- School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
Background: Rilertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), has demonstrated a favorable efficacy and safety profile in adult patients with EGFR T790M + advanced NSCLC. This study examined the cost-effectiveness of rilertinib compared with osimertinib in the second-line treatment for EGFR T790M mutation-positive advanced NSCLC in the Chinese healthcare setting.
Methods: A Markov model was developed to project economic and health outcomes. An unanchored matching-adjusted indirect comparison (MAIC) was used to compare the efficacy of rilertinib with osimertinib. Cost and utility values were obtained from Chinese health system data, public databases, and the published literature. Model robustness was assessed through deterministic and probabilistic sensitivity analyses (PSA).
Results: The incremental life-years (LYs) and quality-adjusted life years (QALYs) for the rilertinib group versus the osimertinib group were 0.34 and 0.30, respectively. The total cost for the rilertinib group was $3,774.60 higher than that for the osimertinib group. The incremental cost-effectiveness ratio (ICER) for rilertinib group compared with osimertinib group was $12,786.08, which is lower than one time the GDP per capita ($13,444.68). Based on the willingness-to-pay (WTP) thresholds in China, rilertinib represented a cost-effective option. Relative efficacy and drug costs parameters were the key drivers of the model outcomes. PSA showed rilertinib’s cost-effective probabilities were 51.6% at one-time GDP per capita and 90.2% at three-times GDP per capita ($40,334.05) WTP threshold.
Conclusion: From a Chinese healthcare system perspective, second-line treatment of EGFR T790M resistance mutation advanced NSCLC with rilertinib may have cost-effectiveness compared with osimertinib.
1 Introduction
Lung cancer is the predominant malignant tumor globally in terms of both incidence and mortality (Bray et al., 2024), posing a great threat to patient health. According to the latest statistics released by the National Cancer Center of China, the age-standardized incidence of lung cancer by the world standard population was 40.78/105 and the age-standardized mortality by the world standard population was 26.66/105 in 2022, both ranking first (Han et al., 2024). Non-small cell lung cancer (NSCLC) constitutes approximately 80%–85% of all lung cancer cases (Planchard et al., 2018; Gou and Wu, 2014; Bareschino et al., 2011), with a global 5-year overall survival rate of merely 16% (Li et al., 2021). Meanwhile, the economic burden on lung cancer patients is severe. In 2017, the aggregate economic burden attributable to lung cancer in China was estimated at 25.07 billion USD, representing 0.121% of China’s gross domestic product (GDP) (Liu et al., 2021). Projections suggest that this burden will increase to 53.40 billion USD by 2030 (Liu et al., 2021). Notably, drug costs constitute a primary component of this economic burden. In 2018, the average annual direct medical cost for lung cancer patients in China ranged from 7,766.15 to 10,874.14 USD, with the average cost per hospitalization spanning from 1,205.34 to 9,208.15 USD (Song et al., 2019). Drug costs accounted for the largest proportion (35.9% and 68.4% (Song et al., 2019)) of average cost per hospitalization.
Epidermal growth factor receptor (EGFR) mutations are the most prevalent driver mutations in Chinese patients with advanced NSCLC, accounting for 50.2% (Shi et al., 2015). Multiple Chinese guidelines recommend the use of EGFR tyrosine kinase inhibitors (TKIs) for the treatment of such patients (Huang et al., 2020; Zhi et al., 2025; Oncology Society of Chinese Medical Association, 2025). Among them, the Chinese Society of Clinical Oncology (CSCO) Guidelines for NSCLC (2025), the most authoritative NSCLC guideline in China, recommends EGFR-TKIs as the first-line therapy for patients with stage IV EGFR-mutant NSCLC (Huang et al., 2020). However, resistance against first- and second-generation EGFR TKIs inevitably emerges, typically resulting in a median progression-free survival (PFS) of only 9–14 months (Cheng et al., 2016). The most common mechanism of resistance is the development of the T790M secondary mutation, found in about 50% of patients treated with first- and second-generation TKIs (Soria et al., 2018). To address this challenge, third-generation EGFR TKIs (such as osimertinib and rilertinib) have been developed to effectively inhibit EGFR T790M–positive tumors and overcome resistance to earlier-generation EGFR-TKIs, extending the patients’ PFS and overall survival (OS) (Wu et al., 2018). Therefore, they are strongly recommended by the CSCO Guidelines for NSCLC (2025) as the second-line treatment. In addition to osimertinib and rilertinib, several other third-generation EGFR-TKIs, including aumolertinib, furmonertinib, befotertinib, and rezivertinib are also recommended in Chinese clinical guidelines for the second-line treatment. This increasingly competitive environment underscores the importance of evaluating the relative value and cost-effectiveness of each new option.
Rilertinib is a Class 1.1 innovative drug and third-generation EGFR-TKI independently developed by a Chinese pharmaceutical company. It has been approved in China by the National Medical Products Administration (NMPA) and was included in the 2024 National Reimbursement Drug List (NRDL). In a recent multicenter, single-arm, open-label Phase II clinical trial (SHC013-II-01, NCT03823807) (Xiong et al., 2022) conducted in China, rilertinib demonstrated promising efficacy in 227 patients with EGFR T790M mutation–positive NSCLC. According to assessments by the independent review committee (IRC), rilertinib achieved an objective response rate (ORR) of 60.8% (95% confidence interval [CI] 54.1%–67.2%), a median PFS of 12.2 months (95% CI 9.7-13.8), and a median OS of 25.9 months (95% CI 25.1-not available [NA]). Besides, the common treatment-emergent adverse events (TEAEs) of grade ≥3 (occurring in ≥1% of patients) included increased serum creatinine phosphokinase (4.5%), diarrhea (2.1%), upper respiratory tract infection (1.0%), and prolonged electrocardiogram QT interval (1.0%).
Cost-effectiveness is one of the most critical drug attributes in addition to the efficacy and safety. Although the clinical efficacy and safety of rilertinib has been established (Xiong et al., 2022), its cost-effectiveness as a scond-line therapy remains uncertain. Consequently, evaluating the cost-effectiveness is critical for understanding the true impact of rilertinib and choosing the optimal option among various alternatives with limited resources. Therefore, in accordance with the CSCO Guidelines for NSCLC (2025) (Guidelines of Chinese Society of Clinical Oncology CSCO, 2025) and the Chinese Guidelines for Pharmacoeconomic Evaluations (Liu et al., 2020), this study selects osimertinib, which has the same indication and a high recommendation level, as the comparator to conduct an economic evaluation of rilertinib. This evaluation aims to inform value-based clinical decision making, reduce patients’ economic burden, and provide a scientific basis for future work such as the renewal of the National Reimbursement Drug List (NRDL).
2 Materials and methods
2.1 Model structure
In accordance with the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) (Husereau et al., 2022), this study conducted a cost-utility analysis based on quality-adjusted life year (QALY), which are widely accepted as the most commonly used outcome measure in cost-utility analysis, to evaluate the cost-effectiveness of rilertinib versus osimertinib from the Chinese healthcare system’s perspective.
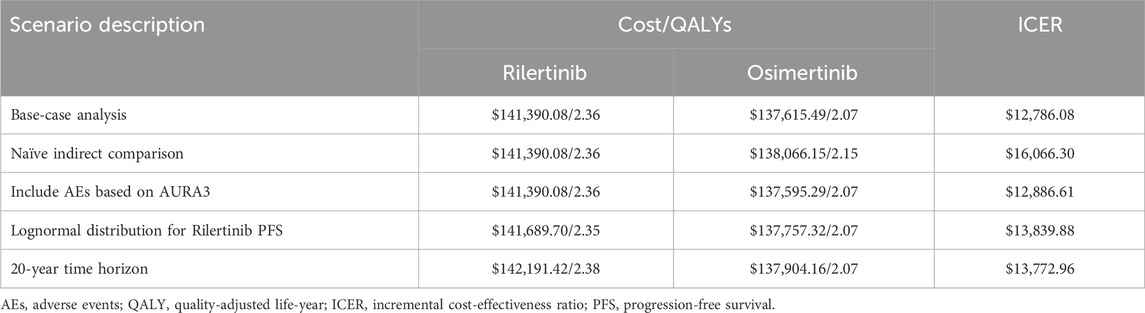
Considering the clinical characteristics of EGFR + NSCLC, such as its long disease course, high propensity for metastasis, and clear treatment sequencing, we have developed a five-state Markov model that considers second-line and sequential treatment regimens using Microsoft Excel for Mac 16.71 (Microsoft Corporation, Redmond, WA). The states included PFS 1, PFS 2, PFS 3, end-stage, and death (Figure 1).
