- 1Department of Pediatrics, Binhai County People’s Hospital, Yancheng, Jiangsu, China
- 2Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Co-Innovation Center of Neuroregeneration, NMPA Key Laboratory for Research and Evaluation of Tissue Engineering Technology Products, Nantong University, Nantong, Jiangsu, China
- 3Department of Orthopaedics, Changshu Hospital Affiliated to Soochow University, First Peoples’ Hospital of Changshu City, Changshu, Jiangsu, China
Cerebral ischemia-reperfusion injury (Cerebral I/R injury) is a critical pathological process following ischemic stroke, closely associated with multiple mechanisms including oxidative stress, neuroinflammation, and neuronal apoptosis. It also involves the alteration and regulation of numerous key genes and non-coding RNAs. Due to its complex regulatory mechanisms, there are currently no Food and Drug Administration (FDA)-approved drugs specifically targeting Cerebral I/R injury. Developing effective therapeutic strategies for Cerebral I/R injury remains a significant challenge in medical research. This review summarizes current treatment approaches for Cerebral I/R injury, which include traditional drugs, antioxidants, neuroprotective agents, exosomes, noncoding RNA therapeutics and combined intervention therapy. Pharmacotherapies exert positive effects on Cerebral I/R injury through antioxidative, anti-inflammatory, and neuroprotective mechanisms. Exosomes and noncoding RNA therapeutics can mitigate brain cell damage and promote neural function recovery by regulating the expression of downstream key genes via miRNAs, demonstrating potential as novel therapeutic options. Emerging evidence indicates that combined therapeutic strategies incorporating nanoparticle-mediated targeting demonstrate efficacy in treating cerebral I/R injury. By exploring the mechanisms of action and clinical application prospects of these different treatment strategies, this review aims to provide new insights and methods for the clinical management of Cerebral I/R injury.
1 Introduction
The brain is one of the most critical organs in the human body, requiring a substantial blood supply to provide oxygen and nutrients. Ischemic stroke, primarily caused by inadequate cerebral blood flow, results in loss of brain function and accounts for approximately 70%–80% of all stroke cases (Sakai and Shichita, 2019; Sommer, 2017). The primary clinical treatment strategy for ischemic stroke involves intravenous thrombolysis with tissue plasminogen activator (tPA) within 4.5 h or mechanical thrombectomy within 24 h to restore cerebral blood flow, a process known as reperfusion (Li M. et al., 2022). However, while reperfusion can alleviate the ischemic condition in brain tissue, it can also cause damage to neurons, glial cells, and other cell types, exacerbating the inflammatory response and brain injury. This phenomenon is referred to as Cerebral Ischemia-Reperfusion Injury (Cerebral I/R injury). The mechanisms underlying Cerebral I/R injury include excitotoxicity from excitatory amino acids, oxidative stress, calcium overload, mitochondrial dysfunction, endoplasmic reticulum stress, neuroinflammation, and disruption of the blood-brain barrier (BBB). The interplay of these mechanisms can ultimately lead to severe neuronal death and neurological dysfunction, further aggravating brain injury. Key regulatory molecules and signaling pathways, as well as non-coding RNAs such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), play crucial roles in the pathogenesis of Cerebral I/R injury.
The treatment strategies for Cerebral I/R injury remain a prominent and challenging focus in medical research. In recent years, numerous therapeutic modalities have been proposed and extensively investigated, including traditional drug therapies, antioxidants, neuroprotective agents, exosomes, noncoding RNA therapeutics and combined intervention therapy. These approaches aim to mitigate or reverse neuronal damage caused by the ischemia/reperfusion process and promote the recovery of neural function (Mao R. et al., 2022; Jurcau and Simion, 2021). Traditional drug therapies protect brain tissue through multiple mechanisms; antioxidants and neuroprotective agents exert relatively specific neuroprotective effects; exosome and noncoding RNA therapeutics represent highly promising emerging approach for repairing and reconstructing damaged tissues (Hamblin and Lee, 2021; Hermann et al., 2024; Winkle et al., 2021). While each of these treatment methods offers distinct advantages, they also face significant challenges and limitations. Promising preclinical findings demonstrate that nanoparticle-based targeting systems combined with adaptive interventions hold significant potential for addressing the dynamic pathological progression of cerebral I/R injury. This review integrates the latest research advancements to provide a comprehensive analysis of the treatment strategies for Cerebral I/R injury, focusing on traditional drug therapies, antioxidants, neuroprotective agents, exosome-based treatments, noncoding RNA therapeutics and combined intervention therapy with the goal of offering novel insights and approaches for the clinical management of Cerebral I/R injury.
2 Cerebral I/R injury mechanisms
2.1 Pathological process of cerebral I/R injury
Cerebral I/R injury is a complex pathological process involving multiple mechanisms (Figure 1). Excitatory amino acids, such as glutamate, play a critical role in Cerebral I/R injury. During ischemia, the excessive release of glutamate from synaptic vesicles leads to an influx of calcium ions, triggering excitotoxicity and subsequent cell death. Upon reperfusion, the restoration of energy metabolism disrupts the balance between glutamate release and uptake, further exacerbating excitotoxicity (Yu et al., 2023). Simultaneously, nitric oxide (NO) production during Cerebral I/R injury may exceed physiological levels, leading to cellular toxicity. NO reacts with superoxide anions to form peroxynitrite, which induces oxidative stress and cellular damage (Wang Y. et al., 2022; Terpolilli et al., 2012; Bolanos and Almeida, 1999). Free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), are closely associated with Cerebral I/R injury. During ischemia, insufficient oxygen supply results in the accumulation of free radicals within cells, causing protein dysfunction, DNA damage, and lipid peroxidation (Sun et al., 2018). Upon reperfusion, the generation of these free radicals increases further, reacting with cell membranes, proteins, and nucleic acids, thereby damaging cellular structure and function. Mitochondria, which produce approximately 90% of cellular ROS, are particularly vulnerable. Cerebral I/R injury enhances mitochondrial ROS production, leading to mitochondrial dysfunction (Kumar Saini and Singh, 2024). Calcium overload is another crucial mechanism in Cerebral I/R injury. During ischemia, intracellular calcium ion concentrations rise, activating calcium-dependent enzymes and disrupting the cytoskeleton. Upon reperfusion, calcium ions flood into cells, exacerbating calcium overload, increasing mitochondrial membrane permeability, and triggering both apoptosis and necrosis (Du et al., 2021; Yin et al., 2019). Additionally, mitochondrial biogenesis, dynamic changes, and abnormal autophagy all contribute to the exacerbation of brain I/R injury. In the early stages of reperfusion, autophagy plays a protective role by clearing damaged organelles and misfolded proteins. However, excessive autophagy in later stages can lead to cell death and secondary tissue damage (Huang et al., 2019). Similarly, endoplasmic reticulum stress (ERS) and the unfolded protein response (UPR) initially provide protection by promoting adaptive responses (Shen et al., 2021; Wang et al., 2020; Li R. et al., 2021). However, prolonged oxidative stress and calcium overload during reperfusion exacerbate misfolded protein accumulation and severe ERS, leading to UPR failure and activation of apoptotic pathways (Jiang et al., 2023; Yang M. et al., 2024). Furthermore, Cerebral I/R injury activates microglia within the central nervous system and induces infiltration and accumulation of peripheral immune cells (Wu et al., 2020a). Damage-associated molecular patterns (DAMPs) released from necrotic tissues trigger the activation of resident microglia, which in turn elevate inflammatory cytokines and promote the recruitment of neutrophils, monocytes, and lymphocytes to the central nervous system, thereby widely activating peripheral immune responses (Chen H. et al., 2020). Concurrently, Cerebral I/R injury damages cerebral microvascular endothelial cells and compromises BBB. Post-injury, endothelial cell permeability increases, leading to BBB disruption, which facilitates further infiltration of peripheral immune cells, exacerbates inflammation, and contributes to more severe tissue damage (Henrot et al., 2023; Shi et al., 2020).
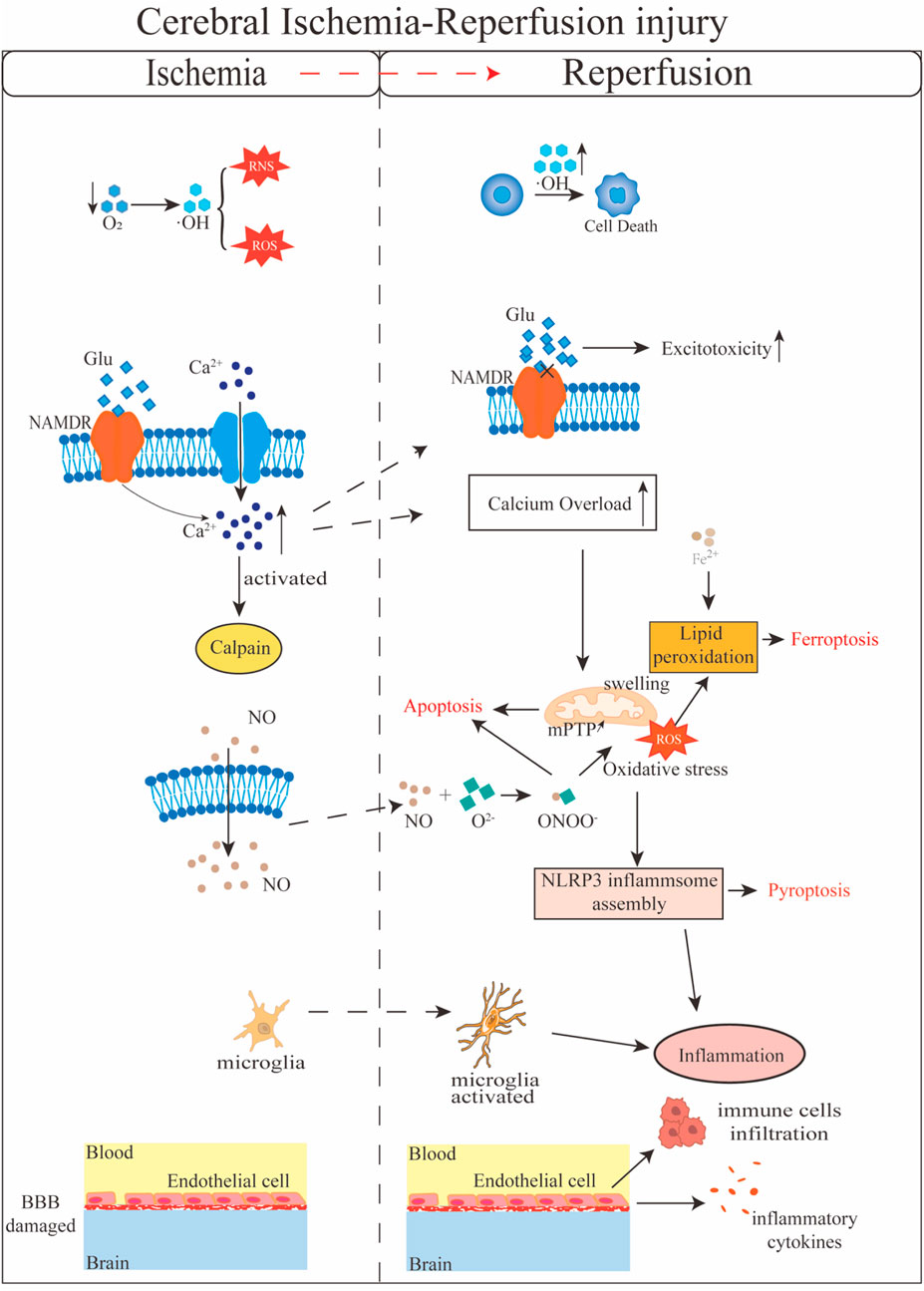
Figure 1. Schematic diagram of the molecular mechanisms involved in cerebral ischemia-reperfusion injury.
Cell death is a critical mechanism underlying I/R injury and serves as an important pathological indicator. In neuronal cells, the restoration of cerebral blood flow can trigger multiple forms of cell death, including apoptosis, necroptosis, pyroptosis, panoptosis, and ferroptosis (Tuo et al., 2022a). During ischemia, mitochondrial dysfunction leads to increased membrane permeability, resulting in the release of cytochrome c and activation of the Caspase signaling cascade (Datta et al., 2020). In Cerebral I/R injury, necroptosis regulated by the RIPK1/RIPK3/MLKL signaling pathway plays a pivotal role (Liao et al., 2020; Li J. et al., 2019; Wang et al., 2018). Pyroptosis is prevalent in the early stages of ischemia-reperfusion and is closely linked to inflammatory responses. The Nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 (NLRP3) inflammasome is a key mediator in this process, and several drugs and inhibitors have been shown to reduce neuronal damage and cerebral infarction following Cerebral I/R injury by inhibiting NLRP3-dependent pyroptosis (Wang L. et al., 2022; Kang et al., 2021; Xiao et al., 2021). Recently, researchers have identified a unique form of inflammatory programmed cell death during Cerebral I/R injury, termed PANoptosis (Yan et al., 2022; Lan et al., 2024). Z-DNA binding protein 1 (ZBP1) and transforming growth factor-β-activated kinase 1 (TAK1) are central regulators of PANoptosis, although the precise mechanisms remain to be fully elucidated (Qiu et al., 2024). Ferroptosis, characterized by lipid peroxidation and iron overload, is also a significant contributor to neuronal death in Cerebral I/R injury (Tuo et al., 2022b). During cerebral ischemia-reperfusion, disruption of the BBB leads to iron dyshomeostasis, while excessive ROS generation further promotes ferroptosis (She et al., 2023). These various cell death pathways act synergistically in Cerebral I/R injury, and ongoing research may identify these signaling pathways as potential therapeutic targets.
