- 1Clinical Medicine, Chongqing Medical University, University of Leicester Joint Institute, Chongqing, China
- 2Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Gastroenterology, Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: Metabolic associated fatty liver disease (MAFLD) is a common liver disease worldwide. However, effective therapies are still lacking. This meta-analysis aimed to compare the efficacy of interventions with or without live combined Bacillus subtilis and Enterococcus faecium (LCBE) enteric-coated capsules in MAFLD patients, thereby providing some reference for clinicians in optimizing treatment strategies.
Methods: Embase, PubMed, Web of Science, Cochrane Library, Wan Fang, China Science and Technology Journal Database, China National Knowledge Infrastructure, and China Biomedical Literature Service System were searched for relevant randomized controlled trials (RCTs). MAFLD patients receiving interventions with or without LCBE enteric-coated capsules were categorized into the experimental or control group, respectively.
Results: This meta-analysis included 21 RCTs with 1783 MAFLD patients. The effective rate was higher in the experimental group than in the control group (P < 0.001). The normal and light fatty liver rate was increased in the experimental group compared to the control group (P = 0.003). Aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase were lower in the experimental group than the control group (all P < 0.01). Body mass index, fasting blood glucose, triglyceride, total cholesterol, and low-density lipoprotein cholesterol were lower, and high-density lipoprotein cholesterol was higher in the experimental group than the control group (all P < 0.05). C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and endotoxin were lower in the experimental group than in the control group (all P < 0.01).
Conclusion: Interventions containing LCBE enteric-coated capsules exhibit satisfactory efficacy, which improve liver function, metabolic status, and inflammation compared to those without LCBE enteric-coated capsules in MAFLD patients.
1 Introduction
Metabolic associated fatty liver disease (MAFLD), characterized by excessive hepatic lipid accumulation, affects over 30% of the global population (Han et al., 2023; Teng et al., 2023). While early-stage MAFLD is usually harmless, it can progress to severe liver damage, including cirrhosis and hepatocellular carcinoma (Grander et al., 2023; Leow et al., 2023). Moreover, persistent hepatic steatosis increases the risks of cardiovascular diseases and type 2 diabetes, which are the leading causes of poor clinical outcomes in MAFLD patients (Powell et al., 2021). The gut-liver axis plays a pivotal role in MAFLD progression (Hsu and Schnabl, 2023). Specifically, gut-derived microbial products (such as lipopolysaccharide) directly influence hepatic inflammation and lipid metabolism through portal circulation, while liver-derived metabolites conversely shape gut microbiota composition (Hsu and Schnabl, 2023; Tilg et al., 2022). This bidirectional communication creates a pathological feedback loop that exacerbates liver injury, which is manifested as elevated liver enzymes, metabolic dysfunction, aggravated inflammation, and impaired liver function (Hsu and Schnabl, 2023). Therefore, interventions that regulate gut dysbiosis, such as probiotic treatment, have emerged as a promising strategy for treating MAFLD patients (Fang et al., 2022).
Currently, several probiotics, such as Lactobacillus, are available in commercial markets, and some previous meta-analyses have explored the efficacy of these probiotics for the treatment of MAFLD (Li et al., 2022; Carpi et al., 2022; Rong et al., 2023; Yang et al., 2021). For instance, a previous meta-analysis reported that probiotics-containing interventions improved energy metabolism biomarkers compared to interventions without probiotics in MAFLD patients (Li et al., 2022). Another previous meta-analysis indicated that probiotics-containing interventions improved liver function and reduced blood lipid levels compared to interventions without probiotics in MAFLD patients (Yang et al., 2021).
Live combined Bacillus subtilis and Enterococcus faecium (LCBE) enteric-coated capsule is a probiotic preparation that consists of two probiotic bacteria, E. faecium R-026 and Bacillus subtilis R-179, at a ratio of 9:1 (Sohail et al., 2018). An in vivo experiment found that LCBE treatment improves liver function, lipid profiles, and inflammation to attenuate MAFLD progression in mice (Jiang et al., 2021). Additionally, some clinical studies have also explored the efficacy of LCBE enteric-coated capsules in MAFLD patients (Yang et al., 2012; Zhao et al., 2013; Luo et al., 2014; Hu et al., 2015; Yang, 2015; Yi and Zeng, 2015; Mei et al., 2016; He et al., 2017; Zhan et al., 2017; Zhao, 2017; Liu et al., 2018; Wang, 2018; Wang et al., 2018; Deng, 2020; Sun et al., 2020; Xu, 2020; Zhang, 2021; Xue et al., 2022; Li et al., 2023; Wang and Dai, 2023; Liu et al., 2024). However, the sample sizes of most studies are relatively small, which limits the statistical power. On the other hand, inconsistent findings exist among previous studies. Therefore, to provide valuable insights for clinical practice, a pooled analysis is required to synthesize data from these studies and evaluate the overall efficacy of LCBE enteric-coated capsules in MAFLD patients.
Accordingly, the current meta-analysis aimed to compare the efficacy of interventions with or without LCBE enteric-coated capsules in MAFLD patients.
2 Methods
2.1 Search strategy
Multiple electronic databases, including Embase, PubMed, Web of Science, Cochrane Library, Wan Fang, China Science and Technology Journal Database (VIP), China National Knowledge Infrastructure (CNKI), and China Biomedical Literature Service System (SinoMed) were searched comprehensively using the following key terms: “Bacillus Subtilis and Enterococcus Faecium”, “live combined Bacillus Subtilis and Enterococcus Faecium Enteric-coated Capsules”, “Medilac-S”, “non-alcoholic fatty liver disease”, “NAFLD”, “non-alcoholic steatohepatitis”, “MAFLD”, “metabolic associated fatty liver disease”, and “NASH”. The search was restricted to studies published before 2 December 2024. Additionally, we manually reviewed the references of selected studies to identify potentially relevant articles.
2.2 Inclusion and exclusion criteria
This meta-analysis was conducted according to populations, interventions, comparators, outcomes, and study designs (PICOS) criteria. The inclusion criteria for eligible studies were: 1) Population (P): patients diagnosed with MAFLD; 2) Interventions (I): patients in the experimental group receiving interventions with LCBE enteric-coated capsules; 3) Comparators (C): patients in the control groups receiving interventions without LCBE enteric-coated capsules; 4) Outcomes (O): efficacy-related results; 5) Study designs (S): randomized controlled trails (RCTs) published in English or Chinese. The exclusion criteria for eligible studies were: 1) reviews, meta-analyses, animal research, or case reports; 2) studies conducted by the same authors and with repeated patients and assessments. The qualities of the final included studies were evaluated by the Risk Of Bias (ROB) 2.0 tool (Sterne et al., 2019).
2.3 Data extraction
The data extracted included details such as the first author, year of publication, sample size, demographics, and intervention. Moreover, the evaluation indicators related to the efficacy of LCBE enteric-coated capsules were screened for system analysis. In this meta-analysis, the efficacy-related indicators involved the improvement of MAFLD (effective rate, and normal and light fatty liver rate), liver function parameters [aspartate transaminase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT)], metabolic parameters [body mass index (BMI), fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)], and inflammation markers [C-reactive protein (CRP), interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and endotoxin]. For the effective rate, the criteria involved in the studies were not uniform, including liver function, blood lipid level, fatty liver improvement, and other aspects, but the efficacy results were divided into three categories: markedly effective, effective, and ineffective. To include as many studies as possible for pooled analysis, this meta-analysis combined the effective rates from different definitions.
