Abstract
Macrophage polarization plays a pivotal role in the pathogenesis and plaque stability of atherosclerosis (AS). In response to microenvironmental cues, macrophages differentiate into pro-inflammatory M1 or anti-inflammatory M2 phenotypes, which respectively exacerbate or mitigate inflammatory responses and influence plaque progression. Emerging evidence highlights the therapeutic potential of targeting macrophage polarization through signaling pathways such as Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB), peroxisome proliferator-activated receptor γ (PPAR-γ), Janus kinase (JAK)-signal transducer and activator of transcription (STAT), phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway, and mitogen-activated protein kinase (MAPK) pathway. Bioactive metabolites derived from traditional Chinese medicine (TCM)—including ginsenosides (e.g., Rb1, Rg3), berberine (BBR), curcumin (CUR), and tanshinone IIA (Tan IIA)—as well as herbal formulas like Bu Yang Huan Wu Decoction (BYHW) and Zhuyu Pill (ZYP), have demonstrated efficacy in promoting M2 polarization and suppressing M1 phenotypes, thereby attenuating AS. This review critically synthesizes the current body of evidence, with a primary focus on preclinical studies (in vitro and in vivo), which comprehensively synthesizes evidence on the targeted modulation of AS-associated macrophage polarization by bioactive metabolites and herbal formulas, with a unique emphasis on the role of TCM as a multi-target regulator of macrophage plasticity. This approach provides novel perspectives for the prevention and treatment of AS.
1 Introduction
Atherosclerosis (AS) is a chronic, progressive inflammatory disease of the arterial wall and is the pathological basis of cardiovascular, cerebrovascular, and peripheral vascular diseases, posing a major global health burden (He et al., 1999; Nedkoff et al., 2023). Its progression is driven by dysregulated lipid and glucose metabolism, hypertension, and lifestyle-related factors such as poor diet, smoking, and physical inactivity (Poznyak et al., 2020; Lechner et al., 2022). These risk factors collectively promote vascular calcification, loss of elasticity, and luminal narrowing. Central to disease progression are endothelial dysfunction, lipid retention, and chronic inflammation, which form a self-amplifying loop via oxidized low-density lipoprotein (ox-LDL) deposition, foam-cell formation, and cytokine release. Endothelial metabolic dysregulation, including altered glycolysis and mitochondrial function, further exacerbates vascular injury and accelerates plaque development (Libby, 2021; Moore et al., 2013; Gimbrone and García-Cardeña, 2016).
Macrophages are the most important immune inflammatory cells in atherosclerotic lesions and play a core role at all stages of the disease process (Bashore et al., 2024; Murray and Wynn, 2011). Following endothelial injury, monocytes are recruited to the lesion site, differentiate into macrophages, and internalize excess lipoproteins to form cholesterol-rich foam cells (Libby, 2021; Moore et al., 2013). These macrophages shape the plaque immune microenvironment and exhibit a spectrum of activation states beyond the classical M1/M2 dichotomy. Single-cell studies have identified inflammatory, lipid-handling, proliferative, and smooth-muscle-like subsets, underscoring their phenotypic plasticity (Bashore et al., 2024). The canonical M1/M2 paradigm, originally derived from the Th1/Th2 framework, describes two extremes of macrophage activation: M1 macrophages, induced by Th1 cytokines, mediate inflammation and tissue injury, whereas M2 macrophages, driven by Th2 cytokines, secrete IL-10 and promote anti-inflammatory and reparative processes (Murray et al., 2014; Gordon, 2003). Dysregulated polarization, characterized by an imbalance in M1/M2 states, is a key mechanism driving AS progression (Makuch et al., 2024; Luo et al., 2024; Yang et al., 2020). Studies have shown that multiple signaling pathways, including TLR4/NF-κB/MAPK (Meng et al., 2023; Huang W. et al., 2024; Wang D. et al., 2023; Zheng Q. et al., 2025), PPAR-γ (Zahr et al., 2023; Bai et al., 2017; Zheng Y. et al., 2025), JAK/STAT (Yu et al., 2025; Wang J. et al., 2024; Chen J. et al., 2025), and PI3K/Akt (Liu et al., 2019; Zhang et al., 2021; Li P. et al., 2023; Fruman et al., 2017), regulate macrophage polarization and influencing the progression of AS. Consequently, targeting the dynamic balance of macrophage polarization through modulation of these signaling pathways represents a promising therapeutic strategy for AS.
Notably, Traditional Chinese Medicine (TCM)-derived bioactive metabolites and herbal formulas have emerged as unique modulators of macrophage plasticity, offering multi-pathway interventions with low toxicity (Zhi et al., 2023; Jian et al., 2019; Liu et al., 2020a; Liu et al., 2020b). We have integrated the available evidence to explore the regulatory mechanisms of key signaling pathways that influence macrophage phenotype during the plaque progression of AS. It is important to note that the vast majority of mechanistic insights and efficacy data discussed in this review are derived from robust preclinical models, including cell cultures and animal studies. These models are indispensable for elucidating complex biological pathways. Furthermore, this paper provides a comprehensive summary of the existing preclinical research and the preliminary clinical evidence regarding the prevention and treatment of AS using bioactive metabolites and herbal formulas. While several bioactive metabolites and herbal formulas have shown promise in early-stage clinical trials, the clinical evidence base remains less extensive than the preclinical foundation. This review comprehensively synthesizes evidence on the targeted modulation of AS-associated macrophage polarization by bioactive metabolites and herbal formulas, with a unique emphasis on the role of TCM as a multi-target regulator of macrophage plasticity. This approach provides novel perspectives for the prevention and treatment of AS.
2 Methods
This review utilized multiple literature search strategies. Authoritative databases were searched, including PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Web of Science (https://www.webofscience.com), China National Knowledge Infrastructure (CNKI, https://www.cnki.net/), and Wanfang Data (http://www.wanfangdata.com.cn/). The search was conducted using a combination of subject terms and free-text words. Keywords included “AS,” “macrophage polarization,” “signaling pathways,” “bioactive metabolites,” and “herbal formulas.” This study only includes results discovered before May 2025. The search strategies were adapted to the characteristics of each database to ensure comprehensiveness and accuracy. Studies were included if they focused on AS and its molecular mechanisms related to macrophage polarization and therapeutic interventions involving bioactive metabolites or herbal formulas. Literature was excluded if it was irrelevant to the topic, lacked sufficient experimental design, contained incomplete data, or was not available in full text (The botanical drugs names were checked at http://mpns.kew.org/mpns-portal/).
3 Overview of AS and macrophage polarization
3.1 Formation of AS
AS is a chronic, progressive inflammatory disease of the arterial wall, driven by immune dysregulation and lipid metabolism disorders (Roy et al., 2022; Engelen et al., 2022; Waksman et al., 2024). Its pathogenesis involves the activation of endothelial cells (ECs), monocytes, macrophages, smooth muscle cells (SMCs), and neutrophils (Doddapattar et al., 2022). Upon exposure to ox-LDL, ECs express chemokines and adhesion molecules, increasing vascular permeability and recruiting leukocytes (Bu et al., 2023). Monocytes infiltrate the intima, differentiate into macrophages or dendritic cells, and—together with SMCs—form foam cells that perpetuate inflammation (Liu W. et al., 2014). Foam cells release pro-inflammatory cytokines, damaging ECs and driving SMC proliferation, plaque growth, and restenosis (Liu X. et al., 2023). SMC-derived extracellular matrix (ECM) forms a fibrous cap over a necrotic, lipid-rich core undergoing calcification (Grootaert and Bennett, 2021). As plaque progresses, foam cells and SMCs undergo apoptosis or necrosis, leading to the accumulation of dead cells, cellular debris, and extracellular materials within the necrotic core (Gimbrone and García-Cardeña, 2016). Continued cell death and immune activation promote matrix metalloproteinases (MMPs)-mediated cap degradation, reducing plaque stability. Neutrophil-derived ECM-degrading enzymes further thin the cap, leading to rupture and thrombosis (Ovchinnikova et al., 2009). The mechanism by which multicellular interactions drive the development of AS plaques (Figure 1).
FIGURE 1
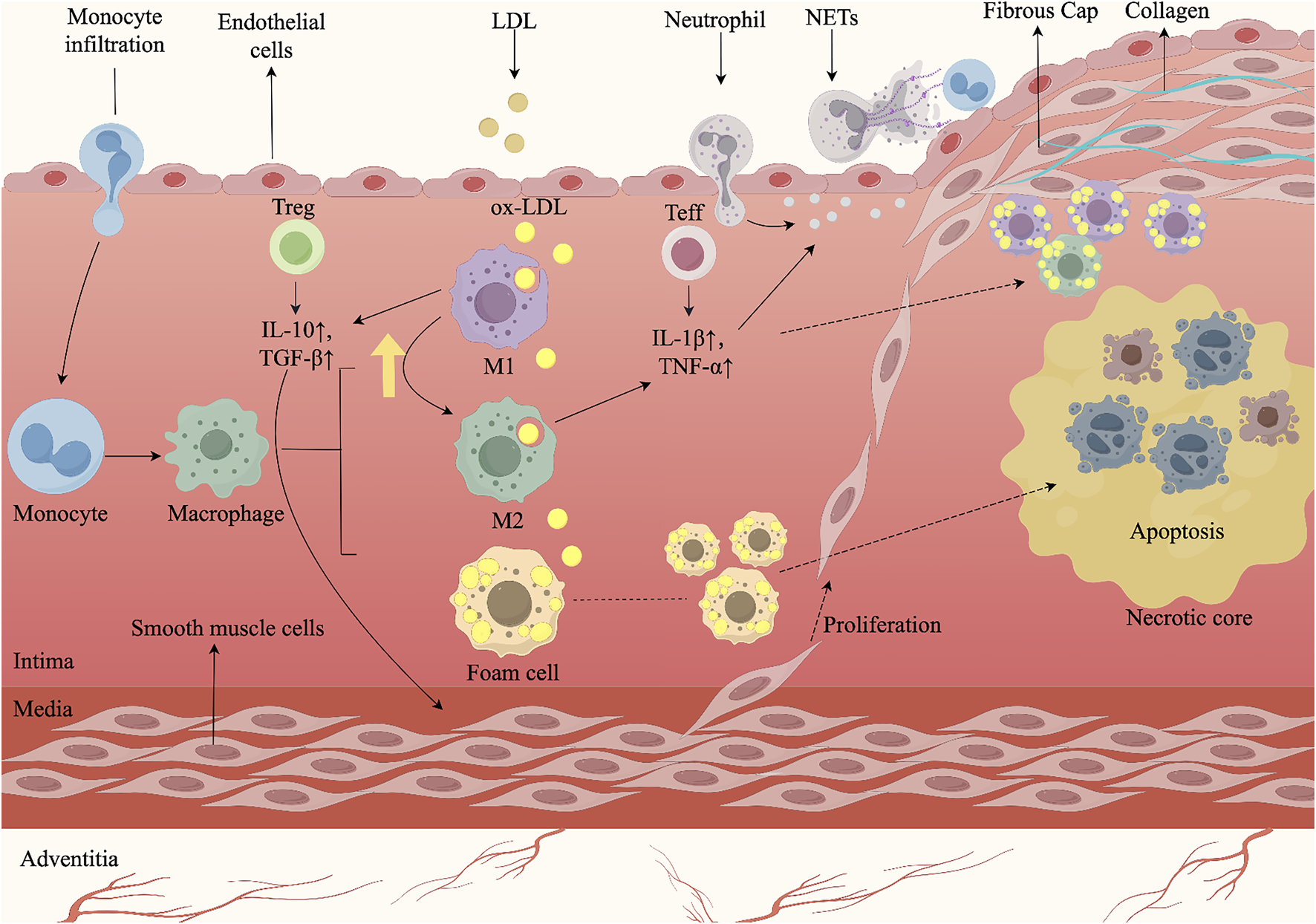
AS arises from multicellular interactions and complex signaling cascades within the arterial wall. Endothelial injury initiates the process by recruiting circulating monocytes, which adhere to activated endothelium, infiltrate into the intima, and differentiate into macrophages. Following uptake of oxLDL, macrophages transform into foam cells, whose accumulation seeds the lipid core and triggers early plaque growth. During lesion progression, macrophages and effector T cells (Teff) secrete pro-inflammatory cytokines such as IL-1β and TNF-α, which stimulate SMCs migration from the media, their proliferation, and subsequent thickening of the vessel wall. M1-polarized macrophages and Teff further amplify inflammatory signaling. By contrast, M2-polarized macrophages and regulatory T cells (Treg) secrete anti-inflammatory mediators including IL-10 and TGF-β, which suppress excessive inflammation and facilitate M1-to-M2 repolarization, thereby contributing to lesion stabilization. In advanced plaques, persistent SMC proliferation and extracellular matrix deposition form a fibrous cap over the necrotic core. However, neutrophil extracellular traps (NETs) exacerbate endothelial injury and promote cell death, destabilizing the plaque and predisposing to cap rupture. Rupture exposes thrombogenic material, triggering platelet aggregation and thrombus formation, which may result in acute cardiovascular events. The figure was created with Figdraw.com.
3.2 Macrophages in AS
Macrophages are key immune cells involved in the pathogenesis of AS. Following endothelial injury, circulating monocytes infiltrate the intima, differentiate into macrophages, and internalize excess apolipoprotein B–containing lipoproteins via scavenger receptors (e.g., SR-A1), generating foam cells that seed the lipid core (Wang B. et al., 2022; Chistiakov et al., 2017; van Tits et al., 2011). Foam-cell–driven inflammatory signaling and matrix remodeling promote smooth muscle cell proliferation and fibrous-cap weakening, reducing plaque stability (Liu Q. et al., 2022; Theofilis et al., 2023; Seifert et al., 2018). Progressive plaque accumulation narrows the lumen and predisposes to rupture, calcification, and thrombosis, causing ischemic injury (Kavurma et al., 2017; Moore and Tabas, 2011). This pathological milieu robustly promotes a shift towards the pro-inflammatory M1 phenotype, which is induced by signals like IFN-γ and LPS (Gordon, 2003). M1 macrophages secrete cytokines (e.g., TNF-α, IL-6) and matrix metalloproteinases (MMPs) that amplify inflammation at lesion sites and compromise fibrous cap integrity, thereby increasing plaque vulnerability (Li P. et al., 2021; Virga et al., 2021; Seifert et al., 2018). They are typically enriched in vulnerable plaque regions, and their apoptosis contributes to the necrotic core expansion (Hou et al., 2023). In contrast, the alternative M2 phenotype, induced by Th2 cytokines such as IL-4 and IL-13, secretes anti-inflammatory cytokines (e.g., IL-10) and profibrotic mediators (e.g., TGF-β) to promote tissue repair, enhance plaque stability, and facilitate lesion regression (Rao et al., 2022; Qiu et al., 2022; Locati et al., 2020). M2 macrophages are predominantly localized to more stable plaque areas (Hou et al., 2023). Local cues—including inflammation, cholesterol crystals, and oxidative stress—shape macrophage activation states beyond the classical M1/M2 dichotomy, revealing IL1hi inflammatory, TREM2+ foam cell–like, proliferative, and ACTA2+ smooth-muscle–like subsets (Bashore et al., 2024; Li B. et al., 2022). The dynamic balance between M1 and M2 macrophages is a critical determinant of AS progression, and targeting macrophage polarization—by inhibiting M1 activation and promoting M2 differentiation—represents a promising therapeutic approach to suppress inflammation, limit necrotic core formation, and stabilize plaques (Wu J. et al., 2023; Wei et al., 2024; Blagov et al., 2023; Jinnouchi et al., 2020; Yu L. et al., 2023). In this context, TCM strategies aim to rebalance M1/M2 phenotypes and modulate foam cell–associated subsets, providing disease-modifying potential in AS.
4 Signaling pathways related to macrophage polarization in AS
During the progression of AS, the activation of multiple signaling pathways leads to dysregulated expression of inflammatory factors (Wu J. et al., 2023; Zhang et al., 2024). Key regulatory axes include Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB), peroxisome proliferator-activated receptor γ (PPAR-γ), Janus kinase (JAK)-signal transducer and activator of transcription (STAT), phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway, and mitogen-activated protein kinase (MAPK) pathway, among others. These cascades drive discrete transcriptional programs that bias macrophages toward pro-inflammatory or pro-resolving states and thereby influence plaque vulnerability, necrotic-core expansion, and fibrous-cap integrity (Meng et al., 2022; Geiß et al., 2022). Accordingly, TCM act on these actionable nodes to re-tune macrophage programs in ways that are mechanistically aligned with AS control.
4.1 TLR4 signaling pathway
TLRs in macrophages recognize pathogen-associated molecular patterns (PAMPs), dimerize, and signal predominantly through MyD88 to activate the interleukin-1 receptor-associated kinase–tumor necrosis factor receptor-associated factor 6 (TRAF6)–IKK (IκB kinase) axis, converging on NF-κB and MAPK cascades (Litak et al., 2020). Cell-surface TLR1/2/4/5/6/10 are engaged by microbial ligands; among them, TLR4 is highly expressed across stages of atherosclerotic plaque formation and couples strongly to pro-inflammatory outputs (Jin M. et al., 2023; Bagheri et al., 2024). Functionally, prototypical agonist LPS activates TLR4–MyD88 signaling to enhance NF-κB p65 phosphorylation and MAPKs, promoting M1 polarisation with increased TNF-α, IL-6, and iNOS; conversely, genetic or pharmacologic reduction of TLR4 favors M2 traits and improves inflammatory conditions such as AS (Yan et al., 2024; Wu et al., 2024; Liu F. et al., 2024; Rumpel et al., 2024; Sun et al., 2022). These mechanisms highlight TLRs as potential therapeutic targets. In the context of TCM, metabolites like Alkaloids and Polyphenols have been shown to inhibit TLR4/NF-κB signaling (Li et al., 2020; Zhou et al., 2015), indicating their potential to modulate macrophage phenotype toward M2, which may contribute to the management of cardiovascular diseases including AS.
4.2 NF-κB signaling pathway
NF-κB is a conserved transcription factor family (p50/p105, p52/p100, RelA/p65, c-Rel, RelB) that is activated predominantly via IKK-dependent phosphorylation and degradation of IκB, enabling p65/p50 nuclear translocation to initiate inflammatory gene programs (Florio et al., 2022). This pathway is autoregulated by NF-κB–driven IκB resynthesis, which limits excessive nuclear residency and maintains immune homeostasis (An et al., 2021). Sustained NF-κB activation upregulates TNF-α, IL-6, and iNOS and drives macrophage polarisation toward the M1 phenotype, whereas attenuation of this axis favors M2-associated anti-inflammatory traits; aberrant stimuli can also reprogram M2 cells back to M1 via NF-κB (Li J. et al., 2023; Huang YM. et al., 2024; Geiß et al., 2022). This is particularly relevant for TCM, where numerous bioactive metabolites and herbal formulas have been reported in preclinical studies to suppress p65 phosphorylation, impede nuclear translocation, or stabilize IκB, thereby curbing M1 polarization bias and highlighting a strategic avenue for TCM to recalibrate macrophage responses and restore immune balance, with implications for AS and related inflammatory pathologies (Chen and Chen, 2019; Fan et al., 2022).
