Introduction
Type 2 diabetes mellitus (T2DM) represents a multifactorial chronic metabolic disorder characterized by persistent hyperglycemia and associated with an escalating global prevalence (Ong et al., 2023). The pathophysiology of T2DM encompasses a network of molecular disturbances, including dysregulated glucose metabolism, heightened oxidative stress, and impaired insulin signaling (Galicia-Garcia et al., 2020; Mlynarska et al., 2025). These underlying mechanisms highlight the need for treatment strategies that target the root molecular pathways of this disease, beyond just glycemic control. The forkhead box O (FOXO) signaling pathway has become a crucial modulator of metabolic homeostasis in this regard. FOXO transcription factors regulate essential cellular processes such as glucose homeostasis, proliferation, apoptosis, and metabolic stress resistance (Wang et al., 2014; Teaney and Cyr, 2023). Dysregulation of this pathway has been linked to the progression and severity of diabetic complications.
Herbal medicine is increasingly recognized for its diverse bioactive compounds that simultaneously modulate multiple signaling pathways (Uti et al., 2025). However, a key challenge in the development of herbal drug candidates is the lack of detailed understanding of their specific molecular mechanisms of action, which frequently impedes their progress toward clinical application. Investigating these mechanisms typically necessitates the use of animal models, which allow for the complex interplay of signaling pathways to be observed in a physiological context. Nevertheless, conventional mammalian models are often constrained by high costs, long experimental timelines, and ethical concerns, limiting their practicality for large-scale mechanistic studies (Nainu et al., 2025). In this context, Drosophila melanogaster emerges as a valuable in vivo model system.
Drosophila possesses a highly conserved FOXO signaling pathway (FOXO-SP) that is orthologous to that of humans, making it a promising platform for translational studies on the effects of candidate drugs (Puig and Mattila, 2011). It supports precise genetic manipulation through its extensive mutant and transgenic resources, making it ideal for studying the interaction of herbal compounds with specific signaling pathways like FOXO (Lopez-Ortiz et al., 2023). Moreover, this model offers experimental efficiency for elucidating both the metabolic and molecular mechanisms of herbal compound activity (Lopez-Ortiz et al., 2023; Baenas and Wagner, 2019). In light of the considerable therapeutic potential, we propose a Drosophila–based high-throughput screening framework to evaluate herbal drug candidates, focusing on the FOXO-SP as a key regulatory node in T2DM. This approach may accelerate the discovery of active compounds and elucidation of their mechanisms, advancing evidence-based herbal therapeutics.
FOXO-SP as a therapeutic target in the pathogenesis of T2DM
The FOXO-SP plays a crucial role in the regulation of cellular metabolism and stress responses, with significant implications in the development of T2DM (Wang et al., 2014). Among the FOXO family members in human, FOXO1 is particularly critical in the regulation of glucose and lipid metabolism (Teaney and Cyr, 2023). It has also been strongly implicated in the pathogenesis of T2DM (Teaney and Cyr, 2023; Marchelek-Mysliwiec et al., 2022). In T2DM, insulin resistance leads to reduced activation of the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling. As a result, the diminished PI3K/AKT signaling fails to suppress FOXO1 activity, causing its accumulation in the nucleus and increasing its transcriptional activity (Batista et al., 2021). This activation of FOXO1 regulates various cellular responses across multiple tissues, including the liver, pancreas, skeletal muscle, and adipose tissue (Teaney and Cyr, 2023; Kousteni, 2012).
FOXO1 functions as a central transcriptional regulator of hepatic glucose metabolism. Under physiological conditions, insulin signaling via PI3K/AKT phosphorylates FOXO1, promoting its cytoplasmic retention and repressing gluconeogenic transcription in the liver (Teaney and Cyr, 2023). In T2DM, this regulation is impaired, thereby increases hepatic glucose production and contributes to chronic hyperglycemia (Zhang et al., 2006). Beyond glucose metabolism, FOXO1 also plays a pivotal role in modulating lipid metabolism and managing oxidative stress responses within hepatocytes. Its hyperactivation promotes the transcription of genes involved in lipolysis, such as adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), leading to increased mobilization and systemic release of free fatty acids (Chakrabarti and Kandror, 2009). Elevated circulating free fatty acids further potentiate peripheral insulin resistance and contribute to lipotoxicity and β-cell dysfunction (da Silva Rosa et al., 2020).