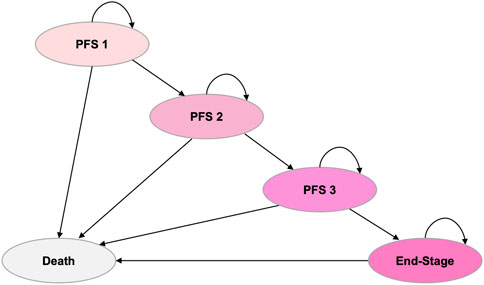
Figure 1. Markov model structure. The model includes five health states: PFS 1, PFS 2, PFS 3, end-stage, and death. Arrows indicate possible transitions between states. PFS, progression-free survival.
All patients entered the model in the PFS one state, where those in the intervention group received rilertinib and those in the control group received osimertinib. The model simulated the disease progression process and was validated through expert consultation to ensure its accuracy in reflecting real-world clinical practice. Both rilertinib and osimertinib are third-generation EGFR-TKIs that share a pyrimidine-based core structure. The structural modification of the indole ring in rilertinib may contribute to a more favorable safety profile. However, at present, there is no clinical evidence suggests that this structural difference leads to a distinct acquired resistance profile compared with osimertinib. Therefore, similar resistance mechanisms were assumed for both treatment groups. Based on clinical guidelines and expert advice, identical subsequent treatment regimens were applied in the PFS two and PFS three states: both groups received bevacizumab combined with pemetrexed and carboplatin in the PFS two state, and anlotinib combined with docetaxel in the PFS three state.
The number of patients in each PFS state (PFS 1–3) during each cycle was determined by the respective PFS curves corresponding to their treatment regimens. Patients who experienced disease progression in any PFS state transitioned to the subsequent state. Mortality in the death state incorporated the natural mortality rate of the Chinese population plus treatment-related adverse event mortality. The end-stage state included patients progressing in PFS 3. At any given cycle, patients occupied only one state.
The base-case analysis adopted a 15-year time horizon, representing a lifetime perspective for these patients, as the mortality rates for both groups were approximately 98.6% (rilertinib) and 99.6% (osimertinib). The model simulation used a starting age of 61 years, consistent with the mean age of patients in the rilertinib trial, with surviving patients projected to approach the average life expectancy of the Chinese population in 2021 (78.2 years) (Xinwen, 2022).
Costs and QALYs were discounted at 5% per annum in accordance with the China Guidelines for Pharmacoeconomics Evaluation 2020 (Liu et al., 2020). Costs incurred in previous years were adjusted using the consumer price index of 2005–2024 and were expressed in 2024 US dollars (1 USD = 7.12 CNY).
In this analysis, a willingness-to-pay (WTP) threshold of $40,334.05 per QALY—equivalent to three times the per capita GDP of China—was used to determine cost-effectiveness. The threshold was based on the China Guidelines for Pharmacoeconomics Evaluation 2020 (Liu et al., 2020) and supported by published studies (Thokala et al., 2018; Cameron et al., 2018; Tzanetakos and Gourzoulidis, 2023), which define treatments as cost-effective if the ICER is less than three times the GDP per capita ($40,334.05). Furthermore, a treatment is considered highly cost-effective if the ICER is less than one times the GDP per capita ($13,444.68). The estimated per capita GDP of China was $13,444.68 (CNY 95,749), derived from the 2024 Statistical Communiqué on National Economic and Social Development of the People’s Republic of China (The Statistical Communiqué, 2025).
Given the treatment cycles of rilertinib and subsequent therapies, and PFS data reported in rilertinib clinical trials at 3-month intervals, the cycle length for this model was set as 3 months. Disease progression was simulated through discrete cycles by dividing time into successive intervals; this discretization process could introduce minor inaccuracies in outcome and cost calculations. To mitigate such errors, a half-cycle correction was applied to refine the estimation.
2.2 Data sources, inputs, and modeling
2.2.1 Efficacy data
PFS data for rilertinib were estimated from the individual patient data (IPD) provided by the Sanhome pharmaceutical company, based the single-arm SHC013-II-01 trial (Xiong et al., 2022) in PFS one state. This study enrolled eligible adult patients with locally advanced or metastatic NSCLC who had experienced disease progression during or after previous EGFR TKIs treatment and were confirmed to have EGFR T790M mutation-positive through testing. Efficacy data for osimertinib were obtained from the AURA3 trial (Mok et al., 2017; Papadimitrakopoulou et al., 2020), the latest phase 3 trial evaluating osimertinib as second-line therapy, using the same eligibility criteria. In PFS two state, efficacy data were derived from the PointBreak trial (Patel et al., 2013), in which all patients received bevacizumab combined with pemetrexed and carboplatin. In PFS three state, efficacy data were derived from the ALTER 0303 trial (Han et al., 2018), in which all patients received anlotinib combined with docetaxel.
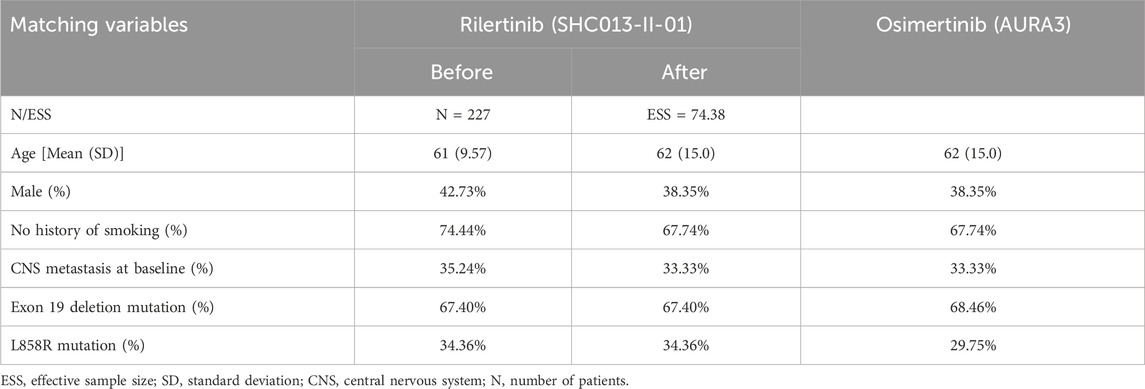
Due to the absence of head-to-head studies comparing rilertinib with osimertinib, indirect comparisons were required in this study. Given the availability of IPD of rilertinib but only aggregate data (AgD) of osimertinib, the unanchored matching-adjusted indirect comparison (MAIC) method (Phillippo et al., 2016) was used, as there was no common comparator in SHC013-II-01 and AURA3. For the selection of matching variables, we referred to the published literature employing MAIC, Cox analysis results, clinical opinions, and the availability of baseline characteristics. Ultimately, six variables were selected for MAIC: age, sex, smoking status, central nervous system (CNS) metastasis at baseline, Exon 19 deletion mutation, and L858R mutation. The Cox analysis results and the rationale for selecting these matching variables were presented in Supplementary Tables S1, S2, respectively. The selection of matching variables was based on the intersection of key prognostic factors reported in both the SHC013-II-01 trial IPD and the published AURA3 trial AgD (Mok et al., 2017; Papadimitrakopoulou et al., 2020). Consequently, variables such as race could not be included, as this information was unavailable in the rilertinib IPD. Similarly, ECOG performance status was not included as it was not reported in the AURA3 publication. However, the inclusion criteria for both trials restricted enrollment to patients with “locally advanced or metastatic NSCLC”, indicating substantial comparability in disease stage between the two cohorts. The final baseline characteristics before and after matching are shown in Table 1. The effective sample size (ESS) was 74.38; this metric is calculated to assess the validity of the matching process, with a larger value indicating a better balance. The detailed principles and calculation formula are described in Supplementary Methods 1.
