Abstract
Background:
Curcumin is a natural polyphenolic compound that originates from turmeric (Curcuma longa L., Linnaeus, Zingiberaceae), a traditional medicinal herb. It is widely recognized for its strong antioxidant properties.
Objective:
This comprehensive review aims to delineate the recent progress in comprehending the role of curcumin in modulating oxidative stress and exerting an anti-fibrotic effect, with a particular focus on liver, renal, myocardial, and pulmonary fibrosis.
Methods:
A systematic review of the literature was conducted via the PubMed, Web of Science, Google Scholar, and China National Knowledge Infrastructure, covering 2000 until 2024. A systematic review identified studies examining curcumin’s regulation of oxidative stress pathways in therapeutic strategies for multiple fibrotic disorders, which were analyzed to synthesize current evidence.
Results:
In recent years, the application of curcumin for the clinical management of fibrotic diseases in a variety of clinical applications has been extensively investigated. Accumulating evidence suggests that curcumin can exert antifibrotic effects by ameliorating oxidative stress through the modulation of various signaling pathways such as regulating reactive oxygen species (ROS), nuclear factor erythroid-2-related factor 2 (NRF2), peroxisome proliferator-activated receptors (PPAR), transforming growth factor- β1 (TGF-β1). In this review, we investigate the pharmacokinetics of curcumin, the relationship between oxidative stress and the pathogenesis of fibrosis, and summarize the related studies of curcumin in the treatment of fibrotic diseases by regulating oxidative stress.
Conclusion:
This comprehensive review elucidates curcumin’s antifibrotic potential and explores its translational applications in developing novel therapeutic strategies to combat fibrotic pathologies, supported by mechanistic evidence that informs safer, more effective treatment paradigms.
1 Introduction
Fibrotic disease is a widespread disease that critically threatens public health, mainly including pulmonary fibrosis, liver fibrosis, renal fibrosis, myocardial fibrosis, peritoneal fibrosis, and other diseases. It has been widely reported to be characterized by high morbidity and mortality (Olson et al., 2007; Pottier et al., 2014). 45% of mortality in developed countries is related to cumulative impairment of tissue-specific function and final organ dysfunction due to fibrosis (Nanthakumar et al., 2015). It is noteworthy that with the acceleration of the aging of population, the above situation will become increasingly serious, and the morbidity and mortality rates will continue to increase (Redente et al., 2011; Kurundkar and Thannickal, 2016). Fibrosis is the procedure of aberrant aggregation of ECM after different types of tissues being damaged. It is a pathological stage of poor repair of all organs and tissues (Henderson et al., 2020; Panizo et al., 2021). Current treatment strategies for fibrotic diseases have many obstacles, including the lack of cell or tissue specificity of anti-fibrotic treatments, the occurrence of adverse events of anti-fibrotic drugs, and limited treatment options (Ramachandran and Henderson, 2016; Nastase et al., 2018; Lamb, 2021). Over the past decade, despite increasing research in the management of fibrotic diseases, its illness and death rate have continued to increase. Therefore, the development of anti-fibrotic therapeutic strategies holds substantial clinical importance. Traditional Chinese medicine (TCM) has a long history which has played a decisive status in the evolution of medicine in China for more than 2,000 years. Existing evidence shows that herbal medicine can serve as an important supplement and alternative method for anti-fibrotic drug treatment (Li et al., 2021; Li et al., 2022). It has the advantages of wide source, low toxicity, and structural diversity (Shan et al., 2019).
Turmeric (Curcuma longa L., Linnaeus, Zingiberaceae) is the rhizome of a perennial herbaceous plant of the ginger family, mainly distributed in southern and southwestern tropical Asia region (Aggarwal et al., 2007; Kocaadam and Şanlier, 2017). In TCM, turmeric was first documented in “New Materia Medica” (Hu Chenxia, 2008). It has the effects of invigorating blood circulation, and relieving depression (Qin et al., 2022). In clinical practice, turmeric is commonly used in a variety of herbal formulas to treat a wide array of diseases and body pathologies (Yang et al., 2019; Wu Yuhong and Liu, 2020; Zhengtao et al., 2022). Curcumin, a phenolic compound isolated from turmeric in modern times, is the main active ingredient responsible for the therapeutic effects of turmeric (Esatbeyoglu et al., 2012; Priyadarsini, 2014). Curcumin is often used as a pigment and spice in food, cosmetics, and textiles to dye and enhance flavor. It can also treat multi-system diseases (Aggarwal et al., 2007; Kocaadam and Şanlier, 2017; Qin et al., 2022), such as dermatologic diseases, infection, stress, and depression. Research on various diseases has proven that curcumin has anti-inflammatory, antioxidant, anti-cancer, antibacterial, anti-parasitic, anti-viral, and immune-modulating effects (Prasad et al., 2014; Kotha and Luthria, 2019; Zheng et al., 2020; Abd El-Hack et al., 2021; Luo et al., 2023). Different concentrations of curcumin have no obvious toxic effects on normal tissues and cells (Aggarwal and Harikumar, 2009). Therefore, curcumin is widely used in clinical research on various diseases.
Curcumin, a lipophilic polyphenolic compound with low molecular mass, demonstrates significant therapeutic efficacy alongside a favorable safety profile. Numerous studies have proved the anti-fibrotic traits of curcumin, primarily in the lung, liver, kidney, and myocardial fibrosis (Kong et al., 2020; Sadoughi et al., 2021) (Figure 1). The mechanism may be related to inhibiting extracellular matrix (ECM) deposition, oxidative stress, and alveolar epithelial cells (AEC) cell apoptosis, reducing inflammatory response, and enhancing autophagy (She et al., 2018; Hernández-Aquino et al., 2020; Fathimath Muneesa et al., 2022; Cui et al., 2023). Among them, oxidative stress plays an important role in the initiation and development of fibrosis by damaging lipids, proteins, and DNA, inducing cell necrosis and apoptosis, amplifying inflammatory responses, stimulating the production of pro-fibrotic mediators, etc (Sánchez-Valle et al., 2012). This article reviews the mechanism of curcumin’s anti-fibrosis effects by inhibiting oxidative stress, to provide a reference for subsequent research.
FIGURE 1
Antifibrotic effects of curcumin.
2 Methods
A comprehensive online search of literature was conducted via Web of Science, Google Scholar, and China National Knowledge Infrastructure, covering 2000 until 2024. The ensuing key concepts were utilized: Curcumin, Turmeric Yellow, Curcumin Phytosome, Diferuloylmethane, Oxidative Stress, Antioxidative Stress, Oxidative Damage, Oxidative Stress Injury, Oxidative Injury, Oxidative Cleavage, Oxidative DNA Damage, ROS, Oxidative and Nitrosative Stress, Oxidative Nitrative Stress, Nitro-Oxidative Stress, Fibrosis, Cicatrix, Fibrosing, Hypertrophic, Keloid, Tissue Adhesions, Cirrhosis, fibrillation, fibration and fibering. A comprehensive examination was also conducted on the bibliographies of all the articles obtained from the search, aiming to incorporate pertinent literature.
3 Extraction, chemical structure, and physicochemical properties of curcumin
Curcumin serves as a natural compound sourced from Zingiberaceae plants, a vital group of medicinal plants (Shakeri et al., 2019). Generally speaking, curcumin can be extracted from the rhizomes or roots of ginger plants (Nan et al., 2023), which are customarily called turmeric (Curcuma) or turmeric in traditional Chinese medicine, respectively (Jianmin et al., 1983). An analysis of the rhizome or root extracts of ginger plants using thin-layer chromatography confirmed that the curcumin content in different plants of the same genus and even in rhizomes and roots of the same plant are significantly different (Jianmin et al., 1983). Qi et al. analyzed the curcumin content in the same species of ginger plants from different regions by HPLC and confirmed that the curcumin content was affected by the species of curcuma (Jianmin et al., 1983; Aidi and Wenyujin, 2002).
In 1953, Srinivasan determined the presence of curcumin and other components in turmeric by chromatography (Kocaadam and Şanlier, 2017). Most crude extracts prepared from turmeric mainly include curcumin (I), desoxymethylcurcumin (II), and dideoxymethylcurcumin (III) (Priyadarsini, 2014). The physicochemical properties of curcumin are presented in Table 1. It can be rapidly degraded when exposed to conditions including an alkaline pH environment (Dellali et al., 2021). Later, Chandrasekhara and Ravindranath et al. synthesized curcumin from vanillin and acetylacetone by condensation using the Pabon method with more than 99% content determined by the rose anthocyanin method (Ravindranath and Chandrasekhara, 1980; 1981a).
TABLE 1
| Content | Description |
|---|---|
| Name | Curcumin |
| CAS number | 458-37-7 |
| Molecular formula | C21H20O6 |
| In vitro studies on the intestinal absorption of curcumin in rats | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione |
| Canonical SMILES | COC1=C(C=CC(=C1)C=CC(=O)CC(=O)C=CC2=CC(=C(C=C2)O)OC)O |
| Molecular weight | 368.37 g/mol |
| Topological Polar SurfaceArea | 93.1Ų |
| Refractive Index | 1.5118 |
| Physical description | A crystalline solid |
| Color | Orange crystalline powder |
| Melting Point | 183 ℃ |
| Solubility | Insoluble in water; very soluble in ethanol, acetic acid |
| Density | 0.9348 at 59°F (NTP, 1992) - Less dense than water; will float |
Physicochemical properties of curcumin.
4 Pharmacokinetics and toxicology of curcumin
Pharmacokinetics is the study of drug absorption, distribution, metabolism, and excretion, which helps us evaluate the properties of specific drugs and their application prospects. At present, there are a large number of literature studies on metabolic process of curcumin in rats, which are mainly administered by intraperitoneal injection, sublingual vein, oral administration, or the pharmacokinetics of curcumin in rats (Holder et al., 1978; Wahlström and Blennow, 1978; Ravindranath and Chandrasekhara, 1980; 1981a; b; Wang et al., 1997). Wahlstrom et al. reported that plasma levels and bile excretion measurements showed malabsorption of curcumin from the intestine in Sprague-Dawley rats at an oral dose of 1 g/kg (Wahlström and Blennow, 1978). Ravindranath et al. used radioactive tritium-labeled drugs to evaluate the tissue distribution of curcumin. The outcomes demonstrated that when Sprague-Dawley rats were given oral curcumin doses of 400 mg, 80 mg, and 10 mg, the percentage of curcumin absorption did not change. This demonstrates a dose-dependent limitation in curcumin absorption bioavailability (Ravindranath and Chandrasekhara, 1981b). Once curcumin is absorbed, it undergoes conjugation effects at different tissue sites. Research has found that curcumin-glucuronide, dihydrocurcumin-glucuronide, tetrahydrocurcumin-glucuronide and tetrahydrocurcumin are the main metabolites of curcumin in the body (Pan et al., 1999; Hoehle et al., 2006). Studies have shown that when rats are administered 100 mg/kg by intraperitoneal injection, curcumin levels are highest in the liver, followed by the spleen (Pan et al., 1999). However, in another human clinical study, 12 patients with colorectal cancer liver metastases received curcumin about 450–3,600 mg of per day 1 week before surgery. At the end, no curcumin was detected in the liver tissue, and only a small amount of reduced turmeric metabolites was detected (Garcea et al., 2004). Moreover, the rate at which curcumin is eliminated from the body serves as another crucial determinant of its biological impacts. Studies have shown that when Sprague-Dawley rats take an oral dose of 1 g/kg of curcumin, 75% of it is defecated in the feces, while the content of curcumin in the urine is negligible (Wahlström and Blennow, 1978). In another study, subsequent to the oral administration of a 400 mg dose of curcumin to rats, no traces of curcumin were discernible in their urinary samples (Ravindranath and Chandrasekhara, 1980). Metabolic studies using radiolabeled curcumin in rats, when administered at 400 mg/animal, about 40% of the unaltered form of curcumin was found in the feces (Ravindranath and Chandrasekhara, 1981b). Regardless of the dosage, the urinary excretion of curcumin is very low. The temporal parameters for the absorption and clearance phases of curcumin (2 g/kg) after oral ingestion in rats are 0.31 ± 0.07 and 1.7 ± 0.5 h respectively (Anand et al., 2007). One clinical study found that patients with advanced colorectal cancer who took turmeric extract orally everyday for 4 months contained 36–180 mg of curcumin and had neither curcumin nor curcuminoids in their urine metabolites (Sharma et al., 2001). Overall study results indicate that oral curcumin has low absorption and rapid clearance (Mirzaei et al., 2017). The above characteristics such as low bioavailability, short plasma half-life, low plasma concentration, and poor oral absorption have seriously limited the clinical development of curcumin (Tomeh et al., 2019; Hao et al., 2023). The study stated that when administered at the maximum recommended dose of 5 g/Kg, there was no obvious toxic effect on Sprague-Dawley rats (Wahlström and Blennow, 1978). Similarly, Aggarwal et al. studied the acute toxicological damage of curcumin-essential oil complex (CEC), an available biological agent, in rats and mice at the uppermost advised dose of 5,000 mg/kg. In contrast to the control group, these animals also showed no symptoms, toxicity, or death (Aggarwal et al., 2016). Findings from Phase I clinical trials involving curcumin indicate its safety in humans, even when administered at elevated doses (12 g/day) (Anand et al., 2007). However, more reliable research is needed to prove whether there is any difference in the toxic damage to internal organs and cells between animal and human bioavailable preparations.
