- 1Genomic Medicine Laboratory UILDM, IRCCS Santa Lucia Foundation, Rome, Italy
- 2Neuropsychiatry Laboratory, Department of Clinical Neuroscience and Neurorehabilitation, IRCCS Santa Lucia Foundation, Rome, Italy
- 3Department of Clinical and Behavioral Neurology, IRCCS Fondazione Santa Lucia, Rome, Italy
- 4Department of Biomedicine and Prevention, Tor Vergata University, Rome, Italy
The integration of pharmacogenetics into personalized medicine enables the optimization of drug selection and dosage, maximizing therapeutic benefits while minimizing the risk of adverse drug reactions. The association between APOE alleles and ARIA, a known adverse reaction in Alzheimer’s disease patients treated with anti-amyloid monoclonal antibodies, has led to the inclusion of APOE genotyping among conventional pharmacogenetic tests. Given the dual role of APOE alleles, the widespread implementation of this genetic test requires caution and should be accompanied by appropriate genetic counselling. APOE genotyping is uniquely positioned at the intersection of pharmacogenetics and germline testing: it provides insight not only into drug safety (specifically the risk of Amyloid-Related Imaging Abnormalities) but also into familial risk for developing Alzheimer’s disease. Carriers of risk alleles, especially homozygotes, face the highest risk and require close monitoring. While APOE genotyping can inform treatment decisions, it also raises ethical concerns due to the broader implications of disclosing genetic risk information for neurodegenerative diseases. Identifying a high-risk APOE genotype in a patient substantially impacts family members. Therefore, patients considered for treatment with anti-amyloid monoclonal antibodies should receive comprehensive pre- and post-test genetic counseling that goes beyond traditional standards, as currently provided for other peculiar tests. Such counseling ensures that patients are adequately informed about potential outcomes, psychological impacts, and familial implications. It also supports ethical decision-making and facilitates truly informed consent, helping to prevent deterministic or overly simplistic interpretations of genetic risk.
1 Introduction
Pharmacogenetics (PGt), the study of how genes affect a person’s response to drugs, is a cornerstone of personalized medicine. Coined in 1959, the term “pharmacogenetics” describes the association between genetic variations and drug response (Vogel, 1959). Actually distinguished in pharmacogenetics (PGt) and pharmacogenomics (PGx), which respectively involve the study of variations in DNA sequence and in DNA/RNA characteristics as they relate to drug response (efficacy, toxicity, adverse drug reactions, etc.) (EMA–European Medicines Agency, 2007). By examining individual genetic variants, PGx aims to tailor medical treatments, optimizing drug selection and dosage to maximize therapeutic benefits while minimizing the risk of adverse drug reactions. The convergence of genetic insights with pharmacological principles holds promise for transforming healthcare by enabling more precise and effective therapeutic interventions (Auwerx et al., 2022; Moyer and Black, 2025).
The Clinical Pharmacogenetics Implementation Consortium (CPIC) reports more than 570 gene-drug interactions, involving 121 genes and 300 drugs (CPIC, 2025). Of these, over 170 drug-gene pairs have prescription guidelines included on FDA labels. Given the widespread use of these drugs and the prevalence of the associated genetic variants, it is estimated that at least one-third of the global population has been exposed to at least one prescription medication with a pharmacogenetic indication (Chanfreau-Coffinier et al., 2019; Auwerx et al., 2022). For these reasons, it has been proposed that pre-emptive pharmacogenetic testing should be implemented to integrate pharmacogenetics in primary care widely (Bryan et al., 2024). While the PGx implementation can significantly advance primary care, these tests must be proposed with adequate pre- and post-test genetic counselling. Recent studies underscore the importance of comprehensive pre-test education and counseling in the context of PGx testing (Allen et al., 2022). Ensuring that patients are well-informed before testing empowers them to make thoughtful, personalized decisions about whether or not to proceed with testing. Additionally, pre-test education enhances their ability to interpret the results accurately and engage in meaningful conversations with their healthcare providers. This collaborative understanding is essential for incorporating PGx insights into medication decisions, ultimately supporting safer, more effective, and individualized treatment strategies (Bagautdinova et al., 2022). While APOE genotyping can inform treatment decisions, it raises ethical concerns due to the broader implications of disclosing genetic risk information for neurodegenerative diseases. Identifying a high-risk APOE genotype in a patient substantially impacts family members. Therefore, patients considered for treatment with anti-amyloid monoclonal antibodies should receive comprehensive pre- and post-test genetic counseling that goes beyond traditional standards (Bagautdinova et al., 2022), as currently provided for other peculiar tests (Pinto et al., 2016).
2 Monoclonal antibodies in Alzheimer’s disease treatment
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common cause of dementia (Valdez-Gaxiola et al., 2024). Characterized by a gradual decline in cognitive abilities—including memory, language, attention, and executive functioning—the disease ultimately results in a significant loss of independence. The key neuropathological features of AD include the accumulation of extracellular amyloid-beta (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein in the brain. For many years, treatment strategies primarily aimed at managing symptoms through cholinesterase inhibitors and NMDA antagonists, providing only limited and temporary relief without addressing the underlying disease pathology (Hales, 2025). The amyloid hypothesis proposes that the accumulation of Aβ peptides in the brain activates a cascade of events leading to neuronal dysfunction, formation of neurofibrillary tangles, neuronal loss and ultimately, cognitive decline (Hales, 2025; Greenberg et al., 2025). The recent approval of several monoclonal antibodies (mAbs) targeting amyloid-beta marks a significant shift in the therapeutic landscape, offering a more direct approach to modifying the disease’s progression (Rentz et al., 2024; van Dyck, 2018). Key mAbs that have been approved or are under investigation to treat AD are Aducanumab (Aduhelm®), Lecanemab (Leqembi®), and Donanemab (Kisunla®) (Wu et al., 2023; Kim et al., 2025). While these therapies offer potential benefits, they also present challenges, particularly the risk of Amyloid-Related Imaging Abnormalities (ARIA), which appear as changes on brain MRI scans. Genetic factors, especially the apolipoprotein E (APOE) genotype, play a significant role in disease progression, treatment response, and the likelihood of ARIA (Greenberg et al., 2025). ARIA typically presents in two main forms: ARIA-E (edema/effusion) and ARIA-H (hemorrhage/hemosiderin deposition). Usually asymptomatic, both ARIA-E and ARIA-H can be revealed by evaluation of Magnetic Resonance Imaging (MRI) scans (van Dyck, 2018; Hales, 2025; Greenberg et al., 2025; Weidauer and Hattingen, 2025).
