- 1Department of Medical Oncology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Medical Oncology, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Department of Chinese Medicine and Integrative Medicine, Shanghai Geriatric Medical Center, Zhongshan Hospital, Fudan University, Shanghai, China
Colorectal cancer (CRC) is a common and aggressive malignancy of the gastrointestinal tract with a severe disease burden. The role of Traditional Chinese Medicine (TCM) and its natural active ingredients in enhancing the therapeutic effects of radiotherapy and chemotherapy and preventing the recurrence and metastasis of CRC has been increasingly recognized. Herba Patriniae has shown significant clinical efficacy for the treatment of CRC. Flavonoids has been found to be one of the main active anticancer components of Herba Patriniae. This review summarizes the latest findings from clinical trials and in vitro studies on anticancer mechanisms of Herba Patriniae, and discusses the role of the flavonoids in combination therapy against CRC. These flavonoids exert anticancer effects through diverse mechanisms. For instance, they prevent the development of precancerous lesions, regulate the cell cycle, modulate CRC cell proliferation, promote tumor cell apoptosis, inhibite epithelial-mesenchymal transition, reverse drug resistance, and modulate gut microbiota by acting on several key signaling pathways, such as PI3K/Akt/mTOR, Wnt/β-catenin, and EGFR/ERK/MAPK. Future research should prioritize clarifying the specific dosage and safety of flavonoids under different pathological conditions, further conducting large-scale, rigorously designed clinical studies to determine the efficacy differences of flavonoids for patients with different pathological types of CRC and simultaneously delving into the mechanisms of their anti-colorectal cancer effects, as well as their interactions with the intestinal microbiota and tumor microenvironment.
Introduction
Based on the latest data from the International Agency for Research on Cancer (IARC), new cases of CRC accounted for 10.2% of all incident malignant neoplasms, making it the third most common cancer worldwide. CRC-associated death accounts for 9.3% of all malignant neoplasms, making it the second deadliest cancer after lung cancer (Bray et al., 2024; Global Nutrition Target Collaborators, 2025). Due to the influence of dietary habits, lifestyle and economic development (Vernia et al., 2021), CRC is becoming more prevalent among younger people. The overall burden of the disease is still increasing, particularly in developing countries (Morgan et al., 2023), this presents a major challenge for the clinical management of CRC. Genetically, colorectal cancer (CRC) can be genetically divided into two main types: hereditary and sporadic. Hereditary CRC primarily includes familial adenomatous polyposis (FAP) and Lynch syndrome. Sporadic CRC, which accounts for approximately 75% of cases, is mainly caused by gene mutations triggered by environmental factors. The occurrence and progression of sporadic CRC frequently involve the inactivation of genes such as APC, DCC, and TP53, over-expression of mutations in genes like KRAS, PIK3CA, and BRAF, as well as deletions in DNA mismatch repair genes. Most cases of CRC cases follow the “adenoma-cancer” sequence of progression, it takes between 5 and 10 years for an adenoma to advance to cancer (Zhu et al., 2020; Sun et al., 2024). Treatment for CRC mainly includes surgical resection, alongside and radiotherapy. However, 25%–30% of patients have metastasis when their initial diagnosis, and 50% of patients experience recurrent metastasis within 5 years of surgery (Ciardiello et al., 2022). Patients diagnosed with advanced CRC face many limitations, such as resistance to chemotherapy and serious adverse reactions. In-depth studies on molecular subtypes have greatly improved the 5-year survival rate of patients receiving molecularly targeted drugs (Ciardiello et al., 2022), such as drugs targeting vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) and epidermal growth factor receptor (EGFR) (Xie et al., 2020). Such studies have also improved the prognosis of patients by detecting their microsatellite instability/mismatch repair (MSI/MMR) and programmed death ligand 1 (PD-L1) status and adding immunotherapy (André et al., 2025). Current pharmacological interventions for CRC primarily focus on single-target agents; however, the therapeutic efficacy of drugs inhibiting individual signaling pathways or biological targets remains limited. In contrast, Traditional Chinese Medicine (TCM) and its bioactive components exhibit distinct advantages through their multi-target, multi-pathway, and multi-effect regulatory mechanisms (Chen et al., 2023; Gou et al., 2023). These compounds can markedly alleviate clinical symptoms, improve quality of life, and stabilize lesions in patients with CRC. In addition, when used in combination therapies, these compounds can enhance treatment outcomes and minimize adverse effects.
Herba Patriniae, a wild plant with recognized medicinal and dietary value, has been utilized for over 2,000 years. It is derived from Patrinia scabiosaefolia or Patrinia villosa of the Valerianaceae family, with its rhizomes, roots, and entire herba employed for therapeutic purposes. Widely distributed across tropical and subtropical regions, Herba Patriniae is rich in bioactive compounds, such as triterpenes, saponins, iridoids, and flavonoids; remarkably, Patrinia villosa contains more flavonoids (Gong et al., 2021). These constituents contribute to its diverse pharmacological properties, including antioxidant, anti-tumor, anti-inflammatory, antimicrobial, and antiviral activities, making it a promising candidate for drug development (Li et al., 2018; Huang et al., 2019; Zou et al., 2021; Liu et al., 2023a; Li K. et al., 2024).
Recently, many studies have supported the efficacy of Herba Patriniae in preventing and treating CRC, although the underlying mechanisms of action remain unclear. The anticancer compounds of this plant have shown significant interactions with target proteins involved in the progression of CRC (Qi et al., 2020). Studies have suggested that Herba Patriniae acts through several signaling pathways to inhibit CRC, with its high flavonoid content playing a pivotal role. These flavonoids suppress cell proliferation (Zhang M. et al., 2015; Yang et al., 2023), induce apoptosis (Liu et al., 2013), mediate cell cycle arrest (Zhang M. et al., 2015), suppress angiogenesis in the tumor microenvironment (Chen et al., 2013), and ameliorate drug resistance (Huang et al., 2019). Despite their potent bioactivity, flavonoids, as polyphenolic compounds, generally have poor oral bioavailability, with only minimal metabolites detectable in urine and blood. Their interaction with gut microbiota is a critical mechanism underlying their biological activity (Liu et al., 2023b). Building on these findings, we reviewed the findings of clinical and preclinical studies focused on effective flavonoids in Herba Patriniae and unraveled their molecular mechanisms in CRC. This review provides a foundation for the development of anticancer small molecules derived from Herba Patriniae and help identify their potential therapeutic targets.
Flavonoids in Herba Patriniae
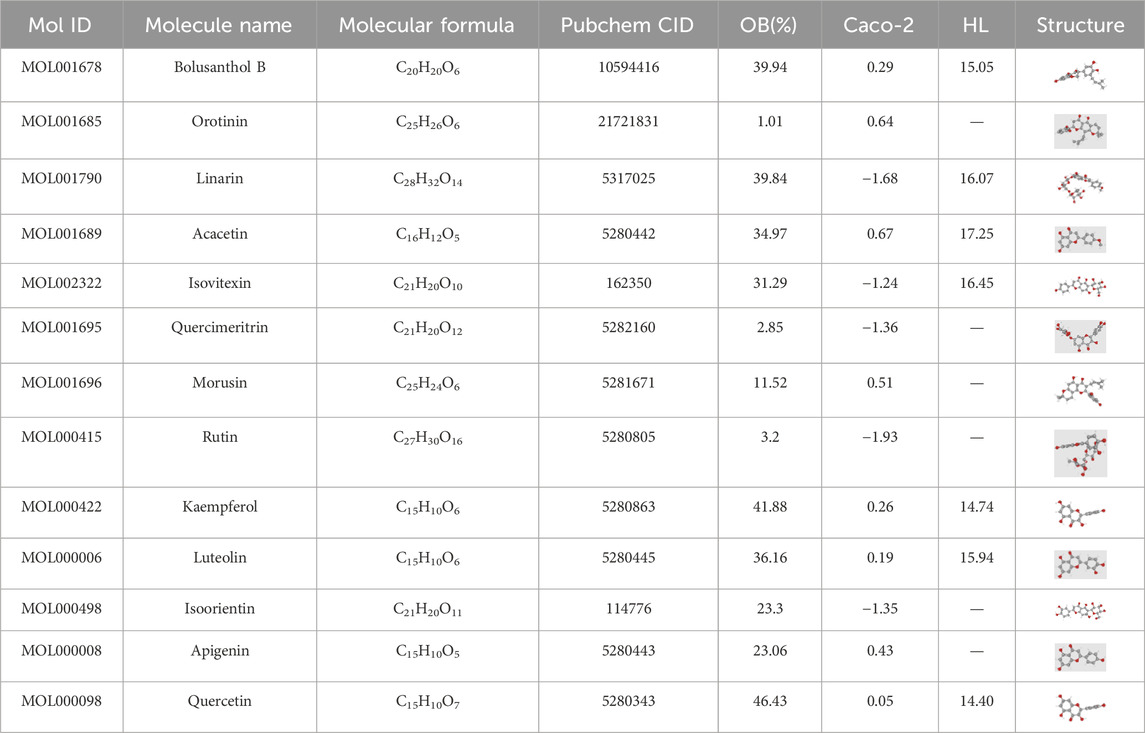
Flavonoids, a class of polyphenolic compounds, have a chemical structure composed of 15 carbon atoms, including two benzene rings (A ring and B ring) and an oxygen-containing ring (C ring). The basic structure of flavonoids is generally C15H10O2, although their molecular formula vary based on their substituent groups. Structural diversity among flavonoids arises from substitutions on the A, B, or C rings, such as amino, methyl, hydroxyl, or glycosyl groups, resulting in derivatives with distinct biological activities (Zhang et al., 2007; Lotito et al., 2011). With a deeper understanding of the role of gut microbiota, studies have shown that flavonoid metabolism in the body relies not only on liver enzyme systems but also on gut microbiota. Through processes such as hydrolysis, reduction, and metabolic transformation, gut microbiota modifies the structure of flavonoids, enhances their biological activity, and affects their bioavailability and pharmacological properties (Kumar Singh et al., 2019; Landete, 2022). Epidemiological studies have indicated that higher flavonoid intake and serum levels are associated with a lower risk of colorectal inflammation and CRC (Chang et al., 2018). Based on an analysis of the TCM pharmacology database, we identified 13 flavonoid compounds from 52 active ingredients in Herba Patriniae that have been widely studied for their anticancer properties (Table 1, https://www.tcmsp-e.com/). A comprehensive understanding of the pharmacological effects of these flavonoids on CRC is critical for advancing therapeutic strategies.
Preclinical studies on the anticancer effects of Herba Patriniae and flavonoids against CRC
Crypts are deep grooves formed by epithelial cells in the colon. Abnormal crypt foci (ACF) appear when these crypts undergo significant morphological changes, such as hyperplasia, irregular shapes, or abnormal cell differentiation. ACF are one of the earliest precancerous lesions in CRC. Early detection and effective intervention can significantly reduce the risk of CRC.
Flavonoid compounds can effectively prevent ACF. For example, the number of abnormal crypts in the colons of stressed mice was significantly reduced after treatment with quercetin (Ritter and Dinh, 1992; Matsukawa et al., 1997; Volate et al., 2005). Interestingly, in an obesity-related carcinogenesis model, quercetin significantly inhibited precancerous lesions and reduced serum leptin levels. Furthermore, in vivo studies revealed that quercetin markedly suppressed the expression of leptin mRNA in differentiated 3T3-L1 mouse adipocytes. These findings suggest that quercetin has the potential to inhibit colorectal cancer induced by obesity (Miyamoto et al., 2010). In the mice model of AOM-induced CRC, a 0.5% quercetin diet effectively inhibited intestinal lesions (Tutino et al., 2018). Similarly, a diet containing 0.1% apigenin reduced the number of high-magnitude ACF (defined as > 4 abnormal crypts per focus) by 57% (P < 0.05) (Au et al., 2006; Leonardi et al., 2010). Luteolin (1.2 mg/kg/day) mitigated AOM-induced intestinal oxidative damage by reducing lipid peroxidation, enhancing antioxidant defenses, and suppressing the formation of precancerous lesions (Ashokkumar and Sudhandiran, 2008). In the APCMin/+ mouse model, apigenin dose-dependent inhibited tumor growth by phosphorylating the p53 protein in tumor tissue, thereby regulating tumorigenesis (Zhong et al., 2010). Treatment with kaempferol reduced tumor burden, restored impaired intestinal barriers, and downregulated the expression of Ki67 and LGR5 in APCMin/+ mouse model (Li X. et al., 2022). However, rutin exhibited no preventive effect in the AOM-induced mouse model, likely due to its low intestinal bioavailability. Improving the solubility of rutin using solid dispersion technology and formulating it into frankincense-based compression-coated tablets enhanced its efficacy in inhibiting the development of CRC in vivo (Ismail et al., 2023).
Chronic inflammation is a significant risk factor for CRC, as it promotes cancer cell proliferation, invasion, and metastasis by modulating the tumor microenvironment, activating oncogenes, immune evasion, and dysbiosis (Yaeger et al., 2016; Chen et al., 2024). Apigenin has been shown to effectively inhibit inflammatory bowel disease (IBD) and colitis-associated cancer (CAC) in mice (Ai et al., 2017). Similarly, vitexin mitigated AOM/DSS-induced chronic colitis-associated carcinogenesis in mice (Chen et al., 2021). Linarin (25 or 50 mg/kg/day) significantly reduced myeloperoxidase activity in the colon and downregulated pro-inflammatory cytokines, such as TNF-α and IL-1β, while upregulating the anti-inflammatory cytokine IL-10. This effect alleviated DSS-induced intestinal damage and the function of the mucosal and intestinal barrier (Jin et al., 2022). In a TNBS-induced experimental colitis model in Wistar rats, morusin (12.5 mg/kg) showed comparable efficacy to sulfasalazine (50 mg/kg) in suppressing inflammation (Vochyánová et al., 2017).
