- Department of Food Science, University of Guelph, Guelph, Canada
Background: Acute myeloid leukemia (AML) is an aggressive hematological malignancy with limited therapeutic options. Despite recent advances in targeted therapies, patients are still faced with poor survival outcomes. Thus, development of novel therapeutic agents with broad efficacy remains an urgent need.
Methods: We conducted a natural compound library screen and identified 6-methoxydihydroavicine, a plant-derived benzophenanthridine alkaloid (BPA) derived from the genus of Macleaya - a perennial herb found in China, North America and Europe - as a potent compound that reduced AML cell viability. We evaluated its cytotoxicity in multiple AML cell lines and investigated its underlying mechanism of action using assays that probed mitochondrial function, and reactive oxygen species (ROS) production.
Results: 6-methoxydihydroavicine significantly reduced cell viability and induced caspase-mediated cell death in AML cell lines in a dose-dependent manner. Mechanistically, 6-methoxydihydroavicine triggered accumulation of mitochondrial ROS and disrupted electron transport chain (ETC) function.
Conclusion: Our findings demonstrate that 6-methoxydihydroavicine exerts strong cytotoxic effects against AML cells through mitochondrial dysfunction and ROS-mediated apoptosis. As a natural, plant-derived compound with distinct anti-AML properties, 6-methoxydihydroavicine represents a promising candidate for further development as a therapeutic agent for AML.
Introduction
Acute myeloid leukemia (AML) is a devastating hematological malignancy characterized by impaired hematopoiesis leading to uncontrolled proliferation and accumulation of undifferentiated myeloid precursors (myeloblasts) in the peripheral blood, bone marrow, and/or other tissues (Döhner et al., 2015; Papaemmanuil et al., 2016; Shimony et al., 2023). Recent data from the Surveillance, Epidemiology, and End Results Program shows that the 5-year survival rate for AML is 31.9% (National Cancer Institute, 2024), while in Canada, the adult 5-year net survival rate is as low as 21% (Statistics Canada, 2020). The standard “7 + 3” chemotherapy regimen, comprising 7 days of cytarabine and 3 days of anthracyclines, has remained largely unchanged since the 1980s (De Kouchkovsky et al., 2011; Newell and Cook, 2021). Although the development of targeted and immunotherapies has introduced new treatment options (Greiner et al., 2022), the prognosis for elderly patients remains poor with a 5-year survival rate of only 7% (Martínez-Cuadrón et al., 2021). AML patients often present with high-risk mutations and/or limited treatment tolerance leaving low-intensity chemotherapy as the main therapeutic strategy. Moreover, the molecular heterogeneity of AML further narrows therapeutic strategies, as existing targeted therapies benefit only a subset of patients harboring specific mutations (Greiner et al., 2022). Thus, new anti-AML drugs are needed to improve patient outcomes.
Natural products have played an important role in drug discovery and cytarabine, the front-line anti-AML therapeutic, was originally derived from natural sources (Chopra and Dhingra, 2021; Katz and Baltz, 2016; Hwang et al., 2019). Benzophenanthridine alkaloids (BPAs) are a class of isoquinoline compounds found widely in Papaveraceae, Corydalis, and Rutaceae families of plants. BPAs have demonstrated anti-tumour (Peng et al., 2023), anti-inflammatory (Lenfeld et al., 1981), anti-viral (Colombo and Bosisio, 1996), and anti-angiogenesis properties (Eun and Koh, 2004). Sanguinarine (SNG) and chelerythrine (CHE) are the most studied (Yang et al., 2012; Laines-Hidalgo et al., 2022), and a recent study showed SNG, CHE, and nitidine (NTD) have activity against gastric cancer cell lines (Liu et al., 2023).
An unbiased, systematic screen of an in-house natural product/nutraceutical library determined that 6-methoxydihydroavicine, a previously uncharacterized BPA, is a potent anti-AML compound that inhibits metabolic processes to impart selective cell death. 6-methoxydihydroavicine is commonly found in Macleaya cordata and Zanthoxylum integrifoliolum. While previous studies have reported anti-cancer activity in solid tumors such as pancreatic (Ma et al., 2022) and ovarian cancers (Zhang et al., 2023), its role in hematological malignancies remains largely unknown. In this study, we sought to investigate the anti-leukemia potential of 6-methoxydihydroavicine and elucidate its underlying mechanism in AML cells. Our results demonstrate that 6-methoxydihydroavicine exerts significant cytotoxicity in AML cell lines through induction of mitochondrial reactive oxygen species (ROS), inhibition of electron transport chain (ETC) activity, and activation of caspase-dependent cell death. These findings position 6-methoxydihydroavicine as a promising candidate for further development as a novel therapeutic agent against AML.
Materials and methods
Cell lines and materials
Ontario Cancer Institute AML2 (OCI-AML2; AML2), and OCI-AML3 (AML3) cell lines were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM; Wisent) supplemented with 10% fetal bovine serum (FBS). The surrogate leukemia stem cell line, TEX, was grown in IMDM supplemented with 15% FBS (Sigma) and 20 ng/mL stem cell factor (SCF; Peprotech), 2 ng/mL interleukin-3 (Peprotech), and 2 mM L-glutamine (Thermo-Fisher). All media were supplemented with an antibiotic solution consisting of 200 μg of streptomycin and 200 units of penicillin per milliliter of media (Wisent). All cells were maintained in an incubator at 37°C with 5% CO2 and 95% relative humidity.