The PFS curve for rilertinib was constructed based on the available IPD. The PFS curve of osimertinib was constructed based on reconstructed IPD data from the clinical trials. To reconstruct the data, this study used GetData Graph Digitizer and the algorithm by Guyot et al. (2012). Subsequently, the hazard ratio (HR) of PFS between the two interventions was calculated using the matching weights. After MAIC adjustment, the PFS-HR of rilertinib versus osimertinib was estimated at 0.763 (standard deviation [SD]: 0.155, 95% CI: 0.527 - 1.104).
To extrapolate the long-term efficacy of each treatment, this study used five parametric survival models recommended by NICE (Latimer, 2011) guidance documents (Exponential, Weibull, Gompertz, Log-logistic, and Lognormal) for curve fitting. Model selection was based on clinical plausibility, visual fit, and statistical goodness-of-fit [Akaike information criterion (AIC) and Bayesian information criterion (BIC)], where lower values indicate a better model fit. For PFS one state, the log-logistic distribution was selected to model the PFS curve for rilertinib, with the osimertinib’s PFS curve was derived by applying the HR adjustment. For PFS two state, the log-normal distribution was selected for the PFS curve for bevacizumab combined with pemetrexed and carboplatin. For PFS three state, the log-logistic distribution was selected for the PFS curve for anlotinib combined with docetaxel (AIC and BIC values for each PFS were shown in Supplementary Table S3; parametric survival curve fittings were presented in Supplementary Figures S1–S3). Additionally, the model integrated age-specific mortality reported in the 2020 China Population Census Yearbook (China Population Census Yearbook, 2020), along with mortality from treatment-related adverse events, to simulate survival outcomes more accurately.
2.2.2 Utility inputs
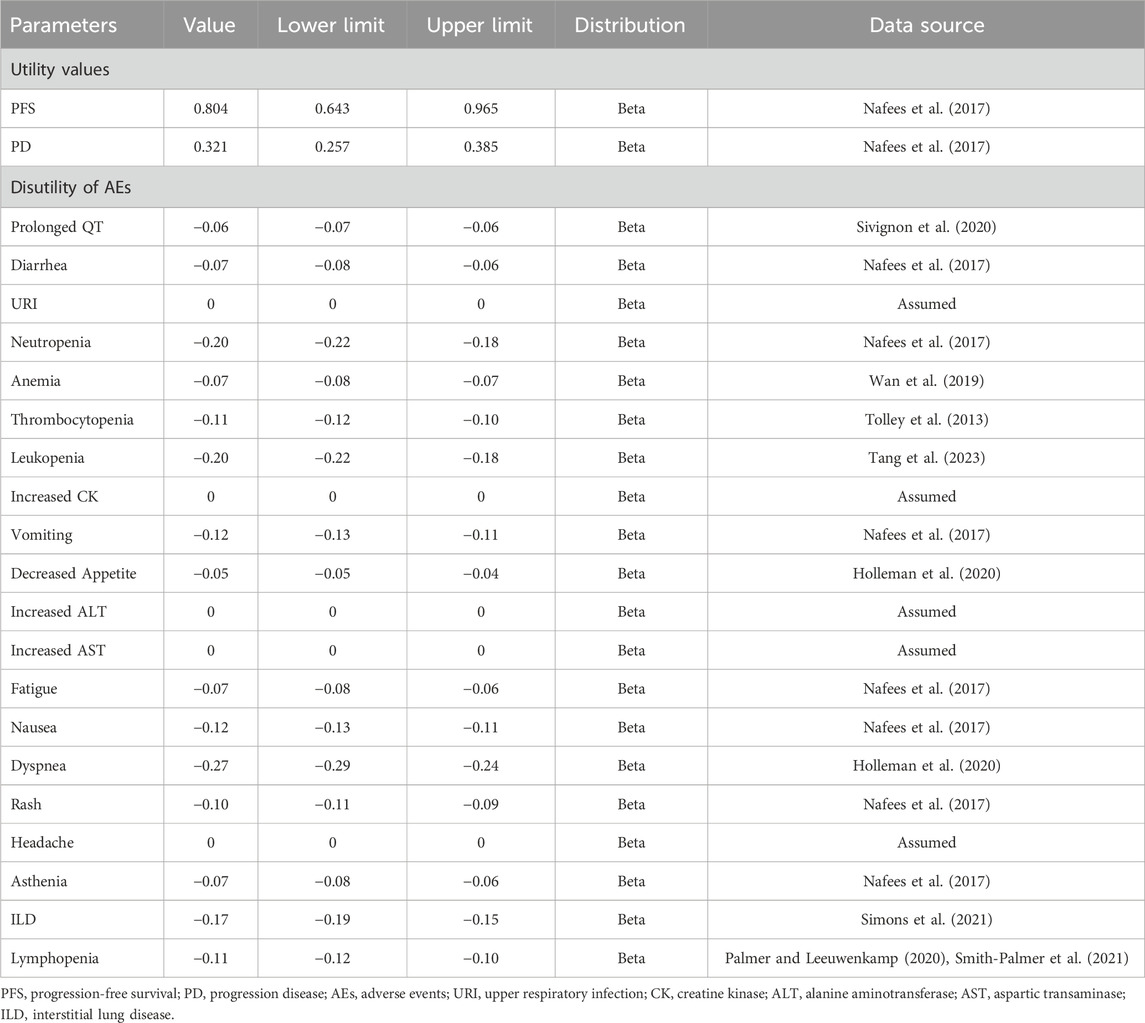
QALYs were determined through health state utility values (HSUVs). These HSU values were individually computed for patients in the PFS and PD states. An assumption was made that utility values remained consistent across different treatment groups. HSU values of PFS and PD were sourced from a study of health state utilities in NSCLC conducted by Nafees et al. (2017), since the original trials of both interventions did not measure patients’ quality of life. We extracted the China-specific utility values, and the values of PFS 1, PFS two and PFS three states were 0.804 while the value of end-stage state was 0.321 in the base–case analysis. Furthermore, disutilities associated with treatment-related adverse events (AEs) with a severity of grade ≥ 3 and an incidence of ≥1% were integrated into the model. Because these AEs were expected to meaningfully reduce the quality of life, grade 1/2 AEs were generally self-limited (Lu et al., 2023). The AEs incidence of rilertinib was provided by Sanhome. Given osimertinib’s extensive clinical use and well-documented safety profile, which are comprehensively reported in its package insert, AEs and their incidence for the osimertinib arm were derived from the drug’s package insert in the base-case analysis.
These disutility values were derived from existing published research and were limited to the initial treatment cycles. Detailed information on utility values used in this study is shown in Table 2. For AEs with unavailable disutility data, a disutility of 0 was assumed.
