- 1Department of Nephrology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
- 2Department of Medical Laboratory Technology and Sciences, School of Allied Health Sciences, Delhi Pharmaceutical Sciences and Research University, New Delhi, India
- 3Department of Women’s Health Services, Henry Ford Hospital, Henry Ford Cancer Institute, Detroit, MI, United States
- 4Department of Nephrology, Sir Ganga Ram Hospital, New Delhi, India
- 5Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
Peritoneal Dialysis (PD) requires a healthy and functional peritoneal membrane for adequate ultrafiltration and fluid balance, making it a vital treatment for patients with end-stage renal disease (ESRD). The spectrum of PD-associated peritoneal fibrosis encompasses a diverse range of collective mechanisms: peritoneal fibrogenesis, epithelial to mesenchymal transition (EMT), peritonitis, angiogenesis, sub-mesothelial immune cells infiltration, and collagen deposition in the sub-mesothelial compact zone of the membrane that accompany deteriorating membrane function. In this narrative review, we summarize the repertoire of current knowledge about the structure, function, and pathophysiology of the peritoneal membrane, focusing on biomolecular mechanisms and signalling pathways that potentiate the development and progression of peritoneal fibrosis. The article suggests future directions that could enhance our comprehension of the relationship between peritoneal membrane dysfunction and its fibrosis to elucidate the promising targets for therapeutic interventions. A thorough understanding of early events in pathophysiology closely associated with the inflammatory events in peritoneal fibrosis is the logical starting point for identifying new targets rather than concentrating on more downstream effects. Biomarkers are essential for monitoring the progression of peritoneal fibrosis and evaluating the effectiveness of therapeutic interventions. Biomarkers are evolving in concert with new targets and novel agents, and biomarker outcomes offer a means of monitoring the peritoneal membrane’s health. Recent approaches to reducing the etiologies of peritoneal membrane dysfunction, the impact of fibroblast switch, and peritoneal membrane events perturbing fibroblast function are explored and suggest using unique, effective therapeutic strategies to target peritoneal fibrosis and associated complications.
Key points
• New data supporting the hypothesis that biomechanical injuries, epigenetics, and gut microbiome leads to peritoneal membrane dysfunction that potentiate systemic peritoneal inflammation and fibrosis.
• Information highlighting the evolving paradigm and outlining the novel modifications in Peritoneal Dialysis solutions supporting dialysis modification by the putative treatment.
• Advances in knowledge about the potential pharmacological/stem cell therapy interventions on canonical/non-canonical pathways involved in peritoneal membrane fibrosis.
• Identifying novel targets and developing corresponding therapeutics offer an essential means of advancing new treatments for Peritoneal Fibrosis.
1 Peritoneal Fibrosis- an overview
Peritoneal dialysis (PD) is a well-known alternative to haemodialysis (HD) and a cost-effective method of renal replacement therapy in patients with end stage renal disease (ESRD), which is a global health burden (Thurlow et al., 2021). Approximately 11% of ESRD patients receive PD worldwide, making PD an important intervention in the management of ESRD (Thurlow et al., 2021; Balzer, 2020; Bello et al., 2022). Long-term PD induces peritoneal fibrosis in approximately 40% of the patients (Baroni et al., 2012). During PD, solute and water transport occur across the peritoneal membrane, which includes peritoneal mesothelial cells (PMCs), an interstitial matrix with fibroblasts and collagen, lymphatics, and a dense microvascular network (Morelle et al., 2023; Aroeira et al., 2007). Progressive damage to the peritoneal membrane during long-term PD impairs solute clearance, fluid balance, and membrane transport properties, often leading to ultrafiltration (UF) failure and technique dropout. Approximately 25% of PD patients experience severe fluid overload, primarily due to membrane failure, mechanical complications, and increased susceptibility to infections such as PD-related peritonitis (Htay et al., 2018; Morelle et al., 2021; Van Biesen et al., 2011; Lan et al., 2016). Long term exposure results in structural and functional alterations, including loss of peritoneal mesothelial cells (PMCs), submesothelial fibrosis, vasculopathy with luminal narrowing, angiogenesis, and persistent inflammation, all contributing to membrane dysfunction and PD failure (Williams et al., 2003). Therefore, the longevity of PD is limited and most PD patients eventually switch to Haemodialysis (HD) within a few years.
Acidic and hypertonic PD solutions trigger biochemical changes in the extracellular matrix and alter PMC phenotypes, promoting peritoneal fibrosis—the final stage of membrane remodeling (Terri et al., 2021; Zhou et al., 2016). A key mechanism in this process is epithelial-to-mesenchymal transition (EMT), where PMCs lose their epithelial characteristics and acquire a mesenchymal, fibroblast-like phenotype with increased motility and invasiveness (Wilson et al., 2020). The transformation of PMCs, fibroblasts into myofibroblasts is mediated by Transforming Growth Factor β1 (TGF-β1) (Wilson et al., 2020). TGF-β1 induces EMT transition primarily through the SMAD2/3 pathway, as well as non-canonical pathways including RAS/RAF/MEK/ERK, PI3K/AKT/mTOR, and STAT3 signalling (Wilson et al., 2020; Wilson, 2018). The above signalling pathways triggers inflammatory cascade leading to the production of numerous inflammatory cytokines, pro-fibrotic molecules, Interleukin (IL)-1ß, IL-6, tumour necrosis factor α (TNF-α) Monocyte Chemoattractant Protein-1 (MCP-1), Connective Tissue Growth Factor (CTGF), Platelet-Derived Growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF) in the membrane (Balzer, 2020; Terri et al., 2021; Strippoli et al., 2016; Mutsaers et al., 2016), The above injurious agents lead to a life-threatening complication of long-term PD, encapsulating peritoneal sclerosis (EPS), occurring in 14%–15% of the patients (Terri et al., 2021; Strippoli et al., 2016). This inflammatory response cause degeneration of peritoneal membrane structure and function (Zhou et al., 2016; Liu et al., 2022; Fung et al., 2020; Kim et al., 2019; Budi et al., 2017). Figure 1 explains the induction of progressive peritoneal fibrosis during long-term exposure to bio-incompatible PD solutions.

Figure 1. Mechanisms involved in peritoneal fibrosis. During long term PD peritoneal membrane is exposed to various insults that cause inflammation and injury such as, PMCs, ECs, macrophages and neutrophils produce various proinflammatory cytokines and growth factors. After activation, recognition process of bacterial pathogens occurs via TLRs, then after they undergo activation of the nuclear factor-kappa B (NF-kB) signalling pathway. This leads to the secretion of various inflammatory cytokines, including IL-6, IL-1β, IL-8, TNF-α, MCP-1, and MIP 2. These factors induce EMT process of PMCs, which results in fibroblast-like cells transformation that secrete ECM. They also elicit chronic inflammation and angiogenesis in the peritoneal cavity. The above processes contribute to the proliferation of fibroblasts and collagen deposition, which cause the thickening and stiffening of the peritoneum leading to peritoneal fibrosis. Abbreviations: PD, Peritoneal Dialysis; PMCs, Peritoneal Mesothelial Cells; ECs, Endothelial Cells; TLRs, Toll Like Receptors; IL, Interleukins; EMT, Epithelial to Mesenchymal Transition; ECM, Extracellular Matrix.
The current comprehensive review provides information that assists in elucidating the recent advances in the pathophysiology involved in peritoneal fibrosis and explores the molecular mechanisms and pathways in treating or preventing the same. Therefore, in view of current medical requirements, herein we have discussed effective therapeutic approaches and pharmacological interventions selectively targeting the molecular activation of fibroblasts during fibrosis which warrant further investigations.
2 Methods
We searched PUBMED for that reported peritoneal membrane inflammation and fibrosis linked to long-term peritoneal dialysis, containing in vitro and in vivo, as well as preclinical and clinical studies. We limited the search to articles published in English and also included previous relevant research (1998–2025).
3 Mechanisms involved Peritoneal Fibrosis
Multiple lines of evidence point to the importance of mechanisms involved in the peritoneal membrane alterations described as EMT of PMCs and generation of myofibroblasts, which play a characteristic role in the subsequent functional deterioration of the peritoneal membrane (Terri et al., 2021; Strippoli et al., 2016). Peritoneal membrane injury can result in EPS, a serious complication of peritoneal fibrosis with potential fatal manifestation characterized by ultrafiltration failure, inflammation, severe peritoneal thickening, fibrin deposition, and calcification (Park et al., 2008). Plasma exudation from peritoneal microvessels causes fibrin deposition, which is the pathological feature of EPS (Kawaguchi et al., 2000; Honda and Oda, 2005). PMCs loss, impaired fibrinolysis, submesothelial myofibroblasts proliferation, collagen and AGE accumulation in the submesothelial layer are the main features of EMT in EPS (Wilson, 2018; Del Peso et al., 2008). At present, the pathogenesis of peritoneal fibrosis has not been fully elucidated. Factors such as activation of myo-fibroblasts, EMT of PMCS, biomechanical injuries, epigenetics and gut microbiota, bioincompatible PD fluid-induced sterile inflammation, peritonitis, peritoneal angiogenesis are all involved in the occurrence of peritoneal fibrosis.
3.1 Peritoneal Fibrosis: the fibroblasts switch and epithelial to mesenchymal transition of peritoneal mesothelial cells
Fibroblast activation is a normal component of wound healing; however, in PD patients, it becomes persistent and leads to the accumulation of activated myofibroblasts that alters the healthy structure and function of the peritoneal membrane, thus hindering the effective treatment (Strippoli et al., 2020; Pap et al., 2020). EMT, a complex biological process in the peritoneal membrane, involves the transformation of PMCs which lose epithelial phenotype (apical, basal polarity) and detach from characteristic basement membrane/ECM attachment, and acquire myofibroblast-like cells characteristics such as invasive ability and mesenchymal phenotype (Figure 2) (Wilson et al., 2020; Yáñez-Mó et al., 2003).
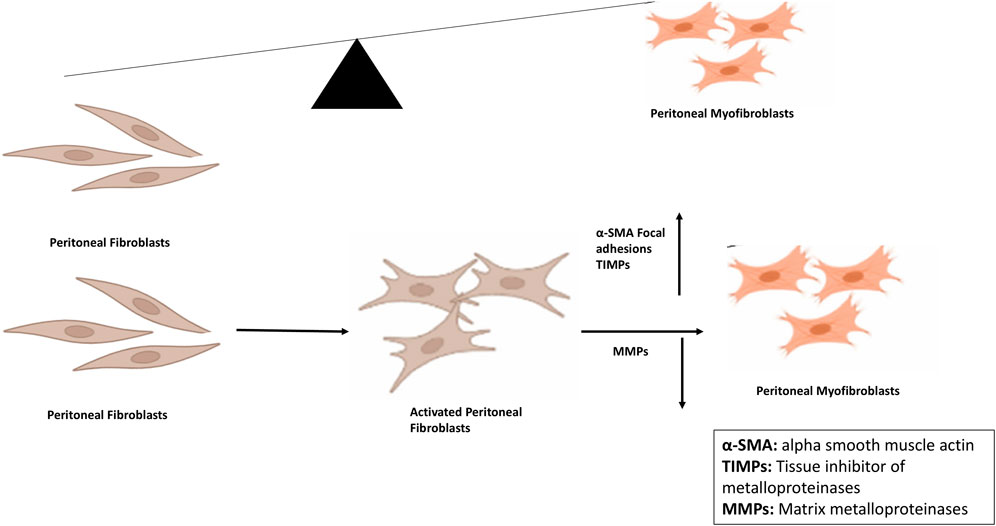
Figure 2. Peritoneal fibroblast-myofibroblast trans differentiation and its implications for peritoneal fibrosis. Conversion is the key process involved in peritoneal fibrosis in which expression of alpha smooth muscle actin as well as of focal adhesions is increased. After conversion, synthesis and secretion of extracellular matrix proteins take place leading to decreased expression of MMPs and increased expression of TIMPs. Abbreviations: MMPs, Matrix metalloproteinases; TIMPs, Tissue inhibitors of metalloproteinases.
Cellular trans-differentiation is a complex phenomenon that converts epithelial cells into mesenchymal cells. This biological process, called EMT, depends on the extracellular environment rather than the genome (Stone et al., 2016). EMT results in the loss of cell polarity and junctions and the gain of fibroblastic shape and invasiveness (Figure 3) (Stone et al., 2016). EMT occurs in both physiological (e.g., organogenesis, development, wound healing, and regeneration) and pathological (e.g., fibrosis, metastasis) conditions (Leggett et al., 2021). PMCs migrate and proliferate on the serosal lining (Tsai et al., 2018) and differentiate into myo-fibroblasts (EMT), which may promote fibrotic conversion (Sandoval et al., 2016) Changes in the gene expression and phenotype of PMCs, making them deposit more collagens and fibronectin and increasing their motility, which facilitate the development of peritoneal fibrosis (Strippoli et al., 2016).
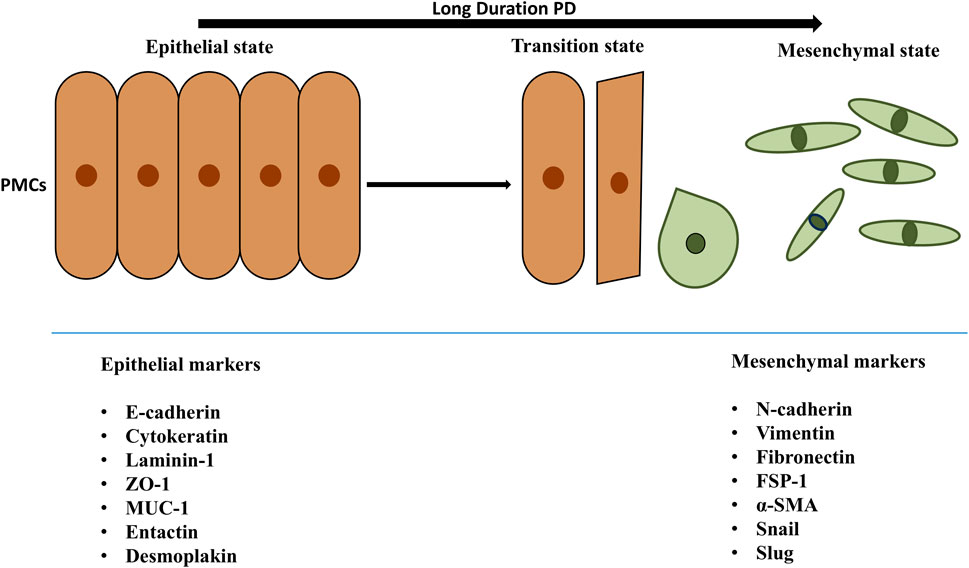
Figure 3. Transition of PMCs from epithelial to mesenchymal state. The epithelial and mesenchymal cell markers are mentioned in the above figure. Peritoneal mesothelial cells of PM explicit epithelial markers such as cytokeratin, ICAM1, E-cadherin. Also, during EMT leading mesenchymal cells are noted because of epithelial markers downregulation and upregulation of mesenchymal markers like α-SMA, fibronectin, vimentin, FSP-1. The epithelial and mesenchymal cell markers are mentioned in the above figure. Peritoneal mesothelial cells of PM explicit epithelial markers such as cytokeratin, ICAM1, E-cadherin. During EMT, mesothelial cells experience a decrease in the expression of epithelial markers, including E-cadherin, enhancing the expression of mesenchymal markers like α-SMA, fibronectin, vimentin, FSP-1. As a consequence, cells acquire invasive capacities and reach the sub-mesothelial stroma, where they produce extracellular matrix—but also inflammatory and angiogenic—factors, promoting peritoneal oxidative stress, inflammation and, finally, fibrosis, affecting peritoneal transport of water and solutes and resulting in ultrafiltration failure. Abbreviations: PD, Peritoneal Dialysis; PMCs, Peritoneal Mesothelial Cells; ZO-1, Zona Occludens 1; ICAM1, Intercellular Adhesion Molecule 1; MUC1, transmembrane glycoprotein mucin 1; α-SMA, Alpha Smooth Muscle Actin; FSP-1, Fibroblast Specific Protein -1.
EMT may play a pivotal role in the early decline of UF capacity, which constitutes the most critical functional impairment during the initial years of PD. A significant correlation has been established between UF efficiency and the dialysate-to-plasma (D/P) creatinine ratio, indicating a shared pathophysiological mechanism (Krediet, 2024; Davies, 2004). However, in many cases, the degree of UF impairment exceeds what would be anticipated based solely on D/P creatinine values (Krediet, 2024). This paradox—characterized by increased solute transport (elevated D/P creatinine) alongside diminished UF—is a hallmark of UF failure secondary to peritoneal fibrosis (Del Peso et al., 2008). The net consequence is fluid overload in PD patients, despite apparent solute equilibration, underscoring the dissociation between solute and water transport in the fibrotic peritoneal membrane.
