- 1Southern Medical Branch of PLA General Hospital, Beijing, China
- 2Department of Pharmacy, Guilin Medical University, Guilin, Guangxi, China
- 3School of Basic Medical Sciences, Yichun University, Yichun, Jiangxi, China
The dried tuber of Gastrodia elata (GE), a perennial orchid with a 2,200-year medicinal history documented in the Shennong Bencaojing (200 BCE), remains a cornerstone of traditional Chinese medicine (TCM) and contemporary integrative therapies across Asia. Initially prescribed for neurological disorders (e.g., epilepsy, stroke prophylaxis) and hypertension, modern research has expanded its therapeutic portfolio to include anti-aging, antitumor, and osteoprotective applications. This systematic review synthesizes 1) traditional ethnopharmacological uses, 2) phytochemical profiling of 100+ identified bioactive metabolites (e.g., gastrodin, parishins), and 3) mechanistic insights into their pharmacokinetic behaviors and pharmacodynamic actions. Notably, botanical drug interactions in TCM formulations enhance gastrodin’s blood-brain barrier penetration, elucidating clinical efficacy. While in vitro/vivo studies validate GE’s antioxidant and neuroprotective effects, translational challenges persist: 1) Limited clinical trials on novel indications (e.g., osteoporosis); 2) Unclear structure-activity relationships of minor metabolites; 3) Standardization needs for industrial applications. This work provides an evidence base to guide future research on GE’s diversified therapeutic development.
1 Introduction
As early as several thousand years ago, the Chinese people recognized that certain natural plants had better therapeutic effects in the treatment of diseases, and had many advantages such as abundant resources and ease of cultivation (Martins and S, 2018; Zhao et al., 2012). In ancient China, based on the experience of using medicinal botanical drugs, natural plants with medicinal properties were compiled into books such as “Shennong’s Classic of Materia Medica,” “Newly Revised Materia Medica,” and “Compendium of Materia Medica.” Various plants were classified according to experience and used for targeted treatment of various diseases (Hu et al., 2019; Tang et al., 2017; Tian et al., 2022). Taking Tianma (Gastrodia elata) as an example, the earliest recorded pharmacological work on Tianma is in “Shennong’s Classic of Materia Medica.” Over 2000 years ago, Tianma was already used for the treatment of cardiovascular, neurological, and nervous system diseases. In addition, a large amount of basic research and clinical studies have shown that Tianma can improve hemodynamics, increase blood flow, enhance vascular elasticity, and have a certain protective effect on the cardiovascular and cerebrovascular systems (Zuowei, 2017).
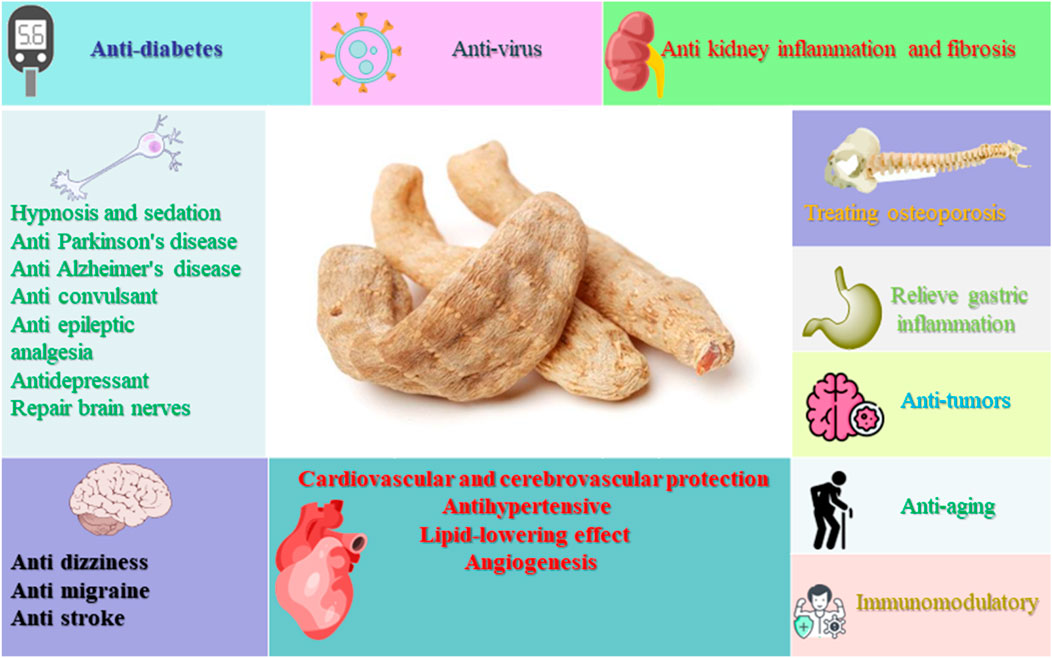
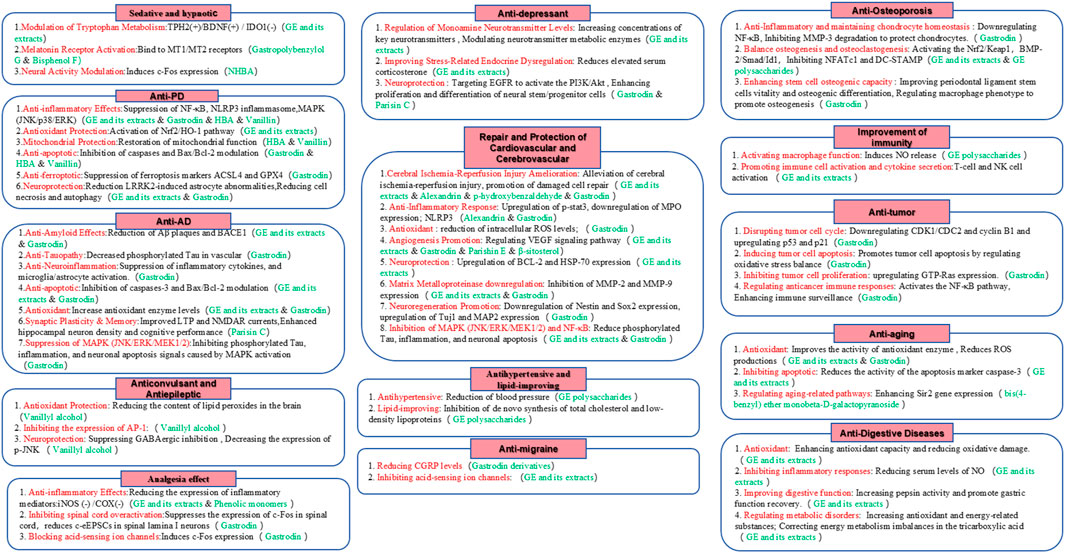
Gastrodia elata is commonly called Tian ma, Red arrow, Dingfeng grass, etc., in China. Gastrodia elata has long been widely studied as a traditional botanical medicine in Asia, especially in China, Korean peninsula, Japan and Russia (Zhan et al., 2016). Gastrodia elata has a wide range of medicinal value and remains popular in Asia. In traditional Chinese Medicine, Rhizoma Gastrodiae is mainly used for hypertension, headache, stroke, limb numbness, hemiplegia, arthritis and other symptoms (Liu et al., 2018; Wang P. H. et al., 2016; Lee et al., 2012). However, with further research, researchers have found that gastrodia also has extensive pharmacological activity such as anti-tumour, anti-aging, improved memory, anti-depression, anti-insomnia and so on (Liu et al., 2018; Farooq et al., 2019; Chen and Sheen, 2011; Liang et al., 2017). The pharmacological activity of gastrodia are shown in Figure 1.
Since the beginning of this century, pharmacologically activemetabolites have been found one after another from Rhizoma Gastrodiae. Clinical studies on gastrodin have been carried out because of its definite therapeutic effect and high safety (Kong et al., 2022; Guo et al., 2015). Although gastrodia has been studied for thousands of years, its active metabolites and pharmacological effects are still waiting for being explored.
In this review, We conducted searches across multiple databases and literature sources, including PubMed, CNKI (China National Knowledge Infrastructure), Chinese Pharmacopoeia, and China Medical Information Platform. The search terms encompassed Gastrodia elata, Rhizoma Gastrodiae, Gastrodin, Neuroprotection, Anti-inflammatory, Immunomodulation, and related keywords and summarized the progress of research on the botanical, phytochemical and pharmacological aspects of Gastrodia elata, in the hope of providing supporting information for the in-depth development and utilisation of Gastrodia elata.
2 Botanical characteristics of Gastrodia elata
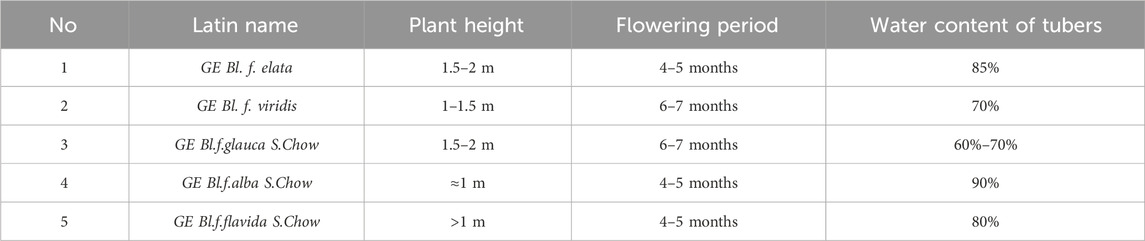
Rhizoma Gastrodiae is the dried tuber of Gastrodia elata Blume of the orchid family, which is a perennial parasitic botanical drug. Currently, gastrodin is recognized as one of the most crucial active monomers in Gastrodia elata Blume. Gastrodia elata cannot carry out photosynthesis because it has no chlorophyll. The seeds need to co-exist with mosmundicola belonging to tricholomataceae for providing nutrition during germination, and after growing to the protocorm, it needs to be associated with honey mushroom of the tricholomidae family to provide nutrition (Chen L. et al., 2019). They grow in the thick humus under the trees at an altitude of 400–3200 m, and are widely distributed in the tropical, subtropical, temperate and cold temperate mountains. Currently, there are mainly five kinds of medicinal Gastrodia elata (Zhan et al., 2016; Zhengyi, 1999) (Table 1). GE Bl. form. glauca S. Chow and GE f. elata have been widely cultivated due to their high adaptability as well as high output.
According to The Botanical Records of China, Gastrodia elata often grows to a height of 1–2 m. The medicinal part of the rhizome is ellipsoid to nearly dumbbell shaped, with the length of 8–12 cm, 3–7 cm in diameter. Usually, the single fruit weighs 0.5–1 kg with denser nodes, which are covered with many triangular broadly ovate sheaths (Zhengyi, 1999).
