- 1School of Integrated Traditional Chinese and Western Medicine, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, China
- 2Key Research Laboratory of Phlegm Stagnation Syndrome and Treatment in Hebei Province, Hebei Academy of Chinese Medicine Sciences, Shijiazhuang, Hebei, China
- 3The Fourth Affiliated Hospital of Hebei University of Chinese Medicine, Shijiazhuang, Hebei, China
- 4The First Affiliated Hospital of Hebei University of Chinese Medicine, Shijiazhuang, Hebei, China
- 5Internal Medicine Department, Hospital of Renmin University of China, Beijing, China
Ethnopharmacological relevance: Baicalin, an extract derived from the dried root of Scutellaria baicalensis Georgi (Huang Qin), has demonstrated neuroprotective properties. Nonetheless, the safety profile of baicalin has not yet been fully elucidated.
Aim of the study: The objective was to characterize the acute and subacute toxicity profiles of baicalin across various organ systems, thereby establishing safe therapeutic windows for its clinical application in the treatment of chronic neurodegenerative disorders.
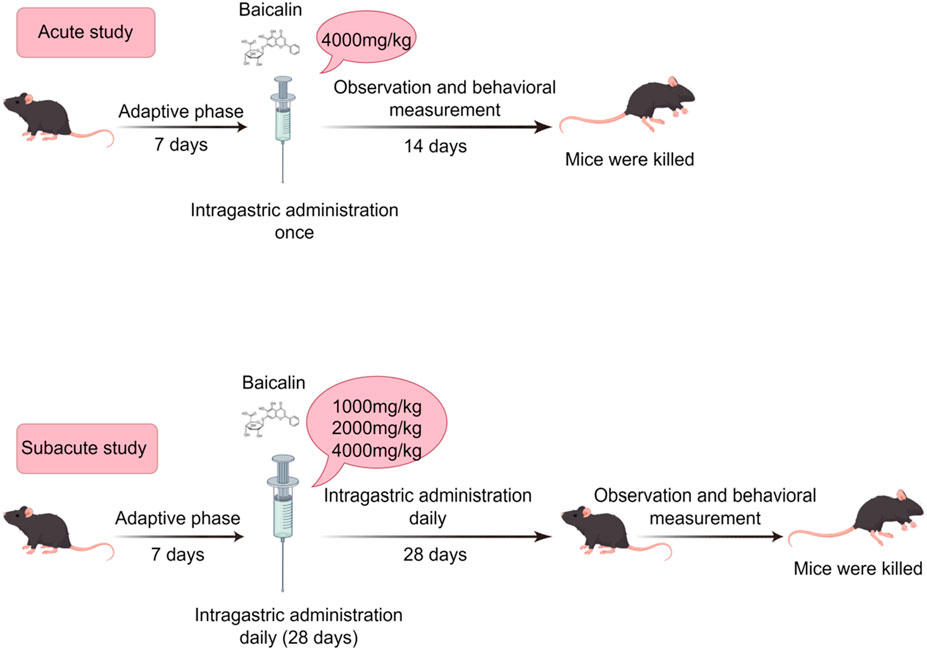
Materials and methods: Acute toxicity was assessed at 4,000 mg/kg (OECD 423), while subacute toxicity evaluated escalating doses (1,000–4,000 mg/kg; OECD 407). Endpoints included survival, general behaviours, behavioral alterations, hematological/biochemical parameters, organ coefficients, and histopathology of brain, liver, and kidney.
Results: Acute exposure showed no mortality (LD50 > 4,000 mg/kg) or lasting physiological effects, with only transient gastrointestinal symptoms in one subject. Subacute administration caused temporary gastrointestinal issues and occasional compulsive behaviors, all resolving within 24 h. Behavioral assessments indicated intact neurocognitive function and emotional stability. Hematological profiles revealed sex-specific responses, with males showing higher lymphocyte percentages and females demonstrating renal changes. Biochemical analyses indicated liver metabolic changes, including alkaline phosphatase suppression and reduced triglycerides, along with mild nephrotoxic signs. Histopathological evaluations confirmed non-necrotic liver stress and unchanged hippocampal structure.
Conclusion: Baicalin showed high acute safety with an LD50 over 4,000 mg/kg in mice, and a subacute no-observed-adverse-effect level (NOAEL) of 2,000 mg/kg, indicating its potential as a neuroprotective agent. However, 4,000 mg/kg doses led to reversible hepatorenal toxicity and biochemical alterations, highlighting the need to monitor organ function during extended high-dose use.
GRAPHICAL ABSTRACT | For acute toxicity assessment, C57BL/6 mice underwent a 7-day acclimation period prior to receiving a single oral gavage dose of 4,000 mg/kg baicalin. Behavioral evaluations were performed for 14 days post-administration. In subacute toxicity studies, different doses of baicalin were administered via daily oral gavage for 28 consecutive days, supplemented by daily behavioral assessments and behavioral testing protocols.
1 Introduction
Scutellaria baicalensis Georgi (Huang-Qin), a important botanical agent in traditional Chinese medicine with a pharmacopeial history dating back to the Shennong Bencao Jing (Divine Farmer’s Materia Medica, c. 200–250 CE), has been systematically documented for its heat-clearing, detoxifying, and pregnancy-stabilizing properties (Wang et al., 2018; Li et al., 2020). Modern pharmacological studies have shown that Huang Qin has anti-inflammatory, antioxidant, neuroprotective and immune-enhancing effects. Baicalin (C21H18O11; 7-d-glucuronic acid-5,6-dihydroxyflavone) (Figure 1), an extract from the dried root of Huang Qin, has been demonstrated multi-target pharmacological efficacy against viral infections, oxidative stress, tumorigenesis, and inflammatory processes (Hu et al., 2022; Hu et al., 2023). Notably, its neuroprotective properties have gained particular therapeutic significance, as evidenced by preclinical studies showing that baicalin significantly reduces levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) while inhibiting microglial overactivation in the model of cerebral ischemia (Yang S. et al., 2019; Wang H. et al., 2024; Li et al., 2025). Similarly, in a mouse model of depression, a 60 mg/kg dose alleviates hippocampal inflammatory damage (Guo et al., 2019). These findings not only provide scientific validation for the herb’s ancient applications in brain disorder treatments but also position Huang-Qin as a promising candidate for developing next-generation neurotherapeutics targeting neurodegeneration, effectively bridging ancient herbal knowledge with modern neuroscience paradigms.
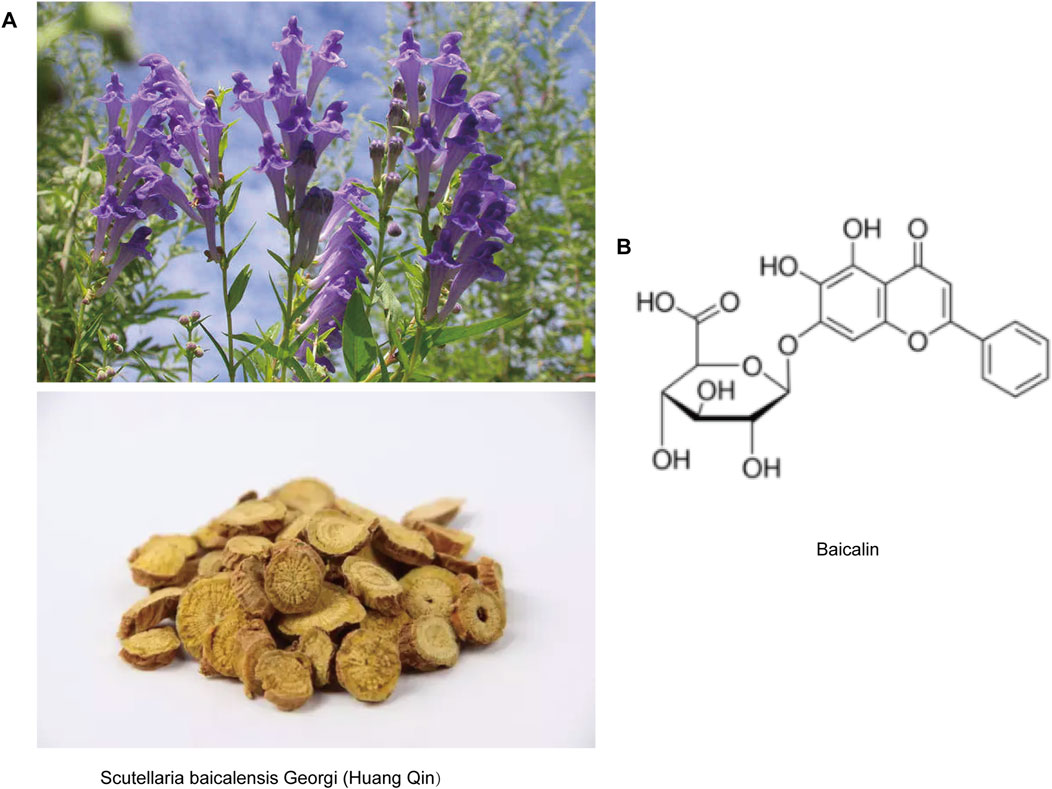
Figure 1. Representative image of baicalin. (A) Pictures of Scutellaria baicalensis Georgi (Huang-Qin) plants and herbal tablets. (B) Molecular structure of baicalin.
Despite extensive investigations into neuroprotective mechanisms of baicalin, its toxicological profile, particularly under high-dose or prolonged exposure scenarios, remains poorly characterized. Existing studies primarily focus on efficacy at low to moderate doses (50–400 mg/kg) (Xi et al., 2016; Zhang et al., 2017; Si et al., 2025), overlooking the cumulative organ-specific toxicity and neurobehavioral sequelae that may emerge during chronic administration—a critical knowledge gap given its proposed clinical use in long-term neurodegenerative therapies. Notably, clinical data shows no signs of hepatic or renal toxicity when assesses with a single oral dose of baicalein in high doses (Li et al., 2014; Si et al., 2025). This knowledge gap is concerning, as structurally analogous flavonoids (e.g., quercetin and silymarin) have demonstrated hepatotoxicity and nephrotoxicity in both preclinical and clinical settings (Harwood et al., 2007; Singh and Semwal, 2024).
Comprehensive toxicity evaluation is an indispensable step in drug development, bridging the gap between therapeutic potential and clinical translatability (Yang et al., 2024). For natural products like baicalin, which are often perceived as inherently safe, but rigorous safety assessments are imperative to define therapeutic windows and mitigate off-target organ damage. Critical questions remain unanswered: What is the maximum tolerated dose of baicalin in acute exposure? Which organs are vulnerable to subacute toxicity? Given its putative neuroprotective properties, might chronic high-dose baicalin exposure paradoxically evoke neurotoxic responses?
In this study, we integrated behavioral and toxicological approaches to conduct a comprehensive analysis of the acute (14-day) and sub-acute (28-day) toxicity of baicalin in C57BL/6J mice for the first time. We simultaneously evaluated: (1) survival, body weight, and general behavior; (2) anxiety/depression-like behaviors and motor function; (3) hematological, hepatic, and renal parameters; (4) histopathology of brain, liver, and kidney. This systematic framework not only identifies baicalin’s toxicity thresholds but also establishes correlations between behavioral deficits and organ-specific damage, providing a blueprint for natural product safety evaluation.
2 Materials and methods
2.1 Animals
A total of sixty 8-week-old C57BL/6J mice (30 males and 30 females) were procured from Beijing Vital River Laboratory Animal Technology Co., Ltd. (License No. SCXK [Jing] 2021-0006). All animals were housed in the Animal Center of the Fourth Affiliated Hospital of Hebei University of Chinese Medicine for 1 week, and then the follow-up experiments were carried out (22°C ± 1°C, 50% ± 10% humidity, 12-h light/dark cycle). This study was approved by the Ethics Committee of Hebei University of Chinese Medicine (Ethics Approval No. DWLL202403137).