In a CRC metastasis models, quercetin (Kee et al., 2016) and luteolin (10 or 50 mg/kg) significantly reduced the number and volume of lung metastases induced by CT26 cells (Kim H. Y. et al., 2013). Moreover, luteolin (IC50 = 5.9 μM, indicating relatively low anticancer activity) suppressed CT26-induced liver metastasis of colon cancer by 24% (Naso et al., 2016).
Clinical studies on the anticancer effects of flavonoids against CRC
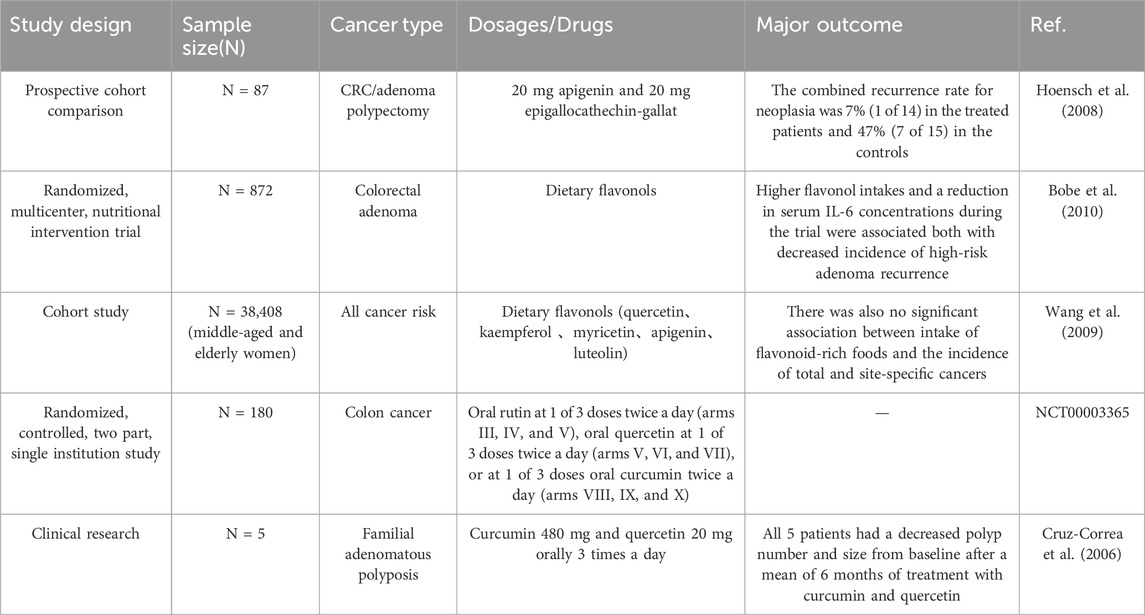
A prospective cohort study involving 87 patients, consisting of 36 patients who underwent CRC surgery and 51 patients who underwent adenoma polypectomy, investigated the preventive effects of a flavonoid mixture (20 mg apigenin and 20 mg epigallocatechin gallate) on tumor recurrence. After 3–4 years of colonoscopy follow-up, the group receiving the flavonoid mixture exhibited a tumor recurrence rate of 7% (1/14, 1 adenoma) compared to a 47% recurrence rate (7/15, including 3 tumor recurrence and 4 adenomas) in a matched untreated control group of patients with CRC (n = 15). These findings suggest that long-term treatment with flavonoids can reduce the recurrence rate of colorectal tumors in patients undergoing surgery (Hoensch et al., 2008). A 4-year nutritional intervention trial featuring a low-fat, high-fiber, high-fruit, and high-vegetable diet found that the intake of flavonoids, such as isoquercitrin, kaempferol, and quercetin, was associated with decreased serum levels of IL-6 and a lower recurrence rate of high-risk adenomas. This finding suggests a potential correlation between flavonoid intake and a decreased risk of adenoma recurrence (Bobe et al., 2010).
Patients with familial adenomatous polyposis (FAP) suffer from a high risk of CRC. In a small clinical study, 6 months of combined treatment with curcumin and quercetin after CRC surgery led to a 60% reduction in the number and size of polyps in the ileum and rectum compared to baseline, indicating that the potential of this combination therapy in reducing polyp burden in patient with FAP (Cruz-Correa et al., 2006).
A Phase I clinical study on quercetin’s resistance and pharmacokinetics revealed the safety of intravenous quercetin. At a specific plasma concentration, it inhibited the activity of lymphocyte tyrosine kinase, showing anti-tumor effects. In nine patients with CRC, treatment with intravenous quercetin for 1 hour suppressed the phosphorylation of serum lymphocyte protein tyrosine for 16 h. Additionally, in a patient with cisplatin-resistant ovarian cancer, treatment with quercetin (420 mg/m2) reduced CA125 levels from 295 units/mL to 55 units/mL. Similarly, another patient with liver cancer showed decreased serum alpha-fetoprotein levels after treatment (Ferry et al., 1996).
However, a large retrospective clinical study with 38,408 participants assessed flavonoid intake using dietary questionnaires and found no significant association between the intake of five common flavonoids (quercetin, kaempferol, myricetin, apigenin, and luteolin) or flavonoid-rich foods and CRC prevention (Wang et al., 2009). This lack of correlation may be attributed to the low flavonoid content in typical diets, antibiotic use, poor dietary habits, etc.
Although Herba Patriniae is widely used in clinical practice and its flavonoid compounds exhibit anti-CRC activity (Table 2), there is currently no large-scale clinical research supporting their efficacy. And most existing clinical studies used the combined administration of two or more flavonoid compounds. Systematic clinical evaluations of flavonoid interventions targeting for CRC pathological diversity and mutational heterogeneity are notably absent in current literature. Future research should also focus on the effective dosage and safety of individual flavonoid compounds from Herba Patriniae.
Mechanisms of action of flavonoids in their anticancer activity against CRC
The majority of colorectal cancer cases follow the classical adenoma-carcinoma sequence: normal colonic epithelium -adenomatous polyp–adenocarcinoma (Li et al., 2021). This carcinogenic progression begins with a mutation in the APC gene, followed by further mutations in KRAS, TP53, DCC and other critical genes (Schumacher et al., 2015). CRC pathogenesis involves the dysregulation of multiple pivotal signaling pathways: The Wnt/β-catenin pathway promotes tumor cell proliferation and differentiation, the PI3K/AKT pathway enhances cancer cell growth, survival, and metabolic adaptation, and the RAS/RAF/MEK/ERK cascade drives cell cycle progression and aberrant proliferation. In contrast, the transforming growth factor-β (TGF-β) pathway exhibits tumor-suppressive effects in the early stages, but promotes tumor invasion and metastasis through epithelial-mesenchymal transition (EMT) in the advanced stages of the disease. Current evidence demonstrates that flavonoids can effectively suppress CRC progression through multi-target mechanisms and the coordinated modulation of these oncogenic pathways (Li Q. et al., 2024).
Inhibition of inflammation-associated tumors
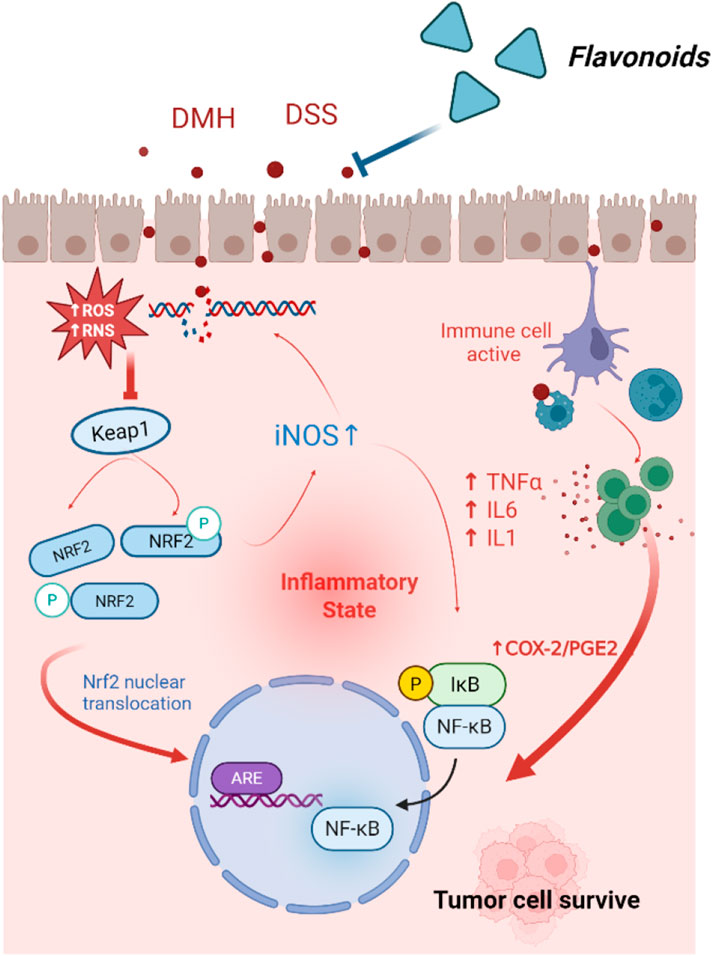
Due to severe oxidative stress, reactive oxygen species (ROS) are released in chronic and sustained inflammation. Elevated levels of ROS can lead to DNA damage, thereby promoting the development of CRC. Interestingly, high levels of ROS in tumor cells present a potential therapeutic target (Lyons et al., 2023). The colon cancer inducer DMH has been shown to elevate ROS levels in the mouse models of CRC. Quercetin, administered at doses of 25 or 50 mg/kg can restore antioxidant response and mitigate membrane damage (Wang et al., 2009). The cytotoxic effects of quercetin on CRC cells are mediated through ROS-induced apoptosis and inhibition of cell survival pathways (Raja et al., 2017). Additionally, quercetin reverses DMH-induced oxidative stress and DNA damage by targeting the NRF2/Keap1signalling pathway. Compared to healthy individuals, patients with CRC exhibit more severe DNA damage in their lymphocytes (Figure 1) (Darband et al., 2020). In vitro studies have shown that quercetin (500 μM) reduces oxidative stress in lymphocytes of patients with CRC. Similarly, luteolin has been reported to suppress azoxymethane-induced CRC by activating the NRF2 signaling pathway (Pandurangan et al., 2014a). Elevated mitochondrial superoxide levels suggest that mitochondrial oxidative damage arises from an imbalance between anti-apoptotic and pro-apoptotic proteins, leading to dose-dependent cellular injury. Prolonged treatment with apigenin at growth-inhibitory doses was shown to induces persistent oxidative stress, ultimately triggering cellular senescence, a natural tumor suppression mechanism. In CRC cell lines HT-29 and HCT-15, apigenin has been shown to induce sustained oxidative stress at growth-inhibitory doses, leading to cellular senescence (Banerjee and Mandal, 2015).
During inflammation, immune cells are recruited to the intestines, where they release ROS and pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6. These factors can alter the intestinal microenvironment, promoting the growth and metastasis of CRC cells. Quercetin has been shown to inhibit the production of TNF-α and IL-6 (Han et al., 2016). Isoorientin, on the other hand, suppresses DSS-induced production of TNF-α, IL-β, and TNF-α induced activation of NF-κB by upregulating AHR, thereby protecting the integrity of the intestinal barrier (Mu et al., 2023).
Pro-inflammatory cytokines secreted by inflammatory cells can also activate the expression of various oncogenes and inhibit tumor suppressor genes. In the AOM/DSS-induced inflammation-associated tumor mouse model, quercetin inhibited the expression of COX-1, COX-2, and iNOS (Warren et al., 2009). Additionally, quercetin (IC50 = 10.5 μmol) effectively downregulated the transcription of COX-2 in human CRC DLD-1 cells (Mutoh et al., 2000).
Inflammation not only induces mutations through DNA damage but can also affect cancer-related genes via epigenetic modification, thereby silencing key tumor suppressor genes. Luteolin was shown to reduce methylation levels in the promoter region of Nrf2 and decreased protein levels and enzyme activities of DNMTs and HDACs in HCT116 cells. This finding suggests that luteolin may exert antitumor effects partly by epigenetically modulating the Nrf2 gene, thereby activating downstream antioxidant stress pathways via the Nrf2/ARE signaling pathway (Zuo et al., 2018). Following a thorough database screening, six key genes targeted by rutin in CRC were identified, including TP53, PCNA, CDK2, LDHA, CDKN1A and CCNB1. Molecular docking studies revealed that rutin exhibited a strong binding affinity for these targets. Following 48 h of rutin treatment in HT29 cells, the mRNA expression levels of the CRC target genes PCNA, CDK2, LDHA and CCNB1 decreased significantly. Conversely, TP53 and CDKN1A expression levels increased. Taken together, these results suggest that rutin treatment exerts regulatory effects in HT-29 cells and is involved in the ROS pathway (Gatasheh, 2024).
