- 1Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2Business Analytics, University of Colorado Denver, Denver, CO, United States
- 3The Third Affiliated Hospital of Beijing University of Chinese Medicine, Beijing, China
Objective: To evaluate the effectiveness and safety of Xiao’er Fengreqing Oral Liquid (XFOL) for pediatric acute pharyngitis/tonsillitis (external wind heat syndrome) through a multi-center, randomized, double-blind, positive-controlled, non-inferiority clinical trial.
Method: A total of 120 participants (60 per group) will be randomized to receive either XFOL or Xiao’er Yanbian Granules (positive control) for 5 days. The primary outcome is the throat pain resolution rate and overall effective rate at Day 5, assessed via the Wong-Baker Faces Pain Rating Scale (WBS). Secondary outcomes include time to symptom onset/resolution, fever resolution time, and traditional Chinese medicine (TCM) syndrome scores. Safety assessments will monitor adverse events, vital signs, and laboratory parameters. Statistical analyses will follow a pre-specified plan, employing non-inferiority testing, survival analysis for time-to-event endpoints, and generalized estimating equations for repeated measures. Missing data will be handled using the last observation carried forward (LOCF) method for effectiveness endpoints, while safety analyses will rely on observed cases.
Conclusion: This trial will provide rigorous evidence on the non-inferiority and safety profile of Fengreqing Oral Liquid, supporting its integration into pediatric care for acute upper respiratory infections. Adherence to a predefined statistical analysis plan ensures transparency and minimizes bias, ultimately guiding evidence-based clinical practice for TCM interventions.
Introduction
Pediatric acute pharyngitis/tonsillitis (PAPT) is an inflammatory condition of the upper respiratory tract, primarily affecting the pharyngeal mucosa and tonsils, with symptoms including sore throat, fever, dysphagia, and cervical lymphadenopathy (Van Driel et al., 2021). Viral pathogens account for 70%–80% of cases, while Group A Streptococcus (GAS) is the predominant bacterial cause, responsible for 15%–30% of cases in children aged 5–15 years (Le Marechal et al., 2013). Globally, PAPT represents a major pediatric health burden, contributing significantly to outpatient visits and antibiotic prescriptions (Carapetis et al., 2005). In high-income countries, the annual incidence ranges from 60 to 90 per 1,000 children, peaking during colder months (Spinks et al., 2021). In the U.S., cross-sectional studies have demonstrated that both acute and chronic tonsillitis contribute significantly to pediatric healthcare burden, with chronic cases requiring more frequent office visits and higher prescription medication use compared to acute cases (Duarte et al., 2015).
Current management of PAPT prioritizes accurate diagnosis to avoid unnecessary antibiotic use while preventing complications such as rheumatic fever. International guidelines, including those from the Infectious Diseases Society of America (IDSA) and the American Academy of Pediatrics (AAP), recommend rapid antigen detection tests (RADTs) or throat cultures to confirm GAS infection before initiating antibiotics (Wald et al., 2013; Shulman et al., 2012). First-line therapy for confirmed GAS pharyngitis remains oral penicillin or amoxicillin due to their narrow spectrum and cost-effectiveness (Van Driel et al., 2021). However, antibiotic overprescription persists globally, with studies reporting 60%–70% of viral cases receiving antimicrobials, exacerbating resistance rates for Streptococcus pneumoniae and Haemophilus influenzae (Klein et al., 2024). Macrolides and cephalosporins are reserved for penicillin-allergic patients, though emerging resistance to these agents has been documented in Asia and Europe (Klein, 1997). Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are widely used for symptomatic relief, but their overuse in children raises concerns about renal and hepatic toxicity (Fashner et al., 2012). Novel strategies, including GAS vaccines targeting M-protein epitopes and phage therapy (Wang et al., 2018), are under investigation but face challenges in effectiveness and scalability.
Emerging evidence supports the role of traditional Chinese medicine (TCM) in managing PAPT, particularly for viral or mild bacterial cases. Xiao’er Fengreqing Oral Liquid (XFOL), a 20-herb formulation including Lonicerae Japonicae Flos (honeysuckle), Forsythiae Fructus (forsythia), and Menthae Haplocalycis Herba (mint), among others, has demonstrated anti-inflammatory, antiviral, and immunomodulatory properties (Zhou et al., 2020). Produced by Handan Pharmaceutical Co., Ltd. (Handan, China), XFOL has been approved by the National Medical Products Administration of China for marketing (Z19990012) in 1995. A multicenter randomized controlled trial (RCT) comparing XFOL with Xiao’er Baotaikang Granules (control) showed comparable effectiveness in fever resolution (median time: 46 h vs. 46 h, P = 0.83) but superior improvement in nasal obstruction (55.95% vs. 39.18%, P < 0.05) and pharyngeal erythema (70.09% vs. 47.62%, P < 0.01) at Day 3 (Feng and Du, 2020). Another trial (n = 65) reported 88.33% clinical cure rates for influenza A-associated pharyngitis with XFOL, achieving viral clearance in 100% of per-protocol cases without severe adverse events (Zhou et al., 2020). Mechanistic studies attribute its effects to inhibition of NF-κB signaling and downregulation of pro-inflammatory cytokines (IL-6, TNF-α) (Jia et al., 2021). Despite promising results, the reliability of these conclusions is reduced due to the low methodological quality of the original studies. Moreover, existing studies lack data from positive-controlled trials. To address this evidence gap, we designed a non-inferiority randomized controlled trial and selected Xiao’er Yanbian Granules (XYG) as the positive control drug due to its proven efficacy in reducing tonsillar inflammation, inflammatory markers (IL-4, IL-1β, TNF-α, CRP), and symptom duration in pediatric pharyngotonsillitis (Wen et al., 2024). Using a non-inferiority margin of Δ = 0.1, based on previous non-inferiority clinical studies and the endorsement of clinical experts, this study will objectively validate XFOL’s effectiveness and safety in PAPT.
