Abstract
Background:
Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide, particularly in low- and middle-income countries. Oxidative stress and excessive inflammation contribute significantly to disease progression and severity. Astaxanthin (ASX), a potent antioxidant and anti-inflammatory carotenoid, has demonstrated protective effects against oxidative damage and immune dysregulation in various conditions. However, its potential role as an adjunctive therapy in CAP remains underexplored. This study aims to evaluate the effects of ASX supplementation on inflammatory cytokines, and clinical outcomes in patients with CAP.
Patients and methods:
A prospective, randomized, double-blind, placebo-controlled study was conducted, in which adult patients diagnosed with CAP were enrolled and assigned to receive either 12 mg/day ASX or a placebo in addition to standard antibiotic therapy for 7 days. Inflammatory markers, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-10 (IL-10), were measured at baseline and post-treatment. Secondary outcomes included Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, as well as length of hospital stay.
Results:
A total of 80 patients (40 per group) completed the study. Patients receiving ASX exhibited significant reductions in pro-inflammatory cytokines compared to the placebo group. Notably, IL-6 and TNF-α levels were significantly lower in the ASX group at the end of the study (P < 0.05). Additionally, SOFA and APACHE II scores showed greater improvements in ASX-treated patients, suggesting a potential role in mitigating disease severity. Although the ASX group had a shorter hospital stay than the placebo group, the difference was not statistically significant (P > 0.05).
Conclusion:
ASX supplementation as an adjunct to standard CAP treatment significantly reduced inflammation while improving disease severity scores. ASX was found to be safe and well-tolerated. These findings highlight its potential therapeutic role in CAP management, warranting further investigation in larger, long-term clinical trials to confirm its benefits and establish optimal dosing strategies.
Clinical Trial Registration:
https://clinicaltrials.gov/study/NCT06334874, identifier NCT06334874.
1 Introduction
Community-acquired pneumonia (CAP) is the most prevalent form of pneumonia, occurring in individuals outside healthcare (Rider and Frazee, 2018). It remains a significant global cause of morbidity and mortality, particularly in low- and middle-income countries, where incidence and fatality rates are substantially higher than in high-income nations (Anderson and Feldman, 2023; Frigati et al., 2024; Atkinson and Mabey, 2025). In the United States, CAP is the leading infectious cause of death and one of the most common reasons for hospitalization, with an estimated 1.5 million hospitalizations annually and a readmission rate exceeding 9% (Kochanek et al., 2016; Ramirez et al., 2017; Dela Cruz et al., 2021).
CAP is caused by a diverse range of pathogens, including bacteria, viruses, and, less commonly, fungi. Among bacterial causes, Streptococcus pneumoniae is the most frequently identified pathogen, accounting for a significant proportion of cases across various severities. Other common bacterial culprits include Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella species. Viral pathogens are also a substantial cause of CAP, with influenza A, rhinovirus, and respiratory syncytial virus being prominent. The specific pathogen responsible can influence the clinical presentation and severity of CAP, highlighting the importance of understanding the etiological landscape for effective management (Jain et al., 2015; Johansson et al., 2010; Musher et al., 2013).
The CAP pathogenesis involves a complex interaction between invading pathogens and the host immune system, often resulting in excessive inflammation and oxidative stress (OS) (Long et al., 2022). A dysregulated immune response, commonly referred to as a “cytokine storm,” plays a critical role in disease progression. Key inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) drive excessive inflammation, while anti-inflammatory cytokines like interleukin-10 (IL-10) and interleukin-1 receptor antagonist (IL-1ra) attempt to counterbalance the response (Kumar, 2020; Keskinidou et al., 2022). However, in CAP, this equilibrium is frequently disrupted, leading to heightened inflammation and oxidative tissue damage (Quinton et al., 2018). The oxidative burden further amplifies inflammation and impairs immune function, perpetuating disease severity (Korkmaz and Traber, 2023; Barakat et al., 2023).
Conventional antibiotic therapy primarily targets the infectious component of CAP but does not directly modulate the inflammatory and oxidative pathways that contribute to disease progression (Muravlyova et al., 2016; Castillo et al., 2013; Gambini and Stromsnes, 2022). Consequently, there is growing interest in adjuvant therapies that mitigate inflammation, enhance immune response, and reduce OS (Gambini and Stromsnes, 2022; Mittal et al., 2014). Antioxidants have emerged as potential therapeutic agents due to their ability to neutralize reactive oxygen species (ROS), regulate cytokine production, and protect against lung tissue damage (Youssef et al., 2024; Lee et al., 2020).
Astaxanthin (ASX), a potent xanthophyll carotenoid derived from microalgae, is renowned for its powerful antioxidant, anti-inflammatory, and immunomodulatory properties (Mo et al., 2023). Its chemical structure, characterized by multiple hydroxyls groups, allows it to effectively neutralize ROS and reactive nitrogen species within cell membranes, providing robust protection against OS (Chang and Xiong, 2020; Pereira et al., 2021). Unlike other carotenoids, ASX’s unique ability to span biological membranes and scavenge free radicals positions it as an exceptional defense against cellular damage (Pereira et al., 2021).
Preclinical and clinical studies have highlighted ASX’s ability to modulate inflammatory pathways, enhance immune function, and protect against oxidative injury in various diseases (Kubo et al., 2019; Yiğit et al., 2019; Kim et al., 2011; Choi et al., 2011). ASX’s potent antioxidant action, coupled with its anti-inflammatory effects, makes ASX a promising therapeutic candidate for managing inflammatory diseases, such as CAP, where OS and inflammation are central to disease progression.
This study aims to evaluate the safety and efficacy of ASX as an adjuvant to standard antibiotic therapy in CAP. Specifically, it investigates the effects of ASX on inflammatory cytokines, and clinical outcomes to determine its potential role in reducing oxidative damage, modulating inflammation, and enhancing CAP management.
2 Patients and methods
2.1 Study design, setting, and ethical considerations
The current prospective, randomized, double-blind, placebo-controlled interventional study was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study took place in the respiratory department of El Matarya Teaching Hospital, Cairo, Egypt, and included patients diagnosed with CAP. The study protocol was approved by the Research Ethics for Experimental and Clinical Studies, Faculty of Pharmacy, Future University in Egypt (REC-FPFUE-32/2023), as well as the General Organization for Teaching Hospitals and Institutes ethical committee (HM000167). Moreover, the study was registered on ClinicalTrials.gov (NCT06334874). The participants were oriented about the possible side effects of their treatment before taking part in the trial, and informed consent was acquired in compliance with the Declaration of Helsinki.
2.2 Methodology
2.2.1 Study population
Eligibility screening was conducted for all participants recruited from the hospital’s respiratory department. Patients (≥18 years) diagnosed with CAP based on the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) criteria were enrolled (Metlay et al., 2019). The diagnosis was confirmed by clinical symptoms (fever ≥38°C, cough, dyspnea, or pleuritic chest pain) and radiological findings.
Exclusion criteria included immunosuppression (HIV, immunosuppressive therapy), cancer, and recent ASX use (within 1 month). Patients with hypersensitivity to ASX, those on warfarin or antioxidant supplements, and those unable to comply with study procedures were also excluded. Additional exclusions encompassed pregnancy, lactation, age ≥70 years, severe comorbidities (e.g., tuberculosis, bronchiectasis, pulmonary edema), and severe pneumonia (CURB-65 ≥ 4).
2.2.2 Study intervention and randomization
Eligible patients were randomly assigned to either the ASX or placebo group using a computer-generated randomization process. Group allocation was concealed in sealed envelopes to ensure blinding for patients, physicians, radiologists, and research personnel involved in evaluations, laboratory analysis, and serum collection.
2.2.2.1 ASX Group
Patients received 12 mg once daily of oral ASX (NOW Foods, Egypt) alongside standard CAP treatment (Brendler and Williamson, 2019; Drugs.com, 2025; WebMD, 2025). The ASX used in this study was sourced from NOW Foods, Egypt, which exclusively utilizes natural ASX from AstaReal®. AstaReal® is renowned for its stringent quality control measures, ensuring high purity, solvent-free, and standardized ASX derived from Haematococcus pluvialis microalgae cultivated under strictly controlled conditions (Foods, 2025).
2.2.2.2 Placebo Group
Patients received a placebo identical in appearance to ASX, in addition to standard CAP treatment.
The study duration was 7 days. Both groups followed standard CAP therapy based on ATS/IDSA guidelines (Metlay et al., 2019).
2.2.3 Study procedures
2.2.3.1 Patient data collection
Baseline data, including demographic information (age, gender, height, weight, body mass index (BMI), complete medication history, and medical history, were recorded for all patients.
2.2.3.2 Vital signs and physical examination
Daily medical assessments were conducted by physicians, while nurses monitored vital signs, including blood pressure (BP), body temperature (Temp), respiratory rate (RR), and heart rate (HR).
2.2.3.3 Laboratory evaluations
Routine laboratory tests were performed daily, measuring hemoglobin (Hgb), blood glucose (BG), Liver enzymes: alanine transaminase (ALT) and aspartate transaminase (AST), kidney function markers: blood urea nitrogen (BUN) and serum creatinine (SCr), C-reactive protein (CRP), white blood cell (WBC) count, and red blood cell (RBC) count were assessed at baseline and after 7 days of therapy.