Inhibition of CRC cell lines proliferation
PI3K/Akt/mTOR signaling
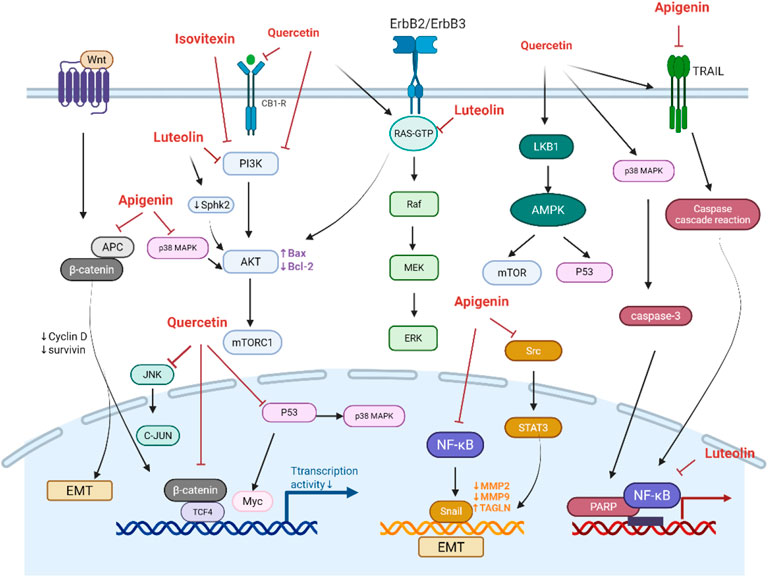
The PI3K/Akt pathway is a critical signaling pathway in CRC development, which promotes cell survival and growth and cell cycle progression. Due to its estrogen-like structure, quercetin can bind to CB1-R, which is an estrogen-responsive receptor. This binding inhibits the PI3K/AKT/mTOR pathway in human colorectal adenocarcinoma cells (Caco2 and DLD-1) and activates the pro-apoptotic JNK/JUN pathway (Zuo et al., 2018). Quercetin also directly interacts with PI3K, reducing the expression of p-PI3K and p-AKT proteins and upregulating Bax and caspase-3 proteins. Consistently, quercetin inhibited cell proliferation and promoted the apoptosis of SW480 cells (Na et al., 2022). Additionally, quercetin downregulates the ErbB2/ErbB3 signaling pathway and the Akt pathway and lowered Bcl-2 levels, which suppressed the growth of HT29 and SW480 cells and induced their apoptosis (Kim et al., 2005). Luteolin exerts cytotoxic effects by inhibiting Akt activation and SphK2, thereby reducing S1P, an activator of Akt (Figure 2) (Kim et al., 2005).
Wnt/β-catenin signaling
In CRC, mutations in the APC gene or excessive activation of β-catenin can lead to its accumulation and nuclear translocation, which promotes the transcriptional activation of oncogenes, such as c-Myc and Cyclin D1, thereby accelerating cell proliferation. The interaction between β-catenin and TCF4 acts as a “molecular switch” in the Wnt signaling pathway. Treatment of SW480 cells with quercetin (160 μmol/L) for 24 h reduced the transcriptional activity of β-catenin/TCF by 18-fold, dose-dependently downregulated the transcription and protein expression of Cyclin D1 and survivin, inhibiting cell proliferation (Shan et al., 2009). Similarly, morusin inhibits Akt, which leads to an increased expression and activation of Gsk-3β. The activated Gsk-3β subsequently reduces the expression of β-catenin, resulting in a decrease in TCF4 expression. Consequently, morusin suppresses key downstream targets of the Wnt/β-catenin pathway (c-Myc and survivin) in a concentration-dependent manner (Zhou et al., 2021). Apigenin-induced dysfunction of APC is a key mechanism responsible for inducing cell cycle arrest in HT29-APC cells. Additionally, apigenin enhances APC expression and promotes apoptosis in wild-type APC cells (Figure 2) (Chung et al., 2007).
EGFR/ERK/MAPK signaling
The activation of EGFR triggers the Ras/Raf/MEK/ERK signaling cascade, which promotes cell proliferation, migration, and invasion. Luteolin was found to mediate the MAPK signaling pathway, thereby inhibit the proliferation of CRC cells (HCT116, HT29), and inducing cell cycle arrest, DNA damage, and apoptosis (Chung et al., 2007). A combination of apigenin (25 μmol) and chrysin (25 μmol) synergistically inhibited CRC cells growth and metastasis by suppressing the P38-MAPK/AKT pathway (Zhang et al., 2021). Quercetin inhibited the ERK/MAPK pathway and targets LKB1 to activate AMPK, thereby enhancing autophagy in radioresistant human CRC cells (HT500) (Russo et al., 2023). Quercetin also enhanced the expression of Sestrin 2 and p53, and activated AMPK/p38, AMPK-mTOR, and AMPK/COX-2 pathways (Lee et al., 2009; Kim et al., 2010; Kim G. T. et al., 2013; Kim et al., 2014).
Tumor cell growth is closely associated with cell cycle progression. Luteolin exhibited potent anti-proliferative properties in human CRC cells by promoting apoptosis, increasing the number of cells in the G1 phase, and reducing the number of cell in the S phase, while enhancing the proliferation of HF cells (Gennari et al., 2011). Furthermore, luteolin shifted oxaliplatin-induced G0/G1 arrest in HCT116 cells to apoptosis (Jang et al., 2019). Phytoestrogens mimic the effects of estradiol and induce apoptosis by interacting with ERα and ERβ. Quercetin modulates hormone receptor ESR2 and GPR30 related signaling pathways, arresting HT-29 cells in the G0/G1 phase, while fermented quercetin (FEQ) extract induces S phase arrest (García-Gutiérrez et al., 2023). The combination of ERβ ligands and tamoxifen (TMX) was found to induce tumor stasis in CRC cells (García-Gutiérrez et al., 2023). Moreover, apigenin reduces ER-mediated YAMC cell growth (Yang et al., 2015).
Induction of apoptosis in CRC cells
Intrinsic Pathway
The intrinsic pathway activates cell death through the release of apoptosis-related factors from the mitochondria. Quercetin has been shown to activate p38 in DLD-1 cells, promoting caspase-3 activation, PARP cleavage, and cell death (Bulzomi et al., 2012). Additionally, quercetin mediates apoptosis in HT-29 cells by modulating Akt phosphorylation and promoting the degradation of CSN6 protein, which affects the expression levels of Myc, p53, Bcl-2, and Bax. This suggests that quercetin-induced apoptosis in HT-29 cells may involve the Akt-CSN6-Myc signaling axis (Yang et al., 2016). Apigenin induces apoptosis in CRC cells by simultaneously inhibiting Bcl-1and Mcl-1 via the STAT3 signaling pathway (Maeda et al., 2018).
Extrinsic Pathway
In the extrinsic pathway, FADD activates caspase-8, finally initiating apoptosis by activating caspase-3. Apigenin enhances the expression and phosphorylation of FADD, potentially leading to CRC cell apoptosis and inhibiting tumor growth (Wang et al., 2011). Tumor necrosis factor ligand superfamily member 10 (TRAIL) triggers apoptotic signaling by binding to its receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2). Quercetin facilitates the redistribution of DR4 and DR5 on the cell surface, enhancing TRAIL-induced caspase cascade (Psahoulia et al., 2007) and the NF-κB pathway to induce apoptosis (Zhang X. A. et al., 2015). A novel dual-targeting oncolytic adenovirus, combining complement CD55-TRAIL, synergistically suppresses tumor growth and induces CRC cell apoptosis both in vitro and in vivo (Xiao et al., 2017). Apigenin disrupts TRAIL resistance in HTLV-1-associated ATL by transcriptionally downregulating c-FLIP (a key inhibitor of death receptor signaling) and upregulating TRAIL-R2 (Ding et al., 2012).
JNK and p38 MAPK Pathways
KRAS-mutant cells are more sensitive to quercetin-induced apoptosis compared to wild-type cells (Xavier et al., 2009). Quercetin selectively activates the c-Jun N-terminal kinase (JNK) pathway in KRAS-mutant cells (Yang et al., 2019). By inhibiting NF-κB, luteolin was shown to enhance TNF-α-induced activation of JNK (Shi et al., 2004).
P53 Pathway, miRNA Regulation, and Others
Isovitexin induces apoptosis and cell cycle arrest via activating p53, thereby protecting against CRC (Li et al., 2023). Apigenin (Lee et al., 2014) and kaempferol (Choi et al., 2018) were shown to induce PARP cleavage and decrease the levels of caspases −8, −9, and −3, finally promoting apoptosis in CRC cells, however, the pro-apoptotic effects of kaempferol were reversed by ROS and p53 signaling.
miRNA-215-5p acts as a tumor suppressor, directly binding to and degrading the mRNA of E2F1 and E2F3, and inhibiting their protein synthesis. E2F1 and E2F3 play key roles in the G1/S phase of the cell cycle. Apigenin was found to downregulates their expression, resulting in cell cycle arrest and reduced cancer cell proliferation of CRC cells (Cheng et al., 2021). Kaempferol inhibits the nonoxidative pentose phosphate pathway (PPP), reducing ribose-5-phosphate (R5P) production and causing DNA damage. Mechanistically, kaempferol upregulates microRNA-195/497 (miR-195/497), which directly binds to the 3′-UTR of PFKFB4 mRNA to suppress PFKFB4 expression. This downregulation inhibits key nonoxidative PPP enzymes transketolase (TKT) and transaldolase (TALDO) (Wu et al., 2025). Apigenin also induces G2/M phase arrest in SW480 and Caco-2 cells (Wang et al., 2004). Concentration-dependent inhibition of HCT116 cell growth by apigenin leads to G2/M arrest, suppression of cyclin B1 and its activation partners cDC2 and CDC25C, and increased expression of the cell cycle inhibitors p53 and p53-dependent p21 (CIP1/WAF1).
Acacetin induces apoptosis in a caspase-independent manner by triggering mitochondrial ROS-mediated cell death through apoptosis-inducing factor in SW480 and HCT-116 CRC cells (Prasad et al., 2020). Luteolin regulates apoptosis signaling in BE CRC cells by downregulating the expression of calpain, UHRF1, and DNMT1 (Krifa et al., 2014). PKM2 is a key enzyme involved in the metabolic reprogramming of cancer cells, and studies have indicated that apigenin can target the K433 site of PKM2, inhibit glycolysis and suppress the proliferation of CRC cells and tumor progression both in vitro and in vivo (Shi et al., 2023).
Inhibition of CRC cell invasion and metastasis
Approximately 90% of cancer-related deaths are attributed to distant metastasis. CRC is known for its high metastatic potential, making the inhibition of metastasis crucial for effective treatment. The mechanisms underlying metastasis are complex, with epithelial-mesenchymal transition (EMT) being a key process. Tumor cells need to undergo EMT and lose their epithelial characteristics to acquire invasive and migratory capabilities. Downregulation of E-cadherin and upregulation of N-cadherin are two hallmarks of EMT.
Quercetin has been identified as a promising therapeutic agent for the treatment of refractory cancers and preventing EMT-mediated metastasis. It modulates EMT markers such as E-cadherin, N-cadherin, β-catenin, and Snail, thereby inhibiting the migration and invasion of Caco-2 and CT26 cells (Han et al., 2016; Kee et al., 2016). TGF-β is a well-known inducer of EMT, and quercetin has been shown to reverse TGF-β1-induced morphological changes and EMT-like phenotypes in SW480 cells by inhibiting the expression of Twist1 (Feng J. et al., 2018). Similarly, luteolin suppresses EMT in CRC cells at the transcriptional level by downregulating CREB1 expression (Liu et al., 2017). Isovitexin reduces the levels of p-PI3K, p-Akt, p-mTOR, and Bcl-2 in tumor tissues, thereby inhibiting the migration, invasion, and EMT of cancer cells (Zhu et al., 2021). Additionally, isoorientin inhibits EMT and reversed cancer stem cell-like traits in oral squamous carcinoma by blocking the Wnt/β-catenin/STAT3 axis (Liu et al., 2021).
Apigenin also exhibited strong anti-EMT properties by inhibiting the Wnt/β-catenin (Xu et al., 2016) and NF-κB/Snail signaling pathways in human CRC cells (Tong et al., 2019). It hindered the migration, invasion, and metastasis of CRC cells through the NEDD9/Src/Akt cascade (Dai et al., 2016). Notably, apigenin upregulates cell surface protein CD26 and enhanced DPPIV activity in HT-29 and HRT-18 human CRC cells, further inhibiting tumor metastasis. The combination of apigenin with chemotherapeutic agents, such as irinotecan, 5-fluorouracil, and oxaliplatin, enhanced the CD26 for advanced CRC (Lefort and Blay, 2011). Pu et al. found that circ_0000345 promotes CRC metastasis by activating the JMJD2C/β-catenin pathway through miR-205-5p (Pu et al., 2024). In addition, they observed that kaempferol suppresses the expression of circ_0000345, effectively blocking JMJD2C/β-catenin signaling and inhibiting the lung metastasis of CRC.
MMPs are critical effectors in the EMT process. Quercetin and luteolin downregulate the expression of metastasis-related proteins MMP-2 and MMP-9 (Kim H. Y. et al., 2013; Han et al., 2016), as well as tissue inhibitors of metalloproteinases (TIMPs) (Pandurangan et al., 2014b; Kee et al., 2016). Apigenin upregulates TAGLN, which in turn downregulates MMP-9 expression and prevents cell proliferation and migration by reducing Akt phosphorylation at Ser473 and particularly at Thr308 (Chunhua et al., 2013).
Enhancing cell sensitivity to drugs or radiation
Recently, natural compounds in cancer treatment has gained increasing attention in clinical practice. Studies have shown that flavonoids found in Patrinia scabiosaefolia, such as quercetin, luteolin, apigenin, and isoorientin, can enhance the cytotoxic effects of various chemotherapeutic agents on tumor cells, which may provide more options for CRC (Özerkan, 2023).
