Abstract
Objective:
To systematically evaluate the efficacy and safety of specific external traditional Chinese medicine therapies (SETCM therapies) versus conventional non-SETCM therapy interventions for improving swallowing function, nutritional status, and reducing complications in adult patients with post-stroke dysphagia (PSD), based on randomized controlled trials (RCTs).
Methods:
A comprehensive search was conducted from inception to present across Chinese [(China National Knowledge Infrastructure (CNKI), VIP Chinese Science and Technology Periodical Database (VIP), Wanfang Data Knowledge Service Platform (WFSD), Chinese Biology Medicine Database (CBM)] and international databases (PubMed, Embase, Web of Science, and Cochrane Library). RCTs investigating validated SETCM therapy modalities (e.g., herbal patches, iontophoresis, compresses, and fumigation) for PSD were included. All herbal components were taxonomically validated using the Kew Medicinal Plant Names Service (MPNS). The control group received conventional therapy (rehabilitation, nursing, and medication). The experimental group received SETCM therapies alone or combined with conventional care. The risk of bias in eligible studies was assessed using the Cochrane risk-of-bias tool. A meta-analysis was performed using RevMan 5.4. Stratified subgroup analyses were conducted based on stroke lesion location (supratentorial vs. brainstem) and intervention type.
Results:
Twenty-five RCTs (n = 2,159 patients; SETCM therapies group = 1,152, control group = 1,007) were included. The meta-analysis demonstrated significantly greater benefits in the SETCM therapies group for: overall response rate (OR = 3.28, 95% CI [2.49, 4.31]); overall cure rate (OR = 2.36, 95% CI [1.84, 3.02]); water swallowing test (WST) score (MD = −0.65, 95% CI [−1.23, −0.06]); and SWAL-quality-of-life (SWAL-QOL) score (MD = 25.61, 95% CI [20.54, 30.67]). The SETCM therapies group also demonstrated superior results in videofluoroscopic swallow study (VFSS) scores, modified Barthel index (MBI), serum albumin (ALB), prealbumin (PA), gugging swallowing screen (GUSS), activities of daily living (ADL), and functional dysphagia scale (FDS) scores, nasogastric tube removal success rate, as well as lower Nutritional Risk Screening 2002 (NRS 2002) scores, reduced aspiration incidence, and shorter nasogastric tube indwelling time. Safety analysis (three studies) reported mild skin irritation (erythema and pruritus) in 2.1% of cases.
Conclusion:
SETCM therapies significantly improved swallowing function, nutritional status, and clinical outcomes in patients with PSD. Efficacy exhibited neuroanatomical specificity: Acupoints are preferred for cerebral hemisphere lesions, while herbal iontophoresis is the best choice for brainstem involvement. These findings support the integration of targeted SETCM therapies into PSD rehabilitation. However, the evidence is limited by methodological biases, necessitating high-quality RCTs for confirmation.
Systematic Review Registration:
https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024599344.
1 Introduction
Stroke, a prevalent neurological disorder resulting from the narrowing, obstruction, or rupture of cerebral blood vessels, is characterized by high morbidity and severe sequelae, significantly impairing patients’ quality of life (Dou, 2009). Among its complications, post-stroke dysphagia (PSD) ranks as one of the most frequent (Martino et al., 2005), with an incidence rate as high as 81% (Alamer et al., 2020). Crucially, a history of stroke is a well-established risk factor for dysphagia (Mourão et al., 2016). Although approximately 60% of patients regain partial swallowing function within 3 months, 11%–50% exhibit persistent dysfunction at 6 months (Song et al., 2024; Xiao et al., 2020). This dysfunction is closely associated with impaired respiratory function (Song et al., 2024; Xiao et al., 2020), increasing the risk of aspiration pneumonia by 3.8-fold and mortality by 11%–16% (Umay et al., 2022; Chang et al., 2022), thereby hindering rehabilitation (Han et al., 2020).
Current modern medical treatments for PSD primarily include oral sensory stimulation training, oral motor exercises, and physical therapy (Hui and Zhang, 2025). Traditional Chinese medicine (TCM) has accumulated extensive experience in managing PSD, achieving favorable outcomes through methods such as swallowing rehabilitation training, oral herbal medicine administration, and acupoint pressure/massage. Although diverse therapeutic approaches for dysphagia exist, the clinical effectiveness of their individual or combined application remains unclear (Yang and Do, 2022). Furthermore, the high cost of treatment imposes a substantial burden on families and the healthcare system (Qureshi et al., 2022).
Consequently, identifying an economical, convenient, effective, and clinically feasible treatment modality capable of enhancing swallowing ability, improving nutritional intake, reducing complications, and facilitating recovery in PSD patients holds significant practical importance. In recent years, guided by TCM meridian theory and the concepts of Qi and blood, and integrated with modern medical technology, research exploring the mechanisms of transdermal drug delivery via acupoints has gained momentum. Specific external traditional Chinese medicine therapies (SETCM therapies), recognized as noninvasive and safe interventions, have consequently garnered increasing attention, offering novel perspectives for PSD rehabilitation (Zheng et al., 2024; Li, 2023). SETCM therapies refer to standardized interventions using pharmacopeia-defined herbal formulations applied via specific modalities (e.g., patching and iontophoresis) at anatomically targeted sites. High-quality randomized controlled trials (RCTs) investigating SETCM therapies for PSD have emerged, progressively focusing on evaluating their efficacy. However, this specific dimension has not been adequately explored in previous meta-analyses, highlighting an urgent need to synthesize the latest evidence using evidence-based medicine (EBM) methodologies.
Therefore, this systematic review assessed RCTs comparing SETCM therapy interventions with non-SETCM therapy controls in adult PSD patients, with a focus on swallowing function, nutritional status, and complications. The aims were to provide more reliable EBM evidence for clinical practice, explore the potential clinical utility of SETCM therapies in PSD management, and thereby offer valuable references for clinicians and rehabilitation therapists.
2 Materials and methods
2.1 Study registration
This study adhered to international guidelines for conducting and reporting meta-analyses concerning the selection and utilization of research methodologies. The study protocol was prospectively registered on the PROSPERO international prospective register of systematic reviews (https://www.crd.york.ac.uk/PROSPERO/), registration number CRD42024599344.
2.2 Literature search strategy
A comprehensive search was conducted across eight electronic databases. Chinese literature databases included China National Knowledge Infrastructure (CNKI), China Biology Medicine disc (SinoMed/CBM), VIP Chinese Science and Technology Periodical Database (VIP), and Wanfang Data Knowledge Service Platform (WFSD). International literature databases included PubMed, Embase, Web of Science, and the Cochrane Library. The search aimed to identify randomized controlled trials (RCTs) investigating specific external traditional Chinese medicine (SETCM) therapy interventions for post-stroke dysphagia (PSD). The search timeframe generally spanned from the inception of each database to the latest available date to ensure the inclusion of the most recent research. Search terms incorporated both medical subject headings (MeSH) and free-text words in Chinese and English: Dysphagia: (“Dysphagia” OR “Swallowing Disorders” OR “Deglutition Disorders” OR “Pseudobulbar Palsy” OR “Oropharyngeal Dysfunction”) Stroke: (“Stroke” OR “Cerebrovascular Accident” OR “Brain Infarction” OR “Cerebral Embolism” OR “Cerebral Ischemia”) SETCM therapy interventions: (“External Traditional Chinese Medicine” OR “Chinese Herbal Patch” OR “Iontophoresis” OR “Hot Compress” OR “Cold Compress” OR “Fumigation” OR “Herbal Steam” OR “Herbal Wash”).
Search strategies utilized Boolean operators (AND, OR), wildcards, and proximity operators where appropriate, combining MeSH terms and free-text keywords. Manual searches of relevant specialized journals and efforts to locate unpublished studies (grey literature) were also performed when necessary.
2.3 Definition, component standardization, and taxonomic validation of SETCM therapy interventions
SETCM therapies are standardized interventions using pharmacopeia-defined herbal formulations applied via specific methods (e.g., patching and iontophoresis) to anatomically defined target sites (typically acupoints). For all included primary studies, detailed information regarding the specific SETCM therapy interventions (including, but not limited to, herbal patching, iontophoresis, fumigation, and medicated stick application) was systematically extracted and re-examined. This encompassed the botanical components, preparation methods, and quality control standards (where mentioned). The botanical names of all reported medicinal plant species were subjected to systematic taxonomic validation using authoritative international databases (Rivera et al., 2014). The Consensus on Phytovigilance for Medicinal Plants (ConPhyMP) tool was employed to evaluate all included studies (Heinrich et al., 2018). This assessment covered: botanical names (scientific name, family, and genus), the plant parts used, functional components, pharmacological effects, mechanism of action, etc.
2.4 Literature inclusion and exclusion criteria
2.4.1 Inclusion criteria
2.4.2 Exclusion criteria
Studies were excluded based on the following criteria:
(1) Dysphagia caused by other underlying conditions (e.g., neurological disorders, head/neck cancer, or congenital abnormalities);
(2) Animal studies, case reports, conference abstracts, reviews, or non-research articles;
(3) Studies where the full text was unavailable or original data were incomplete/unrecoverable;
(4) Studies with major methodological flaws (e.g., lack of randomization and absence of a control group) or meeting any of the following conditions:
a. “High risk” of bias: Studies rated as “high risk” in ≥2 key domains (random sequence generation, allocation concealment, and blinding) using the Cochrane risk-of-bias tool;
b. Insufficient data integrity: Missing data for critical outcome measures where authors could not be contacted for clarification.
(Note: Studies rated as “unclear risk” in only one domain (e.g., allocation concealment) were retained due to the current limited availability of high-quality RCTs in the PSD field. The potential influence of these studies on conclusions was assessed via sensitivity analysis.)
(5) Patients with psychiatric disorders or severe communication barriers affecting informed consent;
(6) Studies lacking predefined primary/secondary outcomes or employing non-standardized assessment tools;
(7) Studies involving duplicate publication or overlapping datasets with prior publications.
2.5 Literature screening and data extraction
Literature screening: Two independent researchers performed the literature search and initial screening based on the predefined search strategy. EndNote 20 was used for reference management (including import, organization, merging, and deduplication). Initial screening involved excluding clearly irrelevant records based on titles and abstracts. Full texts of potentially relevant studies were then retrieved and scrutinized to determine eligibility. Attempts were made to contact authors for missing data from potentially eligible studies; studies were excluded if no response was received. Results from both initial and full-text screening underwent cross-checking by both researchers. Disagreements were resolved through discussion or arbitration by a third reviewer.
Data extraction: Extracted data encompassed: (1) basic study information (authors, year, and journal); (2) patient baseline characteristics (sample size, age, gender, stroke type/location, and time since stroke); (3) intervention details (SETCM therapy type, specific herbs/formulations [validated taxonomically], preparation, dosage, frequency, duration, and concurrent therapies); (4) control intervention details; (5) outcome measures (primary and secondary, as defined in Table 1, including measurement time points and scales used); and (6) quality assessment data. To standardize efficacy assessment, the end of the intervention period was selected as the primary observation endpoint. Continuous outcome data were recorded as mean ± standard deviation ( 7± s). Missing data were requested from the original authors; if unavailable, they were documented as missing.
TABLE 1
| PICOS | Inclusion criteria |
|---|---|
| Population | Adult patients (>18 years old) diagnosed with post-stroke dysphagia (PSD) according to established diagnostic criteria (e.g., criteria from the 4th National Cerebrovascular Disease Conference), with stroke confirmed by CT or MRI |
| Intervention | Experimental group receiving validated external traditional Chinese medicine therapies (SETCM therapies) alone (e.g., herbal patching, TCM iontophoresis, hot compress, fumigation, fumigation-wash, wash-soak, herbal hot compress, cold compress) or SETCM therapies combined with conventional care |
| Comparison | Control group receiving non-SETCM therapy interventions: conventional therapy (e.g., medication), conventional nursing care, or conventional rehabilitation training |
| Outcomes | Primary outcomes: Overall response rate (defined as the percentage of cases achieving comprehensive cure, marked improvement, or improvement); Cure rate (defined as the percentage of cases achieving cure status); Water swallowing test (WST) score; Swallow quality-of-life questionnaire (SWAL-QOL) score; Complication incidence rate. Secondary outcomes: Videofluoroscopic swallow study (VFSS) score; Modified Barthel index (MBI) score; Gugging swallowing screen (GUSS) score; Activities of daily living (ADL) score; Nasogastric tube indwelling duration; Feeding tube removal success rate; Nutritional risk screening 2002 (NRS 2002) score; Fujishima dysphagia scale (FDS) score; Nutritional biomarkers: Serum albumin (ALB), Prealbumin (PA); Safety data: Incidence of skin reactions/systemic adverse events |
| Study design | Randomized controlled trials (RCTs) |
PICOS strategy—inclusion criteria.
Note: P: population; I: intervention; C: comparison; O: outcome; S: study design; CT: computed tomography; MRI: magnetic resonance imaging.
2.6 Assessment of methodological quality (risk of bias)
The methodological quality (risk of bias) of included randomized controlled trials (RCTs) was assessed independently by two reviewers using the Cochrane risk-of-bias tool (RoB 2) for randomized trials. The assessment comprised two parts. In the Descriptive assessment, Detailed descriptions of study methodology relevant to each bias domain were recorded to support the judgment of risk. In the Judgment of Risk assessment, each domain was judged as “Low risk,” “High risk,” or “Unclear risk” of bias.
The specific bias domains evaluated were: Selection Bias: Random sequence generation, Allocation concealment; Performance Bias: Blinding of participants and personnel; Detection Bias: Blinding of outcome assessment; Attrition Bias: Incomplete outcome data; Reporting Bias: Selective outcome reporting; Other Bias: Any other potential sources of bias not covered above.
Disagreements in judgments were resolved through consensus discussion or consultation with a third reviewer.
2.7 Statistical analysis
Statistical analyses of the extracted outcome data were performed using Review Manager (RevMan) software, version 5.4.1, provided by the Cochrane Collaboration. Heterogeneity among studies for each clinical outcome was assessed using the chi2 test (Q statistic) and quantified using the I2 statistic. A significance level of P > 0.10 for the chi2 test indicated acceptable heterogeneity, warranting the use of a fixed-effects model (FEM) for meta-analysis. Conversely, a chi2 test result of P ≤ 0.10 indicated significant heterogeneity, leading to the use of a random-effects model (REM) for meta-analysis. The magnitude of heterogeneity was interpreted based on I2 values: I2 ≤ 25% indicated low heterogeneity, I2 > 50% indicated moderate heterogeneity, and I2 > 75% indicated substantial heterogeneity. Where significant heterogeneity was present, sensitivity analyses were conducted using the study removal method to identify potential sources and exclude studies contributing excessively to heterogeneity. For continuous outcomes, results were pooled using the standardized mean difference (SMD) with 95% confidence intervals (CI). For dichotomous outcomes, the relative risk (RR) with 95% CI was used. Results were presented using forest plots. Publication bias was assessed visually using inverted funnel plots plotting the RR (for dichotomous outcomes) or SMD (for continuous outcomes) against its standard error (SE). Two predefined subgroup analyses were performed: (a) stratified by SETCM therapy intervention type (herbal patching, TCM iontophoresis, medicated stick application); and (b) stratified by stroke lesion location (supratentorial [cerebral hemisphere] vs. brainstem). Studies not reporting specific lesion location were excluded from the latter subgroup analysis.
2.8 GRADE assessment of evidence quality
Two independent researchers assessed the quality of evidence (certainty) for each outcome measure using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt et al., 2011). Assessments were conducted using the online GRADEpro Guideline Development Tool (GRADEpro GDT; https://gradepro.org). The quality of evidence for each outcome was downgraded based on five domains: Risk of bias across included studies; Inconsistency: Unexplained heterogeneity in results; Indirectness: Indirectness of population, intervention, comparator, or outcome; Imprecision: Wide confidence intervals or small sample size; Publication bias: Evidence of publication bias. The initial quality rating for RCTs is “High.” Evidence quality was then categorized as High: No downgrading (we are very confident that the true effect lies close to that of the estimate); Moderate: Downgraded by one level (we are moderately confident in the effect estimate; the true effect is likely close to the estimate, but there is a possibility of being substantially different); or Low: Downgraded by two levels (our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate). Very low: Downgraded by three levels (we have very little confidence in the effect estimate; the true effect is likely substantially different from the estimate).
Disagreements between reviewers were resolved through discussion or arbitration by a third reviewer.
3 Results
3.1 Literature screening results and characteristics of included studies
The initial search identified 1,243 potentially relevant records. After removing 373 duplicate publications, 870 unique records remained. Screening of titles and abstracts excluded 791 records, leaving 79 articles for full-text assessment. Following full-text review, 54 articles were excluded, resulting in the final inclusion of 25 published randomized controlled trials (RCTs). The study selection process is detailed in Figure 1. The baseline characteristics of the included studies are summarized in Table 2. These 25 RCTs enrolled a total of 2,245 participants. Regarding the specific SETCM therapy modalities employed: 16 studies utilized acupoint herbal patching, two studies utilized herbal fumigation, two studies utilized TCM iontophoresis, two studies utilized medicated herbal stick application, and two studies utilized acupoint stimulation with herbal ice-cotton swabs. One study utilized targeted drug delivery therapy. Concerning the intervention durations: 15 studies implemented an intervention period of approximately 4 weeks, six studies implemented an intervention period of 3 weeks, and four studies implemented an intervention period of 2 weeks. The results of the included studies indicated that there were no significant differences in baseline measure scores between the experimental (SETCM therapies) and control groups across all studies (P > 0.05). Post-intervention, outcome measure scores improved significantly compared to baseline within both groups (P < 0.05). The improvement observed in the experimental (SETCM therapies) group was significantly greater than that in the control group (P < 0.05).
FIGURE 1
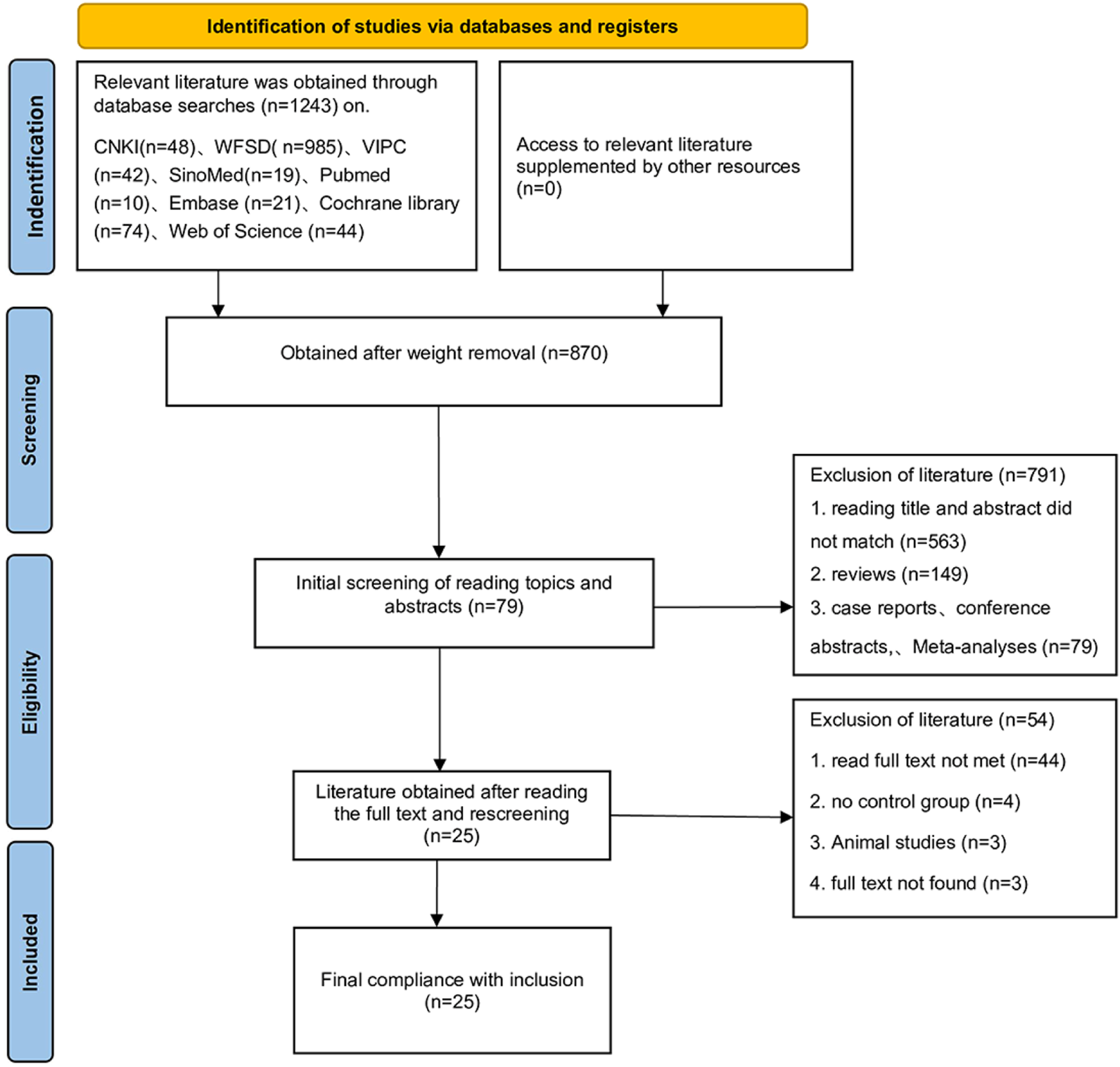
PRISMA flow chart.
