- 1Research Centre of Basic Integrative Medicine, School of Basic Medical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Physiology, School of Basic Medical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 4Department of Gynecology, The Second Clinical School of Guangzhou University of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Institute of Integrated Traditional Chinese and Western Medicine, Sun Yat-sen University, Guangzhou, China
- 6Department of Allergy and Immunology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 7Luzhou Key Laboratory of Research for Integrative on Pain and Perioperative Organ Protection, Department of Anesthesiology and Pain, The Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University, Luzhou, China
Background: Ethanol binge and obesity are the key risk factors for alcohol-related liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD), respectively. The human beings have a habit of drinking alcohol and consuming high calorie foods, these two factors often coexist, and thus contributing to the liver injury. However, the mechanisms of a short-term consumption of high-fat diet (HFD) plus alcohol binge-induced acute liver injury are unclear.
Methods: Male C57BL/6 mice (aged 8–10 weeks) were fed a HFD or HFD Control diet for 3 days. Then, they received a single dose of ethanol or the same volume of distilled water by oral gavage. The liver damage was evaluated after 9 h of ethanol gavage.
Results: Short-term (3 days) HFD feeding plus ethanol binge significantly aggravated liver injury and steatosis in mice as indicated by the increased serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglyceride (TG) levels, the upregulated hepatic TG levels, and Oil Red O staining and H&E staining. Mechanistically, short-term HFD feeding plus ethanol binge disturbed hepatic redox homeostasis by increasing 3-nitrotyrosine (3-NT), malondialdehyde (MDA) and myeloperoxidase (MPO) levels, while decreasing glutathione (GSH) levels. HFD and alcohol co-consumption also increased hepatic TNF-α, IL-1β and IL-18 via enhancing the phosphorylation of MAPK (ERK1/2, p38 and JNK) and NF-κB. The canonical (Caspase-1 to GSDMD) and non-canonical pyroptosis signaling (Caspase-8/11 to GSDMD, and Caspase-3 to GSDME) further contributed to the acute liver injury.
Conclusion: Short-term HFD feeding plus a single dose of ethanol gavage can significantly exacerbate acute liver injury and hepatic fat deposition in mice by enhancing oxidative stress, MAPK and NF-κB signaling, and Caspase-1/8/11-GSDMD and Caspase-3-GSDME pyroptosis signaling.
1 Introduction
Alcohol-related liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD, currently known as metabolic dysfunction-associated steatotic liver disease, MASLD) are the leading causes of chronic liver disease worldwide (Díaz et al., 2023; Matchett et al., 2024). ALD and NAFLD share pathophysiological, histological, and genetic features and both alcohol and metabolic dysfunction coexist as aetiological factors in many patients with hepatic steatosis (Chao et al., 2023; Díaz et al., 2023). Currently, approximately 2 billion people consume alcohol worldwide and upwards of 75 million are diagnosed with alcohol-use disorders and are at risk of ALD, moreover, about 2 billion adults are obese or overweight and over 400 million have diabetes, both of which are the risk factors for NAFLD and hepatocellular carcinoma (Asrani et al., 2019). Although diagnosis of NAFLD requires the exclusion of significant alcohol consumption and other causes of liver disease, significant alcohol consumption is often under-reported in NAFLD patients and that metabolic factors and alcohol interact to exacerbate the progression of liver disease (Díaz et al., 2023).
In modern society, people often drink alcohol to relieve stress and obtain entertainment. At the same time, alcohol culture is also a major means of social interaction. Importantly, binge drinking and high-calorie eating often coexist, these two factors synergistically cause and aggravate the liver damage. Short-term high-fat diet (HFD) feeding plus acute ethanol binge have a synergistic effect on the liver injury (Chang et al., 2015). However, the potential molecular mechanisms of acute liver injury caused by high-calorie eating and alcohol binge consumption still need to be further explored. Here, we used an acute liver injury model in mice fed with HFD for 3 days plus a single binge of ethanol to investigate the synergistic mechanism of HFD plus alcohol binge on acute liver injury.
2 Methods
2.1 Animal models
The male C57BL/6 mice were purchased from Guangdong Medical Laboratory Animal Center (Foshan, China), they were housed in a temperature-controlled animal facility with a 12-h light–dark cycle and allowed to obtain rodent chow and water ad libitum. All experimental procedures of animals in this study were approved by the Institutional Animal Care and Use Committee of Guangzhou University of Chinese Medicine.
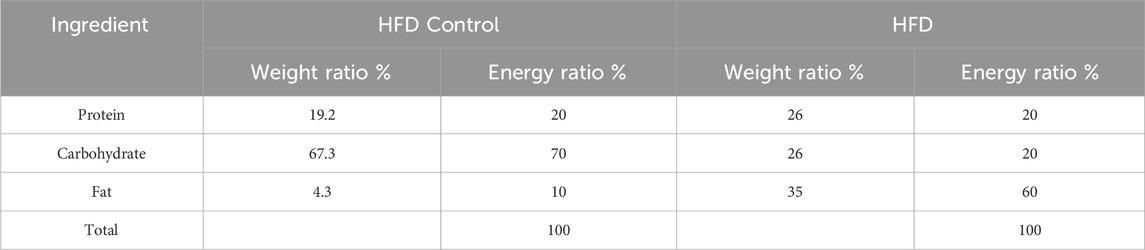
The animal model was established according to Gao’s group previously described with appropriate modifications (Chang et al., 2015; Ding et al., 2010). In detail, the animals were fed a HFD (60% kcal% fat; Cat# GD60, Guangdong Medical Laboratory Animal Center, Foshan, China; Table 1) or a HFD Control diet (10% kcal% fat; Cat# GD450B, Guangdong Medical Laboratory Animal Center, Foshan, China; Table 1) for 3 days, followed by a single gavage of ethanol (as a 31.25% solution in water) at a dose of 5 g/kg body weight (as 0.20 mL per 10 g body weight) or the same volume of distilled water (Chang et al., 2015). The food was not taken away after gavage. After 9 h of ethanol gavage, the animals were deeply anesthetized before blood collection from the orbital sinus for collecting serum and euthanized via cervical dislocation (Mackowiak et al., 2022) (Figure 1A). Then, the liver samples were harvested, and either frozen in −80°C or put in 4% Paraformaldehyde Fix Solution (Cat# G1101, Servicebio, Wuhan, China) before further analysis.
