- 1Department of Biomedical and Biotechnological Sciences, Section of Pharmacology, University of Catania, Catania, Italy
- 2Clinical Toxicology Unit, University Hospital of Catania, Catania, Italy
- 3Department of Biomedical Sciences, Section of Neuroscience and Clinical Pharmacology, University of Cagliari, Cagliari, Italy
- 4Department of Biochemical Engineering, University College London, London, United Kingdom
- 5Unit of Infectious Diseases, Department of Clinical and Experimental Medicine, University of Catania, ARNAS Garibaldi Hospital of Catania, Catania, Italy
- 6Center of Excellence for the Acceleration of HArm Reduction (CoEHAR), University of Catania, Catania, Italy
- 7Unitá Operativa Complessa (UOC) Medicina e Chirurgia d'Accettazione e Urgenza (MCAU), University Teaching Hospital “Policlinico-Vittorio Emanuele”, University of Catania, Catania, Italy
- 8Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
- 9Department of Preventive and Community Medicine, University of The East Ramon Magsaysay Memorial Medical Centre, Quezon City, Philippines
- 10Pulmonology Department, Jinnah Hospital, Lahore, Pakistan
- 11Department of Pulmonology, Allama Iqbal Medical College, Lahore, Pakistan
- 12Departemen Pulmonologi dan Kedokteran Respirasi, Dr. H.A. Rotinsulu Lung Hospital, Bandung, Indonesia
- 13Department of Pulmonary Medicine, All India Institute of Medical Sciences, New Delhi, India
- 14Unit of Infectious Diseases, Department of Medicine and Surgery, “Kore” University of Enna, Enna, Italy
The World Health Organization (WHO) ranks tuberculosis (TB) as one of the top 10 causes of deaths worldwide. Notably, tobacco smoking represents a significant promoting factor in TB progression, being associated with poorer treatment outcomes, delayed conversion to negative smear or culture, and higher dropout rates from treatment plans. Remarkably, high rates of smoking and TB frequently overlaps in the same countries, warranting the need for targeted public health interventions. Prioritising smoking cessation is essential for smokers with TB, as sustained abstinence has been associated with reduced mortality and a more successful cure. This review examines the intricate relationship between cigarette smoking, smoking cessation therapies and anti-TB drugs, focusing on the impact of tobacco smoking compounds on liver detoxifying systems, such as influence of polycyclic aromatic hydrocarbons (PAHs) on hepatic cytochrome P450 (CYP450) enzymes mostly, and on metabolism of antituberculous medications. Integrating smoking cessation and TB treatment programmes must also take into account potential drug-drug interactions between smoking cessation medications and anti-TB drugs, a critical area for patient safety and effective TB management. This review article aims to provide healthcare professionals with the knowledge to better support TB patients who smoke or are intending to quit, to ensure tailored and effective treatment strategies, while highlighting gaps in current research and advocating for further studies to fill these gaps.
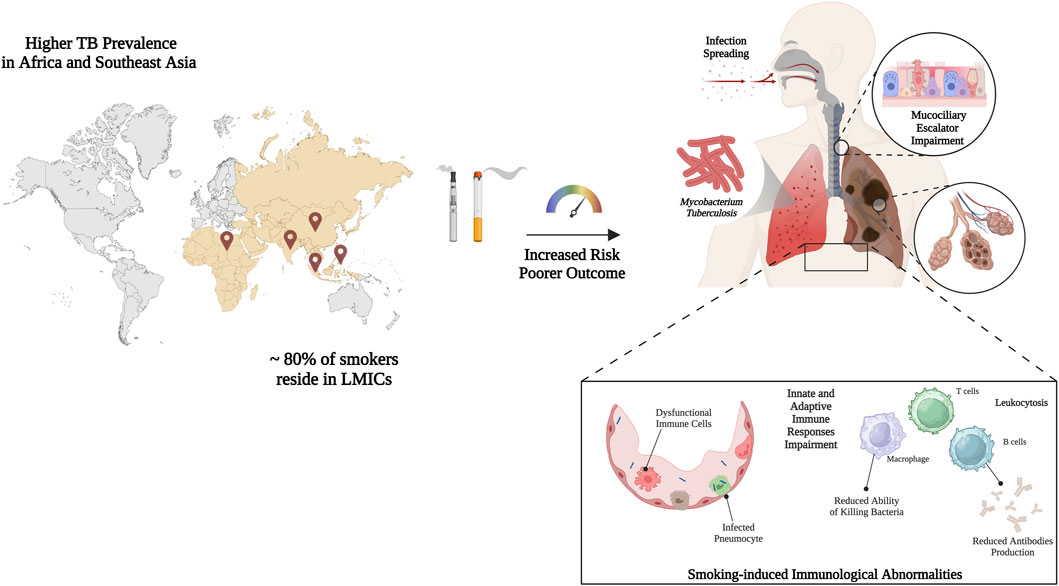
GRAPHICAL ABSTRACT | Graphic representation of the overlap between smoking and tuberculosis prevalence worldwide, especially in low-middle income countries (LMICs), which highlights the existing relationship and the impact of smoking habit in worsening disease outcome. Smoking increases infection odds and accelerates disease course altering immunological parameters and escalating histopathological damages. More specifically, it slows mucociliary clearance, injures the epithelial barrier, and depletes surfactant proteins, facilitating bacillary entry into the alveoli. Created with BioRender.com; accessed on 12 January 2025.
1 Introduction
Tuberculosis (TB) is a preventable, treatable, and curable infectious disease that primarily affects the lungs. Despite advances in medical treatment, TB remains a significant global health challenge (Bloom et al., 2017). In 2022, an estimated 10.6 million people developed TB, with a high prevalence in South-East Asia (notably India, Indonesia, China, the Philippines, Pakistan, and Bangladesh) and Africa (including Nigeria and the Democratic Republic of the Congo). Additionally, approximately 1.3 million people were estimated to have died from TB that same year. Thus, the World Health Organization (WHO) ranks TB as a leading killer infectious disease and one of the top 10 causes of deaths worldwide (Global Tuberculosis Report, 2023).
In Europe, TB incidence has generally decreased over the past decade, with the European Centre for Disease Prevention and Control (ECDC) reporting an average rate of around eight cases per 100,000 people in the European Union (EU)/European Economic Area (EEA) in 2022, with a total of 36,179 TB cases. Eastern European countries such as Romania and Lithuania still face higher TB rates, contributing to the continent’s overall case burden. In Italy, the incidence of TB is relatively low compared to global rates, with around 4.1 cases per 100,000 people in 2022, with 2,439 new cases. However, certain high-risk populations, including recent immigrants, individuals with HIV, and vulnerable urban populations, still experience a notable risk of TB (TB Incidence, 2024).
The susceptibility of these groups is multifactorial. Immigrants often arrive from countries with high TB endemicity and may experience reactivation of latent TB due to stress, malnutrition, or crowded living conditions in the host country (Hayward et al., 2018; Pareek et al., 2016). People living with HIV (PLHIV) are particularly susceptible because of a compromised immune system, as the depletion of CD4+ T-cells critically impairs the body’s ability to control Mycobacterium tuberculosis (Mtb) infection, leading to a significantly higher risk of progression from latent to active disease (Getahun et al., 2010). The WHO estimates that PLHIV are 18 times more likely to develop active TB than HIV-negative individuals (Tuberculosis and HIV, 2025; TB Preventive Treatment, 2025). Vulnerable urban populations, including homeless and those residing in low-income settings, are exposed to a potent mix of biological insults due to factors like poor ventilation in shelters, household crowding, and limited access to healthcare, all of which facilitate disease transmission. A systematic review and meta-analysis investigating the risk of TB among populations living in slum settings, reported an odds ratio of 2.96 (2.84–3.09) for smear-positive TB among dwellers compared with national averages (Noykhovich, Mookherji, and Roess, 2019).
Taken together these data show that each population’s elevated TB burden results from an interplay of biological susceptibility and adverse social conditions. Effective TB control must therefore couple biomedical interventions–systematic Latent Tuberculosis Infection (LTBI) screening of recent migrants, integrated TB–HIV services, smoke-free housing initiatives–with policies that tackle the underlying social determinants of health, such as overcrowding, insecure employment, and inadequate access to care (Zumla et al., 2025; Feldman et al., 2024; Lönnroth et al., 2009).
One significant factor promoting TB disease progression is smoking (Amere et al., 2018). Currently, 1.3 billion people worldwide use tobacco, primarily through smoking, which leads to over seven million deaths annually from smoking-related illnesses (Tobacco Collaborators, 2017 Tobacco Collaborators, 2017; Tobacco Collaborators, 2021 Tobacco Collaborators, 2021; WHO Report on the Global Tobacco Epidemic, 2023).Tobacco smoking is a major risk factor noy only for lung cancer, chronic obstructive pulmonary disease (COPD), and cardiovascular diseases, but also significantly contributes to the global burden of TB, particularly in low- and middle-income countries (LMICs) (Gajalakshmi et al., 2003; Song et al., 2024; Dana et al., 2024; Riccardi et al., 2024; Pampaloni et al., 2021).
Remarkably, high rates of smoking and TB frequently overlaps in the same countries (Global Tuberculosis Report, 2023; WHO Report on the Global Tobacco Epidemic, 2023; TB Disease Burden, 2024; WHO Global, 2025). For instance, countries with a high TB burden such as India, Indonesia, China, the Philippines, and Bangladesh also report high smoking prevalence. This overlap is not coincidental but is rooted in shared socioeconomic determinants. Factors such as poverty, crowded living conditions, limited access to education and healthcare, and malnutrition create a fertile ground for both TB transmission and the adoption of smoking behaviours. Studies have shown a direct correlation between smoking prevalence and TB incidence at a population level, suggesting that tobacco use acts as a significant driver of the TB epidemic in these regions, contributing to increased transmission, disease progression, and mortality (WHO Global, 2024; Jha et al., 2008).
These data suggest that the geographic overlap of high TB burden and intense tobacco use is more than accidental. Indeed, tobacco smoke is likely to amplify TB risk through both direct biological effects (immune suppression, altered drug metabolism) and shared socio-economic determinants.
Approximately 80% of the world’s smokers live in LMICs, where the majority of TB deaths occur. It is estimated that 20% of TB cases are associated with smoking (National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health, 2014).
This co-occurrence presents significant public health challenges, as tobacco use is detrimental to TB. There is sufficient evidence to infer a causal relationship between smoking and increased risk of TB disease, recurrent disease, and mortality (National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health, 2014). Smokers have a TB disease risk approximately twice that of their non-smoking counterparts. It is also possible that smoking impacts the progression to active disease. A recent meta-analysis has shown that smoking is associated with poorer treatment outcomes, delayed conversion to negative smear or culture, and higher dropout rates from treatment plans, ultimately leading to a lower likelihood of treatment success (E. Y. Wang et al., 2020).
Stopping smoking may have significant benefits for individuals with TB. Indeed, successful abstinence from smoking in TB care has been shown to lead to better treatment outcomes for TB patients, including a substantially lower rate of treatment default and failure, better sputum conversion rate, and improved radiological findings (Awaisu et al., 2011). Therefore, prioritizing smoking cessation is vital for smokers with TB. The WHO underscores the significance of smoking cessation for patients with TB (A Guide for Tuberculosis Patients to Quit Smoking, 2025).
When discussing smoking cessation in individuals with TB, it is crucial to acknowledge that the substances in tobacco smoke can alter various liver enzyme detoxification pathways. These alterations can significantly impact the pharmacokinetics of certain antituberculous medications. On the other hand, smoking cessation can lead to a gradual normalization of these metabolic pathways, similarly affecting the pharmacokinetics of drugs. Consequently, during the quitting process, it may be necessary to adjust or lower the dosages of some medications to ensure both effective and safer treatment.
This review article aims to explore the interrelationship between smoking, the process of smoking cessation, and the treatment of TB. An evaluation of the impact of smoking on the effectiveness and metabolism of antituberculosis medications is also conducted. Furthermore, it delves into the potential interactions between pharmaceutical agents employed in smoking cessation programmes and antitubercular treatments. By highlighting these interactions, this review could offer valuable insights for healthcare providers to enhance treatment approaches for TB patients who smoke or have recently stopped, and for researchers to identify potential areas for further study.
2 Methods
A comprehensive review of the literature was conducted from July 2024 to October 2024, encompassing a wide range of research methodologies, including observational studies, randomised and non-randomised clinical trials, experimental studies, case reports, and case series. The primary objective of this review was to identify studies that offered valuable insights into the potential metabolic interactions between smoking, smoking cessation, and smoking cessation medications, with a focus on their impact on the pharmacokinetics and pharmacodynamics of antituberculous drugs. The search was performed in both MEDLINE (PubMed) and EMBASE. Customised search strings were created and employed to locate relevant studies, the details of which are provided in Supplementary Material. Additional specialized databases like DrugBank.com, Knox et al. (2024), PharmGKB, (2024), along with the Summary of Product Characteristics (SmPC), were also examined for each medication. The titles and abstracts of these articles were then reviewed by CMB, SPP, EA and AM to identify studies for a comprehensive evaluation. Studies that appeared suitable for inclusion or could not be definitively excluded based solely on the title and abstract were advanced for further assessment. The Zotero software (Zotero 6.0.30; Roy Rosenzweig Centre for History and New Media (RRCHNM); George Mason University) was employed for the management of records and the elimination of duplicates. Following this, the remaining full-text articles were independently evaluated by other authors, with any disagreements resolved through discussion and consensus. Articles that met the established inclusion criteria were selected for the qualitative synthesis. Additionally, the references cited in the included articles and review papers were further examined to discover any potentially relevant studies. A standardized data extraction form specifically created for this purpose was utilised to gather pertinent data. The extracted details, such as authors, study population, study design, definition of smoking status, median follow-up time and outcomes, were systematically documented and organised in a table. The qualitative synthesis focused on the clinical significance of smoking cessation in relation to TB-related complications.
This review consolidates the evidence on the metabolic and clinical relationships between smoking and TB. It highlights how smoking affects both disease progression and the pharmacokinetics of treatments. The paucity of data on specific enzyme pathways and differences in study populations may affect the applicability of the findings. In addition, the review focuses primarily on pharmacological issues, with less emphasis on behavioural and socioeconomic elements.
3 Impact of tobacco smoke on enzymatic drug metabolism
Tobacco smoke is a complex mixture of polycyclic aromatic hydrocarbons (PAHs), ammonia, aromatic amines, phenols, carbonyls, hydrocyanic acid, and N-nitrosamines (Hoffmann and Hoffmann, 1997). Numerous of these chemicals interact with the enzymes involved in xenobiotic metabolism and various transporters, thus affecting the biotransformation of substances. Therefore, cigarette smoking, as well as quitting, may impact drug metabolism (Maideen, 2019).
Particularly, PAHs formed during incomplete tobacco combustion influence the hepatic cytochrome P450 (CYP450) enzyme system, especially by induction of isoenzymes CYP1A1, 1A2, 1B1, and 2E1, leading to accelerated drug clearance, potentially lowering blood concentrations. As a consequence, therapeutic monitoring and eventually dose adjustment would be required (Stepanov et al., 2010; McDonnell and Dang, 2013; Meech and Mackenzie, 1997). Moreover, mounting evidence points to the possibility that enzyme function may undergo alterations, potentially attributable to epigenetic processes, which could in turn result in a sustained increase in metabolic rate even after the cessation of smoking (Hirota et al., 2008; O’Malley et al., 2014).
Among the isoenzymes, the induction of CYP1A2 is of clinical importance because many drugs are its substrates. A study found a 1.66-fold increase in CYP1A2 activity in smokers of 11–20 cigarettes per day, which is reversed after smoking cessation (Tantcheva-Poór et al., 1999). Within 4 days of quitting, caffeine clearance, a measure of CYP1A2 activity, fell by 36% (Faber and Uwe, 2004). For former heavy smokers taking CYP1A2-metabolized drugs, dose adjustments after cessation are essential to avoid elevated drug levels and adverse drug reactions (ADRs). CYP1A1 metabolic activity has also been shown to be increased by approximately 66%–70% in smokers, mainly due to exposure to aryl hydrocarbons such as benzo [a]pyrene (Vistisen, Loft, and Poulsen, 1991). It also appeared that tobacco use affects the quantitative mRNA expression of CYP1B1, the induction of which may be influenced by genetic polymorphisms (Helmig et al., 2010).
The enhanced CYP2E1 expression and activity (Villard et al., 1998; Seree et al., 1996) in smokers has been demonstrated by accelerated metabolism of substances such as chlorzoxazone of about 24% (Benowitz et al., 2003).
Conversely, the impact of PAHs on uridine diphosphate (UDP)-glucuronosyltransferases (UGTs) appears to be complex and unclear, with a paucity of research in this area. However, given the inconsistency and heterogeneity of findings, it can be inferred that the effects may vary between different UGT isoforms (Collier et al., 2002; Court, 2010; Dragacci et al., 1987).
