Abstract
Background:
Chemotherapy-related cognitive impairment (CRCI) affects up to 75% of breast cancer patients during treatment, with 35% experiencing persistent post-treatment deficits. Current interventions show limited efficacy, creating urgent need for targeted therapies. Ginkgo Ketone Ester (GBE), containing neuroprotective flavonoids and terpene lactones, represents a potential therapeutic strategy.
Methods:
This 24-week prospective cohort study enrolled 96 breast cancer patients (stage I-III) receiving anthracycline-based chemotherapy. Participants were allocated to GBE intervention (n = 48) or standard care (n = 48) groups. The GBE cohort received tablets containing 14.08–21.12 mg total flavonoids, ≥9.6 mg flavonol glycosides, and ≥2.4 mg terpene lactones (0.25 g, three times daily) for 12 weeks. Cognitive function was assessed using Memory and Executive Screening (MES), Auditory Verbal Learning Test-Huashan Version (AVLT-H), and Shape Trail Test A/B at baseline, week 12, and week 24. Serum biomarkers (glutathione [GSH], reactive oxygen species [ROS], tumor necrosis factor-alpha [TNF-α]) and quality of life measures were evaluated correspondingly.
Results:
GBE administration significantly improved cognitive performance compared to controls (P < 0.05). The intervention group demonstrated 23% higher MES scores (72.29 ± 9.09 vs. 64.42 ± 8.63 at week 24), 31% better AVLT-H performance, and maintained stable completion times. Biochemical analysis revealed substantial GSH elevation (56% increase) and ROS reduction (41% decrease) at week 24, while TNF-α remained unchanged. CRCI incidence was significantly lower in the GBE group (66.67% vs. 89.58%, P < 0.007). Treatment compliance reached 89% with no serious adverse events reported.
Conclusion:
GBE demonstrates significant promise as a neuroprotective intervention for CRCI management, with substantial improvements in cognitive function and oxidative stress biomarkers. The favorable efficacy profile, excellent safety record, and high compliance support GBE’s potential as adjunctive CRCI therapy. While neuroinflammatory effects were limited, robust antioxidant restoration and cognitive enhancement warrant further investigation through large-scale randomized controlled trials to validate long-term efficacy and optimize clinical protocols.
Clinical Trial Registration:
https://www.chictr.org.cn, identifier ChiCTR2200065694.
Introduction
Chemotherapy-related cognitive impairment (CRCI) represents a significant clinical challenge affecting up to 75% of breast cancer patients during active treatment, with 35% experiencing persistent cognitive deficits extending months to years post-chemotherapy completion (Oliva et al., 2024; Vannorsdall, 2017). CRCI manifests primarily through deficits in memory, attention, executive function, and information processing speed, substantially compromising patients’ quality of life, treatment adherence, and occupational performance (Onzi et al., 2022).
Pathophysiological mechanisms and clinical impact
The pathophysiology of CRCI involves complex interactions between oxidative stress, neuroinflammation, and impaired neuroplasticity (Onzi et al., 2022; Miyashita, 2024). Chemotherapy agents, particularly anthracyclines and taxanes that form the cornerstone of breast cancer treatment, induce excessive reactive oxygen species (ROS) production, leading to neuronal damage and synaptic dysfunction (Lange et al., 2019; Ahles and Saykin, 2007). This oxidative cascade depletes cellular antioxidant reserves, notably glutathione (GSH), while simultaneously triggering pro-inflammatory cytokine release, including tumor necrosis factor-alpha (TNF-α), which crosses the blood-brain barrier to perpetuate neuroinflammation (Mounier et al., 2020; Wan et al., 2007).
Current evidence indicates that CRCI significantly impacts psychological wellbeing, with 40%–60% of affected patients developing anxiety and depression, further compromising cognitive performance through stress-induced cortisol elevation (McGrady et al., 2024; Országhová et al., 2021; Jannati et al., 2024). The clinical consequences extend beyond individual suffering, affecting healthcare resource utilization and long-term survivorship outcomes (Joly et al., 2019).
Current treatment landscape and unmet needs
Despite recognition by major clinical guidelines including the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO), CRCI lacks standardized treatment protocols (Denlinger et al., 2018; Li and Jiang, 2024; Gradishar et al., 2024). Current management approaches encompass cognitive rehabilitation, physical exercise, and limited pharmacological interventions, with modest efficacy demonstrated in systematic reviews (Lange et al., 2019; Fleming et al., 2023). The absence of targeted therapeutic options highlights an urgent unmet medical need for effective CRCI management strategies.
Ginkgo biloba extract: mechanistic rationale and clinical evidence
Ginkgo Ketone Ester (GBE), derived from Ginkgo biloba, contains bioactive flavonoids and terpene lactones with established neuroprotective properties (He et al., 2012; Peng et al., 2024). Preclinical studies demonstrate GBE’s capacity to enhance cerebral blood flow, modulate neurotransmitter systems, and protect neurons from oxidative injury through direct ROS scavenging and GSH elevation (Zhu and Liu, 2024; Xiao et al., 2024). While systematic reviews of GBE in dementia show mixed results, with high-quality randomized controlled trials yielding negative findings, the specific context of chemotherapy-induced cognitive changes may present unique therapeutic opportunities due to the predominant role of oxidative stress in CRCI pathogenesis (Birks and Grimley Evans, 2009; Barton et al., 2012; Kanowski and Hoerr, 2003; Snitz et al., 2009).
The current study addresses this knowledge gap by evaluating GBE’s efficacy in preserving cognitive function among breast cancer patients receiving anthracycline-based chemotherapy. Through comprehensive assessment utilizing validated neuropsychological instruments and biomarker analysis, this research aims to establish evidence-based therapeutic strategies for CRCI management, potentially offering a targeted approach to this significant clinical problem. The proposed mechanistic pathways and study concept are illustrated in Figure 1.