The incidence of ARIA varies among the different mAbs approved for AD (Kim et al., 2025; Zimmer et al., 2025; Budd Haeberlein et al., 2022; Filippi et al., 2022; van Dyck et al., 2023; Sims et al., 2023).
Several risk factors can modify the likelihood of developing ARIA during treatment with these mAbs. A consistent finding across clinical trials of anti-amyloid mAbs is the significant influence of the APOE genotype on the risk and severity of ARIA (Jessen et al., 2024; Jackson et al., 2024; Cummings, 2023), with a gene-dose effect of the APOEε4 allele (Foley and Wilcock, 2024): homozygotes for APOEε4 (carrying two copies) are at the highest risk, followed by heterozygotes (carrying one copy), while non-carriers have the lowest risk for both ARIA-E and ARIA-H (Hales, 2025; Salloway et al., 2022) (Table 1).
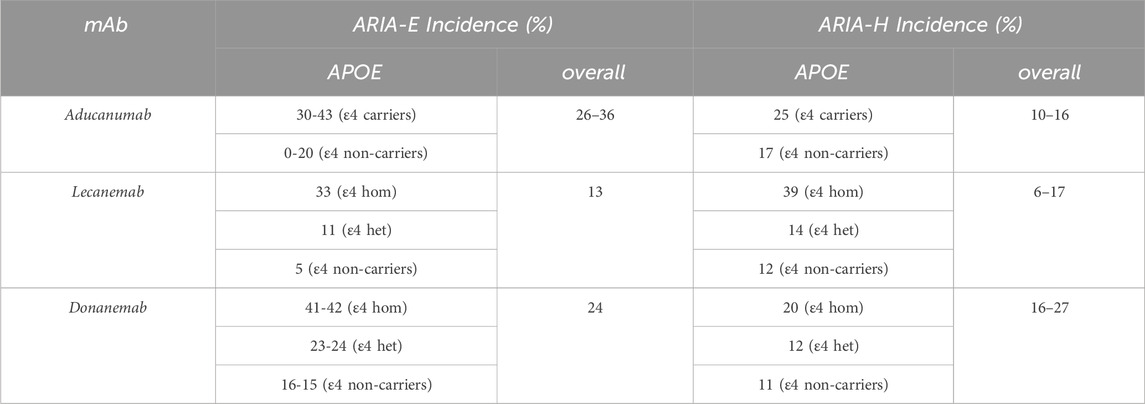
Table 1. Comparison of safety profiles (Filippi et al., 2022; Zimmer et al., 2025).
The increased risk of ARIA in APOEε4 carriers is thought to be due to several factors, including a dysfunction of the neurovascular unit and the consequent dysregulation of the blood-brain barrier. Furthermore, APOEε4 promotes brain inflammation, showing a peculiar neuroinflammatory profile through reactive microglia and proinflammatory cytokine expression. Moreover, APOEε4 is a well-known genetic risk factor for Cerebral Amyloid Angiopathy (CAA), and Aβ plaques formation, increasing neuroinflammation levels that lead to disruptions in the blood-brain barrier (Foley and Wilcock, 2024; Greenberg et al., 2020).
The role of the APOEε2 allele in influencing ARIA risk is less consistently reported than APOEε4. Some studies reported that APOEε2 is associated with specific vasculopathic changes that may lead to vessel rupture, while APOEε4 facilitates vascular amyloid deposition (Greenberg et al., 2025; Lozupone et al., 2025). In this scenario, CAA seems more severe in APOEε2 carriers when compared with APOEε3 homozygotes, but this relationship appears inconsistent among different studies (Nelson et al., 2013; Serrano-Pozo et al., 2015).
Other factors that can increase ARIA risk include higher drug dosages, older age, the presence of pre-existing microhemorrhages on baseline MRI, a history of prior strokes, the use of antithrombotic medications, increased amyloid burden, advanced cerebrovascular disease, and a history of transient ischemic attacks or seizures. The strong association between the APOEε4 allele and ARIA suggests a fundamental connection between the genetic predisposition to AD and the brain’s response to amyloid-clearing therapies, possibly related to the extent of vascular amyloid deposition. Moreover, it lays the basis for a new application of the APOE genotyping test that can be used as a pharmacogenetic test in people candidates for mAb therapy (Cummings, 2023).