3 Phytochemical overview
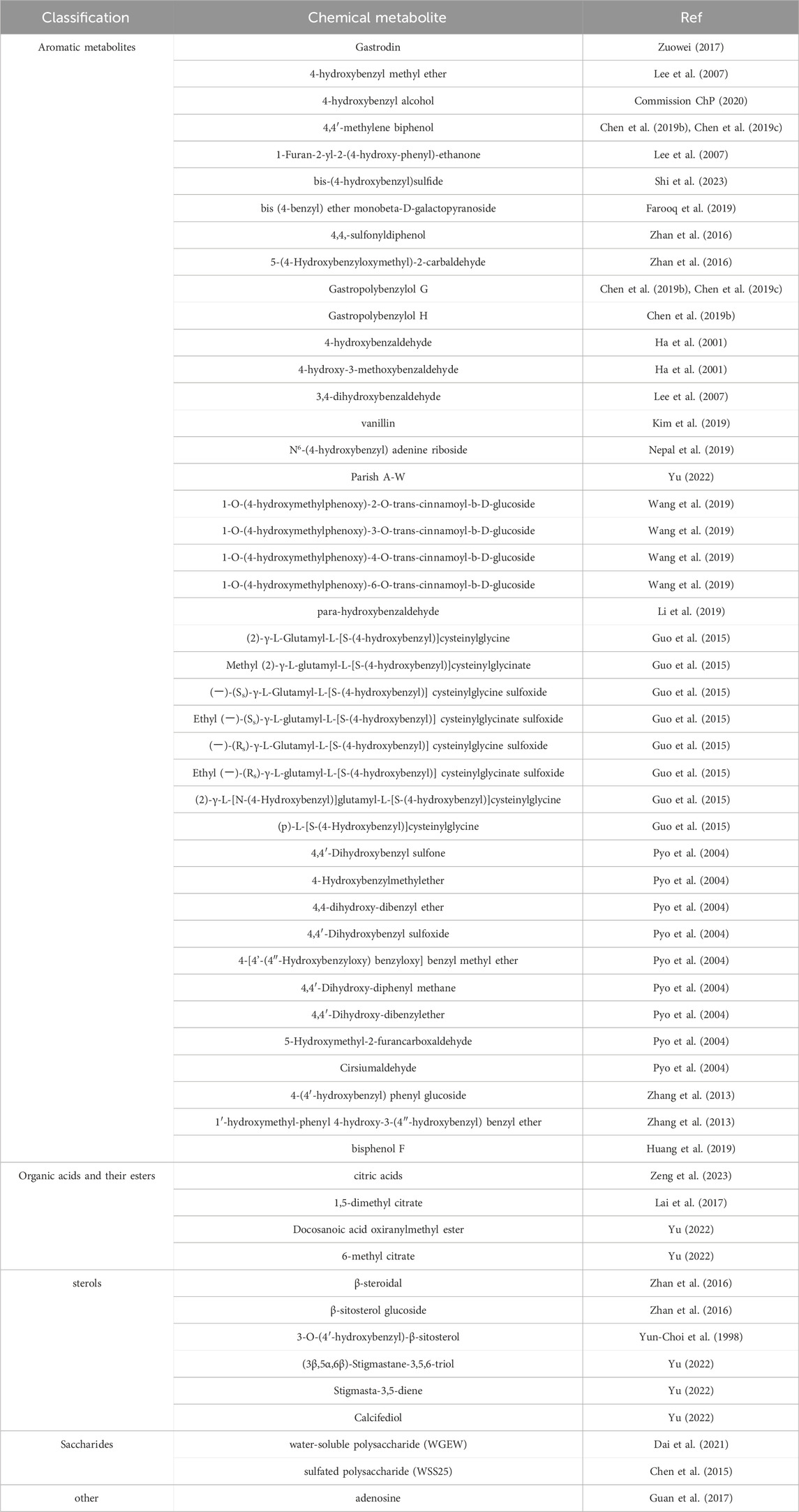
Since the 1950s, the metabolites contained in Rhizoma Gastrodiae have been widely studied. Previous reviews have also reported metabolites from Rhizoma Gastrodiae (Zhan et al., 2016; Guo et al., 2015). Other researchers classified the known chemical composition of Rhizoma Gastrodiae, mainly divided into aromatic metabolites, steroids, organic acids and esters, sugars and their glycosides, and other classes, based on the molecular structure of the parent nucleus of themetabolite (Yu, 2022). Selected bioactive metabolites from Rhizoma Gastrodiae are summarized in Table 2.
3.1 Aromatic metabolites
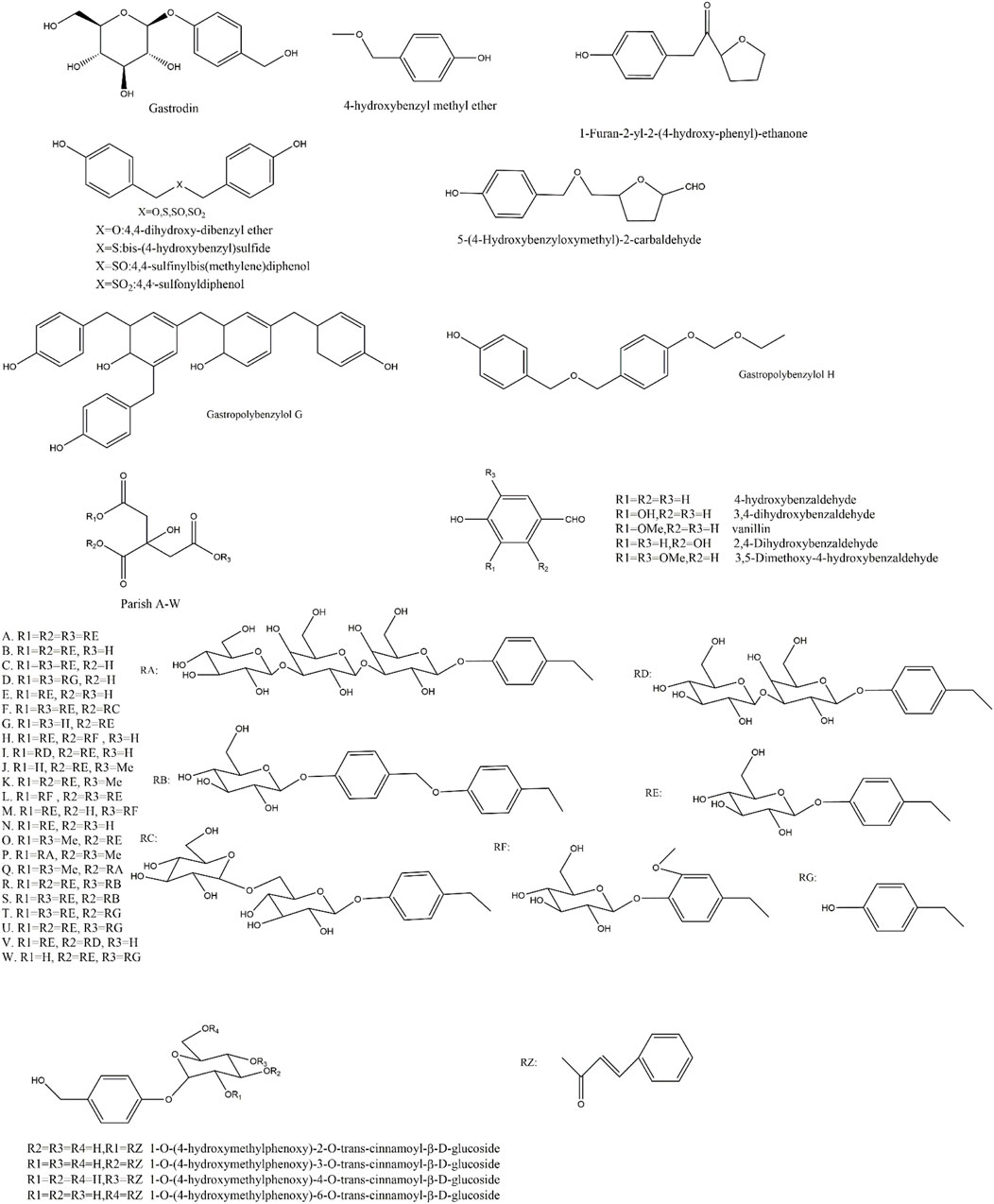
The Aromatic metabolites in Rhizoma Gastrodiae are characterized by at least one benzene ring structure with a delocalized bond. 143 metabolites have been identified to date and there are still a lot to be discovered. Some researchers predicted that there are at least 189 possible parishin-like metabolites in gastrodia. Some researchers have predicted that at least 189 possible parishin-like metabolites are contained in gastrodia by offline two-dimensional liquid-liquid mass spectrometry and graphical similarity comparisons (Yu, 2022; Zhu et al., 2021). Some of the aromatic metabolites of Rhizoma Gastrodiae are shown in Figure 2.
Aromatic metabolites in Rhizoma Gastrodiae play a major role in disease treatment, especially the small molecule monobenzyl analogues. Gastrodin is the most important aromatic molecule in Rhizoma Gastrodiae because of its remarkable pesticide effect. This series of metabolites and their derivatives have a wide range of pharmacological effects either. Among them, Gastrodin and 4-hydroxybenzyl alcohol have stronger ability in eliminating ROS, avoiding oxidative damage to cells and improving the lifespan of the cells (Wang P. H. et al., 2016; He et al., 2021; Zhang et al., 2018; Lai, 2022). In addition, 4-hydroxybenzaldehyde and 4-hydroxy-3-methoxybenzaldehyde in Rhizoma Gastrodiae can effectively inhibit the activity of GABA transaminase, and aldehyde and hydroxyl groups are necessary groups for inhibiting GABA transaminase activity (Ha et al., 2001); The parishin analogues, including parishin A-W, cannot cross the blood-brain barrier to exert central effects due to their high molecular weight, but the hydrolysate p-hydroxybenzyl alcohol is capable of exerting central neuroprotective effects (Lai, 2022). Polybenzyl ethers are metabolites consisting of more than 2 benzyl units linked by oxygen atoms. Gastropolybenzylol H can activate MT1 and MT2 receptors in HEK293 cells, in which MT1 receptor is closely related to the function of the cardiovascular system. It can relax coronary arteries by agonising coronary β2 receptor, which have a certain protective effect on the cardiovascular system. On the other hand, activation of MT1 can alleviate brain inflammation and oxidative damage (Hardeland, 2021; Chen et al., 2019b). In addition, 4,4-dihydroxy-dibenzyl ether in polybenzyl ether showed some anti-platelet aggregation effects (Pyo et al., 2004).
