- 1Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3TCM Prevention and Treatment of Metabolic and Chronic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Diabetic cardiomyopathy (DCM) is one of the most common complications of diabetes, characterized by high morbidity and disability rates, and can lead to heart failure. However, specific therapeutic agents for DCM are currently lacking. Natural compounds derived from traditional Chinese medicine have demonstrated potential in alleviating DCM through multiple mechanisms. Sea buckthorn flavonoids and their derivatives represent a promising class of natural compounds for the treatment of DCM. These compounds have been shown to improve DCM by combating oxidative stress, inhibiting inflammatory responses, regulating epigenetic modifications, modulating autophagy and apoptosis, maintaining mitochondrial homeostasis, alleviating endoplasmic reticulum stress, reducing advanced glycation end products (AGEs) level, and ameliorating cardiomyocyte hypertrophy and myocardial fibrosis. This article provides a brief overview of the pharmacological effects of sea buckthorn flavonoids and their derivatives and systematically reviews their mechanisms in improving DCM. The aim is to promote the effective utilization of herbal medicine and provide insights and references for the development of novel therapeutics for DCM.
1 Introduction
With economic development, changes in lifestyle and dietary habits, and an aging population, diabetes has become an increasingly severe global public health issue. Over the past few decades, the number of diabetic patients and the prevalence of diabetes have been rising at an alarming rate. According to the latest International Diabetes Federation (IDF) Diabetes Atlas, 11th edition, approximately 589 million adults aged 20–79 years worldwide were living with diabetes in 2025, and this number is projected to rise dramatically to 853 million by 2050 (Federation, 2025). Diabetes and its associated complications pose a serious threat to global health and place a tremendous burden on healthcare systems worldwide. Consequently, diabetes has become one of the most urgent public health challenges today (Lin et al., 2020). Diabetes can cause microvascular and macrovascular damage, leading to a variety of diabetes-related complications (Zhang et al., 2024). Cardiovascular diseases induced by diabetes are among the leading causes of mortality associated with diabetes. Moreover, as an independent risk factor, diabetes doubles the risk of adverse outcomes, and when combined with cardiovascular diseases, the mortality rate is even higher (Zhao et al., 2021). It is estimated that patients with type 2 diabetes mellitus (T2DM) have a 75% higher risk of cardiovascular mortality or hospitalization due to heart failure compared to non-diabetic individuals (Søren et al., 2017). Several studies have confirmed that diabetes increases the risk of heart failure, regardless of the presence of common risk factors such as hypertension and coronary artery disease (Lemesle et al., 2021).
Clinical studies have shown that DCM is a major contributor to severe cardiovascular events and worsens the prognosis of diabetic patients. Current data indicate that approximately 12% of diagnosed diabetic patients are affected by DCM. Therefore, DCM has become a critical complication requiring urgent attention in the diabetic population (Lorenzo-Almorós et al., 2017). In 1927, Rubler et al. first proposed the existence of DCM based on observations of four diabetic patients who developed congestive heart failure without common risk factors such as hypertension, myocardial ischemia, coronary artery disease, valvular disease, or peripheral vascular disease (Salvatore et al., 2021). DCM is an organic heart disease caused by diabetes, defined as structural defects and functional abnormalities in the myocardium of diabetic patients in the absence of other cardiovascular diseases, such as coronary artery disease, hypertension, severe valvular disease, and congenital heart disease. It is a common and severe complication of diabetes that can lead to heart failure (Jia et al., 2018). As a typical metabolic cardiomyopathy, the early stages of DCM are marked by left ventricular wall hypertrophy and diastolic dysfunction, followed by the development of widespread myocardial fibrosis and hypertrophy, leading to left ventricular dilation and further exacerbation of diastolic dysfunction. The late stages of DCM are characterized by systolic dysfunction with reduced ejection fraction, accompanied by structural and functional cardiac abnormalities. Ultimately, DCM can result in cardiac insufficiency, heart failure, and even death (Paolillo et al., 2019).
With the increasing prevalence of T2DM, the incidence of DCM is also on the rise. Currently, the treatment of DCM primarily relies on glycemic control, lipid management, dietary adjustments, and increased physical activity, which are part of a comprehensive strategy for diabetes management. However, there are no specific drugs targeting the damaged myocardium in DCM. Furthermore, intensive glucose-lowering therapies have shown limited efficacy in reducing the risk of heart failure and may even increase such risks. This phenomenon is particularly evident with the use of thiazolidinediones, sulfonylureas, certain dipeptidyl peptidase-4 (DPP-4) inhibitors, and related insulin and glucagon-like peptide-1 agonists (Hippisley-Cox and Coupland, 2016; Kenneth B et al., 2016; Ling et al., 2016; Pan et al., 2020; Seferović et al., 2020). Therefore, there is an urgent need for alternative therapies to treat and limit the progression of DCM. In recent years, research on the development of traditional medicines or natural products for the treatment of diabetes and its complications has grown significantly. These natural compounds have become an important source of safe and effective agents for improving glycemic control and alleviating the progression of diabetic complications. Medicinal plants in traditional Chinese medicine serve as a rich repository of natural compounds and are a valuable resource for drug development. Recently, the active components of sea buckthorn have gained increasing attention. Sea buckthorn flavonoids and their derivatives are important natural compounds that have been demonstrated to improve DCM through multiple mechanisms. This review briefly outlines the pharmacological effects of flavonoid compounds derived from sea buckthorn and their major derivatives. Based on preclinical evidence, we systematically elucidate the multi-target mechanisms by which these natural constituents (e.g., catechins, quercetin, myricetin, and anthocyanins) ameliorate DCM, providing novel insights for future drug development and clinical therapeutic strategies.
2 The efficacy of sea buckthorn and the pharmacological effects of its flavonoid derivatives
2.1 The efficacy of sea buckthorn
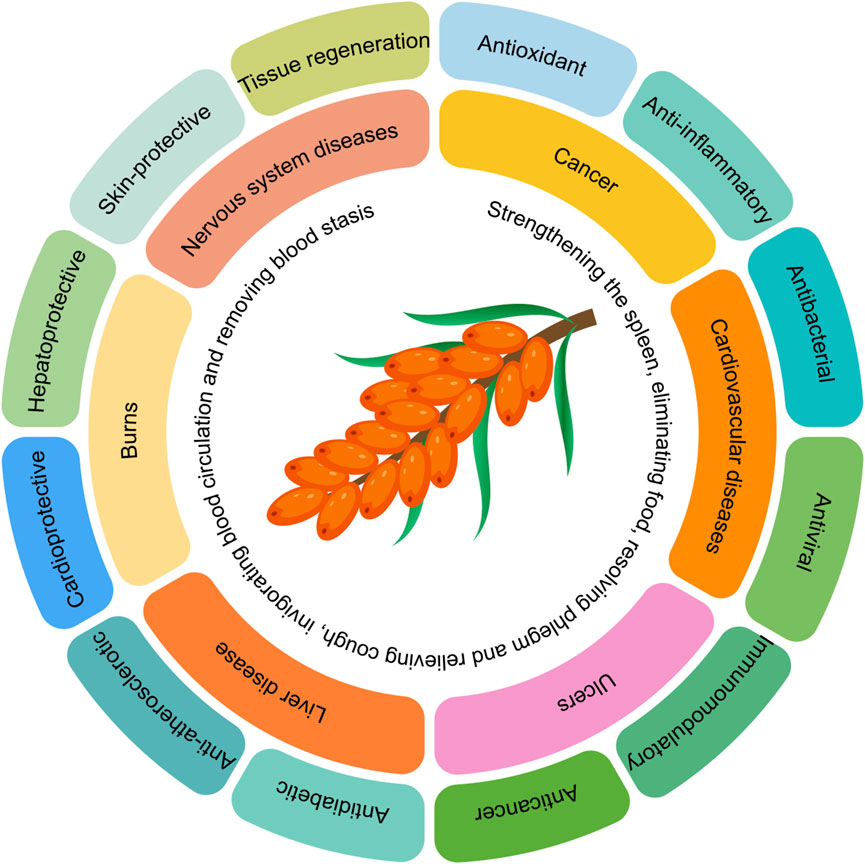
Sea buckthorn is a deciduous shrub belonging to the Elaeagnaceae family. It is estimated to have existed on Earth for approximately 200 million years. Ancient Greek literature records that sea buckthorn was first discovered by the Greeks around 5000 BC, who used it to feed racehorses to enhance their muscle mass (Pundir et al., 2021). Sea buckthorn contains nearly 200 known nutrients and bioactive compounds, giving it high nutritional value (Wang et al., 2022b). The berries of sea buckthorn are rich in proteins, fatty acids, amino acids, vitamins, trace elements, and polysaccharides, as well as bioactive substances such as flavonoids, sterols, triterpenes, tannins, and serotonin (Chen A. et al., 2023). Due to these unique chemical components, sea buckthorn exhibits a wide range of pharmacological activities. Therefore, sea buckthorn is not only a nutrient-rich fruit but also a medicinal plant with significant therapeutic value. In traditional Chinese medicine, sea buckthorn is believed to have multiple functions, including strengthening the spleen, aiding digestion, resolving phlegm, relieving cough, promoting blood circulation, and dispersing blood stasis (Qin-Ge et al., 2020). Modern research has found that sea buckthorn, owing to its antioxidant, anti-inflammatory, antimicrobial, antiviral, immunomodulatory, anticancer, antidiabetic, anti-atherosclerotic, cardioprotective, hepatoprotective, skin-protective, and tissue-regenerative properties, has been widely used in various medical formulations to treat conditions such as cancer, cardiovascular diseases, ulcers, liver diseases, burns, and central nervous system disorders (Christaki, 2012; Krejcarová et al., 2015; Wang et al., 2022b; Danielski and Shahidi, 2024). The summary of the efficacy of sea buckthorn is illustrated in Figure 1.
2.2 Pharmacological effects of sea buckthorn flavonoids and their derivatives
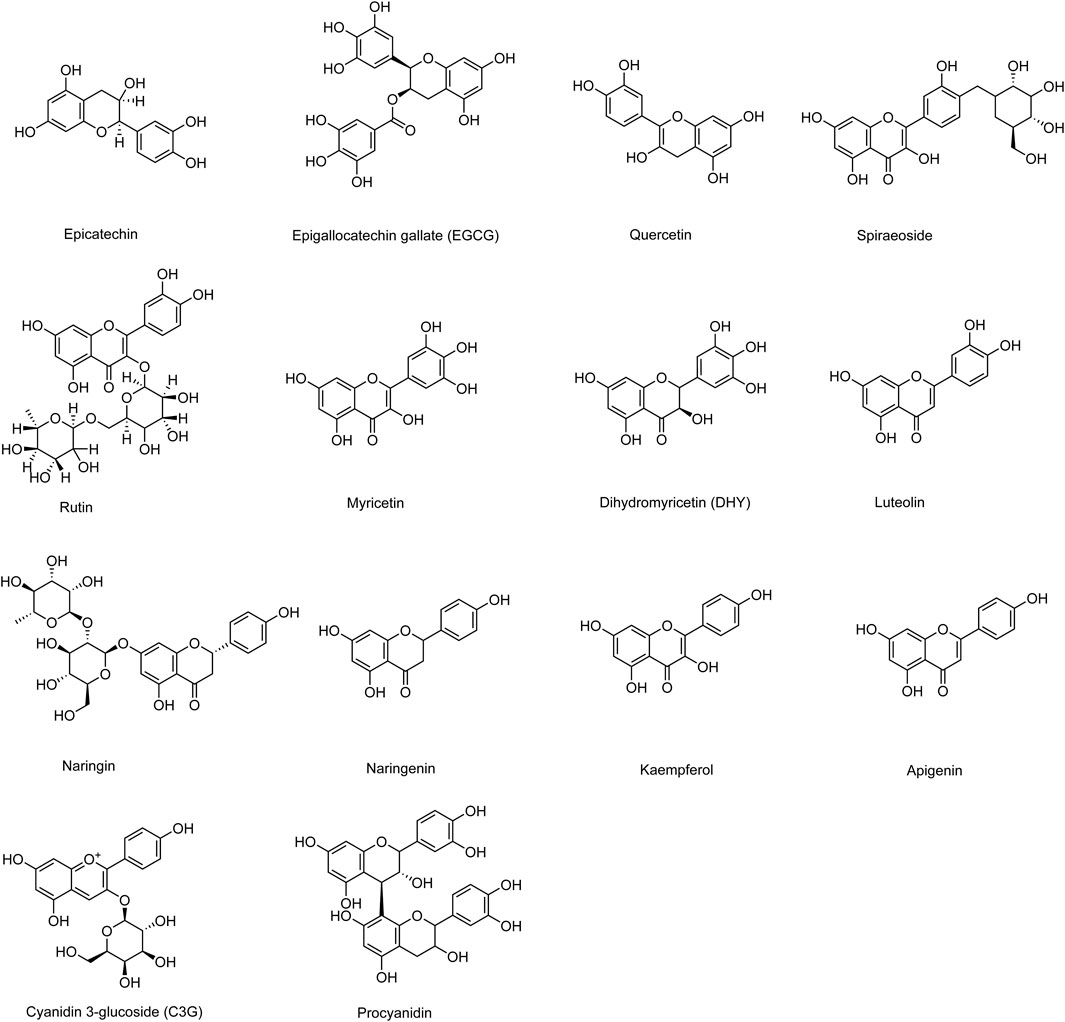
Phytochemical studies have shown that sea buckthorn contains various types of compounds, including flavonoids, phenolic acids, carotenoids, fatty acids, triterpenes, lignans, and vitamins (Tkacz et al., 2021; Xueying et al., 2021). Among these, flavonoids are the most abundant active components in sea buckthorn and are widely distributed in its fruits, leaves, stems, roots, and seeds. Most flavonoids exist as glycosides bound to sugars, while a few are present as free aglycones (Zheng et al., 2022). The flavonoid content in sea buckthorn berries is several times higher than that in other flavonoid-rich plants such as hawthorn, dogwood, cherry, and blueberry. In insulin resistance models, sea buckthorn flavonoids significantly increased glucose consumption in HepG2 cells, indicating their potential for the prevention and treatment of diabetes (He et al., 2023). Additionally, total flavonoids isolated from sea buckthorn have been shown to inhibit high-sucrose diet-induced hypertension, hyperinsulinemia, and dyslipidemia in rats by modulating insulin sensitivity and the angiotensin II signaling pathway (Pang et al., 2008). Furthermore, sea buckthorn total flavonoids have demonstrated lipid-lowering, hypoglycemic, and antioxidant properties in high-fat diet-fed mice, making them effective agents for diabetes and its complications associated with hyperlipidemia and oxidative stress (Zhang et al., 2010). Common sea buckthorn flavonoid components and their derivatives that improve DCM include epicatechin, epigallocatechin gallate (EGCG), quercetin, spiraeoside, rutin, myricetin, dihydromyricetin (DHY), luteolin, naringin, naringenin, kaempferol, apigenin, cyanidin-3-glucoside (C3G), and procyanidin. Their chemical structures are illustrated in Figure 2.
2.2.1 Catechin compounds
Epicatechin, a flavonol found in sea buckthorn, exhibits vasodilatory properties and enhances cellular bioenergetics, thereby reducing the risk of cardiovascular diseases. In endothelial and skeletal muscle cells, epicatechin binds to and activates the G protein-coupled estrogen receptor (GPER), triggering downstream signaling pathways that promote effects such as increased nitric oxide production (Moreno-Ulloa et al., 2015). EGCG, a natural derivative of epicatechin, is formed by the ester bond linkage between epigallocatechin and gallic acid. EGCG has been shown to alleviate lipid-induced insulin resistance, reduce lipid peroxidation, and enhance the activity and expression of intracellular antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), in rats (Li et al., 2011). Furthermore, EGCG possesses potent antioxidant and free radical-scavenging properties, which protect against oxidative damage by reacting with free radicals and inhibiting pro-oxidant enzymes (Potenza et al., 2020). Studies have demonstrated that EGCG can mitigate cardiomyocyte apoptosis by suppressing lipid accumulation, upregulating lipoprotein levels in hypercholesterolemic rats, reducing inflammatory responses, and decreasing lipid peroxidation through its free radical-scavenging activity (Saeed et al., 2015). Additionally, EGCG has been found to ameliorate aging-induced cardiac hypertrophy and fibrosis, thereby improving cardiac function (Ibrahim et al., 2018). Importantly, long-term administration of EGCG has been proven to be safe and well-tolerated, with no significant adverse effects reported (H-H Sherry et al., 2003).
2.2.2 Quercetin compounds
Quercetin is a naturally occurring flavonoid widely distributed in nature, primarily recognized for its potent anti-inflammatory and antioxidant properties. Studies have shown that quercetin can regulate mitochondrial autophagy and endoplasmic reticulum stress through the SIRT1/TMBIM6 pathway, improving mitochondrial energy metabolism and protecting human cardiomyocytes. Additionally, quercetin can delay vascular endothelial dysfunction and terminal cardiac injury (Xing et al., 2021). Quercetin also exhibits regulatory effects in preventing myocardial fibrosis and further modulates pancreatic islet function (Liang et al., 2020). Spiraeoside, a flavonol glycoside formed by the glycosidic linkage of quercetin with a sugar moiety, also possesses anti-inflammatory and antioxidant properties (Nile et al., 2021). Rutin, a flavonol glycoside present in sea buckthorn, also known as rutoside, is formed by the glycosidic linkage of quercetin with rutinose, representing a glycosidic form of quercetin. Research indicates that rutin acts on various tissues and organs through multiple signaling pathways. Rutin demonstrates potent hypoglycemic and antioxidant activities by increasing levels of non-enzymatic antioxidants (glutathione, vitamin C, vitamin E, and ceruloplasmin) and reducing thiobarbituric acid reactive substances (TBARS) and lipid peroxidation levels in diabetic rats (Kamalakkannan and Prince, 2006). Furthermore, rutin inhibits myocardial ischemia-reperfusion-induced apoptosis through the ERK1/2 and PI3K/AKT signaling pathways and protects H9C2 cells from hydrogen peroxide-mediated damage (Jae Ju et al., 2009). Rutin also exhibits significant anti-inflammatory and anti-apoptotic effects by inhibiting nuclear factor-kappa B (NF-κB) and other inflammatory cytokines (TNF-α and IL-1β) (Guangzhi et al., 2016). In traditional Indian medicine, rutin has been used at a dose of 500 mg/day without reported toxicity (Chua, 2013).