Cytotoxicity analysis
To measure cell proliferation and viability, the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) reduction assay (Promega) and the 7-aminoactinomycin (7-AAD; Cayman Chemicals) dye were used. Cells (1.25 × 105 cells/mL) were seeded in a 96-well plate and treated with serial dilutions of 6-methoxydihydroavicine at multiple time points. The untreated groups were incubated in media containing 0.06% dimethyl sulfoxide (matching the solvent volume used in the highest 6-methoxydihydroavicine concentration), serving as the negative control. Following treatment, for the MTS assay, cells were incubated with 20 μL of MTS for 2 h at 37°C and 5% CO2 and absorbance (490 nm) was measured using a SpectraMax M5 spectrophotometer (Molecular Devices; Sunnyvale, CA). For 7-AAD staining, cells were collected and resuspended in 250 μL of staining solution with 1 μg/mL 7-AAD, and the fluorescence was measured with the Guava EasyCyte 8HT Benchtop Flow Cytometer (Merck Millipore).
Reactive oxygen species (ROS) quenching studies
To measure antioxidant effects on 6-methoxydihydroavicine cytotoxicity, leukemia cells (1.25 × 105 cells/mL) were seeded in a 96-well plate and treated with increasing does of 6-methoxydihydroavicine in the absence or presence of 1 mM N-Acetylcysteine (NAC) for 72 h. Cell viability was then assessed using 7AAD (1 µg/mL) and fluorescence was measured using flow cytometry (Guava EasyCyte 8HT; EMD Millipore).
Mitochondrial reactive oxygen species (ROS)
To assess mitochondrial ROS, leukemia cells were seeded in a 12-well plate (1.25 × 105 cells/mL) and treated with increasing doses of 6-methoxydihydroavicine for 3 h. After treatment, cells were collected and stained with 5 μM MitoSOX Red probe (Invitrogen; M36008) according to the manufacturer’s protocol and fluorescence was measured by flow cytometry (Guava EasyCyte 8HT; EMD Millipore).
Caspase-dependent cell survival assay
The pan-caspase inhibitor z-VAD-FMK (Z-VAD; R&D Systems, Minneapolis, MN) was used to study the role of caspase enzymes on 6-methoxydihydroavicine’s cytotoxicity. Here, leukemia cells were treated with 6-methoxydihydroavicine in the presence or absence of 50 μM Z-VAD. Following a 72 hour-incubation, cell viability was measured using the 7AAD assay and flow cytometry.
Oxygen consumption rates
Oxygen consumption rates (OCRs) of leukemia cells were measured on the O2K Oxygraph (Oroboros) using substrate-uncoupler-inhibitor titration (SUIT) protocols (Gnaiger, 2020; Tcheng et al., 2021). Briefly, 5 × 106 cells were treated with or without 1 μM 6-methoxydihydroavicine for 1 h. After treatment, cells were collected and permeabilized using 100 µg/mL digitonin (Sigma) and maintained in Mir05 buffer, as previously described (Tcheng et al., 2021; Makrecka-Kuka et al., 2015). Permeabilized cells were injected into the oxygraphy chamber and once equilibrated, 2.5 mM ADP, 5 mM pyruvate (PYR, Sigma), 10 mM glutamate (GLU; Sigma), and 0.5 mM malate (MAL, Sigma) were added to measure complex I (CI)-supported respiration. 250 nM rotenone (ROT, Sigma) was then added to inhibit CI respiration. To stimulate complex II (CII)-supported respiration, 10 mM succinate was injected. Finally, 250 nM antimycin A (AA; A8674; Sigma) was added to determine non-mitochondrial respiration. Oxygraph chambers were maintained at 37°C, and OCRs were calculated as the negative time derivative of oxygen concentration using the DatLab Software (Oroboros).
Statistical analysis
Statistics were analyzed using GraphPad 8.0 Prism software. Results are presented as a mean ± standard deviation unless otherwise stated. Significance between values was determined by a paired, two-tailed t-test, two-way ANOVA, or by a one-way ANOVA paired with Dunnett’s post hoc analysis for between group comparisons. P < 0.05 was accepted as being statistically significant.
Results
6-Methoxydihydroavicine is a plant-derived anti-AML agent
An in-house chemical library consisting of numerous plant-derived molecules screened against AML cells determined that 6-methoxydihydroavicine was a potent compound. Figure 1A shows the common molecular structure of BPA as well as 6-methoxydihydroavicine and the three additional BPAs included in this study: sanguinarine (SNG), chelerythrine (CHE), and nitidine (NTD). The activity of these BPAs was tested in AML2, AML3, and TEX leukemia cell lines using the MTS assay (Figure 1B), while the cytotoxicity of 6-methoxydihydroavicine was further confirmed using the 7-AAD assay and flow cytometry (Figure 1B). All four BPAs exhibited dose-dependent inhibitory effects on AML cells, with 6-methoxydihydroavicine showing the greatest potency with IC50 values ranging from 0.42 to 1.19 μM (Figure 1C). Cytarabine, a standard AML therapeutic, induces death in these cells at 5–10 μM (Tcheng et al., 2001).