2.2.3.4 Monitoring of adverse effects
Potential adverse effects of ASX supplementation, such as gastrointestinal discomfort, were tracked daily and documented using a standardized adverse event monitoring form.
2.2.3.5 Severity assessment
Disease severity was evaluated using CURB-65, Acute Physiology and Chronic Health Evaluation II (APACHE II), and Sequential organ failure assessment (SOFA) scores to assess the extent of illness and predict clinical outcomes (Asai et al., 2019; Limapichat and Supavajana, 2022; Gursel and Demirtas, 2006). These scores were used to monitor disease progression and response to treatment over the study period.
2.2.3.6 Radiological assessment
Chest radiography (CXR) scans were conducted before enrollment to confirm CAP diagnosis.
2.2.3.7 Monitoring and follow-up
Patients were monitored daily for changes in CAP symptoms, treatment response, and overall clinical progression throughout the study period.
2.2.4 Measurable outcomes
2.2.4.1 Primary outcome
The primary outcome was the change in inflammatory markers, including TNF-α, IL-6, and IL-10 levels, between the ASX and placebo groups. These markers were measured at baseline and after 7 days of treatment to assess the effect of ASX supplementation.
Blood was collected, followed by centrifugation to separate Serum, which was subsequently stored at −80°C until analysis. Quantification of cytokine concentrations was performed using enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s protocol (Elabscience company).
2.2.4.2 Secondary outcomes
• Disease Severity Scores: APACHE II and SOFA scores were assessed at baseline and after 7 days of treatment in both groups.
• Length of Hospital Stay: The duration of hospitalization was compared between the ASX and placebo groups.
2.3 Sample size calculation, data management, and analysis
Sample size was determined using STATA, with a type I error (α) of 0.05 and a power (1-β) of 0.9. Based on prior research showing a significant reduction in IL-6 levels in the supplement group (8.91 ± 4.23 vs. 11.89 ± 1.62 in the placebo group) after 10 days of treatment, the required sample size was calculated as 26 patients per group (Abulmeaty et al., 2021). To account for potential dropouts, 40 patients were included in each group, totaling 80 participants.
Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). The Shapiro-Wilk and Kolmogorov-Smirnov tests were employed to assess normality. For parametric continuous variables, results are presented as means ± standard deviations, whereas non-parametric variables are shown as medians with interquartile ranges. Categorical data are expressed as frequencies and percentages. Between-group comparisons were performed using the unpaired Student’s t-test for normally distributed numerical data, and the Mann-Whitney U test for non-parametric data. Categorical variables were evaluated using either the chi-squared test or Fisher’s exact test. A p-value of <0.05 was deemed statistically significant.
3 Results
Between April and October 2024, patient recruitment was carried out in the respiratory unit. A total of 97 individuals were screened for eligibility, of whom 80 met the inclusion criteria and were randomized in a 1:1 ratio to receive either ASX (n = 40) or placebo (n = 40). All enrolled participants completed the study without any dropouts and were included in the final analysis (Figure 1).
FIGURE 1
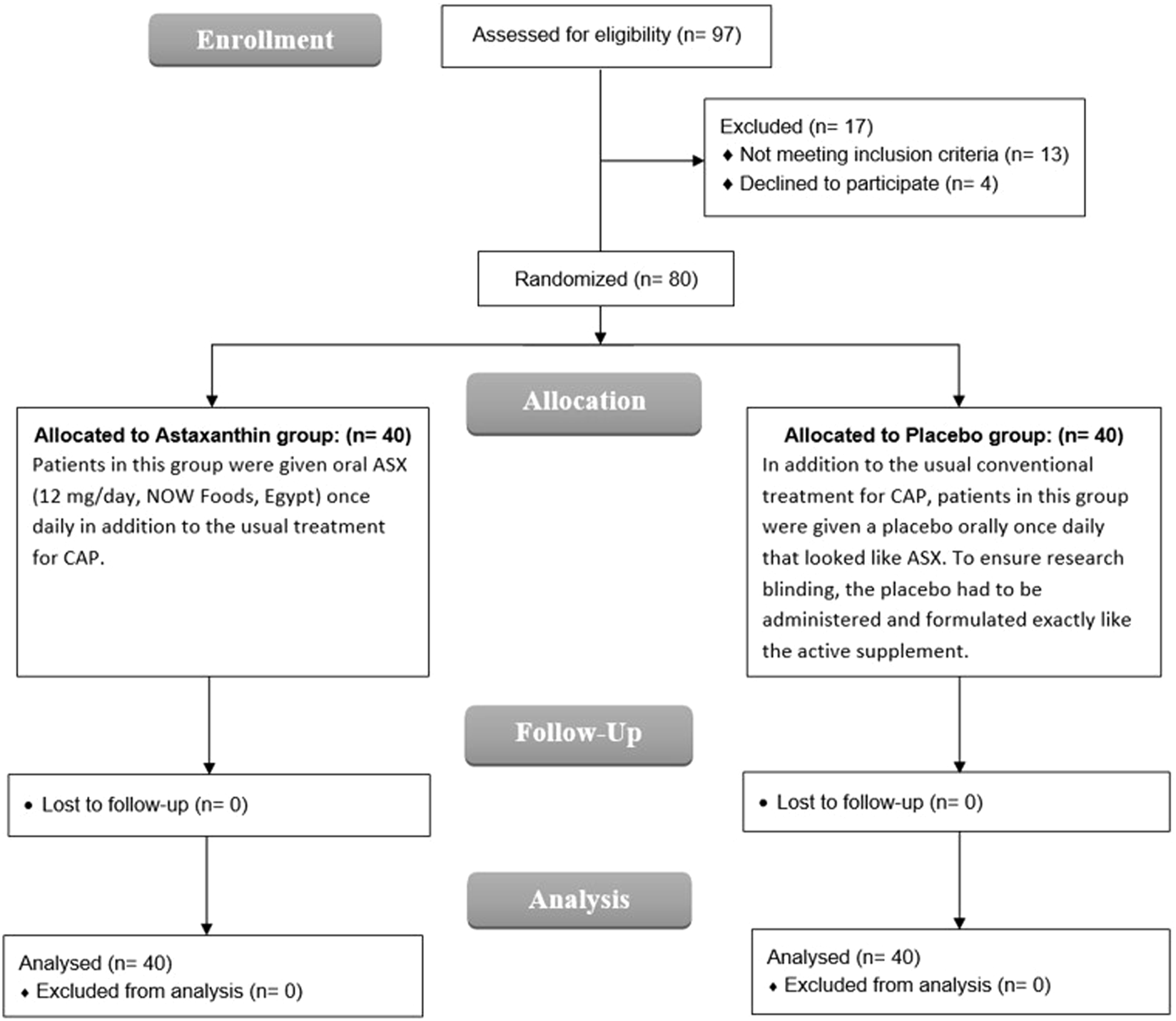
CONSORT flowchart.
3.1 Baseline and demographic characteristics
As shown in Table 1, demographic data of the studied patients including age, height, weight, BMI, Temp and gender were insignificantly different between the two studied groups. The majority of patients (72.5% of ASX group vs. 77.5% of placebo group) showed no comorbidities, while 15% vs. 12.5% were diabetic and 12.5% vs. 10% were hypertensive, with no statistically significant difference between both groups. Moreover, the median CURB-65 score was 1 (IQR 0, 2) in the ASX group and 1 (IQR 1, 2) in the placebo group, and that difference was similar.
TABLE 1
| Item | Astaxanthin group (n = 40) | Placebo group (n = 40) | P-value |
|---|---|---|---|
| Age (years) | 51.6 ± 13.78 | 49 ± 10.66 | 0.348 |
| Gender | |||
| Male | 19 (47.5%) | 21 (52.5%) | 0.655 |
| Female | 21 (52.5%) | 19 (47.5%) | |
| Height (m) | 1.66 ± 0.1 | 1.66 ± 0.09 | 0.953 |
| Weight (kg) | 65.4 ± 11.86 | 67.05 ± 10.04 | 0.504 |
| BMI (kg/m2) | 23.56 ± 2.38 | 24.23 ± 2.58 | 0.229 |
| Temp on admission (°C) | 38.81 ± 0.49 | 38.91 ± 0.43 | 0.374 |
| Comorbidities | |||
| No | 29 (72.5%) | 31 (77.5%) | 0.872 |
| DM | 6 (15%) | 5 (12.5%) | |
| HTN | 5 (12.5%) | 4 (10%) | |
| CURB-65 score | 1 (0, 2) | 1 (1, 2) | 0.640 |
Baseline characteristics of the studied groups.
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant, BMI, body mass index; Temp, Temperature; DM, diabetes mellitus; HTN, hypertension; CURB-65, Confusion, Uremia, Respiratory rate, BP, Age ≥65 years.