2.2.3 Resource use and costs
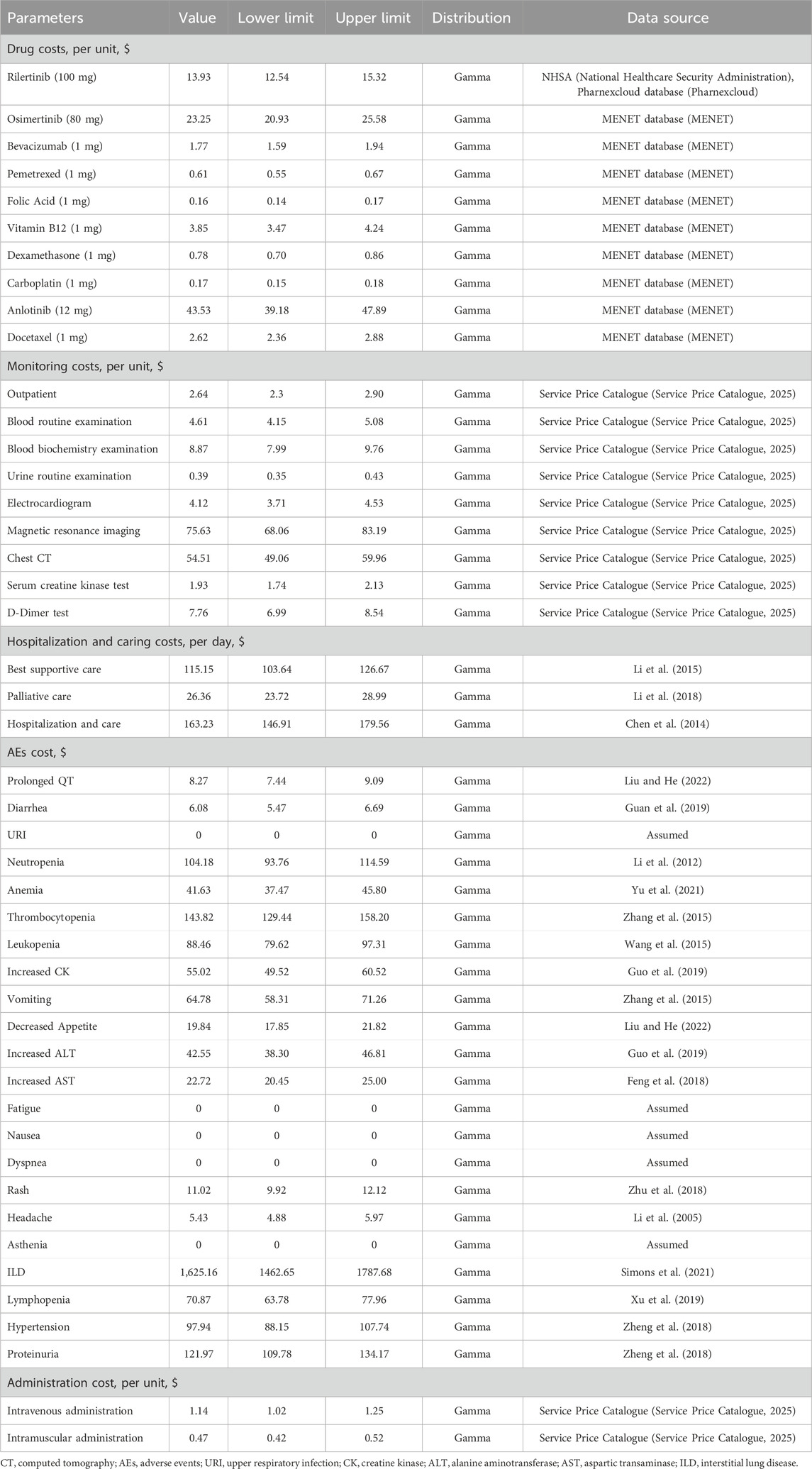
This study calculated costs from a China’s healthcare system perspective, including only direct medical costs (Liu et al., 2020) (Table 3). Most costs were derived from the published literature, and on this basis, opinions of clinical experts were considered. For drugs with multiple specifications, prices were standardized to per-milligram cost to facilitate the calculation.
1 Drug costs
In PFS one state, it was assumed that subjects would not incur additional drug administration costs, as both rilertinib and osimertinib are oral drugs. The latest price of rilertinib was 13.93 USD/100 mg (National Healthcare Security Administration, 2025; Pharnexcloud database, 2025), while the price of osimertinib was 23.25 USD/80 mg (MENET, 2025). In the PFS two state, the drug costs encompassed not only the expenses of bevacizumab injection, pemetrexed disodium injection, and carboplatin, but also covered prophylactic drugs (folic acid, vitamin B12, dexamethasone), along with the costs associated with administering non-oral drugs. In PFS three state, the drug costs involved anlotinib, docetaxel injection, prophylactic dexamethasone, and the charges for non-oral drug administration. Detailed information on drug costs in this study is shown in Table 3.
2 Monitoring, hospitalization and caring costs
This study referenced the CSCO Guidelines for NSCLC (2025) (Guidelines of Chinese Society of Clinical Oncology CSCO, 2025), the SHC013-II-01 clinical trial data provided by the company (Sanhome), the package inserts of rilertinib and osimertinib, and expert surveys to define the items of monitoring in PFS 1. Monitoring items for each treatment were determined based on their respective package inserts. Unit costs for these items were derived from Service Price Catalogue (Table 3). According to clinical expert consensus, patients progressing to PFS two or PFS three states require hospitalization, with examination costs included in “hospitalization and care costs”; best supportive care costs were also incorporated into these two states. Costs in the end-stage state primarily comprised “palliative care costs”. Costs associated with best supportive care, palliative care, and hospitalization and care cost were derived from published literature (Table 3). The frequencies of detailed monitoring, hospitalization and caring are shown in Supplementary Tables S4 and S5.
3 AEs costs
This study included adverse event costs based on the clinical trials and package insert, and only considered the management caused by AEs with a severity of grade ≥3 and an incidence of ≥1% as they were expected to result in significant healthcare utilization (Lu et al., 2023). AE management costs were limited to the initial treatment cycles and calculated once. Unit costs for AEs were derived from published sources or assumed, and the unit cost, and source of AEs are shown in Table 3. The incidence rates of AEs for each drug are shown in Supplementary Table S6.
2.3 Sensitivity analysis
A comprehensive uncertainty assessment was conducted through a series of sensitivity analyses to identify key drivers of model outcomes. For deterministic sensitivity analysis (DSA), each parameter was individually varied within ±10% of the baseline value or within the 95% confidence interval, with the results presented in tornado diagrams. In PSA, multivariate parameter sampling was executed through 1,000 iterations of Monte Carlo simulation. Uncertainty in the HRs of PFS was estimated with normal distributions, health state utility followed beta distributions, and costs were assigned gamma distributions. The results of the PSA were presented through cost-effectiveness acceptability curves (CEACs) and probabilistic scatter plots. This study also conducted scenario analysis, and the scenarios were shown in Table 5.
3 Results
3.1 Base-case analysis
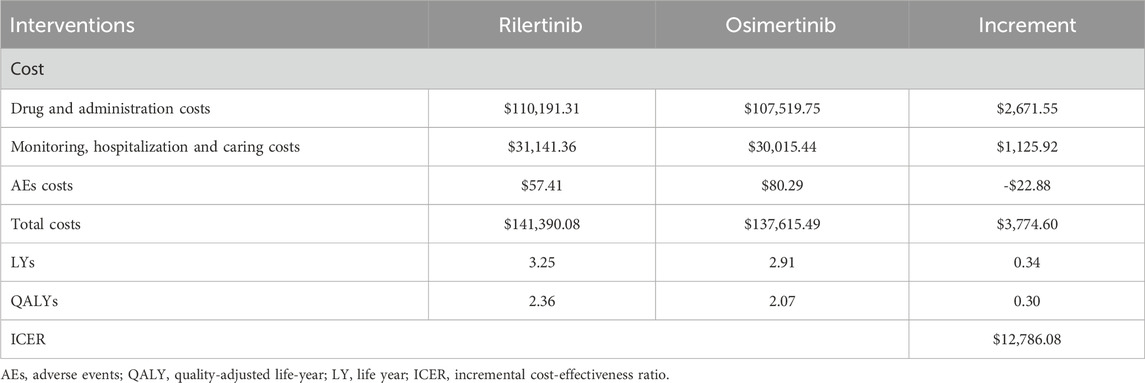
The results of the base–case analysis are presented in Table 4. For rilertinib, the mean costs and QALYs were $141,390.08 and 2.36 respectively, while for osimertinib, the mean costs and QALYs were $137,615.49 and 2.07 respectively. The ICER for rilertinib versus osimertinib is $12,786.08/QALY, which falls below the threshold of 1 times China’s per capita GDP, indicating that rilertinib is highly cost-effective treatment option.