In addition, during EMT, changes in cell membrane receptors, signaling molecules such as TGF-β1, Src, Hypoxia-inducible factor (HIF-1α), and cell morphology and behavior occurs (Wilson et al., 2020). EMT involves complex pathological cross-talk among PMCs, endothelial cells, immune cells, and resident fibroblasts (Strippoli et al., 2020). TGF-β1, is constitutively expressed by the PMCs and plays a major role in the maintenance of a transformed, inflammatory micro-environment in peritoneal membrane (Wilson, 2018). It enhances HIF-1α expression, which drives cell growth, extracellular matrix production and cell migration (Wilson, 2018). Importance of pro-fibrotic, TGF-β1 signalling during EMT of PMCs has been demonstrated by using TGF-β receptor inhibitor GW788388 on the EMT signalling pathway (Lho et al., 2021). Therapeutic Interventions targeting EMT process are detailed in Table 1.
3.2 Biomechanical Lesions
Mechanotransduction, the process of transforming mechanical signals into biochemical events, along with extracellular biochemical factors, regulates various cellular functions and is crucial during development, physiological and pathological conditions (Terri et al., 2021; Strippoli et al., 2020; Orr et al., 2006; Santos and Lagares, 2018). Biomechanical alterations of the ECM, including increased ECM rigidity or forces of varying strengths and dynamic characteristics, modulate form and the functions of cells with an inevitable impact on the cellular behavior and therefore drive distinct fibrotic signaling pathways (Orr et al., 2006; Santos and Lagares, 2018; Chen, 2008). PD requires the infusion of large amounts of PD solutions into the peritoneal cavity, which exposes the peritoneal membrane to biomechanical forces. These forces include mechanical stretch of PMCs and augment intra-abdominal pressure. Additionally, reports state that abdominal surgeries can drive fibrotic conversion pertaining to the vast majority of adhesion myofibroblasts which arise from surface mesothelium followed by EMT process which may induce biomechanical injury to the peritoneal membrane leading to peritoneal adhesions formation (Sandoval et al., 2016; Zindel et al., 2021). Proliferation and migration of mesothelial cells is promoted by receptor tyrosine kinases of the ERBB family, Epidermal Growth Factor Receptor (EGFR), which is documented to be involved in post-surgical peritoneal adhesions (Zindel et al., 2021). Mechanical injury and hypoxia identified by injured surface mesothelium expressing podoplanin (PDPN)/mesothelin (MSLN) and upregulation of hypoxia-inducible factor 1 alpha (HIF1α) are involved in the fibrotic process and the development of peritoneal adhesions suggested in an in vivo study (Tsai et al., 2018).
3.3 Epigenetics and Gut Microbiome: the dynamic duo behind peritoneal fibrosis
Peritoneal fibrosis, which is caused by inflammation, infection, or long-lasting dialysis, leads many patients to discontinue PD, however, its mechanism is unclear. Epigenetic shifts are involved in peritoneal fibrosis, and emerging evidence indicates that epigenetic interventions could help ward off and treat peritoneal fibrosis in practice (Tsai et al., 2018; Wang et al., 2021). Epigenetic regulation in peritoneal fibrosis is complicated, mainly affecting the changes of signalling molecules, transcriptional factors, and genes (Tsai et al., 2018; Wang et al., 2021). The primary epigenetic transformations include, changes in the expression and activity of genes (such as DNA methylation, histone modifications), and different types of non-coding RNA molecules (such as microRNAs (miRNAs), long non-coding RNAs, and circular RNAs) can also play a role in the development of peritoneal fibrosis (Tsai et al., 2018; Sandoval et al., 2016; Wang et al., 2021; Guo et al., 2020). HDAC inhibitors are discussed in Table 1 and 2. The development of peritoneal fibrosis (PF) is significantly influenced by non-coding RNAs, particularly microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). miRNAs are known to regulate key molecular pathways involved in PF and are increasingly being recognized as potential diagnostic biomarkers and therapeutic targets (Sandoval et al., 2016; Guo et al., 2020; Huang et al., 2023). For instance, miR-15a-5p is found to be downregulated in long-term PD patients, suggesting a role in fibrosis progression. Beyond this, a range of other miRNAs—including miR-199a-5p, miR-214-3p, miR-153-3p, miR-129-5p, miR-21, miR-30a, miR-145, miR-30b, miR-200, miR-302c, miR-34a, and miR-29b—have been reported to interact with hypoxia-inducible factor-1α (HIF-1α), a key regulator in fibrotic signaling (Sandoval et al., 2016; Guo et al., 2020). Concurrently, growing evidence suggests that lncRNAs also contribute to the pathogenesis of peritoneal fibrosis, although their precise mechanisms remain under investigation. As research in this field advances, it is crucial to identify practical approaches for targeting these non-coding RNAs to improve peritoneal fibrosis diagnosis and treatment.
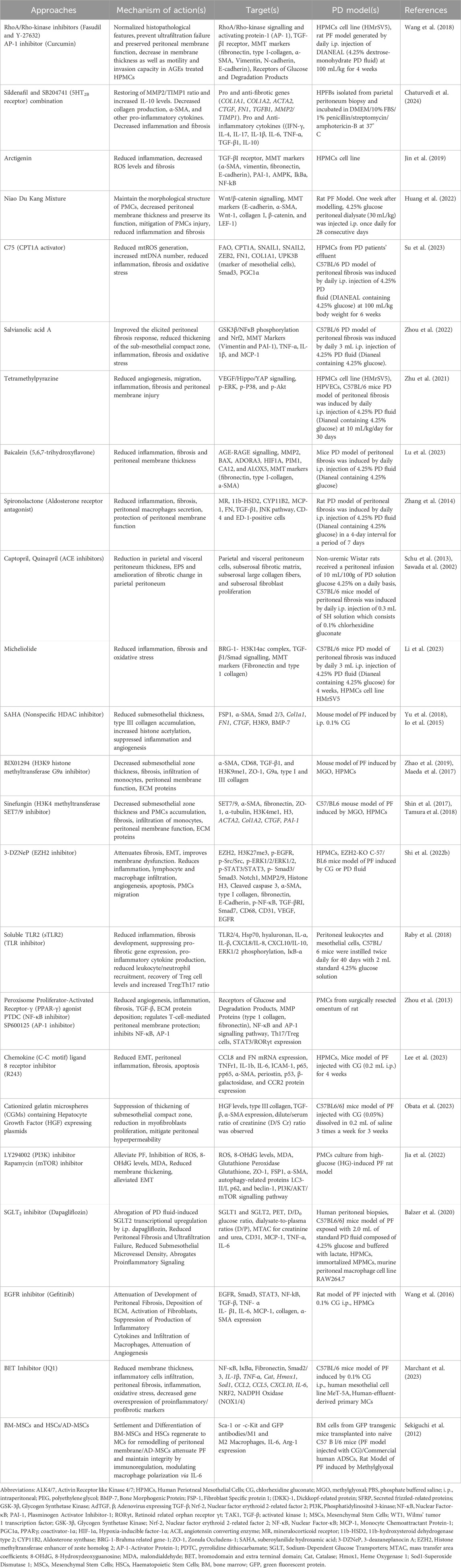
Table 2. Pharmacological interventions in inflammation/membrane thickness/oxidative stress/angiogenesis/fibrosis.
Recent study gives a rational basis and underscores the likely role of gut microbiota dysbiosis in peritoneal fibrosis (Lho et al., 2021; Stepanova, 2023). Compared to healthy controls, PD patients had less Actinobacteria and Firmicutes in their faecal microbiota, with a notable decrease in Bifidobacterium and Lactobacillus, and more Pseudomonas aeruginosa, according to a study by Wang et al. (2012). By lowering the pH through the production of acetic acid and lactic acid, Bifidobacterium and Lactobacillus influence the host positively, either by preventing the growth of pathogenic bacteria or by competing with them for nutrients and adhesion sites (Wang et al., 2012). ESRD induced alterations in the gut microbiota compromise the intestinal barrier and facilitate the translocation of microbial components and endotoxins into the systemic circulation and peritoneal cavity. Dorea, Clostridium, and SMB53 are related to chronic low-grade inflammation, oxidative stress, and immune responses within and beyond the peritoneum, peritonitis in ESRD patients on PD (Wang et al., 2012; Stadlbauer et al., 2017; Luo et al., 2021).
A study revealed, in PD patients, uremic toxins such as indoxyl sulfate (IS) and p-cresol sulfate (PCS) accumulate due to increased gut permeability and incomplete clearance and increase the host’s susceptibility to pathogen invasion. This, along with catheter use and dietary restrictions, disrupts the intestinal microenvironment, promoting pathogenic bacteria and reducing beneficial short chain fatty acids (SCFAs)-producing microbes. Consequently, PD patients exhibit a higher relative abundance of Proteobacteria in their gut microbiota (Simões-Silva et al., 2018). Proteobacteria, a potential marker of gut dysbiosis, shows similar abundance in PD patients and their healthy family members. This suggests that long-term hospitalization, rather than dialysis alone, may contribute to gut microbiome alterations in PD patients (Teixeira et al., 2023).
In PD patients, gut microbiota differs in both taxonomic composition and metabolic function. Those with longer dialysis duration, higher peritoneal glucose exposure, and reduced residual renal function show distinct microbiome profiles and significantly lower fecal levels of SCFAs, including isobutyric and isovaleric acids (Jiang et al., 2021). Identifying these alterations can provide valuable insights for clinicians, enabling early and targeted interventions to reduce complications and mortality risk in patients with ESRD. A study documented, patients on PD had more pathogenic and less beneficial species in their faecal microbiome, which altered the predicted metagenome functions, compared to controls (Stadlbauer et al., 2017).
In recent years, Probiotics, prebiotics, and synbiotics have been reported to reduce uremic toxins such as endotoxins and p-cresol in PD patients. These interventions may also lower mortality rates associated with long-term PD. Additionally, they have been shown to improve gastrointestinal symptoms and enhance the quality of life in PD patients (Li et al., 2025).
Probiotic capsules containing Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus have been shown to enhance gastrointestinal absorption and digestion (Li et al., 2025). They also help reduce inflammatory markers, such as C-reactive protein (CRP) and Interleukin-6 (IL-6) (Pan et al., 2021). Elevated IL-6 levels in PD patients are associated with malnutrition and altered peritoneal small solute transport rates, which can increase mortality. A randomized controlled trial (RCT) noted that PD patients improved serum indoxyl sulfate (IS) levels by consuming 21 g/day of unripe banana flour. Insulin-type fructan decreases Bacteroides thetaiotaomicron, the IS-producing bacterium, by inhibiting its tryptophanase activity. This leads to reduced IS production in the gut of these patients (de Andrade et al., 2021). In severe cases of uremic toxicity related to p-cresol or para-cresol sulfates, synbiotics can promote the growth of Bifidobacterium bifidum strains and increase Lactobacillus abundance in the gut. This intervention effectively reduces intestinal p-cresol levels (Stuivenberg et al., 2022). Although clinical research on gut microbiomes in PD patients remains limited in scale, further studies may validate the use of probiotics, prebiotics, and synbiotics. These interventions have the potential to improve the gut microbiome and reduce complication rates in PD.
4 Pathophysiology behind Peritoneal Membrane deterioration
PD uses the peritoneal membrane as an organ to filter the blood. Chronic exposure of PD solutions induces low-grade inflammation in the peritoneal cavity, which can impair the peritoneal membrane and compromise its function as a dialyzer. However, inflammation can damage the peritoneal membrane over time, causing it to lose its function. Inflammation also triggers peritoneal remodelling and angiogenesis, which are major events that alter the structure and blood vessels of the peritoneal membrane (Catar et al., 2022).
4.1 PD solutions and Chronic Peritoneal Inflammation
Chronic Peritoneal inflammation is considered as an important event during the pathogenesis of PF (Zhang et al., 2017). Peritoneal injury leads to the activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-B (NF-κB), which also promotes the release of multiple proinflammatory cytokines/chemokines (Shi et al., 2021). Peritoneal mesothelium-derived CXC chemokine ligand 1 (CXCL1), a chemokine, associates with peritoneal micro vessel density in uremic patients undergoing PD. Thus, CXCL1 and its receptors may be novel targets for therapeutic intervention to prolong PD therapy (Catar et al., 2022). Other pharmacological interventions targeting receptors involved in inflammation in the peritoneal membrane dysfunction culminating to fibrosis are detailed in Table 2.
A major factor limiting the chronic use of PD remains peritoneal membrane failure due to prolonged exposure to bioincompatible PD solutions leading to the fibrosis of the membrane (Cho et al., 2014). Recent evidences have tried to explain the mechanisms linking inflammation–either infection-induced (peritonitis) or sterile (bioincompatible PD solutions) – involved with the cellular stress and membrane injury in the pathogenesis and regulation of PD related peritoneal fibrosis (Zhou et al., 2016; Honda and Oda, 2005; Fielding et al., 2014; Raby et al., 2017).
The peritoneal cavity has a normal environment that can be disturbed by bio incompatible PD solutions, which can also harm the peritoneal membrane. Traditional (Bio-incompatible) PD solutions have non-physiological features such as low pH (acidic), high lactate and glucose concentrations (hyperglycaemic) (1.5%–4.5%) (Mortier et al., 2002), and hyperosmotic dextrose solutions to achieve a sufficient UF gradient across the peritoneal membrane, and toxin elimination by maintaining electrolyte homoeostasis (Szeto and Johnson, 2017; McIntyre, 2007). However, they also trigger fibrosis, oxidative stress and microinflammation in the peritoneum, leading to changes in its structure and function which involves PMCs depletion and basement membrane breakdown leading to ultrafiltration failure, discontinuation of PD, and an increased risk of developing EPS (Terri et al., 2021; Jagirdar et al., 2019; Kang, 2020). Furthermore, heat sterilisation of PD solutions results in glucose instability generating toxic glucose degradation products (GDPs), glyoxal, 3,4-dideoxyglucosone-3-ene, methylglyoxal (MGO), and others (Jörres, 2012; Catalan et al., 2005). MGO is an extremely toxic GDPs that causes oxidative stress and peritoneal injury as well as reported to enhance the production of vascular endothelial growth factor (VEGF), which may lead to vascular permeability (Hishida et al., 2019). Furthermore, many GDPs such as 3,4-dideoxyglucosone-3-ene (3,4-DGE) are reactive carbonyl compounds which along with glucose form advanced glycation end-products (AGEs), binds to free amino groups on membrane proteins or lipids contributing to pathophysiological alterations in the peritoneal membrane (Linden et al., 2002; Mortier et al., 2004). Bio-incompatible PD solutions recruit immune cells such as Th17, gdT cells, and neutrophils to the sub-mesothelial zone, where they produce inflammatory cytokine, IL-17A.
Most important, the role of IL-17A in peritoneal membrane injury and other PD-related complications has been recently explored (Marchant et al., 2020). This cytokine activates the NF-κB pathway in PMCs, which drives the expression of factors such as IL-6 which then activates the JAK/STAT pathway promoting EMT in PMCs (Marchant et al., 2020).
During peritoneal membrane injury in PD, IL-17A production in the local site stimulates the release of more pro-inflammatory mediators, such as cytokines and chemokines, from infiltrating cells and resident peritoneal cells. This amplifies the inflammatory response promoting fibrosis and angiogenesis in the peritoneal membrane (Marchant et al., 2020).
Oxidative and cellular stress plays a key role in the peritoneal membrane damage. Free radicals in the PD effluent indicate a higher risk of technique failure in stable patients (Morinaga et al., 2012; Xu et al., 2015). Hypertonic PD solutions with high glucose and/or low pH induces oxidative stress and cause apoptosis and autophagy PMCs (Simon et al., 2017; Hara et al., 2017; Wu et al., 2018). Peritoneal membrane damage further involves mitochondrial mechanisms (Ramil-Gómez et al., 2021) pertaining to the Reactive Oxygen Species (ROS) production (López-Armada et al., 2013). In a study the authors measured mitochondrial reactive oxygen species (mtROS) and membrane potential in PMCs with different phenotypes. They found that fibroblast-like PMCs had more mtROS and less membrane potential than epithelial-like PMCs. They also demonstrated that mtROS induced EMT in omental MCs, which was prevented by mitoTEMPO. Moreover, they showed that mitochondrial DNA (mtDNA) levels in PMCs correlated positively with dialysate/plasma creatinine ratio (D/P Creat) and negatively with UF (Ramil-Gómez et al., 2021; López-Armada et al., 2013).