2.2 Key molecules and potential therapeutic targets for cerebral I/R injury
2.2.1 Regulatory molecules and signaling pathways
With our in-depth understanding of the pathological process of cerebral ischemia-reperfusion injury, an increasing number of related molecules involved in the regulation of pathways such as oxidative stress, inflammatory response, and apoptotic signaling have been found to play a crucial role in the occurrence of cerebral ischemia-reperfusion injury. The chemokine (C-C motif) ligand 2/chemokine receptor 2 (CCL2/CCR2) signaling pathway is implicated in acute neuroinflammation and BBB dysfunction following cerebral ischemia-reperfusion. Clinical data indicate that elevated levels of CCL2 in blood or cerebrospinal fluid correlate with clinical symptoms in stroke patients and serve as a prognostic biomarker (Geng et al., 2022; Xu Q. et al., 2024; Liu S. et al., 2023). In recanalized stroke patients, circulating Caveolin-1 (Cav-1) levels are reduced; however, enhancing Cav-1 expression in endothelial cells in middle cerebral artery occlusion/reperfusion (MCAO/R) injury mice significantly reduces infarct volume, decreases vascular permeability, and mitigates thrombo-inflammation (Zhang X. et al., 2022). Platelet-specific deletion of Atypical Chemokine Receptor 3 (ACKR3) enhances platelet activation and thrombosis, exacerbating inflammation and brain tissue damage (Rohlfing et al., 2022). Tripartite Motif Containing 45 (TRIM45) is highly expressed in the peri-infarct region of MCAO/R-treated mice and plays a pivotal role in neuroinflammation via the NF-κB signaling pathway (Xia et al., 2022). Recent studies show that Cav3.2 knockout attenuates oxidative stress, inflammatory response, and neuronal apoptosis through the calcineurin/NFAT3 pathway (Dai et al., 2024). The transient receptor potential cation channel subfamily C member 6 (TRPC6) activation prevents neuronal death, while its blockade increases ischemic sensitivity (Li W. et al., 2019; Liu et al., 2020a; Shekhar et al., 2021; Liu et al., 2020b). Additionally, Rnf213 upregulation correlates with neuronal apoptosis and infarct volume, and RACK1 knockdown exacerbates apoptosis (Li et al., 2023a; Zhao et al., 2024). Furthermore, the latest research has found that some deubiquitinating enzymes (DUBs) play a dual role in cerebral ischemia-reperfusion injury, influencing the extent of injury by regulating processes such as inflammation, oxidative stress, and cell death (Qin X. et al., 2025). In conclusion, as we gain deeper insights into the molecular mechanisms of cerebral ischemia-reperfusion injury, more precise and effective therapeutic strategies can be developed to mitigate brain damage and improve patient outcomes. Future research will continue to explore the interactions among these molecular mechanisms and their specific roles in cerebral ischemia-reperfusion injury, offering new hope for stroke patients.
2.2.2 Non-coding RNAs
Non-coding RNAs, including miRNAs, lncRNAs, and circRNAs, play essential roles in regulating cerebral I/R injury through the modulation of neuroinflammation, oxidative stress, and apoptosis (Table 1). These ncRNA classes synergistically govern BBB integrity and intercellular crosstalk emerging as pivotal therapeutic targets in cerebrovascular disorders. The expression profile of miRNAs in cerebral I/R injury undergoes dynamic alterations, critically contributing to neuroinflammation, oxidative stress, and neuronal apoptosis (Maitrias et al., 2017; Xu et al., 2025; Neag et al., 2022). miR-19a/b-3p exacerbates neuroinflammation by activating the SIRT1/FoxO3/SPHK1 pathway, amplifying pro-inflammatory cytokine production, while miR-328-3p enhances TNF-α and IL-6 expression, promoting neutrophil infiltration (Zhou et al., 2021; Wang S. et al., 2021). miR-670 induces phosphorylation-mediated degradation of Yes-associated protein (YAP), exacerbating neuronal apoptosis and neurological deficits, whereas miR-30a-5p disrupts BBB integrity through ZnT4/zinc dyshomeostasis (Yu et al., 2021; Wang P. et al., 2021). Conversely, miR-532-5p suppresses the CXCL1/CXCR2/NF-κB axis, and miR-652 mitigates oxidative stress by targeting NOX2 (Shi et al., 2021; Zuo et al., 2020). Emerging regulators include miR-124, which inhibits STAT3 phosphorylation to attenuate microglial activation and pyroptosis, and miR-139-5p, activating the Nrf2 antioxidant pathway to counteract ROS/TXNIP-driven NLRP3 activation (Sun et al., 2020; Yao et al., 2022). miR-223 directly binds NLRP3 mRNA to limit cytokine release, while astrocyte-derived exosomal miR-29a-3p suppresses TP53INP1/NF-κB signaling in microglia (Sha et al., 2019; Liu X. et al., 2022). Age-dependent duality is observed in miR-155-5p, where knockdown inhibits DUSP14/NF-κB signaling in young models but requires preservation in aged brains to maintain cognitive stability (Shi Y. et al., 2022; Que et al., 2020). Additionally, macrophage-derived exosomal miR-Novel-3 exacerbates ferroptosis and neuroinflammation by activating microglia (Qin et al., 2024). Collectively, miRNAs orchestrate I/R injury progression through synergistic modulation of inflammatory pathways, redox imbalance, BBB breakdown, and intercellular crosstalk, highlighting their pivotal roles in ischemic pathogenesis.
lncRNAs, non-coding transcripts exceeding 200 nucleotides, regulate ischemic injury through interactions with RNA, DNA, proteins, or RNA-binding proteins. A critical mechanism involves their role as molecular sponges for miRNAs, dynamically modulating gene expression under ischemic stress. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), an evolutionarily conserved lncRNA, is highly expressed in ischemic stroke patients and exacerbates neuroinflammation by interacting with STAT1 to enhance NLRP3 transcription, driving microglial pyroptosis in diabetic models (Khoshnam et al., 2024; Tan et al., 2021; Zhao N. et al., 2023). This interaction amplifies NLRP3-mediated inflammatory cascades, linking MALAT1 dysregulation to poor clinical outcomes (Fathy et al., 2021). Maternally expressed gene 3 (MEG3), upregulated post-ischemia, activates pyroptosis via competitive binding to miR-485, which elevates AIM2 inflammasome components, while simultaneously exacerbating mitochondrial dysfunction and oxidative stress (Li T. H. et al., 2022; Yan et al., 2024; Yao et al., 2024). Potassium voltage-gated channel subfamily Q member one opposite strand 1 (KCNQ1OT1), significantly overexpressed in ischemic stroke, functions as a multi-miRNA sponge (miR-153-3p, miR-140-3p, miR-30b, miR-9) to promote Foxo3-mediated pyroptosis and MMP8-driven neuronal injury. Its knockdown mitigates autophagy via the miR-200a/FOXO3/ATG7 axis, reducing infarct size and endoplasmic reticulum stress (Yu et al., 2019; Yi et al., 2020; Li Y. et al., 2020). X-inactive specific transcript (XIST) exacerbates endothelial pyroptosis by sponging miR-96-5p, leading to NLRP3 inflammasome activation and IL-1β hypersecretion (Zhang et al., 2021). Other lncRNAs, such as taurine upregulated gene 1 (TUG1), disrupt BBB integrity through miR-145/AQP4 dysregulation in astrocytes, while LOC102555978 directly activates NLRP3-dependent microglial pyroptosis, and fetal-lethal noncoding developmental regulatory RNA (FENDRR) amplifies NLRC4 inflammasome signaling to intensify diabetic CIRI (Shan et al., 2020; Yin et al., 2021; Wang L. Q. et al., 2021; Gao et al., 2024). Additional lncRNAs like five prime to XIST (FTX), small nucleolar RNA host gene 12/14/15 (SNHG12/14/15), SOX2 overlapping transcript (SOX2OT), and CCAAT enhancer binding protein alpha antisense RNA 1 (CEBPA-AS1) further modulate ischemic injury via miRNA interactions, influencing oxidative stress, angiogenesis, and neuroinflammatory pathways (Wang J. et al., 2021; Wang et al., 2022c; Wang and Hu, 2021; Tu et al., 2022; Qi et al., 2017; Barangi et al., 2023). Collectively, lncRNAs orchestrate CIRI progression through inflammasome activation, pyroptosis amplification, redox imbalance, and multi-target miRNA sponging, positioning them as pivotal regulators of ischemic pathogenesis.
Circular RNAs (circRNAs), a class of covalently closed non-coding RNAs with enhanced structural stability, are emerging as pivotal regulators in CIRI, where they modulate neuronal injury and repair through miRNA-dependent mechanisms. These molecules, abundant in the nervous system, not only participate in physiological processes like neuronal development and synaptic plasticity but also drive pathological cascades in CIRI (Yang et al., 2018). For instance, circular RNA TLK1 (circTLK1) is upregulated in MCAO models and ischemic stroke patients, where it exacerbates neuronal damage by competitively binding miR-335-3p and promoting TCDD-inducible PARP overexpression (Wu et al., 2019). Similarly, circular RNA HECTD1 (circ-HECTD1) contributes to astrocyte activation and neuronal loss via the miR-142/TIPARP and miR-133b/TRAF3 axes, respectively (Han et al., 2018; Dai et al., 2021). Recent studies further reveal the involvement of circRNAs in inflammatory cell death pathways: circular RNA derived from RIMS1 (circRIMS), previously linked to cancer progression, amplifies NLRP3 inflammasome activity and inflammatory cytokine release by sponging miR-96-5p to activate JAK/STAT1 signaling in CIRI models, while circular RNA from coiled-coil domain-containing 6 (circCCDC6) enhances neuronal injury by suppressing miR-128-3p, leading to TXNIP/NLRP3 pathway activation (Li W. et al., 2023; Wang C. et al., 2023). Beyond these mechanisms, circular RNA ASXL2 (circASXL2), circular RNA CAMK4 (circCAMK4), and circular RNA UCK2 (circUCK2) exacerbate neuronal degeneration through miRNA dysregulation, whereas circular RNA FOXP1 (circFOXP1) and circular RNA FoxO3 (circ-FoxO3) counteract injury by inhibiting STAT3-mediated apoptosis and stabilizing BBB integrity via mTORC1 modulation (Zhang et al., 2024a; Zhang et al., 2020; Tang et al., 2020; Chen W. et al., 2020; Dai et al., 2022; Yang B. et al., 2021; Yang J. et al., 2023; Yang Z. et al., 2022). Notably, circular RNA PTP4A2 (circPTP4A2) drives microglial polarization toward a pro-inflammatory phenotype, aggravating neuroinflammation (Wang X. et al., 2024). The ongoing clinical trial NCT04175691 aims to decode circRNA-miRNA-lncRNA networks in stroke patients, highlighting their potential as diagnostic markers and therapeutic targets. Collectively, circRNAs act as molecular hubs in CIRI, bridging miRNA interactions, inflammatory cascades, and vascular damage, thereby offering multifaceted strategies for neuroprotection.
3 Therapeutic strategies for cerebral I/R injury
Cerebral I/R injury is a serious complication of ischemic stroke. Currently, there are no FDA-approved drugs specifically targeting brain I/R injury, underscoring the urgent need to develop more effective treatments. In recent years, advancements in drug research and development have led to the emergence of new drugs with high efficiency, low toxicity, and high bioavailability, which have shown promising therapeutic effects in treating Cerebral I/R injury (Figure 2). Therapeutic interventions for cerebral I/R injury are time-sensitive and stratified across distinct pathophysiological phases. In the hyperacute window (0–6h), rapid reperfusion with intravenous thrombolysis synergizes with dual-targeted antioxidant therapies to mitigate early oxidative damage. By 6–24h, interventions shift toward neuroinflammation modulation (Xu X. et al., 2024). Subsequently, delayed interventions post-reperfusion focus on modulating chronic inflammation, apoptosis, and neurovascular remodeling (Zhang H. et al., 2022). Active components derived from plants can mitigate the pathological processes induced by ischemia and reperfusion through various signaling pathways. Antioxidants and neuroprotective agents alleviate oxidative stress and promote the release of neurotrophic factors, thereby exerting neuroprotective effects. Exosome and noncoding RNA therapeutics have emerged as a promising research focus due to the therapeutic potential. Currently, the treatment of cerebral I/R injury is transitioning from single-agent therapies to a multi-faceted approach that integrates multiple mechanisms and pathways, aiming to achieve more effective and precise therapeutic outcomes.
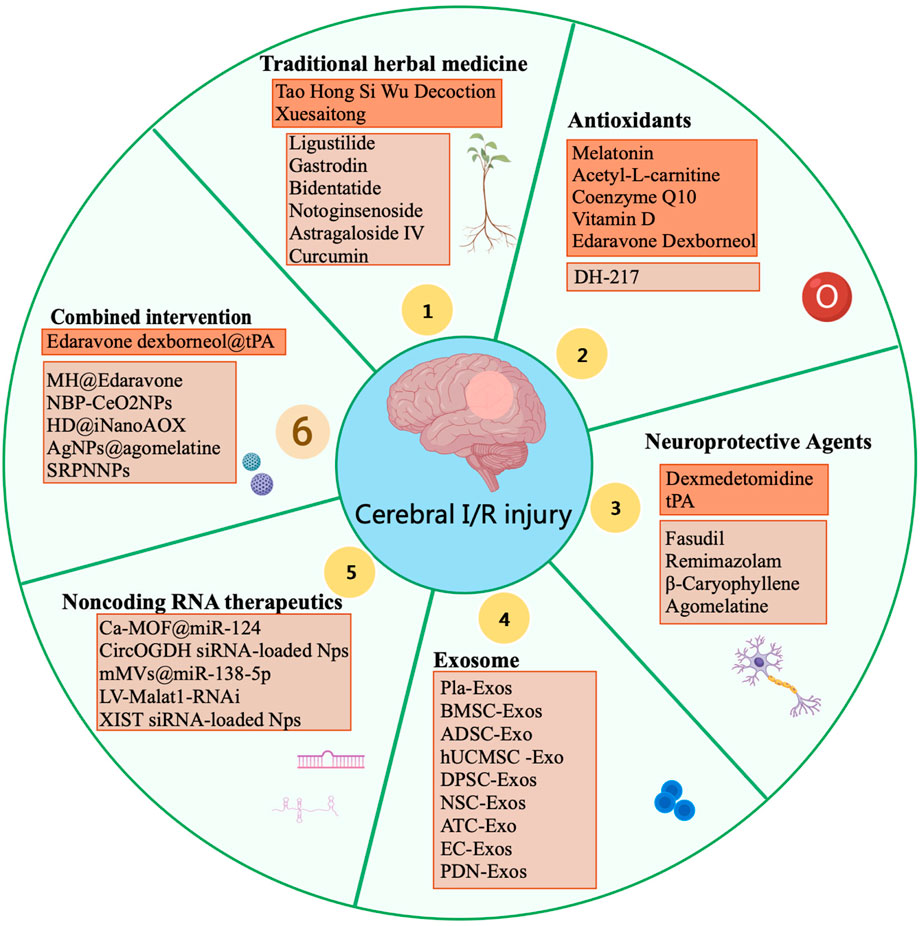
Figure 2. Therapeutic strategies for cerebral ischemia-reperfusion injury. Dark-colored boxes indicate clinically validated treatments, while light-colored boxes represent preclinical studies in animal models.
