- 1Department of Radiation Oncology Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Lung Cancer Center/Lung Cancer Institute, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Medical Oncology Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 4Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Anaplastic lymphoma kinase (ALK) rearrangement is a crucial oncogenic driver in non-small cell lung cancer (NSCLC). ALK tyrosine kinase inhibitors (ALK-TKIs) represent the primary therapeutic option for advanced NSCLC patients with ALK rearrangements. However, the definitive determination of ALK-TKIs sensitivity towards newly identified rare ALK rearrangements remains elusive. Herein, we present a patient with lung adenocarcinoma harboring a novel ALK rearrangement who exhibited major pathological response (MPR) following neoadjuvant treatment with alectinib.
Materials and methods: We conduct immunohistochemistry (IHC) staining with Ventana with D5F3 clone, fluorescence in situ hybridization (FISH, The Vysis ALK Break Apart FISH Probe Kit), and next-generation sequencing (NGS) analyses (Burning Rock, Guangzhou, China) on biopsy sample obtained from the patient.
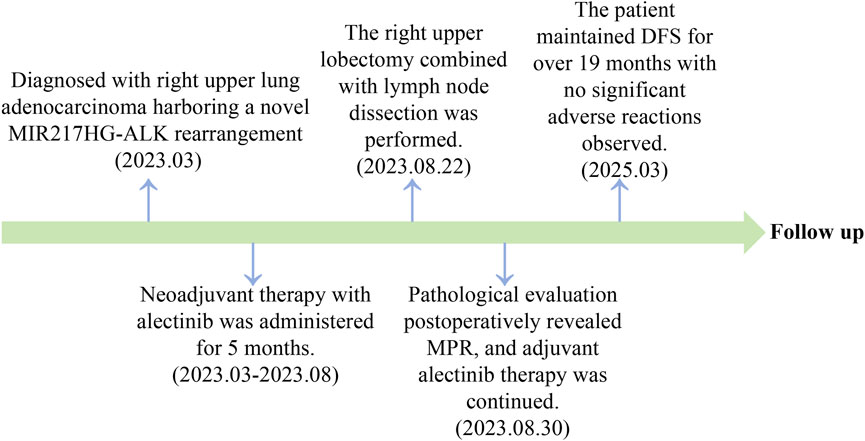
Results: The patient, a 66-year-old female, was diagnosed with stage IIIB-N3 adenocarcinoma in the right upper lobe of the lung. NGS testing revealed a previously unreported MIR217HG-ALK rearrangement, which was subsequently confirmed by IHC and FISH. Following 5 months of neoadjuvant treatment with alectinib, the patient underwent the right upper lobectomy and achieved MPR, resulting in disease-free survival (DFS) exceeding 19 months.
Conclusion: In this study, we present the first documented case of a patient with lung adenocarcinoma harboring a novel MIR217HG-ALK rearrangement who exhibited favorable response to neoadjuvant alectinib. Our findings suggest that alectinib holds promise as an efficacious therapeutic option for individuals with MIR217HG-ALK rearranged lung adenocarcinoma, thereby offering valuable insights for the clinical management of these patients.
Introduction
For advanced non-small cell lung cancer (NSCLC) with anaplastic lymphoma kinase (ALK) positivity, ALK-tyrosine kinase inhibitors (ALK-TKIs) are considered the preferred first-line treatment option (Mok et al., 2020). The Echinoderm microtubule-associated protein-like 4 gene (EML4)-ALK rearrangement is one of the predominant fusion partners in NSCLC, and currently, numerous novel ALK fusion partners have also been identified (Sasaki et al., 2010; Ou et al., 2020). However, considering that these newly discovered ALK fusion partners may exhibit distinct response profiles to ALK-TKIs (Chuang et al., 2021), it is imperative to identify additional rare ALK fusion genes to provide more valuable clinical evidence for patient management. Herein, we present a case of unresectable locally advanced lung adenocarcinoma harboring a novel MIR217HG-ALK rearrangement, and after receiving neoadjuvant treatment with alectinib, the patient achieved major pathological response (MPR) and demonstrated disease-free survival (DFS) exceeding 19 months without notable adverse reactions observed.
