gymnadenia species is notably low. The growth and development of the hand-shaped tubers in the present year are contingent upon the nutritional transfer from the tubers of the preceding year; that is, the growth of a new tuber is maintained by consuming the old ones. This is obviously insufficient to meet the demands of large-scale cultivation. Furthermore, as an orchidaceous plant dependent upon a specific habitat, the germination of its seeds is contingent upon particular mycorrhizal fungi, thereby rendering the process arduous and resulting in an exceedingly low rate of natural germination (Shi, 2023a). Moreover, in the contemporary era, robust market demand, excessive exploitation, and the degradation of indigenous habitats have precipitated a drastic decline in the wild resources of G. conopsea, prompting its classification as an endangered species. Consequently, there is an imperative need to conduct comprehensive research on G. conopsea and to harness its resources efficiently.
5 Traditional uses
G. conopsea (commonly known as hand orchid), a traditional ethnomedicine with a millennium-long history of application, is characterized by a sweet-bitter taste, neutral nature, and heavy, greasy, soft, dilute, and pure properties. It primarily targets the lung, spleen, and stomach meridians, exhibiting therapeutic effects such as invigorating yang, consolidating essence, and nourishing vitality (Shi Y. et al., 2022). For decades, G. conopsea has been documented in the Pharmacopoeia of the People’s Republic of China as a key ingredient in multiple formulations, extensively utilized in Tibetan and Mongolian medical systems for kidney tonification and pulmonary disease management. Additionally, its significance as an aromatic orchid species has attracted research attention in European countries (Lin et al., 2020; Meng et al., 2023). The tuber of G. conopsea is predominantly employed in traditional botanical drug practices across Asia, including China, Nepal, and Japan (Lin et al., 2020; Meng et al., 2023). In China and Russia, its preparations—tinctures and Galenical formulations—are clinically prescribed for treating impotence and alleviating bronchial asthma symptoms, respectively (Nazim et al., 2001; Devkota et al., 2022).
In China, G. conopsea is categorized as a tonic botanical drug, with its medicinal use first recorded in Tibetan Pharmacopoeia Somaratsa (Moon King’s Medicinal Diagnoses), an ancient Tibetan medical text (Pe-Ru Ta-Na, 1985). Yutuobencao further elaborates its efficacy in dispelling cold, treating rheumatic disorders (Tibetan: Huangshui disease), delaying senescence, and promoting semen production (Gyu-Thogrnying-Mayon-Tanmgon-Po, 2006). Dumubencao describes its sweet and greasy taste, highlighting its role as a longevity-enhancing elixir (Santaraksita, 2006). Blue Beryl (Vaidurya Sngon Po), a classical Tibetan medical treatise, emphasizes its capacity to strengthen the body, enhance male fertility, detoxify, and address spleen-related disorders when combined with brown sugar (Mao et al., 2012). The Tibetan Medicinal Materials Standard of Qinghai Province (Vol. 1) identifies G. orchidis (Tibetan: Wangla) as a common Tibetan botanical drug with applications in chronic debility, prolonged hemorrhage, chronic diarrhea, pulmonary deficiency-induced cough, and impotence (Administration, 2019).
According to the Tibetan Formula Database, G. conopsea tubers are included in 104 out of 4,500 traditional Tibetan prescriptions (2.3%), with 33 formulations targeting physical strengthening and anti-aging, 26 for kidney diseases, 12 for gout and musculoskeletal pain, 11 for pulmonary conditions, 7 for ophthalmic disorders, and the remainder for parasitic infections and miscellaneous ailments (Shang et al., 2017).
6 Phytochemistry
The research on the chemical constituents of G. conopsea primarily focuses on its tuberous part. To date, a total of 203 metabolites have been isolated and identified (Tables 1, 2). Among these, glycosides represent the most abundant class of chemical constituents. Additionally, stilbene derivatives, phenanthrenes, aromatic compounds, alkaloids, polysaccharides, lignans, flavones, triterpenoids, steroids, and other compounds have also been isolated and documented (Zi et al., 2010). The diverse array of chemical metabolites found in G. conopsea provides a substantial material basis for its various pharmacological activities. A comprehensive review of these metabolites is instrumental in deepening our understanding of the pharmacological mechanisms underlying G. conopsea and serves as an important reference for investigating the active metabolites present in other species within this genus.
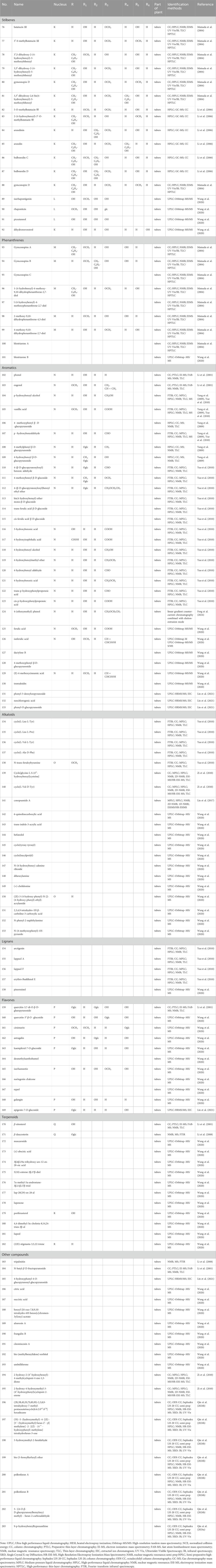
Table 2. Stilbenes, phenanthrenes, aromatics, alkaloids, lignans, flavones, terpenoids and other compounds isolated and identified from G. conopsea.
6.1 Glucosides
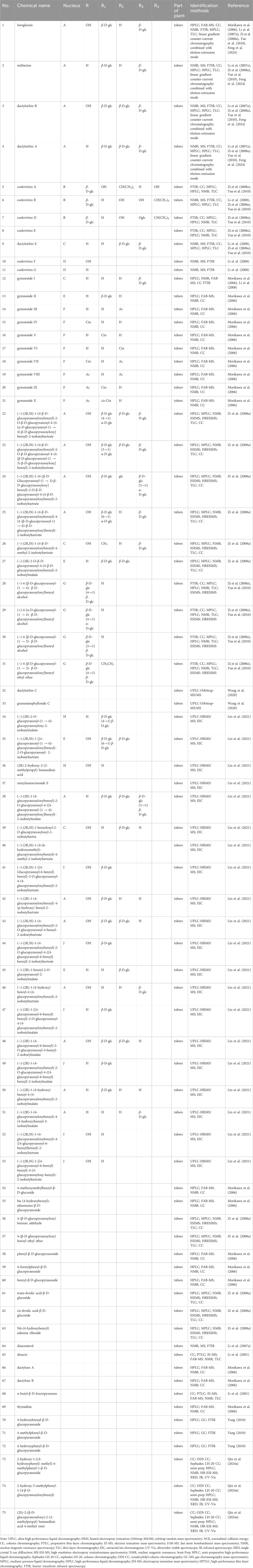
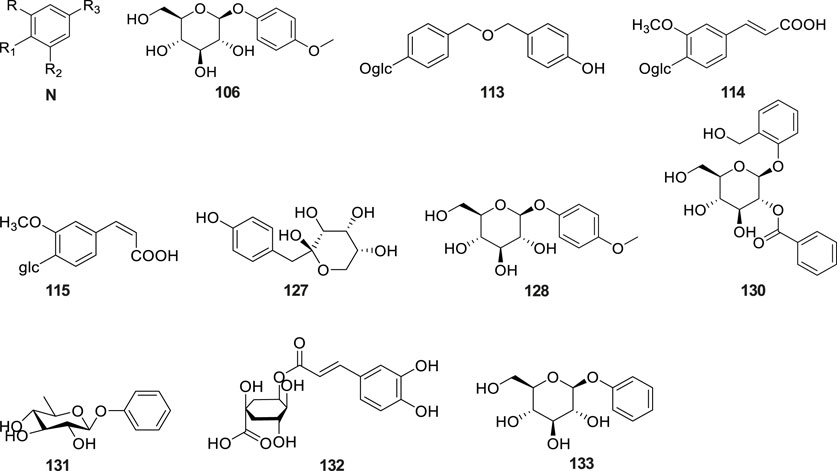
Glucosides represent one of the paramount chemical constituents within the composition of G. conopsea, with a total of 73 distinct compounds having been rigorously investigated and successfully isolated (Table 1; Figure 2). Based on their structural configurations, these glucosides are categorizable into benzylester glucosides and additional varieties of glucosides.
6.1.1 Benzylester glucosides
Fifty-four benzylester glucosides have been successfully isolated and identified from G. conopsea. The fundamental core structures are either 2-isobutyl tartaric acid or 2-isobutyl hydroxysuccinic acid. These acids integrate with one or more gluconyl benzyl alcohols to form a variety of complex compounds (Wang et al., 2020). According to the differences in their organic acids, they are classified as (2R, 3S)-2-isobutyl tartaric acid derivates and (2R)-2-isobutyl hydroxysuccinic acid derivatives. Li et al. (2007a) and Li et al. (2008) were the first to isolate nine benzylester glucosides (No. 1-4, 6, 9–12) from the ethanol extract of the tuber of G. conopsea. In 2006, Morikawa et al. (2006) isolated fourteen benzylester glucosides (No. 1-4, 12–21) from the methanol extraction of G. conopsea tuber. In addition, Zi et al. (2008a) also isolated eleven benzylester glucosides (No. 5-9, 22–27) from the ethanol extract of G. conopsea tuber. Furthermore, Yue et al. (2010) isolated and identified four new benzylester glucosides (No. 28–31) from the ethanolic extract of G. conopsea tuber. The research team headed by Wang et al. (2020) delineated two distinct compounds, dactylorhin C (No. 32) and grammatophylloside C (No. 33), from the 95% methanol extract of the G. conopsea tuber, utilizing UPLC-Orbitrap-MS/MS analytical techniques. Furthermore, an assortment of twenty benzylester glucosides (No. 34–53) was characterized from the ethanol extract of G. conopsea (Lin et al., 2021).
Benzylester glucosides represent a class of compounds found in the tubers of Gymnadenia conopsea (hand orchid) that are not only diverse in structural types but also often present in considerable concentrations. Li (2007b) quantified the content of five specific compounds—loroglossin (No. 1), militarine (No. 2), dactylorhin B (No. 3), dactylorhin A (No. 4), and dactylorhin E (No. 9)—in tubers collected from five distinct geographical regions: Lijiang (Yunnan), Tibet, Yuxian County (Hebei), Kangding (Sichuan), and Xining (Qinghai). The measured concentrations ranged from 0.25 to 3.06 mg/g, 0.10–0.56 mg/g, 0.67–4.073 mg/g, 0.51–2.33 mg/g, and 0.09–0.20 mg/g, respectively. Notably, samples from Xining, Qinghai exhibited the highest content for four of these compounds (No. 1, 2, 3, 4), with dactylorhin E (No. 9) ranking second highest. The total content of these five compounds reached 10.186 mg/g in the Xining samples, followed by the Tibetan samples at 5.458 mg/g. Similarly, He (2023a) employed HPLC to determine the content of three benzylester glucosides—loroglossin (No. 1), militarine (No. 2), and dactylorhin A (No. 4)—in ten batches of G. conopsea samples. The corresponding concentrations ranged from 0.30 to 12.7 mg/g, 0.09–1.76 mg/g, and 0.40–5.59 mg/g, respectively. These findings demonstrate significant variations in the content of these compounds among samples from different origins and batches. Consequently, there is an urgent need to implement standardized cultivation practices to mitigate quality variations arising from factors such as differences in regional soil conditions, climate, and the predominance of wild-sourced material (nearly all current samples).
6.1.2 Other glucosides
A total of 22 other glucoside derivatives (No. 54–75) were successfully isolated and characterized from the tubers of G. conopsea, as demonstrated in Table 1 and Figure 2 (Li et al., 2001; Morikawa et al., 2006; Zi, 2008b; Yang, 2010; Qin et al., 2024a).
6.2 Stilbenes
Stilbene derivatives constitute a specialized class of phytochemicals defined by a 1,2-diphenylethylene skeleton or its polymeric variants as structural backbones. The structural diversity arises from substitution patterns primarily occurring at positions 2, 3, 4, 5, 6, 3′, and 4′ (Figure 3), with hydroxyl (-OH), methoxy (-OCH3), glucosyloxy (-O-glc), and p-hydroxybenzyl groups being the predominant substituents (Table 2; Figure 3). Phytochemical investigations of G. conopsea have yielded significant findings: Li et al. (Li et al., 2006; Matsuda et al., 2004) systematically characterized thirteen stilbenoids (No. 76–88) from methanolic tuber extracts. Subsequent research by Wang et al. (Wang et al., 2020) expanded the chemical repertoire through the isolation of four novel derivatives from 95% methanol extracts, including isorhapontigenin (No. 89), rhaponticin (No. 90), piceatannol (No. 91), and dihydroresveratrol (No. 92).
6.3 Phenanthrenes
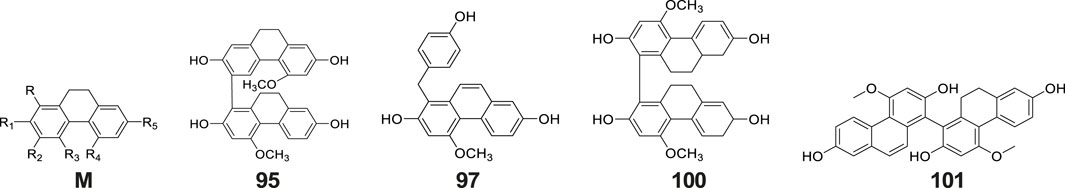
Phenanthrenes represent a class of polycyclic aromatic hydrocarbons comprising three benzene rings, typically featuring dihydrophenanthrene as the fundamental structural core. The substituents are predominantly situated at the positions C-1, C-2, -3, -4, -5 and -7 (Figure 4). The prevalent substituents encompass OH, OCH3 or p-hydroxybenzyl. To date, nine phenanthrene derivatives have been isolated from G. conopsea and structurally characterized, including: gymconopins A-C (No. 93–95), 1-(4-hydrobenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol (No. 96), l-(4-hydroxy benzyl)-4-methoxyphenanthrene-2,7-diol (No. 97), 2-methoxy-9,10- dihydrophenan threne-4,5-diol (No. 98), 4-methoxy-9,10-dihydrophenanthrene-2,7- diol (No. 99), blestriarene A (No. 100), and blestriarene B (No. 101) (Matsuda et al., 2004; Wang et al., 2020). These compounds exhibit variations in hydroxylation patterns and benzyl substitution, as detailed in Table 2 and Figure 4.