Nicotine, a well-known component of cigarette smoke, also alter metabolic pathways particularly by interfering with Organic Cation Transporters (OCTs) in vitro (Bergen et al., 2014; Urakami et al., 1998; Lips et al., 2005). Its impact on CYP2E1, CYP2A1/2A2, and CYP2B1/2B2 has been demonstrated within the central nervous system (Anandatheerthavarada et al., 1993a; Anandatheerthavarada et al., 1993b), though the clinical significance remains unclear (Zevin and Benowitz, 1999). While pharmacokinetics interactions are mainly attributable to PAHs, nicotine does alter and possibly negate drug effects by activating the sympathetic nervous system (N. L. Benowitz, 1997). Additionally, it has been reported that nicotine metabolism is impaired and even reduced by tobacco smoke through the inhibition of CYP2A6, which is mainly responsible for the conversion to cotinine (Lee et al., 1987; Benowitz and Jacob, 1993; 2000). Alongside PAHs and nicotine, other smoke constituents like acetone and carbon monoxide (CO) may affect hepatic enzymes but are considered less impactful (Zevin and Benowitz, 1999; Bellanca et al., 2024).
Beyond the impact on drug-metabolising enzymes, components of tobacco smoke exert various effects at cellular and subcellular level. Many studies have examined the influence of individual components, such as nicotine, aryl hydrocarbon, acetylcholine, and acrolein but the biological consequences stem from prolonged exposure to all components combined.
Cigarette smoke profoundly undermines the host’s capacity to contain Mtb at every stage of infection. In the airways it slows mucociliary clearance, injures the epithelial barrier, and depletes surfactant proteins, facilitating bacillary entry into the alveoli (Tilley et al., 2015; Moré et al., 2010; Honda et al., 1996; Corleis et al., 2023). Once there, smoke-expanded pools of alveolar macrophages are paradoxically less effective: phagocytosis, autophagy, antigen presentation, and production of key cytokines such as tumor necrosis factor-α (TNF-α), interleukin-12 (IL-12), and interferon-γ (IFN-γ) are all attenuated, while a shift toward anti-inflammatory M2 phenotypes and smoke-induced metabolic exhaustion enable intracellular survival of the pathogen (Leemans et al., 2005; Hodge et al., 2007; O’Leary et al., 2014; Chen et al., 2007; Smit et al., 2014).
The innate immune response is further compromised by an influx of hypo-functional neutrophils. Defective efferocytosis leaves bacteria-laden dying cells to rupture, thereby amplifying tissue damage and bacillary spread (Dallenga et al., 2017; Kirkham et al., 2004). Down-stream, dendritic cells exposed to smoke migrate poorly to lymph nodes and bias T-cell priming away from protective Th1 immunity, favouring Th2, Th17, and regulatory T-cell profiles marked by exhaustion receptors such as PD-1 and CTLA-4 (Feng et al., 2011; McNab et al., 2011; Quan et al., 2022).
Emerging evidence indicates that smoking induces gut dysbiosis, altering the composition and function of intestinal bacteria. Since the microbiome contributes to systemic immunity and can metabolise certain drugs, this may represent an indirect mechanism affecting both host susceptibility to TB and the bioavailability of orally administered anti-TB agents (Fan et al., 2023; Leite et al., 2022; Belkaid and Hand, 2014; Zhao et al., 2023).
At a subcellular level, toxins in cigarette smoke, such as cadmium and acrolein, are known to induce mitochondrial dysfunction and increase oxidative stress. This can impair cellular energy production and trigger apoptotic pathways in immune cells, further weakening the host response. Moreover, the added oxidative burden could potentially exacerbate drug-induced toxicities (Zhang et al., 2024; Wang et al., 2017; Aridgides et al., 2019; Lugg et al., 2022).
4 Effects of tobacco smoke on metabolism of antituberculous medications
Therapeutic options for TB encompass both first- and second-line drugs. The current standard for drug-sensitive TB (DS-TB) is a 6-month regimen comprising isoniazid (INH), rifampin (also known as rifampicin) (RIF), pyrazinamide (PZA), and ethambutol (EMB), starting with a 2-month intensive phase of all four drugs, followed by 4 months of INH and RIF (Alsayed and Gunosewoyo, 2023). However, as a result of the rise in antibiotic resistance, second-line drugs are now essential (Drug-Resistant Tuberculosis, 2024). Drug-resistant TB (DR-TB) is defined by resistance to at least one of the first-line drug, while multidrug-resistant TB (MDR-TB) implies resistance to both RIF and INH, and extensively drug-resistant TB (XDR-TB) refers to MDR-TB strains that are resistant to fluoroquinolones (FQ) and at least one aminoglycoside (Johnson et al., 2024). Recent advances, primarily based on three landmark clinical studies, namely, Nix-TB, ZeNix, and TB PRACTECAL (Conradie et al., 2020; 2022; Nyang’wa et al., 2022), led to the introduction of 6–9 months regimens of bedaquiline, pretomanid, linezolid, with or without moxifloxacin (BPaL-M or BPaL, respectively), offering improved outcomes compared to older treatment strategies (Johnson et al., 2024).
On this ground the WHO updated its DR-TB treatment guidelines in 2022 (WHO Consolidated Guidelines on Tuberculosis, 2024), recommending the 6-month BPaL-M regimen for MDR/Rifampicin-Resistant (RR)-TB in patients unexposed to bedaquiline, pretomanid, or linezolid (Johnson et al., 2024). For patients diagnosed with FQ-susceptible strains of Mtb, a 9-month all-oral regimen is advised, while those with FQ resistance may receive BPaL without moxifloxacin. Longer regimens remain viable for patients with additional resistance, intolerance to short-course drugs, severe disease, pregnancy, certain extrapulmonary TB cases, or other complex needs, with alternative agents recommended as needed (i.e., streptomycin, levofloxacin, clofazimine, and delamanid) (Vanino et al., 2023).
There is compelling evidence of an association between TB and smoking habits, in terms of increased risk of poorer outcome and defaulting on antituberculosis treatment (Bates et al., 2007; Batista et al., 2008). Furthermore, there is evidence that tobacco-induced immunological changes are reversible 6 months after smoking cessation (Arcavi and Benowitz, 2004; Miller et al., 1982; Hughes et al., 1985; Sopori, 2002). Therefore, integrating treatment regimens to eradicate Mtb infection and smoking cessation therapies appears to be an effective strategy to act on both fronts and ultimately lead to improved health and reduced healthcare burden (Aryanpur et al., 2016; Novotny, 2008; Schneider and Thomas, 2007). However, one of the major problems is the plausible occurrence of drug-drug interactions (DDIs), as both pathological conditions require long-term medical therapies. Indeed, the shortest treatment regimens for TB last at least 6 months, making the occurrence of DDIs not only likely but probable. Further widening the population at risk of DDIs is the need to provide TB preventive treatment (TPT) to all those at high risk of contracting the disease. Indeed, regimens based on RIF, INH or levofloxacin are recommended for HIV positive patients, cohabitants of affected persons, healthcare workers, and other categories of people engaged in high TB prevalence environments (WHO Consolidated Guidelines on Tuberculosis Module 1, 2024; Tanoglu et al., 2023).
As previously discussed, tobacco smoke is a mixture of substances exhibiting varying abilities to induce or inhibit the activity of hepatic detoxifying enzymes, as well as other transporters involved in the elimination of xenobiotics. In addition, the reversible nature of enzyme induction after smoking cessation emphasizes the need for careful management and dose adjustments of medications in quitters to ensure optimal pharmacotherapy. A further complicating factor is the extensive range of chemotherapeutic antibiotics that are available for the treatment of TB, each of which possesses its own distinctive pharmacokinetic and pharmacodynamic characteristics. It is therefore crucial to ascertain whether the patient is a smoker, bearing in mind the substantial degree of overlap between the two conditions, and whether they are undergoing smoking cessation therapy or intending to start it while simultaneously affected by TB. In this context, it is of utmost importance to analyse the metabolism of individual molecules with a view to establishing a highly personalised therapy.
INH is a prodrug that enters the Mtb cytoplasm via passive diffusion, where it inhibits mycolic acid synthesis by targeting the InhA enzyme, an essential catalyst in an early step of the mycolic acid biosynthetic pathway (Bardou et al., 1998; Mitchison, 1956). INH initially exerts a bacteriostatic effect for the first 24 h of treatment, followed by a bactericidal activity against proliferating Mtb at therapeutic levels (Unissa et al., 2016).
INH is mainly metabolized in the liver through two major pathways to metabolites such as acetyl isoniazid (AcINH), hydrazine (Hz), acetyl hydrazine (AcHz), diacetyl hydrazine (DiAcHz), and isonicotinic acid (INA), primarily mediated by arylamine N-acetyl transferase2 (NAT2) and amidases (Wang et al., 2016; Ellard and Gammon, 1976; McKENNIS et al., 1959). Further oxidation of Hz and AcHz by CYP450 enzymes, particularly CYP2E1, generates reactive intermediates that form covalent adducts with endogenous macromolecules, a process implicated in INH-induced hepatotoxicity (Delaney and Timbrell, 1995; Yue et al., 2004; Metushi and Jack, 2014).
The rifamycins are a family of antibiotics including rifampin, rifabutin, and rifapentine, which bind to the ß-subunit of bacterial DNA-dependent RNA polymerase (RNAP), inhibiting RNA synthesis and causing Mtb death (Abulfathi et al., 2019). Rifamycins are well-absorbed orally, with RIF showing high bioavailability, although food can reduce its absorption. They distribute widely throughout body tissues and fluids, reaching effective concentrations against intracellular pathogens. Specifically, RIF undergoes hepatic metabolism to its active metabolite, 25-desacetyl-rifampicin, and is a well-characterized inducer of multiple CYP450 enzymes, including CYP3A4, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and UGTs. It also affects transporters such as P-glycoprotein (P-gp) and multidrug resistance-associated protein-2 (MRP2), leading to significant DDIs risk. Enzyme induction shortens rifampin’s half-life with prolonged use, making daily dosing necessary. Excreted mainly in bile, with some renal elimination, it can turn body fluids orange-red, a harmless but noticeable ADRs. Hepatotoxicity remains a concern, thus liver function monitoring during therapy is essential (Jamis-Dow et al., 1997; Nakajima et al., 2011; Rae et al., 2001; Sumida et al., 2000; Dalet-Beluche et al., 1992; Niemi et al., 2003; Rifadin, 2023).
Rifabutin, a more hydrophobic rifamycin with reduced enzyme induction potential and fewer DDIs, is particularly valuable in Mtb/HIV coinfections. It also has a longer half-life, allowing less frequent dosing. Of its five identified metabolites, 25-O-desacetyl and 31-hydroxy are the most predominant (Knox et al., 2024; Crabol et al., 2016; Kim et al., 2022; Blaschke and Skinner, 1996). Rifabutin act as a weaker inducer than RIF, with 2–3 times lower effect on CYP3A4, making drug interactions less impactful in clinical practice (AIFA - Ricerca Farmaco, 2024).
Rifapentine, whose half-life is the longest, enables weekly administrations. It is known to induce CYP3A4 and CYP2C8/9 enzymes, starting within 4 days of the first dose and returning to baseline 14 days after discontinuation. Studies suggest that the induction potential of rifapentine is less than that of RIF but greater than that of rifabutin (Priftin, 2000).
PZA is a prodrug that converts to its active form, pyrazinoic acid (PA), under acidic conditions inside Mtb. PA then diffuses back into the bacilli, where it accumulates and exerts multiple effects against Mtb, including inhibition of fatty acid synthase, disruption of membrane potential, and interference with energy production by interacting with ribosomal protein S1 to inhibit trans-translation (Boshoff, Mizrahi, and Barry, 2002; Zimhony et al., 2007; Ngo et al., 2007; Shi et al., 2011).
PZA is primarily metabolized to PA in the liver by amidase. PA can be further oxidized by xanthine oxidase (XO) to form 5-hydroxy-pyrazinoic acid (5-OH-PA), a metabolite thought to be more hepatotoxic than PA. Alternatively, PZA can be initially oxidized to 5-hydroxy-pyrazinamide (5-OH-PZA) by XO, followed by amidase-mediated hydrolysis to 5-OH-PA. PZA and its metabolites are mainly excreted by the kidney (American Medical Association, 1992; Lacroix et al., 1989).
The hepatotoxicity of PZA is dose-dependent, especially at doses above 40 mg/kg, and correlates with its hepatic metabolism, suggesting a direct toxic effect rather than a hypersensitive or immune-mediated mechanism. Experimental studies in Wistar rats treated with PZA or PA showed hepatotoxicity, as demonstrated by elevated serum alanine aminotransferase, aspartate transaminase, and galactose single-point levels (Tostmann et al., 2008; Shih et al., 2013). Recent studies further confirmed 5-OH-PA as the most toxic metabolite, causing liver damage and metabolic shifts in rats (Rawat et al., 2018; Hussain, Zhu, and Ma, 2021).
EMB diffuses into Mtb cells and inhibits the arabinosyltransferases (embA, embB, and embC), thus disrupting the formation of cell wall components like arabinogalactan and lipoarabinomannan, ultimately hindering cell division (Myambutol, 2008; L. Zhang et al., 2020; Goude et al., 2009; Amin et al., 2008). Around 50% of an EMB dose is excreted unchanged in the urine, with an additional 8%–15% appearing as metabolites, and about 20%–22% is found unaltered in faeces. The main metabolic pathway involves oxidation by aldehyde dehydrogenase to an aldehyde metabolite, which is converted to the dicarboxylic acid 2,2'-(ethylenediimino)di-butyric acid (Myambutol, 2008; Peets et al., 1965).
Although EMB metabolism does not involve CYP450 enzymes, a study by Lee S.Y. et al. in 2014 found that it inhibits several CYP isoforms in human liver microsomes. Using liquid chromatography-electrospray ionization tandem mass spectrometry, EMB has been reported to inhibit CYP1A2 and CYP2E1 strongly, CYP2C19 and CYP2D6 moderately, and CYP2A6, CYP2C9, and CYP3A4 weakly (Lee et al., 2014).
Streptomycin, the first drug available for the treatment of Mtb, is now largely a second-line option due to concerns about resistance and toxicity (Waters and Tadi, 2024), indicated in MDR-TB and various non-tuberculosis infections. It is an aminoglycoside which exhibits bactericidal effects both by disrupting cell membranes and impairing protein synthesis through binding to the 16S rRNA in helix 44 (h44) near the A site of the 30S ribosomal subunit. This binding displaces residues A1492 and A1493 in h44, mimicking correct codon-anticodon pairing, which impedes translation and other steps in protein synthesis (Serio et al., 2018; Bulitta et al., 2015). Recent studies indicate that aminoglycosides bind to an additional cryptic site in the 23S rRNA of the 50S subunit, contributing to translation errors that destabilize membrane structure (Sullivan et al., 2018; Ying et al., 2019). Misincorporated proteins may integrate into the cell membrane, enhancing the bactericidal effect by further damaging bacterial integrity (Wallace et al., 1973; Borovinskaya et al., 2007).
Because of poor oral absorption, streptomycin is administered parenterally, typically by intramuscular injection, and less often intravenously. Peak serum concentrations (25–50 mcg/mL) are achieved within an hour after 1 g intramuscular dose (Waters and Tadi, 2024). No significant human metabolites of streptomycin have been identified, with 50%–60% of the drug excreted unchanged in the urine (Chandra Acharya and Kurosu, 2023).
Capreomycin, used primarily as a second-line treatment for MDR-TB, has an unclear mechanism of action. It is thought to inhibit protein synthesis by binding to the 70S ribosomal unit, thereby causing abnormal proteins essential for bacterial survival to be produced, ultimately leading to bacterial cell death (Knox et al., 2024; Capastat, 2019).
Capreomycin is administered parenterally, via intramuscular or intravenous injection, as it cannot be efficiently absorbed if taken orally (Capastat, 2019).
Amikacin is a semi-synthetic aminoglycoside derived from kanamycin A, given as a second-line treatment for MDR-TB and several Gram-negative bacterial infections. It interacts with the bacterial 30S ribosomal subunit, interfering with mRNA binding and tRNA acceptor sites. The disruption of protein synthesis results in the production of non-functional or toxic peptides, leading to bacterial cell death (Arikayce, 2023).
Amikacin exerts bactericidal effects against both Gram-positive and Gram-negative bacteria, including strains resistant to other aminoglycosides like gentamicin and tobramycin. It does not undergo appreciable metabolism, which enhances its stability against bacterial enzymatic deactivation, thereby reducing resistance occurrence (Knox et al., 2024; Arikayce, 2023).
Due to poor oral bioavailability, amikacin is administered parenterally. Roughly 50%–60% of an administered dose is excreted unchanged in the urine, making it mainly cleared through the kidneys (Chandra Acharya and Kurosu, 2023; Arikayce, 2023).
Levofloxacin is a fluoroquinolone antibiotic, specifically the S-(−) isomer of racemic ofloxacin. It inhibits the activity of two key bacterial enzymes, DNA gyrase and topoisomerase IV, which are type II topoisomerases essential for DNA replication, transcription, repair, and recombination (Fish and Chow, 1997).
In humans, levofloxacin is not subject to extensive metabolism, as it is found unchanged in the urine. Indeed, approximately 79.6% of the administered dose is recovered as original drug within 24 h of administration. Three metabolites have been identified at low concentrations, levofloxacin-β-D-glucuronide (M1), desmethyl-levofloxacin (M2), and levofloxacin-N-oxide (M3), although only M2 and M3 were detected in humans (Fish and Chow, 1997; Levaquin, 2008).
At therapeutic plasma concentrations levofloxacin does not alter CYP450 enzymes activity, thereby not being relevant for drug interactions at this level (Levaquin, 2008; Fàbrega et al., 2009).