2.2 Acute oral toxicity study
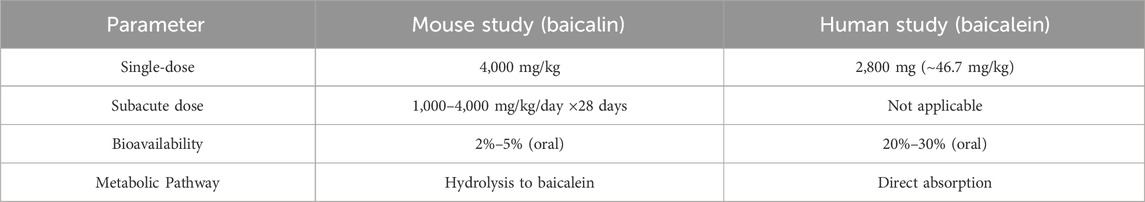
The 4,000 mg/kg dose of baicalin (Shanghai Yuanye Biotechnology Co. B20570, ≥98% purity) was selected in acute oral toxicity study. The acute toxicity assessment was conducted in strict compliance with OECD Guideline 423 (Acute Oral Toxicity–Acute Toxic Class Method) (Liu et al., 2024). A limit test dose of 4,000 mg/kg was selected based on: (1) Preliminary evidence indicates that baicalin, which is formed by the conjugation of the C7 hydroxyl group of baicalein with glucuronic acid, is likely to possess low acute toxicity based on its structural analogy to low-toxicity analogs such as baicalein (Zheng et al., 2017; Dai et al., 2018); (2) Equivalent dose calculations were performed by converting baicalein and baicalin, with adjustments for species-specific metabolic differences and bioavailability variations (Table 1) (Dong et al., 2021); (3) No adverse effects were observed in the subacute toxicity study (e.g., 500 mg/kg in beagle dogs with UP446 formulation containing 60% baicalin) (Yimam et al., 2016); (4) Baicalin is suitable for oral administration but has low bioavailability (Ganguly et al., 2022).
Twenty C57BL/6J mice (10 males and 10 females) were stratified by sex and randomly assigned to two experimental groups (n = 10 per group; five males and five females) through block randomization: Control group was administered a single oral gavage of sterile saline (0.9% NaCl, 20 mL/kg body weight). Baicalin group was received a single high-dose oral suspension of baicalin (4,000 mg/kg in saline, equivalent volume of 20 mL/kg).
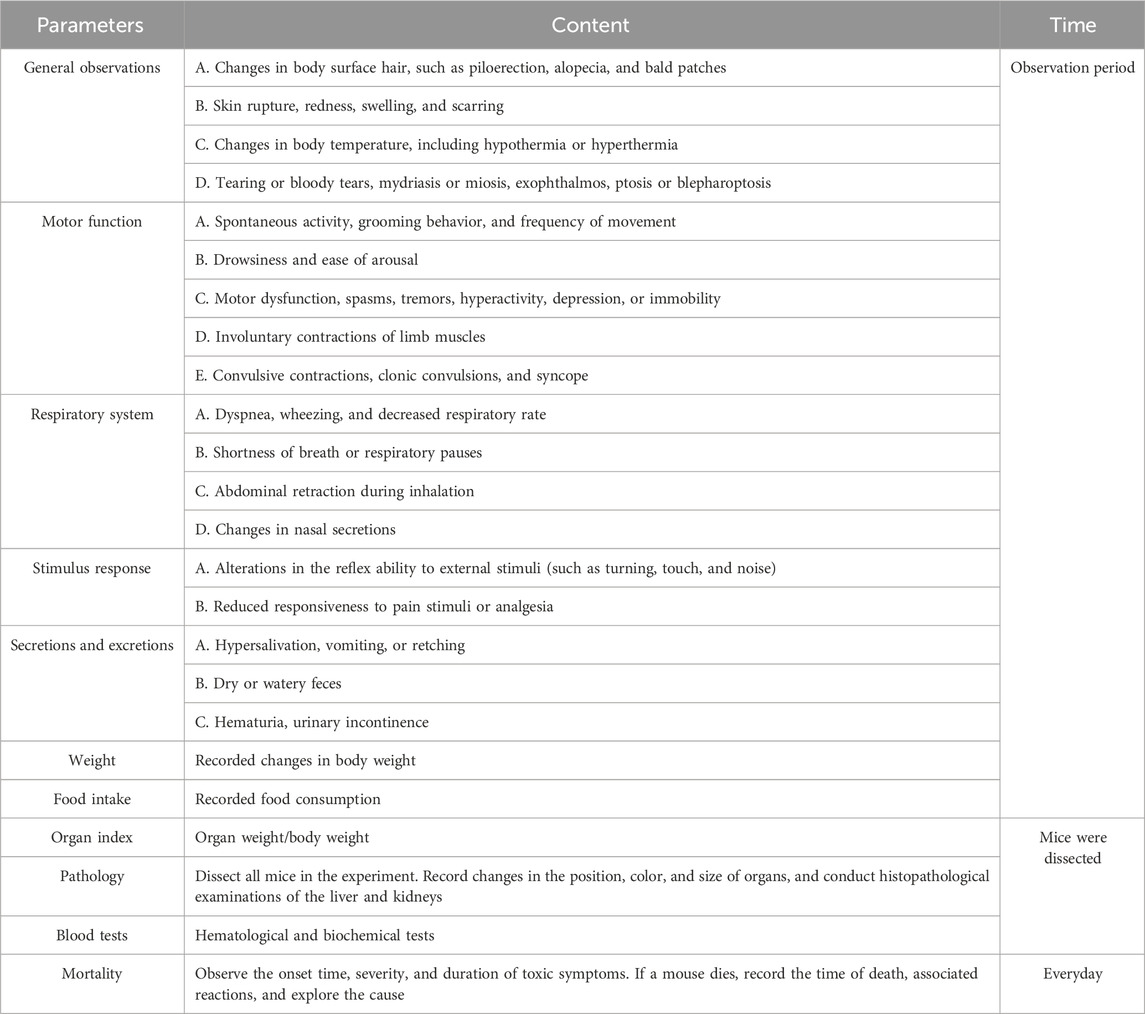
In the experimental protocol, animals underwent a 12-h fasting period with water provided to standardize their metabolic status. To minimize circadian variability, baicalin or sterile saline was administered as a single dose at 8:00–10:00 a.m. Post-administration, 24-h temporary observation period was implemented with evaluations at 0.5, 1, 2, 4, 8, and 24 h focusing on survival, neurological signs (e.g., convulsions, ataxia), and autonomic reactions (e.g., lacrimation, piloerection). Extending beyond the critical phase, a comprehensive observation period from Day 2 to Day 14 involved assessments of mortality, body weight and food intake, as well as the manifestation of clinical symptoms like diarrhea and lethargy. All items to be observed were referred to in Table 2 to ensure continuity and completeness of experimental observations (Ru et al., 2022; Wen et al., 2023). Following the single gavage administration, a comprehensive battery of behavioral assessments was conducted.
2.3 Subacute toxicity study
In accordance with OECD Guideline 407 for Repeated Dose Oral Toxicity Studies in Rodents, a 28-day subacute toxicity assessment was conducted with rigorous attention to experimental design and group (Ramesh and Mandal, 2019). Forty C57BL/6J mice (20 males and 20 females), were randomized into four groups (n = 10 per group, with a sex ratio of five males to five females). The C57BL/6J mouse strain was selected owing to its genetic uniformity, adherence to OECD toxicity testing guidelines, and comprehensive pathological characterization within toxicological research (Yang et al., 2022; Corder et al., 2023). The groups were administered as follows: the Normal group received the vehicle (0.9% NaCl, 20 mL/kg body weight), while the low-dose, mid-dose, and high-dose groups were given 1,000 mg/kg, 2,000 mg/kg, and 4,000 mg/kg of baicalin, respectively. The selection of the dose range was based on acute toxicity findings, which indicated no mortality at 4,000 mg/kg.
Baicalin and NaCl were administered via oral gavage at a volume of 20 mL/kg between 08:00 and 10:00 a.m. for 28 consecutive days. Clinical monitoring included daily recordings of body weight and food intake. Additionally, assessment of general status, including observations of abnormal behaviors (such as hyperactivity and circling), and mortality, were scored according to Table 2. At the end of gavage, a series of behavioral experiments were performed.
At the end of the study, mice were deeply anesthetized with 5% isoflurane for induction, followed by maintenance at 2% (RWD Life Science). Cardiac puncture was conducted to collect whole blood for hematological and biochemical analyses. Necropsy was then carried out, the major organs (liver, kidneys, brain, spleen, and heart) were excised (weighed to the nearest 0.01 g). This comprehensive experimental design ensured the accuracy and reliability of the subacute toxicity assessment of baicalin in mice.
2.4 Behavioral testing
2.4.1 The sucrose preference test (SPT)
Anhedonia was evaluated using the validated sucrose preference test. During the 48-h habituation phase, mice had free access to both 1% sucrose solution and tap water, with bottle positions alternated every 24 h to minimize side bias. 12 h before the start of the official experiment, animals underwent food deprivation while maintaining free water access. The consumption of aqueous sucrose solution and purified water was then measured over a 16-h period, with the bottle position rotated every 8 h to account for positional preference. The sucrose preference rate was calculated as (Sucrose water consumption/(Sucrose water consumption + Pure water consumption)×100%) (Zhu et al., 2024).
2.4.2 The tail suspension test (TST)
TST had been tested to determine depressive-like moods. Mice were suspended by their tails, which were fixed to an iron frame 50 cm above the ground. The test lasted for 6 min, during which the mice were allowed to adapt for 2 min before the time and number of struggles were recorded. After the test, the feces on the ground were removed, and the area was wiped with 75% alcohol to eliminate any odors (Li X. et al., 2018).
2.4.3 The forced swim test (FST)
Despair-like emotions were assessed using the FST. Mice were placed in a vertical transparent plastic cylinder (diameter 30 cm, height 50 cm, water depth 30 cm), and the water temperature will be maintained at 25°C (Agostinho et al., 2019). The test lasted for 6 min, during which the mice were allowed to acclimate for 2 min before the struggle time and frequency (defined as significant movement of all four limbs) were recorded. After each experiment, the water was changed to ensure there were no residual odors (Katrancha et al., 2019).
2.4.4 The marble-burying test (MBT)
Anxiety - like behaviors were assessed using MBT. Twenty clean standard glass marbles (various styles and colors, 15 mm in diameter, weighing approximately 5.2 g) were gently placed on the surface of the bedding material. Mice were placed in one corner of the cage containing the marbles and carefully positioned in the marble - free area of the test cage facing the marbles. After the test, the mice were removed and returned to their home cages. Scoring was performed blind to the treatment conditions or genotypes of the tested mice, with two to three scorers counting the number of buried marbles. A marble was considered buried if at least two-thirds (or sometimes three-quarters) of its surface area was covered by the bedding material (Borgonetti et al., 2020).
2.4.5 The pole test
The pole test is a behavioral test widely used for assessing motor dysfunction in mice. This procedure requires mice to grip and manipulate themselves down to the bottom of the pole, which can evaluate the animals’ motor coordination abilities. During the test, the mouse was placed at the top of the pole with its head facing upwards, and the animal will naturally orient itself downward and descend along the pole to the ground without interruption to return to its home cage. The total time taken for the mouse to descend to the bottom of the pole was recorded (Yu et al., 2020).
2.4.6 The sucrose splash test (SST)
SST is a method used to assess the motivational and self-care behaviors of rodents. In this test, a 10% sucrose solution was sprayed onto the dorsal surface of the rodents’ fur. The grooming behavior, which was triggered by the rodents to remove the solution through licking, biting, or scratching, was then measured. The frequency and duration of grooming behavior were recorded within 5 min after the evaporation of the sucrose solution (Jiang et al., 2019).
2.4.7 The novel object recognition test (NOR)
Exploiting rodents’ innate preference for novel object exploration, NOR is utilized to assess murine learning and memory. The protocol involved three phases: acclimation, training, and testing. During acclimation, mice explored an arena freely for 5 min. In training, two identical objects (A and B) were placed in opposite corners, and mice explored for 5 min. After 24 h, object A was replaced with a new cube (C) for testing, and mice explored again for 5 min. Exploration was defined as the snout being oriented toward or contacting the object within 2 cm. The discrimination index (DI) was calculated as DI (%) = (Time exploring C (TC)/(Time exploring B (TB) + Time exploring C (TC))) × 100%. This protocol effectively measures recognition memory in mice by controlling extraneous variables.
2.4.8 The nest shredding test (NST)
The nest - building assay was conducted by housing mice individually in transparent polycarbonate cages (33 cm × 18 cm × 15 cm) containing 1 cm of autoclaved bedding material. A pre-weighed sterile cotton fiber nestlet (12.7 cm × 12.7 cm, 1.3 cm thickness) was positioned centrally on the bedding surface. After a 2-h acclimation period without disturbance, the remaining intact nest material was carefully collected and reweighed. The percentage of nest material shredded by each mouse was calculated using the formula: [(initial weight–final weight)/initial weight] × 100%. This methodologically standardized protocol allowed for objective quantification of nest-building behavior as a measure of motivational drive and affective state (Martinez Hernandez et al., 2021).