Methods
Ethical approval
This trial was approved by the ethics review committee of the Beijing University of Chinese Medicine Third Affiliated Hospital (ECPJ-BZYSY-2024-03, Beijing, China), and obtained ethical approval from each sub-center. All participants will sign a written informed consent form prior to enrollment. The development and reporting of the trial protocol adhere to the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement (Schulz et al., 2010). The trial has been registered in International Traditional Medicine Clinical Trial Registry (ITMCTR2024000569).
Trial design
This trial employs a multi-center (three-centers), randomized, parallel-group, non-inferiority design. Eligible participants, after signing written informed consent, will be randomly assigned in an equal ratio to either the trial group or the control group. Enrolled participants will receive medication for a 5-day treatment duration. After completing 5 ± 1 day of continuous medication, participants will return to the hospital for follow-up, return the study medication and diary cards, and terminate treatment. The flowchart of the trial design is presented in Figure 1.
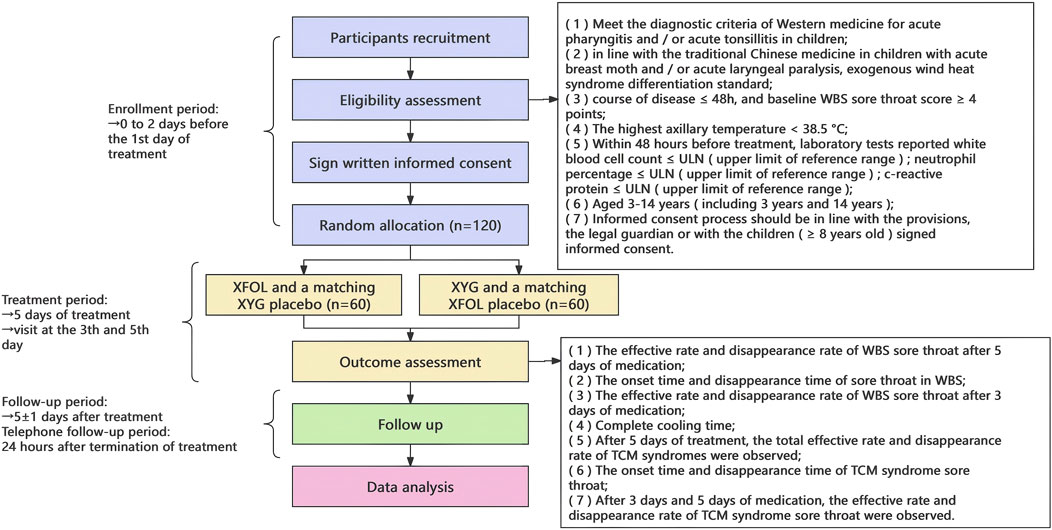
Figure 1. Flowchart of the trial design. XFOL, Xiao’er Fengreqing Oral Liquid; XYG, Xiao’er Yanbian Granules.
Participant and public involvement
Suggested: No participants or members of the public were involved in the design or conduct of this trial.
Study setting
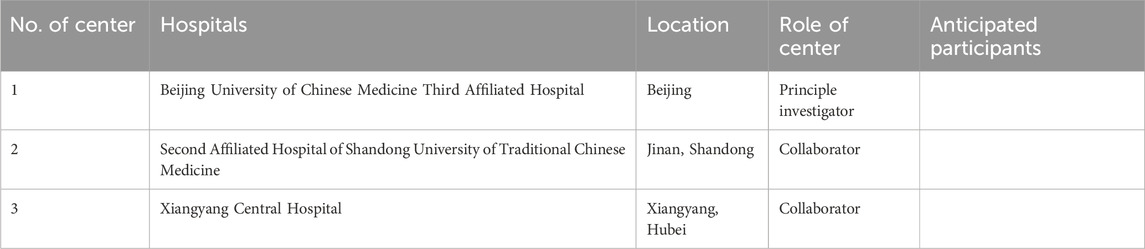
The trial will be conducted at three medical centers in China (detailed in Table 1), with participant recruitment scheduled to begin on 28 October 2024. All patients will be recruited through hospital visits.
Diagnostic criteria
Standard medicine criteria
The diagnosis of acute pharyngitis and tonsillitis follows established guidelines (Chinese Association of Traditional Chinese Medicine, 2020; Hu, 2015; Liu and Gu, 2017; Zheng, 2002). Acute pharyngitis requires: (1) acute-onset sore throat with dysphagia; (2) pharyngeal mucosal hyperemia, swollen lymphoid follicles, or scattered purulent spots; (3) predisposing factors (e.g., viral/bacterial exposure, environmental irritants). Acute tonsillitis is defined by: (1) systemic symptoms (fever ≥38°C, chills, fatigue); (2) severe odynophagia with tonsillar erythema/swelling (Brodsky scale Grade I-III); (3) laboratory evidence (elevated WBC, neutrophils, or CRP for bacterial cases). Infants may present atypically with drooling, abdominal pain, or respiratory distress.
TCM syndrome differentiation
External wind-heat syndrome is diagnosed per TCM guidelines (Chinese Association of Traditional Chinese Medicine, 2020; Zheng, 2002), requiring:
Core features: (a) acute sore throat aggravated by swallowing; (b) erythematous pharynx/tonsils without suppuration.
Supporting signs: Fever (≥37.8°C), dry throat, yellow sputum, rapid floating pulse, red tongue with thin yellow coating.
Exclusion criteria: Purulent tonsils, thick greasy tongue coating, or deep pulse indicating heat-toxin or dampness patterns.
Diagnostic confirmation
The diagnosis is confirmed if the participant presents with one main symptom, one pharyngeal sign, at least two secondary symptoms, and the corresponding tongue and pulse signs.
Inclusion criteria
Eligible participants must satisfy all criteria:
Age 3–12 years, either sex.
Meeting the above diagnostic criteria for external wind heat syndrome.
Symptom onset within 48 h prior to enrollment.
No use of antipyretics, antibiotics, or antiviral drugs within 24 h before enrollment.
