- 1School of Pharmacy and Pharmaceutical Sciences, The University of Queensland, Woolloongabba, QLD, Australia
- 2ARC Training Centre for Environmental and Agricultural Solutions to Antimicrobial Resistance (CEA-StAR), The University of Queensland, Brisbane, QLD, Australia
- 3Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD, Australia
The all-wine industry is projected to generate over US$528 billion in sales globally by 2025, and like many mass-producing industries, it too generates significant waste and by-products, much of which ends up in landfill. Among the various agricultural and industrial by-products, residues from winemaking stand out for their exceptionally rich and diverse bioactive compound content, primarily originating from grape skins, seeds and stems, all of which are rich in polyphenols, organic acids and tannins. These compounds have remarkable antioxidant, antimicrobial and anti-inflammatory properties and can therefore be diverted to agricultural, food preservation, cosmetic and pharmaceutical industries. The mechanism of action of the array of bioactive compounds includes disruption of microbial cell membranes, reduction of oxidative stress, and modulation of inflammatory responses. The current literature is limited to highlights of the scale of waste generated, and the application of its bioactive agents, however, it is notably absent of critical appraisal and discussion in sustainable avenues for development and value-added products, which are comprehensively elaborated herein.
1 Introduction
Grapes (Vitis vinifera) are a widely cultivated fruit globally, especially of significant economic value in the winemaking industry. According to preliminary figures from the International Organisation of Vine and Wine global wine production in 2023 peaked at almost 24 billion litres (Wine, 2024). The waste produced during the winemaking process (Figure 1) represents about 30% of the total weight of the grapes used (Genisheva et al., 2023). By-products mainly consist of pomace (a mixture of skins, seeds and stalks), which, depending on the process, represents about 20% of the total weight of the grapes used (Angelini et al., 2022). These by-products were once considered as waste, but in recent years there has been a growing interest among scientists and industry in transforming wine by-products. By-products contain many bioactive compounds, especially polyphenols such as phenolic acids, flavonoids, and tannins. They have antimicrobial, antioxidant and anti-inflammatory properties, which have significant health benefits (Miklasińska-Majdanik et al., 2018; Corrêa et al., 2017). These by-products present potent candidates for application in agriculture, livestock production, and winemaking and are a potential source of plant-based antibacterial substances (Ferrer-Gallego and Silva, 2022; Nascimento et al., 2000).
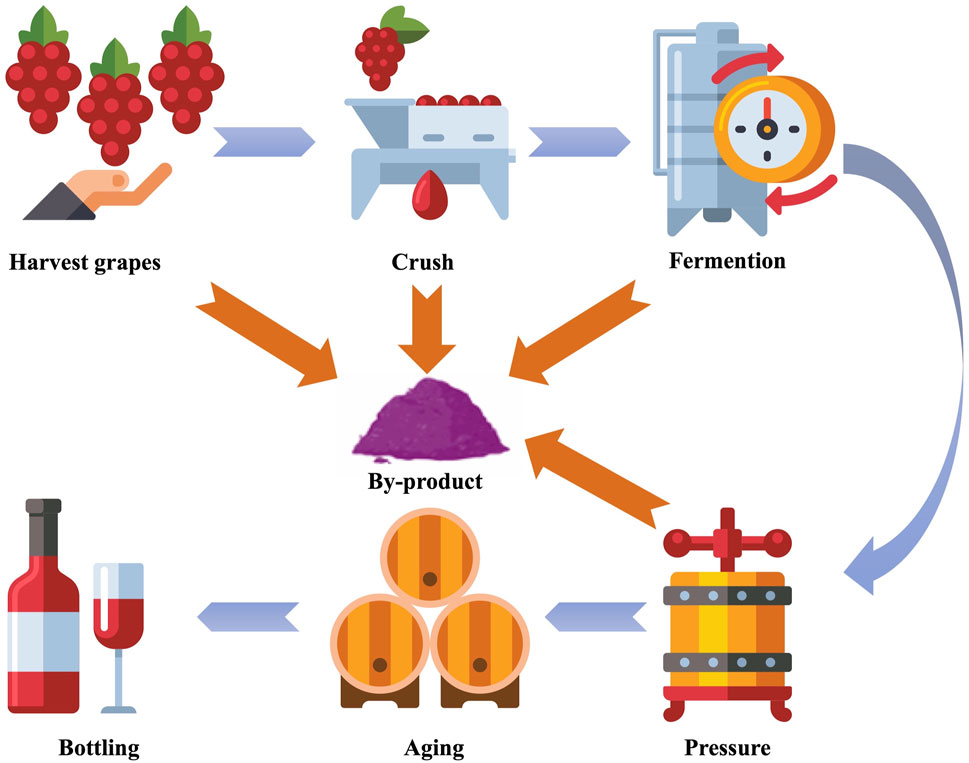
Figure 1. Schematic representation of wine production and by-product formation. Created with reshot.com.
Extensive research has been conducted on grape by-products to enhance human health. It is now becoming a promising solution in the veterinary field, showing potential in addressing the health and wellness challenges faced by the livestock industry (Baydar et al., 2004; Jayaprakasha et al., 2003).
The widespread prevalence of skin infections in domesticated animals and livestock is a cause for concern and the need for safe and effective treatments is on the rise (Joosten et al., 2020; Schrader et al., 2020). Animal skin infections are usually caused by bacterial pathogens such as Staphylococcus aureus (S. aureus), Streptococcus spp., and Pseudomonas aeruginosa (P.aeruginosa), causing serious health problems and significant economic losses to the livestock industries (Scharschmidt and Fischbach, 2013). Conventional treatment usually relies on antibiotics, but the effectiveness of antibiotics is gradually decreasing due to misuse and overuse, leading to antimicrobial resistance (AMR)-a global threat to human and animal health. For this reason, researchers are turning to natural plant alternatives to discover effective solutions that minimize the risk of AMR.
This review highlights the potential of grape by-products as an eco-friendly, sustainable, and naturally sourced alternative for controlling skin infections in animals. By summarizing existing knowledge on the bioactive compounds present in grape by-products, their antibacterial and anti-inflammatory mechanisms and formulation options for practical applications, this review aims to provide a comprehensive resource for future research and product development. Furthermore, it addresses the regulatory and standardization challenges associated with plant-based treatments, while assessing their applicability in large-scale animal healthcare. This exploration is intended to highlight the promise of grape by-products in not only improving animal health outcomes but also contributing to more sustainable livestock practices.
2 Structural basis and pathological implications of skin infections in domesticated animals and livestock
2.1 Structure and function of animal skin
The skin is the largest organ covering the entire body surface of humans and animals. It consists of three main layers: the epidermis, the dermis and the subcutaneous tissue (Souci and Denesvre, 2021). The structure of the skin is represented in Figure 2. The skin is the body’s first line of defence, protecting it from microorganisms, chemicals, and physical damage. The skin prevents the loss of water, electrolytes, and large molecules through the stratum corneum. As a barrier, it provides a stable internal environment for all other organs (Dehdashtian et al., 2018). The skin also plays crucial roles in sensation, temperature regulation, immune surveillance, secretion and excretion, and the production of vitamin D (Scott et al., 2003). Although the skin’s inherent functions enable it to withstand environmental stimuli, it remains susceptible to various forms of damage, compromising its structural and functional integrity. When the skin barrier is compromised, the risk of pollutants (pathogens) entering the body increases, heightening the likelihood of infection (Jonidi Shariatzadeh et al., 2025).
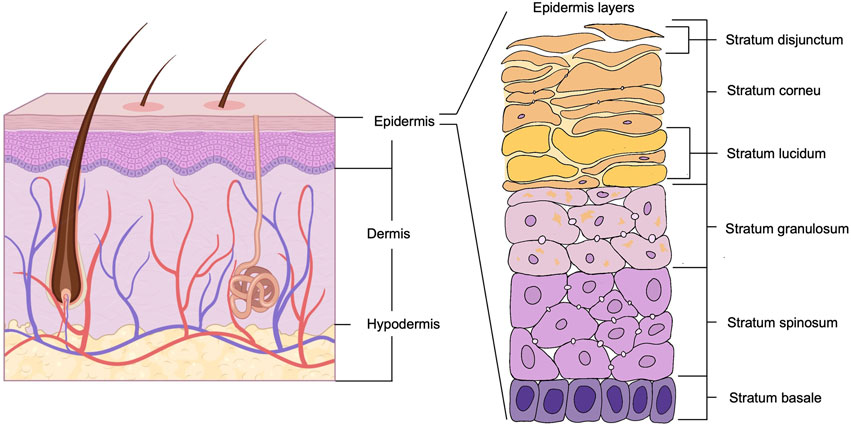
Figure 2. Schematic diagram of the multilayered structure of the skin. Created with BioRender.com.
2.2 Microorganisms hosted on animal skin
Due to its structural characteristics, skin provides an environment conducive to the growth and reproduction of microorganisms. It plays host to millions of bacteria, fungi, viruses, and mites that form the skin’s microbiome (Roth et al., 1988), which plays an important role in protecting the host from pathogens, maintaining skin homeostasis, and promoting immune system development (Byrd et al., 2018; Christensen and Bruggemann, 2014). The microbial diversity on animal skin is much higher than that of humans, with the microbiome of parasitic species on an animal’s skin being influenced primarily by the host’s biological classification and geographic location. Different body parts also exhibit variations in microbial species (Ross et al., 2018). The skin can regulate bacterial composition, with some bacteria becoming dominant. For example, common bacteria found on farm animal skin include Corynebacterium, Staphylococcus, Aerococcaceae, Psychrobacter, Micrococcus, Pseudomonas, Bacillus, and Acinetobacter (Porcellato et al., 2020; O’Shaughnessy-Hunter et al., 2021). They constitute a natural microbiota that protects against pathogens. Companion animals’ skin is dominated by Proteobacteria, followed by Actinobacteria, Firmicutes, Bacteroidetes, and Fusobacteria, which also play important roles in skin health and immune responses (Older et al., 2017; Older et al., 2023). The majority of animal skin infections are caused by coagulase-positive Staphylococci, which are common bacterial pathogens responsible for various skin and soft tissue infections (Foster, 2012).
When the skin’s microbiome is disrupted by external factors (e.g., dysbiosis, reduced bacterial diversity), it negatively impacts skin health, leading to the development and progression of disease (Scharschmidt and Fischbach, 2013; Grice, 2015; Kong and Segre, 2012). In farm and companion animals, infections caused by skin damage are very common (Faccin et al., 2023) Table 1 lists common bacterial infections that lead to animal skin diseases.
2.3 Effects of bacterial infection on the epidermis
The epidermis is the outermost layer of the skin. According to its function and stratification, it is divided into five layers from the outside in: Stratum Corneum, Stratum Lucidum, Stratum Granulosum, Stratum Spinosum and Stratum Basale. Its primary cell type is the keratinocyte (Venus et al., 2010). The detailed structure of the epidermis is shown in the enlarged portion of Figure 2. The stratum corneum is composed of anucleated keratinocytes and lipid matrix (Menon et al., 2012), which has a high barrier function and is the first line of defence against water loss and invasion of external pathogens (Walters and Roberts, 2002). The spinous layer contains Langerhans cells, which are involved in the identification and removal of microorganisms and contribute to the local immune defence of the skin (Hibbs and Clark, 1959). The basal layer contains continuously dividing keratinocytes and melanocytes (Scott et al., 2003), which are involved in skin renewal and ultraviolet (UV) light protection (Plonka et al., 2009; Tapia et al., 2014).
When the epidermal barrier is compromised by trauma, moisture imbalance or immune deficiency, bacteria can easily colonize it and cause infection (Baquero et al., 2021). Common bacterial infections of the epidermis in animals include superficial pyoderma, pustules, and folliculitis, often caused by S. pseudintermedius or S. aureus in other species (Bloom, 2014). These infections usually present as pustules, papules, or epidermal ring of desquamation with clinical signs of itching, erythema, and desquamation (Loeffler and Lloyd, 2018). Although these infections may appear to be minor on the surface, they may develop into deeper dermal or subcutaneous tissue infections if not treated promptly. Chronic or recurrent epidermal infections are also often closely associated with underlying conditions such as atopic dermatitis, endocrine disorders or immunosuppression (Seckerdieck and Mueller, 2018).
2.4 Bacterial infections affecting the dermis
The dermis connects externally to the epidermis and internally to the subcutaneous tissue. It is rich in collagen and elastic fibers, which, together with connective tissue, provide the skin with elasticity and strength (Findlay et al., 2017). The dermis consists of two layers: the superficial papillary dermis and the deeper reticular dermis. It mainly consists of a collagen-based matrix containing a variety of cells, including fibroblasts, endothelial cells, and immune cells (such as macrophages, dendritic cells, lymphocytes, etc.) (Nguyen et al., 2009). The dermis encloses specialized structures like capillaries, nerve endings, sweat glands, and hair follicles, which nourish the skin through blood circulation. When bacterial pathogens breach the epidermal barrier, they invade the dermis, leading to more serious and potential for systemic infections (Chen et al., 2018). Common dermal bacterial infections in animals include abscesses and deep pyoderma, usually caused by anaerobic bacteria such as Bacillus spp., S. spp., and Corynebacterium spp (Lappin, 2012). These infections are typically characterized by pain, swelling, fever, ulceration, and localized bleeding (Loeffler and Lloyd, 2018). In some cases, systemic symptoms such as fever, loss of appetite, and lethargy may also occur, predisposing to sepsis (Mauldin and Peters-Kennedy, 2015).
2.5 Bacterial infections affecting subcutaneous tissue
The subcutaneous tissue, the deepest layer of the skin located beneath the dermis, is mainly composed of fat cells, collagen fibers, and loose connective tissue. It also contains blood vessels, sensory and motor nerves, and lymphatic vessels. The subcutaneous tissue plays a vital role in connecting the skin to underlying muscles and bones, as well as supporting important functions such as energy storage, insulation, and cushioning against external forces (Bichakjian et al., 2007). When this layer of tissue is disrupted by bite wounds, contamination from injections, surgical complications, or systemic immunosuppression, it becomes susceptible to bacterial invasion (Raskin, 2015). Subcutaneous infections in animals usually present as more serious conditions such as necrotising fasciitis, depending on the depth and spread of the infection. Common causative organisms include S. spp. and various anaerobic bacteria (e.g., Clostridium spp.) (Quilli et al., 2022). Unlike epidermal infections, subcutaneous infections usually require systemic antimicrobial therapy and surgical intervention such as drainage or debridement (Nolff and Meyer-Lindenberg, 2015). In addition, antimicrobial resistance of deep-tissue pathogens poses an increasing therapeutic challenge, thus raising concerns.