3.1 Traditional herbal medicine
For thousands of years, herbal medicine has played a significant role in human health. Many herbs have been found to exert protective effects against cerebral I/R injury (Pang et al., 2025; Zhang et al., 2024b). The Tao Hong Si Wu Decoction (THSWD), composed of six herbs—peach kernel, safflower, rehmannia, angelica, peony, and ligusticum—is a widely used traditional Chinese medicine formula known for its functions in promoting blood circulation and regulating immunity. Studies have demonstrated that THSWD can promote angiogenesis, regulate mitochondrial autophagy, reduce ROS production, inhibit NLRP3-mediated inflammatory responses, and decrease neuronal apoptosis, thereby exerting neuroprotective effects in cerebral I/R injury (Chen F. F. et al., 2020; Ji et al., 2022). The main active component of angelica, ligustilide (LIG), has been shown to improve mitochondrial function by activating the AMPK signaling pathway and alleviate cerebral I/R injury through regulation of PINK1/Parkin-mediated mitochondrial autophagy (Wu et al., 2022; Mao Z. et al., 2022). Gastrodin demonstrates multi-mechanistic therapeutic efficacy against cerebral ischemia-reperfusion injury through antioxidative, anti-inflammatory, neuroprotective, and blood-brain barrier stabilizing effects, emerging as an effective natural therapeutic agent for ischemic stroke management (Qin W. et al., 2025). Our research team systematically characterized the neuroprotective profile of Achyranthes bidentata polypeptides, demonstrating their potent anti-apoptotic, anti-inflammatory, and microvascular-protective effects (Cheng et al., 2019). Through bioactivity-guided fractionation combined with HPLC-MS structural elucidation, we identified Bidentatide as the principal bioactive entity (Ding et al., 2021; Ge et al., 2021). Notoginsenoside, derived from the medicinal plant Panax notoginseng, primarily consists of ginsenosides Rb1, Rd, Rg1, Re, and notoginsenoside R1. Xuesaitong soft capsules, formulated from notoginsenoside, have been approved for clinical use in treating ischemic stroke. A recent randomized clinical trial involving 1,200 patients with acute ischemic stroke demonstrated that Xuesaitong significantly improves neurological function at 3 months post-treatment. Specifically, the proportion of patients achieving functional independence was significantly higher in the Xuesaitong group; furthermore, the average reduction in National Institutes of Health Stroke Scale (NIHSS) score was also significantly greater in the Xuesaitong group (Wu et al., 2023). In addition to notoginsenoside and ginsenoside Rg1, astragaloside IV has also shown great potential in treating cerebral I/R injury (Xiao et al., 2021; Wang L. et al., 2023; Xiao et al., 2022; Ling et al., 2024; Xi et al., 2024). It possesses antioxidant, anti-neuroinflammatory, autophagy-regulating, anti-apoptotic, anti-ferroptotic, BBB-protective, neuroregenerative, and angiogenic properties, providing therapeutic benefits during both the early and late stages of cerebral I/R injury (Zeng et al., 2022). A traditional Chinese medicine compound, Qilong Capsule, which contains multiple components such as astragaloside IV, calycosin-7-glucoside, and lumbrokinase, was found in a recent clinical study to significantly increase the proportion of patients achieving functional independence (modified Rankin Scale ≤2) at 12 weeks to 68.5% in the treatment group (Lyu et al., 2024). Curcumin, extracted from the rhizomes of Zingiberaceae plants, acts as a natural iron chelator, increasing GPX4 expression to inhibit ferroptosis, while also regulating autophagy and pyroptosis, thereby improving cerebral I/R injury (Zhang L. et al., 2023; Li F. et al., 2021). Both ginkgetin and muscone converge on PPAR-γ signaling to drive robust microglial M2 polarization, synergistically amplifying neuroprotective effects and functional rehabilitation after cerebral ischemia (Han et al., 2022; Liu F. et al., 2023; Tang et al., 2022). Furthermore, other compounds such as ginkgolide, rhein, quercetin, gomisin N, parthenolide, resveratrol, and formononetin, as well as the herbal formulation Shuanglu Tongnao, have also been found to have protective effects on cerebral I/R injury (Yang Y. et al., 2024; Li L. et al., 2023; Yu et al., 2022; Li R. et al., 2023; Liu H. et al., 2023; Ding et al., 2022; Zhai et al., 2025). These findings suggest that natural plant compounds or their combinations hold significant potential for the treatment of cerebral I/R injury, warranting further clinical investigation.
3.2 Antioxidants
Given the critical role of oxidative stress in the pathogenesis of cerebral I/R injury, antioxidant drugs such as melatonin, acetyl-L-carnitine, vitamin D,and coenzyme Q10 have been evaluated in clinical trials to assess their efficacy in improving neurological function in patients with cerebral ischemia over 1–3 months, demonstrating certain benefits (Brion et al., 2024). Edaravone, a widely used neuroprotective agent in clinical practice, is employed to improve neurological symptoms caused by acute cerebral infarction (Kobayashi et al., 2019; Xu et al., 2021). Recently, Edaravone dexborneol, a novel neuroprotective agent combining edaravone and borneol, has shown excellent efficacy in phase III clinical trials, significantly improving neurological function in patients with acute cerebral ischemia. To be specific, the pivotal trial demonstrated a significantly higher rate of functional independence at 90 days and greater improvement in NIHSS scores. Furthermore, subgroup analysis revealed a 35% increase in neurological recovery rates with sublingual administration (Xu et al., 2021; Fu et al., 2024). Edaravone dexborneol exhibits synergistic anti-inflammatory and antioxidative effects, inhibiting ferroptosis via activation of the Nrf-2/HO-1/GPX4 signaling pathway, regulating extracellular matrix integrin and PDGFB/PDGFR-β signaling, protecting against BBB damage induced by cerebral I/R, and suppressing NF-κB nuclear translocation to block AIM2 inflammasome assembly—thereby suppressing microglial/astrocytic hyperactivation and leukocyte infiltration (Xiao et al., 2024; Sun et al., 2024; Wang D. et al., 2024; Zhang et al., 2025). Recent study has also found that peroxiredoxin-1, an antioxidant enzyme, controls stroke-related microglial responses to mitigate acute ischemic stroke (Kim et al., 2022). Additionally, the novel dual-acting antioxidant DH-217—which exerts neuroprotective effects by modulating the IKKβ/Nrf2/HO-1 signaling pathway to mitigate oxidative stress injury—has demonstrated promising therapeutic benefits in animal models of MCAO/R (Shen et al., 2023). However, the challenge of effectively penetrating the BBB and delivering antioxidants to the brain remains a significant obstacle to translating these findings into clinical applications (Brion et al., 2024). The rapid development of nanotechnology offers new possibilities for overcoming this bottleneck. Nanodrugs that can penetrate the blood-brain barrier and efficiently clear ROS hold promise for addressing the limitations of current drugs for ischemic stroke. Recent studies have shown that various functional nanoparticles capable of ROS removal have exhibited positive effects in Cerebral I/R injury (Yuan et al., 2021; Chen et al., 2024; Hou and Brenner, 2024). For instance, cerium oxide nanoparticles loaded with Dl-3-n-butylphthalide (NBP-CeO2NPs) can maintain BBB integrity, inhibit neuroinflammation and neuronal apoptosis, thereby reducing cerebral infarction and brain edema, providing a potential new treatment for ischemic stroke (Li X. et al., 2022). Moreover, a novel pH-sensitive hirudin-loaded antioxidant nitroxide radical nanoparticle (HD@iNanoAOX) has been shown to effectively improve ischemic brain injury in mice (Mei et al., 2025). Nano delivery of antioxidants has emerged as a promising treatment approach for Cerebral I/R injury, although further clinical validation is required.
3.3 Neuroprotective agents
Some neuroprotective agents can reduce the volume of cerebral infarction after ischemia without causing hemorrhage, alleviate cell damage post-ischemia, and protect the brain from reperfusion injury. Dexmedetomidine (DEX), an α2-adrenergic agonist with sedative properties, exhibits neuroprotective effects by reducing inflammatory responses and oxidative stress, inhibiting apoptosis, protecting the BBB, maintaining coagulation-anticoagulation balance, and preventing vasospasm (Hu Y. et al., 2022). A clinical study demonstrated that dexmedetomidine combined with targeted hemodynamic therapy can reduce neuroinflammation during craniocerebral surgery without adverse effects on hemodynamics (Chen P. H. et al., 2021). Recent studies have also shown that DEX can regulate multiple miRNAs, including miR-324-3p, miR-199a, and miR-205-5p, thereby exerting a protective effect on cerebral I/R injury (Burlacu et al., 2022; Chen Y. et al., 2021; Yang J. J. et al., 2021). The thrombolytic drug tPA exerts neuroprotective effects after reperfusion by increasing AMPK phosphorylation and FUNDC1 expression, thereby improving mitochondrial function in neurons and inhibiting apoptosis (Cai et al., 2021). The novel benzodiazepine agonist remimazolam and the Rho kinase inhibitor fasudil, which target the γ-aminobutyric acid A (GABAa) receptor, can reduce inflammatory responses by inhibiting the NLRP3 and TLR4/NF-κB pathways, promote the secretion of neurotrophic factors, and alleviate cerebral ischemia-reperfusion injury (Shi M. et al., 2022; Guo et al., 2023). β-Caryophyllene, a natural bicyclic sesquiterpene found in essential oils, has significant neuroprotective effects in reducing ischemic stroke damage through the regulation of ferroptosis and activation of the Nrf2/HO1 axis (Hu Q. et al., 2022). Agomelatine is a potential candidate for treating ischemic stroke by protecting the brain from cerebral I/R injury via inhibition of apoptosis (Chumboatong et al., 2017). Citrate-coated silver nanoparticles (AgNPs) loaded with agomelatine can further enhance the formation of the BBB by inhibiting oxidative stress and endoplasmic reticulum stress, providing neuronal protection in acute cerebral ischemia/reperfusion in rats (Gelen et al., 2024). Nano delivery of neuroprotective agents represents a cutting-edge approach to treating cerebral ischemia-reperfusion injury. By overcoming the limitations of conventional therapies and targeting multiple aspects of the injury process, this strategy holds significant promise for improving patient outcomes in the future.
3.4 Exosome
Exosomes contain a variety of bioactive molecules and can transmit complex intercellular signals (Wang et al., 2022d; Wang W. et al., 2021; Miron et al., 2000). Studies have demonstrated that exosomes promote the recovery from cerebral I/R injury by modulating immune responses, cell metabolism, and neuronal plasticity (Table 2) (Hermann et al., 2024; Xu K. et al., 2024). Circulating plasma exosomes, derived from multiple cell sources, are enriched in Heat shock 70 (HSP70) and can regulate ROS, inhibit mitochondrial-mediated neuronal apoptosis, and alleviate blood-brain barrier damage by activating tight junction proteins, thereby improving neurological function in MCAO/R mouse models (Jiang et al., 2020; Kang et al., 2019). Bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos) restore lysosomal function via the mTOR/TFEB pathway and mitigate cerebral I/R injury by targeting DAPK2 with miR-133a-3p (Liu H. et al., 2024; Yang X. et al., 2023). Exosomes from C-X-C chemokine receptor type 4 (CXCR4)-overexpressing BMSCs enhance angiogenesis, protect neurons, and improve acute stroke outcomes (Li X. et al., 2020). Moreover, BMSC-Exos induce microglial polarization from M1 to M2, alleviating neuronal pyroptosis and reducing cerebral I/R injury (Liu et al., 2021; Zhou et al., 2022; Cheng et al., 2021). Adipose-derived mesenchymal stem cell exosomes (ADSC-Exo) inhibit ferroptosis in neurons by targeting CHAC1 with miR-760-3p, thereby reducing cerebral ischemia/reperfusion injury (Wang Y. et al., 2023). ADSC-Exo modified with PD-L1 and HGF target SDF-1α+ expression, creating an anti-inflammatory microenvironment in ischemic brain regions (Lin et al., 2024). A recent study showed that human umbilical cord mesenchymal stem cell-derived exosomes loaded with superparamagnetic iron oxide nanoparticles deliver miR-1228-5p to protect against oxidative damage and improve cognitive dysfunction after cerebral ischemia (Hu et al., 2024). Dental pulp stem cell-derived exosomes (DPSC-Exos) significantly inhibit neuroinflammation caused by I/R, reduce neuronal apoptosis through miR-877-3p, and alleviate brain edema, infarction, and neurological damage in I/R mice (Li S. et al., 2021; Miao et al., 2024). Neural stem cell-derived exosomes (NSC-Exo) improve brain tissue damage such as cerebral infarction, neuronal death, and glial scar formation, promoting motor function recovery. Their neuroprotective effects are associated with inducing M2 polarization of microglia and regulating the secretion of microglia-related inflammatory molecules, thereby alleviating inflammation (Zhang R. et al., 2023; Zhao X. et al., 2023). Astrocyte-derived exosomes (ATC-Exo) alleviate cerebral I/R injury by transporting miR-34c, which targets TLR7 and downregulates the NF-κB/MAPK signaling pathway (Wu et al., 2020b). Brain microvascular endothelial cell-derived exosomes (EC-Exos) play a crucial role in cell communication by promoting neuronal growth, migration, and invasion, directly protecting neurons from I/R injury (Xiao et al., 2017). Further studies show that EC-Exos significantly reduce Bcl2 and Caspase-3 expression while upregulating Bax in MCAO/R mouse brain tissue, weakening ischemia-induced neuronal apoptosis and benefiting ischemic stroke treatment (Sun et al., 2022). The latest research indicates that exercise-induced skeletal muscle-derived exosomes mitigate cerebral ischemic injury by regulating the miR-484/ACSL4 axis (Huang et al., 2024). Panax notoginseng-derived exosomes (PDN) can enter the brain without modification, improve cerebral infarction volume, enhance behavioral outcomes, and maintain BBB integrity. Similar to BMSC-derived exosomes, PDN also reduces cerebral I/R injury by shifting microglial phenotype from pro-inflammatory M1 to anti-inflammatory M2 (Li et al., 2023e). Exosomes from various sources have shown broad therapeutic potential in treating cerebral I/R injury. As a promising drug delivery vehicle, they warrant further investigation for their significant clinical implications.