2.4 Effect size and models
The effect sizes were pooled using the odds ratio (OR) with a 95% confidence interval (CI) or the standard (std.) mean difference (SMD) with a 95% CI. The reason for choosing SMD was due to the inconsistency in units of continuous variables. The former was used to pool the ‘effective rate’ and ‘normal and mild fatty liver rate’; while the latter was used to pool the remaining continuous variables. A random-effects or fixed-effects model was selected according to the I2 test results. When I2 exceeded 50%, indicating significant heterogeneity, the random-effects model was utilized; otherwise, the fixed-effects model was applied.
2.5 Statistics
Publication bias was assessed via Begg’s test. If the P value of Begg’s test was less than 0.05, it indicated that there might be significant publication bias. In this case, the trim-and-filling method was used to adjust the publication bias, and the effect size was recalculated to obtain a more reliable estimate. Sensitivity analyses were conducted by excluding one by one to check the robustness of the overall findings. R software (version 4.4.2) was applied for data analyses. A P value <0.05 indicated significance.
3 Results
3.1 Study screen procedure
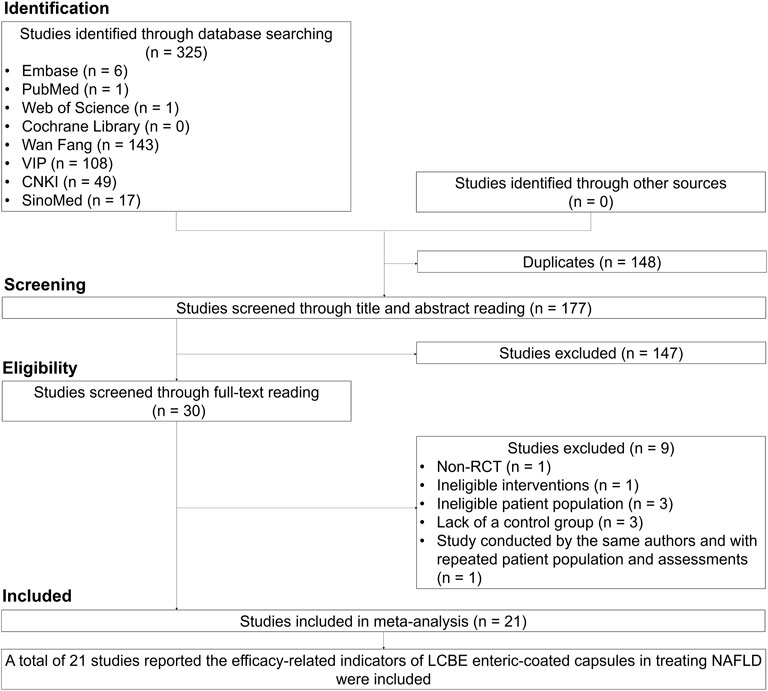
A total of 325 studies were identified through database searching, and 148 duplicates were excluded. Then, 177 studies were screened through title and abstract reading, and 147 studies were excluded. Subsequently, 30 studies were screened through full-text reading, and 9 studies were excluded. Twenty-one studies that reported the efficacy of LCBE enteric-coated capsules in MAFLD patients were finally included (Yang et al., 2012; Zhao et al., 2013; Luo et al., 2014; Hu et al., 2015; Yang, 2015; Yi and Zeng, 2015; Mei et al., 2016; He et al., 2017; Zhan et al., 2017; Zhao, 2017; Liu et al., 2018; Wang, 2018; Wang et al., 2018; Deng, 2020; Sun et al., 2020; Xu, 2020; Zhang, 2021; Xue et al., 2022; Li et al., 2023; Wang and Dai, 2023; Liu et al., 2024; Figure 1).
3.2 Characteristics of included studies
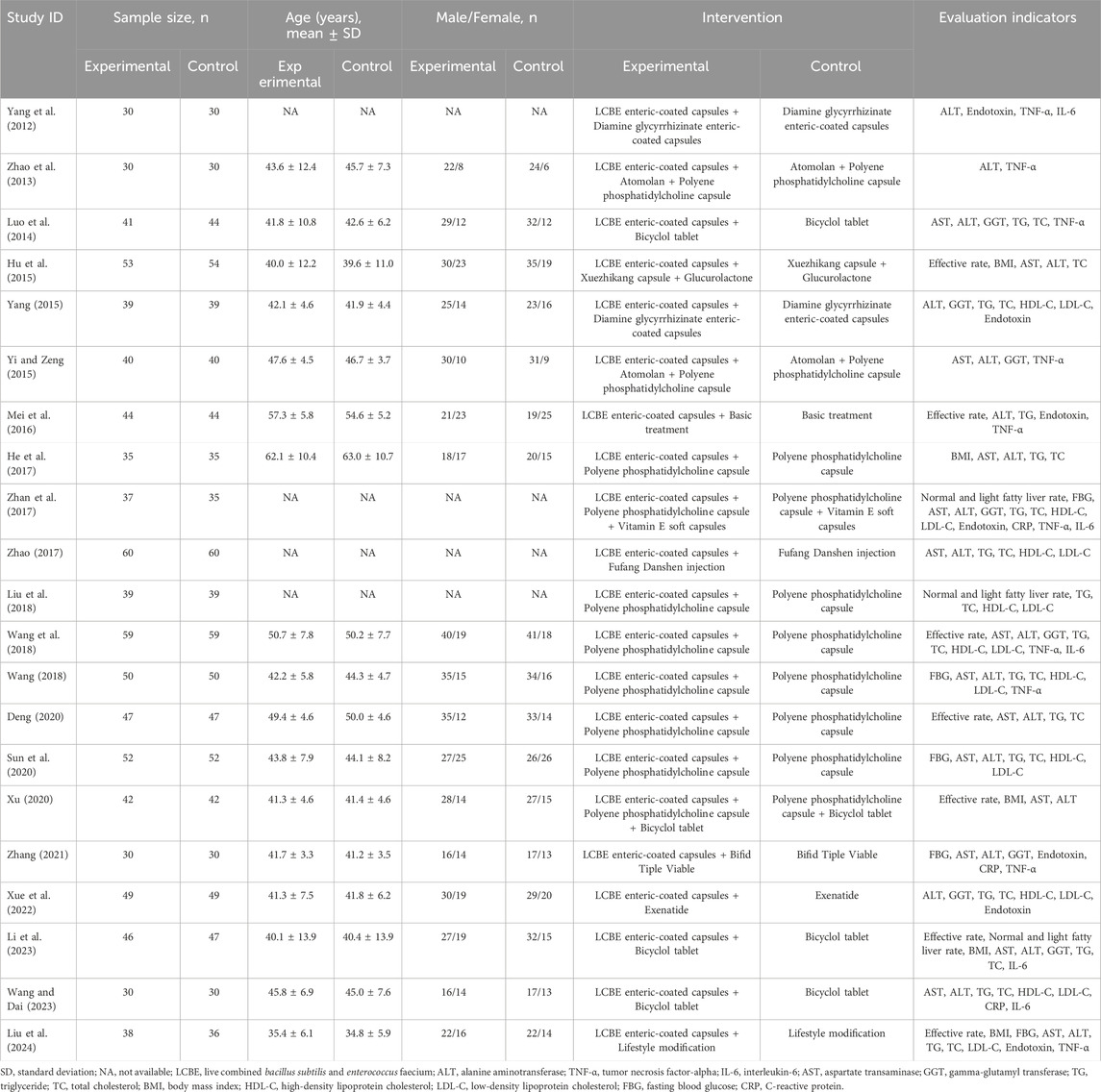
This meta-analysis included 1783 MAFLD patients. Patients who received interventions with LCBE enteric-coated capsules were assigned to the experimental group (N = 891), while patients who received interventions without LCBE enteric-coated capsules were assigned to the control group (N = 892). The mean age of patients in the experimental group ranged from 35.4 to 62.1 years, which was 34.8 to 63.0 years in the control group. Four studies did not provide information on age. The information on sample size, age, sex, interventions, and outcomes of each study is shown in Table 1.
