Abstract
Objective:
To investigate the median effective dose (ED50) and 95% effective dose (ED95) of oliceridine combined with propofol for painless gastroscopy in adults.
Methods:
Patients underwent painless gastroscopy were divided to male and female cohorts. A modified Dixon’s up-and-down sequential method was employed, with an initial oliceridine dose of 20 μg·kg−1 for both cohorts. Subsequent dosing adjustments were determined by the procedural success or failure of the preceding patient. The oliceridine dose was increased or decreased by a ratio of 1:1.2 for positive responses or negative responses. We recorded the time of successful induction, examination time, vital signs (HR, SpO2 and MAP) at predefined phases (including baseline T0, post-induction time T1, completion time T2, and departure time T3), induction dose and total dose of propofol, dose of oliceridine, intraoperative adverse events (including hypoxemia, respiratory depression, hypotension, and bradycardia), postoperative adverse events (including nausea, vomiting, and dizziness), and vasoactive agent administration during the procedure. Probit analysis was subsequently performed to determine the ED50, ED95 and corresponding 95% confidence intervals (CIs) of oliceridine in painless gastroscopy combined with propofol.
Results:
The ED50 and ED95 of oliceridine combined with propofol were determined as 12.63 μg·kg−1 (95% CI: 11.43–13.79) and 14.46 μg·kg−1 (95% CI: 13.41–20.33) in males, and 10.38 μg·kg−1 (95% CI: 9.02–11.96) and 13.19 μg·kg−1 (95% CI: 11.62–28.23) in females. Male negative subgroup required higher oliceridine doses (P < 0.05), while female negative subgroup had lower total propofol dose yet higher oliceridine doses (P < 0.05). Females in the negative subgroup used more propofol (P < 0.05), and both sexes’ negative subgroups consumed more oliceridine (P < 0.05). In males, SpO2 rose at T1 and T2 (P < 0.01), and MAP dropped at T2 and T3 (P < 0.05). In females, HR decreased at T2 (P < 0.05), SpO2 increased at T1 (P < 0.05), and MAP fell at T2 and T3 (P < 0.05). Adverse events included postoperative dizziness (12.50%), nausea (4.17%), and fatigue (4.17%) in females, and vomiting (5.56%) in males.
Conclusion:
The use of oliceridine (13.19–14.46 μg·kg−1) and propofol was associated with safety, efficacy, and lower complication rates during painless gastroscopy.
Clinical Trial Registration:
https://www.chictr.org.cn/showproj.html?proj=249883, identifier ChiCTR2400093609
1 Introduction
Gastroscopy is the gold standard for diagnosing upper gastrointestinal diseases and can effectively detect conditions such as gastritis, gastric polyps, and gastric or esophageal tumors. Relevant epidemiological studies indicate that gastroscopy is recommended for the general population aged ≥45 years, and individuals with normal findings are advised to undergo repeat gastroscopy every 3–5 years, while patients diagnosed with chronic atrophic gastritis or higher-grade lesions should receive annual endoscopic surveillance (Li et al., 2024). Nevertheless, the clinical utility of conventional gastroscopy is compromised by procedure-induced viscerosensory reactions (including emesis, laryngospasm, and autonomic instability), which contribute to suboptimal adherence. Among patients undergoing gastroscopy, 41%–61% exhibited clinically significant procedural discomfort and anxiety (Abraham et al., 2002). With advancements in medical technology and heightened health awareness, painless gastroscopy has gained increasing clinical adoption. Sedated gastroscopy maintains patients in a sleep state throughout the procedure, effectively eliminating anxiety and pain during procedure. This approach not only enhances endoscopic visualization quality but also facilitates the detection of subtle early-stage lesions and improves diagnostic accuracy. The current pharmacological mainstay predominantly utilizes propofol combined with opioids. Propofol demonstrates favorable sedative properties, including rapid onset, short duration of action, and predictable recovery profiles. However, its limited analgesic efficacy and inability to suppress stress responses necessitate dose escalation, which may induce dose-dependent hypotension and respiratory complications (e.g., shallow breathing, bradypnea, or transient apnea) (Martorano et al., 2008). Consequently, adjunct administration with opioid analgesics remains standard practice. Nevertheless, the concomitant use of traditional opioids such as fentanyl elevates risks of opioid-related adverse events, particularly nausea/vomiting and respiratory depression (Tsai et al., 2021). It was demonstrated that the incidence of opioid-related adverse events (ORAE) has been reported to reach 10.6%, which is associated with prolonged hospital stays, increased in-hospital mortality, elevated healthcare expenditures, and higher 30-day readmission rates (Shafi et al., 2018).
Oliceridine is a novel opioid analgesic that functions as a G protein-biased μ-opioid receptor agonist. It provides effective analgesia while demonstrating a reduced incidence of adverse effects such as respiratory depression and gastrointestinal dysfunction compared to conventional opioids (Simons et al., 2023). Based on oliceridines’ pharmacokinetic profile, intravenous bolus administration achieves rapid onset of action within 1–2 min, peaks at 6–12 min, and maintains therapeutic effects for approximately 1–3 h (Kaye et al., 2021). The drug is primarily metabolized by cytochrome P450 (CYP) hepatic enzymes, with inactive metabolites exhibiting negligible pharmacological activity. Pharmacokinetic studies reveal comparable clearance between patients with hepatic or renal impairment and those with normal organ function, supporting its safety in these populations (Nafziger et al., 2020). However, dose reduction may be required for moderate-to-severe hepatic impairment during prolonged administration (Gan and Wase, 2020). These characteristics make oliceridine not only suitable for acute pain management but also advantageous for short-duration procedures such as gynecological interventions and gastrointestinal endoscopy.