3.5 Noncoding RNA therapeutics
miRNA, lncRNA, and circRNA play crucial regulatory roles in cerebral I/R injury and are considered vital biomarkers and therapeutic targets for the early diagnosis and prognosis assessment of stroke. These noncoding RNAs orchestrate complex pathological cascades through interactions with mRNAs, proteins, and signaling pathways, influencing neuroinflammation, oxidative stress, and programmed cell death. Exosomes, natural nanovesicles carrying functional miRNAs, have shown neuroprotective effects in preclinical I/R models by delivering miRNAs like miR-124 and miR-138-5p to neurons and glial cells, modulating apoptosis and mitochondrial function (Wang Y. et al., 2023; Hu et al., 2024; Miao et al., 2024; Wu et al., 2020a; Huang et al., 2024; Chen and Chopp, 2018). However, the clinical translation of noncoding RNA therapies faces challenges including rapid enzymatic degradation, poor BBB penetration, off-target effects, and immunogenicity. To overcome these limitations, innovative delivery systems are being engineered to enhance RNA stability and targeting specificity. For example, Yang et al. designed metal-organic framework nanoparticles (Ca-MOF@miR-124) to efficiently deliver miR-124 into neural stem cells (NSCs), enhancing their differentiation into functional neurons and reducing infarct volume in MCAO mice (Yang H. et al., 2022). Another study utilized microglia membrane-coated vesicles loaded with miR-138-5p (mMVs@miR-138-5p), exploiting the innate inflammatory chemotaxis of microglia to deliver the miRNA to ischemic neurons. This approach improved mitochondrial dynamics via the DNMT3A/Rhebl1 axis, attenuating neuronal death in rat I/R models (Zhu et al., 2024). For circRNA-targeted therapy, circular oxoglutarate dehydrogenase (circOGDH), a neuron-derived circRNA overexpressed in the ischemic penumbra of MCAO mice and human plasma exosomes, was silenced using siRNA-loaded PLGA-PAMAM nanoparticles (Liu Y. et al., 2022; Liu Y. et al., 2024). Systemic administration of these nanoparticles reduced neuronal apoptosis and improved functional recovery in preclinical models.
Clinically, miRNA-based therapies have advanced significantly, with the FDA and European Medicines Agency approving several RNA therapeutics, including anti-miR-92a (for vascular repair) and miR-34 mimics (for cancer), paving the way for stroke applications (Winkle et al., 2021; Liang et al., 2024). In contrast, lncRNA and circRNA therapies remain in early development, though emerging technologies show promise. For instance, CRISPR/Cas9-mediated silencing of MALAT1, a pro-inflammatory lncRNA, reduced neurovascular damage in murine I/R models (Khoshnam et al., 2024; Zhao N. et al., 2023). Similarly, nanoparticle-mediated delivery of XIST siRNA suppressed endothelial pyroptosis by targeting NLRP3 inflammasomes (Zhang et al., 2021). The convergence of gene-editing tools (e.g., CRISPR/dCas9) and advanced nanocarriers (e.g., lipid nanoparticles, exosome mimics) may unlock the therapeutic potential of lncRNAs and circRNAs, enabling precise modulation of neuroinflammatory pathways, BBB integrity, and neuronal survival. In summary, while miRNA therapies lead clinical translation efforts, lncRNA and circRNA-targeted strategies are poised to emerge as next-generation interventions. By integrating optimized delivery platforms with mechanistic insights into ncRNA networks, researchers aim to transform the treatment landscape for cerebral I/R injury, addressing unmet needs in stroke recovery and neuroprotection.
3.6 Combined intervention therapy
Emerging evidence highlights the critical role of multi-mechanistic interventions in addressing the spatiotemporal complexity of cerebral I/R injury. Recent advances in nanomaterial engineering and drug synergy strategies now enable precise targeting of cascading pathological events—from acute oxidative bursts to delayed neuroinflammation and apoptosis—while preserving neurovascular unit integrity. This paradigm shift underscores the therapeutic imperative to integrate phase-specific pharmacological actions with biomimetic drug delivery, harmonizing acute cytoprotection with long-term tissue remodeling.
Co-administration of Edaravone dexborneol enhances free radical scavenging, improving 3-month functional independence rates (modified Rankin Scale score ≤2) by 2.3-fold compared to tPA alone (Dang et al., 2024). Concurrently, the combined application of mild hypothermia (MH) and edaravone (EDA) exerts synergistic neuroprotection against cerebral ischemia-reperfusion injury through Nrf2 pathway activation, effectively reversing neurological deficits and oxidative stress in mice (Yu et al., 2020). Advanced drug delivery systems, such as pH/ROS-responsive nanocarriers, would achieve lesion-specific drug release within ischemic tissue while neutralising ROS in real time (Chen W. et al., 2021). Nanomaterial-engineered systems - including NBP-CeO2NPs, HD@iNanoAOX, and AgNPs delivering agomelatine - demonstrate enhanced neuroprotection by integrating BBB stabilization, hypoxia-responsive drug release, and inhibition of oxidative stress, effectively reducing infarction volume and neuronal apoptosis in preclinical cerebral ischemic models (Li X. et al., 2022; Mei et al., 2025; Gelen et al., 2024). The bioinspired PB-006@MSC nanoplatform integrates a Prussian blue scavenger, neuroprotectant ZL006, and stem cell membrane targeting to achieve precise ischemic penumbra delivery (Zhang Q. et al., 2024). Its multidimensional mechanisms encompass ROS clearance, PSD95 suppression, and inflammatory regulation, culminating in significantly reduced cerebral infarction and improved neurological recovery. Combinatorial therapies utilizing exosomes and anti-apoptotic RNA agents effectively attenuate neuroinflammation, reduce neuronal apoptosis in the ischemic penumbra, improve cognitive function, and restore blood-brain barrier integrity (Yang X. et al., 2023; Lin et al., 2024; Hu et al., 2024). Additionally, the combination of BBB stabilizers and neuroprotective agents also demonstrates promising efficacy in the treatment of cerebral ischemia-reperfusion injury (Ugale et al., 2025). A recent study developed SRPNNPs, a polysorbate 80-modified carrier-free nanoformulation co-assembling ginsenoside Rb1, 3-n-butylphthalide, and probucol, which targets cerebral ischemia-reperfusion injury through apolipoprotein-mediated BBB penetration, neuronal and microglial internalization, and synergistic antioxidant/anti-inflammatory/neuroprotective effects (Guo et al., 2025). Collectively, these stratified therapeutic regimens demonstrate the critical importance of phase-specific multi-target interventions in cerebral I/R injury management, while underscoring the need for further translational research to optimize their synergistic mechanisms and temporal coordination.
4 Perspectives and prospects
With the advancement of medical technology, significant progress has been made in the treatment of cerebral I/R injury. Traditional drug therapy, antioxidants, neuroprotective agents, exosomes, and noncoding RNA therapeutics have each demonstrated their unique advantages and translational challenges. Drug therapy remains one of the primary approaches for treating cerebral I/R injury. Traditional drugs derived from natural plant compounds or compound combinations can alleviate cerebral I/R injury through multiple mechanisms, including antioxidation, anti-neuroinflammation, and regulation of autophagy, yet their multi-target nature complicates mechanistic validation in human trials. Clinical research data have shown the effectiveness of antioxidants and neuroprotective agents, while nano-drug delivery systems enhance their bioavailability through real-time oxidative stress neutralization and lesion-specific release, though scale-up manufacturing and batch-to-batch consistency remain hurdles for clinical adoption.
Exosomes derived from stem cells utilize miRNAs to promote neural repair, but challenges in scalable GMP-compliant production require microfluidic standardization and improved biodistribution tracking techniques to meet regulatory requirements. Noncoding RNA therapeutics targeting key pathways show promise, yet require AI-guided pharmacokinetic modeling to optimize dosing sequences and mitigate hemorrhagic transformation risks, particularly given inter-patient variability in RNA metabolism. The current limitations stem from biological heterogeneity and low blood-brain barrier penetration efficiency. These issues are now expected to be addressed by 3D bioprinted neurovascular units for blood-brain barrier permeability screening and CRISPR-engineered exosome-mimicking vesicles with hypoxia-responsive surface ligands for ischemia targeting. To bridge the preclinical-clinical gap, future strategies must integrate nanomaterials, temporal therapeutic algorithms, and multi-omics profiling to develop closed-loop systems capable of auto-calibrating drug release based on real-time biomarker feedback, validated through multicenter trials with phenotypically stratified cohorts.
Author contributions
MY: Data curation, Investigation, Methodology, Writing – original draft. BL: Investigation, Methodology, Software, Writing – original draft. BC: Investigation, Methodology, Visualization, Writing – original draft. YS: Conceptualization, Resources, Supervision, Writing – review and editing. GL: Conceptualization, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Nos. 81901933), Yancheng Medical Science and Technology Development Plan Project (No. YK2023034), Suzhou Science and Technology Development Planning Project, No. SYW2024048.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barangi, S., Hayes, A. W., and Karimi, G. (2023). The role of lncRNAs/miRNAs/Sirt1 axis in myocardial and cerebral injury. Cell Cycle 22 (9), 1062–1073. doi:10.1080/15384101.2023.2172265
Bolanos, J. P., and Almeida, A. (1999). Roles of nitric oxide in brain hypoxia-ischemia. Biochim. Biophys. Acta 1411 (2-3), 415–436. doi:10.1016/s0005-2728(99)00030-4
Briones-Valdivieso, C., Briones, F., Orellana-Urzúa, S., Chichiarelli, S., Saso, L., and Rodrigo, R. (2024). Novel multi-antioxidant approach for ischemic stroke therapy targeting the role of oxidative stress. Biomedicines 12 (3), 501. doi:10.3390/biomedicines12030501
Burlacu, C. C., Neag, M. A., Mitre, A. O., Sirbu, A. C., Badulescu, A. V., and Buzoianu, A. D. (2022). The role of miRNAs in dexmedetomidine's neuroprotective effects against brain disorders. Int. J. Mol. Sci. 23 (10), 5452. doi:10.3390/ijms23105452
Cai, Y., Yang, E., Yao, X., Zhang, X., Wang, Q., Wang, Y., et al. (2021). FUNDC1-dependent mitophagy induced by tPA protects neurons against cerebral ischemia-reperfusion injury. Redox Biol. 38, 101792. doi:10.1016/j.redox.2020.101792
Chen, F. F., Wang, M. M., Xia, W. W., Peng, D. Y., and Han, L. (2020c). Tao-Hong-Si-Wu Decoction promotes angiogenesis after cerebral ischaemia in rats via platelet microparticles. Chin. J. Nat. Med. 18 (8), 620–627. doi:10.1016/S1875-5364(20)30074-1
Chen, H., He, Y., Chen, S., Qi, S., and Shen, J. (2020a). Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 158, 104877. doi:10.1016/j.phrs.2020.104877
Chen, J., and Chopp, M. (2018). Exosome therapy for stroke. Stroke 49 (5), 1083–1090. doi:10.1161/STROKEAHA.117.018292
Chen, P. H., Tsuang, F. Y., Lee, C. T., Yeh, Y. C., Cheng, H. L., Lee, T. S., et al. (2021a). Neuroprotective effects of intraoperative dexmedetomidine versus saline infusion combined with goal-directed haemodynamic therapy for patients undergoing cranial surgery: a randomised controlled trial. Eur. J. Anaesthesiol. 38 (12), 1262–1271. doi:10.1097/EJA.0000000000001532