2.2.3 Myricetin compounds
Myricetin, a flavonoid found in sea buckthorn, exhibits diverse pharmacological activities, including antioxidant, anti-inflammatory, anti-atherosclerotic, and immunomodulatory properties (Guanhua et al., 2016). Myricetin significantly protects mouse hearts from lipopolysaccharide (LPS)-induced injury, with its underlying mechanism associated with the inhibition of IκBα/NF-κB activity and the reduction of inflammatory cytokines such as IL-1α, IL-1β, TNF-α, and MCP-1 (Liao et al., 2017). Additionally, myricetin alleviates inflammation by modulating the gut microbiota, increasing the abundance of butyrate-producing bacteria, and reducing plasma LPS levels that activate TLR4 (Yang et al., 2024). As a bioactive natural dihydroflavonol, DHY has been shown to possess multiple important pharmacological effects, including free radical scavenging, antioxidant, anti-inflammatory, antithrombotic, antimicrobial, hepatoprotective, and cardioprotective activities (Zhang et al., 2018). Studies have found that DHY improves skeletal muscle insulin resistance by inducing autophagy and promoting glucose and lipid metabolism (Shi et al., 2015). Moreover, DHY mitigates diabetic cardiomyopathy (DCM) by activating sirtuin 3 (SIRT3), suppressing oxidative stress and inflammatory responses, and reducing apoptosis (Chen et al., 2023b).
2.2.4 Anthocyanin compounds
Procyanidin, as antioxidants, exhibit multiple activities, including antioxidative stress, free radical scavenging, anti-inflammatory, anti-advanced glycation end products (AGEs), and cardiovascular protective effects (Zhang L.-M. et al., 2021; Wu et al., 2023). Procyanidin demonstrate anti-nonenzymatic glycation properties, reducing the expression of RAGE protein and decreasing AGE-induced high levels of vascular cell adhesion molecule 1 (VCAM-1) expression. Furthermore, procyanidin prevent AGE-induced ROS production, proliferation, and migration in human aortic smooth muscle cells and alleviate diabetic arterial injury (Luan et al., 2014). Cyanidin-3-glucoside (C3G), a flavonoid formed by the glycosidic linkage of cyanidin with glucose, has been confirmed to possess potent anti-inflammatory and antioxidant effects in both in vitro and in vivo studies. Additionally, research indicates that dietary intake of C3G-rich foods enhances insulin sensitivity in experimental models of insulin resistance (Jia Y. et al., 2020).
2.2.5 Luteolin
Luteolin exhibits various pharmacological properties, including anti-inflammatory and antioxidant effects. It exerts antioxidant activity by protecting or enhancing the activity of endogenous antioxidants such as glutathione reductase (GR), glutathione-S-transferase (GST), SOD, and catalase (CAT) (Liu M. et al., 2020). Luteolin can mitigate LPS-induced inflammatory injury by regulating the mitogen-activated protein kinase (MAPK) pathway and reducing macrophage recruitment. In cardiac tissue, luteolin alleviates ischemia/reperfusion injury by activating the antioxidant Nrf2 signaling pathway, restoring mitochondrial integrity, and inducing pro-survival pathways (Zhou et al., 2021).
2.2.6 Naringin
Naringin, a natural polyphenolic bioflavonoid, is widely found in sea buckthorn, grapefruit, and related citrus fruits. Studies have shown that naringin possesses potent antioxidant and free radical-scavenging properties, anti-apoptotic activity, and hypoglycemic and anti-inflammatory effects in T2DM (Lim et al., 2018; Syed et al., 2020). As an important hypoglycemic phytochemical, naringin can prevent insulin resistance, improve glucose tolerance, and reduce blood glucose levels by decreasing glucose-6-phosphatase activity, inhibiting pro-inflammatory factors, and suppressing gluconeogenesis (Qin et al., 2023). Furthermore, naringin has been demonstrated to protect against high glucose-induced cardiac injury (He et al., 2022).
2.2.7 Naringenin
Naringenin, a dihydroflavonoid, exhibits diverse pharmacological activities, including anti-inflammatory, anti-atherosclerotic, anti-fibrotic, anti-tumor, antiviral, and anti-arrhythmic effects (Salehi et al., 2019; Solanki et al., 2024). Naringenin has potential health benefits for obesity, hypertension, cardiovascular diseases, and metabolic syndrome. Notably, naringenin demonstrates significant cardioprotective effects against pressure overload-induced myocardial hypertrophy and hyperglycemia-induced cardiomyocyte injury (Gao et al., 2018).
2.2.8 Kaempferol
Kaempferol, present in sea buckthorn in its free form or as kaempferol glycosides, has been shown to possess anticancer, anti-inflammatory, antioxidant, anti-fibrotic, and anti-apoptotic properties. Kaempferol can reduce the production of inflammatory cytokines by inhibiting the activation of NF-κB and TGF-β1 and prevent high glucose/hyperglycemia-induced intrinsic cell death in H9c2 cells and STZ-induced rat hearts by stimulating the Nrf2 signaling pathway (Chen et al., 2018).
2.2.9 Apigenin
Apigenin is considered an antidiabetic agent that improves glucose intolerance in transgenic mice overexpressing miRNA103, likely due to the inhibition of TRBP phosphorylation and attenuation of MAPK Erk activation (Ohno et al., 2013).
3 Molecular mechanisms of sea buckthorn flavonoids and their derivatives in ameliorating DCM
3.1 Inhibition of oxidative stress and cellular damage
Hyperglycemia and dyslipidemia stimulate the production of ROS in cardiomyocytes, inducing oxidative stress and disrupting multiple cellular signaling pathways. Accumulating evidence suggests a complex interplay between enhanced oxidative stress and further induction of apoptosis and dysregulated autophagy, which play a critical role in cardiomyocyte death caused by DCM. Therefore, scavenging ROS can effectively prevent diabetes-induced myocardial ischemia-reperfusion injury and the onset or progression of heart failure (Varma et al., 2018; Fatin Farhana et al., 2021).
3.1.1 Enhancement of the AMPK/SIRT1 signaling pathway
In DCM, SIRT1 expression and/or activity are frequently downregulated, which is closely associated with myocardial oxidative damage. As a critical cytoprotective and defense factor, SIRT1 is functionally linked to intracellular ROS levels. Notably, SIRT1 activation has been demonstrated to significantly attenuate ROS accumulation and enhance cardiomyocyte survival (Almalki and Almujri, 2024). In streptozotocin (STZ)-induced diabetic rat models, treatment with EGCG improved cardiac function, reduced myocardial infarct size, apoptosis, and fibrosis, lowered elevated levels of lactate dehydrogenase (LDH) and malondialdehyde (MDA), and alleviated oxidative stress. Pretreatment of H9c2 cardiomyocytes with EGCG before high-glucose culture reduced apoptosis and oxidative stress, increased cell viability, and upregulated the protein expression levels of SIRT1 and manganese superoxide dismutase (MnSOD). The protective effects of EGCG were blocked by SIRT1 inhibitors or siRNA, indicating that EGCG alleviates myocardial ischemia/reperfusion (I/R) injury in diabetic rats by activating the SIRT1 signaling pathway and upregulating MnSOD expression (Wu Y. et al., 2017). SIRT1 is highly expressed in mammalian hearts and plays a key role in cardiac development and protection. Activation of SIRT1 not only maintains cardiac function but also effectively prevents hyperglycemia-induced oxidative stress, inflammation, apoptosis, and fibrosis in the heart (Wenshuai et al., 2020). The protective effects of SIRT1 are attributed to its deacetylation capacity on various mediators and transcription factors, including nuclear factor erythroid 2-related factor 2 (Nrf2), NF-κB p65, and FOXO (Deng et al., 2020; Xu et al., 2021). Kaempferol can reduce ROS, MDA, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) production by upregulating SIRT1 activity and protein levels, while downregulating the nuclear levels of transforming growth factor-beta 1 (TGF-β1) and NF-κB p65. Additionally, kaempferol increases MnSOD and glutathione (GSH) levels in the left ventricle, further upregulating total Bcl2 protein levels, SIRT1 nuclear activity, and Nrf2 nuclear levels, exerting antioxidant, anti-inflammatory, anti-fibrotic, and anti-apoptotic effects. This suggests that kaempferol may protect against DCM by activating SIRT1 and modulating its downstream signaling pathways (Alshehri et al., 2021).
Adenosine monophosphate-activated protein kinase (AMPK) is a key regulator of multiple metabolic pathways and participates in anti-cardiovascular disease by improving endothelial function (Rodríguez et al., 2021). SIRT1 and AMPK respond to changes in energy expenditure and nutrient supply, ultimately regulating cell survival and lifespan through shared signaling pathways. Studies have shown that AMPK can transcriptionally activate nicotinamide phosphoribosyltransferase (NAMPT), thereby inducing SIRT1 activation (Ilbeigi et al., 2020). In human umbilical vein endothelial cells treated with homocysteine, the addition of EGCG significantly increased AMPK and SIRT1 activity, inhibited protein kinase C activation, attenuated homocysteine-induced NADPH oxidase activation, restored endogenous antioxidant enzyme SOD activity, reduced ROS generation, and decreased apoptosis. This indicates that EGCG can protect against endothelial damage-related pathological processes in DCM by enhancing the AMPK/SIRT1 signaling pathway and reducing oxidative stress (Pai et al., 2021).
3.1.2 Activation of the Nrf2/HO-1 signaling pathway
Nrf2 is a transcription factor widely expressed in various tissues and organs. Studies have shown that the Nrf2 signaling pathway is closely related to multiple cardiac diseases and can maintain cardiac homeostasis by regulating antioxidant genes and inhibiting oxidative stress (Zazueta et al., 2022). Under physiological conditions, Nrf2 binds to its inhibitor Kelch-like ECH-associated protein 1 (Keap1) and resides in the cytoplasm. However, when the body produces excessive ROS and undergoes oxidative stress, Nrf2 dissociates from Keap1, translocates to the nucleus, and binds to the promoter region of antioxidant response elements (ARE), thereby activating antioxidant genes and promoting the synthesis of antioxidant enzymes (Tebay et al., 2015). These antioxidant enzymes have anti-inflammatory and antioxidant effects, protecting cardiomyocytes from diabetes- and high-glucose-induced oxidative damage. Activation of Nrf2 also inhibits NF-κB, reduces NLRP3 inflammasome formation, and suppresses apoptosis, ultimately exerting cardioprotective effects (Jiang et al., 2024). Spiraeoside can improve high-glucose-induced reductions in AC16 human cardiomyocyte viability, reduce the release of myocardial enzymes LDH and aspartate aminotransferase (AST), inhibit ROS and MDA generation, increase SOD, glutathione peroxidase (GSH-Px), and catalase (CAT) activity, and significantly reduce high-glucose-induced apoptosis. Notably, spiraeoside upregulates high-glucose-inhibited phosphorylated protein kinase B (p-Akt), Nrf2, and heme oxygenase-1 (HO-1) expression, while the PI3K/Akt signaling pathway inhibitor LY294002 reverses these effects. This suggests that spiraeoside may protect against DCM by activating the PI3K/Akt/Nrf2 signaling pathway, alleviating high-glucose-induced cardiomyocyte oxidative stress, cellular damage, and apoptosis (Liu H. Y. et al., 2020).
HO-1 is a stress-inducible enzyme widely expressed in various tissues and catalyzes the breakdown of heme into bilirubin, carbon monoxide (CO), and iron. The metabolites of heme catabolism exert multiple cytoprotective effects in the heart, with bilirubin having potent antioxidant properties and CO exhibiting vasodilatory, anti-inflammatory, and anti-proliferative functions (Ryter, 2022). In DCM, activation of HO-1 is considered an important mechanism for protecting the heart from oxidative stress and cellular damage. Luteolin can improve cardiac function in DCM rats, reduce serum levels of MDA, creatine kinase (CK), LDH, and myocardial connective tissue growth factor (CTGF), and increase HO-1, SOD, and phosphorylated Akt levels (Wang et al., 2012). Further studies have shown that luteolin activates the Nrf2 signaling pathway, restores mRNA levels of Nrf2 and its downstream target genes HO-1 and NAD(P)H quinone dehydrogenase 1 (NQO-1), and increases SOD activity. Additionally, luteolin alleviates STZ-induced cardiac dysfunction, fibrosis, and hypertrophy in diabetic mice and reduces high-glucose-induced inflammatory phenotypes and oxidative stress in H9C2 cardiomyocytes. This indicates that luteolin can protect against DCM by activating the Nrf2/HO-1-mediated antioxidant response (Li et al., 2019). Quercetin has also been shown to have a similar mechanism of action. Specifically, quercetin improves lipid profiles and blood glucose levels in high-cholesterol diet-induced DCM rats, prevents diastolic dysfunction, increases cardiomyocyte density, and mitigates structural and morphological changes in the heart. Quercetin also enhances the glutathione/oxidized glutathione (GSH/GSSG) ratio in cardiac tissue, promotes Nrf2 nuclear translocation and HO-1 expression, increases SOD, CAT, and GSH-Px activity, reduces ROS accumulation, lowers 8-isoprostane and lipid peroxidation levels, prevents ATP depletion, and regulates the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), uncoupling protein 2 (UCP2), and peroxisome proliferator-activated receptor gamma (PPARγ) (Castillo et al., 2018). The Nrf2 inhibitor ML385 blocks the beneficial effects of quercetin on DCM, suggesting that quercetin improves DCM by promoting Nrf2 nuclear translocation and increasing the expression of antioxidant factors such as HO-1 (Zhang et al., 2022).
3.1.3 Increased expression of Peroxiredoxin-3 (Prx-3)
Mitochondria are the primary source of ROS under hyperglycemic conditions. Most superoxide generated by the electron transport chain is converted to hydrogen peroxide (H2O2) by mitochondrial MnSOD under physiological conditions (Liu et al., 2022). H2O2 can damage cellular macromolecules such as proteins, lipids, and nucleic acids, especially after conversion to hydroxyl radicals via the Haber-Weiss reaction. In mitochondria, H2O2 can be decomposed by glutathione peroxidases (GPxs) 1 and 4 and peroxiredoxins (Prxs) 3 and 5. Kinetic studies have shown that Prx-3 plays a critical role in scavenging H2O2 in mitochondria (Wang et al., 2019). Quercetin can activate the Nrf2/Nrf1 transcriptional pathway, promote peroxiredoxin-3 (Prx-3) expression, reduce 4-hydroxynonenal (4-HNE) and mitochondrial uncoupling protein UCP-3 expression, alleviate cardiac apoptosis, hypertrophy, and fibrosis, and improve cardiac contractile dysfunction. In vitro, Prx-3 overexpression significantly reduces high-glucose-induced mitochondrial oxidative damage and apoptosis. This suggests that quercetin can induce Prx-3 expression in cardiomyocytes, thereby protecting the heart from hyperglycemia-induced oxidative damage (Arkat et al., 2016).
3.1.4 Inhibition of the IκBα/NF-κB signaling pathway
In DCM, antioxidant factors in cardiac tissue, such as SOD, GPx, catalase (CAT), and Nrf2, are reduced (Lu et al., 2022). This leads to the persistent accumulation of ROS, which activates the IκBα/NF-κB pathway, stimulating the expression of pro-inflammatory, pro-fibrotic, and pro-hypertrophic genes, including IL-1β, IL-6, and TNF-α (Xie et al., 2021). Myricetin can inhibit the IκBα/NF-κB signaling pathway, reduce the secretion of inflammatory factors, enhance the Nrf2/HO-1 signaling pathway, restore GPx and SOD activity, improve antioxidant capacity, and ultimately inhibit cardiomyocyte apoptosis. Additionally, myricetin alleviates STZ-induced cardiac hypertrophy and interstitial fibrosis in diabetic mice and improves cardiac function. In a high-glucose environment, myricetin has similar protective effects on neonatal rat cardiomyocytes, and its inhibition of the IκBα/NF-κB pathway is independent of Nrf2. This indicates that myricetin may protect against DCM by inhibiting IκBα/NF-κB and enhancing Nrf2/HO-1 (Liao et al., 2017).
3.2 Suppression of inflammatory responses
3.2.1 Regulation of pro-inflammatory cytokine release
Inflammation is a critical factor in inducing myocardial fibrosis. When DCM is accompanied by acute or chronic myocardial injury, the immune system is activated, releasing a large number of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β. These cytokines not only induce inflammatory responses but also activate cardiac fibroblasts, leading to abnormal collagen metabolism, cardiomyocyte necrosis, and tissue degeneration, ultimately resulting in myocardial fibrosis and heart failure (Hanna and Frangogiannis, 2020). Quercetin has been shown to reduce the expression levels of NLRP3, caspase-1, IL-1β, and IL-18 in mouse DCM models, as well as downregulate the mRNA levels of type I collagen and connective tissue growth factor (CTGF). This indicates that quercetin can alleviate inflammation and improve myocardial fibrosis in DCM by inhibiting the release of pro-inflammatory cytokines (Jiang et al., 2022). In a T2DM rat model induced by STZ and nicotinamide, EGCG treatment improved insulin levels and significantly reduced blood glucose, glycated hemoglobin, insulin resistance index, and lipid profile levels. Additionally, EGCG lowered serum levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and adhesion molecules (ICAM-1 and VCAM-1). Histological examinations also demonstrated its cardioprotective effects, suggesting that EGCG can mitigate myocardial inflammation in DCM by reducing pro-inflammatory cytokine levels (Othman et al., 2017).
Inflammation is a natural response to cellular damage and plays an important role in tissue repair. However, insufficient or excessive inflammatory responses may lead to abnormal elevations in inflammatory cytokines. During acute-phase reactions, inflammatory cytokines such as IL-6 and TNF-α can induce the production of acute-phase proteins, including C-reactive protein (CRP), a biomarker of acute myocardial injury (Yoshizaki, 2011). Rutin has been found to reduce TNF-α and CRP levels in the hearts of STZ-induced diabetic rats, improve electrocardiogram parameters, lower the cardiac hypertrophy index, alleviate histopathological damage, and reduce blood glucose and glycated hemoglobin levels. This suggests that rutin may protect against DCM by modulating TNF-α and CRP levels to attenuate inflammatory responses (Saklani et al., 2016).
