- 1Department of Pharmacology, Faculty of Medicine, Masaryk University, Brno, Czechia
- 2Masaryk Memorial Cancer Institute, Brno, Czechia
- 3Department of Pharmacology and Toxicology, Faculty of Pharmacy, Masaryk University, Brno, Czechia
The use of cannabinoids has a history spanning thousands of years, and their pharmacological and toxicological properties, particularly those of THC and CBD, are well-documented. However, their potential to induce drug-drug interactions remains underexplored. This review aims to provide a comprehensive perspective by contextualizing the historical and pharmacological significance of cannabinoids while focusing on their capacity to modulate the metabolic activity of cytochrome P450 isoforms relevant to drug metabolism. Additionally, we look at the impact of cannabinoids in neuronal circuits impacting the hypothalamic-pituitary hormonal axis, such as the locus coeruleus and raphe nuclei and their possible consequences on the cytochrome P450 system. Recognising potential interactions between cannabinoids and other drugs could enhance understanding of their pharmacological effects, improve the efficacy and safety profiles of cannabinoid-based therapies, and encourage further exploration into this under-researched area of psychopharmacology, with implications for both preclinical research and clinical practice.
1 Background
Cannabis has been used for its medicinal effects for almost five thousand years - the first mention comes from China and dates back to 2737 BC (Zuardi, 2006). Importance was predominantly placed on the nutritional value of the seeds within these regions. In ancient and medieval cultures, it was predominantly used (in addition to its psychoactive effect) to treat a variety of somatic diseases, including headache, fever, bacterial infections, diarrhea, rheumatic pain, and malaria. The 19th century brought extensive medical literature around the use of cannabis, with one of the most notable works being The Hasheesh Eater (1857) of Fitz Hugh Ludlow. The Emperor Wears No Clothes: The Authoritative Historical Record of Cannabis and the Conspiracy Against Marijuana of Jack Herer demonstrates the hysteria around the use of cannabis during this period in the United States of America (USA) “… from 1842 until the end of the 19th century, marijuana, … was one of three substances (after alcohol and opium) more commonly used (in massive doses, generally by oral ingestion)”. The end of the 19th century brought decline and replacement by opium derivates easier to use (Menezes, 2024). A renaissance of its use followed at the beginning of the 20th century with personalities like Queen Victoria and Empress Sissi, who used it for its antitussive properties and appetite stimulation (Crocq, 2020). Protocols for the preparation of extracts and tinctures were incorporated in the third edition of the American Pharmacopoeia with the intention of treatment of mental disorders. However, they were later removed due to the introduction of the Marihuana Tax Act of 1937 in the USA (Aggarwal et al., 2009). Throughout most of the 20th century, cannabis use for medical purposes was limited due to a lack of knowledge about its active substance, (−)-trans-Δ9-tetrahydrocannabinol (THC), which was discovered in 1964 (Gaoni and Mechoulam, 1964), followed by the discovery of cannabinoid receptors and in 1992 also the discovery of endocannabinoids (Devane et al., 1992).
There is an ongoing debate among the botanical community as to whether cannabis exists as a single species (Cannabis sativa with different subspecies and varieties) or whether there are three separate species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. The nomenclature of the plant is based on the organoleptic properties of the plant, the cannabinoid and terpenoid content, and even the habitus of the plant itself, like the shape or size of leaves (Grotenhermen and Russo, 2002). Cannabinoids are a group of compounds present in the plant of cannabis directly alongside others like terpenoids. Grotenhermen and Russo (2002) write that the best-known cannabinoids are THC and cannabidiol (CBD), and depending on the plant, the concentration and ratio can vary.
2 Pharmacology of the cannabinoids and the endocannabinoid system
2.1 Cannabinoids
The group of cannabinoids comprise phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Phytocannabinoids are all cannabinoids isolated from Cannabis sativa, Cannabis indica and Cannabis ruderalis. Endocannabinoids are molecules produced in the human body and fit into different chemical classes (Grotenhermen and Russo, 2002). Synthetic cannabinoids are chemically promiscuous compounds (Roque-Bravo et al., 2023), which we will delve deeper into further. Besides the two best-known phytocannabinoids, THC and CBD over 120 other phytocannabinoids were identified. Monoterpenoids (e.g., myrcene, α-pinene, and limonene) have significant pharmacological effects through the direct activation of the CB1 receptor with variable amplitude response (between 10% and 48%) of 10 µM THC (Raz et al., 2023). Besides the direct interaction with CB1, Raz et al. (2023) determined that monoterpenoids can augment THC-mediated activation of CB1 receptor which, depending on the type of monoterpenoid, can be the result of a summation of effects (e.g., linalool) or potentiation (e.g., limonene).
THC was isolated in 1964 by Raphael Mechoulam and colleagues (Gaoni and Mechoulam, 1964). THC can interact as a partial agonist on cannabinoid receptors (Pertwee, 2008). There are two types of cannabinoid receptors: CB1 and CB2. These are G-protein coupled receptors and have different prevalences in the human body (Nyíri et al., 2005). While CB1 receptors are more readily found in the central nervous system (CNS), the CB2 receptors are in microglia, osteoclasts, and macrophages (Grotenhermen and Russo, 2002; Nyíri et al., 2005; Atakan, 2012; Groce, 2018). The psychoactive properties of THC can be attributed to the partial agonism of CB1 receptors. This interaction causes symptoms like drowsiness, increased appetite, or short-term memory loss (Grotenhermen and Russo, 2002). Reducing the effects of THC merely to its “psychoactive” purposes would be wrong and an understatement. Analgesic, antipruritic, antiemetic, neuroprotective, and bronchodilatory properties have been described so far (Groce, 2018). The effects of THC should not be separated from that of CBD because CBD is a negative allosteric modulator of CB1 (Laprairie et al., 2015). Unlike THC, it does not act directly on the orthosteric site but allosterically decreases the efficacy of orthosteric ligands such as THC. It is believed that it has a neuroprotective effect while avoiding the intoxication caused by THC. This detail can be the main reason why CBD is claimed to be a non-psychoactive molecule, even though it has psychoactive (modulatory) actions (Russo, 2017). Anxiolytic action (Resstel et al., 2009) and anti-convulsant role (Carlini and Cunha, 1981) have also been described. This anxiolytic action possibly results from the agonism of the 5-HT1A receptor (Resstel et al., 2009).
Cannabigerol (CBG) is another non-psychoactive phytocannabinoid found in Cannabis sativa. Like THC, CBG was synthesized and isolated in 1964 (Gaoni and Mechoulam, 1964); however, its clinical significance and research attention have remained relatively limited compared to THC and CBD. The latter compounds have been incorporated into clinical practice, notably in the form of oromucosal spray Sativex® (GW Pharma Ltd.), a registered medicine in the European Union used to alleviate symptoms of multiple sclerosis. In contrast to these more extensively studied phytocannabinoids, CBG exhibits negligible activity at the CB1 receptor and acts as a partial agonist at CB2 (Navarro et al., 2018). Notably, CBG is believed to be a potent agonist of the α2-adrenoceptor at nanomolar concentrations, suggesting potential relevance in the development of antihypertensive therapies and in the treatment of psychiatric conditions such as post-traumatic stress disorder and attention-deficit disorder. Additionally, CBG demonstrates strong antagonistic activity at the 5-HT1A receptor (Cascio et al., 2010). CBG also causes the activation of the PPARγ receptor (Atalay et al., 2019), which contributes to reducing inflammation (Granja et al., 2012).
2.2 Position of the regulatory agencies to the implementation of cannabinoids in clinical use
The integration of cannabinoids into clinical practice is tightly regulated and must meet the same core regulatory requirements related to safety, efficacy, quality, and manufacturing standards as any other drug. However, in practice, cannabinoid-based drugs often face additional scrutiny due to their association with controlled substances and the legal status of cannabis under national and international drug control laws. In the United States, the Food and Drug Administration (FDA) has approved only a few cannabinoid-based medications, such as Epidiolex® (Greenwich Biosciences) for specific seizure disorders, and synthetic cannabinoids like dronabinol for chemotherapy-induced nausea (U.S. Food and Drug Administration, 2020). In the European Union, the European Medicines Agency (EMA) follows similar principles, requiring comprehensive clinical data and adherence to Good Manufacturing Practice (GMP) (European Medicines Agency, 2019). Both agencies emphasize the need for well-controlled studies, pharmacovigilance plans, and clear evidence of benefit over risk. Besides these clinically used drugs, crude dried female flowers of Cannabis sativa or Cannabis indica, known as “medical cannabis,” have been approved in some countries (e.g., the UK, Germany, France—pilot use only, Netherlands, Switzerland, Denmark, Italy, Portugal, Finland, Norway, Poland, and the Czech Republic), while in others it is still prohibited, highly restricted, or approved only in clinical trials (e.g., Slovakia, Bulgaria, Serbia, Hungary, Latvia). Legislation and rules about prescription and reimbursement in different countries were recently revised elsewhere (Baratta et al., 2022). The concentration of active constituents in medical cannabis must be precisely specified, with THC and CBD content typically ranging from approximately 0.1%–20%, as determined by accredited laboratory analysis. Medical cannabis may be clinically used in several indications, including chronic pain (particularly cancer-related pain), neuropathic pain, glaucoma-associated pain, spasticity and spasticity-related pain in multiple sclerosis or spinal cord injury, dyskinesias, and other complications caused by neurological disorders or injuries to the spine or brain, including Parkinsonian tremor (U.S. Food and Drug Administration, 2023; Landa et al., 2018).