Chen, Q., Wang, J., Xiong, X., Chen, J., Wang, B., Yang, H., et al. (2024). Blood-brain barrier-penetrating metal-organic framework antioxidant nanozymes for targeted ischemic stroke therapy. Adv. Healthc. Mater, e2402376. doi:10.1002/adhm.202402376
Chen, W., Jiang, L., Hu, Y., Fang, G., Yang, B., Li, J., et al. (2021c). Nanomedicines, an emerging therapeutic regimen for treatment of ischemic cerebral stroke: a review. J. Control Release 340, 342–360. doi:10.1016/j.jconrel.2021.10.020
Chen, W., Wang, H., Feng, J., and Chen, L. (2020b). Overexpression of circRNA circUCK2 attenuates cell apoptosis in cerebral ischemia-reperfusion injury via miR-125b-5p/GDF11 signaling. Mol. Ther. Nucleic Acids 22, 673–683. doi:10.1016/j.omtn.2020.09.032
Chen, Y., Fan, Z., and Wu, Q. (2021b). Dexmedetomidine improves oxygen-glucose deprivation/reoxygenation (OGD/R) -induced neurological injury through regulating SNHG11/miR-324-3p/VEGFA axis. Bioengineered 12 (1), 4794–4804. doi:10.1080/21655979.2021.1957071
Cheng, C., Chen, X., Wang, Y., Cheng, W., Zuo, X., Tang, W., et al. (2021). MSCs-derived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol. Med. 27 (1), 67. doi:10.1186/s10020-021-00324-0
Cheng, Q., Tong, F., Shen, Y., Wang, C., and Ding, F. (2019). Achyranthes bidentata polypeptide k improves long-term neurological outcomes through reducing downstream microvascular thrombosis in experimental ischemic stroke. Brain Res. 1706, 166–176. doi:10.1016/j.brainres.2018.11.010
Chumboatong, W., Thummayot, S., Govitrapong, P., Tocharus, C., Jittiwat, J., and Tocharus, J. (2017). Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem. Int. 102, 114–122. doi:10.1016/j.neuint.2016.12.011
Dai, F., Hu, C., Li, X., Zhang, Z., Wang, H., Zhou, W., et al. (2024). Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention. Neural Regen. Res. 19 (11), 2480–2487. doi:10.4103/1673-5374.390966
Dai, Q., Ma, Y., Xu, Z., Zhang, L., Yang, H., Liu, Q., et al. (2021). Downregulation of circular RNA HECTD1 induces neuroprotection against ischemic stroke through the microRNA-133b/TRAF3 pathway. Life Sci. 264, 118626. doi:10.1016/j.lfs.2020.118626
Dai, Y., Sheng, Y., Deng, Y., Wang, H., Zhao, Z., Yu, X., et al. (2022). Circ_0000647 promotes cell injury by modulating miR-126-5p/TRAF3 axis in oxygen-glucose deprivation and reperfusion-induced SK-N-SH cell model. Int. Immunopharmacol. 104, 108464. doi:10.1016/j.intimp.2021.108464
Dang, C., Wang, Q., Zhuang, Y., Li, Q., Lu, Y., Xiong, Y., et al. (2024). Synergistic effects of neuroprotective drugs with intravenous recombinant tissue plasminogen activator in acute ischemic stroke: a Bayesian network meta-analysis. PLoS One 19 (12), e0311231. doi:10.1371/journal.pone.0311231
Datta, A., Sarmah, D., Mounica, L., Kaur, H., Kesharwani, R., Verma, G., et al. (2020). Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl. Stroke Res. 11 (6), 1185–1202. doi:10.1007/s12975-020-00806-z
Ding, F., Bai, Y., Cheng, Q., Yu, S., Cheng, M., Wu, Y., et al. (2021). Bidentatide, a novel plant peptide derived from Achyranthes bidentata blume: isolation, characterization, and neuroprotection through inhibition of NR2B-containing NMDA receptors. Int. J. Mol. Sci. 22 (15), 7977. doi:10.3390/ijms22157977
Ding, W., Cai, C., Zhu, X., Wang, J., and Jiang, Q. (2022). Parthenolide ameliorates neurological deficits and neuroinflammation in mice with traumatic brain injury by suppressing STAT3/NF-κB and inflammasome activation. Int. Immunopharmacol. 108, 108913. doi:10.1016/j.intimp.2022.108913
Du, S. J., Zhang, Y., Zhao, Y. M., Dong, Y. J., Tang, J. L., Zhou, X. H., et al. (2021). Astragaloside IV attenuates cerebral ischemia-reperfusion injury in rats through the inhibition of calcium-sensing receptor-mediated apoptosis. Int. J. Mol. Med. 47 (1), 302–314. doi:10.3892/ijmm.2020.4777
Fathy, N., Kortam, M. A., Shaker, O. G., and Sayed, N. H. (2021). Long noncoding RNAs MALAT1 and ANRIL gene variants and the risk of cerebral ischemic stroke: an association study. ACS Chem. Neurosci. 12 (8), 1351–1362. doi:10.1021/acschemneuro.0c00822
Fu, Y., Wang, A., Tang, R., Tian, X., Xia, X., et al. (2024). Sublingual edaravone dexborneol for the treatment of acute ischemic stroke: the TASTE-SL randomized clinical trial. JAMA Neurol. 81 (4), 319–326. doi:10.1001/jamaneurol.2023.5716
Gao, P., Shi, H., Jin, X., Guo, S., Zhou, X., and Gao, W. (2024). Mechanism of astragaloside IV regulating NLRP3 through LOC102555978 to attenuate cerebral ischemia reperfusion induced microglia pyroptosis. Int. Immunopharmacol. 131, 111862. doi:10.1016/j.intimp.2024.111862
Ge, X., Wang, Y., Yu, S., Cao, X., Chen, Y., Cheng, Q., et al. (2021). Anti-inflammatory activity of a polypeptide fraction from Achyranthes bidentate in amyloid β oligomers induced model of alzheimer's disease. Front. Pharmacol. 12, 716177. doi:10.3389/fphar.2021.716177
Gelen, V., Özkanlar, S., Kara, A., and Yeşildağ, A. (2024). Citrate-coated silver nanoparticles loaded with agomelatine provide neuronal therapy in acute cerebral ischemia/reperfusion of rats by inhibiting the oxidative stress, endoplasmic reticulum stress, and P2X7 receptor-mediated inflammasome. Environ. Toxicol. 39 (3), 1531–1543. doi:10.1002/tox.24021
Geng, H., Chen, L., Tang, J., Chen, Y., and Wang, L. (2022). The role of CCL2/CCR2 Axis in cerebral ischemia-reperfusion injury and treatment: from animal experiments to clinical trials. Int. J. Mol. Sci. 23 (7), 3485. doi:10.3390/ijms23073485
Guo, M. F., Zhang, H. Y., Zhang, P. J., Zhao, Y. J., Yu, J. W., Meng, T., et al. (2023). Fasudil alleviates cerebral ischemia-reperfusion injury by inhibiting inflammation and improving neurotrophic factor expression in rats. Acta Neurobiol. Exp. (Wars) 83 (1), 97–110. doi:10.55782/ane-2023-010
Guo, Y., Jia, M., He, Q., Zeng, J., Zhang, Y., Gao, Y., et al. (2025). A carrier-free triple-drug Co-assembled nanoformulation for synergistic treatment of cerebral ischemia-reperfusion injury. ACS Appl. Mater Interfaces 17 (23), 33618–33632. doi:10.1021/acsami.5c05882
Hamblin, M. H., and Lee, J. P. (2021). Neural stem cells for early ischemic stroke. Int. J. Mol. Sci. 22 (14), 7703. doi:10.3390/ijms22147703
Han, B., Zhang, Y., Zhang, Y., Bai, Y., Chen, X., Huang, R., et al. (2018). Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy 14 (7), 1164–1184. doi:10.1080/15548627.2018.1458173
Han, B., Zhao, Y., Yao, J., Fang, T., Wang, Y., et al. (2022). Proteomics on the role of muscone in the consciousness-restoring resuscitation effect of musk on ischemic stroke. J. Ethnopharmacol. 296, 115475. doi:10.1016/j.jep.2022.115475
Henrot, P., Blervaque, L., Dupin, I., Zysman, M., Esteves, P., Gouzi, F., et al. (2023). Cellular interplay in skeletal muscle regeneration and wasting: insights from animal models. J. Cachexia Sarcopenia Muscle 14 (2), 745–757. doi:10.1002/jcsm.13103
Hermann, D. M., Peruzzotti-Jametti, L., Giebel, B., and Pluchino, S. (2024). Extracellular vesicles set the stage for brain plasticity and recovery by multimodal signalling. Brain 147 (2), 372–389. doi:10.1093/brain/awad332
Hou, Z., and Brenner, J. S. (2024). Developing targeted antioxidant nanomedicines for ischemic penumbra: novel strategies in treating brain ischemia-reperfusion injury. Redox Biol. 73, 103185. doi:10.1016/j.redox.2024.103185
Hu, Q., Zuo, T., Deng, L., Chen, S., Yu, W., Liu, S., et al. (2022b). β-Caryophyllene suppresses ferroptosis induced by cerebral ischemia reperfusion via activation of the NRF2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine 102, 154112. doi:10.1016/j.phymed.2022.154112
Hu, W. J., Wei, H., Cai, L. L., Xu, Y. H., Du, R., Zhou, Q., et al. (2024). Magnetic targeting enhances the neuroprotective function of human mesenchymal stem cell-derived iron oxide exosomes by delivering miR-1228-5p. J. Nanobiotechnology 22 (1), 665. doi:10.1186/s12951-024-02941-3
Hu, Y., Zhou, H., Zhang, H., Sui, Y., Zhang, Z., Zou, Y., et al. (2022a). The neuroprotective effect of dexmedetomidine and its mechanism. Front. Pharmacol. 13, 965661. doi:10.3389/fphar.2022.965661
Huang, M., Cheng, S., Li, Z., Chen, J., Wang, C., Li, J., et al. (2024). Preconditioning exercise inhibits neuron ferroptosis and ameliorates brain ischemia damage by skeletal muscle-derived exosomes via regulating miR-484/ACSL4 Axis. Antioxid. Redox Signal 41 (13-15), 769–792. doi:10.1089/ars.2023.0492
Huang, Y. G., Tao, W., Yang, S. B., Wang, J. F., Mei, Z. G., and Feng, Z. T. (2019). Autophagy: novel insights into therapeutic target of electroacupuncture against cerebral ischemia/reperfusion injury. Neural Regen. Res. 14 (6), 954–961. doi:10.4103/1673-5374.250569
Ji, Z. J., Shi, Y., Li, X., Hou, R., Yang, Y., Liu, Z. Q., et al. (2022). Neuroprotective effect of taohong siwu decoction on cerebral ischemia/reperfusion injury via mitophagy-NLRP3 inflammasome pathway. Front. Pharmacol. 13, 910217. doi:10.3389/fphar.2022.910217
Jiang, R. Q., Li, Q. Q., and Sheng, R. (2023). Mitochondria associated ER membranes and cerebral ischemia: molecular mechanisms and therapeutic strategies. Pharmacol. Res. 191, 106761. doi:10.1016/j.phrs.2023.106761
Jiang, Y., He, R., Shi, Y., Liang, J., and Zhao, L. (2020). Plasma exosomes protect against cerebral ischemia/reperfusion injury via exosomal HSP70 mediated suppression of ROS. Life Sci. 256, 117987. doi:10.1016/j.lfs.2020.117987
Jurcau, A., and Simion, A. (2021). Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int. J. Mol. Sci. 23 (1), 14. doi:10.3390/ijms23010014
Kang, J. Y., Park, H., Kim, H., Mun, D., Park, H., Yun, N., et al. (2019). Human peripheral blood-derived exosomes for microRNA delivery. Int. J. Mol. Med. 43 (6), 2319–2328. doi:10.3892/ijmm.2019.4150
Kang, X., Jiang, L., Chen, X., Wang, X., Gu, S., Wang, J., et al. (2021). Exosomes derived from hypoxic bone marrow mesenchymal stem cells rescue OGD-induced injury in neural cells by suppressing NLRP3 inflammasome-mediated pyroptosis. Exp. Cell Res. 405 (1), 112635. doi:10.1016/j.yexcr.2021.112635
Khoshnam, S. E., Moalemnia, A., Anbiyaee, O., Farzaneh, M., and Ghaderi, S. (2024). LncRNA MALAT1 and ischemic stroke: pathogenesis and opportunities. Mol. Neurobiol. 61 (7), 4369–4380. doi:10.1007/s12035-023-03853-3
Kim, S., Lee, W., Jo, H., Sonn, S. K., Jeong, S. J., Seo, S., et al. (2022). The antioxidant enzyme Peroxiredoxin-1 controls stroke-associated microglia against acute ischemic stroke. Redox Biol. 54, 102347. doi:10.1016/j.redox.2022.102347
Kobayashi, S., Fukuma, S., Ikenoue, T., and Fukuhara, S. (2019). Effect of edaravone on neurological symptoms in real-world patients with acute ischemic stroke. Stroke 50 (7), 1805–1811. doi:10.1161/STROKEAHA.118.024351
Kumar Saini, S., and Singh, D. (2024). Mitochondrial mechanisms in cerebral ischemia-reperfusion injury: unravelling the intricacies. Mitochondrion 77, 101883. doi:10.1016/j.mito.2024.101883
Lan, Z., Tan, F., He, J., Liu, J., Lu, M., Hu, Z., et al. (2024). Curcumin-primed olfactory mucosa-derived mesenchymal stem cells mitigate cerebral ischemia/reperfusion injury-induced neuronal PANoptosis by modulating microglial polarization. Phytomedicine 129, 155635. doi:10.1016/j.phymed.2024.155635
Li, F., Xu, Y., Li, X., Wang, X., Yang, Z., Li, W., et al. (2021b). Triblock copolymer nanomicelles loaded with curcumin attenuates inflammation via inhibiting the NF-κB pathway in the rat model of cerebral ischemia. Int. J. Nanomedicine 16, 3173–3183. doi:10.2147/IJN.S300379
Li, J., Zhang, J., Zhang, Y., Wang, Z., Song, Y., Wei, S., et al. (2019a). TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis. Cell Death Dis. 10 (5), 328. doi:10.1038/s41419-019-1558-5