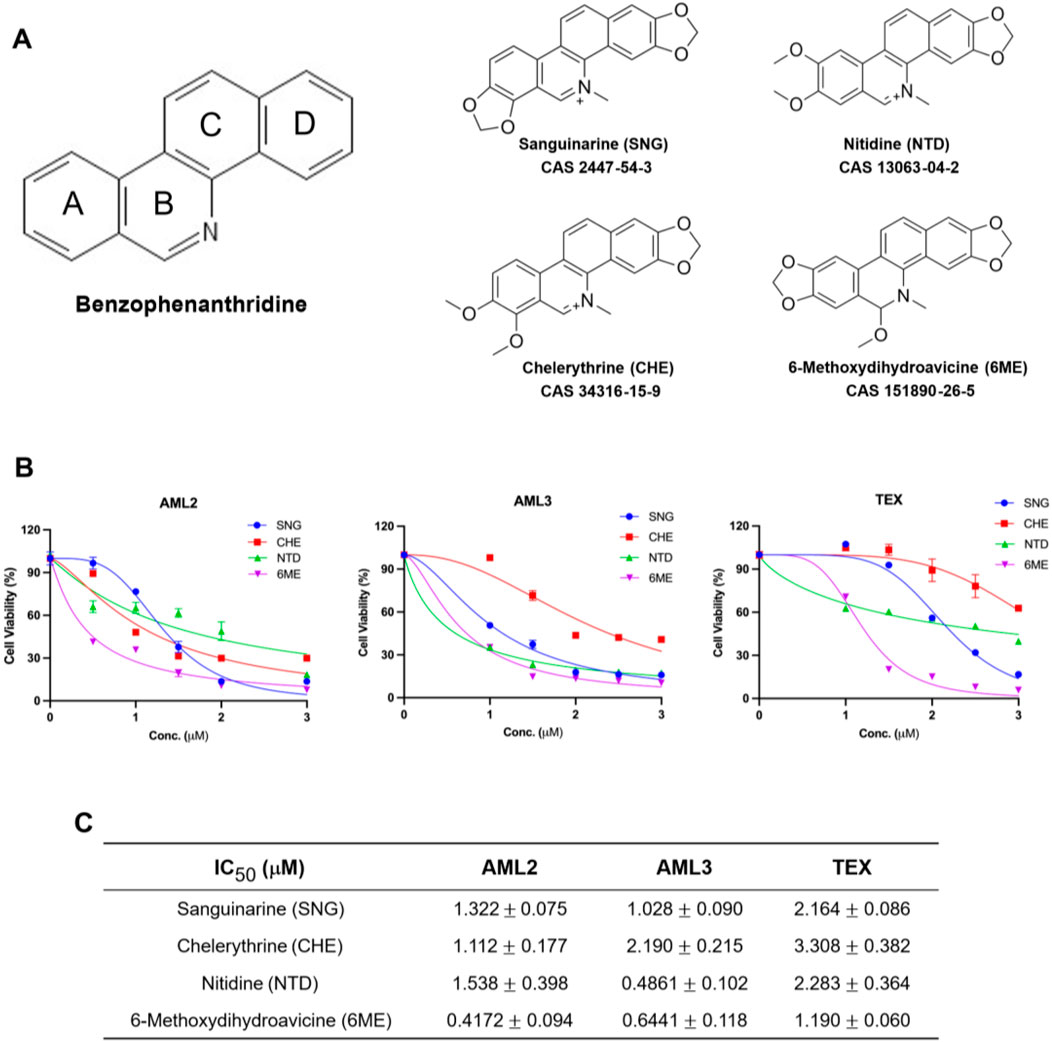
Figure 1. 6-methoxydihydroavicine exhibits strong anti-AML potency. (A) The chemical structure of benzophenanthridine. (B) MTS assay, 7-AAD staining, and flow cytometry analysis of cell viability show the cytotoxic effects of SNG, CHE, NTD, and 6-methoxydihydroavicine in AML cell lines after 72-h treatment. (C) IC50 values of 6-methoxydihydroavicine are lowest among all tested BPAs, ranging from 0.42–1.19 µM. Data presented as % cell viability relative to untreated cells.
6-Methoxydihydroavicine induced ROS participates in AML cell death
ROS, a class of highly reactive molecules that perform numerous biological activities including apoptosis induction (Nakamura and Takada, 2021), was measured in AML cells after 6-methoxydihydroavicine treatment. Using MitoSOX Red fluorogenic probes, 6-methoxydihydroavicine caused a dose-dependent accumulation of mitochondrial ROS (Figure 2A). To determine the functional significance of ROS in 6-methoxydihydroavicine-induced cytotoxicity, cells were also treated with NAC, which regenerates the potent antioxidant glutathione. NAC significantly abrogated 6-methoxydihydroavicine-induced cytotoxicity (Figure 2B), suggesting a functional role of ROS.
