- Department of Dermatology, Zhongshan Second People’s Hospital, Zhongshan, Guangdong, China
Novel therapeutic approaches on molecular pathways are being developed to treat inflammatory and autoimmune cutaneous dermatoses. Apremilast is an orally administered small-molecule phosphodiesterase 4 (PDE4) inhibitor that upregulates intracellular cyclic 3′,5′-adenosine monophosphate (cAMP) levels to mediate a large array of proinflammatory cytokines as well as exerts its anti-inflammatory functions and therapeutic efficacy in skin diseases rather than an immunosuppressive mode of action. Early-phase clinical trials have demonstrated its favorable efficacy such that the United States Food and Drug Administration (USFDA) has approved its use for the treatment of psoriasis, psoriatic arthritis, and Behçet’s syndrome. Compared to conventional immunosuppressive therapies, apremilast has better safety and tolerability profiles that significantly reduce the risk of serious adverse reactions from long-term usage. Apremilast shows easier and faster absorption even by special areas of the body, such as nails, scalp, palms and soles of feet, and genitals, along with clinically meaningful improvements. More recently, accumulating real-world evidence has revealed that it is highly effective for treating multiple immune-mediated inflammatory skin diseases in an off-label manner; it also appears to be useful either alone or as an add-on treatment against some chronic inflammatory skin disorders recalcitrant to conventional therapies. Thus, further large-scale studies and real-life trials are necessary to better elucidate its role in dermatology. The present narrative review provides an overview of apremilast as a novel therapeutic option for skin disorders, including a comprehensive look at its pharmacology, clinical efficacy, and safety profile, with the aim of enlightening clinicians on the broad applications and full potential of this small-molecule drug based on currently available evidence.
1 Introduction
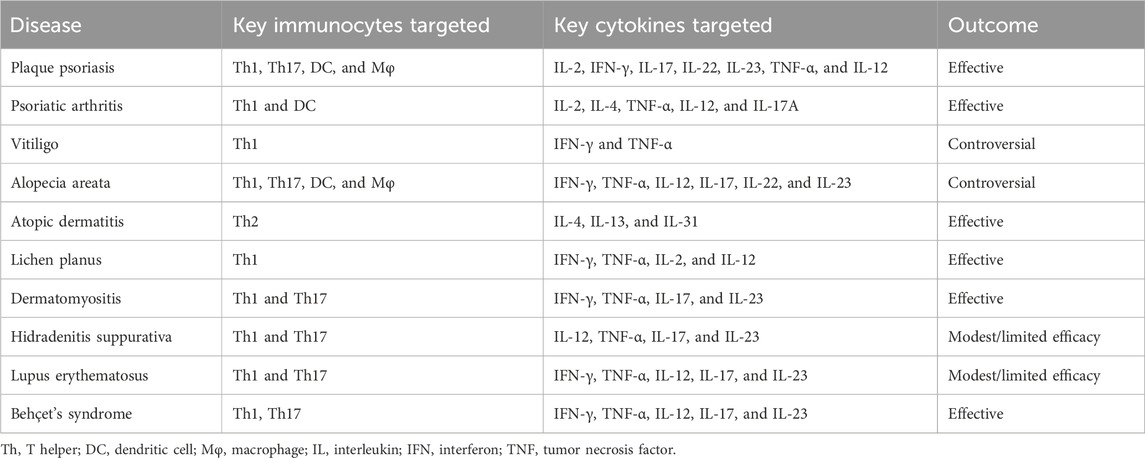
Phosphodiesterase 4 (PDE4) plays a significant role in modulating inflammatory responses by degrading cyclic 3′,5′-adenosine monophosphate (cAMP), which is expressed by various structural cell types. Apremilast is an oral small-molecule PDE4 inhibitor that has shown impressive disease-modifying potency and anti-inflammatory activity in chronic inflammatory dermatoses; hence, it has been approved by the United States Food and Drug Administration (USFDA) for the treatment of moderate-to-severe plaque psoriasis, psoriatic arthritis (PsA), and Behçet’s syndrome in adults. It has been found to accurately inhibit PDE4 isoforms from all four subfamilies (A1A, B1, B2, C1, and D2) as well as selectively regulate the production of inflammatory mediators, including T helper (Th1, Th2, and Th17) cytokines. In T cells and monocytes, apremilast was found to activate phosphorylation of the protein kinase A substrates CREB as well as activate transcription factor-1 (PKA-CREB/ATF-1) pathways while inhibiting NF-κB-driven transcriptional activity, thereby inducing TLR4 signaling dysregulation and reducing type I interferon (IFN)-α production. Unlike immunosuppressors like thalidomide and lenalidomide, apremilast has minimal effects on B-cell differentiation, immunoglobulin and T-cell clonal expansions, as well as in vivo antibody responses, suggesting that its impacts on innate immunity are greater than those on adaptive immunity along with better safety profile (Schafer et al., 2014). In dermatology, preliminary studies have highlighted the potential efficacy of apremilast against various immune-mediated inflammatory skin disorders. In the present review, we summarize the existing clinical practices with regard to apremilast application in immune-mediated inflammatory skin diseases (Table 1), including psoriasis, vitiligo, alopecia areata, atopic dermatitis, lichen planus, dermatomyositis, hidradenitis suppurativa, lupus erythematosus, and Behçet’s syndrome, along with a primary focus on its associated action mechanisms (Figure 1; Table 2).
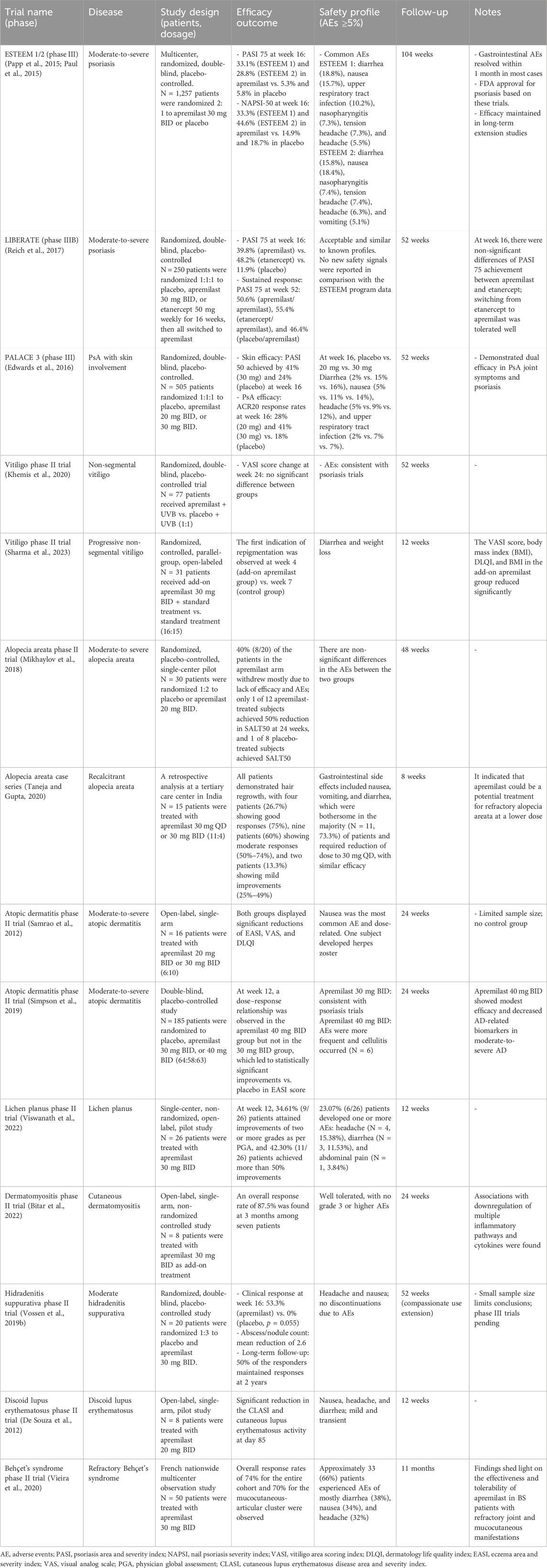
Table 1. Overview of the key clinical trials involving apremilast for immune-mediated skin diseases.
