- 1Research and Innovation Cell, Rayat Bahra University, Mohali, Punjab, India
- 2School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, India
- 3Department of Forensic Science, School of Sciences, JAIN (Deemed to be University), Bangalore, Karnataka, India
- 4School of Applied and Life Sciences, Uttaranchal University, Dehradun, India
- 5Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 6Health and Medical Researches Centre, King Khalid University, Abha, Saudi Arabia
- 7Department of Pathology, College of Korean Medicine, Kyung Hee University, Seoul, Republic of Korea
- 8Department of Humanities and Social Medicine, School of Korean Medicine, Pusan National University, Yangsan-si, Republic of Korea
- 9KM Convergence Research Division, Korea Institute of Oriental Medicine, Daejeon, Republic of Korea
- 10Department of Biotechnology, Era University, Lucknow, Uttar Pradesh, India
- 11Department of Biology, College of Science, University of Hail, Hail, Saudi Arabia
- 12Center for Global Health Research, Saveetha Medical College, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India
Bioactive substances, especially shikonin (naphthoquinone), which is extracted from Lithospermum erythrorhizon, have drawn much attention as promising substitutes for preventing cancer malignancy. Shikonin (SK) has displayed a broad spectrum of anticancer activities, such as necroptosis, cell cycle invasion, Autophagy, apoptosis, Diabetes, DNA damage induction, and suppression of angiogenesis. It reverses drug resistance and inhibited cancer cell growth by altering their metabolism. According to preliminary clinical trials, shikonin may improve the effectiveness of known chemotherapeutic drugs, radiation therapies, and immunotherapies through synergistic and additive interactions in female carcinomas. Despite its potential, additional investigation is required to pinpoint exact processes by which shikonin causes metabolic reprogramming in female cancers. While numerous researches have been reported to understanding the anticancer potential of shikonin, more research is needed to investigate its synergistic effects with conventional cancer therapies and assessing its clinical efficacy in robust trials. Due to less clinical data, more number of clinical trials is vital to establish their efficacy and safety in human patients, while mechanistic experimentation could unveil new therapeutic oncotargets in managing female carcinomas.
1 Introduction
Shikonin (SK), a traditional Chinese herbal medicine (red naphthoquinone compound) extracted from the dried roots of Lithospermum erythrorhizon. SK and its derivatives exhibit various pharmacological effects, such as anti-inflammatory properties, regulation of oxidative balance, and immune system monitoring (Biswal and Biswal, 2024). Recent in vitro studies have shown that SK has potent anti-tumor effects in lung cancer, colon cancer, and other tumors (Ma et al., 2021). Mechanistically, shikonin induces cell death irrespective of p53 status, inhibited ERK-dependent cell growth signals, and halts cell cycle (at G2/M phase). Collectively, these actions contribute to the growth-inhibitory effects of shikonin (Chen et al., 2002). SK induced cell death was significantly reduced via pan-apoptosis inhibitor, highlighting the importance of apoptosis in this phenomenon (Han et al., 2007). Interestingly, Shikonin further initiated autophagy and the protective role of autophagy was evidenced via increased cell death resulting from the suppression of autophagy through the depletion of essential autophagic genes. To elucidate the underlying mode of action behind these effects, shikonin was shown to upregulate p21, autophagy genes and suppression of the genes vital for cell development (Xu et al., 2022). SK has projected a broader range of bioactivities, including anti-inflammatory, wound healing, anti-HIV, and anticancer potential (Chen et al., 2003). It has been broadly considered as an anticancer agent, showing promising results (both in vitro and vivo), as it appears to cause minimal harm to healthy tissues and organs (Wang et al., 2020). The roots of Zicao contain shikonin, a natural red compound with a naphthoquinone structure (Bichave et al., 2024). Shikonin activates signaling pathways that regulated cytoskeleton formation, mitochondrial dysfunction, and oxidative stress responses. When this compound gets accumulated in mitochondria, it produced ROS and disrupted intracellular Ca2+ levels. Thus, cell cycle (cell growth) arrest and apoptosis occur due to microtubules disruption and mitochondrial membrane potential. Therefore, shikonin could serve as a parent compound for developing new gynecological anticancer drugs (Ke et al., 2022). The core parent nuclear structure of shikonin is 5, 8-dihydroxy-1, 4-naphthoquinone with isohexenyl side chains (Shukla et al., 2021). SK compounds further classified into two optical isomers, S and R shikonin (based on their optical activities) (Braun and Bauer 1991). Moreover, the anti-cancer effects of SK are more significant and widespread than those of alkannin. This study elucidated the anti-gynecological potential of SK to broaden its application in gynecological diseases. This study highlighted it as a powerful anti-female cancer therapy candidate by utilizing a variety of databases, such as Web of Science, Scopus, Google Scholar, and PubMed.
2 Shikonin and its pharmacokinetics
Shikonin is a type of 1,4-naphthoquinone (1,4-NQ) with C6–C4 skeleton structure. These compounds are secondary metabolites found in plants, fungi, and microorganisms. Shikonin (deep red) pigment primarily found in L. erythrorhizon roots can be easily extracted (Yazaki, 2017). Researchers have extensively studied shikonin and its both (natural or synthetic) derivatives to develop compounds with enhanced pharmacological properties, such as improved target specificity, increased water solubility, and reduced toxicity to normal tissues (Andújar et al., 2013). Due to shikonin’s high toxicity and low solubility, which limit its use as an anticancer drug, the concept of combinatorial medications has been applied. For instance, α-lipoic acid (cofactor of pyruvate dehydrogenase) has been combined with SK treatment. Eighteen ester derivatives of shikonin hybrids and α-lipoic acid were tested against various cancer cells. Only one compound showed significant PDK1 inhibition and notable cytotoxicity against HeLa cells. This derivative’s enhanced aerobic metabolism led to apoptosis induction, tubulin polymerizationprevention, and G2/M cell cycle arrest (Lin H. Y. et al., 2018). Naphthoquinones substituted with 2, 3-dithiocarbamate was evaluated for their ability to inhibit M2 isoform of pyruvate kinase (PKM2).
Two derivatives exhibited superior PKM2 inhibitory activity compared to shikonin. Most compounds demonstrated IC50 in the nanomolar range when tested against HCT116, B16, MCF7, HeLa, and H1299 cells (Idris et al., 2024). Shikonin coumarin carboxylic acid is another noteworthy compound due to its ability of apoptotic induction by inhibiting HIF-1α expression in HeLa cells (Mishra et al., 2024). SK derivative (β-HIVS) treated HeLa cells displayed reduced expression levels of mTOR, S6 kinases (70-kDa ribosomal protein), AKT, and PI3K were reduced, and cell cycle was arrested in the S phase. Additionally, inhibition of PI3K/AKT/mTOR cell signaling pathway was responsible for inducing apoptosis (Xu et al., 2022). Detailed pharmacokinetics and toxicology of shikonin has been reported in several studies hence we have not included specific section (Yadav et al., 2022; Cui et al., 2025; Olatunde et al., 2024)
3 Effects of shikonin on female malignant carcinoma
Cervical, endometrial, ovarian, and breast carcinomas are widespread malignant carcinomas in females (Kaveh et al., 2016). Additionally, the incidence and mortality of female malignancies have increased recently, putting women’s lives and health in grave danger. These days, surgery, chemotherapy, and radiation therapy are the primary therapeutic modalities utilized for female malignant tumors. Of these, chemotherapy is particularly useful in the treatment of gynecological malignancies (Wright et al., 2016). As a result, we summarized how shikonin affected all prevailing malignant tumors in women.
3.1 Anti-cancer potential of shikonin against cervical carcinoma
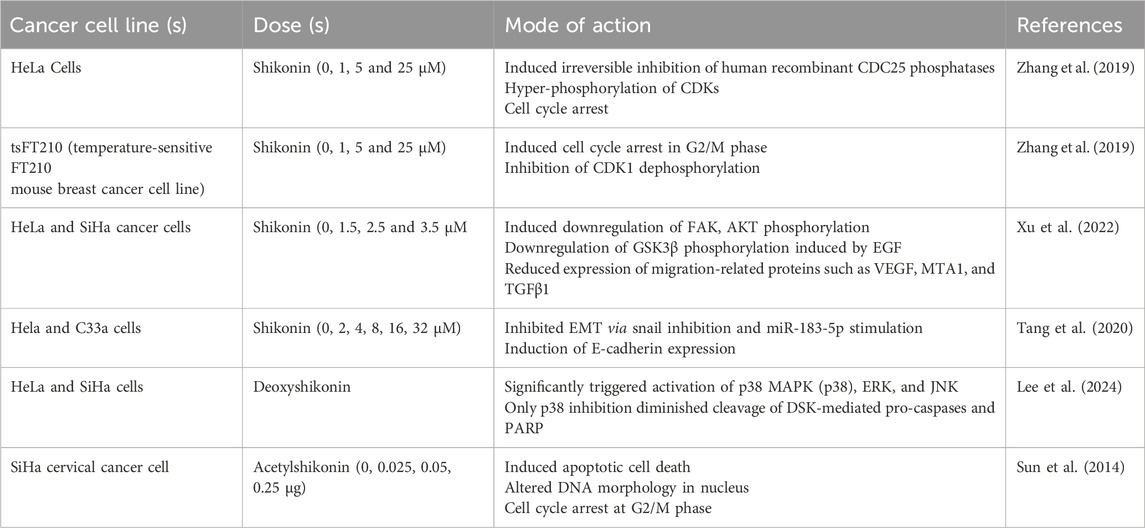
Shikonin, the main component of L. erythrorhizon, has demonstrated anticancer properties by affecting various targets and signaling pathways. It suppresses EMT by downregulating Snail and upregulating miR-183-5p, leading to increased E-cadherin expression. This suggests that shikonin’s anti-cervical cancer effects might stem from its unique ability to inhibit EMT (Tang et al., 2020). Additionally, shikonin targets the dephosphorylation of Cdc25s and CDCK1, thereby halting the cell cycle in cancerous cells. These findings indicate that shikonin can inhibit Cdc25s, causing cellular growth arrest in (both in vitro and vivo) (Zhang et al., 2019). Furthermore, shikonin exhibits significant anti-proliferative effects on HeLa and SiHa cervical cells via inhibiting FAK/AKT/GSK3β signaling pathway (Shukla et al., 2021). It has been shown to exert anticancer effects through multiple signaling pathways and targets. Shikonin reduces the expression of vimentin and snail while enhancing miR-183-5p expression and E-cadherin protein expression and promoter activity (Tang et al., 2020) (Figure 1).
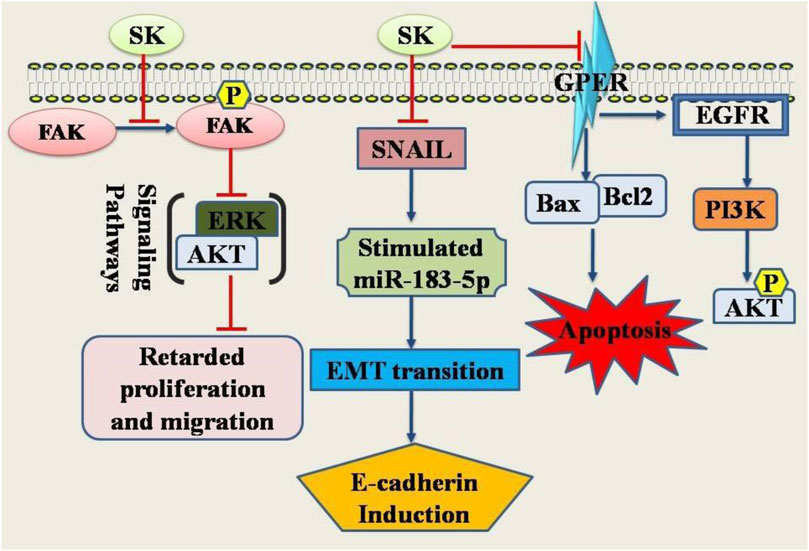
Figure 1. Diagrammatic illustration of the anti-cervical cancer potential of shikonin. Shikonin has been shown its inhibitory or promoting effects on certain genes or proteins required either for the cancer cell death or inhibition of the genes required for apoptosis in cancer cells. Abbreviations: ERK, Extracellular Signal-Regulated Kinase; SNAIL, Snail family transcriptional repressor 1; FAK, Focal Adhesion Kinase; EGFR, Epidermal growth factor receptor; PI3K, Phosphoinositide 3-kinase.