3.3 Quality assessment
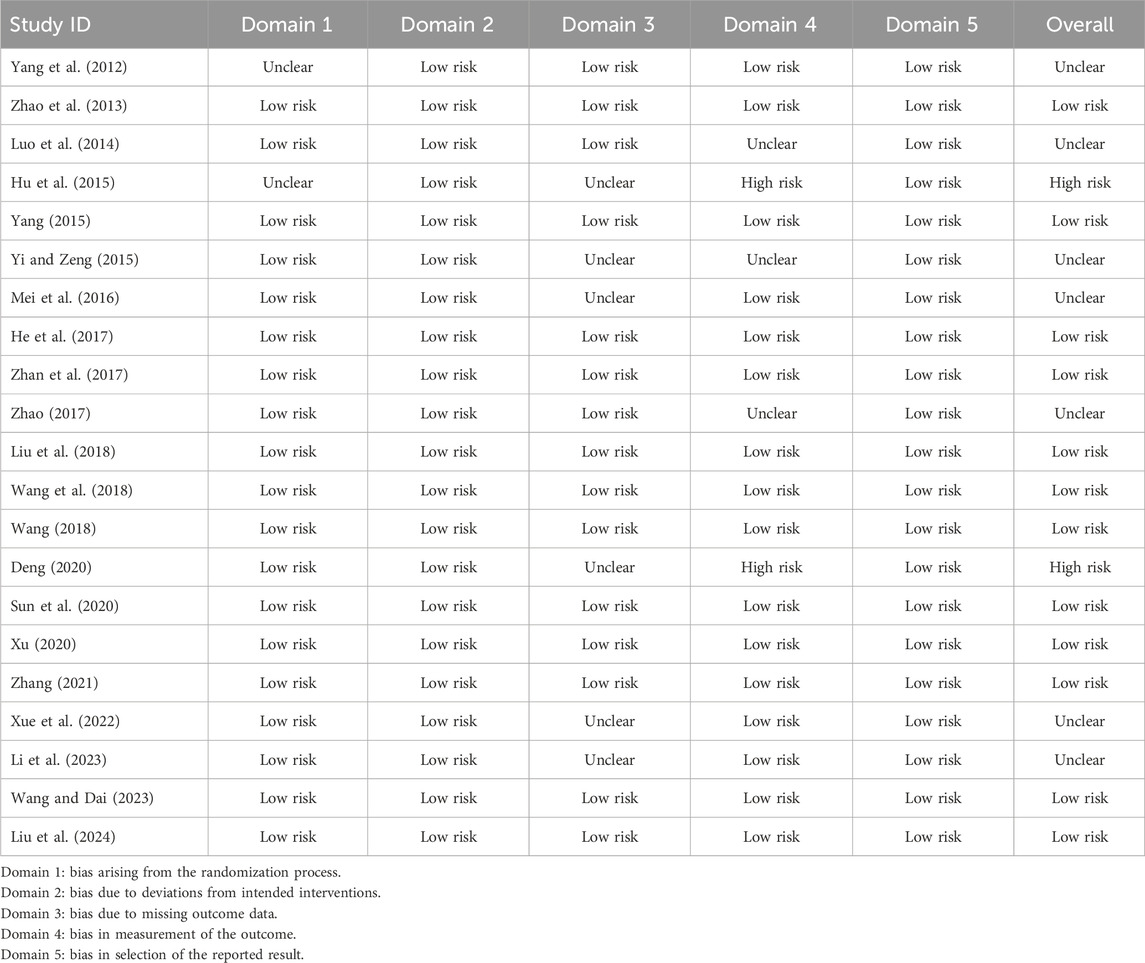
Most studies were assessed as having low risk or unclear risk for 5 domains. However, Hu et al. (2015) and Deng (2020) were assessed as having high risk for bias in the measurement of the outcome. Regarding overall risk of bias, 12 studies were assessed as having low risk, 7 studies were assessed as having unclear risk, and 2 studies were assessed as having high risk (Table 2).
3.4 Comparison of MAFLD improvement between experimental and control groups
Seven studies reported the effective rate, and heterogeneity did not exist among these studies (I2 = 0.000%, P = 0.655). Fixed-effects model suggested that interventions containing LCBE enteric-coated capsules increased the effective rate compared to interventions without LCBE enteric-coated capsules (OR = 2.576; 95% CI: 1.715, 3.870; P < 0.001) (Figure 2A).
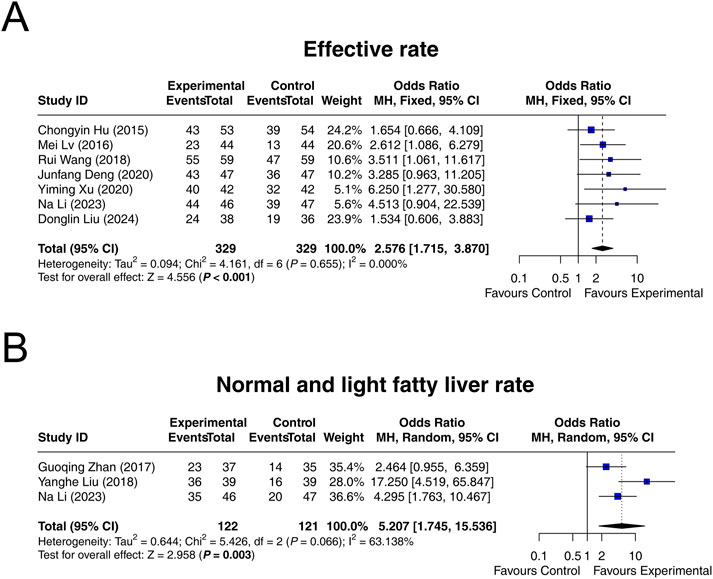
Figure 2. Forest plots of MAFLD improvement. Effective rate (A), as well as normal and light fatty liver rate (B), in the control and experimental groups.
Three studies reported normal and light fatty liver rate after treatment. Heterogeneity was found among these studies (I2 = 63.138%, P = 0.066). Random-effects model indicated that interventions containing LCBE enteric-coated capsules increased the normal and light fatty liver rate compared to interventions without LCBE enteric-coated capsules (OR = 5.207; 95% CI: 1.745, 15.536; P = 0.003) (Figure 2B).