Oliceridine demonstrates potent analgesic efficacy with a favorable adverse effect profile characterized by reduced incidence of respiratory depression and gastrointestinal dysfunction. This pharmacological advantage suggests that its combination with propofol may establish a safer and more effective paradigm for painless diagnostic or therapeutic procedures. However, current evidence on oliceridine utilization in non-operating room anesthesia (NORA) contexts remains limited. The safety and efficacy profiles have not been fully characterized owing to a paucity of data regarding clinically validated dosing regimens, and no studies to date have systematically investigated potential sex-specific variations in therapeutic outcomes. This study therefore quantified the median effective dose (ED50) and 95% effective dose (ED95) of oliceridine-propofol coadministration stratified by biological sex during procedural sedation. The established dose-response profiles provide pharmacodynamic references to optimize gender-tailored oliceridine administration in clinical practice.
2 Methods
2.1 Ethics and trial registration
Written informed consent was obtained from all enrolled participants, with explicit assurance of voluntary withdrawal rights throughout the trial duration. The study protocol received ethical endorsement from the Institutional Review Board (IRB) of the ethics committee at NO.215 Hospital of Shaanxi Nuclear Industry [Approval No. 2024(030)] and was prospectively registered in the ClinicalTrials.gov registry (Registration ID: ChiCTR2400093609).
2.2 Patients
Patients scheduled for painless gastroscopy between January to March 2025 were screened. Inclusion criteria: (1) age 18–64 years; (2) BMI 18.5–29.9 kg/m2; (3) ASA physical status I to II; (4) indicated for diagnostic painless gastroscopy. Exclusion criteria: (1) severe cardiopulmonary, cerebrovascular, hepatic, or renal comorbidities; (2) history of documented hypersensitivity to anesthetic agents or excipients; (3) requirement for advanced endoscopic interventions. Elimination criteria: (1) occurrence of severe anesthesia- or procedure-related complications; (2) voluntary withdrawal from the study.
2.3 Study interventions
All participants adhered to standardized preoperative fasting protocols (8 h for solids, 3 h for clear liquids) without premedication. Upon entering the procedure suite, intravenous access was established with continuous monitoring of non-invasive blood pressure (NIBP), electrocardiogram (ECG), and peripheral oxygen saturation (SpO2). Preoxygenation was administered via an endoscopy-specific facemask at 5 L/min. Sedation was initiated with intravenous (IV) oliceridine (Jiangsu Nhwa Pharmaceutical Co., Ltd., Jiangsu, China) over 2 min, followed by propofol (Guangdong Jiabo Pharmaceutical Co., Ltd., Guangdong, China) delivered at 40 mg/10 s. Endoscopic insertion commenced when the Observer’s Assessment of Alertness/Sedation (OAA/S) scale (score 5: responds readily to verbal commands in normal tone; score 4: lethargic but appropriate response to commands in normal tone; score 3: responds only after name is called repeatedly and/or loudly and requires tactile stimulation to elicit movement; score 2: responds only to mild noxious prodding or shaking; score 1: no response and motor reflexes to painful stimuli) reached ≤1. Preliminary trials indicated that a propofol dosage range of 1–1.5 mg·kg−1 was required to achieve OAA/S score ≤1 in patients. Hemodynamic support: dopamine (1–2 mg IV) was given intravenously for mean arterial pressure (MAP) < 60 mmHg or systolic blood pressure decreased 20% of baseline; Atropine (0.5 mg IV) for heart rate (HR) < 50 bpm; Jaw-thrust maneuver with supplemental oxygen for oxygen saturation (SpO2) <90%. All procedures were performed by a single attending anesthesiologist and the same gastroenterologist.
2.4 Modified Dixon’s up-and-down sequential method
Enrolled patients were stratified into male and female cohorts. Based on pre-trial pharmacokinetic and morphine milligram equivalent (MME) conversion analysis, the initial oliceridine dose was set at 20 μg·kg−1 (equivalent for both sexes), with this regimen demonstrating equipotent analgesic efficacy to 0.1 μg·kg−1 sufentanil. The oliceridine dose was increased or decreased by a ratio of 1:1.2 (Xu et al., 2023) for positive responses (failure) or negative responses (success). Subsequent dosing adjustments were determined by the procedural success/failure of the preceding patient. The formal test commenced at the first crossover point and terminated upon observing seven consecutive reversals (Pace and Stylianou, 2007). Positive response was defined as (Feng et al., 2019): during endoscope insertion or within 3 min of pharyngeal entry, (1) body movement or cough ≥ grade 2 (purposeful limb movement or persistent cough requiring temporary suspension of the procedure); or (2) blood pressure or heart rate exceeding 30% of baseline values. Positive responders requiring sedation initially received propofol (0.5 mg·kg−1 IV bolus; maximum two cumulative doses). If inadequate gastroscopic conditions persisted, a rescue regimen of sufentanil (Yichang Renfu Pharmaceutical Co., Ltd., Hubei, China, 0.05 μg·kg−1 IV) co-administered with propofol (1 mg·kg−1 IV) was administered as an adjunctive analgesic protocol. All patients were transferred to the post-anesthesia care unit (PACU) for recovery, where anesthesia nurses continuously monitored vital signs. Patients were discharged from the PACU upon achieving a modified Aldrete score of ≥9. All cases underwent follow-up within 24 h.
2.5 Statistical analysis
Statistical analyses were performed using SPSS Statistics version 27.0 (IBM Corp.). Normally distributed continuous variables are presented as mean ± standard deviation (SD), with between-group comparisons conducted via independent Student's t-tests. Non-normally distributed data are expressed as median (interquartile range, IQR) and analyzed using Mann-Whitney U tests. The categorical data were subjected to analysis using the chi-square test. Post hoc pairwise comparisons among multiple groups were performed using one-way analysis of variance (ANOVA). Probit regression analysis was employed to calculate the ED50 and ED95 of oliceridine for painless gastroscopy, with corresponding 95% confidence intervals (95% CI). Dose-response curves and sequential trial plots were generated using GraphPad Prism version 5.0 (GraphPad Software Inc.). A two-tailed p-value <0.05 defined statistical significance.