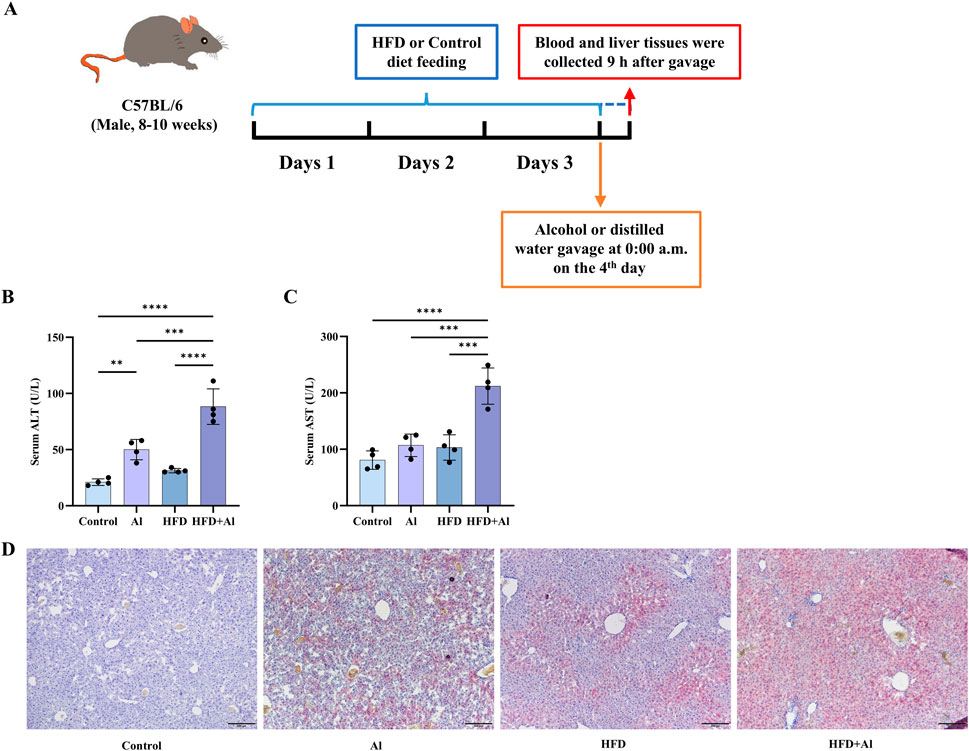
Figure 1. HFD feeding for 3 days plus one acute ethanol binge synergistically induced acute liver injury in mice. (A) Male C57BL/6 wild-type mice started feeding a HFD diet or a Control diet at 9:00 p.m. before the first day, and lasted for 3 days. Then, a single dose of ethanol (5 g/kg body weight as a 31.25% ethanol in water; 0.02 mL/g) was given to mice via gavage at 0:00 a.m. on day 4. The food was not taken away after gavage. The liver and blood were collected at 9:00 a.m. on the fourth day. (B) Serum ALT levels. (C) Serum AST levels. (D) Hepatic Oil Red O staining. All data were expressed as mean ± SD, n = 4 in each group, **p < 0.01, ***p < 0.001, ****p < 0.0001.
2.2 Biochemical analysis
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglycerides (TG) were examined by an automatic biochemical analyzer as we had previously descried (Xu et al., 2024). Hepatic TG, malondialdehyde (MDA), myeloperoxidase (MPO) and the reduced glutathione (GSH) were examined by the commercial assay kits (Cat# A110-1-1, Cat# A003-1-2, Cat# A044-1-1, and Cat# A006-2-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (Xu et al., 2024).
2.3 Histopathology analysis
The fixed liver samples were embedded by the Tissue-Tek® optimum cutting temperature (O.C.T.) compound (Cat# 4583, Sakura, Japan) to perform frozen liver sections for Oil Red O (Cat# G1015, Servicebio, Wuhan, China) staining, or embedded in paraffin to perform sections for staining with hematoxylin (Cat# Ba4097, BaSo, Zhuhai, China) and eosin (Cat# Ba4099, BaSo, Zhuhai, China) (H&E) as we previously described (Zhang et al., 2020; Xu et al., 2024). H&E staining was used to evaluate the morphological change of the liver tissue and lipid accumulation, and Oil Red O staining was used to visualize fat content in the liver samples.
2.4 Immunoblot assay
The total proteins were extracted from the liver samples with lysis buffer, and immunoblot assay was performed according to the standardized processes (Zhang et al., 2020). The antibodies used in this study were described in Table 2. The gray value of protein bands was quantified by the ImageJ software (National Institutes of Health, Bethesda, MD, United States).
2.5 Immunofluorescence staining
Immunofluorescence staining was performed to detect 3-nitrotyrosine (3-NT) in the liver. The frozen sections were subjected to pre-washing treatments as follows: washing with 1 × PBS for 30 min, 1 × TBS for 10 min, and 1 × TBST for 20 min. The liver sections were sealed with goat serum blocking solution (Cat# ZLI-9056, ZSGB-BIO, Beijing, China) at room temperature for 2 h. Then, the 3-NT antibody (Abcam, Cat# ab110282, United Kingdom) was incubated overnight with the liver sections at 4°C, followed by washing with 1 × TBS for 10 min and 1 × TBST for 20 min. The liver sections were incubated with Goat Anti-Mouse IgG (H + L) Fluor 594-conjugated antibody (Cat# S0005, Affinity Biosciences, Beijing, China) under dark conditions for 2 h. The sections were washed with 1 × TBS for 10 min followed by 1 × TBST for 20 min. Finally, the fluorescence intensity of 3-NT was observed under a fluorescence microscope after sealing the sections with anti-fluorescence attenuation sealer (containing DAPI) (Cat# S2110, Solarbio, Beijing, China).
2.6 Statistical analysis
The statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc analysis for data with normal distribution (by Shapiro-Wilk test) and satisfying homogeneity of variance (by Brown-Forsythe test), performed by Brown-Forsythe and Welch ANOVA tests followed by Dunnett T 3 post hoc analysis for data with normal distribution and heteroscedasticity, and performed by Kruskal–Wallis test followed by Dunn’s post hoc analysis for data with skewed distributions (by Shapiro-Wilk test). All data were expressed as the mean ± SD or median ± interquartile range, a value of P < 0.05 was considered as significantly different. All histograms were performed using GraphPad Prism 10.0 (GraphPad Software Inc., San Diego, CA, United States).
3 Results
3.1 Short-term HFD feeding plus acute ethanol binge induced acute liver injury in mice
It has been reported that 3 days of HFD feeding plus acute ethanol binge can induce liver damage in mice (Chang et al., 2015; Babuta et al., 2024c). Here, we established the same animal models (Figure 1A), and like the previous reports (Chang et al., 2015; Babuta et al., 2024c), our data also indicated that HFD feeding for 3 days plus one time of acute ethanol binge on the third day synergistically induced liver injury as indicated by increasing serum levels of ALT and AST (Figures 1B,C) and increasing hepatic fat deposition in mice as indicated by Oil Red O staining (Figure 1D).
In order to further investigate the mechanisms of short-term HFD feeding plus acute ethanol binge on acute liver injury, the other animals were randomly divided into three groups: Control group, HFD group, and short-term HFD feeding plus acute ethanol binge group. Similar to the previous reports that short-term HFD feeding, e.g., for 3–4 days, is sufficient to induce liver steatosis and impair glucose tolerance and hepatic insulin sensitivity (Lee et al., 2011; Ji et al., 2012; Wiedemann et al., 2013; Chang et al., 2015), our data of hepatic H&E staining showed that a small amount of fat vacuoles accumulated in the liver of mice fed a HFD (Figure 2A), hepatic Oil red O staining and hepatic TG levels showed that HFD feeding induced fat deposition in the liver (Figures 2B,C). HFD feeding also increased serum TG levels, and only slightly increase serum ALT and AST levels with no statistical significance (Figures 2D–F). Compared with HFD group, hepatic fat deposition, serum TG, ALT and AST levels were further enhanced in HFD + Al group (Figure 2). Therefore, short term HFD feeding plus ethanol binge synergistically induces acute liver injury and hepatic steatosis in mice.