FOXO1 also plays a complex role in skeletal muscle and adipose tissue. In skeletal muscle, FOXO1 influences both muscle mass and glucose metabolism. Elevated FOXO1 activity leads to muscle atrophy and a reduction in glucose uptake, especially under hyperglycemic conditions and when advanced glycation end-products (AGEs) accumulate (Teaney and Cyr, 2023; Giri et al., 2018). Furthermore, FOXO1 downregulates GLUT4 expression, worsening insulin resistance (Teaney and Cyr, 2023; Karnieli and Armoni, 2008). Studies on mutations in FOXO-SP have shown that inhibiting FOXO1 can enhance muscle mass (Vilchinskaya et al., 2022) and improve insulin sensitivity (Li et al., 2019). In adipose tissue, FOXO1 represses peroxisome proliferator–activated receptor gamma (PPARγ) transcription to inhibit adipogenesis. Excessive suppression of adipogenesis leads to ectopic fat accumulation in non-adipose tissues, such as the liver and muscles, contributing to lipotoxicity and insulin resistance (Peng et al., 2020).
Drosophila as a model organism for screening herbal candidates against T2DM
Drosophila melanogaster has been extensively utilized as a model organism for investigating the molecular mechanisms underlying complex human diseases (Victor Atoki et al., 2025). It has significantly contributed to disease modeling in various contexts, such as neurodegenerative disorders (Nitta and Sugie, 2022), cancer (Bilder et al., 2021), infectious diseases (Harnish et al., 2021), and toxicological assessments (Leao et al., 2019). In recent years, the utility of Drosophila has extended to the study of metabolic disorders, where it offers unique advantages for dissecting the molecular underpinnings of metabolic dysfunction. Building on its established role in disease modeling, Drosophila has emerged as a powerful tool for investigating conserved pathways involved in metabolism.
Numerous studies have demonstrated conserved metabolic regulation between humans and Drosophila, particularly in insulin signaling, nutrient sensing, and energy homeostasis—core pathways in the pathophysiology of T2DM and obesity (Graham and Pick, 2017). Within this conserved signaling cascade, the FOXO-SP plays a pivotal role in metabolic regulation, and its impairment is closely linked to T2DM pathogenesis. Genetically, D. melanogaster contains a single FOXO gene, dFOXO, which serves as the exclusive evolutionary ortholog of the mammalian FOXO transcription factor family (Jünger et al., 2003). Compared to traditional vertebrate models, Drosophila offers advantages such as a rapid life cycle, cost-effectiveness, and advanced genetic tools for modeling disease (Victor Atoki et al., 2025). This is further supported by the extensive availability of mutant and transgenic strains, which significantly expands its applicability for in vivo functional genomics and targeted pathway analyses (Casas-Tinto, 2024).
These conserved features and experimental advantages have enabled the development of robust Drosophila models, which not only replicate T2DM-like phenotypes, but also facilitate the systematic investigation of underlying mechanisms and therapeutic interventions. For instance, silencing the expression of the PI3K catalytic subunit (Dp110) through RNA interference (RNAi) induces diabetes-like characteristics, such as hyperglycemia, reduced body size, and diminished glycogen stores (Mascolo et al., 2022). Mutations in the InRE19 gene, an insulin receptor upstream of the FOXO pathway, also lead to disturbances in glucose and lipid metabolism that mimic diabetes (Murillo-M et al., 2011). The presence of signaling pathways that are homologous to those in mammals, combined with the availability of versatile genetic tools, makes Drosophila a powerful model system. This is relevant in the context of compound screening, especially for therapies targeting the FOXO-SP.