Bibenzyls and phenanthrenes represent characteristic constituents of Orchidaceae plants, extensively documented in Dendrobium species (Zhai et al., 2022), Bletilla striata (Jiang et al., 2021), and tubers of G. conopsea (Meng et al., 2023). Bibenzyl compounds undergo cytochrome P450-catalyzed oxidation to form dihydrophenanthrene intermediates, which subsequently undergo aromatization to yield phenanthrene scaffolds. These compounds, characterized by benzene rings bearing phenolic hydroxyl groups, are classified as plant polyphenols and demonstrate potent free radical-scavenging activities. They constitute key antioxidant metabolites in G. conopsea tubers (Table 4). In their investigation of radical-scavenging activities among phenanthrenes and bibenzyls isolated from G. conopsea tubers, Morikawa et al.(Morikawa et al., 2006) established critical structure-activity relationships: For phenanthrenes, dihydrogenation at positions 9 and 10 enhances bioactivity, while p-hydroxybenzyl substitution at C-1 or C-3 similarly potentiates activity; conversely, for bibenzyl derivatives, methylation at the 3′-position diminishes activity, whereas p-hydroxybenzyl substitution at C-2 and/or C-6 augments efficacy. These structural insights provide a valuable foundation for future applications in synthetic biology aimed at the targeted biosynthesis of high-activity compounds through rational modification of natural product scaffolds.
6.4 Aromatic compounds
Phytochemical studies have revealed that phenolic compounds constitute the predominant class of aromatic constituents isolated from G. conopsea tubers. As ubiquitous secondary metabolites in the plant kingdom, these phenolic derivatives demonstrate significant biological relevance in both plant biochemistry and therapeutic applications. Systematic investigations have identified 32 structurally distinct phenolic derivatives from this orchid species (Table 2; Figure 5). Li et al. (2001) purified phenol (No. 102) and eugenol (No. 103) from an ethanol extract of G. conopsea tubers. Subsequently, Yang et al. (2009) utilized advanced techniques such as reverse-phase column chromatography to isolate and identify six additional compounds (No. 104–109) from the n-butanol extract of G. conopsea tubers. Furthermore, fourteen phenolic compounds (No. 110–123) were separated from the ethanol extract of G. conopsea tubers (Yue et al., 2010). Additionally, Feng et al. (2024) successfully isolated 4-(ethoxymethyl) phenol (No. 124) using linear gradient counter-current chromatography combined with elution-extrusion mode. Moreover, Wang et al. (2020) and Lin et al. (2021) expanded the phenolic profile through 95% methanol and ethanol extractions, yielding six (No. 125–130) and three (No. 131–133) additional compounds, respectively.
These phenolic metabolites are generally present at low levels in G. conopsea tubers, and virtually no reports exist on their in vitro or in vivo pharmacological activities. Future research should prioritize integrating the documented pharmacological effects of G. conopsea in traditional Chinese medicine with computational approaches—such as network pharmacology analysis and molecular docking techniques—to predict and validate the bioactivities of these compounds.
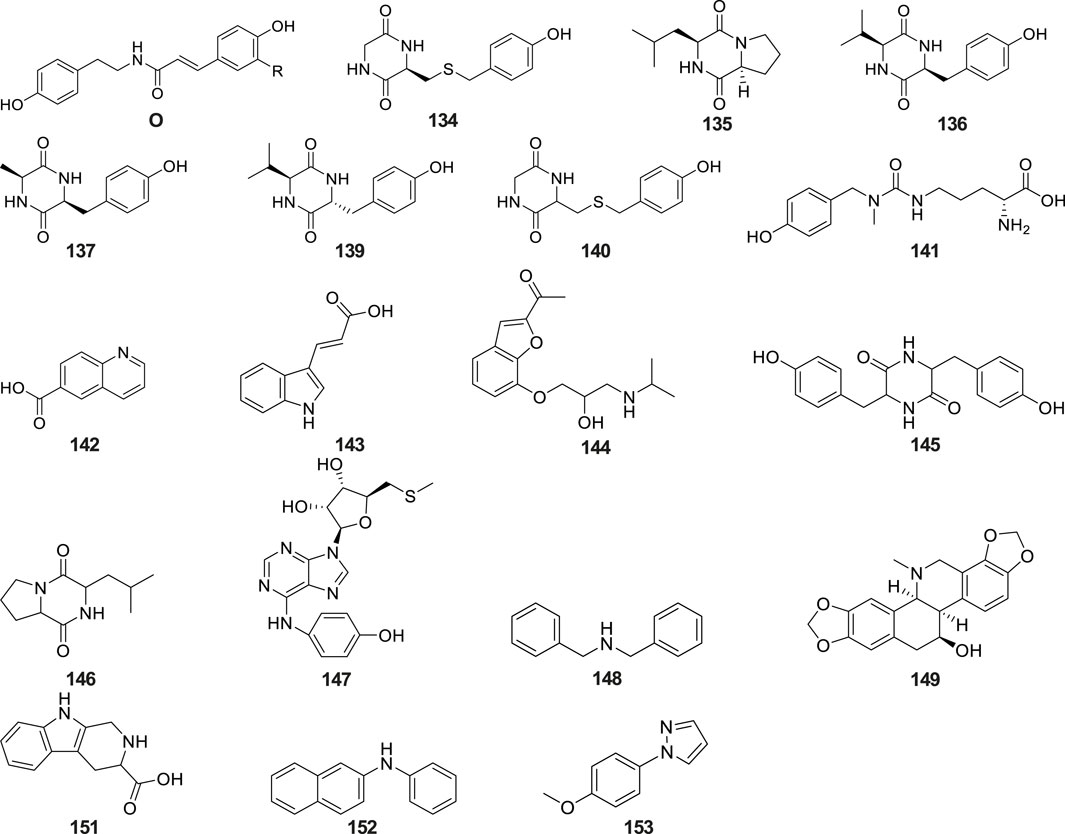
6.5 Alkaloids
Alkaloids are a class of nitrogen-containing alkaline organic compounds with alkali-like properties, historically referred to as pseudoalkaloids due to their alkali-like characteristics. To date, 20 alkaloids have been isolated and identified from G. conopsea (Table 2; Figure 6). Yue et al. (2010) isolated and identified five alkaloids—cyclo(L-Leu-L-Tyr) (No. 134), cyclo(L-Leu-L-Pro) (No. 135), cyclo (L-Val-L-Tyr) (No. 136), cyclo(L-Ala-D-Phe) (No. 137) and N-trans-feruloyltyramine (No. 138) —from the ethanol extract of G. conopsea tubers. Three additional alkaloids, cyclo[glycine-L-S-(4′-hydroxybenzyl)cysteine] (No. 139), cyclo(L-Val- D-Tyr) (No. 140) (Zi et al., 2010) and conopsamide A (No. 141) were isolated from the tubers of G. conopsea (Lin et al., 2017). Furthermore, Wang et al. (2020) identified 12 more alkaloids (No. 142–153) from the 95% methanol aqueous solution of G. conopsea tubers.
6.6 Lignans
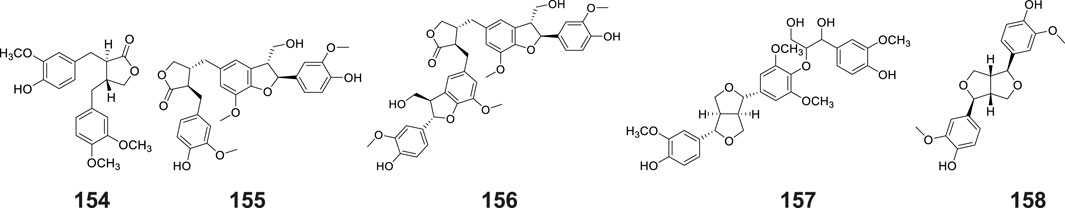
Lignans constitute a class of natural compounds that are synthesized through the dimerization of two phenylpropanoid (C6-C3) units. In their native state, these compounds predominantly occur as free aglycones rather than glycosidically bound forms. Within the phytochemical profile of G. conopsea, lignans exhibit limited distribution, with only five representatives currently documented (Table 2; Figure 7). Notably, phytochemical analysis of the tuber ethanol extract by Yue et al. (2010) identified four furanolignans: arctigenin (No. 154), lappaol A (No. 155), lappaol F (No. 156) and erythro-buddlenol E (No. 157), by Yue et al. (2010). Among these, the first three compounds demonstrated inhibitory effects on Fe2+-Cys-induced MDA formation in rat liver microsomes (Table 4). In a separate investigation, Wang et al. (2020) detected pinoresinol (No. 158) through targeted fractionation of tubers extracted with 95% methanol, marking the first identification of this tetrahydrofuran-type lignan in the genus. These lignans are characteristically abundant in Arctium lappa (burdock seeds) and exhibit diverse biological activities including antitumor, anti-inflammatory, and immunomodulatory effects (Yosri et al., 2023), suggesting their potential contribution to the pharmacological properties of G. conopsea.
6.7 Flavones
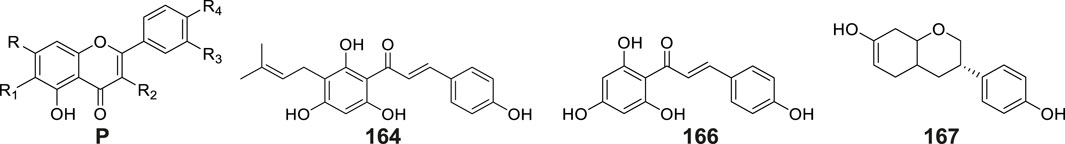
Flavonoids constitute a prominent class of phenylpropanoid derivatives featuring a characteristic C15 skeleton with two aromatic rings (A- and B-rings) interconnected by a heterocyclic pyran moiety (C-ring). These phytochemicals exhibit extensive structural plasticity through hydroxylation, glycosylation, and methylation modifications, contributing to their ecological roles and pharmacological potential. To date, a total of eleven flavonoids have been isolated and characterized from plants of the Gymnadenia species, six of which are glycoside compounds (Table 2; Figure 8). The isolation of quercetin-3,7-di-O-β-D-glucopyranoside (No. 159) was first reported by Li et al. (2001). Subsequent phytochemical profiling of G. conopsea tuber extracts by Wang et al. (2020) employing 95% aqueous methanol extraction led to the identification of nine derivatives: quercetin-3′-β-O-glucoside (No. 160), cirsimarin (No. 161), astragalin (No. 162), kaempferol-7-O-glucoside (No. 163), desmethylxanthohumol (No.164), isorhamnetin (No. 165), naringenin chalcone (No. 166), equol (No. 167) and galangin (No. 168). Furthermore, Lin et al. (2021) successfully elucidated apigenin-7-O-glucoside (No. 169) from the tubers of G. conopsea. While these flavonoids are established bioactive constituents in traditional Chinese medicines, their pharmacological contributions are concentration-dependent. Notably, quantitative data on their abundance in G. conopsea remain unreported in the literature.
6.8 Terpenoids and steroids
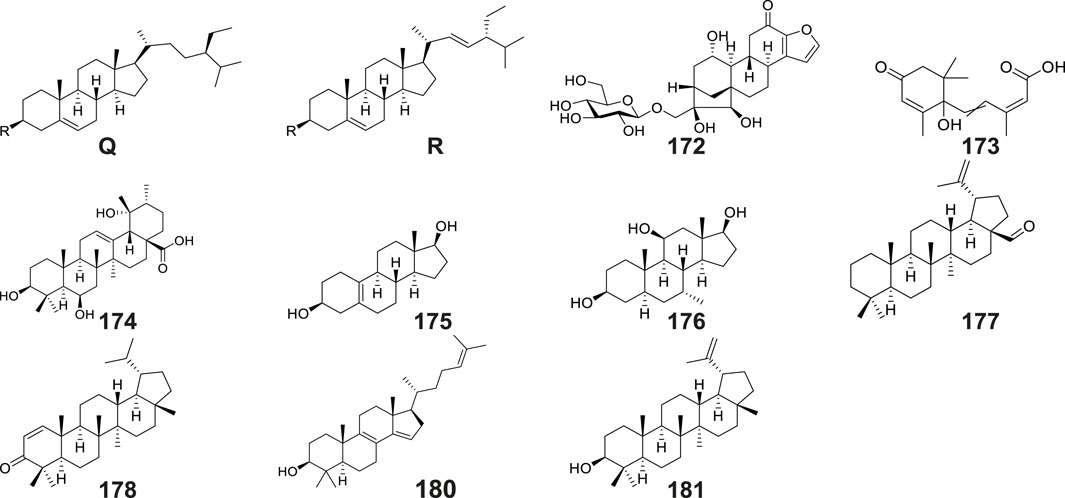
Terpenoids and their derivatives represent a class of secondary metabolites biosynthesized via the mevalonate pathway, featuring isoprene units (C5 units) as their structural backbones. Structurally distinct from terpenoids, steroids constitute a unique family of tetracyclic systems comprising three fused cyclohexane rings and one cyclopentane ring (cyclopentane-perhydrophenanthrene skeleton), which exhibit broad phylogenetic distribution across living organisms. Phytochemical investigations of Gymnadenia species have thus far elucidated 13 terpenoid and steroid derivatives (Table 2; Figure 9), including: β-sitosterol (No. 170) (Li et al., 2001), β-daucosterin (No. 171) (Li et al., 2008), mascaroside (No. 172), (±)-abscisic acid (No. 173), 3β,6β,19α-trihydroxy- urs-12-en- 28-oic acid (No. 174), 5(10)-estrene- 3β,17β-diol (No.175), 7α-methyl-5α- androstane- 3β,11β,17β-triol (176 No.), lup-20(29)-en-28-al (No.177), lupenone (No. 178), poriferasterol (No. 179), 4,4-dimethyl-5α-cholesta-8, 14,24-trien-3β-ol (No. 180), lupeol (No. 181), and (22E)-stigmasta-3,5,22-triene (No. 182) (Wang et al., 2020).
