Abstract
The antifungal activity of the essential oil of Clinopodium chilense (Benth.) Govaerts was investigated against several strains of Candida spp. including clinical isolates and reference strains. Antifungal efficacy was evaluated by determining minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC). The chemical composition of the essential oil, characterized by gas chromatography-mass spectrometry (GC/MS), revealed pulegone (18.5%), thymol (11.0%), and isomenthone (10.0%) as the primary constituents. Clinopodium chilense essential oil (EO) demonstrated broad-spectrum anticandidal activity against all tested Candida spp., with MIC values ranging from 16 to 64 μg/mL and MFC values from 16 to 128 μg/mL. The EO exhibited potent fungicidal activity (MFC/MIC ratio ≤2) against several strains, notably Candida tropicalis (MIC and MFC of 16 μg/mL), and also showed efficacy against C. guilliermondii and C. lusitaniae. Among the major components, thymol generally displayed the lowest MIC values (32–64 μg/mL). Molecular docking studies further suggested thymol as a key contributor to the oil’s antifungal effect, showing strong binding affinities to C. albicans virulence proteins Als9-2 and the enzyme CYP51. Significantly, the essential oil outperformed amphotericin B against all tested clinical isolates. Overall, C. chilense EO exhibits significant fungistatic and fungicidal activity against pathogenic Candida species without affecting normal cell viability. These findings, supported by chemical characterization and in silico analysis of its major components like thymol, highlight its potential as a source of novel natural antifungal agents.
1 Introduction
A global health problem of growing relevance is candidiasis, an opportunistic fungal infection caused by Candida species, with C. albicans being the most common etiological agent (Macias-Paz et al., 2023). This infection can manifest itself in various forms, from superficial mucocutaneous infections to life-threatening invasive systemic infections, particularly in immunocompromised individuals (Lionakis et al., 2023). Conventional treatment of candidiasis is based on the use of synthetic antifungals, such as azoles and echinocandins; however, the increasing prevalence of strains resistant to these drugs (Fisher et al., 2022) has prompted the search for more effective and safer alternative therapies. In this context, essential oils (EOs) have emerged as a promising alternative due to their antimicrobial and antifungal properties and their lower probability of inducing resistance (Sobhy et al., 2025). EOs are complex mixtures of volatile compounds produced by various plants, and their antimicrobial activity has been attributed to the synergy between their components (Ben Miri, 2025). Specifically, EOs derived from plants of the Lamiaceae family have demonstrated remarkable antifungal activity against Candida spp. (Potente et al., 2020).
The Lamiaceae family, also known as the mint family, is one of the largest and most economically important plant families with a global distribution (Panda et al., 2022). In Chile, this family is represented by diverse genera and species, including the genus Clinopodium (Morales, 2018). Although some species of the genus Clinopodium, such as Clinopodium gilliesii, have been studied and the presence of bioactive compounds, including monoterpenes and sesquiterpenes, has been reported in their EO composition (Carvajal et al., 2017), the potential of the EO from the Chilean endemic species Clinopodium chilense remains unexplored.
Clinopodium chilense (Benth.) Govaerts is an evergreen aromatic shrub reaching up to 1.5 m in height. Commonly known as “oreganillo” or “menta de árbol” (tree mint), it is distinguished by its mint-like aroma (Baeza, 1930) and its drought tolerance, behaving as a partially deciduous shrub (Montenegro et al., 1979). Its geographic distribution in Chile spans from the Coquimbo region to the Araucanía region (Muñoz et al., 1981). Over time, this species has been classified under various synonyms, including Gardoquia gilliesii Graham, G. chilensis Benth., Satureja chilensis (Benth.) Briq., and Satureja gilliesii (Graham) Briq (Morales, 2018). The ethnobotanical value of C. chilense lies in its traditional uses. Its leaves and flowers, both dried and ground, are used as a condiment similar to oregano. Furthermore, a mild and pleasant-tasting infusion, similar to tea, is made from the dried leaves and flowers (Cordero et al., 2017; Cordero et al., 2022). In folk medicine, the infusion of “menta de árbol” has been traditionally used to relieve stomach aches, treat indigestion, and as a stimulant (Muñoz et al., 1981). Additionally, its use as an antiseptic in washing pig intestines intended for sausage making has been documented (Cordero et al., 2022). C. chilense is also employed in the restoration of degraded soils and as an ornamental plant in floral gardens (Riedemann et al., 2014). From a chemical perspective, only two studies have been reported on the dichloromethane extract of this species. One study, focusing on the leaves, identified the monoterpenes acetylsaturejol and isoacetylsaturejol, in addition to the sesquiterpenes T-cadinol and (−)-Cadin-4-en-l-ol (Labbe et al., 1993). The other study isolated seven diterpenoids from the flowering aerial parts: one labdane, three isopimaranes, and three rearranged isopimaranes (Labbé et al., 1994). In both studies, the toxicity of the compounds was evaluated using the Artemia salina assay (Labbe et al., 1993; Labbé et al., 1994). However, no prior studies have investigated the composition of the essential oil of C. chilense, nor its potential biological activity, including its antifungal capacity against Candida species. This lack of information represents a significant gap in knowledge, especially considering the potential of essential oils as sources of novel antifungal agents.
Therefore, this research aims to provide the first comprehensive characterization of the essential oil of C. chilense, analyzing its chemical composition and evaluating the antifungal activity of the oil and its major components against five strains of Candida spp. The results of this study could not only reveal the antifungal potential of this endemic Chilean species but also contribute to the discovery of novel bioactive compounds with therapeutic applications in the treatment of candidiasis.
2 Materials and methods
2.1 Chemistry
All reagents, pulegone, thymol, isomenthone, amphotericin B, itraconazole and fluconazole were purchased from Sigma-Aldrich Co. (St. Louis, MO, United States), GIBCO BRL Life Technologies (Grand Island, NY, United States), and Santa Cruz Biotechnology (Santa Cruz, CA, United States).
2.2 Plant material
C. chilense was collected from Viña del Mar, Valparaiso Region, Central Chile (S: 33°02′38″, W: 71°30′04″) during the spring in October 2020. Mr. Patricio Novoa confirmed species authenticity, and a voucher specimen (Cch-1020) was deposited at the Natural Products and Organic Synthesis Laboratory of Universidad de Playa Ancha, Valparaíso, Chile.
2.3 Essential oil and analysis
The EO was extracted from the aerial parts (leaves and branches) of C. chilense (500 g) by hydrodistillation carried out using a Clevenger-type apparatus for 4 h. The EO was collected and dried over anhydrous sodium sulfate and stored in sealed brown vials at 4°C. Subsequently, C. chilense EO was diluted with dichloromethane, and 1 μL of the sample was analyzed using a GC-MS/MS (Hewlett-Packard GC/MS 6890 coupled to a Hewlett-Packard 5973 mass-selective detector) operating in EI mode at 70 eV, equipped with a splitless injector (250°C). The transfer line temperature was 200°C. Helium was used as a carrier gas at a flow rate of 1.2 mL/min, and the capillary column used was a HP-5 ms (60 m × 0.25 mm i.d., film thickness 0.25 μm). The temperature program was 40°C (5 min) to 300°C (5 min) at a rate of 5°C/min. Volatile compounds were identified by comparing their mass spectra, obtained using the Thermo Xcalibur 3.1.66.10 program, with the NIST 2020 library database, and by comparison of their retention index with those reported in the literature. The retention indices were determined under the same operating conditions in relation to a homologous n-alkanes series (C8–C36).
2.4 Antifungal activity
2.4.1 Fungal strains
The antifungal activity of the EO and its main components was evaluated against reference strains and clinical isolates of Candida. The reference strains C. albicans (ATCC 7978) and C. parapsilosis (ATCC 22019) were used, as well as the clinical isolates C. albicans 10,935, C. dubliniensis 3240, Candida glabrata 10,912, C. guilliermondii 12,204, C. lusitaniae 2305, and Candida tropicalis 9841. The clinical isolates were obtained from patients of the Base Hospital of Valdivia, Los Ríos Region, Chile. After identification, the microorganisms were included in the pathogenic fungal collection (Bioassay Laboratory of University of Valparaíso). They are maintained in Sabouraud Dextrose Broth (SDB) with glycerol at −80°C according to established protocols (Madrid et al., 2012).
2.4.2 Fungal growth (MIC and MFC)
The antifungal susceptibility testing was performed in accordance with the guidelines in (CLSI, 2008) reference protocols M27-A3 for yeasts, were used to determine the minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of the EO and their major compounds (pulegone, thymol, isomenthone), as described previously (Madrid et al., 2025). Briefly, cultures of all yeast were placed on Sabouraud Dextrose Agar (SDA) and incubated for 24–72 h at temperature 37°C. Colonies of this culture were suspended in sterile 0.85% NaCl and the inoculum was standardized according to the scale of 0.5 McFarland (1–5 × 106 CFU/mL). The antifungal assay was conducted in 96-well plates. Yeast strains were prepared in sterile water and diluted in RPMI 1640 medium (excluding the sterility control). stock solutions of essential oil and pure compounds were diluted two-fold with RPMI from 256 to 0.03 μg/mL (final volume = 100 µL), with a final DMSO concentration of 1% w/v. A volume of 100 µL of inoculum suspension was added to each well with the exception of the sterility control where sterile water was added to the well instead. Reference antifungal drugs, amphotericin B, itraconazole, and fluconazole, were used as positive controls, whereas DMSO served as the negative control.