TABLE 2
| Author(s) | Year | Sample size | Age | Days | Outcome indicator | Intervention measures | Stroke location | Herbal medicine used | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T | C | Operational area | ||||||||
| Tang and Liu (2024) | 2024 | 34/34 | 64.18 ± 8.09/64.28 ± 8.14 | 30 days | ⑥⑪ ⑫ ⑬ ⑭ | Acupoint application + rehabilitation | Rehabilitation | Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Yu and Wu (2024) | 2024 | 75/75 | 45–78/42–80 | 28 days | ①②④⑤ | Acupoint application + rehabilitation | Rehabilitation | Tiantu point, Renying point, and Lianquan point | Supratentorial | Pinelliae Rhizoma Praeparatum,Acori Tatarinowii Rhizoma, Arisaematis Rhizoma Preparatum, Notoginseng Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Asari Radix et Rhizoma |
| Wang and Sun (2023) | 2023 | 15/15 | 58–82/59–80 | 28 days | ③⑤⑦⑧⑨ | Acupoint application + rehabilitation | Rehabilitation | Tiantu point, Renying point, and Lianquan point | Unspecified | Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Aconiti Lateralis Radix Praeparata, Asari Radix et Rhizoma, Fritillariae Cirrhosae Bulbus, Borneolum Syntheticum |
| Zhu et al. (2023) | 2023 | 32/32 | 77.72 ± 7.87/80.75 ± 6.77 | 21 days | ①② | targeted transdermal medication+ rehabilitation |
Rehabilitation | Dazhui point and Shenshu point | Supratentorial | Rehmanniae Radix Praeparata, Morindae Officinalis Radix, Corni Fructus, Dendrobii Caulis, Cistanches Herba, Aconiti Lateralis Radix Praeparata, Schisandrae Chinensis Fructus, Cinnamomi Cortex, Poria, Ophiopogonis Radix, Acori Tatarinowii Rhizoma, Polygalae Radix |
| Yu and Qiu (2023) | 2023 | 40/40 | 54–77/53–77 | 30 days | ⑥⑪ ⑬ ⑭ | Acupoint application + rehabilitation | Rehabilitation | Acupoint application + rehabilitation | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Wang et al. (2023b) | 2023 | 50/50 | 20–75/20–75 | 14 days | ①②③④ | Acupoint application + medication | Medication | Renying point, Lianquan point, and Fengfu point | Supratentorial | Persicae Semen, Carthami Flos, Angelicae Sinensis Radix, Paeoniae Radix Rubra, Rehmanniae Radix, Scrophulariae Radix, Platycodonis Radix, Aurantii Fructus, Borneolum Syntheticum, Glycyrrhizae Radix et Rhizoma |
| Xu (2022) | 2022 | 60/60 | 45–93/46–93 | 30 days | ⑪ ⑬ ⑭ | Acupoint application + rehabilitation | Rehabilitation | Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Shi et al. (2022) | 2022 | 66/66 | 46–74/43–73 | 30 days | ①②⑫ | Herbal fumigation + rehabilitation | Rehabilitation | neck | Unspecified | Cinnamomi Cortex, Glycyrrhizae Radix et Rhizoma, Poria, Ginseng Radix et Rhizoma, Achyranthis Bidentatae Radix, Eucommiae Cortex, Rehmanniae Radix, Chuanxiong Rhizoma, Paeoniae Radix Rubra, Angelicae Sinensis Radix, Asari Radix et Rhizoma, Saposhnikoviae Radix, Gentianae Macrophyllae Radix, Taxilli Herba |
| Zhou et al. (2022) | 2022 | 75/75 | 33–69/32–65 | 21 days | ①②③④ | Acupoint application + conventional treatment+ rehabilitation |
Conventional treatment + rehabilitation | Renying point, Lianquan point, and Fengfu point | Supratentorial | Persicae Semen, Carthami Flos, Angelicae Sinensis Radix, Paeoniae Radix Rubra, Rehmanniae Radix, Scrophulariae Radix, Platycodonis Radix, Aurantii Fructus, Borneolum Syntheticum, Glycyrrhizae Radix et Rhizoma |
| Jiao (2022) | 2022 | 40/40 | 53–73/52–74 | 28 days | ③⑤⑥ | Acupoint application + conventional treatment | Conventional treatment | Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Wu et al. (2021) | 2021 | 40/40 | 47–83/46–81 | 30 days | ①⑧⑩ | Acupoint application + rehabilitation | Rehabilitation | Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Dong et al. (2021) | 2021 | 46/46 | 52–78/51–79 | 28 days | ①⑧⑨ | Acupoint application + conventional treatment+ rehabilitation |
Conventional treatment +rehabilitation |
Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Shao and Chen (2020) | 2020 | 53/53 | 40–79/41–77 | 28 days | ①②③④⑪ | Herbal medicine stick treatment + conventional treatment | Conventional treatment | Posterior palatal arch, soft palate, pharyngeal palatal arch, posterior pharyngeal wall, and root of tongue | Supratentorial | Moschus, Menthae Haplocalycis Herba, Styrax, Borneolum Syntheticum |
| Zheng et al. (2019) | 2019 | 42/42 | 62.6 ± 7.5/63.4 ± 7.9 | 14 days | ①②③ | Acupoint application + electrical stimulation+ rehabilitation |
Electrical stimulation + rehabilitation | Tiantu point, Renying point, Lianquan point, and Futu point | Supratentorial | Moschus, Ginseng Radix et Rhizoma, Styrax, Bovis Calculus, Bufonis Venenum, Cinnamomi Cortex, Borneolum Syntheticum |
| Hu (2019) | 2019 | 61/61 | 65.3 ± 12.2/65.3 ± 12.2 | 30 days | ①②③④⑤⑦⑧⑨⑩ | Acupoint application + conventional treatment | Conventional treatment | Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Zhou et al. (2018) | 2018 | 40/40 | 36–72/38–70 | 21 days | ①③④ | Herbal medicine stick treatment + conventional treatment | Conventional treatment | Herbal medicine stick treatment + conventional treatment | Brainstem | Bombyx Batryticatus, Acori Tatarinowii Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Moschus, liquor |
| Luo et al. (2018) | 2018 | 35/35 | 43–82/39–85 | 14 days | ①②④ | Acupoint application + rehabilitation | Rehabilitation | Lianquan point | Supratentorial | Kansui Radix, Sinapis Semen, Ephedrae Herba, Asari Radix et Rhizoma |
| Ma et al. (2021) | 2018 | 55/55 | 64.3 ± 11.2/63.5 ± 11.4 | 20 days | ①②③④⑤⑮ | Acupoint application + conventional treatment +rehabilitation |
Conventional treatment + rehabilitation | Lianquan point, Bilateral Gongxue points, and Bilateral Renying points | Unspecified | Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Aconiti Lateralis Radix Praeparata, Asari Radix et Rhizoma, Acori Tatarinowii Rhizoma, Notoginseng Radix et Rhizoma Pulvis |
| Xu and Zhao (2016) | 2016 | 33/33 | 35–77/34–75 | 28 days | ①② | Herbal fumigation + rehabilitation | Rehabilitation | Neck | Unspecified | Cinnamomi Cortex, Glycyrrhizae Radix et Rhizoma, Poria, Ginseng Radix et Rhizoma, Achyranthis Bidentatae Radix, Eucommiae Cortex, Rehmanniae Radix, Chuanxiong Rhizoma, Paeoniae Radix Rubra, Angelicae Sinensis Radix, Asari Radix et Rhizoma, Saposhnikoviae Radix, Gentianae Macrophyllae Radix, Taxilli Herba |
| Que and Bian (2016) | 2016 | 43/43 | 66.88 ± 12.33/65.93 ± 12.61 | 20 days | ①②③ | Herbal medicine stick Treatment + rehabilitation | Rehabilitation | Both palatal arches, posterior pharyngeal wall, posterior root of the tongue, Jinjin points, and Yuyi points | Mixed | Pheretima, Carthami Flos, Liquidambaris Fructus, Menthae Haplocalycis Herba, Borneolum Syntheticum |
| Lai (2015) | 2015 | 50/50 | 44–75/45–72 | 30 days | ① | Acupoint application + rehabilitation | Rehabilitation | Fengchi point, Hegu point, and Jinjin point |
Unspecified | Astragali Radix, Angelicae Sinensis Radix, Paeoniae Radix Rubra, Atractylodis Macrocephalae Rhizoma, Chuanxiong Rhizoma, Carthami Flos, Persicae Semen, Glycyrrhizae Radix et Rhizoma |
| Lin et al. (2014) | 2014 | 30/29 | 60.47 ± 8.07/61.10 ± 9.23 | 30 days | ①②⑤ | Herbal medicine stick treatment + medication+ rehabilitation |
Medication + rehabilitation | Uvula, bilateral palatopharyngeal arches, soft palate, and posterior root of tongue | Brainstem | Arisaema Praeparatum, Pinelliae Rhizoma, Citri Exocarpium Rubrum, Bambusae Caulis in Taenias, Persicae Semen, Aurantii Fructus Immaturus, Carthami Flos, Poria, Acori Tatarinowii Rhizoma, Glycyrrhizae Radix et Rhizoma |
| Zhao et al. (2012) | 2012 | 23/23 | 35–84/35–84 | 30 days | ①⑮ | Acupoint application + rehabilitation | Rehabilitation | Tiantu point, Renying point, and Lianquan point | Unspecified | Asari Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Pinelliae Rhizoma, Arisaematis Rhizoma Preparatum, Borneolum Syntheticum, Fritillariae Cirrhosae Bulbus |
| Zhao et al. (2008) | 2008 | 35/35 | 48–69/50–68 | 20 days | ①② | TCM iontophoresis + rehabilitation | Rehabilitation | Lianquan point, Tiantu point, Fengfu point, Fengchi point, Yifeng point, and Hegu point |
Brainstem | Moschus, Borneolum Syntheticum |
| Huang (2007) | 2007 | 50/50 | 45–74/40–75 | 15 days | ④ | TCM iontophoresis + medication | Medication | Fengfu point, Lianquan point | Brainstem | - |
Baseline characteristics of included studies and details of SETCM therapy interventions.
Notes:
Outcomes assessed: ① Overall response rate; ② Cure rate; ③ Water swallowing test (WST) score; ④ Videofluoroscopic swallow study (VFSS) score; ⑤ Swallow quality-of-life questionnaire (SWAL-QOL) score; ⑥ Post-intervention aspiration rate; ⑦ Modified Barthel index (MBI) score; ⑧ Serum albumin (ALB); ⑨ Prealbumin (PA); ⑩ Nutritional risk screening 2002 (NRS 2002) score; ⑪ Gugging swallowing screen (GUSS) score; ⑫ Activities of daily living (ADL) score; ⑬ Nasogastric tube indwelling duration; ⑭ Feeding tube removal success rate; ⑮ Fujishima dysphagia scale (FDS) score. (See main text for abbreviation definitions).
Stroke lesion location classification:
Supratentorial: Lesions involving cortical/subcortical structures.
Brainstem: Lesions involving medullary/pontine structures.
Mixed/Unspecified: Excluded from lesion location-based efficacy analysis.
3.2 Development of a standardized SETCM therapy intervention database
This study successfully established a standardized database for specific external traditional Chinese medicine therapy (SETCM therapy) interventions. Specifically, through authoritative databases (MPNS v7.3+, POWO) and the Chinese Pharmacopoeia (2020 Edition), rigorous taxonomic validation and name standardization were performed on all medicinal plant species reported in the primary studies, ensuring the accuracy of the botanical origins of the herbs used. These validation results have been compiled and summarized in Table 3: Standardized botanical information.
TABLE 3
| Medicinal Material (Chinese) | Latin name (ChP 2020) | Citrus | Specific Epithet | Named person's initials | Family | Part used |
|---|---|---|---|---|---|---|
| Bajitian | Morindae Officinalis Radix | Morinda | officinalis | How | Rubiaceae | Dried root (Radix) |
| Fuzi | Aconiti Lateralis Radix Praeparata | Aconitum | carmichaelii | Debx. | Ranunculaceae | Processed daughter root |
| Jiangcan | Bombyx Batryticatus | Bombyx | mori | Linnaeus | Bombycidae | Dried larva killed by Beauveria bassiana |
| Jiezi | Sinapis Semen | Sinapis | alba | L. (Carl Linnaeus) | Brassicaceae | Dried ripe seed (Semen) |
| Bingpian | Borneolum Syntheticum | Not mentioned | Not mentioned | Not mentioned | Synthetic | Crystalline product from Cinnamomum branches |
| Bohe | Menthae Haplocalycis Herba | Mentha | haplocalyx | Briq. | Lamiaceae | Dried aerial part (Herba) |
| Baizhu | Atractylodis Macrocephalae Rhizoma | Atractylodes | macrocephala | Koidz. | Asteraceae | Dried rhizome (Rhizoma) |
| Chuanxiong | Chuanxiong Rhizoma | Ligusticum | chuanxiong | Hort. | Apiaceae | Dried rhizome (Rhizoma) |
| Chuanbeimu | Fritillariae Cirrhosae Bulbus | Fritillaria | cirrhosa | D.Don | Liliaceae | Dried bulb (Bulbus) |
| Dannanxing | Arisaema cum Bile | Not mentioned | Not mentioned | Not mentioned | Araceae | Bile-processed tuber of Arisaema |
| Dilong | Pheretima | Pheretima | aspergillum | (E.Perrier) | Megascolecidae | Eviscerated dried body (Lumbricus) |
| Dihuang | Rehmanniae Radix | Rehmannia | glutinosa | Libosch. | Scrophulariaceae | Dried root tuber (Radix) |
| Duzhong | Eucommiae Cortex | Eucommia | ulmoides | Oliv. | Eucommiaceae | Dried bark (Cortex) |
| Fangfeng | Saposhnikoviae Radix | Saposhnikovia | divaricata | (Turcz.)Schischk. | Apiaceae | Dried root (Radix) |
| Fuling | Poria | Poria | cocos | (Schw.) Wolf | Polyporaceae | Dried sclerotium (Sclerotium) |
| Fuzi | Aconiti Lateralis Radix Praeparata | Aconitumm | carmichaelii | Debx. | Ranunculaceae | Dried daughter root (Radix Aconiti Lateralis) |
| Gansui | Kansui Radix | Euphorbia | kansui | T.N.Liou ex T.P.Wang | Euphorbiaceae | Dried root tuber (Radix) |
| Gancao | Glycyrrhizae Radix et Rhizoma | Glycyrrhiza | uralensis | Fisch. | Fabaceae | Dried root and rhizome (Radix et Rhizoma) |
| Honghua | Carthami Flos | Carthamus | tinctorius | L.(Carl Linnaeus) | Asteraceae | Dried flower (Flos) |
| Huangqi | Astragali Radix | Astragalus | membranaceus | (Fisch.) Bge. | Fabaceae | Dried root (Radix) |
| Jiegeng | Platycodonis Radix | Platycodon | grandiflorum | (Jacq.)A.DC. | Campanulaceae | Dried Root (Radix Platycodi) |
| Juhong | Citri Exocarpium Rubrum | Citrus | reticulata | Blanco | Rutaceae | Dried Pericarp (Pericarpium Citri Reticulatae) |
| Lulu tong | Liquidambaris Fructus | Liquidambar | formosana | Hance | Hamamelidaceae | Dried Resin (Resina Liquidambaris) |
| Mahuang | Ephedrae Herba | Ephedra | sinica | Stapf | Ephedraceae | Dried Herbaceous Stem (Herba Ephedrae) |
| Maidong | Ophiopogonis Radix | Ophiopogon | japonicus | (L. f.) Ker-Gawl. | Liliaceae | Dried Root Tuber (Radix Ophiopogonis) |
| Niuxi | Achyranthis Bidentatae Radix | Achyranthes | bidentata | Bl. | Amaranthaceae | Dried Root (Radix Achyranthis) |
| Qinjiao | Gentianae Macrophyllae Radix | Gentiana | macrophylla | Pall. | Gentianaceae | Dried Root (Radix Gentianae) |
| Renshen | Ginseng Radix et Rhizoma | Panax | ginseng | C.A.Mey. | Araliaceae | Dried Root (Radix Ginseng) |
| Roucongrong | Cistanches Herba | Cistanche | deserticola | Y.C.Ma | Orobanchaceae | Dried Fleshy Stem with Scales (Herba Cistanches) |
| Rougui | Cinnamomi Cortex | Cinnamomum | cassia | Presl | Lauraceae | Dried Bark (Cortex Cinnamomi) |
| Sanqi | Notoginseng Radix et Rhizoma | Panax | notoginseng | (Burk.) F. H. Chen | Araliaceae | Dried Root (Radix Notoginseng) |
| Sangjisheng | Taxilli Herba | Taxillus | chinensis | (DC.)Danser | Loranthaceae | Dried Stem with Leaves (Ramulus Taxilli) |
| Shanzhuyu | Corni Fructus | Cornus | officinalis | Sieb. et Zucc. | Cornaceae | Dried Flesh of Fruit (Fructus Corni) |
| Shichangpu | Acori Tatarinowii Rhizoma | Acorus | tatarinowii | Schott | Araceae | Dried Rhizome (Rhizoma Acori Tatarinowii) |
| Shihu | Dendrobii Caulis | Dendrobium | nobile | Lindl. | Orchidaceae | Dried Stem (Caulis Dendrobii) |
| Shexiang | Moschus | Moschus | berezovskii | Flerov | Cervidae | Dried Secretion from Scent Gland (Moschus) |
| Suhexiang | Styrax | Liquidambar | orientalis | Mill. | Hamamelidaceae | Resin from Trunk (Styrax Liquidambaris) |
| Taoren | Persicae Semen | Prunus | persica | (L.)Batsch | Rosaceae | Dried Ripe Seed (Semen Persicae) |
| Wuweizi | Schisandrae Chinensis Fructus | Schisandra | chinensis | (Turcz.)Baill. | Magnoliaceae | Dried Ripe Fruit (Fructus Schisandrae) |
| Xixin | Asari Radix et Rhizoma | Asarum | heterotropoides | Fr.Schmidt | Aristolochiaceae | Dried Root and Rhizome (Radix et Rhizoma Asari) |
| Xuanshen | Scrophulariae Radix | Scrophularia | ningpoensis | Hemsl. | Scrophulariaceae | Dried Root (Radix Scrophulariae) |
| Yuanzhi | Polygalae Radix | Polygala | tenuifolia | Willd. | Polygalaceae | Dried Root (Radix Polygalae) |
| Zhiqiao | Aurantii Fructus | Citrus | aurantium | L. (Carl Linnaeus) | Rutaceae | Dried Immature Whole Fruit (Fructus Immaturus Aurantii) |
| Zhishi | Aurantii Fructus Immaturus | Citrus | aurantium | L. (Carl Linnaeus) | Rutaceae | Dried Nearly Mature Halved Fruit (Fructus Aurantii) |
| Zhuru | Bambusae Caulis in Taenias | Bambusa | tuldoides | Munro | Poaceae | Shavings from Middle Stem Layer (Caulis Bambusae in Taeniam) |
| Danggui | Angelicae Sinensis Radix | Angelica | sinensis | (Oliv.) Diels | Apiaceae | Dried Root (Radix Angelicae Sinensis) |
| Chishao | Paeoniae Radix Rubra | Paeonia | lactiflora | Pall. | Ranunculaceae | Dried Root (Radix Paeoniae Alba) |
| Niuhuang | Bovis Calculus | Bos | taurus domesticus | Gmelin | Bovidae | Dried Gallstone (Calculus Bovis) |
| Chansu | Bufonis Venenum | Bufo | bufo gargarizans | Cantor | Bufonidae | Lyophilized Parotoid Gland Secretion (Venenum Bufonis) |
Standardized botanical information table.
The ConPhyMP tool for plant monitoring of medicinal plants was used to conduct a comprehensive assessment of all included studies and to formulate standardized intervention measures. This assessment covered key elements, including botanical names (scientific name, family, and genus), plant parts used, functional components, pharmacological effects, mechanism of action, etc. Based on this assessment, Table 4: Characteristic metabolites of herbs used were established, clearly presenting the correspondence between each standardized plant entity and its functional components.