3 Outcomes
3.1 Primary outcomes
The ED50 and ED95 of oliceridine with 95% CI combined with propofol for painless gastroscopy in male and female cohort.
3.2 Other outcomes
Demographic and clinical baseline characteristics included age, height, weight, BMI, ASA physical status and basic vital signs.
Induction dose and total administered dose of propofol, dose of oliceridine, time to successful induction, gastroscopy duration (from scope insertion to withdrawal) and administration of vasoactive agents were recorded.
HR, SpO2 and MAP were monitored at predefined intervals: baseline (T0), post-induction time (T1), completion time (T2) and departure time (T3).
Adverse events were categorized as:
1. Intraoperative: hypoxemia (SpO2 <90% for >30s), respiratory depression (respiratory rate <8 breaths/min), hypotension (MAP <60 mmHg or systolic blood pressure decreased >20% of baseline), bradycardia (HR < 50 bpm).
2. Postoperative: nausea, vomiting, dizziness and fatigue after discharge.
4 Results
The study cohort comprised 24 female patients and 19 male patients. One participant in the male group was lost to follow-up after endoscopic procedure (
Figure 1). The baseline characteristics of the patients are summarized in
Table 1. No statistically significant differences in baseline characteristics were observed between positive and negative subgroups within either sex-stratified cohort (P > 0.05). In the male cohort, patients in the negative group required higher doses of oliceridine compared to the positive group (P < 0.05). In the female cohort, the total propofol dose was lower in the negative group than in the positive group (P < 0.05), while oliceridine doses was higher in the negative group (P < 0.05) (
Table 1).
1. Our study was conducted until data for seven crossover points were collected (Figure 2). The ED50 and ED95 values of oliceridine fumarate combined with propofol in painless gastroscopy were 10.38 μg·kg−1 (95% CI: 9.02–11.96) and 13.19 μg·kg-1 (95% CI: 11.62–28.23) in the female group, and 12.63 μg·kg−1 (95% CI: 11.43–13.79) and 14.46 μg·kg−1 (95% CI: 13.41–20.33) in the male group, respectively (Figure 3).
2. No significant differences were observed in the time to successful induction, gastroscopy duration, or propofol induction dose between positive and negative subgroups within male and female cohorts (P > 0.05). In the female cohort, the total propofol dose was significantly higher in the negative subgroup compared to the positive subgroup (P < 0.05). No statistically significant difference in total propofol consumption was observed between negative and positive subgroups within the male cohort(P > 0.05). Oliceridine consumption was significantly higher in negative subgroups compared to positive subgroups within both male and female cohorts (P < 0.05) (Table 1).
