- 1School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Robotic Minimally Invasive Surgery Center, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 3Organ Transplantation Center, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 4Department of Information, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 5Clinical Medical College, Southwest Medical University, Luzhou, China
- 6Department of Nephrology, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Introduction: Effective treatment during the metastatic hormone-sensitive prostate cancer (mHSPC) stage is crucial for delaying disease progression. Due to the lack of a head-to-head comparison of darolutamide (DARO) and abiraterone acetate plus prednisone (AAP) doublet regimen, this study aims to compare the efficacy and safety of DARO + ADT and AAP + ADT in the treatment of mHSPC in the real world.
Methods: This study retrospectively analyzed patients with mHSPC who received DARO or AAP treatment in Sichuan Provincial People’s Hospital from January 2022 to June 2024, with follow-up until December 2024. The clinical data and prostate-specific antigen (PSA) changes of patients were collected. The primary endpoint was time to metastatic castration-resistant prostate cancer (mCRPC), and the secondary endpoints were overall survival (OS), radiological progression-free survival (rPFS), time to PSA progression, time to pain progression, and time to subsequent prostate cancer therapy.
Results: A total of 178 patients were included, with 96 in the DARO group and 82 in the AAP group. The baseline characteristics of the two groups were comparable. The median follow-up time and interquartile ranges of the DARO and AAP groups were 12.0 [7.9–17.6] months and 17.4 [9.3–23.8] months, respectively. For the primary endpoint, DARO significantly delayed the time to mCRPC versus AAP [HR, 0.41 (95%CI, 0.23 to 0.71); P < 0.005]. And the DARO group significantly benefited in all secondary endpoints. DARO significantly led to deeper PSA reduction compared to AAP, with higher median reduction rates, better PSA50 and PSA90 remission rates, and a higher proportion of patients reaching lower PSA values. The incidence of adverse reactions was similar in the two groups, and there was no grade 3 or above drug-related adverse reactions.
Conclusion: In the treatment of mHSPC, DARO + ADT was associated with significant improvement of clinical outcomes versus AAP + ADT, while their safety is comparable.
1 Introduction
Prostate cancer (Pca) is a malignant tumor originating from the epithelial cells of the prostate gland (Rebello et al., 2021). Global cancer statistics indicate that prostate cancer is the second most common cancer in men and the fifth leading cause of cancer-related deaths among males (Smith et al., 2023). Metastatic hormone-sensitive prostate cancer (mHSPC) represents a critical stage, where timely and effective treatment can potentially delay disease progression to metastatic castration-resistant prostate cancer (mCRPC) and improve overall prognosis (Castellan et al., 2018; Chi et al., 2019; Armstrong et al., 2019).
Androgen deprivation therapy (ADT) has long been the cornerstone of treatment for mHSPC (Yu and Aragon-Ching, 2022). However, traditional approaches such as ADT alone or ADT combined with first-generation antiandrogens like bicalutamide have proven insufficient to significantly improve survival or quality of life in mHSPC patients (Ueda et al., 2024; Wenzel et al., 2023). Moreover, nearly all advanced prostate cancer patients eventually develop resistance to ADT and first-generation androgen receptor antagonists, leading to progression to mCRPC (Wang et al., 2023; Watson et al., 2015).
The treatment landscape for mHSPC has undergone significant transformation, with the introduction of novel therapies such as ADT + docetaxel or second-generation antiandrogens demonstrating substantial improvements in survival outcomes (Lam et al., 2024; Hamilou et al., 2018; Kyriakopoulos et al., 2018). The LATITUDE trial was the first to validate the efficacy of the abiraterone acetate plus prednisone (AAP) + ADT doublet regimen (Fizazi et al., 2019a). Studies have shown that this combination leads to a decline in prostate-specific antigen (PSA) levels (Sadaghiani et al., 2022), with the extent of PSA reduction serving as a key early indicator of long-term prognosis (Sadaghiani et al., 2021; Chowdhury et al., 2023). The emergence of the PEACE-1 trial further demonstrated that the triplet regimen of AAP + ADT + docetaxel improves both overall survival (OS) and radiographic progression-free survival (rPFS) in mHSPC patients (Fizazi et al., 2022), establishing this triplet regimen as a standard treatment. With the continuous development of second-generation antiandrogens, darolutamide (DARO) has gained attention. The ARASENS trial highlighted the significant OS benefits of the DARO + ADT + docetaxel triplet regimen (Smith et al., 2022). More recently, the ARANOTE study confirmed the safety and efficacy of the DARO + ADT doublet regimen (Sa et al., 2024), ushering in a new era of dual therapy for mHSPC.
Both DARO and AAP triplet regimen are associated with significantly increased adverse event rates and higher treatment costs (Lee, 2023; Hoeh et al., 2023; Shore et al., 2022). In real-world settings, patients often exhibit reluctance toward chemotherapy due to its side effects, influencing their treatment choices (Jansen et al., 2022; Aparicio et al., 2021). Concerns regarding drug accessibility, tolerability, safety, drug-drug interactions, and health economics have led many mHSPC patients to opt for doublet regimen only (Leith et al., 2022; Freedland et al., 2021; Raval et al., 2024; Schiff, 2022).
Although the efficacy of the doublet and triplet regimen of DARO and AAP has been studied in a controlled environment, real - world data can more comprehensively demonstrate their performance in clinical applications. Patients are resistant to chemotherapy drugs and intolerant to side effects. Meanwhile, there is a lack of head - to - head comparisons of the efficacy and safety of the DARO and AAP doublet regimen in the real world. This study focuses on the real - world setting and compares the efficacy and safety of the DARO + ADT and AAP + ADT doublet regimen for the treatment of mHSPC, with the time to mCRPC as the primary endpoint. The aim is to provide evidence for the clinical application of the DARO + ADT doublet regimen, strive to improve the efficacy and safety, and reduce the economic burden on patients.
