- 1Department of Vascular and Endovascular Surgery, The First Affiliated Hospital of Yangtze University, Jingzhou, Hubei, China
- 2Department of Pharmacology, School of Medicine, Yangtze University, Jingzhou, China
- 3Department of Laboratory Medicine, Jingzhou Hospital Affiliated to Yangtze University, Jingzhou, China
Diabetic cardiomyopathy (DCM) is a major complication of diabetes mellitus, characterized by microvascular dysfunction and progressive structural and functional deterioration of the heart. A central driver of DCM pathogenesis is chronic oxidative stress (OS), primarily resulting from excessive production of reactive oxygen species (ROS) under hyperglycemic conditions. Among the various ROS sources, the NADPH oxidase (NOX) family of enzymes plays a pivotal role in initiating and sustaining oxidative damage. NOX-mediated ROS production contributes to myocardial inflammation, apoptosis, fibrosis, and remodeling, through multiple signaling pathways, including NF-κB, TGF-β/Smad, MAPK, and PI3K/Akt cascades. Despite growing recognition of NOX enzymes as crucial mediators in DCM, therapeutic options specifically targeting NOX-driven oxidative stress remain limited. In this comprehensive review, we summarize current insights into the mechanisms by which NOX regulates cardiac pathology in DCM, highlight the crosstalk between NOX activity and downstream molecular pathways, and discuss potential pharmacological interventions aimed at restoring redox homeostasis. Emerging strategies, such as selective NOX inhibitors, antioxidant therapies, and agents modulating signaling transduction, offer promising avenues for mitigating oxidative injury and improving cardiac function. Furthermore, we emphasize the importance of developing isoform-specific NOX inhibitors to achieve greater efficacy and safety in clinical applications. By providing a detailed overview of NOX-dependent oxidative stress in DCM and associated therapeutic approaches, this review aims to foster further research and innovation toward targeted treatments for diabetic cardiomyopathy.
1 Introduction
DCM refers to structural and functional abnormalities of the heart observed in diabetic patients without coronary artery disease, hypertension, or other forms of heart disease (Zhao et al., 2022). Initially described in 1972, autopsy findings from four diabetic patients revealed unexplained cardiac enlargement and congestive heart failure, offering the first evidence of DCM (Rubler et al., 1972). As a prevalent chronic complication of diabetes, nearly all diabetic patients are at risk of developing cardiac issues, especially those with type 2 diabetes, who exhibit a higher incidence of DCM (Kenny and Abel, 2019). This condition is frequently associated with pathological processes, including oxidative stress, inflammation, and metabolic dysfunction (Marino et al., 2023). Importantly, DCM is usually asymptomatic in its early stages, complicating early diagnosis (Zhang L. et al., 2022). Clinical studies have shown that the myocardium of diabetic patients is more susceptible to damage from acute myocardial infarction and other injuries compared to non-diabetic individuals. Furthermore, diabetic patients exhibit diminished cardiac recovery capacity, leading to poorer rehabilitation outcomes (Shah et al., 2010; Pérez-Cremades et al., 2023). In the absence of timely detection and intervention, DCM can result in severe clinical consequences. Given the increasing global prevalence of diabetes, DCM has emerged as a critical component of diabetes-related cardiovascular diseases and a major contributor to the elevated risk of heart failure and mortality among diabetic patients (Kobayashi and Liang, 2015; Ma et al., 2023). Therefore, identifying effective therapeutic targets for the prevention and management of DCM is crucial.
OS is recognized as a key pathological mechanism in DCM and has emerged as a central focus of research. OS results from an imbalance between excessive ROS production and inadequate antioxidant defense capacity (Ritchie and Abel, 2020). The overproduction of ROS is considered a key mechanism underlying diabetes-induced inflammation and cardiac remodeling (Giacco and Brownlee, 2010; Zhao et al., 2023). Deficiencies in the antioxidant defense system exacerbate OS during the later stages of DCM, further promoting cardiac insulin resistance, disease progression, and heart failure (Zhou and Tian, 2018). Numerous studies suggest that modulating OS signaling pathways and reducing ROS production and accumulation offer promising therapeutic strategies for DCM (Peng et al., 2022; Tang et al., 2022). Accumulated ROS not only directly damage cardiomyocytes but also induce structural and functional alterations in the heart through various signaling pathways. Notably, the NOX family, the only enzymes specifically dedicated to ROS generation, plays a crucial role in the pathogenesis of DCM. NOX family members are transmembrane proteins that catalyze the reduction of oxygen to generate ROS, such as superoxide and hydrogen peroxide, in association with NADPH oxidation (Ogboo et al., 2022; Nabeebaccus et al., 2023). Increased NOX activity leads to excessive ROS production, exacerbating OS and accelerating the onset and progression of DCM.
This review investigates the biological mechanisms of NOX, the pathways through which NOX-mediated OS contributes to cardiac pathological changes, and recent advances in NOX-targeted therapies for DCM. We offer a comprehensive analysis of NOX-related signaling pathways in DCM and explore the potential of targeting NOX-mediated OS as a novel therapeutic approach.
2 NOX: a primary driver of oxidative stress
The development of DCM is strongly associated with oxidative stress, with members of the NOX family acting as key drivers of this process. NOX enzymes generate ROS, which in turn induce inflammation, cellular damage, and cardiac remodeling in heart tissue, thereby accelerating the progression of DCM. NOX activity is markedly elevated in diabetic patients (Daiber et al., 2020; Vilas-Boas et al., 2021), making it a critical factor in cardiac injury and functional deterioration.
2.1 The discovery and fundamental functions of NOX enzymes
The classical nicotinamide adenine dinucleotide phosphate (NADPH) oxidase was first identified in neutrophils in 1933, when Baldridge and Gerrard observed a significant increase in oxygen consumption during bacterial phagocytosis (Cross and Segal, 2004). It was later correctly identified in 1964 by Rossi and Zatti as the key enzyme responsible for initiating the respiratory burst (Rossi and Zatti, 1964). Through extensive research on the different subtypes of NOX, scientists have systematically characterized their structure and function within cells. In 1986, NOX2 was successfully cloned, marking a significant milestone in this field. Over the subsequent decades, other members of the NOX family, such as NOX1, NOX3, and NOX4, were also cloned using advanced molecular cloning techniques. NOX is expressed in phagocytic cells such as neutrophils, eosinophils, monocytes, and macrophages, which are critical components of the innate immune system. NOX is a multi-protein complex that catalyzes the reduction of oxygen and NADPH to produce superoxide (Kleniewska et al., 2012). It comprises two membrane-bound subunits (gp91phox and p22phox), three cytosolic subunits (p47phox, p67phox, and p40phox), and a small G protein (Rac1 or Rac2). In resting phagocytes, NOX remains inactive but becomes activated upon interaction with pathogens and subsequent engulfment by the phagosome (Touyz et al., 2019; Vermot et al., 2021). NOX is crucial for the oxidative stress response in cells, where it generates ROS using NADPH. In parallel, cells depend on reduced glutathione (GSH) as the primary antioxidant to neutralize these ROS. NADPH serves not only as the source of ROS production by NOX but also contributes to the reduction of oxidized glutathione disulfide (GSSH) to GSH through the enzyme glutathione reductase (GR), thereby sustaining the cell’s antioxidant defense. This process enables cells to manage the ROS produced by NOX, preserve redox homeostasis, and avoid the accumulation of oxidative damage (Li et al., 2023; Su et al., 2023).
2.2 Members of the NOX family and their activation mechanisms
The NOX family represents the primary regulated source of ROS formation, comprising seven isoforms with diverse tissue distribution and activation mechanisms: NOX1, NOX2, NOX3, NOX4, NOX5, and the dual oxidases Duox1 and Duox2 (Pecchillo Cimmino et al., 2023). The activation mechanisms of NOX subtypes vary, yet all NOX family members share common structural features, including six conserved transmembrane domains, four conserved heme-binding histidines, an FAD-binding domain, and an NADPH-binding domain (Begum et al., 2022). NOX enzymes transfer electrons from NADPH to the heme group, and subsequently to molecular oxygen, ultimately generating superoxide (O2−), a critical process in cellular oxidative stress. The activation of NOX family members typically requires small GTPases, such as Rac1, and several cytosolic subunits (Ogboo et al., 2022). NOX1 activation involves p22phox, RAC1, p47phox, and/or NOXO1, along with NOXA1. NOX2 activation requires p22phox, RAC1 or RAC2, p47phox, p67phox, and p40phox. NOX4 activation requires p22phox, and its activity can be modulated by POLDIP2. The activation of NOX5 is primarily regulated by Ca2+. DUOX1 and DUOX2 require both DUOXA1 and DUOXA2, along with Ca2+, for activation, and neither is expressed in the cardiovascular system (Zhang et al., 2020).
Their subcellular localization varies, ranging from the plasma membrane to intracellular compartments and the nuclear membrane (Pecchillo Cimmino et al., 2023). Among these, NOX2 and NOX4 are highly expressed in cardiomyocytes, endothelial cells, and fibroblasts (Cadenas, 2018; Jiang et al., 2020; Visnagri et al., 2023). NOX2 is a membrane-bound enzyme activated by stimuli such as angiotensin II (Ang-II), endothelin-1 (ET-1), TNF-α, growth factors, cytokines, and mechanical stress. In contrast, NOX4, located on intracellular membranes, exhibits constitutive activity (Sag et al., 2014; Moris et al., 2017; Bode et al., 2023; Nabeebaccus et al., 2023; Wang et al., 2023). NOX3 is highly expressed in different regions of the inner ear, such as the vestibule, cochlear sensory epithelium, and spiral ganglion, which led to its designation as inner ear NOX (Wang et al., 2023). Duox1 and Duox2 are exclusively expressed in epithelial cells (Brandes et al., 2010).
