- 1Department of Pharmacotherapy and Outcomes Science, School of Pharmacy, Virginia Commonwealth University, Virginia, United States
- 2College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Saudi Arabia
Drug treatment protocols for traumatic brain injury (TBI) that result in long-term, positive outcomes have yet to be determined for various reasons, including diversity of injury and difficulty in measuring outcomes. Brain injury biomarkers are increasingly being used for drug development and treatment research in patients with TBI to supplement pharmacokinetic studies, provide evidence of drug mechanism of action, detect early and long-term clinical outcomes, and homogenize study populations. The use of biomarkers to influence TBI drug development and treatment trials has the potential to lead to more innovative research and personalized patient care. Future TBI clinical trials that utilize these innovative biomarkers study designs and demonstrate strong correlations between biomarkers and clinical outcomes could permit shorter, less expensive, and more successful clinical trials.
1 Introduction
Biomarkers are measurable indicators of biological processes. They vary from molecular to histologic to radiographic markers and have the potential to augment prognostication, diagnosis, and monitoring of patients with various disease states. Importantly for drug development and treatment trials, biomarkers have the potential to be used to measure response for both safety and efficacy endpoints. Biomarkers may decrease the cost and increase efficiency of the drug treatment studies by informing researchers of drug response and toxicity in preclinical and early phase clinical studies (FDA, 2020). This early indication may lead to more rapid determination of drug safety and effectiveness in combination with clinical outcome measures in research trials as well as decrease research-associated costs (Kochanek et al., 2020).
For traumatic brain injury (TBI), biomarkers have increased prognostic capabilities, improved clinical resource utilization, and directed researchers to new drug targets (Wang et al., 2018; Wang et al., 2021). Using biomarkers to guide drug development or treatment of patients with TBI is particularly appealing because little to no progress has been made in the identification of therapies that truly target the pathophysiologic mechanisms of TBI. Additionally, the heterogeneity of patients with TBI makes a single treatment for all patients with TBI unlikely. Biomarkers have the potential to identify patients with similar mechanisms of injury and to target phenotypes likely to respond to specific treatment strategies. Although early objective outcomes, including mortality and acute functional status, are important, the long-term impacts of TBI such as loss of productivity, loss of independence, and prolonged neurological dysfunction are extremely important quality of life measures for those who survive the initial injury. If a biomarker is found to correlate strongly with these long-term TBI outcomes, a targeted approach for drug development and treatment in the acute period after injury may be possible.
