- 1Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmacy, Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Center for Drug Safety and Policy Research, Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 3Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan
- 4Faculty of Pharmaceutical Sciences, Riphah International University, Islamabad, Pakistan
- 5College of Pharmacy, Qatar University, Doha, Qatar
Introduction: Pakistan’s highest diabetes prevalence necessitates equitable access to anti-diabetic medicines. This study evaluated the access to Oral antidiabetics (OADs) and the effect of Pakistan’s recently launched price deregulation policy—applicable to medicines not included on the National Essential Medicines List (non-NEML)—on their prices and affordability by comparing NEML and non-NEML OADs.
Methods: A WHO/HAI methodology-based survey in 30 private pharmacies across six regions gathered prices and availability data of 30 OADs, including the Lowest Price Generic (LPG), Highest Price Generic (HPG), and originator brand (OB). These selected OADs consisted of 11 products from NEML and 19 non-NEML products, comprising 17 single-active ingredient and 13 multi-active ingredient formulations. Published and surveyed retail prices of OADs (in Pakistani Rupees, PKR) before and after deregulation were compared, and the policy’s effect was determined by difference-in-differences (DiD) analysis. Affordability for the lowest-paid employee and medicine availability in percentages were calculated.
Results: The DiD analysis revealed that the unit prices of OADs were significantly increased by PKR 15.08 (OB), PKR 5.89 (HPG), and PKR 2.81 (LPG) (p < 0.05) within just 6 months of the policy’s introduction. Medicines listed on the NEML remained consistently cheaper than non-NEML, with differences of −30.20 for OBs, −9.83 for HPGs, and −7.51 for LPGs in PKR (p < 0.001). As per DiD interaction terms (NEML enlistment status × deregulation), a greater increase in prices of non-NEML OBs was observed compared to NEML counterparts (PKR −10.85, p ≈ 0.05), while differences observed for LPGs (PKR 0.77, p = 0.73) and HPGs (PKR -0.20, p = 0.95) were insignificant. Prices of both single and multi-active ingredient formulations also increased significantly (p < 0.05). Although most OADs had fair availability from 47% to 97% after deregulation, seven out of 30 OADs remained unaffordable at both time points, and the overall affordability declined significantly post-deregulation (p < 0.05).
Conclusion: The study revealed significant price escalations for most OADs, particularly those not enlisted on NEML, highlighting access challenges for diabetic patients and necessitating targeted policy reforms that address key market-related factors to ensure equitable access to OADs.
1 Introduction
Diabetes mellitus is one of the top ten causes of death globally and has emerged as the fastest-growing global health concern (Hossain et al., 2024; World Health Organization, 2024). The World Health Organization (WHO) reports that since 2000, the number of diabetes-related deaths worldwide has increased by 95%. In lower-middle-income countries (LMICs), diabetes is becoming a more common cause of mortality. Since 2000, the death toll from this disease has more than doubled, and it has risen from the 14th to the 8th position (World Health Organization, 2024). According to the International Diabetes Federation, 10.5% of the world’s adult population currently has diabetes, a figure projected to rise to 11.2% by 2045, with nearly 90% of these cases being type 2 diabetes (Kaiser et al., 2018; Sun et al., 2022). Alarmingly, Pakistan has the highest global prevalence of diabetes, with 30.8% of its population affected, and this number is expected to increase to 33.6% by 2045 (Sun et al., 2022).
The high prevalence of diabetes demands an urgent need for access to affordable and effective treatment options. Oral antidiabetics (OADs), crucial for maintaining glycemic control and minimizing complications associated with the disease, are the backbone of diabetes therapy, particularly type 2 diabetes (Qaseem et al., 2017; Yu et al., 2022). Notably, several key OADs are part of the 23rd WHO Model Essential Medicine List (EML) and are also part of Pakistan’s National Essential Medicines List (NEML) 2023 (World Health Organization, 2023; DRA, 2023). Essential medicines are defined as those that meet the priority healthcare needs of a population, warranting their availability in functioning health systems at all times and at affordable prices (World Health Organization, 2023; Wirtz et al., 2017). Furthermore, access to essential medicines is also recognized as a critical component of the Sustainable Development Goals (SDG) by the United Nations (UN), emphasizing the significance of equitable healthcare access for all (United Nations, 2015). In addition to essential medicines, many other OADs, including newer drugs and fixed-dose combinations (FDCs), are frequently prescribed for diabetic patients, especially those with comorbidities, due to their enhanced clinical benefits, such as better glycemic control and improved adherence (Kalra et al., 2014; Bangalore et al., 2007; Ramzan et al., 2019). Thus, ensuring access to these “non-essential” diabetes medicines is also important, especially in countries with high prevalence of diabetes.
Access to diabetes medicines has been reported to be poor in LMICs, where effective disease management is hindered by significant barriers, such as high prices, inadequate accessibility, and deficient healthcare infrastructure (Khatib et al., 2016; Castillo-Laborde et al., 2022; Kibirige et al., 2022; Babar et al., 2019; Butt et al., 2024). Like many other LMICs, Pakistan offers affordable public-sector medications. However, patients frequently turn to private-sector pharmacies to get necessary prescriptions because of their limited availability in the public sector (Saeed et al., 2019; Saeed et al., 2022; Saleem et al., 2021; Saeed et al., 2021; Bibi et al., 2022). This reliance on the private sector led to high out-of-pocket (OOP) expenditure in Pakistan, accounting for 54% of total health spending, underscoring the country’s poor financial protection for healthcare expenditures (Khalid et al., 2021). In February 2024, the federal government of Pakistan approved the proposal for deregulation of medicine prices, permitting market-driven pricing for non-essential medications, including numerous OADs. One of the main goals of this new policy was to reduce medicine prices and enhance their availability through increased market competition (Tribune, 2024). Previously, the Maximum Retail Prices (MRPs) of all medicines in Pakistan were strictly regulated, making the recent shift to a more flexible pricing policy a significant change in the country’s pharmaceutical landscape (Saeed et al., 2020). While price deregulation increases the chances of profitability for manufacturers by allowing them to set the medicine prices, it also poses a greater risk of higher prices, particularly for medicines in markets with poor competition, which could compromise equitable access to medicines. It is important to highlight that prior research has shown that LMICs like Pakistan often lack a systematic approach to medicine price regulations. This underlines the lack of evidence-based interventions and the inability to implement policy measures despite the available literature on medicine pricing issues (Babar, 2022; Babar, 2024; Vogler et al., 2024).
In light of this, monitoring medicine prices, particularly in private pharmacies, where patients pay OOP, is crucial to assess the impact of the recent policy shift. While some studies have indicated that certain OADs are affordable in Pakistan, there is limited data on newer drugs and fixed-dose combinations (FDCs), many of which are frequently prescribed yet are not included on the NEML (Babar et al., 2019; Saeed et al., 2019; Saeed et al., 2022; Butt et al., 2023). Therefore, this study aims to evaluate access to both NEML and non-NEML OADs in Pakistan, by analyzing their prices, affordability, and availability after deregulation, while also comparing the effects of price deregulation on single-active-ingredient (SAI) formulations and FDCs.
2 Materials and methods
2.1 Study design and region
This was a cross-sectional survey-based study conducted using a variant of WHO/Health Action International (HAI) methodology (Health Action International, 2018). A total of 30 OADs were selected for the survey, with different strengths of the same medicine considered as separate products. According to the standard WHO/HAI methodology, the survey region must be split into six areas according to the government-defined levels of administration (e.g., cities, and districts). In this study, six survey areas (cities) across Pakistan were selected for the survey, ensuring representation from each province and the federal capital (Supplementary Figure S1). These included Islamabad (the federal capital), Lahore (the provincial capital of Punjab), Faisalabad (a major city in Punjab), Peshawar (the provincial capital of Khyber Pakhtunkhwa), Karachi (the provincial capital of Sindh), and Quetta (the provincial capital of Baluchistan). Punjab, being the most populous province, was represented by two regions. This selection includes at least one city from each province and a separate inclusion of the federal capital.