4.3 MAPK signaling pathway
The MAPK pathway, comprising extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 modules, is engaged by receptor and stress cues that funnel through a three-tiered kinase cascade (MAPKKK→MAPKK→MAPK) to reprogram transcription and stress responses in vascular cells (Achkar et al., 2018; Binion et al., 2009). Once activated, MAPKs phosphorylate selective substrates and transcription factors, coordinating proliferation–survival decisions and inflammatory gene expression that shape vascular lesion biology (Tang et al., 2022; El-Sayed et al., 2021). Activation of the p38 and JNK pathways is particularly instrumental in driving macrophages toward the pro-inflammatory M1 phenotype by upregulating cytokines like TNF-α and IL-1β, a process evident in AS (Shen et al., 2023). Conversely, certain signals through the ERK pathway can promote the anti-inflammatory M2 phenotype, highlighting the complex role of MAPKs in maintaining polarization balance (Wang Y. et al., 2023). Therefore, targeted modulation of specific MAPK branches represents a viable strategy for treating inflammatory diseases by reprogramming macrophage polarization. Glycosides from TCM have demonstrated efficacy in attenuating inflammation by selectively inhibiting p38 or JNK signaling, approach for the treatment and intervention of inflammatory diseases such as AS (He et al., 2023).
4.4 PPAR-γ signaling pathway
PPAR-γ is a ligand-activated nuclear receptor that integrates lipid/energy metabolism with inflammation control; in macrophages, its activation coordinates cholesterol efflux programs (e.g., ABCA1/ABCG1) and transrepresses pro-inflammatory transcription factors (NF-κB, AP-1, STAT), thereby coupling metabolic homeostasis to immune tone (Chinetti et al., 2003; Pascual et al., 2005; Szanto et al., 2010). AMP-activated protein kinase (AMPK) functions upstream to potentiate PPAR-γ activity and metabolic rewiring, positioning the AMPK→PPAR-γ axis as a nexus between cellular energy status and macrophage gene programs (Cui et al., 2023; Zhou et al., 2023). Functionally, PPAR-γ activation shifts macrophages from an M1 to an M2 phenotype, dampens pro-inflammatory cytokines, and improves the lesional milieu, whereas reduced activity favors inflammatory skewing (Abdalla et al., 2020; Bouhlel et al., 2007). Herbal formula are being explored to modulate the AMPK→PPAR-γ axis (e.g., enhancing AMPK activity, limiting p65 nuclear translocation, or reinforcing cholesterol efflux), which can promote a shift toward the M2 phenotype, underscoring the value of TCM in targeting metabolic-inflammatory crosstalk to mitigate disease progression.
4.5 JAK/STAT signaling pathway
The JAK/STAT pathway couples extracellular cytokine–receptor engagement to transcriptional reprogramming: Upon binding of cytokines to their cognate receptors, receptor-associated JAK kinases are activated and phosphorylate STAT transcription factors (Bi et al., 2019). The phosphorylated STATs then dimerize and translocate to the nucleus to regulate the expression of genes defining macrophage functional phenotypes (Xin et al., 2020; Sarapultsev et al., 2023). Critically, the specific STAT protein activated determines the polarization outcome: STAT1 activation by IFN-γ drives a robust pro-inflammatory M1 phenotype, whereas IL-4/IL-13 signaling via STAT6 induces an anti-inflammatory M2 phenotype (Gong et al., 2017; Zheng et al., 2024; Ding et al., 2024; Li ZH. et al., 2023). The balance between these opposing signals is a key determinant of plaque inflammation and stability. Given this pivotal role, the JAK-STAT pathway represents a promising therapeutic target for modulating macrophage polarization in AS. By tempering STAT1 signaling and/or amplifying STAT6-driven programs, TCM reweight JAK–STAT outputs toward anti-inflammatory macrophage identities, which provides a plausible molecular foundation for the ability of TCM interventions to suppress pro-inflammatory M1 polarization and promote resolution of inflammation in AS.
4.6 PI3K/Akt signaling pathway
The PI3K/Akt pathway integrates receptor and TLR inputs to lipid signaling: class I PI3K generates PIP3, which recruits Akt to the membrane for activation by PDK1 and mTORC2, thereby coordinating metabolism, survival, and inflammatory gene control (Monaci et al., 2021; Abeyrathna and Su, 2015). Akt activation generally promotes an anti-inflammatory M2 phenotype, particularly through the downstream mTORC1 axis, which supports M2-associated metabolic reprogramming (Rocher and Singla, 2013). However, the pathway can also contribute to M1 polarization under specific conditions, for instance, by cross-talking with and enhancing NF-κB signaling (Babaev et al., 2016). The distinct roles of Akt isoforms (e.g., Akt1 vs. Akt2) further add a layer of complexity to this regulation (Arranz et al., 2012). Selected TCM-related products fine-tune PI3K–Akt–mTOR activity—for example, by blunting maladaptive Akt phosphorylation or reshaping metabolic flux—thereby illustrating how TCM can precisely influence immune-metabolic pathways to favor anti-inflammatory macrophage polarization, highlighting their therapeutic potential.
4.7 NRF2 signaling pathway
Nuclear factor erythroid 2–related factor 2 (NRF2) is a redox-sensitive transcription factor that, upon release from Keap1, translocates to the nucleus to bind antioxidant response elements and induce cytoprotective programs (e.g., glutathione synthesis, HO-1), thereby lowering oxidative stress and coupling redox control to lipid handling and foam-cell biology (Meng et al., 2022; Ooi et al., 2017; Wu et al., 2022). Hemodynamic cues (laminar shear), endothelial inflammation, and lipid peroxidation activate NRF2 in vascular beds, linking redox homeostasis to atheroprotection and lesion remodeling (Hosoya et al., 2005; He L. et al., 2024). In foam cells, NRF2 helps balance cholesterol uptake and efflux, integrating oxidative and metabolic signals during atherogenesis (Wu et al., 2022). NRF2 restrains M1 polarisation by repressing pro-inflammatory mediators (IL-6, IL-1β, TNF-α) and can facilitate reparative/M2 traits; enhancing NRF2 stability via U-box containing protein 1 silencing further promotes M2 polarisation and reduces oxidative injury (Kobayashi et al., 2016; Mimura and Itoh, 2015; Li et al., 2025). Context-dependence exists: in certain settings NRF2 activation may favor CD163+ subsets and CD36-mediated lipid uptake, potentially accelerating lesion growth (Liu J. et al., 2020). Overall, evidence supports NRF2’s dominant antioxidant/anti-inflammatory role—NRF2 deficiency aggravates plaque inflammation, calcification, and fibrous-cap thinning—underscoring its importance for plaque stability (Alonso-Piñeiro et al., 2021; Bozaykut et al., 2014; Ruotsalainen et al., 2019). TCM-derived metabolites such as oridonin (activates NRF2 and inhibits NOD-like receptor protein 3 (NLRP3), reducing macrophage infiltration/oxidative stress and stabilising plaques in ApoE−/− mice) and quercetin (binds Keap1 Arg483 to activate NRF2, lower oxidative stress, and suppress macrophage pyroptosis) exemplify tractable NRF2-targeting strategies (Wang L. et al., 2023; Luo et al., 2022); broader classes including flavonoids and terpenoids show similar NRF2-mediated anti-inflammatory/antioxidant effects. A key open question is whether specific metabolites can selectively modulate NRF2 to direct macrophage polarisation, warranting systematic investigation for next-generation AS therapeutics.
5 Research on the intervention of AS through the regulation of macrophage polarization-related pathways by TCM
According to ancient Chinese medical records and modern research, TCM has unique advantages in treating diseases through multi-target, multi-pathway mechanisms with minimal toxicity and side effects. In various pathological stages, from endothelial dysfunction to plaque rupture, TCM regulates macrophage polarization to inhibit excessive inflammatory responses, enhance plaque stability, and thus delay the progression of AS (Wang W. et al., 2024; Jian et al., 2019).
The use of medicinal plants continues as an alternative treatment for various diseases, including cardiovascular disease (Shaito et al., 2020). With the progress of modern society and advances in medical research, various botanical drugs have demonstrated potential application value in the treatment of AS. Bioactive metabolites, isolated from botanical drugs, are structurally defined small molecules with specific biological activities, serving as crucial sources for drug discovery (such as alkaloids, flavonoids, terpenoids, polyphenols, and glycosides) (Pawlita et al., 1985; Cheng et al., 2017). The application value of TCM in the prevention and treatment of AS has been supported by modern pharmacological research. Increasingly, bioactive metabolites and herbal formulas are reported to delay AS, providing a scientific basis for the development of related drugs. This article systematically summarizes bioactive metabolites and herbal formulas regulate macrophage polarization in the treatment of AS. The intervention mechanisms of bioactive metabolites and herbal formulas on macrophage polarization-related signaling pathways (Figure 2).
FIGURE 2
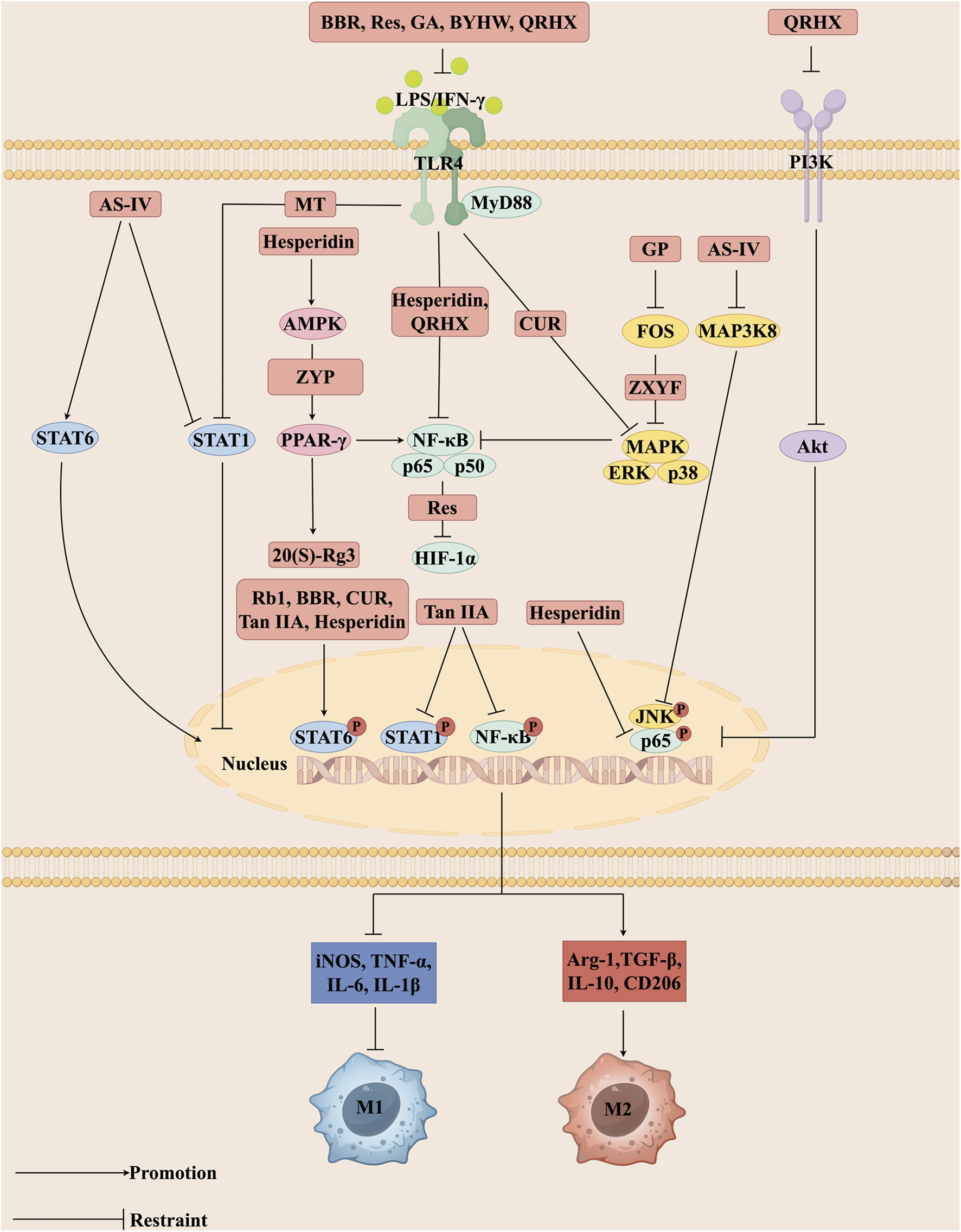
Different bioactive metabolites, including Rb1, Rg3, BBR, CUR, MT, Tan IIA, Res, GA, GP, AS-IV, and Hesperidin as well as herbal formulas such as BYHW, ZYP, ZXYF, and QRHX regulate signaling pathways central to macrophage polarization. These interventions act on key nodes such as TLR4, MAPK, PPAR-γ, and JAK/STAT, thereby modulating intracellular phosphorylation events, including STAT6 and NF-κB nuclear translocation. Through these mechanisms, they alter the expression of inflammatory mediators, promote the polarization of macrophages toward the anti-inflammatory M2 phenotype, and suppress pro-inflammatory M1 activity. Collectively, this rebalancing of macrophage function contributes to the attenuation of vascular inflammation, stabilization of atherosclerotic plaques, and overall improvement in the pathological process of atherosclerosis (AS). The figure was created with Figdraw.com.
5.1 Bioactive metabolites
5.1.1 Ginsenoside
Ginseng refers to the dried root of the species Panax ginseng C.A.Mey. of the Araliaceae family (Liu H et al., 2020), which has both medicinal and dietary value (Jang et al., 2023). Ginsenosides, a class of triterpene metabolites found in P. ginseng, have demonstrated therapeutic effects in atherosclerotic disease. For example, ginsenosides delay AS progression by regulating inflammation-related signaling pathways and lipid metabolism disorders (Sun et al., 2016). To date, ginsenosides extracted from ginseng are classified into two main categories: pentacyclic triterpenes (pentacyclic oleanolic acid type) and tetracyclic triterpenes (tetracyclic dammarane type) (Liang et al., 2024). These structural categories underpin the diverse pharmacological activities of ginsenosides, particularly their immunomodulatory and lipid-regulating effects in AS. Ginsenoside Rb1 (Rb1) is the most abundant bioactive metabolites in ginseng and has multiple effects in preventing and treating AS, including anti-inflammatory, antioxidant, improving myocardial ischemia, and anti-angiogenesis properties (Zhou et al., 2018; Ni et al., 2022). In a study utilizing ApoE−/− mice and primary peritoneal macrophages isolated from C57BL/6 mice, Zhang X. et al. (2018) reported that Rb1 promotes the secretion of IL-4 and IL-13 in peritoneal macrophages. They further showed that Rb1 dose-dependently enhances phosphorylation of STAT6, thereby facilitating a shift in macrophage phenotype toward the anti-inflammatory M2 state. In addition, Rb1 increases IL-10 expression, decreases MMP-9 levels, mitigates AS-associated inflammatory responses, and contributes to enhanced plaque integrity. Rg3, a natural ligand of PPAR-γ, has been shown to specifically bind to this receptor (Kwok et al., 2012). Thus, Rg3 targets PPAR-γ to remodel macrophage polarization from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype, thereby inhibiting AS progression (Kwok et al., 2012). Guo et al. (2018) identified the PPAR-γ signaling pathway as a key mediator of the anti-AS actions of 20(S)-Rg3. In vitro, using bone marrow-derived macrophages (BMDMs), RAW264.7, and THP-1 cells, 20(S)-Rg3 activates PPAR-γ to drive a phenotypic switch in macrophages from M1 to M2, thereby suppressing pro-inflammatory mediators (e.g., iNOS, IL-6 and TNF-α) while enhancing anti-inflammatory factors (e.g., Arg-1, IL-10 and TGF-β). This immunomodulatory effect alleviates advanced glycation end-products (AGEs)-induced inflammation and improves the vascular microenvironment. Moreover, in ApoE−/− mice, Rg3 enhances plaque structural integrity by reducing lipid deposition, promoting smooth muscle cell proliferation, and increasing collagen expression (Xue et al., 2021). In diabetic ApoE−/− mice, Rg3 suppresses pro-inflammatory M1 polarization while fostering anti-inflammatory M2 activation. Notably, these effects are reversed upon co-administration of a PPAR-γ antagonist, which exacerbates inflammatory responses. Consistent with these in vitro findings, studies in diabetic ApoE−/− mice demonstrated that 20(S)-Rg3 reduces lipid accumulation, increases collagen deposition, stabilizes atherosclerotic plaques, and lowers blood glucose levels, ultimately providing integrated protection against atherosclerotic progression through PPAR-γ activation (Guo et al., 2018). Cumulatively, these preclinical findings demonstrate that ginsenosides, especially Rb1 and Rg3, exert strong anti-inflammatory and plaque-stabilizing effects via STAT6 and PPAR-γ signaling. Nevertheless, the translation of these mechanistic insights into clinical benefit remains unproven. Current evidence is largely confined to cell and animal models, while high-quality clinical trials are scarce. Considering the complexity of AS and its clinical consequences, future research should move beyond mechanistic exploration toward rigorous evaluation of clinical endpoints, such as cardiovascular event reduction, long-term plaque stabilization, and metabolic improvement. Only by establishing robust translational frameworks can ginsenosides advance from promising experimental agents to evidence-based therapeutic options.
5.1.2 Berberine
Berberine (BBR) is an isoquinoline present in Tinospora cordifolia and roots, rhizomes, and stem bark of several medicinal plants belonging to the Ranunculaceae, Rutaceae, and Berberidaceae families (Ryuk et al., 2012; Gawel et al., 2020). BBR exerts vascular protective effects, including anti-inflammatory, antioxidant, and lipid metabolism-regulating properties (Wu M. et al., 2020; Wu et al., 2021; Zhu et al., 2022). In addition, the broad pharmacological activities of BBR have been reported to act on multiple diseases, including diabetes, obesity, neurodegenerative, and neuropsychiatric disorders (Gasmi et al., 2024). It modulates multiple signaling pathways, such as STAT, MAPK, NF-κB, PI3K/Akt, and AMPK, to inhibit inflammatory cell infiltration, improve endothelial dysfunction, and enhance vascular remodeling, thereby preventing AS progression (Ai et al., 2021). Notably, the TLR4/MyD88/NF-κB axis is a key pathway through which BBR regulates inflammatory responses and vascular remodeling in AS (den et al., 2010). These mechanistic insights indicate that BBR confers multi-targeted vascular protection, mainly through regulation of inflammation, lipid metabolism, mitochondrial function, and the functional plasticity of macrophages. In vitro studies, such as those using RAW264.7 macrophages, demonstrate that BBR can alter the inflammatory profile of these cells by inhibiting the TLR4/MyD88/NF-κB pathway. This leads to reduced expression of M1-associated markers (e.g., iNOS, TNF-α, and IL-6) and elevated levels of M2-associated markers like CD206 (Li et al., 2020). Furthermore, BBR enhances phosphorylation and activation of the STAT6 signaling pathway, which contributes to its capacity to reprogram macrophage phenotype away from the pro-inflammatory M1 state and toward the anti-inflammatory M2 state (Chen YS. et al., 2024). In AS lesions, mitochondrial dysfunction elevates ROS levels, which can promote a pro-inflammatory macrophage phenotype and compromise plaque structural integrity. BBR helps preserve mitochondrial function, thereby mitigating oxidative stress and contributing to a more stable plaque phenotype (Peng et al., 2019; Tan et al., 2020). These preclinical studies support the anti-inflammatory, lipid-lowering, and plaque-stabilizing roles of BBR in AS models. Nevertheless, most available evidence is derived from experimental settings, and critical questions regarding its pharmacokinetics, optimal dosing, long-term safety, and interactions with standard-of-care therapies remain unanswered. To advance BBR toward clinical utility, future research should not only confirm its vascular protective effects in human subjects but also integrate evaluations of hard clinical endpoints, such as cardiovascular morbidity and mortality. Addressing these translational gaps will be essential to determine whether BBR can evolve from a multi-target experimental agent into a viable therapeutic candidate in the management of atherosclerotic disease.