The MIC determination was conducted with approximately 0.5-2.5 × 103 CFU/mL of the microorganism in each well. Following a 24–48 h incubation at 37°C, the absorbance of the plates was measured at 540 nm using a VERSA Max microplate reader (Molecular Devices, Sunnyvale, CA, United States) for spectrophotometric analysis. The MIC endpoint was calculated as the lowest concentration giving rise to an inhibition of growth equal to or greater than 80% of that of the growth control (MIC), similar to the visual endpoint criterion recommended by the CLSI. After determining the MIC, the minimum fungicidal concentration (MFC) was determined as a sub-culture of 2 μL of each of the wells that showed no growth and were incubated for 72 h at 37°C. The lowest concentration with no visible growth was defined as MFC indicating 99.5% death of the original inoculum. All experiments were performed in triplicate and repeated three times for reproducibility.
2.5 Docking studies
2.5.1 Construction of ligands
To carry out the molecular docking studies, a computer equipped with an Intel® Core™ i7 processor and running the Windows 10 Pro 64-bit operating system was used. The three-dimensional models of the ligands were built using UCSF Chimera 1.18 software. Polar hydrogens were added to each ligand, Gasteiger charges were assigned, and energy minimization was performed using the General AMBER Force Field (GAFF).
2.5.2 Molecular docking
The three-dimensional crystallographic structures of the N-terminal domain of Als9-2 from C. albicans in complex with the gamma peptide of human fibrinogen (PDB ID: 2Y7L; Resolution: 1.49 Å) (Manzano et al., 2024) and Sterol 14-alpha demethylase (CYP51) from C. albicans (PDB ID: 5TZ1; Resolution: 2.0 Å) (Jabba and Jordt, 2019) were retrieved from the RCSB Protein Data Bank (PDB) (https://www.rcsb.org/) and used as docking targets. AutoDock Tools (version 1.5.6) was employed to prepare these protein structures, which involved the removal of water molecules, metal atoms, co-crystallized ligands, and other non-covalently bound substances. Kollman charges and both polar and non-polar hydrogens were added, and the target files were saved in the corresponding pdbqt format. The grid coordinates were set at −1.943 Å (X), −11.223 Å (Y), and 29.743 Å (Z) for PDB 2Y7L, and 67.837 Å (X), 36.744 Å (Y), and 39.072 Å (Z) for PDB 5TZ1. The grid box dimensions were 20 points (X), 20 points (Y), and 20 points (Z) for both targets. The search parameters included 50 runs with a maximum of 25,000,000 evaluations per ligand. The RMSD threshold for multiple clusters was set to <1.6 Å. The results were ranked based on binding energy and potential conformations. The lowest binding energy and the most probable conformation were selected for further analysis. Finally, Discovery Studio Visualizer (version 21.1.0.20298) was used to generate two-dimensional and three-dimensional images of the most stable selected conformation.
2.5.3 Prediction of physicochemical and toxicological parameters
For the obtaining of pharmacokinetic and toxicological parameters, the chemical structures of the analyzed compounds in SMILES format were used on the SwissAdme platform (http://www.swissadme.ch/) and ADMETlab 2.0 (https://ai-druglab.smu.edu/).
2.5.4 Molecular dynamic simulations
For the molecular dynamics simulations, the best poses from the docking experiments between the proteins Als9-2 (PDBid: 2Y7L) (Salgado et al., 2011) and CYP51 PDBid: 5TZ1) (Hargrove et al., 2017) from Candida albicans and the compounds pulegone, thymol and isomenthone were used as a starting point. These best poses were chosen taking into account the most negative binding energies and the lowest RMSD (Bell and Zhang, 2019; Velázquez-Libera et al., 2020) parameter values. Proteins were prepared by adding hydrogen atoms at physiological pH (pH = 7.4) using the UCSF Chimera software version 1.14 (Goddard et al., 2018; Pettersen et al., 2021; Meng et al., 2023). The force field used was CHARMM36 (Vanommeslaeghe and MacKerell, 2012; Gutiérrez et al., 2016; Huang et al., 2017) for proteins, and parameters for organic molecules were obtained from the SwissParam web server (Zoete et al., 2011). The proteins-ligand complexes were placed into a rectangular water box of 15 × 15 × 15 Å3 centered on the mass center of each ligand, using the TIP3P water model (Boonstra et al., 2016). All the complexes were submitted to 5000 steps for energy minimization using the conjugated gradient methodology at a temperature of 298.15 K using the weak coupling algorithm (Berendsen et al., 1984). The Van der Waals cutoff was fixed to 12 Å, under the NPT ensemble (Number of particles, Pressure and Temperature constant). The complexes studied were submitted a 1.0 fs time step under the velocity Verlet algorithm; 2.0 ns of equilibration and 100 ns of molecular dynamics simulation using the NAMD 2.13 software package (Bhandarkar et al., 2003; Phillips et al., 2005; Acun et al., 2018; Phillips et al., 2020).
2.6 Cytotoxicity
2.6.1 Cell lines and culture conditions
The cytotoxicity of C. chilense EO was evaluated in two non-tumorigenic human cell lines: CCD 841 CoN (colon epithelial cells) and HEK-293T (human embryonic kidney). All tested cell lines were obtained from the AmericanType Culture Collection (Rockville, MD, United States). The different cell lines were maintained as monolayers in a culture medium (HAM-F10 + DMEM, 1:1) supplemented with 10% fetal bovine serum, as well as antibiotics (0.01 mg/mL streptomycin and 0.005 mg/mL penicillin). The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere.
2.6.2 Cell viability assay
To determine the effect on cell viability of C. chilense EO, the sulforhodamine B (SRB)assay was used to measure the number of viable cells after each treatment (Silva et al., 2022). Cells were seeded in 96-well plates at 3 × 103 cells/well in 100 μL of culture medium and treated in triplicate with increasing concentrations of essential oil (0.625–100 μg/mL) for 72 h at 37°C and 5% CO2. The cells which received only the medium containing 0.1% DMSO served as the control group. After each incubation, the cells were fixed with trichloroacetic acid, then washed by immersion in distilled water and stained with 50 μL/well of SRB, as well as 0.1% (w/v) in 1% (v/v) acetic acid at room temperature for 30 min. The dye is solubilized with 150 μL/well of 10 mM Tris Base, and the absorbance is then measured at a wavelength of 540 nm in a microplate reader. 5-fluorouracil (5-FU) was used as positive control. Values shown are the mean +SD of three independent experiments in triplicate. Finally, Sigma Plot software (Systat Software, San Jose, CA, United States) was used to calculate the IC50 value.
2.7 Statistical analysis
The data were reported as the mean values ±standard deviation (SD). Due to non-parametric data, a Kruskal–Wallis ANOVA was used with a confidence level of 95% with the STATISTICA 7.0 program.
3 Results and discussion
3.1 EO composition
The EO of C. chilense was obtained with a yield of 1.9% (v/w). The EO of C. chilense fresh leaves is composed mainly by oxygenated monoterpenes (44.6%), followed by oxygenated sesquiterpenes (20.3%), monoterpene esters (11.7%), phenols (11.0%) and hydrocarbon sesquiterpenes (8.6%) (Table 1).
TABLE 1
| No | RT (min) | Components | % Areaa | RIa | RLb | Identification |
|---|---|---|---|---|---|---|
| 1 | 14.56 | cis-sabinol | 1.5 | 1103 | 1105 | RL, MS |
| 2 | 14.95 | Menthone | 2.1 | 1131 | 1131 | RL, MS, Co |
| 3 | 15.03 | Neoisopulegol | 2.2 | 1133 | 1134 | RL, MS |
| 4 | 15.28 | Isomenthone | 10.0 | 1139 | 1139 | RL, MS, Co |
| 5 | 15.35 | Borneol | 0.5 | 1149 | 1149 | RL, MS, Co |
| 6 | 15.66 | 4-terpineol | 3.9 | 1159 | 1160 | RL, MS, Co |
| 7 | 16.82 | α-terpineol | 3.4 | 1197 | 1198 | RL, MS, Co |
| 8 | 17.46 | Pulegone | 18.5 | 1211 | 1211 | RL, MS, Co |
| 9 | 18.38 | isomenthyl acetate | 2.3 | 1250 | 1251 | RL, MS |
| 10 | 18.63 | Isopulegol acetate | 3.2 | 1260 | 1260 | RL, MS, Co |
| 11 | 18.72 | bornyl acetate | 5.3 | 1276 | 1277 | RL, MS, Co |
| 12 | 18.95 | Thymol | 11.0 | 1278 | 1278 | RL, MS, Co |
| 13 | 20.51 | Ascaridole | 0.5 | 1279 | 1280 | RL, MS |
| 14 | 21.15 | α-copaene | 0.6 | 1334 | 1334 | RL, MS |
| 15 | 21.41 | beta-bourbonene | 3.1 | 1338 | 1339 | RL, MS |
| 16 | 22.83 | (R)-(+)-citronellic acid | 2.0 | 1341 | 1341 | RL, MS |
| 17 | 23.02 | Menthofurolactone | 0.9 | 1354 | 1353 | RL, MS |
| 18 | 24.68 | γ-amorphene | 2.5 | 1479 | 1480 | RL, MS |
| 19 | 24.96 | cis-calamenene | 2.4 | 1521 | 1521 | RL, MS |
| 20 | 26.26 | (−)-spathulenol | 1.5 | 1574 | 1576 | RL, MS |
| 21 | 26.39 | caryophyllene oxide | 1.5 | 1580 | 1581 | RL, MS, Co |
| 22 | 27.12 | Epicubenol | 7.9 | 1611 | 1613 | RL, MS |
| 23 | 27.70 | t-cadinol | 8.4 | 1631 | 1630 | RL, MS |
| 24 | 28.01 | 4(15),5,10(14)-germacratrien-1-ol | 1.0 | 1680 | 1680 | RL, MS |
| Total identified | 96.2 | |||||
| Oxygenated monoterpenes | 44.6 | |||||
| Oxygenated sesquiterpenes | 20.3 | |||||
| Monoterpene esters | 11.7 | |||||
| Phenols | 11.0 | |||||
| Hydrocarbon sesquiterpenes | 8.6 |
Essential oil composition of C. chilense.