These results suggest that mitochondrial dysfunction drives EMT in PMCs leading to peritoneal membrane damage. Furthermore, pertaining to cellular stress and membrane damage, mitochondria release damage-associated molecular patterns (DAMPs-mtROS or mtDNA), recognized by the innate immune system, and thus triggering pro-inflammatory and pro-fibrotic responses by activating Toll-like receptors (TLRs), and purinergic receptors (Raby et al., 2018; Mills et al., 2017; Anders and Schaefer, 2014). Raby et al. conducted a study to evaluate the involvement of TLRs and DAMPs in PD solutions-induced membrane fibrosis. They exposed human uremic peritoneal leukocytes, PMCs and mouse peritoneal leukocytes to different PD solutions (bio-incompatible or more bio-compatible) for a prolonged period and measured the pro-inflammatory DAMPs, measured fibrotic responses at the mRNA/protein levels and assessed the role of TLR2/4 in the sterile peritoneal inflammation and fibrosis (Raby et al., 2018). A study has shown that Nucleotide-binding oligomerization domain-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome, a member of the NLR family of intracellular sensors, also mediates sterile inflammation by regulating the release of the pro-inflammatory cytokine IL-1β which promotes peritoneal membrane damage and fibrosis (Hishida et al., 2019). Pharmacological Interventions targeting oxidative stress and their products are detailed in Table 2.
New PD solutions that are more bio-compatible have been made to mitigate the problem. Newer PD solutions, such as icodextrin, and taurin solutions, have been designed to reduce the deleterious effects of bio incompatible PD solutions exposure on the peritoneal membrane (del et al., 2016; Santamaría et al., 2015), and they help to sustain the physiological equilibrium of the peritoneal cavity. Icodextrin, is gradually absorbed from the peritoneal cavity which facilitates a sustained colloid osmotic gradient, resulting in enhanced UF compared to conventional PD solutions. Owing to its superior fluid removal capacity and reduced systemic glucose exposure, icodextrin is particularly advantageous for long-dwell PD exchanges in patients with high peritoneal solute transport rates, where it aids in optimizing volume status while minimizing the metabolic burden associated with glucose absorption (Dousdampanis et al., 2018; Ng et al., 2022). Biocompatible PD solutions, expose the patients to less Glucose Degradation Products (GDPs) than conventional solutions, and better preserve the residual renal function and diuresis with a decrease in peritonitis frequency, are created by using the following approaches: pH adjustment to neutral and GDP minimisation; bicarbonate (±lactate) buffering; glucose polymer substitution for dextrose (which also reduces pH along with GDP); and amino acids as osmotic agents (Htay et al., 2018; Zhou et al., 2016; Yohanna et al., 2015) (Table 3). Recent evidence suggests, sodium-glucose cotransporters (SGLTs) and glucose transporters (GLUTs) in the peritoneal membrane mediate glucose handling and absorption during glucose-based PD (Balzer et al., 2020).
PD solutions act as double edge sword for peritoneal membrane. Biocompatible solutions for PD have some advantages over conventional bio-incompatible solutions, but they also have some drawbacks (Table 4). One of the main challenges is their higher cost in some countries, which may limit their accessibility and affordability. Another issue is the lack of clear evidence on how they affect patient-level clinical outcomes, such as survival and quality of life. Therefore, their role in clinical practice needs to be further defined and evaluated.
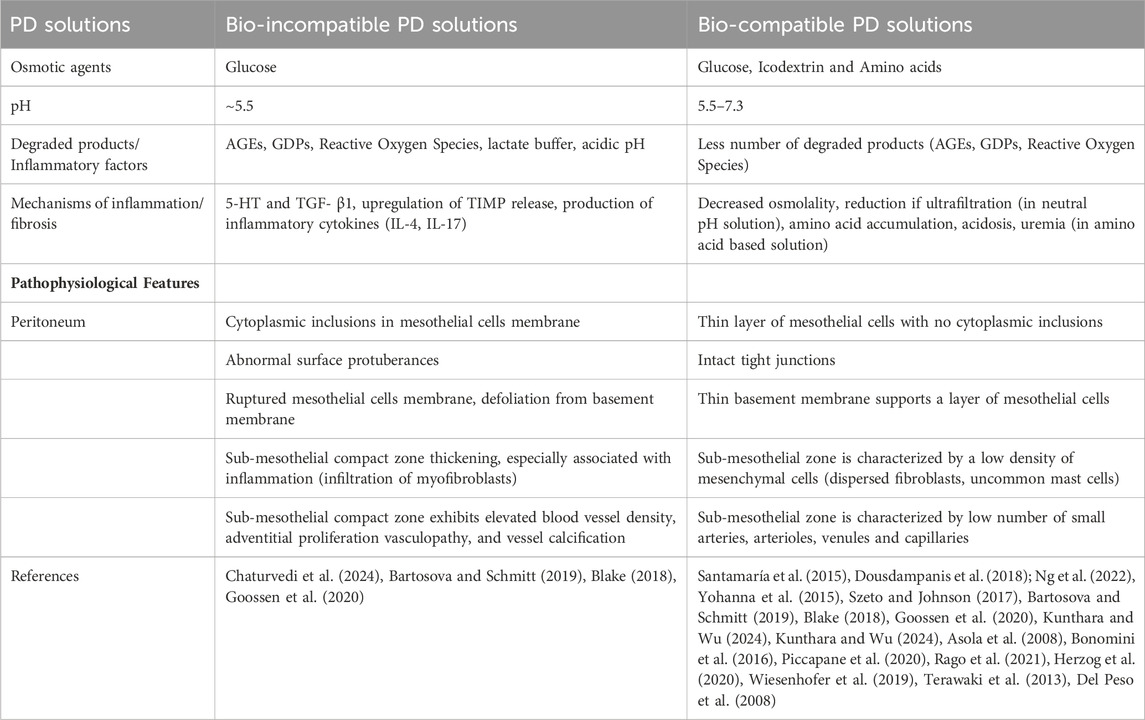
Table 4. Bio-incompatibility and biocompatibility of PD solutions: effects on peritoneal membrane pathophysiology.
4.2 Peritonitis
Peritonitis, a significant side effect, leads to PMCs damage, fibrosis, morbidity, and technical failure (Chou et al., 2022; Perl et al., 2020). Pro-fibrotic growth factors such as TGF-β1 and Fibroblast Growth Factor-2 (FGF-2) and inflammatory cytokines like IL-1β, IL-6, and others are upregulated during peritonitis (Kawaguchi et al., 2000; Virzì et al., 2022). IL-1β expression possibly relates inflammation and peritonitis intimately to the beginning and maintenance phase of the peritoneal fibrosis (Honda and Oda, 2005; Helmke et al., 2019). Likewise, IL-6 has been well connected to inflammation for functioning as solute transport increase across the PM (Wilson, 2018; Yang et al., 2020). Macrophages are reported to be the most abundant cells in PD effluent, which defines a central role in chronic inflammation (Helmke et al., 2019; Yang et al., 2020).
CX3CR1, receptor of CX3CL1 (detected on the peritoneal mesothelium), is expressed on macrophages in the peritoneal membrane (Helmke et al., 2019). A report demonstrated, dialysate exposure induces peritoneal fibrosis through CX3CR1-CX3CL1-mediated macrophage-mesothelial communication, which could be a novel therapeutic strategy for peritoneal fibrosis (Helmke et al., 2019). Based on the current data, peritonitis-induced membrane injury is associated with reduced membrane Cregs and altered complement activation products, such as C and C5b-9. The study also compared peritoneal injuries caused by fungal and P. aeruginosa peritonitis with those caused by Gram-positive bacterial peritonitis. Severe peritoneal membrane injuries due to fungal and P. aeruginosa peritonitis could result in the loss of expression of CRegs in the peritoneum, which increases deposition of complement activation products, suggesting deleterious effects in the membrane and further impaired Creg expression (Fukui et al., 2023). Zindel et al. documented, bacterial contamination activates mesothelial EGFR signalling in postsurgical PF, leading to MC-derived myofibroblasts and peritoneal adhesion (Zindel et al., 2021). Moreover, the authors in a 20-year PD cohort examined the peritoneal membrane characteristics and found that the high peritoneal transport group had a higher risk of peritonitis than the low or intermediate transport groups, even after adjusting for demographics, comorbidities, and biochemical parameters (Chou et al., 2022).
4.3 Peritoneal Angiogenesis
Peritoneal membrane solute transport and angiogenesis are regulated by matrix metalloproteinase 9 (MMP9) via β-catenin signaling. These factors impair ultrafiltration, cause chronic hypervolemia, and increase the risk of technique failure and mortality in PD patients (Padwal et al., 2017). Authors report that MMP9 mRNA expression in PMCs from peritoneal effluent of PD patients correlates with membrane solute transport properties and suggeste suggestes a role for MMP9 in peritoneal membrane injury. They proposed that MMP9 induced peritoneal angiogenesis by cleaving E-cadherin and activating b-catenin signaling, which increased VEGF expression (Padwal et al., 2017). New blood vessels form through angiogenesis, a highly complex process that depends on the balance between growth factors that stimulate or inhibit it (Zhu et al., 2021). Peritoneal angiogenesis plays a key role in developing peritoneal fibrosis (Zhu et al., 2021). Studies showed that TGF-β1 and VEGF-A mediate fibrosis in PD patients (Kinashi et al., 2018; Kariya et al., 2018). TGF-β1 stimulates VEGF-A expression, which in turn promotes angiogenesis (Kinashi et al., 2018; Kariya et al., 2018), indicating that the TGF-β1-VEGF-A pathway plays a key role in fibrosis-associated peritoneal angiogenesis (Kariya et al., 2018).
Twist, a basic helix-loop-helix DNA-binding protein, regulates E-cadherin expression and induces EMT and MMP9 expression in PMCs. The study revealed that peritoneal membrane injury increased Twist expression in the peritoneum (Margetts, 2012).
In a uremic rodent model, angiogenesis and fibrosis have been demonstrated during PD, accompanied by increased expression of angiopoietin (Ang)-2 and reduced expression of Ang receptor Tie2 (Stone et al., 2016; Yuan et al., 2009). With bio-incompatible PD solutions, AGEs and IL-6 can promote the production of VEGF, which has been detected in the effluent of long-term PD patients and involved in inflammation (Stone et al., 2016; Yuan et al., 2009). Therapeutic interventions targeting angiogenesis in the peritoneum are detailed in (Table 2).
5 Molecular Mechanisms and Therapeutic targets of signalling pathways involved in Peritoneal Fibrosis
For targeting treatments that prevent the fibrosis, it is imperative to know the molecular basis of the signalling mechanisms, which contribute to the balance between cell regeneration and activation, and maintenance of activity of myofibroblasts. Several intracellular signal transduction pathways during the process of peritoneal inflammation and angiogenesis associated with the pathophysiology behind fibrosis are depicted in Figure 4.
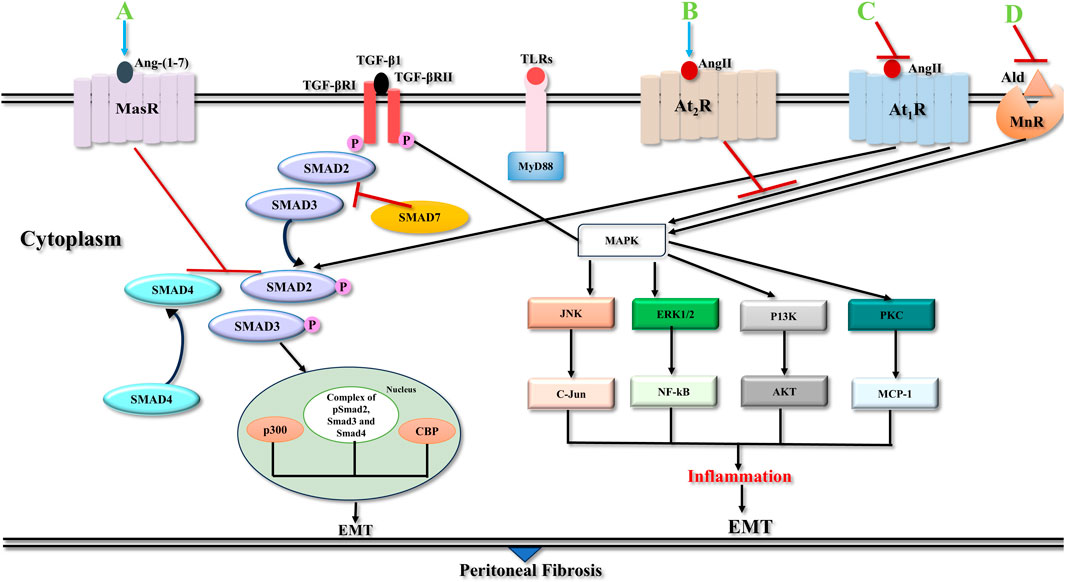
Figure 4. Signalling pathways in peritoneal fibrosis. TGF-βRI phosphorylates Smad2 and Smad3 and liberates them from Smad2/Smad3 receptor complex. Then after they form a Smad2/Smad3/Smad4 complex and relocate to the nuclear segment to regulate the expression of target genes with the help of various transcription factors/co-factors, CBP, p300 and others. Smad7 interferes with phosphorylation of Smad2/Smad3 by restricting their binding with TGF-β1 receptors or their movement to the nucleus. TGF-βRII and TLR ligands induce parallel pathways that culminate in the activation of various non-Smad signalling pathways, JNK, ERK1/2, PI3K, and PKC pathways which are important factors in the emergence of peritoneal fibrosis. Activation of At1R is involved in pro-fibrotic actions via classical RAAS pathway whereas At2R activation through alternative RAAS pathway induces anti-fibrotic actions. Binding of Ang II to At1R receptor activates various MAPKs, with complex of Smad2/3 and Smad4 leading to transcription of pro-fibrotic genes, i.e. CCN2, COl1A1, PAI1, TGF-β and PDGF. Ald activation of MnR receptor induces MAPKs and turns on the expression of pro-fibrotic proteins. Blocking At1R and MnR suppresses receptor activation and stop pro-fibrotic signaling. Activation of At2R/MasR, inhibits TGF-β fibrotic signaling. Pirfenidone might inhibit AT1R and activate MasR. Abbreviations: TGF-β1, Transforming Growth Factor Beta 1; TGF-βRI, Type I TGF-βI receptor; TGF-βRII, Type II TGF-βI receptor; CBP, Creb binding protein; TLRs, Toll like receptors; JNK, C-Jun N-terminal kinase; ERK1/2, Extracellular Signal-Regulated Kinase ½; PI3K, Phosphatidylinositol-3-kinase; PKC, Protein Kinase C; NF-kB, Nuclear Factor-kB; MCP-1, Monocyte Chemoattractant Protein-1; EMT, Epithelial to Mesenchymal transition; Ang II, Angiotensin II; Ald, Aldosterone; At1R, Angiotensin 1 receptor; At2R, Angiotensin 2 receptor; MnR, Mineralocorticoid receptor; MasR, Mas receptor; RAAS, Renin-Angiotensin- Aldosterone system; MAPKs, Mitogen activated protein kinases; CCN2, Cellular communication network factor 2; COL1A1, Alpha-1 type 1 collagen; PAI1, Plasminogen activator inhibitor 1; (A) Ang-(1–7); (B) Ang II; (C) Pirfenidone or Angiotensin convertase enzyme inhibitors (ACEIs) or Angiotensin receptor blocker (ARB); (D) Spironolactone.
TGF-β1 is central in the progression of EMT concluding to peritoneal fibrosis (Gangji et al., 2009; Yao et al., 2008). TGF-β1 is a member of the growth factor family that comprises TGF-βs, activins, and (BMPs) (Xu et al., 2009). PMCs plasticity depends on TGF-β1 and BMP-7, which can induce the epithelial or mesenchymal phenotype of PMCs, respectively (Loureiro et al., 2010). Peritoneal membrane deterioration in PD patients correlates with TGF-β1 levels in PD fluids, is reported in a study (Gangji et al., 2009). Blocking peptides of TGF-β1 protect the peritoneal membrane from PD fluid-induced damage in mice (Loureiro et al., 2011). TGF-β1 signalling employs both Smad-mediated and Smad-independent mechanisms (Suryantoro et al., 2023) (Figure 4).
