- 1Department of Clinical Pharmacy, The First Hospital of Jilin University, Changchun, Jilin, China
- 2School of Pharmaceutical Science, Jilin University, Changchun, Jilin, China
- 3Division of Clinical Research, the First Hospital of Jilin University, Changchun, Jilin, China
Introduction: Adverse drug reactions (ADRs) have posed significant threats to patient safety and could potentially result in adverse clinical outcomes.
Methods: In this study, we analyzed ADRs data reported by a large hospital spanning from 2020 to 2023, with a particular focus on identifying demographic and clinical factors associated with severe ADRs. The dataset encompassed 5,644 cases, incorporating variables such as patient age, gender, smoking and drinking history, allergies, medication type, and administration route.
Results: Among these, 408 cases of severe ADRs underwent detailed examination. Additionally, the study delved into the correlation between adverse drug reactions symptoms (ADRS) and various drug types. According to research statistics, individuals in the middle-aged group (46–65 years) exhibited the highest proportion of severe ADRs at 36.77%. Females were significantly more affected than males, accounting for 66.67% of severe ADRs. Anti-tumor drugs emerged as the primary cause of severe ADRs, responsible for 52.70% of such incidents. Compared with other administration methods, intravenous injection was more prone to causing severe ADRs, with a likelihood of 53.92%. Furthermore, the blood system was identified as the ADRS where severe ADRs occurred at a significantly higher rate than other parts of the body, at 53.19%. Correlation analysis reveals a strong association between medication type and factors such as patient age, administration route, and ADRS. Notably, ADRS was also strongly linked to drug type, gender, and age.
Conclusion: These findings collectively highlight the critical need for personalized treatment plans and targeted monitoring. Particular attention should be directed towards high-risk groups, such as middle-aged females and patients undergoing anti-tumor therapies. By doing so, it is possible to enhance drug safety and minimize the occurrence of severe ADRs.
1 Introduction
Adverse drug reactions (ADRs), defined as “noxious and unintended responses to a drug at doses normally used in humans” by the World Health Organization (WHO) in 1972 (Organization, 1972), remain a significant challenge in the healthcare industry. These ADRs are a leading cause of patients’ morbidity and mortality globally, placing substantial pressure on healthcare systems (Formica et al., 2018).
Europe study have found that 3.5% of hospitalizations are due to ADRs, and 10.1% of hospitalized patients experience ADRs during their stay (Bouvy et al., 2015). More broadly, studies have shown that approximately 16.9% of hospitalized patients experience ADRs (Rothschild et al., 2010), indicating their prevalence worldwide and the serious impact on patient safety (Lee and Chen, 2019; Bailey et al., 2016).
According to WHO standards, a severe ADR is defined as one that results in death, is life-threatening, requires hospitalization or extend the length of stay, causes disability or functional impairment, or has other medically important consequences. Though less common than ordinary ADRs, severe ADRs typically lead to longer hospital stays, additional treatment, and extended recovery periods (Hohl et al., 2011; Dalton and Byrne, 2017). These factors increase medical costs and significantly reduce the patients’ quality of life. Some studies have shown that serious ADRs accounted for 6.7% of hospitalizations and 0.3% of hospital mortality. These findings underscore the importance and urgency of accurately identifying and effectively managing severe ADRs to mitigate their impact on patients and healthcare systems.
Despite extensive international research on ADRs, particularly from the European Union and the United States, data provided by China, especially regarding hospitalized patients, remain limited (Song et al., 2023). There is relatively little research on the incidence and related factors of ADRs, especially severe ADRs, among hospitalized patients in China (Pitt et al., 2016).
To address this gap, we conducted a retrospective analysis of ADRs report data from a large hospital in China spanning 2020 to 2023. The northeastern region of China demonstrates a notably higher prevalence of chronic diseases like hypertension than the national average (Xing et al., 2020), a phenomenon intricately linked to climatic conditions, dietary patterns, and medical resource disparities. This unique convergence of factors not only elevates the complexity of ADRs in the region but also shapes a distinct epidemiological landscape where the cold climate exacerbates vascular stress, high-sodium/high-fat diets drive metabolic risks, and heterogeneous healthcare access influences medication practices. As a leading tertiary referral center, the First Hospital of Jilin University serves a diverse patient population spanning urban and rural areas of Northeast China, uniquely positioned to capture how these environmental, behavioral, and healthcare dynamics intermingle to impact ADRs profiles. The primary objectives were to describe the overall occurrence of ADRs and patient characteristics, and to investigate the association between ADRs severity and clinical factors. By focus on severe ADRs and analyzing their frequency, clinical manifestations, and potential risk factors, this study aims to provide valuable insights for improving medication safety in China’s medical environment.