2.2 Sampling of survey units
According to the standard WHO/HAI methodology, the public sector medicine outlets serve as the sample’s reference point, and other kinds of medicine outlets are selected based on how close they are to these outlets. A list of all public sector medicine outlets was obtained and one biggest public sector hospitals in each survey area was selected as a primary survey anchor (Health Action International, 2018). Subsequently, 4 public sector hospitals were chosen within 3 h of travel from the main hospital. These hospitals were not part of the data collection but served as reference points for selecting nearby private pharmacies. 5 private retail pharmacies were selected, including one pharmacy within 10-km radius of each hospital. The current study applied this sampling strategy consistently across all six survey areas, focusing on data collection from registered private retail pharmacies only. This approach was taken for two main reasons: (1) in Pakistan, the medicines are provided free of charge to the patients in public sector hospital pharmacies, and (2) the availability of medicines in public sector pharmacies is often limited, leading patients to depend mainly on private retail pharmacies, where they pay OOP to obtain these medications (Khalid et al., 2021; Saeed et al., 2020). A similar focused survey in private pharmacies using an adapted WHO/HAI methodology has been conducted previously (Saleem et al., 2021). Finally, a total of 30 retail pharmacies, 5 in each survey area, were selected across the country for this survey.
2.3 Selection of medicines to be surveyed
A total of 30 OADs were systematically selected for inclusion in the survey. First, a comprehensive list of all registered OADs was prepared using publicly available online sources, including drug information system and PharmaGuide (PharmaGuide, 2024; Drug Information System, 2024). This list consisted of a total of 903 brands, comprising 97 generic products with various strengths and dosage forms. The annual procurement lists of three tertiary care hospitals in the survey regions were considered to include the frequently procured OADs (n = 18). All strengths of oral diabetes medicines with therapeutic alternatives from NEML and WHO EML were included in the list (n = 17), and literature was searched to enrich the list further (Butt et al., 2023). A preliminary list of 74 commonly used OADs was prepared following these steps. Finally, six endocrinology consultant physicians and two general physicians working in major hospitals from the survey areas were invited to rate the 74 OADs based on the frequency of prescriptions in their practice setting. They assigned a score from 1-3 to each drug, where: 1 denoted “rarely prescribed”, 2 indicated “occasionally prescribed”, and 3 represented “frequently prescribed”. The top 30 medicines with higher scores were selected for the survey.
The final list included both the essential OADs enlisted in the NEML 2023 (n = 11), and 19 non-NEML products. Of these selected medicines 17 had SAIs and 13 were FDCs. Multiple strengths of 5 frequently used medicines-Metformin, Glimepiride, Empagliflozin, Metformin/Sitagliptin, and Metformin/Glimepiride-were also included. Glibenclamide 5mg, the only antidiabetic medicine included in the global core list of medicines specified in the WHO/HAI survey manual was included (Health Action International, 2018) (See Supplementary Table S1).
2.4 Data collection
The data were collected by trained data collectors, for the Lowest Price Generic (LPG), Highest Price Generic (HPG), and Originator Brand (OB) of the surveyed medicines, using a standardized data collection form during June-July 2024. Where OB is the innovator brand that first received market authorization, HPG is the generic product of selected medicine with the highest price, and LPG is generic with the lowest price available at a survey outlet. Availability and maximum retail prices were noted after physically checking the stock. If a facility had only one generic product of medicine, it was categorized as LPG and HPG for price comparison analysis. A similar approach has been followed in another study (Satheesh et al., 2020). To enable a comparison between current (post-deregulation) prices of all available OAD products in the Pakistani market and their pre-deregulation prices, historical price data were obtained for each product found in the post-deregulation survey, from the PharmaGuide, 31st edition (2024). This edition of the Pharma Guide provides the last drug regulatory authority of Pakistan (DRAP)-regulated prices as of December 1, 2023, immediately before the deregulation of non-NEML medicines (PharmaGuide, 2024). Generally recognized as a reliable public source for medicine pricing, the PharmaGuide has been referenced in several studies for establishing baseline prices, making it a dependable resource for retrospective analyses (Rafi et al., 2021; Kamran et al., 2020; Ayub et al., 2022).
2.5 Data analysis
The data was analyzed by using Microsoft Excel and Stata (version 15; StataCorp LLC, College Station, TX, United States).
2.5.1 Prices
For analysis of prices, the unit prices of each surveyed OADs, defined as price per capsule or tablet, were considered. The unit prices were calculated using the following equation:
The prices were reported as Median Unit Prices (MUPs) categorized by medicine, price type (LPG, HPG, OB), NEML status (NEML and non-NEML medicines), and formulation type (SAI and FDC). The three price types were compared across two primary grouping variables, NEML status, and formulation type, before and after the deregulation policy using the Wilcoxon rank-sum test. Statistical significance was determined at a p-value threshold of 0.05. Observations with missing price data were excluded to ensure accuracy in comparisons. This comparative analysis was performed to set a foundation for the subsequent Difference-in-Differences (DiD) analysis to assess the impact of deregulation policy.
2.5.2 Impact of price deregulation on medicine prices
DiD analysis, a quasi-experimental approach, was applied to evaluate the effects of price deregulation on medicine prices in Pakistan. It is a widely recognized method to measure the impact of health policies (Zhang et al., 2022b; Teng et al., 2024). The analysis compared price changes in NEML medicines (control group/unexposed to deregulation) and non-NEML medicines (treatment group/exposed to deregulation policy), before and after the deregulation policy’s implementation. The data included unit prices of both NEML and Non-NEML OADs across three pricing categories (LPG, HPG, and IB), pre and post price deregulation policy. In the DiD model, we specified three regression equations.
In each equation,
The formulation type variable was not included in the DiD analysis because all of the FDCs were non-NEML i.e., the exposed group. So, the price changes across FDCs and SAIs after deregulation were assessed separately through linear regression models. To analyze whether the price changes varied between these groups, interaction terms were also included, as per the following general model:
Where,
The relationship between the number of registered brands and pre-post prices (including LPG, HPG, and OB) was also evaluated through linear regression models.
2.5.3 Affordability
The affordability of surveyed OADs was calculated in terms of the number of days’ wages (NDWs) required for a lowest-paid government employee to afford the standard treatment course. The defined daily dose (DDD) of each OAD, which is the “assumed average maintenance dose per day for a drug used for its main indication in adults,” was used as a standard dose unit of measurement in the analysis of affordability for standard treatment of each medication (Deressa et al., 2024). The monthly treatment cost and NDWs were calculated by using the following equations:
The salary taken for the analysis was 32000PKR per month, i.e., 1066 PKR or 3.76 USD per day (with effect from July 2023). Notably, this threshold closely aligns with the World Bank’s lower-middle-income poverty line of $3.65/day (2017 PPP)–a metric encompassing 39.4% of Pakistan’s population in fiscal year 2023–24 (World Bank, 2023). According to the standard WHO/HAI methodology, the medicine is considered affordable only if the lowest-paid unskilled government employee spends less than 1 day’s wage to get the standard treatment from that medicine (Health Action International, 2018). The Wilcoxon signed-rank test was used to compare the affordability of surveyed drugs, before and after the deregulation policy, by stratifying the data into two categorical variables—NEML status and formulation type. Descriptive statistics were computed for each variable.
2.5.4 Availability
Availability was reported in two categories OBs and generics (LPG/HPG). It was calculated as the percentage of a particular medicine available at each facility on the day of data collection, using the following equation:
Only post-deregulation availability data were considered in the analysis. The percentage availability was categorized as follows: absent, indicating that 0% of facilities surveyed had the enlisted medicines at the time of the survey; low, indicating that less than 50% of facilities had the surveyed enlisted medicines; fairly high, indicating that 50%–80% of facilities had the surveyed enlisted medicines; high, indicating that more than 80% of facilities had the surveyed enlisted medicines, with them being found in most of the facilities (Saeed et al., 2019). To compare the availability of OADs, we conducted a series of two-sample t-tests with equal variances, comparing mean availability across different groups categorized by NEML status, price category, and formulation type.
3 Results
3.1 Medicines prices before and after the price deregulation policy
Overall, the prices of OADs in Pakistan exhibited a noticeable increase of up to 296% after the launch of the price deregulation policy.
3.1.1 Medicine-specific analysis of prices
The top five OADs with the highest increase in MUPs were Rosiglitazone 4 mg Tabs (LPG: 296.23%), Glipizide 5 mg Tabs (LPG: 174.05%), Gliclazide 80 mg Tabs (LPG: 63.65%; HPG: 87.76%), Glimepiride/Pioglitazone 4mg/15 mg Tabs (LPG: 88.78%; HPG: 131.02%), and Vildagliptin 50 mg Tabs (OB: 70.04%) (See Table 1; Supplementary Table S1).