3.2.2 Inhibition of the NF-κB signaling pathway
NF-κB is a major regulator of inflammatory responses and is activated upon exposure to fatty acids/lipids or glucose overload. Activated NF-κB induces the expression of pro-inflammatory cytokines, including TNF-α, IL-6, IL-1β, and IL-18. These cytokines are involved in various pathological processes, including promoting oxidative stress, altering cardiac cell function, and recruiting other inflammatory cells, further exacerbating myocardial injury (Kim and Rikihisa, 2002; Nishida and Otsu, 2017). Naringenin has been shown to reduce blood glucose levels in diabetic mouse models, inhibit pro-inflammatory cytokine levels and NF-κB expression, and thereby alleviate cardiac fibrosis and cardiomyocyte apoptosis. In high-glucose-treated H9C2 cardiomyocytes, naringenin effectively reduced apoptosis by suppressing pro-inflammatory cytokines. This indicates that naringenin may protect against diabetes-induced myocardial injury by modulating the NF-κB signaling pathway to inhibit inflammatory responses (He et al., 2022). Kaempferol also significantly alleviates high-glucose- and diabetes-induced myocardial inflammation by inhibiting the NF-κB signaling pathway. Pretreatment with kaempferol prevents high-glucose-induced nuclear translocation of the NF-κB p65 subunit, reduces p65 accumulation in the nucleus, and decreases IκB-α degradation, thereby suppressing NF-κB activity. Additionally, kaempferol reduces the infiltration of the macrophage marker F4/80 and TNF-α expression in the cardiac tissue of diabetic mice, mitigating inflammation-induced cardiomyocyte damage (Chen et al., 2018).
As mentioned earlier, hyperglycemia induces excessive ROS production, which activates NF-κB, playing a central role in inflammatory responses. Activated NF-κB promotes the transcription and release of pro-inflammatory mediators such as IL-6 and TNF-α, triggering myocardial inflammation (Yao et al., 2021). Notably, TNF-α-induced oxidative stress can lead to apoptosis through the activation of the NF-κB pathway, creating a vicious cycle where oxidative stress and inflammation mutually reinforce each other, further exacerbating cardiomyocyte apoptosis (Wu et al., 2024). Cyanidin-3-glucoside (C3G) has been shown to improve metabolic abnormalities in diabetic rats, reduce cardiac injury markers (CK, LD, AST), and alleviate oxidative stress. C3G also inhibits the production of inflammatory cytokines TNF-α and IL-6, increases Bcl-2 expression, and downregulates caspase-3 and Bax expression, thereby reducing cardiomyocyte apoptosis (Li et al., 2018). Apigenin similarly exhibits dual anti-inflammatory and antioxidant effects, alleviating cardiac remodeling and fibrosis and improving cardiac function in diabetic mice. Mechanistic studies reveal that apigenin inhibits the NF-κB signaling pathway, reduces the production of inflammatory factors such as TNF-α, increases the activity of antioxidant enzymes (GSH-Px, GPx, MDA, and SOD), and modulates the expression of apoptosis-related proteins (Bax and Bcl-2), thereby mitigating diabetes-induced cardiomyocyte inflammation, oxidative stress, and apoptosis (Liu et al., 2016; Liu et al., 2017).
3.2.3 Inhibition of the p38MAPK signaling pathway
The p38 mitogen-activated protein kinase (p38MAPK) signaling pathway regulates the expression of various genes, including TNF-α and TGF-β. Its activity can also be enhanced by pro-inflammatory cytokines such as TNF-α and IL-6, exacerbating inflammatory responses (Sabio and Davis, 2014). Naringin has been shown to reduce blood glucose, brain natriuretic peptide levels, and the heart weight index in DCM rats, improving cardiomyocyte morphology and myocardial structure. Compared to the normal control group, the expression of phosphorylated p38MAPK was reduced in the myocardial tissue of naringin-treated DCM rats, similar to the effect of the p38MAPK inhibitor SB203580. This suggests that naringin may prevent myocardial remodeling and improve cardiac function by inhibiting the p38MAPK signaling pathway and reducing the accumulation and infiltration of inflammatory cells in the heart (Jie and Wu, 2015).
3.3 Modulation of epigenetic modifications
3.3.1 Regulation of histone deacetylase activity
Epigenetic factors play a critical role in the pathogenesis of diabetic cardiomyopathy (DCM). Diabetes leads to a reduction in the expression of the NAD + -dependent histone deacetylase Sirtuin 1 (SIRT1) (Wang A. J. et al., 2020). SIRT1 exerts multifaceted regulatory roles by deacetylating not only histones but also multiple transcription factors and non-histone substrates, thereby modulating the expression of downstream target genes. Moreover, SIRT1 modulates the sarcoplasmic reticulum calcium ATPase (SERCA2), restoring calcium dyshomeostasis in cardiomyocytes (Przemek A et al., 2019). Epicatechin has been shown to nearly fully restore the significantly impaired contractility in cardiomyocytes of early-stage diabetic rat models. This effect is likely mediated by a marked increase in SIRT1 expression and activity, which regulates the deacetylation of miRNA22 and miRNA34a. Through epigenetic modifications, epicatechin further modulates the expression of SERCA2 and phospholamban, thereby protecting mitochondrial function and energy supply (Vilella et al., 2022).
3.3.2 Regulation of miRNA expression
MicroRNAs (miRNAs) are a class of small non-coding RNAs that primarily exert their biological functions by degrading target genes or inhibiting mRNA translation through binding to the 3′untranslated regions of target genes (Dexheimer and Cochella, 2020). Several miRNAs, including miR-34a, act as barriers in cardiomyocytes of diabetic patients. miR-34a impedes somatic cell reprogramming and negatively regulates autophagy, affecting the degradation of cellular waste, abnormal proteins, and damaged organelles through lysosomal mechanisms (Hwang et al., 2023). In high-glucose-induced cardiomyocytes and cardiac tissues of diabetic mice, miR-34a expression is upregulated, further impairing autophagy. Treatment with DHY reduces miR-34a expression, activates autophagy-related proteins such as Atg7, Beclin-1, and LC3BII/LC3BI, and rescues high-glucose-induced autophagy inhibition. The miR-34a mimic counteracts the protective effects of DHY on DCM by inhibiting autophagy, indicating that DHY improves DCM by reducing miR-34a expression and restoring impaired autophagy (Ni et al., 2020).
As a miRNA, microRNA-30d has been shown to interact with FOXO3A to regulate cardiomyocyte apoptosis, thereby improving DCM (Li et al., 2014). Therefore, microRNA-30d may serve as a novel therapeutic target for DCM. High glucose treatment reduces cardiomyocyte viability and increases apoptosis, while pretreatment with naringenin ameliorates these effects. Additionally, miR-30d-5p expression is significantly reduced in the serum of DCM patients and in high-glucose-treated AC16 cells, and naringenin reverses this downregulation. The use of a miR-30d-5p inhibitor attenuates the protective effects of naringenin against high-glucose-induced cellular damage, suggesting that naringenin alleviates high-glucose-induced injury in human AC16 cardiomyocytes by upregulating miR-30d-5p levels (Jiang et al., 2018).
3.4 Regulation of autophagy and apoptosis
3.4.1 Regulation of autophagy
Autophagy is a lysosome-dependent bulk degradation mechanism that restores cellular homeostasis by degrading and recycling misfolded or aggregated proteins and damaged organelles (Chun and Kim, 2018). Defective autophagy significantly promotes the progression of myocardial interstitial fibrosis and heart failure and exacerbates ventricular dysfunction in certain cardiomyopathies (Dewanjee et al., 2021). Moreover, activation of autophagy can inhibit the TGF-β signaling pathway, thereby alleviating tissue fibrosis (Zou et al., 2016).
3.4.1.1 Regulation of the AMPK signaling pathway
AMPK is recognized as a major energy sensor that regulates autophagy in cells (Wang S. et al., 2022). Sustained hyperglycemia reduces the number of glucose transporters in cardiomyocytes, impairing glucose uptake and leading to insufficient cardiac energy metabolism. Energy or nutrient deprivation promotes AMPK phosphorylation, which inhibits the activity of the mammalian target of rapamycin (mTOR). mTOR is a key component of the mTOR complex and inhibits autophagy activation (Kim et al., 2011). Under hyperglycemic conditions, AMPK is phosphorylated and inactivated, while mTOR is phosphorylated and activated, ultimately suppressing autophagy in DCM hearts (Hiromitsu et al., 2015). EGCG, a well-known activator of AMPK in eukaryotic cells (Cai and Lin, 2009), has been shown to activate myocardial tissue autophagy, alleviate myocardial fibrosis, and improve cardiac contractile function in T2DM rats. EGCG reduces myocardial hydroxyproline, type I collagen, type III collagen, matrix metalloproteinase (MMP)-2, and MMP-9 levels, modulates the expression of TGF-β1, MMP-2, and MMP-9 genes, and upregulates autophagy regulators (e.g., AMPK, mTOR) and autophagy markers (e.g., microtubule-associated protein 1 light chain 3 (LC3), Beclin1). These findings suggest that EGCG protects against DCM-related myocardial fibrosis by activating autophagy through the AMPK/mTOR pathway and inhibiting the TGF-β/MMPs pathway (Jia et al., 2022). Similarly, quercetin also activates autophagy via the AMPK/mTOR signaling pathway to ameliorate cardiac injury in diabetic rats. Quercetin alleviates myocardial fiber disarray, increased myocardial collagen fibers, apoptosis, mitochondrial structural damage, and reduced autophagic vacuoles. It also modulates the AMPK/mTOR pathway, reversing the downregulation of autophagy-related proteins LC3 and Beclin1 and the upregulation of P62, Caspase-3, and Bax/Bcl-2 under high-glucose conditions. Furthermore, immunoprecipitation results show that quercetin inhibits the binding of Beclin1 to Bcl-2, promoting autophagy activation and protecting cardiomyocytes (Chen et al., 2024). mTORC1 inhibits autophagy induction by modulating the Ser637 and Ser757 sites of ULK1 and the Ser258 site of Atg13, thereby attenuating ULK1 complex activity (Nazio et al., 2013). DHY treatment improves cardiac function in diabetic mice, increases LC3 II/LC3 I, Atg7, and Beclin1 protein expression, restores myocardial autophagy. Additionally, DHY enhances the phosphorylation of AMPK and ULK1, indicating that DHY may exert its therapeutic effects on DCM by activating the AMPK/ULK1 signaling pathway to enhance autophagy (Wu B. et al., 2017).
3.4.1.2 Inhibition of the JNK/c-Jun signaling pathway
In diabetic hearts, overexpression of miR-221 inhibits autophagy, leading to heart failure (Su et al., 2014). Studies have shown that the transcription factor c-Jun significantly upregulates miR-221 expression, and the activation of c-Jun N-terminal kinase (JNK) is crucial for this transcriptional upregulation (Kim et al., 2015). Therefore, inhibiting JNK can effectively prevent pathological changes in diabetic hearts. Luteolin improves cardiac function in male SD rats with diabetes, alleviates myocardial injury and fibrosis, and dose-dependently downregulates the expression of JNK, c-Jun, miR-221, and p62 in diabetic hearts. It also increases LC3-II/I and autophagic vacuoles while reducing mitochondrial swelling. These findings suggest that luteolin protects against DCM by inhibiting JNK/c-Jun-regulated miR-221 expression and subsequently relieving autophagy suppression (Xiao et al., 2023).
3.4.2 Inhibition of necroptosis
Recent studies have reported that necroptosis is closely associated with the development of DCM. Necroptosis is a form of programmed cell death mediated by receptor-interacting protein kinase 3 (RIPK3) and RIPK1. RIPK1 binds to RIPK3, recruiting and phosphorylating the mixed lineage kinase domain-like (MLKL) protein to induce necroptosis (Wegner et al., 2017). As a mode of cell death, necroptosis links oxidative stress, inflammatory responses, and the onset of cardiovascular diseases (Royce et al., 2019). RIPK3 is a key regulator of the necroptosis signaling pathway, and its increased expression is a hallmark of necroptosis. Therefore, inhibiting RIPK3 activity helps mitigate myocardial damage caused by necroptosis. DHY reduces the number of TdT-mediated dUTP nick-end labeling (TUNEL)-positive cells and inhibits the expression of RIPK3 and cleaved-caspase 3, thereby alleviating high-glucose-induced necroptosis in cardiomyocytes (Sun et al., 2024). Additionally, the widespread expression of deacetylase 3 (SIRT3) in the heart provides new insights into the regulation of necroptosis. SIRT3 modulates energy metabolism and apoptosis by regulating protein deacetylation (Li et al., 2021). SIRT3 deficiency exacerbates high-glucose-induced mitochondrial damage, increases ROS accumulation, promotes necroptosis, and ultimately aggravates the pathological progression of DCM (Song et al., 2020). By activating SIRT3, DHY inhibits oxidative stress, inflammasome activation, and necroptosis, thereby improving cardiac dysfunction, myocardial hypertrophy, fibrosis, and injury in diabetic mice. In SIRT3 knockout mice, the protective effects of DHY on DCM are not observed, indicating that DHY may exert its therapeutic effects on DCM by modulating SIRT3 to inhibit necroptosis (Chen et al., 2023b).
3.5 Maintenance of mitochondrial homeostasis
Mitochondria are the energy metabolism centers of cells, generating energy through oxidative phosphorylation to meet the high energy demands of the heart. Many critical physiological activities in the heart, such as myocardial contraction and the maintenance of intracellular homeostasis, rely on ATP produced by mitochondria (Manolis et al., 2020). Therefore, the homeostasis of mitochondrial function is essential for cardiac function.
3.5.1 Upregulation of SIRT5 expression
Sirtuin 5 (SIRT5) is widely distributed in the nucleus, cytoplasm, and mitochondria, with its post-translational modifications primarily occurring within mitochondria (Carrico et al., 2018). SIRT5 regulates lysine succinylation and can enter peroxisomes to reduce intracellular H2O2 production, playing a significant role in cellular oxidative stress. Acute myocardial ischemia and hypoxia can upregulate SIRT5 expression through the PGC1α/PPAR-γ pathway, leading to the desuccinylation of key proteins involved in cardiomyocyte energy metabolism, thereby exerting protective effects on these cells (Zou et al., 2018). Quercetin increases SIRT5 expression in cardiomyocytes of mice with transverse aortic constriction, promotes IDH2 desuccinylation, reverses the effects of high glucose on IDH2 expression and succinylation levels in HL-1 cardiomyocytes, and regulates the activity of mitochondrial respiratory complexes I, III, and IV, restoring mitochondrial energy metabolism and membrane permeability transition pore function. Additionally, quercetin modulates the expression of mitochondrial fission/fusion-related genes (e.g., Drp1/Fis1 and Mfn1/Mfn2) and mitochondrial biogenesis-related genes (e.g., Tfam and PGC1α), reduces high-glucose-induced apoptosis and ROS production, increases antioxidant enzyme activity, inhibits NLRP3 expression, and alleviates high-glucose-induced inflammatory injury and myocardial fibrosis in HL-1 cardiomyocytes. These findings suggest that quercetin may protect cardiomyocytes by promoting SIRT5-mediated IDH2 desuccinylation, enhancing mitochondrial tolerance to oxidative stress and inflammation, and maintaining mitochondrial homeostasis (Chang et al., 2021).
3.5.2 Maintenance of mitochondrial calcium homeostasis
Mitochondrial Ca2+ plays a critical role in cardiac excitation-contraction coupling and signal transduction. The mitochondrial calcium uniporter (MCU) is a channel located on the inner mitochondrial membrane responsible for mitochondrial Ca2+ uptake. In diabetic conditions, MCU protein levels are reduced in cardiac mitochondria, disrupting Ca2+ homeostasis and Ca2+ transporters (Suarez et al., 2018). Increasing MCU levels can reverse hyperglycemia-induced metabolic changes, restoring mitochondrial Ca2+ concentration and membrane potential (Díaz-Juárez et al., 2016). Impaired mitochondrial Ca2+ uptake in atrial mitochondria of metabolic syndrome patients and high-fat sucrose diet-fed mice is associated with remodeling of the mitochondrial calcium uniporter complex (MCUC), particularly increased expression of MICU1 and MICU2 subunits, which reduces MCUC activity. This alteration increases susceptibility to atrial fibrillation, while the MCUC agonist kaempferol restores MCUC activity and inhibits atrial fibrillation. These findings indicate that mitochondrial calcium homeostasis imbalance plays a significant role in the pathogenesis of metabolic syndrome-related atrial fibrillation, and kaempferol, with its ability to modulate MCUC activity, may be a potential antiarrhythmic agent (Fossier et al., 2022).
3.5.3 Inhibition of mitochondrial permeability transition pore opening
Apoptosis is a major form of programmed cell death that plays an important role in various diseases involving cardiac pathology (Teringova and Tousek, 2017). Apoptosis can occur through extrinsic, intrinsic, or mitochondrial-mediated pathways (Lossi, 2022). Among the multiple apoptotic pathways, mitochondria-mediated apoptosis is particularly relevant to DCM pathogenesis because hyperglycemia-induced oxidative stress directly impairs mitochondrial integrity. In the mitochondrial-mediated pathway, the opening of the mitochondrial permeability transition pore (mPTP) or increased outer mitochondrial membrane permeability leads to the release of cytochrome c into the cytoplasm, triggering caspase activation and ultimately resulting in cell death (Estaquier et al., 2012). In DCM, quercetin has been shown to attenuate cardiac hemorrhagic lesions and coronary vascular congestion through the reduction of mitochondrial lipid peroxidation specifically targeting mPTP, thereby inhibiting pore opening and cytochrome c-mediated caspase activation. It suggests that quercetin may reduce the risk of mitochondrial pathway-mediated apoptosis in diabetic rat cardiomyocytes by maintaining mitochondrial membrane integrity and inhibiting mitochondrial permeability transition pore opening (Ojo et al., 2022).
