- 1Centre for Drug Research and Development, Guangdong Pharmaceutical University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory for Research and Evaluation of Pharmaceutical Preparations, Guangzhou, China
- 3Guangdong Engineering and Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, Guangzhou, China
- 4School of Chinese Materia Medica, Guangdong Pharmaceutical University, Guangzhou, China
- 5Guangdong Provincial Biotechnology Research Institute (Guangdong Provincial Laboratory Animals Monitoring Center), Guangzhou, China
A Correction on
Anti-inflammatory effect and mechanism of stytontriterpene D on RAW264.7 cells and zebrafish
by Yu C, Qiu G, Liu X, Xie Q, Lin Z, Wang F and Cai L (2025). Front. Pharmacol. 16:1559022. doi: 10.3389/fphar.2025.1559022
There was a mistake in Figures 6–8 as published. An error was made in the concentrations. The corrected Figures 6–8 appear below.
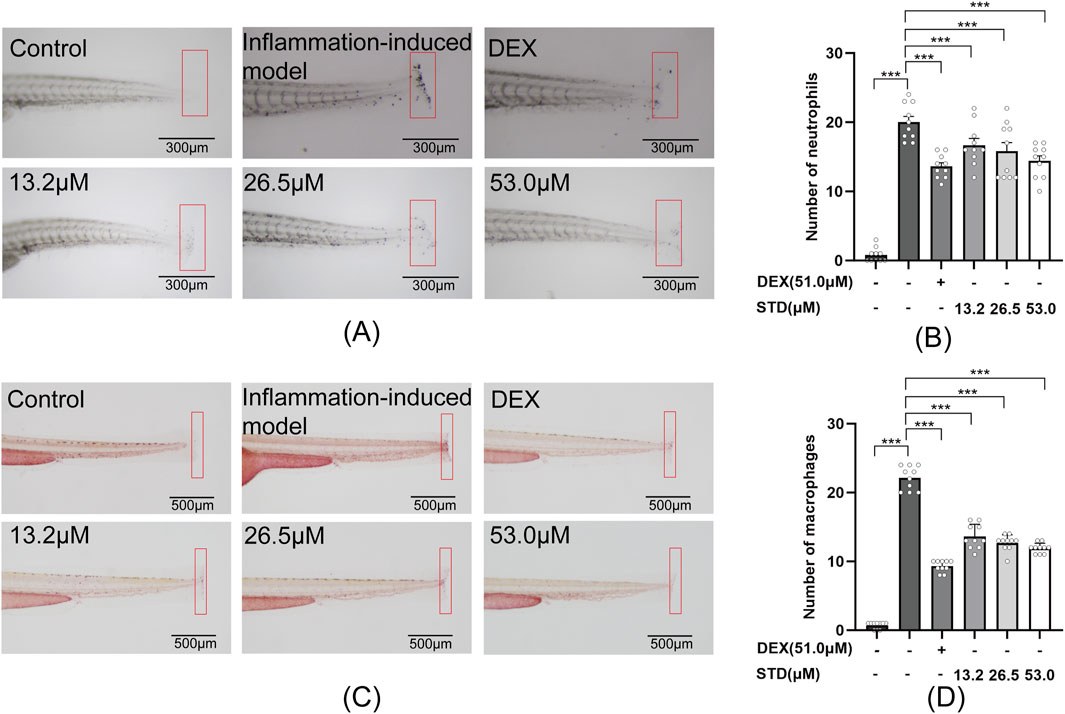
Figure 6. Effect of STD on neutrophils and macrophages recruitment in zebrafish after tail transection (n = 10). (A) The count of neutrophils in the zebrafish tail at ×40 magnification, (B) neutrophil numbers in the zebrafish tail, (C) macrophage recruitment in the zebrafish tail at ×30 magnification, and (D) the quantity of macrophages in the zebrafish tail. Data are shown as average ± SEM values of at least three separate experiments. ***P < 0.001 versus the inflammation-induced model group by one-way ANOVA with Tukey’s test.