Figure 2. Short-term HFD feeding plus acute ethanol gavage induced acute hepatic steatosis in mice. (A) The H&E staining of the liver sample. (B) The Oil Red O staining of the liver sample. (C) Hepatic TG levels in mice. (D) The serum TG levels. (E) The serum ALT levels. (F) The serum AST levels. All data were expressed as mean ± SD, n = 8 in each group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2 HFD plus ethanol binge synergistically induced oxidative stress in the liver
HFD or excessive ethanol intake has been shown to promote the production of reactive oxygen species (ROS), thus, increase oxidative stress in the liver (Ma et al., 2022; Zhu et al., 2022; Park et al., 2023). The high levels of ROS can induce lipid peroxidation. 3-nitrotyrosine (3-NT), a product of reactive-nitrogen species (RNS) with the activated aromatic ring of tyrosine, is another classical biomarker of oxidative stress (Bandookwala and Sengupta, 2020). The immunofluorescence results indicated that hepatic 3-NT levels in HFD group and HFD + Al group were increased when compared with Control group, and hepatic 3-NT levels in HFD + Al group were higher than those in HFD group (Figures 3A,B). MDA, which is a biomarker of lipid peroxidation, has been examined to reflect oxidative stress (Ayala et al., 2014). HFD, and HFD plus ethanol binge increased MDA levels in the liver, and HFD plus ethanol binge upregulated more MDA levels in the liver (Figure 3C). MPO, which is the member of heme peroxidase family in immune cells, can contribute to ROS production (Hawkins and Davies, 2021), and thus, MPO levels also reflects the degree of oxidative stress and inflammation in the organ or tissue. Our data indicated that hepatic MPO levels were increased (Figure 3D), in contrast, GSH (an indicator of antioxidant capacity) levels were decreased in HFD + Al group compared with Control group (Figure 3E). Therefore, HFD plus ethanol binge synergistically contributed to hepatic oxidative stress in mice.
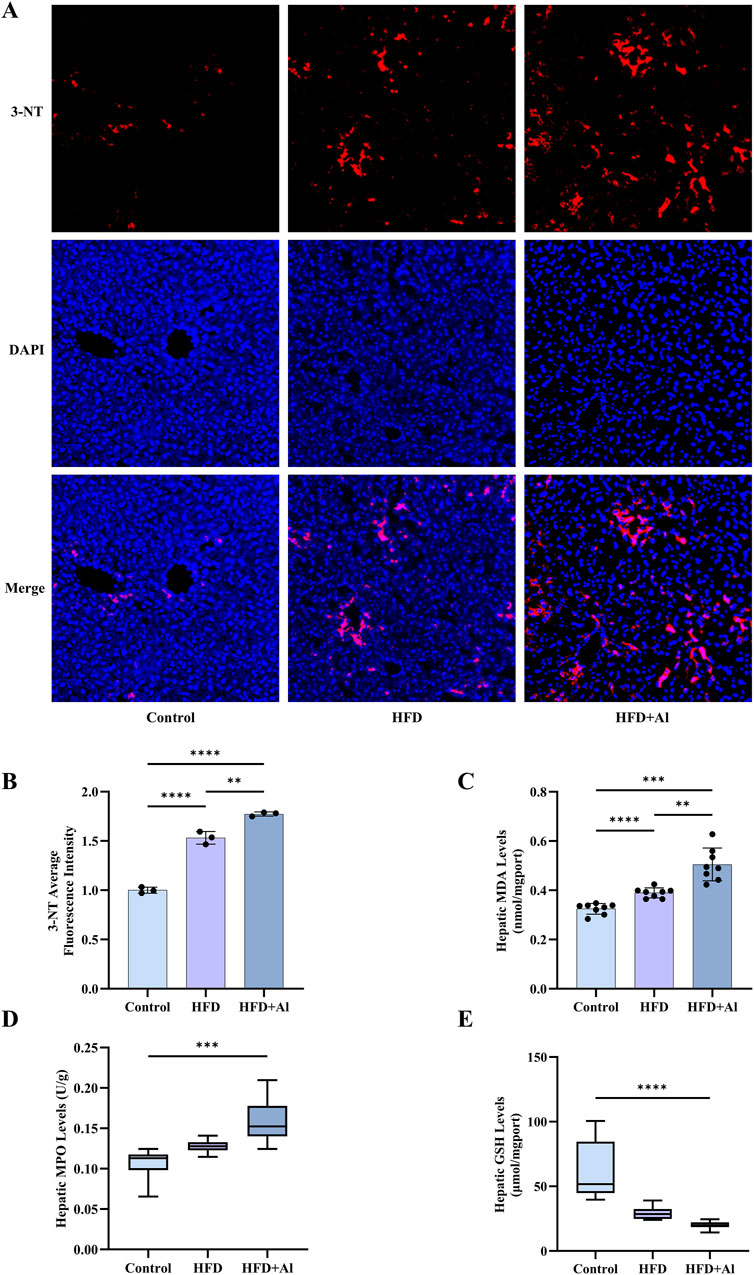
Figure 3. Short-term HFD feeding plus acute ethanol binge induced hepatic oxidative stress in mice. (A) The immunofluorescence staining of 3-NT, 10 ×. (B) The average fluorescence intensity of 3-NT, n = 3 in each group. (C) Hepatic MDA levels; (D) Hepatic MPO levels; (E) Hepatic GSH levels. The data of MPO and GSH were expressed as median ± interquartile range, and the other data were expressed as mean ± SD, n = 8 in each group in C to E, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.3 HFD plus ethanol binge upregulated MAPK and NF-κB phosphorylation in the liver
The increased ROS can further cause acute liver injury after short-term ethanol feeding by activating innate immune signals and inducing sterile inflammation (Tsutsumi et al., 2003; Gao et al., 2024). Here, the phosphorylation of MAPK and NF-κB p65 in the liver were evaluated by Western blotting. Our results showed that the phosphorylation of hepatic ERK, JNK, and NF-κB p65 were elevated in HFD group when compared with Control group, and the phosphorylated hepatic MAPK (p38 and JNK) were further increased in the mice subjected to HFD plus ethanol binge, and ERK and NF-κB p65 also showed increasing trends, however, it was not statistically significant (Figure 4). Therefore, HFD plus ethanol binge significantly increased the phosphorylation levels of MAPK and NF-κB in the liver.