2 Methods
2.1 Data source
This study used the ADRs data in the ADRs reporting system of a large hospital from 2020 to 2023, involving the demographic information of patients and details related to ADRs. All experimental protocols were approved by Ethics Committee of the First Hospital of Jilin University (No. 2023–567).
2.2 Data cleaning and extraction
Initial data cleaning was performed to extract valuable information, focusing on key variables such as age, gender, alcohol history, smoking history, allergy history, severity of adverse reactions, drug types, and route of administration.
2.3 Classifying of severe adverse reactions
The severity of ADRs is determined in accordance with the Reporting and Monitoring of Adverse Drug Reactions (Announced by the Ministry of Health on May 4, 2011 as Order No. 81. Effective from July 1, 2011).
2.4 Analysis of severe adverse reactions
For the analysis of severe adverse reactions, we incorporated each patient’s clinical manifestations to identify specific drug accumulation sites. This information was statistically analyzed to understand patterns and correlations related to the severity of the reactions (Tang and Lu, 2010; Emami Riedmaier et al., 2012).
2.5 Statistical analysis
Statistical analysis was conducted using the software available at SPSS Pro (Chan, 2004a; Chan, 2004b). Descriptive statistics summarized the characteristics of each group. A chi-square test was used to evaluate whether there was a significant association between categorical variables. When the χ2 test result is significant, it indicates that there is a statistical difference in the distribution of the two variables, that is, there is an association between them (but it does not mean causality) (Schober and Vetter, 2019; McHugh, 2013). All methods were carried out in accordance with relevant guidelines and regulations.
3 Results
3.1 Overall situation
Table 1 summarizes the demographic and clinical characteristics related to ADRs recorded from 2020 to 2023.
The analysis of the ADRs data yielded several key insights. The age group most affected was 46–65 years old, accounting for 39.6% of cases. This indicates that middle-aged individuals may have a higher susceptibility to ADRs. Regarding gender distribution, females made up 64.3% of cases, compared to 35.7% for males. A notable 91.1% of cases has no history of drinking or smoking, suggesting that these factors were not primary contributors to the observed ADRs. Similarly, 96.9% of cases had no allergy history, which may imply that allergic reactions are less likely to be the main cause of ADRs in this dataset.
In terms of administration routes, intravenous (IV) injection was the most common at 44.8%, followed by oral administration at 20.8%. This highlights the need for healthcare personnel to closely monitor patients, especially during high-risk administration routes like IV (Wacker, 2013).
Most cases (92.8%) were non-severe, while 7.2% of cases were classified as severe. This aligns with previous relevant research findings. Anti-tumor drugs were the most common type involved, at 35.5% of cases. This underscores the potential risks associated with anti-tumor drugs and suggests a need to enhance surveillances of such drugs (Zhang et al., 2022).
3.2 Overall situation of serious adverse drug reactions
Table 2 categorizes the severe ADRs by age, sex, drinking history, allergy history, drug classes, smoking history, administration routes, and ADRS. The most affected age group for severe ADRs was 46–65 years, accounting for 36.8%. However, this age range represented 39.6% of the overall ADR group, indicating a slight decrease in the proportion of severe cases. The 66–79 years age group had a higher rate of severe reactions compared to their overall share, suggesting that individuals in this age range are more prone to severe ADRs. This emphasizes the need for heightened attention to medication safety in this older population (Zhang et al., 2020; Mangoni, 2012; Cacabelos et al., 2021).
In terms of sex distribution, females accounted for 66.7% of severe ADRs, compared to 64.3% in the overall ADRs group. This indicates that women are more likely to experience severe reactions, suggesting a higher risk of severe outcomes once an ADRs occurs. A large majority (93.4%) of severe ADRs had no history of drinking, and 95.3% had no smoking history, implying that alcohol and smoking have little significant effect on the occurrence of severe ADRs. Additionally, 98.8% of patients had no allergy history, further reinforcing the other factors, such as drug type or individual patient physiology, are more likely to influence the severity of reactions.