Li, L., Jiang, W., Yu, B., Liang, H., Mao, S., Hu, X., et al. (2023c). Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-κB signaling pathway. Biomed. Pharmacother. 168, 115653. doi:10.1016/j.biopha.2023.115653
Li, M., Meng, Z., Yu, S., Li, J., Wang, Y., Yang, W., et al. (2022a). Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem. Biol. Interact. 366, 110137. doi:10.1016/j.cbi.2022.110137
Li, R., Shen, Y., Li, X., Lu, L., Wang, Z., Sheng, H., et al. (2021a). Activation of the XBP1s/O-GlcNAcylation pathway improves functional outcome after cardiac arrest and resuscitation in young and aged mice. Shock 56 (5), 755–761. doi:10.1097/SHK.0000000000001732
Li, R., Zheng, Y., Zhang, J., Zhou, Y., and Fan, X. (2023d). Gomisin N attenuated cerebral ischemia-reperfusion injury through inhibition of autophagy by activating the PI3K/AKT/mTOR pathway. Phytomedicine 110, 154644. doi:10.1016/j.phymed.2023.154644
Li, S., Li, Y., Huang, P., Mao, X., Jiang, K., Chen, R., et al. (2023a). Knockout of Rnf213 ameliorates cerebral ischemic-reperfusion injury by inhibiting neuronal apoptosis through the akt/GSK-3β/β-catenin/bcl-2 pathway. Neuroscience 533, 10–21. doi:10.1016/j.neuroscience.2023.09.018
Li, S., Luo, L., He, Y., Xiang, Y., Xing, Z., et al. (2021c). Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 54 (8), e13093. doi:10.1111/cpr.13093
Li, S., Zhang, R., Wang, A., Li, Y., Zhang, M., Kim, J., et al. (2023e). Panax notoginseng: derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J. Nanobiotechnology 21 (1), 416. doi:10.1186/s12951-023-02161-1
Li, T. H., Sun, H. W., Song, L. J., Yang, B., Zhang, P., Yan, D. M., et al. (2022b). Long non-coding RNA MEG3 regulates autophagy after cerebral ischemia/reperfusion injury. Neural Regen. Res. 17 (4), 824–831. doi:10.4103/1673-5374.322466
Li, W., Xie, L., Wang, L., and Lin, F. (2023b). CircRIMS promotes cerebral ischemia-reperfusion injury through increasing apoptosis and targeting the miR-96-5p/JAK/STAT1 axis. Brain Inj. 37 (11), 1235–1244. doi:10.1080/02699052.2023.2237890
Li, W., Yang, F., Gao, J., Tang, Y., Wang, J., and Pan, Y. (2019b). Over-expression of TRPC6 via CRISPR based synergistic activation mediator in BMSCs ameliorates brain injury in a rat model of cerebral ischemia/reperfusion. Neuroscience 415, 147–160. doi:10.1016/j.neuroscience.2019.06.041
Li, X., Han, Z., Wang, T., Ma, C., Li, H., Lei, H., et al. (2022c). Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials 291, 121904. doi:10.1016/j.biomaterials.2022.121904
Li, X., Zhang, Y., Wang, Y., Zhao, D., Sun, C., Zhou, S., et al. (2020b). Exosomes derived from CXCR4-overexpressing BMSC promoted activation of microvascular endothelial cells in cerebral ischemia/reperfusion injury. Neural Plast. 2020, 8814239. doi:10.1155/2020/8814239
Li, Y., Yi, M., Wang, D., Zhang, Q., Yang, L., and Yang, C. (2020a). LncRNA KCNQ1OT1 regulates endoplasmic reticulum stress to affect cerebral ischemia-reperfusion injury through targeting miR-30b/GRP78. Inflammation 43 (6), 2264–2275. doi:10.1007/s10753-020-01295-w
Liang, J., Zhu, Y., Liu, S., Kuang, B., Tian, Z., Zhang, L., et al. (2024). Progress of exosomal MicroRNAs and traditional Chinese medicine monomers in neurodegenerative diseases. Phytother. Res. 38 (11), 5323–5349. doi:10.1002/ptr.8322
Liao, S., Apaijai, N., Chattipakorn, N., and Chattipakorn, S. C. (2020). The possible roles of necroptosis during cerebral ischemia and ischemia/reperfusion injury. Arch. Biochem. Biophys. 695, 108629. doi:10.1016/j.abb.2020.108629
Lin, S. L., Chang, Y. W., Lee, W., Chiang, C. S., Liu, S. P., Lee, H. T., et al. (2024). Role of STAT3-FOXO3 signaling in the modulation of neuroplasticity by PD-L1-HGF-decorated mesenchymal stem cell-derived exosomes in a murine stroke model. Adv. Sci. (Weinh) 11 (36), e2404882. doi:10.1002/advs.202404882
Ling, G., Zhang, M., Chen, C., Wang, Y., Gao, Q., Li, J., et al. (2024). Progress of Ginsenoside Rb1 in neurological disorders. Front. Pharmacol. 15, 1280792. doi:10.3389/fphar.2024.1280792
Liu, F., Cao, L., Hu, S., Ye, H., Wu, Q., and Wu, L. (2023b). Muscone promotes functional recovery by facilitating microglia polarization into M2 phenotype through PPAR-γ pathway after ischemic stroke. Cell Immunol. 386, 104704. doi:10.1016/j.cellimm.2023.104704
Liu, H., Li, C., Zhang, X., Chen, H., Zhang, Q., Zeng, Y., et al. (2024a). BMSC-Exosomes attenuate ALP dysfunction by restoring lysosomal function via the mTOR/TFEB Axis to reduce cerebral ischemia-reperfusion injury. Exp. Neurol. 376, 114726. doi:10.1016/j.expneurol.2024.114726
Liu, H., Zhang, T. A., Zhang, W. Y., Huang, S. R., Hu, Y., and Sun, J. (2023c). Rhein attenuates cerebral ischemia-reperfusion injury via inhibition of ferroptosis through NRF2/SLC7A11/GPX4 pathway. Exp. Neurol. 369, 114541. doi:10.1016/j.expneurol.2023.114541
Liu, L., Chen, M., Lin, K., Xiang, X., Yang, J., Zheng, Y., et al. (2020b). TRPC6 attenuates cortical astrocytic apoptosis and inflammation in cerebral ischemic/reperfusion injury. Front. Cell Dev. Biol. 8, 594283. doi:10.3389/fcell.2020.594283
Liu, L., Gu, L., Chen, M., Zheng, Y., Xiong, X., and Zhu, S. (2020a). Novel targets for stroke therapy: special focus on TRPC channels and TRPC6. Front. Aging Neurosci. 12, 70. doi:10.3389/fnagi.2020.00070
Liu, S., Zhang, Z., He, Y., Kong, L., Jin, Q., Qi, X., et al. (2023a). Inhibiting leukocyte-endothelial cell interactions by Chinese medicine Tongxinluo capsule alleviates no-reflow after arterial recanalization in ischemic stroke. CNS Neurosci. Ther. 29 (10), 3014–3030. doi:10.1111/cns.14242
Liu, X., Lv, X., Liu, Z., Zhang, M., and Leng, Y. (2022a). MircoRNA-29a in astrocyte-derived extracellular vesicles suppresses brain ischemia reperfusion injury via TP53INP1 and the NF-κB/NLRP3 Axis. Cell Mol. Neurobiol. 42 (5), 1487–1500. doi:10.1007/s10571-021-01040-3
Liu, X., Zhang, M., Liu, H., Zhu, R., He, H., Zhou, Y., et al. (2021). Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 341, 113700. doi:10.1016/j.expneurol.2021.113700
Liu, Y., Li, Y., Zang, J., Zhang, T., Li, Y., Tan, Z., et al. (2022b). CircOGDH is a penumbra biomarker and therapeutic target in acute ischemic stroke. Circ. Res. 130 (6), 907–924. doi:10.1161/CIRCRESAHA.121.319412
Liu, Y., Zhang, T., Zou, X., Yuan, Z., Li, Y., Zang, J., et al. (2024b). Penumbra-targeted CircOGDH siRNA-loaded nanoparticles alleviate neuronal apoptosis in focal brain ischaemia. Stroke Vasc. Neurol. 9 (2), 134–144. doi:10.1136/svn-2022-002009
Lyu, J., Liu, Y., Liu, F., Liu, G., Gao, Y., Wei, R., et al. (2024). Therapeutic effect and mechanisms of traditional Chinese medicine compound (Qilong capsule) in the treatment of ischemic stroke. Phytomedicine 132, 155781. doi:10.1016/j.phymed.2024.155781
Maitrias, P., Metzinger-Le Meuth, V., Nader, J., Reix, T., Caus, T., and Metzinger, L. (2017). The involvement of miRNA in carotid-related stroke. Arterioscler. Thromb. Vasc. Biol. 37 (9), 1608–1617. doi:10.1161/ATVBAHA.117.309233
Mao, R., Zong, N., Hu, Y., Chen, Y., and Xu, Y. (2022a). Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci. Bull. 38 (10), 1229–1247. doi:10.1007/s12264-022-00859-0
Mao, Z., Tian, L., Liu, J., Wu, Q., Wang, N., Wang, G., et al. (2022b). Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomedicine 101, 154111. doi:10.1016/j.phymed.2022.154111
Mei, T., Zhang, P., Hu, Y., Xiao, L., Hou, J., and Nagasaki, Y. (2025). Engineering hirudin encapsulation in pH-responsive antioxidant nanoparticles for therapeutic efficacy in ischemic stroke model mice. Biomaterials 314, 122860. doi:10.1016/j.biomaterials.2024.122860
Miao, Y., Liang, X., Chen, J., Liu, H., He, Z., Qin, Y., et al. (2024). Transfer of miR-877-3p via extracellular vesicles derived from dental pulp stem cells attenuates neuronal apoptosis and facilitates early neurological functional recovery after cerebral ischemia-reperfusion injury through the Bclaf1/P53 signaling pathway. Pharmacol. Res. 206, 107266. doi:10.1016/j.phrs.2024.107266
Miron, R. J., Estrin, N. E., Sculean, A., and Zhang, Y. (2000). Understanding exosomes: Part 2-Emerging leaders in regenerative medicine. Periodontol 94 (1), 257–414. doi:10.1111/prd.12561
Neag, M. A., Mitre, A. O., Burlacu, C. C., Inceu, A. I., Mihu, C., Melincovici, C. S., et al. (2022). miRNA involvement in cerebral ischemia-reperfusion injury. Front. Neurosci. 16, 901360. doi:10.3389/fnins.2022.901360
Pang, C., Zhang, J., Gu, Y., Zhang, Q., and Zhao, Y. (2025). The biological roles of exosome-encapsulated traditional Chinese medicine monomers in neuronal disorders. J. Pharm. Anal. 15 (5), 101131. doi:10.1016/j.jpha.2024.101131
Qi, X., Shao, M., Sun, H., Shen, Y., Meng, D., and Huo, W. (2017). Long non-coding RNA SNHG14 promotes microglia activation by regulating miR-145-5p/PLA2G4A in cerebral infarction. Neuroscience 348, 98–106. doi:10.1016/j.neuroscience.2017.02.002
Qin, C., Dong, M. H., Tang, Y., Chu, Y. H., Zhou, L. Q., Zhang, H., et al. (2024). The foam cell-derived exosomal miRNA Novel-3 drives neuroinflammation and ferroptosis during ischemic stroke. Nat. Aging 4 (12), 1845–1861. doi:10.1038/s43587-024-00727-8
Qin, W., Du, J., Wang, F., and Xu, J. (2025b). Gastrodin: a potential natural product for the prevention and treatment of cerebral ischemia-reperfusion injury. Front. Pharmacol. 16, 1554170. doi:10.3389/fphar.2025.1554170
Qin, X., Zhu, J., Lu, H., Yi, M., Zhao, Z., Zhang, W., et al. (2025a). Research progress of deubiquitinating enzymes in cerebral ischemia-reperfusion injury. Front. Aging Neurosci. 17, 1588920. doi:10.3389/fnagi.2025.1588920
Qiu, Y., Fan, Y., Huang, G., and Liu, J. (2024). N6-methyladenosine demethylase ALKBH5 homologous protein protects against cerebral I/R injury though suppressing SNHG3-mediated neural PANoptosis: involvement of m6A-related macromolecules in the diseases of nervous system. Int. J. Biol. Macromol. 274 (Pt 2), 133815. doi:10.1016/j.ijbiomac.2024.133815
Que, Y. Y., Zhu, T., Zhang, F. X., and Peng, J. (2020). Neuroprotective effect of DUSP14 overexpression against isoflurane-induced inflammatory response, pyroptosis and cognitive impairment in aged rats through inhibiting the NLRP3 inflammasome. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 7101–7113. doi:10.26355/eurrev_202006_21704
Rohlfing, A. K., Kolb, K., Sigle, M., Ziegler, M., Bild, A., Münzer, P., et al. (2022). ACKR3 regulates platelet activation and ischemia-reperfusion tissue injury. Nat. Commun. 13 (1), 1823. doi:10.1038/s41467-022-29341-1
Sakai, S., and Shichita, T. (2019). Inflammation and neural repair after ischemic brain injury. Neurochem. Int. 130, 104316. doi:10.1016/j.neuint.2018.10.013
Sha, R., Zhang, B., Han, X., Peng, J., Zheng, C., Zhang, F., et al. (2019). Electroacupuncture alleviates ischemic brain injury by inhibiting the miR-223/NLRP3 pathway. Med. Sci. Monit. 25, 4723–4733. doi:10.12659/MSM.917213
Shan, W., Chen, W., Zhao, X., Pei, A., Chen, M., Yu, Y., et al. (2020). Long noncoding RNA TUG1 contributes to cerebral ischaemia/reperfusion injury by sponging mir-145 to up-regulate AQP4 expression. J. Cell Mol. Med. 24 (1), 250–259. doi:10.1111/jcmm.14712
She, R., Liu, D., Liao, J., Wang, G., Ge, J., and Mei, Z. (2023). Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: from pathology to therapeutic potential. Front. Cell Neurosci. 17, 1191629. doi:10.3389/fncel.2023.1191629
Shekhar, S., Liu, Y., Wang, S., Zhang, H., Fang, X., Zhang, J., et al. (2021). Novel mechanistic insights and potential therapeutic impact of TRPC6 in neurovascular coupling and ischemic stroke. Int. J. Mol. Sci. 22 (4), 2074. doi:10.3390/ijms22042074