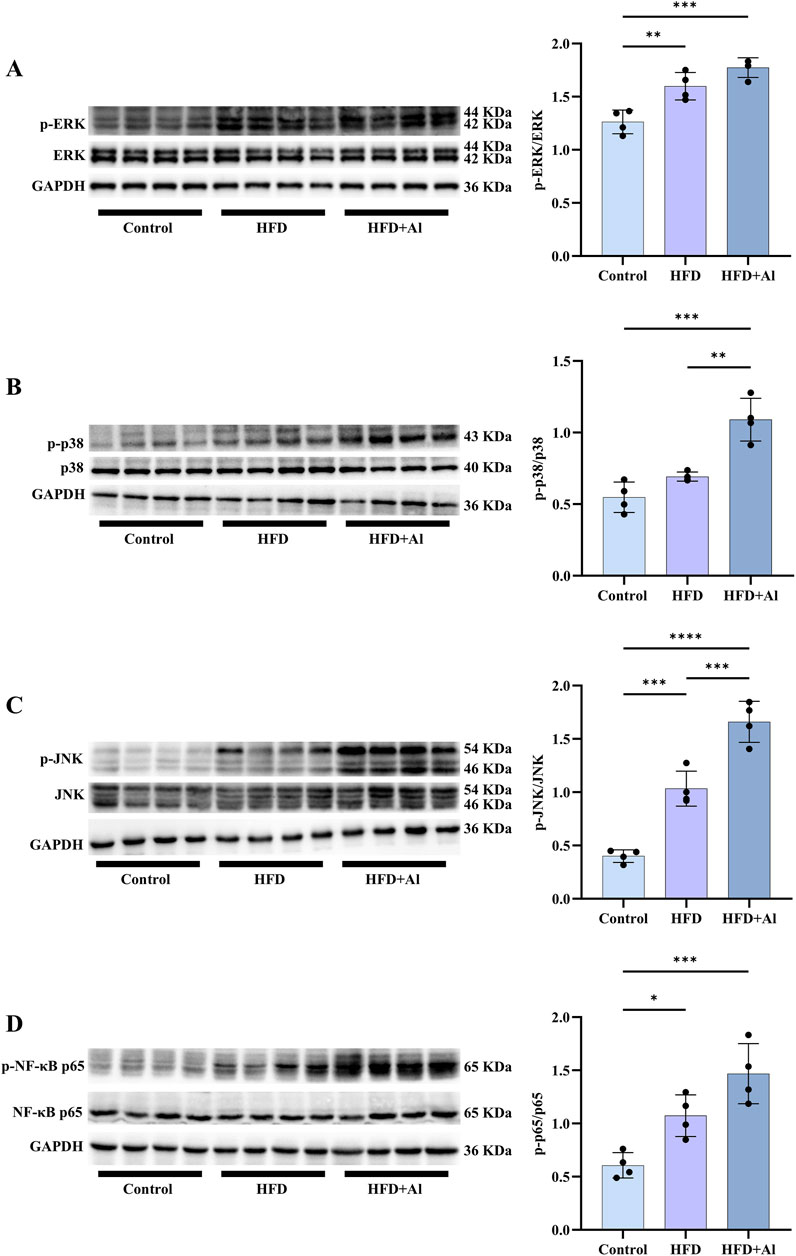
Figure 4. Short-term HFD feeding plus acute ethanol binge enhanced the phosphorylation of MAPK and NF-κB p65 in the liver of mice. Western blotting and quantification of p-ERK/total ERK (A), p-p38/total p38 (B), p-JNK/total JNK (C), and p-NF-κB p65/total NF-κB p65 (D). All data were expressed as mean ± SD, n = 4 in each group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.4 HFD plus ethanol binge aggravated inflammatory cytokines expression in the liver
The phosphorylation of MAPK and NF-κB p65 in the liver can induce the expression of inflammatory cytokines, and thus amplifying inflammation (Zhang et al., 2019; Zhang et al., 2023; Lan et al., 2024). Therefore, TNF-α, IL-1β, and IL-18 levels in the liver were determined by Western blotting. Our results showed that the hepatic protein levels of TNF-α, pro-IL-1β and the cleaved-IL-1β (the maturation form of IL-1β), and pro-IL-18 and the cleaved-IL-18 (the maturation form of IL-18) were increased in HFD + Al group compared with HFD group (IL-18 levels only show upward trends but has no statistical significance) (Figure 5). Therefore, HFD plus ethanol binge induced and amplified hepatic inflammation by aggravating both the expression and maturation of inflammatory cytokines in mice.
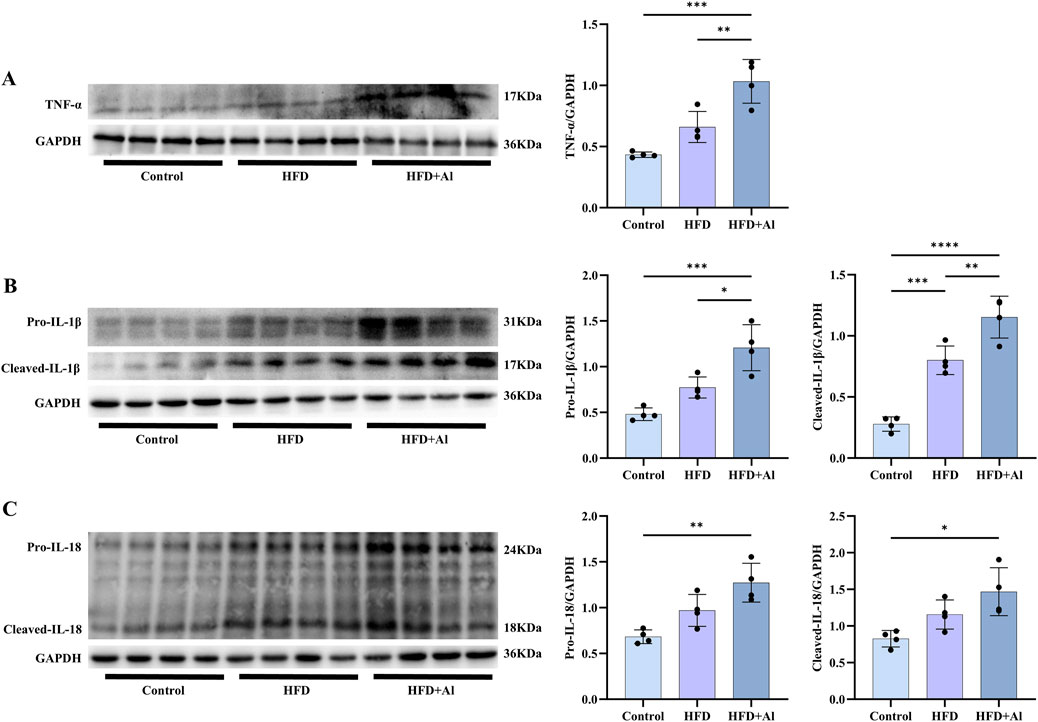
Figure 5. Short-term HFD plus acute ethanol binge enhanced the expression and maturation of hepatic TNF-α, IL-1β and IL-18 in mice. Western blotting and quantification of TNF-α/GAPDH (A), pro-IL-1β/GAPDH and cleaved-IL-1β/GAPDH (B), and pro-IL-18/GAPDH and cleaved-IL-18/GAPDH (C). All data were expressed as mean ± SD, n = 4 in each group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.5 HFD plus ethanol binge synergistically induced hepatic pyroptosis
The pro-IL-1β and pro-IL-18 need to be processed and cleaved by the activated Caspase-1 (the cleaved Caspase-1) to convert into their mature forms (He et al., 2016). Therefore, to investigate whether the effect of HFD plus ethanol binge on the maturation of IL-1β and IL-18 was related to the activation of Caspase-1, we examined the protein levels of Caspase-1. Our results showed that both pro-Caspase-1 and cleaved-Caspase-1 were upregulated in HFD group when compared with Control group, and HFD plus ethanol binge further increased the expression and the activation of Caspase-1 (Figure 6A). Besides the cleavage of IL-1β and IL-18, Caspase-1 can also specifically cleave the linker between the amino-terminal gasdermin-N and carboxy-terminal gasdermin-C domains in gasdermin D (GSDMD), which is required and sufficient for pyroptosis (Shi et al., 2015). To further investigate whether HFD plus ethanol binge-induced acute liver injury is involved in pyroptosis, we examined hepatic GSDMD levels in the liver. Our results showed that both pro-GSDMD and cleaved-GSDMD levels were increased in HFD group and HFD + Al group, and HFD plus ethanol binge facilitated more expression and maturation of GSDMD than HFD alone (Figure 6B).