3.1.2 Prices of generics versus originator brands
In Figure 1, the box plot illustrates the distribution of MUPs for OADs categorized by price types: LPG, HPG, and OB, before and after the deregulation policy. The MUPs for surveyed medicines showed an upward trend across all price categories. For LPGs, the MUP rose moderately from PKR 22.43 to PKR 25.71. HPGs showed an increase in MUPs from PKR 35 to PKR 37.36. For OBs, the MUP showed a marked rise from PKR 21 pre-deregulation to PKR 30.80 post-deregulation. A significant change in price distribution reflected by the IQRs widening following deregulation underscores a wider dispersion of prices across all price types. Supplementary Table S2 provides the summary statistics of MUPs for OADs by price category pre- and post-deregulation.
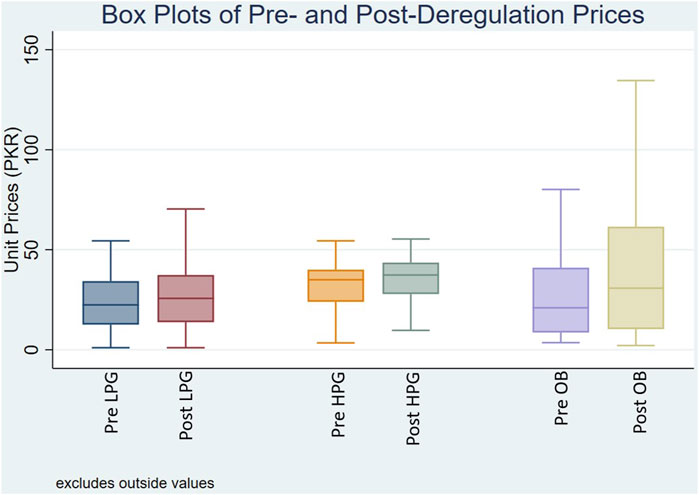
Figure 1. Box plot of median unit prices for oral antidiabetic medicines Pre- and Post-deregulation. Where, Pre/Post LPG: Unit prices of lowest price generics before and after deregulation policy; Pre/Post HPG: Unit prices of highest price generics before and after deregulation policy; Pre/Post OB: Unit prices of originator brands before and after deregulation policy.
3.1.3 Analysis of prices by NEML enlistment status
Overall, the NEML medicines were priced lower than non-NEML medicines both before and after deregulation (p < 0.05). Details on the medians and statistical comparison of MUPs of OADs by NEML status, price, and formulation types, before and after deregulation, can be found in Supplementary Table S3. Interestingly, the price trend for NEML medicines was HPG > LPG > OB, whereas for non-NEML medicines the order reversed to OB > HPG > LPG. The MUPs of non-NEML OBs remained stable at PKR 59.80 post-deregulation, while MUPs of LPGs increased from PKR 26.5 to PKR 30.36, and HPGs rose from PKR 37.7 to PKR 39.29. In contrast, for NEML OBs, the MUPs increased modestly from PKR 13.56 PKR to PKR 15.51, whereas LPGs increased from PKR 18 PKR to PKR 20.39, and HPGs rose from PKR 24.29 to PKR 35. Notably, the average number of products registered for NEML and non-NEML medicines was 37 and 9, respectively.
3.1.4 Impact of price deregulation policy on medicine prices: Results of DiD analysis
The results of DiD analysis confirmed statistically significant price increases for all categories following deregulation (See Table 2). The biggest hike was observed in OBs (PKR 15.05, p = 0.004), followed by HPGs (PKR 5.97, p = 0.02) and LPGs (PKR 2.91, p = 0.01), suggesting that all prices were increased for these categories within 6 months of the policy’s introduction. It also confirmed that the NEML medications were consistently less expensive than non-NEML medications. The price differences between NEML and non-NEML medicines (in PKR) for OBs, HPGs, and LPGs were −30.20 (p < 0.001), −9.83 (p < 0.001), and −7.51 (p < 0.001), respectively, highlighting the protective function of NEML inclusion in preserving affordability. The interaction term (Period × NEML status), expressing the differential price effect of deregulation between NEML and non-NEML medicines, revealed no statistically significant difference in costs between NEML and non-NEML medications for LPGs (β = 0.77, p = 0.35) and HPGs (β = −0.20, p = 0.95) in PKR, after controlling for baseline differences. OBs, on the other hand, a somewhat greater and a marginally significant negative interaction was noted (β = −10.85, p = 0.05), indicating that NEML OB prices rose 10.85 units less after deregulation than non-NEML OB prices. These results suggest that, in contrast to their NEML counterparts, non-NEML OB prices were more significantly raised by the price deregulation, although the effects were less noticeable for LPGs and HPGs. Notably, the lower R-squared values suggest other unmeasured and important factors might have affected the medicine prices.
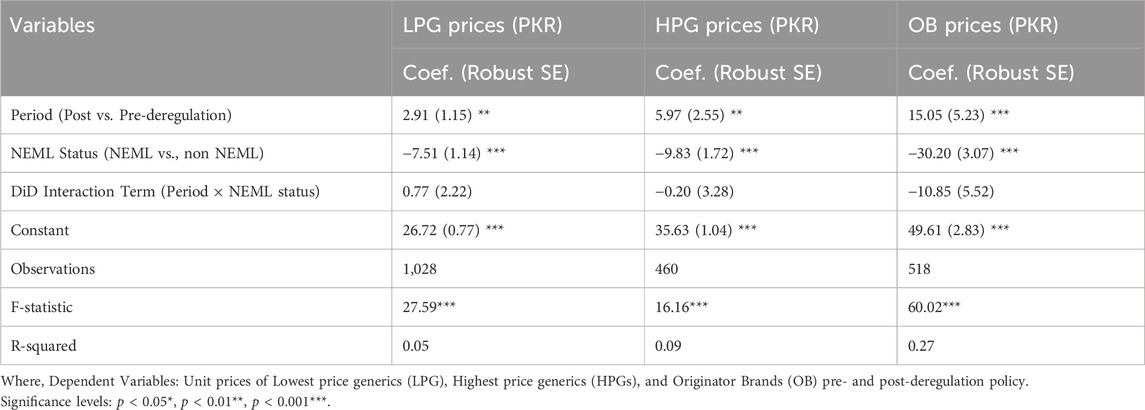
Table 2. Impact of price deregulation on unit prices of OADs: Difference-in-Differences regression analysis.
3.1.5 Prices of single versus multi-active ingredient formulations
Upon stratification by formulation type, the Wilcoxon rank-sum test revealed the prices of LPG and OB of FDCs were significantly higher than those of SAI medicines, while for HPG FDCs the difference was not significant (p < 0.01), both before and after deregulation (See Supplementary Table S3). This trend was further confirmed by the regression results (Table 3), where the baseline unit prices of FDCs were considerably higher than SAI medicines with a difference of PKR 3.05 (p = 0.0009) for LPG, PKR 2.88 (p = 0.09: Not significant), and PKR 14.66 (p < 0.001). The findings also revealed that the prices of both SAI medicines and FDCs increased significantly for all price types (LPG, HPG, and OB) after deregulation. The interaction terms between formulation type and period (pre-post-deregulation) indicated no significant differential impact of deregulation on FDCs compared to SAI medicines across all price types.
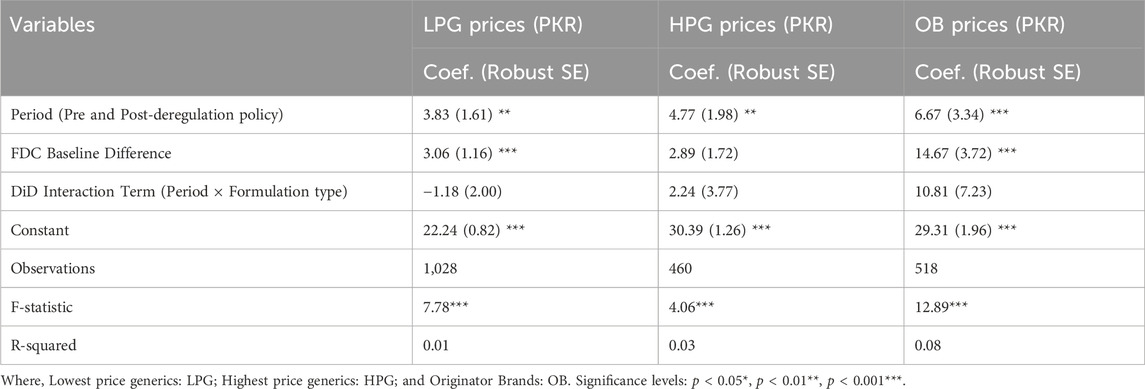
Table 3. Effect of price deregulation on unit prices of OADs (PKR) by formulation type: regression analysis results.
