- 1Kweichow Moutai Distillery Co., Ltd., Renhuai, China
- 2Kweichow Moutai Group, Guizhou Key Laboratory of Microbial Resources Exploration in Fermentation Industry, Zunyi, China
Helicobacter pylori (H. pylori) is considered a key causative agent of gastritis, peptic ulcer, and gastric cancer, affecting more than half of the world’s population. The eradication rate of antibiotic therapy gradually decreases due to the increased risk of resistance. Recent studies have shown that probiotics have good potential in the treatment of H. pylori infection. Several studies involving both human and animal models have demonstrated that probiotic interventions can inhibit H. pylori growth, attenuate H. pylori-induced gastritis, and enhance the eradication rate of antibiotics while reducing side effects. However, there remains some debate regarding the effective benefits of probiotics. The recently published reviews have not systematically elaborated on the differences in outcomes resulting from the use of probiotics of various types and doses, or the combination of probiotics with medications. They have primarily focused on animal studies, without addressing the heterogeneity of results observed in clinical research and the underlying mechanisms, thus failing to provide more high-quality evidence. This review aims to discuss the mechanisms of H. pylori infection in humans, the effects of probiotics in treating H. pylori infection, and the pathways and molecular mechanisms by which probiotics inhibit H. pylori. Future challenges include identifying effective strains, determining optimal doses and treatment durations, standardizing experimental protocols, considering individual variability, and further elucidating the specific molecular mechanisms and long-term impacts of probiotic therapy in H. pylori infection.
1 Introduction
Helicobacter pylori (H. pylori) is a Gram-negative, microaerophilic spiral bacterium, characterized by its unique morphology and flagella, which enable it to traverse the mucus layer in the acidic gastric environment and adhere to epithelial cells, thereby triggering local inflammatory responses. Helicobacter pylori is considered a key pathogenic factor in the development of gastritis, peptic ulcers, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma (Figure 1) (Li et al., 2024). Helicobacter pylori is classified as a group I carcinogen by the World Health Organization. Recently published literature has shown that countries where H. pylori infection rates have declined have also seen a decline in the incidence of gastric cancer (Chen Y-C. et al., 2024). Globally, particularly in developing countries and rural areas, the bacterium is transmitted primarily via the oral-oral or fecal-oral routes, affecting over half of the population (Duan et al., 2023; Stefano et al., 2018). Helicobacter pylori infection typically occurs in childhood, with more than 30% of children being infected (Yuan et al., 2022). Given that children in this early stage of infection are unlikely to develop disease complications, treatment is often considered unnecessary (Saito et al., 2021). Furthermore, H. pylori infection may influence pregnancy outcomes (Masaadeh et al., 2023). Clinical diagnostic methods for H. pylori infection include the urea breath test, stool antigen test, serum antibody test, and gastric biopsy during endoscopy (Figure 1) (Addissouky et al., 2023).
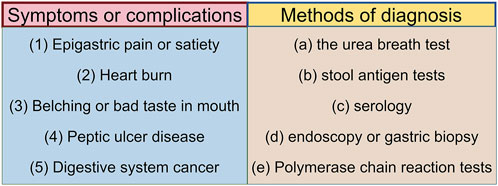
Figure 1. Symptoms after H. pylori infection and corresponding diagnostic tools. The relationship between symptoms and diagnostic tools is as follows: symptom (1), corresponds to diagnostic tools (a), (b), and (d); symptom (2), corresponds to diagnostic tools (a) and (d); symptom (3), corresponds to diagnostic tools (a), (b), and (c); symptom (4), corresponds to diagnostic tools (a), (b), and (d); symptom (5), corresponds to diagnostic tools (d) and (e). (By figdraw).
The standard treatment for H. pylori infection involves a triple therapy or quadruple therapy regimen. Triple therapy typically consists of a proton pump inhibitor (PPI) and two antibiotics administered for 7–14 days, while quadruple therapy adds a bismuth agent to enhance eradication rates. Despite the clinical efficacy of these regimens, significant limitations persist. Firstly, antibiotic resistance, particularly to macrolides such as clarithromycin, has been increasing, leading to a higher treatment failure rate (Malfertheiner et al., 2024). By 2050, it is estimated that 10 million people will die due to antimicrobial resistance infection (de Kraker et al., 2016). Secondly, the complexity of the drug regimen and the extended treatment duration contribute to poor patient adherence. Additionally, common adverse effects, such as gastrointestinal discomfort including diarrhea, further reduce compliance (Chen Z. et al., 2024). In the context of complex gastric lesions and infections, a recent review suggested that traction-assisted endoscopic submucosal dissection may improve clinical treatment efficiency (Niu C. et al., 2024); similarly, microbial modulation potentially improving therapeutic outcomes by promoting gastrointestinal function. In response to these challenges, researchers are developing new anti-H. pylori drugs and exploring simpler and more effective treatment regimens, including new antibacterial drugs, natural plant extracts, anti-H. pylori probiotics and some other anti- H. pylori foods or drugs (Zimmermann and Curtis, 2017; Takeuchi et al., 2014; El et al., 2023; Maria et al., 2023; Shadvar et al., 2024) (Figure 2). Antibiotic choices for children and pregnant women are limited, and probiotic therapy has emerged as a potential alternative approach.
Probiotics are a group of active microorganisms that are beneficial to host health, mainly including lactic acid bacteria and Bifidobacterium. Probiotics exert their beneficial effects through various mechanisms, such as competitive inhibition of pathogenic microorganisms, modulation of the gut microbiota, and enhancement of immune functions. Additionally, probiotics reinforce the mucosal barrier, preventing pathogen invasion or producing metabolites (e.g., short-chain fatty acids and antimicrobial peptides) that inhibit the growth of harmful microorganisms. In the treatment of H. pylori infection, probiotics demonstrate significant adjunctive roles. Certain probiotic strains can directly inhibit the growth of H. pylori or modulate the gastric pH, thereby creating an unfavorable environment for H. pylori survival. Furthermore, probiotics can enhance the host’s immune response, facilitating the clearance of H. pylori. Consequently, probiotics not only reduce the reliance on antibiotics but also alleviate their side effects, making them a promising alternative strategy for the safe and effective treatment of H. pylori infection.
The mechanism of H. pylori infection in human body and the role of probiotics in the treatment of H. pylori infection have been reviewed in some recent literature (Shadvar et al., 2024; Dash et al., 2024; Huang et al., 2024; Liu et al., 2024), which provides a theoretical basis for the feasibility of probiotics in inhibiting H. pylori infection. However, the differences in the results of different types and doses of probiotics, and whether they are combined with drugs have not been systematically described. These reviews focused more on animal experiments and did not sort out the heterogeneous results and related mechanisms produced in clinical research, so as to provide more high-quality evidence and systematic summary. This article aims to systematically review the mechanisms of H. pylori infection in humans, the in vivo effects of probiotics in the treatment of H. pylori infection, and the pathways and molecular mechanisms by which probiotics inhibit H. pylori. Additionally, the article will explore future research directions regarding the use of probiotics in the prevention and control of H. pylori infection.
2 Mechanism and control strategy of Helicobacter pylori infection
The pathways of H. pylori infection in the host include transmission between genetically related individuals and contact with infected individuals of similar socioeconomic status (Stefano et al., 2018). In recent years, significant progress has been made in understanding the mechanisms of H. pylori infection, revealing the complex infection process and immune evasion strategies. The primary process involves H. pylori adapting to the acidic gastric environment, releasing adhesion factors that bind to epithelial cells, and subsequently secreting toxin proteins, thereby establishing a persistent infection (Figure 3). Initially, the TlpB receptor on host cells is triggered by chemical signals, promoting a chemotactic response toward the gastric epithelium (Hanyu et al., 2019). Helicobacter pylori navigates through the acidic environment via its flagella, avoiding direct gastric acid-induced damage, and after traversing the relatively neutral mucus layer, it firmly adheres to gastric epithelial cells (Gu, 2017). Helicobacter pylori secretes urease, which hydrolyzes urea to produce ammonia and carbon dioxide, locally neutralizing gastric acid and creating a microenvironment favorable for its survival (Idowu et al., 2022; Elbehiry et al., 2023). Furthermore, H. pylori utilizes variably expressed adhesins, such as antigen-binding adhesin (BabA), sialic acid-binding adhesins (SabA), outer inflammatory protein (OipA), and outer membrane proteins (OMP), to facilitate the transition from association with the mucus layer to close adhesion to the epithelial cell layer, thereby preventing the bacterium from being affected by host clearance mechanisms (Sharndama and Mba, 2022). Upon tight binding to gastric epithelial cells, adhesins employ a range of effector proteins, including vacuolating cytotoxin A (VacA), cytotoxin-associated gene A (CagA), and cytotoxin-associated gene L protein (CagL), to manipulate host cell signaling and alter the behavior of gastric epithelial cells, thus promoting long-term colonization (Salama et al., 2013). Among these, the CagA pathogenicity island plays a central coordinating role, injecting CagA into host cells via the type IV secretion system, thereby disrupting cellular functions and promoting gastric pathology (Hatakeyama, 2017; Ali and AlHussaini, 2024).