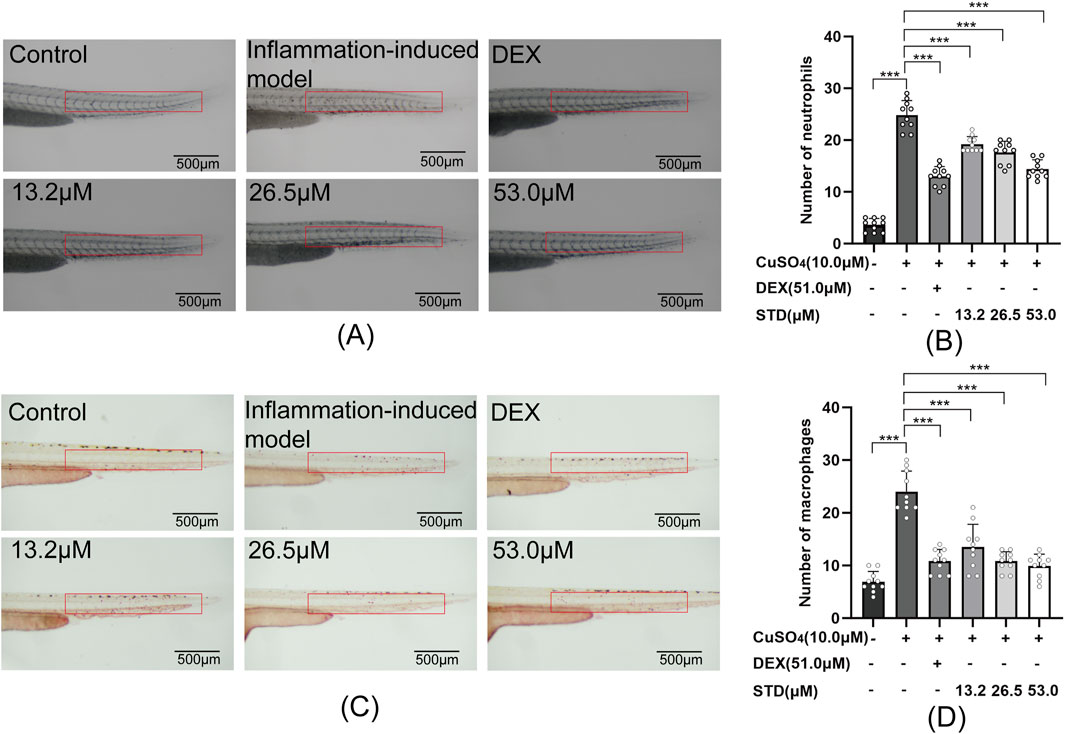
Figure 7. Effect of STD on neutrophils and macrophages in zebrafish induced by copper sulfides (n = 10). (A) Neutrophil recruitment in the lateral line of zebrafish at ×30 magnification, (B) the number of neutrophils in the zebrafish tail, (C) macrophage recruitment in the lateral line at ×30 magnification, and (D) the quantity of macrophages in the zebrafish tail. Data are shown as average ± SEM values of at least three separate experiments. ***P < 0.001 versus the inflammation-induced model group by one-way ANOVA with Tukey’s test.
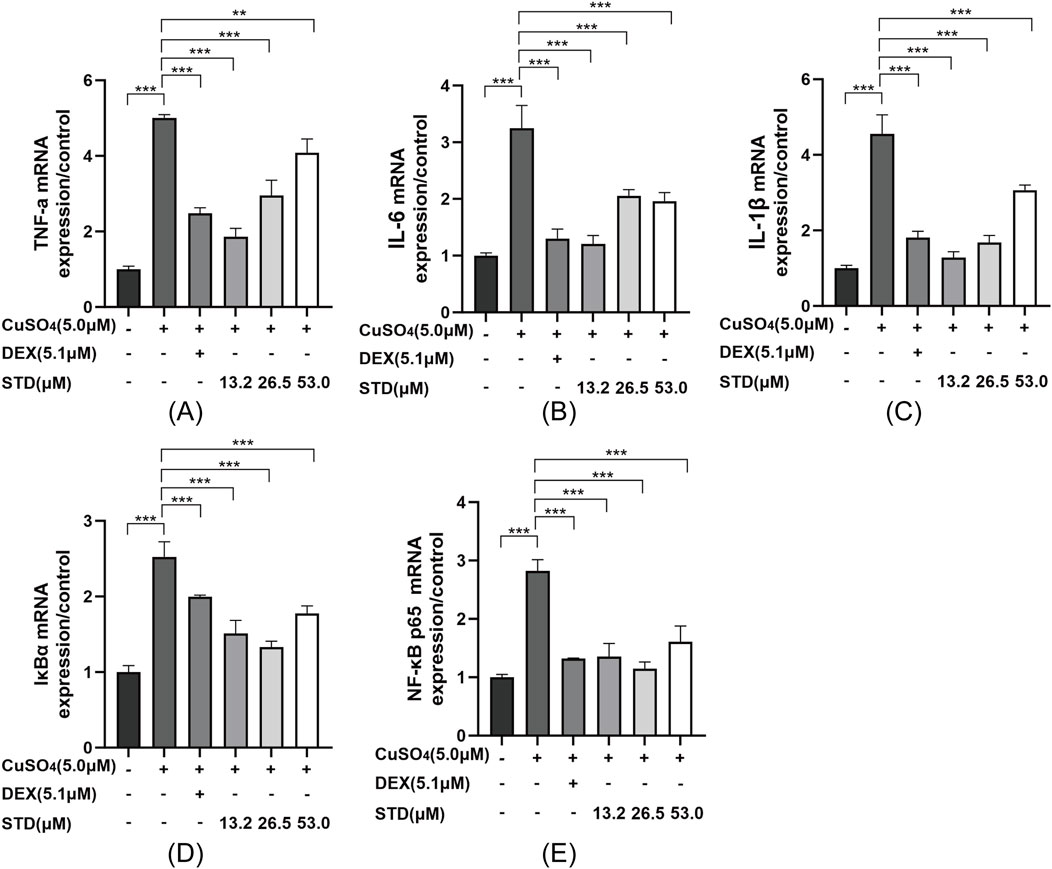
Figure 8. Effect of STD on the expression of inflammation-related genes in copper sulfate–stimulated zebrafish. Groups other than the control were treated with a copper sulfate solution that had 5.1 μM DEX and various amounts of STD (13.2, 26.5, and 53.0 μM). STD was able to suppress the mRNA expression of (A) TNF-α, (B) IL-1β, (C) IL-6, (D) IκBα, and (E) NF-κB p65 in copper sulfate–stimulated zebrafish. Data for three or more separate experiments are given as average ± SEM values of at least three separate experiments. ***P < 0.001 versus the inflammation-induced model group by one-way ANOVA with Tukey’s test.