3.5.4 Restoration of ATP-sensitive potassium (KATP) channels
ATP-sensitive potassium (KATP) channels play a significant cardioprotective role in various cardiovascular diseases. Diabetes reduces the expression and function of mitochondrial KATP channels, impairing KATP channel-mediated vasodilation in vascular smooth muscle cells (Rajković et al., 2024). Cardiomyocytes exposed to high glucose for 24 h exhibit significant damage, including reduced cell viability, increased oxidative stress, elevated apoptosis, and dissipated mitochondrial membrane potential. Pretreatment with naringin significantly mitigates these damages. High glucose decreases the expression levels of Bcl-2 and KATP and SOD activity in cardiomyocytes while increasing Nox4 expression and the ADP/ATP ratio. These changes are inhibited by pretreatment with naringin or PDTC, indicating that naringin protects against high-glucose-induced cardiomyocyte injury by upregulating KATP channel expression (You et al., 2016). Further animal studies reveal that naringin restores the expression of KATP channel subunits Kir6.2, SUR1, and SUR2, reduces calpain activity, and lowers diastolic Ca2+ concentration, improving cardiomyocyte function and alleviating cardiac injury in T2DM mice. The use of the KATP channel inhibitor glibenclamide further increases Ca2+ concentration in T2D cardiomyocytes and abolishes the therapeutic effects of naringin. These findings suggest that naringin may protect against DCM by regulating diastolic Ca2+ concentration through KATP channels (Uryash et al., 2021).
3.6 Inhibition of endoplasmic reticulum stress (ERS)
The endoplasmic reticulum (ER) is a vital organelle involved in protein synthesis, folding, and secretion, maintaining intracellular protein homeostasis. Moderate endoplasmic reticulum stress (ERS) has protective effects, helping cells restore normal function and structure. However, when ERS persists and exceeds the cell’s repair capacity, it triggers apoptotic pathways, leading to cellular damage (Szegezdi et al., 2006). In diabetic rats, changes in myocardial structure and function are closely associated with upregulated ERS, which further activates pro-apoptotic factors and mediates apoptosis (Yang et al., 2016). Glucose-regulated protein 78 (GRP78) is a molecular chaperone located in the ER, playing a crucial role in maintaining ER protein synthesis, proper folding, and calcium homeostasis. GRP78 is considered a protective factor for ER homeostasis, and its increased expression typically indicates the onset and persistence of ERS (Repges et al., 2022). When ERS is too severe or prolonged, it triggers apoptotic signals, inducing the expression and activation of pro-apoptotic factors such as C/EBP homologous protein (CHOP) and caspase-12, leading to apoptosis (Boyce and Yuan, 2006). In H9C2 cardiomyocyte models, high glucose treatment upregulates ERS-related proteins (GRP78, IRE1α, XBP1, ATF6) and apoptosis-related proteins (CHOP, cleaved caspase-12, and caspase-12), inducing ERS, reducing cell viability, increasing cytotoxicity, and promoting apoptosis. Rutin dose-dependently inhibits high-glucose-induced ERS and apoptosis, thereby improving cell viability and reducing cytotoxicity. The ERS activator thapsigargin reverses the inhibitory effects of rutin on high-glucose-induced ERS and apoptosis, suggesting that rutin alleviates high-glucose-induced cardiomyocyte injury by inhibiting ERS and apoptosis (Wang et al., 2021). Additionally, naringin also exhibits potential in alleviating ER stress. Naringin improves myocardial structural damage in diabetic rat models, reduces the accumulation of unfolded or misfolded proteins in the ER, and downregulates the mRNA and protein expression of apoptosis-related factors such as GRP78, CHOP, and caspase-12. This indicates that naringin can reduce cardiomyocyte apoptosis in diabetic rats by inhibiting ERS (Yu et al., 2024).
3.7 Reduction of advanced glycation end products (AGEs) levels
In diabetic patients, hyperglycemia increases oxidative stress and accelerates the accumulation of advanced glycation end products (AGEs). AGEs are closely associated with the accelerated progression of DCM. AGEs exert their effects through receptor-independent and receptor-dependent mechanisms, promoting myocardial injury, fibrosis, and inflammatory responses (Wang X. et al., 2020). The interaction between AGEs and the receptor for advanced glycation end products (RAGE) activates multiple signaling pathways, further inducing the expression of pro-fibrotic and pro-inflammatory genes (Teissier and Boulanger, 2019). Proanthocyanidins reduce AGEs levels in STZ-induced diabetic rats and decrease the mRNA transcription levels of RAGE, NF-κB, and TGF-β in myocardial tissue, alleviating myocardial fibrosis. Additionally, proanthocyanidins reduce the number of degenerated mitochondria and improve the microstructure of the left ventricular myocardium. These findings suggest that proanthocyanidins protect against DCM by reducing AGEs and improving glycation-related cardiac injury (Luan et al., 2014).
3.8 Modulation of gut microbiota
The symbiotic relationship between gut microbiota and the host plays a crucial role in various physiological functions, particularly in nutrient metabolism, immune regulation, and maintenance of the intestinal barrier (Kayama et al., 2020). Accumulating evidence indicates that gut microbiota dysbiosis is closely associated with various cardiovascular and autoimmune diseases, including DCM (Manolis et al., 2021; Belvončíková et al., 2022). Under diabetic conditions, the composition of gut microbiota undergoes significant alterations, characterized by a marked reduction in short-chain fatty acid (SCFA)-producing probiotics (e.g., Clostridium butyricum, Roseburia, Faecalibacterium, and Bifidobacterium) and an increase in opportunistic pathogens, particularly Gram-negative bacteria that produce lipopolysaccharide (LPS) (Letchumanan et al., 2022). These structural and functional changes in gut microbiota can impair intestinal barrier integrity and trigger systemic inflammation, exerting profound effects on myocardial health. First, gut dysbiosis is a key contributor to intestinal barrier dysfunction and endotoxemia. Specifically, microbial imbalance disrupts tight junctions between intestinal epithelial cells, increasing gut permeability. This allows harmful microbial metabolites—particularly LPS and its fragments derived from Gram-negative bacterial cell walls—to translocate across the compromised intestinal barrier into systemic circulation, inducing chronic low-grade inflammation (i.e., metabolic endotoxemia). Circulating LPS activates the Toll-like receptor 4 (TLR4)/myeloid differentiation factor 88 (MyD88) signaling pathway in cardiomyocytes, vascular endothelial cells, and immune cells (e.g., macrophages), thereby initiating downstream NF-κB-mediated inflammatory cascades. This promotes the expression and release of proinflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) and chemokines (e.g., MCP-1). Notably, LPS-TLR4 binding can directly damage cardiomyocytes, impair cardiac function, and exacerbate myocardial inflammation, injury, and fibrosis (Wang et al., 2018; Tahapary et al., 2023). Second, SCFAs play a crucial mediating role in gut microbiota-myocardial inflammation crosstalk. Gut probiotics generate SCFAs (e.g., butyrate, propionate, acetate) through dietary fiber fermentation. Beyond serving as the primary energy source for intestinal epithelial cells and maintaining barrier integrity, SCFAs exert anti-inflammatory, antioxidant, and immunomodulatory effects by activating G protein-coupled receptors (e.g., GPR41, GPR43, GPR109A) or inhibiting histone deacetylases (HDACs) in host cells (including immune cells). Consequently, diabetes-associated dysbiosis with reduced SCFA-producing bacteria diminishes systemic SCFA levels, weakening anti-inflammatory responses and indirectly aggravating myocardial inflammation (Mann et al., 2024). Additionally, gut microbiota critically regulates bile acid metabolism. Dysbiosis may alter bile acid profiles, generating abnormal secondary bile acids—some exhibit anti-inflammatory properties, while others may promote inflammation, thereby modulating myocardial inflammation through microbiota-bile acid interactions (Yntema et al., 2023). These metabolites may affect the metabolic and inflammatory state of the host through direct or indirect means, ultimately having an impact on myocardial health.
Myricetin, a representative sea buckthorn flavonoid, has been demonstrated to modulate gut microbiota composition by increasing the abundance of butyrate-producing bacteria (e.g., Roseburia, Faecalibacterium, and Bifidobacterium). Concurrently, myricetin upregulates intestinal tight junction proteins and goblet cell numbers, further enhancing barrier function and suppressing LPS-induced TLR4/MyD88-mediated systemic inflammation, thereby alleviating cardiac hypertrophy and fibrosis. Fecal microbiota transplantation experiments further revealed that transferring gut contents from myricetin-treated mice significantly improved cardiac function and intestinal barrier integrity in DCM mice, while reducing serum LPS levels and TLR4/MyD88 pathway protein expression. These findings strongly suggest that myricetin may ameliorate DCM progression by restoring gut microbiota structure, repairing intestinal barrier function, and modulating microbial metabolites, thereby attenuating LPS-driven inflammatory pathways, systemic inflammatory burden, and ultimately mitigating myocardial inflammation and fibrosis (Zhu et al., 2024).
3.9 Improvement of myocardial fibrosis and ventricular remodeling
Myocardial fibrosis is a primary cause of ventricular remodeling and leads to reduced myocardial compliance, thereby impairing both systolic and diastolic cardiac function. Chronic volume or pressure overload can ultimately result in heart failure. The progression from DCM to heart failure is accompanied by severe myocardial fibrosis (Cheng et al., 2023). Cardiac interstitial fibrosis is caused by an increase in fibroblasts and excessive deposition of extracellular matrix (ECM) components such as type I collagen, type III collagen, and fibronectin (Kurose, 2021). This excessive ECM deposition is primarily due to overproduction and reduced degradation, often regulated by the transforming growth factor (TGF)-β/matrix metalloproteinase (MMPs) signaling pathway. Ultimately, excessive ECM deposition increases cardiac stiffness, further exacerbating ventricular systolic dysfunction and leading to heart failure (Shah and Prajapati, 2024).
3.9.1 Inhibition of the TGF-β1 signaling pathway
TGF-β1 is one of the primary cytokines promoting fibrosis. It stimulates cardiac interstitial fibroblasts to secrete matrix, leading to myocardial interstitial fibrosis. TGF-β1 not only promotes fibrosis by upregulating fibrosis-related factors but also exacerbates fibrosis by activating the downstream JNK1 signaling pathway (Garg et al., 2021). Phosphorylation of JNK1 plays a critical role in fibroblast proliferation and aggregation and is involved in TGF-β1-induced myofibroblast and ECM transformation. Under diabetic conditions, TGF-β1 can induce fibronectin synthesis by activating the ERK1/2, p38, and JNK signaling pathways, further aggravating myocardial fibrosis (Hou et al., 2021). EGCG reduces the expression of type I and type III collagen in the hearts of diabetic rats, downregulates TGF-β1 and its downstream signaling molecules JNK, phosphorylated JNK, and tissue inhibitor of metalloproteinase-1 (TIMP-1), and upregulates matrix metalloproteinase-9 (MMP-9), thereby improving DCM-related myocardial fibrosis (Gui et al., 2022). In primary cultures of rat cardiac fibroblasts, high-glucose medium activates cardiac fibroblasts, leading to increased TGF-β1 protein, fibronectin, and total collagen. Treatment with epicatechin significantly inhibits these increases. Additionally, high glucose reduces G protein-coupled estrogen receptor levels, which are restored by epicatechin treatment. This effect is associated with c-Src phosphorylation and reverses changes in SMAD levels, indicating that epicatechin blocks the pro-fibrotic phenotype of high-glucose-induced cardiac fibroblasts, at least partially through the inhibition of the TGF-β1/SMAD pathway via the G protein-coupled estrogen receptor (Garate-Carrillo et al., 2021). Rutin also reduces TGF-β1 expression to improve myocardial fibrosis. Rutin decreases the expression of fibrosis-related genes TGF-β1 and fibronectin, ameliorates histopathological damage in cardiomyocytes, alleviates metabolic acidosis and myocardial fibrosis in diabetic rats, and prevents the progression of DCM (Ganesan et al., 2020).
3.9.2 Inhibition of NADPH oxidase activity
Oxidative stress is a key factor in ECM remodeling in diabetic hearts, and most ROS contributing to this enhanced matrix remodeling are believed to originate from NADPH oxidase (Qiu et al., 2022). Furthermore, chronic hyperglycemia promotes myocardial fibrosis by directly activating cardiac fibroblast proliferation and increasing NADPH oxidase activity due to elevated angiotensin II production, further increasing ROS (Faria and Persaud, 2017). Naringin treatment reduces NADPH oxidase activity in STZ-induced diabetic rats, alleviates cardiac fibrosis, and downregulates protein kinase C-β and p38 mitogen-activated protein kinase expression. This suggests that naringin may inhibit NADPH oxidase activity through its antioxidant effects, attenuating cardiac fibrosis in diabetes and limiting cardiac remodeling (Adebiyi et al., 2016).
3.10 Improvement of cardiomyocyte hypertrophy and ventricular hypertrophy
3.10.1 Regulation of the MEF2/HDAC4 signaling pathway
In diabetic patients, left ventricular hypertrophy is a hallmark closely associated with DCM. Obesity and accompanying T2DM are major triggers for the development of left ventricular hypertrophy (De Jong et al., 2017). Hyperglycemia plays a significant role in cardiac remodeling, and myocyte enhancer factor-2 (MEF2) is activated in diabetic hypertrophy, promoting the transcription of cardiac structural genes, leading to cardiac fibroblast proliferation, collagen accumulation, and ventricular wall thickening (Chen et al., 2015). Quercetin improves diastolic dysfunction in Zucker diabetic fatty rats, reduces left ventricular wall thickness, increases left ventricular internal diameter, decreases left ventricular wall collagen content, and inhibits pro-hypertrophic signaling pathways. Further mechanistic studies reveal that quercetin reduces the relative protein expression of the pro-hypertrophic transcription factor MEF2 and its inverse regulator histone deacetylase 4 (HDAC4) and its phosphorylated form (pSer246-HDAC4) in diabetic rat hearts, reducing collagen deposition. This suggests that quercetin may improve cardiomyocyte hypertrophy and ventricular hypertrophy in diabetic rats by regulating the MEF2/HDAC4 pathway (Bartosova et al., 2022).
3.10.2 Regulation of the EETs/PPARs signaling pathway
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear hormone receptor superfamily and regulate cell differentiation, inflammatory responses, and the expression of genes related to glucose and lipid metabolism (Poulsen et al., 2012). PPARs include α, β, and γ subtypes and play important roles in obesity, inflammation, and metabolic syndrome, serving as major drug targets for controlling these conditions (Botta et al., 2018). Epoxyeicosatrienoic acids (EETs), as endogenous ligands of PPARs, have multiple beneficial effects in metabolic diseases, including anti-atherosclerosis, hypertension, myocardial hypertrophy, and diabetes (Wray and Bishop-Bailey, 2008; Romashko et al., 2016). Naringenin upregulates the expression of PPARα, PPARβ, PPARγ, and cytochrome P450 2J3 (CYP2J3) in a concentration-dependent manner, increases 14,15-epoxyeicosatrienoic acid (14,15-EET) levels, and inhibits high-glucose-induced hypertrophy in H9c2 cardiomyocytes. The protective effects of naringenin are diminished by EET-related inhibitors or antagonists, and its upregulation of PPARs expression is also eliminated. This suggests that naringenin may exert anti-diabetic myocardial hypertrophy effects through the EETs/PPARs signaling pathway (Zhang et al., 2019).
4 Conclusions and perspectives
The antioxidant properties of flavonoids suggest that they can reduce the risk of cardiovascular diseases, prevent myocardial ischemia-reperfusion injury, oxidative damage, and aging, and help improve angina and cardiac function (Ciumărnean et al., 2020; Jia J. et al., 2020). The medicinal value of sea buckthorn has been recognized in traditional Chinese medicine for over 3,000 years, dating back to the Tang Dynasty (Beata, 2017). Sea buckthorn is an important plant with both nutritional and medicinal value. As a traditional Chinese medicine, it has the effects of strengthening the spleen, aiding digestion, resolving phlegm, relieving cough, promoting blood circulation, and dispersing blood stasis (He et al., 2023). The flavonoids in sea buckthorn are its primary active components, found in its roots, stems, leaves, flowers, and fruits. The main types include catechins, quercetins, myricetins, and anthocyanins (Xia et al., 2023). Flavonoid derivatives, structurally similar to flavonoids, also exhibit significant biological activity. Numerous studies based on animal models have confirmed that flavonoids and their derivatives from sea buckthorn can delay the progression of DCM. Therefore, we have reviewed recent advances in the research of sea buckthorn flavonoids and their derivatives in improving DCM, aiming to provide a reference for better utilization of these natural compounds in treating DCM.