6.9 Other compounds
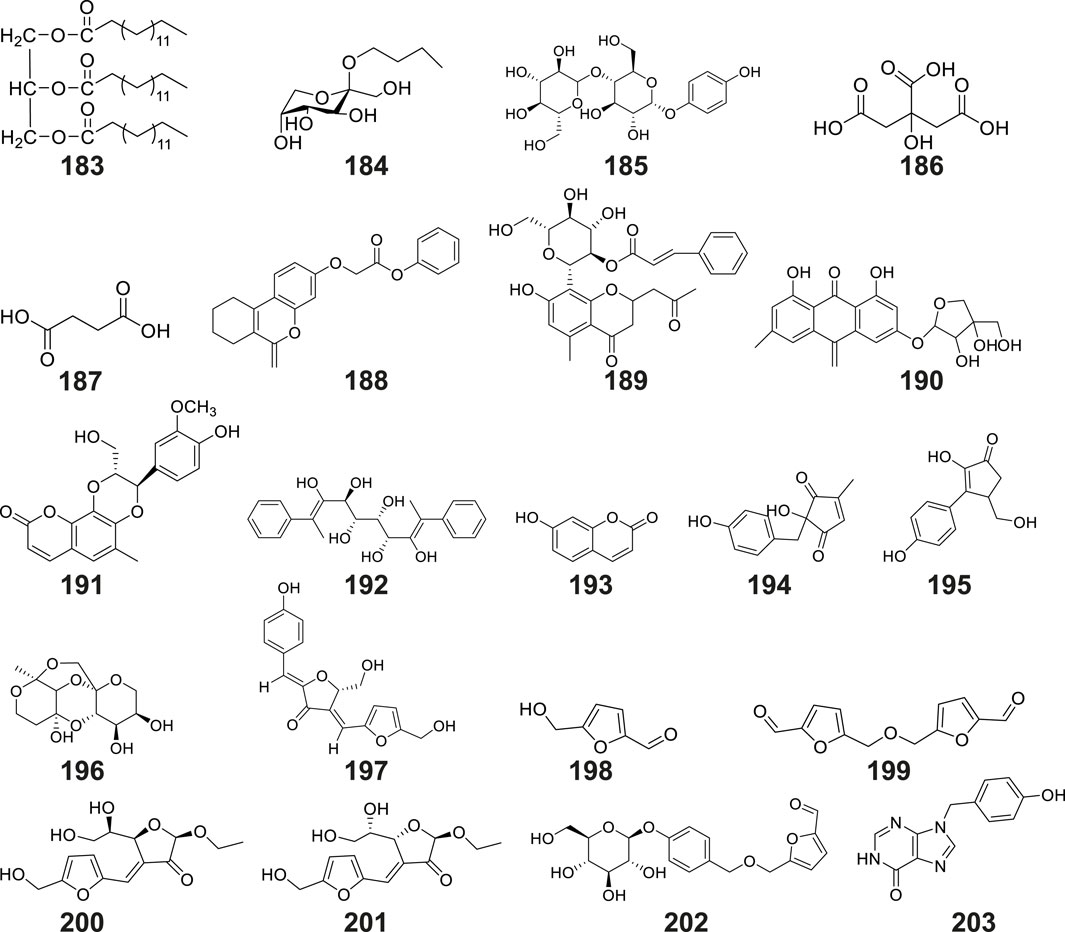
The tubers of G. conopsea were also found to yield a diverse array of other secondary metabolites (Table 2; Figure 10), including: Lipid derivatives: tripalmitin (No. 183) (Li et al., 2008); Saccharides: N-butyl-β-D-fructopyranoside (No. 184) (Li et al., 2001) and 4-Hydroxyphenyl-4-O-glucopyranosyl-glucopyranoside (No. 185) (Lin et al., 2021); Organic acids: citric acid (No. 186) and succinic acid (No. 187); Chromene derivatives: benzyl-[(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3yl) oxy]-acetate (No. 188); Anthraquinones: aloeresin A (No. 189) and frangulin B (No. 190); Coumarin-lignan hybrids: cleomiscosin A (No. 191); Cyclic polyols: bis-(methylbenzylidene)-sorbitol (No. 192) (Wang et al., 2020); Phenolic compounds: umbelliferone (No. 193) (Wang et al., 2020), along with two cyclopentenone derivatives: 2-hydroxy-2-(4′-hydroxybenzyl)-4-methylcyclopent-4- ene-1,3-dione (No. 194), and 2-hydroxy-4-hydroxymethyl-3-(4′-hydroxyphenyl) cyclopent-2-enone (No. 195) (Zi et al., 2010); Complex ethers: (2R,3R,4S,5S,7S,8S,9S)-2,3,8,9- tetrahydroxy-7-methyl-pentaoxatetracyclo[6.6.2.04,5.07,8]hexadecane (No. 196); Furan derivatives: (5S)-5-(hydroxymethyl)-4-[(E)-[5′-(hydroxymethyl)furan-2′-yl]methyl ene]-2-[(Z)-(4″-hydroxyphenyl)methylene] tetrahydrofuran-3-one (No. 197), 5-hydroxymethyl-2-furaldehyde (No. 198), Bis - (5-formylfurfuryl) ether (No. 199), pollenfuran A (No. 200), pollenfuran B (No. 201) and 5- ((4-O - β - D-glucopyranosylbenzyloxy)methyl)-furan-2-carboxaldehyde (No. 202) (Qin et al., 2024b); And finally purine analogs: 9-p-hydroxybenzylhypoxanthine (No. 203) (Qin et al., 2024a).
6.10 Polysaccharides
Beyond its documented small-molecule constituents, G. conopsea has been identified as a significant source of bioactive polysaccharides. A comparative study evaluating five extraction methodologies (hot water, enzyme-assisted, ultrasound- assisted, ultrasound-enzyme hybrid, and microwave-assisted) demonstrated that enzyme-assisted and ultrasound-assisted protocols produced polysaccharides with superior anti-oxidant capacity, establishing these as optimal methods for isolating functional polysaccharides from this species (Li F. W. et al., 2021). Structural characterization by Zhang and Borjihan (2005) revealed that the purified polysaccharide from G. conopsea tubers was predominantly composed of glucose and mannose in a molar ratio of 1:1.5, with a number average molecular weight Mn = 3.21 × 104, a weight average molecular weight Mw = 8.03 × 104, and a polydispersity of 2.5021. Advanced structural analysis employing 13C-NMR and Smith degradation techniques identified (1→3)-linked glycosyl residues as the predominant structural motif, complemented by minor (1→4) linkages. FT-IR and 1H-NMR spectral data further confirmed the β-configuration of glycosidic bonds. Recent multi-analytical investigations utilizing HPSEC-MALLS/RID and PACE- based carbohydrate mapping have characterized water-soluble polysaccharides from G. conopsea tubers across seven Chinese ecoregions. These studies unveiled a complex glycosidic architecture containing α-1,4- and β-1,3(4)-glucosidic bonds, α-1,5-arabinosidic bonds, β-1,4-mannosidic bonds, and α-1,4-D-galactosidic bonds. Notably, bioactivity assessments demonstrated that nitric oxide production in RAW 264.7 macrophages showed significant correlation with specific structural features: α-1,5-arabinosidic and β-1,3(4)-glucosidic bonds exhibited moderate immunomodulatory effects, while α-1,4-D-galactosiduronic and β-1,4-mannosidic bonds displayed particularly pronounced bioactivity (Lin et al., 2015).
Research indicates that polysaccharides from G. conopsea tubers exhibit significant bioactivities, including anti-inflammatory, immunomodulatory, anti-aging, fatigue-alleviating, hypoxia tolerance-enhancing, and anti-radiation effects (Yu, 2017). Their content ranges from 11.07% to 25.05% in crude extracts (Yang, 2010; Yu, 2017; Kong, 2024), establishing polysaccharides as critical functional components of this medicinal plant. However, key structural and mechanistic aspects remain uncharacterized: precise structural features, primary target tissues, and molecular targets of interaction are yet to be elucidated. Intensified research efforts are warranted to advance their development and utilization.
7 Pharmacological activities
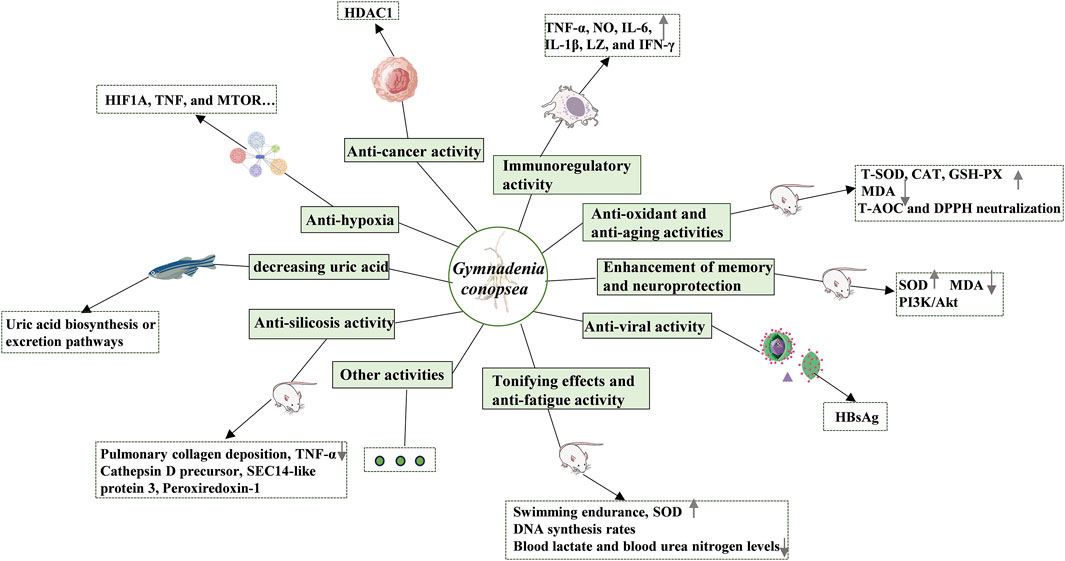
Modern pharmacological studies have established that G. conopsea exhibits a diverse pharmacological profile, encompassing immunomodulation, anti-aging, anti-oxidant activity, neuroprotection, memory enhancement, fatigue resistance, antiviral effects, gastric ulcer mitigation, anti-allergy properties, silicosis inhibition, and sedative-hypnotic functions (Table 3; Figure 11).
7.1 Immunoregulatory activity
As prominent immunomodulators, plant polysaccharides play a critical role in immune system regulation. G. conopsea, containing up to 25.05% polysaccharides (Kong, 2024), serves as a key reservoir of bioactive metabolites. Accumulating evidence indicates that its polysaccharides significantly enhance immune cell functionality—including macrophages and lymphocytes—thereby amplifying host immunity (Yang et al., 2021). Kong (2022) employed an optimized water extraction-alcohol precipitation protocol to isolate G. conopsea polysaccharides. Using RAW264.7 macrophages as an in vitro model, the study demonstrated that these polysaccharides not only upregulated macrophage proliferation and phagocytosis but also activated Fc/C3b receptors and stimulated the secretion of TNF-α, NO, IL-6, IL-1β, LZ, and IFN-γ. Notably, under LPS stimulation, the polysaccharides paradoxically suppressed macrophage phagocytic activity and cytokine release, revealing a bidirectional immunoregulatory mechanism. Complementing these findings, Shang et al. (2015) prepared crude G. conopsea polysaccharides through methanol degreasing, water extraction, and 95% alcohol precipitation. In dexamethasone-induced immunosuppressed mice, medium (100 mg/kg) and high (200 mg/kg) doses of G. conopsea polysaccharides markedly elevated thymic/splenic indices and macrophage phagocytic capacity, confirming dose-dependent immunomodulatory effects. Shang et al. (2014) further reported that G. conopsea polysaccharides augmented peritoneal macrophage activity, increased serum lysozyme levels, promoted delayed-type hypersensitivity, and normalized immune organ weights, suggesting broad-spectrum immunoregulation.
Compared to the extensive research on the in vivo immunomodulatory effects of polysaccharides, studies on their pharmacokinetic profiles, primary target organs, membrane-bound receptors, and downstream signaling pathways remain limited. Additionally, the modulation of gut microbiota—a research hotspot in recent years—is closely linked to systemic immunity (Wang et al., 2023). It is thus imperative to systematically investigate whether G. conopsea polysaccharides exert immunomodulatory effects via gut microbiota regulation in whole-animal models.
7.2 Anti-oxidant and anti-aging activities
Beyond immunomodulation, G. conopsea polysaccharides exhibit potent anti-oxidant and anti-aging properties. A polysaccharide fraction (89.80% purity) obtained through petroleum ether degreasing, ethanol impurity removal, water extraction, and alcohol precipitation demonstrated dose-responsive protective effects in D-galactose-induced aging mice. High-dose administration (0.20 g/kg) over 60 days significantly upregulated T-SOD, CAT, and GSH-PX activities in serum, brain, and liver tissues while suppressing MDA levels—effects attributed to enhanced anti-oxidant enzyme activity and reduced lipid peroxidation (Yu et al., 2018). Sa et al. (2020) isolated a 12.16% pure polysaccharide fraction utilizing an optimized aqueous extraction protocol followed by alcohol precipitation (Yu, 2017) and evaluated its efficacy in Diquat-challenged Small-tailed Han sheep. Dietary supplementation for 15 days elevated SOD/GSH-Px levels and reduced MDA concentrations, effectively counteracting oxidative stress-induced metabolic impairments. Radiation protection studies revealed that polysaccharide administration (150–600 mg/kg) post 60Co-γ irradiation dose-dependently restored hematopoietic function, amplified anti-oxidant defenses, and accelerated repair of radiation-induced damage (Feng et al., 2022).
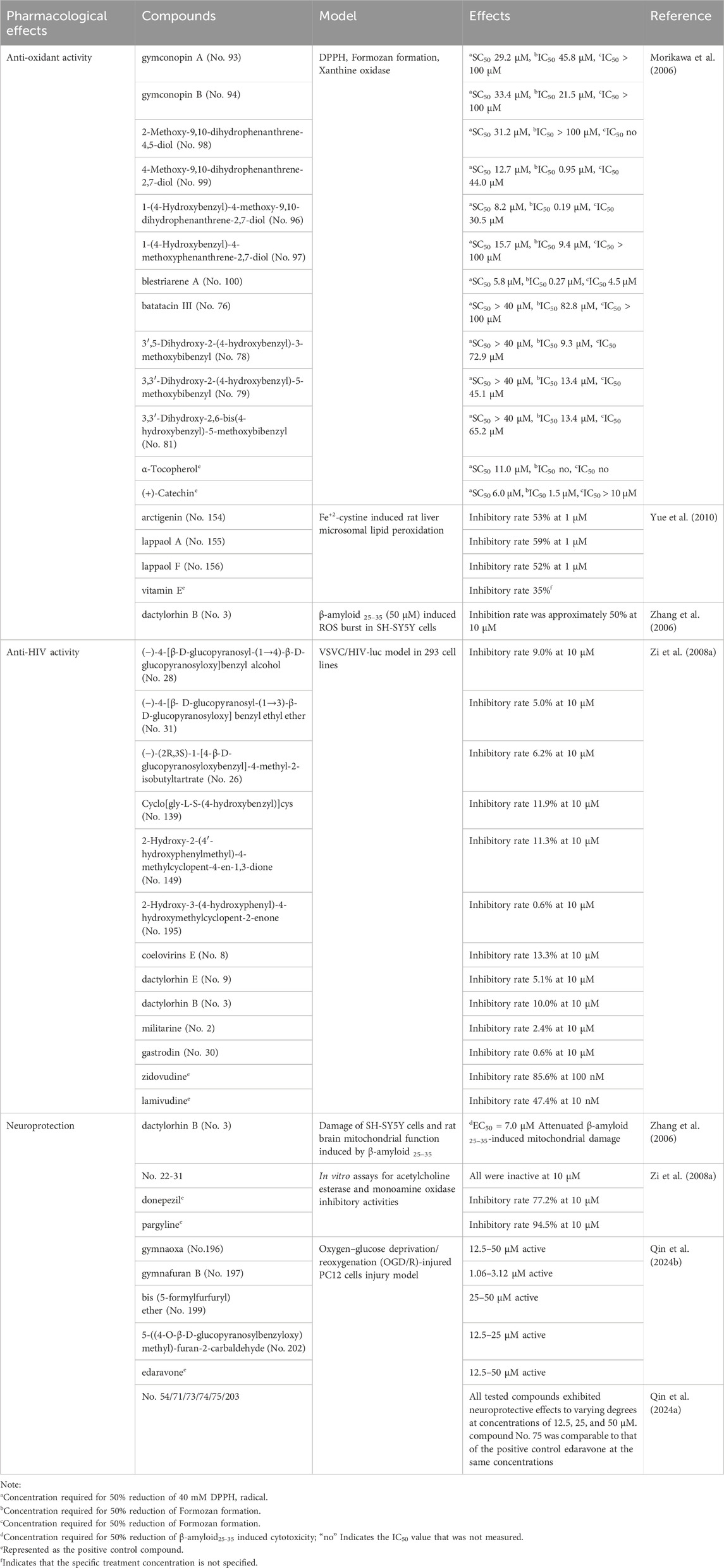
Small-molecule extracts also contribute to anti-oxidant activity. Dose-dependent scavenging of DPPH, ABTS+, and hydroxyl radicals was observed in 69% methanol extracts from 14 plant batches (Kong, 2024). Comparative analysis by Chen and Liu (2024) showed that 95% ethanol extracts possessed superior total anti-oxidant capacity (T-AOC) and DPPH neutralization compared to 75% extracts, though with reduced superoxide anion scavenging efficiency. Similarly, Study by Morikawa et al. (2006) confirmed that the methanol extract of G. conopsea possesses scavenging activity against DPPH and superoxide anion (O2−) radicals. Its major active compounds were enriched in the methanol and acetone eluates from Diaion HP-20 column chromatography, leading to the isolation and identification of 11 compounds with significant activity (see Table 4). Among these, the compound blestriarene A (No. 100) demonstrated potent activity, with SC50 of 5.8 μM for DPPH scavenging, IC50 of 0.27 μM against formazan formation, and 4.5 μM for xanthine oxidase inhibition, outperforming the positive controls α-Tocopherol and (+)-Catechin. Furthermore, at the cellular level, the compound dactylorhin B (No. 3) was reported to alleviate β-amyloid23-35-induced mitochondrial damage and reduce apoptosis in SH-SY5Y cells by inhibiting reactive oxygen species (ROS) (Zhang et al., 2006). Importantly, the principal bioactive component gastrodin (No.30) has been pharmacologically validated as a potent anti-oxidant and anti-aging agent (Shang et al., 2024). Collectively, these findings underscore the robust anti-oxidant and anti-aging activities of G. conopsea.