Moxifloxacin is a fluoroquinolone antibiotic with high potency and superior penetration into tissues and lesions compared to levofloxacin, making it effective for TB treatment. By inhibiting DNA gyrase and topoisomerase IV, moxifloxacin hinders DNA replication and transcription. Its efficacy has been confirmed in a phase 3 clinical trial for RR-TB, thus representing a key component of the shortened treatment regimen for DS-TB (Sarathy et al., 2019; Nunn et al., 2019; Ahmad et al., 2018).
Approximately 52% of an oral or intravenous dose is metabolized via glucuronide and sulfate conjugation. Moxifloxacin is not substrate of the CYP450 system, nor does it affect such enzymes, minimizing its interaction with other drugs. The main metabolites are the sulfate conjugate (M1), eliminated in faeces, and the glucuronide conjugate (M2), excreted in the urine. About 45% of the dose is excreted as unchanged drug (Avelox, 2016; Strydom et al., 2019; Pienaar et al., 2017).
Linezolid is the first member of the oxazolidinone antibiotic class and acts by inhibiting bacterial protein synthesis. It binds to the 50S ribosomal subunit, preventing the formation of the functional 70S initiation complex required for translation (Leach et al., 2011; Shinabarger et al., 1997).
Linezolid undergoes metabolism primarily through non-enzymatic oxidation of its morpholine ring, resulting in two inactive metabolites: aminoethoxyacetic acid (Metabolite A), the predominant one, and hydroxyethyl glycine (Metabolite B). Importantly, the process does not involve the CYP450 enzyme system, showing no inhibitory or inductive effect on clinically significant isoforms such as CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4. The absence of interaction with these major isoforms significantly reduces the risk of CYP450-mediated DDIs (Dryden, 2011; Zyvox, 2014; Slatter et al., 2001). Conversely, a potential for interactions with drugs affecting monoamine levels exists, as it is a reversible, non-selective inhibitor of monoamine oxidase (MAO) (Zyvox, 2014).
Excretion is primarily renal, with approximately 84% of an administered dose excreted in the urine (30% as the unchanged drug, 40% as Metabolite B, and 10% as Metabolite A). Faecal elimination is minimal, accounting for only small amounts of metabolites (Zyvox, 2014; Slatter et al., 2001).
Ethionamide is commonly used in longer regimens, especially when other drugs like bedaquiline, clofazimine, delamanid, or linezolid are not feasible. Its bacteriostatic or bactericidal effects depend on drug concentration at the infection site and organism susceptibility. Ethionamide requires activation by the mycobacterial enzyme flavin monooxygenase (EthA) and the transcriptional repressor EthR to form ethionamide sulfoxide, which binds to NAD+ and inhibits the enoyl-acyl carrier protein reductase (InhA), thereby blocking mycolic acid synthesis, responsible for cell death (Dover et al., 2007).
Ethionamide is extensively metabolized in the liver, mainly through flavin-containing monooxygenase (FMO3). Six metabolites have been identified: 2-ethylisonicotinamide, carbonyl-dihydropyridine, thiocarbonyl-dihydropyridine, S-oxocarbamoyl dihydropyridine, 2-ethylthioisonicotinamide, and ethionamide sulfoxide, the latter having significant antimycobacterial activity (Henderson et al., 2008; Krueger and Williams, 2005; Trecator, 2016).
Clofazimine, originally an anti-leprosy antibiotic, is now recommended as a core drug in both short and long regimens for DR-TB. Although the exact mechanism of action is not fully understood, clofazimine seems to interfere with cellular membrane functions, including ion transport and respiration, ultimately causing Mtb death (Cholo et al., 2017; Barry et al., 1956; Yano et al., 2011; Lechartier and Cole, 2015).
Clofazimine metabolism comprises partial hepatic transformation, with at least eight metabolites identified in human liver microsomes. CYP3A4 and CYP1A2 are primarily involved, with additional contributions from CYP2C8 and CYP2D6. At low concentrations, it is a weak inducer of CYP3A4, but at therapeutic levels shows inhibitory properties, suggesting the potential for both auto-induction and inhibition depending on the concentration (Howlader et al., 2022; Shimokawa et al., 2015). Notably, in vitro studies report that clofazimine also inhibits CYP2C8, CYP2D6, and CYP3A4/5, indicating the likelihood of clinically significant interactions with drugs metabolised by these isoenzymes (Lamprene, 2019; Maartens et al., 2018).
Renal excretion is minimal, with most of clofazimine and its metabolites eliminated through biliary system. The main urinary metabolites, apparently pharmacologically inactive, are produced by hydrolytic dehalogenation and deamination followed by glucuronidation (Banerjee et al., 1974; Levy, 1974; P. C. Feng, Fenselau, and Jacobson, 1981).
Bedaquiline, a diarylquinoline antimycobacterial drug, targets Mtb via inhibition of ATP synthase, essential for bacterial survival. Specifically, it binds to enzyme subunit c. Impeding this crucial mechanism of energy production, bedaquiline is particularly effective against persistent bacilli (Sirturo, 2012; Mesens et al., 2010).
It is predominantly metabolised by CYP3A4 in the liver into the N-monodesmethyl (M2) metabolite, which exhibits anti-tubercular activity, albeit with approximately five times less potency than the parent drug. Minor contributions to drug metabolism have been observed in vitro from CYP1A1, CYP2C8, and CYP2C18, though these enzymes are less relevant in vivo due to their lower hepatic expression compared to CYP3A4 (Shimada et al., 1994). Bedaquiline does not induce or inhibit major CYP isoenzymes, namely, CYP1A2, CYP2C9, CYP2C19, or CYP2D6 at clinically relevant concentrations, minimizing the risk of CYP450-related interactions (Mesens et al., 2010). Moreover, its impact on isoforms CYP1A2, CYP2A6, CYP2C8/9/10, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A4/5, and CYP4A has also been shown to be negligible in vitro.
Excretion is primarily biliary, with a minimal amount appearing in the urine. The drug does not significantly interact with transport proteins like P-gp, further reducing the risk of DDIs (Sirturo, 2012).
Pretomanid is a prodrug used in combination with bedaquiline and linezolid for tackling pulmonary XDR-TB and treatment-intolerant or nonresponsive MDR-TB. It requires activation by Mtb deazaflavin-dependent nitroreductase (Ddn). Under aerobic conditions, pretomanid stops protein and lipid synthesis by reducing the availability of keto mycolic acids, essential for cell wall integrity. In anaerobic environments, it produces nitric oxide (NO), which inhibits cytochrome c oxidase and reduces ATP production in non-replicating Mtb cells (Pstragowski, Zbrzezna, and Bujalska-Zadrozny, 2017; Singh et al., 2008; Malo et al., 2021).
Approximately 20% of drug metabolism is due to partial phase one reactions by CYP3A4. At clinical concentrations, pretomanid does not inhibit major CYP isoenzymes, including CYP1A2, CYP2C8, CYP2C9, CYP2C19 or CYP2D6, and does not induce CYP2C9 or CYP3A4, limiting the potential for DDI trough CYPs. On the other hand, it significantly inhibits the OAT3 transporter, potentially increasing the concentration of OAT3 substrates (Mitnick, McGee, and Peloquin, 2009; Pretomanid, 2019). Of an administered dose, about 53% is excreted in the urine and 38% in faeces, both as unchanged drug and metabolites (Wang et al., 2015).
Delamanid is a prodrug indicated as part of a combination regimen for treating MDR-TB in adults. It requires activation by the mycobacterial F420 coenzyme system, including Ddn, to exert its antimycobacterial activity. Upon activation, it inhibits the synthesis of methoxy-mycolic and keto-mycolic acids, leading to depletion of essential cell wall components and subsequent bacterial death (Lewis and Sloan, 2015; Szumowski and Lynch, 2015).
Delamanid is primarily metabolized by albumin and, to a lesser extent, by CYP3A4 in the liver. Secondary metabolic pathways may involve CYP1A1, CYP2D6, and CYP2E1, though their contributions are minor in comparison to CYP3A4. Its main metabolite, DM-6705 (M1), accounts for 13%–18% of total plasma exposure and has been associated with QT prolongation. The hydrolytic cleavage of its 6-nitro-2,3-dihydroimidazo [2,1-b] oxazole moiety generates M1, which undergoes further transformations involving hydroxylation and oxidation, mediated largely by CYP3A4 (Szumowski and Lynch, 2015; Deltyba, 2014). While M1 and other metabolites do not retain significant antimycobacterial activity, they can still impact cardiac safety. Delamanid is primarily cleared via hepatic metabolism, with negligible renal excretion (Deltyba, 2014; Sasahara et al., 2015).
Among antituberculous medications, INH, RIF, EMB, clofazimine, bedaquiline, and delamanid are known to be CYP450 enzyme system substrates or can impact its metabolic activity by induction or inhibition. Therefore, potential interactions with cigarette smoking compounds may be expected.
Given the activity of PAHs in inducing CYP2E1, smokers taking INH as part of TB therapy may experience increased synthesis of reactive intermediates resulting from further oxidation of oxidised HZ and AcHZ. This could increase the risk of INH-associated hepatotoxicity. However, most studies do not include cigarette smoking as a possible individual risk factor for drug-induced hepatotoxicity (DIH). Interestingly, a retrospective cohort study highlighted that cigarette smoking appears to be negatively associated with DIH, which is surprising as induction of CYP2E1 in smokers is expected to increase exposure to toxic metabolites (Zaverucha-do-Valle et al., 2014). The reduced risk may be related to tobacco smoke compounds in glutathione S-transferase (GST) activity, which is involved in the nicotine detoxification pathway (Pachauri and Flora, 2013). Another explanation for this phenomenon could be a link between smoking habit and NAT2 acetylator status. Indeed, heterocyclic amines in tobacco smoke require activation by CYP1A2 and NAT2 (Voutsinas et al., 2013). Slow acetylator status is associated with reduced ability to detoxify these xenobiotics, but active smokers may have higher NAT2 activity and faster INH metabolism (Kroon, 2007). Based on these premises, smokers who quit may be at increased risk of developing DIH and thus may require dose adjustment to avoid liver damage.
RIF induction of CYP3A4 is mediated by an orphan nuclear receptor known as the pregnane X receptor (PXR). The drug binds to PXR, forming an activated complex, and subsequently combines with the retinoid X receptor (RXR) to form a heterodimer that targets a DNA response element, enhancing CYP3A4 gene transcription. Consequently, CYP3A4 protein synthesis is increased (Lehmann et al., 1998). The expression of several other proteins is induced in a similar manner. Indeed, genes reported to be regulated by known PXR ligands are CYP1A1, CYP2C8, CYP2C9, MDR1, MRP2, and members of the UGT, sulfotransferase, and carboxylesterase families (Goodwin, Redinbo, and Kliewer, 2002). Moreover, in vitro studies have demonstrated that RIF induces various CYP enzymes, including CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A5 (Rae et al., 2001; Dalet-Beluche et al., 1992; Niemi et al., 2003). Concerning CYP1A2, in vivo induction is suggested, as demonstrated by the paraxanthine-to-caffeine ratio rising in healthy subjects (Branch et al., 2000; Fuhr and Rost, 1994).
In light of these findings, the safety and efficacy profile of RIF during TB treatment can be altered in smokers because of the concomitant induction of CYP450 enzymes mediated by PAHs. It is worth mention that RIF clearance increases during multiple-dose therapy, leading to autoinduction of its own metabolism, further complicating therapeutic management (Loos et al., 1987).
According to Lee S.Y. and colleagues, EMB has strong inhibitory potential against CYP1A2 and CYP2E1. Conversely, PAHs are inducers of such cytochromes. Therefore, careful assessment of safety and efficacy profiles of co-administered medications in TB patients who also smoke are warranted to avoid ADRs and achieve therapeutic goals.
Clofazimine is at least partially metabolised in the liver via CYP3A4 and CYP1A2, thereby TB smokers may experience increased metabolite synthesis, potentially necessitating dosage adjustments.
As bedaquiline mainly undergoes phase one reactions catalysed by CYP3A4, its exposure may be either reduced or increased during co-administration with inducers or inhibitors of CYP3A4 respectively (Sirturo, 2012). To a lesser extent, CYP1A1 also participates in drug metabolism, thus co-administering CYP1A1 inducers can decrease bedaquiline concentrations neglecting its effectiveness.
The metabolic pathways of delamanid involve CYP450 isoenzymes as well. In particular, CYP1A1 and 2E1, although playing a minor role in the overall metabolism, can be induced by PAHs making smokers more prone to severe ADRs (e.g., QT prolongation) due to increased metabolite concentrations.
Table 1 and Figure 1 provide an overview of the antituberculous medications known to be CYP450 enzyme system substrates or can impact its metabolic activity and possible interactions with tobacco smoke compounds.
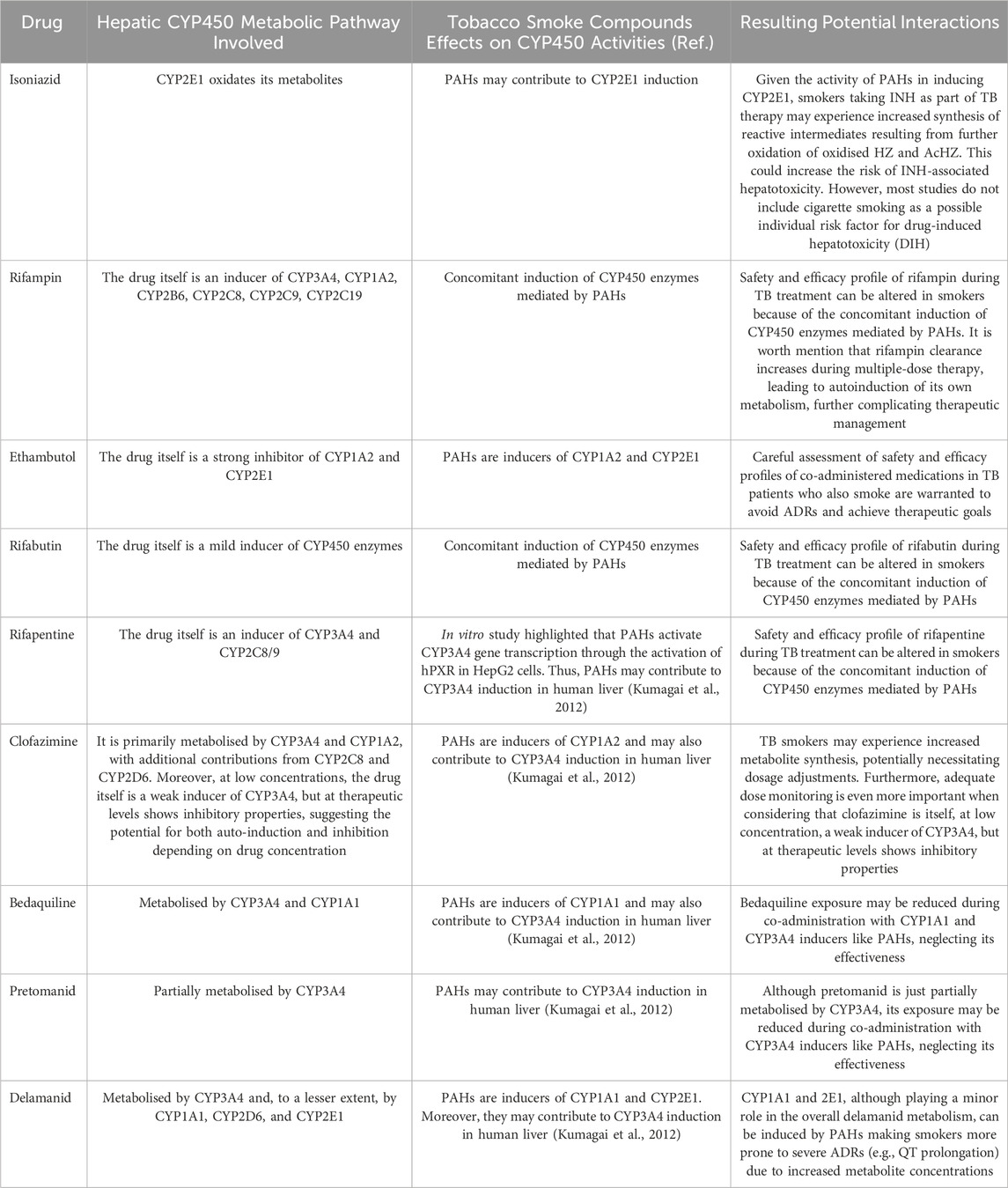
Table 1. Antituberculous medications known to be CYP450 enzyme system substrates or can impact its metabolic activity, possible effects of tobacco smoke compounds, and resulting potential interactions.