2 Methods
2.1 Patients and treatment
This study retrospectively evaluated the efficacy and safety of DARO + ADT versus AAP + ADT in the treatment of mHSPC patients in the real world. The clinical data of mHSPC patients who were treated with DARO + ADT or AAP + ADT and visited Sichuan Provincial People’s Hospital from January 2022 to June 2024 were retrospectively analyzed, and the follow-up lasted until December 2024. In the treatment regimens, the dose of DARO was 600 mg administered orally twice daily in combination with ADT in the DARO + ADT regimen. In contrast, the dose of abiraterone acetate was 1,000 mg administered orally once daily, and the dose of prednisone was 5 mg administered orally twice daily in the AAP + ADT regimen.
The inclusion criteria for patients were as follows: ① Pathologically or cytologically confirmed prostate adenocarcinoma; ② Performance status score of 0–1 according to the Eastern Cooperative Oncology Group (ECOG); ③ Received DARO + ADT or AAP + ADT treatment for at least 1 month and had relatively complete follow-up data; ④ Testosterone was at the castration level during the treatment process (testosterone <50 ng/mL or <1.7 nmol/L). The exclusion criteria were: ① Received docetaxel treatment previously or during the follow-up; ② Had severe underlying diseases that were poorly controlled; ③ Received palliative radiotherapy, palliative surgery or particle implantation simultaneously; ④ Had a history of other primary malignancies, except for patients with in situ carcinoma who had no evidence of disease for 5 years or more and did not require treatment.
2.2 Follow-up observation data and endpoints
We collected the clinical data of patients from the hospital information electronic medical record system (HIS), including: age, Gleason score of the puncture pathology, ECOG score, baseline testosterone, PSA levels (before treatment, 1 month, 3 months, 6 months, 9 months, 12 months after treatment; time to reach PSA50 (defined as the proportion of patients with a 50% decrease in PSA from the baseline value after treatment), PSA90 (defined as the proportion of patients with a 90% decrease in PSA from the baseline value after treatment), PSA <2 ng/mL, PSA <0.02 ng/mL, and PSA <0.008); previous treatment history and drug-related adverse events (AE).
The primary endpoint was the time to mCRPC, and the secondary endpoints included OS, rPFS, time to PSA progression, time to subsequent prostate cancer therapy.
2.3 Data analysis
SPSS 26.0 software was used to conduct statistical analysis of the relevant data. The Kolmogorov-Smirnov method was used to test the normality of the measurement data. The measurement data with non-normal distribution were represented by M (P25, P75) and analyzed by the Mann-Whitney U test. The count data were represented by the number of cases (%) and analyzed by the χ2 test or Fisher’s exact probability method. The Kaplan-Meier estimation and COX regression model were used to analyze the primary and secondary endpoints. A P value less than 0.05 indicated that the difference was statistically significant.
3 Results
3.1 Baseline data
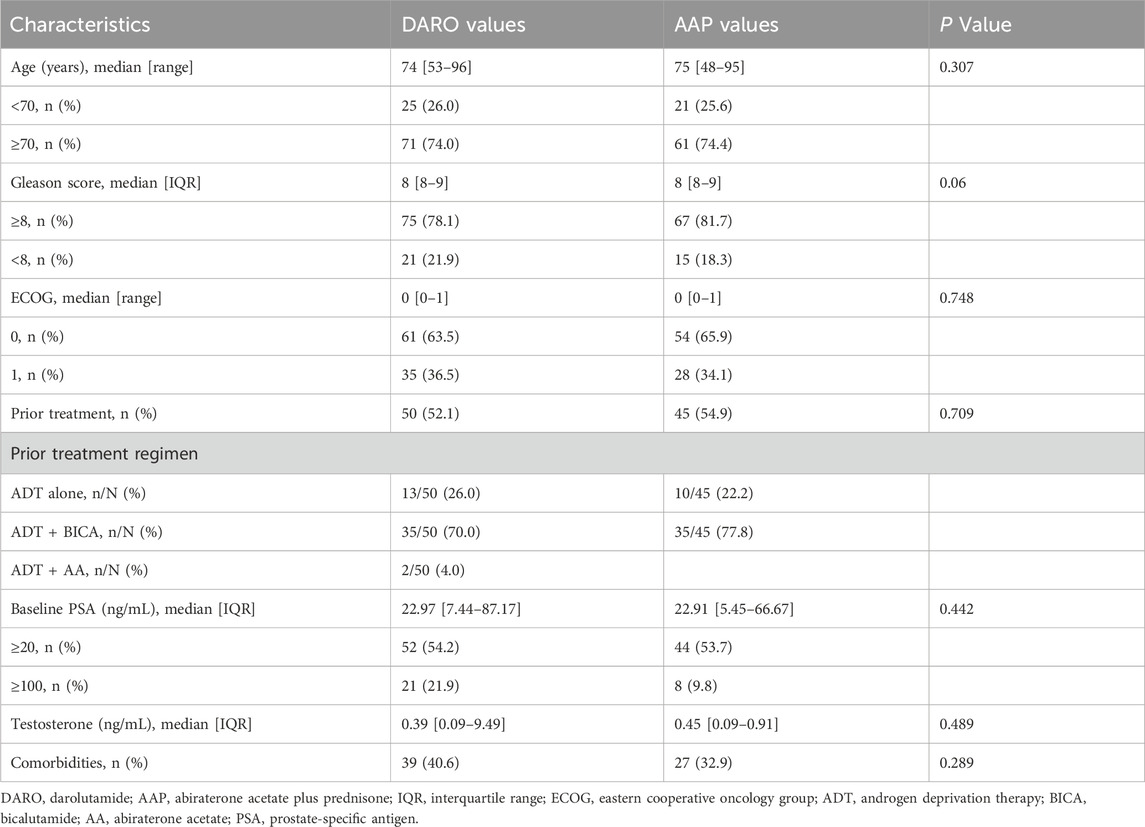
A total of 308 patients were included in the study, and 178 of them met the inclusion criteria. Among them, 96 patients used DARO and 82 patients used AAP. None of the included patients had received docetaxel chemotherapy. The demographic and baseline characteristics of the patients were well balanced between the two groups (P > 0.05) (Table 1). The age distribution was consistent in both groups. The median age in the DARO group was 74 years (53–96 years), and the median age in the AAP group was 75 years (48–75 years). In the DARO group, 75 patients (78.1%) had a Gleason score of ≥8; in the AAP group, 67 patients (81.7%) had a Gleason score of ≥8. In terms of the ECOG score, 61 patients (63.5%) in the DARO group had an ECOG score of 0, and 54 patients (65.9%) in the AAP group had an ECOG score of 0; 35 patients (36.5%) in the DARO group had an ECOG score of 1, and 28 patients (34.1%) in the AAP group had an ECOG score of 1.