Experimental retention index for non-polar column.
bibliographic retention index for non-polar column, MS: mass spectra.
Twenty-four compounds were identified in the EO of C. chilense, which corresponded to 96.2% of the total oil analyzed, and the main components were pulegone (18.5%), thymol (11.0%), and isomenthone (10.0%) (Figure 1).
FIGURE 1

Major compounds present in C. chilense EO.
This is the first study on C. chilense EO, a plant often confused with Clinopodium gilliesii due to shared scientific nomenclature and similar uses, especially in culinary applications. However, a crucial difference lies in the concentration of pulegone, the major compound in both. While C. gilliesii exhibits a pulegone content of 93.8% (Carvajal et al., 2017), C. chilense contains only 18.5%, suggesting a lower risk associated its consumption of the latter. This difference is critical because C. chilense is often confused with C. gilliesii due to similar nomenclature and uses, especially in culinary applications. The lower pulegone concentration, coupled with the presence of thymol and isomenthone (monoterpenes also found in edible plants like thyme and peppermint), suggests a more favorable safety profile for C. chilense (de Sousa Barros et al., 2015; Salehi et al., 2018). In contrast, the essential oil of Clinopodium nubigenum from Ecuador, traditionally used to treat colds and stomach aches, exhibited a high pulegone content (72.8%), with 3,7-dimethyl-1,6-octadien-3-ol (7.0%) and cis-isopulegone (4.7%) as secondary components, and demonstrated antimicrobial activity against C. albicans (Gilardoni et al., 2011). On the other hand, analysis of four wild Clinopodium species, traditionally used in folk medicine from countries of the former Yugoslavia, revealed a predominance of oxygenated monoterpenes: C. thymifolium (35.2% pulegone), C. serpyllifolium (13.0% pulegone, 38.7% piperitenone oxide), C. dalmaticum (27.3% pulegone, 41.7% piperitenone), and C. pulegium (22.9% pulegone, 34.1% piperitenone oxide) (Dunkić et al., 2017). It is important to note that not all Clinopodium species contain pulegone as the primary component. The essential oil of Clinopodium gracile collected in China lacks pulegone in its composition, instead exhibiting germacrene D (20.6%), nootkatone (8.2%), and morillol (7.7%) as major compounds, yet it still demonstrated larvicidal activity against Aedes albopictus (Chen et al., 2013). These variations emphasize the necessity of carefully characterizing each species and its geographical origin to determine their potential uses and safety.
3.2 Antifungal activity
The antifungal activity (MIC and MFC) of EO and their main compounds is presented in Table 2.
TABLE 2
| Sample | A | B | C | D | E | F | G | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| EO | 32 | 64 | 64 | 128 | 16 | 16 | 32 | 32 | 64 | 128 | 64 | 64 | 64 | 128 |
| 1 | 128 | 128 | 128 | 256 | 128 | 256 | 64 | 64 | 64 | 128 | 64 | 128 | 128 | 128 |
| 2 | 64 | 64 | 32 | 64 | 64 | 128 | 32 | 64 | 32 | 32 | 32 | 64 | 64 | 64 |
| 3 | 128 | 128 | 128 | 128 | 64 | 128 | 64 | 64 | 128 | 128 | 128 | 128 | 128 | 128 |
| C1 | 1.0 | 2.0 | 0.5 | 1.0 | 32 | 64 | 64 | 128 | 128 | 256 | 64 | 128 | 128 | 256 |
| C2 | 0.5 | 1.0 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | 4.0 | 2.0 | 4.0 | 0.5 | 1.0 | 32 | 64 |
| C3 | 0.5 | 1.0 | 1.0 | 1.0 | 0.5 | 1.0 | 4.0 | 8.0 | 4.0 | 8.0 | 2.0 | 4.0 | 8 | 16 |
| DMSO | I | I | I | I | I | I | I | |||||||
Minimum inhibitory concentration (MIC) and Minimum Fungicidal Concentration (MFC) of C. chilense EO and and their main compounds against Candida spp. MIC and MFC values are expressed in μg/mL.
A, C. albicans (ATCC, 7978); B, C. parasilopsis (ATCC, 22019); C, C. tropicalis (9841); D, C. lusitaniae (2305); E, C. albicans (10,935); F, C. guilliermondii (12,204); G, C. glabrata (10,912); 1, pulegone; 2, thymol; 3, isomenthone; C1, amphotericin b; C2, fluconazole; C3, itraconazole. Each value represents the mean of three experiments (p < 0.05), performed in quadruplicate. I: inactive.
Table 2 summarizes the activity observed at 48 h for C. chilense EO and its major compounds, specifically those found in abundances exceeding 10%. In terms of results, C. lusitaniae proved to be the most sensitive strain to the different treatments, with Candida tropicalis following closely behind. In contrast, Candida glabrata proved to be the least sensitive species to the various treatments. This observation is consistent with previous research, including a study by Deravi et al. (2021) which also identified C. glabrata as a less susceptible species in clinical isolates of vulvovaginal candidiasis.
Analyzing the relationship between the MFC (Minimum Fungicide Concentration) and MIC (Minimum Inhibitory Concentration) values is essential for a thorough understanding of the mode of action of each treatment. The ratio MFC/MIC was calculated in order to determine if the essential oil or its major compounds had a fungistatic (MFC/MIC ≥4) or fungicidal (MFC/MIC ≤4) activity (Boubrik et al., 2025). In this regard, the C. chilense EO demonstrated broad antifungal activity against all tested Candida spp., with MIC and MFC values ranging from 16 to 128 μg/mL. Notably, the oil exhibited fungicidal activity (MFC/MIC = 1) against C. guilliermondii, C. lusitaniae, and C. tropicalis, with particularly potent activity observed against C. tropicalis at the lowest tested concentration of 16 μg/mL. All compounds exhibited antifungal activity, but the C. chilense EO demonstrated a remarkable ability to inhibit the growth of all strains at significantly lower concentrations compared to most individual compounds. Studies indicate that pulegone, one of the components of the EO, can inhibit the growth of various Candida strains, including C. albicans. Some research also demonstrates that pulegone can affect biofilm formation, a mechanism that helps Candida spp. resist antifungal drugs (Alam and Ahmad Khan, 2021). These results suggest an antifungal potential of pulegone against the analyzed yeast species. Notably, it exhibited a favorable MFC/MIC ratio of 1 against C. lusitaniae at a concentration of 64 μg/mL. However, for the remaining species, the MFC/MIC ratio was 2, requiring higher concentrations to achieve fungicidal effects. Concerning thymol, while it exhibited lower MIC values than pulegone, it demonstrated a superior fungicidal potential with an MFC/MIC ratio of 1 against four strains (C. albicans (ATCC 7978), C. lusitaniae, C. albicans (10,935), and C. glabrata). Notably, this group included C. albicans ATCC and a clinical isolate, with its potency being greater against the clinical isolate at concentrations two times lower than those required for the ATCC strain. For the remaining strains tested, the MFC/MIC ratio was 2. This phenol is well-known for its ability to disrupt the cell membrane and combat biofilm formation (Wang et al., 2017). However, at high concentrations or with prolonged exposure, thymol can be irritating to the skin and mucous membranes (Gavliakova et al., 2013). On the other hand, isomenthone generally inhibited all tested strains at a concentration of 128 μg/mL, exhibiting a fungicidal MFC/MIC ratio of 1, indicating its fungicidal potency at that concentration. The exception was C. lusitaniae, which, similarly to pulegone, was inhibited at a lower concentration of 64 μg/mL. This terpenketone along with other compounds such as menthone, has been shown to inhibit the growth of various fungi. It has been specifically reported that this type of monoterpene exhibits activity against Aspergillus niger (Moghtader, 2013). These findings are consistent with the known mechanism of action of terpenoids, the main components of the essential oil, which destabilize cell membranes, increase their permeability, and consequently disrupt and destroy microorganisms (Gonçalves et al., 2007). Further highlighting the potential of this EO, while amphotericin B proved more effective against ATCC strains, the EO outperformed it against all clinical isolates. While the EO exhibited higher MIC values than the evaluated azoles, it demonstrated superior fungicidal potential, with an MFC/MIC ratio of 1, compared to the value of 2 observed for both azoles across all strains. This MFC/MIC value aligns with that reported by Costa et al. (2017) for C. albicans and C. parapsilopsis. Although fluconazole is prototypically considered a fungistatic agent, our study demonstrated fungicidal activity against diverse Candida spp., with an observed MFC/MIC ratio of 2. This finding, far from being anomalous, is consistent with the growing body of evidence that azole activity is conditional. Our results align with those of Maheronnaghsh et al. (2016), who also reported low MFC/MIC ratios in isolates from cancer patients. Mechanistically, it has been shown that fluconazole, at the high concentrations used to determine the MFC, induces a programmed cell death (apoptosis) cascade in C. albicans (Lee and Lee, 2018), the most prevalent species. Furthermore, the fungicidal activity of this azole is not only dose-dependent but also influenced by the microenvironment. For example, Moosa et al. (2004) demonstrated in vitro that fluconazole becomes fungicidal under the acidic conditions that simulate the vaginal environment. This conditional activity has clear clinical relevance, as it has been validated in vivo by Sobel et al. (2003), who documented the potent efficacy of fluconazole in treating C. albicans vaginitis (Akins, 2005). Therefore, in the face of the conditional efficacy of conventional drugs, these results suggest a promising future for C. chilense EO as a potential alternative against pathogenic yeasts, thus opening new phytopharmacological possibilities.