3.5 Comparison of liver function parameters between experimental and control groups
Fifteen studies reported that AST with heterogeneity existing among them (I2 = 95.859%, P < 0.001). The random-effects model indicated that, compared with interventions without LCBE enteric-coated capsules, interventions containing LCBE enteric-coated capsules reduced AST (SMD: −2.080; 95% CI: −2.736, −1.423, P < 0.001) (Figure 3A).
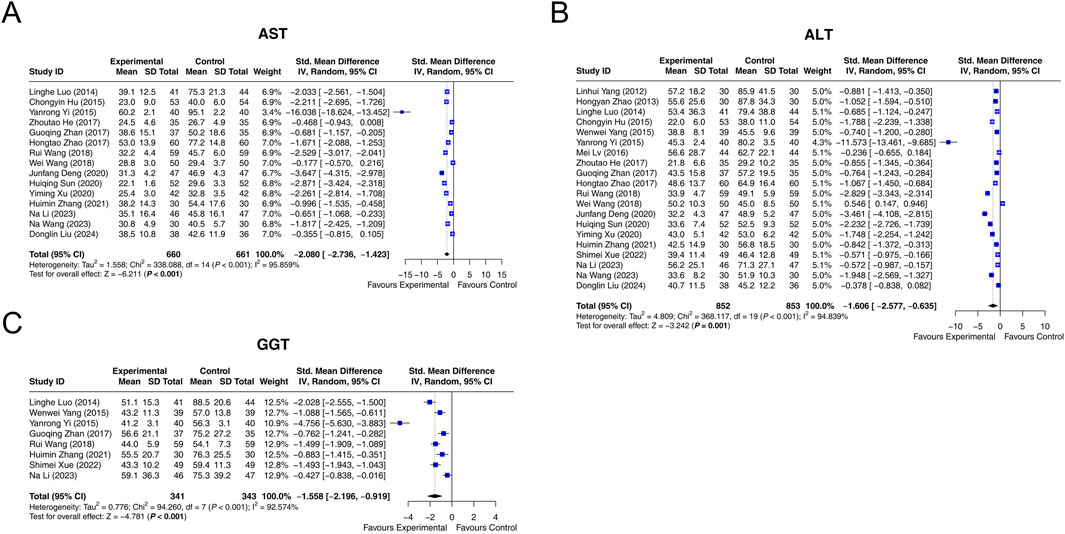
Figure 3. Forest plots of liver function parameters. AST (A), ALT (B), and GGT (C) in the control and experimental groups.
Data on ALT was extracted from 20 studies. Heterogeneity existed among these studies (I2 = 94.839%, P < 0.001). According to the random-effects model, compared with interventions without LCBE enteric-coated capsules, interventions containing LCBE enteric-coated capsules decreased ALT (SMD: −1.606; 95% CI: −2.577, −0.635, P = 0.001) (Figure 3B).
GGT was reported in 8 studies, and heterogeneity existed among them (I2 = 92.574%, P < 0.001). The random-effects model suggested that interventions containing LCBE enteric-coated capsules reduced GGT compared with interventions without LCBE enteric-coated capsules (SMD: −1.558; 95% CI: −2.196, −0.919, P < 0.001) (Figure 3C).
3.6 Comparison of metabolic parameters between experimental and control groups
Five studies reported data on BMI, and heterogeneity was observed among them (I2 = 81.384%, P < 0.001). The random-effects model revealed that interventions containing LCBE enteric-coated capsules reduced BMI compared with interventions without LCBE enteric-coated capsules (SMD: −0.555; 95% CI: −1.010, −0.100, P = 0.017) (Figure 4A).
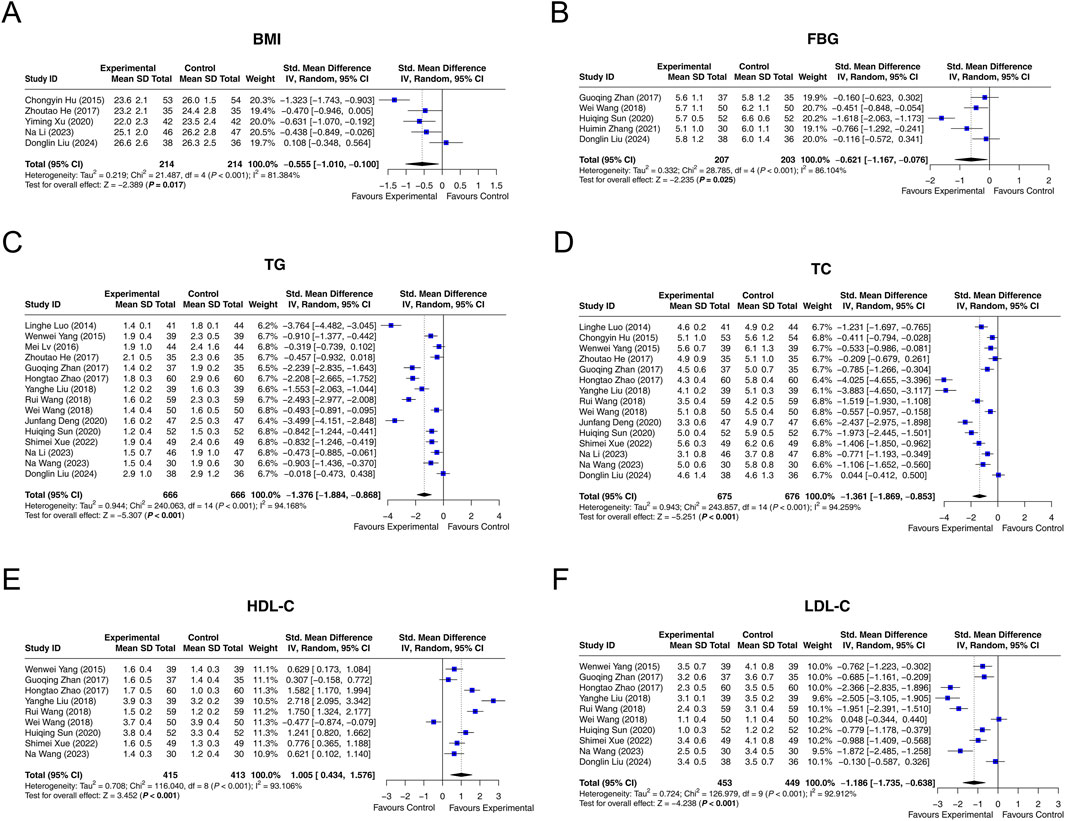
Figure 4. Forest plots of metabolic parameters. BMI (A), FBG (B), TG (C), TC (D), HDL-C (E), and LDL-C (F) in the control and experimental groups.
Among 5 studies that reported FBG, heterogeneity was observed (I2 = 86.104%, P < 0.001). The random-effects model indicated that interventions containing LCBE enteric-coated capsules decreased FBG compared with interventions without LCBE enteric-coated capsules (SMD: −0.621; 95% CI: −1.167, −0.076, P = 0.025) (Figure 4B).
Fifteen studies reported TG. Heterogeneity was found among them (I2 = 94.168%, P < 0.001). According to the random-effects model, interventions containing LCBE enteric-coated capsules reduced TG compared with interventions without LCBE enteric-coated capsules (SMD: −1.376; 95% CI: −1.884, −0.868, P < 0.001) (Figure 4C).