Among all 96 patients who received DARO, 50 patients (52.1%) had a history of previous treatment, and 46 patients (47.9%) received DARO as the first-line treatment. Among 82 patients who received AA, 45 patients (54.9%) had a history of previous treatment, and 37 patients (45.1%) received AA as the first-line treatment.
3.2 Primary end point
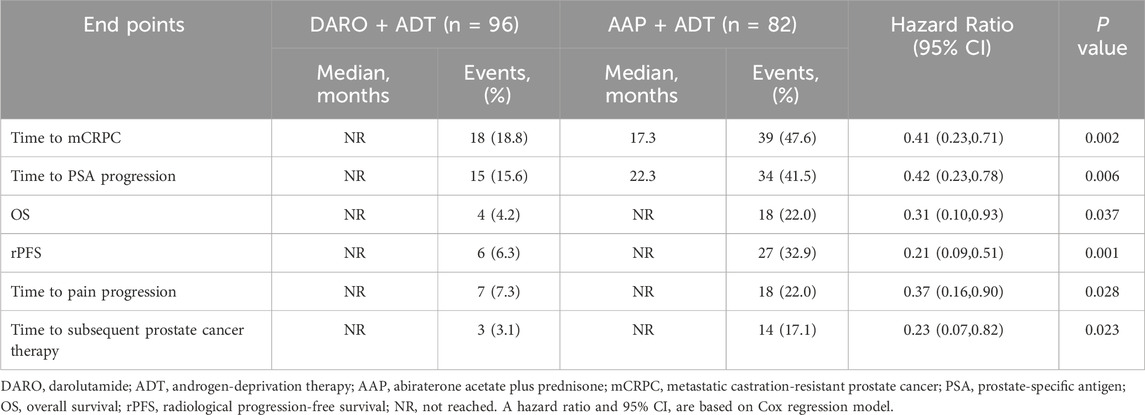
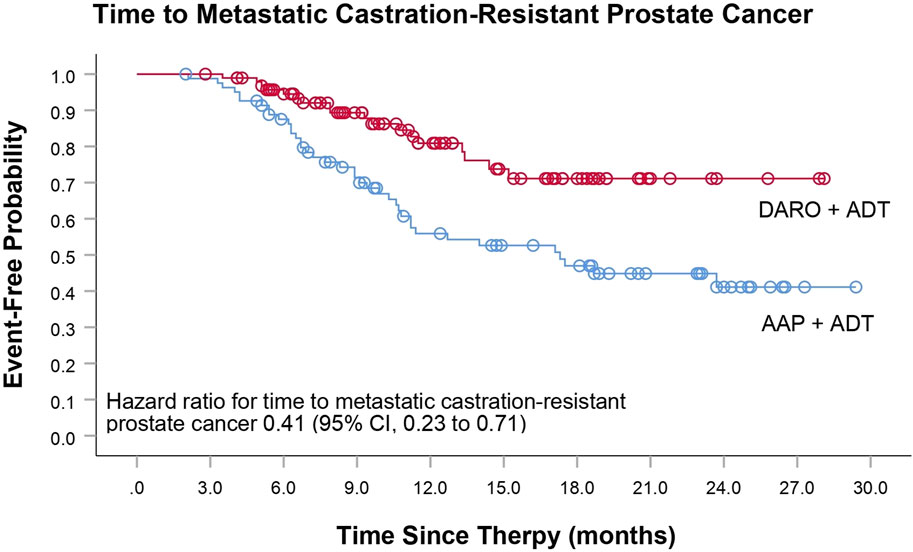
The primary endpoint of this study was the time to mCRPC. The analysis of time to mCRPC demonstrated that the proportion of patients progressing in the DARO group (18/92; 18.8%) was significantly lower than that in the AAP group (39/82; 47.6%). DARO significantly extended the time to progression to mCRPC, with a 59% reduction in the risk of progression to mCRPC compared to the AAP group [HR, 0.41 (95% CI, 0.23 to 0.71); P < 0.005] (Table 2; Figure 1). The median time to progression was not reached in the DARO group, whereas it was 17.3 months in the AAP group.
3.3 Secondary efficacy end points
The DARO group had obvious benefits in all secondary endpoints compared with the AAP group. Among the patients who experienced PSA progression during the follow-up, the proportion of patients in the DARO group (15/96; 15.6%) was less than that in the AAP group (34/82; 41.5%). The risk of PSA progression in the DARO group was 58% lower than that in the AAP group [HR, 0.42 (95% CI, 0.23 to 0.78); P < 0.05]. The median time in the DARO group was not reached, while the median time in the AAP group was 22.3 months. The hazard ratios of OS and rPFS in the DARO group were 69% and 79% lower than those in the AAP group respectively [HR, 0.31 (95% CI, 0.10 to 0.93); P < 0.05 for OS; HR, 0.21 (95% CI, 0.09 to 0.51); P < 0.005 for rPFS] (Table 2; Figure 2). Similarly, compared with the AAP group, the time to pain progression [HR, 0.37 (95% CI, 0.16 to 0.90); P < 0.05] and the time to subsequent prostate cancer therapy [HR, 0.23 (95% CI, 0.07 to 0.82); P < 0.05] were both delayed in the DARO group.
