- 1Department of Gastroenterology, School of Medicine, Recep Tayyip Erdoğan University, Rize, Türkiye
- 2The Global NASH Council, Washington, DC, United States
Metabolic dysfunction-associated steatotic liver disease (MASLD) represents a significant global health concern, with limited pharmacological options despite extensive research efforts. While the recent conditional approval of resmetirom for metabolic dysfunction-associated steatohepatitis with significant or advanced fibrosis has marked a major therapeutic milestone, lifestyle interventions–primarily dietary modifications and structured physical activity–remain the foundation of MASLD management for most patients. However, integrating these non-pharmacological strategies into routine clinical practice remains a significant challenge. In this qualitative evidence synthesis, we searched the PubMed, Scopus, ScienceDirect, and Google Scholar databases to identify and categorize the principal barriers and facilitators influencing the implementation of lifestyle interventions in MASLD care. The analysis identified 67 barriers and 64 facilitators. To address these multifaceted challenges, we propose a multidisciplinary management framework anchored in six core principles: (1) strategic integration of diverse professional expertise with clear role delineation; (2) patient-centered interventions that address both societal and individual barriers while leveraging facilitators; (3) early preventive measures to halt disease progression prior to the development of significant fibrosis; (4) tailored approaches responsive to disease severity and comorbidities; (5) optimized monitoring protocols with specific thresholds for intervention adjustment; and (6) judicious incorporation of digital health technologies, accounting for variability in digital literacy. We conclude that understanding both barriers and facilitators is essential for developing adaptable, patient-centered interventions. Our findings may provide a roadmap for addressing implementation challenges in non-pharmacological MASLD management, emphasizing the importance of preventive, tailored, multidisciplinary approaches that begin early and evolve with disease progression.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is currently recognized as the most common chronic liver disease worldwide, affecting an estimated 38% of the adult population (Stefan et al., 2025; Younossi et al., 2023). This substantial burden has positioned MASLD as a major public health challenge and a leading indication for liver transplantation (Gadiparthi et al., 2020). According to recent data from 204 countries, year 2021 witnessed approximately 1,267.9 million MASLD cases worldwide, resulting in 138.3 thousand deaths and 3,667.3 thousand disability-adjusted life years (DALYs) (Huang et al., 2025). Concurrently, the global age-standardized prevalence reached 15,018.1 per 100,000 population, while mortality and DALYs rates were recorded at 1.6 and 42.4 per 100,000 population, respectively (Huang et al., 2025). These metrics represent significant increases of 24.3% in prevalence and 5.5% in both mortality and DALY rates since 1990, underscoring the accelerating impact of this condition on global health outcomes (Huang et al., 2025). Importantly, this epidemiological trend translates into considerable economic strain, with annual direct medical costs in the United States alone estimated to exceed $100 billion (Miao et al., 2024).
Despite substantial progress in understanding the pathophysiology of MASLD, pharmacological treatment options have remained limited (Puengel and Tacke, 2024). A significant breakthrough has been recently achieved with the conditional FDA approval of resmetirom, a selective thyroid hormone receptor-beta agonist (Keam, 2024). This novel agent induces localized hyperthyroid effects in the liver, effectively reducing lipid accumulation and fibrosis in patients with metabolic dysfunction-associated steatohepatitis (MASH) who have significant or advanced fibrosis (Kaya et al., 2025; Au et al., 2024). However, the clinical use of resmetirom is hindered by several challenges–including restricted distribution to specialty pharmacies, high retail costs, and complex preauthorization requirements from payors, thereby complicating the prescribing process and involving multiple stakeholders (Ravela et al., 2025). In this scenario, non-pharmacological interventions–particularly weight reduction through controlled caloric intake and physical activity–continue to represent the foundation of MASLD management for most affected patients (Rajewski et al., 2025; Ali et al., 2024; Stefano et al., 2023; Ratziu, 2017). Current evidence supports targeted weight reduction of 7%–10% of total body weight for most patients, with the 10% threshold specifically recommended for those with at-risk MASH, as this degree of weight loss consistently correlates with significant improvements in hepatic histology (Diaz et al., 2025). For patients with lean MASLD phenotype, a more modest weight reduction of 3%–5% may also confer measurable hepatic benefits (Diaz et al., 2025). Nutritional interventions with established efficacy include the Mediterranean dietary pattern and carbohydrate-restricted approaches–which emphasize limitation of refined carbohydrates and added sugars, particularly fructose, which has been implicated in hepatic de novo lipogenesis (Simancas-Racines et al., 2025). Specifically, clinical recommendations include minimizing consumption of sugar-sweetened beverages, ultra-processed foods, and products containing high-fructose corn syrup (Parra-Vargas et al., 2020). Notably, the therapeutic efficacy of weight loss can be optimized through the synergistic implementation of both dietary modification and structured physical activity regimens (Chen et al., 2025). Both aerobic exercise modalities (e.g., walking, jogging, cycling, swimming) (Mambrini et al., 2024; Machado, 2021) and resistance training protocols (Medeiros et al., 2025; Askari et al., 2025) have demonstrated efficacy in improving hepatic steatosis and metabolic parameters in patients with MASLD. For adults without cardiovascular or musculoskeletal contraindications, current evidence supports 150–300 min weekly of moderate-intensity aerobic activity, 75–150 min weekly of vigorous-intensity aerobic activity, or an equivalent combination thereof (Diaz et al., 2025). Concurrent resistance training performed ≥2 days weekly can also provide complementary metabolic benefits that improve hepatic and systemic outcomes (Diaz et al., 2025).
Despite robust evidence supporting non-pharmacological interventions for MASLD, their implementation in contemporary clinical settings remains suboptimal due to multiple complex challenges at both societal and individual levels. These hurdles may include–but are not limited to–organizational constraints, limited healthcare provider training, patient adherence difficulties, and insufficient integration into routine clinical workflows (Deshpande et al., 2024; Shibayama et al., 2024; Gu et al., 2023; Heredia et al., 2022; Arora et al., 2021; Stine et al., 2021; Haigh et al., 2019). Building on these premises, we carried out a qualitative evidence synthesis with three primary objectives: 1) to investigate and analyze the societal and individual barriers that may impede the implementation of non-pharmacological interventions for MASLD; 2) to characterize the facilitators that can enhance intervention adherence and effectiveness; and 3) to develop a multidisciplinary framework for integrating lifestyle interventions into MASLD clinical care pathways. Our overarching goals were to inform clinical practice standards, to guide healthcare policy development, and to stimulate targeted research initiatives that may advance the implementation of non-pharmacological management strategies across the MASLD spectrum.
2 Methods
A qualitative evidence synthesis was conducted through searches of PubMed, Scopus, ScienceDirect, and Google Scholar electronic databases. The literature review encompassed original research articles, reviews, meta-analyses, and systematic reviews published in English-language journals between 1 January 2015, and 15 April 2025. Selection criteria specifically targeted non-pharmacological interventions for the prevention and management of MASLD, with emphasis on dietary modification strategies and physical activity interventions. Excluded were conference abstracts, case reports, editorials, letters, and non-peer-reviewed materials (e.g., preprints). The detailed search strategy and keywords are presented in Table 1, while Table 2 describes the construction of search strings and the application of filters for each database. Following an initial screening of titles and abstracts, full-text articles deemed potentially relevant were assessed. Data extraction prioritized identifying barriers and facilitators influencing the implementation of non-pharmacological interventions. A thematic synthesis approach was used to analyze the extracted data on barriers and facilitators. Relevant excerpts from the included studies were inductively coded to identify recurring patterns and concepts related to the non-pharmacological management of MASLD. Codes were compared across studies and refined through an iterative process to ensure consistency and rigor in theme development. Thematic analysis continued until no new themes emerged, indicating thematic saturation. The identified barriers and facilitators were then systematically reviewed and organized using a dual-level taxonomy that distinguishes between individual-level and societal-level determinants. Finally, the findings were synthesized narratively to inform the design of a multidisciplinary framework for integrating lifestyle interventions into MASLD clinical care pathways.