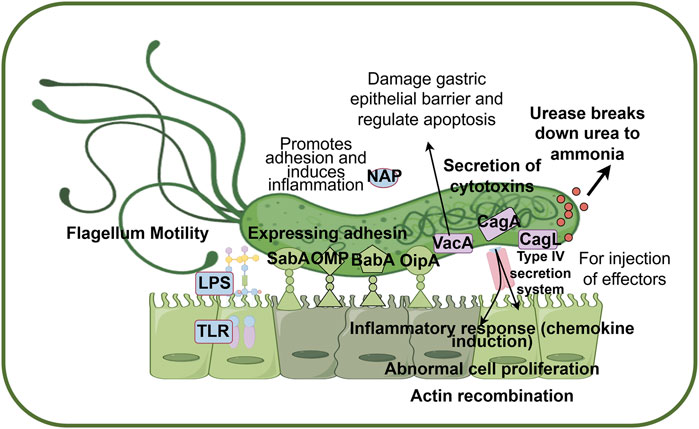
Figure 3. Pathogenic mechanisms of H. pylori infection in hosts. Helicobacter pylori passes through the acidic environment of the stomach by producing ammonia and flagellar movement, and produces adhesin to promote adhesion to host epithelial cells. Finally, H. pylori secreted a series of toxin proteins to destroy epithelial cells and activate the host inflammatory immune response, resulting in persistent infection. Abbreviations: BabA, blood group antigen-binding adhesion; CagA, cytotoxin-associated gene A; CagL, cytotoxin-associated gene L; NAP, neutrophil-activating protein; OipA, outer inflammatory protein A; SabA, sialic acid binding adhesin; VacA, vacuolating cytotoxin A; LPS, lipolyaccharide; TLR, Toll-like receptor; OMP, Outer membrane protein. Factors coded in green are mainly related to adhesion (BabA, SabA, OipA, OMP), factors coded in pink are mainly related to cytotoxin secretion (CagA, CagL, VacA), and factors coded in blue are mainly related to immune response (LPS, TLR, NAP). (By figdraw).
Helicobacter pylori establishes persistent infection by modulating the host immune response. Surface antigens of H. pylori (e.g., lipopolysaccharides) activate the host’s innate immune system through pattern recognition receptors such as TLR2 and TLR4, while its virulence factors (e.g., VacA and CagA) disrupt epithelial cell integrity, induce apoptosis, and trigger inflammatory responses. The condition for CagA to disrupt H. pylori signal transduction pathway is the direct attachment of epithelial cells to integrin-1 (Backert and Tegtmeyer, 2017). After entering the host cell, CagA protein can interact with a variety of signaling molecules, leading to actin reorganization, inflammatory response and abnormal cell proliferation (Shakir et al., 2023). VacA protein is a pore-forming toxin that suddenly kills host cells, interacts with multiple targets, and plays a key role in avoiding immune responses. However, VacA disrupts the integrity of the gastric epithelial barrier by forming channels or pores in the cell membrane, leading to increased permeability, and plays a role in regulating host cell apoptosis according to cell type and environmental conditions (Reyes, 2023; Foegeding et al., 2016). VacA protein also has the function of anti-phagocytosis and producing cytoplasmic vacuoles for H. pylori survival (Elbehiry et al., 2023; Baj et al., 2020). VacA toxin contributes to the formation of vacuoles in host cells and affects the structure and function of cells (Foegeding et al., 2016). In addition, NAP conserved protein can promote the adhesion of H. pylori to gastric mucosa, and induce the synthesis of IL-8, IL-6, TNF-α and other inflammatory substances to damage gastric mucosa (Zhang et al., 2022). Helicobacter pylori colonization, immune escape and causing gastric infection and pathological changes through complex mechanisms. Although the mechanism of infection has been intensively studied, the fine regulation of the interaction between bacteria and the host immune system remains to be revealed.
The detection methods of H. pylori infection include fecal antigen detection, urea breath detection and chemical staining of tissue biopsy (Shakir et al., 2023). The treatment guidelines for H. pylori in Europe, Canada, the United States, and South Korea recommend the use of quadruple therapy in areas with a resistance rate greater than 15%, whereas Proton pump inhibitor based triple therapy is recommended in areas with a clarithromycin resistance rate of <15%, although this is uncommon (Jung et al., 2021; Deane et al., 2024). The use of quadruple therapy is not allowed in Japan because there is insufficient evidence to prove that it is superior to triple therapy for Japanese patients (Cho and Jin, 2022). Fluoroquinolone-containing drugs are commonly used as second-line treatment for H. pylori infection, and a European study involving 5,055 patients receiving second-line treatment showed that 14-day levofloxacin-bismuth treatment was one of the four most effective regimens (Nyssen et al., 2022a). However, due to drug resistance and side effects, the clinical application of fluoroquinolones is limited. Rifabutin therapy is often used as second-,third -, or fourth-line therapy, but it carries the risk of bone marrow suppression and resistance to Mycobacterium (Deane et al., 2024; Gisbert, 2020). A European retrospective analysis of the efficacy of Rifabutin triple therapy for the treatment of H. pylori infection in 500 patients found eradication rates of 66%–80% (Nyssen et al., 2022b). Antibiotic resistance is one of the main reasons for the decline of H. pylori eradication rate. Common resistance mechanisms include some mutations affecting genetically modified drug targets, membrane permeability, efflux pump systems, and biofilm development (Hu et al., 2016; Srisuphanunt et al., 2023). While the gene encoding virulence factor VacA mentioned above was found to be associated with metronidazole resistance, and CagA was associated with levofloxacin resistance (Wang et al., 2019). In the face of increasing antibiotic resistance, it is necessary to revolutionize the eradication treatment of H. pylori infection. It is the future research direction to find personalized, effective and sustainable methods to face this global health challenge (Yamaoka, 2024).
3 Inhibitory effect of probiotics on Helicobacter pylori
Helicobacter pylori infection can affect symptoms such as intestinal discomfort, of which the disorder of intestinal microbiota is considered to be one of the main factors. In particular, some pathogens such as Haemophilus and Streptococcus increased while Faecalibacterium, Lactobacillus, and Akkermansia were reported to decrease significantly (Li et al., 2022; Li et al., 2023). Therefore, probiotic supplementation helps to inhibit H. pylori and is also expected to help regulate intestinal microbiota, which is beneficial to relieve gastrointestinal discomfort and help restore gastrointestinal health. Strains represented by Limosilactobacillus reuteri DSM 17648 have provided substantial scientific evidence. From the perspective of clinical evidence, Limosilactobacillus reuteri DSM 17648 has been proved to enhance the effect of triple and quadruple therapy in the treatment of H. pylori infection and reduce side effects (Liang et al., 2022).
3.1 In vivo and in vitro experiment
Sun et al. isolated four strains of lactic acid bacteria (Lactobacillus sake, Lactobacillus plantarum, Lactobacillus rhamnosus, and Lactobacillus brevis) from fermented foods in Northeast China, all of which were found to inhibit the growth of H. pylori to varying degrees (Sun et al., 2018). Lactobacillus paracasei HP7 demonstrated inhibitory effects against H. pylori both in vitro and in vivo. When combined with extracts of Perilla frutescens and Glycyrrhiza uralensis, it alleviated gastric inflammation and mucosal lesions in H. pylori-infected mice (Lee and Kim, 2020). A study on lactic acid bacteria isolated from fermented cocoa juice showed that 65.52% of the strains exhibited inhibitory effects against H. pylori, with some exerting their action through bacteriocins or organic acids (Kouitcheu Mabeku et al., 2020). Lactobacillus casei T1 and its supernatant exhibited potent inhibition of H. pylori growth, preventing inflammation and dysbiosis caused by H. pylori infection (Wu et al., 2021). The addition of probiotic Lactobacillus salivarius LN12 to amoxicillin and clarithromycin enhanced the therapeutic efficacy of the triple therapy, especially against H. pylori biofilm (Jin and Yang, 2021). The probiotic Lactiplantibacillus pentosus SLC13 has been shown to inhibit H. pylori growth, and its extracellular polysaccharides significantly reduced the expression of interleukin 8 (IL-8) induced by H. pylori infection, demonstrating its potential as an alternative treatment for H. pylori infection and inflammation reduction (Thuy et al., 2022). Lactiplantibacillus plantarum ZJ316 exhibited inhibitory effects on H. pylori both in vitro and in vivo, with mechanisms including the prevention of H. pylori colonization, downregulation of virulence genes, and reduction of IL-8 production (Wu et al., 2023). Recently, Chen et al. isolated five novel gastric-derived Weizmannia coagulans strains from healthy gastric mucosa, among which BCF-01 showed the strongest adhesion and inhibition of H. pylori growth. It effectively restored gastric microbiota, improved H. pylori-mediated mucosal barrier disruption, and alleviated inflammation by inhibiting the macrophage TLR4-NFκB-pyroptosis signaling pathway (Chen Z. et al., 2024). Xu et al. isolated Lactobacillus paragasseri strain LPG-9 from gastric mucosa, which demonstrated good inhibitory effects on H. pylori both in vitro and in vivo. This strain repaired the mucosal barrier by upregulating the expression of mucosal barrier proteins occludin and ZO-1, alleviating gastritis (Xu et al., 2023). Samy M et al. conducted a screening of different probiotic strains antagonistic to H. pylori and found that Bifidobacterium lactis and Lactobacillus acidophilus exhibited the highest inhibitory effects (Abdelhamid et al., 2023). In conclusion, probiotics show good inhibitory effect on H. pylori in vitro and in vivo through a variety of mechanisms, especially Lactobacillus species. Although probiotics have shown promising prospects in the inhibition of H. pylori, more scientific evidences and clinical cohorts are needed to verify their widespread application.
