- 1Department of Infectious Diseases, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Institute for Viral Hepatitis, The Key Laboratory of Molecular Biology for Infectious Diseases, Chinese Ministry of Education, Chongqing Medical University, Chongqing, China
- 3Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Research Institute of Infectious Diseases and Parasitic Disease, Chongqing Key Laboratory of Infectious Diseases and Parasitic Disease, Chongqing, China
Background and aims: The effects of Tenofovir Disoproxil Fumarate (TDF) or Tenofovir Alafenamide (TAF) on lipid profiles have been observed in chronic hepatitis B (CHB) treatment. However, the metabolic features and their impact on cardiovascular risk remain unclear. We conducted a systematic review and meta-analysis to evaluate these effects.
Methods: We searched for studies from four major databases (PubMed, Web of Science, EMBASE, and the Cochrane Library) that reported the effects of TDF or TAF on metabolism and cardiovascular disease risk. The changes in metabolic parameters and 10-year atherosclerotic cardiovascular disease (ASCVD) risk were compared with baseline in the TDF and TAF treatment groups. Extracted data were analyzed with the random-effects model or the fixed-effects model. Potential sources of heterogeneity were investigated using sensitivity and subgroup analyses.
Results: A total of 19 studies including 19,396 CHB patients (12,067 in TDF-only group, 5,423 in TAF-only group, and 1906 in TDF-switched group) were included in this meta-analysis. We found that both TAF and TDF treatment mildly increase the 10-year ASCVD risk. The TAF treatment showed significant increases in body weight, with no significant effects were observed on lipid levels or blood glucose. While TDF treatment has a lipid-lowering effect and caused weight loss. Subanalyses emphasized the impact of changing antiviral treatment strategies on metabolism. We found an increased risk of dyslipidemia and body weight gain after switching from TDF to TAF treatment.
Conclusion: Although TAF and TDF treatments exhibit different metabolic characteristics, both mildly increase the risk of cardiovascular disease.
Clinical Trial Registration: identifier CRD42024595452
Introduction
Hepatitis B virus (HBV) remains a major public health concern, with an estimated 29.6 million infected individuals worldwide in 2019, causing considerable liver-related morbidity and mortality (Sh et al., 2022; Cui et al., 2023). Viral suppression can effectively improve the long-term prognosis of chronic hepatitis B patients, while also reducing the risk of progression to cirrhosis and hepatocellular carcinoma (Wei and Kao, 2017). Therefore, effective antiviral treatment is crucial for patients with chronic hepatitis B.
Tenofovir alafenamide (TAF) and tenofovir disoproxil fumarate (TDF), as first-line oral antiviral agents, are widely used and recommended by international guidelines (The Korean Association for the Study of the Liver KASL, 2022). However, to achieve the ideal treatment goal for chronic hepatitis B patients, which is functional cure, defined as HBsAg seroclearance or seroconversion (Anderson et al., 2021; Terrault et al., 2016). Most patients require lifelong treatment. Therefore, increased attention is being paid to the monitoring and management of comorbidities in the treatment of chronic hepatitis B under long-term antiviral treatment.
The previous studies have investigated whether the use of NA treatment increases the risk of potential complications. TAF was initially used to treat human immunodeficiency virus (HIV) infection before its efficacy was widely recognized (Hamers et al., 2013). However, in patients treated for HIV infection, TAF has been suspected of having potential metabolic side effects, such as elevated TC levels and an increased ASCVD risk (Huhn et al., 2020; Kauppinen et al., 2019). In contrast, TDF has been reported to have lipid-lowering effects in the treatment of HIV patients (Santos et al., 2015). Similarly, during the treatment for CHB, recent cohort studies have shown that TAF and TDF treatments are associated with changes in metabolic parameters (Zhang Q. et al., 2022; Shin et al., 2024). Pin-Nan Cheng et al. showed that the switch from TDF to TAF was associated with weight gain, derangements of lipid profile, and increased insulin resistance in patients with CHB (Cheng et al., 2024). However, dyslipidemia and metabolic syndrome (Mets) is a major risk factor for the development of ASCVD (Atar et al., 2021; Esposito et al., 2012). A recent prospective study reported that TAF had comparable risks of cardiovascular outcomes to those of patients treated with TDF (Fung et al., 2024).
Nevertheless, considerable debate persists in current research regarding whether metabolic parameter changes during NA treatment influence long-term cardiovascular risk in patients with chronic hepatitis B. Similarly, there is also no consensus regarding the effects of NAs on other metabolic parameters such as lipids, glucose, and body weight. Two recent systematic reviews focusing on the impact of NA treatment on lipid levels (Tong et al., 2024; Hwang et al., 2023), But they did not provide a comprehensive assessment of other metabolic parameters and cardiovascular disease risk. Additionally, prior systematic reviews did not include all randomized controlled trials of antiviral treatments.
The long-term cardiovascular outcomes and metabolic effects of antiviral therapies often require extended follow-up periods that are better captured by observational cohort studies. Hence, this meta-analysis incorporates both RCTs and observational studies to comprehensively assess the metabolic features and the risk of cardiovascular disease in patients with CHB under long-term treatment, in order to guide clinical decision-making and develop targeted treatment strategies for different patients.
Methods
This protocol for systematic review and meta-analysis adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) statement and is available on PROSPERO (CRD42024595452).
Literature search
To identify all randomized controlled trials (RCTs) and cohort studies reporting on the treatment of chronic hepatitis B with TDF and TAF, we used the PICOS criteria as a guide for our search strategy. A comprehensive search was conducted across multiple databases, including PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science. We consulted with an experienced medical librarian to refine our search strategies for each database, aiming to identify all relevant studies published from the establishment of each database up to 1 September 2024. The detailed search strategy is provided in the Supplementary Material (Table 1). Additionally, we reviewed the reference lists of the included studies and relevant systematic reviews to identify other potential eligible studies.