3.2 Sensitivity analysis
Ten of the most influential parameters in the DSA are illustrated in Figure 2. Among all parameters, the HR for PFS of rilertinib versus osimertinib has the largest impact on the ICER: when the HR took the lower limit (0.527), the ICER was $8,738.45; when the HR took the upper limit (1.104), the ICER became -$26,080.72, indicating the cost-effectiveness result would reverse. Therefore, the naive indirect comparison results were incorporated into the scenario analysis for comprehensive evaluation. After the PFS HR and discount rate for costs, the ICER is most sensitive to the prices of rilertinib and osimertinib.
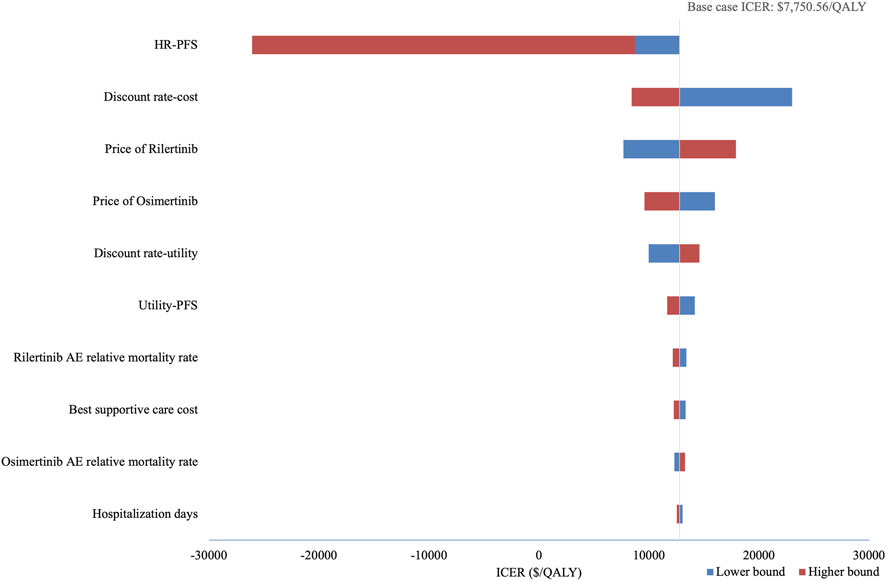
Figure 2. Tornado diagram of deterministic sensitivity analysis. This diagram illustrates the impact of key parameters on the ICER. The vertical line represents the base-case ICER, and horizontal bars show the range of ICERs when varying each parameter. HR, hazard ratio; PFS, progression-free survival; AE, adverse event; ICER, incremental cost-effectiveness ratio.
PSA show an average QALY gain of 0.291 and an incremental cost of $3,886.63, resulting in a probabilistic ICER of $13,361.88/QALY which is consistent with the base-case analysis result. The CEAC is shown in Figure 3, which indicates that when the WTP threshold exceeds $13,146.07, rilertinib has a greater than 50% probability of being the cost-effective option. The scatter plot in Figure 4 visualizes the outcome of all PSA simulations. Most simulations fell within the first quadrant, associating rilertinib with increased costs and QALYs. Notably, 90.20% of these points lie below the three-times GDP per capita threshold (blue line), and 51.60% below the one-time GDP threshold (green line). This distribution provides supportive evidence for the cost-effectiveness of rilertinib.
Figure 3. Cost-effectiveness acceptability curve. The curve shows the probability of rilertinib being cost-effective at various WTP thresholds. WTP, willingness-to-pay; GDP, gross domestic product.
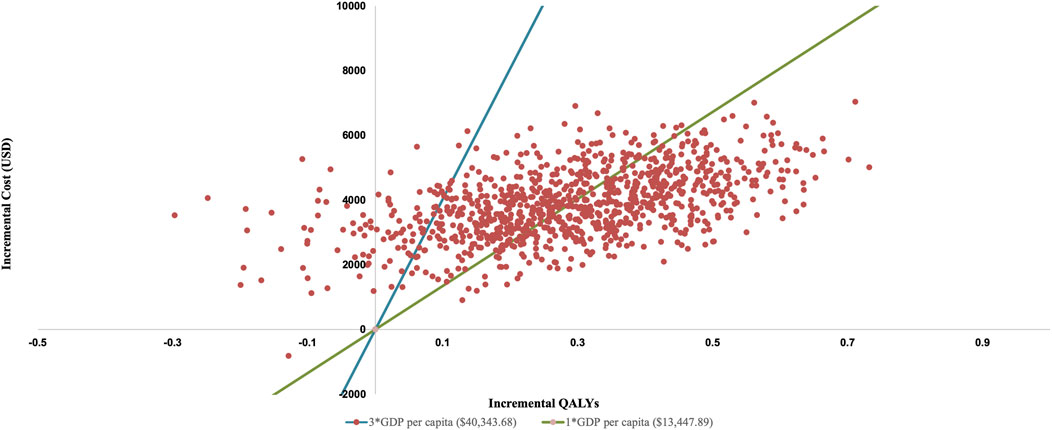
Figure 4. Probabilistic scatter plot of the ICER between rilertinib and osimertinib. Each point represents the results of a single PSA iteration. QALY, quality adjusted life year; GDP, gross domestic product.
The results of the scenario analysis are detailed in Table 5. In all scenarios, the ICERs are below 3 times China’s per capita GDP ($40,343.68), with the ICER in Scenario two falling below 1 times the per capita GDP ($13,447.89). Cost-effectiveness trends are robust across all variables in both the sensitivity and scenario analyses.
4 Discussion
In recent years, China has witnessed rapid development of innovative anticancer drugs, which have significantly improved patient outcomes. According to the Center for Drug Evaluation (CDE) of China (NMPA, 2024), anti-tumor drugs represent the largest category among all approved drugs. However, their high cost may limit patient accessibility (Hao et al., 2021) pose a threat to the long-term sustainability of the healthcare system (Migliorino et al., 2017). Economic evidence has been institutionalized as a critical component in China’s annual national drug reimbursement negotiations, allowing for dynamic updates to the National Reimbursement Drug List (NRDL) and thereby improving the accessibility and affordability of innovative therapies (Gao and Liu, 2017).
In this study, the mean total costs and QALYs for rilertinib were $141,390.08 and 2.36 respectively, compared with $137,615.49 and 2.07 for osimertinib. The ICER for rilertinib versus osimertinib was $12,786.08/QALY, falling below the threshold of one time China’s per capita GDP, indicating that rilertinib is highly cost-effective treatment option. In addition to its favorable economic profile, rilertinib demonstrated robust clinical efficacy.
To the best of our knowledge, this study represents the first cost-effectiveness analysis comparing rilertinib versus osimertinib, both the first-in-class and third-generation EGFR-TKI, for treating locally advanced or metastatic NSCLC in adult patients with confirmed EGFR T790M mutation after progression on prior EGFR TKIs therapy. Given that rilertinib’s approval was based on single-arm trial data, an unanchored MAIC was employed. This approach, recommended by NICE for indirect treatment comparisons, was used to balance the baseline characteristics between two trial populations and reduce potential bias in survival data caused by uneven covariate distribution. Previous studies have shown that NSCLC patients harboring canonical EGFR mutations (Exon 19 deletion or L858R) derive greater benefit from third-generation EGFR-TKIs (Gomez-Randulfe et al., 2025; Gristina et al., 2020; Borgeaud et al., 2024) with mutation subtypes significantly affecting cost-effectiveness estimates (Tian et al., 2024). Therefore, in this study, the proportions of canonical mutations were aligned across treatment groups via the MAIC approach, further mitigating potential efficacy bias.
DSA revealed that the hazard ratio (HR) for PFS had the greatest impact on the model outcomes. Accordingly, a scenario analysis was conducted, including a naïve indirect comparison in Scenario 1, which confirmed that the ICER remained below three times the GDP per capita, with no reversal of cost-effectiveness. PSA further supported the robustness of the base-case findings, showing that rilertinib had a 90.20% probability of being cost-effective at the WTP threshold, reinforcing the robustness of the base-case results. Across all scenario analysis, no ICER exceeded the 3 times GDP per capita, reinforcing the reliability of the conclusions.