3.2 Related clinical study
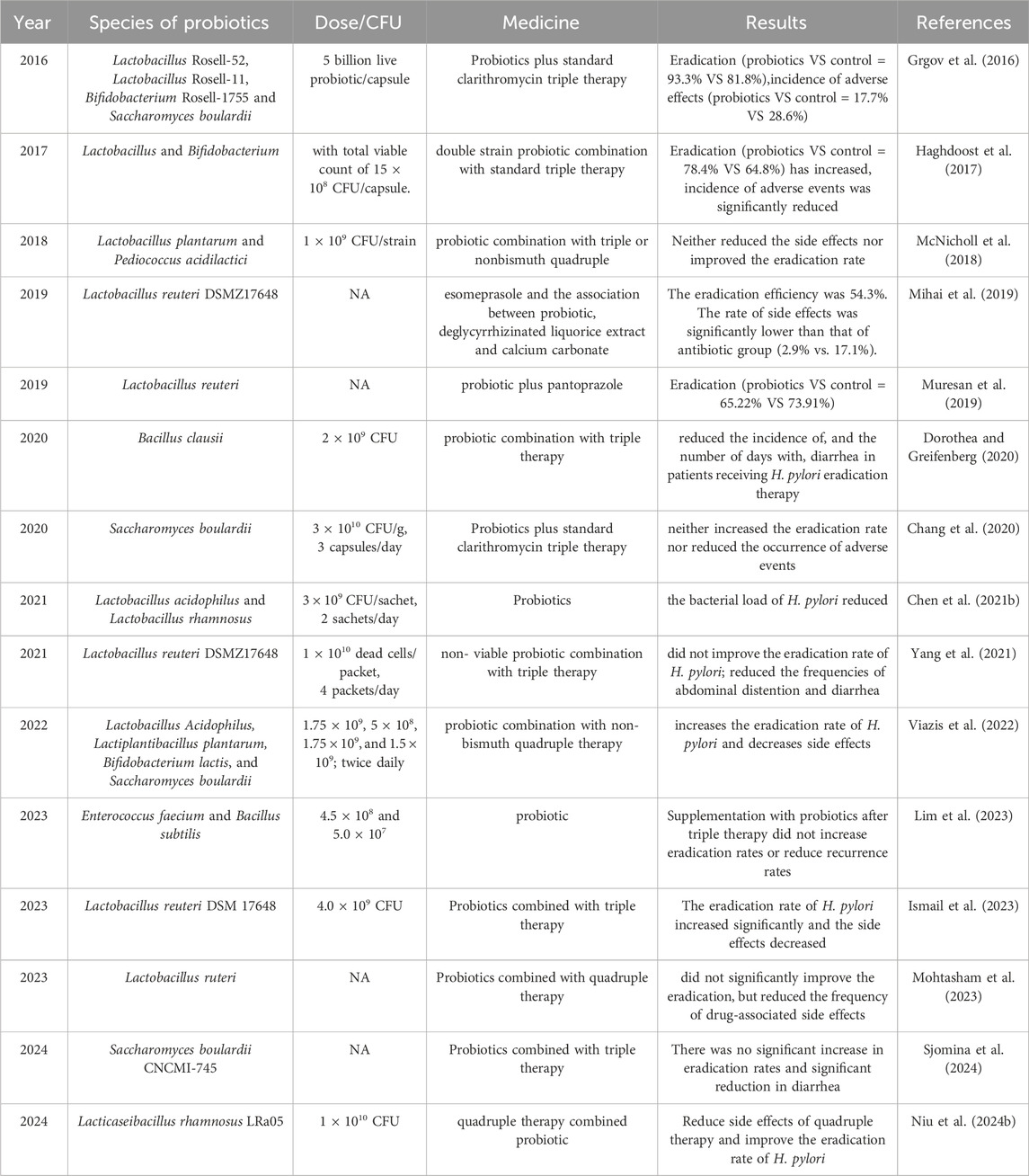
The meta-analysis conducted by Yang et al. demonstrated that supplementation with probiotics as an adjunctive therapy significantly improved eradication rates (RR 1.10, 95% CI 1.06–1.14) and reduced the overall risk of side effects (RR 0.54, 95% CI 0.42–0.70) compared to standard therapy. Among the probiotics, Bifidobacterium spp. exhibited the highest eradication potential, though further high-quality studies are needed (Yang et al., 2024). Another high-quality meta-analysis, based on 40 studies involving 8,924 patients, found an eradication rate of 81.5% following probiotic treatment with various regimens, compared to only 71.6% in the control group (p < 0.001, I2 = 52.1%). Probiotic use before and throughout the eradication treatment, especially when lasting more than 2 weeks, showed superior outcomes, with the best results observed when probiotics were combined with bismuth quadruple therapy (Shi et al., 2019). Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus rhamnosus were found to improve gastritis induced by H. pylori infection to varying degrees L. acidophilus (Asgari et al., 2020). Research by Saracino et al. (2020) showed that Lactobacillus casei, Lactobacillus paracasei, Lactobacillus acidophilus, B. lactis, and Streptococcus thermophilus exhibited antimicrobial and bactericidal activity against H. pylori. However, a meta-analysis of 11 studies involving 403 patients revealed that the average weighted eradication rate for probiotic treatment (including Lactobacilli and Saccharomyces boulardii) was only 14% (95% CI: 2%–25%, p = 0.02) (Losurdo et al., 2018). Overall, as shown in Table 1 (In vivo study), most clinical studies have shown that probiotics increase the eradication rate of H. pylori with antibiotics and attenuate treatment-related side effects. However, some studies suggested that the use of the same kind of probiotics did not increase the eradication rate or reduce the side effects. For example, among the studies using Lactobacillus reuteri (n = 5), some studies showed that eradication rate could be increased to 65.22%, while others showed no significant effect (n = 2). The reasons for these invalid or contradictory findings may be related to the heterogeneity of studies and the different doses of strains used.
In conclusion, current clinical research on the antagonism of H. pylori primarily focuses on certain strains of Lactobacillus, Bifidobacterium, and Saccharomyces. These probiotics have been shown to reduce H. pylori infection rates and attenuate gastrointestinal symptoms, while also enhancing the efficacy of antibiotic treatments for H. pylori infection (Baryshnikova et al., 2023). As one of the most widely used probiotics, Lactobacillus has been demonstrated to decrease H. pylori colonization and attenuate gastrointestinal discomfort. When combined with antibiotics, it can effectively improve the eradication rate of H. pylori and reduce adverse reactions during treatment. Specifically, Lactobacillus reuteri produces potent antimicrobial substances and secretes mucin to strengthen the mucosal barrier, showing promising inhibitory effects against H. pylori (Dargenio et al., 2022; Dargenio et al., 2021). The ability of probiotics to mitigate the side effects of antibiotics may be related to their regulation of the gut microbiota. Li et al.'s analysis revealed that the enrichment of H. pylori in the stomach affects the composition of the gastrointestinal microbiota (Li et al., 2023). Similarly, Bai et al.'s findings suggest that probiotics contribute to the balance of the intestinal microbiota, attenuating gastrointestinal discomfort, although more data are needed to determine whether they can increase eradication rates (Bai et al., 2023). However, it is important to point out that the above conclusions are all based on previously published articles, and there may be heterogeneity among different studies. In particular, the heterogeneity of studies, publication bias or the quality of included studies should be fully considered when referring to meta-analysis (Niu C. et al., 2024).
4 Mechanism of probiotics inhibiting Helicobacter pylori
4.1 Competitive exclusion
Helicobacter pylori colonizes the gastric mucosa by utilizing adhesion factors, such as outer membrane proteins (OMP). While most lactic acid bacteria colonize the human intestine, a few species are capable of colonizing the stomach (Ji and Yang, 2020). Probiotics can compete with H. pylori for adhesion sites on gastric epithelial cells, thereby inhibiting its colonization. For example, S. boulardii can prevent the binding of H. pylori to host cells (mainly through the modification of H. pylori binding sites on duodenal cells by ceramidase (Czerucka and Rampal, 2019). Lactobacillus rhamnosus ATCC 7469, L. acidophilus ATCC 4356, and L. reuteri ATCC 23272 were found to inhibit the adhesion of H. pylori to gastric epithelial cells (Rezaee et al., 2019). In addition, probiotics can modify the expression of epithelial junction proteins and mucins, and release active substances to protect mucosal barrier damage and prevent H. pylori colonization (Qureshi et al., 2019).
4.2 Regulation of gastric acid environment
The urease secreted by H. pylori breaks down urea into ammonia, locally neutralizing the acidic gastric environment to facilitate its survival. Probiotics, on the other hand, counteract this by producing lactic acid or inhibiting urease activity, thereby preventing an increase in gastric pH and weakening H. pylori’s survival mechanisms within the stomach. Lactobacillus plantarum ZJ316 can suppress the expression of the urease gene in H. pylori, thereby preventing its colonization (Wu et al., 2023). Lactobacillus acidophilus ATCC 4356, L. reuteri ATCC 23272 and L. fermentum ATCC 9338 could reduce the urease activity of eight clinical H. pylori strains (Rezaee et al., 2019). Lactobacillus rhamnosus GMNL-74 and Lactobacillus acidophilusGMNL-185 can inhibit the adhesion of H. pylori to gastric epithelium and reduce inflammation caused by infection (Chen et al., 2019). In addition, H. pylori can further weaken gastric mucosal barrier by interfering with intragastric acid-base balance and changing gastric acid secretion, and increase the risk of peptic ulcer and other pathogen infection.