2.3 NOX-induced oxidative stress and its contribution to the pathogenesis of cardiovascular diseases
NOX is the only enzyme whose primary function is the generation of ROS. Its expression and activity in the heart play crucial roles in both physiological and pathological conditions. The ROS produced by NOX can trigger endothelial nitric oxide synthase (eNOS) uncoupling and mitochondrial dysfunction, leading to persistent oxidative stress (OS) and the development of cardiovascular diseases (CVD) (Zhang et al., 2020). NOX has emerged as a key oxidase system contributing to oxidative stress in vascular diseases such as hypertension, aortic aneurysm, hypercholesterolemia, atherosclerosis, and diabetic vascular complications, as well as in cardiovascular diseases including ischemia-reperfusion (IR) injury, myocardial infarction (MI), heart failure, and arrhythmias. Among the NOX family, NOX1, NOX2, and NOX4 are highly expressed in cardiac tissue. Previous studies have demonstrated that inhibition of NOX2 alleviates the harmful effects of hyperglycemia on cells, and that inhibiting NOX2-induced oxidative stress may prevent DCM (Tang et al., 2019; Zhu et al., 2024). NOX4 has been shown to reduce myocardial interstitial fibrosis in diabetic mice by regulating the Akt/mTOR and NF-κB pathways, leading to improved cardiac function (Zhao et al., 2015). NOX1 has been implicated in several cardiovascular diseases, particularly atherosclerosis, hypertension, and ischemia/reperfusion injury (Zhang D. et al., 2022). Increasing experimental evidence suggests that genetic modification of NOX isoforms exerts specific effects on cardiovascular phenotypes in animal models, emphasizing the central role of NOX in CVD progression (Zhou L. et al., 2021; Qiu et al., 2022; Yang et al., 2023; Huang et al., 2024).
In the context of hyperglycemia, NOX is further activated through specific metabolic pathways. Elevated blood glucose increases intracellular glucose levels, resulting in the accumulation of diacylglycerol (DAG), an intermediate in triglyceride synthesis. DAG functions as a critical signaling molecule that activates protein kinase C (PKC) (Khan, 2021). Activated PKC, in turn, stimulates NOX, promoting ROS production (Jubaidi et al., 2022). These ROS exacerbate OS, contributing to cellular damage and the development of cardiovascular and other complications. This signaling pathway has gained attention for its role in regulating angiogenesis, mitigating OS by reducing ROS production, and inducing cell death (Volpe et al., 2018).
2.4 NOX-induced oxidative stress and its impact on cardiac progenitor and stem cells (CPCs)
OS plays a crucial role in the dysfunction of CPCs, particularly in the context of cardiovascular diseases such as DCM (Chen et al., 2018). Excessive production of ROS leads to CPC damage, resulting in impaired proliferation, restricted differentiation potential, and accelerated aging (Molinaro et al., 2022). CPCs are essential for myocardial regeneration. However, under pathological conditions like DCM, their functionality is significantly compromised, limiting cardiac repair and exacerbating heart failure progression. Consequently, modulating oxidative stress to restore CPC function represents a promising therapeutic strategy (Chen et al., 2018).
In this regard, antioxidants, such as resveratrol, or redox modulators have been shown to reduce ROS accumulation, significantly enhancing the proliferative and differentiative capacities of CPCs, thereby promoting cardiac repair (Dudek et al., 2021). This approach not only decelerates the progression of heart failure but also opens new avenues for the clinical management of diabetes and other metabolic heart diseases.
Furthermore, NOX2 and NOX4 are critical in regulating CPC function. Research indicates that NOX2 generates ROS, which promotes angiogenesis and supports cardiac repair. ROS produced by NOX2 activate key factors, including hypoxia-inducible factor (HIF-1α) and vascular endothelial growth factor (VEGF), thereby enhancing angiogenesis and improving the cardiac repair microenvironment, which indirectly supports CPC-mediated repair (Urao and Ushio-Fukai, 2013). Additionally, NOX4 plays a pivotal role in CPC differentiation by modulating ROS levels, thus influencing their differentiation status (Nadworny et al., 2013).
These findings suggest that NOX2 and NOX4 are not only crucial for regulating CPC proliferation and differentiation but also may indirectly facilitate cardiac repair by influencing the cellular microenvironment. Therefore, targeting the OS pathways of NOX provides valuable insights into therapeutic strategies for restoring CPC function and promoting cardiac repair.
2.5 The potential role of NOX in the early diagnosis of DCM
In the early stages of DCM, NOX activity is markedly elevated, making it a potential biological marker for early diagnosis. NOX generates ROS, resulting in oxidative damage and inflammation in cardiomyocytes. These changes can be detected through blood tests for NOX-derived products, such as hydrogen peroxide and superoxide anion, or by directly measuring NOX expression levels to enable early identification (Yu et al., 2007; Nie et al., 2023). Furthermore, as diabetes progresses, the elevation in NOX activity is closely correlated with the severity of cardiac damage (Faria and Persaud, 2017; Maqbool et al., 2020), allowing for more accurate detection of early cardiac injury. Combining traditional cardiac biomarkers (such as BNP and cTnI) with NOX activity measurements can significantly improve the sensitivity and specificity of early diagnosis. By using multiple biomarkers, it is possible to more accurately assess myocardial injury in diabetic patients and detect early changes in heart function.
However, the early diagnosis still faces significant challenges due to the lack of specific biomarkers, subtle early symptoms, insufficient standardized detection methods, and difficulties in detecting early changes. Moreover, the absence of unified screening guidelines further complicates the situation. With advancements in technology and ongoing research, NOX and other biomarkers are expected to provide more precise and efficient methods for the early diagnosis of DCM in the future.
3 The involvement of NOX-regulated signaling pathways in the development and progression of DCM
As diabetes progresses, excessive activation of NOX induces oxidative stress in cardiac cells, which affects cardiac function and structure through multiple signaling pathways. The activation of these pathways not only promotes inflammation but also exacerbates apoptosis, fibrosis, and cardiac remodeling in cardiomyocytes, ultimately contributing to heart failure. Current research suggests that NOX-regulated signaling pathways play a multifaceted role in oxidative stress in DCM and are crucial for the progression of the disease.
3.1 Interaction between NOX and NF-κB signaling pathways
In DCM, NOX activation leads to the generation of ROS, which activates the NF-κB signaling pathway, crucial for regulating immune responses and inflammation. NOX-induced ROS enhances NF-κB activity (Zhang D. et al., 2022), and also activates signaling molecules like IKK and IκB through oxidative stress accumulation. This translocates NF-κB to the nucleus, promoting the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, contributing to inflammation, cardiomyocyte damage, and dysfunction.
For example, NOX1 induces ROS accumulation and activates the TLR2/NF-κB pathway, promoting myocardial fibrosis in DCM (Zhang D. et al., 2022). Under hyperglycemic conditions, NOX-generated ROS enhances the expression of these pro-inflammatory cytokines, triggering inflammatory reactions that lead to cardiac injury and functional decline (Liang et al., 2018). Similarly, NOX4 activates NF-κB signaling through ROS, further exacerbating inflammation and fibrosis in DCM (Fan et al., 2018). Thus, NOX activation of the NF-κB pathway accelerates the progression of DCM, highlighting the potential for NOX-targeted therapies.
3.2 Interaction between NOX and TGF-β/Smad signaling pathway
The TGF-β/Smad signaling pathway plays a crucial role in the development of various cardiovascular diseases, particularly in cardiac fibrosis. NOX4 is key in DCM, where it activates the TGF-β/Smad signaling pathway through ROS generation, promoting the proliferation of cardiac fibroblasts and collagen deposition. ROS activate downstream Smad proteins by interacting with the TGF-β receptor, which leads to fibroblast transformation and worsens the fibrosis process.
For instance, NOX generates ROS, which activates the TGF-β/Smad signaling pathway and promotes myocardial fibrosis in DCM. The accumulation of ROS increases the expression of TGF-β, thereby further activating the Smad2/3 pathway, promoting fibroblast proliferation and collagen deposition, which exacerbates cardiac fibrosis and functional impairment (Fan et al., 2018). NOX activates the TGF-β/Smad pathway through ROS generation, while BCP (a compound) inhibits TGF-β signaling, indirectly reducing NOX-induced ROS production, thereby alleviating cardiac fibrosis and endothelial-mesenchymal transition (Hashiesh et al., 2024). In diabetic patients, cardiac fibrosis is often closely linked to heart failure, loss of contractile function, and decline in cardiac performance. Therefore, NOX-induced activation of the TGF-β/Smad pathway not only promotes fibrosis but also accelerates cardiac remodeling, leading to a vicious cycle of structural damage.
3.3 Interaction between NOX and MAPK signaling pathway
The MAPK signaling pathway, including ERK, JNK, and p38MAPK, is a key regulator of cell proliferation, stress response, and apoptosis (Zhang Q. et al., 2022). In DCM, NOX activates members of the MAPK family through ROS generation, triggering cardiomyocyte proliferation, apoptosis, and cardiac remodeling. ROS activate ERK1/2, JNK, and p38MAPK by altering the intracellular redox environment, and these MAPK family members further regulate cell growth, differentiation, and death (Cui et al., 2022).
In the hyperglycemic environment of diabetes, NOX enhances MAPK activity, leading to a stress response in cardiomyocytes, which induces cytokine secretion and results in inflammation in cardiac tissue. NOX5 generates ROS, which activates the MAPK pathway, particularly the p38, JNK, and ERK1/2 pathways, in DCM. Studies show that NOX5 expression enhances the phosphorylation of these redox-sensitive MAPK pathways, further promoting myocardial hypertrophy, fibrosis, and cardiac dysfunction in DCM, worsening the pathological changes in the heart (Zhao et al., 2020). The interaction between ROS and the MAPK signaling pathway in DCM remains an area for further research. However, current studies have confirmed that NOX, through ROS, regulates the MAPK pathway and plays a pivotal role in cardiac remodeling.
3.4 Interaction between NOX and PI3K/Akt signaling pathway
In the pathogenesis of DCM, the PI3K/Akt signaling pathway is a key regulator of cell survival and anti-apoptotic responses, and its activity is strongly affected by oxidative stress (Ren et al., 2020). NOX influences PI3K/Akt pathway activation through ROS generation, regulating cardiomyocyte survival and anti-apoptotic responses. On one hand, moderate ROS levels can activate the PI3K/Akt pathway, promoting cardiomyocyte survival. On the other hand, under high glucose conditions, NOX upregulation induces ROS overload, which may lead to abnormal activation or depletion of the PI3K/Akt pathway, thereby exacerbating cell apoptosis, myocardial injury, and structural remodeling (Liu and Zhang, 2015). Thus, the interaction between NOX and the PI3K/Akt signaling pathway plays a critical role in cardiac protection and may influence the progression of DCM by regulating the balance of this pathway.
NOX activates several important signaling pathways, such as NF-κB, TGF-β, MAPK, and PI3K/Akt, through ROS generation. These pathways play crucial roles in processes like cardiomyocyte proliferation, apoptosis, and fibrosis. In the following section, we will explore in detail the specific consequences of these signaling pathways in DCM and how they exacerbate cardiac structural and functional changes through inflammatory responses, myocardial fibrosis, and other pathological reactions.