Case report
A 66-year-old non-smoking female presented to our hospital with a 6-month history of persistent cough with sputum production. The patient denied any symptoms of chest tightness, chest pain, fever, or dyspnea. Her medical history was unremarkable, with no evidence of diabetes, hypertension, or cardiac disease, and no family history of malignancies. Chest contrast-enhanced computed tomography (CT) revealed a mass measuring approximately 5 cm × 4.9 cm in the posterior segment of the right upper lobe, accompanied by enlarged lymph nodes in the right hilum and bilateral mediastinum. Meanwhile, the patient underwent a thorough imaging evaluation including enhanced magnetic resonance imaging (MRI) of the head, single-photon emission computed tomography (SPECT) bone scan, and contrast-enhanced CT of the abdomen, which revealed no metastasis in the head, bones, liver, or adrenal glands. Bronchoscopic biopsy was performed and the pathological examination revealed invasive adenocarcinoma on the right upper lobe, and the patient was clinically classified as stage IIIB with involvement of contralateral mediastinal lymph nodes (cT2bN3M0, the eighth edition of the TNM classification for lung cancer). The immunohistochemistry (IHC) staining with Ventana with D5F3 clone demonstrated positive expression of thyroid transcription factor-1 (TTF1) and Napsin A (Figures 1A–C), indicating an original adenocarcinoma of the lung. Subsequently, next-generation sequencing (NGS) testing (Burning Rock, Guangzhou, China) was performed on the lung tissue obtained from bronchoscopy, revealing a novel MIR217HG-ALK fusion variant (M3′UTR: A20; abundance: 3.31%) with an intact ALK kinase domain and no TP53 mutation (Figures 2A,B). The MIR217HG gene, which serves as the host gene for MIR217, is a type of RNA gene classified as a long non-coding RNA (lncRNA). This novel ALK fusion variant was further confirmed through IHC and fluorescence in situ hybridization (FISH, The Vysis ALK Break Apart FISH Probe Kit) analysis (Figures 1D, 2C). Then, oral administration of alectinib dose of 300 mg twice daily was initiated for treatment from 14 March 2023. Three months later, chest contrast-enhanced CT demonstrated a significant reduction in the mass located in the right upper lobe, measuring approximately 3.9 cm × 2.2 cm, accompanied by shrinkage of the right hilum and bilateral mediastinal lymph nodes. Five months later, follow-up chest CT indicated continued reduction in the right upper lobe mass and the involved lymph nodes, with the mass size measuring approximately 3.1 cm × 2.0 cm (Figure 3A). Additionally, both CEA and cytokeratin-19 fragment levels showed a significant decrease (Figure 3B). Consequently, a surgical procedure involving the right upper lobectomy combined with lymph node dissection was performed on the patient on 22 August 2023. Postoperative pathological examination revealed that approximately 10% of the primary lesion consisted of residual tumor tissue, while the remaining portion comprised necrotic tissue, fibrosis, and inflammation (Figures 1E–H). A total of 13 lymph nodes were assessed for treatment response: cancer metastasis was observed in station 2 (R, 1/1), station 3 (3a, 2/2), station 4 (R, 3/3), station 5 (1/1), station 10 (R, 1/1), and station 11 (R, 2/3) with accompanying post-treatment reactions; no cancer metastasis was detected in two lymph nodes from station 7. Therefore, the patient achieved a pathological evaluation of MPR following neoadjuvant alectinib therapy. Moreover, we also performed NGS on the resected specimen to check for possible resistance mechanisms in the remaining active tumor. The NGS result showed MIR217HG-ALK fusion variant and no TP53 mutation, consistent with the finding of NGS analysis before alectinib treatment. Subsequent to surgery, adjuvant alectinib treatment was continued by the patient who has now maintained DFS for over 19 months without obvious adverse reactions noted (Figure 4).
