Abstract
Introduction:
The most severe forms of asthma are characterised by the occurrence of dyspnoea on exertion, impacting on daily activities and quality of life. It has been demonstrated that dynamic hyperinflation (DH) during exercise represents a mechanism of physical activity limitation in severe asthmatics. Inspiratory capacity (IC) at rest may be an indicator of static hyperinflation, and the change in IC during exercise can be regarded as a marker of DH. The study aims to assess whether Benralizumab is able to improve IC and to reduce DH.
Materials and methods:
A pilot study on severe asthmatics was conducted. Assessments of asthma control and quality of life, lung function evaluation and 6-min walk test (6MWT) were carried out on the day of the first drug administration (T0) and after a period of 6 months (T1).
Results:
Twelve severe asthmatics were recruited. Significant improvements of dynamic volumes, asthma control and quality of life were observed after anti IL-5R treatment. At T0, pre-6MWT-IC and post-6MWT-IC were 2.40 ± 0.48 L and 1.60 ± 0.83 L, respectively (p < 0.0001). Minute ventilation (VE) at the beginning of the 6MWT was 13.88 ± 4.44 L·min−1 and post 6MWT was 23.77 ± 12.11 L·min−1 (p < 0.0001). At T1, IC pre 6MWT was higher than IC pre 6MWT at T0 (2.74 ± 1.14, p = 0.010) and did not change after exercise (IC post 6MWT: 2.85 ± 1.22 L, p = 0.53). VE did not change at T1.
Discussion:
These findings show the effect of Benralizumab in improving IC during exercise. The disappearance of DH provides a potential explanation for the beneficial effect of biologics in severe asthmatics.
Introduction
Asthma is a widespread disease throughout the world, which hinders the performance of daily activities and reduces the quality of life of affected patients, especially in the most severe forms (Chung et al., 2014; Wilson et al., 2012). The most common and problematic symptom among asthmatic patients is exertional dyspnea, which has been hypothesized to be associated with the occurrence of dynamic hyperinflation (DH) (Haselkorn et al., 2010). DH is a well-recognized cause of physical exercise limitation among COPD patients, and is sustained by reduced alveolar attachments and consequent impaired lung elastic recoil; on the other hand, its occurrence in asthma is less investigated (Vermeulen et al., 2016), and could be attributed to different causes. In a recent study by van der Meer and colleagues (van der Meer et al., 2021), DH was shown to be associated with features of airway inflammation in moderate to severe asthmatics, and to decrease with systemic corticosteroids, thus being recognized as an important treatable trait. The same authors previously demonstrated that DH is associated with impaired physical activity in severe asthmatics (van der Meer et al., 2019).
Patients affected by severe asthma may require biologic drugs to achieve control of respiratory symptoms. Benralizumab is a monoclonal antibody directed against the IL-5 receptor, which has been proved safe and effective in reducing the number of disease exacerbations, hospitalizations and the dose of oral corticosteroids in patients affected by severe eosinophilic asthma (Nair et al., 2017; Bleecker et al., 2016; FitzGerald et al., 2016). Recent studies (Pelaia et al., 2020; Panettieri et al., 2020; Maniscalco et al., 2024) have shown a tendency to improvement in static lung volumes after treatment with benralizumab, which could further explain the clinical effects on daily activities.
In this pilot study, we sought to demonstrate that anti-IL5 receptor α monoclonal antibody is effective in reducing lung hyperinflation in severe asthmatics. For this purpose, we assessed the changes in inspiratory capacity (IC) in severe asthmatic subjects measured during the 6-min walking test (6MWT), a condition resembling daily physical activities in experimental setting.