3.2 Clinical parameters
As demonstrated in Table 2, there was no statistically significant difference between the two groups regarding HR, RR, and systolic BP. In terms of CXR, it evidenced bilateral lesions in most patients (65% of ASX group vs. 60% of placebo group), and no statistically significant difference was detected. The most frequently reported respiratory symptoms were sputum (72.5% of ASX group vs. 80% of placebo group), followed by dyspnea (70% vs. 75%) and pleuritic chest pain (55% vs. 62.5), and none showed a significant difference between both groups.
TABLE 2
| Item | Astaxanthin group (n = 40) | Placebo group (n = 40) | P-value |
|---|---|---|---|
| HR (bpm) | 100.7 ± 2.5 | 99.68 ± 3.62 | 0.145 |
| RR (breaths/min) | 26.6 ± 1.72 | 26.03 ± 1.83 | 0.152 |
| Systolic BP (mmHg) | 128.13 ± 6.06 | 126.33 ± 11.46 | 0.384 |
| Lesion on CXR | |||
| Unilateral | 14 (35%) | 16 (40%) | 0.644 |
| Bilateral | 26 (65%) | 24 (60%) | |
| Respiratory symptoms | |||
| Dry cough | 2 (5%) | 4 (10%) | 0.675 |
| Sputum | 29 (72.5%) | 32 (80%) | 0.431 |
| Dyspnea | 28 (70%) | 30 (75%) | 0.617 |
| Hemoptysis | 2 (5%) | 1 (2.5%) | >0.999 |
| Pleuritic chest pain | 22 (55%) | 25 (62.5%) | 0.496 |
Clinical parameters of the studied groups.
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant, HR, heart rate; RR, respiratory rate; BP, blood pressure; CXR, chest radiography.
3.3 Hematologic parameters
No significant differences were observed between the ASX and placebo groups in most hematological parameters. BG levels decreased in the ASX group but with no statistically significant difference (P = 0.212) and it nearly remained unchanged in the placebo group (P = 0.685). Additionally, CRP levels significantly declined in both groups by the end of the study (P < 0.001), with a notably greater reduction in the ASX group compared to the placebo group (P = 0.007) (Table 3; Figure 2).
TABLE 3
| Item | Astaxanthin group (n = 40) | Placebo group (n = 40) | P-value |
|---|---|---|---|
| WBCs (103/UL) | |||
| Baseline | 6.64 ± 0.93 | 6.9 ± 0.86 | 0.190 |
| End | 6.87 ± 1.07 | 7.01 ± 1.05 | 0.549 |
| P value (Baseline vs. End) | 0.205 | 0.542 | |
| RBCs (103/UL) | |||
| Baseline | 4.83 ± 0.66 | 4.73 ± 0.56 | 0.466 |
| End | 4.76 ± 0.56 | 4.77 ± 0.68 | 0.943 |
| P value (Baseline vs. End) | 0.297 | 0.596 | |
| ALT (U/L) | |||
| Baseline | 36.5 ± 3.67 | 35.46 ± 4.89 | 0.138 |
| End | 35.21 ± 4.83 | 34.86 ± 4.12 | 0.258 |
| P value (Baseline vs. End) | 0.118 | 0.165 | |
| AST (U/L) | |||
| Baseline | 38.2 ± 2.16 | 39.02 ± 2.19 | 0.097 |
| End | 36.52 ± 3.15 | 37.02 ± 1.93 | 0.394 |
| P value (Baseline vs. End) | 0.654 | 0.435 | |
| BUN (mg/dL) | |||
| Baseline | 9.7 ± 0.7 | 9.5 ± 0.55 | 0.151 |
| End | 9.21 ± 0.68 | 9.23 ± 0.52 | 0.868 |
| P value (Baseline vs. End) | 0.143 | 0.015 | |
| SCr (mg/dL) | |||
| Baseline | 0.45 ± 0.05 | 0.43 ± 0.04 | 0.063 |
| End | 0.42 ± 0.04 | 0.43 ± 0.03 | 0.366 |
| P value (Baseline vs. End) | 0.128 | 0.593 | |
| Hgb (g/dL) | |||
| Baseline | 11.71 ± 1.01 | 11.65 ± 1.2 | 0.809 |
| End | 11.75 ± 0.84 | 11.67 ± 1.14 | 0.722 |
| P value (Baseline vs. End) | 0.672 | 0.825 | |
| BG (mg/dL) | |||
| Baseline | 93 ± 10.09 | 92.03 ± 7.62 | 0.627 |
| End | 92.1 ± 8.56 | 91.7 ± 6 | 0.952 |
| P value (Baseline vs. End) | 0.212 | 0.685 | |
| CRP (mg/dL) | |||
| Baseline | 69.51 ± 4.5 | 70.5 ± 4.17 | 0.312 |
| End | 30.05 ± 5.28 | 33.04 ± 4.33 | 0.007 |
| P value (Baseline vs. End) | <0.001 | <0.001 | |
Hematological parameters of the studied groups.
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant, WBCs, White blood cells; RBCs, Red blood cells; ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; SCr, Serum creatinine; Hgb, Hemoglobin; BG, blood glucose; CRP, C-reactive protein.
The bold numbers indicate statistically significant differences.
FIGURE 2
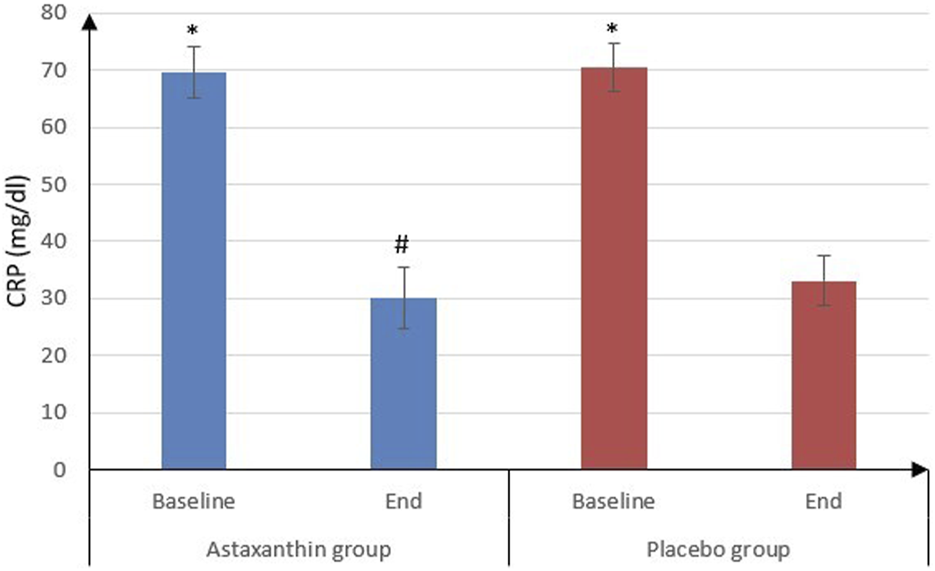
CRP of the studied groups.
3.4 Inflammatory parameters
IL-6 was significantly decreased from 22.09 ± 5.45 pg/mL at the start of study to 9 ± 0.9 pg/mL after treatment in ASX group (P < 0.001) and from 24.03 ± 5.38 to 17.02 ± 4.09 pg/mL in placebo group (P < 0.001), and that drop was deemed to be significantly more in ASX group (13.09 ± 5.41) than the placebo (7.01 ± 3.49), (P < 0.001). However, IL-10 values weren’t statistically significant between the start and end of the study in both groups. As for TNF-α, it was significantly decreased from 45.72 ± 4.03 to 16.28 ± 2.02 pg/mL in ASX group (P < 0.001) and from 46.11 ± 1.65 to 23.14 ± 3.64 pg/mL in placebo group (P < 0.001), and that decrease was significantly more in ASX group (29.45 ± 4.36) than the placebo (22.97 ± 3.99), (P < 0.001) (Table 4; Figures 3, 4).
TABLE 4
| Item | Astaxanthin group (n = 40) | Placebo group (n = 40) | P-value |
|---|---|---|---|
| IL-6 (pg/mL) | |||
| Baseline | 22.09 ± 5.45 | 24.03 ± 5.38 | 0.113 |
| End | 9 ± 0.9 | 17.02 ± 4.09 | <0.001 |
| P value (Baseline vs. End) | <0.001 | <0.001 | |
| Difference | 13.09 ± 5.41 | 7.01 ± 3.49 | <0.001 |
| IL-10 (pg/mL) | |||
| Baseline | 1.11 ± 0.25 | 1.1 ± 0.28 | 0.847 |
| End | 1.14 ± 0.34 | 1.13 ± 0.29 | 0.910 |
| P value (Baseline vs. End) | 0.413 | 0.510 | |
| Difference | −0.03 ± 0.22 | −0.03 ± 0.3 | 0.953 |
| TNF-α (pg/mL) | |||
| Baseline | 45.72 ± 4.03 | 46.11 ± 1.65 | 0.581 |
| End | 16.28 ± 2.02 | 23.14 ± 3.64 | <0.001 |
| P value (Baseline vs. End) | <0.001 | <0.001 | |
| Difference | 29.45 ± 4.36 | 22.97 ± 3.99 | <0.001 |
Inflammatory parameters of the studied groups.