Legal guardian provides written informed consent and agrees to standardized follow-up.
Exclusion criteria
Participants are excluded if any of the following apply:
Alternative diagnoses: (1) Suspected bacterial infection (e.g., tonsillar exudate, cervical lymphadenitis, or fever ≥39.5°C). (2) Herpangina, hand-foot-mouth disease, or other viral exanthems.
Comorbidities: (1) Chronic pharyngitis/tonsillitis (>3 episodes/year). (2) Immunodeficiency, asthma, or severe cardiopulmonary diseases.
Medication factors: (1) Allergy to XFOL components or control drug (e.g., Lianhua Qingwen granules). (2) Use of immunosuppressants or systemic corticosteroids within 4 weeks.
Logistical contraindications: (1) Participation in other clinical trials within 30 days (2) Inability to comply with protocol (e.g., language barriers, geographic instability).
Study discontinuation
Participants may discontinue the trial under the following conditions (documented in Case Report Form, CRF):
Voluntary withdrawal: The participant or their legal guardian chooses to withdraw from the study at any time.
Protocol violations: Incorrect enrollment (e.g., retrospective diagnosis of bacterial infection), or non-adherence to medication (>20% doses missed).
Safety-related termination: (1) Severe adverse events (SAEs) requiring hospitalization. (2) Disease progression necessitating rescue antibiotics.
Investigator decision: (1) Clinical judgment deems discontinuation in the participant’s best interest. (2) Discontinued participants receive appropriate medical management per hospital protocols.
Sample size calculation
Primary endpoints: throat pain resolution rate and overall effective rate
Parameters: Expected throat pain resolution rate: 91.43% (intervention group) vs. 76.92% (control group), non-inferiority margin (Δ) = 0.1 (Zhu et al., 2014), one-sided α = 0.025, power (1-β) = 80% (Chow et al., 2017). Initial calculation using PASS 15 software yielded 34 participants per group.
Adjustments:
A 20% dropout rate adjustment (n = 34/0.8 ≈ 43) required 44 participants per group (total 88).
To account for potential challenges in pediatric compliance and ensure robust statistical power, the final sample size was increased to 120 participants (60 per group). Since non-inferiority must be demonstrated for both primary endpoints to consider the trial successful, no adjustment for multiple testing is required in the analysis phase.
Randomization, allocation concealment, and blinding
This multicenter trial will employ a central block randomization method (block size = 4) to ensure balanced allocation between the two groups, each comprising 60 participants. An independent statistician from the Center for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, will generate the randomization sequence using SAS software, with site-specific coding ranges assigned to each participating center. Sequentially numbered, sealed, opaque envelopes will be used to maintain allocation concealment. Medications will be pre-packaged by personnel uninvolved in trial execution and stored in hospital pharmacies to preserve blinding.
Trained site investigators will enroll eligible participants and obtain informed consent. Upon enrollment, a designated third-party staff member will dispense study medications labeled with unique identifiers corresponding to the randomization list. To maintain blinding, both the participants and investigators will be unaware of treatment assignments throughout the trial. Blinding will also extend to outcome assessors and data analysts to minimize evaluation bias. All site personnel will undergo standardized training to ensure adherence to the trial protocol.
Intervention
Trial group
Intervention: Participants in the trial group will receive XFOL and a matching placebo for XYG (4 g/sachet). Both the active drug and placebo are indistinguishable in appearance, taste, and odor. For ages 3–6 years, 5–10 mL of XFOL (or placebo), administered orally 4 times daily; for ages 6–14 years, 7.5–15 mL of XFOL (or placebo), administered orally 4 times daily. The oral solution and placebo must be shaken thoroughly before use. The color, smell and taste of the placebo for XYG drug are consistent with those of XYG.
XFOL ingredients: Lonicera japonica, Forsythia suspensa, Isatis indigotica, Mentha haplocalyx, Bupleurum chinense, Lophatherum gracile, Arctium lappa, Platycodon grandiflorus, Scutellaria baicalensis, Gardenia jasminoides, Phragmites communis, CaSO4·2H2O, Schizonepeta tenuifolia, Saposhnikovia divaricata, Bombyx batryticatus, Glycyrrhiza uralensis, Massa Medicata Fermentata, Citrus reticulata, Cryptotympana atrata, Paeonia lactiflora, Prunus armeniaca.
Control group
Intervention: Participants in the control group will receive XYG (National Drug Approval Number: Z62020014; 4 g/sachet) and a matching placebo for XFOL (10 mL/bottle). Both the active drug and placebo are identical in appearance, taste, and odor. For ages 3–5 years, 1 sachet (4 g) of XYG (or placebo), administered orally 3 times daily; for ages 6–14 years, 2 sachets (8 g) of XYG (or placebo), administered orally 2–3 times daily. The color, smell and taste of the placebo for XFOL drug are consistent with those of XFOL.
XYG ingredients: Honeysuckle, Belamcanda rhizome, Tinospora pubescens root, Platycodon grandiflorus root, Scrophularia ningpoensis root, Ophiopogon japonicus root, Artificial Bovis Calculus, Borneol.
Treatment course
The treatment duration is 5 days (±1 day) as this period covers the critical progression phase of the disease. Participants will return for follow-up visits after completing the course to return unused medications and logcards. A post-treatment telephone follow-up will be conducted 24 h after discontinuation to assess body temperature, concomitant medications, and adverse events.
Rescue therapy
Acetaminophen oral suspension (brand name: Tylenol; 100 mL:3.2 g) will be administered as antipyretic rescue medication for participants with axillary temperature ≥38.2°C (Liu and Gu, 2017). For dosage, 1–3 years (12–15 kg): 3 mL per dose; 4–6 years (16–21 kg): 5 mL per dose; 7–9 years (22–27 kg): 8 mL per dose; 10–12 years (28–32 kg): 10 mL per dose. For frequency, repeat every 4–6 h if fever persists, with a maximum of 4 doses within 24 h. Persistent high fever unresponsive to rescue therapy requires immediate medical intervention.