3. In male cohorts, compared to T0, SpO2 significantly increased at T1 and T2 (P < 0.01), while MAP decreased at T2 and T3 (P < 0.05). In female cohorts, compared to T0, HR significantly decreased at T2 (P < 0.05), SpO2 increased at T1 (P < 0.05), and MAP decreased at T2 and T3 (P < 0.05). None of the patients required vasoactive drugs during the study period (Table 2).
4. Adverse events
FIGURE 1
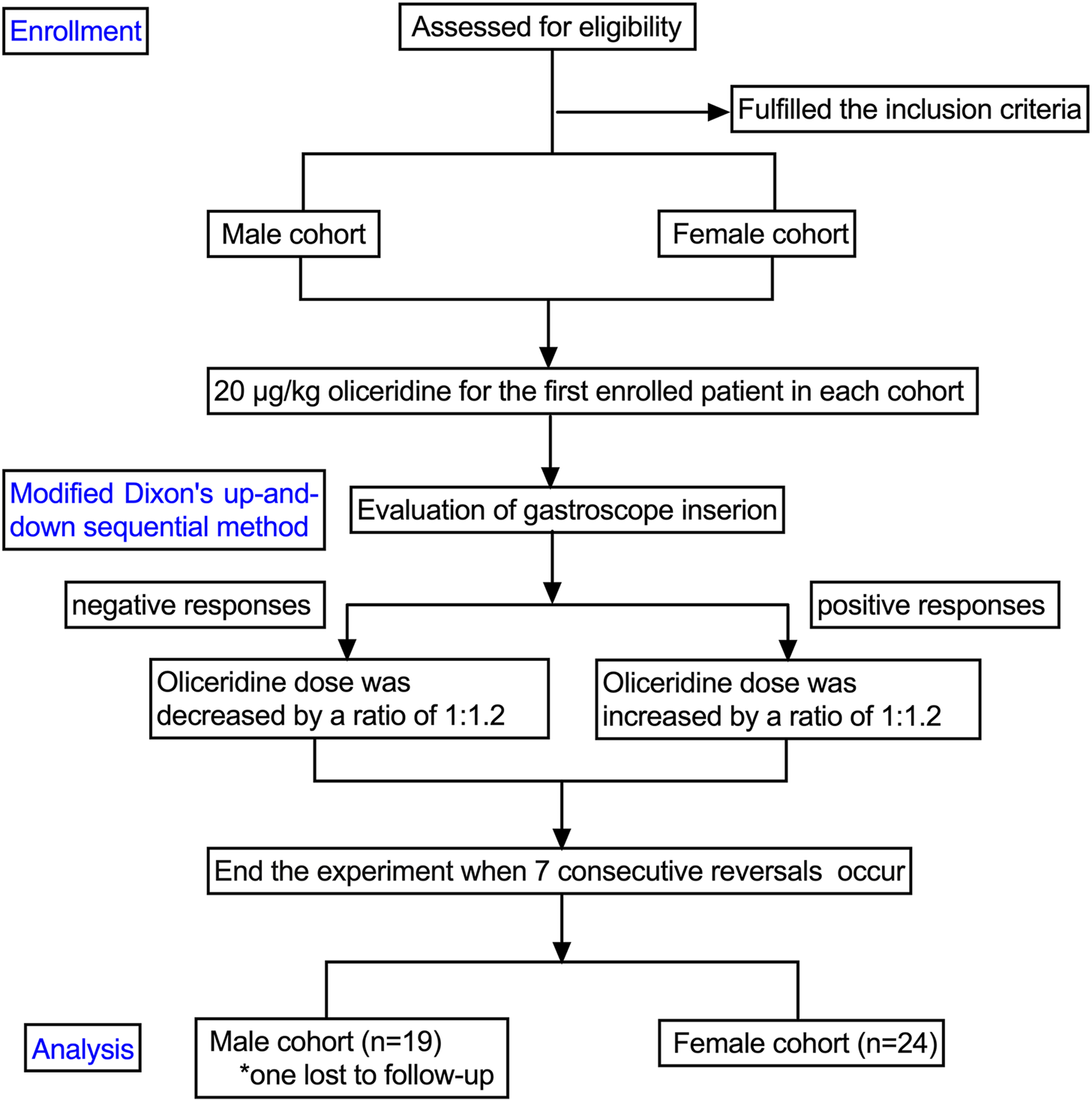
The flowchart of the study. Participants are assessed for eligibility, with those fulling criteria divided into male and female cohorts. Both receive 20 micrograms per kilogram of oliceridine. Using a modified Dixon’s method, responses to gastroscope insertion are evaluated. Negative responses prompt a 1:1.2 dose reduction; positive responses lead to a 1:1.2 increase. The experiment concludes after seven consecutive reversals. Final analysis includes 19 males, with one lost to follow-up, and 24 females.
TABLE 1
| Parameter | Male cohort (n = 19) |
t/Z/χ 2 value | P value | Female cohort (n = 24) |
t/Z/χ 2 value | P value | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| Age (years) | 46.75 (10.22) | 49.45 (13.03) | t (0.49) | 0.63 | 49.80 (11.21) | 45.36 (9.01) | t (1.08) | 0.29 |
| Height (cm) | 172.13 (3.72) | 172.36 (4.13) | t (0.13) | 0.90 | 160.40 (4.72) | 158.50 (3.52) | t (1.13) | 0.27 |
| Weight (kg) | 72.00 (8.21) | 72.45 (5.32) | t (0.15) | 0.89 | 57.70 (4.81) | 56.00 (6.97) | t (0.66) | 0.51 |
| BMI (kg/m2) | 24.24 (2.38) | 24.53 (1.89) | t (0.30) | 0.77 | 22.40 (1.11) | 22.43 (2.53) | t (0.04) | 0.97 |
| ASA | ||||||||
| I (%) | 0 | 2 | χ 2 (1.63) | 0.20 | 2 | 4 | χ2 (0.23) | 0.63 |
| II (%) | 8 | 9 | 8 | 10 | ||||
| Induction time (min), Median (IQR) |
3.00 (0.75) | 3.00 (0) | Z (0.82) | 0.42 | 3.00 (1.25) | 3.00 (1.00) | Z (1.00) | 0.32 |
| Gastroscopy duration (min) | 5.38 (2.20) | 7.45 (3.08) | t (1.63) | 0.12 | 5.50 (2.25) | 5.00 (1.00) | Z (0.62) | 0.54 |
| Induction propofol dosage (mg), Median (IQR) | 88.75 (13.56) | 90.00 (7,75) | t (0.26) | 0.80 | 74.00 (14.30) | 73.93 (8.13) | t (0.02) | 0.99 |
| Total propofol dosage (mg), Median (IQR) | 133.13 (16.68) | 146.36 (33.25) | t (1.03) | 0.32 | 128.00 (39.38) | 99.29 (19.70) | t (2.36) | 0.03* |
| Oliceridine dosage (mg), Median (IQR) | 11.57 (0.00) | 13.89 (2.78) | Z (3.22) | 0.00* | 9.65 (2.09) | 11.57 (4.24) | Z (2.97) | 0.00* |
Characteristics of patients.
Data are presented as the mean ± SD, median (interquartile range) or as the number (proportion) as appropriate. Two-sided t-test, U-text, or Chi-square test, *P < 0.05.