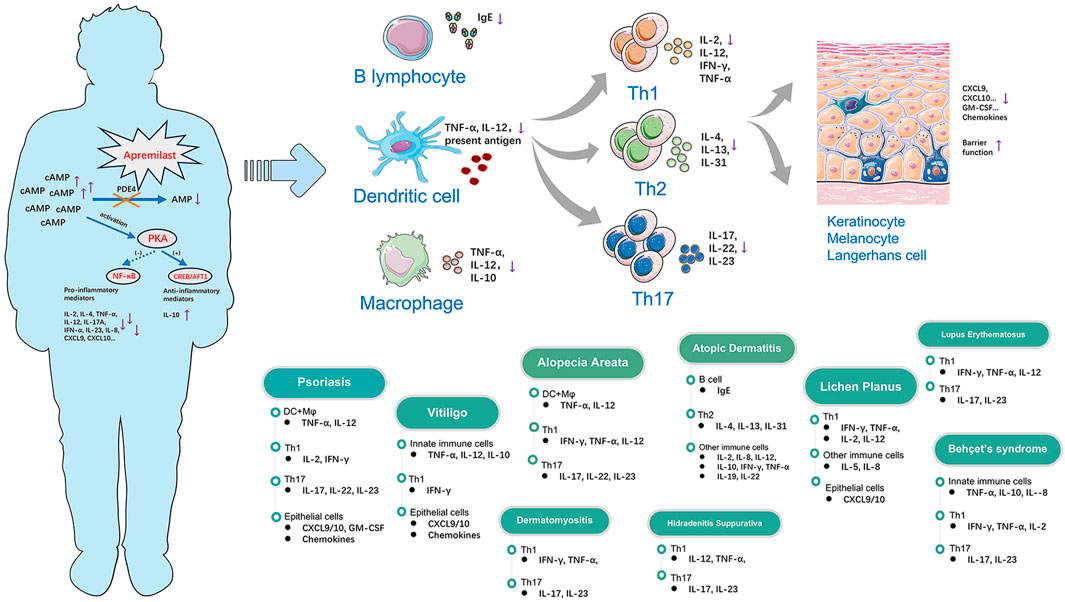
Figure 1. Modes of action of apremilast in the regulation of multiple immune-mediated inflammatory skin diseases through immunocytes and cytokines.
2 Apremilast in immune-mediated inflammatory skin diseases
2.1 Psoriasis and PsA
Psoriasis is a multifactorial chronic inflammatory skin disease that affects approximately 2%–4% of the global population (Parisi et al., 2013); it is associated with several comorbidities, including PsA, with a concurrency rate of over 20% statistically that can seriously harm the physical and mental health of patients. Extant studies have highlighted the critical interplay between Th17-related cells and cytokine cascades in the pathogenesis of psoriasis and PsA. It is well acknowledged that dendritic cells release IL-12, IL-23, and other cytokines to expand the differentiated Th17 cell populations; therefore, the Th1, Th22, and Th17 subsets produce proinflammatory mediators, including IL-17A/F, tumor necrosis factor (TNF)-α, IFN-α, and IL-22, which result in keratinocyte hyperproliferation and immunocyte infiltration in psoriatic skin (Girolomoni et al., 2017). Similarly, in PsA, upregulated expression of TLR2 in the dendritic cells can induce Th1 responses and release proinflammatory cytokines like IL-2, IL-4, TNF-α, IL-12, and IL-17A, thereby triggering inflammatory responses (Ocampo and Gladman, 2019).
Although the innovative biologics available in the market have shown great efficacy in alleviating psoriasis and PsA, they are prone to limitations like primary and secondary failures, safety concerns, and unaffordable costs resulting in treatment discontinuation. In vivo, PDE4 is expressed in various structural cell types in both keratinocytes and the synovium. Along with inhibition of the PDE4 enzyme, apremilast deregulates production of substantial chemokines and cytokines, including CXCL9, CXCL10, TNF-α, IFN-α, IL-2, IL-12, and IL-23, in the human primary peripheral blood mononuclear cells as well as TNF-α in the natural killer cells and epidermal keratinocytes, as suggested in a preclinical model of psoriasis. Moreover, apremilast increases the expression of the anti-inflammatory mediator IL-10 as well as reduces human leukocyte antigen-DR and intercellular cell adhesion molecule-1, thus displaying a broad range of anti-inflammatory activities (Schafer et al., 2010).
Clinical experience with apremilast has demonstrated its potential to alter the landscape of small-molecule immunotherapy of psoriasis. Additionally, it has yielded favorable efficacies at difficult-to-treat sites, such as the scalp, face, nails, and genitals, as well as against palmoplantar pustulosis. As a small-molecule drug, apremilast shows more effective and faster onset in the small blood vessels of the palmoplantar areas as well as demonstrates comparable efficacy to methotrexate; however, there is notable heterogeneity in the outcome measures and risk of bias across studies, which highlights the need for standardized evaluation metrics (Spencer et al., 2023). In the clinic, it is likely employed as a maintenance therapy following conventional treatment; circumstantially, it is even used as a post-biologic or included in combination with other biologics. Additionally, apremilast has been shown to be an alternative approach for PsA patients who failed traditional disease-modifying antirheumatic drugs (DMARDs) for their limited efficacy and considerable toxicity (Sandhu et al., 2020). The long-term efficacy and safety of apremilast in psoriasis and PsA have been evaluated in several large-scale clinical trials, even though its overall effectiveness appears to be less than those of biologic agents (Torres and Puig, 2017). For patients having underlying active or latent infectious diseases, including tuberculosis and viral hepatitis, along with hepatic or renal insufficiency, apremilast is the preferred option. Furthermore, apremilast has been demonstrated to improve the quality of life and have a high safety profile in psoriatic patients with malignancies (Tsentemeidou et al., 2022; Podevin et al., 2023). However, concerns on whether apremilast could inhibit structural joint damage remain undetermined and need to be explored in future clinical trials.
2.2 Vitiligo
Vitiligo is an autoimmune disorder that affects pigmentation and is prevalent in approximately 0.5%–2% of the global population (Bergqvist and Ezzedine, 2020). It is well acknowledged that IFN-γ signaling and C-X-C motif chemokine ligand 9/10 axis contribute to the primary pathogenesis of vitiligo. IFN-γ signals the Langerhans cells and keratinocytes in the epidermis, thereby stimulating the production of chemokines CXCL9/10 in the dermis that are subsequently recognized by CXCR3, resulting in the recruitment of effector CD8+ T cells. The autoreactive T cells induce initial apoptosis of the melanocytes to establish long-lived CD8+ resident memory T cells within the skin (Frisoli and Harris, 2017; Richmond et al., 2017). It has been reported that the CD8+ resident memory T cells could possess cytotoxic effector functions when exposed to inflammatory cytokines and are therefore functionally associated with the disease maintenance of vitiligo (Cheuk et al., 2017). Current therapeutics that suppress autoimmune inflammation could help reverse repigmentation of the skin. By elevating the intracellular cAMP and anti-inflammatory cytokine IL-10 levels, apremilast decreases the production of CXCL9/10 by inhibiting IFN-γ signaling. A case series reported by Majid et al. (2019) showed the positive outcomes of oral apremilast in the disease control of 13 adult patients with onset vitiligo; however, this study lacked a control or comparison group as well as a sufficiently large population size.
A systematic review of apremilast involving 122 participants demonstrated its significant efficacy for the treatment of vitiligo. Furthermore, experimental studies have suggested that the combination of apremilast and narrowband ultraviolet B (UVB) phototherapy is more effective in promoting repigmentation supported by significant decreases in CD8+ T cells, CD11c+ dendritic cells, and Th17-related markers along with upregulation of melanogenesis markers (Kim et al., 2023). Sharma et al. (2023) reported that 30 mg of add-on apremilast twice daily in combination with standard treatment could reduce disease progression and accelerate clinical improvement of vitiligo. However, in a double-blind and placebo-controlled study, 77 patients were randomized 1:1 to apremilast in combination therapy with UVB and placebo with UVB, which showed no significant difference between the groups at week 24 (Sharma et al., 2025). It was suspected that such varying clinical efficacies could be due to a combination of patient-specific factors, including anatomical location, disease stage (progressive/quiescent), variability in immune profiles, and variations in PDE4 subtype expressions; the small sample size, short follow-up, and similar demographic characteristics may also have limited the conclusions. Nevertheless, the results of multiple trials have shown that apremilast is still effective and has anti-inflammatory efficacy in the treatment of vitiligo.
2.3 Alopecia areata
Alopecia areata (AA) is a hair loss disorder the affects approximately 2% of the general population and is characterized by cytokine dysregulation of IFN-γ, IL-15, IL-2, and IL-7 that mediate CD8+ T-cell proliferation, resulting in immune-privilege collapse of the hair follicle. Recent research has shown that PDE4-related cytokine pathways are activated and that PDE4 is upregulated in the scalp lesions in AA patients (Suárez-Fariñas et al., 2015). In animal models, hair regrowth was observed following PDE4 antagonism. Humanized AA mice treated with apremilast showed almost complete absence of CD8+ T cells and decreased levels of proinflammatory cytokines, including IFN-γ and TNF-α, despite the relatively low number of experimental mice owing to difficulties with human scalp transplants (Laufer Britva et al., 2020; Keren et al., 2015). Furthermore, there are cases demonstrating significant improvements (Estebanez et al., 2019; Magdaleno-Tapial et al., 2019). A retrospective analysis involving 15 AA patients showed that apremilast was effective against recalcitrant AA over a follow-up period of 1.5 years (Taneja and Gupta, 2020). Intriguingly, Chhabra and Verma (2019) reported a case of steroid-resistant pediatric alopecia totalis responding to apremilast (30 mg/d) and platelet-rich plasma as an adjuvant, indicating their synergistic effects. Mikhaylov et al. (2018) conducted a randomized placebo-controlled study, in which 40% (8/20) of the patients in the apremilast arm withdrew mostly owing to lack of efficacy and adverse events; here, only one of the 12 apremilast-treated subjects achieved 50% reduction in the severity of alopecia tool (SALT) score (SALT50) at 24 weeks, and only one of the eight placebo-treated subjects achieved SALT50, indicating that apremilast may be ineffective for moderate-to-severe AA (Mikhaylov et al., 2018). Some other similar small-scale clinical studies have shown unsatisfactory results for apremilast treatment efficacy in AA patients, indicating that apremilast is less likely to be effective in severe and recalcitrant AA (Weber et al., 2020; Sakakibara et al., 2019; Liu and King, 2017; Gupta et al., 2023; Rudnicka and Olszewska, 2024). The non-responsiveness in these aforementioned studies may be attributed to the small sample sizes, short durations of therapy, and the fact that most patients had alopecia universalis. Additionally, it is speculated that apremilast is only effective in AA patients with significant PDE4 overexpression. Still, the role of apremilast in refractory AA warrants further evaluation, and the reasons for heterogeneous therapeutic responses in the clinic need to be further delineated in upcoming clinical trials.