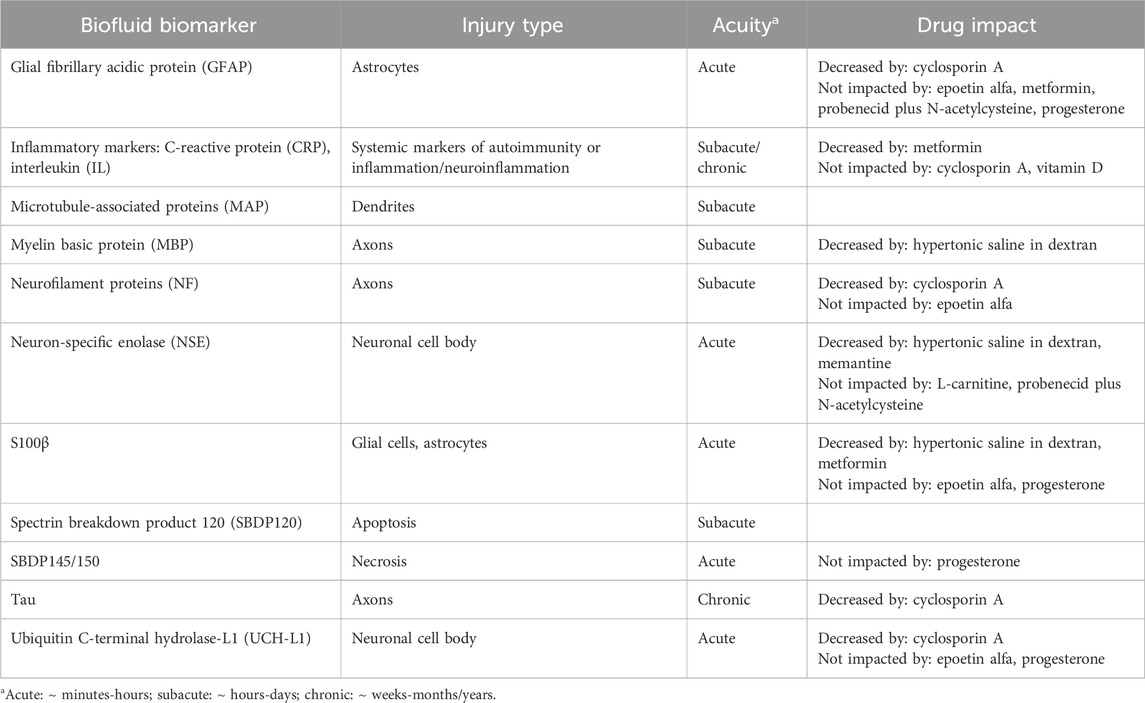
Brain injury biomarkers currently under investigation in patients with TBI have been described and categorized based on physiology in previous publications (Edalatfar et al., 2021). The three most common categories were cytokines, coagulation parameters, and nerve tissue proteins. Nerve tissue proteins include, but are not limited to, S100β, glial fibrillary acidic protein (GFAP), microtubule-associated proteins (MAP), neurofilament light chain proteins (NF-L), and myelin basic proteins (MBP). It has also been postulated that biomarkers can differentiate type of brain injury [e.g., phosphorylated axonal neurofilament heavy chain (pNf-H) indicates axonal injury and ubiquitin C-terminal hydrolase-L1 (UCH-L1) suggests neuronal cell body injury] (Table 1). (Wang et al., 2018; Gutierre et al., 2020)
The use of biomarkers in TBI drug development and treatment research is rapidly growing. This article aims to describe how biomarkers may be used as drug targets to augment the efficiency and effectiveness of drug development and treatment studies for the treatment of patients with TBI.
2 Biomarker applicability in traumatic brain injury clinical studies
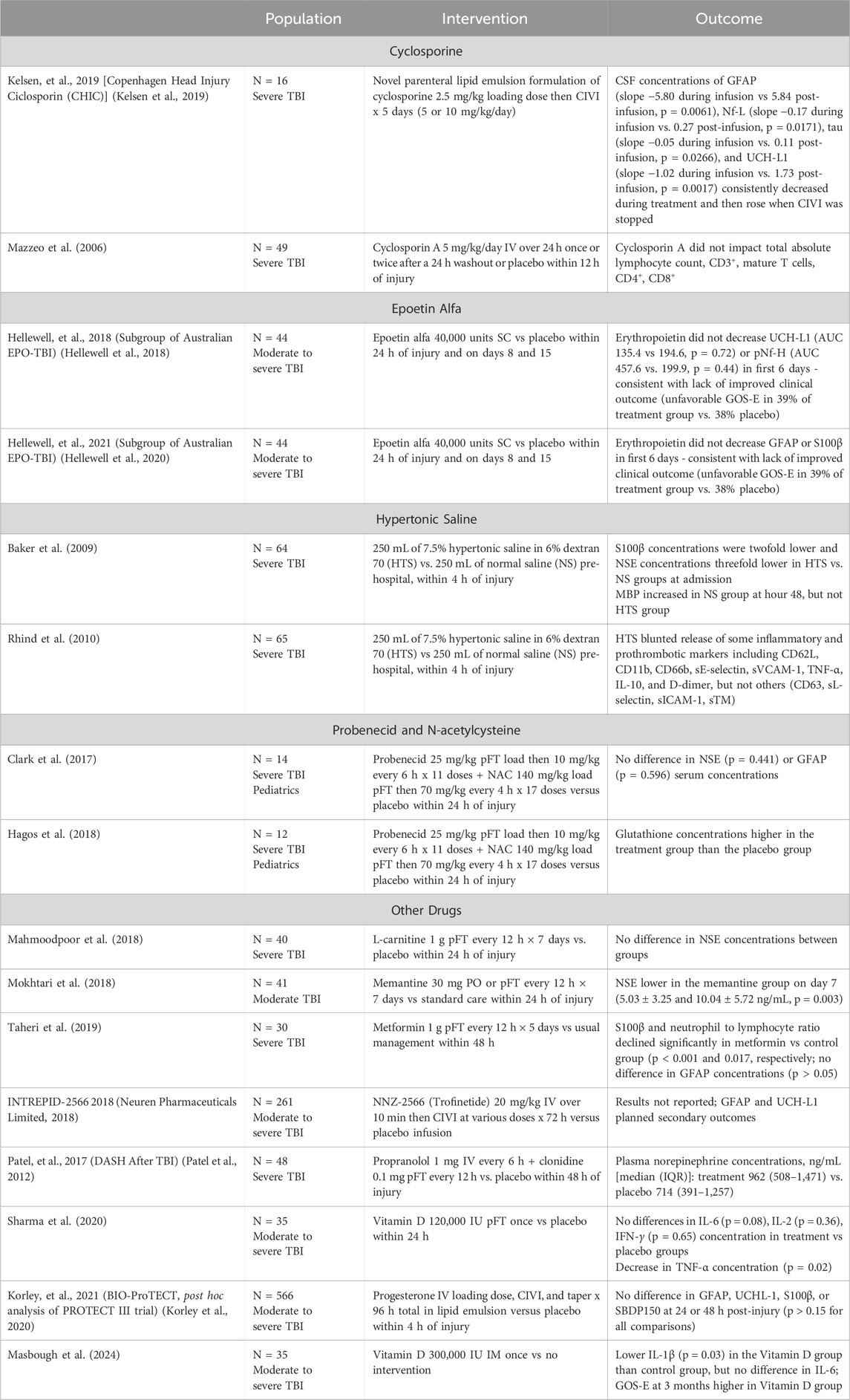
Most pharmacological TBI clinical trials to date have incorporated biomarkers as a secondary endpoint to describe the effect of the drug on injury mechanisms (Tables 1, 2). For example, two clinical studies of treatment with erythropoietin found that biomarker concentrations and profiles were not affected by erythropoietin, suggesting that this drug does not impact the pathophysiologic processes of TBI in the population studied (Hellewell et al., 2018; Hellewell et al., 2020). Trials of hypertonic saline-dextran solution reported reductions in various inflammatory markers, as well as in S100β and neuron-specific enolase (NSE), which correlated with CT findings and clinical outcomes (Baker et al., 2009; Rhind et al., 2010). As including biomarker concentrations becomes more commonplace, the correlation, or lack thereof, between biofluid biomarker concentrations, pharmacokinetics and pharmacodynamics of potential neuroprotective drugs, and clinical outcomes will become more apparent.
2.1 Surrogate biomarkers in combination with pharmacokinetic parameters