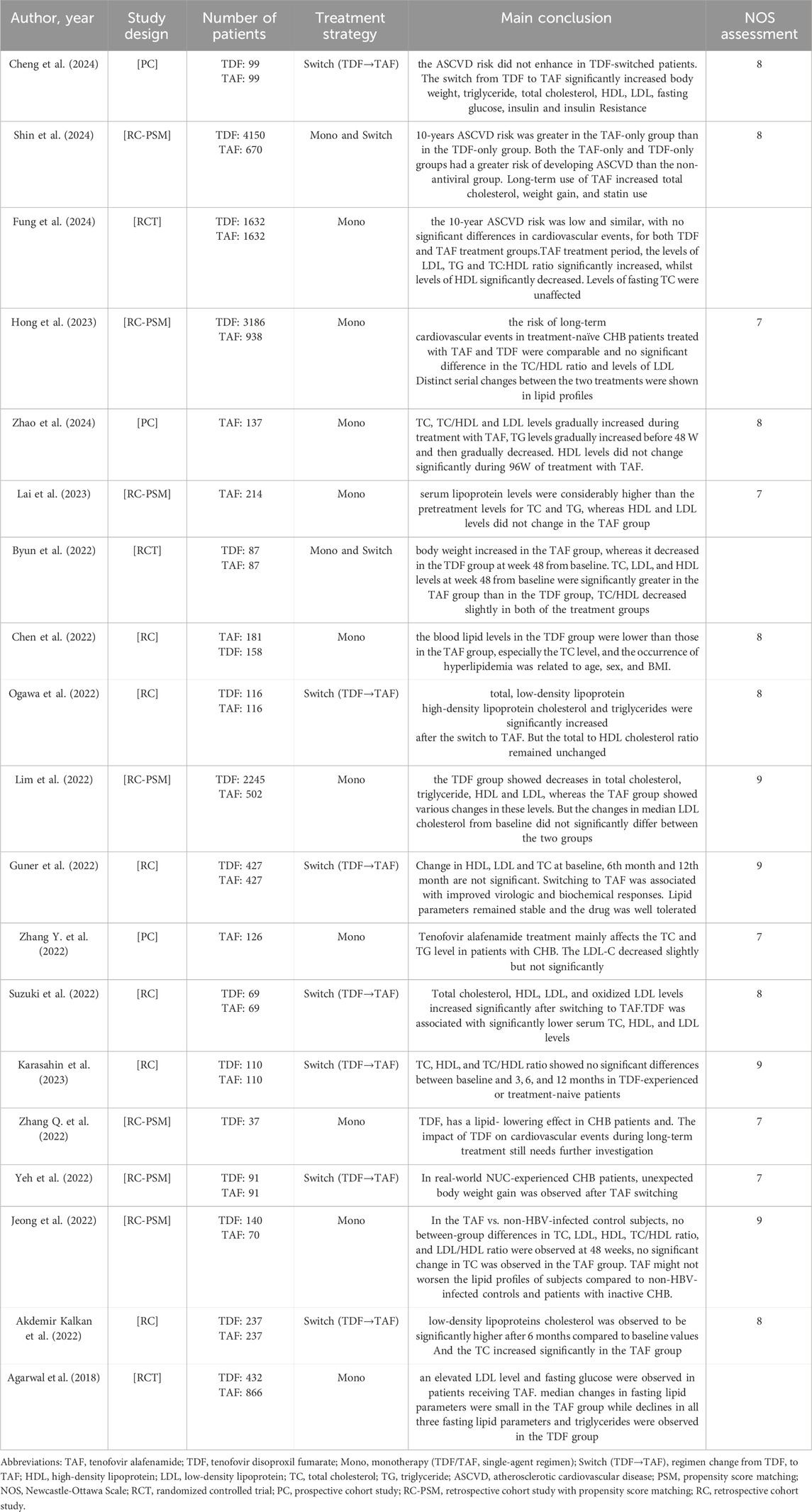
Table 1. Characteristics of the studies showing the Metabolic effects and cardiovascular disease risks of TDF or TAF in patients with chronic hepatitis B.
Eligibility criteria and study selection
In order to incorporate as many high-quality studies as possible, we selected the literature based on the following criteria. The inclusion criteria were as follows: (1) Clinical studies published in English; (2) Patients with chronic hepatitis B virus infection; (3) Randomized controlled trials and cohort studies using TAF or TDF as antiviral drugs. The exclusion criteria were as follows: (1) Full text not available; (2) Review articles or meta-analyses; (3) Case reports; (4) Studies with fewer than 10 patients; (5) Animal studies or experiments; (6) co-infected with HCV or HIV; (7) Articles with unavailable relevant data.
One researcher (Nan Cai) examined the titles and abstracts and removed obviously irrelevant reports. Two researchers (Yuanhai Zhou and Yuxin Chen) reviewed the remaining full-text reports to determine whether the studies met the inclusion criteria. Discrepancies were resolved through discussion or consultation with a third reviewer when necessary.
Data extraction
Two authors (Z. Yuanhai and N. Cai) independently extracted data from eligible studies into a specially designed spreadsheet (Excel, version 16.0; Microsoft Corp), with any discrepancies resolved through discussion. The following data were extracted from each study: (1) Basic study information, including authors, publication year, study type, sample size, and follow-up duration; (2) Patient characteristics, including gender, age, and BMI; (3) Antiviral regimen; (4) All primary and secondary outcome measures; (5) description of the quality assessment. Outcome definitions are the changes in lipid profiles, blood glucose, body weight, and 10-year atherosclerotic cardiovascular disease (ASCVD) risk. The ASCVD risk score was selected as it has been widely adopted as the preferred CV risk stratification tool in chronic disease populations, including patients with liver diseases (Goff et al., 2014). All eligible studies utilized the 10-year ASCVD risk score as their primary analysis endpoint. This methodological consistency enabled standardized data extraction and valid meta-analysis across heterogeneous study designs.
Quality assessment
Two reviewers (Z. Yuanhai and C. Yuxin) independently assessed the risk of bias in all eligible randomized controlled trials (RCTs) using the Cochrane Risk of Bias Tool (Higgins et al., 2011). We conducted quality assessments in 6 domains of this tool. Included the following domains: random sequence generation; allocation concealment; blinding of patients, healthcare practitioners, data collectors, outcome assessors, and data analysts; incomplete outcome data; selective reporting; and other sources of bias. If the review of all individual domains was considered to show a low risk of bias, the trials was assessed as having a low risk of bias. The trials with an uncertain risk of bias or high risk of bias in one or more individual domains were considered to have a high risk of bias. Studies judged to have a high risk of selection bias, performance bias or detection bias were excluded from the meta-analysis.
For eligible cohort studies, the same two reviewers conducted quality assessments based on the Newcastle-Ottawa Scale (NOS) (Ohri, 2025), evaluating 3 domains: selection bias, comparability bias and outcome bias. A NOS score greater than 7 was considered indicative of high-quality studies, and studies with a score below 4 were excluded as low-quality studies. Any disagreements were resolved through discussion or by consulting a third reviewer. The results of the quality assessment can be found in the Supplementary Table S3.