3 Conventional skin treatment methods
The use of antimicrobials and antibiotics is the main approach to treating animal skin infections. Gram-positive bacteria are responsible for most skin and soft tissue infections, especially coagulase-positive Staphylococci, with S. aureus and S. pseudintermedius being the most common pathogens (Faccin et al., 2023). Dependent on the infection site and severity, when topical antimicrobials cannot clear the infection, local antibiotic therapy or systemic treatment is used - Tables 2, 3 show the common treatment methods (Beco et al., 2013). Data on antibiotic dosage were obtained from Merck Veterinary Manual (Mercer, 2022).
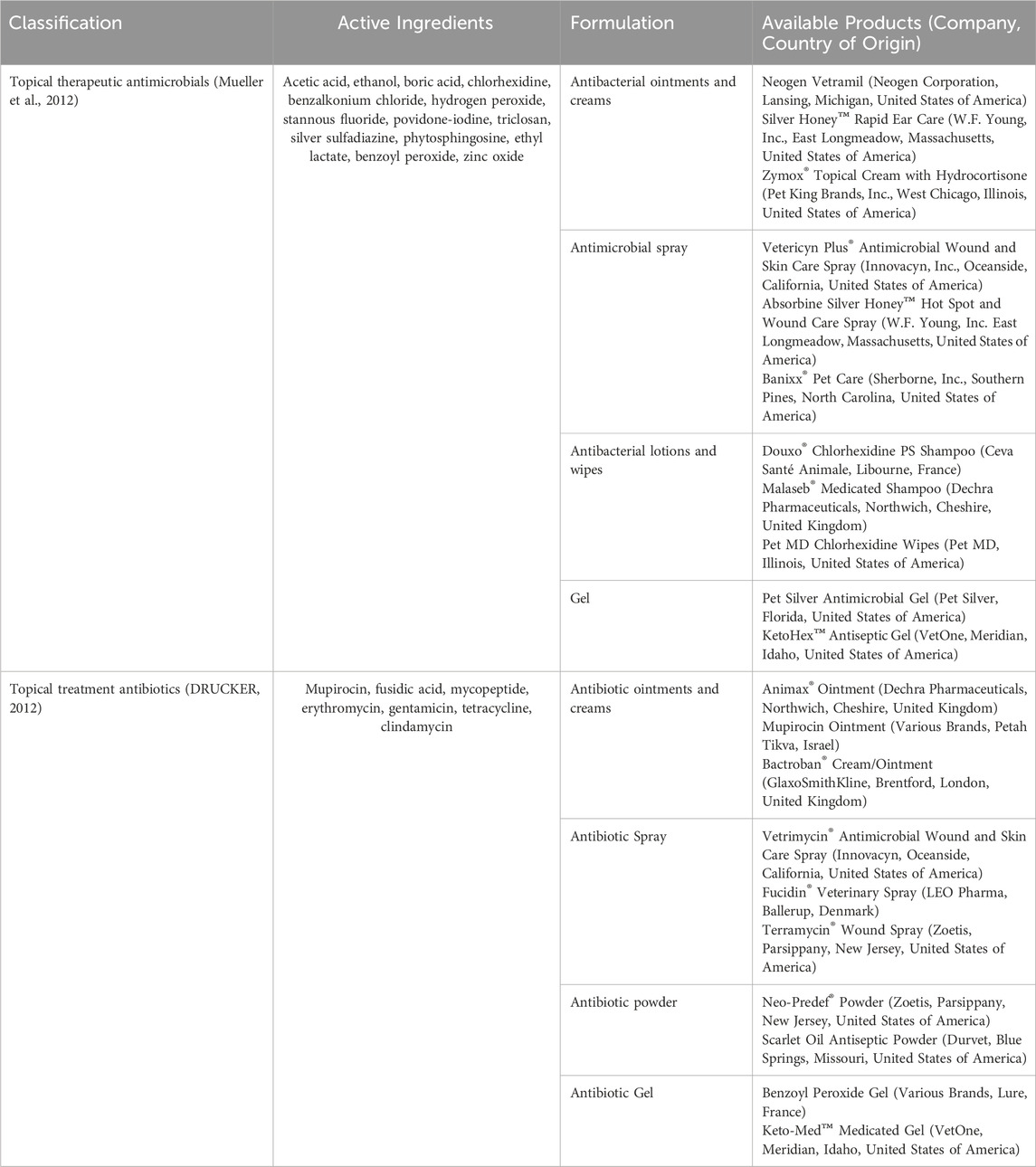
Table 2. Topical antimicrobials commonly used to treat bacterial skin infections in animals, topical antibiotics and products.
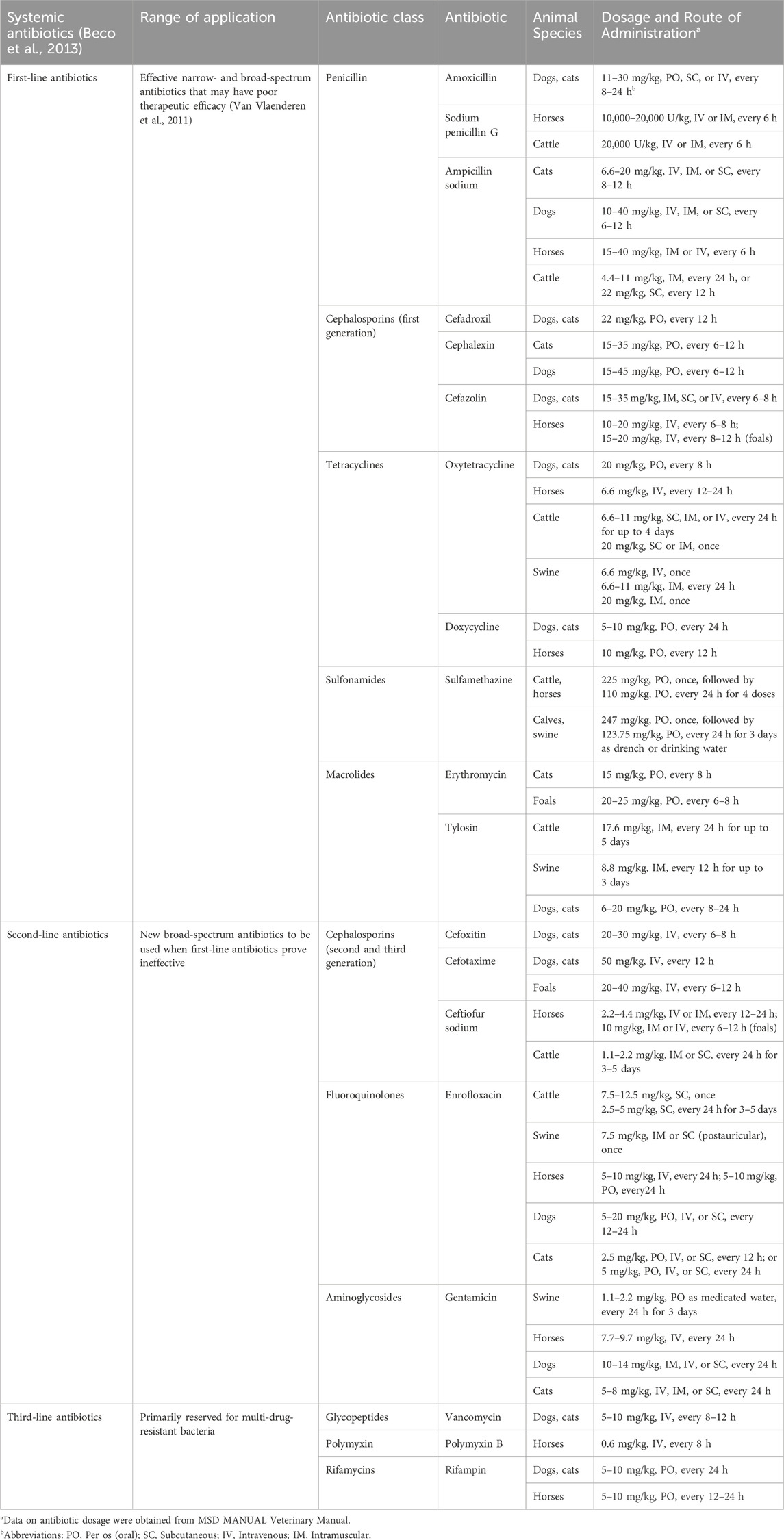
Table 3. Systemic antibiotics, their scope of use in animals along with dosage and administration routes.
Currently, improving the prevention of animal disease, treating infections, and promoting growth to target weights, heavily rely on the use of antibiotics (Woolhouse et al., 2015). Most of the world’s antibiotics are used in animals, not humans. In fact, as of 2017, about 73% of all antibiotics sold globally were used in livestock and other food animals. This reflects the animal industry’s heavy reliance on antibiotics to maintain health and productivity levels. Global consumption of veterinary antibiotics is significant and continues to grow: in 2020, ≈99,000 tonnes of antibiotics were used in animal feed, and, based on current trends, this is expected to increase to more than 8% per year by 2030 (Mulchandani et al., 2023). However, due to their improper use of antibiotic treatments, bacterial pathogens with antibiotic resistance genes have inevitably evolved (Martínez, 2012). In addition to antibiotics used for treatment purposes, the use of antibiotics as antimicrobial growth has greatly contributed to the development of antibiotic resistance in animals (van den Bogaard and Stobberingh, 1999). The spread of resistant bacteria subsequently raises public health issues for humans and the environment, causing significant pressure on animal welfare and the economy. According to the World Organisation for Animal Health, livestock-resistant infections alone could result in an annual loss of up to $1.7 trillion in global gross domestic product by 2050, and up to $5.2 trillion when the transmission of drug-resistant pathogens from livestock to humans is taken into account (through food, direct contact or environmental transmission) (Health WOfA, 2024).
Importantly, the impact of AMR is not limited to food animals; this applies to companion animals (pets) also. Dogs, cats and other pets are treated with antibiotics for infection and pre/post-surgery, and drug-resistant bacteria have become a serious problem in the field of companion animal medicine (Pomba et al., 2016). Antibiotic-resistant infections in pets can lead to long-term illnesses that are both difficult and expensive to treat. In many cases, drug-resistant infections in dogs or cats require extended hospital stays, multiple visits to the veterinarian, and expensive alternative medications or therapies. In addition, pets can be hosts or vectors for drug-resistant bacteria - for example, methicillin-resistant S. aureus (MRSA) and other superbugs can be transmitted between pets and people in the home, further complicating public health efforts (Caneschi et al., 2023). Antimicrobial resistance therefore encompasses both livestock and companion animals, reinforcing the concept of “one health”, where the health of animals, humans and ecosystems are interconnected.
Quantifying antibiotic use, reducing its usage, and finding alternatives are currently the most promising methods to manage antibiotic resistance. Among them, plant-derived bioactives (e.g., polyphenols extracted from winemaking by-products) are gaining attention due to their natural origin, lower risk of resistance development and multifunctional properties.
However, most of these alternatives are still at an early stage of validation and only very few have been converted to veterinary topical formulations. This gap highlights the need for continued innovation and formulation research to develop targeted, animal-safe and environmentally-friendly skin treatment products.
4 Biodiversity of wine by-products
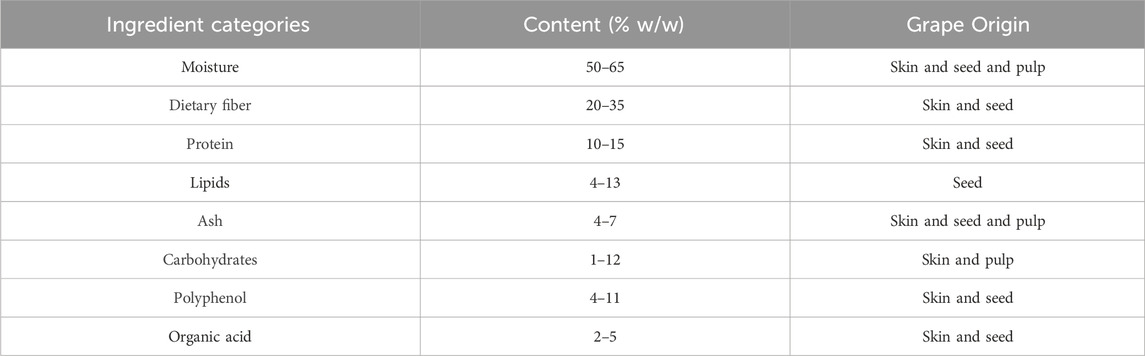
Wine by-products mainly include grape pomace (skins, seeds, pulp and a small amount of grape stems), which are rich and varied in composition and have a high utilization value (Table 4). These by-products contain a large number of polyphenolic compounds, dietary fibers (soluble fibers, insoluble fibers) lipids (mainly unsaturated fatty acids such as linoleic acid and oleic acid) and vegetable proteins. They also contain some organic acids (e.g., tartaric acid, malic acid) and small amounts of residual monosaccharides (glucose and fructose). Minerals such as potassium, calcium and magnesium and trace elements are also present in these by-products (Karastergiou et al., 2024; Nirmal et al., 2025; Jin et al., 2019; Silva et al., 2024).
4.1 Polyphenols in winemaking by-products: composition, bioactivity, and potential applications in skin health
Winemaking is a significant agricultural and industrial activity worldwide, generating large amounts of by-product. The biodiversity in these by-products is influenced by the grape variety, cultivation methods, and the winemaking process. Due to nuances in winemaking techniques, by-products from red and white wine production contain distinct phenolic compounds (Oliveira and Duarte, 2016).
