- 1School of Public and Allied Health, Liverpool John Moores University, Liverpool, United Kingdom
- 2Department of Primary Care and Population Health, University College London, London, United Kingdom
- 3Division of Medicine, University College London, London, United Kingdom
- 4Medway School of Pharmacy, University of Kent, Kent, United Kingdom
- 5Escuela de Medicina, Universidad Cesar Vallejo, Trujillo, Peru
- 6UCL School of Pharmacy, University College London, London, United Kingdom
- 7China Medical University, Taichung, Taiwan
Background: Over-the-counter (OTC) products such as herbal medical products (HMPs) or dietary supplements are a valued part of preventative and supportive self-care for depressive symptoms, but there is a wide array of products available, with differing levels of clinical evidence. It is unclear what the optimal directions for future research in this field are.
Aim: We aimed to explore the size and nature of the evidence base available for OTC products for depression in adults aged 18–60.
Methods: We carried out a scoping review following Joanna Briggs Institute guidance. We searched MEDLINE, Embase, PsycINFO, AMED, and CENTRAL from inception to December 2022, and 10% of the results were screened by two authors and the remainder by one author. We included randomised controlled trials of products commonly available OTC in multiple countries in participants with symptoms or a diagnosis of depression. Results were narratively summarised by the product and volume of evidence available.
Results: Out of 23,933 records found, we screened 1,367 full texts and included 209 trials. The largest volume of evidence was for omega-3s, St John’s Wort, saffron, probiotics, and vitamin D. Among a range of herbal medical products with promising evidence, those most commonly used and thus warranting further research were lavender, lemon balm, chamomile, and Echium. For 41 products, we found only single trials. Few products presented safety issues, whether used alone or adjunctively with antidepressants.
Conclusion: Products with limited but promising evidence included folic acid, lavender, zinc, tryptophan, Rhodiola, and lemon balm, and future research should focus on these products. There is a need for further evaluation of herbal medical products as adjuncts to antidepressants and for exploring their potential benefits when used adjunctively with psychological therapies to support a more integrative approach. Safety reporting in these trials needs to be further improved.
Systematic Review Registration:: https://osf.io/rkm57/.
Introduction
Depression is common—the prevalence of major depressive disorder (MDD) varies between 2% and 21%, depending on the country and measure used (Gutiérrez-Rojas et al., 2020), and prevalence is increasing over time (Moreno-Agostino et al., 2021). MDD is defined according to the DSM-V as the presence of five or more out of nine symptoms experienced frequently within the same two-week period, including depressed mood and loss of pleasure, in addition to other symptoms such as changes in appetite, insomnia, and fatigue. Symptoms must also cause distress or impairment, with the severity of depression determined by the level of impairment, and the condition cannot be better explained by another cause (Uher et al., 2014).
In the United Kingdom (UK), 11.3% report mild depressive symptoms, 4.2% report moderate depressive symptoms, and 3.3% report severe depressive symptoms (Arias De La Torre et al., 2021). MDD is associated with multiple interacting pathogenic factors, including genetics, stressful life events, and chronic diseases (Cui et al., 2024). Underlying pathophysiological theories for depression include 1) deficiencies in monoamine neurotransmitters (e.g., serotonin and dopamine), 2) an increase in hypothalamic–pituitary–adrenal (HPA) axis activity, increasing production of glucocorticoids, which provide negative feedback to the limbic system, hypothalamus, and pituitary, and 3) low-grade inflammation that is self-sustaining, with the presence of reactive oxygen species further triggering inflammatory cytokines, which compromise neuroendocrine activities (Cui et al., 2024). There may also be impaired neurogenesis due to reduced brain-derived neurotrophic factor (BDNF) (Dobrek and Głowacka, 2023) or the effects from the gut microbiota on the function of the HPA axis (Dobrek and Głowacka, 2023).
Depression has a strong impact on individuals, reducing the quality of life and increasing the risk of physical health conditions such as cardiovascular disease and diabetes (Graham et al., 2020; Harshfield et al., 2020). There is also a widespread societal impact as depression increases the risk of sickness absence from employment (Amiri and Behnezhad, 2021). Globally, 16% of disability-adjusted life years can be attributed to depression, reflecting an economic cost of USD$ 4.7 trillion (Arias et al., 2022). There is, therefore, a strong need to find effective methods to prevent, manage, and reduce depressive symptoms in adults.
In the UK, the National Institute for Health and Care Excellence (NICE) guidelines recommend discussions of psychological treatments (e.g., group and individual psychological therapies and guided self-help), exercise, and pharmacological treatment options (e.g., antidepressants), with a decision made jointly with the patient as to which is the most appropriate, considering the least intrusive and least resource-intensive options first (National Institute for Health and Care Excellence, 2022). However, it is estimated that in Great Britain, only 13.4% of individuals reporting depressive symptoms receive some form of treatment (Smits and Huijts, 2015), with particular challenges in accessing psychological therapies due to long waiting times (Baker and Kirk-Wade, 2024). Although antidepressants are more easily accessible, they require help-seeking with a medical practitioner in order to receive them, and views and experiences of their perceived effectiveness, desirability, and side effects are mixed (Crowe et al., 2023).
Consequently, it is unsurprising that prior to, or whilst receiving treatment, people often find ways to self-manage depressive symptoms, most commonly using herbal medical products (HMPs) and vitamins and minerals rather than practitioner-directed modalities (Solomon and Adams, 2015). Homoeopathic preparations are also a possible approach, with one of the most common reasons for using over-the-counter (OTC) homoeopathic preparations in the UK being psychological problems (Reid, 2002). Although there are no specific OTC medicines licensed for depressive symptoms, it is possible that OTC medicines are also used more frequently to mitigate depressive symptoms or symptoms linked to depression (e.g., insomnia).
It is suggested that natural products, such as HMPs or nutrients, may help manage depression through various mechanisms, including inhibiting inflammation, ameliorating oxidative stress, changing the microbiota–gut–brain axis, suppressing hyperactivity in the hypothalamic–pituitary–adrenal axis, and regulating neurotransmitters (Wu et al., 2022). These products are generally derived from different medical traditions and are now an important part of self-care in many countries, either regulated as medicines or supplements/botanicals. Qualitative studies suggest that products such as St John’s Wort can be viewed as more desirable due to perceptions of safety, naturalness, and greater ability to control usage (Pirotta et al., 2014), and they may be used as alternatives to, or in addition to, prescribed antidepressants. There is little mention in the NICE guidelines regarding OTC products for depression, apart from a recommendation not to advise St John’s Wort due to uncertainty about doses and the potential for interactions (National Institute for Health and Care Excellence, 2022).
There has been an abundance of evidence evaluating OTC products for depression over the last few decades, particularly products such as St John’s Wort and omega-3 supplements. These often have relatively conclusive separate systematic reviews and meta-analyses [e.g., St John’s Wort (Ng et al., 2017), saffron (Tóth et al., 2019), and omega-3s (Liao et al., 2019)]. Although this can provide a rigorous assessment of the strength and level of evidence available for each product and a single estimate of effect, it tends to focus the evidence base on a few key products and neglects the wider scope of other promising products that may offer future avenues for prevention and self-care of depressive symptoms.
Where reviews have previously attempted to summarise the wider evidence base beyond single products, they have often relied on narrative summaries (Sarris, 2018) or included only a limited number of studies (Kamat et al., 2023). It is, therefore, valuable to scope the evidence base as a whole, including ongoing trials, to understand where the literature is concentrated and where gaps exist. This allows future research directions to be clearly outlined, broadening the potential field for clinical studies in products for depressive symptoms beyond those with an already high level of evaluation. We, therefore, aimed to
1. Summarise the size and nature of the evidence base that assesses the effectiveness of OTC products for depression in adults aged 18–60.
2. Determine the areas with substantial evidence and those with gaps to identify directions for future research.
Methods
Following Joanna Briggs Institute (JBI) guidance (Peters et al., 2020) and PRISMA guidance for scoping reviews (Tricco et al., 2018), we carried out a scoping review. The review formed part of a larger project summarising the available trial evidence for OTC products for depression, anxiety, and insomnia. It was prospectively registered on the Open Science Framework (https://osf.io/rkm57/). Due to the large volume of results, we synthesised the findings separately for each condition and grouped them according to whether the samples were adults (aged 18–60) or older adults to explore whether an age bias existed in these trials. Two public contributors were involved in developing the review questions, designing the review, and selecting which products to include.
We searched five databases (MEDLINE, Embase, PsycINFO, AMED, and CENTRAL) from inception to December 2022. The CENTRAL searches included trial registry entries. Search terms were grouped into OTC product terms, mental health terms, and randomised controlled trial (RCT) filters (where applicable) and combined using Boolean operators (see Supplementary Material 1). Given the multitude of possible products, product categories rather than individual names were used in searches. The research team piloted searches in MEDLINE and Embase and refined these as needed.
Search results were imported into Rayyan (Ouzzani et al., 2016) after deduplication. Due to the large volume of results, dual title and abstract screening was undertaken for 10% of titles and abstracts by pairs of reviewers (RF, SM, SU, VT, and AS). Reviewers were provided with explicit instructions to maximise consistency, and each pair conducted three batches of screening; discussions and decisions were agreed upon by all five reviewers, and inclusion criteria refined as needed. Only when agreement was 85%–90% between all pairs of reviewers (after the third batch) were the remaining studies screened by a single reviewer. Studies deemed unclear were moved to the full-text stage. The same process was carried out for full-text screening, with decisions documented in MS Excel, and unclear studies at this stage were screened by a second reviewer. As part of screening, studies were labelled according to whether they focussed on anxiety, depression, insomnia, or a combination and whether their sample included people aged 18–60 or 60+. In this article, studies relating to depression in adults aged 18–60 are synthesised.
We included randomised controlled trials (including parallel, factorial, and crossover trials) evaluating products that met the following criteria:
• were likely to be available OTC in a number of countries globally.
• consisted of single chemical medicines, HMPs, homoeopathic products, and/or dietary supplements taken orally. The list of potential products was developed in consultation with our patient and public involvement representatives and so included a wide range of products that may be used by consumers, including homoeopathic products.
• were evaluated alone or as an adjunct treatment.
• included a comparator, without restriction on whether this was a non-pharmacological intervention, prescription medication, another OTC product, no treatment, or placebo.
• were used for at least 1 week.
• typically did not require practitioner input or individualisation (e.g., some traditional Chinese medicine and homoeopathic interventions).
We included studies where the sample mean ages fell between 18 and 60 years and where participants had depressive symptoms established at baseline—either by meeting a threshold on an established depression questionnaire or with a diagnosis of minor or major depressive disorder. Studies that included people with depression and a comorbid condition were eligible, but those focussing on other mental health conditions (e.g., bipolar disorder, dementia, and substance abuse) were excluded. Studies needed to include depressive symptoms or depression remission as an outcome measure. We included trials from any time period and in any language, using Google Lens or a multilingual colleague where available for non-English texts. Subgroup analyses and grey literature presenting result data (e.g., theses) were excluded.
For all identified trial registry entries, published protocols, and conference abstracts, during the period of April–June 2024 we endeavoured to locate the main trial publication through trial number searches, title searches, and “cited by” functions (published protocols only) and by reviewing named author publication lists. Where full texts could not be located, we summarised ongoing trials and published protocols (see Supplementary Material 2) but not conference abstracts. Full texts located through these methods were screened by one author (RF) and were included and extracted if eligible. Reference list screening of relevant reviews was undertaken by SM for 10% (n = 200) reviews; as few new studies were detected and resources were limited, no further reference lists were screened after this point.
Data were extracted using a data extraction form designed according to the JBI data extraction template, including study details such as country, setting, sample size, inclusion criteria, participant characteristics, product characteristics, comparators, effectiveness outcomes for depression, and safety outcomes. Data were extracted by a reviewer (AZ), who also conducted a second eligibility check of included studies. Codings/groupings were applied by RF, who checked any uncertainties by consulting the original papers if needed and confirmed the exclusion of any ineligible studies. RF also extracted data from studies identified through trial registry and conference abstract follow-up. For plants, both common and Latin names were extracted where reported; however, where these were not reported, we avoided imposing potentially incorrect names and instead used the name(s) listed in the study.
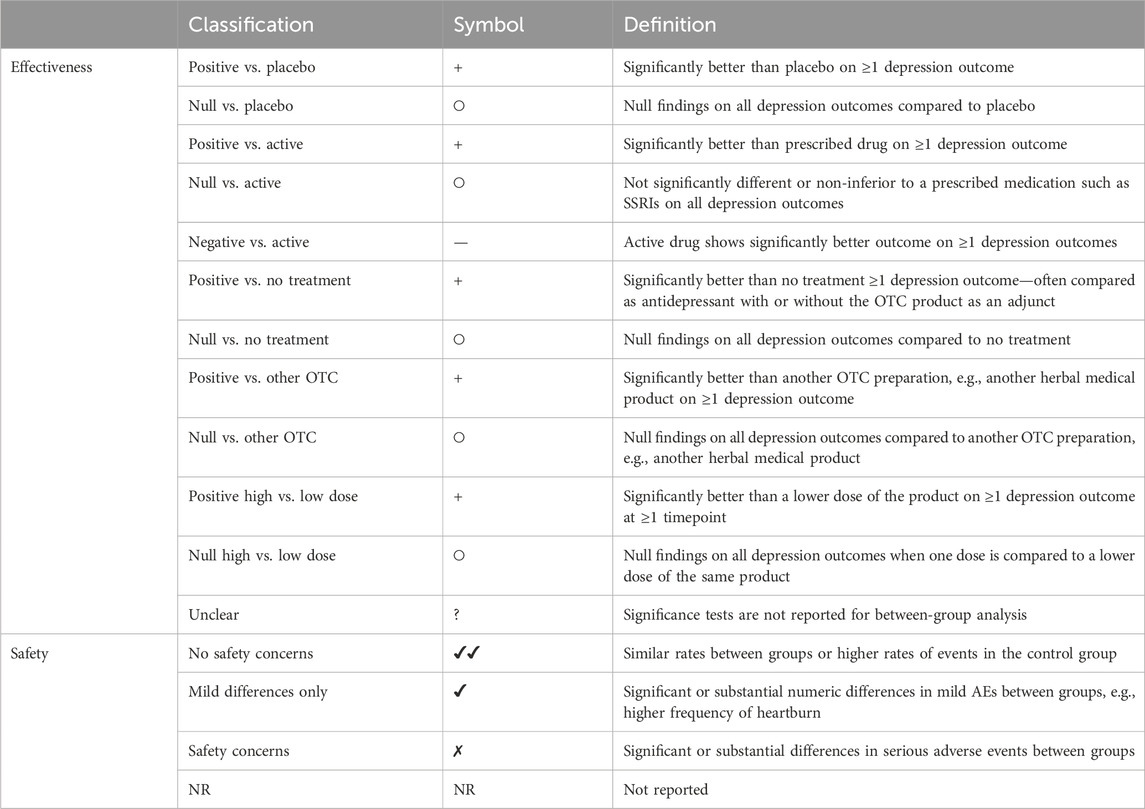
To synthesise the data, we grouped products by overall and specific product type. We summarised the products evaluated, comparators used, comorbid conditions, and whether the products were used as adjuncts or evaluated alone. Due to the large volume of studies, we summarised effectiveness and safety data using vote counting. We classified effectiveness findings according to whether they showed significant effects at ≥ 1 timepoint on any depression trial outcome compared to each type of control treatment, and we classified safety findings by whether there were differences between groups in adverse events (see Table 1 for categorisations applied by RF). Only comparisons relevant to the review were included (e.g., prescribed antidepressant vs. placebo comparisons in a three-arm trial are not presented). We did not carry out a quality assessment as this was not the aim of scoping reviews, which provide a descriptive overview of the evidence base (Peters et al., 2020). We aimed to highlight areas with larger volumes of evidence and identify gaps within the evidence base needing further research.