Significant attention has been devoted to prior studies focused on the synthesis or extraction of high-performance, non-toxic shikonin derivatives. One such naturally occurring derivative is β-hydroxyisovaleryl-shikonin (β-HIVS) (Lu et al., 2013; Rajasekar et al., 2012). This compound induces apoptosis in HeLa cells via altering PI3K/AKT/mTOR signaling (in dose-dependent manner) (Lu et al., 2015). An additional biological component derived from Lithospermum erythrorhizon is deoxyshikonin. Given that the anticancer efficacies of deoxyshikonin on HeLa cancer cells are not well understood, further research was undertaken to explore its anticancer efficacy and the underlying molecular mechanisms. Findings from an apoptosis microarray, which aimed to elucidate the relevant pathways, revealed that DSK reduced cellular inhibitor of apoptosis proteins (cIAP1, cIAP2 and XIAP) (in dose-dependent manner) and triggered PARP cleavage and caspase-8/9/3 activation (Lee et al., 2024). Deoxyshikonin induced apoptosis in HeLa cells via p38-mediated caspase activation and IAP downregulation.
Acetylshikonin (naphthoquinone), derived from Chinese herb (dried purple roots) L. erythrorhizon Sieb. Et Zucc., possess several biological potential, including anti-HIV, antibacterial, antifungal, antipyretic, antimicrobial, anti-inflammatory, antitumor, and analgesic effects (Zhang et al., 2020). It has shown significant antiproliferative effects by halting cells from progressing from the S to the G2/M phase and apoptosis induction in SiHa cells via activating caspases (3 and 8). Consequently, acetylshikonin is considered a promising natural candidate for the efficient treatment of cervical carcinoma (Sun et al., 2014). Table 1 compiles all the significant research on the mechanism of action of shikonin against cervical cell cancer.
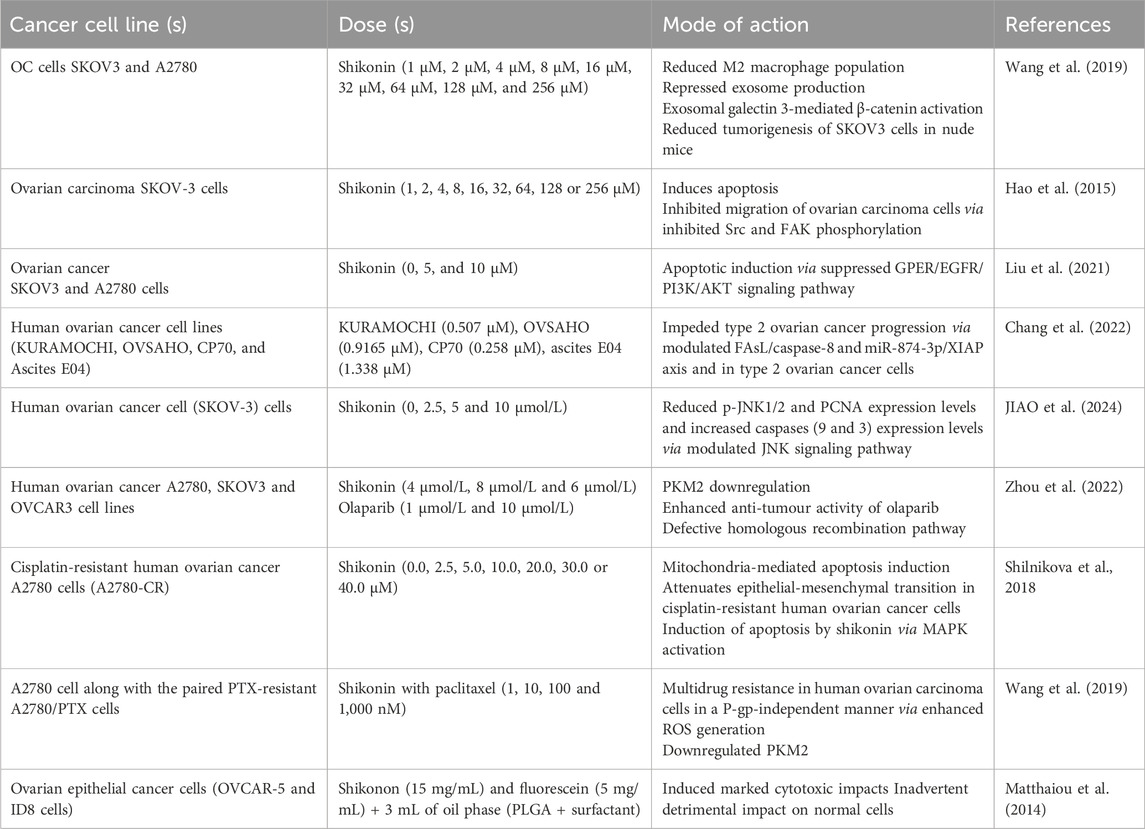
3.2 Anti-cancer potential of shikonin against ovarian carcinoma
Research has shown that the compound shikonin (SK), a type of naphthoquinone with anti-cancer properties, can reduce the production of tumor-associated exosomes (Meng et al., 2024). Shikonin achieves this by inhibiting exosome formation and blocking the activation of β-catenin mediated by exosomal GAL3, which in turn reduces the presence of M2 macrophages in ovarian cancer. Furthermore, shikonin was found to prevent the development of xenograft tumors in mice and limit the infiltration of M2 macrophages into tumor tissues (Wang et al., 2024). In advanced ovarian cancer, the overexpression of Src, a non-receptor tyrosine kinase, is notable, especially since inhibiting Src appears to overcome platinum resistance, partly by enhancing caspase-3-mediated apoptosis. As a result, various Src inhibitors are currently being tested, given Src’s potential as a target in ovarian cancer treatment (Manek et al., 2016). A study indicated that shikonin hindered ovarian cancer cell migration and cell death induction via blockage of two kinases phosphorylation including FAK and Src (Hao et al., 2015). Initial experiments revealed that shikonin was toxic to lymphocytes, normal ovarian IOSE-398 cells, ovarian epithelial cancer cells (OVCAR-5 and ID8 cells), and endothelial MS1 cells. To confine its cytotoxic efficacies to tumor cells within TME, SK was formulated as polymeric NPs with a specific component aimed at tumor microvasculature. The surface of these SK loaded NPs was further modified using carbodiimide/N-hydroxysuccinimide chemistry with PEG and a TME1/endosialin-targeting antibody. SK exhibited significant cytotoxic efficaciesagainst ovarian cancer cells. Consequently, a newly targeted nanomedicine for the treatment of ovarian cancer could be developed using biodegradable SK-loaded PLGA NPs that are PEGylated and equipped with anti-TEM1 scFv (Matthaiou et al., 2014).
A further investigation was designed to assess if shikonin could augment the anticancer efficacy of paclitaxel in human ovarian carcinoma cells that are resistant to drugs, considering its increasing significance in cancer treatment and in overcoming cancer multidrug resistance. The combination of shikonin and paclitaxel resulted in synergistic increase in cytotoxicity and cell death in paclitaxel-resistant ovarian cancer cells, validating that shikonin was able to bypass multidrug resistance associated with paclitaxel. However, in this context of ovarian carcinoma, multidrug resistance reversalby the shikonin/paclitaxel combination was achieved through a P-gp-independent mechanism involving ROS production (Hao et al., 2015).
Cisplatin-based chemotherapy is mainly used to treat ovarian cancer, but it often leads to the development of chemoresistance, which is difficult to overcome (Song et al., 2022; Ranasinghe et al., 2022). Shikonin induced cell death in shikonin treated ovarian cancer cells (SKOV3, A2780, A2780-CR, and SKOV3-PR) for 48 h. Shikonin triggers apoptosis in A2780-CR ovarian cancer cells through a mechanism dependent on mitochondria. Shikonin activated mitogen-activated protein kinases. This study suggests that shikonin aids A2780-CR cells in overcoming chemoresistance by promoting mitochondria-mediated apoptosis and inhibiting EMT (Shilnikova et al., 2018). Proteomic analysis also revealed that the combination of cisplatin and shikonin displayed ferroptosis process as evidenced by decreased glutathione peroxidase 4 (GPX4) and increased levels of Fe2+, ROS, and LPO. Combined impact of these two drugs on ovarian cell viability was reduced by siRNA interference and heme oxygenase 1 (HMOX1) inhibition. siRNA inhibition of HMOX1 led to a reduction in Fe2+accumulation. In vivo, the combination therapy augmented ferroptosis-related proteins expression in tumor tissue and significanttumor growth inhibition the tumor (subcutaneous) in BALB/c nude mice. Cisplatin resistance can be reversed by combined treatment of SK and cisplatinin ovarian cancer via triggering ferroptosis through HMOX1 overexpression, which enhances the accumulation of Fe2+ (Ni et al., 2023).
To ensure shikonin molecules are delivered accurately and effectively to cancer cells, as is necessary for all cytotoxic agents, nanoscale targeted drug delivery systems can be employed. Shikonin-loaded nanoparticles, equipped with antibodies, have been developed and validated as a potent targeted nanomedicine for treating ovarian carcinoma (Matthaiou et al., 2014). Even with chemotherapy, numerous patients suffer from considerable toxicity, frequently because of drug resistance and the unintended build-up of anticancer drugs in healthy cells or tissues. These negative effects can potentially result in the failure of treatment strategies (van den Boogaard et al., 2022). Advanced multifunctional nanomedicines with active and/or passive targeting capabilities have been successfully utilized to avoid the unintended side effects associated with traditional chemotherapy methods. It has been observed that smart targeted nanoparticles/nanosystems accumulate significantly in the tumor microenvironment through active targeting, which involves interaction with specific cancer antigens or molecular markers, and passive targeting, which utilizes increased retention effect and enhanced permeation (Pérez-Herrero and Fernández-Medarde, 2015; Ahmad et al., 2019; Arafat et al., 2024). This results in minimal side effects and maximized therapeutic effects in cancerous cells (62). Among anticancer nanosystems, the potential of biodegradable polymeric nanoparticles to deliver loaded anticancer drugs safely to target cells with minimal side effects has been extensively studied. PLGA is one of the most researched biocompatible polymers among biodegradable options and has been investigated for use as a delivery system (Perinelli et al., 2019).