5.1.3 Curcumin
Curcumin (CUR) is a natural polyphenolic diarylheptanoid extracted from the rhizomes of species belonging to the Zingiberaceae and turmeric families, especially Curcuma longa L (Lv et al., 2020). Extensive preclinical studies in animal models have demonstrated its multi-targeted pharmacological activities, including anti-inflammatory, antithrombotic, antiviral, anticancer, anti-degenerative disease, hepatoprotective, and neuroprotective effects, which are largely mediated through multiple molecular targets (Laurindo et al., 2023; Corrêa Carvalho et al., 2024; Sharifi-Rad et al., 2020). At the mechanistic level, CUR exerts anti-inflammatory effects through multiple pathways, including inhibition of TLR4 overexpression and dimerization, suppression of MAPK pathway phosphorylation, prevention of IκBα degradation, and impairment of nuclear translocation of the NF-κB subunit p65 (Lubbad et al., 2009). Particularly, its interference with the NF-κB pathway—a central regulator of inflammation—extends to attenuating macrophage infiltration within atherosclerotic plaques (Zhang S. et al., 2018). Moreover, CUR modulates macrophage phenotype by directly stabilizing IκBα, thereby restraining the pro-inflammatory M1 state, and concurrently activating PPAR-γ to favor the anti-inflammatory M2 phenotype (Chen and Xu, 2014). These multi-pathway mechanisms suggest that CUR may regulate inflammation and immune balance in AS by modulating macrophage polarization and inflammatory signaling. In cellular models, such as THP-1-derived macrophages, CUR dose-dependently suppresses the production of pro-inflammatory cytokines including TNF-α, IL-6, and IL-12B (p40). This anti-inflammatory effect is linked to inhibition of the TLR4/MAPK/NF-κB axis, which collectively drives a phenotypic shift in macrophages from a pro-inflammatory M1 toward an anti-inflammatory state (Zhou et al., 2015). These findings underscore the potential of CUR as a promising therapeutic agent for AS (Zhou et al., 2015). Moreover, combined treatment with CUR and BBR in RAW264.7 macrophages ameliorates the pathological progression of AS by enhancing STAT6 phosphorylation and suppressing the expression of M1 macrophage markers (Chen YS. et al., 2024). Integrating these preclinical data reveals that CUR not only reduces inflammatory mediator expression but also favors an M2-like functional state in macrophages. Moreover, it exhibits synergistic effects with other natural metabolites such as berberine to exert anti-atherosclerotic actions. Clinical studies have indicated that CUR supplementation may improve surrogate markers, including systemic inflammation and oxidative stress; however, the evidence is largely confined to intermediate endpoints and short-term interventions. Future investigations should therefore move beyond biomarker-based observations and establish whether CUR can achieve durable benefits on hard cardiovascular outcomes, such as plaque stabilization, event reduction, and long-term vascular protection. In addition, issues related to bioavailability, optimal formulation, and integration into standard therapeutic regimens require careful evaluation to clarify its true translational potential in the management of AS.
5.1.4 Matrine
Matrine (MT), also known as oxymatrine or Sophora alkaloid, is an alkaloid obtained from different species of the Sophora genus (Wang X. et al., 2023; Zhang M. et al., 2023). MT plays a crucial role in several pathophysiological processes, including antioxidant, anti-ischemia–reperfusion injury (Lu Q. et al., 2021), anti-sepsis (Wang X. et al., 2023), antiviral (Qiao et al., 2024), intestinal barrier protective (Yu D. et al., 2023), antidepressant (Zhang M. et al., 2023), analgesic, and neuroprotective (Zhu C. et al., 2024) effects. Mechanistically, MT interferes with a network of signaling cascades that drive AS progression. Specifically, MT inhibits the TGF-β1/Smad pathway, leading to suppression of ECM formation and reduced expression of inflammatory factors (Chen C et al., 2024). In addition, in ox-LDL-stimulated vascular smooth muscle cells, MT suppresses the activation of NF-κB, MAPK, and JAK/STAT3 signaling pathways, resulting in the downregulation of pro-inflammatory mediators such as IL-1β, TNF-α, VCAM-1, and ICAM-1, thereby attenuating inflammatory responses and cellular adhesion (Lu et al., 2018). These findings suggest that MT exerts vascular protection by targeting inflammation, oxidative stress, and extracellular matrix remodeling. Treatment of M0-polarized primary mouse peritoneal macrophages with MT effectively inhibited AGEs-induced M1 macrophage polarization through suppression of the TLR4/STAT1 signaling pathway (Cui et al., 2022). Concurrently, MT was found to downregulate the expression of DNA methyltransferases in macrophages, an effect associated with mitigated oxidative stress and delayed AS development (Cui et al., 2022). Additionally, studies have demonstrated that MT ameliorates inflammation and attenuates vascular wall thickening in high-fat diet (HFD)-fed mice (Liu et al., 2016). Collectively, these preclinical observations delineate a role for MT in counteracting AS by modulating macrophage function, restraining pro-inflammatory signaling, and limiting pathological vascular remodeling. However, current evidence remains almost exclusively experimental, and the clinical landscape for MT in AS is largely unexplored. Given its extensive pharmacological spectrum and long history of use in traditional medicine, future research should focus on bridging mechanistic insights with patient-oriented outcomes. Particular attention is needed to clarify its pharmacokinetics, optimal therapeutic window, and long-term safety profile in the context of cardiovascular disease. Establishing whether MT can translate its multi-target anti-atherosclerotic effects into measurable improvements in cardiovascular events will be pivotal for advancing its development as a viable therapeutic candidate.
5.1.5 Resveratrol
Resveratrol (Res) is a natural polyphenolic compound, which is present in a variety of plants and their products, such as Polygonum cuspidatum, grape seeds, peanut and so on (Ma et al., 2023). Res possesses anti-inflammatory, antioxidant, lipid-regulating, and cardioprotective effects, making it effective against chronic inflammatory diseases, including cardiovascular disease (CVD) (Meng et al., 2021; Zhang et al., 2021). These multiple signaling pathways highlight Res as a pleiotropic regulator of vascular inflammation and lipid metabolism in the context of AS. Its ability to broadly suppress pro-inflammatory signaling, as evidenced in both human monocyte-derived M1 and M2 macrophages challenged with 7-oxocholesterol, underscores its potential as a therapeutic agent for AS (Buttari et al., 2014). Guo et al. (2023) demonstrated that Res treatment in LPS+IFN-γ-induced RAW264.7 macrophages suppressed gene and protein expression in the TLR4/NF-κB/HIF-1α pathway. Consequently, Res dose-dependently restricted the pro-inflammatory M1 phenotype, contributing to its anti-AS properties. Furthermore, Res inhibits the degradation of IκB-α and the nuclear translocation of NF-κB p65 induced by TNF-α, thereby reducing arterial macrophage infiltration (Repossi et al., 2020; Shang et al., 2019). Moreover, Res contributes to enhanced plaque integrity and retards AS development. It also reduces ischemia-reperfusion injury and prevents AS-related vascular events (Raj et al., 2021). In addition, Res decreases AS-related markers such as MMP-9 and CD40 ligand in lesion areas, mitigating AS pathology (Ji et al., 2022). Together, these preclinical data indicate that Res orchestrates a multi-faceted anti-atherosclerotic response by tempering pro-inflammatory macrophage activation, dampening key inflammatory cascades, and reinforcing plaque structure, thereby slowing the progression of AS. Nonetheless, most studies remain confined to mechanistic and surrogate outcomes, and whether these effects can be translated into durable cardiovascular protection in humans is still unclear. Given the pleiotropic actions of Res, future investigations should not only validate its impact on clinically relevant endpoints,but also resolve critical challenges including bioavailability, inter-individual variability, and formulation strategies. Addressing these gaps will be essential to determine whether Res can progress from a promising experimental metabolites to a clinically actionable intervention in atherosclerotic disease.
5.1.6 Tanshinone IIA
Salvia miltiorrhiza (Danshen), the dried rhizomes and roots of Salvia miltiorrhiza Bge (Lamiaceae), contains over 200 identified metabolites, including lipophilic diterpene quinones and water-soluble phenolic acids (Petitjean et al., 2022; Guo et al., 2020). Tanshinone IIA (Tan IIA), the most studied diterpene quinone, exhibits anti-inflammatory, anti-thrombotic, anti-apoptotic, and endothelial-protective effects in preclinical models of AS (Wang H. et al., 2020; Yang et al., 2023). Its metabolites, particularly Tan IIA, have also been investigated in studies targeting AS, hepatic steatosis, and diabetic nephropathy (Wu Q. et al., 2023). Specifically, the underlying mechanism is the metabolites of Salvia miltiorrhiza, especially Tan IIA, exert cardiovascular protection through multi-target actions, particularly by regulating inflammation, immune cell function, and plaque stability. Chen et al. (2019) demonstrated in ApoE−/− mice that Tan IIA orchestrates macrophage phenotypic balance by concurrently modulating the STAT6 and NF-κB pathways. It upregulates phosphorylated STAT6, fostering the expression of M2-associated markers (TGF-β, Arg-1, IL-10), while simultaneously inhibiting NF-κB and STAT1 activation to suppress key M1-related mediators (TNF-α, iNOS, IL-12, IL-6), collectively favoring an anti-inflammatory macrophage phenotype and mitigating AS. Furthermore, in ApoE−/− mice, Tan IIA modulates immune cell function and activation, reduces inflammatory factor levels, and restores abnormal signaling pathways (Guo et al., 2020). For example, Tan IIA inhibits the TLR4/MyD88/NF-κB pathway, reducing inflammation in a dose-dependent manner, decreasing macrophage infiltration, increasing collagen content, and stabilizing AS plaques (Chen Z. et al., 2019). Additionally, Tan IIA suppresses NF-κB activation, downregulates PPAR-γ protein expression, and reduces mRNA levels of IL-10, IL-6, and MMP-1, thereby blocking the propagation of inflammatory signals, stabilizing atherosclerotic plaques, and ultimately lowering the risk of plaque rupture (Chen and Xu, 2014). Wang N et al. (2020) further showed that combined treatment of Tan IIA and astragaloside IV (AS-IV) activates PI3K/Akt and inhibits TLR4/NF-κB signaling, significantly reducing IL-6, MMP-9, TNF-α, and C-reactive protein levels, while upregulating endothelial nitric oxide synthase. This combination reduced lipid deposition and stabilized plaques in ApoE−/− mice. In conclusion, these collective preclinical findings position Tan IIA as a multi-faceted agent capable of dampening vascular inflammation, reprogramming macrophage responses, and enhancing plaque structural resilience in AS models. Yet, most available findings remain confined to cellular and animal experiments, and the translation of these benefits into clinical efficacy is far from established. While Tan IIA has demonstrated robust effects on immunomodulation and vascular protection, decisive evidence regarding its ability to alter the natural history of AS in humans is lacking. Future studies should extend beyond mechanistic observations to evaluate clinically relevant outcomes, including plaque regression, stabilization against rupture, and reduction in cardiovascular events. Moreover, careful assessment of pharmacokinetics, dosing strategies, and long-term safety will be essential for determining whether Tan IIA can advance from an experimental metabolites to a clinically applicable therapy in atherosclerotic disease.
5.1.7 Ganoderic acid
Ganoderma lucidum (G. lucidum)is a medicinal mushroom with a long history of use for its tonic and health-promoting properties (Liu C. et al., 2023). G. lucidum is rich in various bioactive metabolites, including polysaccharides, triterpenoids, sterols, and other phytochemicals (Yuan et al., 2022). Ganoderic acid (GA), a triterpenoid metabolite extracted from G. lucidum, exhibits pharmacological activities such as anti-lipid accumulation (Li Y. et al., 2021; Zheng et al., 2023), anticancer (Chen S. et al., 2024), anti-aging (Chen L. et al., 2025), and anti-asthmatic effects (Lu X. et al., 2021). These diverse biological functions suggest that GA may act on multiple signaling pathways to regulate inflammation, lipid metabolism, and vascular homeostasis in the context of AS. Integrated in vivo and in vitro studies (ApoE−/− mice, BMDMs, RAW264.7 cells) have shown that GA targets the TLR4/MyD88/NF-κB axis. This inhibition leads to reduced secretion of key pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, MCP-1) and restrains pro-inflammatory macrophage activation. Consequently, GA administration dose-dependently diminishes the plaque necrotic core, augments collagen deposition, and improves plaque structural integrity (Quan et al., 2024). Additionally, GA regulates the release of inflammatory mediators by modulating key signaling pathways, including PI3K/Akt/mTOR, NF-κB, Neurogenic locus notch homolog protein 1, and JAK3/STAT3, which in turn influences the progression of AS (Wang S. et al., 2024). In general, the preclinical evidence underscores GA’s potential to mitigate AS through concerted anti-inflammatory actions, modulation of macrophage phenotype, and reinforcement of plaque stability. However, clinical investigations remain virtually absent, and the therapeutic significance of GA for human AS is uncertain. Given its long-standing use in traditional medicine and promising mechanistic profile, future work should aim to bridge preclinical efficacy with patient-centered outcomes. Particular emphasis should be placed on evaluating its ability to influence clinically relevant endpoints—such as plaque vulnerability, vascular function, and cardiovascular event rates—while also addressing challenges related to standardization of extracts, pharmacokinetics, and long-term safety. Establishing such evidence will be critical for defining the translational value of GA as a potential adjunctive therapy in atherosclerotic disease.
5.1.8 Geniposide
Gardenia jasminoides Ellis (G. jasminoides) is an evergreen shrub of species belonging to the Rubiaceae family that grows widely in many regions of the world (Shen et al., 2020). Geniposide (GP), the major bioactive metabolite isolated from G. jasminoides, is a cycloartenol glycoside with anti-inflammatory, lipid-regulating, macrophage-modulating, endothelial-protective, and anti-thrombotic properties, which help prevent and treat AS, diabetes, and related complications (Li H. et al., 2022; Li D. et al., 2024). Several studies have demonstrated that GP regulates inflammatory mediator release by modulating key signaling pathways, including MAPK, PI3K, Akt, NF-κB, and TLR, thereby promoting M2 polarization (Chi et al., 2006; Ma et al., 2024). These pleiotropic actions indicate that GP exerts its protective role in AS through simultaneous regulation of inflammation, lipid metabolism, and vascular function. In a study on New Zealand white rabbits with HFD-induced AS, Jin et al. (Jin et al., 2020) demonstrated that GP downregulates the expression of NR4A1, CD14, and IL-1α within the MAPK signaling pathway, while increasing Arg-1 levels and promoting the secretion of IL-10. This collective shift in the inflammatory milieu favors an M2-like functional state in macrophages, an effect mediated through inhibition of the FOS/MAPK pathway, ultimately contributing to reduced plaque burden and ameliorated AS pathology. Furthermore, GP contributes to plaque stabilization by limiting lipid deposition and enhancing collagen fiber content (Chi et al., 2006; Ma et al., 2024). In ApoE−/− mice, GP inhibits foam cell formation and accelerates AS regression, partly through effects on dendritic cell maturation (Liu L. et al., 2014; Liao et al., 2014). Overall, the collective preclinical evidence delineates a role for GP in curtailing atherosclerotic plaque development and reinforcing plaque stability through multi-target mechanisms. At present, the clinical evidence supporting the role of GP in AS is obviously insufficient. Although preclinical studies have elucidated its effects on signaling pathways and macrophage polarization, these mechanisms remain to be clinically validated. Future randomized controlled trials are needed to fill these key evidence gaps and to determine the efficacy and safety of GP in human subjects.
5.1.9 Astragaloside IV
Astragalus mongholicus Bunge (Huangqi) has significant natural antioxidant activity and is effective in reducing the risk of AS (Wang T. et al., 2022). The main bioactive metabolites of Huangqi include polysaccharides, saponins, and flavonoids, among which AS-IV, a tetracyclic triterpenoid saponin (Zheng et al., 2020), is particularly important in cardiovascular protection due to its diverse pharmacological effects, including anti-inflammatory, antioxidant, anti-fibrotic, angiogenic, calcium-regulating, and lipid-lowering effects (Liang et al., 2023; Ou et al., 2023). Mitogen-activated protein kinase kinase 8 (MAP3K8), a key regulator of inflammation and immunity, plays a critical role in inflammation, immune regulation, endothelial function, and cell proliferation (Webb et al., 2019). These mechanistic insights indicate that AS-IV may act through multiple signaling pathways to regulate inflammation, oxidative stress, and endothelial function, thereby conferring protection against AS. Research by He et al. in ApoE−/− mice elucidated that AS-IV reprograms macrophage polarization toward the M2 phenotype by targeting MAP3K8. This intervention resulted in suppressed MAP3K8 expression within the aortic tissue, concomitant inhibition of JNK and NF-κB p65 phosphorylation, and a marked upregulation of M2-associated markers (TGF-β, IL-4, IL-10, Arg-1). This phenotypic shift was further underpinned by the suppression of STAT1 signaling and potentiation of STAT6 activation (He et al., 2023). Beyond macrophage modulation, AS-IV activates PPAR-γ while concurrently suppressing the TLR4/NF-κB and PI3K/Akt pathways, actions that contribute to reduced lipid deposition and confer protection to endothelial cells (Zhang Y. et al., 2022). NRF2, a key antioxidant pathway, maintains redox balance by suppressing pro-inflammatory gene transcription (Lassègue et al., 2012; Kobayashi et al., 2016). In both in vivo AS rat models and in vitro ox-LDL-induced HUVEC models, AS-IV has been shown to activate NRF2—a central transcription factor in the antioxidant defense system—thereby alleviating oxidative stress. This activation contributes to the repair of oxidative stress-induced endothelial damage, suppresses the secretion of inflammatory factors, and ultimately confers therapeutic benefits against AS (Sun et al., 2018; Zhu et al., 2019). In summary, the collective preclinical evidence positions AS-IV as a multi-target agent capable of mitigating AS through coordinated immunomodulation, antioxidant effects, and endothelial protection. The generation of robust clinical evidence is therefore a pivotal next step for advancing AS-IV toward clinical application.