3 Barriers and facilitators to implementing exercise and nutritional strategies in patients with MASLD
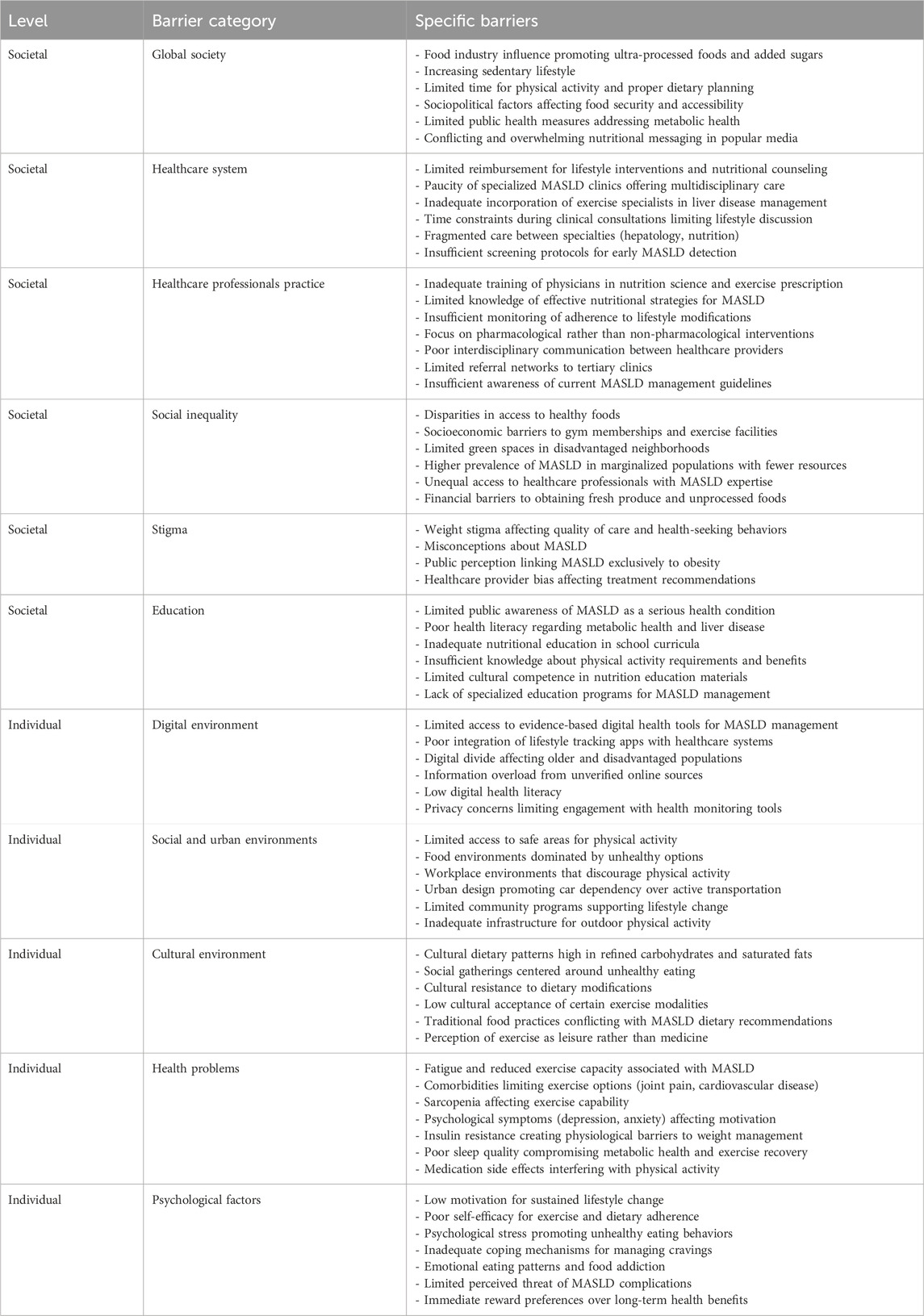
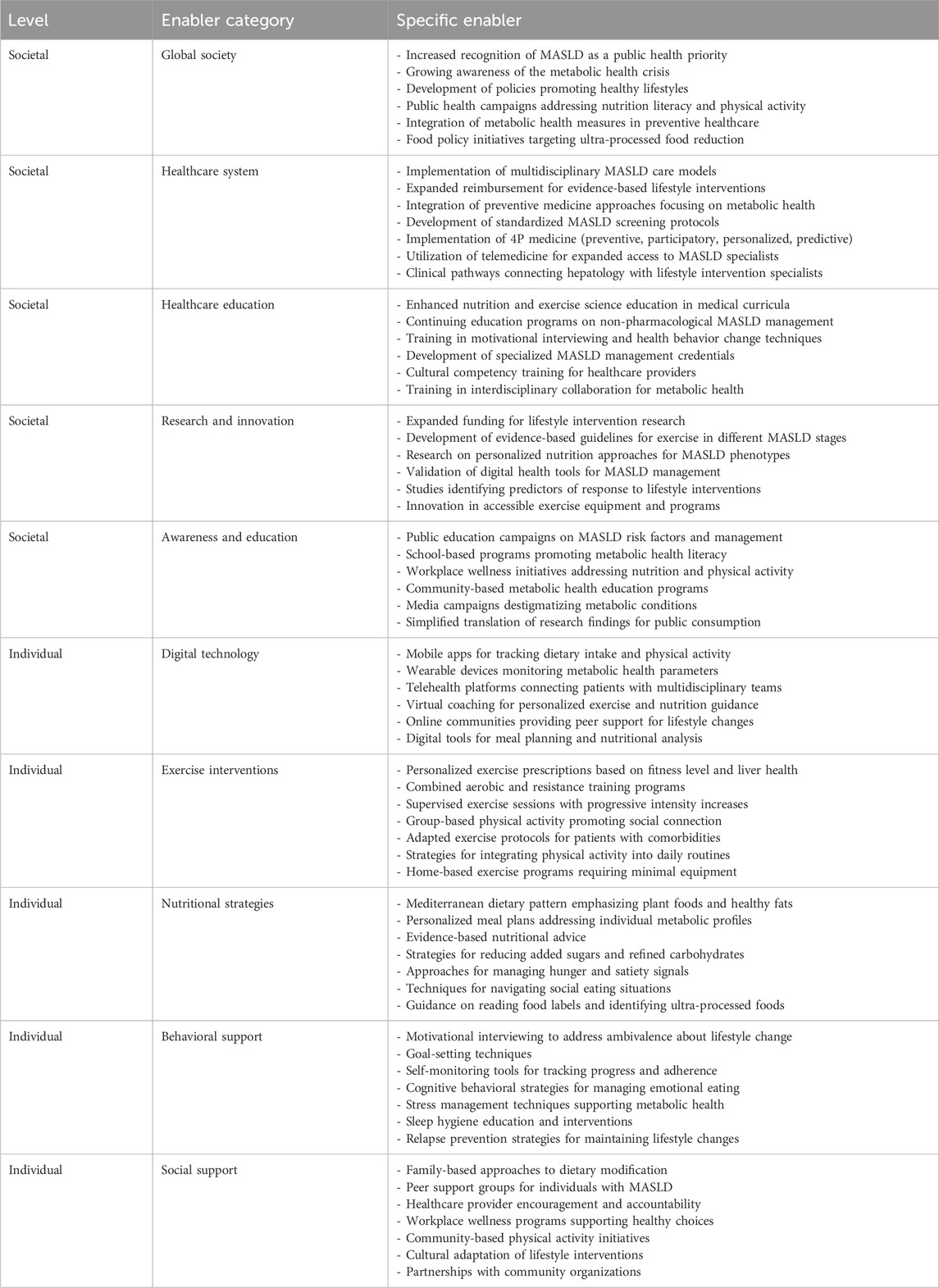
The analysis identified 67 barriers (Table 3) and 64 facilitators (Table 4) relevant to the implementation of non-pharmacological interventions for MASLD. For descriptive clarity, these factors were classified as either societal or individual. Importantly, the relationship between societal and individual factors was characterized by substantial interconnectedness, with numerous elements demonstrating bidirectional influences. In this context, addressing individual-level barriers may contribute to alleviating broader societal challenges, while interventions at the societal level have the potential to diminish individual obstacles. For instance, healthcare system barriers such as “limited reimbursement for lifestyle interventions” (Gobble et al., 2022; Freeman et al., 2021) at the societal level directly impact individual-level treatment accessibility, while psychological factors like “low motivation for sustained lifestyle change” (Tincopa et al., 2021; Hardcastle et al., 2015) at the individual level can undermine the effectiveness of even well-designed societal interventions. Notably, the implementation of non-pharmacological interventions for MASLD revealed a complex interplay where factors frequently function as both barriers and facilitators depending on context, implementation strategy, and specific circumstances. This duality creates a dynamic intervention landscape requiring nuanced understanding for effective clinical practice. The digital environment presents a notable example of this dual role phenomenon. In this regard, digital technologies appear in both tables–as potential barriers when characterized by “limited access to evidence-based digital health tools” (Giebel et al., 2023) and “poor integration of lifestyle tracking apps with healthcare systems” (Magrabi et al., 2019), yet simultaneously as facilitators through “mobile apps for tracking dietary intake and physical activity” (Kamel Boulos and Yang, 2021; Coughlin et al., 2015) and “telehealth platforms connecting patients with multidisciplinary teams” (Belber et al., 2023). This duality highlights how technological solutions can either hinder or enhance MASLD management depending on implementation, accessibility, and digital literacy levels. Cultural factors similarly operate at this intersection, functioning as barriers when “cultural dietary patterns high in refined carbohydrates and saturated fats” (Zhang et al., 2024; Clemente-Suárez et al., 2023) and “social gatherings centered around unhealthy eating” (Buksh et al., 2022; Levy et al., 2021) prevail. However, when cultural elements are integrated into intervention design through “cultural adaptation of lifestyle interventions” (Sevilla-González et al., 2025; Siddiqui et al., 2018) and “partnerships with community organizations,” (Jones et al., 2023; Lazarus et al., 2023) they become powerful facilitators for sustainable behavior change. Health problems themselves demonstrate this complex relationship. While conditions like “fatigue and reduced exercise capacity associated with MASLD” (Younossi et al., 2024) and “comorbidities limiting exercise options” (Deshpande et al., 2024) represent significant barriers, they simultaneously create opportunities for targeted interventions when addressed through “personalized exercise prescriptions based on fitness level and liver health” (Akuta et al., 2024; Glass et al., 2022) and “adapted exercise protocols for patients with comorbidities” (Farrugia et al., 2023). With respect to intervention design and implementation, physical activity programs for MASLD require well-designed approaches that acknowledge both barriers and facilitators. Within this framework, effective exercise programs must transcend the universal barriers of “increasing sedentary lifestyle” (Kim et al., 2020; Hallsworth and Adams, 2019) and “limited time for physical activity” (Patel et al., 2024) through tailored solutions that include “combined aerobic and resistance training programs” (Hashida et al., 2017) and “strategies for integrating physical activity into daily routines” (Arlinghaus and Johnston, 2018). Conversely, the emphasis on “supervised exercise sessions with progressive intensity increases” (Keating et al., 2023) reflects the need to address psychological barriers including “low motivation for sustained lifestyle change” (Livia et al., 2016) and “poor self-efficacy for exercise” (Frith et al., 2010). Nutritional interventions demonstrate similar complexity. Against barriers like “food industry influence promoting ultra-processed foods” (Grinshpan et al., 2023) and “disparities in access to healthy foods” (Kardashian et al., 2022), effective facilitators include adoption of the “Mediterranean dietary pattern emphasizing plant foods and healthy fats” (Zelber-Sagi et al., 2017) and “evidence-based nutritional advice” (Hydes et al., 2020). These approaches must be complemented by “techniques for navigating social eating situations” (Buksh et al., 2022) to overcome the barrier of “social gatherings centered around unhealthy eating” (Levy et al., 2021). The healthcare system itself encompasses significant barriers, including the “paucity of specialized MASLD clinics offering multidisciplinary care” (McPherson et al., 2022). These systemic challenges are compounded by healthcare professional practice barriers such as “inadequate training of physicians in nutrition science” (Mogre et al., 2018) and “focus on pharmacological rather than non-pharmacological interventions” (Hossain et al., 2016). Addressing these challenges requires facilitators that include “implementation of multidisciplinary MASLD care models” (Zoncapè et al., 2022) and “expanded reimbursement for evidence-based lifestyle interventions” (Patel, 2025). Professional education facilitators like “enhanced nutrition and exercise science education in medical curricula” (Khiri and Howells, 2025; Mealy et al., 2019) and “training in motivational interviewing and health behavior change techniques” (Bischof et al., 2021) directly target the identified barriers in clinical practice. Our results also revealed that current digital technologies present paradoxical implications in MASLD management, simultaneously offering therapeutic potential while introducing implementation challenges and possible adverse consequences. Accordingly, while barriers like “low digital health literacy” (Arias López et al., 2023) and “information overload from unverified online sources” (Figueroa et al., 2024) exist, thoughtfully designed technological facilitators can transform patient care through “wearable devices monitoring metabolic health parameters” (Keshet et al., 2023) and “virtual coaching for personalized exercise and nutrition guidance” (Buzcu et al., 2024; Albers et al., 2023) Behavioral support emerges as another critical domain addressing psychological barriers. Against challenges like “emotional eating patterns” (Ley et al., 2021) and “immediate reward preferences over long-term health benefits” (Michaelsen and Esch, 2023) effective facilitators include “motivational interviewing to address ambivalence about lifestyle change” (Almansour et al., 2023) and “cognitive behavioral strategies for managing emotional eating” (Smith et al., 2023). At the broader societal level, factors like “limited public health measures addressing metabolic health” (Díaz et al., 2022) and “socioeconomic barriers to gym memberships and exercise facilities” (Salmi et al., 2023) create substantial impediments to effective MASLD management. These challenges necessitate policy-level facilitators including “development of policies promoting healthy lifestyles” (Lobczowska et al., 2022) and “food policy initiatives targeting ultra-processed food reduction” (Popkin et al., 2021). Social inequalities represent particularly persistent barriers, with “higher prevalence of MASLD in marginalized populations with fewer resources” (Lorek et al., 2025) and “unequal access to healthcare professionals with MASLD expertise” (Miller et al., 2025). Addressing these disparities requires concerted efforts through facilitators like “community-based metabolic health education programs” (Okube et al., 2022) and “expanded funding for lifestyle intervention research” (Pagoto, 2011). The multidimensional nature of MASLD barriers and facilitators suggests that integrated approaches are essential for effective management. The implementation of “4P medicine (preventive, participatory, personalized, predictive)” (Lonardo et al., 2021) as a facilitator directly addresses multiple barriers across both societal and individual levels. Similarly, “personalized meal plans addressing individual metabolic profiles” (Marin-Alejandre et al., 2021) counters barriers related to conflicting nutritional messaging and cultural resistance to dietary modifications. The facilitator of “expanded preventive medicine approaches focusing on metabolic health” (Schattenberg et al., 2023) directly counteracts the barrier of “insufficient screening protocols for early MASLD detection” (Ilagan-Ying et al., 2023). Based on the available evidence, it is imperative to prioritize preventive approaches rather than focusing exclusively on treating patients with MASLD. However, expanding access to preventive care requires addressing fundamental societal barriers including “socioeconomic barriers” (Nasr et al., 2024) and “disparities in access to healthy foods” (Drisdelle et al., 2020). Importantly, the complexity of MASLD management necessitates coordinated efforts across multiple sectors and levels. While individual-level interventions like “self-monitoring tools for tracking progress” (Carpenter et al., 2022) are essential, they must be complemented by societal-level facilitators such as “public education campaigns on MASLD risk factors” (Balakrishnan and Rehm, 2024) and “workplace wellness initiatives addressing nutrition and physical activity” (Peñalvo et al., 2021). The adoption of such an integrated, multidisciplinary framework represents a critical prerequisite for addressing the multifaceted barriers that have historically hindered the real-world translation of evidence-based non-pharmacological strategies in MASLD management.