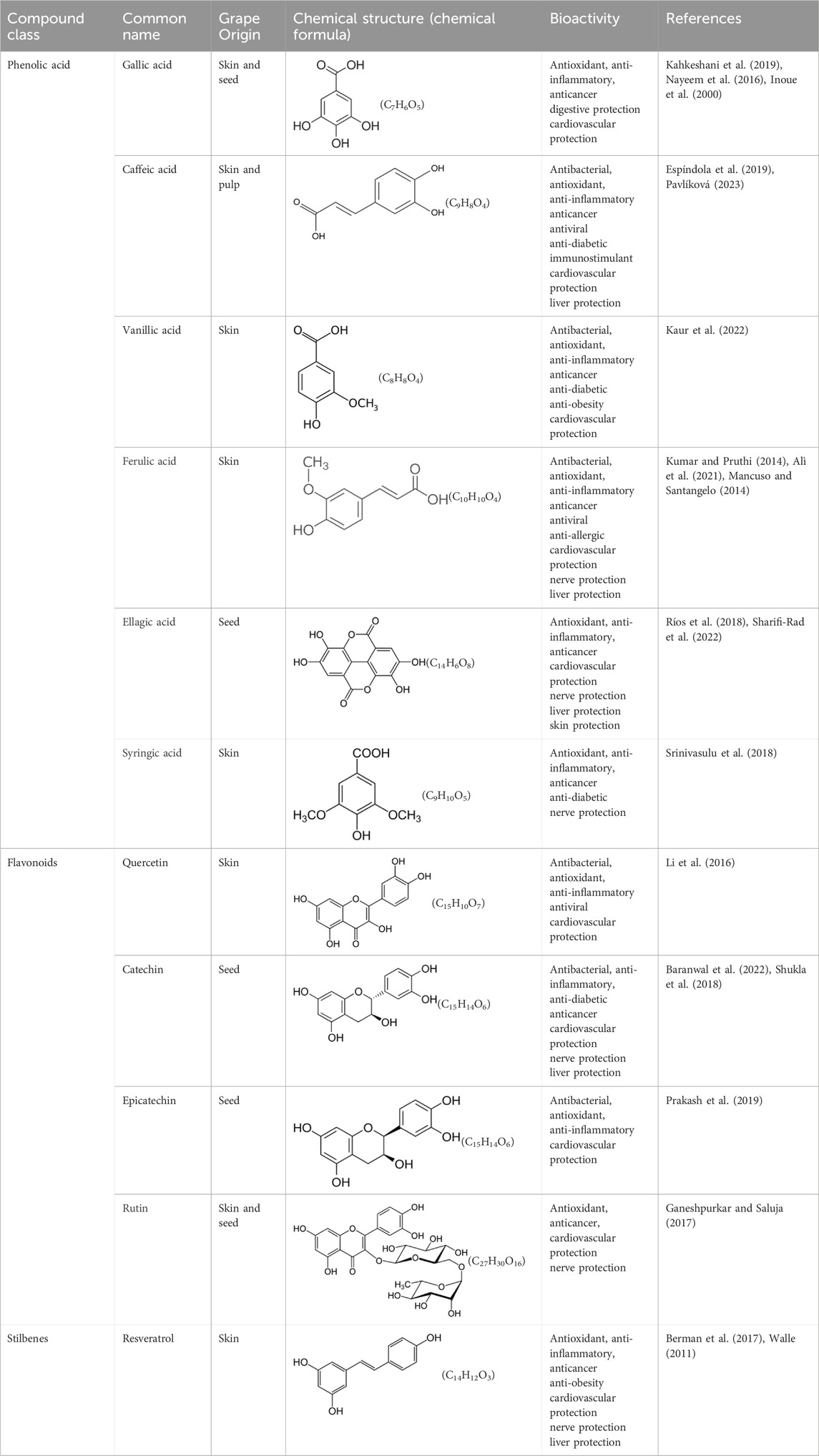
Red wine is typically made from dark-skinned grape varieties (such as Cabernet Sauvignon, Merlot, Pinot Noir), where the skins and juice ferment together. White wine is usually produced from lighter-coloured grape varieties (such as Chardonnay, Sauvignon Blanc), and the grape pomace from white wine production does not undergo maceration, resulting in much lower anthocyanin (which gives the red colour to grape skins) content compared to red wine (Mosele et al., 2023). Grape pomace contains abundant plant secondary metabolites, specifically polyphenols, which make up about 10% of the dry weight of grape pomace (Karastergiou et al., 2024), These phenolic compounds are predominantly found in the seed (60%–70%), followed by skin (30%–35%), and flesh (10%) (Milinčić et al., 2021; Cotea et al., 2018). Most polyphenols in grapes belong to the flavonoid class, and they also include phenolic acids and stilbenes (Hegedüs et al., 2022; Kersh et al., 2023; On et al., 2022). Table 5 below outlines the common polyphenolic agents and their bioactivity as found in grape pomace.
Due to the different brewing processes of red and white wines, especially the pomace fermentation of red wines (Bocsan et al., 2022), the content of polyphenols in red wines is usually higher than that of white wines, resulting in significant differences in antioxidant activity and cardiovascular health (Fernández-Mar et al., 2012; Onache et al., 2023; Di Lorenzo et al., 2016). Polyphenolic compounds in red wine are effective in scavenging free radicals and protecting cells from damage caused by oxidative stress (Vejarano and Luján-Corro, 2022). Components such as resveratrol and quercetin can lower blood pressure, improve blood circulation and prevent atherosclerosis (Zhang et al., 2023). Polyphenols and tannins have an anti-inflammatory effect and help relieve chronic inflammation. Although white wines are lower in polyphenols, they still contain free radical reducing antioxidants (Bertelli et al., 2002). The acids found in white wines (such as tartaric and malic acids) help in the production of stomach acid, which promotes digestion. Its antimicrobial properties also help to maintain intestinal health (Tarko et al., 2020).
Numerous studies have characterized these compounds and underscored their strong antioxidant, anti-inflammatory, and antimicrobial properties. These bioactivities are highly relevant to skin health, where oxidative stress and microbial colonization are key contributors to the development of common conditions such as skin inflammation and wound infections. In vitro studies have demonstrated that polyphenols like pentagalloyl glucose and epigallocatechin gallate can effectively reduce oxidative stress and promote elastin deposition in the extracellular matrix, supporting skin structure and repair (Chowdhury et al., 2021). Polyphenols such as quercetin and resveratrol can rapidly exert antioxidant effects on the skin, mitigating oxidative stress at sites of inflammation by reducing reactive oxygen species activity within the biological barrier (Eskandari et al., 2019). One of the main uses of these polyphenols is the prevention and treatment of bacterial skin infections. Due to frequent exposure of the skin to a variety of pathogens, bacterial infections remain a significant and persistent health challenge. By understanding the structure of the skin and focusing on specific layers and regions that are susceptible to infection, the potential of polyphenol-based interventions for treating skin infections can be better assessed, utilising the unique bioactivities of winemaking by-products to support and maintain skin health.
5 Antimicrobial properties of grapes/wine by-products
Grapes contain a variety of secondary metabolites with antimicrobial properties, including phenolic acids, flavonoids and astragals, which impart a unique flavour to grapes and red wines. The antimicrobial components in grapes are mainly found in the pericarp, seeds and stems. They play a crucial function in plant defence against microbial attack and adaptation to environmental stresses. The diversity and complex structures of these compounds show a wide range of applications in the fields of food preservation, medicine and nutraceuticals. Thus, the antimicrobial activity of grapes and their by-products not only provides a direction for the reuse of their by-products but also offers a potential resource for the development of natural antimicrobial agents.
5.1 Major antimicrobial compounds in grapes and wine by-products
5.1.1 Phenolic acid
Phenolic acids are polyphenolic compounds containing hydroxy acid groups, abundant in plants (including grapes and their by-products). They are renowned for their antioxidant, anti-inflammatory, and antimicrobial properties, benefiting both human health and industrial applications. In grapes, phenolic acids are primarily found in the skin, seeds, and stems, playing a significant role in the health benefits of grape products such as wine and grape extracts. Phenolic acids are mainly classified into two types: hydroxybenzoic acids (such as gallic acid, vanillic acid, and syringic acid) and hydroxycinnamic acids (such as caffeic acid, ferulic acid, p-coumaric acid, and sinapic acid). Phenolic acids are synthesized through the shikimate and phenylpropanoid pathways, which are essential for producing a wide range of plant secondary metabolites (Al et al., 2018; Kumar and Goel, 2019).
5.1.2 Flavonoid
These are secondary metabolites widely present in various parts of plants, with most originating from the pigmented portions of plants. To date, thousands of flavonoid compounds have been identified in a wide range of sources, including fruits, vegetables, flowers, grains, seeds, nuts, herbs, spices, and plant-derived beverages such as tea, coffee, and wine (Daglia, 2012). Flavonoid compounds are characterized by a C15 carbon skeleton with a diphenylpropane structure. The structure includes two benzene rings (rings A and B) linked by a linear three-carbon chain. This central three-carbon chain forms a closed pyran ring (ring C) with the A ring, resulting in a chroman ring structure (the fusion of ring A with the pyran ring C) attached at positions 2, 3, or 4 to the second benzene ring (ring B). This structure defines the various types of flavonoid compounds (Rana and Gulliya, 2019; Kumar and Pandey, 2013). Flavonoids can be categorized into several subclasses—flavanols, flavones, flavanones, anthocyanins, flavanols, and isoflavones—based on the oxidation state of the central pyran ring. In nature, flavonoids protect plants from environmental stressors and serve as powerful antimicrobials or immune enhancers, helping plants defend against various pathogens (Górniak et al., 2019).
5.1.3 Stilbenes
These compounds are synthesized in plants via the phenylpropanoid pathway (Figure 3), beginning with the conversion of phenylalanine to cinnamic acid, which is then activated to cinnamoyl-CoA. Under the catalysis of stilbene synthase, cinnamoyl-CoA condenses with malonyl-CoA to form the stilbene backbone, such as resveratrol (Di et al., 2012; Kaur et al., 2024).
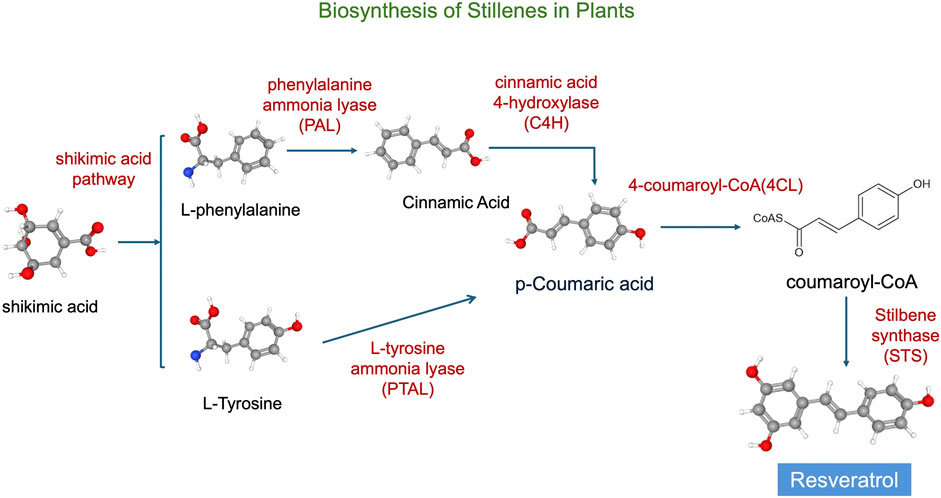
Figure 3. Synthesis pathways of stilbenes. The chemical structures in the figure are adapted from PubChem (National Center for Biotechnology Information, 2025).
In addition, stilbene compounds effectively absorb or scatter UV radiation, reducing photo-oxidative damage (Niesen et al., 2013). Stilbene compounds undergo modifications such as glycosylation, methylation, prenylation and oxidative coupling to further enhance their antioxidant and antimicrobial activities. This synthetic pathway is stimulated under the influence of external factors (e.g., UV radiation, pathogen infection), which increases the accumulation of streptavidin compounds (Akinwumi et al., 2018). Stilbene compounds provide a variety of protective functions to plants under conditions of environmental stress (e.g., drought, low temperature, and UV radiation). They inhibit pathogens such as fungi and bacteria, helping plants to resist infections.
5.2 Mechanisms of antimicrobial action
Polyphenolic compounds in grapes and their derivatives have a wide range of antimicrobial activities. Their antimicrobial mechanisms include disruption of cell membranes, inhibition of biofilms, interference with nucleic acid synthesis, alteration of membrane permeability, inhibition of toxins and restriction of movement (Figure 4). The following section describes these mechanisms in detail.
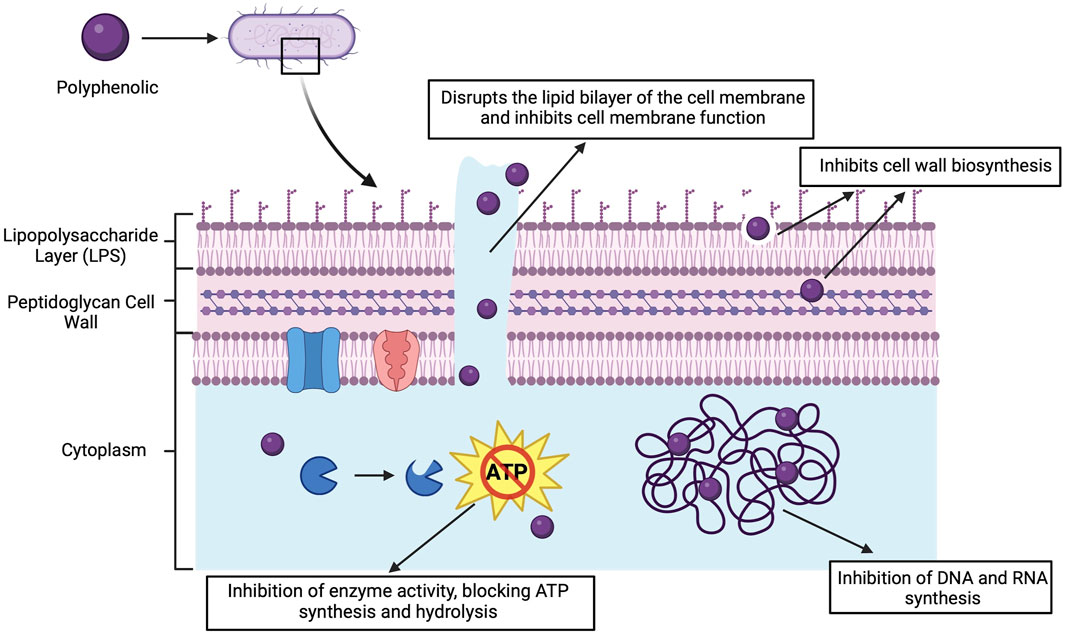
Figure 4. Antimicrobial mechanisms of polyphenolic compounds. Created with BioRender.com.
5.2.1 Destruction of cell membranes
Polyphenolic compounds, represented by flavonoids, can directly insert into the lipid bilayer structure of bacterial cell membranes. Altered the stability of the membrane by insertion, making its structure loose and unstable and destroying the selective permeability of the membrane (Kumar et al., 2021). Leakage of cellular contents (e.g., ions, proteins, and nucleic acids) can occur as a result of damage to the cell membrane. This further affects the osmotic balance and energy metabolism of the bacteria, ultimately leading to bacterial inactivation or death.
Specifically, gallic and ferulic acids induce a non-polar character in P.aeruginosa. Under the influence of phenolic acid, electron acceptors on the surface of Gram-positive bacteria are increased, while the opposite effect is observed for Gram-negative bacteria. At concentrations up to 1,000 μg/mL, phenolic acids significantly enhance membrane damage and cellular content release in bacteria such as E.coli, S. aureus, Listeria monocytogenes (L.monocytogenes), and P.aeruginosa (Borges et al., 2013). In addition, the fatty acid synthase type II (FAS-II) pathway is essential for cell membrane synthesis in Gram-negative bacteria. Various flavonoids such as quercetin, populin, baicalein, which can effectively inhibit FAS-II can prevent the synthesis of phospholipids and lipopolysaccharides by bacteria, thus thwarting cell membrane formation (Li and Tian, 2004).