Results
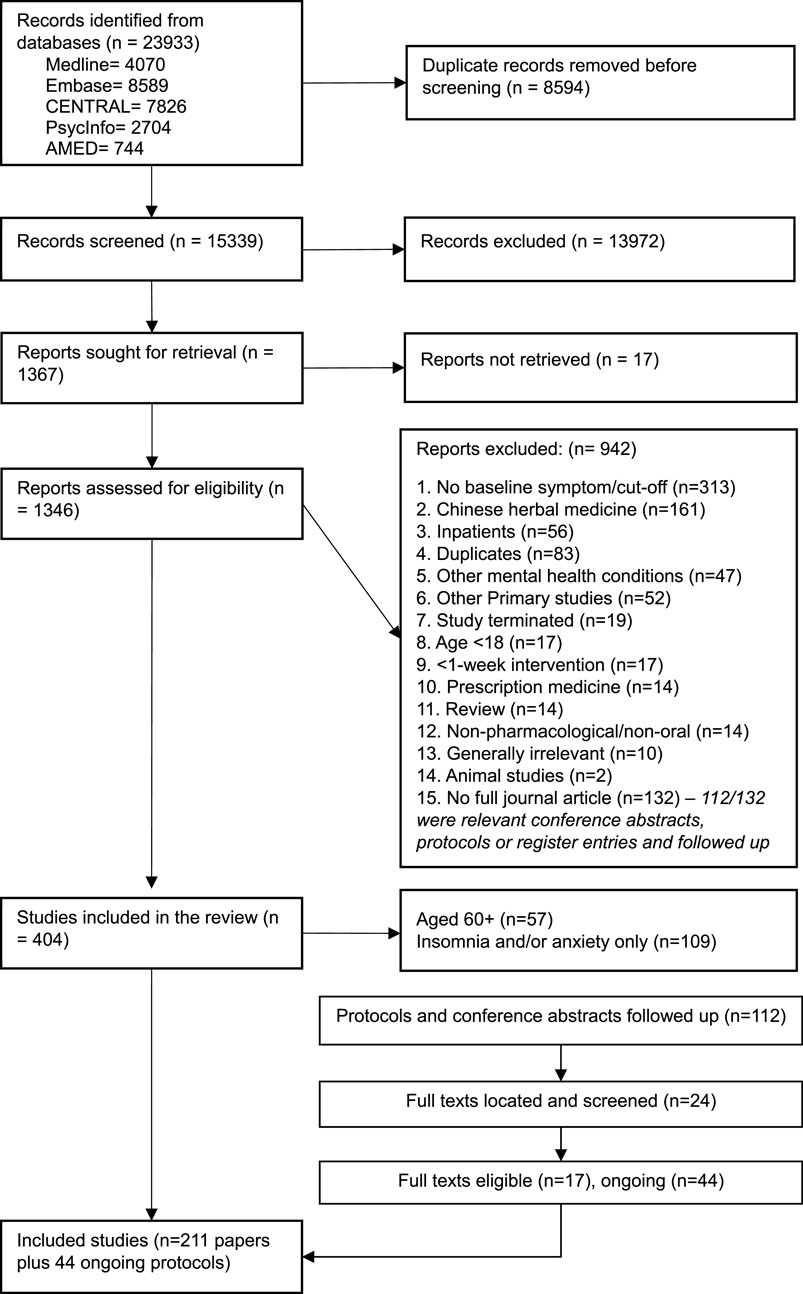
Figure 1 shows the flow of studies through this review. Out of 15,339 records screened, 13,972 were excluded on the basis of title and abstract, and 1,367 full texts were screened. One hundred and ninety papers relating to depression in adults aged 18–60 were included, with a further 16 identified from following up registered protocols and conference abstracts, providing a total of 209 included trials. Of these, 196 evaluated products for depression alone, 10 for depression and anxiety, 2 for depression and insomnia, and 1 for all three conditions. Tables 2–5 summarise all included studies with references.
Summary of included studies
Trials were most commonly carried out in Iran (n = 78), followed by Germany (N = 28), the United States (n = 27), Australia (n = 13), the United Kingdom (n = 9), and China (n = 6). Other countries had five or fewer studies. The vast majority of studies were parallel trials (n = 194), with three factorial trials and four crossover trials. Sample sizes varied widely, with a median size of 70 participants (IQR, 46–123) recruited and a median of 62 participants analysed (IQR 42–106). Most studies were double-blind (n = 187), six were triple-blind, six were single-blind, six were open-label, and four did not report blinding (based on the study details, three were likely to be open-label and one likely single-blind).
In 150 trials, participants were required to have a diagnosis of depression at baseline. In 58 trials, a symptom scale cutoff was used to determine inclusion, with participants needing to meet a minimum threshold. In one study, a diagnosis was implied but not clearly stated, but a scale cutoff was also used. All studies used continuous depression scales as primary or secondary outcomes. Most scales were clinician-rated and tended to be summarised continuously rather than as response or remission; the most common were the Hamilton Depression Rating Scale (106 primary and 13 secondary outcomes), the Montgomery–Åsberg Depression Rating Scale (17 primary and 10 secondary outcomes), and the Clinical Global Impression Scale (8 primary outcomes and 40 secondary outcomes). The self-reported Beck Depression Inventory was also commonly used (61 primary and 33 secondary outcomes). Other scales were used in fewer than 10 studies.
Dietary supplements were evaluated in 114 trials, HMPs in 94 trials, and single chemical medicines in 1 trial. No studies evaluated homoeopathic products. More herbal medical product trials included people with a diagnosis of depression than dietary supplement trials (74/94, 79% vs. 75/114, 66%). Most studies (n = 156) had two trial arms, 42 had three arms, 10 had four, and 1 trial had five arms. Trials with multiple arms could include comparisons with lifestyle changes, other OTC products, prescribed drugs, and placebos. Dietary supplements were more frequently compared to placebo (n = 98/114, 86%) than HMPs (n = 64/94, 67%), whilst HMPs were more frequently compared to prescription drugs (n = 32/94, 32%) than dietary supplements (n = 6/114, 5%).
Dietary supplements (n = 67/114, 59%) were also more likely to be evaluated as adjuncts to other depression treatments (most commonly antidepressants) compared to HMPs (21/94, 20%). Very few studies evaluated products versus psychotherapy-based treatments (n = 4) or as adjuncts to psychotherapy-based treatments (n = 11). Seventeen studies contained comparisons between different OTC products. HMPs were evaluated in people with comorbid health conditions in 22/94 (23%) studies and 32/114 (28%) trials of dietary supplements. Regarding pregnancy status, 4/94 studies evaluated HMPs during the postpartum period, and 9/114 dietary supplements were evaluated during pregnancy or postpartum.
The studies show a general increase in the number of trials evaluating dietary supplements for depression since 1973, peaking in 2019, with an average of 3.2 trials per year. The number of trials evaluating HMPs remained steady, with smaller peaks and troughs and an average of 2.7 trials published per year since 1993. This suggests a sustained interest in OTC products for depression over time.
Products with substantive evidence (>10 trials)
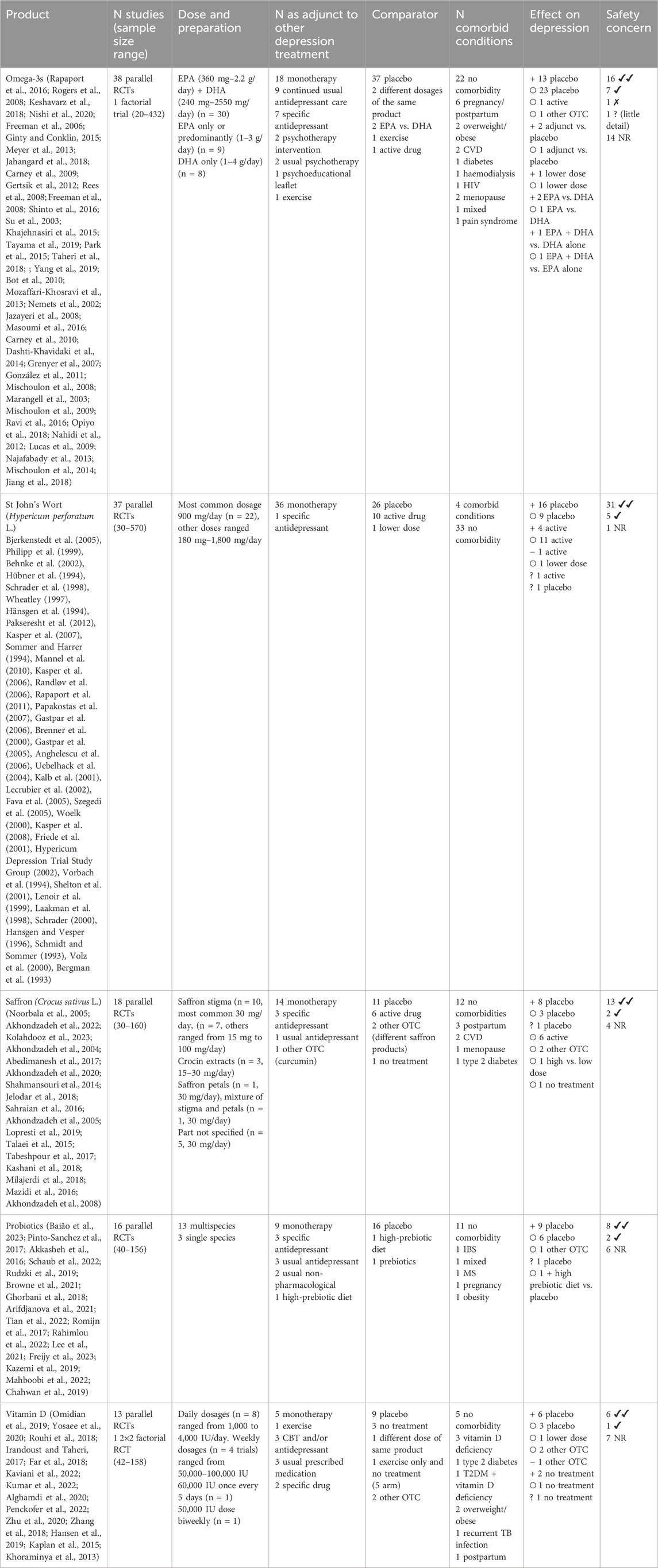
We found that trial evidence was concentrated on a small number of products, namely, omega-3s (n = 39, median sample size: 65), St John’s Wort (n = 38, median sample size: 150), saffron (n = 18, median sample size: 50), probiotics (n = 18, median sample size: 71), and vitamin D (n = 14, median sample size: 67). Within these categories, however, there was considerable variation in the specific products, extracts, and dosages tested (see Table 1 for further details).
The vast majority of omega-3 trials (n = 37/39) compared the supplement to a placebo, with a small number of studies assessing different dosages or comparing eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA). A total of 26 trials were conducted in people with a diagnosis of depression, 12 in those with depressive symptoms, and 1 was unclear. In 11 studies, participants had a range of comorbidities, six of which involved pregnancy/postpartum, and in 16 studies, they were evaluated as an adjunct to antidepressant treatment. On the basis of vote counting, the effects on depression were more frequently null compared to placebo than significantly different (23 vs. 13), with mixed results for other comparisons.
The most commonly studied herbal medical product was St John’s Wort (Hypericum perforatum), which was only evaluated in trials involving people with a depression diagnosis. It was given mainly as a monotherapy to people with no comorbidities. St John’s Wort was compared to a placebo in 26 trials, to an active drug in 10 trials, and to a lower dose in one trial. Using vote counting, the evidence mostly favoured St John’s Wort, with more positive trials compared to placebo (16 positive vs. 9 null) and producing similar or better effects compared to prescription antidepressants (11 similar effects, four positive effects, and one worse effect).
Saffron products were mostly evaluated as a monotherapy compared to placebo. Fourteen trials included participants with a diagnosis of depression, and four focussed on participants with depressive symptoms. A range of extracts were evaluated, including stigma (n = 10), crocin (n = 3), petals (n = 1), mix (n = 1), and unspecified types (n = 5). Saffron was evaluated across a wider range of comorbid conditions than St John’s Wort, including cardiovascular disease, postpartum, menopause, and type 2 diabetes. Generally, positive effects were found compared to placebo (eight trials reported positive effects, and three trials reported null findings), and comparisons with prescription medications indicated similar effects (n = 6) as did comparisons between different saffron products (n = 2).
Probiotics were evaluated mainly as multispecies products (n = 13/16), with three evaluating single strains, compared to a placebo (n = 16). Equal numbers of studies were carried out in people with a diagnosis (n = 8) or symptoms (n = 8) of depression. In five studies, people with a range of comorbidities were included, and in six studies, they were evaluated as an adjunct to antidepressant therapy (n = 7/16). Results by vote counting favoured probiotics vs. placebo (nine positive vs. six null), with similar results to other OTC products and a combination of probiotics plus a high prebiotic diet vs. placebo.
Vitamin D trials encompassed a variety of dosing regimens (daily to weekly) and levels (1,000–100,000 IU). Nine trials were conducted in people with a depression diagnosis, and six trials were conducted in people with depressive symptoms. In most cases, vitamin D was evaluated as an adjunct to prescribed medication and/or Cognitive behavioural therapy (n = 8/14); it was most commonly compared to placebo (n = 9/14) and often assessed in people with comorbid conditions (n = 9/14, mostly vitamin D deficiency). Comparisons with placebo mainly favoured the vitamin D group (six positive vs. three null), but other types of comparisons showed mixed results.
Products with emerging evidence (2–9 trials)
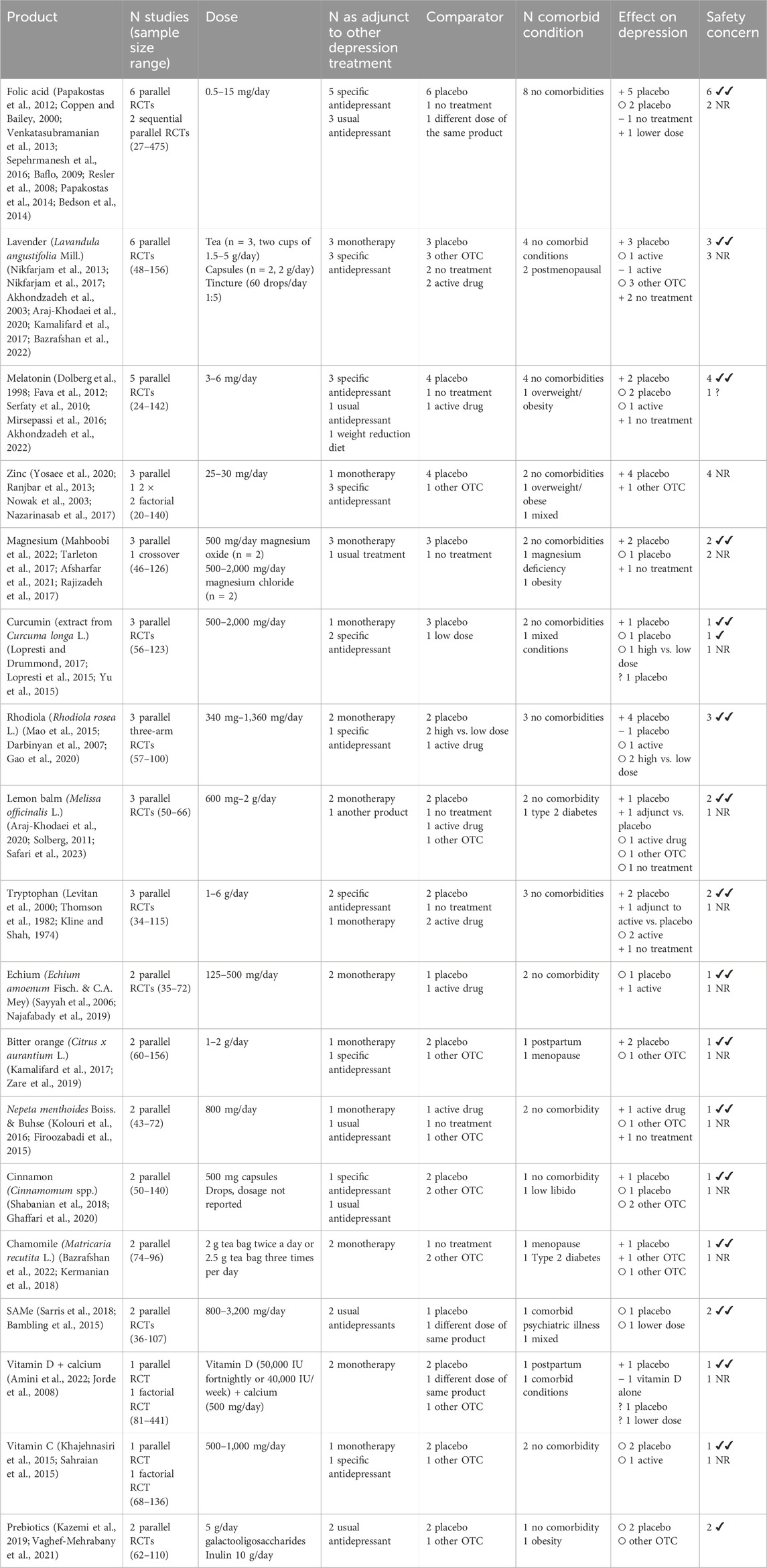
Eighteen products were tested in 2–7 trials per product (see Table 2). Out of these, folic acid (n = 8), lavender (n = 6), zinc (n = 4), tryptophan (n = 3), rhodiola (n = 3), and lemon balm (n = 3) were the most promising. Doses and preparations in these studies were variable—for example, folic acid doses ranged from 0.5 mg to 15 mg per day, whilst lavender was evaluated as a tea, capsules, and tincture. Among the products evaluated in only two trials, positive effects were found for bitter orange, Nepeta menthoides Boiss. & Buhse, and chamomile tea. The vast majority of these trials involved people without comorbidities, with the exceptions being bitter orange (evaluated in one postpartum and one menopause trial) and chamomile tea (evaluated in one menopause and one type 2 diabetes trial). Mixed results were found for melatonin (n = 5), magnesium (n = 4), curcumin (n = 3), cinnamon (n = 2), Echium (n = 2), vitamin C (n = 2), and vitamin D plus calcium (n = 2). Trials found null results for SAMe (n = 2) and prebiotics (galactooligosaccharides or inulin) (n = 2).