3.1.6 Medicine prices and market competition
The findings from the linear regression models revealed a significantly negative association between prices and the number of registered brands (reflecting market competition), both before and after deregulation (See Supplementary Table S4). For LPGs, the addition of each registered brand was associated with a decrease in unit price by PKR 0.26 pre-deregulation and PKR 0.20 post-deregulation (p < 0.001). For HPGs, the unit prices were reduced by PKR 0.33 and PKR 0.39 pre and post-deregulation (p < 0.001). OBs presented a relatively greater reduction in unit prices PKR 0.52 (p < 0.001) before deregulation and PKR 0.72 (p < 0.001) after deregulation.
3.2 Medicines affordability before and after the price deregulation policy
Overall, affordability of OADs in Pakistan significantly worsened post-deregulation, as the Wilcoxon signed-rank test revealed a significant increase in median NDWs from 0.67 to 0.80 for LPG, 0.79 to 1.04 for HPG, and 1.16 to 1.24 for OBs (p < 0.01), indicating a decline in affordability by 19.40%, 31.65%, and 6.89% respectively, after deregulation (See Table 4).
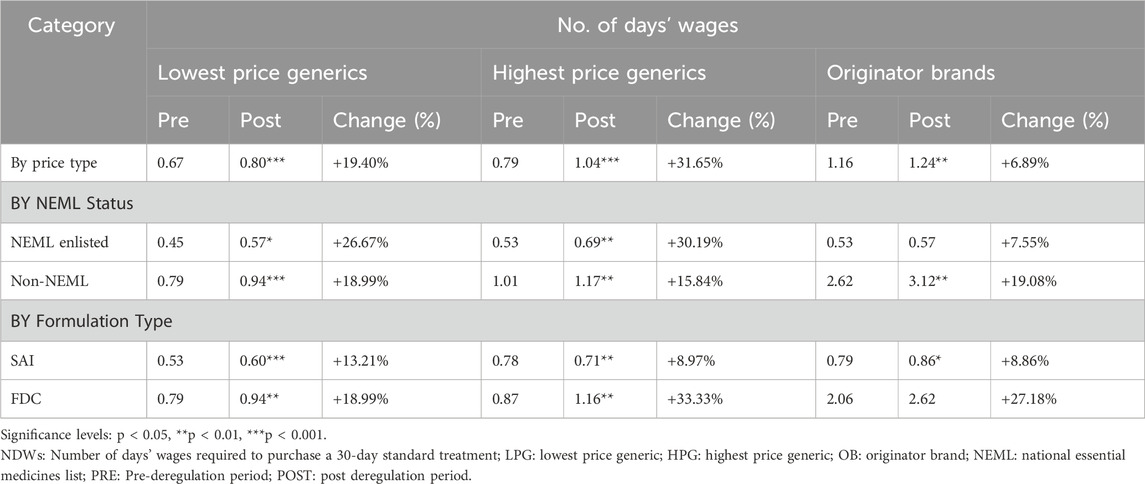
Table 4. Affordability of medicines (no. of days’ wages) pre- and post-deregulation by NEML status, formulation type, and price categories.
3.2.1 Medicine-specific comparison of affordability
All available price types of 7 OADs–Sitagliptin 100 mg Tabs, Vildagliptin 50 mg Tabs, Empagliflozin 10 mg Tabs, Dapagliflozin 5 mg Tabs, Pioglitazone 15 mg Tabs, metformin/sitagliptin 500mg/50 mg Tabs, Vildagliptin/metformin 50 mg/1 g Tabs–remained consistently unaffordable both before and after deregulation. Interestingly, two of these medicines, Empagliflozin 10 mg Tabs and dapagliflozin 5 mg Tabs are included in both NEML and WHO EML 2023. Whereas, the generic products (LPG and HPG) of three OADs–metformin/sitagliptin 1g/50mg, Glimepiride/Pioglitazone 4mg/15mg, and Metformin/Glimepiride 500mg/2 mg–jumped from the affordable to the unaffordable category. Supplementary Table S5 provides the NDWs for each medicine before and after the price deregulation policy.
3.2.2 Affordability of generics versus originator brands
The analysis revealed a significant increase in the NDWs for all price categories post-deregulation. The highest rise in median NDWs was noted for HPGs, increasing significantly by 31.65% from 0.79 to 1.04 (p < 0.001), bringing this category from affordable to unaffordable OADs. Although LPGs remained affordable but experienced a significant 19.40% rise in NDWs (p < 0.001). In contrast, the OBs exhibited minimal change in affordability by 6.89%, with median NDWs rising from 1.16 to 1.24 (p < 0.01). Although the change in the affordability of OBs was minimal, it is noteworthy that OBs remained unaffordable both before and after deregulation with median NDWs greater than 1 day.
3.2.3 Affordability and NEML enlistment status
The affordability of NEML LPG and HPG significantly worsened by 26.67% (p < 0.05) and 30.19% (p < 0.01) respectively. In contrast, the rise in median NDWs was minimal (7.55%) and insignificant for NEML OBs. The non-NEML medicines observed more pronounced rises in median NDWs by 18.99% for LPGs (p < 0.001), 15.84% for HPGs (p < 0.01), and 19.08% for OBs (p < 0.01). Specifically, the median NDWs rose from 0.79 to 0.94 for LPGs, 1.01 to 1.17 for HPGs, and 2.62 to 3.12 for OBs, keeping the non-NEML HPGs and OBs unaffordable. This analysis highlights the need for policies to address affordability issues, particularly for non-NEML OADs.
3.2.4 Affordability based on formulation type
The median NDWs for SAI medicines increased significantly by 13.21% (p < 0.001) for LPG, 8.97% (p < 0.01) for HPG, and 8.86% (p < 0.05) for OB. The median NDWs of FDCs also increased post-deregulation, by 18.99% (p < 0.01) for LPG, 33.33% (p < 0.01) for HPG, and 27.18% (p > 0.05) for OBs. All price types of SAIs remained affordable i.e., NDW<1, before and after deregulation. In the case of FDCs, the LPG remained affordable, the HPG shifted from affordable to the unaffordable category, and OBs remained unaffordable before and after deregulation (See Table 4).
3.3 Availability of oral antidiabetics post-deregulation
3.3.1 Medicine-specific availability
About two-thirds of the surveyed OADs demonstrated moderate to high availability, with rates ranging from 47% to 97%. Metformin 500 mg and Sitagliptin 100 mg were the two most accessible OADs with 97% availability. However, almost half of the OADs (n = 16) fell below the WHO benchmark for availability, set at 80%.
3.3.2 Availability of generics versus originator brands
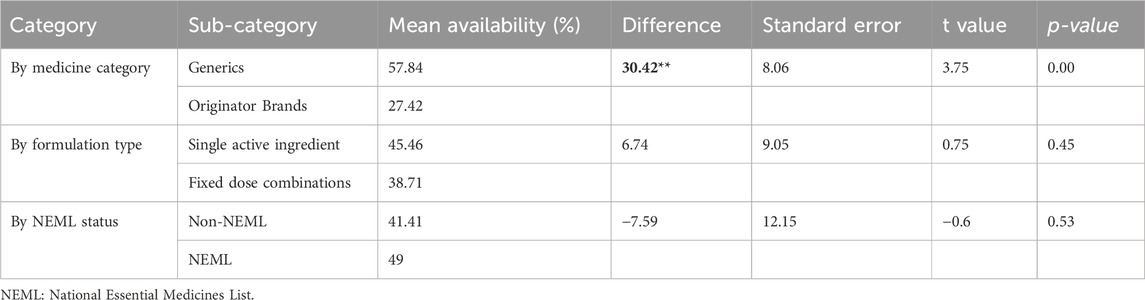
As per the results of independent t-tests to compare mean availability across different medicine categories, the LPGs exhibited significantly higher mean availability (57.85%) compared to OBs (27.42%), with a statistically significant difference of 30.43% (p < 0.05) (See Table 5).
3.3.3 Availability by NEML enlistment status
The NEML medicines were found to be more available than the non-NEML medicines with 49% and 41.41% availability, respectively. However, this difference of 7.59 was insignificant (p = 0.535) (See Table 5). Seven of the 30 surveyed OADs had low availability, including one NEML medicine, Glipizide 5 mg Tabs (23%) (See Supplementary Table S6).
3.3.4 Availability by formulation type
SAI formulations had a mean availability of 45.46%, which was slightly higher than FDCs at 38.72%. However, the difference of 6.75% was not statistically significant (p = 0.459).