In another investigation, PLGA nanoparticles (NPs) were modified with PEG, equipped with a segment of the anti-TEM1 antibody (78Fc), and infused with SK, which triggers necroptosis, resulting in 78Fc-PLGA-SK NPs. In aggressive tumor models such as TC1 murine lung carcinoma models (subcutaneous and intravenous/metastatic), this nanoformulation notably increased cytotoxicity. The 78Fc-PLGA-SK NPs were expelled through urine without accumulating in spleen or liver, yet their administration in MS1-xenograft mice led to a significant build-up and impact on TEM1-positive tumor targets (Matthaiou et al., 2021). A separate study evaluated the IC50 of shikonin and the growth curve for KURAMOCHI, OVSAHO, CP70, and ascites E04 cell lines. It also discovered that type 2 ovarian cancer cells underwent apoptosis due to activated apoptotic signaling pathway (Binju et al., 2019). This approach proved effective in treating type 2 ovarian carcinomas by decreasing the expression of the gene for type 2 ovarian cancer stem cells and reducing tumorigenicity of KURAMOCHI cell cancer stem cells by inducing apoptosis in NOD-SCID mice (Chang et al., 2022).
SK has demonstrated potential in treating ovarian carcinomavia ROS induction. Though, it has limited clinical usage due to its limited bioavailability and poor targeting of tumors, along with the high levels of GSH in tumor cells that diminish its effectiveness (Lu et al., 2024). To address this, ROS-responsive micelles formulation containing SK was developed using hyaluronic acid-phenylboronic acid pinacol ester conjugation (HA-PBAP) for targeted ovarian carcinoma therapeuticsvia disrupting the intracellular redox balance. SK@HA-PBAP formulation accumulated specifically in tumor tissues and concentrates in carcinoma cells via HA/CD44 receptor-mediated endocytosis. Upon encountering high ROS levels, SK@HA-PBAP disintegrates, releasing shikonin and quinone methide from the cancer cells. Quinone methide can deplete glutathione, while the released shikonin promotes ROS production. Ultimately, these processes resulted in a significant redox imbalance that efficiently eradicated tumor cells. Consequently, this ROS-responsive SK@HA-PBA formulation projects a promising viable therapeutic approach for ovarian carcinoma (Hu et al., 2024). Additionally, SK has been shown to effectively trigger apoptosis and proliferation inhibition in SKOV-3 cells. In both medium-dose and high-dose shikonin groups, there was a significant reduction in colony formation and cell survival rates (JIAO et al., 2024).
Another study explored how reducing PKM2 levels affects ovarian cancer cells sensitivity to PARPi that have shown therapeutic success in advanced ovarian carcinomavia inhibiting homologous recombination (HR) pathway (Gomez et al., 2020). PKM2 (key metabolic cancer marker) interacts with DNA damage to directly promote HR. PKM2 suppression using siRNA or the small molecule inhibitor SK increased the anti-cancer effects of olaparib (Ola) in ovarian cancer cells. PKM2 silencing or SK used in combination with Ola decreased cell proliferation, migration, and colony formation while inducing apoptosis. Inhibiting PKM2 disrupted the nuclear accumulation of BRCA1 and increased Ola-induced γH2AX and phospho-ATM (p-ATM) activation. The combined anticancer effects of SK and Ola were also observed in vivo using a xenograft animal model. Furthermore, SK treatment led to decreased expression of BRCA1 and PKM2 and increased DNA damage, as shown by Western blot and immunofluorescence analyses of tissue samples (Zhou et al., 2022). Table 2 summarized all significant studies associated with the mode of action of shikonin against cervical cell carcinoma.
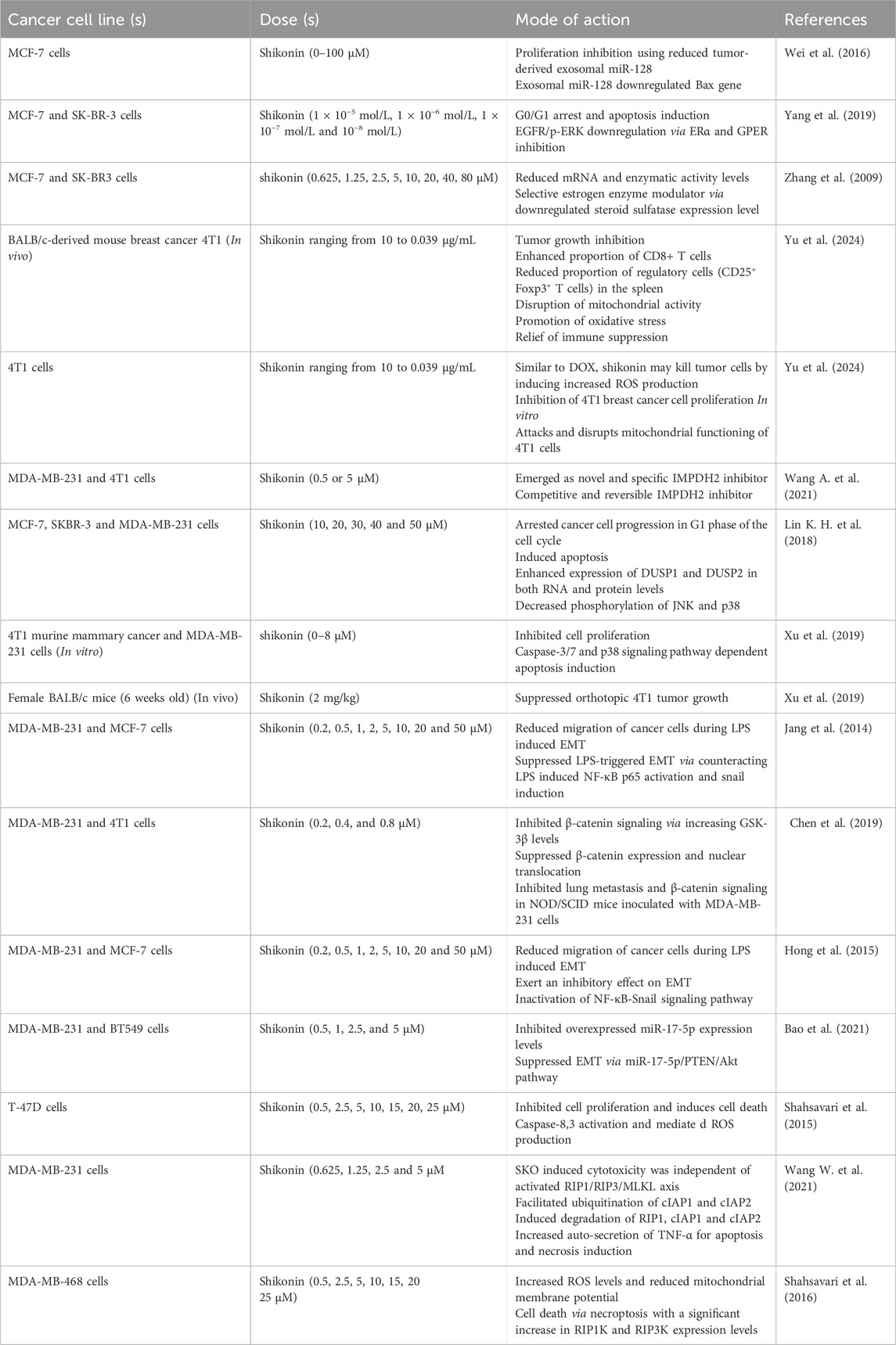
3.3 Anti-cancer potential of shikonin against breast carcinoma
Subtypes of breast cancer (HER2, PR and ER) vary greatly in terms of occurrence, response to chemotherapy, drug resistance, tumor development, and patient survival (Parise et al., 2009; Abhilash et al., 2023). Advances in new BC chemotherapy methods have enhanced their anticancer effects, significantly boosting survival rates (Smolarz et al., 2022). However, these chemotherapeutic strategies still face major challenges, such as insufficient response to treatment and resistance to multiple drugs. Besides chemotherapy, hormone therapy is also utilised as non-targeted treatment for BC, but associated with severe limitations. Resistance to radiation can lead to cancer recurrence after treatment. Immunotherapy and targeted therapies aiming at specific targeting of malignant targets (biochemical) has emerged as a promising approach for BC (Stopeck et al., 2012; Kumari et al., 2023). Therefore, to address these limitations, it is crucial to identify newly developed strategies. Tumor cells can interact with and modify their environment by releasing exosomes, which are vesicles measuring 50–100 nm in diameter (Lowry et al., 2015; Kruger et al., 2014). These exosomes facilitate the transfer of nucleic acids, signals, lipids, and proteins, including microRNAs (miRNAs), from 1 cell to another. Modern research has linked exosomal miRNAs to tumor development, invasion, and progression. Shikonin (0–100 µM) treatment has been found to decrease miR-128 (tumor-derived exosome), thereby inhibiting MCF-7 cells proliferation. Donor Exosomes MCF-7 cells release miR-128 (exosomal) for getting absorbed by recipient MCF-7 cells. In these recipient cells, miR-128 cells can downregulate Bax gene and promote cell division. By reducing exosome secretion, shikonin treatment can thus hinder MCF-7 cell growth. This study demonstrates that SK suppresses MCF-7 cells growth by decreasing tumor-derived exosomes (Wei et al., 2016).
Most individuals with breast cancer display the estrogen receptor (ER) (Haldosén et al., 2014; Duffy, 2006). Metastasis and progression of breast cancer tumors are associated with the membrane-bound ER known as the GP (G protein-coupled) ER (Hsu et al., 2019; Girgert et al., 2019). A study investigated whether the ER/GPER signaling pathway is accountable for shikonin’s ability to trigger apoptosis. Shikonin treatment inhibited MCF-7 cells by inducing apoptosis and cell growth arrest (G0/G1) phase. MCF-7 cells expressed both GPER and ERα, while SK-BR-3 cells were positive for GPER and negative for ERα. Shikonin also reduced expression levels of EGFR and p-ERK in both SK-BR-3 and MCF-7. Downregulation of EGFR/p-ERK through the suppression of GPER and ERα seems to be related to these effects (Yang et al., 2019). The enzyme steroid sulfatase (STS) is crucial in regulating estrogen production in breast cancers. SK altered STS expression in BC cells by blockingMCF-7 and SK-BR-3 cell proliferation. Additionally, STS’s mRNA and enzymatic activity levels were also reduced after shikonin treatment. Thus, by inhibiting STS expression, shikonin may act as a selective regulator of estrogen enzymes. Shikonin (0–80 μM) treatment was given to MCF-7 and SK-BR-3 cells (Zhang et al., 2009). BC in mice derived from BALB/c 4T1 cells was selected for further research due to their growth characteristics and systemic response, which closely resemble those of human breast cancer. Shikonin may inhibit cell division, induce apoptosis, disrupt mitochondrial function, and lead to ROS generation and CRT exposure in vitro (4T1 cells). In vivo, shikonin reduced the percentage of regulatory cells (CD25+ Foxp3+ T cells) in spleen, increased the percentage of CD8+ T cells, and inhibited tumor growth. Through various mechanisms, such as relieving immune suppression, enhancing oxidative stress, and disrupting mitochondrial activity, SK can halt 4T1 BC growth. This study helped inidentifying the optimal dosage and potential limitations of shikonin in vivo for treating breast tumors (Yu et al., 2024).