Statistical analysis
Changes in lipid levels, blood glucose, weight and 10-year ASCVD score in the TAF and TDF treatment groups were expressed as mean differences with 95% confidence intervals. The χ2 test for heterogeneity provided an indication of between-trial heterogeneity. Additionally, to quantify statistical heterogeneity, we used the I2 statistic and the Cochrane Q test, which assess the proportion of variability between studies due to differences rather than chance. Significant heterogeneity was considered when I2 > 50% and p < 0.05, and a random-effects model was used. In the absence of significant heterogeneity, a fixed-effect model was applied.
Sensitivity analysis was conducted to validate the results and further test the robustness of our results. We decided a priori to perform subanalyses included age, sex, antiviral treatment strategies, follow-up duration and different regions. Given the limited number of randomized controlled trials (RCTs, n = 3), we conducted a pooled analysis incorporating both RCTs and observational studies. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (version 6.5) (Cochrane, 2025), subgroup analyses stratified by study design (RCTs vs. observational studies) were performed to minimize heterogeneity arising from combining distinct study types.
Publication bias was visually inspected using a funnel plot and We tested for funnel plot asymmetry with the Egger and Harbord tests. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Stata version 14.0 (Stata Corp, College Station, TX, United States). Quality assessment of RCTs was conducted using Review Manager 5.3.
Results
Characteristics of included studies
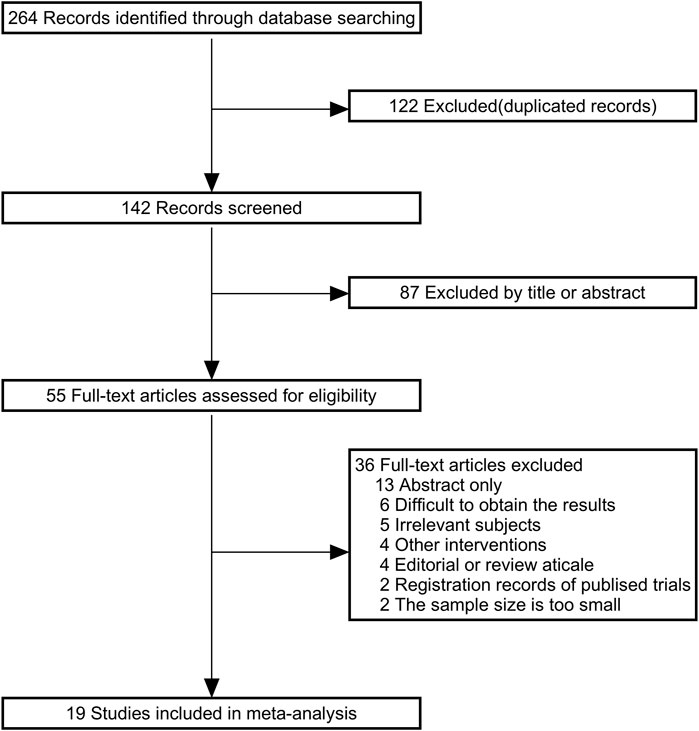
Figure 1 shows the details of the study selection process. A total of 264 articles were identified through the initial bibliographic search strategy. After removing 122 duplicates and excluding 87 articles based on titles and abstracts, 55 studies were considered potentially relevant and underwent full-text review. Among these, 11 studies did not meet the inclusion or exclusion criteria. Of the remaining 44 studies, 13 only reported abstracts, 6 could not provide usable data, 4 were reviews, and 2 were clinical registry records. Finally, 19 studies were included. Information from the included studies is presented in Table 1.
The 19 studies included 19,396 chronic hepatitis B (CHB) patients (TDF group: 12,067 TAF group: 5,423, TDF-switched group: 1906). All studies were reported between 2018 and 2024, with a predominance of male participants. Most enrolled patients were between 40 and 60 years of age. Among the 19 studies, the most common design was retrospective cohort studies (13 studies), followed by 3 prospective cohort studies and 3 randomized controlled trials. 12 studies reported the effects of TAF and TDF on metabolism, while 9 studies showed the outcomes of switching from TDF to TAF. 6 studies discussed the impact of TAF and TDF treatment on cardiovascular disease risk in CHB patients. The quality assessment results of all eligible studies are shown in Supplementary Table S3.
Changes in lipid levels with TAF and TDF
In the TAF group, we focused on analyzing lipid levels at 24, 48, 72 and 96 weeks, including TG, TC, HDL, LDL, and TC/HDL. The results showed that TAF treatment significantly increased TG and TC/HDL levels, while HDL decreased, with minimal effects on LDL and TC levels. During the follow-up period, the mean differences in TG from the baseline after 24, 48, 72 and 96 weeks of treatment were 3.69 mg/dL, 6.40 mg/dL, 6.97 mg/dL, and 9.25 mg/dL in the TAF group and 0.16 mg/dL,0.19 mg/dL, 0.20 mg/dL and 0.24 mg/dL in the TC/HDL ratio, respectively. In contrast, HDL levels showed a decreasing trend, at 24, 48, 72 and 96 weeks were −1.31 mg/dL, −1.72 mg/dL, −2.76 mg/dL and −3.98 mg/dL, respectively. TC levels showed a slight increase compared to baseline at 24 and 48 weeks, with increases of 3.52 mg/dL (95% CI, 0.91–6.12) and 1.83 mg/dL (95% CI, 0.05–3.51), respectively. However, after 72 weeks, there were no significant changes in TC levels. Similarly, LDL changes showed no significant differences after 48 weeks (Table 2). These findings were consistent across study designs in subgroup analyses, where both observational studies (TG: MD +7.39 mg/dL, 95% CI 3.12–11.65; HDL MD −1.03 mg/dL, 95% CI −1.32 to −0.73) and randomized controlled trials (RCTs) (TG MD +6.07 mg/dL, 95% CI 4.68–7.46; HDL MD −3.88 mg/dL, 95% CI −4.30 to −3.47) consistently demonstrated TG increases and HDL declines after 48-week TAF monotherapy (Supplementary Table S12).
