- 1Department of Pathology and Laboratory Medicine, University of California Irvine, Irvine, CA, United States
- 2Department of Medicine, University of California Irvine, Irvine, CA, United States
- 3Department of Neurological Surgery, University of California Irvine, Irvine, CA, United States
- 4Chao Family Comprehensive Cancer Center, University of California Irvine, Irvine, CA, United States
- 5Department of Neurology, University of California Irvine, Irvine, CA, United States
Poly (ADP-ribose) polymerase (PARP) enzymes are critical in repairing DNA damage induced by chemotherapy and/or radiation. Due to PARP’s role in DNA repair, inhibiting PARP leads to genomic instability and accumulation of damaged cells in cell cycle arrest. Previous studies have shown that PARP1 activation contributes to the development of various malignant disorders, and using PARP inhibitors is a promising intervention in these diseases. However, PARP activation is also common in neurological and inflammatory disorders. PARP inhibitors were studied in preclinical models of neurodegenerative disorders such as Parkinson’s, Huntington’s, and Alzheimer’s Disease (AD). In neurodegenerative disorders like AD, activated PARP1 induces Aβ and forms Tau tangles, worsening cognitive symptoms. PARP inhibitors are currently used in combination therapy with chemotherapy drugs, including cisplatin and temozolomide, which are all described as having significant rates of central and peripheral nervous system side-effects, raising the potential question of using PARP inhibition not only as a cancer treatment but as an approach to mitigate the toxicity of the cancer drugs. This review will summarize evidence for the potential use of PARP inhibitors for neurologic disorders and discuss future prospects of how PARP inhibitors could be repurposed as neuroprotective agents against the cognitive complications of chemotherapeutic drugs.
Introduction
Chemotherapy has been the gold standard of care in cancer treatment for decades due to its ability to induce DNA damage in malignant cells. Patients can experience various nervous system complications caused by cancer and its treatments, including chemotherapeutic agents, leading to cognitive impairments, peripheral neuropathy, loss of fine motor skills, and several other symptoms. Chemotherapy-Induced Cognitive Deficits (CICD, chemo brain) is a term that refers to the long-term cognitive impairments induced by chemotherapeutics. With the recent positive trends in patient survival, there is a critical need for effective CICD treatments. Previous CICD therapies have had limited success in the significant alleviation of cognitive impairments due to the complex and poorly understood molecular mechanisms and various confounding variables. Studies have shown that Poly ADP-ribose polymerase (PARP) inhibitors are potential neuronal protectors in multiple pathologies, including neurodegenerative disorders (ND). PARP plays a critical role in repairing that damage, and PARP-1 activation contributes to the pathology of various NDs. PARP inhibitors (PARPi) are a promising targeted therapy for BRCA-mutated cancer patients, while being used in combination with chemotherapy drugs like cisplatin and temozolomide, which cause substantial amounts of central and peripheral nervous system side effects. There are many mechanistic commonalities between CICD and various NDs, including Parkinson’s, Huntington’s, and Alzheimer’s Disease (Table 1). Like many ND, chemotherapy induces reactive oxygen species (ROS) and neuroinflammation. Previous studies have documented that PARPi can prevent cell death induced by oxidative stress and counter-inflammation (Hocsak et al., 2017). Therefore, we propose the use of PARPi not only as a neuroprotective therapy for ND but also as an intervention against the neurotoxicity induced by cancer drugs.