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant, IL, interleukin; TNF α -, tumor necrosis factor alpha.
The bold numbers indicate statistically significant differences.
FIGURE 3
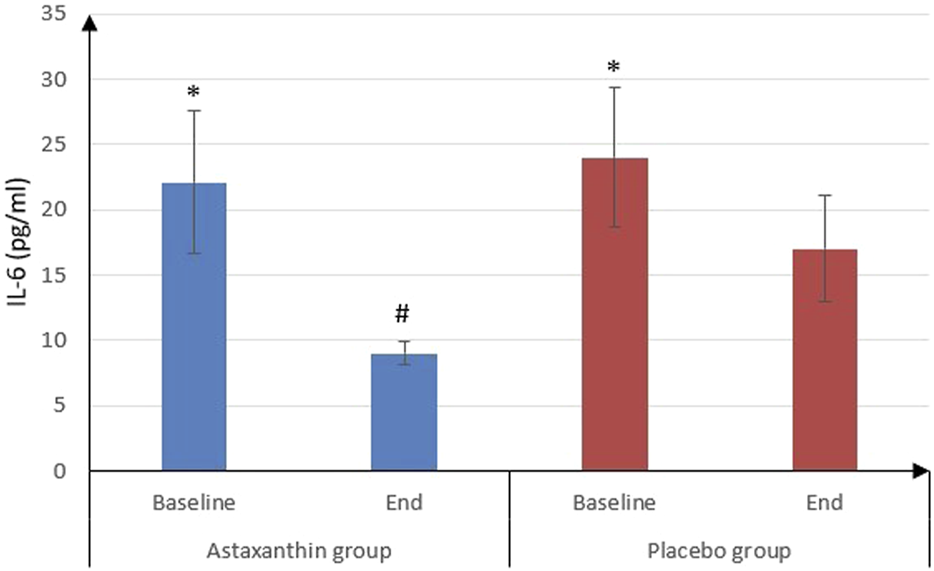
IL-6 of the studied groups.
FIGURE 4
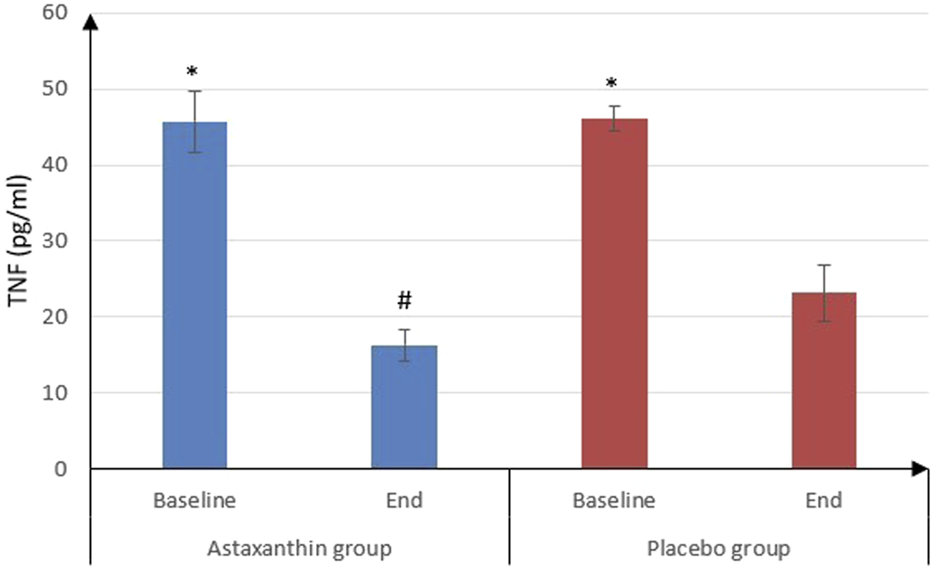
TNF -α of the studied groups.
3.5 Severity scores
Regarding severity scores, the results showed a significant decrease in SOFA and APACHE II scores at the end of study than baseline in both groups (P < 0.001), and by comparing, both scores were significantly decreased in ASX group than the placebo only at the end of study (P = 0.005 and 0.032 respectively) (Table 5; Figures 5, 6).
TABLE 5
| Item | Astaxanthin group (n = 40) | Placebo group (n = 40) | P-value |
|---|---|---|---|
| SOFA score | |||
| Baseline | 8 (7,8) | 8 (7,8) | 0.279 |
| End | 6 (5, 6.75) | 6 (6,7) | 0.005 |
| P value (Baseline vs. End) | <0.001 | <0.001 | |
| APACHE II score | |||
| Baseline | 14 (12.25, 15) | 13.5 (12,15) | 0.411 |
| End | 8 (8,9) | 9 (8,9) | 0.032 |
| P value (Baseline vs. End) | <0.001 | <0.001 | |
Severity scores of the studied groups.
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant, SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation.
The bold numbers indicate statistically significant differences.
FIGURE 5
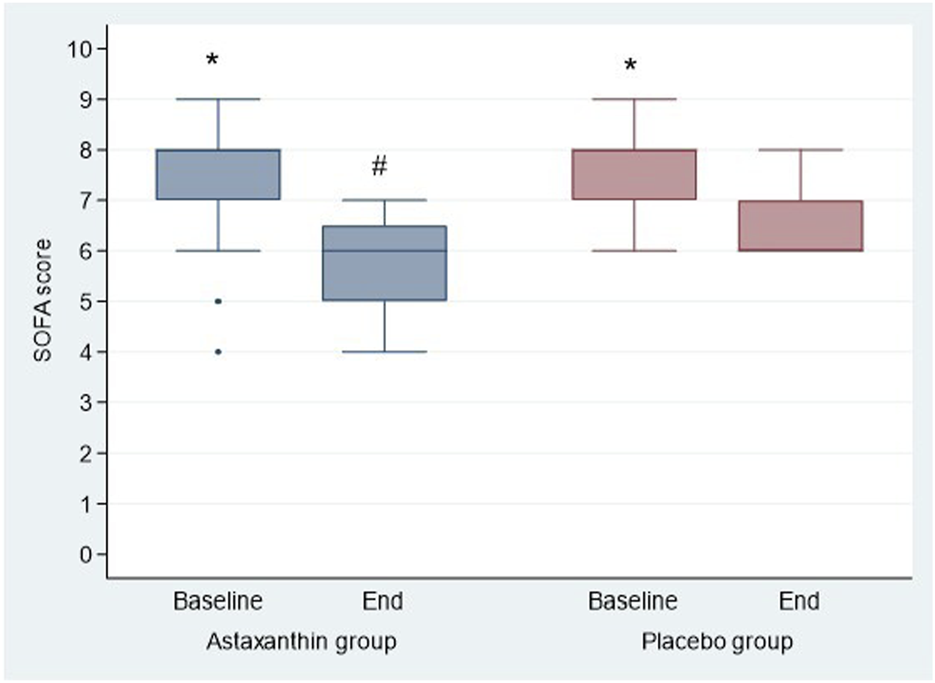
SOFA score of the studied groups.
FIGURE 6
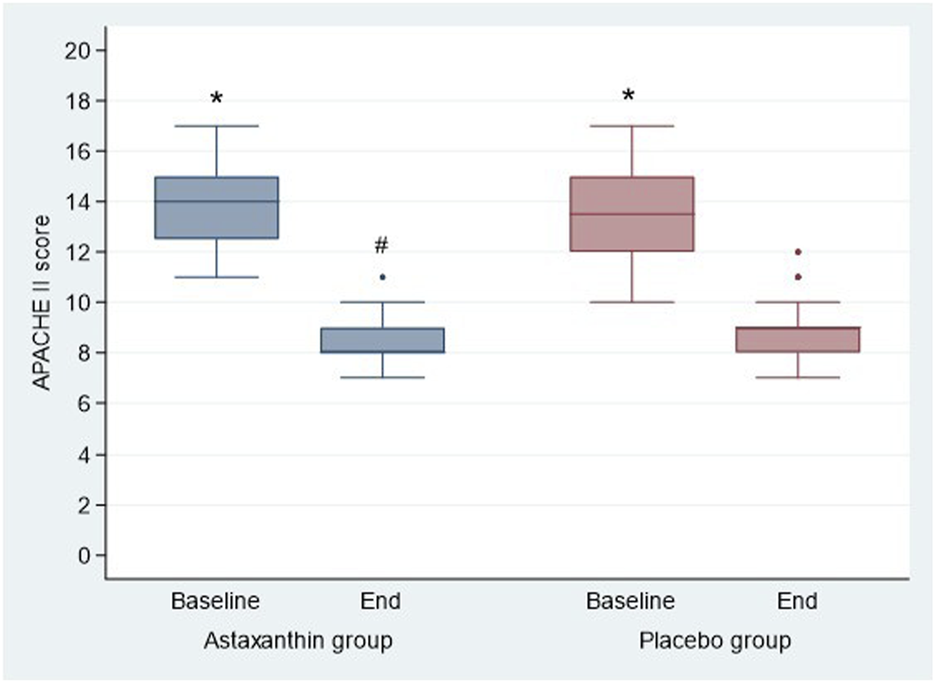
APACHE II score of the studied groups.