Fifteen studies reported TC. Heterogeneity existed among them (I2 = 94.259%, P < 0.001). The random-effects model indicated that interventions containing LCBE enteric-coated capsules reduced TC compared with interventions without LCBE enteric-coated capsules (SMD: −1.361; 95% CI: −1.869, −0.853, P < 0.001) (Figure 4D).
HDL-C was reported in nine studies. There was heterogeneity among them (I2 = 93.106%, P < 0.001). The random-effects model disclosed that interventions containing LCBE enteric-coated capsules increased HDL-C compared with interventions without LCBE enteric-coated capsules (SMD: 1.005; 95% CI: 0.434, 1.576, P < 0.001) (Figure 4E).
Among 10 studies that reported LDL-C, heterogeneity was observed (I2 = 92.912%, P < 0.001). The random-effects model disclosed that interventions containing LCBE enteric-coated capsules decreased LDL-C compared with interventions without LCBE enteric-coated capsules (SMD: −1.186; 95% CI: −1.735, −0.638, P < 0.001) (Figure 4F).
3.7 Comparison of inflammatory markers between experimental and control groups
CRP was reported in 3 studies. Heterogeneity was found among these studies (I2 = 56.179%, P = 0.102). The random-effects model suggested that interventions containing LCBE enteric-coated capsules decreased CRP compared with interventions without LCBE enteric-coated capsules (SMD: −1.860; 95% CI: −2.381, −1.338, P < 0.001) (Figure 5A).
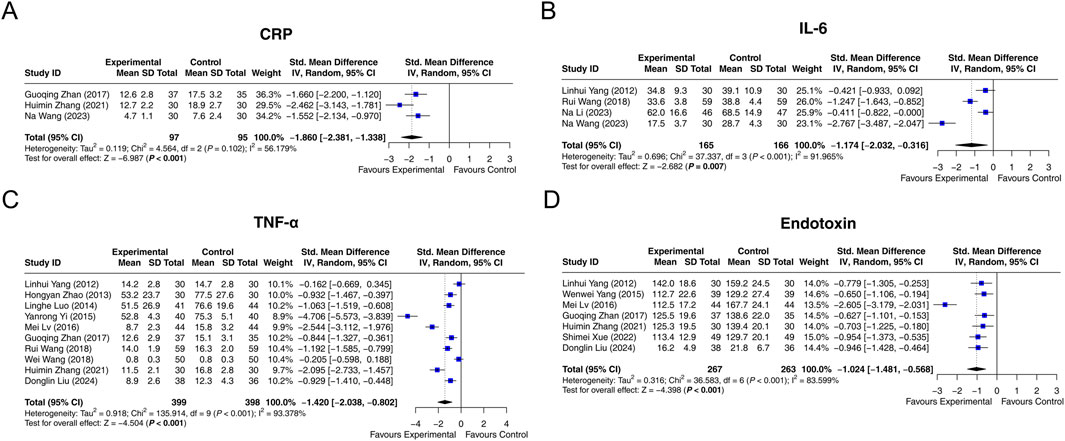
Figure 5. Forest plots of inflammation markers. CRP (A), IL-6 (B), TNF-α (C), and endotoxin (D) in the control and experimental groups.
Four studies had data on IL-6. Heterogeneity was observed among these studies (I2 = 91.965%, P < 0.001). According to the random-effects model, interventions containing LCBE enteric-coated capsules reduced IL-6 compared with interventions without LCBE enteric-coated capsules (SMD: −1.174; 95% CI: −2.032, −0.316, P = 0.007) (Figure 5B).
Ten studies reported TNF-α, and heterogeneity was observed among them (I2 = 93.378%, P < 0.001). The random-effects model indicated that interventions containing LCBE enteric-coated capsules decreased TNF-α compared with interventions without LCBE enteric-coated capsules (SMD: −1.420; 95% CI: −2.038, −0.802, P < 0.001) (Figure 5C).
Endotoxin was reported in 7 studies. Heterogeneity existed among them (I2 = 83.599%, P < 0.001). The random-effects model disclosed that interventions containing LCBE enteric-coated capsules decreased endotoxin compared with interventions without LCBE enteric-coated capsules (SMD: −1.024; 95% CI: −1.481, −0.568, P < 0.001) (Figure 5D).
3.8 Publication bias and sensitivity analysis
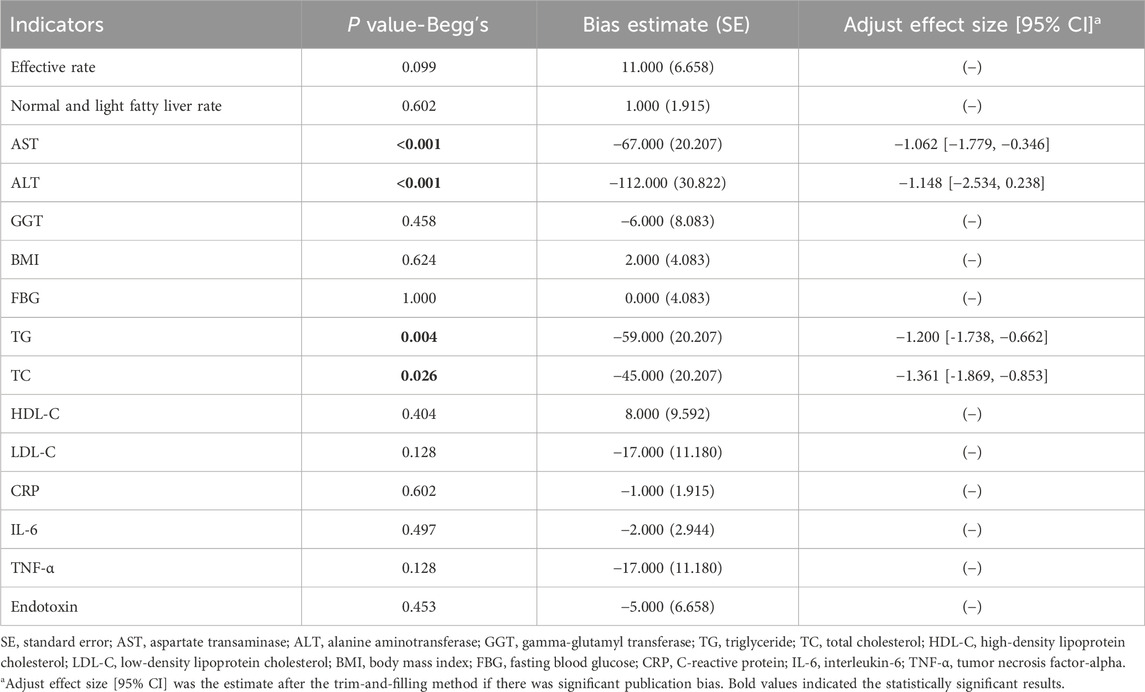
Publication bias did not exist regarding most outcomes (all P > 0.05). However, publication bias existed regarding the outcomes of AST (P < 0.001), ALT (P < 0.001), TG (P = 0.004), and TC (P = 0.026) (Table 3).