2.3 Synthetic cannabinoids
Besides phytocannabinoids, synthetic cannabinoids (SCs) are part of NPS (new psychoactive substances). Roque-Bravo and colleagues (Roque-Bravo et al., 2023) provided an extensive review of these substances. There are numerous SCs with various structures, often non-related to phytocannabinoids or endocannabinoids, and they can be incorporated into different chemical classes: aminoalkylindoles (e.g., WIN55), indazole carboxamides, naphthoylindoles (e.g., JWH-015), pyrazole derivatives (e.g., AM-251) and many more (Suriaga et al., 2023). SCs are lipophilic substances mainly inhaled and rarely consumed by herbal infusions as a tea. From the pharmacological point of view, even taking into consideration structural differences most of the SCs are full agonists of CB1 and CB2, which activation causes a decrease in adenyl cyclase activity and, consequently, a decrease in cAMP and protein kinase A (PKA) in the presynaptic neuron. The activation also contributes to the inhibition of influx of Ca2+ and stimulation of efflux of K+ which hyperpolarizes the membrane and makes the release of neurotransmitters impossible. In the postsynaptic neuron, the binding of SCs to CB1 and CB2 contributes to the activation of kinases such as mitogen-activated protein kinase (MAPK) and extracellular kinases 1 and 2 (ERK1/2). The binding to orphan receptors (e.g., GPR55) leads to increased intracellular Ca2+ concentrations. Interaction with PPAR- γ nuclear receptor leads to regulation of gene transcription (Roque-Bravo et al., 2023). Various partial agonists, antagonists or inverse agonists were also synthesized and studied by Roque-Bravo et al. (2023).
2.4 Endocannabinoids and endocannabinoid system
Endocannabinoids (ECs) are endogenous ligands for cannabinoid receptors. These lipophilic compounds are eicosanoids derived from arachidonic acid. Anandamide (N-arachidonoylethanolamine) and 2-arachidonoyglycerol (2-AG) were the first ECs discovered in 1992 (Devane et al., 1992) and 1995 (Mechoulam et al., 1995). They are an integral part of the endocannabinoid system and act as retrograde regulators of glutamate, gamma-aminobutyric acid (GABA), acetylcholine, and serotonin (Katzung, 2018). Anandamide is a partial agonist (with higher affinity than 2-AG) of cannabinoid receptors but can also bind to other kind of receptors (e.g., TRPV1 and TRPV4) (Pertwee, 2008). 2-AG is a selective full agonist of cannabinoid receptors (Gonsiorek et al., 2000). Other endocannabinoids, such as virodhamine and oleamide, have a chemical structure similar to anandamide, while noladin ether is similar to 2-AG (Rodríguez de Fonseca et al., 2005).
Anandamide is biosynthesized from N-acylphosphatidylethanolamines (NAPEs) through four pathways. The most important is the classic pathway, where the hydrolysis of NAPEs occurs by NAPE-specific phospholipase D (NAPE-PLD). 2-AG is the product of the action of phospholipase-C, phosphatases, and lipases α/β that catalyze the breakdown of diacylglycerols (DAGs). The two main enzymes responsible for the degradation of these endocannabinoids are fatty acid amide hydrolase-1 (FAAH-1) and monoacylglycerol lipase (MAGL). FAAH-1 is responsible for the degradation of anandamide to arachidonate and ethanolamine, while MAGL is responsible for the degradation of 2-AG to arachidonate and glycerol (Deutsch and Chin, 1993; Arreaza et al., 1997). In the metabolism of anandamide, other enzymes such as FAAH-2 and N-acylethanolamine acid amidase (NAAA) also play an active role. In the metabolism of 2-AG, the MAGL and other serine hydrolases make up almost the full extent of the degradation (Evagorou et al., 2010). Interestingly, to a certain extent, FAAH-1, through condensation of arachidonate and ethanolamine, can contribute to the biosynthesis of anandamide, but the importance relative to degradation is negligible. Cyclooxygenase-2 (COX-2) metabolism of anandamide and 2-AG also occurs. This leads to the synthesis of prostaglandin-ethanolamides (or prostamides) and prostaglandins-glycerol, respectively (Simard et al., 2022). Prostamides, although agonists of CB1 and CB2, possess conformational restraints that do not allow for high-affinity binding when compared to anandamide (Berglund et al., 1999). Prostamides, specifically prostamide F2a, have weak activity towards TRPV1 (Matias et al., 2004). A noticeable decrease in the production of interleukin-2 was also reported through PPARγ activation by an unknown anandamide metabolite produced by COX-2 (Rockwell and Kaminski, 2004). Other metabolic pathways of ECs, such as cytochrome P450 and 12/15-lipoxygenases (12/15-LOX), also occur (Simard et al., 2022). More recently, the term “endocannabinoidome” has been coined to describe this group of endocannabinoids, receptors, and biosynthetic and degradation enzymes (Di Marzo, 2018). Modulating the endocannabinoid system involves regulating many physiological functions in the human body, including neurobehavioral processes, hormonal regulation, metabolic pathways, and the proper functioning of the gastrointestinal tract. (Grotenhermen and Russo, 2002; Groce, 2018).
3 The regulation of cytochrome P450 metabolic activity
3.1 Induction and inhibition
Cytochrome P450 (P450) family comprise 18 families and over 50 isoenzymes (Nebert et al., 2013). Besides the substantial influence of genetic polymorphisms, the activity of P450 can be modulated by induction or inhibition. These effects may be mediated by drugs, food supplements, components of the diet, cigarette smoke, herbal compounds and hormones. Induction occurs through the interaction of an inducer with nuclear receptors (sometimes referred to as xenosensors), such as aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), pregnane X receptor (PXR) and the peroxisome proliferator receptors (PPAR) (Knight et al., 2008) or glucocorticoid receptor (Dvorak and Pavek, 2010). Once the interaction occurs, the xenosensors will interfere with P450 gene expression. Commonly, one of the nuclear receptors interferes with another in a process called cross-talk. Understanding the cross-talk between the major nuclear receptors and others, such as NF-κB, is also relevant for understanding the inflammation processes in the human body (Casarett et al., 2008; Zendulka et al., 2016). Inflammation due to the cross-talk between PXR and NF-κB also influences the P450 protein. Inflammation is a trigger for decreased P450 expression and drug metabolization. Inflammatory mediators (such as cytokines and interleukins) activate NF-κB, which inhibits the induction of P450 isoforms (as CYP3A4, CYP2B6, and CYP2C9) by the nuclear receptor PXR (Pavek, 2016; Lenoir et al., 2021). Unlike enzyme induction, which requires multiple doses of the interacting drug to develop, enzyme inhibition occurs immediately after the first administration of the inhibiting drug, as it directly blocks the enzyme’s activity without needing time for upregulation or synthesis. There are two types of inhibition of P450 enzymes: reversible and irreversible, the latter of which is also known as mechanism-based inhibition (Pelkonen et al., 2008). In reversible inhibition, competitive inhibition occurs between the substrate and inhibitor for the enzyme’s active site. The binding is weak, the action is rapid, and it does not inactivate the enzyme. Reversible inhibition can be further divided into uncompetitive inhibition, which occurs when the inhibitor binds to the enzyme-substrate complex, and mixed-inhibition, when both competitive and uncompetitive occur at the same time (Lin and Lu, 1998). Compared to the latter, irreversible or mechanism-based inhibition is of longer duration due to the formation of strong covalent bonds and is dependent on the formation of inhibitor metabolites. This type of binding can only be reverted by the synthesis of a new enzyme, and in some circumstances, this may not be possible due to total enzyme inactivation (Halpert, 1995).
3.2 Cannabinoids as regulators of cytochrome P450 metabolic activity
Yamaori and colleagues demonstrated through multiple studies using human liver microsomes and recombinant P450: CYP1A1, CYP1A2, CYP1B1 (Yamaori et al., 2010), CYP2A6, CYP2B6 (Yamaori et al., 2011a), CYP2C9 (Yamaori et al., 2012), and CYP2D6 (Yamaori et al., 2011b) that THC and its metabolites are reversible inhibitors of several isoforms of P450. The inhibition potencies of the main cannabinoids (THC, CBD, and CBN) are summarized in Table 1 below:
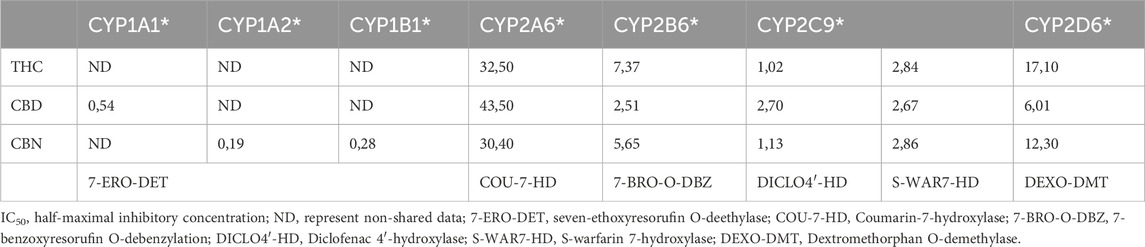
Table 1. The IC50 (µM) of the major cannabinoids investigated in recombinant human CYP enzymes (Yamaori et al., 2010; Yamaori et al., 2011a; Yamaori et al., 2011b; Yamaori et al., 2012).
The inhibition of CYP2B6 by CBD is attributed to the influence of free hydroxyl phenol groups and the pentyl side chain (Yamaori et al., 2011b). THC, CBD, and CBN are also capable of inhibiting CYP2D6, with CBD being the most potent competitive inhibitor. The possibility of structural influence by the hydroxyl groups and pentyl side chains was again discussed due to similar effects caused by the by-product of cannabinoid synthesis in Cannabis sativa, olivetol, which retains the hydroxyl groups and pentyl side chain and cannabidivarin (CBDV). In 2012, Yamaori and colleagues described a strong inhibition of the CYP2C9 isoform by THC, CBD, and CBN (Yamaori et al., 2012).
The same authors also evaluated mechanism-based inhibition (Yamaori et al., 2010; Yamaori et al., 2011a; Yamaori et al., 2011b; Yamaori et al., 2012). Mechanism-based inhibition of the CYP1A1 isoform was observed for THC, CBD, and CBN. CYP1A2 and CYP1B1 were inhibited by CBD (Yamaori et al., 2010). This inhibition is supported by a decrease in the IC50 value after a 20-min pre-incubation. For instance, the IC50 for CYP1A1 decreases from 0.411 μM at 0 min to 0.0767 µM after a 20-min pre-incubation with CBD. This pattern, although strongest for CBD, is observed for THC and CBN regarding the CYP1A1 isoform. Mechanism-dependent inhibition was also pointed out for CYP2A6 due to a marked decrease in IC50 for the various cannabinoids but not for CYP2B6, where the IC50 was similar (Yamaori et al., 2011a).