5.2.2 Inhibits the activity of a number of enzymes
Certain compounds in grapes inhibit toxins and degradative enzymes secreted by bacteria. By limiting the function of these toxins and enzymes, it is possible to reduce bacterial attack on host cells and prevent further development of the infection. Resveratrol reversibly binds to adenosine triphosphate (ATP) synthase and can inhibit ATP hydrolysis and synthesis processes in E. coli, thereby affecting its energy metabolism. This effect was particularly pronounced in the presence of non-fermentable carbon sources (e.g., succinate), which showed a significant limitation of bacterial growth, and in the presence of fermentable carbon sources (e.g., glucose), suggesting an inhibitory effect on the oxidative phosphorylation process (Dadi et al., 2009). Quercetin, catechin and EGCG inhibit bacterial DNA rotamase and block nucleic acid synthesis by binding to the ATP-binding site of the B subunit of the promoter of rotamase (Gradišar et al., 2007).
5.2.3 Inhibition of biofilm formation
The active ingredients in grapes inhibit bacterial adhesion and biofilm maturation. Biofilms are highly organised communities of microorganisms that are widely found in nature. They can form protective structures on human surfaces or medical devices. This protective layer not only provides a stable internal environment for microorganisms to live in, but also greatly enhances their ability to adapt to external stresses (Kang et al., 2023). For example, biofilm formation enhances the resistance of microorganisms to antibiotics and disinfectants, while also enabling effective resistance to mechanical vibration and physical removal (Alonso et al., 2023). Antimicrobial ingredients limit the growth and spread of bacteria by preventing the formation of biofilms, destroying their structure or reducing their stability (Borges et al., 2016). Studies have shown that flavonoids in red wine inhibit the formation of S. aureus biofilms by binding to specific sites in bacterial cell membrane proteins through hydrogen bonding (Cho et al., 2015). Polyphenol extract from Rosa rugosa tea is effective in inhibiting E. coli and P. aeruginosa quorum sensing and biofilm formation (Zhang et al., 2014). Resveratrol can inhibit the biofilm of E. coli at concentrations 2–6 times lower than the minimum inhibitory concentration (MIC), and inhibit the biofilm formation of S. aureus at concentrations 3–4 times lower than the MIC (Lee et al., 2013). Resveratrol disrupts biofilm formation by affecting the expression of genes involved in quorum sensing, surface and secretory proteins, and extracellular polysaccharides essential for biofilm structure (Qin et al., 2014).
5.3 Factors influencing antimicrobial efficacy of wine by-products polyphenols
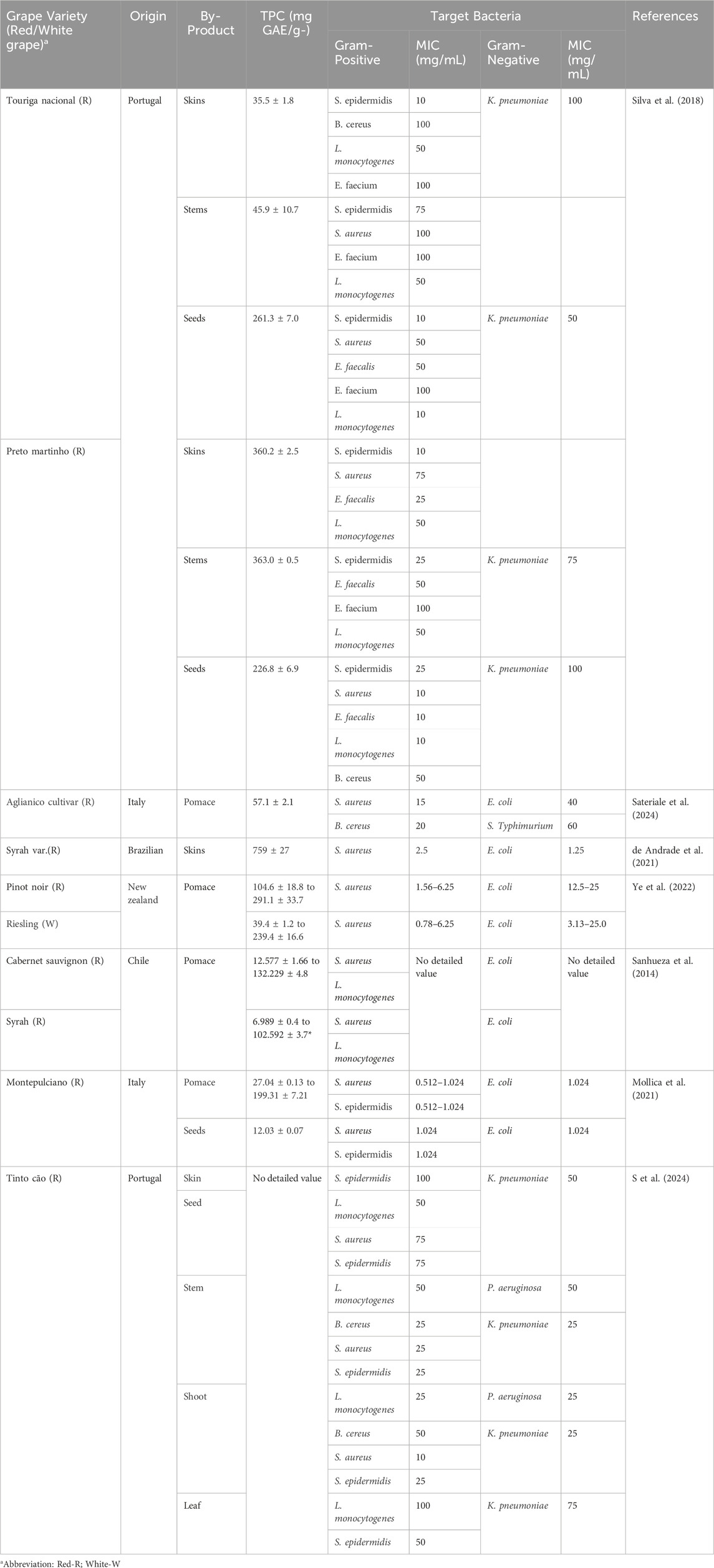
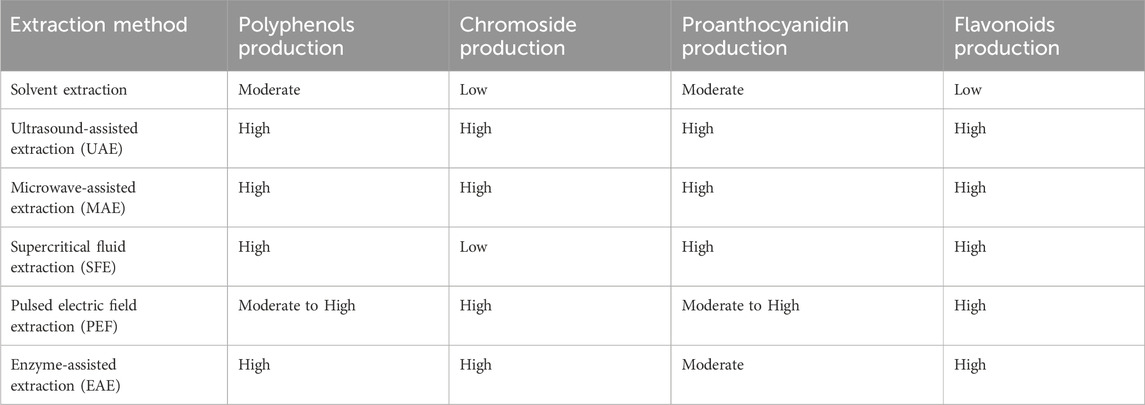
The amount and type of phenolic compounds in grapes (as the main raw material for wine production) and wine (as the final product), as well as in wine by-products (as production waste), are related to several factors. It is influenced by specificities including the variety of grape, growth and vinification, and ageing processes (Xia et al., 2010). Plant polyphenols demonstrate significant antimicrobial activity, and their efficacy varies depending on the type and concentration of polyphenol. The polyphenols recovered from dregs also vary depending on the technology, with dregs containing total phenols ranging from 1,200–4500 mg/L (Landeka et al., 2017; Dóka et al., 2023). Table 6 below lists the antibacterial power of some wine by-products against different bacteria.
In summarizing the aforementioned studies presented in Table 5, the climatic conditions of grape growing regions significantly affect the accumulation of flavonoids in their skin. The diurnal temperature difference affects the composition of compounds such as hydroxy anthocyanins and flavanols, while insufficient water increases the accumulation of anthocyanins (Zhu et al., 2017). Due to the paucity of studies on the antimicrobial activity of wine pomace and the multitude of research methods and research objectives, the correlation between the antimicrobial active compounds in wine pomace and their effects on bacteria has not yet been fully investigated. In particular, the types and content of antimicrobial compounds in wine pomace vary greatly depending on the extraction solvents, extraction procedures, part of the pomace contained, and grape variety have been found to affect the yield, polyphenol composition and antimicrobial activity of the extracts (Cheng et al., 2012; Oliveira et al., 2013). Therefore, it is difficult to draw uniform conclusions from the analysis of the available research data. The antimicrobial activity of phenolic compounds in grape pomace depends not only on the concentration but also on the specificity of the phenolic compounds (Xu et al., 2014).
Extracts isolated from common grape varieties (Table 6) showed strong inhibitory effect on Gram-positive bacteria (S. aureus, S. epidermidis, B. cereus). However, the inhibitory effect on Gram-negative bacteria such as E. coli was weak, and higher concentrations of the extracts were required to achieve an approximate effect. Gram-negative bacteria have a unique cellular structure and defence mechanism. Lipopolysaccharides make up the outer membrane of their cell wall, providing a sturdy shield against penetration of lipophilic solutions, a structure that Gram-positive bacteria lack. In addition, Gram-negative bacteria have an efflux pump system that expels the antimicrobial agent out of the cell, while secreting enzymes that degrade the antimicrobial agent, further diminishing the effect (Piddock, 2006). Gram-negative bacteria are also prone to forming protective biofilms that block antimicrobial and immune system attacks and rapidly spread resistance through gene transfer. These factors make Gram-negative bacteria highly resistant (Tavares et al., 2020).
Considering these findings, future research focuses on further developing the antimicrobial efficacy of phenolic compounds in grape pomace, especially against Gram-negative bacteria. In addition, investigating the synergistic effects of different phenolic compounds on antimicrobial activity, optimizing health-promoting compounds in wine by-products, advancing the use of grape pomace in natural antimicrobials, and supporting sustainable agricultural practices through the valorisation of winemaking waste is warranted.
6 Skin formulations of grape by-products in domesticated animals and livestock
6.1 Products currently used to treat skin ailments
In the scope of animal skin care products, most of the existing natural products are based on either plant extracts or oil components, such as aloe vera, coconut oil, tea tree oil and beeswax, to achieve gentle, symptomatic relief of skin problems. These products are typically applied for mild skin inflammation, dryness, and scratches, and have been validated to some extent against classical effects that promote healing (Table 7).
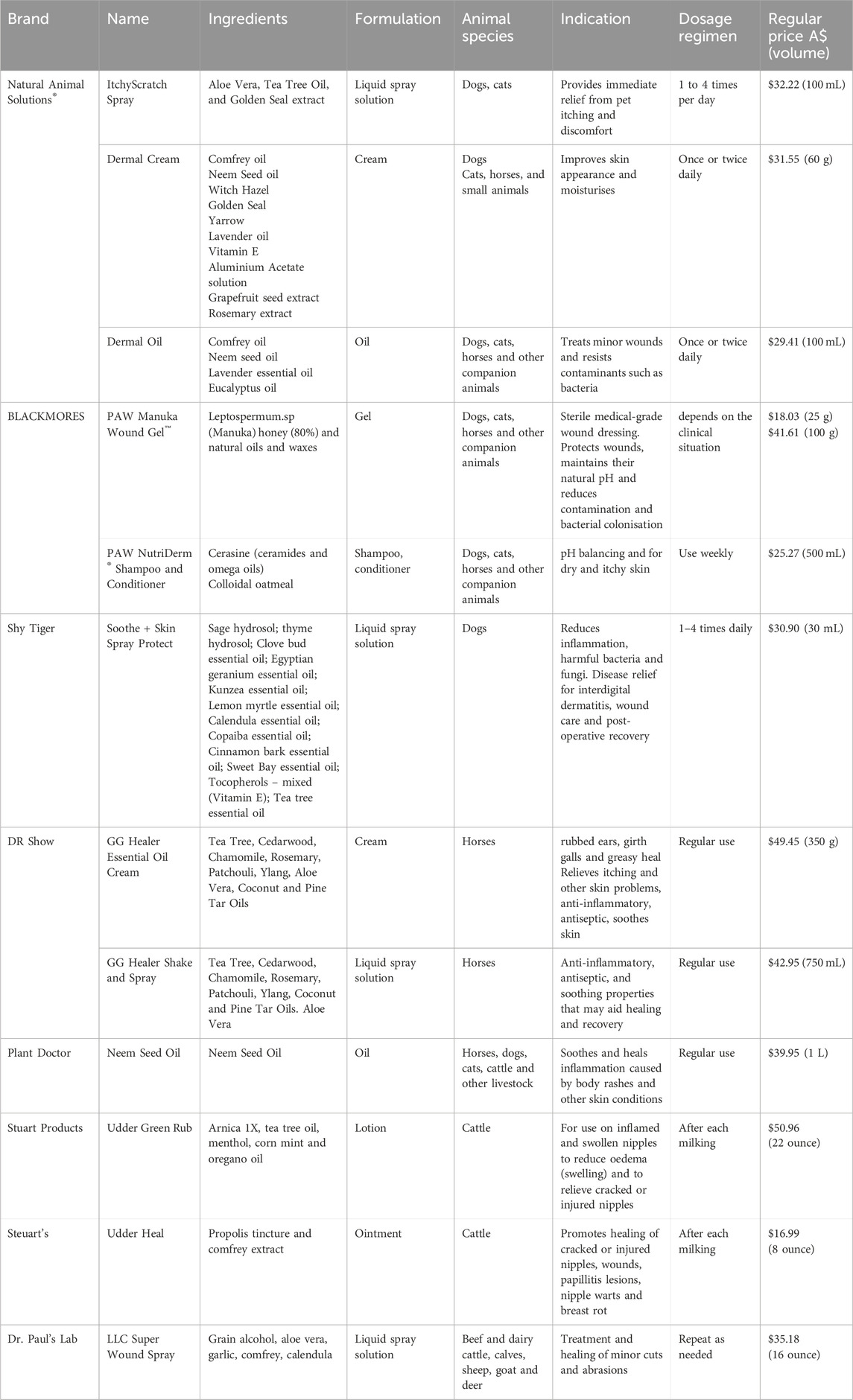
Table 7. Some commercially available products for domesticated and livestock animals. Animal skin health management.