Current guidelines and recommendations for monitoring and managing ARIA in patients receiving Alzheimer’s mAbs treatments emphasize a multi-faceted approach that includes pre-treatment assessment, regular monitoring during treatment, and specific management strategies based on the characteristics of ARIA and the patient’s clinical status. Because ARIA often presents without symptoms, regular MRI monitoring is performed during treatment with mAbs (Hales, 2025; Jessen et al., 2024). Moreover, artificial intelligence tools have been developed to assist clinicians in the identification of ARIA, improving the safety of mAbs therapies (Hales, 2025; Sima et al., 2024). In the pre-treatment assessment of ARIA risks, the characterization of the APOE genotype plays a significant role (Hales, 2025; Jessen et al., 2024). FDA-approved labels for aducanumab, lecanemab, and donanemab reported the suggestion to genotype APOE before initiating the treatment (FDA–U.S. Food and Drug Administration, 2021; FDA–U.S. Food and Drug Administration, 2023; FDA–U.S. Food and Drug Administration, 2024). While APOEε4 status is a significant risk factor, the recommended monitoring schedules are generally the same for all APOE genotypes, although clinicians may exercise more caution with higher-risk individuals.
3 APOE is a non-conventional pharmacogenomic test
Personalized medicine uses PGx data to select therapeutic strategies for the right patient at the right time. The reference clinician performs the pre-test counselling for most PGx tests, not necessarily a geneticist, based on generic guidelines (Bagautdinova et al., 2022). The recent diffusion of some peculiar PGx tests, such as the BRCA test for predicting response to PARP inhibitors, highlights counseling responsibilities (provide appropriate information on genetic aspects, familial risks, etc.) (Pinto et al., 2016). Unlike other PGx tests, APOE genotyping has peculiar implications and should be performed carefully. Firstly, some APOE genotypes are associated with a different risk of disease and an increased frequency of ARIA. Secondarily, APOE alleles can be inherited, and the associated risk of AD can be reflected in family members. For these reasons, the APOE genetic test should be considered not only a PGx test, but also a germline genetic test: it should be considered the same way as conventional germline genetic tests. Germline genetic testing is widely recognized as distinct from other diagnostic and prognostic procedures due to a unique characteristic: the significance of the information it provides for the individual and their offspring, relatives, and extended family. This distinctive aspect, known as genetic exceptionalism, has led to the development of various protocols to ensure the responsible integration of genetic testing into clinical practice (Lorenzo et al., 2023).
The APOE gene on chromosome 19 has three main alleles: ε2, ε3, and ε4. Each individual inherits one APOE allele from each parent, resulting in six possible genotype combinations: ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, and ε4/ε4. These different alleles produce slightly different forms of the ApoE protein, which have varying effects on cholesterol metabolism (Blanchard et al., 2022; Cho et al., 2020) and are implicated in the development and progression of AD (Abondio et al., 2019; Jia et al., 2020; Reiman et al., 2025).
As expected, allele frequency varies among populations. The APOEε2 allele is the least common of the three, found in approximately 5%–10% of the European population (Kolbe et al., 2023), and is associated with a reduced risk of developing AD. It is possible that an individual with the ε2 allele develops AD, in this case the disease tends to manifest later in life (Valdez-Gaxiola et al., 2024; Corder et al., 1994; Serrano-Pozo et al., 2015; Jia et al., 2020). The APOEε3 is the most prevalent, found in around 74%–88% of the European population (Kolbe et al., 2023) and is generally considered to have a neutral effect on the risk of developing AD (Valdez-Gaxiola et al., 2024; Jia et al., 2020). The APOEε4 allele is more common than APOε2, present in approximately 6%–22% of the European population (Kolbe et al., 2023). It is recognized as the strongest genetic risk factor for late-onset AD, significantly increasing the likelihood of developing the disease and often associated with an earlier age of onset (Jia et al., 2020). The lifetime risk (up to 85 years) for mild cognitive impairment (MCI) or AD has been estimated as 30%–55% for APOEε4 homozygotes, 20%–25% for heterozygous APOEε3/APOEε4, and 10%–15% for APOEε3 homozygotes (Jackson et al., 2024). Interestingly, the risk associated with the APOEε4 allele is age-dependent: AD risk increases progressively with age and subsequently declines after 70–79 years in individuals with a family history of AD, and after 65–74 years in those without a history (Jia et al., 2020). APOEε4 is also associated with a more significant accumulation of amyloid-beta deposits in the brain than non-carriers (Jackson et al., 2024). Furthermore, it has been suggested that the ApoE4 protein might have toxic effects beyond its role in amyloid processing, such as an increased response to stress or injury in the brain (Mahley and Huang, 2012). Understanding that the APOE genotype is a risk factor, not a deterministic gene for AD is crucial. Carrying the APOEε4 allele increases susceptibility but does not guarantee that an individual will develop AD. Conversely, individuals without the APOEε4 allele can still develop the disease (Valdez-Gaxiola et al., 2024; Cho et al., 2020). In this context, the APOE test can be utilized on diverse subjects, uncovering various implications: in patients with a family history of AD, the APOE test (along with an analysis of other AD genes such as PSEN1, PSEN2, and APP) can help estimate the recurrence risk for AD; in AD patients, candidates for mAbs therapy can predict adverse events (ARIA); in healthy individuals, regardless of a family history of AD, it can indicate their susceptibility to AD. Otherwise, performing the APOE test in AD patients will provide genetic information about first-degree family members who could be obligate carriers of certain APOE alleles.