5.1 Smad TGF-β1 Signalling
The main signaling pathways responsible for the EMT process in PMCs are induced by TGF-β1 (Davies, 2004). Heterodimeric serine/threonine kinase transmembrane receptor complexes mediate the signaling of TGF-β1 factors. The ligand binds to its primary receptor (receptor type II), which recruits, trans-phosphorylates, and activates the signaling receptor (receptor type I). The receptor type I of TGF-β1, also known as activin receptor-like kinase 5 (ALK5), phosphorylates Smad2 and 3 with its serine-threonine kinase activity. BMP-7 (ALK3) receptor type I phosphorylates Smads (1, 5, and 8). The phosphorylated Smads form heterodimers with Smad4, a common mediator of all Smad pathways (Gangji et al., 2009; Xu et al., 2009; Loureiro et al., 2011). These Smad heterocomplexes move to the nucleus, binding directly to DNA and activating specific target genes. Inhibitory Smad seven limits the Smad signaling triggered by TGF-β1. They prevent the phosphorylation and/or nuclear translocation of Smad2/3 or Smad1/5/8complexes and induce their degradation by recruiting ubiquitin ligases (Gangji et al., 2009; Suryantoro et al., 2023) MMT process arises from the integration of diverse signals triggered by multiple factors, making it difficult to establish a clear hierarchy or prioritize specific pathways (López-Cabrera, 2014).
The contribution of TGF-β1-BMP7-Gremlin-1-Smad pathway cross-talk has recently been reported to be involved in peritoneal fibrosis (Ruiqi et al., 2021). The authors report a possible mechanism, Gremlin-1 enhances the TGF-β1 signalling and suppresses the expression of BMP7 and Smad1/5/8, leading to EMT and peritoneal fibrosis (Ruiqi et al., 2021). In other study, BMP-7 inhibitory effect on EMT is dependent on the activation of Smad1/5/8 proteins that counteract TGF-β1 activated Smad2/3 activity (Raby et al., 2018). Smad3 signalling is essential for TGF-β1 induced EMT and fibrosis, as evidenced by Smad3 knockout mice. These mice display less peritoneal fibrosis, collagen accumulation, and EMT (Patel et al., 2010). EMT and peritoneal membrane fibrotic injury are reduced by inhibitory Smad7, which blocks Smad signaling (Patel et al., 2010).
5.1.1 Transcriptional Regulators
TGF-β1 activates Smad complexes that regulate the expression and activity of EMT transcription factors. Smad3 mediates the transcriptional induction of SNAIL-1 by TGF-β1 (Hoot et al., 2008). Smad3/4 also collaborates with SNAIL1 to repress the genes encoding E-cadherin and occludin in response to TGF-β1 (Vincent et al., 2009). Moreover, TGF-β1 induces ZEB1 expression, which is also modulated by non-Smad pathway (MAPK signalling), and Smad3/4 complexes interact with ZEB1 and ZEB2 to mediate TGFβ-regulated gene expression (Postigo, 2003).
Complete blocking of the Smad pathway does not entirely abolish the fibrosis, proposing that additional pathways are also convoluted in the fibrosis mechanisms (Kinashi et al., 2018).
5.2 Non-Smad TGF-β1 Signalling
Documentation of TGF-β1/Smads signaling help in understanding the fibrosis mechanism partially, but still, the entire phenomenon is incompletely understood. Complementing, the Smad proteins crosstalk with various non-canonical pathways to communicate downstream responses. Non-canonical pathways such as Mitogen activated protein kinases (MAPKs), Extracellular regulated Kinases (ERK1/2), and p-38, along with Jun N-terminal kinases (JNK), Src, phosphatidyl inositol 3 kinase (PI3Kinase)/AKT, Wnt/β-catenin/Ror2 signalling pathways, and NLRP3 inflammasome transduce the pro-fibrotic effects of TGF-β1 concluding to EMT and fibrosis as discussed below.
Map kinase (MEK)-ERK1/2 is a key pathway that mediates the effects of TGF-β1 on EMT and fibrosis (Gui et al., 2012). However, ERK- nuclear factor κ-light chain-enhancer of activated B cells (NF-kB)-Snail-1 pathway inhibition prevents this EMT induction. Moreover, the pathway inhibition also triggers mesenchymal to epithelial transition (MET), reverse process of EMT, in PMCs of patients on long term PD (Strippoli et al., 2008). The AMPK-NF-kB pathway mediates the anti-fibrotic molecule mechanism, which suppresses suppressing PAI-1 expression in PMCs and prevents TGF-β1 induced EMT in peritoneal fibrosis. (Strippoli et al., 2008).
In continuation of non-Smad pathways, TGF-β1 is reported to activate TAK-1 (TGF-β activated kinase 1), which mediates the phosphorylation and activation of p38 and JNK MAPKs pathway (Shim et al., 2005). The p38-mediated pathway controls the EMT process of PMCs through a feedback mechanism that reduces ERK1/2, TAK-1-NF-κB activities. JNK inhibition is stated to preserve E-cadherin expression and prevents EMT conversion (Strippoli et al., 2010). JLP, a (JNK interacting protein) family scaffold protein for MAPK pathway (Dhanasekaran et al., 2007), negatively regulates peritoneal fibrosis by modulating TGF-β1/Smad signalling pathway, EMT, autophagy, and apoptosis (Tian et al., 2022).
PI3K/AKT pathway is another non-Smad mechanism that has been widely investigated in EMT process, contributes pathologically to fibrosis (Lamouille et al., 2014). A report indicated that blocking the PI3K/AKT/mTOR pathway enhanced autophagy and reduced PF during PD (Jia et al., 2022). By reducing intracellular ROS levels, this inhibition enhances the expression of ZO-1 and E-cadherin, and suppresses the expression of p-PI3K/PI3K, p-mTOR/mTOR, fibroblast-specific proteins ferroptosis suppressor protein 1 (FSP1), and α-SMA, which are associated with fibroblast differentiation, thus alleviating fibrosis (Suryantoro et al., 2023). The PI3K/AKT pathway also plays a role in EMT. Wang et al. found that AKT is overactivated during MMT (Jia et al., 2022), and the expression levels of p-AKT and α-SMA in PMCs are significantly inhibited after intervention with the PI3K/AKT pathway blocker wortmannin (Jia et al., 2022). A recent study showed that rat PMCS incorporated adipose-derived mesenchymal stem cell-derived extracellular vesicles (ADSC-EVs) and exhibited increased proliferation and migration via the activation of MAPK-ERK1/2 and PI3K-Akt pathways, which prevented postoperative peritoneal adhesions (Shi M. et al., 2022).
Src activation contributes to peritoneal fibrosis by stimulating the TGF-β1 pathway in a chlorhexidine-stimulated model of peritoneal membrane injury (Wang et al., 2017). Src is required for the phosphorylation of TGF-β receptor (R)-II, which activates TGF-β1. TGF-β1 plays a critical role in EMT programming via Smad and non-Smad RAS/RAF/MEK/ERK pathways; the PI3K/AKT/mTOR pathway; and the STAT3 pathway, which regulate the expression of SNAIL, c-Myc and Cyclin D1 (Wilson et al., 2020).
5.2.1 Transcriptional Regulators
Snail, the master factor of EMT, inhibits E-cadherin expression directly. Both Smad and non-Smad pathways converge on the activation of Snaill (Cano et al., 2000). MEK-ERK1/2-Snail-1 pathway modulates E-cadherin and ZO-1 expression, which are essential for peritoneal membrane integrity. Blocking this pathway restored E-cadherin and ZO-1 levels, decreased peritoneal fibrosis, and enhanced membrane function (Strippoli et al., 2015). SNAIL expression in PMCs is negatively regulated by Smad3, MEK-ERK1/2, and NF-κB signaling pathways (Patel et al., 2010; Strippoli et al., 2008). SNAIL and TWIST, transcription factors that mediate EMT, are under the control of NF-κB, which works together with Snail to turn on the transcription of fibronectin (FN1) (Stanisavljevic et al., 2011; Šošić et al., 2003). Inhibition of AKT decreases the level of SNAIL-1 expression (Lamouille et al., 2012). SNAIL and p38 inhibition have similar effects on PMCs, while TWIST expression increases (Strippoli et al., 2010).
Evidence suggests that Heat Shock Proteins (HSPs) and Notch are involved in EMT other than kinase families. Notch signaling gets activated in the peritoneal membrane of animal fibrotic models through increased HES1/Jagged1 expression (Zehender et al., 2018). In one report involving rat peritoneal membrane (Zehender et al., 2018), HSP70 secured the PMCs from EMT via the involvement of Smad/Non-Smad pathways (Zehender et al., 2018).
5.3 Toll-like Receptor (TLR) ligand-mediated signalling pathways
When peritoneal injury and oxidative stress occurs in PMCs, nuclear high mobility group box 1 (HMGB1) protein, a DAMP, leaks out of the cells. This activates NF-κB, a transcription factor that regulates inflammatory responses. The recognition of HMGB1 by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and receptors for advanced glycation end-products (RAGE), receptors for both DAMPs and pathogen-associated molecular patterns (PAMPs), mediates this activation (Wilson et al., 2020; Liu et al., 2017). DAMPS or PAMPS activate PRRs (Raby and Labéta, 2018), which trigger inflammatory and proliferative events through NF-κB, cytokine and TGF-β1 release and IRAK signaling (IRAK) (Balzer, 2020; Raby and Labéta, 2018). These events activate EMT transcription factors SNAIL, TWIST, and ZEB, repress E-cadherin expression, and induce EMT (Wilson et al., 2020).
PMCs express TLRs such as TLR1, 2 and 5 excluding TLR4, which play an important role in the membrane inflammation process (Colmont et al., 2011). Various cell types, including peritoneal leukocytes and PMCs, express TLRs-2 and 4 in particular, which are major receptors for DAMPs (Raby et al., 2017; Anders and Schaefer, 2014; Colmont et al., 2011). TLRs recognise and respond to a wide range of DAMPs, such as HMGB-1 and HSPs (Anders and Schaefer, 2014; Chen and Nuñez, 2010). TLRs trigger MyD88 dependent signalling pathway upon ligand binding, leading to the activation of ERK1/2, JNK, p38 MAPKs, and NF-kB, as well as the secretion of proinflammatory cytokines, such as IL-6, IL-1β and TNF-α (Colmont et al., 2011). IL-1β induces NF-κB response more strongly than TGF-β1 in PMCs, and their co-stimulation results in an additive effect. NF-κB inhibition prevents EMT induction by TGF-β1/IL-1β costimulation and partially reverses EMT in PMCs obtained from PD patients (Strippoli et al., 2008).
5.4 Vascular Endothelial Growth Factor (VEGF) Signalling
Vascular endothelial growth factor (VEGF) is a specific mitogen of vascular endothelial cells (Lamouille et al., 2014). VEGF belongs to a gene family that includes VEGFA, placental growth factor, VEGFB, C, and D. IL-6, IL-1β, IL-8, MCP-1, TNF-α, and Prostaglandin E2, all involved in angiogenesis, endothelial cells survival, proliferation and capillary tube formation (Colmont et al., 2011). Inhibiting VEGF expression could reduce pathological angiogenesis in the membrane of the long-term PD patients, a report documents (Colmont et al., 2011) Another study documents, inhibiting VEGF attenuates fibrosis, by regulating TGF-β1 expression through the phosphoinositide 3-kinase (PI3K)/Akt pathway (Lee et al., 2008). Peritoneal tissues of PD patients with PF express VEGF significantly more than those of PD patients without PF (Abrahams et al., 2014). Peritoneal fibrosis, involves angiogenesis of human peritoneal vascular endothelial cells (HPVECs) mediated by VEGF/VEGF receptor 2 (VEGFR2) signalling. This signalling pathway also interacts with Hippo/YAP signalling, which regulates cell proliferation and differentiation. Pharmacological inhibition of VEGF/Hippo/YAP signaling reduced peritoneal angiogenesis and prevented further damage to the peritoneal membrane (Zhu et al., 2021). High glucose activates estrogen receptor 1 (ESR1) in PMCs, which induces EMT and peritoneal fibrosis in long term PD. ESR1 transcriptionally regulates H19, a long non-coding RNA that interacts with the transcriptional coactivator p300 to enhance the expression of VEGFA. Targeting the ESR1/H19/VEGFA pathway pharmacologically can attenuate high glucose-induced peritoneal fibrosis (Zhao et al., 2023).
5.5 NOD-like receptor protein 3 (NLRP3)/IL-1β signalling
PM inflammation and fibrosis are associated with the activation of NLRP3 inflammasome, an intracellular complex of the innate immune system. It activates caspase-1 and controls the secretion of cytokines IL-18 and IL-1β in response to PD solutions with high glucose content (Hishida et al., 2019).
5.6 Wnt/β-catenin/Ror2 Signalling
The wingless-type mouse mammary tumor virus integration site family (WNT) signalling pathway has two branches: the canonical branch, which depends on β-catenin, and the non-canonical branch, which does not. The canonical WNT signalling pathway interacts with the TGF-β1 pathway and enhances fibrosis in various organs (He et al., 2009; Guo et al., 2012). Wnt/β-catenin signaling pathway is involved in the EMT of PMCs (Fan et al., 2021). WNT5A is a typical example of a non-canonical WNT protein (Mikels et al., 2009).
5.6.1 Wnt/β-catenin Signalling
The role of WNT signalling in peritoneal membrane injury has been recently explored. WNT signaling and WNT1 expression in MCs are activated by peritoneal fibrosis and correlate with solute transport in PD patients. TGF-β1 enhances WNT2 and WNT4 expression and induces peritoneal fibrosis in mice. Adenovirus expressing TGF-β1 (AdTGF-β) infection increases β-catenin and other WNT signaling components in the peritoneum, indicating TGF-β1/β-catenin crosstalk in peritoneal membrane injury (Padwal et al., 2018). Wnt/β-catenin signaling pathway is upregulated in peritoneal dialysate-induced peritoneal fibrosis; the EMT process is blocked by the use of recombinant human Dickkopf-related protein 1 (Wnt/β-catenin inhibitor) (Guo et al., 2017) and herbal mixture which mitigates peritoneal membrane thickness and fibrosis (Huang et al., 2022).
5.6.2 Wnt/Ror2 Signalling
The non-canonical WNT pathway is poorly understood in the context of peritoneal membrane damage. WNT5A binds to Ror2 and blocks WNT/β-catenin signalling, reducing β-catenin and pGSK3β levels. WNT5A also decreases peritoneal fibrosis and angiogenesis in mice. Ror2 regulates WNT5A-induced fibronectin and VEGF expression in human mesothelial cells. WNT5A prevents peritoneal injury via Ror2 and WNT/β-catenin pathways (Padwal et al., 2020).
6 Strategies to preserve the peritoneal membrane from fibrosis
PD solutions have a vital role in the pathophysiology of peritoneal fibrosis, as mentioned above. However, the interpretation of the above information should be carefully evaluated considering the “caveat” of a possible lack of specificity, the “pharmacological approach” is especially relevant from a translational point of view, since it is possible to hypothesize the design of pharmacological treatments designed to specifically preserve or recuperate the peritoneal membrane homeostasis in PD patients. We have detailed the importance of improving biocompatibility and efficacy of PD solutions, and pharmacological interventions/stem cell treatment strategies in the mechanisms involved during peritoneal membrane fibrosis in Table 3.
6.1 Effluent Biomarkers to assess peritoneal dialysis performance and peritoneal fibrosis
A fibrosis marker that can be accessed in the dialysate would help us identify PD patients with peritoneal membrane deterioration and high risk of complications (Li YC. et al., 2021).