5 Oxidative stress and fibrotic diseases
The process of fibrosis involves excessive deposition of ECM and remodeling of the injured site, leading to the restoration of unnecessary connective tissue and organ dysfunction during the repair process (Lurje et al., 2023). It may be present in virtually all organs of the human organism, such as the liver, kidneys, heart, lungs, and skin. Repeated injury is a general characteristic of fibrotic diseases. There are different causes, such as chronic virus infection, autoimmune, chronic ischemia process, or toxicity (including nicotine, alcohol, drugs, or radiation) (Distler et al., 2019). Fibrosis begins with the triggering of parenchymal cell damage or death and is usually repetitive or persistent. The subsequent repair process is mediated by regulatory processes such as damage recognition, rapid myeloid attraction and fibroblast activation. However, they lack the capacity to restore physiological equilibrium (Lurje et al., 2023). These fibrosis are typically characterized by the chronic inflammatory response coupled with alterations in the invasive immune cell infiltrate (Guillot and Tacke, 2019). Endogenous early-phase proinflammatory mediators including IL-1, IL-6, TNF, and TGF-β derived from macrophages, tissue fibroblasts, and resident stromal populations drive the differentiation of IL-17-producing effector cells. IL-17A promotes tissue damage through the production of ROS that enhances the neutrophilic response and meanwhile increases the expression of TGF-β receptors on fibroblasts, thereby promoting ECM production in the TGF-β response (Henderson et al., 2020). Immune cell subsets further drive fibrotic progression through the release of angiopoietic and fibrogenic mediators that act on resident fibroblasts and vascular endothelial cells, thereby inducing pathological neovascularization, and promoting tissue damage by secreting matrix metalloproteinases and ROS (Pardo et al., 2016; Bartneck et al., 2019; Sutti et al., 2019).
In the pathological mechanism of oxidative stress-induced fibrosis, Oxidative stress drives inflammatory cascades via elevated secretion of proinflammatory cytokines and fibrogenic growth factors, thereby promoting myofibroblast activation and pathological extracellular matrix remodeling. ROS upregulates TGF-β signaling pathways and serve as critical effectors in propagating TGF-β-induced fibrogenic responses, including fibroblast activation, the synthesis of pro-fibrotic mediators, epithelial/endothelial cell apoptosis, and epithelial-mesenchymal transition (EMT) (Liu and Desai, 2015). On the other hand, increased extracellular matrix deposition due to oxidative stress subsequently leads to fibrosis (Jianming and Li, 2016; Makena et al., 2023). In the meanwhile, oxidative stress causes lipid damage to parenchymal cell membranes, disrupts enzyme and protein modifications that are essential for cell metabolism, and promotes DNA mutation, culminating in apoptosis of these cells. In summary, oxidative stress can cause the progress of fibrotic diseases through these damages (Antar et al., 2023) (Figure 2).
FIGURE 2
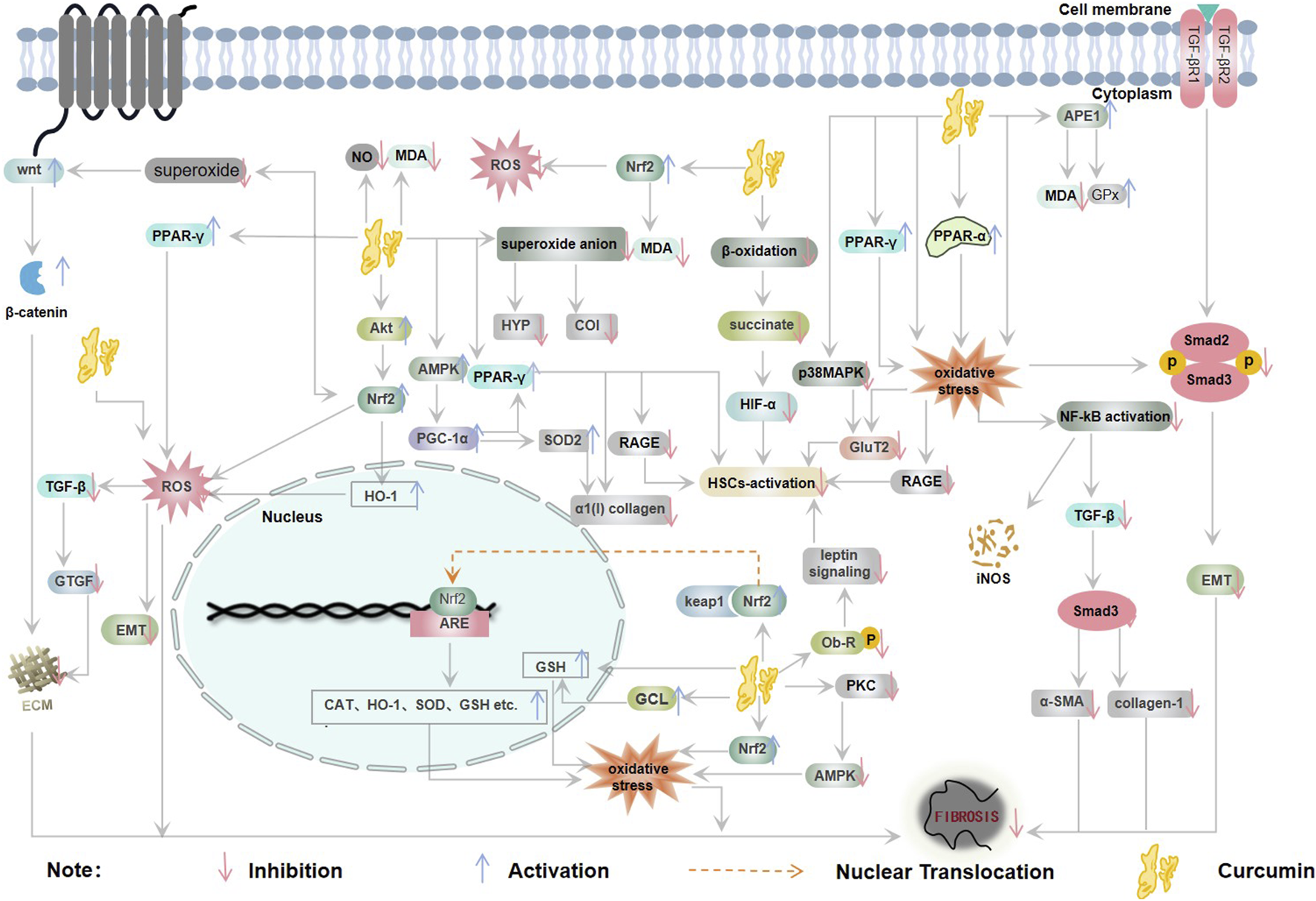
Relationship between oxidative stress and fibrosis (CAT: catalase, ECM: extracellular matrix, EMT: epithelial-mesenchymal transition, NO: nitric oxide, ROS: reactive oxygen species, SOD: superoxide dismutase).
6 Effect of curcumin on fibrotic diseases
The present study systematically elucidates the multifaceted antifibrotic therapeutic mechanisms of curcumin (Figure 3).
FIGURE 3
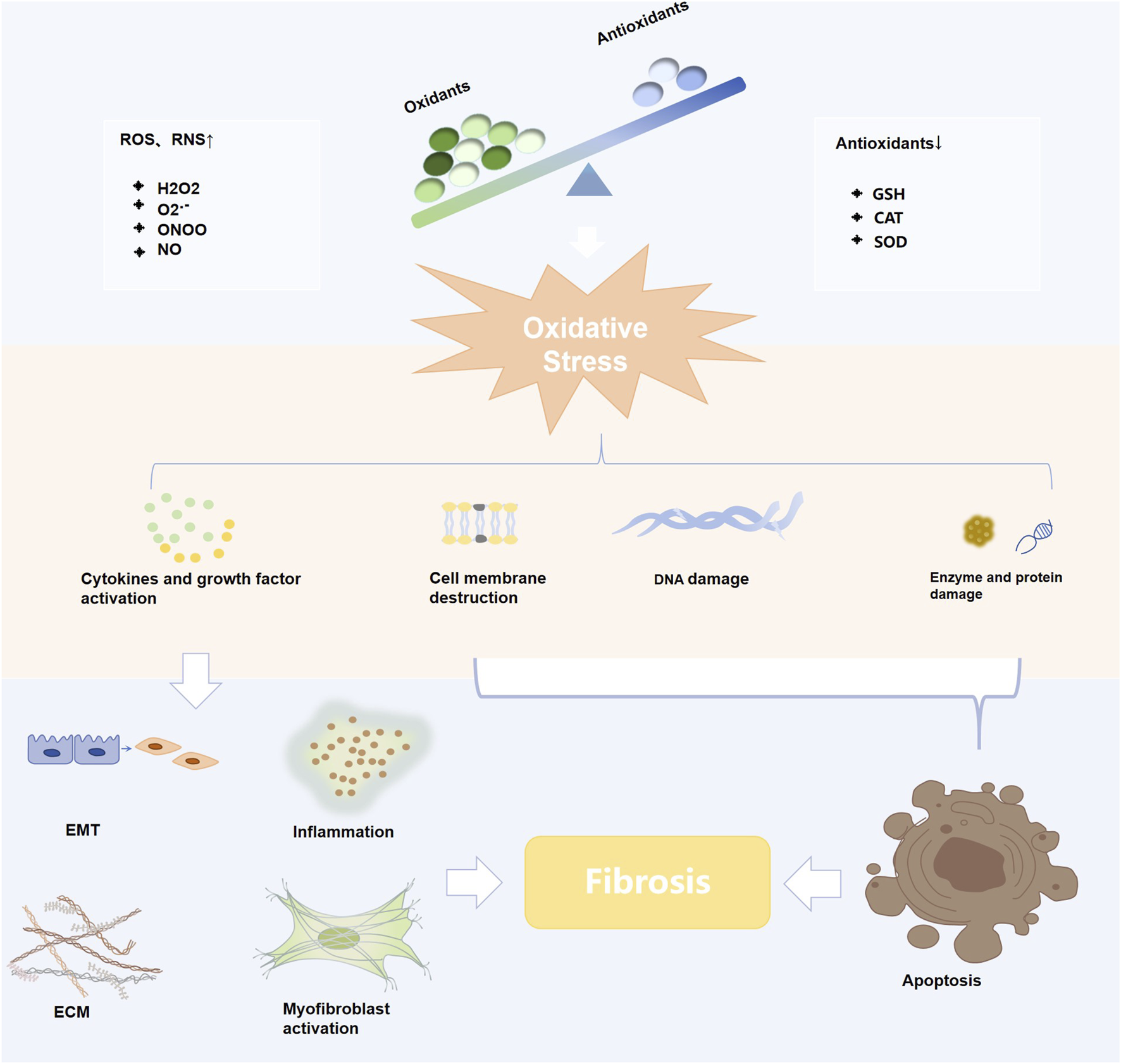
Diagram of the mechanisms of curcumin against various fibrotic diseases.