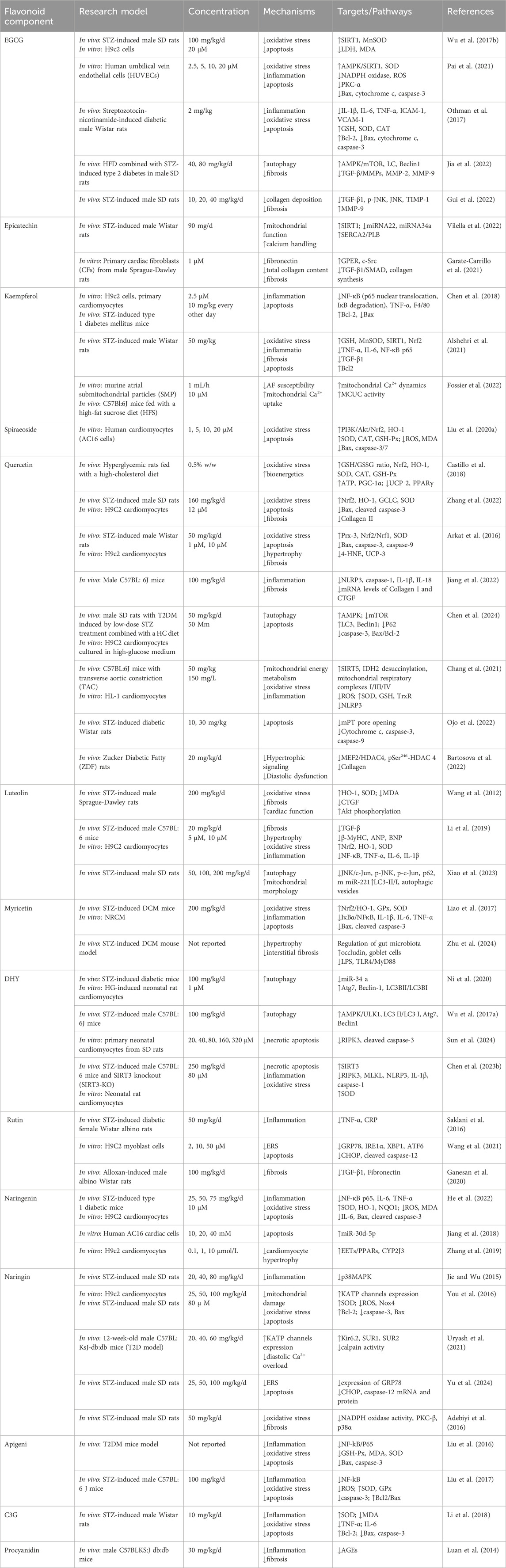
Sea buckthorn flavonoids and their derivatives can treat DCM through multiple pathways. For example, EGCG acts on the AMPK/SIRT1, AMPK/mTOR, SMAD/TGF-β1, TGF-β1/JNK, and TGF-β/MMPs signaling pathways. By modulating these pathways, EGCG ultimately inhibits the release of pro-inflammatory cytokines and adhesion molecules, prevents mitochondrial apoptosis, activates autophagy, alleviates oxidative stress, apoptosis, and fibrosis, and improves DCM. Kaempferol regulates the activity of the mitochondrial calcium uniporter complex and activates SIRT1, modulating downstream signaling pathways to exert antioxidant, anti-inflammatory, anti-fibrotic, and anti-apoptotic effects. Spiraeoside may protect against DCM by activating the PI3K/Akt/Nrf2 signaling pathway, alleviating high-glucose-induced oxidative stress and damage in cardiomyocytes. Quercetin activates the Nrf2/HO-1 and Nrf2/Nrf1 signaling pathways, increases SOD activity, reduces inflammation and oxidative stress, inhibits apoptosis, promotes SIRT5-mediated desuccinylation of isocitrate dehydrogenase (IDH2), inhibits mitochondrial permeability transition pore opening to maintain mitochondrial homeostasis, and modulates the MEF2/HDAC4 pathway to improve cardiomyocyte hypertrophy and fibrosis in DCM. DHY reduces miR-34a expression by activating the AMPK/ULK1 signaling pathway, restores impaired autophagy, and activates SIRT3 to inhibit oxidative stress, inflammasome activation, and necroptosis. Myricetin enhances antioxidant capacity by inhibiting the IκBα/NF-κB signaling pathway and activating the Nrf2/HO-1 signaling pathway. It also modulates gut microbiota composition, improves intestinal barrier function by increasing tight junction protein expression and goblet cell numbers, and inhibits the TLR4/MyD88 pathway to alleviate myocardial hypertrophy and fibrosis. Rutin regulates TNF-α and CRP levels, inhibits ERS and apoptosis, and reduces TGF-β1 expression to improve myocardial fibrosis. Naringenin inhibits pro-inflammatory cytokine levels and NF-κB expression, upregulates miR-30d-5p levels, modulates the EETs/PPARs signaling pathway to alleviate diabetic myocardial hypertrophy, and improves cardiac fibrosis and cardiomyocyte apoptosis. Naringin upregulates KATP channels, inhibits ERS-mediated apoptosis, suppresses the p38 MAPK pathway, inhibits NADPH oxidase activity, reduces inflammatory cell infiltration in the heart, prevents myocardial fibrosis, and improves cardiac remodeling. Luteolin protects against DCM by inhibiting JNK/c-Jun-regulated miR-221 to relieve autophagy suppression, activating the Nrf2 signaling pathway to reduce inflammatory phenotypes and oxidative stress, and inhibiting pyroptosis. Proanthocyanidins reduce AGEs levels, improving glycation-related cardiac damage. C3G and apigenin exert protective effects on DCM through anti-inflammatory actions, improving inflammation and apoptosis. Overall, the sea buckthorn flavonoids and their derivatives used to treat DCM demonstrate multi-target, multi-pathway, broad-spectrum, targeted, and highly effective therapeutic advantages, showing great potential for development and clinical application. The primary mechanisms by which these compounds improve DCM are summarized in Figures 3, 4 and Table 1.
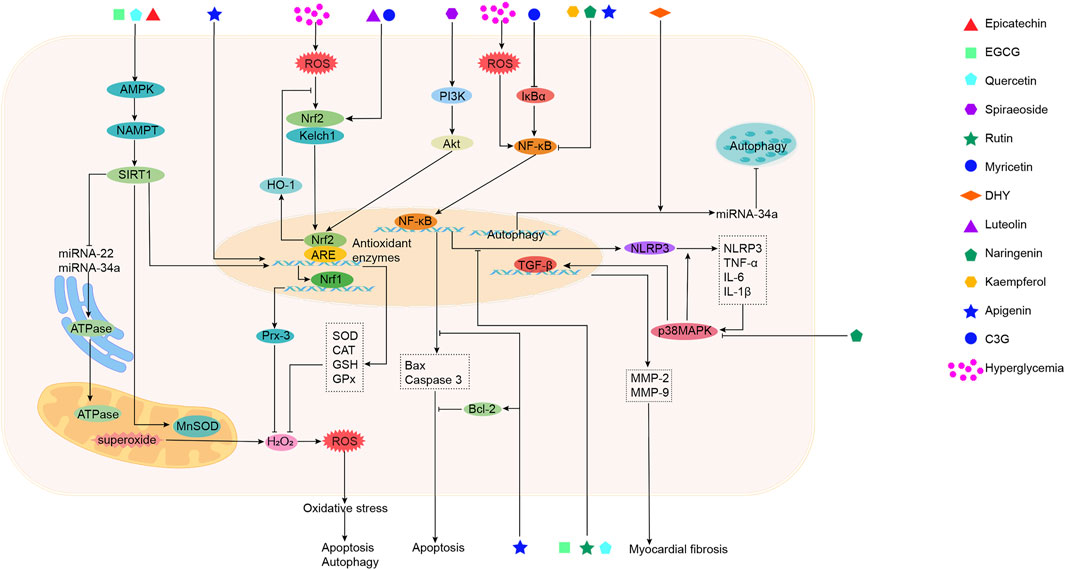
Figure 3. The mechanism of action of sea buckthorn flavonoids and their derivatives in the treatment of DCM: Inhibition of oxidative stress, suppression of inflammatory responses, and regulation of epigenetic modifications.
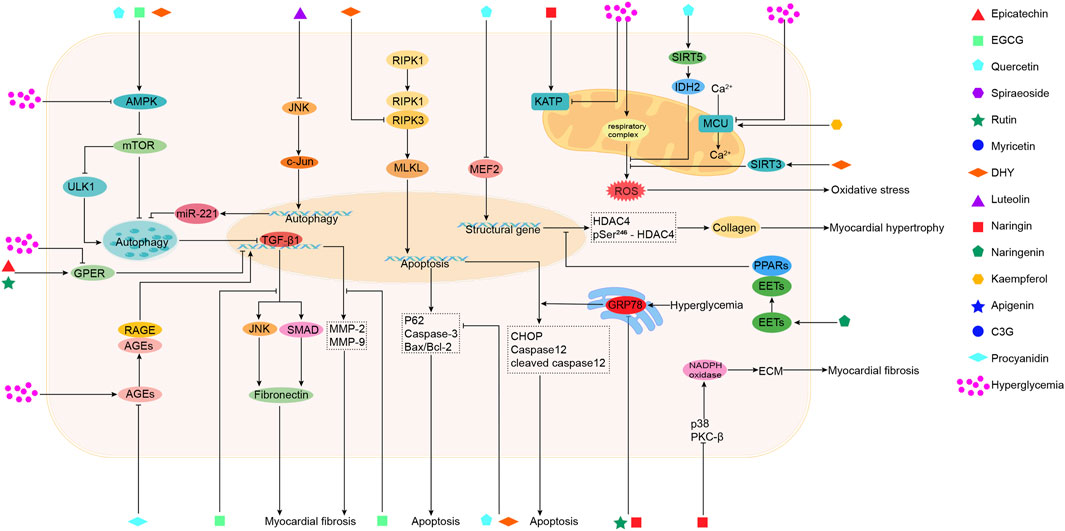
Figure 4. The mechanisms of action of sea buckthorn flavonoids and their derivatives in the treatment of DCM: Regulation of autophagy and apoptosis, maintenance of mitochondrial functional homeostasis, attenuation of endoplasmic reticulum stress, reduction of AGEs levels, and amelioration of cardiomyocyte hypertrophy and myocardial fibrosis.
Although studies have shown that sea buckthorn flavonoids and their derivatives exhibit broad protective effects on multiple targets in DCM, certain limitations remain. First, the generally low water solubility of flavonoids leads to slow dissolution rates in the gastrointestinal tract, severely limiting oral absorption efficiency and resulting in low bioavailability. This not only reduces their in vivo pharmacological activity but also causes fluctuations in pharmacokinetic and pharmacodynamic responses (Zhao et al., 2019). While existing delivery systems such as solid dispersions have demonstrated significant effects in improving solubility, dissolution rates, and oral bioavailability, future research should actively explore more efficient, stable, and safe novel drug delivery strategies (Colombo et al., 2021). These include, but are not limited to Utilizing nanotechnology (e.g., nanoparticles, liposomes, micelles) to construct nanocarrier systems for enhanced solubility, transmembrane transport efficiency, and targeted delivery; Developing flavonoid derivatives or prodrugs with improved water solubility or permeability; Further optimizing physicochemical properties through cocrystallization or inclusion complex techniques (e.g., cyclodextrin encapsulation). Second, poor intestinal membrane permeability poses another major obstacle to flavonoid absorption, particularly for flavonoid glycosides. Due to their high hydrogen-bonding capacity and specific molecular structures, their passage through intestinal epithelial cells is restricted (Fang et al., 2017). Although some aglycone-type flavonoids exhibit relatively better permeability, they are rapidly glucuronidated or sulfated in intestinal cells, further reducing the proportion of active compounds entering systemic circulation. Additionally, certain flavonoids (e.g., luteolin, dihydromyricetin) are substrates for intestinal efflux transporters such as P-glycoprotein (P-gp) and multidrug resistance-associated proteins (MRPs), further reducing their transmembrane transport efficiency and systemic exposure (Różański et al., 2019). Therefore, future research should focus on structural modifications to reduce substrate affinity for efflux pumps or develop novel delivery systems that bypass or inhibit intestinal efflux transporters. At the same time, a deeper understanding of the positive regulatory effects of flavonoids and their metabolites on intestinal barrier function and their application in enhancing absorption is a promising direction for exploration. Finally, significant first-pass metabolism in the gut and liver is another key limiting factor for the systemic availability of active flavonoid components. After oral administration, flavonoids undergo extensive enzymatic conversion in the intestinal wall and liver before reaching systemic circulation (Zhang Z. et al., 2021). Thus, future studies should emphasize the design of prodrug strategies to circumvent first-pass metabolism or the development of stable flavonoid derivatives resistant to metabolic degradation. Furthermore, elucidating the complete absorption, distribution, metabolism, and excretion (ADME) profiles of flavonoids and utilizing pharmacokinetic-pharmacodynamic (PK-PD) correlation models will be crucial for optimizing their design and maximizing pharmacological activity in DCM treatment, thereby enhancing clinical translation potential.
Although most studies indicate that sea buckthorn flavonoids and their derivatives exhibit significant hypoglycemic activity with good safety and tolerability—showing no obvious toxicity or adverse effects—certain flavonoids (e.g., quercetin, apigenin, and luteolin) display weak estrogenic activity and have shown high developmental toxicity in chicken embryo assays, with mortality rates reaching 50% (Zhang and Wu, 2022). Some flavonoids, including apigenin, have also demonstrated negative effects on early-life development and behavior in zebrafish experiments (Bugel et al., 2016). Additionally, modern pharmacological studies have found that high doses of flavonoids may cause liver and kidney toxicity and affect thyroid and reproductive functions (Tang and Zhang, 2022). However, sea buckthorn flavonoids generally do not exhibit significant toxicity and may even protect the liver and kidneys (Xiaoxi et al., 2021; Zili et al., 2021). These differences may be related to factors such as dosage, administration route, individual variability, and interactions among natural components.
Moreover, combining sea buckthorn flavonoids with stem cell transplantation technology may represent an important future research direction. Studies have demonstrated that the combined use of EGCG and mesenchymal stem cell transplantation shows potential in treating DCM. After mesenchymal stem cell transplantation, the microenvironment can induce stem cell differentiation into cardiomyocytes, thereby achieving cardiac regeneration (Szaraz et al., 2017). However, high glucose or diabetic environments reduce the expression of key proteins in stem cells, including vascular endothelial growth factor, insulin-like growth factor-1, and C-X-C chemokine receptor type 4 (Cramer et al., 2010; Li et al., 2023). The reduction of these key proteins leads to decreased proliferation, anti-apoptotic capacity, differentiation, and migration of stem cells. Pre-treatment of adipose-derived stem cells with EGCG can mitigate the effects of these factors on cell viability. For example, in a type 1 diabetic rat model, autologous adipose-derived stem cell transplantation improved cardiac function and reduced blood glucose levels, and oral administration of EGCG further enhanced these effects (Chen et al., 2017). This suggests that EGCG can serve as an adjunct to stem cell transplantation in diabetic patients, enhancing cardiac functional recovery, although further research is needed to elucidate the specific mechanisms by which EGCG enhances the viability of adipose-derived stem cells. Finally, the excellent efficacy of sea buckthorn flavonoids and their derivatives has been demonstrated mainly in cellular or animal models, and there is a paucity of data on clinical trials. These studies have tended to focus on preliminary assessments of efficacy, and there is a need to explore dose-effect relationships, toxicities and side effects, and to conduct further clinical studies.
In summary, through this review, we aim to understand the role and potential of sea buckthorn flavonoids and their derivatives in the prevention and treatment of DCM. This provides insights and references for the development of sea buckthorn flavonoids as novel therapeutic agents for DCM and their further clinical application.
Author contributions
QZ: Formal Analysis, Writing – original draft, Visualization, Methodology, Investigation, Conceptualization, Writing – review and editing. JZ: Project administration, Investigation, Writing – review and editing. YO: Project administration, Writing – review and editing, Investigation. HL: Writing – review and editing, Investigation. CX: Project administration, Writing – review and editing, Supervision. XF: Conceptualization, Writing – review and editing, Supervision, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82374404) and the Natural Science Foundation of Sichuan Province (No. 2024NSFSC1863).