TABLE 4
| Latin name (ChP 2020) | Functional ingredient | Chemical class | Pharmacopoeial content requirement (ChP 2020) | Pharmacological Effect(s) | Mechanism of action | References |
|---|---|---|---|---|---|---|
| Morindae Officinalis Radix | Physicion, Rubiadin | Anthraquinones | Not specified | Anti-osteoporotic | Promotes osteoblast differentiation; Inhibits RANKL/OPG pathway | Ru et al. (2014), Chen et al. (2023) |
| Aconiti Lateralis Radix Praeparata | Aconitine, Mesaconitine | Diterpenoid Alkaloids | Diester-diterpenoid alkaloids ≤0.015% | Cardiotonic, Analgesic | Activates Na+ channels; Enhances myocardial contractility | Ru et al., 2014; Gupta (2020) |
| Bombyx Batryticatus | Fibroin, Sericin | Structural Proteins | Total ash ≤7.0% | Antioxidant, Hypoglycemic | Scavenges ROS; Activates PI3K/Akt signaling pathway | Luo et al. (2024) |
| Sinapis Semen | Sinalbin | Glucosinolates | Not specified | Antibacterial, Anti-inflammatory | Hydrolyzes to generate phenethyl isothiocyanate; Inhibits NF-κB pathway | Chen et al. (2023) |
| Pinelliae Rhizoma | β-Sitosterol, Cavidine | Sterols, Alkaloids | Not specified | Antitumor (Lung cancer) | Induces G2/M phase arrest in cancer cells; Inhibits EGFR phosphorylation | Ru et al. (2014), Choi et al. (2022) |
| Borneolum Syntheticum | d-Borneol | Monoterpenoids | Borneol (C10H18O) ≥55.0% | Penetration enhancer, Anti-inflammatory | Enhances blood-brain barrier permeability; Inhibits COX-2 expression | Gupta (2020) |
| Menthae Haplocalycis Herba | Menthol, Menthone | Monoterpenoids | Volatile oil ≥0.8% | Antispasmodic, Antibacterial | Blocks calcium channels; Inhibits acetylcholine release | Ru et al. (2014) |
| Atractylodis Macrocephalae Rhizoma | Atractylenolide III | Sesquiterpene Lactones | Not specified | Anti-inflammatory, Immunomodulatory | Inhibits TNF-α/IL-6 secretion; Modulates Th1/Th2 balance | Choi et al. (2022) |
| Chuanxiong Rhizoma | Z-Ligustilide, Senkyunolide | Phthalides | Ferulic acid ≥0.10% | Antithrombotic, Neuroprotective | Inhibits TXA2 synthesis; Reduces cerebral ischemia-reperfusion injury | Choi et al. (2022) |
| Fritillariae Cirrhosae Bulbus | Peimine, Peimisine | Steroidal Alkaloids | Total alkaloids ≥0.080% | Antitussive, Expectorant | Inhibits airway sensory C-fibers; Reduces substance P release | Chen et al. (2023) |
| Arisaema cum Bile | Taurine, Cholic Acid | Bile Acids | Not specified | Antipyretic, Anticonvulsant | Modulates GABA_A receptors; Inhibits glutamate excitotoxicity | Gupta (2020) |
| Pheretima | Lumbrokinase | Protease | Not specified | Thrombolytic, Anticoagulant | Degrades fibrinogen; Inhibits platelet aggregation | Luo et al. (2024) |
| Rehmanniae Radix | Catalpol, Acteoside | Iridoid Glycosides | Catalpol ≥0.20% | Hypoglycemic, Anti-inflammatory | Activates GLUT4 translocation; Inhibits NF-κB nuclear translocation | Choi et al. (2022) |
| Saposhnikoviae Radix | Prim-O-Glucosylcimifugin | Chromones | Not specified | Antiallergic, Analgesic | Blocks histamine H1 receptors; Inhibits TRPV1 channel activation | Chen et al. (2023) |
| Poria | Pachymic Acid, Poriacosides | Triterpenoids | Not specified | Diuretic, Immunomodulatory | Inhibits renal tubular Na+-K+-ATPase; Enhances macrophage phagocytosis | Ru et al. (2014), Luo et al. (2024) |
| Kansui Radix | Kansuinin A | Diterpenoids | Not specified | Purgative, Antitumor | Activates enteric plexus NK1 receptors; Induces watery diarrhea | Chen et al. (2023) |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhizin, Liquiritigenin | Triterpenoids, Flavonoids | Glycyrrhizic acid ≥2.0% | Antiulcer, Hepatoprotective | Inhibits 11β-HSD2; Reduces cortisol catabolism | Ru et al. (2014), Cao et al. (2022) |
| Carthami Flos | Hydroxysafflor yellow A | Chalcones | Not specified | Anti-myocardial ischemia, Anticoagulant | Inhibits PAF receptors; Reduces calcium influx | Choi et al. (2022) |
| Astragali Radix | Astragaloside IV, Calycosin | Saponins, Isoflavones | Astragaloside IV ≥ 0.040% | Immunostimulant, Antioxidant | Activates Nrf2 pathway; Enhances SOD activity | Ru et al. (2014), Choi et al. (2022) |
| Platycodonis Radix | Platycodin D | Triterpenoid Saponins | Not specified | Expectorant, Anti-inflammatory | Stimulates respiratory mucus secretion; Inhibits COX-2/PGE2 pathway | Chen et al. (2023) |
| Citri Exocarpium Rubrum | Hesperidin, Nobiletin | Flavonoids | Hesperidin ≥3.0% | Antioxidant, Digestive stimulant | Scavenges free radicals; Enhances gastrointestinal myenteric plexus activity | Ru et al. (2014), Choi et al. (2022) |
| Liquidambaris Fructus | Gallic Acid, Ellagic Acid | Phenolic Acids | Not specified | Antibacterial, Anti-inflammatory | Inhibits bacterial DNA gyrase; Blocks TLR4/MyD88 signaling | Luo et al. (2024) |
| Ephedrae Herba | Ephedrine, Pseudoephedrine | Alkaloids | Ephedrine ≥0.80% | Antiasthmatic, Diaphoretic | Agonizes β2-adrenergic receptors; Activates sweat gland Na+ channels | He et al. (2023) |
| Ophiopogonis Radix | Ophiopogonin D | Steroidal Saponins | Not specified | Anti-myocardial ischemia, Immunomodulatory | Enhances SOD activity; Inhibits TNF-α/IL-1β production | Choi et al. (2022) |
| Achyranthis Bidentatae Radix | β-Ecdysone, Inokosterone | Phytosterols | Not specified | Anti-arthritic, Osteogenic | Inhibits MMP-13 expression; Promotes osteoblast RUNX2 activation | Chen et al. (2023) |
| Gentianae Macrophyllae Radix | Gentiopicroside | Iridoids | Not specified | Anti-inflammatory, Hepatoprotective | Inhibits TGF-β1/Smad3 pathway; Attenuates hepatic fibrosis | Ru et al. (2014) |
| Ginseng Radix et Rhizoma | Ginsenoside Rg1, Rb1 | Triterpenoid Saponins | Total saponins ≥0.30% | Anti-fatigue, Neuroprotective | Enhances mitochondrial complex I activity; Inhibits Aβ42 aggregation | Ru et al. (2014), Cao et al. (2022) |
| Cistanches Herba | Echinacoside | Phenylethanoid Glycosides | Echinacoside ≥0.30% | Aphrodisiac, Neuroprotective | Promotes NO/cGMP pathway; Inhibits tau protein hyperphosphorylation | Choi et al. (2022) |
| Cinnamomi Cortex | Cinnamaldehyde, Coumarin | Phenylpropanoids | Cinnamaldehyde ≥75.0% | Hypoglycemic, Antibacterial | Activates AMPK/GLUT4 pathway; Disrupts microbial cell membranes | Ru et al. (2014), He et al. (2023) |
| Notoginseng Radix et Rhizoma | Notoginsenoside R1 | Triterpenoid Saponins | Ginsenoside Rg1 ≥ 0.20% | Hemostatic, Antithrombotic | Activates P2Y12 receptors; Promotes platelet aggregation | Chen et al. (2023) |
| Taxilli Herba | Quercetin, Avicularin | Flavonoids | Not specified | Anti-inflammatory, Antioxidant | Inhibits NF-κB nuclear translocation; Scavenges hydroxyl radicals | Choi et al. (2022) |
| Corni Fructus | Loganin, Cornuside | Iridoid Glycosides | Morroniside ≥0.20% | Hypoglycemic, Nephroprotective | Inhibits α-glucosidase; Reduces glomerular mesangial cell proliferation | Choi et al. (2022) |
| Acori Tatarinowii Rhizoma | β-Asarone | Phenylpropanoids | Not specified | Antiepileptic, Neuroprotective | Enhances GABAergic neurotransmission; Inhibits NMDA receptor overactivation | Ru et al. (2014) |
| Dendrobii Caulis | Dendrobine, Nobilonine | Alkaloids | Not specified | Immunomodulatory, Antitumor | Activates NK cell cytotoxicity; Induces cancer cell apoptosis | Luo et al. (2024) |
| Moschus | Muscone | Macrocyclic Ketones | Muscone ≥2.0% | Cardiotonic, Anti-inflammatory | Activates cardiomyocyte L-type calcium channels; Inhibits NLRP3 inflammasome | He et al. (2023) |
| Styrax | Styrax | Resin | Total vanillic acid ≥27.0% | Antibacterial, Expectorant | Disrupts bacterial biofilms; Stimulates respiratory ciliary motility | He et al. (2023) |
| Persicae Semen | Amygdalin | Cyanogenic Glycosides | Not specified | Antitumor, Anti-fibrotic | Inhibits TGF-β1/Smad pathway; Blocks EMT process | Choi et al. (2022) |
| Schisandrae Chinensis Fructus | Schisandrin B | Lignans | Schisandrin A ≥0.40% | Hepatoprotective, Antioxidant | Enhances GSH synthesis; Inhibits CYP450 enzyme activity | Ru et al. (2014), Choi et al. (2022) |
| Asari Radix et Rhizoma | Asarinin, Sesamin | Lignans | Volatile oil ≥2.0% | Analgesic, Anti-inflammatory | Inhibits COX-2/PGE2 pathway; Blocks TRPA1 nociceptive receptors | Choi et al. (2022) |
| Scrophulariae Radix | Harpagoside | Iridoid Glycosides | Harpagoside ≥0.050% | Anti-inflammatory, Immunosuppressive | Inhibits NF-κB activation; Reduces Th17 cell differentiation | Cao et al. (2022) |
| Polygalae Radix | Tenuifolin | Saponins | Not specified | Antidepressant, Neuroprotective | Upregulates BDNF/TrkB pathway; Inhibits MAO-A activity | Ru et al. (2014), Choi et al. (2022) |
| Aurantii Fructus | Synephrine, Naringin | Alkaloids, Flavonoids | Synephrine ≥0.30% | Digestive stimulant, Pressor | Agonizes α1-adrenergic receptors; Enhances gastrointestinal motility | Choi et al. (2022) |
| Bambusae Caulis in Taenias | Bambooside A | Flavonoid Glycosides | Not specified | Antioxidant, Antitussive | Scavenges superoxide anions; Inhibits airway hyperresponsiveness | Luo et al. (2024) |
| Angelicae Sinensis Radix | Z-Ligustilide, Ferulic Acid | Phthalides, Phenolic Acids | Ferulic acid ≥0.050% | Hematopoietic, Anticoagulant | Promotes EPO secretion; Inhibits platelet TXA2 synthesis | Choi et al. (2022) |
| Paeoniae Radix Rubra | Paeoniflorin | Monoterpene Glycosides | Paeoniflorin ≥1.8% | Antispasmodic, Anti-inflammatory | Inhibits PGE2 synthesis; Blocks calcium influx | Ru et al. (2014), Choi et al. (2022) |
| Bovis Calculus | Cholic Acid, Tauroursodeoxycholic Acid | Bile Acids | Cholic acid ≥13.0% | Antipyretic, Anticonvulsant | Activates FXR receptors; Inhibits TLR4/NF-κB pathway | He et al. (2023) |
| Bufonis Venenum | Bufalin, Cinobufagin | Steroidal Lactones | Cinobufagin ≥6.0% | Antitumor, Cardiotonic | Inhibits Na+/K+-ATPase; Activates JNK apoptotic pathway | Chen et al. (2023) |
Characteristic metabolite of herbs used.
Collectively, these two tables form the core output of the scientific definition, component standardization, and taxonomic validation of SETCM therapy interventions in this study, providing a reliable, consistent foundation for subsequent analyses (such as efficacy evaluation and mechanistic exploration).
3.3 Quality assessment (risk of bias) of included studies
All 25 included studies (Heinrich et al., 2018; Guyatt et al., 2011; Tang and Liu, 2024; Yu and Wu, 2024; Wang and Sun, 2023; Zhu et al., 2023; Yu and Qiu, 2023; Wang FQ. et al., 2023; Xu, 2022; Shi et al., 2022; Zhou et al., 2022; Jiao, 2022; Wu et al., 2021; Dong et al., 2021; Shao and Chen, 2020; Zheng et al., 2019; Zhou et al., 2018; Luo et al., 2018; Ma et al., 2021; Xu and Zhao, 2016; Que and Bian, 2016; Lai, 2015; Lin et al., 2014; Zhao et al., 2012; Zhao et al., 2008) were published randomized controlled trials (RCTs) involving patients with PSD. The risk of bias across seven domains was rigorously assessed using the Cochrane RoB 1.0 tool, with complete study-level judgments detailed in Table 5. The risk of bias assessment was as follows: Randomization: Five studies (Ma et al., 2021; Lai, 2015; Lin et al., 2014; Zhao et al., 2012; Zhao et al., 2008) did not mention a specific randomization scheme (Unclear risk), while the remainder described randomization methods (Low risk). Allocation concealment: No studies provided sufficient information (Unclear risk). Blinding: Four studies (Wu et al., 2021; Luo et al., 2018; Lai, 2015; Zhao et al., 2012) did not implement blinding (High risk); One study (Tang and Liu, 2024) implemented blinding (Low risk); the remainder were Unclear risk. Attrition bias: All studies reported no dropouts (Low risk). Reporting bias: No selective reporting was detected (Low risk). Other bias: Insufficient data (Unclear risk). See Figures 2, 3. Although the 25 included RCTs collectively showed positive efficacy signals, significant methodological limitations existed: Allocation concealment was inadequately described in all studies (100% “Unclear risk”); Blinding of participants and personnel was High risk in 16% of studies (4/25); Blinding of outcome assessment was Unclear risk in 92% of studies (23/25). These deficiencies, particularly the lack of allocation concealment and blinding, may introduce selection bias and performance/detection bias, potentially inflating the observed treatment effects.
TABLE 5
| Author(s) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
|
Tang and Liu (2024)
Heinrich et al. (2018) |
Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Yu and Wu (2024) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Wang and Sun (2023) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Zhu et al. (2023) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Yu and Qiu (2023) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Wang et al. (2023b) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Xu (2022) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Shi et al. (2022) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Zhou et al. (2022) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Jiao (2022) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Wu et al. (2021) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Dong et al. (2021) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Shao and Chen (2020) | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Zheng et al. (2019) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
|
Zhou et al. (2018)
Shao and Chen (2020) |
Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Luo et al. (2018) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Ma et al. (2021) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Xu and Zhao (2016) | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Que and Bian (2016) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
|
Lai (2015)
Xu and Zhao (2016) |
Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Lin et al. (2014) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Zhao et al. (2012) | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Zhao et al. (2008) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Huang (2007) | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Yuan et al. (2024) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
Bias risk assessment table.
FIGURE 2
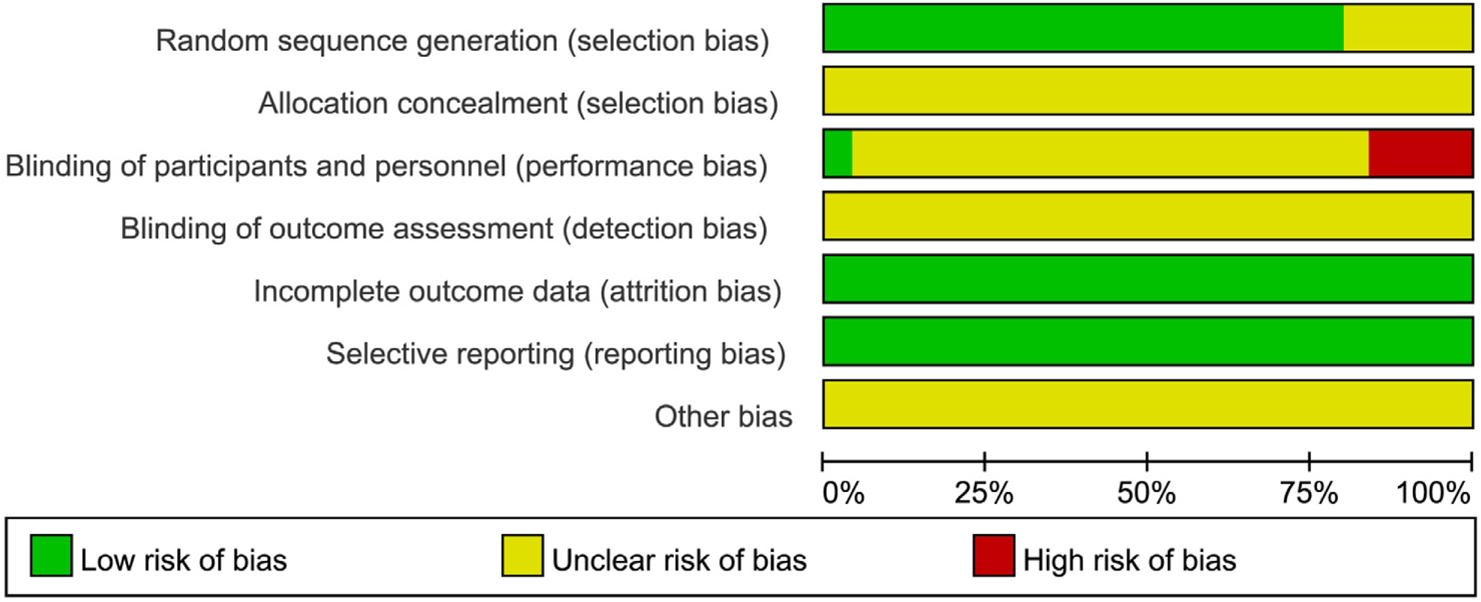
Results of the risk of bias assessment of the included literature.
FIGURE 3
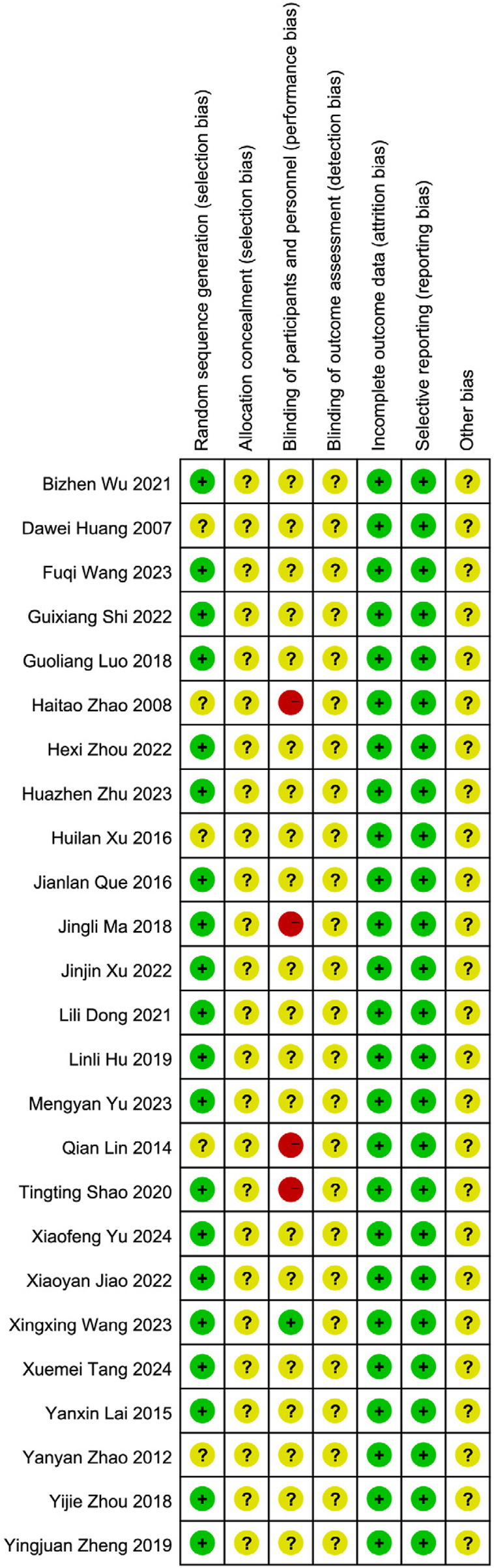
Summary of the risk of bias of the included literature.
3.4 Safety monitoring and adverse event reporting
This study systematically extracted and re-evaluated all reported adverse events (AEs) and serious adverse events (SAEs) from the included primary studies (n = 25 RCTs). Safety data were categorized and graded according to the US National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0). The specific process was as follows.
3.4.1 AE/SAE extraction and verification
Two researchers independently extracted explicitly reported AEs/SAEs from each study, including event type, number of occurrences, severity, causality relationship to the study intervention, and management measures. For vaguely described events (e.g., “local discomfort”), the original authors were contacted for clarification, and if details were unobtainable, the event was marked as “Unspecified.”
3.4.2 CTCAE v5.0 standardized classification
All AEs were classified by organ system and graded for severity into five levels:
Grade 1 (Mild): Asymptomatic or mild symptoms; intervention not indicated. Grade 2 (Moderate): Minimal, local, or noninvasive intervention indicated. Grade 3 (Severe): Hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living (ADL). Grade 4 (Life-threatening): Urgent intervention indicated. Grade 5 (Death): Death related to AE.
