- 1Department of Critical Care Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 2Department of Respiratory and Critical Care Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
Background: Magnesium sulfate is commonly utilized in critical care due to its vasodilatory, bronchodilatory, and neuroprotective properties. However, its impact on mortality outcomes in patients with chronic obstructive pulmonary disease (COPD) requiring intensive care remains inadequately defined.
Methods: A retrospective cohort study was conducted on patients with COPD who were admitted to the ICU at Beth Israel Deaconess Medical Center in Boston from 2008 to 2019. Early administration of magnesium sulfate was considered for intravenous administration within 48 h of ICU admission. Propensity-score-based methods, such as inverse probability weighting, were employed to evaluate the correlation between early use of magnesium sulfate and 28-day mortality.
Results: A total of 3,651 ICU admissions for COPD were included, of which 1,148 (31.4%) patients received magnesium sulfate within the first 48 h. Administering magnesium sulfate early was linked to a reduced 28-day mortality rate (hazard ratio 0.76, 95% confidence interval 0.60–0.95), with consistent results across predefined subgroups. This correlation remained consistent regardless of baseline serum magnesium levels and did not increase the risk of acute kidney injury (AKI). The calculated E-value of 1.96 indicates that significant unmeasured confounding factors would be necessary to fully account for the observed relationship.
Conclusion: In this single-center retrospective cohort, early magnesium sulfate administration in critically ill patients with COPD was associated with lower 28-day mortality without an observed increase in AKI risk. These results advocate for prospective multicenter studies to validate these connections, investigate optimal dosing approaches, and pinpoint the patient subgroups most likely to benefit from this intervention.
Introduction
Chronic obstructive pulmonary disease (COPD) stands as the primary cause of respiratory-related disability and mortality globally. Acute exacerbations often necessitate intensive care unit (ICU) admission for advanced life support, such as mechanical ventilation, due to severe complications like respiratory failure and infection (GBD Chronic Respiratory Disease Collaborators, 2017; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016; Funk et al., 2013). Despite therapeutic progress, ICU patients with COPD exhibit significantly higher mortality rates compared to those without COPD. The prognosis is further compromised by issues like prolonged mechanical ventilation and ventilator-associated pneumonia (VAP) (Nseir et al., 2005). Remarkably, the occurrence of VAP can elevate ICU mortality rates in COPD patients to as high as 64%, while significantly prolonging the duration of mechanical ventilation and hospitalization. Although optimized ventilation strategies, including early invasive–non-invasive sequential therapy, have enhanced clinical outcomes, effective pharmacological interventions targeting mortality reduction remain a pressing area of investigation (Lei et al., 2023; Gungor et al., 2018).
Magnesium sulfate, a compound with bronchodilatory, anti-inflammatory, and electrolyte-modulating properties, has the potential to enhance outcomes in critically ill patients with COPD through various mechanisms (de Baaij et al., 2015). Patients in the ICU with COPD often exhibit hypophosphatemia, electrolyte imbalances, and systemic inflammatory responses that could be mitigated by the therapeutic effects of magnesium sulfate (Wozniak et al., 2022; Panahi et al., 2017). Additionally, noninvasive ventilation (NIV) has been proven to significantly decrease the occurrence of VAP. As a supplementary treatment, magnesium sulfate might indirectly reduce the VAP risk by diminishing the necessity for tracheal intubation (Hess, 2013; Lorente et al., 2007). In cases of respiratory failure, an imbalance between the energy supply and demand of the respiratory muscles contributes to respiratory fatigue. The bronchodilatory properties of magnesium sulfate could potentially alleviate this by lowering airway resistance and decreasing muscle workload (Roussos and Koutsoukou, 2003; Touyz et al., 2024). Previous studies examining the use of Mg in COPD exacerbations have yielded inconsistent results. A systematic review indicated that intravenous magnesium was linked to decreased hospital admission rates, shorter stays, and improved dyspnea scores during acute exacerbations (Jahangir et al., 2022; Liu et al., 2024; Ni et al., 2022). Conversely, other studies have not shown significant enhancements in crucial clinical outcomes. A non-Cochrane review concluded that while magnesium may boost the bronchodilatory effects of beta2-agonists, it does not notably impact dyspnea scores, hospital admission rates, or relapse rates (Shivanthan and Rajapakse, 2014). Given these inconclusive findings, current clinical guidelines do not advocate for the routine use of magnesium sulfate in acute COPD exacerbations.
This uncertainty, particularly in studies involving general ward patients or those with moderate disease, supports our hypothesis that any potential benefit of magnesium sulfate may be context-dependent. Critically ill patients with COPD in the ICU represent a population with more profound physiological derangements and a higher burden of complications, where standard treatments may be insufficient. By specifically evaluating the impact of magnesium sulfate in this high-risk subgroup, our study sought to clarify whether it can confer clinically meaningful benefits, such as reduced duration of ventilation, fewer complications, and lower mortality, which may have been obscured in broader patient populations. Addressing this knowledge gap could help reconcile conflicting evidence and determine whether ICU patients with COPD derive unique benefits from Mg therapy.
Methods
Data sources
This study utilized data from the Medical Information Mart for Intensive Care IV (MIMIC-IV), a comprehensive single-center database containing electronic health records of patients admitted to the Beth Israel Deaconess Medical Center in Boston, Massachusetts, United States, from 2008 to 2019 (Johnson A. E. W. et al., 2023; Johnson et al., 2024). Access to the database was approved by the Institutional Review Boards of the Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology in Cambridge, Massachusetts, United States (record ID: 49780033). Owing to data anonymization, patient consent was not required to adhere to ethical standards and privacy regulations.
Study population
Participants were COPD patients aged 18 years and older who were admitted to the ICU under specific criteria: 1) a confirmed diagnosis of COPD, as indicated by a post-bronchodilator FEV1/FVC ratio of less than 0.70; 2) their first ICU admission; and 3) a minimum ICU stay of 48 h. Data collection included a wide range of parameters such as demographic details (age and sex), disease severity markers (Oxford Acute Severity of Illness Score (OASIS)), comorbidity burden (Charlson Comorbidity Index), organ support interventions (MV and continuous renal replacement therapy (CRRT)), baseline magnesium levels, and infection-related parameters (sepsis status and laboratory biomarkers). Laboratory indicators were initially measured within 24 h of ICU admission. For patients with multiple ICU admissions, only data from the first admission were considered to maintain data independence.
Outcomes
The main endpoint was the effect of magnesium sulfate treatment within 48 h of ICU admission on 28-day mortality. Secondary endpoints comprised the incidence of acute kidney injury (AKI), ICU mortality rate, ICU stay duration, and MV duration.