3.6 Hospital length of stay and medication safety
As shown in Table 6, the duration of hospital stay was shorter in the ASX group compared to the placebo group; however, this difference was not statistically significant. Moreover, no adverse effects were reported in ASX group throughout the study period.
TABLE 6
| Item | Astaxanthin group (n = 40) | Placebo group (n = 40) | P-value |
|---|---|---|---|
| Hospital Length of stay (days) | 7.2 ± 0.91 | 8.03 ± 1.44 | 0.541 |
Hospital stay of the studied groups.
Continuous variables are expressed as mean ± standard deviation or median with interquartile range, as appropriate. Categorical variables are reported as frequencies and percentages. A p-value of less than 0.05 was considered statistically significant.
4 Discussion
This randomized, double-blind, placebo-controlled trial is the first to evaluate the anti-inflammatory and antioxidant effects of ASX as an adjunctive therapy in hospitalized patients with CAP. The results demonstrate that ASX supplementation significantly reduced key inflammatory cytokines, improved clinical severity scores (SOFA and APACHE II), and was safe and well tolerated.
The findings are interpreted in relation to key clinical outcomes: inflammation, OS, disease severity, prognosis, and the potential mechanisms by which ASX may exert its effects.
4.1 Inflammation and cytokine modulation
Inflammation plays a central role in the pathogenesis and clinical progression of CAP. Elevated circulating levels of pro-inflammatory cytokines such as IL-6, TNF-α, and CRP are associated with increased disease severity and poorer outcomes (Siljan et al., 2018; Travlos et al., 2022). In the present study, ASX supplementation significantly reduced all three markers, indicating a substantial attenuation of systemic inflammation.
These findings are consistent with previous clinical and preclinical studies. Shokri-Mashhadi et al. reported that ASX supplementation significantly decreased IL-6 levels in patients with type 2 diabetes (Shokri-Mashhadi et al., 2021). Similarly, Cai et al. demonstrated that ASX inhibited lipopolysaccharide-induced IL-6 and TNF-α expression in lung tissue models (Cai et al., 2019). Rostami et al. observed reductions in inflammatory cytokines and OS in women with endometriosis following ASX use, and a meta-analysis of 14 clinical trials confirmed the efficacy of ASX in lowering CRP levels (Rostami et al., 2023; Xia et al., 2020).
Further supporting evidence comes from studies in other inflammatory contexts. For example, ASX administration has been shown to suppress age-related skin degradation and improve dermatologic outcomes by inhibiting inflammatory cytokine secretion from keratinocytes and reducing OS (Tominaga et al., 2017; Chalyk et al., 2017). Additionally, a randomized controlled trial by Fereidouni et al. found that ASX (6 mg/day for 8 weeks) significantly decreased IL-6 and IL-1β levels in infertile women with polycystic ovary syndrome (Fereidouni et al., 2024).
The anti-inflammatory action of ASX is thought to be mediated by inhibition of the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways—key regulators of cytokine transcription (Wang and Qi, 2022). However, variability in outcomes has been observed across clinical contexts. For instance, studies involving patients with Helicobacter pylori infection and coronary artery disease reported no significant cytokine reductions, suggesting that ASX’s efficacy may depend on disease type, dosage, or treatment duration (Heidari et al., 2023; Andersen et al., 2007).
In our study, levels of IL-10, a key anti-inflammatory cytokine, remained unchanged following ASX supplementation. While preclinical studies have reported ASX-induced increases in IL-10, this effect has not been consistently observed in human trials (Balietti et al., 2016; Akhmad et al., 2025). These findings suggest that ASX primarily acts by suppressing pro-inflammatory mediators, rather than enhancing anti-inflammatory pathways (Wang and Qi, 2022). Such selective modulation may be especially advantageous in CAP, where excessive immune suppression could compromise pathogen clearance.
4.2 Oxidative stress and tissue protection
OS plays a critical role in the pathogenesis of CAP, contributing to alveolar damage and disease progression through the overproduction of ROS (Castillo et al., 2013). Although our study did not directly assess OS biomarkers, the significant reductions observed in IL-6, TNF-α, and CRP may reflect a downstream attenuation of oxidative-inflammatory cascades (Mittal et al., 2014; Biswas, 2016).
The antioxidant potential of ASX has been well documented in both clinical and preclinical studies. Choi et al. and Iwabayashi et al. reported improvements in malondialdehyde levels and total antioxidant capacity following ASX supplementation (Choi et al., 2011; Iwabayashi et al., 2009). In particular, Iwabayashi et al. observed enhanced redox balance in individuals under OS with a daily dose of 12 mg ASX, while Kim et al. demonstrated reduced lipid peroxidation in smokers (Kim et al., 2011; Iwabayashi et al., 2009). These findings are supported by meta-analyses from Hasani et al. and Ma et al., which concluded that ASX significantly increases total antioxidant capacity and enhances endogenous antioxidant enzyme levels, including superoxide dismutase, a key ROS-scavenging enzyme (Hasani et al., 2024; Ma et al., 2022).
Preclinical studies have further reinforced ASX’s antioxidant and cytoprotective capabilities. Research by Song et al. (2014), Zhou et al. (2015), Farruggia et al. (2018), Peng et al. (2020), Gao et al. (2019), and Xu et al. (2019) demonstrated that ASX reduces oxidative damage, modulates inflammatory pathways, and protects against cellular injury across a range of disease models (Song et al., 2014; Zhou et al., 2015; Farruggia et al., 2018; Gao et al., 2019; Xu et al., 2019).
ASX’s efficacy is largely attributed to its unique molecular structure, which enables it to span lipid bilayers and scavenge ROS both within and outside of cellular membranes (Ambati et al., 2014). This structural property is particularly relevant in CAP, where oxidative damage to the respiratory epithelium contributes to inflammation and pulmonary tissue injury (Xu et al., 2020).
In addition to direct ROS scavenging, ASX exerts antioxidant effects through regulation of cellular defense systems. One key mechanism involves the activation of nuclear factor erythroid 2–related factor 2 (Nrf2), a transcription factor that regulates antioxidant response elements and maintains redox homeostasis. Nrf2 is increasingly recognized as a therapeutic target in OS- and inflammation-related diseases. Furthermore, ASX has been shown to modulate mitochondrial function under OS conditions. Pre-treatment with ASX has been reported to restore mitochondrial membrane potential, reduce hydrogen peroxide-induced apoptosis, and enhance mitochondrial activity in redox-challenged states (Wang and Qi, 2022).
Taken together, these properties suggest that ASX may help preserve pulmonary epithelial integrity in CAP by attenuating oxidative injury and limiting secondary inflammatory damage. The clinical improvements observed in our study may, in part, be attributed to these antioxidant mechanisms.
4.3 Clinical severity scores (SOFA and APACHE II)
In this study, ASX supplementation resulted in a significant reduction in both SOFA and APACHE II scores compared to placebo. These scoring systems are well-established tools for assessing multi-organ dysfunction and predicting outcomes in patients with pneumonia and other critical illnesses (Asai et al., 2019; Gursel and Demirtas, 2006).
Our findings are consistent with previous research on antioxidant-based therapies in acute inflammatory conditions. For example, clinical trial evaluating several antioxidants in patients with COVID-19 and sepsis have shown that improvements in OS and inflammatory markers are associated with favorable changes in clinical severity scores (Chavarría et al., 2021). Similarly, studies in patients with OS–related conditions such as septic shock or those undergoing major surgery suggest that early antioxidant supplementation may reduce the risk of organ dysfunction and clinical deterioration (Aisa-Álvarez et al., 2023; Nathens et al., 2002).
The observed improvements in SOFA and APACHE II scores in our study may be attributed to the dual anti-inflammatory and antioxidant actions of ASX. Preclinical studies by Cai et al. support this hypothesis, demonstrating that ASX administration attenuated systemic inflammation and lung injury in experimental models of sepsis and endotoxemia conditions that share key pathophysiological features with severe CAP (Cai et al., 2019).
Collectively, these findings suggest that ASX may help preserve organ function, limit the progression of inflammatory injury, and reduce overall disease severity in hospitalized patients with CAP.
4.4 Prognosis and length of hospital stay
Although the reduction in hospital stay among patients receiving ASX was not statistically significant, a trend toward shorter duration was observed. This finding is consistent with previous studies evaluating antioxidant therapies in acute conditions such as acute pancreatitis and postoperative recovery, where early supplementation has been associated with reduced ICU stays and accelerated clinical improvement (Nathens et al., 2002; Sateesh et al., 2009).
Nonetheless, the evidence regarding the effect of antioxidant supplementation on hospitalization duration remains mixed. While some trials have reported meaningful reductions in hospital stay and enhanced recovery, others have found no significant differences (Alvi et al., 2023; Manzanares et al., 2012). These discrepancies may reflect differences in study design, patient characteristics, antioxidant formulations, dosages, and timing of administration.