There was a mistake in the caption of Figure 8 as published. It was erroneously stated that the control group were treated with a DEX solution, rather than a copper sulfate solution. As such, DEX and copper sulfate were reversed. The corrected caption of Figure 8 appears above.
TNF-α was mistakenly written as TNF-κ and NF-κB was mistakenly written as NF-αB.
A correction has been made to the section Abstract, Paragraph Number 2:
“Methods: In vitro, we evaluated the toxicity of STD to RAW 264.7 cells using the CCK8 method and detected the reactive oxygen species (ROS) and nitric oxide (NO) contents in cells using diacetyldichlorofluorescein (DCFH-DA) and the Griess method. We detected the levels of interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), interleukin-10 (IL-10), and arginase-1 (ARG1) via enzyme-linked immunosorbent assay and measured the expression of related proteins in the NF-κB pathway via western blotting. The toxicity of STD to AB zebrafish was detected in vivo, and the recruitment of neutrophils and macrophages was evaluated in tail cut-induced and copper sulfate-induced zebrafish inflammation models. We used quantitative real-time polymerase chain reaction to study the expression of inflammation-related genes in zebrafish with inflammation induced by copper sulfate.”
Throughout Sections 2.12-2.13, the concentration of DEX was mistakenly written as 61.2 μM, while the correct concentration is “DEX (51.0 μM)”.
All instances have been corrected to “DEX (51.0 μM)” in the following sections:
Materials and methods, 2.12 Tail transection–induced inflammatory model in zebrafish, sub-sections 2.12.1 and 2.12.2; and Materials and methods, 2.13 Copper sulfate–induced inflammatory model in zebrafish, sub-sections 2.13.1 and 2.13.2.
Throughout Section 2.13, the unit for copper sulfate was erroneously written as 1.6 μM, rather than “10.0 μM”. All instances have been corrected to “10.0 μM copper sulfate” in the following sections: Materials and methods, 2.13 Copper sulfate–induced inflammatory model in zebrafish, sub-sections 2.13.1 and 2.13.2.
STD was mistakenly written as DE.
A correction has been made to the section Discussion, Paragraph number 2. The corrected sentence is:
“We found that STD significantly reduced the expression of LPS induced M1 phenotype (IL-1β, IL-6, TNF-a, and iNOS) in a concentration dependent manner, while STD significantly increased the expression of M2 phenotype (ARG1 and IL-10) mRNA, exerting anti-inflammatory effects by modulating macrophage polarization.”
The original article has been updated.
Generative AI statement
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: stytontriterpene D, anti-inflammatory, mechanism, RAW 264.7 cell, zebrafish
Citation: Yu C, Qiu G, Liu X, Xie Q, Lin Z, Wang F and Cai L (2025) Correction: Anti-inflammatory effect and mechanism of stytontriterpene D on RAW264.7 cells and zebrafish. Front. Pharmacol. 16:1620902. doi: 10.3389/fphar.2025.1620902
Received: 30 April 2025; Accepted: 23 September 2025;
Published: 07 October 2025.
Edited and reviewed by:
Chiara Bolego, University of Padua, ItalyCopyright © 2025 Yu, Qiu, Liu, Xie, Lin, Wang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, d2ZlbmcxMjMwQDE2My5jb20=; Lei Cai, Y2FpbGVpMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chuqin Yu
Chuqin Yu Gao Qiu
Gao Qiu Xiangying Liu1,2,3
Xiangying Liu1,2,3 Feng Wang
Feng Wang Lei Cai
Lei Cai