- 1Division of Infectious Diseases in State Key Laboratory of Biotherapy, Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2HitGen Inc., Chengdu, Sichuan, China
The gut-liver axis is a multifaceted system where chemical and biological interactions between gut microbiota-derived metabolites and the liver significantly influence the development and progression of hepatocellular carcinoma Metabolites such as lipopolysaccharide (LPS), bile acids (BAs), and short-chain fatty acids (SCFAs) act as chemical mediators that modulate the tumor microenvironment through immune cell interactions and stromal activation, influencing tumor growth and metastasis. Changes in gut microbiota composition alter these signaling pathways, providing opportunities for therapeutic interventions. Strategies such as prebiotics, probiotics, and natural product-based small molecules have shown promise in modulating the gut-liver axis. Advanced multi-omics, chemical and bioinformatics tools, coupled with in vitro models like organoids, have unveiled intricate molecular interactions, offering insights into novel therapeutic targets. Future research should focus on delineating the pharmacological and immunological mechanisms within the gut-liver axis, developing personalized therapeutic strategies, and translating these findings into clinical applications.
1 Introduction
The etiology and progression of HCC is a complex and multi-stage processes influenced by a variety of factors, including viral infections (e.g., Hepatitis B and C viruses), alcohol consumption, chemical substances (e.g., aflatoxins), and metabolic dysfunction-associated steatotic liver disease (MASLD) resulting from conditions such as obesity and diabetes. All these factors contribute to hepatocyte damage and inflammation. Hepatic fibrosis, primarily driven by the activation of hepatic stellate cells (HSCs), results in the deposition of fibrotic tissue and significant alterations in liver structure (Elpek, 2014). While fibrosis is a defensive response to inflammation or injury, chronic injury leads to excessive accumulation of extracellular matrix (ECM) components, thereby triggering hepatic fibrosis (Robinson et al., 2016). During this process, the liver ECM composition shifts from laminin and type IV collagen to interstitial collagen (Zadorozhna et al., 2020). As fibrosis intensifies and liver structure is disrupted, hepatocytes accumulate genetic mutations and epigenetic alterations, culminating in malignant transformation and HCC formation (Figure 1). Increasing evidence highlights the significant role of gut microbiota in human diseases, with interactions extending to extra-intestinal organs such as the liver (Singhvi et al., 2020). An inseparable bidirectional relationship exists between the liver and the gut, known as gut-liver axis, where bile and its metabolites are transported from the liver to the gut and microbial products are returned via the portal vein, can induce hepatic issues (Albillos et al., 2020). An imbalance in the gut-liver axis can lead to chronic inflammation and contribute to the progression of liver cancer (Arab et al., 2018). In the gut-liver axis, two barriers exist that play crucial roles in maintaining homeostasis and preventing the translocation of harmful substances.
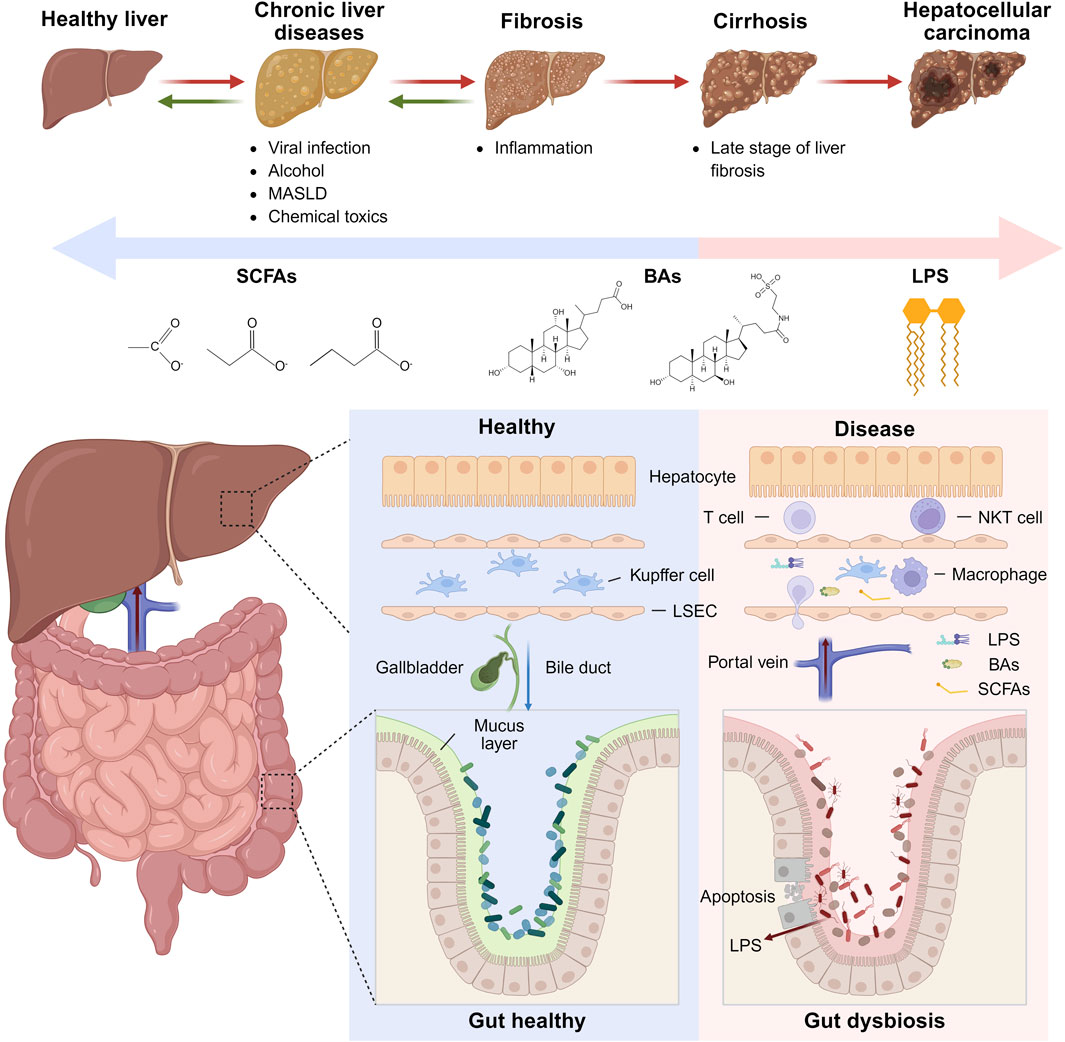
Figure 1. Interactions between gut microbiota and liver cancer progression. Upper Panel: The sequence of liver disease progression from a healthy liver through chronic liver diseases, fibrosis, cirrhosis, to HCC. Lower Panel: Comparison between healthy and diseased gut-liver states, emphasizing alterations in gut barrier integrity, immune cell infiltration in the liver, and the impact of harmful metabolites such as LPS on disease progression. Figure was created with BioRender.com.
The gut barrier consists of a single layer of columnar epithelial cells interconnected by tight junctions (Buckley and Turner, 2018). Goblet cells secrete a mucus layer that serves as the initial physical barrier (Zhang et al., 2022). The gut vascular barrier (GVB) is the innermost physical barrier, controlling the entry of microorganisms and luminal contents into the portal vein, thereby influencing hepatic immunity (Spadoni et al., 2015). Immune cells in the lamina propria, including intestinal macrophages and dendritic cells (DCs), phagocytose invading microorganisms and luminal contents (Luciani et al., 2022). Macrophages prevent pathogen-associated molecular patterns (PAMPs) such as LPS from entering the liver via the portal vein (Mowat et al., 2017). If PAMPs do enter the liver, they can affect the localization of immune cells within the liver (Bi et al., 2021). DCs guide antigens to mesenteric lymph nodes to activate adaptive immunity through resident Treg cells (Esterhazy et al., 2019; Cording et al., 2014). The liver barrier, similar to the gut barrier, comprises endothelial cells, mesenchymal cells, and immune cells, including macrophages, T cells, NK cells, and myeloid-derived suppressor cells (MDSCs), which filter and metabolize blood substances entering the liver via the portal vein (Liang et al., 2022). The liver hosts the largest population of tissue macrophages in the human body, primarily Kupffer cells (Roohani and Tacke, 2021). Gut microbiota and luminal contents reaching the liver are classified, recognized, and captured by liver immune cells (Gola et al., 2021). The gut-liver axis promotes HCC development through alterations in gut microbiota and microbial products, mucosal barrier damage, and GVB disruption (Mouries et al., 2019; Maccioni et al., 2020; Sorribas et al., 2019). Intestinal microbial imbalance and impaired barrier function allow harmful microbial products to enter the liver via the portal vein, triggering inflammation and exacerbating liver disease. Conversely, cirrhosis and portal hypertension alter the gut microbiome composition, facilitating microbial translocation to the liver, altering the hepatic immune environment, intensifying inflammation, and potentially inducing liver cancer.
This review aims to summarize recent advancements in understanding the mechanisms underlying the gut-liver axis in HCC, with a particular focus on the roles of key microbial metabolites such as LPS, BAs, and SCFAs. We will also discuss recent advancements in therapeutic strategies targeting the gut microbiota, including fecal microbiota transplantation (FMT), phage therapy, probiotics, and small molecule drugs. Furthermore, we will highlight the importance of multi-omics analysis techniques, chemical labeling methods, and in vitro models in elucidating the complex interactions within the gut-liver axis. By integrating these insights, this review seeks to offer valuable perspectives on the development of novel diagnostic biomarkers and therapeutic approaches for HCC.
2 The chemical properties and immunological impacts of microbial-derived components on liver cancer progression
The gut microbial produces metabolites such as secondary BAs, SCFAs, lactic acid, and ethanol, as well as microbial components like LPS from Gram-negative bacteria (Lagkouvardos et al., 2023; Yoshimoto et al., 2013). These metabolites and components play crucial roles in modulating hepatic immunity, metabolism, and tumor progression, via the gut-liver axis (Figure 1).
2.1 Lipopolysaccharide (LPS)
2.1.1 LPS in HCC, immune activation and beyond
LPS, a major component of the cell wall of Gram-negative bacteria and a well-known endotoxin, activates the host’s immune response by stimulating hepatic immune cells, particularly macrophages, via Toll-like receptor 4 (TLR4) and is considered a significant factor in promoting HCC progression by inducing liver inflammatory responses that lead to hepatocyte necrosis or apoptosis and subsequent liver injury (Singh et al., 2017; Luo et al., 2023). Specifically, LPS binds to LPS-binding protein (LBP), and the LBP-LPS complex is transferred to TLR4/MD-2 (myeloid differentiation factor 2) by cluster of differentiation 14 (CD14). The lipid A domain of LPS, which is relatively conserved, is responsible for most of its immunological activity and can be detected by the innate immune system at picomolar levels. MD-2 possesses a unique hydrophobic cavity where the acyl chain region of lipid A inserts into a specific binding pocket of MD-2, interacting with hydrophobic residues on both MD-2 and TLR4 to form a hexameric complex (Oblak and Jerala, 2015; Ohto et al., 2012). The binding of LPS induces TLR4 dimerization and recruits downstream signaling molecules, such as the adaptor protein myeloid differentiation factor 88 (MyD88) and TIR-domain-containing adapter inducing interferon-β (TRIF). MyD88 further activates downstream mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB), leading to the release of pro-inflammatory mediators like tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) (Figure 2B).
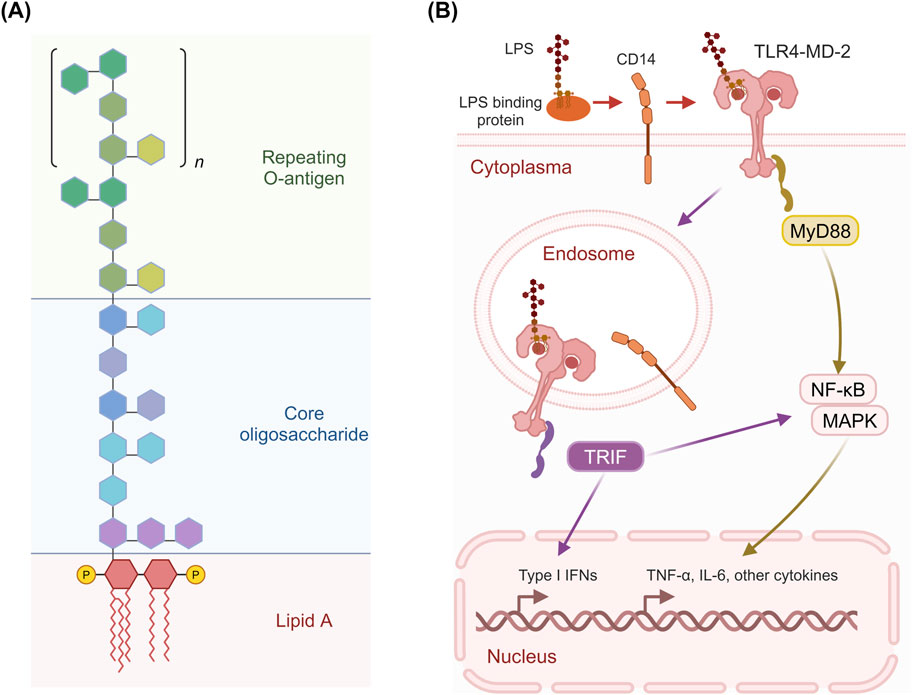
Figure 2. Structure of LPS and its activation of TLR4 signaling pathways. (A) LPS consists of three main parts: the repeating O-antigen, the core oligosaccharide, and Lipid A. The O-antigen is composed of repeating oligosaccharide units, while the core oligosaccharide links the O-antigen to Lipid A, which is embedded in the bacterial membrane and is responsible for the endotoxic activity of LPS. (B) LPS binds to LBP, which facilitates the transfer of LPS to CD14. LPS binds to LBP and is then transferred to CD14, which facilitates its interaction with the TLR4/MD-2 complex. This leads to the activation of two distinct signaling pathways: the MyD88-dependent pathway and the TRIF-dependent pathway. The recruitment of the adaptor protein MyD88 activates downstream signaling cascades involving NF-κB, MAPK, and other signaling molecules, resulting in the production of pro-inflammatory cytokines such as TNF-α and IL-6. The TRIF-dependent pathway leads to the production of Type I IFNs and other cytokines. Figure was created with BioRender.com.
Beyond its direct role in immune activation via TLR4, LPS drives HCC progression through downstream signaling pathways activated by TLR4. Recent studies in mouse tumor models have shown that LPS promotes angiogenesis within tumor tissues by modulating the EGFR/IL8 and VEGFR/STAT3 signaling pathways, thereby enhancing tumor growth, proliferation, and metastasis (Kubo et al., 2024; Wang et al., 2019). Additionally, LPS upregulates the expression of HMGCR, LDLR, and SREBF2 while downregulating PCSK9 expression through the NF-κB pathway, leading to significant intracellular cholesterol accumulation and enhanced pro-inflammatory effects (He et al., 2017). In the context of HCC immunotherapy, LPS has also been reported to induce the expression of PD-1 and PD-L1 in mouse tumor tissues via the METTL14/MIR155HG pathway, providing new insights into HCC immunotherapy (Peng et al., 2022).
Despite the crucial role of TLR4 in HCC development, recent studies have shown that LPS can also act through non-TLR4 mechanisms (Dapito et al., 2012). A recent study has identified Galectin-3 as a potential LPS sensor, through which LPS can modulate glucose metabolism via the LPS/Galectin-3/Rag GTPases/Ragulator-mTORC1 axis, thereby participating in the regulation of HCC (Chen X. et al., 2022).
2.1.2 Structural diversity of LPS and its immune implications
LPS typically consists of a hydrophobic lipid A domain, a more conserved nonrepeating core oligosaccharide, and an O-specific polysaccharide (or O-antigen) (Park and Lee, 2013) (Figure 2A). The lipid A domain comprises a phosphorylated di-glucosamine backbone, typically with phosphorylation at the 1 and 4′ positions, and four acyl groups linked at positions 2, 3, 2′, and 3’. Additionally, two secondary acyl chains are present on the distal glucosamine. The core oligosaccharides typically contain unusual carbohydrate residues, such as 3-deoxy-D-manno-oct-2-ulosonic acid residues, heptoses, and various hexoses, and are relatively conserved among bacterial species. The O-antigen is linked to the core oligosaccharide and typically consists of repeating oligosaccharides made of multiple sugars. It is the most diverse component of LPS, and some Gram-negative bacteria do not even synthesize this part.