Materials and methods
Study population
The study was conducted at the PROMISE Department of the University Hospital of Palermo, Italy. Patients aged between 18 and 65 years suffering from severe eosinophilic asthma according to GINA guidelines (Global Initiative for Asthma, 2024), who were eligible for treatment with an anti-IL-5R monoclonal antibody were recruited. The eligibility criteria (at least 2 out of 3 required) are: a) eosinophil value ≥ 300/mmc in the absence of systemic steroid treatment; b) at least two asthma exacerbations despite maximum inhaled therapy (Step 4–5 GINA) treated with systemic steroid or requiring hospitalization in the previous 12 months; c) continuous therapy with oral steroids, in addition to maximum inhaled therapy in the last year. Patients who smoked or suffered from respiratory diseases other than bronchial asthma were excluded from the study. All subjects were under inhaled corticosteroids/beta-2 adrenergic long-acting bronchodilators (ICS/LABA) therapy in a fixed high-dose combination and a third controller (tiotropium, antileukotriene, doxifylline). Exclusion criteria included contraindications to clinical exercise tests (Holland et al., 2014), comorbidities affecting exercise capacity, treatment with β-blockers and inability to carry out the study protocol. Subjects were in stable clinical conditions at the time of enrollment. The study protocol was approved by Local Ethics Committee and an informed consent was signed by each subject participating in the study.
Study design
Clinical assessments, lung function evaluation and 6MWT were carried out on the day of the first administration of Benralizumab (T0) and after a period of 6 months (T1). Asthma control and quality of life were investigated using the Asthma Control Test (ACT), the Asthma Control Questionnaire (ACQ) and the Asthma Quality of Life Questionnaire (AQLQ).
Forced and slow vital capacity manoeuvres were performed in accordance with ATS/ERS guidelines (Miller et al., 2005; Wanger et al., 2005; Graham et al., 2019). The 6MWT was performed along a flat, straight, 30-m walking course supervised by a well-trained researcher according to ATS guidelines (Holland et al., 2014), using a portable spirometer (Spiropalm; COSMED, Rome, Italy) with integrated pulse oximeter and ventilation (VE) measurement, which allows the measurement of IC at the beginning, at resting conditions (pre-6MWT-IC) and immediately after the end of the test (post-6MWT-IC). DH was defined as a decrease of >150 mL in IC at the end of exercise compared with resting levels (post-6MWT-IC - pre-6MWT-IC) (O’Donnell et al., 2001; Benfante et al., 2018).
Statistical analysis
The results are expressed as mean and standard deviation, unless otherwise specified. Statistical analysis was conducted via RStudio system. The quantitative variables were analyzed using a paired t-test. P-values <0.05 were considered statistically significant.
Results
Twelve patients (8 females and 4 males, age:57 ± 8.6 years) affected by severe eosinophilic asthma, meeting the eligibility criteria for benralizumab treatment, were recruited. All severe asthmatics enrolled in the current study were never smokers. The baseline characteristics of the enrolled patients are depicted in Table 1. No exacerbations were documented during the study period. As expected, significant improvement of dynamic volumes (Figure 1), asthma control and quality of life (Figure 2) was observed after anti IL-5R treatment (Table 2).
TABLE 1
| Characteristic | Total (n=12) |
|---|---|
| Demographic data | |
| Female sex (n) | 8 |
| Age at study enrolment (years) | 57.2 ± 8.63 |
| BMI (kg/m2) | 28.83 ± 6.19 |
| Clinical data | |
| Atopic — yes (%) | 6 (50) |
| CRwNP — yes (%) | 7 (58.3) |
| Bronchiectasis — yes (%) | 2 (17) |
| GERD — yes (%) | 8 (66.7) |
| Obesity — yes (%) | 3 (25) |
| Depression/Anxiety — yes (%) | 3 (25) |
| Osteoporosis — yes (%) | 3 (25) |
| Obstructive sleep apnea syndrome — yes (%) | 2 (17) |
| Pharmacologic therapies | |
| High dose ICS-LABA, n (%) | 12 (100) |
| LAMA, n (%) | 7 (58.3) |
| LTRA, n (%) | 7 (58.3) |
| Previous anti-IgE/anti IL-5 mAbs, n (%) | 4 (33.3) |
| Patients on OCS, n, (%) | 5 (42) |
| OCS, mg/day, mean (SD) | 8.5 (6.5) |
| Biological data | |
| Eosinophils in blood (cells/mm3) | 792.6 ± 466.35 |
| Lung function evaluations | |
| FEV1% predicted | 70.27 ± 24.76 |
| FVC% predicted | 79.45 ± 19.93 |
| FEV1/FVC | 0.73 ± 0.18 |
| FEF 25-75% predicted | 50.64 ± 35.06 |
| Asthma and quality of life scores | |
| ACT | 15 ± 5.71 |
| ACQ | 2.54 ± 1.28 |
| AQLQ | 3.55 ± 1.28 |
Demographic and clinical data, biological and lung function evaluations of the asthmatic subjects at baseline.