5.1.10 Hesperidin
Hesperidin is a flavanone glycoside metabolite extracted from the mature fruit peel of citrus plants belonging to the Rutaceae family, such as orange (Citrus sinensis), grapefruit (Citrus paradise), and lemon (Citrus limon) (Pyrzynska, 2022). It is one of the most widely distributed plant phenolic metabolites in nature (Pyrzynska, 2022). Studies have shown that hesperidin exerts multifunctional pharmacological actions, including antioxidation, anti-inflammation, improvement of endothelial function, blood glucose regulation, and blood pressure reduction (Mas-Capdevila et al., 2020; Ortiz et al., 2022). These mechanisms indicate its potential role in cardiovascular protection and the prevention of AS. Investigations in ApoE−/− mice and RAW264.7 macrophages have revealed that hesperidin activates the AMPK/PPAR-γ pathway and suppresses NF-κB (P65) expression, leading to a reprogramming of macrophage polarization towards an anti-inflammatory M2 phenotype (Fan et al., 2022). This shift in macrophage phenotype is associated with a favorable modulation of the inflammatory milieu, characterized by decreased secretion of TNF-α and IL-6 and increased expression of Arg-1 and IL-10. Consequently, hesperidin treatment reduces lipid deposition, inhibits high-fat diet-induced foam cell formation, and attenuates the development of atherosclerotic plaques (Mas-Capdevila et al., 2020; Fan et al., 2022). Cumulatively, these preclinical findings highlight hesperidin as a multi-target agent with significant potential to ameliorate cardiovascular risk factors and counteract atherosclerotic processes (Ebrahimi et al., 2023). Currently, clinical research on hesperidin primarily focuses on its impact on cardiovascular risk factors such as blood pressure, glycemic control, and systemic inflammation. Direct clinical evidence demonstrating its efficacy in intervening against atherosclerotic disease itself remains limited. While existing human data suggest potential cardiovascular benefits, large-scale, well-designed Randomized Controlled Trials are imperative to definitively establish its therapeutic value for AS. The chemical structure of the bioactive metabolite is shown in Figure 3. This review summarizes the signaling pathways related to the treatment of AS by bioactive metabolites by regulating macrophage polarization (Table 1).
FIGURE 3
Chemical formula structure of the bioactive metabolites.
TABLE 1
| Signaling pathway | Mechanism of action | Bioactive metabolites | Types | Model | Dosage range | Macrophage polarization-related molecular targets | References | |
|---|---|---|---|---|---|---|---|---|
| Upregulation (M2) | Downregulation (M1) | |||||||
| TLR4 | TLR4/MyD88/NF-κB↓ | BBR | Alkaloids | RAW264.7 cell | 5, 10, 20 μmol/L | CD206 | iNOS, IL-6, TNF-α |
Li et al. (2020) |
| TLR4/MAPK/NF-κB↓ | CUR | Polyphenols | THP-1 cell | 0, 7.5, 15, 30 μmol/L | - | TNF-α, IL-6, IL-12B (p40) | Zhou et al. (2015) | |
| TLR4/MyD88/NF-κB↓ | GA | Tterpenoids | ApoE−/− mice, BMDMs and RAW264.7 cell |
in vivo: 1, 5, 25 mg/kg in vitro: 1, 5, 25 μg/mL |
- | TNF-α, IL-6, IL-1β, MCP-1 |
Quan et al. (2024) | |
| TLR4/STAT1↓ | MT | Alkaloids | M0-polarized primary mouse peritoneal macrophages | 2.0 mmol/L | - | iNOS, TNF-α, IL-6, IL-1β | Cui et al. (2022) | |
| NF-κB | TLR4/NF-κB/HIF-1α↓ | Res | Polyphenols | RAW264.7 cell | 1, 5, 10 μmol/L | - | IL-1β, IL-6, TNF-α | Guo et al. (2023) |
| NF-κB↓ | Tan IIA | Tterpenoids | ApoE−/− mice | 10 mg/kg/d | - | IL-6, TNF-α | Chen and Chen (2019) | |
| NF-κB (p65)↓ | Hesperidin | Glycosides | ApoE−/− mice, RAW264.7 cell |
in vivo: 100, 200, 400 mg/kg in vitro: 5, 10, 20 μmol/L |
- | TNF-α, IL-6 | Fan et al. (2022) | |
| MAPK | FOS/MAPK↓ | GP | Glycosides | New Zealand white rabbits with HFD | 1.5 mg/kg/d | IL-10, Arg-1 | iNOS, IL-1β | Jin et al. (2020) |
| MAP3K8↓ | AS-IV | Glycosides | ApoE−/− mice | 20, 50 mg/kg | IL-10, IL-4, TGF-β, Arg-1 | TNF-a | He et al. (2023) | |
| PPAR-γ | PPAR-γ↑ | 20(S)-Rg3 | Glycosides | Diabetic ApoE−/− mice, BMDMs, RAW264.7 and THP-1 cell |
in vivo: 10 mg/kg/2d in vitro: 25 μM |
Arg-1, CD206 | iNOS, CD86 | Guo et al. (2018) |
| AMPK/PPAR-γ↑ | Hesperidin | Glycosides | ApoE−/− mice, RAW264.7 cell |
in vivo: 100, 200, 400 mg/kg in vitro: 5, 10, 20 μmol/L |
IL-10, Arg-1 | - | Fan et al. (2022) | |
| JAK/STAT | p-STAT6↑ | Rb1 | Glycosides | ApoE−/− mice, C57BL/6 mouse peritoneal macrophages |
in vivo: 50 mg/kg/d in vitro: 10, 20, 40, 80 μM |
IL-13, IL-4, IL-10, Arg-1, CD206 | iNOS, MMP-9 | Zhang X. et al. (2018) |
| p-STAT6↑ | BBR | Alkaloids | RAW264.7 cell | 0, 5, 10, 25, 50, 100 μmol/L | - | iNOS, TNF-α, CXCL9 |
Chen YS. et al. (2024) | |
| p-STAT6↑ | CUR | Polyphenols | RAW264.7 cell | 0, 1, 10, 20, 40, 80 μmol/L | - | iNOS, TNF-α, CXCL9 | Chen YS. et al. (2024) | |
| p-STAT6↑, p-STAT1↓ |
Tan IIA | Tterpenoids | ApoE−/− mice | 10 mg/kg/d | IL-10, TGF-β | IL-6, TNF-α | Chen and Chen (2019) | |
Signaling pathways related to the treatment of AS by bioactive metabolites by regulating macrophage polarization.
5.2 Herbal formulas
5.2.1 Bu Yang Huan Wu decoction
Bu Yang Huan Wu Decoction (BYHW) was first recorded in 《Yi Lin Gai Cuo》 during the Qing Dynasty and was composed of seven botanical drug: Astragalus mongholicus Bunge. (Lamiaceae, Huangqin), Paeonia lactiflora Pall. (Paeoniaceae, Chishao), Carthamus tinctorius L. (Asteraceae, Honghua), Ligusticum chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu (Apiaceae, Chuanxiong), Juglans regia L. (Rosaceae, Taoren), Angelica sinensis (Oliv.) Diels (Apiaceae, Danggui), and Lumbricus (Dilong) (Mo et al., 2024). Modern research shows that BYHW provides vascular endothelial protection, regulates blood lipid levels, and exerts anti-AS effects through multi-metabolites and multi-target mechanisms, as well as widely used in the prevention and treatment of atherosclerotic disease, such as CVD (Zhang WW. et al., 2018). These pharmacological activities are largely attributed to its rich composition of bioactive metabolites, which act synergistically on inflammatory, lipid metabolic, and vascular pathways. It is important to note that while clinical application is widespread, the mechanistic understanding of BYHW’s anti-AS effects is predominantly derived from preclinical investigations. For example, in ApoE−/− mice, BYHW inhibits the activation of the TLR4/MyD88/NF-κB signaling pathway, suppresses the expression of pro-inflammatory factors and adhesion molecules, inhibits foam cell formation, and promotes macrophage polarization toward the M2 phenotype (Li WY. et al., 2023). Bioactive metabolites in BYHW with anti-AS properties include AS-IV, paeoniflorin, ligustrazin, amygdalin, luteolin, ferulic acid, safflower yellow, and hydroxy-safflower yellow (Liu W. et al., 2022). Among these, glycosides reduce the release of pro-inflammatory factors and adhesion molecules and inhibit the phosphorylation of JAK2, STAT1, and STAT3, thereby alleviating AS inflammation (Fu et al., 2022). Further studies indicate that AS-IV, paeoniflorin, and amygdalin are among the principal bioactive metabolites in BYHW. These metabolites inhibit AS inflammation by suppressing the expression of STAT3, HIF-1, VEGF, and IκBα, as well as the nuclear translocation of NF-κB (Yan et al., 2023; Liu B. et al., 2020). Collectively, these findings suggest that the synergistic actions of multiple metabolites within BYHW converge on key inflammatory and lipid-regulating pathways, thereby attenuating atherosclerotic progression. BYHW is widely used in the clinical prevention and treatment of atherosclerotic diseases, such as cardiovascular disease. However, despite its long-standing application and anecdotal benefits, high-quality clinical trials specifically confirming its mechanisms, efficacy, and safety in AS remain limited.
5.2.2 Zhuyu Pill
Zhuyu Pill (ZYP) was originally documented in 《Taiping Shenghuifang》 (Zhao Y et al., 2025), which was composed of Coptis deltoidea C.Y.Cheng & P.K.Hsiao (Ranunculaceae, Huanglian) and Tetradium ruticarpum (A.Juss.) T.G.Hartley (Rutaceae, Wuzhuyu) (Zhang X. et al., 2022). ZYP has effects on lowering blood lipids, reducing inflammation, and regulating glucose and lipid metabolism (Zhang X. et al., 2022). The main bioactive metabolites in ZYP are BBR, palmatine, evodiamine, and rutaecarpine (Pan et al., 2023). These metabolites exert synergistic actions on multiple signaling pathways, providing a mechanistic basis for ZYP’s anti-atherosclerotic activity. Mechanistic investigations indicate that ZYP improves AS by inhibiting the TLR4/MyD88/TRAF6 signaling pathway, activating STAT6 phosphorylation, and promoting M2-type macrophage polarization, thereby alleviating lipid metabolism disorders and inflammation (Zhao M. et al., 2025). Song et al. (2025) confirmed that ZYP regulates macrophage polarization, reduces foam cell and inflammatory cell aggregation, and lowers AS plaque deposition. Further studies found that in ApoE−/− mice treated with ZYP, secretion of pro-inflammatory factors related to M1-type macrophages (TNF-α, IL-6) was significantly reduced, while the expression levels of NF-κB and iNOS were decreased. Conversely, anti-inflammatory factors associated with M2-type macrophages (IL-4, IL-13) were increased, along with the expression levels of PPAR-γ and Arg-1 (Song et al., 2024). By network pharmacology and in vivo experiments, ZYP was also found to exert anti-atherosclerotic effects through reduction of IL-6 and TNF-α levels and inhibition of the NF-κB pathway (Pan et al., 2023). Taken together, these findings suggest that ZYP attenuates AS progression via activation of the PPAR-γ/NF-κB signaling axis, suppressing M1 macrophage polarization while promoting M2 differentiation. This results in reduced inflammatory infiltration within arterial tissues and inhibition of plaque formation. Recent clinical trials have begun to evaluate ZYP’s efficacy in patients with hyperlipidemia complicated by carotid AS (Song et al., 2025). While preliminary data are encouraging, mechanistic insights remain predominantly derived from preclinical models, and high-quality randomized controlled trials directly confirming its anti-atherosclerotic effects are still lacking.
5.2.3 ZeXieYin formula
ZeXieYin formula (ZXYF), which can be traced back to 《Huangdi Neijing》, is formulated with three specified botanical drugs: Alisma plantago-aquatica L. (Alismataceae, Zeie), Atractylodes macrocephala Koidz. (Asteraceae, Baizhu), and Pyrola calliantha Andres. (Ericaceae, Luxiancao) (Sun et al., 2024). It is known for its ability to regulate lipid metabolism (Zhu et al., 2020). The traditional use of ZXYF in lipid regulation provides a theoretical foundation for its potential application in the prevention and treatment of AS. Huang Y et al. (2024) demonstrated in ApoE−/− mice that ZXYF significantly reduces inflammation by inhibiting the activation of ERK and p38 in the MAPK family and the expression of p-NF-κB. It also reduces the area of the necrotic lipid core and promotes macrophage polarization toward the M2 phenotype. Additionally, ZXYF inhibits the secretion of MMPs, increases plaque stability, and prevents plaque rupture (Huang R. et al., 2024). ZXYF also modulates the PI3K/Akt/SREBP-1 signaling pathway, reducing the levels of inflammatory cytokines (e.g., IL-1β, TNF-α, IL-10, IL-17, and IL-6) in serum, thereby slowing the progression of AS (Liu R. et al., 2023). Together, these animal studies indicate that ZXYF exerts anti-atherosclerotic effects through multi-target modulation of inflammatory signaling, macrophage polarization, and plaque stabilization. The evidence supporting the use of ZXYF in AS is currently limited to animal studies. Although ZXYF has a long history of clinical use for metabolic regulation in traditional medicine, there is a lack of direct clinical trials confirming its efficacy, mechanisms, and safety in patients with AS.
5.2.4 Qingre Huoxue Decoction
Qingre Huoxue Decoction (QRHX) has been clinically applied for more than two decades, is composed of seven botanical drugs: Astragalus mongholicus Bunge. (Lamiaceae, Huangqin), Paeonia lactiflora Pall. (Paeoniaceae, Chishao), Ilex pubescens Hook. & Arn. (Aquifoliaceae, Maodongqing), Carthamus tinctorius L. (Asteraceae, Honghua), Ligusticum chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu (Apiaceae, Chuanxiong), Dalbergia odorifera T.C.Chen (Fabaceae, Jiangxiang), and Salvia miltiorrhiza Bunge. (Lamiaceae, Danshen) (He W. et al., 2024). QRHX contains high levels of baicalin and salvianolic acid B, both of which exert anti-AS effects by regulating the NF-κB signaling pathway and macrophage polarization (He W. et al., 2024). The multi-metabolite and multi-target nature of QRHX provides a mechanistic basis for its anti-inflammatory, lipid-regulating, and plaque-stabilizing activities. In an in vivo study on ApoE−/− mice, QRHX induces macrophage polarization toward the M2 phenotype by inhibiting the NF-κB signaling pathway, thereby alleviating plaque inflammation. Additionally, QRHX targets and inhibits key proteins in the PI3K/Akt signaling pathway, promotes M2 polarization, and reduces the expression of M1 phenotype-related inflammatory factors (TNF-α, MCP-1, and NLRP3), exerting anti-inflammatory, lipid-regulating, and plaque-stabilizing effects (Jin, 2021). Furthermore, QRHX increases miR-26a-5p expression, decreases PTGS2 expression, and promotes the remodeling of M1-type macrophages to the M2 phenotype, thereby improving AS pathology (He W. et al., 2024). These preclinical findings provide biological plausibility for the clinical effects observed in patients and illustrate a translational pathway from mechanistic studies to therapeutic application. QRHX has demonstrated efficacy in the treatment of AS, showing potential to stabilize plaques and reduce the incidence of adverse cardiovascular events, based on evidence from clinical studies (Zuo et al., 2021). Importantly, QRHX is one of the few traditional formulas for which both clinical and preclinical evidence converge, with clinical observations guiding mechanistic investigations in animal models. This review summarizes the signaling pathways related to the treatment of AS by herbal formulas by regulating macrophage polarization (Table 2).
TABLE 2
| Signaling pathway | Mechanism of action | Herbal formulas name | Botanical drugs | Model | Dosage range | Macrophage polarization-related molecular targets | References | |
|---|---|---|---|---|---|---|---|---|
| Upregulation (M2) | Downregulation (M1) | |||||||
| TLR4 | TLR4/MyD88/NF-κB↓ | BYHW | Astragalus mongholicus, Paeonia lactiflora, Carthamus tinctorius, Ligusticum chuanxiong, Juglans regia, Angelica sinensis, and Lumbricus | ApoE−/− mice | 2.772g/kg/d | - | TNF-α, IL-6, VCAM-1 | Li WY. et al. (2023) |
| NF-κB | NF-κB↓ | QRHX | Astragalus mongholicus, Paeonia lactiflora, Ilex pubescens, Carthamus tinctorius, Ligusticum chuanxiong, Dalbergia odorifera, and Salvia miltiorrhiza | ApoE−/− mice, RAW264.7 cell |
in vivo: 7.5, 15, 30 g/kg/d in vitro: 0, 1, 2.5, 5, 10, 20, 50, 100 μg/mL |
Arg-1, CD163, IL-4, IL-10 | iNOS, TNF-α, MCP-1 | Jin Z. et al. (2023) |
| MAPK | MAPK/NF-κB↓ | ZXYF | Alisma plantago-aquatica, Atractylodes macrocephala, and Pyrola calliantha | ApoE−/− mice | 3.8 g/kg/d | IL-10 | IL-1α, IL-1β, IL-6, TNF-α | Huang R. et al. (2024) |
| PPAR-γ | PPAR-γ/NF-κB↑ | ZYP | Coptis deltoidea and Tetradium ruticarpum | ApoE−/− mice | 130.54, 261.08, 522.16 mg/kg/d | Arg-1, IL-13, IL-4 | iNOS, TNF-α, IL-6 | Song et al. (2024) |
| PI3K/Akt | PI3K/Akt↓ | QRHX | Astragalus mongholicus, Paeonia lactiflora, Ilex pubescens, Carthamus tinctorius, Ligusticum chuanxiong, Dalbergia odorifera, and Salvia miltiorrhiza | ApoE−/− mice | 7.5, 15, 30 g/kg/d | Arg-1 | iNOS, TNF-α, MCP-1, NLRP3 | Jin. (2021) |
Signaling pathways related to the treatment of AS by herbal formulas by regulating macrophage polarization.
6 Discussion
AS is a serious threat to the health of the human cardiovascular system and is closely associated with a variety of acute cardiovascular events. The pathogenesis of AS is complex, involving the interaction of endothelial dysfunction, imbalance of lipid metabolism, and blood flow abnormalities, among which the inflammatory response is the core mechanism driving plaque formation and development. Macrophages are an important part of the human immune function and are involved in the inflammatory response in vivo, and they can be transformed into different cell subtypes according to different environments, which is an important manifestation of macrophage plasticity. Macrophages can be categorized into pro-inflammatory M1-type macrophages and anti-inflammatory M2-type macrophages based on differences in macrophage activation pathways and functions. Macrophage phenotypic differentiation has an important impact on AS regression. Early AS plaques are predominantly M2-type macrophages, but with the progression of the disease, the proportion of M1-type macrophages increases significantly and triggers vascular events, indicating that the regulation of the dynamic balance of M1/M2 polarization is a key link in inhibiting AS plaque formation and stabilizing plaques. Therefore, directing M1-type macrophages to M2-type macrophages has become a novel therapeutic strategy to alleviate the pathological process of AS.