Discussion
Targeting the FOXO-SP in herbal compound screening for T2DM therapy offers several significant scientific advantages and practical applications. This contributes not only to poor glycemic control but also to the progression of diabetic complications such as nephropathy, neuropathy, and retinopathy (Mansour et al., 2023). Supporting this approach, previous studies have demonstrated that certain herbal compounds can modulate FOXO activity in Drosophila (see Figure 1B), indicating their potential to regulate FOXO-dependent signaling pathways (Wang et al., 2022, Bai et al., 2018, Rulifson et al., 2002, Garofalo, 2002, Merigliano et al., 2018). With FOXO gene orthologous to its human counterpart (see Figure 1A), Drosophila exhibits a rapid life cycle and a relatively small compound requirements for testing, making it a suitable model for high-throughput screening.
FIGURE 1
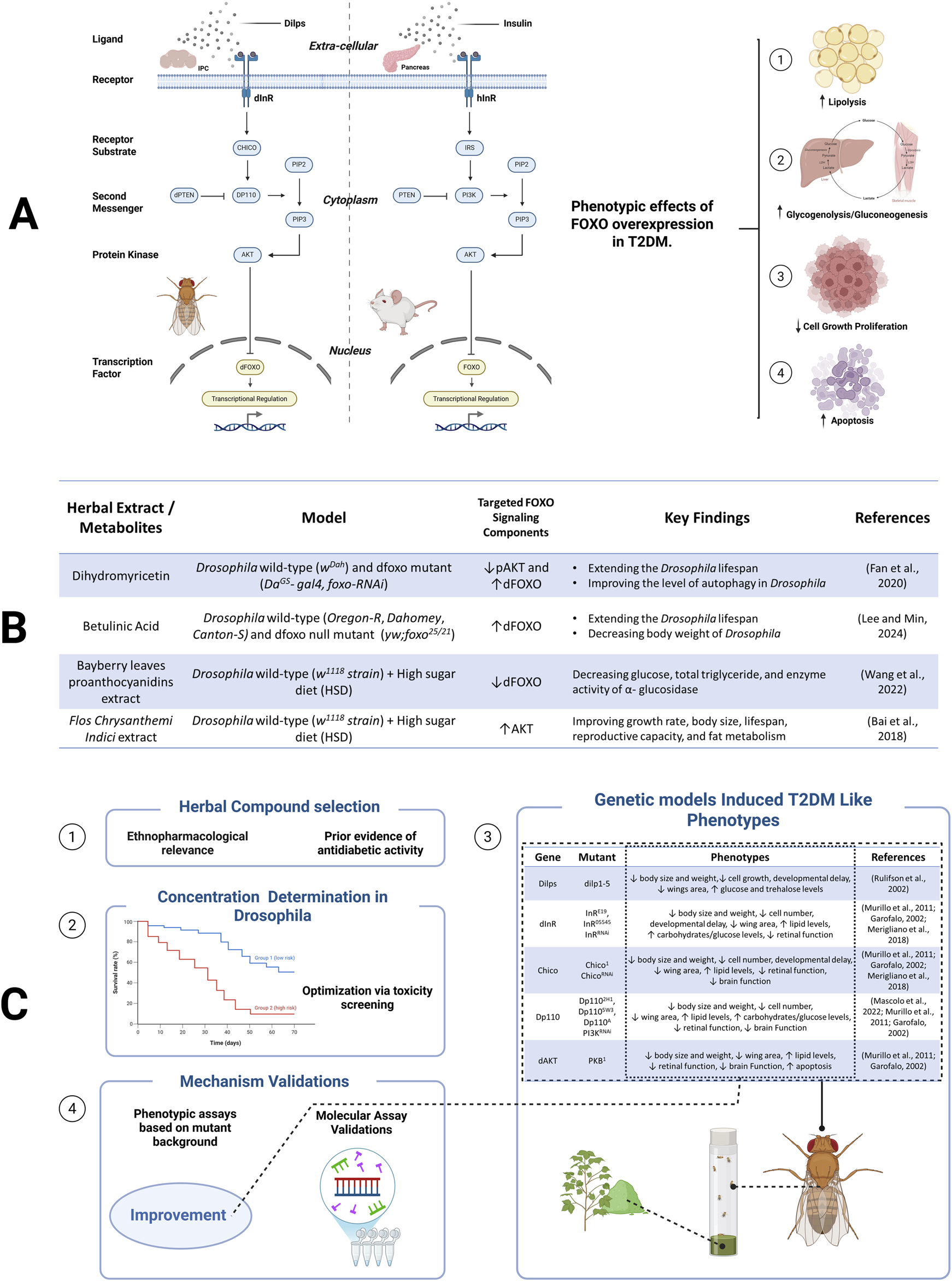
Overview of the FOXO-SP. (A) Conserved FOXO-SP in Drosophila and mammals, from ligand binding to FOXO regulation. (B) Herbal extract/metabolites modulate FOXO-SP. (C) A screening workflow to identify herbal compounds targeting the FOXO-SP.