Data are presented as mean±SD, unless otherwise stated. ACT: Asthma Control Test; ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; BMI, Body Mass Index; FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; FEF25–75%, Forced Expiratory Flow at 25–75% of FVC; LABA, Long-Acting Beta agonist; LAMA, Long-Acting Muscarinic Agonist; LTRA, Leukotriene Receptor Antagonist; OCS, Oral Corticosteroids.
FIGURE 1
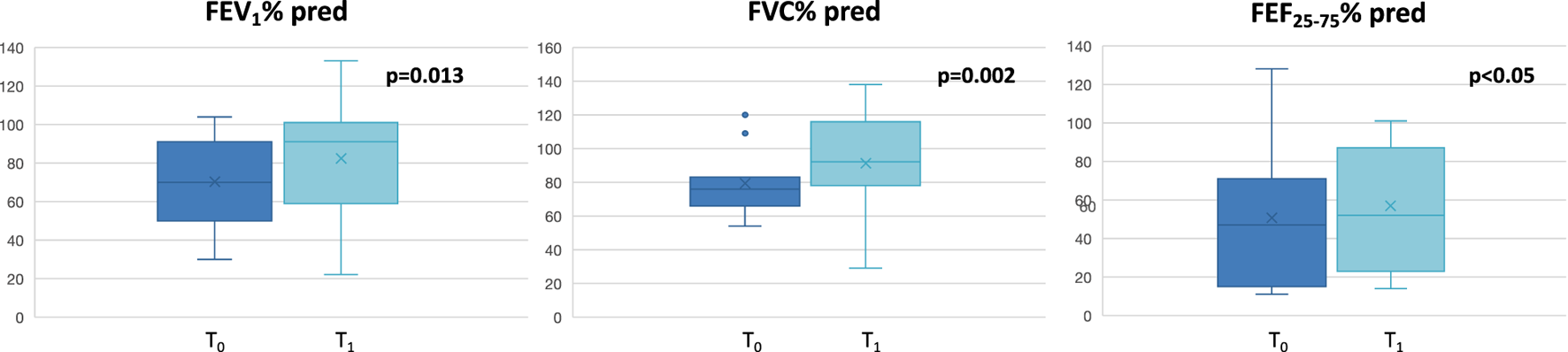
Changes in lung function assessments at T0 and at T1. FEV1: Forced Expiratory Volume in the first second; FVC: forced vital capacity; FEF25-75: Forced expiratory flow at 25%–75% of the vital capacity.
FIGURE 2
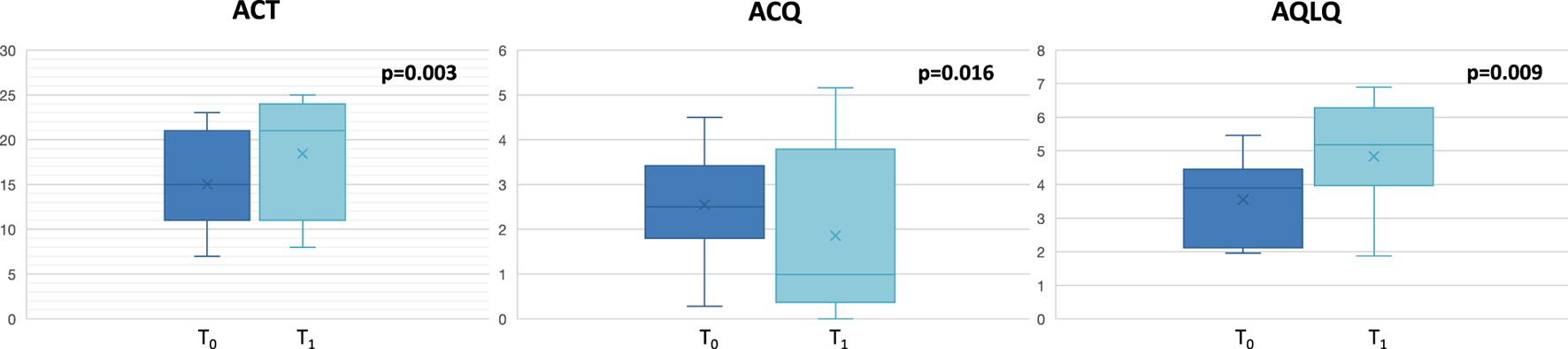
Changes in asthma control and quality of life at T0 and at T1. ACT: Asthma Control Test; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire.