Cisplatin (CP) and oxaliplatin are the most commonly used platinum-based chemotherapeutics agents for treating CRC. However, their application is limited by their toxic effects on normal tissues and drug resistance. Quercetin, when combined with CP, has been shown to reduce ACF while enhancing the efficacy of CP and mitigating its nephrotoxicity (Li et al., 2016). Apigenin inhibits tumorigenesis in cisplatin-resistant CRC cells both in vitro and in vivo by inducing autophagy, and programmed cell death and targeting the mTOR/PI3K/Akt signaling pathway (Chen et al., 2019). Additionally, isoorientin activates the SIRT1/SIRT6/Nrf2 pathway to reduce oxidative stress and apoptosis, thereby alleviating cisplatin-induced nephrotoxicity (Fan et al., 2020). Kaempferol has been shown to inhibit AP-1 transactivation, thereby enhancing the inhibitory effect of oxaliplatin on HCT116 and HT29 cells (Park et al., 2021).
P-glycoprotein (P-gp)-mediated multidrug resistance (MDR) presents a significant challenge to successful chemotherapy. Studies have indicated that quercetin enhances the antiproliferative effects of doxorubicin on P-gp-overexpressing SW620/Ad300 cells by inhibiting ATP-driven transport activity, which increases the intracellular accumulation of doxorubicin. The study also suggests that quercetin may reverse MDR by disrupting D-glutamine and D-glutamate metabolism (Zhou et al., 2020). Moreover, isoorientin reduces doxorubicin-induced cardiotoxicity by activating MAPK, Akt, and caspase-dependent pathways (Li S. et al., 2022).
OCTN2, a member of the solute carrier superfamily and a key determinant for oxaliplatin uptake. Luteolin enhanced oxaliplatin absorption and intracellular accumulation through the PPARγ/OCTN2 pathway, thereby sensitizing SW480 cells to oxaliplatin (Qu et al., 2014). By inhibiting AMPK, luteolin synergistically improves the antitumor efficacy of oxaliplatin in CRC (Jang et al., 2022), and sensitized oxaliplatin-resistant CRC cells to chemotherapy by suppressing the Nrf2 pathway (Chian et al., 2014). Combined treatment with quercetin and oxaliplatin synergistically inhibited glutathione reductase activity, increasing ROS production, and induced glutathione depletion, thereby enhancing oxaliplatin sensitivity in CRC cells (Lee et al., 2023).
5-Fluorouracil (5-FU) is a commonly administered chemotherapeutic agent for treating CRC, but its efficacy is often limited by acquired resistance in advanced stages. Thymidylate synthase (TS), the target protein of 5-FU, is upregulated in CRC and contributes to 5-FU resistance. Apigenin has been shown to enhance the inhibitory effect of 5-FU on cell viability, induce apoptosis in HCT116 cells, and promote cell cycle arrest, likely by inhibiting TS expression (Yang et al., 2021). Likewise, kaempferol synergistically enhanced the effects of 5-FU by suppressing TS expression and inhibiting p-Akt (Li et al., 2019). Additionally, studies have shown that miR-27a can promotes the proliferation of CRC cells through the Wnt/β-catenin pathway. A combination of quercetin and 5-FU exhibitsed stronger cytotoxicity than 5-FU alone. Besides, quercetin enhanced 5-FU sensitivity in CRC by inhibiting the miR-27a/Wnt/β-catenin signaling axis (Terana et al., 2022). Furthermore, quercetin downregulates 5-FU-induced TS levels, upregulates p53 expression, induced ROS production and Ca2+ dysregulation through non-5-FU-dependent pathways in CRC cells. Moreover, quercetin enhanced sensitivity to 5-FU in mice with colitis-associated CRC (Yang et al., 2022). Kaempferol reduced glucose uptake and lactate production in drug-resistant CRC cells, and increased the expression of microRNA-326 (miR-326), which targets PKM2 and inhibits glycolysis, thereby reversing 5-FU resistance (Wu et al., 2022).
Luteolin has also been shown to reverse inflammation and oxidative imbalance induced by irinotecan via PPARγ-dependent downregulation of IL-1β and iNOS (Boeing et al., 2022). Treatment with quercetin enhanced the expression of NKG2D ligands on cancer cells, making them more susceptible to NK cell-mediated cytotoxicity (Bae et al., 2012). Furthermore, the combination of quercetin and ionizing radiation (IR) targeted colon cancer stem cells and inhibits Notch-1 signaling (Li et al., 2020). Both apigenin and luteolin exhibited efficacy in targeting the PI3K/Akt/mTOR axis, making them as promising non-toxic alternatives to synthetic chemical drugs used for the treatment of CRC (Sain et al., 2022).
Interaction of flavonoids with gut microbiota
Gut microbiota plays a crucial role in the progression of CRC, and targeting gut microbiota is an important pharmacological strategy for treating CRC (Jia et al., 2024). Flavonoids are not easily absorbed by the human body after oral intake. Most flavonoids are metabolized by gut microbiota into smaller, more absorbable active metabolites, thus enhancing their bioactivity.
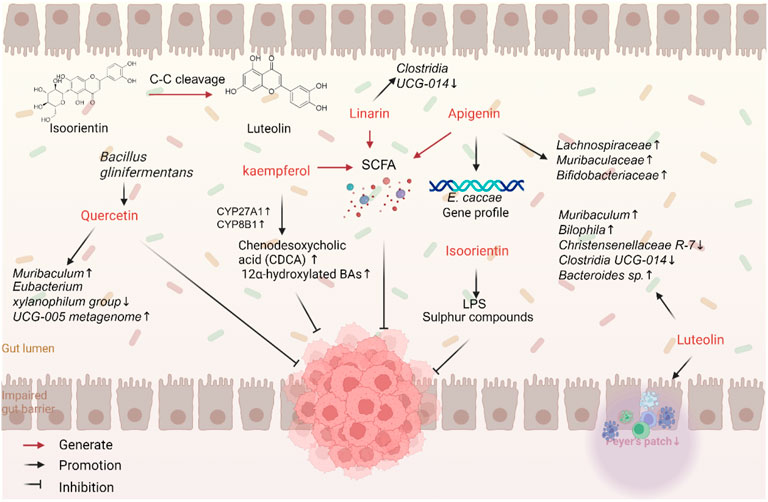
Gut microbiota can transform flavonoids by altering their chemical structure and biological functions, thereby enhancing their anticancer potential (Figure 3). Specific bacterial strains, such as Bifidobacterium and Lactobacillus, play essential roles in this metabolic process. Flavonoids mainly exist as glycosides, with only 5%–10% being directly absorbed, resulting in low bioavailability. The majority of flavonoids are metabolized by gut microbiota into smaller molecules, such as phenolic acids, which increases the abundance of beneficial bacteria and stimulates the production of short-chain fatty acids (SCFAs) (Gonzales et al., 2015; Feng X. et al., 2018). C-glycoside flavonoids are highly metabolized in the gut. For example, enzymes expressed by Caco-2 cells can cleave C-C bonds in vitro, breaking the bonds between sugar and aglycone residues in orientin and isoorientin to produce glucuronidated or sulfated derivatives of luteolin and apigenin (Dihal et al., 2006; Tremmel et al., 2021). Studies have shown that Bacillus glycinifermentans, Flavonifractor plautii, Bacteroides eggerthii, Olsenella scatoligenes, and Eubacterium eligens can degrade quercetin. B. glycinifermentans produces metabolites like 2,4,6-THBA and 3,4-DHBA, which inhibit the proliferation of CRC cells (Sankaranarayanan et al., 2021). 3,4-Dihydroxyphenylacetic acid, a microbial metabolite derived from quercetin, was shown to strongly inhibit the CRC-promoting properties of heme chloride than quercetin itself and prevent heme-induced malignant transformation of colonic epithelial cells and mitochondrial dysfunction (Catalán et al., 2020). The metabolism of flavonoids is a complex process involving various gut microbiota, which can sustain or even enhance their anticancer effects (Zhang et al., 2014; Cattivelli et al., 2023).
On the other hand, flavonoids can regulate the composition of gut microbiota by promoting the growth of beneficial bacteria and inhibiting the proliferation of pathogenic and pro-inflammatory bacteria. In a mouse model of cancer, luteolin has been shown to significantly reduce the abundance of disease-associated or inflammation-associated genera, such as Clostridium UCG-014 and Turicibacter, while increasing the abundance of Muribaculaceae, a health-promoting genus. This finding supports its antitumor effect through microbiota modulation (Pérez-Valero et al., 2024). Similarly, apigenin can mitigate gut dysbiosis by increasing the abundance of beneficial bacteria, such as Lachnospiraceae, Muribaculaceae, and Bifidobacterium (Rithidech et al., 2024), and affecting the growth and gene expression of Enterococcus (Wang et al., 2017). Interestingly, the anticancer effects of apigenin relied on gut microbiota (Bian et al., 2020). Isoorientin has significant advantages in preventing colon damage and gut dysbiosis induced by benzo [a]pyrene (BaP). Isoorientin was found to changes the abundance of gut microbiota, especially Feacalibaculum, Lactobacillus, Acetobacter, Desulfovibrio, and Alistipes, after exposure to BaP. Isoorientin also improves metabolic disorders of gut microbiota after exposure to BaP. Particularly, it improved perturbations in pathways involving LPS and sulfur compounds (He et al., 2019). Linarin reversed DSS-induced gut microbiota dysbiosis, affecting the abundance of different genera, such as Alistipes, Rikenella, and Clostridia UCG-014_norank. It also increased the abundance of SCFA-producing bacteria, like Lactobacillus, Roseburia, Parabacteroides, and Blautia (Jin et al., 2022).
Dysbiosis of gut microbiota can affect the production of metabolites, such as bile acids (BAs), LPS, choline, and SCFAs. Quercetin can modulate the composition of gut microbiota, improve the integrity of the intestinal barrier (Mohammadhasani et al., 2024), and reduce serum levels of hippuric acid (HA), a polyphenol-derived metabolite, observed in patients with Crohn’s disease. HA levels in the gut were found to be positively correlated with polyphenol intake, the abundance of flavonoid-degrading bacteria, and SCFA production (Xiang et al., 2024). Kaempferol can increase the expression of enzymes such as sterol CYP27A1 and sterol CYP8B1, thereby increasing the decreased levels of CDCA and 12α-hydroxylated bile acids and upregulating FXR expression. This finding suggests that kaempferol can downregulate secondary bile acid synthesis pathways, increase G-protein-coupled receptor activity, and decrease NLRs activity, thereby influencing cell differentiation, proliferation, survival, and apoptosis (Li X. et al., 2022). However, further exploration is needed to fully explore the mechanisms through which flavonoids interact with gut microbiota and their metabolites.
Through their bidirectional interaction with gut microbiota, flavonoids promote gut health and inhibit the development of CRC. They are transformed into more active metabolites and modulate the composition of gut microbiota, resulting in anti-inflammatory, antioxidant, and immunomodulatory anticancer effects in cancer. This interaction provides new insights and research directions for the prevention and treatment of CRC.
Construction and application of drug delivery systems
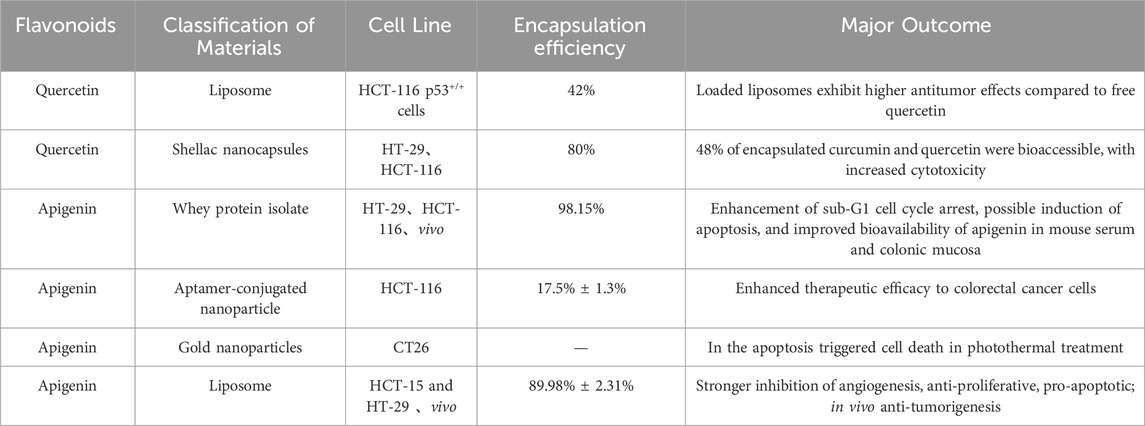
Flavonoids have shown significant potential in the prevention and treatment of CRC. However, their poor water solubility limits both their bioavailability and therapeutic efficacy. Additionally, the metabolism of flavonoids can further reduce their bioavailability and diminish their therapeutic potential. Nanomaterials offer unique advantages in targeted drug delivery. They can protect drugs against degradation, enhance drug solubility, reduce toxicity, and enhance pharmacodynamics and pharmacokinetics. The application of nano-engineered flavonoids not only improves the antitumor effects of chemotherapy drugs but also reduces their systemic toxicity (Table 3).
Quercetin, for example, exhibits low solubility in neutral and hydrophilic fluids. Encapsulation with soybean polysaccharide and chitosan has been shown to enhance the stability and solubility of quercetin-loaded flavonoids. Multiple independent studies have confirmed that quercetin-nanocarriers can improve their pharmacokinetics and absorption, thereby enhancing their anticancer efficacy against CRC. For instance, encapsulating quercetin in nanocapsules potentiated its antioxidant and cytotoxic activity in HT-29 and HCT116 cells (Jain et al., 2023). Additionally, the co-administration of quercetin with nanosynthesized drugs revealed improved antitumor efficacy (Colpan and Erdemir, 2021).