2.4 Atopic dermatitis (AD)
AD is also known as atopic eczema and is a chronic recurrent inflammatory skin disorder characterized by xerotic skin and acute flare-ups of highly pruritic eczematous lesions that affects approximately 2%–4% of adults and up to 20% of children globally (Barbarot et al., 2018; Silverberg et al., 2021). The pathogenesis of AD involves breakdown of the skin barrier due to Th1/Th2 imbalance with cytokine dysregulation, increased immunoglobulin E and eosinophilia through the release of IL-4/5/13, and decreased protection against infection through the release of IL-10. It has been reported that the PDE4 isoforms are overexpressed by up to threefold in the epidermis in AD compared to healthy skin. In murine models, it was observed that AD-associated inflammatory markers were significantly modulated by apremilast (Schafer et al., 2019); the substantial proinflammatory cytokines involved in AD (TNF-α, IL-12, IL-2, IFN-γ, IL-5, and IL-8) were downregulated while the production of anti-inflammatory factors (IL-10) increased. Growing evidence has confirmed the effectiveness and safety of apremilast in the treatment of adults and children with severe AD (Deleanu and Nedelea, 2019; Abrouk et al., 2017). Samrao et al. (2012) conducted an open-label pilot study involving 16 subjects with moderate-to-severe AD; they observed a trend toward improvement even in cohorts who received 20 mg of apremilast twice daily. Farahnik et al. (2017) reported the case of a 55-year-old male patient with a lifelong history of AD recalcitrant to topical steroids and cyclosporine who showed improvements in pruritus and erythema following 10 weeks of treatment with apremilast.
Simpson et al. (2019) conducted a phase II, double-blind, and placebo-controlled trial in 185 adult patients; they observed a dose–response relationship between 40 mg of apremilast administered twice daily and a significant remission in moderate-to-severe AD, with gene array analysis displaying reduced levels of the Th17/Th22-associated biomarkers, including IL-19, IL-22, and S100A, in AD lesions. In another group that was administered 30 mg of apremilast BID, statistically significant improvements were not observed in the EASI score compared to placebo (Simpson et al., 2019). Saporito and Cohen (2016) described the case of an 8-year-old boy with refractory AD who was treated with 30 mg of apremilast daily; here, the boy showed quick improvements of pruritus and inflammation within 2 weeks, indicating that apremilast is a safe and effective medication for the pediatric population. Notably, dupilumab is the first USFDA-approved targeted therapy for AD that blocks IL-4 and IL-13 cytokine signaling (Langan et al., 2020). In patients showing inadequate responses to dupilumab, concomitant apremilast could be promisingly applied for enhanced benefits (Cunningham et al., 2023). In patients at risk of malignancy, apremilast was noted to have a safer profile than JAK inhibitors. Apremilast also shows rapid and complete responses in chronic hand eczema with hepatogenic pruritus or hyperkeratotic hand and foot dermatitis (Navarro-Trivino et al., 2019; Bhat et al., 2024). As novel molecular pathways underlying AD are being uncovered, there are advances in disease management and endotype-based targeted therapies. PDE4 activity is notably increased in AD patients, resulting in intracellular cAMP degradation as well as subsequent increased production of proinflammatory cytokines. Although preclinical studies have lacked active comparators like biologics or standard-of-care therapies that have made it difficult to position apremilast relative to the alternatives, apremilast is undoubtedly a promising targeted therapeutic agent for mild-to-moderate AD in both children and adults.
2.5 Lichen planus (LP)
LP is a chronic multifactorial inflammatory disease with a heterogeneous clinical presentation that predominantly causes severe pruritus and pain in the skin, nails, and mucous membranes; this condition affects approximately 15%–20% of patients with clinical relapse (Tekin et al., 2024). The pathogenesis of LP has been incompletely investigated but is mainly known to involve T-cell-mediated damage of the basal keratinocytes in lesions by various inflammatory cytokines and chemokines, such as TNF-α, IFN-γ, IL-9, IL-17, IL-22, IL-23, CXCL9, and CXCL10. Regulatory T cells have been detected in the blood of LP patients, indicating that the inflammation is not restricted to the skin (Domingues et al., 2016). Apremilast inhibits the enzyme PDE4 and promotes intracellular cAMP to inhibit proinflammatory cytokine transcription and immunocyte chemotaxis, which ultimately decreases the production of inflammatory mediators like TNF-α, IFN-γ, IL-2, IL-5, IL-8, and IL-12. Therefore, it is plausible that apremilast may be effective against LP. Paul et al. (2013) conducted an open-label pilot study of apremilast for the treatment of moderate-to-severe LP; they describe that three out of 10 patients showed improvements of two or more grades in the physician global assessment (PGA) following 12 weeks of apremilast administration at 20 mg twice daily and that all individuals showed statistically significant clinical improvements. Similarly, Viswanath et al. (2022) reported that 34.61% (9/26) of the patients in their study who were prescribed 30 mg of apremilast twice daily attained two or more grades of improvement as per the PGA, while 42.30% (11/26) of the patients achieved more than 50% improvements in the lesions by week 12; the consistent conclusion here is that apremilast is effective and safe in the management of LP (Viswanath et al., 2022). For small sample sizes and studies lacking a control group, it is difficult to generalize the above results convincingly. For oral lichen planus (OLP) that has been reported to have a malignant transformation rate of 2.3% to oral squamous cell carcinoma (Gonzalez-Moles et al., 2021), apremilast is likely a viable option in difficult-to-treat cases (Perschy et al., 2022; Kim-Lim and Thomas, 2023). An earlier report noted that a Th17 phenotype-associated cytokine profile was detected in erosive OLP, while a skew toward the Th2 and Treg phenotypes was found in reticular OLP (Wang et al., 2016; Piccinni et al., 2014). With regard to recalcitrant erosive OLP, oral apremilast at a dose of 30 mg twice daily showed significant improvement in the disease activity (AbuHilal et al., 2016). In the case series reported by Sil et al. (2023) and Bettencourt (2016), subjects with refractory OLP who received apremilast showed significant improvements at 12-week follow-up. A randomized, double-blind, placebo-controlled trial for genital erosive lichen planus based on 42 adult women showed credible evidence for the use of apremilast in such a burdensome genital dermatosis (Skullerud et al., 2021). Nonetheless, there are very little data available for apremilast as a treatment for nail lichen planus. Given its desirable responses in nail psoriasis, it would be an interesting and essential research direction to investigate the activity of apremilast on nail lichen planus in the future.
2.6 Dermatomyositis (DM)
DM is an idiopathic inflammatory myopathy characterized by cutaneous abnormalities that are frequently complicated by interstitial lung disease, which is refractory and recurrent even following treatment with immunosuppressants. Extant studies have revealed the critical roles of the Th1/Th2 pathways in DM, including TNF-α, IFN-γ, IL-4, IL-6, IL-17, IL-18, IL-23, and STAT signaling that have been found to be downregulated with disease improvement (Moneta et al., 2019; Thompson et al., 2018; Giris et al., 2017). In this regard, different case series and small-scale prospective trials have been reported on the off-label use of apremilast in treating DM. A phase IIa, non-randomized, controlled trial of apremilast in eight patients with recalcitrant DM showed that the overall response rate was 87.5% (Bitar et al., 2022); however, the single-arm single-center setting and short intervention course (12 weeks) were the main limitations of this study. Bitar et al. (2019) also reported on three patients with refractory DM who showed 85% improvement in cutaneous DM activity and severity index (CDASI) at 12-week follow-up. In patients with scalp DM, sustained resolution of the scalp pruritus was observed (Kolla et al., 2021; Charlton et al., 2021). In patients with paraneoplastic DM undergoing chemotherapy, immunosuppressants are constantly contraindicated for increased risk of infection, which gives apremilast a distinct advantage (Cabas et al., 2023). Furthermore, emerging evidence has shown that apremilast is a safe and efficient add-on treatment in recalcitrant DM (Elhage et al., 2022; Konishi et al., 2022).