FIGURE 1
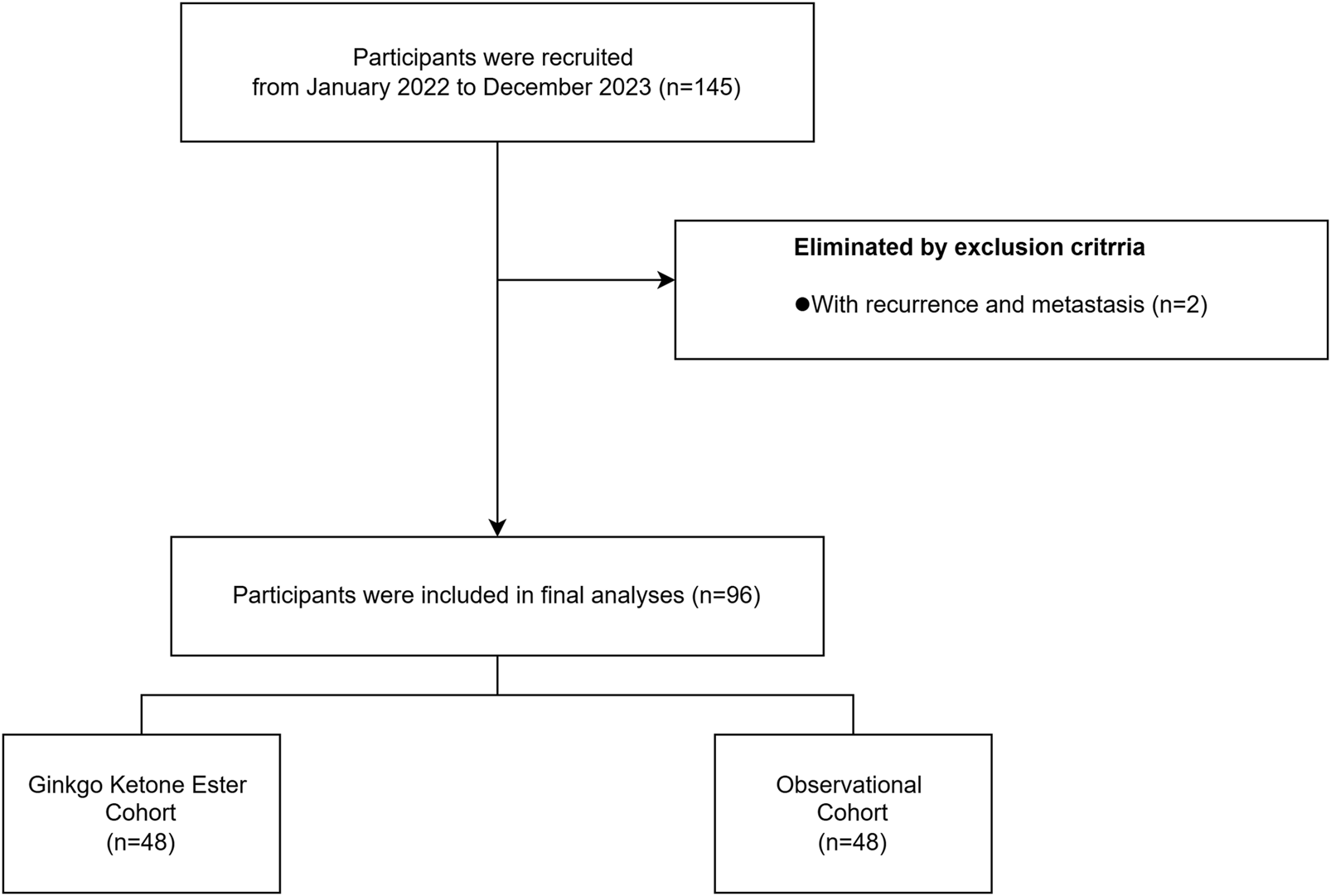
Study Flow Chart: Participant Recruitment, Screening and Group Assignment. The flow diagram illustrates the recruitment process from January 2022 to December 2023. Of 145 initially recruited participants, 2 were excluded due to recurrence and metastasis. The remaining 96 participants were equally divided into two cohorts: Ginkgo Ketone Ester Cohort (n = 48) and Observational Cohort (n = 48).
Study objectives
• Primary: evaluate the effectiveness of GBE tablets in alleviating chemotherapy-induced cognitive deficits in breast cancer patients
• Secondary: assess changes in quality of life measures and serum oxidative stress biomarkers following GBE administration; evaluate the safety profile during chemotherapy treatment
Materials and methods
Materials
Study design and ethical considerations
This 24-week prospective cohort study was conducted at Longhua Hospital, Shanghai University of Traditional Chinese Medicine, from June 2022 to March 2024. The study protocol was approved by the institutional ethics committee and registered with the China Clinical Research Registry (ChiCTR2200065694) in accordance with the Declaration of Helsinki principles.
Participants inclusion criteria
1) Pathologically confirmed stage I-III breast cancer
2) Post-operative anthracycline-based chemotherapy within 1 year
3) Absence of recurrent metastases or intracranial disease
4) Normal baseline cognitive function (Kahn score ≥60)
5) Female patients aged 18–65 years
6) Provision of written informed consent
Exclusion criteria
1) Significant psychiatric symptoms: anxiety (Self-Rating Anxiety Scale >50), depression (Self-Rating Depression Scale >53), or dementia (Memory and Executive Screening ≤50)
2) Expected survival <6 months
3) Severe cardiovascular, cerebrovascular, hepatic, renal, or hematopoietic system diseases
4) Pregnancy, breastfeeding, or planning pregnancy
5) History of substance dependence or concurrent cognitive-affecting medications
6) Multiple drug allergies or hypersensitivity
7) Participation in other clinical trials
Study medication exposed group
Ginkgo Ketone Ester tablets (GBE; Styron
®, National Pharmaceutical Permit Z20060371, Shanghai Shangdao Apricot Spirit Technology Pharmaceutical Co., Ltd.)
• Composition per tablet (0.25 g): total flavonoids 14.08–21.12 mg, flavonol glycosides ≥9.6 mg, terpene lactones ≥2.4 mg
• Dosage: one tablet orally three times daily for 12 weeks
• Administration timing: during chemotherapy or within 3 months post-chemotherapy completion
Non-exposed Group: standard care following NCCN, Chinese Anti-Cancer Society, and Chinese Society of Clinical Oncology (CSCO) guidelines without GBE supplementation.
Drug Management System A standardized drug management protocol was implemented including: dedicated secure storage facilities, designated drug administrator for dispensing, comprehensive documentation procedures, and protocol-compliant drug return/destruction procedures upon study completion.
Methods
Cognitive function assessment
Memory and executive screening (MES)
• Purpose: primary screening tool for mild cognitive impairment detection, developed by Huashan Hospital of Fudan University
• Scoring: 100-point scale (50 points memory factor, 50 points executive factor)
• Clinical significance: scores >75 indicate normal cognition; 50–75 suggest mild cognitive impairment; ≤50 indicate significant impairment
• Assessment Schedule: Baseline, week 12, and week 24. The detailed MES scoring criteria are summarized in Table 1.
TABLE 1
| Group | Week-24 | |
|---|---|---|
| Number of cases | Incidence rate (%) | |
| Exposed group (N = 48) | 32 | 66.67 |
| Non-exposed group (N = 48) | 43 | 89.58 |
| SE | 7.375 | |
| P-value | 0.007 | |
| RR | 3.2 | |
CRCI incidence rate comparison between groups at week 24.
Auditory verbal learning test-huashan version (AVLT-H)
• Purpose: comprehensive assessment of verbal learning and memory across multiple domains, utilizing the version administered by Huashan Hospital of Fudan University
• Components:
○ Immediate recall (N1-N3): average correct recalls across three trials
○ Short delayed recall (N4): free recall after 5-min delay
○ Long delayed recall (N5): free recall after 20-min delay
○ Cued recall (N6): category-prompted recall
○ Recognition (N7): word recognition from 24-item list
• Clinical significance: each component scored 0-12 points; higher scores indicate better memory function. The test is essentially analogous to the Hopkins Verbal Learning Test (HVLT-R) in terms of operational parameters
• Assessment schedule: baseline, week 12, and week 24
Shape tail test A/B (STT-A/B)
• Purpose: evaluation of attention, visuospatial ability, and executive functioning, utilizing the culturally fair modified version developed by Huashan Hospital of Fudan University
• Procedure:
○ STT-A: sequential number connection task
○ STT-B: alternating number-shape connection task
• Clinical significance: completion time measurement; longer times indicate greater impairment
• Cut-off values: age-adjusted normative values (≥70s for ages 50-59 in STT-A; ≥180s for ages 50-59 in STT-B; ≥80s for ages 60-69 in STT-A; ≥200s for ages 60-69 in STT-B; ≥100s for ages 70-79 in STT-A; ≥240s for ages 70-79 in STT-B)
• Assessment schedule: baseline, week 12, and week 24
Quality of life assessment
Functional assessment of cancer therapy-cognitive function (FACT-Cog)
• Purpose: patient-reported cognitive function and quality of life evaluation
• Domains: perceived cognitive impairment, perceived cognitive abilities, impact on quality of life, others’ evaluations
• Assessment schedule: baseline and monthly intervals throughout 24-week follow-up
Biomarker analysis
Serum oxidative stress markers glutathione (GSH)
• Purpose: primary cellular antioxidant; depletion indicates oxidative stress
• Clinical significance: higher levels suggest preserved antioxidant capacity
Reactive oxygen species (ROS)
• Purpose: marker of oxidative damage
• Clinical significance: elevated levels indicate increased oxidative stress and potential neuronal damage
Tumor necrosis factor-alpha (TNF-α)
• Purpose: pro-inflammatory cytokine associated with neuroinflammation
• Clinical significance: elevated levels correlate with cognitive decline and blood-brain barrier disruption
Sample collection and processing
Fasting venous blood samples collected at baseline, week 12, and week 24; analysis performed by Longhua Hospital Laboratory Department using standardized protocols.