Flavonoids also exhibit antioxidant properties by chelating transition metals involved in free radical generation. Metal-flavonoid complexes act as more potent free radical scavengers than isolated flavonoids. For example, a quercetin-ruthenium complex reduced HT29 cell proliferation and induce tumor cell apoptosis by upregulating p53 and Bax and downregulating Bcl2 expression. The radical scavenging ability and antimicrobial activity of isoorientin-Zn were significantly stronger than those of isoorientin alone (Wang et al., 2021). Furthermore, the use of microencapsulated Bifidobacterium bifidum and Lactobacillus gasseri, either alone or in combination with quercetin, inhibited of CRC progression in ApcMin/+ mice (Benito et al., 2021).
Apigenin nanoformulations have been applied for targeted tumor cell treatment. Nano-encapsulation of apigenin enhanced cellular uptake, pro-apoptotic effects, and bioavailability in mouse blood and colonic mucosa (Dutta et al., 2018; Amini et al., 2021; Hong et al., 2022). Liposomal nanocarriers of apigenin exhibited anti-angiogenic properties, reduced cell proliferation, and increased cell apoptosis. Preclinical trials using these formulations in nude mouse xenograft models revealed enhanced antitumor effects (Sen et al., 2019). Luteolin has low oral bioavailability due to its poor water solubility, which maks its intravenous or intraperitoneal administration impossible. Nanoformulations help overcome these challenges, improving the anticancer efficacy of luteolin.
Current challenges and future prospects
The flavonoids found in Herba Patriniae possess extensive anti-tumor properties, and their therapeutic effects in CRC have gained significant attention. These flavonoids show great promise for the development of new therapeutic agents.
Regarding pharmacological mechanisms, current studies suggest that flavonoids exert anti-tumor effects through various pathways (Figure 4). For instance, it was shown that flavonoids can reduce ACF, regulate cell cycle, inhibit the growth and proliferation of CRC cells, promote apoptosis, and suppress EMT, reversing CRC resistance, and modulate the composition of gut microbiota. A growing number of studies have focused on the crucial role of the tumor microenvironment (TME) in cancer progression. Recent findings unveiled that flavonoids from Herba Patriniae can affect the TME, including macrophage phenotypes. However, most studies have primarily observed the effects of flavonoids on stromal cells, and a deeper understanding of their mechanisms of action is still needed.
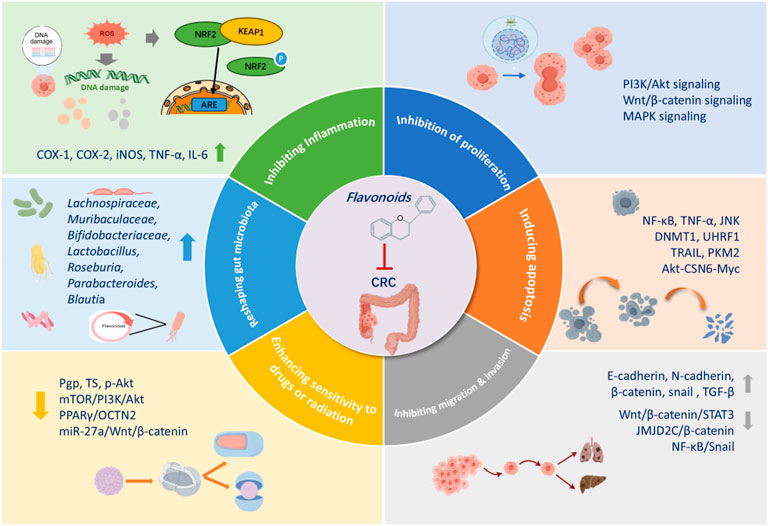
Figure 4. Mechanisms of action of flavonoids from patriniae in the anticancer activity against colorectol cancer.
Well-designed randomized controlled trials are needed to confirm the safety and efficacy of flavonoid-based therapies. Although Herba Patriniae is widely consumed in tropical and subtropical regions, few clinical trials have administered Herba Patriniae and its flavonoids to patients with CRC. Such studies should evaluate the safety and tolerability of Herba Patriniae, and determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT). High-quality Phase II–III clinical studies can provide higher-level evidence regarding efficacy. In addition, large-scale longitudinal interventional studies are important for understanding the metabolic variability of flavonoids for developing personalized therapeutic strategies based on gut microbiota.
Low bioavailability is also a major obstacle limiting the clinical application of flavonoids in Herba Patriniae. However, the development of novel nanoformulations holds promise for improving drug delivery, targeting specific tissues, and enhancing the bioavailability of these compounds. Modifying flavonoids based on their mechanisms of action can also advance the therapeutic use of natural plant compounds in the treatment of CRC.
Although some progress has been made in understanding the interaction between gut microbiota and flavonoid metabolites, there are many unknown aspects. The ability of gut microbes to metabolize flavonoid and the specific metabolites involved in the pathogenesis of CRC are still poorly understood, with limited data from clinical samples. More evidence from sterile mouse models and clinical studies is needed to address these gaps and explore the specific mechanism.
Author contributions
PZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review and editing. RJ: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Supervision, Visualization, Writing – review and editing. YW: Project administration, Software, Supervision, Visualization, Writing – review and editing. YT: Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – review and editing. QL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing – review and editing. FH: Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Shanghai Municipal Health Commission Health Industry Clinical Research Project (202440078, 20214Y0455), National Natural Science Foundation of China (82104619; 82205207).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, X. Y., Qin, Y., Liu, H. J., Cui, Z. H., Li, M., Yang, J. H., et al. (2017). Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB signaling. Oncotarget 8 (59), 100216–100226. doi:10.18632/oncotarget.22145
Amini, S. M., Mohammadi, E., Askarian-Amiri, S., Azizi, Y., Shakeri-Zadeh, A., and Neshastehriz, A. (2021). Investigating the in vitro photothermal effect of green synthesized apigenin-coated gold nanoparticle on colorectal carcinoma. IET Nanobiotechnol 15 (3), 329–337. doi:10.1049/nbt2.12016
André, T., Elez, E., Lenz, H. J., Jensen, L. H., Touchefeu, Y., Van Cutsem, E., et al. (2025). Nivolumab plus ipilimumab versus nivolumab in microsatellite instability-high metastatic colorectal cancer (CheckMate 8HW): a randomised, open-label, phase 3 trial. Lancet 405 (10476), 383–395. doi:10.1016/s0140-6736(24)02848-4
Ashokkumar, P., and Sudhandiran, G. (2008). Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed. Pharmacother. 62 (9), 590–597. doi:10.1016/j.biopha.2008.06.031
Au, A., Li, B., Wang, W., Roy, H., Koehler, K., and Birt, D. (2006). Effect of dietary apigenin on colonic ornithine decarboxylase activity, aberrant crypt foci formation, and tumorigenesis in different experimental models. Nutr. Cancer 54 (2), 243–251. doi:10.1207/s15327914nc5402_11
Bae, J. H., Kim, S. J., Kim, M. J., Oh, S. O., Chung, J. S., Kim, S. H., et al. (2012). Susceptibility to natural killer cell-mediated lysis of colon cancer cells is enhanced by treatment with epidermal growth factor receptor inhibitors through UL16-binding protein-1 induction. Cancer Sci. 103 (1), 7–16. doi:10.1111/j.1349-7006.2011.02109.x
Banerjee, K., and Mandal, M. (2015). Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 5, 153–162. doi:10.1016/j.redox.2015.04.009
Benito, I., Encío, I. J., Milagro, F. I., Alfaro, M., Martínez-Peñuela, A., Barajas, M., et al. (2021). Microencapsulated Bifidobacterium bifidum and Lactobacillus gasseri in combination with quercetin inhibit colorectal cancer development in apc(Min/+) mice. Int. J. Mol. Sci. 22 (9), 4906. doi:10.3390/ijms22094906
Bian, S., Wan, H., Liao, X., and Wang, W. (2020). Inhibitory effects of apigenin on tumor carcinogenesis by altering the gut microbiota. Mediat. Inflamm. 2020, 7141970. doi:10.1155/2020/7141970
Bobe, G., Albert, P. S., Sansbury, L. B., Lanza, E., Schatzkin, A., Colburn, N. H., et al. (2010). Interleukin-6 as a potential indicator for prevention of high-risk adenoma recurrence by dietary flavonols in the polyp prevention trial. Cancer Prev. Res. (Phila) 3 (6), 764–775. doi:10.1158/1940-6207.Capr-09-0161
Boeing, T., Speca, S., de Souza, P., Mena, A. M., Bertin, B., Desreumax, P., et al. (2022). The PPARγ-dependent effect of flavonoid luteolin against damage induced by the chemotherapeutic irinotecan in human intestinal cells. Chem. Biol. Interact. 351, 109712. doi:10.1016/j.cbi.2021.109712
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Bulzomi, P., Galluzzo, P., Bolli, A., Leone, S., Acconcia, F., and Marino, M. (2012). The pro-apoptotic effect of quercetin in cancer cell lines requires ERβ-dependent signals. J. Cell Physiol. 227 (5), 1891–1898. doi:10.1002/jcp.22917
Catalán, M., Ferreira, J., and Carrasco-Pozo, C. (2020). The microbiota-derived metabolite of quercetin, 3,4-Dihydroxyphenylacetic acid prevents malignant transformation and mitochondrial dysfunction induced by Hemin in colon cancer and normal colon Epithelia cell lines. Molecules 25 (18), 4138. doi:10.3390/molecules25184138
Cattivelli, A., Conte, A., and Tagliazucchi, D. (2023). Quercetins, Chlorogenic acids and their colon metabolites inhibit colon cancer cell proliferation at Physiologically Relevant concentrations. Int. J. Mol. Sci. 24 (15), 12265. doi:10.3390/ijms241512265
Chang, H., Lei, L., Zhou, Y., Ye, F., and Zhao, G. (2018). Dietary flavonoids and the risk of colorectal cancer: an Updated Meta-analysis of Epidemiological studies. Nutrients 10 (7), 950. doi:10.3390/nu10070950
Chen, L., Liu, L., Ye, L., Shen, A., Chen, Y., Sferra, T. J., et al. (2013). Patrinia scabiosaefolia inhibits colorectal cancer growth through suppression of tumor angiogenesis. Oncol. Rep. 30 (3), 1439–1443. doi:10.3892/or.2013.2582
Chen, X., Xu, H., Yu, X., Wang, X., Zhu, X., and Xu, X. (2019). Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. buon 24 (2), 488–493.
Chen, Y., Wang, B., Yuan, X., Lu, Y., Hu, J., Gao, J., et al. (2021). Vitexin prevents colitis-associated carcinogenesis in mice through regulating macrophage polarization. Phytomedicine 83, 153489. doi:10.1016/j.phymed.2021.153489
Chen, J. F., Wu, S. W., Shi, Z. M., and Hu, B. (2023). Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action. Chin. Med. 18 (1), 14. doi:10.1186/s13020-023-00719-7
Chen, Y., Liang, J., Chen, S., Lin, N., Xu, S., Miao, J., et al. (2024). Discovery of vitexin as a novel VDR agonist that mitigates the transition from chronic intestinal inflammation to colorectal cancer. Mol. Cancer 23 (1), 196. doi:10.1186/s12943-024-02108-6
Cheng, Y., Han, X., Mo, F., Zeng, H., Zhao, Y., Wang, H., et al. (2021). Apigenin inhibits the growth of colorectal cancer through down-regulation of E2F1/3 by miRNA-215-5p. Phytomedicine 89, 153603. doi:10.1016/j.phymed.2021.153603
Chian, S., Li, Y. Y., Wang, X. J., and Tang, X. W. (2014). Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac J. Cancer Prev. 15 (6), 2911–2916. doi:10.7314/apjcp.2014.15.6.2911
Choi, J. B., Kim, J. H., Lee, H., Pak, J. N., Shim, B. S., and Kim, S. H. (2018). Reactive oxygen species and p53 mediated activation of p38 and caspases is critically involved in kaempferol induced apoptosis in colorectal cancer cells. J. Agric. Food Chem. 66 (38), 9960–9967. doi:10.1021/acs.jafc.8b02656
Chung, C. S., Jiang, Y., Cheng, D., and Birt, D. F. (2007). Impact of adenomatous polyposis coli (APC) tumor supressor gene in human colon cancer cell lines on cell cycle arrest by apigenin. Mol. Carcinog. 46 (9), 773–782. doi:10.1002/mc.20306
Chunhua, L., Donglan, L., Xiuqiong, F., Lihua, Z., Qin, F., Yawei, L., et al. (2013). Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J. Nutr. Biochem. 24 (10), 1766–1775. doi:10.1016/j.jnutbio.2013.03.006
Ciardiello, F., Ciardiello, D., Martini, G., Napolitano, S., Tabernero, J., and Cervantes, A. (2022). Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin. 72 (4), 372–401. doi:10.3322/caac.21728
Colpan, R. D., and Erdemir, A. (2021). Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. J. Microencapsul. 38 (6), 381–393. doi:10.1080/02652048.2021.1948623
Cruz-Correa, M., Shoskes, D. A., Sanchez, P., Zhao, R., Hylind, L. M., Wexner, S. D., et al. (2006). Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 4 (8), 1035–1038. doi:10.1016/j.cgh.2006.03.020
Dai, J., Van Wie, P. G., Fai, L. Y., Kim, D., Wang, L., Poyil, P., et al. (2016). Downregulation of NEDD9 by apigenin suppresses migration, invasion, and metastasis of colorectal cancer cells. Toxicol. Appl. Pharmacol. 311, 106–112. doi:10.1016/j.taap.2016.09.016
Darband, S. G., Sadighparvar, S., Yousefi, B., Kaviani, M., Ghaderi-Pakdel, F., Mihanfar, A., et al. (2020). Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 253, 117584. doi:10.1016/j.lfs.2020.117584
Dihal, A. A., Woutersen, R. A., van Ommen, B., Rietjens, I. M., and Stierum, R. H. (2006). Modulatory effects of quercetin on proliferation and differentiation of the human colorectal cell line Caco-2. Cancer Lett. 238 (2), 248–259. doi:10.1016/j.canlet.2005.07.007
Ding, J., Polier, G., Köhler, R., Giaisi, M., Krammer, P. H., and Li-Weber, M. (2012). Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J. Biol. Chem. 287 (1), 641–649. doi:10.1074/jbc.M111.286526
Dutta, D., Chakraborty, A., Mukherjee, B., and Gupta, S. (2018). Aptamer-conjugated apigenin nanoparticles to target colorectal carcinoma: a promising Safe alternative of colorectal cancer chemotherapy. ACS Appl. Bio Mater 1 (5), 1538–1556. doi:10.1021/acsabm.8b00441
Fan, X., Wei, W., Huang, J., Liu, X., and Ci, X. (2020). Isoorientin attenuates cisplatin-induced nephrotoxicity through the inhibition of oxidative stress and apoptosis via activating the SIRT1/SIRT6/Nrf-2 pathway. Front. Pharmacol. 11, 264. doi:10.3389/fphar.2020.00264
Feng, J., Song, D., Jiang, S., Yang, X., Ding, T., Zhang, H., et al. (2018a). Quercetin restrains TGF-β1-induced epithelial-mesenchymal transition by inhibiting Twist1 and regulating E-cadherin expression. Biochem. Biophys. Res. Commun. 498 (1), 132–138. doi:10.1016/j.bbrc.2018.02.044
Feng, X., Li, Y., Brobbey Oppong, M., and Qiu, F. (2018b). Insights into the intestinal bacterial metabolism of flavonoids and the bioactivities of their microbe-derived ring cleavage metabolites. Drug Metab. Rev. 50 (3), 343–356. doi:10.1080/03602532.2018.1485691
Ferry, D. R., Smith, A., Malkhandi, J., Fyfe, D. W., deTakats, P. G., Anderson, D., et al. (1996). Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 2 (4), 659–668.