Medical treatment has now entered the era of precision medicine. With the development of systems biology and network pharmacology, the molecular mechanisms of Chinese medicine in regulating macrophage polarization-related signaling pathways have gradually been clarified, providing a scientific basis for constructing a precision treatment system that combines Chinese and Western medicine. Exploring the role of Chinese medicine in precisely targeting macrophage polarization via signaling pathways and identifying new therapeutic targets for the prevention and treatment of AS are of great clinical significance. This study systematically reviews the mechanisms by which bioactive metabolites and herbal formulas ameliorate AS through the regulation of macrophage polarization multiple signaling pathways. Several metabolites, including BBR, CUR, Tan IIA, Rb1/Rg3, and GA, as well as the herbal formulas BYHW and ZYP, promote a shift toward the M2 macrophage phenotype, thereby enhancing plaque stability. Accumulated evidence indicates that suppression of the TLR4/MyD88/NF-κB and MAPK pathways, coupled with activation of the PPAR-γ/STAT6 axis, serves as a central mechanism underlying macrophage modulation by these interventions. These findings have been consistently validated in ApoE−/− mouse models as well as in RAW264.7 and THP-1 cellular assays. In contrast, the roles of the PI3K/Akt and certain branches of the MAPK pathway in regulating macrophage polarization within the AS microenvironment remain inconclusive and merit further investigation to elucidate their context-dependent functions (Zhao Y. et al., 2021).
AS is a major pathological basis of CVD, and the preventive and therapeutic effects of TCM on AS contribute to slowing CVD progression. Clinical trials have demonstrated that TCM exerts broad pharmacological actions in alleviating CVD symptoms with a favorable safety profile. Evidence indicates that, compared with conventional Western medicine alone, adjunctive TCM interventions significantly improve treatment outcomes for metabolic syndrome metabolites such as hypertension. High-quality, multicenter randomized controlled trials have further shown that Tongxinluo reduces carotid plaque progression and lowers the risk of major adverse cardiovascular and cerebrovascular events (MACE). Shexiang Baoxin Pill alleviates angina symptoms and reduces the incidence of MACE (Ge et al., 2021). In addition, bioactive metabolites—such as BBR, CUR, and Rb1—not only demonstrate clear efficacy in treating CVD but also are associated with a low incidence of adverse reactions. Overall, from bioactive metabolites to herbal formulas, the growing body of evidence supports the multitarget potential of TCM in the prevention and treatment of CVD.
Rooted in classical TCM theory, the concept of “medicine–food homology” (Yao Shi Tong Yuan) posits that it refers to substances with both nutritional and medicinal value under TCM theory, botanical drugs like Hawthorn are commonly used in both contexts. Consequently, numerous bioactive metabolites derived from these sources hold promise as both therapeutic candidates for CVD and dietary supplements. Preclinical and clinical studies have demonstrated that BBR exerts potent anti-AS effects and significantly improves parameters related to metabolic syndrome (McCubrey et al., 2017). Similarly, CUR has been shown to reduce multiple AS risk factors in large-scale randomized controlled trials, establishing it as an ideal candidate for functional food development (Yaikwawong et al., 2024; Xu et al., 2018). Beyond its nutraceutical benefits, CUR also serves as a natural edible pigment, attracting considerable interest from both the scientific community and the public (Sharifi-Rad et al., 2020). Res has garnered clinical support for its advantages in cardiometabolic protection and lipid regulation (Parsamanesh et al., 2021), with additional promising applications in nutraceuticals, including anti-osteoporosis, anti-vascular aging, and anti-obesity interventions (Cao et al., 2022). Hesperidin mitigates AS by improving endothelial function and modulating lipid profiles; however, its oral bioavailability remains limited. Consumption in the form of citrus juices or specialized supplements may enhance its absorption (Testai and Calderone, 2017; Shylaja et al., 2024; Salden et al., 2016). Hesperidin is also considered a promising dietary supplement. It has been shown to improve muscle metabolic status, thereby facilitating functional recovery and enhancing exercise performance (Martínez-Noguera et al., 2019). Collectively, these bioactive metabolites are increasingly transcending cultural and regional boundaries, gaining recognition within Western medical and nutritional sciences. This trend not only amplifies the global relevance of TCM but also reflects sustained international research interest in the multifunctional potential of natural bioactive metabolites.
As of the prespecified search date, variability in randomization, blinding, sample-size justification, endpoint selection, and reporting transparency persists across studies. Clinical trials are often underpowered with limited follow-up, and many in vitro or single-animal studies lack external calibration against human data. These issues impede comparability and raise risks of bias. These issues impede comparability and raise risks of bias. While in vitro systems are valuable for hypothesis generation and mechanistic testing, their lack of integrated metabolic–immune–multi-organ context warrants cautious interpretation and cross-validation in animal and clinical settings. Substantial variability in extraction procedures, experimental designs, and outcome assessment criteria contributes to pronounced heterogeneity, thereby limiting cross-study comparability and the reliability of integrative analyses. Heterogeneity arises from four domains: 1) models (species, sex, age, comorbidities, diet/induction); 2) interventions/formulations (composition, extraction, chemical fingerprints, active-content consistency); 3) endpoints/assays (definitions and measurements for inflammation, lipid handling, plaque stability); and 4) analytical frameworks (pathway selection and depth of causal validation). Limited incorporation of TCM-specific constructs (e.g., pattern differentiation) into animal models further constrains generalizability.
Although TCM shows considerable potential for the treatment of AS-related diseases, its translation from basic research to clinical application remains fraught with challenges. Bench-to-bedside translation is limited by insufficient pharmacokinetics/toxicology and organ-level safety data, potential synergy/antagonism within multi-herb formulations and batch-to-batch variability. Many of the bioactive metabolites discussed herein, such as CUR and Res, have been identified as ‘pan-assay interfering compounds’ (PAINS) (Magalhães, et al., 2021; Bolz, et al., 2021). This class of compounds may generate false-positive results in in vitro biochemical or cell-based high-throughput screens through non-specific mechanisms, such as redox activity, protein aggregation, fluorescence interference, or membrane disruption (Magalhães, et al., 2021). Consequently, conclusions drawn from in vitro assays, particularly those involving multi-target effects, must be interpreted with extreme caution. Although this review aims to map the network of their potential mechanisms of action rather than directly advocate for clinical translation, we must emphasize that these in vitro findings at best constitute a hypothetical foundation for subsequent research. A significant gap remains between these findings and genuine pharmacological activity or ultimate clinical relevance.
Future studies should integrate in vitro systems, animal models, and clinical data to minimize species-dependent limitations, exclude potential confounding effects, and verify the true biological activity of candidate metabolites, thereby enhancing the clinical relevance and translational value of the findings. To overcome current limitations, future efforts should adopt multidisciplinary approaches and establish standardized frameworks to promote the precision and internationalization of TCM-based strategies for AS prevention and therapy. Key directions include:1) Improving Study Quality and Methodological Standardization: Implement rigorous randomization, blinding, allocation concealment, and transparent reporting; develop internationally recognized quality-control standards, experimental design guidelines, and outcome evaluation frameworks to strengthen study rigor and reproducibility. 2) Reducing Heterogeneity and Enhancing Quality Control: Standardize herbal formulation quality, extraction procedures, and dosing regimens, and define consistent outcome measures to minimize inter-study variability and facilitate multicenter data integration and meta-analyses. 3) Optimizing Clinical Translation and Personalized Therapy: Increase sample sizes, extend follow-up durations, incorporate real-world evidence, and include participants across different ages, sexes, and comorbidity profiles. Integrate patient stratification, genetic background, and metabolic characteristics to explore individualized treatment strategies and enhance generalizability and clinical utility. 4) Multidimensional Evidence Integration and Technological Innovation: Combine in vitro, animal, and clinical investigations, and leverage multi-omics, network pharmacology, and artificial intelligence–based approaches for mechanistic validation and efficacy prediction, thereby improving drug discovery efficiency. Collectively, these strategies are expected to standardize methodologies, improve research quality and reproducibility, reduce heterogeneity, and—together with cutting-edge technologies—drive the development of safer, more effective, and evidence-based TCM interventions, providing a robust scientific foundation for precision prevention and treatment of AS.
Statements
Author contributions
SL: Writing – original draft. JJ: Writing – original draft. YZ: Data curation, Writing – review and editing. JS: Conceptualization, Writing – review and editing. CZ: Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Research Project of the Education Commission of Tianjin Municipality (2021ZD020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AGEs
advanced glycation end-products
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- ApoE−/−
apolipoprotein E knockout
- Arg-1
arginase-1
- AS
atherosclerosis
- AS-IV
Astragaloside IV
- BBR
Berberine
- BYHW
Bu Yang Huan Wu Decoction
- CUR
Curcumin
- CVD
cardiovascular diseases
- ECM
extracellular matrix
- ECs
endothelial cells
- ERK
extracellular signal-regulated kinase
- GA
Ganoderic acid
- G. lucidum
Ganoderma lucidum
- GP
Gardenia glycoside
- HFD
high-fat diet
- HIF-1α
hypoxia-inducible factor
- IFN-γ
interferon-gamma
- IκB
inhibitory κB
- IKK
IκB kinase
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MAP3K8
mitogen-activated protein kinase kinase kinase 8
- MMPs
matrix metalloproteinases
- MT
Matrine
- NF-κB
nuclear factor-kappa B
- NLRP3
NOD-like receptor protein 3
- Nrf2
Nuclear factor E2-related factor 2
- ox-LDL
oxidized low-density lipoprotein
- PAMPs
pathogen-associated molecular patterns
- PI3K
phosphoinositide 3-kinase
- PPAR-γ
peroxisome proliferator-activated receptor γ
- QRHX
Qingre Huoxue Decoction
- Rb1
Ginsenoside Rb1
- Res
Resveratrol
- Rg3
Ginsenoside Rg3
- ROS
reactive oxygen species
- SMCs
smooth muscle cells
- STAT
signal transducer and activator of transcription
- Tan IIA
Tanshinone IIA
- TCM
traditional Chinese medicine
- TGF-β
transforming growth factor-β
- Th1
T Helper 1
- Th2
T Helper 2
- TLR4
Toll-like receptor 4
- TLRs
Toll-like receptors
- TNF-α
tumor necrosis factor-alpha
- TRAF6
tumor necrosis factor receptor-associated factor 6
- VEGF
vascular endothelial growth factor
- ZXYF
ZeXieYin formula
- ZYP
Zhuyu Pill
References
1
Abdalla H. B. Napimoga M. H. Lopes A. H. de Macedo Maganin A. G. Cunha T. M. Van Dyke T. E. et al (2020). Activation of PPAR-γ induces macrophage polarization and reduces neutrophil migration mediated by heme oxygenase 1. Int. Immunopharmacol.84, 106565. 10.1016/j.intimp.2020.106565
2
Abeyrathna P. Su Y. (2015). The critical role of akt in cardiovascular function. Vasc. Pharmacol.74, 38–48. 10.1016/j.vph.2015.05.008
3
Achkar I. W. Abdulrahman N. Al-Sulaiti H. Joseph J. M. Uddin S. Mraiche F. (2018). Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J. Transl. Med.16 (1), 96. 10.1186/s12967-018-1471-1
4
Ai X. Yu P. Peng L. Luo L. Liu J. Li S. et al (2021). Berberine: a review of its pharmacokinetics properties and therapeutic potentials in diverse vascular diseases. Front. Pharmacol.12, 762654. 10.3389/fphar.2021.762654
5
Alonso-Piñeiro J. A. Gonzalez-Rovira A. Sánchez-Gomar I. Moreno J. A. Durán-Ruiz M. C. (2021). Nrf2 and heme Oxygenase-1 involvement in atherosclerosis related oxidative stress. Antioxidants (Basel)10 (9), 1463. 10.3390/antiox10091463
6
An N. Yang J. Wang H. Sun S. Wu H. Li L. et al (2021). Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci.11 (1), 41. 10.1186/s13578-021-00554-z
7
Arranz A. Doxaki C. Vergadi E. Martinez de la Torre Y. Vaporidi K. Lagoudaki E. D. et al (2012). Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. U. S. A.109 (24), 9517–9522. 10.1073/pnas.1119038109
8
Babaev V. R. Ding L. Zhang Y. May J. M. Lin P. C. Fazio S. et al (2016). Macrophage IKKα deficiency suppresses akt phosphorylation, reduces cell survival, and decreases early atherosclerosis. Arterioscler. Thromb. Vasc. Biol.36 (4), 598–607. 10.1161/ATVBAHA.115.306931
9
Bagheri B. Khatibiyan Feyzabadi Z. Nouri A. Azadfallah A. Mahdizade Ari M. Hemmati M. et al (2024). Atherosclerosis and toll-like Receptor4 (TLR4), lectin-like oxidized low-density Lipoprotein-1 (LOX-1), and proprotein convertase subtilisin/kexin Type9 (PCSK9). Mediat. Inflamm.2024, 5830491. 10.1155/2024/5830491
10
Bai L. Li Z. Li Q. Guan H. Zhao S. Liu R. et al (2017). Mediator 1 is atherosclerosis protective by regulating macrophage polarization. Arterioscler. Thromb. Vasc. Biol.37 (8), 1470–1481. 10.1161/ATVBAHA.117.309672
11
Bashore A. C. Yan H. Xue C. Zhu L. Y. Kim E. Mawson T. et al (2024). High-dimensional single-cell multimodal landscape of human carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol.44 (4), 930–945. 10.1161/ATVBAHA.123.320524
12
Bi Y. Chen J. Hu F. Liu J. Li M. Zhao L. (2019). M2 macrophages as a potential target for antiatherosclerosis treatment. Neural Plast.2019, 6724903. 10.1155/2019/6724903
13
Binion D. G. Heidemann J. Li M. S. Nelson V. M. Otterson M. F. Rafiee P. (2009). Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin. Am. J. Physiol. Gastrointest. Liver Physiol.297 (2), G259–G268. 10.1152/ajpgi.00087.2009
14
Blagov A. V. Markin A. M. Bogatyreva A. I. Tolstik T. V. Sukhorukov V. N. Orekhov A. N. (2023). The role of macrophages in the pathogenesis of atherosclerosis. Cells12 (4), 522. 10.3390/cells12040522
15
Bolz S. N. Adasme M. F. Schroeder M. (2021). Toward an understanding of pan-assay interference compounds and promiscuity: a structural perspective on binding modes. J. Chem. Inf. Model61 (5), 2248–2262. 10.1021/acs.jcim.0c01227
16
Bouhlel M. A. Derudas B. Rigamonti E. Dièvart R. Brozek J. Haulon S. et al (2007). PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab.6 (2), 137–143. 10.1016/j.cmet.2007.06.010
17
Bozaykut P. Karademir B. Yazgan B. Sozen E. Siow R. C. Mann G. E. et al (2014). Effects of vitamin E on peroxisome proliferator-activated receptor γ and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radic. Biol. Med.70, 174–181. 10.1016/j.freeradbiomed.2014.02.017
18
Bu L. L. Yuan H. H. Xie L. L. Guo M. H. Liao D. F. Zheng X. L. (2023). New dawn for atherosclerosis: vascular endothelial cell senescence and death. Int. J. Mol. Sci.24 (20), 15160. 10.3390/ijms242015160
19
Buttari B. Profumo E. Segoni L. D'Arcangelo D. Rossi S. Facchiano F. et al (2014). Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: potential therapeutic implications in atherosclerosis. Oxid. Med. Cell Longev.2014, 257543. 10.1155/2014/257543
20
Cao X. Liao W. Xia H. Wang S. Sun G. (2022). The effect of resveratrol on blood lipid profile: a dose-response meta-analysis of randomized controlled trials. Nutrients14 (18), 3755. 10.3390/nu14183755
21
Chen P. Chen W. N. (2019). TanshinoneⅡA regulating macrophage polarization to anti-atherosclerosisand its potential mechanism. Med. J. Liaoning33 (6), 70–74.