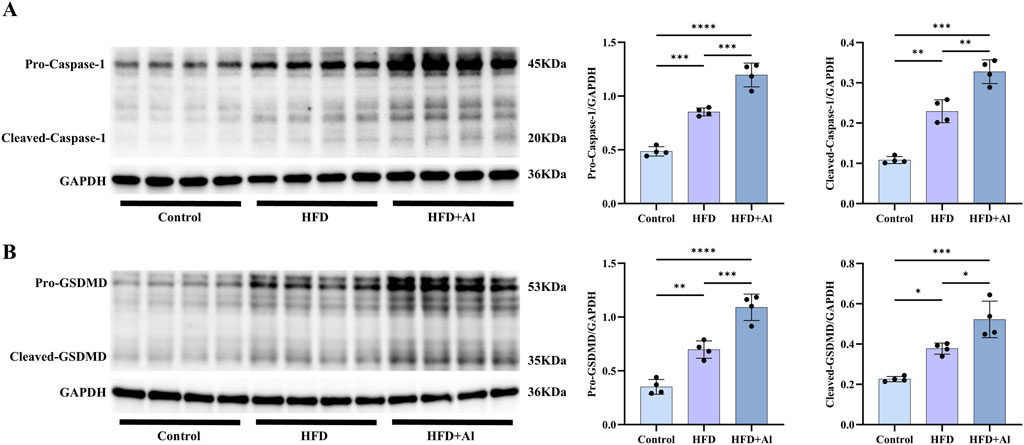
Figure 6. Short-term HFD feeding plus acute ethanol binge activated the canonical Caspase-1 to GSDMD pyroptosis signaling in the liver of mice. Western blotting and quantification of pro-Caspase-1/GAPDH and cleaved-Caspase-1/GAPDH (A), and pro-GSDMD/GAPDH and cleaved-GSDMD/GAPDH (B). All data were expressed as mean ± SD, n = 4 in each group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In addition to the Caspase-1 to GSDMD canonical pyroptosis signaling, Caspase-8 and Caspase-11 can also induce the non-canonical pyroptosis signaling by cleaving GSDMD, and Caspase-3 can induce the non-canonical pyroptosis signaling by cleaving gasdermin E (GSDME) (Shi et al., 2015; Wang Y. et al., 2017). Therefore, we further examined the non-canonical pyroptosis signals. Our results showed that both HFD and HFD plus ethanol binge can increased the hepatic levels of pro-Caspase-11 and cleaved-Caspase-11 (Figure 7A), pro-Caspase-8 and cleaved-Caspase-8 (Figure 7B), pro-Caspase-3 and cleaved-Caspase-3 (Figure 7C), and pro-GSDME and cleaved-GSDME (Figure 7D). Except for the levels of pro-Caspase-8, which has no statistical significance, other non-canonical pyroptosis signals in HFD + Al group were higher than these in HFD group (Figure 7). Therefore, HFD plus ethanol binge synergistically induced hepatic pyroptosis by excessively activating pyroptosis signals.
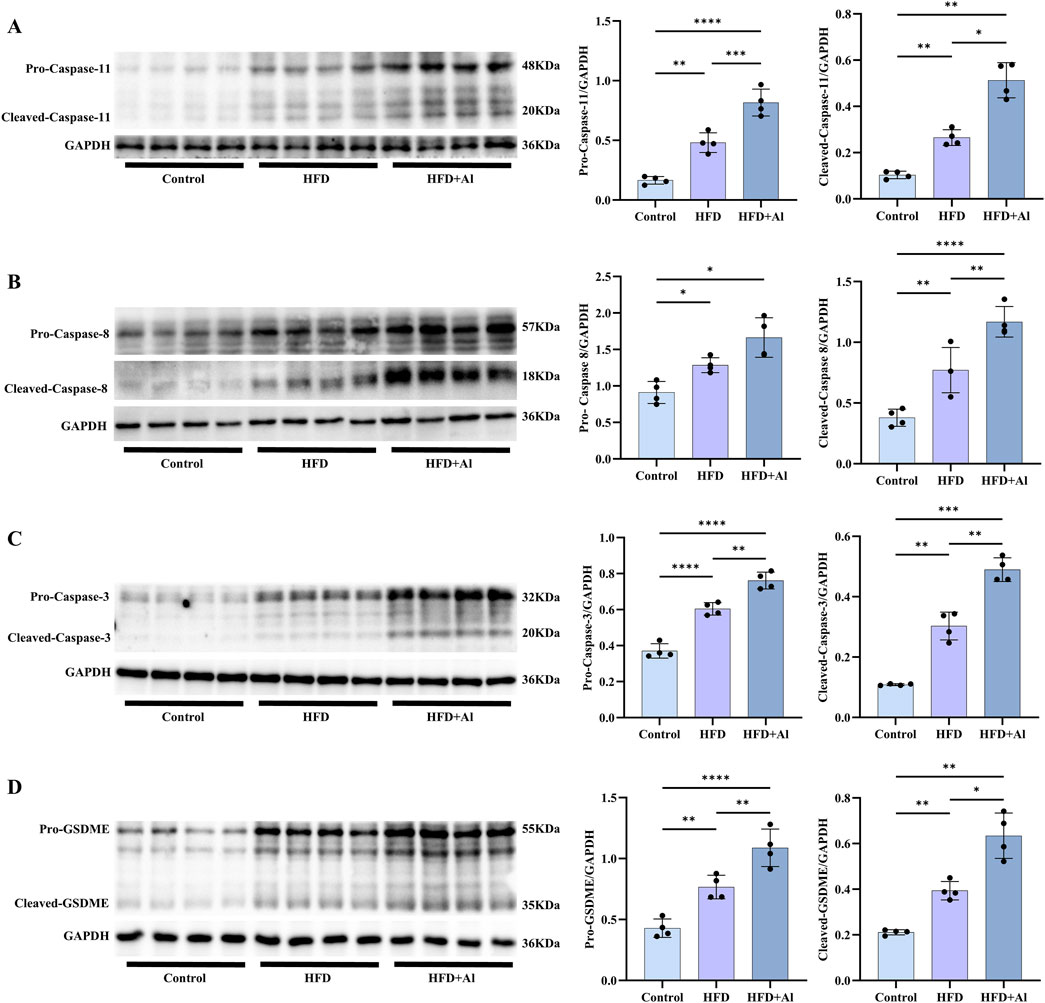
Figure 7. Short-term HFD feeding plus acute ethanol binge increased both the expression and activation of Caspase-11, Caspase-8, Caspase-3 and GSDME in the liver of mice. Western blotting and quantification of pro-Caspase-11/GAPDH and cleaved-Caspase-11/GAPDH (A), pro-Caspase-8/GAPDH and cleaved-Caspase-8/GAPDH (B), pro-Caspase-3/GAPDH and cleaved-Caspase-3/GAPDH (C), and pro-GSDME/GAPDH and cleaved-GSDME/GAPDH (D). All data were expressed as mean ± SD, n = 4 in each group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
4 Discussion
The increased adoption of a Western diet, sedentary habits, and alcohol consumption, lead to a rapid increase in the global prevalence of MASLD and ALD (Babuta et al., 2024b). It has been reported that long-term or short-term HFD feeding plus acute ethanol binge synergistically induced liver injury and hepatic steatosis in mice (Chang et al., 2015; Wang W. et al., 2017). In this study, we confirmed that mice received a single dose of ethanol on the 3rd day of HFD feeding enhanced liver injury when compared with the mice only feeding with a HFD for 3 days. Similar to our study, a recent study also showed that short-term feeding of a metabolic-dysfunction-associated steatohepatitis (MASH) diet (high fat (33 gm%), high cholesterol (10 gm%), and high sucrose (208.4 gm%)) plus daily 5 g/kg alcohol gavage for 3 days can induce liver injury in mice (Babuta et al., 2024c). Male mice feeding the same MASH diet combined with receiving 10% alcohol in drinking water ad libitum and 5 g/kg alcohol gavage weekly for 3 months displayed the key features of severe alcohol-associated hepatitis (Babuta et al., 2024b). Only weekly alcohol binges (5 g/kg) can also exacerbate liver injury in mice model of MASH received the same MASH diet for 3 months (Babuta et al., 2024a). Moreover, Western diet and alcohol consumption coexist as synergistic insults in a substantial proportion of liver disease patient population (Babuta et al., 2024b). Therefore, HFD combined with habitual alcohol consumption can synergistically cause liver damage.