García-Gutiérrez, N., Luna-Bárcenas, G., Herrera-Hernández, G., Campos-Vega, R., Lozano-Herrera, S. J., Sánchez-Tusié, A. A., et al. (2023). Quercetin and its fermented extract as a potential inhibitor of Bisphenol A-Exposed HT-29 colon cancer cells' viability. Int. J. Mol. Sci. 24 (6), 5604. doi:10.3390/ijms24065604
Gatasheh, M. K. (2024). Identifying key genes against rutin on human colorectal cancer cells via ROS pathway by integrated bioinformatic analysis and experimental validation. Comput. Biol. Chem. 112, 108178. doi:10.1016/j.compbiolchem.2024.108178
Gennari, L., Felletti, M., Blasa, M., Angelino, D., Celeghini, C., Corallini, A., et al. (2011). Total extract of Beta vulgaris var. cicla seeds versus its purified phenolic components: antioxidant activities and antiproliferative effects against colon cancer cells. Phytochem. Anal. 22 (3), 272–279. doi:10.1002/pca.1276
Global Nutrition Target Collaborators (2025). Global, regional, and national progress towards the 2030 global nutrition targets and forecasts to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 404 (10471), 2543–2583. doi:10.1016/s0140-6736(24)01821-x
Gong, L., Zou, W., Zheng, K., Shi, B., and Liu, M. (2021). The Herba Patriniae (Caprifoliaceae): a review on traditional uses, phytochemistry, pharmacology and quality control. J. Ethnopharmacol. 265, 113264. doi:10.1016/j.jep.2020.113264
Gonzales, G. B., Smagghe, G., Grootaert, C., Zotti, M., Raes, K., and Van Camp, J. (2015). Flavonoid interactions during digestion, absorption, distribution and metabolism: a sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 47 (2), 175–190. doi:10.3109/03602532.2014.1003649
Gou, H., Su, H., Liu, D., Wong, C. C., Shang, H., Fang, Y., et al. (2023). Traditional medicine pien Tze Huang suppresses colorectal tumorigenesis through restoring gut microbiota and metabolites. Gastroenterology 165 (6), 1404–1419. doi:10.1053/j.gastro.2023.08.052
Han, M., Song, Y., and Zhang, X. (2016). Quercetin suppresses the migration and invasion in human colon cancer Caco-2 cells through regulating Toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn. Mag. 12 (Suppl. 2), S237–S244. doi:10.4103/0973-1296.182154
He, S., Li, X., Li, C., Deng, H., Shao, Y., and Yuan, L. (2019). Isoorientin attenuates benzo[a]pyrene-induced colonic injury and gut microbiota disorders in mice. Food Res. Int. 126, 108599. doi:10.1016/j.foodres.2019.108599
Hoensch, H., Groh, B., Edler, L., and Kirch, W. (2008). Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 14 (14), 2187–2193. doi:10.3748/wjg.14.2187
Hong, S., Dia, V. P., Baek, S. J., and Zhong, Q. (2022). Nanoencapsulation of apigenin with whey protein isolate: physicochemical properties, in vitro activity against colorectal cancer cells, and bioavailability. Leb. Wiss Technol. 154, 112751. doi:10.1016/j.lwt.2021.112751
Huang, S. Z., Liu, W. Y., Huang, Y., Shen, A. L., Liu, L. Y., and Peng, J. (2019). Patrinia scabiosaefolia inhibits growth of 5-FU-resistant colorectal carcinoma cells via induction of apoptosis and suppression of AKT pathway. Chin. J. Integr. Med. 25 (2), 116–121. doi:10.1007/s11655-018-3002-6
Ismail, A., El-Biyally, E., and Sakran, W. (2023). An Innovative approach for formulation of rutin tablets targeted for colon cancer treatment. AAPS PharmSciTech 24 (2), 68. doi:10.1208/s12249-023-02518-7
Jain, S., Lenaghan, S., Dia, V., and Zhong, Q. (2023). Co-delivery of curcumin and quercetin in shellac nanocapsules for the synergistic antioxidant properties and cytotoxicity against colon cancer cells. Food Chem. 428, 136744. doi:10.1016/j.foodchem.2023.136744
Jang, C. H., Moon, N., Oh, J., and Kim, J. S. (2019). Luteolin Shifts oxaliplatin-induced cell cycle arrest at G0/G1 to apoptosis in HCT116 human colorectal carcinoma cells. Nutrients 11 (4), 770. doi:10.3390/nu11040770
Jang, C. H., Moon, N., Lee, J., Kwon, M. J., Oh, J., and Kim, J. S. (2022). Luteolin synergistically enhances antitumor activity of oxaliplatin in colorectal carcinoma via AMPK inhibition. Antioxidants (Basel) 11 (4), 626. doi:10.3390/antiox11040626
Jia, R., Shao, S., Zhang, P., Yuan, Y., Rong, W., An, Z., et al. (2024). PRM1201 effectively inhibits colorectal cancer metastasis via shaping gut microbiota and short-chain fatty acids. Phytomedicine 132, 155795. doi:10.1016/j.phymed.2024.155795
Jin, C., Liu, J., Jin, R., Yao, Y., He, S., Lei, M., et al. (2022). Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier, suppression of inflammatory responses and modulation of gut microbiota. Food Funct. 13 (20), 10574–10586. doi:10.1039/d2fo02128e
Kee, J. Y., Han, Y. H., Kim, D. S., Mun, J. G., Park, J., Jeong, M. Y., et al. (2016). Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine 23 (13), 1680–1690. doi:10.1016/j.phymed.2016.09.011
Kim, W. K., Bang, M. H., Kim, E. S., Kang, N. E., Jung, K. C., Cho, H. J., et al. (2005). Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J. Nutr. Biochem. 16 (3), 155–162. doi:10.1016/j.jnutbio.2004.10.010
Kim, H. J., Kim, S. K., Kim, B. S., Lee, S. H., Park, Y. S., Park, B. K., et al. (2010). Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J. Agric. Food Chem. 58 (15), 8643–8650. doi:10.1021/jf101510z
Kim, G. T., Lee, S. H., and Kim, Y. M. (2013a). Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 colon cancer cells. J. Cancer Prev. 18 (3), 264–270. doi:10.15430/jcp.2013.18.3.264
Kim, H. Y., Jung, S. K., Byun, S., Son, J. E., Oh, M. H., Lee, J., et al. (2013b). Raf and PI3K are the molecular targets for the anti-metastatic effect of luteolin. Phytother. Res. 27 (10), 1481–1488. doi:10.1002/ptr.4888
Kim, G. T., Lee, S. H., Kim, J. I., and Kim, Y. M. (2014). Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 33 (4), 863–869. doi:10.3892/ijmm.2014.1658
Krifa, M., Leloup, L., Ghedira, K., Mousli, M., and Chekir-Ghedira, L. (2014). Luteolin induces apoptosis in BE colorectal cancer cells by downregulating calpain, UHRF1, and DNMT1 expressions. Nutr. Cancer 66 (7), 1220–1227. doi:10.1080/01635581.2014.951729
Kumar Singh, A., Cabral, C., Kumar, R., Ganguly, R., Kumar Rana, H., Gupta, A., et al. (2019). Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery Efficiency. Nutrients 11 (9), 2216. doi:10.3390/nu11092216
Landete, J. M. (2022). Flavone, flavanone and flavonol metabolism from soybean and flaxseed extracts by the intestinal microbiota of adults and infants. J. Sci. Food Agric. 102 (6), 2575–2583. doi:10.1002/jsfa.11599
Lee, Y. K., Park, S. Y., Kim, Y. M., Lee, W. S., and Park, O. J. (2009). AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp. Mol. Med. 41 (3), 201–207. doi:10.3858/emm.2009.41.3.023
Lee, Y., Sung, B., Kang, Y. J., Kim, D. H., Jang, J. Y., Hwang, S. Y., et al. (2014). Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 44 (5), 1599–1606. doi:10.3892/ijo.2014.2339
Lee, J., Jang, C. H., Kim, Y., Oh, J., and Kim, J. S. (2023). Quercetin-induced glutathione depletion sensitizes colorectal cancer cells to oxaliplatin. Foods 12 (8), 1733. doi:10.3390/foods12081733
Lefort, E. C., and Blay, J. (2011). The dietary flavonoid apigenin enhances the activities of the anti-metastatic protein CD26 on human colon carcinoma cells. Clin. Exp. Metastasis 28 (4), 337–349. doi:10.1007/s10585-010-9364-6
Leonardi, T., Vanamala, J., Taddeo, S. S., Davidson, L. A., Murphy, M. E., Patil, B. S., et al. (2010). Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. (Maywood) 235 (6), 710–717. doi:10.1258/ebm.2010.009359
Li, Q. C., Liang, Y., Hu, G. R., and Tian, Y. (2016). Enhanced therapeutic efficacy and amelioration of cisplatin-induced nephrotoxicity by quercetin in 1,2-dimethyl hydrazine-induced colon cancer in rats. Indian J. Pharmacol. 48 (2), 168–171. doi:10.4103/0253-7613.178834
Li, Z., Tang, Y., Zhu, S., Li, D., Han, X., Gu, G., et al. (2018). Ethanol extract of Patrinia scabiosaefolia induces the death of human renal cell carcinoma 786-O cells via SIRT-1 and mTOR signaling-mediated metabolic disruptions. Oncol. Rep. 39 (2), 764–772. doi:10.3892/or.2017.6139
Li, Q., Wei, L., Lin, S., Chen, Y., Lin, J., and Peng, J. (2019). Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 20 (1), 728–734. doi:10.3892/mmr.2019.10296
Li, Y., Wang, Z., Jin, J., Zhu, S. X., He, G. Q., Li, S. H., et al. (2020). Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem. Biophys. Res. Commun. 523 (4), 947–953. doi:10.1016/j.bbrc.2020.01.048
Li, D., Doherty, A. R., Raju, M., Liu, L., Lei, N. Y., Amsden, L. B., et al. (2021). Risk stratification for colorectal cancer in individuals with subtypes of serrated polyps. Gut 71, 2022–2029. doi:10.1136/gutjnl-2021-324301
Li, S., Liu, H., Lin, Z., Li, Z., Chen, Y., Chen, B., et al. (2022a). Isoorientin attenuates doxorubicin-induced cardiac injury via the activation of MAPK, Akt, and Caspase-dependent signaling pathways. Phytomedicine 101, 154105. doi:10.1016/j.phymed.2022.154105
Li, X., Khan, I., Huang, G., Lu, Y., Wang, L., Liu, Y., et al. (2022b). Kaempferol acts on bile acid signaling and gut microbiota to attenuate the tumor burden in ApcMin/+ mice. Eur. J. Pharmacol. 918, 174773. doi:10.1016/j.ejphar.2022.174773
Li, J., Shang, L., Zhou, F., Wang, S., Liu, N., Zhou, M., et al. (2023). Herba Patriniae and its component Isovitexin show anti-colorectal cancer effects by inducing apoptosis and cell-cycle arrest via p53 activation. Biomed. Pharmacother. 168, 115690. doi:10.1016/j.biopha.2023.115690
Li, K., Mi, L., Bai, X., Lu, Y., Zhang, Y., Li, J., et al. (2024a). Induction of apoptosis and autophagy by dichloromethane extract from Patrinia scabiosaefolia Fisch on acute myeloid leukemia cells. Environ. Toxicol. 39 (4), 2123–2137. doi:10.1002/tox.24090
Li, Q., Geng, S., Luo, H., Wang, W., Mo, Y. Q., Luo, Q., et al. (2024b). Signaling pathways involved in colorectal cancer: pathogenesis and targeted therapy. Signal Transduct. Target Ther. 9 (1), 266. doi:10.1038/s41392-024-01953-7
Liu, L., Shen, A., Chen, Y., Wei, L., Lin, J., Sferra, T. J., et al. (2013). Patrinia scabiosaefolia induces mitochondrial-dependent apoptosis in a mouse model of colorectal cancer. Oncol. Rep. 30 (2), 897–903. doi:10.3892/or.2013.2528
Liu, Y., Lang, T., Jin, B., Chen, F., Zhang, Y., Beuerman, R. W., et al. (2017). Luteolin inhibits colorectal cancer cell epithelial-to-mesenchymal transition by suppressing CREB1 expression revealed by comparative proteomics study. J. Proteomics 161, 1–10. doi:10.1016/j.jprot.2017.04.005
Liu, S. C., Huang, C. S., Huang, C. M., Hsieh, M. S., Huang, M. S., Fong, I. H., et al. (2021). Isoorientin inhibits epithelial-to-mesenchymal properties and cancer stem-cell-like features in oral squamous cell carcinoma by blocking Wnt/β-catenin/STAT3 axis. Toxicol. Appl. Pharmacol. 424, 115581. doi:10.1016/j.taap.2021.115581