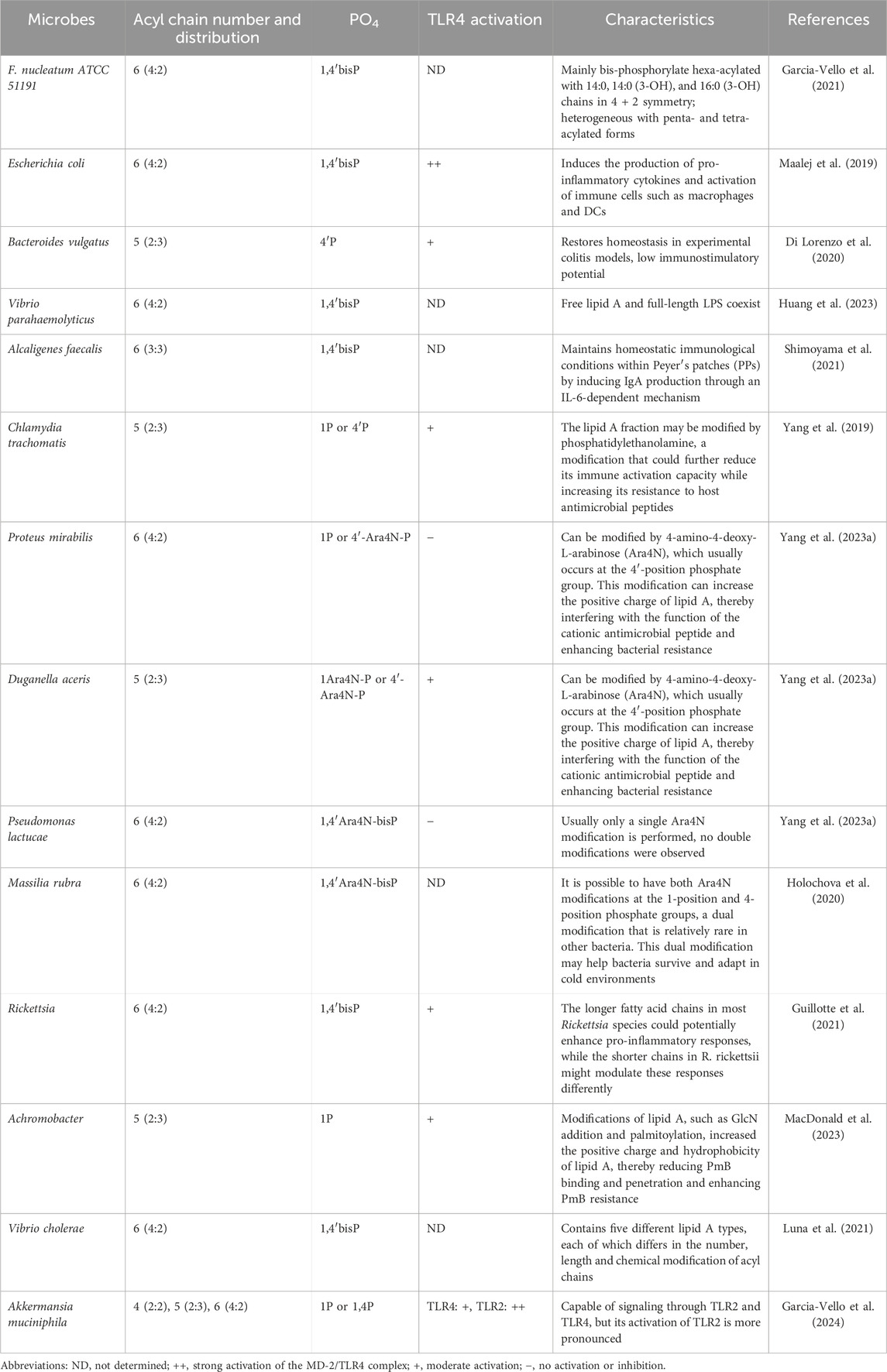
The lipid A structure, while conserved at the species level, undergoes regulated modifications in response to varying environmental conditions, enabling bacteria to evade immune detection (Oblak and Jerala, 2015). For instance, Yersinia pestis and Francisella tularensis alter their lipid A composition based on temperature changes. Notably, lipid A from different bacterial species shows considerable structural diversity, particularly in terms of the number and length of acyl chains and modifications to the phosphate groups. Changes in the number and length of fatty acyl chains may affect their activity on TLR4, potentially acting as agonists or antagonists. The structural diversity of lipid A has been extensively studied, which is beneficial for the development of novel drugs. For example, by synthesizing lipid A analogs with specific structures, it is possible to design drugs that target specific bacteria. Here, we summarize the modifications of lipid A structures and their effects on immune recognition in different bacteria or environments that have been studied in recent years (Table 1).
2.2 Bile acids (BAs)
2.2.1 BAs: structure, synthesis, and metabolism
BAs are cholesterol-derived molecules that play essential roles in lipid digestion and metabolism. Structurally, BAs feature a steroid nucleus composed of four fused rings (three six-membered and one five-membered) and a side chain with multiple carbon atoms (Fuchs et al., 2025). The primary BAs in humans are cholic acid (CA) and chenodeoxycholic acid (CDCA), which differ in their hydroxyl (OH) and methyl (CH3) group arrangements. CA contains two hydroxyl groups (3α, 7α-OH) and a keto group (12-keto), while CDCA lacks the 7α-hydroxyl group.
BA synthesis primarily occurs in the liver through two main pathways: the classical pathway and the alternative pathway. The classical pathway, initiated by CYP7A1 and involving CYP8B1 and CYP27A1, generates CA and CDCA, accounting for about 90% of primary BAs in humans (Grant and DeMorrow, 2020). CYP8B1 determines the CA-to-CDCA ratio. The alternative pathway, involving CYP27A1 and CYP7B1, produces CDCA. In rodents, CDCA is rapidly converted into more hydrophilic α-muricholic acid (α-MCA) and β-muricholic acid (β-MCA) (Asgharpour et al., 2015). Primary BAs are conjugated with glycine or taurine in the liver to form glycocholic acid (GCA), taurocholic acid (TCA), glycochenodeoxycholic acid (GCDCA), and taurochenodeoxycholic acid (TCDCA) (Grant and DeMorrow, 2020). After synthesis, BAs are stored in the gallbladder and secreted into the small intestine via the biliary system postprandially. They facilitate lipid digestion and absorption, with most BAs being reabsorbed by intestinal cells and returned to the liver via the portal vein. A small fraction is excreted in feces (Di Ciaula et al., 2017).
In the gut, BAs are metabolized by microbiota, primarily in the large intestine. Imbalances in gut microbiota can disrupt BA homeostasis, leading to conditions such as hepatitis, fibrosis, and HCC. Common microbial metabolic pathways include 7α-dehydrogenation, epimerization, and deconjugation (Figure 3). In addition to these pathways, BA metabolism also encompasses oxidation, esterification, desulfation, and further isomerization reactions, which collectively give rise to complex chemical structures within the gut (Choudhuri and Klaassen, 2022). Gut bacteria, such as Bacteroides and Clostridium species, convert primary BAs into secondary BAs, for example, deoxycholic acid (DCA) and lithocholic acid (LCA), via 7α-dehydroxylase, which removes the 7α-hydroxyl group (Fiorucci et al., 2021). Eubacterium and Clostridium species can catalyze the conversion of 7α-hydroxy BAs to 7β-hydroxy structures, for example, converting CDCA to ursodeoxycholic acid (UDCA), via 7-hydroxysteroid dehydrogenase (7-HSDH) (Doden et al., 2021). Additionally, gut bacteria such as Bifidobacterium, Lactobacillus, Clostridium, and Enterococcus express bile salt hydrolase (BSH), which deconjugates BAs by hydrolyzing glycine or taurine conjugates, facilitating further microbial metabolism (Doden et al., 2021).
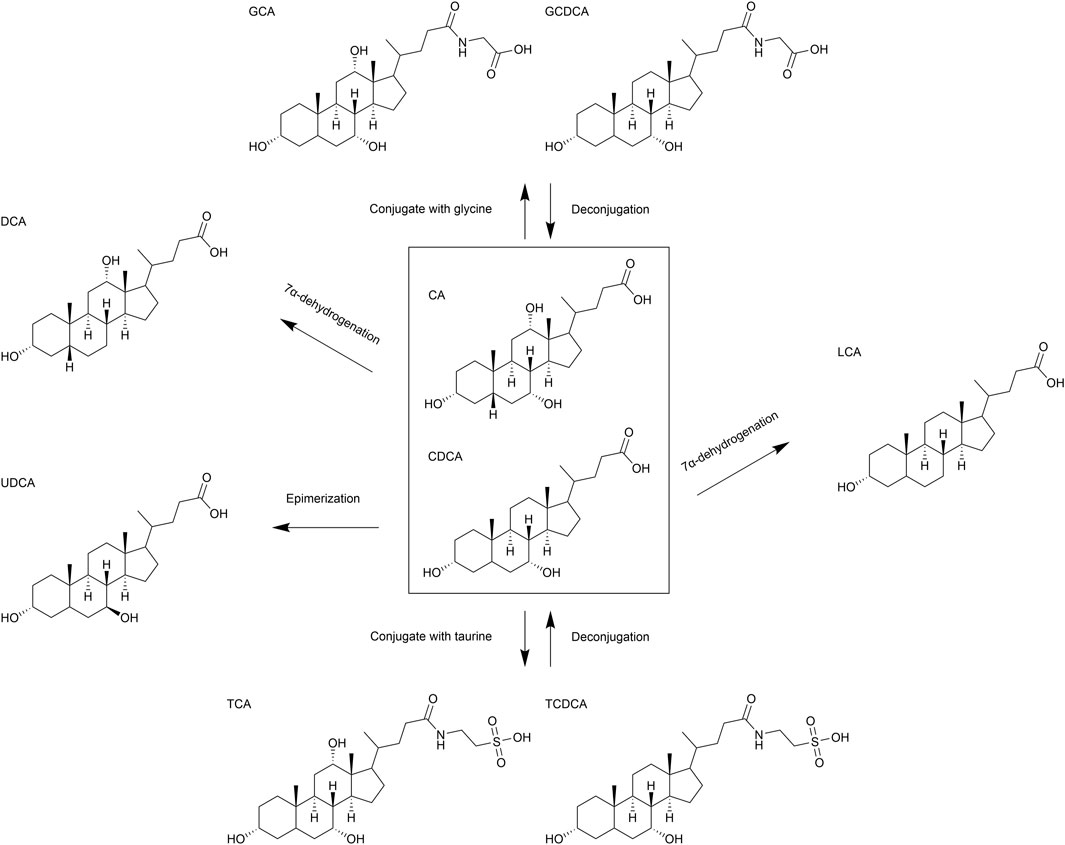
Figure 3. Schematic representation of structure and metabolism of bile acids (BAs). Major biochemical pathways involving the transformation of BAs, including conjugation with glycine and taurine, deconjugation, epimerization, 7α-dehydrogenation.
2.2.2 Impact of BAs on HCC progression and immune modulation
There is a close correlation between the metabolism of BAs in the gut and the progression of HCC as well as its response to immunotherapy. In the years preceding diagnosis, patients with HCC exhibit a significant increase in total BA concentrations, particularly an increased proportion of taurine-conjugated BAs (e.g., TCA and taurodeoxycholic acid), which are closely related to factors such as liver dysfunction and alterations in gut microbiota (Stepien et al., 2022). Another study found differences in the levels of primary and secondary BAs in plasma, liver, and gut in an N-nitrosodiethylamine-induced rat model. Levels of CDCA, CA, UDCA, and hyodeoxycholic acid in plasma can serve as early diagnostic biomarkers for HCC (Xing et al., 2023). Therefore, monitoring changes in the BA profile is of great significance for the early identification of high-risk populations for HCC, assessing disease progression, and guiding therapeutic strategies.
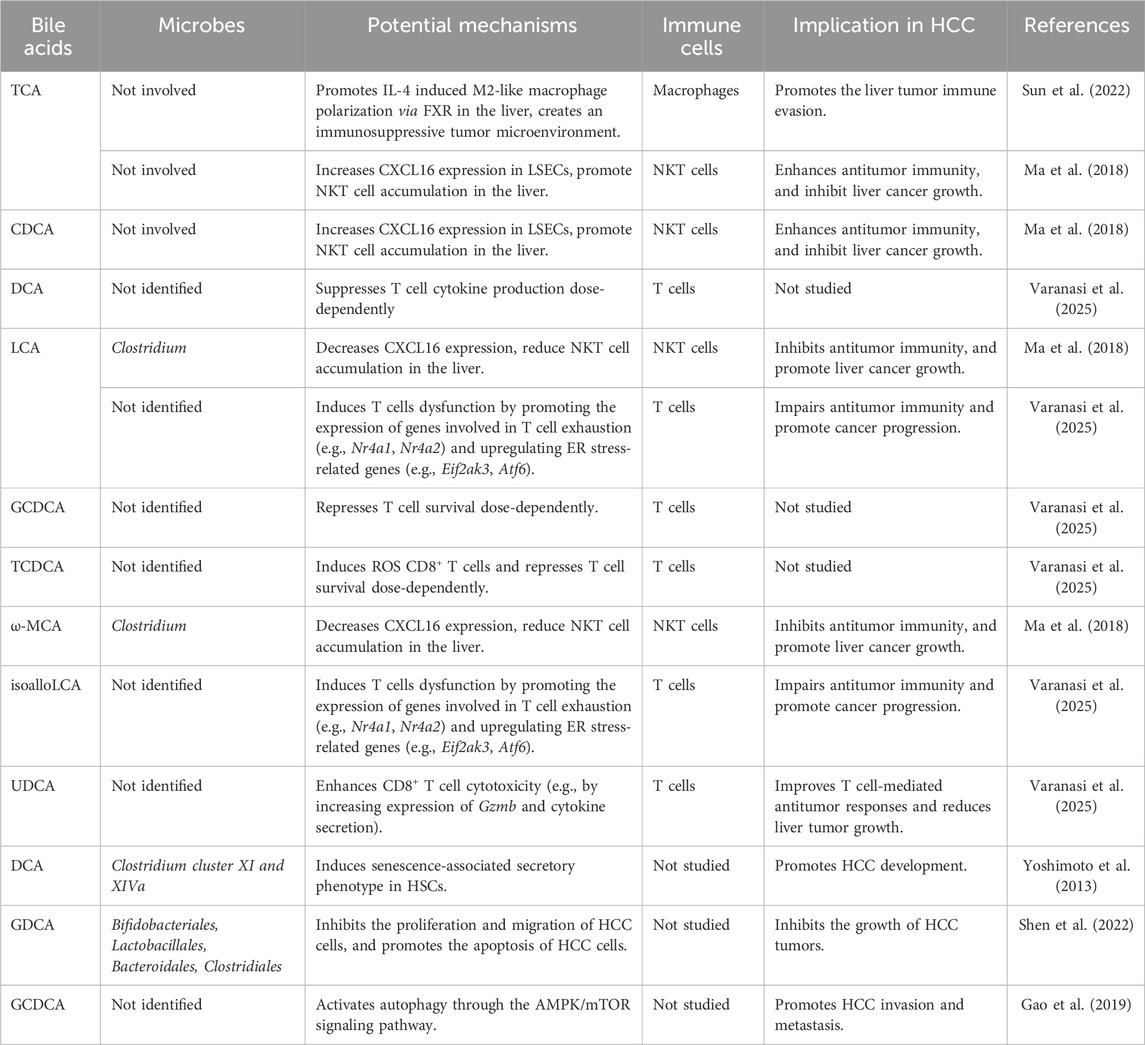
BAs have been shown to exert distinct effects on the development and treatment of HCC by selectively modulating the functions of specific hepatic immune cells such as macrophages and T cells, as well as non-immune cells like HSCs and liver sinusoidal endothelial cells (LSECs) (Table 2). For instance, TCA can promote the polarization of M2-like macrophages in the liver, thereby creating an immunosuppressive tumor microenvironment, which affects tumor immune evasion and growth (Sun et al., 2022). Studies have found that a high-fat diet or genetic obesity alters the gut microbiota, thereby increasing the level of the gut microbial metabolite DCA in the liver. This leads to HSCs senescence and the senescence-associated secretory phenotype, which secrete inflammatory cytokines, chemokines, proteases, and growth factors in the liver, promoting HCC progression (Yoshimoto et al., 2013; Lin et al., 2024). Additionally, certain BAs, such as UDCA, also exhibit potential for the treatment of liver diseases. For example, UDCA has been extensively studied for its efficacy in treating cholestasis and various other chronic liver conditions. UDCA can facilitate liver disease treatment by activating the farnesoid X receptor (FXR)/FGF-15 signaling pathway, regulating apoptosis and autophagy, enhancing intestinal barrier function, and reducing the toxicity of microbe-associated molecular patterns (MAMPs) (Mao et al., 2024). UDCA has also been reported to enhance CD8+ T cell function and inhibit tumor growth in mouse model of liver cancer by modulating bile acid composition and reducing ER stress in the tumor microenvironment (Varanasi et al., 2025). Primary BAs, such as CDCA and TCA, or secondary BAs, such as DCA and LCA, modulate the expression of the chemokine CXCL16 in LSECs. This alteration in CXCL16 expression subsequently influences the accumulation of NKT cells in the liver through CXCR6. Upon antigenic stimulation, NKT cells become activated and produce increased amounts of interferon-γ (IFN-γ), which is crucial for inhibiting liver tumor growth (Ma et al., 2018). Overall, these findings highlight the critical role of BAs in shaping the tumor microenvironment and influencing HCC progression through multiple pathways.