4 Discussion
The study offers a comprehensive evaluation of access to OADs and valuable insight into the immediate effects of the recently adopted medicine price deregulation policy on the prices and affordability of these medicines in the private sector of Pakistan. One of the primary purposes of this price deregulation policy was to foster market competition among non-NEML medicines manufacturers that could cause price reductions and better affordability for patients. Drug price deregulation policy has been adopted in several countries, and variable outcomes have been experienced for different medicine categories, most studies reported either minimal change or increases in medicine prices (Guan et al., 2019; Liu et al., 2017; Costa Font, 2016). In an LMIC like Pakistan, where the prevalence of diabetes is the highest and poor access to medicines is already a critical issue, this study gives critical evidence on current accessibility to antidiabetic medicines post-deregulation policy. It represents an effort to monitor the pharmaceutical market response to this significant policy change in Pakistan (Saeed et al., 2019; Saleem et al., 2016; Bhatti, 2024).
Our study has shown that the overall prices of OADs increased significantly especially for non-NEML medicines, thus aggravating the access challenges for diabetes patients. Notably, this upward trend was observed within just 6 months of the policy’s implementation, raising concerns about the potential for even greater increases over the long term due to the risk of market monopolization. Hence deregulation policy might have inadvertently led to price escalation rather than fostering competitive prices, especially for premium agents with less competitive environments. A study conducted in China found similar results and stated that price changes after policy implementation vary for drug categories (Zhang et al., 2022a). However, the availability for two-thirds of the surveyed OADs ranged from moderate to high, post-deregulation. This finding was partially consistent with the observations by Peruvian researchers who reported improved availability of certain generic medicines following deregulation (Costa Font, 2016).
4.1 Medicines prices before and after price deregulation policy
4.1.1 Prices of generics versus originator brands
Overall, the HPGs were found to be the most expensive, followed by OBs and LPGs, at both time points. Certain generics had higher prices than OBs, possibly due to high generic entrants in the Pakistani market. The results align with the findings by Baber et al., who reported that OBs of some antidiabetic medicines were cheaper than their LPGs in some LMICs, contrary to the observation in a majority of the countries where OBs were priced higher (Babar et al., 2019).
After deregulation, the prices rose significantly across all categories—LPG, HPG, and OB. Similar findings on the effect of deregulation policy on different categories of medicines, with the majority facing price upsurges were reported by Guan et al. and Costa et al. in the Chinese and Peruvian markets (Guan et al., 2019; Costa Font, 2016). Our results highlighted that the policy might have broadly impacted the market, leading to overall price hikes and a possible rise of monopolistic practices among the manufacturers (Zhang and Bian, 2023).
4.1.2 Medicine prices and NEML status
The deregulation policy targeted the non-NEML medicines, ideally, the prices of NEML medicines that were still under regulatory control should have stayed stable within this short period post-deregulation policy. However, the significant price surges of up to 296%, irrespective of their inclusion in the NEML 2023 translate into potentially inefficient implementation of the policy. Another contributing factor behind the lack of significant distinction between both NEML and non-NEML medicines could be the simultaneous price increments of NEML medicines e.g., prices of 146 essential drugs were increased in February 2024 alongside the launch of this deregulation policy for nonessential medicines (Azad, 2024). Nevertheless, the prices of NEML medicines remained lower than the non-NEML medicines at both time points. A multi-country study conducted by Bazarangi et al., also noted that essential medicines have significantly better accessibility than non-essential medicines (Bazargani et al., 2014). Notably, the OBs of NEML medicines were found to be high priced compared to generics while in the case non NEML medicines OBs were the most expensive. Reflecting the premium position of non-NEML OBs in the market. This disparity advocates for switching to the cheapest therapeutically equivalent alternative, which may lead to substantial cost savings (Abdullah et al., 2024).
4.1.3 Impact of price deregulation on medicine prices-insights from DiD analysis
The DiD analysis also confirmed that the NEML-listed medicines, preserved lower prices compared to non-NEML medicines, indicating the protective role of NEML against price hikes. However, this protective effect did not entirely shield essential medicines from price escalation potentially stimulated by deregulation. Liu et al. also detected higher prices for essential medicines after the introduction of the deregulation policy, gravely impacting low-income populations (Liu et al., 2017). The non-NEML medicines especially for highly prevalent diseases like diabetes, have become increasingly unaffordable. This may lead to poor access to innovative treatment options, essential for specific groups of people, thus affecting healthcare equity. The effects of price deregulation differed depending on the type of medication i.e., LPG, HPG, and OB. While LPGs and HPGs did not show any obvious differential effects, the non-NEML OBs had a comparatively larger and significant price increase following the policy than their NEML counterparts. This implies that medications not listed on the NEML may have been disproportionately impacted by price deregulation, especially OBs, which are frequently more expensive and might be less vulnerable to competition. These findings highlight the significance of specific regulatory protections for expensive, non-NEML medications in order to avoid issues with affordability in the private sector.
4.1.4 Medicine prices by formulation type
Increases in prices of both FDCs and SAI medicines were observed across all price categories (LPG, HPG, and OB) after the implementation of the deregulation policy. It was observed that all of the FDCs consistently commanded higher prices than SAI formulations across all price types before and after deregulation, reflecting their superior placement in the market (Hao et al., 2016).
4.1.5 Medicine prices and market competition
The results highlight the significant impact of the number of registered brands representing the market competition on price drops among all categories, i.e., LPG, HPG, and OB. This effect was more prominent in OB prices, possibly due to higher price elasticity in this category. Nguyen et al. also noticed a 20% reduction in price for medicines with about three generic products in the market, and up to 80% price reductions for medicines with ten or more competitors (Nguyen et al., 2022). Chen Yina et al. also reported a price decline due to increasing market competition for drugs in China (Yina et al., 2023). The negative association between prices and market competition was more pronounced in post post-deregulation period, suggesting that the price deregulation policy might have augmented the role of market competition in bringing down the prices of OADs in the Pakistani market. The Peruvian market also showed lower prices for some medicines due to increased generic competition after the implementation of the deregulation policy (Costa Font, 2016). However, other factors related to market dynamics, including product-related, consumer-related, trading strategies, supply-related, and regulatory compliance, should also be considered to explore the impact of market competition along with deregulation on medicine prices (Borges dos Santos et al., 2019).
4.2 Medicines affordability before and after the price deregulation policy
The increase in the number of unaffordable medicines despite NEML’s protective status and the overall rise in NDWs for both NEML and non-NEML medicines highlight issues with the current pricing strategies. Strikingly, despite having more than 5 registered brands each, Empagliflozin 10 mg and Dapagliflozin, NEML medicines, remained among the top seven most unaffordable medicines. Whereas, gliflozins have proven cardiovascular benefits, in addition to effectively controlling blood glucose, by improving heart failure-related outcomes and preventing adverse events (Tornyos et al., 2022). However, Rahul et al. have also reported this particular class of OADs to be high priced and responsible for OOPs (Aggarwal et al., 2022). The reduction in affordability leads to prominent health disparities because patients might opt for alternatives with less efficacy or forego required medicines (Dzudie et al., 2020). A qualitative study of diabetic patients highlighted that patients have to face serious social and financial consequences, like buying cheaper medications and buying from the black market to support their costly treatment (Golder et al., 2021).
Overall, all three price types, i.e., LPG, HPG, and OBs, experienced significant rises in NDWs, worsening the affordability, post deregulation. The generics (LPGs and HPGs) had greater increments in NDWs compared to OBs, reflecting the possible monopolistic behaviour by local manufacturers (Zhang and Bian, 2023). While LPGs were found affordable, HPGs observed the greatest increment in NDWs, taking this category from affordable (pre-deregulation) to unaffordable (post-deregulation). Conversely, OBs experienced relatively minimal change post-deregulation; however, they remained largely unaffordable before and after deregulation, where the lowest-paid worker had to spend more than a day’s wage for monthly treatment with OBs.
The more pronounced decline in affordability observed among the non-NEML medicines compared to NEML medicines underscores the potential risks of blanket deregulation. The medicines for highly prevalent diseases like diabetes should be excluded from this new policy to safeguard patient affordability. A study conducted globally on different income levels for diabetic drugs further supported our finding, stating that availability and affordability were poor in LMICs (Chow et al., 2018).
Affordability declined for both formulation types, SAI and FDC. While all the SAIs and LPG of FDCs remained affordable, the HPG and OB of FDC were found to be unaffordable post-deregulation. The persistent unaffordability of FDC OBs highlights the need for policy intervention to alleviate the impact on diabetic patients who rely on these medicines (Kalra et al., 2020).