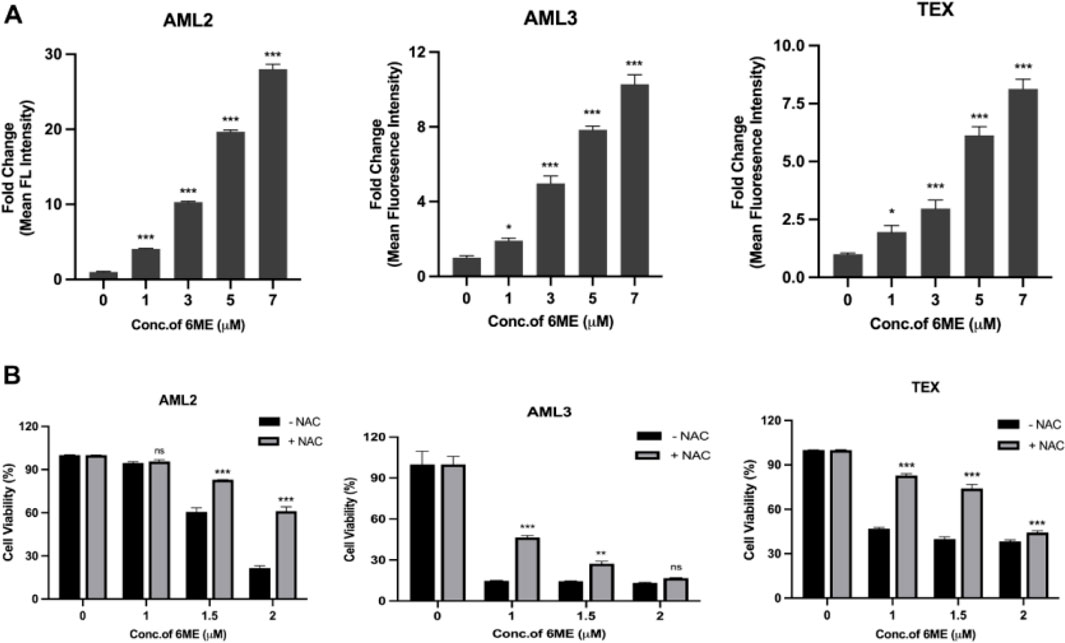
Figure 2. 6-methoxydihydroavicine imparts anti-AML activity through ROS generation. (A) Mitochondrial ROS was measured following treatment with 6-methoxydihydroavicine for 3 h using MitoSOX red probe staining. (B) 6-methoxydihydroavicine activity was tested in the presence or absence of NAC (1 mM) in AML2, AML3, and TEX cells using 7AAD staining. Data presented as mean ± S.D., ns. not significant, *p < 0.05, **p < 0.01, ***p < 0.001 calculated using one-way ANOVA with Dunnett’s multiple comparisons test calculated in GraphPad Prism 8.0.
6-Methoxydihydroavicine imparts anti-AML activity via caspase-mediated death and mitochondrial dysfunction
Mitochondria are a primary source of cellular ROS (Turrens, 2003). Electrons leaking from the mitochondrial electron transport chain (ETC) can react with oxygen and produce ROS. To investigate effects on, ETC, function, respiration rates of complexes I and II were measured in 6-methoxydihydroavicine-treated AML2 and TEX cells using a well-established SUIT protocol and respirometry. 6-methoxydihydroavicine significantly inhibited respiration of both complexes compared to untreated groups in both AML2 and TEX cells (Figure 3A), indicating that 6-methoxydihydroavicine impacts mitochondrial bioenergetics.
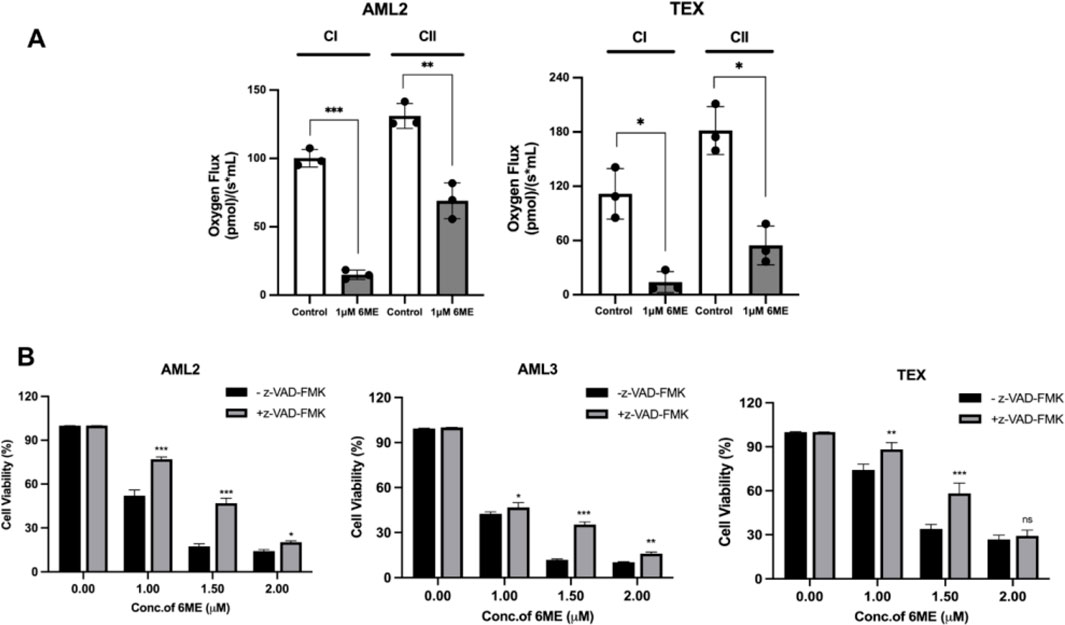
Figure 3. 6-methoxydihydroavicine-induced AML cell death via modulation of caspase activity and mitochondrial dysfunctions. (A) Oxygen consumption rates (OCRs) of complexes I and II were measured AML2 and TEX cells treated with 1 μM 6-methoxydihydroavicine for 1 h following a well-established substrate-uncoupler-inhibitor titration (SUIT) protocol using the Oroboros 2K oxygraphy. (B) 6-methoxydihydroavicine activity was tested in the presence or absence Z-VAD (50 μM) in AML2, AML3, and TEX cells using 7AAD staining. Data presented as mean ± S.D., ns. not significant, *p < 0.05, **p < 0.01, ***p < 0.001 calculated using an unpaired t-test or one-way ANOVA and a Dunnett’s multiple comparisons test in GraphPad Prism 8.0.