7.3 Enhancement of memory and neuroprotection
G. conopsea has demonstrated significant therapeutic potential in neurodegenerative disorders, particularly for Alzheimer’s disease (AD) prophylaxis and cognitive enhancement (Luo, 2021; Guo et al., 2022). Mechanistic studies revealed that a 90% ethanol refluxed extract effectively attenuated β-amyloid25-35(Aβ25-35, 20 μM)-induced apoptosis in PC12 cell model (Cheng, 2024b). Similarly, a 95% ethanol extract alleviated aluminum trichloride-induced behavioral abnormalities in zebrafish by inhibiting neuronal apoptosis (Yu, 2024b), providing preliminary evidence for its anti-AD efficacy. Given the critical role of cholinergic neuron degeneration in cognitive decline, Yu et al. (2013) employed an ibotenic acid-induced cholinergic injury model. They demonstrated that 28-day administration of a 95% ethanol extract (5 mg/kg) significantly ameliorated neuropathological changes and upregulated acetylcholinesterase expression. Ina metabolic-related neural damage model, Shi et al. (2023b) observed that an aqueous extract (0.6–2.4 g/kg) reversed high-fat- diet-induced cognitive deficits in diabetic rats. This effect was mediated by reducing fasting blood glucose, increasing superoxide dismutase (SOD) activity, decreasing malondialdehyde (MDA) levels, and elevating PI3K/Akt protein expression levels. Notably,an ethanol extract (750 mg/kg) exhibited neuroprotective effects against hypoxia by downregulating lipocalin-2 (LCN2) protein expression (Zhang et al., 2020; Afridi et al., 2024). However, most of these studies did not characterize the extracts (e.g., via HPLC) or discuss potential active constituents. Nevertheless, they provide a pharmacological foundation for investigating the neuroprotective components within G. conopsea.
Focusing on specific neuroprotective compounds, Zhang et al. (2006) found that dactylorhin B (No. 3) effectively mitigated SH-SY5Y cell damage induced by Aβ25-35. This protection was achieved by inhibiting reactive oxygen species (ROS) burst and reducing mitochondrial damage. Furthermore, Qin et al. (2024b) reported that compounds NO. 54, 71, 73, 74, 75, 196, 197, 199, 202, and 203 exhibited varying degrees of protective activity in PC12 cells subjected to oxygen-glucose deprivation/reperfusion (OGD/R) injury. Especially, compound (2S)-2-(β-D-pyran-glucosyloxy)-2-(2-methylpropyl)butanedioic acid 4-methyl ester (No. 75) has a significant neuroprotective effect, and its activity is comparable to that of the positive drug edaravone. Further verification through transcriptome, bioinformatics and qPCR suggests that compound No. 75 may exert its protective effect by regulating COL27A1 (Qin et al., 2024a). These findings highlight the potential of these compounds for drug development and establish a solid foundation for elucidating the material basis and underlying molecular mechanisms of G. conopsea’s neuroprotective effects. Notwithstanding, the in vivo neuroprotective effects of these monomeric compounds, along with their in vivo absorption profiles and tissue distribution patterns, particularly the distribution in brain tissue, necessitate further systematic investigations.
7.4 Sedative and hypnotic activities
In the pharmacological compendia of Tibetan and Mongolian medicinal traditions, G. conopsea is documented for its sedative and hypnotic properties. Research indicates that a solution of G. conopsea, prepared with distilled water, demonstrates efficacy in both high-dose (1 mL of solution containing 0.2 g of crude drug, equivalent to a concentration of 0.2 g/mL) and low-dose (1 mL of solution containing 0.1 g of crude drug, equivalent to a concentration of 0.1 g/mL) formulations, in suppressing the spontaneous activity of mice, diminishing the frequency of vertical forelimb lifts, and significantly extending the duration of sleep induced by suprathreshold doses of pentobarbital sodium. Concurrently, it enhances the incidence of mice succumbing to sleep induced by subthreshold doses of pentobarbital sodium, thereby affirming the sedative and hypnotic effects of G. conopsea (Zhou et al., 2009). Moreover, a study conducted by He et al. (2017) assessed the sedative efficacy of three doses (1, 2, and 4 g/kg) of a 75% ethanol extract of G. conopsea using mouse sedation trials. The findings revealed that the suppression rates of spontaneous mouse activities were 22.5%, 44.9%, and 51.2% respectively, signifying that the 75% ethanol extract of G. conopsea exhibits a dose-dependent inhibition of spontaneous mouse activities and possesses notable sedative effects. Although in vivo animal models are effective for evaluating and replicating the clinical efficacy of drugs, they are not the optimal choice for identifying bioactive components and elucidating underlying mechanisms. Existing studies have confirmed the presence of sedative compounds in the tubers of G. conopsea. Consequently, it is necessary to employ well-established in vitro cellular models and molecular docking approaches to further isolate and validate specific bioactive constituents, as well as to clarify their potential molecular mechanisms.
7.5 Tonifying effects and anti-fatigue activity
G. conopsea is widely recognized in traditional Chinese medicine for its dual tonifying and anti-fatigue properties, targeting conditions such as physical debility, pulmonary and renal insufficiency, emaciation, and fatigue-associated syndromes. As a restorative tonic, it demonstrates efficacy in alleviating cough, asthma, and wasting disorders while enhancing Yang energy consolidation. Modern pharmacological studies validate its role in improving energy metabolism and mitigating fatigue through structured experimental models.
The tubers of G. conopsea were powdered and dissolved directly in distilled water to prepare the “G. conopsea solution”. Compared to the control group, administration of this solution at high (40 g/kg), medium (20 g/kg), and low (10 g/kg) doses significantly prolonged weight-loaded swimming time in mice, accompanied by increased activity levels, food intake, and mental alertness (Zhao and Liu, 2011). In a hydrocortisone-induced mouse model of kidney yang deficiency, administration of G. conopsea solutions (0.2 g/mL and 0.1 g/mL concentration, dosed at 10 mL/kg) significantly improved body weight, kidney coefficient, and DNA synthesis rates in renal and splenic tissues, highlighting its kidney-invigorating and body-strengthening effects (Lin, 2009). Medicinal processing (Paozhi) is a distinctive feature of traditional Chinese medicine preparation, serving purposes such as toxicity reduction and efficacy enhancement. Studies have found that G. conopsea processed with goat or cow milk significantly prolongs swimming endurance in mice and elevates serum superoxide dismutase (SOD) activity in rats, with superior effects compared to the crude drug group (Jin and Wang, 2009). Anti-fatigue and hypoxia tolerance tests in mice further confirmed its tonic and invigorating properties (He, 2016). Administration of differently processed G. conopsea preparations (goat milk processing, water processing, and processing with 5% Gardenia jasminoides solution) at various doses (1, 2, and 4 g/kg) all induced dose-dependent increases in weight-loaded swimming time and grid-hanging duration; the goat milk-processed preparation demonstrated the most potent effects.
Studies by Zhao and Liu (2011) and Lin (2009) provided preliminary evidence for the tonic and invigorating effects of the crude G. conopsea drug. Research by Jin and Wang (2009) and He (2016) suggested that milk processing enhances its restorative efficacy. However, some methodological descriptions lack detail: for instance, Jin and Wang (2009). did not specify whether the material was powdered/sieved or the solvent used for suspension; He (2016) did not include a crude drug control group. Furthermore, none of these studies characterized potential bioactive content, HPLC compound profiles, or chemical markers, hindering comparative analysis in subsequent research.
Precise characterization of bioactive metabolites is crucial for promoting the standardization and quality improvement of medicinal plant materials. Research by Yu (2017) identified polysaccharides as the primary bioactive components. Prolonged administration (30 days) of optimally prepared crude G. conopsea polysaccharides (0.05, 0.1, and 0.2 g/kg) not only extended weight-loaded swimming time in mice but also reduced blood lactate and blood urea nitrogen levels while increasing hepatic glycogen reserves, indicating dual mechanisms of energy conservation and metabolic waste clearance. This study confirms polysaccharides as key constituents responsible for the tonic and invigorating effects of G. conopsea. Beyond polysaccharides, further systematic investigation is warranted to determine whether other small-molecule metabolites in G. conopsea—such as benzylester glucosides, stilbenes, and phenanthrenes—contribute to the aforementioned efficacy, and whether synergistic interactions exist between polysaccharides and small molecules.
7.6 Anti-viral activity
In traditional medicine, G. conopsea has been historically employed in managing chronic hepatitis B (Shang et al., 2017). Lu et al. (2002) evaluated the anti-HBV activity of the aqueous extract of G. conopsea. The results showed that the aqueous extract of G. conopsea exhibited moderate inhibition of HBsAg, and higher doses led to more significant inhibition. Moreover, this inhibitory effect was rapid and remained stable over time. Complementary studies further identified antiviral properties in the plant’s methanol extract, which suppressed viral polymerase activity (Kimura, 2003). Moreover, Zi et al. (2008a) evaluated the anti-HIV activity of 11 compounds isolated from G. conopsea tubers using a VSV-G pseudotyped HIV-luc reporter assay in 293 cells. However, these compounds showed only weak activity (0.6%–13.3% inhibition at 10 μM, see Table 4), significantly lower than the positive control drug. Clearly, research on the antiviral properties of G. conopsea remains insufficiently systematic and in-depth. Experience from COVID-19 treatment indicates that while botanical metabolites often exhibit limited direct antiviral potency, they can effectively alleviate symptoms through synergistic mechanisms. These include immune modulation, prevention of cytokine storms, and mitigation of tissue damage (Huang et al., 2021). Therefore, future research should prioritize comprehensive evaluation of G. conopsea’s antiviral activity in whole animal models, coupled with in vitro models targeting specific mechanisms, to enable precise identification of the active metabolites.
7.7 Preventing and treating gastric ulcers
Jiang et al. (2009) established a standardized gastric ulcer model in Sprague-Dawley rats via intragastric administration of hydrochloric acid-ethanol solution (7.5 mL/kg). Their findings revealed that G. conopsea significantly attenuated inflammatory responses in ulcerative lesions by modulating capillary permeability and promoting granulation tissue proliferation. These combined effects enhanced gastric mucosal repair capacity and accelerated ulcer healing, with particularly notable efficacy in acute gastric ulcer management. In another parallel study utilizing the same ulcerogenic protocol (7.5 mL/kg HCl-ethanol), researchers further demonstrated that G. conopsea exerted dual protective effects: it markedly reduced gastric ulcer index scores and suppressed MDA levels, a key biomarker of oxidative stress. These results corroborate the botanical drug’s robust gastroprotective properties, highlighting its potential in both preventing ulcerogenesis and facilitating mucosal recovery (Lin, 2009). G. conopsea whole-component extract demonstrates significant preventive, protective, and reparative effects against gastric ulcers, exhibiting efficacy comparable even to the positive control drug ranitidine (Jiang et al., 2009). This highlights its considerable development potential. However, it is evident that related research reports are limited, and the studies lack depth and systematic rigor. Crucially, the plant material used was not characterized, and no investigation into potential active metabolites was conducted.
7.8 Anti-silicosis activity
Silicosis, a progressive pneumoconiosis characterized by bilateral nodular pulmonary fibrosis, arises from chronic inhalation of crystalline silica (SiO2) particles. Pharmacological studies in silica-exposed rat models demonstrate that the 60% ethanolic extract of G. conopsea (GcAE) effectively mitigates pulmonary fibrosis by upregulating antioxidant enzyme (SOD, GPx) activity, reducing lipid peroxidation product (MDA) levels and lung index, and decreasing Type I/III collagen deposition in lung tissue (Wang et al., 2007; Wang et al., 2008a). GcAE also significantly downregulates TNF-α expression in lung tissue, thereby inhibiting TNF-α-mediated inflammatory cascades and reducing fibrosis (Zeng et al., 2007; Wang et al., 2008b). To further investigate GcAE’s anti-silicosis mechanisms, Chen (2008) employed gene microarray technology to analyze differentially expressed genes in lung tissue. Their data indicate that GcAE counteracts pulmonary fibrosis via multi-target mechanisms, including alleviating oxidative stress, protecting vascular endothelium, and inhibiting lymphocyte-endothelial cell adhesion. Subsequently, Chen (2009) conducted proteomic analysis of differentially expressed proteins in silicotic rat lungs. Results revealed that GcAE intervention significantly upregulated SEC14-like protein 3 (involved in lipid signaling) while downregulating procathepsin D (lysosomal protease regulation) and peroxiredoxin 1 (redox homeostasis), potentially alleviating silica-induced fibrosis by enhancing pulmonary antioxidant defenses and anti-apoptotic capacity. Notably, a comparative pharmacodynamics study by Wu et al. (2010) showed that the 70% ethanolic extract of G. conopsea exhibits anti-fibrotic efficacy comparable to the clinical reference drug tetrandrine in silica-challenged models, highlighting its potential as a phytotherapeutic alternative for silicosis treatment.
