Abstract
Objective:
To provide a comprehensive narrative synthesis of recent advances in the pharmacological actions and therapeutic potential of natural flavonoids in atopic dermatitis (AD), with emphasis on their multi-target pharmacological effects across core pathological mechanisms. The review also addresses pharmacokinetic limitations, formulation challenges, delivery innovations, safety concerns, and emerging clinical evidence to inform translational research and therapeutic development.
Methods:
This narrative review is based on a targeted literature search of PubMed, Web of Science, ScienceDirect, and SpringerLink, covering English-language, peer-reviewed articles published between 2010 and 2025. Search terms included natural flavonoid metabolites (e.g., quercetin, baicalin, epigallocatechin-3-gallate [EGCG]) combined using Boolean operators (e.g., AND, OR) with keywords related to atopic dermatitis, its underlying mechanisms, and therapeutic interventions. Studies focusing on in vitro, in vivo, or clinical evaluations of mechanistic pathways, therapeutic potential, or delivery strategies were included, while those addressing synthetic flavonoids, non-AD models, or lacking mechanistic relevance were excluded. This review does not follow a systematic review protocol.
Results:
Natural flavonoids exert multi-target effects in AD models by restoring skin barrier integrity, modulating immune and chemokine dysregulation, alleviating pruritus, regulating microbial homeostasis and programmed cell death, and attenuating oxidative stress. However, pharmacokinetic and physicochemical limitations such as poor solubility, low bioavailability, metabolic instability, and limited dermal targeting currently constrain clinical application. Potential safety concerns, including hepatotoxicity and endocrine disruption, also warrant careful evaluation. To address these challenges, advanced delivery platforms including microneedles, hydrogels, nanocarriers, microsponges, and liposomes have been explored to improve dermal delivery. Additionally, oral delivery systems developed in other inflammatory and oncological models provide valuable insights for guiding translational strategies in AD. Preliminary clinical evidence suggests potential benefits of flavonoid-based interventions; nevertheless, larger and well-controlled trials are necessary to substantiate their pharmacological effects and evaluate long-term safety.
Conclusion:
Natural flavonoids exhibit multi-target effects in AD by modulating core pathological processes. Although challenges such as limited bioavailability and safety concerns continue to impede clinical translation, these limitations may be addressed through the optimization of delivery strategies, rigorous pharmacokinetic and toxicological assessments, mechanism-driven in vitro, in vivo, ex vivo studies, and well-designed clinical trials.
1 Introduction
Atopic dermatitis (AD) is a complex chronic inflammatory skin disease driven by genetic predisposition (Loset et al., 2019), immune dysregulation (Kim et al., 2019), and environmental factors (Chong et al., 2022). Globally, the prevalence of AD reaches up to 20% in children and approximately 10% in adults (Hadi et al., 2021; Silverberg et al., 2021). Clinically, AD is characterized by intense pruritus and eczematous lesions, frequently accompanied by sleep disturbances and psychological comorbidities (Ariëns et al., 2019; Schmidt and de Guzman Strong, 2021; Casella et al., 2024). These symptoms substantially impair quality of life and impose a significant socioeconomic burden.
Currently, topical corticosteroids, calcineurin inhibitors, and antihistamines remain the mainstay treatments for AD; however, long-term use is associated with skin atrophy, immunosuppression, diminished efficacy, and a high risk of relapse (Broeders et al., 2016). Although biologic agents have improved outcomes for patients with moderate-to-severe AD, therapeutic responses remain heterogeneous, and many patients experience suboptimal long-term efficacy or intolerance (Bangert et al., 2021; David et al., 2023; Rothenberg-Lausell et al., 2024). Moreover, treatment costs, poor adherence, and psychological comorbidities continue to challenge sustained disease management (Guttman-Yassky et al., 2025).
Flavonoids are natural polyphenolic metabolites abundantly found in medicinal herbs, fruits, vegetables, and tea (Hussain et al., 2022). Owing to their antioxidant, anti-inflammatory, and immunomodulatory properties, flavonoids have attracted growing interest in pharmaceutical and biotherapeutic research (Dere and Khan, 2020). Recent studies have highlighted their promising potential in the prevention and treatment of AD (Wu et al., 2021), attributed to their multifaceted activities such as anti-inflammatory (Maleki et al., 2019), antioxidant (Fu K. et al., 2024), immunomodulatory (Zeinali et al., 2017; Ribeiro et al., 2018), and barrier-repairing effects (Park C.-H. et al., 2020).
This review provides a comprehensive narrative overview of recent advances in flavonoid-based interventions for AD (Figure 1). It emphasizes their multi-target pharmacological actions across key pathological processes, including the restoration of skin barrier integrity, modulation of immune and chemokine dysregulation, itch signaling pathways, rebalancing of microbial homeostasis, modulation of programmed cell death pathways, and attenuation of oxidative stress and redox imbalance. Furthermore, the review highlights delivery innovations, safety considerations, and translational strategies, thereby providing a theoretical foundation for future research and clinical application of flavonoid-based therapies in AD. To ensure relevance and thematic focus, the following section outlines the literature search strategy underpinning this review.
FIGURE 1
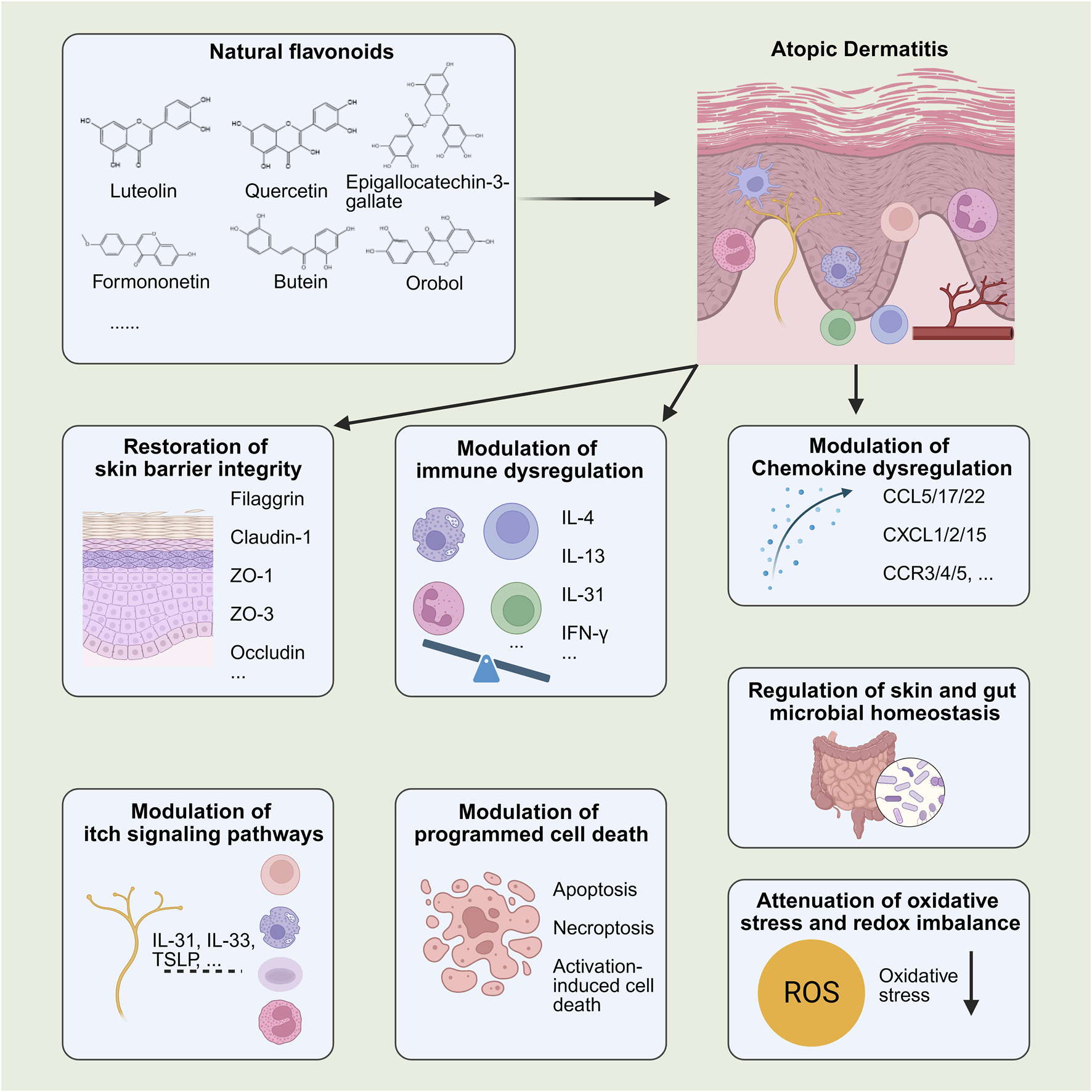
Pharmacological effects of flavonoids in AD. By BioRender. Note: ZO-, Zonula Occludens-; IL-, interleukin; TSLP, thymic stromal lymphopoietin; IFN-γ, interferon-gamma; CXCL, C-X-C motif chemokine ligand; CCL, C-C motif chemokine ligand; CCR-, C-C chemokine receptor type 3.
2 Literature search strategy
To support the narrative synthesis, a focused literature search was conducted across PubMed, Web of Science, ScienceDirect, and SpringerLink, targeting English-language, peer-reviewed articles published between 2010 and 2025. The search strategy employed Boolean operators (e.g., AND, OR) to combine terms for natural flavonoid metabolites (e.g., quercetin, baicalin, epigallocatechin-3-gallate [EGCG]) with keywords related to AD and its underlying mechanisms, including skin barrier dysfunction, immune dysregulation, chemokine imbalance, pruritogenic signaling, microbial dysbiosis, programmed cell death, oxidative stress, and drug delivery.
We included in vitro, in vivo, and clinical studies that investigated the mechanisms of action, therapeutic relevance, or delivery approaches of flavonoids in AD models or patients. Articles were excluded if they centered on synthetic flavonoids, unrelated disease models, or lacked mechanistic or delivery-specific data. The review does not adhere to a systematic review framework but aims to offer a descriptive, theme-based synthesis of current evidence.
3 Classification and pharmacology of flavonoids
Flavonoids are defined by a characteristic C6–C3–C6 skeleton, comprising two aromatic rings connected by an oxygen-containing heterocyclic C-ring. Based on structural features, flavonoids are commonly categorized into several subgroups, including flavones, flavonols, flavanols, isoflavones, chalcones, etc. (Ku et al., 2020; Bo et al., 2024), as shown in Figure 2. Structural modifications such as the type and position of substituents and the degree of hydroxylation can directly influence the physicochemical properties, biological activities, and pharmacological profiles of different flavonoid subclasses (Zouaoui et al., 2021; Abou Baker, 2022).
FIGURE 2
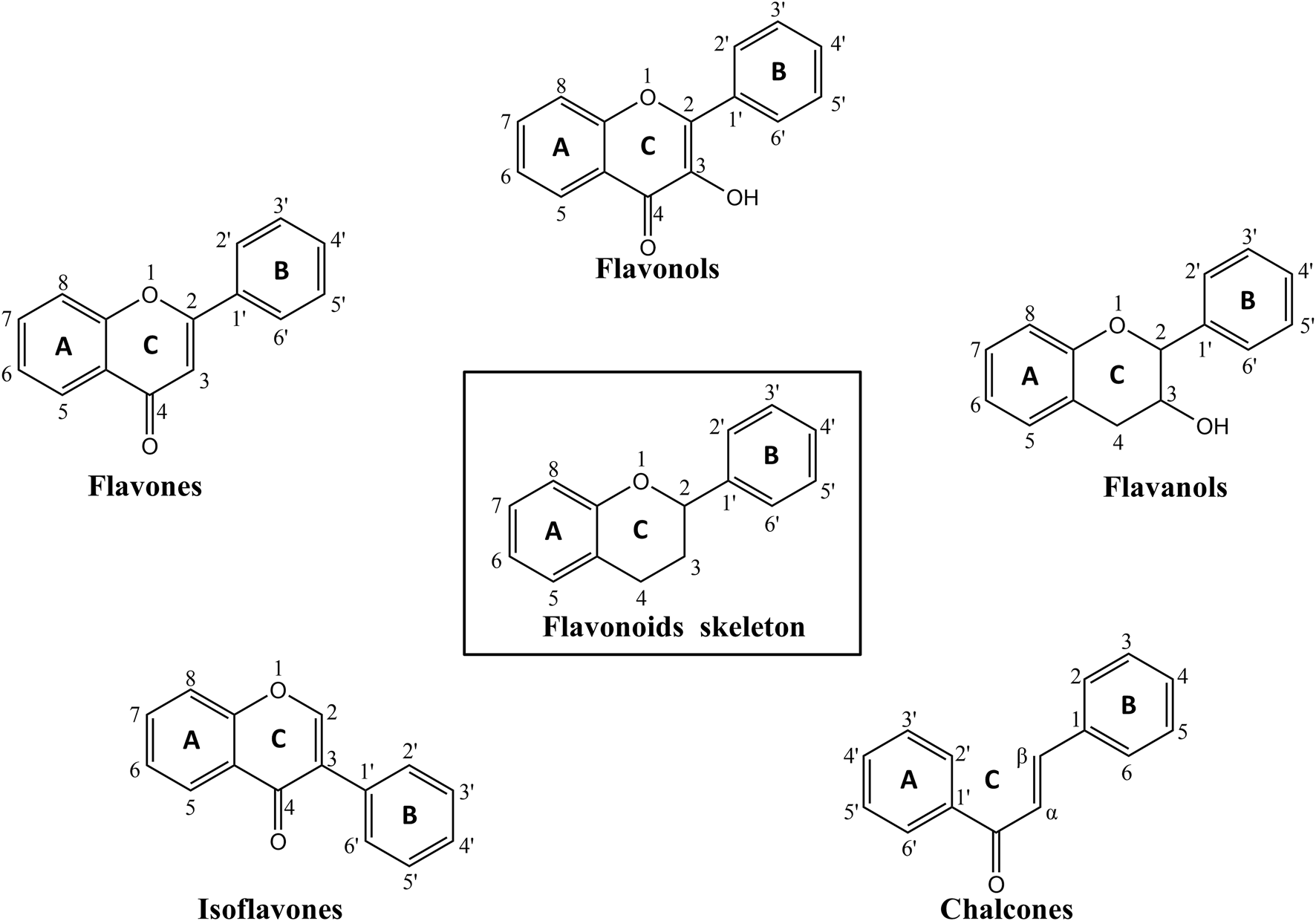
The chemical structures of flavonoids skeleton and corresponding subclasses involved in the paper. By KingDraw.
Flavones are characterized by an unsaturated 2-phenylchromen-4-one backbone, with baicalin and luteolin being prominent representatives (Espíndola, 2023). These flavonoids have demonstrated the ability to reduce neuroinflammation and promote neural plasticity in diverse experimental models (Jin et al., 2019; Shi et al., 2021).
Flavonols feature a C3 hydroxyl group and a C2–C3 double bond, both of which enhance their hydroxyl radical scavenging capacity. These structural features allow flavonols to modulate oxidative stress and inflammation-related signaling pathways, resulting in both antioxidant and anti-inflammatory effects (Treml and Šmejkal, 2016). Representative flavonols such as quercetin and kaempferol have been shown to attenuate oxidative stress (Ji et al., 2015).
Flavanols are defined by multiple hydroxyl groups on the C-ring, with EGCG, a major green tea polyphenol, being a representative metabolite (Bernatoniene and Kopustinskiene, 2018; Payne et al., 2022). Functionally, EGCG selectively induces apoptosis and suppresses tumor cell proliferation (Kumazoe et al., 2022).
Isoflavones, a class of plant-derived phytoestrogens, are predominantly found in Glycine max (L.) Merr. (soybean), Trifolium pratense L. (red clover), and other leguminous plants (Messina, 2014). These flavonoid metabolites exert estrogen-like effects and can ameliorate postmenopausal osteoporosis by modulating estrogen receptor (ER)-mediated signaling pathways (Messina, 2016; Lambert and Jeppesen, 2018; Xiao et al., 2018).
Chalcones are open-chain flavonoids characterized by a 1,3-diphenylprop-2-en-1-one backbone and act as biosynthetic precursors to flavonoids and isoflavonoids. Their α, β-unsaturated carbonyl system contributes to their high reactivity and broad pharmacological potential (Tekale et al., 2020). Chalcones exhibit a wide range of biological activities, including anti-inflammatory, antioxidant, antimicrobial, and anticancer effects, largely through the modulation of signaling pathways such as nuclear factor kappa B (NF-κB), phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), and cyclooxygenase (COX) enzymes (Ugwu et al., 2016).
4 Pharmacological effects of flavonoids in AD
Growing evidence indicates that numerous natural flavonoids (Figure 3) exert therapeutic effects in AD through multi-targeted pharmacological mechanisms (Table 1).
FIGURE 3
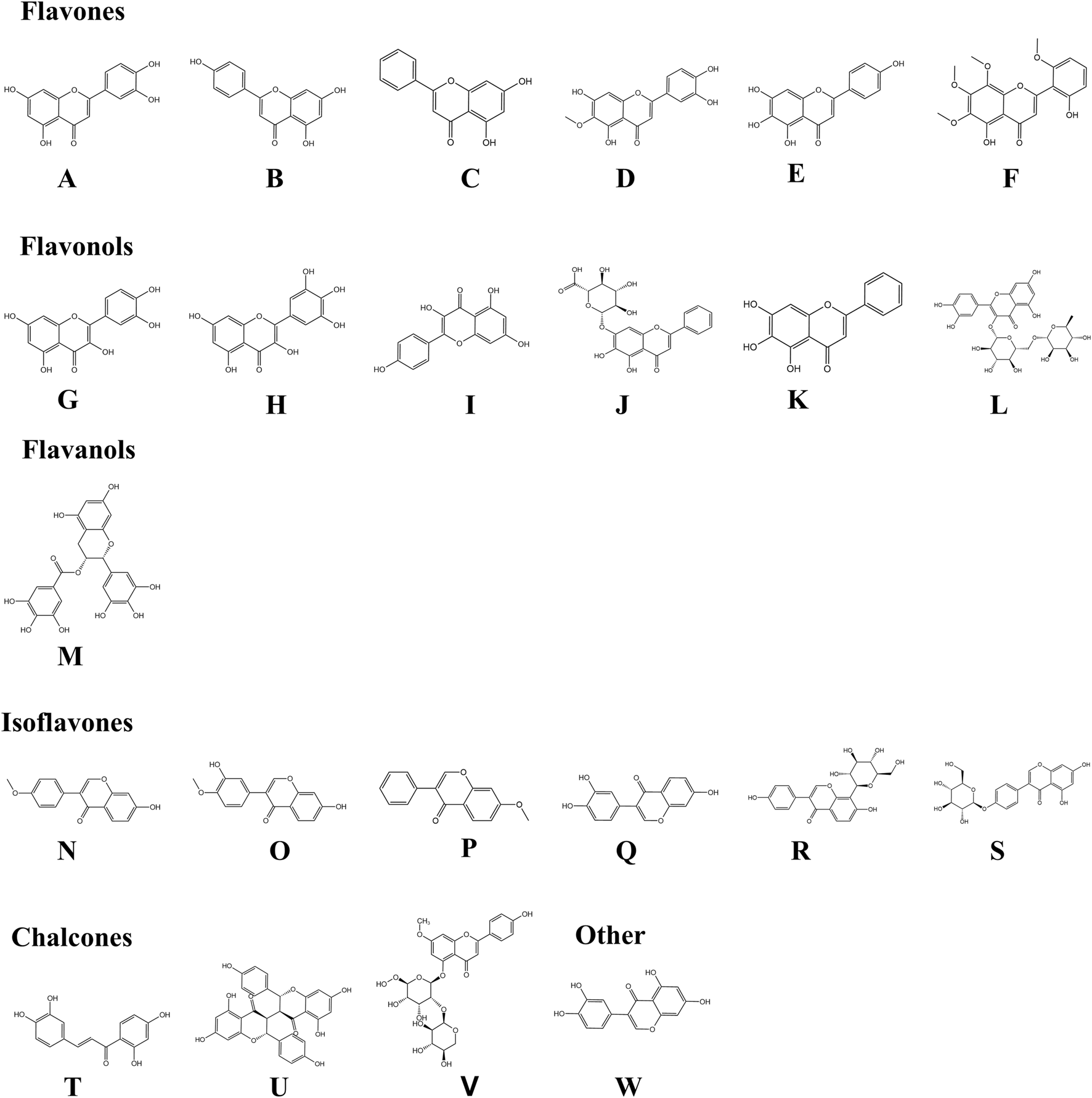
Chemical structures of flavonoids involved in the paper. By KingDraw. Among them, (A) Luteolin (Molecular formula: C15H10O6); (B) Apigenin (Molecular formula: C15H10O5); (C) Chrysin (Molecular formula: C15H10O4); (D) Nepetin (Molecular formula: C16H12O7); (E) Scutellarein (Molecular formula: C15H10O6); (F) Skullcapflavone II (Molecular formula: C19H18O8); (G) Quercetin (Molecular formula: C15H10O7); (H) Myricetin (Molecular formula: C15H10O8); (I) Kaempferol (Molecular formula: C15H10O6); (J) Baicalin (Molecular formula: C21H18O11); (K) Baicalein (Molecular formula: C15H10O5); (L) Rutin (Molecular formula: C27H30O16); (M) Epigallocatechin-3-gallate (Molecular formula: C22H18O11); (N) Formononetin (Molecular formula: C16H12O4); (O) Calycosin (Molecular formula: C16H12O5); (P) 7-Methoxyisoflavone (Molecular formula: C16H12O3); (Q) 7,3′,4′-Trihydroxyisoflavone (Molecular formula: C15H10O5); (R) Puerarin (Molecular formula: C21H20O9); (S) Sophoricoside (Molecular formula: C21H20O10); (T) Butein (Molecular formula: C15H12O5); (U) Chamaejasmine (Molecular formula: C30H22O10); (V) Stechamone (Molecular formula: C27H30O14), and (W) Orobol (Molecular formula: C15H10O6).