All behavioral assessments were performed by trained investigators blinded to treatment groups. To minimize stress accumulation, tests were scheduled with ≥24-h intervals in a randomized order, and environmental variables (noise, lighting) were strictly controlled. Each apparatus was cleaned with ethanol between trials to eliminate olfactory cues.
2.5 Analysis of hematological and biochemical indices
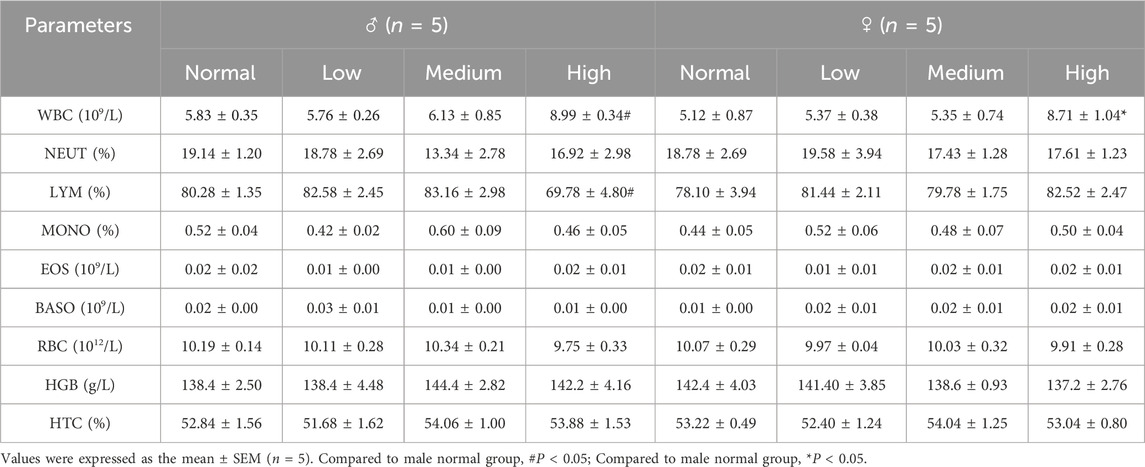
To obtain blood samples, an anticoagulant-containing vessel was utilized to collect approximately 0.5 mL of blood, which was promptly processed using a state-of-the-art fully automated hematological analyzer (Mindray BC6800 plus, Shenzhen, China). This analysis facilitated the determination of various hematological indices, encompassing the white blood cell count (WBC), the percentages of neutrophils (NEU%), the percentages of lymphocytes (LYM%), the percentages of monocytes (MON%), Eosinophil (EOS), basophil (BASO), red blood cell count (RBC), hemoglobin levels (HGB) and hematocrit value (HCT).
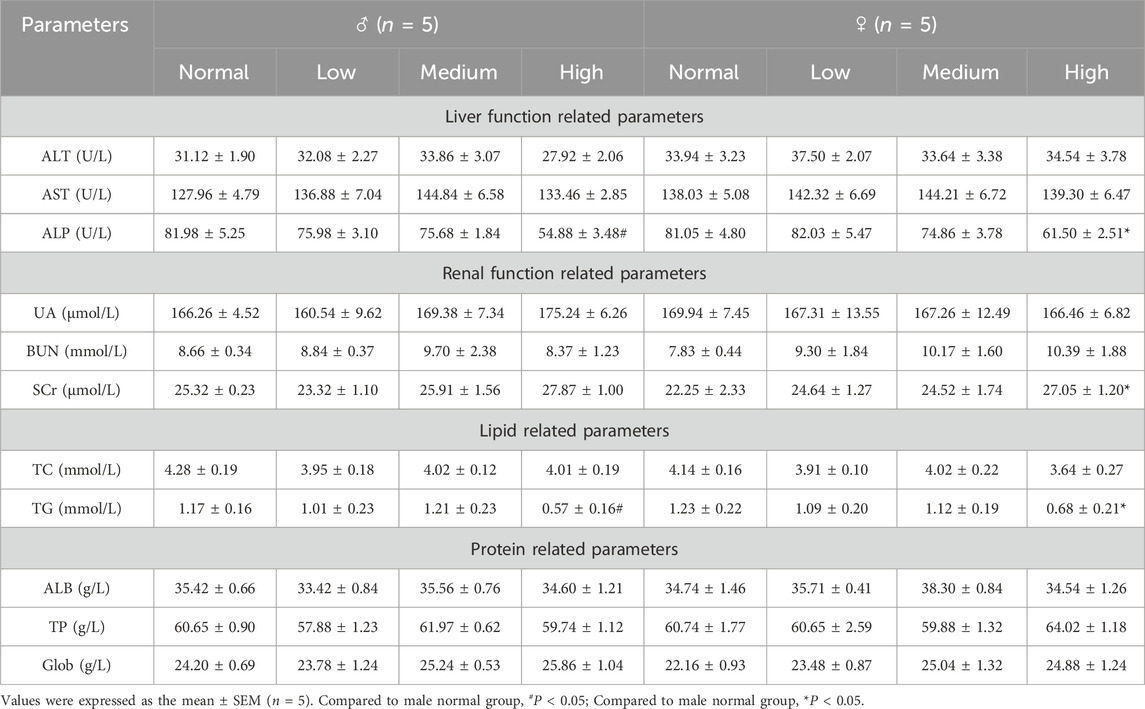
Additionally, approximately 1.8 mL of blood were aspirated and subjected to centrifugation at 3,000 revolutions per minute for 10 min, yielding serum for the subsequent assessment of enzymatic activities employing an advanced analyzer (Rayto Life Technologies, Chemray 800, Shenzhen, China). This evaluation encompassed the measurement of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), uric acid (UA), serum urea nitrogen (BUN), blood creatinine (Scr), total cholesterol (TC), triglycerides (TG), albumin (ALB), total protein (TP) and globulin (Glob).
2.6 Gross anatomy and histopathologic examination
Organ weights were immediately recorded using a precision analytical balance (±0.001 g) to minimize postmortem hydration changes. All measurements were performed by two independent investigators to ensure reproducibility. Two standardized indices were derived to assess dose-dependent organotoxicity: Organ-to-Body Weight Ratio (OBR%) = (Organ Wet Weight (g)/Total Body Weight (g))×100%. Organ-to-Brain Weight Ratio (OBBR%) = (Organ Wet Weight (g)/Brain Wet Weight (g))×100%. OBR normalizes organ mass to whole-body growth, identifying disproportionate organ enlargement/atrophy. OBBR controls for inter-individual neurodevelopmental variability, isolating organ-specific toxicity.
2.7 Hematoxylin-eosin (HE) staining procedure
The sections were dewaxed in xylene for 20 min, followed by sequential hydration in 100%, 95%, 80%, and 75% ethanol for 5 min each. Afterward, the sections were stained with hematoxylin for 5 min, differentiated in hydrochloric acid-ethanol for 30 s, and washed in tap water for 10 min to allow for bluing. Subsequently, the sections were stained with eosin for 3 min, dehydrated through a graded ethanol series, cleared in xylene, and mounted with neutral resin. Finally, the sections were observed under a microscope.
2.8 Statistical analysis
The statistical analysis was performed utilizing GraphPad Prism version and SPSS, with graphical representations created using Adobe Illustrator. For acute toxicity endpoints, intergroup comparisons between control and baicalin group were performed using two-tailed unpaired Student's t-tests following verification of normality (Shapiro-Wilk test) and homogeneity of variance (Levene’s test). Subacute toxicity datasets involving multiple dose groups were analyzed via one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Data were expressed as the mean ± SEM. Statistical significance was determined at a threshold of P < 0.05.
3 Results
3.1 General behaviors of acute oral toxicity in mice
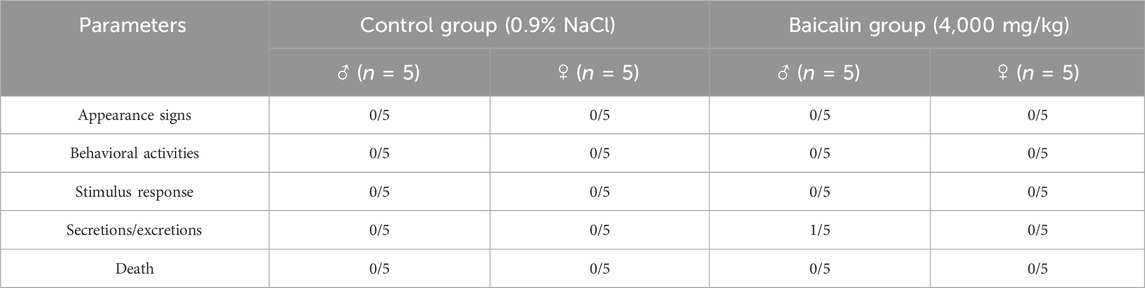
All subjects survived with no treatment-related deaths in the 14-day trial (detailed in Table 3). One male in the baicalin group was observed to have loose stools after gavage, but it disappeared after 24 h. Daily surveillance revealed no detectable pathophysiological alterations in other mice. Longitudinal monitoring showed progressive body weight gain trajectories in all groups, consistent with normal mice growth patterns (Figure 2A, P > 0.05). The results confirmed the absence of significant treatment effects on weekly body mass fluctuations. For 12 h of food intake, there was no significant difference between the groups (Figure 2B, P > 0.05). According to OECD Guideline 420 criteria, the calculated median lethal dose (LD50) of baicalin exceeded 4,000 mg/kg, confirming its favorable acute safety profile even at supratherapeutic doses tested (Nascimento et al., 2020).
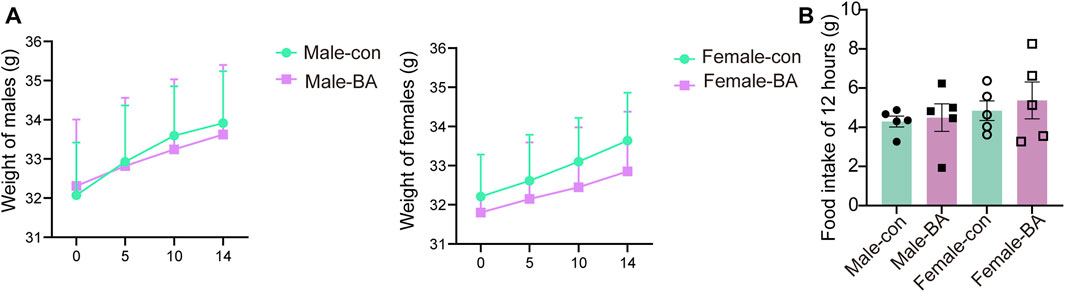
Figure 2. Weight and diet in acute toxicology experiments. (A) Weight gain of male and female mice over 14 days. (B) Food intake of 12 h. Values were expressed as the mean ± SEM (n = 5).
3.2 General behaviors of subacute oral toxicity in mice
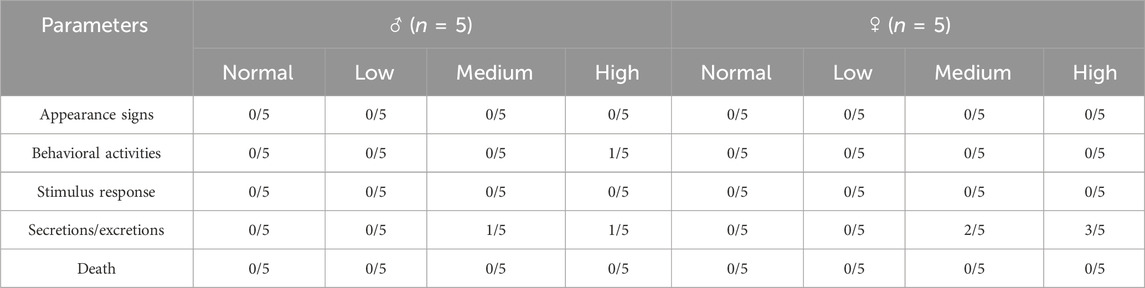
During the 28-day subacute oral toxicity experiment, we closely monitored the mice in all groups. Overall, no significant abnormalities were observed in their fur, behavior, or respiration. Transient gastrointestinal disturbances were observed on day 5, with 4/10 high-dose and 3/10 mid-dose animals exhibiting soft feces. On day 19, one male mouse in the high group demonstrated compulsive grooming behavior, potentially related to minor gavage-induced irritation. Significantly, all aberrant symptoms resolved spontaneously within 24 h without residual effects. Comprehensive behavioral assessments revealed no persistent pathological alterations (detailed in Table 4). The results indicated no significant treatment effects on weekly body weight trajectories (Figure 3A, P > 0.05) or 12-h food intake (Figure 3B, P > 0.05). The basal metabolism remained intact even at the tested dose of 4,000 mg/kg, these findings support the safety profile of baicalin within the evaluated dosage range.