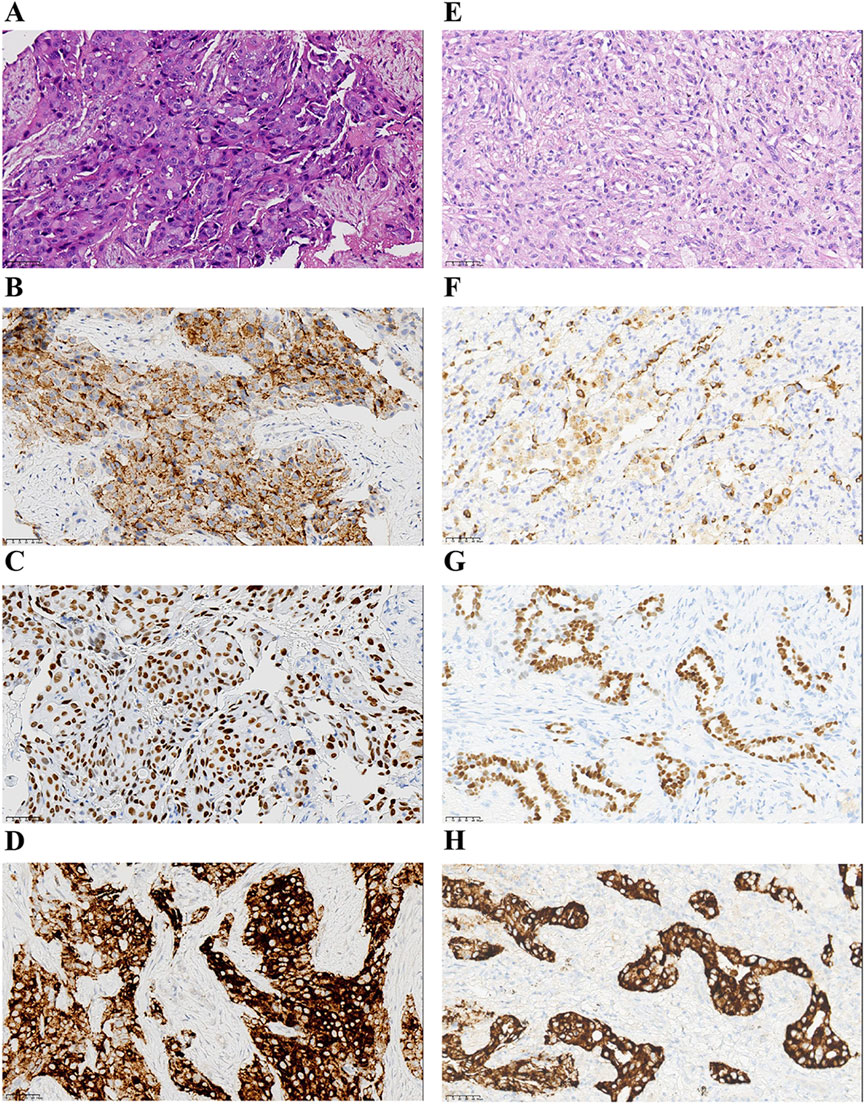
Figure 1. Pathological findings of the patient. (A–D) Hematoxylin-eosin staining and IHC staining at the time of diagnosis (x40). Hematoxylin-eosin staining of lung adenocarcinoma (A). The IHC staining indicated positive for Napsin A (B), TTF-1 (C), and ALK protein expression (D). (E–H) Findings of postoperative pathology examination (x40). Hematoxylin-eosin staining (E), and the IHC staining of Napsin A (F), TTF-1 (G), and ALK protein expression (H). IHC, immunohistochemistry; TTF-1, thyroid transcription factor-1; ALK, anaplastic lymphoma kinase.