4.3 Availability
The post-deregulation analysis of availability for OADs in Pakistan revealed that a considerable proportion of these medicines had moderate to high availability, but a significant number of drugs were below the WHO 80% benchmark, highlighting access issues. This disparity in availability among various drugs underlines market preferences or supply chain efficiencies that might affect certain medicines. Some drugs, for example, Sitagliptin and Metformin, had high availability, probably due to a bigger market demand and a higher number of manufacturers/suppliers. Several other studies have also found Metformin 500 mg to have more than 80% availability in LMICs (Babar et al., 2019; Beran et al., 2018; Osuafor et al., 2021), reflecting not only its popularity but also the positive role of essential medicine lists. Conversely, the low availability of Glipizide, another essential medicine, may indicate reduced manufacturer attention, probably because of either lower demand, profitability, or regulatory challenges. Another study in Bangladesh reported that an inefficient drug supply chain was one suspected reason for low availability (Hakim et al., 2022).
Although the NEML OADs were slightly more available, there was no significant difference between the availability of NEML and non-NEML medicines, which is contrary to the findings by other researchers (Bazargani et al., 2014). This may also be credited to the local manufacturing capacity. The significantly higher availability of generics compared to OBs also underlines the high number of generic entrants in the Pakistani market. As the market is dominated by generics, the pricing of this category is crucial to guarantee access to these medicines for diabetic patients, especially those on low wages.
4.4 Implications
Although the deregulation policy has the potential to boost the pharmaceutical business and innovation in the country, its impact needs to be measured at a large scale, particularly for the poverty-stricken population of Pakistan (Baulch and McCulloch, 2002; Business Recorder, 2024). Our findings recommend that a blanket deregulation policy for non-NEML medicines, based on the supposition that it will uniformly reduce prices, seems to be flawed. A nuanced balance between demand, market competition, and the risk of monopolistic practices by manufacturers must be considered for effective regulation of medicine prices. A targeted approach to deregulation, informed by these market factors, is likely to yield better outcomes in ensuring affordability and accessibility. The regulators and policymakers should consider: i) Re-introducing price control policies for all antidiabetic medicines, so the affordability for diabetics is not compromised in a country like Pakistan with the highest global prevalence of diabetes; ii) Revising the section of medicines to treat diabetes in the NEML, considering the highest global prevalence of diabetes and its associated comorbidities like cardiovascular diseases; iii) Emphasizing the enforcement of NEML-based procurement; iv) employing targeted actions to implement generic prescribing, especially for non-NEML medicines; v) Conducting in-depth granular analysis, to comprehend pricing behaviors and manage regulatory procedures accordingly; vi) Studies should also be conducted to evaluate the treatment adherence as the high cost and compromised access lead to poor adherence, ultimately leading to increased morbidity and mortality (Saraiva et al., 2020).
4.5 Limitations
The present study had several limitations. First, the representativeness of findings may be limited by including only 30 OADs. However, the list of these medicines was meticulously prepared and represents the most frequently prescribed agents. This study is notably the first to include both FDCs and non-NEML medicines, providing novel insights into their access. Second, though the pre-deregulation prices were obtained from a reliable and widely recognized source for retrospective analysis, there may be variability compared to actual market prices before deregulation. This also restricted the availability analysis. Nevertheless, this study provides a baseline assessment of the deregulation policy’s effect and lays the foundation for future research and policy assessments. Third, the relatively low R-squared values in the DiD analysis highlighted that other important factors, such as pharmacy procurement prices, regional economic disparities, and supply chain issues, might have impacted the medicines’ retail prices. Subsequent studies should expand the dataset and include variables such as manufacturers’ policies, wholesale purchase data, regional economic indicators, and supply chain metrics to enhance the robustness of the model and to provide detailed analysis. Moreover, the DiD approach assumes that the treatment and control groups would have followed parallel trends in the absence of the intervention (Zhang et al., 2022b). Due to the use of a single pre-policy data point, we were unable to formally test this assumption. While non-NEML medicines exhibited higher prices than NEML medicines at both time points, this reflects differences in price levels, not trends, and thus the untestable assumption may affect the internal validity of our DiD estimates. This limitation should be considered when interpreting the policy effects. Additionally, macroeconomic variables such as inflation and exchange rate fluctuations were not controlled for in this analysis and may have independently influenced price trends. However, these factors would likely affect both NEML and non-NEML medicines similarly, and thus would not substantially bias our estimated policy impacts. Fourth, our 6-month post-deregulation evaluation may not reflect long-term market stabilization effects. While this timeframe provides crucial initial policy impact data, we strongly recommend future longitudinal studies to understand long-term market dynamics. In future research, it would also be important to account for the date of manufacture when collecting medicine price data, particularly in settings where government-regulated prices are frequently updated. This consideration can help ensure that price comparisons accurately reflect the policy’s impact on medicines manufactured under different pricing regimes.
5 Conclusion
This study highlights the critical challenges concerning access to the key OAD medicines posed by the price deregulation policy in Pakistan. A marked price hike was noted particularly among frequently prescribed non-NEML OB medicines, within just 6 months of the implementation of the policy. This trend has serious implications for diabetic patients, especially those with comorbidities and poor socioeconomic status. The study also highlights the protective but limited role of NEML against these price escalations. Although the availability of OADs was fair in the private sector, the affordability worsened significantly, reflecting the potential monopolistic practices by some manufacturers. These findings call for a policy review targeting a balance between the demand, market competition, and risk for monopolistic practices by manufacturers. Efforts should be made to ensure equitable access to medicines, especially for highly prevalent diseases like diabetes, on a priority basis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. SS: Data curation, Writing – review and editing. NY: Data curation, Software, Writing – original draft. CY: Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review and editing. MJ: Methodology, Writing – review and editing. MA: Data curation, Writing – review and editing. HZ: Data curation, Investigation, Writing – review and editing. MS-U-D: Data curation, Investigation, Writing – review and editing. YF: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review and editing. Z-U-DB: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to extend our sincere gratitude to all the data collectors for their immense support throughout the data collection period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1627735/full#supplementary-material
References
Abdullah, S., Saleem, Z., and Godman, B. (2024). Coping with increasing medicine costs through greater adoption of generic prescribing and dispensing in Pakistan as an exemplar country. Expert Rev. Pharmacoeconomics and Outcomes Res. 24 (2), 167–170. doi:10.1080/14737167.2023.2280802
Aggarwal, R., Vaduganathan, M., Chiu, N., and Bhatt, D. L. (2022). Out-of-Pocket costs for Sglt-2 (Sodium-Glucose transport Protein-2) inhibitors in the United States. Circ. Heart Fail. 15 (3), e009099. doi:10.1161/CIRCHEARTFAILURE.121.009099
Ayub, F., Lee, K. S., Goh, K. W., Nadeem, M. F., Rabbani, I., Sulaiman, A. K., et al. (2022). Price variation among registered brands of anti-cancer medicines available in Pakistan. Malays. J. Pharm. (MJP) 8 (1), 42–56. doi:10.52494/exvw6975
Azad, A. R. (2024). Hike in prices of 146 essential drugs notified: bussiness recorder. Available online at: https://www.brecorder.com/news/40290113 (Accessed December 25, 2024).
Babar, Z. (2024). A conceptual framework to build effective medicine pricing policies for low and middle-income countries (Lmics). Res. Soc. and Adm. Pharm. RSAP S1551-7411 (24), 00199. doi:10.1016/j.sapharm.2024.06.008
Babar, Z.-U.-D. (2022). Forming a medicines pricing policy for low and middle-income countries (lmics): the case for Pakistan. J. Pharm. Policy Pract. 15 (1), 9. doi:10.1186/s40545-022-00413-3
Babar, Z.-U.-D., Ramzan, S., El-Dahiyat, F., Tachmazidis, I., Adebisi, A., and Hasan, S. S. (2019). The availability, pricing, and affordability of essential diabetes medicines in 17 low-middle-and high-income countries. Front. Pharmacol. 10, 1375. doi:10.3389/fphar.2019.01375
Bangalore, S., Kamalakkannan, G., Parkar, S., and Messerli, F. H. (2007). Fixed-dose combinations improve medication compliance: a meta-analysis. Am. J. Med. 120 (8), 713–719. doi:10.1016/j.amjmed.2006.08.033
Baulch, B., and McCulloch, N. (2002). Being poor and becoming poor: poverty status and poverty transitions in rural Pakistan. J. Asian Afr. Stud. 37 (2), 168–185. doi:10.1177/002190960203700208
Bazargani, Y. T., Ewen, M., de Boer, A., Leufkens, H. G., and Mantel-Teeuwisse, A. K. (2014). Essential medicines are more available than other medicines around the globe. PloS one 9 (2), e87576. doi:10.1371/journal.pone.0087576
Beran, D., Ewen, M., Lipska, K., Hirsch, I. B., and Yudkin, J. S. (2018). Availability and affordability of essential medicines: implications for global diabetes treatment. Curr. diabetes Rep. 18, 48–10. doi:10.1007/s11892-018-1019-z
Bhatti, M. W. (2024). Massive increase in medicine prices feared after govt decides to deregulate drug prices: the news. Available online at: https://www.thenews.com.pk/print/1159143-massive-increase-in-medicine-prices-feared-after-govt-decides-to-deregulate-drug-prices (Accessed December 15, 2024).