Products with single trials
Forty-one products were evaluated in one trial each. Nineteen products were of a single substance (13 HMPs, 9 dietary supplements, and 1 chemical (aspirin); see Table 4), and 18 contained mixes of different dietary supplements (n = 11) or mixed HMPs (n = 7). Mixed dietary supplements frequently contained SAMe (n = 4) and various B vitamins in addition to other vitamins and minerals. Mixed HMPs tended to contain different combinations of HMPs that were also evaluated alone, with St John’s Wort included most often (n = 3). The compositions of the mixed products are listed in Table 5.
For single-substance products, positive effects compared to placebo were found for rosemary, green tea, lotus seeds, ulva, basil, chromium, Nigella sativa L., and flavonoid-rich orange juice. Compared to no treatment, Cuscuta and Chlorella showed positive effects. Null effects were found for aspirin, inositol, L-tyrosine, and astaxanthin compared to placebo. L-carnitine showed non-inferiority to amisulpride for depressive symptoms. Inositol showed a tendency to be less effective than imipramine, 4G-beta-D-galactosucrose was less effective than placebo, and asperugo was significantly less effective than fluoxetine. No effects compared to placebo were found for soy extract, sumac, rose, and ginger. Two trials (of fenugreek and vitamin B6) only reported within-group analyses, so their effectiveness was not classified.
Ten mixed products showed significant effects on depressive symptoms, compared to three that showed no effect and one with unclear results (due to conflicting results at different periods of the crossover trial). One supplement showed no interaction with behavioural activation in a 2 × 2 factorial trial, whilst a three-arm trial showed no differences between vitamin D, a vitamin B complex, and a broad-spectrum multivitamin on depressive symptoms. Two trials found effects similar to prescription antidepressants for an Ayurvedic formula and lavender–dodder syrup.
Safety evidence
Most trials (n = 145/209, 69%) reported adverse events (AEs) in sufficient detail to assess safety concerns, but a substantial portion (n = 64/209, 31%) either did not report this or reported in insufficient detail, such as failing to report by trial arm. There were no noticeable trends in the number of trials reporting AEs over time, but dietary supplement trials were less likely to report AE data (44/114, 39%) compared to herbal medical product trials (20/94, 22%). Safety concerns for each product are reported in Tables 2–5.
Of the trials that reported AEs, 123/145 (85%) reported no safety concerns (e.g., similar numbers of AEs between groups), and 21 trials found higher rates of mild AEs in the product group. Higher rates of mild AEs were generally found in products with more substantive evidence, whilst those with emerging evidence or single trials generally reported no safety concerns or did not report AE data. Only one omega-3 trial reported some safety concerns—higher rates of serious adverse events in the EPA group than that in the placebo group, with unclear relatedness (Lesperance, 2011). The proportion of studies reporting increased mild AEs in the intervention group was similar whether products were administered alone (14/121, 12%) or as adjunct therapy (7/89, 8%) (notably, one study included one adjunct and one monotherapy arm, so it is included in both groups). Mixed products showed similar rates of no safety concerns reported (10/19, 53%) to single products (113/190, 59%), and similar proportions did not report on AEs (6/19, 32% vs. 55/190, 29%).
Health economic evidence
We found one trial which assessed the economic impact of folic acid on depression symptoms over 25 weeks. From a National Health Service perspective, folic acid was not more effective than a placebo but was slightly cheaper, with no significant differences in costs or resource use.
Ongoing trials
Forty-seven ongoing trials were found with a protocol or registry entry but no publication, of which 34 evaluated dietary supplements and 13 evaluated HMPs (see Supplementary Material). Most were probiotics (n = 17/34), with four vitamin D trials and three omega-3 trials. A range of HMPs were evaluated. Thirty trials reported evaluating depression alone, with 17 also including people with a range of comorbid conditions, including 6 trials for anxiety/insomnia/stress. Four trials were terminated early—two due to COVID-19, one due to recruitment difficulties, and in one, the reason was not reported.
Most products reflect those that have been evaluated previously; however, new products with no current trials located in this review for depression included resveratrol, hydrangea, cordyceps, dill, 5-HTP, creatine, ashwagandha, Bacopa monnieri (L.) Wettst. + Nardostachys jatamansi (D.Don) DC., three multi-nutrient products, cannabidiol, Platycodon grandiflorum extract + Poncirus trifoliata (likely to be Platycodon grandiflorus (Jacq.) A. DC. + Poncirus trifoliata (L.) Raf.), and Harmal powder (no further details). There were no noticeable emerging trends in the number of trials per product.
Discussion
This review found a total of 209 trials assessing OTC products in people with depressive symptoms or a depression diagnosis. Most products evaluated were dietary supplements or herbal medical products. Only one single chemical medication was found (aspirin), and no homeopathic products were found. We identified notable gaps in the evidence:
• Although a wide range of products have been evaluated, for many of these, we found only a small number of trials, and further replication of results is needed. Ongoing studies currently follow this trend and either evaluate products already with a substantive evidence base or completely new products.
• Evidence is concentrated on five key products (omega-3s, St John’s Wort, saffron, vitamin D, and probiotics). However, these still include wide variations in dose and preparation.
• Many mixed products are available on the market (or are likely to be used simultaneously), but few trials assessed mixed products.
• Few herbal teas have been evaluated, with most herbal medical product trials focussing on capsule formulations.
• Most trials used small to medium sample sizes; more definitive trials are needed as smaller antidepressant trials have been shown to overestimate effects (Hamza et al., 2024).
• Whilst a substantial number of trials have studied OTC products as an addition to antidepressants, few trials have evaluated the effect of OTC products alongside or compared to psychological therapies despite their increasing usage.
• Most products are evaluated against placebo rather than other OTC options, which is likely to hamper patients’ ability to make informed choices.
• A substantial number of trials did not report sufficient safety data, particularly for dietary supplements. However, in those that did, most products had a good safety profile.
• Promising products worthy of further evaluation include folic acid, lavender, zinc, tryptophan, rhodiola, lemon balm, bitter orange, Nepeta menthoides Boiss. & Buhse, and chamomile.
• Products with mixed evidence requiring further evaluation include melatonin, magnesium, curcumin, cinnamon, Echium, vitamin C, and vitamin D plus calcium.
Questionnaires recording the use of products for depression have found that in the US, Serbia, Mexico, and Iran, the most commonly reported HMPs were borage, chamomile, lavender, St John’s Wort, ginseng, ginkgo, orange blossom, valerian, tilia, lemon balm, and peppermint (Roy-Byrne et al., 2005; Ashraf et al., 2021; Alonso-Castro et al., 2021; Stojanović et al., 2017; Niv et al., 2010). There remain notable gaps in the evidence base regarding ginseng, ginkgo, tilia, orange blossom, and peppermint, and there are relatively few studies for products other than St John’s Wort. Borage was commonly used in only one survey in Iran; although the species name was not specified, it is likely to refer to Iranian borage (Echium amoenum Fisch. & C.A.Mey), which was evaluated in two studies included in this review, rather than Borago officinalis L. Echium showed mixed results for its effects on depression and warrants further research. Products containing mixes of herbs and/or dietary supplements were rarely evaluated. This may arise from the fact that standardisation and ensuring supply chain quality are more challenging when multiple extracts are present (Ekor, 2014). Future research should, therefore, focus on individual extracts and evaluating potential additive effects of effective herbal products using methods such as factorial trials, which were rarely used in this review. No homoeopathic products were found that were evaluated for depressive symptoms in this review; this suggests that this is not a good avenue for further research at present.
Products also rarely focused on teas (a small number of lavender and chamomile products), as opposed to capsules, even though in some studies, teas were the most common way of consuming HMPs for depression (Stojanović et al., 2017). Lemon balm (75.9%) and chamomile (60.3%) were the two most commonly used teas in a Portuguese consumer panel (Rocha et al., 2020). Herbal tea consumers closely associated herbal teas with emotions (e.g., calm and relaxation), sensory experiences (e.g., taste and appearance), and perceived effects on health and wellbeing (Rocha et al., 2020). Herbal teas are generally under-researched; a scoping review of the general health benefits of herbal teas found only 16 clinical studies and five observational studies (Poswal et al., 2019). Given their wide availability, low cost, and ability to become part of an everyday lifestyle, herbal teas warrant further clinical and observational research for their role in managing depression.
With regards to dietary supplements, there was a particular gap between the most commonly used products and the evidence base available. One study from New Zealand found that the most common dietary supplement among people with depression was a multivitamin, followed by vitamin B complex (Silvers et al., 2006), while a US study found that people with psychiatric symptoms were more likely to take melatonin compared to those without psychiatric symptoms (Niv et al., 2010). Only a small number of trials evaluated these products for depression, with few positive effects. Rates of omega-3 consumption were not significantly different in a large US survey in those with and without psychiatric symptoms (Niv et al., 2010) despite its substantive evidence base. We found few trials for multivitamins, and these had variable results. Multivitamins in particular present a challenge to evaluate due to the potential for variation between the number, type, and dose of vitamins and minerals included. Melatonin (more commonly used for insomnia) showed mixed effects on depressive symptoms and may warrant further research as a potential treatment for depression.
The use of OTC single-chemical products for depression has been evaluated in relatively few studies. This is not particularly surprising as most single-chemical medications targeting depression are prescription-only. One Swedish study found that adolescents with depressive symptoms were more likely to use OTC analgesics than their non-depressed peers (Hena et al., 2019); however, few studies have explored this relationship in adults. A single trial of aspirin (hypothesised to exert effects through anti-inflammatory mechanisms) found no effects on depressive symptoms. Few randomised trials have assessed aspirin for depression; however, a review of prospective cohort studies found that aspirin use was associated with an increased risk of depression (Kim et al., 2020). Although this may arise from confounding factors such as underlying pain or cardiovascular disease, the existing evidence suggests that aspirin is unlikely to be a promising candidate for future depression research.
Whilst a substantive evidence base was found for a small number of products, existing meta-analyses provide relatively recent conclusions regarding the level and quality of evidence available for these products. A 2019 meta-analysis of nine saffron studies found it to be effective against placebo and not significantly different from antidepressants, with studies mostly having a low or unclear risk of bias (Tóth et al., 2019). A 2017 meta-analysis of 27 studies on St John’s Wort showed similar efficacy and lower dropout rates compared to antidepressants, supported by a moderate-quality evidence base (Ng et al., 2017). Omega-3s showed significant effects in reducing depressive symptoms compared to placebo across 26 studies, with greater effects in EPA-enriched or EPA-only products (Liao et al., 2019). The evidence base for probiotics (19 trials) was generally of high quality and showed that probiotics reduce depressive versus placebo, with greater effects observed in people with major depressive disorder (Goh et al., 2019). Vitamin D (nine trials) showed limited evidence of efficacy to date (Gowda et al., 2015). There are some differences between these meta-analyses and the findings of our review. As included studies had only a small sample size (median 62), it is possible that positive but non-significant effects became significant when pooled, in contrast to our use of vote counting, which has well-established limitations. It may also reflect differences in search strategies and inclusion criteria. Our searches identified more trials than some of these reviews (Tóth et al., 2019; Liao et al., 2019; Gowda et al., 2015) despite similar inclusion criteria, suggesting that future meta-analyses should adopt a more thorough search approach. Other reviews found a large number of studies but employed wider inclusion criteria, including healthy samples (Goh et al., 2019). Generally, products with a more substantive evidence base had more trials showing some differences in mild adverse events; however, this may represent more rigorous detection methods or criteria rather than actual safety differences to products with emerging evidence. Only 10 studies in our review evaluated different doses of the same product; dose-response relationships require further study for all products.
Ongoing trials also focussed on omega-3s, probiotics, and vitamin D. Rather than conducting further trials of these products, future attention needs to be directed towards promising products with fewer trials. Products with fewer studies but with more positive than null results include folic acid, lavender, rhodiola, tryptophan, lemon balm, bitter orange, N. menthoides Boiss. & Buhse, chamomile, and zinc. Lavender, chamomile, and lemon balm are traditionally indicated and used in Western herbal medicine for depression, particularly with comorbid anxiety or stomach complaints (Bell Hunter, 2007; Bone, 2003). Bitter orange (Citrus x aurantium L.) and N. menthoides Boiss. & Buhse are traditionally used for psychological disorders, including depression, particularly in Iran (Eslami-Farouji and Jalili, 2024; Memariani et al., 2019). Rhodiola rosea L. is traditionally used across Europe and Asia for stress, fatigue, and depression (Tao et al., 2019). Most of these products show some evidence of influencing the pathophysiological mechanisms of depression. Lavender oil, rhodiola, lemon balm, N. menthoides Boisse. & Buhse, chamomile, folic acid, tryptophan, and zinc show anti-inflammatory activity in in vitro and in vivo studies (Dobrek and Głowacka, 2023; Zam et al., 2022; Dai et al., 2022; Liwinski and Lang, 2023; Wang et al., 2018; Nayak et al., 2019). Lavender, rhodiola, folic acid, and lemon balm modulate the activity of various neurotransmitters, whilst tryptophan is a precursor molecule for serotonin (Dobrek and Głowacka, 2023; Liwinski and Lang, 2023; Nayak et al., 2019). Bitter orange, N. menthoides Boiss. & Buhse, lemon balm, chamomile, tryptophan, and zinc show antioxidant effects (Dobrek and Głowacka, 2023; Dai et al., 2022; Wang et al., 2018; Nayak et al., 2019; Maksoud et al., 2021). There is also evidence that zinc, lemon balm, and rhodiola moderate HPA axis activity, whilst lavender, chamomile, and zinc showed neuroprotective activity by increasing or restoring BDNF (Dobrek and Głowacka, 2023; Dai et al., 2022; Wang et al., 2018). These potential mechanisms of effect, combined with traditional use and promising clinical evidence, suggest that further, larger trials should concentrate on these products for the treatment of depression. Despite this, few of these HMPs had registered ongoing trials (n = 1 lavender).