4 Downstream effects of OS and their relationship with DCM
Sustained OS in the heart, primarily driven by hyperglycemia, is a well-established contributor to the structural and functional changes observed in DCM. This maladaptive process involves pathological responses such as inflammation and cardiomyocyte apoptosis (Jubaidi et al., 2021). In the diabetic state, excessive production of ROS mediated by NOX serves as a major source of OS. The persistent accumulation of ROS not only directly induces oxidative damage to cardiomyocytes but also activates multiple downstream signaling pathways, resulting in key pathological responses, including inflammation, mitochondrial dysfunction, and myocardial fibrosis. These downstream effects are interconnected and collectively accelerate myocardial dysfunction and structural remodeling (Fiorentino et al., 2013; Zhang et al., 2023).
4.1 Inflammatory response
Inflammation is an adaptive mechanism that helps restore cellular homeostasis under stress (Al-Rasheed et al., 2017; Zhang et al., 2023). However, when stress persists, as in diabetes, this adaptive response becomes maladaptive, resulting in harmful consequences (Jubaidi et al., 2021). Excessive ROS generated by NOX activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and enhance the advanced glycation end-products/receptor for advanced glycation end-products (AGE/RAGE) axis. NF-κB, a redox-sensitive protein complex, plays a pivotal role in inflammation (Shah and Brownlee, 2016). Upon activation, NF-κB promotes the transcription and release of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), amplifying pro-inflammatory cytokine production and exacerbating myocardial injury (Zhuang et al., 2023). Consequently, NF-κB serves as a key initiator of inflammation in DCM (Figure 1).
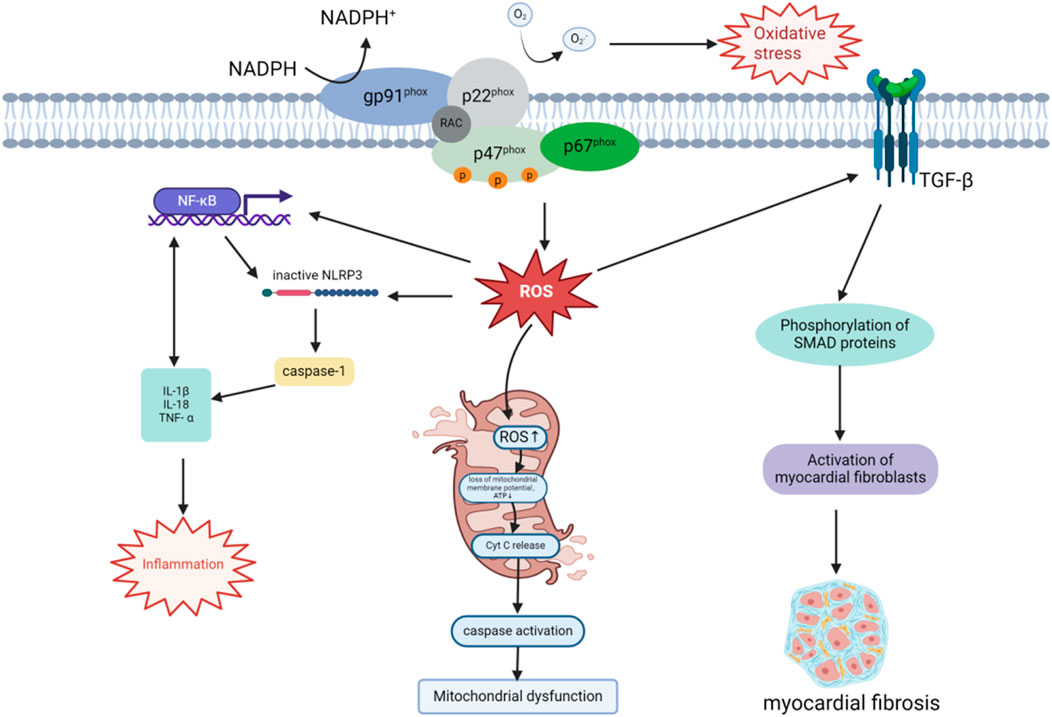
Figure 1. Mechanisms of NOX-derived ROS in DCM progression. The NOX enzyme complex, anchored to the plasma membrane, generates ROS by transferring electrons from NADPH, thereby inducing OS. ROS activate the NF-κB pathway, promote NLRP3 inflammasome assembly, and activate caspase-1, leading to the secretion of pro-inflammatory cytokines such as IL-1β, IL-18, and TNF-α. NLRP3 inflammasome activation further enhances NF-κB signaling through IL-1β release, establishing a positive feedback loop that exacerbates local and systemic inflammation. Additionally, ROS accumulation disrupts mitochondrial membrane potential and ATP synthesis, triggering cytochrome C release and caspase activation, which compromise mitochondrial function and dynamics. ROS also amplify the TGF-β/Smad signaling pathway by enhancing TGF-β receptor activation and downstream Smad protein phosphorylation. This promotes cardiac fibroblast activation and extracellular matrix production, including collagen, driving myocardial remodeling and fibrosis. These interconnected mechanisms collectively contribute to the pathogenesis of DCM.
NOX-induced OS activates the NLRP3 inflammasome through the excessive production of ROS. The NLRP3 inflammasome is composed of NLRP3, the apoptosis-associated speck-like protein containing a CARD domain (ASC), and pro-caspase-1 (Ding et al., 2019). It plays a critical role in regulating the inflammatory response by activating caspase-1, which facilitates the maturation and secretion of pro-inflammatory cytokines such as IL-1β and IL-18. This process intensifies both local and systemic inflammation (Abais et al., 2015; Yang et al., 2019; Zhang L. et al., 2022; Zhao et al., 2024), contributing to the development and progression of DCM (Figure 1). NF-κB enhances NLRP3 inflammasome expression and amplifies inflammation by upregulating the transcription of the NLRP3 gene. Conversely, NLRP3 inflammasome activation increases NF-κB signaling by promoting the production of cytokines such as IL-1β, thereby establishing a positive feedback loop. These interconnected mechanisms sustain chronic inflammation and accelerate the progression of DCM (Luo et al., 2014; Peng et al., 2022).
4.2 Mitochondrial dysfunction
NOX generates excessive ROS, initiating OS within cells and leading to mitochondrial dysfunction (Vásquez-Trincado et al., 2016). NOX enzymes transfer electrons to oxygen molecules via NADPH, producing ROS such as superoxide anion (O2−), which directly damages proteins and lipids, impairing mitochondrial bioenergetic function. This oxidative damage extends to mitochondrial DNA, resulting in promoter inactivation, downregulation of mitochondrial gene expression, and decreased mitochondrial membrane stability, ultimately disrupting ATP synthesis (Singh et al., 2019). Insufficient mitochondrial energy supply not only impairs cellular function but also exacerbates intracellular redox imbalance, further triggering cell death. ROS accumulation also leads to the loss of mitochondrial membrane potential, a key hallmark of mitochondrial dysfunction, and promotes apoptosis by activating intracellular apoptotic pathways such as cytochrome c release and caspase activation (Rehfeldt et al., 2021). These processes exacerbate myocardial cell death. Chronic OS disrupts mitochondrial fusion and fission, impairing mitochondrial dynamics and destabilizing the mitochondrial network (Bhatti et al., 2017). This dysregulation is particularly pronounced in dilated cardiomyopathy DCM. Prolonged ROS-induced mitochondrial damage ultimately results in cardiac dysfunction, characterized by myocardial hypertrophy, fibrosis, and loss of contractile function, which are hallmark pathological features of DCM (Figure 1).
4.3 Myocardial fibrosis
Myocardial fibrosis is a hallmark of cardiac remodeling in DCM (Sung et al., 2015). It occurs alongside hypertrophy as part of the cardiac remodeling process, aiming to preserve the structural integrity of the heart. Fibrosis involves the deposition of fibrotic components, such as collagen I and collagen III, within the extracellular matrix (ECM) to replace dead myocardial cells. However, the stiff nature of collagen tissue reduces the heart’s contractility and flexibility, impairing cardiac function (Frangogiannis, 2021). OS, caused by an imbalance between antioxidant defenses and ROS production, is a central mechanism in DCM and a key regulator of myocardial fibrosis (Zhao et al., 2023). For instance, ROS generated by NOX4 play a pivotal role in promoting myocardial fibrosis through activation of the TGF-β/Smad signaling pathway, which induces excessive collagen deposition and cardiac sclerosis in DCM (Figure 1). In one study, Li and colleagues experimentally demonstrated that NOX4 is a major driver of myocardial OS and fibrosis under diabetic conditions. Diabetes was shown to significantly upregulate NOX4 expression in myocardial tissue, resulting in excessive ROS production. This ROS increase activated the TGF-β/Smad signaling pathway, stimulating cardiac fibroblast activation and promoting collagen deposition, ultimately leading to myocardial fibrosis (Jiang et al., 2014; Li et al., 2019). Furthermore, studies using a prediabetic metabolic syndrome rat model demonstrated that inhibiting OS can effectively reduce myocardial fibrosis (Kusaka et al., 2016).
5 Targeting NOX in the treatment of DCM: experimental studies and clinical applications
The downstream pathological mechanisms triggered by OS, including inflammation, mitochondrial dysfunction, and fibrosis (Peng et al., 2022), form a complex network driving the progression of DCM. These mechanisms interact synergistically, progressively impairing myocardial cell function and compromising the structural integrity of the heart. Importantly, OS not only serves as the central driving force behind these mechanisms but also continuously amplifies them. For instance, the inflammatory response stimulates ROS generation via the release of pro-inflammatory factors (Shah and Brownlee, 2016), while mitochondrial dysfunction exacerbates OS through a feedback loop of mitochondrial ROS (Ren et al., 2010; Singh et al., 2019). Additionally, fibrotic tissue stiffening and collagen deposition accelerate myocardial remodeling (Zhao et al., 2023). This intricate pathological network suggests that effective therapeutic intervention in DCM must target upstream mechanisms to block the source of OS. In this regard, NOX has emerged as a central focus for targeted therapy. Compared to xanthine oxidase and mitochondrial respiration, NOX has been identified as a major source of ROS in cardiac tissue (MacCarthy et al., 2001). Excessive activation of NOX not only initiates OS but also regulates multiple pathological signaling pathways through its various isoforms, such as NOX2 and NOX4. In the diabetic state, NOX-driven overproduction of ROS has been confirmed as a critical trigger for inflammation, mitochondrial dysfunction, and fibrosis. Thus, targeting NOX holds significant therapeutic potential, not only by directly reducing ROS generation but also by interrupting the upstream cascade of pathological mechanisms, offering a novel approach to alleviating DCM.
