- 1School of Life Sciences and Biopharmaceuticals, Shenyang Pharmaceutical University, Shenyang, Liaoning, China
- 2Department of Pharmacy, Jinqiu Hospital of Liaoning Province, Shenyang, Liaoning, China
- 3Department of Pharmacy, General Hospital of Northern Theater Command, Shenyang, Liaoning, China
Background: Depression is a common and severe mental disorder. Quercetin, a natural flavonoid compound, has been shown in several studies through animal experiments to improve depressive symptoms, demonstrating significant antidepressant potential.
Objective: This study represents the first preclinical meta-analysis on quercetin and depression, aiming to systematically evaluate the antidepressant effects of quercetin in animal studies. Methods: This study conducted a systematic search of the PubMed, EMBASE, Cochrane Library, and Web of Science electronic databases, with the search period covering from the inception of the databases to January 2025. Subsequently, the SYRCLE risk of bias assessment tool was used for quality evaluation, and data analysis was performed using RevMan 5.4 software.
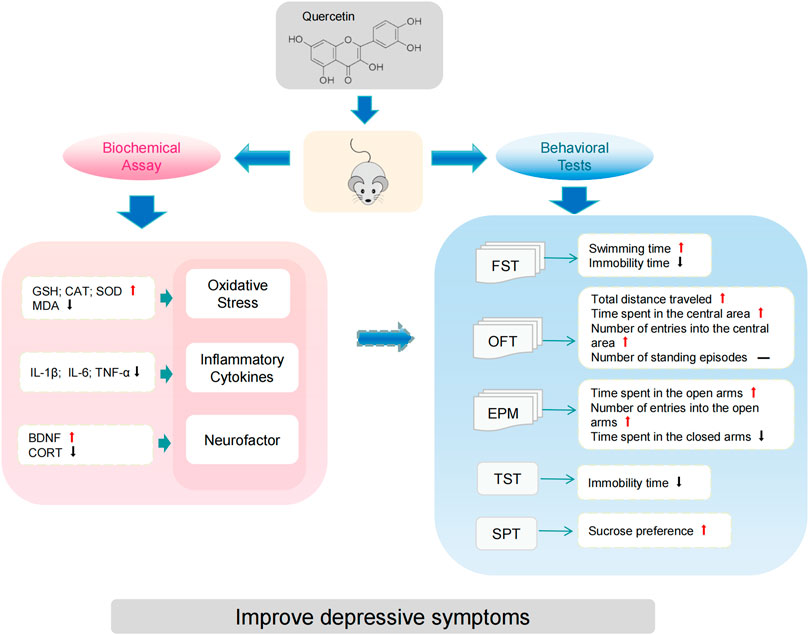
Results: This systematic review included 52 animal studies for random-effects meta-analysis. The results indicated that, compared to the control group, quercetin significantly reduced the immobility time in the forced swimming test and tail suspension test, as well as the time spent in the closed arms of the elevated plus maze. Simultaneously, it increased sucrose preference, swimming time in the forced swimming test, total distance traveled in the open field test, time spent in the central area, and the number of entries into the central area. In the elevated plus maze test, quercetin also increased the time spent in the open arms and the number of entries into the open arms. However, it did not produce a significant effect on the number of standing episodes in the open field test. Moreover, quercetin increased the levels of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and brain-derived neurotrophic factor (BDNF), while reducing the levels of malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and corticosterone (CORT).
Conclusion: This meta-analysis indicates that quercetin significantly improves depressive symptoms. However, further high-quality studies are needed to explore the role of quercetin in antidepressant research.
Systematic Review Registration: http://inplasy.com/, Identifier: INPLASY202530047.
1 Introduction
Depression is a common and highly recurrent mental disorder globally, characterized by persistent low mood, anxiety, anhedonia, and cognitive impairments (Burcusa and Iacono, 2007; Malhi and Mann, 2018). According to reports by the World Health Organization (WHO), over 300 million people worldwide suffer from depression. More than 700 000 people die due to suicide every year (World Health Organization, 2020). It is expected that by 2030, depression will become the leading cause of disability worldwide (Chen et al., 2024), placing a significant psychological and economic burden on individuals, families, and society. In recent years, treatment methods for depression have continuously evolved, including pharmacotherapy, psychotherapy, neuromodulation technologies, AI-assisted diagnosis and treatment, and lifestyle interventions (Sampogna et al., 2024). However, pharmacological intervention remains the cornerstone of depression treatment, with most currently used antidepressants exhibiting limitations such as a single mechanism of action, slow onset, and significant side effects (Pannu et al., 2021). Therefore, there is an urgent need to develop safe and effective antidepressants that target multiple pathways and mechanisms.
Quercetin (3,3′,4′,5,7-hydroxyflavone) is a natural flavonoid compound widely found in plants such as apples, onions, broccoli, wine, green tea, and ginkgo (Williamson and Manach, 2005; Mirza et al., 2023). Many studies have shown that quercetin exhibits various biological activities, including antioxidant (Xu et al., 2019), anti-inflammatory (Wu et al., 2024), and anticancer properties (Maleki et al., 2021), and can protect the nervous system (Cardozo et al., 2021; Fideles et al., 2023) as well as improve cognitive function (Ebrahimpour et al., 2020). In recent years, research on quercetin’s antidepressant effects has been gradually increasing. Current studies indicate that quercetin enhances the expression of BDNF in the prefrontal cortex and hippocampus of mice, improving anxiety, depression, and cognitive deficits induced by psychosocial stress (Ugwu et al., 2022). Other studies suggest that quercetin regulates Acetyl-H3K9 and inhibits astrocyte ferroptosis, significantly improving depressive-like behaviors in a perimenopausal depression rat model (Wang et al., 2024c). Furthermore, Ge et al. discovered that quercetin reduces apoptosis in the hippocampus and prefrontal cortex of chronic unpredictable stress (CUS) model mice, upregulates Nrf2 protein expression, and increases the phosphorylation levels of ERK and CREB, improving depressive behaviors in mice, similar to the effects of the antidepressant fluoxetine (Ge et al., 2024).
Despite increasing evidence confirming the effectiveness of quercetin in treating depression (Chen et al., 2022), some controversial results remain in the published studies. To clarify the clinical indications of quercetin for depression, a comprehensive and scientific evaluation of animal experimental studies is crucial. Therefore, we have summarized the existing evidence and, for the first time, conducted a meta-analysis of preclinical studies on quercetin’s antidepressant effects, which may provide important clues for future clinical research.
2 Materials and methods
This systematic review and meta-analysis were conducted in accordance with the PRISMA guidelines (Page et al., 2021) and the Cochrane Collaboration’s principles. To avoid duplication with ongoing systematic reviews, we first searched for similar reviews on the INPLASY website and subsequently registered our study (registration number: INPLASY202530047).
2.1 Search strategy
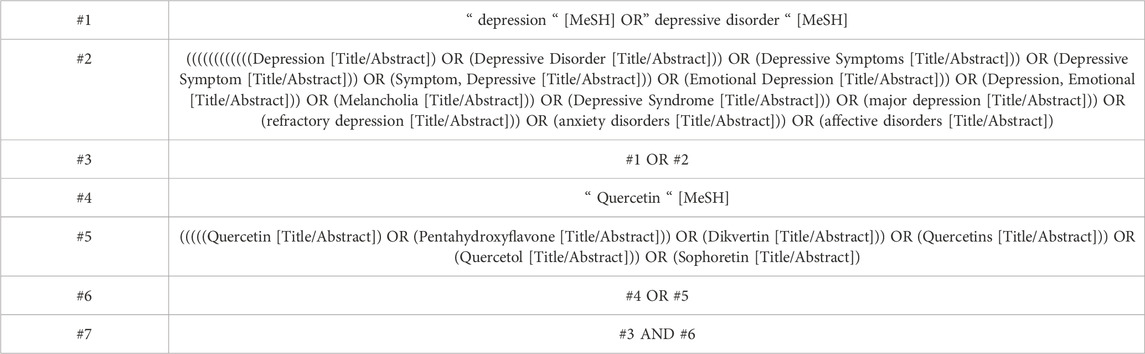
We systematically searched four electronic databases (PubMed, EMBASE, Cochrane Library, Web of Science) for data from the inception of the databases through January 2025. The search strategy utilized terms related to quercetin and depression to identify preclinical studies assessing the impact of quercetin on depression. The search algorithm employed only terms relevant to the topic of interest and filtered unique keywords to database (Reshma et al., 2024). The detailed search strategy is provided in Table 1 (using PubMed as an example).
2.2 Inclusion and exclusion criteria
Based on the PICOS principle, the studies included in this review adhered to the following criteria: (P) Population: Animal studies, with preparation requiring ethical approval, and no restrictions on species, gender, age, or weight of the animals; (I) Intervention: Studies involving quercetin treatment, either alone or in combination, with no restrictions on the route of administration, duration, dosage, or formulation; (C) Comparison: Control groups with either blank controls or standard treatments; (O) Outcome: Primary outcome measures included the forced swimming test (FST), tail suspension test (TST), sucrose preference test (SPT), open field test (OFT), and elevated plus maze (EPM); secondary outcome measures included corticosterone (CORT), brain-derived neurotrophic factor (BDNF), catalase (CAT), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α); (S) Study Design: Animal experiments.
The exclusion criteria were as follows: (1) Review articles, case reports, editorials/letters, patents, abstracts, and other informal journals; (2) In vitro studies, computer simulation studies, and all clinical trials; (3) Republished and irrelevant literature; (4) Studies on quercetin derivatives; (5) Experimental studies lacking a control group; (6) Studies with missing original articles or incomplete original data; (7) Studies where outcome data cannot be extracted or merged.
2.3 Research selection
The literature was screened and excluded using EndNote reference management software. Two researchers independently reviewed the titles and abstracts of the studies, excluded those that did not meet the inclusion criteria, and determined which studies to include. The remaining studies were then read in full by both researchers to further confirm their inclusion. Throughout the screening process, both researchers worked independently, and the final list of included studies was compared. If the studies matched, they were included; if there was a discrepancy, a third researcher discussed and resolved the differences.
2.4 Data extraction
The following information was independently extracted from the included studies by two researchers: (1) First author’s name and publication year; (2) Animal characteristics described in the intervention and control groups, including animal model, species, sex, and the number of animals per group; (3) Treatment information, including the drug dosage, route of administration, and duration of the intervention; (4) Outcome measures: Two researchers independently extracted data from each study, initially attempting to extract numerical data from tables or text. If these were not reported, quantitative data were extracted from graphs using Engauge Digitizer.
2.5 Quality assessment
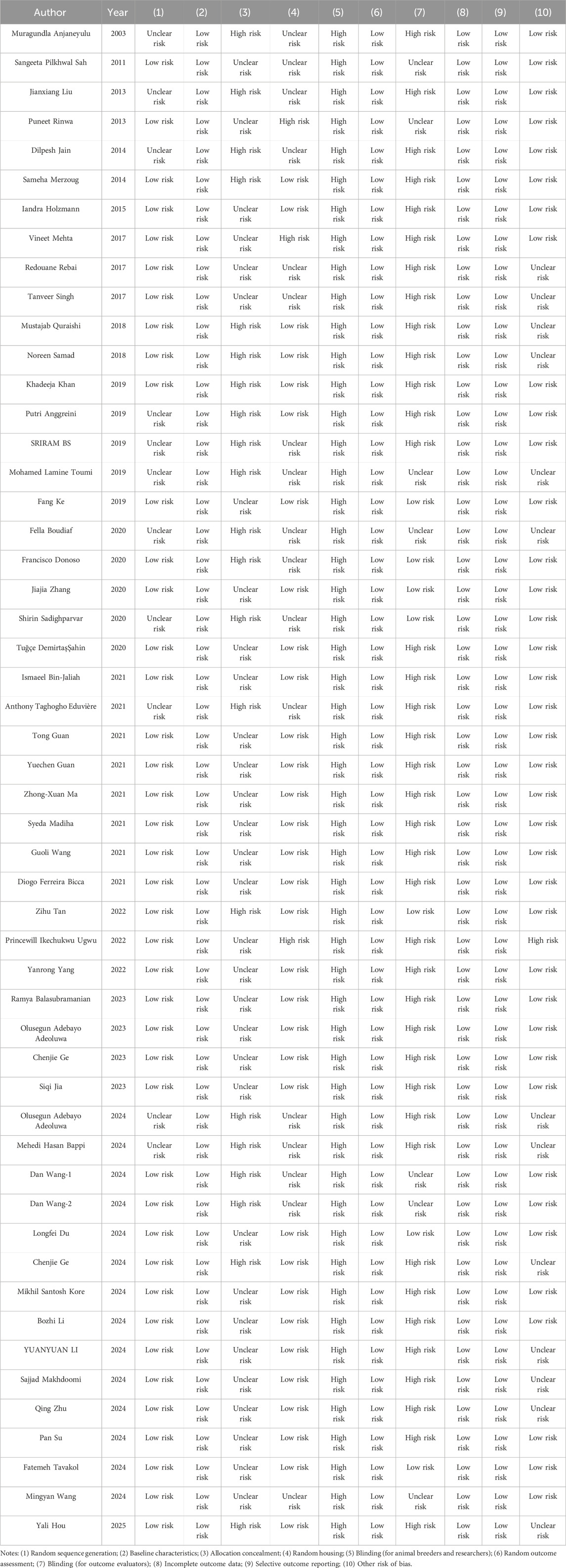
Two researchers independently assessed the risk of bias in each included study using the SYRCLE risk of bias assessment tool (Hooijmans et al., 2014), specifically analyzing the following types of bias: selection bias (random sequence generation, baseline characteristics, allocation concealment), performance bias (random housing, blinding), detection bias (random outcome assessment, blinding), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. Each type of bias was classified as high risk, low risk, or unclear. In the event of a discrepancy, the issue was resolved through discussion with a third researcher.