Anti-tumor drugs were the leading cause of severe ADRs, responsible for 58.3% of cases. Their high toxicity and narrow therapeutic window make them particularly dangerous, necessitating careful monitoring during treatment (Barachini et al., 2023; da Cunha de Medeiros et al., 2023; Pantazi and Tselepis, 2022; Curigliano et al., 2016).
IV administration was the most common route associated with severe ADRs, accounting for 53.9% of cases. This underlines the need for precise dosage control and monitoring due to the rapid and direct delivery of drugs via this method (Marsh et al., 2020; Chionh et al., 2017). Skin and mucous membranes were the next most affected areas, with 19.9% of severe cases.
The hematologic system was the most commonly affected in severe ADRs, representing 53.2% of cases. This indicates that if ADRs involve the hematologic system, there is a high probability they will be severe, thus requiring close monitoring.
3.3 Influence of drug types on the occurrence of severe ADRs
Table 3 demonstrates a significant association between drug category and several factors in severe ADRs. The Pearson chi-square test (χ 2 = 110.022, P = 0.000) revealed a highly significant association between age and drug category. Anti-tumor drugs were predominantly linked to severe ADRs in patients under 18 (118 of 139 cases) and those aged 46–65 (71 of 150 cases). This highlights the higher risk for young and middle-aged patients, particularly those undergoing chemotherapy. In contrast, other drug categories, such as anti-infective and cardiovascular drugs, showed a more even distribution of ADRs across different age groups. This underscores the importance of considering age-specific risks when prescribing these medications.
There was also a highly significant association between drug classes and Adverse Drug Reaction Symptoms (ADRS) (χ2 = 366.906, P < 0.0001). Anti-tumor drugs were mainly associated with hematologic system damage (201 of 238 cases), reflecting their known blood toxicity and emphasizing the need for close hematologic monitoring during chemotherapy. Conversely, anti-infective drugs were more likely to cause skin and mucosal reactions (31 of 42 cases), indicating the need for targeted dermatological monitoring for patients using these drugs. The clear association between different drug classes and specific accumulation sites should guide patient care strategies.
3.4 Characteristics of Adverse Drug Reaction Symptoms in severe ADRs
Table 4’s data analysis reveals significant relationships among age, gender, drug category, and ADRS. The Pearson chi-square test (χ2 = 106.246, P < 0.0001) shows a strong age-ADRS association. Patients under 18 are mainly affected by hematologic system damage (110 of 139 cases). In contrast, patients aged 46–65 show a wider range of ADRS, including the skin/mucosal system (36 cases), digestive system (15 cases), and general systemic damage (15 cases). This suggests younger patients are highly vulnerable to hematologic toxicity (Hardy-Abeloos et al., 2020; Teachey et al., 2022), while middle-aged adults face a broader ADRS. These findings highlight the need for age-specific monitoring: younger patients may need intensive hematologic evaluations, while middle-aged adults require comprehensive systemic assessments.
There’s also a significant sex-ADRS (χ2 = 19.853, P = 0.003). Males are more prone to hematologic reactions (66 of 136 cases), while females tend to have a wider reaction range, especially skin/mucosal system (45 cases) and general systemic damage (20 cases). This implies females may be more susceptible to dermatologic and systemic reactions. These results stress the importance of sex-specific clinical monitoring, particularly for females more prone to severe skin and mucosal reactions.
Moreover, drug classes and ADRS are strongly associated (χ2 = 366.906, P < 0.0001). Anti-tumor drugs are predominantly linked to hematologic damage (201 of 238 cases), reflecting their known blood toxicity. In contrast, anti-infective drugs are more often associated with skin and mucosal reactions (31 of 42 cases).
These results emphasize the need for tailored monitoring based on drug class. Patients undergoing chemotherapy require close hematologic evaluation, while those on anti-infective drugs need vigilant dermatologic monitoring. This approach can mitigate drug-class-specific risks and reduce severe ADRs likelihood.