More recently, in 2020, Bansal and colleagues (Bansal et al., 2020) confirmed the inhibition of CYP1A2, 2C9, 2C19, 2D6, and 3A by THC and CBD (see Table 2). However, these recent studies suggest that the inhibition potencies of THC and CBD may have been underestimated due to methodological limitations, such as inadequate consideration of aqueous solubility or binding of cannabinoids to labware. The study used low-binding microcentrifuge tubes and included 0.2% bovine serum albumin in the reaction mixture to prevent underestimating the inhibition potencies. Thus, the IC50 and Ki values for the isoforms were much lower than those reported by studies of Yamaori and colleagues (Yamaori et al., 2010; Yamaori et al., 2011a; Yamaori et al., 2011b; Yamaori et al., 2012).

Table 2. Comparison of the IC50 (µM) values for inhibition studies of Yamaori et al, Bansal et al and Doohan et al. (Yamaori et al., 2010; Yamaori et al., 2011a; Yamaori et al., 2011b; Yamaori et al., 2012; Bansal et al., 2020; Doohan et al., 2021).
Bansal and colleagues (Bansal et al., 2020) also investigated inhibitory potencies of THC metabolites, specifically 11-COOH-THC and 11-OH-THC. 11-OH-THC was found to be a reversible inhibitor of CYP2C9, 2C19, 2D6, and CYP3A, while 11-COOH-THC did not appear to have any inhibitory action. The clinical relevancy of inhibitory potencies of these metabolites is questionable since they have higher IC50 values than THC for the evaluated P450. Protein binding was again found to play an important role in the strength of time-dependent inhibition of CYP1A2 by CBD. In 2022, Bansal and colleagues (Bansal et al., 2022) expanded previous findings (Bansal et al., 2020) by determining time-dependent inhibition of CBD and its metabolites (7-OH-CBD and 7-COOH-CBD) for CYP2A6, CYP2B6, and CYP2C8 in human liver microsomes. They found that CBD, THC, and their metabolites are potent inhibitors of CYP2B6 and CYP2C8, but no time-dependent inhibition was observed for these P450 isoforms.
In 2020, Nagao and colleagues (Nagao et al., 2020) demonstrated that CBD has a non-linear pharmacokinetic profile due to the saturation of P450 enzyme activities and that it strongly inhibits CYP3A, similar to ketoconazole, in rats. Using a 13C-erythromycin breath test, they confirmed that CBD inhibits the metabolism of erythromycin, a CYP3A substrate, at doses of 10 mg/kg and 50 mg/kg.
In 2021, Doohan and colleagues (Doohan et al., 2021) explored the interaction of twelve cannabinoids with P450 isoforms. For this experiment, Supersomes™ was used, which represents a combination of a single recombinant P450 isoform and NADPH reductase expressed in insect cells. CBN and CBD were found to be inhibitors of caffeine metabolism by CYP1A2 and bupropion metabolism by CYP2B6, respectively, while having weak or no inhibitory effects on triazolam, nifedipine, and testosterone metabolism by CYP3A4 and dextromethorphan metabolism by CYP2D6. Most of cannabinoids inhibited tolbutamide metabolism by CYP2C9, with CBDA (cannabidiolic acid) having a stronger inhibitory effect than sulfaphenazole (Doohan et al., 2021).
Previous studies have shown that cannabinoids can inhibit various P450 isoforms, but there is evidence that they may also induce P450 enzymes. THC was found to induce CYP1A1 in HEPA-1 cells through direct action on the AhR, increasing messenger RNA (mRNA) levels and seven-ethoxyresorufin-o-deethylase (EROD) activity. Interestingly, although marijuana tar induced CYP1A1, the presence of THC in the tar seemed to restrict this induction compared to tobacco tar. However, at the transcriptional level, THC increased CYP1A1 mRNA (Roth et al., 2001). This suggests that THC may act in two ways: as a competitive inhibitor of CYP1A1, reducing the efficiency of binding by other substrates, and as an inducer of CYP1A1 mRNA. The structure of THC is comparable to the prototypical activator of AhR, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which can help explain the activation (although weak) of AhR and mild induction of CYP1A1 (Dauchy et al., 2009). These results were confirmed and expanded upon later when the major phytocannabinoids (THC, CBD, and CBN) were evaluated for their ability to induce CYP1A1 in human HepG2 cells (Yamaori et al., 2015). CBD was revealed to be the most potent inducer of them. The induction of CYP1A1 by CBD occurs via ligand-independent mechanisms through the activation of protein tyrosine kinase and phosphorylation of AhR, much like what happens with omeprazole (Daujat et al., 1992). It’s relevant to mention that crucial for this induction is the pentylresorcinol moiety of CBD (Yamaori et al., 2015). The increase in mRNA expression for CYP1A1 by CBD was independent of the activation of CB1, CB2, and TRPV1 receptors; hence, through docking analysis, the possibility of CBD interacting with the AhR-ARNT complex was put forward (Jang et al., 2023). This differs from the ligand-independent mechanisms mentioned before (Daujat et al., 1992; Yamaori et al., 2015).
While our primary focus was on THC and CBD, a growing body of research has expanded to include additional cannabinoids such as CBG. Doohan and colleagues identified CBG as a strong inhibitor of CYP2C9 metabolic activity (Doohan et al., 2021). Using human liver microsomes, extracts derived from various plant chemotypes with distinct cannabinoid profiles were evaluated for their ability to inhibit cytochrome P450 enzymes. Notably, extracts with high concentrations of CBG demonstrated strong inhibitory effects on CYP2C9 and CYP3A4 isoforms, comparable to those observed with CBD (Treyer et al., 2023). These findings align with those of Roy and colleagues (Roy et al., 2022), who reported CBG as a moderate inhibitor of CYP3A4 and—importantly—of CYP2D6, an interaction not previously documented. In contrast, other cannabinoids like CBC (cannabichromene) and CBDA need to be studied for potential drug-drug interactions.
In the essential oil of Cannabis sativa, there are also present other active ingredients, such as sesquiterpenes β-caryophyllene and trans-nerolidol. In recent years, multiple studies have been conducted to demonstrate their influence on the P450 enzyme system (Nguyen et al., 2017; Lněničková et al., 2018; Šadibolová et al., 2019) and the results are summarized in Table 3. Synergism between these compounds and cannabinoids has been previously suggested, for example, between β-caryophyllene and CBD (Blanton et al., 2022). β-caryophyllene is considered an CB2 agonist (Gertsch et al., 2008). CBD interacts with multiple G protein-coupled receptors including: TRPV1 (Bisogno et al., 2001) and 5-HT1A (Russo et al., 2005). Cross-talk has been suggested to contribute to synergic analgesic effect (Blanton et al., 2022).
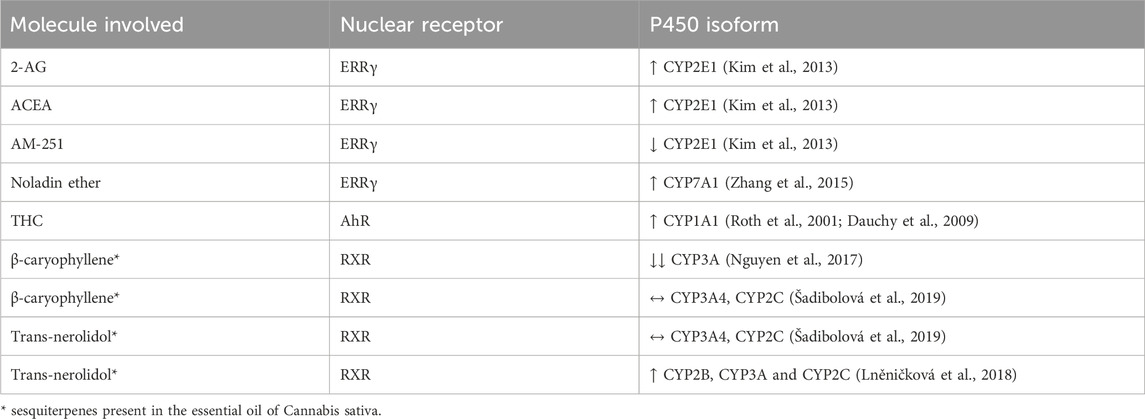
Table 3. Modulation of cytochrome P450 enzymes via nuclear receptors by cannabinoids and sesquiterpenes.
Kim and colleagues studied the influence of endocannabinoids on CYP2E1 (Kim et al., 2013). Authors conclude that mice exposed to alcohol also had high levels of 2-AG, and through its agonism on the CB1 receptors in hepatocytes, 2-AG increased the expression of ERRγ. This nuclear receptor further leads to increased expression of the CYP2E1 enzyme (see Table 3). These findings were confirmed using ACEA (CB1 agonist) and AM251 (CB1 antagonist), in which the latter blocked 2-AG signaling and diminished CYP2E1 induction (Kim et al., 2013). The inverse agonist of ERRγ, GSK5182, decreases CYP2E1 induction. Noladin ether, also known as 2-AGE (2-arachidonyl glyceryl ether), a cannabinoid-like compound, has been shown to induce CYP7A1 through the activation of ERRγ, which results from the prior activation of hepatic CB1 receptors. Although this isoform is not directly related to drug metabolism, it is associated with liver dysfunction due to the accumulation of bile acids, which could disrupt enterohepatic recirculation and, therefore, indirectly affect drug metabolism (Zhang et al., 2015). Recently, in a study about the role of cannabinoids in protection against retina degeneration, it was observed that the intravitreal injection of ACEA, a selective CB1 receptor agonist, and AM251, a selective CB1 antagonist, interfere with the transcription of CYP1A1. While ACEA led to decreased AhR expression, AM251 led to the opposite and, thus, CYP1A1 induction (Soliño et al., 2022).