In the context of our study, the absence of a statistically significant difference may be explained by the short duration of ASX administration (7 days) and the modest sample size, which may have limited the power to detect a true effect. Additionally, the timing of intervention relative to disease progression may have influenced the magnitude of clinical impact observed.
4.5 Comparison with other carotenoids
Several carotenoids, including β-carotene, lycopene, and lutein, have demonstrated antioxidant and anti-inflammatory effects in respiratory conditions such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis. These compounds act primarily by scavenging ROS and modulating pro-inflammatory pathways (Kırkıl et al., 2012; Neuman et al., 2000; Çob et al., 2002; Sagel et al., 2018).
Among this class, ASX is widely regarded as the most potent, owing to its unique molecular structure. Its polar-nonpolar-polar configuration enables it to span lipid bilayers and neutralize ROS both within and outside cellular membranes—an advantage not shared by other carotenoids that tend to localize at the membrane surface (Ambati et al., 2014; Pfander, 1992).
Comparative studies have shown that ASX is up to 550 times more effective than vitamin E and substantially more powerful than vitamin C, lycopene, and green tea catechins in quenching singlet oxygen and other free radicals (Shimidzu et al., 1996; Ablon, 2021). This exceptional antioxidant capacity highlights ASX’s therapeutic potential in diseases characterized by intense oxidative and inflammatory stress, such as CAP.
Given the high oxidative burden and immune dysregulation associated with CAP, ASX’s superior efficacy relative to other carotenoids underscores its promise as a valuable adjunctive therapy in this setting.
4.6 Safety profile
ASX was well tolerated throughout the study period, with no adverse effects reported among hospitalized patients with CAP, including those in the acute phase of infection. This favorable safety outcome supports the potential use of ASX as an adjunctive therapy in acute inflammatory conditions.
These findings are consistent with previous clinical studies, including those by Capelli et al., Miyawaki et al., and Saito et al., which reported no significant adverse effects associated with ASX supplementation across a range of doses and treatment durations (Capelli et al., 2019; Miyawaki et al., 2008; Saito et al., 2012). Importantly, the absence of treatment-related side effects in our trial further confirms the excellent safety profile of ASX, even in acutely ill populations.
Compared to many conventional anti-inflammatory agents, which are often associated with gastrointestinal, renal, or cardiovascular side effects, ASX’s natural origin and strong safety record make it an especially attractive candidate for integration into supportive treatment regimens targeting inflammation and OS (Harirforoosh et al., 2013; Kahraman et al., 2023; Aly et al., 2024).
4.7 Limitations and future directions
Despite the positive findings of this study, some limitations should nevertheless be considered. The relatively small sample size may have limited the statistical power to detect differences in some outcomes and affects the generalizability of the findings. Larger, multicenter trials are needed to validate these results and confirm their clinical relevance.
Microbiological data on CAP pathogens were not collected. As different microorganisms can trigger distinct inflammatory responses, this limits our ability to assess pathogen-specific variations in ASX efficacy. Future studies should incorporate microbiological diagnostics to explore these differences.
A notable limitation of this study is the short follow-up duration, with outcomes assessed only over a 7-day period. While this timeframe offers insight into the acute effects of ASX on inflammatory and OS markers, it does not clarify whether these benefits are sustained beyond the immediate treatment window. Longer follow-up periods are needed to evaluate the long-term efficacy and safety of ASX as an adjunctive therapy in patients with CAP.
The fixed dose of 12 mg once daily may not have maintained optimal therapeutic levels throughout the day. Dose-ranging studies and evaluations of alternative regimens are needed to determine the most effective strategy.
Finally, gene expression analysis was not performed. While protein-level data were informative, molecular studies—such as RT-qPCR for cytokine mRNA—would offer deeper mechanistic insights. Future trials should integrate such analyses to better understand the transcriptional pathways affected by ASX.
5 Conclusion
Adjunctive ASX supplementation significantly attenuated systemic inflammation, as evidenced by reductions in IL-6, TNF-α, and CRP levels, and was associated with improvements in clinical severity scores in hospitalized patients with CAP. ASX was well tolerated, with no adverse effects reported. These findings support its potential role as a safe and effective adjunct to standard CAP therapy, meriting further investigation in larger, longer-term clinical trials to establish optimal dosing and assess sustained clinical benefits.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Research Ethics Committee for Experimental and Clinical Studies, Faculty of Pharmacy, Future University in Egypt. The General Organization for Teaching Hospitals and Institutes ethical committee, General Organization for Teaching Hospitals and Institutes. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FY: Writing – original draft, Writing – review and editing. HA: Writing – review and editing. AH: Writing – review and editing. EE: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the respiratory Department in EL Matarya Teaching Hospital as well as physicians for referring the study participants and for their help and cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- CAP
Community-acquired pneumonia
- OS
Oxidative stress
- IL-6
Interleukin-6
- TNF-α
Tumor necrosis factor-alpha
- IL-10
Interleukin-10
- IL-1ra
Interleukin-1 receptor antagonist
- ROS
Reactive oxygen species
- ASX
Astaxanthin
- (ATS/IDSA)
American Thoracic Society/Infectious Diseases Society of America
- CURB-65
Confusion, Uremia, Respiratory rate, BP, Age ≥65 years
- BMI
Body mass index
- BP
Blood pressure
- TEMP
Body temperature
- RR
Respiratory rate
- HR
Heart rate
- Hgb
Hemoglobin
- BG
Blood glucose
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- BUN
Blood urea nitrogen
- SCr
Serum creatinine
- CRP
C-reactive protein
- WBC
white blood cell
- RBC
Red blood cell
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- SOFA score
Sequential organ failure assessment score
- CXR
Chest radiography
- ELISA
Enzyme-linked immunosorbent assay
- DM
Diabetes mellitus
- HTN
Hypertension
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- MAPK
Mitogen-activated protein kinase
- Nrf2
Nuclear factor erythroid 2–related factor 2.
References
1
Ablon G. (2021). Nutraceuticals. Dermatol. Clin.39 (3), 417–427. 10.1016/j.det.2021.03.006
2
Abulmeaty M. M. A. Aljuraiban G. S. Shaikh S. M. Ne A. L. Mazrou L. R. A. Turjoman A. A. et al (2021). The efficacy of antioxidant oral supplements on the progression of COVID-19 in non-critically ill patients: a randomized controlled trial. Antioxidants (Basel, Switz.)10 (5), 804. 10.3390/antiox10050804
3
Aisa-Álvarez A. Pérez-Torres I. Guarner-Lans V. Manzano-Pech L. Cruz-Soto R. Márquez-Velasco R. et al (2023). Randomized clinical trial of antioxidant therapy patients with septic shock and organ dysfunction in the ICU: SOFA score reduction by improvement of the enzymatic and non-enzymatic antioxidant system. Cells12 (9), 1330. 10.3390/cells12091330
4
Akhmad S. R. Theresia Indah B. Jusak N. Nur Lailatul F. (2025). The effect of astaxanthin on tumor necrosis factor alpha (tnf-α) and interleukin 10 (il-10) expression in uv-b-induced rats model. Folia Medica Indones.61 (1).
5
Alvi M. M. Imtiaz N. Shabbir B. Waheed Z. Atta Ur R. (2023). Evaluating the role of antioxidant therapy in outcome of severe and critical COVID-19 infection requiring high flow oxygen. Lung India official organ Indian Chest Soc.40 (4), 333–338. 10.4103/lungindia.lungindia_369_22
6
Aly M. H. Said A. K. Farghaly A. M. Eldaly D. A. Ahmed D. S. Gomaa M. H. et al (2024). Protective effect of astaxanthin on indomethacin-induced gastric ulcerations in mice. Naunyn-Schmiedeberg's archives Pharmacol.397 (12), 9897–9907. 10.1007/s00210-024-03206-4
7
Ambati R. R. Phang S. M. Ravi S. Aswathanarayana R. G. (2014). Astaxanthin: sources, extraction, stability, biological activities and its commercial applications-a review. Mar. drugs12 (1), 128–152. 10.3390/md12010128
8
Andersen L. P. Holck S. Kupcinskas L. Kiudelis G. Jonaitis L. Janciauskas D. et al (2007). Gastric inflammatory markers and interleukins in patients with functional dyspepsia treated with astaxanthin. FEMS Immunol. Med. Microbiol.50 (2), 244–248. 10.1111/j.1574-695X.2007.00257.x
9
Anderson R. Feldman C. (2023). The global burden of community-acquired pneumonia in adults, encompassing invasive pneumococcal disease and the prevalence of its associated cardiovascular events, with a focus on pneumolysin and macrolide antibiotics in pathogenesis and therapy. Int. J. Mol. Sci.24 (13), 11038. 10.3390/ijms241311038
10
Asai N. Watanabe H. Shiota A. Kato H. Sakanashi D. Hagihara M. et al (2019). Efficacy and accuracy of qSOFA and SOFA scores as prognostic tools for community-acquired and healthcare-associated pneumonia. Int. J. Infect. Dis. IJID official Publ. Int. Soc. Infect. Dis.84, 89–96. 10.1016/j.ijid.2019.04.020
11
Atkinson K. Mabey D. (2025). The burden of communicable diseases in Low- and middle-income countries. Revolutionizing Trop. Medicine2019, 1–36. 10.1002/9781119282686.ch1
12
Balietti M. Giannubilo S. R. Giorgetti B. Solazzi M. Turi A. Casoli T. et al (2016). The effect of astaxanthin on the aging rat brain: gender‐related differences in modulating inflammation. J. Sci. Food Agric.96 (2), 4295–4298. 10.1002/jsfa.7865
13
Barakat M. Elnaem M. H. Al-Rawashdeh A. Othman B. Ibrahim S. Abdelaziz D. H. et al (2023). Assessment of knowledge, perception, experience and phobia toward corticosteroids use among the general public in the era of COVID-19: a multinational studyHealthcare. 11 (2), 255. 10.3390/healthcare11020255
14
Biswas S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox?Oxidative Med. Cell. Longev.2016 (1), 5698931. 10.1155/2016/5698931
15
Brendler T. Williamson E. M. (2019). Astaxanthin: how much is too much? A safety review. Phytotherapy Res. PTR33 (12), 3090–3111. 10.1002/ptr.6514
16
Cai X. Chen Y. Xie X. Yao D. Ding C. Chen M. (2019). Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-κB. Am. J. Transl. Res.11 (3), 1884–1894.