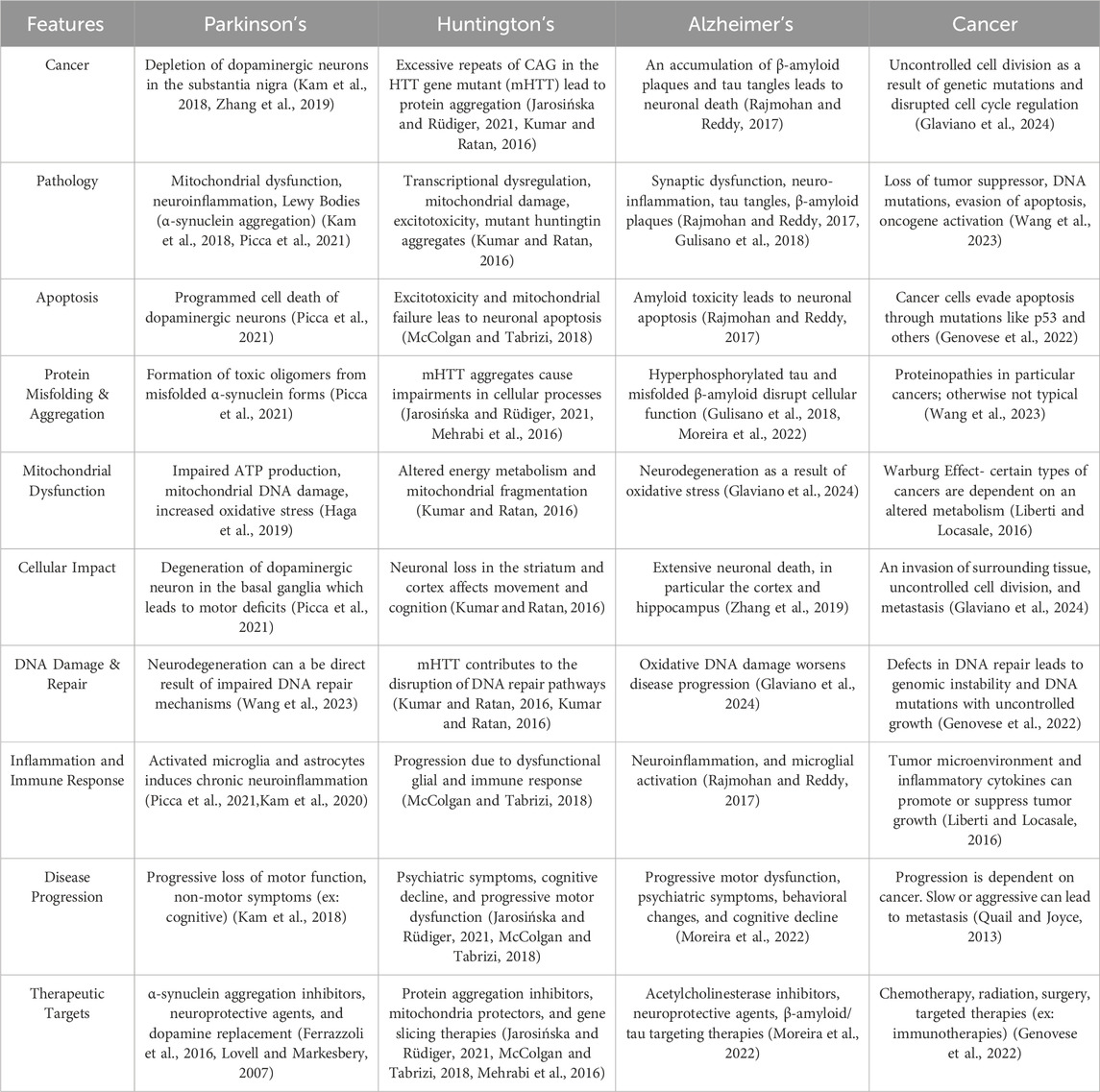
Table 1. Comparative Features of Neurodegenerative Disorders and Cancer. This table illustrates some of the key features of neurodegenerative disorders, such as Parkinson’s, Huntington’s, and Alzheimer’s, in comparison to Cancer. This table was created using BioRender.com (Kam et al., 2018; Jarosińska and Rüdiger, 2021; McColgan and Tabrizi, 2018; Kumar and Ratan, 2016; Mehrabi et al., 2016; Kumar et al., 2023; Rajmohan and Reddy, 2017; Gulisano et al., 2018; Moreira et al., 2022; Zhang et al., 2019; Picca et al., 2021; Haga et al., 2019; Kam et al., 2020; Chen et al., 2022; Genovese et al., 2022; Wang et al., 2023; Kim et al., 2022; Ferrazzoli et al., 2016; Lovell and Markesbery, 2007; Glaviano et al., 2024; Liberti and Locasale, 2016; Quail and Joyce, 2013).
PARP and its role in cancer
The PARP enzymes are a family of 17 members, each with different roles. PARP-1 is the most notable member due to its role in oncology treatments and its first responder-like behavior in DNA damage repair. PARP-1 is a large protein comprised of three main domains: the DNA-binding domain, the catalytic domain, and the auto-modification domain. When PARP-1 becomes overactive, it disrupts the normal regulation of cellular processes such as cell division, apoptosis, and autophagy, optimizing tumor development and growth conditions. PARPi halts proliferation by destabilizing the replication fork by trapping the PARP on DNA lesions, thus preventing the repair of single-strand breaks (SSBs), which would then collide with the replication fork, causing a stall and eventual collapse, leading to double-strand breaks. PARPi has been combined with various other drugs, including anti-angiogenic, PI3K/AKT, epigenetic drugs, immune checkpoint inhibitors, and chemotherapy (Soung and Chung, 2023). Combining chemo drugs like cisplatin with PARPi is a novel cancer strategy because the two compounds can work synergistically to enhance their ability to kill cancer cells, especially in tumors with altered abilities in DNA repair mechanisms like BRCA mutations or compromised homologous recombination (HR) repair mechanisms (McQuade et al., 2018).
Excessive PARP1 activation is associated with chronic inflammation and cancer
Research has shown that chronic inflammation is the foundation of a range of diseases, including cancer, Parkinson’s Disease (PD), Huntington’s Disease (HD), autism spectrum disorder (ASD), Alzheimer’s Disease (AD), diabetes, and cardiovascular disorders. Inflammation is one of the hallmarks of cancer, with a symbiotic relationship, meaning it can promote cancer development and be induced by the development and progression of cancer (Pazzaglia and Pioli, 2019). Interestingly, previous studies indicated that PARP-1 knockout mice were spared from inflammatory/autoimmune diseases or chronic infections involved in cancer development and progression (EZ et al., 2015).
Unfortunately, chemotherapy worsens inflammation by exacerbating the body’s natural systemic inflammatory response. PAR secreted from necrotic cells can stimulate proinflammatory signaling and increase the production of IL-6, IL-8, IL-1β, and TNF-α proinflammatory cytokines (Murata et al., 2019). In addition, excessive activation of PARP1 in microglia leads to glutamate uptake and neuronal injuries, which are associated with chronic neuroinflammation in neurological disorders (Mao and Zhang, 2022).