2.3 Short-chain fatty acids (SCFAs) in liver cancer
SCFAs, which are fermentation byproducts of dietary fiber and other nondigestible carbohydrates by gut microbiota, including acetate, propionate, and butyrate, serve as energy sources for intestinal epithelial cells and modulate the host’s immune response, energy metabolism, and overall gut health. SCFAs primarily exert their biological functions by interacting with G-protein-coupled receptors (GPCRs), particularly GPR41 and GPR43. These receptors play a crucial role in regulating glucose and lipid metabolism and inflammation in intestinal epithelial cells and immune cells. Notably, certain plant bioactive extracts, such as Eucommia bark and leaf extract (Wang Z. et al., 2023) or Arctigenin (Wang et al., 2024), can promote the production of SCFAs and activate the receptors GPR41 and GPR43 by modulating the gut microbiota, thereby alleviating lipid metabolic disorders and elevated blood glucose levels induced by a high-fat diet, as well as hepatic degenerative lesions.
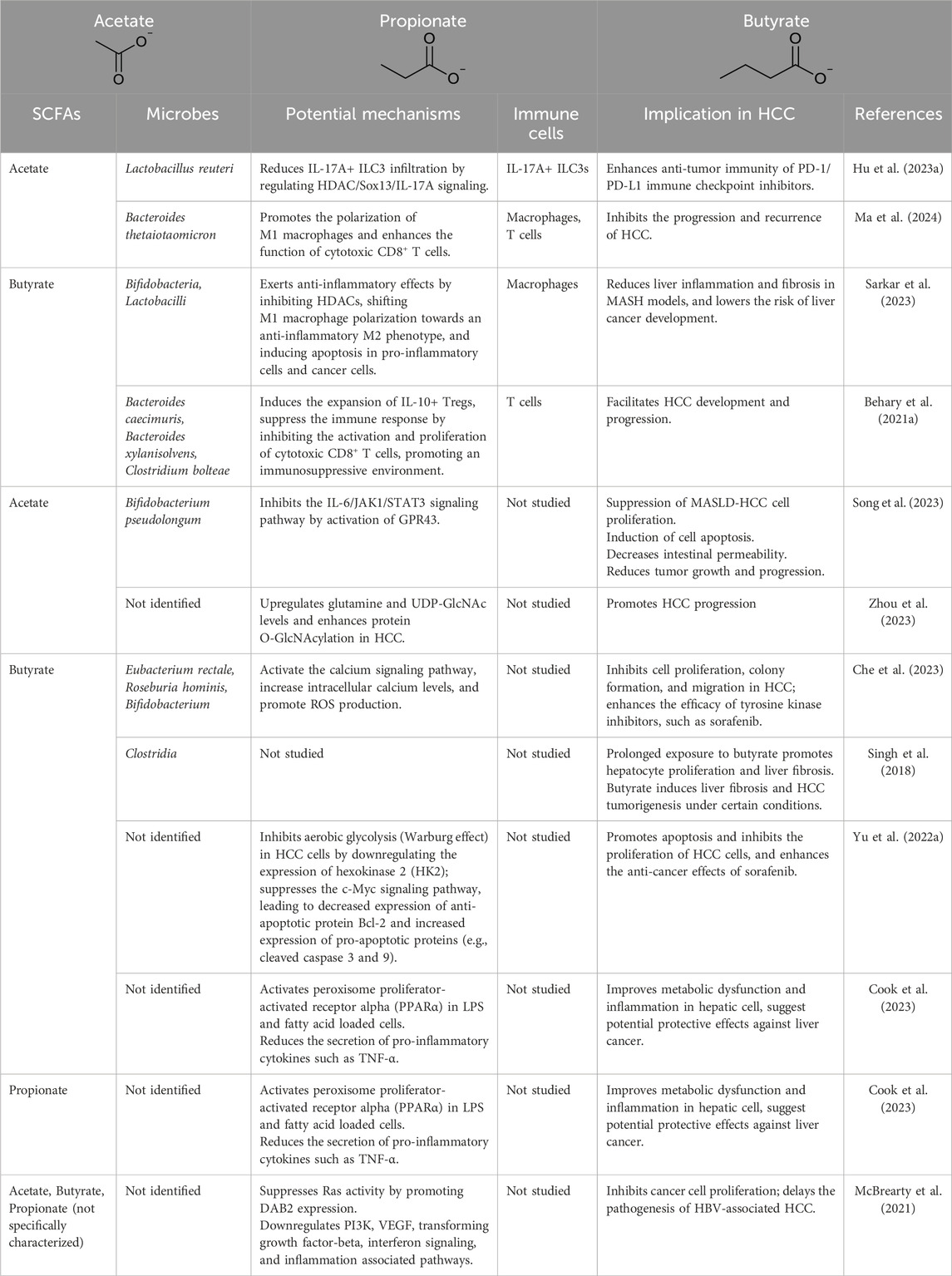
SCFAs also modulate various immune cells, such as macrophages and type 3 innate lymphoid cells (ILC3s), through non-GPCR-dependent mechanisms. Recent studies have highlighted the significant implications of SCFAs in the development and progression of liver cancer (Table 3). For example, in mouse liver cancer models, acetate has been shown to decrease IL-17A levels by increasing Sox13 acetylation via histone deacetylase (HDAC) inhibition, thereby modulating the function of ILC3s and enhancing the efficacy of PD-1/PD-L1 checkpoint inhibition therapy (Hu C. et al., 2023). Similarly, in metabolic dysfunction-associated steatohepatitis (MASH) models, sodium butyrate induces macrophage polarization towards an M2 phenotype through HDAC inhibition, demonstrating anti-inflammatory properties (Sarkar et al., 2023).
Additionally, SCFAs have been implicated in upregulating tumor suppressors such as Disabled homolog 2 (DAB2), inhibiting Ras signaling, and blocking the progression from chronic viral liver disease to HCC (McBrearty et al., 2021). Butyrate’s ability to regulate intracellular calcium homeostasis and reactive oxygen species (ROS) further underscores its potential to enhance the antitumor activity of sorafenib against HCC (Che et al., 2023). The potential detrimental effects of SCFAs in HCC are also noteworthy (Singh et al., 2018; Zhou et al., 2023; Behary et al., 2021a). Long-term intake of large amounts of soluble dietary fiber (such as inulin and fructooligosaccharides) may lead to the production of excessive SCFAs via gut microbiota fermentation. In certain cases, this has been shown to induce cholestasis, liver inflammation, and liver cancer in mice (Singh et al., 2018; Zhou et al., 2023; Behary et al., 2021a). This indicates that under specific conditions, the overproduction of SCFAs may have negative impacts on liver health.
2.4 Other components
In addition to the aforementioned substances, recent studies have explored the effects of other bacterial components or metabolites on liver cancer progression. Lipoteichoic acid, a major component of the cell wall of Gram-positive bacteria, can accumulate in the liver due to obesity. In obese-induced HCC mouse models and MASH-related HCC patients, lipoteichoic acid accumulation activates caspase-11 to cleave gasdermin D, which in turn facilitates the release of IL-33 and IL-1β from stellate cells, thereby promoting cancer progression (Yamagishi et al., 2022).
Specific gut bacteria, such as Enterococcus faecium, enhance the proportion of IFN-γ+CD8+ T cells in the tumor microenvironment by inducing IL-12 and IFN-γ secretion. The exopolysaccharides produced by E. faecium further promote IFN-γ secretion by these T cells, synergistically inducing ferroptosis in HCC cells with sorafenib and enhancing its therapeutic efficacy in advanced HCC (Yu et al., 2024). In HCC patients, the microbiota’s functional profile shifts from carbohydrate metabolism to amino acid metabolism (Behary et al., 2021b). Compared to healthy controls, concentrations of trimethylamine-related metabolites are elevated, along with increases in p-cresol glucuronide, indole-lactic acid, 5-hydroxyindoleacetic acid, and 4-hydroxyphenyllactic acid (Banerjee et al., 2024). Trimethylamine N-oxide, which is a metabolite of trimethylamine produced in the liver, exacerbates chronic hepatic inflammation by inducing vascular endothelial cell damage, increasing M1 macrophages, and reducing M2 macrophages, thereby promoting MASLD progression (Nian et al., 2024; Zhang X. et al., 2023).
Overall, these findings highlight the diverse roles of bacterial components and metabolites in liver cancer progression, emphasizing the need for further research to elucidate their mechanisms and potential therapeutic applications.
3 Designing of therapeutics using the gut-liver axis
3.1 Recent advancements in targeted therapies for gut microbiota modulation
The “core microbiota” in the human intestine comprises genera such as Candida, Saccharomyces, Penicillium, and Aspergillus (Nash et al., 2017). In healthy adults, the gut microbiota is predominantly composed of Bacteroidetes and Firmicutes, with smaller proportions of Proteobacteria, Actinobacteria, and Verrucomicrobia (Mariat et al., 2009). Although bacterial diversity expands rapidly in infancy, it stabilizes in adulthood but continues to evolve due to geographical, nutritional, and lifestyle influences (Cheng et al., 2016).
Recent studies have highlighted alterations in the gut microbiota during various stages of liver cancer development, characterized by a reduction in beneficial bacteria and an increase in potentially harmful bacteria, leading to dysbiosis. For instance, in elderly HCC patients aged 60–80 years, researchers observed significant decreases in beneficial genera such as Blautia, Fusicatenibacter, Anaerostipes, and others, while harmful genera like Escherichia-Shigella, Cronobacter, and Megasphaera were significantly more abundant (Zhang W. et al., 2023). Similar trends were seen in patients with alcoholic liver diseases and HBV-related HCC, where specific bacterial taxa were either depleted or enriched (Lu et al., 2016; Temraz et al., 2021; Ganesan et al., 2024; Huang et al., 2020).
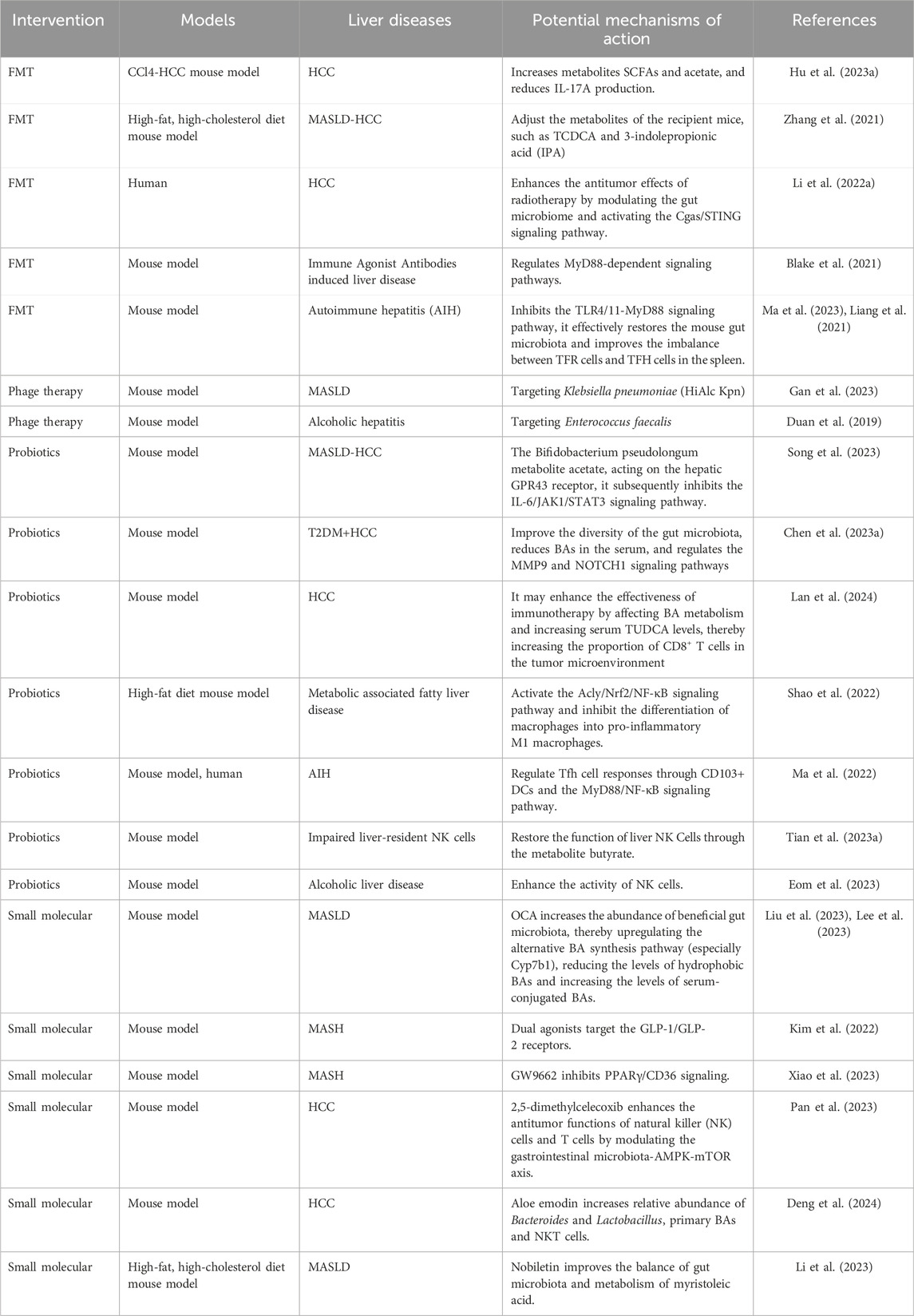
These alterations in gut microbiota and their metabolites play crucial roles in regulating the initiation and progression of liver diseases. They also influence the efficacy and toxicity of cancer therapies through various mechanisms. Given the significant impact of the gut microbiota on disease outcomes, recent advancements in targeted therapies for gut microbiota modulation have shown promise. Current methods include FMT, phage therapy, antibiotics, probiotics, dietary interventions, and small molecules (Woo et al., 2023). These therapies can regulate host inflammation and immune status, thereby affecting cancer treatment outcomes. This summary outlines examples of the latest research progress on study of therapies for the treatment of HCC and other liver diseases (Table 4).
3.1.1 Fecal microbiota transplantation (FMT)
Despite the underlying mechanisms are not fully elucidated, FMT has been shown to restore gut microbiota balance, thereby influencing the tumor immune microenvironment and enhancing antitumor treatment effects (Qin et al., 2022), particularly by enhancing the antitumor effects of immune checkpoint inhibitors.
In addition to its role in cancer therapy, FMT has shown preliminary efficacy in treating MASLD, MASH, and cirrhosis by restoring intestinal barrier function and re-regulating dysbiosis. For example, a recent study in an experimental autoimmune hepatitis (AIH) mouse model found that gut microbiota dysbiosis exacerbated liver damage and imbalanced Tfr/Tfh cells (Ma et al., 2023; Liang et al., 2021). FMT effectively restored gut microbiota dysbiosis caused by antibiotic treatment in AIH mice by inhibiting the TLR4/MyD88 signaling pathway, improved the imbalance between splenic Tfr and Tfh cells, and thereby controlled hepatitis and liver damage progression.
However, the specific mechanisms by which FMT affects liver cancer development through gut microbiota regulation are still not fully understood. Further preclinical studies are needed to elucidate these mechanisms, and well-designed clinical trials are required to verify the efficacy and safety of FMT in treating liver diseases and enhancing cancer immunotherapy.
3.1.2 Phage therapy
Phage therapy, characterized by its high specificity, can modulate the gut microbiota by eliminating specific pathogenic bacteria, thereby improving gut health. Although current research is still in its early stages, studies have indicated that phage-mediated gut microbiota modulation may help suppress liver cancer progression. For instance, research suggests that high-alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) is associated with MASLD. Targeted phage therapy against HiAlc Kpn can regulate hepatic lipid metabolism and inflammation, reduce the expression of genes related to fatty acid synthesis, and mitigate disease progression (Gan et al., 2023). In patients with alcoholic hepatitis, fecal levels of E. faecalis are significantly higher than in non-drinkers, and the expression levels of hemolytic Enterococcus faecalis are positively correlated with increased mortality. Targeted phage therapy against hemolytic E. faecalis has been shown to effectively ameliorate gut microbiota imbalances in mice with alcoholic hepatitis (Duan et al., 2019). These findings suggest that phage therapy holds promise for treating liver diseases by specifically targeting pathogenic bacteria and restoring microbial balance.