6.2 Potential of grape extract in animal skin health maintenance
As the demand for natural ingredients for health applications increases, grape extracts have sparked much interest in the scientific community due to their diverse biological activities (Hegedüs et al., 2022). In recent years, a growing body of research has explored the positive effects of grape extracts on skin health with wound healing promoting effects demonstrated in several animal studies (Ajit et al., 2021). These active ingredients are able to effectively modulate the inflammatory response at the molecular level, inhibit the growth and reproduction of pathogens, and mitigate oxidative damage induced by wounds or UV irradiation through antioxidant mechanisms (Perde-Schrepler et al., 2013; Punzo et al., 2021). The application of grape extracts has shown potential not only in protecting the structural and functional integrity of the skin, but also in promoting tissue regeneration and accelerating wound healing. The studies in Table 8 systematically explores a wide range of possible applications of grape extracts in animal skin care, especially in different types of skin injuries and pathogenic infections.
Although there have been numerous in vivo experiments demonstrating the effectiveness of grape extracts in wound healing, these studies have shown the potential of grape extracts as natural active ingredients in the field of animal skin health. However, most commercially available grape extract products focus on the field of human skin repair, with the main effects being antioxidant, anti-inflammatory and antibacterial, helping to slow down skin ageing, enhance skin barrier function, and improve skin radiance and elasticity (Sharafan et al., 2023; Ferreira and Santos, 2022). These products usually use grape seed or skin extract as the main ingredient and achieve their protective and repairing effects through the abundance of antioxidant actives such as polyphenols, proanthocyanidins and resveratrol (Soto et al., 2015). Despite the effectiveness of grape extracts in promoting skin health, similar products for animal skin treatment are virtually non-existent. This situation reflects the relative limitations in product development and ingredient selection in the current animal skin care field, especially in the utilization of grape extracts.
7 Challenges to use of grape extracts in animal skin treatment
7.1 Lack of in-depth studies on different species
Grape extract is a natural substance rich in bioactive compounds, with many studies having shown they are generally well-tolerated when applied topically to human skin (Fium et al., 2014; Benguechoua et al., 2025). In animal skin tests, resveratrol-containing hydrogels did not cause skin erythema and stratum corneum disruption in mice (Hung et al., 2008). Topical application of 2000 mg/kg of grape seed proanthocyanidin extract to the clipped intact skin of male and female albino rats for 24 h in a one-time dermal contact assay demonstrated that dermal application posed a very low risk of systemic toxicity at this dose (Ray et al., 2001). However, some animal studies have reported mild to moderate irritation in eye tests. Grape extracts, in particular, may induce a mild to moderate irritant response in the cornea or conjunctiva of rabbits, causing temporary discomfort or an inflammatory response (Bagchi et al., 2000). Such reactions are usually attributed to specific active ingredients in the extract, such as high concentrations of tannins or acidic polyphenols, and to the solvents used. Differences in irritation may be due to differences in skin barrier structure, absorption properties and sensitivity to phytochemicals.
The skin of different animal species varies greatly in structure, thickness and barrier properties. For example, the skin of dogs is thinner and more permeable than that of horses and is therefore more susceptible to both favourable and unfavourable effects of topical medications (Panakova et al., 2014). The development of effective products is complicated by the lack of standardised dosage regimens for different animals. Overdosing may lead to toxicity, while underdosing may render the product ineffective. Key factors such as frequency and duration of use can also affect efficacy and safety. Repeated use may cause irritation or sensitisation in animals with sensitive or damaged skin, and cumulative effects must be carefully assessed, particularly in animals prone to chronic skin conditions such as dermatitis.
7.2 Bioavailability and stability challenges
Grape by-products possess a wide range of bioactivities relevant to dermatological applications. Although these extracts are increasingly used in the food industry, their application as bioactive agents in topical formulations remains underexplored (Brown et al., 2006). Many polyphenols derived from wine by-products suffer from unfavourable physicochemical and pharmacokinetic properties, including low aqueous solubility, poor stability under improper storage conditions, and susceptibility to oxidation, all of which can lead to discoloration, odor changes, and diminished efficacy (Soto et al., 2015; Gonçalves et al., 2024). These compounds also tend to exhibit poor systemic bioavailability and limited tissue distribution.
Furthermore, grape extracts present notable formulation challenges. Their hydrophilic nature makes them poorly compatible with the lipid-rich environment of animal skin, impeding their ability to penetrate the stratum corneum and reach deeper dermal layers where therapeutic action is required (Spigno et al., 2007). Effective skin treatment depends on the bioavailability of active compounds—their ability to reach target sites at biologically relevant concentrations. However, the limited solubility and skin permeability of grape extracts, together with possible enzymatic degradation by the skin microbiota or topical metabolic processes, can significantly reduce their efficacy. In addition, the polyphenols in grape extracts are susceptible to oxidation and degradation when exposed to air, light and heat, thus affecting their stability and shelf life in formulations (Amendola et al., 2010). Animal grooming behaviours (e.g., licking or rubbing) and environmental exposures further challenge the stability and adhesion of topical products, and advanced formulations are required to meet these unique needs.
7.3 Insufficient standardisation of dosage and formulation
Inadequate standardisation of dosage and optimal formulation is a major challenge in the conversion of grape extracts into reliable and effective animal skin care products. Grape extracts contain a complex mixture of actives whose concentrations and proportions vary considerably depending on the extraction method, including solvent type, temperature and processing conditions (Table 9) (Barba et al., 2016). Dosage, formulation and frequency of use of extracts have not been standardised in existing studies. Therefore, it is challenging to develop dosages and formulations that are both effective and safe in animal care products. In addition, the bioavailability, skin absorption and stability of grape extracts need to be further optimised.
The production of high-quality grape extracts requires the use of specialised extraction and sealing processes to ensure the concentration and stability of the active ingredient. However, these processes typically increase costs, especially as the active ingredients need to be precisely separated and kept chemically unchanged during the extraction process. In addition, there are significant technical challenges to ensuring the consistency of extracts from different batches. For example, controlling variations in the quality of the raw material and the effect of changes in extraction conditions on the final product. These factors further limit the future need to optimise extraction and processing techniques in order to address these issues, as well as to develop more cost-effective methods to reduce production costs and improve the reliability of quality control.
7.4 Regulatory approval barriers
In many jurisdictions, animal care and treatment products must undergo a rigorous regulatory approval process, particularly when therapeutic claims are made. Grape extract-based formulations, due to their botanical origin and complex composition, are often classified as veterinary medicinal products when intended to prevent or treat disease. This classification necessitates compliance with strict regulatory frameworks. In particular, very few plant-based medicines are approved by the US Food and Drug Administration (US-FDA) for clinical use, because of their complex compositions, which makes it difficult to assess their safety, efficacy and bioavailability (Yo et al., 2022). As grape extracts are still emerging ingredients in animal care products, they require extensive experimental validation and regulatory approval to verify safety and efficacy. The time and economic costs associated with regulatory approvals limit their commercialization progress. To address these challenges and to ensure a more streamlined approach to evaluating veterinary drug products, the FDA provides a standardized set of guidance. FDA provides Target Animal Safety for Veterinary Pharmaceutical Products (FDA, 2009) is a standardized guideline for evaluating the safety of target animals It is referenced worldwide to assist in the development and registration of veterinary products. It aims to reduce the number of animal tests, lower the cost of research and promote the harmonization of global regulations. It also emphasizes the importance of research on scientific data, risk assessment to ensure the safety and efficacy of drugs in target animals. In the European Union, under the provisions of Directive 2001/82/EC (UNION TEPATCOTE, 2001), herbal veterinary products must also demonstrate safety and efficacy through clinical trials and only qualify for the simplified registration route under clearly defined conditions. In Australia, products with therapeutic effects are regulated by the Australian Pesticides and Veterinary Medicines Authority (Authority, 2015). If a grape extract formulation claims to treat skin infections or inflammation in animals, it must be registered as a veterinary medicinal product and undergo pre-market assessment. Despite the existence of these frameworks, there are currently no standardised international guidelines for topical grape extract preparations for veterinary use. The lack of harmonised standards has resulted in longer approval times and increased costs, thus hindering an appetite for product development.
7.5 Lack of marketing and consumer awareness of product effectiveness
The use of grape extracts in animal skin care products faces significant barriers due to limited marketing efforts and low awareness among consumer groups. Currently, most animal skin care products on the market still rely on widely known medicinal or botanical ingredients such as coconut oil, tea tree oil and aloe vera. These ingredients have earned the trust of a wide range of consumers and veterinarians because of the efficacy they have demonstrated over a long period of time during use. In contrast, grape extracts lack similar levels of awareness and trust as relatively new ingredients used in skin care, limiting their acceptance and broader use. A key factor contributing to this situation is the limited availability of established examples that can confirm the safety and efficacy of grape extracts in practical applications and the paucity of published data from animal clinical trials. This has led to a cautious approach to the use of new ingredients that do not have a reliable scientific basis. Although grape extracts have shown encouraging results in preliminary studies, their efficacy and reliability in animal skin care needs to be supported by more clinical trials and practical applications.
8 Future research directions
Future research should include comparative dermal pharmacokinetic and toxicity studies in representative animal species (e.g., dogs, cats, cattle, and horses) considering differences in skin anatomy and drug penetration in different species. This should be accompanied by the development of species-specific topical formulations and standardised dermal safety protocols. Additionally, the extraction process should be optimised to determine safe and effective dosages, eliminate irritating ingredients and support the development of formulations tailored to the specific needs of each species. Rigorous topical testing is necessary to refine these products and future studies should prioritise the assessment of skin tolerance to grape extracts in different animal species to lay the foundation for their use in veterinary dermatology.
To fully utilise the therapeutic efficacy of grape polyphenols in topical applications, the development of advanced delivery systems is essential. These can encompass phytosomes, sol-gel systems, liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) and polymer nanoparticles. Each of these systems offers unique benefits based on specific formulation goals. For example, phytomers enhance the lipophilicity of hydrophilic polyphenols and improve skin penetration through the formation of lipid-compatible complexes (Surini et al., 2018). Lipid-based carriers (e.g., SLN and NLC) provide containment, promote skin retention, protect antioxidants from degradation, and reduce systemic absorption (Santonocito, and Puglia, 2025). Sol-gel systems are FDA-approved and safe for human use, forming a bioadhesive matrix that extends skin contact for sustained and controlled drug release (Salem et al., 2025). At the same time, polymer nanoparticles allow for controlled release and targeted delivery, making them particularly attractive for the treatment of topical dermatological conditions (Salem et al., 2024). These delivery strategies not only improve the solubility and diffusion of polyphenols in the skin but also reduce systemic exposure and improve therapeutic indices.
To ensure consistent product quality, standardised extraction protocols, including solvent type, temperature and extraction time, and quality control markers for key bioactive compounds are required. The development of dosage guidelines specific to species, skin condition and disease severity can also help ensure safety and efficacy. Regulators can support this by promoting good manufacturing practice requirements for botanical veterinary products. Also harmonising international guidelines and creating simplified registration categories for botanical veterinary topicals can support rapid approval, across multiple markets.
To increase market acceptance, stakeholders should invest in publishing preclinical data, demonstrating real-world applications, and communicating the benefits of grape extract formulations to veterinarians and pet owners. Collaboration between academic researchers, veterinary professionals, and industry partners will help generate real-world success stories. Pilot projects or demonstration trials in veterinary clinics would serve to further enhance credibility and consumer confidence.
9 Conclusion
Grape by-products are a highly promising but under-utilised resource for the development of topical preparations aimed at improving animal skin health. These extracts are rich in polyphenols and other bioactive compounds with antioxidant, anti-inflammatory and antimicrobial properties that are highly relevant for dermatological applications. Going forward, more cross-species studies, rigorous safety assessments and optimised formulations are needed to validate the efficacy and safety of these natural compounds. There is also a need to harmonise regulation and increase market awareness to accelerate their widespread use. Through multidisciplinary collaboration and innovation, grape-derived topicals hold the promise of becoming effective and sustainable alternatives in veterinary dermatology.
In addition, the therapeutic effects exhibited by grape by-product polyphenols in animal models suggest that they have high translational value for human applications. Given the pharmacological properties of these natural compounds, their application in the treatment of human skin diseases such as atopic dermatitis, acne and photoaging could be further explored. Therefore, future research should also focus on bridging the gap between veterinary and human dermatology by investigating common skin pathophysiology and conducting comparative studies. Such cross-applications may pave the way for the development of novel plant-derived skincare interventions for both animals and humans, thus enhancing the relevance and impact of sustainable bioactive resources in modern medicine.
Author contributions
WC: Writing – original draft. PP: Writing – review and editing. ZZ: Writing – review and editing. AJ: Writing – review and editing. HP: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank The University of Queensland and ARC Training Centre for Environmental and Agricultural Solutions to Antimicrobial Resistance (CEA-StAR) for providing a Research Training Program Scholarship to WC. The authors would like to acknowledge Edenvale Beverages Pty Ltd. for providing background support for the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalhamed, A. M., Zeedan, G. S. G., and Abou Zeina, H. A. A. (2018). Isolation and identification of bacteria causing mastitis in small ruminants and their susceptibility to antibiotics, honey, essential oils, and plant extracts. Veterinary World 11 (3), 355–362. doi:10.14202/vetworld.2018.355-362
Ajit, A., Vishnu, A. G., and Varkey, P. (2021). Incorporation of grape seed extract towards wound care product development. 3 Biotech. 11 (6), 261. doi:10.1007/s13205-021-02826-4
Akinwumi, B. C., Bordun, K.-A. M., and Anderson, H. D. (2018). Biological activities of stilbenoids. Int. J. Mol. Sci. 19 (3), 792. doi:10.3390/ijms19030792
Al, J. S., Alkhoori, S. A., and Yousef, L. F. (2018). “Chapter 13 - phenolic acids from plants: extraction and application to human health,”. Studies in natural products chemistry. Editor R. Atta ur (Elsevier), 58, 389–417. doi:10.1016/B978-0-444-64056-7.00013-1
Alì, S., Davinelli, S., Accardi, G., Aiello, A., Caruso, C., Duro, G., et al. (2021). Healthy ageing and Mediterranean diet: a focus on hormetic phytochemicals. Mech. Ageing Dev. 200, 111592. doi:10.1016/j.mad.2021.111592
Alice De Quadros, E., Magnino Chaban, N., Bernardes Bizinoto, L., Supranzetti, De R. R., Rodrigues Rosado, I., Martin, I., et al. (2024). Grape seed oil in the treatment of severe wound in a dog - case report. Acta Veterinaria Bras. 18 (1), 25–28. doi:10.21708/avb.2024.18.1.12020
Alonso, V. P. P., Gonçalves, M. P. M., de Brito, F. A. E., Barboza, G. R., Rocha, L. O., and Silva, N. C. C. (2023). Dry surface biofilms in the food processing industry: an overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods. Compr. Rev. Food Sci. Food Saf. 22 (1), 688–713. doi:10.1111/1541-4337.13089
Amendola, D., De Faveri, D. M., and Spigno, G. (2010). Grape marc phenolics: extraction kinetics, quality and stability of extracts. J. Food Eng. 97 (3), 384–392. doi:10.1016/j.jfoodeng.2009.10.033
Angelini, P., Flores, G. A., Piccirilli, A., Venanzoni, R., Acquaviva, A., Di Simone, S. C., et al. (2022). Polyphenolic composition and antimicrobial activity of extracts obtained from grape processing by-products: between green biotechnology and nutraceutical. Process Biochem. 118, 84–91. doi:10.1016/j.procbio.2022.04.019
Authority, A. P. V. M. (2015). Approval of active constituents and registration of agvet chemical products. Available online at: https://www.apvma.gov.au/registrations-and-permits/chemical-product-registration/applying-approvals-registrations-and-variations.