2.7 Hidradenitis suppurativa (HS)
HS is a chronic immune-mediated inflammatory follicular occlusive disease that manifests as inflammatory nodules and abscesses to the formation of sinus tracts and scarring; it is closely associated with various comorbidities, including obesity, hypertension, dyslipidemia, and diabetes mellitus. Its immune dysregulation is predominantly mediated by the TNF-α, IL-1β, IL-10, IL-23/Th17, and IL-12/Th1 pathways. TNF-α inhibitors have been demonstrated to aid with remarkable recovery and ongoing remission in the disease control of HS (Kelly et al., 2015; Moran et al., 2017). In a double-blind, randomized, placebo-controlled trial involving 20 subjects with moderate HS, approximately 53.3% (8/15) of the apremilast-treated patients attained positive clinical responses with significantly lower abscess and nodule counts by 16 weeks (Vossen et al., 2019b); furthermore, prolonged efficacy of the treatment was observed after 2 years (Aarts et al., 2021). In another open-label, phase II trial involving 20 adults with mild-to-moderate HS, 65% of the participants showed significant improvements of HS disease activity (Kerdel et al., 2019); similarly impressive results were also reported for other case series (Weber et al., 2017; Garbayo-Salmons et al., 2021). Paradoxically, Vossen et al. (2019a) found that the inflammatory markers in HS lesions treated with apremilast could not be detected with statistically significant changes, whereas elevated S100A12 and IL-17A levels were found to decrease. Given the efficacy of apremilast in the treatment of psoriasis, HS cases with concomitant psoriasis were successfully treated with the off-label use of apremilast in clinics, even as the molecular crosstalk between HS and psoriasis is actively being studied (Proietti et al., 2020; Lanna et al., 2019). In addition, Garcovich et al. (2020) reported the findings in two apremilast-treated adults having severe HS presenting with concomitant PsA who entered remission successfully. For patients with concomitant HS and Crohn’s disease, the combination of guselkumab and apremilast simultaneously showed clinically validated efficacy (Agud-Dios et al., 2022). The abovementioned small and short-term studies underscore the need for larger and long-term trials to clarify the utility of apremilast in HS therapy.
2.8 Lupus erythematosus (LE)
Apremilast may constitute a safe and effective therapeutic modality for LE. An open-label study reported the use of 20 mg of apremilast twice daily for treating eight patients with discoid LE and noted a significant (p < 0.05) reduction in the chronic LE disease areas and severity index (CLASI) (De Souza et al., 2012). Further observational studies with larger numbers of patients or varying doses of apremilast for treating LE are necessary to verify this pilot open-label study. Moreover, a 44-year-old Japanese woman who developed psoriasis with systemic LE was treated with 30 mg of oral apremilast twice daily and showed improved outcomes with decreased PASI score and systemic LE activity gradually. The pathogenic mechanisms about the Th17 axis have previously been reported together in psoriasis and systemic LE; here, apremilast blocks the production of INF-γ, IL-12, IL-23, and TNF-α, thus suppressing the Th1/Th17-mediated immune responses and exerting anti-inflammatory activity (Ichiyama et al., 2019).
2.9 Behçet’s syndrome (BS)
BS is a chronic recurrent inflammatory skin disease characterized by relapsing mucocutaneous and joint disorders with oral ulcers as the cardinal feature. Here, colchicine or systemic glucocorticoids are the first-line therapies that still show unsatisfactory clinical outcomes. Despite the incompletely investigated action mechanisms of apremilast, its therapeutic benefits in BS are believed to be exerted through modulation of the serum cytokine levels, including those of TNF-α, IL-23, IL-2, IL-8, IL-10, IL-17, and IFN-γ (Wakiya et al., 2022; Fukui et al., 2022). Apremilast could strongly inhibit the activation of surface markers, NETosis, as well as reactive oxygen species production on the neutrophils in BS, resulting in the modulation of innate immunity, intracellular signaling, and chemotaxis (Le Joncour et al., 2023). In the clinic, apremilast has demonstrated efficacies in the refractory mucocutaneous and joint manifestations of BS. In a French nationwide multicenter study involving 50 BS patients, apremilast was administered at 30 mg twice a day with overall response rates of 74% for the entire cohort and 70% for the mucocutaneous-articular cluster; however, the continuation of concomitant therapy with colchicine or DMARDs could represent potential confounders to the efficacy evaluations of this study (Vieira et al., 2020). Additionally, a phase III RELIEF study confirmed significant improvements to the quality of life of the patients (Hatemi et al., 2022), consistent with the findings of preliminary real-world observational studies and randomized placebo-controlled trials (De Luca et al., 2020; Hirahara et al., 2021; Iizuka et al., 2022; Takeno et al., 2022; Lopalco et al., 2019; Ozdede and Hatemi, 2021; Atienza-Mateo et al., 2020). The benefits were also found to be sustained for up to 64 weeks with continued treatment and a known safety profile (Hatemi et al., 2021). For patients with milder disease phenotypes of BS, apremilast may be considered a higher priority than TNF inhibitors (Lopalco et al., 2025).
2.10 Others
Owing to the wide anti-inflammatory effects of apremilast in regulating the immune system with a positive safety record, it has been used in the management of a sufficient number of inflammatory skin disorders in an off-label manner, including pityriasis rubra pilaris (Krase et al., 2016), granuloma annulare (Joshi and Tschen, 2023), autoimmune blistering diseases like pemphigus (Sigmund et al., 2023) and linear IgA bullous disease (Maione et al., 2023), morphea (Higuchi et al., 2023), chronic actinic dermatitis (Kaushik et al., 2020a), sarcoidosis (Baughman et al., 2012), Darier disease (Kleidona et al., 2024), dissecting cellulitis (Bernard et al., 2023), orofacial granulomatosis (Kaushik et al., 2020b), papuloerythroderma (Yin et al., 2023), perforating dermatosis (McMichael and Stoff, 2021), prurigo nodularis (Todberg et al., 2020), pyoderma gangrenosum (Bordeaux et al., 2022), SAPHO syndrome (Adamo et al., 2018), seborrheic dermatitis (Cohen et al., 2020), chronic graft-versus-host disease (Sobral-Costas et al., 2025), Sjögren’s syndrome (Manfrè et al., 2023), and Sneddon–Wilkinson disease (Magdaleno-Tapial et al., 2020). Theoretically, apremilast could be a promising therapy for diseases with PDE4 upregulation, including rare diseases with shared inflammatory pathways. Apremilast also hints at the possibility of a therapeutic window based on small-molecular pathways for multiple immune-mediated inflammatory skin disorders. In recognizing that the action mechanisms of apremilast remain incompletely characterized, large-scale preclinical studies and real-life trials are necessitated to better elucidate the application of apremilast to autoimmune and inflammatory skin disorders, which would be critical to realizing the full potential of this novel treatment approach.
3 Discussion
Apremilast inhibits the enzyme PDE4 to reduce the levels of proinflammatory cytokines and exert its immunomodulatory effects across various diseases in an off-label manner (sometimes) in dermatologic practice. The assessment of its long-term safety is a crucial consideration for extended practical applications. The current guidelines recommend apremilast with no need for laboratory monitoring, but the long-term data on risks remain insufficient. Nausea, vomiting, diarrhea, and gastrointestinal intolerance are some of the commonly observed side effects. Caution should be exercised at the start of treatment for underweight patients, whose body weights should be monitored regularly. Furthermore, long-term safety issues like infections or malignancies may require extended follow-up periods. There are numerous studies on apremilast as a treatment for immune-mediated inflammatory skin diseases; however, the small sample sizes, short follow-up periods, high dropout rates, and/or lack of comparator drugs limit the conclusions of such studies. Data on the responses of specific subgroups (e.g., patients with comorbidities, elderly patients) as well as cumulative safety risks are lacking. Some of the possible study limitations include publication bias (positive results being more likely to be published) and heterogeneity across studies, along with complicated meta-analyses. These contradictory findings may stem from differences in the study design, patient populations, patient heterogeneities, or treatment durations. The variable response rates observed in these studies may be attributed to differences in the genetic polymorphisms, disease severity, or patient adherence. Apremilast works by inhibiting PDE4, which could help explain the variability in the response rates as different diseases or patients exhibit varying PDE4 expression or activities. Gaps in the evidence include findings from pediatric populations, people with specific comorbidities, or the use of concomitant medications. Additionally, most of the reported trials focus on short-term efficacy while lacking long-term safety and efficacy data. Although some extension studies have suggested sustained responses, real-world data with follow-up periods exceeding 5 years are sparse. Addressing these limitations would help identify critical evidence gaps and suggest directions for future research. A critical knowledge gap still remains regarding the role of apremilast in immune-mediated skin diseases; hence, clinical practice guidelines or expert consensus should be formulated to determine the conditions under which apremilast should be recommended.