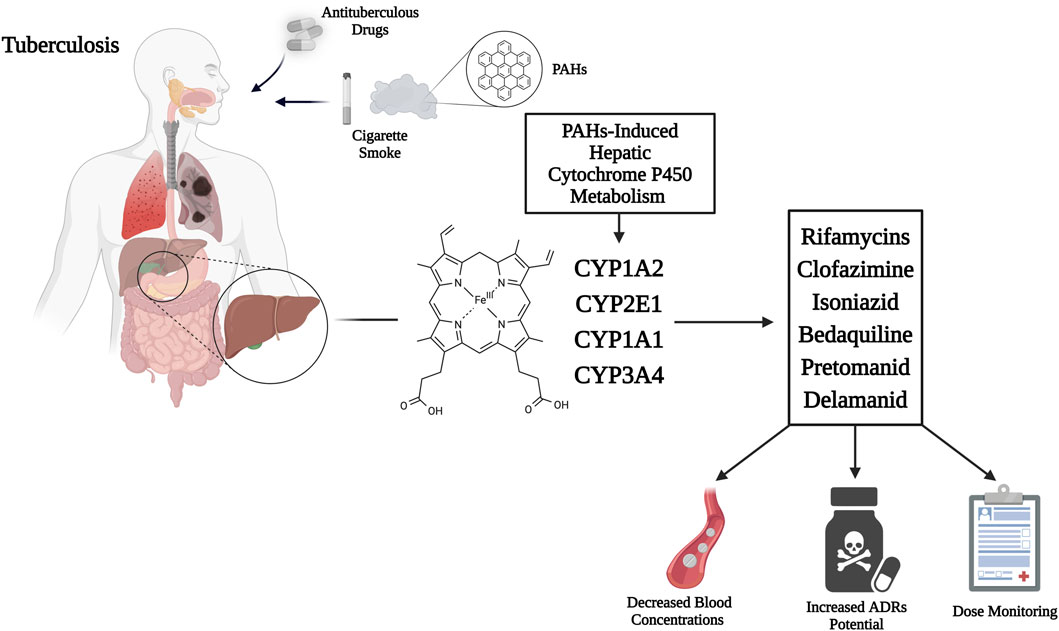
Figure 1. Mechanism of CYP450 hepatic isoenzymes induction by cigarette smoke PAHs, enhancing rifamycins, clofazimine, isoniazid, bedaquiline, pretomanid, and delamanid metabolism, thus decreasing active drug concentrations, increasing toxic metabolites and potential for ADRs. Therefore, dose monitoring appears necessary. Abbreviations: CYP450, cytochrome P450; PAHs, polycyclic aromatic hydrocarbons; ADRs, adverse drug reactions. Created with BioRender.com; accessed on 20 June 2025.
5 Interactions between smoking cessation medications and antituberculous drugs
In the past few years, numerous studies have investigated the association between smoking and TB severity suggesting that the former has a negative impact on patients’ outcome, resulting in delayed culture conversion, treatment extension, and increasing risk of recurrence after pharmacotherapies completion (Slama et al., 2007; Smit et al., 2010; Abal et al., 2005; Nijenbandring de Boer et al., 2014). In this context, Altet N. et al. evaluated the effect of tobacco smoke on radiological manifestations, sputum conversion, and immune response to Mtb by analysing IFN-γ secretion using IFN-γ Release Assays (IGRAs). To this aim, 525 participants were studied: 175 with active pulmonary TB, 350 from contact tracing studies, and 41 with secondary TB cases. Clinical, radiological, and microbiological data were collected for each participant, and they underwent QuantiFERON-TB Gold (QFN-G-IT) and T-SPOT.TB. The authors inferred that smoking had a negative effect on radiological manifestations and delayed the time to sputum conversion, probably related to an attenuated IFN-γ response caused by direct tobacco smoke (Altet et al., 2017).
A prospective cohort study has been performed in India to determine the impact of smoking on TB treatment outcome and the prevalence of smoking habits among newly diagnosed individuals. The investigators enrolled 2,350 patients (1,758 male and 592 female) who were classified as never smokers, current smokers, and former smokers on the basis of self-report. Participants were then started on anti-TB treatment and followed for 2 years. During the observation period, smoking has been associated with more extensive lung disease, cavitation, and positive sputum smear and culture results at baseline. Additionally, both current and former smokers were significantly more likely to have positive microbiological tests after 2 months of treatment. The same groups also showed higher rates of non-adherence, treatment failure, and relapse. In light of these findings, it has been concluded that tobacco smoking is associated with a significantly increased risk of advanced and more severe disease in the form of pulmonary cavitation, positive sputum smear and culture results, and lower conversion rate after treatment initiation (Mahishale et al., 2015).
In 2022, a prospective observational study came to opposite conclusions though. The purpose of the study was to describe the severity of disease and treatment outcomes in TB smokers compared with non-smokers in Guinea-Bissau. It showed that among the 1,780 patients included, 385 of whom were smokers for a median of 10 years, there was no difference in disease severity at diagnosis. In addition, smokers were not more prone to negative treatment outcomes, although a trend was observed (adjusted odds ratio [OR] 1.24, 95% confidence interval [CI] 0.91–1.70), and were more likely to be lost to follow-up, but this was also not significant (adjusted hazard ratio [HR] 2.09, 95% CI 0.89–4.94). Thus, the authors suggested that in a highly endemic TB setting with few tobacco smokers, worse disease severity or outcome due to smoking were not observed, but they highlighted the existence of confounding factors (Bay et al., 2022).
Another study, conducted in Taipei, sought to determine whether tobacco smoking increases the risk of TB relapse in adults who have successfully completed TB treatment, and the underlying factors. A total of 5,567 patients were enrolled, of whom 84 (1.5%) experienced recurrence during follow-up. The incidence of relapses was 4.9 episodes/1,000 person-years of follow-up. In subjects who smoked more than ten cigarettes per day, the risk was twice that of non-smokers/former smokers, according to Cox proportional hazards regression (Yen et al., 2014).
Despite mounting evidence of an association between smoking and TB in terms of risk and influence on outcome, a crucial but often overlooked issue is the interaction between antituberculosis drugs and smoking cessation therapies. Indeed, a significant number of people affected by TB are also smokers in the process of quitting. Unveiling the molecular mechanisms that underscore these events is of utmost importance, as clinically relevant DDIs can complicate TB management and interfere with the success of smoking cessation.
Interactions can also have a significant impact on the safety and efficacy profile of treatments, particularly if they compromise pharmacological activity and increase the odds of ADRs.
Medications for smoking cessation include various forms of nicotine replacement therapy (NRT), bupropion, varenicline, and cytisine (Polosa and Benowitz, 2011; Cohen et al., 2024) that have been shown to improve quit rates in the general population of smokers. Compared with placebo, the likelihood of quitting smoking was roughly doubled with NRT (1.84, 95% CI 1.71–1.99) and bupropion (1.82, 1.60–2.06) and was improved further with varenicline (2.88, 2.40–3.47) (Cahill et al., 2013).
Dogar O. et al. conducted a randomised, double-blind, placebo-controlled trial to assess the effectiveness and safety of cytisine in patients with TB in Bangladesh and Pakistan. 2,472 patients (1,527 from Bangladesh and 945 from Pakistan) smoking daily and willing to quit who had been diagnosed with pulmonary TB within the previous 4 weeks, were enrolled. Subjects were randomly assigned to receive cytisine (n = 1239) at a dose of 9 mg on day 0, gradually tapered to 1.5 mg on day 25, or placebo (n = 1233) for 25 days. The primary endpoint was continuous abstinence at 6 months, defined as self-report and confirmed biochemically by a breath CO reading of less than 10 parts per million (ppm). Primary and safety analysis were done in the intention-to-treat (ITT) population. At 6 months, 401 (32.4%) participants in the cytisine group and 366 (29.7%) in the placebo group had achieved continuous abstinence. Fifty-three (4.3%) patients in the cytisine group and 46 (3.7%) in the placebo group reported serious adverse events (SAEs) (94 events in the cytisine group and 90 events in the placebo group), which included 91 deaths (49 in the cytisine group and 42 in the placebo group). None of the adverse events (AEs) were attributed to the study medication. Authors stated that the add-on of cytisine to brief behavioural support for the treatment of tobacco addiction in TB patients is not supported by their findings (Dogar et al., 2020).
An open-label randomised controlled trial investigated the impact of intensive smoking cessation activities as an add-on to TB treatments on patient-related outcomes. A total of 800 self-reporting smokers with pulmonary TB on standard anti-TB drugs were enrolled in the study and randomly assigned (1:1) to receive either NRT plus behavioural change counselling or counselling alone, delivered at baseline and at two follow-up visits. The primary endpoints were change in TB-score at 24 weeks and culture conversion at 8 weeks. The biochemical smoking cessation rates, defined as serum cotinine levels of less than 10 ng/mL and/or exhaled CO levels of less than 6 ppm, were significantly higher in the intervention group at 24 weeks (47.8% vs. 32.4%) as well as the self-reported cessation rates (69.3% vs. 38.7%). Despite the TB-scores at 24 weeks (95% CI) were lower in the intervention arm (2.07 vs. 2.12), the difference was not clinically meaningful. Patients in the control arm required treatment extension more often than intervention arm (6.4% vs. 2.6%). Authors concluded that combining NRT with behaviour change counselling resulted in a significantly higher quit rates and lower cotinine levels, but significant impact on patient-related or microbiological outcomes were not detected (Sharma et al., 2018).
Safety concerns arise when smoking cessation medications and anti-TB drugs are co-administered, so predicting and assessing potential DDIs would be extremely helpful. To date, many digital tools are available to determine their clinical significance, being the management largely dependent on impact and severity of the interaction. However, there is no consensus among the current resources and a standardised classification method would be warranted. More specifically, the British National Formulary marks with bullet points potentially harmful drug pairs which should be prescribed cautiously, under appropriate monitoring, or avoided altogether (British National Formulary, 2015). Micromedex Drug–Reax System categorizes interactions into three degrees of severity, major, moderate, and minor, and the strength of the reporting into five categories—excellent, good, fair, poor, and unlikely (DRUG-REAX, 2024). Drugs.com Drug Interaction Checker (DDIC) and DrugBank.com classify interactions into four severity levels: major, moderate, minor, and unknown (Knox et al., 2024; Drug Interaction Checker Quickly Check Your Meds, 2024). Vidal’s Interactions médicamenteuses comprises four seriousness grades according to the recommended clinical management—contraindicated, avoid, precaution, and “take into account” (i.e., no specific recommendation) (Les interactions médicamenteuses, 2024). Drug Interaction Facts rates interaction severity into three levels—major, moderate, and minor—and the degree of documentation into five—established, probable, suspected, possible, and unlikely—by combining these two categories. It also ranks each interaction from 1 to 5 in terms of importance (Tatro, 2014). MedScape drug interaction checker sorts DDIs as serious, significant, or minor. Serious interactions impose switching to an alternative molecule, whereas significant ones necessitate close monitoring. Minor DDIs do not require either discontinuation or switching (Drug Interactions Checker - Medscape Drug Reference Database, 2024). Notably, information from digital tools must always be compared with SmPC of interacting drugs in order to avoid errors or misinterpretations.
Concerning potential DDIs between NRT and anti-TB drugs, DrugBank.com reports that the metabolism of nicotine can be decreased when combined with INH or EMB and increased when co-administered with rifabutin. Indeed, when nicotine is administered concurrently with a CYP2A6 inhibitor of unknown strength, the substrate metabolism will be reduced, resulting in increasing serum concentrations and heightened risk, incidence, and/or severity of ADRs. Conversely, concomitant use of nicotine and rifabutin, a CYP2B6 inducer, will result in a modest increase in mediated metabolism of the substrate, potentially leading to a reduction in serum concentration and/or therapeutic effect (DrugBank Clinical API Plugins, 2024).
In vitro research suggests that varenicline does not appear to influence the pharmacokinetics of substances that are primarily metabolised by CYP enzymes. Since less than 10% of its clearance is attributed to varenicline’s metabolism, it is anticipated that agents affecting the CYP450 system will have no effect on its pharmacokinetic profile. This suggests that dose adjustments will not be necessary. Furthermore, at therapeutic levels, varenicline does not inhibit human renal transport proteins, as demonstrated by preclinical studies. Consequently, it is improbable that active substances eliminated by renal secretion will be impacted by varenicline (Champix, 2006). As indicated by online sources, INH, PZA, EMB, and levofloxacin have the potential to reduce its excretion rate. Conversely, varenicline may also impact the clearance of streptomycin, capreomycin, and amikacin. The renal clearance of medications is influenced by various renal functions, including glomerular filtration, passive diffusion, tubular secretion, and reabsorption. It is important to note that two of these processes, namely, tubular secretion and reabsorption, are subject to saturation. As a result, they can be influenced by competition among several substrates for removal. This suggests a significant likelihood that one drug will “out-compete” or saturate the renal excretion pathways prior to the elimination of the other co-administered drugs, leading to inhibited or delayed clearance. The net result of these processes is an increase in serum concentrations, which, by the way, has been demonstrated to escalate the risk and/or severity of ADRs associated to exposure to such drugs (DrugBank Clinical API Plugins, 2024).
Bupropion is subject to metabolic processes involving the CYP450 enzyme system, predominantly through CYP2B6, with contributions from CYP1A2, 2A6, 2C9, 2D6, 2E1, and 3A4 isoforms, though to a lesser degree (Connarn et al., 2015). These enzymes also play a pivotal role in the metabolic pathways of various TB medications, which could lead to alterations in the concentrations and effects of such drugs. It is imperative to adjust dosages and meticulously select treatments for patients who are using bupropion. Furthermore, research from both preclinical and clinical studies indicates that bupropion can inhibit CYP2D6 activity. The oxidation of the bupropion side chain leads to the production of a glycine conjugate of metachlorobenzoic acid, which is primarily expelled as the main urinary metabolite, with glucuronidated metabolites also being found in urine. Although there are discrepancies in the literature regarding their various chiral forms, these metabolites are produced from all three active metabolites by different UGT enzymes. In vitro, the primary enzyme involved in the glucuronidation of hydroxybupropion is UGT2B7, with a minor role played by UGT2B4. UGT2B7 is also chiefly responsible for generating erythrohydrobupropion glucuronide, while UGT1A4, UGT1A3, UGT1A9, and UGT2B4 have lesser involvement. The identification of the UGTs that mediate the conjugation of active bupropion metabolites is crucial for understanding factors that could affect potential DDIs. In vitro research has demonstrated that at concentrations up to 200 mcg/ml, 84% of bupropion binds to proteins in human plasma. Hydroxybupropion exhibits a similar binding rate, whereas the protein binding extent for threohydrobupropion is about half that of bupropion. Consequently, interactions related to protein binding are unlikely to have clinical significance (Jefferson, Pradko, and Muir, 2005).
Digital tools highlight that potential DDIs between bupropion and anti-TB drugs, namely, RIF, INH, PZA, EMB, rifabutin, streptomycin, capreomycin, amikacin, levofloxacin, moxifloxacin, linezolid, and bedaquiline may occur.
DDIC, Drugs.com, MedScape.com, and INTERCheckWeb.it points out the plausible interaction between bupropion and RIF. In particular, according to DDIC and MedScape.com, RIF will decrease the level or effect of bupropion by inducing CYP2B6 activity. Therefore, pharmacological response to bupropion should be monitored more closely whenever a CYP2B6 inducer is added to or withdrawn from therapy, and the bupropion dosage adjusted as necessary. DrugBank.com reports that the metabolism of bupropion can be increased when combined with RIF. Indeed, the latter is an inducer of CYP2C9 while bupropion is substrate of CYP2C9. Concomitant administration of these agents can produce a decrease in the serum concentration of the affected drug which may translate to reduced therapeutic efficacy. Ultimately, INTERCheckWeb.it suggests a reduction in systemic exposure to bupropion with an associated increase in clearance of 203% and reductions in Cmax and AUC of 39% and 43% respectively, due to the rifampin-related induction of bupropion metabolism.
Two digital tools identified the potential interaction between bupropion and INH. DrugBank.com reports that the risk or severity of seizure can be increased when bupropion is combined with INH. Indeed, bupropion carries a dose-dependent risk of seizure further exacerbated when combined with other medications that can reduce the seizure threshold. According to Drug.com, combining INH with other medications known to cause liver toxicity, such as bupropion, could increase the likelihood of liver injury. INH is subject to metabolism through the actions of N-acetyltransferase and CYP2E1. The acetylation process of INH varies genetically and is generally categorized as either slow or rapid, with slow acetylators showing a relative deficiency in N-acetyltransferase. While the rate of acetylation does not significantly impact the drug’s effectiveness, it may result in elevated levels of INH in the bloodstream and a higher occurrence of ADRs. Furthermore, INH has been found to act as an in vitro inhibitor for several isoenzymes, including CYP450 (2C9, 2C19, 2E1, and 3A4). Consequently, concurrent administration of hepatotoxic medications that are metabolised via these pathways may result in elevated concentrations of the concomitant drug, which can potentially culminate in hepatic injury.
PZA, EMB and levofloxacin have been demonstrated to reduce the renal clearance rate of bupropion, with the potential observation of elevated plasma levels. Consequently, this may result in an escalation of the risk, occurrence, and/or severity of ADRs associated with bupropion exposure. Conversely, bupropion has been observed to inhibit the renal elimination of streptomycin, capreomycin, and amikacin, which could result in elevated serum concentrations and an augmented risk of ADRs associated with aminoglycosides (DrugBank Clinical API Plugins, 2024).
Regarding the co-administration of bupropion with linezolid, four tools indicate an increased risk of serotonergic syndrome (agitation, mental confusion, cognitive deficits, diaphoresis, myoclonus, hyperreflexia, hypertension, palpitations, and muscle rigidity). Therefore, it would be recommended to use the combination with caution and to monitor the onset of early signs such as anxiety, confusion, disorientation and, if necessary, to discontinue drug treatment (Drug Interaction Checker Quickly Check Your Meds, 2024; Drug Interactions Checker - Medscape Drug Reference Database, 2024; DrugBank Clinical API Plugins, 2024; Intercheck WEB, 2024).