22
Chen Z. Xu H. (2014). Anti-inflammatory and immunomodulatory mechanism of tanshinone IIA for atherosclerosis. Evid. Based Complement. Altern. Med.2014, 267976. 10.1155/2014/267976
23
Chen C. Wu S. Lin B. (2024). The potential therapeutic value of the natural plant compounds matrine and oxymatrine in cardiovascular diseases. Front. Cardiovasc Med.11, 1417672. 10.3389/fcvm.2024.1417672
24
Chen F. Guo N. Cao G. Zhou J. Yuan Z. (2014). Molecular analysis of curcumin-induced polarization of murine RAW264.7 macrophages. J. Cardiovasc Pharmacol.63 (6), 544–552. 10.1097/FJC.0000000000000079
25
Chen Z. Gao X. Jiao Y. Qiu Y. Wang A. Yu M. et al (2019). Tanshinone IIA exerts anti-inflammatory and immune-regulating effects on vulnerable atherosclerotic plaque partially via the TLR4/MyD88/NF-κB signal pathway. Front. Pharmacol.10, 850. 10.3389/fphar.2019.00850
26
Chen J. Chen G. Li J. Wang D. Liang W. Zhao S. (2025). NLRC5 in macrophages promotes atherosclerosis in acute coronary syndrome by regulating STAT3 expression. Cardiovasc Toxicol.25 (3), 365–378. 10.1007/s12012-024-09957-z
27
Chen L. Wu B. Mo L. Chen H. Yin X. Zhao Y. et al (2025). High-content screening identifies ganoderic acid A as a senotherapeutic to prevent cellular senescence and extend healthspan in preclinical models. Nat. Commun.16 (1), 2878. 10.1038/s41467-025-58188-5
28
Chen S. Chen K. Lin Y. Wang S. Yu H. Chang C. et al (2024). Ganoderic acid T, a ganoderma triterpenoid, modulates the tumor microenvironment and enhances the chemotherapy and immunotherapy efficacy through downregulating galectin-1 levels. Toxicol. Appl. Pharmacol.491, 117069. 10.1016/j.taap.2024.117069
29
Chen Ys Y. S. Wang T. T. Han X. Y. Hua C. J. Jin B. Y. Shang S. S. et al (2024). Effect of M1 macrophage polarization regulated by berberine combined with curcumin on atherosclerosis. J. Pract. Med.40 (14), 1915–1921. 10.3969/j.issn.1006-5725.2024.14.003
30
Cheng H. M. Koutsidis G. Lodge J. K. Ashor A. Siervo M. Lara J. (2017). Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis257, 100–108. 10.1016/j.atherosclerosis.2017.01.009
31
Chi H. Barry S. P. Roth R. J. Wu J. J. Jones E. A. Bennett A. M. et al (2006). Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. U. S. A.103 (7), 2274–2279. 10.1073/pnas.0510965103
32
Chinetti G. Fruchart J. C. Staels B. (2003). Peroxisome proliferator-activated receptors: new targets for the pharmacological modulation of macrophage gene expression and function. Curr. Opin. Lipidol.14 (5), 459–468. 10.1097/00041433-200310000-00006
33
Chistiakov D. A. Melnichenko A. A. Myasoedova V. A. Grechko A. V. Orekhov A. N. (2017). Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. Berl.95 (11), 1153–1165. 10.1007/s00109-017-1575-8
34
Corrêa Carvalho G. Marena G. D. Gaspar Gonçalves Fernandes M. Ricci Leonardi G. Santos H. A. Chorilli M. (2024). Curcuma longa: nutraceutical use and association with nanotechnology. Adv. Healthc. Mater13 (22), e2400506. 10.1002/adhm.202400506
35
Cui Q. Du H. Ma Y. Wang T. Zhu H. Zhu L. et al (2022). Matrine inhibits advanced glycation end products-induced macrophage M1 polarization by reducing DNMT3a/b-mediated DNA methylation of GPX1 promoter. Eur. J. Pharmacol.926, 175039. 10.1016/j.ejphar.2022.175039
36
Cui Y. Chen J. Zhang Z. Shi H. Sun W. Yi Q. (2023). The role of AMPK in macrophage metabolism, function and polarisation. J. Transl. Med.21 (1), 892. 10.1186/s12967-023-04772-6
37
Ding Y. Sun Y. Wang H. Zhao H. Yin R. Zhang M. et al (2024). Atherosis-associated lnc_000048 activates PKR to enhance STAT1-mediated polarization of THP-1 macrophages to M1 phenotype. Neural Regen. Res.19 (11), 2488–2498. 10.4103/NRR.NRR-D-23-01355
38
Doddapattar P. Dev R. Ghatge M. Patel R. B. Jain M. Dhanesha N. et al (2022). Myeloid cell PKM2 deletion enhances efferocytosis and reduces atherosclerosis. Circ. Res.130 (9), 1289–1305. 10.1161/CIRCRESAHA.121.320704
39
Ebrahimi F. Ghazimoradi M. M. Fatima G. Bahramsoltani R. (2023). Citrus flavonoids and adhesion molecules: potential role in the management of atherosclerosis. Heliyon9 (11), e21849. 10.1016/j.heliyon.2023.e21849
40
El-Sayed N. Mostafa Y. M. AboGresha N. M. Ahmed A. A. M. Mahmoud I. Z. El-Sayed N. M. (2021). Dapagliflozin attenuates diabetic cardiomyopathy through erythropoietin up-regulation of AKT/JAK/MAPK pathways in streptozotocin-induced diabetic rats. Chem. Biol. Interact.347, 109617. 10.1016/j.cbi.2021.109617
41
Engelen S. E. Robinson A. J. B. Zurke Y. X. Monaco C. (2022). Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed?Nat. Rev. Cardiol.19 (8), 522–542. 10.1038/s41569-021-00668-4
42
Fan H. J. Lai Y. G. Jiang X. F. Wang Q. Li K. C. Fu Y. C. et al (2022). Study on the mechanism of hesperidin in improving atherosclerosis in ApoE-/-Mice. Henan Tradit. Chin. Med.42 (03), 497–402. 10.16367/j.issn.1003-5028.2022.03.0086
43
Florio T. J. Lokareddy R. K. Yeggoni D. P. Sankhala R. S. Ott C. A. Gillilan R. E. et al (2022). Differential recognition of canonical NF-κB dimers by importin α3. Nat. Commun.13 (1), 1207. 10.1038/s41467-022-28846-z
44
Fruman D. A. Chiu H. Hopkins B. D. Bagrodia S. Cantley L. C. Abraham R. T. (2017). The PI3K pathway in human disease. Cell170 (4), 605–635. 10.1016/j.cell.2017.07.029
45
Fu X. Sun Z. Long Q. Tan W. Ding H. Liu X. et al (2022). Glycosides from buyang huanwu decoction inhibit atherosclerotic inflammation via JAK/STAT signaling pathway. Phytomedicine105, 154385. 10.1016/j.phymed.2022.154385
46
Gasmi A. Asghar F. Zafar S. Oliinyk P. Khavrona O. Lysiuk R. et al (2024). Berberine: pharmacological features in health, disease and aging. Curr. Med. Chem.31 (10), 1214–1234. 10.2174/0929867330666230207112539
47
Gawel K. Kukula-Koch W. Nieoczym D. Stepnik K. Ent W. V. Banono N. S. et al (2020). The influence of palmatine isolated from berberis sibiricaRadix on pentylenetetrazole-induced seizures in zebrafish. Cells9 (5), 1233. 10.3390/cells9051233
48
Ge J. B. Fan W. H. Zhou J. M. Shi H. M. Ji F. S. Wu Y. et al (2021). Efficacy and safety of Shexiang Baoxin pill (MUSKARDIA) in patients with stable coronary artery disease: a multicenter, double-blind, placebo-controlled phase IV randomized clinical trial. Chin. Med. J. Engl.134 (2), 185–192. 10.1097/CM9.0000000000001257
49
Geiß C. Salas E. Guevara-Coto J. Régnier-Vigouroux A. Mora-Rodríguez R. A. (2022). Multistability in macrophage activation pathways and metabolic implications. Cells11 (3), 404. 10.3390/cells11030404
50
Gimbrone M. A. Jr García-Cardeña G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res.118 (4), 620–636. 10.1161/CIRCRESAHA.115.306301
51
Gong M. Zhuo X. Ma A. (2017). STAT6 upregulation promotes M2 macrophage polarization to suppress atherosclerosis. Med. Sci. Monit. Basic Res.23, 240–249. 10.12659/msmbr.904014
52
Gordon S. (2003). Alternative activation of macrophages. Nat. Rev. Immunol.3 (1), 23–35. 10.1038/nri978
53
Grootaert M. O. J. Bennett M. R. (2021). Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res.117 (11), 2326–2339. 10.1093/cvr/cvab046
54
Guo M. Xiao J. Sheng X. Zhang X. Tie Y. Wang L. et al (2018). Ginsenoside Rg3 mitigates atherosclerosis progression in diabetic apoE-/- mice by skewing macrophages to the M2 phenotype. Front. Pharmacol.9, 464. 10.3389/fphar.2018.00464
55
Guo R. Li L. Su J. Li S. Duncan S. E. Liu Z. et al (2020). Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Des. Devel Ther.14, 4735–4748. 10.2147/DDDT.S266911
56
Guo L. Zhang X. Lv N. Wang L. Gan J. Jiang X. et al (2023). Therapeutic role and potential mechanism of resveratrol in atherosclerosis: TLR4/NF-κB/HIF-1α. Mediat. Inflamm.2023, 1097706. 10.1155/2023/1097706
57
He J. Vupputuri S. Allen K. Prerost M. R. Hughes J. Whelton P. K. (1999). Passive smoking and the risk of coronary heart disease-a meta-analysis of epidemiologic studies. N. Engl. J. Med.340 (12), 920–926. 10.1056/NEJM199903253401204
58
He X. Y. Zhang Z. Jia L. Q. Yang G. L. Wang J. Y. Zhang M. X. (2023). Mechanism of astragaloside IV in preventing atherosclerosis by regulating MAP3K8 mediated interaction between pyroptosis and macrophage polarization. China J. Traditional Chin. Med. Pharm.38 (05), 2311–2316.
59
He L. Chen Q. Wang L. Pu Y. Huang J. Cheng C. K. et al (2024). Activation of Nrf2 inhibits atherosclerosis in ApoE-/- mice through suppressing endothelial cell inflammation and lipid peroxidation. Redox Biol.74, 103229. 10.1016/j.redox.2024.103229
60
He W. Zhao H. Xue W. Luo Y. Yan M. Li J. et al (2024). Qingre huoxue decoction alleviates atherosclerosis by regulating macrophage polarization through exosomal miR-26a-5p. Drug Des. Devel Ther.18, 6389–6411. 10.2147/DDDT.S487476
61
Hosoya T. Maruyama A. Kang M. I. Kawatani Y. Shibata T. Uchida K. et al (2005). Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem.280 (29), 27244–27250. 10.1074/jbc.M502551200
62
Hou P. Fang J. Liu Z. Shi Y. Agostini M. Bernassola F. et al (2023). Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis.14 (10), 691. 10.1038/s41419-023-06206-z
63
Huang R. Sun Y. Liu R. Zhu B. Zhang H. Wu H. (2024). ZeXieYin formula alleviates atherosclerosis by inhibiting the MAPK/NF-κB signaling pathway in APOE-/- mice to attenuate vascular inflammation and increase plaque stability. J. Ethnopharmacol.327, 117969. 10.1016/j.jep.2024.117969
64
Huang Y. M. Wu Y. S. Dang Y. Y. Xu Y. M. Ma K. Y. Dai X. Y. (2024). Par3L, a polarity protein, promotes M1 macrophage polarization and aggravates atherosclerosis in mice via p65 and ERK activation. Acta Pharmacol. Sin.45 (1), 112–124. 10.1038/s41401-023-01161-z
65
Huang W. Zhang L. Wang Z. Xing J. Lai X. Huang Z. et al (2024). Emodin suppresses NLRP3/GSDMD-induced inflammation via the TLR4/MyD88/NF-κB signaling pathway in atherosclerosis. Cardiovasc Drugs Ther.24. 10.1007/s10557-024-07659-w
66
Jang W. Y. Hwang J. Y. Cho J. Y. (2023). Ginsenosides from Panax ginseng as key modulators of NF-κB signaling are powerful anti-inflammatory and anticancer agents. Int. J. Mol. Sci.24 (7), 6119. 10.3390/ijms24076119
67
Ji W. Sun J. Hu Z. Sun B. (2022). Resveratrol protects against atherosclerosis by downregulating the PI3K/AKT/mTOR signaling pathway in atherosclerosis model mice. Exp. Ther. Med.23 (6), 414. 10.3892/etm.2022.11341
68
Jian X. Liu Y. Zhao Z. Zhao L. Wang D. Liu Q. (2019). The role of traditional Chinese medicine in the treatment of atherosclerosis through the regulation of macrophage activity. Biomed. Pharmacother.118, 109375. 10.1016/j.biopha.2019.109375
69
Jin Z. (2021). The study on the mechanism of promoting macrophage M2Polarization in the prevention and treatment of atherosclerosiswith qingre huoxue formula. GuangzhouUniversity Chin. Med. 10.27044/d.cnki.ggzzu.2021.000632
70
Jin Z. Li J. Pi J. Chu Q. Wei W. Du Z. et al (2020). Geniposide alleviates atherosclerosis by regulating macrophage polarization via the FOS/MAPK signaling pathway. Biomed. Pharmacother.125, 110015. 10.1016/j.biopha.2020.110015
71
Jin M. Fang J. Wang J. J. Shao X. Xu S. W. Liu P. Q. et al (2023). Regulation of toll-like receptor (TLR) signaling pathways in atherosclerosis: from mechanisms to targeted therapeutics. Acta Pharmacol. Sin.44 (12), 2358–2375. 10.1038/s41401-023-01123-5
72
Jin Z. Luo Y. Zhao H. Cui J. He W. Li J. et al (2023). Qingre huoxue decoction regulates macrophage polarisation to attenuate atherosclerosis through the inhibition of NF-κB signalling-mediated inflammation. J. Ethnopharmacol.301, 115787. 10.1016/j.jep.2022.115787
73
Jinnouchi H. Guo L. Sakamoto A. Torii S. Sato Y. Cornelissen A. et al (2020). Diversity of macrophage phenotypes and responses in atherosclerosis. Cell Mol. Life Sci.77 (10), 1919–1932. 10.1007/s00018-019-03371-3
74
Kavurma M. M. Rayner K. J. Karunakaran D. (2017). The walking dead: macrophage inflammation and death in atherosclerosis. Curr. Opin. Lipidol.28 (2), 91–98. 10.1097/MOL.0000000000000394
75
Kobayashi E. H. Suzuki T. Funayama R. Nagashima T. Hayashi M. Sekine H. et al (2016). Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun.7, 11624. 10.1038/ncomms11624
76
Kwok H. H. Guo G. L. Lau J. K. Cheng Y. K. Wang J. R. Jiang Z. H. et al (2012). Stereoisomers ginsenosides-20(S)-Rg3 and -20(R)-Rg3 differentially induce angiogenesis through peroxisome proliferator-activated receptor-gamma. Biochem. Pharmacol.83 (7), 893–902. 10.1016/j.bcp.2011.12.039
77
Lassègue B. San Martín A. Griendling K. K. (2012). Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res.110 (10), 1364–1390. 10.1161/CIRCRESAHA.111.243972
78
Laurindo L. F. de Carvalho G. M. de Oliveira Zanuso B. Figueira M. E. Direito R. de Alvares Goulart R. et al (2023). Curcumin-based nanomedicines in the treatment of inflammatory and immunomodulated diseases: an evidence-based comprehensive review. Pharmaceutics15 (1), 229. 10.3390/pharmaceutics15010229
79
Lechner K. von Schacky C. McKenzie A. L. Worm N. Nixdorff U. Lechner B. et al (2022). Lifestyle factors and high-risk atherosclerosis: pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol.27 (4), 394–406. 10.1177/2047487319869400
80
Li B. Liu F. Ye J. Cai X. Qian R. Zhang K. et al (2022). Regulation of macrophage polarization through periodic photo-thermal treatment to facilitate osteogenesis. Small18 (38), e2202691. 10.1002/smll.202202691
81
Li J. G. Sun W. X. Liu J. Y. Li X. S. Xue W. Q. Luo C. J. (2020). Intervention effects of berberine on mice macrophage polarization based on TLR4/MyD88/NF-κB signaling pathway. China Pharm.31 (15), 1804–1809. 10.6039/j.issn.1001-0408.2020.15.03
82
Li D. Li X. Zhang X. Chen J. Wang Z. Yu Z. et al (2024). Geniposide for treating atherosclerotic cardiovascular disease: a systematic review on its biological characteristics, pharmacology, pharmacokinetics, and toxicology. Chin. Med.19 (1), 111. 10.1186/s13020-024-00981-3
83
Li Y. Ai S. Li Y. Ye W. Li R. Xu X. et al (2025). The role of natural products targeting macrophage polarization in sepsis-induced lung injury. Chin. Med.20 (1), 19. 10.1186/s13020-025-01067-4
84
Li H. Yang D. H. Zhang Y. Zheng F. Gao F. Sun J. et al (2022). Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury. Chin. Med.17 (1), 73. 10.1186/s13020-022-00616-5
85
Li J. Ye F. Xu X. Xu P. Wang P. Zheng G. et al (2023). Correction: targeting macrophage M1 polarization suppression through PCAF inhibition alleviates autoimmune arthritis via synergistic NF-κB and H3K9Ac blockade. J. Nanobiotechnology21 (1), 336. 10.1186/s12951-023-02079-8
86
Li P. Hao Z. Wu J. Ma C. Xu Y. Li J. et al (2021). Comparative proteomic analysis of polarized human THP-1 and mouse RAW264.7 macrophages. Front. Immunol.12, 700009. 10.3389/fimmu.2021.700009
87
Li P. Li H. Li X. Li S. Xu H. Cui J. et al (2023). San Jie Tong Mai Fang protects against atherosclerosis progression by regulating macroautophagy through the PI3K/AKT/mTOR signaling pathway. J. Cardiovasc Pharmacol.82 (4), 333–343. 10.1097/FJC.0000000000001452
88
Li W. Y. Long Q. Y. Fu X. Y. Ma L. Tan W. Li Y. L. et al (2023). Effects of Buyang Huanwu decoction and astragali radix-angelicae sinensis radix combination on inflammatory responses in atherosclerotic mice. Zhongguo Zhong Yao Za Zhi48 (15), 4164–4172. 10.19540/j.cnki.cjcmm.20230418.401
89
Li Y. Tang J. Gao H. Xu Y. Han Y. Shang H. et al (2021). Ganoderma lucidum triterpenoids and polysaccharides attenuate atherosclerotic plaque in high-fat diet rabbits. Nutr. Metab. Cardiovasc Dis.31 (6), 1929–1938. 10.1016/j.numecd.2021.03.023
90
Li Z. H. Chen J. F. Zhang J. Lei Z. Y. Wu L. L. Meng S. B. et al (2023). Mesenchymal stem cells promote polarization of M2 macrophages in mice with acute-on-chronic liver failure via Mertk/JAK1/STAT6 signaling. Stem Cells41 (12), 1171–1184. 10.1093/stmcls/sxad069
91
Liang J. Yang C. Li P. Zhang M. Xie X. Xie X. et al (2023). Astragaloside IV inhibits AOM/DSS-induced colitis-associated tumorigenesis via activation of PPARγ signaling in mice. Phytomedicine121, 155116. 10.1016/j.phymed.2023.155116
92
Liang Y. Fu J. Shi Y. Jiang X. Lu F. Liu S. (2024). Integration of 16S rRNA sequencing and metabolomics to investigate the modulatory effect of ginsenoside Rb1 on atherosclerosis. Heliyon10 (6), e27597. 10.1016/j.heliyon.2024.e27597
93
Liao P. Liu L. Wang B. Li W. Fang X. Guan S. (2014). Baicalin and geniposide attenuate atherosclerosis involving lipids regulation and immunoregulation in ApoE-/- mice. Eur. J. Pharmacol.740, 488–495. 10.1016/j.ejphar.2014.06.039
94
Libby P. (2021). The changing landscape of atherosclerosis. Nature592 (7855), 524–533. 10.1038/s41586-021-03392-8
95