Gastropolybenzylol G, 4,4′-methylene biphenol, as one of the polybenzyl metabolites, is able to activate MT1 and MT2 receptors in HEK293 cells, and the two p-hydroxy groups in methylene biphenols, are the key pharmacophore for the activation of MT1 and MT2 receptors (Chen et al., 2019b; Chen et al., 2019c). Moreover, MT1 is involved in the regulation of circadian rhythms, and activating MT1 can improve sleep. After MT2 receptor being activated, the expression of neurotrophic factor mRNA in the hippocampus region increased, the number of mitochondria increased, and nerve growth was promoted, which lead to improving cognition and anti-depression. Some of the metabolites have neuroprotective, anti-inflammatory and antioxidant effects (Zhang et al., 2013; Huang et al., 2019). Heteroatom aromatic metabolites formed by linking heteroatoms to each other had activities of anti-inflammatory, apoptosis-inhibiting, and neuroleptic-reducing. The same could be found when the benzyl unit was replaced by the alcohol hydroxyl group (Guo et al., 2015; Wang et al., 2019). In addition, It showed significant inhibition on topoisomerase I and II when the aromatic benzene ring connected to furan ring through the carbon chain or the oxygen atom, but indicated no obvious damage to HT-29, MCF-7, HEPPG 2 cells (Lee et al., 2007).
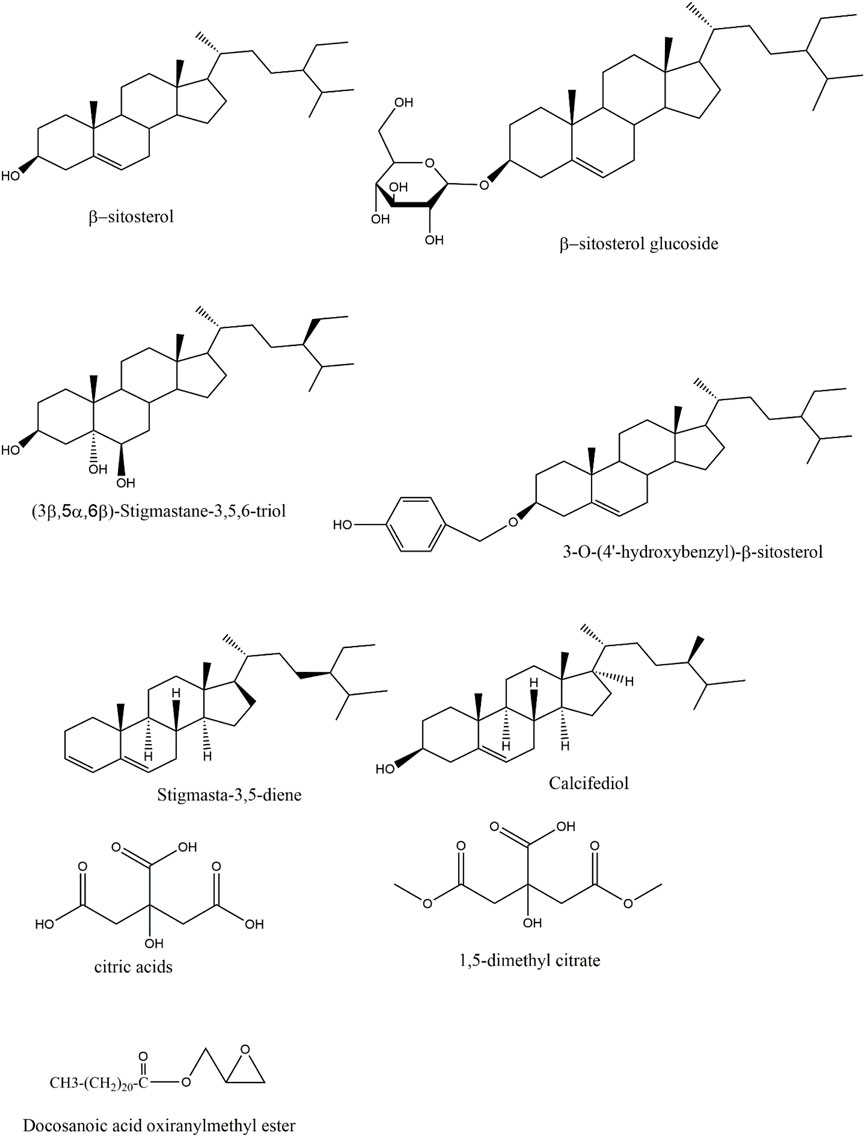
The steroidal metabolites in Rhizoma Gastrodiae are featuring a perhydrocyclopentanophenanthrene skeleton with two angular methyl groups and a C-17 side chain. Few studies have been conducted on the steroid in Rhizoma Gastrodiae. Steroid metabolites in Rhizoma Gastrodiae that have been found include 3-O-(4′-hydroxybenzyl)-beta-sitosterol, β-sitosterol, β-sitosterol glucoside, 3β,5α,6β-Trihydroxystigmastane, stigmasta-3,5-diene, calcifediol and so on (Zhan et al., 2016; Wu et al., 2023). The related structures are shown in Figure 3. Among them, β-sitosterol has been proved to having a wide range of biological activities, such as anti-anxiety, sedation, analgesia, immune regulation, antibacterial, anti-cancer, anti-inflammatory, lipid-lowering, liver protection, heart protection, anti-oxidant, anti-diabetic activity (Babu et al., 2020; Khan et al., 2022). Most of the other steroids metabolites in Rhizoma Gastrodiae were also able to penetrate though the blood-brain barrier and demonstrated certain central and peripheral anti-inflammatory effects. Based on this evidence, it can be inferred that steroidal metabolites may also be a vital type of metabolites in Rhizoma Gastrodiae, which is responsible for the medicinal efficacy.
3.2 Organic acids and their esters of Rhizoma Gastrodiae
Themetabolites such as Docosanoic acid oxiranylmethyl ester, 6-methyl citrate, 1,5-dimethyl citrate, and citric acids have been isolated from GE (Zhan et al., 2016; Lai et al., 2017), and the related structures are shown in Figure 3. A study had confirmed that citric acid being given to mice ig could reduce brain inflammation and oxidative damage, while also exhibited certain protective effects on the liver (Abdel-Salam et al., 2014).
3.3 Saccharides and glycosides
Rhizoma Gastrodiae polysaccharides often has side chains formed by benzyl, which is one of the most important active metabolites. It has pharmacological effects such as regulating immunity, anti-cardiovascular and cerebrovascular diseases, anti-tumor, regulating intestinal flora, and improving osteoporosis (Chen et al., 2016; Zhao et al., 2022; Kim et al., 2012; Chen et al., 2012; Chen et al., 2015). Among them, the sulfated derivatives of water-soluble polysaccharide extracted from gastrodia polysaccharides (WGEW) are essential groups for inhibiting angiogenesis. It has been verified that the optimal degree of sulfation is between 0.173 and 0.194 (Chen et al., 2012). It was found that Rhizoma Gastrodiae polysaccharides with spherical conformations and dense structures are more effective in inducing late apoptosis of MCF-7 cells, thereby exerting anti-tumor effects after the analysis via using asymmetric flow field-flow fractionation (AF4), multi-angle light scattering (MALS), and differential refractive index (dRI) detectors in combination (AF4-MALS-dRI) (Dai et al., 2021).
Other categories Here, primarily composed of amino acids, nucleotides, and other metabolites that do not fit into the aforementioned categories. Rhizoma Gastrodiae contains a diverse array of amino acids and nucleotides. Pyroglutamic acid exhibits immunomodulatory effects, while adenosine demonstrates certain antiviral properties (Zhan et al., 2016).
In addition to the aforementioned five types of metabolites, Rhizoma Gastrodiae also contains trace elements such as La (Lanthanum), Sr (Strontium), and Zn (Zinc), which can serve as nutritional supplements for deficiencies associated with cardiovascular and cerebrovascular diseases (Xu and Xu, 2009).
4 Pharmacological effects of Rhizoma Gastrodiae
The bioactive metabolites of Rhizoma Gastrodiae are complex and include aromatic metabolites, carbohydrates and glycosides, volatile oils, proteins, organic acids and their esters, amino acids, vitamins, and trace elements. However, the most extensively studied metabolites of Rhizoma Gastrodiae are aromatic metabolites and plant polysaccharides (Heese, 2020; Zhu et al., 2019). These metabolites can enhance and protect the functions of the central nervous system, cardiovascular system, skeletal system, digestive system, endocrine system, urinary system, and respiratory system. Additionally, they exhibit a wide range of pharmacological effects, including boosting immunity, anti-tumor activities, antimicrobial properties, and delaying aging (Wu et al., 2023; Sun et al., 2023; Schloss et al., 2021). Detailed information is shown in Figure 4.
4.1 The pharmacological effects of Rhizoma Gastrodiae
The pharmacological effects of Rhizoma Gastrodiae on the nervous system include sedative and hypnotic effects, anti-Parkinson’s effects, anti-Alzheimer’s effects, antipsychotic effects, anticonvulsant effects, anti-vertigo effects, anti-epileptic effects, anti-stroke effects, and analgesic effects (Liu et al., 2018; Chen and Sheen, 2011; Schloss et al., 2021).