With 89 trials evaluating products as adjuncts alongside conventional treatment (mostly antidepressants), this represents a more realistic approach to evaluation (Solomon and Adams, 2015) and is welcomed. However, very few trials assessed the additional value of these therapies to psychological therapies. As these are considered potential first-line treatments in the UK (National Institute for Health and Care Excellence, 2022), this needs to be evaluated further. The substantive nature of the evidence base for some products also suggests that NICE guidelines should formally review these in future updates and provide clarity as to whether to recommend the use of these products for depression. Likewise, 54 trials included people with comorbidities. As depression is more common in people with long-term conditions (Gutiérrez-Rojas et al., 2020), this is heartening. Thirteen studies were also carried out in pregnant or postpartum women. Preliminary evidence indicated most products were safe, whether administered alone or alongside antidepressants. This will provide confidence to users of OTC products for depression, particularly those already taking antidepressants. However, 64 studies did not report adverse events, and reporting did not improve over time. Authors are strongly recommended to clearly report adverse events (or lack thereof) within OTC product trials, particularly as these products are most frequently used without clinical supervision. Further comprehensive analysis of adverse event data for each product is also recommended. Only one economic evaluation was found; further work on whether OTC products could provide savings in healthcare resource use for depression is needed.
Strengths of our review include the fact that we located a much larger number of trials than a previous review—focussing on products for all ages and for anxiety and insomnia (n = 76) (Kamat et al., 2023)—and more than some of the individual product meta-analyses discussed above, demonstrating the value of a scoping review methodology and a thorough approach to searching and follow up. We also summarised ongoing trials. We brought together multiple available products rather than focussing on a single product to provide an overall map of the evidence. We focussed on samples where participants had baseline depression symptoms or a diagnosis rather than those that examined depressive symptoms in non-depressed samples.
However, there were some key limitations to our review. Despite attempts to search exhaustively, some trials did not use product class keywords that were used in our searches, and we were unable to search each potential product name. It is, therefore, likely that some relevant studies were missed—for example, a meta-analysis of St John’s Wort (Ng et al., 2017) located more comparisons with SSRIs than those found in our review, and two potentially eligible trials of aspirin were found in another review (Dominiak et al., 2022). Future studies need to report product class, descriptors and names. Searches were also only carried out up to 2022. Follow-up of trial protocols and conference abstracts detected 16 further studies, some of which were published after the search dates. However, trial registry entries were not always up to date, and sometimes, a lack of detail limited further searching. Likewise, multiple publications from the same studies were not always clearly labelled. Only 10% of studies were screened by two authors, which may have introduced bias. In our protocol, we originally planned to have two reviewers screening all titles, abstracts, and full texts and to perform citation tracking and reference list screening of included studies; however, the large volume of studies identified and our limited resources precluded this. Future reviews should use a higher proportion of dual screening or have all studies dual-screened. Although we included non-English literature, we did not search using multi-language terms, and some studies were translated using Google Lens, which may not provide fully accurate details for data extraction. Future research should also consider including traditional Chinese medicine approaches to provide a more global perspective.
As the review was intended to be descriptive, we used simple vote counting to summarise the study’s effectiveness. This could be difficult to classify when different results occurred in multiple measures of depression or at different timepoints, and we did not account for sample size and, therefore, study power. We also did not explore the quality of statistical analyses or the use of intention-to-treat vs. per-protocol analysis, which were often poorly described and inconsistently labelled across studies. Results are, therefore, less precise than a meta-analysis or narrative review with consideration of study quality, and as such, they should be viewed as preliminary and not definitive. Safety data were variably reported and were not always tested for significance between adverse event incidences between groups; therefore, we took a cautious approach, and imbalances of more than a few between groups were classed as differences. As almost no serious adverse events were reported in trials; this is unlikely to affect review safety conclusions substantially. Including such a large number of products and trials necessarily resulted in some loss of nuance regarding dosage and preparation type.
Conclusion
The largest volume of evidence exists for omega-3s, St John’s Wort, saffron, probiotics, and vitamin D, all of which are relatively established products. However, a multitude of other products are promising, including folic acid, lavender, zinc, tryptophan, rhodiola, and lemon balm. Likewise, several products have mixed evidence, requiring further study such as melatonin, magnesium, curcumin, cinnamon, Echium, vitamin C, and vitamin D plus calcium. Among these, chamomile, lavender, lemon balm, and Echium represent commonly used products and should be prioritised for further research into their safety and effectiveness.
Most studies reporting on safety presented no safety issues, with a small number showing differences in mild adverse effects. One study on an omega-3 product reported a higher incidence of serious adverse events in the product group. Safety data were consistent whether the products were used alone or as adjuncts to antidepressants. However, trial authors should ensure that such information is reported completely and clearly in papers. There is a need for further evaluation of HMPs as adjuncts to antidepressants and for exploring their potential benefits when used adjunctively with psychological therapies to reflect likely clinical usage.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further enquiries can be directed to the corresponding author.
Author contributions
RF: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. AZ: Data curation, Writing – review and editing. SM: Data curation, Investigation, Methodology, Project administration, Validation, Writing – review and editing. AS: Data curation, Investigation, Validation, Writing – review and editing. CB: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. SB: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. JB-A: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. MH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. KW: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review was linked to a project funded by the National Institute National Institute for Health and Care Research (NIHR) School for Primary Care Research (project reference 635). Aiman Zamri was funded by an NIHR SPCR Internship Placement.
Acknowledgments
The authors would like to thank Tanya Cohen and Christine Vial, public contributors, who had input in the research questions, study design, and included products in this review. The authors would also like to thank Sayem Uddin and Verity Thomas for their involvement in screening titles, abstracts, and full texts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1609605/full#supplementary-material
References
Abedimanesh, N., Ostadrahimi, A., Bathaie, S. Z., Abedimanesh, S., Motlagh, B., Asghari, J. M., et al. (2017). Effects of saffron aqueous extract and its main constituent, crocin, on health-Related quality of life, depression, and sexual Desire in coronary Artery disease patients: a double-blind, placebo-controlled, randomized clinical trial. Iran. Red. Crescent Med. J. 19. doi:10.5812/ircmj.13676
Adalat, M., Khalili, M., Ayromlou, H., Haririan, S., Fazljou, S. M. B., Rezaeizadeh, H., et al. (2019). Antidepressant effects of a Persian medicine Remedy on multiple sclerosis patients: a double-blinded randomized clinical trial. GMJ 8, e1212. doi:10.31661/gmj.v8i0.1212
Adams, P., Rose, D. P., Folkard, J., Wynn, V., Seed, M., and Strong, R. (1973). Effect of pyridoxine hydrochloride (vitamin B6) upon depression associated with oral contraception. Lancet 28, 897–904. doi:10.1016/s0140-6736(73)91359-7
Afsharfar, M., Shahraki, M., Shakiba, M., Asbaghi, O., and Dashipour, A. (2021). The effects of magnesium supplementation on serum level of brain derived neurotrophic factor (BDNF) and depression status in patients with depression. Clin. Nutr. ESPEN 42, 381–386. doi:10.1016/j.clnesp.2020.12.022
Akhondzadeh, B. A., Ghoreishi, S., Noorbala, A., Akhondzadeh, S., and Rezazadeh, S. (2008). Petal and stigma of crocus sativus l. in the treatment of depression: a pilot double - blind randomized trial. J. Med. Plants 7. doi:10.1016/j.pnpbp.2006.11.010
Akhondzadeh, S., Fallah-Pour, H., Afkham, K., Jamshidi, A.-H., and Khalighi-Cigaroudi, F. (2004). Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement. Altern. Med. 4, 12. doi:10.1186/1472-6882-4-12
Akhondzadeh, S., Kashani, L., Fotouhi, A., Jarvandi, S., Mobaseri, M., Moin, M., et al. (2003). Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Biol. Psychiatry 27, 123–127. doi:10.1016/s0278-5846(02)00342-1
Akhondzadeh, S., Mostafavi, S., Keshavarz, S. A., Mohammadi, M. R., Hosseini, S., and Eshraghian, M. R. (2020). A placebo controlled randomized clinical trial of Crocus sativus L. (saffron) on depression and food craving among overweight women with mild to moderate depression. J. Clin. Pharm. Ther. 45, 134–143. doi:10.1111/jcpt.13040
Akhondzadeh, S., Mostafavi, S.-A., Keshavarz, S. A., Mohammadi, M. R., and Chamari, M. (2022). Melatonin effects in women with comorbidities of overweight, depression, and sleep disturbance: a randomized placebo controlled clinical trial. Sleep. Med. Res. 13, 22–30. doi:10.17241/smr.2021.01130
Akhondzadeh, S., Tahmacebi-Pour, N., Noorbala, A., Amini, H., Fallah-Pour, H., Jamshidi, A., et al. (2005). Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytotherapy Res. 19, 148–151. doi:10.1002/ptr.1647
Akkasheh, G., Kashani-Poor, Z., Tajabadi-Ebrahimi, M., Jafari, P., Akbari, H., Taghizadeh, M., et al. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320. doi:10.1016/j.nut.2015.09.003
Alghamdi, S., Alsulami, N., Khoja, S., Alsufiani, H., Tayeb, H. O., and Tarazi, F. I. (2020). Vitamin D supplementation Ameliorates severity of major depressive disorder. J. Mol. Neurosci. 70, 230–235. doi:10.1007/s12031-019-01461-2
Allaert, F.-A., Demais, H., and Collén, P. N. (2018). A randomized controlled double-blind clinical trial comparing versus placebo the effect of an edible algal extract (Ulva Lactuca) on the component of depression in healthy volunteers with anhedonia. BMC Psychiatry 18, 215. doi:10.1186/s12888-018-1784-x
Alonso-Castro, A. J., Ruiz-Padilla, A. J., Ortiz-Cortes, M., Carranza, E., Ramírez-Morales, M. A., Escutia-Gutiérrez, R., et al. (2021). Self-treatment and adverse reactions with herbal products for treating symptoms associated with anxiety and depression in adults from the central-western region of Mexico during the Covid-19 pandemic. J. Ethnopharmacol. 272, 113952. doi:10.1016/j.jep.2021.113952
Amini, S., Amani, R., Jafarirad, S., Cheraghian, B., Sayyah, M., and Hemmati, A. A. (2022). The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: a randomized double-blind clinical trial. Nutr. Neurosci. 25, 22–32. doi:10.1080/1028415X.2019.1707396
Amiri, S., and Behnezhad, S. (2021). Depression symptoms and risk of sick leave: a systematic review and meta-analysis. Int. Arch. Occup. Environ. Health 94, 1495–1512. doi:10.1007/s00420-021-01703-0
Anghelescu, I., Kohnen, R., Szegedi, A., Klement, S., and Kieser, M. (2006). Comparison of Hypericum extract WS ® 5570 and paroxetine in ongoing treatment after recovery from an episode of moderate to severe depression: results from a randomized multicenter study. Pharmacopsychiatry 39, 213–219. doi:10.1055/s-2006-951388
Araj-Khodaei, M., Noorbala, A. A., Yarani, R., Emadi, F., Emaratkar, E., Faghihzadeh, S., et al. (2020). A double-blind, randomized pilot study for comparison of Melissa officinalis L. and Lavandula angustifolia Mill. with Fluoxetine for the treatment of depression. BMC Complement. Med. Ther. 20, 207. doi:10.1186/s12906-020-03003-5
Arias, D., Saxena, S., and Verguet, S. (2022). Quantifying the global burden of mental disorders and their economic value. eClinicalMedicine 54, 101675. doi:10.1016/j.eclinm.2022.101675
Arias De La Torre, J., Vilagut, G., Ronaldson, A., Dregan, A., Ricci-Cabello, I., Hatch, S. L., et al. (2021). Prevalence and age patterns of depression in the United Kingdom. A population-based study. J. Affect. Disord. 279, 164–172. doi:10.1016/j.jad.2020.09.129
Arifdjanova, S. R., Abrurakhmanova, Z. Z., Bizulya, E. S., Gumenyuk, L. N., Sorokina, L. E., and Gerbali, O. Y. (2021). The role of probiotics in combination therapy of depressive disorders. Russ. Open Med. J. 10, e0109. doi:10.15275/rusomj.2021.0109
Ashraf, H., Salehi, A., Sousani, M., and Sharifi, M. H. (2021). Use of complementary alternative medicine and the associated factors among patients with depression. Evidence-Based Complementary Altern. Med. 2021, 6626394–6626398. doi:10.1155/2021/6626394
Azizi, S., Mohamadi, N., Sharififar, F., Dehghannoudeh, G., Jahanbakhsh, F., and Dabaghzadeh, F. (2022). Rosemary as an adjunctive treatment in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Complementary Ther. Clin. Pract. 49, 101685. doi:10.1016/j.ctcp.2022.101685
Baflo, C. (2009). Adjuvant folate with Escitalopram treatment and Homocystein, folate, vitamin B-12 levels in patients with major depressive disorder. Vitam. B 19. Available online at: https://psychiatry-psychopharmacology.com/en/adjuvant-folate-with-escitalopram-treatment-and-homocystein-folate-vitamin-b-12-levels-in-patients-with-major-depressive-disorder-131628
Baião, R., Capitão, L. P., Higgins, C., Browning, M., Harmer, C. J., and Burnet, P. W. J. (2023). Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: a randomised, double-blind, placebo-controlled study. Psychol. Med. 53, 3437–3447. doi:10.1017/S003329172100550X
Baker, C., and Kirk-Wade, E. (2024). Mental health statistics: prevalence, services and funding in England. House Common Libr. Available online at: https://researchbriefings.files.parliament.uk/documents/SN06988/SN06988.pdf.
Bambling, M., Parham, S. C., Coulson, S., and Vitetta, L. (2015). S-adenosylmethionine (SAMe) and Magnesium Orotate as adjunctives to SSRIs in sub-optimal treatment response of depression in adults: a pilot study. Adv. Integr. Med. 2, 56–62. doi:10.1016/j.aimed.2015.04.003
Bazrafshan, M.-R., Masmouei, B., Soufi, O., and Delam, H. (2022). Comparison of the effectiveness of lavender and chamomile herbal tea on anxiety and depression in Postmenopausal women: a randomized controlled trial. Women’s Health Bull. 9. doi:10.30476/whb.2022.94844.1172
Bedson, E., Bell, D., Carr, D., Carter, B., Hughes, D., Jorgensen, A., et al. (2014). Folate augmentation of treatment – evaluation for depression (FolATED): randomised trial and economic evaluation. Health Technol. Assess. 18, 1–160. doi:10.3310/hta18480
Behnke, K., Jensen, G. S., Graubaum, H.-J., and Gruenwald, J. (2002). Hypericum perforatum versus fluoxetine in the treatment of mild to moderate depression. Adv. Ther. 19, 43–52. doi:10.1007/BF02850017
Bell Hunter, K. A. (2007). Beyond Hypericum: perceptions of treatments by Herbalists for depression. Available online at: https://researchcommons.waikato.ac.nz/server/api/core/bitstreams/2c649bdf-d70b-4deb-91ec-e3084fe51bb7/content.
Bergman, R., Nuessner, J., and Demling, J. (1993). Behandlung leichter bis mittelschwerer Depressionen: Vergleich von Hypericum perforatum mit Amtitriptylin. TW Neurol. Psychiatr. 7, 235–240.
Berk, M., Mohebbi, M., Dean, O. M., Cotton, S. M., Chanen, A. M., Dodd, S., et al. (2020). Youth depression Alleviation with anti-inflammatory Agents (YoDA-A): a randomised clinical trial of rosuvastatin and aspirin. BMC Med. 18, 16. doi:10.1186/s12916-019-1475-6
Bhargava, J., and Khan, Z. Y. (2012). Comparative evaluation of the efficacy and side effects of imipramine, sertraline and an Ayurvedic Formulation in patients of depression. J. Clin. Diagnostic Res. 6, 220–225.