Chemotherapy-induced cognitive deficits (CICD)
Chemotherapeutic drugs were designed to cause DNA damage in rapidly dividing cells, particularly cancer cells, but healthy cells are also affected. “Chemo-brain” or “Chemo-fog” is a term used to describe the cancer-related cognitive impairments that occur as a consequence of chemotherapeutic treatments. These cognitive impairments can affect patients for weeks, years, or lifetimes during the treatment and even after the discontinuation of chemotherapy (Figure 1). In response to DNA damage, PARP induction causes a depletion of NAD + cellular levels, leading to energy deficiency and cell death, ultimately contributing to cognitive impairments (Murata et al., 2019). An adverse side effect of chemotherapy is an inflammatory response in the brain (Schroyen et al., 2021). PARP regulates the inflammatory responses, and hyperactivation of PARP is associated with the production of pro-inflammatory cytokines by microglia and astrocytes, affecting neuronal function and contributing to cognitive deficits. Previous clinical trials have proposed pharmacotherapeutic interventions like central nervous system stimulants as a way to manage CICD but have had limited success (Karschnia et al., 2019). In the absence of effective pharmacologic treatments to mitigate CICD, the use of PARPi is hypothesized to potentially open new avenues in preventing neuronal and neural stem cell damage and the ensuing cognitive impairments.
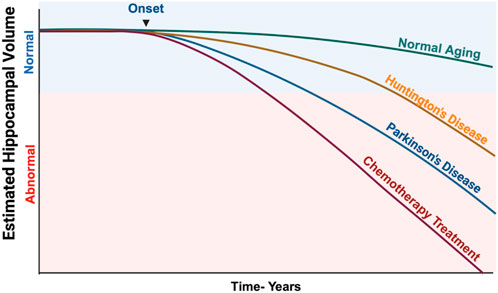
Figure 1. Estimated Hippocampal Volume Loss in Various Neurodegenerative Diseases and Chemotherapy-Treated Patients. This illustration shows how dramatically chemotherapeutic agents can impact the hippocampal volume, which directly correlates with significant cognitive impairments. Data was derived from several research studies that analyzed volumetric changes in the hippocampus over a prolonged period. Illustration created using BioRender.com (Pieperhoff et al., 2022; Nolen et al., 2016; Wilkes et al., 2023).
Sex differences in CICD
There is limited research on the sex differences on the effects of chemotherapy-induced cognitive deficits. Both men and women can experience CICD, but females are more frequently affected and studied, particularly the subset of breast cancer patients. In addition, a study conducted on long-term survivors of childhood cancer showed that females were at greater risk of developing CICD and having long-term side effects (Jacola et al., 2016).
PARP’s role in various neurological disorders
In neurological disorders like AD, PD, and HD, the common driving factors to disease progression are DNA damage, mitochondrial impairment, neuroinflammation, and oxidative stress, as represented in Figure 2 (Yan et al., 2022). Although PARP1 activation is essential in facilitating the DNA repair mechanism, excessive amounts of PARP activation exacerbate cell health and further contribute to these neurological disorders (Hu et al., 2023/11). Slightly elevated levels of PARP1 activity are initiated in response to minuscule amounts of DNA damage induced by the early stages of disease. As the disease progresses, so does extensive DNA damage, causing a cascade of predisposed factors that trigger heightened PARP1 activation. These events can induce parthanatos, or PARP1-mediated cell death, neuroinflammation, and aggravate disease pathology.
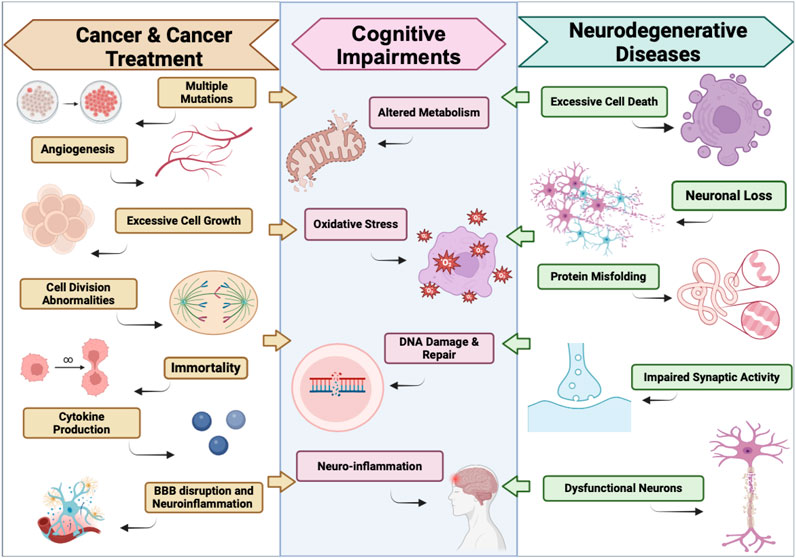
Figure 2. Parallels between the effects of Cancer & Cancer Treatments on the Brain and Neurodegenerative Diseases. This illustration defines some key similarities between the Pathology of Cancer & Cancer Treatments Induced Neurotoxicity, and neurodegenerative diseases that lead to the development of cognitive impairments. Illustration created using BioRender.com.
Influence of aging on PARP effects
Age is defined as the phenomenon of adaptation to our body’s ever-changing physical and psychological environment. Aging significantly increases the risk of developing neurodegenerative diseases (Mao and Zhang, 2022) and cancer. With age comes inadequate telomerase expression, as well as telomere shortening (Kesäniemi et al., 2019). Previous studies have shown the relationship between p53 and PARP1 and their role in telomere shortening (Maresca et al., 2023). P53 is responsible for cell cycle arrest, promoting apoptotic activity, and telomere shortening (Mao and Zhang, 2022). In aging, PARP1 binding is critical for p53 transcriptional activity because it interferes with the p53 and chromosomal region maintenance one protein binding, leading to the nuclear accumulation of p53 (Ying et al., 2016). As one ages, PARP’s role becomes more integrated in the development of NDs and cancer. DNA repair is a shared hallmark of the two (Clarke and Mostoslavsky, 2022).