Omitting any of the studies would not alter the outcomes of the effective rate, GGT, TG, TC, HDL-C, LDL-C, CRP, TNF-α, and endotoxin. However, omitting Na Li (2023) would affect the outcomes of normal and light fatty liver rate, omitting Yi and Zeng (2015) would affect the outcomes of AST, omitting filled Yi and Zeng (2015) would alter the outcomes of ALT, omitting Xu (2020) and Li et al. (2023) would affect the outcomes of BMI, omitting Wang et al. (2018) and Zhang (2021) would affect the outcomes of FBG, and omitting Wang (2018) would affect the outcomes of IL-6 (Supplementary Figures S1A–O).
4 Discussion
As a gut microbiota intervention, probiotics exhibit satisfactory efficacy in treating MAFLD patients, according to previous meta-analyses (Ma et al., 2013; Xiao et al., 2019). However, relevant evidence regarding LCBE enteric-coated capsules is scarce. This meta-analysis discovered that the effective rate and normal and light fatty liver rate in MAFLD patients receiving interventions containing LCBE enteric-coated capsules were 2.576 and 5.207 times, respectively, of those in patients receiving interventions without LCBE enteric-coated capsules. A potential reason might be that LCBE enteric-coated capsules could enhance liver function, improve metabolic status, reduce inflammation, restore the intestinal barrier, and modulate gut microbiota composition, thereby attenuating MAFLD progression (Jiang et al., 2021).
Probiotics possess the ability to improve liver function by regulating intestinal microbiota and systemic inflammation (Milosevic et al., 2019; Safari and Gerard, 2019; Di Vincenzo et al., 2024). Several meta-analyses have reported the effect of probiotics on liver function parameters in MAFLD patients (Ma et al., 2013; Musazadeh et al., 2022; Kanchanasurakit et al., 2022). For example, a previous meta-analysis indicated that probiotic therapies reduced ALT and AST in MAFLD patients (Ma et al., 2013). Another previous meta-analysis discovered that probiotics could lower ALT, AST, and GGT levels in MAFLD patients (Musazadeh et al., 2022). In line with these previous meta-analyses (Ma et al., 2013; Musazadeh et al., 2022), we also discovered that interventions containing LCBE enteric-coated capsules decreased AST, ALT, and GGT compared to those without LCBE enteric-coated capsules in MAFLD patients. Our findings suggested that interventions containing LCBE enteric-coated capsules could improve liver function in MAFLD patients.
The interplay between metabolic dysfunction and gut microbiota drives the progression of MAFLD (Tilg et al., 2021). Previous meta-analyses have disclosed the effect of probiotics on metabolic parameters in MAFLD patients (Kanchanasurakit et al., 2022; Liu et al., 2019; Kazeminasab et al., 2024). For instance, probiotics decreased TG compared to placebo in MAFLD patients (Kanchanasurakit et al., 2022). Another previous meta-analysis reported that probiotics plus exercise reduced LDL-C and TC compared to exercise alone in MAFLD patients (Kazeminasab et al., 2024). In accordance with these previous meta-analyses, we discovered that BMI, FBG, TG, TC, and LDL-C were reduced, and HDL-C was increased by interventions containing LCBE enteric-coated capsules compared to those without LCBE enteric-coated capsules in MAFLD patients. Our findings indicated that interventions containing LCBE enteric-coated capsules were beneficial in improving metabolic status in MAFLD patients.
Gut dysbiosis can facilitate the production of endotoxins, which further promotes the release of proinflammatory cytokines, thereby accelerating the progression of MAFLD (Buzzetti et al., 2016). Previous meta-analyses revealed that gut microbiota interventions could reduce inflammation, as evidenced by reduced TNF-α, IL-6, lipopolysaccharides, and CRP in MAFLD patients (Carpi et al., 2022; Pan et al., 2024; Pan et al., 2020). Consistent with the findings of these previous meta-analyses, we found that inflammatory markers, including CRP, IL-6, TNF-α, and endotoxin, were reduced by interventions containing LCBE enteric-coated capsules compared to those without LCBE enteric-coated capsules in MAFLD patients. As explained by a previous study, the effect of LCBE on lowering inflammation might be through the toll-like receptor 4/nuclear factor kappa-B pathway (Jiang et al., 2021). Considering that aggravated inflammation could promote MAFLD progression, LCBE enteric-coated capsules could be given prophylactically in patients with early-stage MAFLD, which might be beneficial in attenuating disease progression.
In this meta-analysis, several liver function parameters, metabolic parameters, and inflammation markers were extracted from the enrolled studies. Clinically, liver function parameters, including ALT, AST, and GGT, have greater importance in clinical practice due to their ability to directly reflect the degree of liver injury. The abnormal metabolic parameters or inflammatory markers may be relevant, but may not be directly related to liver injury. Therefore, ALT, AST, and GGT possess higher priority in the evaluation of MAFLD.
Gut dysbiosis plays a crucial role in the progression of MAFLD by inducing liver injury, increasing liver inflammation and fibrosis, and causing metabolic dysfunction (Leung et al., 2016). Without proper control, gut dysbiosis can accelerate the progression of MAFLD to cirrhosis and hepatocellular carcinoma (Fang et al., 2022). Therefore, regulating gut dysbiosis is a promising strategy to improve the management of MAFLD patients. This meta-analysis discovered that interventions containing LCBE enteric-coated capsules improved liver function and metabolic status, and reduced inflammation compared to interventions without LCBE enteric-coated capsules in MAFLD patients. In clinical practice, this probiotic may be recommended for the treatment of MAFLD. However, Enterococcal species, such as Enterococcus faecalis and E. faecium, exhibit pathogenic genes, potentially leading to severe illness, disability, and death (Boeder et al., 2024; Wei et al., 2024). Considering pathogenic genes of E. faecium may impact the treatment outcomes, careful monitoring is required for MAFLD patients receiving LCBE enteric-coated capsules.
Several limitations existed among the included studies. (Han et al., 2023). To better understand the relative advantages of LCBE enteric-coated capsules, the comparison of this regimen with other probiotics in MAFLD patients, such as Lactobacillus and Bifidobacterium, could be further investigated. (Teng et al., 2023). Liver fibrosis plays a crucial role in the progression of MAFLD. However, only two included studies reported liver fibrosis-related markers, which hindered us from conducting a pooled analysis. Therefore, further studies could consider exploring the effect of LCBE enteric-coated capsules on liver fibrosis by evaluating liver fibrosis-related markers, such as liver stiffness and fibrosis scores, in MAFLD patients. (Grander et al., 2023). The included studies only involved adult MAFLD patients. Therefore, the efficacy of interventions containing LCBE enteric-coated capsules in pediatric MAFLD patients should be further investigated. (Leow et al., 2023). According to the quality assessment, some studies were assessed as having unclear or high risks of bias, particularly in bias due to missing outcome data and bias in the measurement of the outcome. These factors might affect the precision of the findings of this meta-analysis. (Powell et al., 2021). Since no enrolled studies reported the results stratified by gender, the impact of gender on the efficacy of LCBE enteric-coated capsules in MAFLD patients was unclear. This aspect could be further explored.