Bagchi, D., Bagchi, M., Stohs, S., Das, D., Ray, S., Kuszynski, C., et al. (2000). Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology 148, 187–197. doi:10.1016/s0300-483x(00)00210-9
Baquero, F., Saralegui, C., Marcos-Mencía, D., Ballestero, L., Vañó-Galván, S., Moreno-Arrones, Ó. M., et al. (2021). Epidermis as a platform for bacterial transmission. Front. Immunol. 12, 12–2021. doi:10.3389/fimmu.2021.774018
Baranwal, A., Aggarwal, P., Rai, A., and Kumar, N. (2022). Pharmacological actions and underlying mechanisms of catechin: a review. Mini Rev. Med. Chem. 22 (5), 821–833. doi:10.2174/1389557521666210902162120
Barba, F. J., Zhu, Z., Koubaa, M., Sant'Ana, A. S., and Orlien, V. (2016). Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci. and Technol. 49, 96–109. doi:10.1016/j.tifs.2016.01.006
Baydar, N. G., Özkan, G., and Sağdiç, O. (2004). Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food control. 15 (5), 335–339. doi:10.1016/s0956-7135(03)00083-5
Beco, L., Guaguère, E., Méndez, C. L., Noli, C., Nuttall, T., and Vroom, M. (2013). Suggested guidelines for using systemic antimicrobials in bacterial skin infections: part 2— antimicrobial choice, treatment regimens and compliance. Veterinary Rec. 172 (6), 156–160. doi:10.1136/vr.101070
Benguechoua, M. I., Benguechoua, M., Benarous, K., Kaouka, A., and Yousfi, M. (2025). Enhanced formulation and comprehensive analysis of novel natural ointments with grape seed and pomegranate peel infused in olive oil. Turk J. Biol. 49 (1), 28–39. doi:10.55730/1300-0152.2721
Berman, A. Y., Motechin, R. A., Wiesenfeld, M. Y., and Holz, M. K. (2017). The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis. Oncol. 1 (1), 35. doi:10.1038/s41698-017-0038-6
Bertelli, A. A., Migliori, M., Panichi, V., Longoni, B., Origlia, N., Ferretti, A., et al. (2002). Oxidative stress and inflammatory reaction modulation by white wine. Ann. N. Y. Acad. Sci. 957 (1), 295–301. doi:10.1111/j.1749-6632.2002.tb02929.x
Bichakjian, C. K., and Johnson, T. M. (2007). “CHAPTER 1 - anatomy of the skin,” in Local flaps in facial reconstruction. Editor S. R. Baker Second Edition (Edinburgh: Mosby), 3–13. doi:10.1016/j.joms.2008.05.311
Bloom, P. (2014). Canine superficial bacterial folliculitis: current understanding of its etiology, diagnosis and treatment. Veterinary J. 199 (2), 217–222. doi:10.1016/j.tvjl.2013.11.014
Bocsan, I. C., Măgureanu, D. C., Pop, R. M., Levai, A. M., Macovei, Ș. O., Pătraşca, I. M., et al. (2022). Antioxidant and anti-inflammatory actions of polyphenols from red and white grape pomace in ischemic heart diseases. Biomedicines 10 (10), 2337. doi:10.3390/biomedicines10102337
Borges, A., Abreu, A. C., Dias, C., Saavedra, M. J., Borges, F., and Simões, M. (2016). New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 21 (7), 877. doi:10.3390/molecules21070877
Borges, A., Ferreira, C., Saavedra, M. J., and Simões, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 19 (4), 256–265. doi:10.1089/mdr.2012.0244
Bralley, E. E., Hargrove, J. L., Greenspan, P., and Hartle, D. K. (2007). Topical anti-inflammatory activities of vitis rotundifolia (muscadine grape) extracts in the tetradecanoylphorbol acetate model of ear inflammation. J. Med. Food 10 (4), 636–642. doi:10.1089/jmf.2006.244
Brown, M. B., Martin, G. P., Jones, S. A., and Akomeah, F. K. (2006). Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 13 (3), 175–187. doi:10.1080/10717540500455975
Byrd, A. L., Belkaid, Y., and Segre, J. A. (2018). The human skin microbiome. Nat. Rev. Microbiol. 16 (3), 143–155. doi:10.1038/nrmicro.2017.157
Caneschi, A., Bardhi, A., Barbarossa, A., and Zaghini, A. (2023). The use of antibiotics and antimicrobial resistance in veterinary medicine, a complex phenomenon: a narrative review. Antibiot. (Basel) 12 (3), 487. doi:10.3390/antibiotics12030487
Chen, Y. E., Fischbach, M. A., and Belkaid, Y. (2018). Skin microbiota–host interactions. Nature 553 (7689), 427–436. doi:10.1038/nature25177
Cheng, V. J., Bekhit, A. E.-D. A., McConnell, M., Mros, S., and Zhao, J. (2012). Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 134 (1), 474–482. doi:10.1016/j.foodchem.2012.02.103
Cho, H. S., Lee, J.-H., Cho, M. H., and Lee, J. (2015). Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 31 (1), 1–11. doi:10.1080/08927014.2014.991319
Chowdhury, A., Nosoudi, N., Karamched, S., Parasaram, V., and Vyavahare, N. (2021). Polyphenol treatments increase elastin and collagen deposition by human dermal fibroblasts; Implications to improve skin health. J. Dermatological Sci. 102 (2), 94–100. doi:10.1016/j.jdermsci.2021.03.002
Christensen, G. J., and Bruggemann, H. (2014). Bacterial skin commensals and their role as host guardians. Benef. Microbes 5 (2), 201–215. doi:10.3920/BM2012.0062
Collado, R., Prenafeta, A., González-González, L., Pérez-Pons, J. A., and Sitjà, M. (2016). Probing vaccine antigens against bovine mastitis caused by Streptococcus uberis. Vaccine 34 (33), 3848–3854. doi:10.1016/j.vaccine.2016.05.044
Corrêa, R. C., Haminiuk, C. W., Barros, L., Dias, M. I., Calhelha, R. C., Kato, C. G., et al. (2017). Stability and biological activity of Merlot (Vitis vinifera) grape pomace phytochemicals after simulated in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 36, 410–417. doi:10.1016/j.jff.2017.07.030
Cotea, V. V., Luchian, C., Niculaua, M., Zamfir, C. I., Moraru, I., Nechita, B. C., et al. (2018). Evaluation of phenolic compounds content in grape seeds. Environ. Eng. and Manag. J. (EEMJ) 17 (4), 795–802. doi:10.30638/eemj.2018.080
Dadi, P. K., Ahmad, M., and Ahmad, Z. (2009). Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 45 (1), 72–79. doi:10.1016/j.ijbiomac.2009.04.004
Daglia, M. (2012). Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 23 (2), 174–181. doi:10.1016/j.copbio.2011.08.007
de Andrade, R. B., Machado, B. A. S., Barreto, G. A., Nascimento, R. Q., Corrêa, L. C., Leal, I. L., et al. (2021). Syrah grape skin residues has potential as source of antioxidant and anti-microbial bioactive compounds. Biology 10 (12), 1262. doi:10.3390/biology10121262
Dehdashtian, A., Stringer, T. P., Warren, A. J., Mu, E. W., Amirlak, B., and Shahabi, L. (2018). “Anatomy and physiology of the skin,” in Melanoma: a modern multidisciplinary approach, 15–26. doi:10.1007/978-3-319-78310-9_2
Di, L., Wei, Z., and Shujin, Z. (2012). Relevant enzymes, genes and regulation mechanisms in biosynthesis pathway of stilbenes. Open J. Med. Chem. 2012. doi:10.4172/2155-9821.1000119
Di Lorenzo, A., Bloise, N., Meneghini, S., Sureda, A., Tenore, G. C., Visai, L., et al. (2016). Effect of winemaking on the composition of red wine as a source of polyphenols for anti-infective biomaterials. Mater. (Basel) 9 (5), 316. doi:10.3390/ma9050316
Dóka, O., Ficzek, G., Simon, G., Zsófi, Z., Villangó, S., and Végvári, G. (2023). Quantification of total polyphenol content in wine lees by conventional optical and photoacoustic spectroscopy. OENO One 57 (2), 257–264. doi:10.20870/oeno-one.2023.57.2.7178
Drucker, C. R. (2012). Update on topical antibiotics in dermatology. Dermatol. Ther. 25 (1), 6–11. doi:10.1111/j.1529-8019.2012.01493.x
Elshaer, E. E., Elwakil, B. H., Eskandrani, A., Elshewemi, S. S., and Olama, Z. A. (2022). Novel Clotrimazole and Vitis vinifera loaded chitosan nanoparticles: antifungal and wound healing efficiencies. Saudi J. Biol. Sci. 29 (3), 1832–1841. doi:10.1016/j.sjbs.2021.10.041
Eskandari, M., Rembiesa, J., Startaitė, L., Holefors, A., Valančiūtė, A., Faridbod, F., et al. (2019). Polyphenol-hydrogen peroxide reactions in skin: in vitro model relevant to study ROS reactions at inflammation. Anal. Chim. Acta 1075, 91–97. doi:10.1016/j.aca.2019.05.032
Espíndola, K. M. M., Ferreira, R. G., Narvaez, L. E. M., Silva Rosario, A. C. R., Da Silva, A. H. M., Silva, A. G. B., et al. (2019). Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 9, 541. doi:10.3389/fonc.2019.00541
Faccin, M., Wiener, D. J., Rech, R. R., Santoro, D., and Rodrigues Hoffmann, A. (2023). Common superficial and deep cutaneous bacterial infections in domestic animals: a review. Veterinary Pathol. 60 (6), 796–811. doi:10.1177/03009858231176558
FDA (2009). Target animal safety for veterinary pharmaceutical products. Available online at: https://www.fda.gov/media/70438/download.
Fernández-Mar, M., Mateos, R., Garcia-Parrilla, M. C., Puertas, B., and Cantos-Villar, E. (2012). Bioactive compounds in wine: resveratrol, hydroxytyrosol and melatonin: a review. Food Chem. 130 (4), 797–813. doi:10.1016/j.foodchem.2011.08.023
Ferreira, S. M., and Santos, L. (2022). A potential valorization strategy of wine industry by-products and their application in cosmetics-case study: grape pomace and grapeseed. Molecules 27 (3), 969. doi:10.3390/molecules27030969
Ferrer-Gallego, R., and Silva, P. (2022). The wine industry by-products: applications for food industry and health benefits. Antioxidants 11 (10), 2025. doi:10.3390/antiox11102025
Filip, A., Daicoviciu, D., Clichici, S., Bolfa, P., Catoi, C., Baldea, I., et al. (2011). The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J. Photochem. Photobiol. B Biol. 105 (2), 133–142. doi:10.1016/j.jphotobiol.2011.08.002
Findlay, M. W., and Gurtner, G. C. (2017). “Chapter 35 - engineering niches for skin and wound healing,” in Biology and engineering of stem cell niches. Editors A. Vishwakarma, and J. M. Karp (Boston: Academic Press), 559–579. doi:10.1016/B978-0-12-802734-9.00035-4
Fiume, M. M., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D. C., et al. (2014). Safety assessment of Vitis vinifera (Grape)-Derived ingredients as used in cosmetics. Int. J. Toxicol. 33 (3_Suppl. l), 48S–83S. doi:10.1177/1091581814545247
Foster, A. P. (2012). Staphylococcal skin disease in livestock. Veterinary dermatol. 23 (4), 342–351. doi:10.1111/j.1365-3164.2012.01093.x
Ganeshpurkar, A., and Saluja, A. K. (2017). The pharmacological potential of rutin. Saudi Pharm. J. 25 (2), 149–164. doi:10.1016/j.jsps.2016.04.025
Genisheva, Z., Soares, M., Oliveira, J., and Carvalho, J. (2023). Wine production wastes, valorization, and perspectives. doi:10.5772/intechopen.1003184
Gonçalves, M. B. S., Marques, M. P., Correia, F., Pires, P. C., Correia, M., Makvandi, P., et al. (2024). Wine industry by-products as a source of active ingredients for topical applications. Phytochem. Rev. doi:10.1007/s11101-024-10030-4
Górniak, I., Bartoszewski, R., and Króliczewski, J. (2019). Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 18, 241–272. doi:10.1007/s11101-018-9591-z
Gradišar, H., Pristovšek, P., Plaper, A., and Jerala, R. (2007). Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 50 (2), 264–271. doi:10.1021/jm060817o
Grice, E. A. (2015). The intersection of microbiome and host at the skin interface: genomic-and metagenomic-based insights. Genome Res. 25 (10), 1514–1520. doi:10.1101/gr.191320.115
Hammam, M., Mansour, H., and Hassan, W. (2022). Grape seed extract promotes Staphyloccous aureus infected skin wound Healing in diabetic rat model. Egypt. J. Med. Microbiol. 31 (4), 15–23. doi:10.21608/ejmm.2022.262676
Health WOfA (2024). Superbugs could jeopardise food security for over two billion people and increase annual health care costs by US$ 159 billion annually by 2050, finds most extensive modelling to date. Available online at: https://www.woah.org/en/superbugs-could-jeopardise-food-security-for-over-two-billion-people-and-increase-annual-health-care-costs-by-us-159-billion-annually-by-2050-finds-most-extensive-modelling-to-date/.