4 Genetic counselling
As anticipated, defining the APOE genotype has consequences that involve the entire family, not limited to the patient. While almost all PGx tests are performed to determine the dosage or the risk of side effects of treatments, APOE genotyping can also reveal an individual’s heritable AD risk, besides the risk of ARIA when treated with mAbs. Therefore, APOE genetic test should be considered not only a PGx test but also a germline genetic test: the significance of the information it provides for the individual and their offspring, relatives, and extended family (genetic exceptionalism). In this scenario, administering APOE genotyping for therapeutic purposes reveals genetic risks for AD that affect the whole family of the AD patient. Given the well-established association between the APOEε4 allele and an increased risk for AD, it is understandable that some laboratories and healthcare providers express caution regarding the ordering or offering of APOE testing (i.e., in dyslipidemia genetic panels) (Ison et al., 2024). For over 2 decades, clinicians have raised ethical and practical concerns about disclosing such information to patients, particularly considering its limited clinical utility and the potential for psychological distress (Bird, 1995). Indeed, some individuals have described receiving these results as distressing or even traumatic, particularly in the absence of appropriate genetic counseling (Zallen, 2018). Nevertheless, some studies report that most patients can tolerate this information and derive meaningful benefits, such as guiding decisions related to health behavior modifications and financial planning. The Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) studies, which examined psychological responses to APOEε4 genetic test, found that participants experienced minimal long-term psychological harm (Cassidy et al., 2008; Green et al., 2009). In one arm of the study, the disclosure of APOEε4 status as a risk factor for coronary artery disease was also well tolerated (Christensen et al., 2016). Moreover, evidence suggests that learning one’s genotype has motivated some individuals to adopt healthier lifestyles, including improvements in diet and physical activity (Zallen, 2018).
In all cases where APOEε4 status will be disclosed, pre-test genetic counseling is critical, regardless of context (Green et al., 2009). Clinicians and genetic counsellors should engage in thorough discussions with patients, addressing the clinical and psychosocial implications of testing, clarifying motivations for pursuing genetic information (i.e., antiMAbs administration), and ensuring informed consent that encompasses both the potential benefits and limitations of knowing one’s APOE status. An adequate pre-test genetic counselling reduces the risk of anxiety and depression, helping patients deal with possible results, risks associated with one’s genotype, and its heritability. This approach deals with the psychological impact of learning one’s APOE status, improving individual knowledge about it and promoting healthier lifestyles. In this context, comprehensive pre-test genetic counselling is essential to equip individuals and caregivers with the necessary information to make informed decisions about APOE testing and to prepare them for the potential implications of the results. The information provided should be tailored to the individual’s specific situation, motivations for considering testing, and level of understanding. Furthermore, genetic counselors should discuss the ARIA risks associated with APOEε4 status to provide useful information and possible treatment strategies.
In AD patients, the decision to undergo APOE genetic testing involves several critical ethical considerations, each requiring careful attention during the pre-test counselling process. One of the first areas to discuss in genetic counselling is the potential implications for the patient. The genetic counselor ensures that the individual fully understands the purpose of the test, which in this context includes assessing the risk for AD and the potential risk for ARIA if considering treatment with anti-amyloid mAbs (Filippi et al., 2022). The counselling should clearly explain the implications of potential test results, such as an increased or decreased heritable risk for AD and how the APOE genotype might influence eligibility for or management during treatment with these emerging therapies. It is also crucial to emphasize the limitations of APOE testing, particularly its non-diagnostic nature for AD (Mayeux et al., 1998). Individuals must be informed that testing is entirely voluntary, and they have the right to refuse the test. The discussion should also cover the potential benefits of testing, including informing lifestyle choices, facilitating future planning, determining eligibility for clinical trials, and providing a clearer understanding of ARIA risk associated with specific treatments (in Table 2, propositus-specific considerations are listed). Conversely, the potential risks, such as psychological distress and the possibility of genetic discrimination, should also be thoroughly discussed (Bird, 1995; Zallen, 2018). Providing this information in a culturally and linguistically appropriate format is essential to ensure proper understanding and autonomous decision-making (Uhlmann and Roberts, 2018). The complexity surrounding the dual purpose of APOE testing in this context—risk assessment for both the disease and a treatment side effect—adds a significant layer to the informed consent process, requiring comprehensive and tailored explanations. Due to the nature of the AD, it may be necessary that the caregiver and/or family members assist the patient in genetic counselling. In fact, pre-test counselling should also include a discussion about the potential implications of an individual’s APOE results for family members. Explaining the inheritance patterns of APOE alleles can help family members understand their own potential risk. In this scenario, it is essential to consistently emphasize the probabilistic nature of genetic risk, avoiding deterministic language that might wrongly suggest a definitive outcome. In this context, key issues of genetic counseling have to be adapted to these new applications of APOE testing (Goldman et al., 2011; Goldman, 2012; Uhlmann and Roberts, 2018) (Table 2).