4 Discussion
Our analysis of ADRs from 2020 to 2023 reveals the complex patterns of severe ADRs, with a focus on demographic and clinical factors. Middle-aged adults (46–65 years old)are the most affected, comprising 39.58% of all ADRs and 36.77% of severe cases. This heightened vulnerability may stem from increased health issues, polypharmacy, and age-related alterations in drug metabolism (Budnitz et al., 2021; Cardelli et al., 2012; Ragoonanan et al., 2021; Figure 1).
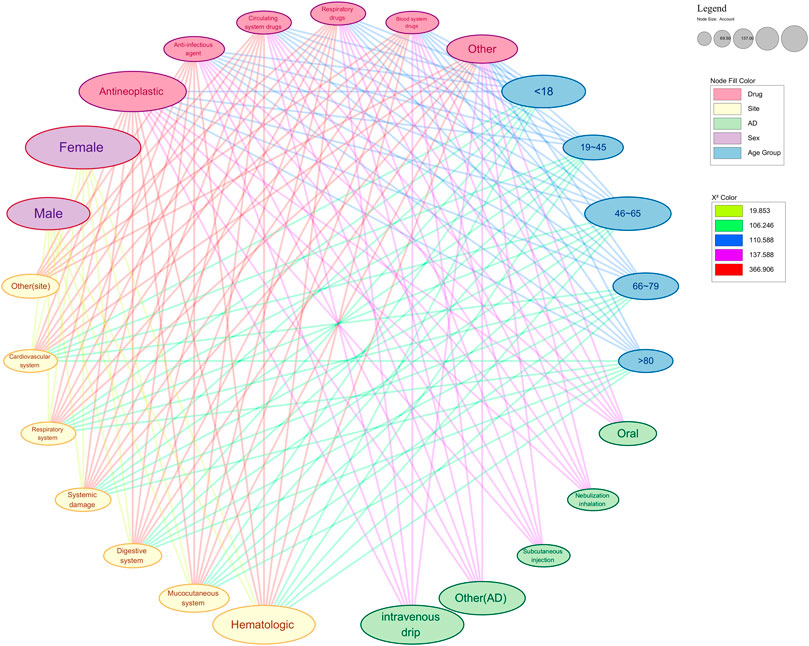
Figure 1. Network diagram of significant analysis results of various factors of serious ARDs. This figure shows the significance analysis results with drug type and drug accumulation site as independent variables. The color of the nodes in the graph represents the group they belong to. The color of the edge reflects the strength of saliency. Nodes connected to each other imply a significant correlation and mutual influence between the two. AD: administration.
The study also shows a higher incidence of ADRs in women, who account for 64.26% of all cases and 66.67% of severe ADRs. This suggests that gender differences in drug metabolism and response are significant, underscoring the need to consider biological and social factors when assessing patient risks. Factors such as body composition, hormonal differences, and healthcare access may contribute to women’s increased risk of severe reactions (Moyer et al., 2019; de Vries et al., 2019). It was reported that gender significantly influences drug metabolism through sex-specific differences in cytochrome P450 enzyme activities (e.g., higher CYP3A4 activity in females and CYP1A2/CYP2E1 in males), hormonal regulation (estrogen and testosterone modulating glucuronidation and CYP expression), and pharmacokinetics (absorption, distribution, and renal excretion) (Scandlyn et al., 2008). These mechanisms lead to clinical implications such as gender-specific dosing requirements and varying rates of drug-induced toxicity (Schmetzer and Flörcken, 2012).
A notable finding is the high rate of ADRs associated with IV administration, which accounts for 53.92% of severe ADRs. The rapid delivery of drugs via IV can lead to immediate reactions, particularly with potent agents like anti-tumor drugs. This emphasizes the need for vigilant monitoring during IV therapy, especially for chemotherapy patients who are already at a higher risk of severe ADRs (Wacker, 2013).
Interestingly, the study also establishes links between drug classes and ADRS, helping to pay attention to the occurrence of ADRs. Younger patients, particularly those under 18, show a higher incidence of blood-related ADRs, highlighting the need for careful hematologic monitoring in this group. In contrast, middle-aged adults experience a broader range of reactions, likely due to their underlying health conditions and the multiple medications they take.