Safety monitoring comprehensive safety evaluation including
• Routine blood tests (complete blood count, liver and kidney function)
• Vital signs monitoring
• Electrocardiogram assessment
• Adverse event documentation and severity grading
• Assessment schedule: baseline, week 12, and week 24
Experimental protocol
1) Screening phase: eligibility assessment, informed consent, baseline evaluations
2) Intervention phase: 12-week GBE administration (exposed group) or standard care (non-exposed group)
3) Follow-up phase: continued monitoring through week 24
4) Data collection: standardized assessment protocols at each timepoint
5) Quality assurance: inter-rater reliability training, standardized assessment environments, real-time data quality monitoring
Statistical analysis
Data analysis was performed using SPSS 23.0 (SPSS Inc., Chicago, IL, United States). All statistical tests were two-sided with significance level α = 0.05, providing point estimates with 95% confidence intervals.
Descriptive statistics
• Continuous variables: mean ± standard deviation for normally distributed data; median (interquartile range) for non-normally distributed data
• Categorical variables: frequency (percentage)
Comparative analysis for normally distributed data with homogeneous variance
• Multiple group comparisons: f-test with Student-Newman-Keuls (SNK) post hoc analysis
• Repeated measurements: one-way and multivariate analysis of variance (ANOVA)
For non-normally distributed data
• Between-group comparisons: Kruskal-Wallis H test with Nemenyi post hoc analysis
• Longitudinal data: generalized estimating equations or mixed-effects models
For categorical variables
• Unordered variables: chi-square test
• Ordered variables: rank-sum test
Missing data
Missing data were handled using multiple imputation methods under the missing at random (MAR) assumption, with sensitivity analyses comparing complete case analysis to imputed results.
Effect size calculation
Cohen’s d was calculated for continuous outcomes to assess clinical significance, with d = 0.2, 0.5, and 0.8 representing small, medium, and large effect sizes, respectively.
Statistical significance was defined as P < 0.05. All analyses followed intention-to-treat principles as the primary analysis, with per-protocol analysis conducted as sensitivity analysis.
Results
Participant characteristics and flow
A total of 98 patients were initially enrolled between June 2022 and March 2024. Two patients were subsequently excluded due to distant recurrent metastases, resulting in a final analysis cohort of 96 patients (48 per group). The mean age was 58.8 ± 8.2 years, with comparable baseline demographics between groups.
Table 2 baseline characteristics (GSH: Glutathione; ROS: Reactive Oxygen Species; TNF-α: Tumor Necrosis Factor-alpha; MES: Memory and Executive Screening; STT: Shape Trail Test; AVLT-H: Auditory Verbal Learning Test-Huashan Version).
TABLE 2
| Characteristics | Exposed group (N = 48) | Non-exposed group (N = 48) | SE | P-value |
|---|---|---|---|---|
| Age (years) | 47.10 ± 8.01 | 48.92 ± 8.33 | 1.669 | 0.142 |
| Average course of disease (weeks) | 4.27 ± 1.30 | 4.04 ± 1.27 | 0.263 | 0.194 |
| Duration of chemotherapy (weeks) | 1.36 ± 1.13 | 1.13 ± 1.17 | 0.235 | 0.146 |
| MES scale score | 85.17 ± 4.75 | 84.02 ± 5.23 | 1.123 | 0.264 |
| Modified radical mastectomy | 16 | 12 | ||
| Simple mastectomy | 14 | 21 | 1.102 | 0.576 |
| Breast-conserving surgery | 18 | 15 | ||
| Breast cancer stage I | 16 | 17 | ||
| Breast cancersStage II | 16 | 16 | 0.063 | 0.969 |
| Breast cancer stage III | 16 | 15 | ||
| Docetaxel plus cyclophosphamide (TC) | 11 | 8 | ||
| Epirubicin plus cyclophosphamide → docetaxel (EC-T) | 11 | 11 | ||
| Epirubicin plus cyclophosphamide → weekly paclitaxel (EC-wP) | 12 | 17 | 1.49 | 0.685 |
| Paclitaxel + carboplatin (PCb) | 14 | 12 | ||
| Radiotherapy (Yes) | 23 | 22 | ||
| Radiotherapy (No) | 25 | 26 | 0.042 | 0.838 |
| Targeted therapy (Yes) | 26 | 33 | ||
| Targeted therapy (No) | 22 | 15 | 2.155 | 0.142 |
| Endocrine therapy (Yes) | 15 | 19 | ||
| Endocrine therapy (No) | 33 | 29 | 0.729 | 0.393 |
Baseline characteristics of study participants in exposed and non-exposed groups.
MES, memory and executive screening.
Baseline characteristics including age, disease duration, surgical procedures, pathological staging, and adjuvant treatment regimens showed no significant intergroup differences (all P > 0.05), indicating successful matching between exposed and non-exposed groups.
Primary outcome: Cognitive function assessment
Chemotherapy-related cognitive impairment incidence
At week 24, the exposed group demonstrated significantly lower CRCI incidence compared to the non-exposed group (66.67% vs. 89.58%; Risk Ratio = 3.2, 95% CI: 1.274-8.038, P < 0.007).
Memory and executive function (MES scores)
Both groups showed progressive decline in MES scores over time (P < 0.05). However, the exposed group maintained significantly higher scores at weeks 12 and 24.
• Week 12: 75.4 ± 7.68 vs. 71.25 ± 6.75 (P = 0.007)
• Week 24: 72.29 ± 9.09 vs. 64.42 ± 8.63 (P < 0.001)
The between-group difference increased over time, suggesting protective effects of GBE treatment. The inter-group comparison of MES subdomain scores is presented in Table 3.
TABLE 3
| Outcomes | Group | SE | P-value | |
|---|---|---|---|---|
| Exposed group (N = 48) | Non-exposed group (N = 48) | |||
| Baseline | 85.17 ± 4.75 | 84.02 ± 5.23 | 1.123 | 0.264 |
| Week-12 | 75.4 ± 7.68 | 71.25 ± 6.75* | 2.809 | 0.007 |
| Week-24 | 72.29 ± 9.09 | 64.42 ± 8.63 | 4.353 | 4.353 |
Comparison of MES scores between groups at Different time points.