Acknowledgments
This paper has been greatly improved with the suggestions of the reviewers. The authors sincerely thank the reviewers and all the authors of the references.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adebiyi, O. A., Adebiyi, O. O., and Owira, P. M. O. (2016). Naringin reduces hyperglycemia-induced cardiac fibrosis by relieving oxidative stress. PLos One 11 (3), e0149890. doi:10.1371/journal.pone.0149890
Almalki, W., and Almujri, S. S. (2024). Oxidative stress and senescence in aging kidneys: the protective role of SIRT1. EXCLI J. 23, 1030–1067. doi:10.17179/excli2024-7519
Alshehri, A. S., El-Kott, A. F., Eleawa, S. M., El-Gerbed, M. S. A., Khalifa, H. S., El-Kenawy, A. E., et al. (2021). Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J. Physiology Pharmacol. 72 (3). doi:10.26402/jpp.2021.3.04
Arkat, S., Umbarkar, P., Singh, S., and Sitasawad, S. L. (2016). Mitochondrial Peroxiredoxin-3 protects against hyperglycemia induced myocardial damage in diabetic cardiomyopathy. Free Radic. Biol. Med. 97, 489–500. doi:10.1016/j.freeradbiomed.2016.06.019
Bartosova, L., Horvath, C., Galis, P., Ferenczyova, K., Kalocayova, B., Szobi, A., et al. (2022). Quercetin alleviates diastolic dysfunction and suppresses adverse pro-hypertrophic signaling in diabetic rats. Front. Endocrinol. 13, 1029750. doi:10.3389/fendo.2022.1029750
Beata, O. (2017). The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 213 (0). doi:10.1016/j.jep.2017.11.022
Belvončíková, P., Marônek, M., and Gardlík, R. (2022). Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int. J. Mol. Sci. 23, 10729. doi:10.3390/ijms231810729
Botta, M., Audano, M., Sahebkar, A., Sirtori, C., Mitro, N., and Ruscica, M. (2018). PPAR agonists and metabolic syndrome: an established role? Int. J. Mol. Sci. 19, 1197. doi:10.3390/ijms19041197
Boyce, M., and Yuan, J. (2006). Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 13, 363–373. doi:10.1038/sj.cdd.4401817
Bugel, S. M., Bonventre, J. A., and Tanguay, R. L. (2016). Comparative developmental toxicity of flavonoids using an integrative zebrafish system. Toxicol. Sci. 154 (1), 55–68. doi:10.1093/toxsci/kfw139
Cai, E. P., and Lin, J. K. (2009). Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 57 (20), 9817–9827. doi:10.1021/jf902618v
Carrico, C., Meyer, J., He, W., Gibson, B., and Verdin, E. (2018). The mitochondrial acylome emerges: proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab. 27 (3), 497–512. doi:10.1016/j.cmet.2018.01.016
Castillo, R. L., Herrera, E. A., Gonzalez-Candia, A., Reyes-Farias, M., de la Jara, N., Peña, J. P., et al. (2018). Quercetin prevents diastolic dysfunction induced by a high-cholesterol diet: role of oxidative stress and bioenergetics in hyperglycemic rats. Oxidative Med. Cell. Longev. 2018, 7239123. doi:10.1155/2018/7239123
Chang, X., Zhang, T., Wang, J. Y., Liu, Y., Yan, P. Z., Meng, Q. Y., et al. (2021). SIRT5-Related desuccinylation modification contributes to quercetin-induced protection against heart failure and high-glucose-prompted cardiomyocytes injured through regulation of mitochondrial quality surveillance. Oxidative Med. Cell. Longev. 2021, 2021. doi:10.1155/2021/5876841
Chen, A., Feng, X., Dorjsuren, B., Chimedtseren, C., Damda, T.-A., and Zhang, C. (2023a). Traditional food, modern food and nutritional value of sea buckthorn (Hippophae rhamnoides L.): a review. J. Future Foods 3 (3), 191–205. doi:10.1016/j.jfutfo.2023.02.001
Chen, T. S., Liou, S. Y., Kuo, C. H., Pan, L. F., Yeh, Y. L., Liou, J., et al. (2017). Green tea epigallocatechin gallate enhances cardiac function restoration through survival signaling expression in diabetes mellitus rats with autologous adipose tissue-derived stem cells. J. Appl. Physiol. 123 (5), 1081–1091. doi:10.1152/japplphysiol.00471.2016
Chen, X., Qian, J., Wang, L., Li, J., Zhao, Y., Han, J., et al. (2018). Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine 60, 83–94. doi:10.1007/s12020-018-1525-4
Chen, X.-Y., Liu, G.-L., Zhang, W., Zhang, J.-N., Yan, Y., Dong, W.-Q., et al. (2015). Inhibition of MEF2A prevents hyperglycemia-induced extracellular matrix accumulation by blocking akt and TGF-β1/Smad activation in cardiac fibroblasts. Int. J. Biochem. Cell Biol. 69, 52–61. doi:10.1016/j.biocel.2015.10.012
Chen, Y., Zheng, Y., Chen, R., Shen, J., Zhang, S., Gu, Y., et al. (2023b). Dihydromyricetin attenuates diabetic cardiomyopathy by inhibiting oxidative stress, inflammation and necroptosis via sirtuin 3 activation. Antioxidants 12, 200. doi:10.3390/antiox12010200
Chen, Y. F., Qiu, Q., Wang, L., Li, X. R., Zhou, S., Wang, H., et al. (2024). Quercetin ameliorates myocardial injury in diabetic rats by regulating autophagy and apoptosis through AMPK/mTOR signaling pathway. Am. J. Chin. Med. 52 (03), 841–864. doi:10.1142/s0192415x24500344
Cheng, Y., Wang, Y., Yin, R.-L., Xu, Y., Zhang, L., Zhang, Y., et al. (2023). Central role of cardiac fibroblasts in myocardial fibrosis of diabetic cardiomyopathy. Front. Endocrinol. 14, 1162754. doi:10.3389/fendo.2023.1162754
Christaki, E. (2012). Hippophae rhamnoides L. (sea buckthorn): a potential source of nutraceuticals. Food Public Health 2, 69–72. doi:10.5923/J.FPH.20120203.02
Chua, L. S. (2013). A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 150 (3), 805–817. doi:10.1016/j.jep.2013.10.036
Chun, Y., and Kim, J. (2018). Autophagy: an essential degradation program for cellular homeostasis and life. Cells 7, 278. doi:10.3390/cells7120278
Ciumărnean, L., Milaciu, M., Runcan, O., Vesa, Ș., Rãchişan, A., Negrean, V., et al. (2020). The effects of flavonoids in cardiovascular diseases. Molecules 25, 4320. doi:10.3390/molecules25184320
Colombo, M., Michels, L., Teixeira, H., and Koester, L. (2021). Flavonoid delivery by solid dispersion: a systematic review. Phytochem. Rev. 21, 783–808. doi:10.1007/s11101-021-09763-3
Cramer, C., Freisinger, E., Jones, R., Slakey, D., Dupin, C., Newsome, E., et al. (2010). Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 19 (12), 1875–1884. doi:10.1089/scd.2010.0009
Danielski, R., and Shahidi, F. (2024). Phenolic composition and bioactivities of sea buckthorn (Hippophae rhamnoides L.) fruit and seeds: an unconventional source of natural antioxidants in North America. J. Sci. food Agric. 104, 5553–5564. doi:10.1002/jsfa.13386
De Jong, K., Czeczor, J., Sithara, S., McEwen, K., Lopaschuk, G., Appelbe, A., et al. (2017). Obesity and type 2 diabetes have additive effects on left ventricular remodelling in normotensive patients-a cross sectional study. Cardiovasc. Diabetol. 16, 21. doi:10.1186/s12933-017-0504-z
Deng, H.-J., Zhou, C.-H., Huang, L.-T., Wen, L., Zhou, M., and Wang, C.-X. (2020). Activation of silent information regulator 1 exerts a neuroprotective effect after intracerebral hemorrhage by deacetylating NF-κB/p65. J. Neurochem. 157, 574–585. doi:10.1111/jnc.15258
Dewanjee, S., Vallamkondu, J., Kalra, R., John, A., Reddy, P., and Kandimalla, R. (2021). Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 68, 101338. doi:10.1016/j.arr.2021.101338
Dexheimer, P., and Cochella, L. (2020). MicroRNAs: from mechanism to organism. Front. Cell Dev. Biol. 8, 409. doi:10.3389/fcell.2020.00409
Díaz-Juárez, J., Suarez, J., Cividini, F., Scott, B., Diemer, T., Dai, A., et al. (2016). Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. Am. J. Physiol. Cell Physiol. 311, 6. doi:10.1152/ajpcell.00236.2016
Estaquier, J., Vallette, F., Vayssiere, J., and Mignotte, B. (2012). The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 942, 157–183. doi:10.1007/978-94-007-2869-1_7
Fang, Y., Cao, W., Xia, M., Pan, S., and Xu, X. (2017). Study of structure and permeability relationship of flavonoids in Caco-2 cells. Nutrients 9, 1301. doi:10.3390/nu9121301
Faria, A., and Persaud, S. (2017). Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol. Ther. 172, 50–62. doi:10.1016/j.pharmthera.2016.11.013
Fatin Farhana, J., Satirah, Z., Izatus Shima, T., Zariyantey Abd, H., and Siti Balkis, B. (2021). The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int. J. Mol. Sci. 22 (10), 5094. doi:10.3390/ijms22105094
Federation, I. D. (2025). IDF diabetes atlas. 11th ed. International Diabetes Federation. Available online at: https://diabetesatlas.org (Accessed June 6, 2025).
Fossier, L., Panel, M., Butruille, L., Colombani, S., Azria, L., Woitrain, E., et al. (2022). Enhanced mitochondrial calcium uptake suppresses atrial fibrillation associated with metabolic syndrome. J. Am. Coll. Cardiol. 80 (23), 2205–2219. doi:10.1016/j.jacc.2022.09.041
Ganesan, D., Albert, A., Paul, E., Ananthapadmanabhan, K., Andiappan, R., and Sadasivam, S. G. (2020). Rutin ameliorates metabolic acidosis and fibrosis in alloxan induced diabetic nephropathy and cardiomyopathy in experimental rats. Mol. Cell. Biochem. 471 (1-2), 41–50. doi:10.1007/s11010-020-03758-y
Gao, Y., Wang, Z., Zhang, Y., Liu, Y., Wang, S., Sun, W., et al. (2018). Naringenin inhibits NG-nitro-L-arginine methyl ester-induced hypertensive left ventricular hypertrophy by decreasing angiotensin-converting enzyme 1 expression. Exp. Ther. Med. 16, 867–873. doi:10.3892/etm.2018.6258
Garate-Carrillo, A., Ramirez-Sanchez, I., Nguyen, J., Gonzalez, J., Ceballos, G., and Villarreal, F. (2021). Antifibrotic effects of (-)-Epicatechin on high glucose stimulated cardiac fibroblasts. J. Med. Food 24 (11), 1177–1185. doi:10.1089/jmf.2020.0210
Garg, R., Kumariya, S., Katekar, R., Verma, S., Goand, U., and Gayen, J. (2021). JNK signaling pathway in metabolic disorders: an emerging therapeutic target. Eur. J. Pharmacol. 901, 174079. doi:10.1016/j.ejphar.2021.174079
Guangzhi, H., Yushu, D., Rentao, H., Kai, W., Yinsong, Z., and Guobiao, L. (2016). Rutin inhibits neuroinflammation and provides neuroprotection in an experimental rat model of subarachnoid hemorrhage, possibly through suppressing the RAGE-NF-κB inflammatory signaling pathway. Neurochem. Res. 41 (6), 1496–1504. doi:10.1007/s11064-016-1863-7
Guanhua, D., Lan, S., Rui, Z., Lida, D., Junke, S., Li, Z., et al. (2016). Polyphenols: potential source of drugs for the treatment of ischaemic heart disease. Pharmacol. Ther. 162 (0), 23–34. doi:10.1016/j.pharmthera.2016.04.008
Gui, L. M., Wang, F. X., Hu, X. K., Liu, X. J., Yang, H., Cai, Z. X. R., et al. (2022). Epigallocatechin gallate protects diabetes mellitus rats complicated with cardiomyopathy through TGF-β1/JNK signaling pathway. Curr. Pharm. Des. 28 (33), 2758–2770. doi:10.2174/1381612828666220902115437
Hanna, A., and Frangogiannis, N. (2020). Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 34, 849–863. doi:10.1007/s10557-020-07071-0
He, N., Wang, Q., Huang, H., Chen, J., Wu, G., Zhu, M., et al. (2023). A comprehensive review on extraction, structure, detection, bioactivity, and metabolism of flavonoids from sea buckthorn (Hippophae rhamnoides L.). J. Food Biochem. 2023 (1), 1–27. doi:10.1155/2023/4839124
He, Y., Wang, S., Sun, H., Li, Y., and Feng, J. (2022). Naringenin ameliorates myocardial injury in STZ-Induced diabetic mice by reducing oxidative stress, inflammation and apoptosis via regulating the Nrf2 and NF-κB signaling pathways. Front. Cardiovasc. Med. 9, 946766. doi:10.3389/fcvm.2022.946766
H-H Sherry, C., Yan, C., Iman A, H., James A, C., Farah, S., Chris A, B., et al. (2003). Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 9 (9), 3312–3319.
Hippisley-Cox, J., and Coupland, C. (2016). Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. Bmj-British Med. J. 354, i3477. doi:10.1136/bmj.i3477
Hiromitsu, K., Genzou, T., Kazuko, G., Akiko, T., Atsushi, M., Atsushi, O., et al. (2015). Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 11 (7), 1146–1160. doi:10.1080/15548627.2015.1051295
Hou, T.-Y., Wu, S.-B., Kau, H., and Tsai, C.-C. (2021). JNK and p38 inhibitors prevent transforming growth Factor-β1-Induced myofibroblast transdifferentiation in human graves’ orbital fibroblasts. Int. J. Mol. Sci. 22, 2952. doi:10.3390/ijms22062952
Hwang, T., Cuiu, Y.-C., Chen, Y.-C., Chen, P.-C., Tsai, T., Chou, K., et al. (2023). Tumor suppressive functions of hsa-miR-34a on cell cycle, migration and protective autophagy in bladder cancer. Int. J. Oncol. 62, 66. doi:10.3892/ijo.2023.5514
Ibrahim, M., Suruthi, S., and Sakthivel, G. (2018). Ameliorative effect of epigallocatechin gallate on cardiac hypertrophy and fibrosis in aged rats. J. Cardiovasc. Pharmacol. 71 (2), 65–75. doi:10.1097/FJC.0000000000000545
Ilbeigi, D., Nourbakhsh, M., Pasalar, P., Meshkani, R., Afra, H. S., Panahi, G., et al. (2020). Nicotinamide phosphoribosyltransferase knockdown leads to lipid accumulation in HepG2 cells through the SIRT1-AMPK pathway. Cell J. (Yakhteh) 22, 125–132. doi:10.22074/cellj.2020.7013
Jae Ju, J., Yu Mi, H., Yong Chun, J., Eun Ju, L., Ju Sun, K., Hye Jung, K., et al. (2009). Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food Chem. Toxicol. 47 (7), 1569–1576. doi:10.1016/j.fct.2009.03.044
Jia, G. H., Hill, M. A., and Sowers, J. R. (2018). Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circulation Res. 122 (4), 624–638. doi:10.1161/circresaha.117.311586
Jia, J., Zang, E.-H., Lv, L.-J., Li, Q.-Y., Zhang, C., Xia, Y., et al. (2020a). Flavonoids in myocardial ischemia-reperfusion injury: therapeutic effects and mechanisms. Chin. Herb. Med. 13, 49–63. doi:10.1016/j.chmed.2020.09.002
Jia, Q., Yang, R., Mehmood, S., and Li, Y. (2022). Epigallocatechin-3-gallate attenuates myocardial fibrosis in diabetic rats by activating autophagy. Exp. Biol. Med. 247 (17), 1591–1600. doi:10.1177/15353702221110646
Jia, Y., Wu, C., Kim, Y.-S., Yang, S., Kim, Y., Kim, J.-S., et al. (2020b). A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 3, 514. doi:10.1038/s42003-020-01231-6
Jiang, C. J., Li, D., Chen, L., Liu, Y., Zhao, Y., Mei, G. B., et al. (2022). Quercetin ameliorated cardiac injury via reducing inflammatory actions and the glycerophospholipid metabolism dysregulation in a diabetic cardiomyopathy mouse model. Food Funct. 13 (14), 7847–7856. doi:10.1039/d2fo00912a
Jiang, J. M., Liang, G. B., Wu, Z. J., Mo, H. L., You, Q., Wang, Z. Q., et al. (2018). Naringenin alleviates high D-glucose-induced injuries through upregulation of microRNA-30d-5p level in human AC16 cardiac cells. J. Appl. Biomed. 16 (4), 274–280. doi:10.1016/j.jab.2018.02.005
Jiang, X.-S., Liu, T., Xia, Y.-F., Gan, H., Ren, W., and Du, X.-G. (2024). Activation of the Nrf2/ARE signaling pathway ameliorates hyperlipidemia-induced renal tubular epithelial cell injury by inhibiting mtROS-mediated NLRP3 inflammasome activation. Front. Immunol. 15, 1342350. doi:10.3389/fimmu.2024.1342350
Jie, H., and Wu, K. (2015). GW26-e4786 protective effects of naringin on diabetic cardiomyopathy in rats through inhibiting p38MAPK pathway. J. Am. Coll. Cardiol. 66 (16), C76. doi:10.1016/j.jacc.2015.06.302
Kamalakkannan, N., and Prince, P. (2006). Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 98 (1), 97–103. doi:10.1111/J.1742-7843.2006.PTO_241.X
Kayama, H., Okumura, R., and Takeda, K. (2020). Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 38, 23–48. doi:10.1146/annurev-immunol-070119-115104
Kenneth, B. M., Adrian F, H., Margaret, M. R., Michael, M. G., Guilherme, H. O., Robert, C., et al. (2016). Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. J. Am. Med. Assoc. 316 (5), 500–508. doi:10.1001/jama.2016.10260
Kim, H., Shen, Q., Eun, J., Shin, W., Yang, H., Park, W., et al. (2015). Abstract 3111: jnk/c-jun- and NF-κB-mediated microRNA-221 governs tumor suppressor HDAC6 to potentiate malignant progression of liver cancer. Cancer Res. 75, 3111. doi:10.1158/1538-7445.AM2015-3111
Kim, H.-Y., and Rikihisa, Y. (2002). Roles of p38 mitogen-activated protein kinase, NF-kappaB, and protein kinase C in proinflammatory cytokine mRNA expression by human peripheral blood leukocytes, monocytes, and neutrophils in response to anaplasma phagocytophila. Infect. Immun. 70, 4132–4141. doi:10.1128/IAI.70.8.4132-4141.2002
Kim, J., Kundu, M., Viollet, B., and Guan, K. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141. doi:10.1038/ncb2152
Krejcarová, J., Strakova, E., Suchý, P., Herzig, I., and Karásková, K. (2015). Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities - a review. Acta Veterinaria Brno 84, 257–268. doi:10.2754/AVB201584030257