Covariate filtering methods
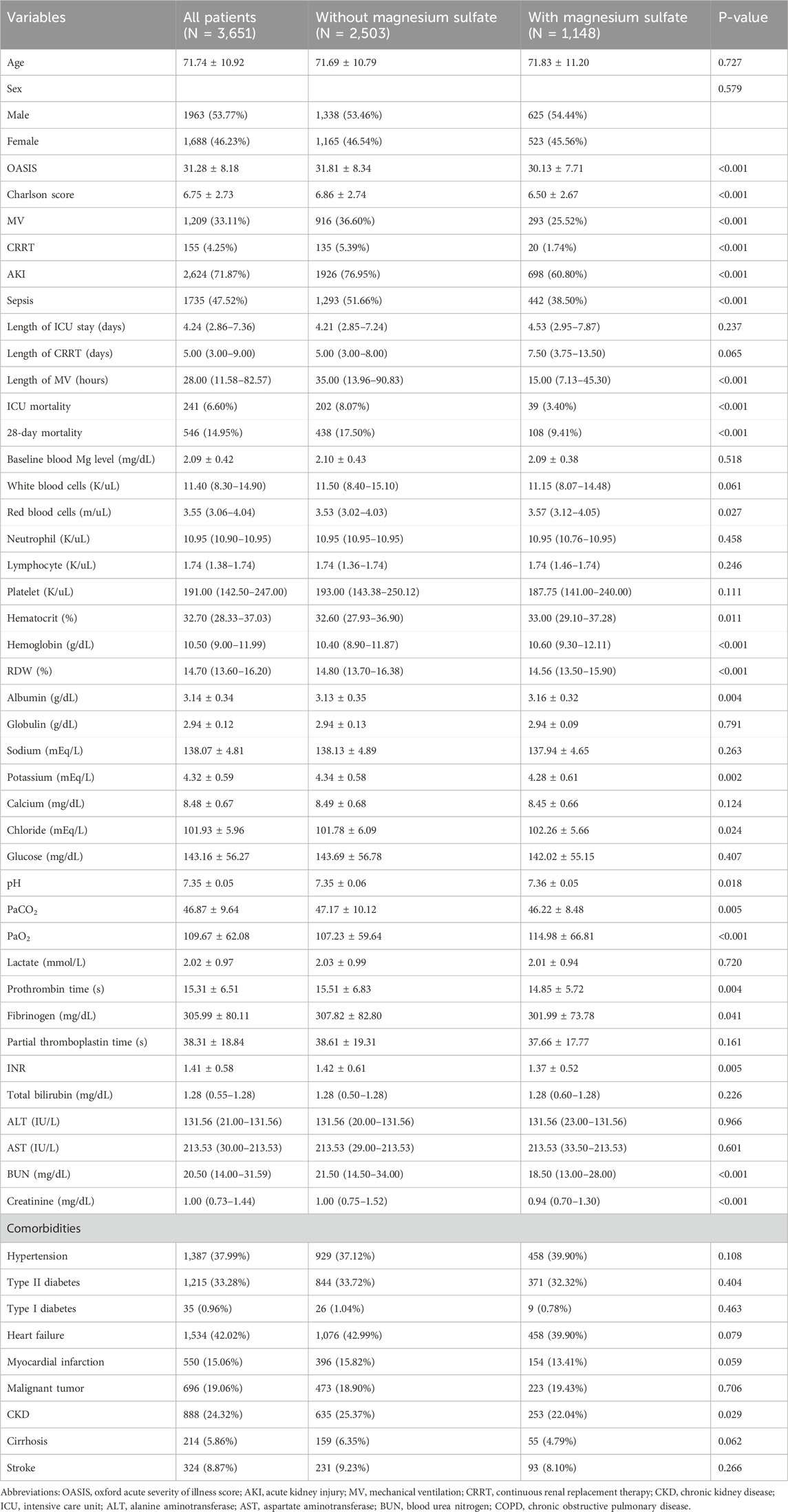
The covariate selection utilized the Boruta algorithm, least absolute shrinkage, and selection operator (LASSO) regression. LASSO regression employs L1 regularization to reduce certain feature coefficients to zero, aiding in both feature selection and parameter estimation. The Boruta algorithm functions as a robust wrapper method that statistically assesses original features against manually generated “shadow features” (randomized arrangements of the original data) using a Z-score test (Lin et al., 2025), effectively minimizing the false-positive rate. Ultimately, the variables “age, OASIS score, AKI, sepsis, MV, CRRT, blood urea nitrogen (BUN), creatinine” were incorporated into the regression model (Figure 1).
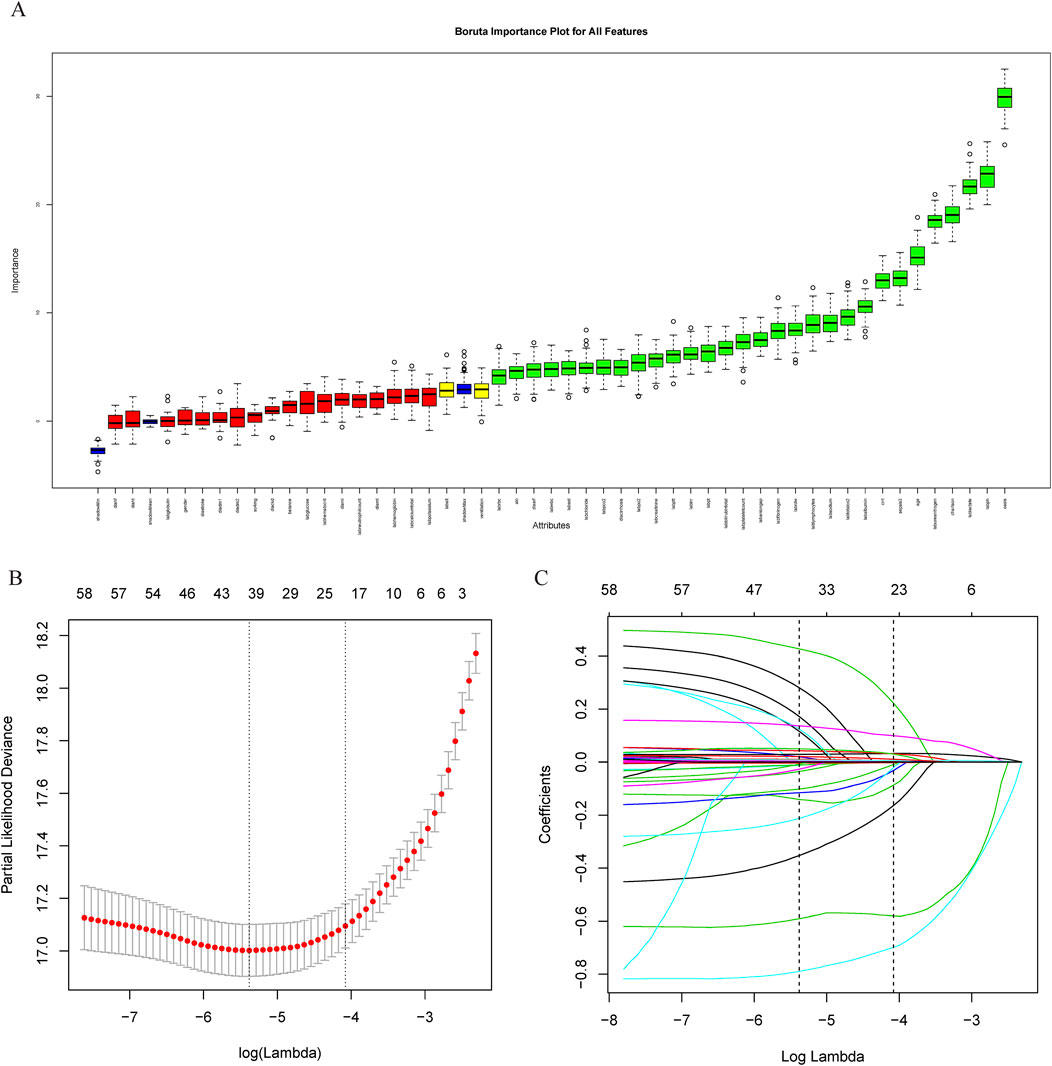
Figure 1. Feature variable screening. (A) Feature variable screening using Boruta; (B, C) Feature variable screening using LASSO. Abbreviations: LASSO, least absolute shrinkage and selection operator.