Bjerkenstedt, L., Edman, G. V., Alken, R. G., and Mannel, M. (2005). Hypericum extract LI 160 and fluoxetine in mild to moderate depression: a randomized, placebo?controlled multi?center study in outpatients. Eur. Arch. Psychiatry Clin. Neurosci. 255, 40–47. doi:10.1007/s00406-004-0532-z
Bot, M., Brouwer, I. A., Roca, M., Kohls, E., Penninx, BWJH, Watkins, E., et al. (2019). Effect of multinutrient supplementation and food-Related Behavioral activation therapy on prevention of major depressive disorder among overweight or obese adults with Subsyndromal depressive symptoms: the MooDFOOD randomized clinical trial. JAMA 321, 858–868. doi:10.1001/jama.2019.0556
Bot, M., Pouwer, F., Assies, J., Jansen, EHJM, Diamant, M., Snoek, F. J., et al. (2010). Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study. J. Affect. Disord. 126, 282–286. doi:10.1016/j.jad.2010.04.008
Brenner, R., Azbel, V., Madhusoodanan, S., and Pawlowska, M. (2000). Comparison of an extract of hypericum (LI 160) and sertraline in the treatment of depression: a double-blind, randomized pilot study. Clin. Ther. 22, 411–419. doi:10.1016/S0149-2918(00)89010-4
Browne, P. D., Bolte, A. C., Besseling-van Der Vaart, I., Claassen, E., and De Weerth, C. (2021). Probiotics as a treatment for prenatal maternal anxiety and depression: a double-blind randomized pilot trial. Sci. Rep. 11, 3051. doi:10.1038/s41598-021-81204-9
Carney, R. M., Freedland, K. E., Rubin, E. H., Rich, M. W., Steinmeyer, B. C., and Harris, W. S. (2009). Omega-3 augmentation of sertraline in treatment of depression in patients with coronary Heart disease: a randomized controlled trial. JAMA 302, 1651–1657. doi:10.1001/jama.2009.1487
Carney, R. M., Freedland, K. E., Stein, P. K., Steinmeyer, B. C., Harris, W. S., Rubin, E. H., et al. (2010). Effect of omega-3 fatty acids on Heart rate Variability in depressed patients with coronary Heart disease. Psychosom. Med. 72, 748–754. doi:10.1097/PSY.0b013e3181eff148
Chahwan, B., Kwan, S., Isik, A., Van Hemert, S., Burke, C., and Roberts, L. (2019). Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 253, 317–326. doi:10.1016/j.jad.2019.04.097
Coppen, A., and Bailey, J. (2000). Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J. Affect. Disord. 60, 121–130. doi:10.1016/s0165-0327(00)00153-1
Crowe, M., Inder, M., and McCall, C. (2023). Experience of antidepressant use and discontinuation: a qualitative synthesis of the evidence. Psychiatr. Ment. Health Nurs. 30, 21–34. doi:10.1111/jpm.12850
Cui, L., Li, S., Wang, S., Wu, X., Liu, Y., Yu, W., et al. (2024). Major depressive disorder: hypothesis, mechanism, prevention and treatment. Sig Transduct. Target Ther. 9, 30. doi:10.1038/s41392-024-01738-y
Dai, Y.-L., Li, Y., Wang, Q., Niu, F.-J., Li, K.-W., Wang, Y.-Y., et al. (2022). Chamomile: a review of its traditional Uses, chemical Constituents, pharmacological activities and quality control studies. Molecules 28, 133. doi:10.3390/molecules28010133
Darbinyan, V., Aslanyan, G., Amroyan, E., Gabrielyan, E., Malmström, C., and Panossian, A. (2007). Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nordic J. Psychiatry 61, 343–348. doi:10.1080/08039480701643290
Dashti-Khavidaki, S., Gharekhani, A., Khatami, M.-R., Miri, E.-S., Khalili, H., Razeghi, E., et al. (2014). Effects of omega-3 fatty acids on depression and quality of life in maintenance Hemodialysis patients. Am. J. Ther. 21, 275–287. doi:10.1097/MJT.0000000000000078
Davidson, J. R. T., Abraham, K., Connor, K. M., and McLeod, M. N. (2003). Effectiveness of chromium in atypical depression: a placebo-controlled trial. Biol. Psychiatry 53, 261–264. doi:10.1016/s0006-3223(02)01500-7
De Sousa-Muñoz, R. L., and Filizola, R. G. (2009). Efficacy of soy isoflavones for depressive symptoms of the climacteric syndrome. Maturitas 63, 89–93. doi:10.1016/j.maturitas.2009.02.008
Ditzier, K., Gessner, B., and Schatton, W. F. H. (1994). Clinical trial on Neurapas® versus placebo in patients with mild to moderate depressive symptoms: a placebo-controlled, randomised double-blind study Phase IV: clinical trial. Complementary Ther. Med. 2, 5–13. doi:10.1016/0965-2299(94)90153-8
Djokic, G., Korcok, D., Djordjevic, V., Agic, A., Rankovic, A., and Djukic Dejanovic, S. (2017). The effects of S-adenosyl-L-methionine-vitamin B complex on mild and moder-ate depressive symptoms. Hippokratia 21, 140–143.
Dobrek, L., and Głowacka, K. (2023). Depression and its Phytopharmacotherapy—a narrative review. IJMS 24, 4772. doi:10.3390/ijms24054772
Dolberg, O. T., Hirschmann, S., and Grunhaus, L. (1998). Melatonin for the treatment of sleep Disturbances in major depressive disorder. AJP 155, 1119–1121. doi:10.1176/ajp.155.8.1119
Dominiak, M., Gędek, A., Sikorska, M., Mierzejewski, P., Wojnar, M., and Antosik-Wójcińska, A. Z. (2022). Acetylsalicylic acid and mood disorders: a systematic review. Pharmaceuticals 16, 67. doi:10.3390/ph16010067
Ekor, M. (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4, 177. doi:10.3389/fphar.2013.00177
Eslami-Farouji, A., and Jalili, F. (2024). Exploring the miracles toward the Ethnobotany of Citrus aurantium L. (Rutaceae) in Iran (hitherto unknown uses of the plant), alongside an overview worldwide. Ethn. Res. Appl. 29. doi:10.32859/era.29.47.1-52
Far, Z. M., Rahnema, M., and Qafelehbashi, H. (2018). The effect of vitamin D3 on depression in Iranian Women. JCDR. doi:10.7860/JCDR/2018/35716.11870
Fava, M., Alpert, J., Nierenberg, A. A., Mischoulon, D., Otto, M. W., Zajecka, J., et al. (2005). A double-blind, randomized trial of St John’s wort, fluoxetine, and placebo in major depressive disorder. J. Clin. Psychopharmacol. 25, 441–447. doi:10.1097/01.jcp.0000178416.60426.29
Fava, M., Targum, S. D., Nierenberg, A. A., Bleicher, L. S., Carter, T. A., Wedel, P. C., et al. (2012). An exploratory study of combination buspirone and melatonin SR in Major Depressive Disorder (MDD): a possible role for neurogenesis in drug discovery. J. Psychiatric Res. 46, 1553–1563. doi:10.1016/j.jpsychires.2012.08.013
Firoozabadi, A., Zarshenas, M. M., Salehi, A., Jahanbin, S., and Mohagheghzadeh, A. (2015). Effectiveness of Cuscuta planiflora ten. And Nepeta menthoides Boiss. & Buhse in major depression: a triple-blind randomized controlled trial study. J. Evid. Based Complement. Altern. Med. 20, 94–97. doi:10.1177/2156587214557359
Firoozeei, T. S., Barekatain, M., Karimi, M., Zargaran, A., Akhondzadeh, S., and Rezaeizadeh, H. (2020). Lavender and dodder combined herbal syrup versus citalopram in major depressive disorder with anxious distress: a double-blind randomized trial. J. Integr. Med. 18, 409–415. doi:10.1016/j.joim.2020.06.002
Fontan, J. M. (1991). Beneficios del uso de una asociacion de aminoacidos neurtroficos y vitamina B12 como tratamiento coadyuvante del sindrome depresivo. Prensa medica argent. 78, 415–419.
Freeman, M. P., Davis, M., Sinha, P., Wisner, K. L., Hibbeln, J. R., and Gelenberg, A. J. (2008). Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J. Affect. Disord. 110, 142–148. doi:10.1016/j.jad.2007.12.228
Freeman, M. P., Hibbeln, J. R., Wisner, K. L., Brumbach, B. H., Watchman, M., and Gelenberg, A. J. (2006). Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr. Scand. 113, 31–35. doi:10.1111/j.1600-0447.2005.00660.x
Freijy, T. M., Cribb, L., Oliver, G., Metri, N.-J., Opie, R. S., Jacka, F. N., et al. (2023). Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: the “Gut Feelings” randomised controlled trial. Front. Neurosci. 16, 1097278. doi:10.3389/fnins.2022.1097278
Friede, M., Henneicke Von Zepelin, H.-H., and Freudenstein, J. (2001). Differential therapy of mild to moderateDepressive episodes (ICD-10 F 32.0; F 32.1) with St. John’s wort. Pharmacopsychiatry 34, 38–41. doi:10.1055/s-2001-15459
Gao, L., Wu, C., Liao, Y., and Wang, J. (2020). Antidepressants effects of Rhodiola capsule combined with sertraline for major depressive disorder: a randomized double-blind placebo-controlled clinical trial. J. Affect. Disord. 265, 99–103. doi:10.1016/j.jad.2020.01.065
Gastpar, M., Singer, A., and Zeller, K. (2005). Efficacy and tolerability of Hypericum extract STW3 in long-term treatment with a Once-daily dosage in comparison with sertraline. Pharmacopsychiatry 38, 78–86. doi:10.1055/s-2005-837807
Gastpar, M., Singer, A., and Zeller, K. (2006). Comparative efficacy and safety of a Once-daily dosage of Hypericum extract STW3-VI and citalopram in patients with moderate depression: a double-blind, randomised, multicentre, placebo-controlled study. Pharmacopsychiatry 39, 66–75. doi:10.1055/s-2006-931544
Gelenberg, A. J., Wojcik, J. D., Falk, W. E., Baldessarini, R. J., Zeisel, S. H., Schoenfeld, D., et al. (1990). Tyrosine for depression: a double-blind trial. J. Affect. Disord. 19, 125–132. doi:10.1016/0165-0327(90)90017-3
Gertsik, L., Poland, R. E., Bresee, C., and Rapaport, M. H. (2012). Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 32, 61–64. doi:10.1097/JCP.0b013e31823f3b5f
Ghaffari, S., Ghobadi, A., Jamshidi, A. H., Mortazavi, S. H., Naderi, S., Aqamolaei, A., et al. (2020). Cinnamomum tamala as an adjuvant therapy in the treatment of major depressive disorder: a double-blind, randomized, placebo-controlled clinical trial with placebo control. Adv. Integr. Med. 7, 141–147. doi:10.1016/j.aimed.2019.12.002
Ghorbani, Z., Nazari, S., Etesam, F., Nourimajd, S., Ahmadpanah, M., and Razeghi Jahromi, S. (2018). The effect of Synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch. Neurosci. 5. doi:10.5812/archneurosci.60507
Ginty, A. T., and Conklin, S. M. (2015). Short-term supplementation of acute long-chain omega-3 polyunsaturated fatty acids may alter depression status and decrease symptomology among young adults with depression: a preliminary randomized and placebo controlled trial. Psychiatry Res. 229, 485–489. doi:10.1016/j.psychres.2015.05.072
Goh, K. K., Liu, Y.-W., Kuo, P.-H., Chung, Y.-C. E., Lu, M.-L., and Chen, C.-H. (2019). Effect of probiotics on depressive symptoms: a meta-analysis of human studies. Psychiatry Res. 282, 112568. doi:10.1016/j.psychres.2019.112568
González, A., Mata, S., Sánchez, P., González, D., Urbina, M., Fazzino, F., et al. (2011). como coadyuvantes del tratamiento antidepresivo y sus efectos sobre el factor neurotrófico derivado del cerebro en suero y en células mononucleares.
Gowda, U., Mutowo, M. P., Smith, B. J., Wluka, A. E., and Renzaho, A. M. N. (2015). Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition 31, 421–429. doi:10.1016/j.nut.2014.06.017
Graham, E. A., Deschênes, S. S., Khalil, M. N., Danna, S., Filion, K. B., and Schmitz, N. (2020). Measures of depression and risk of type 2 diabetes: a systematic review and meta-analysis. J. Affect. Disord. 265, 224–232. doi:10.1016/j.jad.2020.01.053
Grenyer, B. F. S., Crowe, T., Meyer, B., Owen, A. J., Grigonis-Deane, E. M., Caputi, P., et al. (2007). Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog. Neuro-Psychopharmacology Biol. Psychiatry 31, 1393–1396. doi:10.1016/j.pnpbp.2007.06.004
Gutiérrez-Rojas, L., Porras-Segovia, A., Dunne, H., Andrade-González, N., and Cervilla, J. A. (2020). Prevalence and correlates of major depressive disorder: a systematic review. Braz J. Psychiatry 42, 657–672. doi:10.1590/1516-4446-2020-0650
Hamza, T., Furukawa, T. A., Orsini, N., Cipriani, A., Iglesias, C. P., and Salanti, G. (2024). A dose–effect network meta-analysis model with application in antidepressants using restricted cubic splines. Stat. Methods Med. Res. 33, 1461–1472. doi:10.1177/09622802211070256
Hansen, J. P., Pareek, M., Hvolby, A., Schmedes, A., Toft, T., Dahl, E., et al. (2019). Vitamin D3 supplementation and treatment outcomes in patients with depression (D3-vit-dep). BMC Res. Notes 12, 203. doi:10.1186/s13104-019-4218-z
Hansgen, K.-D., and Vesper, J. (1996). Antidepressive Wirksamkeit eines hochdosierten Hypericum-Extraktes. Münchener Med. Wochenschr. 138, 35–39.
Hänsgen, K.-D., Vesper, J., and Ploch, M. (1994). Multicenter double-blind study Examining the antidepressant effectiveness of the Hypericum extract LI 160. J. Geriatr. Psychiatry Neurol. 7, 15–18. doi:10.1177/089198879400700106
Hariri, N., Darafshi Ghahroudi, S., Jahangiri, S., Borumandnia, N., Narmaki, E., and Saidpour, A. (2020). The beneficial effects of sumac (Rhus coriaria L.) supplementation along with restricted calorie diet on anthropometric indices, oxidative stress, and inflammation in overweight or obese women with depression: a randomized clinical trial. Phytotherapy Res. 34, 3041–3051. doi:10.1002/ptr.6737
Harshfield, E., Pennells, L., Schwartz, J., Willeit, P., Kaptoge, S., Bell, S., et al. (2020). Association between depressive symptoms and incident cardiovascular diseases. JAMA 324, 2396–2405. doi:10.1001/jama.2020.23068
Hayashi, M., Kawamura, M., Kawashima, Y., Uemura, T., and Maoka, T. (2020). Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on the status of stress and sleep in adults. J. Clin. Biochem. Nutr. 66, 92–102. doi:10.3164/jcbn.19-113
Hena, M., Leung, C., Clausson, E. K., and Garmy, P. (2019). Association of depressive symptoms with consumption of analgesics among adolescents. J. Pediatr. Nurs. 45, e19–e23. doi:10.1016/j.pedn.2018.12.008
Hübner, W.-D., Lande, S., and Podzuweit, H. (1994). Hypericum treatment of mild depressions with Somatic symptoms. J. Geriatr. Psychiatry Neurol. 7, 12–14. doi:10.1177/089198879400700105
Hypericum Depression Trial Study Group (2002). Effect of Hypericum perforatum (St John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 287, 1807–1814. doi:10.1001/jama.287.14.1807
Irandoust, K., and Taheri, M. (2017). The effect of vitamin D supplement and Indoor Vs Outdoor physical activity on depression of obese depressed women. Asian J. Sports Med. doi:10.5812/asjsm.13311
Jahangard, L., Sadeghi, A., Ahmadpanah, M., Holsboer-Trachsler, E., Sadeghi Bahmani, D., Haghighi, M., et al. (2018). Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders - results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatric Res. 107, 48–56. doi:10.1016/j.jpsychires.2018.09.016
Jazayeri, S., Tehrani-Doost, M., Keshavarz, S. A., Hosseini, M., Djazayery, A., Amini, H., et al. (2008). Comparison of Therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry 42, 192–198. doi:10.1080/00048670701827275
Jelodar, G., Javid, Z., Sahraian, A., and Jelodar, S. (2018). Saffron improved depression and reduced homocysteine level in patients with major depression: a Randomized, double-blind study. Avicenna J. Phytomed. 8, 43–50.