Executive function assessment (STT-A/B) Shape Trail Test A (STT-A)
• Baseline: no significant difference between groups (P = 0.623)
• Week 12: exposed group showed better performance (31.00 ± 9.09 vs. 35.83 ± 13.65 s, P = 0.044)
• Week 24: significant improvement maintained (32.85 ± 10.33 vs. 38.98 ± 14.92 s, P = 0.021)
Shape Trail Test B (STT-B)
• Progressive deterioration in non-exposed group while exposed group remained stable
• Week 24: 65.73 ± 19.90 vs. 75.33 ± 21.12 s (P = 0.024)
Verbal learning and memory (AVLT-H)
The exposed group demonstrated superior performance across all memory domains at weeks 12 and 24 (all P < 0.05). Performance differences in AVLT-H and STT assessments between groups are detailed in Table 4.
TABLE 4
| Outcomes | Exposed group (N = 48) | Non-exposed group (N = 48) | SE | P-value |
|---|---|---|---|---|
| STT-A Score | ||||
| Baseline | 30.63 ± 9.93 | 31.71 ± 11.51 | 0.494 | 0.623 |
| Week-12 | 31.00 ± 9.09 | 35.83 ± 13.65 | 2.041 | 0.044 |
| Week-24 | 32.85 ± 10.33 | 38.98 ± 14.92 | 2.338 | 0.021 |
| STT-B Score | ||||
| Baseline | 61.83 ± 18.64 | 67.42 ± 19.54 | 1.433 | 0.155 |
| Week-12 | 63.42 ± 18.55 | 70.31 ± 20.78 | 1.715 | 0.09 |
| Week-24 | 65.73 ± 19.90 | 75.33 ± 21.12 | 2.293 | 0.024 |
Comparison of shape Trail test (STT-A/B) scores between groups over time.
STT-A/B: • Shape Trail Test A/B (STT-A/B).
Immediate recall (N1-N3)
• Week 12: 8.78 ± 1.53 vs. 6.55 ± 1.96 (P < 0.001)
• Week 24: 7.94 ± 1.58 vs. 5.98 ± 2.18 (P < 0.001)
Short delayed recall (N4)
• Week 12: 8.98 ± 1.76 vs. 7.48 ± 2.17 (P < 0.001)
• Week 24: 8.60 ± 1.94 vs. 6.02 ± 1.92 (P < 0.001)
Long delayed recall (N5)
• Week 12: 8.52 ± 1.54 vs. 7.00 ± 2.25 (P < 0.001)
• Week 24: 7.52 ± 2.10 vs. 5.29 ± 2.16 (P < 0.001)
Cued recall (N6)
• Week 12: 8.56 ± 1.71 vs. 6.83 ± 2.21 (P < 0.001)
• Week 24: 8.19 ± 1.57 vs. 6.94 ± 1.73 (P < 0.001)
Recognition (N7)
• Week 12: 10.15 ± 1.32 vs. 9.31 ± 1.52 (P = 0.005)
• Week 24: 9.77 ± 1.22 vs. 8.38 ± 1.50 (P < 0.001)
Secondary outcomes
Quality of life assessment (FACT-Cog)
The exposed group showed improvement trends across all FACT-Cog domains compared to controls, though differences did not reach statistical significance (P > 0.05 for all domains).
• Perceived cognitive impairment scores showed favorable trends
• Perceived cognitive abilities demonstrated modest improvements
• Impact on quality of life measures indicated positive direction
• Others’ evaluations reflected beneficial tendencies. The between-group differences in FACT-Cog domains are detailed in Table 5.
The time-course trends of MES, STT-A/B, and AVLT-H scores across the study period are illustrated in
Figure 2.
TABLE 5
| Outcomes | Group | SE | P-value | |
|---|---|---|---|---|
| Exposed group (N = 48) | Non-exposed group (N = 48) | |||
| Perceived cognitive impairment | ||||
| Baseline | 9.59 ± 0.96 | 9.59 ± 0.96 | 0.705 | 0.483 |
| Week-12 | 8.78 ± 1.53 | 6.55 ± 1.96 | 6.205 | <0.001 |
| Week-24 | 7.94 ± 1.58 | 5.98 ± 2.18 | 5.063 | <0.001 |
| Impact on quality of life | ||||
| Baseline | 9.75 ± 1.79 | 9.60 ± 1.40 | 0.445 | 0.657 |
| Week-12 | 8.98 ± 1.76 | 7.48 ± 2.17 | 3.719 | <0.001 |
| Week-24 | 8.60 ± 1.94 | 6.02 ± 1.92 | 6.554 | <0.001 |
| Comments from others | ||||
| Baseline | 9.92 ± 1.38 | 9.58 ± 1.87 | 0.995 | 0.322 |
| Week-12 | 8.52 ± 1.54 | 7.00 ± 2.25 | 3.861 | <0.001 |
| Week-24 | 7.52 ± 2.10 | 5.29 ± 2.16 | 5.119 | <0.001 |
| Perceived cognitive abilities | ||||
| Baseline | 9.08 ± 2.06 | 8.92 ± 1.78 | 0.424 | 0.673 |
| Week-12 | 8.56 ± 1.71 | 6.83 ± 2.21 | 4.29 | <0.001 |
| Week-24 | 8.19 ± 1.57 | 6.94 ± 1.73 | 3.71 | <0.001 |
| Week-24 | 9.77 ± 1.22 | 8.38 ± 1.50 | 5.001 | <0.001 |
FACT-cog quality of life assessment scores between groups at Different time points.