Statistical analysis
Continuous variables are reported as mean ± standard deviation or median interquartile range, depending on their distribution. Groups of variables were compared using Student's t-test for normally distributed data and the Wilcoxon rank-sum test for non-normally distributed data. Categorical variables are presented as counts and percentages and were analyzed using the chi-square test.
Three models were constructed to evaluate the impact of early magnesium sulfate administration on 28-day mortality: Model 1 remained unadjusted, Model 2 was adjusted for characteristic variables, and Model 3 included a propensity score methodology involving matching, covariate-adjusted scores, and inverse probability weighting. E-values were computed to assess the potential impact of unmeasured confounders on the relationship between early magnesium sulfate use and 28-day mortality (VanderWeele and Ding, 2017; Haneuse et al., 2019). Survival disparities were examined utilizing the Kaplan–Meier method and evaluated through time-series tests. Stratified analyses and interaction tests were carried out to confirm the consistency of associations across various subgroups, encompassing demographics, therapeutic interventions, sepsis, AKI, baseline magnesium levels, and OASIS scores. Data analysis was conducted using R software (version 4.4.2), with p-values below 0.05 considered statistically significant.
Results
Basic characteristics of the study population
In this study, we analyzed a cohort of 3,651 patients with severe COPD. Within the first 48 h of ICU admission, 31.44% of the patients (1,148) received magnesium sulfate treatment, as detailed in Table 1. Our findings indicated that sepsis was diagnosed in 47.52% of the cohort (1,735 patients), and 71.87% (2,624 patients) developed AKI after admission. Both comorbidities occurred less frequently in the magnesium sulfate group than in the untreated group. This group also had significantly lower CRRT and invasive mechanical ventilation rates, as shown in Table 1.
Clinical outcomes in critically ill patients with COPD
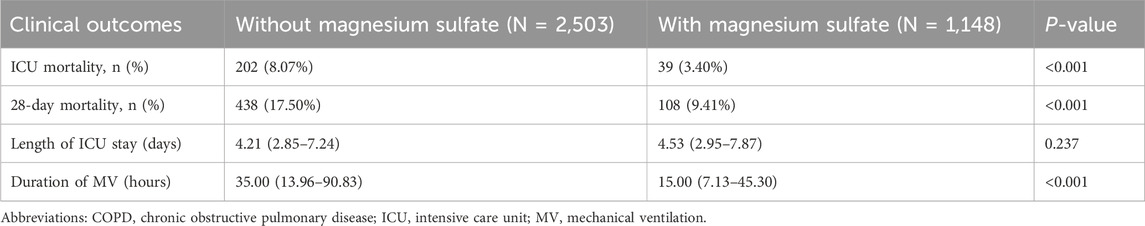
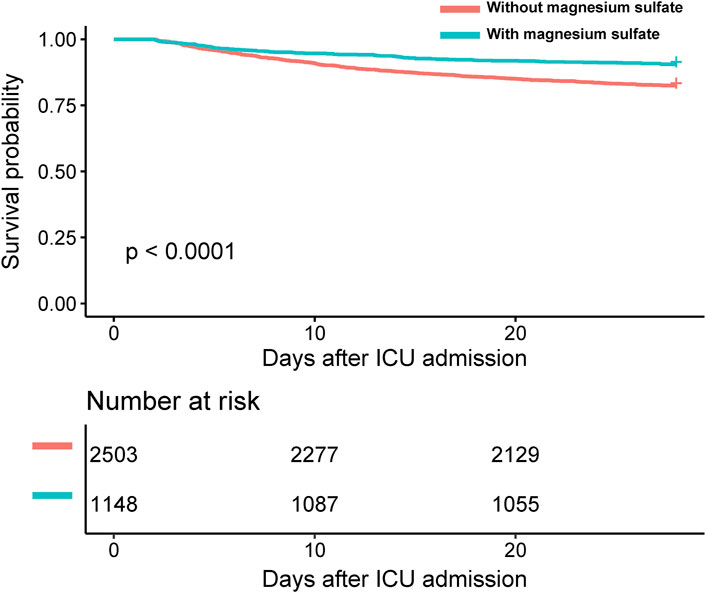
Clinically, patients treated with magnesium sulfate demonstrated significantly lower mortality rates both in the ICU and at 28 days post-admission compared to those who did not receive the treatment, with p-values less than 0.05 for both endpoints (Table 2). Kaplan–Meier survival curves further emphasized a significantly higher 28-day survival rate among patients treated with magnesium sulfate, confirmed by a time-series test with a p-value of less than 0.05 (Figure 2). Additionally, the mean duration of MV was significantly shorter in the magnesium-treated group (p < 0.001). However, no significant difference was observed in the length of ICU stay between the two groups (p = 0.237).
Impact of magnesium sulfate use on clinical outcomes
Table 3 demonstrates a consistent link between lower 28-day mortality rates in all examined models for patients receiving magnesium sulfate treatment. Particularly, in the model utilizing propensity score inverse probability weighting, the use of magnesium sulfate was linked to a 24% reduction in 28-day mortality compared to non-users (hazard ratio [HR] 0.76 [95% confidence interval {CI} 0.60–0.95]). The calculated E-value of 1.96 (upper confidence limit 2.89) indicates that an unmeasured confounding factor would need to have a relative risk of at least 1.96 to negate the observed association.