TABLE 2
| Characteristic | T0 (baseline) | T1 (6 months) | p-value |
|---|---|---|---|
| Lung function evaluations | |||
| FEV1% predicted | 70.27 ± 24.76 | 82.45 ± 32.8 | 0.013 |
| FVC% predicted | 79.45 ± 19.93 | 91.36 ± 30.49 | 0.002 |
| FEV1/FVC | 0.73 ± 0.18 | 0.72 ± 0.12 | ns |
| FEF 25-75% predicted | 50.64 ± 35.06 | 56.91 ± 32.25 | <0.05 |
| Asthma and quality of life scores | |||
| ACT | 15 ± 5.71 | 17.33 ± 7.23 | 0.003 |
| ACQ | 2.54 ± 1.28 | 1.85 ± 1.89 | 0.016 |
| AQLQ | 3.55 ± 1.28 | 4.83 ± 1.66 | 0.009 |
| 6-min walk test | |||
| IC pre 6MWT test (L) | 2.40 ± 0.48 | 2.74 ± 1.14 | 0.010 |
| IC post 6MWT test (L) | 1.60 ± 0.83 | 2.85 ± 1.22 | <0.05 |
| VE pre 6MWT test (L·min−1) | 13.88 ± 4.44 | 11.35 ± 7.34 | <0.05 |
| VE post 6MWT test (L·min−1) | 23.77 ± 12.11 | 22.34 ± 7.06 | <0.05 |
| 6MWD (meters) | 393 ± 221 | 375 ± 186 | ns |
| SpO2 pre 6MWT test (%) | 95.91 ± 1.08 | 96.54 ± 1.17 | ns |
| SpO2 post 6MWT test (%) | 95 ± 2.73 | 96.36 ± 2.38 | ns |
Change in clinical and lung function evaluations of the study subjects at T0 and T1.
Data are presented as mean ±SD, unless otherwise stated. ACT, Asthma Control Test; ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25–75%, forced expiratory flow at 25–75% of FVC; IC, inspiratory capacity; SpO2, peripheral oxygen saturation; VE, minute ventilation; 6MWD, 6-min walk distance; 6MWT, 6-min walk test.
At T0, pre-6MWT-IC and post-6MWT-IC were 2.40 ± 0.48 L and 1.60 ± 0.83 L, respectively (p < 0.0001). Minute ventilation (VE) at the beginning of the 6MWT was 13.88 ± 4.44 L·min−1and post 6MWT was 23.77 ± 12.11 L·min−1 (p < 0.0001). The distance walked was 393 ± 221 m.
At T1, pre-6MWT-IC was 2.74 ± 1.14 L and post-6MWT-IC was 2.85 ± 1.22 L (p = NS); similarly, VE did not change (pre-6MWT vs. post-6MWT: 11.35 ± 7.34 L·min−1 vs. 22.34 ± 7.06 L·min−1). The average walked distance was 375 ± 186 m).
When comparing pre-6MWT-IC between T0 and T1, IC significantly increased after treatment period, indicating the beneficial effect of benralizumab on static hyperinflation (pre-6MWT-IC, T0 vs. T1: 2.40 ± 0.48 L vs 2.74 ± 1.14 L; p = 0.01). In addition, at T1 IC did not change after exercise (p = 0.53) suggesting that DH disappeared after treatment with benralizumab (Figure 3).