3.3 Endocannabinoid system and modulation of neurotransmitter systems
The importance of different neurotransmitter systems in the regulation of P450 enzymes has been previously described (Wójcikowski and Daniel, 2011; Kuban and Daniel, 2021; Haduch et al., 2022; 2023; Pukło et al., 2023). Endocannabinoid system is a retrograde regulator of the other neurotransmitter systems, particularly CB1 receptors are predominantly localized on presynaptic neuronal terminals, where they regulate the release of various neurotransmitters (Marsicano and Lutz, 1999; Schlicker and Kathmann, 2001). Endocannabinoids are synthesized and released by postsynaptic neurons and act retrogradely on presynaptic CB1 receptors to inhibit the release of GABA, glutamate, acetylcholine, serotonin, and noradrenaline Additional complexity arises from the ability of CB1 receptors to form heteromers with other G-protein coupled receptors (Hermann et al., 2002; Carriba et al., 2007; Chen et al., 2016). Interactions between the endocannabinoid and dopaminergic systems may also occur indirectly via GABAergic (Sperlágh et al., 2009) and glutamatergic pathways (Melis et al., 2004). Given that the endocannabinoid system is closely linked with these systems, it is possible to hypothesize that it influences drug metabolism by modulating the expression of P450 enzymes (Zendulka et al., 2016).
Neurotransmitters such as noradrenaline, dopamine, serotonin, and glutamate are indirectly involved in regulating the P450 enzymes, and cannabinoid signaling affects these neurotransmitters. This action on the P450 protein levels and activity is mediated by the hypothalamic-pituitary axis, which can stimulate or suppress hormone release, including growth hormone, corticosterone, and thyroid hormones. The hypothalamic paraventricular (PVN) and arcuate nucleus (ARC) are crucial in this process. The PVN contains neuroendocrine neurons that produce thyrotropin-releasing hormone (TRH), corticotropin-releasing hormone (CRH), and somatostatin (SRIH). The ARC is responsible for the production of growth hormone-releasing hormone (GHRH), which leads to the production of growth hormone (GH) by the pituitary (see Figure 1).
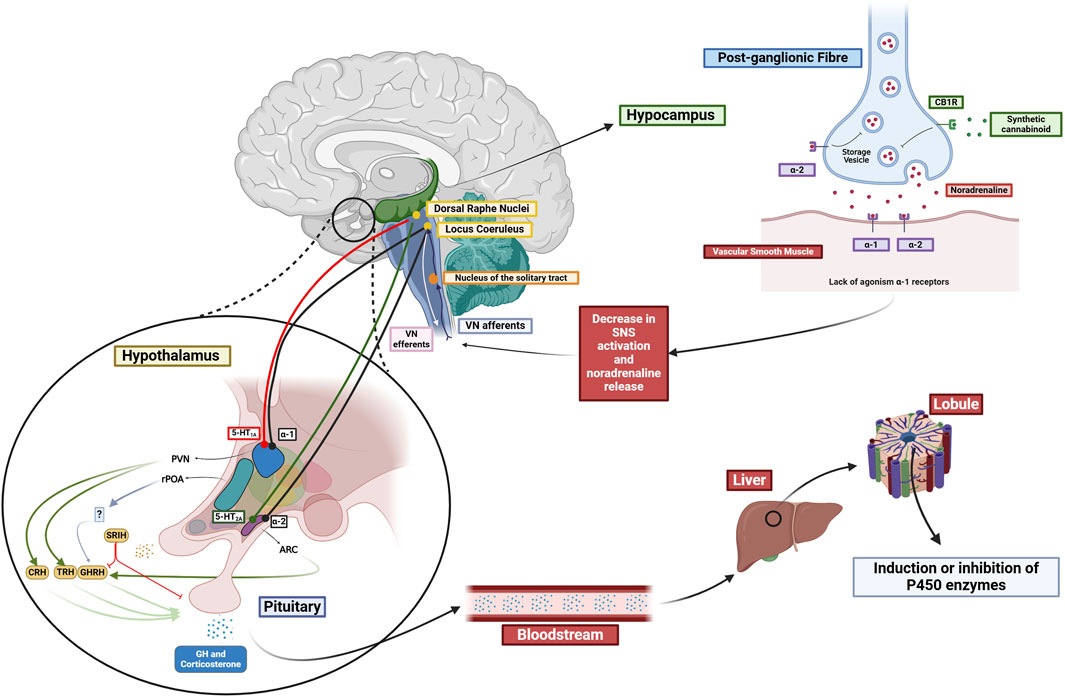
Figure 1. Proposed influence of the different neurotransmitter systems in the regulation of P450 enzymes. Cannabinoids interaction with CB1 receptors influences the modulation of various neurotransmitter systems (NA, DA, 5-HT). Possible hypothalamic influence in the PVN and ARC interfering with the release of several hormones, which, in turn, interact with nuclear receptors (PPARα, GR, PXR, and CAR), thereby affecting the inhibition or induction of P450 enzymes. Created in BioRender (https://BioRender.com/j18d041).
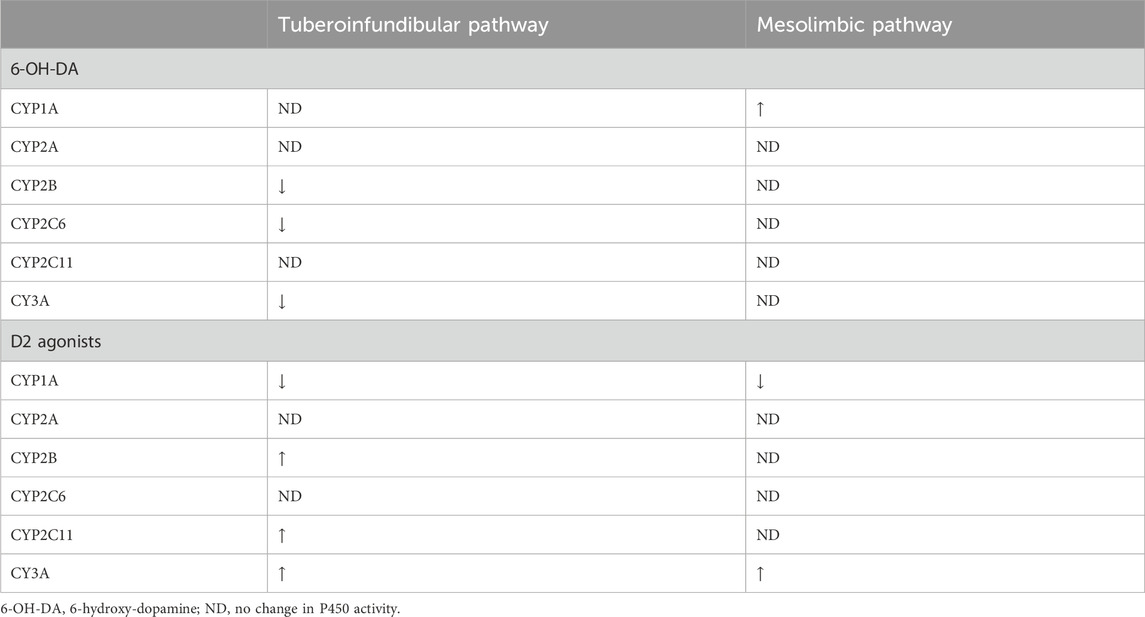
Since 2007, Daniel and colleagues (Wójcikowski et al., 2007; 2008; Wójcikowski and Daniel, 2008; 2009) have demonstrated the relation between the brain dopaminergic system and the regulation of P450 enzymes. Using 6-hydroxydopamine (6-OH-DA), they caused dopaminergic pathways (tuberoinfundibular, mesolimbic, nigrostriatal) lesions. The lesion of the tuberoinfundibular pathway resulted in the inhibition of CYP2B, CYP3A and CYP2C6. The lesion of the mesolimbic pathway resulted in modest induction of CYP1A. The lack of a neuronal connection between the tuberoinfundibular pathway and the pituitary gland, and consequently the lack of endogenous hormone release, explains some of the unchanged P450 activities. Nuclear receptors (GR, PXR, CAR, PPARα) are responsible for the regulation of P450 activity. PPARα seems to be activated by thyroid hormones, leading to an induction of CYP1A. Functional crosstalk between GR, PXR and CAR seems to regulate the activation of CYP2B and CYP3A (see Table 4).
Complementing previous findings activation of D2 receptors (e.g., apomorphine, amphetamine, quinpirole and SKF82958) led to an increase in GH and a decrease in triiodothyronine (T3) in the pituitary, which led to induction of CYP2B, CYP2C11, and CYP3A and inhibition of CYP1A. D2 receptor activation in the nucleus accumbens increased corticosterone and decreased T3, causing inhibition of CYP1A activity and induction of CYP3A (see Table 4) (Wójcikowski et al., 2008).