4 Conclusion
Overall, the efficacy and safety of apremilast in dermatological therapies are supported by clinical evidence; recently, apremilast has been applied in the treatment of various immune-mediated inflammatory skin diseases in an off-label manner. However, it remains to be determined whether this small-molecule PDE4 inhibitor could achieve durable long-term responses, for which future clinical trials are still under development. It is anticipated that apremilast deserves greater value and may have more promising prospects in clinical applications.
5 Future directions
The broader roles of apremilast in immune-mediated skin diseases are still under investigation. The extant trials on its efficacy and safety are underpowered, with promising yet inconclusive results. Addressing the limitations of these apremilast studies requires focusing on extended sample sizes, methodological rigor, extended follow-up durations, and better alignment with real-world clinical needs. It is worth noting that although apremilast acts by inhibiting PDE4, the understanding of its interactions with disease-specific pathways remains incomplete. This means that mechanistic drivers including the baseline PDE4 expression or cytokine profiles of variable responses across diseases should be investigated. By combining larger, longer, and more diverse trials with standardized endpoints, mechanistic research, and real-world validation, apremilast could be applied in a more personalized and effective manner to immune-mediated skin conditions.
Author contributions
XL: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review and editing. JZ: Conceptualization, Visualization, Writing – original draft, Writing – review and editing. XH: Visualization, Writing – original draft, Writing – review and editing. MT: Conceptualization, Writing – original draft, Writing – review and editing. JL: Conceptualization, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to the reviewers for their careful review and constructive comments, which have significantly improved the quality of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarts, P., Vossen, A., van der Zee, H. H., Prens, E. P., and van Straalen, K. R. (2021). Long-term treatment with apremilast in hidradenitis suppurativa: a 2-year follow-up of initial responders. J. Am. Acad. Dermatol. 85 (1), 258–260. doi:10.1016/j.jaad.2020.08.113
Abrouk, M., Farahnik, B., Zhu, T. H., Nakamura, M., Singh, R., Lee, K., et al. (2017). Apremilast treatment of atopic dermatitis and other chronic eczematous dermatoses. J. Am. Acad. Dermatol. 77 (1), 177–180. doi:10.1016/j.jaad.2017.03.020
AbuHilal, M., Walsh, S., and Shear, N. (2016). Treatment of recalcitrant erosive oral Lichen planus and desquamative gingivitis with oral apremilast. J. Dermatol. Case Rep. 10 (3), 56–57. doi:10.3315/jdcr.2016.1232
Adamo, S., Nilsson, J., Krebs, A., Steiner, U., Cozzio, A., French, L. E., et al. (2018). Successful treatment of SAPHO syndrome with apremilast. Br. J. Dermatol. 179 (4), 959–962. doi:10.1111/bjd.16071
Agud-Dios, M., Arroyo-Andrés, J., Rubio-Muñiz, C., and Postigo-Lorente, C. (2022). Successful treatment of hidradenitis suppurativa and Crohn's disease with combined guselkumab and apremilast. Dermatol. Ther. 35 (10), e15743. doi:10.1111/dth.15743
Atienza-Mateo, B., Martín-Varillas, J. L., Graña, J., Espinosa, G., Moriano, C., Pérez-Sandoval, T., et al. (2020). “Apremilast in refractory orogenital ulcers and other manifestations of Behçet's disease,” in National multicenter study of 51 cases in clinical practice.
Barbarot, S., Auziere, S., Gadkari, A., Girolomoni, G., Puig, L., Simpson, E. L., et al. (2018). Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 73 (6), 1284–1293. doi:10.1111/all.13401
Baughman, R. P., Judson, M. A., Ingledue, R., Craft, N. L., and Lower, E. E. (2012). Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch. Dermatol. 148 (2), 262–264. doi:10.1001/archdermatol.2011.301
Bergqvist, C., and Ezzedine, K. (2020). Vitiligo: a review. Dermatology 236 (6), 571–592. doi:10.1159/000506103
Bernard, J. W., Reddy, S., and Flowers, R. H. (2023). The successful use of oral apremilast for a case of dissecting cellulitis. JAAD Case Rep. 39, 122–124. doi:10.1016/j.jdcr.2023.06.033
Bettencourt, M. (2016). Oral Lichen planus treated with apremilast. J. Drugs Dermatol. 15 (8), 1026–1028.
Bhat, K., Patra, S., Bhardwaj, A., Singh, S., Budania, A., Bains, A., et al. (2024). Efficacy of apremilast in hyperkeratotic hand and foot dermatitis: results from a randomized observer-blinded comparative study. Int. J. Dermatol. 63 (11), 1535–1540. doi:10.1111/ijd.17185
Bitar, C., Maghfour, J., Ho-Pham, H., Stumpf, B., and Boh, E. (2019). Apremilast as a potential treatment for moderate to severe dermatomyositis: a retrospective study of 3 patients. JAAD Case Rep. 5 (2), 191–194. doi:10.1016/j.jdcr.2018.11.019
Bitar, C., Ninh, T., Brag, K., Foutouhi, S., Radosta, S., Meyers, J., et al. (2022). Apremilast in recalcitrant cutaneous dermatomyositis: a nonrandomized controlled trial. JAMA Dermatol. 158 (12), 1357–1366. doi:10.1001/jamadermatol.2022.3917
Bordeaux, Z. A., Kwatra, S. G., and West, C. E. (2022). Treatment of pyoderma gangrenosum with apremilast monotherapy. JAAD Case Rep. 30, 8–10. doi:10.1016/j.jdcr.2022.10.001
Cabas, N., Turina, M., Pizzolitto, S., De Vita, S., and Quartuccio, L. (2023). Efficacy and safety of apremilast in a patient with paraneoplastic dermatomyositis with resistant skin disease. Clin. Exp. Rheumatol. 41 (2), 396–397. doi:10.55563/clinexprheumatol/e3gjax
Charlton, D., Moghadam-Kia, S., Smith, K., Aggarwal, R., English III, J. C., and Oddis, C. V. (2021). Refractory cutaneous dermatomyositis with severe scalp pruritus responsive to apremilast. J. Clin. Rheumatol. 27 (8S), S561–S562. doi:10.1097/RHU.0000000000000999
Cheuk, S., Schlums, H., Gallais Serezal, I., Martini, E., Chiang, S. C., Marquardt, N., et al. (2017). CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity 46 (2), 287–300. doi:10.1016/j.immuni.2017.01.009
Chhabra, G., and Verma, P. (2019). Steroid-resistant alopecia totalis in a child successfully treated with oral apremilast and platelet-rich plasma therapy. Dermatol. Ther. 32 (6), e13082. doi:10.1111/dth.13082
Cohen, S. R., Gordon, S. C., Lam, A. H., and Rosmarin, D. (2020). Recalcitrant seborrheic dermatitis successfully treated with apremilast. J. Cutan. Med. Surg. 24 (1), 90–91. doi:10.1177/1203475419878162
Cunningham, K. N., Alsukait, S., Cohen, S. R., Learned, C., Gao, D. X., Kachuk, C., et al. (2023). Apremilast 30 mg twice daily combined with dupilumab for the treatment of recalcitrant moderate-to-severe atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 37 (6), e765–e767. doi:10.1111/jdv.18906
De Luca, G., Cariddi, A., Campochiaro, C., Vanni, D., Boffini, N., Tomelleri, A., et al. (2020). Efficacy and safety of apremilast for Behçet's syndrome: a real-life single-centre Italian experience. Rheumatol. Oxf. 59 (1), 171–175. doi:10.1093/rheumatology/kez267
De Souza, A., Strober, B. E., Merola, J. F., Oliver, S., and Franks Jr, A. G. (2012). Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J. Drugs Dermatol. 11 (10), 1224–1226. doi:10.1111/j.1524-4725.2012.02539.x
Deleanu, D., and Nedelea, I. (2019). Biological therapies for atopic dermatitis: an update. Exp. Ther. Med. 17 (2), 1061–1067. doi:10.3892/etm.2018.6989
Domingues, R., de Carvalho, G. C., Aoki, V., da Silva Duarte, A. J., and Sato, M. N. (2016). Activation of myeloid dendritic cells, effector cells and regulatory T cells in Lichen planus. J. Transl. Med. 14 (1), 171. doi:10.1186/s12967-016-0938-1