Lemesle, G., Puymirat, E., Bonello, L., Simon, T., Steg, P., Ferrières, J., et al. (2021). Compared impact of diabetes on the risk of heart failure from acute myocardial infarction to chronic coronary artery disease. Diabetes metabolism 48, 101265. doi:10.1016/j.diabet.2021.101265
Letchumanan, G., Abdullah, N., Marlini, M., Baharom, N., Lawley, B., Omar, M. R., et al. (2022). Gut microbiota composition in prediabetes and newly diagnosed type 2 diabetes: a systematic review of observational studies. Front. Cell. Infect. Microbiol. 12, 943427. doi:10.3389/fcimb.2022.943427
Li, G., Wang, J., Wu, W., Wang, M., Han, X., Zhang, Z., et al. (2023). Proteomic analysis of the supernatant from bone marrow mesenchymal stem cells under high glucose conditions. J. proteome Res. 23, 344–355. doi:10.1021/acs.jproteome.3c00588
Li, L., Luo, W., Qian, Y., Zhu, W., Qian, J., Li, J., et al. (2019). Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 59, 152774. doi:10.1016/j.phymed.2018.11.034
Li, L., Zeng, H., He, X., and Chen, J. X. (2021). Sirtuin 3 alleviates diabetic cardiomyopathy by regulating TIGAR and cardiomyocyte metabolism. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 10, e018913. doi:10.1161/JAHA.120.018913
Li, W. Z., Chen, S. W., Zhou, G. Q., Li, H. L., Zhong, L., and Liu, S. W. (2018). Potential role of cyanidin 3-glucoside (C3G) in diabetic cardiomyopathy in diabetic rats: an in vivo approach. Saudi J. Biol. Sci. 25 (3), 500–506. doi:10.1016/j.sjbs.2016.11.007
Li, X., Du, N., Zhang, Q., Li, J., Chen, X., Liu, X., et al. (2014). MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 5, e1479. doi:10.1038/cddis.2014.430
Li, Y., Zhao, S., Zhang, W., Zhao, P., He, B., Wu, N., et al. (2011). Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res. Clin. Pract. 93 (2), 205–214. doi:10.1016/j.diabres.2011.03.036
Liang, W., Aiping, T., Xiangbo, A., Yunlong, X., and Yunpeng, X. (2020). Quercetin dihydrate inhibition of cardiac fibrosis induced by angiotensin II in vivo and in vitro. Biomed. Pharmacother. 127 (0), 110205. doi:10.1016/j.biopha.2020.110205
Liao, H. H., Zhu, J. X., Feng, H., Ni, J., Zhang, N., Chen, S., et al. (2017). Myricetin possesses potential protective effects on diabetic cardiomyopathy through inhibiting Iκ B α/NF κ B and enhancing Nrf2/HO-1. Oxidative Med. Cell. Longev. 2017, 8370593. doi:10.1155/2017/8370593
Lim, Y., Kim, J., Pan, J., Kim, J. K., Park, T.-S., Kim, Y., et al. (2018). Naringin protects pancreatic β-Cells against oxidative stress-induced apoptosis by inhibiting both intrinsic and extrinsic pathways in insulin-deficient diabetic mice. Mol. Nutr. Food Res. 62. doi:10.1002/mnfr.201700810
Lin, X., Xu, Y., Pan, X., Xu, J., Ding, Y., Sun, X., et al. (2020). Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci. Rep. 10, 14790. doi:10.1038/s41598-020-71908-9
Ling, L., Sheyu, L., Ke, D., Jiali, L., Per Olav, V., Pujing, Z., et al. (2016). Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. Bmj-British Med. J. 352 (0), i610. doi:10.1136/bmj.i610
Liu, H. J., Fan, Y. L., Liao, H. H., Liu, Y., Chen, S., Ma, Z. G., et al. (2017). Apigenin alleviates STZ-Induced diabetic cardiomyopathy. Mol. Cell. Biochem. 428 (1-2), 9–21. doi:10.1007/s11010-016-2913-9
Liu, H. J., Yang, Z., Deng, W., and Tang, Q. Z. (2016). GW27-e0367 apigenin attenuates the cardiac remodeling in experimental diabetic cardiomyopathy. J. Am. Coll. Cardiol. 68 (16), C14. doi:10.1016/j.jacc.2016.07.054
Liu, H. Y., Zhang, Z. Y., Zhang, L., Yao, X. L., Zhong, X. M., Cheng, G. C., et al. (2020a). Spiraeoside protects human cardiomyocytes against high glucose-induced injury, oxidative stress, and apoptosis by activation of PI3K/Akt/Nrf2 pathway. J. Biochem. Mol. Toxicol. 34 (10), e22548. doi:10.1002/jbt.22548
Liu, M., Cheng, C., Li, X., Zhou, S., Hua, J., Huang, J., et al. (2020b). Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1α pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. Int. J. Publ. Br. Industrial Biol. Res. Assoc. 141, 111436. doi:10.1016/j.fct.2020.111436
Liu, M., Sun, X.-H., Chen, B., Dai, R., Xi, Z., and Xu, H. (2022). Insights into manganese superoxide dismutase and human diseases. Int. J. Mol. Sci. 23, 15893. doi:10.3390/ijms232415893
Lorenzo-Almorós, A., Tuñón, J., Orejas, M., Cortés, M., Egido, J., and Lorenzo, O. (2017). Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 16, 28. doi:10.1186/s12933-017-0506-x
Lossi, L. (2022). The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 479 (3), 357–384. doi:10.1042/BCJ20210854
Lu, Y., An, L., Taylor, M., and Chen, Q. (2022). Nrf2 signaling in heart failure: expression of Nrf2, Keap1, antioxidant and detoxification genes in dilated or ischemic cardiomyopathy. Physiol. Genomics 54, 115–127. doi:10.1152/physiolgenomics.00079.2021
Luan, S. S., Yu, F., Li, B. Y., Qin, R. J., Li, X. L., Cai, Q., et al. (2014). Quantitative proteomics study of protective effects of grape seed procyanidin B2 on diabetic cardiomyopathy in Db/Db mice. Biosci. Biotechnol. Biochem. 78 (9), 1577–1583. doi:10.1080/09168451.2014.930320
Mann, E. R., Lam, Y. K., and Uhlig, H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24 (8), 577–595. doi:10.1038/s41577-024-01014-8
Manolis, A., Manolis, A., Manolis, T., Apostolaki, N., Apostolopoulos, E., Melita, H., et al. (2020). Mitochondrial dysfunction in cardiovascular disease: current status of translational research/clinical and therapeutic implications. Med. Res. Rev. 41, 275–313. doi:10.1002/med.21732
Manolis, A., Manolis, T., Melita, H., and Manolis, A. (2021). Gut microbiota and cardiovascular disease: symbiosis versus dysbiosis. Curr. Med. Chem. 29, 4050–4077. doi:10.2174/0929867328666211213112949
Moreno-Ulloa, A., Mendez-Luna, D., Beltran-Partida, E., Castillo, C., Guevara, G., Ramirez-Sanchez, I., et al. (2015). The effects of (-)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER). Pharmacol. Res. 100, 309–320. doi:10.1016/j.phrs.2015.08.014
Nazio, F., Strappazzon, F., Antonioli, M., Bielli, P., Cianfanelli, V., Bordi, M., et al. (2013). mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 15, 406–416. doi:10.1038/ncb2708
Ni, T. J., Lin, N., Lu, W. Q., Sun, Z. Z., Lin, H., Chi, J. F., et al. (2020). Dihydromyricetin prevents diabetic cardiomyopathy via miR-34a suppression by activating autophagy. Cardiovasc. Drugs Ther. 34 (3), 291–301. doi:10.1007/s10557-020-06968-0
Nile, A., Nile, S., Cespedes-Acuna, C., and Oh, J.-W. (2021). Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food Chem. Toxicol. Int. J. Publ. Br. Industrial Biol. Res. Assoc. 154, 112327. doi:10.1016/j.fct.2021.112327
Nishida, K., and Otsu, K. (2017). Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 113 (4), 389–398. doi:10.1093/cvr/cvx012
Ohno, M., Shibata, C., Kishikawa, T., Yoshikawa, T., Takata, A., Kojima, K., et al. (2013). The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci. Rep. 3, 2553. doi:10.1038/srep02553
Ojo, O. O., Obaidu, I. M., Obigade, O. C., and Olorunsogo, O. O. (2022). Quercetin and vitamin E ameliorate cardio-apoptotic risks in diabetic rats. Mol. Cell. Biochem. 477 (3), 793–803. doi:10.1007/s11010-021-04332-w
Othman, A. I., El-Sawi, M. R., El-Missiry, M. A., and Abukhalil, M. H. (2017). Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 94, 362–373. doi:10.1016/j.biopha.2017.07.129
Pai, P. Y., Chou, W. C., Chan, S. H., Wu, S. Y., Chen, H. I., Li, C. W., et al. (2021). Epigallocatechin gallate reduces homocysteine-caused oxidative damages through modulation SIRT1/AMPK pathway in endothelial cells. Am. J. Chin. Med. 49 (01), 113–129. doi:10.1142/s0192415x21500063
Pan, X., Xu, S., Li, J., and Tong, N. (2020). The effects of DPP-4 inhibitors, GLP-1RAs, and SGLT-2/1 inhibitors on heart failure outcomes in diabetic patients with and without heart failure history: insights from CVOTs and drug mechanism. Front. Endocrinol. 11, 599355. doi:10.3389/fendo.2020.599355
Pang, X., Zhao, J., Zhang, W., Zhuang, X., Wang, J., Xu, R., et al. (2008). Antihypertensive effect of total flavones extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J. Ethnopharmacol. 117 (2), 325–331. doi:10.1016/j.jep.2008.02.002
Paolillo, S., Marsico, F., Prastaro, M., Renga, F., Esposito, L., De Martino, F., et al. (2019). Diabetic cardiomyopathy: definition, diagnosis, and therapeutic implications. Heart Fail. Clin. 15 (3), 341–347. doi:10.1016/j.hfc.2019.02.003
Potenza, M., Iacobazzi, D., Sgarra, L., and Montagnani, M. (2020). The intrinsic virtues of EGCG, an extremely good cell guardian, on prevention and treatment of diabesity complications. Molecules 25, 3061. doi:10.3390/molecules25133061
Poulsen, L., Siersbæk, M., and Mandrup, S. (2012). PPARs: fatty acid sensors controlling metabolism. Seminars Cell Dev. Biol. 23 (6), 631–639. doi:10.1016/j.semcdb.2012.01.003
Przemek A, G., Seung Pil, J., Dongtak, J., Ahyoung, L., Philyoung, L., Jae Gyun, O., et al. (2019). Role of SIRT1 in modulating acetylation of the sarco-endoplasmic reticulum Ca(2+)-ATPase in heart failure. Circulation Res. 124 (9), e63–e80. doi:10.1161/circresaha.118.313865
Pundir, S., Garg, P., Dviwedi, A., Ali, A., Kapoor, V. K., Kapoor, D., et al. (2021). Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: a review. J. Ethnopharmacol. 266, 113434. doi:10.1016/j.jep.2020.113434
Qin, G., He, N.-X., Huang, H., Fu, X., Zhang, Z.-L., Shu, J., et al. (2023). Hippophae rhamnoides L.: a comprehensive review on the botany, traditional uses, phytonutrients, health benefits, quality markers, and applications. J. Agric. food Chem. 71, 4769–4788. doi:10.1021/acs.jafc.2c06916
Qin-Ge, M., Rong-Rui, W., Dong-Li, S., Zhi-Pei, S., and Jiang-Hong, D. (2020). Structurally diverse flavonolignans with immunosuppressive and neuroprotective activities from the fruits of Hippophae rhamnoides L. J. Agric. Food Chem. 68 (24), 6564–6575. doi:10.1021/acs.jafc.0c01432
Qiu, J., Liu, D., Li, P., Zhou, L., Zhou, L., Liu, X., et al. (2022). NADPH oxidase mediates oxidative stress and ventricular remodeling through SIRT3/FOXO3a pathway in diabetic mice. Antioxidants 11, 1745. doi:10.3390/antiox11091745
Rajković, J., Perić, M., Stanišić, J., Gostimirovic, M., Novaković, R., Djokic, V., et al. (2024). Effect of Type-2 diabetes mellitus on the expression and function of smooth muscle ATP-sensitive potassium channels in human internal mammary artery grafts. Pharmaceuticals 17, 857. doi:10.3390/ph17070857
Repges, E., Zaidi, M. A. Z. A., Jansen, F., Zimmer, S., Tiyerlili, V., Nickenig, G., et al. (2022). The endoplasmic reticulum (ER) chaperone GRP78 is secreted during ER stress and alleviates endothelial cell inflammation. Eur. Heart J. 43. doi:10.1093/eurheartj/ehac544.2938
Rodríguez, C., Muñoz, M., Contreras, C., and Prieto, D. (2021). AMPK, metabolism, and vascular function. FEBS J. 288, 3746–3771. doi:10.1111/febs.15863
Romashko, M., Schragenheim, J., Abraham, N., and McClung, J. (2016). Epoxyeicosatrienoic acid as therapy for diabetic and ischemic cardiomyopathy. Trends Pharmacol. Sci. 37 (11), 945–962. doi:10.1016/j.tips.2016.08.001
Royce, G., Brown-Borg, H., and Deepa, S. (2019). The potential role of necroptosis in inflammaging and aging. GeroScience 41, 795–811. doi:10.1007/s11357-019-00131-w
Różański, M., Studzian, M., and Pulaski, L. (2019). Direct measurement of kinetic parameters of ABCG2-dependent transport of natural flavonoids using a fluorogenic substrate. J. Pharmacol. Exp. Ther. 371, 309–319. doi:10.1124/jpet.119.261347
Ryter, S. (2022). Heme oxygenase-1: an anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants 11, 555. doi:10.3390/antiox11030555
Sabio, G., and Davis, R. (2014). TNF and MAP kinase signalling pathways. Seminars Immunol. 26 (3), 237–245. doi:10.1016/j.smim.2014.02.009
Saeed, N. M., El-Naga, R. N., El-Bakly, W. M., Abdel-Rahman, H. M., Salah ElDin, R. A., and El-Demerdash, E. (2015). Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: a mechanistic study. Biochem. Pharmacol. 95 (3), 145–155. doi:10.1016/j.bcp.2015.02.006
Saklani, R., Gupta, S. K., Mohanty, I. R., Kumar, B., Srivastava, S., and Mathur, R. (2016). Cardioprotective effects of rutin via alteration in TNF-α, CRP, and BNP levels coupled with antioxidant effect in STZ-Induced diabetic rats. Mol. Cell. Biochem. 420 (1-2), 65–72. doi:10.1007/s11010-016-2767-1
Salehi, B., Fokou, P., Sharifi-Rad, M., Zucca, P., Pezzani, R., Martins, N., et al. (2019). The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals 12, 11. doi:10.3390/ph12010011
Salvatore, T., Pafundi, P. C., Galiero, R., Albanese, G., Di Martino, A., Caturano, A., et al. (2021). The diabetic cardiomyopathy: the contributing pathophysiological mechanisms. Front. Med. 8, 695792. doi:10.3389/fmed.2021.695792
Seferović, P. M., Coats, A. J. S., Ponikowski, P., Filippatos, G., Huelsmann, M., Jhund, P. S., et al. (2020). European society of cardiology/heart failure association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur. J. Heart Fail. 22 (2), 196–213. doi:10.1002/ejhf.1673
Shah, G., and Prajapati, A. (2024). The role of TGF-β in cardiac fibrosis and heart failure: a review. IP Int. J. Compr. Adv. Pharmacol. doi:10.18231/j.ijcaap.2024.001
Shi, L., Zhang, T., Liang, X., Hu, Q., Huang, J., Zhou, Y., et al. (2015). Dihydromyricetin improves skeletal muscle insulin resistance by inducing autophagy via the AMPK signaling pathway. Mol. Cell. Endocrinol. 409, 92–102. doi:10.1016/j.mce.2015.03.009
Solanki, S., Vig, H., Khatri, N., Singh, B. P., Khan, M. S., Devgun, M., et al. (2024). Naringenin: a promising immunomodulator for anti-inflammatory, neuroprotective, and anti-cancer applications. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 24, 1–25. doi:10.2174/0118715230320007240708074939
Song, S., Ding, Y., Dai, G., Zhang, Y., Xu, M.-T., Shen, J., et al. (2020). Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 42, 230–241. doi:10.1038/s41401-020-0490-7
Søren, L. K., Ulrik, M. M., Pardeep S, J., Mark C, P., David, P., Sithu, W., et al. (2017). Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the I-preserve trial (irbesartan in heart failure with preserved ejection fraction). Circulation 135 (8), 728. doi:10.1161/CIRCULATIONAHA.116.024593
Su, M., Wang, J., Wang, C., Wang, X., Dong, W., Qiu, W., et al. (2014). MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ. 22, 986–999. doi:10.1038/cdd.2014.187
Suarez, J., Cividini, F., Scott, B., Lehmann, K., Díaz-Juárez, J., Diemer, T., et al. (2018). Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J. Biol. Chem. 293, 8182–8195. doi:10.1074/jbc.RA118.002066
Sun, L. L., Xiao, Y. J., San, W. Q., Chen, Y., and Meng, G. L. (2024). Dihydromyricetin regulates RIPK3-CaMKII to prevent necroptosis in high glucose-stimulated cardiomyocytes. Heliyon 10 (7), e28921. doi:10.1016/j.heliyon.2024.e28921
Syed, A., Reza, M., Shafiq, M., Kumariya, S., Singh, P., Husain, A., et al. (2020). Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 257, 118118. doi:10.1016/j.lfs.2020.118118
Szaraz, P., Gratch, Y., Iqbal, F., and Librach, C. (2017). In vitro differentiation of human mesenchymal stem cells into functional cardiomyocyte-like cells. J. Vis. Exp. 126, 55757. doi:10.3791/55757
Szegezdi, E., Logue, S., Gorman, A., and Samali, A. (2006). Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7, 880–885. doi:10.1038/sj.embor.7400779
Tahapary, D., Fatya, A., Kurniawan, F., Marcella, C., Rinaldi, I., Tarigan, T., et al. (2023). Increased intestinal-fatty acid binding protein in obesity-associated type 2 diabetes mellitus. PLoS One 18, e0279915. doi:10.1371/journal.pone.0279915
Tang, Z., and Zhang, Q. (2022). The potential toxic side effects of flavonoids. BIOCELL 46 (2), 357–366. doi:10.32604/biocell.2022.015958
Tebay, L., Robertson, H., Durant, S., Vitale, S., Penning, T., Dinkova-Kostova, A., et al. (2015). Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 88 Pt B, 108–146. doi:10.1016/j.freeradbiomed.2015.06.021
Teissier, T., and Boulanger, E. (2019). The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology 20, 279–301. doi:10.1007/s10522-019-09808-3
Teringova, E., and Tousek, P. (2017). Apoptosis in ischemic heart disease. J. Transl. Med. 15 (1), 87. doi:10.1186/s12967-017-1191-y