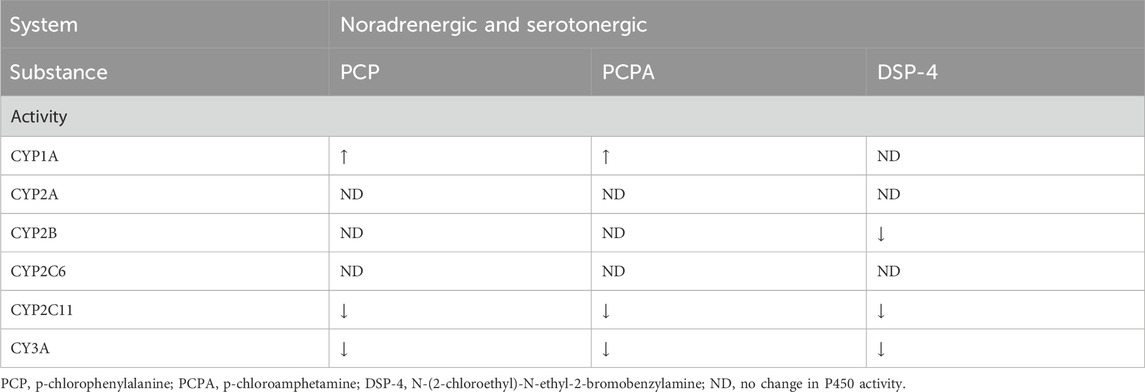
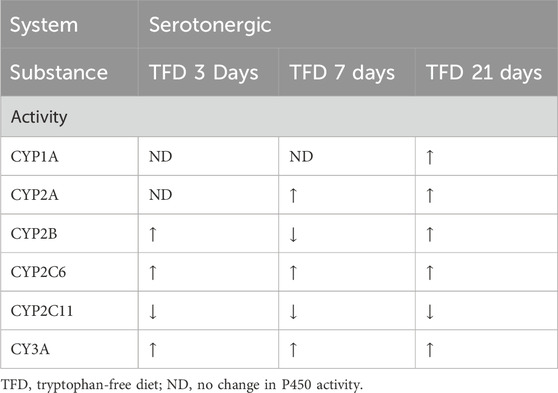
The researchers also investigated the interaction of neurotransmitter systems and hypothalamic/pituitary hormones through the pharmacological depletion of the respective neurotransmitters (Kot and Daniel, 2011). N - (2 - chloroethyl) - N -ethyl- 2 - bromobenzylamine (DSP-4) is an inhibitor of the reuptake of noradrenaline (Ross et al., 1973) and leads to a diminution of dopamine-β-hydroxylase activity (Ross, 1976), therefore functioning as a noradrenergic toxin. Similarly, p-chloroamphetamine (PCA) act as an inhibitor of serotonin uptake and an inhibitor of tryptophan hydroxylase (Sanders-Bush et al., 1975). The p-chlorophenylalanine (PCPA) inhibits tryptophan hydroxylase, therefore acting as a serotonergic toxin (Jéquier et al., 1967) (see Table 5). To expand on these previous results, a tryptophan-free diet was used as an alternative approach to test the influence of serotonin on the P450 enzyme system (see Table 6) (Kot et al., 2012). These experiments revealed that increased hypothalamic serotonin levels led to increased activity of the various CYP1A, CYP2A, CYP2B, CYP2C6, and CYP3A isoforms and decreased activity of CYP2C11. The deviation for CYP2A and CYP2B when comparing the tryptophan-free diet after 3 and 7 days is due to increased serotonin levels in the plasma and a plateau in the brain, which might be a consequence of an induced release of serotonin from the platelets acting as a reservoir for serotonin. This might be explained through the activation of 5-HT1A, (hypothalamic) 5-HT2A, 5-HT2B (pituitary) and 5-HT2C (adrenal cortex) receptors which increase adrenocorticotropic hormone (ACTH), GH and corticosterone levels (see Figure 1). After 3 weeks, a drop in serotonin, dopamine and respective metabolites was observed in the CNS and plasma. As a result, CYP1A, CYP2A, CYP2B, CYP2C6 and CYP3A had increased activity while the opposite was true for CYP2C11.
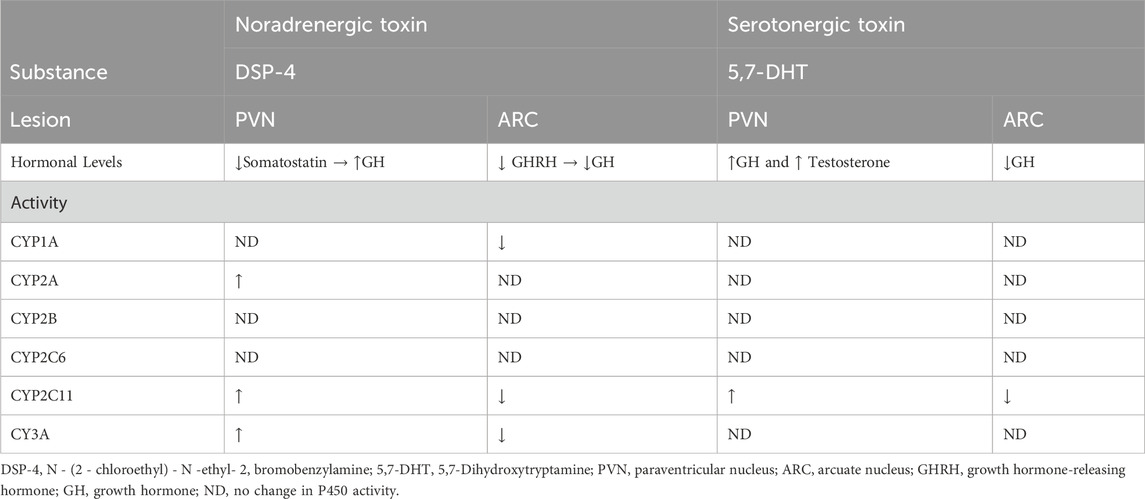
The intracerebral administration of DSP-4, a noradrenergic toxin, and 5,7-dihydroxytryptamine (5,7-DHT), a serotonergic toxin, changed P450 enzyme activity (see Table 7). These changes seem to be directly associated with changes in growth hormone levels (Bromek et al., 2013; Rysz et al., 2016).
More recently, in 2018, Bromek et al. identified that 5-HT1A receptors in PVN play a crucial role in the serotonergic regulation of P450 enzymes (Bromek et al., 2018). The agonist of the 5-HT1A receptor led to an increase in somatostatin, which in turn suppresses growth hormone release and, consequently, decreases CYP2C11 and CYP3A expression and enzyme activity. A decrease in corticotropin-releasing (CRT) was also observed. In 2019, Bromek and colleagues (Bromek et al., 2019), using the same experimental design, proved the importance of 5-HT2 in the regulation of P450. Both single and repeated administration of a selective 5-HT2 agonist 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropan increased CYP2C11 and CYP3A activity in the liver, resulting from increased synthesis of GHRH and, consequently, GH.
Considering the results of the previous studies, it is possible to outline a hypothesis about the involvement of cannabinoids in the HPA axis and subsequent regulation of the P450 enzymes (see Figure 1 below). Since CBD is known as an agonist of 5-HT1A (Russo et al., 2005), it would technically mediate similar effects as 5-carboxyamidotryptamine (5-CT) and 8-OH-DPAT (a 5HT1 serotonergic agonists) in the previous experiment (Bromek et al., 2018). Besides the direct interactions of cannabinoid ligands with DA, 5-HT or adrenergic receptors, endocannabinoids act as retrograde regulators of the above-mentioned systems (see Figure 1).
By this, various homotropic and heterotropic neuronal inter-regulations exist (Hermann et al., 2002; Gonzalez et al., 2009; Sperlágh et al., 2009). It has been hypothesised that at least a part of the effects of cannabinoids is regulated via dopamine (Di Marzo, 2009; Di Giovanni, 2010; Fernández-Ruiz et al., 2010; Kuepper et al., 2010; El Khoury et al., 2012). It is known that CB1 receptor stimulation increases dopamine release in the nucleus accumbens (Sperlágh et al., 2009). CB1 receptors are, similarly to α1 receptors, present on presynaptic elements in the nucleus accumbens, an integral part of the mesolimbic neuronal pathway (Chevaleyre et al., 2006; Mitrano et al., 2012). By this, nucleus accumbens also receives 5-HT signalling (Brown and Molliver, 2000).
In light of published papers (Sperlágh et al., 2009; Di Giovanni, 2010; Fernández-Ruiz et al., 2010; Kuepper et al., 2010; El Khoury et al., 2012), the overall effects of CB ligands could also be mediated via the cross-talk between endocannabinoid system and above-mentioned hormones, neuroregulatory pathways and subsequently influence P450 metabolic activity.
Another possible regulatory pathway of P450 by cannabinoids involves indirect influence on the noradrenergic and serotonergic transmission in specific brain regions, such as the locus coeruleus (major noradrenergic brainstem nucleus) and the dorsal raphe nuclei (the largest serotonergic nucleus) (Michelsen et al., 2008; Breton-Provencher et al., 2021). The influence on these sites could potentially have more significant consequences in the hypothalamus, affecting P450 expression.
Synthetic cannabinoids WIN 55212-2, and CP55940 increase noradrenergic activity in vivo (Mendiguren and Pineda, 2006). This effect does not occur locally by acting on CB1 receptors in the locus coeruleus. Instead, it is mediated through the systemic administration of CB1 agonists acting on peripheral CB1 receptors, resulting in hypotension due to the inhibition of noradrenaline release from postganglionic sympathetic neurons (Niederhoffer et al., 2003). The resulting hypotension leads to the activation of the neurons in the locus coeruleus through the vagus nerve (see Figure 1).
Regarding glutamatergic neurotransmission, activation of CB1 receptors by anandamide and synthetic cannabinoids WIN 55212-2 and CP55940 increase glutamatergic signaling to the PVN and ARC by activating NMDA receptors in locus coeruleus (Mendiguren and Pineda, 2004). This is important because locus coeruleus has innervations to the PVN and ARC in the hypothalamus (Szabadi, 2013). If an increase in the locus coeruleus activity were to occur, it could result in NA release and activation of parvocellular neurons in the PVN through α1-adrenoreceptors. These neurons are responsible for producing TRH, CRH, and SRIH. Similarly, if the ARC were to be activated through α2-adrenoreceptors consequently, it would lead to the release of growth hormone, which could subsequently interfere with the expression of specific P450 isoforms.
4 Future perspectives
The endocannabinoid system has been the focus of extensive research due to its promising therapeutic potential, with studies conducted in various fields, including psychiatry, inflammatory diseases and cancer (Groce, 2018). It is important to note that the function of the endocannabinoid system can be altered not only by cannabinoid receptor ligands but also indirectly by affecting the synthesis and degradation of endogenous cannabinoids. This offers a variety of pharmacological mechanisms for new drugs targeting the alteration of the activity of this important regulator of physiological and pathophysiological processes. Notwithstanding the elevated clinical expectations that pertain to the development of drugs based on the modulation of the endocannabinoid system, setbacks are also present (Di Marzo, 2018). It is also important to note the rising abuse of synthetic cannabinoids (Alzu’bi et al., 2024) and the legalization of cannabis for recreational use in many countries, both of which contribute to a growing population of cannabinoid users.
To better assess the interaction potential of existing or novel cannabinoids and other compounds affecting endocannabinoid system activity, it would be advisable to incorporate additional tests beyond the standard in vitro inhibition studies currently required by the EMA and FDA during preclinical drug evaluation. These supplementary assays could provide a more comprehensive understanding of potential effects on P450, including mechanisms beyond direct enzyme inhibition. Such tests should focus on the influence of studied drugs on the activation/inhibition of nuclear receptors regulating P450 hepatic activity, namely, CAR, AhR, and PXR. Because the endocannabinoid system is dynamic with many feedbacks and interplays with other neurotransmitters that change in time, the time factor should also be involved in the P450 testing. Different effects could be seen in both acute and chronic exposition to cannabinoids, similarly to modulation of glutamatergic neurotransmission by cannabis (Chowdhury et al., 2024).