While our findings are valuable, we must acknowledge several limitations. The study is based on passive surveillance data from a hospital reporting system, which inherently suffers from underreporting and reporting bias due to its reliance on voluntary submissions. Mild or non-life-threatening ADRs are likely underrepresented, while severe ADRs may be overemphasized, skewing the observed distribution of ADRs severity and demographic associations. For example, the lower proportion of elderly individuals (80+) in severe ADRs might not reflect their true risk but rather underreporting due to under recognition or under documentation of ADRs in clinical settings. Additionally, the retrospective design may introduce biases from missing data, unaccounted comorbidities, and drug interactions, potentially affecting the accuracy of our analyses. The focus on specific age and gender groups also risks overlooking other at-risk populations. Reporting bias further arises from variations in healthcare-seeking behaviors or institutional reporting practices, introducing systematic errors in data collection. These limitations mean our results may not fully capture real-world ADR prevalence, especially among underrepresented groups. When interpreting findings, it is critical to recognize that observed associations could be influenced by incomplete data, and conclusions about risk profiles require cautious extrapolation to broader populations.
Future research should adopt a longitudinal approach to investigate the effects of high-risk drugs, particularly anti-tumor drugs, across more diverse patient populations. Exploring genetic and physical factors influencing drug metabolism could enhance our understanding of individual-level risks. By implementing personalized treatment and monitoring strategies that account for patient demographics, drug types, and administration methods, we can improve medication safety and patients’ outcomes.
5 Conclusion
The occurrence of ADRs is significantly influenced by various demographic and clinical factors, with middle-aged adults, female, individuals undergoing intravenous drug administration, and those on anti-tumor drugs facing elevated risks. Recognizing the complex interplay of these factors in severe ADRs is essential for enhancing the efficacy of monitoring practices and developing personalized treatments strategies. This approach can ultimately improve patient safety and clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JZ: Methodology, Conceptualization, Writing – review and editing, Supervision, Writing – original draft, Funding acquisition. HoY: Writing – original draft, Project administration. JZ: Writing – review and editing, Methodology, Investigation, Data curation. HuY: Writing – review and editing, Validation, Visualization, Funding acquisition. JM: Data curation, Writing – review and editing, Resources. SZ: Writing – review and editing, Supervision, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Project of Science and Technology Department of Jilin Province, China [YDZJ202501ZYTS138], Education Department of Jilin Province, China [JJKH20250215KJ], Special health personnel of Jilin Province [JLSWSRCZX 2023-42, JLSRCZX 2025-138, JLSRCZX 2025-025], Jilin Provincial Health Commission [J2023JC006].
Acknowledgments
The authors acknowledge the First Hospital of Jilin University, for providing information to carry out this work. All authors thank Dr. Xiuzhu Gao in core facility of The First Hospital of Jilin University, for training and guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bailey, C., Peddie, D., Wickham, M. E., Badke, K., Small, S. S., Doyle-Waters, M. M., et al. (2016). Adverse drug event reporting systems: a systematic review. Br. J. Clin. Pharmacol. 82 (1), 17–29. doi:10.1111/bcp.12944
Barachini, S., Ghelardoni, S., Varga, Z. V., Mehanna, R. A., Montt-Guevara, M. M., Ferdinandy, P., et al. (2023). Antineoplastic drugs inducing cardiac and vascular toxicity - an update. Vasc. Pharmacol. 153, 107223. doi:10.1016/j.vph.2023.107223
Bouvy, J. C., De Bruin, M. L., and Koopmanschap, M. A. (2015). Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 38 (5), 437–453. doi:10.1007/s40264-015-0281-0
Budnitz, D. S., Shehab, N., Lovegrove, M. C., Geller, A. I., Lind, J. N., and Pollock, D. A. (2021). US emergency department visits attributed to medication harms, 2017-2019. Jama 326 (13), 1299–1309. doi:10.1001/jama.2021.13844
Cacabelos, R., Naidoo, V., Corzo, L., Cacabelos, N., and Carril, J. C. (2021). Genophenotypic factors and pharmacogenomics in adverse drug reactions. Int. J. Mol. Sci. 22 (24), 13302. doi:10.3390/ijms222413302
Cardelli, M., Marchegiani, F., Corsonello, A., Lattanzio, F., and Provinciali, M. (2012). A review of pharmacogenetics of adverse drug reactions in elderly people. Drug Saf. 35 (Suppl. 1), 3–20. doi:10.1007/BF03319099
Chan, Y. H. (2004a). Biostatistics 301A. Repeated measurement analysis (mixed models). Singap. Med. J. 45 (10), 456–461.