3.1.3 Probiotics
Probiotics, such as Bifidobacterium and Lactobacillus species, enhance immunity by regulating gut microbiota balance. In HCC patients receiving PD-1 monoclonal antibody immunotherapy, Akkermansia muciniphila improves treatment efficacy by modulating BA metabolism and increasing serum TUDCA levels, thereby increasing CD8+ T cells infiltration in the tumor microenvironment (Lan et al., 2024). While Clostridium butyricum alone does not alleviate metabolic-associated fatty liver disease in mice, its combination with soluble dietary fiber significantly increases gut microbiota diversity, interferes with lipid synthesis, inhibits macrophage differentiation towards M1, and exerts anti-inflammatory effects, thereby inhibiting disease progression (Shao et al., 2022). Clinical data analysis shows that Tfh, Tfr, and Treg cell levels, as well as the Tfr-Tfh index, are higher in HCC patients than in those with chronic hepatitis and healthy controls, with a high Tfr-Tfh index significantly associated with HCC recurrence (Wang B. et al., 2020). Lactobacillus enhances the suppressive effect of prednisone on Tfh responses via CD103+ DCs and the MyD88/NF-κB pathway, improving therapeutic outcomes in AIH patients and animal models (Ma et al., 2022). Probiotics, including Phocaeicola dorei, Lactobacillus helveticus, and C. butyricum, can also enhance NK cell activity by improving gut microbiota balance (Tian P. et al., 2023; Eom et al., 2023). Early antibiotic treatment disrupts IL-18/IL-18R signaling in Kupffer cells and hepatocytes, inhibiting the functional maturation of NK cells in the liver, but this can be restored by supplementing with specific butyrate-producing bacteria such as C. butyricum (Tian P. et al., 2023). The acetate produced by Bifidobacterium pseudolongum inhibits the IL-6/JAK1/STAT3 signaling cascade via the GPR43, blocking tumor cell cycle progression and enhancing apoptosis, which may attenuate the progression of MASLD to HCC (Song et al., 2023).
In addition to their immunomodulatory effects, probiotics regulate host metabolic functions. For example, Lactobacillus gasseri LA39 enhances primary BA synthesis in the liver by regulating proteins like CYP27A1 and promotes secondary BA levels in the gut, thereby modulating BA metabolism along the liver-gut axis (Hu J. et al., 2023). Lactobacillus brevis ameliorates the pathological transition from type 2 diabetes to liver cancer in mouse models by modulating serum BA concentrations and regulating matrix metalloproteinase-9 (MMP9) and NOTCH1 signaling pathways (Chen S. et al., 2023). These findings highlight the multifaceted roles of probiotics in modulating gut microbiota, enhancing immune responses, and regulating metabolic functions, thereby offering potential therapeutic benefits for liver diseases and cancer.
3.1.4 Small molecules
Small molecules that target specific receptors and modulate gut microbiota balance have emerged as promising therapeutic agents for liver diseases. For example, dual agonists targeting the GLP-1/GLP-2 receptors significantly improve gut microbiota composition in MASH model mice, enhance intestinal barrier tight junctions, and thus alleviate disease phenotypes (Kim et al., 2022). Similarly, the PPARγ antagonist GW9662 increases beneficial bacteria (e.g., Dubosiella and Lactobacillus) and reduces harmful bacteria (e.g., Helicobacteraceae, Desulfovibriaceae, and Rickenaceae), thereby improving gut microbiota disorders and alleviating MASH progression (Xiao et al., 2023).
Natural product compounds also contribute to the modulation of gut microbiota and the treatment of liver diseases. For instance, 2,5-dimethylcelecoxib, a celecoxib derivative, regulates gut microbiota (e.g., Bacteroides acidifaciens, Odoribacter laneus, and Odoribacter splanchnicus) and activates the AMPK/mTOR signaling pathway in leukocytes. This activation enhances the antitumor capabilities of NK cells and T cells, inhibits immune cell exhaustion, and effectively suppresses liver cancer growth, improving HCC prognosis in mouse models (Pan et al., 2023). Nobiletin, a natural flavonoid compound from citrus fruits, reverses gut microbiota dysbiosis in MASLD mouse models and alleviates MASLD symptoms by improving myristic acid metabolism (Li et al., 2023). Aloe emodin exhibits anti-HCC activity by increasing the relative abundance of Bacteroides and Lactobacillus, promoting BSH accumulation, increasing primary BA content, and triggering IFN-γ production in NKT cells in the liver (Deng et al., 2024).
3.2 Immune-based therapies progress focusing on TLR4 and FXR as representative targets
Immune-based therapies targeting the gut-liver axis hold significant promise for the treatment of HCC. Strategies such as FMT and probiotics have been shown to modulate the gut microbiota and enhance the efficacy of immune checkpoint inhibitors. Additionally, the use of small molecule drugs that target specific immune-regulatory pathways, such as TLR4 and FXR, has shown potential in liver diseases studies (Supplementary Table 1).
TLR4 promotes inflammatory responses and liver fibrosis progression by activating downstream signaling pathways, such as the MyD88/NF-κB axis, and plays a significant role in various liver diseases, including MASLD, hepatic fibrosis, cirrhosis, and HCC (Tang et al., 2023). Inhibiting TLR4 signaling triggered by PAMPs like LPS to reduce inflammation and slow fibrosis development may represent a promising therapeutic opportunity. Several natural products have been identified as TLR4 inhibitors. For example, studies in mouse liver cancer models induced by dimethyl nitrosamine and CCl4 have shown that ginsenoside Rk3 can significantly inhibit LPS-induced TLR4 signaling, reduce inflammatory cytokine expression, and thus alleviate liver fibrosis (Qu et al., 2021). Echinacea purpurea polysaccharide (EPP) and red rice seed coat extract have also been shown to inhibit the growth of mouse liver cancer cells by modulating the TLR4-mediated NF-κB pathway (Jing et al., 2024; Chen Y. et al., 2023). Nimbolide, another plant extract, has been shown in mouse liver cancer models to alleviate gut microbiota imbalance by increasing the relative abundance of Bifidobacterium and Lactobacillus, enhance intestinal barrier integrity by upregulating tight junction-related protein ZO-1 expression, and reduce inflammation by downregulating the TLR4-mediated NF-κB pathway activity, thereby inhibiting tumorigenesis and development (Ram et al., 2022).
BAs exert their effects through various receptors, such as FXR and Takeda G protein-coupled receptor 5 (TGR5), which play crucial roles in modulating liver cancer progression. FXR, a nuclear receptor primarily expressed in the intestines, liver, and other tissues, regulates BAs, glucose, and lipid metabolism. Activation of FXR by BAs can modulate downstream signaling pathways, such as NF-κB, thereby reducing the release of inflammatory cytokines and exerts anti-inflammatory effects. Recent research has explored the potential of FXR agonists in treating liver cancer and related conditions. For example, obeticholic acid (OCA), an FXR agonist, has been shown to ameliorate MASLD symptoms by modulating gut microbiota composition, enhancing the abundance of beneficial intestinal microbes such as A. muciniphila, Bifidobacterium spp., and Bacteroides spp., and regulating BA synthesis and serum levels (Liu et al., 2023; Lee et al., 2023; Gou et al., 2022). Natural products have also been studied for their ability to regulate FXR function. For instance, celastrol, a well-known natural product, has been found in rat liver cancer models to reduce the relative abundance of Bacteroides fragilis, increase glycine binding to BAs, and alleviate HCC by regulating the (FXR/RXRα)/mTOR axis (Zeng et al., 2023).
Recent advancements in research methodologies, such as molecular docking and molecular dynamics simulations, combined with AI-driven screening technologies, hold great potential for revolutionizing the discovery of small molecule drugs targeting receptors like TLR4 and FXR. Researchers have used molecular docking and molecular dynamics simulations to study the tetramer formation of the TLR4/MD2 complex and the key residues involved, which aids in discovering small molecule antagonists that can block TLR4 signaling (Tafazzol and Duan, 2019). Based on structural similarity to OCA, 109 FDA-approved drugs were selected from the PubChem database. Molecular docking combined with GROMACS software for molecular dynamics simulations was employed to evaluate the stability of drug-protein complexes and their ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties, ultimately revealing that Montelukast and Alvimopan are more druggable for further development (Jose et al., 2023). In a recent study, a team from Tsinghua University used AI technology, employing next-generation virtual screening platforms HelixVS and HelixDock, to identify a novel pyrazolo [1,5-a]pyrimidine derivative, TH023, which targets the TLR4-TLR4 homodimerization interface and inhibits TLR4 signaling (Jiang et al., 2024).
4 Multi-omics analysis technique and in vitro models in deciphering the interactions of the gut-liver axis
4.1 Utilization of multi-omics analysis technique to understand and characterize the gut-liver axis
The gut-liver axis represents a complex, bidirectional communication network. With the advancement of multi-omics analysis techniques, such as genomics, proteomics, and metabolomics, gut microbes and their metabolites can be identified with greater precision. This has significantly deepened our understanding of their roles in the pathogenesis of HCC. Multi-omics analysis methods can be applied to the diagnosis, treatment, and prognosis prediction of cancer. By comparing the small molecule metabolic profiles of healthy individuals with those of patients with liver disease, researchers can identify specific metabolic pathways or metabolites associated with disease progression, thereby revealing changes in the metabolic activities of the gut microbiota and their impact on liver diseases. For instance, in several studies, researchers have conducted microbiome and transcriptome analyses on fecal samples, tumor tissues, and adjacent non-tumor tissues from a large number of HBV-associated HCC patients. These studies found that various bacteria, including Bacteroides, Clostridium XIVa, Dialister, Veillonella, and Streptococcus pneumoniae, were more abundant in HCC patients. These bacteria may influence the occurrence, progression, and prognosis of HCC by regulating metabolites such as serum BAs, acetate, glutamate, and arachidonic acid (Huang et al., 2020; Zheng et al., 2023).
Bioinformatic tools can assist in the analysis of omics data. For example, in the study of the gut-liver axis, MetaboAnalyst can be used to process and analyze metabolomics data, including the processing of liquid chromatography-mass spectrometry (LC-MS) data, functional annotation, statistical analysis, and integration of multi-omics data. Differential expression analysis of metabolites using MetaboAnalyst can help researchers identify metabolic biomarkers related to the gut-liver axis and further explore the role of these metabolites in the pathogenesis of liver diseases (Hu et al., 2021).
By integrating bioinformatics methods to conduct in-depth analyses of multi-omics results, we can more comprehensively elucidate the pathogenesis and provide new insights into the mechanisms of HCC (Shi et al., 2023; Cui et al., 2023). In a recent study focusing on the mRNA expression and clinical follow-up information of HCC patients, the TCGA dataset and regression analysis were utilized to identify 18 BAs metabolism-related genes significantly associated with HCC prognosis. A risk model was developed to predict the prognosis of liver cancer patients and their response to immunotherapy (Shi et al., 2023). Long non-coding RNAs (lncRNAs) data have also garnered attention in constructing predictive models for the treatment response and prognosis of HCC patients (Cui et al., 2023).
Multi-omics analysis also provides a more comprehensive understanding of the molecular mechanisms within the gut-liver axis, elucidating how specific microbes contribute to liver diseases. A study conducted in a murine model of Type 2 Diabetes Mellitus (T2DM) and HCC employed 16S rRNA sequencing and gas chromatography-mass spectrometry (GC-MS) to analyze the regulatory effects of gut microbiota, particularly Actinomycetes, as well as microbial metabolites, notably total BAs, following treatment with L. brevis (Chen S. et al., 2023). Furthermore, the research delved deeper through transcriptomic analysis to investigate the impact of the treatment on gene expression, revealing that MMP9 and NOTCH1 signaling pathways may play pivotal roles in disease alleviation.
Alternatively, multi-omics analysis techniques have also made substantial contributions to clarifying the mechanisms through which some traditional Chinese medicines or natural compounds exert their therapeutic effects (Deng et al., 2024; Jing et al., 2024; Chen Y. et al., 2023; Zeng et al., 2023). For example, recent studies have utilized 16S rRNA gene sequencing and untargeted metabolomics to investigate the intervention mechanism of EPP on HCC (Jing et al., 2024). The results indicate that EPP modulates the gut microbiota, particularly by increasing the abundance of gut microorganisms that produce propionate and butyrate (such as Coprococcus, Clostridium, and Roseburia). This enhances the expression of intestinal tight junction proteins, thereby repairing the intestinal barrier, controlling the leakage of LPS, and inhibiting the TLR4/NF-κB signaling pathway. In another study investigating the therapeutic effects of the traditional Chinese medicine “Xiayuxue decoction” (XYXD) on HCC, researchers employed ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) to identify the major active components in XYXD (Deng et al., 2024). Among these, aloe emodin was found to be the most effective. Furthermore, metagenomic and metabolomic analyses revealed that the active components promote the production of BSH by increasing the abundance of Bacteroides and Lactobacillus. BSH, in turn, regulates the metabolism of primary BAs, which triggers the production of IFN-γ by NKT cells in the liver, thereby exerting an immunotherapeutic effect against HCC.
4.2 Utilization of in vitro models to understand and characterize the gut-liver axis
Organoid models, which simulate the three-dimensional structure and function of tissues, offer a more physiologically relevant experimental platform for studying intestinal and liver diseases (Gunther et al., 2022; Shek et al., 2021). For example, liver organoids have been employed to elucidate the pathophysiological mechanisms of liver diseases such MASLD and MASH (He et al., 2023). To better mimic the complex interactions between the intestine and liver, researchers have developed cell-based Caco-2/HepG2 co-culture models to simulate the metabolic processes of sugars in the human digestive system and their impact on liver health (van Laar et al., 2022).
In recent years, advances in microfluidic technology have led to significant progress in developing in vitro models of the gut-liver axis. The Gut-liver chip, also known as Organoids/organs-on-a-chip, represents one of the important breakthroughs in this field. This technology employs microfluidic techniques to construct co-culture organoid models, recapitulating the physiological structure and function of the intestine and liver (Wu et al., 2023; Guo et al., 2023). For instance, Choe et al. used microfluidic technology to co-culture intestinal epithelial cells and liver cells, simulating intestinal absorption and liver metabolism processes and reproducing first-pass metabolism dynamics (Choe et al., 2017). The Gut-liver chip also allows researchers to investigate how microbial metabolites affect hepatocyte functions, including BA synthesis, glucose and lipid metabolism (Choe et al., 2017), and the secretion of albumin and urea (Kang et al., 2023). Additionally, this technology can be utilized to study the interactions of the gut-liver axis under specific disease conditions, such as fatty liver disease and acute liver failure (Yang J. et al., 2023; Liu et al., 2024).
However, despite the great potential of these new in vitro models in gut-liver axis research, several challenges remain. For example, accurately simulating the complex intercellular signaling and microenvironment remains a critical issue (Kang et al., 2023). Compared to organoids derived from healthy tissues, those from HCC and cholangiocarcinoma exhibit downregulated expression of pattern recognition receptors such as TLRs, which is inconsistent with in vivo observations (Shek et al., 2021). Therefore, there is a need to develop in vitro models that more closely reflect in vivo characteristics for drug screening and therapeutic applications.
4.3 Application of chemical labeling and analytical techniques used in characterize the gut-liver axis
The gut microbiota and its metabolites play a crucial role in the gut-liver axis, highlighting the urgent need for comprehensive and in-depth studies on their composition and distribution. The application of novel chemical labeling and analytical techniques provides strong support for this research endeavor.
Chen et al. employed fluorescence labeling to observe the encapsulation and release of probiotics in the gut (Chen QW. et al., 2022), thereby aiding in the optimization of probiotic therapies. They labeled different probiotics with fluorescein coumarin and Cy5, respectively, and examined the distribution of these labeled probiotics within microspheres using fluorescence microscopy. This approach confirmed successful encapsulation and simulated their release in a gut environment. In recent years, a series of D-amino acid (DAA) based chemical probes have been developed to study the metabolism and activity of gut microbiota (Lin et al., 2020a; Lin et al., 2020b; Chen D. et al., 2023; Wang W. et al., 2020) (Figure 4). These probes, constructed by linking fluorescent groups, isotopes, and near-infrared (NIR-II) groups to DAAs, are incorporated into peptidoglycan structures during bacterial metabolism. This enables non-invasive and non-perturbative bacterial labeling and allows for the visualization of metabolic activities, such as the growth and division of different gut bacteria, in vivo.
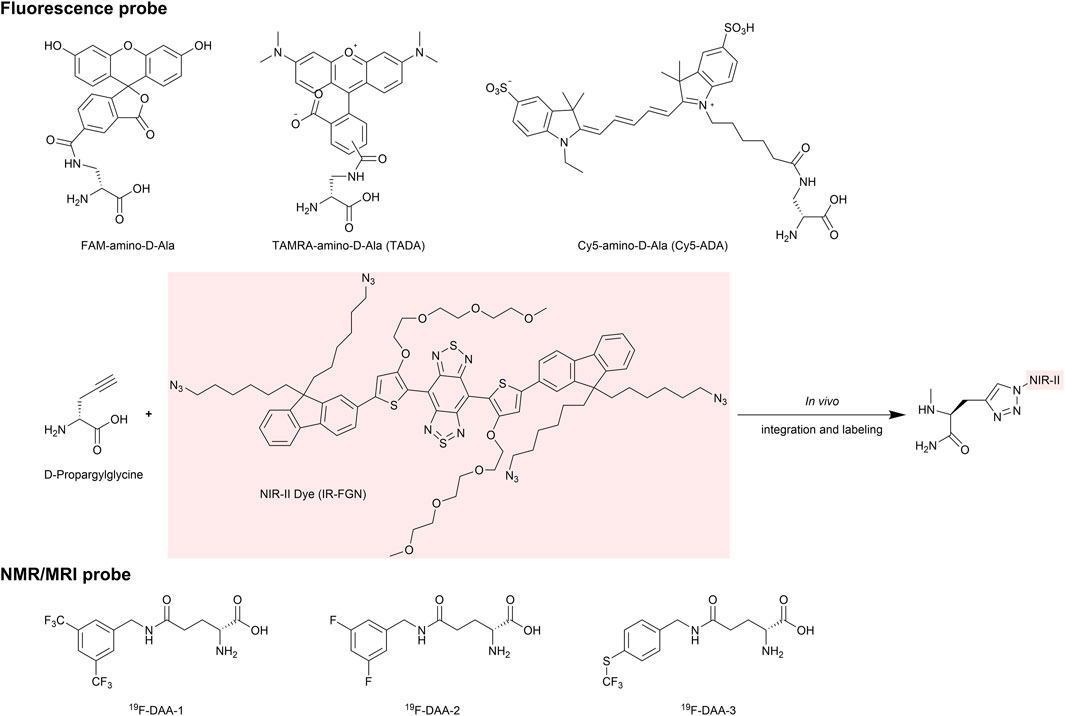
Figure 4. Representative D-amino acid (DAA) based chemical probes. The chemical structures of probes used for in vivo imaging are shown, including D-Ala probes labeled with fluorophores such as FAM, TAMRA, and Cy5. Additionally, the D-Propargylglycine probe is designed for in vivo labeling with NIR-II dyes via an azide group. By replacing the fluorescent label with a 19F modification, the probe can be used for higher sensitivity and deeper penetration in NMR imaging.