IL-6, a chronic peritoneal inflammation marker, is the main biomarker used in PD and measured in dialysis fluid (Suryantoro et al., 2023; Li YC. et al., 2021). Bacterial clearance and infection (acute or subclinical) in PD patients can be assessed by IL-6 levels in PD effluent. Certain biomarkers present in dialysis fluid, such as IL-6, IL-8, and cancer antigen-125 (CA-125), are employed to estimate the quantity of peritoneal macrophages (PMCs) and assess the overall health of the peritoneal membrane (Masola et al., 2022). Some are emerging, like inflammatory markers (Th17 secreted IL-17, T reg cells, and M1/M2 macrophages), some are excreted by PMCs such as miRNAs, aquaporin-1 (AQP-1) (Lopes Barreto and Krediet, 2013) and also markers of cell stress/ageing/senescence, advanced oxidized protein products (AOPPs) (Corciulo et al., 2019). The European Training and Research in PD Network (EuTRiPD) network covers them all (Aufricht et al., 2017). Furthermore, one study provides evidence for the role of AQP-1 as the ultrafiltration mediator in the human peritoneal membrane (Corciulo et al., 2019). Tweaking of Th17 response and boosting the Treg response, might save the peritoneal membrane from damage as explicated in the study (Corciulo et al., 2019). Exposure to hyperglycemic dialysate induces oxidative stress-mediated cellular senescence. Therefore, dialysate advanced oxidized protein products levels may reflect PMC senescence and injury. Moreover, dialysate toxicity and inflammation followed by MMT process modulate the expression of Hsp27 and Hsp72. Dialysate levels of VEGF, MMP-2, and Plasminogen activator inhibitor-1 (PAI-1), associated with PD duration, may serve as indicators of PD-linked peritoneal fibrosis, according to prospective cohort studies (Corciulo et al., 2019). Proteomics and metabolomics could find new biomarkers in PD effluent that could signal PM problems. The metabolic state in PD effluent could tell how healthy the membrane is and how long it will survive. Metabolic profiling of serum and PD effluent may facilitate the evaluation of PM permeability and the early detection of PM dysfunction. Furthermore, metabolite-enriched PD solutions may prevent membrane inflammation and fibrosis, thereby preserving the peritoneal membrane’s permeability (Devuyst et al., 1998). One study suggested that effluent decoy receptor-2 (eDcR2) levels may reflect peritoneal fibrosis in PD patients (Kondou et al., 2022). Moreover, serum α-Klotho and galectin-3 levels were associated with peritoneal membrane thickness and PD duration (Yang et al., 2022). A study has shown that increased dialysate-to-plasma (D/P) creatinine ratio is associated with increased risk of mortality in PD patients (Padwal et al., 2018). The same study has demonstrated that the WNT1 gene and protein expression are correlated with peritoneal solute transport rate (PSTR) measured by D/P creatinine. Therefore, WNT1 may be a biomarker for clinical outcomes in PD patients (Padwal et al., 2018). Osteopontin, a phosphorylated glycoprotein involved in inflammation, EMT, and fibrosis may also be a useful indicator of peritoneal deterioration in long term PD patients (Li J. et al., 2021). Regression analysis of 109 PD patients showed Osteopontin levels in peritoneal effluents were an independent predictive factor for the increased PSTR (Li J. et al., 2021; Kothari et al., 2016) Effluent dialysate mtDNA levels could serve as a prognostic biomarker of peritoneal membrane damage induced by PD (Ramil-Gómez et al., 2021; Xie et al., 2019). High CCL8 levels in PD effluents may be associated with an increased risk of PD failure, and the CCL8 pathway is associated with PF (Lee et al., 2023). A study, using Fourier transform infrared (FTIR) spectroscopy, characterized the molecular profiles of PD effluents and clinical data, and used machine learning to assess the potential of this proof-of-principle study for low-cost, high-speed diagnosis in PD (Grunert et al., 2020). A recent study, highlighting that MMT associated biomarkers and clinical data under Machine Learning models (MAUXI software) can predict endurance and different PD technique failures, opening new avenues to individual treatments (Arriero-País et al., 2025). List of Markers are detailed in Table 5.
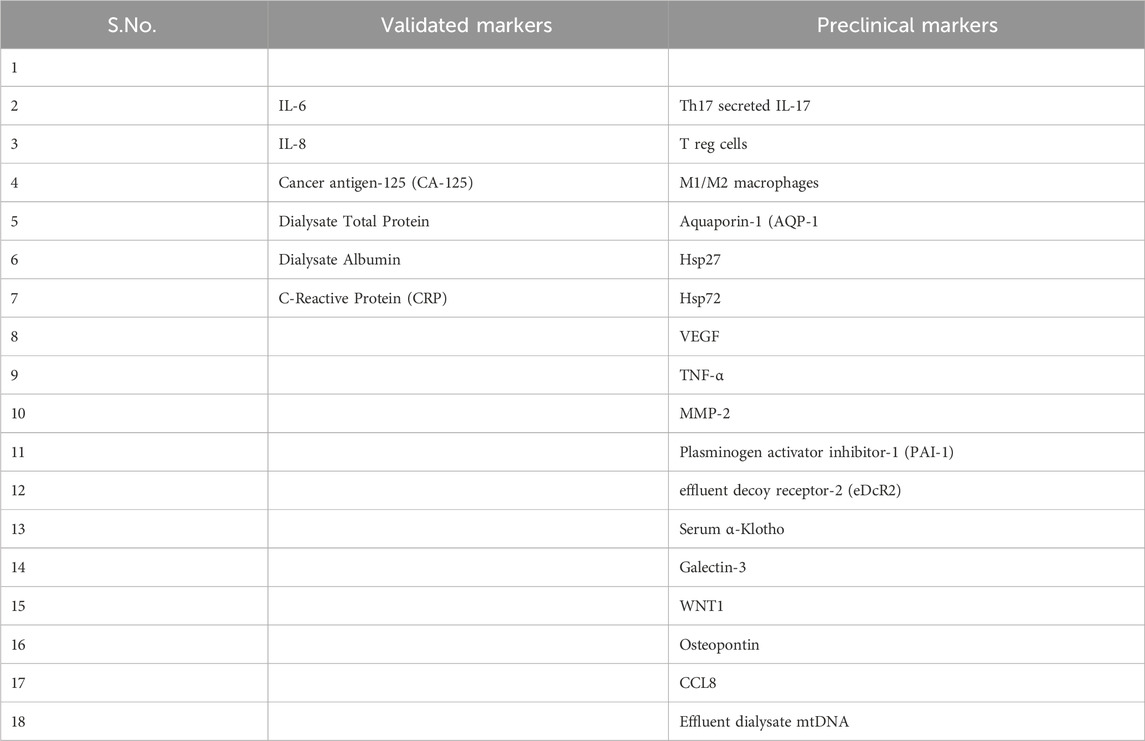
Table 5. List of validated and Pre-clinical targets for peritoneal fibrosis during peritoneal dialysis.
7 Conclusion
Long-term PD leads to peritoneal membrane thickening and, ultimately, fibrosis. The peritoneal membrane suffers less harm from PD nowadays, as catheter complications have decreased and PD solutions have become more compatible with the body. It is important to note that the development of pharmacological interventions targeting peritoneal fibrosis in PD patients is still an active area of research, and much work remains to fully understand the molecular mechanisms in peritoneal fibrosis and membrane survival. Effective therapies to prevent this process remain to be developed. Other non-pharmacological strategies, such as optimizing dialysis prescription, use of biocompatible PD solutions, and individualized patient care, also play crucial roles in managing peritoneal fibrosis and improving patient outcomes. The success and safety of the newer interventions mentioned in this narrative review need to be further evaluated through clinical trials before they can be implemented in routine clinical practice.
8 Future Directions
Notably, future directions can be aimed at gathering information on single-cell transcriptomic studies and knockout models to enhance the peritoneal membrane viability and developing new ways to predict, detect or monitor how the peritoneal membrane works or gets injured. This could include: 1) More sophisticated mathematical models to support the peritoneal membrane function testing, such as detailed descriptions of icodextrin, macromolecular or trans-capillary aspects of peritoneal membrane function, 2) Peritoneal Equilibration Test (PET), which measures the solute and water transfer across the PM to semi-quantitatively, a non-invasive method, assess PD performance, that should be established in the clinics, 3) Better biomarkers need to be validated and used to create reliable models of prognosis, 4) Omics-based biomarkers in PD effluent of long term PD patients have the potential to predict or diagnose peritoneal membrane dysfunction, enabling the establishment of reliable early prognostic tools and, possibly, the discovery of novel therapeutic targets, 5) Peritoneal microbiota needs to be investigated in fibrogenesis, to elucidate the molecular mechanisms developmental pathways of peritoneal fibrosis, Testing treatments that protect the peritoneal membrane could include: 1) New therapies use antibodies or proteins to change the expression of these molecules that cause inflammation, which can help stop the PMCs from transforming into fibrous tissue. For example: Based on the current knowledge and recent data, pharmacological modulation of GLUTs may antagonize glucose absorption and improve peritoneal ultrafiltration which represents a major goal for future research in PD., 2) Oral or dialysate additives that prevent inflammation or scarring of peritoneal membrane, and 3) New dialysis solutions with adjusted pH. Other targets aimed at sustaining PMCs viability and regulating intricate interplay between the peritoneal membrane immune system and PMCs may help in delaying peritoneal fibrosis. By elucidating the cellular and molecular mechanisms of peritoneal membrane fibrosis, both the fundamental and translational research can be advanced, as it may enable the development of therapeutic interventions to prevent the damage and restore the homeostasis of the peritoneal membrane. Collectively, the above documented information may open the avenue for developing a novel therapy in regulating peritoneal fibrosis in PD.
The review has some limitations, such as possible publication bias that favours positive results and distorts the evaluation of therapeutic approaches. More extensive studies are needed to identify effective targets for PD patients, especially to protect the peritoneal membrane integrity and prevent EMT. Overcoming these limitations is essential for developing safe and efficacious therapies in diverse clinical scenarios of peritoneal fibrosis.
Author contributions
NP: Conceptualization, Validation, Writing – review and editing, Supervision, Funding acquisition, Resources, Project administration, Writing – original draft. SC: Visualization, Formal analysis, Writing – original draft, Methodology, Conceptualization, Writing – review and editing. HS: Formal analysis, Writing – original draft, Visualization, Validation, Writing – review and editing, Conceptualization, Methodology. MU: Methodology, Formal analysis, Writing – review and editing. AR: Methodology, Formal analysis, Writing – review and editing. MJ: Investigation, Methodology, Writing – review and editing, Formal analysis. AJ: Writing – review and editing, Writing – original draft, Investigation, Methodology. SK: Formal analysis, Methodology, Writing – review and editing, Investigation. VA: Visualization, Validation, Writing – review and editing, Investigation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We appreciate the funding from the Indian Council of Medical Research, New Delhi, India (Grant number 5/4/7-4/15/NCD-II).
Acknowledgments
We acknowledge the students and post-doctoral fellows for their critical comments and contributions in finalizing this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrahams, A. C., Habib, S. M., Dendooven, A., Riser, B. L., van der Veer, J. W., Toorop, R. J., et al. (2014). Patients with encapsulating peritoneal sclerosis have increased peritoneal expression of connective tissue growth factor (CCN2), transforming growth factor-β1, and vascular endothelial growth factor. PLoS One 9, e112050. doi:10.1371/journal.pone.0112050
Anders, H. J., and Schaefer, L. (2014). Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 25, 1387–1400. doi:10.1681/ASN.2014010117
Aroeira, L. S., Aguilera, A., Sánchez-Tomero, J. A., Bajo, M. A., del Peso, G., Jiménez-Heffernan, J. A., et al. (2007). Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J. Am. Soc. Nephrol. 18, 2004–2013. doi:10.1681/ASN.2006111292
Arriero-País, E. M., Bajo-Rubio, M. A., Arrojo-García, R., Sandoval, P., González-Mateo, G. T., Albar-Vizcaíno, P., et al. (2025). Biomarker and clinical data–based predictor tool (MAUXI) for ultrafiltration failure and cardiovascular outcome in peritoneal dialysis patients: a retrospective and longitudinal study. BMJ Health Care Inf. 32 (1), e101138. doi:10.1136/bmjhci-2024-101138
Asola, M., Virtanen, K., Någren, K., Helin, S., Taittonen, M., Kastarinen, H., et al. (2008). Amino-acid-based peritoneal dialysis solution improves amino-acid transport into skeletal muscle. Kidney Int. Suppl. 108, S131–S136. doi:10.1038/sj.ki.5002614
Aufricht, C., Beelen, R., Eberl, M., Fischbach, M., Fraser, D., Jörres, A., et al. (2017). Biomarker research to improve clinical outcomes of peritoneal dialysis: consensus of the European Training and Research in Peritoneal Dialysis (EuTRiPD) network. Kidney Int. 92, 824–835. doi:10.1016/j.kint.2017.02.037
Balzer, M. S. (2020). Molecular pathways in peritoneal fibrosis. Cell Signal 75, 109778. doi:10.1016/j.cellsig.2020.109778
Balzer, M. S., Rong, S., Nordlohne, J., Zemtsovski, J. D., Schmidt, S., Stapel, B., et al. (2020). SGLT2 inhibition by intraperitoneal dapagliflozin mitigates peritoneal fibrosis and ultrafiltration failure in a mouse model of chronic peritoneal exposure to high-glucose dialysate. Biomolecules 10, 1573. doi:10.3390/biom10111573
Baroni, G., Schuinski, A., de Moraes, T. P., Meyer, F., and Pecoits-Filho, R. (2012). Inflammation and the peritoneal membrane: causes and impact on structure and function during peritoneal dialysis. Mediat. Inflamm. 2012, 912595. doi:10.1155/2012/912595
Bartosova, M., and Schmitt, C. P. (2019). Biocompatible peritoneal dialysis: the target is still way off. Front. Physiol. 9, 1853. doi:10.3389/fphys.2018.01853
Bello, A. K., Okpechi, I. G., Osman, M. A., Cho, Y., Cullis, B., Htay, H., et al. (2022). Epidemiology of peritoneal dialysis outcomes. Nat. Rev. Nephrol. 18, 779–793. doi:10.1038/s41581-022-00623-7
Blake, P. G. (2018). Is the peritoneal dialysis biocompatibility hypothesis dead? Kidney Int. 94, 246–248. doi:10.1016/j.kint.2018.04.014
Bonomini, M., Di Silvestre, S., Di Tomo, P., Di Pietro, N., Mandatori, D., Di Liberato, L., et al. (2016). Effect of peritoneal dialysis fluid containing osmo-metabolic agents on human endothelial cells. Drug Des. Devel. Ther. 10, 3925–3932. doi:10.2147/DDDT.S117078
Budi, E. H., Duan, D., and Derynck, R. (2017). Transforming growth Factor-β receptors and smads: regulatory complexity and functional versatility. Trends Cell Biol. 27, 658–672. doi:10.1016/j.tcb.2017.04.005
Cano, A., Pérez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., et al. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83. doi:10.1038/35000025
Catalan, M. P., Santamaría, B., Reyero, A., Ortiz, A., Egido, J., and Ortiz, A. (2005). 3,4-di-deoxyglucosone-3-ene promotes leukocyte apoptosis. Kidney Int. 68, 1303–1311. doi:10.1111/j.1523-1755.2005.00528.x
Catar, R. A., Bartosova, M., Kawka, E., Chen, L., Marinovic, I., Zhang, C., et al. (2022). Angiogenic role of mesothelium-derived chemokine CXCL1 during unfavorable peritoneal tissue remodeling in patients receiving peritoneal dialysis as renal replacement therapy. Front. Immunol. 13, 821681. doi:10.3389/fimmu.2022.821681
Chaturvedi, S., Singh, H., Agarwal, V., Jaiswal, A., and Prasad, N. (2024). Unravelling the role of Sildenafil and SB204741 in suppressing fibrotic potential of peritoneal fibroblasts obtained from PD patients. Front. Pharmacol. 14, 1279330. doi:10.3389/fphar.2023.1279330
Chen, C. S. (2008). Mechanotransduction - a field pulling together? J. Cell Sci. 121, 3285–3292. doi:10.1242/jcs.023507
Chen, G. Y., and Nuñez, G. (2010). Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837. doi:10.1038/nri2873
Cho, Y., Hawley, C. M., and Johnson, D. W. (2014). Clinical causes of inflammation in peritoneal dialysis patients. Int. J. Nephrol. 2014, 909373. doi:10.1155/2014/909373