6.1 Anti-hepatic fibrosis effect of curcumin
PPARα (NR1C1), a ligand-responsive nuclear hormone receptor, demonstrates pronounced hepatic tissue enrichment, was originally characterized as a pharmacological target for heterogenin that induces peroxisome proliferation in rodents (Issemann and Green, 1990). Besides PPARα, this receptor subclass includes two additional variants specified by the PPARβ/δ (NR1C2) and PPARγ (NR1C3) genes, with each exhibiting organ-specific expression profiles and distinct physiological roles (Kliewer et al., 1994). Hepatic PPARα serves as a master regulator of β-oxidation processes and systemic lipid/energy equilibrium (Janovick et al., 2022; Qiu et al., 2023). Nuclear receptor PPARα is predominantly activated under energy-deprived states, orchestrating mitochondrial energetic reprogramming that culminates in enhanced oxidative phosphorylation for ATP synthesis. Curcumin inhibits autophagy induced by oxidative damage in hepatic cells via PPAR-α activation, thereby reducing the occurrence of EMT, decreasing ROS and MDA, and inhibiting the production of ECM, thus playing a crucial part in improving hepatic fibrosis (Elmansi et al., 2017; Kong et al., 2020).
PPARγ serves as a differentiation marker for hepatic stellate cells (HSCs), with its transcriptional activity declining during their transition to myofibroblasts, whereas agonist-mediated inhibition suppresses HSC activation (Li et al., 2015). Curcumin upregulates PGC-1α via AMPK signaling, subsequently enhancing PPARγ activity and Superoxide Dismutase-2 (SOD-2) transcription/activity, thereby suppressing α1(I) collagen expression in cultured HSCs (Zhai et al., 2015). Within HSCs, curcumin enhances PPARγ functionality while mitigating oxidative stress through Ob-R dephosphorylation, subsequently suppressing Ob-R transcription and blocking leptin-mediated signaling, thereby abolishing leptin’s pro-activation effects on HSCs (Tang et al., 2009). It additionally suppressed receptor of advanced glycation endproducts (RAGE) transcriptional activity via enhanced PPARγ functionality and attenuated oxidative stress, thereby abolishing receptor of advanced glycation endproduct (AGE)-mediated stimulation of HSC activation (Lin et al., 2012). Furthermore, curcumin mitigates liver fibrosis progression through PPAR-γ activation, leading to elevated glutathione levels and diminished oxidative stress within activated hepatic stellate cells (Zheng and Chen, 2006).
GSH, serving as the primary intracellular redox buffer, demonstrates particularly high concentration within hepatic tissue (Vairetti et al., 2021). Curcumin can upregulate glutathione, GSH/GSSG ratio and total glutathione levels thus improving hepatic fibrosis in Wistar rats (Reyes-Gordillo et al., 2008; Hernández-Aquino et al., 2020). Research demonstrates curcumin suppresses GLUT2 transcriptional activity via PPARγ activation and promotes de novo glutathione biosynthesis, thereby eliminating HSC activation and ultimately ameliorating hyperglycemia-associated liver fibrosis (Lin and Chen, 2011).
Glutamate cysteine ligase (GCL) serves as a critical regulatory enzyme governing GSH biosynthesis. Curcumin induces GCL expression to increase GSH and reduce oxidative stress, thereby preventing liver fibrosis formation in HSCs and Sprague-Dawley rats (Zheng et al., 2007; Fu et al., 2008).
NRF2 mediates the expression of multiple genes and influences various physiological processes, including substance metabolism, ROS clearance, and glutathione synthesis (Hayes and Dinkova-Kostova, 2014). Curcumin can protect HSCs from oxidative stress by upregulating NRF2 and inhibiting the activation and secretion of glucose oxidase (Go) -induced ECM molecules (Liu et al., 2016; Gowifel et al., 2020).
Nitrotyrosine is a product of protein oxidation and is considered a marker of oxidative damage. Curcumin may prevent liver fibrosis through the decrease of nitrotyrosine staining in thioacetamide (TAA) -treated rats (Bruck et al., 2007). Overproduction of ROS oxidizes guanine residues to 8-OHdG, and these adducts can cause DNA damage when added to DNA (Singh et al., 2011). Curcumin significantly diminishes the proportion of 8-OH-dG-immunopositive nuclei, serving as an established biomarker for oxidative stress-induced DNA damage (Vizzutti et al., 2010). Heme oxygenase-1 (HO-1) serves as the primary regulatory enzyme in heme metabolism, exhibiting pronounced antioxidant and anti-inflammatory properties (Yachie, 2021). Curcumin may ameliorate hepatic fibrosis by increasing HO-1 and decreasing oxidative stress in Male Sprague–Dawley rats (Öner-İyidoğan et al., 2014). Apurinic/apyrimidinic endonuclease 1 (APE1) plays a crucial part in the base excision repair (BER) pathway of ROS-induced damaged bases and DNA single-strand breaks and is a key element of proteins upregulated by oxidative stress (Weaver et al., 2022). Curcumin may protect the liver from oxidative stress via upregulating APE1 (Bassiouny et al., 2011). AGEs and RAGE play a pivotal role in NASH- associated hepatic fibrosis (Lohwasser et al., 2009). Curcumin ameliorates oxidative stress-induced effects by suppressing AGEs-induced leptin signaling activation, thereby blocking HSC activation (Tang and Chen, 2014).
In addition, curcumin inhibits MDA formation and significantly improves liver antioxidant status (Wu et al., 2008). In N-(4-hydroxyphenyl) acetamide (NHPA) -treated rats, curcumin attenuated hepatic collagen III accumulation and fibrogenesis by inhibiting nitric oxide and MDA production. At the same time, curcumin can improve the levels of GSH, SOD, and CAT in liver fibrosis induced by thioacetamide and bisphenol a, and reduce MDA (Elswefy et al., 2020; Radwan et al., 2024). Abnormal accumulation of succinate can induce ROS production (Chouchani et al., 2014). Hypoxia inducible factor- 1α (HIF-1α) is involved in cell cycle change, extracellular matrix deposition, and myofibroblast transition (Senavirathna et al., 2018; Aquino-Gálvez et al., 2019). Curcumin prevents HSC upregulation by suppressing the succinic acid/HIF-1α signaling cascade and reducing succinic acid accumulation by countering fatty acid oxidation (She et al., 2018).
In summary, the anti-fibrotic action of curcumin in the liver is characterized by its multi-pronged attack on the hepatic triad of injury: it dampens Kupffer cell activation, directly suppresses HSC activation, and enhances hepatoprotective signaling. The targeting of resident liver cells like Kupffer cells and HSCs presents a distinct, liver-specific strategy not seen in other organs. This contrasts with its role in pulmonary fibrosis, where its primary cellular targets are likely activated fibroblasts and alveolar epithelial cells, highlighting how curcumin’s efficacy is shaped by the unique cellular ecosystem of each diseased organ.
6.2 Anti-renal fibrosis effect of curcumin
High glucose induces superoxide imbalance in glomerular mesangial cells and causes the accumulation of ECM in diabetic glomeruli. Renal tubular EMT is a factor in the accumulation of renal matrix proteins, and oxidative stress may predispose to the development of EMT in renal tubular epithelial cells in diabetic nephropathy. Evidence indicates curcumin ameliorates renal fibrosis via Wnt/β-catenin modulation coupled with superoxide suppression (Ho et al., 2016). It can also protect NRK-52E cells from high glucose-stimulated EMT by enabling NRF2 and HO-1 (Ho et al., 2016).
NRF2 is a cytoprotective transcription factor that can induce the expression of multiple antioxidants and phase II detoxification enzymes and plays an essential part in adjusting cellular detoxification and redox homeostasis. Evidence indicates curcumin ameliorates cisplatin-induced EMT and renal fibrosis via NRF2 activation (Trujillo et al., 2016). Soetikno et al. suggested that curcumin can also alleviate redox imbalance and renal fibrosis by governing the NRF2-Keap1 signaling cascade (Soetikno et al., 2013). Lia et al. suggested that curcumin could attenuate oxidative stress and renal fibrosis caused by acetaldehyde by activating the NRF2 signaling cascade to reduce MDA content and increase the levels of SOD, CAT, glutathione peroxidase (GPX), glutathione reductase (GR) and GSH (Li et al., 2019). Furthermore, it has also been suggested that curcumin can not only reverse the suppressive effect of redox imbalance on GPx efficacy, thereby alleviating the ROS accumulation in rats resulted by Ochratoxin A, but also alleviate the renal fibrosis caused by Ochratoxin A (Damiano et al., 2020).
The landscape of renal fibrosis underscores a central role for oxidative stress in driving mitochondrial dysfunction and epithelial-mesenchymal transition (EMT). Curcumin’s intervention in this organ demonstrates a distinctive emphasis on rectifying metabolic derangements within highly metabolic renal tubular cells. The significant amelioration of renal fibrosis through the Wnt/β-catenin and Nrf2-Keap1 pathways highlights a therapeutic strategy that is particularly crucial in the context of diabetic nephropathy. This metabolic-centric approach presents a contrast to its action in hepatic fibrosis, where the primary battleground involves the activation of quiescent hepatic stellate cells by inflammatory signals from Kupffer cells. Thus, in the kidney, curcumin appears to function not only as an antioxidant but also as a metabolic regulator, protecting the energy-fragile tubular epithelium from oxidative injury and subsequent fibrotic transformation.
6.3 Anti-myocardial fibrosis effect of curcumin
Cardiac fibrosis serves as a hallmark characteristic of pathological hypertrophy, manifesting as extracellular matrix proliferation driven by collagen deposition. Treatment of H9C2 cells with palmitate significantly increased ROS and redox imbalance, curcumin activated the NRF2 signaling cascade, thereby significantly increasing the expression of downstream genes glutamate-cysteine ligase catalytic (Gclc), HO-1, and NAD(P)H quinone oxidoreductase 1 (NQO-1), and antioxidant response inhibited myocardial fibrosis (Zeng et al., 2015). Studies have shown that the protein kinase C (PKC) is a serine/threonine-associated protein kinase (Newton, 2003). Hyperglycemia leads to PKC activation, and increased PKC activity can lead to alterations in the ECM (Sheetz and King, 2002), leading to cardiomyocyte hypertrophy and interstitial fibrosis, PKC activation can also induce mitogen-activated protein kinase (MAPK). Vivian Soetikno and Flori R. Sari et al. suggested that curcumin could ameliorate myocardial fibrosis in diabetic rats by suppressing the PKC-MAPK signaling cascade and attenuating oxidative stress (Soetikno et al., 2012). It is also reported that curcumin can reduce redox imbalance by activating the PPAR-γ pathway, thereby inhibiting inflammation and fibrosis (Gbr et al., 2021).
6.4 Anti-pulmonary fibrosis effect of curcumin
ROS/RNS produced either endogenous or extrinsic may damage alveolar epithelium directly (Kinnula et al., 2005). Oxidative stress occurred by activating transcription factors that trigger cell-mediated cell signaling pathways and induce inflammatory cytokines, while also damaging DNA and lipids (Bezerra et al., 2023; Liu et al., 2023). Research indicates that curcumin augments antioxidant capacity via upregulating HO-1 expression in fibroblasts and primary pulmonary endothelial cells while suppressing radiation-triggered (Lee et al., 2010). In murine LMSCs, curcumin may have an antioxidant effect on it through the Akt/Nrf2/HO-1 pathway, thereby playing a role in anti-pulmonary fibrosis (Ke et al., 2020). Animal experiments showed that curcumin inhibited hydroxyproline content, collagen type I, TGF-β1 expression, myeloperoxidase (MPO), and superoxide generation in the lungs of amiodarone rats, thereby improving pulmonary fibrosis (Punithavathi et al., 2003). According to previous studies that TGF-β1 can induce NADPH oxidase 4 (NOX4)-dependent ROS production, thereby promoting fibroblast migration (Amara et al., 2010), and can upregulate mitochondrial ROS to induce lung epithelial cell senescence to promote fibrosis (Yoon et al., 2005; Taslidere et al., 2014). Furthermore, curcumin and nanocurcumin inhibited ROS production and alleviated paraquat (PQ) -induced pulmonary fibrosis by modulating gene expression of the kelch-like ECH-associated protein 1 (KEAP1), HO-1, NQO1, and glutathione-S-transferase (GST) in lung tissue (Tyagi et al., 2016; Mahlooji et al., 2022). We summarized the effects of curcumin on various fibrotic diseases in Table 2 (Table 2).