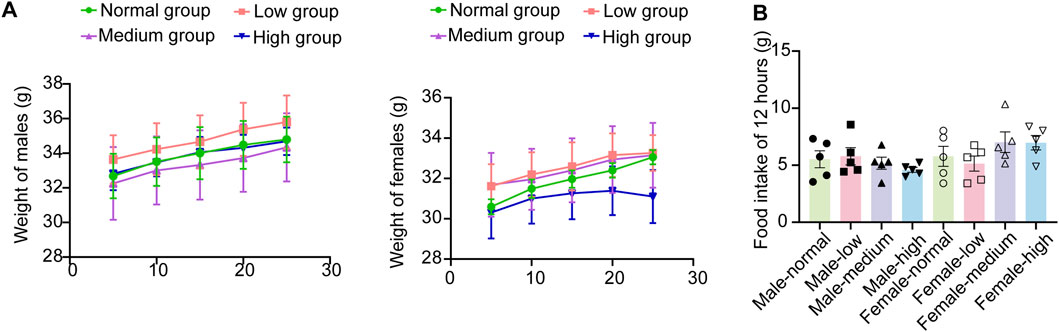
Figure 3. Weight and diet in subacute toxicology experiments. (A) Weight gain of male and female mice over 28 days. (B) Food intake of 12 h. Values were expressed as the mean ± SEM (n = 5).
3.3 Changes in behavioral parameters
3.3.1 Behavioral correlates of depression
To investigate the potential behavioral impacts of prolonged drug administration, this study integrated behavioral data from both acute and subacute toxicity. Whether in the acute or subacute toxicity stages, the sucrose preference ratios of mice in all groups did not have significant differences (Figures 4A,B, P > 0.05). During the experiment, two mice were excluded from the SPT due to leaks in their water bottles. The findings suggested that extended administration did not significantly impact the fundamental emotional state of the mice, as evidenced by the stability in their perception and preference for pleasurable stimuli.
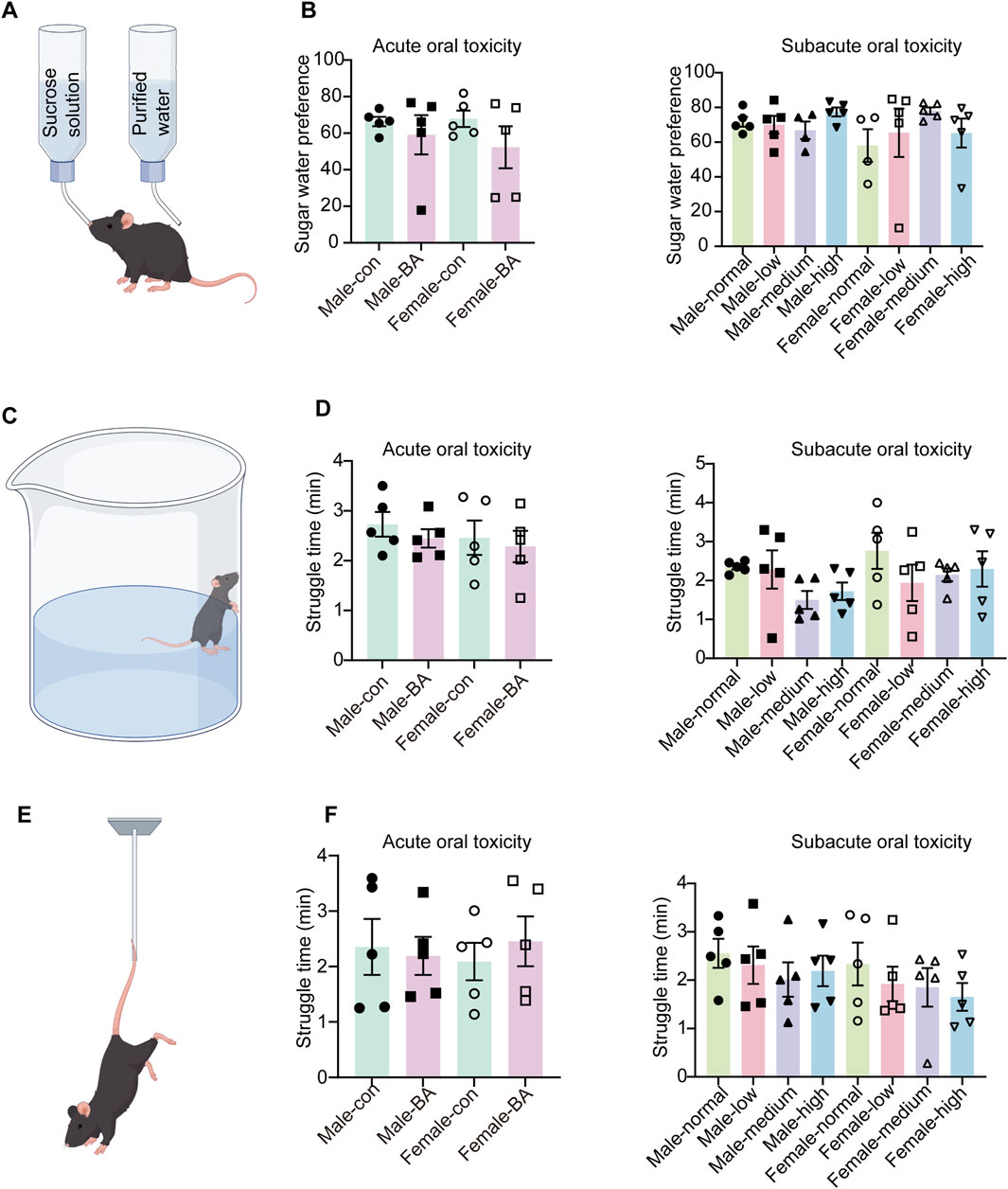
Figure 4. Alterations in depression-related behaviors of all groups. (A) Schematic diagram of the sucrose preference test. (B) Sugar water preference values of mice in each group (During the experiment, two mice were excluded from the SPT due to leaks in their water bottles). (C) Schematic diagram of the forced swim test. (D) Struggle time of mice in each group. (E) Schematic diagram of the tail suspension test. (F) Struggle time of mice in each group. Values were expressed as the mean ± SEM (n = 5).
Similarly, there were no significant differences in struggle time among mice during FST and TST (Figures 4B,C–E, P > 0.05). This phenomenon indicated the preserved behavioral despair resistance and psychological resilience following extended pharmacological exposure.
3.3.2 Anxiety and compulsive behavior profiling
MBT is widely recognized as a key indicator of compulsive-like behavior in rodents. No significant differences in marble-burying counts were observed across all groups in both acute and subacute toxicity phases (Figures 5A,B, P > 0.05). Comparable frequencies of self-grooming were observed across all groups in the SST (Figures 5C,D, P > 0.05). This suggested that acute and subacute administration did not evoke significant alterations in stress responses or self-grooming behavior. Motor function assessments via the pole test revealed no dose-dependent effects of baicalin (Figures 5E,F, P > 0.05). Baicalin did not alter compulsive behaviors, stress reactivity, or motor function in either acute or subacute phases. These findings collectively validate its dose-independent neurobehavioral safety.
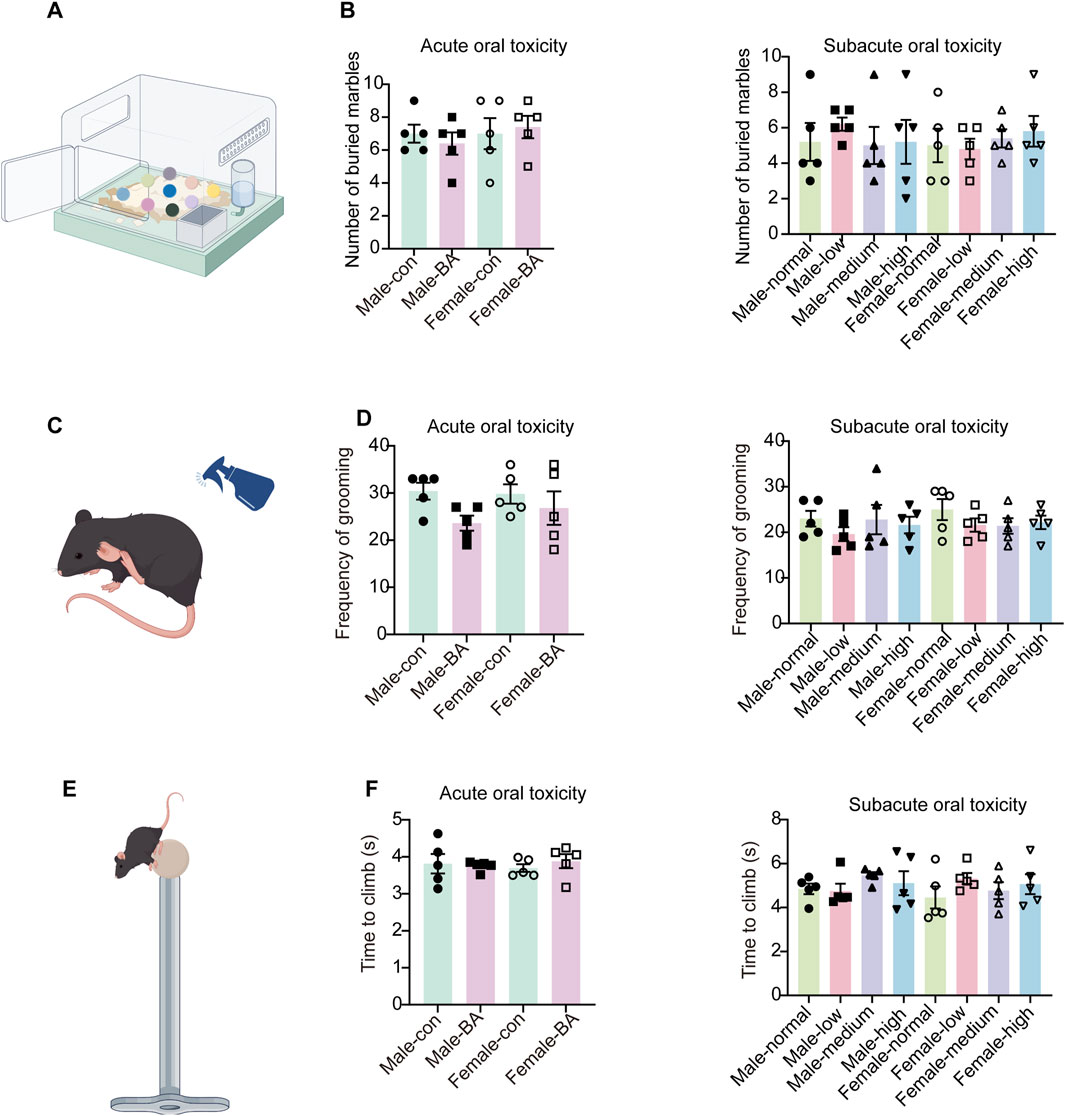
Figure 5. Anxiety and compulsive behavior profiling of all groups. (A) Schematic diagram of the marble-burying test. (B) Number of marbles buried in each group. (C) Schematic diagram of the sucrose splash test. (D) Frequency of grooming in each group. (E) Schematic diagram of the pole test. (F) Total time taken to climb down in each group. Values were expressed as the mean ± SEM (n = 5).