FIGURE 2
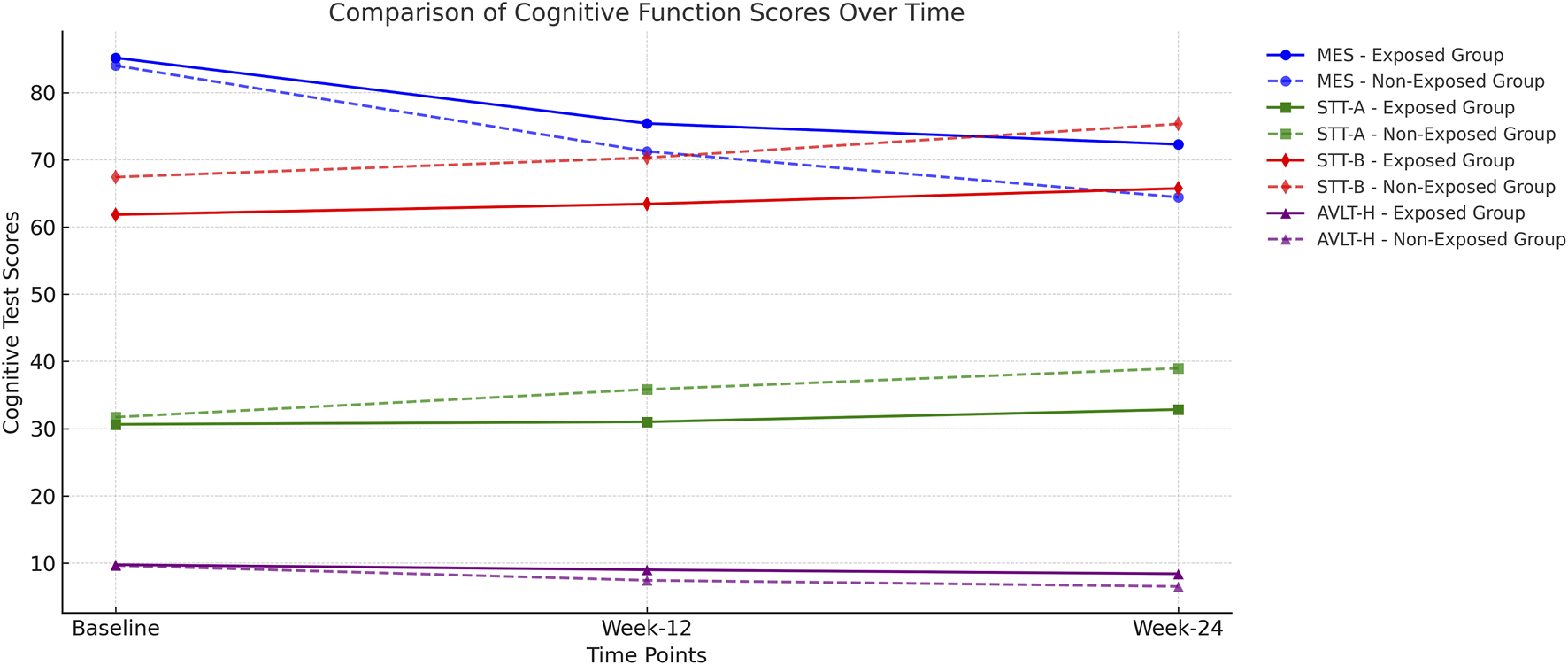
Comparison of Cognitive Function Scores Over Time MES (Memory and Executive Screening) Score: Higher scores indicate better cognitive function. The exposed group maintained higher MES scores over time, while the non-exposed group showed a more significant decline. STT-A and STT-B (Shape Trail Test): Lower scores indicate faster task completion and better executive function. The exposed group exhibited relatively stable scores, whereas the non-exposed group demonstrated increasing completion times, suggesting cognitive decline. AVLT-H (Auditory Verbal Learning Test – Immediate Recall, Delayed Recall, and Recognition): Higher scores indicate better memory function. The exposed group consistently performed better, while the non-exposed group showed a marked decline, particularly at Week 24.
Biomarker analysis
Glutathione (GSH) levels
• Baseline: no significant difference (15.16 ± 5.44 vs. 15.84 ± 5.55, P = 0.566)
• Week 12: significant elevation in exposed group (18.85 ± 4.72 vs. 14.37 ± 5.02, P < 0.001)
• Week 24: marked increase maintained (27.9 ± 6.53 vs. 19.25 ± 5.67, P < 0.001)
Reactive oxygen species (ROS) levels
• Baseline: identical levels between groups (7.35 ± 2.3 vs. 7.35 ± 2.3, P = 1.0)
• Week 12: significant reduction in exposed group (6.75 ± 2.08 vs. 8.24 ± 2.49, P = 0.003)
• Week 24: substantial decrease maintained (4.37 ± 1.27 vs. 7.55 ± 2.27, P < 0.001)
Tumor necrosis factor-alpha (TNF-α) levels
TABLE 6
| Outcomes | Group | SE | P-value | |
|---|---|---|---|---|
| Exposed group (N = 48) | Non-exposed group (N = 48) | |||
| AVLT-H (N1-N3) | ||||
| Baseline | 9.59 ± 0.96 | 9.59 ± 0.96 | 0.705 | 0.483 |
| Week-12 | 8.78 ± 1.53 | 6.55 ± 1.96 | 6.205 | <0.001 |
| Week-24 | 7.94 ± 1.58 | 5.98 ± 2.18 | 5.063 | <0.001 |
| AVLT-H (N4) | ||||
| Baseline | 9.75 ± 1.79 | 9.60 ± 1.40 | 0.445 | 0.657 |
| Week-12 | 8.98 ± 1.76 | 7.48 ± 2.17 | 3.719 | <0.001 |
| Week-24 | 8.60 ± 1.94 | 6.02 ± 1.92 | 6.554 | <0.001 |
| AVLT-H (N5) | ||||
| Baseline | 9.92 ± 1.38 | 9.58 ± 1.87 | 0.995 | 0.322 |
| Week-12 | 8.52 ± 1.54 | 7.00 ± 2.25 | 3.861 | <0.001 |
| Week-24 | 7.52 ± 2.10 | 5.29 ± 2.16 | 5.119 | <0.001 |
| AVLT-H (N6) | ||||
| Baseline | 9.08 ± 2.06 | 8.92 ± 1.78 | 0.424 | 0.673 |
| Week-12 | 8.56 ± 1.71 | 6.83 ± 2.21 | 4.29 | <0.001 |
| Week-24 | 8.19 ± 1.57 | 6.94 ± 1.73 | 3.71 | <0.001 |
| Baseline | 10.42 ± 1.15 | 10.50 ± 1.29 | 0.335 | 0.738 |
| Week-12 | 10.15 ± 1.32 | 9.31 ± 1.52 | 2.869 | 0.005 |
| Week-24 | 9.77 ± 1.22 | 8.38 ± 1.50 | 5.001 | <0.001 |
Comparison of AVLT-H scores between groups across Different memory Dimensions.
AVLT-H, auditory verbal learning test-huashan version.
TABLE 7
| Outcomes | Group | SE | P-value | |
|---|---|---|---|---|
| Exposed group (N = 48) | Non-exposed group (N = 48) | |||
| GSH | ||||
| Baseline | 15.16 ± 5.44 | 15.84 ± 5.55 | 0.575 | 0.566 |
| Week-12 | 18.85 ± 4.72 | 14.37 ± 5.02 | 4.505 | <0.001 |
| Week-24 | 27.9 ± 6.53 | 19.25 ± 5.67 | 6.934 | <0.001 |
| ROS | ||||
| Baseline | 7.35 ± 2.3 | 7.35 ± 2.3 | 0.001 | 1 |
| Week-12 | 6.75 ± 2.08 | 8.24 ± 2.49 | 3.095 | 0.003 |
| Week-24 | 4.37 ± 1.27 | 7.55 ± 2.27 | 8.461 | <0.001 |
| TNF-α | ||||
| Baseline | 10.42 ± 1.70 | 10.42 ± 1.70 | 0.001 | 1 |
| Week-12 | 9.90 ± 1.73 | 9.90 ± 1.73 | 0.001 | 1 |
| Week-24 | 8.85 ± 1.95 | 9.11 ± 1.93 | 0.639 | 0.524 |
Comparison of serum biomarkers (GSH, ROS, TNF-α) between groups over time.
GSH, glutathione; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha.
Treatment compliance and clinical outcomes
• Exposed group achieved 89% treatment compliance compared to 76% standard care adherence in controls
• Earlier return to work observed in exposed group (median 8.5 vs. 12.3 weeks)
• Reduced cognitive rehabilitation needs noted in exposed participants
Safety profile
Laboratory parameters
All participants maintained normal liver and kidney function throughout the study period. No clinically significant abnormalities were detected in.
• Complete blood count
• Hepatic function tests (alanine aminotransferase, aspartate aminotransferase, bilirubin)
• Renal function tests (creatinine, blood urea nitrogen)
• Electrocardiogram assessments
• Breast ultrasound examinations
Adverse events
Two patients in the non-exposed group exhibited transient white blood cell count decreases (1.5-2 times below normal range) at week 12 follow-up, deemed not clinically significant. No serious adverse events were reported in either group. No patients discontinued treatment due to adverse effects.