TABLE 2
| Disease | Animals/cell lines | Upregulation | Downregulation | Inducers | Concentration | Duration | References |
|---|---|---|---|---|---|---|---|
| Pulmonary Fibrosis | Female C57BL/6 mice | — | HYP | RT | 1% or 5% curcumin | 4 months | Lee et al. (2010) |
| PMVEC | — | ROS | RT | 5, 10, 25, 50, 100 μM | 4 h | ||
| Primary fibroblasts | — | — | — | 5, 10, 25, 50, 100 μM | 4 h | ||
| Murine LMSCs | p-Akt/Akt, NRF2, HO-1 | ROS | H2O2 | 2.5, 5, 10 μM | 6 h | Ke et al. (2020) | |
| Male Fischer 344 rats | — | MPO, HYP, Type I Collagen, Superoxide anion,TGF-β1 | Amiodarone | 200 mg/kg | 5 weeks | Punithavathi et al. (2003) | |
| Parke’s strain of mice | — | HYP, ROS, TIMP-1, a-SMA | PQ | 5 mg/kg | 49 h | Tyagi et al. (2016) | |
| Female mice | — | ROS, α-SMA, HYP, Nitrite, MPO, EPO | SiO2 | 5 mg/kg | 21 days | Kumari and Singh (2022) | |
| Male Wistar rats | NRF2, HO-1, NQO1, TAC, TTG, GST | KEAP1, Hydroxyproline | PQ | 30 mg/kg/day | 7 days | Hosseini et al. (2021) | |
| Hepatic Fibrosis | Male Sprague-Dawley rats | — | HYP, HA, PC III, Collagen IV, α-SMA | CCl4 | 100, 200, 400 mg/kg | 8 weeks | Kong et al. (2020) |
| BNL CL.2 cells | PPAR-α, GSH | α-SMA, ROS | TGF-β1 | 10, 20, 30 μM/L | 24 h | ||
| Male Wistar rats | GSH | α-SMA, Col-I, Smad3, CTGF, TGF-β | CCl4 | 100 mg/kg | 4 weeks | Hernández-Aquino et al. (2020) | |
| Male ICR mice | — | α-SMA, HIF-1α, Col1α, Col3α, FN, TGF-β1, SDH, succinate | HFD | 50 mg/kg | 10 weeks | She et al. (2018) | |
| HSCs | — | Col1α, Col3α, FN, α-SMA, TGF-β1, ROS | dimethyl succinate | 10 μM | 8 h | ||
| HSCs | — | SDH, HIF-1α | PA | 10 μM | 8 h | ||
| Male Wistar rats | GSH, GSSG, GSH+GSSG, GSH/GSSG | collagen, TGF-β | CCl 4 | 100 mg/kg | 2 months | Reyes-Gordillo et al. (2008) | |
| Male Wistar rats | — | Nitrotyrosine | TAA | 300 mg/kg | 12 weeks | Bruck et al. (2007) | |
| Male Sprague Dawely rats | SOD, HO-1 | ROS, MDA | TAA | 100, 200 mg/kg | 18 weeks | Elmansi et al. (2017) | |
| Male C57BL/6 mice | — | α-SMA,TIMP-1, Procollagen type I, ROS, 8-OH-dG | MCD | 25 μg | 4, 8, 10 weeks | Vizzutti et al. (2010) | |
| Male Sprague–Dawley rats | SOD, HO-1 | MDA, ROS | HFD | 1 g/kg | 16 weeks | Öner-İyidoğan et al. (2014) | |
| HSCs | PGC-1α, AMPKα, SOD2, PPAR-γ | α1(I) collagen | — | 5, 10, 15 μm | 24 h | Zhai et al. (2015) | |
| Sprague-Dawley rats | GSH, GCL, GSH/GSSG, PPARγ | α-SMA, αI(I) collagen, FN, Tβ-RII, Tβ-RI, Lipid hydroperoxide, HYP, PDGF, TGF-β | CCl4 | 200, 400 mg/kg | 8 weeks | Fu et al. (2008) | |
| HSCs | PPAR-γ, GSH, GCL, GSH/GSSG | α-SMA, αI(I) collagen, TGF-βRI, TGF-βR II, ROS, LPO, Ob-R, PDGF-βR, CTGF | Leptin | 5, 10, 20, 30 μM | 24 h | Tang et al. (2009) | |
| HSCs | PPARγ, GSH, GSH/GSSG, GCL | p38 MAPK, αI(I) procollagen, α-SMA, Tβ-RI, Tβ-II, CTGF, ROS, LPO, GLUT2, PDGF-βR | Glucose | 10, 20, 30 μM | 24 h | Lin and Chen (2011) | |
| HSCs | PPAR-γ, GSH, GSH/GSSG, GCL | Tß-RI, Tß-RII, ROS, LPO, αI(I) collagen | — | 5, 10, 15, 20, 30 µM | 24 h | Zheng et al. (2007) | |
| Male Sprague-Dawley rats | GSH, GPX, APE1, PPARγ | MDA, TGF-β, CTGF, TIMP-1, α-SMA, STAP | CCl4 | 200 mg/kg | 4 or 8 weeks | Bassiouny et al. (2011) | |
| HSCs | PPAR-γ, GSH, GSH/GSSG | Tβ-RI, Tβ-RII, CTGF, αI(I)-procollagen, α-SMA | — | 20 μM | 24 h | Zheng and Chen (2006) | |
| Male Sprague-Dawley rats | SOD, GSH | MDA | CCl4 | 0.005% curcumin in feed | 8 weeks | Wu et al. (2008) | |
| Male Wistar rats | NRF2, SOD, GSH, HO-1 | MPO, MDA, iNOS, Collagen type I, α-SMA, NF-ĸB-p65, TGF-β, p-Smad3 | TAA | 200 mg/kg | 8 weeks | Gowifel et al. (2020) | |
| HSCs | PPARγ, GSH, GSH/GSSG, GCL | RAGE, ROS, LPO | AGEs | 5, 10, 20, 25, 30 µM | 24 h | Lin et al. (2012) | |
| HSCs | NRF2, GCL, GSH, AGE-R1 | Leptin, RAGE | AGEs | 20 mM | — | Tang and Chen (2014) | |
| Male Wistar albino rats | CAT, GSH, TIMP-2 | MDA | BPA | 100 mg/kg | 8 weeks | Elswefy et al. (2020) | |
| HSC-T6 | NRF2, GSH | ROS, MDA, α-SMA | GO | 0.15 µM | 3 h | Liu et al. (2016) | |
| Male Wistar albino rats | SOD | NO, MDA, α-SMA, Collagen III | NHPA | 200 mg/kg | 22 h | Alhusain et al. (2022) | |
| Male Rattus norvigicus | CAT, SOD, GSH | MDA | TAA | Curcumin group:50 mg/kg; Curcumin NPs group:15 mg/kg | 2 weeks | Radwan et al. (2024) | |
| Renal Fibrosis | Male Wistar rats | Wnt5a, β-catenin | Superoxide, 8-OH-dG, Fibronectin, TGF-β1 | STZ | 10 mg/kg/day | 56 days | Ho et al. (2016) |
| Rat mesangial cells | Wnt5a, β-catenin | Superoxide, TGF-β1, Fibronectin | D-glucose | 10 μM | 48 h | ||
| Male Wistar rats | NRF2, CAT, GR | TGF β1, Collagen I, Collagen IV, a-SMA, MDA, 3-NT, p47phox, gp91phox, PKCβ2 | CIS | 200 mg/kg | 72 h | Trujillo et al. (2016) | |
| The NRK-52E normal rat/kidney tubular epithelial cell line | NRF2, HO-1 | α-SMA | HG | 5, 10, 20 µM | 24 h | Zhang et al. (2015) | |
| Male Sprague-Dawley rats | NRF2, HO-1, GPx, CCr | Keap1, p67phox, p22phox, MDA, NF-κB, TNF-α, TGF-β1, Fibronectin | 5/6 nephrectomy | 75 mg/kg/day | 8 weeks | Soetikno et al. (2013) | |
| Male Sprague Dawley rats | SOD, CAT, Gpx | MDA | OTA | 100 mg/kg | 14 days | Damiano et al. (2020) | |
| Male C57BL/6 mice | GSH, NRF2, HO-1, NQO1, UGT, SOD, CAT, GPx, GR | MDA, α-SMA, Collagen I | Glyoxylate | 50,100 mg/kg | 14 days | Li et al. (2019) | |
| Myocardial Fibrosis | Male adult Sprague Dawley rats | GSH, TAC, PPAR-γ | MDA, TGF-β1, CaMKII | STZ | 100 mg/kg/day | 6 weeks | Gbr et al. (2021) |
| Male C57BL/6 mice | NRF2, HO-1, NQO-1 | CTGF, TGF-β | HFD | 50 mg/kg/day | 8 weeks | Zeng et al. (2015) | |
| H9C2 embryonic rat heart-derived cell line | NRF2, HO-1, GCLC, NQO-1 | ROS, TGF-β | PA | 20 μM | 15 h | ||
| Male Sprague–Dawley rats | GPx | MDA, p22phox, p67phox, gp91phox, PKC-α, PKC-β2, p-P38MAPK/P38MAPK, p-ERK1/2/ERK1/2, TGF-β | STZ | 100 mg/kg/day | 8 weeks | Soetikno et al. (2012) |
Effect of curcumin on fibrotic diseases.
The evidence presented above positions curcumin as a modulator of the oxidative milieu that drives fibroblast differentiation and ECM deposition in the lungs. A key comparative insight emerges when contrasting lung and liver fibrosis: while both conditions involve TGF-β signaling, the upstream triggers and key effector cells differ substantially. In the liver, curcumin’s interception of damage signals from hepatocytes to Kupffer cells and HSCs is critical. In the lung, however, its ability to mitigate epithelial cell injury and its consequent signaling to fibroblasts may be of paramount importance. This underlines the concept that while core pathways like Nrf2 and TGF-β are shared therapeutic targets, the cellular “entry point” for curcumin’s action is organ-specific.
6.5 Anti-fibrotic effect of curcumin analogues and formulations
Removal of unstable molecular groups and retention of active molecular groups by curcumin analogues can enhance their instability. A13, classified among curcumin analogues, shares fundamental pharmacological properties with curcumin yet demonstrates enhanced efficacy compared to curcumin regarding metabolic stability and oral bioavailability. It reduces oxidative stress and improves myocardial fibrosis in diabetic rats by activating the Nrf2/ARE pathway (Xiang et al., 2020). In addition, curcumin analog Y20 has shown better pharmacokinetic profile in vivo and can exert dual anti-inflammatory and antioxidant activities. It activates Nrf2 expression and thereby regulates oxidative stress, which may mediate high-fat diet-induced cardiomegaly, apoptosis, and fibrosis (Qian et al., 2015). Curc-mPEG454, a curcumin conjugate functionalized with short-chain polyethylene glycol (PEG), demonstrates elevated serum concentrations of curcumin while preserving its anti-inflammatory efficacy. Curc-mPEG454 augments cellular redox homeostasis through stimulation of de novo biosynthesis of Nrf2-mediated GSH (Xiao et al., 2021). The main metabolite of curcumin is tetrahydrocurcumin (THC), which is superior in inducing glutathione peroxidase and quenching free radicals, and is more stable and has better intestinal absorption than curcumin. Dietary tetrahydrocurcumin can improve renal fibrosis by reducing copper-zinc superoxide dismutase (CuZn SOD) and glutathione peroxidase (GPX-1) (Lau et al., 2018). Dehydrogingerone (DHZ), a polyphenolic constituent isolated from the rhizome of Zingiber officinale, which is a semi-analog of curcumin. DHZ ameliorated the TAA-mediated downregulation of catalase activity, thereby alleviating hepatic fibrosis progression (Sharma et al., 2022). C66, a recently developed curcumin analogue, exhibits a significantly reduced effective dosage. It stimulates miR-200a, downregulates Keap1 expression, activates NRF2, alleviates redox imbalance, and thus anti-renal fibrosis (Wu et al., 2016). Curcumin nanoparticles can improve the bioavailability and biodistribution, which significantly decreased MDA and significantly improved CAT, SOD and GSH in thioacetamide (TAA) -stimulated hepatic fibrosis in rats (Radwan et al., 2024) (Figure 4). These structural and delivery innovations directly address curcumin’s pharmacokinetic limitations. A13 and C66 analogues enable lower dosing in fibrosis models, while nanoformulations like Curc-mPEG454 achieve sustained plasma exposure critical for chronic fibrosis management. This paves the way for human trials targeting organ-specific accumulation.