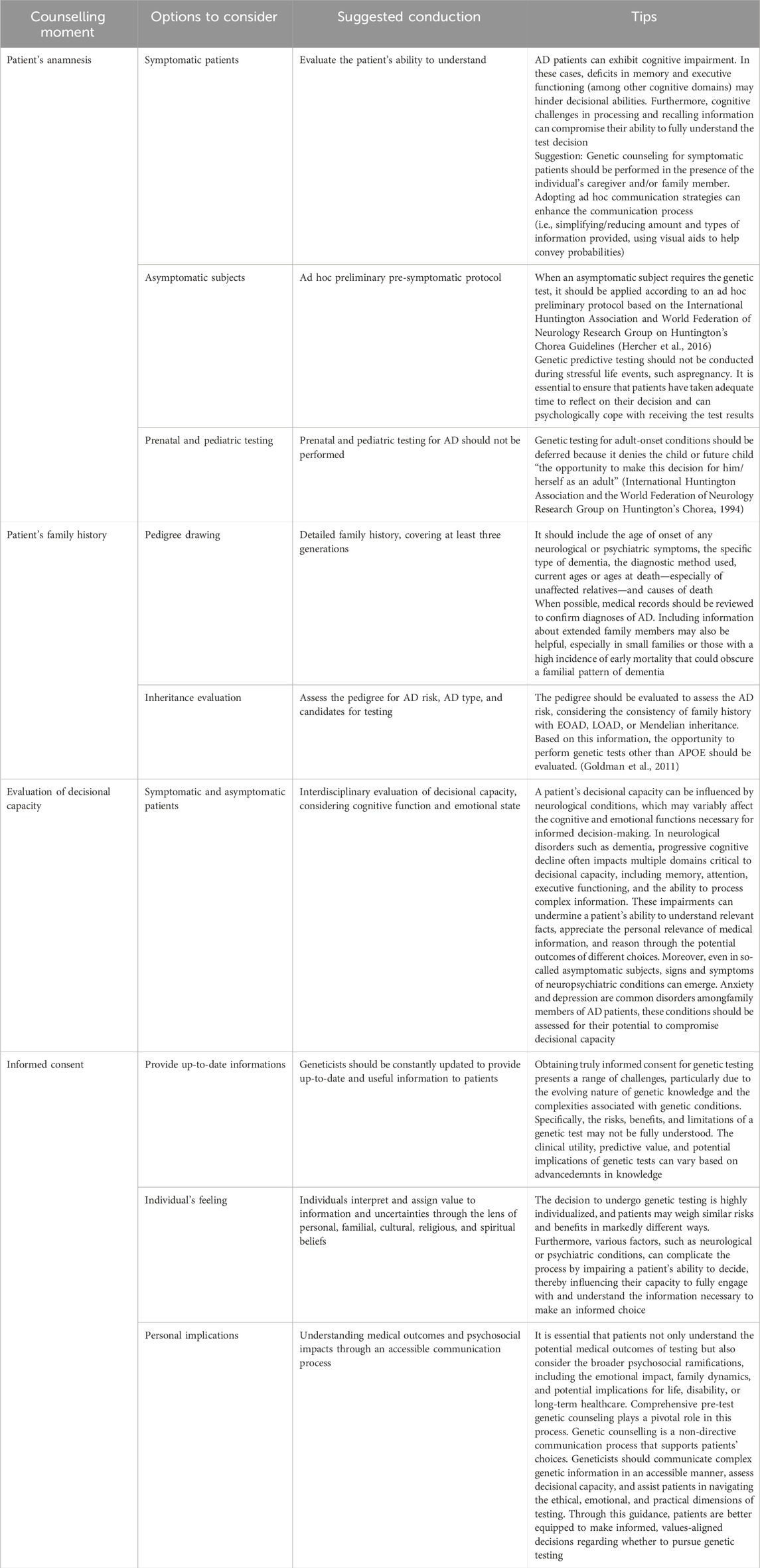
Table 2. Key issues for genetic counseling (Goldman et al., 2011; Goldman, 2012; Uhlmann and Roberts, 2018).
5 Conclusion
The emergence of mAbs targeting amyloid-beta represents a significant advancement in potential AD treatment. Aducanumab, Lecanemab, and Donanemab all function by reducing amyloid plaque burden in the brain, albeit through slightly different mechanisms. Current guidelines for managing patients on these therapies emphasize the importance of pre-treatment assessment, including confirmation of amyloid pathology and APOE genotyping, as well as baseline MRI to identify pre-existing conditions. The APOE genotype plays a dual role in AD: risk factor and PGx test. In this scenario, the APOE genetic test should not be considered only as a PGx test, because it has the potential to harm patients’ health and their whole family. The administration of APOE genetic test should be performed in a dedicated genetic counselling process, as appropriate for neurological late-onset genetic disorders. The growing need to perform APOE analyses on many patients (ideally all patients who are candidates for mAbs) should encourage the establishment of genetic counseling centers specifically trained for these conditions.
The increasing diffusion of telemedicine can support genetic counselling improvements, making the evaluation of several patients who cannot reach the medical center available. Telemedicine allows healthcare professionals to conduct real-time virtual consultations, perform clinical assessments, monitor patient health metrics remotely, and provide individualized treatment recommendations without requiring the patient to be physically present in a healthcare facility (Weiskirchen S. and Weiskirchen R., 2025; Griffith et al., 2024).
The update of clinical protocols and the development of optimized network of medical centers in the territory will improve the diagnosis and the management of AD patients (Marra et al., 2025). In this context, the application of telemedicine to genetic counselling for APOE will promote an adequate administration, ensuring that the ethical standards required for this complex (pharmacogenetic with several secondary implications) genetic test are met. As healthcare systems increasingly adopt telemedicine as a complement to traditional in-person care, its role in promoting health equity, optimizing resource utilization, and improving overall patient outcomes continues to grow.
Author contributions
SZ: Conceptualization, Data curation, Writing – original draft, Writing – review and editing. CrP: Writing – original draft, Writing – review and editing. JF: Writing – original draft, Writing – review and editing. FP: Writing – original draft, Writing – review and editing. ClP: Writing – original draft, Writing – review and editing. CC: Writing – original draft, Writing – review and editing. EG: Conceptualization, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially funded by CNR [A multifactorial intervention for successful aging (MUSA) - fase 3 (FOE 2021-2022-2023)] (cod. CNRTRE cdc 870) and the BIT Foundation (cod. BIT–cdc 860) to SZ. This study was supported by the Ministry of Health (Ricerca Corrente) to CC.