During the trial, the use of antibiotics, antiviral agents, or other TCM with purported effectiveness for pediatric acute pharyngitis/tonsillitis is strictly prohibited. Additionally, non-protocol treatments such as infrared therapy, cupping, and acupuncture are disallowed throughout the study and follow-up periods. Acetaminophen is permitted for fever management (axillary temperature ≥38.2°C), and its dosage must adhere to the product instructions. All concomitant medications, including rescue therapies, must be meticulously documented in the CRF and original medical records, specifying the generic drug names, dosages, administration times, and reasons for use to ensure comprehensive data integrity and protocol adherence.
Outcome measures
Pain measurement
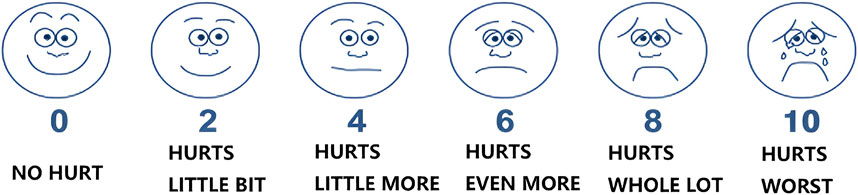
The Wong-Baker Faces Pain Rating Scale (WBS) employs six facial expressions (0–10 scale) to quantify throat pain intensity (Figure 2). Children aged ≥3 years select the face best representing their current pain, with 0 indicating no pain (smiling face) and 10 reflecting severe pain (crying face) (Garra et al., 2010).
TCM symptom evaluation
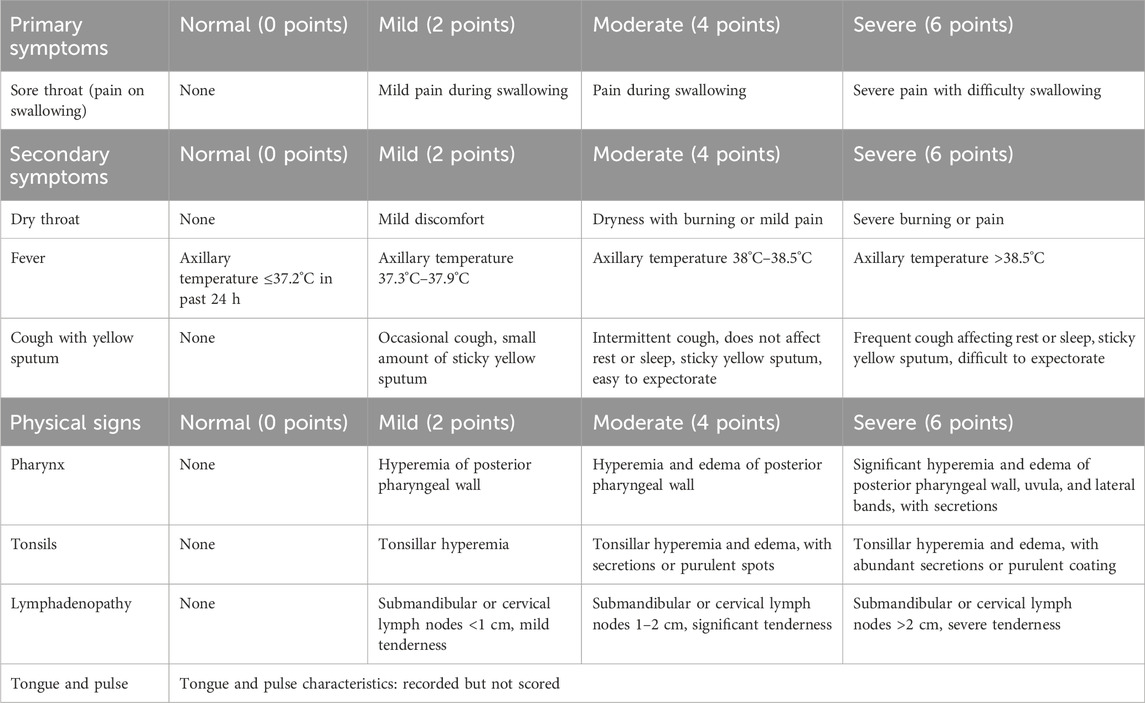
The following table outlines the grading and quantification criteria for TCM syndrome symptoms, including primary symptoms (e.g., sore throat), secondary symptoms (e.g., dry throat, fever, cough), and physical signs (e.g., pharyngeal and tonsillar findings). Symptoms are scored on a severity scale (0–6 points for primary symptoms; 0–6 points for secondary symptoms and physical signs), with higher scores indicating greater severity. Tongue and pulse characteristics are recorded but not scored (Chinese Association of Traditional Chinese Medicine, 2020) (detailed in Table 2).
Baseline data
Prior to treatment initiation, comprehensive baseline data will be collected, including demographic characteristics (sex, age, height, weight, ethnicity), medical history (disease duration, past medical conditions, allergies, pre-trial medications), and diagnostic indicators (symptoms such as sore throat and fever, physical signs including pharyngeal/tonsillar erythema, and laboratory results).
Primary outcomes
The primary effectiveness endpoint is the throat pain resolution rate and overall effective rate at Day 5, assessed using the WBS score. Participants will record pain severity daily in logcards, with scores ranging from 0 (no pain) to 10 (severe pain). Effectiveness is defined as a ≥50% reduction in WBS score from baseline, while resolution requires a score of 0. The trial will be considered successful only if non-inferiority is demonstrated for both primary endpoints simultaneously, with the test treatment non-inferior to the control treatment.
Secondary outcomes
1. Time to Onset and Resolution of WBS-Assessed Sore Throat
Participants will record throat pain severity using the WBS score in daily logcards at baseline and every 24 h post-treatment. Onset time is defined as the first time period during which the WBS score decreases by ≥ 1-grade from baseline, while resolution time refers to the interval when the score decreases to 0.