TABLE 1
| Metabolites | Methods | Models | Routes of administration | Targets | Signal pathways | Mechanisms | References |
|---|---|---|---|---|---|---|---|
| Flavones | |||||||
| Luteolin | In vivo | NC/Nga mice, Kun Ming mice | Topical | Skin thickness↓, mast cell number↓, granulated mast cells infiltration↓, degranulated mast cells infiltration↓, mast cell degranulation↓, lymphocyte infiltration↓, skin hydration↑, Filaggrin↑, IL-4↓, IL-6↓, IL-17↓, TNF-α↓, IgE↓, IFN-γ↑, TEWL↓ | JAK/STAT | Modulate immune imbalance, improve skin barrier function | Choi et al. (2010), Tang et al. (2022) |
| Apigenin | In vitro/In vivo | RAW264.7, RBL-2H3, HaCaT, HMC-1, SKH-1 hairless mice (hr/hr); ICR mice | Topical | Lamellar body↑, ABCA12↑, Filaggrin↑, TEWL↓, HMG-CoA reductase↑, SPT1↑, FAS↑, CAMP↑, mBD3↑, stratum corneum hydration↓, Loricrin↑, AQP3↑, HA↑, HAS-1↑, HAS-2↑, HAS-3↑, HBD-1↑, HBD-2↑, HBD-3↑, LL-37↑, NO↓, IL-1β↓, IL-6↓, COX-2↓, iNOS↓, β-hexosaminidase↓, TNF-α↓, IL-4↓, IL-5↓, IL-13↓, IL-31↓, Tryptase↓, FcεRIα↓, FcεRIγ↓, p-Lyn↓, p-Syk↓, p-PLCγ1↓ | MAPK | Modulate immune imbalance, improve skin barrier function, attenuate pruritus, modulate skin and gut microbiota | Hou et al. (2013), Park et al. (2020a), Che et al. (2019) |
| Chrysin | In vitro/In vivo | HaCaT, BALB/c mice | Topical/Oral | Skin thickness↓, mast cell infiltration↓, CCL5+ cells↓, CCL5↓, IgE↓, TSLP↓, EGR1↓ | NF-κB, MAPK | Regulate chemokines, attenuate pruritus | Yeo et al. (2020), Yeo et al. (2021) |
| Nepetin | In vitro/In vivo | HaCaT, BALB/c mice | Oral | Epidermal hyperplasia↓, immune cell infiltration↓, apoptosis rate↓, IL-1β↓, IL-6↓, TNF-α↓, ROS↓, iNOS↓, COX-2↓, PGES2↓, NO↓ | MyD88–MKK3/6–Akt | Modulate oxidative stress, regulate programmed cell death | Gong et al. (2024) |
| Scutellarein | In vitro/In vivo | HaCaT, C57BL/6J mice | Subcutaneous injection | Skin thickness↓, TRPV3 current↓, TRPV3 open probability↓, TRPV3-mediated Ca2+ influx↓, BrdU incorporation↓, PBMCs chemotaxis↓, IgE↓, IL-1β↓, TNF-α↓, IL-4↓, IL-6↓, CXCL15↓ | TRPV3 channels | Regulate chemokines, attenuate pruritus | Wang et al. (2022c) |
| Skullcapflavone II | In vitro/In vivo | BMDCs, Mouse CD4+ T cells, HaCaT, Human primary keratinocytes, BALB/c mice | Topical | Skin thickness↓, CD4+ T cell proliferation↓, CD4+ T cell infiltration↓, Gr-1+ neutrophil infiltration↓, mast cell infiltration↓, eosinophil infiltration↓, CTSS↓, pro-CTSS↓, active-CTSS↓, CXCL1↓, CCL17/22↓, TSLP↓, IL-4↓, IFN-γ↓, IL-6↓, IL-12↓, IL-17A↓, IgE↓, S100A8↓, Mcpt8↓ | STAT, NF-κB, MAPK | Regulate chemokines, attenuate pruritus | Lee et al. (2022b) |
| Flavonols | |||||||
| Quercetin | In vitro/In vivo | HaCaT, RAW264.7, human mast cells (HMC-1), NC/Nga mice, C57BL/6 mice | Topical/Oral | Skin thickness↓, mast cell infiltration↓, eosinophil levels↓, IL-1β↓, IL-1α↓, IL-4↓, IL-5↓, IL-6↓, IL-8↓, IL-10↑, IL-13↓, IL-15↓, IL-33↓, TNF-α↓, IFN-γ↓, IgE↓, TSLP↓, CCL2/3/5/7/11/17/22/27↓, TLR2↓, TLR6↓, HMGB1↓, COX-2↓, iNOS↓, ICAM-1↓, VEGF↓, caspase-3↓, caspase-8↓, Bid↓, Nrf2↑, HO-1↑, PPARα↑, PPARγ↑, glutathione↑, MMP-1↓, MMP-2↓, MMP-9↓, SOD1↑, SOD2↑, catalase↑, GPx↑, Twist↑, Snail↑, E-cadherin↑, Occludin↑ | HMGB1/RAGE/NF-κB, Nrf2/HO-1, MAPK, JAK/STAT, TLR2/TLR6 | Improve skin barrier function, regulate chemokines, modulate oxidative stress | Karuppagounder et al. (2016), Hou et al. (2019), Beken et al. (2020) |
| Myricetin | In vitro/In vivo | HaCaT, Balb/c Mice, Kunming mice | Topical | Skin thickness↓, mast cell infiltration↓, CD4+ T cell infiltration↓, keratinocyte integrity↑, lamellar body secretion area↑, TEWL↓, Filaggrin↑, IL-1β↓, IL-4↓, IL-17↓, IFN-γ↓, TNF-α↓, IgE↓, histamine↓, TSLP↓, CCL17/22↓, T-bet↓, GATA-3↓, TGF-β↓, iNOS↓, COX-2↓ | NF-κB, STAT1 | Improve skin barrier function, modulate immune imbalance | Hou et al. (2022), Gao et al. (2023) |
| Kaempferol | In vitro/In vivo | Jurkat T cells, CD4+ T cells and splenocytes from BALB/c mice, BALB/c mice, C57BL/6 mice | Oral/Intraperitoneal injection, i.p | Skin thickness↓, CD3+ T cells↓, CD4+ T cells↓, CD68+ macrophages↓, mast cell infiltration↓, TEWL↓, Filaggrin↑, Loricrin↑, Involucrin↑, IL-2↓, IL-4↓, IL-6↓, IL-13↓, IL-17↓, IL-31↓, IFN-γ↓, TSLP↓, CD69↓, cleaved caspase-3/7/9↓, MRP-1 activity↓, Bcl-2↑, HO-1↓ | NF-κB, MAPK | Improve skin barrier function, modulate immune imbalance, regulate programmed cell death | Lee and Jeong (2021), Nasanbat et al. (2023) |
| Baicalin | In vivo | BALB/c mice, Pseudo germ-free mice | Oral | Skin thickness↓, mast cell infiltration↓, TEWL↓, TSLP↓, MCP-1↓, IL-4↓, IFN-γ↑, IgE↓, Filaggrin↑, Loricrin↑, Involucrin↑, histamine↓, Alistipes spp.↓, Parabacteroides spp.↓, Mycoplasma spp.↓, Lactobacillus spp.↑, Coprococcus 1 spp.↑, Ruminiclostridium 6 spp.↑ | JAK/STAT, NF-κB | Improve skin barrier function, modulate immune imbalance, modulate skin and gut microbiota | Wang et al. (2022a) |
| Baicalein | In vitro | HaCaT | — | K1/K10↑ | MAPK, Akt | Improve skin barrier function | Huang et al. (2016a) |
| Rutin | In vivo | BALB/c J mice | Topical | Skin thickness↓, eosinophil infiltration↓, mast cell infiltration↓, IL-4↓, IL-5↓, IL-13↓, IL-31↓, IL-32↓, IFN-γ↓, IgE↓, histamine↓ | — | Modulate immune imbalance, attenuate pruritus | Choi and Kim (2013) |
| Flavanols | |||||||
| Epigallocatechin-3-gallate | In vivo | Kunming mice, Nc/Nga mice | Topical | Skin thickness↓, mast cell number↓, IFN-γ↓, IL-4↓, IL-5↓, IL-6↓, IL-13↓, IL-17A↓, TNF-α↓, IgE↓, histamine↓, LDH activity↓, MDA↓, SOD↑, GSH↑, T-AOC↑, RIP1↓, RIP3↓, MLKL↓ | NF-κB, MAPK | Modulate oxidative stress, regulate programmed cell death, modulate skin and gut microbiota | Chiu et al. (2021), Han et al. (2022) |
| Isoflavones | |||||||
| Formononetin | In vitro/In vivo | HaCaT, RBL-2H3, BMMCs, BALB/c mice | Oral/Intraperitoneal injection, i.p | FcεRIγ, p-Lyn, p-Syk, p-PLCγ, IL-13, TNF-α, Filaggrin, Loricrin, IgE, β-hexosaminidase, histamine, TSLP, E-cadherin, IgE | NF-κB | Improve skin barrier function, attenuate pruritus | Li et al. (2018), Zhou et al. (2023) |
| Calycosin | In vitro/In vivo | HaCaT, C57BL/6 mice | Oral | Occludin↑, ZO-1↑, TSLP↓, IL-33↓ | TLR4/MyD88/NF-κB | Improve skin barrier function, attenuate pruritus | Tao et al. (2017) |
| 7-Methoxyisoflavone | In vivo | BALB/c mice | Topical | Skin thickness↓, keratinocyte hyperproliferation↓, mast cell infiltration↓, neutrophil infiltration↓, IL-17+ Th17 cells↓, spleen index↓, IL-4↓, IL-17A↓, IgE↓, IFN-γ↓, TSLP↓, CXCL1/2/3↓, CCL17/22↓ | MAPK-AP-1, NF-κB, IL-17/STAT3 | Modulate immune imbalance, regulate chemokines, attenuate pruritus | Dong et al. (2022) |
| 7,3′,4′-Trihydroxyisoflavone | In vitro/In vivo | HaCaT, NC/Nga mice | Topical | Skin thickness↓, eosinophil infiltration↓, mast cell infiltration↓, IgE↓, CCL17/22↓ | MAPK | Modulate oxidative stress, regulate chemokines | Park et al. (2020b) |
| Puerarin | In vitro/In vivo | HaCaT, BALB/c mice | Oral | Skin thickness↓, mast cell infiltration↓, IL-1β↓, IL-4↓, IL-5↓, IL-6↓, IL-31↓, TNF-α↓, IgE↓, PAR2↓, TSLP↓, CCL2/5/17↓, CXCL8/9/10/11↓ | MAPK, NF-κB, Akt, STAT1, PAR2–NF-κB–TSLP axis | Modulate immune imbalance, regulate chemokines, attenuate pruritus | Lee et al. (2018) |
| Sophoricoside | In vitro/In vivo | C57BL/6 mice naïve CD4+ T, BALB/c mice | Topical | Skin thickness↓, mast cell infiltration↓, IgE↓, IFN-γ↓, TNF-α↓, IL-2↓, IL-4↓, IL-5↓, IL-6↓, IL-12↓, IL-17A↓, T-bet↓, GATA-3↓, RORγt↓ | — | Modulate immune imbalance, attenuate pruritus | Kim and Lee (2021) |
| Chalcones | |||||||
| Butein | In vitro | HaCaT, human acute monocytic leukemia cell line THP-1 (THP-1 cells) | Topical | ICAM-1↓, monocyte adhesion↓, IL-6↓, IP-10↓, MCP-1↓, ROS↓ | MAPK, NF-κB | Modulate oxidative stress | Seo et al. (2015) |
| Chamaejasmine | In vitro/In vivo | RBL-2H3, SKH-1 hairless mice | Topical | Skin thickness↓, skin hydration↑, TEWL↓, mast cells↓, IgE↓, IL-4↓, β-hexosaminidase↓ | — | Improve skin barrier function, modulate immune imbalance, attenuate pruritus | Kim et al. (2019) |
| Stechamone | In vitro/In vivo | RBL-2H3, SKH-1 hairless mice | Topical | Skin thickness↓, mast cell↓, lymphocyte infiltration↓, TEWL↓, β-hexosaminidase↓, IgE↓, IL-4↓ | — | Improve skin barrier function, modulate immune imbalance, attenuate pruritus | Jo et al. (2018) |
| Others | |||||||
| Orobol | In vitro/In vivo | HaCaT, NC/Nga mice | Topical | Skin thickness↓, skin hydration↑, TEWL↓, eosinophil infiltration↓, mast cell infiltration↓, CCL17/22↓, IgE↓, IL-4↓, IL-13↓ | MAPK, NF-κB | Improve skin barrier function, regulate chemokines, modulate immune imbalance, attenuate pruritus | Lee et al. (2022a) |
Pharmacological effects of flavonoids in AD.
Note: BMDCs, bone marrow-derived dendritic cells; BMMCs, bone marrow-derived mast cells; CD4+ T cells, cluster of differentiation 4 positive T lymphocytes; HaCaT, human immortalized keratinocyte cell line; HMC-1, human mast cell line-1; Jurkat T cells, human T lymphocyte leukemia cell line; RBL-2H3, rat basophilic leukemia cells; RAW264.7, murine macrophage-like cell line; SKH-1, hairless mice (hr/hr), hairless mouse strain used for dermatological research; ICR, mice, Institute of Cancer Research mice; THP-1, human acute monocytic leukemia cell line; ABCA12, ATP-binding cassette subfamily A member 12; AQP3, aquaporin 3; Bcl-2, B-cell lymphoma 2; Bid, BH3-interacting domain death agonist; BrdU, 5-bromo-2′-deoxyuridine; CAMP, cathelicidin antimicrobial peptide; CAT, catalase; CCL, C-C motif chemokine ligand; CD69, cluster of differentiation 69; COX-2, cyclooxygenase-2; CTSS, Cathepsin S; CXCL-, C-X-C motif chemokine ligand; EGR1, early growth response 1; FAS, fatty acid synthase; FcεRIα, high-affinity IgE receptor subunit alpha; FcεRIγ, high-affinity IgE receptor subunit gamma; GATA-3, GATA-binding protein 3; GPx, glutathione peroxidase; GSH, glutathione; HAS, hyaluronic acid synthase; HBD-, human beta-defensin; HMGB1, high mobility group box 1; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule 1; IFN-γ, interferon-gamma; IgE, immunoglobulin E; IL-, interleukin; iNOS, inducible nitric oxide synthase; K1/K10, keratin 1 and keratin 10; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; Mcpt8, mast cell protease 8; MLKL, mixed lineage kinase domain-like protein; mBD3, mouse beta-defensin 3; MMP-, matrix metalloproteinases, MRP-1, multidrug resistance-associated protein 1; MyD88, myeloid differentiation primary response 88; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; Occludin, tight junction protein occludin; PAR2, protease-activated receptor 2; PGES2, prostaglandin E synthase 2; PGE2, prostaglandin E2; p-Lyn, phosphorylated Lyn; p-Syk, phosphorylated spleen tyrosine kinase; p-PLCγ1, phosphorylated phospholipase C gamma 1; PPARα, peroxisome proliferator-activated receptor alpha; PPARγ, peroxisome proliferator-activated receptor gamma; RAGE, receptor for advanced glycation end-products; RIP1, receptor-interacting serine/threonine-protein kinase 1; RIP3, receptor-interacting serine/threonine-protein kinase 3; RORγt, RAR-related orphan receptor gamma t; ROS, reactive oxygen species; S100A8, S100 calcium-binding protein A8; Snail, zinc finger protein SNAI1; SOD, superoxide dismutase; SPT1, serine-palmitoyl transferase 1; STAT, signal transducer and activator of transcription; TEWL, transepidermal water loss; T-bet, T-box transcription factor (TBX21); TGF-β, transforming growth factor beta; TLR-, Toll-like receptor; TNF-α, tumor necrosis factor-alpha; TRPV3, transient receptor potential vanilloid 3; TSLP, thymic stromal lymphopoietin; Twist, twist-related protein 1; VEGF, vascular endothelial growth factor; ZO-, zonula occludens; Akt, protein kinase B; AP-1, activator protein 1; JAK, janus kinase; MAPK, mitogen-activated protein kinase; MKK3/6, mitogen-activated protein kinase kinase 3/6; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor kappa B; TRPV3, transient receptor potential vanilloid 3 channel.
4.1 Restoration of skin barrier integrity
Disruption of the skin barrier, comprising the stratum corneum, lamellar lipids, tight junctions, and cutaneous microbiota, is a central pathophysiological hallmark of AD, as part of the broader mechanisms illustrated in Figure 4. This dysfunction is characterized by downregulated structural protein expression, disorganized intercellular junctions, lipid imbalance, and microbial dysbiosis (Melnik, 2015; Fujii, 2020). These alterations result in increased transepidermal water loss (TEWL) and a diminished protective barrier, which together permit the transcutaneous entry of allergens and pro-inflammatory mediators. Consequently, keratinocyte hyperactivation and immune dysregulation are initiated, establishing a self-perpetuating cycle of barrier disruption and inflammatory amplification that ultimately drives disease progression (Katsarou et al., 2023; Çetinarslan et al., 2023).
FIGURE 4
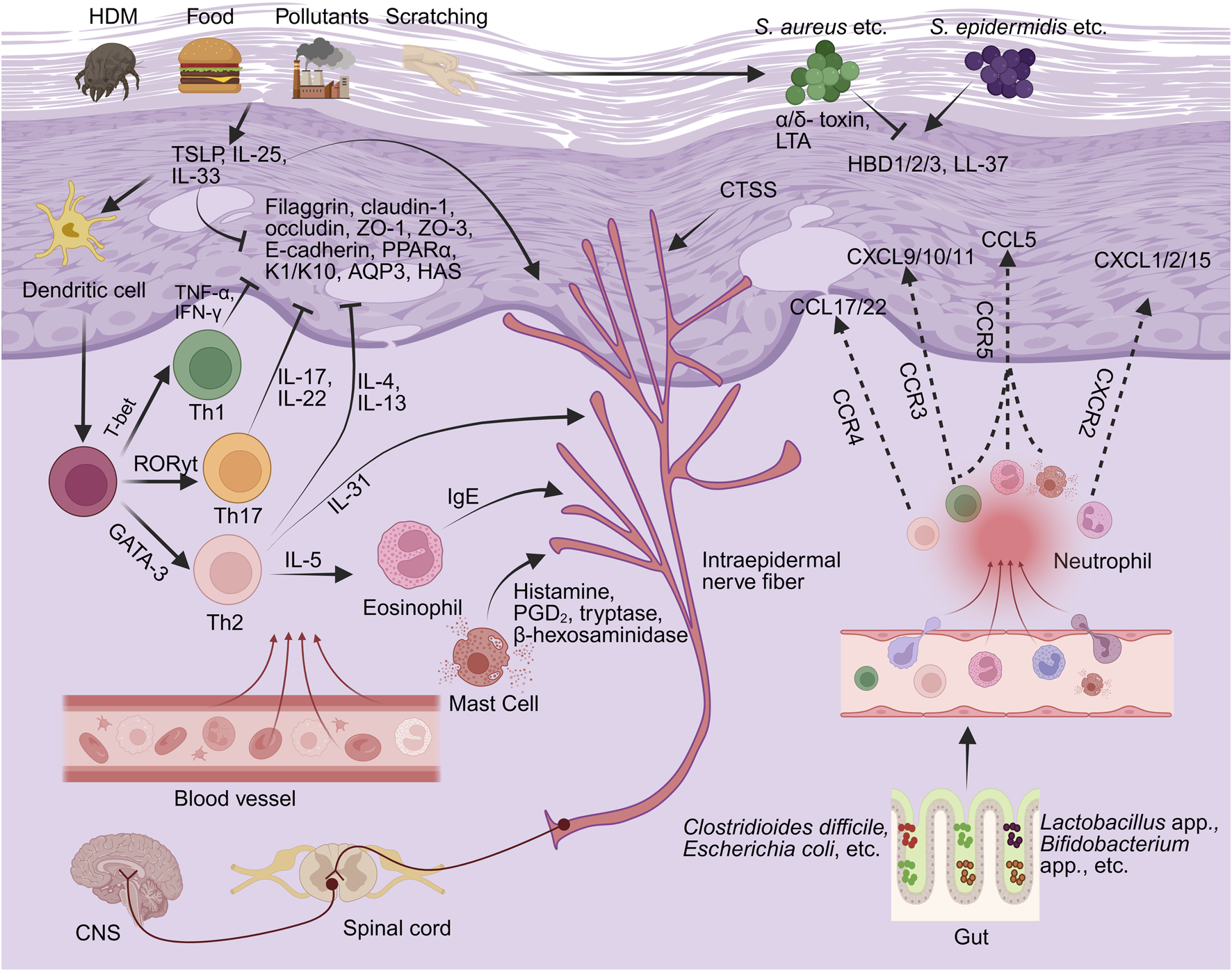
The pathological mechanisms underlying AD (the part that flavonoids can intervene in). By BioRender. Note: HDM, house dust mite; TSLP, thymic stromal lymphopoietin; IL-, interleukin; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor-alpha; ZO-, Zonula Occludens-; K1/K10, keratin 1 and keratin 10; PPARα, peroxisome proliferator-activated receptor alpha; AQP3, aquaporin 3; HAS, hyaluronic acid synthase; Th-, T helper; T-bet, T-box transcription factor (TBX21); GATA3, GATA-binding protein 3; RORγt, retinoic acid-related orphan receptor gamma t; CCR-, C-C chemokine receptor; CCL, C-C motif chemokine ligand; CXCL-, C-X-C motif chemokine ligand; HBD, human beta-defensin; Staphylococcus aureus, Staphylococcus aureus; Staphylococcus epidermidis, Staphylococcus epidermidis; LTA, lipoteichoic acid; IgE, immunoglobulin E; PGD2, prostaglandin D2; CGRP, calcitonin gene-related peptide; CTSS, Cathepsin S.
At the molecular level, AD skin lesions exhibit significant downregulation of structural proteins such as filaggrin and claudin-1 (Melnik, 2015; Callou et al., 2022), as well as tight junction-associated proteins, among which Zonula Occludens- (ZO-) 1 and occludin shown to be significantly reduced in AD skin lesions (Yuki et al., 2016), and ZO-3 expression diminished in keratinocyte-based AD models (Hu et al., 2023; Trujillo-Paez et al., 2024). These alterations collectively result in impaired keratinocyte cohesion and elevated TEWL. Notably, these alterations occur prior to the onset of inflammation and induce the upregulation of epithelial-derived cytokines such as thymic stromal lymphopoietin (TSLP) and interleukin-33 (IL-33), which subsequently activate the Th2 immune axis in a sustained manner (Dai et al., 2022). Several flavonoid metabolites have demonstrated potential to modulate barrier function through multiple mechanisms. Chamaejasmine, stechamone, and orobol have been shown to reduce TEWL, increase skin hydration, mitigate erythema, pruritus, and xerosis, attenuate epidermal hyperplasia and mast cell infiltration, and attenuate stratum corneum damage (Jo et al., 2018; Kim et al., 2019; Lee C. H. et al., 2022). Baicalin and formononetin significantly upregulate the expression of filaggrin and loricrin, reduce TEWL, suppress mast cell infiltration, and ameliorate both epidermal disruption and modified Eczema Area and Severity Index (EASI) scores in AD mouse models (Wang L. et al., 2022; Zhou et al., 2023). Experimental studies indicate that calycosin may modulate the Toll-like receptor (TLR) 4/NF-κB signaling pathway, suppress the expression of TSLP and IL-33, and increase tight junction proteins such as ZO-1 and occludin in both human immortalized keratinocyte cell line (HaCaT) and mouse AD models (Tao et al., 2017).
Adherens junctions (AJs) play a critical role in maintaining epidermal integrity, with E-cadherin serving as a central adhesion molecule. In AD, downregulation of E-cadherin disrupts intercellular cohesion, resulting in elevated TEWL and increased permeability to environmental allergens (Dong et al., 2024). In a fluorescein isothiocyanate (FITC)-induced AD mouse model, formononetin was observed to improve E-cadherin localization, suppress the expression of TSLP and IL-33, reinforce the epidermal barrier integrity, and attenuate immune activation (Li et al., 2018). In HaCaT model, quercetin treatment was associated with increased expression of E-cadherin and occludin, upregulation of antioxidant enzymes including superoxide dismutase 1 (SOD1) and superoxide dismutase 2 (SOD2) and catalase, and reduced levels of pro-inflammatory cytokines and matrix metalloproteinases (MMPs), which may support improved keratinocyte migration and in vitro wound closure (Beken et al., 2020).