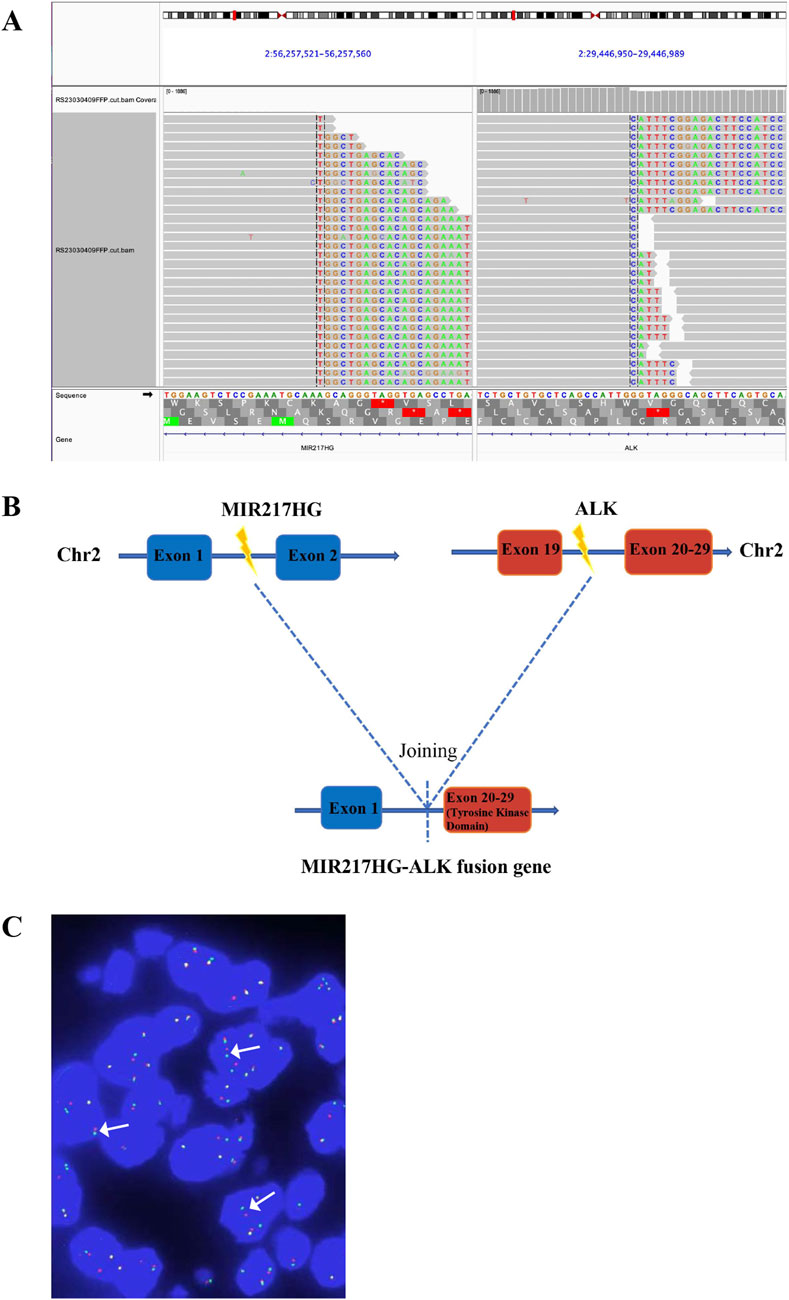
Figure 2. Identification of MIR217HG-ALK fusion by NGS. (A) The Integrative Genomics Viewer (IVG) snapshot of MIR217HG-ALK fusion. (B) Illustration of MIR217HG-ALK fusion (the new variant consists of MIR217HG exon 1 and ALK exon 20–29, positivity threshold: ≥15%). (C) FISH result. ALK gene isolation probe successfully identified the presence of ALK fusion (the percentage of the rearranged cells: 30.27%), displaying a split signal (either green or red, indicated by arrows) at both the 5′and 3′ends of the ALK gene. The yellow signals represent intact ALK gene. NGS, next-generation sequencing; ALK, anaplastic lymphoma kinase; FISH, fluorescence in situ hybridization.