FIGURE 4
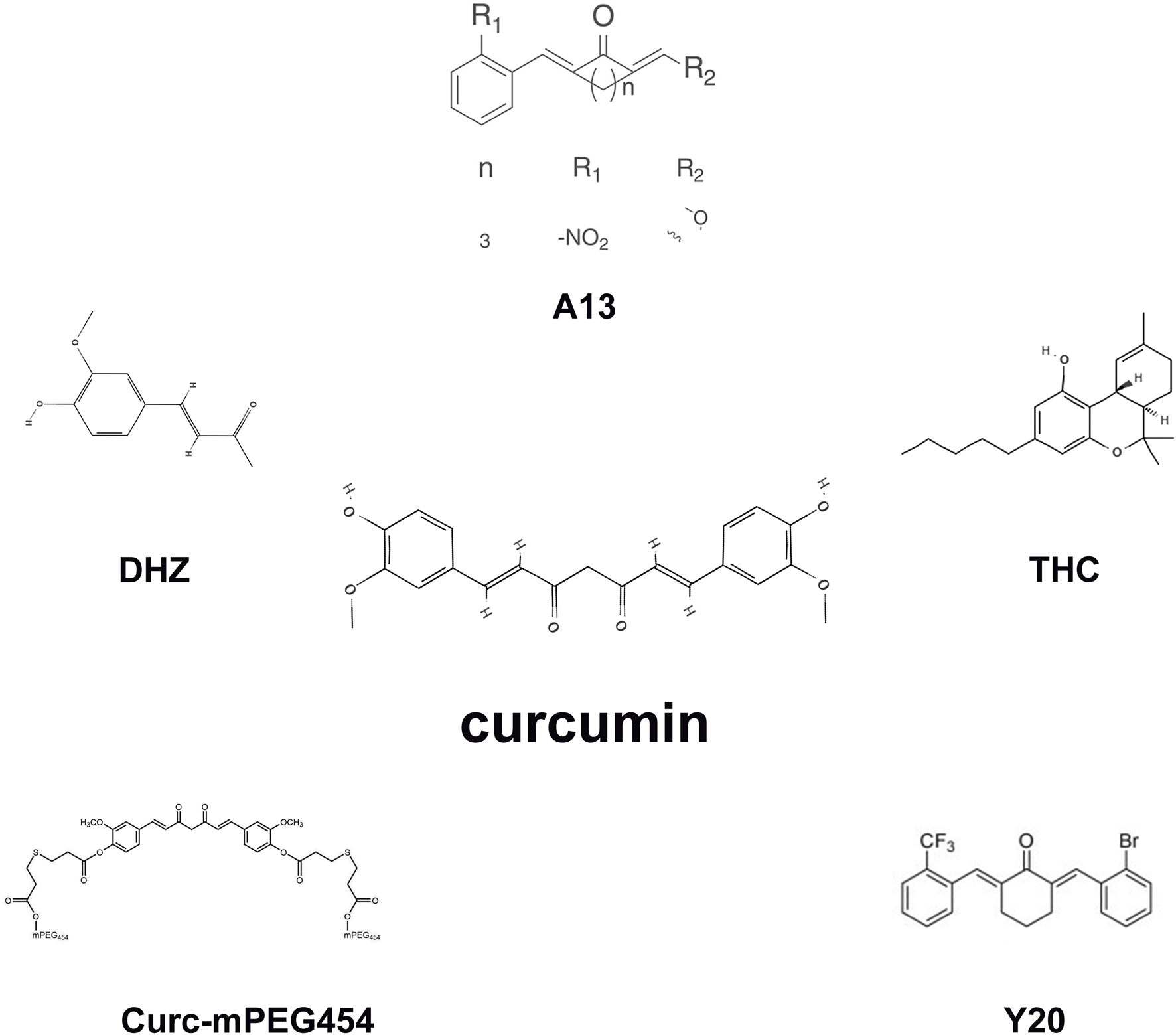
The molecular structures of the curcumin analogues.
6.6 Transcriptional reprogramming of fibrotic pathways by curcumin
Curcumin orchestrates transcriptional suppression of fibrotic genes through interconnected mechanisms. In hepatic fibrosis models, it inhibits succinate accumulation by blocking succinate dehydrogenase activity, thereby preventing HIF-1α-mediated upregulation of Col1α, Col3α (She et al., 2018). Simultaneously, it reprograms AGE receptor expression in hepatic stellate cells—downregulating pro-fibrotic RAGE while upregulating detoxifying AGE-R1—via interruption of leptin signaling and Nrf2 activation (Tang and Chen, 2014). Furthermore, curcumin (200 mg/kg) induces APE1 expression in fibrotic livers, enhancing DNA repair while suppressing NF-κB-driven transcription of TNF-α and IL-6 (Bassiouny et al., 2011).
7 Clinical evidences supporting the anti-fibrotic effects of curcumin
In patients with non-alcoholic fatty liver disease (NAFLD), a randomized controlled trial (RCT) found that 12 weeks of curcumin supplementation (1,500 mg/day) significantly reduced hepatic fibrosis scores compared to baseline, although it was not superior to lifestyle modification alone in ameliorating systemic inflammation (Saadati et al., 2019). The disparity in outcomes underscores the potential need for longer intervention periods or more bioavailable formulations, a notion strongly supported by a 12-month RCT in patients with metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes, where 1,500 mg/day of curcumin led to significant reductions in both hepatic steatosis and liver stiffness, alongside marked improvements in systemic inflammation and oxidative stress (Yaikwawong et al., 2025).
The most compelling evidence for curcumin’s direct anti-fibrotic action in human tissue comes from a 72-week, double-blind RCT using the phospholipid formulation Meriva (2 g/day) in patients with biopsy-proven non-alcoholic steatohepatitis (NASH) (Musso et al., 2025). This study reported that a remarkable 42% of patients on Meriva achieved regression of significant liver fibrosis, with 62% experiencing NASH resolution, effects potentially mediated by the inhibition of hepatic NF-κB. Importantly, the same trial also observed a significant regression of concomitant chronic kidney disease in 50% of the Meriva group, suggesting systemic anti-fibrotic benefits (Musso et al., 2025). To specifically validate these findings in renal fibrosis, the large-scale, multicenter MPAC-CKD-1 trial was designed to evaluate the efficacy of a micro-particle curcumin formulation on albuminuria and estimated glomerular filtration rate (eGFR) in patients with chronic kidney disease, with results anticipated to provide crucial evidence on its potential to slow disease progression (Weir et al., 2018).
8 Future perspectives
Curcumin exhibits a broad spectrum of positive effects in mediating oxidative stress for therapeutic intervention in fibrotic diseases. In the present investigation, the molecular mechanism of curcumin-mediated oxidative stress against fibrotic diseases was reviewed, such as PPAR, NRF2, HO-1, TGF-β, AGE-RAGE, and other signaling pathways. Curcumin functions as a free radical scavenger through its phenolic substances, beta-diones, and methoxys, acting on the activity of ROS (Farzaei et al., 2018). In liver fibrosis, damage to liver cells causes the formation of apoptotic bodies (AB), the leakage of mitochondrial DNA, or the generation of ROS. These factors initiate the stimulation of Kupffer cells and the conversion of dormant hepatic stellate cells into myofibroblastic phenotypes. Leptin, Angiotensin II, TGF-β, Platelet-derived growth factor (PDGF), and other mediators help activate NOXs in macrophages and stellate cells and further accelerate matrix deposition. Reaserches have found that curcumin has shown prophylactic and curative effects on oxidization-related liver disease through multiple cells signaling cascades. These pathways include upregulated PPAR (Zhai et al., 2015; Elmansi et al., 2017; Kong et al., 2020), NRF2 (Liu et al., 2016; Gowifel et al., 2020) and HO-1 (Öner-İyidoğan et al., 2014) signaling pathways, and downregulated RAGE (Lin et al., 2012) and AGEs (Tang and Chen, 2014) signaling pathways. In pulmonary fibrosis, ROS generation contributes to fibroblast phenotype acquisition, including differentiation, contraction, apoptotic resistance, and ECM deposition (Hecker et al., 2009; Hosseinzadeh et al., 2018). Evidence suggests that curcumin can activate the protein kinase B (Akt)/Nrf2/HO-1 (Lee et al., 2010; Ke et al., 2020) signaling pathway and attenuate the TGF-β1, MPO (Li et al., 2019) and ROS (Kumari and Singh, 2022) to inhibit redox imbalance in pulmonary fibrosis models. In myocardial fibrosis, the cardiac source of ROS is mainly nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (Li et al., 2002), which exacerbates cardiac fibrosis and modulates gap junction function, resulting in diminished myocyte coupling and facilitating arrhythmogenic reentry (Sovari, 2016). Curcumin reduces cardiac fibrosis by activating SIRT1, increasing NRF2 (Zeng et al., 2015), increasing NADPH oxidase subunits, weakening PKC-MAPK (Soetikno et al., 2012) signaling pathway, and reducing SOD and MDA (Sadoughi et al., 2021). In renal fibrosis, redox imbalance can cause the reduction of ATP production after mitochondrial dysfunction, and may also lead to the development of EMT in diabetic nephropathy, resulting in renal fibrosis (Lv et al., 2018). Emerging evidence indicates that curcumin attenuates renal fibrosis through Wnt/β-catenin (Ho et al., 2016), HO-1 (Zhang et al., 2015) and Nrf2-Keap1 (Soetikno et al., 2013) signaling pathways. These provide references for the pharmacology and clinical application of curcumin for therapeutic intervention in fibrotic diseases. However, there are some questions that need to be further clarified in future studies before this natural compound can be used clinically.
Synthesizing the evidence from liver, renal, myocardial, and pulmonary fibrosis, a coherent model for curcumin’s pleiotropic actions comes into focus. We propose a “Core Pathway - Specific Branch” model to conceptualize its effects. At the heart of this model lies the consistent activation of the Nrf2 antioxidant pathway and the concerted suppression of the TGF-β signaling axis. These two core pathways, addressing the universal pillars of oxidative stress and pro-fibrotic signaling, form the foundational mechanism of curcumin’s efficacy across all organs studied.
Firstly, human pharmacokinetics of curcumin remain incompletely characterized, with low absorption and fast metabolism (Mirzaei et al., 2017). Secondly, limited absorption of curcumin severely constrains its clinical utility. Fortunately, chemical modification of curcumin significantly enhances its therapeutic efficacy, target selectivity, and safety profile. In addition to this, sophisticated drug delivery platforms, including liposomes, nanoparticles, and phospholipid complexes formulated with diverse synthetic/natural biomaterials (proteins, lipids, polymers), have demonstrated enhanced bioavailability and formulation stability for curcumin derivatives. The promising work on phospholipid complexes and nanoparticles must be advanced towards “smart” targeted delivery. Strategies should include designing nanoparticles that home to activated stellate cells or profibrotic fibroblasts, engineered for stimulus-responsive release in the high-ROS microenvironment of the fibrotic niche. This would ensure precise spatiotemporal delivery, enhancing efficacy while minimizing off-target effects. Combining curcumin with established anti-fibrotic drugs in a single nano-formulation could also create powerful synergistic therapies, where curcumin acts as a “sensitizing” agent to enhance the primary drug’s efficacy. Although extensive research efforts have addressed formulation limitations and optimized physicochemical attributes, critical gaps persist in curcumin’s therapeutic efficacy, target specificity, and pharmacokinetic performance—issues that warrant urgent attention from the scientific community (Bisht et al., 2007). Third, due to the multi-target properties of curcumin, its anti-fibrosis mechanism has not been fully illustrated and more validation is needed. The majority of contemporary investigations primarily emphasize cellular and animal-based models, yet scarce clinical evidence exists to assess curcumin’s anti-fibrotic therapeutic potential and its corresponding dosage requirements. More studies should provide more conclusive evidence, especially those with large samples and multi-center prospective cohort studies. The most critical frontier is the design of definitive clinical trials. The scarcity of clinical evidence, highlighted earlier, must be addressed through hypothesis-driven, biomarker-enriched trials. These trials should incorporate. Validated Redox and ECM Biomarkers: Moving beyond standard serum enzymes to include direct markers of oxidative stress (e.g., specific lipid peroxidation adducts) and ECM turnover (e.g., PRO-C3) to objectively quantify anti-fibrotic efficacy. Precision Enrollment: Focusing on specific fibrotic disease etiologies and potentially stratifying patients based on their baseline redox or inflammatory status. Long-term Safety and Efficacy Assessment: Rigorously evaluating the long-term safety profile, a concern we previously raised, and the ability of curcumin to halt or reverse fibrosis progression in large-scale, multi-center, randomized controlled trials. Fourth, concerning safety evaluation, extended-duration human studies are required to rigorously evaluate the therapeutic safety profile of curcumin in clinical populations.