4 Multidisciplinary framework for addressing barriers and leveraging enablers
The conceptual foundation of a multidisciplinary framework for non-pharmacological MASLD management is predicated upon several interdependent core principles–including the strategic integration of diverse professional expertise with clear role delineation; patient-centered interventions that address both societal and individual barriers while leveraging facilitators; early preventive measures to halt disease progression prior to the development of significant fibrosis; tailored approaches responsive to disease severity and comorbidities; optimized monitoring protocols with specific thresholds for intervention adjustment; and judicious incorporation of digital health technologies, accounting for variability in digital literacy (Lara-Romero and Romero-Gómez, 2024). The subsequent analysis examines these fundamental aspects in detail.
4.1 Strategic integration of diverse professional expertise
An optimal multidisciplinary team for non-pharmacological MASLD management should comprise healthcare professionals from various disciplines, each with precisely delineated roles and responsibilities (Figure 1). Specifically, the core team should encompass a hepatologist who performs non-invasive assessment of hepatic steatosis and fibrosis staging, and determines the potential necessity for liver biopsy (Anstee et al., 2022); a primary care physician who orchestrates patient care coordination and ensures longitudinal follow-up (Brandman, 2019); a registered dietitian who designs personalized nutrition plans and delivers practical dietary counseling (Policarpo et al., 2021); an exercise physiologist who formulates individualized physical activity prescriptions (Haus et al., 2013); a behavioral health specialist who addresses psychological barriers to treatment adherence (Balakrishnan et al., 2023); and a nurse coordinator who facilitates patient education and monitors intervention adherence (Vacca, 2020). Extended team members may also be incorporated as dictated by specific patient requirements. These specialists can include an endocrinologist for management of diabetes and related metabolic disorders (Lomonaco et al., 2011); a cardiologist for comprehensive cardiovascular risk assessment (Sîrbu et al., 2016); a bariatric specialist for advanced weight management interventions in patients with morbid obesity (Głuszyńska et al., 2021); and a social worker to address socioeconomic determinants of health (Ye et al., 2024). This comprehensive interprofessional structure may ultimately ensure that all dimensions of MASLD management are addressed through the synchronized efforts of specialized experts operating within a cohesive framework.
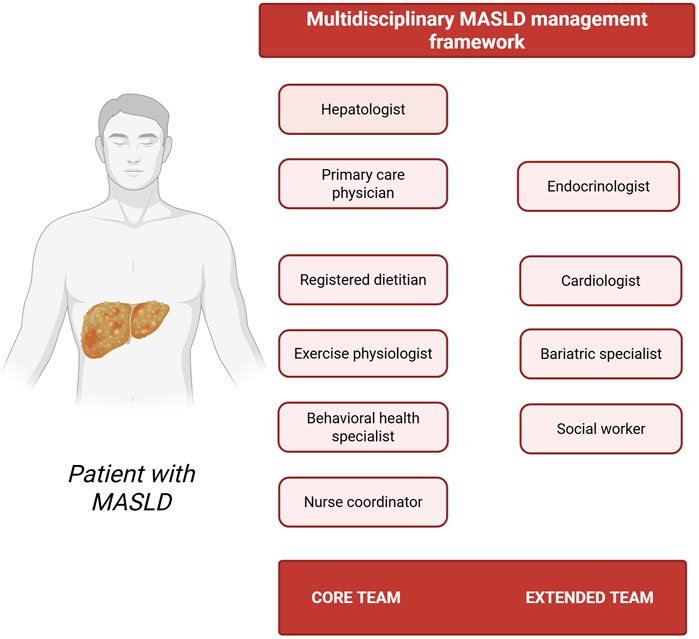
Figure 1. Multidisciplinary MASLD management framework. The schematic representation highlights both core and extended team members, including hepatologist, primary care physician, registered dietitian, exercise physiologist, behavioral health specialist, nurse coordinator, endocrinologist, cardiologist, bariatric specialist, and social worker–emphasizing the importance of coordinated, patient-centered care (Created with BioRender.com).
4.2 Patient-centered interventions
For individuals with MASLD, patient-centered care necessitates understanding the complex interplay of barriers and facilitators that influence adherence and outcomes to non-pharmacological management (Eskridge et al., 2023). Unfortunately, healthcare system barriers significantly impact patient-centered care delivery–including limited reimbursement for lifestyle interventions (Gobble et al., 2022; Freeman et al., 2021), fragmented care between specialties (Brahmania et al., 2024), and insufficient screening protocols (Ilagan-Ying et al., 2023). Potential solutions may include developing cost-effectiveness data supporting lifestyle interventions (Schulz et al., 2014), implementing shared electronic health records (Li et al., 2022), and developing standardized screening pathways that include comprehensive metabolic and liver health assessments (Arora et al., 2024). Professional practice barriers such as inadequate training in nutrition and exercise prescription (Mogre et al., 2018), excessive focus on pharmacological rather than behavioral interventions (Hossain et al., 2016), and insufficient monitoring of adherence (Zeng et al., 2024) also require targeted solutions. These challenges can be overcome through continuing education programs (Donghia et al., 2025), established referral pathways (Chan et al., 2023), and digital tracking tools integrated with electronic health records (Kirilov, 2024). Importantly, the provision of effective patient-centered care for MASLD necessitates the systematic identification and targeted remediation of individual-level barriers. Health-related hurdles affecting exercise capability–including fatigue, depression, sleep disorders (Mostafa et al., 2024), reduced exercise capacity (Deshpande et al., 2024), and sarcopenia (Li et al., 2024) – necessitate tailored adaptations. These can be addressed through low-intensity, short-duration activities with gradual progression, personalized exercise prescriptions accommodating joint pain and functional limitations, and incorporation of resistance training with appropriate progression protocols. Psychological barriers–including low motivation for lifestyle change and poor self-efficacy for exercise and diet adherence–can be mitigated through motivational interviewing techniques, setting small achievable goals, and providing guided experiences with supportive feedback (Hardcastle et al., 2015). Another aspect that should be carefully considered is that patient-centered approaches must account for social and cultural contexts. As previously discussed, limited public awareness of MASLD (Kopka et al., 2024), poor health literacy (Donghia et al., 2025), and challenging food environments (Paik et al., 2024) represent significant barriers. Addressing these aspects may require public education campaigns highlighting the reversibility of MASLD through lifestyle changes, development of culturally appropriate educational materials, and strategic partnerships with community organizations to improve healthy food access (Allen et al., 2025). On a separate note, environmental constraints such as limited access to exercise facilities (Hillsdon et al., 2007) and food environments promoting unhealthy choices (Paik et al., 2024) necessitate creative solutions. Home-based exercise programs requiring minimal equipment, identification of safe walking areas, and guidance for meal planning and preparation strategies that incorporate cultural food preferences can potentially address these challenges. Additionally, social support enablers–including family-based approaches to dietary modification, peer support groups for individuals with MASLD, and community partnerships–can significantly enhance patient-centered care. Effective implementation may involve engaging family members in nutrition education sessions and establishing both in-person and virtual peer support groups to broaden the spectrum of available resources.
4.3 Early preventive measures
Early detection of MASLD during pre-fibrotic stages permits capitalizing on preserved hepatic regenerative potential before the establishment of architectural changes such as fibrous septa and regenerative nodule formation (Albert and Wood, 2024). Evidence-based nutritional strategies represent a fundamental cornerstone of preventive anti-fibrotic strategies (Gao et al., 2023). Specifically, Mediterranean dietary patterns (Miryan et al., 2023), personalized nutritional approaches (Marin-Alejandre et al., 2021), as well as a high intake of vegetables, fruits, vegetable oils, low-fat dairy products, white meat, and nuts (Soleimani et al., 2019) may confer substantial preventive benefits when implemented during the initial stages of the disease trajectory. To optimize efficacy, these nutritional interventions should be ideally tailored to individual preferences and operationalized through practical meal plans that incorporate cultural considerations and sustainable dietary modifications. In parallel, early implementation of structured physical activity programs may facilitate the establishment of sustainable habits prior to disease progression that might otherwise limit exercise capacity (Chun et al., 2023). The utilization of validated rating of perceived exertion scales ensures appropriate exercise intensity, while individualized modifications accommodate patients with pre-existing functional limitations (Glass et al., 2022). We believe that comprehensive early preventive approaches must systematically address modifiable risk factors–including excessive alcohol consumption, suboptimally controlled diabetes mellitus, dyslipidemia, and obesity (Xie et al., 2023). Proactive management of these factors can mitigate the cumulative effects of multiple metabolic derangements before significant hepatocellular damage manifests. In this scenario, prevention-focused educational interventions should be strategically directed toward both the general population and high-risk clinical cohorts (Schattenberg et al., 2023). These endeavors should be coupled with culturally appropriate informational resources and robust community partnerships to optimize their preventive efficacy and population-level impact.