Finally, to assess whether caspase enzymes are involved in cell death, we co-incubated 6-methoxydihydroavicine with the pan-caspase inhibitor Z-VAD (50 μM). After incubation for 72 h, Z-VAD significant reduced 6-methoxydihydroavicine-induced reductions of AML cell viability (Figure 3B), implicating the role of caspase activity in 6-methoxydihydroavicine-induced cell death.
Discussion
A high-throughput screen identified 6-methoxydihydroavicine as a novel anti-AML compound, and validation studies determined that this alkaloid, derived from a plant found in North America, was capable of inducing cytotoxicity in AML through metabolic alterations. In this study, 6-methoxydihydroavicine triggered AML cell death through ROS induction, caspase activity, and mitochondrial dysfunction.
6-methoxydihydroavicine is a BPA with reported anti-cancer activity in solid tumors (Ma et al., 2022; Zhang et al., 2023); however, its role in AML and its mechanism of action in hematologic malignancies remain largely unknown. In pancreatic and ovarian cancer cells, 6-methoxydihydroavicine activates the RIPK1/caspase axis leading to disruption of oxaloacetic acid metabolism (Ma et al., 2022) and alters mitochondrial respiration to induce MAPK-mediated apoptosis (Ma et al., 2022; Zhang et al., 2016). Previous work by our group showed that 6-ME bound to and decreased activity of PPAR-γ leading to decreased fatty acid oxidation (FAO) and selective death of AML cells. In the current study, we extended these findings and build upon the work of others to show that 6-methoxydihydroavicine-induced cell death is associated with ROS and caspase activity. The observed cytotoxicity of 6-methoxydihydroavicine is related to elevated mitochondrial ROS, which was mitigated when co-incubated with the glutathione regenerating molecule NAC (Figure 2B). These results are in line with previous findings that FAO inhibition, through decreased NADPH leading to reduced glutathione production, results in ROS-induced cell death that was mitigated by NAC co-incubation (Lee et al., 2015; Pike et al., 2011). In this study, 6-methoxydihydroavicine also decreased CI and CII activity and CI or II inhibition results in ROS-mediated death of cancer cells (Basit et al., 2017; Li et al., 2003; Quinlan et al., 2012; Dröse, 2013; Guzy et al., 2008; Roma et al., 2022), which aligns with a previous report that targeting oxidative stress could be an effective approach for cancer treatment (Ma et al., 2018). Whether this change in complex activity is related to PPARγ targeting or an off-target effect will be the focus of future studies. Additional work is also needed to confirm the functional importance of CI and CII in 6-methoxydihydroavicine-induced cytotoxicity as well as the link between complex inhibition, PPAR inhibition and ROS generation.