17
Capelli B. Talbott S. Ding L. (2019). Astaxanthin sources: suitability for human health and nutrition. Funct. Foods Health Dis.9, 430–445. 10.31989/ffhd.v9i6.584
18
Castillo R. L. Carrasco R. Álvarez P. I. Ruiz M. Luchsinger V. Zunino E. et al (2013). Relationship between severity of adult community-acquired pneumonia and impairment of the antioxidant defense system. Biol. Res.46 (2), 207–213. 10.4067/S0716-97602013000200013
19
Chalyk N. E. Klochkov V. A. Bandaletova T. Y. Kyle N. H. Petyaev I. M. (2017). Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res.48, 40–48. 10.1016/j.nutres.2017.10.006
20
Chang M. X. Xiong F. (2020). Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: recent advances and future directions. Mol. (Basel, Switz.)25 (22), 5342. 10.3390/molecules25225342
21
Chavarría A. P. Vázquez R. R. V. Cherit J. G. D. Bello H. H. Suastegui H. C. Moreno-Castañeda L. et al (2021). Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput. Struct. Biotechnol. J.19, 1379–1390. 10.1016/j.csbj.2021.02.009
22
Choi H. D. Kim J. H. Chang M. J. Kyu-Youn Y. Shin W. G. (2011). Effects of astaxanthin on oxidative stress in overweight and Obese adults. Phytotherapy Res. PTR25 (12), 1813–1818. 10.1002/ptr.3494
23
Çobanogcarlu N. Özçelik U. Göçmen A. Kiper N. Doǧru D. (2002). Antioxidant effect of β‐carotene in cystic fibrosis and bronchiectasis: clinical and laboratory parameters of a pilot study. Acta Paediatr.91 (7), 793–798. 10.1080/08035250213212
24
Dela Cruz C. S. Evans S. E. Restrepo M. I. Dean N. Torres A. Amara-Elori I. et al (2021). Understanding the host in the management of pneumonia. An official American thoracic society workshop report. Ann. Am. Thorac. Soc.18 (7), 1087–1097. 10.1513/AnnalsATS.202102-209ST
25
Drugs.com (2025). Astaxanthin. Available online at: https://www.drugs.com/drp/astaxanthin-capsules-and-oral-powder.html.
26
Farruggia C. Kim M. B. Bae M. Lee Y. Pham T. X. Yang Y. et al (2018). Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem.62, 202–209. 10.1016/j.jnutbio.2018.09.005
27
Fereidouni F. Kashani L. Amidi F. Khodarahmian M. Zhaeentan S. Ardehjani N. A. et al (2024). Astaxanthin treatment decreases pro-inflammatory cytokines and improves reproductive outcomes in patients with polycystic ovary syndrome undergoing assisted reproductive technology: a randomized clinical trial. Inflammopharmacology32 (4), 2337–2347. 10.1007/s10787-024-01504-0
28
Foods N. (2025). Astaxanthin - the aquatic carotenoid for cellular health and inner beauty. Available online at: https://www.nowfoods.com/healthy-living/articles/astaxanthin-aquatic-carotenoid-cellular-health-and-inner-beauty.
29
Frigati L. Greybe L. Andronikou S. Eber E. Sunder B. V. S. Goussard P. (2024). Respiratory infections in low and middle-income countries. Paediatr. Respir. Rev.54, 43–51. 10.1016/j.prrv.2024.08.002
30
Gambini J. Stromsnes K. (2022). Oxidative stress and inflammation: from mechanisms to therapeutic approaches. Biomedicines10 (4), 753. 10.3390/biomedicines10040753
31
Gao D. Yao S. Sun Y. Zheng D. Zhang Q. Li W. (2019). Astaxanthin attenuate iohexol-induced human proximal renal tubular epithelial cells injury via the ROS/NLRP3 inflammasome signal pathway. SDRP J. Food Sci. and Technol.4, 687–695. 10.25177/jfst.4.3.ra.503
32
Gursel G. Demirtas S. (2006). Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Int. Rev. Thorac. Dis.73 (4), 503–508. 10.1159/000088708
33
Harirforoosh S. Asghar W. Jamali F. (2013). Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J. Pharm. and Pharm. Sci. a Publ. Can. Soc. Pharm. Sci. Soc. Can. des Sci. Pharm.16 (5), 821–847. 10.18433/j3vw2f
34
Hasani M. Arabpour Z. Hasani M. Saeedi A. Khodabakhshi A. (2024). Effect of astaxanthin on physical activity factors, lipid profile, inflammatory markers, and antioxidants indices in athletic men: a systematic review and meta-analysis. J. Funct. Foods122, 106477. 10.1016/j.jff.2024.106477
35
Heidari M. Chaboksafar M. Alizadeh M. Sohrabi B. Kheirouri S. (2023). Effects of astaxanthin supplementation on selected metabolic parameters, anthropometric indices, Sirtuin1 and TNF-α levels in patients with coronary artery disease: a randomized, double-blind, placebo-controlled clinical trial. Front. Nutr.10, 1104169. 10.3389/fnut.2023.1104169
36
Iwabayashi M. Fujioka N. Nomoto K. Miyazaki R. Takahashi H. Hibino S. et al (2009). Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. ANTI-AGING Med.6 (4), 15–21. 10.3793/jaam.6.15
37
Jain S. Self W. H. Wunderink R. G. Fakhran S. Balk R. Bramley A. M. et al (2015). Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med.373 (5), 415–427. 10.1056/NEJMoa1500245
38
Johansson N. Kalin M. Tiveljung-Lindell A. Giske C. G. Hedlund J. (2010). Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis.50 (2), 202–209. 10.1086/648678
39
Kahraman C. Kaya Bilecenoglu D. Sabuncuoglu S. Cankaya I. T. (2023). Toxicology of pharmaceutical and nutritional longevity compounds. Expert Rev. Mol. Med.25, e28. 10.1017/erm.2023.18
40
Keskinidou C. Vassiliou A. G. Dimopoulou I. Kotanidou A. Orfanos S. E. (2022). Mechanistic understanding of lung inflammation: recent advances and emerging techniques. J. Inflamm. Res.15, 3501–3546. 10.2147/JIR.S282695
41
Kim J. H. Chang M. J. Choi H. D. Youn Y. K. Kim J. T. Oh J. M. et al (2011). Protective effects of haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. food14 (11), 1469–1475. 10.1089/jmf.2011.1626
42
Kırkıl G. Hamdi M. Sancaktar E. Kaman D. Şahin K. Kucuk O. (2012). The effect of lycopene supplementation on chronic obstructive lung disease. Nobel Med.8, 98–104.
43
Kochanek K. D. Murphy S. L. Xu J. Tejada-Vera B. (2016). Deaths: final data for 2014. Natl. Cent. Health Statistics, Natl. Vital Statistics Syst.65 (4), 1–122.