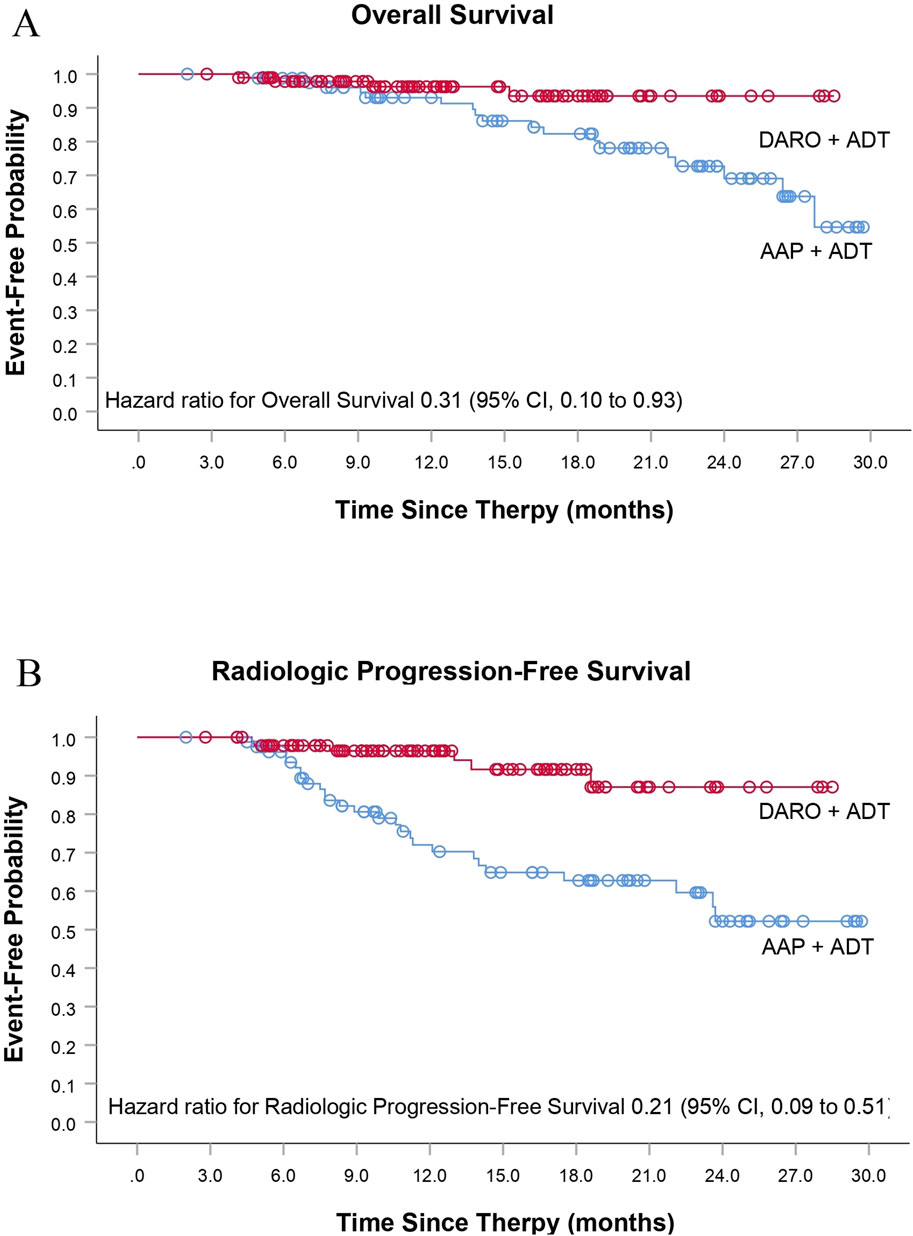
Figure 2. Additional secondary time-to-event end points. (A) Overall Survival and (B) Radiologic Progression-Free Survival.
3.4 PSA response rate
This study retrospectively analyzed changes in PSA levels following treatment. Compared to baseline, the median PSA reduction rates in the DARO group at 1, 3, 6, 9, and 12 months were 95.1%, 96.4%, 92.8%, 84.2%, and 61.7%, respectively, while those in the AAP group were 34.7%, 71.2%, 70.5%, 50.0%, and 45.9%, respectively. The median follow-up times [IQR] for the DARO and AAP groups were 12.0 [7.9–17.6] months and 17.4 [9.3–23.8] months, respectively. The Mann-Whitney U test indicated no significant difference in baseline PSA levels between the two groups (P > 0.05), confirming comparability.