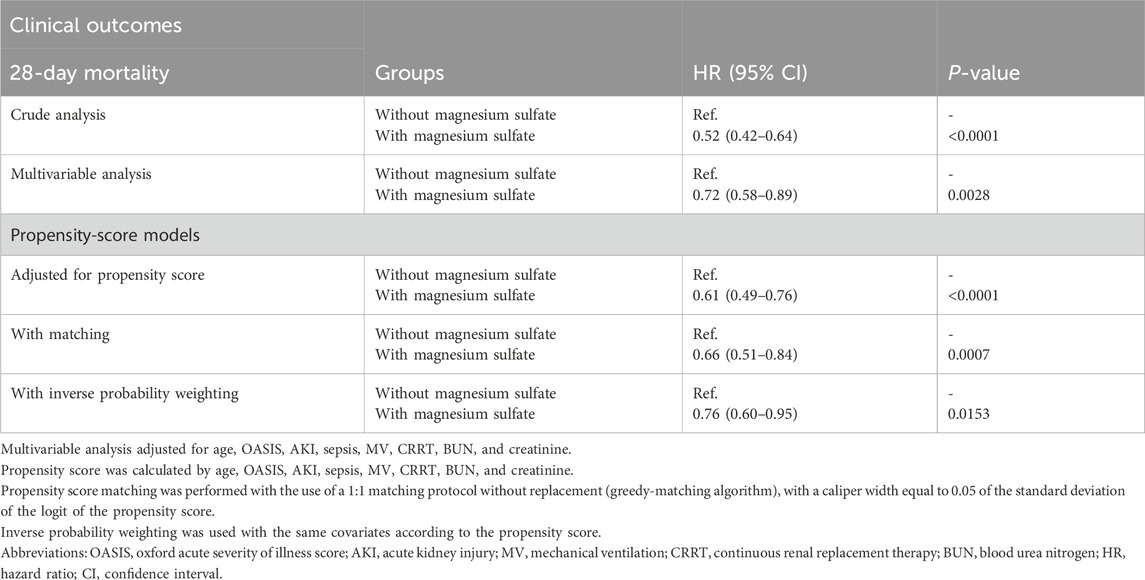
Table 3. Associations between magnesium sulfate use and clinical outcomes in the crude analysis, multivariable Cox analysis, and propensity score analyses.
Stratified analyses and interaction tests
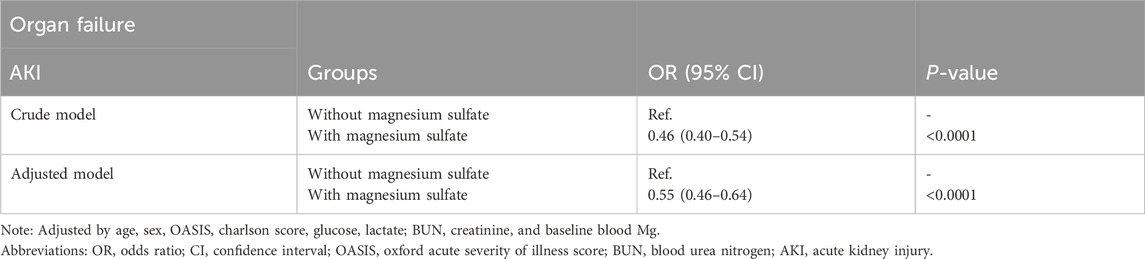
The beneficial effects of magnesium sulfate on reducing 28-day mortality remained consistent across all subgroups analyzed Figure 3. Significant interactions were observed between magnesium sulfate administration and both BUN levels and mechanical ventilation use, with interaction p-values below 0.05, indicating statistical significance (Table 4). Specifically, patients with BUN levels under 24 mg/dL who received magnesium sulfate treatment exhibited a significantly reduced 28-day mortality rate (HR 0.48 [95% CI: 0.33–0.71]) compared to untreated patients. Similarly, a positive effect was noted in patients on mechanical ventilation who were treated with magnesium sulfate versus those who were not (HR = 0.54 [95% CI: 0.37–0.79]). These results highlight the substantial impact of magnesium sulfate in various clinical scenarios within ICU settings. Furthermore, while the relationship between magnesium sulfate use and 28-day mortality remained consistent across different baseline blood magnesium levels, no significant association was found in the subgroup with blood magnesium levels ≥2.4 mg/dL. Subgroup analyses of the neutrophil-to-lymphocyte ratio (NLR) and duration of mechanical ventilation indicated that patients treated with magnesium sulfate experienced lower 28-day mortality as NLR or duration of mechanical ventilation increased (Figure 4). Finally, we did not observe an increased risk of AKI associated with the early use of magnesium sulfate (Table 5).
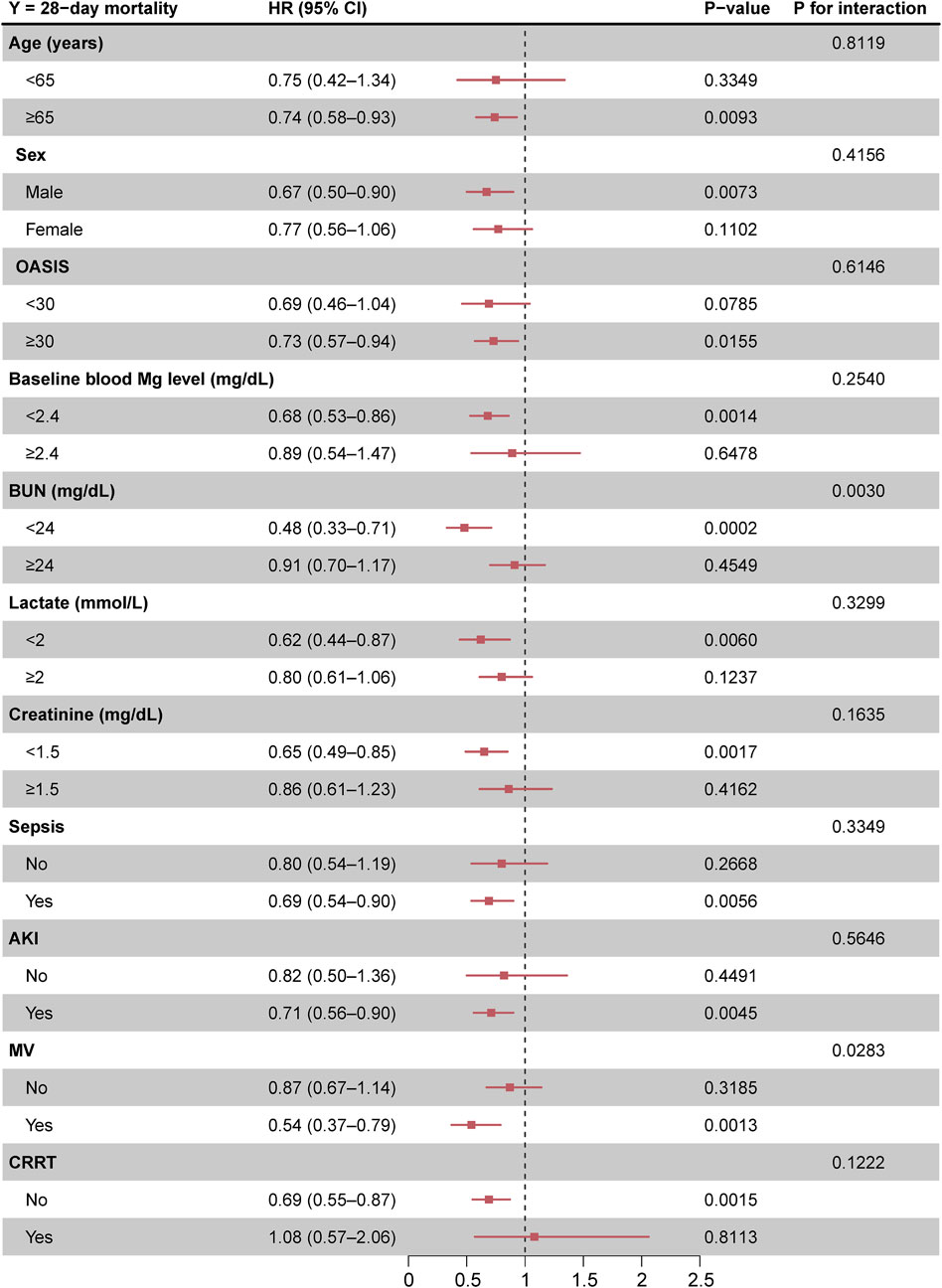
Figure 3. Forest plot of magnesium sulfate use on 28-day mortality in prespecified and exploratory subgroups in each subgroup. Abbreviations: HR, hazard ratio; CI, confidence interval; OASIS, Oxford acute severity of illness score; AKI, acute kidney injury; MV, mechanical ventilation; CRRT, continuous renal replacement therapy.