Finally, co-administration of bedaquiline with bupropion may cause an increased risk of hepatotoxicity, so it is better to avoid this association whenever possible, especially in patients who already have impaired liver function (Drug Interaction Checker Quickly Check Your Meds, 2024).
Possible interactions between antituberculous drugs and smoking cessation medications are summarised in Table 2.
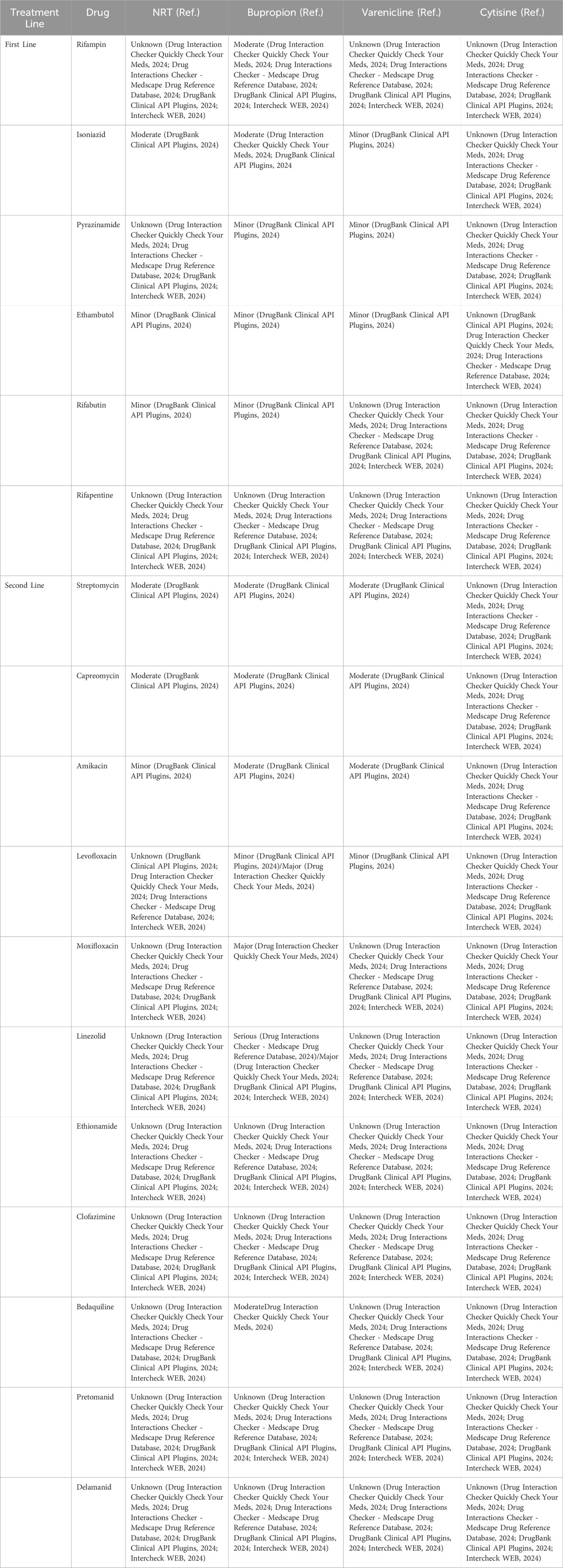
Table 2. Possible interactions between antituberculous drugs and smoking cessation medications, classified according to severity level. Major/Serious: highly clinically significant. Avoid combinations; the risk of the interaction outweighs the benefit. Moderate: moderately clinically significant. Usually avoid combinations; use it only under special circumstances. Minor: minimally clinically significant. Minimize risk; assess risk and consider an alternative drug, take steps to circumvent the interaction risk and/or institute a monitoring plan. Unknown: no interaction information available.
Table 3 summarise the role of non-CYP450 proteins involved in drug metabolism/transport and impact of cigarette smoking.
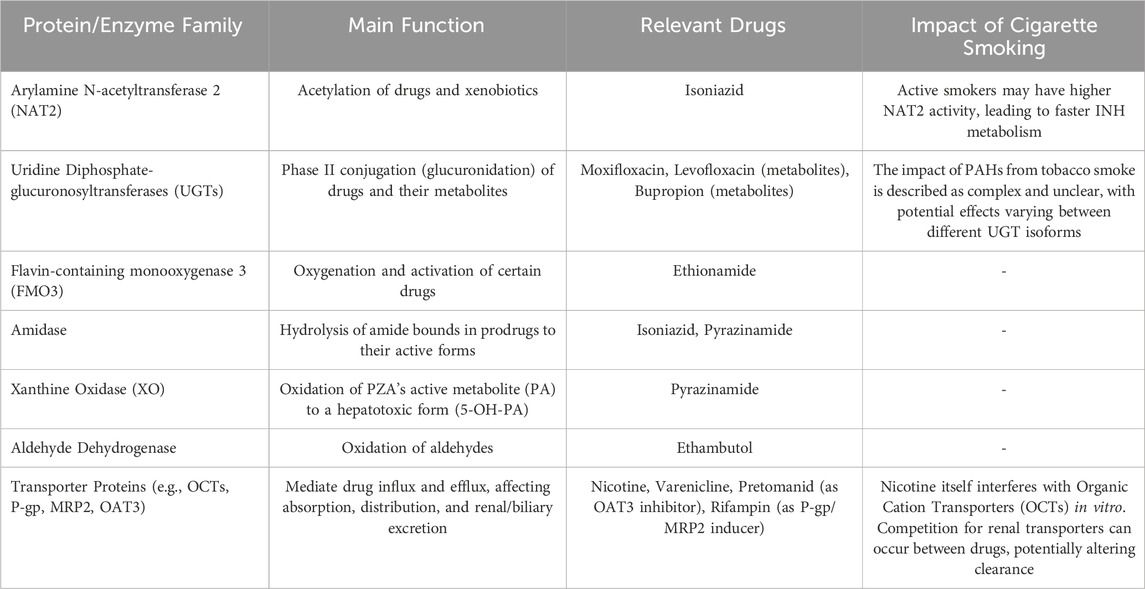
Table 3. Role of non-CYP450 proteins involved in drug metabolism/transport and impact of cigarette smoking on their activity.
6 Discussion
To our knowledge this scoping review is the first to comprehensively explore the intricate relationship between tobacco smoking, smoking cessation, and TB treatment. Smoking plays a significant role in accelerating TB progression, worsening treatment outcomes, and increasing mortality rates. Furthermore, the impact of tobacco smoke on the enzymatic metabolism of antituberculous drugs highlights a critical challenge in optimizing treatment for TB patients who smoke. Smoking cessation, while beneficial for improving treatment outcomes, presents its own set of complexities, particularly in adjusting therapeutic regimens as enzymatic activity normalizes post-cessation. Careful monitoring of patients during this transition is therefore essential.
Tobacco smoke, particularly through its PAHs, alters the pharmacokinetics and pharmacodynamics of antituberculous drugs. By inducing hepatic enzyme activity, tobacco smoke accelerates drug metabolism, potentially reducing the efficacy of TB medications. Conversely, smoking cessation reverses this enzyme induction, necessitating tailored drug dosing to maintain therapeutic effectiveness while minimizing adverse reactions.
Evidence highlights the importance of integrated care strategies that combine TB treatment with smoking cessation efforts. Quitting smoking not only supports better TB outcomes but also reduces the risk of chronic conditions like cardiovascular disease and COPD. However, careful management of potential DDIs between anti-TB therapies and smoking cessation medications is essential to ensure both safety and efficacy. Monitoring interactions is critical when prescribing smoking cessation therapies alongside TB medications. Specifically, avoiding combinations such as bupropion with linezolid or bedaquiline is recommended due to the risk of serotonin syndrome or hepatotoxicity.
The present work has certain limitations, firstly because it is based on data from the existing literature, which appears to be fragmentary and inconsistent. Despite a careful search and selection of sources, the evidence often comes from works that were not designed to explore the field of drug-drug interactions and, in particular, with tobacco combustion compounds. Additionally, there is a paucity of contemporary studies that seek to quantify the metabolisms of specific molecules and the extent of the impact of changes in enzyme activity induced by cigarette smoke. A comprehensive evaluation of the individual associations, based on pharmacokinetic studies that also consider individual variability, is still a long way off.
Given the complexity of these interactions, a patient-centered approach is essential. This includes assessing individual smoking status, metabolic responses, and drug regimens to tailor treatment effectively. Collaborative care involving TB specialists and smoking cessation experts can enhance personalization, improve adherence, and minimize ADRs.
The overlap of high TB prevalence and smoking rates, especially in LMICs, highlights the need for targeted public health interventions. Policies that integrate smoking cessation into TB control programs could significantly reduce the global TB burden. Routine incorporation of smoking cessation into TB care has the potential to enhance treatment adherence, reduce drug resistance, and improve patient outcomes. Global frameworks already exist but are often implemented in silos. The WHO’s MPOWER package offers a set of evidence-based tobacco control measures (Monitor tobacco use, Protect people from smoke, Offer help to quit, Warn about dangers, Enforce bans on advertising, and Raise taxes) that can be synergistically aligned with the WHO’s End TB Strategy. For example, raising tobacco taxes not only reduces smoking prevalence but can also generate revenue to fund TB control programs. Similarly, integrating “Offer help to quit” services directly into TB clinics is a high-impact intervention. However, policy implementation in LMICs faces substantial barriers, including limited resources, and weak health systems. Successful country models, such as integrating brief tobacco interventions into national TB programs, demonstrate that these challenges can be overcome with political commitment and tailored strategies (WHO Report on the Global Tobacco Epidemic, 2019; The End TB Strategy, 2025; A WHO the Union Monograph on TB and Tobacco Control, 2005).
Despite these promising directions, significant knowledge gaps remain. Understanding the long-term effects of smoking cessation on drug metabolism and TB outcomes requires further investigation. Future research should focus on elucidating the specific mechanisms of DDIs, including the roles of PAHs and nicotine in enzyme modulation. Clinical trials are needed to develop optimal dosing strategies during smoking cessation for TB patients, particularly in relation to newer antituberculous drugs. Additionally, the influence of genetic polymorphisms on enzyme activity among TB smokers and quitters warrants exploration.
A concerted effort to integrate smoking cessation with TB care offers an opportunity to optimize treatment outcomes, reduce healthcare burdens, and enhance the quality of life for affected populations. These efforts should be prioritized in public health strategies to combat the dual burden of TB and tobacco use effectively.
Author contributions
CMB: Methodology, Conceptualization, Writing – original draft. SPP: Conceptualization, Writing – original draft, Methodology. EA: Conceptualization, Methodology, Writing – original draft. GD: Writing – review and editing. CB: Writing – original draft, Data curation. AC: Writing – original draft, Data curation. GG: Writing – original draft, Data curation. GN: Investigation, Writing – review and editing. DC: Investigation, Writing – review and editing. JN: Formal Analysis, Writing – review and editing, Software. HS: Software, Writing – review and editing, Formal Analysis. RT: Writing – review and editing, Formal Analysis, Software. AMo: Formal Analysis, Software, Writing – review and editing. MC: Writing – review and editing, Visualization. RB: Writing – review and editing, Supervision, Validation. AMa: Supervision, Writing – original draft, Conceptualization. GC: Validation, Writing – review and editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1606150/full#supplementary-material
References
Abal, A. T., Jayakrishnan, B., Parwer, S., El Shamy, A., Abahussain, E., and Sharma, P. N. (2005). Effect of cigarette smoking on sputum smear conversion in adults with active pulmonary tuberculosis. Respir. Med. 99 (4), 415–420. doi:10.1016/j.rmed.2004.08.016
Abulfathi, A. A., Decloedt, E. H., Svensson, E. M., Diacon, A. H., Donald, P., and Reuter, H. (2019). Clinical pharmacokinetics and pharmacodynamics of rifampicin in human tuberculosis. Clin. Pharmacokinet. 58 (9), 1103–1129. doi:10.1007/s40262-019-00764-2
A Guide for Tuberculosis Patients to Quit Smoking (2025). A Guide for tuberculosis patients to quit smoking. Available online at: https://www.who.int/publications/i/item/9241506922 (Accessed November 29, 2024).
Ahmad, N., Ahuja, S. D., Akkerman, O. W., Alffenaar, J.-W. C., Anderson, L. F., Baghaei, P., et al. (2018). Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet London, Engl. 392 (10150), 821–834. doi:10.1016/S0140-6736(18)31644-1
AIFA - Ricerca Farmaco. (2024). AIFA - Ricerca Farmaco. Available online at: https://medicinali.aifa.gov.it/it/#/it/dettaglio/0000022408 (Accessed November 25, 2024).
Alsayed, S. S. R., and Gunosewoyo, H. (2023). Tuberculosis: pathogenesis, current treatment regimens and new drug targets. Int. J. Mol. Sci. 24 (6), 5202. doi:10.3390/ijms24065202
Altet, N., Latorre, I., Jiménez-Fuentes, M. Á., Maldonado, J., Molina, I., González-Díaz, Y., et al. (2017). Assessment of the influence of direct tobacco smoke on infection and active TB management. PloS One 12 (8), e0182998. doi:10.1371/journal.pone.0182998
Amere, G. A., Nayak, P., Salindri, A. D., Narayan, K. M. V., and Magee, M. J. (2018). Contribution of smoking to tuberculosis incidence and mortality in high-tuberculosis-burden countries. Am. J. Epidemiol. 187 (9), 1846–1855. doi:10.1093/aje/kwy081
American Medical Association (1992). “Division of drugs and toxicology,” in Drug evaluations annual 1994. American Medical Association.
Amin, A. G., Goude, R., Shi, L., Zhang, J., Chatterjee, D., and Parish, T. (2008). EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiol. Read. Engl. 154 (Pt 1), 240–248. doi:10.1099/mic.0.2007/012153-0
Anandatheerthavarada, H. K., Williams, J. F., and Wecker, L. (1993a). Differential effect of chronic nicotine administration on brain cytochrome P4501a1/2 and P4502E1. Biochem. Biophysical Res. Commun. 194 (1), 312–318. doi:10.1006/bbrc.1993.1821
Anandatheerthavarada, H. K., Williams, J. F., and Wecker, L. (1993b). The chronic administration of nicotine induces cytochrome P450 in rat brain. J. Neurochem. 60 (5), 1941–1944. doi:10.1111/j.1471-4159.1993.tb13424.x
Arcavi, L., and Benowitz, N. L. (2004). Cigarette smoking and infection. Archives Intern. Med. 164 (20), 2206–2216. doi:10.1001/archinte.164.20.2206
Aridgides, D. S., Mellinger, D. L., Armstrong, D. A., Hazlett, H. F., Dessaint, J. A., Hampton, T. H., et al. (2019). Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress. Sci. Rep. 9 (1), 9624. doi:10.1038/s41598-019-46045-7
Arikayce (2023). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/207356s012lbl.pdf.
Aryanpur, M., Hosseini, M., Reza Masjedi, M., Mortaz, E., Tabarsi, P., Hamid, S., et al. (2016). A randomized controlled trial of smoking cessation methods in patients newly-diagnosed with pulmonary tuberculosis. BMC Infect. Dis. 16, 369. doi:10.1186/s12879-016-1727-4
Avelox (2016). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021085s063lbl.pdf.
Awaisu, A., Mohamed, M. H. N., Noordin, N. M., Aziz, N. A., Sulaiman, S. A. S., Muttalif, A. R., et al. (2011). The SCIDOTS project: evidence of benefits of an integrated tobacco cessation intervention in tuberculosis care on treatment outcomes. Subst. Abuse Treat. Prev. Policy 6, 26. doi:10.1186/1747-597X-6-26
A WHO the Union Monograph on TB and Tobacco Control (2005). Joining efforts to control two related global epidemics. Available online at: https://www.who.int/publications/i/item/WHO-HTM-TB-2007.390 (Accessed June 19, 2025).