Inosine 5′-monophosphate dehydrogenase 2 (IMPDH2) is an enzyme that regulate the speed of de novo guanine nucleotide synthesis and therefore considered a potential target for cancer therapeutics due to its consistent overexpression in various TNBCs (Fotie, 2018). Enzymatic studies using the Lineweaver-Burk plot have shown that SK acts as competitive inhibitor of IMPDH2. Shikonin treatment effectively curtails the proliferation of the human TNBC cell line MDA-MB-231 and the murine TNBC cell line 4T1 (dose-dependent manner), although this effect is mitigated by the addition of exogenous guanosine, a component of the purine nucleotide salvage pathway. The overall findings of the research suggest that shikonin is a specific inhibitor of IMPDH2 with anti-TNBC properties, warranting further clinical trials (Wang A. et al., 2021). In breast cancer tissues, there is an abnormal overexpression of PDK1 (key enzyme in the glucose metabolism pathway) promote tumor growth and metastasis (Du et al., 2016). PDHC/PDK axis and PDK1 are significant targets for regulatingglucose metabolism and anti-tumor and activity. Several semi-synthesized shikonin (SK) derivatives were evaluated for their anti-tumor effects on human BC cells. The findings indicated that SK derivatives significantly blocked the growth of MDA-MB-231 cells. E2 and E5 (novel SK derivatives) disrupted tumor glycolysis and induced apoptosis by specifically targeting PDK1. Consequently, E2 emerges as a promising lead drug (new PDK1 inhibitor) used for TNBC treatment (Chen et al., 2022).
RNA-seq transcriptome analysis was employed to explore how shikonin affects cell proliferation of different BC cells. Shikonin induced apoptosis in MDA-MB-231 cells and halted progression of cell cycle. It also increased the RNA and protein levels of dual specificity phosphatase (DUSP1 and DUSP2). Additionally, shikonin inhibited p38 and JNK, which are downstream signaling molecules of DUSP1 and DUSP2. These findings suggested cell cycle arrest and apoptosis induction in SK treated BC cells via upregulated DUSP1 and DUSP2, thereby inhibiting the p38/MAPK/JNK pathways (Lin K. H. et al., 2018). In vitro, shikonin triggered p38-dependent apoptosis in both MDA-MB-231 human BC cells and murine mammary cancer cells, resulting in anti-tumor effect. The anti-tumor effects of shikonin were also examined in orthotopic mouse models. Tumor volumes in SK treated group began to differ from the control group on the seventh day after 4T1 cells were injected into mice. By day 13, SK suppressed tumor growth in the orthotopic 4T1 model. Shikonin emerged as an anti-tumor agent for BC cells, including MDA-MB-231 cells and 4T1 murine mammary carcinoma (Xu et al., 2019). Further studies investigated shikonin’s impact on invasion and migration of human BC. Shikonin inhibited MMP-9 production and its proteolytic and promoter activity, thereby preventing phorbol 12-myristate 13-acetate (PMA) from inducing cell invasion in MCF-7 BC cells. Moreover, shikonin reduced MMP-9 promoter activation in MDA-MB-231 cells. These findings indicate that SK inhibited migration and progression in human BC cells by modulating MMP-9. Thus, SK may be a promising anti breast cancer drug (Jang et al., 2014).
EMT is regarded as the most detrimental phase in metastasis, and thus pharmacologically targeting EMT could be a viable strategy to enhance the therapeutic effectiveness of TNBC (Neophytou et al., 2018; Li et al., 2018). Moreover, shikonin has demonstrated considerable success in inhibiting EMT. By suppressing glycogen synthase kinase 3β mediated β-catenin signaling, SK reversed EMT transition and hindered the metastasis of TNBC. Shikonin altered arrangement of cytoskeletal proteins (F-actin and vimentin), reduced cell migration, elevated E-cadherin levels, and lowered levels of N-cadherin and Snail. Histological analysis revealed that shikonin decreased vimentin and β-catenin levels in lung metastatic sites while increasing GSK-3β, E-cadherin, and phosphorylated β-catenin. These findings underscore SK potential as promising candidate for novel anti TNBC therapies, as it effectively inhibits TNBC metastasis viaEMT targeting through reduction of β-catenin signaling (GSK-3β-regulated) (Chen et al., 2019). Similarly, another study examined SK impact on EMT. LPS enhanced cell motility and invasion by inducing phenotypic changes akin to EMT. SK significantly increased expression levels of E-cadherin in MCF-7 cells and reduced LPS-induced EMT markers expression such as N-cadherin in MDA-MB-231 cells. In vitro, SK also inhibited cell invasion and migration. SK mediated its effects on LPS-induced EMT via inactivated NF-κB-Snail signaling pathway. These findings provided new evidence aboutSKmediated EMT inhibition to prevent BC cells from invading and migrating. Therefore, SK may serve as an effective anticancer treatment for BC by preventing metastases (Hong et al., 2015). Moreover, SK was found to impede the migration in BT549 and MDA-MB-231 cells. Concurrently, SK treated MDA-MB-231 cells exhibited similar alterations in EMT biomarkers. Shikonin also reduced the miR-17-5p expression, which is typically elevated in BC. EMT and metastasis of TNBC cells were inhibited by PTEN. Additionally, shikonin hindered EMT and migration of BC cells by engaging Akt and p-Akt (Ser473). SK effectively suppressed EMT viamiR-17-5p/PTEN/Akt pathway thereby preventing TNBC cell migration (Bao et al., 2021) (Figure 2).
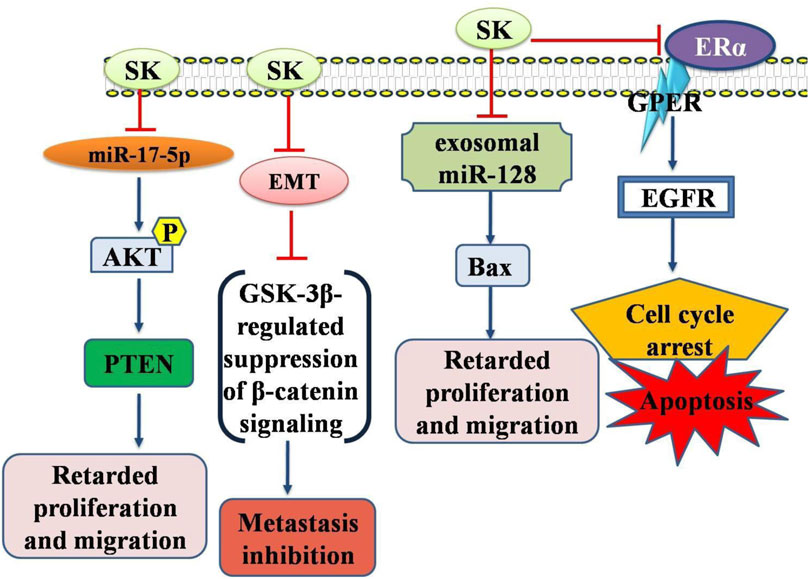
Figure 2. Diagrammatic illustration of the anti-breast cancer potential of shikonin. Shikonin has been shown its inhibitory or promoting effects on certain genes or proteins required either for the cancer cell death or inhibition of the genes required for apoptosis in cancer cells. Abbreviations: EGFR, Epidermal growth factor receptor; AKT, Protein Kinase B (PKB); PTEN, Phosphatase and TENsin homolog deleted on chromosome 10.
Targeted therapeutic drugs can eliminate cancer cells that resist apoptosis by utilizing necrotic signaling pathways (Carneiro and El-Deiry., 2020). To evaluate shikonin’s effect on inducing necroptosis or apoptosis, the T-47D breast cancer cell line was employed. Necroptosis was identified as the main mechanism of cell death. Shikonin treated cells in the presence of Nec-1 exhibited caspase-3-mediated apoptosis. Shikonin facilitates necroptosis or apoptosis by triggering ROS production in T-47D mitochondrial cells. Inducing necroptosis, a secondary programmed cell death process activated by ROS, offers a new strategy for breast cancer treatment (Shahsavari et al., 2015). In a similar study, SK effect on RIPK1-RIPK3-mediated necroptosis and apoptosis was examined in MCF-7 (ER + breast cancer) cells. SK induced both necroptosis and apoptosis, with a notable increase in expression levels of both RIPK1 and RIPK3. However, necroptosis was dominant pathway in MCF-7 cells. SK significantly increased cell growth arrest at the sub-G1 and later cell cycle stages, indicating increased necroptosis and apoptosis. Nec-1 prevented shikonin-induced necroptosis when Z-VAD-FMK inhibited caspase which ultimately resulted in reducedMMP and enhanced ROS levels (Shahsavari et al., 2018). Furthermore, SK significantly induced apoptosis and necrosis in MDA-MB-231 cells by enhancing autoubiquitination levels and promoting the proteasome-dependent degradation of cellular inhibitor of apoptosis protein 1 (cIAP1 and cIAP2). SKinduced degradation of cIAP1 and cIAP2 proteins and autoubiquitination led to a significant decrease in RIP1 inactivation and ubiquitination that played a vital role in inhibiting pro-survival pathways and augmenting necrosis in MDA-MB-231 cells. Consequently, shikonin could potentially be further investigated as novel therapeutic option for TNBC treatment (Wang W. et al., 2021).
In cancerous cells, resistance to cell death and metabolic reprogramming are essential factors. The primary challenges in effectively treating triple negative breast cancer are heightened resistance to apoptosis and tumor recurrence. It is thought that ROS production and mitochondrial dysfunction contribute to necroptosis process in cancerous cells (Hsu et al., 2020; Gong et al., 2019). SK treated MDA-MB-468 cells exhibitedreduced MMP and an increased ROS levels. Recent studies suggest that SK induces augmented ROS levels in the mitochondria of the TNBS cells, which can act as double-edged sword in the context of apoptosis or necroptosis. SK primarily induces cell death through RIP1K-RIP3K-mediated necroptosis; however, in the presence of Nec-1, apoptosis becomes predominant. These findings offer new perspectives on treating drug-resistant TNBC (Shahsavari et al., 2016). Table 3 summarized all significant studies associated with the mode of action of shikonin against breast carcinoma. One clinical trial (NCT01287468) has been reported against breast cancer includes “Academia Cinica investigator award” by shikonin in the year 2010.
Triple-negative breast cancer (TNBC) metastases and recurrences can be addressed by activating the human immune system through necrotic immunogenic cell death (ICD) (Kim and Kin., 2021; Cheng et al., 2023). However, the primary challenge lies in developing a necroptotic inducer and its accurate delivering to the tumor site. Significant necroptosis mediated ICD was observed in 4T1 cells that were treated with SK and chitosan silver nanoparticles (Chi-Ag NPs). A MUC1 aptamer-targeted nanocomplex, known as MUC1@Chi-Ag@CPB@SK or MUC1@ACS, was designed to co-deliver SK and Chi-Ag NPs. MUC1@ACS NPs accumulation at the tumor site was 6.02 times greater than that of free drug. At tumor site, MUC1@ACS NPs released SK and Chi-Ag NPs in response to acidic environment, causing tumor cell necrosis by increasing expression levels of tetrameric MLKL, RIPK3, p-RIPK3, and, which subsequently triggered ICD. This process led to Treg cells inhibition and enhanced infiltration of T cells (both CD8+ and CD4+) within tumors, effectively preventing TNBC metastasis via treating both primary tumor and distant tumor growth (Liang et al., 2024). These findings highlighted the vital role of nanoparticles in facilitating drug to target interactions during necroptotic ICD (Figure 3).