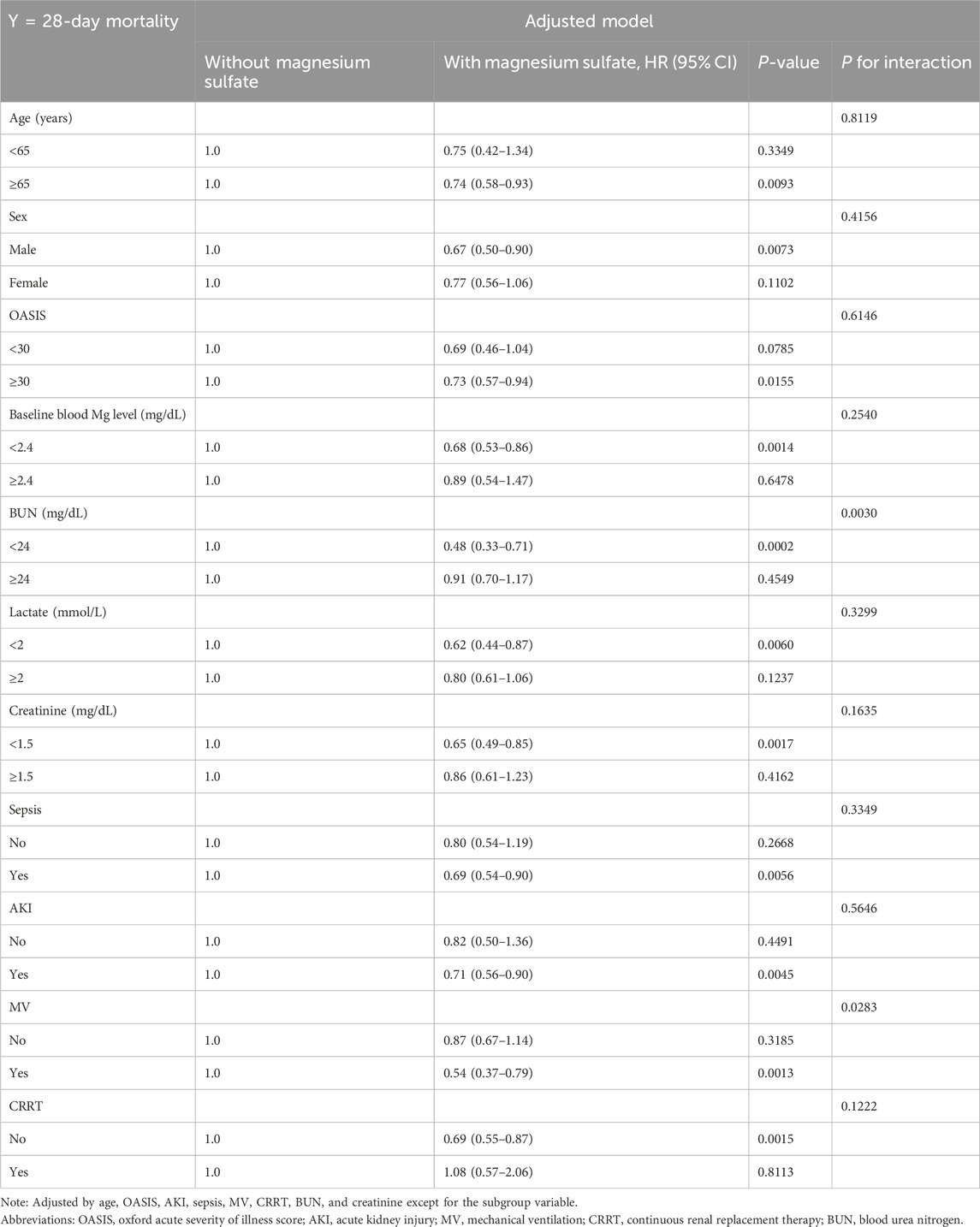
Table 4. Effect size of magnesium sulfate use on 28-day mortality in prespecified and exploratory subgroups in each subgroup.
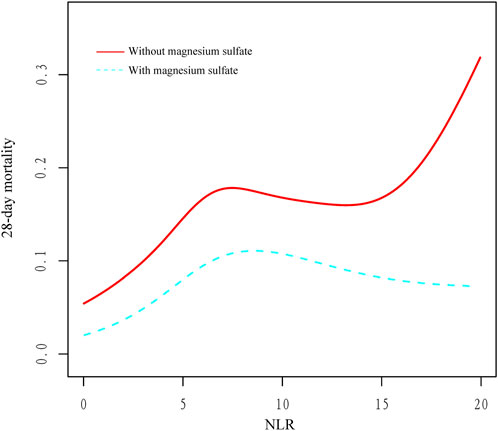
Figure 4. Relationship between NLR and 28-day mortality in critically ill patients with COPD. Abbreviations: NLR, neutrophil-to-lymphocyte ratio; COPD, chronic obstructive pulmonary disease.
Discussion
In this study, we analyzed data from critically ill patients with COPD admitted to the ICU to investigate the relationship between magnesium sulfate administration within 48 h of ICU admission and clinical outcomes. The early administration of magnesium sulfate was associated with a lower 28-day mortality rate, without an increased risk of AKI. While the length of ICU stays showed no significant variance between the groups, patients receiving magnesium sulfate had a shorter duration of mechanical ventilation. These results imply that the benefits observed may be more closely tied to survival and respiratory improvement rather than disparities in overall resource utilization.
Current clinical uses and research on magnesium sulfate
Magnesium sulfate is extensively utilized in conditions like preeclampsia, eclampsia, and specific arrhythmias. Its role in critical care has expanded recently, driven by emerging evidence of broader benefits. For example, magnesium has been investigated as a treatment for atrial fibrillation (to aid in restoring sinus rhythm), kidney protection, and neuroprotection in ICU patients (Gu et al., 2023; Davey and Teubner, 2005; Saver et al., 2015). A retrospective cohort study utilizing the MIMIC-IV database discovered that magnesium sulfate usage in patients with sepsis was linked to significantly lower 28-day mortality, a decreased incidence of AKI, and the necessity for renal replacement therapy (Roussos and Koutsoukou, 2003). These benefits are ascribed to magnesium’s anti-inflammatory and antioxidant properties, which might mitigate organ damage seen in critical illness. Importantly, these advantages seem to depend on maintaining magnesium within an optimal range. Another analysis observed that excessive magnesium supplementation resulting in hypermagnesemia (serum magnesium >1.07 mmol/L) was associated with increased 28- and 90-day mortality (Li et al., 2024). This highlights that while magnesium sulfate is generally well tolerated, oversupplementation (particularly in patients with renal impairment) can lead to adverse effects such as hypotension and respiratory depression. Therefore, precise dosing and monitoring of serum magnesium levels are crucial when using magnesium in critical care.