2. Effectiveness and Resolution Rates of WBS-Assessed Sore Throat at Day 3
Daily WBS scores will be analyzed to calculate the proportion of participants achieving ≥50% reduction (effectiveness) or complete resolution (score = 0) by Day 3.
3. Complete Fever Resolution Time
Participants will record axillary temperature three times daily (morning, noon, evening) until fever subsides. Complete resolution is defined as sustained temperature <37.2°C for ≥24 h.
4. TCM Syndrome Effectiveness and Resolution Rates at Day 5
Investigators will evaluate total TCM symptom scores (primary and secondary symptoms, physical signs) at baseline and Day 5. Effectiveness requires ≥50% score reduction, while resolution is defined as a score of 0.
5. Time to Onset and Resolution of TCM-Assessed Sore Throat
Participants will self-report TCM-specific sore throat severity in logcards every 24 h. Onset and resolution are determined using the same thresholds as WBS score (≥1-grade reduction and score = 0, respectively).
6. TCM-Based Effectiveness and Resolution Rates on Days 3 and 5
TCM sore throat scores from daily logcards will be analyzed to calculate effectiveness (≥50% reduction) and resolution (score = 0) rates at both timepoints.
7. TCM Symptom-Specific Effectiveness and Resolution at Day 5
Individual TCM symptoms (e.g., dry throat, cough, pharyngeal signs) will be assessed by investigators. Effectiveness and resolution rates for each symptom/parameter are calculated based on score reductions (≥50% or to 0) at Day 5.
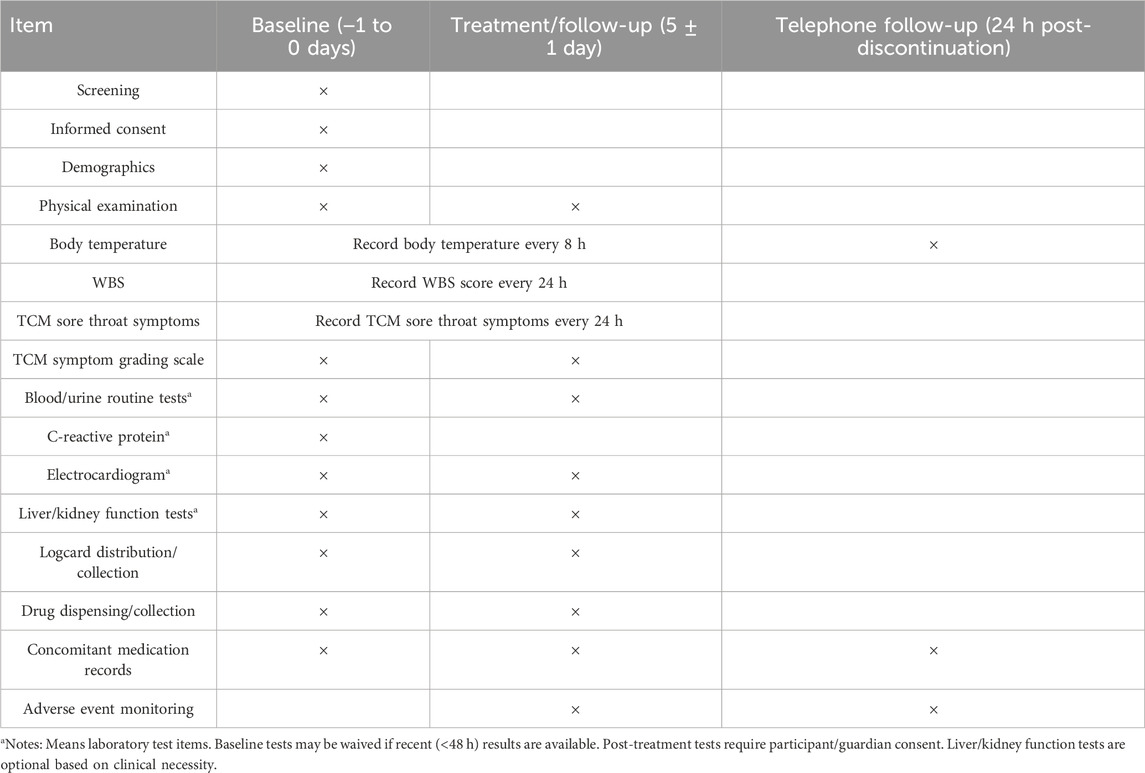
The trial procedures are divided into three phases: baseline (−1 to 0 days), treatment/follow-up (5 ± 1 day after treatment initiation), and post-treatment telephone follow-up (24 h after discontinuation). Key assessments and interventions across these phases are summarized in Table 3.
Safety assessments
Safety evaluation metrics
Safety assessments encompass three components: (1) clinical adverse reactions, monitored continuously post-treatment; (2) vital signs (blood pressure, respiratory rate, body temperature, and heart rate), measured at baseline and treatment endpoint; (3) laboratory tests, including complete blood count (WBC, RBC, HGB, PLT, NEUT, NEUT%, LYM%), C-reactive protein, urinalysis (WBC, RBC, GLU, PRO), liver function (ALT, AST, ALP, GGT/γ-GT, TBIL), renal function (BUN, Cr), and electrocardiogram. Pre-existing laboratory results within 48 h prior to enrollment may exempt retesting. Post-treatment laboratory tests (after 5 ± 1 day of medication) will be performed with participant/guardian consent. Liver and kidney function tests are optional based on clinical necessity. The incidence of adverse reactions serves as the primary safety endpoint.
Adverse event (AE) management
Definition: AEs refer to any unfavorable medical occurrences post-treatment, regardless of causality. Severity is classified using the NCI-CTCAE v5.0 criteria: Grade 1 (mild), Grade 2 (moderate), Grade 3 (severe), Grade 4 (life-threatening), and Grade 5 (fatal) (U.S. Department of Health and Human Services, 2025). Management includes: (1) continuing treatment with observation for mild AEs; (2) discontinuing treatment without intervention for moderate AEs; or (3) halting treatment and administering rescue therapy for severe AEs. For serious AEs (SAEs), immediate trial termination, emergency unblinding (if required), and prompt reporting to sponsors and ethics committees are mandated. All AEs are documented with details on onset/resolution time, severity, interventions, and outcomes.