Although there are many reasons a drug may fail in clinical trials, one explanation may be that the drug did not achieve therapeutic concentrations at the site of action to achieve the desired pharmacodynamic effect. Currently, drug pharmacokinetic parameters are assessed to determine what doses will produce the concentrations needed to achieve the therapeutic effect observed in preclinical trials. There are multiple pitfalls in using pharmacokinetic parameters in this way. For one, although trials often report the drug concentration achieved, this is hard to interpret because therapeutic CSF or blood concentration ranges are not known for most drugs. Additionally, although assays are available for some drugs to measure concentrations, most drugs do not have commercially available assays. Lastly, even if an assay is available, it is not always feasible to collect a sample from the site of action (e.g., CSF from the brain or brain tissue); therefore, it is not known if the drug reached the site of action.
Biomarker concentrations as a surrogate for drug concentrations could be more clinically relevant than a drug concentration if the biomarker concentration correlates closely with clinical outcomes. Like traditional dose-finding studies, the therapeutic dose of a drug could be determined by the largest decrease or increase in a biomarker suggesting that it had the largest pharmacodynamic effect. Using biomarkers in this way would decrease the need for the development of a multitude of drug assays as a single biomarker assay could be used for multiple drugs acting at the same site.
Another obstacle that may be overcome by using biomarkers in this way is the translation from animal models to humans. Currently, body surface area or weight based dosing is used to estimate the effective dose in humans; instead, change in biomarker concentration may more effectively identify an effective dose.
The phase I study of probenecid plus NAC illustrates the use of known drug pharmacokinetic parameters in combination with a surrogate biomarker (Hagos et al., 2018; Clark et al., 2017). Due to concern for NAC reaching therapeutic concentrations at its site of action in the CSF, it was administered in combination with probenecid to decrease active transport out of the brain. Although the study directly measured drug concentrations in both the blood and CSF, the post hoc analysis measured glutathione concentrations in the CSF which should increase when NAC is administered. Because these CSF concentrations were increased in the treatment group, the authors concluded that NAC was achieving therapeutic concentrations in the brain. Future studies can use this method of measuring a surrogate biomarker known to be impacted by the drug being studied to ensure that effective drug concentrations are achieved at the site of action.