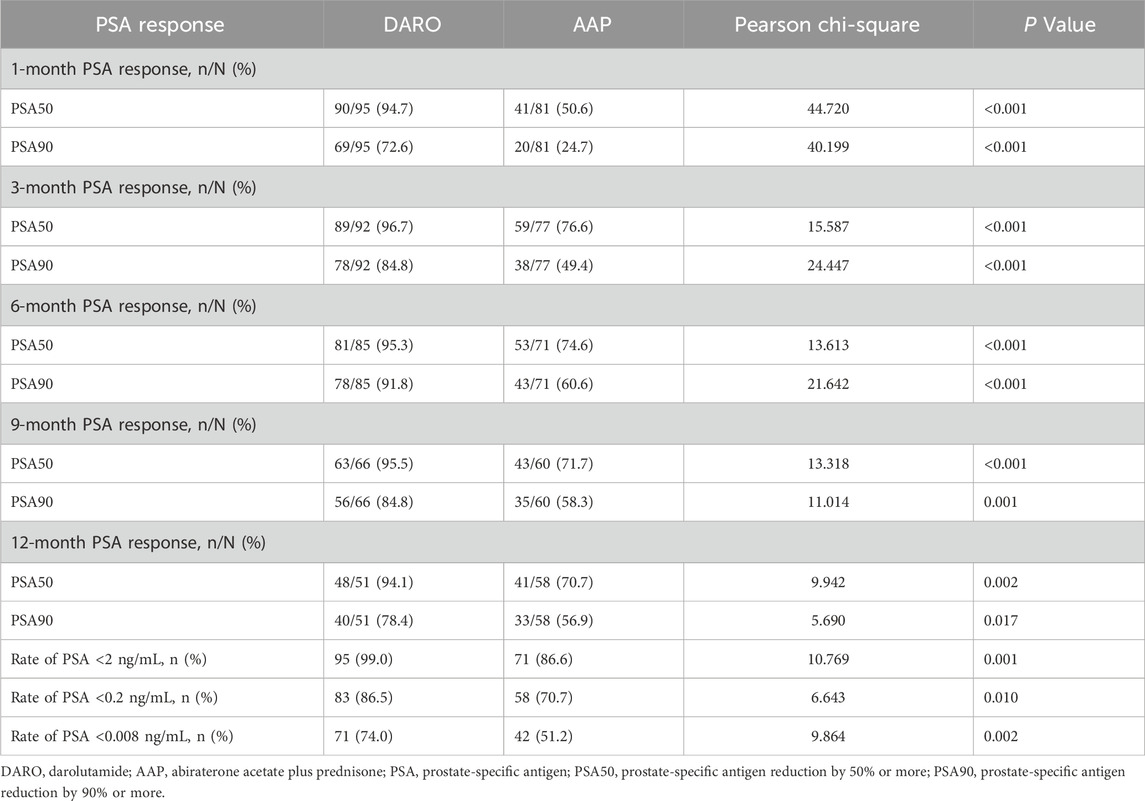
In the DARO group (n = 96), 95, 92, 85, 66, and 51 patients had PSA follow-up data at 1, 3, 6, 9, and 12 months, respectively. The PSA50 response rates were 94.7%, 96.7%, 95.3%, 95.5%, and 94.1%, while the PSA90 response rates were 72.6%, 84.8%, 91.8%, 84.8%, and 78.4%, respectively. In the AAP group (n = 82), 81, 77, 71, 60, and 58 patients had PSA follow-up data at the corresponding time points. The PSA50 response rates were 50.6%, 76.6%, 74.6%, 71.7%, and 70.7%, while the PSA90 response rates were 24.7%, 49.4%, 60.6%, 58.3%, and 56.9%, respectively. Pearson’s chi-square test revealed that the DARO group had significantly higher PSA50 and PSA90 response rates at all time points compared to the AAP group (P < 0.05).
Furthermore, the proportions of patients achieving PSA <2 ng/mL, <0.2 ng/mL, and <0.008 ng/mL in the DARO group were 99%, 86.5%, and 74.0%, respectively, compared to 86.6%, 70.7%, and 51.2% in the AAP group. The differences between the two groups were statistically significant (P < 0.05), with the DARO group demonstrating superior outcomes (Table 3).
3.5 Drug-related adverse reactions
According to the previous medical records of patients in this study, the incidence of adverse reactions during the follow-up was similar between the two groups. In the DARO group, it was 22/96 (22.9%), and in the AAP group, it was 27/82 (32.9%). The adverse reactions with relatively high incidences in the DARO group included gastrointestinal reactions, rash, constipation, and abnormal liver function, etc.; the adverse reactions with relatively high incidences in the AAP group included abnormal liver function, hypertension, fatigue, and gastrointestinal reactions, etc. No drug-related adverse reactions above grade 3 were reported in either group.
4 Discussion
This study evaluated the efficacy and safety of DARO + ADT in patients with mHSPC. In the assessment of the primary endpoint, we compared the time to mCRPC. The results showed that the time to mCRPC in the DARO group was significantly prolonged. This finding indicates that DARO has a favorable effect in delaying disease progression compared to AAP. This contradicts the meta-analysis by Lin Wang et al., who concluded that abiraterone acetate had the lowest risk of metastasis and death (Wang et al., 2022). However Rana McKay concluded that in the real-world, patients receiving DARO have better outcomes (Rana et al., 2025), which is consistent with this study. In addition, the COX regression analysis between the DARO group and the AAP group also showed a significant difference, suggesting that the DARO doublet regimen has an advantage in reducing the risk of mHSPC progressing to mCRPC. DARO significantly improved OS with consistent safety in phase III trials involving patients with non - metastatic castration resistant prostate cancer (nmCRPC) and mHSPC (in combination with ADT and Docetaxel) (Smith et al., 2022; Fizazi et al., 2019b). These findings highlight that in real - world data, the efficacy of the DARO doublet regimen may be superior to that of the AAP doublet regimen.
In the assessment of secondary efficacy endpoints, the comparison of the time to PSA progression is equally important. The study found that the DARO group showed a significant advantage in the time to PSA progression, which may be related to its stronger anti-tumor activity (Sung et al., 2021). The analysis results of OS and rPFS also support this view, indicating that patients in the DARO group can maintain a progression-free state for a longer time after treatment (Gillessen et al., 2023). The comparison of the time to pain progression provides a basis for evaluating the safety of treatment, showing that the DARO group has an advantage in reducing bone-related symptoms (Sagaram and Rao, 2021). The comparison of the time to subsequent prostate cancer therapy reflects the impact of different treatment regimens on patients' subsequent treatment choices, suggesting that clinicians need to comprehensively consider patients’ long-term management needs when formulating treatment plans (Wei et al., 2025).