3.4.3 Safety analysis results
Only three studies (12% of included studies) reported AEs. All reported events were local skin reactions, with a cumulative occurrence of 24 cases (Total sample size N = 2,159), yielding an overall incidence rate of 2.1%. The detailed classification is as follows (Table 6).
TABLE 6
| Organ system | Type of AE | CTCAE terminology | Severity | Number of occurrences | Source of study | Did it lead to the interruption of the intervention |
|---|---|---|---|---|---|---|
| Skin and subcutaneous tissues | Localized erythema | Rash maculo-papular | Grade 1 | 12 | Yu Mengyan, 2023 | No |
| Skin and subcutaneous tissues | Pruritus | Pruritus | Grade 1 | 8 | Shao Tingting, 2020 | No |
| Skin and subcutaneous tissues | Contact dermatitis | Dermatitis contact | Grade 2 | 4 | Zhu Huazhen, 2023 | Yes (2 cases with patch suspension) |
Adverse events associated with external herbal treatment method (classified according to CTCAE v5.0).
Key Findings: The only reported AE type: Skin and subcutaneous tissue disorders (100%). Severity distribution:
Grade 1 (83.3%, n = 20): Transient erythema/pruritus; resolved spontaneously without intervention.
Grade 2 (16.7%, n = 4): Contact dermatitis; required temporary intervention suspension and topical corticosteroid application.
No SAEs reported: No Grade 3–5 events or systemic reactions (e.g., allergy, hepatic/renal dysfunction) occurred.
3.4.4 Evidence limitations
Incomplete reporting: 88% of studies (22/25) failed to describe AE monitoring procedures or explicitly state “no adverse reactions observed.”
Unclear causality: No studies provided laboratory-confirmed causal links (e.g., patch testing) between AEs and specific herbal constituents/excipients.
Lack of long-term safety data: All studies omitted follow-up for delayed reactions (>4 weeks post-intervention). Most (88%) studies lacked prospective safety monitoring protocols, precluding definitive conclusions about long-term tolerability.
3.5 Meta-analysis results
3.5.1 Meta-analysis of overall response rate
Nineteen studies (Yu and Wu, 2024; Zhu et al., 2023; Wang FQ. et al., 2023; Shi et al., 2022; Zhou et al., 2022; Wu et al., 2021; Dong et al., 2021; Shao and Chen, 2020; Zheng et al., 2019; Zhou et al., 2018; Luo et al., 2018; Ma et al., 2021; Xu and Zhao, 2016; Que and Bian, 2016; Lai, 2015; Lin et al., 2014; Zhao et al., 2012; Zhao et al., 2008; Huang, 2007) reported the post-treatment clinical overall response rate, involving 1,766 patients (SETCM therapies group: n = 884, effective cases = 799; Control group: n = 882, effective cases = 659). Heterogeneity testing (I2 = 0%, P = 0.87) indicated homogeneity among the selected studies, warranting the use of a fixed-effects model (FEM) for meta-analysis. The analysis revealed a statistically significant difference in the overall response rate between the SETCM therapies group and the control group [OR = 3.28, 95% CI: (2.49, 4.31), P < 0.00001]. This indicates that the application of SETCM therapies significantly improved the clinical overall response rate compared to the control group (Figure 4). Sensitivity analysis, excluding three studies (Shao and Chen, 2020; Xu and Zhao, 2016; Huang, 2007) rated as high risk for performance bias (blinding of participants), showed that the direction and statistical significance of the pooled effect estimate remained materially unchanged [OR = 3.09, 95% CI: (2.26, 4.21), P < 0.00001], demonstrating the robustness of the meta-analysis results (Figure 5).
FIGURE 4
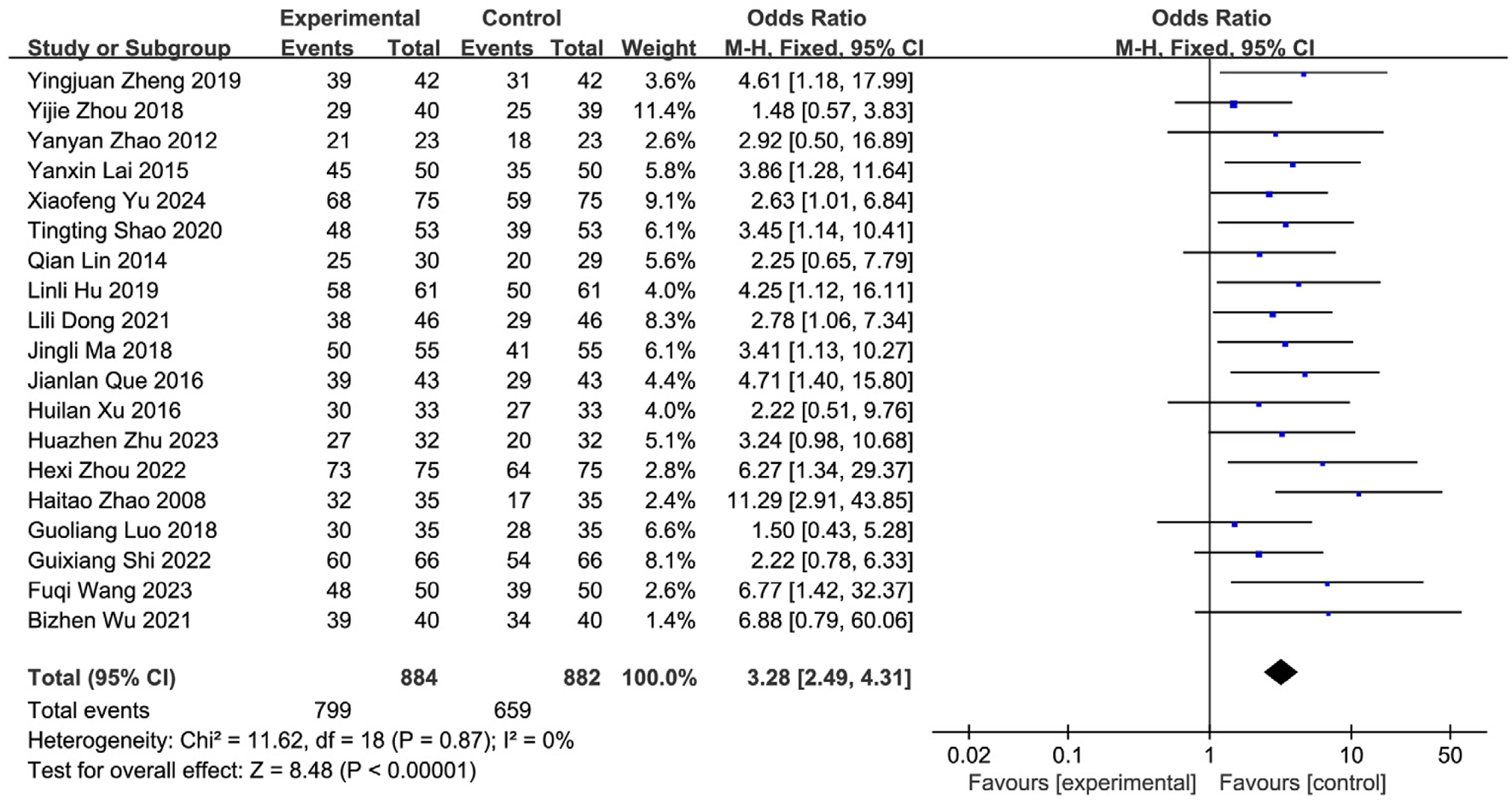
Meta-analysis of the total clinical effectiveness rate of the 19 studies of SETCM therapies for PSD included.
FIGURE 5
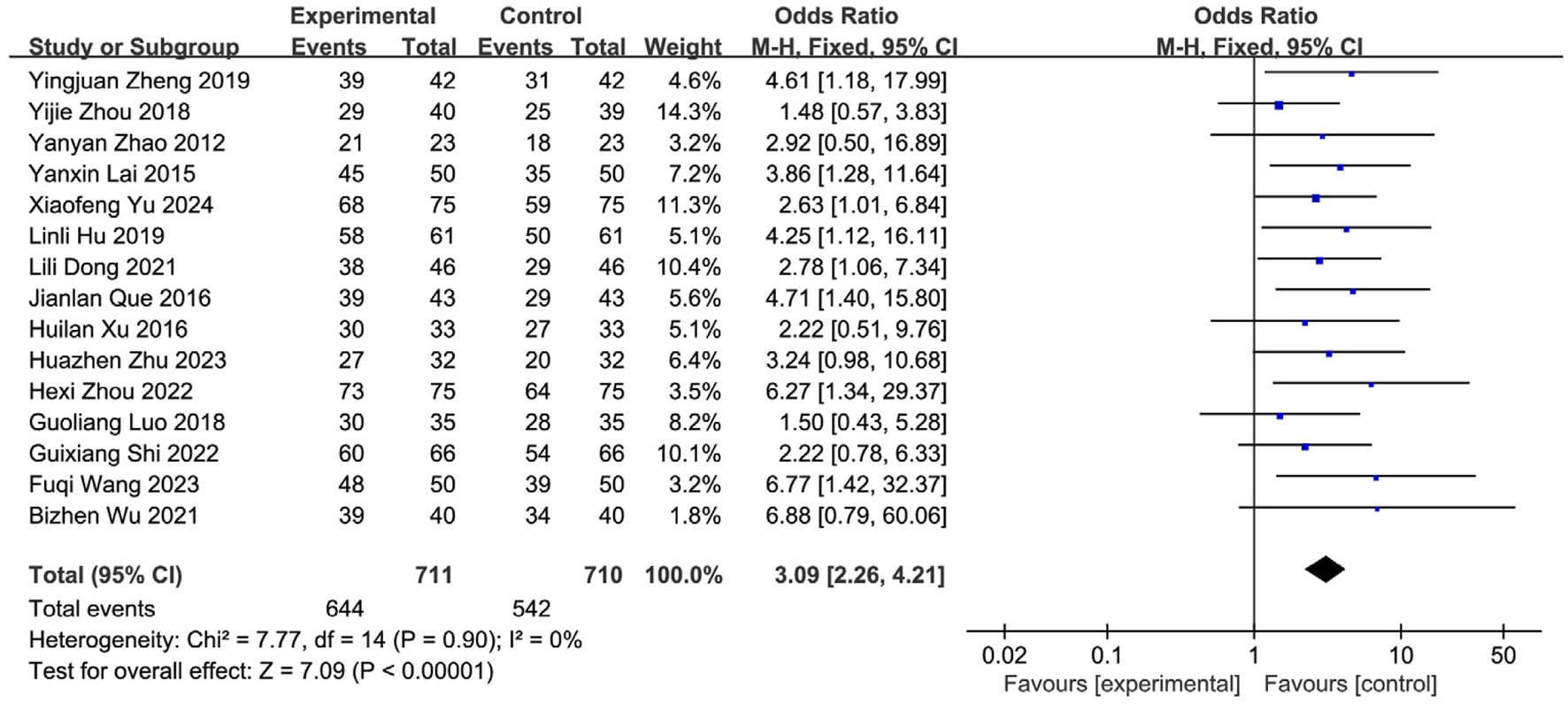
Sensitivity analysis of the total clinical effectiveness rate of the 15 studies of SETCM therapies for PSD included.
3.5.2 Meta-analysis of cure rate
Fourteen studies (Yu and Wu, 2024; Zhu et al., 2023; Wang FQ. et al., 2023; Shi et al., 2022; Zhou et al., 2022; Shao and Chen, 2020; Zheng et al., 2019; Zhou et al., 2018; Ma et al., 2021; Xu and Zhao, 2016; Que and Bian, 2016; Lai, 2015; Zhao et al., 2012; Huang, 2007) reporting the post-treatment clinical cure rate were included, involving 1,369 patients (SETCM therapies group: n = 685, cured cases = 272; Control group: n = 684, cured cases = 158). Heterogeneity testing (I2 = 0%, P = 0.89) indicated homogeneity among the selected studies, warranting the use of an FEM for meta-analysis. The analysis revealed a statistically significant difference in the cure rate between the SETCM therapies group and the control group [OR = 2.36, 95% CI: (1.84, 3.02), P < 0.00001]. This indicates that the application of SETCM therapies significantly improved the clinical cure rate compared to the control group (Figure 6).
FIGURE 6
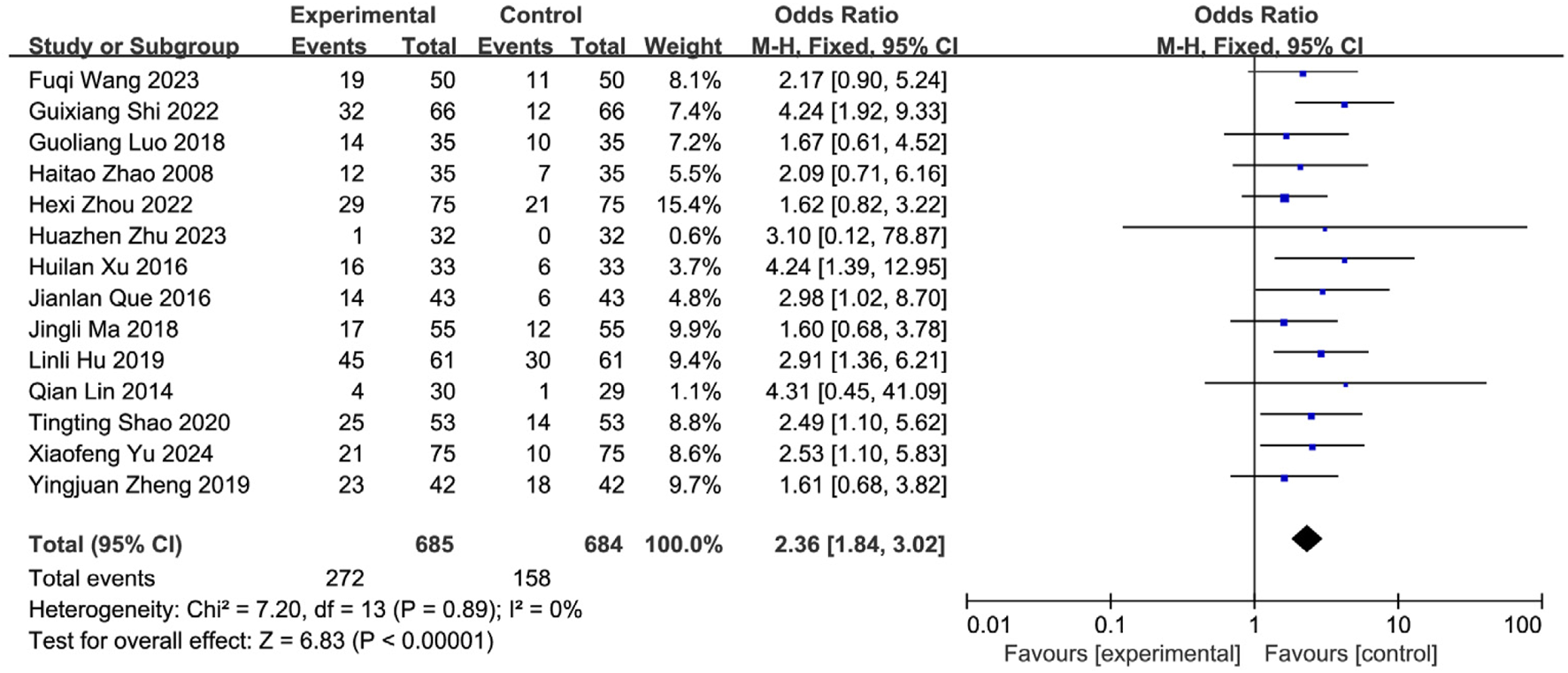
Meta-analysis of the cure rate of the 14 included studies of SETCM therapies for PSD.
3.5.3 Meta-analysis of water swallowing test (WST) scores
Ten studies (Wang and Sun, 2023; Wang FQ. et al., 2023; Jiao, 2022; Shao and Chen, 2020; Zheng et al., 2019; Zhou et al., 2018; Luo et al., 2018; Xu and Zhao, 2016; Que and Bian, 2016; Lai, 2015) reported post-treatment WST scores, involving 947 patients (SETCM therapies group: n = 474; Control group: n = 473). Overall heterogeneity testing indicated substantial heterogeneity (I2 = 98%, P < 0.00001). Sequential removal of individual studies failed to significantly reduce heterogeneity, suggesting intervention modality as a primary source. A random-effects model (REM) was employed for meta-analysis. Results demonstrated that SETCM therapies significantly reduced WST scores compared to controls [MD = −0.65, 95%CI: (−1.23, −0.06), P = 0.03] (Figure 7). High heterogeneity likely originated from variations in intervention type, duration, assessment timing, and combination therapies.
FIGURE 7
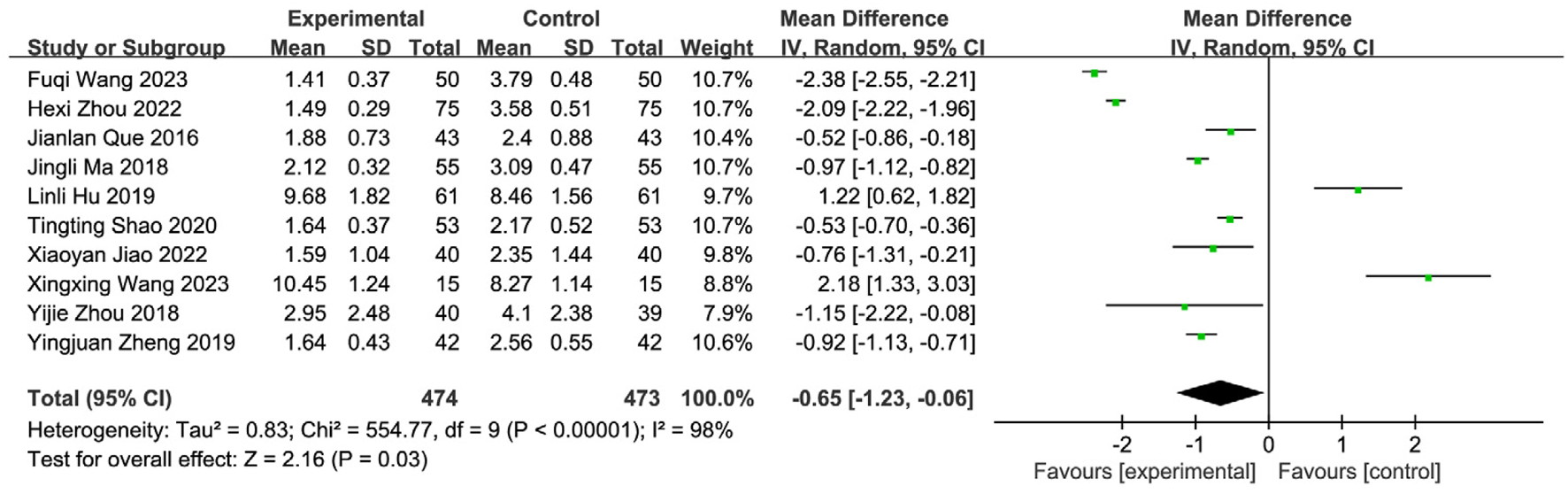
Meta-analysis of WST scores of 10 included studies of SETCM therapies for PSD.
Subgroup analysis by intervention type revealted significant residual heterogeneity (I2 = 89.8%, P = 0.002), necessitating REM. Results showed: Herbal patching: Significantly reduced WST scores [MD = −1.79, 95%CI: (−2.56, −1.02), P < 0.00001]. Medicated stick stimulation: Significantly reduced WST scores [MD = −0.54, 95%CI: (−0.69, −0.39), P < 0.00001] (Figure 8). While all subgroups demonstrated efficacy, unresolved heterogeneity suggests contributions from technical factors (operator skill, application site, session duration, intervention timing).