5.1 Dapagliflozin
Dapagliflozin is a highly effective, reversible, and selective sodium-glucose co-transporter-2 (SGLT2) inhibitor widely used in the treatment of type 2 diabetes (Dhillon, 2019). While its primary action is in the kidneys, clinical data highlight the cardiovascular benefits of SGLT2 inhibitors, emphasizing their potential to prevent cardiovascular events and heart failure (Arow et al., 2020). Clinical studies have shown that patients receiving dapagliflozin therapy experience a reduced risk of heart failure exacerbation and cardiovascular-related mortality (McMurray et al., 2019). Animal studies further support these findings. In BTBR mice, dapagliflozin slows the progression of DCM, inhibits NLRP3 inflammasome activation and fibrosis, and promotes activation of the mTORC2 pathway in cardiac tissue (Ye et al., 2017; Chen et al., 2020). Additionally, dapagliflozin exerts cardioprotective effects in diabetic mice under angiotensin II stress by reducing ROS levels and calcium transport activity in membrane channels, thereby decreasing OS and providing antioxidant protection to myocardial cells (Arow et al., 2020).
In a study by Xing et al., experimental results demonstrated that dapagliflozin protects myocardial cells from hyperglycemia-induced damage by inhibiting NOX-mediated OS (Xing et al., 2021). Chen et al. further confirmed that dapagliflozin reduces the levels of NOX subunits p67phox and NOX4, preventing ROS accumulation (Chen et al., 2019). Specifically, dapagliflozin treatment decreased the expression of gp91phox and p22phox in the membrane NOX complex and inhibited the translocation of the p67^phox subunit to the membrane. Although superoxide dismutase (SOD) levels were reduced following dapagliflozin treatment, the production of superoxide anions was significantly suppressed (Xing et al., 2021). These findings suggest that dapagliflozin inhibits the recruitment of cytosolic p67phox and reduces its binding to p22phox, suppressing activation of the gp91phox isoform and correcting the imbalance between oxidants and antioxidants, thereby improving myocardial OS (Figure 2). While further exploration of the underlying mechanisms is needed, the existing evidence indicates that dapagliflozin holds promise as a targeted NOX therapy for DCM.
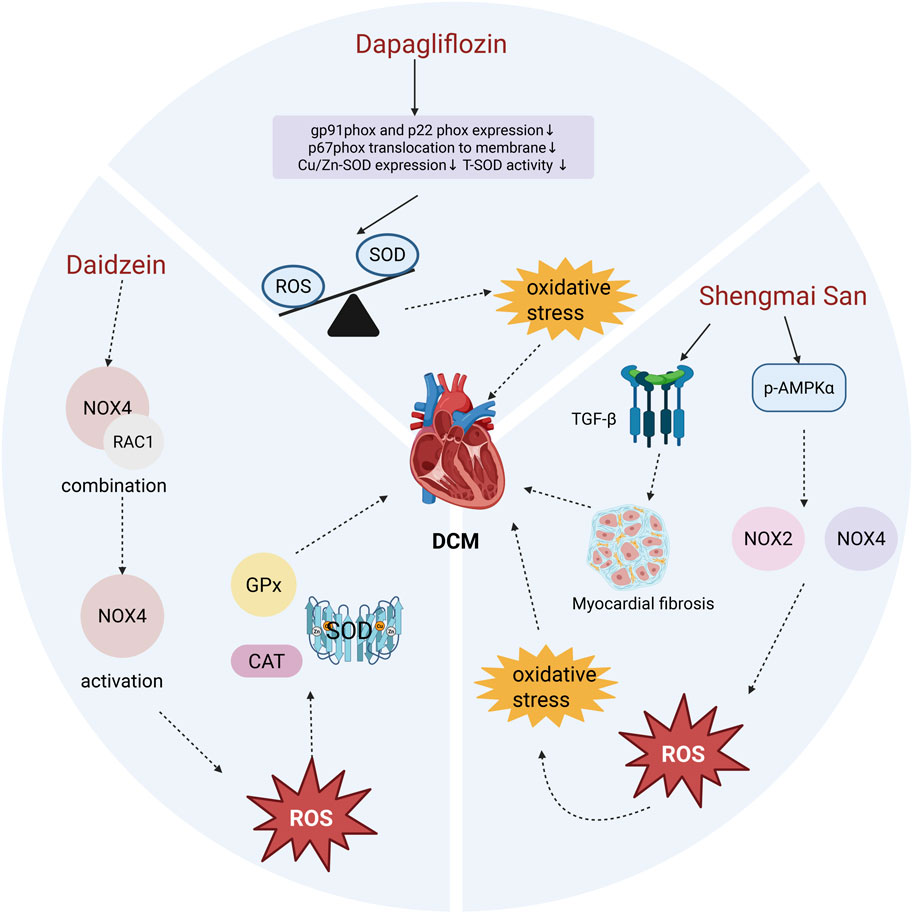
Figure 2. Effects of Dapagliflozin, Daidzein, and Shengmai San on Myocardial Oxidative Stress in DCM. Dapagliflozin treatment significantly reduced the compensatory increase in T-SOD activity and the expression of Cu/Zn-SOD, without altering the levels of Mn-SOD, GPx, or CAT. Although overall SOD levels decreased following Dapagliflozin treatment, it effectively suppressed the production of superoxide anions, restoring the balance between oxidants and antioxidants and improving myocardial OS. Daidzein prevents the interaction between RAC1 and NOX4, thereby inhibiting NOX4 activation. This reduction in NOX4 activity lowers ROS production, maintains antioxidant enzyme activity, and alleviates DCM. Shengmai San enhances the phosphorylation of AMPKα (p-AMPKα), which inhibits the expression and activity of NOX2 and NOX4. This leads to reduced ROS generation and mitigates myocardial OS and fibrosis.
The pharmacological profile of dapagliflozin indicates that it is rapidly absorbed from the gastrointestinal tract following oral administration, typically reaching peak plasma concentrations within 2 h under fasting conditions. Its bioavailability is approximately 78%, meaning the majority of the drug enters the bloodstream after oral administration (Anderson, 2014). The average steady-state volume of distribution is 118 L, with approximately 91% of the drug bound to plasma proteins. Dapagliflozin is primarily excreted via urine (approximately 75%), mainly as the parent compound and metabolites, with the remaining 21% eliminated through feces. After a single 10 mg dose in healthy subjects, the mean terminal elimination half-life of dapagliflozin is approximately 12.9 h (Dhillon, 2019). In patients with type 2 diabetes, dapagliflozin is generally well-tolerated over extended periods (Jabbour et al., 2018). These pharmacokinetic characteristics make it effective in maintaining therapeutic effects and convenient for daily management. While its safety profile is generally favorable, adverse effects and contraindications remain, including urinary tract infections, hypoglycemia, hypotension, and diabetic ketoacidosis (Plosker, 2014). Dapagliflozin has demonstrated significant cardiovascular protection in patients with heart failure and diabetes. However, the specific mechanisms and long-term effects of this action in diverse patient populations require further investigation.
5.2 Daidzein
Daidzein is a naturally occurring plant-derived estrogen, classified as a non-steroidal estrogen, with diverse pharmacological properties, including anti-hemolytic, antioxidant, and anti-inflammatory effects (Dwiecki et al., 2009; Zhou D. D. et al., 2021). Ankit P. Laddha and colleagues investigated the effects of daidzein in a rat model of DCM and found that daidzein treatment decreased oxidative damage to cardiomyocytes by maintaining AMPK and SIRT1 levels (Laddha and Kulkarni, 2021). Furthermore, daidzein treatment prevented necrotic damage in cardiac tissue and inhibited myocardial fibrosis. Notably, compared to normal control animals, the diabetic control group exhibited significantly increased expression of NOX4 and RAC1, which was markedly reduced following daidzein treatment (Laddha and Kulkarni, 2021).
NOX4 consists of transmembrane and cytosolic subunits. Under hyperglycemic conditions, the cytosolic subunit becomes phosphorylated and associates with the membrane subunit to form a functional oxidase complex. This complex transfers electrons from NADPH to oxygen, generating superoxide radicals that promote OS and cellular damage. Activation of the NOX4 complex also depends on the small GTPase RAC1, which exists in the cytoplasm as a dimer with Rho GDP-dissociation inhibitor (Rho-GDI). When RAC1 binds to GTP, it associates with the NOX4 membrane subunit, further activating the complex and generating ROS (Gorin et al., 2003; Acevedo and González-Billault, 2018). Studies have demonstrated that flavonoids inhibit RAC1 expression and prevent its binding to the NOX4 membrane subunit, thereby preventing NOX4 activation and alleviating OS. This inhibition also stabilizes antioxidant enzyme levels, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) (Figure 2). Thus, daidzein alleviates the progression of DCM primarily by inhibiting NOX4-induced OS in cardiac tissue, making it a promising therapeutic approach for DCM.
Daidzein shows a small plasma peak approximately 1 h after intake, indicating its initial absorption in the small intestine. A larger peak appears 5–8 h post-ingestion, reflecting the recirculation of conjugates and absorption in the colon. Plasma concentration reaches its maximum around 7 h after ingestion. Nearly all of the daidzein is rapidly absorbed and metabolized, with minimal excretion in feces and urine, although up to 30% of the ingested daidzein can be recovered in urine (Elksnis et al., 2023). In terms of bioavailability, studies have shown that daidzein in its glucoside form has higher bioavailability compared to its aglycone form (Rüfer et al., 2008). Additionally, insoluble fibers such as inulin have been found to enhance the absorption of soybean isoflavones (Piazza et al., 2007). These findings provide insights into optimizing therapeutic applications of daidzein. However, further clinical trials are needed to evaluate potential adverse effects and ensure safety. Common side effects include gastrointestinal discomfort, sore throat, weight gain, rash, and breast tenderness. In rare cases, vaginal bleeding has been reported 2–3 days after intake during the third year post-menopause (Akhlaghipour et al., 2024). These side effects underscore the importance of careful consideration in the clinical use of daidzein, particularly for specific patient populations.