The results of this study showed that compared with the AAP group, the PSA level in the DARO group was significantly reduced. In the comparison of PSA response rates, we observed changes in PSA levels at different time points, especially the differences between the DARO group and the AAP group. This finding is consistent with previous research results, indicating that DARO treatment may significantly reduce PSA levels in the early stage (Jafari et al., 2024; Tombal et al., 2022). At the same time, the comparison of PSA50 and PSA90 remission rates showed that the remission rates in the DARO group were significantly higher than those in the AAP group, which further supports the potential advantages of DARO in treatment. For the achievement of PSA <2 ng/mL, <0.02 ng/mL, and <0.008 ng/mL, our statistical analysis results also showed significant differences. These results are consistent with previous studies that emphasized the importance of early PSA reduction in improving the prognosis and survival rate of mHSPC patients (Liu et al., 2024; Myint et al., 2022; Wenzel et al., 2024), and also indicate that the DARO doublet regimen is more effective than AAP in reducing PSA levels and can provide a new treatment option for clinical practice.
This study aims to compare the efficacy of DARO + ADT versus AAP + ADT in the treatment of mHSPC in a real-world setting, addressing a significant gap in clinical evidence. The results demonstrated that the DARO group significantly outperformed the AAP group in both primary and secondary endpoints, including PSA changes and time to mCRPC (P < 0.05), highlighting the beneficial role of the DARO + ADT combination in mHSPC management, offering the possibility of choosing the DARO + ADT doublet in patients who are unable to choose the DARO triple therapy and supporting its doublet potential as a first-line treatment option. However, these results will only concern patients ineligible for the triplet. And the retrospective design of the study may introduce bias, and the limited follow-up duration precludes a comprehensive assessment of long-term efficacy and safety.
Despite Laila A. Gharzai’s assertion that no endpoint has been validated as a surrogate endpoint for OS, that caution is essential when employing rPFS as a surrogate endpoint in clinical trial design, and that follow-up metrics must be optimized for enhanced assessment in future studies (Gharzai et al., 2023), the use of rPFS as the primary endpoint is not unprecedented. In numerous large-scale randomized controlled trials (RCTs), rPFS has also been used as a primary endpoint in some studies, although OS is often selected as the primary endpoint. In the present study, the relatively low number of patient deaths observed during the follow-up period, coupled with the unique characteristics of mHSPC led to the selection of rPFS as the primary endpoint. Future research should involve larger-scale, longer-term, multicenter, prospective studies with appropriate follow-up endpoints selected to further validate these findings and explore their implications for personalized treatment strategies.
5 Conclusion
This real-world study demonstrates that DARO + ADT significantly improves clinical outcomes compared to AAP + ADT in the treatment of mHSPC. DARO + ADT delayed the time to mCRPC and showed superior PSA reduction rates, higher PSA50 and PSA90 response rates, and better secondary endpoints, including OS and rPFS. Both regimens exhibited comparable safety profiles, with no grade 3 or higher adverse events. These findings support DARO + ADT as a promising first-line treatment for mHSPC, though larger, prospective studies are needed to confirm long-term efficacy and safety.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sichuan Provincial People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TH: Data curation, Formal Analysis, Software, Writing – original draft, Writing – review and editing. FZ: Methodology, Writing – original draft. YaZ: Data curation, Investigation, Writing – review and editing. BL: Software, Writing – original draft. YoZ: Data curation, Investigation, Writing – review and editing. CG: Conceptualization, Formal Analysis, Writing – review and editing. GW: Investigation, Methodology, Writing – review and editing. JZ: Data curation, Project administration, Writing – original draft. JT: Methodology, Resources, Writing – original draft. YN: Formal Analysis, Resources, Writing – review and editing. YF: Software, Writing – review and editing. SR: Funding acquisition, Resources, Validation, Visualization, Writing – review and editing. WD: Validation, Visualization, Writing – review and editing. DW: Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Network and Data Security Key Laboratory of Sichuan Province (NDS 2024-4) and Chuan Gan Yan (2024-216).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aparicio, J., Sternberg, C. N., and de Wit, R. (2021). Cabazitaxel in metastatic prostate cancer: current status and future perspectives. Cancer Treat. Rev. 97, 102245. doi:10.1016/j.ctrv.2021.102245
Armstrong, A. J., Szmulewitz, R. Z., Petrylak, D. P., Holzbeierlein, J., Villers, A., Azad, A., et al. (2019). ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 37 (32), 2974–2986. doi:10.1200/JCO.19.00799
Castellan, P., Marchioni, M., Castellucci, R., De Francesco, P., Iantorno, R., Schips, L., et al. (2018). Abiraterone acetate for early stage metastatic prostate cancer: patient selection and special considerations. Ther. Clin. Risk Manag. 14, 2341–2347. doi:10.2147/TCRM.S159824
Chi, K. N., Agarwal, N., Bjartell, A., Chung, B. H., Pereira de Santana Gomes, A. J., Given, R., et al. (2019). Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381 (13), 13–24. doi:10.1056/NEJMoa1903307
Chowdhury, S., Bjartell, A., Agarwal, N., Chung, B. H., Pereira de Santana Gomes, A. J., Given, R., et al. (2023). Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann. Oncol. 34 (5), 477–485. doi:10.1016/j.annonc.2023.02.009
Fizazi, K., Foulon, S., Carles, J., Roubaud, G., McDermott, R., Fléchon, A., et al. (2022). Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 399 (10336), 1695–1707. doi:10.1016/S0140-6736(22)00367-1