At the cytoskeletal level, keratins keratin 1 and keratin 10 (K1/K10) are critical structural proteins that contribute to the maintenance of epidermal mechanical integrity and barrier stability; however, their expression is markedly suppressed in AD skin lesions (Dai et al., 2021). In HaCaT model, baicalein was associated with promoting the structural maturation of keratinocytes by upregulating the expression of K1/K10 (Huang K.-F. et al., 2016).
Lamellar bodies, specialized organelles in the granular layer, secrete polar lipids and enzymes to form the stratum corneum’s intercellular lipid matrix (Elias and Wakefield, 2020; Shin et al., 2020); their impaired formation contributes to barrier dysfunction in AD (Cork et al., 2009). In addition, aquaporin 3 (AQP3) and hyaluronic acid synthase (HAS) are essential regulators of skin hydration and hyaluronic acid synthesis (Camilion et al., 2022; Tricarico et al., 2022). Myricetin has been reported to increase lamellar body secretion and support the structural integrity of the epidermal lipid matrix in AD mouse model (Gao et al., 2023), while apigenin has been reported to increase the expression of AQP3, HAS, and filaggrin, upregulates skin hydration and lipid synthesis, increase lamellar body density, and increase the expression of lipid-synthesizing enzymes, indicating potential roles in epidermal hydration and barrier maintenance in cell models (Hou et al., 2013; Park C.-H. et al., 2020).
Peroxisome proliferator-activated receptor alpha (PPARα), a key nuclear transcription factor that regulates keratinocyte differentiation and epidermal lipid metabolism, is markedly downregulated in AD (Kuai et al., 2024), leading to impaired barrier integrity and defective lipid synthesis, which further supported by PPARα agonist-induced barrier restoration in ex vivo skin model (Majewski et al., 2021). In a house dust mite-induced NC/Nga mouse model (a spontaneous AD model), quercetin has been reported to activate the PPARα signaling pathway, which may contribute to the improvements in lipid biosynthesis, inflammatory status, and barrier integrity (Karuppagounder et al., 2016).
4.2 Modulation of immune dysregulation
CD4+ T lymphocytes (CD4+ T cells) are central to adaptive immunity and differentiate into functionally subsets such as T helper (Th)1, Th2, Th17, and regulatory T cells (Tregs), which mediate distinct immune responses. These lineages maintain immune homeostasis through cytokine- and transcription factor-driven cross-regulation. Among these, Th1, Th2, and Th17 subsets are particularly implicated in the initiation and chronic progression of AD (Su et al., 2017), as part of the broader mechanisms illustrated in Figure 4.
In AD, an imbalance between Th1 and Th2 cells is recognized as one of the most prominent immunological hallmarks. Th1 cells differentiate in response to IL-12 and interferon-gamma (IFN-γ), a process regulated by the transcription factor T-bet (Jankovic and Feng, 2015). These cells predominantly secrete IFN-γ and tumor necrosis factor-alpha (TNF-α). In contrast, Th2 cells are induced by IL-4, which promotes the expression of GATA-binding protein 3 (GATA3) and leads to the production of IL-4, IL-5, and IL-13, all of which play key roles in driving allergic inflammation (Ruterbusch et al., 2020). During the acute phase of AD, Th2 cytokines are markedly upregulated, facilitating immunoglobulin E (IgE) production, eosinophil infiltration, and suppression of Th1 responses, thereby amplifying Th1/Th2 imbalance (Spergel et al., 1999). Among these, IL-4 and IL-13 have been identified as central pathogenic mediators that induce inflammation, impair skin barrier integrity, and provoke pruritus (Sehra et al., 2010; Bieber, 2020). In the chronic phase, Th1-related activity becomes predominant, characterized by increased production of IFN-γ and TNF-α (Moss et al., 2004), which further contribute to tissue damage and sustained immune dysregulation (Salama and Fowell, 2020). Several flavonoids have been reported to restore Th1/Th2 balance by targeting key signaling pathways. Apigenin has been reported to modulate allergic responses in both in vitro and in vivo models. In IgE-sensitized rat basophilic leukemia cells (RBL-2H3) cell model, apigenin treatment was associated with the suppression of FcεRI-mediated activation and downstream MAPK signaling, accompanied by reduced levels of Th2 cytokines (IL-4, IL-5, IL-6, IL-13) and TNF-α (Park C.-H. et al., 2020). Complementary findings in a compound 48/80-induced AD mouse model demonstrated attenuation of mast cell activation and IL-31-associated signaling pathways (Che et al., 2019). Similarly, in HaCaT model, myricetin treatment was associated with the downregulation of T-bet and GATA3 expression, accompanied by decreased levels of IL-4 and IFN-γ, suggesting a potential role in modulating Th1/Th2-associated immune signaling (Hou et al., 2022).
In addition to Th1 and Th2 subsets, Th17 cells and their associated cytokines are also involved in the immunopathogenesis of AD. In acute AD lesions, Th17-derived cytokines such as IL-17 and IL-22 are markedly elevated (Simon et al., 2014), which aggravates skin inflammation and disrupts epidermal barrier integrity (Asarch et al., 2008; Korn et al., 2009). The differentiation of Th17 cells is regulated by the lineage-specific transcription factor retinoic acid-related orphan receptor gamma t (RORγt) (Zhao et al., 2021; Hall et al., 2022). Luteolin has been observed to inhibit the Janus kinase 2 (JAK2)/STAT3 signaling pathway in AD mouse model, along with reduced IL-17 expression and diminished Th17-mediated inflammatory responses (Tang et al., 2022). Experimental evidence from AD mouse models suggests that 7-methoxyisoflavone exhibits dual immunomodulatory effects in both Th2- and Th17-predominant settings. In the Th2-driven FITC-induced model, it is associated with the downregulation of IL-4 and IFN-γ, while in the Th17-driven oxazolone (OXZ) model, it suppresses phosphorylated STAT3 (p-STAT3) and IL-17A expression, ultimately contributing to the coordinated regulation of Th1, Th2, and Th17 immune axes (Dong et al., 2022). Experimental studies integrating both in vitro and in vivo models have investigated the immunomodulatory properties of sophoricoside. In vitro, sophoricoside was shown to inhibit CD4+ T cell differentiation into Th1 (T-bet), Th2 (GATA3), and Th17 (RORγt) subsets, accompanied by reduced expression of IFN-γ, IL-4, and IL-17A. In a corresponding AD mouse model, sophoricoside treatment was associated with attenuated inflammatory cell infiltration and cytokine production in lesional skin, thereby mitigating AD-like pathological manifestations (Kim and Lee, 2021).
4.3 Modulation of chemokine dysregulation
Chemokines are small, secreted proteins produced by both structural and immune cells that regulate immune responses at sites of inflammation by directing immune cell migration through interactions with G protein-coupled receptors, including CC chemokine receptors (CCR) and CXC chemokine receptors (CXCR) (Liu J. et al., 2021). In AD, chemokines serve as critical mediators bridging innate and adaptive immunity (Sun et al., 2021), as part of the broader mechanisms illustrated in Figure 4. They coordinate the temporal recruitment of immune cell subsets and facilitate the progression from acute to chronic inflammation (Nedoszytko et al., 2014). In addition, chemokines play essential roles in driving Th2-skewed immune responses, disrupting skin barrier function, and amplifying downstream inflammatory signaling cascades (Kitahata et al., 2022).
During the acute phase of AD, Th2-associated chemokines play a dominant role in promoting inflammation. C-C motif chemokine ligand 17 and 22 (CCL17 and CCL22), predominantly secreted by keratinocytes and dendritic cells, bind to C-C chemokine receptor 4 (CCR4) and selectively recruit Th2 cells to lesional skin. These chemokines are widely recognized as key biomarkers of disease activity (Carola et al., 2021; He et al., 2021). Quercetin has been reported to attenuate Th2-mediated inflammation in MC903-induced AD mouse model along with reduced expression of CCL17 and CCL22, which may contribute to reduced Th2 cell infiltration. In HaCaT model, quercetin treatment was associated with decreased secretion of CCL17 and CCL22 secretion, potentially through modulation of the TLR2/6 and MAPK signaling pathways (Hou et al., 2019). Similarly, other flavonoids such as 7,3′,4′-trihydroxyisoflavone, skullcapflavone II, and orobol have been reported to downregulate CCL17 and CCL22 expression both in mouse and HaCaT AD models. These effects appear to be primarily associated with the modulation of signaling pathways such as MAPK, NF-κB, and STAT. By interfering with chemokine-mediated immune cell recruitment, these flavonoid metabolites have been associated with attenuated cutaneous inflammation, including erythema, pruritus, and barrier disruption (Park S. H. et al., 2020; Lee Y. et al., 2022; Lee et al., 2022 C. H.).
In addition to Th2-associated chemokines, CCL5 is also significantly upregulated in AD, although it is not Th2-specific. This chemokine acts on Th1 cells, eosinophils, and mast cells to promote immune cell infiltration and epidermal hyperplasia, which functions as a mediator linking acute and chronic phases, as supported by its upregulation across disease stages (Yu et al., 2020; Matsui et al., 2022). Recent studies have reported that chrysin mitigates inflammatory cell infiltration and histopathological alterations in AD mouse model, potentially via suppression of the NF-κB signaling pathway and downregulation of CCL5 expression (Yeo et al., 2020).
As inflammation progresses, neutrophil-associated CXC chemokines play an increasingly prominent role in the pathogenesis of AD. Among them, C-X-C motif chemokine ligand 1 and 2 (CXCL1 and CXCL2) are significantly upregulated in lesional skin and primarily mediate neutrophil recruitment through interaction with the CXCR2 receptor, thereby aggravating tissue damage and acute symptoms such as erythema and edema (Sakai et al., 2021). Transcriptomic analyses by Chen et al. revealed that CXCL1 and CXCL2 are highly enriched in IL-17-related signaling pathways, which participate in acute immune responses triggered by microbial coinfections (Chen et al., 2019). Experimental evidence indicates that skullcapflavone II downregulates CXCL1 expression in MC903-induced AD mouse model, which is associated with reduced infiltration of CD4+ T cells, eosinophils, and neutrophils (Lee Y. et al., 2022). In addition, 7-methoxyisoflavone has been reported to significantly suppress CXCL1, CXCL2, and CXCL3 expression in both FITC- and oxazolone-induced AD models, which was associated with reduced neutrophil infiltration and attenuated skin erythema and edema (Dong et al., 2022).
CXCL15, a murine neutrophil chemotactic factor, is markedly upregulated during the early inflammatory stage in 2,4-dinitrochlorobenzene (DNCB)-induced model of AD. It acts synergistically with CXCL1 and CXCL2 to promote neutrophil infiltration and enhance inflammatory cell accumulation in the skin (Zheng et al., 2023). In carvacrol- and 2,4-dinitrofluorobenzene (DNFB)-induced mouse models, scutellarein treatment was associated with reduced secretion of CXCL15 and related pro-inflammatory cytokines, thereby alleviating AD-like symptoms (Wang Y. et al., 2022).
As AD progresses into the chronic phase, Th1-mediated immune responses become increasingly dominant, characterized by the sustained overexpression of IFN-γ-induced chemokines such as CXCL9, CXCL10, and CXCL11. These chemokines play a critical role in maintaining chronic inflammation and driving tissue damage (Drozhdina and Suslova, 2021). Studies by Renert-Yuval et al. (2021) and Kibalina et al. (2022) have shown that CXCL9, CXCL10, and CXCL11 are significantly upregulated and co-expressed with IFN-γ in the lesional skin of adult AD patients, further substantiating their role in the Th1-dominant inflammatory axis. Puerarin has been reported to suppress the expression of Th1-associated chemokine (CXCL9, CXCL10, CXCL11) in TNF-α/IFN-γ-stimulated HaCaT model, and to attenuate inflammation and skin tissue damage in a DNCB-induced AD mouse model (Lee et al., 2018).
4.4 Modulation of itch signaling pathways
Persistent or recurrent pruritus is a hallmark symptom of AD, and its pathogenesis is driven by complex interactions among upstream inflammatory mediators, neuronal sensitization mechanisms, and immune-driven processes (Tominaga and Takamori, 2022). Recent evidence suggests that AD-associated itch is mediated by a broad spectrum of signaling molecules, including pro-inflammatory cytokines such as IL-31 (Duca et al., 2022), IL-33 (Nakajima et al., 2021) and TSLP (Meng J. et al., 2021); sensory ion channels such as members of the transient receptor potential (TRP) family (Meng J. et al., 2021); immune effector events such as IgE-mediated mast cell degranulation (Mollanazar et al., 2016); and proteolytic mediators including Cathepsin S (CTSS) (Ruppenstein et al., 2021). These factors may act synergistically amplify peripheral itch perception and contribute to the persistence and exacerbation of chronic pruritus through multiple signaling cascades, as partially illustrated in Figure 4 and in greater detail in Figure 5.
FIGURE 5
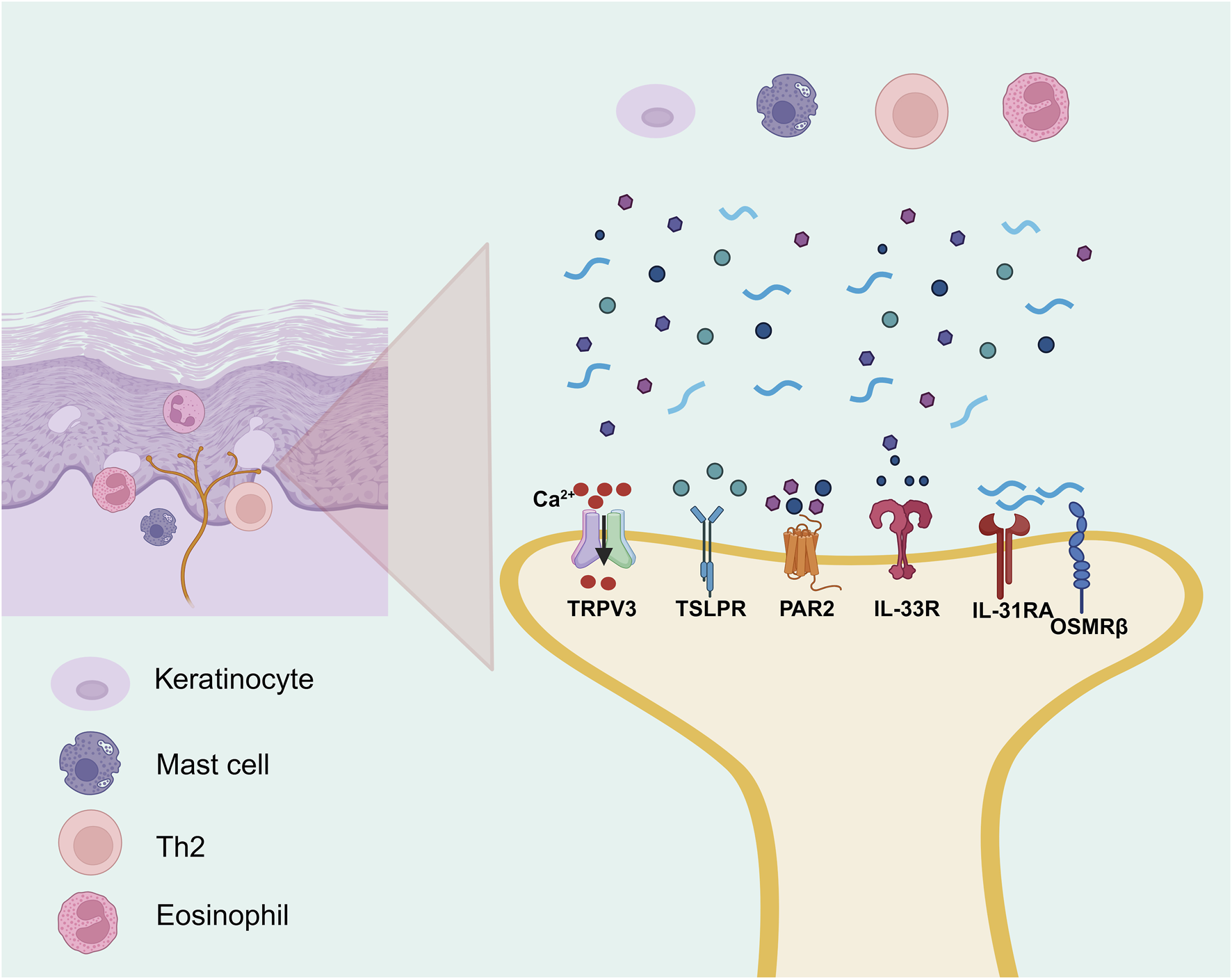
The itch signaling underlying AD (the part that flavonoids can intervene in). By BioRender. Note: Th2, T helper 2 cell; TSLPR, thymic stromal lymphopoietin receptor; IL-13RA, interleukin-13 receptor alpha; OSMRβ, oncostatin M receptor beta; IL-33R, interleukin-33 receptor; PAR2, protease-activated receptor-2; TRPV3, transient receptor potential vanilloid 3.
TSLP and IL-33 serve as upstream alarmins in AD, initiating both immune and sensory signaling. TSLP is abundantly expressed in keratinocytes of AD lesions and promotes Th2-type inflammation while directly activating transient receptor potential ankyrin 1 (TRPA1) channels on sensory neurons, enhancing pruritus (Wilson et al., 2013; Meng J. et al., 2021). It also upregulates IL-33 and synergizes to suppress barrier proteins such as filaggrin and claudin-1, exacerbating epidermal dysfunction (Dai et al., 2022). Upon cellular damage, IL-33 activates type 2 innate lymphoid cells (ILC2s) and basophils to produce IL-5 and IL-13, and simultaneously enhances IL-31 signaling, forming a self-reinforcing loop of inflammation and itch (Imai, 2023; Yamamura et al., 2024). As a Th2 effector, IL-31 correlates with itch severity and signals via IL-31 receptor alpha (IL-31RA)/Oncostatin M receptor beta (OSMRβ) receptors on sensory nerves to release calcitonin gene-related peptide and drive neurogenic inflammation (Fassett et al., 2023). Several flavonoids have been reported exhibit pharmacological effects in alleviating itch-associated inflammatory responses in AD models. In DNCB-induced AD mouse model, puerarin treatment was associated with reduced expression of pruritogenic cytokines including IL-31 and TSLP, indicating its potential anti-inflammatory activity (Lee et al., 2018). In addition, in a Dermatophagoides farinae extract and DNCB-induced AD mouse model, rutin was reported to downregulate Th2-type cytokines including IL-31, IL-4, and IL-13, along with decreased serum histamine levels and suppressing mast cell infiltration in lesional skin (Choi and Kim, 2013).
In addition to classical Th2 cytokines, non-canonical sensory pathways are essential contributors to chronic pruritus in AD. CTSS, a protease strongly associated with itch, is markedly upregulated in AD lesions and sustains neuroimmune activation by cleaving PAR2 or directly stimulating sensory nerve endings (Ruppenstein et al., 2021). In parallel, the transient receptor potential vanilloid 3 (TRPV3) calcium channel, overexpressed in keratinocytes and sensory neurons, increases neuronal excitability and contributes to persistent itch (Meng J. et al., 2021). In HaCaT model, skullcapflavone II was reported to suppress the expression of CTSS, a pruritogenic protease, through inhibition of the STAT1/NF-κB/p38 MAPK signaling pathway (Lee Y. et al., 2022). Likewise, in AD mouse model, scutellarein was reported to inhibit TRPV3 channel activity, accompanied by reduced, accompanied by reduced neural hyperexcitability, lower serum IgE levels, and decreased expression of proinflammatory cytokines including IL-1β, TNF-α, IL-4, IL-6, and CXCL15 (Wang Y. et al., 2022).
During the acute phase of AD, the interplay between immune cells and the peripheral nervous system plays a pivotal role in initiating and amplifying pruritus (Steinhoff et al., 2022). Among these mechanisms, IgE-mediated mast cell degranulation serves as a key driver of immediate itch responses. Upon activation, mast cells rapidly release pruritogenic mediators such as histamine, prostaglandin D2 (PGD2), tryptase, and β-hexosaminidase (Poto et al., 2022), which collectively stimulate C-type sensory nerve fibers and trigger neuroimmune amplification (Siiskonen and Harvima, 2019). Tryptase can further potentiate itch signal transmission by activating sensory neurons through PAR2. Several natural metabolites have shown inhibitory effects on mast cell-mediated pruritic signaling. For instance, Daphnopsis costaricensis Barringer and Grayum extract identified with 11 flavonoid metabolites and stechamone were observed to suppresse β-hexosaminidase release, reduce mast cell degranulation and IL-4 production, and were associated with attenuated scratching behavior in mouse AD models (Jo et al., 2018; Bae et al., 2022). Chamaejasmine was also observed to attenuate degranulation in IgE-sensitized RBL-2H3 and was associated with reduced serum levels of histamine, IgE, and IL-4 in AD mouse model (Jo et al., 2018; Kim et al., 2019). Moreover, luteolin has been shown to reduce serum IgE levels and attenuate scratching behavior in AD mouse model (Choi et al., 2010), with additional evidence showing its pharmacological effects in various pruritus models suggests that luteolin may inhibit mast cell degranulation and suppression of IL-4/IgE and PGD2–IL-33 signaling pathways (Gendrisch et al., 2021).
4.5 Regulation of skin and gut microbial homeostasis
In AD, the disruption of skin and gut microbial homeostasis is increasingly recognized as a critical factor contributing to the initiation and persistence of chronic inflammation and epithelial barrier dysfunction (Pessôa et al., 2023; Chen et al., 2024), as part of the broader mechanisms illustrated in Figure 4. Under normal conditions, commensal skin microbiota help preserve homeostasis by regulating cutaneous pH, promoting barrier protein expression, and shaping local immune responses (Naik et al., 2012). Among them, Staphylococcus epidermidis and other resident species strengthen skin integrity and immune tolerance by producing antimicrobial peptides, maintaining an acidic microenvironment, and preventing pathogenic colonization (Olesen et al., 2021).
In patients with AD, cutaneous microbial diversity is markedly reduced, often accompanied by predominant colonization by S. aureus (Staphylococcus aureus), which contributes significantly to recurrent inflammation and skin barrier dysfunction (Demessant-Flavigny et al., 2023). Notably, the hands, as characteristically dry sites, harbor distinct microbial communities that help maintain acidic pH and microbial balance (Huang et al., 2025); however, repeated scratching disrupts this equilibrium, promotes pH neutralization, and facilitates S. aureus adhesion and inflammatory responses (Bay et al., 2025).