5.3 Shengmai San
Shengmai San (SMS) is a traditional Chinese medicine formula composed of Panax ginseng (Araliaceae, promotes Qi and disperses stagnation), Ophiopogon japonicus (Liliaceae, nourishes Yin and generates fluids), and Schisandra chinensis (Schisandraceae, tonifies Qi) (Ma et al., 2016; Ouyang et al., 2022; Wang et al., 2022). Clinically, SMS is considered a typical formula for tonifying Qi and nourishing Yin, traditionally used to treat ischemic diseases and diabetes, primarily by targeting OS and inflammation (Mo et al., 2015). Recent evidence suggests that SMS has significant protective effects on the heart and vasculature. Studies by Zhao et al. demonstrated that SMS alleviates cardiac hypertrophy and fibrosis through the TGF-β-dependent pathway (Zhao et al., 2016) and exhibits anti-myocardial fibrosis effects in high-fat diet and streptozotocin (STZ)-induced rat models (Ni et al., 2011). Additionally, SMS possesses antioxidant and anti-inflammatory properties and acts as a regulator of lipid metabolism (Li et al., 2013). SMS also mitigates myocardial oxidative damage by activating AMPKα and inhibiting NOX signaling, showing therapeutic potential in treating DCM (Lu et al., 2021).
As a traditional herbal compound, SMS intervenes in NOX-mediated OS through multiple pathways. It reverses the elevation of cardiac NOX2 and NOX4 protein levels while inhibiting the translocation of p47phox and p67phox to the cell membrane. Moreover, ginsenoside Rg1, an active component of Panax ginseng, has been shown to downregulate NOX2 and reduce ROS production in hippocampal neurons treated with H2O2 (Xu et al., 2019). Furthermore, AMPK plays a pivotal antioxidant role in the cardiovascular system by inhibiting NOX expression and activity (Li et al., 2020). Experimental results indicate that SMS significantly enhances p-AMPKα protein levels in diabetic hearts (Tian et al., 2018; Lu et al., 2021) (Figure 2). In conclusion, SMS enhances overall antioxidant capacity, effectively alleviates OS damage in DCM, and demonstrates its potential as a promising therapeutic strategy for this condition.
With the expansion of clinical applications, SMS has been developed into various formulations, including Shengmai oral liquid, Shengmai capsules, Shengmai granules, Shengmai injection, and Dengzhan Shengmai capsules (Ouyang et al., 2022). The Schisandra lignans in Shengmai granules are rapidly absorbed and cleared in both volunteers and mice, with an absorption half-life of 0.03–0.04 h and a clearance half-life of 0.86–0.88 h. Studies on SMI revealed that 11 components, including ginsenosides and Schisandra lignans, are rapidly distributed to various tissues in rat serum. Schisandra lignans quickly accumulate in tissues, while Ophiopogon saponin D is cleared within 4 h. Ginsenosides Rg1, Re, Rf, and Rg2 are excreted within 8 h, whereas Rb1, Rd, and Rc remain at higher concentrations for up to 96 h (Ouyang et al., 2022). In China, Panax ginseng, Ophiopogon japonicus, and Schisandra chinensis have long been used as raw materials for health products, with well-established safety profiles. Except for Shengmai injection, there have been no reports of clinical toxicity or side effects associated with the oral Shengmai San compound. However, careful attention should be given to dosage and potential interactions with other medications.
A detailed discussion of the pharmacological properties of these three drugs (Dapagliflozin, Daidzein, and Shengmai San) is provided above. Additional drugs targeting NOX to alleviate oxidative stress and aid in the treatment of DCM are summarized in Table 1.
Most existing treatment strategies regulate NOX activity through indirect pathways, which can somewhat alleviate the pathological manifestations of DCM. However, due to the limited research on the direct inhibition of NOX, it remains uncertain whether NOX is a core therapeutic target in DCM. Therefore, future research should focus on investigating and validating the direct effects of NOX inhibitors in DCM treatment, and determine whether NOX can serve as a more targeted therapeutic target.
Previous studies have demonstrated that direct NOX inhibitors, such as Apocynin and DPI, can significantly reduce the pathological damage and improve cardiac function in animal models of DCM (Privratsky et al., 2003; Li et al., 2010). Compounds such as Apocynin and DPI are widely used as research tools to inhibit NADPH oxidase activity. These findings provide substantial evidence supporting the role of NOX as a potential target for DCM treatment, suggesting that NOX may play a critical role in the improvement of diabetic cardiomyopathy. Future studies should focus on further evaluating the clinical efficacy of direct NOX inhibition and compare these results with existing therapeutic strategies to more definitively assess the potential of NOX in DCM treatment.
Therefore, the potential and application prospects of NOX as a therapeutic target for DCM clearly warrant further investigation. Direct research targeting NOX could clarify its unique role in DCM treatment, thus enhancing treatment precision and efficacy, and advancing the clinical application of NOX-targeted therapeutic strategies.
6 Outlook and conclusion
DCM is one of the most common and severe cardiovascular complications of diabetes. With the increasing number of diabetic patients, DCM has become a leading cause of heart failure and death. Its development is closely associated with chronic oxidative stress, where the overproduction of ROS and the imbalance of the antioxidant defense system are key contributors to the progression of DCM. In recent years, the NOX family, as a primary source of ROS, has been identified as playing a pivotal role in the pathogenesis of DCM. Overactivation of NOX not only directly damages cardiomyocytes but also contributes to inflammation, myocardial fibrosis, and apoptosis through multiple signaling pathways, further exacerbating structural and functional changes in the heart.
Current research suggests that NOX-targeted therapies hold significant promise for reducing oxidative stress and improving heart function. In particular, targeting specific NOX isoforms may provide more precise drug interventions for treating diabetic cardiomyopathy. However, despite preliminary studies showing the efficacy of NOX inhibitors in animal models, translating these findings into clinically applicable therapies remains a significant challenge. Future research should focus on the selective targeting of NOX isoforms, the selectivity of drugs, and their safety profiles to develop NOX inhibitors with clinical potential, particularly for early diagnosis and intervention in diabetic cardiomyopathy.
Moreover, with the advancement of big data, genomics, and proteomics technologies, personalized treatment will become an important direction in managing diabetic cardiomyopathy. By precisely identifying individual variations in NOX activity, ROS levels, and associated signaling pathways, more personalized treatment plans can be developed, maximizing therapeutic outcomes while minimizing side effects.
Although traditional antioxidants play a role in reducing oxidative stress, their clinical effectiveness is limited, often accompanied by side effects. Therefore, future research should prioritize the development of novel antioxidants, particularly those that specifically regulate NOX activity. Additionally, combining NOX inhibitors with existing anti-inflammatory and anti-fibrotic drugs could significantly enhance treatment efficacy for diabetic cardiomyopathy.
NOX represents a critical target for therapeutic intervention in diabetic cardiomyopathy, offering substantial clinical research value. By deepening our understanding of NOX-mediated oxidative stress and its role in DCM, more precise and effective therapeutic strategies are expected to be developed, providing new solutions for the early diagnosis, intervention, and treatment of diabetic cardiomyopathy.
Author contributions
ZY: Conceptualization, Validation, Visualization, Writing – original draft. SW: Writing – original draft, Writing – review and editing. ZW: Validation, Writing – original draft. BH: Supervision, Writing – review and editing. JG: Funding acquisition, Resources, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (grant no. 82300526).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abais, J. M., Xia, M., Zhang, Y., Boini, K. M., and Li, P. L. (2015). Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal 22 (13), 1111–1129. doi:10.1089/ars.2014.5994
Acevedo, A., and González-Billault, C. (2018). Crosstalk between Rac1-mediated actin regulation and ROS production. Free Radic. Biol. Med. 116, 101–113. doi:10.1016/j.freeradbiomed.2018.01.008
Akhlaghipour, I., Nasimi Shad, A., Askari, V. R., and Baradaran Rahimi, V. (2024). Daidzin and its de-glycosylated constituent daidzein as a potential therapeutic for cardiovascular diseases: a review from bench to bed. Phytother. Res. 38 (8), 3973–3985. doi:10.1002/ptr.8261
Al-Rasheed, N. M., Al-Rasheed, N. M., Hasan, I. H., Al-Amin, M. A., Al-Ajmi, H. N., Mohamad, R. A., et al. (2017). Simvastatin ameliorates diabetic cardiomyopathy by attenuating oxidative stress and inflammation in rats. Oxid. Med. Cell Longev. 2017, 1092015. doi:10.1155/2017/1092015
Anderson, S. L. (2014). Dapagliflozin efficacy and safety: a perspective review. Ther. Adv. Drug Saf. 5 (6), 242–254. doi:10.1177/2042098614551938
Arow, M., Waldman, M., Yadin, D., Nudelman, V., Shainberg, A., Abraham, N. G., et al. (2020). Sodium-glucose cotransporter 2 inhibitor dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 19 (1), 7. doi:10.1186/s12933-019-0980-4
Begum, R., Thota, S., Abdulkadir, A., Kaur, G., Bagam, P., and Batra, S. (2022). NADPH oxidase family proteins: signaling dynamics to disease management. Cell Mol. Immunol. 19 (6), 660–686. doi:10.1038/s41423-022-00858-1