In this perspective, we emphasize the use of genetic Drosophila models that manipulate the components of FOXO-SP as representations of T2DM. Although non-genetic Drosophila models such as those induced by high-sugar or high-fat diets have also successfully recapitulated aspects of insulin resistance and metabolic dysfunction, genetic models offer significant advantages in phenotypic consistency, reproducibility, and the ability to directly investigate the molecular roles of specific pathways. This approach enables more detailed and efficient mechanistic studies, especially in screening drug candidates targeting specific components of the FOXO-SP. Moving forward, integrating diet-induced models could complement this perspective by enhancing clinical relevance in T2DM. To support the utility of these genetic models, the application of advanced molecular tools is essential for dissecting the FOXO-SP with high spatial and temporal resolution. The use of advanced genetic tools in Drosophila, such as the GAL4/Upstream Activating Sequence (GAL4/UAS system), RNAi, and reporter transgenics, enables precise manipulation of the dFOXO component pathways.
A systematic screening workflow was designed to implement this approach (see Figure 1C). Candidate compounds—including both purified phytochemicals and crude plant extracts—were selected based on their ethnopharmacological relevance and prior evidence of antidiabetic activity. Initial toxicity assays were recommended to identify sublethal and pharmacologically relevant dose ranges, thereby ensuring that observed phenotypic effects reflect bioactivity rather than toxicity. To mimic diabetic-like conditions, we can employ Drosophila mutants with impaired function in key components of the FOXO-SP. These mutants exhibit metabolic phenotypes analogous to T2DM, including reduced body size, impaired growth, elevated lipid and carbohydrate accumulation, and developmental delay (see Figure 1C). To evaluate therapeutic effects, herbal candidates could subsequently be administered via several routes (e.g., oral feeding or systemic injection). The phenotypic traits served as quantifiable readouts for assessing the metabolic effects of the candidate compounds. Interestingly, the same mutant models also facilitated mechanistic investigation by linking phenotypic improvements to modulation of the FOXO-SP. To validate this, RNA sequencing can be employed to see profile transcriptomic changes in response to treatment. This step enabling the identification of differentially expressed genes associated with the modulation of the FOXO-SP. By linking molecular insights to measurable phenotypes, this approach offers a methodologically robust and high-throughput platform that targets the FOXO-SP.
However, despite its prospects, several challenges remain in targeting the FOXO-SP and in utilizing Drosophila as a model organism. For example, FOXO activation has been associated with beneficial effects, such as lifespan extension and suppression of tumorigenesis in specific tissue contexts (Farhan et al., 2020; Fan et al., 2020; Lee and Min, 2024). These findings introduce complexity and raise concerns regarding the broad inhibition of FOXO, as therapeutic outcomes may vary depending on specific tissue and disease context. Therefore, understanding therapeutics goals and comorbid risks is essential when considering FOXO-targeted interventions. Moving forward, selective activation or inhibition of FOXO, when integrated with advanced drug delivery systems, may hold considerable opportunity in the optimization of T2DM therapy while minimizing off-target effects.
When employing Drosophila as a model, the evaluation of herbal compounds particularly in terms of solubility and pharmacokinetic profiling remains a challenge. The solubility of herbal compounds is a well-recognized consideration across all in vivo model organisms, as it directly impacts bioavailability, including in Drosophila. Notably, Drosophila requires only relatively low concentrations to elicit measurable biological responses due to its small body size and high sensitivity to compounds. This low-dose requirement can partially compensate for the limitations imposed by poor aqueous solubility. Furthermore, several organic solvents such as ethanol and DMSO have often been used in Drosophila at specific concentrations, making them suitable carriers for hydrophobic herbal constituents. However, preliminary studies are strongly recommended to optimize solvent selection and confirm non-toxicity before conducting experiments.