Plants are a significant source of clinically relevant anti-cancer drugs. Indeed, the most common clinical AML therapeutic, cytarabine, was derived from Cryptotheca crypta, a Caribbean sponge (Schwartsmann et al., 2001). BPAs, which are isolated form Papaveraceae, Corydalis, and Rutaceae families of plants, are noted for their anti-inflammatory, anti-tumor, and anti-bacterial activities (Peng et al., 2023). From the genus of Macleaya (Papaveraceae), these plants are primarily found in China, Europe and North America and have been used historically for medicinal effects. SNG exerts an anti-proliferative activity on murine melanoma cells and A375 human melanoma xenografts in vitro and in vivo (De Stefano et al., 2009). Mechanically, SNG induces H2O2-dependent cell ferroptosis in human cervical cancer (HeLa) cells by downregulating SLC7A11 and glutathione (Alakkal et al., 2022). Other BPAs, like CHE, inhibit non-small cell lung cells by downregulating β-Catenin (Heng and Cheah, 2020), while NTD suppresses epithelial mesenchymal transformation and glioma stem cells by targeting the JAK2/STAT3 signaling pathway (Jia et al., 2021). Moreover, a synthetic BPA, NK109, demonstrated strong anti-leukemia activity (Nakanishi et al., 1999), with its metabolites showing reduced toxicity to host cells while retaining significant anti-tumor effects in clinical trials (Nakanishi et al., 2000). Notably, several natural BPAs show superior activity compared to marketed drugs in vitro. Burgenine is more potent than doxorubicin in drug-resistant CEM/ADR5000 cell lines (Sandjo et al., 2014), while oxynitidine derivatives display greater inhibitory effects against DU145 prostate cancer cells compared to camptothecin (Tang et al., 2019). While clinical data on natural BPAs remain limited, early-stage trials and preclinical evidence suggest a favorable therapeutic window (Peng et al., 2023; Tavares de et al., 2014; Xu et al., 2025; Pica et al., 2012), with certain compounds demonstrating selective cytotoxicity against cancer cells and manageable toxicity profiles in animal models. In this study, other BPAs demonstrated potency with low IC50 values and 6 ME was selected as the lead compound, as it was consistently the most potent. Future studies will determine which structural features contribute to 6 ME’s superior activity. These findings collectively highlight the therapeutic promise of BPAs and support their further investigation as potential anti-cancer agents.
In summary, found in plants growing in North America, 6-methoxydihydroavicine is a novel compound belonging to the BPA family that exhibited potent anti-AML activity. Mechanistically, it induces mitochondrial ROS, impairs CI and CII respiration, and imparts caspase-dependent cell death. These findings provide new insights into 6-methoxydihydroavicine as a potential anti-leukemia therapeutic.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
YY: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing. PS: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding provided by the University of Guelph.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alakkal, A., Thayyullathil, F., Pallichankandy, S., Subburayan, K., Cheratta, A. R., and Galadari, S. (2022). Sanguinarine induces H2O2-dependent apoptosis and ferroptosis in human cervical cancer. Biomedicines 10 (8), 1795. doi:10.3390/biomedicines10081795
Basit, F., van Oppen, L. M., Schöckel, L., Bossenbroek, H. M., van Emst-de Vries, S. E., Hermeling, J. C., et al. (2017). Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 8 (3), e2716. doi:10.1038/cddis.2017.133
Chopra, B., and Dhingra, A. K. (2021). Natural products: a lead for drug discovery and development. Phytother. Res. 35 (9), 4660–4702. Epub 2021 Apr 13. PMID: 33847440. doi:10.1002/ptr.7099
Colombo, M. L., and Bosisio, E. (1996). Pharmacological activities of chelidonium majus L. (Papaveraceae). Pharmacol. Res. 33 (2), 127–134. doi:10.1006/phrs.1996.0019
De Kouchkovsky, N. R. A., Stocker, C. J., Roy, A. G., Hislop, D., Wargent, E., Bell, R., et al. (2011). A new, highly selective marine peroxisome proliferator-activated receptor delta agonist increases responsiveness to thermogenic stimuli and glucose uptake in skeletal muscle in obese mice. Diab Obes. Metab. 13 (5), 455–464. doi:10.1111/j.1463-1326.2011.01371.x
De Stefano, I., Raspaglio, G., Zannoni, G. F., Travaglia, D., Prisco, M. G., Mosca, M., et al. (2009). Antiproliferative and antiangiogenic effects of the benzophenanthridine alkaloid sanguinarine in melanoma. Biochem. Pharmacol. 78, 1374–1381. doi:10.1016/j.bcp.2009.07.011
Döhner, H., Weisdorf, D. J., and Bloomfield, C. D. (2015). Acute myeloid leukemia. N. Engl. J. Med. 373 (12), 1136–1152. doi:10.1056/NEJMra1406184