2.6 Data analysis
In this meta-analysis, the outcome measures were continuous data. Due to differences in animal species and models, standardized mean differences (SMD) and 95% confidence intervals (CIs) were used for analysis. The heterogeneity of the data was assessed using the I2 statistic. When I2 <50% and p > 0.05, low heterogeneity was assumed, and a fixed-effect model was used; otherwise, a random-effects model was applied. Sensitivity analysis was performed when more than 10 studies were included in an outcome measure, and funnel plots, along with Begg’s and Egger’s tests, were used to assess publication bias. A p-value <0.05 was considered statistically significant. All data analyses were performed using Review Manager 5.4 and STATA 15.1 software.
3 Results
3.1 Study selection
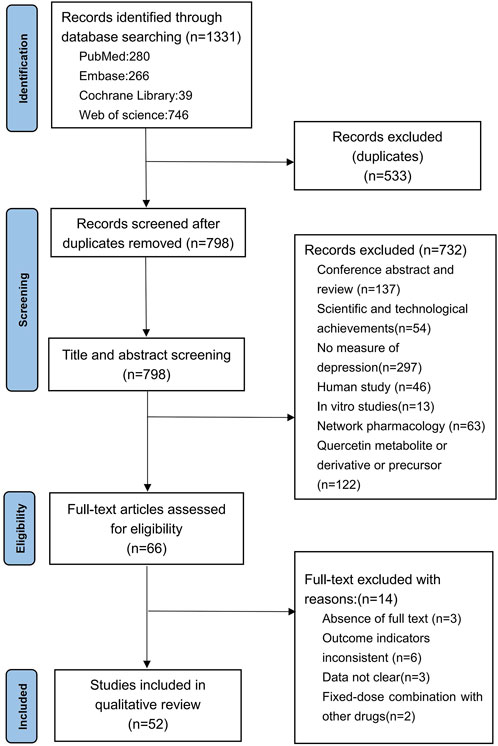
Based on the predefined search strategy, a total of 1,331 articles were retrieved. After excluding 533 duplicates, 798 articles remained. By reviewing the titles and abstracts, we further excluded conference abstracts and reviews (n = 137), technological achievements (n = 54), studies not measuring depression (n = 297), human studies (n = 46), in vitro studies (n = 13), network pharmacology studies (n = 63), and studies on quercetin metabolites, derivatives, or precursors (n = 122). Finally, 66 articles were selected for full-text screening. Afterward, we excluded studies with no full text (n = 3), studies with inconsistent outcome measures (n = 6), studies with unclear data (n = 3), and studies involving fixed-dose combinations with other drugs (n = 2), resulting in the inclusion of 52 articles (Anjaneyulu et al., 2003; Sah et al., 2011; Liu et al., 2013; Rinwa and Kumar, 2013; Jain and Gangshettiwar, 2014; Merzoug et al., 2014; Holzmann et al., 2015; Mehta et al., 2017; Rebai et al., 2017; Singh et al., 2017; Quraishi et al., 2018; Samad et al., 2018; Anggreini et al., 2019; Fang et al., 2019; Khan et al., 2019; Sriram and Ravichandra, 2019; Toumi et al., 2019; Boudia et al., 2020; Donoso et al., 2020; Sadighparvar et al., 2020; Ahin et al., 2020; Zhang et al., 2020; Bicca et al., 2021; Bin-Jaliah, 2021; Eduviere et al., 2021; Guan T. et al., 2021; Guan Y. et al., 2021; Ma et al., 2021; Madiha et al., 2021; Wang et al., 2021; Tan et al., 2022; Ugwu et al., 2022; Yang et al., 2022; Adeoluwa et al., 2023; Balasubramanian et al., 2023; Ge et al., 2023; Jia et al., 2023; Adeoluwa et al., 2024; Bappi et al., 2024; Du et al., 2024; Ge et al., 2024; Kore et al., 2024; Li B. et al., 2024; Li Y. et al., 2024; Makhdoomi et al., 2024; Su et al., 2024; Tavakol et al., 2024; Wang et al., 2024c; Wang et al., 2024a; Wang et al., 2024b; Zhu et al., 2024; Hou et al., 2025) for the comprehensive analysis. The detailed literature search and screening process is shown in Figure 1.
3.2 Characteristics and quality of included studies
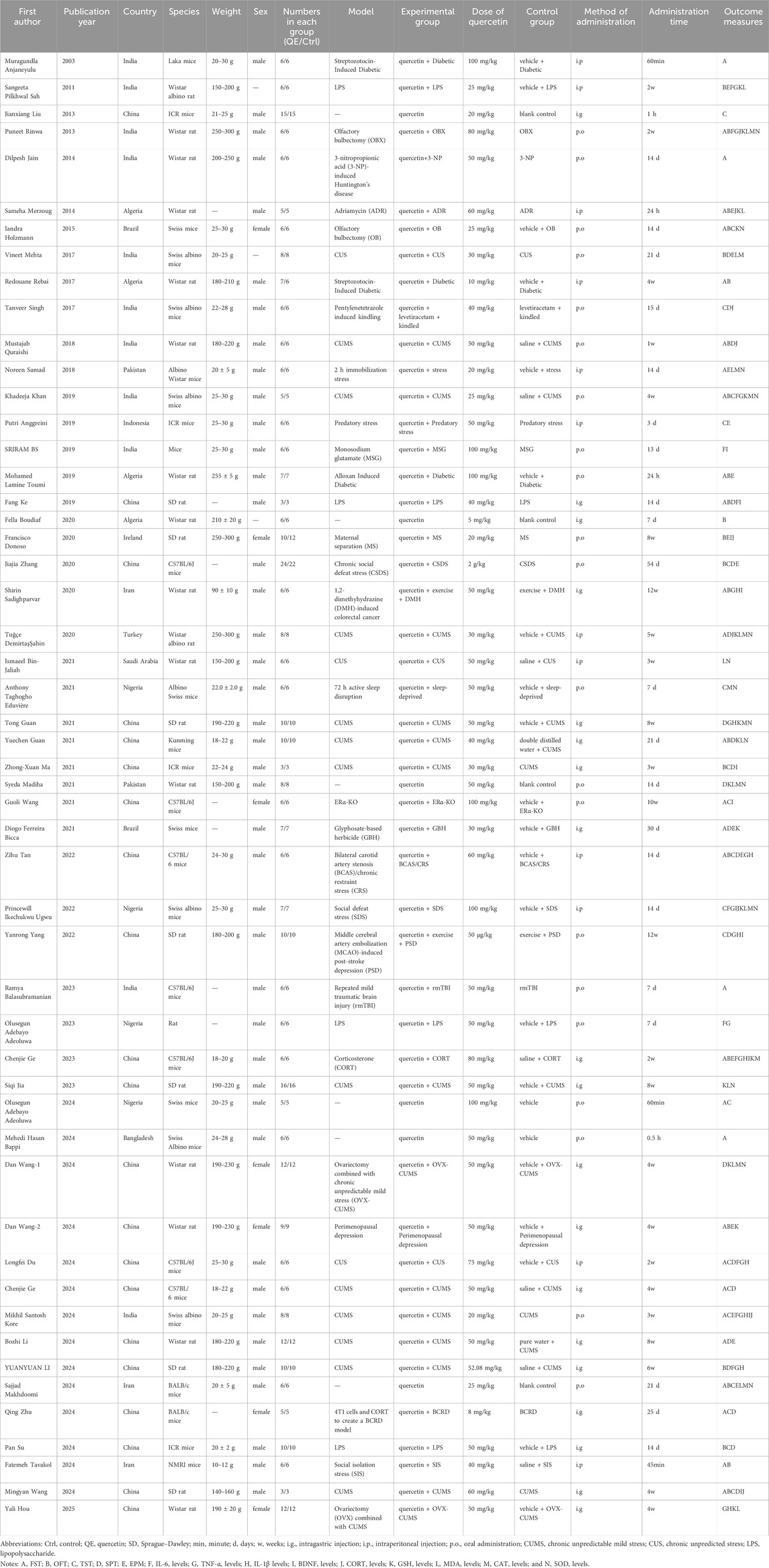
A total of 52 studies included in this meta-analysis were published between 2003 and 2025, and their main characteristics are summarized in Table 2. Of these, 21 studies were conducted in China, 11 in India, 4 in Nigeria and Algeria, 3 in Iran, 2 in Brazil and Pakistan, and one each in Indonesia, Bangladesh, Saudi Arabia, Ireland, and Turkey. Twenty-five studies induced depression in animals through stress, 15 through chemical induction, 5 through surgical induction, and 1 through genetic knockout. The sample size of each study ranged from 3 to 24 animals. Of the included studies, 28 used mice (weighing 10–30 g), and 24 used rats (weighing 80–300 g). Except for 10 studies, the remaining all used male animals. The quercetin dosage ranged from 50 μg/kg to 2 g/kg. Twenty-one studies administered quercetin orally, 19 via gavage, and the remaining 12 via intraperitoneal injection. The duration of quercetin supplementation ranged from 0.5 h to 12 weeks. Among the 52 included studies, the baseline characteristics were evenly distributed between the experimental and control groups in all studies. Random sequence generation was reported in 41 studies (78.8%), but none of the studies mentioned allocation concealment or blinding of animal breeders and researchers. Seven studies performed blinded outcome assessment. In addition, all studies reported the completeness of outcome data, with all animals undergoing the same assessments during the experiment, and no selective reporting was detected. Overall, the quality of the included studies primarily ranged from moderate to low risk of bias. There was a high number of “unclear risk” or “high risk” bias sources in allocation concealment and blinding, but most studies showed a low risk of bias in random sequence generation, outcome assessment, and other biases, providing a certain level of reliability to the meta-analysis results. However, caution should still be taken when interpreting and applying these results due to the potential impact of these biases. The detailed information on the quality assessment of the literature is presented in Table 3.
3.3 Behavioral tests
3.3.1 FST
The meta-analysis of FST, 29 studies (Anjaneyulu et al., 2003; Rinwa and Kumar, 2013; Jain and Gangshettiwar, 2014; Merzoug et al., 2014; Holzmann et al., 2015; Rebai et al., 2017; Quraishi et al., 2018; Samad et al., 2018; Fang et al., 2019; Khan et al., 2019; Toumi et al., 2019; Ahin et al., 2020; Sadighparvar et al., 2020; Bicca et al., 2021; Guan T. et al., 2021; Wang et al., 2021; Tan et al., 2022; Ge et al., 2023; Adeoluwa et al., 2024; Bappi et al., 2024; Du et al., 2024; Ge et al., 2024; Kore et al., 2024; Li B. et al., 2024; Makhdoomi et al., 2024; Tavakol et al., 2024; Wang et al., 2024c; Wang et al., 2024b; Zhu et al., 2024) on immobility time (involving 435 animals) and 6 studies (Merzoug et al., 2014; Rebai et al., 2017; Khan et al., 2019; Toumi et al., 2019; Balasubramanian et al., 2023; Ge et al., 2023) on swimming time (involving 77 animals) were included. The results indicated that, compared to the control group, quercetin treatment significantly reduced immobility time (SMD = −2.65; 95% CI = [−3.22, −2.08]; p < 0.001; I2 = 74%) and increased swimming time (SMD = 3.83; 95% CI = [2.51, 5.15]; p < 0.001; I2 = 56%). The forest plot showing the effect of quercetin on FST is presented in Figure 2.