Chemical labeling techniques, such as isotope labeling, can enhance the sensitivity of metabolomics analysis (Yuan et al., 2018; Kaur et al., 2023; Lin et al., 2021; Garg et al., 2018; Conway et al., 2019; Lin et al., 2023). In a recent study, researchers developed a method for analyzing fecal metabolites through integrated chemical isotope labeling combined with liquid chromatography-mass spectrometry (CIL-LC-MS). Different chemical isotope labeling reagents were used to label metabolites containing carboxyl, carbonyl, amino, and thiol groups, respectively. Using specific mass spectrometry methods, a systematic analysis of the metabolites in mouse feces was conducted, revealing significant differences in 211 metabolites between model mice and wild-type (WT) mice (Yuan et al., 2018).
The use of specific metabolite-binding probes for the separation and analysis of analytes can also help enhance analytical sensitivity. For instance, bicyclobutane, serving as a selective probe, is conjugated to magnetic beads via diethylenetriamine (Kaur et al., 2023). This probe-magnetic bead system labels and separates thiol metabolites, improving the sensitivity and specificity of thiol metabolite detection by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Lin et al. also employed a novel selective probe for the absolute quantification of SCFAs at low nanomolar concentrations (Lin et al., 2023). The probe consists of a magnetic bead substrate, a selective reaction group (N-methylbenzylamine), and a bioorthogonal cleavable group (p-nitrocinnamyloxy-carbonyl, Noc). SCFAs react with the selective reactive group through a nucleophilic addition-elimination mechanism to form stable covalent bonds, allowing for their separation. Coupled with a13C6 isotope-labeled internal standard of SCFAs and UHPLC-MS, this method achieves high-sensitivity, high-reproducibility quantitative analysis of SCFAs. The limit of detection (LOD) and limit of quantification (LOQ) of the assay were very low, 1 nM and 10 nM, respectively.
Chemical labeling techniques can also be employed to enhance the capabilities of proteomic analysis (Han et al., 2025). Gut microbes express a variety of BSH, which regulate the composition of the host’s BA pool. Researchers designed activity-based probes based on the cores of different BAs, such as CA, CDCA, DCA, and LCA. These probes incorporate a fluoromethylketone (FMK) warhead to covalently label the active site of BSH and an azide group as a click chemistry handle for subsequent detection and analysis. This enables the identification and assessment of BSH substrate specificity towards various BA substrates. The probes enrich and identify BSHs with different substrate specificities through Cu-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry. This approach provides a new perspective for developing precise microbial community intervention strategies or novel drugs to regulate BA metabolism.
5 Summary and prospects
The gut-liver axis is a complex, bidirectional communication network involving chemical and biological interactions. This review summarizes the intricate relationship between the gut-liver axis and HCC, highlighting the significant roles of gut microbiota and its derived molecules, such as LPS, BAs, and SCFAs, in disease progression and treatment.
Understanding the compositional, chemical, and immunological changes underlying the gut-liver axis is crucial, as these alterations can serve as potential diagnostic biomarkers and therapeutic targets. The application of multi-omics analysis techniques, chemical labeling and analytical technologies, coupled with bioinformatics tools and in vitro models, has greatly enhanced our understanding of the molecular mechanisms of the gut-liver axis in HCC and facilitated the discovery of potential biomarkers. These novel research findings bring hope to therapeutic strategies targeting liver diseases via the gut-liver axis. Approaches such as FMT, phage therapy, probiotics, and small molecule drugs aim to restore the balance of the gut microbiota, enhance immune responses, and mitigate the deleterious effects of dysregulated microbial metabolites. Natural products or small molecule drugs identified through high-throughput screening and chemical structural optimization have also shown potential in reducing inflammation, fibrosis, and tumor growth by interfering with key signaling pathways involved in HCC development.
In conclusion, the gut-liver axis represents a promising research and therapeutic avenue for HCC. However, the complex interaction mechanisms among the gut microbiota, host immune system, and hepatocytes are not yet fully elucidated and require further investigation. Existing studies mostly rely on animal models, which may not fully reflect the complexity of human diseases. Future research should focus on elucidating the complex interactions among the gut microbiota, microbial metabolites, immune system, and liver diseases, with a particular emphasis on identifying novel immunotherapeutic targets. Given individual differences, more targeted intervention measures, such as biological therapies and small molecule therapies targeting specific bacteria or metabolic pathways, should be formulated. Additionally, larger-scale clinical trials are essential to validate the efficacy and safety of therapies targeting the gut-liver axis and to explore potential synergistic effects when combined with existing HCC treatments. The continuous investigation of the chemical interactions and biological effects of gut microbiota-derived components will undoubtedly provide valuable insights and lay the foundation for the development of innovative therapeutic strategies to combat liver cancer.
Author contributions
YH: Conceptualization, Visualization, Writing – original draft, Writing – review and editing. MG: Visualization, Writing – original draft. JC: Visualization, Writing – original draft. RB: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Number 32370187).
Acknowledgments
We are very grateful to Qian Sun for providing the idea for this study and for collecting and organizing some of the materials.
Conflict of interest
Yongjian Hu was employed by HitGen Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1595853/full#supplementary-material
References
Albillos, A., de Gottardi, A., and Rescigno, M. (2020). The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72 (3), 558–577. doi:10.1016/j.jhep.2019.10.003
Anfuso, B., Tiribelli, C., Adorini, L., and Rosso, N. (2020). Obeticholic acid and Int-767 modulate collagen deposition in a Nash in vitro model. Sci. Rep. 10 (1), 1699. doi:10.1038/s41598-020-58562-x
Arab, J. P., Martin-Mateos, R. M., and Shah, V. H. (2018). Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol. Int. 12 (Suppl. 1), 24–33. doi:10.1007/s12072-017-9798-x
Asgharpour, A., Kumar, D., and Sanyal, A. (2015). Bile acids: emerging role in management of liver diseases. Hepatol. Int. 9 (4), 527–533. doi:10.1007/s12072-015-9656-7
Banerjee, R., Wehrle, C. J., Wang, Z., Wilcox, J. D., Uppin, V., Varadharajan, V., et al. (2024). Circulating gut microbe-derived metabolites are associated with hepatocellular carcinoma. Biomedicines 12 (9), 1946. doi:10.3390/biomedicines12091946
Behary, J., Amorim, N., Jiang, X. T., Raposo, A., Gong, L., McGovern, E., et al. (2021a). Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 12 (1), 187. doi:10.1038/s41467-020-20422-7
Behary, J., Raposo, A. E., Amorim, N. M. L., Zheng, H., Gong, L., McGovern, E., et al. (2021b). Defining the temporal evolution of gut dysbiosis and inflammatory responses leading to hepatocellular carcinoma in Mdr2 −/− mouse model. BMC Microbiol. 21 (1), 113. doi:10.1186/s12866-021-02171-9
Bi, C., Xiao, G., Liu, C., Yan, J., Chen, J., Si, W., et al. (2021). Molecular immune mechanism of intestinal microbiota and their metabolites in the occurrence and development of liver cancer. Front. Cell Dev. Biol. 9, 702414. doi:10.3389/fcell.2021.702414
Blake, S. J., James, J., Ryan, F. J., Caparros-Martin, J., Eden, G. L., Tee, Y. C., et al. (2021). The immunotoxicity, but not anti-tumor efficacy, of anti-Cd40 and anti-Cd137 immunotherapies is dependent on the gut microbiota. Cell Rep. Med. 2 (12), 100464. doi:10.1016/j.xcrm.2021.100464
Buckley, A., and Turner, J. R. (2018). Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 10 (1), a029314. doi:10.1101/cshperspect.a029314
Cai, Z., Wang, B., Zhou, Z., Zhao, X., Hu, L., Ren, Q., et al. (2022). Discovery of a novel and orally active farnesoid X receptor agonist for the protection of acetaminophen-induced hepatotoxicity. Chem. Biol. Drug Des. 99 (3), 483–495. doi:10.1111/cbdd.14014
Canovai, E., Farre, R., Accarie, A., Lauriola, M., De Hertogh, G., Vanuytsel, T., et al. (2023). Int-767-a dual farnesoid-X receptor (Fxr) and Takeda G protein-coupled receptor-5 (Tgr5) agonist improves survival in rats and attenuates intestinal ischemia reperfusion injury. Int. J. Mol. Sci. 24 (19), 14881. doi:10.3390/ijms241914881
Cao, S., Yang, X., Zhang, Z., Wu, J., Chi, B., Chen, H., et al. (2022). Discovery of a tricyclic farnesoid X receptor agonist Hec96719, a clinical candidate for treatment of non-alcoholic steatohepatitis. Eur. J. Med. Chem. 230, 114089. doi:10.1016/j.ejmech.2021.114089
Che, Y., Chen, G., Guo, Q., Duan, Y., Feng, H., and Xia, Q. (2023). Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology 78 (1), 88–102. doi:10.1097/HEP.0000000000000047
Chen, D., Guo, J., Li, A., Sun, C., Lin, H., Lin, H., et al. (2023c). Metabolic fluorine labeling and hotspot imaging of dynamic gut microbiota in mice. Sci. Adv. 9 (4), eabg6808. doi:10.1126/sciadv.abg6808
Chen, Q. W., Li, Q. R., Cao, M. W., Yan, J. H., and Zhang, X. Z. (2022b). Hierarchy-assembled dual probiotics system ameliorates cholestatic drug-induced liver injury via gut-liver axis modulation. Adv. Sci. (Weinh) 9 (17), e2200986. doi:10.1002/advs.202200986
Chen, S., Han, P., Zhang, Q., Liu, P., Liu, J., Zhao, L., et al. (2023a). Lactobacillus brevis alleviates the progress of hepatocellular carcinoma and type 2 diabetes in mice model via interplay of gut microflora, bile acid and Notch 1 signaling. Front. Immunol. 14, 1179014. doi:10.3389/fimmu.2023.1179014
Chen, X., Yu, C., Liu, X., Liu, B., Wu, X., Wu, J., et al. (2022a). Intracellular galectin-3 is a lipopolysaccharide sensor that promotes glycolysis through Mtorc1 activation. Nat. Commun. 13 (1), 7578. doi:10.1038/s41467-022-35334-x
Chen, Y., Zhao, Z., Guo, S., Li, Y., Yin, H., Tian, L., et al. (2023b). Red rice seed coat targeting Sphk2 ameliorated alcoholic liver disease via restored intestinal barrier and improved gut microbiota in mice. Nutrients 15 (19), 4176. doi:10.3390/nu15194176
Cheng, J., Ringel-Kulka, T., Heikamp-de Jong, I., Ringel, Y., Carroll, I., de Vos, W. M., et al. (2016). Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 10 (4), 1002–1014. doi:10.1038/ismej.2015.177
Chianelli, D., Rucker, P. V., Roland, J., Tully, D. C., Nelson, J., Liu, X., et al. (2020). Nidufexor (Lmb763), a novel Fxr modulator for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 63 (8), 3868–3880. doi:10.1021/acs.jmedchem.9b01621
Choe, A., Ha, S. K., Choi, I., Choi, N., and Sung, J. H. (2017). Microfluidic gut-liver chip for reproducing the first pass metabolism. Biomed. Microdev. 19 (1), 4. doi:10.1007/s10544-016-0143-2
Choudhuri, S., and Klaassen, C. D. (2022). Molecular regulation of bile acid homeostasis. Drug Metab. Dispos. 50 (4), 425–455. doi:10.1124/dmd.121.000643
Conway, L. P., Garg, N., Lin, W., Vujasinovic, M., Lohr, J. M., and Globisch, D. (2019). Chemoselective probe for detailed analysis of ketones and aldehydes produced by gut microbiota in human samples. Chem. Commun. (Camb) 55 (62), 9080–9083. doi:10.1039/c9cc04605d
Cook, K. J., Coulter, A., Keenan, M., Greenway, F., and Losso, J. N. (2023). Sodium propionate or sodium butyrate promotes fatty acid oxidation in Hepg2 cells under oxidative stress. J. Med. Food 26 (1), 74–79. doi:10.1089/jmf.2021.0120
Cording, S., Wahl, B., Kulkarni, D., Chopra, H., Pezoldt, J., Buettner, M., et al. (2014). The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 7 (2), 359–368. doi:10.1038/mi.2013.54
Cui, H., Lian, J., Xu, B., Yu, Z., Xiang, H., Shi, J., et al. (2023). Identification of a bile acid and bile salt metabolism-related Lncrna signature for predicting prognosis and treatment response in hepatocellular carcinoma. Sci. Rep. 13 (1), 19512. doi:10.1038/s41598-023-46805-6
Dai, M., Peng, W., Lin, L., Wu, Z. E., Zhang, T., Zhao, Q., et al. (2023). Celastrol as an intestinal fxr inhibitor triggers tripolide-induced intestinal bleeding: underlying mechanism of gastrointestinal injury induced by Tripterygium Wilfordii. Phytomedicine 121, 155054. doi:10.1016/j.phymed.2023.155054
Dapito, D. H., Mencin, A., Gwak, G. Y., Pradere, J. P., Jang, M. K., Mederacke, I., et al. (2012). Promotion of hepatocellular carcinoma by the intestinal microbiota and Tlr4. Cancer Cell 21 (4), 504–516. doi:10.1016/j.ccr.2012.02.007
Deng, Z., Ouyang, Z., Mei, S., Zhang, X., Li, Q., Meng, F., et al. (2024). Enhancing Nkt cell-mediated immunity against hepatocellular carcinoma: role of Xyxd in promoting primary bile acid synthesis and improving gut microbiota. J. Ethnopharmacol. 318 (Pt B), 116945. doi:10.1016/j.jep.2023.116945
Di Ciaula, A., Garruti, G., Lunardi Baccetto, R., Molina-Molina, E., Bonfrate, L., Wang, D. Q., et al. (2017). Bile acid physiology. Ann. Hepatol. 16 (Suppl. 1), s4–s14. doi:10.5604/01.3001.0010.5493
Di Lorenzo, F., Pither, M. D., Martufi, M., Scarinci, I., Guzman-Caldentey, J., Lakomiec, E., et al. (2020). Pairing bacteroides vulgatus Lps structure with its immunomodulatory effects on human cellular models. ACS Cent. Sci. 6 (9), 1602–1616. doi:10.1021/acscentsci.0c00791
Doden, H. L., Wolf, P. G., Gaskins, H. R., Anantharaman, K., Alves, J. M. P., and Ridlon, J. M. (2021). Completion of the gut microbial Epi-Bile acid pathway. Gut Microbes 13 (1), 1–20. doi:10.1080/19490976.2021.1907271