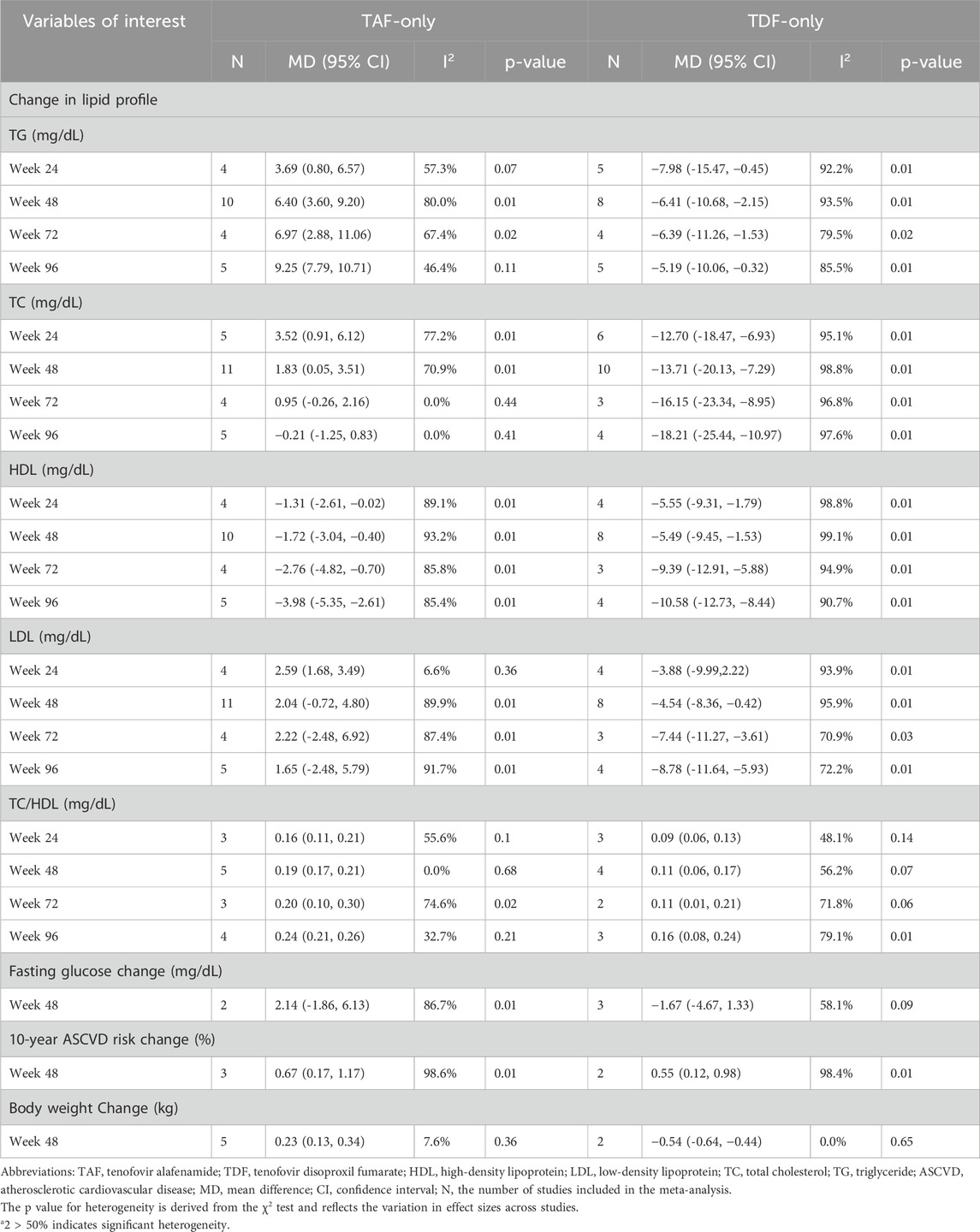
Table 2. The changes of metabolic profile and ASCVD risk during TAF-only or TDF-only treatment (vs. baseline).
In the TDF group, we observed a significant decrease in TG, TC, HDL and LDL levels throughout the entire follow-up period. At 48 weeks of treatment, the levels of TG, TC, HDL and LDL were −6.41 mg/dL (95% CI, −10.68 to −2.15), −13.71 mg/dL (95% CI, −20.13 to −7.29), −5.49 mg/dL (95% CI, −9.45 to −1.53), and −4.54 mg/dL (95% CI, −8.36 to −0.42), respectively (Table 2). Interestingly, similar to TAF, we also observed a slight increase in the TC/HDL ratio, which was +0.11 mg/dL (95% CI, 0.06–0.17) at 48 weeks. Importantly, this lipid-lowering profile of TDF remained consistent across study designs, with both RCTs and observational studies demonstrating concordant reductions in all lipid parameters (Supplementary Table S12).
Changes in body weight and blood glucose with TAF and TDF
In view of the impact of TDF and TAF on blood lipids in CHB patients, it is important to explore whether they may also lead to other metabolic changes. Current research mainly focuses on whether TAF and TDF influence changes in body weight and blood glucose. We conducted a meta-analysis of existing studies, and the results showed that after 48 weeks of TAF treatment, the change in body weight compared to baseline was 0.23 kg (95% CI, 0.13–0.34). In the TDF treatment cohort, body weight slightly decreased (mean difference −0.54, 95% CI -0.64 to −0.44).
However, in our study, the effects of TAF and TDF on blood glucose were not significant. At 48 weeks, the changes in blood glucose were 2.14 mg/dL (95% CI, −1.86–6.13) in the TAF treatment group and −1.67 mg/dL (95% CI, −4.67 to 1.33) in the TDF treatment group (Table 2).
Switch-TAF group
Given the current concerns regarding the safety of switching from TDF to TAF treatment, this study included a total of 9 studies involving 1,906 patients, aiming to analyze the changes in blood lipids and body weight after switching to TAF treatment.
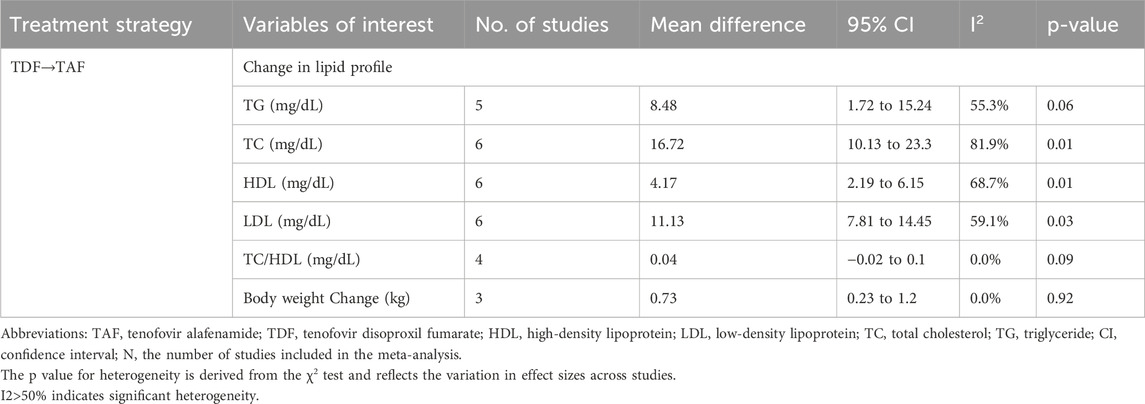
Compared to the baseline levels before switching, after 24 weeks of TAF treatment, serum TG, TC, HDL and LDL levels significantly increased, with changes of 8.48 mg/dL (95% CI, 1.72–15.24), 16.72 mg/dL (95% CI, 10.13–23.3), 4.17 mg/dL (95% CI, 2.19–6.15), and 11.13 mg/dL (95% CI, 7.81–14.45), respectively (Table 3). However, after switching to TAF treatment, the TC/HDL ratio did not show any increase (mean difference 0.04 mg/dL, 95% CI -0.02–0.1).