FIGURE 2
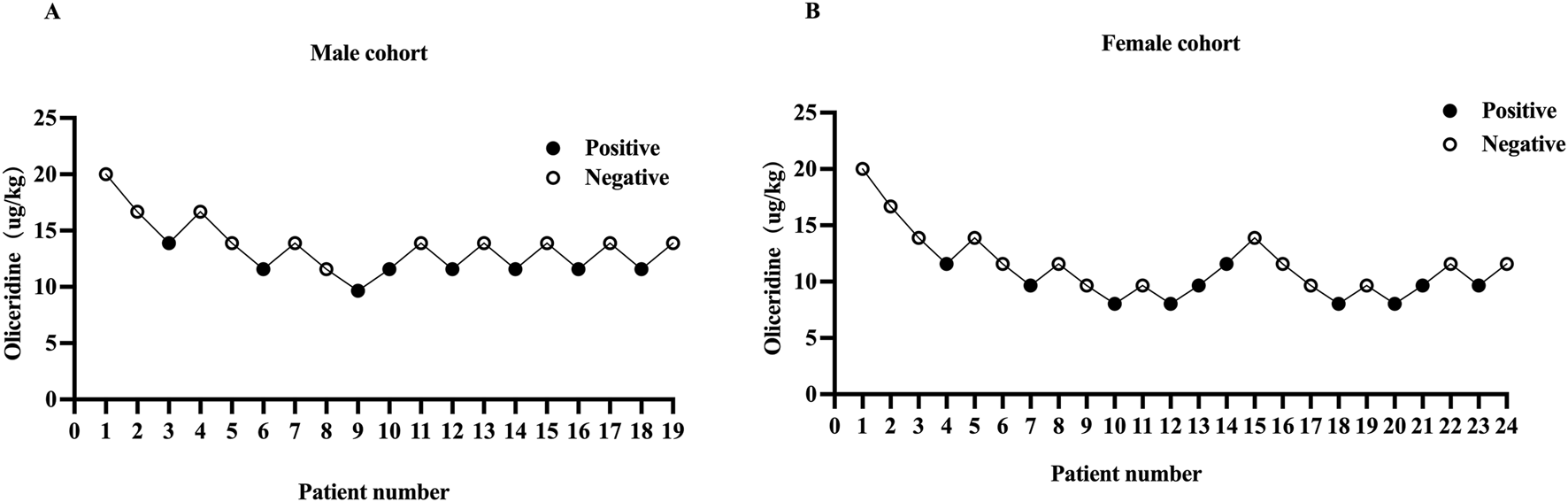
Individual responce to the stimulus of gastroscopy placement in male (A) and female (B) cohorts. The black dots represent “positive” responses and the white dots represent “negative” responses.
FIGURE 3
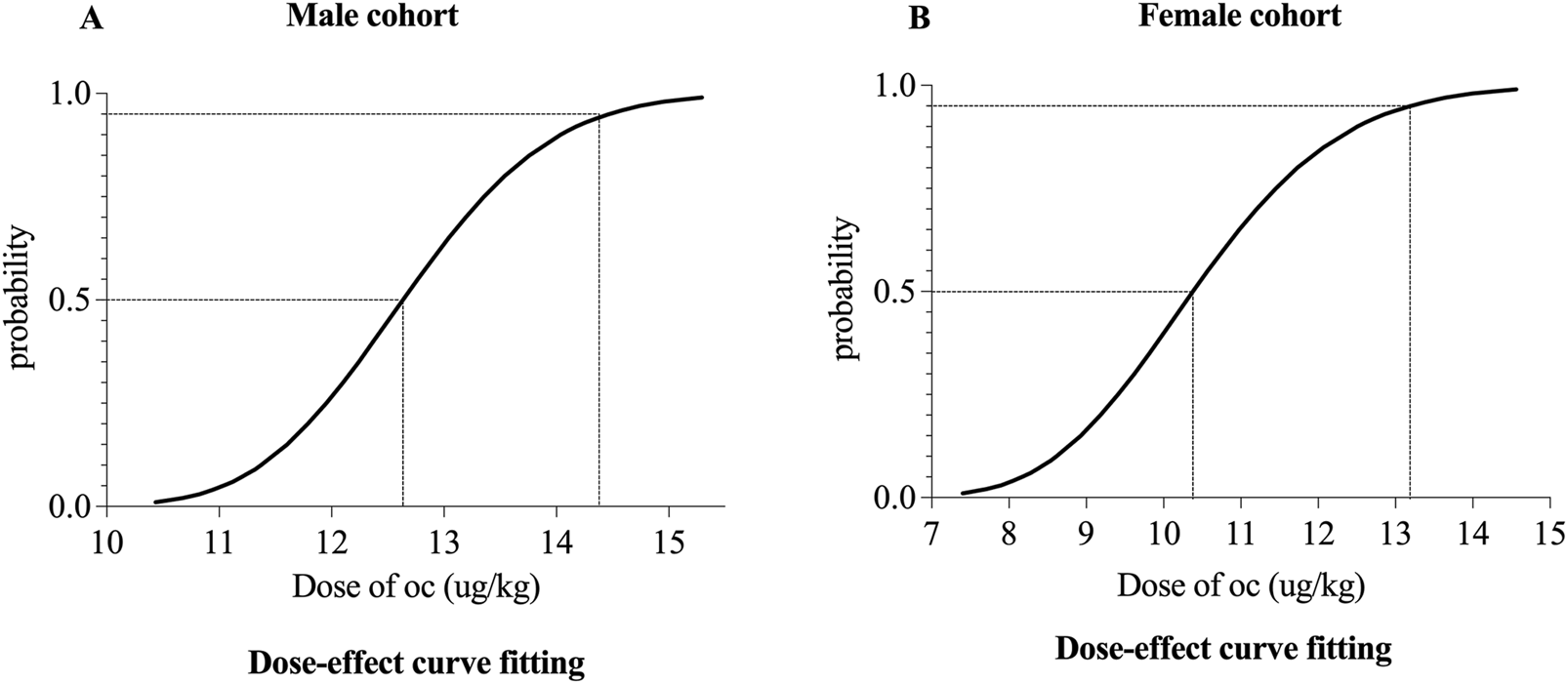
Dose–response curve of oliceridine in painless gastroscopy for male (A) and female (B) cohorts.
TABLE 2
| Parameter | Male cohort (n = 19) | P | Female cohort (n = 24) | P |
|---|---|---|---|---|
| HR (bmp) | ||||
| T0 | 77.63 (10.53) | 80.50 (8.72) | ||
| T1 | 75.00 (8.76) | 1.00 | 73.15 (9.13) | 0.08 |
| T2 | 75.89 (8.41) | 1.00 | 72.25 (6.22) | 0.03* |
| T3 | 76.63 (7.89) | 1.00 | 76.17 (8.78) | 1.00 |
| SpO2 (%) | ||||
| T0 | 97.21 (1.58) | 97.71 (1.38) | ||
| T1 | 99.16 (1.54) | 0.00* | 99.18 (1.29) | 0.02* |
| T2 | 98.84 (1.54) | 0.02* | 98.96 (1.30) | 0.11 |
| T3 | 97.42 (1.71) | 1.00 | 97.63 (2.04) | 1.00 |
| MAP (mmHg) | ||||
| T0 | 92.68 (11.04) | 90.79 (10.81) | ||
| T1 | 84.21 (13.07) | 0.21 | 87.00 (12.93) | 1.00 |
| T2 | 77.00 (9.86) | 0.00* | 74.46 (11.02) | 0.00* |
| T3 | 78.42 (10.22) | 0.00* | 76.96 (10.21) | 0.00* |
HR, SpO2 and MAP at different time points for patients.