Banerjee, D. K., Ellard, G. A., Gammon, P. T., and Waters, M. F. (1974). Some observations on the pharmacology of clofazimine (B663). Am. J. Trop. Med. Hyg. 23 (6), 1110–1115. doi:10.4269/ajtmh.1974.23.1110
Bardou, F., Raynaud, C., Ramos, C., Lanéelle, M. A., and Lanŕelle, G. (1998). Mechanism of isoniazid uptake in Mycobacterium tuberculosis. Microbiol. Read. Engl. 144, 2539–2544. doi:10.1099/00221287-144-9-2539
Barry, V. C., Belton, J. G., O’Sullivan, J. F., and Twomey, D. (1956). 650. The oxidation of derivatives of o-phenylenediamine. Part IV. A new series of glyoxalinophenazines derived from anilinoaposafranines and their behaviour on hydrogenation. J. Chem. Soc. 3347–3350. doi:10.1039/JR9560003347
Bates, M. N., Khalakdina, A., Pai, M., Chang, L., Lessa, F., and Smith, K. R. (2007). Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Archives Intern. Med. 167 (4), 335–342. doi:10.1001/archinte.167.4.335
Batista, L., d’Arc, J., and Rodrigues, L. C. (2008). Smoking increases the risk of relapse after successful tuberculosis treatment. Int. J. Epidemiol. 37 (4), 841–851. doi:10.1093/ije/dyn113
Bay, J. G., Patsche, C. B., Marie Svendsen, N., Gomes, V. F., Rudolf, F., and Wejse, C. (2022). Tobacco smoking impact on tuberculosis treatment outcome: an observational study from west Africa. Int. J. Infect. Dis. IJID Official Publ. Int. Soc. Infect. Dis. 124 (Suppl. 1), S50–S55. doi:10.1016/j.ijid.2022.07.067
Belkaid, Y., and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell. 157 (1), 121–141. doi:10.1016/j.cell.2014.03.011
Bellanca, C. M., Augello, E., Di Benedetto, G., Burgaletto, C., Flavia Cantone, A., Cantarella, G., et al. (2024). A web-based scoping review assessing the influence of smoking and smoking cessation on antidiabetic drug meabolism: implications for medication efficacy. Front. Pharmacol. 15, 1406860. doi:10.3389/fphar.2024.1406860
Benowitz, N. L. (1997). The role of nicotine in smoking-related cardiovascular disease. Prev. Med. 26 (4), 412–417. doi:10.1006/pmed.1997.0175
Benowitz, N. L., and Jacob, P. (1993). Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin. Pharmacol. Ther. 53 (3), 316–323. doi:10.1038/clpt.1993.27
Benowitz, N. L., and Jacob, P. (2000). Effects of cigarette smoking and carbon monoxide on nicotine and cotinine metabolism. Clin. Pharmacol. Ther. 67 (6), 653–659. doi:10.1067/mcp.2000.107086
Benowitz, N. L., Peng, M., and Jacob, P. (2003). Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin. Pharmacol. Ther. 74 (5), 468–474. doi:10.1016/j.clpt.2003.07.001
Bergen, A. W., Javitz, H. S., Krasnow, R., Michel, M., Nishita, D., Conti, D. V., et al. (2014). Organic cation transporter variation and response to smoking cessation therapies. Nicotine and Tob. Res. Official J. Soc. Res. Nicotine Tob. 16 (12), 1638–1646. doi:10.1093/ntr/ntu161
Blaschke, T. F., and Skinner, M. H. (1996). The clinical pharmacokinetics of rifabutin. Clin. Infect. Dis. An Official Publ. Infect. Dis. Soc. Am. 22, S15–S22. doi:10.1093/clinids/22.supplement_1.s15
Bloom, B. R., Atun, R., Cohen, T., Dye, C., Fraser, H., Gomez, G. B., et al. (2017). “Tuberculosis,” in Major infectious diseases. Editors K. K. Holmes, S. Bertozzi, B. R. Bloom, and P. Jha, 3rd ed. (Washington (DC): The International Bank for Reconstruction and Development/The World Bank). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK525174/.
Borovinskaya, M. A., Pai, R. D., Zhang, W., Schuwirth, B. S., Holton, J. M., Hirokawa, Go, et al. (2007). Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat. Struct. and Mol. Biol. 14 (8), 727–732. doi:10.1038/nsmb1271
Boshoff, H. I., Mizrahi, V., and Barry, C. E. (2002). Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 184 (8), 2167–2172. doi:10.1128/JB.184.8.2167-2172.2002
Branch, R. A., Adedoyin, A., Frye, R. F., Wilson, J. W., and Romkes, M. (2000). In vivo modulation of CYP enzymes by quinidine and rifampin. Clin. Pharmacol. Ther. 68 (4), 401–411. doi:10.1067/mcp.2000.110561
British National Formulary (BNF) (2015). British national formulary (BNF). Pharmaceutical Press. Available online at: https://www.pharmaceuticalpress.com/products/british-national-formulary/ (Accessed March 12, 2024).
Bulitta, J. B., Ly, N. S., Landersdorfer, C. B., Wanigaratne, N. A., Velkov, T., Yadav, R., et al. (2015). Two mechanisms of killing of Pseudomonas aeruginosa by tobramycin assessed at multiple inocula via mechanism-based modeling. Antimicrob. Agents Chemother. 59 (4), 2315–2327. doi:10.1128/AAC.04099-14
Cahill, K., Stevens, S., Perera, R., and Lancaster, T. (2013). Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev. 2013 (5), CD009329. doi:10.1002/14651858.CD009329.pub2
Capastat (2019). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/050095s072lbl.pdf.
Champix (2006). Champix. European Medicines Agency EMA. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/champix.
Chandra Acharya, P., and Kurosu, M. (2023). Medicinal chemistry of chemotherapeutic agents: a comprehensive resource of anti-infective and anti-cancer drugs. 1st edition. Academic Press. doi:10.1016/C2020-0-03892-2
Chen, H., Cowan, M. J., Hasday, J. D., Vogel, S. N., and Medvedev, A. E. (2007). Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, P38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J. Immunol. Baltim. Md 179, 6097–6106. doi:10.4049/jimmunol.179.9.6097
Cholo, M. C., Mothiba, M. T., Fourie, B., and Anderson, R. (2017). Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J. Antimicrob. Chemother. 72 (2), 338–353. doi:10.1093/jac/dkw426
Cohen, G., Bellanca, C. M., Bernardini, R., Rose, J. E., and Polosa, R. (2024). Personalized and adaptive interventions for smoking cessation: emerging trends and determinants of efficacy. iScience 27 (11), 111090. doi:10.1016/j.isci.2024.111090
Collier, A. C., Tingle, M. D., Paxton, J. W., Mitchell, M. D., and Keelan, J. A. (2002). Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum. Reprod. Oxf. Engl. 17 (10), 2564–2572. doi:10.1093/humrep/17.10.2564
Connarn, J. N., Zhang, X., Babiskin, A., and Sun, D. (2015). Metabolism of bupropion by carbonyl reductases in liver and intestine. Drug Metabolism Dispos. Biol. Fate Chem. 43 (7), 1019–1027. doi:10.1124/dmd.115.063107
Conradie, F., Bagdasaryan, T. R., Borisov, S., Howell, P., Mikiashvili, L., Ngubane, N., et al. (2022). Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N. Engl. J. Med. 387 (9), 810–823. doi:10.1056/NEJMoa2119430
Conradie, F., Diacon, A. H., Ngubane, N., Howell, P., Everitt, D., Crook, A. M., et al. (2020). Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 382 (10), 893–902. doi:10.1056/NEJMoa1901814
Corleis, B., Tzouanas, C. N., Wadsworth, M. H., Cho, J. L., Linder, A. H., Schiff, A. E., et al. (2023). Tobacco smoke exposure recruits inflammatory airspace monocytes that establish permissive lung niches for Mycobacterium tuberculosis. Sci. Transl. Med. 15 (725), eadg3451. doi:10.1126/scitranslmed.adg3451
Court, M. H. (2010). Interindividual variability in hepatic drug glucuronidation: studies into the role of age, sex, enzyme inducers, and genetic polymorphism using the human liver bank as a model system. Drug Metab. Rev. 42 (1), 209–224. doi:10.3109/03602530903209288
Crabol, Y., Catherinot, E., Veziris, N., Vincent, J., and Olivier, L. (2016). Rifabutin: where do we stand in 2016? J. Antimicrob. Chemother. 71 (7), 1759–1771. doi:10.1093/jac/dkw024
Dalet-Beluche, I., Boulenc, X., Fabre, G., Maurel, P., and Bonfils, C. (1992). Purification of two cytochrome P450 isozymes related to CYP2A and CYP3A gene families from monkey (baboon, Papio Papio) liver microsomes. Cross reactivity with human forms. Eur. J. Biochem. 204 (2), 641–648. doi:10.1111/j.1432-1033.1992.tb16677.x
Dallenga, T., Repnik, U., Corleis, B., Eich, J., Reimer, R., Griffiths, G. W., et al. (2017). Tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages. Cell. Host and Microbe 22 (4), 519–530.e3. doi:10.1016/j.chom.2017.09.003
Dana, N. R., Rika, S., Pudia M, I., Alexander, M. B., Muthia, S., Linda, R., et al. (2024). Modifiable and non-modifiable risk factors for tuberculosis among adults in Indonesia: a systematic review and meta-analysis. Afr. J. Infect. Dis. 18 (2), 19–28. doi:10.21010/Ajidv18i2.3
Delaney, J., and Timbrell, J. A. (1995). Role of cytochrome P450 in hydrazine toxicity in isolated hepatocytes in vitro. Xenobiotica; Fate Foreign Compd. Biol. Syst. 25 (12), 1399–1410. doi:10.3109/00498259509061927
Deltyba (2014). Deltyba. European Medicines Agency EMA. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/deltyba.
Dogar, O., Keding, A., Gabe, R., Marshall, A.-M., Huque, R., Barua, D., et al. (2020). Cytisine for smoking cessation in patients with tuberculosis: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet 8 (11), e1408–e1417. doi:10.1016/S2214-109X(20)30312-0
Dover, L. G., Alahari, A., Paul, G., Gomes, J. M., Bhowruth, V., Reynolds, R. C., et al. (2007). EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob. Agents Chemother. 51 (3), 1055–1063. doi:10.1128/AAC.01063-06
Dragacci, S., Hamar-Hansen, C., Fournel-Gigleux, S., Lafaurie, C., Magdalou, J., and Siest, G. (1987). Comparative study of clofibric acid and bilirubin glucuronidation in human liver microsomes. Biochem. Pharmacol. 36 (22), 3923–3927. doi:10.1016/0006-2952(87)90459-x
DrugBank Clinical API Plugins (2024). DrugBank clinical API Plugins. Available online at: https://dev.drugbank.com/demo/ddi_checker (Accessed March 12, 2024).
Drug Interaction Checker Quickly Check Your Meds (2024). Drug interaction checker quickly Check Your Meds. Drugs. Com. Available online at: https://www.drugs.com/drug_interactions.html (Accessed November 26, 2024).
Drug Interactions Checker - Medscape Drug Reference Database (2024). Drug interactions checker - Medscape drug reference database. Available online at: https://reference.medscape.com/drug-interactionchecker (Accessed November 26, 2024).
DRUG-REAX (2024). University of technology sydney. Available online at: https://search.lib.uts.edu.au/discovery/fulldisplay/alma991001040579705671/61UTS_INST:61UTS (Accessed March 12, 2024).
Drug-Resistant Tuberculosis (2024). Key updates to the treatment of drug-resistant tuberculosis: rapid communication. Available online at: https://www.who.int/publications/i/item/B09123 (Accessed November 25, 2024).
Dryden, M. S. (2011). Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J. Antimicrob. Chemother. 66, 7–15. doi:10.1093/jac/dkr072
Ellard, G. A., and Gammon, P. T. (1976). Pharmacokinetics of isoniazid metabolism in man. J. Pharmacokinet. Biopharm. 4 (2), 83–113. doi:10.1007/BF01086149
Faber, M. S., and Uwe, F. (2004). Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin. Pharmacol. and Ther. 76 (2), 178–184. doi:10.1016/j.clpt.2004.04.003
Fàbrega, A., Madurga, S., Giralt, E., and Vila, J. (2009). Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2 (1), 40–61. doi:10.1111/j.1751-7915.2008.00063.x
Fan, J., Yuan, Z., Ran, M., Tang, J., Zhu, J., Aldrich, M. C., et al. (2023). Cross-talks between gut microbiota and tobacco smoking: a two-sample mendelian randomization study. BMC Med. 21 (1), 163. doi:10.1186/s12916-023-02863-1
Feldman, C., Theron, A. J., Cholo, M. C., and Anderson, R. (2024). Cigarette smoking as a risk factor for tuberculosis in adults: epidemiology and aspects of disease pathogenesis. Pathog. Basel, Switz. 13 (2), 151. doi:10.3390/pathogens13020151
Feng, P. C., Fenselau, C. C., and Jacobson, R. R. (1981). Metabolism of clofazimine in leprosy patients. Drug Metabolism Dispos. Biol. Fate Chem. 9 (6), 521–524. doi:10.1016/s0090-9556(25)06563-8
Feng, Y., Kong, Y., Barnes, P. F., Huang, F.-F., Klucar, P., Wang, X., et al. (2011). Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect. Immun. 79 (1), 229–237. doi:10.1128/IAI.00709-10
Fish, D. N., and Chow, A. T. (1997). The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32 (2), 101–119. doi:10.2165/00003088-199732020-00002
Fuhr, U., and Rost, K. L. (1994). Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics 4 (3), 109–116. doi:10.1097/00008571-199406000-00001
Gajalakshmi, V., Peto, R., Kanaka, T. S., and Jha, P. (2003). Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet London, Engl. 362 (9383), 507–515. doi:10.1016/S0140-6736(03)14109-8
Getahun, H., Gunneberg, C., Granich, R., and Paul, N. (2010). HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. An Official Publ. Infect. Dis. Soc. Am. 50 (Suppl. 3), S201–S207. doi:10.1086/651492
Global Tuberculosis Report (2023). Glob. Tuberc. Rep.Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (Accessed 25 November 2024).
Goodman, L. S., Goodman Gilman, A., Hardman, L., Limbird, L. E., and Goodman Gilman, A. (2001). Goodman and gilman’s the pharmacological basis of therapeutics.
Goodwin, B., Redinbo, M. R., and Kliewer, S. A. (2002). Regulation of Cyp3a gene transcription by the pregnane x receptor. Annu. Rev. Pharmacol. Toxicol. 42, 1–23. doi:10.1146/annurev.pharmtox.42.111901.111051
Goude, R., Amin, A. G., Chatterjee, D., and Parish, T. (2009). The arabinosyltransferase EmbC is inhibited by ethambutol in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 53 (10), 4138–4146. doi:10.1128/AAC.00162-09
Hardman, J. G., Limbird, L. E., Gilman, A. G., and McGraw, H. (2001). Goodman and gilman’s the pharmacological basis of therapeutics. 10th Edition (New York: McGraw-Hill), 1392–1393.
Hayward, S., Harding, R. M., McShane, H., and Tanner, R. (2018). Factors influencing the higher incidence of tuberculosis among migrants and ethnic minorities in the UK. F1000Research 7, 461. doi:10.12688/f1000research.14476.2
Helmig, S., Udo Seelinger, J., Philipp-Gehlhaar, M., Döhrel, J., and Schneider, J. (2010). Cyp1B1 mRNA expression in correlation to cotinine levels with respect to the Cyp1B1 L432V gene polymorphism. Eur. J. Epidemiol. 25 (12), 867–873. doi:10.1007/s10654-010-9505-x
Henderson, M. C., Siddens, L. K., Morré, J. T., Krueger, S. K., and Williams, D. E. (2008). Metabolism of the anti-tuberculosis drug ethionamide by mouse and human FMO1, FMO2 and FMO3 and mouse and human lung microsomes. Toxicol. Appl. Pharmacol. 233 (3), 420–427. doi:10.1016/j.taap.2008.09.017
Hirota, T., Takane, H., Higuchi, S., and Ieiri, I. (2008). Epigenetic regulation of genes encoding drug-metabolizing enzymes and transporters; DNA methylation and other mechanisms. Curr. Drug Metab. 9 (1), 34–38. doi:10.2174/138920008783331130
Hodge, S., Hodge, G., Ahern, J., Jersmann, H., Holmes, M., and Reynolds, P. N. (2007). Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 37 (6), 748–755. doi:10.1165/rcmb.2007-0025OC
Hoffmann, D. H., and Hoffmann, I. (1997). The changing cigarette, 1950-1995. J. Toxicol. Environ. Health 50 (4), 307–364. doi:10.1080/009841097160393
Honda, Y., Takahashi, H., Kuroki, Y., Akino, T., and Abe, S. (1996). Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest 109 (4), 1006–1009. doi:10.1378/chest.109.4.1006
Howlader, S., Kim, M.-J., Jony, M. R., Long, N. P., Cho, Y.-S., Kim, D.-H., et al. (2022). Characterization of clofazimine metabolism in human liver microsomal incubation in vitro. Antimicrob. Agents Chemother. 66 (10), e0056522. doi:10.1128/aac.00565-22
Hughes, D. A., Haslam, P. L., Townsend, P. J., and Turner-Warwick, M. (1985). Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clin. Exp. Immunol. 61 (2), 459–466.
Hussain, Z., Zhu, J., and Ma, X. (2021). Metabolism and hepatotoxicity of pyrazinamide, an antituberculosis drug. Drug Metabolism Dispos. Biol. Fate Chem. 49 (8), 679–682. doi:10.1124/dmd.121.000389
INTERCheck WEB (2024). INTERCheck WEB. Available online at: https://intercheckweb.marionegri.it/home#/main (Accessed November 26, 2024).