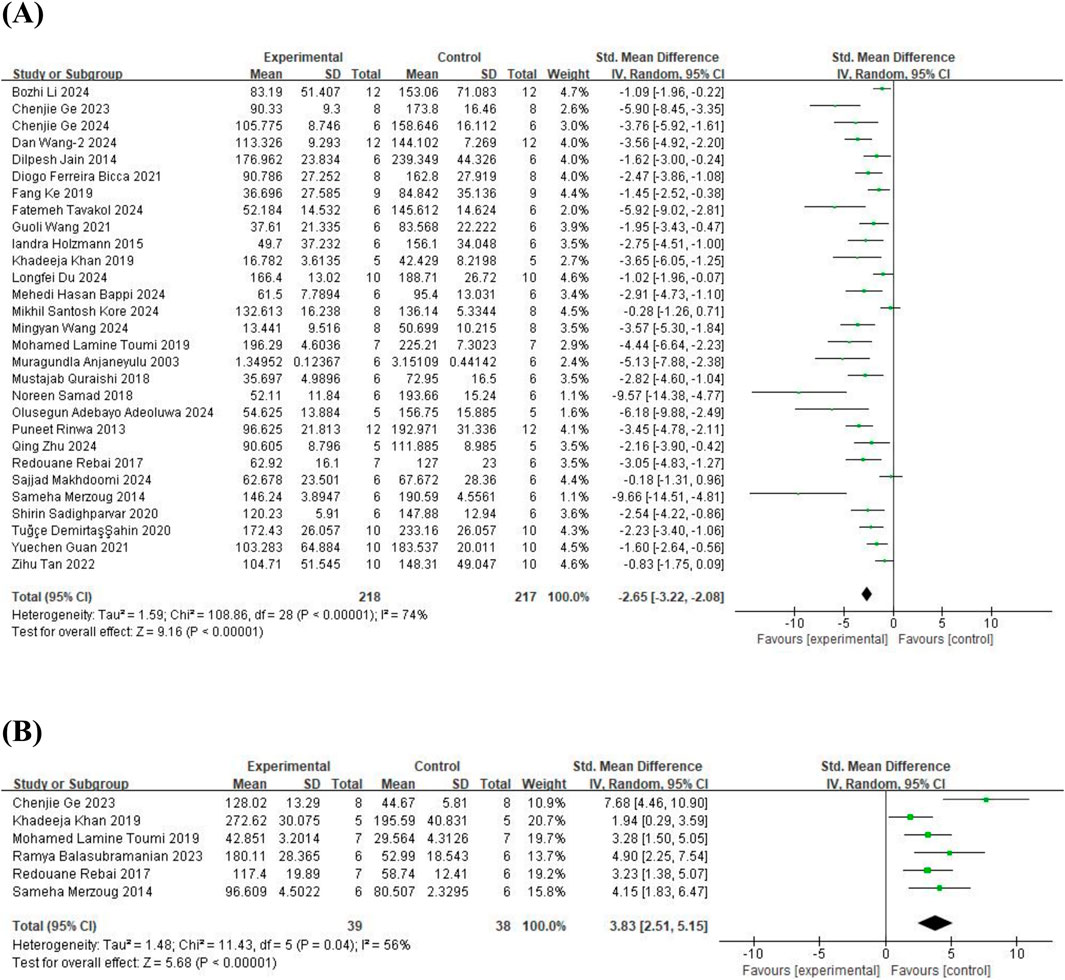
Figure 2. Forest plot for the effect of quercetin on the forced swimming test. (A) the immobility time; (B) the swimming time.
3.3.2 OFT
As for the OFT, 11 studies (Merzoug et al., 2014; Rebai et al., 2017; Quraishi et al., 2018; Fang et al., 2019; Toumi et al., 2019; Boudia et al., 2020; Donoso et al., 2020; Makhdoomi et al., 2024; Su et al., 2024; Wang et al., 2024c; Wang et al., 2024b) on total distance traveled (involving 175 animals), 13 studies (Merzoug et al., 2014; Mehta et al., 2017; Quraishi et al., 2018; Fang et al., 2019; Khan et al., 2019; Toumi et al., 2019; Donoso et al., 2020; Zhang et al., 2020; Ma et al., 2021; Tan et al., 2022; Ge et al., 2023; Li B. et al., 2024; Su et al., 2024) on time spent in the central area (involving 242 animals), 6 studies (Mehta et al., 2017; Rebai et al., 2017; Quraishi et al., 2018; Donoso et al., 2020; Makhdoomi et al., 2024; Wang et al., 2024c) on the number of entries into the central area (involving 91 animals), and 10 studies (Sah et al., 2011; Rinwa and Kumar, 2013; Merzoug et al., 2014; Holzmann et al., 2015; Fang et al., 2019; Khan et al., 2019; Sadighparvar et al., 2020; Guan T. et al., 2021; Li B. et al., 2024; Tavakol et al., 2024) on the number of standing episodes (involving 152 animals) were included. The results showed that, compared to the control group, quercetin treatment significantly increased the total distance traveled (SMD = 1.12; 95% CI = [0.30, 1.94]; p = 0.008; I2 = 81%), time spent in the central area (SMD = 1.88; 95% CI = [1.14, 2.63]; p < 0.001; I2 = 79%), and the number of entries into the central area (SMD = 1.18; 95% CI = [0.44, 1.92]; p = 0.002; I2 = 58%). However, quercetin treatment did not show a statistically significant effect on the number of standing episodes (SMD = 0.98; 95% CI = [−0.01, 1.98]; p = 0.05; I2 = 85%). The forest plot showing the effect of quercetin on OFT is presented in Figure 3.
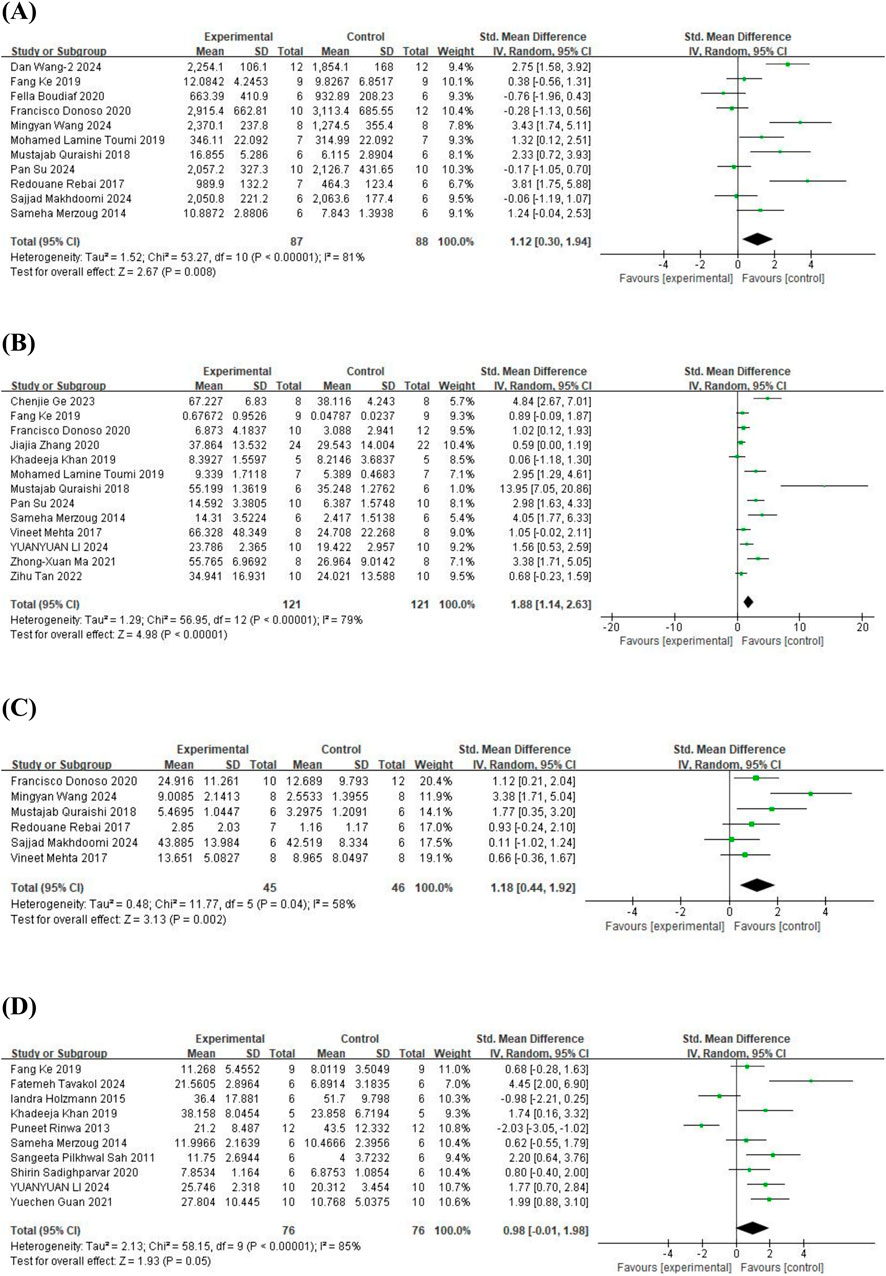
Figure 3. Forest plot for the effect of quercetin on the open field test. (A) Total distance traveled; (B) the time spent in the central area; (C) the number of entries into the central; (D) the number of standing episodes.
3.3.3 TST
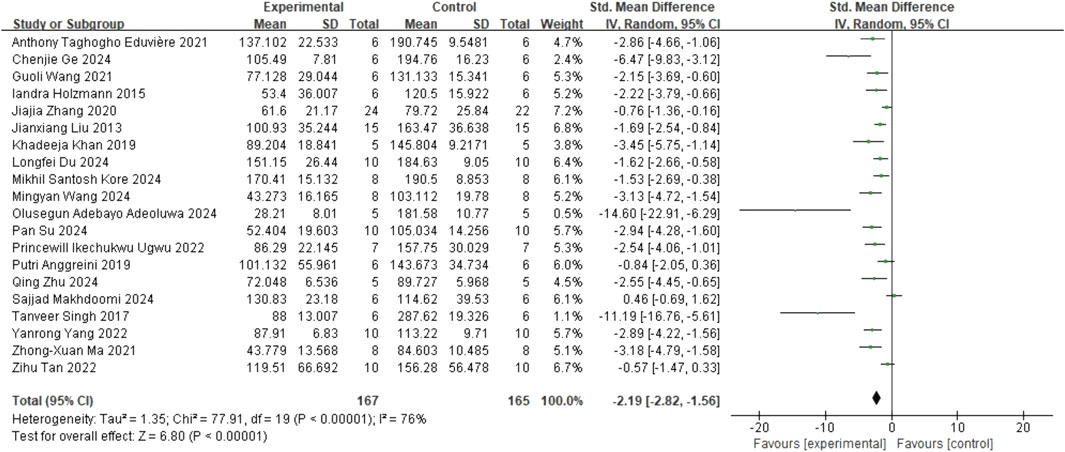
In the TST, 20 studies (Liu et al., 2013; Holzmann et al., 2015; Singh et al., 2017; Anggreini et al., 2019; Khan et al., 2019; Zhang et al., 2020; Eduviere et al., 2021; Ma et al., 2021; Wang et al., 2021; Tan et al., 2022; Ugwu et al., 2022; Yang et al., 2022; Adeoluwa et al., 2024; Du et al., 2024; Ge et al., 2024; Kore et al., 2024; Makhdoomi et al., 2024; Su et al., 2024; Wang et al., 2024c; Zhu et al., 2024) on immobility time (involving 332 animals) were included. The results indicated that, compared to the control group, quercetin treatment significantly reduced immobility time (SMD = −2.19; 95% CI = [-2.82, −1.56]; p < 0.001; I2 = 76%). The forest plot showing the effect of quercetin on TST is presented in Figure 4.