The Decreasing Adrenergic or Sympathetic Hyperactivity After Traumatic Brain Injury (DASH after TBI) trial illustrates the idea of measuring pharmacodynamic effect as well (Patel et al., 2012). Propranolol and clonidine were administered to patients with severe TBI to block detrimental sympathetic storming associated with high catecholamine concentrations which translates to poor clinical outcomes. Rather than measuring drug concentrations, norepinephrine concentrations were compared to assess pharmacodynamic effect. The combination of propranolol and clonidine was not shown to decrease norepinephrine concentrations compared to placebo which aligned with lack of difference in the clinical primary outcome of ventilator-free days. Although the lack of change in norepinephrine concentrations could have been related to this drug combination being ineffective, there are other factors to consider, such as sample timing and rapid norepinephrine degradation, that influence norepinephrine concentrations.
2.2 Evidence of drug mechanism of action
Biomarkers may provide indications as to the mechanism by which drugs achieve a clinical benefit. For drugs that have a known mechanism of action, biomarkers that relate to this mechanism can be used to measure the effect of the drug on its target. An example of this was done in a phase I study of probenecid and N-acetylcysteine (NAC) in pediatric patients with severe TBI (Hagos et al., 2018). Both drugs are known to increase concentrations of glutathione (an antioxidant) through various mechanisms independently and synergistically. This exploratory trial found that CSF glutathione concentrations were higher in the treatment group than in the placebo group thus providing evidence that these drugs were achieving drug concentrations sufficient to influence glutathione concentrations in the CSF. This confirms the known mechanism of action and encourages further pursuit of this investigational treatment strategy.
If the mechanism of action of a drug is not known, the relationship between a biomarker and a specific pathophysiological pathway or brain component can be used to connect the drug with that pathway. By observing the change in biomarker concentration in response to a drug, one can presume that the drug is impacting that pathway or component. Although not yet explored in clinical trials, preclinical models have suggested this type of relationship. In rats, levetiracetam was found to attenuate the rise in phospho-neurofilament-H (pNF-H) compared to placebo (Yang et al., 2019). The mechanism by which levetiracetam improve outcomes in TBI is not known, but considering pNF-H is specific to axonal damage, it was suggested that levetiracetam diminished axonal injury. Similarly, high-dose valproic acid in swine decreased levels of GFAP and NF-L compared to placebo, which suggests valproic acid might preserve astrocytes and axons after TBI (Korley et al., 2018).
2.3 Generalizability of pre-clinical and early clinical outcomes
A significant barrier to TBI drug development is the inconsistent results in preclinical models and human subjects (Kochanek et al., 2020). Large clinical trials could be avoided if data suggested a low likelihood that the drug-disease effect found in preclinical trials would occur in humans. Before expensive clinical phase II/III studies are designed, biomarkers could be used alone or in combination with clinical outcome parameters in smaller and shorter clinical trials to determine the likelihood of a drug achieving the pharmacodynamic effect needed to produce a long-term clinically significant improvement.
The Biomarkers of Injury and Outcome (BIO)-Progesterone for Traumatic Brain Injury, Experimental Clinical Treatment (ProTECT) trial is an example of how biomarkers could have been used to assess the pharmacodynamic effect of a drug prior to a large clinical trial. This trial was performed to investigate the negative results of the ProTECT III trial (Korley et al., 2020; Wright et al., 2014). ProTECT III was a large (N = 882), randomized clinical trial of progesterone versus placebo in patients with moderate to severe TBI. It was designed after preclinical studies suggested neuroprotective effects of progesterone, including decreased cerebral edema and neuronal loss. Although theoretically sound, early administration of progesterone in ProTECT III did not result in improved outcomes at 6 months. As a post hoc analysis, BIO-ProTECT used biomarkers [GFAP, UCHL-1, S100β, and spectrin breakdown product (SBDP)150] to demonstrate that progesterone did not decrease brain cell death as desired and suggested that this lack of pharmacodynamic effect in humans may have contributed to the fact that ProTECT III was a negative trial. Other factors including trial design, patient adherence, and patient population certainly contributed to the negative outcome as well, but completion of this large, expensive clinical trial may not have been done if the information from BIO-ProTECT was available prior to beginning ProTECT III.