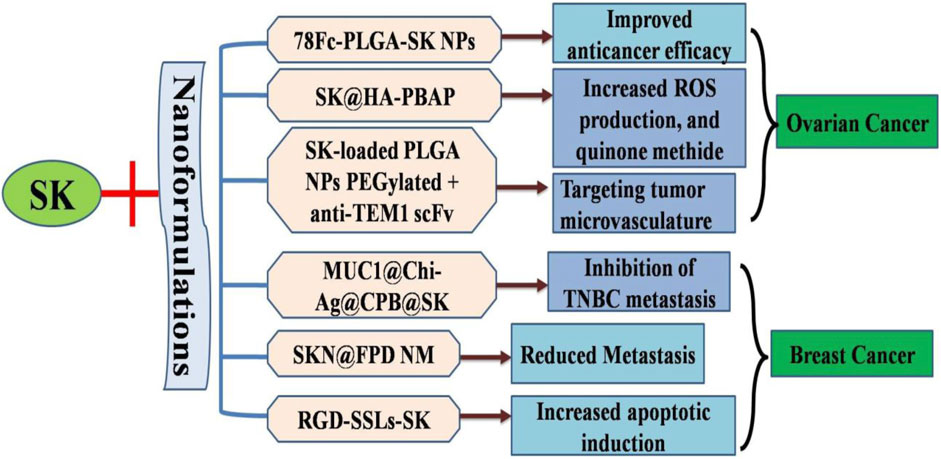
Figure 3. Diagrammatic illustration of nanoformulatios of shikonin to increase targeted drug delivery and increased apoptotic efficiency.
The widespread application of this compound in clinical settings is limited due to its inadequate water solubility and lower bioavailability. In this research, RGD-modified shikonin-loaded liposomes (RGD-SSLs-SK) were successfully developed to address these challenges. These liposomes exhibited remarkable physicochemical characteristics, such asextended release time, particle size, encapsulation efficiency, and zeta potential (Wen et al., 2018). It is evident that RGD-SSLs-SK can induce apoptosis via modulating Bax (increase) and Bcl-2 (decrease) expression levels. Additionally, it may inhibit cell migration, adhesion, and proliferation via reducing NF-κB p65 and MMP-9 expression levels, although it did not affect MMP-2 expression in MDA-MB-231 BC cells. Consequently, these findings suggested the usage of RGD-modified liposomes as carriers for targeted SK delivery and projected a highly viable approach for targeted breast cancer therapy. The results demonstrated that free SK, RGD-SSLs-SK, and shikonin-loaded liposomes (SSLs-SK) all have the ability to reduce cellular growth sand free SK could swiftly penetrate the cytomembrane and enter the intracellular space, whereas the liposomes were internalized through endocytosis after a certain period (90).
In recent years, chemoimmunotherapy has proven to be an effective cancer treatment, yet TNBC remains without definitive cure (Jia et al., 2017; Liu et al., 2023). Theseunsatisfactoryfindings are probably due to insufficient tumor immunogenicity and tumor metastasis (immunosuppressive TME). To overcome these challenges, a successful TNBC chemoimmunotherapy was developed by integrating an efficient delivery system with siTGF-β, SK, and TGF-β. The SK/siTGF-β nanoparticles (NPs) (approximately 110 nm) demonstrated good stability and a uniform structure. Silencing TGF-β reduced inhibited EMT and TGF-β-mediated ITM, which contributed to limiting Treg proliferation, enhancing CTL infiltration, and preventing lung metastasis. Consequently, SK/siTGF-β NPs exhibited highest therapeutic efficacy by delaying tumor progression and restraining lung metastasis. Additionally, the codelivery approach blocked tumor recurrence by inducing a long-lasting immune memory response. SK/siTGF-β NPs, which focus on boosting IR and inhibiting the ITM, present a potential strategy for TNBC treatment (Li J. et al., 2022). A significant challenge in breast cancer endocrine therapy is tamoxifen resistance. LNC RNAs plays vital role in tumor growth. Compared to the original MCF-7 cells, tamoxifen-resistant MCF-7R cells showed increased BCL11A (mRNA and protein) expression levels and decreased uc.57 levels. Moreover, breast cancer tissues exhibited higher BCL11A mRNA and lower uc.57 mRNA levels compared to precancerous breast cells. SK treatment reduced tamoxifen resistance in MCF-7R cells via targeting uc.57/BCL11A. Overexpressed Uc.57 levels reduced tamoxifen resistance and downregulated BCL11A in MCF-7R cells. Furthermore, knocking down BCL11A decreased tamoxifen resistance via blockingPI3K/AKT/MAPK cell signaling pathway. Thus, it appears that SK reduces tamoxifen resistance in MCF-7R BC cells via activating uc.57, which suppressedPI3K/AKT/MAPK signaling pathways via BCL11A downregulation (Zhang et al., 2017).
The combined action of metformin intensified the significant reduction in cell viability caused by shikonin. This drug pairing completely halted cell migration, reversed epithelial-mesenchymal transition (EMT), and triggered both apoptosis and increased ROS levels. Augmented BAX and PTEN expression and reduced BCL-2 expression levels were shown by in vitro experimentation. Metformin encouraged apoptotic cell morphology and mitigated damage, whereas extended exposure to shikonin led to the total disintegration of the nuclear membrane. Real-time PCR analysis indicated an upregulation of the anti-EMT gene CDH1, while EMT gene levels were suppressed. Furthermore, the combination therapy reduced CD44/CD24 ratios, enhancing chemosensitivity. Shikonin’s interactions with growth-signaling molecules were significantly enhanced by binding energies. Together, shikonin and metformin inhibit the tumorigenic characteristics of MCF-7 cells, such as proliferation, invasion, and EMT, and they may also help prevent multidrug resistance (Tabari et al., 2022). The creation, synthesis, and nuclear magnetic resonance analysis of a novel copolymer with adjustable block lengths of poly (N-isopropylacrylamide) and polylactic acid have been accomplished. Subsequently, a thermosensitive nanomicelle (TN) with a unique core-shell configuration is assembled in an aqueous solution. A shikonin-loaded thermosensitive nanomicelle (STN) is formed by incorporating shikonin into a biodegradable inner core. Shikonin specifically triggers programmed cell death (PCD), enhancing the therapeutic impact. Notably, PCD and the inhibition of proliferation synergize when T > VPTT (temperature-regulated passive targeting). Consequently, STNs accumulation within tumors is significantly augmented when T > VPTT during intravenous administration to BALB/c nude mice with BC, further validating improved synergist therapeutic effectiveness. Thus, STN could be an effective nanoformulation for clinical cancer therapy (Su et al., 2017).
Research has shown enhanced oxidative stress is effective against cancer (Klaunig, 2018; Hayes et al., 2020). SK helps regulate oxidative stress. To treat TNBC, hyaluronic acid-coated shikonin liposomes (HA-SK-Lip) were developed. These liposomes exhibited slow drug release and high drug encapsulation efficiency. Studies on anticancer properties of HA-SK-Lip revealed that they significantly blocked MDA-MB-231 cells growth. HA-SK-Lip uptake via CD44 receptor-mediated endocytosis pathway in BC cells was significantly elevated compared to SK-Lip and frees SK. Further analysis indicated that HA-SHK-Lip could significantly reduce intracellular glutathione (GSH) levels and enhance ROS production. In BALB/C mice with MDA-MB-231 tumors, anticancer efficacy of HA-SK-Lip was notably superior to that of free SK and SK-Lip. These results suggest that HA-SK-Lip mediated targeting MDA-MB-231 tumor cells and augmenting cellular oxidative stress could be a strong therapeutic approach for TNBC management (Meng et al., 2022).
One potential target for treating TNBC is the mitochondria (Weiner-Gorzel and Murphy., 2021). SK targets polymerase gamma (POLG) to exert strong inhibitory potential on mitochondrial biogenesis. Due to SK’s poor water solubility and stability, biomimetic micelle is designed to improve the poor water solubility thereby facilitatingmitochondria-targeted delivery and tumor lesion formation. To create a “right-side-out” RBCm-camouflaged cationic micelle (ThTM/SK@FP-RBCm), a folic acid (FA) conjugated polyethylene glycol derivative (FA-PEG-FA) is applied to outer membranes of red blood cells (FP-RBCm). Triphenylphosphine (TPP) moiety and FP-RBCm coating on the micelle’s surface enhance tumor lesion distribution, electrostatic attraction-dependent mitochondrial targeting, and receptor-mediated cellular uptake thereby enhancing the inhibitory potential on mitochondrial biosynthesis in TNBC cells. Administered intravenously, ThTM/SK@FP-RBCm significantly reduces lung metastasis and tumor growth in a TNBC animal model without noticeable harm. These outcomes displayed suppressed mitochondrial biogenesis and improved therapeutic outcomes for TNBC (Peng et al., 2022). Mammaglobin-modified liposomes loaded with shikonin (MGB-SK-LPs) are designed for targeted breast cancer treatment. The MGB-SK-LPs developed in this study can selectively target breast cancer cells, concentrate drugs on the tumor cell surface, and release them gradually. They also have the potential to significantly enhance SK’s anticancer therapeutic efficacy in vivo, providing a promising option for targeted breast cancer therapy (Zhang et al., 2022).
Polypeptide nanogel (effective against tumor microenvironment) holds significant promise as an effective anticancer treatment (Liu et al., 2021; Li Z. et al., 2022). A one-step ring-opening polymerization process was employed to synthesize a GSH responsive methylated PEG-poly (phenylalanine)-poly (cystine) block copolymer (mPPC). SK, referred to as mPPC/SHK, was encapsulated within the nanogel. Due to its enhanced permeability, retention effects and biocompatibility, mPPC accumulate at the tumor site, leading to the efficient release of SK when triggered by elevated GSH level. GSH responsive polypeptide nanogel encapsulating SK shows effective potential as tumor nanotherapy (Li et al., 2025). TNBC which often has poor prognosis is susceptible to metastasis and drug resistance. SK inhibits the epithelial-mesenchymal transition (EMT) pathway, which is typically linked to the significant activation of these TNBC characteristics. To load the SK, a folic acid-linked PEG nanomicelle (NM) was created and combined with DOX (referred to as FPD). They developed the SK@FPD NM using the drug loadings of DOX and SK and their dual drug effective ratio. Consequently, the combination of SK and doxorubicin (DOX) is expected to enhance anti-tumor activity and reduce metastasis (Zhong et al., 2023).
4 Efficacy of shikonin against endometrial cancer
Shikonin plant exhibits anticancer effects against numerous cancers. It is aneffective therapeutic agent for endometrioid endometrial cancer with its significant antiproliferative and apoptosis-inducing effects via modulating the miR-106b/phosphatase and tensin homolog/AKT/mTOR signaling pathway (Huang and Hu., 2018). Endometriosis, a common condition in women of reproductive age, is characterized by the infiltration of mononuclear cells into lesions (Ahn et al., 2015). Shikonin has been shown to inhibit the progression of endometriosis through several mechanisms, including reduced migration of mononuclear cells to lesions and reduced RANTES (chemokine for mononuclear macrophages) expression. In mouse/human chimera models, shikonin may prevent the growth of ectopic endometrial tissue. Its therapeutic effects might be attributed to the reduction of peritoneal inflammation, downregulated RANTES expression and inhibited monocyte recruitment in the peritoneal cavity of females with endometriosis. Further investigation into this compound could lay the groundwork for developing new treatments for endometriosis (Yuan et al., 2014). Altogether, more clinical investigations are needed to be done to elucidate potent treatment for female cancers.
5 Conclusion and future perspective
Shikonin (prominent bioactive substance) found in Lithospermum erythrorhizon, has been recognized for its ability to effectively destroy numerous carcinomas. Its antitumor properties target multiple pathways and mechanisms, attracting considerable interest and research in recent years. This review reports all possible advancements in elucidating the anticancer potential of SK and its nanoformulations via emphasizing its effect on different cell signaling pathways. This encompasses boosting ROS production, inhibiting angiogenesis, and triggering necroptosis and apoptosis. In summary, our review underscores the potential of shikonin and proposes strategies for employing it and its nanoformulations in the effective treatment of cancer. Due to much unlimited data, potential clinical translation and research are need in this direction. However, more in vivo and potential clinical translation and clinical studies are needed to validate its candidature to be utilized as anti female cancer lead candidate.