4.1.1 Sedative and hypnotic effects
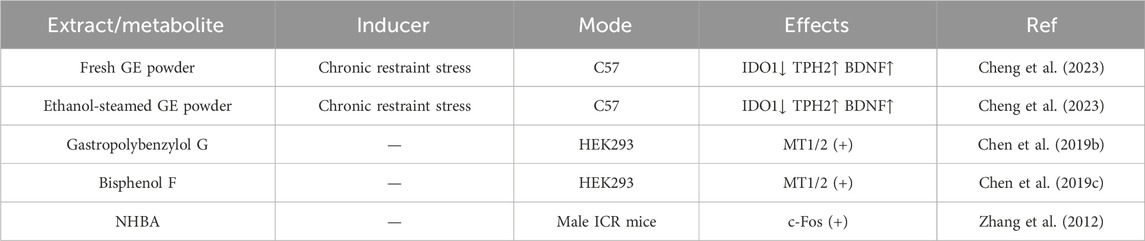
With increasing stress, insomnia has become a common condition in modern society. Its main triggers include disruptions in the biological clock caused by irregular daily routines, imbalances in sleep-related neurotransmitters, and dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis (de Feijter et al., 2022). Research has shown that Gastrodin, p-hydroxybenzyl alcohol, and Parishin A can promote sleep by upregulating the sleep-related neurotransmitters 5-HT and GABA, while inhibiting the wake-promoting neurotransmitter DA (Zhou et al., 2024). Oral administration of fresh Rhizoma Gastrodiae powder and ethanol-steamed GE powder to C57 mice can reduce IDO 1, increase TPH 2 and BDNF levels, and elevate tryptophan levels, thereby enhancing the production of 5-HT and melatonin. This, in turn, improves the abundance of sleep-related neurotransmitters, promoting sedative, calming, and hypnotic effects in mice (Cheng et al., 2023). One of the active metabolites in GE, Gastropolybenzylol G, can activate melatonin receptors MT1 and MT2 in HEK293 cells, thereby further promoting sleep (Chen et al., 2019b; Chen et al., 2019c). Rhizoma Gastrodiae extract N6-(4-hydroxybenzyl) adenine riboside (NHBA) increases the expression of the proto-oncogene protein c-Fos in the ventrolateral preoptic area GABAergic neurons. Studies have confirmed that the expression of c-Fos significantly increases during rapid eye movement (REM) sleep, suggesting that NHBA activates the sleep center in the anterior hypothalamus to promote sleep. Some studies have also shown that gastrodin and p-hydroxybenzyl alcohol can improve sleep by affecting the HPA axis (Zhang et al., 2012). Detailed information is shown in Table 3.
4.1.2 Anti-Parkinson effect
Parkinson’s disease is a neurodegenerative disorder characterized by the death of dopaminergic neurons, leading to dopamine deficiency in the brain. The specific pathogenesis remains unclear, but its main features include mitochondrial dysfunction and increased reactive oxygen species (ROS), abnormal folding and aggregation of α-synuclein in synapses, elevated levels of pro-inflammatory factors in the brain microenvironment, ferroptosis, and the presence of pathogenic genes associated with Parkinson’s disease (Lu et al., 2022).
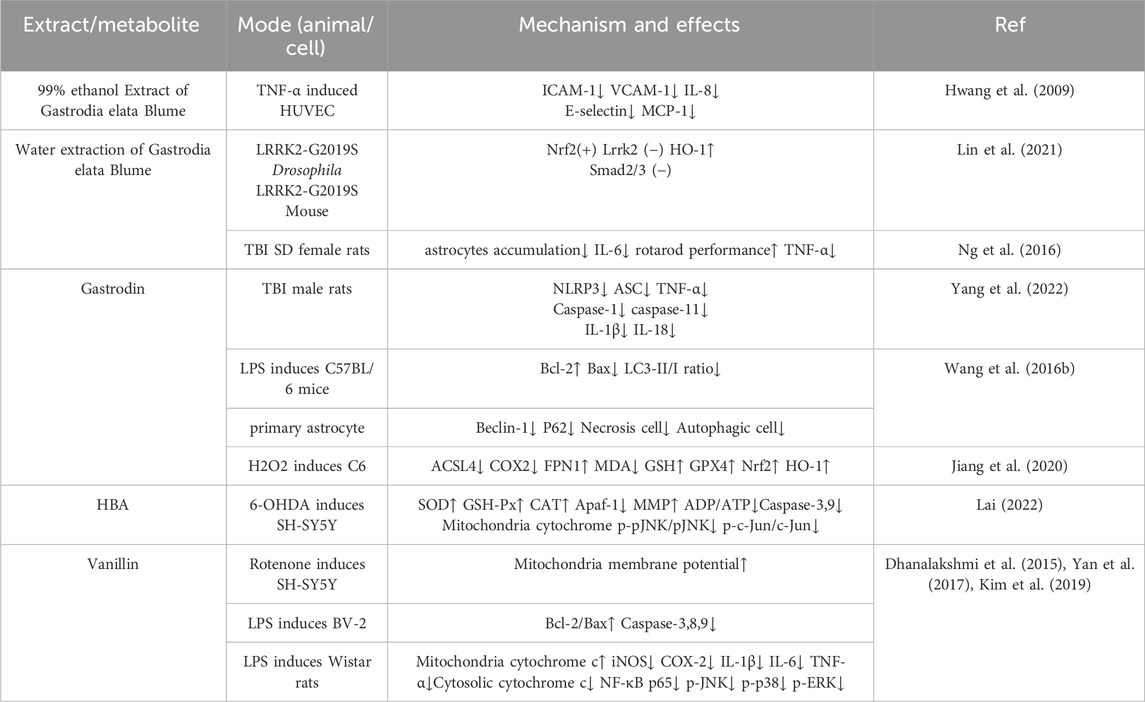
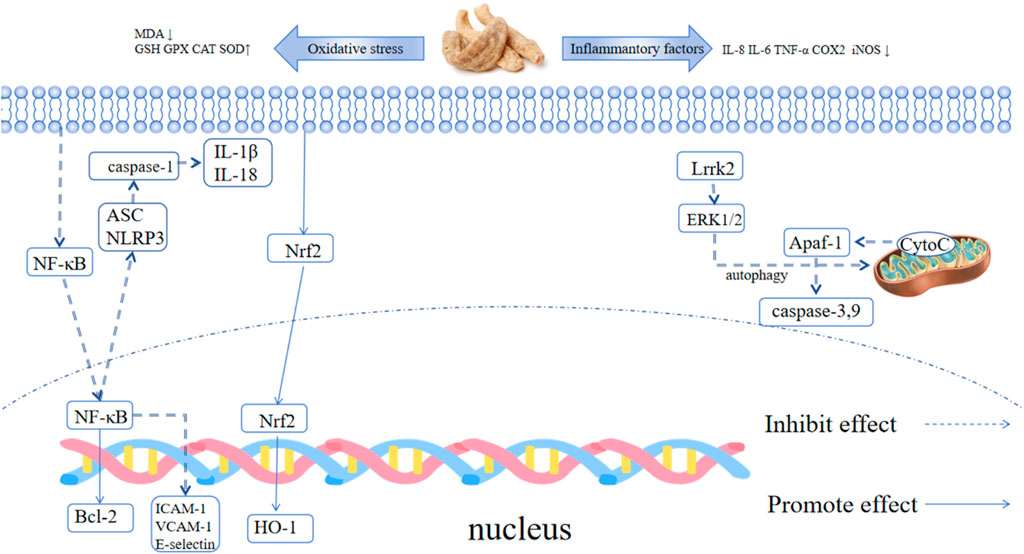
Studies on rats with brain injury have shown that gastrodin can modulate the NLRP3 signaling pathway, reduce the levels of ASC, TNF-α, IL-6, IL-1β, and IL-18, thereby alleviating inflammation and decreasing astrocyte accumulation. It promotes an increase in Bcl-2 and a decrease in Bax, and it also reduces Beclin-1, LC3-II, and P62 levels to prevent astrocyte apoptosis (Yang et al., 2022; Ng et al., 2016; Wang X. S. et al., 2016). Vanillin also possesses good antioxidant and anti-inflammatory properties. It can cross the blood-brain barrier (Salau et al., 2020), reducing the expression of p-JNK, p-P38, and p-ERK in rotenone induced human SH-SY5Y cells, thereby improving mitochondrial dysfunction, oxidative stress, and apoptotic cascades. It can inhibit cellular inflammation by suppressing the elevation of LPS induced ERK1/2, p38, NF-κB p65, JNK, IL-1β, IL-6, iNOS, and COX-2 (Dhanalakshmi et al., 2015; Yan et al., 2017; Kim et al., 2019).
Rhizoma Gastrodiae ethanol extract can inhibit TNF-α-induced vascular inflammation in HUVEC cells by suppressing oxidative stress and NF-κB activation. This is demonstrated by the reduced mRNA expression of ICAM-1, VCAM-1, E-selectin, macrophage chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8), showcasing its anti-inflammatory and anti-ROS effects (Hwang et al., 2009). Another report suggests that 0.1% Rhizoma Gastrodiae water extract can counteract the upregulation of Smad2/3 signaling caused by LRRK2 overactivation in Parkinson’s fruit flies with the LRRK2-G2019S mutation (the most common familial Parkinson’s disease mutation) by activating the Nrf2 signaling pathway. This restores normal microglial function and improves their motor condition,. In individuals with Parkinson’s, where abnormal folding and aggregation of α-Syn are typically observed, Lrrk2 overactivation is often found. It is therefore hypothesized that the Rhizoma Gastrodiae water extract may improve the Parkinson’s condition by alleviating the abnormal folding and aggregation of α-Syn, thereby protecting neurons (Lin et al., 2021). HBA can induce the increased expression of antioxidant enzymes in SH-SY5Y cells, thereby reducing oxidative stress, exerting mitochondrial protection, and inhibiting apoptosis. It inhibits the ROS-dependent JNK/Jun/caspase-3 signaling pathway, effectively protecting dopaminergic neurons, reducing oxidative damage and death of neurons, and improving Parkinson’s symptoms (Lai, 2022).
H2O2 treatment downregulated the protein expression of Nrf2, HO-1, GPX4 and FPN1 in rat C6 cells, while pretreatment with 25 μM gastrodin significantly reversed this trend (the protein expression of Nrf2, HO-1 and GPX4 was 1.8-fold, 2.1-fold and 1.5-fold higher than that in the H2O2 group, respectively, p < 0.01). In addition, gastrodin inhibited the H2O2-induced upregulation of ACSL4 and COX2 protein expression (the expression of ACSL4 and COX2 in the 25 μM gastrodin group was approximately 30% lower than that in the H2O2 group, p < 0.05), and reduced intracellular iron accumulation (the intracellular iron concentration in the 25 μM gastrodin group was approximately 25% lower than that in the H2O2 group, p < 0.01) (Jiang et al., 2020). Gastrodin can regulate neurotransmitters, exhibit both antioxidant and anti-inflammatory properties, inhibit the activation of microglial cells, manage mitochondrial cascade reactions, and enhance neurotrophic factor levels (Liu et al., 2018). Table 4 summarizes the effects of GE and its extracts on Parkinson’s disease, while Figure 5 illustrates the mechanism of action of GE.
4.1.3 Anti-Alzheimer’s disease (AD)
Alzheimer’s disease is an age-related neurodegenerative disorder, with 95% of cases being non-hereditary sporadic cases. Its pathological mechanisms are complex, with major hypotheses including β-amyloid (Aβ) deposition, neuroinflammation, excessive phosphorylation of tau proteins, and synaptic neuronal loss (Jiang et al., 2023). It is specifically manifested as cognitive impairment and memory decline (Lindsay et al., 2002; Sulistio and Heese, 2016).