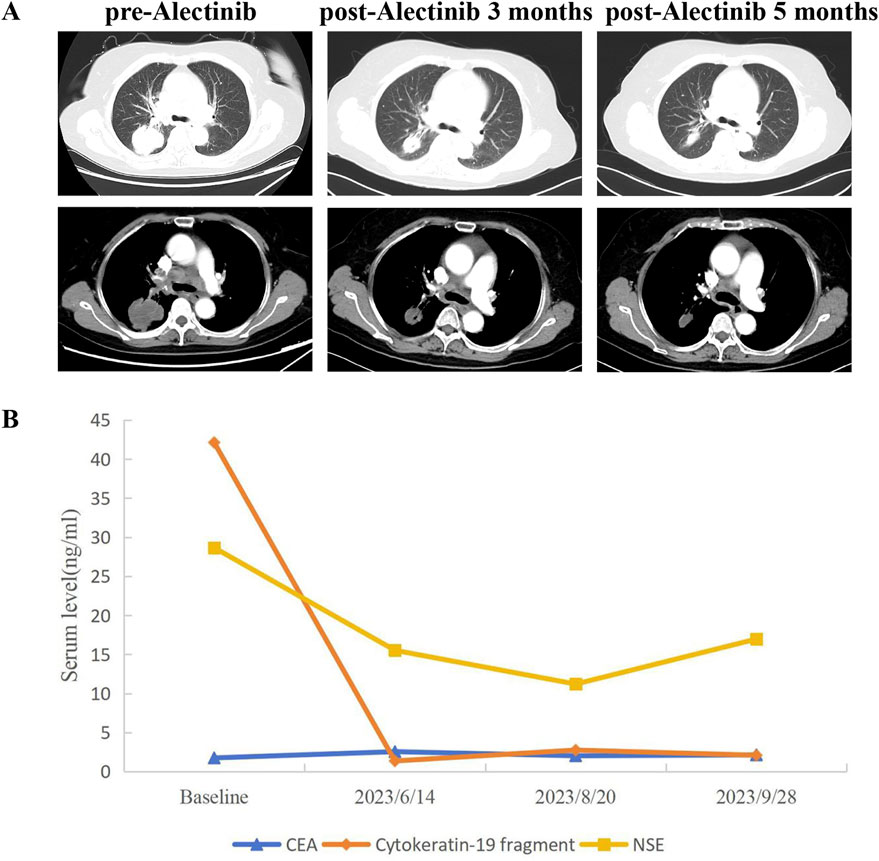
Figure 3. CT scans and changes of tumor markers during treatment. (A) Chest CT images of right lung adenocarcinoma before and after neoadjuvant alectinib (evaluated according to RECIST 1.1 criteria). (B) Dynamic monitoring of tumor markers during neoadjuvant alectinib. CT, Computed tomography.
Discussion
A retrospective study conducted by Tatsuya Yoshida et al. unveiled that crizotinib exhibits varying sensitivity towards different ALK fusion variants (Yoshida et al., 2016), indicating the presence of heterogeneity in the response of ALK-TKIs to distinct ALK fusion partners. Thus, it holds immense significance to ascertain the specific types and breakpoints of ALK fusion variants for guiding clinical treatment decisions for patients. There are numerous techniques available for the detection of ALK fusion, and in comparison to traditional methods such as IHC, FISH, and RT-PCR, NGS detection enables more comprehensive detection of ALK fusion partners, facilitating the identification of a broader spectrum of novel ALK rearrangements, and is therefore a critical method for thorough ALK fusion analysis (Letovanec et al., 2018). RNA-based sequencing and whole transcriptome sequencing can serve as complementary tools to DNA-based NGS for the detection of rare ALK fusion variants (Benayed et al., 2019). Furthermore, the structural domain of ALK tyrosine kinase plays a pivotal role in driving tumorigenesis (Ou et al., 2020; Soda et al., 2007). In this case, a novel MIR217HG-ALK rearrangement was identified through NGS testing, which retained the intact kinase domain of ALK and potentially activated tumorigenesis, leading to sensitivity to ALK-TKIs. We have demonstrated that the patient harboring MIR217HG-ALK rearrangement exhibit a favorable response to neoadjuvant alectinib, achieving MPR, with a DFS exceeding 19 months.
Currently, alectinib has demonstrated superior clinical efficacy compared to crizotinib in advanced ALK-positive NSCLC (Camidge et al., 2019). However, the investigation of alectinib as neoadjuvant therapy for locally advanced ALK-positive NSCLC remains inadequate. A retrospective study revealed remarkable pathological response rate and safety profile of crizotinib in patients with ALK rearrangement undergoing neoadjuvant treatment (Zhang et al., 2019). Moreover, several clinical cases have reported the feasibility of utilizing alectinib as neoadjuvant therapy (Cao et al., 2023; Wang et al., 2023). The ongoing ALNEO clinical trial is also to evaluate the efficacy and safety of alectinib as neoadjuvant therapy in surgically resectable stage III ALK-positive NSCLC (Leonetti et al., 2021). These studies present promising prospects for the application of ALK-TKIs in neoadjuvant treatment.