Shen, M., Zheng, Y., Li, G., Chen, Y., Huang, L., Wu, J., et al. (2023). Dual antioxidant DH-217 mitigated cerebral ischemia-reperfusion injury by targeting ikkβ/nrf2/HO-1 signal Axis. Neurochem. Res. 48 (2), 579–590. doi:10.1007/s11064-022-03783-x
Shen, Y., Li, R., Yu, S., Zhao, Q., Wang, Z., Sheng, H., et al. (2021). Activation of the ATF6 (activating transcription factor 6) signaling pathway in neurons improves outcome after cardiac arrest in mice. J. Am. Heart Assoc. 10 (12), e020216. doi:10.1161/JAHA.120.020216
Shi, M., Chen, J., Liu, T., Dai, W., Zhou, Z., Chen, L., et al. (2022b). Protective effects of remimazolam on cerebral ischemia/reperfusion injury in rats by inhibiting of NLRP3 inflammasome-dependent pyroptosis. Drug Des. Devel Ther. 16, 413–423. doi:10.2147/DDDT.S344240
Shi, Y., Li, Z., Li, K., and Xu, K. (2022a). miR-155-5p accelerates cerebral ischemia-reperfusion inflammation injury and cell pyroptosis via DUSP14/TXNIP/NLRP3 pathway. Acta Biochim. Pol. 69 (4), 787–793. doi:10.18388/abp.2020_6095
Shi, Y., Yi, Z., Zhao, P., Xu, Y., and Pan, P. (2021). MicroRNA-532-5p protects against cerebral ischemia-reperfusion injury by directly targeting CXCL1. Aging (Albany NY) 13 (8), 11528–11541. doi:10.18632/aging.202846
Shi, Y., Zhang, L., Mao, L., Hu, X., Jiang, X., et al. (2020). Publisher Correction: rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 11 (1), 4335. doi:10.1038/s41467-020-18263-5
Sommer, C. J. (2017). Ischemic stroke: experimental models and reality. Acta Neuropathol. 133 (2), 245–261. doi:10.1007/s00401-017-1667-0
Sun, H., Li, J. J., Feng, Z. R., Liu, H. Y., and Meng, A. G. (2020). MicroRNA-124 regulates cell pyroptosis during cerebral ischemia-reperfusion injury by regulating STAT3. Exp. Ther. Med. 20 (6), 227. doi:10.3892/etm.2020.9357
Sun, J., Yuan, Q., Guo, L., Xiao, G., Zhang, T., Liang, B., et al. (2022). Brain microvascular endothelial cell-derived exosomes protect neurons from ischemia-reperfusion injury in mice. Pharm. (Basel) 15 (10), 1287. doi:10.3390/ph15101287
Sun, M. S., Jin, H., Sun, X., Huang, S., Zhang, F. L., Guo, Z. N., et al. (2018). Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell Longev. 2018, 3804979. doi:10.1155/2018/3804979
Sun, Z., Zhao, H., Yang, S., Liu, R., Yi, L., Gao, J., et al. (2024). Edaravone Dexborneol protects against blood-brain barrier disruption following cerebral ischemia/reperfusion by upregulating pericyte coverage via vitronectin-integrin and PDGFB/PDGFR-β signaling. Free Radic. Biol. Med. 225, 758–766. doi:10.1016/j.freeradbiomed.2024.10.309
Tan, X., Guo, W., Peng, Z., Gu, C., Xiang, P., Tu, Y., et al. (2021). LncRNA-Malat1 down-regulates miR-211-5p expression to promote neuronal damage from cerebral ischemia reperfusion injury. Biochem. Pharmacol. 192, 114694. doi:10.1016/j.bcp.2021.114694
Tang, C., Ou, J., Kou, L., Deng, J., and Luo, S. (2020). Circ_016719 plays a critical role in neuron cell apoptosis induced by I/R via targeting miR-29c/Map2k6. Mol. Cell Probes 49, 101478. doi:10.1016/j.mcp.2019.101478
Tang, T., Wang, X., Qi, E., Li, S., and Sun, H. (2022). Ginkgetin promotes M2 polarization of microglia and exert neuroprotection in ischemic stroke via modulation of PPARγ pathway. Neurochem. Res. 47 (10), 2963–2974. doi:10.1007/s11064-022-03583-3
Terpolilli, N. A., Kim, S. W., Thal, S. C., Kataoka, H., Zeisig, V., Nitzsche, B., et al. (2012). Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res. 110 (5), 727–738. doi:10.1161/CIRCRESAHA.111.253419
Tu, X., Zhang, H., Chen, S., Ding, Y. H., Wu, X., Liang, R., et al. (2022). LncRNA CEBPA-AS1 alleviates cerebral ischemia-reperfusion injury by sponging miR-340-5p regulating APPL1/LKB1/AMPK pathway. FASEB J. 36 (1), e22075. doi:10.1096/fj.202100826RR
Tuo, Q. Z., Liu, Y., Xiang, Z., Yan, H. F., Zou, T., Shu, Y., et al. (2022b). Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct. Target Ther. 7 (1), 59. doi:10.1038/s41392-022-00917-z
Tuo, Q. Z., Zhang, S. T., and Lei, P. (2022a). Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 42 (1), 259–305. doi:10.1002/med.21817
Ugale, R., Vatte, S., Girdhar, P., and Anandani, D. (2025). Deferoxamine prevents BBB disruption, neuroinflammation and apoptotic changes in early hours of ischemic reperfusion injury. Neurochem. Int. 188, 106009. doi:10.1016/j.neuint.2025.106009
Wang, C., Dong, M., Zhang, X., Wang, X., Zhao, Y., and Cao, Y. (2023a). Competitive binding of circCCDC6 to microRNA-128-3p activates TXNIP/NLRP3 pathway and promotes cerebral ischemia-reperfusion defects. Acta Biochim. Pol. 70 (4), 807–815. doi:10.18388/abp.2020_6552
Wang, C., and Hu, F. (2021). Long noncoding RNA SOX2OT silencing alleviates cerebral ischemia-reperfusion injury via miR-135a-5p-mediated NR3C2 inhibition. Brain Res. Bull. 173, 193–202. doi:10.1016/j.brainresbull.2021.05.018
Wang, D., Wang, Y., Shi, J., Jiang, W., Huang, W., Chen, K., et al. (2024b). Edaravone dexborneol alleviates ischemic injury and neuroinflammation by modulating microglial and astrocyte polarization while inhibiting leukocyte infiltration. Int. Immunopharmacol. 130, 111700. doi:10.1016/j.intimp.2024.111700
Wang, J., Fu, Z., Wang, M., Lu, J., Yang, H., and Lu, H. (2021d). Knockdown of XIST attenuates cerebral ischemia/reperfusion injury through regulation of miR-362/ROCK2 Axis. Neurochem. Res. 46 (8), 2167–2180. doi:10.1007/s11064-021-03354-6
Wang, L., Liu, C., and Tang, B. (2023b). Astragaloside IV mitigates cerebral ischaemia-reperfusion injury via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis. Eur. J. Pharmacol. 944, 175516. doi:10.1016/j.ejphar.2023.175516
Wang, L., Ren, W., Wu, Q., Liu, T., Wei, Y., Ding, J., et al. (2022b). NLRP3 inflammasome activation: a therapeutic target for cerebral ischemia-reperfusion injury. Front. Mol. Neurosci. 15, 847440. doi:10.3389/fnmol.2022.847440
Wang, L. Q., Zheng, Y. Y., Zhou, H. J., Zhang, X. X., Wu, P., and Zhu, S. M. (2021c). LncRNA-Fendrr protects against the ubiquitination and degradation of NLRC4 protein through HERC2 to regulate the pyroptosis of microglia. Mol. Med. 27 (1), 39. doi:10.1186/s10020-021-00299-y
Wang, P., Pan, R., Weaver, J., Jia, M., Yang, X., Yang, T., et al. (2021b). MicroRNA-30a regulates acute cerebral ischemia-induced blood-brain barrier damage through ZnT4/zinc pathway. J. Cereb. Blood Flow. Metab. 41 (3), 641–655. doi:10.1177/0271678X20926787
Wang, S., Jun, J., Cong, L., Du, L., and Wang, C. (2021a). miR-328-3p, a predictor of stroke, aggravates the cerebral ischemia-reperfusion injury. Int. J. Gen. Med. 14, 2367–2376. doi:10.2147/IJGM.S307392
Wang, W., Hu, Y., and Zhang, Y. (2022c). FTX attenuates cerebral ischemia-reperfusion injury by inhibiting apoptosis and oxidative stress via miR-186-5p/MDM4 pathway. Neurotox. Res. 40 (2), 542–552. doi:10.1007/s12640-022-00485-8
Wang, W., Li, M., Chen, Z., Xu, L., Chang, M., Wang, K., et al. (2022d). Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem. Pharmacol. 198, 114954. doi:10.1016/j.bcp.2022.114954
Wang, W., Shen, D., Zhang, L., Ji, Y., Xu, L., Chen, Z., et al. (2021e). SKP-SC-EVs mitigate denervated muscle atrophy by inhibiting oxidative stress and inflammation and improving microcirculation. Antioxidants (Basel) 11 (1), 66. doi:10.3390/antiox11010066
Wang, X., Zhang, S., Lv, B., Chen, H., Zhang, W., Dong, L., et al. (2024a). Circular RNA PTP4A2 regulates microglial polarization through STAT3 to promote neuroinflammation in ischemic stroke. CNS Neurosci. Ther. 30 (4), e14512. doi:10.1111/cns.14512
Wang, Y., Hong, F., and Yang, S. (2022a). Roles of nitric oxide in brain ischemia and reperfusion. Int. J. Mol. Sci. 23 (8), 4243. doi:10.3390/ijms23084243
Wang, Y., Niu, H., Li, L., Han, J., Liu, Z., Chu, M., et al. (2023c). Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J. Nanobiotechnology 21 (1), 109. doi:10.1186/s12951-023-01862-x
Wang, Y. C., Li, X., Shen, Y., Lyu, J., Sheng, H., Paschen, W., et al. (2020). PERK (protein kinase RNA-like ER kinase) branch of the unfolded protein response confers neuroprotection in ischemic stroke by suppressing protein synthesis. Stroke 51 (5), 1570–1577. doi:10.1161/STROKEAHA.120.029071
Wang, Z., Guo, L. M., Wang, Y., Zhou, H. K., Wang, S. C., Chen, D., et al. (2018). Inhibition of HSP90α protects cultured neurons from oxygen-glucose deprivation induced necroptosis by decreasing RIP3 expression. J. Cell Physiol. 233 (6), 4864–4884. doi:10.1002/jcp.26294
Winkle, M., El-Daly, S. M., Fabbri, M., and Calin, G. A. (2021). Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 20 (8), 629–651. doi:10.1038/s41573-021-00219-z
Wu, F., Han, B., Wu, S., Yang, L., Leng, S., Li, M., et al. (2019). Circular RNA TLK1 aggravates neuronal injury and neurological deficits after ischemic stroke via miR-335-3p/TIPARP. J. Neurosci. 39 (37), 7369–7393. doi:10.1523/JNEUROSCI.0299-19.2019
Wu, L., Song, H., Zhang, C., Wang, A., Zhang, B., Xiong, C., et al. (2023). Efficacy and safety of Panax notoginseng saponins in the treatment of adults with ischemic stroke in China: a randomized clinical trial. JAMA Netw. Open 6 (6), e2317574. doi:10.1001/jamanetworkopen.2023.17574
Wu, L., Xiong, X., and Wu, X. (2020a). Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front. Mol. Neurosci. 13, 28. doi:10.3389/fnmol.2020.00028
Wu, Q., Liu, J., Mao, Z., Tian, L., Wang, N., Wang, G., et al. (2022). Ligustilide attenuates ischemic stroke injury by promoting Drp1-mediated mitochondrial fission via activation of AMPK. Phytomedicine 95, 153884. doi:10.1016/j.phymed.2021.153884
Wu, W., Liu, J., Yang, C., Xu, Z., and Huang, J. (2020b). Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res. Bull. 163, 84–94. doi:10.1016/j.brainresbull.2020.07.013
Xi, Z. C., Ren, H. G., Ai, L., Wang, Y., Liu, M. F., Qiu, Y. F., et al. (2024). Author Correction: ginsenoside Rg1 mitigates cerebral ischaemia/reperfusion injury in mice by inhibiting autophagy through activation of mTOR signalling. Acta Pharmacol. Sin. 46, 1799. doi:10.1038/s41401-025-01502-0
Xia, Q., Zhan, G., Mao, M., Zhao, Y., and Li, X. (2022). TRIM45 causes neuronal damage by aggravating microglia-mediated neuroinflammation upon cerebral ischemia and reperfusion injury. Exp. Mol. Med. 54 (2), 180–193. doi:10.1038/s12276-022-00734-y
Xiao, B., Chai, Y., Lv, S., Ye, M., Wu, M., Xie, L., et al. (2017). Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 40 (4), 1201–1209. doi:10.3892/ijmm.2017.3106
Xiao, L., Dai, Z., Tang, W., Liu, C., and Tang, B. (2021). Astragaloside IV alleviates cerebral ischemia-reperfusion injury through NLRP3 inflammasome-mediated pyroptosis inhibition via activating Nrf2. Oxid. Med. Cell Longev. 2021, 9925561. doi:10.1155/2021/9925561
Xiao, P., Huang, H., Zhao, H., Liu, R., Sun, Z., Liu, Y., et al. (2024). Edaravone dexborneol protects against cerebral ischemia/reperfusion-induced blood-brain barrier damage by inhibiting ferroptosis via activation of nrf-2/HO-1/GPX4 signaling. Free Radic. Biol. Med. 217, 116–125. doi:10.1016/j.freeradbiomed.2024.03.019
Xiao, Q., Kang, Z., Liu, C., and Tang, B. (2022). Panax notoginseng saponins attenuate cerebral ischemia-reperfusion injury via mitophagy-induced inhibition of NLRP3 inflammasome in rats. Front. Biosci. Landmark Ed. 27 (11), 300. doi:10.31083/j.fbl2711300
Xu, J., Wang, A., Meng, X., Yalkun, G., Xu, A., Gao, Z., et al. (2021). Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a phase III, randomized, double-blind, comparative trial. Stroke 52 (3), 772–780. doi:10.1161/STROKEAHA.120.031197