Regarding formulation and dosing for clinical translation, pharmacokinetic profiling evaluation in Drosophila is an emerging area. While the absence of a mammalian-like hepatic system limits direct comparison, Drosophila possesses mammalian-like xenobiotic detoxification systems, including cytochrome P450s, phase II enzymes, and transporters. These components support functional absorption, systemic distribution, and metabolism of small molecules. The feasibility of conducting pharmacokinetic and biodistribution analyses in Drosophila has been demonstrated in a recent study (Sadova et al., 2024), providing a foundation for its use in early-stage pharmacokinetic research. However, further characterization and the establishment of standardized dose-conversion metrics are needed to strengthen translational relevance. Nevertheless, Drosophila offers distinct advantages for early-phase drug discovery, including pathway-specific mechanistic elucidation, target identification, and detection of off-target effects within a whole-organism context. These features are particularly valuable for prioritizing bioactive compounds prior to validation in mammalian systems. To bridge the translational gap, a tiered experimental framework integrating Drosophila with complementary mammalian models and clinical data is warranted. Such an approach not only accelerates the identification of lead compounds but also reinforces the scientific rigor of preclinical development pipelines.
Statements
Author contributions
MM: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing. NL: Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing. YO: Conceptualization, Supervision, Writing – original draft, Writing – review and editing. FN: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Baenas N. Wagner A. E. (2019). Drosophila melanogaster as an alternative model organism in nutrigenomics. Genes Nutr.14, 14. 10.1186/s12263-019-0641-y
2
Bai Y. Li K. Shao J. Luo Q. Jin L. H. (2018). Flos chrysanthemi indici extract improves a high-sucrose diet-induced metabolic disorder in drosophila. Exp. Ther. Med.16 (3), 2564–2572. 10.3892/etm.2018.6470
3
Batista T. M. Haider N. Kahn C. R. (2021). Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia64 (5), 994–1006. 10.1007/s00125-021-05415-5
4
Bilder D. Ong K. Hsi T. C. Adiga K. Kim J. (2021). Tumour-host interactions through the lens of drosophila. Nat. Rev. Cancer21 (11), 687–700. 10.1038/s41568-021-00387-5
5
Casas-Tinto S. (2024). Drosophila as a model for human disease: insights into rare and ultra-rare diseases. Insects15 (11), 870. 10.3390/insects15110870
6
Chakrabarti P. Kandror K. V. (2009). FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J. Biol. Chem.284 (20), 13296–13300. 10.1074/jbc.C800241200
7
da Silva Rosa S. C. Nayak N. Caymo A. M. Gordon J. W. (2020). Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep.8 (19), e14607. 10.14814/phy2.14607
8
Fan X. Zeng Y. Fan Z. Cui L. Song W. Wu Q. et al (2020). Dihydromyricetin promotes longevity and activates the transcription factors FOXO and AOP in drosophila. Aging13, 460–476. 10.18632/aging.202156
9
Farhan M. Silva M. Li S. Yan F. Fang J. Peng T. et al (2020). The role of FOXOs and autophagy in cancer and metastasis-Implications in therapeutic development. Med. Res. Rev.40 (6), 2089–2113. 10.1002/med.21695
10
Galicia-Garcia U. Benito-Vicente A. Jebari S. Larrea-Sebal A. Siddiqi H. Uribe K. B. et al (2020). Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci.21 (17), 6275. 10.3390/ijms21176275
11