Alzheimer’s disease
The pathogenesis of AD involves the accumulation of two neurotoxic protein aggregates in the central nervous system: amyloid-β (Αβ) peptide and hyperphosphorylated tau proteins (Maggiore et al., 2022). PARP-1 serves as a DNA repair enzyme, but when the cell suffers extensive DNA damage, it becomes depleted of NAD+ and ATP, which leads to parthanatos (Salech et al., 2020). Degenerating tissues and the deposition of highly insoluble materials stimulate inflammatory responses (Akiyama et al., 2000). PARP-1 overactivation is critical in the Αβ deposition and tau tangle formation. The use of PARP-1 inhibitors exerts a protective effect against neurodegeneration by diminishing neuroinflammation and microglial activation (Salech et al., 2020).
Parkinson’s disease
PD is characterized by an accumulation of α-synuclein in Lewy bodies and Lewy neurites (Bloem et al., 2021). α-synuclein pre-formed fibrils (α-syn PFF) kill neurons by activating PARP-1 in cell death through parthanatos (Hocsak et al., 2017; Galluzzi et al., 2018). Inhibition of PARP prevents neurodegeneration and behavioral deficits caused by introducing α-syn PFF (Kam et al., 2018). α-syn PFF induces inflammatory mediator activation, which can contribute to cell death and neuroprotection by a caspase inhibitor (Codolo et al., 2013). The PARP-1 inhibitors in PD have neuroprotective effects against neurodegeneration (Outeiro et al., 2007).
Huntington’s disease
HD is defined as a CAG nucleotide expansion correlated with length and age at onset (Author Anonymous, 1993). The excessive CAG repeats and glutamine residues lead to protein misfolding and accumulation of inclusions that trigger neuronal dysfunction and eventual neurodegeneration. These neuronal intranuclear inclusions occur because of mutated huntingtin, thus interacting and impairing several cellular functions (DiFiglia et al., 1997; Sugars and Rubinsztein, 2003). Significantly high PARP expression has been detected in neurons and glial cells in patients with HD (Vis et al., 2005). PARPi can block the formation of Poly-ADP ribose (PAR), reducing inflammation and protecting against cell death.
Autism
ASD encompasses a range of neurodevelopmental disorders involving impairments in communication, repetitive behaviors, and social interactions. PARP-1 activity is increased in response to oxidative stress, which is often found in individuals with autism. Increased oxidative stress often leads to neuroinflammation, which is associated with autism (Sriram et al., 2015). By regulating oxidative stress responses, PARP-1 can indirectly influence the neuroinflammatory pathways in ASD. The inhibition of PARP-1 has been shown to prevent neurobehavioral and neurochemical abnormalities (Sriram et al., 2015).
A study conducted by Ahmad et al., 2020 (Ahmad et al., 2020), demonstrated that 5-AIQ, a PARPi, reduced repetitive behavior and increased social interactions, which could be indicative of the restoration of immune function. The use of PARPi has been shown to be a promising strategy for new molecular targets involved in the development of neuroimmune dysfunctions in ASD.
Role of the other PARP members in CICD and neurodegeneration
There are various members of the PARP family, each with unique roles in cellular processes. The roles of PARP2 closely overlap with those of PARP1. PARP3 is a critical player in the repair of double-stranded breaks and genomic stability in neurons, but its role in neurodegeneration is less well known (Boehler et al., 2011). PARP6, on the other hand, is important in neuronal development, axon formation, and various mutations that are linked to neurodevelopmental disorders (Huang et al., 2016). PARP7, 9, 10, 12, and 14 have various roles in inflammatory signaling (Ke et al., 2019; Dhoonmoon and Nicolae, 2023; Welsby et al., 2014). Despite the structural similarities between the multitude of PARP family members, they differ in catalytic activity and cellular localization, which might differentially impact neuronal survival, repair, and neuroinflammation.
The therapeutic window and timing of PARP inhibitors as an approach to preventing chemotherapy-induced cognitive disorders
PARPi are FDA-approved treatments for several types of cancer with evidence of homologous recombination deficiency (HRD). Niraparib was approved in 2017 as a maintenance therapy for platinum-based chemotherapy patients with recurrent epithelial ovarian cancer who responded positively to cisplatin (Mirza et al., 2016-12). It is typically recommended to start niraparib maintenance within 8 weeks of the last dose of the platinum chemotherapy. The current standard of care suggests that patients stay on maintenance therapy for a minimum of 36 months, unless there is disease progression or unacceptable toxicities (Lee, 2021). In a study conducted by Póti et al., it was suggested that the long-term use of a PARP inhibitor, niraparib, did not cause significant mutagenicity in cell line models and tumor xenografts (Póti et al., 2018). Despite the established benefits of PARPi, the increased use of PARPi in the clinical setting has raised awareness about PARPi resistance. These resistance mechanisms include BRCA reversion mutations, HR restoration, drug efflux increase, and the stabilization of the DNA replication fork (Giudice et al., 2022). The most common adverse events that occur with prolonged use of PARPi include gastrointestinal toxicity, hematological issues, and fatigue (Tian et al., 2022).