Regarding the changes in body weight, the results showed that after 24 weeks of switching to TAF treatment, body weight significantly increased compared to baseline (MD, 0.73; 95% CI, 0.23–1.2).
Changes in 10-year cardiovascular risk
To determine whether the impact of TAF and TDF on lipid profiles during long-term treatment of CHB patients could exacerbate the risk of cardiovascular diseases, we systematically reviewed the existing literature and identified 6 relevant studies. Of these, 3 studies including a total of 8,282 patients were included in a meta-analysis. After 48 weeks of treatment with TAF and TDF, the estimated 10-year ASCVD risk change for the TAF-only treatment group was 0.67 (95% CI, 0.17–1.17), while for the TDF-only treatment group, it was 0.55 (95% CI, 0.12–0.98). The results clearly indicate that both TAF and TDF treatments are associated with an increased risk of cardiovascular disease (Table 2).
Discussion
To our knowledge, the present meta-analysis is the first study that clearly addresses the impact of NA drugs on host metabolism and cardiovascular disease risk during CHB treatment. A total of 16 cohort studies and 3 randomized controlled trials (RCTs), involving 19,396 CHB patients, were evaluated. The results indicate that both TAF and TDF treatments can slightly increase the risk of ASCVD. Except for being associated with weight gain, TAF does not have a significant effect on other metabolic parameters. However, TDF treatment is associated with reductions in weight and lipid levels. Interestingly, the subgroup analysis of patients switched to TAF treatment revealed a marked increase in lipid levels.
Previous studies have shown that HBV infection, unlike HCV infection, is negatively correlated with lipid metabolism and may therefore have a protective effect on Mets, hepatic steatosis, and cardiovascular diseases (Wong et al., 2012; Wang et al., 2020). However, numerous studies have found that NA treatment have different effects on metabolic functions during long-term antiviral treatment. The impact of TAF on lipid profiles remains controversial. A cohort study found that TAF increased the levels of TC, TG and LDL, with no significant effect on HDL (Zhao et al., 2024). Similarly, in a recent RCT involving 1,632 participants, it was found that TAF treatment increased LDL, TG and TC/HDL levels while decreasing HDL levels, with no impact on TC (Fung et al., 2024). Consistent with these findings, our meta-analysis confirmed that TAF treatment lowers HDL levels without markedly influencing TC levels.
However, A previous meta-analysis showed that, compared with other antiviral drugs, TAF worsened lipid profiles after 6 months of treatment (Hwang et al., 2023). The reason why their findings regarding changes in Serum lipid levels, especially HDL, are inconsistent with ours may be that the number of studies included in the previous meta-analysis of each lipoprotein type was limited. A recent network meta-analysis reported no significant difference in lipid levels between TAF-treated and untreated CHB patients (Tong et al., 2024). This finding is consistent with our results regarding the impact of TAF on lipid levels. But our systematic review is updated and more comprehensive, providing more accurate evidence. We assessed changes in lipid levels over time and further explored the impact on risk of cardiovascular disease and other metabolic characteristics.
For patients with high cardiovascular (CV) risk, the 2016 ESC/EAS Guidelines recommend achieving either an LDL level <1.8 mmol/L (70 mg/dL) or a ≥50% reduction from baseline when baseline LDL ranges between 1.8 and 3.5 mmol/L (70–135 mg/dL) (Catapano et al., 2016). However, while multiple studies—including our meta-analysis—demonstrate statistically significant metabolic effects of TDF (e.g., LDL reduction: −8.78 mg/dL [95% CI: −11.64 to −5.93]; weight reduction: −0.54 kg [95% CI: −0.64 to −0.44]), these modest changes may be insufficient to achieve the ESC/EAS-recommended treatment targets for high-risk patients. Therefore, we conclude that TDF’s impact on metabolic parameters, though statistically significant, remains clinically marginal for high-CV-risk populations.
Metabolic disorders are the main characteristic of metabolic syndrome (Mets), which is a clustering of obesity, dysglycemia, dyslipidaemia (Esposito et al., 2012). Thus, our meta-analysis primarily concentrated on the changes in these metabolic parameters. As in previous studies (Shin et al., 2024; Cheng et al., 2024; Yeh et al., 2022; Byun et al., 2022), our results indicate that TAF significantly increases body weight, while weight decreases with TDF treatment. Weight management has gained increasing clinical attention. Although our meta-analysis demonstrated a weight-reducing effect of TDF, the observed magnitude of change failed to reach the ideal target range specified in the 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity (Jensen et al., 2014), the mechanisms underlying the differential effects of TDF and TAF on body weight remain an important area of investigation, particularly given their implications for cardiovascular disease risk. Hill A et al. demonstrated that TDF is associated with a 5% weight loss risk in HIV-negative individuals receiving pre-exposure prophylaxis (PrEP) (Shah et al., 2021; Bosch et al., 2023), which mechanistically parallels the weight reduction effect (Δ = −0.54 kg) observed in HBV-infected patients receiving TDF in our study. This bidirectional phenomenon may originate from two distinct mechanisms. First, gastrointestinal toxicity could play a role, as TDF may induce nausea and malabsorption in non-infected populations through its prodrug metabolism pathway. Second, regarding adipogenic regulation, TAF appears to enhance adipocyte differentiation via PPARγ pathway activation in HIV-infected states, while TDF may partially counteract this effect through PPARγ suppression (Dulion et al., 2025).