Staphylococcus aureus in AD skin has also been shown to accumulate in keratinocyte lysosomes and trigger IL-1α secretion via TLR9 activation, thereby promoting inflammation (Moriwaki et al., 2019). It also secretes multiple virulence factors such as superantigens, α- and δ-toxins, and lipoteichoic acid (LTA), which activate T cells to produce IL-4, IL-5, TSLP, and IL-31 (Chung et al., 2022); collectively promote IgE class switching and Th2 polarization (Gough et al., 2022); and contribute to heightened pruritus and inflammation in AD (Aziz et al., 2024). In parallel, S. aureus downregulates the expression of key barrier-associated proteins such as filaggrin and loricrin (Cau et al., 2021), compromising keratinocyte cohesion and exacerbating skin barrier dysfunction (Demessant-Flavigny et al., 2023). A mechanism-oriented systematic review identified 85 flavonoid metabolites with anti-MRSA activity, approximately 70% of which demonstrated potent in vitro pharmacological effects (MIC ≤16 μg/mL) via membrane disruption, biofilm inhibition, and efflux pump suppression, suggesting their potential suitability for topical application in managing S. aureus-associated skin microbiota dysbiosis (Xu et al., 2024). Among them, a study reported that pure EGCG, at a concentration of 80 μg/mL, achieved a 99.999% (log5) reduction in clinical MRSA isolates within 4 h, indicating potent antibacterial effects (Aljuffali et al., 2022; Feilcke et al., 2023). Given EGCG’s multi-target pharmacological effects in AD models (Śladowska et al., 2025), incorporation of an S. aureus-induced model may facilitate investigation into its potential microbiota-modulating mechanisms. This model is characterized by pronounced Th1/Th17 inflammation and microbial dysbiosis, thereby providing a relevant experimental platform to examine how flavonoids contribute to restoring gut–skin axis homeostasis in AD (Zhang et al., 2024).
In addition to its local cutaneous effects, gut microbiota dysbiosis plays a significant role in systemic immune regulation via the gut–skin axis (Melli et al., 2020). Patients with AD frequently exhibit reduced gut microbial diversity (Melli et al., 2020), characterized by decreased abundance of beneficial genera such as Lactobacillus app. and Bifidobacterium app., along with increased abundance of pro-inflammatory taxa including Clostridioides difficile (Ahn, 2023). This dysbiosis leads to impaired production of short-chain fatty acids, disruption of the intestinal epithelial barrier (Pessôa et al., 2023), reduced differentiation of Tregs, and exaggerated Th2-type immune responses, all of which contribute to heightened skin sensitivity and inflammation (Chen et al., 2024). Emerging studies have demonstrated that glycosylated flavonoids exert microbiota-modulatory effects by selectively enriching beneficial bacterial taxa and suppressing pathogenic bacteria, thereby contributing to the reestablishment of gut microbial homeostasis (Pan et al., 2023; Xiong et al., 2023). Recent study suggests that the flavonoid baicalin may modulate gut microbiota composition and is associated with the alleviation of AD-like phenotypes. In DNCB-induced AD mouse model, baicalin was found to modulate gut microbial balance by increasing the abundance of Lactobacillus app. and Coprococcus 1 app., while reducing Parabacteroides app. and Alistipes app., an effect further supported by fecal microbiota transplantation experiments (Wang L. et al., 2022).
The downregulation of antimicrobial peptides (AMPs) is an important factor contributing to the increased susceptibility of AD patients to microbial infections (Ong et al., 2002). In lesional skin, the expression of key AMPs, including human β-defensin 1, 2, and 3 (HBD-1, HBD-2, HBD-3), the cathelicidin LL-37 (Szabó et al., 2023), and its encoding gene cathelicidin antimicrobial peptide (CAMP) (Ma et al., 2022), is markedly reduced. This reduction compromises the skin’s innate immune defense capacity. Apigenin has been shown to robustly upregulate AMP expression in both in vivo and in vitro models. In a C57BL/6J mouse model of AD, apigenin significantly upregulated the expression of CAMP and mouse β-defensin 3 (mBD3), which may contribute to increased local antimicrobial activity (Hou et al., 2013). Similarly, in HaCaT model, apigenin was found to upregulate the expression of HBD-1, HBD-2, HBD-3, and LL-37, which may enhance antimicrobial peptide-mediated defenses under inflammatory conditions (Park C.-H. et al., 2020).
4.6 Modulation of programmed cell death
In AD pathogenesis, programmed cell death maintains immune homeostasis, modulates inflammation, and supports skin barrier integrity (Lossi, 2022; Tanaka et al., 2022). Beyond classical apoptosis, necroptosis (Luo C.-H. et al., 2024) and activation-induced cell death (AICD) (Cencioni et al., 2015) have also been implicated in AD progression and the regulation of immune and epithelial cell function (Figure 6).
FIGURE 6
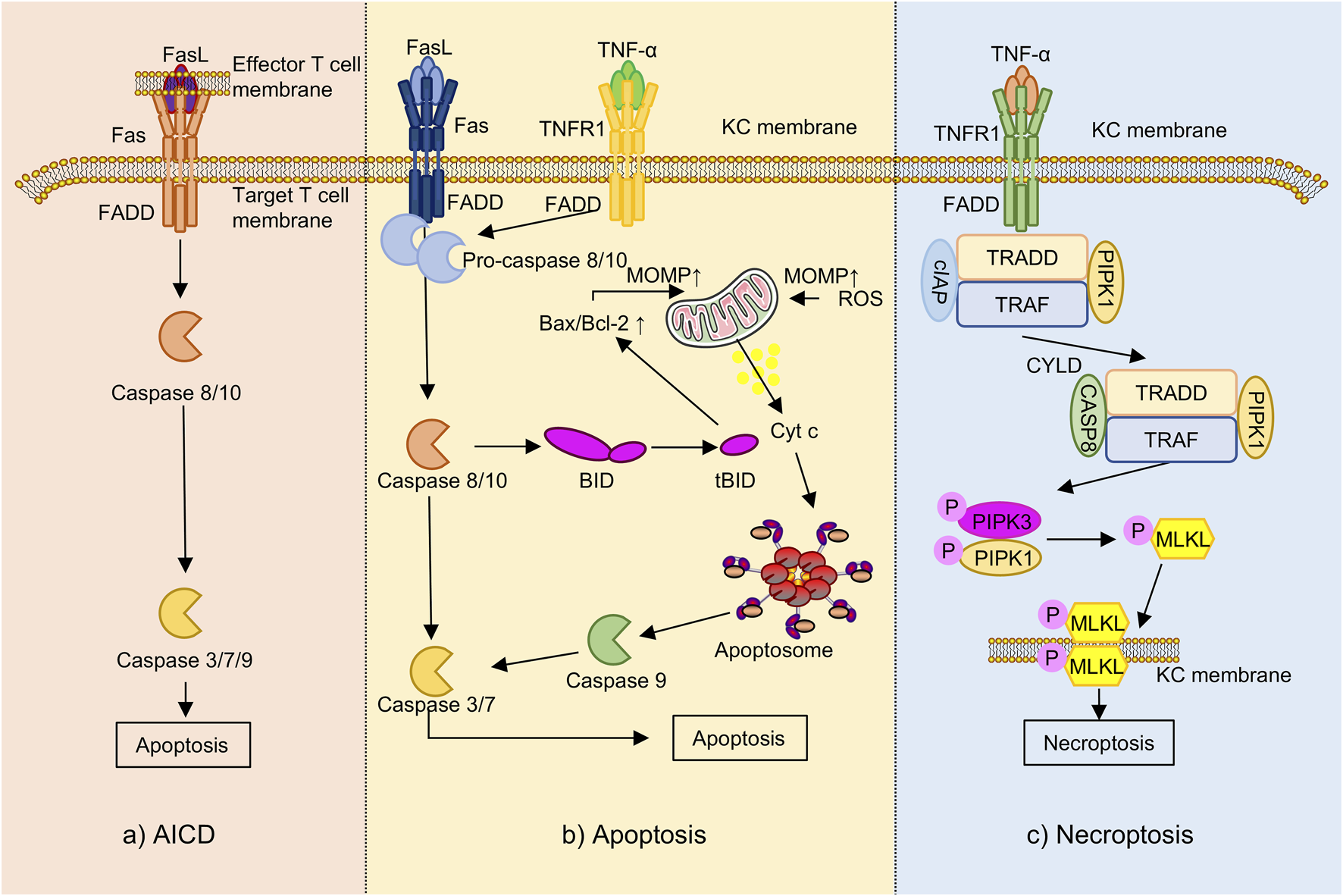
The programmed cell death underlying AD (the part that flavonoids can intervene in). Note: Fas, Fas cell surface death receptor; FasL, Fas ligand; FADD, Fas-associated death domain; TNFR1, tumor necrosis factor receptor 1; TRAF, TNF receptor-associated factor; cIAP, cellular inhibitor of apoptosis protein; KC, keratinocyte; BID, BH3-interacting domain death agonist; tBID, truncated BID; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; MOMP, mitochondrial outer membrane permeabilization; Cytc, cytochrome c; ROS, reactive oxygen species; RIPK-, receptor-interacting protein kinase; MLKL, mixed lineage kinase domain-like protein; TRADD, TNFR1-associated death domain protein; CYLD, cylindromatosis tumor suppressor; AICD, activation-induced cell death.
Apoptosis is a caspase-dependent form of programmed cell death that proceeds through two major pathways: the extrinsic Fas cell surface death receptor/Fas ligand (Fas/FasL) axis and the intrinsic mitochondrial pathway (Rebane et al., 2012). In the extrinsic pathway, IFN-γ secreted by cutaneous lymphocyte-associated antigen-positive (CLA+) T cells upregulates Fas expression on keratinocytes (Trautmann et al., 2000), rendering them sensitive to FasL-mediated signaling. This cascade activates caspase-8 and downstream caspase-3 (Takahashi et al., 1999), leading to DNA fragmentation and keratinocyte apoptosis (Simon et al., 2006).
The intrinsic pathway is typically activated by TNF-α or oxidative stress, which disrupts the Bcl-2-associated X protein to B-cell lymphoma 2 (Bax/Bcl-2) ratio, promoting mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release (Tait and Green, 2010), which subsequently activates caspase-9 and initiates the apoptotic cascade (Martinou and Youle, 2011). In AD, elevated expression of Fas and FasL increases keratinocyte vulnerability to IFN-γ-induced apoptosis (Rebane et al., 2012), thereby contributing to skin barrier breakdown and exacerbation of eczematous inflammation (Szymanski et al., 2018). In the TNF-α-stimulated HaCaT model, baicalin treatment was associated with attenuation of inflammatory injury via inhibition of the STAT3/NF-κB signaling pathways. This effect involved reduced production of pro-inflammatory cytokines and modulation of apoptotic regulators including Bax, Bcl-2, and caspase-3 (Wu et al., 2020).
Necroptosis is a regulated, caspase-independent form of programmed cell death mediated by the receptor-interacting protein kinase 1 (RIPK1), RIPK3, and mixed lineage kinase domain-like protein (MLKL) signaling axis (Fritsch et al., 2019). Although mechanistically distinct, it mimics necrosis morphologically, including membrane rupture and inflammatory content release (Karlowitz and van Wijk, 2023). Under caspase-8 inhibition, RIPK1/RIPK3 phosphorylation initiates necrosome formation and MLKL activation, triggering membrane breakdown and the release of pro-inflammatory damage-associated molecular patterns (DAMPs) (Fritsch et al., 2019; Meng Y. et al., 2021). In AD, TNF-α, oxidative stress, or microbial cues induce keratinocyte necroptosis, resulting in IL-33 release and ILC2 activation, thereby promoting Th2-skewed inflammation. This necroptotic axis is significantly elevated in AD lesions and correlates with disease severity, highlighting its role in chronic inflammation (Luo C.-H. et al., 2024). In a mouse model of AD, treatment with EGCG-loaded nanoparticles (EGCG-NPs) was associated with decreased expression of necroptosis-related markers including RIPK1, RIPK3, and MLKL, as well as a reduction in Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling- (TUNEL-) positive keratinocytes, suggesting potential inhibition of keratinocyte necroptosis (Han et al., 2022). Additionally, the flavonoid nepetin has been reported to attenuate oxidative stress, suppress pro-inflammatory cytokines production, and reduce cell death by modulating the myeloid differentiation primary response 88 (MyD88) –mitogen-activated protein kinase kinase 3/6 (MKK3/6) –Akt signaling pathway, a potential role in the regulation of necroptosis or other non-apoptotic forms of programmed cell death (Gong et al., 2024).
AICD is a specialized form of programmed cell death that occurs in chronically stimulated T cells following prolonged antigen exposure, serving as a critical mechanism to maintain immune homeostasis and peripheral tolerance (Arakaki et al., 2014). AICD is primarily mediated through the Fas/FasL signaling axis. Upon sustained activation, T cells upregulate FasL, which binds to Fas receptors on themselves or neighboring cells, initiating caspase-8 activation and leading to apoptosis (Zhang et al., 2000; Nakano et al., 2007). This leads to the downstream activation of effector caspases, including caspase-3, -7, and -9, ultimately resulting in apoptotic cell death. Both in vitro and ex vivo studies have shown that Th1 cells exhibit greater susceptibility to AICD than Th2 cells, a difference largely attributed to more efficient Fas ligand surface expression in Th1 cells, which in turn contributes to the Th2-biased immune profile observed in AD (Oberg et al., 1997; Akdis et al., 2003). Recent research has reported that in DNCB and dust mite-induced AD mouse models, kaempferol attenuates disease symptoms, potentially through modulation of T cell overactivation and apoptosis. Mechanistic in vitro findings suggest that kaempferol preserves the expression of anti-apoptotic and apoptotic regulatory proteins, including Bcl-2 and caspase-3, -7, and -9, thereby reducing AICD-induced cell death in T cells (Lee and Jeong, 2021). Additionally, in vivo evidence indicates that kaempferol improves skin barrier function and attenuates oxidative stress (Nasanbat et al., 2023).
4.7 Attenuation of oxidative stress and redox imbalance
Oxidative stress is increasingly recognized as a central pathogenic factor in the progression of AD (Galiniak et al., 2022), as shown in Figure 7. In lesional skin, excessive accumulation of ROS compromises the epidermal barrier, facilitates TEWL and allergen penetration, activates pro-inflammatory signaling cascades, and disrupts immune homeostasis (Raimondo et al., 2023). Together, these processes establish a self-perpetuating cycle of oxidative damage, inflammation, and immune dysregulation that contributes to the chronicity and severity of the disease.
FIGURE 7
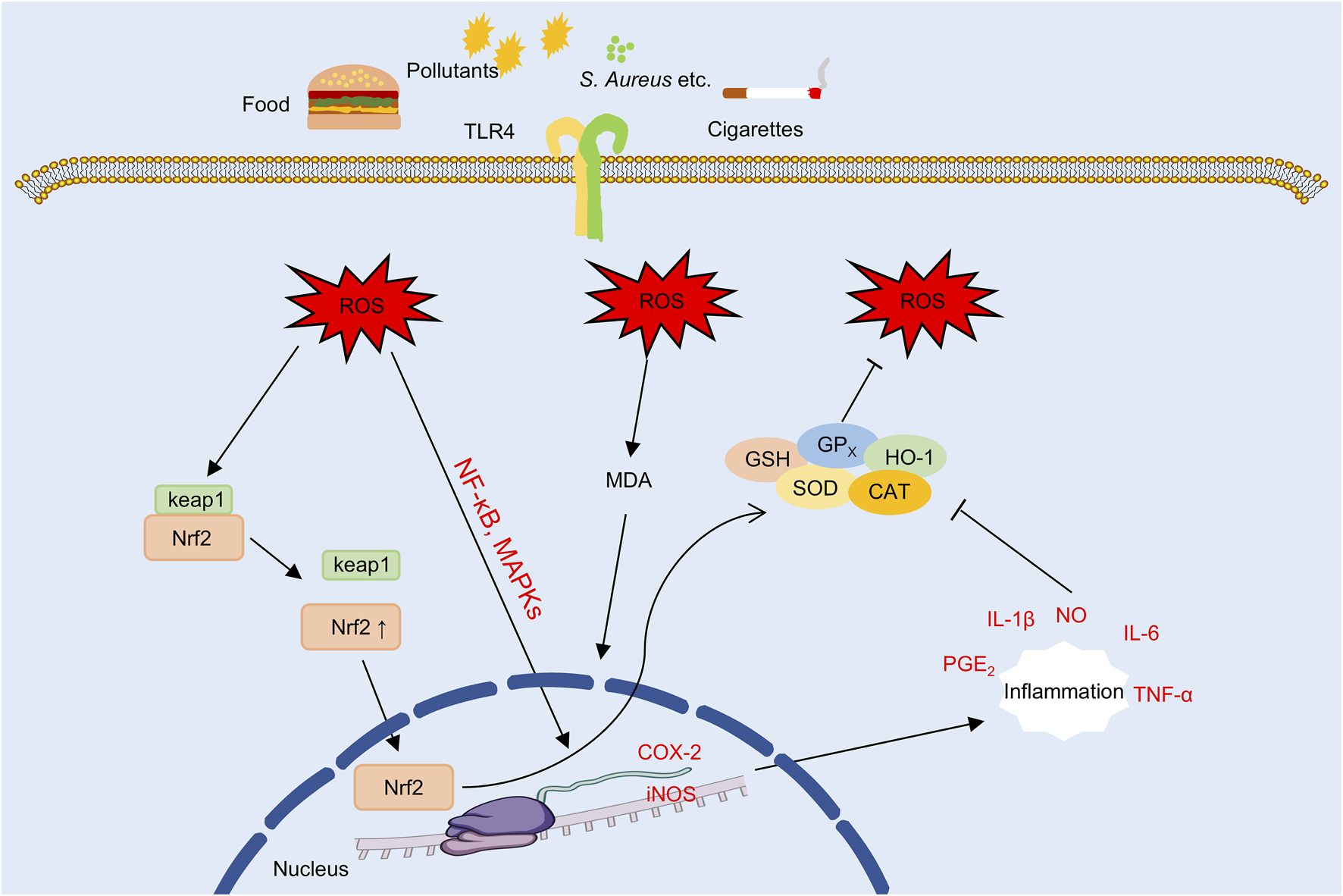
The oxidative stress underlying AD (the part that flavonoids can intervene in). Note: TLR4, Toll-like receptor 4; ROS, reactive oxygen species; MDA, malondialdehyde; NF-κB, nuclear factor kappa B; MAPK, mitogen-activated protein kinase; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; GSH, glutathione; GPX, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; HO-1, heme oxygenase-1; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; NO, nitric oxide; PGE2, prostaglandin E2.
Persistent accumulation of ROS induces lipid peroxidation in keratinocyte membranes and structural proteins, resulting in impaired barrier function and increased TEWL (Yang et al., 2022; Berdyshev, 2024). In parallel, ROS activate inflammatory signaling cascades such as the NF-κB and MAPK pathways, which promote the expression of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α, thereby reinforcing inflammation through a self-amplifying loop (Yang et al., 2022; Herath et al., 2024). Experimental evidence indicates that butein reduces ROS generation and suppresses IL-6 and intercellular adhesion molecule-1 (ICAM-1) expression in TNF-α-stimulated HaCaT model. These effects are associated with inhibition of MAPK and NF-κB pathway activation, suggesting potential antioxidant and skin barrier-protective properties activities in vitro (Seo et al., 2015). Similarly, in LPS-stimulated HaCaT model, nepetin has been shown to attenuate ROS accumulation and reduce the production of inflammatory cytokine, potentially through modulation of the MyD88-MKK3/6–Akt–NF-κB signaling pathway (Gong et al., 2024).
Downstream of ROS accumulation, COX-2 and iNOS are key pro-inflammatory enzymes that amplify oxidative and immune-mediated damage. COX-2 catalyzes the synthesis of prostaglandin E2 (PGE2) (Ilari et al., 2020), while iNOS produces nitric oxide (NO), which together contribute to the formation of reactive nitrogen species (RNS) (Zhao et al., 2022), thereby exacerbating tissue inflammation and oxidative injury. In addition, ROS promote Th2-skewed immune responses and impair Treg function (Nakajima et al., 2021), resulting in the upregulation of type 2 cytokines such as IL-4 and IL-13 (Fu Z. et al., 2024), which further exacerbate chronic allergic inflammation in AD. Among natural antioxidants, in LPS-stimulated RAW264.7 macrophages, apigenin has been shown to suppresses the expression of COX-2, iNOS, as well as the inhibition of the MAPK signaling pathway and in IgE-sensitized RBL-2H3 cells, it suppresses of the FcεRI signaling pathway, suggesting potential anti-allergic and anti-inflammatory activities (Park C.-H. et al., 2020). Similarly, quercetin and its derivatives have been shown to downregulate the expression of iNOS, COX-2, and Th2-associated cytokines (IL-4, IL-5, and TSLP), while reducing serum IgE levels and eosinophil counts in AD mouse model, suggesting that they may concurrently mitigate oxidative stress and modulate immune responses (Jafarinia et al., 2020).
To counteract the toxicity of ROS, the body depends on a complex network of endogenous antioxidant systems, including SOD, glutathione (GSH), glutathione peroxidase (GPx), and heme oxygenase-1 (HO-1) (Jegadheeshwari et al., 2024), which collectively maintain cellular redox homeostasis (Meca et al., 2021). Among these, SOD and GPx function cooperatively to neutralize ROS (Bhat et al., 2014; Ighodaro and Akinloye, 2018), GSH preserves the intracellular reducing environment, and HO-1 expression is upregulated by the nuclear factor erythroid 2-related factor 2 (Nrf2) (Liu F. et al., 2021; Peng et al., 2024), thereby enhancing cytoprotective responses. In contrast, malondialdehyde (MDA), a terminal product of lipid peroxidation, serves as a widely recognized biomarker of oxidative stress and cellular injury (Mas-Bargues et al., 2021). In DNCB-induced AD mouse model, EGCG-NPs were associated with increased activity of antioxidant enzymes such as SOD and GSH, decreased MDA levels, and enhanced total antioxidant capacity (T-AOC), collectively contributing to the restoration of epidermal redox balance (Han et al., 2022). In addition, quercetin has been reported to exert similar protective effects by activating the Nrf2/HO-1 signaling pathway, enhancing antioxidant enzyme expression, and mitigating hydrogen peroxide-induced oxidative stress in keratinocytes (Jafarinia et al., 2020).
5 Limitations and safety concerns in conventional oral and topical delivery of flavonoids
5.1 Pharmacokinetic limitations to the oral bioavailability of flavonoids
Flavonoids exhibit broad pharmacological activities, particularly in dermatological disorders. Nonetheless, their clinical translation is substantially limited by pharmacokinetic barriers. The low oral bioavailability of flavonoids results primarily from structural characteristics, transport mechanisms, metabolic clearance, and tissue distribution.
Most natural flavonoids exist predominantly as O-glycosides and C-glycosides, whose high hydrophilicity and limited lipophilicity restrict their ability to traverse the intestinal epithelium and enter systemic circulation. It is increasingly recognized that these glycosides are generally believed to require hydrolysis in the intestinal lumen by host enzymes or gut microbiota into more membrane-permeable aglycones for efficient absorption (Nakamura et al., 2020). Animal studies further confirm that unmetabolized glycosides, such as quercetin-3-O-sophoroside, cannot penetrate the intestinal epithelium (Nakamura et al., 2018). O-glycosides (e.g., isoquercitrin) are typically hydrolyzed in the upper gastrointestinal tract to release aglycones like quercetin, while C-glycosides (e.g., orientin, vitexin), due to their structural stability, are only metabolized into absorbable forms in the colon by gut microbiota (Xie et al., 2022). Notably, human gut microbiota can transform C-glycosides such as orientin and vitexin into their corresponding aglycones, luteolin and apigenin, suggesting an important role of the gut microbiota as a key contributor to their metabolic conversion (Wang S. et al., 2022).