Several challenges present themselves in translating the use of PARPi in preclinical settings to clinical use, like optimizing dosing and timing, drug delivery to the brain, identifying predictive biomarkers, understanding resistance mechanisms, and establishing efficacy as a single agent or in combination (Vinayak and Ford, 2010). It is essential that we continue researching drug delivery and targeting strategies to maximize the therapeutic potential of PARPi while minimizing their toxicity.
Conclusion
PARPi have shown tremendous potential in the cancer field, with their anti-tumor effectiveness when combined with chemotherapeutic drugs (Figure 3), and tremendous potential in the treatment of various neurodegenerative disorders. This review introduces the idea of repurposing PARP inhibitors to mitigate the CICD due to the benefits already shown in preclinical models of various non-oncological neurodegenerative disorders. The neuroprotective and anti-inflammatory activity of PARPi in targeting neuronal injury in neurodegenerative conditions shows the potential of PARPi in preventing and/or mitigating CICD.
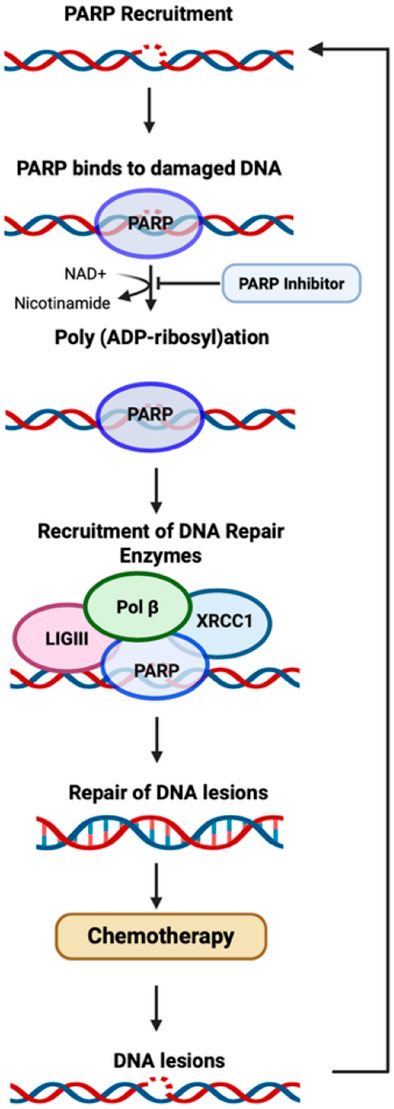
Figure 3. Mechanism of PARP. A schematic representation of PARP recruitment after the detection of DNA damage, and the proposed mechanism of action for PARP inhibitors (Demin et al., 2021).
Author contributions
DO: Conceptualization, Investigation, Writing – review and editing, Validation, Formal Analysis, Writing – original draft, Software. KG: Writing – review and editing, Writing – original draft. DB: Supervision, Visualization, Conceptualization, Methodology, Software, Project administration, Investigation, Resources, Formal Analysis, Funding acquisition, Validation, Writing – original draft, Writing – review and editing, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. National Cancer Institute award R01 CA263806 to DAB and UCI Cancer Center Award [P30CA062203] from the National Cancer Institute to DAB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, S. F., Ansari, M. A., Nadeem, A., Bakheet, S. A., Alqahtani, F., Alhoshani, A. R., et al. (2020). 5-aminoisoquinolinone attenuates social behavior deficits and immune abnormalities in the BTBR T+ Itpr3tf/J mouse model for autism. Pharmacol. Biochem. Behav. 189-172859. doi:10.1016/j.pbb.2020.172859
Akiyama, H., Barger, S., Barnum, S., Bradt, B., Bauer, J., Cole, G. M., et al. (2000). Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421. doi:10.1016/s0197-4580(00)00124-x
Author anonymous, ,(1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 72, 971–983. doi:10.1016/0092-8674(93)90585-e
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson's disease. Lancet 397, 2284–2303. doi:10.1016/s0140-6736(21)00218-x
Boehler, C., Gauthier, L. R., Mortusewicz, O., Biard, D. S., Saliou, J. M., Bresson, A., et al. (2011). Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc. Natl. Acad. Sci. U. S. A. 108, 2783–2788. doi:10.1073/pnas.1016574108
Chen, X., Zhang, T., Su, W., Dou, Z., Zhao, D., Jin, X., et al. (2022). Mutant p53 in cancer: from molecular mechanism to therapeutic modulation. Cell Death & Dis. 13 (11), 974. doi:10.1038/s41419-022-05408-1
Clarke, T. L., and Mostoslavsky, R. (2022). DNA repair as a shared hallmark in cancer and ageing. Mol. Oncol. 16, 3352–3379. doi:10.1002/1878-0261.13285
Codolo, G., Plotegher, N., Pozzobon, T., Brucale, M., Tessari, I., Bubacco, L., et al. (2013). Triggering of inflammasome by aggregated α–synuclein, an inflammatory response in synucleinopathies. PLOS ONE 8, e55375. doi:10.1371/journal.pone.0055375
Demin, A. A., Hirota, K., Tsuda, M., Adamowicz, M., Hailstone, R., Brazina, J., et al. (2021). XRCC1 prevents toxic PARP1 trapping during DNA base excision repair. Mol. Cell 81 (2021 Jul 15), 3018–3030.e5. doi:10.1016/j.molcel.2021.05.009
Dhoonmoon, A., and Nicolae, C. M. (2023). Mono-ADP-ribosylation by PARP10 and PARP14 in genome stability. Nar. Cancer 5, zcad009. doi:10.1093/narcan/zcad009
DiFiglia, M., Sapp, E., Chase, K. O., Davies, S. W., Bates, G. P., Vonsattel, J. P., et al. (1997). Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain - PubMed. Science 277. doi:10.1126/science.277.5334.1990