The differential effects of TAF and TDF on metabolic profiles and estimated ASCVD risk have attracted considerable attention. Hyunjae Shin et al. showed that both TAF and TDF increased the risk of cardiovascular disease during long-term CHB treatment, with TAF having a more significant effect (Shin et al., 2024). However, an RCT from Canada and a large cohort study from South Korea found that the 10-year ASCVD risk in CHB patients treated with TAF and TDF was low and similar, with no significant difference in cardiovascular events (Fung et al., 2024; Hong et al., 2023). Pin-Nan Cheng et al. also found that the 10-year ASCVD risk did not increase after switching from TDF to TAF treatment (Cheng et al., 2024). To address the current controversy, we included six studies, three of which were included in the meta-analysis. Due to the lack of comparative data between the TAF and TDF groups, we did not analyze the differences in their effects on ASCVD risk. Our meta-analysis indicates that both TAF and TDF mildly increase the 10-year ASCVD risk. But the change remains within acceptable limits.
High LDL levels and low HDL levels are considered independent risk factors for cardiovascular disease (Assmann and Gotto, 2004), Recent studies have reported that the TC:HDL ratio has greater predictive value than individual parameters (Gimeno-Orna et al., 2005; Calling et al., 2019), and an increased TC:HDL ratio is associated with a higher risk of cardiovascular disease (Manubolu et al., 2022). Although there is significant heterogeneity among the included studies, our results show that both TDF and TAF increase the TC/HDL ratio and decrease HDL levels, thereby supporting our conclusion that both TDF and TAF treatment may increase the ASCVD risk. The 2018 AHA/ACC Guideline on the Management of Blood Cholesterol classifies a 10-year ASCVD risk <5% as low-risk (Grundy et al., 2019). Based on our findings, we recommend regular monitoring of CVD risk factors (e.g., hypertension, dyslipidemia) in CHB patients during treatment to identify those at elevated CVD risk.
Our systematic review and meta-analysis also provide the latest evidence on the safety of the treatment regimen of switching from TDF to TAF, in our subanalyses, we found a sharp increase in blood lipid levels after the switch to TAF treatment. Although we did not perform a meta-analysis of cardiovascular disease risk in the subgroup analysis, current studies suggest that ASCVD risk does not change after switching to TAF treatment (Cheng et al., 2024). However, we found that LDL levels, which are considered a risk factor for cardiovascular disease (Assmann and Gotto, 2004), rise sharply after switching to TAF treatment. Therefore, for populations with CVD risk factors, such as smoking, obesity and dyslipidemia, switching to TAF treatment requires comprehensive evaluation and careful consideration.
Our study had several limitations. First, consistent with prior meta-analytic approaches, we incorporated both randomized controlled trials (RCTs) and non-randomized observational studies to comprehensively synthesize all available evidence. While this approach may introduce significant heterogeneity and potential publication bias across studies, we mitigated these limitations through four key methodological strategies: (1) application of a random-effects model to enhance the generalizability of inferences beyond the study populations, (2) pre-specified subgroup analyses stratified by study design (RCTs vs. observational studies) to explore sources of heterogeneity, (3) we conducted a rigorous methodological quality assessment of the included studies, and only cohort studies with a NOS score greater than 6 were included in this meta-analysis, and (4) sensitivity analyses to verify the robustness of pooled estimates. Second, although multiple algorithms exist for estimating 10-year cardiovascular risk, our meta-analysis specifically employed the Pooled Cohort Equations (PCE) model—derived from U.S. populations—to calculate ASCVD risk scores, as it currently represents the most validated tool, and all included studies adopted this method as their analytical endpoint. However, this approach may limit the generalizability of our findings to other ethnic groups or populations with substantially different demographic characteristics. Third, during the data collection phase, lipid and cholesterol data were presented using median and interquartile range in some studies due to the non-normal distribution of these variables. To avoid losing high-quality studies, we addressed this issue by extracting data using the sample mean and standard deviation estimation tools recommended by Luo et al. (2018). This may affect the precision of the results. Fourth, because of insufficient data, we were unable to perform the subgroup analysis as planned, and the effects of NA treatment on different genders and age groups have not been fully assessed. There is a strong need for a meta-analysis in the future when sufficient data become available.
In conclusion, long-term TAF treatment is associated with weight gain, but has no significant effect on blood lipids and blood glucose. In contrast, long-term TDF treatment is associated with lipid-lowering effects and weight loss. However, both TDF and TAF increase the TC/HDL ratio and decrease HDL levels, thereby mildly raising the ASCVD risk. Clinicians should consider the potential risks of metabolic disorders and CVD during long-term NA treatment, especially when switching to TAF therapy. Future research should include more high-quality RCTs to further validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Y-HZ: Conceptualization, Formal Analysis, Methodology, Writing – original draft. NC: Data curation, Software, Visualization, Writing – review and editing. Y-XC: Data curation, Software, Visualization, Writing – review and editing. Y-LS: Data curation, Software, Visualization, Writing – review and editing. PH: Funding acquisition, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded in part by grants from the Chongqing Talents Project (cstc2021ycjh-bgzxm0150), Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJZD-K202300404), the First batch of key Disciplines on Public Health in Chongqing of Health Commission of Chongqing, the Remarkable Innovation–Clinical Research Project of The Second Affiliated Hospital of Chongqing Medical University.