Jamis-Dow, C. A., Katki, A. G., Collins, J. M., and Klecker, R. W. (1997). Rifampin and rifabutin and their metabolism by human liver esterases. Xenobiotica; Fate Foreign Compd. Biol. Syst. 27 (10), 1015–1024. doi:10.1080/004982597239994
Jefferson, J. W., Pradko, J. F., and Muir, K. T. (2005). Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin. Ther. 27 (11), 1685–1695. doi:10.1016/j.clinthera.2005.11.011
Jha, P., Jacob, B., Gajalakshmi, V., Gupta, P. C., Dhingra, N., Kumar, R., et al. (2008). A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. 358 (11), 1137–1147. doi:10.1056/NEJMsa0707719
Johnson, T. M., Rivera, C. G., Lee, G., and Zeuli, J. D. (2024). Pharmacology of emerging drugs for the treatment of multi-drug resistant tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 37, 100470. doi:10.1016/j.jctube.2024.100470
Kim, M., Johnson, C. E., Schmalstig, A. A., Annis, A., Wessel, S. E., Van Horn, B., et al. (2022). A long-acting formulation of rifabutin is effective for prevention and treatment of Mycobacterium tuberculosis. Nat. Commun. 13 (1), 4455. doi:10.1038/s41467-022-32043-3
Kirkham, P. A., Spooner, G., Rahman, I., and Rossi, A. G. (2004). Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophysical Res. Commun. 318 (1), 32–37. doi:10.1016/j.bbrc.2004.04.003
Knox, C., Wilson, M., Klinger, C. M., Franklin, M., Oler, E., Wilson, A., et al. (2024). DrugBank 6.0: the DrugBank knowledgebase for 2024. Nucleic Acids Res. 52 (D1), D1265–D1275. doi:10.1093/nar/gkad976
Kroon, L. A. (2007). Drug interactions with smoking. Am. J. Health-System Pharm. AJHP Official J. Am. Soc. Health-System Pharm. 64 (18), 1917–1921. doi:10.2146/ajhp060414
Krueger, S. K., and Williams, D. E. (2005). Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. and Ther. 106 (3), 357–387. doi:10.1016/j.pharmthera.2005.01.001
Kumagai, T., Suzuki, H., Sasaki, T., Sakaguchi, S., Miyairi, S., Yamazoe, Y., et al. (2012). Polycyclic aromatic hydrocarbons activate CYP3A4 gene transcription through human pregnane X receptor. Drug Metabolism Pharmacokinet. 27 (2), 200–206. doi:10.2133/dmpk.dmpk-11-rg-094
Lacroix, C., Hoang, T. P., Nouveau, J., Guyonnaud, C., Laine, G., Duwoos, H., et al. (1989). Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur. J. Clin. Pharmacol. 36 (4), 395–400. doi:10.1007/BF00558302
Lamprene (2019). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/019500s014lbl.pdf.
Leach, K. L., Brickner, S. J., Noe, M. C., and Miller, P. F. (2011). Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 1222, 49–54. doi:10.1111/j.1749-6632.2011.05962.x
Lechartier, B., and Cole, S. T. (2015). Mode of action of clofazimine and combination therapy with benzothiazinones against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 59 (8), 4457–4463. doi:10.1128/AAC.00395-15
Lee, B. L., Benowitz, N. L., and Jacob, P. (1987). Influence of tobacco abstinence on the disposition kinetics and effects of nicotine. Clin. Pharmacol. Ther. 41 (4), 474–479. doi:10.1038/clpt.1987.59
Lee, S. Y., Jang, H., Lee, J.-Y., Kwon, K., Oh, S. J., and Kim, S. K. (2014). Inhibition of cytochrome P450 by ethambutol in human liver microsomes. Toxicol. Lett. 229 (1), 33–40. doi:10.1016/j.toxlet.2014.06.006
Leemans, J. C., Thepen, T., Weijer, S., Florquin, S., van Rooijen, N., van de Winkel, J. G., et al. (2005). Macrophages play a dual role during pulmonary tuberculosis in mice. J. Infect. Dis. 191 (1), 65–74. doi:10.1086/426395
Lehmann, J. M., McKee, D. D., Watson, M. A., Willson, T. M., Moore, J. T., and Kliewer, S. A. (1998). The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investigation 102 (5), 1016–1023. doi:10.1172/JCI3703
Leite, G., Barlow, G. M., Hosseini, A., Parodi, G., Pimentel, M. L., Wang, J., et al. (2022). Smoking has disruptive effects on the small bowel luminal microbiome. Sci. Rep. 12 (1), 6231. doi:10.1038/s41598-022-10132-z
Les interactions médicamenteuses (2024). “Les interactions médicamenteuses,” in Vidal. Available online at: https://www.vidal.fr/medicaments/utilisation/prendre-traitement/interactions-medicamenteuses.html (Accessed March 12, 2024).
Levaquin (2008). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf.
Levy, L. (1974). Pharmacologic studies of clofazimine. Am. J. Trop. Med. Hyg. 23 (6), 1097–1109. doi:10.4269/ajtmh.1974.23.1097
Lewis, J. M., and Sloan, D. J. (2015). The role of delamanid in the treatment of drug-resistant tuberculosis. Ther. Clin. Risk Manag. 11, 779–791. doi:10.2147/TCRM.S71076
Lips, K. S., Volk, C., Schmitt, B. M., Pfeil, U., Arndt, P., Miska, D., et al. (2005). Polyspecific cation transporters mediate luminal Release of acetylcholine from bronchial epithelium. Am. J. Respir. Cell. Mol. Biol. 33 (1), 79–88. doi:10.1165/rcmb.2004-0363OC
Lönnroth, K., Jaramillo, E., Williams, B. G., Dye, C., and Raviglione, M. (2009). Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc. Sci. and Med. 68 (12), 2240–2246. doi:10.1016/j.socscimed.2009.03.041
Loos, U., Musch, E., Jensen, J. C., Schwabe, H. K., and Eichelbaum, M. (1987). Influence of the enzyme induction by rifampicin on its presystemic metabolism. Pharmacol. and Ther. 33 (1), 201–204. doi:10.1016/0163-7258(87)90052-0
Lugg, S. T., Scott, A., Parekh, D., Naidu, B., and Thickett, D. R. (2022). Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax 77 (1), 94–101. doi:10.1136/thoraxjnl-2020-216296
Maartens, G., Brill, M. J. E., Pandie, M., and Svensson, E. M. (2018). Pharmacokinetic interaction between bedaquiline and clofazimine in patients with drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. Official J. Int. Union Against Tuberc. Lung Dis. 22 (1), 26–29. doi:10.5588/ijtld.17.0615
Mahishale, V., Patil, B., Lolly, M., Eti, A., and Khan, S. (2015). Prevalence of smoking and its impact on treatment outcomes in newly diagnosed pulmonary tuberculosis patients: a hospital-based prospective study. Chonnam Med. J. 51 (2), 86–90. doi:10.4068/cmj.2015.51.2.86
Maideen, N. M. P. (2019). Tobacco smoking and its drug interactions with comedications involving CYP and UGT enzymes and nicotine. World J. Pharmacol. 8 (2), 14–25. doi:10.5497/wjp.v8.i2.14
Malo, A., Kellermann, T., Ignatius, E. H., Dooley, K. E., Dawson, R., Joubert, A., et al. (2021). A validated liquid chromatography tandem mass spectrometry assay for the analysis of pretomanid in plasma samples from pulmonary tuberculosis patients. J. Pharm. Biomed. Analysis 195, 113885. doi:10.1016/j.jpba.2020.113885
McDonnell, A. M., and Dang, C. H. (2013). Basic review of the cytochrome P450 system. J. Adv. Pract. Oncol. 4 (4), 263–268. doi:10.6004/jadpro.2013.4.4.7
McKENNIS, H., Yard, A. S., Weatherby, J. H., and Hagy, J. A. (1959). Acetylation of hydrazine and the formation of 1,2-diacetylhydrazine in vivo. J. Pharmacol. Exp. Ther. 126 (2), 109–116. doi:10.1016/s0022-3565(25)25600-2
McNab, F. W., Berry, M. P. R., Graham, C. M., Bloch, S. A. A., Oni, T., Wilkinson, K. A., et al. (2011). Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur. J. Immunol. 41 (7), 1941–1947. doi:10.1002/eji.201141421
Meech, R., and Mackenzie, P. I. (1997). Structure and function of uridine diphosphate glucuronosyltransferases. Clin. Exp. Pharmacol. Physiology 24 (12), 907–915. doi:10.1111/j.1440-1681.1997.tb02718.x
Mesens, N., Steemans, M., Hansen, E., Verheyen, G. R., Van Goethem, F., and Van Gompel, J. (2010). Screening for phospholipidosis induced by central nervous drugs: comparing the predictivity of an in vitro assay to high throughput in silico Assays. Toxicol. Vitro An Int. J. Publ. Assoc. BIBRA 24 (5), 1417–1425. doi:10.1016/j.tiv.2010.04.007
Metushi, I. G., and Jack, U. (2014). Isoniazid-induced liver injury and immune response in mice. J. Immunotoxicol. 11 (4), 383–392. doi:10.3109/1547691X.2013.860644
Miller, L. G., Goldstein, G., Murphy, M., and Ginns, L. C. (1982). Reversible alterations in immunoregulatory T cells in smoking. Analysis by monoclonal antibodies and flow cytometry. Chest 82 (5), 526–529. doi:10.1378/chest.82.5.526
Mitchison, J. M. (1956). The thickness of the cortex of the sea-urchin egg and the problem of the vitelline membrane. J. Cell. Sci. s3-97 (37), 109–121. doi:10.1242/jcs.s3-97.37.109
Mitnick, C. D., Bryan, M. G., and Peloquin, C. A. (2009). Tuberculosis pharmacotherapy: strategies to optimize patient care. Expert Opin. Pharmacother. 10 (3), 381–401. doi:10.1517/14656560802694564
Moré, J. M., Voelker, D. R., Silveira, L. J., Edwards, M. G., Chan, E. D., and Bowler, R. P. (2010). Smoking reduces surfactant protein D and phospholipids in patients with and without chronic obstructive pulmonary disease. BMC Pulm. Med. 10, 53. doi:10.1186/1471-2466-10-53
Myambutol (2008). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/016320s063lbl.pdf.
Nakajima, A., Fukami, T., Kobayashi, Y., Watanabe, A., Nakajima, M., and Yokoi, T. (2011). Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine. Biochem. Pharmacol. 82 (11), 1747–1756. doi:10.1016/j.bcp.2011.08.003
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health (2014). The health Consequences of smoking—50 Years of progress: a Report of the surgeon general. Reports of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention US. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK179276/.
Ngo, S. C., Zimhony, O., Chung, W. J., Sayahi, H., Jacobs, W. R., and Welch, J. T. (2007). Inhibition of isolated Mycobacterium tuberculosis fatty acid synthase I by pyrazinamide analogs. Antimicrob. Agents Chemother. 51 (7), 2430–2435. doi:10.1128/AAC.01458-06
Niemi, M., Backman, J. T., Fromm, M. F., Neuvonen, P. J., and Kivistö, K. T. (2003). Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42 (9), 819–850. doi:10.2165/00003088-200342090-00003
Nijenbandring de Boer, R., Cobelens, F., Ramalho, D., Miranda, P. F. C., Logo, K., Oliveira, H., et al. (2014). Delayed culture conversion due to cigarette smoking in active pulmonary tuberculosis patients. Tuberc. Edinb. Scotl. 94 (1), 87–91. doi:10.1016/j.tube.2013.10.005
Novotny, T. E. (2008). Smoking cessation and tuberculosis: connecting the DOTS. Int. J. Tuberc. Lung Dis. Official J. Int. Union Against Tuberc. Lung Dis. 12 (10), 1103.
Noykhovich, E., Mookherji, S., and Roess, A. (2019). The risk of tuberculosis among populations living in slum settings: a systematic review and meta-analysis. J. Urban Health Bull. N. Y. Acad. Med. 96 (2), 262–275. doi:10.1007/s11524-018-0319-6
Nunn, A. J., Phillips, P. P. J., Meredith, S. K., Chiang, C.-Y., Conradie, F., Dalai, D., et al. (2019). A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 380 (13), 1201–1213. doi:10.1056/NEJMoa1811867
Nyang'wa, B. T., Berry, C., Kazounis, E., Motta, I., Parpieva, N., Tigay, Z., et al. (2022). A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 387 (25), 2331–2343. doi:10.1056/NEJMoa2117166
O’Leary, S. M., Coleman, M. M., Mei Chew, W., Morrow, C., McLaughlin, A. M., Gleeson, L. E., et al. (2014). Cigarette smoking impairs human pulmonary immunity to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 190 (12), 1430–1436. doi:10.1164/rccm.201407-1385OC
O’Malley, M., King, A. N., Conte, M., Ellingrod, V. L., and Ramnath, N. (2014). Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J. Thorac. Oncol. 9 (7), 917–926. doi:10.1097/JTO.0000000000000191
Pachauri, V., and Flora, S. J. S. (2013). Effect of nicotine pretreatment on arsenic-induced oxidative stress in male wistar rats. Hum. and Exp. Toxicol. 32 (9), 972–982. doi:10.1177/0960327112474833
Pampaloni, A., Locatelli, M. E., Venanzi Rullo, E., Alaimo, S., Cosentino, F., Marino, A., et al. (2021). “Diagnosis on the dock” project: a proactive screening program for diagnosing pulmonary tuberculosis in disembarking refugees and new sei model. Int. J. Infect. Dis. 106, 98–104. doi:10.1016/j.ijid.2021.03.032
Pareek, M., Greenaway, C., Noori, T., Munoz, J., and Zenner, D. (2016). The impact of migration on tuberculosis epidemiology and control in high-income countries: a review. BMC Med. 14 (1), 48. doi:10.1186/s12916-016-0595-5
Peets, E. A., Sweeney, W. M., Place, V. A., and Buyske, D. A. (1965). The absorption, excretion, and metabolic fate of ethambutol in man. Am. Rev. Respir. Dis. 91, 51–58. doi:10.1164/arrd.1965.91.1.51
PharmGKB (2024). PharmGKB. Available online at: https://www.pharmgkb.org/(Accessed November 27, 2024).
Pienaar, E., Sarathy, J., Prideaux, B., Dietzold, J., Dartois, V., Kirschner, D. E., et al. (2017). Comparing efficacies of moxifloxacin, levofloxacin and gatifloxacin in tuberculosis granulomas using a multi-scale systems pharmacology approach. PLoS Comput. Biol. 13 (8), e1005650. doi:10.1371/journal.pcbi.1005650
Polosa, R., and Benowitz, N. L. (2011). Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol. Sci. 32 (5), 281–289. doi:10.1016/j.tips.2010.12.008
Pretomanid (2019). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212862s000lbl.pdf.
Priftin (2000). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21024S5_Priftin.cfm.
Pstragowski, M., Zbrzezna, M., and Bujalska-Zadrozny, M. (2017). Advances in pharmacotherapy of tuberculosiS. Acta Pol. Pharm. 74 (1), 3–11.
Quan, D. H., Kwong, A. J., Hansbro, P. M., and Britton, W. J. (2022). No smoke without fire: the impact of cigarette smoking on the immune control of tuberculosis. Eur. Respir. Rev. An Official J. Eur. Respir. Soc. 31 (164), 210252. doi:10.1183/16000617.0252-2021
Rae, J. M., Johnson, M. D., Lippman, M. E., and Flockhart, D. A. (2001). Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J. Pharmacol. Exp. Ther. 299 (3), 849–857. doi:10.1016/s0022-3565(24)29202-8
Rawat, A., Chaturvedi, S., Singh, A. K., Guleria, A., Dubey, D., Keshari, A. K., et al. (2018). Metabolomics approach discriminates toxicity index of pyrazinamide and its metabolic products, pyrazinoic acid and 5-hydroxy pyrazinoic acid. Hum. and Exp. Toxicol. 37 (4), 373–389. doi:10.1177/0960327117705426
Riccardi, N., Monari, C., Antonello, R. M., Saderi, L., Occhineri, S., Pontarelli, A., et al. (2024). TB outpatient care in a high-income, low-incidence country. Int. J. Tuberc. Lung Dis. Official J. Int. Union Against Tuberc. Lung Dis. 28 (10), 513–515. doi:10.5588/ijtld.24.0059
Rifadin (2023). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/050420s089,050627s034lbl.pdf.