Ez, C., Ks, S., Mk, S., F, A., and G, S. (2015). Analysis of the intricate relationship between chronic inflammation and cancer - PubMed. Biochem. J. 468 (05/15). doi:10.1042/BJ20141337
Ferrazzoli, D., Carter, A., Ustun, F. S., Palamara, G., Ortelli, P., Maestri, R., et al. (2016). Dopamine replacement therapy, learning and reward prediction in Parkinson’s disease: implications for rehabilitation. Front. Behav. Neurosci. 10, 121. doi:10.3389/fnbeh.2016.00121
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death & Differ. 25, 486–541. doi:10.1038/s41418-017-0012-4
Genovese, I., Fornetti, E., and Ruocco, G. (2022). Mitochondria inter-organelle relationships in cancer protein aggregation. Front. Cell Dev. Biol. 10, 1062993. doi:10.3389/fcell.2022.1062993
Giudice, E., Gentile, M., Salutari, V., Ricci, C., Musacchio, L., Carbone, M. V., et al. (2022). PARP inhibitors resistance: mechanisms and perspectives. Cancers 14, 1420. doi:10.3390/cancers14061420
Glaviano, A., Singh, S. K., Lee, E. H. C., Okina, E., Lam, H. Y., Carbone, D., et al. (2024). Cell cycle dysregulation in cancer - PubMed. Pharmacol. Rev. 77. doi:10.1016/j.pharmr.2024.100030
Gulisano, W., Maugeri, D., Baltrons, M. A., Amato, A., Palmeri, A., et al. (2018). Role of amyloid-β and tau proteins in Alzheimer’s disease: confuting the amyloid cascade. J. Alzheimer's Dis. JAD 64, S611-S631. doi:10.3233/JAD-179935
Haga, H., Matsuo, K., Yabuki, Y., Zhang, C., Han, F., and Fukunaga, K. (2019). Enhancement of ATP production ameliorates motor and cognitive impairments in a mouse model of MPTP−induced Parkinson's disease. Neurochem. Int. 129 (2019/10/01), 104492. doi:10.1016/j.neuint.2019.104492
Hocsak, E., Szabo, V., Kalman, N., Antus, C., Cseh, A., Sumegi, K., et al. (2017). PARP inhibition protects mitochondria and reduces ROS production via PARP-1-ATF4-MKP-1-MAPK retrograde pathway. Free Radic. Biol. Med. 108, 770–784. doi:10.1016/j.freeradbiomed.2017.04.018
Hu, M.-L., Pan, Y. R., Yong, Y. Y., Liu, Y., Yu, L., Qin, D. L., et al. (2023). Poly (ADP-ribose) polymerase 1 and neurodegenerative diseases: past, present, and future. Ageing Res. Rev. 91, 102078. doi:10.1016/j.arr.2023.102078
Huang, J. Y., Wang, K., Vermehren-Schmaedick, A., Adelman, J. P., and Cohen, M. S. (2016). PARP6 is a regulator of hippocampal dendritic morphogenesis. Sci. Rep. 6 (1), 18512. doi:10.1038/srep18512
Jacola, L. M., Krull, K. R., Pui, C. H., Pei, D., Cheng, C., Reddick, W. E., et al. (2016). Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J. Clin. Oncol. 34, 1239–1247. doi:10.1200/JCO.2015.64.3205
Jarosińska, O. D., and Rüdiger, S. G. D. (2021). Frontiers | molecular strategies to target protein aggregation in Huntington’s disease. Front. Mol. Biosci. 8. doi:10.3389/fmolb.2021.769184
Kam, T.-I., Mao, X., Park, H., Chou, S. C., Karuppagounder, S. S., Umanah, G. E., et al. (2018). Poly (ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease. Sci. (New York, N.Y.) 362, eaat8407. doi:10.1126/science.aat8407
Kam, T.-I., Hinkle, J. T., Dawson, T. M., and Dawson, V. L. (2020). Microglia and astrocyte dysfunction in Parkinson’s disease. Neurobiol. Dis. 144, 105028. doi:10.1016/j.nbd.2020.105028
Karschnia, P., Parsons, M. W., and Dietrich, J. (2019). Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet. Oncol. 20. doi:10.1016/S1470-2045(18)30938-0
Ke, Y., Wang, C., Zhang, J., Zhong, X., Wang, R., Zeng, X., et al. (2019). The role of PARPs in inflammation—and metabolic—related diseases: molecular mechanisms and beyond. Cells 8, 1047. doi:10.3390/cells8091047
Kesäniemi, J., Lavrinienko, A., Tukalenko, E., Boratyński, Z., Kivisaari, K., Mappes, T., et al. (2019). Exposure to environmental radionuclides associates with tissue-specific impacts on telomerase expression and telomere length. Sci. Rep. 9 (1), 850. doi:10.1038/s41598-018-37164-8
Kim, S., Choi, J. G., Kang, Y. R., and Park, D. S. (2022). Inhibition of α-synuclein aggregation by MT101-5 is neuroprotective in mouse models of Parkinson’s disease. Biomed. & Pharmacother. 154 (2022/10/01), 113637. doi:10.1016/j.biopha.2022.113637
Kumar, A., and Ratan, R. R. (2016). Oxidative stress and Huntington’s disease: the good, the bad, and the ugly. J. Huntingt. Dis. 5, 217–237. doi:10.3233/JHD-160205