Acknowledgments
The authors are grateful to all research and clinic staff contributing to our work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1604972/full#supplementary-material
References
Agarwal, K., Brunetto, M., Seto, W. K., Lim, Y.-S., Fung, S., Marcellin, P., et al. (2018). 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol. 68, 672–681. doi:10.1016/j.jhep.2017.11.039
Akdemir Kalkan, I., Karasahin, O., Sarigul, F., Altunisik Toplu, S., Aladag, M., Akgul, F., et al. (2022). Comparison of tenofovir alafenamide and entecavir therapy in patients with chronic hepatitis B initially treated with tenofovir disoproxil: a retrospective observational survey. Hepat. Mon. 21, 21. doi:10.5812/hepatmon.118721
Anderson, R. T., Choi, H. S. J., Lenz, O., Peters, M. G., Janssen, H. L. A., Mishra, P., et al. (2021). Association between seroclearance of hepatitis B surface antigen and long-term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 19, 463–472. doi:10.1016/j.cgh.2020.05.041
Assmann, G., and Gotto, A. M. (2004). HDL cholesterol and protective factors in atherosclerosis. Circulation 109, III8–III14. doi:10.1161/01.CIR.0000131512.50667.46
Atar, D., Jukema, J. W., Molemans, B., Taub, P. R., Goto, S., Mach, F., et al. (2021). New cardiovascular prevention guidelines: how to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis 319, 51–61. doi:10.1016/j.atherosclerosis.2020.12.013
Bosch, B., Akpomiemie, G., Chandiwana, N., Sokhela, S., Hill, A., McCann, K., et al. (2023). Weight and metabolic changes after switching from tenofovir alafenamide/emtricitabine (FTC)+Dolutegravir (DTG), tenofovir disoproxil fumarate (TDF)/FTC + DTG, and TDF/FTC/Efavirenz to TDF/Lamivudine/DTG. Clin. Infect. Dis. 76, 1492–1495. doi:10.1093/cid/ciac949
Byun, K. S., Choi, J., Kim, J.-H., Lee, Y. S., Lee, H. C., Kim, Y. J., et al. (2022). Tenofovir alafenamide for drug-resistant hepatitis B: a randomized trial for switching from tenofovir disoproxil fumarate. Clin. Gastroenterol. Hepatol. 20, 427–437.e5. doi:10.1016/j.cgh.2021.04.045
Calling, S., Johansson, S.-E., Wolff, M., Sundquist, J., and Sundquist, K. (2019). The ratio of total cholesterol to high density lipoprotein cholesterol and myocardial infarction in Women’s health in the lund area (WHILA): a 17-year follow-up cohort study. BMC Cardiovasc Disord. 19, 239. doi:10.1186/s12872-019-1228-7
Catapano, A. L., Graham, I., De Backer, G., Wiklund, O., Chapman, M. J., Drexel, H., et al. (2016). 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 37, 2999–3058. doi:10.1093/eurheartj/ehw272
Chen, J. W., Cao, X. Y., Qi, X., and Zhang, J. M. (2022). Effect of nucleos(t)ide analogues on blood lipid profiles in patients with chronic hepatitis B: a cross-sectional survey. Med. Baltim. 101, e31980. doi:10.1097/MD.0000000000031980
Cheng, P., Feng, I., Chen, J., Kuo, H., Lee, P., Yu, M., et al. (2024). Body weight increase and metabolic derangements after tenofovir disoproxil fumarate switch to tenofovir alafenamide in patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 59, 230–238. doi:10.1111/apt.17765
Cochrane. (2025). Cochrane Handbook for Systematic reviews of interventions. Available online at: https://training.cochrane.org/handbook/current (Accessed May 20, 2025).
Cui, F., Blach, S., Manzengo Mingiedi, C., Gonzalez, M. A., Sabry Alaama, A., Mozalevskis, A., et al. (2023). Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol. Hepatol. 8, 332–342. doi:10.1016/S2468-1253(22)00386-7
Dulion, B., Olali, A. Z., Patel, N., Virdi, A. K., Naqib, A., Wallace, J., et al. (2025). Tenofovir alafenamide promotes weight gain and impairs fatty acid metabolism-related signaling pathways in visceral fat tissue compared to tenofovir disoproxil fumarate. Antivir. Res. 237, 106151. doi:10.1016/j.antiviral.2025.106151
Esposito, K., Chiodini, P., Colao, A., Lenzi, A., and Giugliano, D. (2012). Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35, 2402–2411. doi:10.2337/dc12-0336
Fung, S. K., Pan, C. Q., Wong, G. L., Seto, W., Ahn, S. H., Chen, C., et al. (2024). Atherosclerotic cardiovascular disease risk profile of patients with chronic hepatitis B treated with tenofovir alafenamide or tenofovir disoproxil fumarate for 96 weeks. Aliment. Pharmacol. Ther. 59, 217–229. doi:10.1111/apt.17764
Gimeno-Orna, J. A., Faure-Nogueras, E., and Sancho-Serrano, M. A. (2005). Usefulness of total cholesterol/HDL-cholesterol ratio in the management of diabetic dyslipidaemia. Diabet. Med. 22, 26–31. doi:10.1111/j.1464-5491.2004.01341.x
Goff, D. C., Lloyd-Jones, D. M., Bennett, G., Coady, S., D’Agostino, R. B., Gibbons, R., et al. (2014). 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/american heart association task force on practice guidelines. Circulation 129, S49–S73. doi:10.1161/01.cir.0000437741.48606.98
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., et al. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. Circulation 139, e1082–e1143. doi:10.1161/CIR.0000000000000625
Guner, R., Yoruk, G., Karabay, O., Yamazhan, T., Baykam, N., Yildirim, M., et al. (2022). Real life efficacy, renal and lipid profile data of tenofovir alafenamide in switched chronic hepatitis B patients. J. Hepatol. 77, S858–S859. doi:10.1016/S0168-8278(22)02013-X
Hamers, R. L., Zaaijer, H. L., Wallis, C. L., Siwale, M., Ive, P., Botes, M. E., et al. (2013). HIV–HBV coinfection in Southern Africa and the effect of Lamivudine- Versus tenofovir-containing cART on HBV outcomes. JAIDS J. Acquir Immune Defic. Syndr. 64, 174–182. doi:10.1097/QAI.0b013e3182a60f7d
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hong, H., Choi, W.-M., Lee, D., Shim, J. H., Kim, K. M., Lim, Y.-S., et al. (2023). Cardiovascular risk in chronic hepatitis B patients treated with tenofovir disoproxil fumarate or tenofovir alafenamide. Clin. Mol. Hepatol. 30, 49–63. doi:10.3350/cmh.2023.0328
Huhn, G. D., Shamblaw, D. J., Baril, J.-G., Hsue, P. Y., Mills, B. L., Nguyen-Cleary, T., et al. (2020). Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide Versus tenofovir disoproxil fumarate. Open Forum Infect. Dis. 7, ofz472. doi:10.1093/ofid/ofz472
Hwang, E. G., Jung, E.-A., Yoo, J.-J., Kim, S. G., and Kim, Y. S. (2023). Risk of dyslipidemia in chronic hepatitis B patients taking tenofovir alafenamide: a systematic review and meta-analysis. Hepatol. Int. 17, 860–869. doi:10.1007/s12072-023-10528-7
Jensen, M. D., Ryan, D. H., Apovian, C. M., Ard, J. D., Comuzzie, A. G., Donato, K. A., et al. (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American college of cardiology/american heart association task force on practice guidelines and the obesity society. Circulation 129, S102–S138. doi:10.1161/01.cir.0000437739.71477.ee
Jeong, J., Shin, J. W., Jung, S. W., Park, E. J., and Park, N. H. (2022). Tenofovir alafenamide treatment may not worsen the lipid profile of chronic hepatitis B patients: a propensity score-matched analysis. Clin. Mol. Hepatol. 28, 254–264. doi:10.3350/cmh.2021.0314
Karasahin, O., Kalkan, I. A., Dal, T., Toplu, S. A., Harputluoglu, M., Mete, A. O., et al. (2023). First year real life experience with tenofovir alafenamide fumarate: the pythagorean cohort. Hepatol. Forum 4, 61–68. doi:10.14744/hf.2022.2022.0043
Kauppinen, K. J., Kivelä, P., and Sutinen, J. (2019). Switching from tenofovir disoproxil fumarate to tenofovir alafenamide significantly worsens the lipid profile in a real-world setting. AIDS Patient Care STDs 33, 500–506. doi:10.1089/apc.2019.0236
Lai, R.-M., Lin, S., Wang, M.-M., Li, N., Zhou, J.-H., Lin, X.-Y., et al. (2023). Tenofovir alafenamide significantly increased serum lipid levels compared with entecavir therapy in chronic hepatitis B virus patients. World J. Hepatol. 15, 964–972. doi:10.4254/wjh.v15.i8.964
Lim, J., Choi, W., Shim, J. H., Lee, D., Kim, K. M., Lim, Y., et al. (2022). Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in treatment-naïve chronic hepatitis B. Liver Int. 42, 1517–1527. doi:10.1111/liv.15261
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27, 1785–1805. doi:10.1177/0962280216669183
Manubolu, V. S., Verghese, D., Lakshmanan, S., Alalawi, L., Kinninger, A., Bitar, J. A., et al. (2022). Coronary computed tomography angiography evaluation of plaque morphology and its relationship to HDL and total cholesterol to HDL ratio. J. Clin. Lipidol. 16, 715–724. doi:10.1016/j.jacl.2022.06.003
Ogawa, E., Nakamuta, M., Koyanagi, T., Ooho, A., Furusyo, N., Kajiwara, E., et al. (2022). Switching to tenofovir alafenamide for nucleos(t)ide analogue-experienced patients with chronic hepatitis B: week 144 results from a real-world, multi-centre cohort study. Aliment. Pharmacol. Ther. 56, 713–722. doi:10.1111/apt.17107
Ohri. (2025). The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 20, 2025).