2.4 Early indication of long-term outcomes
TBI is not just an acute injury but often results in long-term impairment that requires chronic follow up. Therefore, clinical trials must demonstrate a prolonged effect beyond the treatment interval on clinical outcomes, which is commonly measured by Glasgow Outcome Scale Extended (GOS-E) at 6 months post-injury. This lengthy follow-up period is susceptible to attrition, thereby requiring more patients to be enrolled to avoid reducing power, which increases cost. Biomarkers measured at shorter intervals (e.g., days to weeks) after injury may decrease the follow-up time needed in clinical drug trials. In the future, if the correlation between biomarker concentrations and long-term outcomes strengthens, clinical trials could strongly suggest long-term benefits of drug treatment by measuring the impact on biomarkers at 24–48 hours rather than conducting lengthy, expensive trials that require 6 months follow-up. Recent studies attempt to demonstrate the connection between blood biomarkers and long-term outcomes, but admit the limitations, including the heterogeneity found among patients with TBI (Yue et al., 2023; Whitehouse et al., 2025; Korley et al., 2022; Crichton et al., 2021; Helmrich et al., 2022; Svingos et al., 2022; Trifilio et al., 2024; Schneider et al., 2023).
2.5 Homogenization of study populations and personalization of therapy
The clinical presentation, hospital course and clinical outcomes among patients with TBI are often extremely heterogeneous despite similar presenting GCS scores, imaging, and laboratory results. GCS scores have traditionally been used to classify the severity of a TBI, but this scale is flawed for many reasons including the influence of other factors such as drugs, alcohol, hypotension and hypoxemia. Additionally, GCS does not account for the diverse mechanisms of TBI (e.g., penetrating versus blunt) which more strongly influences clinical intervention and better stratifies patients.
The ability to classify patients into endophenotypes using biomarkers could assist in the development of drugs, particularly monoclonal antibodies, used to target a specific pathophysiology (Wang et al., 2024). Biomarkers can be used to identify which structure of a neuron is damaged or which pathophysiologic mechanism is causing harm. This information may subsequently be used to identify which drugs are most likely to be effective based on its mechanism of action. For example, high concentrations of inflammatory biomarkers may suggest neuroinflammation and the patient may be best treated with immunomodulators. Currently, evidence of targeted and personalized therapy such as this does not exist, which is oftentimes suggested as a reason for the large number of TBI negative trials.
This application of biomarkers would be similar to how monoclonal antibodies (e.g., lecanemab, donanemab) were developed to treat patients with Alzheimer’s disease. For example, donanemab is an amyloid-beta directed monoclonal antibody. Trials of donanemab included only patients with amyloid-beta pathology (Sims et al., 2023). This inclusion criteria increased the likelihood that only patients who would benefit from donanemab would be included. By including only this population, rather than the heterogeneous population of all patients with Alzheimer’s disease, the clinical trial was more likely to be successful. This use is also similar to the identification of genetic mutations in cancer (e.g., HER2, ALK, EGFR) that are targeted by drugs.
Future clinical trials may use biomarkers to select a homogenous patient population likely to benefit from the drug intervention; so far, biomarkers have been used to justify drug studies based on mechanism of action and type of injury. For example, one group chose to study glyburide, a sulfonylurea receptor 1 (SUR1) antagonist, because CSF concentrations of SUR1 predict swelling and outcome in patients with TBI (Eisenberg et al., 2020). To increase the likelihood that patients enrolled in a future trial of glyburide would benefit from SUR1 antagonism, use of SUR1 concentrations as inclusion criteria could be considered. However, the practical implications of obtaining hyperacute CSF sample analysis and results prior to enrollment would first need to be addressed. Similarly, a study in rats suggested that levetiracetam diminished axonal injury because concentrations of pNF-H were lower in the drug treatment group than in the placebo group (Yang et al., 2019). This evidence could be used to justify a study of levetiracetam in patients with evidence of diffuse axonal injury after TBI with the hope that the endophenotype of TBI most likely to benefit was chosen.
3 Conclusion
As a growing component of TBI research, innovative methods will be employed to incorporate biomarkers into preclinical and clinical trial design. Key areas for research include using population biomarker kinetics to determine when it is best to start drug therapy in a clinical trial, initiating or discontinuing drug treatment as a reaction to a rise or fall in individual biomarker concentrations, determining a biomarker threshold as an inclusion or exclusion criterion, and identifying when a secondary injury is occurring using biomarker concentrations and subsequently intervening at that point.