FIGURE 3
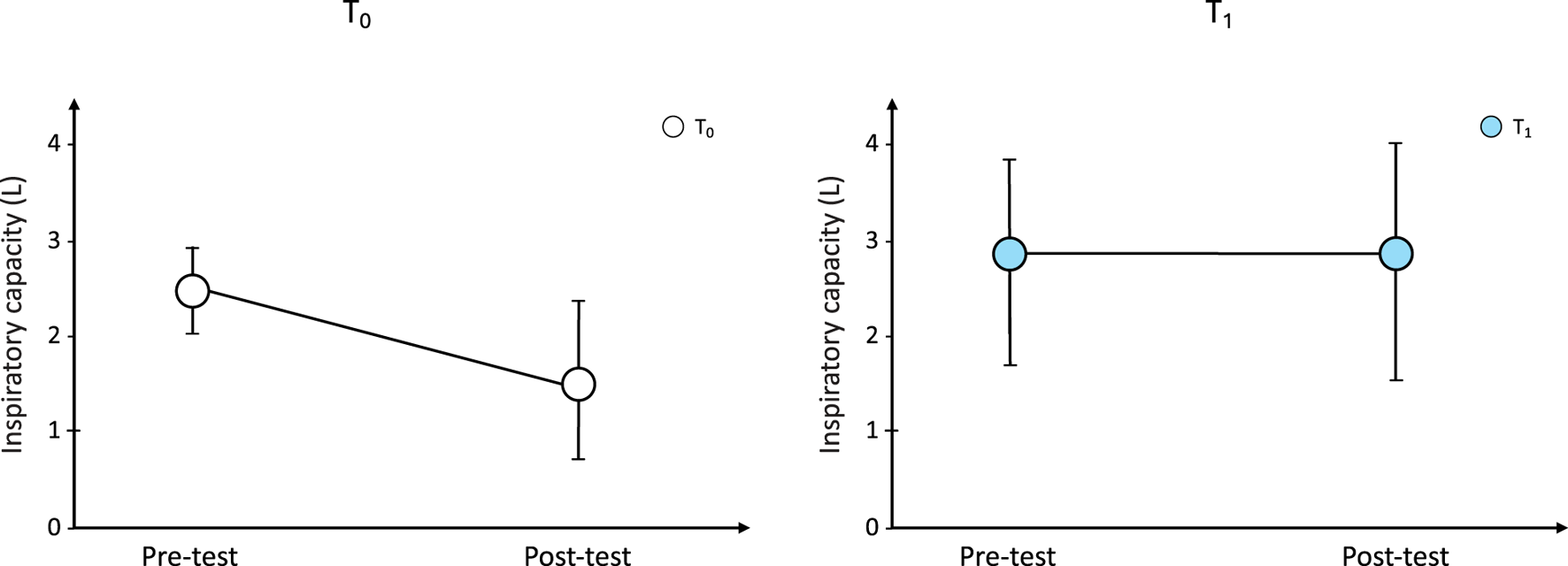
Changes from baseline in IC during the 6-min walk test at T0 and after bernalizumab treatment. IC: inspiratory capacity.
No difference in terms of percutaneous oxygen saturation and meters walked in the 6MWT was observed after the treatment period.
Discussion
DH is considered one of the main factors contributing to asthma symptoms and impaired daily life activity (van der Meer et al., 2019); for this reason, it is candidate to become an important treatable trait in moderate to severe asthma. In this exploratory study, we measured the IC changes to evaluate the occurrence of static and dynamic hyperinflation in experimental settings resembling daily activities. The current findings confirm the occurrence of DH in severe asthmatics, and demonstrate that a 6-month period of treatment with biologic drug is able to 1) significant increase IC at resting condition, suggesting a beneficial action on static hyperinflation, and 2) reduce the occurrence of DH, which disappeared in 10 out of 12 patients.
Static hyperinflation is a functional condition primarily caused by abnormally increased static (or quasi-static) pulmonary compliance, and is expressed by reduced IC. Although this is common in emphysema, there is evidence suggesting that static pulmonary compliance can also increase in severe asthmatics, especially during exacerbation, and return to normal condition after antinflammatory treatment (Gold et al., 1967; Gelb et al., 1998). In subjects with severe airway obstruction and high respiratory rates, with the consequent occurrence of expiratory flow-limitation, DH may occur. It is plausible to conceive that, following pharmacological treatment capable of reducing airway obstruction, IC increases because of lung deflation towards resting conditions of the respiratory system. The current findings support this phenomenon, showing an increase in IC at resting (pre-WT) conditions after treatment with biologic, which paralleled the increase in FEV1. As mentioned above, the possibility that the biologic treatment has acted on lung compliance through unknown mechanisms cannot be excluded.