The synergistic effects of short-term HFD feeding plus acute ethanol binge-induced acute liver injury were involved in hepatic oxidative stress, as that hepatic 3-NT and MDA, which are essential biomarkers of oxidative injury, were enhanced in HFD + Al group when compared with HFD group. In contrast, the antioxidant reduced GSH was downregulated by HFD, or HFD plus acute ethanol binge, and the GSH levels was slightly low in HFD + Al group than HFD group with no statistical significance. These data indicated that short-term HFD feeding plus acute ethanol binge enhanced oxidative stress but decreased the antioxidant capacity in the liver. MPO, which is a member of heme peroxidase family in neutrophils, can generate powerful oxidizing species including hypochlorous acid (HOCl) (Hawkins and Davies, 2021), which also reflects the degree of oxidative stress and inflammation in the organ or tissue. We found that hepatic MPO levels increased in HFD group and HFD + Al group, and there was statistical significance between HFD + Al group and Control group. This was consistent with a previous report that MPO+ neutrophils were diffused in the parenchymal regions at 9 h post ethanol gavage in 3d-HFD-fed mice (Chang et al., 2015). Therefore, short-term HFD feeding plus acute ethanol binge may synergistically contribute to liver oxidative stress and inflammation.
The excessive ROS can contribute to the activation of innate immune signals, such as MAPK and NF-κB (Ryan et al., 2004; Tsung et al., 2007; Zhang et al., 2017). Our results showed that short-term HFD feeding plus acute ethanol binge can increase the phosphorylation of ERK, p38, JNK and NF-κB-p65 in the liver. The phosphorylation of MAPK and NF-κB may initiate the production of inflammatory cytokines (Zhang et al., 2017). Our results demonstrated that 3 days HFD feeding plus acute ethanol binge significantly enhanced the protein levels of TNF-α, IL-1β, and IL-18 in the liver of mice. Similar to this, the increased hepatic free fatty acids (FFAs) may contribute to the elevation of Cxcl1 mRNA in hepatocytes (and to a lesser extent in hepatic stellate cells and sinusoidal endothelial cells) via activating ERK1/2, JNK or NF-κB in mice with 3-day HFD-plus-ethanol binge feeding (Chang et al., 2015). Therefore, the inflammatory cytokines TNF-α, IL-1β, and IL-18 may combine with CXCL1 to synergistically induce liver injury and steatosis.
The inactive precursors of IL-1β and IL-18 should undergo cleavage and activation by the cleaved-Caspase-1, resulting in the formation of mature cleaved forms—cleaved-IL-1β and cleaved-IL-18, which subsequently release from cells to trigger an inflammatory response (Zhang et al., 2017). Our data showed that both the full length of IL-1β and IL-18 (pro-IL-1β and pro-IL-18) and the cleaved IL-1β and IL-18 were increased by HFD plus acute ethanol binge. Szabo et al. found that 3 days combined insult of a MASH-inducing diet and alcohol binges activated hepatic NLRP3 inflammasome, as indicated by a significant increase in the levels of cleaved-Caspase-1 and cleaved-IL-Iβ in the liver (Babuta et al., 2024c). Here, we showed that a short-term HFD feeding with acute ethanol binge markedly elevated the protein levels of both precursor and mature forms of Caspase-1 in the liver of mice. In addition, the activated Caspase-1 may also cleave GSDMD, which is the common effector for cytokine secretion and the typical pyroptosis trigger that follows the activation of inflammasomes (Du et al., 2024). Both GSDMD and GSDMD-N were upregulated in the liver tissues of human MASLD/MASH, and GSDMD plays a key role in the pathogenesis of steatohepatitis, by controlling cytokine secretion, NF-κB activation, and lipogenesis (Xu et al., 2018). Here, we showed that both short-term HFD feeding and short-term HFD feeding plus acute ethanol binge can increase the precursor and maturation of GSDMD in the liver of mice. However, a recent study showed that 3 days MASH diet feeding (which was composed of high fat (33 gm%), high cholesterol (10 gm%), and high sucrose (208.4 gm%)) plus daily acute alcohol binges for 3 days fail to activate GSDMD (Babuta et al., 2024c). This difference may be related to the composition of diet, frequency of alcohol consumption, and even the living environment of mice.
It is well-known that Caspase-1 is activated after various typical inflammasome recognizing ligands, moreover, human Caspase-4 and the mouse homologue Caspase-11 and human Caspase-5 can directly recognize bacterial lipopolysaccharide (LPS), both of which trigger pyroptosis via GSDMD (Shi et al., 2015; Liu et al., 2016). Caspase-11-GSDMD pathway in the liver was activated in a hybrid feeding mouse model of alcoholic hepatitis and patients (Khanova et al., 2018; Wang et al., 2018). Caspase-11 promotes 12 weeks HFD feeding induced NAFLD in mice by increasing glycolysis, oxidative phosphorylation, and pyroptosis in macrophages (Drummer et al., 2023). Our results indicated that both the full-length and cleaved forms of Caspase-11 was enhanced by short-term HFD feeding and short-term HFD feeding plus acute ethanol binge. Caspase-8 activation during TAK1 inhibition results in cleavage of both GSDMD and GSDME (Sarhan et al., 2018). Moreover, GSDME can also be cleaved by Caspase-3 in its linker, generating a GSDME-N fragment that perforates membranes and thereby induces pyroptosis (Wang Y. et al., 2017). Both the precursors and mature forms of Caspase-8, Caspase-3 and GSDME were increased by short-term HFD feeding or short-term HFD feeding plus acute ethanol binge. Moreover, short-term HFD feeding plus acute ethanol binge enhanced more hepatic cleaved-Caspase-8, pro- and cleaved-Caspase-3 and pro- and cleaved-GSDME than only short-term HFD feeding in mice. Thus, both the canonical and non-canonical pyroptosis signaling may be an important mechanism for acute liver injury induced by short-term HFD feeding plus acute alcohol binge.