Magnesium sulfate in COPD exacerbations: evidence and new insights
The effectiveness of magnesium sulfate in acute COPD exacerbation has been explored in various trials and reviews with varying outcomes (Jahangir et al., 2022; Shivanthan and Rajapakse, 2014; Skorodin et al., 1995). A recent Cochrane systematic review and other meta-analyses on intravenous magnesium in COPD indicated some modest advantages: magnesium therapy enhanced lung function (e.g., increased FEV1 and peak expiratory flow) and was linked to reduced odds of hospital admission (Ni et al., 2022). For instance, Jahangir et al. found that IV magnesium resulted in significantly improved FEV1/PEF outcomes and approximately a 55% decrease in the risk of hospitalization in COPD exacerbation patients compared to placebo (Jahangir et al., 2022). Magnesium has long been recognized for its beneficial effects on severe asthma attacks by alleviating bronchospasm and enhancing FEV1. However, it demonstrates limited efficacy in milder asthma exacerbations—a trend that appears to be somewhat mirrored in COPD research.
Despite these improvements in intermediate outcomes, previous randomized trials have not consistently shown improvements in the most critical endpoints of COPD exacerbation. Multiple RCTs, often small and single-center studies, have not demonstrated significant reductions in the need for ICU admission, intubation, or mortality with IV magnesium. For example, a trial involving 124 patients by Nouira et al. found no significant differences in intubation rates or 90-day mortality between the magnesium and control groups (Nouira et al., 2014). Similarly, an earlier trial by Skorodin et al. observed improved peak flow after magnesium administration but no significant impact on dyspnea scores or hospitalization rates (Skorodin et al., 1995). These inconsistent findings likely result from variations in study designs, such as differences in magnesium dosage (ranging from 1.2 g to 2.5 g), timing of administration, and patient populations, all of which can affect outcomes. Many RCTs have excluded the sickest COPD patients, such as those needing invasive mechanical ventilation or those with life-threatening respiratory failure. Therefore, previous trials may have lacked the power to detect survival benefits, particularly as they focused on moderate exacerbations treated in an emergency setting.
Our findings offer new insights in this context. In our analysis, encompassing a wide cohort of ICU patients with COPD exacerbation, the use of magnesium was linked to enhanced survival, particularly in the most severe cases necessitating mechanical ventilation. This differs from previous interventional trials that did not show a clear mortality benefit, underscoring that the life-saving potential of magnesium might only manifest in critical patient subgroups. In other words, magnesium sulfate could provide benefits in severe COPD exacerbations that were not evident in earlier studies focusing on less critical patients. By specifically investigating outcomes in mechanically ventilated individuals, our study indicates that magnesium might lower mortality rates where standard therapies often fall short. This new discovery warrants further exploration as it suggests that magnesium’s impact goes beyond minor improvements in lung function or hospital stay, potentially changing the trajectory of life-threatening COPD exacerbations.
Potential mechanisms and benefits of magnesium sulfate
The physiological effects of magnesium sulfate offer plausible explanations for its potential benefits in critically ill patients with COPD. Magnesium is a well-known smooth muscle relaxant in the airways, acting by antagonizing calcium influx into bronchial smooth muscle cells and inhibiting acetylcholine release, resulting in bronchodilation (Barbagallo et al., 2021; Knightly et al., 2017). During severe exacerbations, magnesium administration can alleviate bronchospasm, reducing airway resistance and dynamic hyperinflation, thus enhancing ventilation efficiency. This effect likely contributes to improved gas exchange and may lower the risk of barotrauma or ventilator-induced lung injury by decreasing the pressure needed to ventilate stiff, constricted lungs. Maintaining adequate magnesium levels has been linked to a shorter duration of mechanical ventilation in ICU patients, suggesting that magnesium-facilitated bronchodilation and lung mechanics can expedite respiratory recovery (Altun et al., 2019; Santosh Raju et al., 2023). Additionally, magnesium exhibits central nervous system and neuromuscular effects that could enhance patient-ventilator synchrony. By blocking N-methyl-D-aspartic acid receptors and reducing acetylcholine release at the neuromuscular junction, magnesium imparts mild sedative and muscle relaxant properties (Wang et al., 2011; Laires et al., 2004). These effects may help prevent episodes of dyssynchrony in mechanically ventilated patients. By promoting a calmer respiratory drive and relaxing respiratory muscles, magnesium might reduce the need for deep sedation or paralytics to achieve ventilator synchrony. Supporting this, a clinical study demonstrated that adding magnesium infusion to standard sedatives in critically ill patients significantly shortened the duration of mechanical ventilation and decreased sedative requirements (Altun et al., 2019). Improved ventilator synchrony and earlier weaning can lead to fewer ventilator-associated complications (such as ventilator-induced lung injury, nosocomial pneumonia, and prolonged sedation delirium) and ultimately enhance survival. Furthermore, the anti-inflammatory and antioxidant properties of magnesium are particularly relevant in COPD exacerbations, which are often characterized by intense airway and systemic inflammation. Magnesium can inhibit the release of pro-inflammatory cytokines such as TNF-α and IL-6, thereby attenuating the inflammatory cascade (Aryana et al., 2014; Cross et al., 2018). In patients with sepsis, this immunomodulation has been associated with improved organ function and reduced mortality; similar benefits may be observed in COPD exacerbations, where inflammation contributes to respiratory failure (Jain et al., 2024). Magnesium also acts as a cofactor for enzymes involved in scavenging reactive oxygen species, thereby reducing oxidative stress and tissue damage in the lungs (Liu and Dudley, 2020). By mitigating inflammation and oxidative injury, magnesium can help protect against multiorgan dysfunction during severe exacerbations and enhance the overall resilience of the patient.
In summary, the multifaceted actions of magnesium sulfate, including bronchodilation, modulation of the respiratory drive, and anti-inflammatory and antioxidant effects, provide a strong theoretical basis for the observed mortality reduction in patients with mechanically ventilated COPD. It is likely to improve acute respiratory physiology (facilitating ventilation and oxygenation) while addressing the systemic factors (inflammation and oxidative stress) that influence recovery. Careful monitoring is imperative, and even as we harness the therapeutic effects of magnesium, we must avoid hypermagnesemia (>2.4 mg/dL, or >1.0 mmol/L), which can cause hypotension, bradyarrhythmia, and respiratory depression. When used judiciously, magnesium sulfate has emerged as a promising adjunct in the management of severe COPD exacerbations, especially in patients requiring invasive ventilation, by improving both the pulmonary and systemic conditions that determine their outcomes. This hypothesis, supported by our data, adds a new dimension to the discussion on magnesium in COPD care and encourages future prospective trials targeting this high-risk population.