The most interesting observation is that DH disappeared after treatment with benralizumab. A real-life investigation on the effect of benralizumab on lung volumes (Pelaia et al., 2020) demonstrated clinically relevant changes in static lung volumes after 24 weeks of treatment in 22 severe asthmatics. The tendency to reduction in lung hyperinflation was also shown in a larger cohort of severe asthmatics in a phase IIIB study on benralizumab (the SOLANA study) (Panettieri et al., 2020). Maniscalco and coworkers (Maniscalco et al., 2024) conducted a retrospective observational study on the effect of biologics on lung hyperinflation, showing a tendency towards efficacy in reducing lung hyperinflation of biological agents. The novelty of our findings lies in the fact that lung volume changes were assessed with a portable spirometer during the walking test, in a condition that mimics daily common activities. In a previous study, Benfante et al. (2018) highlighted this phenomenon by studying two groups of patients (subject with severe asthma and individuals suffering from COPD) with a similar degree of obstruction. The study showed that severe asthmatics developed dynamic hyperinflation to the same extent of COPD, by means of similar reductions in IC during the WT. Another study showed that patients with history of severe asthma, in particular the “near-fatal” patients, underwent a significant decline of IC during cardiopulmonary exercise test (Rinaldo et al., 2023). These observations clearly confirm that DH occurs in the most severe forms of asthma. Recent observations demonstrated that, unlike to COPD, DH in asthmatics is strictly associated with airway inflammation and can be reduced by systemic anti-inflammatory treatment (van der Meer et al., 2021). In this context, it is possible to consider that a treatment that selectively blocks the inflammatory cascade has beneficial effects on inflamed airways. Taken together, these results suggest that systemic anti-inflammatory treatments, including monoclonal antibodies, may have the potential to positively impact on daily life activities and improve exercise capacity by decreasing DH. Available data support this assumption, by showing an effect of benralizumab on functional indices of peripheral airways (Pelaia et al., 2020; Maniscalco et al., 2024; Chan and Lipworth, 2023).
The increase of IC appears to be associated with important clinical implications in terms of exercise tolerance and perception of dyspnea, more so than the FEV1 parameter. The IC represents an indirect parameter for measuring end-expiratory lung volume, being on the other hand an extremely simple test to perform. It has been shown that the increase in IC, associated with bronchodilator therapy, significantly correlates with dyspnea at rest, with dyspnea during exercise and with physical performance and consequently with quality of life (Pretto et al., 2007). It is known that in asthmatic subjects, poor control of the symptoms of the disease, including dyspnea, is associated with a greater risk of limitation of physical activity, with a negative impact on work and daily life activities (Haselkorn et al., 2010). Carpagnagno et al. investigated the physical activity levels in patients suffering from severe asthma and treated with biologic therapy, and demonstrated that these therapies could be valuable in augmenting the physical activity level in severe asthma (Carpagnano et al., 2019). Lombardi et al. (Lombardi et al., 2023) assessed the clinical response to mepolizumab using the 6MWT, showing the potential of this test to complement the assessment of severe asthma. In this study, 6MWT showed sensitivity to change after asthma treatment and good correlations with asthma symptoms, quality of life and small airway dysfunction.