However, the aforementioned studies exhibit notable limitations requiring future refinement: (1) Limited dose-response assessment: Most utilized only a single dose, leaving dose-dependent effects unconfirmed; (2) Insufficient extract characterization: Lack of qualitative/quantitative profiling of bioactive constituents; (3) Inconsistent positive controls: Absence of reference drug groups in some studies (see Table 4); (4) Inadequate mechanistic validation: Superficial pathway analysis without functional validation of targets.
7.9 Other activities
Beyond its primary applications, G. conopsea exhibits multifaceted pharmacological activities, including lipid-lowering and hepatoprotective effects, as evidenced by studies demonstrating that 70% ethanolic extracts significantly alleviate hyperlipidemia-induced hepatic damage in rat models through the regulation of lipid metabolism (Zhang et al., 2013). The plant also demonstrates notable hypouricemic properties, likely mediated by its diverse phytochemical metabolites such as flavonoids, polyphenols, alkaloids, terpenes, and phenylpropanoids, which may interfere with uric acid biosynthesis or excretion pathways (Chen T. et al., 2022). Concurrently, network pharmacology analyses by Liang et al. (2021) propose that its bioactive components combat hypoxia via multi-target modulation of hypoxia-inducible factor 1α, TNF, and mTOR (mechanistic target of rapamycin) signaling axes. In anticancer research, conopsamide A (No. 141), a unique ureido-substituted amino acid isolated from tuber ethanol extracts, has emerged as a potential HDAC1 (histone deacetylase 1) inhibitor with epigenetic regulatory capabilities (Lin et al., 2017). Additionally, methanol extracts of the tubers exhibit tranilast-comparable anti-allergic activity, particularly in fractions purified by reverse-phase silica gel chromatography, though the precise bioactive molecules remain to be fully characterized (Matsuda et al., 2004).
8 Toxicity
Toxicity is closely associated with the safety of drug administration, representing a pivotal element in pharmaceutical research. In acute oral toxicity tests, BALB/C mice (17–20 g) and SD rats (180–220 g) were administered Wangla (prepared from G. conopsea tuber) at doses of 1.00, 2.15, 4.64, and 10.00 g/kg body weight. No mortality or abnormal symptoms occurred, with LD50 values exceeding 10.00 g/kg for all groups, classifying the substance as practically non-toxic. Genotoxicity assessments—including mouse bone marrow micronucleus assays (BALB/C mice, 25–30 g body weight, dosage at 1.25, 2.50, 5.00 g/kg) and sperm abnormality tests (BALB/C mice, 25–35 g body weight, dosage at 1.25, 2.50, 5.00 g/kg)—showed no significant increases in micronucleated polychromatic erythrocytes or sperm abnormalities (P > 0.05), contrasting sharply with positive controls (cyclophosphamide at 40 mg/kg and 30 mg/kg, respectively; P < 0.01). In a 30-day subchronic study, SD rats (95.3 ± 9.4 g) received dietary doses of 1.67, 3.33, and 6.67 g/kg (50–200× human intake). No adverse effects were observed on body weight, food utilization, hematology (hemoglobin, RBC/WBC counts), blood biochemistry (ALT, AST, BUN, creatinine, lipids, glucose, proteins), or organ coefficients (liver, kidney, spleen, gonads), except for isolated focal hepatic necrosis in 5/10 male rats at the highest dose (6.67 g/kg). This finding was deemed spontaneous due to the absence of dose dependency, corroborated histopathologically by normal kidney, stomach, and duodenal tissues. Collectively, the data support the safety of Wangla for oral use as both a medicinal agent and food supplement (Bai and Zheng, 2007).
In another study, He et al. (2023b) conducted a 90-day long-term toxicity study, SPF SD rats (n = 120, 180–200 g, equal sex distribution) were administered G. conopsea via daily gavage and medicated feed at three doses: 5.1 g/kg (10×), 10.2 g/kg (20×), and 15.4 g/kg (30× clinical human equivalent dose), followed by a 15-day recovery period. Throughout the experiment, no mortality or behavioral abnormalities (e.g., secretions, altered feces/urine) occurred, and body weight/food intake remained unchanged versus controls. Hematologically, transient elevation of MPV (mean platelet volume) was observed in the 20× and 30× groups at day 45 (P < 0.01), but all values normalized by day 90 and recovery. Biochemical analysis revealed significantly decreased creatinine (Crea) and total protein (TP) in the 20× and 30× groups at day 90 (P < 0.01), which reversed post-recovery; however, cholesterol (CHO) reduction persisted in the 20× group during recovery (P < 0.05). No alterations occurred in ALT, AST, glucose, triglycerides, or other metabolic markers. Critically, organ coefficients (liver, heart, kidneys, spleen, gonads, etc.) showed no differences from controls (P ≥ 0.05), and histopathology of 12 organs (including liver, kidneys, heart, lungs, and reproductive tissues) confirmed absence of lesions at all timepoints (45 days, 90 days, 105 days). The study employed no positive toxin controls, as its design focused exclusively on dose-dependent toxicity assessment. These results indicate no evidence of cumulative organ damage or irreversible toxicity, supporting the safety of long-term clinical use; the isolated CHO decrease warrants further investigation but lacks pathological correlation.
The findings of acute and long - term toxicity experiments indicate that G. conopsea exhibits no toxicity, which is in line with the outcomes of long - term clinical applications. Its functions, including tonifying effects and neuroprotective properties, endow it with broad prospects for application in the development of future pharmaceuticals and health products.
9 Breeding research
Current research on G. conopsea cultivation technology remains in its nascent stage, with limited progress in scaling artificial propagation systems. Existing efforts primarily focus on foundational techniques including symbiotic fungal isolation, seed germination optimization, and callus induction protocols. Systematic evaluation of these methodologies is critical for establishing scalable cultivation frameworks to support industrial applications.
9.1 Introduction and domestication of wild resources
Wild resource domestication has emerged as a strategic approach to address medicinal resource scarcity and enable artificial cultivation transitions. However, few studies specifically address G. conopsea domestication dynamics. Pioneering work by Song et al. (2011) demonstrated that successful transplantation requires microenvironmental fidelity to native habitats, recommending wild plants transfer with intact soil into shaded shelters, coupled with stringent hydration, nutrient, and pest management. Their parallel attempts at seed propagation via direct sowing or sand-burial methods proved unsuccessful due to orchidaceous seed underdevelopment, highlighting reproductive challenges.
Vegetative propagation techniques show partial success: autumn-harvested tubers with multiple buds can undergo ramet division when each segment retains nascent buds, achieving medicinal harvest readiness after 3–5 years (Song et al., 2011). Spring equinox division protocols involve sectioning rhizomes into 1–2 bud-eye segments with retained fibrous roots, followed by plant ash treatment and furrow planting (30 cm row spacing, 8–10 cm plant spacing, 5–6 cm depth), achieving full emergence within 15 days under moisture-controlled conditions (Bao et al., 2024). The research conducted by Bao (2023) revealed that transplanted G. conopsea tends to enter a state of dormancy at an earlier stage. The researchers posited that this phenomenon might be attributable to the diminished soil nutrient quality in the transplantation site compared to the native habitat soil of G. conopsea. Consequently, they emphasize the necessity for an intensified investigation into the microenvironmental characteristics of the soil habitat for this species. Field studies have demonstrated that plants thriving in organically rich soil exhibit enhanced vigor and higher population densities (Yang et al., 2017). These findings underscore the imperative for soil microenvironment optimization—particularly organic matter enrichment—combined with scientific irrigation, fertilization, and biotic stress management to reduce cultivation costs and enhance breeding efficiency.
In October 2023, we conducted an introduction trial, transplanting wild G. conopsea plants from 3,600 m altitude to cultivated fields at 3,200 m. Seedlings emerged in mid-May 2024 but displayed generalized leaf yellowing, potentially linked to transplant injury (Figure 12A). Most plants flowered and fruited normally (Figure 12B). However, by May 2025, many exhibited leaves wilting and mortality (Figure 12C). Excavation revealed root rot and heavy nematode infestation in affected individuals (Figures 12D,E). Field surveys corroborated grower reports of progressive size reduction and population decline in transplanted G. conopsea, suggesting nematode-related pathology. Furthermore, most transplants were placed on bare ground with thorough weed removal in soils deficient in organic matter. This likely diminished weed-derived nematode-suppressive exudates and created a suboptimal microenvironment for growth-promoting mycorrhizal fungi (Gai, 2015), leading to malnutrition, weakened resistance, and severe nematode-induced rot. Thus, pre-transplant preparations for G. conopsea must include: (1) Soil amendment with organic matter fermented with beneficial mycorrhizal fungi specific to this species. (2) Pre-emptive soil sterilization against pests. (3) Intercropping with nematode-suppressive plants. (4) Installation of shading and humidification systems. These measures will establish a suitable microenvironment for growth. Given current limitations in tissue-culture rapid propagation and seed-based reproduction, strategically integrating vegetative propagation with habitat-mimicking cultivation systems may accelerate breakthroughs in artificial cultivation and ensure sustainable medicinal material supply.

Figure 12. Artificial introduction and cultivation of G. conopsea. (A) Growth status of plants transplanted in October 2023, documented on 10 May 2024; (B) Flowering and fruiting observed on 15 July 2024; (C) Plants exhibiting leaf yellowing and desiccation observed on 8 June 2025; (D) Decayed tubers from yellowed and desiccated plants; (E) Dense colonization of white nematodes in partially decayed tuber tissues (indicated by red arrows). Data sourced from the authors’ original research (unpublished).
9.2 Tissue culture-based rapid propagation
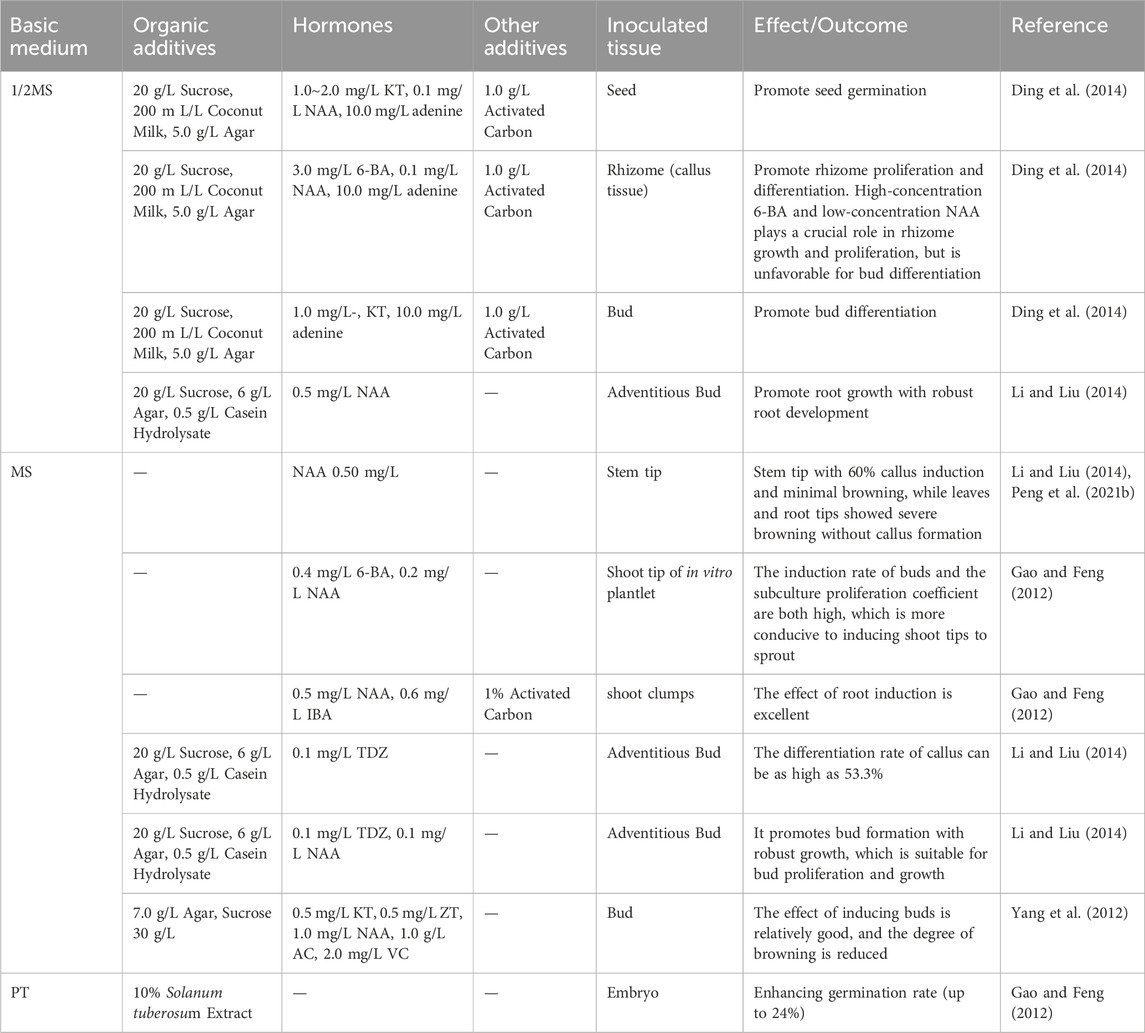
Plant tissue culture technology offers distinct advantages over conventional propagation methods for G. conopsea, including accelerated breeding cycles and enhanced multiplication efficiency of elite genotypes (Li et al., 2024). The seeds serve as the quintessential explant materials for tissue culture. While mature seeds from Chinese (Xinglong Mountain, Gansu) and Russian (Novosibirsk) populations exhibit poor germination (Ding et al., 2014; Nabieva et al., 2020), immature seeds demonstrate improved viability (up to 20% germination) (Ding et al., 2014), with embryos at 1–4 months post-anthesis showing optimal germination-seedling transition in PT medium supplemented with 10% potato extract and 1% activated carbon (Gao and Feng, 2012). Notably, Western European ecotypes achieve 40% germination in mature seeds, suggesting geographic genetic divergence in germination physiology (Waes and Debergh, 2010). Seed-based protocols have been refined through optimized media formulations (Table 5): 1/3 MS medium with 0.3 mg/L NAA + 1.0 mg/L 2-iP + 10% coconut water enhances protocorm formation (Nabieva et al., 2020), while 1/2MS medium containing 1.0–2.0 mg/L KT + 0.1 mg/L NAA + 10 mg/L adenine + 20 g/L sucrose + 200 mL/L coconut milk promotes rhizome differentiation via synergistic regulation of cell division (adenine), carbon metabolism (sucrose), and nutrient supply (coconut milk) (Ding et al., 2014). Seed preservation strategies leverage orchidaceous desiccation tolerance, with 4°C storage of dried seeds proving effective for medium-term germplasm conservation, though systematic studies on moisture content and thermal drying impacts remain lacking (Magrini et al., 2019; Jiang, 2022a; Shi, 2023a).