Metagenomic analyses reveal that while O-glycosidases are widely expressed in the gut microbiota, their expression levels vary significantly among individuals, leading to highly personalized hydrolysis efficiency and absorption outcomes (Goris et al., 2021). This is supported by the observation that glycosylated flavonoids are nearly undetectable in plasma, with aglycones generally considered to be the predominant bioactive forms (Chen et al., 2022).
Flavonoid absorption is also influenced by transmembrane transport mechanisms. Studies using the Caco-2 Brush Border Expressing clone 1 cell line (Caco-2 BBe1) model demonstrate that glycosylated flavonoids are primarily absorbed via Sodium-dependent glucose transporter 1- and Glucose transporter 2-mediated active transport, whereas aglycones are mainly taken up by passive diffusion (Zhang et al., 2020). These mechanisms not only affect transepithelial flux but also play a key role in determining oral bioavailability.
Even after successful absorption into the circulatory system, flavonoids face significant first-pass metabolic barriers. In both the intestinal epithelium and liver, they are rapidly transformed into hydrophilic metabolites through phase II enzymatic reactions such as glucuronidation, sulfation, and methylation, catalyzed by enzymes including uridine 5′-diphospho-glucuronosyltransferase, sulfotransferase, and catechol-O-methyltransferase. These transformations are generally associated with reduced bioactivity and lower plasma concentrations (Najmanová et al., 2020). For instance, the oral bioavailability of morin is only 0.45%, whereas systemic exposure reaches 92.9% following intravenous administration, clearly illustrating the limiting impact of first-pass metabolism on the pharmacological potential of oral administration (Li et al., 2019).
In addition, flavonoids also undergo phase I metabolism mediated by hepatic cytochrome P450 (CYP450) enzymes. For example, EGCG can be oxidatively metabolized by CYP450 enzymes (Zhao et al., 2019), while quercetin undergoes glucuronidation by uridine 5′-diphospho-glucuronosyltransferase 1A1 to form quercetin-3-glucuronide, which can re-enter systemic circulation via enterohepatic recycling (Zhang et al., 2020). Although this recycling prolongs the residence time of flavonoids in the body, it is often accompanied by reduced bioactivity, posing challenges for maintaining consistent pharmacological activity.
Furthermore, flavonoids predominantly circulate in conjugated forms and exhibit high plasma protein binding affinity, which limits their distribution to target tissues. Although flavonoids are detectable in organs such as the liver, kidneys, and lungs, their concentrations at disease sites are typically low and transient, thereby limiting their therapeutic potential (Teng et al., 2023). Additionally, several flavonoids exhibit remarkably short plasma half-lives. For example, ikarisoside A exhibits a half-life of 3.15 h and a clearance rate of 42.9 L/h/kg (Cong et al., 2018), while isoformononetin has a half-life of just 1.9 h and an oral bioavailability of 21.6% (Raju et al., 2019). These pharmacokinetic features, characterized by rapid clearance and short systemic exposure, further challenge their preclinical-to-clinical applicability.
5.2 Limitations of conventional topical flavonoid delivery
Although certain flavonoids such as naringenin and flavanone exhibit enhanced skin permeability under inflamed or barrier-compromised conditions, their transdermal absorption remains limited in intact skin, often necessitating the use of penetration enhancers to achieve therapeutic depth (Alalaiwe et al., 2020). The stratum corneum, with its tightly packed lipid matrix, serves as a major barrier impeding the cutaneous deposition of natural bioactive metabolites including flavonoids (Wang et al., 2024).
Moreover, key flavonoids such as quercetin (Khursheed et al., 2020) and fisetin (Szymczak and Cielecka-Piontek, 2023) exhibit extremely poor aqueous solubility and high lipophilicity, resulting in limited dispersibility in topical formulations and thereby potentially limiting skin permeation. As a result, their unformulated free forms demonstrate minimal cutaneous bioavailability and markedly limited topical pharmacological potential.
In addition, flavonoid molecules generally exhibit poor physicochemical stability under conditions such as light exposure, elevated temperature, and pH fluctuations, making them prone to degradation and potentially affecting their pharmacological activity. In particular, the phenolic hydroxyl groups are highly reactive and susceptible to oxidation, cleavage, and photochemical transformation. A spectroscopic analysis involving 177 flavonoid metabolites revealed their pronounced instability under ultraviolet irradiation, with this issue being particularly relevant in topical application settings (Taniguchi et al., 2023). For example, free quercetin displays structural instability under light or oxidative stress, resulting in a substantial decline in both antioxidant and anti-inflammatory activities, potentially rendering it therapeutically ineffective (Vale et al., 2021).
5.3 Potential toxicities and safety considerations of oral flavonoids
While flavonoids are valued for their therapeutic effects, accumulating evidence indicates that high doses or prolonged exposure can result in toxicity, highlighting the need for a comprehensive safety assessment in future research.
With respect to hepatotoxicity, animal studies have demonstrated that high doses of quercetin can markedly elevate serum transaminase levels and induce lipid peroxidation as well as glutathione depletion in hepatic tissues (Singh et al., 2021), indicating that excessive quercetin intake may contribute to hepatocellular injury by activating oxidative stress pathways. Similarly, under oxidative stress, EGCG not only failed to provide protection in hydrogen peroxide-induced model but also worsened mitochondrial membrane potential loss, caspase-3 activation, and DNA fragmentation, suggesting potential pro-apoptotic and mitochondria-damaging effects under such conditions (Sahadevan et al., 2023).
In terms of genotoxicity, isoquercitrin, a quercetin derivative, has demonstrated mild mutagenic activity in the Ames test, indicating that potential DNA damage may occur under certain structural or metabolic conditions (Kapoor et al., 2022). Regarding endocrine disruption, common flavonoids such as genistein, quercetin, and apigenin have been reported to activate estrogen receptor signaling pathways at low concentrations, thereby exhibiting phytoestrogen-like activity (Zhang and Wu, 2022). These findings indicate that, at certain exposure levels, these metabolites have the potential to disrupt endocrine homeostasis and increase the risk of reproductive or developmental toxicity.
In vitro studies have shown that certain flavonoids, at high concentrations, can inhibit the growth of probiotic bacteria such as Lactobacillus app., while promoting the proliferation of opportunistic pathogens and inducing the accumulation of harmful metabolites, including H2S and NH3 (Pan et al., 2023), which may adversely affect gut microbial homeostasis under specific conditions.
Moreover, quercetin and its metabolites have been shown in vitro to exert weak to moderate inhibitory effects on key drug-metabolizing enzymes, including cytochrome P450 isoforms CYP3A4 and CYP2C19, as well as several major drug transporters (Mohos et al., 2020). These findings raise concerns that high-dose quercetin supplementation could potentially interfere with the metabolism and clearance of co-administered drugs, thereby raising the possibility of clinically relevant drug-drug interactions.
Given the documented risks associated with high-dose or long-term use of flavonoids, including hepatotoxicity, genotoxicity, endocrine disruption, gut microbiota dysbiosis, and drug interactions, it is imperative to develop optimized oral and topical delivery strategies. Rational modulation of dosage and release kinetics is equally essential to facilitate the clinical translation and standardized application of flavonoid-based therapies.
6 Delivery of flavonoids using novel strategies
6.1 Topical delivery strategies for flavonoids
Innovative transdermal delivery systems have demonstrated measurable improvements in enhancing the topical bioavailability of poorly soluble agents. For example, a micelle-in-hydrogel formulation containing only 0.075% hydrocortisone achieved a 9.2-fold increase in skin flux and a 50-fold improvement in cumulative permeation compared to a 1% commercial cream, revealing the advantage of follicular targeting in transdermal drug delivery (Yuan et al., 2020). Similarly, dissolvable microneedles loaded with 0.25% dexamethasone enabled rapid intradermal release and effectively attenuated AD symptoms, reducing epidermal thickness by >70% and spleen index by over 50%, without observable toxicity (Ben David et al., 2023). Moreover, accumulating evidence supports the potential of novel transdermal systems to enhance flavonoid delivery and therapeutic effects in preclinical AD models (Table 2).
TABLE 2
| Delivery system | Flavonoid(s) | Carrier materials | Key results | References |
|---|---|---|---|---|
| Microneedles | EGCG + Ascorbic acid | Poly (γ-glutamic acid), dissolvable microneedle | 95% EGCG retention after 4 weeks; skin delivery up to 600 μm; serum IgE reduced from 12156 to 5555 ng/mL | Chiu et al. (2021) |
| Polymeric nanoparticles | EGCG | PEG-PLGA nanoparticles | Reduced epidermal thickness from 109.4 μm to 43.6 μm; improved antioxidant enzymes (SOD, GSH, T-AOC) by 35%–60% | Han et al. (2022) |
| Hydrogel | Naringenin | Carboxymethyl cellulose/2-hydroxyethyl acrylate hydrogel | Enhanced release at pH 8.5 (73%) vs. pH 5.5 (42%); 58.8% skin permeation; >90% HaCaT cell viability | Park et al. (2018) |
| Microsponge | Naringenin | Ethyl cellulose-based microsponge, Carbopol gel | 92.3% cumulative release; skin deposition 802.9 μg/cm2; reduced ear thickness and WBC count in vivo | Nagula and Wairkar (2020) |
| Liposome | Taxifolin | Liposome | Clinical score reduction: 72.3% (TAX) vs. 82.2% (TAX + L); greater IgE suppression and hydration restoration with TAX + L | Kim et al. (2015) |
Topical delivery strategies for flavonoids in AD.
Note: EGCG, epigallocatechin-3-gallate; IgE, immunoglobulin E; PEG, polyethylene glycol; PLGA, poly (lactic-co-glycolic acid); SOD, superoxide dismutase; GSH, glutathione; T-AOC, total antioxidant capacity; WBC, white blood cells; TAX, taxifolin; TAX + L, taxifolin + liposome.
6.1.1 Microneedles
A promising approach to enhance the topical delivery of flavonoids is the use of microneedle systems, which painlessly penetrate the stratum corneum and enable direct intradermal deposition of active metabolites (Luo Z. W. et al., 2024). For example, dissolvable poly (γ-glutamic acid) microneedles co-loaded with EGCG and L-ascorbic acid retained 95% EGCG and 93% antioxidant activity after 4 weeks at 4 °C, and delivered 63% EGCG and 62% ascorbic acid up to 600 μm into the skin. A once-weekly dose (358 μg EGCG, 438 μg ascorbic acid) reduced serum IgE from 12,156 to 5,555 ng/mL and histamine from 81 to 40 pg/mL, highlighting their low-dose, high-efficiency therapeutic potential (Chiu et al., 2021).
6.1.2 Polymeric nanoparticles
Another widely explored approach for enhancing the delivery efficiency of flavonoids is nanoscale encapsulation, which enhances solubility, physicochemical stability, and skin-targeting efficiency (Huang P.-H. et al., 2016). A recent study showed polyethylene glycol-poly (lactic-co-glycolic acid) (PEG–PLGA) nanoencapsulation of EGCG was shown to reduce epidermal thickness from 109.4 μm to 43.6 μm after 21 days of topical application, accompanied by decreases in lactate dehydrogenase and MDA, and increases SOD, GSH, and T-AOC by 35%–60%. Compared to free EGCG, the nanoparticles showed greater suppression of IL-4, TNF-α, and IL-17A, which may contribute to modulating Th1/Th2/Th17 immune balance (Han et al., 2022).
6.1.3 Hydrogels
As semi-solid matrices with excellent biocompatibility and hydration properties, hydrogel-based systems have been proposed as a favorable platform for controlled flavonoid delivery and enhanced dermal deposition (Arora and Nanda, 2019). Notably, a pH-responsive carboxymethyl cellulose/2-hydroxyethyl acrylate hydrogel was reported to enhance the stability and transdermal delivery of naringenin. After 30 days at 40 °C and 75% humidity, the formulation maintained stable release. Drug release reached 73% at pH 8.5, compared to 42% at pH 5.5. Skin permeation efficiency increased to 58.8%, significantly outperforming conventional 1,3-butylene glycol systems (43.5% and 42.4%), with reduced stratum corneum retention and improved dermal deposition. No cytotoxicity was observed in HaCaT cells, with cell viability maintained above 90%, and the system achieved high dermal delivery efficiency at a moderate drug loading level of 51.5% (Park et al., 2018).
6.1.4 Microsponges
Microsponge-based carriers have been investigated as a potential platform for transdermal flavonoid delivery, providing a porous structure conducive to prolonged release and enhanced skin deposition (Grillo et al., 2019). Ethyl cellulose-based microsponges loaded with naringenin achieved a cumulative in vitro release of 92.3% within 24 h. Compared to the plain gel, the Carbopol gel formulation (NGMSG1%) exhibited a 2.1-fold release (87.5% vs. 42.4%) and achieved a skin deposition of 802.9 μg/cm2, approximately 3.7 times greater than that of the conventional formulation. In vivo, the formulation significantly reduced ear thickness and white blood cell counts, indicating sustained-release performance, improved skin retention, and notable anti-inflammatory effects (Nagula and Wairkar, 2020).
6.1.5 Liposomes
In addition, liposomal carriers have been shown to enhance transdermal absorption and immunomodulatory effects of flavonoids (Zhang and Michniak-Kohn, 2020). Topical application of 1% taxifolin (TAX) glycoside significantly attenuated TNCB-induced AD-like lesions, with clinical scores reduced by 72.3% and 82.2% in the TAX and TAX + L (liposome) groups, respectively. TAX treatment decreased TEWL and increased skin hydration, while TAX + L restored hydration to normal levels, with a strong inverse correlation between TEWL and hydration (r = −0.61). Both treatments markedly reduced serum IgE levels, with greater suppression observed in the TAX + L group, accompanied by downregulation of IL-4 and upregulation of IFN-γ. Liposomal encapsulation significantly enhanced transdermal delivery and barrier recovery, suggesting potential benefits in terms of stability, permeability, and immune modulation (Kim et al., 2015).
Taken together, these innovative delivery platforms have been shown to enhance the transdermal bioavailability of flavonoids and improve their pharmacological effects and biocompatibility. Such multi-dimensional advantages may support the rational design of flavonoid-based topical formulations with potential for clinical translation in AD and related inflammatory skin disorders.
6.2 Oral delivery strategies for flavonoids
Despite their diverse pharmacological activities, the systemic application of flavonoids remains constrained by limited aqueous solubility, low oral bioavailability, rapid metabolic clearance, and insufficient targeting to skin and immune tissues. While direct evidence in AD models remains scarce, oral delivery systems developed in oncology and inflammatory conditions may provide mechanistic insights with potential translational relevance (Vazhappilly et al., 2021).
For example, poly (lactic-co-glycolic acid) nanoparticles co-loaded with baicalin and CpG oligodeoxynucleotides promoted macrophage repolarization and T cell-mediated cytotoxicity in tumors (Han et al., 2021), whereas dual-drug nanostructured lipid carriers functionalized with R9dGR peptide achieved >73% tumor inhibition without systemic toxicity (Ma et al., 2020). In inflammatory models, cerium oxide–quercetin nanocomposites (CeO2@QU) effectively scavenged ROS and contributed to the maintenance of redox homeostasis (Wang et al., 2021). Exosome-based systems further expand this potential, providing biocompatible and targetable platforms for oral flavonoid delivery (Ferreira et al., 2022). Notably, hesperidin-loaded bovine milk exosomes enhanced oral bioavailability by approximately 2.5-fold and significantly suppressed melanoma progression in vivo with no apparent systemic toxicity (Kumar et al., 2024).
Taken together, these findings suggest a mechanistic basis for exploring for oral flavonoid delivery in AD, although targeted preclinical validation remains essential.
6.3 Limitations of novel delivery systems
Although recent studies have reported substantial improvements in the delivery efficiency and therapeutic performance of flavonoids using advanced delivery systems, including enhanced stability, bioavailability, and targeting efficiency (Costa et al., 2021; Delgado-Pujol et al., 2025), several practical and clinical limitations have been identified, which merit further investigation and refinement.
For topical administration routes, including dermal and ocular delivery, hydrogels and nanogels are often limited by suboptimal skin permeability, poor adhesiveness, and challenges in large-scale manufacturing (Grillo et al., 2019; Labie and Blanzat, 2023). While microneedle technology effectively overcomes the stratum corneum barrier, its clinical application remains constrained by low drug-loading capacity and insufficient mechanical strength (Han et al., 2025). Liposomal formulations, commonly used for local and ocular delivery, may still be affected by particle size heterogeneity, suboptimal encapsulation efficiency, and difficulties in industrial-scale production (Akar et al., 2024). Even with chitosan surface modification, such systems have shown limitations in overcoming mucosal barriers, which may reduce their retention and permeability (Khalil et al., 2020). Moreover, targeted microsponge-based gels developed for dermal diseases have exhibited variable pharmacodynamic effects and inconsistent release profiles (Nagula and Wairkar, 2020).
For oral administration, polymeric nanoparticles offer controlled release and high drug-loading potential, but are prone to protein corona formation in vivo, which compromises targeting capabilities. In addition, degradation within the gastrointestinal tract and first-pass metabolism substantially reduce systemic bioavailability, and preclinical animal findings may not consistently predict human pharmacokinetics and efficacy (Beach et al., 2024). Although hydrogels and nanogels hold promise in oral formulations, their physicochemical stability and delivery accuracy under dynamic digestive conditions remain suboptimal (Delgado-Pujol et al., 2025). Therefore, despite notable advances in enhancing flavonoid pharmacokinetics, both topical and oral delivery systems still require further optimization to meet clinical standards. In particular, greater emphasis is required on material design, biological barrier modulation, scalable fabrication, and in vivo transformation mechanisms to meet the stringent demands for safety, stability, reproducibility, and standardization necessary for clinical translation.
7 Clinical experimental research
Recent clinical investigations have begun to explore the therapeutic potential of natural flavonoids in AD, with early-phase studies suggesting possible reductions in inflammation, pruritus, and skin barrier impairment. Notably, flavonoids such as quercetin, silymarin, EGCG, and grape seed proanthocyanidins have shown reported to exhibit beneficial effects in topical and systemic interventions, with preliminary data indicating acceptable safety profiles (Table 3).
TABLE 3
| Metabolites/Drugs | Population | Design | Intervention | Outcomes | References |
|---|---|---|---|---|---|
| Silymarin + Fumaria officinalis L. extract | 40 eczema patients | Randomized, double-blind controlled clinical trial | Topical cream with Fumaria officinalis L. extract and silymarin vs. mometasone 0.1% | SCORAD score reduced from 26.05 ± 7.1 to 6.94 ± 2.6 in botanical drug group (p = 0.04); comparable to mometasone group (27.66 ± 5.9 to 4.77 ± 1.6, p = 0.03); no adverse effects reported | Iraji et al. (2022) |
| Quercetin | 30 healthy volunteers; UV, histamine, SLS, GA-induced skin stress | Single-blind clinical study | Topical Quercevita® (1% quercetin phytosome) | Quercetin 1% significantly reduced erythema (−10.05%, p = 0.00329), wheal diameter (−13.25%), and itch VAS score after histamine stimulation; improved hydration and reduced TEWL vs. placebo | Maramaldi et al. (2016) |
| Silymarin | Number of subjects not specified; topical application in AD | Formulation development and clinical application | Silymarin-loaded (PLO) | Optimized PLO (20% pluronic, 3% lecithin) showed enhanced silymarin skin penetration; In vivo topical application significantly reduced erythema, swelling, and inflammation in AD patients | Mady et al. (2016) |
| EGCG + Vitamin E + Grape seed procyanidins | 44 patients with mild-to-moderate AD (face/neck) | Randomized, controlled, double-blind clinical study | MD2011001 cream (vitamin E, EGCG, grape seed procyanidins) | MD2011001 reduced Investigator’s Global Assessment (IGA) score significantly over 28 days; faster lesion area reduction vs. placebo; well tolerated on face and periocular regions (n = 44) | Patrizi et al. (2016) |
| Whey protein + Dodder (Cuscuta campestris Yunck.) seed extract | 52 adults with moderate-to-severe AD | Randomized, double-blind, placebo-controlled clinical trial | Oral whey protein with Cuscuta campestris Yunck. extract | After 15-day treatment, skin moisture and elasticity significantly increased (p < 0.001); pruritus and sleep disturbance significantly reduced at day 15 (p < 0.05) and day 30 (p < 0.001); pigmentation also decreased (p < 0.001); no serious adverse effects | Mehrbani et al. (2015) |
Clinical experimental research.
Note: EGCG, epigallocatechin-3-gallate; GA, glycolic acid; PLO, pluronic-lecithin organogel; SCORAD, SCORing, Atopic Dermatitis; SLS, sodium lauryl sulfate; UV, ultraviolet radiation; VAS, visual analog scale; TEWL, transepidermal water loss.
As a classical flavonoid complex, silymarin presents notable challenges in transdermal delivery, which have prompted formulation innovations. A preclinical study developed a Pluronic lecithin organogel (PLO) system to enhance the dermal delivery of silymarin. Topical application in AD patients resulted in visible reductions in erythema and inflammation (Mady et al., 2016). Although the study lacked a control group and primarily focused on formulation optimization and percutaneous absorption, the observed outcomes may support the need for further clinical investigation. A randomized, double-blind, controlled clinical trial evaluated the clinical therapeutic efficacy of a topical botanical drug cream containing Fumaria officinalis L. extract and silymarin in patients with mild to moderate AD (Iraji et al., 2022). A total of 40 patients were enrolled and randomly assigned to receive either the botanical drug cream or 0.1% mometasone cream for 2 weeks. Both groups exhibited significant reductions in SCORing Atopic Dermatitis (SCORAD) scores, with no significant difference between them, indicating that the botanical drug formulation was non-inferior to low-potency corticosteroid treatment. These findings support the anti-inflammatory potential of silymarin and its synergistic plant-derived metabolites in clinical settings.
In another single-blind human study using a skin irritation model, the soothing and antipruritic effects of 1% Quercevita® cream, a phytosomal formulation of quercetin, were assessed (Maramaldi et al., 2016). Thirty healthy volunteers were subjected to induced skin irritation via UV exposure, histamine, sodium lauryl sulfate, and fruit acids. The quercetin formulation significantly reduced erythema, wheal formation, and subjective itch scores, exhibiting anti-inflammatory effects comparable to 1% desloratadine cream and supporting its potential utility as a topical anti-inflammatory agent. A double-blind randomized controlled trial also investigated the therapeutic efficacy and safety of MD2011001, a non-steroidal combination cream containing EGCG, grape seed proanthocyanidins, and vitamin E, in 44 patients with mild to moderate AD involving the face and neck (Patrizi et al., 2016). After 28 days of treatment, significant reductions in lesion size and improvements in Investigator Global Assessment (IGA) scores were observed. The formulation was well tolerated, with no serious adverse events reported.