Kumar, D., Hasan, G. M., Islam, A., and Hassan, M. I. (2023). Therapeutic targeting of Huntington's disease: molecular and clinical approaches. Biochem. Biophysical Res. Commun. 655 (2023/05/07), 18–24. doi:10.1016/j.bbrc.2023.02.075
Lee, A. (2021). Niraparib: a review in first-line maintenance therapy in advanced ovarian cancer. Target. Oncol. 16, 839–845. doi:10.1007/s11523-021-00841-2
Liberti, M. V., and Locasale, J. W. (2016). The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218. doi:10.1016/j.tibs.2015.12.001
Lovell, M. A., and Markesbery, W. R. (2007). Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 35, 7497–7504. doi:10.1093/nar/gkm821
Maggiore, A., Casale, A. M., Toscanelli, W., Cappucci, U., Rotili, D., Grieco, M., et al. (2022). Neuroprotective effects of PARP inhibitors in Drosophila models of Alzheimer’s disease. Cells 11, 1284. doi:10.3390/cells11081284
Mao, K., and Zhang, G. (2022). The role of PARP1 in neurodegenerative diseases and aging. FEBS J. 289, 2013–2024. doi:10.1111/febs.15716
Maresca, C., Dello Stritto, A., D'Angelo, C., Petti, E., Rizzo, A., Vertecchi, E., et al. (2023). PARP1 allows proper telomere replication through TRF1 poly (ADP-ribosyl)ation and helicase recruitment. Commun. Biol. 6, 234. doi:10.1038/s42003-023-04596-6
McColgan, P., and Tabrizi, S. J. (2018). Huntington's disease: a clinical review. Eur. J. Neurology 25 (2018/01/01), 24–34. doi:10.1111/ene.13413
McQuade, R. M., Stojanovska, V., Bornstein, J. C., and Nurgali, K. (2018). PARP inhibition in platinum-based chemotherapy: chemopotentiation and neuroprotection. Pharmacol. Res. 137, 104–113. doi:10.1016/j.phrs.2018.09.031
Mehrabi, N. F., Waldvogel, H. J., Tippett, L. J., Hogg, V. M., Synek, B. J., and Faull, R. L. M. (2016). Symptom heterogeneity in Huntington's disease correlates with neuronal degeneration in the cerebral cortex. Neurobiol. Dis. 96, 67–74. doi:10.1016/j.nbd.2016.08.015
Mirza, M. R., Monk, B. J., Herrstedt, J., Oza, A. M., Mahner, S., Redondo, A., et al. (2016). Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 375, 2154–2164. doi:10.1056/nejmoa1611310
Moreira, N. C. d. S., Lima, J. E. B. d. F., Marchiori, M. F., Carvalho, I., and Sakamoto-Hojo, E. T. (2022). Neuroprotective effects of cholinesterase inhibitors: current scenario in therapies for Alzheimer’s disease and future perspectives. J. Alzheimer's Dis. Rep. 6, 177–193. doi:10.3233/ADR-210061
Murata, M. M., Kong, X., Moncada, E., Chen, Y., Imamura, H., Wang, P., et al. (2019). NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol. Biol. Cell 30, 2584–2597. doi:10.1091/mbc.E18-10-0650
Nolen, S. C., Lee, B., Shantharam, S., Yu, H. J., Su, L., Billimek, J., et al. (2016). The effects of sequential treatments on hippocampal volumes in malignant glioma patients. J. neuro-oncology 129, 433–441. doi:10.1007/s11060-016-2188-8
Outeiro, T. F., Grammatopoulos, T. N., Altmann, S., Amore, A., Standaert, D. G., Hyman, B. T., et al. (2007). Pharmacological inhibition of PARP-1 reduces α-synuclein- and MPP+-induced cytotoxicity in Parkinson’s disease in vitro models. Biochem. Biophysical Res. Commun. 357, 596–602. doi:10.1016/j.bbrc.2007.03.163
Pazzaglia, S., and Pioli, C. (2019). Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells 9, 41. doi:10.3390/cells9010041
Picca, A., Guerra, F., Calvani, R., Romano, R., Coelho-Júnior, H. J., Bucci, C., et al. (2021). Mitochondrial dysfunction, protein misfolding and neuroinflammation in Parkinson's disease: roads to biomarker discovery - PubMed. Biomolecules 11, 10/13. doi:10.3390/biom11101508
Pieperhoff, P., Südmeyer, M., Dinkelbach, L., Hartmann, C. J., Ferrea, S., Moldovan, A. S., et al. (2022). Regional changes of brain structure during progression of idiopathic Parkinson's disease – a longitudinal study using deformation based morphometry. Cortex 151 (2022/06/01), 188–210. doi:10.1016/j.cortex.2022.03.009
Póti, Á., Berta, K., Xiao, Y., Pipek, O., Klus, G. T., Ried, T., et al. (2018). Long-term treatment with the PARP inhibitor niraparib does not increase the mutation load in cell line models and tumour xenografts - PubMed. Br. J. cancer 119. doi:10.1038/s41416-018-0312-6