3.3 The molecular docking study
A molecular docking study assessed the major compounds of C. chilense EO against two key receptors involved in Candida spp. pathogenicity; the results are presented in Table 3.
TABLE 3
| Compound | Enzyme (PDB code) | Binding energy (kcal/mol) | Key interactions |
|---|---|---|---|
| Pulegone (1) | 2Y7L | −6.38 | Val22; Pro29; Arg171; Lys59; Ile172 |
| 5TZ1 | −7.02 | Hse327; Phe233; Pro230; Phe380; Met508; Leu121; Leu373 | |
| Thymol (2) | 2Y7L | −6.98 | Val22; Arg171; Pro29; Tyr297; Glu27 |
| 5TZ1 | −7.86 | Ser378; Phe380; Leu376; Pro230; Phe233 | |
| Isomenthone (3) | 2Y7L | −6.57 | Val23; Pro30; Tyr298; Ile173; Lys60 |
| 5TZ1 | −6.62 | Ser334; His333; Leu332; Ser334; Pro186; Met464; Leu77 | |
| Amphotericin B | 2Y7L | −8.95 | Trp295; Trp296; ser181; Tyr271; Arg172; Lys60; Phe59 |
| 5TZ1 | −10.38 | His333; Tyr74; Phe419; Ile427; Gly428; Arg337 | |
| Fluconazole | 2Y7L | −7.97 | Ser170; Trp295; Glu27; Gly296; Ile172; Leu179; Pro29 Arg171; Val22; Trp294 |
| 5TZ1 | −6.90 | Leu121; Pro230; Met508; Tyr64; Pro375; Leu376; Arg377 | |
| Itraconazole | 2Y7L | −9.96 | Tyr21,Val22; Arg171; Ile167; Val161; Ser169; Trp223 Thr168; Tyr23; Asn224; Ser159 |
| 5TZ1 | −10.51 | Ala61; Phe58; Leu87; Leu121; Leu376; Ile304; Pro230 Ile471; Met508; Pro462; Gly472 |
Binding energies and interactions of the main compounds present in Clinopodium chilense oil with the N-terminal domain of Candida albicans adhesin Als9-2 (PDB ID: 2Y7L) and sterol 14-alpha demethylase (CYP51) of Candida albicans.
The interactions detailed in Table 3 were assessed against key receptors critical for Candida spp. virulence. One such receptor, Als9-2, is an adhesin of C. albicans that facilitates fungal attachment to host cells by interacting with fibrinogen. This interaction contributes to biofilm formation and virulence in mucosal, systemic, and device-related infections. Sterol 14-alpha demethylase (CYP51) is a key enzyme in ergosterol biosynthesis, essential for fungal cell membrane integrity. Inhibition of CYP51 disrupts the membrane, enhancing the effectiveness of antifungal treatments. Both represent important therapeutic targets against C. albicans (Lim et al., 2025; Mesileya et al., 2025).
DMSO was used as the solvent control in the molecular docking experiments. As expected, no relevant binding affinities or specific interactions were observed. Although recent evidence indicates that DMSO may influence certain physical or kinetic properties—such as medium viscosity or protein stability—under specific conditions, it does not directly interact with the active site nor act as a competitive ligand (Tunçer and Gurbanov, 2023; Feoli et al., 2024).
The lowest binding energies were obtained for the compounds used as positive controls—amphotericin B, fluconazole, and itraconazole—with values of −8.95, −7.97, and −9.96 kcal/mol, respectively, for protein 2Y7L, and −10.38, −6.90, and −10.51 kcal/mol, respectively, for protein 5TZ1. These results indicate that the major compounds of C. chilense EO have binding affinity for two key proteins associated with the pathogenicity of C. albicans: the Als9-2 adhesin (PDB ID: 2Y7L) and the sterol 14α-demethylase enzyme (CYP51, PDB ID: 5TZ1). As expected, the reference compounds exhibited the best binding energies at both receptors, validating the docking methodology used.
Among the natural compounds evaluated, thymol (compound 2) showed the strongest binding affinities toward both proteins, outperforming pulegone (1) and isomenthone (3). This greater affinity suggests that thymol may significantly contribute to the antifungal activity of the essential oil. Several studies have demonstrated that thymol possesses potent antifungal activity against Candida albicans, including fluconazole-resistant strains. For instance, thymol has been shown to inhibit biofilm formation and reduce fungal cell viability. Moreover, when combined with conventional antifungals such as fluconazole, thymol exhibits synergistic effects that enhance treatment efficacy (Jafri and Ahmad, 2020).
Studies on Cryptococcus neoformans, another human fungal pathogen, have investigated the mode of action of thymol. Results indicate that thymol disrupts ergosterol biosynthesis—an essential component of the fungal cell membrane—by modulating the expression of genes involved in this pathway, such as ERG1, ERG11, and HMG1. Additionally, thymol induces intracellular calcium homeostasis imbalance and interferes with protein glycosylation, processes that are critical for fungal cell viability (Jung et al., 2021).
Pulegone and isomenthone also showed interactions with both molecular targets, but with higher (i.e., less favorable) binding energies, suggesting lower affinity and, likely, a reduced contribution to the overall antifungal activity. A study by Miri et al. (2022) reported that the essential oil of Mentha pulegium, containing 74.81% pulegone, exhibited significant antimicrobial activity against several microorganisms, including C. albicans. These findings suggest that pulegone may be responsible for the observed antifungal activity, although its precise mechanisms of action remain unknown.
The compounds tested exhibited binding energies against 2Y7L ranging from −6.38 kcal/mol (pulegone, compound 1) to −6.98 kcal/mol (thymol, compound 2). For the 5TZ1 protein, binding energies ranged from −6.62 kcal/mol (isomenthone, compound 3) to −7.86 kcal/mol (thymol, compound 2). In both cases, thymol demonstrated the strongest binding affinities. Among the three compounds evaluated against different Candida strains, compound 2 (thymol) showed the highest antifungal activity. Molecular docking studies suggest that this compound binds efficiently to both target proteins (Figure 2).
FIGURE 2
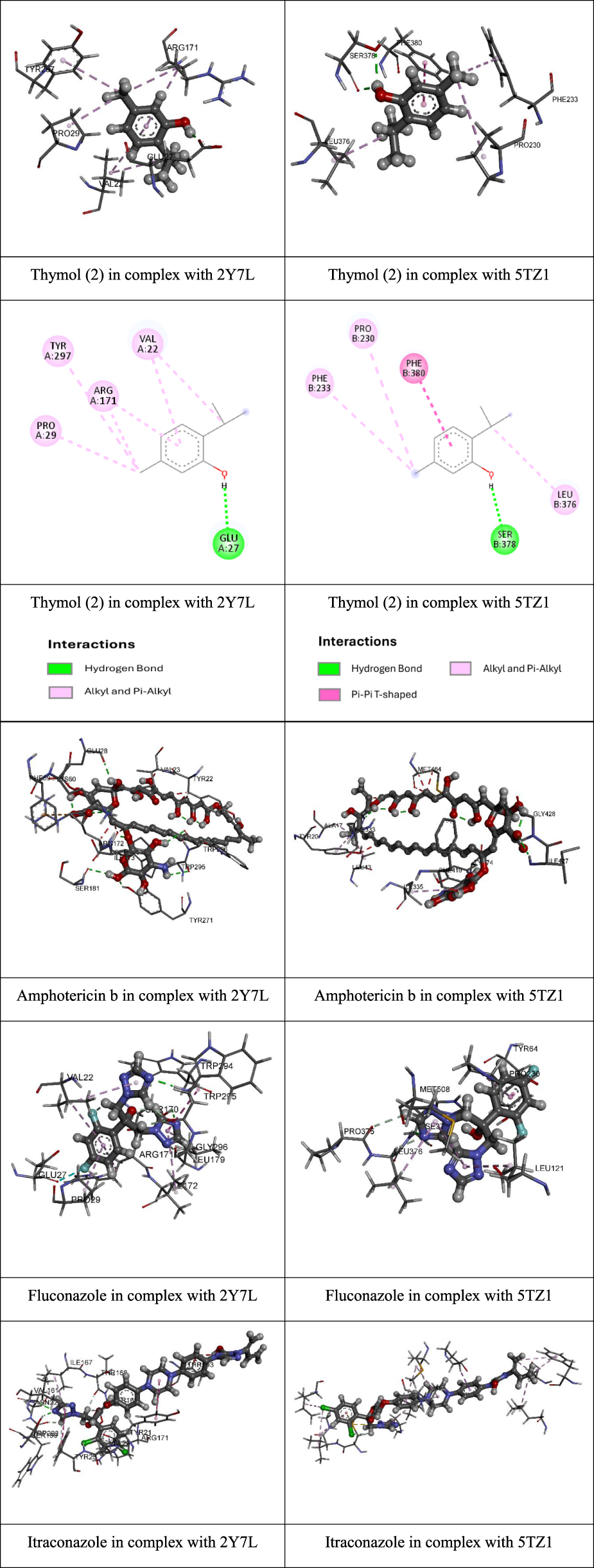
2D and 3D images representing the most stable complexes between thymol and the target proteins.
In the case of the Als9-2 adhesin (PDB ID: 2Y7L), thymol formed a hydrogen bond with residue Glu27 at a distance of 1.87 Å, along with multiple alkyl interactions involving residues Val22, Arg171, Pro29, and Tyr297. These interactions suggest a stable binding to the N-terminal domain of the adhesin, potentially interfering with C. albicans adhesion mechanisms to host cells.