However, neoadjuvant ALK-TKI therapy for patients with locally advanced unresectable N3-stage NSCLC harboring ALK rearrangement has only been reported in isolated cases (Zhai et al., 2023). Zhai X et al. documented a stage IIIB-N3 patient achieving qPCR after 5 months of neoadjuvant alectinib14, paralleling the treatment duration in our case. Additionally, Shi L et al. described two patients with stage IIB lung adenocarcinoma who achieved qPCR following long-course neoadjuvant alectinib (Shi et al., 2023). Given the advanced clinical stage (IIIB-N3) of the patient and the lack of established neoadjuvant treatment regimens or drug selection for novel ALK-rearranged NSCLC, the multidisciplinary team (MDT) at our institution opted for neoadjuvant alectinib, which has demonstrated superior efficacy over crizotinib in advanced ALK-rearranged disease (Camidge et al., 2019). After 3 months of alectinib treatment, imaging evaluation was performed. The surgeon recommended continued treatment to enable radical surgical resection. Consequently, the patient received alectinib for 5 months and met the surgical criteria. Surgery was performed successfully, and postoperative pathology revealed a MPR. However, residual cancer cells were still present in the primary lesion and lymph nodes of the resected specimen, and the patient had been initially diagnosed at a relatively advanced clinical stage. To potentially prolong the patient’s DFS and OS, the patient, after thorough discussion, opted for adjuvant alectinib treatment. Currently, DFS has exceeded 19 months. Unfortunately, financial constraints led the patient to forgo potential biomarker testing, such as circulating tumor DNA (ctDNA), opting solely for imaging evaluation, thereby limiting our study. In future clinical practice, a combined approach incorporating both imaging examinations and biomarker detection should be prioritized to enable earlier identification of disease recurrence or metastasis, thereby facilitating timely clinical interventions.
Our study, being a single-case report, necessitates further collection of clinical data from patients with the MIR217HG-ALK rearrangement to summarize clinical characteristics, treatment patterns, and conduct prognosis analyses. The current follow-up period for this case is 19 months, and we will continue to monitor the patient’s DFS and OS, along with treatment strategies post-recurrence or metastasis. Although we validated the findings with NGS on residual tissue, single-cell sequencing was not performed, limiting our exploration of the carcinogenic mechanisms of this novel fusion. Future efforts will focus on patients with MIR217HG-ALK rearrangement, aiming for a multi-center case study, dynamic monitoring of ctDNA in similar cases, and the development of a patient-derived xenograft (PDX) model to further investigate the oncogenic mechanisms of this ALK rearrangement.
Conclusion
In conclusion, we have identified a novel MIR217HG-ALK rearrangement in NSCLC for the first time, thereby expanding the spectrum of ALK fusion variants. Moreover, we have provided preliminary evidence demonstrating the feasibility and safety of neoadjuvant alectinib in locally advanced unresectable NSCLC harboring MIR217HG-ALK rearrangement. We anticipate that prospective studies investigating the application of ALK-TKI in rare ALK-rearranged NSCLC, thereby offering more precise personalized treatment options for patients’ clinical management.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the West China Hospital, Sichuan University ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YX: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. WL: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. KH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. QZ: Conceptualization, Resources, Supervision, Writing – review and editing. QW: Conceptualization, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Youth Project of Sichuan Natural Science Foundation (Nos 2023NSFSC1892, 2024NSFSC1918) and Postdoctoral Research Project of West China Hospital, Sichuan University (No. 2020HXBH132).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Benayed, R., Offin, M., Mullaney, K., Sukhadia, P., Rios, K., Desmeules, P., et al. (2019). High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin. Cancer Res. 25 (15), 4712–4722. doi:10.1158/1078-0432.CCR-19-0225