3.3.4 SPT
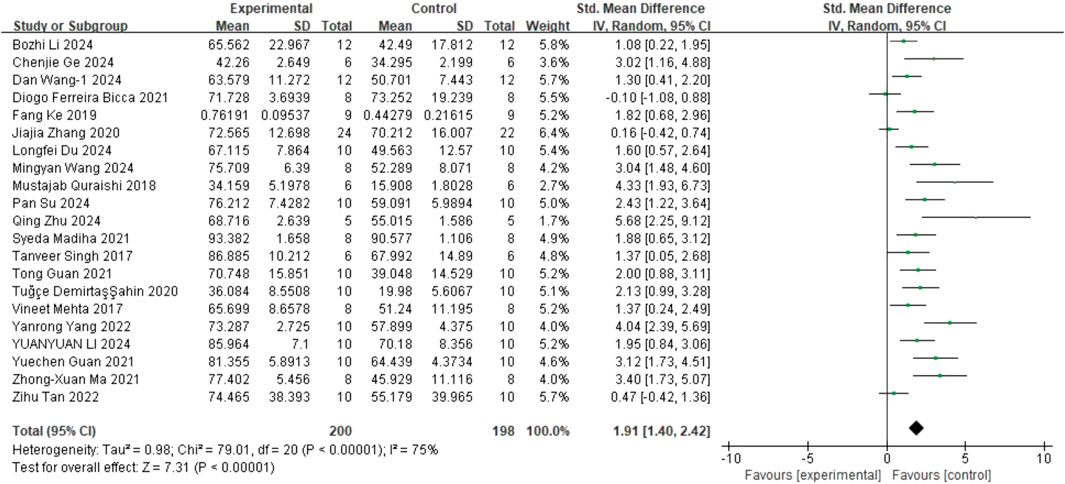
Regarding SPT, 21 studies (Mehta et al., 2017; Singh et al., 2017; Quraishi et al., 2018; Fang et al., 2019; Ahin et al., 2020; Zhang et al., 2020; Bicca et al., 2021; Guan T. et al., 2021; Guan Y. et al., 2021; Ma et al., 2021; Madiha et al., 2021; Tan et al., 2022; Yang et al., 2022; Du et al., 2024; Ge et al., 2024; Li B. et al., 2024; Li B. et al., 2024; Su et al., 2024; Wang et al., 2024c; Wang et al., 2024a; Zhu et al., 2024) on sucrose preference (involving 398 animals) were included. The results indicated that, compared to the control group, quercetin treatment significantly increased sucrose preference in animals (SMD = 1.91; 95% CI = [1.40, 2.42]; p < 0.001; I2 = 75%). The forest plot showing the effect of quercetin on SPT is presented in Figure 5.
3.3.5 EPM
In terms of the EPM, 15 studies (Sah et al., 2011; Merzoug et al., 2014; Mehta et al., 2017; Samad et al., 2018; Anggreini et al., 2019; Toumi et al., 2019; Donoso et al., 2020; Zhang et al., 2020; Bicca et al., 2021; Tan et al., 2022; Ge et al., 2023; Kore et al., 2024; Li B. et al., 2024; Makhdoomi et al., 2024; Wang et al., 2024b) on time spent in the open arms (involving 274 animals), 9 studies (Sah et al., 2011; Merzoug et al., 2014; Anggreini et al., 2019; Donoso et al., 2020; Bicca et al., 2021; Tan et al., 2022; Ge et al., 2023; Makhdoomi et al., 2024; Wang et al., 2024b) on the number of entries into the open arms (involving 146 animals), and 5 studies (Merzoug et al., 2014; Toumi et al., 2019; Donoso et al., 2020; Kore et al., 2024; Wang et al., 2024b) on time spent in the closed arms (involving 88 animals) were included. The results showed that, compared to the control group, quercetin treatment significantly increased the time spent in the open arms (SMD = 1.53; 95% CI = [0.91, 2.15]; p < 0.001; I2 = 76%), the number of entries into the open arms (SMD = 1.58; 95% CI = [0.72, 2.44]; p < 0.001; I2 = 78%), and decreased the time spent in the closed arms (SMD = −1.99; 95% CI = [-3.39, −0.58]; p = 0.006; I2 = 84%). The forest plot showing the effect of quercetin on EPM is presented in Figure 6.
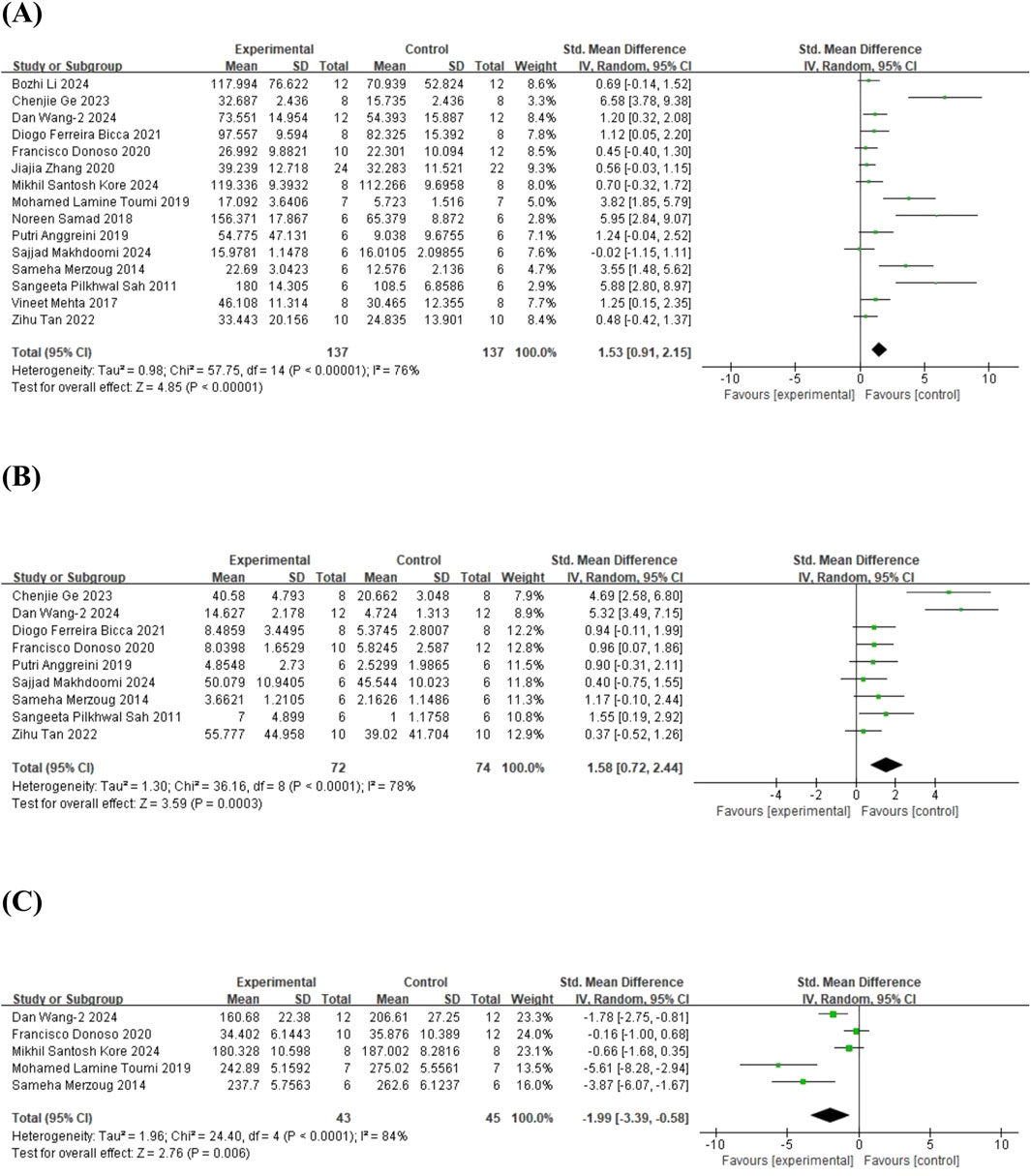
Figure 6. Forest plot for the effect of quercetin on the elevated plus maze. (A) The time spent in the open arms; (B) the number of entries into the open arms; (C) the time spent closed arms.
3.4 Biochemical assay
3.4.1 Oxidative stress
To investigate the antioxidant effects of quercetin treatment, 16 studies (Sah et al., 2011; Rinwa and Kumar, 2013; Merzoug et al., 2014; Holzmann et al., 2015; Khan et al., 2019; Ahin et al., 2020; Bicca et al., 2021; Guan T. et al., 2021; Guan Y. et al., 2021; Madiha et al., 2021; Ugwu et al., 2022; Ge et al., 2023; Jia et al., 2023; Wang et al., 2024a; Wang et al., 2024b; Hou et al., 2025) on glutathione (GSH) (involving 266 animals), 14 studies (Rinwa and Kumar, 2013; Holzmann et al., 2015; Samad et al., 2018; Khan et al., 2019; Ahin et al., 2020; Bin-Jaliah, 2021; Eduviere et al., 2021; Guan T. et al., 2021; Guan Y. et al., 2021; Madiha et al., 2021; Ugwu et al., 2022; Jia et al., 2023; Makhdoomi et al., 2024; Wang et al., 2024a) on superoxide dismutase (SOD) (involving 224 animals), 12 studies (Rinwa and Kumar, 2013; Mehta et al., 2017; Samad et al., 2018; Khan et al., 2019; Ahin et al., 2020; Eduviere et al., 2021; Guan T. et al., 2021; Madiha et al., 2021; Ugwu et al., 2022; Ge et al., 2023; Makhdoomi et al., 2024; Wang et al., 2024a) on catalase (CAT) (involving 176 animals), and 14 studies (Sah et al., 2011; Rinwa and Kumar, 2013; Merzoug et al., 2014; Mehta et al., 2017; Samad et al., 2018; Ahin et al., 2020; Bin-Jaliah, 2021; Guan T. et al., 2021; Madiha et al., 2021; Ugwu et al., 2022; Jia et al., 2023; Makhdoomi et al., 2024; Wang et al., 2024a; Hou et al., 2025) on malondialdehyde (MDA) (involving 232 animals) were included. The results indicated that, compared to the control group, quercetin treatment significantly increased GSH levels in animals (SMD = 2.85; 95% CI = [2.02, 3.67]; p < 0.001; I2 = 79%), SOD levels (SMD = 2.57; 95% CI = [1.63, 3.50]; p < 0.001; I2 = 82%), and CAT levels (SMD = 2.36; 95% CI = [1.32, 3.40]; p < 0.001; I2 = 82%), while decreasing MDA levels (SMD = −2.42; 95% CI = [-3.08, −1.76]; p < 0.001; I2 = 67%). The forest plot showing the effect of quercetin on oxidative stress markers is presented in Figure 7.