Acknowledgments
This study was supported by the Ministry of Health (Ricerca Corrente).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used to refine the English language in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abondio, P., Sazzini, M., Garagnani, P., Boattini, A., Monti, D., Franceschi, C., et al. (2019). The genetic variability of APOE in different human populations and its implications for longevity. Genes (Basel) 10 (3), 222. doi:10.3390/genes10030222
Allen, J. D., Pittenger, A. L., and Bishop, J. R. (2022). A scoping review of attitudes and experiences with pharmacogenomic testing among patients and the general public: implications for patient counseling. J. Pers. Med. 12 (3), 425. doi:10.3390/jpm12030425
Auwerx, C., Sadler, M. C., Reymond, A., and Kutalik, Z. (2022). From pharmacogenetics to pharmaco-omics: milestones and future directions. HGG Adv. 16 (2), 100100. doi:10.1016/j.xhgg.2022.100100
Bagautdinova, D., Lteif, C., Eddy, E., Terrell, J., Fisher, C. L., and Duarte, J. D. (2022). Patients' perspectives of factors that influence pharmacogenetic testing uptake: enhancing patient counseling and results dissemination. J. Pers. Med. 11 (12), 2046. doi:10.3390/jpm12122046
Bird, T. D. (1995). Apolipoprotein E genotyping in the diagnosis of alzheimer's disease: a cautionary view. Ann. Neurology 38 (1), 2–4. doi:10.1002/ana.410380103
Blanchard, J. W., Akay, L. A., Davila-Velderrain, J., von Maydell, D., Mathys, H., Davidson, S. M., et al. (2022). APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611 (7937), 769–779. doi:10.1038/s41586-022-05439-w
Bryan, E. G., Lunsford, K., Mullis, M. D., McFarlane, A., Elwood, E., Gawronski, B. E., et al. (2024). Enhancing the integration of pre-emptive pharmacogenetic (PGx) testing in primary care: prioritizing underserved patients' preferences in implementation. J. Pers. Med. 29 (12), 1128. doi:10.3390/jpm14121128
Budd Haeberlein, S., Aisen, P. S., Barkhof, F., Chalkias, S., Chen, T., Cohen, S., et al. (2022). Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9 (2), 197–210. doi:10.14283/jpad.2022.30
Cassidy, M. R., Roberts, J. S., Bird, T. D., Steinbart, E. J., Cupples, L. A., Chen, C. A., et al. (2008). Comparing test-specific distress of susceptibility versus deterministic genetic testing for alzheimer's disease. Alzheimer's Dementia 4 (6), 406–413. doi:10.1016/j.jalz.2008.04.007
Chanfreau-Coffinier, C., Hull, L. E., Lynch, J. A., DuVall, S. L., Damrauer, S. M., Cunningham, F. E., et al. (2019). Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US veterans health administration pharmacy users. JAMA Netw. Open 5 (6), e195345. doi:10.1001/jamanetworkopen.2019.5345
Cho, H., Kim, Y. E., Chae, W., Kim, K. W., Kim, J. W., Kim, H. J., et al. (2020). Distribution and clinical impact of apolipoprotein E4 in subjective memory impairment and early mild cognitive impairment. Sci. Rep. 10 (1), 13365. doi:10.1038/s41598-020-69603-w
Christensen, K. D., Roberts, J. S., Whitehouse, P. J., Royal, C. D., Obisesan, T. O., Cupples, L. A., et al. (2016). Disclosing pleiotropic effects during genetic risk assessment for Alzheimer disease: a randomized trial. Ann. Intern. Med. 164 (3), 155–163. doi:10.7326/m15-0187
Corder, E. H., Saunders, A. M., Risch, N. J., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., et al. (1994). Protective effect of apolipoprotein E type 2 allele for late onset alzheimer disease. Nat. Genet. 7 (2), 180–184. doi:10.1038/ng0694-180
CPIC (Clinical Pharmacogenetics Implementation Consortium) (2025). Available online at: https://cpicpgx.org/genes-drugs/ (Accessed April 10, 2025).
Cummings, J. (2023). Anti-amyloid monoclonal antibodies are transformative treatments that redefine alzheimer's disease therapeutics. Drugs 83 (7), 569–576. doi:10.1007/s40265-023-01858-9
EMA – European Medicines Agency (2007). ICH topic E15 definitions for genomic biomarkers. pharmacogenomics, pharmacogenetics, genomic data sample coding Categ. Available online at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-15-definitions-genomic-biomarkers-pharmacogenomics-pharmacogenetics-genomic-data-and-sample-coding-categories-step-4_en.pdf (Accessed June 03, 2025).
FDA – U.S. Food and Drug Administration (2021). Aducanumab (marketed as aduhelm) information. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s003lbl.pdf (Accessed March 20, 2025).
FDA – U.S. Food and Drug Administration (2023). Drug trials snapshots: LEQEMBI. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269Orig1s001lbl.pdf (Accessed March 20, 2025).
FDA – U.S. Food and Drug Administration (2024). FDA approves treatment for adults with Alzheimer’s disease. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761248s000lbl.pdf (Accessed March 20, 2025).