Duan, Y., Llorente, C., Lang, S., Brandl, K., Chu, H., Jiang, L., et al. (2019). Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 575 (7783), 505–511. doi:10.1038/s41586-019-1742-x
Elpek, G. O. (2014). Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J. Gastroenterol. 20 (23), 7260–7276. doi:10.3748/wjg.v20.i23.7260
Eom, J. A., Jeong, J. J., Han, S. H., Kwon, G. H., Lee, K. J., Gupta, H., et al. (2023). Gut-microbiota prompt activation of natural killer cell on alcoholic liver disease. Gut Microbes 15 (2), 2281014. doi:10.1080/19490976.2023.2281014
Espinoza-Culupu, A., Vazquez-Ramirez, R., Farfan-Lopez, M., Mendes, E., Notomi Sato, M., da Silva Junior, P. I., et al. (2020). Acylpolyamine mygalin as a Tlr4 antagonist based on molecular docking and in vitro analyses. Biomolecules 10 (12), 1624. doi:10.3390/biom10121624
Esterhazy, D., Canesso, M. C. C., Mesin, L., Muller, P. A., de Castro, T. B. R., Lockhart, A., et al. (2019). Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569 (7754), 126–130. doi:10.1038/s41586-019-1125-3
Farias-Pereira, R., Kim, E., and Park, Y. (2020). Cafestol increases fat oxidation and energy expenditure in Caenorhabditis elegans via Daf-12-dependent pathway. Food Chem. 307, 125537. doi:10.1016/j.foodchem.2019.125537
Feng, Z., Zhou, P., Wu, X., Zhang, J., and Zhang, M. (2022). Hydroxysafflor yellow A protects against ulcerative colitis via suppressing TLR4/NF-κB signaling pathway. Chem. Biol. Drug Des. 99 (6), 897–907. doi:10.1111/cbdd.14045
Finamore, C., Festa, C., Fiorillo, B., Leva, F. S. D., Roselli, R., Marchiano, S., et al. (2023). Expanding the library of 1,2,4-oxadiazole derivatives: discovery of new farnesoid X receptor (fxr) antagonists/pregnane X receptor (Pxr) agonists. Molecules 28 (6), 2840. doi:10.3390/molecules28062840
Fiorucci, S., Carino, A., Baldoni, M., Santucci, L., Costanzi, E., Graziosi, L., et al. (2021). Bile acid signaling in inflammatory bowel diseases. Dig. Dis. Sci. 66 (3), 674–693. doi:10.1007/s10620-020-06715-3
Fuchs, C. D., Simbrunner, B., Baumgartner, M., Campbell, C., Reiberger, T., and Trauner, M. (2025). Bile acid metabolism and signalling in liver disease. J. Hepatol. 82 (1), 134–153. doi:10.1016/j.jhep.2024.09.032
Gan, L., Feng, Y., Du, B., Fu, H., Tian, Z., Xue, G., et al. (2023). Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae. Nat. Commun. 14 (1), 3215. doi:10.1038/s41467-023-39028-w
Ganesan, R., Gupta, H., Jeong, J. J., Sharma, S. P., Won, S. M., Oh, K. K., et al. (2024). Characteristics of microbiome-derived metabolomics according to the progression of alcoholic liver disease. Hepatol. Int. 18 (2), 486–499. doi:10.1007/s12072-023-10518-9
Gao, L., Lv, G., Li, R., Liu, W. T., Zong, C., Ye, F., et al. (2019). Glycochenodeoxycholate promotes hepatocellular carcinoma invasion and migration by Ampk/Mtor dependent autophagy activation. Cancer Lett. 454, 215–223. doi:10.1016/j.canlet.2019.04.009
Garcia-Vello, P., Di Lorenzo, F., Lamprinaki, D., Notaro, A., Speciale, I., Molinaro, A., et al. (2021). Structure of the O-antigen and the lipid a from the lipopolysaccharide of Fusobacterium nucleatum Atcc 51191. Chembiochem 22 (7), 1252–1260. doi:10.1002/cbic.202000751
Garcia-Vello, P., Tytgat, H. L. P., Elzinga, J., Van Hul, M., Plovier, H., Tiemblo-Martin, M., et al. (2024). The lipooligosaccharide of the gut symbiont Akkermansia muciniphila exhibits a remarkable structure and Tlr signaling capacity. Nat. Commun. 15 (1), 8411. doi:10.1038/s41467-024-52683-x
Garg, N., Conway, L. P., Ballet, C., Correia, M. S. P., Olsson, F. K. S., Vujasinovic, M., et al. (2018). Chemoselective probe containing a unique bioorthogonal cleavage site for investigation of gut microbiota metabolism. Angew. Chem. Int. Ed. Engl. 57 (42), 13805–13809. doi:10.1002/anie.201804828
Gola, A., Dorrington, M. G., Speranza, E., Sala, C., Shih, R. M., Radtke, A. J., et al. (2021). Commensal-driven immune zonation of the liver promotes host defence. Nature 589 (7840), 131–136. doi:10.1038/s41586-020-2977-2
Gou, H., Liu, S., Liu, L., Luo, M., Qin, S., He, K., et al. (2022). Obeticholic acid and 5β-cholanic acid 3 exhibit anti-tumor effects on liver cancer through CXCL16/CXCR6 pathway. Front. Immunol. 13, 1095915. doi:10.3389/fimmu.2022.1095915
Grant, S. M., and DeMorrow, S. (2020). Bile acid signaling in neurodegenerative and neurological disorders. Int. J. Mol. Sci. 21 (17), 5982. doi:10.3390/ijms21175982
Guillotte, M. L., Chandler, C. E., Verhoeve, V. I., Gillespie, J. J., Driscoll, T. P., Rahman, M. S., et al. (2021). Lipid a structural divergence in Rickettsia pathogens. mSphere 6 (3), 001844-e221. doi:10.1128/mSphere.00184-21
Gunther, C., Winner, B., Neurath, M. F., and Stappenbeck, T. S. (2022). Organoids in gastrointestinal diseases: from experimental models to clinical translation. Gut 71 (9), 1892–1908. doi:10.1136/gutjnl-2021-326560
Guo, F., Gao, Y., Li, X., and Lei, X. (2022). Natural product 2-oxokolavenol is a novel Fxr agonist. Molecules 27 (24), 8968. doi:10.3390/molecules27248968
Guo, Y., Chen, X., Gong, P., Li, G., Yao, W., and Yang, W. (2023). The gut-organ-axis concept: advances the application of gut-on-chip technology. Int. J. Mol. Sci. 24 (4), 4089. doi:10.3390/ijms24044089
Han, L., Pendleton, A., Singh, A., Xu, R., Scott, S. A., Palma, J. A., et al. (2025). Chemoproteomic profiling of substrate specificity in gut microbiota-associated bile salt hydrolases. Cell Chem. Biol. 32 (1), 145–156.e9. doi:10.1016/j.chembiol.2024.05.009
He, C., Lu, D., Lin, Z., Chen, H., Li, H., Yang, X., et al. (2023). Liver organoids, novel and promising modalities for exploring and repairing liver injury. Stem Cell Rev. Rep. 19 (2), 345–357. doi:10.1007/s12015-022-10456-3
He, M., Zhang, W., Dong, Y., Wang, L., Fang, T., Tang, W., et al. (2017). Pro-inflammation NF-κB signaling triggers a positive feedback via enhancing cholesterol accumulation in liver cancer cells. J. Exp. Clin. Cancer Res. 36 (1), 15. doi:10.1186/s13046-017-0490-8
Hollenback, D., Hambruch, E., Fink, G., Birkel, M., Schulz, A., Hornberger, M., et al. (2024). Development of cilofexor, an intestinally-biased farnesoid X receptor agonist, for the treatment of fatty liver disease. J. Pharmacol. Exp. Ther. 389 (1), 61–75. doi:10.1124/jpet.123.001900
Holochova, P., Maslanova, I., Sedlacek, I., Svec, P., Kralova, S., Kovarovic, V., et al. (2020). Description of Massilia rubra sp. nov., Massilia aquatica sp. nov., Massilia mucilaginosa sp. nov., Massilia frigida sp. nov., and one Massilia genomospecies isolated from antarctic streams, lakes and regoliths. Syst. Appl. Microbiol. 43 (5), 126112. doi:10.1016/j.syapm.2020.126112
Hu, C., Wang, T., Zhuang, X., Sun, Q., Wang, X., Lin, H., et al. (2021). Metabolic analysis of early nonalcoholic fatty liver disease in humans using liquid chromatography-mass spectrometry. J. Transl. Med. 19 (1), 152. doi:10.1186/s12967-021-02820-7
Hu, C., Xu, B., Wang, X., Wan, W. H., Lu, J., Kong, D., et al. (2023a). Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in hcc. Hepatology 77 (1), 48–64. doi:10.1002/hep.32449
Hu, J., Hou, Q., Zheng, W., Yang, T., and Yan, X. (2023b). Lactobacillus gasseri La39 promotes hepatic primary bile acid biosynthesis and intestinal secondary bile acid biotransformation. J. Zhejiang Univ. Sci. B 24 (8), 734–748. doi:10.1631/jzus.B2200439
Huang, D., Ji, F., Tan, X., Qiao, J., Li, H., Wang, Z., et al. (2023). Free lipid a and full-length lipopolysaccharide coexist in vibrio parahaemolyticus Atcc33846. Microb. Pathog. 174, 105889. doi:10.1016/j.micpath.2022.105889
Huang, H., Ren, Z., Gao, X., Hu, X., Zhou, Y., Jiang, J., et al. (2020). Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in hbv-related hepatocellular carcinoma. Genome Med. 12 (1), 102. doi:10.1186/s13073-020-00796-5
Huang, W., Cao, Z., Wang, W., Yang, Z., Jiao, S., Chen, Y., et al. (2024). Discovery of Lh10, a novel fexaramine-based Fxr agonist for the treatment of liver disease. Bioorg Chem. 143, 107071. doi:10.1016/j.bioorg.2023.107071
Huo, X., Li, D., Wu, F., Li, S., Qiao, Y., Wang, C., et al. (2022). Cultivated human intestinal fungus Candida metapsilosis M2006b attenuates colitis by secreting acyclic sesquiterpenoids as Fxr agonists. Gut 71 (11), 2205–2217. doi:10.1136/gutjnl-2021-325413
Jiang, J., Ma, Y., Liu, Y., Lu, D., Gao, X., Krausz, K. W., et al. (2022). Glycine-beta-muricholic acid antagonizes the intestinal farnesoid X receptor-ceramide axis and ameliorates nash in mice. Hepatol. Commun. 6 (12), 3363–3378. doi:10.1002/hep4.2099
Jiang, Y. Y., Yan, S. T., Zhang, S. Z., Wang, M., Diao, W. M., Li, J., et al. (2024). Discovery of pyrazolo[1,5-a]Pyrimidine derivatives targeting Tlr4-Tlr4 * homodimerization via Ai-powered next-generation screening. Eur. J. Med. Chem. 280, 116945. doi:10.1016/j.ejmech.2024.116945
Jing, G., Xu, W., Ma, W., Yu, Q., Zhu, H., Liu, C., et al. (2024). Echinacea purpurea polysaccharide intervene in hepatocellular carcinoma via modulation of gut microbiota to inhibit TLR4/NF-κB pathway. Int. J. Biol. Macromol. 261 (Pt 2), 129917. doi:10.1016/j.ijbiomac.2024.129917
Jose, S., Devi, S. S., Sajeev, A., Girisa, S., Alqahtani, M. S., Abbas, M., et al. (2023). Repurposing Fda-approved drugs as fxr agonists: a structure based in silico pharmacological study. Biosci. Rep. 43 (3). doi:10.1042/BSR20212791
Kang, S. G., Choi, Y. Y., Mo, S. J., Kim, T. H., Ha, J. H., Hong, D. K., et al. (2023). Effect of gut microbiome-derived metabolites and extracellular vesicles on hepatocyte functions in a gut-liver axis chip. Nano Converg. 10 (1), 5. doi:10.1186/s40580-022-00350-6
Kaur, A., Lin, W., Dovhalyuk, V., Driutti, L., Di Martino, M. L., Vujasinovic, M., et al. (2023). Chemoselective bicyclobutane-based mass spectrometric detection of biological thiols uncovers human and bacterial metabolites. Chem. Sci. 14 (20), 5291–5301. doi:10.1039/d3sc00224a
Khedr, L. H., Rahmo, R. M., Farag, D. B., Schaalan, M. F., and El Magdoub, H. M. (2020). Crocin attenuates cisplatin-induced hepatotoxicity via TLR4/NF-κBp50 signaling and BAMBI modulation of TGF-β activity: involvement of miRNA-9 and miRNA-29. Food Chem. Toxicol. 140, 111307. doi:10.1016/j.fct.2020.111307
Kim, E. R., Park, J. S., Kim, J. H., Oh, J. Y., Oh, I. J., Choi, D. H., et al. (2022). A Glp-1/Glp-2 receptor dual agonist to treat Nash: targeting the gut-liver axis and microbiome. Hepatology 75 (6), 1523–1538. doi:10.1002/hep.32235
Kubo, T., Nishimura, N., Kaji, K., Tomooka, F., Shibamoto, A., Iwai, S., et al. (2024). Role of epiregulin on lipopolysaccharide-induced hepatocarcinogenesis as a mediator via Egfr signaling in the cancer microenvironment. Int. J. Mol. Sci. 25 (8), 4405. doi:10.3390/ijms25084405
Lagkouvardos, I., Intze, E., Schaubeck, M., Rooney, J. P., Hecht, C., Piloquet, H., et al. (2023). Early life gut microbiota profiles linked to synbiotic formula effects: a randomized clinical trial in European infants. Am. J. Clin. Nutr. 117 (2), 326–339. doi:10.1016/j.ajcnut.2022.11.012
Lan, X., Ma, J., Huang, Z., Xu, Y., and Hu, Y. (2024). Akkermansia muciniphila might improve anti-Pd-1 therapy against hcc by changing host bile acid metabolism. J. Gene Med. 26 (1), e3639. doi:10.1002/jgm.3639
Lee, S. M., Jun, D. W., Yoon, E. L., Oh, J. H., Roh, Y. J., Lee, E. J., et al. (2023). Discovery biomarker to optimize obeticholic acid treatment for non-alcoholic fatty liver disease. Biol. Direct 18 (1), 50. doi:10.1186/s13062-023-00407-4
Li, H., Xi, Y., Liu, H., and Xin, X. (2022b). Gypenosides ameliorate high-fat diet-induced non-alcoholic steatohepatitis via farnesoid X receptor activation. Front. Nutr. 9, 914079. doi:10.3389/fnut.2022.914079
Li, S. Z., Zhang, N. N., Yang, X., Huang, T. Q., Lin, Y., Jiang, Z. M., et al. (2023). Nobiletin ameliorates nonalcoholic fatty liver disease by regulating gut microbiota and myristoleic acid metabolism. J. Agric. Food Chem. 71 (19), 7312–7323. doi:10.1021/acs.jafc.2c08637
Li, Y., Chen, H., Ke, Z., Huang, J., Huang, L., Yang, B., et al. (2020). Identification of isotschimgine as a novel farnesoid X receptor agonist with potency for the treatment of obesity in mice. Biochem. Biophys. Res. Commun. 521 (3), 639–645. doi:10.1016/j.bbrc.2019.10.169
Li, Y., Deng, X., Tan, X., Li, Q., Yu, Z., Wu, W., et al. (2024a). Protective role of curcumin in disease progression from non-alcoholic fatty liver disease to hepatocellular carcinoma: a meta-analysis. Front. Pharmacol. 15, 1343193. doi:10.3389/fphar.2024.1343193
Li, Y., Xu, T., Zhao, Y., Zhang, H., Liu, Z., Wang, H., et al. (2024b). Discovery and optimization of novel nonbile acid Fxr agonists as preclinical candidates for the treatment of inflammatory bowel disease. J. Med. Chem. 67 (7), 5642–5661. doi:10.1021/acs.jmedchem.3c02304
Li, Z., Zhang, Y., Hong, W., Wang, B., Chen, Y., Yang, P., et al. (2022a). Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma via sting signaling. Gut Microbes 14 (1), 2119055. doi:10.1080/19490976.2022.2119055
Liang, M., Liwen, Z., Jianguo, S., Juan, D., Fei, D., Yin, Z., et al. (2021). Fecal microbiota transplantation controls progression of experimental autoimmune hepatitis in mice by modulating the Tfr/Tfh immune imbalance and intestinal microbiota composition. Front. Immunol. 12, 728723. doi:10.3389/fimmu.2021.728723
Liang, Y., Kaneko, K., Xin, B., Lee, J., Sun, X., Zhang, K., et al. (2022). Temporal analyses of postnatal liver development and maturation by single-cell transcriptomics. Dev. Cell 57 (3), 398–414 e5. doi:10.1016/j.devcel.2022.01.004
Lin, J., Rao, D., Zhang, M., and Gao, Q. (2024). Metabolic reprogramming in the tumor microenvironment of liver cancer. J. Hematol. Oncol. 17 (1), 6. doi:10.1186/s13045-024-01527-8
Lin, L., Song, J., Du, Y., Wu, Q., Gao, J., Song, Y., et al. (2020b). Quantification of bacterial metabolic activities in the gut by D-amino acid-based in vivo labeling. Angew. Chem. Int. Ed. Engl. 59 (29), 11923–11926. doi:10.1002/anie.202004703
Lin, L., Wu, Q., Song, J., Du, Y., Gao, J., Song, Y., et al. (2020a). Revealing the in vivo growth and division patterns of mouse gut bacteria. Sci. Adv. 6 (36), eabb2531. doi:10.1126/sciadv.abb2531