Quail, D., and Joyce, J. (2013). Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437. doi:10.1038/nm.3394
Rajmohan, R., and Reddy, P. H. (2017). Amyloid beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J. Alzheimer's Dis. JAD 57, 975–999. doi:10.3233/JAD-160612
Salech, F., Ponce, D. P., Paula-Lima, A. C., SanMartin, C. D., and Behrens, M. I. (2020). Nicotinamide, a poly [ADP-Ribose] polymerase 1 (PARP-1) inhibitor, as an adjunctive therapy for the treatment of Alzheimer’s disease. Front. Aging Neurosci. 12, 255. doi:10.3389/fnagi.2020.00255
Schroyen, G., Blommaert, J., van Weehaeghe, D., Sleurs, C., Vandenbulcke, M., Dedoncker, N., et al. (2021). Neuroinflammation and its association with cognition, neuronal markers and peripheral inflammation after chemotherapy for breast cancer. Cancers 13, 4198. doi:10.3390/cancers13164198
Soung, Y.-H., and Chung, J. (2023). Combination treatment strategies to overcome PARP inhibitor resistance. Biomolecules 13, 1480. doi:10.3390/biom13101480
Sriram, C. S., Jangra, A., Gurjar, S. S., Hussain, M. I., Borah, P., Lahkar, M., et al. (2015). Poly (ADP-ribose) polymerase-1 inhibitor, 3-aminobenzamide pretreatment ameliorates lipopolysaccharide-induced neurobehavioral and neurochemical anomalies in mice. Pharmacol. Biochem. Behav. 133, 83–91. doi:10.1016/j.pbb.2015.03.022
Sugars, K. L., and Rubinsztein, D. C. (2003). Transcriptional abnormalities in Huntington disease. Trends Genet. 19, 233–238. doi:10.1016/S0168-9525(03)00074-X
Tian, X., Chen, L., Gai, D., He, S., Jiang, X., and Zhang, N. (2022). Adverse event profiles of PARP inhibitors: Analysis of spontaneous reports submitted to FAERS. Front. Pharmacol. 13, 851246. doi:10.3389/fphar.2022.851246
Vinayak, S., and Ford, J. M. (2010). PARP inhibitors for the treatment and prevention of breast cancer. Curr. breast cancer Rep. 2, 190–197. doi:10.1007/s12609-010-0026-0
Vis, J. C., Schipper, E., de Boer-van Huizen, R. T., Verbeek, M. M., de Waal, R. M. W., Wesseling, P., et al. (2005). Expression pattern of apoptosis-related markers in Huntington’s disease. Acta Neuropathol. 109, 321–328. doi:10.1007/s00401-004-0957-5
Wang, Z.-X., Li, Y.-L., Pu, J.-L., and Zhang, B.-R. (2023). DNA damage-mediated neurotoxicity in Parkinson’s disease. Int. J. Mol. Sci. 24, 6313. doi:10.3390/ijms24076313
Welsby, I., Hutin, D., Gueydan, C., Kruys, V., Rongvaux, A., and Leo, O. (2014). PARP12, an interferon-stimulated gene involved in the control of protein translation and inflammation. J. Biol. Chem. 289 (2014/09/19), 26642–26657. doi:10.1074/jbc.M114.589515
Wilkes, F. A., Jakabek, D., Walterfang, M., Velakoulis, D., Poudel, G. R., Stout, J. C., et al. (2023). Hippocampal morphology in Huntington's disease, implications for plasticity and pathogenesis: the IMAGE-HD study. Psychiatry Res. Neuroimaging 335 (2023/10/01), 111694. doi:10.1016/j.pscychresns.2023.111694
Yan, L., Guo, M. S., Zhang, Y., Yu, L., Wu, J. M., Tang, Y., et al. (2022). Dietary plant polyphenols as the potential drugs in neurodegenerative diseases: current evidence, advances, and opportunities. Oxidative Med. Cell. Longev. 2022, 1–40. doi:10.1155/2022/5288698
Ying, Y., Padanilam, B. J., Ying, Y., and Padanilam, B. J. (2016). Regulation of necrotic cell death: p53, PARP1 and cyclophilin D-overlapping pathways of regulated necrosis? Cell. Mol. Life Sci. 73 (11), 2309–2324. doi:10.1007/s00018-016-2202-5
Keywords: PARP (poly(ADP-ribose), PARP inhibitors (PARPi), chemo brain, neuroinflamamation, chemotherapy
Citation: Ordaz DA, Gupta K and Bota DA (2025) The role of Poly-ADP ribose polymerase (PARP) enzymes in chemotherapy-induced cognitive impairments – parallels with other neurodegenerative disorders. Front. Pharmacol. 16:1615843. doi: 10.3389/fphar.2025.1615843
Received: 22 April 2025; Accepted: 23 May 2025;
Published: 09 June 2025.
Edited by:
Jacob Raber, Oregon Health and Science University, United StatesReviewed by:
Narendran Annadurai, University of Nebraska Medical Center, United StatesPeng Wen Tan, Yale University, United States
Copyright © 2025 Ordaz, Gupta and Bota. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela A. Bota, ZGJvdGFAdWNpLmVkdQ==
 Dahlia A. Ordaz
Dahlia A. Ordaz Kalpna Gupta
Kalpna Gupta Daniela A. Bota
Daniela A. Bota