Our study indicated that short-term HFD feeding plus acute ethanol binge induced acute liver injury in mice through increasing oxidative stress, inflammation, and the canonical and non-canonical pyroptosis signaling. In addition to the well-known of oxidative stress and inflammation, pyroptosis may act as a novel therapeutic target for treating liver damage induced by high calorie diet with excessive alcohol consumption in human beings. ROS can activate MAPK and NF-κB, thereby inducing inflammatory cytokines expression, and the maturation of inflammatory cytokines, such IL-1β and IL-18, requires Caspase-1 (Averill-Bates, 2024; Yang et al., 2024). Classically, Caspase-1 can also induce pyroptosis by cleavage of GSDMD (Shi et al., 2015). GSDMD is a member of gasdermins (GSDMs) family, which consist of GSDMA, GSDMB, GSDMC, GSDMD, GSDME and DFNB 59 (also known as pejvakin (PJVK)) in humans (Zheng et al., 2020). Until now, it has been shown that these GSDMs, except DFNB 59, are inducers of pyroptosis (Wang Y. et al., 2017; Hou et al., 2020; Zheng et al., 2020; Zhou et al., 2020; Deng et al., 2022; Privitera et al., 2023; Zhou et al., 2024). However, it is unclear the roles of GSDMA, GSDMB, and GSDMC in acute liver injury induced by short-term HFD feeding plus acute alcohol binge. Moreover, it is currently uncertain which of these three mechanisms, including oxidative stress, inflammation, and pyroptosis, is more important. It should be noted that MAPK and NF-κB can also be activated independently of ROS, such as by the increased LPS (Carpino et al., 2020). Therefore, it is still uncertain which of these three mechanisms is the main one? More likely, they have both upstream-downstream relationships and can independently play a role in liver damage.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of Guangzhou University of Chinese Medicine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YD: Investigation, Methodology, Visualization, Writing – original draft. XC: Writing – original draft, Visualization, Investigation. WG: Writing – original draft, Investigation. YC: Writing – original draft, Investigation. LX: Writing – original draft, Investigation, Formal Analysis. WS: Writing – original draft, Investigation. WL: Supervision, Writing – review and editing. JD: Investigation, Writing – original draft. KW: Writing – original draft, Investigation, Resources. QL: Writing – original draft, Investigation. CL: Investigation, Writing – original draft. MD: Funding acquisition, Supervision, Writing – review and editing. JeX: Writing – review and editing, Supervision, Resources. JnX: Supervision, Writing – review and editing, Resources. HZ: Writing – review and editing, Supervision, Methodology, Software. ZK: Supervision, Writing – review and editing. YZ: Writing – review and editing, Supervision, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Natural Science Foundation of Guangdong Province (Grant Number: 2023A1515012828), Flagship Department Construction Project for Collaborative Traditional Chinese Medicine (TCM) and Western Medicine (The comprehensive letter of TCM [2024] NO. 221, Grant Number: 2025TGL0370000014), Research Project of Guangdong Provincial Bureau of TCM (Grant Numbers: 20231103, 20241081 and 20241055), Special Project for Key Fields of Guangdong Provincial Ordinary Colleges and Universities (Grant Number: 2024ZDZX 2042), Construction Project of Inheritance Studio of National Famous and Old TCM Expert Yang Hongzhi (Grant Number: 140000020162), Medical Research Foundation of Guangdong Province (B2025183), and Guangdong Province Key Discipline Construction Project of TCM (20220104).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asrani, S. K., Devarbhavi, H., Eaton, J., and Kamath, P. S. (2019). Burden of liver diseases in the world. J. Hepatol. 70 (1), 151–171. doi:10.1016/j.jhep.2018.09.014
Averill-Bates, D. (2024). Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 1871 (2), 119573. doi:10.1016/j.bbamcr.2023.119573
Ayala, A., Muñoz, M. F., and Argüelles, S. (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 360438. doi:10.1155/2014/360438
Babuta, M., Morel, C., de Carvalho Ribeiro, M., Calenda, C., Ortega-Ribera, M., Thevkar Nagesh, P., et al. (2024a). Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis. Gut 73 (11), 1854–1869. doi:10.1136/gutjnl-2023-331447
Babuta, M., Morel, C., de Carvalho Ribeiro, M., Datta, A. A., Calenda, C., Copeland, C., et al. (2024b). A novel experimental model of MetALD in male mice recapitulates key features of severe alcohol-associated hepatitis. Hepatol. Commun. 8 (7), e0450. doi:10.1097/hc9.0000000000000450
Babuta, M., Nagesh, P. T., Datta, A. A., Remotti, V., Zhuang, Y., Mehta, J., et al. (2024c). Combined insults of a MASH diet and alcohol binges activate intercellular communication and neutrophil recruitment via the NLRP3-IL-1β Axis in the liver. Cells 13 (11), 960. doi:10.3390/cells13110960
Bandookwala, M., and Sengupta, P. (2020). 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 130 (10), 1047–1062. doi:10.1080/00207454.2020.1713776
Carpino, G., Del Ben, M., Pastori, D., Carnevale, R., Baratta, F., Overi, D., et al. (2020). Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 72 (2), 470–485. doi:10.1002/hep.31056
Chang, B., Xu, M. J., Zhou, Z., Cai, Y., Li, M., Wang, W., et al. (2015). Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1. Hepatology 62 (4), 1070–1085. doi:10.1002/hep.27921
Chao, X., Wang, S., Ma, X., Zhang, C., Qian, H., Williams, S. N., et al. (2023). Persistent mTORC1 activation due to loss of liver tuberous sclerosis complex 1 promotes liver injury in alcoholic hepatitis. Hepatology 78 (2), 503–517. doi:10.1097/hep.0000000000000373
Deng, W., Bai, Y., Deng, F., Pan, Y., Mei, S., Zheng, Z., et al. (2022). Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602 (7897), 496–502. doi:10.1038/s41586-021-04384-4
Díaz, L. A., Arab, J. P., Louvet, A., Bataller, R., and Arrese, M. (2023). The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 20 (12), 764–783. doi:10.1038/s41575-023-00822-y
Ding, W. X., Li, M., Chen, X., Ni, H. M., Lin, C. W., Gao, W., et al. (2010). Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139 (5), 1740–1752. doi:10.1053/j.gastro.2010.07.041
Drummer, C. t., Saaoud, F., Jhala, N. C., Cueto, R., Sun, Y., Xu, K., et al. (2023). Caspase-11 promotes high-fat diet-induced NAFLD by increasing glycolysis, OXPHOS, and pyroptosis in macrophages. Front. Immunol. 14, 1113883. doi:10.3389/fimmu.2023.1113883
Du, G., Healy, L. B., David, L., Walker, C., El-Baba, T. J., Lutomski, C. A., et al. (2024). ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature 630 (8016), 437–446. doi:10.1038/s41586-024-07373-5
Gao, H., Jiang, Y., Zeng, G., Huda, N., Thoudam, T., Yang, Z., et al. (2024). Cell-to-cell and organ-to-organ crosstalk in the pathogenesis of alcohol-associated liver disease. eGastroenterology 2 (4), e100104. doi:10.1136/egastro-2024-100104
Hawkins, C. L., and Davies, M. J. (2021). Role of myeloperoxidase and oxidant formation in the extracellular environment in inflammation-induced tissue damage. Free Radic. Biol. Med. 172, 633–651. doi:10.1016/j.freeradbiomed.2021.07.007
He, Y., Hara, H., and Núñez, G. (2016). Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41 (12), 1012–1021. doi:10.1016/j.tibs.2016.09.002