4.4 Tailored approaches responsive to disease severity and comorbidities
The optimal implementation of non-pharmacological strategies in MASLD management necessitates systematic adaptation of therapeutic modalities based on a comprehensive assessment of disease severity, fibrosis stage, and comorbid conditions. This personalized therapeutic paradigm ensures that interventions specifically target the underlying pathophysiological mechanisms while accommodating the limitations imposed by coexisting conditions (Lonardo et al., 2021). For patients exhibiting simple steatosis without significant inflammatory activity or fibrosis, the therapeutic emphasis should remain on lifestyle modifications coupled with vigilant monitoring protocols (Lonardo et al., 2021; Valenzuela-Vallejo et al., 2023). As disease severity progresses, more intensive interventions become imperative, including structured dietary regimens, supervised exercise protocols, and judicious consideration of pharmacological adjuncts when clinically indicated (Valenzuela-Vallejo et al., 2023). Notably, MASLD frequently manifests within a constellation of metabolic comorbidities requiring coordinated management strategies (Targher et al., 2024). In this context, the previously described multidisciplinary care team must meticulously adapt interventions to address concurrent conditions including diabetes mellitus, cardiovascular disease, dyslipidemia, and visceral adiposity, ensuring therapeutic approaches maintain coherence rather than introducing contradictory elements. This necessitates specific vigilance regarding potential pharmacological interactions, contraindications to specific exercise modalities, and nutritional considerations that should simultaneously address multiple metabolic derangements. The presence and extent of hepatic fibrosis necessitates specific modifications to non-pharmacological interventions (Semmler et al., 2021). For patients with advanced fibrosis or established cirrhosis, nutritional interventions must address protein requirements that differ between compensated and decompensated liver disease, sodium restrictions in the presence of ascites, and micronutrient deficiencies characteristic of advanced cirrhosis (Mendez-Guerrero et al., 2024). Therapeutic adaptations must also account for physical and functional limitations that may impact intervention adherence (Glass et al., 2022). For patients with sarcopenia, mobility restrictions, or cardiovascular disease, exercise prescriptions should incorporate modified modalities such as seated exercises, aquatic therapy, or adapted movements that preserve therapeutic efficacy while minimizing risk. These adaptations should follow a progressive implementation model, commencing with supervised sessions that gradually transition to modified home-based programs with appropriate safety parameters. Additionally, the complexity of non-pharmacological interventions must be calibrated against the patient’s capacity to implement recommendations. For individuals with multiple comorbidities already managing complex treatment regimens, therapeutic approaches may require strategic prioritization and phased implementation to prevent exceeding adherence capacity. This strategic approach may ultimately ensure that interventions remain feasible while maximizing their potential benefit across the disease spectrum and comorbidity profile.
4.5 Optimized monitoring protocols
An optimized management of MASLD through non-pharmacological interventions necessitates the implementation of comprehensive monitoring frameworks that systematically evaluate intervention adherence, physiological adaptations, and biomarkers of disease progression (Noureddin et al., 2024). Such protocols should incorporate evidence-derived thresholds for therapeutic modifications while retaining sufficient flexibility to accommodate heterogeneity in individual treatment responses. Rigorous monitoring frameworks may encompass multiple assessment domains–including anthropometric parameters, metabolic indices, non-invasive fibrosis assessment tools, functional capacity metrics, and patient-reported outcomes (Figure 2). This multidimensional approach ensures comprehensive evaluation of both disease-specific parameters and global health indices. Appropriate assessment intervals that optimize the balance between timely intervention modifications and minimization of resource utilization and patient burden are also advisable. Initial comprehensive assessments should establish baseline values across all relevant parameters, followed by more frequent evaluations during the initial intervention phase to capture rapid modifications in modifiable factors. As clinical stability is achieved, monitoring intervals may be judiciously extended while maintaining vigilance for indicators of disease progression or suboptimal response. The monitoring framework should also incorporate systematic assessment of intervention adherence across nutritional, physical activity, and behavioral domains. Upon identification of adherence barriers (Castera et al., 2025), the monitoring protocol should trigger specific supportive interventions including therapeutic goal recalibration, systematic barrier analysis, and provision of supplementary resources or educational interventions. Importantly, contemporary monitoring frameworks must incorporate systematic assessment of patient-reported outcomes including treatment satisfaction indices and health-related quality of life metrics (Younossi et al., 2022). These subjective measures provide essential context for the interpretation of physiological parameters and guide therapeutic adjustments to ensure interventions remain congruent with patient priorities and capabilities (Barberá et al., 2024). Regular assessment of these outcomes may ultimately facilitate collaborative decision-making regarding management modifications and supports sustained patient engagement throughout the therapeutic continuum.

Figure 2. Comprehensive monitoring protocol for patients with MASLD. Overview of a structured monitoring protocol for patients with MASLD, detailing five key domains: (1) anthropometric parameters (e.g., body mass index, waist circumference, fat mass indices), (2) metabolic indices (e.g., serum glucose, lipid profile, liver function biomarkers, insulin resistance), (3) non-invasive fibrosis assessment (e.g., transient elastography, fibrosis scores), (4) functional capacity metrics (e.g., cardiorespiratory fitness, muscle strength), and (5) patient-reported outcomes (e.g., health-related quality of life, disease-specific questionnaires) (Created with BioRender.com).
4.6 Judicious incorporation of digital health technologies
Digital health technologies offer significant potential to improve non-pharmacological MASLD management through enhanced monitoring capabilities, expanded access to specialized care, and delivery of personalized interventions (Allen et al., 2024). As previously discussed, digital technologies may serve as significant enablers through mobile health applications for dietary intake documentation and physical activity quantification, telehealth platforms facilitating patient-specialist connectivity, and wearable biosensors monitoring physiological and behavioral parameters (Buzcu et al., 2024; Albers et al., 2023; Keshet et al., 2023). These technological modalities can extend the therapeutic reach of healthcare providers beyond traditional clinical environments, enabling continuous monitoring and supportive interventions between scheduled clinical encounters. For MASLD management specifically, digital tools may facilitate precise dietary assessment (Zuppinger et al., 2022) and objective physical activity quantification (Kamel Boulos and Yang, 2021). Nonetheless, the reliance on technological solutions must acknowledge and systematically address variations in digital literacy across diverse patient populations. In this setting, effective implementation approaches should include structured orientation protocols for recommended applications, establishment of tiered support systems for users with limited technological proficiency, development of standardized protocols for virtual consultations, and incorporation of progressive technology adoption strategies calibrated to individual capability and comfort. For patients with limited digital literacy, hybrid implementation models combining traditional intervention delivery with incremental technological integration may optimize engagement while progressively building technological self-efficacy. The selection of technological interventions should be guided by robust evidence regarding their clinical effectiveness, user interface accessibility, and appropriateness for specific patient populations. Additionally, implementation strategies should prioritize technologies with demonstrated efficacy in MASLD management (Kaewdech et al., 2024; Alkhouri et al., 2023) while considering multifaceted factors including cost implications, accessibility across socioeconomic strata, data security protocols, and interoperability capabilities with existing healthcare information systems. This evidence-based selection process helps ensure that technological interventions deliver measurable clinical benefits rather than merely increasing system complexity without corresponding improvements in patient outcomes. While technological solutions offer considerable advantages, we believe that they must complement rather than supplant interpersonal connections in healthcare delivery. Finally, the implementation of novel technological solutions–including artificial intelligence–necessitates careful consideration of ethical dimensions including data privacy protections, informed consent processes, and potential exacerbation of existing healthcare disparities (Pugliese et al., 2025). These ethical considerations should be systematically addressed through structured clinical educational frameworks that equip healthcare professionals with both technological competencies and ethical awareness in digital health implementation.
5 Discussion
This qualitative synthesis of the available evidence has provided a comprehensive elucidation of barriers and facilitators influencing the real-world implementation of non-pharmacological strategies for MASLD management. Our findings suggest that, to optimize effectiveness in real-world settings, nutritional and physical activity interventions require coordinated, multilevel strategies that concurrently address both societal and individual barriers, while strategically harnessing facilitating factors. In response, the multidisciplinary framework presented herein outlines a systematic implementation approach encompassing the full spectrum from primary prevention to the management of advanced hepatic pathology. This framework emphasizes the critical need for therapeutic customization based on disease severity, individual patient contexts, and sociocultural determinants. Moreover, it highlights the essential shift from fragmented care models toward integrated, patient-centered paradigms, wherein hepatologists, primary care providers, nutritionists, exercise physiologists, and behavioral health specialists operate collaboratively with harmonized objectives and well-defined communication pathways.
Several critical insights emerge from the reviewed evidence. Foremost among these is the recognition that early intervention is of paramount importance. Although growing evidence indicates that even cirrhosis may be reversible under certain conditions (Jung and Yim, 2017; Sohrabpour et al., 2012), initiating dietary and physical activity interventions prior to the development of significant fibrosis in patients with MASLD represents a more rational and effective strategy than attempting to reverse advanced liver pathology. Early intervention may also benefit from a lower burden of MASLD-associated comorbidities, such as fatigue and sarcopenia, which are known to impede successful lifestyle modifications. However, barriers to implementation extend beyond the individual patient level, permeating healthcare systems, professional education curricula, and broader societal structures. These observations underscore the necessity for coordinated, multi-tiered interventions. In this context, digital health technologies can offer both opportunities and challenges, presenting a complex landscape that demands meticulous implementation strategies. It is also noteworthy that cultural determinants and psychological factors might exert a profound influence on adherence to lifestyle modifications. This highlights the need for personalized, culturally sensitive approaches, rather than reliance on standardized recommendations, to enhance the effectiveness and sustainability of interventions.
The operational agenda delineated herein addresses critical knowledge gaps, particularly concerning personalized intervention strategies and implementation methodologies across diverse healthcare environments. Future research should prioritize the development and rigorous validation of cost-effective, scalable delivery models for evidence-based lifestyle interventions that can be systematically integrated into routine clinical practice within heterogeneous healthcare systems. Health policymakers and healthcare leadership should recognize that meaningful advancements in MASLD management require substantial investment in preventive strategies and lifestyle intervention infrastructure. This includes expanding reimbursement frameworks for evidence-based lifestyle programs, systematically incorporating nutrition specialists and exercise physiologists into hepatology care teams, enhancing professional education in nutritional science and physical activity prescription, and implementing public health initiatives that improve food environment quality and opportunities for physical activity. For clinicians, the current evidence synthesis offers pragmatic guidance for the implementation of multidisciplinary care models and for systematically overcoming common barriers to sustainable lifestyle modification. The proposed framework underscores that, although weight reduction remains a key therapeutic objective, an exclusive focus on anthropometric outcomes may be counterproductive. Instead, prioritizing improvements in metabolic health through sustainable dietary patterns and structured physical activity regimens represents a more constructive and patient-centered approach for many individuals with MASLD.