4.3 Production of antimicrobial substances
Probiotics can produce various antimicrobial substances through biological metabolism. Lactobacilli, for example, metabolize carbohydrates to generate short-chain fatty acids such as acetic acid, propionic acid, butyric acid, and other organic acids. They can also produce bacteriocins and hydrogen peroxide, all of which exhibit significant antimicrobial properties (Kim et al., 2003; Homan and Orel, 2015). The organic acids metabolized by lactobacilli not only lower the gastric pH but also inhibit the activity of urease, thereby hindering the growth of H. pylori (Rezaee et al., 2019). Research by Rezaee et al., 2019 confirmed that Lactobacillus reuteri ATCC 23272 can inhibit H. pylori by producing antimicrobial acids. A recent study demonstrated that Weizmannia coagulans BC99 improved inflammation and oxidative stress after H. pylori infection by modulating gut microbiota-derived metabolites such as valeric acid (Zhai et al., 2024). Hydrogen peroxide produced by probiotics can cause oxidative damage of H. pylori cells by inducing the production of peroxide ions and interfering with H. pylori activity (Ji and Yang, 2020; Bai et al., 2022). The antioxidant system within H. pylori itself can produce enzymes such as superoxide dismutase to counteract host immune responses (Bereswill et al., 2000). Bacteriocins can disrupt the cell wall and membrane structures of H. pylori. Hu et al. (2021) reported that extracellular polysaccharides produced by Lactobacillus plajomi PW-7 effectively inhibit the growth of H. pylori and compromise its cell membrane integrity.
Helicobacter pylori incubation with supernatant metabolites from L. gasseri resulted in downregulation of acid resistance related gene arsS and flagella regulatory gene flgR, thereby reducing H. pylori activity. In addition, H. pylori iron absorption regulatory genes were downregulated. Results in a significant increase in sensitivity to antimicrobial peptide LL-37 (Zuo et al., 2022). In conclusion, probiotics can produce a variety of anti-H. pylori antibiotics, but which substances play the main role and whether multiple substances have synergistic effects need further identification and experimental verification. With the vigorous development of synthetic biology, future studies can try to use cloning strategies such as red/ET-mediated homologous recombination and transformation-related recombination to synthesize probiotic metabolites with antagonistic H. pylori (Alam et al., 2021).
4.4 Immunoregulatory effects
The persistent inflammatory response following H. pylori infection may induce inflammatory diseases (de Brito et al., 2019). The regulation of the host immune system by probiotics is conducive to reducing the immune escape of H. pylori. Probiotics enhance the host resistance to H. pylori chronic infection by regulating dendritic cells to induce B cells to produce Immunoglobulin A (IgA) (Dash et al., 2024). Lactobacillus gasseri Kx110A1, isolated from the human stomach, inhibits the expression of tumor necrosis factor-α (TNF-α) converting enzyme on host macrophages, consequently reducing the release of TNF-α and interleukin-6 (IL-6) (Gebremariam et al., 2019). IL-8 induces the migration of neutrophils and monocytes to the mucosa. Lactobacillus plantarum ZJ316 protects the host from inflammatory injury by inhibiting immune cell infiltration and IL-8 production during H. pylori infection (Wu et al., 2023). Research evidence suggests that probiotics may also enhance the expression of anti-inflammatory cytokine interleukin-10 (IL-10) (Zhao et al., 2018). A study by Park et al. (2020) demonstrated that, compared to the H. pylori infection group, the group of mice treated with Lactobacillus plantarum APSulloc 331,261 exhibited a significant downregulation of inflammatory cytokines such as TNF-α, interleukin-1β (IL-1β), and interleukin-4 (IL-4). Lactobacillus plantarum ZJ316 was found to significantly reduce the levels of interferon-γ and IL-6, increase the level of IL-10, and repair mucosal damage, thereby reducing H. pylori abundance and attenuating gastric inflammation caused by H. pylori infection (Zhou et al., 2021). Lactobacillus acidophilus NCFM and Lactiplantibacillus plantarum Lp-115 improved H. pylori eradication rate and attenuated gastric inflammation. This result is associated with an immunomodulatory process (reduced expression of cytokines such as IL-8 and TNF-α) (Shen et al., 2023). Lactobacillus fermentum UCO-979C enhances resistance to H. pylori infection by modulating the gastric innate immune response, significantly reducing the levels of TNF-α, IL-8, and Monocyte Chemotactic Protein 1 (MCP-1) in the gastric mucosa of infected mice, while increasing the expression of Interferon-gamma (IFN-γ) and IL-10 (Garcia-Castillo et al., 2020). Lactobacillus gasseri ATCC 33323 inhibits the secretion of IL-8 in human gastric adenocarcinoma cells infected with H. pylori, and significantly reduces the mRNA expression of genes such as Bcl-2, β-catenin, integrin α5, and integrin β1 (Yarmohammadi et al., 2021). Lactobacillus rhamnosus JB3 suppresses IL-8 secretion, as well as the mRNA levels of vacA, sabA, and fucT, and the expression of Lewis (Le)x antigens and Toll-like receptor 4 (TLR4) in H. pylori-infected AGS cells (Do et al., 2021). Both live and pasteurized Lactobacillus crispatus strain RIGLD-1 regulate H. pylori-induced inflammation by downregulating the mRNA expression of IL-1β, IL-6, IL-8, and TNF-α, and upregulating the expression of IL-10 and Transforming Growth Factor Beta (TGF-β) cytokines in AGS cells (Fakharian et al., 2023). Lin et al. (2020) investigated the effects of multiple Lactobacillus species on the immune response and metabolic balance of H. pylori infected mice. The results showed that the intervention of multiple Lactobacillus species could restore the levels of alanine, arginine, aspartic acid, glycine and tryptophan in serum, and increase the contents of butyric acid, valeric acid, palmitic acid, palmitic acid, stearic acid and oleic acid. These are important indicators related to immunity and metabolism (Lin et al., 2020).
In conclusion, probiotics promote the activation of immune cells in the host gastric mucosa, increase the secretion of cytokines such as IL-10 and IgA, and enhance the host immune defense function. In addition, probiotics such as Lactobacillus and Bifidobacterium can also help to clear H. pylori by regulating innate immunity, enhancing immune response and strengthening mucosal barrier.
4.5 Regulation of gastric microecology
The analysis of data from GEO revealed significant differences in the microbiota between healthy individuals and those infected with H. pylori. Furthermore, 11 bacterial populations that were significantly negatively correlated with H. pylori infection and notably enriched in the HP- group were identified, the majority of which were probiotic species, including Lactobacillus and Enterococcus (Chen Z. et al., 2021). Compared with gut microbiota, gastric microbiota is characterized by low density, poor specificity, and large fluctuations. The number of bacteria in the gastric microecosystem of healthy adults varies significantly, which may be affected by many factors such as region, culture and dietary habits (Xu et al., 2022; Stewart et al., 2020). He et al. found that the combination of Lactobacillus salivarius and Lactobacillus rhamnosus could improve the gastric and intestinal microecology in the H. pylori infected group. n particular, H. pylor-induced reduction of anti-inflammatory bacteria Faecalibaculum in the intestine was restored, and inflammatory infiltration and the incidence of precancerous lesions were reduced (He et al., 2022). The supplementation of probiotics can also regulate the structure of gastric flora, and the recovery of gastric microecology is considered to be related to the eradication of H. pylori (Nabavi-Rad et al., 2022). The quadruple therapy of antibiotics in the treatment of H. pylori infection can aggravate the gastrointestinal microecological disorder. While probiotics restore the balance of intestinal flora through different mechanisms, improve the eradication rate, and reduce the occurrence of adverse reactions (Xu et al., 2022). In conclusion, probiotics play an important role in the occurrence and development of gastrointestinal diseases by antagonizing H. pylori colonization by regulating gastric pH, secreting antibacterial substances, stimulating immune responses and regulating gastrointestinal flora (Figure 4).