44
Korkmaz F. T. Traber K. E. (2023). Innate immune responses in pneumonia. Pneumonia (Nathan Qld)15 (1), 4. 10.1186/s41479-023-00106-8
45
Kubo H. Asai K. Kojima K. Sugitani A. Kyomoto Y. Okamoto A. et al (2019). Astaxanthin suppresses cigarette smoke-induced emphysema through Nrf2 activation in mice. Mar. drugs17 (12), 673. 10.3390/md17120673
46
Kumar V. (2020). Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front. Immunol.11, 1722. 10.3389/fimmu.2020.01722
47
Lee S. F. Harris R. Stout-Delgado H. W. (2020). Targeted antioxidants as therapeutics for treatment of pneumonia in the elderly. Transl. Res.220, 43–56. 10.1016/j.trsl.2020.03.002
48
Limapichat T. Supavajana S. (2022). Comparison between the severity scoring systems A-DROP and CURB-65 for predicting safe discharge from the emergency department in patients with community-acquired pneumonia. Emerg. Med. Int.2022, 6391141. 10.1155/2022/6391141
49
Long M. E. Mallampalli R. K. Horowitz J. C. (2022). Pathogenesis of pneumonia and acute lung injury. Clin. Sci. Lond. Engl.136 (10), 747–769. 10.1042/CS20210879
50
Ma B. Lu J. Kang T. Zhu M. Xiong K. Wang J. (2022). Astaxanthin supplementation mildly reduced oxidative stress and inflammation biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutr. Res. (New York, NY)99, 40–50. 10.1016/j.nutres.2021.09.005
51
Manzanares W. Dhaliwal R. Jiang X. Murch L. Heyland D. K. (2012). Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit. care (London, Engl.)16 (2), R66. 10.1186/cc11316
52
Metlay J. P. Waterer G. W. Long A. C. Anzueto A. Brozek J. Crothers K. et al (2019). Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med.200 (7), e45–e67. 10.1164/rccm.201908-1581ST
53
Mittal M. Siddiqui M. R. Tran K. Reddy S. P. Malik A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants and Redox Signal.20 (7), 1126–1167. 10.1089/ars.2012.5149
54
Miyawaki H. Takahashi J. Tsukahara H. Takehara I. (2008). Effects of astaxanthin on human blood rheology. J. Clin. Biochem. Nutr.43 (2), 69–74. 10.3164/jcbn.2008048
55
Morilla M. J. Ghosal K. Romero E. L. (2023). More than pigments: the potential of astaxanthin and bacterioruberin-based nanomedicines. Pharmaceutics15 (7), 1828. 10.3390/pharmaceutics15071828
56
Muravlyova L. Molotov-Luchankiy V. Bakirova R. Klyuyev D. Demidchik L. Lee V. (2016). Characteristic of the oxidative stress in blood of patients in dependence of community-acquired pneumonia severity. Open access Macedonian J. Med. Sci.4 (1), 122–127. 10.3889/oamjms.2016.040
57
Musher D. M. Roig I. L. Cazares G. Stager C. E. Logan N. Safar H. (2013). Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J. Infect.67 (1), 11–18. 10.1016/j.jinf.2013.03.003
58
Nathens A. B. Neff M. J. Jurkovich G. J. Klotz P. Farver K. Ruzinski J. T. et al (2002). Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann. Surg.236 (6), 814–822. 10.1097/00000658-200212000-00014
59
Neuman I. Nahum H. Ben-Amotz A. (2000). Reduction of exercise-induced asthma oxidative stress by lycopene, a natural antioxidant. Allergy55 (12), 1184–1189. 10.1034/j.1398-9995.2000.00748.x
60
Pereira C. P. M. Souza A. C. R. Vasconcelos A. R. Prado P. S. Name J. J. (2021). Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (review). Int. J. Mol. Med.47 (1), 37–48. 10.3892/ijmm.2020.4783
61
Pfander H. (1992). [1] carotenoids: an overview. Methods Enzym.213, 3–13. 10.1016/0076-6879(92)13105-7
62
Quinton L. J. Walkey A. J. Mizgerd J. P. (2018). Integrative physiology of pneumonia. Physiol. Rev.98 (3), 1417–1464. 10.1152/physrev.00032.2017
63
Ramirez J. A. Wiemken T. L. Peyrani P. Arnold F. W. Kelley R. Mattingly W. A. et al (2017). Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am.65 (11), 1806–1812. 10.1093/cid/cix647
64
Rider A. C. Frazee B. W. (2018). Community-acquired pneumonia. Emerg. Med. Clin. N. Am.36 (4), 665–683. 10.1016/j.emc.2018.07.001
65
Rostami S. Alyasin A. Saedi M. Nekoonam S. Khodarahmian M. Moeini A. et al (2023). Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: a randomized, triple-blind placebo-controlled clinical trial. Front. Endocrinol.14, 1144323. 10.3389/fendo.2023.1144323
66
Sagel S. D. Khan U. Jain R. Graff G. Daines C. L. Dunitz J. M. et al (2018). Effects of an antioxidant-enriched multivitamin in cystic fibrosis. A randomized, controlled, multicenter clinical trial. Am. J. Respir. Crit. care Med.198 (5), 639–647. 10.1164/rccm.201801-0105OC
67
Saito M. Yoshida K. Saito W. Fujiya A. Ohgami K. Kitaichi N. et al (2012). Astaxanthin increases choroidal blood flow velocity. Graefe's archive Clin. Exp. Ophthalmol. = Albrecht von Graefes Archiv fur klinische und Exp. Ophthalmol.250 (2), 239–245. 10.1007/s00417-011-1843-1
68
Sateesh J. Bhardwaj P. Singh N. Saraya A. (2009). Effect of antioxidant therapy on hospital stay and complications in patients with early acute pancreatitis: a randomised controlled trial. Trop. Gastroenterol.30 (4), 201–206.
69
Shimidzu N. Goto M. Miki W. (1996). Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci.62 (1), 134–137. 10.2331/fishsci.62.134
70
Shokri-Mashhadi N. Tahmasebi M. Mohammadi-Asl J. Zakerkish M. Mohammadshahi M. (2021). The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract.75 (5), e14022. 10.1111/ijcp.14022
71
Siljan W. W. Holter J. C. Nymo S. H. Husebye E. Ueland T. Aukrust P. et al (2018). Cytokine responses, microbial aetiology and short-term outcome in community-acquired pneumonia. Eur. J. Clin. investigation48 (1), e12865. 10.1111/eci.12865
72
Song X. Wang B. Lin S. Jing L. Mao C. Xu P. et al (2014). Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. J. Cell. Mol. Med.18 (11), 2198–2212. 10.1111/jcmm.12347
73
Tominaga K. Hongo N. Fujishita M. Takahashi Y. Adachi Y. (2017). Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr.61 (1), 33–39. 10.3164/jcbn.17-35
74
Travlos A. Bakakos A. Vlachos K. F. Rovina N. Koulouris N. Bakakos P. (2022). C-Reactive protein as a predictor of survival and length of hospital stay in community-acquired pneumonia. J. personalized Med.12 (10), 1710. 10.3390/jpm12101710
75
Wang S. Qi X. (2022). The putative role of astaxanthin in neuroinflammation modulation: mechanisms and therapeutic potential. Front. Pharmacol.13, 916653. 10.3389/fphar.2022.916653
76
WebMD (2025). Health benefits of astaxanthin. Available online at: https://www.webmd.com/diet/health-benefits-astaxanthin.
77
Xia W. Tang N. Kord-Varkaneh H. Low T. Y. Tan S. C. Wu X. et al (2020). The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: a meta-analysis of randomized controlled trials. Pharmacol. Res.161, 105113. 10.1016/j.phrs.2020.105113
78
Xu W. Wang M. Cui G. Li L. Jiao D. Yao B. et al (2019). Astaxanthin protects OTA-induced lung injury in mice through the Nrf2/NF-κB pathway. Toxins (Basel).11 (9), 540. 10.3390/toxins11090540
79
Xu W. Zhao T. Xiao H. (2020). The implication of oxidative stress and AMPK-Nrf2 antioxidative signaling in pneumonia pathogenesis. Front. Endocrinol.11, 400. 10.3389/fendo.2020.00400
80
Yiğit M. Güneş A. Uğuz C. Yalçın T. Tök L. Öz A. et al (2019). Effects of astaxanthin on antioxidant parameters in ARPE-19 cells on oxidative stress model. Int. J. Ophthalmol.12 (6), 930–935. 10.18240/ijo.2019.06.08
81
Youssef F. M. Elmokadem E. M. Samy A. E. H. Ateyya H. (2024). Antioxidants as adjuvant therapy in the treatment of community-acquired pneumonia. Future J. Pharm. Sci.10 (1), 106. 10.1186/s43094-024-00674-6
82
Zhou L. Gao M. Xiao Z. Zhang J. Li X. Wang A. (2015). Protective effect of astaxanthin against multiple organ injury in a rat model of sepsis. J. Surg. Res.195 (2), 559–567. 10.1016/j.jss.2015.02.026
Summary
Keywords
community-acquired pneumonia, antioxidant, astaxanthin, oxidative stress, adjuvant therapy, inflammatory markers, SOFA, APACHE
Citation
Youssef FM, Ateyya H, Hanna Samy AE and Elmokadem EM (2025) The anti-inflammatory and antioxidant effects of astaxanthin as an adjunctive therapy in community-acquired pneumonia: a randomized controlled trial. Front. Pharmacol. 16:1621308. doi: 10.3389/fphar.2025.1621308
Received
12 June 2025
Accepted
18 July 2025
Published
07 August 2025
Volume
16 - 2025
Edited by
Gabriela Cristina Fernandez, National Scientific and Technical Research Council (CONICET), Argentina
Reviewed by
Ubashini Vijakumaran, Universiti Kuala Lumpur, Malaysia
Ruihua Xin, Chinese Academy of Agricultural Sciences, China
Updates
Copyright
© 2025 Youssef, Ateyya, Hanna Samy and Elmokadem.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatma Makram Youssef, fatma.aboelhassan@fue.edu.eg
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.