9 Conclusion
In summary, numerous investigations have validated that the anti-fibrotic effect of curcumin through mediating oxidative stress, and more studies are needed to further confirm the anti-fibrotic effect of curcumin. It is hoped that with further research, the therapeutic effect of curcumin on fibrotic diseases may be understood and applied clinically.
Statements
Author contributions
SY: Writing – review and editing, Formal Analysis, Visualization, Writing – original draft, Project administration, Methodology, Investigation, Software, Data curation, Conceptualization. JP: Visualization, Data curation, Project administration, Writing – review and editing, Software, Writing – original draft, Methodology, Conceptualization, Formal Analysis, Investigation. KL: Software, Methodology, Formal Analysis, Data curation, Visualization, Writing – original draft, Conceptualization, Writing – review and editing, Investigation. CH: Writing – original draft, Writing – review and editing, Investigation, Formal Analysis, Visualization, Methodology. FY: Writing – review and editing, Investigation, Methodology, Visualization, Formal Analysis, Writing – original draft. KC: Writing – review and editing, Investigation, Methodology, Formal Analysis, Writing – original draft, Visualization. XY: Writing – review and editing, Formal Analysis, Validation, Methodology. YZ: Formal Analysis, Methodology, Writing – review and editing, Validation. JJ: Writing – original draft, Writing – review and editing, Supervision, Conceptualization. ML: Writing – review and editing, Methodology, Formal Analysis, Validation. XL: Resources, Funding acquisition, Writing – original draft, Project administration, Conceptualization, Writing – review and editing, Supervision. CZ: Writing – original draft, Writing – review and editing, Resources, Supervision, Funding acquisition, Project administration, Conceptualization. YH: Supervision, Writing – original draft, Project administration, Conceptualization, Writing – review and editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The National Natural Science Foundation of China (82374291), 2022 “Tianfu Qingcheng Plan” Tianfu Science and Technology Leading Talents Project (Chuan Qingcheng No. 1090), the National TCM Clinical Excellent Talents Training Program (National TCM Renjiao Letter [2022] No. 1), Special subject of scientific research of Sichuan Administration of Traditional Chinese Medicine (2024zd005), Sichuan Science and Technology Program (2024NSFJQ0059), Joint Innovation Fund of Health Commission of Chengdu and Chengdu University of Traditional Chinese Medicine (WXLH202403091).
Acknowledgments
We thank Microsoft Office PowerPoint for providing visualization support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abd El-Hack M. E. El-Saadony M. T. Swelum A. A. Arif M. Abo Ghanima M. M. Shukry M. et al (2021). Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric.101 (14), 5747–5762. 10.1002/jsfa.11372
2
Aggarwal B. B. Harikumar K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol.41 (1), 40–59. 10.1016/j.biocel.2008.06.010
3
Aggarwal B. B. Sundaram C. Malani N. Ichikawa H. (2007). Curcumin: the Indian solid gold. Adv. Exp. Med. Biol.595, 1–75. 10.1007/978-0-387-46401-5_1
4
Aggarwal M. L. Chacko K. M. Kuruvilla B. T. (2016). Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: a bioavailable turmeric formulation. Mol. Med. Rep.13 (1), 592–604. 10.3892/mmr.2015.4579
5
Aidi Q. (2002). Analysis of curcumin in Curcuma longa, C. wenyujin, C. kwangsiensis by HPLC. Chin. Traditional Herb. Drugs (06), 33–35.
6
Alhusain A. Fadda L. Sarawi W. Alomar H. Ali H. Mahamad R. et al (2022). The potential protective effect of curcumin and α-lipoic acid on N-(4-Hydroxyphenyl) acetamide-induced hepatotoxicity through downregulation of α-SMA and collagen III expression. Dose-Response20 (1), 15593258221078394.
7
Amara N. Goven D. Prost F. Muloway R. Crestani B. Boczkowski J. (2010). NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFβ1-induced fibroblast differentiation into myofibroblasts. Thorax65 (8), 733–738. 10.1136/thx.2009.113456
8
Anand P. Kunnumakkara A. B. Newman R. A. Aggarwal B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm.4 (6), 807–818. 10.1021/mp700113r
9
Antar S. A. Ashour N. A. Marawan M. E. Al-Karmalawy A. A. (2023). Fibrosis: types, effects, markers, mechanisms for disease progression, and its relation with oxidative stress, immunity, and inflammation. Int. J. Mol. Sci.24 (4), 4004. 10.3390/ijms24044004
10
Aquino-Gálvez A. González-Ávila G. Jiménez-Sánchez L. L. Maldonado-Martínez H. A. Cisneros J. Toscano-Marquez F. et al (2019). Dysregulated expression of hypoxia-inducible factors augments myofibroblasts differentiation in idiopathic pulmonary fibrosis. Respir. Res.20 (1), 130. 10.1186/s12931-019-1100-4
11
Bartneck M. Schrammen P. L. Möckel D. Govaere O. Liepelt A. Krenkel O. et al (2019). The CCR2(+) macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers. Cell Mol. Gastroenterol. Hepatol.7 (2), 371–390. 10.1016/j.jcmgh.2018.10.007
12
Bassiouny A. R. Zaky A. Kandeel K. M. (2011). Alteration of AP-endonuclease1 expression in curcumin-treated fibrotic rats. Ann. Hepatol.10 (4), 516–530. 10.1016/s1665-2681(19)31521-2
13
Bezerra F. S. Lanzetti M. Nesi R. T. Nagato A. C. Silva C. P. E. Kennedy-Feitosa E. et al (2023). Oxidative stress and inflammation in acute and chronic lung injuries. Antioxidants (Basel)12 (3), 548. 10.3390/antiox12030548
14
Bisht S. Feldmann G. Soni S. Ravi R. Karikar C. Maitra A. et al (2007). Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J. Nanobiotechnology5, 3. 10.1186/1477-3155-5-3
15
Bruck R. Ashkenazi M. Weiss S. Goldiner I. Shapiro H. Aeed H. et al (2007). Prevention of liver cirrhosis in rats by curcumin. Liver Int.27 (3), 373–383. 10.1111/j.1478-3231.2007.01453.x
16
Chouchani E. T. Pell V. R. Gaude E. Aksentijević D. Sundier S. Y. Robb E. L. et al (2014). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature515 (7527), 431–435. 10.1038/nature13909
17
Cui Y. Wang Q. Zhang X. Yang X. Shi Y. Li Y. et al (2023). Curcumin alleviates aflatoxin B(1)-Induced liver pyroptosis and fibrosis by regulating the JAK2/NLRP3 signaling pathway in ducks. Foods12 (5), 1006. 10.3390/foods12051006
18
Damiano S. Andretta E. Longobardi C. Prisco F. Paciello O. Squillacioti C. et al (2020). Effects of curcumin on the renal toxicity induced by ochratoxin A in rats. Antioxidants (Basel)9 (4), 332. 10.3390/antiox9040332
19
Dellali M. Iurciuc Tincu C. E. Savin C. L. Spahis N. Djennad M. Popa M. (2021). Hydrogel films based on chitosan and oxidized carboxymethylcellulose optimized for the controlled release of curcumin with applications in treating dermatological conditions. Molecules26 (8), 2185. 10.3390/molecules26082185
20
Distler J. H. W. Györfi A. H. Ramanujam M. Whitfield M. L. Königshoff M. Lafyatis R. (2019). Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol.15 (12), 705–730. 10.1038/s41584-019-0322-7
21
Elmansi A. M. El-Karef A. A. Shishtawy M. Eissa L. A. (2017). Hepatoprotective effect of curcumin on hepatocellular carcinoma through autophagic and apoptic pathways. Ann. Hepatol.16 (4), 607–618. 10.5604/01.3001.0010.0307
22
Elswefy S. E. Abdallah F. R. Wahba A. S. Hasan R. A. Atteia H. H. (2020). Antifibrotic effect of curcumin, N-acetyl cysteine and propolis extract against bisphenol A-induced hepatotoxicity in rats: prophylaxis versus co-treatment. Life Sci.260, 118245. 10.1016/j.lfs.2020.118245
23
Esatbeyoglu T. Huebbe P. Ernst I. M. Chin D. Wagner A. E. Rimbach G. (2012). Curcumin--from molecule to biological function. Angew. Chem. Int. Ed. Engl.51 (22), 5308–5332. 10.1002/anie.201107724
24
Farzaei M. H. Zobeiri M. Parvizi F. El-Senduny F. F. Marmouzi I. Coy-Barrera E. et al (2018). Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients10 (7), 855. 10.3390/nu10070855
25
Fathimath Muneesa M. Barki R. R. Shaikh S. B. Bhandary Y. P. (2022). Curcumin intervention during progressive fibrosis controls inflammatory cytokines and the fibrinolytic system in pulmonary fibrosis. Toxicol. Appl. Pharmacol.449, 116116. 10.1016/j.taap.2022.116116
26
Fu Y. Zheng S. Lin J. Ryerse J. Chen A. (2008). Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol.73 (2), 399–409. 10.1124/mol.107.039818
27
Garcea G. Jones D. J. Singh R. Dennison A. R. Farmer P. B. Sharma R. A. et al (2004). Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer90 (5), 1011–1015. 10.1038/sj.bjc.6601623
28
Gbr A. A. Abdel Baky N. A. Mohamed E. A. Zaky H. S. (2021). Cardioprotective effect of pioglitazone and curcumin against diabetic cardiomyopathy in type 1 diabetes mellitus: impact on CaMKII/NF-κB/TGF-β1 and PPAR-γ signaling pathway. Naunyn Schmiedeb. Arch. Pharmacol.394 (2), 349–360. 10.1007/s00210-020-01979-y
29
Gowifel A. M. H. Khalil M. G. Nada S. A. Kenawy S. A. Ahmed K. A. Salama M. M. et al (2020). Combination of pomegranate extract and curcumin ameliorates thioacetamide-induced liver fibrosis in rats: impact on TGF-β/Smad3 and NF-κB signaling pathways. Toxicol. Mech. Methods30 (8), 620–633. 10.1080/15376516.2020.1801926
30
Guillot A. Tacke F. (2019). Liver macrophages: old dogmas and new insights. Hepatol. Commun.3 (6), 730–743. 10.1002/hep4.1356
31
Hao M. Chu Y. Lei J. Yao Z. Wang P. Chen Z. et al (2023). Pharmacological mechanisms and clinical applications of curcumin: update. Aging Dis.14 (3), 716–749. 10.14336/ad.2022.1101
32
Hayes J. D. Dinkova-Kostova A. T. (2014). The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci.39 (4), 199–218. 10.1016/j.tibs.2014.02.002
33
Hecker L. Vittal R. Jones T. Jagirdar R. Luckhardt T. R. Horowitz J. C. et al (2009). NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med.15 (9), 1077–1081. 10.1038/nm.2005
34
Henderson N. C. Rieder F. Wynn T. A. (2020). Fibrosis: from mechanisms to medicines. Nature587 (7835), 555–566. 10.1038/s41586-020-2938-9
35
Hernández-Aquino E. Quezada-Ramírez M. A. Silva-Olivares A. Ramos-Tovar E. Flores-Beltrán R. E. Segovia J. et al (2020). Curcumin downregulates smad pathways and reduces hepatic stellate cells activation in experimental fibrosis. Ann. Hepatol.19 (5), 497–506. 10.1016/j.aohep.2020.05.006
36
Ho C. Hsu Y. C. Lei C. C. Mau S. C. Shih Y. H. Lin C. L. (2016). Curcumin rescues diabetic renal fibrosis by targeting superoxide-mediated wnt signaling pathways. Am. J. Med. Sci.351 (3), 286–295. 10.1016/j.amjms.2015.12.017
37
Hoehle S. I. Pfeiffer E. Sólyom A. M. Metzler M. (2006). Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem.54 (3), 756–764. 10.1021/jf058146a
38
Holder G. M. Plummer J. L. Ryan A. J. (1978). The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica8 (12), 761–768. 10.3109/00498257809069589
39
Hosseini A. Rasaie D. Asl S. Nili-Ahmadabadi A. (2021). Evaluation of the protective effects of curcumin and nanocurcumin against lung injury induced by sub-acute exposure to paraquat in rats. Toxin reviews40 (4), 1233–1241.