Regarding the sterol 14α-demethylase (CYP51, PDB ID: 5TZ1), thymol also exhibited a favorable interaction pattern, forming two conventional hydrogen bonds with residue Ser378 (at 2.00 and 2.26 Å), a T-shaped π–π interaction with Phe380 (at 5.08 Å), and additional alkyl interactions with residues Leu376, Pro230, and Phe233. These findings indicate that thymol may interfere with ergosterol biosynthesis, a key function targeted by azole antifungal agents.
The analysis of the physicochemical and toxicological parameters of the major compounds found in the essential oil of Clinopodium chilense is presented in Tables 4, 5. These data support the evaluation of their viability as candidates with pharmacological potential.
TABLE 4
| Compound | Log Po/w | Water solubility | GI | BBB permeant | P-gp substrate | Log Kp [cm/s] |
|---|---|---|---|---|---|---|
| Pulegone (1) | 2.71 | Poorly soluble | High | Yes | No | −5.04 |
| Thymol (2) | 2.82 | Poorly soluble | High | Yes | No | −4.87 |
| Isomenthone (3) | 2.65 | Poorly soluble | High | Yes | No | −5.08 |
Pharmacokinetics parameters.
Log Po/w: Logarithm of the octanol/water partition coefficient (P); GI, absorption: Gastrointestinal absorption; BBB, permeant: Permeability of the blood-brain barrier; P-gp substrate: Transport by P-glycoprotein; Log Kp: Logarithm of the cutaneous permeability coefficient.
TABLE 5
| Compound | hERG Blockers % | Ames % | DILI % | LD50 -log (mol/kg) | Lipinski ruler |
|---|---|---|---|---|---|
| Pulegone (1) | 33.39 | 42.56 | 44.66 | 1.84 | Yes; 0 violation |
| Thymol (2) | 33.56 | 41.24 | 42.9 | 1.99 | Yes; 0 violation |
| Isomenthone (3) | 34.35 | 52.88 | 46.06 | 2.01 | Yes; 0 violation |
Prediction of toxicological parameters.
hERG, Blockers %: The human ether-a-go-go related gene; Ames %: Ames test for mutagenicity. DILI %: Drug-induced liver injury. LD50: Lethal Dose 50.
The three compounds analyzed exhibited similar Log P values, indicating comparable lipophilicity profiles. This property favors permeability across biological membranes without compromising aqueous solubility (Chloe, 2024). Moreover, a high predicted gastrointestinal absorption and the ability to cross the blood–brain barrier was observed, suggesting good oral bioavailability and the potential to exert effects on the central nervous system, depending on the therapeutic application.
None of the compounds showed affinity for P-glycoproteins, thus reducing the risk of active efflux and enhancing their potential for intracellular accumulation. The skin permeability coefficient (Kp) was similar for all three compounds, with values ranging from −4.87 to −5.09 cm/s, suggesting limited dermal absorption—an important consideration for the development of topical formulations.
Regarding toxicological parameters, all compounds showed approximately 30% inhibition of the hERG channel, indicating a moderate but not alarming cardiotoxicity risk. Ames (mutagenicity) and DILI (drug-induced liver injury) tests yielded values below 50%, except for isomenthone, which showed an Ames result of 52.88%. This finding may warrant further evaluation in future studies. Additionally, the lethal dose 50 (LD50) values indicated relatively low acute toxicity, ranging from 1.84 mol/kg for pulegone to 2.01 mol/kg for isomenthone.
Although pulegone has been identified as a potential carcinogen in some toxicological studies involving prolonged high-dose exposure in rodents (Jabba and Jordt, 2019; Voigt, et al., 2024), these findings do not necessarily preclude its use in therapeutic contexts. The carcinogenic effects observed are highly dose-dependent and were associated with sustained oral intake, not short-term or localized administration. In our case, pulegone is considered for antifungal use, which could involve topical or controlled-release formulations that minimize systemic absorption and toxicological risks. Furthermore, many clinically approved drugs carry similar concerns but remain in use when the therapeutic benefits outweigh potential adverse effects. As such, the potential of pulegone as an antifungal agent remains valid, though further preclinical toxicological assessment will be necessary to ensure its safe medical application.
Finally, it is worth noting that all three compounds meet the criteria set by Lipinski’s Rule of Five, supporting their potential as bioactive molecules with pharmacokinetic properties suitable for oral administration. To validate their effectiveness at the molecular level, molecular dynamics simulations were performed, providing information on the dynamic behavior of the complexes, as well as details at the molecular level during the simulation period. The first parameter to analyze is the RMSD, which provides a criterion for stability throughout the simulation period. As can be seen in Figure 3, all the complexes studied stabilized after 2 ns of simulation time. It is important to note that this parameter remained low throughout the 100 ns of the trajectory, with RMSD values below 2 Å, indicating high stability of the ligands in the active sites of both proteins. This suggests that the compounds could be good inhibitors of Als9-2 and CYP51 proteins.
FIGURE 3

Graphical representation of the RMSD parameter during the simulation time.
From the graph shown in Figure 3, we can see that the lowest RMSD values were found in the complexes formed by the Als9-2 protein with pulegone and thymol, with values of 1.2663 ± 0.1628 Å and 1.2656 ± 0.1843 Å, respectively. These values indicate no significant differences between these complexes and the others, so it is necessary to analyze other variables obtained from the trajectory.
Hydrogen bonding interactions between the ligand and the protein confer stability to the complex over time. In our case, and as seen in Figure 4, this parameter’s behavior was weak during the 100 ns of simulation time.
FIGURE 4

Number of hydrogen between the complexes studied during 100 ns of simulation time.
The largest number of hydrogen bonding interactions (H-Bond) were found in the complexes formed by Isomenthone-CYP51 and thymol-Asl9-2. It is important to note that the standard deviation of this parameter was much higher than the mean for all simulated systems, indicating that this type of interaction was not stable over time. Based on the results obtained for this parameter, we can conclude that hydrogen bonding interactions do not explain the stability of the complexes studied.
To corroborate the behavior of the hydrogen bond interactions obtained during the simulation, we will analyze the interaction occupancy term. This term calculates the percentage of hydrogen bond interactions that remained below 3 Å during the simulation. This parameter is shown in Table 6.
TABLE 6
| Complex | H-bond interaction | Occupancy (%) |
|---|---|---|
| Isomenthone-CIP51 | TYR88-Side | 12.53% |
| THR78-Side | 7.19% | |
| Pulegone-CIP51 | TYR64-Side | 0.07% |
| GLN66-Side | 0.02% | |
| Thymol-CIP51 | ARG469-Main | 2.87% |
| THR311-Side | 2.29% | |
| Isomenthone-Als9-2 | ILE172-Main | 1.37% |
| LYS59-Side | 1.18% | |
| Pulegone-Als9-2 | ILE172-Main | 0.44% |
| ASN299-Side | 0.20% | |
| Thymol-Als9-2 | GLU27-Side | 9.02% |
| GLU277-Side | 8.28% |
H-bond occupancies analysis from 100 ns of simulation time.
For a hydrogen bond interaction to be considered stable during the trajectory, the occupancy percentage should be above 50% (Tsujimura et al., 2025). As can be seen in Table 6, in none of the complexes did the occupancy percentage exceed 15%. This corroborates the previously stated finding that these interactions were not stable during the 100 ns of simulation time, which does not explain the stability found in the RMSD parameter. Therefore, it is necessary to analyze other parameters such as radius of gyration (Rg) (Figure 5).
FIGURE 5
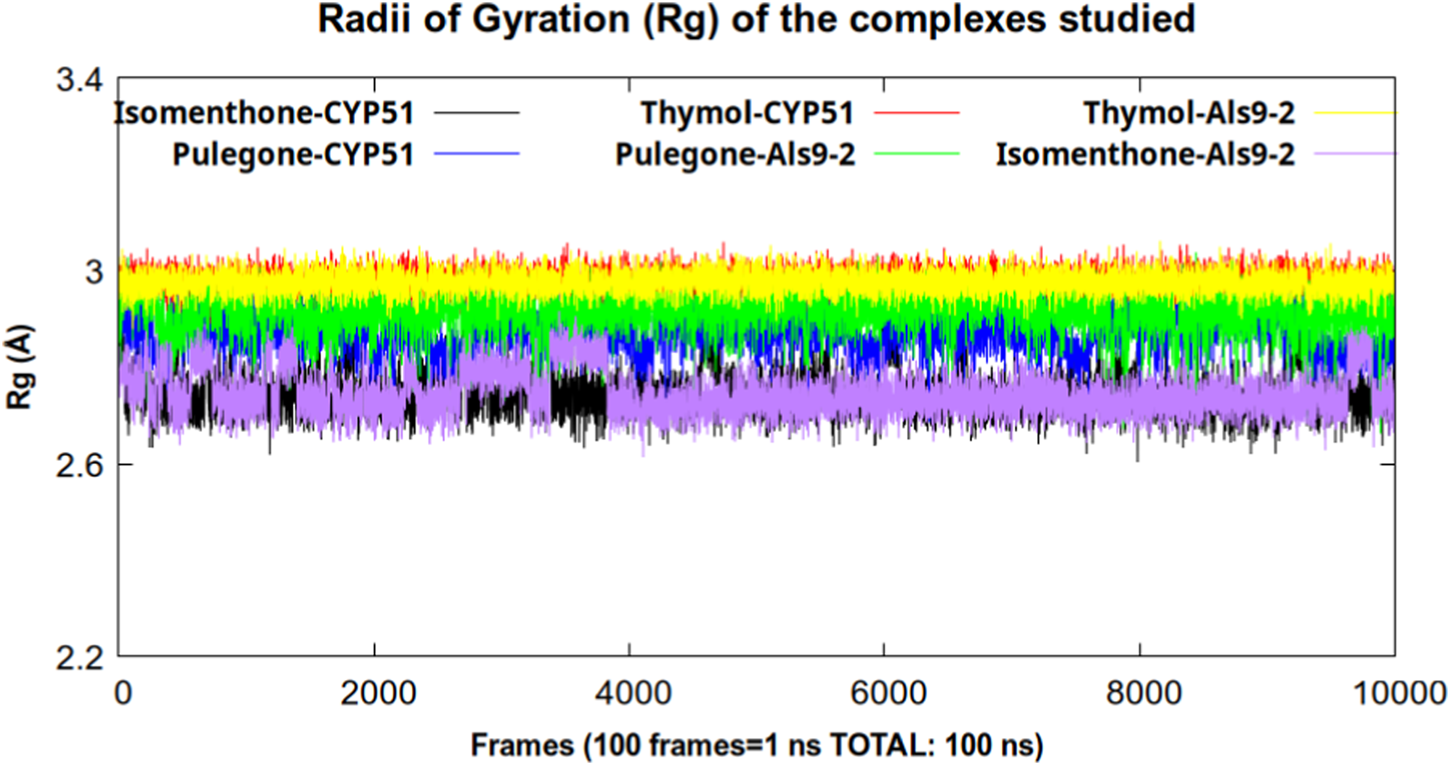
Radius of Gyration (Rg) of the Cα atoms in the complexes studied during 100 ns of simulation time.