However, our findings need to be interpreted in the context of several limitations. First, we recognize that socioeconomic determinants may play a fundamental role in shaping adherence to non-pharmacological interventions for MASLD, as they influence access to resources, health literacy, and the ability to sustain lifestyle changes. Factors such as income disparities, limited access to healthy foods, and financial barriers to exercise facilities can exacerbate disease progression, particularly in marginalized populations. To enhance adherence, our proposed framework integrates targeted strategies like including social workers in multidisciplinary teams to address these determinants directly, alongside community partnerships and policy advocacy for equitable resource distribution. While we acknowledge that stratifying barriers and facilitators by specific socioeconomic factors would provide further insight into intervention tailoring, this level of granularity was not feasible within the scope of our current synthesis. Future research should prioritize detailed stratification to better understand how socioeconomic subgroups experience distinct challenges and to develop even more targeted approaches. Second, the effectiveness of any management framework depends on its adaptation to specific regional and cultural contexts. As each community presents a unique combination of barriers and facilitators, a one-size-fits-all approach to non-pharmacological interventions cannot be considered satisfactory. In the future, it will be essential to conduct regional studies and culturally adapt research to address the distinct socioeconomic conditions, healthcare access, and social determinants of health that characterize different populations. Third, when implementing nutritional interventions–particularly those based on the Mediterranean dietary pattern–it is crucial to emphasize to both patients and healthcare providers that the primary goal is to select foods with comparable nutritional profiles, rather than sourcing items exclusively from Mediterranean countries. This includes an emphasis on plant-based foods and minimally processed ingredients. To enhance feasibility, sustainability, and adherence, the dietary approaches should be adapted to local food availability and culinary traditions. Furthermore, recommendations to increase fruit intake should be accompanied by careful guidance. Although fruits are excellent sources of vitamins, fiber, and beneficial phytochemicals, some varieties are also high in fructose, which–when consumed in excess–may contribute to hepatic fat accumulation and disease progression. Clinical advice should therefore emphasize moderation, encourage the selection of whole, fiber-rich fruits over fruit juices or sweetened fruit products, and be individualized to consider each patient’s metabolic and clinical profile. An additional limitation of this synthesis is that the barriers and facilitators identified were not stratified according to disease duration. It is likely that patients with longstanding MASLD/MASH may experience distinct challenges–such as intervention fatigue, cumulative comorbidities, or adaptive behaviors–compared to those who are recently diagnosed, whose primary barriers may relate more to health literacy or initial motivation. Future research should specifically address how barriers and facilitators to non-pharmacological management change over the disease trajectory, to inform the design of stage-specific interventions that support sustained adherence. We also acknowledge that the evidence base synthesized in this review is derived from studies exhibiting substantial heterogeneity in design, population characteristics, intervention modalities, and outcome measures. This variability may constrain the direct comparability of findings and limit the strength of our synthesized conclusions. As with all qualitative syntheses, the potential for selection and interpretation bias during coding, theme development, and synthesis remains an inherent limitation. Furthermore, the transferability of these results to health systems and countries with different MASLD burdens may be restricted. Future research should aim to validate these identified barriers, facilitators, and proposed frameworks in diverse international contexts, especially in regions facing a high or increasing prevalence of MASLD.
In conclusion, while non-pharmacological strategies are widely acknowledged as foundational in MASLD management, their full therapeutic potential remains unrealized without systematic operationalization that addresses implementation barriers and leverages facilitators. This necessitates progressing beyond broad conceptual acceptance toward the adoption of evidence-based frameworks that afford lifestyle interventions methodological rigor comparable to pharmacotherapies–including standardized protocols, longitudinal outcome monitoring, and integration into clinical care pathways. Future efforts should concentrate on bridging persistent implementation gaps through precision nutrition strategies, culturally adapted exercise prescriptions, and digital health solutions that enhance scalability. Concurrently, advancing biomarker-driven monitoring frameworks and interdisciplinary training programs will strengthen the evidence-based application of these interventions. Collectively, these measures will enable the hepatology community to transition from viewing lifestyle interventions as theoretically foundational to delivering them as practically transformative, thereby substantially reducing the global burden of MASLD-related morbidity.
Author contributions
YY: Conceptualization, Project administration, Writing – review and editing, Writing – original draft, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Recep Tayyip Erdogan University Development Foundation (grant number: 02025004021438).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akuta, N., Kawamura, Y., Fujiyama, S., Nakamichi, K., Saegusa, E., Ogura, H., et al. (2024). Impact of genetic polymorphism on personalized diet and exercise program for steatotic liver disease. Hepatol. Res. 54 (1), 54–66. doi:10.1111/hepr.13968
Albers, N., Hizli, B., Scheltinga, B. L., Meijer, E., and Brinkman, W. P. (2023). Setting physical activity goals with a virtual coach: vicarious experiences, personalization and acceptance. J. Med. Syst. 47 (1), 15. doi:10.1007/s10916-022-01899-9
Albert, S. G., and Wood, E. M. (2024). FIB-4 as a screening and disease monitoring method in pre-fibrotic stages of metabolic dysfunction-associated fatty liver disease (MASLD). J. Diabetes Complicat. 38 (7), 108777. doi:10.1016/j.jdiacomp.2024.108777
Ali, H., Shahzil, M., Moond, V., Shahzad, M., Thandavaram, A., Sehar, A., et al. (2024). Non-pharmacological approach to diet and exercise in metabolic-associated fatty liver disease: bridging the gap between research and clinical practice. J. Pers. Med. 14 (1), 61. doi:10.3390/jpm14010061
Alkhouri, N., Edwards, K., Berman, M., Finn, H., Escandon, R., Lupinacci, P., et al. (2023). A novel prescription digital therapeutic option for the treatment of metabolic dysfunction-associated steatotic liver disease. Gastro. Hep. Adv. 3 (1), 9–16. doi:10.1016/j.gastha.2023.08.019
Allen, A. M., Arab, J. P., and Wong, V. W. (2024). MASLD: a disease in flux. Nat. Rev. Gastroenterol. Hepatol. 21 (11), 747–750. doi:10.1038/s41575-024-00990-5
Allen, M. J., Tulleners, R., Brain, D., O'Beirne, J., Powell, E. E., Barnett, A., et al. (2025). Implementation of a nurse-delivered, community-based liver screening and assessment program for people with metabolic dysfunction-associated steatotic liver disease (LOCATE-NAFLD trial). BMC Health Serv. Res. 25 (1), 421. doi:10.1186/s12913-025-12580-5
Almansour, M., AlQurmalah, S. I., and Abdul Razack, H. I. (2023). Motivational interviewing-an evidence-based, collaborative, goal-oriented communication approach in lifestyle medicine: a comprehensive review of the literature. J. Taibah Univ. Med. Sci. 18 (5), 1170–1178. doi:10.1016/j.jtumed.2023.03.011
Anstee, Q. M., Castera, L., and Loomba, R. (2022). Impact of non-invasive biomarkers on hepatology practice: past, present and future. J. Hepatol. 76 (6), 1362–1378. doi:10.1016/j.jhep.2022.03.026
Arias López, M. D. P., Ong, B. A., Borrat Frigola, X., Fernández, A. L., Hicklent, R. S., Obeles, A. J. T., et al. (2023). Digital literacy as a new determinant of health: a scoping review. PLOS Digit. Health 2 (10), e0000279. doi:10.1371/journal.pdig.0000279
Arlinghaus, K. R., and Johnston, C. A. (2018). The importance of creating habits and routine. Am. J. Lifestyle Med. 13 (2), 142–144. doi:10.1177/1559827618818044
Arora, C., Malhotra, A., Ranjan, P., Vikram, N. K., Dwivedi, S. N., Singh, N., et al. (2021). Perceived barriers and facilitators for adherence to lifestyle prescription: perspective of obese patients with non alcoholic fatty liver disease from north India. Diabetes Metab. Syndr. 15 (4), 102138. doi:10.1016/j.dsx.2021.05.011
Arora, U., Biswas, S., Aggarwal, S., Duseja, A., and Shalimar, S. (2024). MASLD screening and diagnostic algorithms are interchangeable with existing NAFLD literature. J. Hepatol. 80 (2), e89–e91. doi:10.1016/j.jhep.2023.10.032
Askari, R., Rabani, N., Marefati, H., Azarnive, M. S., Pusceddu, M., and Migliaccio, G. M. (2025). Aerobic-resistance training with royal jelly supplementation has a synergistic effect on paraoxonase 1 changes and liver function in women with MASLD. Med. Kaunas. 61 (2), 349. doi:10.3390/medicina61020349
Au, K., Zheng, M. H., Lee, W. J., Ghanem, O. M., Mahawar, K., Shabbir, A., et al. (2024). Resmetirom and metabolic dysfunction-associated steatohepatitis: perspectives on multidisciplinary management from global healthcare professionals. Curr. Obes. Rep. 13 (4), 818–830. doi:10.1007/s13679-024-00582-z
Balakrishnan, M., and Rehm, J. (2024). A public health perspective on mitigating the global burden of chronic liver disease. Hepatology 79 (2), 451–459. doi:10.1097/HEP.0000000000000679
Balakrishnan, M., Liu, K., Schmitt, S., Heredia, N. I., Sisson, A., Montealegre, J. R., et al. (2023). Behavioral weight-loss interventions for patients with NAFLD: a systematic scoping review. Hepatol. Commun. 7 (8), e0224. doi:10.1097/HC9.0000000000000224
Barberá, A., White, T. M., Arora, A. K., Henry, L., Lazarus, J. V., and Younossi, Z. M. (2024). Patient-reported outcomes in metabolic dysfunction-associated steatotic liver disease. Semin. Liver Dis. 45, 210–220. doi:10.1055/a-2435-2091
Belber, G. S., Vasconcelos, R. O., Agreli, H. L. F., Haddad, A. E., Peduzzi, M., and Leonello, V. M. (2023). Telehealth use in primary healthcare collaborative interprofessional practice: protocol for a scoping review. BMJ Open 13 (3), e069163. doi:10.1136/bmjopen-2022-069163
Bischof, G., Bischof, A., and Rumpf, H. J. (2021). Motivational interviewing: an evidence-based approach for use in medical practice. Dtsch. Arztebl. Int. 118 (7), 109–115. doi:10.3238/arztebl.m2021.0014
Brahmania, M., Rogal, S., Serper, M., Patel, A., Goldberg, D., Mathur, A., et al. (2024). Pragmatic strategies to address health disparities along the continuum of care in chronic liver disease. Hepatol. Commun. 8 (5), e0413. doi:10.1097/HC9.0000000000000413
Brandman, D. (2019). Who should treat fatty liver disease: primary care or hepatology? Clin. Liver Dis. Hob. 13 (6), 158–161. doi:10.1002/cld.766
Buksh, S. M., de Wit, J. B. F., and Hay, P. (2022). Sociocultural influences contribute to overeating and unhealthy eating: creating and maintaining an obesogenic social environment in Indigenous communities in urban Fiji. Nutrients 14 (14), 2803. doi:10.3390/nu14142803
Buzcu, B., Tessa, M., Tchappi, I., Najjar, A., Hulstijn, J., Calvaresi, D., et al. (2024). Towards interactive explanation-based nutrition virtual coaching systems. Auton. Agent Multi Agent Syst. 38 (1), 5. doi:10.1007/s10458-023-09634-5
Carpenter, C. A., Ugwoaba, U. A., Cardel, M. I., and Ross, K. M. (2022). Using self-monitoring technology for nutritional counseling and weight management. Digit. Health 8, 20552076221102774. doi:10.1177/20552076221102774
Castera, L., Alazawi, W., Bugianesi, E., Caussy, C., Federici, M., Romero-Gómez, M., et al. (2025). A european survey to identify challenges in the management of metabolic dysfunction-associated steatotic liver disease. Liver Int. 45 (2), e16224. doi:10.1111/liv.16224
Chan, W. K., Chuah, K. H., Rajaram, R. B., Lim, L. L., Ratnasingam, J., and Vethakkan, S. R. (2023). Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J. Obes. Metab. Syndr. 32 (3), 197–213. doi:10.7570/jomes23052
Chen, M. J., Chen, Y., Lin, J. Q., Hu, R., Liu, D., Chen, J. Y., et al. (2025). Evidence summary of lifestyle interventions in adults with metabolic dysfunction-associated steatotic liver disease. Front. Nutr. 11, 1421386. doi:10.3389/fnut.2024.1421386
Chun, H. S., Lee, M., Lee, H. A., Oh, S. Y., Baek, H. J., Moon, J. W., et al. (2023). Association of physical activity with risk of liver fibrosis, sarcopenia, and cardiovascular disease in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 21 (2), 358–369.e12. doi:10.1016/j.cgh.2021.12.043
Clemente-Suárez, V. J., Beltrán-Velasco, A. I., Redondo-Flórez, L., Martín-Rodríguez, A., and Tornero-Aguilera, J. F. (2023). Global impacts of Western diet and its effects on metabolism and health: a narrative review. Nutrients 15 (12), 2749. doi:10.3390/nu15122749
Coughlin, S. S., Whitehead, M., Sheats, J. Q., Mastromonico, J., Hardy, D., and Smith, S. A. (2015). Smartphone applications for promoting healthy diet and nutrition: a literature review. Jacobs J. Food. Nutr. 2 (3), 021.