Garofalo R. S. (2002). Genetic analysis of insulin signaling in drosophila. TRENDS Endocrinol. and Metabolism13 (4), 156–162. 10.1016/s1043-2760(01)00548-3
12
Giri B. Dey S. Das T. Sarkar M. Banerjee J. Dash S. K. (2018). Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed. Pharmacother.107, 306–328. 10.1016/j.biopha.2018.07.157
13
Graham P. Pick L. (2017). Drosophila as a model for diabetes and diseases of insulin resistance. Curr. Top. Dev. Biol.121, 397–419. 10.1016/bs.ctdb.2016.07.011
14
Harnish J. M. Link N. Yamamoto S. (2021). Drosophila as a model for infectious diseases. Int. J. Mol. Sci.22 (5), 2724. 10.3390/ijms22052724
15
Jünger M. A. R. F. Stocker H. Wasserman J. D. Végh M. Radimerski T. Greenberg M. E. et al (2003). The drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol.2 (3), 20. 10.1186/1475-4924-2-20
16
Karnieli E. Armoni M. (2008). Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: from physiology to pathology. Am. J. Physiol. Endocrinol. Metab.295 (1), E38–E45. 10.1152/ajpendo.90306.2008
17
Kousteni S. (2012). FoxO1, the transcriptional chief of staff of energy metabolism. Bone50 (2), 437–443. 10.1016/j.bone.2011.06.034
18
Leao M. B. Goncalves D. F. Miranda G. M. da Paixao G. M. X. Dalla Corte C. L. (2019). Toxicological evaluation of the herbicide palace® in Drosophila melanogaster. J. Toxicol. Environ. Health A82 (22), 1172–1185. 10.1080/15287394.2019.1709109
19
Lee H. Y. Min K. J. (2024). Betulinic acid increases the lifespan of Drosophila melanogaster via Sir2 and FoxO activation. Nutrients16 (3), 441. 10.3390/nu16030441
20
Li Y. Pan H. Zhang X. Wang H. Liu S. Zhang H. et al (2019). Geniposide improves glucose homeostasis via regulating FoxO1/PDK4 in skeletal muscle. J. Agric. Food Chem.67 (16), 4483–4492. 10.1021/acs.jafc.9b00402
21
Lopez-Ortiz C. Gracia-Rodriguez C. Belcher S. Flores-Iga G. Das A. Nimmakayala P. et al (2023). Drosophila melanogaster as a translational model system to explore the impact of phytochemicals on human health. Int. J. Mol. Sci.24 (17), 13365. 10.3390/ijms241713365
22
Mansour A. Mousa M. Abdelmannan D. Tay G. Hassoun A. Alsafar H. (2023). Microvascular and macrovascular complications of type 2 diabetes mellitus: exome wide association analyses. Front. Endocrinol. (Lausanne)14, 1143067. 10.3389/fendo.2023.1143067
23
Marchelek-Mysliwiec M. Nalewajska M. Turon-Skrzypinska A. Kotrych K. Dziedziejko V. Sulikowski T. et al (2022). The role of forkhead box O in pathogenesis and therapy of diabetes mellitus. Int. J. Mol. Sci.23 (19), 11611. 10.3390/ijms231911611
24
Mascolo E. Liguori F. Merigliano C. Schiano L. Gnocchini E. Pilesi E. et al (2022). Vitamin B6 rescues insulin resistance and glucose-induced DNA damage caused by reduced activity of drosophila PI3K. J. Cell Physiol.237 (9), 3578–3586. 10.1002/jcp.30812
25
Merigliano C. Mascolo E. La Torre M. Saggio I. Verni F. (2018). Protective role of vitamin B6 (PLP) against DNA damage in drosophila models of type 2 diabetes. Sci. Rep.8 (1), 11432. 10.1038/s41598-018-29801-z
26
Mlynarska E. Czarnik W. Dzieza N. Jedraszak W. Majchrowicz G. Prusinowski F. et al (2025). Type 2 diabetes mellitus: new pathogenetic mechanisms, treatment and the Most important complications. Int. J. Mol. Sci.26 (3), 1094. 10.3390/ijms26031094
27
Murillo-Maldonado J. M. Sanchez-Chavez G. Salgado L. M. Salceda R. Riesgo-Escovar J. R. (2011). Drosophila insulin pathway mutants affect visual physiology and brain function besides growth, lipid, and carbohydrate metabolism. Diabetes60 (5), 1632–1636. 10.2337/db10-1288
28