The variable radius of gyration is defined as the root mean square distance of the mass center of atom or collection atoms from a common mass centers and shows us the degree of compaction that a system presents during the trajectory (Lobanov et al., 2008).
As we can see in Figure 5, the lowest values for this parameter were found in the complexes formed by isomenthone and pulegone with CYP51, indicating greater compaction of these systems and, consequently, greater stability. The complex formed by isomenthone-CYP51 had the highest number of hydrogen bonds calculated and the highest occupancy, indicating that this compound could be a good inhibitor of the CYP51 enzyme.
3.4 Cytotoxicity assay
Due to the side effects and resistance that can arise from synthetic drugs used to treat Candida infections, both cutaneous and systemic, the search for alternatives in natural products, such as essential oils, has intensified for effective therapies against candidiasis or candidemia. In this context, it is crucial to know the cytotoxic effect that these products may have on healthy cells, in order to ensure their safe application in therapies for these pathologies. Consequently, in this study, the cytotoxic effect of C. chilensis EO on two non-tumorigenic human cell lines was investigated using an SRB assay (Table 7).
TABLE 7
| Sample | CCD 841 CoN IC50 (µg/mL) | HEK-293 IC50 (µg/mL) |
|---|---|---|
| C. chilense EO | 93.45 ± 0.89 | 96.78 ± 0.24 |
| 5-FU | 56.4 ± 0.31 | 18.32 ± 0.56 |
Half-maximal inhibitory concentration (IC50) values of C. chilensis EO on two non-tumorigenic human cell lines.
Data are reported as mean values ±SD of three different experiments with samples in triplicate. p < 0.05 vs control (ethanol-treated cells).
In contrast to the chemotherapeutic agent 5-fluorouracil (5-FU), the C. chilense EO exhibited low cytotoxicity against the non-tumorigenic cell line, as indicated by its IC50 value. This finding suggests a favorable safety profile for the EO, indicating a minimal impact on normal cells. This finding aligns with observations from other essential oils containing similar major components. As an example, a commercial Mentha piperita EO (commonly known as peppermint, a sterile hybrid of watermint and spearmint, widely cultivated for its menthol flavor) containing approximately 29.90% isomenthone, exhibited very low toxicity in human epidermal keratinocyte (HaCaT) cells up to a concentration of 400 μg/mL (Silva et al., 2022). Similarly, Thymus caramanicus Jalas (commonly called Iranian thyme, a thyme species endemic to Iran whose leaves have been used traditionally in Iranian medicine to treat various conditions and whose extracts have demonstrated antibacterial, neuroprotective, antinociceptive, and anticancer properties), its EO with 20.84% thymol, was non-toxic to the normal gingival HGF1-PI1 cell line but highly cytotoxic to KB (oral carcinoma) cells at low concentrations (Fekrazad et al., 2017). Furthermore, a study on Minthostachys verticillata EO (a wild plant from Argentina and Paraguay, commonly known as peperina or peppermint, primarily consumed as an infusion and to flavor mate and tereré, and used for medicinal purposes as a digestive, antispasmodic, and anti-inflammatory), which contains 60.0% pulegone, demonstrated no in vitro cytotoxicity or in vivo cytogenotoxicity at both low and high concentrations (Escobar et al., 2012). Notably, Minthostachys mollis (a wild plant commonly known as muña, peperina, poleo de Quito, tifo or tipo is a species of woody shrub, native to Peru, Bolivia, Colombia, Ecuador, Argentina and Venezuela), whose essential oil possesses the same three major components as the C. chilense EO and a similar pulegone concentration (12.4%, with 1.0% thymol and isomenthone), showed minimal cytotoxic effects on healthy HEK.230T cells at 687.60 ± 33.50 ug/mL (Benites et al., 2018). These observations support the existing literature, which indicates that thymol, pulegone, and isomenthone are effective inhibitors of cancer cells with a relative cytotoxic activity in normal cells (Da Rocha et al., 2012; Jung et al., 2012; Ezatpour et al., 2023). Moreover, numerous studies suggest that minor components can play a significant role in antibacterial activity, potentially through synergistic effects with other constituents (Miladinović et al., 2021). This synergistic effect is likely also relevant to cytotoxic activities in cells. Therefore, further investigation is warranted to evaluate the cytotoxic potential of these minor components, both individually and in various combinations, to fully elucidate their role(s) in toxicity against both tumor and non-tumor cells. Based on the results obtained and the consulted literature, C. chilense EO shows promising potential as a therapeutic agent. While the toxicity of its main components, such as pulegone, is acknowledged, the levels present in the oil would not reach the intake doses considered risky (Voigt et al., 2024), suggesting a negligible or very low health risk. Nevertheless, given that its application is envisioned in a dermatological solution and considering its apparent lack of cytotoxicity in non-tumor cells, the importance of a careful evaluation of the dosage and of further research to identify potential synergistic or antagonistic effects before its therapeutic use is emphasized.
4 Conclusion
This study demonstrated that Clinopodium chilense EO possesses broad-spectrum antifungal activity against Candida, even superior to amphotericin B in clinical isolates. Thymol, one of its main components, is the most promising due to its strong binding to key Candida virulence proteins and its favorable pharmacokinetic and toxicological properties. Their potential for oral use could be realized through formulations or encapsulations designed to minimize health risks. Therefore, C. chilense EO is presented as a source of bioactive molecules and a promising natural antifungal.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
IM: Investigation, Data curation, Methodology, Writing – review and editing. CV: Writing – original draft, Investigation, Formal Analysis. EM: Formal Analysis, Methodology, Investigation, Writing – original draft, Software. KM-U: Investigation, Methodology, Software, Formal Analysis, Writing – original draft. VS: Writing – original draft, Investigation. AM: Project administration, Funding acquisition, Formal Analysis, Methodology, Data curation, Supervision, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors thank FONDECYT Regular, Grant No. 1230311 from the Agencia Nacional de Investigación y Desarrollo (ANID) of Chile.
Acknowledgments
To Beca Doctorado Nacional N° 21240311 from Agencia Nacional de Investigación y Desarrollo (ANID) of Chile, Núcleo Milenio de Bioproductos, Genómica y Microbiología Ambiental (BioGEM) Código BioGEM NCN2023_054 and the supercomputing infrastructure of the NLHPC (ECM-02) of Universidad de Chile.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Acun B. Hardy D. J. Kale L. V. Li K. Phillips J. C. Stone J. E. (2018). Scalable molecular dynamics with NAMD on the summit system. IBM J. Res. Dev.62 (6), 1–9. 10.1147/JRD.2018.2888986
2
Akins R. A. (2005). An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol.43 (4), 285–318. 10.1080/13693780500138971
3
Alam M. Z. Ahmad Khan M. S. (2021). Phytomedicine from Middle Eastern countries: an alternative remedy to modern medicine against candida spp infection. Evid. Based Complement. Altern. Med.2021, 6694876. 10.1155/2021/6694876
4
Baeza V. M. (1930). Los nombres vulgares de las plantas silvestres de Chile y su concordancia con los nombres científicos y observaciones sobre la aplicación técnica y medicinal de algunas especies. Santiago, Chile: Imprenta «El Globo».
5
Bell E. W. Zhang Y. (2019). DockRMSD: an open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J. Cheminform.11, 40. 10.1186/s13321-019-0362-7
6
Ben Miri Y. (2025). Essential oils: chemical composition and diverse biological activities: a comprehensive review. Nat. Prod. Comm.20 (1), 1–29. 10.1177/1934578X241311790
7
Benites J. Guerrero-Castilla A. Salas F. Martinez J. L. Jara-Aguilar R. Venegas-Casanova E. A. et al (2018). Chemical composition, in vitro cytotoxic and antioxidant activities of the essential oil of Peruvian Minthostachys mollis griseb. Bol. Latinoam. Caribe Plant Med. Aromat.17 (6), 566–574.