Deshpande, K., Olynyk, J., Ayonrinde, O., and Nosaka, K. (2024). Barriers to exercise in patients with metabolic dysfunction-associated steatotic liver disease: a patient survey. J. Clin. Med. Res. 16 (2-3), 94–105. doi:10.14740/jocmr5113
Díaz, L. A., Fuentes-López, E., Ayares, G., Idalsoaga, F., Arnold, J., Márquez-Lomas, A., et al. (2022). The establishment of public health policies and the burden of non-alcoholic fatty liver disease in the americas. Lancet Gastroenterol. Hepatol. 7 (6), 552–559. doi:10.1016/S2468-1253(22)00008-5
Diaz, L. A., Arab, J. P., Idalsoaga, F., Perelli, J., Vega, J., Dirchwolf, M., et al. (2025). Updated recommendations for the management of metabolic dysfunction-associated steatotic liver disease (MASLD) by the Latin American working group. Ann. Hepatol. 30, 101903. doi:10.1016/j.aohep.2025.101903
Donghia, R., Bonfiglio, C., Giannelli, G., and Tatoli, R. (2025). Impact of education on metabolic dysfunction-associated steatotic liver disease (MASLD): a southern Italy cohort-based study. J. Clin. Med. 14 (6), 1950. doi:10.3390/jcm14061950
Drisdelle, C., Kestens, Y., Hamelin, A. M., and Mercille, G. (2020). Disparities in access to healthy diets: how food security and food shopping behaviors relate to fruit and vegetable intake. J. Acad. Nutr. Diet. 120 (11), 1847–1858. doi:10.1016/j.jand.2020.03.020
Eskridge, W., Cryer, D. R., Schattenberg, J. M., Gastaldelli, A., Malhi, H., Allen, A. M., et al. (2023). Metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis: the patient and physician perspective. J. Clin. Med. 12 (19), 6216. doi:10.3390/jcm12196216
Farrugia, M. A., Le Garf, S., Chierici, A., Piche, T., Gual, P., Iannelli, A., et al. (2023). Therapeutic physical exercise programs in the context of NASH cirrhosis and liver transplantation: a systematic review. Metabolites 13 (3), 330. doi:10.3390/metabo13030330
Figueroa, G., Castañeda, S., McLean, H., Dukandar, J., Wilson, S., Martin, P., et al. (2024). Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig. Dis. Sci. 69 (2), 384–398. doi:10.1007/s10620-023-08212-9
Freeman, K. J., Grega, M. L., Friedman, S. M., Patel, P. M., Stout, R. W., Campbell, T. M., et al. (2021). Lifestyle medicine reimbursement: a proposal for policy priorities informed by a cross-sectional survey of lifestyle medicine practitioners. Int. J. Environ. Res. Public Health. 18 (21), 11632. doi:10.3390/ijerph182111632
Frith, J., Day, C. P., Robinson, L., Elliott, C., Jones, D. E., and Newton, J. L. (2010). Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J. Hepatol. 52 (1), 112–116. doi:10.1016/j.jhep.2009.10.010
Gadiparthi, C., Spatz, M., Greenberg, S., Iqbal, U., Kanna, S., Satapathy, S. K., et al. (2020). NAFLD epidemiology, emerging pharmacotherapy, liver transplantation implications and the trends in the United States. J. Clin. Transl. Hepatol. 8 (2), 215–221. doi:10.14218/JCTH.2020.00014
Gao, V., Long, M. T., Singh, S. R., Kim, Y., Zhang, X., Rogers, G., et al. (2023). A healthy diet is associated with a lower risk of hepatic fibrosis. J. Nutr. 153 (5), 1587–1596. doi:10.1016/j.tjnut.2023.03.038
Giebel, G. D., Speckemeier, C., Abels, C., Plescher, F., Börchers, K., Wasem, J., et al. (2023). Problems and barriers related to the use of digital health applications: scoping review. J. Med. Internet Res. 25, e43808. doi:10.2196/43808
Glass, O., Liu, D., Bechard, E., Guy, C. D., Pendergast, J., Mae Diehl, A., et al. (2022). Perceptions of exercise and its challenges in patients with nonalcoholic fatty liver disease: a survey-based study. Hepatol. Commun. 6 (2), 334–344. doi:10.1002/hep4.1808
Głuszyńska, P., Lemancewicz, D., Dzięcioł, J. B., and Razak Hady, H. (2021). Non-alcoholic fatty liver disease (NAFLD) and bariatric/metabolic surgery as its treatment option: a review. J. Clin. Med. 10 (24), 5721. doi:10.3390/jcm10245721
Gobble, J., Donohue, D., and Grega, M. (2022). Reimbursement as a catalyst for advancing lifestyle medicine practices. J. Fam. Pract. 71 (Suppl. 1 Lifestyle), eS105–eS109. doi:10.12788/jfp.0255
Grinshpan, L. S., Eilat-Adar, S., Ivancovsky-Wajcman, D., Kariv, R., Gillon-Keren, M., and Zelber-Sagi, S. (2023). Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: a systematic review. JHEP Rep. 6 (1), 100964. doi:10.1016/j.jhepr.2023.100964
Gu, Y., Zhou, R., Kong, T., Zhang, W., Chen, Y., Wang, C., et al. (2023). Barriers and enabling factors in weight management of patients with nonalcoholic fatty liver disease: a qualitative study using the COM-B model of behaviour. Health Expect. 26 (1), 355–365. doi:10.1111/hex.13665
Haigh, L., Bremner, S., Houghton, D., Henderson, E., Avery, L., Hardy, T., et al. (2019). Barriers and facilitators to mediterranean diet adoption by patients with nonalcoholic fatty liver disease in northern Europe. Clin. Gastroenterol. Hepatol. 17 (7), 1364–1371. doi:10.1016/j.cgh.2018.10.044
Hallsworth, K., and Adams, L. A. (2019). Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 1 (6), 468–479. doi:10.1016/j.jhepr.2019.10.008
Hardcastle, S. J., Hancox, J., Hattar, A., Maxwell-Smith, C., Thøgersen-Ntoumani, C., and Hagger, M. S. (2015). Motivating the unmotivated: how can health behavior be changed in those unwilling to change? Front. Psychol. 6, 835. doi:10.3389/fpsyg.2015.00835
Hashida, R., Kawaguchi, T., Bekki, M., Omoto, M., Matsuse, H., Nago, T., et al. (2017). Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J. Hepatol. 66 (1), 142–152. doi:10.1016/j.jhep.2016.08.023
Haus, J. M., Solomon, T. P., Kelly, K. R., Fealy, C. E., Kullman, E. L., Scelsi, A. R., et al. (2013). Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 98 (7), E1181–E1188. doi:10.1210/jc.2013-1229
Heredia, N. I., Thrift, A. P., and Balakrishnan, M. (2022). Perceived barriers to weight loss among Hispanic patients with non-alcoholic fatty liver disease. Hisp. Health Care Int. 20 (3), 171–178. doi:10.1177/15404153211043885
Hillsdon, M., Panter, J., Foster, C., and Jones, A. (2007). Equitable access to exercise facilities. Am. J. Prev. Med. 32 (6), 506–508. doi:10.1016/j.amepre.2007.02.018
Hossain, N., Kanwar, P., and Mohanty, S. R. (2016) A comprehensive updated review of pharmaceutical and nonpharmaceutical treatment for NAFLD. Gastroenterol. Res. Pract. 2016:7109270. doi:10.1155/2016/7109270
Huang, M., Chen, H., Wang, H., Zhang, Y., Li, L., Lan, Y., et al. (2025). Global burden and risk factors of MASLD: trends from 1990 to 2021 and predictions to 2030. Intern. Emerg. Med. 20, 1013–1024. doi:10.1007/s11739-025-03895-6
Hydes, T. J., Ravi, S., Loomba, R., and Gray, M. E. (2020). Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin. Mol. Hepatol. 26 (4), 383–400. doi:10.3350/cmh.2020.0067
Ilagan-Ying, Y. C., Banini, B. A., Do, A., Lam, R., and Lim, J. K. (2023). Screening, diagnosis, and staging of non-alcoholic fatty liver disease (NAFLD): application of society guidelines to clinical practice. Curr. Gastroenterol. Rep. 25 (10), 213–224. doi:10.1007/s11894-023-00883-8
Jones, P. D., Lai, J. C., Bajaj, J. S., and Kanwal, F. (2023). Actionable solutions to achieve health equity in chronic liver disease. Clin. Gastroenterol. Hepatol. 21 (8), 1992–2000. doi:10.1016/j.cgh.2023.03.043
Jung, Y. K., and Yim, H. J. (2017). Reversal of liver cirrhosis: current evidence and expectations. Korean J. Intern. Med. 32 (2), 213–228. doi:10.3904/kjim.2016.268
Kaewdech, A., Assawasuwannakit, S., Churuangsuk, C., Chamroonkul, N., and Sripongpun, P. (2024). Effect of smartphone-assisted lifestyle intervention in MASLD patients: a randomized controlled trial. Sci. Rep. 14 (1), 13961. doi:10.1038/s41598-024-64988-4
Kamel Boulos, M. N., and Yang, S. P. (2021). Mobile physical activity planning and tracking: a brief overview of current options and desiderata for future solutions. Mhealth 7, 13. doi:10.21037/mhealth.2020.01.01
Kardashian, A., Dodge, J. L., and Terrault, N. A. (2022). Racial and ethnic differences in diet quality and food insecurity among adults with fatty liver and significant fibrosis: a U.S. population-based study. Aliment. Pharmacol. Ther. 56 (9), 1383–1393. doi:10.1111/apt.17219
Kaya, E., Yilmaz, Y., and Alkhouri, N. (2025). Clinical insights on resmetirom: clinical indications, patient selection, and monitoring response to therapy. J. Clin. Gastroenterol. 59 (5), 412–419. doi:10.1097/MCG.0000000000002150
Keam, S. J. (2024). Resmetirom: first approval. Drugs 84 (6), 729–735. doi:10.1007/s40265-024-02045-0
Keating, S. E., Sabag, A., Hallsworth, K., Hickman, I. J., Macdonald, G. A., Stine, J. G., et al. (2023). Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: a position statement from exercise and sport science Australia. Sports Med. 53 (12), 2347–2371. doi:10.1007/s40279-023-01918-w
Keshet, A., Reicher, L., Bar, N., and Segal, E. (2023). Wearable and digital devices to monitor and treat metabolic diseases. Nat. Metab. 5 (4), 563–571. doi:10.1038/s42255-023-00778-y
Khiri, N., and Howells, K. (2025). Nutritional education in medical curricula and clinical practice: a scoping review on the knowledge deficit amongst medical students and doctors. J. Hum. Nutr. Diet. 38 (2), e70031. doi:10.1111/jhn.70031