Filippi, M., Cecchetti, G., Spinelli, E. G., Vezzulli, P., Falini, A., and Agosta, F. (2022). Amyloid-related imaging abnormalities and β-Amyloid-Targeting antibodies: a systematic review. JAMA Neurol. 79 (3), 291–304. doi:10.1001/jamaneurol.2021.5205
Foley, K. E., and Wilcock, D. M. (2024). Three major effects of APOEε4 on Aβ immunotherapy induced ARIA. Front. Aging Neurosci. 16, 1412006. doi:10.3389/fnagi.2024.1412006
Goldman, J. S. (2012). New approaches to genetic counseling and testing for alzheimer's disease and frontotemporal degeneration. Curr. Neurol. Neurosci. Rep. 12 (5), 502–510. doi:10.1007/s11910-012-0296-1
Goldman, J. S., Hahn, S. E., Catania, J. W., LaRusse-Eckert, S., Butson, M. B., Rumbaugh, M., et al. (2011). Genetic counseling and testing for alzheimer disease: joint practice guidelines of the American college of medical genetics and the national society of genetic counselors. Genet. Med. 13 (6), 597–605. doi:10.1097/GIM.0b013e31821d69b8
Green, R. C., Roberts, J. S., Cupples, L. A., Relkin, N. R., Whitehouse, P. J., Brown, T., et al. (2009). Disclosure of APOE genotype for risk of alzheimer's disease. N. Engl. J. Med. 361 (3), 245–254. doi:10.1056/NEJMoa0809578
Greenberg, S. M., Bacskai, B. J., Hernandez-Guillamon, M., Pruzin, J., Sperling, R., and van Veluw, S. J. (2020). Cerebral amyloid angiopathy and alzheimer disease - one peptide, two pathways. Nat. Rev. Neurol. 16, 30–42. doi:10.1038/s41582-019-0281-2
Greenberg, S. M., Bax, F., and van Veluw, S. J. (2025). Amyloid-related imaging abnormalities: manifestations, metrics and mechanisms. Nat. Rev. Neurol. 21, 193–203. doi:10.1038/s41582-024-01053-8
Griffith, A., Chande, C., Kulkarni, S., Morel, J., Cheng, Y. H., Shimizu, E., et al. (2024). Point-of-care diagnostic devices for periodontitis - current trends and urgent need. Sens. Diagn 3 (7), 1119–1134. doi:10.1039/d3sd00317e
Hales, C. M. (2025). Alzheimer's disease diagnosis and management in the age of amyloid monoclonal antibodies. Med. Clin. North Am. 109 (2), 463–483. doi:10.1016/j.mcna.2024.10.003
Hercher, L., Uhlmann, W. R., Hoffman, E. P., Gustafson, S., and Chen, K. M. Public Policy Committee of NSGC. (2016). Prenatal testing for adult-onset conditions: the position of the national society of genetic counselors. J. Genet. Couns. 25 (6), 1139–1145. doi:10.1007/s10897-016-9992-3
International Huntington Association and the World Federation of Neurology Research Group on Huntington's Chorea. (1994). Guidelines for the molecular genetics predictive test in Huntington's disease. J. Med. Genet. 31 (7), 555–559. doi:10.1136/jmg.31.7.555
Ison, H. E., Mowaswes, M., Durst, R., Leucker, T., Knowles, J. W., and Brown, E. E. (2024). Is it time for a paradigm shift? Inclusion of APOE on genetic dyslipidemia panels. J. Genet. Couns. 34 (1), e1889. doi:10.1002/jgc4.1889
Jackson, R. J., Hyman, B. T., and Serrano-Pozo, A. (2024). Multifaceted roles of APOE in alzheimer disease. Nat. Rev. Neurol. 20 (8), 457–474. doi:10.1038/s41582-024-00988-2
Jessen, F., Kramberger, M. G., Angioni, D., Aarsland, D., Balasa, M., Bennys, K., et al. (2024). Progress in the treatment of alzheimer's disease is needed - position statement of european Alzheimer's disease consortium (EADC) investigators. J. Prev. Alzheimers Dis. 11 (5), 1212–1218. doi:10.14283/jpad.2024.153
Jia, L., Xu, H., Chen, S., Wang, X., Yang, J., Gong, M., et al. (2020). The APOE ε4 exerts differential effects on familial and other subtypes of alzheimer's disease. Alzheimers Dement. 16 (12), 1613–1623. doi:10.1002/alz.12153
Kim, B. H., Kim, S., Nam, Y., Park, Y. H., Shin, S. M., and Moon, M. (2025). Second-generation anti-amyloid monoclonal antibodies for alzheimer's disease: current landscape and future perspectives. Transl. Neurodegener. 14 (1), 6. doi:10.1186/s40035-025-00465-w
Kolbe, D., da Silva, N. A., Dose, J., Torres, G. G., Caliebe, A., Krause-Kyora, B., et al. (2023). Current allele distribution of the human longevity gene APOE in Europe can mainly be explained by ancient admixture. Aging Cell 22 (5), e13819. doi:10.1111/acel.13819
Lorenzo, D., Esquerda, M., Bofarull, M., Cusi, V., Roig, H., Bertran, J., et al. (2023). The reuse of genetic information in research and informed consent. Eur. J. Hum. Genet. 31 (12), 1393–1397. doi:10.1038/s41431-023-01457-y
Lozupone, M., Dibello, V., Daniele, A., and Panza, F. (2025). An update on emerging anti-amyloid-β monoclonal antibodies for treating alzheimer's disease: the role of apolipoprotein E. Expert Opin. Emerg. Drugs 26, 77–81. doi:10.1080/14728214.2025.2481847
Mahley, R. W., and Huang, Y. (2012). Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron 76 (5), 871–885. doi:10.1016/j.neuron.2012.11.020
Marra, C., Beccia, F., Caffarra, P., L'Abbate, F., Agosta, F., Benussi, A., et al. (2025). Towards a new Value-based scenario for the management of dementia in Italy: a SINdem Delphi consensus study. Neurol. Sci. 46, 2913–2923. doi:10.1007/s10072-025-08143-5
Mayeux, R., Saunders, A. M., Shea, S., Mirra, S., Evans, D., Roses, A. D., et al. (1998). Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s disease centers consortium on apolipoprotein E and Alzheimer’s disease. N. Engl. J. Med. 338, 506–511. doi:10.1056/NEJM199802193380804