Edwards, C. J., Blanco, F. J., Crowley, J., Birbara, C. A., Jaworski, J., Aelion, J., et al. (2016). Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase iii, randomised, controlled trial (palace 3). Ann. Rheum. Dis. 75 (6), 1065–1073. doi:10.1136/annrheumdis-2015-207963
Elhage, K. G., Zhao, R., and Nakamura, M. (2022). Advancements in the treatment of cutaneous lupus erythematosus and dermatomyositis: a review of the literature. Clin. Cosmet. Investig. Dermatol. 15, 1815–1831. doi:10.2147/CCID.S382628
Estebanez, A., Estebanez, N., Martin, J. M., and Montesinos, E. (2019). Apremilast in refractory alopecia areata. Int. J. Trichol. 11 (5), 213–215. doi:10.4103/ijt.ijt_59_19
Farahnik, B., Beroukhim, K., Nakamura, M., Abrouk, M., Zhu, T. H., Singh, R., et al. (2017). Use of an oral phosphodiesterase-4 inhibitor (apremilast) for the treatment of chronic, severe atopic dermatitis: a case report. Dermatol. Online J. 23 (5). doi:10.5070/d3235034926
Frisoli, M. L., and Harris, J. E. (2017). Vitiligo: mechanistic insights lead to novel treatments. J. Allergy Clin. Immunol. 140 (3), 654–662. doi:10.1016/j.jaci.2017.07.011
Fukui, S., Kawaai, S., Tamaki, H., Fukuda, K., and Okada, M. (2022). A novel combination treatment with apremilast and tumor necrosis factor inhibitor for a patient with refractory intestinal Behcet's disease. Scand. J. Rheumatol. 51 (1), 81–82. doi:10.1080/03009742.2021.1929458
Garbayo-Salmons, P., Expósito-Serrano, V., Ribera Pibernat, M., and Romaní, J. (2021). Hidradenitis suppurativa treated with apremilast: a case series. Actas Dermosifiliogr. English Ed. 112 (10), 936–939. doi:10.1016/j.adengl.2021.09.009
Garcovich, S., Giovanardi, G., Malvaso, D., De Simone, C., and Peris, K. (2020). Apremilast for the treatment of hidradenitis suppurativa associated with psoriatic arthritis in multimorbid patients: case report and review of literature. Med. Baltim. 99 (5), e18991. doi:10.1097/MD.0000000000018991
Giris, M., Durmus, H., Yetimler, B., Tasli, H., Parman, Y., and Tuzun, E. (2017). Elevated IL-4 and IFN-Gamma levels in muscle tissue of patients with dermatomyositis. Vivo 31 (4), 657–660. doi:10.21873/invivo.11108
Girolomoni, G., Strohal, R., Puig, L., Bachelez, H., Barker, J., Boehncke, W. H., et al. (2017). The role of IL-23 and the IL-23/T(H)17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 31 (10), 1616–1626. doi:10.1111/jdv.14433
Gonzalez-Moles, M. A., Ramos-Garcia, P., and Warnakulasuriya, S. (2021). An appraisal of highest quality studies reporting malignant transformation of oral Lichen planus based on a systematic review. Oral Dis. 27 (8), 1908–1918. doi:10.1111/odi.13741
Gupta, A. K., Wang, T., Bamimore, M. A., Piguet, V., and Tosti, A. (2023). The relative efficacy of monotherapy with janus kinase inhibitors, dupilumab and apremilast in adults with alopecia areata: network meta-analyses of clinical trials. J. Cosmet. Dermatol. 22 (9), 2553–2559. doi:10.1111/jocd.15903
Hatemi, G., Mahr, A., Takeno, M., Kim, D. Y., Saadoun, D., Direskeneli, H., et al. (2021). Apremilast for oral ulcers associated with active Behçet’s syndrome over 68 weeks: long-term results from a phase 3 randomised clinical trial. Clin. Exp. Rheumatol. 39 (Suppl. 132), 80–87. doi:10.55563/clinexprheumatol/ra8ize
Hatemi, G., Mahr, A., Takeno, M., Kim, D., Melikoglu, M., Cheng, S., et al. (2022). Impact of apremilast on quality of life in Behçet's syndrome: analysis of the phase 3 RELIEF study. RMD Open 8 (2), e002235. doi:10.1136/rmdopen-2022-002235
Higuchi, T., Takagi, K., Tochimoto, A., Ichimura, Y., Hirose, H., Sawada, T., et al. (2023). Antifibrotic effect of apremilast in systemic sclerosis dermal fibroblasts and bleomycin-induced mouse model. Sci. Rep. 13 (1), 19378. doi:10.1038/s41598-023-46737-1
Hirahara, L., Kirino, Y., Soejima, Y., Takeno, M., Takase-Minegishi, K., Yoshimi, R., et al. (2021). Efficacy and safety of apremilast for 3 months in behçet's disease: a prospective observational study. Mod. Rheumatol. 31 (4), 856–861. doi:10.1080/14397595.2020.1830504
Ichiyama, S., Komatsu, T., Hoashi, T., Kanda, N., Nagai, K., Yamada, Y., et al. (2019). Treatment with apremilast was beneficial for chronic graft-versus-host disease skin lesion in a patient with psoriasis. J. Dermatol. 46 (6), e218–e219. doi:10.1111/1346-8138.14766
Iizuka, Y., Takase-Minegishi, K., Hirahara, L., Kirino, Y., Soejima, Y., Namkoong, H. O., et al. (2022). Beneficial effects of apremilast on genital ulcers, skin lesions, and arthritis in patients with behçet's disease: a systematic review and meta-analysis. Mod. Rheumatol. 32 (6), 1153–1162. doi:10.1093/mr/roab098
Joshi, T. P., and Tschen, J. (2023). Apremilast for the treatment of generalized granuloma annulare: a case series of 8 patients. JAAD Case Rep. 38, 59–60. doi:10.1016/j.jdcr.2023.06.011
Kaushik, A., Narang, T., and Handa, S. (2020a). Successful use of apremilast as a steroid-sparing agent in chronic actinic dermatitis. Dermatol. Ther. 33 (6), e13809. doi:10.1111/dth.13809
Kaushik, A., Raj, D., Chatterjee, D., and Vinay, K. (2020b). Apremilast in orofacial granulomatosis—A report of five cases. Dermatol. Ther. 33 (6), e14345. doi:10.1111/dth.14345
Kelly, G., Hughes, R., McGarry, T., van den Born, M., Adamzik, K., Fitzgerald, R., et al. (2015). Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 173 (6), 1431–1439. doi:10.1111/bjd.14075
Kerdel, F. R., Azevedo, F. A., Kerdel Don, C., Don, F. A., Fabbrocini, G., and Kerdel, F. A. (2019). Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J. Drugs Dermatol. 18 (2), 170–176.
Keren, A., Shemer, A., Ullmann, Y., Paus, R., and Gilhar, A. (2015). The PDE4 inhibitor, apremilast, suppresses experimentally induced alopecia areata in human skin in vivo. J. Dermatol. Sci. 77 (1), 74–76. doi:10.1016/j.jdermsci.2014.11.009
Khemis, A., Fontas, E., Moulin, S., Montaudie, H., and Passeron, T. (2020). Apremilast in combination with narrowband UVB in the treatment of vitiligo: a 52-week monocentric prospective randomized placebo-controlled study. J. Invest. Dermatol. 140 (8), 1533–1537. doi:10.1016/j.jid.2019.11.031
Kim, H. J., Del Duca, E., Pavel, A. B., Singer, G. K., Abittan, B. J., Chima, M. A., et al. (2023). Apremilast and narrowband ultraviolet B combination therapy suppresses Th17 axis and promotes melanogenesis in vitiligo skin: a randomized, split-body, pilot study in skin types IV–VI. Arch. Dermatol. Res. 315 (2), 215–221. doi:10.1007/s00403-022-02343-1
Kim-Lim, P., and Thomas, C. (2023). Crushed apremilast for the treatment of oral Lichen planus. JAAD Case Rep. 37, 114–115. doi:10.1016/j.jdcr.2023.05.013
Kleidona, I. A., Agiasofitou, E., Tsiogka, A., Chorti, M., Gregoriou, S., Stratigos, A., et al. (2024). Darier disease responding to apremilast: report of two cases. Indian J. Dermatol. Venereol. Leprol. 90 (2), 240–243. doi:10.25259/IJDVL_83_2023
Kolla, A. M., Liu, L., Shaw, K., Shapiro, J., Femia, A., and Lo Sicco, K. (2021). A narrative review of therapies for scalp dermatomyositis. Dermatol. Ther. 34 (6), e15138. doi:10.1111/dth.15138
Konishi, R., Tanaka, R., Inoue, S., Ichimura, Y., Nomura, T., and Okiyama, N. (2022). Evaluation of apremilast, an oral phosphodiesterase 4 inhibitor, for refractory cutaneous dermatomyositis: a phase 1b clinical trial. J. Dermatol. 49 (1), 118–123. doi:10.1111/1346-8138.16179
Krase, I. Z., Cavanaugh, K., and Curiel-Lewandrowski, C. (2016). Treatment of refractory Pityriasis rubra pilaris with novel phosphodiesterase 4 (PDE4) inhibitor apremilast. JAMA Dermatol. 152 (3), 348–350. doi:10.1001/jamadermatol.2015.3405
Langan, S. M., Irvine, A. D., and Weidinger, S. (2020). Atopic dermatitis. Lancet 396 (10247), 345–360. doi:10.1016/S0140-6736(20)31286-1