Hegedüs, I., Andreidesz, K., Szentpéteri, J. L., Kaleta, Z., Szabó, L., Szigeti, K., et al. (2022). The utilization of physiologically active molecular components of grape seeds and grape marc. Int. J. Mol. Sci. 23 (19), 11165. doi:10.3390/ijms231911165
Hemmati, A. A., Aghel, N., Rashidi, I., and Gholampur-Aghdami, A. (2011). Topical grape (Vitis vinifera) seed extract promotes repair of full thickness wound in rabbit. Int. Wound J. 8 (5), 514–520. doi:10.1111/j.1742-481X.2011.00833.x
Hibbs, R. G., and Clark, Jr W. H. (1959). Electron microscope studies of the human epidermis: the cell boundaries and topography of the stratum malpighii. J. Cell Biol. 6 (1), 71–76. doi:10.1083/jcb.6.1.71
Hung, C.-F., Lin, Y.-K., Huang, Z.-R., and Fang, J.-Y. (2008). Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol. Pharm. Bull. 31 (5), 955–962. doi:10.1248/bpb.31.955
Inoue, M., Sakaguchi, N., IsuzuGAwA, K., Tani, H., and Ogihara, Y. (2000). Role of reactive oxygen species in gallic acid-induced apoptosis. Biol. Pharm. Bull. 23 (10), 1153–1157. doi:10.1248/bpb.23.1153
Jayaprakasha, G., Selvi, T., and Sakariah, K. (2003). Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 36 (2), 117–122. doi:10.1016/s0963-9969(02)00116-3
Jin, Q., O’Hair, J., Stewart, A. C., O’Keefe, S. F., Neilson, A. P., Kim, Y.-T., et al. (2019). Compositional characterization of different industrial white and red grape pomaces in Virginia and the potential valorization of the major components. Foods 8 (12), 667. doi:10.3390/foods8120667
Jonidi Shariatzadeh, F., Currie, S., Logsetty, S., Spiwak, R., and Liu, S. (2025). Enhancing wound healing and minimizing scarring: a comprehensive review of nanofiber technology in wound dressings. Prog. Mater. Sci. 147, 101350. doi:10.1016/j.pmatsci.2024.101350
Joosten, P., Ceccarelli, D., Odent, E., Sarrazin, S., Graveland, H., Van Gompel, L., et al. (2020). Antimicrobial usage and resistance in companion animals: a cross-sectional study in three European countries. Antibiot. (Basel) 9 (2), 87. doi:10.3390/antibiotics9020087
Kahkeshani, N., Farzaei, F., Fotouhi, M., Alavi, S. S., Bahramsoltani, R., Naseri, R., et al. (2019). Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran. J. basic Med. Sci. 22 (3), 225–237. doi:10.22038/ijbms.2019.32806.7897
Kang, X., Yang, X., He, Y., Guo, C., Li, Y., Ji, H., et al. (2023). Strategies and materials for the prevention and treatment of biofilms. Mater. Today Bio 23, 100827. doi:10.1016/j.mtbio.2023.100827
Karastergiou, A., Gancel, A.-L., Jourdes, M., and Teissedre, P.-L. (2024). Valorization of grape pomace: a review of phenolic composition, bioactivity, and therapeutic potential. Antioxidants 13 (9), 1131. doi:10.3390/antiox13091131
Kaur, G., Kaur, R., Sodhi, G. K., George, N., Rath, S. K., Walia, H. K., et al. (2024). Stilbenes: a journey from folklore to pharmaceutical innovation. Archives Microbiol. 206 (5), 229. doi:10.1007/s00203-024-03939-z
Kaur, J., Gulati, M., Singh, S. K., Kuppusamy, G., Kapoor, B., Mishra, V., et al. (2022). Discovering multifaceted role of vanillic acid beyond flavours: nutraceutical and therapeutic potential. Trends Food Sci. and Technol. 122, 187–200. doi:10.1016/j.tifs.2022.02.023
Kersh, D. M. E., Hammad, G., Donia, M. S., and Farag, M. A. (2023). A comprehensive review on grape juice beverage in context to its processing and composition with future perspectives to maximize its value. Food Bioprocess Technol. 16 (1), 1–23. doi:10.1007/s11947-022-02858-5
Kong, H. H., and Segre, J. A. (2012). Skin microbiome: looking back to move forward. J. Investigative Dermatology 132 (3, Part 2), 933–939. doi:10.1038/jid.2011.417
Kumar, H., Bhardwaj, K., Cruz-Martins, N., Nepovimova, E., Oleksak, P., Dhanjal, D. S., et al. (2021). Applications of fruit polyphenols and their functionalized nanoparticles against foodborne bacteria: a mini review. Molecules 26 (11), 3447. doi:10.3390/molecules26113447
Kumar, N., and Goel, N. (2019). Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 24, e00370. doi:10.1016/j.btre.2019.e00370
Kumar, N., and Pruthi, V. (2014). Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 4, 86–93. doi:10.1016/j.btre.2014.09.002
Kumar, S., and Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: an overview. Sci. world J. 2013 (1), 162750. doi:10.1155/2013/162750
Landeka, I., Jurčević, D. M., Guberović, I., Petras, M., Rimac, S., Brnčić, , et al. (2017). Polyphenols from wine lees as a novel functional bioactive compound in the protection against oxidative stress and hyperlipidaemia. Food Technol. Biotechnol. 55 (1), 109–116. doi:10.17113/ftb.55.01.17.4894
Lappin, M. R. (2012). Microbiology and infectious disease. Small Anim. Clin. Diagn. by Lab. Methods, 315–336. doi:10.1016/b978-1-4377-0657-4.00015-6
Lee, J.-H., Cho, H. S., Joo, S. W., Chandra Regmi, S., Kim, J.-A., Ryu, C.-M., et al. (2013). Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157: H7. Biofouling 29 (10), 1189–1203. doi:10.1080/08927014.2013.832223
Li, B. H., and Tian, W. X. (2004). Inhibitory effects of flavonoids on animal fatty acid synthase. J. Biochem. 135 (1), 85–91. doi:10.1093/jb/mvh010
Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M. T., Wang, S., et al. (2016). Quercetin, inflammation and immunity. Nutrients 8 (3), 167. doi:10.3390/nu8030167
Loeffler, A., and Lloyd, D. H. (2018). What has changed in canine pyoderma? A narrative review. Veterinary J. 235, 73–82. doi:10.1016/j.tvjl.2018.04.002
Mancuso, C., and Santangelo, R. (2014). Ferulic acid: pharmacological and toxicological aspects. Food Chem. Toxicol. 65, 185–195. doi:10.1016/j.fct.2013.12.024
Martínez, J. L. (2012). Natural antibiotic resistance and contamination by antibiotic resistance determinants: the two ages in the evolution of resistance to antimicrobials. Front. Microbiol. 3, 1. doi:10.3389/fmicb.2012.00001
Mauldin, E. A., and Peters-Kennedy, J. (2015). Integumentary system. Jubb, kennedy and palmer's pathology of domestic animals, 1, 509. doi:10.1016/B978-0-7020-5317-7.00006-0
Mauldin, E. A., and Peters-Kennedy, J. (2016). Integumentary system. Jubb, Kennedy and Palmer's Pathology Domest. Animals 1, 509. doi:10.1016/B978-0-7020-5317-7.00006-0
Menon, G. K., Cleary, G. W., and Lane, M. E. (2012). The structure and function of the stratum corneum. Int. J. Pharm. 435 (1), 3–9. doi:10.1016/j.ijpharm.2012.06.005
Mercer, M. A. (2022). Antibacterial agents MSD veterinary manual: MSD veterinary manual. Available online at: https://www.msdvetmanual.com/pharmacology/antibacterial-agents/use-of-penicillins-in-animals.
Miklasińska-Majdanik, M., Kępa, M., Wojtyczka, R. D., Idzik, D., and Wąsik, T. J. (2018). Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. public health 15 (10), 2321. doi:10.3390/ijerph15102321
Milinčić, D. D., Stanisavljević, N. S., Až, K., Bajić, S. S., Kojić, M. O., Gašić, U. M., et al. (2021). Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT. 138, 110739. doi:10.1016/j.lwt.2020.110739
Mollica, A., Scioli, G., Della Valle, A., Cichelli, A., Novellino, E., Bauer, M., et al. (2021). Phenolic analysis and in vitro biological activity of red wine, pomace and grape seeds oil derived from Vitis vinifera L. Cv. Montepulciano d’Abruzzo. Antioxidants 10 (11), 1704. doi:10.3390/antiox10111704
Mosele, J., da Costa, B. S., Bobadilla, S., and Motilva, M. J. (2023). Phenolic composition of red and white wine byproducts from different grapevine cultivars from La rioja (Spain) and how this is affected by the winemaking process. J. Agric. Food Chem. 71 (48), 18746–18757. doi:10.1021/acs.jafc.3c04660
Mueller, R. S., Bergvall, K., Bensignor, E., and Bond, R. (2012). A review of topical therapy for skin infections with bacteria and yeast. Veterinary Dermatol. 23 (4), 330–341. doi:10.1111/j.1365-3164.2012.01057.x
Mulchandani, R., Wang, Y., Gilbert, M., and Van Boeckel, T. P. (2023). Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob. Public Health 3 (2), e0001305. doi:10.1371/journal.pgph.0001305
Nagase, N., Sasaki, A., Yamashita, K., Shimizu, A., Wakita, Y., Kitai, S., et al. (2002). Isolation and species distribution of staphylococci from animal and human skin. J. Veterinary Med. Sci. 64 (3), 245–250. doi:10.1292/jvms.64.245
Nascimento, G. G., Locatelli, J., Freitas, P. C., and Silva, G. L. (2000). Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 31, 247–256. doi:10.1590/s1517-83822000000400003
Nayak, B. S., Ramdath, D. D., Marshall, J. R., Isitor, G. N., Eversley, M., Xue, S., et al. (2010). Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phytotherapy Res. 24 (8), 1151–1157. doi:10.1002/ptr.2999
Nayeem, N., Asdaq, S., Salem, H., and Ahel-Alfqy, S. (2016). Gallic acid: a promising lead molecule for drug development. J. Appl. Pharm. 8 (2), 1–4. doi:10.4172/1920-4159.1000213
Nguyen, D. T., Orgill, D. P., and Murphy, G. F. (2009). “4 - the pathophysiologic basis for wound healing and cutaneous regeneration,” in Biomaterials for treating skin loss. Editors D. Orgill, and C. Blanco (United Kingdom, Europe: Woodhead Publishing), 25–57.
Niesen, D. B., Hessler, C., and Seeram, N. P. (2013). Beyond resveratrol: a review of natural stilbenoids identified from 2009–2013. J. Berry Res. 3 (4), 181–196. doi:10.3233/jbr-130062
Nirmal, N., Mahale, K. R., Rathod, N. B., Siddiqui, S. A., and Dhar, B. K. (2025). Winery waste: a sustainable approach for bioactive compound extraction and various industrial applications. Process Saf. Environ. Prot. 193, 760–771. doi:10.1016/j.psep.2024.11.106
Nolff, M. C., and Meyer-Lindenberg, A. (2015). Necrotising fasciitis in a domestic shorthair cat – negative pressure wound therapy assisted debridement and reconstruction. J. Small Animal Pract. 56 (4), 281–284. doi:10.1111/jsap.12275
Older, C. E., Diesel, A., Patterson, A. P., Meason-Smith, C., Johnson, T. J., Mansell, J., et al. (2017). The feline skin microbiota: the bacteria inhabiting the skin of healthy and allergic cats. PLoS One 12 (6), e0178555. doi:10.1371/journal.pone.0178555
Older, C. E., Hoffmann, A. R., and Diesel, A. B. (2023). The feline skin microbiome: interrelationship between health and disease. J. Feline Med. Surg. 25 (7), 1098612X231180231. doi:10.1177/1098612x231180231
Oliveira, D. A., Salvador, A. A., Smânia, A., Smânia, E. F. A., Maraschin, M., and Ferreira, S. R. S. (2013). Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 164 (3), 423–432. doi:10.1016/j.jbiotec.2012.09.014
Oliveira, M., and Duarte, E. (2016). Integrated approach to winery waste: waste generation and data consolidation. Front. Environ. Sci. and Eng. 10 (1), 168–176. doi:10.1007/s11783-014-0693-6
Onache, P. A., Florea, A., Geana, E.-I., Ciucure, C. T., Ionete, R. E., Sumedrea, D. I., et al. (2023). Assessment of bioactive phenolic compounds in musts and the corresponding wines of white and red grape varieties. Appl. Sci. 13 (9), 5722. doi:10.3390/app13095722
Onache, P. A., Geana, E.-I., Ciucure, C. T., Florea, A., Sumedrea, D. I., Ionete, R. E., et al. (2022). Bioactive phytochemical composition of grape pomace resulted from different white and red grape cultivars. Separations 9 (12), 395. doi:10.3390/separations9120395
O’Shaughnessy-Hunter, L. C., Yu, A., Rousseau, J. D., Foster, R. A., and Weese, J. S. (2021). Longitudinal study of the cutaneous microbiota of healthy horses. Veterinary Dermatol. 32 (5), 467-e128. doi:10.1111/vde.12983
Panakova, L., and Szalai, K. (2014). “Surface, barrier, and interface zone: comparative aspects of the skin,” in Comparative medicine: anatomy and physiology. Editor E. Jensen-Jarolim (Vienna: Springer Vienna), 103–117. doi:10.1007/978-3-7091-1559-6_7
Pavlíková, N. (2023). Caffeic acid and diseases—mechanisms of action. Int. J. Mol. Sci. 24 (1), 588. doi:10.3390/ijms24010588
Perde-Schrepler, M., Chereches, G., Brie, I., Tatomir, C., Postescu, I. D., Soran, L., et al. (2013). Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B Biol. 118, 16–21. doi:10.1016/j.jphotobiol.2012.10.008
Piddock, L. J. (2006). Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19 (2), 382–402. doi:10.1128/CMR.19.2.382-402.2006
Plonka, P. M., Passeron, T., Brenner, M., Tobin, D. J., Shibahara, S., Thomas, A., et al. (2009). What are melanocytes really doing all day long. Exp. Dermatol 18 (9), 799–819. doi:10.1111/j.1600-0625.2009.00912.x
Pomba, C., Rantala, M., Greko, C., Baptiste, K. E., Catry, B., van Duijkeren, E., et al. (2016). Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 72 (4), 957–968. doi:10.1093/jac/dkw481
Porcellato, D., Meisal, R., Bombelli, A., and Narvhus, J. A. (2020). A core microbiota dominates a rich microbial diversity in the bovine udder and may indicate presence of dysbiosis. Sci. Rep. 10 (1), 21608. doi:10.1038/s41598-020-77054-6
Prakash, M., Basavaraj, B., and Murthy, K. C. (2019). Biological functions of epicatechin: plant cell to human cell health. J. Funct. Foods 52, 14–24. doi:10.1016/j.jff.2018.10.021
Punzo, A., Porru, E., Silla, A., Simoni, P., Galletti, P., Roda, A., et al. (2021). Grape Pomace for Topical Application: green NaDES sustainable extraction, skin permeation studies, antioxidant and anti-inflammatory activities characterization in 3D human keratinocytes. Biomolecules 11 (8), 1181. doi:10.3390/biom11081181
Qin, N., Tan, X., Jiao, Y., Liu, L., Zhao, W., Yang, S., et al. (2014). RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 4 (1), 5467. doi:10.1038/srep05467
Quilling, L. L., Outerbridge, C. A., White, S. D., and Affolter, V. K. (2022). Retrospective case series: necrotising fasciitis in 23 dogs. Veterinary Dermatol. 33 (6), 534–544. doi:10.1111/vde.13113
Rana, A. C., and Gulliya, B. (2019). Chemistry and pharmacology of flavonoids-A review. Indian J. Pharm. Educ. and Res. 53 (1), 8–20. doi:10.5530/ijper.53.1.3
Raskin, R. E. (2015). “Skin and subcutaneous tissue,” in Canine and feline cytology: a color atlas and interpretation guide, 34–90. doi:10.1016/C2011-0-06737-6
Ray, S., Bagchi, D., Lim, P. M., Bagchi, M., Gross, S. M., Kothari, S. C., et al. (2001). Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res. Commun. Mol. Pathol. Pharmacol. 109 (3-4), 165–197.