Bhatti, J. S., Bhatti, G. K., and Reddy, P. H. (2017). Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 1863 (5), 1066–1077. doi:10.1016/j.bbadis.2016.11.010
Bode, K., Hauri-Hohl, M., Jaquet, V., and Weyd, H. (2023). Unlocking the power of NOX2: a comprehensive review on its role in immune regulation. Redox Biol. 64, 102795. doi:10.1016/j.redox.2023.102795
Brandes, R. P., Weissmann, N., and Schröder, K. (2010). NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 49 (5), 687–706. doi:10.1016/j.freeradbiomed.2010.04.030
Cadenas, S. (2018). ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 117, 76–89. doi:10.1016/j.freeradbiomed.2018.01.024
Chen, H., Tran, D., Yang, H. C., Nylander, S., Birnbaum, Y., and Ye, Y. (2020). Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: an AMPK-mTOR interplay. Cardiovasc Drugs Ther. 34 (4), 443–461. doi:10.1007/s10557-020-06978-y
Chen, W., Ju, J., Yang, Y., Wang, H., Chen, W., Zhao, X., et al. (2018). Astragalus polysaccharides protect cardiac stem and progenitor cells by the inhibition of oxidative stress-mediated apoptosis in diabetic hearts. Drug Des. Devel Ther. 12, 943–954. doi:10.2147/dddt.S155686
Chen, Y. Y., Wu, T. T., Ho, C. Y., Yeh, T. C., Sun, G. C., Kung, Y. H., et al. (2019). Dapagliflozin prevents NOX- and SGLT2-Dependent oxidative stress in lens cells exposed to fructose-induced diabetes mellitus. Int. J. Mol. Sci. 20 (18), 4357. doi:10.3390/ijms20184357
Cross, A. R., and Segal, A. W. (2004). The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta 1657 (1), 1–22. doi:10.1016/j.bbabio.2004.03.008
Cui, B., Wang, Y., Jin, J., Yang, Z., Guo, R., Li, X., et al. (2022). Resveratrol treats UVB-induced photoaging by Anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxid. Med. Cell Longev. 2022, 6037303. doi:10.1155/2022/6037303
Daiber, A., Steven, S., Vujacic-Mirski, K., Kalinovic, S., Oelze, M., Di Lisa, F., et al. (2020). Regulation of vascular function and inflammation via© cross talk of reactive oxygen and nitrogen species from mitochondria or NADPH oxidase-implications for diabetes progression. Int. J. Mol. Sci. 21 (10), 3405. doi:10.3390/ijms21103405
Dhillon, S. (2019). Correction to: dapagliflozin: a review in type 2 diabetes. Drugs 79 (18), 2013. doi:10.1007/s40265-019-01239-1
Ding, S., Xu, S., Ma, Y., Liu, G., Jang, H., and Fang, J. (2019). Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules 9 (12), 850. doi:10.3390/biom9120850
Dudek, J., Kutschka, I., and Maack, C. (2021). Metabolic and redox regulation of cardiovascular stem cell biology and pathology. Antioxid. Redox Signal 35 (3), 163–181. doi:10.1089/ars.2020.8201
Dwiecki, K., Neunert, G., Polewski, P., and Polewski, K. (2009). Antioxidant activity of daidzein, a natural antioxidant, and its spectroscopic properties in organic solvents and phosphatidylcholine liposomes. J. Photochem Photobiol. B 96 (3), 242–248. doi:10.1016/j.jphotobiol.2009.06.012
Elksnis, A., Welsh, N., Wikström, P., Lau, J., and Carlsson, P. O. (2023). The selective NOX4 inhibitor GLX7013159 decreases blood glucose concentrations and human beta-cell apoptotic rates in diabetic NMRI Nu/Nu mice transplanted with human islets. Free Radic. Res. 57 (6-12), 460–469. doi:10.1080/10715762.2023.2284637
Fan, L., Xiao, Q., Zhang, L., Wang, X., Huang, Q., Li, S., et al. (2018). CAPE-pNO(2) attenuates diabetic cardiomyopathy through the NOX4/NF-κB pathway in STZ-Induced diabetic mice. Biomed. Pharmacother. 108, 1640–1650. doi:10.1016/j.biopha.2018.10.026
Faria, A., and Persaud, S. J. (2017). Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol. Ther. 172, 50–62. doi:10.1016/j.pharmthera.2016.11.013
Fiorentino, T. V., Prioletta, A., Zuo, P., and Folli, F. (2013). Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 19 (32), 5695–5703. doi:10.2174/1381612811319320005
Frangogiannis, N. G. (2021). Cardiac fibrosis. Cardiovasc Res. 117 (6), 1450–1488. doi:10.1093/cvr/cvaa324
García-Díez, E., López-Oliva, M. E., Caro-Vadillo, A., Pérez-Vizcaíno, F., Pérez-Jiménez, J., Ramos, S., et al. (2022). Supplementation with a cocoa-carob blend, alone or in combination with metformin, attenuates diabetic cardiomyopathy, cardiac oxidative stress and inflammation in zucker diabetic rats. Antioxidants (Basel) 11 (2), 432. doi:10.3390/antiox11020432
Giacco, F., and Brownlee, M. (2010). Oxidative stress and diabetic complications. Circ. Res. 107 (9), 1058–1070. doi:10.1161/circresaha.110.223545
Gorin, Y., Ricono, J. M., Kim, N. H., Bhandari, B., Choudhury, G. G., and Abboud, H. E. (2003). Nox4 mediates angiotensin II-induced activation of akt/Protein kinase B in mesangial cells. Am. J. Physiol. Ren. Physiol. 285 (2), F219–F229. doi:10.1152/ajprenal.00414.2002
Hashiesh, H. M., Azimullah, S., Nagoor Meeran, M. F., Saraswathiamma, D., Arunachalam, S., Jha, N. K., et al. (2024). Cannabinoid 2 receptor activation protects against diabetic cardiomyopathy through inhibition of AGE/RAGE-Induced oxidative stress, fibrosis, and inflammasome activation. J. Pharmacol. Exp. Ther. 391 (2), 241–257. doi:10.1124/jpet.123.002037
Hiromura, M., Mori, Y., Terasaki, M., Kushima, H., Saito, T., Osaka, N., et al. (2021). Glucose-dependent insulinotropic polypeptide inhibits cardiac hypertrophy and fibrosis in diabetic mice via suppression of TGF-β2. Diab Vasc. Dis. Res. 18 (2), 1479164121999034. doi:10.1177/1479164121999034
Huang, Y. Z., Wu, J. C., Lu, G. F., Li, H. B., Lai, S. M., Lin, Y. C., et al. (2024). Pulmonary hypertension induces serotonin hyperreactivity and metabolic reprogramming in coronary arteries via NOX1/4-TRPM2 signaling pathway. Hypertension 81 (3), 582–594. doi:10.1161/hypertensionaha.123.21345
Jabbour, S., Seufert, J., Scheen, A., Bailey, C. J., Karup, C., and Langkilde, A. M. (2018). Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes. Metab. 20 (3), 620–628. doi:10.1111/dom.13124
Jiang, F., Liu, G. S., Dusting, G. J., and Chan, E. C. (2014). NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses. Redox Biol. 2, 267–272. doi:10.1016/j.redox.2014.01.012
Jiang, J., Huang, K., Xu, S., Garcia, J. G. N., Wang, C., and Cai, H. (2020). Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 36, 101638. doi:10.1016/j.redox.2020.101638
Jubaidi, F. F., Zainalabidin, S., Taib, I. S., Abdul Hamid, Z., Mohamad Anuar, N. N., Jalil, J., et al. (2022). The role of PKC-MAPK signalling pathways in the development of hyperglycemia-induced cardiovascular complications. Int. J. Mol. Sci. 23 (15), 8582. doi:10.3390/ijms23158582
Jubaidi, F. F., Zainalabidin, S., Taib, I. S., Hamid, Z. A., and Budin, S. B. (2021). The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int. J. Mol. Sci. 22 (10), 5094. doi:10.3390/ijms22105094
Kenny, H. C., and Abel, E. D. (2019). Heart failure in type 2 diabetes mellitus. Circ. Res. 124 (1), 121–141. doi:10.1161/circresaha.118.311371
Khan, S. (2021). Wogonin and alleviation of hyperglycemia via inhibition of DAG mediated PKC expression. A brief insight. Protein Pept. Lett. 28 (12), 1365–1371. doi:10.2174/0929866528666211027113349
Kleniewska, P., Piechota, A., Skibska, B., and Gorąca, A. (2012). The NADPH oxidase family and its inhibitors. Arch. Immunol. Ther. Exp. Warsz. 60 (4), 277–294. doi:10.1007/s00005-012-0176-z
Kobayashi, S., and Liang, Q. (2015). Autophagy and mitophagy in diabetic cardiomyopathy. Biochim. Biophys. Acta 1852 (2), 252–261. doi:10.1016/j.bbadis.2014.05.020
Kusaka, H., Koibuchi, N., Hasegawa, Y., Ogawa, H., and Kim-Mitsuyama, S. (2016). Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol. 15 (1), 157. doi:10.1186/s12933-016-0473-7
Kyriazis, I. D., Hoffman, M., Gaignebet, L., Lucchese, A. M., Markopoulou, E., Palioura, D., et al. (2021). KLF5 is induced by FOXO1 and causes oxidative stress and diabetic cardiomyopathy. Circ. Res. 128 (3), 335–357. doi:10.1161/circresaha.120.316738
Laddha, A. P., and Kulkarni, Y. A. (2021). Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 284, 119664. doi:10.1016/j.lfs.2021.119664
Li, C., Song, Z., Gao, P., Duan, W., Liu, X., Liang, S., et al. (2023). Transaldolase inhibits CD36 expression by modulating glutathione-p38 signaling, exerting protective effects against macrophage foam cell formation. Acta Biochim. Biophys. Sin. (Shanghai) 55 (9), 1496–1505. doi:10.3724/abbs.2023146
Li, C., Zhang, J., Xue, M., Li, X., Han, F., Liu, X., et al. (2019). SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 18 (1), 15. doi:10.1186/s12933-019-0816-2
Li, J., Zhu, H., Shen, E., Wan, L., Arnold, J. M., and Peng, T. (2010). Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59 (8), 2033–2042. doi:10.2337/db09-1800
Li, L. H., Wang, J. S., and Kong, L. Y. (2013). Protective effects of shengmai san and its three fractions on cerebral ischemia-reperfusion injury. Chin. J. Nat. Med. 11 (3), 222–230. doi:10.1016/s1875-5364(13)60020-5
Li, T., Mu, N., Yin, Y., Yu, L., and Ma, H. (2020). Targeting AMP-activated protein kinase in aging-related cardiovascular diseases. Aging Dis. 11 (4), 967–977. doi:10.14336/ad.2019.0901
Liang, E., Liu, X., Du, Z., Yang, R., and Zhao, Y. (2018). Andrographolide ameliorates diabetic cardiomyopathy in mice by blockage of oxidative damage and NF-κB-Mediated inflammation. Oxid. Med. Cell Longev. 2018, 9086747. doi:10.1155/2018/9086747
Liu, R., Duan, T., Yu, L., Tang, Y., Liu, S., Wang, C., et al. (2023). Acid sphingomyelinase promotes diabetic cardiomyopathy via NADPH oxidase 4 mediated apoptosis. Cardiovasc Diabetol. 22 (1), 25. doi:10.1186/s12933-023-01747-1
Liu, Y., and Zhang, J. (2015). Nox2 contributes to cardiac fibrosis in diabetic cardiomyopathy in a transforming growth factor-β dependent manner. Int. J. Clin. Exp. Pathol. 8 (9), 10908–10914. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4637621/pdf/ijcep0008-10908.pdf.