40
Hosseinzadeh A. Javad-Moosavi S. A. Reiter R. J. Yarahmadi R. Ghaznavi H. Mehrzadi S. (2018). Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin. Ther. Targets22 (12), 1049–1061. 10.1080/14728222.2018.1541318
41
Hu Chenxia L. Y. W. Q. (2008). Discussion on the use of turmeric in the treatment of Bi syndrome. New Chin. Med. (05), 106–107. 10.13457/j.cnki.jncm.2008.05.052
42
Issemann I. Green S. (1990). Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature347 (6294), 645–650. 10.1038/347645a0
43
Janovick N. A. Dann H. M. Loor J. J. Drackley J. K. (2022). Prepartum dietary energy intake alters hepatic expression of genes related to peroxisome proliferator-activated receptor and inflammation in peripartal dairy cows. J. Dairy Sci.105 (10), 8069–8086. 10.3168/jds.2021-21669
44
Jianmin C. Yuheng C. Jingguang Y. (1983). Research on turmeric plants in China (IV.) determination of curcumin compounds in rhizomes and tubers of turmeric genus. Chin. Traditional Herb. Drugs14 (02), 11–15.
45
Jianming T. Li H. (2016). Research advances in regulation of extracellular matrix metabolism by oxidative stress. China Med. Her.13 (04), 36–40.
46
Ke S. Zhang Y. Lan Z. Li S. Zhu W. Liu L. (2020). Curcumin protects murine lung mesenchymal stem cells from H(2)O(2) by modulating the Akt/Nrf2/HO-1 pathway. J. Int. Med. Res.48 (4), 300060520910665. 10.1177/0300060520910665
47
Kinnula V. L. Fattman C. L. Tan R. J. Oury T. D. (2005). Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med.172 (4), 417–422. 10.1164/rccm.200501-017PP
48
Kliewer S. A. Forman B. M. Blumberg B. Ong E. S. Borgmeyer U. Mangelsdorf D. J. et al (1994). Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. U. S. A.91 (15), 7355–7359. 10.1073/pnas.91.15.7355
49
Kocaadam B. Şanlier N. (2017). Curcumin, an active component of turmeric (curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr.57 (13), 2889–2895. 10.1080/10408398.2015.1077195
50
Kong D. Zhang Z. Chen L. Huang W. Zhang F. Wang L. et al (2020). Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol.36, 101600. 10.1016/j.redox.2020.101600
51
Kotha R. R. Luthria D. L. (2019). Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules24 (16), 2930. 10.3390/molecules24162930
52
Kumari S. Singh R. (2022). Protective effects of intranasal curcumin on silica-induced lung damage. Cytokine157, 155949. 10.1016/j.cyto.2022.155949
53
Kurundkar A. Thannickal V. J. (2016). Redox mechanisms in age-related lung fibrosis. Redox Biol.9, 67–76. 10.1016/j.redox.2016.06.005
54
Lamb Y. N. (2021). Nintedanib: a review in fibrotic interstitial lung diseases. Drugs81 (5), 575–586. 10.1007/s40265-021-01487-0
55
Lau W. L. Khazaeli M. Savoj J. Manekia K. Bangash M. Thakurta R. G. et al (2018). Dietary tetrahydrocurcumin reduces renal fibrosis and cardiac hypertrophy in 5/6 nephrectomized rats. Pharmacol. Res. Perspect.6 (2), e00385. 10.1002/prp2.385
56
Lee J. C. Kinniry P. A. Arguiri E. Serota M. Kanterakis S. Chatterjee S. et al (2010). Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res.173 (5), 590–601. 10.1667/rr1522.1
57
Li J. M. Gall N. P. Grieve D. J. Chen M. Shah A. M. (2002). Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension40 (4), 477–484. 10.1161/01.hyp.0000032031.30374.32
58
Li X. Chen Y. Wu S. He J. Lou L. Ye W. et al (2015). microRNA-34a and microRNA-34c promote the activation of human hepatic stellate cells by targeting peroxisome proliferator-activated receptor γ. Mol. Med. Rep.11 (2), 1017–1024. 10.3892/mmr.2014.2846
59
Li Y. Zhang J. Liu H. Yuan J. Yin Y. Wang T. et al (2019). Curcumin ameliorates glyoxylate-induced calcium oxalate deposition and renal injuries in mice. Phytomedicine61, 152861. 10.1016/j.phymed.2019.152861
60
Li X. Li L. Lei W. Chua H. Z. Li Z. Huang X. et al (2021). Traditional Chinese medicine as a therapeutic option for cardiac fibrosis: pharmacology and mechanisms. Biomed. Pharmacother.142, 111979. 10.1016/j.biopha.2021.111979
61
Li W. Q. Liu W. H. Qian D. Liu J. Zhou S. Q. Zhang L. et al (2022). Traditional Chinese medicine: an important source for discovering candidate agents against hepatic fibrosis. Front. Pharmacol.13, 962525. 10.3389/fphar.2022.962525
62
Lin J. Chen A. (2011). Curcumin diminishes the impacts of hyperglycemia on the activation of hepatic stellate cells by suppressing membrane translocation and gene expression of glucose transporter-2. Mol. Cell Endocrinol.333 (2), 160–171. 10.1016/j.mce.2010.12.028
63
Lin J. Tang Y. Kang Q. Feng Y. Chen A. (2012). Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. Br. J. Pharmacol.166 (8), 2212–2227. 10.1111/j.1476-5381.2012.01910.x
64
Liu R. M. Desai L. P. (2015). Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol.6, 565–577. 10.1016/j.redox.2015.09.009
65
Liu Z. Dou W. Zheng Y. Wen Q. Qin M. Wang X. et al (2016). Curcumin upregulates Nrf2 nuclear translocation and protects rat hepatic stellate cells against oxidative stress. Mol. Med. Rep.13 (2), 1717–1724. 10.3892/mmr.2015.4690
66
Liu X. Wang X. Chang J. Zhang H. Cao P. (2023). Landscape analysis and overview of the literature on oxidative stress and pulmonary diseases. Front. Pharmacol.14, 1190817. 10.3389/fphar.2023.1190817
67
Lohwasser C. Neureiter D. Popov Y. Bauer M. Schuppan D. (2009). Role of the receptor for advanced glycation end products in hepatic fibrosis. World J. Gastroenterol.15 (46), 5789–5798. 10.3748/wjg.15.5789
68
Luo M. Zheng Y. Tang S. Gu L. Zhu Y. Ying R. et al (2023). Radical oxygen species: an important breakthrough point for botanical drugs to regulate oxidative stress and treat the disorder of glycolipid metabolism. Front. Pharmacol.14, 1166178. 10.3389/fphar.2023.1166178
69
Lurje I. Gaisa N. T. Weiskirchen R. Tacke F. (2023). Mechanisms of organ fibrosis: emerging concepts and implications for novel treatment strategies. Mol. Asp. Med.92, 101191. 10.1016/j.mam.2023.101191
70
Lv W. Booz G. W. Fan F. Wang Y. Roman R. J. (2018). Oxidative stress and renal fibrosis: recent insights for the development of novel therapeutic strategies. Front. Physiol.9, 105. 10.3389/fphys.2018.00105
71
Mahlooji M. A. Heshmati A. Kheiripour N. Ghasemi H. Asl S. S. Solgi G. et al (2022). Evaluation of protective effects of curcumin and nanocurcumin on aluminium phosphide-induced subacute lung injury in rats: modulation of oxidative stress through SIRT1/FOXO3 signalling pathway. Drug Res. (Stuttg)72 (2), 100–108. 10.1055/a-1647-2418
72
Makena P. Kikalova T. Prasad G. L. Baxter S. A. (2023). Oxidative stress and lung fibrosis: towards an adverse outcome pathway. Int. J. Mol. Sci.24 (15), 12490. 10.3390/ijms241512490
73
Mirzaei H. Shakeri A. Rashidi B. Jalili A. Banikazemi Z. Sahebkar A. (2017). Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother.85, 102–112. 10.1016/j.biopha.2016.11.098
74
Musso G. Pinach S. Mariano F. Saba F. De Michieli F. Framarin L. et al (2025). Effect of phospholipid curcumin meriva on liver histology and kidney disease in nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Hepatology81 (2), 560–575. 10.1097/hep.0000000000000937
75
Nan Z. Nan Z. Ming L. Gaijing F. Chunfang H. (2023). Analysis of curcumin patent information layout. China Sci. Technol. Inf. (07), 22–24.
76
Nanthakumar C. B. Hatley R. J. Lemma S. Gauldie J. Marshall R. P. Macdonald S. J. (2015). Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug Discov.14 (10), 693–720. 10.1038/nrd4592
77
Nastase M. V. Zeng-Brouwers J. Wygrecka M. Schaefer L. (2018). Targeting renal fibrosis: mechanisms and drug delivery systems. Adv. Drug Deliv. Rev.129, 295–307. 10.1016/j.addr.2017.12.019
78
Newton A. C. (2003). Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J.370 (Pt 2), 361–371. 10.1042/bj20021626
79
Olson A. L. Swigris J. J. Lezotte D. C. Norris J. M. Wilson C. G. Brown K. K. (2007). Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med.176 (3), 277–284. 10.1164/rccm.200701-044OC
80
Öner-İyidoğan Y. Tanrıkulu-Küçük S. Seyithanoğlu M. Koçak H. Doğru-Abbasoğlu S. Aydin A. F. et al (2014). Effect of curcumin on hepatic heme oxygenase 1 expression in high fat diet fed rats: is there a triangular relationship?Can. J. Physiol. Pharmacol.92 (10), 805–812. 10.1139/cjpp-2014-0174
81
Pan M. H. Huang T. M. Lin J. K. (1999). Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos.27 (4), 486–494. 10.1016/s0090-9556(24)15211-7
82
Panizo S. Martínez-Arias L. Alonso-Montes C. Cannata P. Martín-Carro B. Fernández-Martín J. L. et al (2021). Fibrosis in chronic kidney disease: pathogenesis and consequences. Int. J. Mol. Sci.22 (1), 408. 10.3390/ijms22010408
83
Pardo A. Cabrera S. Maldonado M. Selman M. (2016). Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res.17, 23. 10.1186/s12931-016-0343-6
84
Pottier N. Cauffiez C. Perrais M. Barbry P. Mari B. (2014). FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol. Sci.35 (3), 119–126. 10.1016/j.tips.2014.01.003
85
Prasad S. Gupta S. C. Tyagi A. K. Aggarwal B. B. (2014). Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol. Adv.32 (6), 1053–1064. 10.1016/j.biotechadv.2014.04.004
86
Priyadarsini K. I. (2014). The chemistry of curcumin: from extraction to therapeutic agent. Molecules19 (12), 20091–20112. 10.3390/molecules191220091
87
Punithavathi D. Venkatesan N. Babu M. (2003). Protective effects of curcumin against amiodarone-induced pulmonary fibrosis in rats. Br. J. Pharmacol.139 (7), 1342–1350. 10.1038/sj.bjp.0705362
88
Qian Y. Zhong P. Liang D. Xu Z. Skibba M. Zeng C. et al (2015). A newly designed curcumin analog Y20 mitigates cardiac injury via anti-inflammatory and anti-oxidant actions in obese rats. PLoS One10 (3), e0120215. 10.1371/journal.pone.0120215
89
Qin Y. W. Fei C. H. Zhang W. Li Y. Xu Z. Su L. L. et al (2022). Efficacy-related substances of blood-activating and stasis-resolving medicinals derived from curcuma plants: a review. Zhongguo Zhong Yao Za Zhi47 (1), 24–35. 10.19540/j.cnki.cjcmm.20210817.603
90
Qiu Y. Y. Zhang J. Zeng F. Y. Zhu Y. Z. (2023). Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol. Res.192, 106786. 10.1016/j.phrs.2023.106786
91
Radwan A. M. Fatoh S. A. Massoud A. Tousson E. (2024). Effectiveness of curcumin nanoparticles in rat liver fibrosis caused by thioacetamide. Environ. Toxicol.39 (1), 388–397. 10.1002/tox.23984
92
Ramachandran P. Henderson N. C. (2016). Antifibrotics in chronic liver disease: tractable targets and translational challenges. Lancet Gastroenterol. Hepatol.1 (4), 328–340. 10.1016/s2468-1253(16)30110-8
93
Ravindranath V. Chandrasekhara N. (1980). Absorption and tissue distribution of curcumin in rats. Toxicology16 (3), 259–265. 10.1016/0300-483x(80)90122-5
94
Ravindranath V. Chandrasekhara N. (1981a). In vitro studies on the intestinal absorption of curcumin in rats. Toxicology20 (2-3), 251–257. 10.1016/0300-483x(81)90056-1
95
Ravindranath V. Chandrasekhara N. (1981b). Metabolism of curcumin--studies with [3H]curcumin. Toxicology22 (4), 337–344. 10.1016/0300-483x(81)90027-5
96
Redente E. F. Jacobsen K. M. Solomon J. J. Lara A. R. Faubel S. Keith R. C. et al (2011). Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol.301 (4), L510–L518. 10.1152/ajplung.00122.2011
97
Reyes-Gordillo K. Segovia J. Shibayama M. Tsutsumi V. Vergara P. Moreno M. G. et al (2008). Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam. Clin. Pharmacol.22 (4), 417–427. 10.1111/j.1472-8206.2008.00611.x
98
Saadati S. Sadeghi A. Mansour A. Yari Z. Poustchi H. Hedayati M. et al (2019). Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterol.19 (1), 133. 10.1186/s12876-019-1055-4
99
Sadoughi F. Hallajzadeh J. Mirsafaei L. Asemi Z. Zahedi M. Mansournia M. A. et al (2021). Cardiac fibrosis and curcumin: a novel perspective on this natural medicine. Mol. Biol. Rep.48 (11), 7597–7608. 10.1007/s11033-021-06768-1
100
Sánchez-Valle V. Chávez-Tapia N. C. Uribe M. Méndez-Sánchez N. (2012). Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem.19 (28), 4850–4860. 10.2174/092986712803341520
101
Senavirathna L. K. Huang C. Yang X. Munteanu M. C. Sathiaseelan R. Xu D. et al (2018). Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Sci. Rep.8 (1), 2709. 10.1038/s41598-018-21073-x
102
Shakeri A. Panahi Y. Johnston T. P. Sahebkar A. (2019). Biological properties of metal complexes of curcumin. Biofactors45 (3), 304–317. 10.1002/biof.1504
103
Shan L. Liu Z. Ci L. Shuai C. Lv X. Li J. (2019). Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol.75, 105765. 10.1016/j.intimp.2019.105765
104
Sharma R. A. McLelland H. R. Hill K. A. Ireson C. R. Euden S. A. Manson M. M. et al (2001). Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin. Cancer Res.7 (7), 1894–1900.