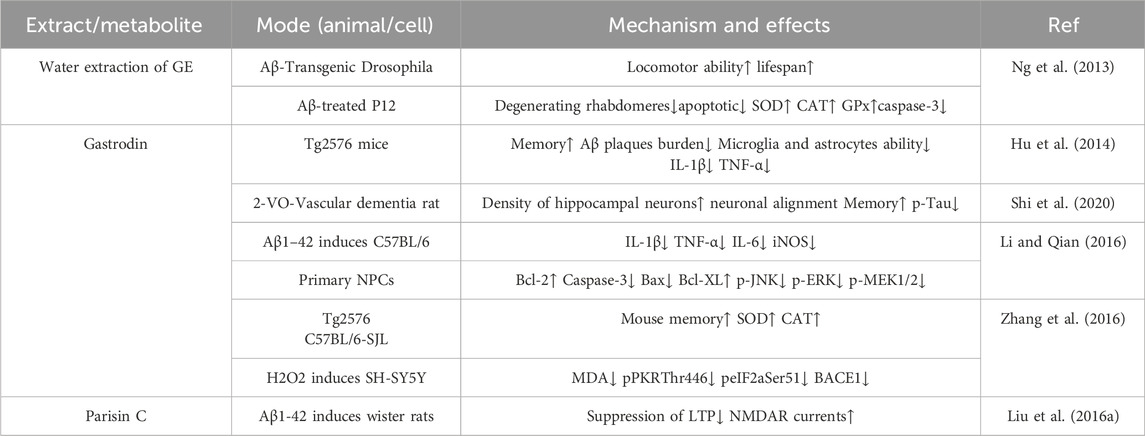
The water extract of Rhizoma Gastrodiae (WGE) significantly ameliorates the pathological conditions in Aβ-induced Alzheimer’s disease model Drosophila. In the lifespan assay, treatment with 5 mg/g WGE extended the median lifespan of Aβ42 Drosophila by 7 days (26.9% prolongation) and the maximum lifespan by 7 days (both P < 0.001). Climbing experiments showed that 5 mg/g WGE enhanced the climbing ability of Drosophila by 14.4% (P < 0.001), 11.6% (P < 0.01), and 9.74% (P < 0.05) on days 12, 19, and 23, respectively. In retinal degeneration assays, WGE (5 mg/g) increased the number of rhabdomeres per ommatidium by 0.97, comparable to the effect of 10 μmol/g donepezil (P < 0.001). In vitro experiments further confirmed that WGE alleviates Aβ-induced apoptosis in PC12 cells by enhancing the activities of antioxidant enzymes such as CAT, SOD, and GPX (increased to 120%, 150%, and 160%, respectively), reducing ROS production (decreased to 80%), and inhibiting Caspase-3 activity (31.8% reduction). Moreover, 1000 μg/mL GE completely reverses Aβ-induced cytotoxicity (Lin et al., 2021; Ng et al., 2013). Rhizoma Gastrodiae extract improved the viability of Aβ-treated PC12 cells in a dose-dependent manner, attenuating Aβ-induced oxidative and apoptotic stress. Rhizoma Gastrodiae also significantly upregulated the enzymatic activities of catalase, superoxide dismutase, and glutathione peroxidase, leading to a reduction in reactive oxygen species production and the activity of the apoptotic marker caspase-3 (Ng et al., 2013). It also alleviate vascular cognitive impairment by increasing the acetylcholine content and stabilizing the structure and function of mitochondria (Li et al., 2018).
There is also evidence indicating that Gastrodin can improve memory in mice, reduce the deposition of Aβ amyloid plaques, the number of astrocytes and activated microglial cells, and decrease the expression of TNF-α and IL-1β in N9 cells. At the same time, it regulates the TLR4/TRAF6/NF-κB pathway to alleviate neuroinflammation and microglial activation in the AD model, thereby exerting anti-AD effects (He et al., 2021; Hu et al., 2014). It can also downregulate the expression of amyloid precursor protein (APP) cleaving enzymes to inhibit the accumulation of Aβ and the abnormal phosphorylation of Tau protein, improve the neurofibrillary tangles (NFTs) caused by abnormal Tau phosphorylation, and drive the non-amyloidogenic pathway to prevent Alzheimer’s disease (AD) (Shi et al., 2020; Li and Qian, 2016; Zhang et al., 2016). The extract of Rhizoma Gastrodiae, Parisin C, can inhibit the abnormal activation of N-methyl-D-aspartate receptors (NMDAR) in the Wistar rat AD model induced by Aβ1-42, thereby exerting anti-AD effects.
Intracerebroventricular (i.c.v.) injection of Aβ1-42 oligomers (2 μmol/L) significantly inhibited NMDAR-dependent long-term potentiation (LTP) in the hippocampal dentate gyrus of rats, reducing the LTP amplitude from 195.1% ± 9.6% in the control group to 148.7% ± 6.5% (P < 0.05). Pretreatment with Parishin C (20 mg/kg, intraperitoneal injection) restored the LTP amplitude to 179.0% ± 8.4% (P < 0.05), while i. c.v. administration of 10 μmol/L Parishin C further increased the LTP amplitude to 210.2% ± 22.1% (P < 0.05). Electrophysiological experiments further confirmed that Aβ1-42 oligomers (2 μmol/L) reduced the NMDAR current in hippocampal neurons to 71.0% ± 5.0% of the pre-administration level (P < 0.05), and the current continued to decrease to 44.1% ± 7.1% after drug washout. Pretreatment with Parishin C (10 μmol/L) significantly antagonized this inhibitory effect (P < 0.05). Abnormal activation of NMDAR may lead to neuronal hyperexcitability and disrupt neuroprotective mechanisms. Aβ1-42-induced inhibition of NMDAR function impairs synaptic plasticity of hippocampal neurons. By specifically protecting NMDAR current, Parishin C improves Aβ-induced hippocampal neuronal injury, thereby protecting cells, maintaining information processing ability, and ultimately improving advanced cognitive functions such as learning and memory (Liu Z. et al., 2016). The relevant information is shown in Table 5.
4.1.4 Anticonvulsant and antiepileptic effects
Kainic acid is a neurotoxic substance that acts as an agonist for glutamate receptors in the central nervous system to increase oxidative stress and neuronal damage. It can be used for establishing rat models of epilepsy (Chen and Sheen, 2011). Rhizoma Gastrodiae and Uncaria rhynchophylla used in combination for kainic acid (KA)-induced seizures in SD rats significantly delayed the onset time of wet dog shakes in rats compared to the use of U. rhynchophylla alone, suggesting that Rhizoma Gastrodiae has a certain anticonvulsant effect (Hsieh et al., 1999). Subsequent research confirmed that vanillyl alcohol in Rhizoma Gastrodiae can reduce seizures induced by ferric chloride in rats. It can relieve symptoms of wet dog-like shaking by reducing the content of lipid peroxides in the brain, inhibiting the expression of AP-1, suppressing GABAergic inhibition, and decreasing the expression of p-JNK (Hsieh et al., 2007).
4.1.5 Analgesia effect
Rhizoma Gastrodiae has been applied in analgesia, yet the underlying mechanism of its pain-relieving effect remains poorly investigated. The ethanol extract of GE (GEE) and phenolic monomers can reduce the expression of iNOS and COX-2 in RAW264.7 cells and decrease the writhing times in mice. Additionally, GEE can reduce COX-I and COX-II in RBL 2H3 cells, thereby demonstrating a certain analgesic effect (Liu et al., 2018; Lee et al., 2006). In 2016, researchers confirmed that gastrodin inhibits the expression of c-Fos in the spinal cord of mice, as well as C-fiber evoked EPSCs (c-eEPSCs) in spinal lamina I neurons. It has an analgesic effect on peripheral inflammation induced spinal spontaneous pain, mechanical and thermal hypersensitivity induced pain. This hypersensitivity is not dependent on opioid receptors and does not develop tolerance (Qiu et al., 2014). The analgesic mechanism may function by partially blocking acid-sensing ion channels, thereby inhibiting the presynaptic enhancement effect in the spinal cord caused by inflammation (Qiu et al., 2014; Xiao et al., 2016).
4.1.6 Antidepressant effects
Rhizoma Gastrodiae exhibits therapeutic efficacy not only in neurodegenerative diseases but also demonstrates specific effects on psychiatric disorders. After administering WGE to Sprague-Dawley (SD) rats, the concentration of 5-hydroxytryptamine (5-HT) in the prefrontal cortex and dopamine (DA) in the striatum significantly increased. This led to a reduction in immobility time during the Forced Swim Test (FST) for the rats. It also decreased the levels of 5-HT and serum corticosterone in rats subjected to the unpredictable chronic mild stress (UCMS) model and improved grooming behavior and activity levels (Huang et al., 2021). Moreover, WGE can exert antidepressant effects by reducing the activity of monoamine oxidase (MAO-A) in PC12 cells and increasing the activity of tyrosine hydroxylase (TH) (Chen et al., 2009; Lin et al., 2016). Metabolomics (UPLC-QTOF-MS) combined with transcriptomics, network pharmacology, and molecular docking have confirmed that gastrodin and Parishin C, the key bioactive metabolites of Gastrodia elata, target the epidermal growth factor receptor (EGFR), activate the PI3K/Akt signaling pathway, and promote the proliferation of hippocampal neural stem/progenitor cells (NSPCs, ∼30% increase in BrdU + cells vs. model group) and neuronal differentiation (∼25% increase in BrdU + NeuN + cells). These effects alleviate chronic mild stress (CMS)-induced depressive-like behaviors, as evidenced by a ∼20% increase in sucrose preference and a ∼25% reduction in immobility time in the tail suspension test. These findings suggest that bioactive factors such as gastrodin and Parishin C exert antidepressant effects via the “EGFR-PI3K/Akt” axis (Huang et al., 2021; Liu et al., 2025).
4.2 Effects of gastrodia on cardiovascular
Rhizoma Gastrodiae has cardiovascular effects, including repair and protection of the cardiovascular system (Yang et al., 2022; Zhu et al., 2018; Baral et al., 2015), improvement of dizziness symptoms (Liu et al., 2018), antihypertensive effects (Lee et al., 2012), relief of migraines and hemiplegia (Wang P. H. et al., 2016), and anti-arteriosclerosis effects (Lee et al., 2012; Kim et al., 2012). The effects of Rhizoma Gastrodiae and its extracts on the cardiovascular system are shown in Table 6.