To date, no published study has evaluated the cost-effectiveness of rilertinib as a second-line treatment for EGFR T790M-positive advanced NSCLC. Although previous research has established osimertinib’s cost-effectiveness in the second-line setting (Guan et al., 2019; Shi et al., 2022), even after several rounds of price reduction in China, this analysis demonstrates that rilertinib maintains cost-effectiveness. These findings the economic value of rilertinib for Chinese patients with EGFR T790M-positive advanced NSCLC.
This study has several advantages. First, given that rilertinib’s second-line indication has already been included in the NRDL, this analysis provides timely pharmacoeconomic evidence supporting its continued inclusion, offering guidance for clinical decision-making and reimbursement policy. Second, a five-state sequential Markov model was developed based on real-world clinical pathways, informed by expert consultation, and covering multiple lines of therapy through to death. This approach provides a comprehensive economic framework for evaluating long-term treatment outcomes. Third, treatment efficacy was compared using a rigorous MAIC methodology, ensuring balanced comparison in the absence of head-to-head trials. Fourth, extensive sensitivity analyses confirmed the robustness of the model.
Nonetheless, this study has several limitations. First, although MAIC was used to adjust for observed baseline differences between rilertinib and osimertinib trial populations, residual confounding from unobserved prognostic factors may remain. For instance, variables such as ECOG performance status and race could not be matched, as they were not reported in the AURA3 or were unavailable in the IPD of rilertinib. Future head-to-head randomized controlled trials are needed to validate these results. Second, utility data were derived from literature, using methods consistent with prior studies (Wu et al., 2018; Guan et al., 2019; Wu et al., 2019), which may introduce uncertainty. However, sensitivity analysis demonstrated that the ICER remained stable and below the threshold. Third, the model adopted protocol-specified dosing rather than real-world practices patterns, potentially affecting resource use estimates. Despite this, a series of sensitivity analysis confirmed the robustness of the model across a broad range of parameter values. Fourth, subgroup analyses based on potential differences in acquired resistance mechanisms or patient histology were not performed due to limited evidence. Nevertheless, the unified post-progression pathway in our model reflects the standard of care for the overall T790M-positive population, an approach supported by major clinical guidelines as well as expert consultation. These assumptions are considered reasonable and are unlikely to affect the study’s main conclusions.
Based on the findings and limitations, future research should focus on the following aspects. First, as the clinical use of rilertinib increases, it will be essential to incorporate emerging real-world data to update and validate the simulation, for providing more comprehensive evidence. Second, real-world evidence collection should specifically investigate potential differences in resistance mechanisms between third-generation EGFR-TKIs and their impacts on clinical outcomes to further refine treatment pathways and economic evaluations. Third, evidence on key patient subgroups should be accumulated to enable future subgroup analyses and provide more nuanced cost-effectiveness evidence.
5 Conclusion
This study utilized clinical trial data (Xiong et al., 2022; Mok et al., 2017; Papadimitrakopoulou et al., 2020) to perform a robust indirect comparison of efficacy and incorporated local resource utilization and unit cost data to model the cost-effectiveness of rilertinib. The results suggest that, compared with osimertinib, rilertinib may be associated with longer PFS and yielded greater LYs and QALYs. For adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC who progressed after prior EGFR TKIs, rilertinib could represent a cost-effective treatment option based on the available evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XL: Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review and editing. YnL: Data curation, Formal Analysis, Funding acquisition, Writing – review and editing. YqL: Data curation, Formal Analysis, Funding acquisition, Writing – review and editing. MZ: Data curation, Formal Analysis, Writing – review and editing. XX: Data curation, Formal Analysis, Writing – review and editing. YZ: Data curation, Formal Analysis, Writing – review and editing. JQ: Data curation, Formal Analysis, Writing – review and editing. ZD: Data curation, Formal Analysis, Methodology, Writing – review and editing. FC: Data curation, Formal Analysis, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Jiangsu Funding Program for Excellent Postdoctoral Talent (2023ZB105). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1628024/full#supplementary-material
References
Bareschino, M. A., Schettino, C., Rossi, A., Maione, P., Sacco, P. C., Zeppa, R., et al. (2011). Treatment of advanced non small cell lung cancer. J. Thorac. Dis. 3 (2), 122–133. doi:10.3978/j.issn.2072-1439.2010.12.08
Borgeaud, M., Parikh, K., Banna, G. L., Kim, F., Olivier, T., Le, X., et al. (2024). Unveiling the landscape of uncommon EGFR mutations in NSCLC-A systematic review. J. Thorac. Oncol. 19 (7), 973–983. doi:10.1016/j.jtho.2024.03.016
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Cameron, D., Ubels, J., and Norström, F. (2018). On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob. Health Action 11 (1), 1447828. doi:10.1080/16549716.2018.1447828
Chen, Z., Leng, J., and Gao, G. (2014). Analysis on hospitalization expense of five kinds of cancers and its influencing factors. Chin. Health Econ. 33 (07), 57–60. doi:10.7664/CHE20140717
Cheng, H., Nair, S. K., and Murray, B. W. (2016). Recent progress on third generation covalent EGFR inhibitors. Bioorg Med. Chem. Lett. 26 (8), 1861–1868. doi:10.1016/j.bmcl.2016.02.067
China Population Census Yearbook (2020). Office of the leading Group of the State Council for the seventh national population census. China population census yearbook 2020. Available online at: http://www.stats.gov.cn/sj/pcsj/rkpc/7rp/zk/indexch.htm (Accessed March 28, 2025).
Feng, X., Feng, Z., and Ma, Y. (2018). Clinical trial of compound glycyrrhizin tablets in the treatment of autoimmune hepatitis-primary biliary cirrhosis overlap syndrome. Chin. J. Clin. Pharmacol. 34 (03), 240–243. doi:10.13699/j.cnki.1001-6821.2018.03.015
Gao, S., and Liu, G. (2017). The application of pharmacoeconomics in the field of medicine and health. Chin. J. phar Econ. 12, 16–18. doi:10.12010/j.issn.1673-5846.2017.08.003
Gomez-Randulfe, I., Scanlon, L. A., Carter, M., Moliner, L., Cil, E., Califano, R., et al. (2025). First-line osimertinib compared to earlier generation TKIs in advanced EGFR-mutant NSCLC: a real-world survival analysis. Lung Cancer 200, 108084. doi:10.1016/j.lungcan.2025.108084
Gou, L. Y., and Wu, Y. L. (2014). Prevalence of driver mutations in non-small-cell lung cancers in the People'S Republic of China. Lung Cancer (Auckl) 5, 1–9. doi:10.2147/LCTT.S40817
Gristina, V., Malapelle, U., Galvano, A., Pisapia, P., Pepe, F., Rolfo, C., et al. (2020). The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: a systematic review and critical appraisal. Cancer Treat. Rev. 85, 101994. doi:10.1016/j.ctrv.2020.101994
Guan, H., Liu, G., Xie, F., Sheng, Y., and Shi, L. (2019). Cost-effectiveness of osimertinib as a second-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer in China. Clin. Ther. 41 (11), 2308–2320. doi:10.1016/j.clinthera.2019.09.008
Guidelines of Chinese Society of Clinical Oncology (CSCO) (2025). Non-small cell lung cancer. Beijing: People’s Medical Publishing House.
Guo, P., Chen, X., and Wang, X. (2019). Progress in the mechanism and clinical treatment of antiplatelet resistance of aspirin and clopidogrel. Chin. J. Clin. Neurosci. 27 (03), 321–328.
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-Meier survival curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., et al. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 4 (11), 1569–1575. doi:10.1001/jamaoncol.2018.3039
Han, B., Zheng, R., Zeng, H., Wang, S., Sun, K., Chen, R., et al. (2024). Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 4 (1), 47–53. doi:10.1016/j.jncc.2024.01.006
Hao, X., Shen, A., and Wu, B. (2021). Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced non-small-cell lung cancer. Front. Pharmacol. 12, 573852. doi:10.3389/fphar.2021.573852
Holleman, M. S., Al, M. J., Zaim, R., Groen, H. J. M., and Uyl-de Groot, C. A. (2020). Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur. J. Health Econ. 21 (1), 153–164. doi:10.1007/s10198-019-01117-3
Huang, L., Jiang, S., and Shi, Y. (2020). Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J. Hematol. Oncol. 13 (1), 143. doi:10.1186/s13045-020-00977-0
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 25 (1), 3–9. doi:10.1016/j.jval.2021.11.1351
Latimer, N. (2011). NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK395885/pdf/Bookshelf_NBK395885.pdf (Accessed April 16, 2025).