5 Conclusion
This review addresses the critical knowledge gap regarding how cannabinoids interact with cytochrome P450 enzymes. As global cannabis use continues to rise, particularly among older adults, and with increasing THC concentrations in cannabis products (Manthey et al., 2021), understanding these interactions is essential for ensuring safe and effective use. This significance is further highlighted by the fact that older age groups are often polymedicated. While some studies highlight the inhibition of various P450 isoforms by THC, CBD, CBN, and CBDA, few explain the underlying mechanisms. Based on the existing literature, we hypothesize how the endocannabinoid system interacts with the monoaminergic and glutamatergic systems, its impact on the HPA axis, and how these interactions may ultimately influence P450 enzyme expression. Phytocannabinoids or synthetic cannabinoids are capable of direct drug-drug interactions at the level of P450 enzymes, as well as possibly capable of triggering a change in 5-HT, DA or NA signaling, which, in turn, might influence the liver P450 activity via hormones and nuclear receptors. Since the studies that have been published so far have not investigated the “net” central contribution of cannabinoid ligands to the “overall” change in the liver P450 activity, this hypothesis needs to be proved or disproved by well-designed experiments. This narrative review compels to provide further insights and to motivate research in this understudied topic.
Author contributions
CDF: Data curation, Writing – original draft, Resources, Visualization. OZ: Project administration, Writing – review and editing, Conceptualization. JJ: Formal Analysis, Writing – review and editing, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Authors acknowledge financial support from the Start Up Grant of Masaryk University MUNI/LF-SUp/1365/2023 and specific research at Masaryk University MUNI/A/1580/2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, S. K., Carter, G. T., Sullivan, M. D., ZumBrunnen, C., Morrill, R., and Mayer, J. D. (2009). Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J. Opioid Manag. 5, 153–168. doi:10.5055/jom.2009.0016
Alzu’bi, A., Almahasneh, F., Khasawneh, R., Abu-El-Rub, E., Baker, W. B., and Al-Zoubi, R. M. (2024). The synthetic cannabinoids menace: a review of health risks and toxicity. Eur. J. Med. Res. 29, 49. doi:10.1186/s40001-023-01443-6
Arreaza, G., Devane, W. A., Omeir, R. L., Sajnani, G., Kunz, J., Cravatt, B. F., et al. (1997). The cloned rat hydrolytic enzyme responsible for the breakdown of anandamide also catalyzes its formation via the condensation of arachidonic acid and ethanolamine. Neurosci. Lett. 234, 59–62. doi:10.1016/s0304-3940(97)00673-3
Atalay, S., Jarocka-Karpowicz, I., and Skrzydlewska, E. (2019). Antioxidative and anti-inflammatory properties of Cannabidiol. Antioxidants (Basel) 9, 21. doi:10.3390/antiox9010021
Atakan, Z. (2012). Cannabis, a complex plant: different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2, 241–254. doi:10.1177/2045125312457586
Bansal, S., Maharao, N., Paine, M. F., and Unadkat, J. D. (2020). Predicting the potential for cannabinoids to precipitate pharmacokinetic drug interactions via reversible inhibition or inactivation of major cytochromes P450. Drug Metab. Dispos. 48, 1008–1017. doi:10.1124/dmd.120.000073
Bansal, S., Paine, M. F., and Unadkat, J. D. (2022). Comprehensive predictions of cytochrome P450 (P450)-mediated in vivo cannabinoid-drug interactions based on reversible and time-dependent P450 inhibition in human liver microsomes. Drug Metab. Dispos. 50, 351–360. doi:10.1124/dmd.121.000734
Baratta, F., Pignata, I., Ravetto Enri, L., and Brusa, P. (2022). Cannabis for medical use: analysis of recent clinical trials in view of current legislation. Front. Pharmacol. 13, 888903. doi:10.3389/fphar.2022.888903
Berglund, B. A., Boring, D. L., and Howlett, A. C. (1999). Investigation of structural analogs of prostaglandin amides for binding to and activation of CB1 and CB2 cannabinoid receptors in rat brain and human tonsils. Adv. Exp. Med. Biol. 469, 527–533. doi:10.1007/978-1-4615-4793-8_77
Bisogno, T., Hanus, L., De Petrocellis, L., Tchilibon, S., Ponde, D. E., Brandi, I., et al. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852. doi:10.1038/sj.bjp.0704327
Blanton, H., Yin, L., Duong, J., and Benamar, K. (2022). Cannabidiol and beta-caryophyllene in combination: a therapeutic functional interaction. Int. J. Mol. Sci. 23, 15470. doi:10.3390/ijms232415470
Breton-Provencher, V., Drummond, G. T., and Sur, M. (2021). Locus coeruleus norepinephrine in learned behavior: anatomical modularity and spatiotemporal integration in targets. Front. Neural Circuits 15, 638007. doi:10.3389/fncir.2021.638007
Bromek, E., Rysz, M., Haduch, A., and Daniel, W. A. (2019). Serotonin receptors of 5-HT2 type in the hypothalamic arcuate nuclei positively regulate liver cytochrome P450 via stimulation of the growth hormone-releasing hormone/growth hormone hormonal pathway. Drug Metab. Dispos. 47, 80–85. doi:10.1124/dmd.118.083808
Bromek, E., Rysz, M., Haduch, A., Wójcikowski, J., and Daniel, W. A. (2018). Activation of 5-ht1a receptors in the hypothalamic paraventricular nuclei negatively regulates cytochrome P450 expression and activity in rat liver. Drug Metab. Dispos. 46, 786–793. doi:10.1124/dmd.117.079632
Bromek, E., Wójcikowski, J., and Daniel, W. A. (2013). Involvement of the paraventricular (PVN) and arcuate (ARC) nuclei of the hypothalamus in the central noradrenergic regulation of liver cytochrome P450. Biochem. Pharmacol. 86, 1614–1620. doi:10.1016/j.bcp.2013.09.006
Brown, P., and Molliver, M. E. (2000). Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J. Neurosci. 20, 1952–1963. doi:10.1523/JNEUROSCI.20-05-01952.2000
Carlini, E. A., and Cunha, J. M. (1981). Hypnotic and antiepileptic effects of cannabidiol. J. Clin. Pharmacol. 21, 417S-427S–427S. doi:10.1002/j.1552-4604.1981.tb02622.x
Carriba, P., Ortiz, O., Patkar, K., Justinova, Z., Stroik, J., Themann, A., et al. (2007). Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32, 2249–2259. doi:10.1038/sj.npp.1301375
L. J. Casarett, J. Doull, and C. D. Klaassen (2008). Casarett and Doull’s toxicology: the basic science of poisons. 7th ed. (New York: McGraw-Hill).
Cascio, M. G., Gauson, L. A., Stevenson, L. A., Ross, R. A., and Pertwee, R. G. (2010). Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 159, 129–141. doi:10.1111/j.1476-5381.2009.00515.x
Chen, J., Varga, A., Selvarajah, S., Jenes, A., Dienes, B., Sousa-Valente, J., et al. (2016). Spatial distribution of the cannabinoid type 1 and capsaicin receptors may contribute to the complexity of their crosstalk. Sci. Rep. 6, 33307. doi:10.1038/srep33307
Chevaleyre, V., Takahashi, K. A., and Castillo, P. E. (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 29, 37–76. doi:10.1146/annurev.neuro.29.051605.112834
Chowdhury, K. U., Holden, M. E., Wiley, M. T., Suppiramaniam, V., and Reed, M. N. (2024). Effects of cannabis on glutamatergic neurotransmission: the interplay between cannabinoids and glutamate. Cells 13, 1130. doi:10.3390/cells13131130
Crocq, M.-A. (2020). History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 22, 223–228. doi:10.31887/DCNS.2020.22.3/mcrocq
Dauchy, S., Miller, F., Couraud, P.-O., Weaver, R. J., Weksler, B., Romero, I.-A., et al. (2009). Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem. Pharmacol. 77, 897–909. doi:10.1016/j.bcp.2008.11.001
Daujat, M., Peryt, B., Lesca, P., Fourtanier, G., Domergue, J., and Maurel, P. (1992). Omeprazole, an inducer of human CYP1A1 and 1A2, is not a ligand for the Ah receptor. Biochem. Biophysical Res. Commun. 188, 820–825. doi:10.1016/0006-291X(92)91130-I
Deutsch, D. G., and Chin, S. A. (1993). Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 46, 791–796. doi:10.1016/0006-2952(93)90486-G
Devane, W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., et al. (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949. doi:10.1126/science.1470919
Di Giovanni, G. (2010). Dopamine interaction with other neurotransmitter systems: relevance in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 16, 125–126. doi:10.1111/j.1755-5949.2010.00143.x
Di Marzo, V. (2009). The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 60, 77–84. doi:10.1016/j.phrs.2009.02.010
Di Marzo, V. (2018). New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 17, 623–639. doi:10.1038/nrd.2018.115
Doohan, P. T., Oldfield, L. D., Arnold, J. C., and Anderson, L. L. (2021). Cannabinoid interactions with cytochrome P450 drug metabolism: a full-spectrum characterization. AAPS J. 23, 91. doi:10.1208/s12248-021-00616-7
Dvorak, Z., and Pavek, P. (2010). Regulation of drug-metabolizing cytochrome P450 enzymes by glucocorticoids. Drug Metab. Rev. 42, 621–635. doi:10.3109/03602532.2010.484462
El Khoury, M.-A., Gorgievski, V., Moutsimilli, L., Giros, B., and Tzavara, E. T. (2012). Interactions between the cannabinoid and dopaminergic systems: evidence from animal studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 36–50. doi:10.1016/j.pnpbp.2011.12.005
European Medicines Agency (2019). European medicines agency. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex (Accessed May 5, 2025).