Liu, X., An, L., Zhou, Y., Peng, W., and Huang, C. (2023a). Antibacterial mechanism of Patrinia scabiosaefolia against Methicillin resistant Staphylococcus epidermidis. Infect. Drug Resist 16, 1345–1355. doi:10.2147/idr.S398227
Liu, X., Ma, S., Zhang, Y., Fu, Y., and Cai, S. (2023b). Transmembrane behaviors and quantitative structure-activity relationship of dietary flavonoids in the presence of intestinal digestive products from different carbohydrate sources based on in vitro and in silico analysis. Food Chem. X 20, 100994. doi:10.1016/j.fochx.2023.100994
Lotito, S. B., Zhang, W. J., Yang, C. S., Crozier, A., and Frei, B. (2011). Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic. Biol. Med. 51 (2), 454–463. doi:10.1016/j.freeradbiomed.2011.04.032
Lyons, N. J., Giri, R., Begun, J., Clark, D., Proud, D., He, Y., et al. (2023). Reactive oxygen species as mediators of disease progression and therapeutic response in colorectal cancer. Antioxid. Redox Signal 39 (1-3), 186–205. doi:10.1089/ars.2022.0127
Maeda, Y., Takahashi, H., Nakai, N., Yanagita, T., Ando, N., Okubo, T., et al. (2018). Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int. J. Oncol. 52 (5), 1661–1673. doi:10.3892/ijo.2018.4308
Matsukawa, Y., Nishino, H., Okuyama, Y., Matsui, T., Matsumoto, T., Matsumura, S., et al. (1997). Effects of quercetin and/or restraint stress on formation of aberrant crypt foci induced by azoxymethane in rat colons. Oncology 54 (2), 118–121. doi:10.1159/000227674
Miyamoto, S., Yasui, Y., Ohigashi, H., Tanaka, T., and Murakami, A. (2010). Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem. Biol. Interact. 183 (2), 276–283. doi:10.1016/j.cbi.2009.11.002
Mohammadhasani, K., Vahedi Fard, M., Mottaghi Moghaddam Shahri, A., and Khorasanchi, Z. (2024). Polyphenols improve non-alcoholic fatty liver disease via gut microbiota: a comprehensive review. Food Sci. Nutr. 12 (8), 5341–5356. doi:10.1002/fsn3.4178
Morgan, E., Arnold, M., Gini, A., Lorenzoni, V., Cabasag, C. J., Laversanne, M., et al. (2023). Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72 (2), 338–344. doi:10.1136/gutjnl-2022-327736
Mu, J., Song, J., Li, R., Xue, T., Wang, D., and Yu, J. (2023). Isovitexin prevents DSS-induced colitis through inhibiting inflammation and preserving intestinal barrier integrity through activating AhR. Chem. Biol. Interact. 382, 110583. doi:10.1016/j.cbi.2023.110583
Mutoh, M., Takahashi, M., Fukuda, K., Komatsu, H., Enya, T., Matsushima-Hibiya, Y., et al. (2000). Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: structure-activity relationship. Jpn. J. Cancer Res. 91 (7), 686–691. doi:10.1111/j.1349-7006.2000.tb01000.x
Na, S., Ying, L., Jun, C., Ya, X., Suifeng, Z., Yuxi, H., et al. (2022). Study on the molecular mechanism of nightshade in the treatment of colon cancer. Bioengineered 13 (1), 1575–1589. doi:10.1080/21655979.2021.2016045
Naso, L. G., Badiola, I., Marquez Clavijo, J., Valcarcel, M., Salado, C., Ferrer, E. G., et al. (2016). Inhibition of the metastatic progression of breast and colorectal cancer in vitro and in vivo in murine model by the oxidovanadium(IV) complex with luteolin. Bioorg Med. Chem. 24 (22), 6004–6011. doi:10.1016/j.bmc.2016.09.058
Özerkan, D. (2023). The Determination of cisplatin and luteolin synergistic effect on colorectal cancer cell apoptosis and mitochondrial dysfunction by Fluorescence Labelling. J. Fluoresc. 33 (3), 1217–1225. doi:10.1007/s10895-023-03145-y
Pandurangan, A. K., Ananda Sadagopan, S. K., Dharmalingam, P., and Ganapasam, S. (2014a). Luteolin, a bioflavonoid inhibits Azoxymethane-induced colorectal cancer through activation of Nrf2 signaling. Toxicol. Mech. Methods 24 (1), 13–20. doi:10.3109/15376516.2013.843111
Pandurangan, A. K., Dharmalingam, P., Sadagopan, S. K., and Ganapasam, S. (2014b). Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum. Exp. Toxicol. 33 (11), 1176–1185. doi:10.1177/0960327114522502
Park, J., Lee, G. E., An, H. J., Lee, C. J., Cho, E. S., Kang, H. C., et al. (2021). Kaempferol sensitizes cell proliferation inhibition in oxaliplatin-resistant colon cancer cells. Arch. Pharm. Res. 44 (12), 1091–1108. doi:10.1007/s12272-021-01358-y
Pérez-Valero, Á., Magadán-Corpas, P., Ye, S., Serna-Diestro, J., Sordon, S., Huszcza, E., et al. (2024). Antitumor effect and gut microbiota modulation by quercetin, luteolin, and Xanthohumol in a rat model for colorectal cancer prevention. Nutrients 16 (8), 1161. doi:10.3390/nu16081161
Prasad, N., Sharma, J. R., and Yadav, U. C. S. (2020). Induction of growth cessation by acacetin via β-catenin pathway and apoptosis by apoptosis inducing factor activation in colorectal carcinoma cells. Mol. Biol. Rep. 47 (2), 987–1001. doi:10.1007/s11033-019-05191-x
Psahoulia, F. H., Drosopoulos, K. G., Doubravska, L., Andera, L., and Pintzas, A. (2007). Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol. Cancer Ther. 6 (9), 2591–2599. doi:10.1158/1535-7163.Mct-07-0001
Pu, Y., Han, Y., Ouyang, Y., Li, H., Li, L., Wu, X., et al. (2024). Kaempferol inhibits colorectal cancer metastasis through circ_0000345 mediated JMJD2C/β-catenin signalling pathway. Phytomedicine 128, 155261. doi:10.1016/j.phymed.2023.155261
Qi, L., Zhang, Y., Song, F., and Ding, Y. (2020). Chinese herbal medicine promote tissue differentiation in colorectal cancer by activating HSD11B2. Arch. Biochem. Biophys. 695, 108644. doi:10.1016/j.abb.2020.108644
Qu, Q., Qu, J., Guo, Y., Zhou, B. T., and Zhou, H. H. (2014). Luteolin potentiates the sensitivity of colorectal cancer cell lines to oxaliplatin through the PPARγ/OCTN2 pathway. Anticancer Drugs 25 (9), 1016–1027. doi:10.1097/cad.0000000000000125
Raja, S. B., Rajendiran, V., Kasinathan, N. K., P, A., Venkatabalasubramanian, S., Murali, M. R., et al. (2017). Differential cytotoxic activity of Quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food Chem. Toxicol. 106 (Pt A), 92–106. doi:10.1016/j.fct.2017.05.006
Rithidech, K. N., Peanlikhit, T., Honikel, L., Li, J., Liu, J., Karakach, T., et al. (2024). Consumption of apigenin prevents radiation-induced gut dysbiosis in male C57BL/6J mice exposed to Silicon Ions. Radiat. Res. 201 (4), 317–329. doi:10.1667/rade-23-00110.1
Ritter, S., and Dinh, T. T. (1992). Age-related changes in capsaicin-induced degeneration in rat brain. J. Comp. Neurol. 318 (1), 103–116. doi:10.1002/cne.903180108
Russo, M., Moccia, S., Luongo, D., and Russo, G. L. (2023). Senolytic flavonoids enhance type-I and type-II cell death in human radioresistant colon cancer cells through AMPK/MAPK pathway. Cancers (Basel) 15 (9), 2660. doi:10.3390/cancers15092660
Sain, A., Kandasamy, T., and Naskar, D. (2022). In silico approach to target PI3K/Akt/mTOR axis by selected Olea europaea phenols in PIK3CA mutant colorectal cancer. J. Biomol. Struct. Dyn. 40 (21), 10962–10977. doi:10.1080/07391102.2021.1953603
Sankaranarayanan, R., Sekhon, P. K., Ambat, A., Nelson, J., Jose, D., Bhat, G. J., et al. (2021). Screening of human gut bacterial Culture Collection identifies species that Biotransform quercetin into metabolites with anticancer properties. Int. J. Mol. Sci. 22 (13), 7045. doi:10.3390/ijms22137045
Schumacher, F. R., Schmit, S. L., Jiao, S., Edlund, C. K., Wang, H., Zhang, B., et al. (2015). Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat. Commun. 6, 7138. doi:10.1038/ncomms8138
Sen, K., Banerjee, S., and Mandal, M. (2019). Dual drug loaded liposome bearing apigenin and 5-Fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf. B Biointerfaces 180, 9–22. doi:10.1016/j.colsurfb.2019.04.035
Shan, B. E., Wang, M. X., and Li, R. Q. (2009). Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Invest 27 (6), 604–612. doi:10.1080/07357900802337191
Shi, R. X., Ong, C. N., and Shen, H. M. (2004). Luteolin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells. Oncogene 23 (46), 7712–7721. doi:10.1038/sj.onc.1208046
Shi, J., Ji, X., Shan, S., Zhao, M., Bi, C., and Li, Z. (2023). The interaction between apigenin and PKM2 restrains progression of colorectal cancer. J. Nutr. Biochem. 121, 109430. doi:10.1016/j.jnutbio.2023.109430
Sun, Y., Zhang, X., Hang, D., Lau, H. C., Du, J., Liu, C., et al. (2024). Integrative plasma and fecal metabolomics identify functional metabolites in adenoma-colorectal cancer progression and as early diagnostic biomarkers. Cancer Cell 42 (8), 1386–1400.e8. doi:10.1016/j.ccell.2024.07.005
Terana, G. T., Abd-Alhaseeb, M. M., Omran, G. A., and Okda, T. M. (2022). Quercetin potentiates 5-fluorouracil effects in human colon cancer cells through targeting the Wnt/β-catenin signalling pathway: the role of miR-27a. Contemp. Oncol. Pozn. 26 (3), 229–238. doi:10.5114/wo.2022.120361
Tong, J., Shen, Y., Zhang, Z., Hu, Y., Zhang, X., and Han, L. (2019). Apigenin inhibits epithelial-mesenchymal transition of human colon cancer cells through NF-κB/Snail signaling pathway. Biosci. Rep. 39 (5). doi:10.1042/bsr20190452
Tremmel, M., Kiermaier, J., and Heilmann, J. (2021). In vitro metabolism of six C-glycosidic flavonoids from Passiflora incarnata L. Int. J. Mol. Sci. 22 (12), 6566. doi:10.3390/ijms22126566
Tutino, V., V, D. E. N., Tafaro, A., Bianco, G., Gigante, I., Scavo, M. P., et al. (2018). Cannabinoid receptor-1 up-regulation in azoxymethane (AOM)-treated mice after dietary treatment with quercetin. Anticancer Res. 38 (8), 4485–4491. doi:10.21873/anticanres.12752
Vernia, F., Longo, S., Stefanelli, G., Viscido, A., and Latella, G. (2021). Dietary factors modulating colorectal carcinogenesis. Nutrients 13 (1), 143. doi:10.3390/nu13010143
Vochyánová, Z., Pokorná, M., Rotrekl, D., Smékal, V., Fictum, P., Suchý, P., et al. (2017). Prenylated flavonoid morusin protects against TNBS-induced colitis in rats. PLoS One 12 (8), e0182464. doi:10.1371/journal.pone.0182464
Volate, S. R., Davenport, D. M., Muga, S. J., and Wargovich, M. J. (2005). Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis 26 (8), 1450–1456. doi:10.1093/carcin/bgi089
Wang, W., VanAlstyne, P. C., Irons, K. A., Chen, S., Stewart, J. W., and Birt, D. F. (2004). Individual and interactive effects of apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma cell lines. Nutr. Cancer 48 (1), 106–114. doi:10.1207/s15327914nc4801_14
Wang, L., Lee, I. M., Zhang, S. M., Blumberg, J. B., Buring, J. E., and Sesso, H. D. (2009). Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 89 (3), 905–912. doi:10.3945/ajcn.2008.26913
Wang, Q. R., Yao, X. Q., Wen, G., Fan, Q., Li, Y. J., Fu, X. Q., et al. (2011). Apigenin suppresses the growth of colorectal cancer xenografts via phosphorylation and up-regulated FADD expression. Oncol. Lett. 2 (1), 43–47. doi:10.3892/ol.2010.215
Wang, M., Firrman, J., Zhang, L., Arango-Argoty, G., Tomasula, P., Liu, L., et al. (2017). Apigenin Impacts the growth of the gut microbiota and alters the gene expression of Enterococcus. Molecules 22 (8), 1292. doi:10.3390/molecules22081292