Tkacz, K., Wojdyło, A., Turkiewicz, I., and Nowicka, P. (2021). Triterpenoids, phenolic compounds, macro- and microelements in anatomical parts of sea buckthorn (Hippophaë rhamnoides L.) berries, branches and leaves. J. Food Compos. Analysis 103, 104107. doi:10.1016/J.JFCA.2021.104107
Uryash, A., Mijares, A., Flores, V., Adams, J. A., and Lopez, J. R. (2021). Effects of naringin on cardiomyocytes from a rodent model of type 2 diabetes. Front. Pharmacol. 12, 719268. doi:10.3389/fphar.2021.719268
Varma, U., Koutsifeli, P., Benson, V., Mellor, K., and Delbridge, L. (2018). Molecular mechanisms of cardiac pathology in diabetes - experimental insights. Biochimica Biophysica Acta-Molecular Basis Dis. 1864, 1949–1959. doi:10.1016/j.bbadis.2017.10.035
Vilella, R., Izzo, S., Naponelli, V., Savi, M., Bocchi, L., Dallabona, C., et al. (2022). In vivo treatment with a standardized green tea extract restores cardiomyocyte contractility in diabetic rats by improving mitochondrial function through SIRT1 activation. Pharmaceuticals 15 (11), 1337. doi:10.3390/ph15111337
Wang, A. J., Wang, S., Wang, B. J., Xiao, M., Guo, Y., Tang, Y., et al. (2020a). Epigenetic regulation associated with sirtuin 1 in complications of diabetes mellitus. Front. Endocrinol. (Lausanne) 11, 598012. doi:10.3389/fendo.2020.598012
Wang, G. G., Li, W., Lu, X. H., Bao, P. J., and Zhao, X. (2012). Luteolin ameliorates cardiac failure in type I diabetic cardiomyopathy. J. Diabetes Its Complicat. 26 (4), 259–265. doi:10.1016/j.jdiacomp.2012.04.007
Wang, S., Ge, W., Harns, C., Meng, X., Zhang, Y., and Ren, J. (2018). Ablation of toll-like receptor 4 attenuates aging-induced myocardial remodeling and contractile dysfunction through NCoRI-HDAC1-mediated regulation of autophagy. J. Mol. Cell. Cardiol. 119, 40–50. doi:10.1016/j.yjmcc.2018.04.009
Wang, S., Li, H., Yuan, M., Fan, H., and Cai, Z. (2022a). Role of AMPK in autophagy. Front. Physiology 13, 1015500. doi:10.3389/fphys.2022.1015500
Wang, J., Wang, R., Li, J. L., and Yao, Z. H. (2021). Rutin alleviates cardiomyocyte injury induced by high glucose through inhibiting apoptosis and endoplasmic reticulum stress. Exp. Ther. Med. 22 (3), 944. doi:10.3892/etm.2021.10376
Wang, X., Chen, X., Yu, H.-T., Tan, Y., Lin, Q., Keller, B., et al. (2020b). Engineered cardiac tissues: a novel in vitro model to investigate the pathophysiology of mouse diabetic cardiomyopathy. Acta Pharmacol. Sin. 42, 932–941. doi:10.1038/s41401-020-00538-8
Wang, Z., Sun, R., Wang, G., Chen, Z., Li, Y., Zhao, Y., et al. (2019). SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 28, 101343. doi:10.1016/j.redox.2019.101343
Wang, Z., Zhao, F., Wei, P., Chai, X., Hou, G., and Meng, Q. (2022b). Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): a comprehensive review. Front. Nutr. 9, 1036295. doi:10.3389/fnut.2022.1036295
Wegner, K., Saleh, D., and Degterev, A. (2017). Complex pathologic roles of RIPK1 and RIPK3: moving beyond necroptosis. Trends Pharmacol. Sci. 38 (3), 202–225. doi:10.1016/j.tips.2016.12.005
Wenshuai, M., Guo, W.-G., Shang, F., Li, Y., Li, W., Liu, J., et al. (2020). Bakuchiol alleviates hyperglycemia-induced diabetic cardiomyopathy by reducing myocardial oxidative stress via activating the SIRT1/Nrf2 signaling pathway. Oxidative Med. Cell. Longev., 2020. doi:10.1155/2020/3732718
Wray, J., and Bishop-Bailey, D. (2008). Epoxygenases and peroxisome proliferator-activated receptors in Mammalian vascular biology. Exp. Physiol. 93, 148–154. doi:10.1113/expphysiol.2007.038612
Wu, B., Lin, J., Luo, J., Han, D., Fan, M. M., Guo, T., et al. (2017a). Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. Biomed Res. Int. 2017, 3764370. doi:10.1155/2017/3764370
Wu, H., Liu, Y., and Liu, C. (2024). The interregulatory circuit between non-coding RNA and apoptotic signaling in diabetic cardiomyopathy. Noncoding RNA Res. 9 (4), 1080–1097. doi:10.1016/j.ncrna.2024.06.011
Wu, Q., Zhang, F., Niu, M., Yan, J., Shi, L., Liang, Y., et al. (2023). Extraction methods, properties, functions, and interactions with other nutrients of lotus procyanidins: a review. J. Agric. food Chem. 71, 14413–14431. doi:10.1021/acs.jafc.3c05305
Wu, Y., Xia, Z. Y., Zhao, B., Leng, Y., Dou, J., Meng, Q. T., et al. (2017b). (-)-Epigallocatechin-3-gallate attenuates myocardial injury induced by ischemia/reperfusion in diabetic rats and in H9c2 cells under hyperglycemic conditions. Int. J. Mol. Med. 40 (2), 389–399. doi:10.3892/ijmm.2017.3014
Xia, C., Gao, A., Zhu, Y., Dong, T., and Tsim, K. (2023). Flavonoids from seabuckthorn (Hippophae rhamnoides L.) restore CUMS-Induced depressive disorder and regulate the gut microbiota in mice. Food Funct. 14, 7426–7438. doi:10.1039/d3fo01332d
Xiao, C., Chen, M. Y., Han, Y. P., Liu, L. J., Yan, J. L., and Qian, L. B. (2023). The protection of luteolin against diabetic cardiomyopathy in rats is related to reversing JNK-Suppressed autophagy. Food Funct. 14 (6), 2740–2749. doi:10.1039/d2fo03871d
Xiaoxi, S., Yiming, Z., Ziyi, Z., Chenhui, S., and Hongli, S. (2021). Study on antioxidant activity of quercetin from seabuckthorn meal in vitro and its protective effect on aging mice. Food Industry Sci. Technol. 42, 350–356. doi:10.13386/J.ISSN1002-0306.2021020011
Xie, W., Huang, W., Cai, S., Chen, H., Fu, W.-J., Chen, Z., et al. (2021). NF-κB/IκBα signaling pathways are essential for resistance to heat stress-induced ROS production in pulmonary microvascular endothelial cells. Mol. Med. Rep. 24, 814. doi:10.3892/mmr.2021.12454
Xing, C., Tian, Z., Qingyan, M., Wang, X., Peizheng, Y., Xue, W., et al. (2021). Quercetin improves cardiomyocyte vulnerability to hypoxia by regulating SIRT1/TMBIM6-Related mitophagy and endoplasmic reticulum stress. Oxidative Med. Cell. Longev. 2021 (0), 5529913. doi:10.1155/2021/5529913
Xu, J.-J., Cui, J., Lin, Q., Chen, X., Zhang, J., Gao, E., et al. (2021). Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 342, 82–93. doi:10.1016/j.ijcard.2021.08.007
Xueying, M., Wei, Y., Heikki, K., and Baoru, Y. (2021). Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn (Hippophaë rhamnoides). Crit. Rev. Food Sci. Nutr. 62 (14). doi:10.1080/10408398.2020.1869921
Yang, L., Gao, Y., Wang, H., Zhong, W., Gong, J., Farag, M., et al. (2024). Myricetin attenuates the inflammatory bowel disease in prediabetic mice via inflammation inhibition and gut microbiota modulation. Food Saf. Health 2, 303–317. doi:10.1002/fsh3.12041
Yang, Q., Gao, H., Dong, R., and Wu, Y. (2016). Sequential changes of endoplasmic reticulum stress and apoptosis in myocardial fibrosis of diabetes mellitus-induced rats. Mol. Med. Rep. 13, 5037–5044. doi:10.3892/mmr.2016.5180
Yao, J., Li, Y., Jin, Y., Chen, Y., Tian, L., and He, W. (2021). Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-κB/NLRP3 and PGC1a/SIRT3 pathways. Int. Immunopharmacol. 96, 107728. doi:10.1016/j.intimp.2021.107728
Yntema, T., Koonen, D. P. Y., and Kuipers, F. (2023). Emerging roles of gut microbial modulation of bile acid composition in the etiology of cardiovascular diseases. Nutrients 15 (8), 1850. doi:10.3390/nu15081850
Yoshizaki, K. (2011). Pathogenic role of IL-6 combined with TNF-α or IL-1 in the induction of acute phase proteins SAA and CRP in chronic inflammatory diseases. Adv. Exp. Med. Biol. 691, 141–150. doi:10.1007/978-1-4419-6612-4_15
You, Q., Wu, Z., Wu, B., Liu, C., Huang, R., Yang, L., et al. (2016). Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 230 (2), 197–214. doi:10.1530/joe-16-0004
Yu, M. G., Gordin, D., Fu, J., Park, K., Li, Q., and King, G. L. (2024). Protective factors and the pathogenesis of complications in diabetes. Endocr. Rev. 45 (2), 227–252. doi:10.1210/endrev/bnad030
Zazueta, C., Jiménez-Uribe, A., Pedraza-Chaverri, J., and Buelna-Chontal, M. (2022). Genetic variations on redox control in cardiometabolic diseases: the role of Nrf2. Antioxidants 11, 507. doi:10.3390/antiox11030507
Zhang, J., Chen, Y., Luo, H., Sun, L.-L., Xu, M., Yu, J., et al. (2018). Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 9, 1204. doi:10.3389/fphar.2018.01204
Zhang, J., Qiu, H. M., Huang, J. J., Ding, S. M., Huang, B., Zhou, P., et al. (2019). EETs/PPARs activation together mediates the preventive effect of naringenin in high glucose-induced cardiomyocyte hypertrophy. Biomed. Pharmacother. 109, 1498–1505. doi:10.1016/j.biopha.2018.10.176
Zhang, L.-M., Lv, S.-S., Fu, S., Wang, J.-Q., Liang, L.-Y., Li, R., et al. (2021a). Procyanidins inhibit fine particulate matter-induced vascular smooth muscle cells apoptosis via the activation of the Nrf2 signaling pathway. Ecotoxicol. Environ. Saf. 223, 112586. doi:10.1016/j.ecoenv.2021.112586
Zhang, W., Zhao, J., Wang, J., Pang, X., Zhuang, X., Zhu, X., et al. (2010). Hypoglycemic effect of aqueous extract of seabuckthorn (Hippophae rhamnoides L.) seed residues in streptozotocin-induced diabetic rats. Phytotherapy Res. 24 (2), 228–232. doi:10.1002/ptr.2917
Zhang, W., Zhou, J., Kang, P. F., Shi, C., and Qian, S. H. (2022). Quercetin inhibits pyroptosis in diabetic cardiomyopathy through the Nrf2 pathway. J. Diabetes Res. 2022, 9723632. doi:10.1155/2022/9723632
Zhang, X., and Wu, C. (2022). In silico, in vitro, and in vivo evaluation of the developmental toxicity, estrogenic activity, and mutagenicity of four natural phenolic flavonoids at low exposure levels. ACS Omega 7 (6), 4757–4768. doi:10.1021/acsomega.1c04239
Zhang, X., Zhao, S.-Z., Huang, Y., Mingming, , Li, B., Li, C., et al. (2024). Diabetes-related macrovascular complications are associated with an increased risk of diabetic microvascular complications: a prospective study of 1518 patients with type 1 diabetes and 20 802 patients with type 2 diabetes in the UK biobank. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 13, e032626. doi:10.1161/JAHA.123.032626
Zhang, Z., Lu, Y., Qi, J., and Wu, W. (2021b). An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm. Sin. B 11, 2449–2468. doi:10.1016/j.apsb.2020.12.022
Zhao, J., Yang, J., and Xie, Y. (2019). Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int. J. Pharm. 570, 118642. doi:10.1016/j.ijpharm.2019.118642
Zhao, M., Song, L., Sun, L., Wang, M., Wang, C., Yao, S., et al. (2021). Associations of type 2 diabetes onset age with cardiovascular disease and mortality: the kailuan study. Diabetes Care 44, 1426–1432. doi:10.2337/DC20-2375
Zheng, W., Wenqian, W., Changlong, Z., Xiangdong, G., and Weihua, C. (2022). Evaluation of antioxidative and neuroprotective activities of total flavonoids from sea buckthorn (Hippophae rhamnoides L.). Front. Nutr. 9 (0), 861097. doi:10.3389/fnut.2022.861097
Zhou, X.-R., Ru, X.-C., Xiao, C., Pan, J., Lou, Y.-Y., Tang, L.-H., et al. (2021). Sestrin2 is involved in the Nrf2-regulated antioxidative signaling pathway in luteolin-induced prevention of the diabetic rat heart from ischemia/reperfusion injury. Food Funct. 12 (8), 3562–3571. doi:10.1039/d0fo02942d
Zhu, J. X., Bao, Z. J., Hu, Z. Q., Wu, S. L., Tian, C. H., Zhou, Y. R., et al. (2024). Myricetin alleviates diabetic cardiomyopathy by regulating gut microbiota and their metabolites. Nutr. Diabetes 14 (1), 10. doi:10.1038/s41387-024-00268-4
Zili, G., Jingya, C., Lei, Z., Wenhao, X., and Yuanyuan, X. (2021). Mechanochemical-assisted extraction and hepatoprotective activity research of flavonoids from sea buckthorn (Hippophaë rhamnoides L.) pomaces. Molecules 26 (24), 7615. doi:10.3390/molecules26247615
Zou, M., Wang, F., Gao, R., Wu, J., Ou, Y., Chen, X., et al. (2016). Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced fibrogenesis in human cardiac fibroblasts. Sci. Rep. 6, 24747. doi:10.1038/srep24747
Zou, R., Shi, W., Tao, J., Li, H., Lin, X., Yang, S., et al. (2018). SIRT5 and post-translational protein modifications: a potential therapeutic target for myocardial ischemia-reperfusion injury with regard to mitochondrial dynamics and oxidative metabolism. Eur. J. Pharmacol. 818, 410–418. doi:10.1016/j.ejphar.2017.11.005
Glossary
4-HNE 4-Hydroxynonenal
AGEs Advanced Glycation End Products
Akt Protein Kinase B
AMPK Adenosine Monophosphate-Activated Protein Kinase
ANP Atrial Natriuretic Peptide
ARE Antioxidant Response Element
Atg7 Autophagy-Related Gene 7
ATPase Adenosine Triphosphatase
Bax Bcl-2-Associated X Protein
Bcl-2 B-cell Lymphoma 2
BNP Brain Natriuretic Peptide
CAT Catalase
Caspase-3 Cysteine-Aspartic Acid Protease 3
Caspase-12 Cysteine-Aspartic Protease 12
CFs Cardiac Fibroblasts
CHOP C/EBP-Homologous Protein
C3G Cyanidin 3-glucoside
CTGF Connective Tissue Growth Factor
CYP2J3 Cytochrome P450 Family 2 Subfamily J Member 3
DHY Dihydromyricetin
ECM Extracellular Matrix
EETs Epoxyeicosatrienoic Acids
EGCG (−)-Epigallocatechin Gallate
eIF2α Eukaryotic Translation Initiation Factor 2α
ERS Endoplasmic Reticulum Stress
F4/80 EGF-like Module-Containing Mucin-Like Hormone Receptor-Like 1
FoxO1 Forkhead Box Protein O1
GCLC Glutamate-Cysteine Ligase Catalytic Subunit
GPER G Protein-Coupled Estrogen Receptor
GPX Glutathione Peroxidase
GRP78 Glucose-Regulated Protein 78
GSH Glutathione
GSH-Px Glutathione Peroxidase
GSSG Glutathione Disulfide
HDAC4 Histone Deacetylase 4
HFD High-Fat Diet
HO-1 Heme Oxygenase 1
HUVECs Human Umbilical Vein Endothelial Cells
ICAM-1 Intercellular Adhesion Molecule 1
IDH2 Isocitrate Dehydrogenase 2
IL-1β Interleukin-1 Beta
IL-6 Interleukin-6
IL-8 Interleukin-8
IL-18 Interleukin-18
IκBα Inhibitor of Nuclear Factor Kappa B Alpha
IRE1α Inositol-Requiring Enzyme 1 Alpha
JNK c-Jun N-terminal Kinase
KATP ATP-Sensitive Potassium Channel
LC3 Microtubule-Associated Protein 1 Light Chain 3
LDH Lactate Dehydrogenase
LPS Lipopolysaccharide
MDA Malondialdehyde
MEF2 Myocyte Enhancer Factor 2
miR-34a MicroRNA-34a
miR-221 MicroRNA-221
miR-30d-5p MicroRNA-30d-5p
MLKL Mixed Lineage Kinase Domain-Like Pseudokinase
MMP-2 Matrix Metalloproteinase-2
MMP-9 Matrix Metalloproteinase-9
MnSOD Manganese Superoxide Dismutase
mTOR Mechanistic Target of Rapamycin
NADPH Nicotinamide Adenine Dinucleotide Phosphate
NADPH oxidase Nicotinamide Adenine Dinucleotide Phosphate Oxidase
NAMPT Nicotinamide Phosphoribosyltransferase
NF-κB Nuclear Factor Kappa B
NLRP3 NLR Family Pyrin Domain Containing 3
NOX NADPH Oxidase
NQO1 NAD(P)H Quinone Dehydrogenase 1
Nrf2 Nuclear Factor Erythroid 2–Related Factor 2
PGC-1α Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha
PI3K Phosphoinositide 3-Kinase
PKC-α Protein Kinase C Alpha
PKC-β Protein Kinase C Beta
PLB Phospholamban
PPARγ Peroxisome Proliferator-Activated Receptor Gamma
PPARs Peroxisome Proliferator-Activated Receptors
Prx-3 Peroxiredoxin-3
pSer246 - HDAC4 Phosphorylated Serine 246 on Histone Deacetylase 4
RAGE Receptor for Advanced Glycation End Products
RIPK1 Receptor-Interacting Serine/Threonine-Protein Kinase 1
RIPK3 Receptor-Interacting Serine/Threonine-Protein Kinase 3
ROS Reactive Oxygen Species
SERCA2 Sarco/Endoplasmic Reticulum Calcium ATPase 2
SIRT1 Sirtuin 1
SIRT3 Sirtuin 3
SIRT5 Sirtuin 5
SMAD Small Mothers Against Decapentaplegic
SMP Submitochondrial Particles
SOD Superoxide Dismutase
STZ Streptozotocin
TAC Transverse Aortic Constriction
TGF-β Transforming Growth Factor Beta
TGF-β1 Transforming Growth Factor-beta 1
TLR4 Toll-Like Receptor 4
TIMP-1 Tissue Inhibitor of Metalloproteinase-1
TNF-α Tumor Necrosis Factor Alpha
TrxR Thioredoxin Reductase
UCP-2 Uncoupling Protein 2
UCP-3 Uncoupling Protein 3
ULK1 Unc-51 Like Autophagy Activating Kinase 1
VCAM-1 Vascular Cell Adhesion Molecule 1
XBP1 X-Box Binding Protein 1
Keywords: sea buckthorn, flavonoids, natural compounds, diabetic cardiomyopathy, molecular mechanisms
Citation: Zhang Q, Zhang J, Ouyang Y, Liu H, Xie C and Fu X (2025) Sea buckthorn flavonoids and their derivatives: potential natural compounds for the treatment of diabetic cardiomyopathy. Front. Pharmacol. 16:1599756. doi: 10.3389/fphar.2025.1599756
Received: 25 March 2025; Accepted: 04 July 2025;
Published: 17 July 2025.
Edited by:
Ashwell Rungano Ndhlala, University of Limpopo, South AfricaReviewed by:
Chuan Wang, Shaanxi University of Chinese Medicine, ChinaZhihua Geng, Shihezi University, China
Copyright © 2025 Zhang, Zhang, Ouyang, Liu, Xie and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxu Fu, ZnV4aWFveHVAY2R1dGNtLmVkdS5jbg==
 Quan Zhang
Quan Zhang Jiahong Zhang
Jiahong Zhang Yujie Ouyang
Yujie Ouyang Hongyan Liu
Hongyan Liu Chunguang Xie
Chunguang Xie Xiaoxu Fu
Xiaoxu Fu