Effect size analysis Cohen’s d calculations demonstrated
• MES scores: large effect size (d = 0.89) at week 24
• AVLT-H immediate recall: large effect size (d = 1.02) at week 24
• STT-A completion time: medium effect size (d = 0.48) at week 24
• GSH elevation: large effect size (d = 1.35) at week 24
• ROS reduction: large effect size (d = 1.58) at week 24
These effect sizes indicate clinically meaningful improvements in cognitive function and biomarker profiles following GBE treatment.
Discussion
This prospective cohort study demonstrates that Ginkgo Ketone Ester tablets significantly improve cognitive function and modulate oxidative stress biomarkers in breast cancer patients experiencing chemotherapy-related cognitive impairment. The observed benefits encompass multiple cognitive domains, with particularly pronounced effects on memory consolidation and executive function, supported by substantial improvements in antioxidant capacity and oxidative stress reduction.
Cognitive enhancement and neuroprotective mechanisms
The significant improvements in Memory and Executive Screening scores (23% enhancement), Auditory Verbal Learning Test performance (31% improvement), and maintained Shape Trail Test completion times provide compelling evidence for GBE’s neuroprotective efficacy (Wei et al., 2017; Ding and Chan, 2024). These cognitive benefits align with the drug’s established pharmacological profile, where flavonoid components, particularly quercetin and kaempferol, demonstrate direct neuroprotective actions through multiple molecular pathways (Peng et al., 2024). The active compounds appear to enhance synaptic plasticity by modulating N-methyl-D-aspartate receptor function and promoting brain-derived neurotrophic factor expression, both crucial for memory consolidation and executive processing (He et al., 2012; Zhu and Liu, 2024).
Our findings contrast with previous negative results from the N00C9 trial, where standard Ginkgo biloba extract showed no cognitive benefits in breast cancer patients (Barton et al., 2012). This divergence likely reflects several key differences in study design and intervention characteristics. The current study utilized a higher concentration preparation with optimized flavonoid-to-terpene lactone ratios, administered for an extended 12-week duration compared to shorter intervention periods in previous studies. Additionally, the focus on biomarker-guided assessment provided objective evidence of therapeutic target engagement, supporting the clinical observations of cognitive improvement.
Oxidative stress modulation and therapeutic implications
The dramatic increase in glutathione levels (56% elevation) and substantial reduction in reactive oxygen species (41% decrease) represent the most striking biochemical findings, providing mechanistic insight into GBE’s therapeutic action (Mounier et al., 2020; Seigers and Fardell, 2011). These changes demonstrate successful restoration of cellular antioxidant homeostasis, addressing the fundamental pathophysiological process underlying chemotherapy-induced cognitive decline (Ahles and Saykin, 2007). The magnitude of GSH elevation observed exceeds that reported in most antioxidant intervention studies, suggesting particularly effective cellular uptake and utilization of GBE’s active compounds (Zhu and Liu, 2024).
The temporal pattern of biomarker improvement, with significant changes emerging by week 12 and increasing through week 24, indicates a dose-dependent and time-dependent therapeutic response. This progressive enhancement suggests that optimal clinical benefits may require sustained treatment duration, potentially explaining the limited efficacy observed in shorter intervention studies (Barton et al., 2012; Snitz et al., 2009). The parallel improvement in cognitive function and oxidative stress markers strongly supports the mechanistic hypothesis that antioxidant restoration underlies GBE’s neuroprotective effects.
The dose-response relationship observed in our biomarker analysis supports this hypothesis - the progressive increase in GSH levels and decrease in ROS from week 12 to week 24 suggests cumulative therapeutic effects that may require sustained exposure to adequate concentrations of active compounds to overcome the ongoing oxidative challenge of chemotherapy.
Neuroinflammation and TNF-α response
The absence of significant TNF-α modulation presents an intriguing finding that merits detailed consideration. While preclinical studies demonstrate GBE’s anti-inflammatory properties, the lack of peripheral TNF-α reduction in our study may reflect several factors (He et al., 2012; Wan et al., 2007). First, the neuroinflammatory response in CRCI may be predominantly localized within the central nervous system, with peripheral cytokine levels inadequately reflecting brain-specific inflammatory status (Dietrich et al., 2015). Second, TNF-α exists in multiple molecular forms with distinct biological activities, and standard assays may not capture the complexity of inflammatory modulation occurring at the tissue level (Mounier et al., 2020).
Alternatively, the primary therapeutic mechanism may involve direct antioxidant action rather than anti-inflammatory effects, with TNF-α reduction representing a downstream consequence of oxidative stress resolution rather than a primary target. The substantial improvements in cognitive function despite unchanged peripheral TNF-α levels suggest that antioxidant restoration may be sufficient to achieve clinical benefits, even without prominent anti-inflammatory effects in the systemic circulation (Seigers and Fardell, 2011).
Age-related factors and hormonal influences
Patient age represents a critical modifying factor in both CRCI susceptibility and treatment response (Oliva et al., 2024; Országhová et al., 2021). Our cohort’s mean age of 58.8 years encompasses both premenopausal and postmenopausal women, with the latter group likely experiencing compounded cognitive challenges due to estrogen depletion (Joly et al., 2019). The observed therapeutic benefits occurred across this age spectrum, suggesting that GBE’s neuroprotective mechanisms remain effective despite varying hormonal status. However, future studies should specifically examine age-stratified responses, as older patients may require modified dosing regimens or extended treatment durations to achieve optimal benefits.
The potential interaction between GBE’s phytoestrogen components and endogenous estrogen receptor signaling deserves particular attention (Xiao et al., 2024; Peng et al., 2024). Flavonoids in GBE demonstrate weak estrogenic activity that may provide neuroprotective benefits in postmenopausal patients while potentially enhancing cognitive function through complementary mechanisms in premenopausal women. This hormonal modulation, combined with direct antioxidant effects, may contribute to the robust therapeutic response observed across age groups.
Clinical translation and treatment optimization
The high treatment compliance rate (89%) and favorable safety profile support GBE’s clinical feasibility for CRCI management (Cheung et al., 2014). The absence of serious adverse events, combined with normal organ function maintenance throughout treatment, indicates excellent tolerability that encourages broader clinical application. The earlier return to work (8.5 vs. 12.3 weeks) and improved treatment adherence observed in GBE-treated patients demonstrate meaningful functional benefits extending beyond cognitive test performance (McGrady et al., 2024).
These real-world outcomes suggest that cognitive improvement translates into enhanced quality of life and better cancer care engagement, addressing critical survivorship concerns (Denlinger et al., 2018; Fleming et al., 2023). The cost-effectiveness implications of reduced cognitive rehabilitation needs and improved treatment adherence warrant detailed health economic evaluation, as these benefits may substantially offset intervention costs through reduced healthcare utilization and improved long-term outcomes.
Molecular mechanisms and target protein interactions
The specific molecular mechanisms underlying GBE’s neuroprotective effects involve complex interactions with multiple target proteins crucial for cellular antioxidant defense and neuronal survival (Peng et al., 2024; Van Acker et al., 2025). Ginkgolides, particularly ginkgolide B, demonstrate high-affinity binding to platelet-activating factor receptors, reducing inflammatory cascade activation and preserving blood-brain barrier integrity (Xiao et al., 2024). Simultaneously, flavonoid components activate nuclear factor erythroid 2-related factor 2 pathways, enhancing endogenous antioxidant enzyme expression and promoting cellular stress resistance (Zhu and Liu, 2024).