Fizazi, K., Shore, N., Tammela, T. L., Ulys, A., Vjaters, E., Polyakov, S., et al. (2019b). Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 380 (13), 1235–1246. doi:10.1056/NEJMoa1815671
Fizazi, K., Tran, N., Fein, L., Matsubara, N., Rodriguez-Antolin, A., Alekseev, B. Y., et al. (2019a). Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 20 (5), 686–700. doi:10.1016/S1470-2045(19)30082-8
Freedland, S. J., Sandin, R., Sah, J., Emir, B., Mu, Q., Ratiu, A., et al. (2021). Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US Veterans Health Administration. Cancer Med. 10 (23), 8570–8580. doi:10.1002/cam4.4372
Gharzai, L. A., Jiang, R., Jaworski, E. M., Morales Rivera, K., Dess, R. T., Jackson, W. C., et al. (2023). Meta - analysis of candidate surrogate end points in advanced prostate cancer. NEJM Evid. 2 (4), EVIDoa2200195. doi:10.1056/EVIDoa2200195
Gillessen, S., Bossi, A., Davis, I. D., de Bono, J., Fizazi, K., James, N. D., et al. (2023). Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur. J. Cancer 185, 178–215. doi:10.1016/j.ejca.2023.02.018
Hamilou, Z., Saad, F., and Fizazi, K. (2018). Treatment of hormone-naïve metastatic prostate cancer. Curr. Opin. Support Palliat. Care 12 (3), 334–338. doi:10.1097/SPC.0000000000000359
Hoeh, B., Garcia, C. C., Wenzel, M., Tian, Z., Tilki, D., Steuber, T., et al. (2023). Triplet or doublet therapy in metastatic hormone-sensitive prostate cancer: updated network meta-analysis stratified by disease volume. Eur. Urol. Focus 9 (5), 838–842. doi:10.1016/j.euf.2023.03.024
Jafari, A., Hosseini, F. A., and Jalali, F. S. (2024). A systematic review of the economic burden of colorectal cancer. Health Sci. Rep. 7 (8), e70002. doi:10.1002/hsr2.70002
Jansen, L., van der Leest, A., van Vulpen, M., Pos, F. J., van Moorselaar, R. J. A., van der Poel, H. G., et al. (2022). Patient preferences and treatment decisions in men with metastatic prostate cancer: a systematic review. Crit. Rev. Oncol. Hematol. 174, 103644. doi:10.1016/j.critrevonc.2022.103644
Kyriakopoulos, C. E., Chen, Y. H., Carducci, M. A., Liu, G., Jarrard, D. F., Hahn, N. M., et al. (2018). Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 36 (11), 1080–1087. doi:10.1200/JCO.2017.75.3657
Lam, A. P., Cahn, D. J., and Bupathi, M. (2024). Managing patients with metastatic hormone-sensitive prostate cancer: a shared-care approach to combination therapy. Clin. Adv. Hematol. Oncol. 22 (9.0), 1–20.
Lee, A. (2023). Darolutamide: a review in metastatic hormone-sensitive prostate cancer. Target Oncol. 18 (5), 793–800. doi:10.1007/s11523-023-00984-4
Leith, A., Ribbands, A., Kim, J., Clayton, E., Gillespie-Akar, L., Yang, L., et al. (2022). Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: a real-world study from the United States, five European countries and Japan. BMC Urol. 22 (1), 33. doi:10.1186/s12894-022-00979-9
Liu, J., Wang, S., Yang, Y., Wang, S., Campobasso, D., Tan, Y. G., et al. (2024). Real-world retrospective study of prostate-specific antigen and safety assessment with darolutamide plus androgen deprivation therapy for metastasis hormone-sensitive prostate cancer. Transl. Androl. Urol. 13 (3), 433–441. doi:10.21037/tau-24-96
Myint, Z. W., Kolesar, J. M., McCorkle, J. R., Wu, J., Ellis, C. S., Otto, D. E., et al. (2022). Correlation between trough level of abiraterone and prostate-specific antigen (PSA) response in metastatic hormone-sensitive prostate cancer. Med. Sci. Monit. 28, e938091. doi:10.12659/MSM.938091
Rana, R. M. K., Morgans, A. K., Khan, N., Constantinovici, N., Chen, G., Ghadessi, M., et al. (2025). Clinical use and outcomes of androgen-receptor pathway inhibitors triplet therapy for metastatic hormone-sensitive prostate cancer (ARAAT). JCO 43, 66. doi:10.1200/JCO.2025.43.5_suppl.66
Raval, A. D., Chen, S., Littleton, N., Constantinovici, N., and Goebell, P. J. (2024). Underutilization of androgen deprivation therapy (ADT) intensification for the treatment of men with metastatic hormone-sensitive prostate cancer (mHSPC): asystematic review of real-world database studies. J. Clin. Oncol. 42, 66. doi:10.1200/jco.2024.42.4_suppl.66
Rebello, R. J., Oing, C., Knudsen, K. E., Loeb, S., Johnson, D. C., Reiter, R. E., et al. (2021). Prostate cancer. Prostate Cancer. Nat. Rev. Dis. Prim. 7 (1), 9. doi:10.1038/s41572-020-00243-0
Saad, F., Vjaters, E., Shore, N., Olmos, D., Xing, N., Pereira de Santana Gomes, A. J., et al. (2024). Darolutamide in combination with androgen-deprivation therapy in patients with metastatic hormone-sensitive prostate cancer from the phase III ARANOTE trial. J. Clin. Oncol. 42 (36), 4271–4281. doi:10.1200/JCO-24-01798
Sadaghiani, M. S., Sheikhbahaei, S., Werner, R. A., Pienta, K. J., Pomper, M. G., Gorin, M. A., et al. (2022). 177 Lu-PSMA radioligand therapy effectiveness in metastatic castration-resistant prostate cancer: an updated systematic review and meta-analysis. Prostate 82 (7), 826–835. doi:10.1002/pros.24325
Sadaghiani, M. S., Sheikhbahaei, S., Werner, R. A., Pienta, K. J., Pomper, M. G., Solnes, L. B., et al. (2021). A systematic review and meta-analysis of the effectiveness and toxicities of lutetium-177-labeled prostate-specific membrane antigen-targeted radioligand therapy in metastatic castration-resistant prostate cancer. Eur. Urol. 80 (1), 82–94. doi:10.1016/j.eururo.2021.03.004
Sagaram, S., and Rao, A. (2021). Rapidly evolving treatment paradigm and considerations for sequencing therapies in metastatic prostate cancer-a narrative review. Transl. Androl. Urol. 10 (7), 3188–3198. doi:10.21037/tau-20-1383
Schiff, H. I. (2022). Management of patients with metastatic castration-sensitive prostate cancer in the real-world setting in the United States. Letter. Lett. J. Urol. 207 (4), 939. doi:10.1097/JU.0000000000002456
Shore, N., Jiang, S., Garcia-Horton, V., Lopes, G., Higano, C. S., Crespo, M., et al. (2022). The hospitalization-related costs of adverse events for novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer: an indirect comparison. Adv. Ther. 39 (11), 5025–5042. doi:10.1007/s12325-022-02245-8
Smith, A., Johnson, B., and Brown, C. (2023). Prostate cancer epidemiology in 2022: a global perspective. J. Oncol. 30, 150–165.