Chou, Y. H., Chen, Y. T., Chen, J. Y., Tarng, D. C., and Lin, C. C. (2022). Baseline peritoneal membrane transport characteristics are associated with peritonitis risk in incident peritoneal dialysis patients. Membranes 12, 276. doi:10.3390/membranes12030276
Colmont, C. S., Raby, A. C., Dioszeghy, V., Lebouder, E., Foster, T. L., Jones, S. A., et al. (2011). Human peritoneal mesothelial cells respond to bacterial ligands through a specific subset of toll-like receptors. Nephrol. Dial. Transpl. 26, 4079–4090. doi:10.1093/ndt/gfr217
Corciulo, S., Nicoletti, M. C., Mastrofrancesco, L., Milano, S., Mastrodonato, M., Carmosino, M., et al. (2019). AQP1-Containing exosomes in peritoneal dialysis effluent as biomarker of dialysis efficiency. Cells 8, 330. doi:10.3390/cells8040330
Davies, S. J. (2004). Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int. 66 (6), 2437–2445. doi:10.1111/j.1523-1755.2004.66021.x
de Andrade, L. S., Sardá, F. A. H., Pereira, N. B. F., Teixeira, R. R., Rodrigues, S. D., de Lima, J. D., et al. (2021). Effect of unripe banana flour on gut-derived uremic toxins in individuals undergoing peritoneal dialysis: a randomized, double-blind, placebo-controlled, crossover trial. Nutrients 13 (2), 646. doi:10.3390/nu13020646
del, P. G., Jiménez-Heffernan, J. A., Selgas, R., Remón, C., Ossorio, M., Fernández-Perpén, A., et al. (2016). Biocompatible dialysis solutions preserve peritoneal mesothelial cell and vessel wall integrity. A case-control study on human biopsies. Perit. Dial. Int. 36 (2), 129–134. doi:10.3747/pdi.2014.00038
Del Peso, G., Jiménez-Heffernan, J. A., Bajo, M. A., Aroeira, L. S., Aguilera, A., Fernández-Perpén, A., et al. (2008). Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int. Suppl. 108, S26–S33. doi:10.1038/sj.ki.5002598
Devuyst, O., Nielsen, S., Cosyns, J. P., Smith, B. L., Agre, P., Squifflet, J. P., et al. (1998). Aquaporin-1 and endothelial nitric oxide synthase expression in capillary endothelia of human peritoneum. Am. J. Physiol. 275, H234–H242. doi:10.1152/ajpheart.1998.275.1.H234
Dhanasekaran, D. N., Kashef, K., Lee, C. M., Xu, H., and Reddy, E. P. (2007). Scaffold proteins of MAP-Kinase modules. Oncogene 26, 3185–3202. doi:10.1038/sj.onc.1210411
Dousdampanis, P., Musso, C. G., and Trigka, K. (2018). Icodextrin and peritoneal dialysis: advantages and new applications. Int. Urol. Nephrol. 50 (3), 495–500. doi:10.1007/s11255-017-1647-2
Fan, Y., Zhao, X., Ma, J., and Yang, L. (2021). LncRNA GAS5 competitively combined with miR-21 regulates PTEN and influences EMT of peritoneal mesothelial cells via Wnt/β-Catenin signaling pathway. Front. Physiol. 12, 654951. doi:10.3389/fphys.2021.654951
Fielding, C. A., Jones, G. W., McLoughlin, R. M., McLeod, L., Hammond, V. J., Uceda, J., et al. (2014). Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40, 40–50. doi:10.1016/j.immuni.2013.10.022
Fukui, S., Mizuno, M., Tawada, M., Suzuki, Y., Kojima, H., Matsukawa, Y., et al. (2023). Peritoneal expression of membrane complement regulators is decreased in peritoneal dialysis patients with infected peritonitis. Int. J. Mol. Sci. 24, 9146. doi:10.3390/ijms24119146
Fung, W. W., Poon, P. Y. K., Ng, J. K. C., Kwong, V. W. K., Pang, W. F., Kwan, B. C. H., et al. (2020). Longitudinal changes of NF-κB downstream mediators and peritoneal transport characteristics in incident peritoneal dialysis patients. Sci. Rep. 10, 6440. doi:10.1038/s41598-020-63258-3
Gangji, A. S., Brimble, K. S., and Margetts, P. J. (2009). Association between markers of inflammation, fibrosis and hypervolemia in peritoneal dialysis patients. Blood Purif. 28, 354–358. doi:10.1159/000232937
George Kunthara, M. (2024). Preservation of Peritoneal Membrane Structure and Function in Peritoneal Dialysis. Updates on Renal Replacement Therapy. IntechOpen. Available online at: http://dx.doi.org/10.5772/intechopen.111586
González-Mateo, G. T., Aguirre, A. R., Loureiro, J., Abensur, H., Sandoval, P., Sánchez-Tomero, J. A., et al. (2015). Rapamycin protects from Type-I peritoneal membrane failure inhibiting the angiogenesis, lymphangiogenesis, and Endo-MT. Biomed. Res. Int. 2015, 989560. doi:10.1155/2015/989560
Goossen, K., Becker, M., Marshall, M. R., Bühn, S., Breuing, J., Firanek, C. A., et al. (2020). Icodextrin versus glucose solutions for the once-daily long dwell in peritoneal dialysis: an enriched systematic review and meta-analysis of randomized controlled trials. Am. J. Kidney Dis. 75, 830–846. doi:10.1053/j.ajkd.2019.10.004
Grunert, T., Herzog, R., Wiesenhofer, F. M., Vychytil, A., Ehling-Schulz, M., and Kratochwill, K. (2020). Vibrational spectroscopy of peritoneal dialysis effluent for rapid assessment of patient characteristics. Biomolecules 10, 965. doi:10.3390/biom10060965
Gui, T., Sun, Y., Shimokado, A., and Muragaki, Y. (2012). The roles of mitogen-activated protein kinase pathways in TGF-β-Induced epithelial-mesenchymal transition. J. Signal Transduct. 2012, 289243. doi:10.1155/2012/289243
Guo, Y., Sun, L., Xiao, L., Gou, R., Fang, Y., Liang, Y., et al. (2017). Aberrant Wnt/beta-catenin pathway activation in dialysate-induced peritoneal fibrosis. Front. Pharmacol. 8, 774. doi:10.3389/fphar.2017.00774
Guo, Y., Wang, L., Gou, R., Tang, L., and Liu, P. (2020). Noncoding RNAs in peritoneal fibrosis: background, mechanism, and therapeutic approach. Biomed. Pharmacother. 129, 110385. doi:10.1016/j.biopha.2020.110385
Guo, Y., Wang, L., Gou, R., Wang, Y., Shi, X., Zhang, Y., et al. (2021). Ameliorative role of SIRT1 in peritoneal fibrosis: an in vivo and in vitro study. Cell Biosci. 11, 79. doi:10.1186/s13578-021-00591-8
Guo, Y., Xiao, L., Sun, L., and Liu, F. (2012). Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol. Res. 61, 337–346. doi:10.33549/physiolres.932289
Hara, K., Hamada, C., Wakabayashi, K., Kanda, R., Kaneko, K., Horikoshi, S., et al. (2017). Scavenging of reactive oxygen species by astaxanthin inhibits epithelial-mesenchymal transition in high glucose-stimulated mesothelial cells. PLoS One 12, e0184332. doi:10.1371/journal.pone.0184332
He, W., Dai, C., Li, Y., Zeng, G., Monga, S. P., and Liu, Y. (2009). Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776. doi:10.1681/ASN.2008060566
Helmke, A., Nordlohne, J., Balzer, M. S., Dong, L., Rong, S., Hiss, M., et al. (2019). CX3CL1-CX3CR1 interaction mediates macrophage-mesothelial cross talk and promotes peritoneal fibrosis. Kidney Int. 95, 1405–1417. doi:10.1016/j.kint.2018.12.030
Herzog, R., Bartosova, M., Tarantino, S., Wagner, A., Unterwurzacher, M., Sacnun, J. M., et al. (2020). Peritoneal dialysis fluid supplementation with alanyl-glutamine attenuates conventional dialysis fluid-mediated endothelial cell injury by restoring perturbed cytoprotective responses. Biomolecules 10, 1678. doi:10.3390/biom10121678
Herzog, R., Sacnun, J. M., González-Mateo, G., Bartosova, M., Bialas, K., Wagner, A., et al. (2021). Lithium preserves peritoneal membrane integrity by suppressing mesothelial cell αB-crystallin. Sci. Transl. Med. 13 (608), eaaz9705. doi:10.1126/scitranslmed.aaz9705
Hishida, E., Ito, H., Komada, T., Karasawa, T., Kimura, H., Watanabe, S., et al. (2019). Crucial role of NLRP3 inflammasome in the development of peritoneal dialysis-related peritoneal fibrosis. Sci. Rep. 9, 10363. doi:10.1038/s41598-019-46504-1
Honda, K., and Oda, H. (2005). Pathology of encapsulating peritoneal sclerosis. Perit. Dial. Int. 25, S19–S29. doi:10.1177/089686080502504s04
Hoot, K. E., Lighthall, J., Han, G., Lu, S. L., Li, A., Ju, W., et al. (2008). Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J. Clin. Invest. 118, (8), 2722–32. doi:10.1172/JCI33713
Htay, H., Johnson, D. W., Wiggins, K. J., Badve, S. V., Craig, J. C., Strippoli, G. F., et al. (2018). Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Sys Rev. 10, CD007554. doi:10.1002/14651858.CD007554.pub3
Huang, L., Wang, Z., and Li, Y. (2022). Intervention mechanism of Niao Du Kang mixture on the EMT process of peritoneal fibrosis based on the Wnt/β-Catenin signaling pathway. Evid. Based Complement. Altern. Med. 2022, 2089483. doi:10.1155/2022/2089483
Huang, Y., Ma, J., Fan, Y., and Yang, L. (2023). Mechanisms of human umbilical cord mesenchymal stem cells-derived exosomal lncRNA GAS5 in alleviating EMT of HPMCs via Wnt/β-catenin signaling pathway. Aging 15, 4144–4158. doi:10.18632/aging.204719
Ichihara, M., Sobue, S., Ito, M., Ito, M., Hirayama, M., and Ohno, K. (2015). Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med. Gas. Res. 5, 12. doi:10.1186/s13618-015-0035-1
Io, K., Nishino, T., Obata, Y., Kitamura, M., Koji, T., and Kohno, S. (2015). SAHA suppresses peritoneal fibrosis in mice. Perit. Dial. Int. 35, 246–258. doi:10.3747/pdi.2013.00089
Jagirdar, R. M., Bozikas, A., Zarogiannis, S. G., Bartosova, M., Schmitt, C. P., and Liakopoulos, V. (2019). Encapsulating peritoneal sclerosis: pathophysiology and current treatment options. Int. J. Mol. Sci. 20, 5765. doi:10.3390/ijms20225765
Jia, M., Qiu, H., Zhang, S., Li, D., and Jin, D. (2022). Inhibition of PI3K/AKT/mTOR signalling pathway activates autophagy and suppresses peritoneal fibrosis in the process of peritoneal dialysis. Front. Physiol. 13, 778479. doi:10.3389/fphys.2022.778479
Jiang, N., Zhang, C., Feng, H., Yuan, J., Ding, L., Fang, W., et al. (2021). Clinical characteristics associated with the properties of gut microbiota in peritoneal dialysis patients. Perit. Dial. Int. 41 (3), 298–306. doi:10.1177/0896860820976983
Jiao, T., Huang, Y., Sun, H., and Yang, L. (2023). Exosomal lnc-CDHR derived from human umbilical cord mesenchymal stem cells attenuates peritoneal epithelial-mesenchymal transition through AKT/FOXO pathway. Aging 15, 6921–6932. doi:10.18632/aging.204883
Jin, G., Su, Y., Dong, Q., Zhao, X., Zhang, L., and Yan, X. (2019). Arctigenin alleviates TGF-β1-induced epithelial-mesenchymal transition and PAI-1 expression via AMPK/NF-κB pathway in peritoneal mesothelial cells. Biochem. Biophys. Res. Commun. 520, 413–419. doi:10.1016/j.bbrc.2019.09.130
Jörres, A. (2012). Novel peritoneal dialysis solutions--what are the clinical implications? Blood Purif. 33, 153–159. doi:10.1159/000334151
Kang, D. H. (2020). Loosening of the mesothelial barrier as an early therapeutic target to preserve peritoneal function in peritoneal dialysis. Kidney Res. Clin. Pract. 39, 136–144. doi:10.23876/j.krcp.20.052
Kang, S. H., Kim, S. O., Cho, K. H., Park, J. W., Yoon, K. W., and Do, J. Y. (2014). Paricalcitol ameliorates epithelial-to-mesenchymal transition in the peritoneal mesothelium. Nephron Exp. Nephrol. 126, 1–7. doi:10.1159/000357156
Kariya, T., Nishimura, H., Mizuno, M., Suzuki, Y., Matsukawa, Y., Sakata, F., et al. (2018). TGF-β1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am. J. Physiol. Ren. Physiol. 314, F167–F180. doi:10.1152/ajprenal.00052.2017
Kawaguchi, Y., Kawanishi, H., Mujais, S., Topley, N., and Oreopoulos, D. G. (2000). Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. Perit. Dial. Int. 20, S43–S55. doi:10.1177/089686080002004s04
Kim, Y. C., Kim, K. H., Lee, S., Jo, J. W., Park, J. Y., Park, M. S., et al. (2019). ST2 blockade mitigates peritoneal fibrosis induced by TGF-β and high glucose. J. Cell Mol. Med. 23, 6872–6884. doi:10.1111/jcmm.14571
Kinashi, H., Ito, Y., Sun, T., Katsuno, T., and Takei, Y. (2018). Roles of the TGF-β-VEGF-C pathway in fibrosis-related lymphangiogenesis. Int. J. Mol. Sci. 19, 2487. doi:10.3390/ijms19092487
Kondou, A., Begou, O., Dotis, J., Karava, V., Panteris, E., Taparkou, A., et al. (2022). Impact of metabolomics technologies on the assessment of peritoneal membrane profiles in peritoneal dialysis patients: a systematic review. Metabolites 12, 145. doi:10.3390/metabo12020145
Kothari, A. N., Arffa, M. L., Chang, V., Blackwell, R. H., Syn, W. K., Zhang, J., et al. (2016). Osteopontin-A master regulator of epithelial-mesenchymal transition. J. Clin. Med. 5, 39. doi:10.3390/jcm5040039
Krediet, R. T. (2024). Physiology of peritoneal dialysis; pathophysiology in long-term patients. Front. Physiol. 15, 1322493. doi:10.3389/fphys.2024.1322493
Lamouille, S., Connolly, E., Smyth, J. W., Akhurst, R. J., and Derynck, R. (2012). TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 125, 1259–1273. doi:10.1242/jcs.095299
Lamouille, S., Xu, J., and Derynck, R. (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196. doi:10.1038/nrm3758
Lan, P. G., Clayton, P. A., Johnson, D. W., McDonald, S. P., Borlace, M., Badve, S. V., et al. (2016). Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit. Dial. Int. 36, 623–630. doi:10.3747/pdi.2015.00218
Lee, K. S., Park, S. J., Kim, S. R., Min, K. H., Lee, K. Y., Choe, Y. H., et al. (2008). Inhibition of VEGF blocks TGF-beta1 production through a PI3K/Akt signalling pathway. Eur. Respir. J. 31 (3), 523–31. doi:10.1183/09031936.00125007
Lee, Y., Lee, J., Park, M., Seo, A., Kim, K. H., Kim, S., et al. (2023). Inflammatory chemokine (C-C motif) ligand 8 inhibition ameliorates peritoneal fibrosis. FASEB J. 37, e22632. doi:10.1096/fj.202200784R
Leggett, S. E., Hruska, A. M., Guo, M., and Wong, I. Y. (2021). The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems. Cell Commun. Signal 19, 32. doi:10.1186/s12964-021-00713-2
Lho, Y., Do, J. Y., Heo, J. Y., Kim, A. Y., Kim, S. W., and Kang, S. H. (2021). Effects of TGF-β1 receptor inhibitor GW788388 on the epithelial to mesenchymal transition of peritoneal mesothelial cells. Int. J. Mol. Sci. 22, 4739. doi:10.3390/ijms22094739
Li, C., Lin, X., Li, Y., Duan, J., and Cai, X. (2025). Gut microbiome dynamics of patients on dialysis: implications for complications and treatment. Front. Pharmacol. 16, 1470232. doi:10.3389/fphar.2025.1470232
Li, J., Lan, J., Qiao, Q., Shen, L., and Lu, G. (2021a). Effluent osteopontin levels reflect the peritoneal solute transport rate. Open Med. (Wars) 16, 847–853. doi:10.1515/med-2021-0302
Li, S., Luo, C., Chen, S., Zhuang, Y., Ji, Y., Zeng, Y., et al. (2023). Brahma-related gene 1 acts as a profibrotic mediator and targeting it by micheliolide ameliorates peritoneal fibrosis. J. Transl. Med. 21, 639. doi:10.1186/s12967-023-04469-w