The use of biomarkers to influence TBI drug development and treatment trials has the potential to lead to more innovative research and personalized patient care. In the future, strong correlations between biomarkers and clinical outcomes could permit shorter, less expensive, and more successful clinical trials.
Author contributions
MS: Writing – review and editing, Writing – original draft. SA: Writing – review and editing. GB: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. KFU251992).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baker, A. J., Rhind, S. G., Morrison, L. J., Black, S., Crnko, N. T., Shek, P. N., et al. (2009). Resuscitation with hypertonic saline–dextran reduces serum biomarker levels and correlates with outcome in severe traumatic brain injury patients. J. Neurotrauma 26 (8), 1227–1240. doi:10.1089/neu.2008.0868
Clark, R. S. B., Empey, P. E., Bayır, H., Rosario, B. L., Poloyac, S. M., Kochanek, P. M., et al. (2017). Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS ONE 12 (7), e0180280. doi:10.1371/journal.pone.0180280
Crichton, A., Ignjatovic, V., Babl, F. E., Oakley, E., Greenham, M., Hearps, S., et al. (2021). Interleukin-8 predicts fatigue at 12 Months post-injury in children with traumatic brain injury. J. Neurotrauma 38 (8), 1151–1163. doi:10.1089/neu.2018.6083
Edalatfar, M., Piri, S. M., Mehrabinejad, M. M., Mousavi, M. S., Meknatkhah, S., Fattahi, M. R., et al. (2021). Biofluid biomarkers in traumatic brain injury: a systematic scoping review. Neurocrit Care 35, 559–572. doi:10.1007/s12028-020-01173-1
Eisenberg, H. M., Shenton, M. E., Pasternak, O., Simard, J. M., Okonkwo, D. O., Aldrich, C., et al. (2020). Magnetic resonance imaging pilot study of intravenous glyburide in traumatic brain injury. J. Neurotrauma 37 (1), 185–193. doi:10.1089/neu.2019.6538
FDA (2020). Research C for DE and. About biomarkers and qualification. FDA. Available online at: https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification.
Gutierre, M. U., Telles, J. P. M., Welling, L. C., Rabelo, N. N., Teixeira, M. J., and Figueiredo, E. G. (2020). Biomarkers for traumatic brain injury: a short review. Neurosurg. Rev. 44, 2091–2097. doi:10.1007/s10143-020-01421-0
Hagos, F. T., Empey, P. E., Wang, P., Ma, X., Poloyac, S. M., Bayir, H., et al. (2018). Exploratory application of neuropharmacometabolomics in severe childhood traumatic brain injury. Crit. Care Med. 46 (9), 1471–1479. doi:10.1097/CCM.0000000000003203
Hellewell, S. C., Conquest, A., Little, L., Vallance, S., Board, J., Bellomo, R., et al. (2020). EPO treatment does not alter acute serum profiles of GFAP and S100B after TBI: a brief report on the Australian EPO-TBI clinical trial. J. Clin. Neurosci. 76, 5–8. doi:10.1016/j.jocn.2020.04.081
Hellewell, S. C., Mondello, S., Conquest, A., Shaw, G., Madorsky, I., Deng, J. V., et al. (2018). Erythropoietin does not alter serum profiles of neuronal and axonal biomarkers after traumatic brain injury: findings from the Australian EPO-TBI clinical trial. Crit. Care Med. 46 (4), 554–561. doi:10.1097/CCM.0000000000002938