Dröse, S. (2013). Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning. Biochim. Biophys. Acta 1827 (5), 578–587. doi:10.1016/j.bbabio.2013.01.004
Eun, J. P., and Koh, G. Y. (2004). Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem. Biophys. Res. Commun. 317 (2), 618–624. doi:10.1016/j.bbrc.2004.03.077
Gnaiger, E. (2020). Mitochondrial pathways and respiratory control: an introduction to OXPHOS analysis. Bioenerg. Commun., 2020: 2–2. doi:10.26124/bec:2020-0002
Greiner, J., Götz, M., and Wais, V. (2022). Increasing role of targeted immunotherapies in the treatment of AML. Int. J. Mol. Sci. 23 (6), 3304. doi:10.3390/ijms23063304
Guzy, R. D., Sharma, B., Bell, E., Chandel, N. S., and Schumacker, P. T. (2008). Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell Biol. 28 (2), 718–731. doi:10.1128/MCB.01338-07
Heng, W. S., and Cheah, S. C. (2020). Chelerythrine chloride downregulates β-catenin and inhibits stem cell properties of non-small cell lung carcinoma. Molecules 25 (1), 224. doi:10.3390/molecules25010224
Hwang, D., Kim, M., Park, H., Jeong, M. I., Jung, W., and Kim, B. (2019). Natural products and acute myeloid leukemia: a review highlighting mechanisms of action. Nutrients 11 (5), 1010. doi:10.3390/nu11051010
Jia, M., Wang, Y., Guo, Y., Yu, P., Sun, Y., Song, Y., et al. (2021). Nitidine chloride suppresses epithelial-mesenchymal transition and stem cell-like properties in glioblastoma by regulating JAK2/STAT3 signaling. Cancer Med. 10 (9), 3113–3128. doi:10.1002/cam4.3869
Katz, L., and Baltz, R. H. (2016). Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol. 43 (2-3), 155–176. doi:10.1007/s10295-015-1723-5
Laines-Hidalgo, J. I., Muñoz-Sánchez, J. A., Loza-Müller, L., and Vázquez-Flota, F. (2022). An update of the sanguinarine and benzophenanthridine alkaloids' biosynthesis and their applications. Molecules 27 (4), 1378. doi:10.3390/molecules27041378
Lee, E. A., Angka, L., Rota, S. G., Hanlon, T., Mitchell, A., Hurren, R., et al. (2015). Targeting mitochondria with avocatin B induces selective leukemia cell death. Cancer Res. 75 (12), 2478–2488. doi:10.1158/0008-5472.CAN-14-2676
Lenfeld, J., Kroutil, M., Marsálek, E., Slavík, J., Preininger, V., and Simánek, V. (1981). Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 43 (2), 161–165. doi:10.1055/s-2007-971493
Li, N., Ragheb, K., Lawler, G., Sturgis, J., Rajwa, B., Melendez, J. A., et al. (2003). Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278 (10), 8516–8525. doi:10.1074/jbc.M210432200
Liu, M., Sun, S., Meng, Y., Wang, L., Liu, H., Shi, W., et al. (2023). Benzophenanthridine alkaloid chelerythrine elicits necroptosis of gastric cancer cells via selective conjugation at the redox hyperreactive C-terminal Sec498 residue of cytosolic selenoprotein thioredoxin reductase. Molecules 28 (19), 6842. doi:10.3390/molecules28196842
Ma, D., Gilbert, T., Pignanelli, C., Tarade, D., Noel, M., Mansour, F., et al. (2018). Exploiting mitochondrial and oxidative vulnerabilities with a synthetic analog of pancratistatin in combination with piperlongumine for cancer therapy. FASEB J. 32 (1), 417–430. doi:10.1096/fj.201700275R
Ma, N., Shangguan, F., Zhou, H., Huang, H., Lei, J., An, J., et al. (2022). 6-methoxydihydroavicine, the alkaloid extracted from Macleaya cordata (Willd.) R. Br. (Papaveraceae), triggers RIPK1/Caspase-dependent cell death in pancreatic cancer cells through the disruption of oxaloacetic acid metabolism and accumulation of reactive oxygen species. Phytomedicine 102, 154164. doi:10.1016/j.phymed.2022.154164
Makrecka-Kuka, M., Krumschnabel, G., and Gnaiger, E. (2015). High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules 5 (3), 1319–1338. doi:10.3390/biom5031319
Martínez-Cuadrón, D., Serrano, J., Gil, C., Tormo, M., Martínez-Sánchez, P., Pérez-Simón, J. A., et al. (2021). Evolving treatment patterns and outcomes in older patients (≥60 years) with AML: changing everything to change nothing? Leukemia 35 (6), 1571–1585. doi:10.1038/s41375-020-01058-4
Nakamura, H., and Takada, K. (2021). Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 112 (10), 3945–3952. doi:10.1111/cas.15068
Nakanishi, T., Masuda, A., Suwa, M., Akiyama, Y., Hoshino-Abe, N., and Suzuki, M. (2000). Synthesis of derivatives of NK109, 7-OH benzo[c]phenanthridine alkaloid, and evaluation of their cytotoxicities and reduction-resistant properties. Bioorg Med. Chem. Lett. 10 (20), 2321–2323. doi:10.1016/s0960-894x(00)00467-4
Nakanishi, T., Suzuki, M., Saimoto, A., and Kabasawa, T. (1999). Structural considerations of NK109, an antitumor benzo[c]phenanthridine alkaloid. J. Nat. Prod. 62 (6), 864–867. doi:10.1021/np990005d
National Cancer Institute. (2024). Acute Myeloid Leukemia — cancer Stat Facts. net survival estimates for primary sites of cancer, by sex, three years combined.