Nainu F. Bahar M. A. Habibie H. Najib A. Zubair M. S. Arba M. et al (2025). Exploring the antidiabetic potential of Sulawesi ethnomedicines: a study of Cordia myxa and Syzygium malaccense in a drosophila model of hyperglycemia. Narra J.5 (1), e1972. 10.52225/narra.v5i1.1972
29
Nitta Y. Sugie A. (2022). Studies of neurodegenerative diseases using drosophila and the development of novel approaches for their analysis. Fly. (Austin).16 (1), 275–298. 10.1080/19336934.2022.2087484
30
Ong K. L. Stafford L. K. McLaughlin S. A. Boyko E. J. Vollset S. E. Smith A. E. et al (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet402 (10397), 203–234. 10.1016/S0140-6736(23)01301-6
31
Peng S. Li W. Hou N. Huang N. (2020). A review of FoxO1-Regulated metabolic diseases and related drug discoveries. Cells9 (1), 184. 10.3390/cells9010184
32
Puig O. M. J. Mattila J. (2011). Understanding forkhead box class O function: lessons from Drosophila melanogaster. Antioxidants and Redox Signal.14 (4), 635–647. 10.1089/ars.2010.3407
33
Rulifson E. J. Kim S. K. Nusse R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science.296 (5570), 1118–1120. 10.1126/science.1070058
34
Sadova N. Blank-Landeshammer B. Curic D. Iken M. Weghuber J. (2024). Sex-specific pharmacokinetic response to phytoestrogens in Drosophila melanogaster. Biomed. Pharmacother.175, 116612. 10.1016/j.biopha.2024.116612
35
Teaney N. A. Cyr N. E. (2023). FoxO1 as a tissue-specific therapeutic target for type 2 diabetes. Front. Endocrinol. (Lausanne)14, 1286838. 10.3389/fendo.2023.1286838
36
Uti D. E. Atangwho I. J. Alum E. U. Egba S. I. Ugwu O. P.-C. Ikechukwu G. C. (2025). Natural antidiabetic agents: current evidence and development pathways from medicinal plants to clinical use. Nat. Product. Commun.20 (3). 10.1177/1934578x251323393
37
Victor Atoki A. Aja P. M. Shinkafi T. S. Ondari E. N. Adeniyi A. I. Fasogbon I. V. et al (2025). Exploring the versatility of Drosophila melanogaster as a model organism in biomedical research: a comprehensive review. Fly. (Austin).19 (1), 2420453. 10.1080/19336934.2024.2420453
38
Vilchinskaya N. Altaeva E. Lomonosova Y. (2022). Gaining insight into the role of FoxO1 in the progression of disuse-induced skeletal muscle atrophy. Adv. Biol. Regul.85, 100903. 10.1016/j.jbior.2022.100903
39
Wang M. Mao H. Chen J. Qi L. Wang J. (2022). Ameliorative effect of bayberry leaves proanthocyanidins on high sugar diet induced Drosophila melanogaster. Front. Pharmacol.13, 1008580. 10.3389/fphar.2022.1008580
40
Wang Y. Zhou Y. Graves D. T. (2014). FOXO transcription factors: their clinical significance and regulation. Biomed. Res. Int.2014, 925350. 10.1155/2014/925350
41
Zhang W. Patil S. Chauhan B. Guo S. Powell D. R. Le J. et al (2006). FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem.281 (15), 10105–10117. 10.1074/jbc.M600272200
Summary
Keywords
Drosophila , Foxo, T2DM, herbal medicine, preclinical assay
Citation
Mudjahid M, Latada NP, Ophinni Y and Nainu F (2025) Drosophila-based screening of herbal compounds targeting FOXO signaling pathway in type 2 diabetes mellitus. Front. Pharmacol. 16:1621414. doi: 10.3389/fphar.2025.1621414
Received
01 May 2025
Accepted
15 July 2025
Published
31 July 2025
Volume
16 - 2025
Edited by
Yongsheng Chen, Jinan University, China
Reviewed by
Fatimawali Fatimawali, Sam Ratulangi University, Indonesia
Himanshu Pawankumar Gupta, Columbia University, United States
Zeshan Ali, Bohai University, China
Updates
Copyright
© 2025 Mudjahid, Latada, Ophinni and Nainu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Firzan Nainu, firzannainu@unhas.ac.id
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.