Author contributions
PP: Conceptualization, Formal Analysis, Validation, Visualization, Writing – original draft, Writing – review and editing. SL: Conceptualization, Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review and editing. JK: Formal Analysis, Investigation, Validation, Writing – review and editing, Data curation, Resources. AS: Formal Analysis, Investigation, Supervision, Writing – review and editing, Validation, Visualization. MA: Investigation, Project administration, Supervision, Writing – review and editing, Formal Analysis, Resources. MP: Resources, Validation, Writing – review and editing, Conceptualization, Formal Analysis, Funding acquisition, Methodology. S-WS: Data curation, Investigation, Resources, Validation, Writing – review and editing, Visualization. HK: Formal Analysis, Writing – review and editing, Data curation, Investigation, Resources, Validation. MZ: Conceptualization, Visualization, Writing – review and editing, Formal Analysis, Methodology. MS: Resources, Writing – review and editing, Conceptualization, Project administration, Supervision, Visualization. FK: Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. BK: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: RS-2020-KH087790), National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT): 2022R1G1A1007716, The National Research Foundation of Korea. grant numbers: 2021R1C1C2014229, and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00350362).
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (RGP2/356/46).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abhilash, N., Ajay, S. K., Ramesh, K. V., and Math, R. K. (2023). Lasiosiphon glaucus a potent ethnobotanical medicinal plant against breast cancer targeting multiple pathways: an invitro study. Adv. Traditional Med. 23 (2), 557–564. doi:10.1007/s13596-021-00624-0
Ahmad, A., Khan, F., Mishra, R. K., and Khan, R. (2019). Precision cancer nanotherapy: evolving role of multifunctional nanoparticles for cancer active targeting. J. Med. Chem. 62 (23), 10475–10496. doi:10.1021/acs.jmedchem.9b00511
Ahn, S. H., Monsanto, S. P., Miller, C., Singh, S. S., Thomas, R., and Tayade, C. (2015). Pathophysiology and immune dysfunction in endometriosis. BioMed Res. Int. 2015 (1), 795976. doi:10.1155/2015/795976
Andújar, I., Ríos, J. L., Giner, R. M., and Recio, M. C. (2013). Pharmacological properties of shikonin–a review of literature since 2002. Planta Medica 79 (18), 1685–1697. doi:10.1055/s-0033-1350934
Arafat, M., Sakkal, M., Beiram, R., and &AbuRuz, S. (2024). Nanomedicines: emerging platforms in smart chemotherapy treatment—A recent review. Pharmaceuticals 17 (3), 315. doi:10.3390/ph17030315
Bao, C., Liu, T., Qian, L., Xiao, C., Zhou, X., Ai, H., et al. (2021). Shikonin inhibits migration and invasion of triple-negative breast cancer cells by suppressing epithelial-mesenchymal transition via miR-17-5p/PTEN/Akt pathway. J. Cancer 12 (1), 76–88. doi:10.7150/jca.47553
Bichave, A., Vishwakarma, R., Hajatay, S. F., and Patil, S. (2024). Shikonin: hope for innovative and targeted cancer treatments. Am. J. Pharm. Res. 14 (04). doi:10.5281/zenodo.11100817
Binju, M., Amaya-Padilla, M. A., Wan, G., Gunosewoyo, H., SuryoRahmanto, Y., and Yu, Y. (2019). Therapeutic inducers of apoptosis in ovarian cancer. Cancers 11 (11), 1786. doi:10.3390/cancers11111786
Biswal, S., and Biswal, B. K. (2024). Bioavailability and pharmacological properties of Shikonin-A phytocompound from Lithospermum erythrorhizon. ChemistrySelect 9 (45), e202403504. doi:10.1002/slct.202403504
Braun, M., and Bauer, C. (1991). Synthesis of shikonin and alkannin. Liebigs Ann. Chem. 1991 (11), 1157–1164. doi:10.1002/jlac.1991199101199
Carneiro, B. A., and El-Deiry, W. S. (2020). Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17 (7), 395–417. doi:10.1038/s41571-020-0341-y
Chang, Y. H., Lin, Y. J., Huang, C. Y., Harnod, T., and Ding, D. C. (2022). Shikonin impedes type 2 ovarian cancer progression via FasL/caspase-8 and mir-874-3p/XIAP axis and prohibits the properties of stemness. Am. J. Cancer Res. 12 (10), 4584–4601.
Chen, Q., Han, H., Lin, F., Yang, L., Feng, L., Lai, X., et al. (2022). Novel shikonin derivatives suppress cell proliferation, migration and induce apoptosis in human triple-negative breast cancer cells via regulating PDK1/PDHC axis. Life Sci. 310, 121077. doi:10.1016/j.lfs.2022.121077
Chen, X., Yang, L., Oppenheim, J. J., and Howard, O. Z. (2002). Cellular pharmacology studies of shikonin derivatives. Phytotherapy Res. 16 (3), 199–209. doi:10.1002/ptr.1100
Chen, X., Yang, L., Zhang, N., Turpin, J. A., Buckheit, R. W., Osterling, C., et al. (2003). Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 47 (9), 2810–2816. doi:10.1128/aac.47.9.2810-2816.2003
Chen, Y., Chen, Z. Y., Chen, L., Zhang, J. Y., Fu, L. Y., Tao, L., et al. (2019). Shikonin inhibits triple-negative breast cancer-cell metastasis by reversing the epithelial-to-mesenchymal transition via glycogen synthase kinase 3β-regulated suppression of β-catenin signaling. Biochem. Pharmacol. 166, 33–45. doi:10.1016/j.bcp.2019.05.001
Cheng, X. Y., Liang, Y., Zhang, H. F., Qian, F. Z., Sun, X. H., and Liu, X. A. (2023). An immunogenic cell death-related classification predicts response to immunotherapy and prognosis in triple-negative breast cancer. Am. J. Transl. Res. 15 (4), 2598–2609.
Cui, J., Xiang, S., Zhang, Q., Xiao, S., Yuan, G., Liu, C., et al. (2025). Design, synthesis, and biological evaluation of 5, 8-Dimethyl shikonin oximes as SARS-CoV-2 mpro inhibitors. Molecules 30 (6), 1321. doi:10.3390/molecules30061321
Du, J., Yang, M., Chen, S., Li, D., Chang, Z., and Dong, Z. (2016). PDK1 promotes tumor growth and metastasis in a spontaneous breast cancer model. Oncogene 35 (25), 3314–3323. doi:10.1038/onc.2015.393
Duffy, M. J. (2006). Estrogen receptors: role in breast cancer. Crit. Rev. Clin. Laboratory Sci. 43 (4), 325–347. doi:10.1080/10408360600739218
Fotie, J. (2018). Inosine 5′-monophosphate dehydrogenase (IMPDH) as a potential target for the development of a new generation of antiprotozoan agents. Mini Rev. Med. Chem. 18 (8), 656–671. doi:10.2174/1389557516666160620065558
Girgert, R., Emons, G., and Gründker, C. (2019). Estrogen signaling in ERα-negative breast cancer: erβ and GPER. Front. Endocrinol. 9, 781. doi:10.3389/fendo.2018.00781
Gomez, M. K., Illuzzi, G., Colomer, C., Churchman, M., Hollis, R. L., O’Connor, M. J., et al. (2020). Identifying and overcoming mechanisms of PARP inhibitor resistance in homologous recombination repair-deficient and repair-proficient high grade serous ovarian cancer cells. Cancers 12 (6), 1503. doi:10.3390/cancers12061503
Gong, Y., Fan, Z., Luo, G., Yang, C., Huang, Q., Fan, K., et al. (2019). The role of necroptosis in cancer biology and therapy. Mol. cancer 18, 100–117. doi:10.1186/s12943-019-1029-8
Haldosén, L. A., Zhao, C., and Dahlman-Wright, K. (2014). Estrogen receptor beta in breast cancer. Mol. Cell. Endocrinol. 382 (1), 665–672. doi:10.1016/j.mce.2013.08.005
Han, W., Li, L., Qiu, S., Lu, Q., Pan, Q., Gu, Y., et al. (2007). Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol. Cancer Ther. 6 (5), 1641–1649. doi:10.1158/1535-7163.MCT-06-0511
Hao, Z., Qian, J., and Yang, J. (2015). Shikonin induces apoptosis and inhibits migration of ovarian carcinoma cells by inhibiting the phosphorylation of Src and FAK. Oncol. Lett. 9 (2), 629–633. doi:10.3892/ol.2014.2771
Hayes, J. D., Dinkova-Kostova, A. T., and Tew, K. D. (2020). Oxidative stress in cancer. Cancer Cell 38 (2), 167–197. doi:10.1016/j.ccell.2020.06.001
Hong, D., Jang, S. Y., Jang, E. H., Jung, B., Cho, I. H., Park, M. J., et al. (2015). Shikonin as an inhibitor of the LPS-induced epithelial-to-mesenchymal transition in human breast cancer cells. Int. J. Mol. Med. 36 (6), 1601–1606. doi:10.3892/ijmm.2015.2373
Hsu, L. H., Chu, N. M., Lin, Y. F., and Kao, S. H. (2019). G-protein coupled estrogen receptor in breast cancer. Int. J. Mol. Sci. 20 (2), 306. doi:10.3390/ijms20020306
Hsu, S. K., Chang, W. T., Lin, I. L., Chen, Y. F., Padalwar, N. B., Cheng, K. C., et al. (2020). The role of necroptosis in ROS-mediated cancer therapies and its promising applications. Cancers (Basel) 12 (8), 2185. doi:10.3390/cancers12082185
Hu, K., Li, X., Tan, Z., and Shi, Y. (2024). Simple ROS-responsive micelles loaded Shikonin for efficient ovarian cancer targeting therapy by disrupting intracellular redox homeostasis. Eur. J. Pharm. Biopharm. 204, 114525. doi:10.1016/j.ejpb.2024.114525
Huang, C., and Hu, G. (2018). Shikonin suppresses proliferation and induces apoptosis in endometrioid endometrial cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling pathway. Biosci. Rep. 38 (2), BSR20171546. doi:10.1042/BSR20171546
Idris, M., Purnomo, A. S., Martak, F., and Fatmawati, S. (2024). “Phytochemical screening and cytotoxicity of Melastomamalabathricum L. leaves extracts against MCF-7, HeLa, A549, B16, and HT29 cells,” in AIP conference proceedings (Vol. 3071, No. 1). Surabaya, Indonesia: AIP Publishing. doi:10.1063/5.0207722
Jang, S. Y., Lee, J. K., Jang, E. H., Jeong, S. Y., and Kim, J. H. (2014). Shikonin blocks migration and invasion of human breast cancer cells through inhibition of matrix metalloproteinase-9 activation. Oncol. Rep. 31 (6), 2827–2833. doi:10.3892/or.2014.3159
Jia, H., Truica, C. I., Wang, B., Wang, Y., Ren, X., Harvey, H. A., et al. (2017). Immunotherapy for triple-negative breast cancer: existing challenges and exciting prospects. Drug Resist. Updat. 32, 1–15. doi:10.1016/j.drup.2017.07.002
Jiao, P., DU, Q., Li, N., and Wang, J. (2024). Effect of Shikonin on proliferation and apoptosis of ovarian cancer cells# br## br. J. Med. Mol. Biol. 21 (5), 475–480. doi:10.3870/j.issn.1672-8009.2024.05.014