Lu, Y., Zhu, S., Wang, X., Liu, J., Li, Y., Wang, W., et al. (2021). ShengMai-San attenuates cardiac remodeling in diabetic rats by inhibiting NOX-mediated oxidative stress. Diabetes Metab. Syndr. Obes. 14, 647–657. doi:10.2147/dmso.S287582
Luo, B., Li, B., Wang, W., Liu, X., Xia, Y., Zhang, C., et al. (2014). NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 9 (8), e104771. doi:10.1371/journal.pone.0104771
Ma, S., Li, X., Dong, L., Zhu, J., Zhang, H., and Jia, Y. (2016). Protective effect of sheng-mai yin, a traditional Chinese preparation, against doxorubicin-induced cardiac toxicity in rats. BMC Complement. Altern. Med. 16, 61. doi:10.1186/s12906-016-1037-9
Ma, X. M., Geng, K., Law, B. Y., Wang, P., Pu, Y. L., Chen, Q., et al. (2023). Lipotoxicity-induced mtDNA release promotes diabetic cardiomyopathy by activating the cGAS-STING pathway in obesity-related diabetes. Cell Biol. Toxicol. 39 (1), 277–299. doi:10.1007/s10565-021-09692-z
MacCarthy, P. A., Grieve, D. J., Li, J. M., Dunster, C., Kelly, F. J., and Shah, A. M. (2001). Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation 104 (24), 2967–2974. doi:10.1161/hc4901.100382
Maqbool, A., Watt, N. T., Haywood, N., Viswambharan, H., Skromna, A., Makava, N., et al. (2020). Divergent effects of genetic and pharmacological inhibition of Nox2 NADPH oxidase on insulin resistance-related vascular damage. Am. J. Physiol. Cell Physiol. 319 (1), C64–c74. doi:10.1152/ajpcell.00389.2019
Marino, F., Salerno, N., Scalise, M., Salerno, L., Torella, A., Molinaro, C., et al. (2023). Streptozotocin-induced type 1 and 2 diabetes mellitus mouse models show different functional, cellular and molecular patterns of diabetic cardiomyopathy. Int. J. Mol. Sci. 24 (2), 1132. doi:10.3390/ijms24021132
McMurray, J. J. V., Solomon, S. D., Inzucchi, S. E., Køber, L., Kosiborod, M. N., Martinez, F. A., et al. (2019). Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381 (21), 1995–2008. doi:10.1056/NEJMoa1911303
Mo, W. L., Chai, C. Z., Kou, J. P., Yan, Y. Q., and Yu, B. Y. (2015). Sheng-mai-san attenuates contractile dysfunction and structural damage induced by chronic intermittent hypoxia in mice. Chin. J. Nat. Med. 13 (10), 743–750. doi:10.1016/s1875-5364(15)30074-1
Molinaro, C., Salerno, L., Marino, F., Scalise, M., Salerno, N., Pagano, L., et al. (2022). Unraveling and targeting myocardial regeneration deficit in diabetes. Antioxidants (Basel) 11 (2), 208. doi:10.3390/antiox11020208
Moris, D., Spartalis, M., Tzatzaki, E., Spartalis, E., Karachaliou, G. S., Triantafyllis, A. S., et al. (2017). The role of reactive oxygen species in myocardial redox signaling and regulation. Ann. Transl. Med. 5 (16), 324. doi:10.21037/atm.2017.06.17
Nabeebaccus, A. A., Reumiller, C. M., Shen, J., Zoccarato, A., Santos, C. X. C., and Shah, A. M. (2023). The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc Res. 118 (17), 3305–3319. doi:10.1093/cvr/cvac030
Nadworny, A. S., Guruju, M. R., Poor, D., Doran, R. M., Sharma, R. V., Kotlikoff, M. I., et al. (2013). Nox2 and Nox4 influence neonatal c-kit(+) cardiac precursor cell status and differentiation. Am. J. Physiol. Heart Circ. Physiol. 305 (6), H829–H842. doi:10.1152/ajpheart.00761.2012
Ni, Q., Wang, J., Li, E. Q., Zhao, A. B., Yu, B., Wang, M., et al. (2011). Study on the protective effect of shengmai san (see text) on the myocardium in the type 2 diabetic cardiomyopathy model rat. J. Tradit. Chin. Med. 31 (3), 209–219. doi:10.1016/s0254-6272(11)60044-7
Nie, Z., Zhang, K., Chen, X., Wang, J., Gao, H., Zheng, B., et al. (2023). A multifunctional integrated metal-free MRI agent for early diagnosis of oxidative stress in a mouse model of diabetic cardiomyopathy. Adv. Sci. (Weinh) 10 (7), e2206171. doi:10.1002/advs.202206171
Ogboo, B. C., Grabovyy, U. V., Maini, A., Scouten, S., van der Vliet, A., Mattevi, A., et al. (2022). Architecture of the NADPH oxidase family of enzymes. Redox Biol. 52, 102298. doi:10.1016/j.redox.2022.102298
Ouyang, Y., Tang, L., Hu, S., Tian, G., Dong, C., Lai, H., et al. (2022). Shengmai san-derived compound prescriptions: a review on chemical constituents, pharmacokinetic studies, quality control, and pharmacological properties. Phytomedicine 107, 154433. doi:10.1016/j.phymed.2022.154433
Pecchillo Cimmino, T., Ammendola, R., Cattaneo, F., and Esposito, G. (2023). NOX dependent ROS generation and cell metabolism. Int. J. Mol. Sci. 24 (3), 2086. doi:10.3390/ijms24032086
Peng, M. L., Fu, Y., Wu, C. W., Zhang, Y., Ren, H., and Zhou, S. S. (2022). Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front. Endocrinol. (Lausanne) 13, 907757. doi:10.3389/fendo.2022.907757
Pérez-Cremades, D., Chen, J., Assa, C., and Feinberg, M. W. (2023). MicroRNA-mediated control of myocardial infarction in diabetes. Trends Cardiovasc Med. 33 (4), 195–201. doi:10.1016/j.tcm.2022.01.004
Piazza, C., Privitera, M. G., Melilli, B., Incognito, T., Marano, M. R., Leggio, G. M., et al. (2007). Influence of inulin on plasma isoflavone concentrations in healthy postmenopausal women. Am. J. Clin. Nutr. 86 (3), 775–780. doi:10.1093/ajcn/86.3.775
Plosker, G. L. (2014). Dapagliflozin: a review of its use in patients with type 2 diabetes. Drugs 74 (18), 2191–2209. doi:10.1007/s40265-014-0324-3
Privratsky, J. R., Wold, L. E., Sowers, J. R., Quinn, M. T., and Ren, J. (2003). AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42 (2), 206–212. doi:10.1161/01.Hyp.0000082814.62655.85
Qiu, J., Liu, D., Li, P., Zhou, L., Zhou, L., Liu, X., et al. (2022). NADPH oxidase mediates oxidative stress and ventricular remodeling through SIRT3/FOXO3a pathway in diabetic mice. Antioxidants (Basel) 11 (9), 1745. doi:10.3390/antiox11091745
Rehfeldt, S. C. H., Laufer, S., and Goettert, M. I. (2021). A highly selective in vitro JNK3 inhibitor, FMU200, restores mitochondrial membrane potential and reduces oxidative stress and apoptosis in SH-SY5Y cells. Int. J. Mol. Sci. 22 (7), 3701. doi:10.3390/ijms22073701
Ren, B. C., Zhang, Y. F., Liu, S. S., Cheng, X. J., Yang, X., Cui, X. G., et al. (2020). Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell Mol. Med. 24 (21), 12355–12367. doi:10.1111/jcmm.15725
Ren, J., Pulakat, L., Whaley-Connell, A., and Sowers, J. R. (2010). Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. Berl. 88 (10), 993–1001. doi:10.1007/s00109-010-0663-9
Ritchie, R. H., and Abel, E. D. (2020). Basic mechanisms of diabetic heart disease. Circ. Res. 126 (11), 1501–1525. doi:10.1161/circresaha.120.315913
Rossi, F., and Zatti, M. (1964). Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia 20 (1), 21–23. doi:10.1007/bf02146019
Rubler, S., Dlugash, J., Yuceoglu, Y. Z., Kumral, T., Branwood, A. W., and Grishman, A. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 30 (6), 595–602. doi:10.1016/0002-9149(72)90595-4
Rüfer, C. E., Bub, A., Möseneder, J., Winterhalter, P., Stürtz, M., and Kulling, S. E. (2008). Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. Am. J. Clin. Nutr. 87 (5), 1314–1323. doi:10.1093/ajcn/87.5.1314
Sag, C. M., Santos, C. X., and Shah, A. M. (2014). Redox regulation of cardiac hypertrophy. J. Mol. Cell Cardiol. 73, 103–111. doi:10.1016/j.yjmcc.2014.02.002
Shah, A. M., Uno, H., Køber, L., Velazquez, E. J., Maggioni, A. P., MacDonald, M. R., et al. (2010). The inter-relationship of diabetes and left ventricular systolic function on outcome after high-risk myocardial infarction. Eur. J. Heart Fail 12 (11), 1229–1237. doi:10.1093/eurjhf/hfq179
Shah, M. S., and Brownlee, M. (2016). Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 118 (11), 1808–1829. doi:10.1161/circresaha.116.306923
Singh, A., Kukreti, R., Saso, L., and Kukreti, S. (2019). Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24 (8), 1583. doi:10.3390/molecules24081583
Su, L., Zhang, J., Gomez, H., Kellum, J. A., and Peng, Z. (2023). Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 19 (2), 401–414. doi:10.1080/15548627.2022.2084862
Sun, J., Zhang, L., Fang, J., Yang, S., and Chen, L. (2020). Galectin-3 mediates high-glucose-induced cardiomyocyte injury by the NADPH oxidase/reactive oxygen species pathway. Can. J. Physiol. Pharmacol. 98 (11), 826–833. doi:10.1139/cjpp-2019-0708
Sung, M. M., Hamza, S. M., and Dyck, J. R. (2015). Myocardial metabolism in diabetic cardiomyopathy: potential therapeutic targets. Antioxid. Redox Signal 22 (17), 1606–1630. doi:10.1089/ars.2015.6305
Tan, Y. Y., Chen, L. X., Fang, L., and Zhang, Q. (2020). Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of NADPH oxidase and NF-κB activities. BMC Complement. Med. Ther. 20 (1), 378. doi:10.1186/s12906-020-03177-y
Tang, S. G., Liu, X. Y., Wang, S. P., Wang, H. H., Jovanović, A., and Tan, W. (2019). Trimetazidine prevents diabetic cardiomyopathy by inhibiting Nox2/TRPC3-induced oxidative stress. J. Pharmacol. Sci. 139 (4), 311–318. doi:10.1016/j.jphs.2019.01.016
Tang, Z., Wang, P., Dong, C., Zhang, J., Wang, X., and Pei, H. (2022). Oxidative stress signaling mediated pathogenesis of diabetic cardiomyopathy. Oxid. Med. Cell Longev. 2022, 5913374. doi:10.1155/2022/5913374