Bibi, M., Haq, N. U., Kareem, A., Ullah, H., Baloch, N., Rehman, G., et al. (2022). Evaluation of availability, prices, and affordability of selected essential medicines in Balochistan, Pakistan. Int. J. Public Health 67, 1604375. doi:10.3389/ijph.2022.1604375
Borges dos Santos, M. A., dos Santos Dias, L. L., Santos Pinto, C. D. B., da Silva, R. M., and Osorio-de-Castro, C. G. S. (2019). Factors influencing pharmaceutical Pricing-a scoping review of academic literature in health science. J. Pharm. Policy Pract. 12, 24–12. doi:10.1186/s40545-019-0183-0
Business Recorder (2024). Price deregulation: Pakistan’s pharma sector sales hit $3.3bn in Fy24, 22% higher year-on-year. Available online at: https://www.brecorder.com/news/40318578/price-deregulation-pakistans-pharma-sector-sales-hit-33bn-in-fy24-22-higher-year-on-year (Accessed December 24, 2024).
Butt, M. D., Ong, S. C., Rafiq, A., Kalam, M. N., Sajjad, A., Abdullah, M., et al. (2024). A systematic review of the economic burden of diabetes mellitus: contrasting perspectives from high and low middle-income countries. J. Pharm. Policy Pract. 17 (1), 2322107. doi:10.1080/20523211.2024.2322107
Butt, M. D., Ong, S. C., Rafiq, A., Malik, T., Sajjad, A., Batool, N., et al. (2023). An observational multi-center study on type 2 diabetes treatment prescribing pattern and patient adherence to treatment. Sci. Rep. 13 (1), 23037. doi:10.1038/s41598-023-50517-2
Castillo-Laborde, C., Hirmas-Adauy, M., Matute, I., Jasmen, A., Urrejola, O., Molina, X., et al. (2022). Barriers and facilitators in access to diabetes, hypertension, and dyslipidemia medicines: a scoping review. Public Health Rev. 43, 1604796. doi:10.3389/phrs.2022.1604796
Chow, C. K., Ramasundarahettige, C., Hu, W., AlHabib, K. F., Avezum, A., Cheng, X., et al. (2018). Availability and affordability of essential medicines for diabetes across high-income, middle-income, and low-income countries: a prospective epidemiological study. Lancet Diabetes and Endocrinol. 6 (10), 798–808. doi:10.1016/S2213-8587(18)30233-X
Costa Font, J. (2016). Deregulation and access to medicines: the Peruvian experience. J. Int. Dev. 28 (6), 997–1005. doi:10.1002/jid.3096
Deressa, H. D., Abuye, H., Adinew, A., Ali, M. K., Kebede, T., and Habte, B. M. (2024). Access to essential medicines for diabetes care: availability, price, and affordability in central Ethiopia. Glob. Health Res. Policy 9 (1), 12. doi:10.1186/s41256-024-00352-3
DRA (2023). National essential medicines list. Available online at: https://www.dra.gov.pk/publications/national-essential-medicine-lists/ (Accessed November 09, 2024).
Drug Information System (2024). Online drug information system. Available online at: https://www.druginfosys.com (Accessed December 12, 2024).
Dzudie, A., Njume, E., Abanda, M., Aminde, L., Hamadou, B., Dzekem, B., et al. (2020). Availability, cost and affordability of essential cardiovascular disease medicines in the south west region of Cameroon: preliminary findings from the Cameroon science for disease study. PLoS One 15 (3), e0229307. doi:10.1371/journal.pone.0229307
Golder, S., Bach, M., O'Connor, K., Gross, R., Hennessy, S., and Hernandez, G. G. (2021). Public perspectives on anti-diabetic drugs: exploratory analysis of Twitter posts. JMIR diabetes 6 (1), e24681. doi:10.2196/24681
Government of Pakistan (2024). Specialized healthcare and medical education department. Available online at: https://health.punjab.gov.pk/Index.aspx (Accessed December 12, 2024).
Guan, X., Wushouer, H., Yang, M., Han, S., Shi, L., Ross-Degnan, D., et al. (2019). Influence of government price regulation and deregulation on the price of antineoplastic medications in China: a controlled interrupted time series study. BMJ open 9 (11), e031658. doi:10.1136/bmjopen-2019-031658
Hakim, S., Chowdhury, M. A. B., Ahmed, N. U., and Uddin, M. J. (2022). The availability of essential medicines for diabetes at health facilities in Bangladesh: evidence from 2014 and 2017 national surveys. BMC Health Serv. Res. 22 (1), 377. doi:10.1186/s12913-022-07738-4
Hao, J., Rodriguez-Monguio, R., and Seoane-Vazquez, E. (2016). Fixed-dose combination and single active ingredient drugs: a comparative cost analysis. Expert Rev. Pharmacoeconomics and Outcomes Res. 16 (1), 127–134. doi:10.1586/14737167.2015.1068690
Health Action International (2018). Measuring medicine prices, availability, affordability and price components (2nd edition). Available online at: https://haiweb.org/publication/measuring-medicine-prices-availability-affordability-and-price-components-2nd-ed/ (Accessed October 14, 2024).
Hossain, M. J., Al-Mamun, M., and Islam, M. R. (2024). Diabetes mellitus, the fastest growing global public health concern: early detection should be focused. Health Sci. Rep. 7 (3), e2004. doi:10.1002/hsr2.2004
Kaiser, A. B., Zhang, N., and Der Pluijm, W. V. (2018). Global prevalence of type 2 diabetes over the next ten years (2018-2028). Diabetes 67 (Suppl. 1). doi:10.2337/db18-202-lb
Kalra, S., Baruah, M. P., and Das, A. K. (2014). From vicious to virtuous: changing the cycle of diabetes care. J. Soc. Health Diabetes 2 (02), 062–063. doi:10.4103/2321-0656.130784
Kalra, S., Das, A., Priya, G., Ghosh, S., Mehrotra, R., Das, S., et al. (2020). Fixed-dose combination in management of type 2 diabetes mellitus: expert opinion from an international panel. J. Fam. Med. Prim. Care 9 (11), 5450–5457. doi:10.4103/jfmpc.jfmpc_843_20
Kamran, S., Saqlain, M., Khan, Z., Mahmood, S., Ali, H., Ahmad, N., et al. (2020). Cost analysis of registered brands of oral antidepressant drugs in Pakistan: a descriptive analysis. Expert Rev. Pharmacoeconomics and Outcomes Res. 20 (5), 473–479. doi:10.1080/14737167.2019.1666001
Khalid, F., Raza, W., Hotchkiss, D. R., and Soelaeman, R. H. (2021). Health services utilization and out-of-pocket (Oop) expenditures in public and private facilities in Pakistan: an empirical analysis of the 2013–14 Oop health expenditure survey. BMC health Serv. Res. 21, 178–14. doi:10.1186/s12913-021-06170-4
Khatib, R., McKee, M., Shannon, H., Chow, C., Rangarajan, S., Teo, K., et al. (2016). Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the pure study data. Lancet 387 (10013), 61–69. doi:10.1016/S0140-6736(15)00469-9
Kibirige, D., Olum, R., Kyazze, A. P., Bongomin, F., and Sanya, R. E. (2022). Availability and affordability of essential medicines and diagnostic tests for diabetes mellitus in Africa. Trop. Med. and Int. Health 27 (11), 942–960. doi:10.1111/tmi.13819
Liu, J., Wang, L., Liu, C., and Zhang, X. (2017). Impact of price deregulation policy on the affordability of essential medicines for women’s health: a panel data analysis. Expert Rev. Pharmacoeconomics and Outcomes Res. 17 (6), 625–631. doi:10.1080/14737167.2017.1330151
Nguyen, N. X., Sheingold, S. H., Tarazi, W., and Bosworth, A. (2022). Effect of competition on generic drug prices. Appl. health Econ. health policy 20 (2), 243–253. doi:10.1007/s40258-021-00705-w
Osuafor, N. G., Ukwe, C. V., and Okonta, M. (2021). Evaluation of availability, price, and affordability of cardiovascular, diabetes, and global medicines in Abuja, Nigeria. PLoS One 16 (8), e0255567. doi:10.1371/journal.pone.0255567