Jiang, W., Whellan, D. J., Adams, K. F., Babyak, M. A., Boyle, S. H., Wilson, J. L., et al. (2018). Long-chain omega-3 fatty acid supplements in depressed Heart Failure Patients: results of the OCEAN trial. JACC Heart Fail. 6, 833–843. doi:10.1016/j.jchf.2018.03.011
Jorde, R., Sneve, M., Figenschau, Y., Svartberg, J., and Waterloo, K. (2008). Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J. Intern. Med. 264, 599–609. doi:10.1111/j.1365-2796.2008.02008.x
Kalb, R., Trautmann-Sponsel, R. D., and Kieser, M. (2001). Efficacy and tolerability of Hypericum extract WS 5572 versus placebo in mildly to moderately depressed patients - a randomized double-blind multicenter clinical trial -. Pharmacopsychiatry 34, 96–103. doi:10.1055/s-2001-14280
Kamalifard, M., Farshbaf Khalili, A., Namadian, M., Herizchi, S., and Ranjbar, Y. (2017). Comparison of the effect of lavender and bitter orange on depression in menopausal women: a triple-blind randomized controlled trial. Int. J. Women’s Health Reproduction Sci. 5, 224–230. doi:10.15296/ijwhr.2017.40
Kamat, D., Al-Ajlouni, Y. A., and Hall, R. C. W. (2023). The Therapeutic impact of plant-based and Nutritional supplements on anxiety, depressive symptoms and sleep quality among adults and Elderly: a systematic review of the literature. IJERPH 20, 5171. doi:10.3390/ijerph20065171
Kaplan, B. J., Rucklidge, J. J., Romijn, A. R., and Dolph, M. (2015). A randomised trial of nutrient supplements to minimise psychological stress after a natural disaster. Psychiatry Res. 228, 373–379. doi:10.1016/j.psychres.2015.05.080
Karimi, F., Hosseini, H., Mazloom, S., and Rakhshandeh, H. (2021). بررسی تأثیر کپسول خوراکی عصاره برگ ریحان بر افسردگی زنان یائسه: یک کارآزمایی سازی شده بالینی تصادفی. IJOGI 24. doi:10.22038/ijogi.2021.18172
Kashani, L., Esalatmanesh, S., Eftekhari, F., Salimi, S., Foroughifar, T., Etesam, F., et al. (2018). Efficacy of Crocus sativus (saffron) in treatment of major depressive disorder associated with post-menopausal hot flashes: a double-blind, randomized, placebo-controlled trial. Arch. Gynecol. Obstet. 297, 717–724. doi:10.1007/s00404-018-4655-2
Kasper, S., Anghelescu, I.-G., Szegedi, A., Dienel, A., and Kieser, M. (2006). Superior efficacy of St John’s wort extract WS®5570 compared to placebo in patients with major depression: a randomized, double-blind, placebo-controlled, multi-center trial [ISRCTN77277298]. BMC Med. 4, 14. doi:10.1186/1741-7015-4-14
Kasper, S., Anghelescu, I.-G., Szegedi, A., Dienel, A., and Kieser, M. (2007). Placebo controlled continuation treatment with Hypericum extract WS® 5570 after recovery from a mild or moderate depressive episode. Wien Med. Wochenschr 157, 362–366. doi:10.1007/s10354-007-0441-7
Kasper, S., Volz, H. P., Möller, H. J., Dienel, A., and Kieser, M. (2008). Continuation and long-term maintenance treatment with Hypericum extract WS® 5570 after recovery from an acute episode of moderate depression — a double-blind, randomized, placebo controlled long-term trial. Eur. Neuropsychopharmacol. 18, 803–813. doi:10.1016/j.euroneuro.2008.06.006
Kaviani, M., Nikooyeh, B., Etesam, F., Behnagh, S. J., Kangarani, H. M., Arefi, M., et al. (2022). Effects of vitamin D supplementation on depression and some selected pro-inflammatory biomarkers: a double-blind randomized clinical trial. BMC Psychiatry 22, 694. doi:10.1186/s12888-022-04305-3
Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., and Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 38, 522–528. doi:10.1016/j.clnu.2018.04.010
Kermanian, S., Mozaffari-Khosravi, H., Dastgerdi, G., Zavar-Reza, J., and Rahmanian, M. (2018). The effect of chamomile tea versus Black tea on Glycemic control and Blood lipid profiles in depressed patients with type 2 diabetes: a randomized clinical trial.
Keshavarz, S. A., Mostafavi, S.-A., Akhondzadeh, S., Mohammadi, M. R., Hosseini, S., Eshraghian, M. R., et al. (2018). Omega-3 supplementation effects on body weight and depression among dieter women with co-morbidity of depression and obesity compared with the placebo: a randomized clinical trial. Clin. Nutr. ESPEN 25, 37–43. doi:10.1016/j.clnesp.2018.03.001
Khajehnasiri, F., Akhondzadeh, S., Mortazavi, S. B., Allameh, A., Sotoudeh, G., Khavanin, A., et al. (2015). Are supplementation of omega-3 and Ascorbic acid effective in reducing oxidative stress and depression among depressed Shift workers? Int. J. Vitam. Nutr. Res. 85, 299–310. doi:10.1024/0300-9831/a000249
Khoraminya, N., Tehrani-Doost, M., Jazayeri, S., Hosseini, A., and Djazayery, A. (2013). Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust. N. Z. J. Psychiatry 47, 271–275. doi:10.1177/0004867412465022
Kim, H., Kim, J., and Jung, J. (2020). The association between aspirin use and depression: a systematic review and meta-analysis of observational studies. Pharmacoepidemiol. Drug 29, 613–622. doi:10.1002/pds.5011
Kline, N. S., and Shah, B. K. (1974). A Pattern of antidepressive effect of tryptophan & imipramine in Males & Females. Dis. Nerv. Syst. 35, 481–483.
Kolahdooz, G., Vosough, I., Sepahi, S., and Mohajeri, S. A. (2023). The effect of crocin versus sertraline in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Int. Clin. Psychopharmacol. 38, 9–15. doi:10.1097/YIC.0000000000000426
Kolouri, S., Firoozabadi, A., Salehi, A., Zarshenas, M. M., Dastgheib, S. A., Heydari, M., et al. (2016). Nepeta menthoides Boiss. & Buhse freeze-dried aqueous extract versus sertraline in the treatment of major depression: a double blind randomized controlled trial. Complementary Ther. Med. 26, 164–170. doi:10.1016/j.ctim.2016.03.016
Kumar, P. N. S., Menon, V., and Andrade, C. (2022). A randomized, double-blind, placebo-controlled, 12-week trial of vitamin D augmentation in major depressive disorder associated with vitamin D deficiency. J. Affect. Disord. 314, 143–149. doi:10.1016/j.jad.2022.07.014
Laakman, G., Schuele, C., Baghai, T., and Kieser, M. (1998). St. John’sWort in mild to moderate depression: the relevance of Hyperforin for the clinical efficacy. Pharmacopsychiatry 31, 54–59. doi:10.1055/s-2007-979346
Lecrubier, Y., Clerc, G., Didi, R., and Kieser, M. (2002). Efficacy of St. John’s wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am. J. Psychiatry 159, 1361–1366. doi:10.1176/appi.ajp.159.8.1361
Lee, H. J., Hong, J. K., Kim, J.-K., Kim, D.-H., Jang, S. W., Han, S.-W., et al. (2021). Effects of probiotic NVP-1704 on mental health and sleep in healthy adults: an 8-week randomized, double-blind, placebo-controlled trial. Nutrients 13, 2660. doi:10.3390/nu13082660
Lenoir, S., Degenring, F. H., and Sailer, R. (1999). A double-blind randomised trial to investigate three different concentrations of a standardised fresh plant extract obtained from the shoot tips of Hypericum perforatum L. Phytomedicine 6, 141–146. doi:10.1016/s0944-7113(99)80001-4
Levine, J., Mishori, A., Susnosky, M., Martin, M., and Belmaker, R. H. (1999). Combination of inositol and serotonin reuptake inhibitors in the treatment of depression. Biol. Psychiatry 45, 270–273. doi:10.1016/s0006-3223(98)00145-0
Levitan, R. D., Shen, J.-H., Jindal, R., and Driver, H. S. (2000). Controlled trial of tryptophan combined with fluoxetine to treat major depressive disorder: antidepressant and hypnotic effects.
Liao, Y., Xie, B., Zhang, H., He, Q., Guo, L., Subramanieapillai, M., et al. (2019). Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl. Psychiatry 9, 190. doi:10.1038/s41398-019-0515-5
Liwinski, T., and Lang, U. E. (2023). Folate and its significance in depressive disorders and Suicidality: a comprehensive narrative review. Nutrients 15, 3859. doi:10.3390/nu15173859
Lopresti, A. L., and Drummond, P. D. (2017). Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J. Affect. Disord. 207, 188–196. doi:10.1016/j.jad.2016.09.047
Lopresti, A. L., Maes, M., Meddens, M. J. M., Maker, G. L., Arnoldussen, E., and Drummond, P. D. (2015). Curcumin and major depression: a randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur. Neuropsychopharmacol. 25, 38–50. doi:10.1016/j.euroneuro.2014.11.015
Lopresti, A. L., Smith, S. J., Hood, S. D., and Drummond, P. D. (2019). Efficacy of a standardised saffron extract (affron®) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: a randomised, double-blind, placebo-controlled study. J. Psychopharmacol. 33, 1415–1427. doi:10.1177/0269881119867703
Lucas, M., Asselin, G., Mérette, C., Poulin, M.-J., and Dodin, S. (2009). Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am. J. Clin. Nutr. 89, 641–651. doi:10.3945/ajcn.2008.26749
Mahboobi, S., Ghasvarian, M., Ghaem, H., Alipour, H., Alipour, S., and Eftekhari, M. H. (2022). Effects of probiotic and magnesium co-supplementation on mood, cognition, intestinal barrier function and inflammation in individuals with obesity and depressed mood: a randomized, double-blind placebo-controlled clinical trial. Front. Nutr. 9, 1018357. doi:10.3389/fnut.2022.1018357
Maksoud, S., Abdel-Massih, R. M., Rajha, H. N., Louka, N., Chemat, F., Barba, F. J., et al. (2021). Citrus aurantium L. Active Constituents, Biological effects and extraction methods. An updated review. Molecules 26, 5832. doi:10.3390/molecules26195832
Mannel, M., Kuhn, U., Schmidt, U., Ploch, M., and Murck, H. (2010). St. John’s wort extract LI160 for the treatment of depression with atypical features – a double-blind, randomized, and placebo-controlled trial. J. Psychiatric Res. 44, 760–767. doi:10.1016/j.jpsychires.2010.01.010
Manshadi Seyed Ali, D., Seyed, A. M., Mohammad, R. S., Jayran, Z., SeyedAhmad, S., Shams Ali, R., et al. (2021). Effect of green tea consumption in treatment of mild to moderate depression in Iranian patients living with HIV: a double-blind randomized clinical trial. Chin. Herb. Med. 13, 136–141. doi:10.1016/j.chmed.2020.08.002
Mao, J. J., Xie, S. X., Zee, J., Soeller, I., Li, Q. S., Rockwell, K., et al. (2015). Rhodiola rosea versus sertraline for major depressive disorder: a randomized placebo-controlled trial. Phytomedicine 22, 394–399. doi:10.1016/j.phymed.2015.01.010
Marangell, L. B., Martinez, J. M., Zboyan, H. A., Kertz, B., Kim, H. F. S., and Puryear, L. J. (2003). A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. AJP 160, 996–998. doi:10.1176/appi.ajp.160.5.996
Masoumi, S. Z., Kazemi, F., Tavakolian, S., Rahimi, A., Oshvandi, K., Soltanian, A., et al. (2016). Effect of citalopram in combination with omega-3 on depression in post-menopausal women: a triple blind randomized controlled trial. Control. Trial 10, QC01-QC05. doi:10.7860/JCDR/2016/19487.8597
Mazidi, M., Shemshian, M., Mousavi, S. H., Norouzy, A., Kermani, T., Moghiman, T., et al. (2016). A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complementary Integr. Med. 13, 195–199. doi:10.1515/jcim-2015-0043
Mech, A. W., and Farah, A. (2016). Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C Polymorphism: a randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 77, 668–671. doi:10.4088/JCP.15m10166
Memariani, Z., Rahimi, A., Farzaei, M. H., and Zakaria Nejad, N. (2019). Nepeta menthoides Boiss. & Buhse, an endemic species in Iran: a review of traditional uses, phytochemistry and pharmacology. J. Herbmed Pharmacol. 8, 194–204. doi:10.15171/jhp.2019.29
Meyer, B. J., Grenyer, B. F. S., Crowe, T., Owen, A. J., Grigonis-Deane, E. M., and Howe, P. R. C. (2013). Improvement of major depression is associated with increased Erythrocyte DHA. Lipids 48, 863–868. doi:10.1007/s11745-013-3801-7
Milajerdi, A., Jazayeri, S., Shirzadi, E., Hashemzadeh, N., Azizgol, A., Djazayery, A., et al. (2018). The effects of alcoholic extract of saffron (Crocus satious L.) on mild to moderate comorbid depression-anxiety, sleep quality, and life satisfaction in type 2 diabetes mellitus: a double-blind, randomized and placebo-controlled clinical trial. Complementary Ther. Med. 41, 196–202. doi:10.1016/j.ctim.2018.09.023
Mirsepassi, Z., Saedi, S., Behpournia, H., Shadloo, B., Artounian, V., Yahyavi, S. T., et al. (2016). Comparing effects of melatonin versus Trazodone on sleep quality in major depressed patients receiving sertraline.
Mischoulon, D., Best-Popescu, C., Laposata, M., Merens, W., Murakami, J. L., Wu, S. L., et al. (2008). A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur. Neuropsychopharmacol. 18, 639–645. doi:10.1016/j.euroneuro.2008.04.011
Mischoulon, D., Papakostas, G. I., Dording, C. M., Farabaugh, A. H., Sonawalla, S. B., Agoston, A. M., et al. (2009). A double-blind, randomized controlled trial of Ethyl-Eicosapentaenoate for major depressive disorder. J. Clin. Psychiatry 70, 1636–1644. doi:10.4088/JCP.08m04603
Mischoulon, D., Price, L. H., Carpenter, L. L., Tyrka, A. R., Papakostas, G. I., Baer, L., et al. (2014). A double-blind, randomized, placebo-controlled clinical trial of S -adenosyl- l -methionine (SAMe) versus Escitalopram in major depressive disorder. J. Clin. Psychiatry 75, 370–376. doi:10.4088/JCP.13m08591
Moreno-Agostino, D., Wu, Y.-T., Daskalopoulou, C., Hasan, M. T., Huisman, M., and Prina, M. (2021). Global trends in the prevalence and incidence of depression:a systematic review and meta-analysis. J. Affect. Disord. 281, 235–243. doi:10.1016/j.jad.2020.12.035
Mozaffari-Khosravi, H., Yassini-Ardakani, M., Karamati, M., and Shariati-Bafghi, S.-E. (2013). Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 23, 636–644. doi:10.1016/j.euroneuro.2012.08.003
Nahidi, F., Taghizade, S., Sadr, S., and Alavi Majd, H. (2012). بررسی تأثیر اسیدهای چرب امگا-3 بر افسردگی پس از زایمان. IJOGI 14. doi:10.22038/ijogi.2012.6006
Najafabady, M. T., Baghbadoranee, P. Y., Moghimipour, E., Haghighizadeh, M., and Boostani, H. (2019). Comparison the effect of echium amoenum extract with fluoxetine on depression in menopausal women. a double-blind randomized controlled trial. AJP 13. doi:10.22377/ajppmds
Najafabady, T., Shalikar, Z., Abedi, P., and Bamshad, Z. (2013). The impact of omega-3 fatty acids on depression of menopausal women: a randomized double-blind clinical trial. Arak Med. Univ. J. 16, 16–23.