Data are presented as mean ± SD or number. Significance for post hoc analysis after analysis of variance (ANOVA) was corrected with Bonferroni’s method. Compared with T0 *P < 0.05. T0 is the time before the administration of oliceridine (baseline); T1 is the time of post-induction; T2 is the time of completion; T3 is the time of departure.
No significant intraoperative complications, including hypotension, bradycardia, or respiratory depression, were observed in any patient during the procedure. Male patients exhibited vomiting (1/18, 5.56%) and one male participant was lost to follow-up during the study period. Among female, postoperative adverse events included dizziness (3/24, 12.50%), nausea (1/24, 4.17%), and fatigue (1/24, 4.17%) (Table 3).
TABLE 3
| Adverse event | Male cohort (n = 18) | Female cohort (n = 24) | P |
|---|---|---|---|
| Dizziness | 0 | 3 (12.50%) | 0.25 |
| Nausea | 0 | 1 (4.17%) | 1.00 |
| Vomiting | 1 (5.56%) | 0 | 0.43 |
| Fatigue | 0 | 1 (4.17%) | 1.00 |
Postoperative adverse events data.
Chi-squared test and Fisher’s exact test were used to evaluate the differences between two cohorts.
5 Discussion
Our study established the effective dose of oliceridine combined with propofol for gastroscopic procedures. Probit regression analysis determined the ED50 and ED95 of oliceridine to suppress endoscope insertion responses as 10.38 and 13.19 μg·kg−1 in females, 12.63 and 14.46 μg·kg−1 in males. All patients safely and effectively completed the examination without serious complications. Jia et al. (2024) demonstrated that the ED50 of oliceridine combined with propofol for suppressing gastroscope insertion responses was 15 μg·kg−1 in patients aged 18–65 years, compared with 12 μg·kg−1 in those aged >65 years. These findings are similar with our observations. The study by Tang et al. (Tang et al., 2025) reported ED90 and ED99 values of 22.5 and 23.8 μg·kg−1 for oliceridine. The observed discrepancies in our findings may be attributed to methodological variations, particularly their use of the biased coin design (BCD), which potentially enhances the precision of ED90 determination compared to our approach.
Previous studies have demonstrated sex-related differences in the analgesic efficacy of various opioid analgesics. Our findings revealed that both ED50 and ED95 values were significantly lower in the female cohort compared to the male cohort. The mechanisms underlying these sex-based disparities in opioid efficacy are multifactorial. Key contributors include differential pain sensitivity, sex-specific nociceptive signal processing, and variations in drug concentration requirements, receptor sensitivity, and gonadal hormone modulation (Bradbury, 2003; Chaudhary et al., 2017). Notably, neuroimaging evidence indicates that females exhibit stronger μ-opioid receptor binding capacity in cortical and subcortical regions, enabling enhanced analgesic responses to equivalent opioid doses (Bodnar and Kest, 2010). Furthermore, heightened opioid sensitivity in females may stem from gonadal hormone-mediated regulation of opioid receptors (Joe et al., 2016). These hormonal interactions potentiate the inhibitory effects of opioids on stress responses in female patients, underscoring the necessity for sex-specific considerations in clinical analgesic regimens. In clinical practice, individualized dose adjustments of oliceridine should be implemented based on patients’ sex, body weight, pain intensity, and previous medication responses, with particular attention to the potential requirement for lower initial doses in female populations. Close monitoring of therapeutic responses, especially in female patients, remains imperative. These tailored therapeutic strategies and vigilant surveillance constitute the cornerstone for ensuring optimal drug safety and efficacy.
Oliceridine dosage requirements exhibit significant variation across different clinical procedures. Zhang et al. (2024) reported that the ED50 of oliceridine combined with 2 mg·kg−1 propofol for analgesia during induced abortion was 19 μg·kg−1 (95% CI: 15–23 μg·kg−1) in patients with a history of vaginal delivery, compared to 26 μg·kg−1 (95% CI: 21–31 μg·kg−1) in those without such history. Compared to gastroscopy, induced abortion procedures induce more intense nociceptive stimulation, thus necessitating higher oliceridine doses to achieve adequate pain control.
Oliceridine demonstrates approximately 5-fold greater analgesic potency than morphine, effectively suppressing gag reflex, cough, and cardiovascular adverse responses triggered by gastroscope passage through the oropharynx. When combined with propofol, it reduces propofol requirements while mitigating the risk of severe respiratory depression. No procedure-related adverse events (respiratory depression, hypotension, or bradycardia) were observed in the study cohort. This safety profile may be attributed to two key factors: (1) oliceridine’s minimal respiratory depressive effects, as evidenced by prior studies showing comparable respiratory depression rates between oliceridine and placebo even in high-risk populations (BMI ≥30 kg/m2) (Brzezinski et al., 2021); (2) All patients in this study were administered oxygen via dedicated endoscopic masks, which potentially contributed to the reduced incidence of hypoxemia.