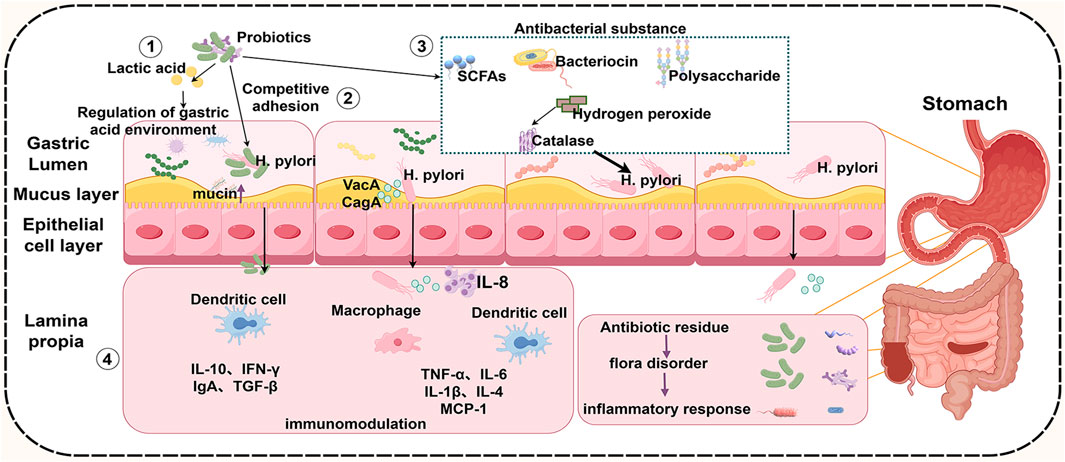
Figure 4. Mechanisms by which probiotics inhibit H. pylori. Probiotics improve the gastrointestinal microecological environment by changing the gastric pH value ①, competitive adhesion with H. pylori ②, secreting antibacterial substances③, increasing the secretion of anti-inflammatory factors and reducing the secretion of pro-inflammatory factors ④, thereby attenuating H. pylori infection and reducing related complications. SCFAs, short chain fatty acids; IL-10, interleukin-10; IFN-γ, Interferon-gamma; IgA, Immunoglobulin A; TGF-β, Transforming Growth Factor Beta; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; IL-4, interleukin-4; MCP-1, Monocyte Chemotactic Protein-1; VacA, vacuolated cytotoxin), CagA, bacterial toxin-associated gene A. (By figdraw).
5 Conclusions and future perspectives
This review systematically summarizes the mechanisms of H. pylori (H. pylori) infection and its prevention and treatment strategies, with a particular focus on the latest research advancements regarding the role of probiotics in the management of H. pylori infection. Emphasis is placed on the mechanisms by which probiotics inhibit H. pylori and the directions for future research. Numerous studies have indicated that probiotic supplementation is beneficial in preventing and treating H. pylori infection. However, the effectiveness of probiotic therapies in eradicating H. pylori has been inconsistent, with low eradication rates, which may be attributed to the unique gastric environment that hampers the colonization of probiotics derived from the intestinal tract. Therefore, it is crucial to identify probiotics that can withstand the acidic gastric environment and effectively eradicate H. pylori. Autologous probiotics are considered a promising new approach in microbial therapy, demonstrating good efficacy in inhibiting H. pylori, but further validation through large, multicenter, randomized controlled trials is still required (Baryshnikova et al., 2023). Although probiotics have been shown to be beneficial in selected settings (e.g., antibiotic-associated diarrhea and certain types of inflammatory bowel disease), their routine use or as adjunctive therapy for H. pylori in healthy individuals is not supported by strong clinical evidence. Given the uncertainties regarding the effectiveness of probiotics in treating H. pylori infection, some meta-analyses have even reached contradictory conclusions. The Maastricht VI/Florence Consensus Report (2021) does not recommend routine probiotic supplementation due to inconsistent clinical evidence regarding efficacy (Malfertheiner et al., 2022). The 2020 guidelines of the American Gastroenterological Association also state that probiotics are not recommended for most gastrointestinal diseases (Su et al., 2020).
Probiotic treatment may also lead to adverse effects, such as exacerbated constipation and bloating, and its safety profile requires further investigation. Moreover, although Enterococcus spp. is widely used in food and feed, its ability to harbor virulence factor and inherent antimicrobial resistance raises significant safety concerns. Of note, Enterococcus are excluded from the US FDA GRAS list and the EU QPS list (Franz et al., 2011). Therefore, strain identification and description, production process and quality control, clinical trials and efficacy verification, safety assessment, clear labeling and instructions, and continuous monitoring and feedback mechanisms need to be further improved in the regulatory aspect of the safety of probiotics use.
Nevertheless, for patients who experience severe side effects from antibiotic therapy or have limited antibiotic options, as well as those with susceptible gastrointestinal microbiota, probiotic therapy offers a promising alternative. Future research should differentiate between symptomatic and asymptomatic infected individuals, select specific probiotics or their fermented products, utilize widely studied strains, standardize experimental protocols (including dosage, duration, and clinical endpoints), and assess the long-term effects of probiotic interventions.
Author contributions
LY: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. CY: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – review and editing. YH: Conceptualization, Formal Analysis, Methodology, Writing – review and editing. FY: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – review and editing. HT: Conceptualization, Methodology, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was jointly funded by Moutai Group Research and Development Project (MTGF2025011), Guizhou Science and Technology Support Program [Qian Ke He (2022) 021].
Conflict of interest
Authors LY, CY, YH, FY, and HT were employed by Kweichow Moutai Distillery Co., Ltd.
The authors declare that this study received funding from Moutai Group. The funder had the following involvement in the study: designing the study, collecting and analyzing the data, writing the article, and deciding to publish it.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhamid, S. M., Edris, A. E., and Sadek, Z. (2023). Novel approach for the inhibition of Helicobacter pylori contamination in yogurt using selected probiotics combined with eugenol and cinnamaldehyde nanoemulsions. Food Chem. 417, 135877. doi:10.1016/j.foodchem.2023.135877
Addissouky, T., Ali, M., Sayed, I., and Wang, Y. (2023). Recent advances in diagnosing and treating Helicobacter pylori through botanical extracts and advanced technologies. Archives Pharmacol. Ther. 5, 53–66. doi:10.33696/Pharmacol.4.045
Alam, K., Hao, J., Zhang, Y., and Li, A. (2021). Synthetic biology-inspired strategies and tools for engineering of microbial natural product biosynthetic pathways. Biotechnol. Adv. 49, 107759. doi:10.1016/j.biotechadv.2021.107759
Ali, A., and AlHussaini, K. I. (2024). Helicobacter pylori: a contemporary perspective on pathogenesis, diagnosis and treatment strategies. Microorganisms 12 (1), 222. doi:10.3390/microorganisms12010222
Asgari, B., Kermanian, F., Hedayat Yaghoobi, M., Vaezi, A., Soleimanifar, F., and Yaslianifard, S. (2020). The anti-Helicobacter pylori effects of Lactobacillus acidophilus, L. plantarum, and L. rhamnosus in stomach tissue of C57BL/6 mice. Visc. Med. 36 (2), 137–143. doi:10.1159/000500616
Backert, S., and Tegtmeyer, N. (2017). Type IV secretion and signal transduction of Helicobacter pylori CagA through interactions with host cell receptors. Toxins (Basel) 9, 115. doi:10.3390/toxins9040115
Bai, X., Zhu, M., He, Y., Wang, T., Tian, D., and Shu, J. (2022). The impacts of probiotics in eradication therapy of Helicobacter pylori. Archives Microbiol. 204 (12), 692. doi:10.1007/s00203-022-03314-w
Bai, X.-F., Tian, D., Wang, T.-Y., Shu, J.-C., He, Y.-J., and Zhu, M.-J. (2023). The impact of probiotics on gut microbiota in the eradication of Helicobacter pylori infection: a systematic review. Eur. Rev. Med. Pharmacol. Sci. 27 (14), 6736–6743. doi:10.26355/eurrev_202307_33144
Baj, J., Forma, A., Sitarz, M., Portincasa, P., Garruti, G., Krasowska, D., et al. (2020). Helicobacter pylori virulence Factors—Mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 10 (1), 27. doi:10.3390/cells10010027
Baryshnikova, N. V., Ilina, A. S., Ermolenko, E. I., Uspenskiy, Y. P., and Suvorov, A. N. (2023). Probiotics and autoprobiotics for treatment of Helicobacter pylori infection. World J. Clin. Cases 11 (20), 4740–4751. doi:10.12998/wjcc.v11.i20.4740
Bereswill, S., Neuner, O., Strobel, S., and Kist, M. (2000). Identification and molecular analysis of superoxide dismutase isoforms in Helicobacter pylori. FEMS Microbiol. Lett. 183 (2), 241–245. doi:10.1111/j.1574-6968.2000.tb08965.x
Chang, Y. W., Park, Y. M., Oh, C. H., Oh, S. J., Cho, J.-H., Kim, J.-W., et al. (2020). Effects of probiotics or broccoli supplementation on Helicobacter pylori eradication with standard clarithromycin-based triple therapy. Korean J. Intern Med. 35 (3), 574–581. doi:10.3904/kjim.2019.139