Wang, X., He, S., Yuan, L., Deng, H., and Zhang, Z. (2021). Synthesis, structure Characterization, and antioxidant and Antibacterial activity study of Iso-orientin-Zinc complex. J. Agric. Food Chem. 69 (13), 3952–3964. doi:10.1021/acs.jafc.0c06337
Warren, C. A., Paulhill, K. J., Davidson, L. A., Lupton, J. R., Taddeo, S. S., Hong, M. Y., et al. (2009). Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J. Nutr. 139 (1), 101–105. doi:10.3945/jn.108.096271
Wu, H., Du, J., Li, C., Li, H., Guo, H., and Li, Z. (2022). Kaempferol can reverse the 5-Fu resistance of colorectal cancer cells by inhibiting PKM2-mediated glycolysis. Int. J. Mol. Sci. 23 (7), 3544. doi:10.3390/ijms23073544
Wu, H., Du, J., Hu, P., Wang, P., Wang, Z., Ma, J., et al. (2025). Kaempferol induces DNA damage in colorectal cancer cells by regulating the MiR-195/miR-497-PFKFB4-mediated nonoxidative pentose phosphate pathway. J. Agric. Food Chem. 73 (14), 8312–8322. doi:10.1021/acs.jafc.4c13123
Xavier, C. P., Lima, C. F., Preto, A., Seruca, R., Fernandes-Ferreira, M., and Pereira-Wilson, C. (2009). Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett. 281 (2), 162–170. doi:10.1016/j.canlet.2009.02.041
Xiang, L., Zhuo, S., Luo, W., Tian, C., Xu, S., Li, X., et al. (2024). Decoding polyphenol metabolism in patients with Crohn's disease: insights from diet, gut microbiota, and metabolites. Food Res. Int. 192, 114852. doi:10.1016/j.foodres.2024.114852
Xiao, B., Qin, Y., Ying, C., Ma, B., Wang, B., Long, F., et al. (2017). Combination of oncolytic adenovirus and luteolin exerts synergistic antitumor effects in colorectal cancer cells and a mouse model. Mol. Med. Rep. 16 (6), 9375–9382. doi:10.3892/mmr.2017.7784
Xie, Y. H., Chen, Y. X., and Fang, J. Y. (2020). Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target Ther. 5 (1), 22. doi:10.1038/s41392-020-0116-z
Xu, M., Wang, S., Song, Y. U., Yao, J., Huang, K., and Zhu, X. (2016). Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 11 (5), 3075–3080. doi:10.3892/ol.2016.4331
Yaeger, R., Shah, M. A., Miller, V. A., Kelsen, J. R., Wang, K., Heins, Z. J., et al. (2016). Genomic Alterations observed in colitis-associated cancers are distinct from those found in sporadic colorectal cancers and vary by type of inflammatory bowel disease. Gastroenterology 151 (2), 278–287. doi:10.1053/j.gastro.2016.04.001
Yang, L., Allred, K. F., Dykes, L., Allred, C. D., and Awika, J. M. (2015). Enhanced action of apigenin and naringenin combination on estrogen receptor activation in non-malignant colonocytes: implications on sorghum-derived phytoestrogens. Food Funct. 6 (3), 749–755. doi:10.1039/c4fo00300d
Yang, L., Liu, Y., Wang, M., Qian, Y., Dong, X., Gu, H., et al. (2016). Quercetin-induced apoptosis of HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis. Mol. Med. Rep. 14 (5), 4559–4566. doi:10.3892/mmr.2016.5818
Yang, Y., Wang, T., Chen, D., Ma, Q., Zheng, Y., Liao, S., et al. (2019). Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol. Int. 43 (2), 117–124. doi:10.1002/cbin.11055
Yang, C., Song, J., Hwang, S., Choi, J., Song, G., and Lim, W. (2021). Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 47, 102144. doi:10.1016/j.redox.2021.102144
Yang, C., Song, J., Park, S., Ham, J., Park, W., Park, H., et al. (2022). Targeting thymidylate synthase and tRNA-derived non-Coding RNAs improves therapeutic sensitivity in colorectal cancer. Antioxidants (Basel) 11 (11), 2158. doi:10.3390/antiox11112158
Yang, H., Yue, G. G., Yuen, K. K., Gao, S., Leung, P. C., Wong, C. K., et al. (2023). Mechanistic insights into the anti-tumor and anti-metastatic effects of Patrinia villosa aqueous extract in colon cancer via modulation of TGF-β R1-smad2/3-E-cadherin and FAK-RhoA-cofilin pathways. Phytomedicine 117, 154900. doi:10.1016/j.phymed.2023.154900
Zhang, Y., Tie, X., Bao, B., Wu, X., and Zhang, Y. (2007). Metabolism of flavone C-glucosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats. Br. J. Nutr. 97 (3), 484–494. doi:10.1017/s0007114507336830
Zhang, Z., Peng, X., Zhang, N., Liu, L., Wang, Y., and Ou, S. (2014). Cytotoxicity comparison of quercetin and its metabolites from in vitro fermentation of several gut bacteria. Food Funct. 5 (9), 2152–2156. doi:10.1039/c4fo00418c
Zhang, M., Sun, G., Shen, A., Liu, L., Ding, J., and Peng, J. (2015a). Patrinia scabiosaefolia inhibits the proliferation of colorectal cancer in vitro and in vivo via G1/S cell cycle arrest. Oncol. Rep. 33 (2), 856–860. doi:10.3892/or.2014.3663
Zhang, X. A., Zhang, S., Yin, Q., and Zhang, J. (2015b). Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 11 (42), 404–409. doi:10.4103/0973-1296.153096
Zhang, X., Zhang, W., Chen, F., and Lu, Z. (2021). Combined effect of chrysin and apigenin on inhibiting the development and progression of colorectal cancer by suppressing the activity of P38-MAPK/AKT pathway. IUBMB Life 73 (5), 774–783. doi:10.1002/iub.2456
Zhong, Y., Krisanapun, C., Lee, S. H., Nualsanit, T., Sams, C., Peungvicha, P., et al. (2010). Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur. J. Cancer 46 (18), 3365–3374. doi:10.1016/j.ejca.2010.07.007
Zhou, Y., Zhang, J., Wang, K., Han, W., Wang, X., Gao, M., et al. (2020). Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 881, 173185. doi:10.1016/j.ejphar.2020.173185
Zhou, Y., Li, X., and Ye, M. (2021). Morusin inhibits the growth of human colorectal cancer HCT116-derived sphere-forming cells via the inactivation of Akt pathway. Int. J. Mol. Med. 47 (4), 1. doi:10.3892/ijmm.2021.4884
Zhu, M., Dang, Y., Yang, Z., Liu, Y., Zhang, L., Xu, Y., et al. (2020). Comprehensive RNA sequencing in adenoma-cancer transition identified Predictive biomarkers and therapeutic targets of human CRC. Mol. Ther. Nucleic Acids 20, 25–33. doi:10.1016/j.omtn.2020.01.031
Zhu, H., Zhao, N., and Jiang, M. (2021). Isovitexin attenuates tumor growth in human colon cancer cells through the modulation of apoptosis and epithelial-mesenchymal transition via PI3K/Akt/mTOR signaling pathway. Biochem. Cell Biol. 99 (6), 741–749. doi:10.1139/bcb-2021-0045
Zou, W., Gong, L., Zhou, F., Long, Y., Li, Z., Xiao, Z., et al. (2021). Anti-inflammatory effect of traditional Chinese medicine preparation Penyanling on pelvic inflammatory disease. J. Ethnopharmacol. 266, 113405. doi:10.1016/j.jep.2020.113405
Zuo, Q., Wu, R., Xiao, X., Yang, C., Yang, Y., Wang, C., et al. (2018). The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell Biochem. 119 (11), 9573–9582. doi:10.1002/jcb.27275
Glossary
ACF Abnormal crypt foci
AHR Aryl hydrocarbon receptor
AKT protein kinase B or PKB
AOM Azoxymethane
APC Adenomatous Polyposis Coli gene
AP-1 Activator protein-1
ARE AU-rich element
ATL Adult T-cell leukemia/lymphoma
Bax Bcl-2-associated X protein
Bcl-2 B-cell lymphoma 2
CAC Colitis-associated cancer
CA125 Cancer antigen 125
CB1-R Cannabinoid receptor 1
CDCA Chenodeoxycholic acid
cDC2 Type 2 conventional dendritic cell
CDC25C Cell division cycle 25C
CDK6 Cyclin-dependent kinase 6
CD55 Decay-accelerating factor,DAF
c-FLIP Cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein
COX-1 Cyclooxygenase-1
COX-2 Cyclooxygenase-2
CP Cisplatin
CRC CRC
CREB1 cyclic-AMP response binding protein 1
CSCs Cancer stem cells
CSN6 Constitutive photomorphogenesis 9 signalosome subunit 6
c-Myc Myc proto-oncogene protein
CCND1 Cyclin D1
CYP8B1 Sterol 12-alpha-hydroxylase
CYP27A1 Cytochrome P450 Family 27 Subfamily A Member 1
DPPIV Dipeptidyl peptidase IV
TRAIL-R1 TNF-related apoptosis-inducing ligand receptor 1,DR4
TRAIL-R2 TNF-related apoptosis-inducing ligand receptor 2,DR5
DSS Dextran sulfate sodium
DMH 1,2-dimethylhydrazine
DNA DeoxyriboNucleic Acid
DNMTs DNA methyltransferases
DNMT1 DNA methyltransferase1
EGFR Epidermal Growth Factor Receptor
EMT Epithelial-mesenchymal transition
ERα Estrogen receptor α
ERβ Estrogen receptor β,ESR2
ErbB2 Human epidermal growth factor receptor 2
ErbB3 Human epidermal growth factor receptor 3
ERK Extracellular regulated protein kinases
E2F1 E2F Transcription Factor 1
E2F3 E2F Transcription Factor 3
FADD Fas-associated protein with death domain
FAP Familial adenomatous polyposis
FEQ Fermented quercetin
FXR Farnesoid X Receptor
GPR30 G protein-couple receptor 30,GPER
HDACs Histone deacetylases
HTLV-1 Human T-lymphotropic virus 1
IBD Inflammatory bowel disease
IL-1β Interleukin-1 beta
IL-6 Interleukin-6
IL-10 Interleukin-10
iNOS Inducible nitric oxide synthase
JMJD2C Jumonji domain 2c
JNK c-Jun N-terminal kinase
JUN Jun proto-oncogen
Ki67 marker of proliferation
KRAS Kirsten rat sarcoma viral oncogene
LGR5 Leucine-rich repeat-containing G protein-coupled receptor 5
LKB1 Liver kinase B
LPS lipopolysaccharides
MAPK Mitogen-activated protein kinase
MDR Multidrug resistance
MEK Mitogen-activated protein kinase kinase, MAPKK
mTOR Mammalian target of rapamycin
MMP Matrix metalloproteinases
NEDD9 Neural Precursor Cell Expressed, Developmentally Downregulated 9
NLRs Nucleotide-binding and oligomerization domain-like receptors
NF-κB Nuclear factor kappa-B
NK Natural killer
NKG2D Natural killer group 2D
NRF2 Nuclear factor erythroid 2-related factor 2
OCTN2 Organic cation/carnitine transporter 2
PARP Poly ADP-ribose polymerase
p-Akt Phospho-protein kinase B
P-gp P-glycoprotein
PI3K Phosphatidylinositol 3-kinase
p-PI3K Phospho- Phosphatidylinositol 3-kinase
PKM2 Pyruvate kinase M2
PPARγ Peroxisome proliferators-activated receptorsγ
Ras Rat sarcoma
Raf Raf-1 proto-Oncogene
ROS Reactive oxygen species
SCFAs Short-chain fatty acids
SIRT1 Silent information regulator 1
SIRT6 Silent information regulator 6
SphK2 sphingosine kinase2
SRC SRC Proto-Oncogene
STAT3 Signal transducer and activator of transcription 3
S1P Sphingosine-1-phosphate
TAGLN Transgelin
TCF4 T-cell factor 4
TIMPs Tissue inhibitors of metalloproteinases
TNBS 2,4,6-trinitrobenzenesulfonic acid sol
TNF-α Tumor necrosis factor-α
TRAIL TNF Superfamily Member 10, TNFSF1O
TS Thymidylate synthase
UHRF1 Ubiquitin-like with PHD and RING finger domains 1
Wnt Wingless/Integrated
Keywords: Herba Patriniae, flavonoids, colorectal cancer, gut microbiota, anticancer
Citation: Zhang P, Jia R, Wang Y, Tang Y, Li Q and Hou F (2025) Clinical research, mechanisms, and prospects of flavonoids from Herba Patriniae in the treatment of colorectal cancer. Front. Pharmacol. 16:1633286. doi: 10.3389/fphar.2025.1633286
Received: 22 May 2025; Accepted: 05 August 2025;
Published: 28 August 2025.
Edited by:
Olu Israel Oyewole, Osun State University, NigeriaReviewed by:
Leena Dhoble, University of Florida, United StatesMingyue Zhou, Macau University of Science and Technology, Macao SAR, China
Elif Günalan, Istanbul Health and Technology University, Türkiye
Copyright © 2025 Zhang, Jia, Wang, Tang, Li and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Li, THp3ZkBob3RtYWlsLmNvbQ==; FengGang Hou, Zmdob3U1NTVAMTI2LmNvbQ==
 PingPing Zhang1
PingPing Zhang1 Qi Li
Qi Li FengGang Hou
FengGang Hou