In addition to topical applications, the oral administration of flavonoids has also received preliminary clinical investigation. A randomized, double-blind, placebo-controlled clinical trial involving 52 adults with moderate to severe AD assessed the efficacy of oral supplementation with whey protein combined with Cuscuta campestris Yunck. seed extract (Mehrbani et al., 2015). This combination contains multiple flavonoids including quercetin, kaempferol, and rutin. After 15 days of administration, patients exhibited notable improvements in skin hydration, elasticity, pruritus, and sleep disturbances. These improvements persisted for at least 2 weeks after treatment cessation, supporting a sustained symptomatic relief and skin barrier-restorative effects.
Collectively, current clinical studies suggest that natural flavonoids, particularly quercetin, silymarin, EGCG, and grape seed proanthocyanidins, may exert therapeutic effects in both topical and systemic interventions for AD, with generally favorable safety profiles. However, most trials to date have involved small sample sizes, lacked placebo controls, or utilized multi-metabolite formulations, limiting the interpretability of individual flavonoid effects. Future studies should prioritize large-scale, well-controlled clinical trials with optimized dosage forms and advanced delivery systems to validate efficacy, assess safety, and define appropriate treatment strategies.
8 Discussion and perspectives
AD is a chronic multifactorial inflammatory skin disease resulting from the interplay of genetic, immunological, environmental, and microbial factors. Although corticosteroids and biologics have shown clinical benefits, their long-term use is often constrained by high costs, adverse effects, heterogeneous patient responses, and limited applicability across AD subtypes. Consequently, these limitations have catalyzed increasing interest in natural metabolites with multi-target pharmacological properties and favorable safety profiles.
As highlighted in this review, flavonoids have emerged as promising therapeutic candidates due to their broad-spectrum actions on key pathophysiological processes in AD, including immune modulation, oxidative stress attenuation, barrier restoration, microbial regulation, pruritus relief, and regulation of programmed cell death. In particular, we extend current understanding by incorporating underexplored yet mechanistically relevant dimensions, such as the skin–gut axis, necroptosis, and AICD, thus providing a more integrated and updated perspective compared with existing literature.
However, despite robust pharmacodynamic potential in preclinical studies, the clinical translation of flavonoids remains hindered by multiple challenges, including poor solubility, low oral and dermal bioavailability, rapid systemic clearance, and insufficient toxicological profiling in humans. Moreover, the lack of standardized formulations, dose–response analyses, and long-term safety data significantly impedes regulatory progress and scientific reproducibility.
Although flavonoids are widely explored as multi-target natural metabolites in the pharmacological interventions for AD, some structurally reactive metabolites may function as pan-assay interference compounds (PAINS), thereby confounding mechanistic interpretation. PAINS often cause false-positive results through nonspecific redox activity, metal chelation, covalent protein modification, or membrane disruption (Baell, 2016; Bolz et al., 2021). For example, EGCG and quercetin can oxidize into ortho-quinones that non-selectively react with nucleophilic residues, leading to broad, non-targeted activity (Baell, 2016). Other flavonoids without classical redox structures, such as genistein and resveratrol, have also been shown to disturb membrane dipole potential or lower energy barriers, which can alter protein conformation and interfere with signaling (de Matos et al., 2021; Magalhães et al., 2022). Without orthogonal or in vivo validation, such effects may mislead interpretation, especially in in vitro models evaluating membrane-related functions (Bolz et al., 2021). Given that many mechanistic insights presented in this review are primarily derived from in vitro studies, and rigorously designed in vivo studies remain limited, future research should aim to reduce potential PAINS-related artifacts and enhance translational relevance by integrating pharmacokinetic and pharmacodynamic data, systematically evaluating membrane-related interference, and rigorously validating target specificity.
Furthermore, to address translational bottlenecks, we propose three strategic directions for future research. First, advanced delivery platforms, including microneedles, nanoparticles, and hydrogel-based systems, should be designed to enhance cutaneous retention, epithelial penetration, and pharmacokinetic stability. Concurrently, oral bioavailability can be improved through structural optimization, bioenhancer co-administration, or targeted nanoformulations that increase gastrointestinal absorption and metabolic resistance. Second, well-powered and mechanistically informed clinical trials are required to validate therapeutic efficacy, define human dosing thresholds, and ensure long-term safety. Third, the implementation of standardized manufacturing and quality control systems will be critical to ensure consistency, scalability, and regulatory compliance. Together, these approaches will be instrumental in transforming flavonoids from preclinical candidates to standardized therapeutic options for AD, while minimizing confounding assay artifacts and enhancing translational relevance.
9 Conclusion
In summary, flavonoids constitute a promising multi-target therapeutic platform for AD, providing a robust mechanistic foundation for translational advancement. Addressing pharmacokinetic limitations and safety concerns, alongside the advances in delivery strategies, could support the development of flavonoid-based therapies as disease-modifying interventions. These efforts align with precision dermatology’s emphasis on targeted, mechanism-informed care that integrates epithelial, immune, and microbial dimensions. Realizing this potential will require sustained collaboration across dermatology, pharmacology, toxicology, and pharmaceutical science to ensure the safe, efficacious, and scalable clinical application of flavonoid-based interventions.
Statements
Author contributions
DL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Visualization, Writing – original draft. YH: Conceptualization, Formal Analysis, Methodology, Writing – original draft. JZ: Conceptualization, Formal Analysis, Writing – original draft. JC: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – review and editing. HT: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Writing – review and editing. TT: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the China Scholarship Council (Grant No. 202308230308).
Acknowledgments
Figures 1, 4, 5 were created and licensed for academic use by BioRender (https://BioRender.com) and chemical structures in Figures 2, 3 were drawn by using KingDraw software (version 3.0; Qingdao KingAgroot, China), for which we are grateful.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abou Baker D. H. (2022). An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: a comprehensive review based on up to date knowledge. Toxicol. Rep.9, 445–469. 10.1016/j.toxrep.2022.03.011
2
Ahn K. (2023). The effect of prebiotics on atopic dermatitis. Allergy Asthma Immunol. Res.15, 271–275. 10.4168/aair.2023.15.3.271
3
Akar S. Fardindoost S. Hoorfar M. (2024). High throughput microfluidics-based synthesis of PEGylated liposomes for precise size control and efficient drug encapsulation. Colloids Surf. B Biointerfaces238, 113926. 10.1016/j.colsurfb.2024.113926
4
Akdis M. Trautmann A. Klunker S. Daigle I. Küçüuksezer U. C. Deglmann W. et al (2003). T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J.17, 1026–1035. 10.1096/fj.02-1070com
5
Alalaiwe A. Lin C.-F. Hsiao C.-Y. Chen E.-L. Lin C.-Y. Lien W.-C. et al (2020). Development of flavanone and its derivatives as topical agents against psoriasis: the prediction of therapeutic efficiency through skin permeation evaluation and cell-based assay. Int. J. Pharm.581, 119256. 10.1016/j.ijpharm.2020.119256
6
Aljuffali I. A. Lin C.-H. Yang S.-C. Alalaiwe A. Fang J.-Y. (2022). Nanoencapsulation of tea catechins for enhancing skin absorption and therapeutic efficacy. AAPS Pharmscitech23, 187. 10.1208/s12249-022-02344-3
7
Arakaki R. Yamada A. Kudo Y. Hayashi Y. Ishimaru N. (2014). Mechanism of activation-induced cell death of T cells and regulation of FasL expression. Crit. Rev. Immunol.34, 301–314. 10.1615/CritRevImmunol.2014009988
8
Ariëns L. F. M. Van Nimwegen K. J. M. Shams M. de Bruin D. T. van der Schaft J. van Os-Medendorp H. et al (2019). Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm. Venereol.99, 762–768. 10.2340/00015555-3212
9
Arora D. Nanda S. (2019). Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int. J. Pharm.567, 118448. 10.1016/j.ijpharm.2019.118448
10
Asarch A. Barak O. Loo D. S. Gottlieb A. B. (2008). Th17 cells: a new therapeutic target in inflammatory dermatoses. J. Dermatol. Treat.19, 318–326. 10.1080/09546630802206660
11
Aziz F. Hisatsune J. Ono H. K. Kajimura J. Yu L. Masuda K. et al (2024). Genomic analysis and identification of a novel superantigen, SargEY, in Staphylococcus argenteus isolated from atopic dermatitis lesions. Msphere9, e0050524. 10.1128/msphere.00505-24
12
Bae Y. Kim T. Park N. Choi S. Yi D. Soto S. et al (2022). Ameliorative effects of Daphnopsis costaricensis extract against oxazolone-induced atopic dermatitis-like lesions in BALB/c mice. Nutrients14, 4521. 10.3390/nu14214521
13
Baell J. B. (2016). Feeling nature’s PAINS: natural products, natural product drugs, and pan assay interference compounds (PAINS). J. Nat. Prod.79, 616–628. 10.1021/acs.jnatprod.5b00947
14
Bangert C. Rindler K. Krausgruber T. Alkon N. Thaler F. M. Kurz H. et al (2021). Persistence of mature dendritic cells, TH2A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Rα blockade. Sci. Immunol.6, eabe2749. 10.1126/sciimmunol.abe2749
15
Bay L. Barnes C. J. Fritz B. G. Ravnborg N. Ruge I. F. Halling-Sønderby A.-S. et al (2025). Unique dermal bacterial signature differentiates atopic dermatitis skin from healthy. Msphere10, e00156-25. 10.1128/msphere.00156-25
16
Beach M. A. Nayanathara U. Gao Y. Zhang C. Xiong Y. Wang Y. et al (2024). Polymeric nanoparticles for drug delivery. Chem. Rev.124, 5505–5616. 10.1021/acs.chemrev.3c00705
17
Beken B. Serttas R. Yazicioglu M. Turkekul K. Erdogan S. (2020). Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol.33, 69–79. 10.1089/ped.2019.1137
18
Ben David N. Richtman Y. Gross A. Ibrahim R. Nyska A. Ramot Y. et al (2023). Design and evaluation of dissolvable microneedles for treating atopic dermatitis. Pharmaceutics15, 1109. 10.3390/pharmaceutics15041109
19
Berdyshev E. (2024). Skin lipid barrier: structure, function and metabolism. Allergy Asthma Immunol. Res.16, 445–461. 10.4168/aair.2024.16.5.445
20
Bernatoniene J. Kopustinskiene D. M. (2018). The role of catechins in cellular responses to oxidative stress. Mol. Basel Switz.23, 965. 10.3390/molecules23040965
21
Bhat S. A. Bhat B. A. Khan M. A. Majid S. Mir M. R. Ali A. (2014). A study on the antioxidant defense system of psoriasis patients in the ethnic population of kashmir-India. Comp. Clin. Pathol.23, 547–549. 10.1007/s00580-012-1649-5
22
Bieber T. (2020). Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy75, 54–62. 10.1111/all.13954
23
Bo S. T. Chang S. K. Chen Y. P. Sheng Z. L. Jiang Y. M. Yang B. (2024). The structure characteristics, biosynthesis and health benefits of naturally occurring rare flavonoids. Crit. Rev. Food Sci. Nutr.64, 2490–2512. 10.1080/10408398.2022.2124396
24
Bolz S. N. Adasme M. F. Schroeder M. (2021). Toward an understanding of pan-assay interference compounds and promiscuity: a structural perspective on binding modes. J. Chem. Inf. Model.61, 2248–2262. 10.1021/acs.jcim.0c01227
25
Broeders J. A. Ali U. A. Fischer G. (2016). Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: a 15-year experience. J. Am. Acad. Dermatol.75, 410–419.e3. 10.1016/j.jaad.2016.02.1228
26
Callou T. M. P. Orfali R. L. Sotto M. N. Pereira N. V. Zaniboni M. C. Aoki V. et al (2022). Increased expression of filaggrin and claudin-1 in the ocular surface of patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. JEADV36, 247–254. 10.1111/jdv.17768
27
Camilion J. V. Khanna S. Anasseri S. Laney C. Mayrovitz H. N. (2022). Physiological, pathological, and circadian factors impacting skin hydration. Cureus14, e27666. 10.7759/cureus.27666
28
Carola C. Salazar A. Rakers C. Himbert F. Do Q.-T. Bernard P. et al (2021). A cornflower extract containing N-feruloylserotonin reduces inflammation in human skin by neutralizing CCL17 and CCL22 and inhibiting COX-2 and 5-LOX. Mediat. Inflamm.2021, 6652791. 10.1155/2021/6652791
29
Casella R. Miniello A. Buta F. Yacoub M. R. Nettis E. Pioggia G. et al (2024). Atopic dermatitis and autism spectrum disorders: common role of environmental and clinical Co-factors in the onset and severity of their clinical course. Int. J. Mol. Sci.25, 8936. 10.3390/ijms25168936
30
Cau L. Williams M. R. Butcher A. M. Nakatsuji T. Kavanaugh J. S. Cheng J. Y. et al (2021). Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol.147, 955–966.e16. 10.1016/j.jaci.2020.06.024
31
Cencioni M. T. Santini S. Ruocco G. Borsellino G. De Bardi M. Grasso M. G. et al (2015). Erratum: FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis.6, e1785. 10.1038/cddis.2015.164
32
Çetinarslan T. Kümper L. Fölster-Holst R. (2023). The immunological and structural epidermal barrier dysfunction and skin microbiome in atopic dermatitis-an update. Front. Mol. Biosci.10, 1159404. 10.3389/fmolb.2023.1159404
33
Che D. N. Cho B. O. Shin J. Y. Kang H. J. Kim J.-S. Oh H. et al (2019). Apigenin inhibits IL-31 cytokine in human mast cell and mouse skin tissues. Mol. Basel Switz.24, 1290. 10.3390/molecules24071290
34
Chen L. Liang J. Zhang X. Shao L. Pan Q. He S. et al (2019). Identification of differentially expressed genes in lesional versus nonlesional skin of patients with atopic dermatitis by using high-throughput transcriptome-wide RNA sequencing. Chin. J. Dermatol., 729–735. 10.35541/cjd.20190001
35
Chen L. Cao H. Huang Q. Xiao J. Teng H. (2022). Absorption, metabolism and bioavailability of flavonoids: a review. Crit. Rev. Food Sci. Nutr.62, 7730–7742. 10.1080/10408398.2021.1917508
36
Chen M. Wang R. Wang T. (2024). Gut microbiota and skin pathologies: mechanism of the gut-skin axis in atopic dermatitis and psoriasis. Int. Immunopharmacol.141, 112658. 10.1016/j.intimp.2024.112658
37
Chiu Y.-H. Wu Y.-W. Hung J.-I. Chen M.-C. (2021). Epigallocatechin gallate/L-ascorbic acid-loaded poly-γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis. Acta Biomater.130, 223–233. 10.1016/j.actbio.2021.05.032
38
Choi J. K. Kim S.-H. (2013). Rutin suppresses atopic dermatitis and allergic contact dermatitis. Exp. Biol. Med. Maywood N. J.238, 410–417. 10.1177/1535370213477975
39
Choi M.-J. Lee Y.-M. Jin B.-S. Kim B.-H. (2010). Inhibitory effect of luteolin liposome solution by animal model for atopic dermatitis in NC/nga mice. Lab. Anim. Res.26, 47–53. 10.5625/lar.2010.26.1.47
40
Chong A. C. Visitsunthorn K. Ong P. Y. (2022). Genetic/environmental contributions and immune dysregulation in children with atopic dermatitis. J. Asthma Allergy23, 1681–1700. 10.2147/JAA.S293900
41
Chung E. J. Luo C.-H. Thio C. L.-P. Chang Y.-J. (2022). Immunomodulatory role of Staphylococcus aureus in atopic dermatitis. Pathog. Basel Switz.11, 422. 10.3390/pathogens11040422
42
Cong Y. Wang R. Zhang C. Lin H. (2018). A simple and selective LC-MS/MS method for quantification of ikarisoside a in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr.32, e4245. 10.1002/bmc.4245
43
Cork M. J. Danby S. G. Vasilopoulos Y. Hadgraft J. Lane M. E. Moustafa M. et al (2009). Epidermal barrier dysfunction in atopic dermatitis. J. Invest. Dermatol.129, 1892–1908. 10.1038/jid.2009.133
44
Costa R. Costa Lima S. A. Gameiro P. Reis S. (2021). On the development of a cutaneous flavonoid delivery system: advances and limitations. Antioxidants10, 1376. 10.3390/antiox10091376
45
Dai X. J. Utsunomiya R. Shiraishi K. Mori H. Muto J. Murakami M. et al (2021). Nuclear IL-33 plays an important role in the suppression of FLG, LOR, keratin 1, and keratin 10 by IL-4 and IL-13 in human keratinocytes. J. Invest. Dermatol.141, 2646–2655.e6. 10.1016/j.jid.2021.04.002
46
Dai X. Muto J. Shiraishi K. Utsunomiya R. Mori H. Murakami M. et al (2022). TSLP impairs epidermal barrier integrity by stimulating the formation of nuclear IL-33/phosphorylated STAT3 complex in human keratinocytes. J. Invest. Dermatol.142, 2100–2108.e5. 10.1016/j.jid.2022.01.005
47
David E. Ungar B. Renert-Yuval Y. Facheris P. Del Duca E. Guttman-Yassky E. (2023). The evolving landscape of biologic therapies for atopic dermatitis: present and future perspective. Clin. Exp. Allergy53, 156–172. 10.1111/cea.14263
48
de Matos A. M. Blázquez-Sánchez M. T. Sousa C. Oliveira M. C. De Almeida R. F. M. Rauter A. P. (2021). C-glucosylation as a tool for the prevention of PAINS-induced membrane dipole potential alterations. Sci. Rep.11, 4443. 10.1038/s41598-021-83032-3
49
Delgado-Pujol E. J. Martínez G. Casado-Jurado D. Vázquez J. León-Barberena J. Rodríguez-Lucena D. et al (2025). Hydrogels and nanogels: pioneering the future of advanced drug delivery systems. Pharmaceutics17, 215. 10.3390/pharmaceutics17020215
50
Demessant-Flavigny A.-L. Connétable S. Kerob D. Moreau M. Aguilar L. Wollenberg A. (2023). Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: a narrative review. J. Eur. Acad. Dermatol. Venereol. JEADV37 (Suppl. 5), 3–17. 10.1111/jdv.19125
51
Dere M. D. Khan A. A. (2020). Potentials of encapsulated flavonoids in biologics: a review. Am. J. Biomed. Life Sci.8, 97–113. 10.11648/j.ajbls.20200804.16
52
Dong H. Feng C. Cai X. Hao Y. Gu X. Cai L. et al (2022). 7-methoxyisoflavone ameliorates atopic dermatitis symptoms by regulating multiple signaling pathways and reducing chemokine production. Sci. Rep.12, 8760. 10.1038/s41598-022-12695-3
53
Dong S. T. Li D. M. Shi D. M. (2024). Skin barrier-inflammatory pathway is a driver of the psoriasis-atopic dermatitis transition. Front. Med.11, 1335551. 10.3389/fmed.2024.1335551
54
Drozhdina M. Suslova E. (2021). Immune response in atopic dermatitis: main pathogenetic mechanisms and stage-dependent correlations with age in regard to dermatological and non-dermatological systemic processes. Med. Immunol.23, 237–244. 10.15789/1563-0625-IRI-2138
55
Duca E. Sur G. Armat I. Samasca G. Sur L. (2022). Correlation between interleukin 31 and clinical manifestations in children with atopic dermatitis: an observational study. Allergol. Immunopathol. (Madr.)50, 75–79. 10.15586/aei.v50i1.521
56
Elias P. M. Wakefield J. S. (2020). Could cellular and signaling abnormalities converge to provoke atopic dermatitis?J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG18, 1215–1223. 10.1111/ddg.14232
57
Espíndola C. (2023). Some nanocarrier’s properties and chemical interaction mechanisms with flavones. Mol. Basel Switz.28, 2864. 10.3390/molecules28062864
58
Fassett M. S. Braz J. M. Castellanos C. A. Salvatierra J. J. Sadeghi M. Yu X. et al (2023). IL-31-dependent neurogenic inflammation restrains cutaneous type 2 immune response in allergic dermatitis. Sci. Immunol.8, eabi6887. 10.1126/sciimmunol.abi6887
59
Feilcke R. Bär V. Wendt C. Imming P. (2023). Antibacterial and disinfecting effects of standardised tea extracts on more than 100 clinical isolates of methicillin-resistant Staphylococcus aureus. Plants12, 3440. 10.3390/plants12193440
60
Ferreira D. Moreira J. N. Rodrigues L. R. (2022). New advances in exosome-based targeted drug delivery systems. Crit. Rev. Oncol. Hematol.172, 103628. 10.1016/j.critrevonc.2022.103628
61
Fritsch M. Günther S. D. Schwarzer R. Albert M.-C. Schorn F. Werthenbach J. P. et al (2019). Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature575, 683–687. 10.1038/s41586-019-1770-6
62
Fu K. Li X. Wei X. He J. Jia M. Li Y. et al (2024a). Research progress on pharmacological action and mechanism of hedysari radix flavones. Zhongcaoyao55, 3906–3915.