Chen, M. J., Chen, C. C., Huang, Y. C., Tseng, C. C., Hsu, J. T., Lin, Y. F., et al. (2021b). The efficacy of Lactobacillus acidophilus and rhamnosus in the reduction of bacterial load of Helicobacter pylori and modification of gut Microbiota—A double-blind, placebo-controlled, randomized trial. Helicobacter 26 (6), e12857. doi:10.1111/hel.12857
Chen, Y.-C., Malfertheiner, P., Yu, H.-T., Kuo, C.-L., Chang, Y.-Y., Meng, F.-T., et al. (2024a). Global prevalence of Helicobacter pylori infection and incidence of gastric cancer between 1980 and 2022. Gastroenterology 166 (4), 605–619. doi:10.1053/j.gastro.2023.12.022
Chen, Y.-H., Tsai, W.-H., Wu, H.-Y., Chen, C.-Y., Yeh, W.-L., Chen, Y.-H., et al. (2019). Probiotic lactobacillus spp. act against Helicobacter pylori -induced inflammation. J. Clin. Med. 8 (1), 90. doi:10.3390/jcm8010090
Chen, Z., Chen, H., Yu, L., Xin, H., Kong, J., Bai, Y., et al. (2021a). Bioinformatic identification of key pathways, hub genes, and microbiota for therapeutic intervention in Helicobacter pylori infection. J. Cell Physiol. 236 (2), 1158–1183. doi:10.1002/jcp.29925
Chen, Z., Tang, Z., Li, W., Deng, X., Yu, L., Yang, J., et al. (2024b). Weizmannia coagulans BCF-01: a novel gastrogenic probiotic for Helicobacter pylori infection control. Gut Microbes 16 (1), 2313770. doi:10.1080/19490976.2024.2313770
Cho, J.-H., and Jin, S.-Y. (2022). Current guidelines for Helicobacter pylori treatment in east Asia 2022: differences among China, Japan, and South Korea. World J. Clin. Cases 10 (19), 6349–6359. doi:10.12998/wjcc.v10.i19.6349
Czerucka, D., and Rampal, P. (2019). Diversity of saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J. Gastroenterol. 25 (18), 2188–2203. doi:10.3748/wjg.v25.i18.2188
Dargenio, C., Dargenio, V. N., Bizzoco, F., Indrio, F., Francavilla, R., and Cristofori, F. (2021). Limosilactobacillus reuteri strains as adjuvants in the management of Helicobacter pylori infection. Medicina 57 (7), 733. doi:10.3390/medicina57070733
Dargenio, V., Cristofori, F., Dargenio, C., Giordano, P., Indrio, F., Celano, G., et al. (2022). Use of Limosilactobacillus reuteri DSM 17938 in paediatric gastrointestinal disorders: an updated review. Benef. Microbes 13 (3), 221–242. doi:10.3920/BM2021.0151
Dash, D., Mishra, V., Panda, M. K., and Pathak, S. K. (2024). Effects of lactobacillus spp. on Helicobacter pylori: a promising frontier in the era of antibiotic resistance. Probiotics Antimicrob. Proteins, 1–20. doi:10.1007/s12602-024-10396-z
Deane, C., Kelly, O., and O’Morain, C. (2024). Current and future perspectives on the management of Helicobacter pylori: a narrative review. Antibiotics 13 (6), 541. doi:10.3390/antibiotics13060541
de Brito, B. B., da Silva, F. A. F., Soares, A. S., Pereira, V. A., Santos, M. L. C., Sampaio, M. M., et al. (2019). Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 25 (37), 5578–5589. doi:10.3748/wjg.v25.i37.5578
de Kraker, M. E., Stewardson, A. J., and Harbarth, S. (2016). Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 13 (11), e1002184. doi:10.1371/journal.pmed.1002184
Do, A. D., Chang, C. C., Su, C. H., and Hsu, Y. M. (2021). Lactobacillus rhamnosus JB3 inhibits Helicobacter pylori infection through multiple molecular actions. Helicobacter 26 (3), e12806. doi:10.1111/hel.12806
Dorothea, MPMIP, and Greifenberg, M. (2020). Effect of Bacillus clausii capsules in reducing adverse effects associated with Helicobacter pylori eradication therapy: a randomized, double-blind, controlled trial. Infect. Dis. Ther. 9 (4), 879. doi:10.1007/s40121-020-00333-2
Duan, M., Li, Y., Liu, J., Zhang, W., Dong, Y., Han, Z., et al. (2023). Transmission routes and patterns of Helicobacter pylori. Helicobacter 28 (1), e12945. doi:10.1111/hel.12945
Elbehiry, A., Marzouk, E., Aldubaib, M., Abalkhail, A., Anagreyyah, S., Anajirih, N., et al. (2023). Helicobacter pylori infection: current status and future prospects on diagnostic, therapeutic and control challenges. Antibiotics 12 (2), 191. doi:10.3390/antibiotics12020191
Elbestawy, M. K. M., El-Sherbiny, G. M., Moghannem, S. A., and Farghal, E. E. (2023). Antibacterial, antibiofilm, and anti-inflammatory activities of ginger extract against Helicobacter pylori. Microbiol. Res. (Pavia). 14 (3), 1124–1138. doi:10.3390/microbiolres14030075
Fakharian, F., Sadeghi, A., Pouresmaeili, F., Soleimani, N., and Yadegar, A. (2023). Immunomodulatory effects of live and pasteurized Lactobacillus crispatus strain RIGLD-1 on Helicobacter pylori -triggered inflammation in gastric epithelial cells in vitro. Mol. Biol. Rep. 50 (8), 6795–6805. doi:10.1007/s11033-023-08596-x
Foegeding, N. J., Caston, R. R., McClain, M. S., Ohi, M. D., and Cover, T. L. (2016). An overview of Helicobacter pylori VacA toxin biology. Toxins 8 (6), 173. doi:10.3390/toxins8060173
Franz, CMAP, Huch, M., Abriouel, H., Holzapfel, W., and Gálvez, A. (2011). Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 151 (2), 125–140. doi:10.1016/j.ijfoodmicro.2011.08.014
Garcia-Castillo, V., Marcial, G., Albarracín, L., Tomokiyo, M., Clua, P., Takahashi, H., et al. (2020). The exopolysaccharide of Lactobacillus fermentum UCO-979C is partially involved in its immunomodulatory effect and its ability to improve the resistance against Helicobacter pylori infection. Microorganisms 8 (4), 479. doi:10.3390/microorganisms8040479
Gebremariam, H. G., Qazi, K. R., Somiah, T., Pathak, S. K., Sjölinder, H., Sverremark Ekström, E., et al. (2019). Lactobacillus gasseri suppresses the production of proinflammatory cytokines in Helicobacter pylori -infected macrophages by inhibiting the expression of ADAM17. Front. Immunol. 10, 2326. doi:10.3389/fimmu.2019.02326
Gisbert, J. P. (2020). Rifabutin for the treatment of Helicobacter pylori infection: a review. Pathogens 10 (1), 15. doi:10.3390/pathogens10010015
Grgov, S., Tasić, T., Radovanović-Dinić, B., and Benedeto-Stojanov, D. (2016). Can probiotics improve efficiency and safety profile of triple Helicobacter pylori eradication therapy? A prospective randomized study. Vojnosanit. Pregl. 73 (11), 1044–1049. doi:10.2298/VSP150415127G
Gu, H. (2017). Role of flagella in the pathogenesis of Helicobacter pylori. Curr. Microbiol. 74, 863–869. doi:10.1007/s00284-017-1256-4
Haghdoost, M., Taghizadeh, S., Montazer, M., Poorshahverdi, P., Ramouz, A., and Fakour, S. (2017). Double strain probiotic effect on Helicobacter pylori infection treatment: a double-blinded randomized controlled trial. Casp. J. Intern Med. 8 (3), 165–171. doi:10.22088/cjim.8.3.165
Hanyu, H., Engevik, K. A., Matthis, A. L., Ottemann, K. M., Montrose, M. H., and Aihara, E. (2019). Helicobacter pylori uses the TlpB receptor to sense sites of gastric injury. Infect. Immun. 87 (9), e00202-19. doi:10.1128/IAI.00202-19
Hatakeyama, M. (2017). Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B 93 (4), 196–219. doi:10.2183/pjab.93.013
He, C., Peng, C., Xu, X., Li, N., Ouyang, Y., Zhu, Y., et al. (2022). Probiotics mitigate Helicobacter pylori -induced gastric inflammation and premalignant lesions in INS-GAS mice with the modulation of gastrointestinal microbiota. Helicobacter 27 (4), e12898. doi:10.1111/hel.12898
Homan, M., and Orel, R. (2015). Are probiotics useful in Helicobacter pylori eradication? World J. Gastroenterol. 21 (37), 10644–10653. doi:10.3748/wjg.v21.i37.10644
Hu, J., Tian, X., Wei, T., Wu, H., Lu, J., Lyu, M., et al. (2021). Anti- Helicobacter pylori activity of a Lactobacillus sp. PW-7 exopolysaccharide. Foods 10 (10), 2453. doi:10.3390/foods10102453
Hu, Y., Zhang, M., Lu, B., and Dai, J. (2016). Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter 21 (5), 349–363. doi:10.1111/hel.12299
Huang, T.-T., Cao, Y.-X., and Cao, L. (2024). Novel therapeutic regimens against Helicobacter pylori: an updated systematic review. Front. Microbiol. 15, 1418129. doi:10.3389/fmicb.2024.1418129