Lanna, C., Mazzilli, S., Zangrilli, A., Bianchi, L., and Campione, E. (2019). One drug and two diseases: a case of multidrug-resistant hidradenitis suppurativa and psoriasis treated with apremilast. Dermatol. Ther. 32 (6), e13089. doi:10.1111/dth.13089
Laufer Britva, R., Keren, A., Paus, R., and Gilhar, A. (2020). Apremilast and tofacitinib exert differential effects in the humanized mouse model of alopecia areata. Br. J. Dermatol. 182 (1), 227–229. doi:10.1111/bjd.18264
Le Joncour, A., Regnier, P., Maciejewski-Duval, A., Charles, E., Barete, S., Fouret, P., et al. (2023). Reduction of neutrophil activation by phosphodiesterase 4 blockade in Behcet's disease. Arthritis Rheumatol. 75 (9), 1628–1637. doi:10.1002/art.42486
Liu, L. Y., and King, B. A. (2017). Lack of efficacy of apremilast in 9 patients with severe alopecia areata. J. Am. Acad. Dermatol. 77 (4), 773–774. doi:10.1016/j.jaad.2017.05.034
Lopalco, G., Venerito, V., Leccese, P., Emmi, G., Cantarini, L., Lascaro, N., et al. (2019). Real-world effectiveness of apremilast in multirefractory mucosal involvement of behçet's disease. Ann. Rheum. Dis. 78 (12), 1736–1737. doi:10.1136/annrheumdis-2019-215437
Lopalco, G., Morrone, M., Venerito, V., Cantarini, L., Emmi, G., Espinosa, G., et al. (2025). Exploring relief for behçet's disease refractory oral ulcers: a comparison of TNF inhibitors versus apremilast. Rheumatol. Oxf. 64 (3), 1302–1308. doi:10.1093/rheumatology/keae274
Magdaleno-Tapial, J., Valenzuela-Onate, C., Sanchez-Carazo, J. L., and Alegre-de Miquel, V. (2019). Improvement of alopecia areata with apremilast. Australas. J. Dermatol. 60 (2), 144–145. doi:10.1111/ajd.12934
Magdaleno-Tapial, J., Valenzuela-Onate, C., Alonso-Carpio, M., Garcia-Legaz, M., Alegre-de Miquel, V., Zaragoza-Ninet, M. G., et al. (2020). Improvement of recalcitrant sneddon-wilkinson disease with apremilast. Australas. J. Dermatol. 61 (2), 185–186. doi:10.1111/ajd.13253
Maione, V., Bettolini, L., Venturuzzo, A., Tonon, F., Battocchio, S., and Calzavara-Pinton, P. (2023). Efficacy of apremilast in a case of resistant linear IgA bullous disease. Int. J. Dermatol. 62 (5), e300–e302. doi:10.1111/ijd.16548
Majid, I., Imran, S., and Batool, S. (2019). Apremilast is effective in controlling the progression of adult vitiligo: a case series. Dermatol. Ther. 32 (4), e12923. doi:10.1111/dth.12923
Manfrè, V., Parisi, S., Caligiuri, I., Repetto, O., Zabotti, A., Pegolo, E., et al. (2023). Secretagogue effect of PDE4 inhibitor apremilast on human salivary gland organoids obtained from primary Sjögren’s syndrome patients. Clin. Exp. Rheumatol. 41 (12), 2493–2501. doi:10.55563/clinexprheumatol/7f4fzu
McMichael, J., and Stoff, B. K. (2021). Treatment of a perforating dermatosis with apremilast. JAAD Case Rep. 16, 155–157. doi:10.1016/j.jdcr.2021.08.031
Mikhaylov, D., Pavel, A., Yao, C., Kimmel, G., Nia, J., Hashim, P., et al. (2018). A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch. Dermatol. Res. 311 (1), 29–36. doi:10.1007/s00403-018-1876-y
Moneta, G. M., Pires Marafon, D., Marasco, E., Rosina, S., Verardo, M., Fiorillo, C., et al. (2019). Muscle expression of type I and type II interferons is increased in juvenile dermatomyositis and related to clinical and histologic features. Arthritis Rheumatol. 71 (6), 1011–1021. doi:10.1002/art.40800
Moran, B., Sweeney, C. M., Hughes, R., Malara, A., Kirthi, S., Tobin, A. M., et al. (2017). Hidradenitis suppurativa is characterized by dysregulation of the Th17:Treg cell axis, which is corrected by anti-TNF therapy. J. Investig. Dermatol. 137 (11), 2389–2395. doi:10.1016/j.jid.2017.05.033
Navarro-Trivino, F. J., Cuenca-Barrales, C., Vega-Castillo, J. J., and Ruiz-Villaverde, R. (2019). Chronic hand eczema and hepatogenic pruritus with good response to apremilast. Dermatol. Ther. 32 (3), e12879. doi:10.1111/dth.12879
Ocampo, D. V., and Gladman, D. (2019). Psoriatic arthritis. F1000Res 8, F1000 Faculty Rev-1665. doi:10.12688/f1000research.19144.1
Ozdede, A., and Hatemi, G. (2021). An evaluation of apremilast for the treatment of adult patients with oral ulcers associated with Behçet's syndrome. Expert Opin. Pharmacother. 22 (12), 1533–1537. doi:10.1080/14656566.2021.1939307
Papp, K., Reich, K., Leonardi, C. L., Kircik, L., Chimenti, S., Langley, R. G. B., et al. (2015). Apremilast, an oral phosphodiesterase 4 (pde4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase iii, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [esteem] 1). J. Am. Acad. Dermatol. 73 (1), 37–49. doi:10.1016/j.jaad.2015.03.049
Parisi, R., Symmons, D. P., Griffiths, C. E., Ashcroft, D. M., Identification, Management of, P., and Associated ComorbidiTy project, t. (2013). Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Investig. Dermatol. 133 (2), 377–385. doi:10.1038/jid.2012.339
Paul, J., Foss, C. E., Hirano, S. A., Cunningham, T. D., and Pariser, D. M. (2013). An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J. Am. Acad. Dermatol. 68 (2), 255–261. doi:10.1016/j.jaad.2012.07.014
Paul, C., Cather, J., Gooderham, M., Poulin, Y., Mrowietz, U., Ferrandiz, C., et al. (2015). Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase iii, randomized controlled trial (esteem 2). Br. J. Dermatol. 173 (6), 1387–1399. doi:10.1111/bjd.14164
Perschy, L., Anzengruber, F., Rappersberger, K., Itzlinger-Monshi, B., Aichelburg, M. C., Graf, V., et al. (2022). Apremilast in oral Lichen planus - a multicentric, retrospective study. J. Dtsch. Dermatol. Ges. 20 (3), 343–346. doi:10.1111/ddg.14696
Piccinni, M. P., Lombardelli, L., Logiodice, F., Tesi, D., Kullolli, O., Biagiotti, R., et al. (2014). Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral Lichen planus. Oral Dis. 20 (2), 212–218. doi:10.1111/odi.12094
Podevin, P., Cottencin, A. C., Becquart, C., Azib, S., Duparc, A., Darras, S., et al. (2023). Real-life use of apremilast for the treatment of psoriasis in patients with oncological comorbidities: a retrospective descriptive multicentre study in France. Eur. J. Dermatol. 33 (1), 34–40. doi:10.1684/ejd.2023.4407
Proietti, I., Michelini, S., Mambrin, A., Di Fraia, M., Tolino, E., Balduzzi, V., et al. (2020). A case of hidradenitis suppurativa successfully treated with apremilast in a patient with psoriasis and SAMPUS. Dermatol. Ther. 33 (3), e13448. doi:10.1111/dth.13448
Reich, K., Gooderham, M., Green, L., Bewley, A., Zhang, Z., Khanskaya, I., et al. (2017). The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-Week results from a phase iiib, randomized, placebo-controlled trial (Liberate). J. Eur. Acad. Dermatol. Venereol. 31 (3), 507–517. doi:10.1111/jdv.14015
Richmond, J. M., Bangari, D. S., Essien, K. I., Currimbhoy, S. D., Groom, J. R., Pandya, A. G., et al. (2017). Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and May serve as biomarkers of disease. J. Investig. Dermatol. 137 (2), 350–358. doi:10.1016/j.jid.2016.09.016
Rudnicka, L., and Olszewska, M. (2024). A ranking of treatment efficacy in alopecia areata is not possible without head-to-head studies. J. Eur. Acad. Dermatol. Venereol. 38 (5), 786–787. doi:10.1111/jdv.19964
Sakakibara, M., Shimoyama, H., Nomura, M., Nakashima, C., Hirabayashi, M., Sei, Y., et al. (2019). Efficacy of the phosphodiesterase-4 inhibitor, apremilast, in a patient with severe alopecia areata. Eur. J. Dermatol. 29 (4), 436–437. doi:10.1684/ejd.2019.3576
Samrao, A., Berry, T. M., Goreshi, R., and Simpson, E. L. (2012). A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch. Dermatol. 148 (8), 890–897. doi:10.1001/archdermatol.2012.812