FIGURE 8
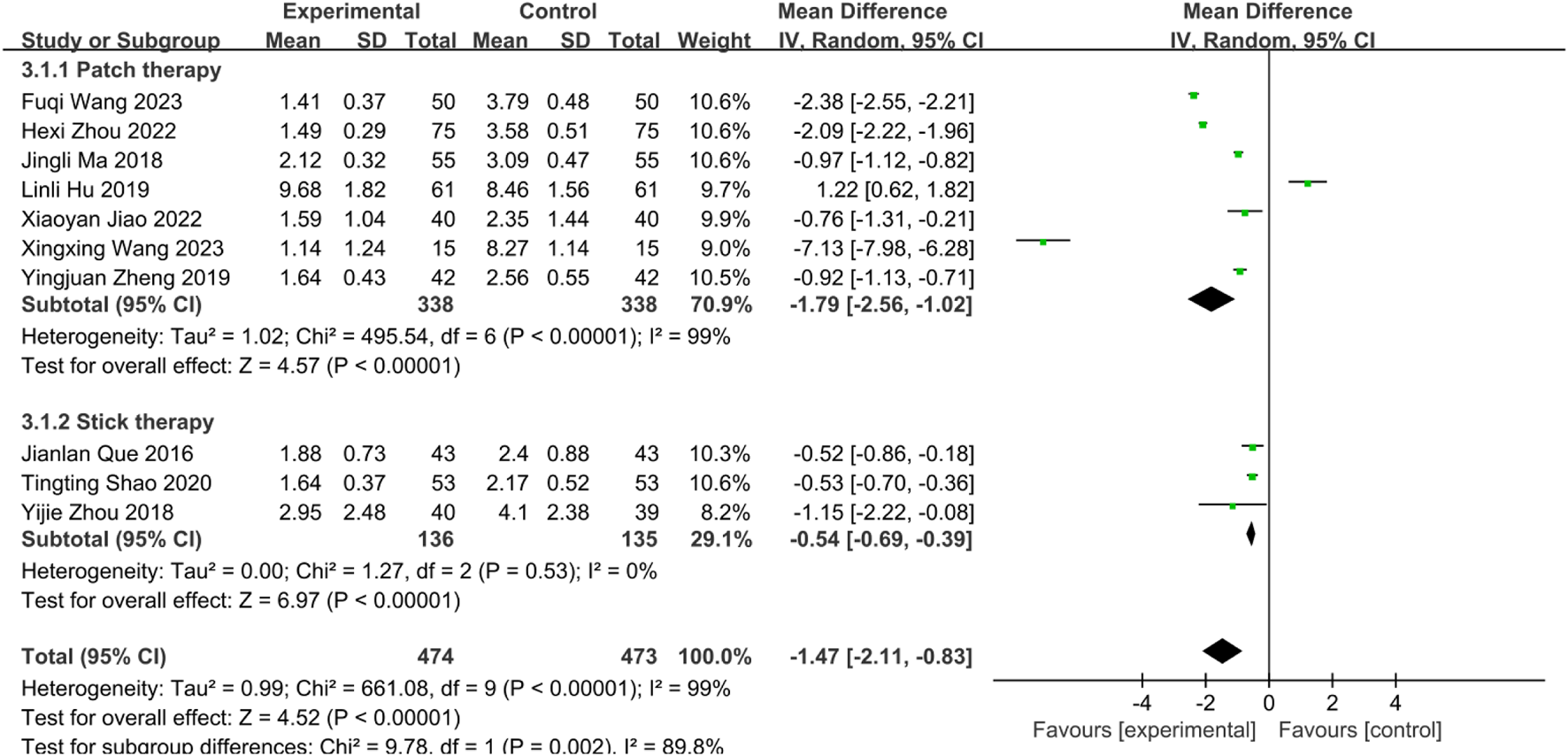
Subgroup analysis of WST scores of 10 included studies of SETCM therapies for PSD.
3.5.4 Meta-analysis of videofluoroscopic swallow study (VFSS) scores
Nine studies (Yu and Wu, 2024; Wang FQ. et al., 2023; Zhou et al., 2022; Shao and Chen, 2020; Zhou et al., 2018; Luo et al., 2018; Ma et al., 2021; Xu and Zhao, 2016; Yuan et al., 2024) involving 988 PSD patients (SETCM therapies: n = 494; Control: n = 494) evaluated swallowing function using VFSS scores. Random-effects meta-analysis indicated SETCM therapies significantly improved VFSS scores versus controls [MD = 2.07, 95%CI (1.72, 2.41), Z = 11.70, P < 0.00001], with substantial heterogeneity (I2 = 74%, P = 0.0001; Figure 9). Subgroup analysis by intervention type (Figure 10).
FIGURE 9
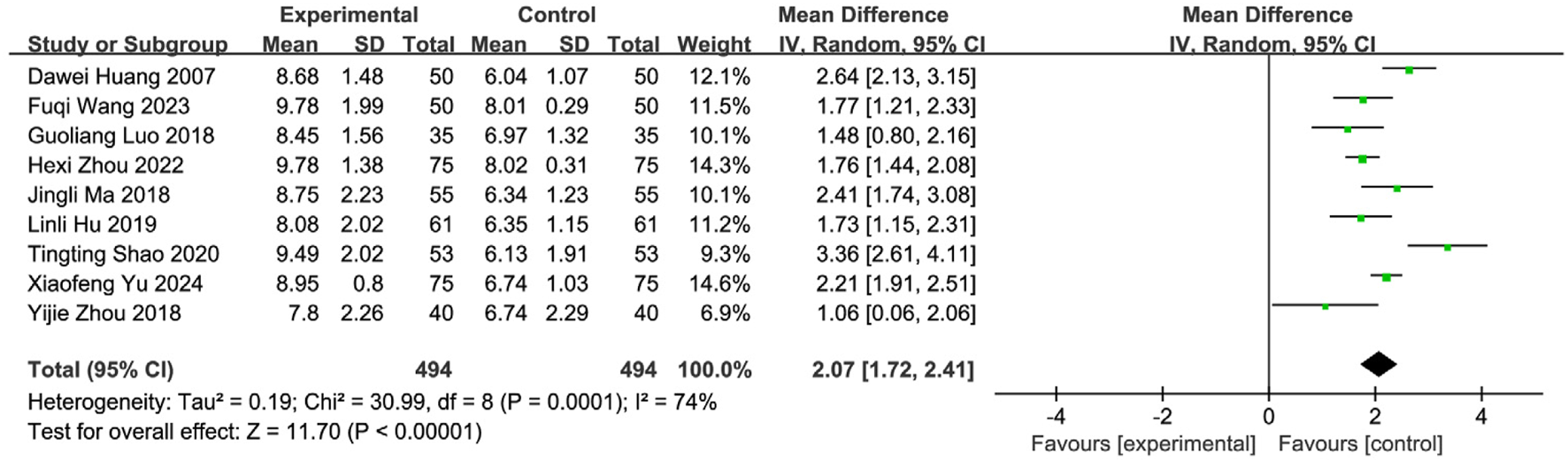
Meta-analysis of VFSS scores of nine included studies examining SETCM therapies for PSD.
FIGURE 10
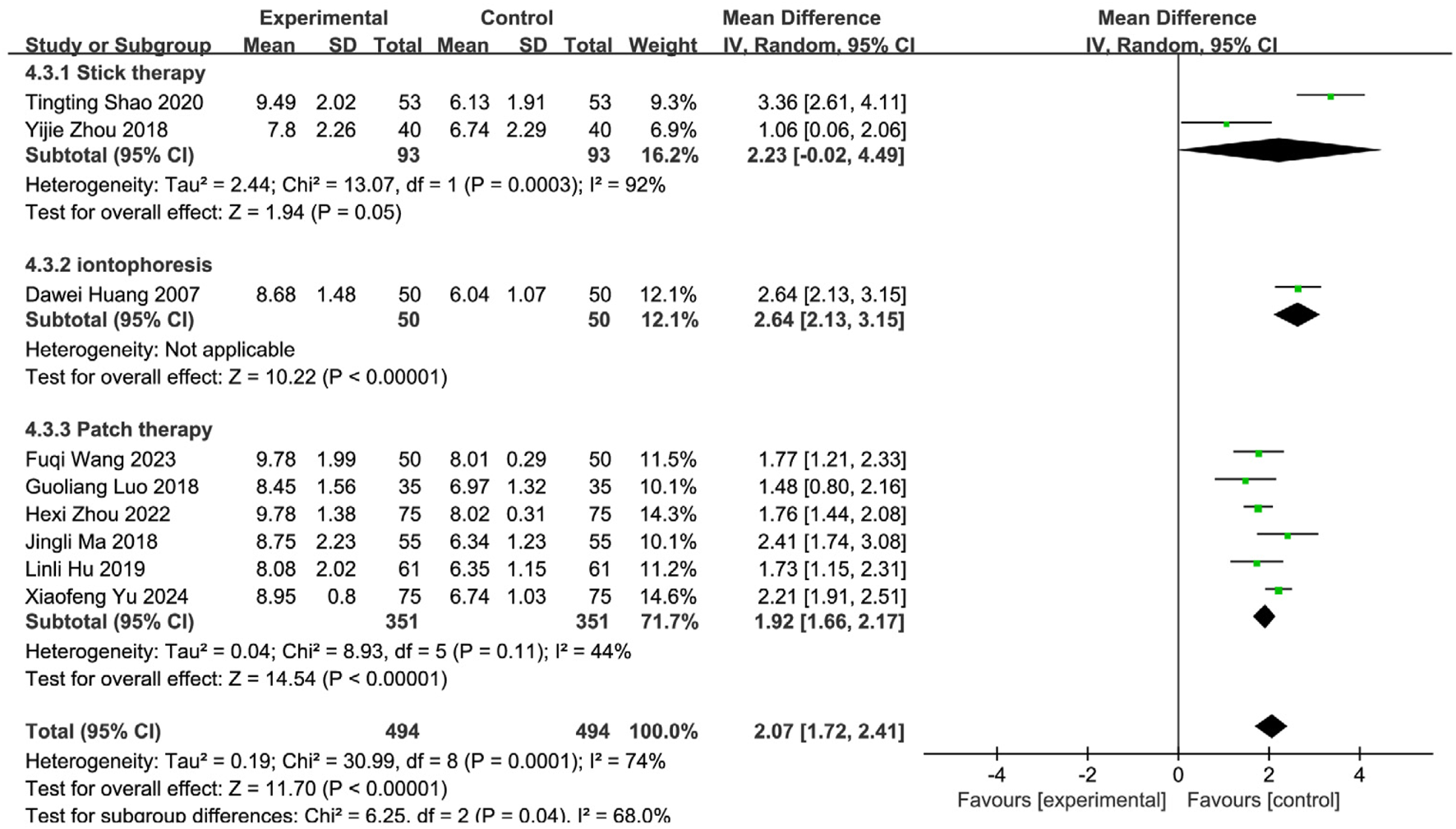
Subgroup analysis of VFSS scores of nine included studies of SETCM therapies for PSD.
Medicated stick (two studies): Moderate improvement [MD = 2.23, 95%CI (−0.02, 4.49)], high heterogeneity (I2 = 92%, P = 0.0003). Iontophoresis (one study): Significant improvement [MD = 2.64, 95%CI (2.13, 3.15)]. Herbal patching (six studies): Consistent improvement [MD = 1.92, 95%CI (1.66, 2.17)], moderate heterogeneity (I2 = 44%, P = 0.11). Subgroup differences were significant (P = 0.04, I2 = 68.0%), confirming that intervention type partially explained heterogeneity. Sensitivity analysis identified three influential studies (Yu and Wu, 2024; Shao and Chen, 2020; Yuan et al., 2024) (potential confounders: patient age, operator technique, treatment duration/timing). Their exclusion reduced heterogeneity (I2 = 19%, P = 0.29). Fixed-effects analysis affirmed robust efficacy [MD = 1.75, 95%CI (1.55, 1.98), Z = 15.95, P < 0.00001; Figure 11]. SETCM therapies significantly enhanced VFSS scores and accelerated swallowing recovery.
FIGURE 11
![Forest plot showing mean differences between experimental and control groups across six studies. Each study lists means, standard deviations, and sample sizes. Confidence intervals are indicated with horizontal lines. The overall effect size is 1.76, favoring the experimental group, with a 95% confidence interval of [1.55, 1.98]. The plot indicates low heterogeneity with an I-squared value of 19%.](https://www.frontiersin.org/files/Articles/1635090/xml-images/fphar-16-1635090-g011.webp)
SETCM therapy sensitivity analysis of VFSS scores of six included studies of the treatment of PSD.
3.5.5 Meta-analysis of SWAL-QOL scores
Six studies (Yu and Wu, 2024; Wang and Sun, 2023; Jiao, 2022; Zhou et al., 2018; Xu and Zhao, 2016; Zhao et al., 2012) reported SWAL-QOL scores, involving 551 patients (SETCM therapies: n = 275; Control: n = 276). Heterogeneity testing (I2 = 29%, P = 0.22) indicated homogeneity, supporting an FEM. Meta-analysis demonstrated a statistically significant improvement in the SETCM therapies group [MD = 25.61, 95%CI (20.54, 30.67), Z = 9.91, P < 0.00001], indicating SETCM therapies significantly reduced SWAL-QOL scores (i.e., improved quality of life) (Figure 12).
FIGURE 12
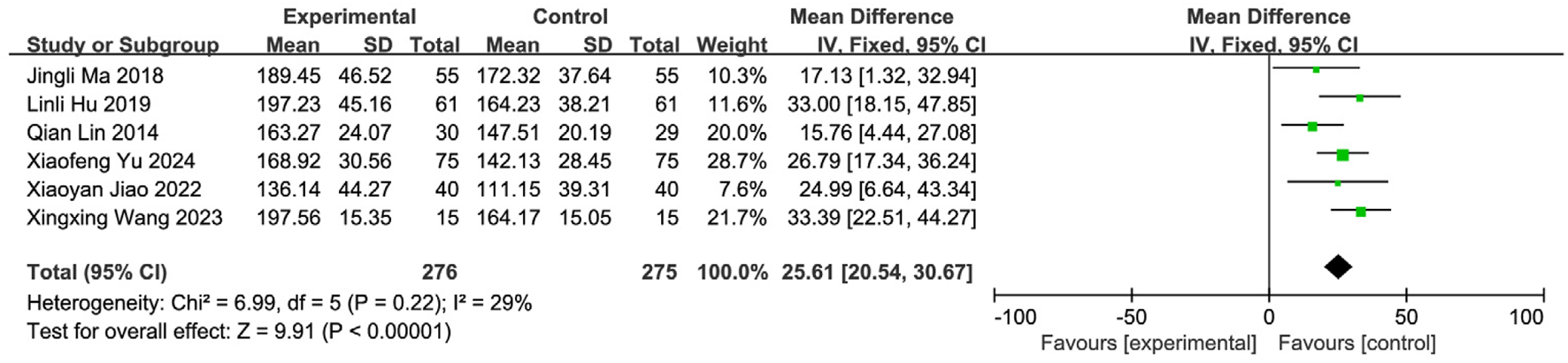
Meta-analysis of SWAL-QOL scores of six included studies of the treatment of PSD using SETCM therapies.
3.5.6 Meta-analysis of post-intervention aspiration
Three studies (Tang and Liu, 2024; Yu and Qiu, 2023; Jiao, 2022) reported aspiration events, involving 228 patients (SETCM therapies: n = 114, aspiration = 6; Control: n = 114, aspiration = 20). Homogeneity was confirmed (I2 = 0%, P = 0.87), warranting an FEM. SETCM therapies significantly reduced aspiration risk [OR = 0.21, 95%CI (0.08, 0.59), Z = 2.96, P = 0.003] (Figure 13).
FIGURE 13
![Forest plot displaying the odds ratios from three studies comparing experimental and control groups. The studies are Mengyan Yu 2023, Xiaoyan Jiao 2022, and Xuemei Tang 2024. Each study shows events and weights. The studies cumulatively favor the experimental group with an overall odds ratio of 0.21, 95% CI [0.08, 0.59]. Heterogeneity is minimal, and the overall effect is significant.](https://www.frontiersin.org/files/Articles/1635090/xml-images/fphar-16-1635090-g013.webp)
Meta-analysis of misabsorption rates of three included studies on the treatment of PSD by SETCM therapies.
3.5.7 Supplementary meta-analyses: Activities of daily living
3.5.7.1 MBI score meta-analysis
Two studies (Wang and Sun, 2023; Zhou et al., 2018) reported MBI scores (n = 152; SETCM therapies:76, Control:76). Homogeneity (I2 = 0%, P = 0.72) supported FEM. SETCM therapies significantly improved MBI scores [MD = 12.71, 95%CI (9.68, 15.73), Z = 8.23, P < 0.00001], indicating enhanced daily living independence (Figure 14).
FIGURE 14
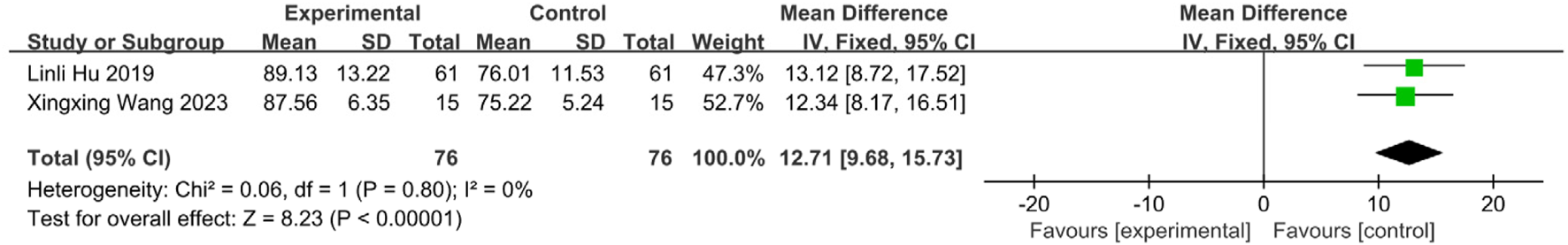
Meta-analysis of MBI scores of two included studies on the treatment of PSD by SETCM therapies.
3.5.7.2 ADL score meta-analysis
Two studies (Tang and Liu, 2024; Shi et al., 2022) reported ADL scores (n = 200; SETCM therapies:100, Control:100). Homogeneity (I2 = 12%, P = 0.29) supported FEM. SETCM therapies significantly improved ADL scores [MD = 16.57, 95%CI (12.16, 20.98), Z = 7.36, P < 0.00001], confirming better self-care capacity (Figure 15).
FIGURE 15

Meta-analysis of two included studies reporting ADL scores for treatment of PSD with SETCM therapies.
3.5.8 Meta-analysis of serum albumin (ALB) levels
Four studies (Wang and Sun, 2023; Wu et al., 2021; Dong et al., 2021; Zhou et al., 2018) involving 324 patients (intervention: n = 162; control: n = 162) reported serum albumin (ALB) levels. Homogeneity was confirmed (I2 = 0%, P = 0.72), and an FEM was applied. Results demonstrated significantly higher ALB levels in the intervention group than in the controls [MD = 4.67, 95% CI (2.86, 6.47), Z = 5.07, P < 0.00001], indicating that SETCM therapies increase serum albumin concentrations in patients with PSD (Figure 16).
FIGURE 16
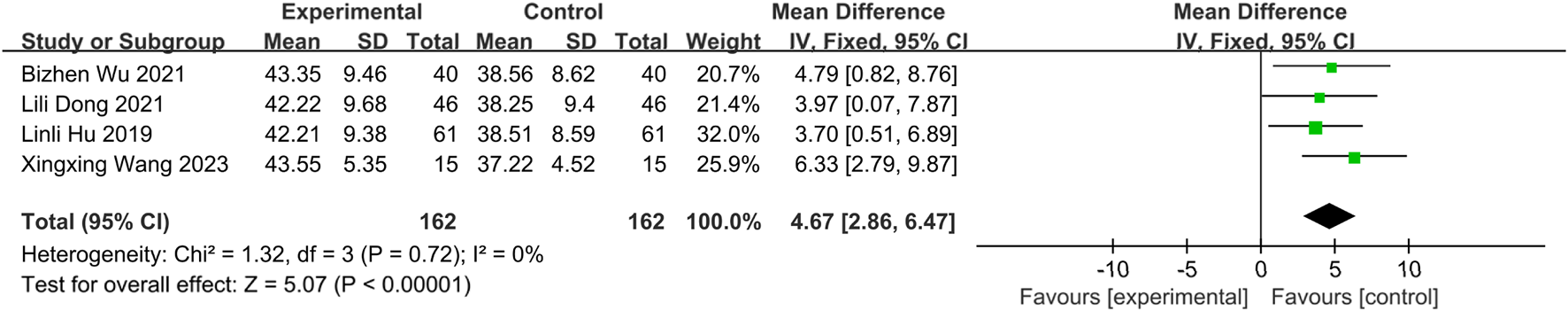
Meta-analysis of four included studies of ALB levels for SETCM therapy treatment of PSD.
3.5.9 Meta-analysis of prealbumin (PA) levels
Three studies (Wang and Sun, 2023; Dong et al., 2021; Zhou et al., 2018) involving 244 patients (intervention: n = 122; control: n = 122) reported prealbumin (PA) levels. Significant heterogeneity was detected (I2 = 64%, P = 0.06), necessitating a random-effects model (REM) (Figure 17). Sensitivity analysis identified Wang Xingxing’s study (Wang and Sun, 2023) as the source of heterogeneity; its exclusion resulted in homogeneity (I2 = 0%, P = 0.69). The observed heterogeneity likely originated from the relatively small sample size in Wang and Sun (2023), which may compromise statistical power. An FEM was applied to the remaining two homogeneous studies. Results showed a statistically significant increase in PA levels for the intervention group [MD = 22.10, 95% CI (10.65, 33.54), Z = 3.78, P = 0.0002], demonstrating that SETCM therapies elevate prealbumin levels in PSD patients (Figure 18).
FIGURE 17
![Forest plot showing the mean differences between experimental and control groups in three studies by Lili Dong 2021, Linli Hu 2019, and Xingxing Wang 2023. The mean differences and 95% confidence intervals are presented for each study, with a pooled estimate at the bottom: 30.75 [12.76, 48.74]. The plot indicates heterogeneity with Tau squared equals 159.21, Chi squared equals 5.51, degrees of freedom equals 2, P equals 0.06, and I squared equals 64%. A test for overall effect shows Z equals 3.35, P equals 0.0008, favoring the experimental intervention.](https://www.frontiersin.org/files/Articles/1635090/xml-images/fphar-16-1635090-g017.webp)
Meta-analysis of three included studies on PA levels during SETCM therapy treatment of PSD.