Sarathy, J., Blanc, L., Alvarez-Cabrera, N., Brien, P., Dias-Freedman, I., Mina, M., et al. (2019). Fluoroquinolone efficacy against tuberculosis is driven by penetration into lesions and activity against resident bacterial populations. Antimicrob. Agents Chemother. 63 (5), e02516-18–18. doi:10.1128/AAC.02516-18
Sasahara, K., Shimokawa, Y., Hirao, Y., Koyama, N., Kitano, K., Shibata, M., et al. (2015). Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: importance of albumin metabolism in vivo. Drug Metabolism Dispos. 43, 1267–1276. doi:10.1124/dmd.115.064527
Schneider, N. K., and Thomas, E. N. (2007). Addressing smoking cessation in tuberculosis control. Bull. World Health Organ. 85 (10), 820–821. doi:10.2471/blt.07.034797
Seree, E. M., Villard, P. H., Re, J. L., De Meo, M., Lacarelle, B., Attolini, L., et al. (1996). High inducibility of mouse renal CYP2E1 gene by tobacco smoke and its possible effect on DNA single strand breaks. Biochem. Biophysical Res. Commun. 219 (2), 429–434. doi:10.1006/bbrc.1996.0250
Serio, A. W., Keepers, T., Andrews, L., and Krause, K. M. (2018). Aminoglycoside revival: review of a historically important class of antimicrobials undergoing rejuvenation. EcoSal Plus 8 (1). doi:10.1128/ecosalplus.ESP-0002-2018
Sharma, S. K., Mohan, A., Singh, A. D., Mishra, H., Jhanjee, S., Pandey, R. M., et al. (2018). Impact of nicotine replacement therapy as an adjunct to anti-tuberculosis treatment and behaviour change counselling in newly diagnosed pulmonary tuberculosis patients: an open-label, randomised controlled trial. Sci. Rep. 8 (1), 8828. doi:10.1038/s41598-018-26990-5
Shi, W., Zhang, X., Jiang, X., Yuan, H., Lee, J. S., Barry, C. E., et al. (2011). Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Sci. (New York, N.Y.) 333 (6049), 1630–1632. doi:10.1126/science.1208813
Shih, T.-Y., Pai, C.-Y, Yang, P., Chang, W.-L., Wang, N.-C., and Hu, O. (2013). A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob. Agents Chemother. 57 (4), 1685–1690. doi:10.1128/AAC.01866-12
Shimada, T., Yamazaki, H., Mimura, M., Inui, Y., and Guengerich, F. P. (1994). Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 caucasians. J. Pharmacol. Exp. Ther. 270 (1), 414–423. doi:10.1016/s0022-3565(25)22379-5
Shimokawa, Y., Yoda, N., Kondo, S., Yamamura, Y., Takiguchi, Y., and Umehara, K. (2015). Inhibitory potential of twenty five anti-tuberculosis drugs on CYP activities in human liver microsomes. Biol. and Pharm. Bull. 38 (9), 1425–1429. doi:10.1248/bpb.b15-00313
Shinabarger, D. L., Marotti, K. R., Murray, R. W., Lin, A. H., Melchior, E. P., Swaney, S. M., et al. (1997). Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41 (10), 2132–2136. doi:10.1128/aac.41.10.2132
Singh, R., Manjunatha, U., Boshoff, H. I. M., Ha, Y. H., Niyomrattanakit, P., Ledwidge, R., et al. (2008). PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO Release, New York, N.Y. Science 322, 1392–1395. doi:10.1126/science.1164571
Sirturo (2012). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf.
Slama, K., Chiang, C.-Y., Enarson, D. A., Hassmiller, K., Fanning, A., Gupta, P., et al. (2007). Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. Official J. Int. Union Against Tuberc. Lung Dis. 11 (10), 1049–1061.
Slatter, J. G., Stalker, D. J., Feenstra, K. L., Welshman, I. R., Bruss, J. B., Sams, J. P., et al. (2001). Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metabolism Dispos. Biol. Fate Chem. 29 (8), 1136–1145.
Smit, Z., van, R. N., Binder, A., Meldau, R., Semple, P. L., Evans, A., et al. (2014). Cigarette smoke impairs cytokine responses and BCG containment in alveolar macrophages. Thorax 69 (4), 363–370. doi:10.1136/thoraxjnl-2013-204229
Smit, Z., van, R. N., Pai, M., Yew, W. W., Leung, C. C., Zumla, A., et al. (2010). Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur. Respir. J. 35 (1), 27–33. doi:10.1183/09031936.00072909
Song, M., Zhang, M., Han, J., and Fu, W. (2024). Construction and validation of a nomogram to identify the risk of cavitation in pulmonary tuberculosis. Infect. Drug Resist. 17, 2803–2813. doi:10.2147/IDR.S459330
Sopori, M. (2002). Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2 (5), 372–377. doi:10.1038/nri803
Stepanov, I., Villalta, P. W., Knezevich, A., Jensen, J., Hatsukami, D., and Hecht, S. S. (2010). Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem. Res. Toxicol. 23 (1), 66–73. doi:10.1021/tx900281u
Strydom, N., Gupta, S. V., Fox, W. S., Via, L. E., Bang, H., Lee, M., et al. (2019). Tuberculosis drugs’ distribution and emergence of resistance in patient’s lung lesions: a mechanistic model and tool for regimen and dose optimization. PLoS Med. 16 (4), e1002773. doi:10.1371/journal.pmed.1002773
Sullivan, O., Poitevin, F., Sierra, R. G., Gati, C., Han Dao, E., Rao, Y., et al. (2018). Aminoglycoside ribosome interactions reveal novel conformational States at ambient temperature. Nucleic Acids Res. 46 (18), 9793–9804. doi:10.1093/nar/gky693
Sumida, A., Fukuen, S., Yamamoto, I., Matsuda, H., Naohara, M., and Azuma, J. (2000). Quantitative analysis of constitutive and inducible CYPs mRNA expression in the HepG2 cell line using reverse transcription-competitive PCR. Biochem. Biophysical Res. Commun. 267 (3), 756–760. doi:10.1006/bbrc.1999.2029
Szumowski, J. D., and Lynch, J. B. (2015). Profile of delamanid for the treatment of multidrug-resistant tuberculosis. Drug Des. Dev. Ther. 9, 677–682. doi:10.2147/DDDT.S60923
Tanoglu, A., Erdem, H., Friedland, J. S., Ankaralı, H., Garcia-Goez, J. F., Albayrak, A., et al. (2023). Clinicopathological profile of peritoneal tuberculosis and a new scoring model for predicting mortality: an international ID-IRI study. Eur. J. Clin. Microbiol. and Infect. Dis. 42 (8), 981–992. Official Publication of the European Society of Clinical Microbiology. doi:10.1007/s10096-023-04630-9
Tantcheva-Poór, I., Zaigler, M., Rietbrock, S., and Fuhr, U. (1999). Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy caucasians using a saliva-based caffeine test. Pharmacogenetics 9 (2), 131–144.
TB Disease Burden (2024). TB disease burden. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023/tb-disease-burden (Accessed November 29, 2024).
TB Incidence (2024). TB incidence. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023/tb-disease-burden/1-1-tb-incidence (Accessed November 29, 2024).
TB Preventive Treatment (2025). Identifying populations for TB preventive treatment TB knowledge sharing. Available online at: https://tbksp.who.int/en/node/631 (Accessed June 19, 2025).
The End TB Strategy (2025). The End TB strategy. Available online at: https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19 (Accessed June 19, 2025).
Tilley, A. E., Walters, M. S., Shaykhiev, R., and Crystal, R. G. (2015). Cilia dysfunction in lung disease. Annu. Rev. Physiology 77, 379–406. doi:10.1146/annurev-physiol-021014-071931
Tobacco Collaborators (2017). Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the global burden of disease study 2015. Lancet London, Engl. 389 (10082), 1885–1906. doi:10.1016/S0140-6736(17)30819-X
Tobacco Collaborators (2021). Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet London, Engl. 397 (10292), 2337–2360. doi:10.1016/S0140-6736(21)01169-7
Tostmann, A., Boeree, M. J., Aarnoutse, R. E., de Lange, W. C. M., van der Ven, A. J. A. M., and Dekhuijzen, R. (2008). Antituberculosis drug-induced hepatotoxicity: concise up-to-Date review. J. Gastroenterology Hepatology 23 (2), 192–202. doi:10.1111/j.1440-1746.2007.05207.x
Trecator (2016). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/013026s029lbl.pdf.
Tuberculosis and HIV (2025). Tuberculosis and HIV. Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/tuberculosis-hiv (Accessed June 19, 2025).
Unissa, A. N., Subbian, S., Hanna, L. E., and Selvakumar, N. (2016). Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 45, 474–492. doi:10.1016/j.meegid.2016.09.004
Urakami, Y., Okuda, M., Masuda, S., Saito, H., and Inui, K. I. (1998). Functional Characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J. Pharmacol. Exp. Ther. 287 (2), 800–805. doi:10.1016/s0022-3565(24)37859-0
Vanino, E., Granozzi, B., Akkerman, O. W., Munoz-Torrico, M., Palmieri, F., Seaworth, B., et al. (2023). Update of drug-resistant tuberculosis treatment guidelines: a turning point. Int. J. Infect. Dis. IJID Official Publ. Int. Soc. Infect. Dis. 130 (Suppl. 1), S12–S15. doi:10.1016/j.ijid.2023.03.013
Villard, P.-H., Hcrber, R., Sérée, E. M., Attolini, L., Magdalou, J., and Bruno, L. (1998). Effect of cigarette smoke on UDP-glucuronosyltransferase activity and cytochrome P450 content in liver, lung and kidney microsomes in mice. Pharmacol. and Toxicol. 82 (2), 74–79. doi:10.1111/j.1600-0773.1998.tb01401.x
Vistisen, K., Loft, S., and Poulsen, H. E. (1991). Cytochrome P450 IA2 activity in man measured by caffeine metabolism: effect of smoking, broccoli and exercise. Adv. Exp. Med. Biol. 283, 407–411. doi:10.1007/978-1-4684-5877-0_55
Voutsinas, J., Wilkens, L. R., Adrian, F., Vogt, T. M., Yokochi, L. A., Decker, R., et al. (2013). Heterocyclic amine intake, smoking, cytochrome P450 1A2 and N-acetylation phenotypes, and risk of colorectal adenoma in a multiethnic population. Gut 62 (3), 416–422. doi:10.1136/gutjnl-2011-300665
Wallace, B. J., Tai, P. C., Herzog, E. L., and Davis, B. D. (1973). Partial inhibition of polysomal ribosomes of Escherichia coli by streptomycin. Proc. Natl. Acad. Sci. U. S. A. 70 (4), 1234–1237. doi:10.1073/pnas.70.4.1234
Wang, E. Y., Arrazola, R. A., Mathema, B., Ahluwalia, I. B., and Mase, S. R. (2020). The impact of smoking on tuberculosis treatment outcomes: a meta-analysis. Int. J. Tuberc. Lung Dis. Official J. Int. Union Against Tuberc. Lung Dis. 24 (2), 170–175. doi:10.5588/ijtld.19.0002
Wang, H.-T., Lin, J.-H., Yang, C.-H., Haung, C.-H., Weng, C.-W., Lin, A.M.-Y., et al. (2017). Acrolein induces mtDNA damages, mitochondrial fission and mitophagy in human lung cells. Oncotarget 8 (41), 70406–70421. doi:10.18632/oncotarget.19710
Wang, L., Ma, Y., Duan, H., Yao, J., Liang, Li, Zhang, R., et al. (2015). Pharmacokinetics and tissue distribution study of PA-824 in rats by LC-MS/MS. J. Chromatogr. B, Anal. Technol. Biomed. Life Sci. 1006, 194–200. doi:10.1016/j.jchromb.2015.10.039
Wang, P., Pradhan, K., Zhong, X., and Ma, X. (2016). Isoniazid metabolism and hepatotoxicity. Acta Pharm. Sin. B 6 (5), 384–392. doi:10.1016/j.apsb.2016.07.014
Waters, M., and Tadi, P. (2024). “Streptomycin,” in StatPearls. Treasure island (FL) (StatPearls Publishing). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK555886/.
WHO Consolidated Guidelines on Tuberculosis (2024). WHO consolidated guidelines on tuberculosis: Module 4: treatment: drug-resistant tuberculosis treatment. Available online at: https://www.who.int/publications/i/item/9789240007048 (Accessed November 25, 2024).
WHO Consolidated Guidelines on Tuberculosis Module 1 (2024). WHO consolidated guidelines on tuberculosis Module 1: prevention - tuberculosis preventive treatment, Second Edition. Available online at: https://www.who.int/publications/i/item/9789240096196 (Accessed November 29, 2024).
WHO Global (2024). Report on trends in prevalence of tobacco use 2000-2025, Fourth Edition. Available online at: https://www.who.int/publications/i/item/9789240039322 (Accessed November 25, 2024).
WHO Global (2025). Report on trends in prevalence of tobacco use 2000–2030. Available online at: https://www.who.int/publications/i/item/9789240088283 (Accessed June 19, 2025).
WHO Report on the Global Tobacco Epidemic (2019). Offer help to quit tobacco use. Available online at: https://www.who.int/publications/i/item/9789241516204 (Accessed June 19, 2025).
WHO Report on the Global Tobacco Epidemic (2023). Protect people from tobacco smoke. Available online at: https://www.who.int/publications/i/item/9789240077164 (Accessed November 25, 2024).
Yano, T., Kassovska-Bratinova, S., Shin Teh, J., Winkler, J., Sullivan, K., Isaacs, A., et al. (2011). Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J. Biol. Chem. 286 (12), 10276–10287. doi:10.1074/jbc.M110.200501
Yen, Y.-F., Yen, M.-Y., Lin, Y.-S., Lin, Y.-P., Shih, H.-C., Li, L.-H., et al. (2014). Smoking increases risk of recurrence after successful anti-tuberculosis treatment: a population-based study. Int. J. Tuberc. Lung Dis. Official J. Int. Union Against Tuberc. Lung Dis. 18 (4), 492–498. doi:10.5588/ijtld.13.0694
Ying, L., Zhu, H., Shoji, S., and Fredrick, K. (2019). Roles of specific aminoglycoside-ribosome interactions in the inhibition of translation. RNA (New York, N.Y.) 25 (2), 247–254. doi:10.1261/rna.068460.118
Yue, J., Peng, R., Yang, J., Kong, R., and Liu, J. (2004). CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol. Sin. 25 (5), 699–704.
Zaverucha-do-Valle, C., Monteiro, S. P., El-Jaick, K. B., Rosadas, L. A., Costa, M. J. M., Quintana, M. S. B., et al. (2014). The role of cigarette smoking and liver enzymes polymorphisms in anti-tuberculosis drug-induced hepatotoxicity in Brazilian patients. Tuberc. Edinb. Scotl. 94 (3), 299–305. doi:10.1016/j.tube.2014.03.006
Zevin, S., and Benowitz, N. L. (1999). Drug interactions with tobacco smoking. An update. Clin. Pharmacokinet. 36 (6), 425–438. doi:10.2165/00003088-199936060-00004
Zhang, C., Ou, A., Jin, L., Yang, N., Deng, P., Guan, C., et al. (2024). Cadmium exposure triggers alveolar epithelial cell pyroptosis by inducing mitochondrial oxidative stress and activating the cGAS-STING pathway. Cell. Commun. Signal. CCS 22 (1), 566. doi:10.1186/s12964-024-01946-7
Zhang, L., Zhao, Y., Gao, Y., Wu, L., Gao, R., Qi, Z., et al. (2020). Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Sci. (New York, N.Y.) 368 (6496), 1211–1219. doi:10.1126/science.aba9102
Zhao, Q., Chen, Y., Huang, W., Zhou, H., and Zhang, W. (2023). Drug-microbiota interactions: an emerging priority for precision medicine. Signal Transduct. Target. Ther. 8 (1), 386. doi:10.1038/s41392-023-01619-w
Zimhony, O., Vilchèze, C., Arai, M., Welch, J. T., and Jacobs, W. R. (2007). Pyrazinoic acid and its N-propyl ester inhibit fatty acid synthase type I in replicating tubercle bacilli. Antimicrob. Agents Chemother. 51 (2), 752–754. doi:10.1128/AAC.01369-06
Zumla, A., Sahu, S., Ditiu, L., Singh, U., Park, Y.-J., Yeboah-Manu, D., et al. (2025). Inequities underlie the alarming resurgence of tuberculosis as the world’s top cause of death from an infectious disease - breaking the silence and addressing the underlying root causes. IJID Reg. 14 (Suppl. 2), 100587. doi:10.1016/j.ijregi.2025.100587
Zyvox (2014). U. S. Food Drug Adm. (FDA). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021130s032,021131s026,021132s031lbl.pdf.
Keywords: cytochrome P450, drug-drug interactions, adverse drug reactions, antibiotics, nicotine, smoking cessation
Citation: Bellanca CM, Polosa SP, Augello E, Di Benedetto G, Burgaletto C, Cantone AF, Gaudio G, Nunnari G, Campagna D, Nailes JM, Shahbaz H, Tanuwihardja RK, Mohan A, Ceccarelli M, Bernardini R, Marino A and Cantarella G (2025) A scoping review about smoking, smoking cessation and their effects on anti-tuberculosis agents: insights into drug metabolisms, safety, and effectiveness. Front. Pharmacol. 16:1606150. doi: 10.3389/fphar.2025.1606150
Received: 07 April 2025; Accepted: 26 June 2025;
Published: 16 July 2025.
Edited by:
Robert L. Lins, Independent Researcher, Antwerp, BelgiumReviewed by:
Hyunchan Lee, National Eye Institute (NIH), United StatesYaroslav Faletrov, Belarusian State University, Belarus
Copyright © 2025 Bellanca, Polosa, Augello, Di Benedetto, Burgaletto, Cantone, Gaudio, Nunnari, Campagna, Nailes, Shahbaz, Tanuwihardja, Mohan, Ceccarelli, Bernardini, Marino and Cantarella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Di Benedetto, Z2l1bGlhLmRpYmVuZWRldHRvQHVuaWN0Lml0
†These authors have contributed equally to this work
‡These authors share senior authorship
 Carlo Maria Bellanca
Carlo Maria Bellanca Simone Pietro Polosa4†
Simone Pietro Polosa4† Egle Augello
Egle Augello Giulia Di Benedetto
Giulia Di Benedetto Chiara Burgaletto
Chiara Burgaletto Anna Flavia Cantone
Anna Flavia Cantone Gabriella Gaudio
Gabriella Gaudio Davide Campagna
Davide Campagna Hamza Shahbaz
Hamza Shahbaz Reza Kurniawan Tanuwihardja
Reza Kurniawan Tanuwihardja Renato Bernardini
Renato Bernardini Andrea Marino
Andrea Marino Giuseppina Cantarella
Giuseppina Cantarella