Drug accountability and medication compliance
Drug inventory management
During each follow-up visit, investigators will count the remaining medication or empty packaging returned by participants and inquire about adherence to the prescribed regimen (e.g., missed doses, dosage deviations). This information will be documented in the CRF to evaluate compliance.
Compliance assessment
Medication adherence is defined as strict adherence to the prescribed dosing schedule without unauthorized use of additional therapies. Compliance will be calculated using the medication counting method, supplemented by participant interviews when necessary:
Data management
Data entry and modification
Prior to data entry, stringent controls are implemented to identify and correct errors, with all modifications documented and retained. Data managers collaborate with the principal investigator to establish data range checks and logic validation rules, encompassing automated system checks and manual medical reviews. Post-entry corrections require approval by the site principal investigator, ensuring traceability of all system operations through audit trails.
Database setup and data verification
This trial utilizes an electronic Case Report Form (eCRF) and Electronic Data Capture (EDC) system, designed to align with the protocol. Data managers implement automated logic checks and validation rules, supported by SAS software for comprehensive data verification. This includes identifying missing data, ensuring values fall within predefined ranges, and validating logical consistency. Investigators or clinical research coordinators (CRCs) input source data into the EDC, followed by multi-tiered verification (investigator, CRC, and monitor) and electronic signatures. Discrepancies are resolved through iterative queries, with all modifications documented in audit trails to ensure data integrity and traceability.
Blind review and locking
Unresolved queries are addressed during a blind review by sponsors, investigators, and statisticians to finalize the statistical analysis plan. The database is locked after joint authorization and undergoes initial unblinding for analysis. Post-trial, all data and documentation are archived for ≥2 years, with prior sponsor notification required before disposal.
Statistical analysis data set
Full Analysis Set (FAS): Includes all randomized participants who received at least one dose of the study drug and provided post-baseline effectiveness data. Missing primary outcomes are imputed using the last observation carried forward (LOCF) method under the intention-to-treat (ITT) principle. FAS serves as the primary population for effectiveness analyses.
Per-Protocol Set (PPS): A subset of FAS comprising participants with protocol adherence, complete baseline data, no major protocol deviations, and measurable primary endpoints. PPS is used for sensitivity analyses of primary outcomes.
Safety Set (SS): Encompasses all participants who received ≥1 dose and had safety assessments. Adverse event rates are calculated using SS.
Statistical analysis principles
No interim analysis will be conducted in this study. Following comprehensive data verification, missing values will be addressed, and erroneous entries or logical inconsistencies will be corrected to ensure data quality and completeness prior to formal statistical analyses. All analyses will be performed by investigators and independent biostatisticians using SAS 9.4 (SAS Institute Inc., Cary, NC) or equivalent software, with analysts blinded to treatment allocation. Hypothesis testing will employ two-tailed significance tests (except for pre-specified non-inferiority comparisons), with results reported as 95% confidence intervals (CI) and a significance threshold of α = 0.05. Statistical uncertainties and variability will be rigorously quantified to support robust clinical interpretations.
Missing values
For effectiveness endpoints, missing values are addressed using the LOCF method for the FAS. LOCF assumes that the last observed value remains unchanged for subsequent missing timepoints, providing a conservative estimate of treatment effects. This approach is widely used in clinical trials to minimize bias from participant dropout and ensure the robustness of ITT analyses.
For safety endpoints, no imputation is applied; analyses rely solely on observed data to avoid overestimating or underestimating adverse event rates. This ensures a more accurate representation of safety outcomes.
Demographic and baseline characteristics
Demographic and clinical baseline characteristics will be summarized by treatment and control group. Categorical variables (e.g., sex, ethnicity, medical history) will be reported as frequency counts (n) and percentages (%), with denominators reflecting available data. Continuous variables (e.g., age, height, weight, symptom duration) will be presented as means ± standard deviations (SD) for normally distributed data or medians with interquartile ranges (IQR) for non-normally distributed data. Missing data counts will be explicitly noted for all variables.
The following baseline parameters will be analyzed:
1. Demographics: Age (years), sex (male/female %), height (cm), weight (kg), ethnicity.
2. Medical History: Disease duration (days), allergies, pre-trial medications, prior episodes of pharyngitis/tonsillitis.
3. Clinical Metrics: Baseline axillary temperature (°C), pharyngeal/tonsillar erythema severity (graded 0–3), laboratory values (WBC, CRP, ALT, AST, Scr).
4. TCM Syndrome Scores: Baseline scores for sore throat, dry throat, cough, and pharyngeal signs using the TCM symptom grading scale.
No formal hypothesis testing will be performed for baseline comparisons; results will be interpreted descriptively to evaluate randomization balance.
Analysis of primary outcome
Non-inferiority will be evaluated using a predefined margin (Δ = 10%), with the lower bound of the one-sided 97.5% CI for the risk difference (trial-control) between groups compared against −Δ. If this lower bound exceeds −Δ, non-inferiority will be established. The sample size was calculated based on this non-inferiority hypothesis with a one-sided significance level of 0.025, maintaining consistency between design and analysis phases. Between-group comparisons will utilize Chi-square tests for categorical outcomes, supplemented by mixed-effects logistic regression adjusted for baseline covariates (age, baseline WBS score). To ensure consistency in statistical inference, both FAS and PPS analyses must demonstrate non-inferiority to confirm the robustness of conclusions. The trial will be considered successful only when non-inferiority is established for both primary endpoints; therefore, no adjustment for multiple testing is necessary as this represents a conservative approach requiring success on all endpoints. Should non-inferiority be established, superiority testing will be conducted as a hierarchical testing procedure by assessing whether the lower bound of the 95% CI exceeds zero. Results will be reported as proportions, risk differences, and adjusted odds ratios with 95% CIs, with forest plots used to visually represent the treatment effects and their confidence intervals relative to both the non-inferiority margin and zero.