Oliceridine has gained significant interest for demonstrating a reduced risk of adverse events (e.g., respiratory depression and gastrointestinal complications) relative to conventional opioids, thereby positioning itself as a potential therapeutic breakthrough to mitigate the clinical challenges associated with existing opioid regimens (Wang et al., 2024). A systematic review and meta-analysis of randomized controlled trials has shown that oliceridine exhibits robust therapeutic efficacy and a favorable safety profile as an intravenous analgesic for postoperative pain management, delivering prompt pain relief with superior tolerability and a significantly lower risk of adverse events relative to morphine and hydromorphone (Biskupiak et al., 2024; Niu et al., 2023). In our study, nausea and vomiting were reported in both male and female cohorts, with a higher incidence observed among female participants. This disparity may be associated with sex-specific physiological characteristics in females, potentially linked to differential pharmacokinetic responses or estrogen-mediated visceral sensitivity modulation.
Postoperative adverse events were infrequent: dizziness (3/24, 12.5%), nausea (1/24, 4.2%), and fatigue (1/24, 4.2%) in females; vomiting (1/19, 5.3%) and one loss to follow-up in males. These findings align with oliceridine’s biased μ-opioid receptor agonism-preferentially activating G protein-coupled signaling pathways while minimizing β-arrestin recruitment-thereby reducing classic opioid-related adverse effects without compromising analgesia (Bergese et al., 2019; Sun et al., 2023).
This study has the following limitations. Firstly, the research was constrained by the modified sequential method and its single-center design, leading to a relatively limited final sample size. Adopting multicenter studies with larger sample sizes would enhance the accuracy of the findings. Secondly, patients with comorbid chronic conditions were not included in this investigation. The anesthetic efficacy and safety of propofol combined with oliceridine in populations with underlying chronic diseases require further exploration. Thirdly, this study did not employ end-tidal carbon dioxide (PetCO2) monitoring. Future research incorporating PetCO2 monitoring could more sensitively and precisely reveal the dynamic changes in respiratory depression during sedation and its independent association with adverse reactions such as hemodynamic changes. Fourthly, additional randomized controlled trials are warranted to investigate the clinical applications of oliceridine in anesthesia practice.
6 Conclusion
The combination of oliceridine and propofol effectively suppresses the response to gastroscope insertion during painless gastroscopy, with high safety and minimal complications. In the female group, the ED50 of oliceridine was 10.38 μg·kg−1, and the ED95 was 13.19 μg·kg−1. In the male group, the ED50 of oliceridine was 12.63 μg·kg−1, and the ED95 was 14.46 μg·kg−1.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of the ethics committee at NO. 215 Hospital of Shaanxi Nuclear Industry [Approval No. 2024(030)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Methodology, Conceptualization, Writing – original draft. XG: Visualization, Writing – review and editing, Formal Analysis, Software. XZ: Data curation, Methodology, Writing – original draft. YC: Writing – original draft, Data curation. LJ: Funding acquisition, Writing – review and editing, Resources, Conceptualization, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Beijing Kangmeng Charity Foundation (YXHA-2024091-003).
Acknowledgments
We sincerely appreciate the academic guidance and intellectual support from Professor Chunling Jiang throughout this research endeavor. Special recognition is extended to the endoscopic team for their proficient execution of clinical procedures. Their commitment to maintaining optimal operational standards under high-throughput clinical conditions was instrumental in ensuring the reliability of research outcomes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abraham N. Barkun A. Larocque M. Fallone C. Mayrand S. Baffis V. et al (2002). Predicting which patients can undergo upper endoscopy comfortably without conscious sedation. Gastrointest. Endosc.56 (2), 180–189. 10.1016/s0016-5107(02)70175-2
2
Bergese S. D. Brzezinski M. Hammer G. B. Beard T. L. Pan P. H. Mace S. E. et al (2019). ATHENA: a phase 3, open-label study of the safety and effectiveness of oliceridine (TRV130), A G-Protein selective agonist at the µ-Opioid receptor, in patients with moderate to severe acute pain requiring parenteral opioid therapy. J. Pain Res.12, 3113–3126. 10.2147/JPR.S217563
3
Biskupiak J. Oderda G. Brixner D. Wandstrat T. L. (2024). Gastrointestinal adverse effects associated with the use of intravenous oliceridine compared with intravenous hydromorphone or fentanyl in acute pain management utilizing adjusted indirect treatment comparison methods. J. Comp. Eff. Res.13 (5), e230041. 10.57264/cer-2023-0041
4
Bodnar R. J. Kest B. (2010). Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm. Behav.58 (1), 72–81. 10.1016/j.yhbeh.2009.09.012
5
Bradbury J. (2003). Why do men and women feel and react to pain differently? Research suggests men and women May not process pain signals the same way. Lancet361 (9374), 2052–2053. 10.1016/s0140-6736(03)13679-3
6
Brzezinski M. Hammer G. B. Candiotti K. A. Bergese S. D. Pan P. H. Bourne M. H. et al (2021). Low incidence of opioid-induced respiratory depression observed with oliceridine regardless of age or body mass index: exploratory analysis from a phase 3 open-label trial in postsurgical pain. Pain Ther.10 (1), 457–473. 10.1007/s40122-020-00232-x
7
Chaudhary M. A. Schoenfeld A. J. Harlow A. F. Ranjit A. Scully R. Chowdhury R. et al (2017). Incidence and predictors of opioid prescription at discharge after traumatic injury. JAMA Surg.152 (10), 930–936. 10.1001/jamasurg.2017.1685
8
Feng A. M. He S. S. Wang J. W. Xing F. Li J. Lu X. H. (2019). Effects of different concentrations of oxycodone on propofol ED50/ED90 during painless gastroscopy. J. Pract. Med.35 (07), 1141–1145. 10.3969/j.issn.1006⁃5725.2019.07.029
9
Gan T. J. Wase L. (2020). Oliceridine, a G protein-selective ligand at the μ-opioid receptor, for the management of moderate to severe acute pain. Drugs Today (Barc)56 (4), 269–286. 10.1358/dot.2020.56.4.3107707
10
Jia J. Li G. S. Zhang B. B. Zhang J. Q. Zhang W. (2024). Effect of age factor on potency of oliceridine in inhibiting responses to gastroscopic implantation when combined with propofol. Chin. J. Anesthesiol.44 (12), 1456–1459. 10.3760/cma.j.cn131073.20240715.01211
11
Joe H. B. Kim J. Y. Kwak H. J. Oh S. E. Lee S. Y. Park S. Y. (2016). Effect of sex differences in remifentanil requirements for the insertion of a laryngeal mask airway during propofol anesthesia: a prospective randomized trial. Medicine95 (39), e5032. 10.1097/MD.0000000000005032
12
Kaye A. D. Edinoff A. N. Babin K. C. Hebert C. M. Hardin J. L. Cornett E. M. et al (2021). Pharmacological advances in opioid therapy: a review of the role of oliceridine in pain management. Pain Ther.10 (2), 1003–1012. 10.1007/s40122-021-00313-5
13
Li J. Y. Shen P. Zhu Z. H. Tang M. L. Shui L. M. Sun Y. X. et al (2024). Chin. J. Epidemiol.45 (9), 1244–1250. 10.3760/cma.j.cn112338-20240322-00147
14
Martorano P. P. Aloj F. Baietta S. Fiorelli A. Munari M. Paccagnella F. et al (2008). Sufentanil-propofol vs remifentanil-propofol during total intravenous anesthesia for neurosurgery. A multicentre study. Minerva Anestesiol.74 (6), 233–243.