3.3.3 Cognitive and innate behavioral assessments
To examine the effects of baicalin on the cognitive and instinctive behaviors of mice, this study employed the NOR and NST experiment. During the acute studies, no statistically significant difference emerged in the frequency of new objects (Figures 6A,B, P > 0.05). This finding tentatively indicated that short-term exposure to baicalin does not significantly alter in recognition-related behaviors. However, as the experiment progressed into the subacute toxicological studies, there was a marked increase in the frequency of recognition of both old and new objects (Figure 6C, P < 0.05). This observation suggested that prolonged exposure to baicalin may enhance the responsiveness of mice to novel stimuli at the behavioral level, which potentially indicated an increase in exploratory behavior. Nonetheless, it was important to note that further analysis of the recognition indices revealed no significant differences in recognition indices during either the acute or subacute toxicological phases (Figure 6D, P > 0.05).
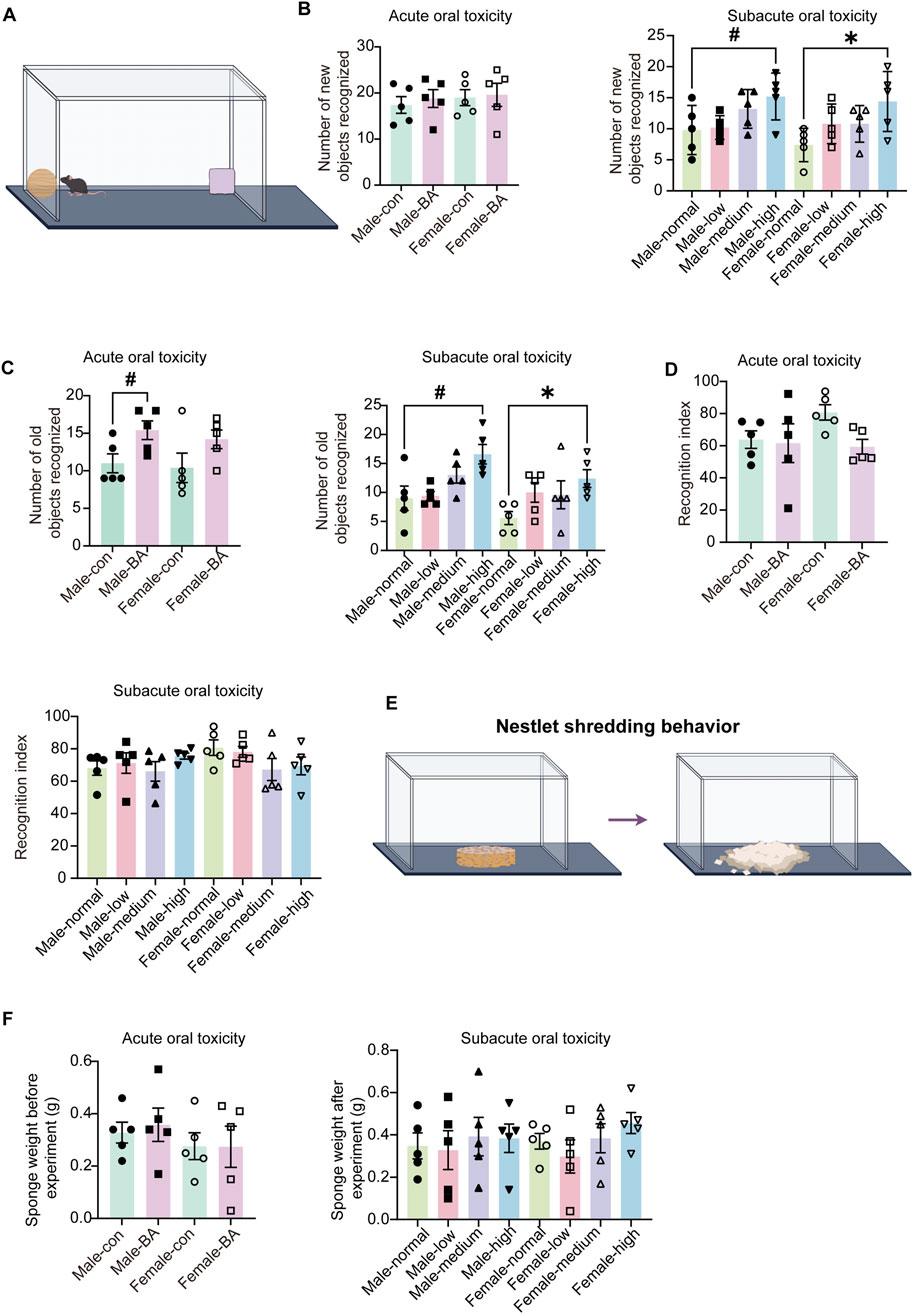
Figure 6. Cognitive and innate behavioral assessments of all groups. (A) Schematic diagram of the novel object recognition test. (B) Number of new objects recognized of mice in each group. (C) Number of old objects recognized of mice in each group. (D) Recognition index of mice in each group. (E) Schematic diagram of the nest shredding test. (F) Sponge weight in each group. Values were expressed as the mean ± SEM (n = 5). Compared to male normal group, #P < 0.05; Compared to female normal group, *P < 0.05.
NST demonstrated no significant changes in nest material disruption weights among all groups (Figures 6E,F, P > 0.05). These findings collectively demonstrated that baicalin does not impair cognitive processes or instinctual behaviors within the tested dose range, that supported safety profile of baicalin for chronic use.
3.4 Evaluation of organ coefficients
In order to gain a deeper understanding of the toxicological effects of baicalin, we employed organ-body weight ratios and organ-brain weight ratios as key indices. These parameters were selected for their potential to comprehensively reflect the impact of baicalin on the physiological state of experimental organisms at the organ level.
No significant differences were observed among all groups in terms of either the organ-body weight ratios or the organ-brain weight ratios (Figure 7, P > 0.05). This finding implied that baicalin might not cause pronounced adverse effects in various organs. It provided valuable insights into the potential safety profile of baicalin during subacute exposure, suggested that its impact on organ-level physiological relationships might be minimal within the scope of our experimental design.
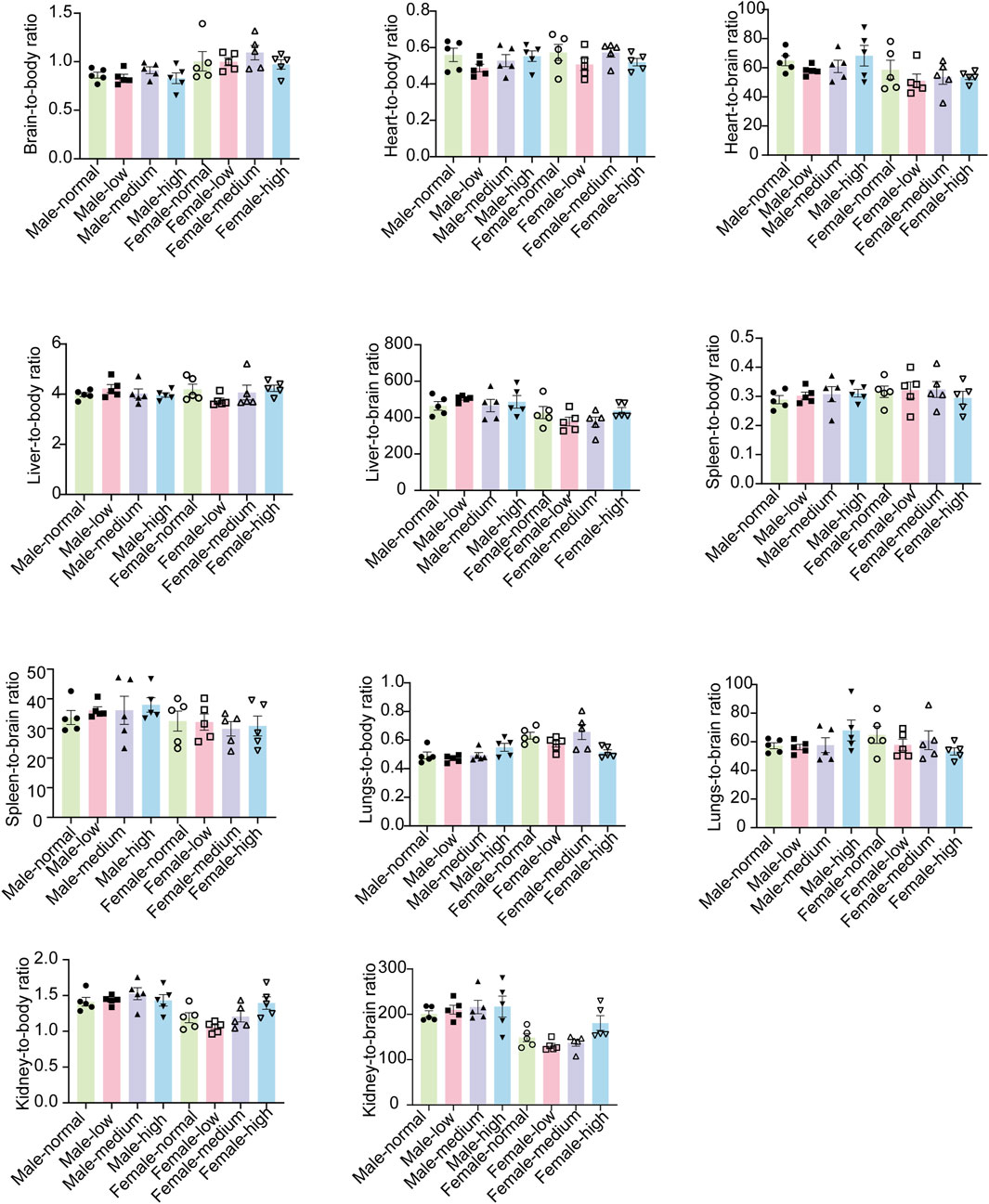
Figure 7. Organ-body weight ratios and the organ-brain weight ratios of mice in each group. Values were expressed as the mean ± SEM (n = 5).
3.5 Routine blood assessment
The hematological analysis of mice demonstrated alterations in WBC and LYM%. Administration of a high dose of baicalin resulted in a statistically significant increase in WBC counts compared to the normal group (Table 5, P < 0.05). Male mice in the high-dose group showed a significant decrease in LYM% (P < 0.05), whereas female mice exhibited a non-significant trend (Table 5, P > 0.05). The hematological observations indicated that baicalin exerts dose-dependent immunomodulatory effects, as evidenced by leukocytosis and gender-specific lymphopenia. Collectively, these findings affirmed the safety profile of baicalin within the tested dosage range.
3.6 Assessment of biochemical results
Liver function assessments demonstrated a dose-dependent decrease in ALP activity, with a particularly significant reduction observed in female mice within the high-dose group (Table 6, P < 0.05). This finding suggested a generalized modulation of ALP by baicalin. The observed decline may indicate altered hepatic metabolic activity, suggesting a pharmacological perturbation of liver physiology.
Renal function analysis showed significantly elevated SCr levels in female mice of the high-dose group (Table 6, P < 0.05), a biomarker of glomerular filtration impairment, which may indicated potential nephrotoxicity. Lipid profile evaluations demonstrated a significant reduction in TG levels in the high-dose group (Table 6, P < 0.05), consistent with baicalin’s putative lipid-lowering effects.
These biochemical findings showed that baicalin affected liver, kidney, and lipid metabolism. Reduced ALP and TG levels suggested beneficial metabolic effects, but increased Scr raised concerns about kidney safety.
3.7 Organ-specific histomorphometric analyses
To assess the toxicity profile of chronic baicalin exposure, we performed organ-specific histomorphometric analyses on hippocampal, hepatic and renal tissues. Considering the susceptibility of the hippocampus to neurotoxic damage, we conducted a systematic evaluation of its subregional integrity following prolonged exposure to high dose. Remarkably, our findings revealed no pathological changes in the CA1, CA3, or dentate gyrus (DG) neurons or glial cells. Histological analyses corroborated these findings, demonstrating preserved hippocampal cytoarchitecture and neuronal morphology (Figure 8).