Lin, W., Conway, L. P., Vujasinovic, M., Lohr, J. M., and Globisch, D. (2021). Chemoselective and highly sensitive quantification of gut microbiome and human metabolites. Angew. Chem. Int. Ed. Engl. 60 (43), 23232–23240. doi:10.1002/anie.202107101
Lin, W., Garcia, F. R., Norin, E. L., Kart, D., Engstrand, L., Du, J., et al. (2023). Sensitive quantification of short-chain fatty acids combined with global metabolomics in microbiome cultures. Chem. Commun. (Camb) 59 (39), 5843–5846. doi:10.1039/d3cc01223a
Liu, J., Du, Y., Xiao, X., Tan, D., He, Y., and Qin, L. (2024). Construction of in vitro liver-on-a-chip models and application progress. Biomed. Eng. Online 23 (1), 33. doi:10.1186/s12938-024-01226-y
Liu, J., Sun, J., Yu, J., Chen, H., Zhang, D., Zhang, T., et al. (2023). Gut microbiome determines therapeutic effects of oca on Nafld by modulating bile acid metabolism. NPJ Biofilms Microbiomes 9 (1), 29. doi:10.1038/s41522-023-00399-z
Liu, K., Chen, X., Ren, Y., Liu, C., Yuan, A., Zheng, L., et al. (2022). Identification of a novel farnesoid X receptor agonist, Kaempferol-7-O-rhamnoside, a compound ameliorating drug-induced liver injury based on virtual screening and in vitro validation. Toxicol. Appl. Pharmacol. 454, 116251. doi:10.1016/j.taap.2022.116251
Liu, M., Zhang, G., Song, M., Wang, J., Shen, C., Chen, Z., et al. (2020). Activation of farnesoid X receptor by schaftoside ameliorates acetaminophen-induced hepatotoxicity by modulating oxidative stress and inflammation. Antioxid. Redox Signal 33 (2), 87–116. doi:10.1089/ars.2019.7791
Liu, T., Xing, S., Du, J., Wang, M., Han, J., and Li, Z. (2021). Synthesis and evaluation of the anti-inflammatory activity of Novel 8-quinolinesulfonamide derivatives as Tlr4/Md-2 inhibitors with efficacy in adjuvant-induced arthritis. Bioorg Chem. 114, 105037. doi:10.1016/j.bioorg.2021.105037
Lu, H., Ren, Z., Li, A., Zhang, H., Jiang, J., Xu, S., et al. (2016). Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci. Rep. 6, 33142. doi:10.1038/srep33142
Luan, Z. L., Ming, W. H., Sun, X. W., Zhang, C., Zhou, Y., Zheng, F., et al. (2021). A naturally occurring FXR agonist, alisol B 23-acetate, protects against renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 321 (5), F617–F628. doi:10.1152/ajprenal.00193.2021
Luciani, C., Hager, F. T., Cerovic, V., and Lelouard, H. (2022). Dendritic cell functions in the inductive and effector sites of intestinal immunity. Mucosal Immunol. 15 (1), 40–50. doi:10.1038/s41385-021-00448-w
Luna, E., Kim, S., Gao, Y., Widmalm, G., and Im, W. (2021). Influences of Vibrio cholerae lipid a types on lps bilayer properties. J. Phys. Chem. B 125 (8), 2105–2112. doi:10.1021/acs.jpcb.0c09144
Luo, J. Q., Ren, H., Chen, M. Y., Zhao, Q., Yang, N., Liu, Q., et al. (2023). Hydrochlorothiazide-induced glucose metabolism disorder is mediated by the gut microbiota via Lps-Tlr4-related macrophage polarization. iScience 26 (7), 107130. doi:10.1016/j.isci.2023.107130
Ma, C., Han, M., Heinrich, B., Fu, Q., Zhang, Q., Sandhu, M., et al. (2018). Gut microbiome-mediated bile acid metabolism regulates liver cancer via Nkt cells. Science 360 (6391), eaan5931. doi:10.1126/science.aan5931
Ma, H., Yang, L., Liang, Y., Liu, F., Hu, J., Zhang, R., et al. (2024). B. thetaiotaomicron-derived acetic acid modulate immune microenvironment and tumor growth in hepatocellular carcinoma. Gut Microbes 16 (1), 2297846. doi:10.1080/19490976.2023.2297846
Ma, L., Song, J., Chen, X., Dai, D., Chen, J., and Zhang, L. (2023). Fecal microbiota transplantation regulates Tfh/Tfr cell imbalance via Tlr/Myd88 pathway in experimental autoimmune hepatitis. Heliyon 9 (10), e20591. doi:10.1016/j.heliyon.2023.e20591
Ma, L., Zhang, L., Zhuang, Y., Ding, Y., and Chen, J. (2022). Lactobacillus improves the effects of prednisone on autoimmune hepatitis via gut microbiota-mediated follicular helper T cells. Cell Commun. Signal 20 (1), 83. doi:10.1186/s12964-021-00819-7
Maalej, M., Forgione, R. E., Marchetti, R., Bulteau, F., Thepaut, M., Lanzetta, R., et al. (2019). Human macrophage galactose-type Lectin (Mgl) recognizes the outer core of Escherichia coli lipooligosaccharide. Chembiochem 20 (14), 1778–1782. doi:10.1002/cbic.201900087
Maccioni, L., Gao, B., Leclercq, S., Pirlot, B., Horsmans, Y., De Timary, P., et al. (2020). Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 12 (1), 1782157. doi:10.1080/19490976.2020.1782157
MacDonald, L., Keenan, S., Di Lorenzo, F., Adade, N. E., Kenna, D. T. D., Millar, B. C., et al. (2023). Polymyxin resistance and heteroresistance are common in clinical isolates of Achromobacter species and correlate with modifications of the lipid a Moiety of lipopolysaccharide. Microbiol. Spectr. 11 (1), e0372922. doi:10.1128/spectrum.03729-22
Mao, Q., Lin, B., Zhang, W., Zhang, Y., Zhang, Y., Cao, Q., et al. (2024). Understanding the role of ursodeoxycholic acid and gut microbiome in non-alcoholic fatty liver disease: current evidence and perspectives. Front. Pharmacol. 15, 1371574. doi:10.3389/fphar.2024.1371574
Mariat, D., Firmesse, O., Levenez, F., Guimaraes, V., Sokol, H., Dore, J., et al. (2009). The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9, 123. doi:10.1186/1471-2180-9-123
Marzano, M., Fosso, B., Colliva, C., Notario, E., Passeri, D., Intranuovo, M., et al. (2022). Farnesoid X receptor activation by the novel agonist TC-100 (3α, 7α, 11β-Trihydroxy-6α-ethyl-5β-cholan-24-oic Acid) preserves the intestinal barrier integrity and promotes intestinal microbial reshaping in a mouse model of obstructed bile acid flow. Biomed. Pharmacother. 153, 113380. doi:10.1016/j.biopha.2022.113380
McBrearty, N., Arzumanyan, A., Bichenkov, E., Merali, S., Merali, C., and Feitelson, M. (2021). Short chain fatty acids delay the development of hepatocellular carcinoma in hbx transgenic mice. Neoplasia 23 (5), 529–538. doi:10.1016/j.neo.2021.04.004
Mo, C., Xu, X., Zhang, P., Peng, Y., Zhao, X., Chen, S., et al. (2023). Discovery of Hpg1860, a structurally novel nonbile acid fxr agonist currently in clinical development for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 66 (14), 9363–9375. doi:10.1021/acs.jmedchem.3c00456
Mouries, J., Brescia, P., Silvestri, A., Spadoni, I., Sorribas, M., Wiest, R., et al. (2019). Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71 (6), 1216–1228. doi:10.1016/j.jhep.2019.08.005
Mowat, A. M., Scott, C. L., and Bain, C. C. (2017). Barrier-tissue macrophages: functional adaptation to environmental challenges. Nat. Med. 23 (11), 1258–1270. doi:10.1038/nm.4430
Nara, S. J., Jogi, S., Cheruku, S., Kandhasamy, S., Jaipuri, F., Kathi, P. K., et al. (2022). Discovery of Bms-986339, a pharmacologically differentiated farnesoid X receptor agonist for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 65 (13), 8948–8960. doi:10.1021/acs.jmedchem.2c00165
Nash, A. K., Auchtung, T. A., Wong, M. C., Smith, D. P., Gesell, J. R., Ross, M. C., et al. (2017). The gut mycobiome of the human microbiome project healthy cohort. Microbiome 5 (1), 153. doi:10.1186/s40168-017-0373-4
Nian, F., Chen, Y., Xia, Q., Zhu, C., Wu, L., and Lu, X. (2024). Gut microbiota metabolite trimethylamine N-oxide promoted nafld progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int. Immunopharmacol. 142 (Pt B), 113173. doi:10.1016/j.intimp.2024.113173
Oblak, A., and Jerala, R. (2015). The molecular mechanism of species-specific recognition of lipopolysaccharides by the Md-2/Tlr4 receptor complex. Mol. Immunol. 63 (2), 134–142. doi:10.1016/j.molimm.2014.06.034
Ohto, U., Fukase, K., Miyake, K., and Shimizu, T. (2012). Structural basis of species-specific endotoxin sensing by innate immune receptor Tlr4/Md-2. Proc. Natl. Acad. Sci. U. S. A. 109 (19), 7421–7426. doi:10.1073/pnas.1201193109
Pan, B., Chen, Z., Zhang, X., Wang, Z., Yao, Y., Wu, X., et al. (2023). 2,5-Dimethylcelecoxib alleviated Nk and T-cell exhaustion in hepatocellular carcinoma via the gastrointestinal microbiota-Ampk-Mtor axis. J. Immunother. Cancer 11 (6), e006817. doi:10.1136/jitc-2023-006817
Park, B. S., and Lee, J. O. (2013). Recognition of lipopolysaccharide pattern by Tlr4 complexes. Exp. Mol. Med. 45 (12), e66. doi:10.1038/emm.2013.97
Peng, L., Pan, B., Zhang, X., Wang, Z., Qiu, J., Wang, X., et al. (2022). Lipopolysaccharide facilitates immune escape of hepatocellular carcinoma cells via M6a modification of Lncrna Mir155hg to upregulate Pd-L1 expression. Cell Biol. Toxicol. 38 (6), 1159–1173. doi:10.1007/s10565-022-09718-0
Plunk, M. A., Alaniz, A., Olademehin, O. P., Ellington, T. L., Shuford, K. L., and Kane, R. R. (2020). Design and catalyzed activation of Tak-242 prodrugs for localized inhibition of Tlr4-induced inflammation. ACS Med. Chem. Lett. 11 (2), 141–146. doi:10.1021/acsmedchemlett.9b00518
Qin, H., Yuan, B., Huang, W., and Wang, Y. (2022). Utilizing gut microbiota to improve hepatobiliary tumor treatments: recent advances. Front. Oncol. 12, 924696. doi:10.3389/fonc.2022.924696
Qin, T., Gao, X., Lei, L., Zhang, W., Feng, J., Wang, X., et al. (2023). Structural optimization and biological evaluation of 1-adamantylcarbonyl-4-phenylpiperazine derivatives as Fxr agonists for Nafld. Eur. J. Med. Chem. 245 (Pt 1), 114903. doi:10.1016/j.ejmech.2022.114903
Qu, L., Ma, X., and Fan, D. (2021). Ginsenoside Rk3 suppresses hepatocellular carcinoma development through targeting the gut-liver Axis. J. Agric. Food Chem. 69 (35), 10121–10137. doi:10.1021/acs.jafc.1c03279
Ram, A. K., Vairappan, B., and Srinivas, B. H. (2022). Nimbolide attenuates gut dysbiosis and prevents bacterial translocation by improving intestinal barrier integrity and ameliorating inflammation in hepatocellular carcinoma. Phytother. Res. 36 (5), 2143–2160. doi:10.1002/ptr.7434
Rapacciuolo, P., Finamore, C., Giorgio, C. D., Fiorillo, B., Massa, C., Urbani, G., et al. (2024). Design, synthesis, and pharmacological evaluation of Dual Fxr-Lifr modulators for the treatment of liver fibrosis. J. Med. Chem. 67 (20), 18334–18355. doi:10.1021/acs.jmedchem.4c01651
Rinella, M. E., Dufour, J. F., Anstee, Q. M., Goodman, Z., Younossi, Z., Harrison, S. A., et al. (2022). Non-invasive evaluation of response to obeticholic acid in patients with Nash: results from the regenerate study. J. Hepatol. 76 (3), 536–548. doi:10.1016/j.jhep.2021.10.029
Robinson, M. W., Harmon, C., and O'Farrelly, C. (2016). Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 13 (3), 267–276. doi:10.1038/cmi.2016.3
Roohani, S., and Tacke, F. (2021). Liver injury and the macrophage issue: molecular and mechanistic facts and their clinical relevance. Int. J. Mol. Sci. 22 (14), 7249. doi:10.3390/ijms22147249
Sarkar, A., Mitra, P., Lahiri, A., Das, T., Sarkar, J., Paul, S., et al. (2023). Butyrate limits inflammatory macrophage Niche in Nash. Cell Death Dis. 14 (5), 332. doi:10.1038/s41419-023-05853-6
Shah, D., Das, P., Acharya, S., Agarwal, B., Christensen, D. J., Robertson, S. M., et al. (2021). Small immunomodulatory molecules as potential therapeutics in experimental Murine models of acute lung injury (Ali)/acute respiratory distress syndrome (Ards). Int. J. Mol. Sci. 22 (5), 2573. doi:10.3390/ijms22052573
Shao, J., Ge, T., Wei, Y., Zhou, Y., Shi, M., Liu, H., et al. (2022). Co-interventions with Clostridium butyricum and soluble dietary fiber targeting the gut microbiota improve MAFLD via the Acly/Nrf2/NF-κB signaling pathway. Food Funct. 13 (10), 5807–5819. doi:10.1039/d1fo04224f
Shek, D., Chen, D., Read, S. A., and Ahlenstiel, G. (2021). Examining the gut-liver axis in liver cancer using organoid models. Cancer Lett. 510, 48–58. doi:10.1016/j.canlet.2021.04.008
Shen, R., Ke, L., Li, Q., Dang, X., Shen, S., Shen, J., et al. (2022). Abnormal bile acid-microbiota crosstalk promotes the development of hepatocellular carcinoma. Hepatol. Int. 16 (2), 396–411. doi:10.1007/s12072-022-10299-7
Shi, M., Tang, J., Zhang, T., and Han, H. (2022). Swertiamarin, an active iridoid glycoside from Swertia pseudochinensis H. Hara, protects against alpha-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and bile acid excretion pathway. J. Ethnopharmacol. 291, 115164. doi:10.1016/j.jep.2022.115164
Shi, Q., Yuan, X., Xue, C., Gu, X., and Li, L. (2023). Establishment and validation of a novel risk score for hepatocellular carcinoma based on bile acid and bile salt metabolism-related genes. Int. J. Mol. Sci. 24 (10), 8597. doi:10.3390/ijms24108597
Shi, Y., Tang, Y., Sun, Z., Sui, P., Shao, Y., Wang, Z., et al. (2025). Scutellarein inhibits osteosarcoma growth by targeting the TLR4/TRAF6/NF-κB pathway. Drug Des. Devel Ther. 19, 51–64. doi:10.2147/DDDT.S489092
Shimoyama, A., Di Lorenzo, F., Yamaura, H., Mizote, K., Palmigiano, A., Pither, M. D., et al. (2021). Lipopolysaccharide from gut-associated lymphoid-tissue-resident alcaligenes faecalis: complete structure determination and chemical synthesis of its lipid A. Angew. Chem. Int. Ed. Engl. 60 (18), 10023–10031. doi:10.1002/anie.202012374
Singh, A., Koduru, B., Carlisle, C., Akhter, H., Liu, R. M., Schroder, K., et al. (2017). Nadph oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by Toll-like receptor-4. Sci. Rep. 7 (1), 14346. doi:10.1038/s41598-017-14574-8
Singh, V., Yeoh, B. S., Chassaing, B., Xiao, X., Saha, P., Aguilera, O. R., et al. (2018). Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 175 (3), 679–694.e22. doi:10.1016/j.cell.2018.09.004
Singhvi, N., Gupta, V., Gaur, M., Sharma, V., Puri, A., Singh, Y., et al. (2020). Interplay of human gut microbiome in health and wellness. Indian J. Microbiol. 60 (1), 26–36. doi:10.1007/s12088-019-00825-x
Song, J., Cui, Z. Y., Lian, L. H., Han, X., Hou, L. S., Wang, G., et al. (2020). 20s-Protopanaxatriol ameliorates hepatic fibrosis, potentially involving Fxr-mediated inflammatory signaling cascades. J. Agric. Food Chem. 68 (31), 8195–8204. doi:10.1021/acs.jafc.0c01978
Song, Q., Zhang, X., Liu, W., Wei, H., Liang, W., Zhou, Y., et al. (2023). Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Hepatol. 79 (6), 1352–1365. doi:10.1016/j.jhep.2023.07.005
Sorribas, M., Jakob, M. O., Yilmaz, B., Li, H., Stutz, D., Noser, Y., et al. (2019). Fxr modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J. Hepatol. 71 (6), 1126–1140. doi:10.1016/j.jhep.2019.06.017