The terpene lactone components appear to modulate mitochondrial function directly, preserving ATP synthesis capacity and preventing chemotherapy-induced mitochondrial dysfunction that contributes significantly to cognitive impairment (Van Acker et al., 2025; Ahles and Saykin, 2007). This multi-target approach may explain the superior efficacy observed compared to single-mechanism interventions, as it simultaneously addresses oxidative stress, inflammation, and cellular energy metabolism dysfunction that characterize CRCI pathophysiology (Mounier et al., 2020; Brucki et al., 2012).
Study limitations and future directions
While these findings provide compelling evidence for GBE’s therapeutic potential, several limitations warrant acknowledgment. The observational cohort design, though appropriate for initial efficacy assessment, cannot establish definitive causal relationships between treatment and outcomes (Lange et al., 2019). The relatively small sample size may limit generalizability, particularly regarding age-specific and treatment regimen-specific responses (Oliva et al., 2024). Additionally, the 24-week follow-up period, while adequate for demonstrating short-term benefits, provides limited insight into long-term cognitive outcomes and potential treatment discontinuation effects (Országhová et al., 2021).
Future research should prioritize large-scale, multi-center randomized controlled trials with extended follow-up periods to validate these promising findings (Fleming et al., 2023). Mechanistic studies incorporating neuroimaging and cerebrospinal fluid biomarker analysis would provide deeper insight into central nervous system effects and optimal therapeutic targets (Dietrich et al., 2015). Investigation of combination therapies, integrating GBE with cognitive training or physical exercise interventions, may yield synergistic benefits exceeding single-modality approaches (Lange et al., 2019).
Clinical implications and therapeutic positioning
These results position GBE as a promising adjunctive therapy for CRCI management, offering a biologically rational intervention with demonstrated efficacy and excellent safety profile (Gradishar et al., 2024; Liu et al., 2021). The observed improvements across multiple cognitive domains, supported by objective biomarker evidence, suggest clinical utility for patients experiencing significant functional impairment from chemotherapy-induced cognitive changes (Vannorsdall, 2017; Onzi et al., 2022). However, GBE should be viewed as complementary to, rather than replacement for, established cognitive rehabilitation and psychosocial support interventions (Lange et al., 2019; Fleming et al., 2023).
The integration of GBE into clinical practice requires careful patient selection, with particular attention to baseline cognitive function, chemotherapy regimen, and individual risk factors for CRCI development (Miyashita, 2024). Biomarker monitoring may prove valuable for optimizing treatment duration and assessing therapeutic response, potentially enabling personalized treatment approaches that maximize benefits while minimizing costs and treatment burden (Baghdadli et al., 2023; Cheung et al., 2014).
This study establishes GBE as a scientifically supported option for CRCI management while highlighting the need for continued research to optimize its clinical application and define its role within comprehensive survivorship care (Denlinger et al., 2018). The substantial improvements in both cognitive function and quality of life measures suggest meaningful clinical benefits that warrant serious consideration in evidence-based CRCI treatment protocols.
Conclusion
This prospective cohort study establishes Ginkgo Ketone Ester as a promising therapeutic intervention for chemotherapy-related cognitive impairment in breast cancer patients. The comprehensive analysis demonstrates significant improvements across multiple cognitive domains, with 23% enhancement in Memory and Executive Screening scores, 31% improvement in verbal learning performance, and maintained executive function capabilities. These clinical benefits are supported by substantial biochemical evidence, including 56% elevation in glutathione levels and 41% reduction in reactive oxygen species, indicating successful restoration of cellular antioxidant homeostasis.
The therapeutic effects extend beyond cognitive test performance to encompass meaningful functional outcomes, including improved treatment compliance, earlier return to work, and enhanced quality of life measures. The excellent safety profile, with no serious adverse events reported and normal organ function maintenance throughout treatment, supports the clinical feasibility of GBE as an adjunctive intervention for CRCI management.
While TNF-α modulation showed limited response, the robust improvements in cognitive function and oxidative stress parameters suggest that antioxidant restoration represents a sufficient therapeutic mechanism for achieving clinically meaningful benefits. The observed effects demonstrate large effect sizes across primary outcomes, indicating substantial clinical significance beyond statistical significance.
However, several limitations must be acknowledged. The observational study design limits causal inference, and the relatively small sample size may restrict generalizability across diverse patient populations and treatment regimens. The 24-week follow-up period, while adequate for demonstrating acute benefits, provides limited insight into long-term cognitive outcomes and potential treatment discontinuation effects.
Future research priorities should focus on large-scale, multi-center randomized controlled trials with extended follow-up periods to validate these findings and establish optimal treatment protocols. Mechanistic studies incorporating neuroimaging and cerebrospinal fluid biomarker analysis would provide deeper understanding of central nervous system effects and therapeutic target engagement. Investigation of combination therapies and age-stratified treatment approaches may further optimize clinical outcomes.
Despite these limitations, this study provides compelling evidence for GBE’s potential as an effective, safe, and well-tolerated intervention for CRCI management. The substantial improvements in cognitive function, supported by objective biomarker evidence and meaningful functional outcomes, warrant serious consideration for integration into evidence-based survivorship care protocols. The findings contribute significantly to addressing the critical unmet need for effective CRCI treatments while establishing a foundation for future therapeutic development in this important clinical area.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Validation, Data curation, Writing – original draft. LC: Validation, Writing – original draft, Methodology. KJ: Validation, Data curation, Writing – original draft. DW: Data curation, Writing – original draft, Formal Analysis, Software. CW: Writing – original draft, Methodology, Data curation. YQ: Writing – original draft, Visualization, Validation. SL: Writing – review and editing, Project administration, Validation, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82474508) and the Shanghai Science and Technology Commission (Grant No. 23Y11920700).