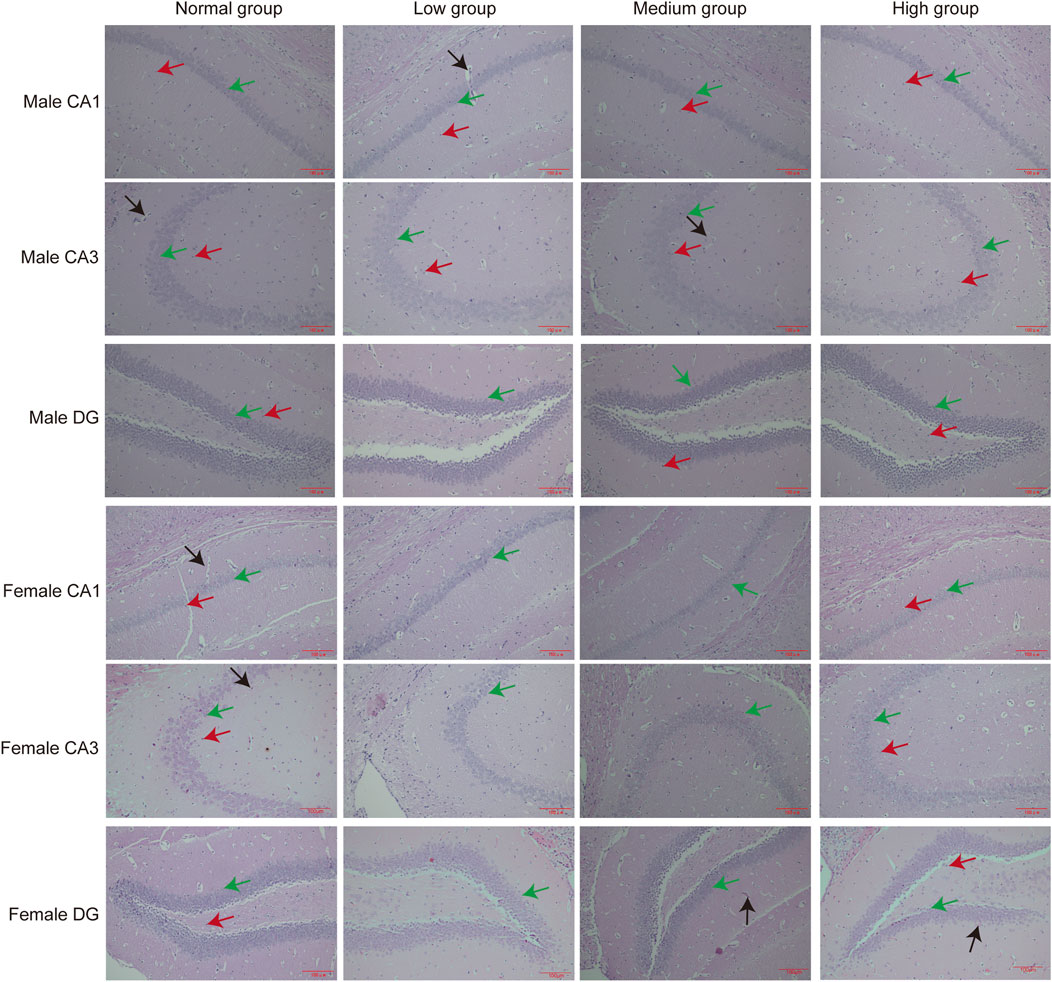
Figure 8. Pathologic results of hippocampal sections of mice in each group. Green arrows point to neuronal cells, red arrows point to glial cells, black arrows point to capillaries (n = 3).
In the morphological examination of liver histopathological sections, our analysis concentrated on alterations in hepatic sinusoidal endothelial cells (HSECs), Kupffer cells, and hepatocytes. The findings indicated that the group receiving a high-dose baicalin intervention exhibited pathological changes, characterized by hepatocytes displaying pyknosis and acidophilic chromatin condensation, which were indicative of an early metabolic stress state (Figure 9A).
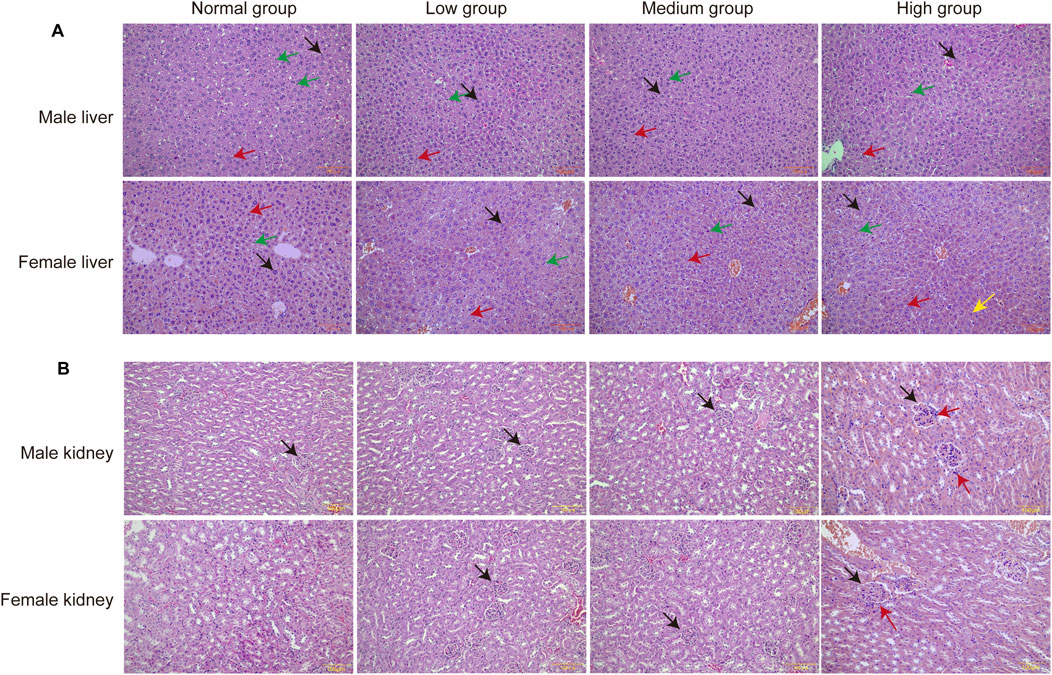
Figure 9. Pathologic results of liver and kidney sections of mice in each group. (A) Pathologic section of the liver. Green arrows point to Kupffer cells, red arrows point to endothelial cells, black arrows point to hepatocytes. (B) Pathologic section of the kidney. Black arrows point to glomeruli and red arrows point to inflammatory cell infiltration (n = 3).
Upon examination of the pathological sections of the kidney, it was observed that the glomeruli remained structurally intact with well-defined borders. However, a slight infiltration of inflammatory cells was noted surrounding the glomeruli following 28 days of exposure to a high dose of baicalin. This finding suggested that administration of baicalin at a dosage of 4,000 mg/kg may induce mild inflammation in the renal tissue (Figure 9B). Notably, these dose-dependent histopathological changed lacked progression to overt necrosis or fibrosis, aligning with transient SCr elevations.
4 Discussion
Baicalin, a flavonoid compound characterized by broad-spectrum biological activities, exhibits reduced membrane permeability due to its glycosylated structure, consequently attenuating its cytotoxicity (Chen et al., 2022; Froldi et al., 2022). The remarkable tolerance observed at 4,000 mg/kg is attributed to its unique pharmacokinetic characteristics, particularly its limited oral bioavailability. The extremely low aqueous solubility of baicalin (merely 16.82 μg/mL) restricts its dissolution rate and extent in the gastrointestinal tract (Li W. et al., 2018). Furthermore, its suboptimal lipid-water partition coefficient impedes passive transmembrane diffusion (Akao et al., 2000). Compounded by weak mucosal permeability, these factors collectively contribute to its poor oral bioavailability (Chen et al., 2022). The pharmacokinetic characteristics of baicalin are primarily reflected in carrier-mediated transport, enzyme-regulated metabolism, and enterohepatic circulation. Its intestinal absorption mainly relies on active transport mediated by specific transporters such as organic anion-transporting polypeptides (OATPs), rather than solely relying on passive diffusion. This transport mechanism renders its absorption susceptible to competitive inhibition by other drugs or compounds, thereby reducing bioavailability (Feng et al., 2019; Huang et al., 2019). Baicalin metabolism primarily depends on UDP-glucuronosyltransferases (UGTs). UGTs predominate in glucuronidation reactions (phase Ⅱ metabolism), generating water-soluble metabolites (e.g., baicalein glucuronide) to facilitate excretion (Yang et al., 2017; Wang R. et al., 2024). Furthermore, following biliary excretion, baicalin is hydrolyzed into aglycones (e.g., baicalein) by intestinal hydrolases (Xing et al., 2005). These aglycones are then reabsorbed into the circulation, thereby forming enterohepatic recycling (Figure 10) (Chen et al., 2014; Zhang et al., 2018). The absence of mortality and significant histopathological alterations at the 4,000 mg/kg aligns with OECD Guideline 420 criteria. Notably, transient fecal looseness in a few animals resolved spontaneously within 24 h, suggesting self-limiting gastrointestinal adaptation rather than systemic toxicity.
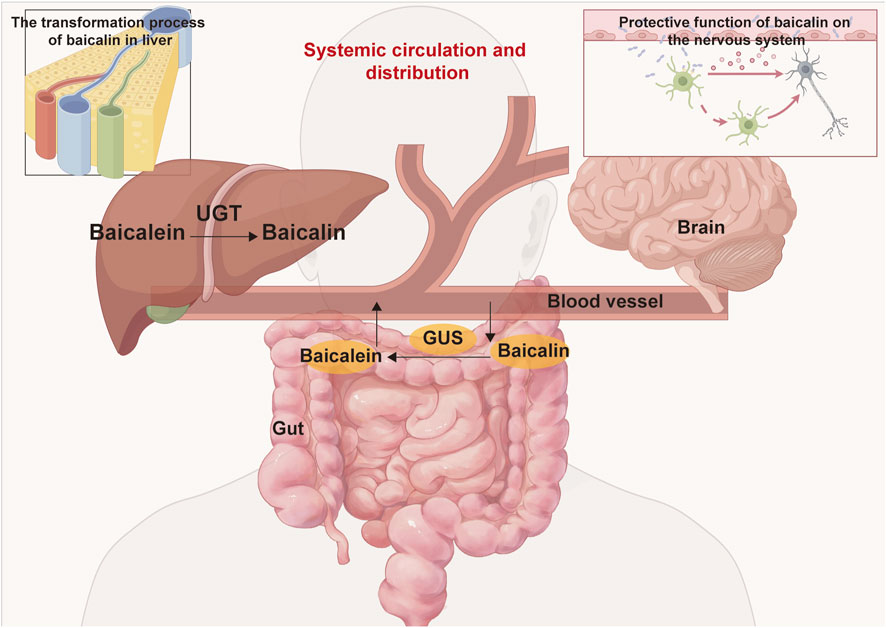
Figure 10. The metabolism and distribution of baicalin and baicalein within the body involve several key processes. Following oral administration, baicalin is transported to the intestine, where is enzymatically converted into baicalein by β-glucuronidase (GUS). Subsequently, the absorbed baicalein undergoes conversion back into baicalin via UDP-glucuronosyltransferase (UGT) in the liver, entering the enterohepatic circulation. Throughout this metabolic pathway, baicalin is capable of being distributed to various organs, including the brain, liver, gut, and others.
Sucrose preference indices were maintained, and struggle time in FST/TST remained unchanged, indicating that baicalin did not induce emotional dysregulation. While subacute treatment increased exploratory behavior towards novel stimuli, the intact object discrimination indicated preserved recognition memory, which aligns with serotonergic enhancement rather than cognitive impairment (Mustafa et al., 2025). These robust negative findings addressed critical regulatory concerns about phytochemical CNS liabilities, positioning baicalin as a preferential candidate for neuropsychiatric applications where synthetic antidepressants exhibit narrow therapeutic indices (e.g., SSRI-induced suicidal ideation).
Treatment did not significantly affect organ coefficients in male or female subjects, ruling out major structural adaptations like atrophy or compensatory hypertrophy. But the results of the blood test were the focus of our attention. Male mice treated with high-dose baicalin showed a statistically significant reduction in LYM%, whereas female mice exhibited no significant changes. This sex-based discrepancy may be attributed to baicalin-induced increases in estrogen and progesterone levels, with estrogen known for its immunomodulatory properties in females (Li et al., 2023). Estrogen potentially mitigates baicalin-induced immunosuppression by inhibiting pro-inflammatory cytokines or preserving lymphoid tissues (Al-Kuraishy et al., 2021; Fu et al., 2023; Li et al., 2023). Consequently, the endogenous hormonal environment in female mice helps maintain stable LYM% levels even under high-dose baicalin exposure, thereby preventing significant alterations. In contrast, male mice, lacking estrogen-mediated protective mechanisms, exhibited increased susceptibility to baicalin’s immunosuppressive effects, resulting in a marked decline in LYM%. Notably, numerous studies utilizing male mouse models, such as C57BL/6J or ICR strains, have documented baicalin-induced immunological changes, whereas comparative studies involving female mice remain limited (Wei et al., 2025).