In conclusion, interventions containing LCBE enteric-coated capsules show satisfactory efficacy, which can improve liver function, metabolic status, and inflammation compared to those without LCBE enteric-coated capsules in MAFLD patients. Among the evaluated parameters, ALT, AST, and GGT should be given priority in clinical practice due to their ability to directly reflect the degree of liver injury. Further studies could consider optimizing the study design, increasing the sample size, evaluating the long-term efficacy, and comparing LCBE enteric-coated capsules with other probiotics to better guide clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. HW: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review and editing. HY: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Sichuan Science and Technology Program (Grant No. 22ZDYF0621).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1610426/full#supplementary-material
References
Boeder, A. M., Spiller, F., Carlstrom, M., and Izidio, G. S. (2024). Enterococcus faecalis: implications for host health. World J. Microbiol. Biotechnol. 40 (6), 190. doi:10.1007/s11274-024-04007-w
Buzzetti, E., Pinzani, M., and Tsochatzis, E. A. (2016). The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65 (8), 1038–1048. doi:10.1016/j.metabol.2015.12.012
Carpi, R. Z., Barbalho, S. M., Sloan, K. P., Laurindo, L. F., Gonzaga, H. F., Grippa, P. C., et al. (2022). The effects of probiotics, prebiotics and synbiotics in non-alcoholic Fat liver disease (NAFLD) and non-alcoholic steatohepatitis (nash): a systematic review. Int. J. Mol. Sci. 23 (15), 8805. doi:10.3390/ijms23158805
Deng, J. (2020). Effects of live combined Bacillus subtilis and Bacillus subtilis enteric-coated capsules combined with polyene phosphatidylcholine on liver function and blood lipid levels in patients with non-alcoholic fatty liver disease. Cap. Food Med. 27 (20), 90. doi:10.3969/j.issn.1005-8257.2020.20.063
Di Vincenzo, F., Del Gaudio, A., Petito, V., Lopetuso, L. R., and Scaldaferri, F. (2024). Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg. Med. 19 (2), 275–293. doi:10.1007/s11739-023-03374-w
Fang, J., Yu, C. H., Li, X. J., Yao, J. M., Fang, Z. Y., Yoon, S. H., et al. (2022). Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front. Cell Infect. Microbiol. 12, 997018. doi:10.3389/fcimb.2022.997018
Grander, C., Grabherr, F., and Tilg, H. (2023). Non-alcoholic fatty liver disease: pathophysiological concepts and treatment options. Cardiovasc. Res. 119 (9), 1787–1798. doi:10.1093/cvr/cvad095
Han, S. K., Baik, S. K., and Kim, M. Y. (2023). Non-alcoholic fatty liver disease: definition and subtypes. Clin. Mol. Hepatol. 29 (Suppl. l), S5–S16. doi:10.3350/cmh.2022.0424
He, Z., Xu, C., Han, X., and Chen, Y. (2017). Effects of probiotics on the expression of cytokines and intestinal flora in patients with non-alcoholic fatty liver disease. Chin. J. Gerontology 37 (23), 5878–5880. doi:10.3969/j.issn.1005-9202.2017.23.060
Hsu, C. L., and Schnabl, B. (2023). The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21 (11), 719–733. doi:10.1038/s41579-023-00904-3
Hu, C., Hu, Y., and Li, B. (2015). Efficacy of Medilac-s combined with Xuezhikang and Glucurolactone in the treatment of non-alcoholic fatty liver disease. World Latest Med. Inf. (6), 94. doi:10.3969/j.issn.1671-3141.2015.06.0079
Jiang, J., Xiong, J., Ni, J., Chen, C., and Wang, K. (2021). Live combined B. Subtilis and E. Faecium alleviate liver inflammation, improve intestinal barrier function, and modulate gut microbiota in mice with non-alcoholic fatty liver disease. Med. Sci. Monit. 27, e931143. doi:10.12659/MSM.931143
Kanchanasurakit, S., Kositamongkol, C., Lanoi, K., Nunta, M., Saetuan, T., Chaiyakunapruk, N., et al. (2022). Effects of synbiotics, probiotics, and prebiotics on liver enzymes of patients with non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Front. Nutr. 9, 880014. doi:10.3389/fnut.2022.880014
Kazeminasab, F., Miraghajani, M., Mokhtari, K., Karimi, B., Rosenkranz, S. K., and Santos, H. O. (2024). The effects of probiotic supplementation and exercise training on liver enzymes and cardiometabolic markers in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. (Lond) 21 (1), 59. doi:10.1186/s12986-024-00826-8
Leow, W. Q., Chan, A. W., Mendoza, P. G. L., Lo, R., Yap, K., and Kim, H. (2023). Non-alcoholic fatty liver disease: the pathologist's perspective. Clin. Mol. Hepatol. 29 (Suppl. l), S302–S318. doi:10.3350/cmh.2022.0329
Leung, C., Rivera, L., Furness, J. B., and Angus, P. W. (2016). The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13 (7), 412–425. doi:10.1038/nrgastro.2016.85
Li, Na, Wang, M., Wang, Le, Xiaomin, L. V., Ji, Y., Huilian, H., et al. (2023). Clinical observation of probiotics combined with bicyclol in the treatment of non- alcoholic fatty liver disease. China Med. 32 (9), 91–94. doi:10.3969/j.issn.1006-4931.2023.09.021
Li, S., Liu, J., Wang, Z., Duan, F., Jia, Z., Chen, X., et al. (2022). The promising role of probiotics/prebiotics/synbiotics in energy metabolism biomarkers in patients with NAFLD: a systematic review and meta-analysis. Front. Public Health 10, 862266. doi:10.3389/fpubh.2022.862266
Liu, D., Wang, Z., Chen, P., Wang, A., Wu, X., and Chen, J. (2024). Study on the clinical effect of microecologics on patients with nonalcoholic steatohepatitis. Jiangxi Med. J. 59 (4), 340–344. doi:10.3969/j.issn.1006-2238.2024.04.002
Liu, L., Li, P., Liu, Y., and Zhang, Y. (2019). Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: a meta-analysis. Dig. Dis. Sci. 64 (12), 3402–3412. doi:10.1007/s10620-019-05699-z
Liu, Y., Long, D., Bei, Z., Ling, M., Zhang, Y., and Cai, D. (2018). Efficacy of bacillus subtilis duplex and polyene phosphatidylcholine combination in treatments of patients with nonalcoholic fatty liver diseases. J. Pract. Hepatology 21 (4), 573–576. doi:10.3969/j.issn.1672-5069.2018.04.022
Luo, L., Chen, J., Liu, H., and Xia, L. (2014). Intervention effect of live combined Bacillus subtilis and Enterococcus on patients with nonalcoholic steatohepatitis. Chin. J. Dig. 34 (12), 852–853. doi:10.3760/cma.j.issn.0254-1432.2014.12.018
Ma, Y. Y., Li, L., Yu, C. H., Shen, Z., Chen, L. H., and Li, Y. M. (2013). Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J. Gastroenterol. 19 (40), 6911–6918. doi:10.3748/wjg.v19.i40.6911
Mei, Lv, Lang, X., Wang, F., Cui, L., and Li, Q. (2016). Clinical study of microecologics in improving insulin resistance of patients with non-alcoholic fatty liver disease. Prog. Mod. Biomed. 16 (6), 1073–5138. doi:10.13241/j.cnki.pmb.2016.06.020
Milosevic, I., Vujovic, A., Barac, A., Djelic, M., Korac, M., Radovanovic Spurnic, A., et al. (2019). Gut-liver Axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature. Int. J. Mol. Sci. 20 (2), 395. doi:10.3390/ijms20020395
Musazadeh, V., Roshanravan, N., Dehghan, P., and Ahrabi, S. S. (2022). Effect of probiotics on liver enzymes in patients with non-alcoholic fatty liver disease: an umbrella of systematic review and meta-analysis. Front. Nutr. 9, 844242. doi:10.3389/fnut.2022.844242
Pan, X., Wen, S. W., Kaminga, A. C., and Liu, A. (2020). Gut metabolites and inflammation factors in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sci. Rep. 10 (1), 8848. doi:10.1038/s41598-020-65051-8
Pan, Y., Yang, Y., Wu, J., Zhou, H., and Yang, C. (2024). Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 24 (1), 283. doi:10.1186/s12876-024-03356-y
Powell, E. E., Wong, V. W., and Rinella, M. (2021). Non-alcoholic fatty liver disease. Lancet 397 (10290), 2212–2224. doi:10.1016/S0140-6736(20)32511-3
Rong, L., Ch'ng, D., Jia, P., Tsoi, K. K. F., Wong, S. H., and Sung, J. J. Y. (2023). Use of probiotics, prebiotics, and synbiotics in non-alcoholic fatty liver disease: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 38 (10), 1682–1694. doi:10.1111/jgh.16256
Safari, Z., and Gerard, P. (2019). The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol. Life Sci. 76 (8), 1541–1558. doi:10.1007/s00018-019-03011-w
Sohail, G., Xu, X., Christman, M. C., and Tompkins, T. A. (2018). Probiotic Medilac-S® for the induction of clinical remission in a Chinese population with ulcerative colitis: a systematic review and meta-analysis. World J. Clin. Cases 6 (15), 961–984. doi:10.12998/wjcc.v6.i15.961
Sterne, J. A. C., Savovic, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sun, H., Cui, S., and Han, X. (2020). Efficacy of intestinal probiotics in the treatment of nonalcoholic fatty liver disease. Med. J. Liaoning 34 (2), 1–3.