8
Berendsen H. J. Postma J. Van Gunsteren W. F. DiNola A. Haak J. R. (1984). Molecular dynamics with coupling to an external Bath. J. Chem. Phys.81, 3684–3690. 10.1063/1.448118
9
Bhandarkar M. Brunner R. Chipot C. Dalke A. Dixit S. Grayson P. et al (2003). “NAMD user’s guide,”, 51. Urbana.61801
10
Boonstra S. Onck P. R. van der Giessen E. (2016). CHARMM TIP3P water model suppresses peptide folding by solvating the unfolded state. J. Phys. Chem. B120, 3692–3698. 10.1021/acs.jpcb.6b01316
11
Boubrik F. Boubellouta T. Benyoucef N. Bellik Y. Gali L. Akdoğan A. et al (2025). Investigating the chemical composition and antifungal effect of cinnamomum Cassia essential oil against Saccharomyces cerevisiae and acremonium sp. Sci. Rep.15 (1), 10195. 10.1038/s41598-025-94785-6
12
Carvajal F. Huanca A. González-Teuber M. Urzúa A. Echeverría J. (2017). Uses of hazardous medicinal plants: composition of the essential oil of Clinopodium gilliesii (benth.) kuntze (lamiaceae), collected in Chile. Bol. Latinoam. Caribe Plant Med. Aromat.16 (5), 486–492.
13
Chen X. B. Liu X. C. Zhou L. Liu Z. L. (2013). Essential oil composition and larvicidal activity of Clinopodium gracile (benth) matsum (labiatae) aerial parts against the Aedes albopictus mosquito. Trop. J. Pharm. Res.12 (5), 799–804. 10.4314/tjpr.v12i5.21
14
Chloe W. (2024). Lipophilicity: understanding the role of lipid affinity in drug design and absorption. J. Pharmacokinet. Exp. Ther.8, 278. 10.4172/jpet.1000278
15
CLSI (2008). Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A3. 3rd Edn.Wayne, PA: CLSI.
16
Cordero S. Abello L. Galvez F. (2017). “Plantas silvestres comestibles y medicinales de Chile y otras partes del mundo,” in Corporación Chilena de la Madera, Concepción, Chile.
17
Cordero S. Abello L. Gálvez F. (2022). Rizoma: a new comprehensive database on traditional uses of Chilean native plants. Biodivers. Data J.10, e80002. 10.3897/BDJ.10.e80002
18
Costa M. D. C. M. F. d. Silva A. G. d. Silva A. P. S. d. Lima V. L. d. M. Bezerra-Silva P. C. Rocha S. K. L. d. et al (2017). Essential oils from leaves of medicinal plants of Brazilian flora: chemical composition and activity against candida species. Medicines4 (2), 27. 10.3390/medicines4020027
19
Da Rocha M. S. Dodmane P. R. Arnold L. L. Pennington K. L. Anwar M. M. Adams B. R. et al (2012). Mode of action of pulegone on the urinary bladder of F344 rats. Toxicol. Sci.128 (1), 1–8. 10.1093/toxsci/kfs135
20
Deravi N. Fathi M. Tabatabaeifar S. N. Pooransari P. Ahmadi B. Shokoohi G. et al (2021). Azole antifungal resistance in Candida albicans and Candida glabrata isolated from vulvovaginal candidiasis patients. Arch. Clin. Infect. Dis.16 (2), e106360. 10.5812/archcid.106360
21
de Sousa Barros A. de Morais S. M. Ferreira P. A. T. Vieira Í. G. P. Craveiro A. A. dos Santos Fontenelle R. O. et al (2015). Chemical composition and functional properties of essential oils from mentha species. Ind. Crops Prod.76, 557–564. 10.1016/j.indcrop.2015.07.004
22
Dunkić V. Kremer D. Grubešić R. J. Rodríguez J. V. Ballian D. Bogunić F. et al (2017). Micromorphological and phytochemical traits of four clinopodium L. species (lamiaceae). S. Afr. J. Bot.111, 232–241. 10.1016/j.sajb.2017.03.013
23
Escobar F. M. Cariddi L. N. Sabini M. C. Reinoso E. Sutil S. B. Torres C. V. et al (2012). Lack of cytotoxic and genotoxic effects of Minthostachys verticillata essential oil: studies in vitro and in vivo. Food Chem. Toxicol.50 (9), 3062–3067. 10.1016/j.fct.2012.06.018
24
Ezatpour B. Dorosti N. Rezaee E. Ghaziani F. (2023). Comparison of the chemical composition and biological activities of essential oils from two satureja species: molecular docking studies. J. Mex. Chem. Soc.67 (1), 70–81. 10.29356/jmcs.v67i1.1816
25
Fekrazad R. Afzali M. Pasban-Aliabadi H. Esmaeili-Mahani S. Aminizadeh M. Mostafavi A. (2017). Cytotoxic effect of Thymus caramanicus jalas on human oral epidermoid carcinoma KB cells. Braz. Dent. J.28 (1), 72–77. 10.1590/0103-6440201700737
26
Feoli A. Sarno G. Castellano S. Sbardella G. (2024). DMSO‐related effects on ligand‐binding properties of lysine methyltransferases G9a and SETD8. Chembiochem25 (4), e202300809. 10.1002/cbic.202300809
27
Fisher M. C. Alastruey-Izquierdo A. Berman J. Bicanic T. Bignell E. M. Bowyer P. et al (2022). Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol.20 (9), 557–571. 10.1038/s41579-022-00720-1
28
Gavliakova S. Biringerova Z. Buday T. Brozmanova M. Calkovsky V. Poliacek I. et al (2013). Antitussive effects of nasal thymol challenges in healthy volunteers. Respir. Physiol. Neurobiol.187 (1), 104–107. 10.1016/j.resp.2013.02.011
29
Gilardoni G. Malagon O. Morocho V. Negri R. Tosi S. Guglielminetti M. et al (2011). Phytochemical researches and antimicrobial activity of Clinopodium nubigenum kunth (kuntze) raw extracts. Rev. Bras. Farmacogn.21, 850–855. 10.1590/S0102-695X2011005000139
30
Goddard T. D. Huang C. C. Meng E. C. Pettersen E. F. Couch G. S. Morris J. H. et al (2018). UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci.27, 14–25. 10.1002/pro.3235
31
Gonçalves M. J. Vicente A. M. Cavaleiro C. Salgueiro L. (2007). Composition and antifungal activity of the essential oil of Mentha cervina from Portugal. Nat. Prod. Res.21 (10), 867–871. 10.1080/14786410701482244
32
Gutiérrez I. S. Lin F. Y. Vanommeslaeghe K. Lemkul J. A. Armacost K. A. Brooks C. L. et al (2016). Parametrization of halogen bonds in the CHARMM general force field: improved treatment of ligand–protein interactions. Bioorg. Med. Chem.24, 4812–4825. 10.1016/j.bmc.2016.06.034
33
Hargrove T. Y. Friggeri L. Wawrzak Z. Qi A. Hoekstra W. J. Schotzinger R. J. et al (2017). Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem.292 (16), 6728–6743. 10.1074/jbc.M117.778308
34
Huang J. Rauscher S. Nawrocki G. Ran T. Feig M. de Groot B. L. et al (2017). CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods14, 71–73. 10.1038/nmeth.4067
35
Jabba S. V. Jordt S. E. (2019). Risk analysis for the carcinogen pulegone in mint-and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern. Med.179 (12), 1721–1723. 10.1001/jamainternmed.2019.3649
36
Jafri H. Ahmad I. (2020). Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med.30 (1), 100911. 10.1016/j.mycmed.2019.100911
37
Jung E. Byun S. Kim S. Kim M. Park D. Lee J. (2012). Isomenthone protects human dermal fibroblasts from TNF-α-induced death possibly by preventing activation of JNK and p38 MAPK. Food Chem. Toxicol.50 (10), 3514–3520. 10.1016/j.fct.2012.07.002
38
Jung K. W. Chung M. S. Bai H. W. Chung B. Y. Lee S. (2021). Investigation of antifungal mechanisms of thymol in the human fungal pathogen, Cryptococcus neoformans. Molecules26 (11), 3476. 10.3390/molecules26113476
39
Labbe C. Castillo M. Connolly J. D. (1993). Mono and sesquiterpenoids from Satureja gilliesii. Phytochemistry34 (2), 441–444. 10.1016/0031-9422(93)80026-O
40
Labbé C. Castillo M. Fainia F. Coll J. Connolly J. D. (1994). Rearranged isopimarenes and other diterpenoids from Satureja gilliesii. Phytochemistry36 (3), 735–738. 10.1016/S0031-9422(00)89807-6
41
Lee W. Lee D. G. (2018). A novel mechanism of fluconazole: fungicidal activity through dose-dependent apoptotic responses in Candida albicans. Microbiology164 (2), 194–204. 10.1099/mic.0.000589
42
Lim S. J. Muhd Noor N. D. Sabri S. Mohamad Ali M. S. Salleh A. B. Oslan S. N. (2025). Features of the rare pathogen Meyerozyma guilliermondii strain SO and comprehensive in silico analyses of its adherence-contributing virulence factor agglutinin-like sequences. J. Biomol. Struct. Dyn.43 (7), 3728–3748. 10.1080/07391102.2023.2300757
43
Lionakis M. S. Drummond R. A. Hohl T. M. (2023). Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol.23 (7), 433–452. 10.1038/s41577-022-00826-w
44
Lobanov M. Y. Bogatyreva N. Galzitskaya O. (2008). Radius of gyration as an indicator of protein structure compactness. Mol. Biol.42, 623–628. 10.1134/S0026893308040195
45