Study limitations and future directions
This study has several limitations. First, its retrospective single-center design inherently carries the risk of selection bias, limiting its generalizability to other institutions or healthcare systems. Despite applying multiple propensity-score-based methods and calculating E-values, our results remained susceptible to residual confounding from unmeasured variables. Specifically, the E-value analysis indicated that an unmeasured confounder would require a risk ratio of at least 1.96 with both treatment allocation and outcome to fully explain the observed association, suggesting that a substantial hidden bias would be necessary to nullify our findings. Second, detailed data on the exact timing of magnesium sulfate administration relative to key interventions, such as the initiation of mechanical ventilation, were not systematically recorded, precluding a more granular temporal analysis. Additionally, the administered dose of magnesium sulfate was not standardized; most patients received approximately 2 g intravenously; however, variations in dosing and duration prevented a meaningful dose–response evaluation. Third, we only extracted baseline serum magnesium levels measured within the first 24 h of ICU admission without information on intratherapy or post-therapy levels. Consequently, we could not assess dynamic changes in serum magnesium levels or the incidence of hypermagnesemia over the course of treatment. Fourth, potentially relevant concomitant medications such as corticosteroids, bronchodilators, antibiotics, and diuretics were not consistently available in the database and were thus not included in our propensity score models. Furthermore, certain disease severity indicators beyond the OASIS score, such as PaO2/FiO2 ratios or detailed ventilator parameters, were either incomplete or unavailable. Although our machine learning-based covariate selection and propensity-based adjustments were designed to mitigate such imbalances, the absence of these variables may introduce bias. Finally, the observational nature of the study precludes causal inferences. The findings should be interpreted as hypothesis-generating rather than definitive evidence of benefits.
Future research should aim to validate these results in multicenter prospective trials that incorporate standardized dosing protocols, serial magnesium concentration monitoring, and detailed temporal mapping of administration relative to critical interventions. Such studies should also explore subgroup effects, particularly in mechanically ventilated patients and those with varying baseline magnesium levels or renal functions, to refine patient selection and optimize the efficacy and safety profile of magnesium sulfate for ICU-managed COPD exacerbations.
Conclusion
In this single-center retrospective cohort study, the early administration of magnesium sulfate in critically ill patients with COPD was associated with lower 28-day mortality. This association remained consistent across baseline serum magnesium levels and did not increase the risk of AKI. These findings imply a potential therapeutic role for magnesium sulfate in this population, particularly in severe cases necessitating intensive care. However, causal inferences cannot be drawn due to the observational design. Further multicenter prospective studies with standardized dosing, serial magnesium monitoring, and comprehensive subgroup analyses are needed to validate these associations and establish evidence-based guidelines for the optimal use of magnesium sulfate in critically ill patients with COPD.
Data availability statement
The raw data supporting the conclusions of this article are from a third-party dataset available from MIMIC-IV, a freely accessible critical care database. Reproduction of these data is not permitted according to the Data Use Agreement of the database, but access can be requested here: https://mimic.physionet.org/gettingstarted/access.
Author contributions
MX: Writing – original draft, Writing – review and editing, Conceptualization. MC: Writing – review and editing, Writing – original draft, Conceptualization. XD: Writing – original draft, Funding acquisition, Formal Analysis, Writing – review and editing. SL: Writing – review and editing, Writing – original draft, Funding acquisition, Conceptualization, Data curation, Formal Analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Project of the Affiliated Hospital of North Sichuan Medical College (2023ZD008), the Project of the Doctoral Initiation Fund (2023GC002), Scientific Research and Development Program Project (2024PTZK008), and Sichuan Province Clinical Key Specialty Construction Project (2023GZZKP002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altun, D., Eren, G., Cetingok, H., Hergünsel, G. O., and Çukurova, Z. (2019). Can we use magnesium for sedation in the intensive care unit for critically ill patients: is it as effective as other sedatives? Yoğun bakım ünitesinde magnezyum'u kritik hastalar için sedasyon amaçlı kullanabilir miyiz? Diğer sedatifler kadar etkili mi?. Agri 31 (2), 86–92. doi:10.14744/agri.2019.59244
Aryana, P., Rajaei, S., Bagheri, A., Karimi, F., and Dabbagh, A. (2014). Acute effect of intravenous administration of magnesium sulfate on serum levels of Interleukin-6 and tumor necrosis Factor-α in patients undergoing elective coronary bypass graft with cardiopulmonary bypass. Anesth. Pain Med. 4 (3), e16316. doi:10.5812/aapm.16316
Barbagallo, M., Veronese, N., and Dominguez, L. J. (2021). Magnesium in aging, health and diseases. Nutrients 13 (2), 463. doi:10.3390/nu13020463
Cross, S. N., Nelson, R. A., Potter, J. A., Norwitz, E. R., and Abrahams, V. M. (2018). Magnesium sulfate differentially modulates fetal membrane inflammation in a time-dependent manner. Am. J. Reprod. Immunol. 80 (1), e12861. doi:10.1111/aji.12861
Davey, M. J., and Teubner, D. (2005). A randomized controlled trial of magnesium sulfate, in addition to usual care, for rate control in atrial fibrillation. Ann. Emerg. Med. 45 (4), 347–353. doi:10.1016/j.annemergmed.2004.09.013
de Baaij, J. H., Hoenderop, J. G., and Bindels, R. J. (2015). Magnesium in man: implications for health and disease. Physiol. Rev. 95 (1), 1–46. doi:10.1152/physrev.00012.2014
Funk, G. C., Bauer, P., Burghuber, O. C., Fazekas, A., Hartl, S., Hochrieser, H., et al. (2013). Prevalence and prognosis of COPD in critically ill patients between 1998 and 2008. Eur. Respir. J. 41 (4), 792–799. doi:10.1183/09031936.00226411
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease Study 2015. Lancet 388 (10053), 1545–1602. doi:10.1016/S0140-6736(16)31678-6
GBD Chronic Respiratory Disease Collaborators (2017). Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir. Med. 5 (9), 691–706. doi:10.1016/S2213-2600(17)30293-X