Analysis of secondary outcomes
Time-to-event endpoints
For time to onset/resolution of sore throat (WBS or TCM-assessed) and complete fever resolution time, survival analyses will be conducted using Kaplan-Meier curves to estimate cumulative event probabilities, with between-group comparisons via log-rank tests. Results will be reported as median event times and adjusted hazard ratios (HR) derived from Cox proportional hazards models, accounting for baseline severity and age. Participants lost to follow-up will be censored at their last recorded observation.
Repeated measures and binary outcomes
Effective/resolution rates at Days 3 and 5 (WBS or TCM-assessed) and TCM symptom-specific scores will be analyzed using generalized estimating equations (GEE) with an exchangeable correlation structure to address repeated measurements. Binary outcomes (e.g., resolution yes/no) will be modeled via log-binomial regression, reporting risk ratios (RR) and 95% CI. Continuous TCM scores will use linear mixed-effects models, adjusted for baseline values and study center.
Sensitivity and subgroup analyses
Analyses will be repeated in the per-protocol set to confirm robustness. Missing data will be handled via multiple imputations for sensitivity checks. Subgroup analyses will stratify by age (3–6 vs. 7–12 years) and baseline symptom severity (WBS score ≥7 vs. <7).
Safety outcomes and adverse events
Safety analyses will focus on AE incidence, categorized by severity (NCI-CTCAE v5.0 grades 1–5), system organ class, and causality (definite/probable/possible/unlikely related to treatment). AE rates will be summarized descriptively as frequencies (%) within each treatment group, with no formal statistical testing due to expected low event rates. SAEs will undergo detailed case-by-case assessment for potential treatment relationships, with timelines and outcomes documented. All safety data will be analyzed in the SS, comprising participants who received ≥1 dose of the study drug.
Discussion
This trial aims to provide robust evidence on the effectiveness and safety of XFOL for pediatric acute pharyngitis/tonsillitis (external wind heat syndrome), addressing a critical gap in evidence-based TCM interventions for pediatric upper respiratory infections. By employing a multicenter, double-blind, positive-controlled non-inferiority design with standardized training across sites, the study ensures methodological rigor and generalizability. The integration of both subjective (WBS, TCM symptom scores) and objective outcomes (fever resolution time, laboratory parameters) balances participant-reported experiences with clinically measurable endpoints. Predefined statistical methods, including survival analysis and repeated measures models, enhance the reliability of conclusions.
This trial has several limitations. First, primary outcomes (e.g., WBS-assessed sore throat) rely on subjective participant-reported measures, potentially introducing bias. However, objective metrics such as fever resolution time and laboratory-confirmed inflammatory markers (e.g., CRP) are incorporated to balance subjective assessments. Additionally, though non-inferiority tests can be used to confirm XFOL efficacy relative to the comparator, it is also necessary to conduct an analysis of the advantages and disadvantages of the XFOL. Compared with the positive control drug XYG, the advantage of XFOL lies in its composition, which enables it to comprehensively exert the effects of clearing heat and dispersing wind, as well as to reduce swelling and fever, relieve sore throat, and improve tonsil enlargement. On the other hand, its limitations include relatively weak efficacy for other types of throat diseases and limited research data on its long-term efficacy and safety. Despite these limitations, the trial’s multicenter design, standardized training across sites, and predefined statistical analysis plan enhance methodological rigor. Results will provide critical evidence for integrating TCM into pediatric care, addressing unmet needs in acute upper respiratory infection management.
Our study makes contribution to supplement current international reporting in the following three aspects. Firstly, it establishes a more scientific and rigorous scientifically rigorous statistical analysis framework for international reports, especially in the context of traditional Chinese medicine clinical trials, by providing solutions for reasonable sample size estimation, analysis methods for primary and secondary endpoints, and handling of missing data. Subsequently, this study establishes a novel hybrid evaluation model by integrating an efficacy framework that combines TCM syndrome scoring with pediatric-specific tools including the Wong-Baker FACES scale and age-stratified dosing. Finally, our study will supply extensive data and analysis report on the safety of XFOL in treating pediatric acute pharyngitis/tonsillitis.
Conclusion
In summary, this multi-center, randomized, double-blind, positive-controlled, non-inferiority trial will evaluate the effectiveness and safety of XFOL for pediatric acute pharyngitis/tonsillitis. By implementing a pre-registered statistical analysis plan, the study will ensure methodological transparency and minimize the risk of outcome reporting bias, providing a robust framework for assessing Fengreqing Oral Liquid. Predefining endpoints, analysis populations, and non-inferiority criteria will safeguard against data-driven conclusions and enhance reproducibility.
We argue that this study will significantly contribute to the evidence-based integration of TCM into pediatric care.
Author contributions
KL: Writing – original draft. LS: Writing – review and editing. Y-KS: Writing – review and editing. YD: Writing – review and editing. YY: Writing – review and editing. Z-YS: Writing – review and editing. J-PL: Writing – review and editing. H-LL: Writing – review and editing. Z-LL: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Backup Leader Support Program of Beijing University of Chinese Medicine (90010960920033); Double First-Class Construction Support Program - Key Discipline of Traditional Chinese Medicine Evidence-based Medicine of Beijing University of Chinese Medicine - Evidence-Based Medicine in Traditional Chinese Medicine (90010951310169).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Carapetis, J. R., Steer, A. C., Mulholland, E. K., and Weber, M. (2005). The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5 (11), 685–694. doi:10.1016/S1473-3099(05)70267-X
Chinese Association of Traditional Chinese Medicine (2020). Technical guidelines for the design and evaluation of clinical trials of traditional Chinese medicine for pediatric common diseases, part 13: acute pharyngitis and tonsillitis. Beijing: CATCM Press.