Smith, M. R., Hussain, M., Saad, F., Fizazi, K., Sternberg, C. N., Crawford, E. D., et al. (2022). Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl. J. Med. 386 (12), 1132–1142. doi:10.1056/NEJMoa2119115
Sung, W. W. Y., Choi, H. C. W., Luk, P. H. Y., and So, T. H. (2021). A cost-effectiveness analysis of systemic therapy for metastatic hormone-sensitive prostate cancer. Front. Oncol. 11, 627083. doi:10.3389/fonc.2021.627083
Tombal, B., Borre, M., Rathenborg, P., Werbrouck, P., Van Poppel, H., Heidenreich, A., et al. (2022). Long-term efficacy and safety of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: updated results from the ARAMIS trial. Eur. Urol. 81 (4), 331–340. doi:10.1016/j.eururo.2021.12.017
Ueda, T., Shiraishi, T., Miyashita, M., Kayukawa, N., Gabata, Y., Sako, S., et al. (2024). Apalutamide versus bicalutamide in combination with androgen deprivation therapy for metastatic hormone sensitive prostate cancer. Sci. Rep. 14 (1), 705. doi:10.1038/s41598-024-51389-w
Wang, L., Paller, C., Hong, H., Rosman, L., De Felice, A., Brawley, O., et al. (2022). Comparison of treatments for nonmetastatic castration-resistant prostate cancer: matching-adjusted indirect comparison and network meta-analysis. J. Natl. Cancer Inst. 114 (2), 191–202. doi:10.1093/jnci/djab071
Wang, S. H., Gu, Y. F., and Xu, S. (2023). Adionuclide therapy for metastatic castration-resistant prostate cancer: new advances. Zhonghua Nan Ke Xue 29 (3), 275–281.
Watson, P. A., Arora, V. K., and Sawyers, C. L. (2015). Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15 (12), 701–711. doi:10.1038/nrc4016
Wei, X., Zhao, J., Nie, L., Shi, Y., Zhao, F., Shen, Y., et al. (2025). Assessing the predictive value of intraductal carcinoma of the prostate (IDC-P) in determining abiraterone efficacy for metastatic hormone-sensitive prostate cancer (mHSPC) patients. Prostate 85 (2), 130–139. doi:10.1002/pros.24809
Wenzel, M., Cano Garcia, C., Humke, C., Hoeh, B., Steuber, T., Tilki, D., et al. (2024). Prostate-specific antigen nadir and cancer-control outcomes in real-world apalutamide-treated metastatic hormone-sensitive prostate cancer patients: a single-center analysis. Eur. Urol. Oncol. 31, 364–371. doi:10.1016/j.euo.2024.08.007
Wenzel, M., Hoeh, B., Chun, F. K. H., and Mandel, P. (2023). Androgen deprivation therapy plus chemotherapy ± androgen receptor-targeting agents for metastatic hormone-sensitive prostate cancer. Urologie 62 (4), 360–368. doi:10.1007/s00120-023-02029-0
Keywords: metastatic hormone-sensitive prostate cancer, darolutamide, abiraterone acetate, androgen deprivation therapy, efficacy, safety
Citation: Hu T, Zhou F, Zheng Y, Luo B, Zhang Y, Gu C, Wang G, Zhang J, Tian J, Nie Y, Feng Y, Ren S, Di W and Wang D (2025) Efficacy and safety of darolutamide versus abiraterone acetate plus prednisone in combination with ADT for mHSPC: a real-world clinical retrospective study. Front. Pharmacol. 16:1608339. doi: 10.3389/fphar.2025.1608339
Received: 08 April 2025; Accepted: 05 June 2025;
Published: 19 June 2025.
Edited by:
Nor Eddine Sounni, University of Liège, BelgiumReviewed by:
Tatsuya Shimomura, Jikei University School of Medicine, JapanCharles Pottier, University Hospital of Liège, Belgium
Copyright © 2025 Hu, Zhou, Zheng, Luo, Zhang, Gu, Wang, Zhang, Tian, Nie, Feng, Ren, Di and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Wang, d2FuZ2RvbmcxOTY5MDUzMEAxNjMuY29t; Wenjia Di, ZGl3ZW5qaWFAdWVzdGMuZWR1LmNu; Shangqing Ren, cnNxMDUxNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ting Hu1,2†
Ting Hu1,2† Fang Zhou
Fang Zhou Bohan Luo
Bohan Luo Jingzhi Tian
Jingzhi Tian Yunlin Feng
Yunlin Feng Dong Wang
Dong Wang