Spadoni, I., Zagato, E., Bertocchi, A., Paolinelli, R., Hot, E., Di Sabatino, A., et al. (2015). A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350 (6262), 830–834. doi:10.1126/science.aad0135
Stepien, M., Lopez-Nogueroles, M., Lahoz, A., Kuhn, T., Perlemuter, G., Voican, C., et al. (2022). Prediagnostic alterations in circulating bile acid profiles in the development of hepatocellular carcinoma. Int. J. Cancer 150 (8), 1255–1268. doi:10.1002/ijc.33885
Su, L., Athamna, M., Wang, Y., Wang, J., Freudenberg, M., Yue, T., et al. (2021). Sulfatides are endogenous ligands for the Tlr4-Md-2 complex. Proc. Natl. Acad. Sci. U. S. A. 118 (30), e2105316118. doi:10.1073/pnas.2105316118
Sun, R., Kong, B., Yang, N., Cao, B., Feng, D., Yu, X., et al. (2021). The hypoglycemic effect of berberine and berberrubine involves modulation of intestinal farnesoid X receptor signaling pathway and inhibition of hepatic gluconeogenesis. Drug Metab. Dispos. 49 (3), 276–286. doi:10.1124/dmd.120.000215
Sun, R., Zhang, Z., Bao, R., Guo, X., Gu, Y., Yang, W., et al. (2022). Loss of Sirt5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J. Hepatol. 77 (2), 453–466. doi:10.1016/j.jhep.2022.02.030
Tafazzol, A., and Duan, Y. (2019). Key residues in Tlr4-Md2 tetramer formation identified by free energy simulations. PLoS Comput. Biol. 15 (10), e1007228. doi:10.1371/journal.pcbi.1007228
Tang, Y. L., Zhu, L., Tao, Y., Lu, W., and Cheng, H. (2023). Role of targeting Tlr4 signaling axis in liver-related diseases. Pathol. Res. Pract. 244, 154410. doi:10.1016/j.prp.2023.154410
Temraz, S., Nassar, F., Kreidieh, F., Mukherji, D., Shamseddine, A., and Nasr, R. (2021). Hepatocellular carcinoma immunotherapy and the potential influence of gut microbiome. Int. J. Mol. Sci. 22 (15), 7800. doi:10.3390/ijms22157800
Teno, N., Iguchi, Y., Oda, K., Yamashita, Y., Masuda, A., Fujimori, K., et al. (2021). Discovery of orally active and nonsteroidal farnesoid X receptor (Fxr) antagonist with propensity for accumulation and responsiveness in ileum. ACS Med. Chem. Lett. 12 (3), 420–425. doi:10.1021/acsmedchemlett.0c00640
Therdtatha, P., Song, Y., Tanaka, M., Mariyatun, M., Almunifah, M., Manurung, N. E. P., et al. (2021). Gut microbiome of Indonesian adults associated with obesity and type 2 diabetes: a cross-sectional study in an Asian city, Yogyakarta. Microorganisms 9 (5), 897. doi:10.3390/microorganisms9050897
Tian, F., Chen, T., Xu, W., Fan, Y., Feng, X., Huang, Q., et al. (2023b). Curcumin compensates glp-1 deficiency via the microbiota-bile acids axis and modulation in functional crosstalk between Tgr5 and Fxr in Ob/Ob mice. Mol. Nutr. Food Res. 67 (22), e2300195. doi:10.1002/mnfr.202300195
Tian, P., Yang, W., Guo, X., Wang, T., Tan, S., Sun, R., et al. (2023a). Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate-Il-18 axis. Nat. Commun. 14 (1), 1710. doi:10.1038/s41467-023-37419-7
van Laar, A., Grootaert, C., Van Nieuwerburgh, F., Deforce, D., Desmet, T., Beerens, K., et al. (2022). Metabolism and health effects of rare sugars in a Caco-2/Hepg2 coculture model. Nutrients 14 (3), 611. doi:10.3390/nu14030611
Varanasi, S. K., Chen, D., Liu, Y., Johnson, M. A., Miller, C. M., Ganguly, S., et al. (2025). Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science 387 (6730), 192–201. doi:10.1126/science.adl4100
Wang, B., Zhu, J., Ma, X., Qiu, S., Pan, B., Zhou, J., et al. (2020a). Tfr-Tfh index: a new predicator for recurrence of hepatocellular carcinoma patients with Hbv infection after curative resection. Clin. Chim. Acta 511, 282–290. doi:10.1016/j.cca.2020.10.017
Wang, N., Li, C., and Zhang, Z. (2024). Arctigenin ameliorates high-fat diet-induced metabolic disorders by reshaping gut microbiota and modulating GPR/HDAC3 and TLR4/NF-κB pathways. Phytomedicine 135, 156123. doi:10.1016/j.phymed.2024.156123
Wang, W., Cao, Z., Yang, Z., Chen, Y., Yao, H., Zhou, D., et al. (2023b). Design, synthesis, and biological studies of novel sulfonamide derivatives as farnesoid X receptor agonists. Eur. J. Med. Chem. 258, 115614. doi:10.1016/j.ejmech.2023.115614
Wang, W., Yang, Q., Du, Y., Zhou, X., Du, X., Wu, Q., et al. (2020b). Metabolic labeling of peptidoglycan with Nir-ii dye enables in vivo imaging of gut microbiota. Angew. Chem. Int. Ed. Engl. 59 (7), 2628–2633. doi:10.1002/anie.201910555
Wang, Z., Yan, M., Li, J., Long, J., Li, Y., and Zhang, H. (2019). Dual functions of Stat3 in Lps-induced angiogenesis of hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Cell Res. 1866 (4), 566–574. doi:10.1016/j.bbamcr.2018.11.016
Wang, Z., Yao, W., Sun, Y., Han, Y., Chen, X., Gong, P., et al. (2023a). Eucommia bark/leaf extract improves lipid metabolism disorders by affecting intestinal microbiota and microbiome-host interaction in Hfd mice. J. Agric. Food Chem. 71, 3297–3314. doi:10.1021/acs.jafc.2c07239
Wen, F., Bian, D., Wu, X., Liu, R., Wang, C., and Gan, J. (2022). Su5, a new auraptene analog with improved metabolic stability, ameliorates nonalcoholic fatty liver disease in methionine- and choline-deficient diet-fed Db/Db mice. Chem. Biol. Drug Des. 99 (3), 504–511. doi:10.1111/cbdd.14021
Woo, A. Y. M., Aguilar Ramos, M. A., Narayan, R., Richards-Corke, K. C., Wang, M. L., Sandoval-Espinola, W. J., et al. (2023). Targeting the human gut microbiome with small-molecule inhibitors. Nat. Rev. Chem. 7 (5), 319–339. doi:10.1038/s41570-023-00471-4
Wu, L., Ai, Y., Xie, R., Xiong, J., Wang, Y., and Liang, Q. (2023). Organoids/Organs-on-a-Chip: new frontiers of intestinal pathophysiological models. Lab. Chip 23 (5), 1192–1212. doi:10.1039/d2lc00804a
Xi, L., Shi, A., Shen, T., Wang, G., Wei, Y., and Guo, J. (2023). Licraside as novel potent Fxr agonist for relieving cholestasis: structure-based drug discovery and biological evaluation studies. Front. Pharmacol. 14, 1197856. doi:10.3389/fphar.2023.1197856
Xiao, J., Xiang, H., Xiang, H., Sun, Z., Xu, J., Ren, H., et al. (2023). GW9662 ameliorates nonalcoholic steatohepatitis by inhibiting the PPARγ/CD36 pathway and altering the gut microbiota. Eur. J. Pharmacol. 960, 176113. doi:10.1016/j.ejphar.2023.176113
Xie, S., Li, J., Lyu, F., Xiong, Q., Gu, P., Chen, Y., et al. (2023). Novel tripeptide rkh derived from Akkermansia muciniphila protects against lethal sepsis. Gut 73 (1), 78–91. doi:10.1136/gutjnl-2023-329996
Xing, L., Zhang, Y., Li, S., Tong, M., Bi, K., Zhang, Q., et al. (2023). A dual coverage monitoring of the bile acids profile in the liver-gut axis throughout the whole inflammation-cancer transformation progressive: reveal hepatocellular carcinoma pathogenesis. Int. J. Mol. Sci. 24 (5), 4258. doi:10.3390/ijms24054258
Xiong, H., Zhang, C., Han, L., Xu, T., Saeed, K., Han, J., et al. (2022). Suppressed farnesoid X receptor by iron overload in mice and humans potentiates iron-induced hepatotoxicity. Hepatology 76 (2), 387–403. doi:10.1002/hep.32270
Yamagishi, R., Kamachi, F., Nakamura, M., Yamazaki, S., Kamiya, T., Takasugi, M., et al. (2022). Gasdermin D-mediated release of il-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci. Immunol. 7 (72), eabl7209. doi:10.1126/sciimmunol.abl7209
Yang, C., Briones, M., Chiou, J., Lei, L., Patton, M. J., Ma, L., et al. (2019). Chlamydia trachomatis lipopolysaccharide evades the canonical and noncanonical inflammatory pathways to subvert innate immunity. mBio 10 (2), 005955-e619. doi:10.1128/mBio.00595-19
Yang, H., Sherman, M. E., Koo, C. J., Treaster, L. M., Smith, J. P., Gallaher, S. G., et al. (2023a). Structure determination of lipid a with multiple glycosylation sites by Tandem Ms of lithium-adducted negative ions. J. Am. Soc. Mass Spectrom. 34 (6), 1047–1055. doi:10.1021/jasms.3c00014
Yang, J., Hirai, Y., Iida, K., Ito, S., Trumm, M., Terada, S., et al. (2023b). Integrated-Gut-Liver-on-a-Chip platform as an in vitro human model of non-alcoholic fatty liver disease. Commun. Biol. 6 (1), 310. doi:10.1038/s42003-023-04710-8
Yang, Y. L., Zhou, W. W., Wu, S., Tang, W. L., Wang, Z. W., Zhou, Z. Y., et al. (2021). Intestinal flora is a key factor in insulin resistance and contributes to the development of polycystic ovary syndrome. Endocrinology 162 (10), bqab118. doi:10.1210/endocr/bqab118
Yao, Z., Chen, L., Hu, M., Meng, F., Chen, M., and Wang, G. (2024). The discovery of a new potent Fxr agonist based on natural product screening. Bioorg Chem. 143, 106979. doi:10.1016/j.bioorg.2023.106979
Yoshimoto, S., Loo, T. M., Atarashi, K., Kanda, H., Sato, S., Oyadomari, S., et al. (2013). Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499 (7456), 97–101. doi:10.1038/nature12347
Yu, G. R., Lim, D. W., Karunarathne, W., Kim, G. Y., Kim, H., Kim, J. E., et al. (2022b). A non-polar fraction of Saponaria officinalis L. Acted as a Tlr4/Md2 complex antagonist and inhibited Tlr4/Myd88 signaling in vitro and in vivo. FASEB J. 36 (7), e22387. doi:10.1096/fj.202200018RR
Yu, H., Lin, G., Jiang, J., Yao, J., Pan, Z., Xie, H., et al. (2024). Synergistic activity of Enterococcus Faecium-induced ferroptosis via expansion of IFN-γ+CD8+ T cell population in advanced hepatocellular carcinoma treated with sorafenib. Gut Microbes 16 (1), 2410474. doi:10.1080/19490976.2024.2410474
Yu, Q., Dai, W., Ji, J., Wu, L., Feng, J., Li, J., et al. (2022a). Sodium butyrate inhibits aerobic glycolysis of hepatocellular carcinoma cells via the C-Myc/Hexokinase 2 pathway. J. Cell Mol. Med. 26 (10), 3031–3045. doi:10.1111/jcmm.17322
Yuan, B. F., Zhu, Q. F., Guo, N., Zheng, S. J., Wang, Y. L., Wang, J., et al. (2018). Comprehensive profiling of fecal metabolome of mice by integrated chemical isotope labeling-mass spectrometry analysis. Anal. Chem. 90 (5), 3512–3520. doi:10.1021/acs.analchem.7b05355
Yun, C., Yan, S., Liao, B., Ding, Y., Qi, X., Zhao, M., et al. (2024). The microbial metabolite agmatine acts as an Fxr agonist to promote polycystic ovary syndrome in female mice. Nat. Metab. 6 (5), 947–962. doi:10.1038/s42255-024-01041-8
Zadorozhna, M., Di Gioia, S., Conese, M., and Mangieri, D. (2020). Neovascularization is a key feature of liver fibrosis progression: anti-angiogenesis as an innovative way of liver fibrosis treatment. Mol. Biol. Rep. 47 (3), 2279–2288. doi:10.1007/s11033-020-05290-0
Zeng, D., Zhang, L., and Luo, Q. (2023). Celastrol-regulated gut microbiota and bile acid metabolism alleviate hepatocellular carcinoma proliferation by regulating the interaction between FXR and RXRα in vivo and in vitro. Front. Pharmacol. 14, 1124240. doi:10.3389/fphar.2023.1124240
Zeng, L., Wang, Y., Li, N., Niu, M., Wang, Y., and Chen, P. (2020). Protective effect of a novel (2s, 3r, 4s)-chromene-3-carboxamide derivative, Z20 against sepsis-induced organ injury. Inflammation 43 (4), 1222–1232. doi:10.1007/s10753-019-01174-z
Zhang, C., Wang, N., Tan, H. Y., Guo, W., Chen, F., Zhong, Z., et al. (2020a). Direct inhibition of the TLR4/MyD88 pathway by geniposide suppresses HIF-1α-independent VEGF expression and angiogenesis in hepatocellular carcinoma. Br. J. Pharmacol. 177 (14), 3240–3257. doi:10.1111/bph.15046
Zhang, G., Sun, X., Wen, Y., Shi, A., Zhang, J., Wei, Y., et al. (2020b). Hesperidin alleviates cholestasis via activation of the farnesoid X receptor in vitro and in vivo. Eur. J. Pharmacol. 885, 173498. doi:10.1016/j.ejphar.2020.173498
Zhang, J., Yu, Q., Jiang, D., Yu, K., Yu, W., Chi, Z., et al. (2022). Epithelial gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci. Immunol. 7 (68), eabk2092. doi:10.1126/sciimmunol.abk2092
Zhang, N., Fan, T., Zhao, L., Li, Y., Bao, Y., Ma, X., et al. (2023d). Discovery and development of palmatine analogues as anti-Nash agents by activating farnesoid X receptor (Fxr). Eur. J. Med. Chem. 245 (Pt 1), 114886. doi:10.1016/j.ejmech.2022.114886
Zhang, T., Xing, S., Du, J., Xia, J., Dong, S., Li, Z., et al. (2023c). Discovery of novel Tlr4/Md-2 inhibitors: receptor structure-based virtual screening studies and anti-inflammatory evaluation. Bioorg Chem. 141, 106880. doi:10.1016/j.bioorg.2023.106880
Zhang, W., Xu, X., Cai, L., and Cai, X. (2023b). Dysbiosis of the gut microbiome in elderly patients with hepatocellular carcinoma. Sci. Rep. 13 (1), 7797. doi:10.1038/s41598-023-34765-w
Zhang, X., Coker, O. O., Chu, E. S., Fu, K., Lau, H. C. H., Wang, Y. X., et al. (2021). Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 70 (4), 761–774. doi:10.1136/gutjnl-2019-319664
Zhang, X., Liang, Y., Jiang, J., Lu, C., Shi, F., Cao, Q., et al. (2023a). A high-salt diet exacerbates liver fibrosis through enterococcus-dependent macrophage activation. Microbiol. Spectr. 11 (2), e0340322. doi:10.1128/spectrum.03403-22
Zheng, C., Lu, F., Chen, B., Yang, J., Yu, H., Wang, D., et al. (2023). Gut microbiome as a biomarker for predicting early recurrence of Hbv-related hepatocellular carcinoma. Cancer Sci. 114 (12), 4717–4731. doi:10.1111/cas.15983
Zhong, Y., Chen, Y., Pan, Z., Tang, K., Zhong, G., Guo, J., et al. (2022). Ginsenoside rc, as an Fxr activator, alleviates acetaminophen-induced hepatotoxicity via relieving inflammation and oxidative stress. Front. Pharmacol. 13, 1027731. doi:10.3389/fphar.2022.1027731
Zhou, J. X., Li, C. N., Liu, Y. M., Lin, S. Q., Wang, Y., Xie, C., et al. (2022). Discovery of 9,11-seco-cholesterol derivatives as novel Fxr antagonists. ACS Omega 7 (20), 17401–17405. doi:10.1021/acsomega.2c01567
Keywords: gut-liver axis, hepatocellular carcinoma, gut microbiota-derived metabolites, tumor microenvironment, therapeutic interventions, multi-omics analysis
Citation: Hu Y, Gao M, Chenghuang J and Bao R (2025) Insights into the gut-liver axis: mechanisms and emerging therapies in hepatocellular carcinoma. Front. Pharmacol. 16:1595853. doi: 10.3389/fphar.2025.1595853
Received: 21 March 2025; Accepted: 23 April 2025;
Published: 19 May 2025.
Edited by:
Ralf Weiskirchen, RWTH Aachen University, GermanyReviewed by:
Alessia Provera, University of Eastern Piedmont, ItalyWeiyu Bai, Yunnan University, China
Copyright © 2025 Hu, Gao, Chenghuang and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Bao, YmFvcnVpQHNjdS5lZHUuY24=
 Yongjian Hu
Yongjian Hu Mingming Gao
Mingming Gao Jiajin Chenghuang
Jiajin Chenghuang Rui Bao
Rui Bao