National Institute for Health and Care Excellence (2022). Depression in adults: treatment and management. London, United Kingdom National Institute for Health and Care Excellence. Available online at: https://www.nice.org.uk/guidance/ng222/chapter/recommendations#table-1.
Nayak, B. N., Singh, R. B., and Buttar, H. S. (2019). Role of tryptophan in health and disease: systematic review of the anti-Oxidant, anti-inflammation, and Nutritional Aspects of tryptophan and its Metabolites. World Heart J.
Nazarinasab, M., Behrouzian, F., and Salmanpour, R. (2017). Evaluating the effectiveness of zinc sulfate in improving depression symptoms in patients treated with selective serotonin reuptake inhibitors in Golestan Hospital in Ahvaz, Iran. Minerva Psychiatry 58. doi:10.23736/S0391-1772.17.01934-3
Nemets, B., Stahl, Z., and Belmaker, R. H. (2002). Addition of omega-3 fatty acid to maintenance medication treatment for Recurrent unipolar depressive disorder. AJP 159, 477–479. doi:10.1176/appi.ajp.159.3.477
Ng, Q. X., Venkatanarayanan, N., and Ho, C. Y. X. (2017). Clinical use of Hypericum perforatum (St John’s wort) in depression: a meta-analysis. J. Affect. Disord. 210, 211–221. doi:10.1016/j.jad.2016.12.048
Nikfarjam, M., Parvin, N., Assarzadegan, N., and Asghari, S. (2013). The effects of Lavandula angustifolia Mill Infusion on depression in patients using citalopram: a comparison study. Iran. Red. Crescent Med. J. 15, 734–739. doi:10.5812/ircmj.4173
Nikfarjam, M., Rakhshan, R., and Ghaderi, H. (2017). Comparison of effect of Lavandula officinalis and Venlafaxine in treating depression: a double blind clinical trial. JCDR 11, KC01-KC04. doi:10.7860/JCDR/2017/20657.10233
Nishi, D., Su, K.-P., Usuda, K., Chang, J. P.-C., Hamazaki, K., Ishima, T., et al. (2020). Plasma estradiol levels and antidepressant effects of omega-3 fatty acids in pregnant women. Brain, Behav. Immun. 85, 29–34. doi:10.1016/j.bbi.2019.02.014
Niv, N., Shatkin, J. P., Hamilton, A. B., Unützer, J., Klap, R., and Young, A. S. (2010). The Use of herbal medications and dietary supplements by people with mental Illness. Community Ment. Health J. 46, 563–569. doi:10.1007/s10597-009-9235-2
Noorbala, A. A., Akhondzadeh, S., Tahmacebi-Pour, N., and Jamshidi, A. H. (2005). Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J. Ethnopharmacol. 97, 281–284. doi:10.1016/j.jep.2004.11.004
Nowak, G., Siwek, M., Dudek, D., Ziêba, A., and Pilc, A. (2003). Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol. J. Pharmacol. 55, 1143–1147.
Omidian, M., Mahmoudi, M., Abshirini, M., Eshraghian, M. R., Javanbakht, M. H., Zarei, M., et al. (2019). Effects of vitamin D supplementation on depressive symptoms in type 2 diabetes mellitus patients: randomized placebo-controlled double-blind clinical trial. Diabetes & Metabolic Syndrome Clin. Res. & Rev. 13, 2375–2380. doi:10.1016/j.dsx.2019.06.011
Opiyo, R. O., Nyasulu, P. S., Koigi, R. K., Obondo, A., Ogoyi, D., and Kogi-Makau, W. (2018). Effect of fish oil omega-3 fatty acids on reduction of depressive symptoms among HIV-seropositive pregnant women: a randomized, double-blind controlled trial. Ann. Gen. Psychiatry 17, 49. doi:10.1186/s12991-018-0220-4
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan - a web and mobile app for systematic reviews. Syst. Rev. 5, 210. doi:10.1186/s13643-016-0384-4
Pakseresht, S., Boustani, H., Azemi, M. E., Nilsaz, J., Babapour, R., and Haghdust, M. R. (2012). Evaluation of Pharmaceutical products of St. John’s wort efficacy added on Tricyclic antidepressants in treating major depressive disorder: a double blind randomized control trial. Jundishapur J. Nat. Pharm. Prod. 7, 106–110. doi:10.5812/jjnpp.4318
Panahi, Y., Badeli, R., Karami, G.-R., Badeli, Z., and Sahebkar, A. (2015). A randomized controlled trial of 6-week Chlorella vulgaris supplementation in patients with major depressive disorder. Complementary Ther. Med. 23, 598–602. doi:10.1016/j.ctim.2015.06.010
Papakostas, G. I., Crawford, C. M., Scalia, M. J., and Fava, M. (2007). Timing of clinical improvement and symptom resolution in the treatment of major depressive disorder. A replication of findings with the use of a double-blind, placebo-controlled trial of Hypericum perforatum versus fluoxetine. Neuropsychobiology 56, 132–137. doi:10.1159/000115779
Papakostas, G. I., Shelton, R. C., Zajecka, J. M., Bottiglieri, T., Roffman, J., Cassiello, C., et al. (2014). Effect of adjunctive l -methylfolate 15 mg among Inadequate Responders to SSRIs in depressed patients who were Stratified by biomarker levels and Genotype: results from a randomized clinical trial. J. Clin. Psychiatry 75, 855–863. doi:10.4088/JCP.13m08947
Papakostas, G. I., Shelton, R. C., Zajecka, J. M., Etemad, B., Rickels, K., Clain, A., et al. (2012). L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. AJP 169, 1267–1274. doi:10.1176/appi.ajp.2012.11071114
Park, M., Choi, J., and Lee, H.-J. (2020). Flavonoid-rich orange Juice Intake and altered gut microbiome in young adults with depressive symptom: a randomized controlled study. Nutrients 12, 1815. doi:10.3390/nu12061815
Park, Y., Park, Y.-S., Kim, S. H., Oh, D. H., and Park, Y.-C. (2015). Supplementation of n-3 polyunsaturated fatty acids for major depressive disorder: a randomized, double-blind, 12-week, placebo-controlled trial in Korea. Ann. Nutr. Metab. 66, 141–148. doi:10.1159/000377640
Penckofer, S., Ridosh, M., Adams, W., Grzesiak, M., Woo, J., Byrn, M., et al. (2022). Vitamin D supplementation for the treatment of depressive symptoms in women with type 2 diabetes: a randomized clinical trial. J. Diabetes Res. 2022, 4090807–4090810. doi:10.1155/2022/4090807
Peters, M., Godfrey, C., McInerney, P., Munn, Z., Tricco, A., and Khalil, H. (2020). Chapter 11: scoping reviews. JBI Manual for Evidence Synthesis. doi:10.46658/JBIMES-20-12
Philipp, M., Kohnen, R., Hiller, K.-O., Linde, K., and Berner, M. (1999). Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks Commentary: has hypericum found its place in antidepressant treatment? BMJ 319, 1534–1539. doi:10.1136/bmj.319.7224.1534
Pinto-Sanchez, M. I., Hall, G. B., Ghajar, K., Nardelli, A., Bolino, C., Lau, J. T., et al. (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression Scores and Alters brain activity: a pilot study in patients with Irritable Bowel syndrome. Gastroenterology 153, 448–459. doi:10.1053/j.gastro.2017.05.003
Pirotta, M., Willis, K., Carter, M., Forsdike, K., Newton, D., and Gunn, J. (2014). Less like a drug than a drug’: the use of St John’s wort among people who self-identify as having depression and/or anxiety symptoms. Complementary Ther. Med. 22, 870–876. doi:10.1016/j.ctim.2014.07.007
Poswal, F. S., Russell, G., Mackonochie, M., MacLennan, E., Adukwu, E. C., and Rolfe, V. (2019). Herbal teas and their health benefits: a scoping review. Plant Foods Hum. Nutr. 74, 266–276. doi:10.1007/s11130-019-00750-w
Rafiee, Z. A., Foroughi Eghbal, A., Mirghazanfari, S., Ghasemzadeh, M., Nassireslami, E., and Donyavi, V. (2022). Nigella sativa extract in the treatment of depression and serum Brain-Derived Neurotrophic Factor (BDNF) levels. J. Res. Med. Sci. 27, 28. doi:10.4103/jrms.jrms_823_21
Rahimlou, M., Hosseini, S. A., Majdinasab, N., Haghighizadeh, M. H., and Husain, D. (2022). Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr. Neurosci. 25, 411–422. doi:10.1080/1028415X.2020.1758887
Rajizadeh, A., Mozaffari-Khosravi, H., Yassini-Ardakani, M., and Dehghani, A. (2017). Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: a randomized, double-blind, placebo-controlled trial. Nutrition 35, 56–60. doi:10.1016/j.nut.2016.10.014
Randløv, C., Mehlsen, J., Thomsen, C. F., Hedman, C., Von Fircks, H., and Winther, K. (2006). The efficacy of St. John’s Wort in patients with minor depressive symptoms or dysthymia – a double-blind placebo-controlled study. Phytomedicine 13, 215–221. doi:10.1016/j.phymed.2005.11.006
Ranjbar, E., Kasaei, M. S., Mohammad-Shirazi, M., Nasrollahzadeh, J., Rashidkhani, B., Shams, J., et al. (2013). Effects of zinc supplementation in patients with major depression: a randomized clinical trial. Iran. J. Psychiatry 8, 73–79.
Ranjbar, M., Firoozabadi, A., Salehi, A., Ghorbanifar, Z., Zarshenas, M. M., Sadeghniiat-Haghighi, K., et al. (2018). Effects of Herbal combination (Melissa officinalis L. and Nepeta menthoides Boiss. & Buhse) on insomnia severity, anxiety and depression in insomniacs: randomized placebo controlled trial. Integr. Med. Res. 7, 328–332. doi:10.1016/j.imr.2018.08.001
Rapaport, M. H., Nierenberg, A. A., Howland, R., Dording, C., Schettler, P. J., and Mischoulon, D. (2011). The treatment of minor depression with St. John’s Wort or citalopram: Failure to show benefit over placebo. J. Psychiatric Res. 45, 931–941. doi:10.1016/j.jpsychires.2011.05.001
Rapaport, M. H., Nierenberg, A. A., Schettler, P. J., Kinkead, B., Cardoos, A., Walker, R., et al. (2016). Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol. Psychiatry 21, 71–79. doi:10.1038/mp.2015.22
Ravi, S., Khalili, H., Abbasian, L., Arbabi, M., and Ghaeli, P. (2016). Effect of omega-3 fatty acids on depressive symptoms in HIV-positive individuals: a randomized, placebo-controlled clinical trial. Ann. Pharmacother. 50, 797–807. doi:10.1177/1060028016656017
Rees, A.-M., Austin, M.-P., and Parker, G. B. (2008). Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust. N. Z. J. Psychiatry 42, 199–205. doi:10.1080/00048670701827267
Reid, S. (2002). A survey of the use of over-the-counter homeopathic medicines purchased in health stores in central Manchester. Homeopathy 91, 225–229. doi:10.1054/homp.2002.0053
Resler, G., Lavie, R., Campos, J., Mata, S., Urbina, M., García, A., et al. (2008). Effect of folic acid combined with fluoxetine in patients with major depression on Plasma homocysteine and vitamin B12, and serotonin levels in Lymphocytes. Neuroimmunomodulation 15, 145–152. doi:10.1159/000151527
Rocha, C., Moura, A. P., and Cunha, L. M. (2020). Consumers’ associations with herbal infusions and home preparation practices. Food Qual. Prefer. 86, 104006. doi:10.1016/j.foodqual.2020.104006
Rogers, P. J., Appleton, K. M., Kessler, D., Peters, T. J., Gunnell, D., Hayward, R. C., et al. (2008). No effect of n -3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br. J. Nutr. 99, 421–431. doi:10.1017/S0007114507801097
Romijn, A. R., Rucklidge, J. J., Kuijer, R. G., and Frampton, C. (2017). A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 51, 810–821. doi:10.1177/0004867416686694
Rouhi, M., Rouhi, N., Mohamadpour, S., and Tajrishi, H. P.-R. (2018). Vitamin D reduces postpartum depression and fatigue among Iranian women. Br. J. Midwifery 26, 787–793. doi:10.12968/bjom.2018.26.12.787
Roy-Byrne, P. P., Bystritsky, A., Russo, J., Craske, M. G., Sherbourne, C. D., and Stein, M. B. (2005). Use of herbal medicine in primary care patients with mood and anxiety disorders. Psychosomatics 46, 117–122. doi:10.1176/appi.psy.46.2.117
Rudzki, L., Ostrowska, L., Pawlak, D., Małus, A., Pawlak, K., Waszkiewicz, N., et al. (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. doi:10.1016/j.psyneuen.2018.10.010
Saccarello, A., Montarsolo, P., Massardo, I., Picciotto, R., Pedemonte, A., Castagnaro, R., et al. (2020). Oral administration of S-adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 improves the mild-to-moderate symptoms of depression: a randomized, double-blind, placebo-controlled study. Prim. Care Companion CNS Disord. 22, 19m02578. doi:10.4088/PCC.19m02578
Safari, M., Asadi, A., Aryaeian, N., Huseini, H. F., Shidfar, F., Jazayeri, S., et al. (2023). The effects of melissa officinalis on depression and anxiety in type 2 diabetes patients with depression: a randomized double-blinded placebo-controlled clinical trial. BMC Complement. Med. Ther. 23, 140. doi:10.1186/s12906-023-03978-x
Sahraian, A., Ghanizadeh, A., and Kazemeini, F. (2015). Vitamin C as an adjuvant for treating major depressive disorder and suicidal behavior, a randomized placebo-controlled clinical trial. Trials 16, 94. doi:10.1186/s13063-015-0609-1
Sahraian, A., Jelodar, S., Javid, Z., Mowla, A., and Ahmadzadeh, L. (2016). Study the effects of saffron on depression and lipid profiles: a double blind comparative study. Asian J. Psychiatry 22, 174–176. doi:10.1016/j.ajp.2015.10.012
Sarris, J. (2018). Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytotherapy Res. 32, 1147–1162. doi:10.1002/ptr.6055
Sarris, J., Byrne, G. J., Bousman, C., Stough, C., Murphy, J., MacDonald, P., et al. (2018). Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: an 8-week double-blind, randomized, controlled trial<sup/>. Eur. Neuropsychopharmacol. 28, 1126–1136. doi:10.1016/j.euroneuro.2018.07.098
Sarris, J., Byrne, G. J., Stough, C., Bousman, C., Mischoulon, D., Murphy, J., et al. (2019). Nutraceuticals for major depressive disorder-more is not merrier: an 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 245, 1007–1015. doi:10.1016/j.jad.2018.11.092
Sarris, J., Kavanagh, D. J., Deed, G., and Bone, K. M. (2009). St. John’s wort and Kava in treating major depressive disorder with comorbid anxiety: a randomised double-blind placebo-controlled pilot trial. Hum. Psychopharmacol. Clin. Exp. 24, 41–48. doi:10.1002/hup.994
Sayyah, M., Sayyah, M., and Kamalinejad, M. (2006). A preliminary randomized double blind clinical trial on the efficacy of aqueous extract of Echium amoenum in the treatment of mild to moderate major depression. Prog Neuropsychopharmacol Biol Psychiatry. Available online at: https://pubmed.ncbi.nlm.nih.gov/16309809/
Schaub, A.-C., Schneider, E., Vazquez-Castellanos, J. F., Schweinfurth, N., Kettelhack, C., Doll, J. P. K., et al. (2022). Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl. Psychiatry 12, 227. doi:10.1038/s41398-022-01977-z
Schmidt, V., and Sommer, H. (1993). Johanniskraut-Extrakt zur ambulenten Therapie der Depression Aufmerksamkeit und Reaktionsvermoegen bleiben erhalten. Fortschritte Med. 111, 37–40.