Helmrich, IRAR, Czeiter, E., Amrein, K., Büki, A., Lingsma, H. F., Menon, D. K., et al. (2022). Incremental prognostic value of acute serum biomarkers for functional outcome after traumatic brain injury (CENTER-TBI): an observational cohort study. Lancet Neurol. 21 (9), 792–802. doi:10.1016/S1474-4422(22)00218-6
Kelsen, J., Karlsson, M., Hansson, M. J., Yang, Z., Fischer, W., Hugerth, M., et al. (2019). Copenhagen head injury ciclosporin study: a phase IIa safety, pharmacokinetics, and biomarker study of ciclosporin in severe traumatic brain injury patients. J. Neurotrauma 36 (23), 3253–3263. doi:10.1089/neu.2018.6369
Kochanek, P. M., Jackson, T. C., Jha, R. M., Clark, R. S. B., Okonkwo, D. O., Bayır, H., et al. (2020). Paths to successful translation of new therapies for severe traumatic brain injury in the golden age of traumatic brain injury research: A Pittsburgh vision. J. Neurotrauma 37 (22), 2353–2371. doi:10.1089/neu.2018.6203
Korley, F., Pauls, Q., Yeatts, S. D., Jones, C. M. C., Corbett-Valade, E., Silbergleit, R., et al. (2020). Progesterone treatment does not decrease serum levels of biomarkers of glial and neuronal cell injury in moderate and severe traumatic brain injury subjects: a secondary analysis of the progesterone for traumatic brain injury, experimental clinical treatment (ProTECT) III trial. J. Neurotrauma 38, 1953–1960. doi:10.1089/neu.2020.7072
Korley, F. K., Jain, S., Sun, X., Puccio, A. M., Yue, J. K., Gardner, R. C., et al. (2022). Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: an observational cohort study. Lancet Neurol. 21 (9), 803–813. doi:10.1016/S1474-4422(22)00256-3
Korley, F. K., Nikolian, V. C., Williams, A. M., Dennahy, I. S., Weykamp, M., and Alam, H. B. (2018). Valproic acid treatment decreases serum glial fibrillary acidic protein and neurofilament light chain levels in swine subjected to traumatic brain injury. J. Neurotrauma 35 (10), 1185–1191. doi:10.1089/neu.2017.5581
Mahmoodpoor, A., Shokouhi, G., Hamishehkar, H., Soleimanpour, H., Sanaie, S., Porhomayon, J., et al. (2018). A pilot trial of l-carnitine in patients with traumatic brain injury: effects on biomarkers of injury. J. Crit. Care 45, 128–132. doi:10.1016/j.jcrc.2018.01.029
Masbough, F., Kouchek, M., Koosha, M., Salarian, S., Miri, M., Raoufi, M., et al. (2024). Investigating the effect of high-dose vitamin D3 administration on inflammatory biomarkers in patients with moderate to severe traumatic brain injury: a randomized clinical trial. Iran. J. Med. Sci. 49 (10), 643–651. doi:10.30476/ijms.2023.99465.3156
Mazzeo, A. T., Kunene, N. K., Gilman, C. B., Hamm, R. J., Hafez, N., and Bullock, M. R. (2006). Severe human traumatic brain injury, but not cyclosporin A treatment, depresses activated T lymphocytes early after injury. J. Neurotrauma 23 (6), 962–975. doi:10.1089/neu.2006.23.962
Mokhtari, M., Nayeb-Aghaei, H., Kouchek, M., Miri, M. M., Goharani, R., Amoozandeh, A., et al. (2018). Effect of memantine on serum levels of neuron-specific enolase and on the Glasgow coma scale in patients with moderate traumatic brain injury. J. Clin. Pharmacol. 58 (1), 42–47. doi:10.1002/jcph.980
Neuren Pharmaceuticals Limited (2018). A randomized, double-blind, placebo-controlled, dose-escalation study of NNZ-2566 in patients with traumatic brain injury. clinicaltrials.gov. Available online at: https://clinicaltrials.gov/ct2/show/NCT00805818.