Evagorou, A., Anagnostopoulos, D., Farmaki, E., and Siafaka-Kapadai, A. (2010). Hydrolysis of 2-arachidonoylglycerol in Tetrahymena thermophila. Identification and partial characterization of a Monoacylglycerol Lipase-like enzyme. Eur. J. Protistol. 46, 289–297. doi:10.1016/j.ejop.2010.06.001
Fernández-Ruiz, J., Hernández, M., and Ramos, J. A. (2010). Cannabinoid–dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 16, e72–e91. doi:10.1111/j.1755-5949.2010.00144.x
Gaoni, Y., and Mechoulam, R. (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647. doi:10.1021/ja01062a046
Gertsch, J., Leonti, M., Raduner, S., Racz, I., Chen, J.-Z., Xie, X.-Q., et al. (2008). Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A. 105, 9099–9104. doi:10.1073/pnas.0803601105
Gonsiorek, W., Lunn, C., Fan, X., Narula, S., Lundell, D., and Hipkin, R. W. (2000). Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol. Pharmacol. 57, 1045–1050. doi:10.1016/s0026-895x(24)26516-0
Gonzalez, B., Paz, F., Florán, L., Aceves, J., Erlij, D., and Florán, B. (2009). Cannabinoid agonists stimulate [3H]GABA release in the globus pallidus of the rat when G(i) protein-receptor coupling is restricted: role of dopamine D2 receptors. J. Pharmacol. Exp. Ther. 328, 822–828. doi:10.1124/jpet.108.145425
Granja, A. G., Carrillo-Salinas, F., Pagani, A., Gómez-Cañas, M., Negri, R., Navarrete, C., et al. (2012). A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 7, 1002–1016. doi:10.1007/s11481-012-9399-3
Groce, E. (2018). The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. J. Med. Regul. 104, 32. doi:10.30770/2572-1852-104.4.32
Grotenhermen, F., and Russo, E. B. (2002). Cannabis and cannabinoids: pharmacology, toxicology, and therapeutic potential. 1st Edn. New York, NY: Routledge.
Haduch, A., Bromek, E., Kuban, W., Basińska-Ziobroń, A., Danek, P. J., Alenina, N., et al. (2023). The effect of brain serotonin deficit (TPH2-KO) on the expression and activity of liver cytochrome P450 enzymes in aging male Dark Agouti rats. Pharmacol. Rep. 75, 1522–1532. doi:10.1007/s43440-023-00540-x
Haduch, A., Bromek, E., Pukło, R., Jastrzębska, J., Danek, P. J., and Daniel, W. A. (2022). The effect of the selective N-methyl-D-aspartate (NMDA) receptor GluN2B subunit antagonist CP-101,606 on cytochrome P450 2D (CYP2D) expression and activity in the rat liver and brain. Int. J. Mol. Sci. 23, 13746. doi:10.3390/ijms232213746
Halpert, J. R. (1995). Structural basis of selective cytochrome P450 inhibition. Annu. Rev. Pharmacol. Toxicol. 35, 29–53. doi:10.1146/annurev.pa.35.040195.000333
Hermann, H., Marsicano, G., and Lutz, B. (2002). Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109, 451–460. doi:10.1016/s0306-4522(01)00509-7
Jang, Y. su, Jeong, S., Kim, A.-ram, Mok, B. R., Son, S. J., Ryu, J., et al. (2023). Cannabidiol mediates epidermal terminal differentiation and redox homeostasis through aryl hydrocarbon receptor (AhR)-dependent signaling. J. Dermatological Sci. 109, 61–70. doi:10.1016/j.jdermsci.2023.01.008
Jéquier, E., Lovenberg, W., and Sjoerdsma, A. (1967). Tryptophan hydroxylase inhibition: the mechanism by which p-chlorophenylalanine depletes rat brain serotonin. Mol. Pharmacol. 3, 274–278. doi:10.1016/s0026-895x(25)14792-5
B. G. Katzung (2018). Basic and clinical pharmacology. Fourteenth edition (New York Chicago San Francisco Athens London Madrid Mexico City Milan New Delhi Singapore Sydney Toronto: McGraw-Hill Education).
Kim, D.-K., Kim, Y.-H., Jang, H.-H., Park, J., Kim, J. R., Koh, M., et al. (2013). Estrogen-related receptor γ controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 62, 1044, 1054. doi:10.1136/gutjnl-2012–303347
Knight, T. R., Choudhuri, S., and Klaassen, C. D. (2008). Induction of hepatic glutathione S-transferases in male mice by prototypes of various classes of microsomal enzyme inducers. Toxicol. Sci. 106, 329–338. doi:10.1093/toxsci/kfn179
Kot, M., and Daniel, W. A. (2011). Cytochrome P450 is regulated by noradrenergic and serotonergic systems. Pharmacol. Res. 64, 371–380. doi:10.1016/j.phrs.2011.06.020
Kot, M., Pilc, A., and Daniel, W. A. (2012). Simultaneous alterations of brain and plasma serotonin concentrations and liver cytochrome P450 in rats fed on a tryptophan-free diet. Pharmacol. Res. 66, 292–299. doi:10.1016/j.phrs.2012.06.009
Kuban, W., and Daniel, W. A. (2021). Cytochrome P450 expression and regulation in the brain. Drug Metab. Rev. 53, 1–29. doi:10.1080/03602532.2020.1858856
Kuepper, R., Morrison, P. D., van Os, J., Murray, R. M., Kenis, G., and Henquet, C. (2010). Does dopamine mediate the psychosis-inducing effects of cannabis? A review and integration of findings across disciplines. Schizophr. Res. 121, 107–117. doi:10.1016/j.schres.2010.05.031
Landa, L., Juřica, J., Slíva, J., Pechacková, M., and Demlová, R. (2018). Medical cannabis in the treatment of cancer pain and spastic conditions and options of drug delivery in clinical practice. Biomed. Pap. 162 (1), 18–25. doi:10.5507/bp.2018.007
Laprairie, R. B., Bagher, A. M., Kelly, M. E. M., and Denovan-Wright, E. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 172, 4790–4805. doi:10.1111/bph.13250
Lenoir, C., Rollason, V., Desmeules, J. A., and Samer, C. F. (2021). Influence of inflammation on cytochromes P450 activity in adults: a systematic review of the literature. Front. Pharmacol. 12, 733935. doi:10.3389/fphar.2021.733935
Lin, J. H., and Lu, A. Y. (1998). Inhibition and induction of cytochrome P450 and the clinical implications. Clin. Pharmacokinet. 35, 361–390. doi:10.2165/00003088-199835050-00003
Lněničková, K., Svobodová, H., Skálová, L., Ambrož, M., Novák, F., and Matoušková, P. (2018). The impact of sesquiterpenes β-caryophyllene oxide and trans-nerolidol on xenobiotic-metabolizing enzymes in mice in vivo. Xenobiotica 48, 1089–1097. doi:10.1080/00498254.2017.1398359
Manthey, J., Freeman, T. P., Kilian, C., López-Pelayo, H., and Rehm, J. (2021). Public health monitoring of cannabis use in Europe: prevalence of use, cannabis potency, and treatment rates. Lancet Regional Health - Eur. 10, 100227. doi:10.1016/j.lanepe.2021.100227
Marsicano, G., and Lutz, B. (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 11, 4213–4225. doi:10.1046/j.1460-9568.1999.00847.x
Matias, I., Chen, J., Petrocellis, L. D., Bisogno, T., Ligresti, A., Fezza, F., et al. (2004). Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J. Pharmacol. Exp. Ther. 309, 745–757. doi:10.1124/jpet.103.061705
Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N. E., Schatz, A. R., et al. (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90. doi:10.1016/0006-2952(95)00109-D
Melis, M., Pistis, M., Perra, S., Muntoni, A. L., Pillolla, G., and Gessa, G. L. (2004). Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 24, 53–62. doi:10.1523/JNEUROSCI.4503-03.2004
Mendiguren, A., and Pineda, J. (2004). Cannabinoids enhance N-methyl-d-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci. Lett. 363, 1–5. doi:10.1016/j.neulet.2004.02.073
Mendiguren, A., and Pineda, J. (2006). Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur. J. Pharmacol. 534, 83–88. doi:10.1016/j.ejphar.2006.01.002
Michelsen, K. A., Prickaerts, J., and Steinbusch, H. W. M. (2008). The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog. Brain Res. 172, 233–264. doi:10.1016/S0079-6123(08)00912-6
Mitrano, D. A., Schroeder, J. P., Smith, Y., Cortright, J. J., Bubula, N., Vezina, P., et al. (2012). α-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology 37, 2161–2172. doi:10.1038/npp.2012.68
Nagao, M., Nakano, Y., Tajima, M., Sugiyama, E., Sato, V. H., Inada, M., et al. (2020). Nonlinear disposition and metabolic interactions of cannabidiol through CYP3A inhibition in vivo in rats. Cannabis Cannabinoid Res. 5, 318–325. doi:10.1089/can.2019.0098
Navarro, G., Varani, K., Reyes-Resina, I., Sánchez de Medina, V., Rivas-Santisteban, R., Sánchez-Carnerero Callado, C., et al. (2018). Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1–CB2 heteroreceptor complexes. Front. Pharmacol. 9, 632. doi:10.3389/fphar.2018.00632
Nebert, D. W., Wikvall, K., and Miller, W. L. (2013). Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond B Biol. Sci. 368, 20120431. doi:10.1098/rstb.2012.0431
Nguyen, L. T., Myslivečková, Z., Szotáková, B., Špičáková, A., Lněničková, K., Ambrož, M., et al. (2017). The inhibitory effects of β-caryophyllene, β-caryophyllene oxide and α-humulene on the activities of the main drug-metabolizing enzymes in rat and human liver in vitro. Chemico-Biological Interact. 278, 123–128. doi:10.1016/j.cbi.2017.10.021
Niederhoffer, N., Schmid, K., and Szabo, B. (2003). The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Schmiedeb. Arch. Pharmacol. 367, 434–443. doi:10.1007/s00210-003-0755-y
Nyíri, G., Cserép, C., Szabadits, E., MacKie, K., and Freund, T. F. (2005). CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 136, 811–822. doi:10.1016/j.neuroscience.2005.01.026
Pavek, P. (2016). Pregnane X receptor (PXR)-Mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions. Front. Pharmacol. 7, 456. doi:10.3389/fphar.2016.00456
Pelkonen, O., Turpeinen, M., Hakkola, J., Honkakoski, P., Hukkanen, J., and Raunio, H. (2008). Inhibition and induction of human cytochrome P450 enzymes: current status. Arch. Toxicol. 82, 667–715. doi:10.1007/s00204-008-0332-8
Pertwee, R. G. (2008). Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol. 13, 147–159. doi:10.1111/j.1369-1600.2008.00108.x
Pukło, R., Bromek, E., Haduch, A., Basińska-Ziobroń, A., Kuban, W., and Daniel, W. A. (2023). Molecular mechanisms of the regulation of liver cytochrome P450 by brain NMDA receptors and via the neuroendocrine pathway—a significance for new psychotropic therapies. Int. J. Mol. Sci. 24, 16840. doi:10.3390/ijms242316840
Raz, N., Eyal, A. M., Zeitouni, D. B., Hen-Shoval, D., Davidson, E. M., Danieli, A., et al. (2023). Selected cannabis terpenes synergize with THC to produce increased CB1 receptor activation. Biochem. Pharmacol. 212, 115548. doi:10.1016/j.bcp.2023.115548
Resstel, L. B., Tavares, R. F., Lisboa, S. F., Joca, S. R., Corrêa, F. M., and Guimarães, F. S. (2009). 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 156, 181–188. doi:10.1111/j.1476-5381.2008.00046.x
Rockwell, C. E., and Kaminski, N. E. (2004). A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J. Pharmacol. Exp. Ther. 311, 683–690. doi:10.1124/jpet.104.065524
Rodríguez de Fonseca, F., Del Arco, I., Bermudez-Silva, F., Bilbao, A., Cippitelli, A., and Navarro, M. (2005). The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol. 40, 2–14. doi:10.1093/alcalc/agh110
Roque-Bravo, R., Silva, R. S., Malheiro, R. F., Carmo, H., Carvalho, F., da Silva, D. D., et al. (2023). Synthetic cannabinoids: a pharmacological and toxicological overview. Annu. Rev. Pharmacol. Toxicol. 63, 187–209. doi:10.1146/annurev-pharmtox-031122-113758
Ross, S. B. (1976). Long-term effects of N-2-chlorethyl-N-ethyl-2-bromobenzylamine hydrochloride on noradrenergic neurones in the rat brain and heart. Br. J. Pharmacol. 58, 521–527. doi:10.1111/j.1476-5381.1976.tb08619.x
Ross, S. B., Johansson, J. G., Lindborg, B., and Dahlbom, R. (1973). Cyclizing compounds. I. Tertiary N-(2-bromobenzyl)-N-haloalkylamines with adrenergic blocking action. Acta Pharm. Suec. 10, 29–42.