Tian, J., Tang, W., Xu, M., Zhang, C., Zhao, P., Cao, T., et al. (2018). Shengmai san alleviates diabetic cardiomyopathy through improvement of mitochondrial lipid metabolic disorder. Cell Physiol. Biochem. 50 (5), 1726–1739. doi:10.1159/000494791
Touyz, R. M., Anagnostopoulou, A., Camargo, L. L., Rios, F. J., and Montezano, A. C. (2019). Vascular biology of superoxide-generating NADPH oxidase 5-Implications in hypertension and cardiovascular disease. Antioxid. Redox Signal 30 (7), 1027–1040. doi:10.1089/ars.2018.7583
Urao, N., and Ushio-Fukai, M. (2013). Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic. Biol. Med. 54, 26–39. doi:10.1016/j.freeradbiomed.2012.10.532
Vásquez-Trincado, C., García-Carvajal, I., Pennanen, C., Parra, V., Hill, J. A., Rothermel, B. A., et al. (2016). Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 594 (3), 509–525. doi:10.1113/jp271301
Vermot, A., Petit-Härtlein, I., Smith, S. M. E., and Fieschi, F. (2021). NADPH oxidases (NOX): an overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants (Basel) 10 (6), 890. doi:10.3390/antiox10060890
Vilas-Boas, E. A., Almeida, D. C., Roma, L. P., Ortis, F., and Carpinelli, A. R. (2021). Lipotoxicity and β-Cell failure in type 2 diabetes: oxidative stress linked to NADPH oxidase and ER stress. Cells 10 (12), 3328. doi:10.3390/cells10123328
Visnagri, A., Oexner, R. R., Kmiotek-Wasylewska, K., Zhang, M., Zoccarato, A., and Shah, A. M. (2023). Nicotinamide adenosine dinucleotide phosphate oxidase-mediated signaling in cardiac remodeling. Antioxid. Redox Signal 38 (4-6), 371–387. doi:10.1089/ars.2022.0176
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., and Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 9 (2), 119. doi:10.1038/s41419-017-0135-z
Wang, D., Li, J., Luo, G., Zhou, J., Wang, N., Wang, S., et al. (2023). Nox4 as a novel therapeutic target for diabetic vascular complications. Redox Biol. 64, 102781. doi:10.1016/j.redox.2023.102781
Wang, H. J., Chia-Hui Tan, E., Chiang, T. Y., Chen, W. C., Shen, C. C., and Ueng, Y. F. (2022). Effect of repeated shengmai-san administration on nifedipine pharmacokinetics and the risk/benefit under co-treatment. J. Food Drug Anal. 30 (1), 111–127. doi:10.38212/2224-6614.3401
Wang, L., Bi, X., and Han, J. (2020). Silencing of peroxisome proliferator-activated receptor-alpha alleviates myocardial injury in diabetic cardiomyopathy by downregulating 3-hydroxy-3-methylglutaryl-coenzyme A synthase 2 expression. IUBMB Life 72 (9), 1997–2009. doi:10.1002/iub.2337
Weng, L., Li, L., Zhao, K., Xu, T., Mao, Y., Shu, H., et al. (2022). Non-invasive local acoustic therapy ameliorates diabetic heart fibrosis by suppressing ACE-mediated oxidative stress and inflammation in cardiac fibroblasts. Cardiovasc Drugs Ther. 36 (3), 413–424. doi:10.1007/s10557-021-07297-6
Xing, Y. J., Liu, B. H., Wan, S. J., Cheng, Y., Zhou, S. M., Sun, Y., et al. (2021). A SGLT2 inhibitor dapagliflozin alleviates diabetic cardiomyopathy by suppressing high glucose-induced oxidative stress in vivo and in vitro. Front. Pharmacol. 12, 708177. doi:10.3389/fphar.2021.708177
Xu, T. Z., Shen, X. Y., Sun, L. L., Chen, Y. L., Zhang, B. Q., Huang, D. K., et al. (2019). Ginsenoside Rg1 protects against H2O2-induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int. J. Mol. Med. 43 (2), 717–726. doi:10.3892/ijmm.2018.4005
Yang, K., Velagapudi, S., Akhmedov, A., Kraler, S., Lapikova-Bryhinska, T., Schmiady, M. O., et al. (2023). Chronic SIRT1 supplementation in diabetic mice improves endothelial function by suppressing oxidative stress. Cardiovasc Res. 119 (12), 2190–2201. doi:10.1093/cvr/cvad102
Yang, Y., Wang, H., Kouadir, M., Song, H., and Shi, F. (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10 (2), 128. doi:10.1038/s41419-019-1413-8
Ye, Y., Bajaj, M., Yang, H. C., Perez-Polo, J. R., and Birnbaum, Y. (2017). SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 31 (2), 119–132. doi:10.1007/s10557-017-6725-2
Yu, X., Tesiram, Y. A., Towner, R. A., Abbott, A., Patterson, E., Huang, S., et al. (2007). Early myocardial dysfunction in streptozotocin-induced diabetic mice: a study using in vivo magnetic resonance imaging (MRI). Cardiovasc Diabetol. 6, 6. doi:10.1186/1475-2840-6-6
Zhang, D., Li, Y., Wang, W., Lang, X., Zhang, Y., Zhao, Q., et al. (2022). NOX1 promotes myocardial fibrosis and cardiac dysfunction via activating the TLR2/NF-κB pathway in diabetic cardiomyopathy. Front. Pharmacol. 13, 928762. doi:10.3389/fphar.2022.928762
Zhang, L., Ai, C., Bai, M., Niu, J., and Zhang, Z. (2022). NLRP3 inflammasome/pyroptosis: a key driving force in diabetic cardiomyopathy. Int. J. Mol. Sci. 23 (18), 10632. doi:10.3390/ijms231810632
Zhang, Q., Wang, L., Wang, S., Cheng, H., Xu, L., Pei, G., et al. (2022). Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target Ther. 7 (1), 78. doi:10.1038/s41392-022-00925-z
Zhang, X. J., Han, X. W., Jiang, Y. H., Wang, Y. L., He, X. L., Liu, D. H., et al. (2023). Impact of inflammation and anti-inflammatory modalities on diabetic cardiomyopathy healing: from fundamental research to therapy. Int. Immunopharmacol. 123, 110747. doi:10.1016/j.intimp.2023.110747
Zhang, Y., Murugesan, P., Huang, K., and Cai, H. (2020). NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat. Rev. Cardiol. 17 (3), 170–194. doi:10.1038/s41569-019-0260-8
Zhao, G. J., Zhao, C. L., Ouyang, S., Deng, K. Q., Zhu, L., Montezano, A. C., et al. (2020). Ca(2+)-Dependent NOX5 (NADPH oxidase 5) exaggerates cardiac hypertrophy through reactive oxygen species production. Hypertension 76 (3), 827–838. doi:10.1161/hypertensionaha.120.15558
Zhao, J., Cao, T. T., Tian, J., Chen, H. H., Zhang, C., Wei, H. C., et al. (2016). Shengmai san ameliorates myocardial dysfunction and fibrosis in diabetic Db/Db mice. Evid. Based Complement. Altern. Med. 2016, 4621235. doi:10.1155/2016/4621235
Zhao, L., Hu, H., Zhang, L., Liu, Z., Huang, Y., Liu, Q., et al. (2024). Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions. MedComm (2020) 5 (4), e516. doi:10.1002/mco2.516
Zhao, M. X., Zhou, B., Ling, L., Xiong, X. Q., Zhang, F., Chen, Q., et al. (2017). Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 8 (3), e2690. doi:10.1038/cddis.2017.106
Zhao, Q. D., Viswanadhapalli, S., Williams, P., Shi, Q., Tan, C., Yi, X., et al. (2015). NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation 131 (7), 643–655. doi:10.1161/circulationaha.114.011079
Zhao, X., Liu, S., Wang, X., Chen, Y., Pang, P., Yang, Q., et al. (2022). Diabetic cardiomyopathy: clinical phenotype and practice. Front. Endocrinol. (Lausanne) 13, 1032268. doi:10.3389/fendo.2022.1032268
Zhao, Y., Pan, B., Lv, X., Chen, C., Li, K., Wang, Y., et al. (2023). Ferroptosis: roles and molecular mechanisms in diabetic cardiomyopathy. Front. Endocrinol. (Lausanne) 14, 1140644. doi:10.3389/fendo.2023.1140644
Zhong, H., Tang, H., Wang, Y., Tang, S., and Zhu, H. (2024). MiR-29c alleviates hyperglycemia-induced inflammation via targeting TGF-β in cardiomyocytes. Mol. Cell Biochem. 479 (8), 2047–2054. doi:10.1007/s11010-023-04813-0
Zhou, B., and Tian, R. (2018). Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Invest 128 (9), 3716–3726. doi:10.1172/jci120849
Zhou, D. D., Luo, M., Shang, A., Mao, Q. Q., Li, B. Y., Gan, R. Y., et al. (2021). Antioxidant food components for the prevention and treatment of cardiovascular diseases: effects, mechanisms, and clinical studies. Oxid. Med. Cell Longev. 2021, 6627355. doi:10.1155/2021/6627355
Zhou, L., Liu, Y., Wang, Z., Liu, D., Xie, B., Zhang, Y., et al. (2021). Activation of NADPH oxidase mediates mitochondrial oxidative stress and atrial remodeling in diabetic rabbits. Life Sci. 272, 119240. doi:10.1016/j.lfs.2021.119240
Zhu, T., Ye, Z., Song, J., Zhang, J., Zhao, Y., Xu, F., et al. (2024). Effect of extracellular matrix stiffness on efficacy of dapagliflozin for diabetic cardiomyopathy. Cardiovasc Diabetol. 23 (1), 273. doi:10.1186/s12933-024-02369-x
Keywords: diabetic cardiomyopathy, NADPH oxidase, reactive oxygen species, oxidative stress, redox signaling pathways
Citation: Ye Z, Wang S, Wan Z, Huang B and Guo J (2025) Targeting NADPH oxidase-driven oxidative stress in diabetic cardiomyopathy: mechanisms and therapeutic perspectives. Front. Pharmacol. 16:1610429. doi: 10.3389/fphar.2025.1610429
Received: 12 April 2025; Accepted: 18 June 2025;
Published: 03 July 2025.
Edited by:
Marcel Henrique Marcondes Sari, Federal University of Paraná, BrazilReviewed by:
Fabiola Marino, University of Magna Graecia, ItalyAdeniyi Adebesin, University of Texas Southwestern Medical Center, United States
Copyright © 2025 Ye, Wang, Wan, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birun Huang, aGJyXzE5ODFAc2luYS5jb20=; Jiawei Guo, Z3Vvanc5QG1haWwyLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
 Zilv Ye
Zilv Ye Sutong Wang3†
Sutong Wang3† Jiawei Guo
Jiawei Guo