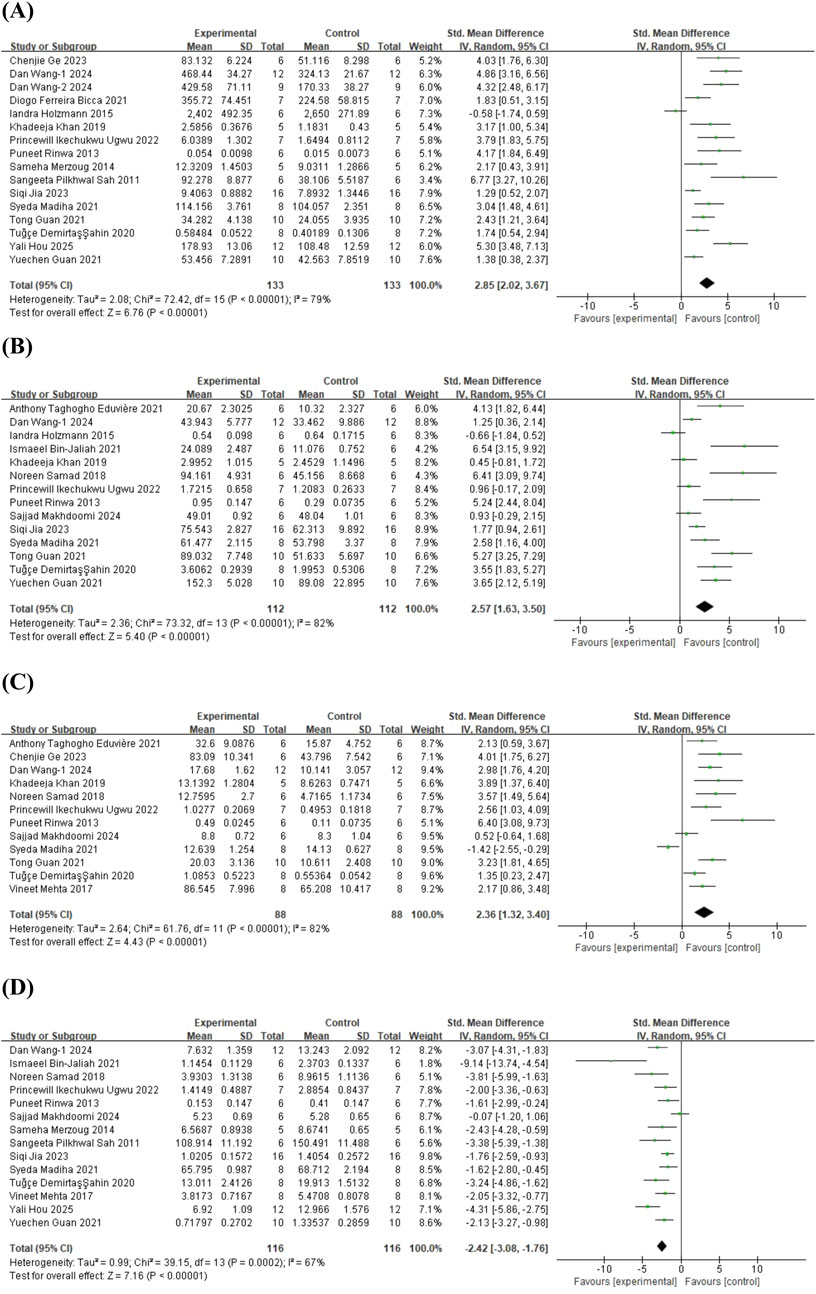
Figure 7. Forest plot for the effect of quercetin on the antioxident effects. (A) GSH; (B) SOD; (C) CAT; (D) MDA.
3.4.2 Inflammatory cytokines
In the case of inflammatory cytokines, 14 studies (Sah et al., 2011; Rinwa and Kumar, 2013; Khan et al., 2019; Sadighparvar et al., 2020; Guan T. et al., 2021; Tan et al., 2022; Ugwu et al., 2022; Yang et al., 2022; Adeoluwa et al., 2023; Ge et al., 2023; Du et al., 2024; Kore et al., 2024; Li B. et al., 2024; Hou et al., 2025) on tumor necrosis factor-α (TNF-α) (involving 208 animals), 11 studies (Sah et al., 2011; Rinwa and Kumar, 2013; Fang et al., 2019; Khan et al., 2019; Sriram and Ravichandra, 2019; Ugwu et al., 2022; Adeoluwa et al., 2023; Ge et al., 2023; Du et al., 2024; Kore et al., 2024; Li B. et al., 2024) on interleukin-6 (IL-6) (involving 150 animals), and 10 studies (Sah et al., 2011; Sadighparvar et al., 2020; Guan T. et al., 2021; Tan et al., 2022; Yang et al., 2022; Ge et al., 2023; Du et al., 2024; Kore et al., 2024; Li B. et al., 2024; Hou et al., 2025) on interleukin-1β (IL-1β) (involving 160 animals) were included in the meta-analysis. The results indicated that, compared to the control group, quercetin treatment significantly reduced TNF-α levels in animals (SMD = −4.16; 95% CI = [-5.39, −2.93]; p < 0.001; I2 = 82%), IL-6 levels (SMD = −2.49; 95% CI = [-3.48, −1.50]; p < 0.001; I2 = 76%), and IL-1β levels (SMD = −2.17; 95% CI = [-3.09, −1.24]; p < 0.001; I2 = 77%). These results suggest that quercetin has significant anti-inflammatory effects. The forest plot showing the effect of quercetin on inflammatory cytokine levels is presented in Figure 8.
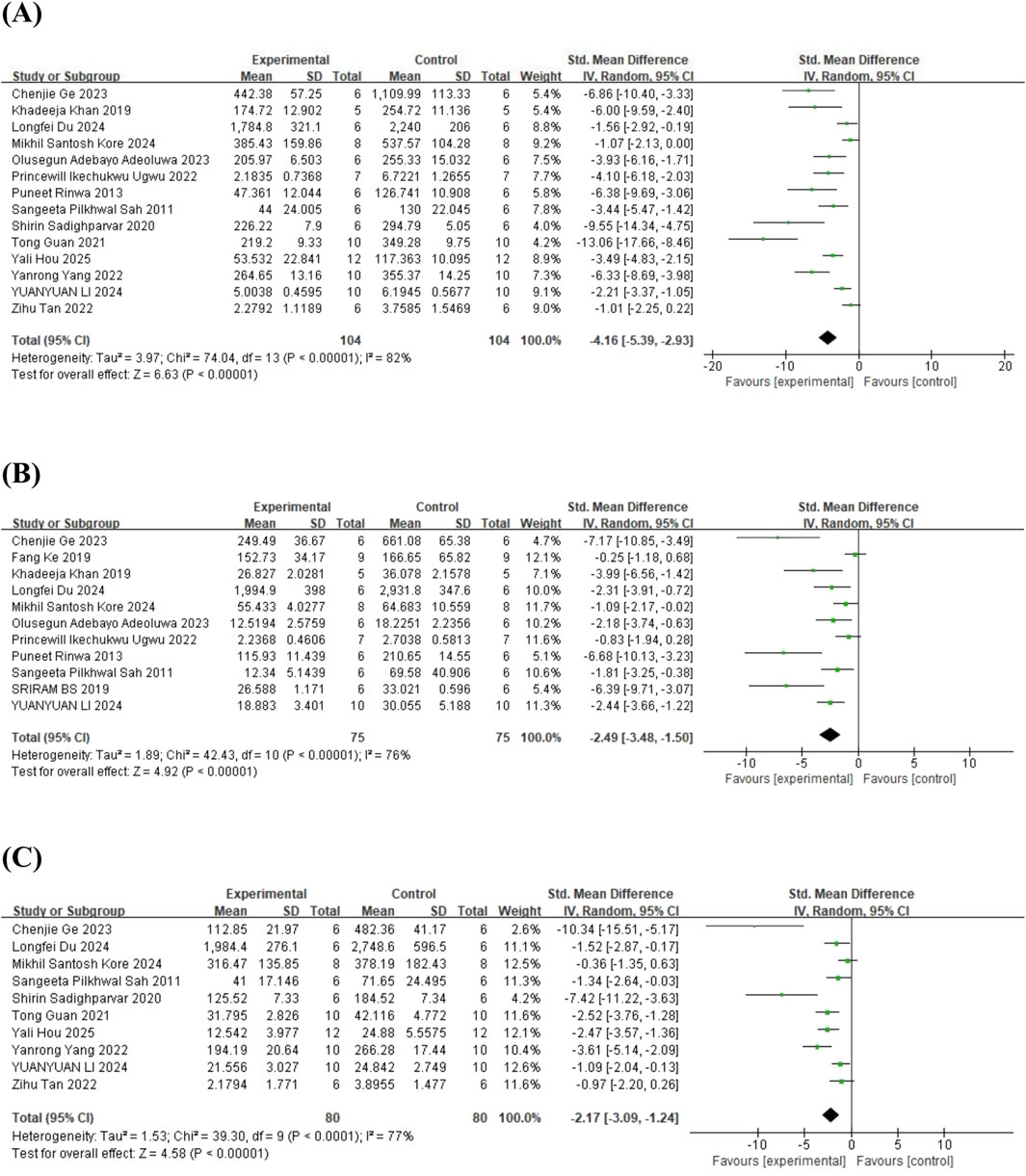
Figure 8. Forest plot for the effect of quercetin on the inflammatory cytokines. (A) TNF-α; (B) IL-6; (C) IL-1β.
3.4.3 BDNF and CORT
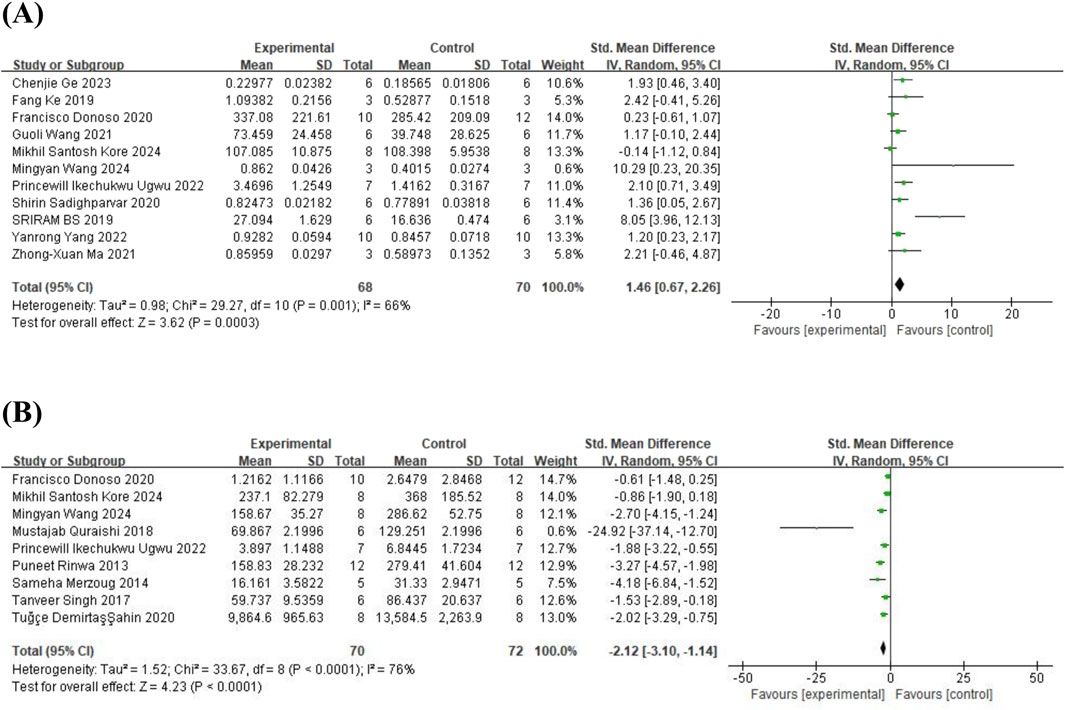
Eleven studies (Fang et al., 2019; Sriram and Ravichandra, 2019; Donoso et al., 2020; Sadighparvar et al., 2020; Ma et al., 2021; Wang et al., 2021; Ugwu et al., 2022; Yang et al., 2022; Ge et al., 2023; Kore et al., 2024; Wang et al., 2024c) on brain-derived neurotrophic factor (BDNF) (involving 138 animals) and 9 studies (Rinwa and Kumar, 2013; Merzoug et al., 2014; Singh et al., 2017; Quraishi et al., 2018; Donoso et al., 2020; Ahin et al., 2020; Ugwu et al., 2022; Kore et al., 2024; Wang et al., 2024c) on corticosterone (CORT) (involving 142 animals) were included. The results indicated that, compared to the control group, quercetin treatment significantly increased BDNF levels (SMD = 1.46; 95% CI = [0.67, 2.26]; p < 0.001; I2 = 66%) and decreased CORT levels in animals (SMD = −2.12; 95% CI = [-3.10, −1.14]; p < 0.001; I2 = 76%). The forest plot showing the effect of quercetin on BDNF and CORT levels is presented in Figure 9. The effects of quercetin on all behavioral and biochemical endpoints are summarized in Table 4.