FIGURE 18
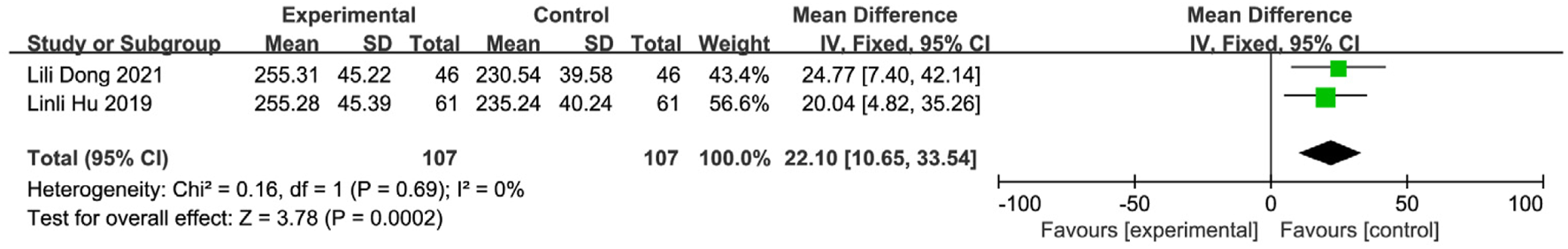
Sensitivity analysis of two included studies on PA levels during SETCM therapy treatment of PSD.
3.5.10 Meta-analysis of NRS2002 scores
Two studies (Wu et al., 2021; Zhou et al., 2018) involving 202 patients (intervention: n = 101; control: n = 101) reported Nutritional Risk Screening 2002 (NRS 2002) scores. Homogeneity was confirmed (I2 = 0%, P = 0.54), supporting an FEM. Results revealed a statistically significant reduction in nutritional risk scores for the intervention group [MD = −0.28, 95% CI (−0.42, −0.14), Z = 3.85, P = 0.0001], suggesting that SETCM therapies mitigate malnutrition risk in PSD patients (Figure 19).
FIGURE 19
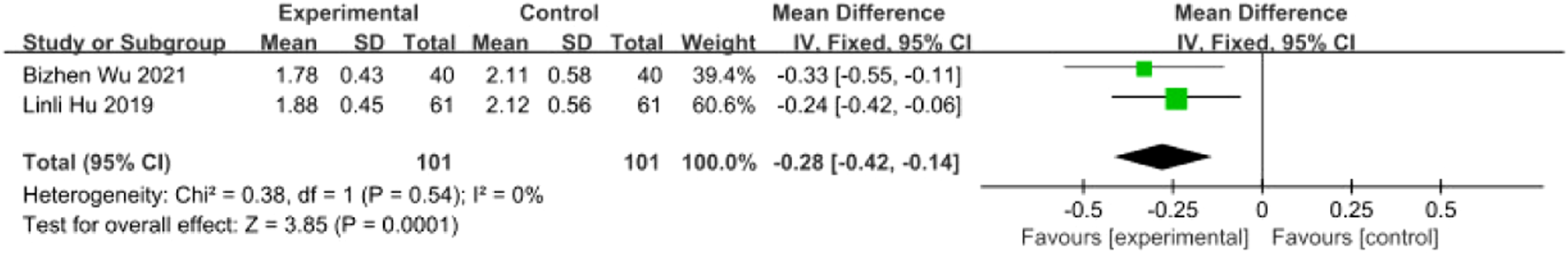
Meta-analysis of two included studies of SETCM therapy treatment of PSD, Meta-analysis of literature NRS2002 scores.
3.5.11 Meta-analysis of GUSS scores
Four studies (Tang and Liu, 2024; Yu and Qiu, 2023; Xu, 2022; Shao and Chen, 2020) involving 374 patients (intervention: n = 187; control: n = 187) reported GUSS scores. Heterogeneity testing indicated substantial variability among studies (I2 = 71%, P = 0.01), warranting a random-effects model (REM) for meta-analysis (Figure 20). Sensitivity analysis via sequential exclusion revealed that removal of Shao Tingting’s study (Shao and Chen, 2020) significantly reduced heterogeneity (I2 = 0%, P = 0.74). This observed heterogeneity likely stemmed from divergent intervention protocols in Shao and Chen (2020), which increased the dispersion of effect estimates. The remaining three homogeneous studies were analyzed using an FEM. Results demonstrated a statistically significant improvement in GUSS scores for the intervention group [MD = 2.39, 95% CI (1.89, 2.88), Z = 9.44, P < 0.00001], indicating that SETCM therapies significantly enhance swallowing function in PSD patients (Figure 21).
FIGURE 20
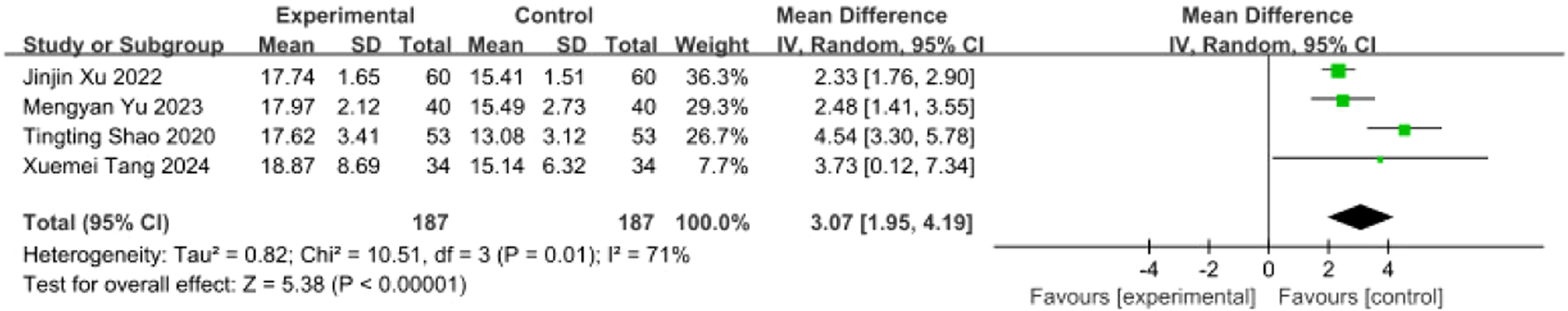
Meta-analysis of GUSS scores from four included studies of SETCM therapies for PSD.
FIGURE 21
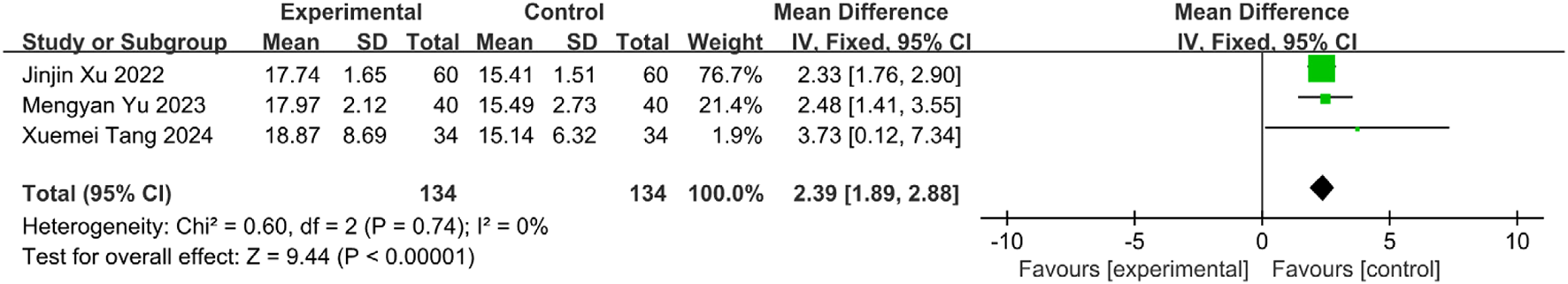
Sensitivity analysis of GUSS scores from three included studies of SETCM therapies for PSD.
3.5.12 Meta-analysis of enteral nutrition tube duration
Three studies (Tang and Liu, 2024; Yu and Qiu, 2023; Xu, 2022) involving 268 patients (intervention: n = 134; control: n = 134) reported enteral nutrition tube duration. Heterogeneity testing confirmed homogeneity (I2 = 0%, P = 0.72), supporting an FEM. Results showed a statistically significant reduction in tube duration for the intervention group [MD = −2.83, 95% CI (−3.25, −2.41), Z = 13.19, P < 0.00001], indicating that SETCM therapies shorten enteral nutrition dependency in PSD patients (Figure 22).
FIGURE 22
![Forest plot showing mean differences between experimental and control groups across three studies: Jinjin Xu 2022, Mengyan Yu 2023, and Xuemei Tang 2024. The plot demonstrates statistical significance favoring the experimental group, with a total mean difference of -2.83 and a 95% confidence interval of [-3.25, -2.41]. Heterogeneity is low with Chi² = 0.65, df = 2, P = 0.72, and I² = 0%. Overall effect significance is Z = 13.19, P < 0.00001.](https://www.frontiersin.org/files/Articles/1635090/xml-images/fphar-16-1635090-g022.webp)
Meta-analysis of gastric tube retention duration of three included studies of SETCM therapies for PSD.
3.5.13 Meta-analysis of tube removal success rate
Three studies (Tang and Liu, 2024; Yu and Qiu, 2023; Xu, 2022) involving 268 patients (intervention: n = 134; control: n = 134) reported tube removal success rates. Homogeneity was confirmed (I2 = 0%, P = 0.82), and an FEM was applied. Results demonstrated a statistically significant increase in success rates for the intervention group [OR = 4.56, 95% CI (2.44, 8.53), Z = 4.76, P < 0.00001], indicating that SETCM therapies improve enteral nutrition tube removal outcomes in PSD patients (Figure 23).
FIGURE 23
![Forest plot showing odds ratios for three studies: Jinjin Xu 2022, Mengyan Yu 2023, and Xuemei Tang 2024. The experimental groups had significantly higher events compared to control groups. Odds ratios with 95% confidence intervals for each study are displayed: Jinjin Xu 6.33 [1.73, 23.23], Mengyan Yu 3.81 [1.45, 10.02], Xuemei Tang 4.34 [1.49, 12.65]. The total odds ratio is 4.56 [2.44, 8.53]. Heterogeneity is low with Chi² = 0.39, I² = 0%. Overall effect test Z = 4.76, P < 0.00001.](https://www.frontiersin.org/files/Articles/1635090/xml-images/fphar-16-1635090-g023.webp)
Gastric tube removal success rates reported in three included studies on SETCM therapies for PSD. Meta-analysis of gastric tube removal success rates in the literature.
3.5.14 Meta-analysis of FDS scores
Two studies (Xu and Zhao, 2016; Zhao et al., 2008) involving 156 patients (intervention: n = 78; control: n = 78) reported functional dysphagia scale (FDS) scores. Heterogeneity testing confirmed homogeneity (I2 = 0%, P = 0.50), supporting an FEM. Results demonstrated statistically significant between-group differences [MD = 2.25, 95% CI (1.79, 2.72), Z = 9.51, P < 0.00001], indicating that SETCM therapies improve swallowing function in PSD patients (Figure 24).
FIGURE 24
![Forest plot showing the effect size of two studies, Jingli Ma 2018 and Yanyan Zhao 2012, comparing experimental and control groups. Mean differences with 95% confidence intervals are given: 2.07 [1.37, 2.77] and 2.39 [1.77, 3.01] respectively. The overall effect is 2.25 [1.79, 2.72]. Heterogeneity indicates Chi² = 0.45, df = 1 (P = 0.50); I² = 0%. The test for overall effect shows Z = 9.51 (P < 0.00001). Results favor the experimental group.](https://www.frontiersin.org/files/Articles/1635090/xml-images/fphar-16-1635090-g024.webp)
Meta-analysis of FDS dysphagia efficacy score reported by two included studies of SETCM therapies for PSD hemispheres.
3.5.15 Stratified efficacy analysis by stroke location and intervention modality
Considering potential effect variations across interventions and lesion locations, efficacy outcomes were stratified by stroke site (supratentorial vs. brainstem) and intervention type. Supratentorial subgroup analysis: Acupoint application showed significant efficacy: Pooled OR = 2.73 (95% CI: 1.65–4.52, P < 0.0001) with low heterogeneity (I2 = 46%, P = 0.10). Wang Fuqi (2023): OR = 6.77 [1.42, 32.37]; Yu Xiaofeng (2024): OR = 2.63 [1.01, 6.84] Medicated stick therapy demonstrated complementary efficacy: Shao Tingting (2020) OR = 3.45 (P = 0.03), with no statistical difference versus acupoint application (subgroup difference: P = 0.71). Overall effect: OR = 2.84 (95% CI: 1.80–4.50, P < 0.00001), supporting acupoint application as primary therapy for supratentorial lesions (Figure 25).
FIGURE 25
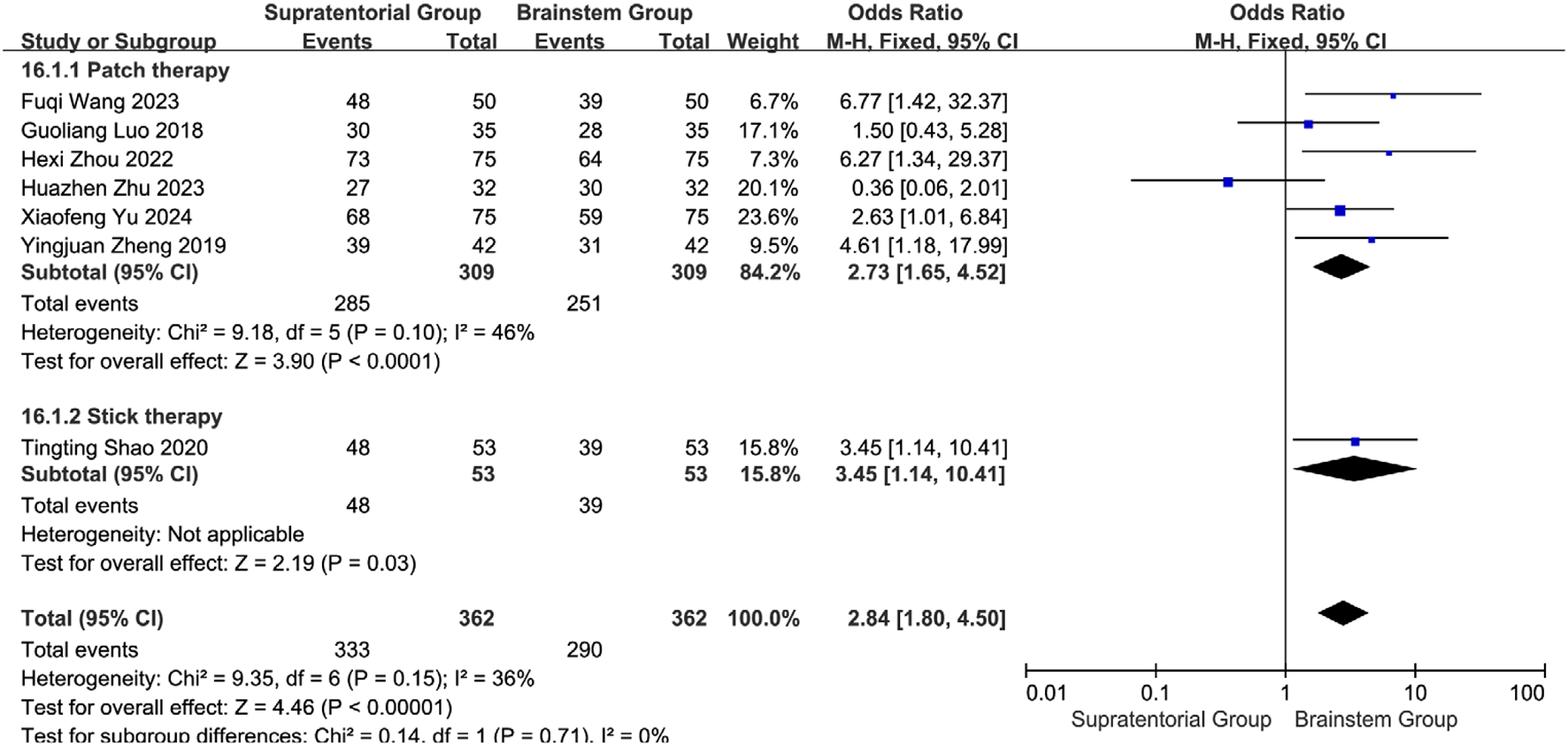
Subgroup analysis of the efficacy of different interventions in patients with stroke foci located in the cerebral hemispheres.
Brainstem subgroup analysis: Iontophoresis: OR = 11.29 (95% CI: 2.91–43.85, P = 0.0005); Acupoint application: OR = 1.73 (95% CI: 0.81–3.68, P = 0.16; I2 = 0%). Overall effect: Pooled OR = 3.12 (95% CI: 0.97–10.05, P = 0.06) with moderate heterogeneity (I2 = 66%). Subgroup comparison: Iontophoresis showed 6.52-fold superiority over acupoint application (RR = 6.52, 95% CI: 1.35–31.48; subgroup difference: P = 0.02, I2 = 82.2%), indicating its preferential use for brainstem lesions (Figure 26). Efficacy exhibited neuroanatomical specificity: Acupoints are preferred for cerebral hemisphere lesions, while herbal iontophoresis is the best choice for brainstem involvement. This confirms that there is no universal intervention for all PSD mechanisms and establishes the first evidence-based framework for personalized SETCM therapy application.
FIGURE 26
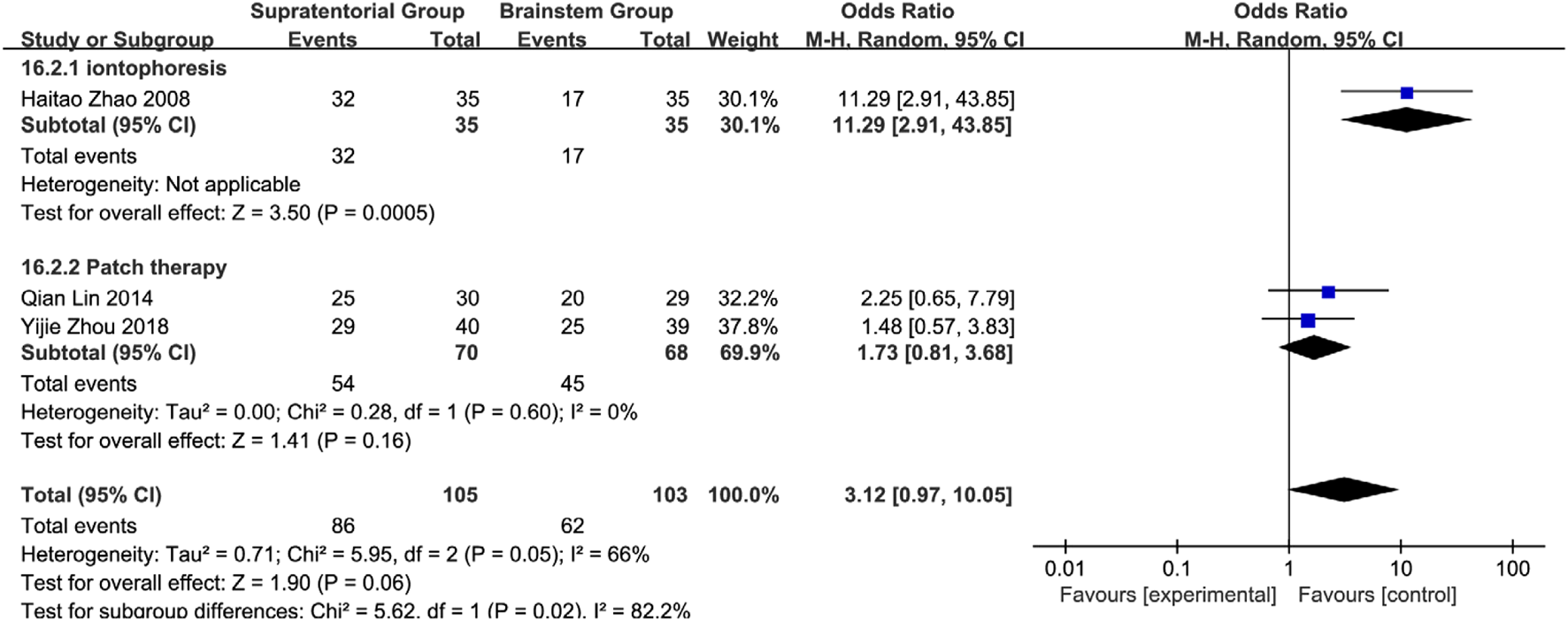
Subgroup analysis of the efficacy of different interventions in patients with stroke foci located in the brain stem.
3.6 Publication bias analysis
Funnel plot asymmetry (Figure 27) suggests potential publication bias, where smaller studies with non-significant results may be underrepresented. This could inflate the pooled effect estimate (e.g., overall response rate OR = 3.28) and limit its generalizability to real-world settings (actual treatment effects may be smaller). Although trim-and-fill analysis was precluded due to limited study numbers per outcome, the results should be interpreted as optimistic estimates.