Histopathological evaluations of the high-dose baicalin group showed that hepatocytes displayed pyknosis and acidophilic chromatin condensation, which were indicative of an early metabolic stress state. However, these changes failed to progress to overt necrosis or fibrosis. Biochemically, a dose-dependent decrease in ALP activity was observed, particularly significant in female mice of the high-dose group (Kimura et al., 2018). Studies have demonstrated that once the causative agent is eliminated, the liver can restore normal cellular morphology and enzyme activities (Porukala and Vinod, 2022; Xu et al., 2022). This is based on the liver’s well-known regenerative capacity. Regarding the kidneys, in the high-dose baicalin group, a slight infiltration of inflammatory cells was noted surrounding the glomeruli after 28 days of exposure. Elevated SCr levels were observed in female mice of the high-dose group. Similar to the liver, in related toxicology studies on compounds causing mild renal inflammation and transient changes in renal function markers, when the exposure is terminated, the renal tissue has been shown to resolve the inflammation, and the renal function markers return to normal levels (Yang M. et al., 2019; Ou et al., 2021). In our study, while we lacked post-exposure follow-up to directly demonstrate reversibility in the present experimental design, the absence of progression to more severe pathological states—such as glomerular damage, tubular necrosis, or chronic fibrosis—strongly indicates that the observed hepatic and renal changes are reversible.
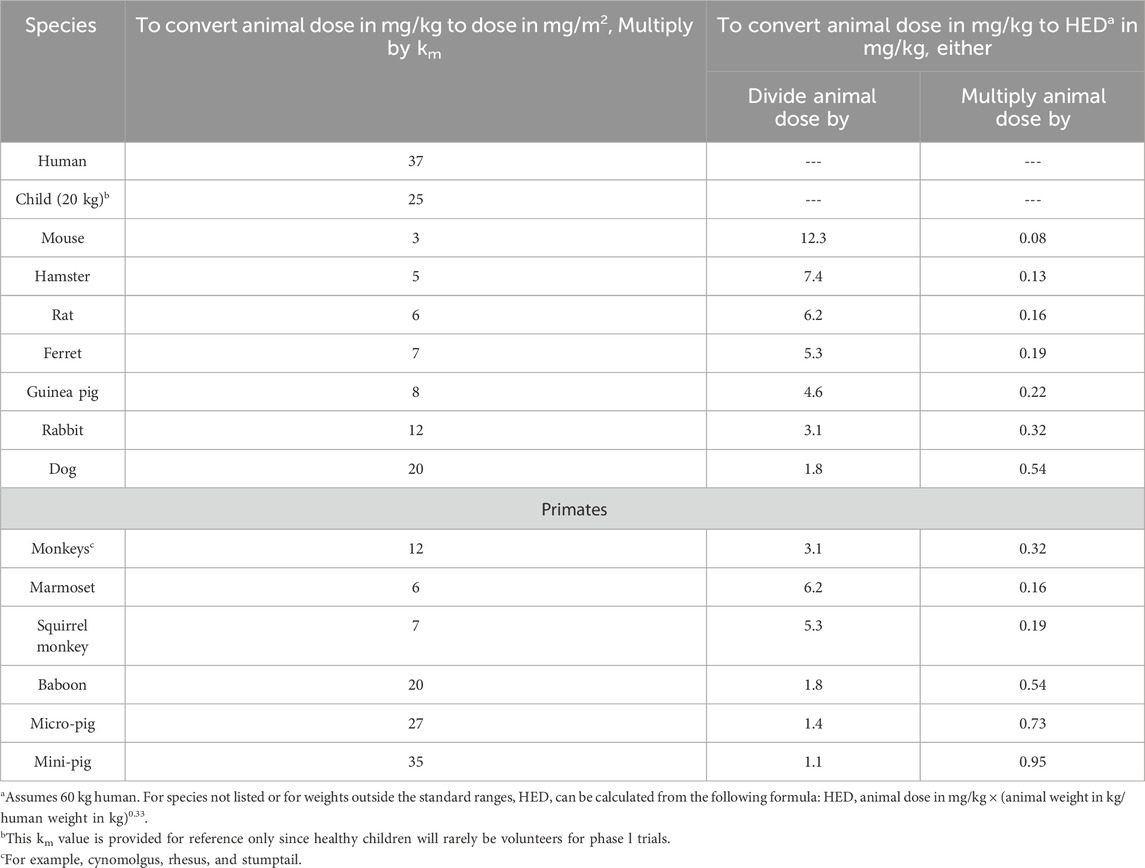
Based on the translation of these findings into clinical practice, we suggest a murine no-observed-adverse-effect level (NOAEL) of 2,000 mg/kg/day, which corresponds to a human equivalent dose of approximately 162.16 mg/kg, as determined by the conversion factors presented in Table 7. This proposed dosage is consistent with clinical trials of baicalein, which have demonstrated safety at a dosage of 2,800 mg/day in humans, taking into account the lower bioavailability of baicalin (Li et al., 2014). To mitigate toxicity risks, nanoformulations (e.g., baicalin-loaded liposomes) could enhance bioavailability.
By integrating behavioral, biochemical, and pathological endpoints, this study not only redefines baicalin’s safety landscape but also establishes a translatable framework for evaluating natural product toxicity. As the quest for neuroprotective phytochemicals intensifies, our findings served as a timely reminder that “natural” does not equate to “harmless,” and rigorous safety pharmacology must accompany efficacy studies. It is noteworthy that due to interspecies metabolic disparities, these findings require validation in higher-order animal models. Long-term studies in neurodegenerative disease models are also essential to confirm their clinical translational value. Despite being a natural product, rigorous safety evaluation remains indispensable, and baicalin warrants further investigation as a low-risk candidate for neuroprotective applications.
5 Conclusion
This study showed that baicalin was safe for mice at dose up to 4,000 mg/kg, causing no deaths, major organ damage, or lasting neurobehavioral issues. Temporary liver and kidney changes were reversible, blood alterations indicated dose-related immune effects without reaching toxic levels. The NOAEL was 2,000 mg/kg suggesting its potential for long-term neurodegenerative treatments. Despite being natural, thorough safety checks was crucial, confirming baicalin as a low-risk neuroprotective option.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by The Ethics Committee of Hebei University of Traditional Chinese Medicine (Ethics Approval No. DWLL202403137). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JY: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review and editing. MC: Conceptualization, Investigation, Validation, Writing – review and editing. WZ: Data curation, Investigation, Methodology, Writing – review and editing. JL: Data curation, Formal Analysis, Validation, Writing – review and editing. JZ: Methodology, Project administration, Writing – review and editing. XP: Data curation, Investigation, Writing – review and editing. YL: Conceptualization, Funding acquisition, Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – review and editing. PH: Data curation, Formal Analysis, Methodology, Project administration, Writing – review and editing. LP: Conceptualization, Funding acquisition, Resources, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the State Administration of Traditional Chinese Medicine of the People’s Republic of China Funding Project: National Prestigious Chinese Physicians Inheritance Studio [State TCM Human Education Letter (2022) No. 75]. Hebei Provincial Administration of Traditional Chinese Medicine Funding Project (2024075, 2022113, 2022112, and 2025429). Hebei Province Graduate Innovation Funding Project (CXZZBS2024153). Funded Project of Hebei Province Three Three Three Talent Project (C20231022). Hebei Province Natural Science Foundation (H2023423049).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agostinho, A. S., Mietzsch, M., Zangrandi, L., Kmiec, I., Mutti, A., Kraus, L., et al. (2019). Dynorphin-based “release on demand” gene therapy for drug-resistant temporal lobe epilepsy. EMBO Mol. Med. 11 (10), e9963. doi:10.15252/emmm.201809963
Akao, T., Kawabata, K., Yanagisawa, E., Ishihara, K., Mizuhara, Y., Wakui, Y., et al. (2000). Baicalin, the predominant flavone glucuronide of scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J. Pharm. Pharmacol. 52 (12), 1563–1568. doi:10.1211/0022357001777621
Al-Kuraishy, H. M., Al-Gareeb, A. I., Faidah, H., Al-Maiahy, T. J., Cruz-Martins, N., and Batiha, G. E. (2021). The looming effects of estrogen in Covid-19: a rocky rollout. Front. Nutr. 8, 649128. doi:10.3389/fnut.2021.649128
Borgonetti, V., Les, F., López, V., and Galeotti, N. (2020). Attenuation of anxiety-like behavior by Helichrysum stoechas (L.) moench methanolic extract through Up-Regulation of ERK signaling pathways in noradrenergic neurons. Pharm. (Basel) 13 (12), 472. doi:10.3390/ph13120472
Chen, H., Gao, Y., Wu, J., Chen, Y., Chen, B., Hu, J., et al. (2014). Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 354 (1), 5–11. doi:10.1016/j.canlet.2014.08.003
Chen, S., Xie, Q., Yang, M., Shi, Y., Shi, J., and Zeng, X. (2022). Scutellaria baicalensis extract-phospholipid complex: preparation and initial pharmacodynamics research in rats. Curr. Pharm. Biotechnol. 23 (6), 847–860. doi:10.2174/1389201022666210729142257
Corder, K. M., Hoffman, J. M., Sogorovic, A., and Austad, S. N. (2023). Behavioral comparison of the C57BL/6 inbred mouse strain and their CB6F1 siblings. Behav. Process. 207, 104836. doi:10.1016/j.beproc.2023.104836
Dai, J., Liang, K., Zhao, S., Jia, W., Liu, Y., Wu, H., et al. (2018). Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. U. S. A. 115 (26), E5896–e5905. doi:10.1073/pnas.1801745115
Dong, R., Li, L., Gao, H., Lou, K., Luo, H., Hao, S., et al. (2021). Safety, tolerability, pharmacokinetics, and food effect of baicalein tablets in healthy Chinese subjects: a single-center, randomized, double-blind, placebo-controlled, single-dose phase I study. J. Ethnopharmacol. 274, 114052. doi:10.1016/j.jep.2021.114052
Feng, W., Ao, H., Peng, C., and Yan, D. (2019). Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 142, 176–191. doi:10.1016/j.phrs.2019.02.024
Froldi, G., Djeujo, F. M., Bulf, N., Caparelli, E., and Ragazzi, E. (2022). Comparative evaluation of the antiglycation and Anti-α-Glucosidase activities of baicalein, baicalin (baicalein 7-O-Glucuronide) and the antidiabetic drug metformin. Pharmaceutics 14 (10), 2141. doi:10.3390/pharmaceutics14102141
Fu, L., Adu-Amankwaah, J., Sang, L., Tang, Z., Gong, Z., Zhang, X., et al. (2023). Gender differences in GRK2 in cardiovascular diseases and its interactions with estrogen. Am. J. Physiol. Cell Physiol. 324 (2), C505–c516. doi:10.1152/ajpcell.00407.2022
Ganguly, R., Gupta, A., and Pandey, A. K. (2022). Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: a review. World J. Gastroenterol. 28 (26), 3047–3062. doi:10.3748/wjg.v28.i26.3047
Guo, L. T., Wang, S. Q., Su, J., Xu, L. X., Ji, Z. Y., Zhang, R. Y., et al. (2019). Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation 16 (1), 95. doi:10.1186/s12974-019-1474-8
Harwood, M., Danielewska-Nikiel, B., Borzelleca, J. F., Flamm, G. W., Williams, G. M., and Lines, T. C. (2007). A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 45 (11), 2179–2205. doi:10.1016/j.fct.2007.05.015
Hu, Q., Hou, S., Xiong, B., Wen, Y., Wang, J., Zeng, J., et al. (2023). Therapeutic effects of baicalin on diseases related to gut-brain axis dysfunctions. Molecules 28 (18), 6501. doi:10.3390/molecules28186501
Hu, Z., Guan, Y., Hu, W., Xu, Z., and Ishfaq, M. (2022). An overview of pharmacological activities of baicalin and its aglycone baicalein: new insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 25 (1), 14–26. doi:10.22038/ijbms.2022.60380.13381
Huang, T., Liu, Y., and Zhang, C. (2019). Pharmacokinetics and bioavailability enhancement of baicalin: a review. Eur. J. Drug Metab. Pharmacokinet. 44 (2), 159–168. doi:10.1007/s13318-018-0509-3
Jiang, C., Sakakibara, E., Lin, W. J., Wang, J., Pasinetti, G. M., and Salton, S. R. (2019). Grape-derived polyphenols produce antidepressant effects via VGF- and BDNF-dependent mechanisms. Ann. N. Y. Acad. Sci. 1455 (1), 196–205. doi:10.1111/nyas.14098
Katrancha, S. M., Shaw, J. E., Zhao, A. Y., Myers, S. A., Cocco, A. R., Jeng, A. T., et al. (2019). Trio haploinsufficiency causes neurodevelopmental disease-associated deficits. Cell Rep. 26 (10), 2805–2817.e9. doi:10.1016/j.celrep.2019.02.022