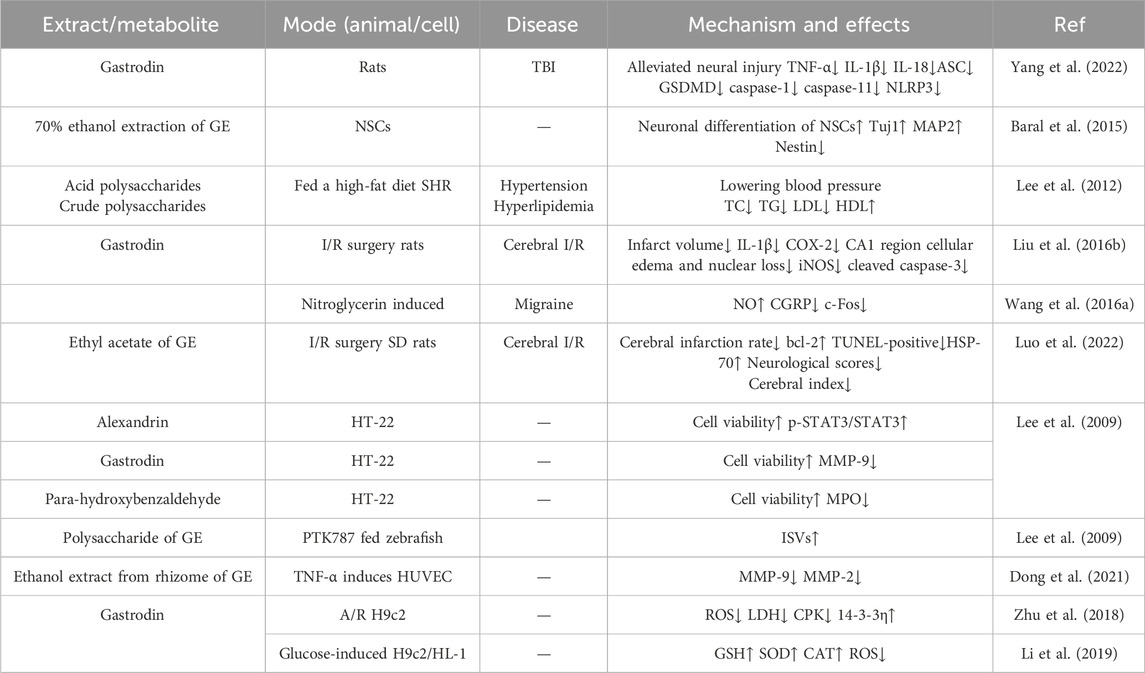
Table 6. Rhizoma Gastrodiae and its extracts’ effects on the cardiovascular and cerebrovascular systems.
4.2.1 Repair and protection of cardiovascular and cerebrovascular systems
Rhizoma Gastrodiae has been shown to exert potent therapeutic and protective effects against cardiovascular diseases. Emerging evidence from current studies suggests that its extracts, including alexandrin, para-hydroxybenzaldehyde, and gastrodin, are capable of ameliorating cerebral ischemia-reperfusion injury and facilitating repair of damaged cells (Luo et al., 2022; Li et al., 2019). These three types of metabolites can improve the viability of HT22 cells after oxygen-glucose deprivation/reperfusion (OGD/R) treatment. Western blot (WB) experiments also confirmed that alexandrin can upregulate the expression of p-stat3 and downregulate the expression of MPO, thereby alleviating oxidative stress and inflammatory responses in the cardiovascular system caused by abnormal MPO(myeloperoxidase) expression. In addition, Gastrodin downregulates the expression of MMP-9, which is a high-risk marker for cardiovascular diseases. Elevated levels of MMP-9 indicate a poor prognosis for cardiovascular diseases (Luo et al., 2022). The study on gastrodin ameliorating cerebral ischemia-reperfusion injury, despite verifying the expression changes of p-STAT3 and MPO via Western blot, lacked a positive control drug (such as edaravone, a commonly used neuroprotective agent), making it impossible to comparatively assess gastrodin’s therapeutic superiority. Moreover, the evaluation of brain injury severity relied solely on histopathological scoring without incorporating functional indicators like neurological deficit scores, thus failing to comprehensively reflect its therapeutic efficacy. Intraperitoneal injection of Gastrodin in TBI (Traumatic brain injury) rats can alleviate the reduction in the number of neurons, nuclear shrinkage, and degeneration in the brainstem area caused by TBI. It also reduces the expression of inflammatory factors TNF-α, IL-1β, and IL-18, and downregulates the expression of pyroptosis-related proteins GSDMD, NLRP3, ASC, caspase-1, and caspase-11. This suggests that Gastrodin may improve brain injury by inhibiting the NLRP3 inflammasome signaling pathway to affect pyroptosis (Yang et al., 2022). GE ethyl acetate extract also exhibits certain neuroprotective effects. Administering it to SD rats with ischemia-reperfusion injury can increase the expression of BCL-2 and HSP-70, enhance brain cell survival rate, and improve brain damage (Duan et al., 2015). However, this study did not clearly determine the content ratio of active components in the extract, making it impossible to ascertain whether a single component or multiple components synergistically contribute to the effects, thereby affecting the accuracy of mechanism analysis.
The 95% ethanol extract of GE has been demonstrated to promote angiogenesis in zebrafish models and exhibit protective effects against ischemic cardiovascular diseases and atherosclerosis when administered in vivo (Liu et al., 2020). Using metabolomics technology (LC-TOF-MS), combined with zebrafish models and grey correlation analysis, ten metabolites highly correlated with pro-angiogenic activity (correlation coefficient >0.9) were identified, including gastrodin, parishin E, β-sitosterol, etc. Experiments confirmed that the extract of Gastrodia elata showed the optimal pro-angiogenic effect at 100 μg/mL, significantly promoting the growth of intersegmental vessels in zebrafish. Network pharmacology analysis revealed that these metabolites exert their effects by targeting VEGFA, TNF and other targets, and regulating signaling pathways such as VEGF, MAPK, and NF-κB (Liu et al., 2020). Thus, GE demonstrates a positive effect in the therapeutic intervention and protective management of cardiovascular diseases.
Furthermore, experimental evidence has demonstrated that in vitro co-culture of neural stem cells (NSCs) with gastrodin leads to a significant downregulation of Nestin and Sox2 expression, accompanied by an upregulation of Tuj1 and MAP2. These findings suggest that gastrodin exhibits neuroregenerative properties in NSCs, facilitating the repair of brain neural injuries (Baral et al., 2015). Administration of gastrodin to A/R H9c2 cells can reduce intracellular ROS levels, decrease the release of LDH and CPK, and enhance the expression of 14-3-3η, thereby reducing cell apoptosis and exerting a protective effect on the cells (Zhu et al., 2018). The licorice saponin can also protect high glucose-induced H9c2 and HL-1 cardiomyocyte from toxicity, oxidative stress, and apoptosis by enhancing the nuclear translocation of Nrf2 mediated by GSK-3β, increasing GSH, SOD, and CAT levels, and reducing ROS. This suggests that licorice saponin may also be used as a potential treatment for diabetic cardiomyopathy (Dong et al., 2021).
4.2.2 Reduce hypertension
Researchers have discovered that Rhizoma Gastrodiae acidic polysaccharides and crude polysaccharides can reduce hypertension in spontaneously hypertensive rats (SHR). These metabolites simultaneously increase high-density lipoprotein cholesterol (HDL-C) levels while lowering total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, . They also inhibit de novo synthesis of total cholesterol and low-density lipoproteins in rats, thereby improving hemorheology through multiple pathways and reducing the incidence of cardiovascular diseases and atherosclerosis (Lee et al., 2012; Kim et al., 2012).
4.2.3 Anti-migraine
Calcitonin gene-related peptide (CGRP) is significantly elevated in migraines (Russo and Hay, 2023). Researchers have found that using gastrodin to synthesize gastrodin derivatives (Gastrodin-D) can reduce plasma CGRP levels in SD rats, exerting an anti-migraine effect (Wang P. H. et al., 2016). There are also reports indicating that Rhizoma Gastrodiae inhibits acid-sensing ion channels, blocks pain transmission, and alleviates migraines (Feng, 2022).
4.3 Other effects of Rhizoma Gastrodiae
Rhizoma Gastrodiae is commonly used for neurological disorders (Liu et al., 2018), brain injuries (Baral et al., 2015) and the treatment of cardiovascular diseases (Lee et al., 2012). However, the medical applications of Rhizoma Gastrodiae are not confined to the aforementioned uses. As ongoing research deepens the understanding of its pharmacological mechanisms, investigators have continued to uncover novel therapeutic effects of Rhizoma Gastrodiae, with osteoporosis treatment being a notable example (Chen et al., 2015), boosting immunity (Chen et al., 2016), treating gastritis (Lee et al., 2009), delaying aging, and anti-tumor effects (Farooq et al., 2019; Liang et al., 2017; Ahn et al., 2007).
4.3.1 Reducing osteoporosis
Current studies indicate that both gastrodin and Rhizoma Gastrodiae polysaccharide WSS25 exhibit specific effects on osteoporosis. Gastrodin works by inhibiting the nuclear translocation of NF-κB in chondrocytes, downregulating the expression of TNF-α and IL-1β, and inhibiting MMP-3 degradation to maintain chondrocyte homeostasis. In a model of LPS-induced human periodontal ligament stem cells, Gastrodin can also mitigate the attack of inflammatory factors such as TNF-α on these cells, enhance the vitality and osteogenic capacity of human periodontal ligament stem cells, increase the M2/M1 ratio, and promote the differentiation and formation of osteoblasts. In the glucocorticoid-induced osteoporosis rat model, the use of Gastrodin was found to improve osteoporosis status by activating the Nrf2/Keap1 pathway and upregulating the expression of OCN, BMP-2, and RUNX2. Moreover, Gastrodin also ameliorates osteoporosis by blocking the formation, maturation, and migration of osteoclasts through the inhibition of the NFATc1 gene and specific transmembrane protein DC-STAMP (Chen et al., 2018).
WSS25 is a sulfated polysaccharide extracted from the rhizome of Rhizoma Gastrodiae. It binds to bone morphogenetic protein 2 (BMP-2) in hepatocellular carcinoma cells, and BMP-2 may simultaneously regulate both osteoclasts and osteoblasts. WSS25 effectively inhibits the expression of TRAP, NFATc1, MMP-9, and cathepsin K in RAW264.7 or BMMs cells induced by RANKL, thereby suppressing the formation and differentiation of osteoclasts and alleviating bone resorption. On the other hand, WSS25 promotes the expression of osteogenic markers such as OCN, BMP-2, and RUNX2, enhancing osteoblast differentiation and improving bone strength. Long-term administration of WSS25 significantly reduces bone loss in ovariectomized mice and mitigates the inhibition of the BMP-2/Smad/Id1 signaling pathway caused by the BMP-2 antagonist noggin. These findings suggest that Rhizoma Gastrodiae has potential applications in the treatment of osteoporosis, warranting further exploration (Chen et al., 2015).