Li, Y. C., Sung, P. H., Yang, Y. H., Chiang, J. Y., Yip, H. K., and Yang, C. C. (2021b). Dipeptidyl peptidase 4 promotes peritoneal fibrosis and its inhibitions prevent failure of peritoneal dialysis. Commun. Biol. 4, 144. doi:10.1038/s42003-021-01652-x
Linden, T., Cohen, A., Deppisch, R., Kjellstrand, P., and Wieslander, A. (2002). 3,4-Dideoxyglucosone-3-ene (3,4-DGE): a cytotoxic glucose degradation product in fluids for peritoneal dialysis. Kidney Int. 62, 697–703. doi:10.1046/j.1523-1755.2002.00490.x
Liu, L., Sun, Q., Davis, F., Mao, J., Zhao, H., and Ma, D. (2022). Epithelial-mesenchymal transition in organ fibrosis development: current understanding and treatment strategies. Burns Trauma 10, tkac011. doi:10.1093/burnst/tkac011
Liu, T., Zhang, L., Joo, D., and Sun, S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2, 17023. doi:10.1038/sigtrans.2017.23
Lopes Barreto, D., and Krediet, R. T. (2013). Current status and practical use of effluent biomarkers in peritoneal dialysis patients. Am. J. Kidney Dis. 62, 823–833. doi:10.1053/j.ajkd.2013.01.031
López-Armada, M. J., Riveiro-Naveira, R. R., Vaamonde-García, C., and Valcárcel-Ares, M. N. (2013). Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13, 106–118. doi:10.1016/j.mito.2013.01.003
López-Cabrera, M. (2014). Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv. Med. 473134. doi:10.1155/2014/473134
Loureiro, J., Aguilera, A., Selgas, R., Sandoval, P., Albar-Vizcaíno, P., Pérez-Lozano, M. L., et al. (2011). Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J. Am. Soc. Nephrol. 22, 1682–1695. doi:10.1681/ASN.2010111197
Loureiro, J., Sandoval, P., del Peso, G., Gónzalez-Mateo, G., Fernández-Millara, V., Santamaria, B., et al. (2013). Tamoxifen ameliorates peritoneal membrane damage by blocking mesothelial to mesenchymal transition in peritoneal dialysis. PLoS One 8, e61165. doi:10.1371/journal.pone.0061165
Loureiro, J., Schilte, M., Aguilera, A., Albar-Vizcaíno, P., Ramírez-Huesca, M., Pérez-Lozano, M. L., et al. (2010). BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol. Dial. Transpl. 25, 1098–1108. doi:10.1093/ndt/gfp618
Lu, X., Wu, K., Jiang, S., Li, Y., Wang, Y., Li, H., et al. (2023). Therapeutic mechanism of baicalein in peritoneal dialysis-associated peritoneal fibrosis based on network pharmacology and experimental validation. Front. Pharmacol. 14, 1153503. doi:10.3389/fphar.2023.1153503
Luo, D., Zhao, W., Lin, Z., Wu, J., Lin, H., Li, Y., et al. (2021). The effects of hemodialysis and peritoneal dialysis on the gut microbiota of end-stage renal disease patients, and the relationship between gut microbiota and patient prognoses. Front. Cell Infect. Microbiol. 11, 579386. doi:10.3389/fcimb.2021.579386
Maeda, K., Doi, S., Nakashima, A., Nagai, T., Irifuku, T., Ueno, T., et al. (2017). Inhibition of H3K9 methyltransferase G9a ameliorates methylglyoxal-induced peritoneal fibrosis. PLoS One 12, e0173706. doi:10.1371/journal.pone.0173706
Marchant, V., Tejera-Muñoz, A., Marquez-Expósito, L., Rayego-Mateos, S., Rodrigues-Diez, R. R., Tejedor, L., et al. (2020). IL-17A as a potential therapeutic target for patients on peritoneal dialysis. Biomolecules 10, 1361. doi:10.3390/biom10101361
Marchant, V., Trionfetti, F., Tejedor-Santamaria, L., Rayego-Mateos, S., Rotili, D., Bontempi, G., et al. (2023). BET protein inhibitor JQ1 ameliorates experimental peritoneal damage by inhibition of inflammation and oxidative stress. Antioxidants 12, 2055. doi:10.3390/antiox12122055
Margetts, P. J. (2012). Twist: a new player in the epithelial-mesenchymal transition of the peritoneal mesothelial cells. Nephrol. Dial. Transpl. 27, 3978–3981. doi:10.1093/ndt/gfs172
Masola, V., Bonomini, M., Borrelli, S., Di Liberato, L., Vecchi, L., Onisto, M., et al. (2022). Fibrosis of peritoneal membrane as target of new therapies in peritoneal dialysis. Int. J. Mol. Sci. 23, 4831. doi:10.3390/ijms23094831
McIntyre, C. W. (2007). Update on peritoneal dialysis solutions. Kidney Int. 71, 486–490. doi:10.1038/sj.ki.5002109
Mikels, A., Minami, Y., and Nusse, R. (2009). Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 284, 30167–30176. doi:10.1074/jbc.M109.041715
Mills, E. L., Kelly, B., and O'Neill, L. A. J. (2017). Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498. doi:10.1038/ni.3704
Morelle, J., Lambie, M., Öberg, C. M., and Davies, S. (2023). The peritoneal membrane and its role in peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 19, 244–253. doi:10.2215/CJN.0000000000000282
Morelle, J., Stachowska-Pietka, J., Öberg, C., Gadola, L., La Milia, V., Yu, Z., et al. (2021). ISPD recommendations for the evaluation of peritoneal membrane dysfunction in adults: classification, measurement, interpretation and rationale for intervention. Perit. Dial. Int. 41, 352–372. doi:10.1177/0896860820982218
Morinaga, H., Sugiyama, H., Inoue, T., Takiue, K., Kikumoto, Y., Kitagawa, M., et al. (2012). Effluent free radicals are associated with residual renal function and predict technique failure in peritoneal dialysis patients. Perit. Dial. Int. 32, 453–461. doi:10.3747/pdi.2011.00032
Mortier, S., De Vriese, A. S., Van de Voorde, J., Schaub, T. P., Passlick-Deetjen, J., and Lameire, N. H. (2002). Hemodynamic effects of peritoneal dialysis solutions on the rat peritoneal membrane: role of acidity, buffer choice, glucose concentration, and glucose degradation products. J. Am. Soc. Nephrol. 13, 480–489. doi:10.1681/ASN.V132480
Mortier, S., Faict, D., Schalkwijk, C. G., Lameire, N. H., and De Vriese, A. S. (2004). Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int. 66, 1257–1265. doi:10.1111/j.1523-1755.2004.00879.x
Mutsaers, S. E., Prêle, C. M., Pengelly, S., and Herrick, S. E. (2016). Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 106, 1018–1024. doi:10.1016/j.fertnstert.2016.09.005
Ng, J. K., Chan, G. C., and Li, P. K. (2022). Icodextrin in peritoneal dialysis: implications on clinical practice and survival outcome. Kidney360 3 (5), 793–795. doi:10.34067/KID.0001902022
Obata, Y., Abe, K., Miyazaki, M., Koji, T., Tabata, Y., and Nishino, T. (2023). The transfer of the hepatocyte growth factor gene by macrophages ameliorates the progression of peritoneal fibrosis in mice. Int. J. Mol. Sci. 24, 6951. doi:10.3390/ijms24086951
Orr, A. W., Helmke, B. P., Blackman, B. R., and Schwartz, M. A. (2006). Mechanisms of mechanotransduction. Dev. Cell 10, 11–20. doi:10.1016/j.devcel.2005.12.006
Padwal, M., Cheng, G., Liu, L., Boivin, F., Gangji, A. S., Brimble, K. S., et al. (2018). WNT signaling is required for peritoneal membrane angiogenesis. Am. J. Physiol. Ren. Physiol. 314, F1036–F1045. doi:10.1152/ajprenal.00497.2017
Padwal, M., Liu, L., and Margetts, P. J. (2020). The role of WNT5A and Ror2 in peritoneal membrane injury. J. Cell Mol. Med. 24, 3481–3491. doi:10.1111/jcmm.15034
Padwal, M., Siddique, I., Wu, L., Tang, K., Boivin, F., Liu, L., et al. (2017). Matrix metalloproteinase 9 is associated with peritoneal membrane solute transport and induces angiogenesis through β-catenin signaling. Nephrol. Dial. Transpl. 32, 50–61. doi:10.1093/ndt/gfw076
Pan, Y., Yang, L., Dai, B., Lin, B., and Lin, S. (2021). Effects of probiotics on malnutrition and health-related quality of life in patients undergoing peritoneal dialysis: a randomized controlled trial. J. Ren. Nutr. 31 (2), 199–205. doi:10.1053/j.jrn.2020.04.008
Pap, D., Veres-Székely, A., Szebeni, B., Rokonay, R., Ónody, A., Lippai, R., et al. (2020). Characterization of IL-19, -20, and -24 in acute and chronic kidney diseases reveals a pro-fibrotic role of IL-24. J. Transl. Med. 18, 172. doi:10.1186/s12967-020-02338-4
Park, S. H., Kim, Y. L., and Lindholm, B. (2008). Experimental encapsulating peritoneal sclerosis models: pathogenesis and treatment. Perit. Dial. Int. 28, S21–S28. doi:10.1177/089686080802805s05
Patel, P., Sekiguchi, Y., Oh, K. H., Patterson, S. E., Kolb, M. R. J., and Margetts, P. J. (2010). Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 77, 319–328. doi:10.1038/ki.2009.436
Perl, J., Fuller, D. S., Bieber, B. A., Boudville, N., Kanjanabuch, T., Ito, Y., et al. (2020). Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am. J. Kidney Dis. 76, 42–53. doi:10.1053/j.ajkd.2019.09.016
Piccapane, F., Bonomini, M., Castellano, G., Gerbino, A., Carmosino, M., Svelto, M., et al. (2020). A novel formulation of glucose-sparing peritoneal dialysis solutions with l-Carnitine improves biocompatibility on human mesothelial cells. Int. J. Mol. Sci. 22, 123. doi:10.3390/ijms22010123
Postigo, A. A. (2003). Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. EMBO J. 22, 2443–2452. doi:10.1093/emboj/cdg225
Raby, A. C., Colmont, C. S., Kift-Morgan, A., Köhl, J., Eberl, M., Fraser, D., et al. (2017). Toll-like receptors 2 and 4 are potential therapeutic targets in peritoneal dialysis-associated fibrosis. J. Am. Soc. Nephrol. 28, 461–478. doi:10.1681/ASN.2015080923
Raby, A. C., González-Mateo, G. T., Williams, A., Topley, N., Fraser, D., López-Cabrera, M., et al. (2018). Targeting toll-like receptors with soluble toll-like receptor 2 prevents peritoneal dialysis solution-induced fibrosis. Kidney Int. 94, 346–362. doi:10.1016/j.kint.2018.03.014
Raby, A. C., and Labéta, M. O. (2018). Preventing peritoneal dialysis-associated fibrosis by therapeutic blunting of peritoneal toll-like receptor activity. Front. Physiol. 9, 1692. doi:10.3389/fphys.2018.01692
Rago, C., Lombardi, T., Di Fulvio, G., Di Liberato, L., Arduini, A., Divino-Filho, J. C., et al. (2021). A new peritoneal dialysis solution containing L-carnitine and xylitol for patients on continuous ambulatory peritoneal dialysis: first clinical experience. Toxins 13, 174. doi:10.3390/toxins13030174
Ramil-Gómez, O., Rodríguez-Carmona, A., Fernández-Rodríguez, J. A., Pérez-Fontán, M., Ferreiro-Hermida, T., López-Pardo, M., et al. (2021). Mitochondrial dysfunction plays a relevant role in pathophysiology of peritoneal membrane damage induced by peritoneal dialysis. Antioxidants 10, 447. doi:10.3390/antiox10030447
Rossi, L., Battistelli, C., de Turris, V., Noce, V., Zwergel, C., Valente, S., et al. (2018). HDAC1 inhibition by MS-275 in mesothelial cells limits cellular invasion and promotes MMT reversal. Sci. Rep. 8, 8492. doi:10.1038/s41598-018-26319-2
Ruiqi, L., Ming, P., Qihang, S., Yangyang, L., Junli, C., Wei, L., et al. (2021). Saikosaponin D inhibits peritoneal fibrosis in rats with renal failure by regulation of TGFβ1/BMP7/Gremlin1/smad pathway. Front. Pharmacol. 12, 628671. doi:10.3389/fphar.2021.628671
Sandoval, P., Jiménez-Heffernan, J. A., Guerra-Azcona, G., Pérez-Lozano, M. L., Rynne-Vidal, Á., Albar-Vizcaíno, P., et al. (2016). Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J. Pathol. 239, 48–59. doi:10.1002/path.4695
Santamaría, B., Ucero, A. C., Benito-Martin, A., Vicent, M. J., Orzáez, M., Celdrán, A., et al. (2015). Biocompatibility reduces inflammation-induced apoptosis in mesothelial cells exposed to peritoneal dialysis fluid. Blood Purif. 39, 200–209. doi:10.1159/000374103
Santos, A., and Lagares, D. (2018). Matrix stiffness: the conductor of organ fibrosis. Curr. Rheumatol. Rep. 20, 2. doi:10.1007/s11926-018-0710-z
Sawada, T., Ishii, Y., Tojimbara, T., Nakajima, I., Fuchinoue, S., and Teraoka, S. (2002). The ACE inhibitor, quinapril, ameliorates peritoneal fibrosis in an encapsulating peritoneal sclerosis model in mice. Pharmacol. Res. 46, 505–510. doi:10.1016/s1043661802002281
Schuinski, A. F., Baroni, G., Pecoits Filho, R. F., Meyer, F., Cerqueira, M. L. W., da Silva, M. A. A., et al. (2013). Evaluation of the use of captopril on peritoneal fibrosis induced in rats by the use of glucose solution 4.25%. J. Bras. Nefrol. 35, 273–278. doi:10.5935/0101-2800.20130046
Sekiguchi, Y., Hamada, C., Ro, Y., Nakamoto, H., Inaba, M., Shimaoka, T., et al. (2012). Differentiation of bone marrow-derived cells into regenerated mesothelial cells in peritoneal remodeling using a peritoneal fibrosis mouse model. J. Artif. Organs 15, 272–282. doi:10.1007/s10047-012-0648-2
Shi, M., Liu, H., Zhang, T., Zhang, M., Tang, X., Zhang, Z., et al. (2022c). Extracellular vesicles derived from adipose mesenchymal stem cells promote peritoneal healing by activating MAPK-ERK1/2 and PI3K-Akt to alleviate postoperative abdominal adhesion. Stem Cells Int. 2022, 1940761. doi:10.1155/2022/1940761
Shi, Y., Hu, Y., Wang, Y., Ma, X., Tang, L., Tao, M., et al. (2021). Blockade of autophagy prevents the development and progression of peritoneal fibrosis. Front. Pharmacol. 12, 724141. doi:10.3389/fphar.2021.724141
Shi, Y., Li, J., Chen, H., Hu, Y., Tang, L., Wang, Y., et al. (2022b). Inhibition of EZH2 suppresses peritoneal angiogenesis by targeting a VEGFR2/ERK1/2/HIF-1α-dependent signaling pathway. J. Pathol. 258, 164–178. doi:10.1002/path.5987
Shi, Y., Li, J., Chen, H., Hu, Y., Tang, L., Zhou, X., et al. (2022a). Pharmacologic inhibition of histone deacetylase 6 prevents the progression of chlorhexidine gluconate-induced peritoneal fibrosis by blockade of M2 macrophage polarization. Front. Immunol. 13, 899140. doi:10.3389/fimmu.2022.899140
Shim, J. H., Xiao, C., Paschal, A. E., Bailey, S. T., Rao, P., Hayden, M. S., et al. (2005). TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681. doi:10.1101/gad.1360605
Shin, H. S., Ko, J., Kim, D. A., Ryu, E. S., Ryu, H. M., Park, S. H., et al. (2017). Metformin ameliorates the phenotype transition of peritoneal mesothelial cells and peritoneal fibrosis via a modulation of oxidative stress. Sci. Rep. 7, 5690. doi:10.1038/s41598-017-05836-6
Silva, F. M. O., Costalonga, E. C., Silva, C., Carreira, A. C. O., Gomes, S. A., Sogayar, M. C., et al. (2019). Tamoxifen and bone morphogenic protein-7 modulate fibrosis and inflammation in the peritoneal fibrosis model developed in uremic rats. Mol. Med. 25, 41. doi:10.1186/s10020-019-0110-5
Simões-Silva, L., Araujo, R., Pestana, M., Soares-Silva, I., and Sampaio-Maia, B. (2018). The microbiome in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis. Pharmacol. Res. 130, 143–151. doi:10.1016/j.phrs.2018.02.011
Simon, F., Tapia, P., Armisen, R., Echeverria, C., Gatica, S., Vallejos, A., et al. (2017). Human peritoneal mesothelial cell death induced by high-glucose hypertonic solution involves Ca2+ and Na+ ions and oxidative stress with the participation of PKC/NOX2 and PI3K/Akt pathways. Front. Physiol. 8, 379. doi:10.3389/fphys.2017.00379