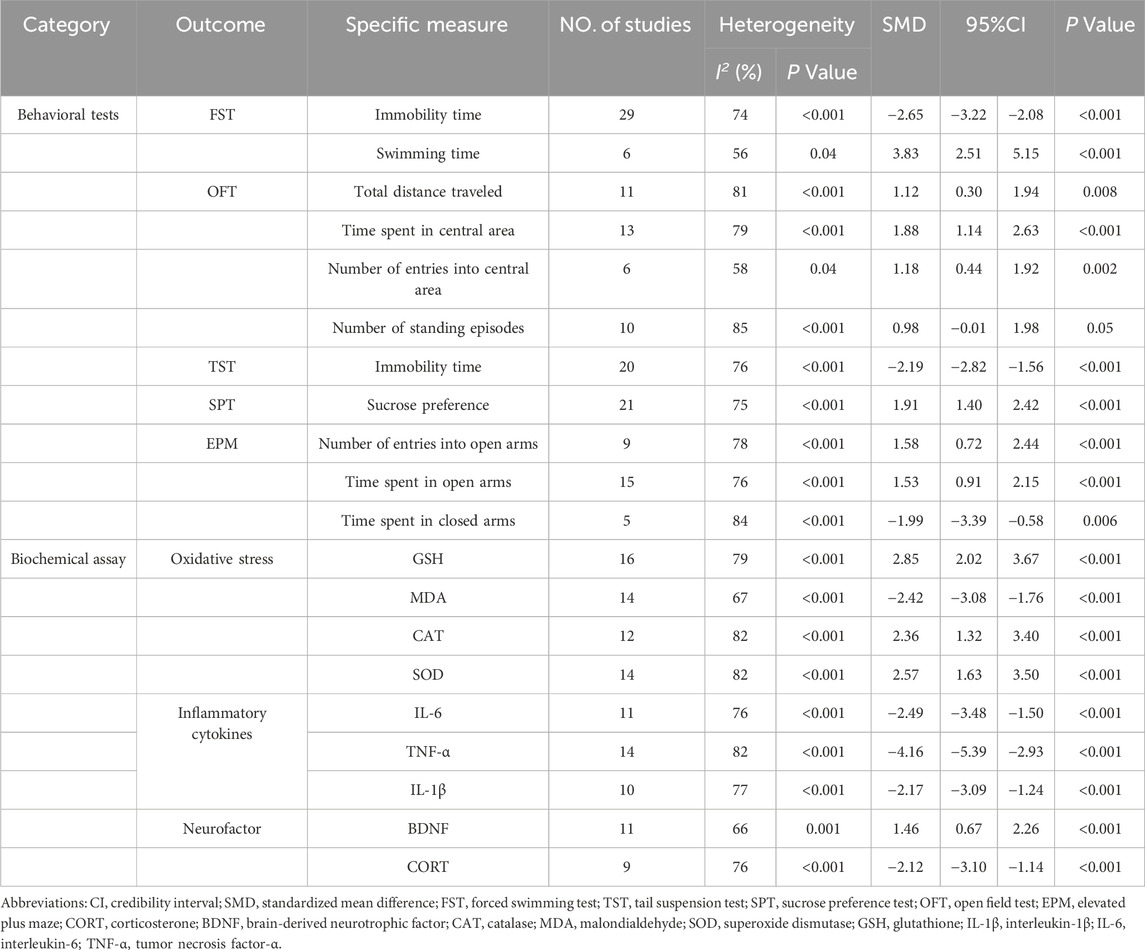
Table 4. Summary of the effects of quercetin vs. control on all behavioral and biochemical endpoints.
3.5 Sensitivity analysis and publication bias
In the sensitivity analysis with the exclusion of one study, the overall effects for all outcomes remained consistent, except for the number of standing episodes in the open field test. In the analysis of standing episodes, after excluding the study by Iandra Holzmann et al., the results changed. Heterogeneity decreased, and a significant effect was observed (SMD = 1.21; 95% CI = [0.17, 2.25]; p = 0.02; I2 = 84%). After excluding the study by Puneet Rinwa et al., the results also changed. Heterogeneity decreased, and a significant effect was observed (SMD = 1.28; 95% CI = [0.51, 2.06]; p = 0.001; I2 = 70%). The detailed results of the sensitivity analysis are presented in Supplementary Figure S1.
Funnel plots, Begg’s test, and Egger’s test were conducted for 15 outcome measures, including FST immobility time, total distance traveled in OFT, number of standing episodes in OFT, time spent in the central area of OFT, TST immobility time, sucrose preference in SPT, time spent in the open arms of the EPM, GSH, SOD, CAT, MDA, TNF-α, IL-6, IL-1β, and BDNF, to assess publication bias. The results indicated that there is some publication bias in the study (see Supplementary Figures S2-S3). Despite the positive findings, further research is needed to validate these results due to the potential publication bias in this meta-analysis.
3.6 Subgroup analysis
Due to the high heterogeneity among the meta-analyses, we further conducted subgroup analyses of all behavioral tests and biochemical markers according to animal species, dose of quercetin intervention, and duration of treatment. Subgroup analysis demonstrated that, compared to the control group, quercetin administration at a dose of ≥60 mg/kg significantly increased the total distance traveled in the open field test (OFT) (SMD = 1.88; 95% CI = [0.63, 3.12]; p = 0.003; I2 = 59%). Conversely, no statistically significant differences were observed in the total distance traveled in the OFT at doses of ≤10 mg/kg or 10–60 mg/kg. The influence of shorter treatment durations (≤1 week) on total distance traveled was not significant (SMD = 0.97; 95% CI = [-0.29, 2.24]; p = 0.13; I2 = 73%). In contrast, longer treatment durations (2–4 weeks) significantly increased the total distance traveled (SMD = 2.39; 95% CI = [0.52, 4.25]; p = 0.01; I2 = 85%). In subgroup analyses by species, treatment with quercetin significantly increased the total distance traveled in rats (SMD = 1.44; 95% CI = [0.46, 2.43]; p = 0.004; I2 = 83%), whereas no significant effect was observed in inbred mice (SMD = −0.06; 95% CI = [-1.19, 1.07]; p = 0.92). Detailed results of the subgroup analyses are shown in Supplementary Table S1.
4 Discussion
In this meta-analysis, we summarized the evidence from 52 published animal studies, which investigated the effects of quercetin supplementation on antidepressant effects across various behavioral and biochemical parameters. The results indicate that quercetin significantly affected animal behavior measures, including immobility time and swimming time in the FST, total distance traveled, time spent in the central area, and the number of entries into the central area in the OFT, immobility time in the TST, sucrose preference in the SPT, the number of entries into the open arms, time spent in the open arms, and time spent in the closed arms in the EPM, as well as biochemical markers such as GSH, SOD, CAT, MDA, TNF-α, IL-6, IL-1β, BDNF, and CORT. However, no significant effect was found on the number of standing episodes in the OFT.
This study shows that, compared to the control group, quercetin significantly increased sucrose preference in the sucrose preference test, reduced immobility time in the forced swimming test (FST) and tail suspension test (TST), and decreased time spent in the closed arms in the elevated plus maze (EPM). Additionally, quercetin increased total distance traveled and had significant effects on central area time and the number of entries into the open arms in the open field test (OFT) and EPM, showing the great effciency of quercetin for relieving depressive symptoms in animal studies. Depressive behavior test indicators are crucial for assessing the progression and therapeutic response of depression. Typically, the more severe the depression, the longer the immobility time, the shorter the distance traveled, and the less time spent in the central area and open arms, with lower sucrose preference (Tong et al., 2023; Ma et al., 2024). As reported by Anggreini, mice under stress showed a reduction in the time spent in open arms and the number of open-arm entries, indicating reduced exploratory activity, which is characteristic of higher anxiety in mice (Anggreini et al., 2019). Ma et al. found that chronic quercetin supplementation enhanced sucrose preference in mice subjected to chronic unpredictable mild stress, alleviating one of the key factors of depressive behavior—anhedonia (Ma et al., 2021). Furthermore, Samad et al. reported that quercetin can prevent stress-induced anxiety and depressive-like behaviors, as well as improve memory in male mice (Samad et al., 2018). In addition, Anjaneyu Lu et al.'s research on the antidepressant activity of quercetin in diabetic rats showed that quercetin reduced immobility time in a dose-dependent manner, which was similar to the effect of the antipsychotic drug fluoxetine and imipramine (Anjaneyulu et al., 2003). Moreover, the antidepressant and anxiolytic-like effects observed in quercetin-treated animals in our study are consistent with the findings of Sameha Merzoug, who reported that quercetin alleviates doxorubicin-induced anxiety-like behaviors and motor dysfunction in the open field and elevated plus maze tests, except for vertical exploration (i.e., the number of standing episodes) (Merzoug et al., 2014).
This study shows that quercetin significantly increases the levels of GSH, SOD, and CAT in animals, and decreases the levels of MDA, TNF-α, IL-6, and IL-1β. The anti-inflammatory and antioxidant effects of quercetin may be one of the important mechanisms underlying its antidepressant effects. Many studies suggest that the anti-inflammatory effects of quercetin may help alleviate neuropsychiatric symptoms. Sah et al. demonstrated that quercetin significantly reduced the levels of IL-1β and IL-6 in rats treated with lipopolysaccharide (LPS), thereby improving anxiety-like symptoms (Sah et al., 2011). These results were also verified in mice subjected to chronic unpredictable stress (CUS) (Mehta et al., 2017; Du et al., 2024). Due to the presence of both catechol and hydroxyl functional groups in its molecular structure, quercetin can directly exert antioxidant effects (Qi et al., 2022). Previously, Şahin et al. showed that quercetin, when administered via intraperitoneal injection, increased the SOD activity in the striatum of rats in the CUMS model (Ahin et al., 2020). One study found that quercetin had a significant effect on the perimenopausal depression rat model, as quercetin treatment significantly increased GSH levels in the brain and reduced the level of the oxidative stress marker MDA (Hou et al., 2025). Another study showed that quercetin alleviated oxidative stress and inflammation in rats by upregulating antioxidant mechanisms and downregulating the expression of COX2 and NF-κB (Bahar et al., 2017).
This study demonstrates that quercetin significantly increases BDNF levels and decreases CORT levels in animals, further supporting the biological basis of its antidepressant action. BDNF is a protein abundantly present in the human brain, which plays a critical role in protecting dendrites and axons, promoting synaptic plasticity, and regulating neuronal survival and intracellular signaling pathways (Kowiański et al., 2018). Clinical studies have shown that BDNF levels are significantly reduced in patients diagnosed with major depressive disorder (Kishi et al., 2017; Shi et al., 2020). CORT is an important glucocorticoid that regulates the body’s response to various stressors, such as psychological and physiological stress. A large body of evidence indicates that patients with depression or those who are chronically stressed typically exhibit overactivation of the hypothalamic-pituitary-adrenal (HPA) axis and elevated cortisol levels (Cubała and Landowski, 2014; Fischer et al., 2017; Druzhkova et al., 2022). Furthermore, existing studies have demonstrated that quercetin and its derivatives may exert neuroprotective effects by interacting with NMDA receptors, thereby reducing neuronal hyperexcitability and damage (Subramanian et al., 2023). Additionally, the modulation of NMDA receptors by quercetin could help restore neurotransmitter balance and ameliorate depressive-like behaviors (Wang et al., 2024c).
The findings of this study may provide multifaceted reference information for the future design of clinical trials of quercetin, which could facilitate the development of quercetin as a potential antidepressant and offer more therapeutic options for patients with depression. First, regarding the selection of subjects, the animal models included in this study cover a variety of methods for inducing depression, suggesting that future clinical trials may consider including patient groups with different etiologies or clinical manifestations of depression to more comprehensively evaluate the efficacy of quercetin. Second, in terms of dosing regimen design, this study has a wide range of quercetin doses and diverse routes of administration. Future clinical trials can refer to this information and, in combination with human pharmacokinetic characteristics, design rational human dosing regimens and routes of administration. Finally, regarding the evaluation of efficacy, this study involves multiple behavioral tests and biochemical indicators as outcome measures. Future clinical trials can draw on these indicators and, in combination with clinical practice, select more sensitive and specific assessment tools to measure the symptom improvement of patients with depression.
5 Strengths
To our knowledge, the strength of this study lies in its being the first systematic review and meta-analysis of quercetin’s antidepressant effects in preclinical research. Through a comprehensive analysis of multiple behavioral tests and biochemical indicators, the ameliorative effects of quercetin on depressive-like symptoms in animal experiments were evaluated. Our findings provide practical value for translating animal data to clinical evidence.
6 Limitations
However, there are several limitations in our systematic review and meta-analysis. First, the number of studies included is limited, and the total sample size is relatively small. Second, the included studies lacked standardized protocols regarding animal species, depression modeling methods, treatment interventions, and outcome assessment methods, which led to higher heterogeneity. Third, most of the studies included did not implement effective allocation concealment measures, nor did they blind the researchers or outcome assessors, which may have introduced selection, performance, and detection biases. Finally, although the sensitivity analysis indicated that the results were relatively stable, the significant risk of bias remains a concern. Therefore, further high-quality studies are urgently needed to validate the results presented in this study.