Newell, L. F., and Cook, R. J. (2021). Advances in acute myeloid leukemia. BMJ 375, n2026. doi:10.1136/bmj.n2026
Papaemmanuil, E., Gerstung, M., Bullinger, L., Gaidzik, V. I., Paschka, P., Roberts, N. D., et al. (2016). Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374 (23), 2209–2221. doi:10.1056/NEJMoa1516192
Pelcovits, A., and Niroula, R. (2020). Acute myeloid leukemia: a review. R. I. Med. J. (2013) 103 (3), 38–40. Available online at: https://pubmed.ncbi.nlm.nih.gov/32236160/
Peng, R., Xu, M., Xie, B., Min, Q., Hui, S., Du, Z., et al. (2023). Insights on antitumor activity and mechanism of natural benzophenanthridine alkaloids. Molecules 28 (18), 6588. doi:10.3390/molecules28186588
Pica, F., Balestrieri, E., Serafino, A., Sorrentino, R., Gaziano, R., Moroni, G., et al. (2012). Antitumor effects of the benzophenanthridine alkaloid sanguinarine in a rat syngeneic model of colorectal cancer. Anticancer Drugs 23 (1), 32–42. doi:10.1097/CAD.0b013e32834a0c8e
Pike, L. S., Smift, A. L., Croteau, N. J., Ferrick, D. A., and Wu, M. (2011). Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta 1807 (6), 726–734. doi:10.1016/j.bbabio.2010.10.022
Quinlan, C. L., Orr, A. L., Perevoshchikova, I. V., Treberg, J. R., Ackrell, B. A., and Brand, M. D. (2012). Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 287 (32), 27255–27264. doi:10.1074/jbc.M112.374629
Roma, A., Tcheng, M., Ahmed, N., Walker, S., Jayanth, P., Minden, M. D., et al. (2022). Shikonin impairs mitochondrial activity to selectively target leukemia cells. Phytomed. Plus 2, 100300. doi:10.1016/j.phyplu.2022.100300
Rota, S. G., Roma, A., Dude, I., Ma, C., Stevens, R., MacEachern, J., et al. (2017). Estrogen receptor β is a novel target in acute myeloid leukemia. Mol. Cancer Ther. 16 (11), 2618–2626. doi:10.1158/1535-7163.MCT-17-0292
Sandjo, L. P., Kuete, V., Tchangna, R. S., Efferth, T., and Ngadjui, B. T. (2014). Cytotoxic benzophenanthridine and furoquinoline alkaloids from Zanthoxylum buesgenii (Rutaceae). Chem. Cent. J. 8 (1), 61. doi:10.1186/s13065-014-0061-4
Schwartsmann, G., Brodani da Rocha, A., Berlinck, R. G., and Jimeno, B. J. (2001). Marine organisms as a source of new anticancer agents. Lancet Oncol. 2 (4), 221–225. doi:10.1016/s1470-2045(00)00292-8
Shimony, S., Stahl, M., and Stone, R. M. (2023). Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 98 (3), 502–526. doi:10.1002/ajh.26822
Tang, W. L., Zhang, Y., Hu, D. X., Yang, H., Yu, Q., Chen, J. W., et al. (2019). Synthesis and biological evaluation of 5-aminoethyl benzophenanthridone derivatives as DNA topoisomerase IB inhibitors. Eur. J. Med. Chem. 178, 81–92. doi:10.1016/j.ejmech.2019.05.074
Tavares de, C., Zanon, G., Weber, A. D., Neto, A. T., Mostardeiro, C. P., Da Cruz, I. B., et al. (2014). Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS One 9 (5), e97000. doi:10.1371/journal.pone.0097000
Tcheng, M., Roma, A., Ahmed, N., Smith, R. W., Jayanth, P., Minden, M. D., et al. (2021). Very long chain fatty acid metabolism is required in acute myeloid leukemia. Blood 137 (25), 3518–3532. doi:10.1182/blood.2020008551
Turrens, J. F. (2003). Mitochondrial formation of reactive oxygen species. J. Physiol. 552 (Pt 2), 335–344. doi:10.1113/jphysiol.2003.049478
Xu, Q., Liu, P., Nie, Q., Chu, Y., Yao, X., Fang, J., et al. (2025). Structural simplification of quaternary benzophenanthridine alkaloids generating a candidate for the treatment of non-small cell lung cancer. Eur. J. Med. Chem. 290, 117551. doi:10.1016/j.ejmech.2025.117551
Yang, X. J., Miao, F., Yao, Y., Cao, F. J., Yang, R., Ma, Y. N., et al. (2012). In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules 17 (11), 13026–13035. doi:10.3390/molecules171113026
Zhang, H., Shangguan, F., Zhang, L., Ma, N., Song, S., Ma, L., et al. (2023). A novel mechanism of 6-methoxydihydroavicine in suppressing ovarian carcinoma by disrupting mitochondrial homeostasis and triggering ROS/MAPK mediated apoptosis. Front. Pharmacol. 14, 1093650. doi:10.3389/fphar.2023.1093650
Keywords: benzophenanthridine alkaloid, 6-methoxydihydroavicine, acute myeloid leukemia, ROS, metabolism
Citation: Yang Y and Spagnuolo PA (2025) 6-Methoxydihydroavicine is a benzophenanthridine alkaloid with anti-leukemia activity. Front. Pharmacol. 16:1621050. doi: 10.3389/fphar.2025.1621050
Received: 30 April 2025; Accepted: 09 June 2025;
Published: 24 June 2025.
Edited by:
John Thor Arnason, University of Ottawa, CanadaReviewed by:
Siyaram Pandey, University of Windsor, CanadaLaura Flores-Bocanegra, National Autonomous University of Mexico, Mexico
Copyright © 2025 Yang and Spagnuolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul A. Spagnuolo, cGF1bC5zcGFnbnVvbG9AdW9ndWVscGguY2E=
 Yingying Yang
Yingying Yang Paul A. Spagnuolo
Paul A. Spagnuolo