Kaveh, F., Baumbusch, L. O., Nebdal, D., Børresen-Dale, A. L., Lingjærde, O. C., Edvardsen, H., et al. (2016). A systematic comparison of copy number alterations in four types of female cancer. BMC Cancer 16, 913–915. doi:10.1186/s12885-016-2899-4
Ke, L. N., Kong, L. Q., Xu, H. H., Chen, Q. H., Dong, Y., Li, B., et al. (2022). Research progress on structure and anti-gynecological malignant tumor of shikonin. Front. Chem. 10, 935894. doi:10.3389/fchem.2022.935894
Kim, R., and Kin, T. (2021). Current and future therapies for immunogenic cell death and related molecules to potentially cure primary breast cancer. Cancers (Basel) 13 (19), 4756. doi:10.3390/cancers13194756
Klaunig, J. E. (2018). Oxidative stress and cancer. Curr. Pharm. Des. 24 (40), 4771–4778. doi:10.2174/1381612825666190215121712
Kruger, S., Elmageed, Z. Y. A., Hawke, D. H., Wörner, P. M., Jansen, D. A., Abdel-Mageed, A. B., et al. (2014). Molecular characterization of exosome-like vesicles from breast cancer cells. BMC Cancer 14, 44–10. doi:10.1186/1471-2407-14-44
Kumari, L., Mishra, L., Patel, P., Sharma, N., Gupta, G. D., and Kurmi, B. D. (2023). Emerging targeted therapeutic strategies for the treatment of triple-negative breast cancer. J. Drug Target. 31 (9), 889–907. doi:10.1080/1061186X.2023.2245579
Lee, C. Y., Chen, P. N., Kao, S. H., Wu, H. H., Hsiao, Y. H., Huang, T. Y., et al. (2024). Deoxyshikonin triggers apoptosis in cervical cancer cells through p38 MAPK-mediated caspase activation. Environ. Toxicol. 39 (9), 4308–4317. doi:10.1002/tox.24323
Li, J., Gong, X., Jiang, R., Lin, D., Zhou, T., Zhang, A., Li, H., et al. (2018). Fisetin inhibited growth and metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via PTEN/Akt/GSK3β signal pathway. Front. Pharmacol. 31 (9), 772. doi:10.3389/fphar.2018.00772
Li, J., Zhao, M., Liang, W., Wu, S., Wang, Z., and Wang, D. (2022). Codelivery of Shikonin and siTGF-β for enhanced triple negative breast cancer chemo-immunotherapy. J. Control. Release 342, 308–320. doi:10.1016/j.jconrel.2022.01.015
Li, S., Wang, Q., Li, Z., Zhang, J., Jiang, X., Liu, S., et al. (2025). Glutathione-responsive polypeptide nanogel encapsulates Shikonin for breast cancer therapy. Nanomedicine Nanotechnol. Biol. Med. 64, 102802. doi:10.1016/j.nano.2025.102802
Li, Z., Xu, W., Yang, J., Wang, J., Wang, J., Zhu, G., et al. (2022). A tumor microenvironments-adapted polypeptide hydrogel/nanogel composite boosts antitumor molecularly targeted inhibition and immunoactivation. Adv. Mater. 34 (21), 2200449. doi:10.1002/adma.202200449
Liang, J., Tian, X., Zhou, M., Yan, F., Fan, J., Qin, Y., et al. (2024). Shikonin and chitosan-silver nanoparticles synergize against triple-negative breast cancer through RIPK3-triggered necroptotic immunogenic cell death. Biomaterials 309, 122608. doi:10.1016/j.biomaterials.2024.122608
Lin, H. Y., Han, H. W., Sun, W. X., Yang, Y. S., Tang, C. Y., Lu, G. H., et al. (2018). Design and characterization of α-lipoic acyl shikonin ester twin drugs as tubulin and PDK1 dual inhibitors. Eur. J. Med. Chem. 144, 137–150. doi:10.1016/j.ejmech.2017.12.019
Lin, K. H., Huang, M. Y., Cheng, W. C., Wang, S. C., Fang, S. H., Tu, H. P., et al. (2018). RNA-seq transcriptome analysis of breast cancer cell lines under shikonin treatment. Sci. Rep. 8 (1), 2672. doi:10.1038/s41598-018-21065-x
Liu, Y., Chen, L., Shi, Q., Zhao, Q., and Ma, H. (2021). Tumor microenvironment–responsive polypeptide nanogels for controlled antitumor drug delivery. Front. Pharmacol. 12, 748102. doi:10.3389/fphar.2021.748102
Liu, Y., Hu, Y., Xue, J., Li, J., Yi, J., Bu, J., et al. (2023). Advances in immunotherapy for triple-negative breast cancer. Mol. Cancer 22 (1), 145. doi:10.1186/s12943-023-01850-7
Lowry, M. C., Gallagher, W. M., and O'Driscoll, L. (2015). The role of exosomes in breast cancer. Clin. Chem. 61 (12), 1457–1465. doi:10.1373/clinchem.2015.240028
Lu, C., Zhang, Z., Fan, Y., Wang, X., Qian, J., and Bian, Z. (2024). Shikonin induces ferroptosis in osteosarcomas through the mitochondrial ROS-regulated HIF-1α/HO-1 axis. Phytomedicine 135, 156139. doi:10.1016/j.phymed.2024.156139
Lu, D., Qian, J., Li, W., Feng, Q., Pan, S., and Zhang, S. (2015). β-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol. Lett. 10 (6), 3434–3442. doi:10.3892/ol.2015.3769
Lu, J. J., Bao, J. L., Wu, G. S., Xu, W. S., Huang, M. Q., Chen, X. P., et al. (2013). Quinones derived from plant secondary metabolites as anti-cancer agents. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chemistry-Anti-Cancer Agents 13 (3), 456–463. doi:10.2174/187152013804910389
Ma, S. X., Tang, L. B., Chen, Z. H., Wei, M. L., Tang, Z. J., Zheng, Y. H., et al. (2021). Effects of shikonin on the development of ovarian follicles and female germline stem cells. J. Int. Med. Res. 49 (7), 03000605211029461. doi:10.1177/03000605211029461
Manek, R., Pakzamir, E., Mhawech-Fauceglia, P., Pejovic, T., Sowter, H., Gayther, S. A., et al. (2016). Targeting Src in endometriosis-associated ovarian cancer. Oncogenesis 5 (8), e251. doi:10.1038/oncsis.2016.54
Matthaiou, E. I., Barar, J., Sandaltzopoulos, R., Li, C., Coukos, G., and Omidi, Y. (2014). Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int. J. nanomedicine 9, 1855–1870. doi:10.2147/IJN.S51880
Matthaiou, E. I., Guo, Y., Barar, J., Sandaltzopoulos, R., Kandalaft, L. E., Li, C., et al. (2021). TEM1-targeting PEGylated PLGA shikonin nanoformulation for immunomodulation and eradication of ovarian cancer. BioImpacts BI 12 (1), 65–86. doi:10.34172/bi.2021.23511
Meng, L., Ren, J., Liu, Z., and Zhao, Y. (2022). Hyaluronic acid-coated shikonin liposomes for the treatment of triple-negative breast cancer via targeting tumor cells and amplification of oxidative stress. J. Drug Deliv. Sci. Technol. 70, 103193. doi:10.1016/j.jddst.2022.103193
Meng, L., Zhang, C., and Yu, P. (2024). Treating cancer through modulating exosomal protein loading and function: the prospects of natural products and traditional Chinese medicine. Pharmacol. Res. 203, 107179. doi:10.1016/j.phrs.2024.107179
Mishra, P. S., Mishra, R., Patil, V. M., and &Dewangan, S. (2024). Role of natural secondary metabolites as HIF-1 inhibitors in cancer therapy. Med. Chem. Res. 33 (5), 721–734. doi:10.1007/s00044-024-03219-x
Neophytou, C., Boutsikos, P., and &Papageorgis, P. (2018). Molecular mechanisms and emerging therapeutic targets of triple-negative breast cancer metastasis. Front. Oncol. 8, 31. doi:10.3389/fonc.2018.00031
Ni, M., Zhou, J., Zhu, Z., Xu, Q., Yin, Z., Wang, Y., et al. (2023). Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe2+ accumulation. Phytomedicine 112, 154701. doi:10.1016/j.phymed.2023.154701
Olatunde, O. Z., Yong, J., Lu, C., and Ming, Y. (2024). A review on shikonin and its derivatives as potent anticancer agents targeted against topoisomerases. Curr. Med. Chem. 31 (8), 920–937. doi:10.2174/0929867330666230208094828
Parise, C. A., Bauer, K. R., Brown, M. M., and Caggiano, V. (2009). Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. breast J. 15 (6), 593–602. doi:10.1111/j.1524-4741.2009.00822.x
Peng, J., Hu, X., Fan, S., Zhou, J., Ren, S., Sun, R., et al. (2022). Inhibition of mitochondrial biosynthesis using a “right-side-out” membrane-camouflaged micelle to facilitate the therapeutic effects of shikonin on triple-negative breast cancer. Adv. Healthc. Mater. 11 (18), 2200742. doi:10.1002/adhm.202200742
Pérez-Herrero, E., and Fernández-Medarde, A. (2015). Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 93, 52–79. doi:10.1016/j.ejpb.2015.03.018
Perinelli, D. R., Cespi, M., Bonacucina, G., and Palmieri, G. F. (2019). PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid)(PLGA) copolymers for the design of drug delivery systems. J. Pharm. investigation 49, 443–458. doi:10.1007/s40005-019-00442-2
Rajasekar, S., Park, C., Park, S., Park, Y. H., Kim, S. T., Choi, Y. H., et al. (2012). In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J. Ethnopharmacol. 144 (2), 335–345. doi:10.1016/j.jep.2012.09.017
Ranasinghe, R., Mathai, M. L., and Zulli, A. (2022). Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon 8 (9), e10608. doi:10.1016/j.heliyon.2022.e10608
Shahsavari, Z., Karami-Tehrani, F., and Salami, S. (2015). Shikonin induced necroptosis via reactive oxygen species in the T-47D breast cancer cell line. Asian Pac. J. Cancer Prev. 16 (16), 7261–7266. doi:10.7314/apjcp.2015.16.16.7261
Shahsavari, Z., Karami-Tehrani, F., and Salami, S. (2018). Targeting cell necroptosis and apoptosis induced by shikonin via receptor interacting protein kinases in estrogen receptor positive breast cancer cell line, MCF-7. Anti-Cancer Agents Med. Chemistry-Anti-Cancer Agents 18 (2), 245–254. doi:10.2174/1871520617666170919164055
Shahsavari, Z., Karami-Tehrani, F., Salami, S., and Ghasemzadeh, M. (2016). RIP1K and RIP3K provoked by shikonin induce cell cycle arrest in the triple negative breast cancer cell line, MDA-MB-468: necroptosis as a desperate programmed suicide pathway. Tumor Biol. 37, 4479–4491. doi:10.1007/s13277-015-4258-5
Shilnikova, K., Piao, M. J., Kang, K. A., Ryu, Y. S., Park, J. E., Hyun, Y. J., et al. (2018). Shikonin induces mitochondria-mediated apoptosis and attenuates epithelial-mesenchymal transition in cisplatin-resistant human ovarian cancer cells. Oncol. Lett. 15 (4), 5417–5424. doi:10.3892/ol.2018.8065
Shukla, L. I., Vardhan, P. V., Devika, T. K., Roy, S., and &Bhatacharya, S. (2021). “Generation of plant mutant lines using gamma radiation with enhanced secondary metabolite contents,” in Biotechnological approaches to enhance plant secondary metabolites (Boca Raton, FL, USA: CRC Press), 27–48.