Kim, D., Vazquez-Montesino, L. M., Li, A. A., Cholankeril, G., and Ahmed, A. (2020). Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 72 (5), 1556–1568. doi:10.1002/hep.31158
Kirilov, N. (2024). Capture of real-time data from electronic health records: scenarios and solutions. Mhealth 10, 14. doi:10.21037/mhealth-24-2
Kopka, C. J., Pugliese, N., Brennan, P. N., and Lazarus, J. V. (2024). We must address the MASLD awareness gap, improve educational quality and prepare for the digitally quantified self. Liver Int. 44 (9), 2099–2101. doi:10.1111/liv.15951
Lara-Romero, C., and Romero-Gómez, M. (2024). Treatment options and continuity of care in metabolic-associated fatty liver disease: a multidisciplinary approach. Eur. Cardiol. 19, e06. doi:10.15420/ecr.2023.34
Lazarus, J. V., Kopka, C. J., Younossi, Z. M., and Allen, A. M. (2023). It is time to expand the fatty liver disease community of practice. Hepatology 78 (5), 1325–1328. doi:10.1097/HEP.0000000000000411
Levy, D. E., Pachucki, M. C., O’Malley, A. J., Porneala, B., Yaqubi, A., and Thorndike, A. N. (2021). Social connections and the healthfulness of food choices in an employee population. Nat. Hum. Behav. 5 (10), 1349–1357. doi:10.1038/s41562-021-01103-x
Ley, S. L., Zeller, M. H., Reiter-Purtill, J., Kleiner, D. E., Dixon, J., Xanthakos, S., et al. (2021). Unhealthy eating, psychopathology, and nonalcoholic fatty liver disease in youth presenting for bariatric surgery. J. Pediatr. Gastroenterol. Nutr. 73 (6), 670–676. doi:10.1097/MPG.0000000000003253
Li, E., Clarke, J., Ashrafian, H., Darzi, A., and Neves, A. L. (2022). The impact of electronic health record interoperability on safety and quality of care in high-income countries: systematic review. J. Med. Internet Res. 24 (9), e38144. doi:10.2196/38144
Li, X., He, J., and Sun, Q. (2024). The prevalence and effects of sarcopenia in patients with metabolic dysfunction-associated steatotic liver disease (MASLD): a systematic review and meta-analysis. Clin. Nutr. 43 (9), 2005–2016. doi:10.1016/j.clnu.2024.07.006
Livia, B., Elisa, R., Claudia, R., Roberto, P., Cristina, A., Emilia, S. T., et al. (2016). Stage of change and motivation to a healthier lifestyle before and after an intensive lifestyle intervention. J. Obes. 2016, 6421265. doi:10.1155/2016/6421265
Lobczowska, K., Banik, A., Romaniuk, P., Forberger, S., Kubiak, T., Meshkovska, B., et al. (2022). Frameworks for implementation of policies promoting healthy nutrition and physically active lifestyle: systematic review. Int. J. Behav. Nutr. Phys. Act. 19 (1), 16. doi:10.1186/s12966-021-01242-4
Lomonaco, R., Chen, J., and Cusi, K. (2011). An endocrine perspective of nonalcoholic fatty liver disease (NAFLD). Ther. Adv. Endocrinol. Metab. 2 (5), 211–225. doi:10.1177/2042018811419157
Lonardo, A., Arab, J. P., and Arrese, M. (2021). Perspectives on precision medicine approaches to NAFLD diagnosis and management. Adv. Ther. 38 (5), 2130–2158. doi:10.1007/s12325-021-01690-1
Lorek, D., Łupina, K., Bisaga, W., Malicki, D., Stępień, W., Kumor, L., et al. (2025). The socioeconomic and environmental determinants of metabolic dysfunction-associated steatotic liver disease: understanding inequalities in prevalence and outcomes. Korean J. Fam. Med. 46 (2), 61–69. doi:10.4082/kjfm.25.0027
Machado, M. V. (2021). Aerobic exercise in the management of metabolic dysfunction associated fatty liver disease. Diabetes Metab. Syndr. Obes. 14, 3627–3645. doi:10.2147/DMSO.S304357
Magrabi, F., Habli, I., Sujan, M., Wong, D., Thimbleby, H., Baker, M., et al. (2019). Why is it so difficult to govern mobile apps in healthcare? BMJ Health Care Inf. 26 (1), e100006. doi:10.1136/bmjhci-2019-100006
Mambrini, S. P., Grillo, A., Colosimo, S., Zarpellon, F., Pozzi, G., Furlan, D., et al. (2024). Diet and physical exercise as key players to tackle MASLD through improvement of insulin resistance and metabolic flexibility. Front. Nutr. 11, 1426551. doi:10.3389/fnut.2024.1426551
Marin-Alejandre, B. A., Cantero, I., Perez-Diaz-Del-Campo, N., Monreal, J. I., Elorz, M., Herrero, J. I., et al. (2021). Effects of two personalized dietary strategies during a 2-year intervention in subjects with nonalcoholic fatty liver disease: a randomized trial. Liver Int. 41 (7), 1532–1544. doi:10.1111/liv.14818
McPherson, S., Armstrong, M. J., Cobbold, J. F., Corless, L., Anstee, Q. M., Aspinall, R. J., et al. (2022). Quality standards for the management of non-alcoholic fatty liver disease (NAFLD): consensus recommendations from the British association for the study of the liver and British society of gastroenterology NAFLD special interest group. Lancet Gastroenterol. Hepatol. 7 (8), 755–769. doi:10.1016/S2468-1253(22)00061-9
Mealy, R. N., Richardson, L. A., Miller, B., Smith, M., and Juvancic-Heltzel, J. A. (2019). Exercise is medicine®: knowledge and awareness among exercise science and medical school students. Int. J. Exerc. Sci. 12 (3), 505–514. doi:10.70252/GVFF3004
Medeiros, D. G., Ferreira, L. F., Lamp, J. D. S., and Telles da Rosa, L. H. (2025). The impact of resistance training in patients diagnosed with metabolic dysfunction-associated steatotic liver disease: a systematic review. Eur. J. Gastroenterol. Hepatol. 37 (2), 129–136. doi:10.1097/MEG.0000000000002887
Mendez-Guerrero, O., Carranza-Carrasco, A., Chi-Cervera, L. A., Torre, A., and Navarro-Alvarez, N. (2024). Optimizing nutrition in hepatic cirrhosis: a comprehensive assessment and care approach. World J. Gastroenterol. 30 (10), 1313–1328. doi:10.3748/wjg.v30.i10.1313
Miao, L., Targher, G., Byrne, C. D., Cao, Y. Y., and Zheng, M. H. (2024). Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 35 (8), 697–707. doi:10.1016/j.tem.2024.02.007
Michaelsen, M. M., and Esch, T. (2023). Understanding health behavior change by motivation and reward mechanisms: a review of the literature. Front. Behav. Neurosci. 17, 1151918. doi:10.3389/fnbeh.2023.1151918
Miller, K. C., Geyer, B., Alexopoulos, A. S., Moylan, C. A., and Pagidipati, N. (2025). Disparities in metabolic dysfunction-associated steatotic liver disease prevalence, diagnosis, treatment, and outcomes: a narrative review. Dig. Dis. Sci. 70 (1), 154–167. doi:10.1007/s10620-024-08722-0
Miryan, M., Darbandi, M., Moradi, M., Najafi, F., Soleimani, D., and Pasdar, Y. (2023). Relationship between the mediterranean diet and risk of hepatic fibrosis in patients with non-alcoholic fatty liver disease: a cross-sectional analysis of the RaNCD cohort. Front. Nutr. 10, 1062008. doi:10.3389/fnut.2023.1062008
Mogre, V., Stevens, F. C. J., Aryee, P. A., Amalba, A., and Scherpbier, A. J. J. A. (2018). Why nutrition education is inadequate in the medical curriculum: a qualitative study of students’ perspectives on barriers and strategies. BMC Med. Educ. 18 (1), 26. doi:10.1186/s12909-018-1130-5
Mostafa, A. M., Hafez, S. M., Abdullah, N. M., and Fouad, Y. (2024). Fatigue, depression, and sleep disorders are more prevalent in patients with metabolic-associated fatty liver diseases. Eur. J. Gastroenterol. Hepatol. 36 (5), 665–673. doi:10.1097/MEG.0000000000002752
Nasr, P., Shang, Y., Wester, A., Strandberg, R., Widman, L., Lazarus, J. V., et al. (2024). Socioeconomic factors associated with the presence of and outcomes in metabolic dysfunction-associated steatotic liver disease. Liver Int. 44 (11), 3050–3059. doi:10.1111/liv.16091
Noureddin, N., Copur-Dahi, N., and Loomba, R. (2024). Monitoring disease progression in metabolic dysfunction-associated steatotic liver disease. Aliment. Pharmacol. Ther. 59 (Suppl. 1), S41–S51. doi:10.1111/apt.17752
Okube, O. T., Kimani, S., and Mirie, W. (2022). Community-based lifestyle intervention improves metabolic syndrome and related markers among Kenyan adults. J. Diabetes Metab. Disord. 21 (1), 607–621. doi:10.1007/s40200-022-01023-1
Pagoto, S. (2011). The current state of lifestyle intervention implementation research: where do we go next? Transl. Behav. Med. 1 (3), 401–405. doi:10.1007/s13142-011-0071-x
Paik, A., Henry, L., de Avila, L., AlQahtani, S., Nader, F., Paik, J. M., et al. (2024). Food swamps and food deserts impact on metabolic dysfunction-associated steatotic liver disease mortality in U.S. counties. Clin. Gastroenterol. Hepatol. S1542-3565 (24), 997–1007.e5. doi:10.1016/j.cgh.2024.08.053
Parra-Vargas, M., Rodriguez-Echevarria, R., and Jimenez-Chillaron, J. C. (2020). Nutritional approaches for the management of nonalcoholic fatty liver disease: an evidence-based review. Nutrients 12 (12), 3860. doi:10.3390/nu12123860
Patel, P. (2025). Lifestyle medicine reimbursement is improving. Am. J. Lifestyle Med. 19, 682–683. doi:10.1177/15598276251321430
Patel, S., Kim, R. G., Shui, A. M., Magee, C., Lu, M., Chen, J., et al. (2024). Fatty liver education promotes physical activity in vulnerable groups, including those with unhealthy alcohol use. Gastro Hep. Adv. 3 (1), 84–94. doi:10.1016/j.gastha.2023.09.012