In conclusion, DH is an important target and an important treatable trait in severe asthma. The current study confirmed that severe asthmatic subjects develop DH during exercise and showed that a 6-month period of treatment with benralizumab in severe eosinophilic asthmatics is able to increase IC significantly at resting condition, suggesting a beneficial action on static hyperinflation, and to reduce significantly the occurrence of DH, impacting on daily activities and quality of life. Further studies are needed to explore these preliminary findings in a larger population of severe asthmatic subjects.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by AOUP Giaccone Policlinico Palermo, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AB: Conceptualization, Formal Analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review and editing. AT: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. LG: Data curation, Investigation, Validation, Visualization, Writing – review and editing. AL: Data curation, Investigation, Writing – review and editing. PM: Data curation, Investigation, Visualization, Writing – review and editing. SB: Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review and editing. NS: Conceptualization, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
AB and NS conceived and designed the study; AB, AT, and PM were involved in the acquisition of data; SB performed the statistical analyses. All authors were involved in drafting the work or revising it critically for data analysis and interpretation; and all authors agreed to the submission and to be accountable for all aspects of the work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Benfante A. Di Marco F. Terraneo S. Centanni S. Scichilone N. (2018). Dynamic hyperinflation during the 6-min walk test in severely asthmatic subjects. ERJ Open Res.4, 00143-2017–2017. 10.1183/23120541.00143-2017
2
Bleecker E. R. FitzGerald J. M. Chanez P. Papi A. Weinstein S. F. Baker P. et al (2016). Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β(2)-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet388 (10056), 2115–2127. 10.1016/S0140-6736(16)31324-1
3
Carpagnano G. E. Sessa F. Scioscia G. Lacedonia D. Foschino M. P. Venuti M. O. P. et al (2019). Physical activity as a new tool to evaluate the response to omalizumab and mepolizumab in severe asthmatic patients: a pilot study. Front. Pharmacol.10, 1630. 10.3389/fphar.2019.01630
4
Chan R. Lipworth B. J. (2023). Real-life effects of benralizumab on airway oscillometry in severe eosinophilic asthma. BMJ Open Respir. Res.10 (1), e001472. 10.1136/bmjresp-2022-001472
5
Chung F. K. Wenzel S. E. Brozek J. L. Bush A. Castro M. Sterk P. J. et al (2014). International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J.43, 343–373. 10.1183/09031936.00202013
6
FitzGerald J. M. Bleecker E. R. Nair P. Korn S. Ohta K. Lommatzsch M. et al (2016). Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet388 (10056), 2128–2141. 10.1016/S0140-6736(16)31322-8
7
Gelb A. F. Zamel N. Hogg J. C. Müller N. L. Schein M. J. (1998). Pseudophysiologic emphysema resulting from severe small-airways disease. Am. J. Respir. Crit. Care Med.158 (3), 815–819. 10.1164/ajrccm.158.3.9801045
8
Global Initiative for Asthma (2024). Global strategy for asthma management and prevention. Available online at: www.ginasthma.org.
9
Gold W. M. Kaufman H. S. Nadel J. A. (1967). Elastic recoil of the lungs in chronic asthmatic patients before and after therapy. J. Appl. Physiol.23 (4), 433–438. 10.1152/jappl.1967.23.4.433
10
Graham B. L. Steenbruggen I. Miller M. R. Barjaktarevic I. Z. Cooper B. G. Hall G. L. H. et al (2019). Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am. J. Respir. Crit. Care Med.200 (8), 70–88. 10.1164/rccm.201908-1590ST
11
Haselkorn T. Chen H. Miller D. P. Fish J. E. Peters S. P. Weiss S. T. et al (2010). Asthma control and activity limitations: insights from the real-world evaluation of asthma control and treatment (REACT) study. Ann. Allergy Asthma Immunol.104 (6), 471–477. 10.1016/j.anai.2010.04.006
12
Holland A. E. Spruit M. A. Troosters T. Puhan M. A. Pepin V. Saey D. et al (2014). An official european respiratory society/american thoracic society technical standard: field walking tests in chronic respiratory disease. Eur. Respir. J.44, 1428–1446. 10.1183/09031936.00150314