Schrader, E. (2000). Equivalence of St John’s wort extract (Ze 117) and fluoxetine: a randomized, controlled study in mild-moderate depression. Int. Clin. Psychopharmacol. 15, 61–68. doi:10.1097/00004850-200015020-00001
Schrader, E., Meier, B., and Brattström, A. (1998). Hypericum treatment of mild-moderate depression in a placebo-controlled study. A prospective, double-blind, randomized, placebo-controlled, multicentre study. Hum. Psychopharmacol. Clin. Exp. 13, 163–169. doi:10.1002/(sici)1099-1077(199804)13:3<163::aid-hup5>3.3.co;2-9
Sepehrmanesh, Z., Omidi, A., and Gholampoor, N. (2016). Acid folic supplementation in major depressive disorder treatment: a double-blind randomized clinical trial. Iran. Red. Crescent Med. J. 19. doi:10.5812/ircmj.33243
Serfaty, M. A., Osborne, D., Buszewicz, M. J., Blizard, R., and Raven, P. W. (2010). A randomized double-blind placebo-controlled trial of treatment as usual plus exogenous slow-release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. Int. Clin. Psychopharmacol. 25, 132–142. doi:10.1097/YIC.0b013e32832c260b
Shabanian, S., Ebrahimbabaei, M., Safavi, P., and Lotfizadeh, M. (2018). Comparing the effect of rose drop, ginger, and cinnamon on sexual function in depressed women with sexual dysfunction. Phcog Res. 10, 314. doi:10.4103/pr.pr_150_17
Shahmansouri, N., Farokhnia, M., Abbasi, S.-H., Kassaian, S. E., Noorbala Tafti, A.-A., Gougol, A., et al. (2014). A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 155, 216–222. doi:10.1016/j.jad.2013.11.003
Shahmoradi, N., Davarinejad, O., Brühl, A. B., and Brand, S. (2023). Effects of Aphrodite an herbal Compound on SSRI-Induced sexual Dysfunctions and depression in Females with major depressive disorder: findings from a randomized clinical trial. Medicina 59, 1663. doi:10.3390/medicina59091663
Shelton, R. C., Keller, M. B., Gelenberg, A., Dunner, D. L., Hirschfeld, R., Thase, M. E., et al. (2001). Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA 285, 1978–1986. doi:10.1001/jama.285.15.1978
Shinto, L., Marracci, G., Mohr, D. C., Bumgarner, L., Murchison, C., Senders, A., et al. (2016). Omega-3 fatty acids for depression in multiple sclerosis: a randomized pilot study. PLoS ONE 11, e0147195. doi:10.1371/journal.pone.0147195
Silvers, K. M., Woolley, C. C., and Hedderley, D. (2006). Dietary supplement use in people being treated for depression. Asia Pac J Clin Nutr. 15, 30–34.
Smits, F., and Huijts, T. (2015). Treatment for depression in 63 countries worldwide: Describing and explaining cross-national differences. Health & Place 31, 1–9. doi:10.1016/j.healthplace.2014.10.002
Solberg, E. (2011). The effects of powdered Fertilized Eggs on depression. J. Med. Food 14, 870–875. doi:10.1089/jmf.2010.0146
Solomon, D., and Adams, J. (2015). The use of complementary and alternative medicine in adults with depressive disorders. A critical integrative review. J. Affect. Disord. 179, 101–113. doi:10.1016/j.jad.2015.03.031
Sommer, H., and Harrer, G. (1994). Placebo-controlled double-blind study Examining the effectiveness of an Hypericum preparation in 105 mildly depressed patients. J. Geriatr. Psychiatry Neurol. 7, 9–11. doi:10.1177/089198879400700104
Stojanović, N. M., Samardžić, L., Randjelović, P. J., and Radulović, N. S. (2017). Prevalence of self-medication practice with herbal products among non-psychotic psychiatric patients from southeastern Serbia: a cross-sectional study. Saudi Pharm. J. 25, 884–890. doi:10.1016/j.jsps.2017.02.002
Su, K.-P., Huang, S.-Y., Chiu, C.-C., and Shen, W. W. (2003). Omega-3 fatty acids in major depressive disorder A preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 13, 267–271. doi:10.1016/s0924-977x(03)00032-4
Szegedi, A., Kohnen, R., Dienel, A., and Kieser, M. (2005). Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John’s wort): randomised controlled double blind non-inferiority trial versus paroxetine. BMJ 330, 503. doi:10.1136/bmj.38356.655266.82
Tabeshpour, J., Sobhani, F., Sadjadi, S. A., Hosseinzadeh, H., Mohajeri, S. A., Rajabi, O., et al. (2017). A double-blind, randomized, placebo-controlled trial of saffron stigma (Crocus sativus L.) in mothers suffering from mild-to-moderate postpartum depression. Phytomedicine 36, 145–152. doi:10.1016/j.phymed.2017.10.005
Taheri, M., Irandoust, K., Sadeghi, A., and Yari, S. (2018). The effect of OMEGA-3 fatty acid supplement and aerobic exercise on lipid profile and depression in obese women. Acta Medica Mediterr. 34, 865.
Talaei, A., Hassanpour Moghadam, M., Sajadi Tabassi, S. A., and Mohajeri, S. A. (2015). Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord. 174, 51–56. doi:10.1016/j.jad.2014.11.035
Tao, H., Wu, X., Cao, J., Peng, Y., Wang, A., Pei, J., et al. (2019). Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 39, 1779–1850. doi:10.1002/med.21564
Tarleton, E. K., Littenberg, B., MacLean, C. D., Kennedy, A. G., and Daley, C. (2017). Role of magnesium supplementation in the treatment of depression: a randomized clinical trial. PLoS ONE 12, e0180067. doi:10.1371/journal.pone.0180067
Tarutani, S., Omori, M., Ido, Y., Yano, M., Komatsu, T., and Okamura, T. (2022). Effects of 4G-beta-D-Galactosylsucrose in patients with depression: a randomized, double-blinded, placebo-controlled, parallel-group comparative study. J. Psychiatric Res. 148, 110–120. doi:10.1016/j.jpsychires.2022.01.059
Tayama, J., Ogawa, S., Nakaya, N., Sone, T., Hamaguchi, T., Takeoka, A., et al. (2019). Omega-3 polyunsaturated fatty acids and psychological intervention for workers with mild to moderate depression: a double-blind randomized controlled trial. J. Affect. Disord. 245, 364–370. doi:10.1016/j.jad.2018.11.039
Thomson, J., Rankin, H., Ashcroft, G. W., Yates, C. M., McQueen, J. K., and Cummings, S. W. (1982). The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo. Psychol. Med. 12, 741–751. doi:10.1017/s0033291700049047
Tian, P., Chen, Y., Zhu, H., Wang, L., Qian, X., Zou, R., et al. (2022). Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain, Behav. Immun. 100, 233–241. doi:10.1016/j.bbi.2021.11.023
Torkestani, N., Atahra, M., Heidari, T., Farahani, L., and Roozbehani, N. (2013). Effect of fenugreek seed on menopausal depression: a randomized double-blind clinical trial. J. Arak Univ. Med. Sci. 16, 1–7.
Tóth, B., Hegyi, P., Lantos, T., Szakács, Z., Kerémi, B., Varga, G., et al. (2019). The efficacy of saffron in the treatment of mild to moderate depression: a meta-analysis. Planta Med. 85, 24–31. doi:10.1055/a-0660-9565
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern Med. 169, 467–473. doi:10.7326/M18-0850
Tsujita, N., Akamatsu, Y., Nishida, M. M., Hayashi, T., and Moritani, T. (2019). Effect of tryptophan, vitamin B6, and Nicotinamide-containing supplement Loading between Meals on mood and Autonomic Nervous system activity in young adults with Subclinical depression: a randomized, double-blind, and placebo-controlled study. J. Nutr. Sci. Vitaminol. 65, 507–514. doi:10.3177/jnsv.65.507
Uebelhack, R., Gruenwald, J., Graubaum, H.-J., and Busch, R. (2004). Efficacy and tolerability of Hypericum extract STW 3-VI in patients with moderate depression: a double-blind, randomized, placebo-controlled clinical trial. Adv. Ther. 21, 265–275. doi:10.1007/BF02850158
Uher, R., Payne, J., Pavlova, B., and Perlis, R. (2014). Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress. Anxiety 31, 459–471. doi:10.1002/da.22217
Ullah, H., Di Minno, A., Esposito, C., El-Seedi, H. R., Khalifa, S. A. M., Baldi, A., et al. (2022). Efficacy of a food supplement based on S-adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild-to-moderate depression: a monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. Biomed. & Pharmacother. 156, 113930. doi:10.1016/j.biopha.2022.113930
Vaghef-Mehrabany, E., Ranjbar, F., Asghari-Jafarabadi, M., Hosseinpour-Arjmand, S., and Ebrahimi-Mameghani, M. (2021). Calorie restriction in combination with prebiotic supplementation in obese women with depression: effects on metabolic and clinical response. Nutr. Neurosci. 24, 339–353. doi:10.1080/1028415X.2019.1630985
Venkatasubramanian, R., Kumar, C. N., and Pandey, R. S. (2013). A randomized double-blind comparison of fluoxetine augmentation by high and low dosage folic acid in patients with depressive episodes. J. Affect. Disord. 150, 644–648. doi:10.1016/j.jad.2013.02.029
Volz, H.-P., Eberhardt, R., and Grill, G. (2000). Wirksamkeit und Vertraeglichkeit des Johanniskrautextraktes D-0496 bei leichten bis mittelschweren depressiven Episoden: Plazebokontrollierte Doppelblindstudie ueber 6 Wochen. Nervenheilkunde 19, 401–405.
Vorbach, E.-U., Hübner, W.-D., and Arnoldt, K.-H. (1994). Effectiveness and Tolerance of the Hypericum extract LI 160 in comparison with imipramine: randomized double-blind study with 135 outpatients. J. Geriatr. Psychiatry Neurol. 7, 19–23. doi:10.1177/089198879400700107
Wang, J., Um, P., Dickerman, B. A., and Liu, J. (2018). Zinc, magnesium, Selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients 10, 584. doi:10.3390/nu10050584
Wheatley, D. (1997). LI 160, an extract of St. John’s wort, versus amitriptyline in mildly to moderately depressed outpatients - a controlled 6-week clinical trial. Pharmacopsychiatry 30, 77–80. doi:10.1055/s-2007-979523
Woelk, H. (2000). Comparison of St John’s wort and imipramine for treating depression: randomised controlled trial. BMJ 321, 536–539. doi:10.1136/bmj.321.7260.536
Wu, S.-X., Li, J., Zhou, D.-D., Xiong, R.-G., Huang, S.-Y., Saimaiti, A., et al. (2022). Possible effects and mechanisms of dietary natural products and nutrients on depression and anxiety: a narrative review. Antioxidants 11, 2132. doi:10.3390/antiox11112132
Yang, B., Lin, L., Bazinet, R. P., Chien, Y.-C., Chang, J. P.-C., Satyanarayanan, S. K., et al. (2019). Clinical efficacy and Biological Regulations of ω–3 PUFA-derived Endocannabinoids in major depressive disorder. Psychother. Psychosom. 88, 215–224. doi:10.1159/000501158
Ye, M., Bae, H., Park, S., Lew, J., Soo Kim, K., and Shim, I. (2022). A randomized double-blind, placebo-controlled study on anti-stress effects of Nelumbinis Semen. IJERPH 19, 7963. doi:10.3390/ijerph19137963
Yosaee, S., Soltani, S., Esteghamati, A., Motevalian, S. A., Tehrani-Doost, M., Clark, C. C. T., et al. (2020). Effects of zinc, vitamin D, and their co-supplementation on mood, serum cortisol, and brain-derived neurotrophic factor in patients with obesity and mild to moderate depressive symptoms: a phase II, 12-wk, 2 × 2 factorial design, double-blind, randomized, placebo-controlled trial. Nutrition 71, 110601. doi:10.1016/j.nut.2019.110601
Yu, J.-J., Pei, L.-B., Zhang, Y., Wen, Z.-Y., and Yang, J.-L. (2015). Chronic supplementation of curcumin Enhances the efficacy of antidepressants in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J. Clin. Psychopharmacol. 35, 406–410. doi:10.1097/JCP.0000000000000352
Zam, W., Quispe, C., Sharifi-Rad, J., López, M. D., Schoebitz, M., Martorell, M., et al. (2022). An updated review on the Properties of Melissa officinalis L.: not Exclusively anti-anxiety. Front. Biosci. Sch. Ed. 14, 16. doi:10.31083/j.fbs1402016
Zanardi, R., and Smeraldi, E. (2006). A double-blind, randomised, controlled clinical trial of acetyl-l-carnitine vs. amisulpride in the treatment of dysthymia. Eur. Neuropsychopharmacol. 16, 281–287. doi:10.1016/j.euroneuro.2005.10.005
Zare, Z., Nakhee, M., Amin, B., Neghabi, F., Yazdani, S., and Bidel, Z. (2019). The effect of Citrus aurantium capsule on postpartum depression in women. Iran. J. Obstretics, Gynecol. Infertil. 22, 26–34. Available online at: https://civilica.com/doc/954281.
Zarghami, M., Chabra, A., Azadbakht*, M., Khalilian, A., and Asghar Hoseini, A. (2018). Antidepressant effect of asperugo procumbens L. In comparison with fluoxetine: a randomized double blind clinical trial. Res. J. Pharmacogn. 5. doi:10.22127/rjp.2018.64860
Zhang, L., Wang, S., Zhu, Y., and Yang, T. (2018). Vitamin D3 as adjunctive therapy in the treatment of depression in tuberculosis patients: a short-term pilot randomized double-blind controlled study. NDT 14, 3103–3109. doi:10.2147/NDT.S183039
Keywords: depression, scoping review, herbal medicine, dietary (food) supplements, major depressive disorder
Citation: Frost R, Zamri A, Mathew S, Salame A, Bhanu C, Bhamra SK, Bazo-Alvarez JC, Heinrich M and Walters K (2025) Understanding the research landscape of over-the-counter herbal products, dietary supplements, and medications evaluated for depressive symptoms in adults: a scoping review. Front. Pharmacol. 16:1609605. doi: 10.3389/fphar.2025.1609605
Received: 10 April 2025; Accepted: 19 May 2025;
Published: 15 July 2025.
Edited by:
Andrea Orellana-Manzano, Facultad de Ciencias de la Vida (FCV), EcuadorReviewed by:
Xiaoyu Dong, Shengjing Hospital of China Medical University, ChinaMudassir Alam, Aligarh Muslim University, India
Xuanpeng Wang, Dalian Polytechnic University, China
Copyright © 2025 Frost, Zamri, Mathew, Salame, Bhanu, Bhamra, Bazo-Alvarez, Heinrich and Walters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachael Frost, ci5oLmZyb3N0QGxqbXUuYWMudWs=
 Rachael Frost
Rachael Frost Aiman Zamri2
Aiman Zamri2 Sukvinder K. Bhamra
Sukvinder K. Bhamra Michael Heinrich
Michael Heinrich