Peñalvo, J. L., Sagastume, D., Mertens, E., Uzhova, I., Smith, J., Wu, J. H. Y., et al. (2021). Effectiveness of workplace wellness programmes for dietary habits, overweight, and cardiometabolic health: a systematic review and meta-analysis. Lancet Public Health 6 (9), e648–e660. doi:10.1016/S2468-2667(21)00140-7
Policarpo, S. R. O., Machado, M. V., Barreira, D., and Cortez-Pinto, H. (2021). NAFLD nutritional management: results from a multidisciplinary approach. GE Port. J. Gastroenterol. 29 (6), 401–408. doi:10.1159/000519932
Popkin, B. M., Barquera, S., Corvalan, C., Hofman, K. J., Monteiro, C., Ng, S. W., et al. (2021). Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 9 (7), 462–470. doi:10.1016/S2213-8587(21)00078-4
Puengel, T., and Tacke, F. (2024). Pharmacotherapeutic options for metabolic dysfunction-associated steatotic liver disease: where are we today? Expert Opin. Pharmacother. 25 (9), 1249–1263. doi:10.1080/14656566.2024.2374463
Pugliese, N., Bertazzoni, A., Hassan, C., Schattenberg, J. M., and Aghemo, A. (2025). Revolutionizing MASLD: how artificial intelligence is shaping the future of liver care. Cancers (Basel) 17 (5), 722. doi:10.3390/cancers17050722
Rajewski, P., Cieściński, J., Rajewski, P., Suwała, S., Rajewska, A., and Potasz, M. (2025). Dietary interventions and physical activity as crucial factors in the prevention and treatment of metabolic dysfunction-associated steatotic liver disease. Biomedicines 13 (1), 217. doi:10.3390/biomedicines13010217
Ratziu, V. (2017). Non-pharmacological interventions in non-alcoholic fatty liver disease patients. Liver Int. 37 (Suppl. 1), 90–96. doi:10.1111/liv.13311
Ravela, N., Shackelford, P., Blessing, N., Yoder, L., Chalasani, N., and Samala, N. (2025). Early experience with resmetirom to treat metabolic dysfunction-associated steatohepatitis with fibrosis in a real-world setting. Hepatol. Commun. 9 (4), e0670. doi:10.1097/HC9.0000000000000670
Salmi, L., Hasanen, E., Simula, M., Virmasalo, I., and Muukkonen, P. (2023). Perceived barriers to physical activity in the social spaces of low socioeconomic status suburbs. Wellb. Space Soc. 5, 100164. doi:10.1016/j.wss.2023.100164
Schattenberg, J. M., Allen, A. M., Jarvis, H., Zelber-Sagi, S., Cusi, K., Dillon, J. F., et al. (2023). A multistakeholder approach to innovations in NAFLD care. Commun. Med. (Lond). 3 (1), 1. doi:10.1038/s43856-022-00228-y
Schulz, D. N., Smit, E. S., Stanczyk, N. E., Kremers, S. P., de Vries, H., and Evers, S. M. (2014). Economic evaluation of a web-based tailored lifestyle intervention for adults: findings regarding cost-effectiveness and cost-utility from a randomized controlled trial. J. Med. Internet Res. 16 (3), e91. doi:10.2196/jmir.3159
Semmler, G., Datz, C., Reiberger, T., and Trauner, M. (2021). Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int. 41 (10), 2249–2268. doi:10.1111/liv.15024
Sevilla-González, M., González-Ortiz, A., Landa-Anell, M. V., Melgarejo-Hernández, M. A., Arias-Marroquín, A. T., Del Razo-Olvera, F. M., et al. (2025). Adaptation of the nutrition care process for metabolic diseases in the Mexican population. Front. Nutr. 12, 1513747. doi:10.3389/fnut.2025.1513747
Shibayama, K., Furushima, C., Saka, M., Sakamoto, T., and Takahashi, H. (2024). Barriers to lifestyle modification in patients with non-alcoholic fatty liver disease: a scoping review. J. Rural. Med. 19 (1), 1–9. doi:10.2185/jrm.2023-026
Siddiqui, F., Koivula, R. W., Kurbasic, A., Lindblad, U., Nilsson, P. M., and Bennet, L. (2018). Physical activity in a randomized culturally adapted lifestyle intervention. Am. J. Prev. Med. 55 (2), 187–196. doi:10.1016/j.amepre.2018.04.016
Simancas-Racines, D., Annunziata, G., Verde, L., Fascì-Spurio, F., Reytor-González, C., Muscogiuri, G., et al. (2025). Nutritional strategies for battling obesity-linked liver disease: the role of medical nutritional therapy in metabolic dysfunction-associated steatotic liver disease (MASLD) management. Curr. Obes. Rep. 14 (1), 7. doi:10.1007/s13679-024-00597-6
Sîrbu, O., Floria, M., Dăscălița, P., Şorodoc, V., and Şorodoc, L. (2016). Non-alcoholic fatty liver disease-from the cardiologist perspective. Anatol. J. Cardiol. 16 (7), 534–541. doi:10.14744/AnatolJCardiol.2016.7049
Smith, J., Ang, X. Q., Giles, E. L., and Traviss-Turner, G. (2023). Emotional eating interventions for adults living with overweight or obesity: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 20 (3), 2722. doi:10.3390/ijerph20032722
Sohrabpour, A. A., Mohamadnejad, M., and Malekzadeh, R. (2012). Review article: the reversibility of cirrhosis. Aliment. Pharmacol. Ther. 36 (9), 824–832. doi:10.1111/apt.12044
Soleimani, D., Ranjbar, G., Rezvani, R., Goshayeshi, L., Razmpour, F., and Nematy, M. (2019). Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 12, 315–324. doi:10.2147/DMSO.S198744
Stefan, N., Yki-Järvinen, H., and Neuschwander-Tetri, B. A. (2025). Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 13 (2), 134–148. doi:10.1016/S2213-8587(24)00318-8
Stefano, J. T., Duarte, S. M. B., Ribeiro Leite Altikes, R. G., and Oliveira, C. P. (2023). Non-pharmacological management options for MAFLD: a practical guide. Ther. Adv. Endocrinol. Metab. 14, 20420188231160394. doi:10.1177/20420188231160394
Stine, J. G., Soriano, C., Schreibman, I., Rivas, G., Hummer, B., Yoo, E., et al. (2021). Breaking down barriers to physical activity in patients with nonalcoholic fatty liver disease. Dig. Dis. Sci. 66 (10), 3604–3611. doi:10.1007/s10620-020-06673-w
Targher, G., Byrne, C. D., and Tilg, H. (2024). MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut 73 (4), 691–702. doi:10.1136/gutjnl-2023-330595
Tincopa, M. A., Wong, J., Fetters, M., and Lok, A. S. (2021). Patient disease knowledge, attitudes and behaviours related to nonalcoholic fatty liver disease: a qualitative study. BMJ Open Gastroenterol. 8, e000634. doi:10.1136/bmjgast-2021-000634
Vacca, V. M. (2020). Nonalcoholic fatty liver disease: what nurses need to know. Nursing 50 (3), 32–39. doi:10.1097/01.NURSE.0000654028.01550.56
Valenzuela-Vallejo, L., Sanoudou, D., and Mantzoros, C. S. (2023). Precision medicine in fatty liver disease/non-alcoholic fatty liver disease. J. Pers. Med. 13 (5), 830. doi:10.3390/jpm13050830
Xie, J., Huang, H., Liu, Z., Li, Y., Yu, C., Xu, L., et al. (2023). The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology 77 (3), 949–964. doi:10.1002/hep.32728
Ye, S., Liu, J., and Zhou, R. (2024). Letter: enhancing cirrhosis management-the critical role of social workers in supporting NAFLD surveillance. Aliment. Pharmacol. Ther. 60 (8), 1150–1151. doi:10.1111/apt.18245
Younossi, Z. M., Yilmaz, Y., Yu, M. L., Wai-Sun Wong, V., Fernandez, M. C., Isakov, V. A., et al. (2022). Clinical and patient-reported outcomes from patients with nonalcoholic fatty liver disease across the world: data from the global non-alcoholic steatohepatitis (NASH)/non-alcoholic fatty liver disease (NAFLD) registry. Clin. Gastroenterol. Hepatol. 20 (10), 2296–2306.e6. doi:10.1016/j.cgh.2021.11.004
Younossi, Z. M., Golabi, P., Paik, J. M., Henry, A., Van Dongen, C., and Henry, L. (2023). The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77 (4), 1335–1347. doi:10.1097/HEP.0000000000000004
Younossi, Z. M., Kremer, A. E., Swain, M. G., Jones, D., Bowlus, C., Trauner, M., et al. (2024). Assessment of fatigue and its impact in chronic liver disease. J. Hepatol. 81 (4), 726–742. doi:10.1016/j.jhep.2024.04.008
Zelber-Sagi, S., Salomone, F., and Mlynarsky, L. (2017). The mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 37 (7), 936–949. doi:10.1111/liv.13435
Zeng, M. H., Shi, Q. Y., Xu, L., and Mi, Y. Q. (2024). Establishment and validation of an adherence prediction system for lifestyle interventions in non-alcoholic fatty liver disease. World J. Gastroenterol. 30 (10), 1393–1404. doi:10.3748/wjg.v30.i10.1393
Zhang, X., Daniel, C. R., Soltero, V., Vargas, X., Jain, S., Kanwal, F., et al. (2024). A study of dietary patterns derived by cluster analysis and their association with metabolic dysfunction-associated steatotic liver disease severity among Hispanic patients. Am. J. Gastroenterol. 119 (3), 505–511. doi:10.14309/ajg.0000000000002508
Zoncapè, M., Liguori, A., and Tsochatzis, E. A. (2022). Multi-disciplinary clinic models for the management of non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 11 (4), 586–591. doi:10.21037/hbsn-22-58
Keywords: metabolic dysfunction-associated steatotic liver disease, lifestyle interventions, barriers, facilitators, qualitative evidence synthesis
Citation: Yilmaz Y (2025) Barriers and facilitators to non-pharmacological management of metabolic dysfunction-associated steatotic liver disease: a qualitative evidence synthesis. Front. Pharmacol. 16:1615809. doi: 10.3389/fphar.2025.1615809
Received: 21 April 2025; Accepted: 19 August 2025;
Published: 29 August 2025.
Edited by:
Carla Carvalho, University of São Paulo, BrazilReviewed by:
Evelyn Nunes Goulart Da Silva Pereira, Oswaldo Cruz Foundation (Fiocruz), BrazilSantiago Rodríguez Villafuerte, Hospital Vozandes, Ecuador
Copyright © 2025 Yilmaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusuf Yilmaz, ZHJ5dXN1ZnlpbG1hekBnbWFpbC5jb20=
 Yusuf Yilmaz
Yusuf Yilmaz