Beyond seed explants, Li and Liu (2014) evaluated multiple tissues (young leaves, root meristems, axillary buds, shoot tips, and floral pedicels) for G. conopsea micropropagation, identifying shoot tips as optimal with 60% callus induction and minimal browning on MS medium containing 0.5 mg/L NAA (Table 5), while leaves and root tips showed severe browning without callus formation. Yang et al. (2012) developed an integrated anti-browning protocol: sequential sterilization (75% ethanol for 20 s → 0.1% HgCl2 for 10 min), 1 g/L activated carbon (higher concentrations suppressed axillary bud growth), and 5–10 days dark pre-culture under 2000 lx light with 3-day subcultures, achieving 60% browning reduction despite 2 mg/L vitamin C’s growth inhibition. Peng K. et al. (2021) confirmed hormonal specificity, showing 0.5 mg/L NAA in MS medium maximized shoot tip callus induction (59.5%), contrasting sharply with 1.0 mg/L 2,4-D’s inefficacy (11.5%), while 6-BA exhibited concentration-dependent effects—0.4 mg/L enhanced clustered bud proliferation (3.8-fold) but >0.4 mg/L reduced subculture capacity by 40% (Table 5; Gao and Feng, 2012). Root induction reached 80% success in 1/2MS + 0.5 mg/L NAA (Li and Liu, 2014). Current limitations necessitate phase-specific media optimization and advanced anti-browning strategies to enable scalable cultivation of this orchid species.
9.3 Symbiotic seed germination and seedling cultivation
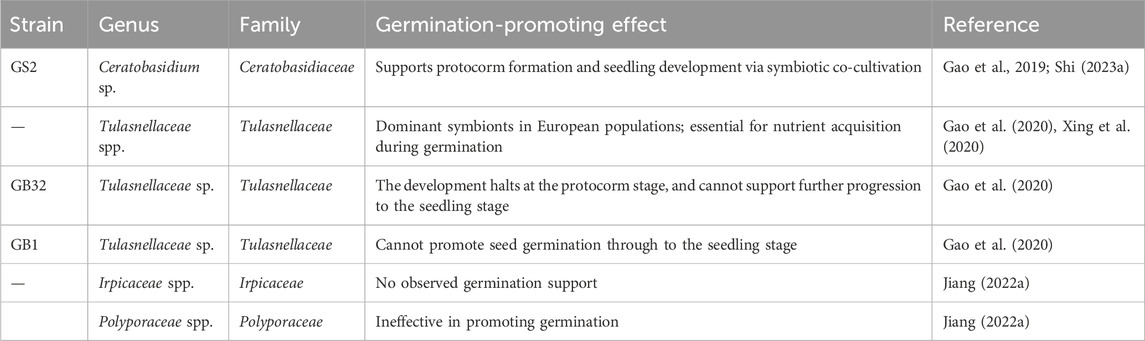
The dust-like, endosperm-deficient seeds of G. conopsea exhibit obligate mycoheterotrophic germination, relying exclusively on symbiotic fungal colonization (typically Ceratobasidiaceae or Tulasnellaceae) for nutrient acquisition in natural ecosystems (Shi L. et al., 2022; Yao et al., 2024). These mycorrhizal partners supply critical resources—water, micronutrients, phytohormones, and antimicrobial compounds—that simultaneously suppress competing microbes and activate embryonic development (Stark et al., 2009; Steinfort et al., 2010). However, the stochastic distribution of compatible fungi in soil matrices, combined with limited seed dispersal efficiency, creates ecological bottlenecks, resulting in <1% natural germination success and severely constraining population recruitment (Mccormick et al., 2018; Li T. et al., 2021). To address this, targeted isolation of germination-promoting fungi has emerged as a key strategy for enhancing propagation efficiency. Xing et al. (Gao et al., 2019) pioneered this approach by identifying Ceratobasidium sp. GS2 from root endophytes, demonstrating its capacity to drive protocorm formation and seedling development via in situ seed-fungus co-cultivation (Table 6; Yue, 2020). Fungal specificity studies reveal narrow symbiotic compatibility: among 102 isolates from protocorms and seedlings, only Ceratobasidiaceae strains supported full germination-to-seedling transitions, while Schizophyllaceae, Irpicaceae, and Polyporaceae showed negligible efficacy, suggesting ecological niche partitioning (Jiang et al., 2022b). Table 6 summarizes the main endomycorrhizal fungal strains implicated in studies on the symbiotic germination of G. conopsea seeds and their effects in promoting seed germination and growth.
Geographic divergence in mycorrhizal partnerships further complicates propagation strategies. European G. conopsea populations predominantly associate with Tulasnellaceae fungi, whereas Asian ecotypes rely on Ceratobasidiaceae symbionts, reflecting potential co-evolutionary adaptations to regional soil microbiomes (Xing et al., 2020). This biogeographic specificity underscores the necessity for location-tailored fungal isolation protocols. For instance, Xing’s strain-mixing technique achieved preliminary success in naturalized germination but requires refinement for cross-regional applicability (Yue, 2020). Current limitations in scalable symbiotic systems highlight unmet needs: 1) systematic screening of germination-active fungi across diverse habitats, 2) optimization of the symbiotic germination system between fungi and seeds, and 3) elucidation of molecular mechanisms governing fungal recognition and nutrient exchange. Addressing these gaps will enable engineered symbiotic germination platforms to bypass natural recruitment bottlenecks, facilitating large-scale conservation and cultivation of this ecologically vulnerable orchid.
10 Conclusion and future prospects
G. conopsea has emerged as a critically important medicinal resource, validated by its ethnopharmacological legacy, phytochemical richness, and diverse pharmacological activities. Phytochemical studies have identified 203 bioactive compounds, including benzyl ester glucosides, stilbenoids, and polysaccharides, which collectively underpin its anti-oxidant, immunomodulatory, neuroprotective, and anti-fatigue properties. Despite these advancements, pharmacological researches remain largely limited to crude aqueous/alcoholic extracts (Table 3). Few isolated compounds (Tables 1, 4) have subjected to systematic in vitro bioactivity validation, and in vivo pharmacological testing is notably scarce. Consequently, the mechanistic links between specific compounds and their therapeutic effects remain inadequately derexplored, impeding the development of standardized preparations and quality control protocols.
Current literature indicates that G. conopsea polysaccharides are abundant and exhibit immunomodulatory, tonic, and anti-fatigue activities. However, critical knowledge gaps persist regarding their branching structures, in vivo absorption/distribution, and molecular targets. Benzylester glucosides—present at high levels in alcoholic extracts—demonstrate neuroprotective effects consistent with the G. conopsea’s documented enhancement of memory and cognitive function. Nevertheless, these compounds lack validation in animal models and identification of specific molecular targets. Despite being the most abundant bioactive constituents, standardized studies investigating potential synergistic interactions between polysaccharides and benzylester glucosides are exceptionally limited. In antioxidant research, in vitro evaluations have primarily focused on free radical scavenging capacity, which shows minimal correlation with in vivo animal studies demonstrating modulation of endogenous antioxidant enzyme systems. Future work should employ validated cellular models to identify active metabolites responsible for upregulating antioxidant enzymes. For antiviral screening, expanded use of viral molecular target models and advanced techniques—such as reporter gene systems, surface plasmon resonance (SPR), protein microarrays, and molecular docking—is essential. Given that viral infections trigger excessive immune responses (e.g., cytokine storms), immune dysfunction, and multi-organ failure, botanical medicines offer advantages through their multi-component/multi-target nature and synergistic interactions. These properties support efficacy in modulating inflammatory responses, mitigating tissue damage, and alleviating symptoms (e.g., cough, fatigue) (Bai et al., 2025), providing strategic directions for evaluating G. conopsea’s antiviral potential and elucidating its active metabolites and mechanisms.
Progress in pharmacological research and clinical applications of G. conopsea is contingent upon sustainable raw material supply. However, wild resources are nearly depleted, and artificial cultivation remains unrealized—a challenge demanding urgent technological innovation. Current cultivation systems are immature, with domestication efforts hampered by unresolved bottlenecks such as progressive dwarfism and yield reduction in successive plantings. Key priorities include addressing nematode infestations and establishing a conducive microenvironment for mutualistic growth between G. conopsea and its symbiotic mycorrhizal fungi. Tissue culture faces challenges including tissue browning, low multiplication rates, protracted culture cycles, and high costs. Optimization requires refined culture media formulations to improve proliferation efficiency and reduce browning incidence, alongside automation to lower production costs. Symbiotic germination represents a promising solution for resource scarcity but necessitates further screening for high-efficiency endophytic fungal strains and optimization of symbiotic seedling systems (e.g., substrate composition, environmental controls) to enable scalable seedling production and ultimately resolve critical supply limitations.
Author contributions
J-YW: Data curation, Writing – original draft. RT: Writing – original draft, Writing – review and editing. J-WW: Software, Writing – original draft. WC: Formal Analysis, Writing – original draft. XL: Formal Analysis, Writing – review and editing. Ji-WW: Data curation, Writing – review and editing. ML: Writing – review and editing. FJ: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the technology development program established through collaboration between Zhejiang Chinese Medical University and industry partners (No. 2023-HT-190).
Conflict of interest
Authors XL and Ji-WW were employed by Shanghai Hope-tec Biotechnology Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Administration, Q. H. D. (2019). Qinghai Tibetan medicinal materials standards, 1. Lanzhou: Gansu Nationalities Publishing House, 40–48.
Afridi, R., Kim, J., Bhusal, A., Lee, W., and Suk, K. (2024). Lipocalin-2 as a mediator of neuroimmune communication. J. Leukoc. Biol. 116, 357–368. doi:10.1093/jleuko/qiad157
Anghelescu, N. E., Balogh, L., Balogh, M., Kigyossy, N., Georgescu, M. I., Petra, S. A., et al. (2024). Gymnadenia winkeliana-A new orchid species to Romanian flora. Plants 13, 1363. doi:10.3390/plants13101363
Anghelescu, N. E., Balogh, M., Balogh, L., Kigyossy, N., Georgescu, M. I., Petra, S. A., et al. (2023). Epipactis bucegensis-A separate autogamous species within the E. helleborine alliance. Plants, 12. doi:10.3390/plants12091761
Bai, D., and Zheng, Y. (2007). Toxicological evaluation of the safty of Tibetan drug wangla. Chin. J. Comp. Med., 740–742. doi:10.3969/j.issn.1671-7856.2007.12.015
Bai, Y., Liu, T., Zhang, S., Shi, Y., Yang, Y., Ding, M., et al. (2025). Traditional Chinese medicine for viral pneumonia therapy: pharmacological basis and mechanistic insights. Int. J. Biol. Sci. 21, 989–1013. doi:10.7150/ijbs.105086
Bao, J. H., Bai, Y. F., Cao, W. J. S. G., Wang, X. L., Bao, Y. T., He, C. L., et al. (2024). Research progress on Gymnadenia conopsea. West China J. Pharm. Sci. 39, 223–230. doi:10.13375/j.cnki.wcjps.2024.02.022
Bao, Y. T. (2023). Study on tissue culture and acclimation of endangered Mongolian medicine plant Gymnadenia conopsea. Tongliao: Inner Mongolia Minzu University. doi:10.27228/d.cnki.gnmmu.2023.000410
Bateman, R. M. (2021b). Phenotypic versus genotypic disparity in the Eurasian orchid genus gymnadenia: exploring the limits of phylogeny reconstruction. Syst. Biodivers. 4, 400–422. doi:10.1080/14772000.2021.1877845
Bateman, R. M., Rudall, P. J., and Denholm, I. (2021a). In situ morphometric survey elucidates the evolutionary systematics of the orchid genus gymnadenia in the British isles. Syst. Biodivers. 19, 571–600. doi:10.1080/14772000.2021.1877848
Brandrud, M. K., Paun, O., Lorenz, R., Baar, J., and Hedrén, M. (2019). Restriction-site associated DNA sequencing supports a sister group relationship of nigritella and gymnadenia (orchidaceae). Mol. Phylogenet. Evol. 136, 21–28. doi:10.1016/j.ympev.2019.03.018
Cha, S. N., Qi, B., Hu, H. X., A, L., Yong, X., Ao, W. L. J., et al. (2024). Prediction of the potential distribution area of endangered medicinal plant Gymnadenia conopsea in China under the background of climate change. Chin. J. Appl. Ecol. 35, 3023–3030. doi:10.13287/j.1001-9332.202411.023
Chen, J. J. (2009). Differentially expressed proteomics during generation of silicosis and effects of Gymnadenia conopsea achohol extract on it. Chongqing: Chongqing Medical University. doi:10.7666/d.Y1546437
Chen, J. Y., Du, W. B., and Su, X. (2022a). A taxonomic inventory of national key protected wild plants in Qinghai province, based on the national checklist of key protected wild plants (2021). Acta pratacult. Sin. 31, 12. doi:10.11686/cyxb2021502
Chen, L. (2008). Study on differential gene expression profiling of lung tissue in the early stage of rats exposed to silica and effects of Gymnadenia conopsea achohol extract on IT. Chongqing: Chongqing Medical University.