Sandhu, V. K., Eder, L., and Yeung, J. (2020). Apremilast and its role in psoriatic arthritis. G. Ital. Dermatol. Venereol. 155 (4), 386–399. doi:10.23736/S0392-0488.20.06640-7
Saporito, R. C., and Cohen, D. J. (2016). Apremilast use for moderate-to-severe atopic dermatitis in pediatric patients. Case Rep. Dermatol. 8 (2), 179–184. doi:10.1159/000446836
Schafer, P. H., Parton, A., Gandhi, A. K., Capone, L., Adams, M., Wu, L., et al. (2010). Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 159 (4), 842–855. doi:10.1111/j.1476-5381.2009.00559.x
Schafer, P. H., Parton, A., Capone, L., Cedzik, D., Brady, H., Evans, J. F., et al. (2014). Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell. Signal 26 (9), 2016–2029. doi:10.1016/j.cellsig.2014.05.014
Schafer, P. H., Adams, M., Horan, G., Truzzi, F., Marconi, A., and Pincelli, C. (2019). Apremilast normalizes gene expression of inflammatory mediators in human keratinocytes and reduces antigen-induced atopic dermatitis in mice. Drugs R. D. 19 (4), 329–338. doi:10.1007/s40268-019-00284-1
Sharma, S., Bhardwaj, A., Dwivedi, P., Yadav, S. S., Shamim, M. A., Singh, S., et al. (2023). Apremilast add-on benefits over conventional drugs (ABCD) in unstable non-segmental vitiligo: a 12-week single-center randomized controlled trial. Cureus 15 (4), e37180. doi:10.7759/cureus.37180
Sharma, A., Gupta, V., Bhatia, S., Upadhyay, A., Challa, A., and Gupta, S. (2025). Apremilast versus betamethasone oral mini-pulse in the treatment of progressive non-segmental vitiligo: a randomised pilot trial. Indian J. Dermatol. Venereol. Leprol. 91 (3), 398–401. doi:10.25259/IJDVL_799_2024
Sigmund, A. M., Winkler, M., Engelmayer, S., Kugelmann, D., Egu, D. T., Steinert, L. S., et al. (2023). Apremilast prevents blistering in human epidermis and stabilizes keratinocyte adhesion in pemphigus. Nat. Commun. 14 (1), 116. doi:10.1038/s41467-022-35741-0
Sil, S., Shome, S., Saha, N., and Ghosh, S. (2023). Apremilast in oral lichen planus: report of two cases and review of literature. Indian J. Dermatol. 68 (6), 728. doi:10.4103/ijd.ijd_499_23
Silverberg, J. I., Barbarot, S., Gadkari, A., Simpson, E. L., Weidinger, S., Mina-Osorio, P., et al. (2021). Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 126 (4), 417–428.e2. doi:10.1016/j.anai.2020.12.020
Simpson, E. L., Imafuku, S., Poulin, Y., Ungar, B., Zhou, L., Malik, K., et al. (2019). A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J. Investig. Dermatol. 139 (5), 1063–1072. doi:10.1016/j.jid.2018.10.043
Skullerud, K. H., Gjersvik, P., Pripp, A. H., Qvigstad, E., and Helgesen, A. L. O. (2021). Apremilast for genital erosive Lichen planus in women (the AP-GELP study): study protocol for a randomised placebo-controlled clinical trial. Trials 22 (1), 469. doi:10.1186/s13063-021-05428-w
Sobral-Costas, T. G., Escudero-Tornero, R., Maseda, R., Mozo Del Castillo, Y., and Feito-Rodriguez, M. (2025). Apremilast in chronic graft-versus-host disease: a paediatric case report. Clin. Exp. Dermatol. 50 (5), 1029–1031. doi:10.1093/ced/llae494
Spencer, R. K., Elhage, K. G., Jin, J. Q., Davis, M. S., Hakimi, M., Bhutani, T., et al. (2023). Apremilast in palmoplantar psoriasis and palmoplantar pustulosis: a systematic review and meta-analysis. Dermatol. Ther. Heidelb. 13 (2), 437–451. doi:10.1007/s13555-022-00877-w
Suárez-Fariñas, M., Ungar, B., Noda, S., Shroff, A., Mansouri, Y., Fuentes-Duculan, J., et al. (2015). Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J. Allergy Clin. Immunol. 136 (5), 1277–1287. doi:10.1016/j.jaci.2015.06.032
Takeno, M., Dobashi, H., Tanaka, Y., Kono, H., Sugii, S., Kishimoto, M., et al. (2022). Apremilast in a Japanese subgroup with Behçet's syndrome: results from a phase 3, randomised, double-blind, placebo-controlled study. Mod. Rheumatol. 32 (2), 413–421. doi:10.1093/mr/roab008
Taneja, N., and Gupta, S. (2020). Apremilast is efficacious in refractory alopecia areata. J. Dermatol. Treat. 31 (7), 727–729. doi:10.1080/09546634.2019.1616046
Tekin, B., Xie, F., and Lehman, J. S. (2024). Lichen planus: what is new in diagnosis and treatment? Am. J. Clin. Dermatol. 25 (5), 735–764. doi:10.1007/s40257-024-00878-9
Thompson, C., Piguet, V., and Choy, E. (2018). The pathogenesis of dermatomyositis. Br. J. Dermatol. 179 (6), 1256–1262. doi:10.1111/bjd.15607
Todberg, T., Skov, L., Simonsen, S., Kainu, K., and Zachariae, C. (2020). Efficacy of apremilast in patients with prurigo nodularis: a proof-of-concept study. Acta Derm. Venereol. 100 (8), adv00118. doi:10.2340/00015555-3461
Torres, T., and Puig, L. (2017). Apremilast: a novel oral treatment for psoriasis and psoriatic arthritis. Am. J. Clin. Dermatol. 19 (1), 23–32. doi:10.1007/s40257-017-0302-0
Tsentemeidou, A., Sotiriou, E., Sideris, N., Bakirtzi, K., Papadimitriou, I., Lallas, A., et al. (2022). Apremilast in psoriasis patients with serious comorbidities: a case series and systematic review of literature. Dermatol. Pract. Concept 12 (4), e2022179. doi:10.5826/dpc.1204a179
Vieira, M., Buffier, S., Vautier, M., Le Joncour, A., Jamilloux, Y., Gerfaud-Valentin, M., et al. (2020). Apremilast in refractory behçet's syndrome: a multicenter observational study. Front. Immunol. 11, 626792. doi:10.3389/fimmu.2020.626792
Viswanath, V., Joshi, P., Dhakne, M., Dhoot, D., Mahadkar, N., and Barkate, H. (2022). Evaluation of the efficacy and safety of apremilast in the management of Lichen planus. Clin. Cosmet. Investig. Dermatol. 15, 2593–2600. doi:10.2147/CCID.S390591
Vossen, A., van der Zee, H. H., Davelaar, N., Mus, A. M. C., van Doorn, M. B. A., and Prens, E. P. (2019a). Apremilast for moderate hidradenitis suppurativa: no significant change in lesional skin inflammatory biomarkers. J. Eur. Acad. Dermatol. Venereol. 33 (4), 761–765. doi:10.1111/jdv.15354
Vossen, A., van Doorn, M. B. A., van der Zee, H. H., and Prens, E. P. (2019b). Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J. Am. Acad. Dermatol. 80 (1), 80–88. doi:10.1016/j.jaad.2018.06.046
Wakiya, R., Ushio, Y., Ueeda, K., Kameda, T., Shimada, H., Nakashima, S., et al. (2022). Efficacy and safety of apremilast and its impact on serum cytokine levels in patients with Behçet's disease. Dermatol. Ther. 35 (8), e15616. doi:10.1111/dth.15616
Wang, H., Zhang, D., Han, Q., Zhao, X., Zeng, X., Xu, Y., et al. (2016). Role of distinct CD4+ T helper subset in pathogenesis of oral Lichen planus. J. Oral Pathol. Med. 45 (6), 385–393. doi:10.1111/jop.12405
Weber, P., Seyed Jafari, S. M., Yawalkar, N., and Hunger, R. E. (2017). Apremilast in the treatment of moderate to severe hidradenitis suppurativa: a case series of 9 patients. J. Am. Acad. Dermatol. 76 (6), 1189–1191. doi:10.1016/j.jaad.2017.02.026
Weber, B., Radakovic, S., and Tanew, A. (2020). Apremilast for extensive and treatment-resistant alopecia areata: a retrospective analysis of five patients. Eur. J. Dermatol. 30, 165–168. doi:10.1684/ejd.2020.3749
Keywords: apremilast, phosphodiesterase 4 inhibitor, immune-mediated inflammatory skin disease, cytokines, small-molecule drug
Citation: Liang X, Zheng J, Huang X, Tan M and Liao J (2025) Apremilast treatment of immune-mediated inflammatory skin diseases: a narrative review. Front. Pharmacol. 16:1633426. doi: 10.3389/fphar.2025.1633426
Received: 22 May 2025; Accepted: 24 July 2025;
Published: 21 August 2025.
Edited by:
Sarmistha Saha, GLA University, IndiaReviewed by:
Gang Li, Chinese Academy of Agricultural Sciences, ChinaManish Kumar Jeengar, Amrita Vishwa Vidyapeetham University, India
Copyright © 2025 Liang, Zheng, Huang, Tan and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Liao, bGlhb2ppYTEwMTRAMTYzLmNvbQ==
 Xiaoqian Liang
Xiaoqian Liang Jiecheng Zheng
Jiecheng Zheng