Idowu, S., Bertrand, P. P., and Walduck, A. K. (2022). Gastric organoids: advancing the study of H. pylori pathogenesis and inflammation. Helicobacter 27 (3), e12891. doi:10.1111/hel.12891
Ismail, N. I., Nawawi, K. N. M., Hsin, D. C. C., Hao, K. W., Mahmood, NRKN, Chearn, G. L. C., et al. (2023). Probiotic containing Lactobacillus reuteri DSM 17648 as an adjunct treatment for Helicobacter pylori infection: a randomized, double-blind, placebo-controlled trial. Helicobacter 28 (6), e13017. doi:10.1111/hel.13017
Ji, J., and Yang, H. (2020). Using probiotics as supplementation for Helicobacter pylori antibiotic therapy. Int. J. Mol. Sci. 21 (3), 1136. doi:10.3390/ijms21031136
Jin, F., and Yang, H. (2021). Effects of Lactobacillus salivarius LN12 in combination with amoxicillin and clarithromycin on Helicobacter pylori biofilm in vitro. Int. J. Mol. Sci. 9 (8), 1611. doi:10.3390/microorganisms9081611
Jung, H. K., Kang, S. J., Lee, Y. C., Yang, H. J., Park, S. Y., Shin, C. M., et al. (2021). Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver 15 (2), 168–195. doi:10.5009/gnl20288
Kim, T.-S., Hur, J.-W., Yu, M.-A., Cheigh, C.-I., Kim, K.-N., Hwang, J.-K., et al. (2003). Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J. Food Prot. 66 (1), 3–12. doi:10.4315/0362-028x-66.1.3
Kouitcheu Mabeku, L. B., Ngue, S., Bonsou Nguemo, I., and Leundji, H. (2020). Potential of selected lactic acid bacteria from Theobroma cacao fermented fruit juice and cell-free supernatants from cultures as inhibitors of Helicobacter pylori and as good probiotic. BMC Res. notes 13, 64–67. doi:10.1186/s13104-020-4923-7
Lee, H.-A., and Kim, O. (2020). Synergistic effect of probiotic HP7 and the extracts of Perilla frutescens and Glycyrrhiza glabra to alleviate Helicobacter pylori -induced damages. Transl. Res. 21 (3), 101–108. doi:10.12729/jbtr.2020.21.3.101
Li, M., Shao, D., Zhou, J., Gu, J., Qin, J., Li, X., et al. (2022). Microbial diversity and composition in six different gastrointestinal sites among participants undergoing upper gastrointestinal endoscopy in Henan, China. Microbiol. Spectr. 10 (3), e0064521–e0064521. doi:10.1128/spectrum.00645-21
Li, M., Wang, X., Dong, X., Teng, G., Dai, Y., and Wang, W. (2024). Lactobacillus reuteri compared with placebo as an adjuvant in Helicobacter pylori eradication therapy: a meta-analysis of randomized controlled trials. Ther. Adv. Gastroenterol. 17, 17562848241258021. doi:10.1177/17562848241258021
Li, Y., Ouyang, Y., and He, C. (2023). Research trends on the relationship between Helicobacter pylori and microbiota: a bibliometric analysis. Helicobacter 28 (6), e13021. doi:10.1111/hel.13021
Liang, B., Yuan, Y., Peng, X.-J., Liu, X.-L., Hu, X.-K., and Xing, D.-M. (2022). Current and future perspectives for Helicobacter pylori treatment and management: from antibiotics to probiotics. Front. Cell. Infect. Microbiol. 12, 1042070. doi:10.3389/fcimb.2022.1042070
Lim, N. R., Choi, S. Y., and Chung, W. C. (2023). Probiotic supplementation for treatment of Helicobacter pylori infection: a double-blind randomized clinical trial. Korean J. Helicobacter Up. Gastrointest. Res. 23 (1), 34–41. doi:10.7704/kjhugr.2022.0051
Lin, C.-C., Huang, W.-C., Su, C.-H., Lin, W.-D., Wu, W.-T., Yu, B., et al. (2020). Effects of multi-strain probiotics on immune responses and metabolic balance in Helicobacter pylori -infected mice. Nutrients 12 (8), 2476. doi:10.3390/nu12082476
Liu, M., Gao, H., Miao, J., Zhang, Z., Zheng, L., Li, F., et al. (2024). Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: a review. Front. Microbiol. 14, 1330029. doi:10.3389/fmicb.2023.1330029
Losurdo, G., Cubisino, R., Barone, M., Principi, M., Leandro, G., Ierardi, E., et al. (2018). Probiotic monotherapy and Helicobacter pylori eradication: a systematic review with pooled-data analysis. World J. Gastroenterol. 24 (1), 139–149. doi:10.3748/wjg.v24.i1.139
Malfertheiner, P., Megraud, F., Rokkas, T., Gisbert, J. P., Liou, J.-M., Schulz, C., et al. (2022). Management of Helicobacter pylori infection: the maastricht VI/Florence consensus report. Gut 71 (9), 1724–1762. doi:10.1136/gutjnl-2022-327745
Malfertheiner, P., Schulz, C., and Hunt, R. H. H. (2024). Pylori infection: a 40-year journey through shifting the paradigm to transforming the management. Dig. Dis. 10 (000538079), 10–1159. doi:10.1053/j.gastro.2023.12.022
Maria, T. G., Jesus Manuel, G.-V., Luis Antonio Gómez, F., Pedro, C., and Ignacio, G. (2023). Garlic extracts: effect of pH on inhibition of Helicobacter pylori. J. Life 13 (7), 1434. doi:10.3390/life13071434
Masaadeh, A. H., Mathias, P. C., Ford, B. A., and Bosch, D. E. (2023). Helicobacter pylori exposure in nausea and vomiting of pregnancy increases risk of preterm delivery. Infect. Dis. Obstet. Gynecol. 2023 (1), 6612268. doi:10.1155/2023/6612268
McNicholl, A. G., Molina-Infante, J., Lucendo, A. J., Calleja, J. L., Pérez-Aisa, Á., Modolell, I., et al. (2018). Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: a randomized, double-blind, placebo-controlled trial. Helicobacter 23 (5), e12529. doi:10.1111/hel.12529
Mihai, C., Mihai, B. M., Dranga, M., Cardoneanu, A., and Prelipcean, C. C. (2019). Lactobacillus reuteri–An alternative in the first-line of Helicobacter pylori eradication. Farmacia 67 (5), 871–876. doi:10.31925/farmacia.2019.5.17
Mohtasham, M., Joukar, F., Maroufizadeh, S., Mojtahedi, K., Asgharnezhad, M., and Mansour-Ghanaei, F. (2023). Lactobacillus ruteri compared with placebo as an adjuvant in quadruple therapy for Helicobacter pylori eradication: a randomized, double-blind, controlled trial. Arab. J. Gastroenterol. 24 (1), 40–44. doi:10.1016/j.ajg.2022.10.004
Muresan, I. A. P., Pop, L. L., and Dumitrascu, D. L. (2019). Lactobacillus reuteri versus triple therapy for the eradication of Helicobacter pylori in functional dyspepsia. Med. Pharm. Rep. 92 (4), 352–355. doi:10.15386/mpr-1375
Nabavi-Rad, A., Sadeghi, A., Asadzadeh Aghdaei, H., Yadegar, A., Smith, S. M., and Zali, M. R. (2022). The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut microbes 14 (1), 2108655. doi:10.1080/19490976.2022.2108655
Niu, C., Zhang, J., Vallabhajosyula, S., E-Xin, B., and Napel, M.Okolo 3rd PIJJoGC (2024a). The impact of traction methods on endoscopic submucosal dissection efficacy for gastric neoplasia: a systematic review and meta-analysis. J. Gastrointest. Cancer 55 (1), 129–142. doi:10.1007/s12029-023-00982-9
Niu, Y., Li, J., Qian, H., Liang, C., Shi, X., and Bu, S. (2024b). Evaluation of efficacy and safety of Lacticaseibacillus rhamnosus LRa05 in the eradication of Helicobacter pylori: a randomized, double-blind, placebo-controlled trial. Front. Immunol. 15, 1450414. doi:10.3389/fimmu.2024.1450414
Nyssen, O. P., Vaira, D., Aísa, Á. P., Rodrigo, L., Castro-Fernandez, M., Jonaitis, L., et al. (2022a). Empirical second-line therapy in 5000 patients of the European registry on Helicobacter pylori management (Hp-EuReg). Clin. Gastroenterol. Hepatol. 20 (10), 2243–2257. doi:10.1016/j.cgh.2021.12.025
Nyssen, O. P., Vaira, D., Saracino, I. M., Fiorini, G., Caldas, M., Bujanda, L., et al. (2022b). Experience with rifabutin-containing therapy in 500 patients from the European Registry on Helicobacter pylori management (Hp-EuReg). J. Clin. Med. 11 (6), 1658. doi:10.3390/jcm11061658
Park, H., Cho, D., Huang, E., Seo, J. Y., Kim, W. G., Todorov, S. D., et al. (2020). Amelioration of alcohol induced gastric ulcers through the administration of Lactobacillus plantarum APSulloc 331261 isolated from green tea. Front. Microbiol. 11, 420. doi:10.3389/fmicb.2020.00420
Qureshi, N., Li, P., and Gu, Q. (2019). Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen? Appl. Microbiol. Biotechnol. 103, 1573–1588. doi:10.1007/s00253-018-09580-3
Reyes, V. E. (2023). Helicobacter pylori and its role in gastric cancer. Microorganisms 11 (5), 1312. doi:10.3390/microorganisms11051312
Rezaee, P., Kermanshahi, R. K., and Falsafi, T. (2019). Antibacterial activity of Lactobacilli probiotics on clinical strains of Helicobacter pylori. Iran. J. Basic Med. Sci. 22 (10), 1118–1124.