63
Fu Z. Gu Q. Wang L. Chen L. Zhou L. Jin Q. et al (2024b). Cell-free fat extract regulates oxidative stress and alleviates Th2-mediated inflammation in atopic dermatitis. Front. Bioeng. Biotechnol.12, 1373419. 10.3389/fbioe.2024.1373419
64
Fujii M. (2020). Current understanding of pathophysiological mechanisms of atopic dermatitis: interactions among skin barrier dysfunction, immune abnormalities and pruritus. Biol. Pharm. Bull.43, 12–19. 10.1248/bpb.b19-00088
65
Galiniak S. Mołoń M. Biesiadecki M. Bożek A. Rachel M. (2022). The role of oxidative stress in atopic dermatitis and chronic urticaria. Antioxid. Basel Switz.11, 1590. 10.3390/antiox11081590
66
Gao J.-F. Tang L. Luo F. Chen L. Zhang Y.-Y. Ding H. (2023). Myricetin treatment has ameliorative effects in DNFB-induced atopic dermatitis mice under high-fat conditions. Toxicol. Sci. Off. J. Soc. Toxicol.191, 308–320. 10.1093/toxsci/kfac138
67
Gendrisch F. Esser P. R. Schempp C. M. Wölfle U. (2021). Luteolin as a modulator of skin aging and inflammation. Biofactors Oxf. Engl.47, 170–180. 10.1002/biof.1699
68
Gong G. Ganesan K. Zheng Y. Xiao J. Dong T. Tsim K. (2024). Nepetin attenuates atopic dermatitis in HaCaT cells and BALB/c mice through MyD88-MKK3/6-akt signaling. Curr. Med. Chem.32, 2775–2791. 10.2174/0109298673244967231101114033
69
Goris T. Cuadrat R. R. C. Braune A. (2021). Flavonoid-modifying capabilities of the human gut microbiome—an in silico study. Nutrients13, 2688. 10.3390/nu13082688
70
Gough P. Khalid M. B. Hartono S. Myles I. A. (2022). Microbial manipulation in atopic dermatitis. Clin. Transl. Med.12, e828. 10.1002/ctm2.828
71
Grillo R. Dias F. V. Querobino S. M. Alberto-Silva C. Fraceto L. F. De Paula E. et al (2019). Influence of hybrid polymeric nanoparticle/thermosensitive hydrogels systems on formulation tracking and in vitro artificial membrane permeation: a promising system for skin drug-delivery. Colloids Surf. B Biointerfaces174, 56–62. 10.1016/j.colsurfb.2018.10.063
72
Guttman-Yassky E. Renert-Yuval Y. Brunner P. M. (2025). Atopic dermatitis. Lancet405, 583–596. 10.1016/S0140-6736(24)02519-4
73
Hadi H. A. Tarmizi A. I. Khalid K. A. Gajdács M. Aslam A. Jamshed S. (2021). The epidemiology and global burden of atopic dermatitis: a narrative review. Life Basel11, 936. 10.3390/life11090936
74
Hall J. A. Pokrovskii M. Kroehling L. Kim B.-R. Kim S. Y. Wu L. et al (2022). Transcription factor RORα enforces stability of the Th17 cell effector program by binding to a rorc cis-regulatory element. Immunity55, 2027–2043.e9. 10.1016/j.immuni.2022.09.013
75
Han S. Wang W. Wang S. Yang T. Zhang G. Wang D. et al (2021). Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics11, 2892–2916. 10.7150/thno.50928
76
Han M. Wang X. Wang J. Lang D. Xia X. Jia Y. et al (2022). Ameliorative effects of epigallocatechin-3-gallate nanoparticles on 2,4-dinitrochlorobenzene induced atopic dermatitis: a potential mechanism of inflammation-related necroptosis. Front. Nutr.9, 953646. 10.3389/fnut.2022.953646
77
Han Y. Qin X. Lin W. Wang C. Yin X. Wu J. et al (2025). Microneedle-based approaches for skin disease treatment. Nano Micro Lett.17, 132. 10.1007/s40820-025-01662-y
78
He H. Del Duca E. Diaz A. Kim H. J. Gay-Mimbrera J. Zhang N. et al (2021). Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J. Allergy Clin. Immunol.147, 1369–1380. 10.1016/j.jaci.2020.08.041
79
Herath H. M. U. L. Piao M. J. Kang K. A. Fernando P. D. S. M. Hyun J. W. (2024). Rosmarinic acid protects skin keratinocytes from particulate matter 2.5-induced apoptosis. Int. J. Med. Sci.21, 681–689. 10.7150/ijms.90814
80
Hou M. H. Sun R. Hupe M. Kim P. L. Park K. Crumrine D. et al (2013). Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol.22, 210–215. 10.1111/exd.12102
81
Hou D.-D. Zhang W. Gao Y.-L. Sun Y.-Z. Wang H.-X. Qi R.-Q. et al (2019). Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol.74, 105676. 10.1016/j.intimp.2019.105676
82
Hou D.-D. Gu Y.-J. Wang D.-C. Niu Y. Xu Z.-R. Jin Z.-Q. et al (2022). Therapeutic effects of myricetin on atopic dermatitis in vivo and in vitro. Phytomedicine Int. J. Phytother. Phytopharm.102, 154200. 10.1016/j.phymed.2022.154200
83
Hu X. Q. Zhou Y. Shi J. X. Qi M. X. Li X. Yang Y. et al (2023). Osthole relieves skin damage and inhibits chronic itch through modulation of akt/ZO-3 pathway in atopic dermatitis. Eur. J. Pharmacol.947, 175649. 10.1016/j.ejphar.2023.175649
84
Huang K.-F. Ma K.-H. Liu P.-S. Chen B.-W. Chueh S.-H. (2016a). Baicalein increases keratin 1 and 10 expression in HaCaT keratinocytes via TRPV4 receptor activation. Exp. Dermatol.25, 623–629. 10.1111/exd.13024
85
Huang P.-H. Hu S. C.-S. Lee C.-W. Yeh A.-C. Tseng C.-H. Yen F.-L. (2016b). Design of acid-responsive polymeric nanoparticles for 7,3′,4′-trihydroxyisoflavone topical administration. Int. J. Nanomedicine11, 1615–1627. 10.2147/IJN.S100418
86
Huang C. Zhuo F. Guo Y. Wang S. Zhang K. Li X. et al (2025). Skin microbiota: pathogenic roles and implications in atopic dermatitis. Front. Cell. Infect. Microbiol.14, 1518811. 10.3389/fcimb.2024.1518811
87
Hussain D. S. Mirza W. Murtaza M. Nazir A. Hanif I. Ahmad M. (2022). Sources and chemistry of flavonoids; their biological and therapeutic potential. Sci. Inq. Rev.6. 10.32350/sir.62.03
88
Ighodaro O. M. Akinloye O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex. J. Med.54, 287–293. 10.1016/j.ajme.2017.09.001
89
Ilari S. Dagostino C. Malafoglia V. Lauro F. Giancotti L. A. Spila A. et al (2020). Protective effect of antioxidants in nitric oxide/COX-2 interaction during inflammatory pain: the role of nitration. Antioxid. Basel Switz.9, 1284. 10.3390/antiox9121284
90
Imai Y. (2023). ILC2s in skin disorders. Allergol. Int. Off. J. Jpn. Soc. Allergol.72, 201–206. 10.1016/j.alit.2023.01.002
91
Iraji F. Sharif Makhmalzadeh B. Abedini M. Aghaei A. Siahpoush A. (2022). Effect of herbal cream containing Fumaria officinalis and silymarin for treatment of eczema: a randomized double-blind controlled clinical trial. Avicenna J. Phytomedicine12, 155–162. 10.22038/AJP.2022.19492
92
Jafarinia M. Sadat Hosseini M. Kasiri N. Fazel N. Fathi F. Ganjalikhani Hakemi M. et al (2020). Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol.16, 36. 10.1186/s13223-020-00434-0
93
Jankovic D. Feng C. G. (2015). CD4(+) T cell differentiation in infection: amendments to the Th1/Th2 axiom. Front. Immunol.6, 198. 10.3389/fimmu.2015.00198
94
Jegadheeshwari S. Santhi J. J. Velayutham M. Issac P. K. Kesavan M. (2024). DbGTi protein attenuates chromium (VI)-induced oxidative stress via activation of the Nrf2/HO-1 signalling pathway in zebrafish (danio rerio) larval model. Int. J. Biol. Macromol.280, 136099. 10.1016/j.ijbiomac.2024.136099
95
Ji L.-L. Sheng Y.-C. Zheng Z.-Y. Shi L. Wang Z.-T. (2015). The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med.85, 12–23. 10.1016/j.freeradbiomed.2015.03.035
96
Jin X. Liu M.-Y. Zhang D.-F. Zhong X. Du K. Qian P. et al (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther.25, 575–590. 10.1111/cns.13086
97
Jo B.-G. Park N.-J. Jegal J. Choi S. Lee S. W. Jin H. et al (2018). A new flavonoid from stellera chamaejasme L., stechamone, alleviated 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in a murine model. Int. Immunopharmacol.59, 113–119. 10.1016/j.intimp.2018.04.008
98
Kapoor M. Moriwaki M. Timm D. Satomoto K. Minegawa K. (2022). Genotoxicity and mutagenicity evaluation of isoquercitrin-γ-cyclodextrin molecular inclusion complex using ames test and a combined micronucleus and comet assay in rats. J. Toxicol. Sci.47 (6), 221–235. 10.2131/jts.47.221
99
Karlowitz R. van Wijk S. J. L. (2023). Surviving death: emerging concepts of RIPK3 and MLKL ubiquitination in the regulation of necroptosis. FEBS J.290, 37–54. 10.1111/febs.16255
100
Karuppagounder V. Arumugam S. Thandavarayan R. A. Sreedhar R. Giridharan V. V. Watanabe K. (2016). Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today21, 632–639. 10.1016/j.drudis.2016.02.011
101
Katsarou S. Makris M. Vakirlis E. Gregoriou S. (2023). The role of tight junctions in atopic dermatitis: a systematic review. J. Clin. Med.12, 1538. 10.3390/jcm12041538
102
Khalil M. Hashmi U. Riaz R. Rukh Abbas S. (2020). Chitosan coated liposomes (CCL) containing triamcinolone acetonide for sustained delivery: a potential topical treatment for posterior segment diseases. Int. J. Biol. Macromol.143, 483–491. 10.1016/j.ijbiomac.2019.10.256
103
Khursheed R. Singh S. K. Wadhwa S. Gulati M. Awasthi A. (2020). Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discov. Today25, 209–222. 10.1016/j.drudis.2019.11.001
104
Kibalina I. V. Tsybikov N. N. Fefelova E. V. Sholokhov L. F. (2022). Pathophysiological role of chemokine MIG/CXCL9 in the development of atopic dermatitis. Acta Biomed. Sci.7, 99–104. 10.29413/ABS.2022-7.2.11
105
Kim B.-H. Lee S. (2021). Sophoricoside from Styphnolobium japonicum improves experimental atopic dermatitis in mice. Phytomedicine Int. J. Phytother. Phytopharm.82, 153463. 10.1016/j.phymed.2021.153463
106
Kim J. Y. Lee O. S. Ha S. Kim J. H. Park G. Kim J. K. et al (2015). In vivo assessment of the effect of taxifolin glycoside on atopic dermatitis-like skin lesions using biomedical tools in NC/nga mice. Clin. Exp. Dermatol.40, 547–555. 10.1111/ced.12522
107
Kim T.-Y. Park N.-J. Jegal J. Choi S. Lee S. W. Hang J. et al (2019). Chamaejasmine isolated from Wikstroemia dolichantha diels suppresses 2,4-dinitrofluoro-benzene-induced atopic dermatitis in SKH-1 hairless mice. Biomolecules9, 697. 10.3390/biom9110697
108
Kitahata K. Matsuo K. Sato M. Susami Y. Hara Y. Morikawa T. et al (2022). Anti-allergic effect of ascorbic acid derivative DDH-1 in a mouse model of atopic dermatitis. Exp. Dermatol.31, 1234–1242. 10.1111/exd.14578
109
Korn T. Bettelli E. Oukka M. Kuchroo V. K. (2009). IL-17 and Th17 cells. Annu. Rev. Immunol.27, 485–517. 10.1146/annurev.immunol.021908.132710
110
Ku Y.-S. Ng M.-S. Cheng S.-S. Lo A. W.-Y. Xiao Z. X. Shin T.-S. et al (2020). Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients12, 1717. 10.3390/nu12061717
111
Kuai L. Huang F. Mao L. J. Ru Y. Jiang J. S. Song J. K. et al (2024). Single-atom catalysts with isolated Cu1-N4 sites for atopic dermatitis cascade catalytic therapy via activating PPAR signaling. Small Weinh. Bergstr. Ger.20, e2407365. 10.1002/smll.202407365
112
Kumar D. N. Chaudhuri A. Dehari D. Gamper A. M. Kumar D. Agrawal A. K. (2024). Enhanced therapeutic efficacy against melanoma through exosomal delivery of hesperidin. Mol. Pharm.21, 3061–3076. 10.1021/acs.molpharmaceut.4c00490
113
Kumazoe M. Fujimura Y. Yoshitomi R. Shimada Y. Tachibana H. (2022). Fustin, a flavanonol, synergically potentiates the anticancer effect of green tea catechin epigallocatechin-3-O-gallate with activation of the eNOS/cGMP axis. J. Agric. Food Chem.70, 3458–3466. 10.1021/acs.jafc.1c07567
114
Labie H. Blanzat M. (2023). Hydrogels for dermal and transdermal drug delivery. Biomater. Sci.11, 4073–4093. 10.1039/D2BM02070J
115
Lambert M. N. T. Jeppesen P. B. (2018). Isoflavones and bone health in perimenopausal and postmenopausal women. Curr. Opin. Clin. Nutr. Metab. Care21, 475–480. 10.1097/MCO.0000000000000513
116
Lee H.-S. Jeong G.-S. (2021). Therapeutic effect of kaempferol on atopic dermatitis by attenuation of T cell activity via interaction with multidrug resistance-associated protein 1. Br. J. Pharmacol.178, 1772–1788. 10.1111/bph.15396
117
Lee J.-H. Jeon Y.-D. Lee Y.-M. Kim D.-K. (2018). The suppressive effect of puerarin on atopic dermatitis-like skin lesions through regulation of inflammatory mediators in vitro and in vivo. Biochem. Biophys. Res. Commun.498, 707–714. 10.1016/j.bbrc.2018.03.018
118
Lee C. H. Yang H. E. Yoon Park J. H. Kim J.-E. Lee K. W. (2022a). Orobol from enzyme biotransformation attenuates Dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/nga mice. Food Funct.13, 4592–4599. 10.1039/d1fo04362e
119
Lee Y. Oh J.-H. Li N. Jang H.-J. Ahn K.-S. Oh S.-R. et al (2022b). Topical skullcapflavone II attenuates atopic dermatitis in a mouse model by directly inhibiting associated cytokines in different cell types. Front. Immunol.13, 1064515. 10.3389/fimmu.2022.1064515
120
Li L. Q. Wang Y. Wang X. Y. Tao Y. Bao K. F. Hua Y. Q. et al (2018). Formononetin attenuated allergic diseases through inhibition of epithelial-derived cytokines by regulating E-cadherin. Clin. Immunol. Orl. Fla195, 67–76. 10.1016/j.clim.2018.07.018
121
Li J. Yang Y. Ning E. Peng Y. Zhang J. (2019). Mechanisms of poor oral bioavailability of flavonoid morin in rats: from physicochemical to biopharmaceutical evaluations. Eur. J. Pharm. Sci.128, 290–298. 10.1016/j.ejps.2018.12.011
122
Liu F. Feng M. Xing J. Zhou X. (2021a). Timosaponin alleviates oxidative stress in rats with high fat diet-induced obesity via activating Nrf2/HO-1 and inhibiting the NF-κB pathway. Eur. J. Pharmacol.909, 174377. 10.1016/j.ejphar.2021.174377
123
Liu J. Zhang X. Cheng Y. Cao X. (2021b). Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol.18, 2461–2471. 10.1038/s41423-021-00726-4
124
Loset M. Brown S. J. Saunes M. Hveem K. (2019). Genetics of atopic dermatitis: from DNA sequence to clinical relevance. Dermatol. Int. J. Clin. Investig. Dermatol.235, 355–364. 10.1159/000500402
125
Lossi L. (2022). The concept of intrinsic versus extrinsic apoptosis. Biochem. J.479, 357–384. 10.1042/BCJ20210854
126
Luo C.-H. Lai A. C.-Y. Tsai C.-C. Chen W.-Y. Chang Y.-S. Chung E. J.-C. et al (2024a). Staphylococcus aureus exacerbates dermal IL-33/ILC2 axis activation through evoking RIPK3/MLKL-mediated necroptosis of dry skin. JCI Insight9, e185878. 10.1172/jci.insight.185878
127
Luo Z. W. Peng Z. Z. Cao X. P. Chen M. J. You X. Q. (2024b). Dissolvable microneedle particles for enhanced topical drug delivery. J. Drug Deliv. Sci. Technol.91, 105209. 10.1016/j.jddst.2023.105209
128
Ma Z. Li N. Zhang B. Hui Y. Zhang Y. Lu P. et al (2020). Dual drug-loaded nano-platform for targeted cancer therapy: toward clinical therapeutic efficacy of multifunctionality. J. Nanobiotechnology18, 123. 10.1186/s12951-020-00681-8
129
Ma X. Pan Y. Zhao W. Sun P. Zhao J. Yan S. et al (2022). Bifidobacterium infantis strain YLGB-1496 possesses excellent antioxidant and skin barrier-enhancing efficacy in vitro. Exp. Dermatol.31, 1089–1094. 10.1111/exd.14583
130
Mady F. M. Essa H. El-Ammawi T. Abdelkader H. Hussein A. K. (2016). Formulation and clinical evaluation of silymarin pluronic-lecithin organogels for treatment of atopic dermatitis. Drug Des. devel. Ther.10, 1101–1110. 10.2147/DDDT.S103423
131
Magalhães P. R. Reis P. B. P. S. Vila-Viçosa D. Machuqueiro M. Victor B. L. (2022). Optimization of an in silico protocol using probe permeabilities to identify membrane pan-assay interference compounds. J. Chem. Inf. Model.62, 3034–3042. 10.1021/acs.jcim.2c00372
132
Majewski G. Craw J. Falla T. (2021). Accelerated barrier repair in human skin explants induced with a plant-derived PPAR-α activating complex via cooperative interactions. Clin. Cosmet. Investig. Dermatol.14, 1271–1293. 10.2147/CCID.S325967
133
Maleki S. J. Crespo J. F. Cabanillas B. (2019). Anti-inflammatory effects of flavonoids. Food Chem.299, 125124. 10.1016/j.foodchem.2019.125124
134
Maramaldi G. Togni S. Pagin I. Giacomelli L. Cattaneo R. Eggenhöffner R. et al (2016). Soothing and anti-itch effect of quercetin phytosome in human subjects: a single-blind study. Clin. Cosmet. Investig. Dermatol.9, 55–62. 10.2147/CCID.S98890
135
Martinou J.-C. Youle R. J. (2011). Mitochondria in apoptosis: bcl-2 family members and mitochondrial dynamics. Dev. Cell21, 92–101. 10.1016/j.devcel.2011.06.017
136
Mas-Bargues C. Escrivá C. Dromant M. Borrás C. Viña J. (2021). Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys.709, 108941. 10.1016/j.abb.2021.108941
137
Matsui K. Shibata R. Mogi K. (2022). Influence of the Th1 cytokine environment on CCL5 production from langerhans cells. Biol. Pharm. Bull.45, 491–496. 10.1248/bpb.b21-00985
138
Meca A.-D. Turcu-Stiolica A. Stanciulescu E. C. Andrei A. M. Nitu F. M. Banita I. M. et al (2021). Variations of serum oxidative stress biomarkers under first-line antituberculosis treatment: a pilot study. J. Pers. Med.11, 112. 10.3390/jpm11020112
139
Mehrbani M. Choopani R. Fekri A. Mehrabani M. Mosaddegh M. Mehrabani M. (2015). The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: a randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol.172, 325–332. 10.1016/j.jep.2015.07.003
140
Melli L. C. F. L. Carmo-Rodrigues M. S. do Araújo-Filho H. B. Mello C. S. Tahan S. Pignatari A. C. C. et al (2020). Gut microbiota of children with atopic dermatitis: controlled study in the metropolitan region of são paulo, Brazil. Allergol. Immunopathol. (Madr.)48, 107–115. 10.1016/j.aller.2019.08.004
141
Melnik B. C. (2015). The potential role of impaired notch signalling in atopic dermatitis. Acta Derm. Venereol.95, 5–11. 10.2340/00015555-1898
142
Meng J. Li Y. Fischer M. J. M. Steinhoff M. Chen W. Wang J. (2021a). Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front. Immunol.12, 696784. 10.3389/fimmu.2021.696784
143
Meng Y. Sandow J. J. Czabotar P. E. Murphy J. M. (2021b). The regulation of necroptosis by post-translational modifications. Cell Death Differ.28, 861–883. 10.1038/s41418-020-00722-7
144
Messina M. (2014). Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr.100 (Suppl. 1), 423S–30S. 10.3945/ajcn.113.071464
145
Messina M. (2016). Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients8, 754. 10.3390/nu8120754
146
Mohos V. Fliszár-Nyúl E. Ungvári O. Kuffa K. Needs P. W. Kroon P. A. et al (2020). Inhibitory effects of quercetin and its main methyl, sulfate, and glucuronic acid conjugates on cytochrome P450 enzymes, and on OATP, BCRP and MRP2 transporters. Nutrients12, 2306. 10.3390/nu12082306
147
Mollanazar N. K. Smith P. K. Yosipovitch G. (2016). Mediators of chronic pruritus in atopic dermatitis: getting the itch out?Clin. Rev. Allergy Immunol.51, 263–292. 10.1007/s12016-015-8488-5
148
Moriwaki M. Iwamoto K. Niitsu Y. Matsushima A. Yanase Y. Hisatsune J. et al (2019). Staphylococcus aureus from atopic dermatitis skin accumulates in the lysosomes of keratinocytes with induction of IL ‐1α secretion via TLR 9. Allergy74, 560–571. 10.1111/all.13622
149
Moss R. B. Moll T. El-Kalay M. Kohne C. Soo Hoo W. Encinas J. et al (2004). Th1/Th2 cells in inflammatory disease states: therapeutic implications. Expert Opin. Biol. Ther.4, 1887–1896. 10.1517/14712598.4.12.1887
150
Nagula R. L. Wairkar S. (2020). Cellulose microsponges based gel of naringenin for atopic dermatitis: design, optimization, in vitro and in vivo investigation. Int. J. Biol. Macromol.164, 717–725. 10.1016/j.ijbiomac.2020.07.168
151
Naik S. Bouladoux N. Wilhelm C. Molloy M. J. Salcedo R. Kastenmuller W. et al (2012). Compartmentalized control of skin immunity by resident commensals. Science337, 1115–1119. 10.1126/science.1225152
152
Najmanová I. Vopršalová M. Saso L. Mladěnka P. (2020). The pharmacokinetics of flavanones. Crit. Rev. Food Sci. Nutr.60, 3155–3171. 10.1080/10408398.2019.1679085
153
Nakajima S. Tie D. Nomura T. Kabashima K. (2021). Novel pathogenesis of atopic dermatitis from the view of cytokines in mice and humans. Cytokine148, 155664. 10.1016/j.cyto.2021.155664
154
Nakamura T. Kinjo C. Nakamura Y. Kato Y. Nishikawa M. Hamada M. et al (2018). Lymphatic metabolites of quercetin after intestinal administration of quercetin-3-glucoside and its aglycone in rats. Arch. Biochem. Biophys.645, 126–136. 10.1016/j.abb.2018.03.024
155
Nakamura K. Zhu S. Komatsu K. Hattori M. Iwashima M. (2020). Deglycosylation of the isoflavone C-glucoside puerarin by a combination of two recombinant bacterial enzymes and 3-oxo-glucose. Appl. Environ. Microbiol.86, e00607-20. 10.1128/AEM.00607-20
156
Nakano K. Saito K. Mine S. Matsushita S. Tanaka Y. (2007). Engagement of CD44 up-regulates fas ligand expression on T cells leading to activation-induced cell death. Apoptosis12, 45–54. 10.1007/s10495-006-0488-8
157
Nasanbat B. Uchiyama A. Amalia S. N. Inoue Y. Yokoyama Y. Ogino S. et al (2023). Kaempferol therapy improved MC903 induced-atopic dermatitis in a mouse by suppressing TSLP, oxidative stress, and type 2 inflammation. J. Dermatol. Sci.111, 93–100. 10.1016/j.jdermsci.2023.06.008
158
Nedoszytko B. Sokołowska-Wojdyło M. Ruckemann-Dziurdzińska K. Roszkiewicz J. Nowicki R. J. (2014). Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Adv. Dermatol. Allergol. Dermatol. Alergol.31, 84–91. 10.5114/pdia.2014.40920