Subgroup analyses revealed that the effects of quercetin on the total distance traveled in the open field test were significantly influenced by dose, treatment duration, and animal species. Specifically, high doses (≥60 mg/kg) and prolonged treatment durations (2–4 weeks) significantly increased the total distance traveled in rats. However, no significant effects were observed in mice or under conditions of low doses and short treatment durations. Collectively, these findings indicate that treatment duration, dosage, and animal species significantly influence the therapeutic efficacy of quercetin. Therefore, future studies should further standardize experimental protocols.
7 Conclusion
In this study, we conducted a Meta-analysis for the first time to demonstrate that quercetin exhibits significant antidepressant effects in animal studies. The underlying mechanisms may involve the regulation of oxidative stress, inflammatory responses, neurotrophic factors, and HPA axis function. Although the results are limited by the heterogeneity of animal models and the risk of bias, the multitarget properties of quercetin provide a theoretical basis for its potential as an antidepressant agent. Future research should focus on standardized preclinical studies and explore its clinical translation, including dose optimization, sex differences, and combination therapy strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YY: Data curation, Software, Formal Analysis, Writing – original draft, Visualization, Conceptualization. YZ: Writing – original draft, Methodology, Software, Data curation, Visualization, Formal Analysis. LC: Software, Visualization, Writing – original draft, Methodology, Formal Analysis. ZL: Formal Analysis, Writing – original draft, Methodology, Data curation. QZ: Validation, Supervision, Conceptualization, Software, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1598053/full#supplementary-material
References
Adeoluwa, O. A., Eduviere, A. T., Adeoluwa, G. O., Otomewo, L. O., and Adeniyi, F. R. (2024). The monoaminergic pathways are involved in the antidepressant-like effect of quercetin. Naunyn. Schmiedeb. Arch. Pharmacol. 397 (4), 2497–2506. doi:10.1007/s00210-023-02789-8
Adeoluwa, O. A., Olayinka, J. N., Adeoluwa, G. O., Akinluyi, E. T., Adeniyi, F. R., Fafure, A., et al. (2023). Quercetin abrogates lipopolysaccharide-induced depressive-like symptoms by inhibiting neuroinflammation via microglial NLRP3/NFκB/iNOS signaling pathway. Behav. Brain Res. 450, 114503. doi:10.1016/j.bbr.2023.114503
Ahin, T. D., Gocmez, S. S., Duruksu, G., Yazir, Y., and Utkan, T. (2020). Resveratrol and quercetin attenuate depressive-like behavior and restore impaired contractility of Vas deferens in chronic stress-exposed rats: involvement of oxidative stress and inflammation. Naunyn. Schmiedeb. Arch. Pharmacol. 393 (5), 761–775. doi:10.1007/s00210-019-01781-5
Anggreini, P., Ardianto, C., Rahmadi, M., and Khotib, J. (2019). Quercetin attenuates acute predator stress exposure-evoked innate fear and behavioral perturbation. J. Basic Clin. Physiol. Pharmacol. 30 (6). doi:10.1515/jbcpp-2019-0242
Anjaneyulu, M., Chopra, K., and Kaur, I. (2003). Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. J. Med. Food. 6 (4), 391–395. doi:10.1089/109662003772519976
Bahar, E., Kim, J. Y., and Yoon, H. (2017). Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. Int. J. Mol. Sci. 18 (9), 1989. doi:10.3390/ijms18091989
Balasubramanian, R., Bazaz, M. R., Pasam, T., Sharief, N., Velip, L., Samanthula, G., et al. (2023). Involvement of microbiome gut-brain axis in neuroprotective effect of quercetin in mouse model of repeated mild traumatic brain injury. Neuromolecular Med. 25 (2), 242–254. doi:10.1007/s12017-022-08732-z
Bappi, M. H., Mia, M. N., Ansari, S. A., Ansari, I. A., Prottay, A., Akbor, M. S., et al. (2024). Quercetin increases the antidepressant-like effects of sclareol and antagonizes diazepam in thiopental sodium-induced sleeping mice: a possible GABAergic transmission intervention. Phytother. Res. 38 (5), 2198–2214. doi:10.1002/ptr.8139
Bicca, D. F., Spiazzi, C. C., Ramalho, J. B., Soares, M. B., and Cibin, F. (2021). A subchronic low-dose exposure of a glyphosate-based herbicide induces depressive and anxious-like behavior in mice: quercetin therapeutic approach. Environ. Sci. Pollut. Res. 28 (47), 67394–67403. doi:10.1007/s11356-021-15402-3
Bin-Jaliah, I. (2021). Quercetin inhibits chronic stress-induced depression associated with the inhibition of nitrosative stress and apoptosis. Int. J. Pharmacol. 17 (5), 319–327. doi:10.3923/ijp.2021.319.327
Boudia, F., Chouba, I., Amri, N., and Tahraoui, A. (2020). Anti-diabetic role of quercetin and cinnamon on neurobehavioral alterations and biochemical parameters of induced diabetics rats. J. Anim. Behav. Biometeorol. 8 (3), 190–195. doi:10.31893/jabb.20025
Burcusa, S. L., and Iacono, W. G. (2007). Risk for recurrence in depression. Clin. Psychol. Rev. 27 (8), 959–985. doi:10.1016/j.cpr.2007.02.005
Cardozo, V., Vaamonde, L., Parodi-Talice, A., Zuluaga, M. J., Agrati, D., Portela, M., et al. (2021). Multitarget neuroprotection by quercetin: changes in gene expression in two perinatal asphyxia models. Neurochem. Int. 147, 105064. doi:10.1016/j.neuint.2021.105064
Chen, S., Sun, H., Song, Y., Zhang, M., Huang, W., Zhao, C., et al. (2024). Transition and trend analysis of the burden of depression in China and different income countries: based on GBD database and joinpoint regression model. J. Affect. Disord. 362, 437–449. doi:10.1016/j.jad.2024.06.067
Chen, S., Tang, Y., Gao, Y., Nie, K., Wang, H., Su, H., et al. (2022). Antidepressant potential of quercetin and its glycoside derivatives: a comprehensive review and update. Front. Pharmacol. 13, 865376. doi:10.3389/fphar.2022.865376
Cubała, W. J., and Landowski, J. (2014). C-reactive protein and cortisol in drug-naïve patients with short-illness-duration first episode major depressive disorder: possible role of cortisol immunomodulatory action at early stage of the disease. J. Affect. Disord. 152-154, 534–537. doi:10.1016/j.jad.2013.10.004
Donoso, F., Egerton, S., Bastiaanssen, T., Fitzgerald, P., Gite, S., Fouhy, F., et al. (2020). Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 116, 104673. doi:10.1016/j.psyneuen.2020.104673
Druzhkova, T. A., Yakovlev, A. A., Rider, F. K., Zinchuk, M. S., Guekht, A. B., and Gulyaeva, N. V. (2022). Elevated serum cortisol levels in patients with focal epilepsy, depression, and comorbid epilepsy and depression. Int. J. Mol. Sci. 23 (18), 10414. doi:10.3390/ijms231810414
Du, L., Fan, X., Yang, Y., Wu, S., and Liu, Y. (2024). Quercetin ameliorates cognitive impairment in depression by targeting HSP90 to inhibit NLRP3 inflammasome activation. Mol. Neurobiol. 61 (9), 6628–6641. doi:10.1007/s12035-024-03926-x
Ebrahimpour, S., Zakeri, M., and Esmaeili, A. (2020). Crosstalk between obesity, diabetes, and Alzheimer'S disease: introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 62, 101095. doi:10.1016/j.arr.2020.101095
Eduviere, A. T., Moke, E. G., Omogbiya, A. I., Otomewo, L. O., Olayinka, J. N., Aboyewa, F. E., et al. (2021). Quercetin modulates behavioural and biochemical alterations in stressed mice. Biosci. Biotechnol. Res. Asia 18 (4), 681–689. doi:10.13005/bbra/2951
Fang, K., Li, H. R., Chen, X. X., Gao, X. R., Huang, L. L., Du, A. Q., et al. (2019). Quercetin alleviates LPS-induced depression-like behavior in rats via regulating BDNF-related imbalance of copine 6 and TREM1/2 in the hippocampus and PFC. Front. Pharmacol. 10, 1544. doi:10.3389/fphar.2019.01544
Fideles, S., de Cássia, O. A., Buchaim, D. V., de Souza, B. M. P. E., Parreira, M., de Oliveira, R. J., et al. (2023). Influence of the neuroprotective properties of quercetin on regeneration and functional recovery of the nervous system. Antioxidants 12 (1), 149. doi:10.3390/antiox12010149
Fischer, S., Strawbridge, R., Vives, A. H., and Cleare, A. J. (2017). Cortisol as a predictor of psychological therapy response in depressive disorders: systematic review and meta-analysis. Br. J. Psychiatry 210 (2), 105–109. doi:10.1192/bjp.bp.115.180653
Ge, C., Wang, S., Wu, X., and Lei, L. (2023). Quercetin mitigates depression-like behavior via the suppression of neuroinflammation and oxidative damage in corticosterone-induced mice. J. Chem. Neuroanat. 132, 102313. doi:10.1016/j.jchemneu.2023.102313
Ge, C., Wang, S., Wu, X., and Lei, L. (2024). Quercetin attenuates brain apoptosis in mice with chronic unpredictable mild stress-induced depression. Behav. Brain Res. 465, 114934. doi:10.1016/j.bbr.2024.114934
Guan, T., Cao, C., Hou, Y., Li, Y., Wei, X., Li, S., et al. (2021). Effects of quercetin on the alterations of serum elements in chronic unpredictable mild stress-induced depressed rats. Biometals 34 (3), 589–602. doi:10.1007/s10534-021-00298-w
Guan, Y., Wang, J., Wu, X., Song, L., Wang, Y., Gong, M., et al. (2021). Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 1772, 147661. doi:10.1016/j.brainres.2021.147661
Holzmann, I., Da Silva, L. M., Corrêa Da Silva, J. A., Steimbach, V. M. B., and de Souza, M. M. (2015). Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol. Biochem. Behav. 136, 55–63. doi:10.1016/j.pbb.2015.07.003
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14 (1), 43. doi:10.1186/1471-2288-14-43
Hou, Y., Qian, H., Yao, R., Yu, Z., Wang, J., Dai, J., et al. (2025). Untargeted metabolomics revealed that quercetin inhibited ferroptosis by improving metabolic disorder in the hippocampus of perimenopausal depression model rats. Mol. Neurobiol. 62 (3), 2872–2888. doi:10.1007/s12035-024-04445-5
Jain, D., and Gangshettiwar, A. (2014). Combination of lycopene, quercetin and poloxamer 188 alleviates anxiety and depression in 3-nitropropionic acid-induced huntington's disease in rats. J. Intercult. Ethnopharmacol. 3 (4), 186–191. doi:10.5455/jice.20140903012921
Jia, S., Wang, R., Zhang, D., Guan, Z., Ding, T., Zhang, J., et al. (2023). Quercetin modulates the liver metabolic profile in a chronic unpredictable mild stress rat model based on metabolomics technology. Food Funct. 14 (3), 1726–1739. doi:10.1039/d2fo03277e
Khan, K., Najmi, A. K., and Akhtar, M. (2019). A natural phenolic compound quercetin showed the usefulness by targeting inflammatory, oxidative stress markers and augment 5-HT levels in one of the animal models of depression in mice. Drug Res. 69 (7), 392–400. doi:10.1055/a-0748-5518
Kishi, T., Yoshimura, R., Ikuta, T., and Iwata, N. (2017). Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front. Psychiatry. 8, 308. doi:10.3389/fpsyt.2017.00308
Kore, M. S., Godad, A. P., and Doshi, G. M. (2024). Evaluation of the combinatory anti-depressant approach of metformin and quercetin in mice exposed to chronic unpredictable mild stress behavorial alteration. Indian J. Pharm. Educ. Res. 58 (4), 1130–1138. doi:10.5530/ijper.58.4.124