Li, J., Gu, X., and Xv, Y. (2005). Clinical trial of ibuprofen soft capsule in treating migraine. Pharm. Clin. Res. (06), 29–31. doi:10.13664/j.cnki.pcr.2005.06.014
Li, H., Fan, C., and Huang, X. (2012). Pharmacoeconomic evaluation of gefitinib in the treatment of advanced non-small cell lung cancer. Chin. J. New Drugs. 21 (17), 2077–2085.
Li, X., Wang, Y., Wang, Y., Chen, J., Wu, S., Hu, C., et al. (2015). Supportive care costs associated with second-line chemotherapy in Chinese patients with advanced non-squamous non-small cell lung cancer: a retrospective cohort study. Drugs Real World Outcomes 2 (1), 87–97. doi:10.1007/s40801-015-0017-6
Li, Z., Jiang, S., He, R., Dong, Y., Pan, Z., Xu, C., et al. (2018). Trajectories of hospitalization cost among patients of end-stage lung cancer: a retrospective study in China. Int. J. Environ. Res. Public Health 15 (12), 2877. doi:10.3390/ijerph15122877
Li, S., Li, J., Peng, L., Li, Y., and Wan, X. (2021). Cost-effectiveness of lorlatinib as a first-line therapy for untreated advanced anaplastic lymphoma kinase-positive non-small cell lung cancer. Front. Oncol. 11, 684073. doi:10.3389/fonc.2021.684073
Liu, J., and He, X. (2022). Cost-utility analysis of osimertinib for adjuvant treatment ofepidermal grouth factor receptor mutation-positive non-small cell lung cancer. World Clin. Drug 43 (06), 736–745. doi:10.13683/j.wph.2022.06.015
Liu, G., Hu, S., Wu, J., Wu, J., Dong, C., and Li, H. (2020). China guidelines for pharmacoeconomic evaluations (Chinese-English version). Beijing: China Market Press.
Liu, C., Shi, J., Wang, H., Yan, X., Wang, L., Ren, J., et al. (2021). Population-level economic burden of lung cancer in China: provisional prevalence-based estimations, 2017-2030. Chin. J. Cancer Res. 33 (1), 79–92. doi:10.21147/j.issn.1000-9604.2021.01.09
Lu, Y., Dai, Z., Chang, F., Wang, L., He, J., Shi, P., et al. (2023). Whether and how disutilities of adverse events were used in the economic evaluation of drug therapy for cancer treatment. PharmacoEconomics 41, 295–306. doi:10.1007/s40273-022-01232-9
MENET (2025). MENET database. Available online at: https://www.menet.com.cn/(Accessed March 28, 2025).
Migliorino, M. R., Santo, A., Romano, G., Cortinovis, D., Galetta, D., Alabiso, O., et al. (2017). Economic burden of patients affected by non-small cell lung cancer (NSCLC): the LIFE study. J. Cancer Res. Clin. Oncol. 143 (5), 783–791. doi:10.1007/s00432-016-2326-x
Mok, T. S., Wu, Y.-L., Ahn, M.-J., Garassino, M. C., Kim, H. R., Ramalingam, S. S., et al. (2017). Osimertinib or platinum-pemetrexed in EGFR T790M-Positive lung cancer. N. Engl. J. Med. 376 (7), 629–640. doi:10.1056/NEJMoa1612674
Nafees, B., Lloyd, A. J., Dewilde, S., Rajan, N., and Lorenzo, M. (2017). Health state utilities in non-small cell lung cancer: an international study. Asia Pac J. Clin. Oncol. 13, e195–e203. doi:10.1111/ajco.12477
National Healthcare Security Administration (2025). National healthcare security administration. Available online at: http://ybj.hunan.gov.cn/ybj/first113541/firstF/f3113607/202501/t20250124_33574687.html (Accessed March 28, 2025).
NMPA (2024). Center for drug evaluation, NMPA. Available online at: https://www.cde.org.cn/main/news/viewInfoCommon/54538c67b7e764fc51666567fc620241 (Accessed March 28, 2025).
Oncology Society of Chinese Medical Association (2025). Chinese medical association guideline for clinical diagnosis and treatment of lung cancer for patients (2024 edition). Chin. J. Oncol. 47 (01), 26–38. doi:10.3760/cma.j.cn112152-20241024-00464
Palmer, J., and Leeuwenkamp, O. R. (2020). Cost-effectiveness of lutetium (177Lu) oxodotreotide vs everolimus in gastroenteropancreatic neuroendocrine tumors in Norway and Sweden. World J. Clin. Cases 8 (20), 4793–4806. doi:10.12998/wjcc.v8.i20.4793
Papadimitrakopoulou, V. A., Mok, T. S., Han, J. Y., Ahn, M. J., Delmonte, A., Ramalingam, S. S., et al. (2020). Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-Tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann. Oncol. 31 (11), 1536–1544. doi:10.1016/j.annonc.2020.08.2100
Patel, J. D., Socinski, M. A., Garon, E. B., Reynolds, C. H., Spigel, D. R., Olsen, M. R., et al. (2013). PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 31 (34), 4349–4357. doi:10.1200/JCO.2012.47.9626
Pharnexcloud database (2025). Pharnexcloud database. Available online at: https://www.pharnexcloud.com/ (Accessed April 8, 2025).
Phillippo, D. M., Ades, A. E., Dias, S., Palmer, S., Abrams, K. R., and Welton, N. J. (2016). NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submission to NICE. Available online at: https://research-information.bris.ac.uk/ws/portalfiles/portal/94868463e/Population_adjustment_TSD_FINAL.pdf (Accessed March 28, 2025).
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E. F., Faivre-Finn, C., et al. (2018). Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29 (Suppl. 4), 192–237. doi:10.1093/annonc/mdy275
Service Price Catalogue (2025). Service price catalogue of five provinces and municipalities: jiangsu, beijing, Hubei, sichuan, Guangdong. Available online at: https://m12333.cn/policy/miwur.html (Accessed March 28, 2025).
Shi, Y., Li, J., Zhang, S., Wang, M., Yang, S., et al. (2015). Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology - mainland China subset analysis of the PIONEER study. PLoS One 10 (11), e0143515. doi:10.1371/journal.pone.0143515
Shi, Y., Pei, R., and Liu, S. (2022). Osimertinib versus platinum-pemetrexed in patients with previously treated EGFR T790M advanced non-small cell lung cancer: an updated AURA3 trial-based cost-effectiveness analysis. Front. Oncol. 12, 833773. doi:10.3389/fonc.2022.833773
Simons, C. L., Malone, D., Wang, M., Maglinte, G. A., Inocencio, T., Wade, S. W., et al. (2021). Cost-effectiveness for KTE-X19 CAR T therapy for adult patients with relapsed/refractory mantle cell lymphoma in the United States. J. Med. Econ. 24 (1), 421–431. doi:10.1080/13696998.2021.1894158
Sivignon, M., Monnier, R., Tehard, B., and Roze, S. (2020). Cost-effectiveness of alectinib compared to crizotinib for the treatment of first-line ALK+ advanced non-small-cell lung cancer in France. PLoS One 15 (1), e0226196. doi:10.1371/journal.pone.0226196
Smith-Palmer, J., Leeuwenkamp, O. R., Virk, J., and Reed, N. (2021). Lutetium oxodotreotide (177Lu-Dotatate) for the treatment of unresectable or metastatic progressive gastroenteropancreatic neuroendocrine tumors: a cost-effectiveness analysis for Scotland. BMC Cancer 21 (1), 10. doi:10.1186/s12885-020-07710-7
Song, J., Guan, H., and Liu, G. (2019). Direct medical cost of lung cancer in China: a systematic review. Chin. J. Evidence-Based Med. 19 (01), 44–53. doi:10.7507/1672-2531.201808083
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378 (2), 113–125. doi:10.1056/NEJMoa1713137
Tang, Y., Zhao, M., and Tang, W. (2023). Pharmacoeconomic evaluation of sintilimab versus camrelizumab in the First-LineTreatment of patients with non-squamous advanced non-small cell lung cancer in China. Health Econ. Res. 40 (02), 34–40. doi:10.14055/j.cnki.33-1056/f.2023.02.005
The Statistical Communiqué (2025). The statistical communiqué of the people’s Republic of China on the 2024 national economic and social development. Available online at: https://www.gov.cn/lianbo/bumen/202502/content_7008605.htm (Accessed March 28, 2025).