Saito, H., Nishikawa, Y., Masuzawa, Y., Tsubokura, M., and Mizuno, Y. (2021). Helicobacter pylori infection mass screening for children and adolescents: a systematic review of observational studies. J. Gastrointest. Cancer 52, 489–497. doi:10.1007/s12029-021-00630-0
Salama, N. R., Hartung, M. L., and Müller, A. (2013). Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 11 (6), 385–399. doi:10.1038/nrmicro3016
Saracino, I. M., Pavoni, M., Saccomanno, L., Fiorini, G., Pesci, V., Foschi, C., et al. (2020). Antimicrobial efficacy of five probiotic strains against Helicobacter pylori. Antibiotics 9 (5), 244. doi:10.3390/antibiotics9050244
Shadvar, N., Akrami, S., Mousavi Sagharchi, S.-M.-A., Askandar, R. H., Merati, A., Aghayari, M., et al. (2024). A review for non-antibiotic treatment of Helicobacter pylori: new insight. Front. Microbiol. 15, 1379209. doi:10.3389/fmicb.2024.1379209
Shakir, S. M., Shakir, F. A., and Couturier, M. R. (2023). Updates to the diagnosis and clinical management of Helicobacter pylori infections. Clin. Chem. 69 (8), 869–880. doi:10.1093/clinchem/hvad081
Sharndama, H. C., and Mba, I. E. (2022). Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J. Microbiol. 53 (1), 33–50. doi:10.1007/s42770-021-00675-0
Shen, S., Ren, F., Qin, H., Bukhari, I., Yang, J., Gao, D., et al. (2023). Lactobacillus acidophilus NCFM and Lactiplantibacillus plantarum Lp-115 inhibit Helicobacter pylori colonization and gastric inflammation in a murine model. Front. Cell. Infect. Microbiol. 13, 1196084. doi:10.3389/fcimb.2023.1196084
Shi, X., Zhang, J., Mo, L., Shi, J., Qin, M., and Huang, X. (2019). Efficacy and safety of probiotics in eradicating Helicobacter pylori: a network meta-analysis. Medicine 98 (15), e15180. doi:10.1097/MD.0000000000015180
Sjomina, O., Poļaka, I., Suhorukova, J., Vangravs, R., Paršutins, S., Knaze, V., et al. (2024). Randomised clinical trial: efficacy and safety of H. pylori eradication treatment with and without saccharomyces boulardii supplementation. Eur. J. Cancer Prev. 33 (3), 217–222. doi:10.1097/CEJ.0000000000000858
Srisuphanunt, M., Wilairatana, P., Kooltheat, N., Duangchan, T., Katzenmeier, G., and Rose, J. B. (2023). Molecular mechanisms of antibiotic resistance and novel treatment strategies for Helicobacter pylori infections. Trop. Med. Infect. Dis. 8 (3), 163. doi:10.3390/tropicalmed8030163
Stefano, K., Marco, M., Federica, G., Laura, B., Barbara, B., Gioacchino, L., et al. (2018). Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Bio Medica Atenei Parm. 89 (Suppl. 8), 72–76. doi:10.23750/abm.v89i8-S.7947
Stewart, O. A., Wu, F., and Chen, Y. (2020). The role of gastric microbiota in gastric cancer. Gut microbes 11 (5), 1220–1230. doi:10.1080/19490976.2020.1762520
Su, G. L., Ko, C. W., Bercik, P., Falck-Ytter, Y., Sultan, S., Weizman, A. V., et al. (2020). AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 159 (2), 697–705. doi:10.1053/j.gastro.2020.05.059
Sun, L., Zhao, H., Liu, L., Wu, X., Gao, Q., and Zhao, Y. (2018). Effects of Lactobacillus on the inhibition of Helicobacter pylori growth. Biotechnol. Biotechnol. Equip. 32 (6), 1533–1540. doi:10.1080/13102818.2018.1515599
Takeuchi, H., Trang, V. T., Morimoto, N., Nishida, Y., Matsumura, Y., and Sugiura, T. (2014). Natural products and food components with anti- Helicobacter pylori activities. World J. Gastroenterol. 20 (27), 8971–8978. doi:10.3748/wjg.v20.i27.8971
Thuy, T. T. D., Kuo, P.-Y., Lin, S.-M., and Kao, C.-Y. (2022). Anti- Helicobacter pylori activity of potential probiotic Lactiplantibacillus pentosus SLC13. BMC Microbiol. 22 (1), 277. doi:10.1186/s12866-022-02701-z
Viazis, N., Argyriou, K., Kotzampassi, K., Christodoulou, D. K., Apostolopoulos, P., Georgopoulos, S. D., et al. (2022). A four-probiotics regimen combined with a standard Helicobacter pylori -eradication treatment reduces side effects and increases eradication rates. Nutrients 14 (3), 632. doi:10.3390/nu14030632
Wang, D., Guo, Q., Yuan, Y., and Gong, Y. (2019). The antibiotic resistance of Helicobacter pylori to five antibiotics and influencing factors in an area of China with a high risk of gastric cancer. BMC Microbiol. 19, 152–10. doi:10.1186/s12866-019-1517-4
Wu, D., Cao, M., Zhou, J., Yan, S., Peng, J., Yu, Z., et al. (2021). Lactobacillus casei T1 from kurut against Helicobacter pylori -induced inflammation and the gut microbial disorder. J. Funct. Foods 85, 104611. doi:10.1016/j.jff.2021.104611
Wu, S., Xu, Y., Chen, Z., Chen, Y., Wei, F., Xia, C., et al. (2023). Lactiplantibacillus plantarum ZJ316 reduces Helicobacter pylori adhesion and inflammation by inhibiting the expression of adhesin and urease genes. Mol. Nutr. and food Res. 67 (18), 2300241. doi:10.1002/mnfr.202300241
Xu, B., Kong, J., Lin, Y., Tang, Z., Liu, J., Chen, Z., et al. (2023). Anti- Helicobacter pylori activity and gastroprotective effects of human stomach-derived Lactobacillus paragasseri strain LPG-9. Food Funct. 14 (24), 10882–10895. doi:10.1039/d3fo03562j
Xu, W., Xu, L., and Xu, C. (2022). Relationship between Helicobacter pylori infection and gastrointestinal microecology. Front. Cell. Infect. Microbiol. 12, 938608. doi:10.3389/fcimb.2022.938608
Yamaoka, Y. (2024). Revolution of Helicobacter pylori treatment. J. Gastroenterol. Hepatol. 39, 1016–1026. doi:10.1111/jgh.16526
Yang, C., Liang, L., Lv, P., Liu, L., Wang, S., Wang, Z., et al. (2021). Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: a randomized double-blind placebo-controlled trial. Helicobacter 26 (6), e12856. doi:10.1111/hel.12856
Yang, Z., Zhou, Y., Han, Z., He, K., Zhang, Y., Wu, D., et al. (2024). The effects of probiotics supplementation on Helicobacter pylori standard treatment: an umbrella review of systematic reviews with meta-analyses. Sci. Rep. 14 (1), 10069. doi:10.1038/s41598-024-59399-4
Yarmohammadi, M., Yadegar, A., Ebrahimi, M. T., and Zali, M. R. (2021). Effects of a potential probiotic strain Lactobacillus gasseri ATCC 33323 on Helicobacter pylori -induced inflammatory response and gene expression in coinfected gastric epithelial cells. Probiotics Antimicrob. Proteins 13 (3), 751–764. doi:10.1007/s12602-020-09721-z
Yuan, C., Adeloye, D., Luk, T. T., Huang, L., He, Y., Xu, Y., et al. (2022). The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child. Adolesc. Health 6 (3), 185–194. doi:10.1016/S2352-4642(21)00400-4
Zhai, S., Gao, Y., Jiang, Y., Li, Y., Fan, Q., Tie, S., et al. (2024). Weizmannia coagulans BC99 affects valeric acid production via regulating gut microbiota to ameliorate inflammation and oxidative stress responses in Helicobacter pylori mice. J. Food Sci. 89, 9985–10002. doi:10.1111/1750-3841.17514
Zhang, Y., Li, X., Shan, B., Zhang, H., and Zhao, L. (2022). Perspectives from recent advances of Helicobacter pylori vaccines research. Helicobacter 27 (6), e12926. doi:10.1111/hel.12926
Zhao, K., Xie, Q., Xu, D., Guo, Y., Tao, X., Wei, H., et al. (2018). Antagonistics of Lactobacillus plantarum ZDY2013 against Helicobacter pylori SS1 and its infection in vitro in human gastric epithelial AGS cells. J. Biosci. Bioeng. 126 (4), 458–463. doi:10.1016/j.jbiosc.2018.04.003
Zhou, Q., Xue, B., Gu, R., Li, P., and Gu, Q. (2021). Lactobacillus plantarum ZJ316 attenuates Helicobacter pylori -induced gastritis in C57BL/6 mice. J. Agric. Food Chem. 69 (23), 6510–6523. doi:10.1021/acs.jafc.1c01070
Zimmermann, P., and Curtis, N. (2017). Antimicrobial effects of antipyretics. Antimicrob. agents Chemother. 61 (4), e02268-16. doi:10.1128/aac.02268-16
Keywords: Helicobacter pylori, antibiotic resistance, probiotic, lactobacillus, eradication
Citation: Yuan L, Yang C, Han Y, Yang F and Tu H (2025) Beyond antibiotics: probiotics as a promising ally against Helicobacter pylori. Front. Pharmacol. 16:1620870. doi: 10.3389/fphar.2025.1620870
Received: 06 May 2025; Accepted: 25 June 2025;
Published: 11 July 2025.
Edited by:
Kefang Sun, Rochester General Hospital, United StatesReviewed by:
Irena Mladenova, Trakia University, BulgariaChengu Niu, Rochester Regional Health, United States
Hsi-Chang Shih, Johns Hopkins University, United States
Copyright © 2025 Yuan, Yang, Han, Yang and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huabing Tu, dHVtb3V0YWkyMDIzQDE2My5jb20=
 Lin Yuan1,2
Lin Yuan1,2 Huabing Tu
Huabing Tu