Hou, J., Zhao, R., Xia, W., Chang, C. W., You, Y., Hsu, J. M., et al. (2020). PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22 (10), 1264–1275. doi:10.1038/s41556-020-0575-z
Ji, Y., Sun, S., Xia, S., Yang, L., Li, X., and Qi, L. (2012). Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J. Biol. Chem. 287 (29), 24378–24386. doi:10.1074/jbc.M112.371807
Khanova, E., Wu, R., Wang, W., Yan, R., Chen, Y., French, S. W., et al. (2018). Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 67 (5), 1737–1753. doi:10.1002/hep.29645
Lan, T., Chen, B., Hu, X., Cao, J., Chen, S., Ding, X., et al. (2024). Tianhuang formula ameliorates liver fibrosis by inhibiting CCL2-CCR2 axis and MAPK/NF-κB signaling pathway. J. Ethnopharmacol. 321, 117516. doi:10.1016/j.jep.2023.117516
Lee, Y. S., Li, P., Huh, J. Y., Hwang, I. J., Lu, M., Kim, J. I., et al. (2011). Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60 (10), 2474–2483. doi:10.2337/db11-0194
Liu, X., Zhang, Z., Ruan, J., Pan, Y., Magupalli, V. G., Wu, H., et al. (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535 (7610), 153–158. doi:10.1038/nature18629
Ma, J., Guillot, A., Yang, Z., Mackowiak, B., Hwang, S., Park, O., et al. (2022). Distinct histopathological phenotypes of severe alcoholic hepatitis suggest different mechanisms driving liver injury and failure. J. Clin. Invest 132 (14), e157780. doi:10.1172/jci157780
Mackowiak, B., Xu, M., Lin, Y., Guan, Y., Seo, W., Ren, R., et al. (2022). Hepatic CYP2B10 is highly induced by binge ethanol and contributes to acute-on-chronic alcohol-induced liver injury. Alcohol Clin. Exp. Res. 46 (12), 2163–2176. doi:10.1111/acer.14954
Matchett, K. P., Paris, J., Teichmann, S. A., and Henderson, N. C. (2024). Spatial genomics: mapping human steatotic liver disease. Nat. Rev. Gastroenterol. Hepatol. 21 (9), 646–660. doi:10.1038/s41575-024-00915-2
Park, S. H., Seo, W., Xu, M. J., Mackowiak, B., Lin, Y., He, Y., et al. (2023). Ethanol and its nonoxidative metabolites promote acute liver injury by inducing ER stress, adipocyte death, and lipolysis. Cell Mol. Gastroenterol. Hepatol. 15 (2), 281–306. doi:10.1016/j.jcmgh.2022.10.002
Privitera, G., Rana, N., Armuzzi, A., and Pizarro, T. T. (2023). The gasdermin protein family: emerging roles in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 20 (6), 366–387. doi:10.1038/s41575-023-00743-w
Ryan, K. A., Smith, M. F., Sanders, M. K., and Ernst, P. B. (2004). Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect. Immun. 72 (4), 2123–2130. doi:10.1128/iai.72.4.2123-2130.2004
Sarhan, J., Liu, B. C., Muendlein, H. I., Li, P., Nilson, R., Tang, A. Y., et al. (2018). Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. U. S. A. 115 (46), E10888–e10897. doi:10.1073/pnas.1809548115
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526 (7575), 660–665. doi:10.1038/nature15514
Tsung, A., Klune, J. R., Zhang, X., Jeyabalan, G., Cao, Z., Peng, X., et al. (2007). HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204 (12), 2913–2923. doi:10.1084/jem.20070247
Tsutsumi, T., Suzuki, T., Moriya, K., Shintani, Y., Fujie, H., Miyoshi, H., et al. (2003). Hepatitis C virus core protein activates ERK and p38 MAPK in cooperation with ethanol in transgenic mice. Hepatology 38 (4), 820–828. doi:10.1053/jhep.2003.50399
Wang, S., Wang, H., and Ding, W. X. (2018). Pyroptosis, A novel player for alcoholic hepatitis? Hepatology 67 (5), 1660–1662. doi:10.1002/hep.29725
Wang, W., Xu, M. J., Cai, Y., Zhou, Z., Cao, H., Mukhopadhyay, P., et al. (2017a). Inflammation is independent of steatosis in a murine model of steatohepatitis. Hepatology 66 (1), 108–123. doi:10.1002/hep.29129
Wang, Y., Gao, W., Shi, X., Ding, J., Liu, W., He, H., et al. (2017b). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547 (7661), 99–103. doi:10.1038/nature22393
Wiedemann, M. S., Wueest, S., Item, F., Schoenle, E. J., and Konrad, D. (2013). Adipose tissue inflammation contributes to short-term high-fat diet-induced hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 305 (3), E388–E395. doi:10.1152/ajpendo.00179.2013
Xu, B., Jiang, M., Chu, Y., Wang, W., Chen, D., Li, X., et al. (2018). Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 68 (4), 773–782. doi:10.1016/j.jhep.2017.11.040
Xu, L., Guo, W., Dai, J., Cheng, Y., Chen, Y., Liu, W., et al. (2024). Hydrogen gas alleviates acute ethanol-induced hepatotoxicity in mice via modulating TLR4/9 innate immune signaling and pyroptosis. Int. Immunopharmacol. 127, 111399. doi:10.1016/j.intimp.2023.111399
Yang, S., Liu, H., Fang, X. M., Yan, F., and Zhang, Y. (2024). Signaling pathways in uric acid homeostasis and gout: from pathogenesis to therapeutic interventions. Int. Immunopharmacol. 132, 111932. doi:10.1016/j.intimp.2024.111932
Zhang, K., Zhang, M. X., Meng, X. X., Zhu, J., Wang, J. J., He, Y. F., et al. (2023). Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways. Mil. Med. Res. 10 (1), 56. doi:10.1186/s40779-023-00494-4
Zhang, T., Hu, J., Wang, X., Zhao, X., Li, Z., Niu, J., et al. (2019). MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway. J. Hepatol. 70 (1), 87–96. doi:10.1016/j.jhep.2018.08.026
Zhang, Y., Huang, Z., and Li, H. (2017). Insights into innate immune signalling in controlling cardiac remodelling. Cardiovasc Res. 113 (13), 1538–1550. doi:10.1093/cvr/cvx130
Zhang, Y., Zhou, G., Chen, Z., Guan, W., Zhang, J., Bi, M., et al. (2020). Si-Wu-Tang alleviates nonalcoholic fatty liver disease via blocking TLR4-JNK and caspase-8-GSDMD signaling pathways. Evid. Based Complement. Altern. Med. 2020, 8786424. doi:10.1155/2020/8786424
Zheng, Z., Deng, W., Lou, X., Bai, Y., Wang, J., Zeng, H., et al. (2020). Gasdermins: pore-forming activities and beyond. Acta Biochim. Biophys. Sin. (Shanghai) 52 (5), 467–474. doi:10.1093/abbs/gmaa016
Zhou, B., Jiang, Z. H., Dai, M. R., Ai, Y. L., Xiao, L., Zhong, C. Q., et al. (2024). Full-length GSDME mediates pyroptosis independent from cleavage. Nat. Cell Biol. 26 (9), 1545–1557. doi:10.1038/s41556-024-01463-2
Zhou, Z., He, H., Wang, K., Shi, X., Wang, Y., Su, Y., et al. (2020). Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368 (6494), eaaz7548. doi:10.1126/science.aaz7548
Keywords: high-fat diet feeding, acute ethanol binge, oxidative stress, caspase-1, caspase-8, caspase-11, GSDMD, GSDME
Citation: Deng Y, Chen X, Guo W, Chen Y, Xu L, Suo W, Liu W, Dai J, Wang K, Li Q, Lu C, Dai M, Xu J, Xu J, Zhu H, Kuang Z and Zhang Y (2025) Short-term high-fat diet feeding plus acute ethanol binge induced acute liver injury in mice via oxidative stress, inflammation and pyroptosis. Front. Pharmacol. 16:1602280. doi: 10.3389/fphar.2025.1602280
Received: 29 March 2025; Accepted: 30 May 2025;
Published: 18 June 2025.
Edited by:
Ruichao Yue, China Agricultural University, ChinaReviewed by:
Lanlan Fan, Guangxi University of Chinese Medicine, ChinaYuanzhi Wang, China Agricultural University, China
Copyright © 2025 Deng, Chen, Guo, Chen, Xu, Suo, Liu, Dai, Wang, Li, Lu, Dai, Xu, Xu, Zhu, Kuang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaxing Zhang, emhhbmd5YXhpbmdAZ3p1Y20uZWR1LmNu; Zaoyuan Kuang, enlrdWFuZ0BnenVjbS5lZHUuY24=; Hequan Zhu, aHF1YW56aHVAb3V0bG9vay5jb20=
‡Lead contact
†These authors contribute equal to this work
 Yao Deng1†
Yao Deng1† Wenhai Guo
Wenhai Guo Wei Liu
Wei Liu Min Dai
Min Dai Jiean Xu
Jiean Xu Jinwen Xu
Jinwen Xu Hequan Zhu
Hequan Zhu Yaxing Zhang
Yaxing Zhang