Roth, M. D., Marques-Magallanes, J. A., Yuan, M., Sun, W., Tashkin, D. P., and Hankinson, O. (2001). Induction and regulation of the carcinogen-metabolizing enzyme CYP1A1 by marijuana smoke and delta (9)-tetrahydrocannabinol. Am. J. Respir. Cell Mol. Biol. 24, 339–344. doi:10.1165/ajrcmb.24.3.4252
Roy, P., Dennis, D. G., Eschbach, M. D., Anand, S. D., Xu, F., Maturano, J., et al. (2022). Metabolites of cannabigerol generated by human cytochrome P450s are bioactive. Biochemistry 61, 2398–2408. doi:10.1021/acs.biochem.2c00383
Russo, E. B. (2017). Cannabidiol claims and misconceptions. Trends Pharmacol. Sci. 38, 198–201. doi:10.1016/j.tips.2016.12.004
Russo, E. B., Burnett, A., Hall, B., and Parker, K. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. doi:10.1007/s11064-005-6978-1
Rysz, M., Bromek, E., Haduch, A., Liskova, B., Wójcikowski, J., and Daniel, W. A. (2016). The reverse role of the hypothalamic paraventricular (PVN) and arcuate (ARC) nuclei in the central serotonergic regulation of the liver cytochrome P450 isoform CYP2C11. Biochem. Pharmacol. 112, 82–89. doi:10.1016/j.bcp.2016.04.017
Šadibolová, M., Zárybnický, T., Smutný, T., Pávek, P., Šubrt, Z., Matoušková, P., et al. (2019). Sesquiterpenes are agonists of the pregnane X receptor but do not induce the expression of phase I drug-metabolizing enzymes in the human liver. Int. J. Mol. Sci. 20, 4562. doi:10.3390/ijms20184562
Sanders-Bush, E., Bushing, J. A., and Sulser, F. (1975). Long-term effects of p-chloroamphetamine and related drugs on central serotonergic mechanisms. J. Pharmacol. Exp. Ther. 192, 33–41. doi:10.1016/s0022-3565(25)30027-3
Schlicker, E., and Kathmann, M. (2001). Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 22, 565–572. doi:10.1016/s0165-6147(00)01805-8
Simard, M., Archambault, A.-S., Lavoie, J.-P. C., Dumais, É., Di Marzo, V., and Flamand, N. (2022). Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochem. Pharmacol. 205, 115261. doi:10.1016/j.bcp.2022.115261
Soliño, M., Larrayoz, I. M., López, E. M., Rey-Funes, M., Bareiro, M., Loidl, C. F., et al. (2022). CB1 cannabinoid receptor is a target for neuroprotection in light induced retinal degeneration. Adv. Drug Alcohol Res. 2, 10734. doi:10.3389/adar.2022.10734
Sperlágh, B., Windisch, K., Andó, R. D., and Sylvester Vizi, E. (2009). Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem. Int. 54, 452–457. doi:10.1016/j.neuint.2009.01.017
Suriaga, A., Tappen, R. M., Aston, E. R., Chiang-Hanisko, L., and Newman, D. (2023). Cannabinoids and synthetic cannabinoids as a cause of death: trends and their healthcare implications. J. Nurs. Scholarsh. 55, 623–636. doi:10.1111/jnu.12817
Szabadi, E. (2013). Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. 27, 659–693. doi:10.1177/0269881113490326
Treyer, A., Reinhardt, J. K., Eigenmann, D. E., Oufir, M., and Hamburger, M. (2023). Phytochemical comparison of medicinal cannabis extracts and study of their CYP-mediated interactions with coumarinic oral anticoagulants. Med. Cannabis Cannabinoids 6, 21–31. doi:10.1159/000528465
U.S. Food and Drug Administration (2020). FDA. Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms (Accessed May 5, 2025).
U.S. Food and Drug Administration (2023). FDA. Available online at: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (Accessed May 5, 2025).
Wójcikowski, J., and Daniel, W. A. (2008). Identification of factors mediating the effect of the brain dopaminergic system on the expression of cytochrome P450 in the liver. Pharmacol. Rep. 60, 966–971.
Wójcikowski, J., and Daniel, W. A. (2009). The brain dopaminergic system as an important center regulating liver cytochrome P450 in the rat. Expert Opin. drug metabolism and Toxicol. 5, 631–645. doi:10.1517/17425250902973703
Wójcikowski, J., and Daniel, W. A. (2011). The role of the nervous system in the regulation of liver cytochrome p450. Curr. Drug Metab. 12, 124–138. doi:10.2174/138920011795016908
Wójcikowski, J., Gołembiowska, K., and Daniel, W. A. (2007). The regulation of liver cytochrome P450 by the brain dopaminergic system. CDM 8, 631–638. doi:10.2174/138920007781368872
Wójcikowski, J., Gołembiowska, K., and Daniel, W. A. (2008). Regulation of liver cytochrome P450 by activation of brain dopaminergic system: physiological and pharmacological implications. Biochem. Pharmacol. 76, 258–267. doi:10.1016/j.bcp.2008.04.016
Yamaori, S., Kinugasa, Y., Jiang, R., Takeda, S., Yamamoto, I., and Watanabe, K. (2015). Cannabidiol induces expression of human cytochrome P450 1A1 that is possibly mediated through aryl hydrocarbon receptor signaling in HepG2 cells. Life Sci. 136, 87–93. doi:10.1016/j.lfs.2015.07.007
Yamaori, S., Koeda, K., Kushihara, M., Hada, Y., Yamamoto, I., and Watanabe, K. (2012). Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab. Pharmacokinet. 27, 294–300. doi:10.2133/dmpk.dmpk-11-rg-107
Yamaori, S., Kushihara, M., Yamamoto, I., and Watanabe, K. (2010). Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem. Pharmacol. 79, 1691–1698. doi:10.1016/j.bcp.2010.01.028
Yamaori, S., Maeda, C., Yamamoto, I., and Watanabe, K. (2011a). Differential inhibition of human cytochrome P450 2A6 and 2B6 by major phytocannabinoids. Forensic Toxicol. 29, 117–124. doi:10.1007/s11419-011-0112-7
Yamaori, S., Okamoto, Y., Yamamoto, I., and Watanabe, K. (2011b). Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab. Dispos. 39, 2049–2056. doi:10.1124/dmd.111.041384
Zendulka, O., Dovrtělová, G., Nosková, K., Turjap, M., Šulcová, A., Hanuš, L., et al. (2016). Cannabinoids and cytochrome P450 interactions. Curr. Drug Metab. 17, 206–226. doi:10.2174/1389200217666151210142051
Zhang, Y., Kim, D.-K., Lee, J.-M., Park, S. B., Jeong, W.-I., Kim, S. H., et al. (2015). Orphan nuclear receptor oestrogen-related receptor γ (ERRγ) plays a key role in hepatic cannabinoid receptor type 1-mediated induction of CYP7A1 gene expression. Biochem. J. 470, 181–193. doi:10.1042/BJ20141494
Keywords: cannabinoids, endocannabinoid system, cytochrome P450, metabolism, hypothalamic-pituitary axis, cannabinoid-based therapies
Citation: Fonseca CDF, Zendulka O and Juřica J (2025) Cannabinoids and the endocannabinoid system in the regulation of cytochrome P450 metabolic activity-a review. Front. Pharmacol. 16:1599012. doi: 10.3389/fphar.2025.1599012
Received: 24 March 2025; Accepted: 19 May 2025;
Published: 05 June 2025.
Edited by:
Momir Mikov, University of Novi Sad, SerbiaReviewed by:
Zvi Loewy, Touro University, United StatesLirit Franks, The University of Utah, United States
Copyright © 2025 Fonseca, Zendulka and Juřica. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Juřica, anVyaWNhQG1lZC5tdW5pLmN6
 Carlos D.F. Fonseca
Carlos D.F. Fonseca Ondřej Zendulka
Ondřej Zendulka Jan Juřica
Jan Juřica