Conflict of interest
Author DW was employed by Shanghai XingLing Technology Pharmaceutical Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahles T. A. Saykin A. J. (2007). Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer7 (3), 192–201. 10.1038/nrc2073
2
Baghdadli A. Arcuri G. G. Green C. G. Gauthier L. R. Gagnon P. Gagnon B. (2023). The fast cognitive evaluation (FaCE): a screening tool to detect cognitive impairment in patients with cancer. BMC Cancer23 (1), 35. 10.1186/s12885-022-10470-1
3
Barton D. L. Burger K. Novotny P. J. Fitch T. R. Kohli S. Soori G. et al (2012). The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support. Care Cancer21 (4), 1185–1192. 10.1007/s00520-012-1647-9
4
Birks J. Grimley Evans J. (2009). Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev.1, Cd003120. 10.1002/14651858.CD003120.pub3
5
Brucki S. Zhao Q. Lv Y. Zhou Y. Hong Z. Guo Q. (2012). Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS ONE7 (12), e51157. 10.1371/journal.pone.0051157
6
Cheung Y. T. Foo Y. L. Shwe M. Tan Y. P. Fan G. Yong W. S. et al (2014). Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J. Clin. Epidemiol.67 (7), 811–820. 10.1016/j.jclinepi.2013.12.011
7
Denlinger C. S. Sanft T. Baker K. S. Broderick G. Demark-Wahnefried W. Friedman D. L. et al (2018). Survivorship, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw.16 (10), 1216–1247. 10.6004/jnccn.2018.0078
8
Dietrich J. Prust M. Kaiser J. (2015). Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience309, 224–232. 10.1016/j.neuroscience.2015.06.016
9
Ding Z. Chan A. S. (2024). The shape trail test is sensitive in differentiating older adults with mild cognitive impairment: a culture-neutral five-minute test. J. Prev. Alzheimer's Dis.11 (4), 1166–1176. 10.14283/jpad.2024.80
10
Fleming B. Edison P. Kenny L. (2023). Cognitive impairment after cancer treatment: mechanisms, clinical characterization, and management. Bmj380, e071726. 10.1136/bmj-2022-071726
11
Gradishar W. J. Moran M. S. Abraham J. Abramson V. Aft R. Agnese D. et al (2024). Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw.22 (5), 331–357. 10.6004/jnccn.2024.0035
12
He G. Xu Y. Wu L. Zhang Z. (2012). Regulating effect of Ginkgo biloba extract 50 on hippocampal inflammation-related cytokines in senile rats. China J. Chin. Materia Medica37 (14), 2130–2134. 10.4268/cjcmm20121421
13
Jannati A. Toro-Serey C. Gomes-Osman J. Banks R. Ciesla M. Showalter J. et al (2024). Digital clock and recall is superior to the mini-mental state examination for the detection of mild cognitive impairment and mild dementia. Alzheimer's Res. and Ther.16 (1), 2. 10.1186/s13195-023-01367-7
14
Joly F. Lange M. Dos Santos M. Vaz-Luis I. Di Meglio A. (2019). Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers (Basel)11 (12), 1896. 10.3390/cancers11121896
15
Kanowski S. Hoerr R. (2003). Ginkgo biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry36 (6), 297–303. 10.1055/s-2003-45117
16
Lange M. Joly F. Vardy J. Ahles T. Dubois M. Tron L. et al (2019). Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol.30 (12), 1925–1940. 10.1093/annonc/mdz410
17
Li J. Jiang Z. (2024). Chinese society of clinical oncology breast cancer (CSCO BC) guidelines in 2024: International contributions from China. Cancer Biol. and Med.21, 838–843. 10.20892/j.issn.2095-3941.2024.0374
18
Liu M. Wang C.-B. Xie F. Peng Y. Wang S. Chinese Society of Breast Surgery (2021). Clinical practice guidelines for diagnosis and treatment of invasive breast cancer: chinese society of breast surgery (CSBrS) practice guidelines 2021. Chin. Med. J.134 (9), 1009–1013. 10.1097/cm9.0000000000001498
19
McGrady M. E. Willard V. W. Williams A. M. Brinkman T. M. (2024). Psychological outcomes in adolescent and young adult cancer survivors. J. Clin. Oncol.42 (6), 707–716. 10.1200/jco.23.01465
20
Miyashita M. (2024). Chemotherapy-related cognitive impairment: what we need to know and what we can do. Asia-Pacific J. Oncol. Nurs.11 (1), 100334. 10.1016/j.apjon.2023.100334
21
Mounier N. M. Abdel-Maged A. E. Wahdan S. A. Gad A. M. Azab S. S. (2020). Chemotherapy-induced cognitive impairment (CICI): an overview of etiology and pathogenesis. Life Sci.258, 118071. 10.1016/j.lfs.2020.118071
22
Oliva G. Giustiniani A. Danesin L. Burgio F. Arcara G. Conte P. (2024). Cognitive impairment following breast cancer treatments: an umbrella review. Oncol.29 (7), e848–e863. 10.1093/oncolo/oyae090
23
Onzi G. R. D'Agustini N. Garcia S. C. Guterres S. S. Pohlmann P. R. Rosa D. D. et al (2022). Chemobrain in breast cancer: mechanisms, clinical manifestations, and potential interventions. Drug Saf.45 (6), 601–621. 10.1007/s40264-022-01182-3
24
Országhová Z. Mego M. Chovanec M. (2021). Long-term cognitive dysfunction in cancer survivors. Front. Mol. Biosci.8, 770413. 10.3389/fmolb.2021.770413
25
Peng Y. Chen Q. Xue Y. H. Jin H. Liu S. Du M. Q. et al (2024). Ginkgo biloba and its chemical components in the management of alzheimer's disease. Am. J. Chin. Med.52 (3), 625–666. 10.1142/s0192415x24500277
26
Seigers R. Fardell J. E. (2011). Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci. Biobehav Rev.35 (3), 729–741. 10.1016/j.neubiorev.2010.09.006
27
Snitz B. E. O'Meara E. S. Carlson M. C. Arnold A. M. Ives D. G. Rapp S. R. et al (2009). Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. Jama302 (24), 2663–2670. 10.1001/jama.2009.1913
28
Van Acker Z. P. Leroy T. Annaert W. (2025). Mitochondrial dysfunction, cause or consequence in neurodegenerative diseases?Bioessays47 (1), e2400023. 10.1002/bies.202400023
29
Vannorsdall T. D. (2017). Cognitive changes related to cancer therapy. Med. Clin. N. Am.101 (6), 1115–1134. 10.1016/j.mcna.2017.06.006
30
Wan Y. Xu J. Ma D. Zeng Y. Cibelli M. Maze M. (2007). Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology106 (3), 436–443. 10.1097/00000542-200703000-00007
31
Wei M. Shi J. Li T. Ni J. Zhang X. Li Y. et al (2017). Diagnostic accuracy of the Chinese version of the trail‐making test for screening cognitive impairment. J. Am. Geriatrics Soc.66 (1), 92–99. 10.1111/jgs.15135
32
Xiao L. Tang J. Tan H. Xie Y. Wang S. Xie L. et al (2024). Efficacy and safety of Ginkgo biloba extract combined with donepezil hydrochloride in the treatment of Chinese patients with vascular dementia: a systematic review meta-analysis. Front. Pharmacol.15, 1374482. 10.3389/fphar.2024.1374482
33
Zhu Q. Liu D. (2024). Clinical efficacy and mechanism of Ginkgo biloba extract in the treatment of elderly ischemic cerebrovascular disease. Pak J. Pharm. Sci.37 (3), 705–713.
Summary
Keywords
breast cancer, chemotherapy-related cognitive impairment, ginkgo ketone ester, neuroprotection, oxidative stress
Citation
Chen J, Chen L, Jiang K, Wang D, Wu C, Qin Y and Liu S (2025) Ginkgo ketone ester tablets for the treatment of cognitive impairment associated with chemotherapy for breast cancer - a prospective cohort study. Front. Pharmacol. 16:1615505. doi: 10.3389/fphar.2025.1615505
Received
28 April 2025
Accepted
03 July 2025
Published
21 July 2025
Volume
16 - 2025
Edited by
Ruiwen Zhang, University of Houston, United States
Reviewed by
Medhat Taha, Mansoura University, Egypt
Owona Pascal Emmanuel, University of Yaounde I, Cameroon
İmdat Eroğlu, Gazi University, Türkiye
Updates
Copyright
© 2025 Chen, Chen, Jiang, Wang, Wu, Qin and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Liu, lhrxktcm@163.com
‡These authors have contributed equally to this work
ORCID: Sheng Liu, orcid org/0000-0002-5243-4519
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.