Thokala, P., Ochalek, J., Leech, A. A., and Tong, T. (2018). Cost-Effectiveness thresholds: the past, the present and the future. Pharmacoeconomics 36 (5), 509–522. doi:10.1007/s40273-017-0606-1
Tian, W., Niu, L., Zhou, R., Wang, Z., Ning, J., Lu, R., et al. (2024). Cost-effectiveness analysis of osimertinib plus chemotherapy for patients with EGFR-mutated advanced non-small cell lung cancer. Cancer Med. 13 (16), e70083. doi:10.1002/cam4.70083
Tolley, K., Goad, C., Yi, Y., Maroudas, P., Haiderali, A., and Thompson, G. (2013). Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur. J. Health Econ. 14 (5), 749–759. doi:10.1007/s10198-012-0419-2
Tzanetakos, C., and Gourzoulidis, G. (2023). Does a standard cost-effectiveness threshold exist? The case of Greece. Value Health Reg. Issues 36, 18–26. doi:10.1016/j.vhri.2023.02.006
Wan, X., Luo, X., Tan, C., Zeng, X., Zhang, Y., and Peng, L. (2019). First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer 125 (20), 3526–3534. doi:10.1002/cncr.32368
Wang, X., Shou, T., and Hu, J. (2015). A clinical study of rhG-CSF of different dosages in preventing leukopenia after chemotherapy inpatients with advanced non-small cell lung cancer. China Oncol. 25 (10), 823–827. doi:10.3969/j.issn.1007-3969.2015.10.012
Wu, B., Gu, X., and Zhang, Q. (2018). Cost-Effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapy. J. Thorac. Oncol. 13 (2), 184–193. doi:10.1016/j.jtho.2017.10.012
Wu, B., Gu, X., Zhang, Q., and Xie, F. (2019). Cost-Effectiveness of osimertinib in treating newly diagnosed, advanced EGFR-Mutation-Positive non-small cell lung cancer. Oncologist 24 (3), 349–357. doi:10.1634/theoncologist.2018-0150
Xinwen (2022). The central people's government of the People'S Republic of China. Available online at: https://www.gov.cn/xinwen/2022-07/12/content_5700670.htm (Accessed March 28, 2025).
Xiong, A., Ren, S., Liu, H., Miao, L., Wang, L., Chen, J., et al. (2022). Efficacy and safety of SH-1028 in patients with EGFR T790M-Positive NSCLC: a multicenter, Single-Arm, Open-Label, phase 2 trial. J. Thorac. Oncol. 17 (10), 1216–1226. doi:10.1016/j.jtho.2022.06.013
Xu, Y., Mao, N., Chirikov, V., Du, F., Yeh, Y. C., Liu, L., et al. (2019). Cost-effectiveness of teriflunomide compared to interferon Beta-1b for relapsing multiple sclerosis patients in China. Clin. Drug Investig. 39 (3), 331–340. doi:10.1007/s40261-019-00750-3
Yu, Y. F., Luan, L., Zhu, F. F., Dong, P., Li, L. T., et al. (2021). Modelled economic analysis for Dacomitinib-A cost effectiveness analysis in treating patients with EGFR-Mutation-Positive non-small cell lung cancer in China. Front. Oncol. 11, 564234. doi:10.3389/fonc.2021.564234
Zhang, C., Zhang, H., and Shi, J. (2015). Pharmacoeconomic analysis of erlotinib versus pemetrexedin the treatment of advanced non-small cell lung cancer. Chin. J. New Drugs. 24 (14), 1616–1623.
Zheng, H., Xie, L., Zhan, M., Wen, F., Xu, T., and Li, Q. (2018). Cost-effectiveness analysis of the addition of bevacizumab to chemotherapy as induction and maintenance therapy for metastatic non-squamous non-small-cell lung cancer. Clin. Transl. Oncol. 20 (3), 286–293. doi:10.1007/s12094-017-1715-1
Zhi, X., Wang, J., and Zhao, J. (2025). Clinical Practice Guidelines for the management of brain metastases from non-small cell lung cancer with actionable gene alterations in China (2025 edition). Chin. J. Lung Cancer 28 (01), 1–21. doi:10.3779/j.issn.1009-3419.2024.102.42
Zhu, J., He, W., Ye, M., Fu, J., Chu, Y. B., Zhao, Y. Y., et al. (2018). Cost-effectiveness of afatinib and erlotinib as second-line treatments for advanced squamous cell carcinoma of the lung. Future Oncol. 14 (27), 2833–2840. doi:10.2217/fon-2018-0321
Glossary
EGFR Epidermal growth factor receptor
AE Adverse event
AgD Aggregate data
AIC Akaike information criterion
BIC Bayesian information criterion
CDE Center for Drug Evaluation
CEAE Cost-effectiveness acceptability curves
CI Confidence interval
CNS Central nervous system
DSA Deterministic sensitivity analysis
ESS Effective sample size
GDP Gross domestic product
HR Hazard ratio
HSUV health state utility value
ICER Incremental cost-effectiveness ratio
IPD Individual patient data
IRC Independent review committee
MAIC Matching-adjusted indirect comparison
NA Not available
NHSA National Healthcare Security Administration
NMPA National Medical Products Administration
NRDL National Reimbursement Drug List
NSCLC Non-small cell lung cancer
ORR Objective response rate
OS Overall survival
PFS Progression-free survival
PSA Probabilistic sensitivity analyses
QALY Quality-adjusted life years
SD Standardized deviation
TEAE Treatment-emergent adverse events
TKI Tyrosine kinase inhibitor
TTO Time trade-off
WTP Willingness-to-pay
Keywords: rilertinib, osimertinib, cost-effectiveness, non-small cell lung cancer, epidermal growth factor receptor, China
Citation: Li X, Lu Y, Lu Y, Zhou M, Xie X, Zhou Y, Qiu J, Dai Z and Chang F (2025) Cost-effectiveness of rilertinib versus osimertinib in second-line treatment in EGFR T790M resistance mutation advanced non-small cell lung cancer in China. Front. Pharmacol. 16:1628024. doi: 10.3389/fphar.2025.1628024
Received: 13 May 2025; Accepted: 29 September 2025;
Published: 09 October 2025.
Edited by:
Johanna Lister, Takeda Pharmaceuticals International GmbH, SwitzerlandReviewed by:
Yuhao Xie, St. John’s University, United StatesJang Ho Lee, Asan Medical Center, Republic of Korea
Copyright © 2025 Li, Lu, Lu, Zhou, Xie, Zhou, Qiu, Dai and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanjing Dai, a2F5ZGFpempAMTYzLmNvbQ==; Feng Chang, Y3B1Y2ZAMTYzLmNvbQ==
†Present addresses: Zhanjing Dai, School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, Jiangsu, China
Feng Chang, School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, Jiangsu, China
‡These authors have contributed equally to this work and share first authorship
 Xiangcheng Li
Xiangcheng Li Yun Lu
Yun Lu Yuqiong Lu
Yuqiong Lu Mengyuan Zhou
Mengyuan Zhou Xiaoxi Xie
Xiaoxi Xie Yang Zhou
Yang Zhou Feng Chang
Feng Chang