Chen, T., Pubu, D., Zhang, W., Meng, S., Yu, C., Yin, X., et al. (2022b). Optimization of the extraction process and metabonomics analysis of uric acid-reducing active substances from gymnadenia r.Br. And its protective effect on hyperuricemia zebrafish. Front. Nutr. 9, 1054294. doi:10.3389/fnut.2022.1054294
Chen, T. R., and Liu, J. L. (2024). Analysis of volatile components and antioxidant effects of the different ethanol concentrations of gymnadenia Conopsea(L.)R.Br. China Food Saf. Mag., 55–63. doi:10.16043/j.cnki.cfs.2024.30.058
Chen, Y. S., Song, Z. Q., Wei, R., Luo, Y., Chen, W., Yang, F., et al. (2023). A dataset on inventory and geographical distribution of vascular plants in xizang, China. Biodivers. Sci. 31, 23188–77. doi:10.17520/biods.2023188
Cheng, H. X. Z. (2024b). Inhibition of AChE activity by ethanol extract of Gymnadenia crassinervis and protection against Aβ25-35-induced cell damage. Changsha: Central South University of Forestry and Technology. doi:10.27662/d.cnki.gznlc.2024.001124
Cheng, Y. S., Wang, T. Y., Xi, W. M., and Yan, M. (2024a). Ecological niches and interspecific associations of dominant species within an endangered Gymnadenia conopsea (L.) R. Br. Community. Plant Sci. J. 42, 444–453. doi:10.11913/PSJ.2095-0837.23229
Devkota, H. P., Adhikari-Devkota, A., Logesh, R., Belwal, T., and Pant, B. (2022). Orchids of genus vanda: traditional uses, phytochemistry, bioactivities, and commercial importance. Reference Ser. Phytochemistry, 591–605. doi:10.1007/978-3-030-38392-3_37
Ding, L., Zhang, L., Guo, L., Sang, J., Qin, L. X., and Wang, B. Q. (2014). Asymbiotic seed germination and rapid seedling regeneration of endangered Gymnadenia conopsea (L.) R. Br. Plant Physiol. J. 50, 77–82. doi:10.13592/j.cnki.ppj.2014.01.009
Feng, Q., Zhang, Z., Sun, M., Wang, Z., Xu, X., Chen, Q., et al. (2024). Separation and purification of benzylester glucosides and derivatives from tubers of Gymnadenia conopsea (L.) R. Br. By linear gradient counter-current chromatography combined with elution-extrusion mode. J. Sep. Sci. 47, e2400118. doi:10.1002/jssc.202400118
Feng, Y. Y., Li, H. Y., Zhang, L. Z., Liu, Q. Q., Wu, D. Y., Liang, H. C., et al. (2022). Therapeutic effect of Tibetan medicine Gymnadenia conopsea polysaccharide on hematopoietic and anti-oxidative damage in mice irradiated with 60 Co-γ rays. J. Nucl. Agric. Sci. 36, 1362–1370. doi:10.11869/j.issn.100-8551.2022.07.1362
Gai, X. G. (2015). Correlation between growth forms of Orchidaceae plants and the composition of mycorrhizal fungal communities. Beijin: Peking Union Medical College. doi:10.7666/d.Y2817962
Gao, X. L., and Feng, H. (2012). Tissue culture and plant regeneration of Gymnadenia conopsea. Southwest China J. Agric. Sci. 25, 1841–1844. doi:10.16213/j.cnki.scjas.2012.05.023
Gao, Y., Chen, Y. H., and Xing, X. K. (2019). Symbiotic fungi inducing seed germination of medicinal Gymnadenia conopsea of Orchidaceae. Mycosystema 38, 1948–1957. doi:10.13346/j.mycosystema.190208
Gao, Y., Zhao, Z., Li, J., Liu, N., Jacquemyn, H., Guo, S., et al. (2020). Do fungal associates of co-occurring orchids promote seed germination of the widespread orchid species gymnadenia conopsea? Mycorrhiza 30, 221–228. doi:10.1007/s00572-020-00943-1
Guo, Z., Li, X., Yang, J. Y., Hu, Y., and Qin, X. Y. (2022). Effects of the active ingredients of Tibetan medicine wangla on senile plaques formation and inflammatory response in APP/PS1 transgenic 5×FAD mice. J. Chin. Med. Mater. 45, 1444–1450. doi:10.13863/j.issn1001-4454.2022.06.030
Gyu-Thogrnying-Mayon-Tanmgon-Po, (2006). Yutuobencao. Tibetan edition. Beijing: The Ethnic Publishing House, 25.
He, K. L. (2016). Influence of different processing methods on gymnadenia R.br anti-fatigue effect and antioxidant activity. Shaanxi J. Tradit. Chin. Med. 37, 754–755. doi:10.3969/j.issn.1000-7369.2016.06.051
He, K. L., Li, B., and Ji, M. C. (2017). Pharmacodynamics research on the activity of Gymnadenia conopsea. Shaanxi J. Tradit. Chin. Med. 38, 1308–1310. doi:10.3969/j.issn.1000-7369.2017.09.068
He, W. (2023a). Study on quality standard and long-term toxicity test of Mongolian medicine Gymnadenia conopsea. Tongliao: Inner Mongolia Minzu University. doi:10.27228/d.cnki.gnmmu.2023.000229
He, W., Wang, X., A, R., Heye, M., and Yong, Q. (2023b). Study on the long-term toxicity of Mongolian medicine Gymnadenia conopsea. J. Inn. Mong. Minzu Univ. Sci. Ed. 38, 366–378. doi:10.14045/j.cnki.15-1220.2023.04.013
Huang, K., Zhang, P., Zhang, Z., Youn, J. Y., Wang, C., Zhang, H., et al. (2021). Traditional chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol. Ther. 225, 107843. doi:10.1016/j.pharmthera.2021.107843
Jiang, L. Z., Xiong, P., and Zeng, W. F. (2009). Study on the effect of tuber gymnadenia powderon treating the acute gastric ulcer. J. Chin. Med. Mater. 32, 1119–1122. doi:10.13863/j.issn1001-4454.2009.07.020
Jiang, S., Wang, M., Jiang, L., Xie, Q., Yuan, H., Yang, Y., et al. (2021). The medicinal uses of the genus bletilla in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 280, 114263. doi:10.1016/j.jep.2021.114263
Jiang, X., Zhao, Z., Jacquemyn, H., Ding, G., Ding, W., and Xing, X. (2022b). Addition of fungal inoculum increases seed germination and protocorm formation in a terrestrial orchid. Glob. Ecol. Conserv. 38, e02235. doi:10.1016/j.gecco.2022.e02235
Jiang, X. L. (2022a). Symbiotic fungi diversity of germination stage and symbiotic germination mechanism of Gymnadenia conopsea seeds. Beijing: Peking Union Medical College. doi:10.27648/d.cnki.gzxhu.2022.000346
Jin, L., and Wang, X. L. (2009). Study on the strengthening with tonics activity of the different processing products of G. conopsea. J. Med. Pharm. Chin. Minor. 15, 28–29. doi:10.16041/j.cnki.cn15-1175.2009.01.022
Kimura, T., Jyo, M., Nakamura, N., Komatsu, K., Hattori, M., Shimotohno, K., et al. (2003). Inhibitory effect of Tibetan medicinal plants on viral polymerases. J. Trad. Med. 20, 243.–250.
Kong, X. Y. (2024). Research on the establishment of fingerprint of Gymnadenia conopsea in Qinghai-Tibet region and comprehensive evaluation of its quality. Xining: Qinghai Normal University. doi:10.27778/d.cnki.gqhzy.2024.000477
Kong, Y. (2022). Effect of Gymnadenia conopsea polysaccharide on the function of monocyte macrophages. Chengdu: Southwest Minzu University. doi:10.27417/d.cnki.gxnmc.2022.000036
Li, F. W., Peng, M. L., Zhu, Y. P., Gao, W. B., and Yu, X. H. (2021a). Effect of extraction method on the physicochemical properties and antioxidant activity of polysaccharides from Gymnadenia conopsea R. Br. Food Ferment. Ind. 47, 150–155. doi:10.13995/j.cnki.11-1802/ts.027159
Li, M. (2007b). Studies on the chemical constituents and quantitative determination of glucosyloxybenzyl 2-isobutylmalates in the tubers of Gymnadenia conopsea. Beijing: Peking Union Medical College. Available online at: https://d.wanfangdata.com.cn/thesis/Y1126923.
Li, M., Guo, S. X., Wang, C. L., and Xiao, P. G. (2007a). Studies on chemical constituents of tubers of Gymnadenia conopsea. Chin. Pharm. J. 42, 1696–1698. doi:10.3321/j.issn:1001-2494.2007.22.005
Li, M., Guo, S. X., Wang, C. L., Yang, J. S., and Xiao, P. G. (2008). Studies on chemical constituents of the tubers of Gymnadenia conopsea. Chin. Pharm. J. 43, 409–412. doi:10.3321/j.issn:1001-2494.2008.06.003
Li, M., Wang, C. L., Guo, S. X., Yang, J. S., and Xiao, P. G. (2006). Advances in studies on chemical constituents and pharmacological activities for plants of gymnadenia R. Br. Chin. Tradit. Herb. Drugs. 37, 1264–1268. doi:10.7501/j.issn.0253-2670.2006.8.550
Li, M. F., and Liu, J. J. (2014). In vitro culture of rare and endangered orchid: gymnadenia conopsea. J. Northwest For. Univ. 29, 110–113. doi:10.3969/j.issn.1001-7461.2014.06.21
Li, M. J., He, R. X., Liao, L. Q., He, B. Z., and Guo, L. J. (2024). Analysis of environmental impact factors of tissue culture and propagation of Goodyera schlechtendaliana rchb. F. Mol. Plant Breed., 1–12. Available online at: https://link.cnki.net/urlid/46.1068.S.20240314.1041.002.
Li, S., Wang, D., Kuang, H. X., Gang, T. J. R., and Ao, S. C. (2001). The chemical constituents of Gymnadenia conopsea R. Br. Chin. Tradit. Herb. Drugs, 20+40. doi:10.7501/j.issn.0253-2670.2001.1.010
Li, T., Wu, S., Yang, W., Selosse, M., and Gao, J. (2021b). How mycorrhizal associations influence orchid distribution and population dynamics. Front. Plant Sci. 12, 647114. doi:10.3389/fpls.2021.647114
Liang, C. T., Liu, L., Zhong, H., Bai, M. Z. M., and Zhang, Y. C. (2021). Study on the anti-hypoxia mechanism of Tibet medicine Gymnadenia conopsea (l.)R.Br based on network pharmacology. J. Med. Pharm. Chin. Minor. 27, 54–59. doi:10.3969/j.issn.1006-6810.2021.12.025
Lin, M., Xiong, H., Xiang, X., Zhou, Z., Liang, L., and Mei, Z. (2020). The effect of plant geographical location and developmental stage on root-associated microbiomes of Gymnadenia conopsea. Front. Microbiol. 11, 1257. doi:10.3389/fmicb.2020.01257
Lin, P., Wang, X., Zhong, X., Zhou, N., Wu, L., Li, J., et al. (2021). Chemical characterization of a PD-1/PD-L1 inhibitory activity fraction of the ethanol extract from Gymnadenia conopsea. J. Asian Nat. Prod. Res. 23, 235–249. doi:10.1080/10286020.2020.1844190
Lin, P., Yao, J., Wu, J., Tian, J., Bao, Y., and Lin, S. (2017). A new ureido-substituted amino acid from the tubers of Gymnadenia conopsea. Chin. Chem. Lett. 28, 257–259. doi:10.1016/j.cclet.2016.08.005
Lin, P. C., Wu, D. T., Xie, J., Zhao, J., and Li, S. P. (2015). Characterization and comparison of bioactive polysaccharides from the tubers of Gymnadenia conopsea. Food Hydrocoll. 43, 199–206. doi:10.1016/j.foodhyd.2014.05.015
Lin, Z. C. (2009). The pharmacology of Gymnadenia conopsea. Guangzhou: Guangzhou University of Chinese Medicine. Available online at: https://d.wanfangdata.com.cn/thesis/D01321603.
Liu, S. D., Chun, X., Ma, X. Y., Mai, L. S., Liu, C. H., and Si, Q. T. (2022). Research progress on anti-aging and clinical application of palmistra chinensis. Chin. J. Aesthetic Med. 31, 178–181. doi:10.15909/j.cnki.cn61-1347/r.004901
Long, H. L., Cai, H. F., Yang, Y., Li, H. B., Pan, Y. Q., Li, K. Q., et al. (2019). Study on microscopic and chemical identification of shou shen. Guangdong Chem. Ind. 46, 51–52. doi:10.3969/j.issn.1007-1865.2019.06.021
Lu, W., Zhang, H., and Wang, W. (2002). Pharmacology of Gymnadenia conopsea. Res. Tradit. Chin. Med. 18, 43–44.
Luo, K. X. (2021). The therapeutic effect and mechanism of focused ultrasound combined with gastrodin on Aβ1-42-induced AD-like mice. Kunming: Kunming Medical University. doi:10.27202/d.cnki.gkmyc.2021.000554
Magrini, S., De Vitis, M., Torelli, D., Santi, L., and Zucconi, L. (2019). Seed banking of terrestrial orchids: evaluation of seed quality in anacamptis following 4-year dry storage. Plant Biol. 21, 544–550. doi:10.1111/plb.12936
Mao, J., Carlo, , and Mao, S. (2012). Blue beryl: commentary on the four medical tantras. Shanghai: Shanghai Scientific and Technical Publishers, 530–531.
Matsuda, H., Morikawa, T., Xie, H., and Yoshikawa, M. (2004). Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Med. 70, 847–855. doi:10.1055/s-2004-827234
Mccormick, M. K., Whigham, D. F., and Canchani-Viruet, A. (2018). Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 219, 1207–1215. doi:10.1111/nph.15223
Meekers, T., Hutchings, M. J., Honnay, O., and Jacquemyn, H. (2012). Biological flora of the British isles: gymnadenia conopsea s.l. J. Ecol. 100, 1269–1288. doi:10.1111/j.1365-2745.2012.02006.x
Meng, X., Wang, M., and Lin, P. (2023). Gymnadenia conopsea (L.) R. br.: comprehensive review of propagation and breeding, traditional uses, chemical composition, pharmacology, quality control, and processing. J. Ethnopharmacol. 306, 116205. doi:10.1016/j.jep.2023.116205
Morikawa, T., Xie, H., Matsuda, H., Wang, T., and Yoshikawa, M. (2006). Bioactive constituents from Chinese natural medicines. XVII. Constituents with radical scavenging effect and new glucosyloxybenzyl 2-isobutylmalates from Gymnadenia conopsea. Chem. Pharm. Bull. (Tokyo) 54, 506–513. doi:10.1248/cpb.54.506
Nabieva, A., Zhmud, E., and Zaytseva, Y. (2020). Initial stages of Gymnadenia conopsea (orchidaceae) morphogenesis in in vitro culture. BIO Web Conf. 24, 00059. doi:10.1051/bioconf/20202400059
Nazim, M., Lyle, E., and Craker, (2001). Medicinal plants used for the treatment of bronchial asthma in Russia and central Asia. J. Herbs, Spices and Med. Plants 8, 91–117. doi:10.1300/j044v08n02_03
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10, 89. doi:10.1186/s13643-021-01626-4
Peng, K., Liu, Y., Lan, C., Liu, H., Wu, F., Xia, M., et al. (2021b). Effects of different explant types and plant growth regulators on tissue culture of Gymnadenia conopsea. Mod. Agric. Sci. Technol., 62–64. doi:10.3969/j.issn.1007-5739.2021.07.024
Peng, K. Z., Yu, H., Xia, M., Liu, H., Lan, C. J., Wu, F., et al. (2021a). Study on quality evaluation of Gymnadenia conopsea from different regions besed on 1H-NMR metabolomics. J. Sichuan For. Sci. Technol. 42, 126–131. doi:10.12172/202010070001
Pe-Ru Ta-Na, M. M. (1985). Tibetan pharmacopoeia somaratsa. Beijing: The Ethnic Publishing House, 160–161.