FIGURE 27
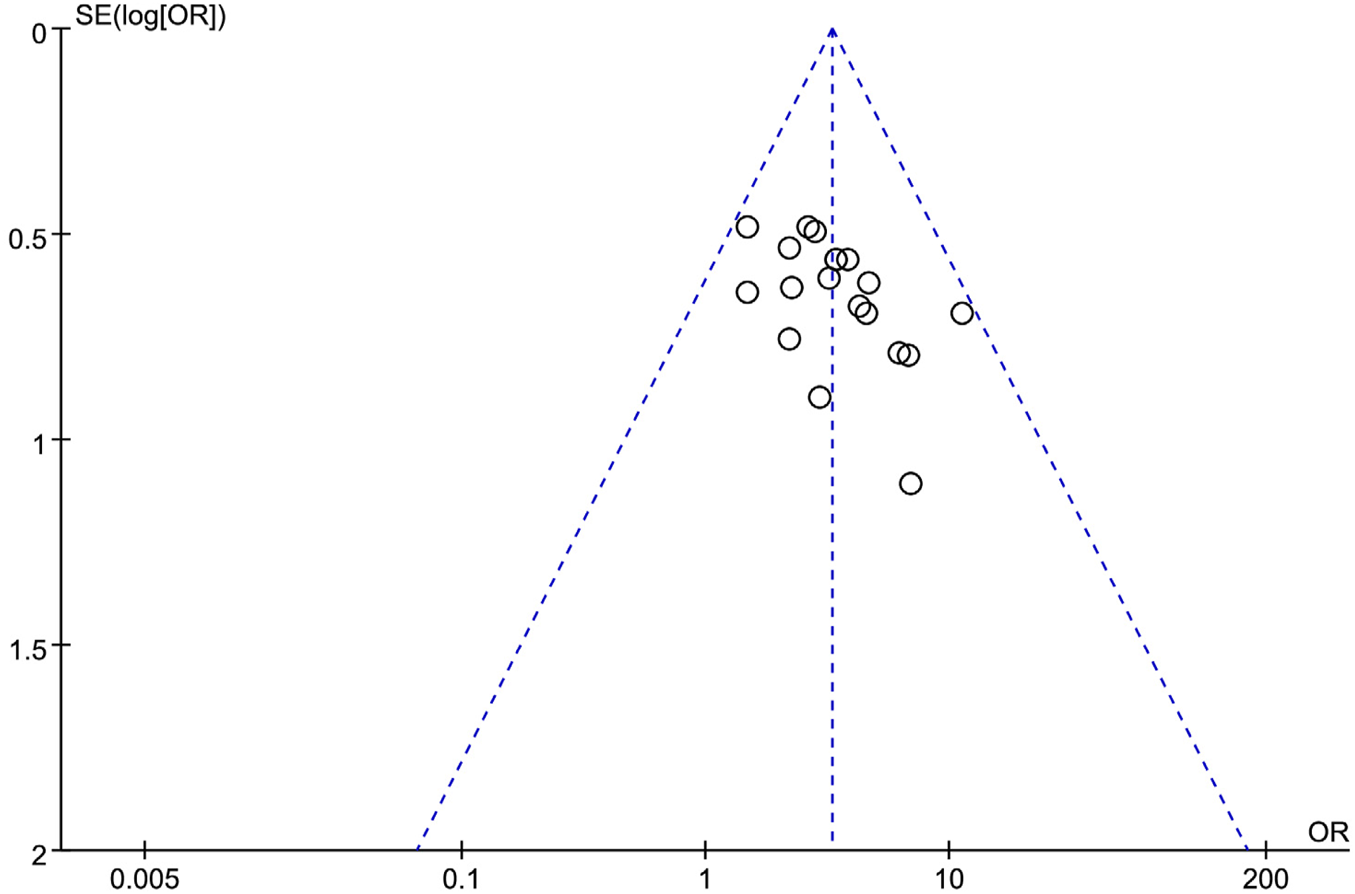
An inverted funnel plot of the effectiveness reported by 19 studies using SETCM therapies to treat PSD.
3.7 GRADE evidence quality
Using GRADEpro, evidence quality was rated as: Moderate: Overall response rate, Cure rate, SWAL-QOL scores. Low: VFSS scores, Aspiration rate, MBI, ALB, NRS 2002, ADL, Enteral tube duration, Tube removal success rate, FDS. Very low: WST scores, PA levels, GUSS scores. Downgrading factors included allocation concealment/blinding bias, substantial heterogeneity, and small sample sizes (Table 7). Bias risk was the primary downgrading factor for most outcomes (e.g., VFSS, WST, and GUSS).
TABLE 7
| Author(s): Jingwen Zhang Question: Traditional Chinese medicine external application compared to conventional treatment for post-stroke swallowing function Setting: Bibliography: Topical use of Chinese herbal medicine in the treatment of post-stroke dysphagia. Cochrane Database of Systematic Reviews [Year], Issue [Issue No.] |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Traditional Chinese medicine external application | Conventional treatment | Relative (95% CI) | Absolute (95% CI) | ||
| Overall response rate | ||||||||||||
| 19 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | none | 799/884 (90.4%) | 659/882 (74.7%) | OR 3.28 (2.49–4.31) | 159 more per 1,000 (from 133 more to 180 more) | ⊕⊕⊕○ Moderatea |
IMPORTANT |
| Cure rate | ||||||||||||
| 14 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 272/685 (39.7%) | 158/684 (23.1%) | OR 2.36 (1.84–3.02) | 184 more per 1,000 (from 125 more to 245 more) | ⊕⊕⊕○ Moderatea |
IMPORTANT |
| WST score | ||||||||||||
| 10 | Randomized trials | Seriousa | Very seriousb | Not serious | Not serious | None | 474 | 473 | - | MD 0.65 lower (1.23 lower to 0.06 lower) | ⊕○○○ Very lowa,b |
IMPORTANT |
| VFSS score | ||||||||||||
| 9 | Randomized trials | Seriousa | Seriousb | Not serious | Not serious | None | 494 | 494 | - | MD 2.07 higher (1.72 higher to 2.41 higher) | ⊕⊕○○ Lowa,b |
IMPORTANT |
| SWAL-QOL score | ||||||||||||
| 6 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 276 | 275 | - | MD 25.61 higher (20.54 higher to 30.67 higher) | ⊕⊕⊕○ Moderatea |
NOT IMPORTANT |
| Post-intervention aspiration rate | ||||||||||||
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 5/114 (4.4%) | 20/114 (17.5%) | OR 0.21 (0.08–0.59) | 133 fewer per 1,000 (from 159 fewer to 64 fewer) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
| MBI score | ||||||||||||
| 2 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 76 | 76 | - | MD 12.71 higher (9.68 higher to 15.73 higher) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
| Alb | ||||||||||||
| 4 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 162 | 162 | - | MD 4.67 higher (2.86 higher to 6.47 higher) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
| Pa | ||||||||||||
| 3 | Randomized trials | Seriousa | Seriousb | Not serious | Seriousc | None | 122 | 122 | - | MD 30.75 higher (12.76 higher to 48.74 higher) | ⊕○○○ Very lowa,b,c |
NOT IMPORTANT |
| NRS2002 score | ||||||||||||
| 2 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 101 | 101 | - | MD 0.28 lower (0.42 lower to 0.14 lower) | ⊕⊕○○ Lowa,c |
IMPORTANT |
| GUSS score | ||||||||||||
| 4 | Randomized trials | Seriousa | Seriousb | Not serious | Seriousc | None | 187 | 187 | - | MD 3.07 higher (1.95 higher to 4.19 higher) | ⊕○○○ Very lowa,b,c |
NOT IMPORTANT |
| ADL score | ||||||||||||
| 2 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 100 | 100 | - | MD 16.57 higher (12.16 higher to 20.98 higher) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
| Nasogastric tube indwelling duration | ||||||||||||
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 134 | 134 | - | MD 2.83 lower (3.25 lower to 2.41 lower) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
| Feeding tube removal success rate | ||||||||||||
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 96/134 (71.6%) | 61/134 (45.5%) | OR 4.56 (2.44–8.53) | 337 more per 1,000 (from 216 more to 422 more) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
| FDS score | ||||||||||||
| 2 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | None | 78 | 78 | - | MD 2.25 higher (1.79 higher to 2.72 higher) | ⊕⊕○○ Lowa,c |
NOT IMPORTANT |
Grading of quality of evidence of outcome metrics.
CI: confidence interval; MD: mean difference; OR: odds ratio.
Explanations.
Large bias in randomization, allocation concealment, and blinding in included studies.
Greater heterogeneity across studies.
Small sample size.
3.8 SETCM therapy safety analysis
Pooled safety data indicated a relatively favorable safety profile for SETCM therapies (primarily acupoint application) among limited studies reporting safety information. Mild local skin irritation was the main adverse reaction observed in three studies (Zhu et al., 2023; Yu and Qiu, 2023; Shao and Chen, 2020) (12% of included studies), easily managed with standard care. This aligns with transdermal delivery’s advantage in bypassing first-pass metabolism and gastrointestinal adverse effects, theoretically offering superior safety over oral herbs, which can be particularly beneficial for PSD patients with impaired gastrointestinal function or medication adherence issues. However, it must be strongly emphasized that 88% of included studies lacked systematic prospective safety monitoring and reporting. Consequently, robust evidence remains lacking regarding long-term SETCM therapy safety, tolerability in special populations (e.g., sensitive skin and elderly), or potential interactions with specific Western medications (e.g., anticoagulants). Future high-quality RCTs must incorporate predefined safety endpoints (including detailed AE/SAE collection and laboratory monitoring) as core components with adequate reporting.
3.9 SETCM therapy high frequency herbal combination laws
A total of 50 herbs were prescribed in the 25 studies included in this review. The cumulative frequency of the top eight herbs was 50.63%, indicating a higher concentration of these herbs in the formulary. Table 2 provides a separate list of herbs used in each study.
The eight most commonly prescribed herbs for the treatment of PSD are:
Arisaematis Rhizoma Preparatum, Pinelliae Rhizoma, Asari Radix et Rhizoma, Borneolum Syntheticum, Aconiti Lateralis Radix Praeparata, Glycyrrhizae Radix et Rhizoma, Fritillariae Cirrhosae Bulbus, and Acori Tatarinowii Rhizoma.
Table 8 shows the frequency distribution of the herbs.
TABLE 8
| Herb | Frequency | Relative frequency (%) | Cumulative frequency (%) |
|---|---|---|---|
| Arisaematis Rhizoma Preparatum | 16 | 10.13 | 10.13 |
| Pinelliae Rhizoma | 15 | 9.49 | 19.62 |
| Asari Radix et Rhizoma | 12 | 7.59 | 27.21 |
| Borneolum Syntheticum | 12 | 7.59 | 34.80 |
| Aconiti Lateralis Radix Praeparata | 7 | 4.43 | 39.23 |
| Glycyrrhizae Radix et Rhizoma | 6 | 3.80 | 43.03 |
| Fritillariae Cirrhosae Bulbus | 6 | 3.80 | 46.83 |
| Acori Tatarinowii Rhizoma | 6 | 3.80 | 50.63 |
The frequency distribution of the herbs.
4 Discussion
4.1 Strengths and limitations
This review strictly adhered to Cochrane guidelines, systematically searching four Chinese and four international databases, ultimately including 25 RCTs with 2,245 PSD patients to comprehensively evaluate SETCM therapy efficacy. Results indicate that SETCM therapies demonstrate potential benefits in improving swallowing function as a complementary intervention. Specifically, SETCM therapies combined with conventional therapy significantly outperformed controls in: improving overall response rate, cure rate, VFSS scores, SWAL-QOL scores, MBI scores, ALB levels, PA levels, GUSS scores, ADL scores, tube removal success rate, and FDS scores. Reducing: WST scores, NRS2002 scores, aspiration incidence, and enteral tube duration.
Nutritional biomarker improvements (serum albumin and prealbumin) further confirm benefits in reversing negative nitrogen balance. The 82.2% subgroup difference (P = 0.02) for brainstem lesions demonstrates that iontophoresis uniquely overcomes impaired corticobulbar pathways via direct brainstem drug delivery, unachievable by topical application alone. Based on current evidence, we recommend: Short-term adjunct therapy: SETCM therapies (especially iontophoresis) for acute brainstem PSD (Evidence: Moderate) Non-replacement role: No evidence supports replacing standard swallowing training (e.g., electrical stimulation and compensatory strategies) Contraindication: Herbal application contraindicated in patients with skin lesions (irritation risk: 2.1%) Seven key limitations warrant consideration: ①Language bias: 95% of included studies were Chinese-language publications from mainland China, limiting generalizability to Western populations with distinct stroke etiologies, rehabilitation protocols, and herbal access. ②Intervention heterogeneity: Significant variability existed across eight herbal formulas (e.g., Huoxue Huayu patch vs. Tianma Xibi paste), treatment duration (2–4 weeks), and, critically—non-standardized acupoint selection. Divergent acupoint combinations represent a major source of heterogeneity, potentially yielding differential effects and complicating cross-study comparisons. ③Long-term evidence gap: No studies reported outcomes beyond 3 months; sustainability for chronic PSD (>6 months) remains unknown. ④Mechanistic uncertainty: While preclinical data suggest neuroplasticity modulation (Dou et al., 2021; Savović et al., 2012), no trials measured biomarkers (e.g., serum cytokines and fMRI activation) to validate mechanisms. ⑤Risk of bias: Lack of allocation concealment may introduce selection bias (e.g., preferential assignment), while inadequate blinding likely inflated subjective outcomes (e.g., WST scores). Empirical bias models (Lapa et al., 2021) suggest these limitations may exaggerate effects by 15%–30%, necessitating cautious interpretation of efficacy claims. Future high-quality RCTs adhering to CONSORT guidelines (particularly on randomization, allocation concealment, and blinding) are essential. Our GRADE downgrading (e.g., “Low”/”Very low” for VFSS/WST/GUSS) due to risk of bias and imprecision further supports this caution. The predominance of Chinese-language studies may reflect regional reporting norms where AE documentation is often minimal unless the condition is severe. We mitigated this through a stringent CTCAE v5.0 reappraisal but acknowledge potential under-detection. ⑥Publication bias: Funnel plot asymmetry suggests underrepresentation of small studies with non-significant results, potentially overestimating effect sizes and limiting real-world generalizability. ⑦Herbal reporting heterogeneity: While species names were standardized (Table 2), missing key details (e.g., exact composition ratios, bioactive compound quantification) impede reproducibility and clinical translation.
4.2 Mechanistic research
Stroke, a highly prevalent acute neurological disorder globally, constitutes a triple public health burden: First, its sudden onset and high fatality rate make it one of the top three causes of death; Second, recurrence rates up to 40% lead to cumulative neurological damage, creating high-disability populations (Gu et al., 2021; Guo et al., 2023); Third, brain tissue damage from vascular events often leaves permanent dysfunction (e.g., hemiplegia and aphasia) (Mbalinda et al., 2024), with 37% lower survival in recurrent versus first-ever stroke patients (Chen et al., 2013). Among complications, PSD ranks first with 22%–65% prevalence. Its clinical heterogeneity manifests in temporal evolution: 51%–86% at 1 week post-stroke, 29%–40% at 1–3 months, and 8%–13% persisting at 6 months (Song et al., 2024; Xiao et al., 2020). This progressive injury directly causes aspiration pneumonia and malnutrition while inducing psychological impairments (e.g., phagophobia, social withdrawal), forming a dysfunction-stress vicious cycle that worsens prognosis (Karisik et al., 2024). Karisik et al. (Mohtasebi et al., 2023) found 2.3-fold higher 12-month mortality in 236 PSD patients versus recovered controls, exacerbated by metabolic imbalances (dehydration, electrolyte disorders) (Karisik et al., 2024). Neuroanatomically, swallowing relies on multilevel regulation by corticobulbar tracts, brainstem nuclei, and peripheral nerves. Modern research indicates unilateral hemispheric (especially left frontal/opercular) or brainstem lesions cause delayed swallow initiation and impaired pharyngeal phase coordination. Pharmacokinetic evidence shows that tetramethylpyrazine (the primary alkaloid of Chuanxiong Rhizoma [Apiaceae]) achieves significantly enhanced acupoint absorption with permeation enhancers (e.g., menthol from Menthae Haplocalycis Herba [Lamiaceae]) (Xing et al., 2024). Experimental studies suggest that tetramethylpyrazine may activate TRPV1 receptors in the nucleus tractus solitarius (Chen et al., 2011), upregulating c-Fos expression to modulate neuroplasticity. Additionally, phenolic metabolites (e.g., salvianolic acid B from Salvia miltiorrhiza Bunge [Lamiaceae] in “blood-activating” patches) demonstrate anti-inflammatory/antioxidant effects by scavenging ROS and inhibiting proinflammatory cytokines (TNF-α and IL-1β), potentially mitigating post-stroke edema and neuronal damage to indirectly restore swallowing pathways (Chinese Academy of Traditional Chinese Medicine and Chinese Acupuncture and Moxibustion Association, 2011). SETCM therapies (e.g., acupoint application and iontophoresis) for PSD are rooted in TCM theory (Tang et al., 2023; Zhang, 2024). PSD correlates with pathological changes, including local circulatory impairment, inflammation, and neural dysfunction (Yao et al., 2022). TCM-derived strategies focus on principles to improve neural function, enhance local circulation, and regulate physiological processes (Ma et al., 2022). Transdermal therapy at specific acupoints (e.g., Lianquan [CV23], Tiantu [CV22], Renying [ST9]) represents a noninvasive approach. This method utilizes transdermal absorption of herbal metabolites (e.g., circulation-promoting, secretion-modulating compounds) at target sites to stimulate local responses and regulate physiological functions for swallowing improvement (Zheng et al., 2024). Key advantages include non-invasiveness and potentially reduced systemic side effects versus oral administration. The 2020 Chinese Pharmacopoeia includes these acupoints and topical herbs in its transdermal section, providing regulatory support. This integration of TCM and biomedical theories reflects PSD’s complexity, suggesting multidimensional interventions. Based on meridian theory, SETCM therapies regulate channel Qi-blood by stimulating acupoints/local skin to restore swallowing. “The neural reflex pathway” is a potential auxiliary mechanism of SETCM therapies, rather than a substitute for transdermal absorption. Preclinical studies indicate dual mechanisms: a. Biomechanical stimulation: Acupoint pressure upregulates cortical swallowing centers via somatosensory input (Yao et al., 2022; Ma et al., 2022); b. Biochemical modulation: TRPV1 activation enhances pharyngeal afferents, while anti-inflammatory compounds (e.g., flavonoids) resolve neural edema (Liu et al., 2000; Cui et al., 2024; Cheng et al., 2017). However, clinical evidence remains indirect. Observed efficacy may reflect synergy between transdermal drug delivery and neural pathway stimulation, but human neuroplasticity data are lacking. Future trials should validate mechanisms via fMRI and neurotransmitter assays.
4.3 Innovation points
Compared with previous studies, this study is innovative in three aspects: 1. Systematic integration of evidence of efficacy: This is the first review to quantify the impact of TCM external treatment on multidimensional outcome indicators such as functional rehabilitation, living ability, and complication control in PSD patients, which makes up for the limitation of previous studies that only focused on a single indicator. (2) In-depth expansion of mechanism research: By integrating modern pharmacological and neuroimaging evidence, the two-pathway hypothesis of “TCM external treatment may enhance sensory afferents and regulate central neural plasticity in the medulla oblongata swallowing by activating TRPV1 receptor” is proposed, which provides a biological rational explanation for the therapeutic effect. 3. Methodological innovation in clinical transformation: a synergistic intervention model of external treatment of traditional Chinese medicine and modern rehabilitation technology (such as transcranial magnetic stimulation) was constructed, and its effective rate was significantly higher than that of a single rehabilitation group, providing a new paradigm for multimodal treatment. 4. Innovation of hierarchical analysis of brain regions: The neuroanatomical hierarchical analysis framework of stroke lesions was introduced for the first time in the systematic evaluation of the external TCM treatments in PSD. Cerebral hemisphere lesions: OR = 2.73 [1.65–4.52], brain stem lesions: The OR of iontophoresis was 11.29 [2.91–43.85] (6.5 times higher than that of plaster therapy), which provided a methodological paradigm to solve the problem of “too wide range leading to ambiguous conclusions” in previous meta-analyses.