4.3.2 Improvement of immunity
Rhizoma Gastrodiae polysaccharide GDP has been shown to induce NO release in RAW264.7 cells, thereby activating immune cells and significantly enhancing the phagocytic activity of RAW264.7 macrophages (Chen et al., 2016). In tumor-bearing mice, administration of the Rhizoma Gastrodiae water extract has been shown to upregulate serum levels of IL-2 and IFN-γ, induce T-cell activation, and enhance immune responses. Additionally, separate studies have demonstrated that the silkie chicken Rhizoma Gastrodiae nutrient solution significantly increases thymus weight ratio in mice, promotes NK cell activation, and potently enhances immune system function (Li et al., 2019; Russo and Hay, 2023).
4.3.3 Anti-tumour effect
Studies have demonstrated that Rhizoma Gastrodiae exhibits antitumor activity, with underlying mechanisms involving multiple aspects. Specifically, gastrodin treatment leads to an increase in the proportion of subG1 and G2/M phase cells, accompanied by a decrease in G0/G1 phase cells, in DBTRG-05MG glioma cells. This is associated with downregulation of CDK1 (cyclin-dependent kinase 1)/CDC2 and cyclin B1, as well as upregulation of p53 and p21, thereby disrupting the tumor cell cycle. Gastrodin increases intracellular ROS levels without raising mitochondrial ROS levels, decreases GSH levels in DBTRG-05MG cells, increases SOD levels, and reduces GPx and CAT levels, thereby promoting glioma cell apoptosis (Liang et al., 2017). Additionally, the ethyl ether extract of Rhizoma Gastrodiae inhibits the proliferation of B16 cells by upregulating GTP-Ras expression (Heo et al., 2007). Other studies have indicated that gastrodin, the main metabolite of Gastrodia elata, attenuates H22 tumor cell transplantation-induced decrease in CD4+ T cells. This is accompanied by a dose-dependent reduction in IFN-γ and IL-2 expression, inhibition of IL-4 upregulation, modulation of CD4+ T cell subpopulation ratios, and activation of the NF-κB pathway, thereby exerting an antitumor effect through anticancer immune responses (Shu et al., 2013).
4.3.4 Anti-aging effect
In addition to its broad pharmacological effects, Rhizoma Gastrodiae and its extracts have been shown to possess remarkable anti-aging properties. Gastrodin can enhance the antioxidant capacity (SOD, CAT) of black fruit flies, extending their lifespan and improving their oxidative resistance (He et al., 2021). The aqueous extract of Rhizoma Gastrodiae significantly upregulates the enzyme activities of catalase, superoxide dismutase, and glutathione peroxidase, leading to the production of reactive oxygen species and a decrease in the activity of the apoptosis marker caspase-3, resulting in αβ-induced fruit flies having longer lifespans, better motor function, and fewer small eye degenerations. The mechanism of action may involve alleviating αβ-induced oxidative and apoptotic stress (Ng et al., 2013). A new metabolite, “bis (4-benzyl) ether monobeta-D-galactopyranoside”, ametabolite isolated from the rhizome of Gastrodia elata (GE), has been shown to significantly extend the lifespan of two yeast strains, K6001 and YOM36. Additionally, experimental evidence indicates that this metabolite reduces reactive oxygen species (ROS) and malondialdehyde (MDA) levels in BY4741 yeast cells while upregulating the expression of catalase (CAT) and thiol peroxidase (CPx). Moreover, in yeast strain K6001, the metabolite enhances Sir2 gene expression and inhibits the Uth1/TOR signaling pathway, thereby promoting lifespan extension and exerting anti-aging effects (Farooq et al., 2019).
4.3.5 Treatment of digestive diseases
GE extract has been demonstrated to mitigate oxidative stress in the stomach, enhance energy and amino acid metabolism, alleviate inflammatory responses, and thereby alleviate gastritis Using 1H NMR metabolomics, we intervened rats with chronic atrophic gastritis model with water extract (T1), n-butanol extract (T2), ethyl acetate extract (T3) and petroleum ether extract (T4) of Gastrodia elata continuously for 21 days. It was found that compared with the model group, T1 group significantly reduced the level of malondialdehyde (MDA) in gastric tissue (p < 0.001), significantly increased the activities of superoxide dismutase (SOD) and glutathione (GSH), decreased the levels of serum nitric oxide (NO) and xanthine oxidase (XOD), and increased pepsin activity by 28.5%. Metabolomic analysis showed that it could regulate 34 differential metabolites, including decreasing the levels of branched-chain amino acids such as leucine (p < 0.01), isoleucine and acetate, increasing antioxidant substances such as glucose (p < 0.01) and taurine, and correcting the energy metabolism disorder of tricarboxylic acid cycle. The study confirmed that the high-polarity metabolites of Gastrodia elata (T1) exerted the best therapeutic effect through anti-oxidation, anti-inflammation and repair of energy and amino acid metabolism disorders, in which water-soluble phenols and polysaccharides were the key active metabolites, providing a scientific basis at the metabolomic level for the application of Gastrodia elata in the treatment of gastritis (Xu et al., 2020).
5 The pharmacokinetics of Rhizoma Gastrodiae and its active metabolites
Detailed pharmacokinetic studies can well explain the pharmacokinetic characteristics of various active metabolites in GE in vivo. Understanding the pharmacokinetic characteristics of active metabolites in GE helps further investigate the interactions between GE and various chemical metabolites, and provides deeper insights into the influence of external factors on the efficacy of GE. Pharmacokinetic properties of Gastrodia elata metabolites are detailed in Table 7.
5.1 Absorption
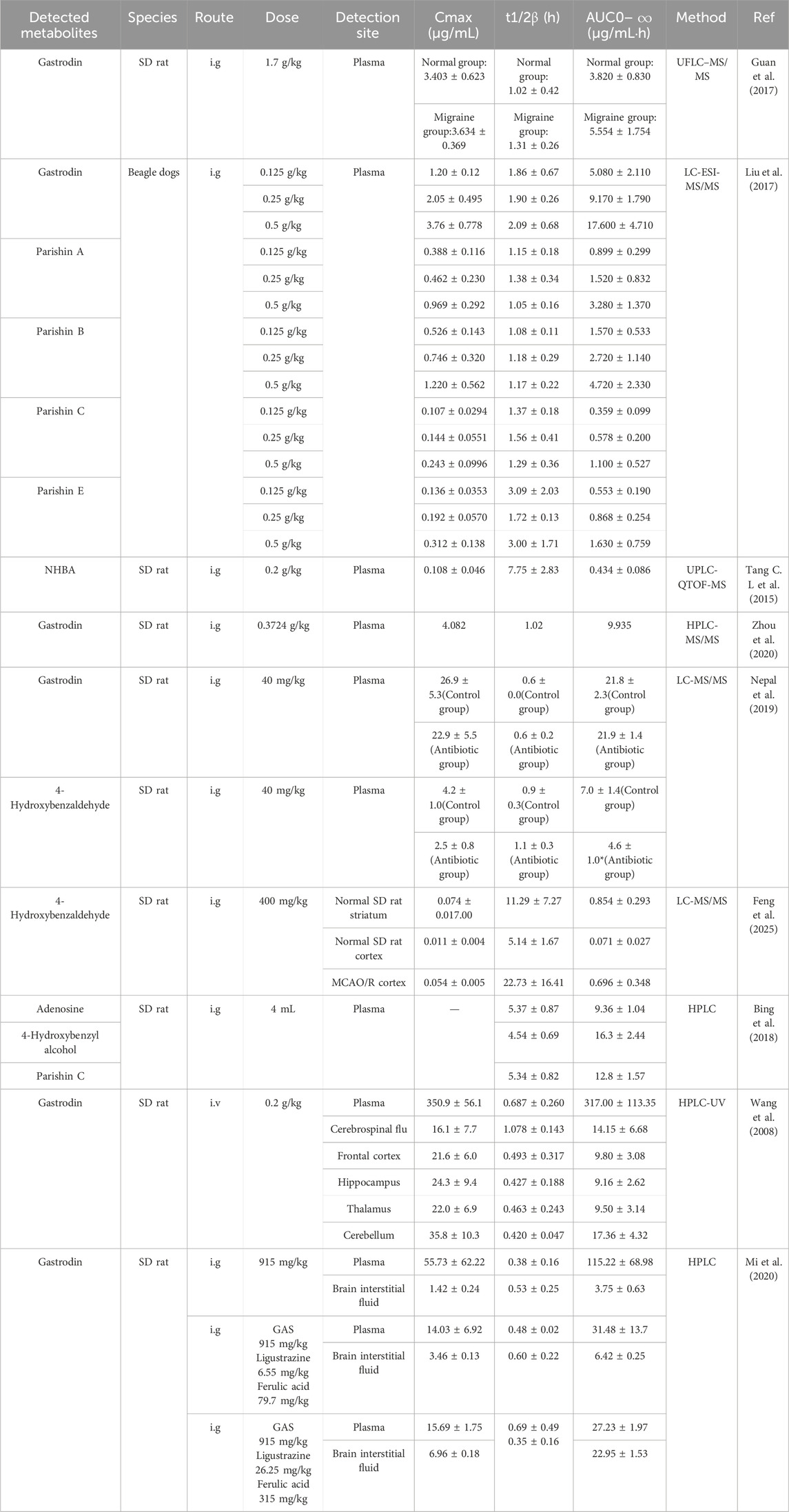
Gastrodin was detectable in plasma within 10 min after intragastric administration to beagle dogs, with a time to peak concentration (Tmax) of 1.10–2.00 h, which prolonged with increasing doses. This is consistent with the rapid absorption observed in rats (detectable at 5 min post-gavage), though the Tmax was slightly longer (Tang C et al., 2015; Liu et al., 2017). This rapid absorption of gastrodin is primarily mediated by sodium-dependent glucose transporters (SGLTs). The SGLT inhibitor phlorizin (0.2 mM) significantly suppressed gastrodin absorption in rat perfused intestinal segments, reducing effective permeability to ∼30% in the duodenum and jejunum, and ∼10% in the ileum. In contrast, the facilitative glucose transporter (GLUT) inhibitor phloretin (0.2 mM) had no significant effect on gastrodin absorption, indicating that gastrodin uptake is predominantly mediated by SGLT1 rather than GLUT family transporters (Cai et al., 2013).