Kimura, A., Kunimatsu, R., Yoshimi, Y., Tsuka, Y., Awada, T., Horie, K., et al. (2018). Baicalin promotes osteogenic differentiation of human cementoblast lineage cells via the Wnt/β catenin signaling pathway. Curr. Pharm. Des. 24 (33), 3980–3987. doi:10.2174/1381612824666181116103514
Li, M., Shi, A., Pang, H., Xue, W., Li, Y., Cao, G., et al. (2014). Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 156, 210–215. doi:10.1016/j.jep.2014.08.031
Li, W., Pi, J., Zhang, Y., Ma, X., Zhang, B., Wang, S., et al. (2018a). A strategy to improve the oral availability of baicalein: the baicalein-theophylline cocrystal. Fitoterapia 129, 85–93. doi:10.1016/j.fitote.2018.06.018
Li, X., Liang, S., Li, Z., Li, S., Xia, M., Verkhratsky, A., et al. (2018b). Leptin increases expression of 5-HT(2B) receptors in astrocytes thus enhancing action of fluoxetine on the depressive behavior induced by sleep deprivation. Front. Psychiatry 9, 734. doi:10.3389/fpsyt.2018.00734
Li, X., Yue, J., Kumar, Y., and Ma, Y. (2023). Chemoprotective effect of baicalin against cyclophosphamide induced ovarian toxicity in mice via inhibition of TGF-β. Heliyon 9 (12), e22079. doi:10.1016/j.heliyon.2023.e22079
Li, Y., Song, K., Zhang, H., Yuan, M., An, N., Wei, Y., et al. (2020). Anti-inflammatory and immunomodulatory effects of baicalin in cerebrovascular and neurological disorders. Brain Res. Bull. 164, 314–324. doi:10.1016/j.brainresbull.2020.08.016
Li, Y. F., Zhang, Y. F., Huang, C., and Jiang, J. M. (2025). Baicalin improves neurological outcomes in mice with ischemic stroke by inhibiting astrocyte activation and neuroinflammation. Int. Immunopharmacol. 149, 114186. doi:10.1016/j.intimp.2025.114186
Liu, J., Ping, X., Sun, S. J., Yang, J., Lu, Y., and Pei, L. (2024). Safety assessment of acori tatarinowii rhizoma: acute and subacute oral toxicity. Front. Pharmacol. 15, 1377876. doi:10.3389/fphar.2024.1377876
Martinez Hernandez, A., Silbern, I., Geffers, I., Tatenhorst, L., Becker, S., Urlaub, H., et al. (2021). Low-expressing synucleinopathy mouse models based on oligomer-forming mutations and C-Terminal truncation of α-Synuclein. Front. Neurosci. 15, 643391. doi:10.3389/fnins.2021.643391
Mustafa, N., Afroz, R., Batool, Z., Salman, T., Nawaz, S., and Haleem, D. J. (2025). Exploring Serotonin-1A receptor function in the effects of buspirone on cognition by molecular receptor expression and EEG analytical studies. Eur. J. Pharmacol. 990, 177275. doi:10.1016/j.ejphar.2025.177275
Nascimento, C. P., Luz, D. A., da Silva, C. C. S., Malcher, C. M. R., Fernandes, L. M. P., Dalla Santa, H. S., et al. (2020). Ganoderma lucidum ameliorates neurobehavioral changes and oxidative stress induced by ethanol binge drinking. Oxid. Med. Cell Longev. 2020, 2497845. doi:10.1155/2020/2497845
Ou, Y., Zhang, W., Chen, S., and Deng, H. (2021). Baicalin improves podocyte injury in rats with diabetic nephropathy by inhibiting PI3K/Akt/mTOR signaling pathway. Open Med. (Wars) 16 (1), 1286–1298. doi:10.1515/med-2021-0335
Porukala, M., and Vinod, P. K. (2022). Systems-level analysis of transcriptome reorganization during liver regeneration. Mol. Omics 18 (4), 315–327. doi:10.1039/d1mo00382h
Ramesh, N., and Mandal, A. K. A. (2019). Pharmacokinetic, toxicokinetic, and bioavailability studies of epigallocatechin-3-gallate loaded solid lipid nanoparticle in rat model. Drug Dev. Ind. Pharm. 45 (9), 1506–1514. doi:10.1080/03639045.2019.1634091
Ru, L., Liu, R., Xing, H., Yuan, Y., Yuan, Z., Xu, Z., et al. (2022). Acute and subacute oral toxicity assessment of gancao xiexin decoction in sprague-dawley rats. Front. Pharmacol. 13, 1078665. doi:10.3389/fphar.2022.1078665
Si, L., An, Y., Zhou, J., and Lai, Y. (2025). Neuroprotective effects of baicalin and baicalein on the central nervous system and the underlying mechanisms. Heliyon 11 (1), e41002. doi:10.1016/j.heliyon.2024.e41002
Singh, B., and Semwal, B. C. (2024). A compressive review on source, toxicity and biological activity of flavonoid. Curr. Top. Med. Chem. 24 (24), 2093–2116. doi:10.2174/0115680266316032240718050055
Wang, H., Ma, J., Li, X., Peng, Y., and Wang, M. (2024a). FDA compound library screening baicalin upregulates TREM2 for the treatment of cerebral ischemia-reperfusion injury. Eur. J. Pharmacol. 969, 176427. doi:10.1016/j.ejphar.2024.176427
Wang, R., Wang, C., Lu, L., Yuan, F., and He, F. (2024b). Baicalin and baicalein in modulating tumor microenvironment for cancer treatment: a comprehensive review with future perspectives. Pharmacol. Res. 199, 107032. doi:10.1016/j.phrs.2023.107032
Wang, Z. L., Wang, S., Kuang, Y., Hu, Z. M., Qiao, X., and Ye, M. (2018). A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 56 (1), 465–484. doi:10.1080/13880209.2018.1492620
Wei, H., Xia, D., Li, L., Liang, L., Ning, L., Gan, C., et al. (2025). Baicalin modulates glycolysis via the PKC/Raf/MEK/ERK and PI3K/AKT signaling pathways to attenuate IFN-I-Induced neutrophil NETosis. Mediat. Inflamm. 2025, 8822728. doi:10.1155/mi/8822728
Wen, J., Yang, Y., and Hao, J. (2023). Acori tatarinowii rhizoma: a comprehensive review of its chemical composition, pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 14, 1090526. doi:10.3389/fphar.2023.1090526
Xi, Y. L., Li, H. X., Chen, C., Liu, Y. Q., Lv, H. M., Dong, S. Q., et al. (2016). Baicalin attenuates high fat diet-induced insulin resistance and ectopic fat storage in skeletal muscle, through modulating the protein kinase B/Glycogen synthase kinase 3 beta pathway. Chin. J. Nat. Med. 14 (1), 48–55. doi:10.3724/sp.J.1009.2016.00048
Xing, J., Chen, X., and Zhong, D. (2005). Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 78 (2), 140–146. doi:10.1016/j.lfs.2005.04.072
Xu, Q., Deng, Y., Ming, J., Luo, Z., Chen, X., Chen, T., et al. (2022). Methyl 6-O-cinnamoyl-α-d-glucopyranoside ameliorates acute liver injury by inhibiting oxidative stress through the activation of Nrf2 signaling pathway. Front. Pharmacol. 13, 873938. doi:10.3389/fphar.2022.873938
Yang, M., Kan, L., Wu, L., Zhu, Y., and Wang, Q. (2019a). Effect of baicalin on renal function in patients with diabetic nephropathy and its therapeutic mechanism. Exp. Ther. Med. 17 (3), 2071–2076. doi:10.3892/etm.2019.7181
Yang, N., Sun, R., Liao, X., Aa, J., and Wang, G. (2017). UDP-Glucuronosyltransferases (UGTs) and their related metabolic cross-talk with internal homeostasis: a systematic review of UGT isoforms for precision medicine. Pharmacol. Res. 121, 169–183. doi:10.1016/j.phrs.2017.05.001
Yang, R., Wang, R., Xu, A., Zhang, J., and Ma, J. (2024). Mitigating neurodegenerative diseases: the protective influence of baicalin and baicalein through neuroinflammation regulation. Front. Pharmacol. 15, 1425731. doi:10.3389/fphar.2024.1425731
Yang, S., Wang, H., Yang, Y., Wang, R., Wang, Y., Wu, C., et al. (2019b). Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. 117, 109102. doi:10.1016/j.biopha.2019.109102
Yang, W., Yang, X., Jiang, L., Song, H., Huang, G., Duan, K., et al. (2022). Combined biological effects and lung proteomics analysis in mice reveal different toxic impacts of electronic cigarette aerosol and combustible cigarette smoke on the respiratory system. Arch. Toxicol. 96 (12), 3331–3347. doi:10.1007/s00204-022-03378-z
Yimam, M., Lee, Y. C., and Jia, Q. (2016). 26-week repeated oral dose toxicity study of UP446, a combination of defined extracts of Scutellaria baicalensis and Acacia catechu, in beagle dogs. Regul. Toxicol. Pharmacol. 78, 66–77. doi:10.1016/j.yrtph.2016.04.007
Yu, R., Li, J., Lin, Z., Ouyang, Z., Huang, X., Reglodi, D., et al. (2020). TAT-Tagging of VIP exerts positive allosteric modulation of the PAC1 receptor and enhances VIP neuroprotective effect in the MPTP mouse model of parkinson's disease. Biochim. Biophys. Acta Gen. Subj. 1864 (8), 129626. doi:10.1016/j.bbagen.2020.129626
Zhang, C. L., Xu, Y. J., Xiang, D., Yang, J. Y., Lei, K., and Liu, D. (2018). Pharmacokinetic characteristics of baicalin in rats with 17α-ethynyl-estradiol-induced intrahepatic cholestasis. Curr. Med. Sci. 38 (1), 167–173. doi:10.1007/s11596-018-1861-x
Zhang, X., Yang, Y., Du, L., Zhang, W., and Du, G. (2017). Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int. Immunopharmacol. 50, 38–47. doi:10.1016/j.intimp.2017.06.007
Zheng, L., Zhang, C., Li, L., Hu, C., Hu, M., Sidikejiang, N., et al. (2017). Baicalin ameliorates renal fibrosis via inhibition of transforming growth factor β1 production and downstream signal transduction. Mol. Med. Rep. 15 (4), 1702–1712. doi:10.3892/mmr.2017.6208
Keywords: baicalin, acute toxicity, subacute toxicity, behavioral tests, histopathology, hematology abstract
Citation: Yang J, Chen M, Zhang W, Liu J, Zhao J, Ping X, Lu Y, He P and Pei L (2025) Integrated neurobehavioral and organ-specific safety profiling of baicalin: acute/subacute toxicity studies. Front. Pharmacol. 16:1607919. doi: 10.3389/fphar.2025.1607919
Received: 08 April 2025; Accepted: 14 July 2025;
Published: 21 August 2025.
Edited by:
Gladys Ouedraogo, L'Oreal, FranceReviewed by:
Xueliang Shang, North China University of Science and Technology, ChinaWenchao Tang, Guizhou University of Traditional Chinese Medicine, China
An Yongkang, First Affiliated Hospital of Henan University of Traditional Chinese Medicine, China
Copyright © 2025 Yang, Chen, Zhang, Liu, Zhao, Ping, Lu, He and Pei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Lu, MzE0NzQ5MTY0QHFxLmNvbQ==; Pei He, aGVwZWlfODVAMTI2LmNvbQ==; Lin Pei, cGVpbGluMTM4MzExOTAzMDlAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jiali Yang
Jiali Yang Mengyu Chen1,2,3,4†
Mengyu Chen1,2,3,4† Jia Liu
Jia Liu Ye Lu
Ye Lu Pei He
Pei He Lin Pei
Lin Pei