Ríos, J.-L., Giner, R. M., Marín, M., and Recio, M. C. (2018). A pharmacological update of ellagic acid. Planta medica. 84 (15), 1068–1093. doi:10.1055/a-0633-9492
Ross, A. A., Müller, K. M., Weese, J. S., and Neufeld, J. D. (2018). Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proc. Natl. Acad. Sci. 115 (25), E5786–E5795. doi:10.1073/pnas.1801302115
Roth, R. R., and James, W. D. (1988). Microbial ecology of the skin. Annu. Rev. Microbiol. 42, 441–464. doi:10.1146/annurev.mi.42.100188.002301
Salem, Y., Sunoqrot, S., Hammad, A., Rajha, H. N., Alzaghari, L. F., Abusulieh, S., et al. (2025). Oxidation-driven assembly of phenolic compounds from grape seeds waste into nanoparticles as potential anti-inflammatory and wound healing therapies. ACS Appl. Bio Mater. 8, 2275–2286. doi:10.1021/acsabm.4c01800
Salem, Y., Sunoqrot, S., Rajha, H. N., Abusulieh, S., Afif, C., Francis, H., et al. (2024). Grape seed phenolic extracts encapsulation in polymeric nanoparticles: characterization and in vitro evaluation against skin melanoma. J. Drug Deliv. Sci. Technol. 100, 106094. doi:10.1016/j.jddst.2024.106094
Sanhueza, L., Tello, M., Vivanco, M., Mendoza, L., and Wilkens, M. (2014). Relation between antibacterial activity against food transmitted pathogens and total phenolic compounds in grape pomace extracts from Cabernet Sauvignon and Syrah varieties. Adv. Microbiol. 2014. doi:10.4236/aim.2014.45029
Sateriale, D., Forgione, G., Di Rosario, M., Pagliuca, C., Colicchio, R., Salvatore, P., et al. (2024). Vine-Winery byproducts as precious resource of natural antimicrobials: in vitro antibacterial and antibiofilm activity of grape pomace extracts against foodborne pathogens. Microorganisms 12 (3), 437. doi:10.3390/microorganisms12030437
Santonocito, D., and Puglia, C. (2025). Lipid Nanoparticles and Skin: Discoveries and Advances. Cosmetics 12 (22). doi:10.3390/cosmetics12010022
Scharschmidt, T. C., and Fischbach, M. A. (2013). What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov. Today Dis. Mech. 10 (3-4), e83–e89. doi:10.1016/j.ddmec.2012.12.003
Schrader, S. M., Vaubourgeix, J., and Nathan, C. (2020). Biology of antimicrobial resistance and approaches to combat it. Sci. Transl. Med. 12 (549). doi:10.1126/scitranslmed.aaz6992
Scott, D. W., and Miller, W. H. (2003). “Chapter 1 - structure and function of the skin,” in Equine dermatology. Editors D. W. Scott, and W. H. Miller (W.B. Saunders), 1–58. Saint Louis.
Seckerdieck, F., and Mueller, R. S. (2018). Recurrent pyoderma and its underlying primary diseases: a retrospective evaluation of 157 dogs. Veterinary Rec. 182 (15), 434. doi:10.1136/vr.104420
Sharafan, M., Malinowska, M. A., Ekiert, H., Kwasniak, B., Sikora, E., and Szopa, A. (2023). Vitis vinifera (vine grape) as a valuable cosmetic raw material. Pharmaceutics 15 (5), 1372. doi:10.3390/pharmaceutics15051372
Sharifi-Rad, J., Quispe, C., Castillo, C. M. S., Caroca, R., Lazo-Vélez, M. A., Antonyak, H., et al. (2022). [Retracted] ellagic acid: a review on its natural sources, chemical stability, and therapeutic potential. Oxidative Med. Cell. Longev. 2022 (1), 3848084. doi:10.1155/2022/3848084
Shukla, A. S., Jha, A. K., Kumari, R., Rawat, K., Syeda, S., and Shrivastava, A. (2018). “Chapter 9 - role of catechins in chemosensitization,”. Role of nutraceuticals in cancer chemosensitization. Editors A. C. Bharti, and B. B. Aggarwal (Academic Press), 2, 169–198. doi:10.1016/B978-0-12-812373-7.00009-7
Silva, A., Martins, R., Silva, V., Fernandes, F., Carvalho, R., Aires, A., et al. (2024). Red grape by-products from the demarcated douro region: chemical analysis, antioxidant potential and antimicrobial activity against food-borne pathogens. Molecules 29 (19), 4708. doi:10.3390/molecules29194708
Silva, V., Igrejas, G., Falco, V., Santos, T. P., Torres, C., Oliveira, A. M. P., et al. (2018). Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food control. 92, 516–522. doi:10.1016/j.foodcont.2018.05.031
Silva, V., Ribeiro, J., Singh, R. K., Aires, A., Carvalho, R., Falco, V., et al. (2024). Exploring winemaking by-products of tinto cão grapes: antioxidant and antimicrobial activity against multiresistant bacteria. Med. Sci. Forum 24 (1), 7. doi:10.3390/ECA2023-16399
Soto, M. L., Falqué, E., and Domínguez, H. (2015). Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2 (3), 259–276. doi:10.3390/cosmetics2030259
Souci, L., and Denesvre, C. (2021). 3D skin models in domestic animals. Veterinary Res. 52 (1), 21. doi:10.1186/s13567-020-00888-5
Spigno, G., Tramelli, L., and De Faveri, D. M. (2007). Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 81 (1), 200–208. doi:10.1016/j.jfoodeng.2006.10.021
Srinivasulu, C., Ramgopal, M., Ramanjaneyulu, G., Anuradha, C., and Kumar, C. S. (2018). Syringic acid (SA)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. and Pharmacother. 108, 547–557. doi:10.1016/j.biopha.2018.09.069
Sura, R., Hinckley, L., Risatti, G., and Smyth, J. (2008). Fatal necrotising fasciitis and myositis in a cat associated with Streptococcus canis. Veterinary Rec. 162 (14), 450–453. doi:10.1136/vr.162.14.450
Surini, S., Mubarak, H., and Ramadon, D. (2018). Cosmetic serum containing grape (Vitis vinifera L.) seed extract phytosome: formulation and in vitro penetration study. J. young Pharm. 10 (2s), S51–S55. doi:10.5530/jyp.2018.2s.10
Tapia, C. V., Falconer, M., Tempio, F., Falcon, F., Lopez, M., Fuentes, M., et al. (2014). Melanocytes and melanin represent a first line of innate immunity against Candida albicans. Med. Mycol. 52 (5), 445–454. doi:10.1093/mmy/myu026
Tarko, T., Duda-Chodak, A., and Soszka, A. (2020). Changes in phenolic compounds and antioxidant activity of fruit musts and fruit wines during simulated digestion. Molecules 25 (23), 5574. doi:10.3390/molecules25235574
Tavares, T. D., Antunes, J. C., Padrão, J., Ribeiro, A. I., Zille, A., Amorim, M. T. P., et al. (2020). Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 9 (6), 314. doi:10.3390/antibiotics9060314
UNION TEPATCOTE (2001). Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32001L0082.
van den Bogaard, A. E., and Stobberingh, E. E. (1999). Antibiotic usage in animals: impact on bacterial resistance and public health. Drugs 58 (4), 589–607. doi:10.2165/00003495-199958040-00002
Van Duijkeren, E., Jansen, M. D., Flemming, S. C., de Neeling, H., Wagenaar, J. A., Schoormans, A. H., et al. (2007). Methicillin-resistant Staphylococcus aureus in pigs with exudative epidermitis. Emerg. Infect. Dis. 13 (9), 1408–1410. doi:10.3201/eid1309.061268
Van Vlaenderen, I., Nautrup, B. P., and Gasper, S. M. (2011). Estimation of the clinical and economic consequences of non-compliance with antimicrobial treatment of canine skin infections. Prev. Vet. Med. 99 (2-4), 201–210. doi:10.1016/j.prevetmed.2011.01.006
Vejarano, R., and Luján-Corro, M. (2022). Red wine and health: approaches to improve the phenolic content during winemaking. Front. Nutr. 9, 890066. doi:10.3389/fnut.2022.890066
Venus, M., Waterman, J., and McNab, I. (2010). Basic physiology of the skin. Surg. Oxf. 28 (10), 469–472. doi:10.1016/j.mpsur.2010.07.011
Walle, T. (2011). Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 1215 (1), 9–15. doi:10.1111/j.1749-6632.2010.05842.x
Walters, K. A., and Roberts, M. S. (2002). “The structure and function of skin,” in Dermatological and transdermal formulations. NY, United States: CRC Press, 19–58. doi:10.1201/9780824743239.ch1
WHO (2023). Antimicrobial resistance. Available online at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Woolhouse, M., Ward, M., van Bunnik, B., and Farrar, J. (2015). Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. Lond B Biol. Sci. 370 (1670), 20140083. doi:10.1098/rstb.2014.0083
Xia, E.-Q., Deng, G.-F., Guo, Y.-J., and Li, H.-B. (2010). Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 11 (2), 622–646. doi:10.3390/ijms11020622
Xu, C., Yagiz, Y., Hsu, W.-Y., Simonne, A., Lu, J., and Marshall, M. R. (2014). Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (vitis rotundifolia michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 62 (28), 6640–6649. doi:10.1021/jf501073q
Ye, Z., Qin, Y., Harrison, R., Hider, R., and Bekhit, A. E.-D. A. (2022). Characterization of bioactive compounds in lees from New Zealand wines with different vinification backgrounds. Antioxidants 11 (12), 2335. doi:10.3390/antiox11122335
You, L., Liang, K., An, R., and Wang, X. (2022). The path towards FDA approval: a challenging journey for Traditional Chinese Medicine. Pharmacol. Res. 182, 106314. doi:10.1016/j.phrs.2022.106314
Zhang, J., Rui, X., Wang, L., Guan, Y., Sun, X., and Dong, M. (2014). Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food control. 42, 125–131. doi:10.1016/j.foodcont.2014.02.001
Zhang, W., Zheng, Y., Yan, F., Dong, M., and Ren, Y. (2023). Research progress of quercetin in cardiovascular disease. Front. Cardiovasc Med. 10, 1203713. doi:10.3389/fcvm.2023.1203713
Zhu, L., Huang, Y., Zhang, Y., Xu, C., Lu, J., and Wang, Y. (2017). The growing season impacts the accumulation and composition of flavonoids in grape skins in two-crop-a-year viticulture. J. food Sci. Technol. 54, 2861–2870. doi:10.1007/s13197-017-2724-3
Glossary
AMR Antimicrobial Resistance
ATP Adenosine Triphosphate
C. difficile Clostridioides difficile
C. perfringens Clostridium perfringens
C. septicum Clostridium septicum
E. coli Escherichia coli
EGCG Epigallocatechin Gallate
FAS-II Fatty Acid Synthase Type II
IM Intramuscular
IV Intravenous
K. pneumoniae Klebsiella pneumoniae
L. monocytogenes Listeria monocytogenes
MIC Minimum Inhibitory Concentration
MRSA methicillin-resistant Staphylococcus aureus
NLCs Nanostructured lipid carriers
P. aeruginosa Pseudomonas aeruginosa
PO Per os (oOral)
S. agalactiae Streptococcus agalactiae
S. aureus Staphylococcus aureus
S. canis Streptococcus canis
S. capitis Staphylococcus capitis
S. chromogenes Staphylococcus chromogenes
S. epidermidis Staphylococcus epidermidis
S. hyicus Staphylococcus hyicus
S. pseudintermedius Staphylococcus pseudintermedius
S. schleiferi Staphylococcus schleiferi
S. sciuri Staphylococcus sciuri
SC Subcutaneous
SLNs Solid lipid nanoparticles
US-FDA US Food and Drug Administration
UV Ultraviolet
Keywords: wine by-products, antimicrobial, animal skin infections, polyphenols, antimicrobial resistance, animal healthcare applications
Citation: Chen W, Pandey P, Ziora ZM, Jayasree A and Parekh HS (2025) Grape by-products from the wine industry – an untapped sustainable resource for application in animal skin health preservation and treatment. Front. Pharmacol. 16:1620087. doi: 10.3389/fphar.2025.1620087
Received: 29 April 2025; Accepted: 21 July 2025;
Published: 26 August 2025.
Edited by:
Antonella Smeriglio, University of Messina, ItalyReviewed by:
Federica Turrini, University of Genoa, ItalyRoberta Hoskin, North Carolina State University, United States
Adriana Maite Fernández-Fernández, University of the Republic, Uruguay
Copyright © 2025 Chen, Pandey, Ziora, Jayasree and Parekh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anjana Jayasree, YS5qYXlhc3JlZUB1cS5lZHUuYXU=; Harendra S. Parekh, aC5wYXJla2hAdXEuZWR1LmF1
†ORCID: Wanting Chen, orcid.org/0009-0005-9432-048X; Preeti Pandey, orcid.org/0000-0001-7826-2375; Zyta M. Ziora, orcid.org/0000-0002-9664-3101; Anjana Jayasree, orcid.org/0000-0003-4719-0174; Harendra S. Parekh, orcid.org/0000-0001-8792-4318
 Wanting Chen
Wanting Chen Preeti Pandey
Preeti Pandey Zyta M. Ziora2,3†
Zyta M. Ziora2,3† Anjana Jayasree
Anjana Jayasree Harendra S. Parekh
Harendra S. Parekh