Chow, S. C., Shao, J., Wang, H., and Lokhnygina, Y. (2017). Sample size calculations in clinical research. 3rd ed. New York: Taylor and Francis Group.
Duarte, V. M., McGrath, C. L., Shapiro, N. L., and Bhattacharrya, N. (2015). Healthcare costs of acute and chronic tonsillar conditions in the pediatric population in the United States. Int. J. Pediatr. Otorhinolaryngol. 79 (6), 921–925. doi:10.1016/j.ijporl.2015.04.019
Fashner, J., Ericson, K., and Werner, S. (2012). Treatment of the common cold in children and adults. Am. Fam. Physician 86 (2), 153–159.
Feng, Z., and Du, Y. (2020). Observation on the efficacy of xiaoer fengreqing in treating children with influenza A. J. Tradit. Chin. Med. Pharm. 32 (07), 1296–1299.
Garra, G., Singer, A. J., Taira, B. R., Chohan, J., Cardoz, H., Chisena, E., et al. (2010). Validation of the wong-baker FACES pain rating scale in pediatric emergency department patients. Acad. Emerg. Med. 17 (1), 50–54. doi:10.1111/j.1553-2712.2009.00620.x
Jia, R. K., Liu, H. Y., and Sui, J. L. (2021). Network pharmacology study of xiaozifeng reqing against novel coronavirus pneumonia. Mod. Tradit. Chin. Med. 41 (06), 22–33.
Klein, E. Y., Impalli, I., Poleon, S., Denoel, P., Cipriano, M., Van Boeckel, T. P., et al. (2024). Global trends in antibiotic consumption during 2016-2023 and future projections through 2030. Proc. Natl. Acad. Sci. U. S. A. 121 (49), e2411919121. doi:10.1073/pnas.2411919121
Klein, J. O. (1997). History of macrolide use in pediatrics. Pediatr. Infect. Dis. J. 16 (4), 427–431. doi:10.1097/00006454-199704000-00025
Le Marechal, F., Martinot, A., Duhamel, A., Pruvost, I., and Dubos, F. (2013). Streptococcal pharyngitis in children: a meta-analysis of clinical decision rules and their clinical variables. BMJ Open 3 (3), e001482. doi:10.1136/bmjopen-2012-001482
Liu, D. B., and Gu, Q. L. (2017). Diagnosis and treatment of acute tonsillitis in children—clinical practice guidelines (established in 2016). Chin. J. Pract. Pediatr. 32 (03), 161–164.
Schulz, K. F., Altman, D. G., and Moher, D.CONSORT Group (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern Med. 152 (11), 726–732. doi:10.7326/0003-4819-152-11-201006010-00232
Shulman, S. T., Bisno, A. L., Clegg, H. W., Gerber, M. A., Kaplan, E. L., Lee, G., et al. (2012). Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the infectious diseases society of America. Clin. Infect. Dis. 55 (10), e86–e102. doi:10.1093/cid/cis629
Spinks, A., Glasziou, P. P., and Del Mar, C. B. (2021). Antibiotics for treatment of sore throat in children and adults. Cochrane Database Syst. Rev. 12 (12), Cd000023. doi:10.1002/14651858.CD000023.pub5
U.S. Department of Health and Human Services (2025). Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Van Driel, M. L., De Sutter, A. I., Thorning, S., and Christiaens, T. (2021). Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst. Rev. 3 (3), Cd004406. doi:10.1002/14651858.CD004406.pub5
Wald, E. R., Applegate, K. E., Bordley, C., Darrow, D. H., Glode, M. P., Marcy, S. M., et al. (2013). Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 132 (1), e262–e280. doi:10.1542/peds.2013-1071
Wang, Z., Kong, L., Liu, Y., Fu, Q., Cui, Z., Wang, J., et al. (2018). A phage lysin fused to a cell-penetrating peptide kills intracellular methicillin-resistant Staphylococcus aureus in keratinocytes and has potential as a treatment for skin infections in mice. Appl. Environ. Microbiol. 84 (12), e00380-18. doi:10.1128/aem.00380-18
Wen, Q., Wang, X. B., and Li, R. L. (2024). Quality evaluation and regulatory recommendations for xiaoer yanbian granules. Drug Eval. 21 (7), 819–824.
Zheng, X. Y. (2002). Guidelines for clinical research of new traditional Chinese medicine. Beijing: China Medical Science and Technology Press.
Zhou, C., Wang, J., and Chen, Z. (2020). Multicenter clinical trial of xiaoer fengreqing mixture in treatment of acute upper respiratory tract infection in children. Drug Eval. Res. 43 (12), 2450–2456.
Keywords: pharyngitis, tonsillitis, Xiao’er Fengreqing oral Liquid, randomized controlled trial, non-inferiority design, trial protocol, statistical analysis plan
Citation: Liu K, Shi L, Song Y-K, Du Y, Yuan Y, Shi Z-Y, Liu J-P, Liu H-L and Liu Z-L (2025) Xiao’er Fengreqing oral liquid versus Xiao’er Yanbian granules for pediatric acute pharyngitis/tonsillitis (external wind heat syndrome): protocol and statistical analysis plan for a multi-center, randomized, double-blind, active drug-controlled trial. Front. Pharmacol. 16:1625547. doi: 10.3389/fphar.2025.1625547
Received: 09 May 2025; Accepted: 30 June 2025;
Published: 18 July 2025.
Edited by:
Abhijit Chakraborty, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jianning Guo, China-Japan Friendship Hospital, ChinaHaisheng Hu, First Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2025 Liu, Shi, Song, Du, Yuan, Shi, Liu, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao-Lan Liu, bHpsMTAxOUAxNjMuY29t
†These authors contributed equally to this work and share the first authorship
 Kai Liu
Kai Liu Lei Shi1†
Lei Shi1† Yi-Ke Song
Yi-Ke Song Yi Yuan
Yi Yuan Ze-Yang Shi
Ze-Yang Shi Jian-Ping Liu
Jian-Ping Liu Zhao-Lan Liu
Zhao-Lan Liu