Chan, Y. H. (2004b). Biostatistics 301. Repeated measurement analysis. Singap. Med. J. 45 (8), 354–369.
Chionh, F., Lau, D., Yeung, Y., Price, T., and Tebbutt, N. (2017). Oral versus intravenous fluoropyrimidines for colorectal cancer. Cochrane Database Syst. Rev. 7 (7), Cd008398. doi:10.1002/14651858.CD008398.pub2
Curigliano, G., Cardinale, D., Dent, S., Criscitiello, C., Aseyev, O., Lenihan, D., et al. (2016). Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J. Clin. 66 (4), 309–325. doi:10.3322/caac.21341
da Cunha de Medeiros, P., Nascimento, C. C., and Perobelli, J. E. (2023). Antineoplastic drugs in environmentally relevant concentrations cause endocrine disruption and testicular dysfunction in experimental conditions. Environ. Toxicol. Pharmacol. 100, 104122. doi:10.1016/j.etap.2023.104122
Dalton, K., and Byrne, S. (2017). Role of the pharmacist in reducing healthcare costs: current insights. Integr. Pharm. Res. Pract. 6, 37–46. doi:10.2147/IPRP.S108047
de Vries, S. T., Denig, P., Ekhart, C., Burgers, J. S., Kleefstra, N., Mol, P. G. M., et al. (2019). Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in The Netherlands: an explorative observational study. Br. J. Clin. Pharmacol. 85 (7), 1507–1515. doi:10.1111/bcp.13923
Emami Riedmaier, A., Nies, A. T., Schaeffeler, E., and Schwab, M. (2012). Organic anion transporters and their implications in pharmacotherapy. Pharmacol. Rev. 64 (3), 421–449. doi:10.1124/pr.111.004614
Formica, D., Sultana, J., Cutroneo, P. M., Lucchesi, S., Angelica, R., Crisafulli, S., et al. (2018). The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin. Drug Saf. 17 (7), 681–695. doi:10.1080/14740338.2018.1491547
Hardy-Abeloos, C., Pinotti, R., and Gabrilove, J. (2020). Ibrutinib dose modifications in the management of CLL. J. Hematol. Oncol. 13 (1), 66. doi:10.1186/s13045-020-00870-w
Hohl, C. M., Nosyk, B., Kuramoto, L., Zed, P. J., Brubacher, J. R., Abu-Laban, R. B., et al. (2011). Outcomes of emergency department patients presenting with adverse drug events. Ann. Emerg. Med. 58 (3), 270–279.e4. doi:10.1016/j.annemergmed.2011.01.003
Lee, C. Y., and Chen, Y. P. (2019). Machine learning on adverse drug reactions for pharmacovigilance. Drug Discov. Today 24 (7), 1332–1343. doi:10.1016/j.drudis.2019.03.003
Mangoni, A. A. (2012). Predicting and detecting adverse drug reactions in old age: challenges and opportunities. Expert Opin. Drug Metab. Toxicol. 8 (5), 527–530. doi:10.1517/17425255.2012.665874
Marsh, N., Webster, J., Ullman, A. J., Mihala, G., Cooke, M., Chopra, V., et al. (2020). Peripheral intravenous catheter non-infectious complications in adults: a systematic review and meta-analysis. J. Adv. Nurs. 76 (12), 3346–3362. doi:10.1111/jan.14565
McHugh, M. L. (2013). The chi-square test of independence. Biochem. Med. Zagreb. 23 (2), 143–149. doi:10.11613/bm.2013.018
Moyer, A. M., Matey, E. T., and Miller, V. M. (2019). Individualized medicine: sex, hormones, genetics, and adverse drug reactions. Pharmacol. Res. Perspect. 7 (6), e00541. doi:10.1002/prp2.541
Organization, W. H. (1972). International drug monitoring: the role of national centres, report of a WHO meeting [held in Geneva from 20 to 25 September 1971]. Geneva, Switzerland; World Health Organization.