Smolarz, B., Nowak, A. Z., and Romanowicz, H. (2022). Breast cancer—epidemiology, classification, pathogenesis and treatment (review of literature). Cancers (Basel) 14 (10), 2569. doi:10.3390/cancers14102569
Song, M., Cui, M., and Liu, K. (2022). Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 232, 114205. doi:10.1016/j.ejmech.2022.114205
Stopeck, A. T., Brown-Glaberman, U., Wong, H. Y., Park, B. H., Barnato, S. E., Gradishar, W. J., et al. (2012). The role of targeted therapy and biomarkers in breast cancer treatment. Clin. Exp. metastasis 29, 807–819. doi:10.1007/s10585-012-9496-y
Su, Y., Huang, N., Chen, D., Zhang, L., Dong, X., Sun, Y., et al. (2017). Successful in vivo hyperthermal therapy toward breast cancer by Chinese medicine shikonin-loaded thermosensitive micelle. Int. J. Nanomedicine 12, 4019–4035. doi:10.2147/IJN.S132639
Sun, H., Xu, H., Zhang, A., Wang, P., Han, Y., and Wang, X. (2014). In vitro anticancer activity of acetylshikonin action against cervical cancer. Plant Sci. Today 1, 39–45. doi:10.14719/pst.2014.1.2.15
Tabari, A. R., Gavidel, P., Sabouni, F., and &Gardaneh, M. (2022). Synergy between sublethal doses of shikonin and metformin fully inhibits breast cancer cell migration and reverses epithelial-mesenchymal transition. Mol. Biol. Rep. 49 (6), 4307–4319. doi:10.1007/s11033-022-07265-9
Tang, Q., Liu, L., Zhang, H., Xiao, J., and Hann, S. S. (2020). Regulations of miR-183-5p and snail-mediated shikonin-reduced epithelial-mesenchymal transition in cervical cancer cells. Drug Des. Dev. Ther. 14, 577–589. doi:10.2147/DDDT.S236216
van den Boogaard, W. M., Komninos, D. S., and &Vermeij, W. P. (2022). Chemotherapy side-effects: not all DNA damage is equal. Cancers (Basel) 14 (3), 627. doi:10.3390/cancers14030627
Wang, A., Liu, J., Yang, Y., Chen, Z., Gao, C., Wang, Z., et al. (2021). Shikonin promotes ubiquitination and degradation of cIAP1/2-mediated apoptosis and necrosis in triple negative breast cancer cells. Chin. Med. 16, 16–15. doi:10.1186/s13020-021-00426-1
Wang, F., Mayca Pozo, F., Tian, D., Geng, X., Yao, X., Zhang, Y., et al. (2020). Shikonin inhibits cancer through P21 upregulation and apoptosis induction. Front. Pharmacol. 11, 861. doi:10.3389/fphar.2020.00861
Wang, M., Sun, Y., Gu, R., Tang, Y., Han, G., and Zhao, S. (2024). Shikonin reduces M2 macrophage population in ovarian cancer by repressing exosome production and the exosomal galectin 3-mediated β-catenin activation. J. Ovarian Res. 17 (1), 101. doi:10.1186/s13048-024-01430-3
Wang, W., Wu, Y., Chen, S., Liu, X., He, J., Wang, S., et al. (2021). Shikonin is a novel and selective IMPDH2 inhibitor that target triple-negative breast cancer. Phytotherapy Res. 35 (1), 463–476. doi:10.1002/ptr.6825
Wang, Z., Yin, J., Li, M., Shen, J., Xiao, Z., Zhao, Y., et al. (2019). Combination of shikonin with paclitaxel overcomes multidrug resistance in human ovarian carcinoma cells in a P-gp-independent manner through enhanced ROS generation. Chin. Med. 14, 1–11. doi:10.1186/s13020-019-0231-3
Wei, Y., Li, M., Cui, S., Wang, D., Zhang, C. Y., Zen, K., et al. (2016). Shikonin inhibits the proliferation of human breast cancer cells by reducing tumor-derived exosomes. Molecules 21 (6), 777. doi:10.3390/molecules21060777
Weiner-Gorzel, K., and Murphy, M. (2021). Mitochondrial dynamics, a new therapeutic target for triple negative breast cancer. Biochimica Biophysica Acta (BBA)-Reviews Cancer 1875 (2), 188518. doi:10.1016/j.bbcan.2021.188518
Wen, X., Li, J., Cai, D., Yue, L., Wang, Q., Zhou, L., et al. (2018). Anticancer efficacy of targeted shikonin liposomes modified with RGD in breast cancer cells. Molecules 23 (2), 268. doi:10.3390/molecules23020268
Wright, A. A., Bohlke, K., Armstrong, D. K., Bookman, M. A., Cliby, W. A., Coleman, R. L., et al. (2016). Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 34 (28), 3–15. doi:10.1016/j.ygyno.2016.05.022
Xu, J., Koizumi, K., Liu, M., Mizuno, Y., Suzaki, M., Iitsuka, H., et al. (2019). Shikonin induces an anti-tumor effect on murine mammary cancer via p38-dependent apoptosis. Oncol. Rep. 41 (3), 2020–2026. doi:10.3892/or.2019.6966
Xu, Z., Huang, L., Zhang, T., Liu, Y., Fang, F., Wu, X., et al. (2022). Shikonin inhibits the proliferation of cervical cancer cells via FAK/AKT/GSK3β signalling. Oncol. Lett. 24 (3), 304. doi:10.3892/ol.2022.13424
Yadav, S., Sharma, A., Nayik, G. A., Cooper, R., Bhardwaj, G., Sohal, H. S., et al. (2022). Review of shikonin and derivatives: isolation, chemistry, biosynthesis, pharmacology and toxicology. Front. Pharmacol. 13, 905755. doi:10.3389/fphar.2022.905755
Yang, Y., Gao, W., Tao, S., Wang, Y., Niu, J., Zhao, P., et al. (2019). ER-mediated anti-tumor effects of shikonin on breast cancer. Eur. J. Pharmacol. 863, 172667. doi:10.1016/j.ejphar.2019.172667
Yazaki, K. (2017). Lithospermum erythrorhizon cell cultures: present and future aspects. Plant Biotechnol. 34 (3), 131–142. doi:10.5511/plantbiotechnology.17.0823a
Yu, C., Xing, H., Fu, X., Zhang, Y., Yan, X., Feng, J., et al. (2024). Effect and mechanisms of shikonin on breast cancer cells in vitro and in vivo. BMC Complementary Med. Ther. 24 (1), 389. doi:10.1186/s12906-024-04671-3
Yuan, D. P., Gu, L., Long, J., Chen, J., Ni, J., Qian, N., et al. (2014). Shikonin reduces endometriosis by inhibiting RANTES secretion and mononuclear macrophage chemotaxis. Exp. Ther. Med. 7 (3), 685–690. doi:10.3892/etm.2013.1458
Zhang, C. H., Wang, J., Zhang, L. X., Lu, Y. H., Ji, T. H., Xu, L., et al. (2017). Shikonin reduces tamoxifen resistance through long non-coding RNA uc. 57. Oncotarget 8 (51), 88658–88669. doi:10.18632/oncotarget.20809
Zhang, Q., Zhang, M., and Wang, W. (2022). Construction of shikonin-loaded mammaglobin-modified liposomes for breast cancer targeted therapy. Nano Life 12 (04), 2250010. doi:10.1142/s1793984422500106
Zhang, S., Gao, Q., Li, W., Zhu, L., Shang, Q., Feng, S., et al. (2019). Shikonin inhibits cancer cell cycling by targeting Cdc25s. BMC cancer 19, 20–29. doi:10.1186/s12885-018-5220-x
Zhang, Y., Qian, R. Q., and Li, P. P. (2009). Shikonin, an ingredient of Lithospermum erythrorhizon, down-regulates the expression of steroid sulfatase genes in breast cancer cells. Cancer Lett. 284 (1), 47–54. doi:10.1016/j.canlet.2009.04.008
Zhang, Z., Bai, J., Zeng, Y., Cai, M., Yao, Y., Wu, H., et al. (2020). Pharmacology, toxicity and pharmacokinetics of acetylshikonin: a review. Pharm. Biol. 58 (1), 950–958. doi:10.1080/13880209.2020.1818793
Zhong, W., Shen, Z., Wang, M., Wang, H., Sun, Y., Tao, X., et al. (2023). Tumor microenvironment responsive nanomicelle with folic acid modification co-delivery of doxorubicin/shikonin for triple negative breast cancer treatment. Pharmaceuticals 16 (3), 374. doi:10.3390/ph16030374
Zhou, S., Li, D., Xiao, D., Wu, T., Hu, X., Zhang, Y., et al. (2022). Inhibition of PKM2 enhances sensitivity of olaparib to ovarian cancer cells and induces DNA damage. Int. J. Biol. Sci. 18 (4), 1555–1568. doi:10.7150/ijbs.62947
Glossary
SK Shikonin
ERK Extracellular Signal-Regulated Kinas
ROS Reactive oxygen species
PKM2 Pyruvate kinase
EMT Epithelial-mesenchymal transition
β-HIVS β-hydroxyisovaleryl-shikonin
GAL3 Galectin-3
FAK Focal Adhesion Kinase
GPX4 Glutathione peroxidase 4
HMOX1 Heme oxygenase 1
HA-PBAP hyaluronic acid-phenylboronic acid pinacol ester conjugation
PKM2 Pyruvate Kinase M2
HR Homologous recombination
Ola Olaparib
BRCA1 Breast Cancer 1, Early Onset
STS Steroid sulfatase
CRT Calreticulin protein
IMPDH2 5′-monophosphate dehydrogenase 2
PDK1 3-phosphoinositide-dependent protein kinase 1
PTEN Phosphatase and tensin homolog
ICD Immunogenic cell death (ICD)
TGF-β Transforming growth factor-beta
STN Shikonin-loaded thermosensitive nanomicelle
TNBC Triple-Negative Breast Cancer
POLG Polymerase gamma
TPP Triphenylphosphine
MGB-SK-LPs Mammaglobin-modified liposomes loaded with shikonin
DOX Doxorubicin
DUSP Dual specificity phosphatase
Keywords: shikonin, female cancer, anticancer, phytochemical, naphthoquinone
Citation: Pandey P, Lakhanpal S, Jamuna KV, Singh A, Abohassan M, Park MN, Shin S-W, Kang HN, Zahera M, Saeed M, Khan F and Kim B (2025) Review projecting shikonin as a therapeutic candidate in female carcinomas: a preclinical perspective. Front. Pharmacol. 16:1627124. doi: 10.3389/fphar.2025.1627124
Received: 12 May 2025; Accepted: 24 June 2025;
Published: 04 July 2025.
Edited by:
Johnson O. Oladele, Royal Scientific Research Institute, NigeriaReviewed by:
Leena Dhoble, University of Florida, United StatesOluwaseyi Emilola Okoro, Ministry of Health and Wellness, Jamaica
Copyright © 2025 Pandey, Lakhanpal, Jamuna, Singh, Abohassan, Park, Shin, Kang, Zahera, Saeed, Khan and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahad Khan, ZmFoYWRpbnRlZ3JhbGlhbkBnbWFpbC5jb20=; Bonglee Kim, Ym9uZ2xlZWtpbUBraHUuYWMua3I=
 Pratibha Pandey
Pratibha Pandey Sorabh Lakhanpal2
Sorabh Lakhanpal2 Moon Nyeo Park
Moon Nyeo Park Han Na Kang
Han Na Kang Manaal Zahera
Manaal Zahera Mohd Saeed
Mohd Saeed Fahad Khan
Fahad Khan Bonglee Kim
Bonglee Kim