Camidge, D. R., Dziadziuszko, R., Peters, S., Mok, T., Noe, J., Nowicka, M., et al. (2019). Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 14 (7), 1233–1243. doi:10.1016/j.jtho.2019.03.007
Cao, P., Zhao, Q., Li, Y., Shi, R., Zhu, G., Zhang, Z., et al. (2023). Case report: ALK rearranged locally advanced lung adenocarcinoma showing inconsistent radiographic findings and pathological responses during neoadjuvant alectinib therapy. Front. Pharmacol. 14, 1140894. doi:10.3389/fphar.2023.1140894
Chuang, C. H., Chen, H. L., Chang, H. M., Tsai, Y. C., Wu, K. L., Chen, I. H., et al. (2021). Systematic review and network meta-analysis of anaplastic lymphoma kinase (ALK) inhibitors for treatment-naïve ALK-positive lung cancer. Cancers (Basel) 13 (8), 1966. doi:10.3390/cancers13081966
Leonetti, A., Minari, R., Boni, L., Gnetti, L., Verzè, M., Ventura, L., et al. (2021). Phase II, open-label, single-arm, multicenter study to assess the activity and safety of alectinib as neoadjuvant treatment in surgically resectable stage III ALK-Positive NSCLC: ALNEO trial. Clin. Lung Cancer 22 (5), 473–477. doi:10.1016/j.cllc.2021.02.014
Letovanec, I., Finn, S., Zygoura, P., Smyth, P., Soltermann, A., Bubendorf, L., et al. (2018). Evaluation of NGS and RT-PCR methods for ALK rearrangement in european NSCLC patients: results from the european thoracic oncology platform lungscape project. J. Thorac. Oncol. 13 (3), 413–425. doi:10.1016/j.jtho.2017.11.117
Mok, T., Camidge, D. R., Gadgeel, S. M., Rosell, R., Dziadziuszko, R., Kim, D. W., et al. (2020). Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-Positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 31 (8), 1056–1064. doi:10.1016/j.annonc.2020.04.478
Ou, S. I., Zhu, V. W., and Nagasaka, M. (2020). Catalog of 5' fusion partners in ALK-Positive NSCLC circa 2020. JTO Clin. Res. Rep. 1 (1), 100015. doi:10.1016/j.jtocrr.2020.100015
Sasaki, T., Rodig, S. J., Chirieac, L. R., and Jänne, P. A. (2010). The biology and treatment of EML4-ALK non-small cell lung cancer. Eur. J. Cancer 46 (10), 1773–1780. doi:10.1016/j.ejca.2010.04.002
Shi, L., Gao, S., Tong, L., Meng, Q., Zhou, S., Yu, D., et al. (2023). Pathological complete response to long-course neoadjuvant alectinib in lung adenocarcinoma with EML4-ALK rearrangement: report of two cases and systematic review of case reports. Front. Oncol. 13, 1120511. doi:10.3389/fonc.2023.1120511
Soda, M., Choi, Y. L., Enomoto, M., Takada, S., Yamashita, Y., Ishikawa, S., et al. (2007). Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448 (7153), 561–566. doi:10.1038/nature05945
Wang, L. M., Zhao, P., Sun, X. Q., Yan, F., and Guo, Q. (2023). Pathological complete response to neoadjuvant alectinib in unresectable anaplastic lymphoma kinase positive non-small cell lung cancer: a case report. World J. Clin. Cases 11 (22), 5322–5328. doi:10.12998/wjcc.v11.i22.5322
Yoshida, T., Oya, Y., Tanaka, K., Shimizu, J., Horio, Y., Kuroda, H., et al. (2016). Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 34 (28), 3383–3389. doi:10.1200/JCO.2015.65.8732
Zhai, X., Wang, T., Lin, Y., Zhang, J., Wang, Y., Wang, W., et al. (2023). Case report: complete pathological admission in N3 unresectable locally advanced lung adenocarcinoma with a novel INTS10-ALK and EML4-ALK fusion after neoadjuvant crizotinib. Front. Oncol. 13, 1104910. doi:10.3389/fonc.2023.1104910
Keywords: lung adenocarcinoma, MIR217HG-ALK, neoadjuvant alectinib, major pathological response (MPR), stage IIIB-N3
Citation: Xue Y, Li W, Huang K, Zhou Q and Wu Q (2025) Case Report: Locally advanced lung adenocarcinoma with a novel MIR217HG-ALK rearrangement responding to neoadjuvant alectinib. Front. Pharmacol. 16:1602654. doi: 10.3389/fphar.2025.1602654
Received: 30 March 2025; Accepted: 18 July 2025;
Published: 11 August 2025.
Edited by:
Ying-Hong Feng, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Meng Fu, University of Science and Technology of China (USTC), ChinaQuanying Tang, Tianjin Medical University General Hospital, China
Copyright © 2025 Xue, Li, Huang, Zhou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Zhou, cHJvZl9xaF96aG91QDEyNi5jb20=; Qiang Wu, dGVhbXdvcmsyMzA3MTFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yinyin Xue
Yinyin Xue Wen Li
Wen Li Kaili Huang
Kaili Huang Qinghua Zhou2,4*
Qinghua Zhou2,4*