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., and Moryś, J. (2018). BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 38 (3), 579–593. doi:10.1007/s10571-017-0510-4
Li, B., Yan, Y., Zhang, T., Xu, H., Wu, X., Yao, G., et al. (2024). Quercetin reshapes gut microbiota homeostasis and modulates brain metabolic profile to regulate depression-like behaviors induced by CUMS in rats. Front. Pharmacol. 15, 1362464. doi:10.3389/fphar.2024.1362464
Li, Y., Zhang, B., Cui, Z., Fan, P., and Wang, S. (2024). Quercetin regulates depression-like behavior in CUMS rat models via TLR4/NF-κB signaling. Biocell 48 (5), 731–744. doi:10.32604/biocell.2024.048820
Liu, J., Fang, Y., Wei, Z., Yang, X., and Zeng, L. (2013). Synergic antidepressive effect of quercetin and Hypericum perforatum extract in mice. Zhejiang Da Xue Xue Bao Yi Xue Ban. 42 (6), 615–619. doi:10.3785/j.issn.1008-9292.2013.06.004
Ma, Y., Zhou, X., Zhang, F., Huang, C., Yang, H., Chen, W., et al. (2024). The effect of Scutellaria baicalensis and its active ingredients on major depressive disorder: a systematic review and meta-analysis of literature in pre-clinical research. Front. Pharmacol. 15, 1313871. doi:10.3389/fphar.2024.1313871
Ma, Z. X., Zhang, R. Y., Rui, W. J., Wang, Z. Q., and Feng, X. (2021). Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/BDNF signaling pathway. Behav. Brain Res. 406, 113245. doi:10.1016/j.bbr.2021.113245
Madiha, S., Batool, Z., Tabassum, S., Liaquat, L., Sadir, S., Shahzad, S., et al. (2021). Quercetin exhibits potent antioxidant activity, restores motor and non-motor deficits induced by rotenone toxicity. PLoS One 16 (11), e0258928. doi:10.1371/journal.pone.0258928
Makhdoomi, S., Fadaiie, A., Mohammadi, M., Ranjbar, A., and Haddadi, R. (2024). Quercetin's restorative properties in Male mice with 3-Nitropropionic acid-induced huntington-like symptoms: molecular docking, behavioral, and biochemical assessment. Cell biochem. Biophys. 82 (2), 1489–1502. doi:10.1007/s12013-024-01302-z
Maleki, D. P., Sadoughi, F., Asemi, Z., and Yousefi, B. (2021). Anti-cancer properties of quercetin in osteosarcoma. Cancer Cell Int. 21 (1), 349. doi:10.1186/s12935-021-02067-8
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet. 392 (10161), 2299–2312. doi:10.1016/S0140-6736(18)31948-2
Mehta, V., Parashar, A., and Udayabanu, M. (2017). Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 171, 69–78. doi:10.1016/j.physbeh.2017.01.006
Merzoug, S., Toumi, M. L., and Tahraoui, A. (2014). Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn. Schmiedeb. Arch. Pharmacol. 387 (10), 921–933. doi:10.1007/s00210-014-1008-y
Mirza, M. A., Mahmood, S., Hilles, A. R., Ali, A., Khan, M. Z., Zaidi, S., et al. (2023). Quercetin as a therapeutic product: evaluation of its pharmacological action and clinical Applications-A review. Pharmaceuticals 16 (11), 1631. doi:10.3390/ph16111631
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189. doi:10.1016/j.jclinepi.2021.03.001
Pannu, A., Sharma, P. C., Thakur, V. K., and Goyal, R. K. (2021). Emerging role of flavonoids as the treatment of depression. Biomolecules 11 (12), 1825. doi:10.3390/biom11121825
Qi, W., Qi, W., Xiong, D., and Long, M. (2022). Quercetin: its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules 27 (19), 6545. doi:10.3390/molecules27196545
Quraishi, M., Mokale, S. N., and Sakle, N. S. (2018). Ameliorative effect of quercetin and rutin via modulation o f hypothalamic-pituitary-adrenal axis and regulation of fasting glucose in chronic stress -Induced prediabetes. Pharmacogn. Mag. 14 (55), S65–S71. doi:10.4103/pm.pm_323_17
Rebai, R., Boudah, A., and Derri, N. (2017). Melatonin and quercetin improve depressive-like behavior and dyslipidemia in streptozotocin-induced diabetes in rats. J. ANIMAL PLANT Sci. 27 (6), 2081–2085.
Reshma, A., Subramanian, A., Kumarasamy, V., Tamilanban, T., Sekar, M., Gan, S. H., et al. (2024). Neurocognitive effects of proanthocyanidin in alzheimer's disease: a systematic review of preclinical evidence. Braz. J. Med. Biol. Res. 57, e13587. doi:10.1590/1414-431X2024e13587
Rinwa, P., and Kumar, A. (2013). Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 255, 86–98. doi:10.1016/j.neuroscience.2013.09.044
Sadighparvar, S., Darband, S. G., Yousefi, B., Kaviani, M., Ghaderi-Pakdel, F., Mihanfar, A., et al. (2020). Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: possible involvement of inflammation and BDNF signalling. Exp. Physiol. 105 (9), 1598–1609. doi:10.1113/EP088605
Sah, S. P., Tirkey, N., Kuhad, A., and Chopra, K. (2011). Effect of quercetin on lipopolysaccharide induced-sickness behavior and oxidative stress in rats. Indian J. Pharmacol. 43 (2), 192–196. doi:10.4103/0253-7613.77365
Samad, N., Saleem, A., Yasmin, F., and Shehzad, M. A. (2018). Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in Male mice. Physiol. Res. 67 (5), 795–808. doi:10.33549/physiolres.933776
Sampogna, G., Toni, C., Catapano, P., Rocca, B. D., Di Vincenzo, M., Luciano, M., et al. (2024). New trends in personalized treatment of depression. Curr. Opin. Psychiatr. 37 (1), 3–8. doi:10.1097/YCO.0000000000000903
Shi, Y., Luan, D., Song, R., and Zhang, Z. (2020). Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 41, 40–51. doi:10.1016/j.euroneuro.2020.09.633
Singh, T., Kaur, T., and Goel, R. K. (2017). Adjuvant quercetin therapy for combined treatment of epilepsy and comorbid depression. Neurochem. Int. 104, 27–33. doi:10.1016/j.neuint.2016.12.023
Sriram, B. S., and Ravichandra, V. (2019). An experimental study evaluating the influence of quercetin on monosodium glutamate-induced depression in Swiss albino Male mice. Asian J. Pharm. Clin. Res. 12 (5), 292–294. doi:10.22159/ajpcr.2019.v12i5.32986
Su, P., Li, Z., Yan, X., Wang, B., Bai, M., Li, Y., et al. (2024). Quercetin and kaempferol inhibit HMC-1 activation via SOCE/NFATc2 signaling and suppress hippocampal mast cell activation in lipopolysaccharide-induced depressive mice. Inflamm. Res. 73 (6), 945–960. doi:10.1007/s00011-024-01876-7
Subramanian, A., Tamilanban, T., Sekar, M., Begum, M. Y., Atiya, A., Ramachawolran, G., et al. (2023). Neuroprotective potential of Marsilea quadrifolia linn against monosodium glutamate-induced excitotoxicity in rats. Front. Pharmacol. 14, 1212376. doi:10.3389/fphar.2023.1212376
Tan, Z., Yang, G., Qiu, J., Yan, W., Liu, Y., Ma, Z., et al. (2022). Quercetin alleviates demyelination through regulating microglial phenotype transformation to mitigate neuropsychiatric symptoms in mice with vascular dementia. Mol. Neurobiol. 59 (5), 3140–3158. doi:10.1007/s12035-021-02712-3
Tavakol, F., Amini-Khoei, H., Sureda, A., Zarean, E., and Lorigooini, Z. (2024). Exploring the anti-depressant effects and nitric oxide modulation of quercetin: a preclinical study in socially isolated mice. World J. Biol. Psychiatry. 25 (10), 592–603. doi:10.1080/15622975.2024.2424162
Tong, Y., Dong, L., Shu, H., Yang, Y., Bai, Y., and Wen, J. (2023). Preclinical evidence evaluation of xiaoyao san in treating chronic unpredictable mild stress model of depression based on meta-analysis. Phytomedicine 119, 154991. doi:10.1016/j.phymed.2023.154991
Toumi, M. L., Merzoug, S., and Boutefnouchet, A. (2019). Ameliorating effects of quercetin in a diabetic rat model with social anxiety disorder. Environ. Health Sci. 11 (2), 132–143. doi:10.1007/s13530-019-0398-0
Ugwu, P. I., Ben-Azu, B., Ugwu, S. U., Uruaka, C. I., Nworgu, C. C., Okorie, P. O., et al. (2022). Preventive putative mechanisms involved in the psychopathologies of mice passively coping with psychosocial defeat stress by quercetin. Brain Res. Bull. 183, 127–141. doi:10.1016/j.brainresbull.2022.03.004
Wang, D., Wang, J., Yu, Z., Yao, R., Zhang, J., and Zhao, X. (2024a). Quercetin alleviates perimenopausal depression induced by ovariectomy combined with chronic unpredictable mild stress through regulating serum elements and inhibiting ferroptosis in prefrontal cortex of rats. Biol. Trace Elem. Res. 202 (12), 5596–5611. doi:10.1007/s12011-024-04106-7
Wang, D., Yu, Z., Yao, R., Zhang, J., Cui, W., Dai, J., et al. (2024b). Quercetin alleviates depressive-like behavior by modulating acetyl-H3K9 mediated ferroptosis pathway in hypothalamus of perimenopausal depression rat model. Biomed. Pharmacother. 179, 117369. doi:10.1016/j.biopha.2024.117369
Wang, G., Li, Y., Lei, C., Lei, X., Zhu, X., Yang, L., et al. (2021). Quercetin exerts antidepressant and cardioprotective effects in estrogen receptor α-deficient female mice via BDNF-AKT/ERK1/2 signaling. J. Steroid. Biochem. Mol. Biol. 206, 105795. doi:10.1016/j.jsbmb.2020.105795
Wang, M., Wei, X., Jia, Y., Wang, C., Wang, X., Zhang, X., et al. (2024c). Quercetin alleviates chronic unpredictable mild stress-induced depression-like behavior by inhibiting NMDAR1 with α2δ-1 in rats. CNS Neurosci. Ther. 30 (4), e14724. doi:10.1111/cns.14724
Williamson, G., and Manach, C. (2005). Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 81 (1 Suppl. l), 243S–255S. doi:10.1093/ajcn/81.1.243S
World Health Organization (2020). Depression. Available online at: https://www.who.int/news-room/fact-sheets/detail/depression.
Wu, J., Lv, T., Liu, Y., Liu, Y., Han, Y., Liu, X., et al. (2024). The role of quercetin in NLRP3-associated inflammation. Inflammopharmacology 32 (6), 3585–3610. doi:10.1007/s10787-024-01566-0
Xu, D., Hu, M. J., Wang, Y. Q., and Cui, Y. L. (2019). Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24 (6), 1123. doi:10.3390/molecules24061123
Yang, Y., Zhao, Y., Wang, L., Tong, J., and Li, J. (2022). The effects of quercetin treatment combined with exercise training on post-stroke depression like behavior in rats. Chin. J. Neuroanat. 38 (3), 293–299. doi:10.16557/j.cnki.1000-7547.2022.03.008
Zhang, J., Ning, L., and Wang, J. (2020). Dietary quercetin attenuates depressive-like behaviors by inhibiting astrocyte reactivation in response to stress. Biochem. Biophys. Res. Commun. 533 (4), 1338–1346. doi:10.1016/j.bbrc.2020.10.016
Keywords: quercetin, depression, systematic review, meta-analysis, preclinical studies
Citation: Yang Y, Zhang Y, Chen L, Li Z and Zhao Q (2025) Therapeutic potential of quercetin in depressive symptoms: a systematic review and meta-analysis of preclinical studies. Front. Pharmacol. 16:1598053. doi: 10.3389/fphar.2025.1598053
Received: 22 March 2025; Accepted: 13 June 2025;
Published: 08 July 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Mohamed Addi, Mohamed Premier University, MoroccoArunkumar Subramanian, SRM Institute of Science and Technology, India
Copyright © 2025 Yang, Zhang, Chen, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingchun Zhao, emhhb3FpbmdjaHVuMTk2N0AxNjMuY29t
 Yang Yang
Yang Yang Yingshi Zhang
Yingshi Zhang Lixin Chen2
Lixin Chen2 Qingchun Zhao
Qingchun Zhao