Patel, M. B., McKenna, J. W., Alvarez, J. M., Sugiura, A., Jenkins, J. M., Guillamondegui, O. D., et al. (2012). Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH after TBI Study): study protocol for a randomized controlled trial. Trials 13, 177. doi:10.1186/1745-6215-13-177
Rhind, S. G., Crnko, N. T., Baker, A. J., Morrison, L. J., Shek, P. N., Scarpelini, S., et al. (2010). Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J. Neuroinflammation 7, 5. doi:10.1186/1742-2094-7-5
Schneider, A. L. C., Huie, J. R., Jain, S., Sun, X., Ferguson, A. R., Lynch, C., et al. (2023). Associations of microvascular injury-related biomarkers with traumatic brain injury severity and outcomes: a transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) pilot study. J. Neurotrauma 40 (15–16), 1625–1637. doi:10.1089/neu.2022.0442
Sharma, S., Kumar, A., Choudhary, A., Sharma, S., Khurana, L., Sharma, N., et al. (2020). Neuroprotective role of oral vitamin D supplementation on consciousness and inflammatory biomarkers in determining severity outcome in acute traumatic brain injury patients: a double-blind randomized clinical trial. Clin. Drug Investig. 40 (4), 327–334. doi:10.1007/s40261-020-00896-5
Sims, J. R., Zimmer, J. A., Evans, C. D., Lu, M., Ardayfio, P., Sparks, J., et al. (2023). Donanemab in early symptomatic alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330 (6), 512–527. doi:10.1001/jama.2023.13239
Svingos, A. M., Robicsek, S. A., Hayes, R. L., Wang, K. K., Robertson, C. S., Brophy, G. M., et al. (2022). Predicting clinical outcomes 7-10 Years after severe traumatic brain injury: exploring the prognostic utility of the IMPACT lab model and cerebrospinal fluid UCH-L1 and MAP-2. Neurocrit Care 37 (1), 172–183. doi:10.1007/s12028-022-01461-y
Taheri, A., Emami, M., Asadipour, E., Kasirzadeh, S., Rouini, M. R., Najafi, A., et al. (2019). A randomized controlled trial on the efficacy, safety, and pharmacokinetics of metformin in severe traumatic brain injury. J. Neurol. 266 (8), 1988–1997. doi:10.1007/s00415-019-09366-1
Trifilio, E., Bottari, S., McQuillan, L. E., Barton, D. J., Lamb, D. G., Robertson, C., et al. (2024). Temporal profile of serum neurofilament light (NF-L) and heavy (pNF-H) level associations with 6-month cognitive performance in patients with moderate-severe traumatic brain injury. J. Head. Trauma Rehabil. 39 (6), E470–E480. doi:10.1097/HTR.0000000000000932
Wang, K. K., Munoz Pareja, J. C., Mondello, S., Diaz-Arrastia, R., Wellington, C., Kenney, K., et al. (2021). Blood-based traumatic brain injury biomarkers - clinical utilities and regulatory pathways in the United States, Europe and Canada. Expert Rev. Mol. Diagn 21 (12), 1303–1321. doi:10.1080/14737159.2021.2005583
Wang, K. K., Yang, Z., Zhu, T., Shi, Y., Rubenstein, R., Tyndall, J. A., et al. (2018). An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn 18 (2), 165–180. doi:10.1080/14737159.2018.1428089
Wang, P., Okada-Rising, S., Scultetus, A. H., and Bailey, Z. S. (2024). The relevance and implications of monoclonal antibody therapies on traumatic brain injury pathologies. Biomedicines 12 (12), 2698. doi:10.3390/biomedicines12122698
Whitehouse, D. P., Wilson, L., Czeiter, E., Buki, A., Wang, K. K. W., von Steinbüchel, N., et al. (2025). Association of blood-based biomarkers and 6-month patient-reported outcomes in patients with mild TBI: a CENTER-TBI analysis. Neurology 104 (1), e210040. doi:10.1212/WNL.0000000000210040
Wright, D. W., Yeatts, S. D., Silbergleit, R., Palesch, Y. Y., Hertzberg, V. S., Frankel, M., et al. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371 (26), 2457–2466. doi:10.1056/NEJMoa1404304
Yang, Z., Zhu, T., Mondello, S., Akel, M., Wong, A. T., Kothari, I. M., et al. (2019). Serum-based phospho-neurofilament-heavy protein as theranostic biomarker in three models of traumatic brain injury: an operation brain trauma therapy study. J. Neurotrauma 36 (2), 348–359. doi:10.1089/neu.2017.5586
Keywords: biomarkers, traumatic brain injury, drug development, drug discovery, pharmacology
Citation: Sandler M, Almohaish S and Brophy GM (2025) Brain injury biomarkers as targets for drugs development and personalized treatment for traumatic brain injury patients. Front. Pharmacol. 16:1606174. doi: 10.3389/fphar.2025.1606174
Received: 04 April 2025; Accepted: 09 May 2025;
Published: 26 May 2025.
Edited by:
Rebekah Mannix, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Nagaraja Sethuraman Balakathiresan, National Institute on Alcohol Abuse and Alcoholism (NIH), United StatesHakan Aldskogius, Uppsala University, Sweden
Copyright © 2025 Sandler, Almohaish and Brophy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sulaiman Almohaish, c2FsbW9oYWlzaEBrZnUuZWR1LnNh; Gretchen M. Brophy, Z2Jyb3BoeUB2Y3UuZWR1
 Melissa Sandler
Melissa Sandler Sulaiman Almohaish
Sulaiman Almohaish Gretchen M. Brophy
Gretchen M. Brophy