105
Sharma N. Shaikh T. B. Eedara A. Kuncha M. Sistla R. Andugulapati S. B. (2022). Dehydrozingerone ameliorates thioacetamide-induced liver fibrosis via inhibition of hepatic stellate cells activation through modulation of the MAPK pathway. Eur. J. Pharmacol.937, 175366. 10.1016/j.ejphar.2022.175366
106
She L. Xu D. Wang Z. Zhang Y. Wei Q. Aa J. et al (2018). Curcumin inhibits hepatic stellate cell activation via suppression of succinate-associated HIF-1α induction. Mol. Cell Endocrinol.476, 129–138. 10.1016/j.mce.2018.05.002
107
Sheetz M. J. King G. L. (2002). Molecular understanding of hyperglycemia's adverse effects for diabetic complications. Jama288 (20), 2579–2588. 10.1001/jama.288.20.2579
108
Singh S. K. Szulik M. W. Ganguly M. Khutsishvili I. Stone M. P. Marky L. A. et al (2011). Characterization of DNA with an 8-oxoguanine modification. Nucleic Acids Res.39 (15), 6789–6801. 10.1093/nar/gkr275
109
Soetikno V. Sari F. R. Sukumaran V. Lakshmanan A. P. Mito S. Harima M. et al (2012). Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC-MAPK signaling pathway. Eur. J. Pharm. Sci.47 (3), 604–614. 10.1016/j.ejps.2012.04.018
110
Soetikno V. Sari F. R. Lakshmanan A. P. Arumugam S. Harima M. Suzuki K. et al (2013). Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol. Nutr. Food Res.57 (9), 1649–1659. 10.1002/mnfr.201200540
111
Sovari A. A. (2016). Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol. Res. Pract.2016, 9656078. 10.1155/2016/9656078
112
Sutti S. Bruzzì S. Heymann F. Liepelt A. Krenkel O. Toscani A. et al (2019). CX(3)CR1 mediates the development of monocyte-derived dendritic cells during hepatic inflammation. Cells8 (9), 1099. 10.3390/cells8091099
113
Tang Y. Chen A. (2014). Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab. Invest94 (5), 503–516. 10.1038/labinvest.2014.42
114
Tang Y. Zheng S. Chen A. (2009). Curcumin eliminates leptin's effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology150 (7), 3011–3020. 10.1210/en.2008-1601
115
Taslidere E. Esrefoglu M. Elbe H. Cetin A. Ates B. (2014). Protective effects of melatonin and quercetin on experimental lung injury induced by carbon tetrachloride in rats. Exp. Lung Res.40 (2), 59–65. 10.3109/01902148.2013.866181
116
Tomeh M. A. Hadianamrei R. Zhao X. (2019). A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci.20 (5), 1033. 10.3390/ijms20051033
117
Trujillo J. Molina-Jijón E. Medina-Campos O. N. Rodríguez-Muñoz R. Reyes J. L. Loredo M. L. et al (2016). Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: relation to oxidative stress. Food Funct.7 (1), 279–293. 10.1039/c5fo00624d
118
Tyagi N. Dash D. Singh R. (2016). Curcumin inhibits paraquat induced lung inflammation and fibrosis by extracellular matrix modifications in mouse model. Inflammopharmacology24 (6), 335–345. 10.1007/s10787-016-0286-z
119
Vairetti M. Di Pasqua L. G. Cagna M. Richelmi P. Ferrigno A. Berardo C. (2021). Changes in glutathione content in liver diseases: an update. Antioxidants (Basel)10 (3), 364. 10.3390/antiox10030364
120
Vizzutti F. Provenzano A. Galastri S. Milani S. Delogu W. Novo E. et al (2010). Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab. Invest90 (1), 104–115. 10.1038/labinvest.2009.112
121
Wahlström B. Blennow G. (1978). A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh)43 (2), 86–92. 10.1111/j.1600-0773.1978.tb02240.x
122
Wang Y. J. Pan M. H. Cheng A. L. Lin L. I. Ho Y. S. Hsieh C. Y. et al (1997). Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal.15 (12), 1867–1876. 10.1016/s0731-7085(96)02024-9
123
Weaver T. M. Hoitsma N. M. Spencer J. J. Gakhar L. Schnicker N. J. Freudenthal B. D. (2022). Structural basis for APE1 processing DNA damage in the nucleosome. Nat. Commun.13 (1), 5390. 10.1038/s41467-022-33057-7
124
Weir M. A. Walsh M. Cuerden M. S. Sontrop J. M. Chambers L. C. Garg A. X. (2018). Micro-particle curcumin for the treatment of chronic kidney Disease-1: study protocol for a multicenter clinical trial. Can. J. Kidney Health Dis.5, 2054358118813088. 10.1177/2054358118813088
125
Wu S. J. Lin Y. H. Chu C. C. Tsai Y. H. Chao J. C. (2008). Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J. Med. Food11 (2), 224–229. 10.1089/jmf.2007.555
126
Wu H. Kong L. Tan Y. Epstein P. N. Zeng J. Gu J. et al (2016). C66 ameliorates diabetic nephropathy in mice by both upregulating NRF2 function via increase in miR-200a and inhibiting miR-21. Diabetologia59 (7), 1558–1568. 10.1007/s00125-016-3958-8
127
Wu Yuhong H. M. Liu C. (2020). Clinical Application of Gegen Jianghuang Powder to Treat Cervical Spondylosis on the Basis of Prescription and Syndrome Corresponding: study on Academic Thoughts and Clinical Experience of National TCM Master XIONG Jibo. J. Hunan Univ. Chin. Med.40 (08), 918–921.
128
Xiang L. Zhang Q. Chi C. Wu G. Lin Z. Li J. et al (2020). Curcumin analog A13 alleviates oxidative stress by activating Nrf2/ARE pathway and ameliorates fibrosis in the myocardium of high-fat-diet and streptozotocin-induced diabetic rats. Diabetol. Metab. Syndr.12, 1. 10.1186/s13098-019-0485-z
129
Xiao S. Deng Y. Shen N. Sun Y. Tang H. Hu P. et al (2021). Curc-mPEG454, a PEGylated curcumin derivative, as a multi-target anti-fibrotic prodrug. Int. Immunopharmacol.101 (Pt A), 108166. 10.1016/j.intimp.2021.108166
130
Yachie A. (2021). Heme Oxygenase-1 deficiency and oxidative stress: a review of 9 independent human cases and animal models. Int. J. Mol. Sci.22 (4), 1514. 10.3390/ijms22041514
131
Yaikwawong M. Jansarikit L. Jirawatnotai S. Chuengsamarn S. (2025). Curcumin for inflammation control in individuals with type 2 diabetes mellitus and metabolic dysfunction-associated steatotic liver disease: a randomized controlled trial. Nutrients17 (12), 1972. 10.3390/nu17121972
132
Yang J. B. Zhu D. Q. Shao M. Li A. W. Liu Z. R. Gao R. J. et al (2019). Effects of shengmai jianghuang san on intestinal flora in nude mice with radio resistant cells of nasopharyngeal carcinoma. Zhongguo Zhong Yao Za Zhi44 (3), 553–558. 10.19540/j.cnki.cjcmm.20181203.001
133
Yoon Y. S. Lee J. H. Hwang S. C. Choi K. S. Yoon G. (2005). TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene24 (11), 1895–1903. 10.1038/sj.onc.1208262
134
Zeng C. Zhong P. Zhao Y. Kanchana K. Zhang Y. Khan Z. A. et al (2015). Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J. Mol. Cell Cardiol.79, 1–12. 10.1016/j.yjmcc.2014.10.002
135
Zhai X. Qiao H. Guan W. Li Z. Cheng Y. Jia X. et al (2015). Curcumin regulates peroxisome proliferator-activated receptor-γ coactivator-1α expression by AMPK pathway in hepatic stellate cells in vitro. Eur. J. Pharmacol.746, 56–62. 10.1016/j.ejphar.2014.10.055
136
Zhang X. Liang D. Guo L. Liang W. Jiang Y. Li H. et al (2015). Curcumin protects renal tubular epithelial cells from high glucose-induced epithelial-to-mesenchymal transition through Nrf2-mediated upregulation of heme oxygenase-1. Mol. Med. Rep.12 (1), 1347–1355. 10.3892/mmr.2015.3556
137
Zheng S. Chen A. (2006). Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am. J. Physiol. Gastrointest. Liver Physiol.290 (5), G883–G893. 10.1152/ajpgi.00450.2005
138
Zheng S. Yumei F. Chen A. (2007). De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic. Biol. Med.43 (3), 444–453. 10.1016/j.freeradbiomed.2007.04.016
139
Zheng D. Huang C. Huang H. Zhao Y. Khan M. R. U. Zhao H. et al (2020). Antibacterial mechanism of curcumin: a review. Chem. Biodivers.17 (8), e2000171. 10.1002/cbdv.202000171
140
Zhengtao J. F. C. Zhicong Z. X. Z. Meiling S. Y. T. Baoyi H. (2022). Target Prediction and Mechanism Investigation of Gegen Jianghuang Powder for Treating Cerebral Infarction. Traditional Chin. Drug Res. Clin. Pharmacol.33 (05), 633–644. 10.19378/j.issn.1003-9783.2022.05.010
Summary
Keywords
reactive oxygen species, reactive nitrogen species, curcuma, anti-fibrosis effects, traditional chinese medicine, signaling pathways
Citation
Yu S, Pu J, Liu K, He C, Yang F, Chen K, Yang X, Zhu Y, Jiang J, Luo M, Liu X, Zhang C and He Y (2025) Curcumin mediates oxidative stress to play an anti-fibrotic role, focusing on liver, renal, myocardial and pulmonary fibrosis. Front. Pharmacol. 16:1636538. doi: 10.3389/fphar.2025.1636538
Received
28 May 2025
Revised
29 October 2025
Accepted
30 October 2025
Published
21 November 2025
Volume
16 - 2025
Edited by
Javier Echeverria, University of Santiago, Chile
Reviewed by
Sara Taha Elazab, Mansoura University, Egypt
Glaucia Maria Pastore, State University of Campinas, Brazil
Updates
Copyright
© 2025 Yu, Pu, Liu, He, Yang, Chen, Yang, Zhu, Jiang, Luo, Liu, Zhang and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujiao He, heyujiao@cdu.edu.cn; Chuantao Zhang, zhangchuantao@cdutcm.edu.cn; Xiao Liu, lxzl111@yeah.net
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.