15
Nafziger A. N. Arscott K. A. Cochrane K. Skobieranda F. Burt D. A. Fossler M. J. (2020). The influence of renal or hepatic impairment on the pharmacokinetics, safety, and tolerability of oliceridine. Clin. Pharmacol. Drug Dev.9 (5), 639–650. Epub 2019 Nov 7. 10.1002/cpdd.750
16
Niu J. Hu W. Lu Y. Tang H. (2023). Efficacy and safety of oliceridine treatment in patients with postoperative pain: a systematic review and meta-analysis of randomized controlled trials. Expert Rev. Clin. Pharmacol.16 (6), 589–599. 10.1080/17512433.2023.2213889
17
Pace N. L. Stylianou M. P. (2007). Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology107 (1), 144–152. 10.1097/01.anes.0000267514.42592.2a
18
Shafi S. Collinsworth A. W. Copeland L. A. Ogola G. O. Qiu T. Kouznetsova M. et al (2018). Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg.153 (8), 757–763. 10.1001/jamasurg.2018.1039
19
Simons P. van der Schrier R. van Lemmen M. Jansen S. Kuijpers K. W. K. van Velzen M. et al (2023). Respiratory effects of biased ligand oliceridine in older volunteers: a pharmacokinetic-pharmacodynamic comparison with morphine. Anesthesiology138 (3), 249–263. 10.1097/ALN.0000000000004473
20
Sun Q. Li Z. Wang Z. Wang Q. Qin F. Pan H. et al (2023). Immunosuppression by opioids: mechanisms of action on innate and adaptive immunity. Biochem. Pharmacol.209, 115417. 10.1016/j.bcp.2023.115417
21
Tang Z. Yin G. Yu Y. Luo Y. Tian S. Zhang Q. et al (2025). Determination of ED90 and ED99 of oliceridine combined with propofol in inhibiting responses to gastroscope insertion: a biased coin up-and-down design. BMC Anesthesiol.25 (1), 175. 10.1186/s12871-025-03052-8
22
Tsai S. H. L. Yolcu Y. U. Hung S. W. Kurian S. J. Alvi M. A. Fu T. S. et al (2021). The analgesic effect of intravenous lidocaine versus intrawound or epidural bupivacaine for postoperative opioid reduction in spine surgery: a systematic review and meta-analysis. Clin. Neurol. Neurosurg.201, 106438. 10.1016/j.clineuro.2020.106438
23
Wang C. Liu L. Bai X. (2024). Global trends in oliceridine (TRV130) research from 2013 to 2024: a bibliometrics and knowledge graph analysis. Drug Des. Devel Ther.18, 4681–4692. 10.2147/DDDT.S475205
24
Xu C. Peng R. Qian X. Feng S. Yuan H. (2023). Modified dixon sequential method to determine the effective dose of alfentanil compounded with propofol for day-case hysteroscopy. Ther. Adv. Drug Saf.14, 20420986231214992. 10.1177/20420986231214992
25
Zhang B. B. Zhang W. Jia J. Wang Z. L. Li G. S. Zhang J. Q. (2024). Median effective dose of oliceridine combined with propofol for analgesia during induced abortion in patients with different labor histories. Chin. J. Anesthesiol.44 (12), 1437–1440. 10.3760/cma.j.cn131073.20240919.01207
Summary
Keywords
oliceridine, painless gastroscopy, propofol, effective dose 50, effective dose 95
Citation
Cao J, Gu X, Zhang X, Cheng Y and Jiang L (2025) Determination of the effective dose of oliceridine combined with propofol using the modified Dixon’s up-and-down method in painless gastroscopy. Front. Pharmacol. 16:1620158. doi: 10.3389/fphar.2025.1620158
Received
29 April 2025
Accepted
15 October 2025
Published
27 October 2025
Volume
16 - 2025
Edited by
Angelo A. Izzo, University of Naples Federico II, Italy
Reviewed by
Marcelo Adrian Estrin, Interamerican Open University, Argentina
Hong Fu, Chongqing Emergency Medical Center, China
Updates
Copyright
© 2025 Cao, Gu, Zhang, Cheng and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liuqin Jiang, jlq215hp@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.