Gu, W. J., Duan, X. J., Liu, X. Z., Cen, Y., Tao, L. Y., Lyu, J., et al. (2023). Association of magnesium sulfate use with mortality in critically ill patients with sepsis: a retrospective propensity score-matched cohort study. Br. J. Anaesth. 131 (5), 861–870. doi:10.1016/j.bja.2023.08.005
Gungor, S., Kargin, F., Irmak, I., Ciyiltepe, F., Acartürk Tunçay, E., Atagun Guney, P., et al. (2018). Severity of acidosis affects long-term survival in COPD patients with hypoxemia after intensive care unit discharge. Int. J. Chron. Obstruct Pulmon Dis. 13, 1495–1506. doi:10.2147/COPD.S159504
Haneuse, S., VanderWeele, T. J., and Arterburn, D. (2019). Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321 (6), 602–603. doi:10.1001/jama.2018.21554
Hess, D. R. (2013). Noninvasive ventilation for acute respiratory failure. Respir. Care 58 (6), 950–972. doi:10.4187/respcare.02319
Jahangir, A., Zia, Z., Niazi, M. R. K., Sahra, S., Sharif, M. A., Jahangir, A., et al. (2022). Efficacy of magnesium sulfate in the chronic obstructive pulmonary disease population: a systematic review and meta-analysis. Adv. Respir. Med. 90, 125–133. doi:10.5603/ARM.a2022.0012
Jain, A., Singam, A., and Mudiganti, VNKS (2024). Recent advances in immunomodulatory therapy in sepsis: a comprehensive review. Cureus 16 (3), e57309. doi:10.7759/cureus.57309
Johnson A. E. W., A. E. W., Bulgarelli, L., Shen, L., Gayles, A., Shammout, A., Horng, S., et al. (2023). MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10 (1), 1. doi:10.1038/s41597-022-01899-x
Johnson, A., Bulgarelli, L., Pollard, T., Gow, B., Moody, B., Horng, S., et al. (2024). MIMIC-IV (version 3.1). PhysioNet. doi:10.13026/kpb9-mt58
Knightly, R., Milan, S. J., Hughes, R., Knopp-Sihota, J. A., Rowe, B. H., Normansell, R., et al. (2017). Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst. Rev. 11 (11), CD003898. doi:10.1002/14651858.CD003898.pub6
Laires, M. J., Monteiro, C. P., and Bicho, M. (2004). Role of cellular magnesium in health and human disease. Front. Biosci. 9, 262–276. doi:10.2741/1223
Lei, Y., He, J., Hu, F., Zhu, H., Gu, J., Tang, L., et al. (2023). Sequential inspiratory muscle exercise-noninvasive positive pressure ventilation alleviates oxidative stress in COPD by mediating SOCS5/JAK2/STAT3 pathway. BMC Pulm. Med. 23 (1), 385. doi:10.1186/s12890-023-02656-5
Li, L., Li, L., Zhao, Q., Liu, X., Liu, Y., Guo, K., et al. (2024). High serum magnesium level is associated with increased mortality in patients with sepsis: an international, multicenter retrospective study. MedComm (2020) 5 (10), e713. doi:10.1002/mco2.713
Lin, S., Zhang, J., Dang, X., and Zhan, Q. (2025). Association of early enoxaparin prophylactic anticoagulation with ICU mortality in critically ill patients with chronic obstructive pulmonary disease: a machine learning-based retrospective cohort study. Front. Pharmacol. 16, 1588846. doi:10.3389/fphar.2025.1588846
Liu, M., and Dudley, S. C. (2020). Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants (Basel) 9 (10), 907. doi:10.3390/antiox9100907
Liu, B., Li, M., Wang, J., Zhang, F., Wang, F., Jin, C., et al. (2024). The role of magnesium in cardiac arrest. Front. Nutr. 11, 1387268. doi:10.3389/fnut.2024.1387268
Lorente, L., Blot, S., and Rello, J. (2007). Evidence on measures for the prevention of ventilator-associated pneumonia. Eur. Respir. J. 30 (6), 1193–1207. doi:10.1183/09031936.00048507
Ni, H., Aye, S. Z., and Naing, C. (2022). Magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 5 (5), CD013506. doi:10.1002/14651858.CD013506.pub2
Nouira, S., Bouida, W., Grissa, M. H., Beltaief, K., Trimech, M. N., Boubaker, H., et al. (2014). Magnesium sulfate versus ipratropium bromide in chronic obstructive pulmonary disease exacerbation: a randomized trial. Am. J. Ther. 21 (3), 152–158. doi:10.1097/MJT.0b013e3182459a8e
Nseir, S., Di Pompeo, C., Soubrier, S., Cavestri, B., Jozefowicz, E., Saulnier, F., et al. (2005). Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest 128 (3), 1650–1656. doi:10.1378/chest.128.3.1650
Panahi, Y., Mojtahedzadeh, M., Najafi, A., Ghaini, M. R., Abdollahi, M., Sharifzadeh, M., et al. (2017). The role of magnesium sulfate in the intensive care unit. EXCLI J. 16, 464–482. doi:10.17179/excli2017-182
Roussos, C., and Koutsoukou, A. (2003). Respiratory failure. Eur. Respir. J. Suppl. 47, 3s–14s. doi:10.1183/09031936.03.00038503
Santosh Raju, K., BhaskaraRao, J. V., Naidu, B. T. K., and Sunil Kumar, N. (2023). A study of hypomagnesemia in patients admitted to the ICU. Cureus 15 (7), e41949. doi:10.7759/cureus.41949
Saver, J. L., Starkman, S., Eckstein, M., Stratton, S. J., Pratt, F. D., Hamilton, S., et al. (2015). Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 372 (6), 528–536. doi:10.1056/NEJMoa1408827
Shivanthan, M. C., and Rajapakse, S. (2014). Magnesium for acute exacerbation of chronic obstructive pulmonary disease: a systematic review of randomised trials. Ann. Thorac. Med. 9 (2), 77–80. doi:10.4103/1817-1737.128844
Skorodin, M. S., Tenholder, M. F., Yetter, B., Owen, K. A., Waller, R. F., Khandelwahl, S., et al. (1995). Magnesium sulfate in exacerbations of chronic obstructive pulmonary disease. Arch. Intern Med. 155 (5), 496–500. doi:10.1001/archinte.1995.00430050072008
Touyz, R. M., de Baaij, J. H. F., and Hoenderop, J. G. J. (2024). Magnesium disorders. N. Engl. J. Med. 390 (21), 1998–2009. doi:10.1056/NEJMra1510603
VanderWeele, T. J., and Ding, P. (2017). Sensitivity analysis in observational research: introducing the E-Value. Ann. Intern Med. 167 (4), 268–274. doi:10.7326/M16-2607
Wang, H., Liang, Q. S., Cheng, L. R., Li, X. h., Fu, W., Dai, W. t., et al. (2011). Magnesium sulfate enhances non-depolarizing muscle relaxant vecuronium action at adult muscle-type nicotinic acetylcholine receptor in vitro. Acta Pharmacol. Sin. 32 (12), 1454–1459. doi:10.1038/aps.2011.117
Keywords: magnesium sulfate, COPD, critical care, prognosis, MIMIC-IV
Citation: Xiao M, Chen M, Ding X and Lin S (2025) Early administration of magnesium sulfate and its impact on clinical outcomes in ICU-admitted patients with COPD: a retrospective cohort study. Front. Pharmacol. 16:1616294. doi: 10.3389/fphar.2025.1616294
Received: 22 April 2025; Accepted: 26 August 2025;
Published: 04 September 2025.
Edited by:
Yonggang Zhang, Sichuan University, ChinaReviewed by:
John Sieh Dumbuya, Affiliated Hospital of Guangdong Medical University, ChinaNoorollah Tahery, Abadan University of Medical Sciences, Iran
Copyright © 2025 Xiao, Chen, Ding and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Lin, ZHIuc2hhbmxpbkBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Min Xiao1†
Min Xiao1† Shan Lin
Shan Lin