Litak J. Grochowski C. Litak J. Osuchowska I. Gosik K. Radzikowska E. et al (2020). TLR-4 signaling vs. immune checkpoints, miRNAs molecules, cancer stem cells, and wingless-signaling interplay in glioblastoma multiforme-future perspectives. Int. J. Mol. Sci.21 (9), 3114. 10.3390/ijms21093114
96
Liu B. Song Z. Yu J. Li P. Tang Y. Ge J. (2020). The atherosclerosis-ameliorating effects and molecular mechanisms of BuYangHuanWu decoction. Biomed. Pharmacother.123, 109664. 10.1016/j.biopha.2019.109664
97
Liu C. Song X. Li Y. Ding C. Li X. Dan L. et al (2023). A comprehensive review on the chemical composition, pharmacology and clinical applications of ganoderma. Am. J. Chin. Med.51 (8), 1983–2040. 10.1142/S0192415X23500878
98
Liu J. Zhang L. Ren Y. Gao Y. Kang L. Lu S. (2016). Matrine inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Mol. Med. Rep.13 (3), 2313–2319. 10.3892/mmr.2016.4767
99
Liu Y. Wang X. Pang J. Zhang H. Luo J. Qian X. et al (2019). Attenuation of atherosclerosis by protocatechuic acid via inhibition of M1 and promotion of M2 macrophage polarization. Agric. Food Chem.67 (3), 807–818. 10.1021/acs.jafc.8b05719
100
Liu X. Mi X. Wang Z. Zhang M. Hou J. Jiang S. et al (2020a). Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis.11 (6), 454. 10.1038/s41419-020-2597-7
101
Liu X. Wu J. Tian R. Su S. Deng S. Meng X. (2020b). Targeting foam cell formation and macrophage polarization in atherosclerosis: the therapeutic potential of rhubarb. Biomed. Pharmacother.129, 110433. 10.1016/j.biopha.2020.110433
102
Liu F. Yang Y. Dong H. Zhu Y. Feng W. Wu H. (2024). Essential oil from cinnamomum cassia presl bark regulates macrophage polarization and ameliorates lipopolysaccharide-induced acute lung injury through TLR4/MyD88/NF-κB pathway. Phytomedicine129, 155651. 10.1016/j.phymed.2024.155651
103
Liu H. Lv C. Lu J. (2020). Panax ginseng C. A. meyer as a potential therapeutic agent for organ fibrosis disease. Chin. Med.15 (1), 124. 10.1186/s13020-020-00400-3
104
Liu J. Yang B. Wang Y. Wu Y. Fan B. Zhu S. et al (2020). Polychlorinated biphenyl quinone promotes macrophage polarization to CD163+ cells through Nrf2 signaling pathway. Environ. Pollut.257, 113587. 10.1016/j.envpol.2019.113587
105
Liu L. Liao P. Wang B. Fang X. Li W. Guan S. (2014). Oral administration of baicalin and geniposide induces regression of atherosclerosis via inhibiting dendritic cells in ApoE-knockout mice. Int. Immunopharmacol.20 (1), 197–204. 10.1016/j.intimp.2014.02.037
106
Liu Q. Pan J. Bao L. Xu C. Qi Y. Jiang B. et al (2022). Major vault protein prevents atherosclerotic plaque destabilization by suppressing macrophage ASK1-JNK signaling. Arterioscler. Thromb. Vasc. Biol.42 (5), 580–596. 10.1161/ATVBAHA.121.316662
107
Liu R. Sun Y. Di D. Zhang X. Zhu B. Wu H. (2023). PI3K/AKT/SERBP-1 pathway regulates Alisma orientalis beverage treatment of atherosclerosis in APOE-/- high-fat diet mice. Pharm. Biol.61 (1), 473–487. 10.1080/13880209.2023.2168020
108
Liu W. Yin Y. Zhou Z. He M. Dai Y. (2014). OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm. Res.63 (1), 33–43. 10.1007/s00011-013-0667-3
109
Liu W. Zeng K. Zhou X. Zhang Y. Nie C. (2022). Comparative study on brain pharmacokinetics of Buyang Huanwu decoction in normal and cerebral ischemia rats using brain microdialysis combined with LC-MS/MS. Chin. Herb. Med.14 (4), 630–637. 10.1016/j.chmed.2022.03.007
110
Liu X. Luo P. Zhang W. Zhang S. Yang S. Hong F. (2023). Roles of pyroptosis in atherosclerosis pathogenesis. Biomed. Pharmacother.166, 115369. 10.1016/j.biopha.2023.115369
111
Locati M. Curtale G. Mantovani A. (2020). Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol.15, 123–147. 10.1146/annurevpathmechdis-012418-012718
112
Lu Y. H. Wang D. Jing H. Y. (2018). Effect and related mechanism on inflammatory response, proliferation and apoptosis of oxLDL-induced vascular smooth muscle cell of matrine. Chin. J. Immunol.34 (04), 537–543. 10.3969/j.issn.1000-484X.2018.04.012
113
Lu Q. Lin X. Wu J. Wang B. (2021). Matrine attenuates cardiomyocyte ischemia-reperfusion injury through activating AMPK/Sirt3 signaling pathway. J. Recept Signal Transduct. Res.41 (5), 488–493. 10.1080/10799893.2020.1828914
114
Lu X. Xu C. Yang R. Zhang G. (2021). Ganoderic acid A alleviates OVA-induced asthma in mice. Inflammation44 (5), 1908–1915. 10.1007/s10753-021-01468-1
115
Lubbad A. Oriowo M. A. Khan I. (2009). Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol. Cell Biochem.322 (1-2), 127–135. 10.1007/s11010-008-9949-4
116
Luo X. Weng X. Bao X. Bai X. Lv Y. Zhang S. et al (2022). A novel anti-atherosclerotic mechanism of quercetin: competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol.57, 102511. 10.1016/j.redox.2022.102548
117
Luo M. Zhao F. Cheng H. Su M. Wang Y. (2024). Macrophage polarization: an important role in inflammatory diseases. Front. Immunol.15, 1352946. 10.3389/fimmu.2024.1352946
118
Lv Y. L. Jia Y. Wan Z. An Z. L. Yang S. Han F. F. et al (2020). Curcumin inhibits the formation of atherosclerosis in ApoE-/- mice by suppressing cytomegalovirus activity in endothelial cells. Life Sci.257, 117658. 10.1016/j.lfs.2020.117658
119
Ma H. Z. Chen Y. Guo H. H. Wang J. Xin X. L. Li Y. C. et al (2023). Effect of resveratrol in gestational diabetes mellitus and its complications. World J. Diabetes14 (6), 808–819. 10.4239/wjd.v14.i6.808
120
Ma G. Dong Q. Li F. Jin Z. Pi J. Wu W. et al (2024). Network pharmacology and in vivo evidence of the pharmacological mechanism of geniposide in the treatment of atherosclerosis. BMC Complement. Med. Ther.24 (1), 53. 10.1186/s12906-024-04356-x
121
Magalhães P. R. Reis P. B. P. S. Vila-Viçosa D. Machuqueiro M. Victor B. L. (2021). Identification of pan-assay INterference compoundS (PAINS) using an MD-Based protocol. Methods Mol. Biol.2315, 263–271. 10.1007/978-1-0716-1468-6_15
122
Makuch M. Stepanechko M. Bzowska M. (2024). The dance of macrophage death: the interplay between the inevitable and the microenvironment. Front. Immunol.15, 1330461. 10.3389/fimmu.2024.1330461
123
Martínez-Noguera F. J. Marín-Pagán C. Carlos-Vivas J. Rubio-Arias J. A. Alcaraz P. E. (2019). Acute effects of hesperidin in oxidant/antioxidant state markers and performance in amateur cyclists. Nutrients11 (8), 1898. 10.3390/nu11081898
124
Mas-Capdevila A. Teichenne J. Domenech-Coca C. Caimari A. Del Bas J. M. Escoté X. et al (2020). Effect of hesperidin on cardiovascular disease risk factors: the role of intestinal microbiota on hesperidin bioavailability. Nutrients12 (5), 1488. 10.3390/nu12051488
125
McCubrey J. A. Lertpiriyapong K. Steelman L. S. Abrams S. L. Yang L. V. Murata R. M. et al (2017). Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY)9 (6), 1477–1536. 10.18632/aging.101250
126
Meng T. Xiao D. Muhammed A. Deng J. Chen L. He J. (2021). Anti-inflammatory action and mechanisms of resveratrol. Molecules26 (1), 229. 10.3390/molecules26010229
127
Meng T. Li X. Li C. Liu J. Chang H. Jiang N. et al (2022). Natural products of traditional Chinese medicine treat atherosclerosis by regulating inflammatory and oxidative stress pathways. Front. Pharmacol.13, 997598. 10.3389/fphar.2022.997598
128
Meng D. Deng X. Wu Y. Wu J. Zhang Y. Zhang J. et al (2023). Corilagin ameliorates macrophages inflammation in atherosclerosis through TLR4-NFκB/MAPK pathway. Heliyon9 (6), e16960. 10.1016/j.heliyon.2023.e16960
129
Mimura J. Itoh K. (2015). Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med.88 (Pt B), 221–232. 10.1016/j.freeradbiomed.2015.06.019
130
Mo J. Liao W. Du J. Huang X. Li Y. Su A. et al (2024). Buyang huanwu decoction improves synaptic plasticity of ischemic stroke by regulating the cAMP/PKA/CREB pathway. J. Ethnopharmacol.335, 118636. 10.1016/j.jep.2024.118636
131
Monaci S. Coppola F. Giuntini G. Roncoroni R. Acquati F. Sozzani S. et al (2021). Hypoxia enhances the expression of RNASET2 in human monocyte-derived dendritic cells: role of PI3K/AKT pathway. Int. J. Mol. Sci.22 (14), 7564. 10.3390/ijms22147564
132
Moore K. J. Tabas I. (2011). Macrophages in the pathogenesis of atherosclerosis. Cell145 (3), 341–355. 10.1016/j.cell.2011.04.005
133
Moore K. J. Sheedy F. J. Fisher E. A. (2013). Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol.13 (10), 709–721. 10.1038/nri3520
134
Murray P. J. Wynn T. A. (2011). Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol.11 (11), 723–737. 10.1038/nri3073
135
Murray P. J. Allen J. E. Biswas S. K. Fisher E. A. Gilroy D. W. Goerdt S. et al (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity41 (1), 14–20. 10.1016/j.immuni.2014.06.008
136
Nedkoff L. Briffa T. Zemedikun D. Herrington S. Wright F. L. (2023). Global trends in atherosclerotic cardiovascular disease. Clin. Ther.45 (11), 1087–1091. 10.1016/j.clinthera.2023.09.020
137
Ni X. C. Wang H. F. Cai Y. Y. Yang D. Alolga R. N. Liu B. et al (2022). Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol.54, 102363. 10.1016/j.redox.2022.102363
138
Ooi B. K. Goh B. H. Yap W. H. (2017). Oxidative stress in cardiovascular diseases: involvement of Nrf2 antioxidant redox signaling in macrophage foam cells formation. Int. J. Mol. Sci.18 (11), 2336. 10.3390/ijms18112336
139
Ortiz A. C. Fideles S. O. M. Reis C. H. B. Bellini M. Z. Pereira E. S. B. M. Pilon J. P. G. et al (2022). Therapeutic effects of citrus flavonoids neohesperidin, hesperidin and its aglycone, hesperetin on bone health. Biomolecules12 (5), 626. 10.3390/biom12050626
140
Ou Z. Wang Y. Yao J. Chen L. Miao H. Han Y. et al (2023). Astragaloside IV promotes angiogenesis by targeting SIRT7/VEGFA signaling pathway to improve brain injury after cerebral infarction in rats. Biomed. Pharmacother.168, 115598. 10.1016/j.biopha.2023.115598
141
Ovchinnikova O. Robertson A. K. Wågsäter D. Folco E. J. Hyry M. Myllyharju J. et al (2009). T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe(-/-) mice. Am. J. Pathol.174 (2), 693–700. 10.2353/ajpath.2009.080561
142
Pan Y. Feng X. Song W. Zhou X. Zhou Z. Chen G. et al (2023). Effects and potential mechanism of Zhuyu pill against atherosclerosis: network pharmacology and experimental validation. Drug Des. Devel Ther.17, 597–612. 10.2147/DDDT.S398808
143
Parsamanesh N. Asghari A. Sardari S. Tasbandi A. Jamialahmadi T. Xu S. et al (2021). Resveratrol and endothelial function: a literature review. Pharmacol. Res.170, 105725. 10.1016/j.phrs.2021.105725
144
Pascual G. Fong A. L. Ogawa S. Gamliel A. Li A. C. Perissi V. et al (2005). A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature437 (7059), 759–763. 10.1038/nature03988
145
Pawlita M. Clad A. zur Hausen H. (1985). Complete DNA sequence of lymphotropic papovavirus: prototype of a new species of the polyomavirus genus. Virology143 (1), 196–211. 10.1016/0042-6822(85)90108-4
146
Peng W. Cai G. Xia Y. Chen J. Wu P. Wang Z. et al (2019). Mitochondrial dysfunction in atherosclerosis. DNA Cell Biol.38 (7), 597–606. 10.1089/dna.2018.4552
147
Petitjean S. J. L. Lecocq M. Lelong C. Denis R. Defrère S. Mariage P. A. et al (2022). Salvia miltiorrhiza bunge as a potential natural compound against COVID-19. Cells11 (8), 1311. 10.3390/cells11081311
148
Poznyak A. Grechko A. V. Poggio P. Myasoedova V. A. Alfieri V. Orekhov A. N. (2020). The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci.21 (5), 1835. 10.3390/ijms21051835
149
Pyrzynska K. (2022). Hesperidin: a review on extraction methods, stability and biological activities. Nutrients14 (12), 2387. 10.3390/nu14122387
150
Qi L. F. Liu S. Fang Q. Qian C. Peng C. Liu Y. et al (2024). Ginsenoside Rg3 restores mitochondrial cardiolipin homeostasis via GRB2 to prevent Parkinson's Disease. Adv. Sci. (Weinh)11 (39), e2403058. 10.1002/advs.202403058
151
Qiao W. Yao X. Lu W. Zhang Y. Malhi K. Li H. et al (2024). Matrine exhibits antiviral activities against PEDV by directly targeting spike protein of the virus and inducing apoptosis via the MAPK signaling pathway. Int. J. Biol. Macromol.270 (Pt 2), 132408. 10.1016/j.ijbiomac.2024.132408
152
Qiu S. Xie L. Lu C. Gu C. Xia Y. Lv J. et al (2022). Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J. Exp. Clin. Cancer Res.41 (1), 296. 10.1186/s13046-022-02499-8
153
Quan Y. Z. Ma A. Ren C. Q. An Y. P. Qiao P. S. Gao C. et al (2024). Ganoderic acids alleviate atherosclerosis by inhibiting macrophage M1 polarization via TLR4/MyD88/NF-κB signaling pathway. Atherosclerosis391, 117478. 10.1016/j.atherosclerosis.2024.117478
154
Raj P. Thandapilly S. J. Wigle J. Zieroth S. Netticadan T. (2021). A comprehensive analysis of the efficacy of resveratrol in atherosclerotic cardiovascular disease, myocardial infarction and heart failure. Molecules26 (21), 6600. 10.3390/molecules26216600
155
Rao X. Zhou X. Wang G. Jie X. Xing B. Xu Y. et al (2022). NLRP6 is required for cancer-derived exosome-modified macrophage M2 polarization and promotes metastasis in small cell lung cancer. Cell Death Dis.13 (10), 891. 10.1038/s41419-022-05336-0
156
Repossi G. Das U. N. Eynard A. R. (2020). Molecular basis of the beneficial actions of resveratrol. Arch. Med. Res.51 (2), 105–114. 10.1016/j.arcmed.2020.01.010
157
Rocher C. Singla D. K. (2013). SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One8 (12), e84009. 10.1371/journal.pone.0084009
158
Roy P. Orecchioni M. Ley K. (2022). How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol.22 (4), 251–265. 10.1038/s41577-021-00584-1
159
Rumpel N. Riechert G. Schumann J. (2024). miRNA-Mediated fine regulation of TLR-induced M1 polarization. Cells13 (8), 701. 10.3390/cells13080701
160
Ruotsalainen A. K. Lappalainen J. P. Heiskanen E. Merentie M. Sihvola V. Näpänkangas J. et al (2019). Nuclear factor E2-related factor 2 deficiency impairs atherosclerotic lesion development but promotes features of plaque instability in hypercholesterolaemic mice. Cardiovasc Res.115 (1), 243–254. 10.1093/cvr/cvy143
161
Ryuk J. A. Zheng M. S. Lee M. Y. Seo C. S. Li Y. Lee S. H. et al (2012). Discrimination of Phellodendron amurense and P. chinense based on DNA analysis and the simultaneous analysis of alkaloids. Arch. Pharm. Res.35 (6), 1045–1054. 10.1007/s12272-012-0612-y
162
Salden B. N. Troost F. J. de Groot E. Stevens Y. R. Garcés-Rimón M. Possemiers S. et al (2016). Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr.104 (6), 1523–1533. 10.3945/ajcn.116.136960
163
Sarapultsev A. Gusev E. Komelkova M. Utepova I. Luo S. Hu D. (2023). JAK-STAT signaling in inflammation and stress-related diseases: implications for therapeutic interventions. Mol. Biomed.4 (1), 40. 10.1186/s43556-023-00151-1
164
Seifert R. Kuhlmann M. T. Eligehausen S. Kiefer F. Hermann S. Schäfers M. (2018). Molecular imaging of MMP activity discriminates unstable from stable plaque phenotypes in shear-stress induced murine atherosclerosis. PLoS One13 (10), e0204305. 10.1371/journal.pone.0204305
165
Shaito A. Thuan D. T. B. Phu H. T. Nguyen T. H. D. Hasan H. Halabi S. et al (2020). Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front. Pharmacol.11, 422. 10.3389/fphar.2020.00422
166
Shang X. Lin K. Yu R. Zhu P. Zhang Y. Wang L. et al (2019). Resveratrol protects the myocardium in sepsis by activating the phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian target of rapamycin (mTOR) pathway and inhibiting the nuclear Factor-κB (NF-κB) signaling pathway. Med. Sci. Monit.25, 9290–9298. 10.12659/MSM.918369
167
Sharifi-Rad J. Rayess Y. E. Rizk A. A. Sadaka C. Zgheib R. Zam W. et al (2020). Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol.11, 01021. 10.3389/fphar.2020.01021
168
Shen B. Feng H. Cheng J. Li Z. Jin M. Zhao L. et al (2020). Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J. Cell Mol. Med.24 (9), 5097–5108. 10.1111/jcmm.15139
169
Shen S. Huang Z. Lin L. Fang Z. Li W. Luo W. et al (2023). Tussilagone attenuates atherosclerosis through inhibiting MAPKs-mediated inflammation in macrophages. Int. Immunopharmacol.119, 110066. 10.1016/j.intimp.2023.110066
170
Shylaja H. Viswanatha G. L. Sunil V. Hussain S. M. Farhana S. A. (2024). Effect of hesperidin on blood pressure and lipid profile: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res.38 (5), 2560–2571. 10.1002/ptr.8174
171
Song W. Zhang Z. Y. Wang K. Qiu H. R. Zhang X. B. Shen T. (2024). Zhuyu pills promote polarization of macrophages toward M2 phenotype to prevent atherosclerosis via PPARγ/NF-κB signaling pathway. Zhongguo Zhong Yao Za Zhi49 (1), 243–250. 10.19540/j.cnki.cjcmm.20230823.501
172
Song W. Yang L. L. Zhang Z. Y. Shen T. (2025). Zhuyuwan in treatment of hyperlipidemia complicated with carotid atherosclerosis: a randomized controlled trial. Chin. J. Exp. Traditional Med. Formulae, 1–10. 10.13422/j.cnki.syfjx.20242326