4.4 Outlook
This study provided a relatively comprehensive assessment of the efficacy of SETCM therapies in the treatment of dysphagia after stroke through a systematic literature search and comprehensive analysis. The results showed significant improvement in swallowing function with external TCM treatment. However, researchers should be aware of two opposing pieces of evidence: ① There was no effect of the application of therapy in the brainstem subgroup: There was no significant benefit of acupoint application in PSD patients with brainstem lesions (OR = 1.73, 95%CI [0.81–3.68], P = 0.16), suggesting that this therapy has neuroanatomical specific limitations. ② Lack of evidence of long-term efficacy: the intervention period of all included studies was no more than 4 weeks, and there were no data to support the sustainability of efficacy (>3 months), which is far from the goal of long-term functional remodeling emphasized by modern rehabilitation medicine. In terms of study scope and quality control, there may be publication bias because most studies were published in Chinese, which may lead to biased interpretation of the results. Future studies need to expand the scope of literature, especially by strengthening the systematic screening and integration of high-quality literature in English and other languages. In terms of standardization, the existing TCM external treatment interventions (such as acupoint application and TCM iontophoresis) lack unified standards in the key parameters, such as drug composition, dose, and treatment duration, which affects the comparison and repeatability of treatment effects. In terms of mechanism research, although the mechanism of external TCM treatment has been preliminarily revealed, the specific molecular mechanism and neural regulatory network still need to be further explored. Variability in herbal formulations and application techniques limits direct comparability. Future randomized controlled trials (RCTS) of SETCM therapies for PSD are strongly recommended to strictly comply with the CONSORT Extended Statement of Herbal Medicines and the ConPhyMP reporting standards (Hu, 2019), and to report the detailed information of the herbs used, including: 1) the full scientific name, medicinal site and source of the herbs; 2) the detailed process of extraction and preparation method; 3) quality control standards for key active ingredients or indicator ingredients (e.g., content determination); and 4) drug stability data to improve the transparency and reproducibility of evidence. Monitoring long-term safety (>3 months) and conducting multicenter RCTs to verify the superiority of iontoimplantation in brainstem lesions will greatly improve the scientific validity, transparency, and strength of evidence. Future research needs to enhance the pharmacokinetic studies of transdermal absorption (such as using tracer techniques) to directly quantify the absorption, distribution, and target tissue concentration of the active ingredients after application at the acupoints, thereby providing more direct pharmacological evidence for “acupoints as effective drug delivery sites.”
5 Conclusion
In conclusion, SETCM therapies—when adhering to ChP 2020 standards documented in Table 2 and Supplementary Tables S2, S3—offer clinically meaningful benefits. However, the limitations of the available evidence include heterogeneity and regional bias, which require caution when it is used as an adjunct to conventional treatment. Standardized interventions and multi-country RCTs are imperative before widespread clinical adoption.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
JZ: Project administration, Writing – original draft, Data curation, Supervision, Writing – review and editing, Conceptualization, Software, Methodology, investigation. XK: Writing – review and editing, Software. JZ: Writing – review and editing, Formal analysis. JN: Validation, Writing – review and editing. SC: Writing – review and editing, Resources. SS: Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank all other authors for consulting and sorting out the relevant literature, and for the support of the above funds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1635090/full#supplementary-material
References
1
Alamer A. Melese H. Nigussie F. (2020). Effectiveness of neuromuscular electrical stimulation on post-stroke dysphagia: a systematic review of randomized controlled trials. Clin. Interv. Aging15, 1521–1531. 10.2147/CIA.S262596
2
Arnold M. Liesirova K. Broeg-Morvay A. Meisterernst J. Schlager M. Mono M. L. et al (2016). Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLOS ONE11 (2), e0148424. 10.1371/journal.pone.0148424
3
Chang M. C. Choo Y. J. Seo K. C. Yang S. Y. (2022). The relationship between dysphagia and pneumonia in acute stroke patients: a systematic review and meta-analysis. Front. Neurol.13, 834240. 10.3389/fneur.2022.834240
4
Chen T. Liu W. Chao X. Zhang L. Qu Y. Huo J. et al (2011). Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res. Bull.84 (2), 163–168. 10.1016/j.brainresbull.2010.11.015
5
Chen Y. Chen G. G. Huang S. M. (2013). Study on the relationship between dysphagia and focal site of acute stroke. China Med. Innov.10 (34), 48–49. 10.3969/j.issn.1674-4985.2013.34.022
6
Cheng C. W. Wu T. X. Shang H. C. Li Y. P. Altman D. G. Moher D. et al (2017). CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann. Intern Med.167 (2), 112–121. 10.7326/M16-2977
7
Chinese Academy of Traditional Chinese Medicine, Chinese Acupuncture and Moxibustion Association (2011). Evidence-based clinical practice guidelines for Chinese medicine: acupuncture and moxibustion. Beijing: China Traditional Chinese Medicine Press, 126.
8
Cui Y. R. Jing R. Yang L. Zhang B. B. (2024). Research progress in the treatment of patients with dysphagia after stroke. Adv. Clin. Med.14 (4), 1928–1933. 10.12677/acm.2024.1441246
9
Dong L. L. Lin T. Jiang Y. Q. (2021). The effect of acupoint patch combined with rehabilitation nursing care on swallowing function and nutritional status of patients with post-stroke dysphagia. J. Chronic Dis.22 (04), 575–577. 10.16440/j.cnki.1674-8166.2021.04.028
10
Dou Z. L. (2009). Evaluation and treatment of dysphagia. Beijing: People's Health Press.
11
Dou B. Li Y. Ma J. Xu Z. Fan W. Tian L. et al (2021). Role of neuroimmune crosstalk in mediating the anti-inflammatory and analgesic effects of acupuncture on inflammatory pain. Front. Neurosci.15, 695670. 10.3389/fnins.2021.695670
12
Gu H. Q. Yang X. Wang C. J. Zhao X. Q. Wang Y. L. Liu L. P. et al (2021). Clinical characteristics, management, and in-hospital outcomes in patients with stroke or transient ischemic attack in China. JAMA Netw. Open4 (8), e2120745. 10.1001/jamanetworkopen.2021.20745
13
Guo Y. Jiang J. Cao F. Yan J. (2023). Disease burden based on gender and age and risk factors for stroke in China, 2019. Zhong Nan Da Xue Xue Bao Yi Xue Ban.48 (8), 1217–1224. 10.11817/j.issn.1672-7347.2023.220561
14
Guyatt G. Oxman A. D. Akl E. A. Kunz R. Vist G. Brozek J. et al (2011). GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol.64 (4), 383–394. 10.1016/j.jclinepi.2010.04.026
15
Hamidon B. B. Nabil I. Raymond A. A. (2006). Risk factors and outcome of dysphagia after an acute ischaemic stroke. Med. J. Malays.61 (5), 553–557.
16
Han C. H. Kim J. H. Kim M. Kim H. R. Kim S. Y. Choi H. Y. et al (2020). Electroacupuncture for post-stroke dysphagia: a protocol for systematic review and meta-analysis of randomized controlled trials. Med. Baltim.99 (38), e22360. 10.1097/MD.0000000000022360
17
Heinrich M. Lardos A. Leonti M. Weckerle C. Willcox M. Applequist W. et al (2018). Best practice in research: consensus statement on ethnopharmacological field studies - ConSEFS. J. Ethnopharmacol.211, 329–339. 10.1016/j.jep.2017.08.015
18
Hu L. L. (2019). Study on the application effect of acupoint patch combined with swallowing training in patients with post-stroke dysphagia. Chin. general Med.22 (S1), 176–179.
19
Huang D. W. (2007). Observation on the therapeutic effect of ultrasonic medium-frequency ion implantation in the treatment of post-stroke dysphagia. China Med. J.4 (20), 24. 10.3969/j.issn.1673-7210.2007.20.012
20
Hui Y. P. Zhang Q. J. (2025). Current status and prospect of the development of dysphagia rehabilitation. Chin. J. Rehabilitation Med.40 (3), 321–325. 10.3969/j.issn.1001-1242.2025.03.001
21
Jiao X. Y. (2022). The application of acupoint paste combined with a swallowing therapeutic instrument and swallowing function training in the rehabilitation of post-stroke patients with dysphagia. Chin. foreign Med. Res.20 (13), 157–160. 10.14033/j.cnki.cfmr.2022.13.042
22
Karisik A. Bader V. Moelgg K. Buergi L. Dejakum B. Komarek S. et al (2024). Intensified post-stroke care improves long-term dysphagia recovery after acute ischemic stroke. Results from the STROKE CARD trial. Eur. Stroke J.10, 568–575. 10.1177/23969873241284123
23
Lai Y. X. (2015). The effect of Chinese medicine acupoint dressing on the rehabilitation of patients with dysphagia in stroke. China Pharmacoeconomics (z1), 53–54.
24
Lapa S. Foerch C. Singer O. C. Hattingen E. Luger S. (2021). Ischemic lesion location based on the ASPECT score for risk assessment of neurogenic dysphagia. Dysphagia36 (5), 882–890. 10.1007/s00455-020-10204-0
25
Li Q. L. (2023). Effectiveness of Chinese medicine acupoints in treating patients with diabetic peripheral neuropathy. Renren Health (32), 24–26.
26
Lin X. Chen M. Y. Lin X. Y. (2014). Observations on the efficacy of cold pharyngeal stimulation with traditional Chinese medicine ice lollipop in the treatment of post-stroke dysphagia. China Rehabil. (6), 409–411. 10.3870/zgkf.2014.06.002
27
Liu J. P. Li Y. M. Qiu Y. F. Lin Y. N. Zhang Y. Gao X. D. (2000). Study on the transdermal absorption and acupoint effect of aminophylline membrane agent. J. China Pharm. Univ. (05), 32–34. 10.3321/j.issn:1000-5048.2000.05.008
28
Luo G. L. Yang S. W. Lu Q. Z. He G. Z. Chen G. X. Lou L. et al (2018). Observation on the therapeutic effect of Tianmoxibustion paste applied to Lianquan point on dysphagia after ischemic stroke. J. Guangzhou Univ. Traditional Chin. Med.35 (3), 435–439. 10.13359/j.cnki.gzxbtcm.2018.03.012
29
Ma J. L. Li Y. J. Meng Y. Wang J. H. (2021). Observation on the efficacy of acupoint patch of expectorant and choking cure formula in treating post-stroke dysphagia. Chin. J. Traditional Chin. Med.36 (7), 4364–4367.
30
Ma L. Y. Xu Z. H. Jing S. Wang D. D. Wang L. Zhang X. (2022). Current status of research on Chinese medicine treatment and rehabilitation technology of swallowing disorders in stroke. J. Changchun Univ. Traditional Chin. Med.38 (09), 1054–1057. 10.13463/j.cnki.cczyy.2022.09.026
31
Martino R. Foley N. Bhogal S. Diamant N. Speechley M. Teasell R. (2005). Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke36 (12), 2756–2763. 10.1161/01.STR.0000190056.76543.eb
32
Mbalinda S. N. Kaddumukasa M. Najjuma J. N. Kaddumukasa M. Nakibuuka J. Burant C. J. et al (2024). Stroke recurrence rate and risk factors among stroke survivors in sub-saharan africa: a systematic review. Neuropsychiatr. Dis. Treat.20, 783–791. 10.2147/NDT.S442507
33
Mohtasebi M. Irwin D. Singh D. Mazdeyasna S. Liu X. Haratbar S. R. et al (2023). Detection of low-frequency oscillations in neonatal piglets with speckle contrast diffuse correlation tomography. J. Biomed. Opt.28 (12), 121204. 10.1117/1.JBO.28.12.121204
34
Mourão A. M. Lemos S. M. Almeida E. O. Vicente L. C. Teixeira A. L. (2016). Frequency and factors associated with dysphagia in stroke. Codas28 (1), 66–70. 10.1590/2317-1782/20162015072
35
Que J. L. Bian X. M. (2016). Observation on the efficacy of Chinese medicine ice-cotton stick acupoint stimulation to improve dysphagia after stroke. China TCM Emerg.25 (3), 510–512. 10.3969/j.issn.1004-745X.2016.03.045
36
Qureshi A. I. Suri M. F. K. Huang W. Akinci Y. Chaudhry M. R. Pond D. S. et al (2022). Annual direct cost of dysphagia associated with acute ischemic stroke in the United States. J. Stroke Cerebrovasc. Dis.31 (5), 106407. 10.1016/j.jstrokecerebrovasdis.2022.106407
37
Rivera D. Allkin R. Obón C. Alcaraz F. Verpoorte R. Heinrich M. (2014). What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol.152 (3), 393–402. 10.1016/j.jep.2013.12.022
38
Savović J. Jones H. E. Altman D. G. Harris R. J. Jüni P. Pildal J. et al (2012). Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann. Intern Med.157 (6), 429–438. 10.7326/0003-4819-157-6-201209180-00537
39
Shao T. T. Chen L. (2020). Clinical study on swallowing disorders in the oral phase of stroke intervened by traditional Chinese medicine rehabilitation nursing care combined with pharyngeal stimulation of traditional Chinese medicine sticks. New Chin. Med.52 (24), 157–160. 10.13457/j.cnki.jncm.2020.24.048
40
Shi G. X. Shi S. H. Han S. M. (2022). Clinical effect of rehabilitation intervention combined with traditional Chinese medicine fumigation in patients with stroke-complicated dysphagia. Chin. Health Care40 (11), 32–34.
41
Song W. Wu M. Wang H. Pang R. Zhu L. (2024). Prevalence, risk factors, and outcomes of dysphagia after stroke: a systematic review and meta-analysis. Front. Neurol.15, 1403610. 10.3389/fneur.2024.1403610
42
Tang X. M. Liu S. J. (2024). Intervention effect of acupoint paste Chinese medicine care combined with rehabilitation training on post-stroke dysphagia and its impact on daily life function. Chin. Sci. Technol. J. Database Full Text Ed. Med. Health (9), 0166–0169.
43
Tang Y. L. Li J. W. Bai D. Q. Zhang Y. M. Liu L. Chen Y. X. et al (2023). Effect of acupressure combined with motor imagery on the rehabilitation effect of patients with swallowing dysfunction after ischemic stroke. Pract. Hosp. Clin. J.20 (2), 28–33. 10.3969/j.issn.1672-6170.2023.02.006
44
Umay E. Eyigor S. Ertekin C. Unlu Z. Selcuk B. Bahat G. et al (2022). Best practice recommendations for stroke patients with dysphagia: a delphi-based consensus study of experts in Turkey. -Part I: management, diagnosis, and follow-up. Dysphagia37 (2), 217–236. 10.1007/s00455-021-10273-9
45
Wang X. X. Sun A. Y. (2023). Improvement of swallowing function of patients with post-stroke dysphagia by acupressure combined with rehabilitation nursing care. Electron. J. Pract. Clin. Nurs.8 (44), 63–66.
46
Wang L. N. He D. K. Shao Y. R. Lv J. Wang P. F. Ge Y. et al (2023a). Early platelet level reduction as a prognostic factor in intensive care unit patients with severe aspiration pneumonia. Front. Physiol.14, 1064699. 10.3389/fphys.2023.1064699
47
Wang F. Q. Qin H. W. Wang Y. (2023b). Clinical efficacy of acupoint plastering with butylphthalide-associated anorexia and blood stasis formula in the treatment of dysphagia after acute cerebral infarction. J. Cardiovasc. Cerebrovasc. Dis. Chin. West. Med.21 (20), 3827–3830. 10.12102/j.issn.1672-1349.2023.20.028
48
Wilson R. D. (2012). Mortality and cost of pneumonia after stroke for different risk groups. J. Stroke Cerebrovasc. Dis.21 (1), 61–67. 10.1016/j.jstrokecerebrovasdis.2010.05.002
49
Wu B. Z. Liu Q. Liao X. J. (2021). The effect of acupoint patch combined with swallowing training on the nutritional status of post-stroke patients with dysphagia. Chin. Pract. Med.16 (16), 163–165. 10.14163/j.cnki.11-5547/r.2021.16.063
50
Xiao L. J. Guo Q. Huang F. Y. Liao M. X. Zhang L. L. Yan T. B. (2020). Correlation between swallowing function and pulmonary ventilation function, and respiratory muscle strength in patients with dysphagia after stroke. Zhonghua Yi Xue Za Zhi100 (7), 504–508. 10.3760/cma.j.issn.0376-2491.2020.07.005
51
Xing Z. Chen Y. Chen J. Peng C. Peng F. Li D. (2024). Metabolomics integrated with mass spectrometry imaging reveals novel action of tetramethylpyrazine in migraine. Food Chem.460 (Pt 2), 140614. 10.1016/j.foodchem.2024.140614
52
Xu J. J. (2022). Discussion on the effect of acupoint patch to improve post-stroke dysphagia. Pract. Clin. Nurs. Electron. J.7 (24), 55–57.
53
Xu H. L. Zhao X. H. (2016). Clinical observation of rehabilitation nursing combined with traditional Chinese medicine fumigation on the recovery of swallowing function in patients with stroke-complicated dysphagia. Shizhen Guojian Guojian27 (08), 1935–1936. 10.3969/j.issn.1008-0805.2016.08.053
54
Yang C. Do Z. L. (2022). Meta-analysis of the efficacy of non-invasive brain stimulation technology in the treatment of post-stroke dysphagia. Chin. J. Rehabilitation Med.37 (07), 948–954. 10.3969/j.issn.1001-1242.2022.07.015
55
Yao S. Guo P. F. Xu D. H. Wang A. P. Sun M. Li J. et al (2022). Clinical effect of Xiang Congzhi therapy in treating post-stroke swallowing dysfunction. Chin. Med. Her.19 (25), 119–123. 10.20047/j.issn1673-7210.2022.25.27
56
Yu M. Y. Qiu Q. C. (2023). Intervention value of acupoint paste Chinese medicine care combined with rehabilitation training on post-stroke dysphagia. Jilin Med. Sci.44 (03), 839–842. 10.3969/j.issn.1004-0412.2023.03.081
57
Yu X. F. Wu X. M. (2024). Clinical study on the treatment of dysphagia during the recovery period of cerebral infarction by acupoint plastering combined with dysphagia therapeutic instrument. New Chin. Med.56 (3), 176–180. 10.13457/j.cnki.jncm.2024.03.034
58
Yuan S. Qiu B. Liang Y. Deng B. Xu J. Tang X. et al (2024). Role of TRPV1 in electroacupuncture-mediated signal to the primary sensory cortex during regulation of the swallowing function. CNS Neurosci. Ther.30 (3), e14457. 10.1111/cns.14457
59
Zhang Y. (2024). Application of the PESTLE and needle technique in post-stroke dysphagia. Sichuan: Chengdu University of Traditional Chinese Medicine.
60
Zhao H. T. Yuan S. C. Liu Q. R. (2008). Clinical analysis of current pulse Chinese medicine ionization acupoint penetration in the treatment of post-stroke dysphagia. Chin. J. Pract. Neurological Dis.11 (3), 28–30. 10.3969/j.issn.1673-5110.2008.03.012
61
Zhao Y. Y. Kou J. Huang D. Bai Y. J. Feng X. D. (2012). Observations on the effect of acupoint patch with rehabilitation training on post-stroke dysphagia. Chin. J. Rehabilitation Med.27 (8), 771–772.
62
Zheng Y. J. Hu S. X. Dang W. K. Zhu S. J. (2019). Observation on the efficacy of musk cardioprotective pill acupoint patch combined with swallowing therapeutic instrument in the treatment of swallowing disorder after cerebral infarction. China Pract. Med.14 (12), 1–3. 10.14163/j.cnki.11-5547/r.2019.12.001
63
Zheng J. Q. Zhang S. J. Wu X. Y. Yu J. H. Liu Z. T. Lei K. J. et al (2024). Progress in the evaluation of the effects of acupoint drug delivery and its application. Chin. J. Traditional Chin. Med.49 (14), 3706–3713. 10.19540/j.cnki.cjcmm.20240401.601
64
Zhou Y. J. Hu Q. Y. Wei H. J. Liao M. R. Wang P. L. Su D. et al (2018). Effect of Tongguan San medicated stick on swallowing function in patients with post-stroke dysphagia. Jiangxi Tradit. Chin. Med.49 (5), 37–39.
65
Zhou H. X. Qin H. W. Kong Y. F. (2022). Clinical observation on the treatment of dysphagia after acute cerebral infarction by acupoint application of Huixiahuayuifang and acupuncture. J. Pract. Chin. Med.38 (3), 399–401.
66
Zhu H. Z. Wang P. Chen D. X. Yang H. L. (2023). Efficacy of directional drug delivery of dihuang drink in treating patients with post-stroke dysphagia of kidney deficiency and phlegm stasis type and its effect on survival quality. Med. Clin. Res.40 (6), 838–840. 10.3969/j.issn.1671-7171.2023.06.011
Summary
Keywords
deglutition disorders, external traditional Chinese medicine therapies, stroke, therapeutic efficacy, systematic review
Citation
Zhang J, Kang X, Zhao J, Niu J, Chen S and Shi S (2025) Systematic review and meta-analysis of specific external Chinese herbal medicines for post-stroke dysphagia: efficacy and clinical implications. Front. Pharmacol. 16:1635090. doi: 10.3389/fphar.2025.1635090
Received
27 May 2025
Accepted
14 July 2025
Published
01 September 2025
Volume
16 - 2025
Edited by
Annalisa Chiavaroli, University of Studies G.d'Annunzio Chieti and Pescara, Italy
Reviewed by
Pragna Landge, Drs. Kiran and Pallavi Patel Global University, India
Bülent Alyanak, TC Saglik Bakanligi Golcuk Necati Celik Devlet Hastanesi, Türkiye
Heriviyatno Julika Siagian, Universitas Sembilanbelas November Kolaka, Indonesia
Updates
Copyright
© 2025 Zhang, Kang, Zhao, Niu, Chen and Shi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Shi, ss18955386@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.