13
Lombardi C. Berti A. Cottini M. Roca E. Ventura L. (2023). Using the 6-min walk test to assess the clinical response to mepolizumab and conventional therapy in severe eosinophilic asthma. ERJ Open Res.9 (5), 00114–2023. 10.1183/23120541.00114-2023
14
Maniscalco M. Candia C. Calabrese C. D’Amato M. Matera M. G. Molino A. et al (2024). Impact of biologics on lung hyperinflation in patients with severe asthma. Respir. Med.225, 107578. 10.1016/j.rmed.2024.107578
15
Miller M. R. Hankinson J. Brusasco V. Burgos F. Casaburi R. Coates A. et al (2005). Standardisation of spirometry. Eur. Respir. J.26, 319–338. 10.1183/09031936.05.00034805
16
Nair P. Wenzel S. Rabe K. F. Bourdin A. Lugogo N. L. Kuna P. et al (2017). Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N. Engl. J. Med.376 (25), 2448–2458. 10.1056/NEJMoa1703501
17
O’Donnell D. E. Revill S. M. Webb K. A. (2001). Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.164, 770–777. 10.1164/ajrccm.164.5.2012122
18
Panettieri R. A. Jr Welte T. Shenoy K. V. Korn S. Jandl M. Kerwin E. M. et al (2020). Onset of effect, changes in airflow obstruction and lung volume, and health-related quality of life improvements with benralizumab for patients with severe eosinophilic asthma. Phase IIIb randomized, controlled trial (SOLANA). J. Asthma Allergy13, 115–126. 10.2147/JAA.S240044
19
Pelaia C. Busceti M. T. Crimi C. Carpagnano E. G. Lombardo N. Terracciano R. et al (2020). Real-life effects of benralizumab on exacerbation number and lung hyperinflation in atopic patients with severe eosinophilic asthma. Biomed. and Pharmacother.129, 110444. 10.1016/j.biopha.2020.110444
20
Pretto J. J. McMahon M. A. Rochford P. D. Berlowitz D. J. Jones S. M. Brazzale D. J. et al (2007). A pilot study of inspiratory capacity and resting dyspnea correlations in exacerbations of COPD and asthma. Int. J. Chron. Obstruct Pulmon Dis.2 (4), 651–656.
21
Rinaldo R. F. Imeri G. Mondoni M. Parazzini E. M. Vigo B. Masseroni A. et al (2023). Does the severity of asthma affect exercise capacity and daily physical activity?J. Asthma60 (8), 1622–1631. 10.1080/02770903.2023.2169932
22
van der Meer A.-N. de Jong K. Hoekstra-Kuik A. Bel E. H. ten Brinke A. (2019). Dynamic hyperinflation impairs daily life activity in asthma. Eur. Respir. J.53, 1801500. 10.1183/13993003.01500-2018
23
van der Meer A.-N. de Jong K. Hoekstra-Kuik A. Bel E. H. ten Brinke A. (2021). Targeting dynamic hyperinflation in moderate-to-severe asthma: a randomised controlled trial. ERJ Open Res.7, 00738–2020. 10.1183/23120541.00738-2020
24
Vermeulen F. Garcia G. Ninane V. Laveneziana P. (2016). Activity limitation and exertional dyspnea in adult asthmatic patients: what do we know?Respir. Med.117, 122–130. 10.1016/j.rmed.2016.06.003
25
Wanger J. Clausen J. L. Coates A. Pedersen O. F. Brusasco V. Burgos F. et al (2005). Standardisation of the measurement of lung volumes. Eur. Respir. J.26, 511–522. 10.1183/09031936.05.00035005
26
Wilson S. R. Rand C. S. Cabana M. D. Foggs M. B. Halterman J. S. Olson L. et al (2012). Asthma outcomes: quality of life. J. Allergy Clin. Immunol.129, S88–S123. 10.1016/j.jaci.2011.12.988
Summary
Keywords
dynamic hyperinflation, inspiratory capacity, severe asthma, exercise, benralizumab
Citation
Benfante A, Tomasello A, Gentile L, Lisotta AL, Marasà P, Battaglia S and Scichilone N (2025) The effects of benralizumab on lung volume changes during exercise in experimental setting in severe asthmatics: a pilot study. Front. Pharmacol. 16:1611168. doi: 10.3389/fphar.2025.1611168
Received
13 April 2025
Accepted
04 July 2025
Published
23 July 2025
Volume
16 - 2025
Edited by
Izolde Bouloukaki, University of Crete, Greece
Reviewed by
Corrado Pelaia, University of Magna Graecia, Italy
Fabio Perrotta, University of Campania Luigi Vanvitelli, Italy
Ersin Demirer, Istanbul Kartal Dr. Lutfi Kirdar Education and Research Hospital, Türkiye
Updates
Copyright
© 2025 Benfante, Tomasello, Gentile, Lisotta, Marasà, Battaglia and Scichilone.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alida Benfante, alida.benfante@unipa.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.