173
Sun Y. Liu Y. Chen K. (2016). Roles and mechanisms of ginsenoside in cardiovascular diseases: progress and perspectives. Sci. China Life Sci.59 (3), 292–298. 10.1007/s11427-016-5007-8
174
Sun B. Rui R. Pan H. Zhang L. Wang X. (2018). Effect of combined use of astragaloside IV (AsIV) and atorvastatin (AV) on expression of PPAR-γ and inflammation-associated cytokines in atherosclerosis rats. Med. Sci. Monit.24, 6229–6236. 10.12659/MSM.908480
175
Sun J. Zhao P. Ding X. Li F. Jiang J. Huang H. et al (2022). Cayratia japonica prevents ulcerative colitis by promoting M2 macrophage polarization through blocking the TLR4/MAPK/NF-κB pathway. Mediat. Inflamm.2022, 1108569. 10.1155/2022/1108569
176
Sun Y. Zhang H. Liu R. Wang Y. Zhang X. Huang R. et al (2024). Zexieyin formula alleviates Alzheimer's disease via post-synaptic CaMKII modulating AMPA receptor: involved in promoting neurogenesis to strengthen synaptic plasticity in mice hippocampus. Phytomedicine131, 155802. 10.1016/j.phymed.2024.155802
177
Szanto A. Balint B. L. Nagy Z. S. Barta E. Dezso B. Pap A. et al (2010). STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity33 (5), 699–712. 10.1016/j.immuni.2010.11.009
178
Tan W. Wang Y. Wang K. Wang S. Liu J. Qin X. et al (2020). Improvement of endothelial dysfunction of berberine in atherosclerotic mice and mechanism exploring through TMT-based proteomics. Oxid. Med. Cell Longev.2020, 8683404. 10.1155/2020/8683404
179
Tang K. Zhong B. Luo Q. Liu Q. Chen X. Cao D. et al (2022). Phillyrin attenuates norepinephrine-induced cardiac hypertrophy and inflammatory response by suppressing p38/ERK1/2 MAPK and AKT/NF-kappaB pathways. Eur. J. Pharmacol.927, 175022. 10.1016/j.ejphar.2022.175022
180
Testai L. Calderone V. (2017). Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients9 (5), 502. 10.3390/nu9050502
181
Theofilis P. Oikonomou E. Tsioufis K. Tousoulis D. (2023). The role of macrophages in atherosclerosis: Pathophysiologic mechanisms and treatment considerations. Int. J. Mol. Sci.24 (11), 9568. 10.3390/ijms24119568
182
van Tits L. J. Stienstra R. van Lent P. L. Netea M. G. Joosten L. A. Stalenhoef A. F. (2011). Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis214 (2), 345–349. 10.1016/j.atherosclerosis.2010.11.018
183
Virga F. Cappellesso F. Stijlemans B. Henze A. T. Trotta R. Van Audenaerde J. et al (2021). Macrophage miR-210 induction and metabolic reprogramming in response to pathogen interaction boost life-threatening inflammation. Sci. Adv.7 (19), eabf0466. 10.1126/sciadv.abf0466
184
Waksman R. Merdler I. Case B. C. Waksman O. Porto I. (2024). Targeting inflammation in atherosclerosis: overview, strategy and directions. EuroIntervention20 (1), 32–44. 10.4244/EIJ-D-23-00606
185
Wang B. Tang X. Yao L. Wang Y. Chen Z. Li M. et al (2022). Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J. Clin. Invest132 (10), e154217. 10.1172/JCI154217
186
Wang D. Tan Z. Yang J. Li L. Li H. Zhang H. et al (2023). Perfluorooctane sulfonate promotes atherosclerosis by modulating M1 polarization of macrophages through the NF-κB pathway. Ecotoxicol. Environ. Saf.249, 114384. 10.1016/j.ecoenv.2022.114384
187
Wang H. Zhong L. Mi S. Song N. Zhang W. Zhong M. (2020). Tanshinone IIA prevents platelet activation and down-regulates CD36 and MKK4/JNK2 signaling pathway. BMC Cardiovasc Disord.20 (1), 81. 10.1186/s12872-019-01289-z
188
Wang J. Wang J. Zhong J. Liu H. Li W. Chen M. et al (2024). LRG1 promotes atherosclerosis by inducing macrophage M1-like polarization. Proc. Natl. Acad. Sci. U. S. A.121 (35), e2405845121. 10.1073/pnas.2405845121
189
Wang L. Zhao X. Ding J. Liu Y. Liu H. Zheng L. et al (2023). Oridonin attenuates the progression of atherosclerosis by inhibiting NLRP3 and activating Nrf2 in apolipoprotein E-deficient mice. Inflammopharmacology31 (4), 1993–2005. 10.1007/s10787-023-01161-9
190
Wang N N. Zhang X. Ma Z. Niu J. Ma S. Wenjie W. et al (2020). Combination of tanshinone IIA and astragaloside IV attenuate atherosclerotic plaque vulnerability in ApoE(-/-) mice by activating PI3K/AKT signaling and suppressing TRL4/NF-κB signaling. Biomed. Pharmacother.123, 109729. 10.1016/j.biopha.2019.109729
191
Wang S. Wang L. Shangguan J. Jiang A. Ren A. (2024). Research progress on the biological activity of ganoderic acids in Ganoderma lucidum over the last five years. Life (Basel)14 (10), 1339. 10.3390/life14101339
192
Wang T. Zhou Y. Wang K. Jiang X. Wang J. Chen J. (2022). Prediction and validation of potential molecular targets for the combination of Astragalus membranaceus and Angelica sinensis in the treatment of atherosclerosis based on network pharmacology. Med. Baltim.101 (26), e29762. 10.1097/MD.0000000000029762
193
Wang W. Li H. Shi Y. Zhou J. Khan G. J. Zhu J. et al (2024). Targeted intervention of natural medicinal active ingredients and traditional Chinese medicine on epigenetic modification: possible strategies for prevention and treatment of atherosclerosis. Phytomedicine122, 155139. 10.1016/j.phymed.2023.155139
194
Wang X. Wu F. P. Huang Y. R. Li H. D. Cao X. Y. You Y. et al (2023). Matrine suppresses NLRP3 inflammasome activation via regulating PTPN2/JNK/SREBP2 pathway in sepsis. Phytomedicine109, 154574. 10.1016/j.phymed.2022.154574
195
Wang Y. Chen L. Zhang M. Li X. Yang X. Huang T. et al (2023). Exercise-induced endothelial Mecp2 lactylation suppresses atherosclerosis via the Ereg/MAPK signalling pathway. Atherosclerosis375, 45–58. 10.1016/j.atherosclerosis.2023.05.009
196
Webb L. V. Ventura S. Ley S. C. (2019). ABIN-2, of the TPL-2 signaling complex, modulates mammalian inflammation. Trends Immunol.40 (9), 799–808. 10.1016/j.it.2019.07.001
197
Wei J. Shen S. Tian Y. Kang P. Sun G. (2024). Correlation analysis of macrophage distribution and pathological features of carotid atherosclerotic plaque. Ann. Vasc. Surg.98, 355–364. 10.1016/j.avsg.2023.08.030
198
Wu M. Yang S. Wang S. Cao Y. Zhao R. Li X. et al (2020). Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front. Pharmacol.11, 223. 10.3389/fphar.2020.00223
199
Wu C. Zhao Y. Zhang Y. Yang Y. Su W. Yang Y. et al (2021). Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR's cholesterol-decreasing efficacy in patients. J. Adv. Res.37, 197–208. 10.1016/j.jare.2021.07.011
200
Wu W. Hendrix A. Nair S. Cui T. (2022). Nrf2-Mediated dichotomy in the vascular system: mechanistic and therapeutic perspective. Cells11 (19), 3042. 10.3390/cells11193042
201
Wu J. Li K. Zhou M. Gao H. Wang W. Xiao W. (2024). Natural compounds improve diabetic nephropathy by regulating the TLR4 signaling pathway. J. Pharm. Anal.14 (8), 100946. 10.1016/j.jpha.2024.01.014
202
Wu J. He S. Song Z. Chen S. Lin X. Sun H. et al (2023). Macrophage polarization states in atherosclerosis. Front. Immunol.14, 1185587. 10.3389/fimmu.2023.1185587
203
Wu Q. Guan Y. B. Zhang K. J. Li L. Zhou Y. (2023). Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J. Ethnopharmacol.316, 116667. 10.1016/j.jep.2023.116667
204
Xin P. Xu X. Deng C. Liu S. Wang Y. Zhou X. et al (2020). The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol.80, 106210. 10.1016/j.intimp.2020.106210
205
Xu X. Y. Meng X. Li S. Gan R. Y. Li Y. Li H. B. (2018). Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients10 (10), 1553. 10.3390/nu10101553
206
Xue Q. He N. Wang Z. Fu X. Aung L. H. H. Liu Y. et al (2021). Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res.45 (1), 22–31. 10.1016/j.jgr.2020.07.002
207
Yaikwawong M. Jansarikit L. Jirawatnotai S. Chuengsamarn S. (2024). The effect of curcumin on reducing atherogenic risks in Obese patients with type 2 diabetes: a randomized controlled trial. Nutrients16 (15), 2441. 10.3390/nu16152441
208
Yan F. Ding H. Sun Z. Liu J. Li J. Zhou D. et al (2023). Glycoside combinations of buyang huanwu decoction ameliorate atherosclerosis via STAT3, HIF-1, and VEGF. Naunyn Schmiedeb. Arch. Pharmacol.396 (6), 1187–1203. 10.1007/s00210-023-02389-6
209
Yan Q. Song C. Liu H. Li Y. Ma J. Zhao Y. et al (2024). Adipose-derived stem cell exosomes loaded with icariin attenuated M1 polarization of macrophages via inhibiting the TLR4/Myd88/NF-κB signaling pathway. Int. Immunopharmacol.137, 112448. 10.1016/j.intimp.2024.112448
210
Yang S. Yuan H. Q. Hao Y. M. Ren Z. Qu S. L. Liu L. S. et al (2020). Macrophage polarization in atherosclerosis. Clin. Chim. Acta501, 142–146. 10.1016/j.cca.2019.10.034
211
Yang C. Mu Y. Li S. Zhang Y. Liu X. Li J. (2023). Tanshinone IIA: a Chinese herbal ingredient for the treatment of atherosclerosis. Front. Pharmacol.14, 1321880. 10.3389/fphar.2023.1321880
212
Yu D. Su D. Liu Z. (2023). Matrine protects intestinal barrier function via MicroRNA-155 through ROCK1-Signaling pathway. Turk J. Gastroenterol.34 (8), 831–838. 10.5152/tjg.2023.21884
213
Yu Y. Chen H. Wang R. Xu F. Yin J. Zang T. et al (2025). sFRP5 ameliorates atherosclerosis by suppressing the JNK/TLR9 pathway in macrophages. Transl. Res.281, 1–13. 10.1016/j.trsl.2025.05.004
214
Yu L. Zhang Y. Liu C. Wu X. Wang S. Sui W. et al (2023). Heterogeneity of macrophages in atherosclerosis revealed by single-cell RNA sequencing. FASEB J.37 (3), e22810. 10.1096/fj.202201932RR
215
Yuan H. Xu Y. Luo Y. Zhang J. R. Zhu X. X. Xiao J. H. (2022). Ganoderic acid D prevents oxidative stress-induced senescence by targeting 14-3-3ε to activate CaM/CaMKII/NRF2 signaling pathway in mesenchymal stem cells. Aging Cell21 (9), e13686. 10.1111/acel.13686
216
Zahr T. Liu L. Chan M. Zhou Q. Cai B. He Y. et al (2023). PPARγ (peroxisome proliferator-activated receptor γ) deacetylation suppresses aging-associated atherosclerosis and hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol.43 (1), 30–44. 10.1161/ATVBAHA.122.318061
217
Zhang X. Qin Y. Wan X. Liu H. Lv C. Ruan W. et al (2021). Rosuvastatin exerts anti-atherosclerotic effects by improving macrophage-related foam cell formation and polarization conversion via mediating autophagic activities. J. Transl. Med.19 (1), 62. 10.1186/s12967-021-02727-3
218
Zhang L. Li J. Kou Y. Shen L. Wang H. Wang Y. et al (2024). Mechanisms and treatment of atherosclerosis: focus on macrophages. Front. Immunol.15, 1490387. 10.3389/fimmu.2024.1490387
219
Zhang M. Li A. Yang Q. Li J. Zheng L. Wang G. et al (2023). Matrine alleviates depressive-like behaviors via modulating microbiota-gut-brain axis in CUMS-induced mice. J. Transl. Med.21 (1), 145. 10.1186/s12967-023-03993-z
220
Zhang S. Zou J. Li P. Zheng X. Feng D. (2018). Curcumin protects against atherosclerosis in apolipoprotein E-Knockout mice by inhibiting toll-like receptor 4 expression. J. Agric. Food Chem.66 (2), 449–456. 10.1021/acs.jafc.7b04260
221
Zhang W. W. Xu F. Wang D. Ye J. Cai S. Q. (2018). Buyang Huanwu decoction ameliorates ischemic stroke by modulating multiple targets with multiple components: in vitro evidences. Chin. J. Nat. Med.16 (3), 194–202. 10.1016/S1875-5364(18)30047-5
222
Zhang X X. Liu M. H. Qiao L. Zhang X. Y. Liu X. L. Dong M. et al (2018). Ginsenoside Rb1 enhances atherosclerotic plaque stability by skewing macrophages to the M2 phenotype. J. Cell Mol. Med.22 (1), 409–416. 10.1111/jcmm.13329
223
Zhang X. Gao R. Zhou Z. Sun J. Tang X. Li J. et al (2022). Uncovering the mechanism of Huanglian-Wuzhuyu herb pair in treating nonalcoholic steatohepatitis based on network pharmacology and experimental validation. J. Ethnopharmacol.296, 115405. 10.1016/j.jep.2022.115405
224
Zhang Y. Du M. Wang J. Liu P. (2022). Astragaloside IV relieves atherosclerosis and hepatic steatosis via MAPK/NF-κB signaling pathway in LDLR-/- mice. Front. Pharmacol.13, 828161. 10.3389/fphar.2022.828161
225
Zhao Y. Qian Y. Sun Z. Shen X. Cai Y. Li L. et al (2021). Role of PI3K in the progression and regression of atherosclerosis. Front. Pharmacol.12, 632378. 10.3389/fphar.2021.632378
226
Zhao M. He Q. Shu X. Xu R. Zhang Z. Mou Y. et al (2025). Zhuyu pill attenuates metabolic-associated fatty liver disease by regulating macrophage polarization through TLR4 signaling pathway. Phytomedicine138, 156439. 10.1016/j.phymed.2025.156439
227
Zhao Y. Wu J. Liu X. Chen X. Wang J. (2025). Decoding nature: multi-target anti-inflammatory mechanisms of natural products in the TLR4/NF-κB pathway. Front. Pharmacol.15, 1467193. 10.3389/fphar.2024.1467193
228
Zheng Y. Ren W. Zhang L. Zhang Y. Liu D. Liu Y. (2020). A review of the pharmacological action of astragalus polysaccharide. Front. Pharmacol.11, 349. 10.3389/fphar.2020.00349
229
Zheng G. Zhao Y. Li Z. Hua Y. Zhang J. Miao Y. et al (2023). GLSP and GLSP-derived triterpenes attenuate atherosclerosis and aortic calcification by stimulating ABCA1/G1-mediated macrophage cholesterol efflux and inactivating RUNX2-mediated VSMC osteogenesis. Theranostics13 (4), 1325–1341. 10.7150/thno.80250
230
Zheng Y. Wei K. Jiang P. Zhao J. Shan Y. Shi Y. et al (2024). Macrophage polarization in rheumatoid arthritis: signaling pathways, metabolic reprogramming, and crosstalk with synovial fibroblasts. Front. Immunol.15, 1394108. 10.3389/fimmu.2024.1394108
231
Zheng Q. Lin Y. Zeng L. Chen S. Chen L. Lin X. et al (2025). ITE-mediated AhR activation attenuates atherosclerosis by promoting macrophage M2-like polarization through NF-κB/LCN2 pathway suppression. Life Sci.375, 123715. 10.1016/j.lfs.2025.123715
232
Zheng Y. Shao M. Zheng Y. Sun W. Qin S. Sun Z. et al (2025). PPARs in atherosclerosis: the spatial and temporal features from mechanism to druggable targets. J. Adv. Res.69, 225–244. 10.1016/j.jare.2024.03.020
233
Zhi W. Liu Y. Wang X. Zhang H. (2023). Recent advances of traditional Chinese medicine for the prevention and treatment of atherosclerosis. J. Ethnopharmacol.301, 115749. 10.1016/j.jep.2022.115749
234
Zhou Y. Zhang T. Wang X. Wei X. Chen Y. Guo L. et al (2015). Curcumin modulates macrophage polarization through the inhibition of the toll-like receptor 4 expression and its signaling pathways. Cell Physiol. Biochem.36 (2), 631–641. 10.1159/000430126
235
Zhou P. Xie W. Luo Y. Lu S. Dai Z. Wang R. et al (2018). Inhibitory effects of ginsenoside Rb1 on early atherosclerosis in ApoE-/- mice via inhibition of apoptosis and enhancing autophagy. Molecules23 (11), 2912. 10.3390/molecules23112912
236
Zhou Z. Luo G. Li C. Zhang P. Chen W. Li X. et al (2023). Metformin induces M2 polarization via AMPK/PGC-1α/PPAR-γ pathway to improve peripheral nerve regeneration. Am. J. Transl. Res.15 (5), 3778–3792.
237
Zhu Z. Li J. Zhang X. (2019). Astragaloside IV protects against oxidized low-density lipoprotein (ox-LDL)-Induced endothelial cell injury by reducing oxidative stress and inflammation. Med. Sci. Monit.25, 2132–2140. 10.12659/MSM.912894
238
Zhu B. Zhai Y. Ji M. Wei Y. Wu J. Xue W. et al (2020). Alisma orientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE-/- mice. Front. Pharmacol.11, 570555. 10.3389/fphar.2020.570555
239
Zhu C. Li K. Peng X. X. Yao T. J. Wang Z. Y. Hu P. et al (2022). Berberine a traditional Chinese drug repurposing: its actions in inflammation-associated ulcerative colitis and cancer therapy. Front. Immunol.13, 1083788. 10.3389/fimmu.2022.1083788
240
Zhu C. Zhang M. Gong S. Du J. Ma L. Liu Y. et al (2024). Identification of matrine as a Kirsten rats arcomaviral oncogene homolog inhibitor alleviating chemotherapy-induced neuropathic pain. Phytomedicine132, 155841. 10.1016/j.phymed.2024.155841
241
Zuo Q. Chu Q. Jin Z. Li J. Wu W. (2021). Qingre huoxue decoction for treatment of acute st segment elevation myocardial infarction: a prospective multicenter cohort study[in Chinese]. J. Tradit. Chin. Med.62 (3), 229–234. 10.13288/i.112166/r.2021.03.010
Summary
Keywords
atherosclerosis, macrophage polarization, signaling pathways, bioactive metabolites, herbal formulas, research progress
Citation
Liu S, Jing J, Zhang Y, Sun J and Zhu C (2025) Potential of herbal formulas and bioactive metabolites in treating atherosclerosis: targeted modulation of macrophage polarization. Front. Pharmacol. 16:1631274. doi: 10.3389/fphar.2025.1631274
Received
19 May 2025
Revised
14 October 2025
Accepted
17 October 2025
Published
06 November 2025
Volume
16 - 2025
Edited by
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Reviewed by
Brigitta Buttari, National Institute of Health (ISS), Italy
Zhengtian Lv, Zhejiang Chinese Medical University, China
Klaudia Kocsy, The University of Sheffield, United Kingdom
Nicola Laera, University of Brescia and ASST-Spedali Civili di Brescia, Italy
Updates
Copyright
© 2025 Liu, Jing, Zhang, Sun and Zhu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaojun Zhu, zcj0706@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.