Macias-Paz I. U. Pérez-Hernández S. Tavera-Tapia A. Luna-Arias J. P. Guerra-Cárdenas J. E. Reyna-Beltrán E. (2023). Candida albicans the main opportunistic pathogenic fungus in humans. Rev. Argent. Microbiol.55 (2), 189–198. 10.1016/j.ram.2022.08.003
46
Madrid A. Espinoza L. González C. Mellado M. Villena J. Santander R. et al (2012). Antifungal study of the resinous exudate and of meroterpenoids isolated from Psoralea glandulosa (fabaceae). J. Ethnopharmacol.144 (3), 809–811. 10.1016/j.jep.2012.10.027
47
Madrid A. Venegas C. Olave V. Silva V. Ferreira C. Gutiérrez C. et al (2025). Anti-yeast activity of the essential oil of Peumus boldus mol. From the coastal drylands of central Chile against strains that cause uropharyngeal and vulvovaginal candiadiasis. Bol. Latinoam. Caribe Plant Med. Aromat.24 (2), 298–307. 10.37360/blacpma.25.24.2.22
48
Maheronnaghsh M. Tolouei S. Dehghan P. Chadeganipour M. Yazdi M. (2016). Identification of candida species in patients with oral lesion undergoing chemotherapy along with minimum inhibitory concentration to fluconazole. Adv. Biomed. Res.5 (1), 132. 10.4103/2277-9175.187394
49
Manzano J. A. H. Brogi S. Calderone V. Macabeo A. P. G. Austriaco N. (2024). Globospiramine exhibits inhibitory and fungicidal effects against Candida albicans via apoptotic mechanisms. Biomolecules14 (6), 610. 10.3390/biom14060610
50
Meng E. C. Goddard T. D. Pettersen E. F. Couch G. S. Pearson Z. J. Morris J. H. et al (2023). UCSF ChimeraX: tools for structure building and analysis. Protein Sci.32, e4792. 10.1002/pro.4792
51
Mesileya K. T. Onyeka P. C. Adaramola I. M. Igbalaye Q. O. Bodun D. S. Alao W. K. et al (2025). Tinospora cordifolia bioactive compounds as a novel sterol 14a-demethylase (CYP51) inhibitor: an in silico study. Silico Pharmacol.13 (1), 28–16. 10.1007/s40203-025-00312-w
52
Miladinović D. L. Dimitrijević M. V. Mihajilov-Krstev T. M. Marković M. S. Ćirić V. M. (2021). The significance of minor components on the antibacterial activity of essential oil via chemometrics. LWT136, 110305. 10.1016/j.lwt.2020.110305
53
Miri Y. B. Taoudiat A. Mahdid M. (2022). Antioxidant and antifungal activities in vitro of essential oils and extracts of twelve Algerian species of thymus against some mycotoxigenic aspergillus Genera. J. Biol. Res.25, 10299. 10.4081/jbr.2022.10299
54
Moghtader M. (2013). In vitro antifungal effects of the essential oil of Mentha piperita L. and its comparison with synthetic menthol on Aspergillus Niger. Afr. J. Plant Sci.7 (11), 521–527. 10.5897/AJPS2013.1027
55
Montenegro G. Hoffmann A. J. Aljaro M. E. Hoffman A. E. (1979). Satureja gilliesii, a poikilohydric shrub from the Chilean mediterranean vegetation. Can. J. Bot.57, 1206–1213. 10.1139/b79-145
56
Moosa M. Y. S. Sobel J. D. Elhalis H. Du W. Akins R. A. (2004). Fungicidal activity of fluconazole against Candida albicans in a synthetic vagina-simulative medium. Antimicrob. Agents Chemother.48 (1), 161–167. 10.1128/aac.48.1.161-167.2004
57
Morales R. (2018). Las labiadas (Lamiaceae) de Chile. An. Jard. Bot. Madr.75 (1), e067. 10.3989/ajbm.2480
58
Muñoz M. Barrera E. Meza I. (1981). El uso medicinal y alimenticio de plantas nativas y naturalizadas en Chile. Mus. Nac. Hist. Nat. 33, 91.
59
Panda S. K. Van Puyvelde L. Mukazayire M. J. Gazim Z. C. (2022). Editorial: ethnopharmacology of the lamiaceae: opportunities and challenges for developing new medicines. Front. Pharmacol.13, 961486. 10.3389/fphar.2022.961486
60
Pettersen E. F. Goddard T. D. Huang C. C. Meng E. C. Couch G. S. Croll T. I. et al (2021). UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci.30, 70–82. 10.1002/pro.3943
61
Phillips J. C. Braun R. Wang W. Gumbart J. Tajkhorshid E. Villa E. et al (2005). Scalable molecular dynamics with NAMD. J. Comput. Chem.26, 1781–1802. 10.1002/jcc.20289
62
Phillips J. C. Hardy D. J. Maia J. D. Stone J. E. Ribeiro J. V. Bernardi R. C. et al (2020). Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys.153, 044130. 10.1063/5.0014475
63
Potente G. Bonvicini F. Gentilomi G. A. Antognoni F. (2020). Anti-candida activity of essential oils from lamiaceae plants from the mediterranean area and the Middle East. Antibiotics9 (7), 395. 10.3390/antibiotics9070395
64
Riedemann P. Aldunate G. Teillier S. (2014). “Arbustos nativos de la zona centro-sur de Chile,” in Corporación Chilena de la Madera, Concepción, Chile. Editor de CampoG.
65
Salehi B. Mishra A. P. Shukla I. Sharifi‐Rad M. Contreras M. D. M. Segura‐Carretero A. et al (2018). Thymol, thyme, and other plant sources: health and potential uses. Phytother. Res.32 (9), 1688–1706. 10.1002/ptr.6109
66
Salgado P. S. Yan R. Taylor J. D. Burchell L. Jones R. Hoyer L. L. et al (2011). Structural basis for the broad specificity to host-cell ligands by the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. U.S.A.108 (38), 15775–15779. 10.1073/pnas.1103496108
67
Silva W. M. F. Bona N. P. Pedra N. S. Cunha K. F. D. Fiorentini A. M. Stefanello F. M. et al (2022). Risk assessment of in vitro cytotoxicity, antioxidant and antimicrobial activities of Mentha piperita L. essential oil. J. Toxicol. Environ. Health A.85 (6), 230–242. 10.1080/15287394.2021.1999875
68
Sobel J. D. Zervos M. Reed B. D. Hooton T. Soper D. Nyirjesy P. et al (2003). Fluconazole susceptibility of vaginal isolates obtained from women with complicated candida vaginitis: clinical implications. Antimicrob. Agents Chemother.47, 34–38. 10.1128/AAC.47.1.34-38.2003
69
Sobhy M. Abdelkarim E. A. Hussein M. A. Aziz T. Al-Asmari F. Alabbosh K. F. et al (2025). Essential oils as antibacterials against multidrug-resistant foodborne pathogens: mechanisms, recent advances, and legal considerations. Food Biosci.64, 105937. 10.1016/j.fbio.2025.105937
70
Tsujimura M. Ishikita H. Saito K. (2025). Determinants of hydrogen bond distances in proteins. Phys. Chem. Chem. Phys.27, 9794–9805. 10.1039/D5CP00511F
71
Tunçer S. Gurbanov R. (2023). Non-growth inhibitory doses of dimethyl sulfoxide alter gene expression and epigenetic pattern of bacteria. Appl. Microbiol. Biotechnol.107 (1), 299–312. 10.1007/s00253-022-12296-0
72
Vanommeslaeghe K. MacKerell A. D. (2012). Automation of the CHARMM general force field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. Model.52, 3144–3154. 10.1021/ci300363c
73
Velázquez-Libera J. L. Durán-Verdugo F. Valdés-Jiménez A. Núñez-Vivanco G. Caballero J. (2020). LigRMSD: a web server for automatic structure matching and RMSD calculations among identical and similar compounds in protein-ligand docking. Bioinform36, 2912–2914. 10.1093/bioinformatics/btaa018
74
Voigt V. Franke H. Lachenmeier D. W. (2024). Risk assessment of pulegone in foods based on benchmark dose–response modeling. Foods13 (18), 2906. 10.3390/foods13182906
75
Wang L. Zhao X. Zhu C. Xia X. Qin W. Li M. et al (2017). Thymol kills bacteria, reduces biofilm formation, and protects mice against a fatal infection of actinobacillus Pleuropneumoniae strain L20. Vet. Microbiol.203, 202–210. 10.1016/j.vetmic.2017.02.021
76
Zoete V. Cuendet M. A. Grosdidier A. Michielin O. (2011). SwissParam: a fast force field generation tool for small organic molecules. J. Comput. Chem.32, 2359–2368. 10.1002/jcc.21816
Summary
Keywords
Clinopodium chilense, essential oil, pulegone, thymol, Candida spp
Citation
Montenegro I, Villarroel C, Muñoz E, Mena-Ulecia K, Silva V and Madrid A (2025) Anticandidal activity of Clinopodium chilense essential oil. Front. Pharmacol. 16:1634250. doi: 10.3389/fphar.2025.1634250
Received
23 May 2025
Accepted
27 June 2025
Published
10 July 2025
Volume
16 - 2025
Edited by
Karim Hosni, Institut National de Recherche et d’Analyse Physico-Chimique (INRAP), Tunisia
Reviewed by
Oscar Herrera-Calderon, Universidad Nacional Mayor de San Marcos, Peru
Ganeshkumar Arumugam, São Paulo State University, Brazil
Ana Laura Esquivel Campos, Universidad Autónoma Metropolitana, Mexico
Joe Anthony Manzano, University of Santo Tomas, Philippines
Updates
Copyright
© 2025 Montenegro, Villarroel, Muñoz, Mena-Ulecia, Silva and Madrid.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alejandro Madrid, alejandro.madrid@upla.cl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.