Teng, M. L., Ng, C. H., Huang, D. Q., Chan, K. E., Tan, D. J., Lim, W. H., et al. (2023). Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 29 (Suppl. l), S32–S42. doi:10.3350/cmh.2022.0365
Tilg, H., Adolph, T. E., Dudek, M., and Knolle, P. (2021). Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat. Metab. 3 (12), 1596–1607. doi:10.1038/s42255-021-00501-9
Tilg, H., Adolph, T. E., and Trauner, M. (2022). Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34 (11), 1700–1718. doi:10.1016/j.cmet.2022.09.017
Wang, Na, and Dai, Q. (2023). Investigate on the effects of probiotics combined with bicyclol tablets on inflammatory factors and liver fibrosis-related cytokines in patients with nonalcoholic steatohepatitis. Mod. Med. Health Res. Electron. J. 7 (12), 85–88. doi:10.3969/j.issn.2096-3718.2023.12.028
Wang, R. (2018). Efficacy capsule containing bacillus subtilis and enterococcus combined with phosphatidylcholine in treating non-alcoholic steatohepatitis. Anhui Med. Pharm. J. 22 (5), 940–943. doi:10.3969/j.issn.1009-6469.2018.05.041
Wang, W., Shi, L., Shi, L., and Xu, Le (2018). Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Chin. J. Intern. Med. 57 (2), 101–106. doi:10.3760/cma.j.issn.0578-1426.2018.02.004
Wei, Y., Palacios Araya, D., and Palmer, K. L. (2024). Enterococcus faecium: evolution, adaptation, pathogenesis and emerging therapeutics. Nat. Rev. Microbiol. 22 (11), 705–721. doi:10.1038/s41579-024-01058-6
Xiao, M. W., Lin, S. X., Shen, Z. H., Luo, W. W., and Wang, X. Y. (2019). Systematic review with meta-analysis: the effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2019, 1484598. doi:10.1155/2019/1484598
Xu, Y. (2020). Effects of bicyclol combined with intestinal probiotics in treatment of patients with nonalcoholic fatty liver disease. Med. J. Chin. People's Health 32 (20), 1–3. doi:10.3969/j.issn.1672-0369.2020.20.001
Xue, S., Hu, Y., and Liu, Y. (2022). Curative effect of live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules combined with exenatide on patients with non-alcoholic steatohepatitis. Chin. J. Drug Abuse Prev. Treat. 28 (5), 607–610. doi:10.15900/j.cnki.zylf1995.2022.05.030
Yang, L., Guo, H., Cai, J., Cai, X., Liu, G., and Chen, D. (2012). Intervention effect of microbiological capsules containing Bacillus subtilis and Enterococcus on intestinal flora in patients with NASH. World Chin. J. Dig. 20 (20), 1873–1878. doi:10.11569/wcjd.v20.i20.1873
Yang, R., Shang, J., Zhou, Y., Liu, W., Tian, Y., and Shang, H. (2021). Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 15 (12), 1401–1409. doi:10.1080/17474124.2022.2016391
Yang, W. (2015). Influence of lived combined B. subtilis and E. faecium enteric-coated capsules on serum endotoxin and intestinal microflora of patients with NASH. Chin. J. Microecology 27 (7), 816–823. doi:10.13381/j.cnki.cjm.201507019
Yi, Y., and Zeng, Ya (2015). Effects of probiotics on liver function and carbohydrate metabolism in patients with nonalcoholic fatty liver disease. J. Clin. Res. (6), 1132–1133. doi:10.3969/j.issn.1671-7171.2015.06.031
Zhan, G., Fang, Li, Li, R., Liu, X., Zhang, W., Guo, P., et al. (2017). Effects of B.subtilis-E. faecium capsule on non-alcoholic steatohepatitis. Chin. J. Microecology 29 (1), 74–77. doi:10.13381/j.cnki.cjm.201701018
Zhang, H. (2021). Curative effect of Bifidobacterium combined with Enterococcus subtilis in treatment of non-alcoholic fatty liver disease. Chin. J. Sch. Dr. 35 (1), 50–1,9.
Zhao, H. (2017). Effect of live combined Bacillus subtilis and Enterococcus in the treatment of non-alcoholic fatty liver disease. Pract. Clin. J. Integr. Traditional Chin. West. Med. 17 (5), 56–57. doi:10.13638/j.issn.1671-4040.2017.05.034
Keywords: metabolic associated fatty liver disease, live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules, liver function, metabolic status, inflammation
Citation: Wu Y, Wu H and Yi H (2025) Efficacy of interventions with live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules in metabolic associated fatty liver disease patients: a meta-analysis of randomized controlled trials. Front. Pharmacol. 16:1610426. doi: 10.3389/fphar.2025.1610426
Received: 12 April 2025; Accepted: 12 May 2025;
Published: 27 May 2025.
Edited by:
Ralf Weiskirchen, RWTH Aachen University, GermanyReviewed by:
Jijun Tan, Yichun University, ChinaKiran Kumar Bhukya, Osmania University, India
Amal Dwidar, Tanta University, Egypt
Yongfang Xie, Chongqing university of post and telecommunications, China
Copyright © 2025 Wu, Wu and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wu, d3VoYW9odWF4aUAxNjMuY29t; Hang Yi, aGVpemhpNzBAMTYzLmNvbQ==
 Yutong Wu1
Yutong Wu1 Hao Wu
Hao Wu