Moyer, A. M., and Black, J. L. (2025). Pharmacogenomic testing in the clinical laboratory: historical progress and future opportunities. Ann. Lab. Med. 1 (3), 247–258. doi:10.3343/alm.2024.0652
Nelson, P. T., Pious, N. M., Jicha, G. A., Wilcock, D. M., Fardo, D. W., Estus, S., et al. (2013). Apoe-ε2 and apoe-ε4 correlate with increased amyloid accumulation in cerebral vasculature. J. Neuropathol. Exp. Neurol. 72 (7), 708–715. doi:10.1097/NEN.0b013e31829a25b9
Pinto, C., Bella, M. A., Capoluongo, E., Carrera, P., Clemente, C., Colombo, N., et al. (2016). Recommendations for the implementation of BRCA testing in the care and treatment pathways of ovarian cancer patients. Future Oncol. 12 (18), 2071–2075. doi:10.2217/fon-2016-0189
Reiman, E. M., Ghisays, V., and Langbaum, J. B. (2025). The risk of alzheimer disease in APOE4 homozygotes. JAMA Neurol. doi:10.1001/jamaneurol.2025.0639
Rentz, D. M., Aisen, P. S., Atri, A., Hitchcock, J., Irizarry, M., Landen, J., et al. (2024). Benefits and risks of FDA-Approved amyloid-targeting antibodies for treatment of early alzheimer's disease: navigating clinician-patient engagement. Alzheimers Dement. 20 (11), 8162–8171. doi:10.1002/alz.14199
Salloway, S., Chalkias, S., Barkhof, F., Burkett, P., Barakos, J., Purcell, D., et al. (2022). Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early alzheimer disease. JAMA Neurol. 79, 13–21. doi:10.1001/jamaneurol.2021.4161
Serrano-Pozo, A., Qian, J., Monsell, S. E., Betensky, R. A., and Hyman, B. T. (2015). APOEε2 is associated with milder clinical and pathological alzheimer disease. Ann. Neurol. 77 (6), 917–929. doi:10.1002/ana.24369
Sima, D. M., Phan, T. V., Van Eyndhoven, S., Vercruyssen, S., Magalhães, R., Liseune, A., et al. (2024). Artificial intelligence assistive software tool for automated detection and quantification of amyloid-related imaging abnormalities. JAMA Netw. Open 7 (2), e2355800. doi:10.1001/jamanetworkopen.2023.55800
Sims, J. R., Zimmer, J. A., Evans, C. D., Lu, M., Ardayfio, P., Sparks, J., et al. (2023). Donanemab in early symptomatic alzheimer disease: the TRAIL-BLAZER-ALZ 2 randomized clinical trial. JAMA 330 (6), 512–527. doi:10.1001/jama.2023.13239
Uhlmann, W. R., and Roberts, J. S. (2018). Ethical issues in neurogenetics. Handb. Clin. Neurol. 147, 23–36. doi:10.1016/B978-0-444-63233-3.00003-8
Valdez-Gaxiola, C. A., Rosales-Leycegui, F., Gaxiola-Rubio, A., Moreno-Ortiz, J. M., and Figuera, L. E. (2024). Early- and late-onset alzheimer's disease: two sides of the same coin? Diseases 12 (6), 110. doi:10.3390/diseases12060110
van Dyck, C. H. (2018). Anti-Amyloid-β monoclonal antibodies for alzheimer's disease: pitfalls and promise. Biol. Psychiatry 83 (4), 311–319. doi:10.1016/j.biopsych.2017.08.010
van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., et al. (2023). Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388 (1), 9–21. doi:10.1056/NEJMoa2212948
Vogel, F. (1959). “Moderne probleme der Humangenetik,” in Ergebnisse der Inneren Medizin und Kinderheilkunde (Springer), 52–125.
Weidauer, S., and Hattingen, E. (2025). Cerebral amyloid angiopathy: clinical presentation, sequelae and neuroimaging Features-An update. Biomedicines 13 (3), 603. doi:10.3390/biomedicines13030603
Weiskirchen, S., and Weiskirchen, R. (2025). Unraveling the future: hot topics shaping molecular diagnostics today. Expert Rev. Mol. Diagn 25 (4), 111–116. doi:10.1080/14737159.2025.2467969
Wu, W., Ji, Y., Wang, Z., Wu, X., Lim, J., Gu, F., et al. (2023). The FDA-Approved anti-amyloid-β monoclonal antibodies for the treatment of alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Med. Res. 28 (1), 544. doi:10.1186/s40001-023-01512-w
Zallen, D. T. (2018). “Well, good luck with that”: reactions to learning of increased genetic risk for alzheimer disease. Genet. Med. 20 (11), 1462–1467. doi:10.1038/gim.2018.13
Zimmer, J. A., Ardayfio, P., Wang, H., Khanna, R., Evans, C. D., Lu, M., et al. (2025). Amyloid-related imaging abnormalities with donanemab in early symptomatic alzheimer disease: secondary analysis of the TRAILBLAZER-ALZ and ALZ 2 randomized clinical trials. JAMA Neurol. 82, 461–469. doi:10.1001/jamaneurol.2025.0065
Keywords: pharmacogenetics, predictive test, genetic counselling, alzheimer disease, monoclonal antibodies, APOE, ARIA
Citation: Zampatti S, Peconi C, Farro J, Piras F, Pellicano C, Caltagirone C and Giardina E (2025) Pharmacogenetics or predictive genetics? APOE testing blurs the lines. Front. Pharmacol. 16:1627239. doi: 10.3389/fphar.2025.1627239
Received: 12 May 2025; Accepted: 25 June 2025;
Published: 21 July 2025.
Edited by:
Lei-Yun Wang, Wuhan No. 1 Hospital, ChinaReviewed by:
Emilio Di Maria, University of Genoa, ItalyCopyright © 2025 Zampatti, Peconi, Farro, Piras, Pellicano, Caltagirone and Giardina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emiliano Giardina, ZW1pbGlhbm8uZ2lhcmRpbmFAdW5pcm9tYTIuaXQ=
 Stefania Zampatti
Stefania Zampatti Cristina Peconi
Cristina Peconi Juliette Farro
Juliette Farro Fabrizio Piras
Fabrizio Piras Clelia Pellicano
Clelia Pellicano Carlo Caltagirone
Carlo Caltagirone Emiliano Giardina
Emiliano Giardina