Qaseem, A., Barry, M. J., Humphrey, L. L., Forciea, M. A., Fitterman, N., Horwitch, C., et al. (2017). Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American college of physicians. Ann. Intern. Med. 166 (4), 279–290. doi:10.7326/M16-1860
Rafi, S., Rasheed, H., Usman, M., Nawaz, H. A., Anjum, S. M., Chaudhry, M., et al. (2021). Availability of essential medicines in Pakistan—a comprehensive document analysis. PloS one 16 (7), e0253880. doi:10.1371/journal.pone.0253880
Ramzan, S., Timmins, P., Hasan, S. S., and Babar, Z. U. D. (2019). Trends in global prescribing of antidiabetic medicines in primary care: a systematic review of literature between 2000–2018. Prim. Care Diabetes 13 (5), 409–421. doi:10.1016/j.pcd.2019.05.009
Saeed, A., Lambojon, K., Saeed, H., Saleem, Z., Anwer, N., Aziz, M. M., et al. (2022). Access to insulin products in Pakistan: a national scale cross-sectional survey on prices, availability, and affordability. Front. Pharmacol. 13, 820621. doi:10.3389/fphar.2022.820621
Saeed, A., Saeed, F., Saeed, H., Saleem, Z., Yang, C., Chang, J., et al. (2021). Access to essential cardiovascular medicines in Pakistan: a national survey on the availability, price, and affordability, using Who/Hai methodology. Front. Pharmacol. 11, 595008. doi:10.3389/fphar.2020.595008
Saeed, A., Saeed, H., Saleem, Z., Fang, Y., and Babar, Z.-U.-D. (2019). Evaluation of prices, availability and affordability of essential medicines in lahore division, Pakistan: a cross-sectional survey using Who/Hai methodology. PloS one 14 (4), e0216122. doi:10.1371/journal.pone.0216122
Saeed, A., Saeed, H., Saleem, Z., Yang, C., Jiang, M., Zhao, M., et al. (2020). Impact of national drug pricing policy 2018 on access to medicines in lahore division, Pakistan: a pre-post survey study using Who/Hai methodology. BMJ open 10 (10), e034720. doi:10.1136/bmjopen-2019-034720
Saleem, F., Hassali, M. A., Iqbal, Q., Baloch, M., and Shanker, P. R. (2016). Uncontrollable medicine prices in Pakistan. Lancet 388 (10060), 2602. doi:10.1016/S0140-6736(16)32120-1
Saleem, Z., Saeed, H., Akbar, Z., Saeed, A., Khalid, S., Farrukh, L., et al. (2021). Who key access antibiotics prices, availability and affordability in private sector pharmacies in Pakistan. Cost Eff. Resour. Allocation 19, 10. doi:10.1186/s12962-021-00263-x
Saraiva, E. M. S., Coelho, J. L. G., dos Santos Figueiredo, F. W., and do Souto, R. P. (2020). Medication non-adherence in patients with type 2 diabetes mellitus with full access to medicines. J. Diabetes and Metabolic Disord. 19, 1105–1113. doi:10.1007/s40200-020-00612-2
Satheesh, G., Sharma, A., Puthean, S., Ansil, M. T. P., Jareena, E., Raj Mishra, S., et al. (2020). Availability, price and affordability of essential medicines for managing cardiovascular diseases and diabetes: a statewide survey in Kerala, India. Trop. Med. and Int. Health 25 (12), 1467–1479. doi:10.1111/tmi.13494
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). Idf diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Teng, J., Li, Q., Song, G., and Han, Y. (2024). Does the diagnosis-intervention packet payment reform impact medical costs, quality, and medical service capacity in secondary and tertiary hospitals? A difference-in-differences analysis based on a province in northwest China. Risk Manag. Healthc. Policy 17, 2055–2065. doi:10.2147/RMHP.S467471
Tornyos, D., Meuer, M., Lukács, R., El Alaoui El Abdallaoui, O., Kupó, P., Faludi, R., et al. (2022). Cardiovascular outcomes in patients treated with sodium-glucose transport protein 2 inhibitors, a network meta-analysis of randomized trials. Front. Cardiovasc. Med. 9, 1041200. doi:10.3389/fcvm.2022.1041200
Tribune (2024). Cabinet approves drug price deregulation. Available online at: https://tribune.com.pk/story/2455673/cabinet-approves-drug-price-deregulation (Accessed November 10, 2024).
United Nations (2015). Sustainable development goals. Available online at: https://sdgs.un.org/goals (Accessed November 09, 2024).
Vogler, S., Zimmermann, N., Knoll, V., Salcher-Konrad, M., Windisch, F., Espin, J., et al. (2024). We need to be part of the solution’: lessons from the 2024 ppri conference on ensuring access to affordable medicines through innovative policies. J. Pharm. Policy Pract. 17 (Suppl. 1), 2442002. doi:10.1080/20523211.2024.2442002
Wirtz, V. J., Hogerzeil, H. V., Gray, A. L., Bigdeli, M., de Joncheere, C. P., Ewen, M. A., et al. (2017). Essential medicines for universal health coverage. Lancet 389 (10067), 403–476. doi:10.1016/S0140-6736(16)31599-9
World Bank (2023). Poverty and inequality. Available online at: https://datatopics.worldbank.org/world-development-indicators/themes/poverty-and-inequality.html (Accessed July 02, 2025).
World Health Organization (2023). Who model list of essential medicines - 23rd list. Available online at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02 (Accessed November 09, 2024).
World Health Organization (2024). The top 10 causes of death. Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed November 09, 2024).
Yina, C., Pengcheng, L., Haomiao, N., and Yang, C. (2023). An empirical study of the impact of generic drug competition on drug market prices in China. Front. Public Health 11, 1146531. doi:10.3389/fpubh.2023.1146531
Yu, J., Lee, S.-H., and Kim, M. K. (2022). Recent updates to clinical practice guidelines for diabetes mellitus. Endocrinol. Metabolism 37 (1), 26–37. doi:10.3803/EnM.2022.105
Zhang, Y.-J., Ren, Y., Zheng, Q., Tan, J., Yao, M.-H., Huang, Y.-X., et al. (2022a). The impact of national centralized drug procurement on health expenditures for lung cancer inpatients: a difference-in-differences analysis in a large tertiary hospital in China. Front. Public Health 10, 956823. doi:10.3389/fpubh.2022.956823
Zhang, Z., Bai, Q., and Bian, Y. (2022b). “Cause analysis of high price differences drugs before and after the deregulation of drug price control: empirical study with big data on drug price data of 16 provinces in China,” in 2022 3rd International Conference on Education, Knowledge and Information Management (ICEKIM), Harbin, China, 21-23 January 2022 (IEEE).
Keywords: medicine price-deregulation, anti-diabetic medicines, access to medicines, pharmaceutical policy, national essential medicines list
Citation: Saeed A, Shukar S, Yasin NA, Yang C, Jiang M, Aziz MM, Zahoor H, Sunnan-Ud-Din M, Fang Y and Babar Z-U-D (2025) Pakistan’s first medicine price deregulation policy: assessing its impact on prices, affordability, and availability of oral anti-diabetic medicines in private pharmacies. Front. Pharmacol. 16:1627735. doi: 10.3389/fphar.2025.1627735
Received: 27 May 2025; Accepted: 07 July 2025;
Published: 16 July 2025.
Edited by:
Marcus Tolentino Silva, University of Brasilia, BrazilReviewed by:
Muhammad Usman, University of Veterinary and Animal Sciences, PakistanPatricia Vella Bonanno, Faculty of Health Sciences, University of Malta, Malta
Copyright © 2025 Saeed, Shukar, Yasin, Yang, Jiang, Aziz, Zahoor, Sunnan-Ud-Din, Fang and Babar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amna Saeed, ZHIuYW1uYXNhZWVkOTJAeGp0dS5lZHUuY24=; Yu Fang, eXVmYW5nQG1haWwueGp0dS5lZHUuY24=
 Amna Saeed
Amna Saeed Sundus Shukar
Sundus Shukar Najwa Ali Yasin
Najwa Ali Yasin Caijun Yang
Caijun Yang Minghuan Jiang
Minghuan Jiang Muhammad Majid Aziz3
Muhammad Majid Aziz3 Haris Zahoor
Haris Zahoor Muhammad Sunnan-Ud-Din
Muhammad Sunnan-Ud-Din Yu Fang
Yu Fang