Santos, J. R., Saumoy, M., Curran, A., Bravo, I., Llibre, J. M., Navarro, J., et al. (2015). The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin. Infect. Dis. 61, 403–408. doi:10.1093/cid/civ296
Shah, S., Pilkington, V., and Hill, A. (2021). Is tenofovir disoproxil fumarate associated with weight loss? AIDS 35, S189–S195. doi:10.1097/QAD.0000000000003083
Sheena, B. S., Hiebert, L., Han, H., Ippolito, H., Abbasi-Kangevari, M., Abbasi-Kangevari, Z., et al. (2022). Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol. Hepatol. 7, 796–829. doi:10.1016/S2468-1253(22)00124-8
Shin, H., Lim, G. S., Yoon, J. W., Ko, Y., Park, Y., Park, J., et al. (2024). Metabolic effects and cardiovascular disease risks of antiviral treatments in patients with chronic hepatitis B. J. Med. Virol. 96, e29760. doi:10.1002/jmv.29760
Suzuki, K., Suda, G., Yamamoto, Y., Abiko, S., Kinoshita, K., Miyamoto, S., et al. (2022). Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on lipid profiles in patients with hepatitis B. PLOS ONE 17, e0261760. doi:10.1371/journal.pone.0261760
Terrault, N. A., Bzowej, N. H., Chang, K., Hwang, J. P., Jonas, M. M., Murad, M. H., et al. (2016). AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63, 261–283. doi:10.1002/hep.28156
The Korean Association for the Study of the Liver (KASL) (2022). KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 28, 276–331. doi:10.3350/cmh.2022.0084
Tong, K., Chen, M., Wang, D., Dai, H., Peng, J., Zhang, J., et al. (2024). Effects of first-line nucleot(s)ide analogues on lipid profiles in patients with chronic hepatitis B: a network meta-analysis. Eur. J. Clin. Pharmacol. 80, 335–354. doi:10.1007/s00228-023-03616-y
Wang, C., Cheng, P., and Kao, J. (2020). Systematic review: chronic viral hepatitis and metabolic derangement. Aliment. Pharmacol. Ther. 51, 216–230. doi:10.1111/apt.15575
Wei, L., and Kao, J.-H. (2017). Benefits of long-term therapy with nucleos(t)ide analogues in treatment-naïve patients with chronic hepatitis B. Curr. Med. Res. Opin. 33, 495–504. doi:10.1080/03007995.2016.1264932
Wong, V. W.-S., Wong, G. L.-H., Chu, W. C.-W., Chim, A. M.-L., Ong, A., Yeung, D. K.-W., et al. (2012). Hepatitis B virus infection and fatty liver in the general population. J. Hepatol. 56, 533–540. doi:10.1016/j.jhep.2011.09.013
Yeh, M.-L., Liang, P.-C., Trinh, S., Huang, C.-I., Huang, C.-F., Hsieh, M.-Y., et al. (2022). Body weight changes in treated hepatitis B patients switching to tenofovir alafenamide. J. Formos. Med. Assoc. 121:1273–1282. doi:10.1016/j.jfma.2021.09.009
Zhang, Q., Liang, J., Yin, J., Jiang, Y., Yu, N., Liao, X., et al. (2022). Real-life impact of tenofovir disoproxil fumarate and entecavir therapy on lipid profile, glucose, and uric acid in chronic hepatitis B patients. J. Med. Virol. 94, 5465–5474. doi:10.1002/jmv.27977
Zhang, Y., Li, Z., Luo, Q., Xu, W., Wang, L., Zhu, S., et al. (2022). Changes in blood lipids in patients with chronic hepatitis B after 48 weeks of tenofovir alafenamide treatment: a prospective real-world clinical study. Antivir. Ther. 27, 13596535221082399. doi:10.1177/13596535221082399
Keywords: dyslipidemia, metabolic profile, cardiovascular risk, tenofovir, drug safety, chronic hepatitis B
Citation: Zhou Y-H, Cai N, Chen Y-X, Su Y-L and Hu P (2025) Metabolic effects and cardiovascular disease risks of TDF or TAF in patients with chronic hepatitis B: a systematic review and meta-analysis. Front. Pharmacol. 16:1604972. doi: 10.3389/fphar.2025.1604972
Received: 02 April 2025; Accepted: 17 June 2025;
Published: 30 June 2025.
Edited by:
Nicola Squillace, IRCCS San Gerardo dei Tintori Foundation, ItalyReviewed by:
Diego Ripamonti, Papa Giovanni XXIII Hospital, ItalyAysun Yakut, Istanbul Medipol University, Türkiye
Copyright © 2025 Zhou, Cai, Chen, Su and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Hu, aHBfY3FAMTYzLmNvbQ==
 Yuan-Hai Zhou
Yuan-Hai Zhou Nan Cai2,3
Nan Cai2,3 Peng Hu
Peng Hu