Šošić, D., Richardson, J. A., Yu, K., Ornitz, D. M., and Olson, E. N. (2003). Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell 112, 169–180. doi:10.1016/s0092-8674(03)00002-3
Stadlbauer, V., Horvath, A., Ribitsch, W., Schmerböck, B., Schilcher, G., Lemesch, S., et al. (2017). Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci. Rep. 7, 15601. doi:10.1038/s41598-017-15650-9
Stanisavljevic, J., Porta-de-la-Riva, M., Batlle, R., de Herreros, A. G., and Baulida, J. (2011). The p65 subunit of NF-κB and PARP1 assist Snail1 in activating fibronectin transcription. J. Cell Sci. 124, 4161–4171. doi:10.1242/jcs.078824
Stepanova, N. (2023). The gut-peritoneum axis in peritoneal dialysis and peritoneal fibrosis. Kidney Med. 5, 100645. doi:10.1016/j.xkme.2023.100645
Stone, R. C., Pastar, I., Ojeh, N., Chen, V., Liu, S., Garzon, K. I., et al. (2016). Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 365, 495–506. doi:10.1007/s00441-016-2464-0
Strippoli, R., Benedicto, I., Foronda, M., Perez-Lozano, M. L., Sánchez-Perales, S., López-Cabrera, M., et al. (2010). p38 maintains E-cadherin expression by modulating TAK1-NF-kappa B during epithelial-to-mesenchymal transition. J. Cell Sci. 123, 4321–4331. doi:10.1242/jcs.071647
Strippoli, R., Benedicto, I., Pérez Lozano, M. L., Cerezo, A., López-Cabrera, M., and del Pozo, M. A. (2008). Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis. Model Mech. 1, 264–274. doi:10.1242/dmm.001321
Strippoli, R., Loureiro, J., Moreno, V., Benedicto, I., Lozano, M. L. P., Barreiro, O., et al. (2015). Caveolin-1 deficiency induces a MEK-ERK1/2-Snail-1-dependent epithelial-mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Mol. Med. 7, 357. doi:10.15252/emmm.201570010
Strippoli, R., Moreno-Vicente, R., Battistelli, C., Cicchini, C., Noce, V., Amicone, L., et al. (2016). Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int. 2016, 3543678. doi:10.1155/2016/3543678
Strippoli, R., Sandoval, P., Moreno-Vicente, R., Rossi, L., Battistelli, C., Terri, M., et al. (2020). Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 11 (8), 647. doi:10.1038/s41419-020-02822-1
Stuivenberg, G. A., Chmiel, J. A., Akouris, P. P., Burton, J. P., and Reid, G. (2022). Probiotic bifidobacteria mitigate the deleterious effects of para-Cresol in a Drosophila melanogaster toxicity model. mSphere 7 (6), e0044622. doi:10.1128/msphere.00446-22
Su, W., Hu, Z., Zhong, X., Cong, A., Zhang, Y., Zhou, Z., et al. (2023). Restoration of CPT1A-mediated fatty acid oxidation in mesothelial cells protects against peritoneal fibrosis. Theranostics 13, 4482–4496. doi:10.7150/thno.84921
Suryantoro, S. D., Thaha, M., Sutanto, H., and Firdausa, S. (2023). Current insights into cellular determinants of peritoneal fibrosis in peritoneal dialysis: a narrative review. J. Clin. Med. 12, 4401. doi:10.3390/jcm12134401
Szeto, C. C., and Johnson, D. W. (2017). Low GDP solution and glucose-sparing strategies for peritoneal dialysis. Semin. Nephrol. 37, 30–42. doi:10.1016/j.semnephrol.2016.10.005
Tamura, R., Doi, S., Nakashima, A., Sasaki, K., Maeda, K., Ueno, T., et al. (2018). Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PLoS One 13, e0196844. doi:10.1371/journal.pone.0196844
Teixeira, R. R., de Andrade, L. S., Pereira, N. B. F., Montenegro, H., Hoffmann, C., and Cuppari, L. (2023). Gut microbiota profile of patients on peritoneal dialysis: comparison with household contacts. Eur. J. Clin. Nutr. 77 (1), 90–97. doi:10.1038/s41430-022-01190-7
Terawaki, H., Hayashi, Y., Zhu, W. J., Matsuyama, Y., Terada, T., Kabayama, S., et al. (2013). Transperitoneal administration of dissolved hydrogen for peritoneal dialysis patients: a novel approach to suppress oxidative stress in the peritoneal cavity. Med. Gas. Res. 3, 14. doi:10.1186/2045-9912-3-14
Terri, M., Trionfetti, F., Montaldo, C., Cordani, M., Tripodi, M., Lopez-Cabrera, M., et al. (2021). Mechanisms of peritoneal fibrosis: focus on immune cells-peritoneal stroma interactions. Front. Immunol. 12, 607204. doi:10.3389/fimmu.2021.607204
Thurlow, J. S., Joshi, M., Yan, G., Norris, K. C., Agodoa, L. Y., Yuan, C. M., et al. (2021). Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am. J. Nephrol. 52, 98–107. doi:10.1159/000514550
Tian, M., Zhang, L., Wang, Y., Deng, M., Peng, C., Liang, W., et al. (2022). Loss of JNK-associated leucine zipper protein promotes peritoneal dialysis-related peritoneal fibrosis. Kidney Dis. 8, 168–179. doi:10.1159/000521564
Tsai, J. M., Sinha, R., Seita, J., Fernhoff, N., Christ, S., Koopmans, T., et al. (2018). Surgical adhesions in mice are derived from mesothelial cells and can be targeted by antibodies against mesothelial markers. Sci. Transl. Med. 10, eaan6735. doi:10.1126/scitranslmed.aan6735
Van Biesen, W., Study Group, E. B. C. M., Covic, A. C., Fan, S., Claes, K., Lichodziejewska-Niemierko, M., et al. (2011). Fluid status in peritoneal dialysis patients: the european Body Composition Monitoring (EuroBCM) study cohort. PLoS One 6, e17148. doi:10.1371/journal.pone.0017148
Vincent, T., Neve, E. P. A., Johnson, J. R., Kukalev, A., Rojo, F., Albanell, J., et al. (2009). A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat. Cell Biol. 11, 943–950. doi:10.1038/ncb1905
Virzì, G. M., Milan Manani, S., Marturano, D., Clementi, A., Lerco, S., Tantillo, I., et al. (2022). Eryptosis in peritoneal dialysis-related peritonitis: the potential role of inflammation in mediating the increase in eryptosis in PD. J. Clin. Med. 11, 6918. doi:10.3390/jcm11236918
Wang, I. K., Lai, H. C., Yu, C. J., Liang, C. C., Chang, C. T., Kuo, H. L., et al. (2012). Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl. Environ. Microbiol. 78, 1107–1112. doi:10.1128/AEM.05605-11
Wang, J., Wang, L., Xu, L., Shi, Y., Liu, F., Qi, H., et al. (2017). Targeting Src attenuates peritoneal fibrosis and inhibits the epithelial to mesenchymal transition. Oncotarget 8, 83872–83889. doi:10.18632/oncotarget.20040
Wang, L., Liu, N., Xiong, C., Xu, L., Shi, Y., Qiu, A., et al. (2016). Inhibition of EGF receptor blocks the development and progression of peritoneal fibrosis. J. Am. Soc. Nephrol. 27, 2631–2644. doi:10.1681/ASN.2015030299
Wang, Q., Yang, X., Xu, Y., Shen, Z., Cheng, H., Cheng, F., et al. (2018). RhoA/Rho-kinase triggers epithelial-mesenchymal transition in mesothelial cells and contributes to the pathogenesis of dialysis-related peritoneal fibrosis. Oncotarget 9, 14397–14412. doi:10.18632/oncotarget.24208
Wang, Y., Shi, Y., Tao, M., Zhuang, S., and Liu, N. (2021). Peritoneal fibrosis and epigenetic modulation. Perit. Dial. Int. 41, 168–178. doi:10.1177/0896860820938239
Wiesenhofer, F. M., Herzog, R., Boehm, M., Wagner, A., Unterwurzacher, M., Kasper, D. C., et al. (2019). Targeted metabolomic profiling of peritoneal dialysis effluents shows anti-oxidative capacity of alanyl-glutamine. Front. Physiol. 9, 1961. doi:10.3389/fphys.2018.01961
Williams, J. D., Craig, K. J., Topley, N., and Williams, G. T. (2003). Peritoneal dialysis: changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int. Suppl. 84, S158–S161. doi:10.1046/j.1523-1755.63.s84.46.x
Wilson, R. B. (2018). Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Perit. 3, 20180103. doi:10.1515/pp-2018-0103
Wilson, R. B., Archid, R., and Reymond, M. A. (2020). Reprogramming of mesothelial-mesenchymal transition in chronic peritoneal diseases by estrogen receptor modulation and TGF-β1 inhibition. Int. J. Mol. Sci. 21, 4158. doi:10.3390/ijms21114158
Wu, J., Xing, C., Zhang, L., Mao, H., Chen, X., Liang, M., et al. (2018). Autophagy promotes fibrosis and apoptosis in the peritoneum during long-term peritoneal dialysis. J. Cell Mol. Med. 22, 1190–1201. doi:10.1111/jcmm.13393
Xie, X., Wang, J., Xiang, S., Chen, Z., Zhang, X., and Chen, J. (2019). Dialysate cell-free mitochondrial DNA fragments as a marker of intraperitoneal inflammation and peritoneal solute transport rate in peritoneal dialysis. BMC Nephrol. 20, 128. doi:10.1186/s12882-019-1284-3
Xu, H., Watanabe, M., Qureshi, A. R., Heimbürger, O., Bárány, P., Anderstam, B., et al. (2015). Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit. Dial. Int. 35, 206–215. doi:10.3747/pdi.2013.00259
Xu, J., Lamouille, S., and Derynck, R. (2009). TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172. doi:10.1038/cr.2009.5
Yáñez-Mó, M., Lara-Pezzi, E., Selgas, R., Ramírez-Huesca, M., Domínguez-Jiménez, C., Jiménez-Heffernan, J. A., et al. (2003). Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 348, 403–413. doi:10.1056/nejmoa020809
Yang, J., Cai, M., Wan, J., Wang, L., Luo, J., Li, X., et al. (2022). Effluent decoy receptor 2 as a novel biomarker of peritoneal fibrosis in peritoneal dialysis patients. Perit. Dial. Int. 42, 631–639. doi:10.1177/08968608211067866
Yang, X., Bao, M., Fang, Y., Yu, X., Ji, J., and Ding, X. (2021). STAT3/HIF-1α signaling activation mediates peritoneal fibrosis induced by high glucose. J. Transl. Med. 19, 283. doi:10.1186/s12967-021-02946-8
Yang, X., Yan, H., Jiang, N., Yu, Z., Yuan, J., Ni, Z., et al. (2020). IL-6 trans-signaling drives a STAT3-dependent pathway that leads to structural alterations of the peritoneal membrane. Am. J. Physiol. Ren. Physiol. 318, F338–F353. doi:10.1152/ajprenal.00319.2019
Yao, Q., Pawlaczyk, K., Ayala, E. R., Styszynski, A., Breborowicz, A., Heimburger, O., et al. (2008). The role of the TGF/smad signaling pathway in peritoneal fibrosis induced by peritoneal dialysis solutions. Nephron Exp. Nephrol. 109, e71–e78. doi:10.1159/000142529
Yohanna, S., Alkatheeri, A. M. A., Brimble, S. K., McCormick, B., Iansavitchous, A., Blake, P. G., et al. (2015). Effect of Neutral-pH, low-glucose degradation product peritoneal dialysis solutions on residual renal function, urine volume, and ultrafiltration: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 10, 1380–1388. doi:10.2215/CJN.05410514
Yu, M., Shi, J., Sheng, M., Gao, K., Zhang, L., Liu, L., et al. (2018). Astragalus inhibits epithelial-to-mesenchymal transition of peritoneal mesothelial cells by down-regulating β-Catenin. Cell Physiol. Biochem. 51, 2794–2813. doi:10.1159/000495972
Yu, M. A., Shin, K. S., Kim, J. H., Kim, Y. I., Chung, S. S., Park, S. H., et al. (2009). HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J. Am. Soc. Nephrol. 20, 567–581. doi:10.1681/ASN.2008040424
Yuan, J., Fang, W., Ni, Z., Dai, H., Lin, A., Cao, L., et al. (2009). Peritoneal morphologic changes in a peritoneal dialysis rat model correlate with angiopoietin/Tie-2. Pediatr. Nephrol. 24, 163–170. doi:10.1007/s00467-008-0944-5
Zehender, A., Huang, J., Györfi, A. H., Matei, A. E., Trinh-Minh, T., Xu, X., et al. (2018). The tyrosine phosphatase SHP2 controls TGFβ-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat. Commun. 9, 3259. doi:10.1038/s41467-018-05768-3
Zhang, L., Hao, J. B., Ren, L. S., Ding, J. L., and Hao, L. R. (2014). The aldosterone receptor antagonist spironolactone prevents peritoneal inflammation and fibrosis. Lab. Invest. 94, 839–850. doi:10.1038/labinvest.2014.69
Zhang, Z., Jiang, N., and Ni, Z. (2017). Strategies for preventing peritoneal fibrosis in peritoneal dialysis patients: new insights based on peritoneal inflammation and angiogenesis. Front. Med. 11, 349–358. doi:10.1007/s11684-017-0571-2
Zhao, J., Shi, J., Shan, Y., Yu, M., Zhu, X., Zhu, Y., et al. (2020). Asiaticoside inhibits TGF-β1-induced mesothelial-mesenchymal transition and oxidative stress via the Nrf2/HO-1 signaling pathway in the human peritoneal mesothelial cell line HMrSV5. Cell Mol. Biol. Lett. 25, 33. doi:10.1186/s11658-020-00226-9
Zhao, J. L., Guo, M. Z., Zhu, J. J., Zhang, T., and Min, D. Y. (2019). Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1). Cell Mol. Biol. Lett. 24, 32. doi:10.1186/s11658-019-0157-x
Zhao, T., Sun, Z., Lai, X., Lu, H., Liu, L., Li, S., et al. (2023). Tamoxifen exerts anti-peritoneal fibrosis effects by inhibiting H19-activated VEGFA transcription. J. Transl. Med. 21, 614. doi:10.1186/s12967-023-04470-3
Zhou, F., Yao, L., Lu, X., Li, Y., Han, X., and Wang, P. (2022). Therapeutic targeting of GSK3β-Regulated Nrf2 and NFκB signaling pathways by salvianolic acid A ameliorates peritoneal fibrosis. Front. Med. 9, 804899. doi:10.3389/fmed.2022.804899
Zhou, G., Su, X., Ma, J., Wang, L., and Li, D. (2013). Pioglitazone inhibits high glucose-induced synthesis of extracellular matrix by NF-κB and AP-1 pathways in rat peritoneal mesothelial cells. Mol. Med. Rep. 7, 1336–1342. doi:10.3892/mmr.2013.1309
Zhou, Q., Bajo, M. A., Del Peso, G., Yu, X., and Selgas, R. (2016). Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 90, 515–524. doi:10.1016/j.kint.2016.03.040
Zhu, X., Shan, Y., Yu, M., Shi, J., Tang, L., Cao, H., et al. (2021). Tetramethylpyrazine ameliorates peritoneal angiogenesis by regulating VEGF/Hippo/YAP signaling. Front. Pharmacol. 12, 649581. doi:10.3389/fphar.2021.649581
Keywords: peritoneal fibrogenesis, epithelial to mesenchymal transition, biomechanical injury in peritoneal membrane, epigenetics and altered gut microbiome, peritoneal dialysis solutions and inflammation, peritonitis and peritoneal angiogenesis, Smad/non-Smad signaling, biomarkers
Citation: Prasad N, Chaturvedi S, Singh H, Udumula MP, Rawat A, Jeyakumar M, Jaiswal A, Kumar S and Agarwal V (2025) Peritoneal Dialysis -Associated Fibrosis: Emerging Mechanisms and Therapeutic Opportunities. Front. Pharmacol. 16:1635624. doi: 10.3389/fphar.2025.1635624
Received: 26 May 2025; Accepted: 14 July 2025;
Published: 22 August 2025.
Edited by:
Marco Allinovi, Careggi University Hospital, ItalyReviewed by:
Manuel Lopez-Cabrera, Spanish National Research Council (CSIC), SpainLuciano D'Apolito, BioGeM Institute, Italy
Copyright © 2025 Prasad, Chaturvedi, Singh, Udumula, Rawat, Jeyakumar, Jaiswal, Kumar and Agarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narayan Prasad, bmFyYXlhbi5uZXBocm9AZ21haWwuY29t; Saurabh Chaturvedi, c2F1cmFiaGNoYXR1cnZlZGkyNjdAZ21haWwuY29t; Harshit Singh, cmFqYXRoYXJzaEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Narayan Prasad
Narayan Prasad Saurabh Chaturvedi
Saurabh Chaturvedi Harshit Singh
Harshit Singh Mary Priyanka Udumula3
Mary Priyanka Udumula3 Atul Rawat
Atul Rawat Akhilesh Jaiswal
Akhilesh Jaiswal Vikas Agarwal
Vikas Agarwal