Qin, J., Xue, S., Xu, C., Jin, J., Wang, J., Yuan, H., et al. (2024a). Bioactivity-guided isolation of antistroke compounds from Gymnadenia conopsea (L.) R. Br. Molecules 29, 4389. doi:10.3390/molecules29184389
Qin, J., Xue, S. Y., Wang, J. B., Yuan, H. L., and Liu, L. (2024b). Chemical constituents and neuroprotective effects of Gymnadenia conopsea. Chin. Tradit. Herb. Drugs. 55, 7923–7931. doi:10.7501/j.issn.1674-5515.2024.23.001
Sa, R. L., Yang, B., Ge, G. H. S., Qian, L. N., and Zhao, S. H. (2020). Effects of Gymnadenia conopsea polysaccharide extract on growth performance, antioxidant function and meat quality of house-fed mutton sheep under oxidative stress. Acta Vet. Zootech. Sin. 51, 2187–2196. doi:10.11843/j.issn.0366-6964.2020.09.016
Shang, J., Li, J. J., and Zhang, G. Y. (2014). Effects of coeloglossum polysaccharide on immunoregulatory function of mice. arXiv 27, 1305–1308. doi:10.16213/j.cnki.scjas.2014.03.083
Shang, L., Zhang, G. Y., Li, J. J., and Shang, J. (2015). The effects of Tibet drug wangla polysaccharide on immune functions of immunosuppressed mice. Pharm. Clin. Chin. Mater. Med. 6, 43–45+49.
Shang, X., Guo, X., Liu, Y., Pan, H., Miao, X., and Zhang, J. (2017). Gymnadenia conopsea (L.) R. br.: a systemic review of the ethnobotany, phytochemistry, and pharmacology of an important Asian folk medicine. Front. Pharmacol. 8, 24. doi:10.3389/fphar.2017.00024
Shang, Y. J., Zhang, Q., Han, Y. B., and Liang, Z. S. (2024). Pharmacological effects of chemical components of gastrodia rhizoma and its product development. Acta Chin. Med. Pharmacol. 52, 115–121. doi:10.19664/j.cnki.1002-2392.240169
Shi, L., Han, L., Zhao, Z., Li, Q., Wang, Y., Ding, G., et al. (2022b). Furanoids from the Gymnadenia conopsea (orchidaceae) seed germination supporting fungus ceratobasidium sp. (GS2). Front. Microbiol. 13, 1037292. doi:10.3389/fmicb.2022.1037292
Shi, L. X. (2023a). Study on the metabolites during seed symbiotic germination of endangered medicinal plant Gymnadenia conopsea (Orchidaceae). Beijing: Peking Union Medical College. doi:10.27648/d.cnki.gzxhu.2023.000862
Shi, Y., Cao, S. S., Zhang, R. H., Zhang, X., Francky, R., and Liu, J. P. (2022a). Research progress on chemical composition, pharmacological effect and clinical application status of palm ginseng. Shaanxi J. Tradit. Chin. Med. 43, 1150–1153. doi:10.3969/j.issn.1000-7369.2022.08.039
Shi, Y., Cao, S. S., Zhang, R. H., Zhang, Y. H., Lei, S. L., Wang, X., et al. (2023b). Effect of Gymnadenia conopsea on rats with type 2 diabetes cognitive impairment and related mechanism. Cent. South Pharm. 21, 3179–3185. doi:10.7539/j.issn.1672-2981.2023.12.013
Song, X. M., Wan, Q. F., and Chen, B. (2011). Study on artificial breeding technology of Gymnadenia conopsea. New Technol. New Prod. China, 365–366. doi:10.13612/j.cnki.cntp.2011.04.158
Stark, C., Babik, W. A., and Durka, W. (2009). Fungi from the roots of the common terrestrial orchid Gymnadenia conopsea. Mycol. Res. 113, 952–959. doi:10.1016/j.mycres.2009.05.002
Steinfort, U., Verdugo, G., Besoain, X., and Cisternas, M. A. (2010). Mycorrhizal association and symbiotic germination of the terrestrial orchid Bipinnula fimbriata (poepp.) johnst (orchidaceae). Flora 205, 811–817. doi:10.1016/j.flora.2010.01.005
Waes, J. M. V., and Debergh, P. C. (2010). In vitro germination of some Western European orchids. Physiol. Plant. 67, 253–261. doi:10.1111/j.1399-3054.1986.tb02452.x
Wang, J., Liu, Q., Zeng, J. B., and Wang, S. (2008a). Effects of Gymnadenia conopsea ethanol extract on synthesis of types I and lll collagen in lungs of rats exposed to silica. Med. J. Chin. People's Armed Police Force, 9–11. doi:10.14010/j.cnki.wjyx.2008.01.002
Wang, J., Xu, X., Zeng, J. B., and Wang, S. (2008b). Using the tissue microarray to explore the effects of GcAE on TNF-α expression of lung in rats induced by silica. arXiv, 485–487. doi:10.3969/j.issn.1009-6469.2008.06.003
Wang, J., Zeng, J. B., Zhao, X. F., Li, Q., and Wang, S. X. (2007). Effects of Gymnadenia conopsea alcohol extract on collagen synthesis in rat lungs exposed to silica and its mechanism of antioxidative stress. J. Integr. Med. 5, 50–55. doi:10.3736/jcim20070110
Wang, J., Zhu, N., Su, X., Gao, Y., and Yang, R. (2023). Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells 12, 793. doi:10.3390/cells12050793
Wang, X., Zhong, X., Zhou, N., Cai, N., Xu, J., Wang, Q., et al. (2020). Rapid characterizaiton of chemical constituents of the tubers of Gymnadenia conopsea by UPLC-Orbitrap-MS/MS analysis. Molecules 25, 898. doi:10.3390/molecules25040898
Wu, Y. X., Li, P., and Zhang, J. Z. (2010). Study of anti-fibrosis effect of Gymnadenia conopsea. Med. J. Chin. People's Armed Police Forces 21, 676–680. doi:10.14010/j.cnki.wjyx.2010.08.010
Xing, X., Gao, Y., Zhao, Z., Michael, W., J, D. K., André, S. M., et al. (2020). Similarity in mycorrhizal communities associating with two widespread terrestrial orchids decays with distance. J. Biogeogr. 47, 421–433. doi:10.1111/jbi.13728
Xue, S. Y. (2023). Study on the anti-aging effect and components of Gymnadenia conopsea based on Caenorhabditis elegans and UPLC-QTOF-MS. Yangzhou: Yangzhou University. doi:10.27441/d.cnki.gyzdu.2023.002458
Yang, C. L., Zhong, Z. C., Luo, J., Yang, S., Chen, Z. J., Cui, J. Z., et al. (2018a). Geographic distribution, habitat and analysis on endangered cause of gymnadenia R. Br. In Tibet. Tibet. J. Agric. Sci. 40, 32–36. doi:10.3969/j.issn.1005-2925.2018.01.009
Yang, F., Liu, D. L., Qiu, F., and Wang, S. X. (2009). Study on the hydrosoiuble constituents in the tuber of Gymnadenia conopsea r.Br. Food Res. Dev. 30, 125–128. doi:10.3969/j.issn.1005-6521.2009.07.036
Yang, M. M. (2010). Study on polysaccharides charaeteristies from Gymnadenia conopsea r.Br. Tianjin: Tianjin University of Science and Technology. doi:10.7666/d.y2082830
Yang, S. (2018b). Investigation and anaiysis on soil environment of the gymnadenia R.BR. Habitats in Tibet. Yangling: Northwest A&F University.
Yang, S., Chen, Z. J., Zhong, Z. C., Yang, C. L., Cui, J. Z., Zhao, G. Q., et al. (2017). Habitats of soil nutrients status of wild gymnadenia in Tibet. Guizhou Agric. Sci. 45, 56–60.
Yang, S., Fang, J. P., and Wang, Y. (2012). Study on influencing factors on browning rate of explants in tissue culture of Gymnadenia conopsea. Guizhou Agric. Sci. 40, 22–25. doi:10.3969/j.issn.1001-3601.2012.01.008
Yang, X. H., Zhao, M. X., Chen, H., Gao, D. D., and Ding, G. T. (2021). Advances in research on the mechanism of immunomodulatory effects of plant polysaccharides. J. Food Saf. Qual. 12, 5349–5355. doi:10.19812/j.cnki.jfsq11-5956/ts.2021.13.037
Yao, N., Wang, T., Wang, Y., Sun, M. L., Zheng, B. Q., and Cao, X. L. (2024). Diversity and functions of endophytic microorganisms in three medicinal orchids. Microbiol. China, 1–18. doi:10.13344/j.microbiol.china.240455
Yosri, N., Alsharif, S. M., Xiao, J., Musharraf, S. G., Zhao, C., Saeed, A., et al. (2023). Arctium lappa (burdock): insights from ethnopharmacology potential, chemical constituents, clinical studies, pharmacological utility and nanomedicine. Biomed. Pharmacother. 158, 114104. doi:10.1016/j.biopha.2022.114104
Yu, C. Y., Shi, J. G., and Zhang, J. J. (2013). Effects of coeloglossum extract on cholinesterase changes in cholinergic impaired rats. J. Logist. Univ. Pap. Med. Sci. 22, 583–585+626+670.
Yu, L. (2024b). The study of the therapeutic effect and mechanism of Mongolian medicine Gymnadenia conopsea R.Br et extract on the improvement of behavioral disorders induced by aluminum chloride in zebrafish models of Alzheimer's disease. Tongliao Inn. Mong. Minzu Univ. doi:10.27228/d.cnki.gnmmu.2024.000473
Yu, L., Dai, X. M., Jiang, S. D., Bao, S. L., Sa, R. G., Chen, Y. L., et al. (2024a). Comprehensive assessment of the quality of Gymnadenia conopsea p.Br from various origins, utilizing UPLC-Q-TOF-MS in conjunction with a multivariate statistical approach. Asia-Pacific Tradit. Med. 20, 72–77. doi:10.11954/ytctyy.202407014
Yu, P., Han, H. P., Shang, J., and Chen, Z. (2018). Study on the anti-aging effect of polysaccharides from Gymnadenia conopsea. Northwest Pharm. J. 33, 46–49. doi:10.3969/j.issn.1004-2407.2018.01.012
Yu, P. Z. (2017). Study on extract techniques and pharmacological activities of polysaccharide from Gymnadenia conopsea. Xining: Qinghai Normal University. doi:10.7666/d.D01373959
Yu, Y. P., Zhang, R. J., Yuan, X., Chen, X. D., Wan, L. S., Xing, Z., et al. (2024c). Investigation and analysis of the germplasm resources on the gymnadenia genus in the sejila Mountain area in southeastern xizang. Chin. Wild Plant Res. 43, 117–122. doi:10.3969/j.issn.1006-9690.2024.10.016
Yue, G. (2020). Study on fungal symbiotic germination of seeds of endangered orchid medicinal plant Gymnadenia conopsea. Beijing: Peking Union Medical College. doi:10.27648/d.cnki.gzxhu.2020.000782
Yue, Z. G., Zi, J. C., Zhu, C. G., Lin, S., Yang, Y. C., and Shi, J. G. (2010). Constituents of Gymnadenia conopsea. China J. Chin. Mater. Med. 35, 2852–2861. doi:10.4268/cjcmm20102114
Zeng, J. B., Wang, J., Du, H. K., Zhao, X. F., and Wang, S. X. (2007). Effect of Gymnadenia conopsea alcohol extract on pulmonary fibrosis of rats exposed to silica and the expression of tumor necrosis factor-alpha. J. Hyg. Res. 36, 674–678. doi:10.3969/j.issn.1000-8020.2007.06.006
Zhai, D., Lv, X., Chen, J., Peng, M., and Cai, J. (2022). Recent research progress on natural stilbenes in dendrobium species. Molecules 27, 7233. doi:10.3390/molecules27217233
Zhang, D., Zhang, Y., Liu, G., and Zhang, J. (2006). Dactylorhin B reduces toxic effects of beta-amyloid fragment (25-35) on neuron cells and isolated rat brain mitochondria. Naunyn. Schmiedeb. Arch. Pharmacol. 374, 117–125. doi:10.1007/s00210-006-0095-9
Zhang, T. E., Chen, C. Y., Li, S. H., Chen, C., Liu, W. W., and Yan, Z. Y. (2013). Effect of the extract of Gymnadenia conopsea on the blood lipid and liver function in experimental hyperlipidemia rats. Lishizhen Med. Mater. Med. Res. 24, 865–867. doi:10.3969/j.issn.1008-0805.2013.04.042
Zhang, X. H., and Borjihan, G. (2005). Molecular weight and composition determination of Gymnadenia conopsea polysaccharide. Acta Sci. Nat. Univ. Neimongol. 36 (4). doi:10.3969/j.issn.1000-1638.2005.01.009
Zhang, Y., Liu, L., Liang, C., Zhou, L., Tan, L., Zong, Y., et al. (2020). Expression profiles of long noncoding RNAs in mice with high-altitude hypoxia-induced brain injury treated with Gymnadenia conopsea (L.) R. Br. Neuropsychiatr. Dis. Treat. 16, 1239–1248. doi:10.2147/NDT.S246504
Zhao, L., and Liu, G. Q. (2011). Experimental study of shouzhang shen liquids on anti-fatigue effects in mice. Clin. J. Chin. Med. 3, 17. doi:10.3969/j.issn.1674-7860.2011.22.011
Zhou, X. X., Xiong, P., Lin, Z. C., and Chen, Y. (2009). Sedative and hypnotic effects by gymnadenia conopsea's experimental study. Mod. Chin. Med. 11, 33–35. doi:10.13313/j.issn.1673-4890.2009.09.010
Zi, J., Li, S., Liu, M., Gan, M., Lin, S., Song, W., et al. (2008a). Glycosidic constituents of the tubers of Gymnadenia conopsea. J. Nat. Prod. 71, 799–805. doi:10.1021/np070670j
Zi, J., Lin, S., Zhu, C., Yang, Y., and Shi, J. (2010). Minor constituents from the tubers of Gymnadenia conopsea. J. Asian Nat. Prod. Res. 12, 477–484. doi:10.1080/10286020.2010.491476
Zi, J. C. (2008b). Chemical constituents of Gymnadenia conopsea. Beijing: Peking Union Medical College. Available online at: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIIWTEzMTk5MjIaCHY1bWtkdzJl.
Keywords: Gymnadenia conopsea, phytochemistry, pharmacological activity, symbiotic germination, sustainable conservation
Citation: Wu J-Y, Tang R-N, Wang J-W, Chen W-Y, Liu X, Wang J-W, Li M-Y and Jiang F-S (2025) Gymnadenia conopsea orchid: a systematic review. Front. Pharmacol. 16:1595714. doi: 10.3389/fphar.2025.1595714
Received: 18 March 2025; Accepted: 09 July 2025;
Published: 06 August 2025.
Edited by:
Fabien Schultz, Bernhard Nocht Institute for Tropical Medicine (BNITM), GermanyReviewed by:
Timothy Omara, University of Natural Resources and Life Sciences Vienna, AustriaSabreena Safuan, Universiti Sains Malaysia Health Campus, Malaysia
Copyright © 2025 Wu, Tang, Wang, Chen, Liu, Wang, Li and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fu-Sheng Jiang, amZzMTAyMEAxNjMuY29t; Mei-Ya Li, bG1laXlhQDEyNi5jb20=; Ji-Wen Wang, amFzb25AaHRjaGVtLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jing-Yi Wu1,2†
Jing-Yi Wu1,2† Mei-Ya Li
Mei-Ya Li Fu-Sheng Jiang
Fu-Sheng Jiang