Xu, K., Zhao, X., He, Y., Guo, H., and Zhang, Y. (2024c). Stem cell-derived exosomes for ischemic stroke: a conventional and network meta-analysis based on animal models. Front. Pharmacol. 15, 1481617. doi:10.3389/fphar.2024.1481617
Xu, Q., Liu, Y., Tian, X., Xia, X., Zhang, Y., Zhang, X., et al. (2024a). Monocyte chemoattractant protein-1, inflammatory biomarkers, and prognosis of patients with ischemic stroke or transient ischemic attack: fndings from a nationwide registry study. J. Am. Heart Assoc. 13 (16), e035820. doi:10.1161/JAHA.124.035820
Xu, R., Peng, Q., Chen, W., Cheng, X., and Wang, G. (2025). ncRNAs-mediated pyroptosis in cerebral ischemia-reperfusion injury: pathophysiology, mechanisms, and therapeutic perspectives. Curr. Issues Mol. Biol. 47 (3), 141. doi:10.3390/cimb47030141
Xu, X., Chen, M., and Zhu, D. (2024b). Reperfusion and cytoprotective agents are a mutually beneficial pair in ischaemic stroke therapy: an overview of pathophysiology, pharmacological targets and candidate drugs focusing on excitotoxicity and free radical. Stroke Vasc. Neurol. 9 (4), 351–359. doi:10.1136/svn-2023-002671
Yan, H., Huang, W., Rao, J., Yan, D., and Yuan, J. (2024). Demethylase FTO-mediated m6A modification of lncRNA MEG3 activates neuronal pyroptosis via NLRP3 signaling in cerebral ischemic stroke. Mol. Neurobiol. 61 (2), 1023–1043. doi:10.1007/s12035-023-03622-2
Yan, W. T., Yang, Y. D., Hu, X. M., Ning, W. Y., Liao, L. S., Lu, S., et al. (2022). Do pyroptosis, apoptosis, and necroptosis (PANoptosis) exist in cerebral ischemia? Evidence from cell and rodent studies. Neural Regen. Res. 17 (8), 1761–1768. doi:10.4103/1673-5374.331539
Yang, B., Zang, L., Cui, J., and Wei, L. (2021a). Circular RNA TTC3 regulates cerebral ischemia-reperfusion injury and neural stem cells by miR-372-3p/TLR4 axis in cerebral infarction. Stem Cell Res. Ther. 12 (1), 125. doi:10.1186/s13287-021-02187-y
Yang, H., Han, M., Li, J., Ke, H., Kong, Y., Wang, W., et al. (2022b). Delivery of miRNAs through metal-organic framework nanoparticles for assisting neural stem cell therapy for ischemic stroke. ACS Nano 16 (9), 14503–14516. doi:10.1021/acsnano.2c04886
Yang, J., Chen, M., Cao, R. Y., Li, Q., and Zhu, F. (2018). The role of circular RNAs in cerebral ischemic diseases: ischemic stroke and cerebral ischemia/reperfusion injury. Adv. Exp. Med. Biol. 1087, 309–325. doi:10.1007/978-981-13-1426-1_25
Yang, J., He, W., Zhu, L., Liang, T., Liang, X., et al. (2023a). CircFOXP1 alleviates brain injury after acute ischemic stroke by regulating STAT3/apoptotic signaling. Transl. Res. 257, 15–29. doi:10.1016/j.trsl.2023.01.007
Yang, J. J., Zhao, Y. H., Yin, K. W., Zhang, X. Q., and Liu, J. (2021b). Dexmedetomidine inhibits inflammatory response and oxidative stress through regulating miR-205-5p by targeting HMGB1 in cerebral ischemic/reperfusion. Immunopharmacol. Immunotoxicol. 43 (4), 478–486. doi:10.1080/08923973.2021.1942901
Yang, M., Wang, K., Liu, B., Shen, Y., and Liu, G. (2024a). Hypoxic-ischemic encephalopathy: pathogenesis and promising therapies. Mol. Neurobiol. 62, 2105–2122. doi:10.1007/s12035-024-04398-9
Yang, X., Xu, J., Lan, S., Tong, Z., Chen, K., Liu, Z., et al. (2023b). Exosomal miR-133a-3p derived from BMSCs alleviates cerebral ischemia-reperfusion injury via targeting DAPK2. Int. J. Nanomedicine 18, 65–78. doi:10.2147/IJN.S385395
Yang, Y., Wu, Q., Shan, X., Zhou, H., Wang, J., Hu, Y., et al. (2024b). Ginkgolide B attenuates cerebral ischemia-reperfusion injury via inhibition of ferroptosis through disrupting NCOA4-FTH1 interaction. J. Ethnopharmacol. 318 (Pt B)), 116982. doi:10.1016/j.jep.2023.116982
Yang, Z., Huang, C., Wen, X., Liu, W., Huang, X., Li, Y., et al. (2022a). Circular RNA circ-FoxO3 attenuates blood-brain barrier damage by inducing autophagy during ischemia/reperfusion. Mol. Ther. 30 (3), 1275–1287. doi:10.1016/j.ymthe.2021.11.004
Yao, L., Peng, P., Ding, T., Yi, J., and Liang, J. (2024). m(6)A-Induced lncRNA MEG3 promotes cerebral ischemia-reperfusion injury via modulating oxidative stress and mitochondrial dysfunction by hnRNPA1/sirt2 Axis. Mol. Neurobiol. 61 (9), 6893–6908. doi:10.1007/s12035-024-04005-x
Yao, Y., Hu, S., Zhang, C., Zhou, Q., Wang, H., Yang, Y., et al. (2022). Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int. Immunopharmacol. 105, 108582. doi:10.1016/j.intimp.2022.108582
Yi, M., Li, Y., Wang, D., Zhang, Q., Yang, L., and Yang, C. (2020). KCNQ1OT1 exacerbates ischemia-reperfusion injury through targeted inhibition of miR-140-3P. Inflammation 43 (5), 1832–1845. doi:10.1007/s10753-020-01257-2
Yin, B., Hou, X. W., and Lu, M. L. (2019). Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol. Sin. 40 (5), 599–607. doi:10.1038/s41401-018-0082-y
Yin, M., Chen, W. P., Yin, X. P., Tu, J. L., Hu, N., and Li, Z. Y. (2021). LncRNA TUG1 demethylated by TET2 promotes NLRP3 expression, contributes to cerebral ischemia/reperfusion inflammatory injury. ASN Neuro 13, 17590914211003247. doi:10.1177/17590914211003247
Yu, H., Wu, Z., Wang, X., Gao, C., Liu, R., Kang, F., et al. (2020). Protective effects of combined treatment with mild hypothermia and edaravone against cerebral ischemia/reperfusion injury via oxidative stress and Nrf2 pathway regulation. Int. J. Oncol. 57 (2), 500–508. doi:10.3892/ijo.2020.5077
Yu, L., Zhang, Y., Chen, Q., He, Y., Zhou, H., Wan, H., et al. (2022). Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed. Pharmacother. 149, 112836. doi:10.1016/j.biopha.2022.112836
Yu, S., Yu, M., He, X., Wen, L., Bu, Z., and Feng, J. (2019). KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell 18 (3), e12940. doi:10.1111/acel.12940
Yu, S. J., Yu, M. J., Bu, Z. Q., He, P. P., and Feng, J. (2021). MicroRNA-670 aggravates cerebral ischemia/reperfusion injury via the Yap pathway. Neural Regen. Res. 16 (6), 1024–1030. doi:10.4103/1673-5374.300455
Yu, S. P., Jiang, M. Q., Shim, S. S., Pourkhodadad, S., and Wei, L. (2023). Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: implications for preventive treatments of ischemic stroke and late-onset Alzheimer's disease. Mol. Neurodegener. 18 (1), 43. doi:10.1186/s13024-023-00636-1
Yuan, J., Li, L., Yang, Q., Ran, H., Wang, J., Hu, K., et al. (2021). Targeted treatment of ischemic stroke by bioactive nanoparticle-derived reactive oxygen species responsive and inflammation-resolving nanotherapies. ACS Nano 15 (10), 16076–16094. doi:10.1021/acsnano.1c04753
Zeng, M., Zhang, R., Yang, Q., Guo, L., Zhang, X., Yu, B., et al. (2022). Pharmacological therapy to cerebral ischemia-reperfusion injury: focus on saponins. Biomed. Pharmacother. 155, 113696. doi:10.1016/j.biopha.2022.113696
Zhai, Y., Pang, X., Mei, X., Pang, Y., Shu, J., Xiao, Y., et al. (2025). Shuanglu tongnao formula alleviates cerebral ischemia/reperfusion injury by rebuilding inflammatory microenvironment after cerebral ischemia. J. Ethnopharmacol. 346, 119640. doi:10.1016/j.jep.2025.119640
Zhang, H., Xie, Q., and Hu, J. (2022b). Neuroprotective effect of physical activity in ischemic stroke: focus on the neurovascular unit. Front. Cell Neurosci. 16, 860573. doi:10.3389/fncel.2022.860573
Zhang, H., Zhu, C., Zhou, X., Wang, L., Deng, L., He, B., et al. (2025). Edaravone dexborneol protected neurological function by targeting NRF2/ARE and NF-κB/AIM2 pathways in cerebral ischemia/reperfusion injury. Front. Pharmacol. 16, 1581320. doi:10.3389/fphar.2025.1581320
Zhang, L., Han, Y., Wu, X., Chen, B., Liu, S., Huang, J., et al. (2023a). Research progress on the mechanism of curcumin in cerebral ischemia/reperfusion injury: a narrative review. Apoptosis 28 (9-10), 1285–1303. doi:10.1007/s10495-023-01869-7
Zhang, M., Yang, J. K., and Ma, J. (2021). Regulation of the long noncoding RNA XIST on the inflammatory polarization of microglia in cerebral infarction. Exp. Ther. Med. 22 (3), 924. doi:10.3892/etm.2021.10356
Zhang, Q., Chen, H., Yin, J., Chen, Y., Liu, L., et al. (2024c). Reduction of oxidative stress and excitotoxicity by mesenchymal stem cell biomimetic Co-delivery system for cerebral ischemia-reperfusion injury treatment. Small 20 (43), e2401045. doi:10.1002/smll.202401045
Zhang, R., Mao, W., Niu, L., Bao, W., Wang, Y., Wang, Y., et al. (2023b). NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. Elife 12, e84493. doi:10.7554/eLife.84493
Zhang, W., Liu, D., and Fan, J. (2024a). Downregulation of circAsxl2 relieves neuronal injury induced by oxygen-glucose deprivation/reperfusion. Mol. Neurobiol. 61 (2), 812–820. doi:10.1007/s12035-023-03532-3
Zhang, W., Xu, H., Li, C., Han, B., and Zhang, Y. (2024b). Exploring Chinese herbal medicine for ischemic stroke: insights into microglia and signaling pathways. Front. Pharmacol. 15, 1333006. doi:10.3389/fphar.2024.1333006
Zhang, X., Gong, P., Zhao, Y., Wan, T., Yuan, K., Xiong, Y., et al. (2022a). Endothelial caveolin-1 regulates cerebral thrombo-inflammation in acute ischemia/reperfusion injury. EBioMedicine 84, 104275. doi:10.1016/j.ebiom.2022.104275
Zhang, Z. H., Wang, Y. R., Li, F., Liu, X. L., Zhang, H., Zhu, Z. Z., et al. (2020). Circ-camk4 involved in cerebral ischemia/reperfusion induced neuronal injury. Sci. Rep. 10 (1), 7012. doi:10.1038/s41598-020-63686-1
Zhao, L., Chen, Y., Li, H., Ding, X., and Li, J. (2024). Deciphering the neuroprotective mechanisms of RACK1 in cerebral ischemia-reperfusion injury: pioneering insights into mitochondrial autophagy and the PINK1/Parkin axis. CNS Neurosci. Ther. 30 (8), e14836. doi:10.1111/cns.14836
Zhao, N., Hua, W., Liu, Q., Wang, Y., Liu, Z., Jin, S., et al. (2023a). MALAT1 knockdown alleviates the pyroptosis of microglias in diabetic cerebral ischemia via regulating STAT1 mediated NLRP3 transcription. Mol. Med. 29 (1), 44. doi:10.1186/s10020-023-00637-2
Zhao, X., Zhu, J., Chen, S., Liu, R., and Long, T. (2023b). Neural stem cell-derived exosomes improve neurological function in rats with cerebral ischemia-reperfusion injury by regulating microglia-mediated inflammatory response. J. Inflamm. Res. 16, 3079–3092. doi:10.2147/JIR.S414121
Zhou, F., Wang, Y. K., Zhang, C. G., and Wu, B. Y. (2021). miR-19a/b-3p promotes inflammation during cerebral ischemia/reperfusion injury via SIRT1/FoxO3/SPHK1 pathway. J. Neuroinflammation 18 (1), 122. doi:10.1186/s12974-021-02172-5
Zhou, H., Zhou, J., Teng, H., Yang, H., Qiu, J., and Li, X. (2022). MiR-145 enriched exosomes derived from bone marrow-derived mesenchymal stem cells protects against cerebral ischemia-reperfusion injury through downregulation of FOXO1. Biochem. Biophys. Res. Commun. 632, 92–99. doi:10.1016/j.bbrc.2022.09.089
Zhu, X., Liu, Q., Zhu, F., Jiang, R., Lu, Z., Wang, C., et al. (2024). An engineered cellular carrier delivers miR-138-5p to enhance mitophagy and protect hypoxic-injured neurons via the DNMT3A/Rhebl1 axis. Acta Biomater. 186, 424–438. doi:10.1016/j.actbio.2024.07.059
Keywords: cerebral ischemia-reperfusion injury, traditional drugs, antioxidants, neuroprotection, exosomes, non-coding RNA
Citation: Yang M, Liu B, Chen B, Shen Y and Liu G (2025) Cerebral ischemia-reperfusion injury: mechanisms and promising therapies. Front. Pharmacol. 16:1613464. doi: 10.3389/fphar.2025.1613464
Received: 17 April 2025; Accepted: 07 July 2025;
Published: 16 July 2025; Corrected: 22 July 2025.
Edited by:
Marcos Roberto De Oliveira, Federal University of Rio Grande do Sul, BrazilReviewed by:
Huifang Xing, Tianjin Medical University General Hospital, ChinaOla Elazazy, Badr University in Cairo, Egypt
Copyright © 2025 Yang, Liu, Chen, Shen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangliang Liu, MjQyODg1MTI0OUBxcS5jb20=; Yuntian Shen, c3l0NTE3QG50dS5lZHUuY24=
†These authors have contributed equally to this work
 Mingming Yang1†
Mingming Yang1† Yuntian Shen
Yuntian Shen