Pantazi, D., and Tselepis, A. D. (2022). Cardiovascular toxic effects of antitumor agents: pathogenetic mechanisms. Thromb. Res. 213 (Suppl. 1), S95–s102. doi:10.1016/j.thromres.2021.12.017
Pitts, P. J., Louet, H. L., Moride, Y., and Conti, R. M. (2016). 21st century pharmacovigilance: efforts, roles, and responsibilities. Lancet Oncol. 17 (11), e486–e492. doi:10.1016/S1470-2045(16)30312-6
Ragoonanan, D., Khazal, S. J., Abdel-Azim, H., McCall, D., Cuglievan, B., Tambaro, F. P., et al. (2021). Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nat. Rev. Clin. Oncol. 18 (7), 435–453. doi:10.1038/s41571-021-00474-4
Rothschild, J. M., Churchill, W., Erickson, A., Munz, K., Schuur, J. D., Salzberg, C. A., et al. (2010). Medication errors recovered by emergency department pharmacists. Ann. Emerg. Med. 55 (6), 513–521. doi:10.1016/j.annemergmed.2009.10.012
Scandlyn, M. J., Stuart, E. C., and Rosengren, R. J. (2008). Sex-specific differences in CYP450 isoforms in humans. Expert Opin. Drug Metab. Toxicol. 4 (4), 413–424. doi:10.1517/17425255.4.4.413
Schmetzer, O., and Flörcken, A. (2012). Sex differences in the drug therapy for oncologic diseases. Handb. Exp. Pharmacol. (214), 411–442. doi:10.1007/978-3-642-30726-3_19
Schober, P., and Vetter, T. R. (2019). Chi-square tests in medical research. Anesth. and Analgesia 129 (5), 1193. doi:10.1213/ANE.0000000000004410
Song, H., Pei, X., Liu, Z., Shen, C., Sun, J., Liu, Y., et al. (2023). Pharmacovigilance in China: evolution and future challenges. Br. J. Clin. Pharmacol. 89 (2), 510–522. doi:10.1111/bcp.15277
Tang, W., and Lu, A. Y. (2010). Metabolic bioactivation and drug-related adverse effects: current status and future directions from a pharmaceutical research perspective. Drug Metab. Rev. 42 (2), 225–249. doi:10.3109/03602530903401658
Teachey, D. T., Devidas, M., Wood, B. L., Chen, Z., Hayashi, R. J., Hermiston, M. L., et al. (2022). Children's oncology group trial AALL1231: a phase III clinical trial testing bortezomib in newly diagnosed T-cell acute lymphoblastic leukemia and lymphoma. J. Clin. Oncol. 40 (19), 2106–2118. doi:10.1200/JCO.21.02678
Wacker, M. (2013). Nanocarriers for intravenous injection--the long hard road to the market. Int. J. Pharm. 457 (1), 50–62. doi:10.1016/j.ijpharm.2013.08.079
Xing, L., Jing, L., Tian, Y., Yan, H., Zhang, B., Sun, Q., et al. (2020). Epidemiology of dyslipidemia and associated cardiovascular risk factors in northeast China: a cross-sectional study. Nutr. Metab. Cardiovasc Dis. 30 (12), 2262–2270. doi:10.1016/j.numecd.2020.07.032
Zhang, L., Ma, L., Sun, F., Tang, Z., and Chan, P. (2020). A multicenter study of multimorbidity in older adult inpatients in China. J. Nutr. Health Aging 24 (3), 269–276. doi:10.1007/s12603-020-1311-x
Keywords: adverse drug reactions, severe adverse drug reactions, clinical factors, personalized treatment, anti-tumor drug
Citation: Zhai J, Yan H, Zhang J, Yan H, Ma J and Zhang S (2025) A comprehensive analysis of adverse drug reactions in 2020–2023: case studies. Front. Pharmacol. 16:1628347. doi: 10.3389/fphar.2025.1628347
Received: 15 May 2025; Accepted: 15 September 2025;
Published: 02 October 2025.
Edited by:
Yao Liu, Daping Hospital, ChinaReviewed by:
Mariana Linhares Pereira, Universidade Federal de São João Del Rei, BrazilYashpal Manchanda, Ministry of Health, Kuwait
Copyright © 2025 Zhai, Yan, Zhang, Yan, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sixi Zhang, c2l4aUBqbHUuZWR1LmNu
 Jinghui Zhai
Jinghui Zhai Hongzhe Yan1,2
Hongzhe Yan1,2 Sixi Zhang
Sixi Zhang