159
Oberg H.-H. Lengl-Janßen B. Kabelitz D. Janssen O. (1997). Activation-induced T cell death: resistance or susceptibility correlate with cell surface fas ligand expression and T helper phenotype. Cell. Immunol.181, 93–100. 10.1006/cimm.1997.1200
160
Olesen C. M. Ingham A. C. Thomsen S. F. Clausen M.-L. Andersen P. S. Edslev S. M. et al (2021). Changes in skin and nasal microbiome and staphylococcal species following treatment of atopic dermatitis with dupilumab. Microorganisms9, 1487. 10.3390/microorganisms9071487
161
Ong P. Y. Ohtake T. Brandt C. Strickland I. Boguniewicz M. Ganz T. et al (2002). Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med.347, 1151–1160. 10.1056/NEJMoa021481
162
Pan L. Ye H. Pi X. Liu W. Wang Z. Zhang Y. et al (2023). Effects of several flavonoids on human gut microbiota and its metabolism by in vitro simulated fermentation. Front. Microbiol.14, 1092729. 10.3389/fmicb.2023.1092729
163
Park S. H. Shin H. S. Park S. N. (2018). A novel pH-responsive hydrogel based on carboxymethyl cellulose/2-hydroxyethyl acrylate for transdermal delivery of naringenin. Carbohydr. Polym.200, 341–352. 10.1016/j.carbpol.2018.08.011
164
Park C.-H. Min S.-Y. Yu H.-W. Kim K. Kim S. Lee H.-J. et al (2020a). Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int. J. Mol. Sci.21, 4620. 10.3390/ijms21134620
165
Park S. H. Lee C. H. Lee J. Y. Yang H. Kim J. H. Park J. H. Y. et al (2020b). Topical application of 7,3’,4’-trihydroxyisoflavone alleviates atopic dermatitis-like symptoms in NC/nga mice. Planta Med.86, 190–197. 10.1055/a-1068-7983
166
Patrizi A. Raone B. Neri I. Gurioli C. Carbonara M. Cassano N. et al (2016). Randomized, controlled, double-blind clinical study evaluating the safety and efficacy of MD2011001 cream in mild-to-moderate atopic dermatitis of the face and neck in children, adolescents and adults. J. Dermatol. Treat.27, 346–350. 10.3109/09546634.2015.1115814
167
Payne A. Nahashon S. Taka E. Adinew G. M. Soliman K. F. A. (2022). Epigallocatechin-3-gallate (EGCG): new therapeutic perspectives for neuroprotection, aging, and neuroinflammation for the modern age. Biomolecules12, 371. 10.3390/biom12030371
168
Peng S. Liao L. Deng H. Liu X. Lin Q. Wu W. (2024). Alleviating effect of lipid phytochemicals in seed oil (Brassica napus L.) on oxidative stress injury induced by H2O2 in HepG2 cells via Keap1/Nrf2/ARE signaling pathway. Nutrients16, 2820. 10.3390/nu16172820
169
Pessôa R. Clissa P. B. Sanabani S. S. (2023). The interaction between the host genome, epigenome, and the gut–skin axis microbiome in atopic dermatitis. Int. J. Mol. Sci.24, 14322. 10.3390/ijms241814322
170
Poto R. Quinti I. Marone G. Taglialatela M. de Paulis A. Casolaro V. et al (2022). IgG autoantibodies against IgE from atopic dermatitis can induce the release of cytokines and proinflammatory mediators from basophils and mast cells. Front. Immunol.13, 880412. 10.3389/fimmu.2022.880412
171
Raimondo A. Serio B. Lembo S. (2023). Oxidative stress in atopic dermatitis and possible biomarkers: present and future. Indian J. dermatol.68, 657–660. 10.4103/ijd.ijd_878_22
172
Raju K. S. R. Rashid M. Gundeti M. Taneja I. Malik M. Y. Singh S. K. et al (2019). LC-ESI-MS/MS method for the simultaneous determination of isoformononetin, daidzein, and equol in rat plasma: application to a preclinical pharmacokinetic study. J. Chromatogr. B1129, 121776. 10.1016/j.jchromb.2019.121776
173
Rebane A. Zimmermann M. Aab A. Baurecht H. Koreck A. Karelson M. et al (2012). Mechanisms of IFN-γ–induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J. Allergy Clin. Immunol.129, 1297–1306. 10.1016/j.jaci.2012.02.020
174
Renert-Yuval Y. Del Duca E. Pavel A. B. Fang M. Lefferdink R. Wu J. et al (2021). The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J. Allergy Clin. Immunol.148, 148–163. 10.1016/j.jaci.2021.01.001
175
Ribeiro D. Proenca C. Rocha S. Lima J. L. F. C. Carvalho F. Fernandes E. et al (2018). Immunomodulatory effects of flavonoids in the prophylaxis and treatment of inflammatory bowel diseases: a comprehensive review. Curr. Med. Chem.25, 3374–3412. 10.2174/0929867325666180214121734
176
Rothenberg-Lausell C. Bar J. Del Duca E. Guttman-Yassky E. (2024). Diversity of atopic dermatitis and selection of immune targets. Ann. Allergy Asthma Immunol.132, 177–186. 10.1016/j.anai.2023.11.020
177
Ruppenstein A. Limberg M. M. Loser K. Kremer A. E. Homey B. Raap U. (2021). Involvement of neuro-immune interactions in pruritus with special focus on receptor expressions. Front. Med.8, 627985. 10.3389/fmed.2021.627985
178
Ruterbusch M. Pruner K. B. Shehata L. Pepper M. (2020). In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu. Rev. Immunol.38, 705–725. 10.1146/annurev-immunol-103019-085803
179
Sahadevan R. Binoy A. Shajan I. Sadhukhan S. (2023). Mitochondria-targeting EGCG derivatives protect H9c2 cardiomyocytes from H2O2-induced apoptosis: design, synthesis and biological evaluation. RSC Adv.13, 29477–29488. 10.1039/d3ra04527g
180
Sakai K. Sanders K. M. Pavlenko D. Lozada T. Akiyama T. (2021). Crisaborole prevents infiltration of neutrophils to suppress itch in a mouse model of atopic dermatitis. Itch6, e53. 10.1097/itx.0000000000000053
181
Salama N. A. Fowell D. J. (2020). Retention and egress of Th1 cells in the inflamed skin. J. Immunol.204, 220–10. 10.4049/jimmunol.204.Supp.220.10
182
Schmidt A. D. de Guzman Strong C. (2021). Current understanding of epigenetics in atopic dermatitis. Exp. Dermatol.30, 1150–1155. 10.1111/exd.14392
183
Sehra S. Yao Y. Howell M. D. Nguyen E. T. Kansas G. S. Leung D. Y. M. et al (2010). IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J. Immunol. Balt. Md 1950184, 3186–3190. 10.4049/jimmunol.0901860
184
Seo W. Y. Youn G. S. Choi S. Y. Park J. (2015). Butein, a tetrahydroxychalcone, suppresses pro-inflammatory responses in HaCaT keratinocytes. BMB Rep.48, 495–500. 10.5483/bmbrep.2015.48.9.259
185
Shi J. Li Y. Y. Zhang Y. Chen J. Gao J. Q. Zhang T. Y. et al (2021). Baicalein ameliorates aβ-induced memory deficits and neuronal atrophy via inhibition of PDE2 and PDE4. Front. Pharmacol.12, 794458. 10.3389/fphar.2021.794458
186
Shin K.-O. Ha D. H. Kim J. O. Crumrine D. A. Meyer J. M. Wakefield J. S. et al (2020). Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells9, 680. 10.3390/cells9030680
187
Siiskonen H. Harvima I. (2019). Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front. Cell. Neurosci.13, 422. 10.3389/fncel.2019.00422
188
Silverberg J. I. Barbarot S. Gadkari A. Simpson E. L. Weidinger S. Mina-Osorio P. et al (2021). Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol.126, 417–428.e2. 10.1016/j.anai.2020.12.020
189
Simon D. Lindberg R. L. P. Kozlowski E. Braathen L. R. Simon H.-U. (2006). Epidermal caspase-3 cleavage associated with interferon-γ-expressing lymphocytes in acute atopic dermatitis lesions. Exp. Dermatol.15, 441–446. 10.1111/j.0906-6705.2006.00428.x
190
Simon D. Aeberhard C. Erdemoglu Y. Simon H.-U. (2014). Th17 cells and tissue remodeling in atopic and contact dermatitis. Allergy69, 125–131. 10.1111/all.12351
191
Singh P. Sharma S. Rath S. (2021). A versatile flavonoid quercetin: study of its toxicity and differential gene expression in the liver of mice. Phytomedicine Plus2, 100148. 10.1016/j.phyplu.2021.100148
192
Śladowska K. Moćko P. Brzostek T. Malinowska-Lipień I. Owca M. Kawalec P. (2025). Efficacy and safety of epigallocatechin gallate in the treatment and prevention of dermatitis: a systematic review. Biomedicines13, 1458. 10.3390/biomedicines13061458
193
Spergel J. M. Mizoguchi E. Oettgen H. Bhan A. K. Geha R. S. (1999). Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Invest.103, 1103–1111. 10.1172/JCI5669
194
Steinhoff M. Ahmad F. Pandey A. Datsi A. AlHammadi A. Al-Khawaga S. et al (2022). Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol.149, 1875–1898. 10.1016/j.jaci.2022.03.010
195
Su C. Yang T. Wu Z. Zhong J. Huang Y. Huang T. et al (2017). Differentiation of T-helper cells in distinct phases of atopic dermatitis involves Th1/Th2 and Th17/treg. Eur. J. Inflamm.15, 46–52. 10.1177/1721727X17703271
196
Sun Z. Vattepu R. Zhang S. (2021). Chemokines and innate lymphoid cells in skin inflammation. Cells10, 3074. 10.3390/cells10113074
197
Szabó L. Kapitány A. Somogyi O. Alhafez I. Gáspár K. Palatka R. et al (2023). Antimicrobial peptide loss, except for LL-37, is not characteristic of atopic dermatitis. Acta Derm. Venereol.103, adv9413. 10.2340/actadv.v103.9413
198
Szymanski L. Cios A. Lewicki S. Szymanski P. Stankiewicz W. (2018). Fas/FasL pathway and cytokines in keratinocytes in atopic dermatitis - manipulation by the electromagnetic field. PLOS One13, e0205103. 10.1371/journal.pone.0205103
199
Szymczak J. Cielecka-Piontek J. (2023). Fisetin-in search of better bioavailability-from macro to nano modifications: a review. Int. J. Mol. Sci.24, 14158. 10.3390/ijms241814158
200
Tait S. W. G. Green D. R. (2010). Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol.11, 621–632. 10.1038/nrm2952
201
Takahashi H. Nakamura S. Asano K. Kinouchi M. Ishida-Yamamoto A. Iizuka H. (1999). Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp. Cell Res.249, 291–298. 10.1006/excr.1999.4476
202
Tanaka R. Ichimura Y. Kubota N. Saito A. Nakamura Y. Ishitsuka Y. et al (2022). Differential involvement of programmed cell death ligands in skin immune responses. J. Invest. Dermatol.142, 145–154.e8. 10.1016/j.jid.2021.06.026
203
Tang L. Gao J. Li X. Cao X. Zhou B. (2022). Molecular mechanisms of luteolin against atopic dermatitis based on network pharmacology and in vivo experimental validation. Drug Des. devel. Ther.16, 4205–4221. 10.2147/DDDT.S387893
204
Taniguchi M. LaRocca C. A. Bernat J. D. Lindsey J. S. (2023). Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod.86, 1087–1119. 10.1021/acs.jnatprod.2c00720
205
Tao Y. Wang Y. Wang X. Y. Wang C. Bao K. F. Ji L. et al (2017). Calycosin suppresses epithelial derived initiative key factors and maintains epithelial barrier in allergic inflammation via TLR4 mediated NF-κB pathway. Cell. Physiol. biochem. Int. J. Exp. Cell. Physiol. biochem. Pharmacol.44, 1106–1119. 10.1159/000485416
206
Tekale S. Mashele S. Pooe O. Thore S. Kendrekar P. Pawar R. et al (2020). “Biological role of chalcones in medicinal chemistry,” in Vector-borne diseases - recent developments in Epidemiology and control. (IntechOpen). 10.5772/intechopen.91626
207
Teng H. Zheng Y. Cao H. Huang Q. Xiao J. Chen L. (2023). Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: a review. Crit. Rev. Food Sci. Nutr.63, 378–393. 10.1080/10408398.2021.1947772
208
Tominaga M. Takamori K. (2022). Peripheral itch sensitization in atopic dermatitis. Allergol. Int. Off. J. Jpn. Soc. Allergol.71, 265–277. 10.1016/j.alit.2022.04.003
209
Trautmann A. Akdis M. Kleemann D. Altznauer F. Simon H.-U. Graeve T. et al (2000). T cell–mediated fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J. Clin. Invest.106, 25–35. 10.1172/JCI9199
210
Treml J. Šmejkal K. (2016). Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf.15, 720–738. 10.1111/1541-4337.12204
211
Tricarico P. M. Mentino D. De Marco A. Del Vecchio C. Garra S. Cazzato G. et al (2022). Aquaporins are one of the critical factors in the disruption of the skin barrier in inflammatory skin diseases. Int. J. Mol. Sci.23, 4020. 10.3390/ijms23074020
212
Trujillo-Paez J. V. Peng G. Le Thanh Nguyen H. Nakamura M. Umehara Y. Yue H. N. et al (2024). Calcitriol modulates epidermal tight junction barrier function in human keratinocytes. J. Dermatol. Sci.114, 13–23. 10.1016/j.jdermsci.2024.02.001
213
Ugwu D. I. Ezema B. E. Okoro U. C. Eze F. U. Ekoh O. C. Egbujor M. C. et al (2016). ChemInform abstract: syntheses and pharmacological applications of chalcones: a review. Cheminform47, chin.201617216. 10.1002/chin.201617216
214
Vale D. L. Martinez R. M. Medeiros D. C. da Rocha C. Sfeir N. Lopez R. F. V. et al (2021). A topical formulation containing quercetin-loaded microcapsules protects against oxidative and inflammatory skin alterations triggered by UVB irradiation: enhancement of activity by microencapsulation. J. Drug Target.29, 983–997. 10.1080/1061186X.2021.1898621
215
Vazhappilly C. G. Amararathna M. Cyril A. C. Linger R. Matar R. Merheb M. et al (2021). Current methodologies to refine bioavailability, delivery, and therapeutic efficacy of plant flavonoids in cancer treatment. J. Nutr. Biochem.94, 108623. 10.1016/j.jnutbio.2021.108623
216
Wang Y. Li C. Wan Y. Qi M. Chen Q. Sun Y. et al (2021). Quercetin-loaded ceria nanocomposite potentiate dual-directional immunoregulation via macrophage polarization against periodontal inflammation. Small17, 2101505. 10.1002/smll.202101505
217
Wang L. Xian Y.-F. Loo S. K. F. Ip S. P. Yang W. Chan W. Y. et al (2022a). Baicalin ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice through modulating skin barrier function, gut microbiota and JAK/STAT pathway. Bioorg. Chem.119, 105538. 10.1016/j.bioorg.2021.105538
218
Wang S. Liu S. Wang J. Tao J. Wu M. Ma W. et al (2022b). A newly isolated human intestinal strain deglycosylating flavonoid C-glycosides. Arch. Microbiol.204, 310–318. 10.1007/s00203-022-02881-2
219
Wang Y. Tan L. Jiao K. Xue C. Tang Q. Jiang S. et al (2022c). Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol.179, 4792–4808. 10.1111/bph.15913
220
Wang Z. Chen H. Liang T. Hu Y. Xue Y. Wu Y. et al (2024). The implications of lipid mobility, drug-enhancers (surfactants)-skin interaction, and TRPV1 activation on licorice flavonoid permeability. Drug Deliv. Transl. Res.14, 1582–1600. 10.1007/s13346-023-01473-x
221
Wilson S. R. Thé L. Batia L. M. Beattie K. Katibah G. E. McClain S. P. et al (2013). The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell155, 285–295. 10.1016/j.cell.2013.08.057
222
Wu X. Deng X. Wang J. Li Q. (2020). Baicalin inhibits cell proliferation and inflammatory cytokines induced by tumor necrosis factor α (TNF-α) in human immortalized keratinocytes (HaCaT) human keratinocytes by inhibiting the STAT3/nuclear factor kappa B (NF-κB) signaling pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res.26, e919392. 10.12659/MSM.919392
223
Wu S. Pang Y. He Y. Zhang X. Peng L. Guo J. et al (2021). A comprehensive review of natural products against atopic dermatitis: flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed. Pharmacother. Biomedecine Pharmacother.140, 111741. 10.1016/j.biopha.2021.111741
224
Xiao Y. Q. Zhang S. Tong H. B. Shi S. R. (2018). Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother. Res. PTR32, 384–394. 10.1002/ptr.5966
225
Xie L. Deng Z. Zhang J. Dong H. Wang W. Xing B. et al (2022). Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods11, 882. 10.3390/foods11060882
226
Xiong H.-H. Lin S.-Y. Chen L.-L. Ouyang K.-H. Wang W.-J. (2023). The interaction between flavonoids and intestinal microbes: a review. Foods12, 320. 10.3390/foods12020320
227
Xu S. Kang A. Tian Y. Li X. Qin S. Yang R. et al (2024). Plant flavonoids with antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA). ACS Infect. Dis.10, 3086–3097. 10.1021/acsinfecdis.4c00292
228
Yamamura Y. Nakashima C. Otsuka A. (2024). Interplay of cytokines in the pathophysiology of atopic dermatitis: insights from murin models and human. Front. Med.11, 1342176. 10.3389/fmed.2024.1342176
229
Yang C.-Y. Pan C.-C. Tseng C.-H. Yen F.-L. (2022). Antioxidant, anti-inflammation and antiaging activities of artocarpus altilis methanolic extract on urban particulate matter-induced HaCaT keratinocytes damage. Antioxidants11, 2304. 10.3390/antiox11112304
230
Yeo H. Lee Y. H. Koh D. Lim Y. Shin S. Y. (2020). Chrysin inhibits NF-κB-dependent CCL5 transcription by targeting IκB kinase in the atopic dermatitis-like inflammatory microenvironment. Int. J. Mol. Sci.21, 7348. 10.3390/ijms21197348
231
Yeo H. Lee Y. H. Ahn S. S. Jung E. Lim Y. Shin S. Y. (2021). Chrysin inhibits TNFα-induced TSLP expression through downregulation of EGR1 expression in keratinocytes. Int. J. Mol. Sci.22, 4350. 10.3390/ijms22094350
232
Yu Y. Lin D. Cai X. Cui D. Fang R. Zhang W. et al (2020). Enhancement of chemokine mRNA expression by toll-like receptor 2 stimulation in human peripheral blood mononuclear cells of patients with atopic dermatitis. Biomed. Res. Int.2020, 1497175. 10.1155/2020/1497175
233
Yuan L. Pan M. Lei M. Zhou X. Hu D. Liu Q. et al (2020). A novel composite of micelles and hydrogel for improving skin delivery of hydrocortisone and application in atopic dermatitis therapy. Appl. Mater. Today19, 100593. 10.1016/j.apmt.2020.100593
234
Yuki T. Tobiishi M. Kusaka-Kikushima A. Ota Y. Tokura Y. (2016). Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17. PLOS One11, e0161759. 10.1371/journal.pone.0161759
235
Zeinali M. Rezaee S. A. Hosseinzadeh H. (2017). An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. Biomedecine Pharmacother.92, 998–1009. 10.1016/j.biopha.2017.06.003
236
Zhang Z. J. Michniak-Kohn B. (2020). Flavosomes, novel deformable liposomes for the co-delivery of anti-inflammatory compounds to skin. Int. J. Pharm.585, 119500. 10.1016/j.ijpharm.2020.119500
237
Zhang X. Wu C. (2022). In silico, in vitro, and in vivo evaluation of the developmental toxicity, estrogenic activity, and mutagenicity of four natural phenolic flavonoids at low exposure levels. ACS Omega7, 4757–4768. 10.1021/acsomega.1c04239
238
Zhang J. Gao J.-X. Salojin K. Shao Q. Grattan M. Meagher C. et al (2000). Regulation of FAS ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and C-jun Nh2-terminal kinase. J. Exp. Med.191, 1017–1030. 10.1084/jem.191.6.1017
239
Zhang H. Hassan Y. I. Liu R. Mats L. Yang C. Liu C. et al (2020). Molecular mechanisms underlying the absorption of aglycone and glycosidic flavonoids in a caco-2 BBe1 cell model. ACS Omega5, 10782–10793. 10.1021/acsomega.0c00379
240
Zhang S. Fang X. Xu B. Zhou Y. Li F. Gao Y. et al (2024). Comprehensive analysis of phenotypes and transcriptome characteristics reveal the best atopic dermatitis mouse model induced by MC903. J. Dermatol. Sci.114, 104–114. 10.1016/j.jdermsci.2024.05.003
241
Zhao J. Yang J. Xie Y. (2019). Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int. J. Pharm.570, 118642. 10.1016/j.ijpharm.2019.118642
242
Zhao Y. Liu Z. Qin L. Wang T. Bai O. (2021). Insights into the mechanisms of Th17 differentiation and the yin-yang of Th17 cells in human diseases. Mol. Immunol.134, 109–117. 10.1016/j.molimm.2021.03.010
243
Zhao Y. Yang Y. Liu M. Qin X. Yu X. Zhao H. et al (2022). COX-2 is required to mediate crosstalk of ROS-dependent activation of MAPK/NF-κB signaling with pro-inflammatory response and defense-related NO enhancement during challenge of macrophage-like cell line with giardia duodenalis. PLoS Negl. Trop. Dis.16, e0010402. 10.1371/journal.pntd.0010402
244
Zheng C. Cao T. Ye C. Zou Y. (2023). Neutrophil recruitment by CD4 tissue-resident memory T cells induces chronic recurrent inflammation in atopic dermatitis. Clin. Immunol. Orl. Fla256, 109805. 10.1016/j.clim.2023.109805
245
Zhou Z. W. Zhu X. Y. Li S. Y. Lin S. E. Zhu Y. H. Ji K. M. et al (2023). Formononetin inhibits mast cell degranulation to ameliorate compound 48/80-induced pseudoallergic reactions. Mol. Basel Switz.28, 5271. 10.3390/molecules28135271
246
Zouaoui S. Farman M. Semmar N. (2021). Review on structural trends and chemotaxonomical aspects of pharmacologically evaluated flavonoids. Curr. Top. Med. Chem.21, 628–648. 10.2174/1568026621666210113165007
Summary
Keywords
natural flavonoids, atopic dermatitis, botanical drugs, multi-target pharmacological actions, novel drug delivery systems
Citation
Li D, Han Y, Zhou J, Chen J, Tey HL and Tan TTY (2025) Flavonoids in atopic dermatitis: mechanisms, delivery innovations, and translational strategies. Front. Pharmacol. 16:1631977. doi: 10.3389/fphar.2025.1631977
Received
20 May 2025
Accepted
07 August 2025
Published
22 August 2025
Volume
16 - 2025
Edited by
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Reviewed by
Patricia Quintero Rincón, University of Antioquia, Colombia
Chao Yi Huang, Kaohsiung Armed Forces General Hospital, Taiwan
Sabyasachi Banerjee, Gupta College of Technological Sciences, India
Madhur Kulkarni, Indira College of Pharmacy, India
Updates
Copyright
© 2025 Li, Han, Zhou, Chen, Tey and Tan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Chen, chenjing6385@163.com; Hong Liang Tey, teyhongliang@ntu.edu.sg; Timothy T. Y. Tan, tytan@ntu.edu.sg
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.