5.2 Distribution
Studies have shown that after intravenous injection of 100 mg/kg gastrodin in rats, it rapidly and widely distributes in a free state due to its water solubility, detectable in visceral tissues within 2 min. The brain-to-blood distribution ratio is only 0.007, leading researchers to suggest that the metabolic product of gastrodin, HBA, exerts therapeutic effects in the brain. HBA reaches peak cerebrospinal fluid concentration at 40 min post-oral administration, with a brain-to-blood ratio of ∼20%, indicating efficient blood-brain barrier (BBB) permeability. In vitro hCMEC/D3 model experiments show that 32.91% of HBA penetrates the barrier within 240 min, facilitated by its lipophilicity (XlogP3 = 0.2) and passive diffusion (Lin et al., 2007; Yan, 2024). This conclusion was later challenged: in a migraine rat model, oral gastrodin capsules increased AUC0‒
5.3 Metabolism
In phase I metabolism mediated by cytochrome P450 (CYP) enzymes, rats and dogs showed higher metabolic capacity for gastrodin, with remaining amounts of 29% and 24%, respectively, while humans and monkeys exhibited weaker metabolism (remaining amounts. 70% and 71%). In phase II metabolism mediated by uridine diphosphate glucuronosyltransferase (UGT), the remaining amounts in dogs and rats were 34% and 42%, compared to 67% and 63% in humans and monkeys. Molecular docking showed that gastrodin binds to CYP3A4 and CYP2C19 via hydrogen bonds, confirming these enzymes as potential major metabolic enzymes (in vitro metabolic differences of gastrodin in liver microsomes of different species detected by high-performance liquid chromatography). Stability of results also influences metabolism: compared to gastrodin, N6-hydroxybenzyladenosine has a significantly longer half-life (gastrodin t1/2β: ∼1.86–2.09 h), likely attributed to the metabolic stability of its nucleoside structure (determined by ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for N6-hydroxybenzyladenosine in rat plasma). Intestinal microbiota affect metabolism: after oral administration, gastrodin is rapidly absorbed, with no significant differences in Cmax and AUC between control and antibiotic groups. However, pharmacokinetic parameters of the gastrodin metabolite 4-HBA changed significantly: antibiotic treatment reduced 4-HBA Cmax by 40.5% and AUC by 34.3%, while volume of distribution (Vd) and clearance (CL) increased by 100% and 55.2%, respectively, indicating that intestinal microbiota inhibition significantly reduces conversion of gastrodin to 4-HBA (Nepal et al., 2019). Pharmacokinetic parameters (e.g., Tmax, AUC) of Parishin metabolites (e.g., Parishin B, C, E) differ from those of gastrodin. Pearson correlation coefficients between Parishin A and Parishin B/C/E range from 0.76 to 0.95 (e.g., 0.95 for Parishin A and E), significantly higher than those with gastrodin (0.54–0.57), suggesting that Parishin metabolites may interconvert via metabolism, whereas gastrodin shows weak correlation with Parishins, possibly exerting effects through different metabolic pathways (Liu et al., 2017).
5.4 Excretion
Gastrodin is primarily excreted in urine as the parent metabolite. In normal rats, the clearance (CL) of gastrodin after oral administration of 200 mg/kg was 5.73 ± 1.59 mL/min, indicating rapid elimination (Cai et al., 2013; Lin et al., 2007). In diabetic rats with upregulated intestinal sodium-dependent glucose transporter 1 (SGLT1) expression, the Tmax of gastrodin was significantly shortened to 20.0 ± 0.0 min, suggesting accelerated absorption. However, the elimination rate constant (k) was 0.033 ± 0.013 L/min, not significantly different from normal rats (0.040 ± 0.014 L/min), indicating that increased SGLT1 expression did not alter elimination rate (Cai et al., 2013). When comparing single extract and metabolite formulations, the elimination rate constant (Ke) of gastrodin in rats administered pure Gastrodia elata extract was 0.0204 ± 0.004 min-1. In the Tian Gou Jiang Ya Capsule metabolite formulation, Ke decreased to 0.017 ± 0.001 min-1, 0.0151 ± 0.003 min-1, and 0.013 ± 0.001 min-1 with increasing doses (low, medium, high), indicating that the metabolite formulation delayed gastrodin elimination and prolonged its residence time in vivo. Although the metabolite formulation altered elimination rate and distribution of gastrodin, the area under the curve (AUC) showed no significant difference from the single extract: the medium-dose metabolite group had an AUC of 5187.2 ± 871.5 μg/mL, compared to 5462.1 ± 281.2 μg/mL in the extract group, without statistical significance. This suggests that the metabolite formulation did not significantly affect the total absorption of gastrodin, only altering its elimination kinetics (Cai et al., 2013; Song et al., 2017).
6 Clinical efficacy of marketed preparations of Gastrodia elata
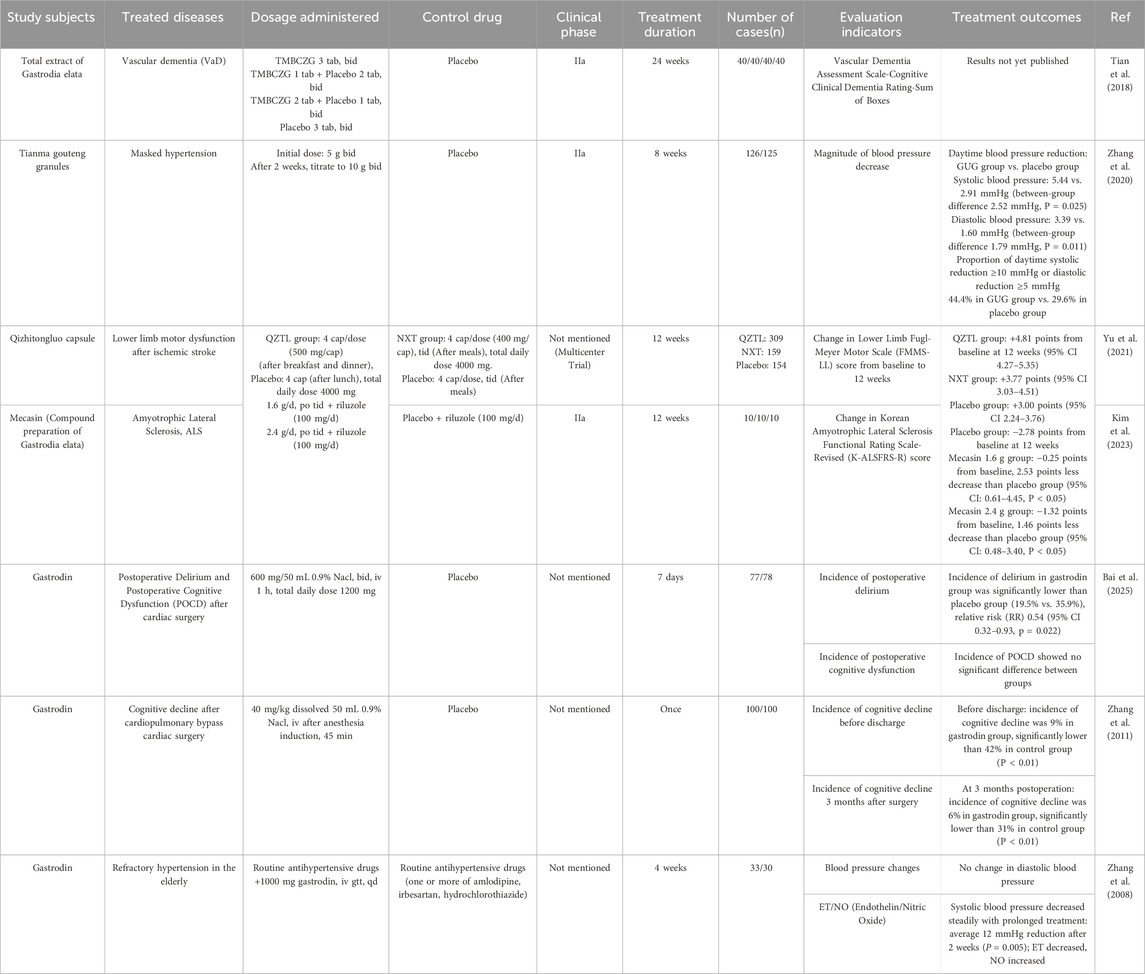
Gastrodia elata has demonstrated definite therapeutic effects, leading to the successive market launch of related products. The only marketed monomeric drugs are gastrodin and its derivative acetylgastrodin, while other products are formulations prepared from Gastrodia elata extracts combined with other botanical drugal medicines. As of 3 July 2025, a search of China’s medical information platform using “Gastrodia elata” as a keyword identified 163 registered preparations and related formulations (China Medical Information Platform, 2025). Clinical studies on these preparations primarily focus on cardiovascular and cerebrovascular diseases and neuroprotection. Most studies adopt a placebo-controlled design, while a few use positive drug controls to observe enhanced efficacy. The corresponding clinical effects are shown in Table 8. The following clinical comparative experiments confirm the efficacy of Gastrodia elata preparations.
7 Conclusion
Despite the fact that Gastrodia elata has been researched and used for thousands of years in China, its level of utilization remains relatively low. Among the five commonly used medicinal varieties of Rhizoma Gastrodiae, only two types are widely cultivated and utilized (Zhan et al., 2016; Zhengyi, 1999). At present, the primary method of using Gastrodia elata is still traditional decoction, and related formulations mainly consist of traditional Chinese medicine, with only gastrodin being used in clinical single-agent preparations (Tian et al., 2022; Kong et al., 2022). This situation is not conducive to the utilization and modernization of Rhizoma Gastrodiae, indicating a need for further research to fill the gap. The reason for this situation is the insufficient research on the extracted metabolites. Transcriptomics (RNA-seq) and targeted metabolomics (HPIC-MS/MS) technologies can be used to systematically explore the therapeutic mechanisms of other active metabolites of Gastrodia elata in diseases, which is conducive to the development of monomer drug research (Song et al., 2024).
Through literature surveys, it has been found that more than a hundred metabolites have been extracted from Rhizoma Gastrodiae to date, with the most extensively studied being gastrodin, which is the main active substance known to treat hypertension, headaches, and other brain-related injuries. It has been shown to exhibit significant antioxidant, anti-aging, and neuroprotective effects (Kong et al., 2022; Yu, 2022). However, there has been relatively little research on other metabolites such as polysaccharides, sterols, and organic acids. Although some studies have confirmed that such metabolites possess certain pharmacological effects (Zhan et al., 2016; Lai et al., 2017; Abdel-Salam et al., 2014; Chen et al., 2016; Chen et al., 2015), the evidence in this area still suggests that the pharmacological research on Gastrodia elata remains limited. Through multi-omics integration, such as transcriptomics (RNA-seq) and targeted metabolomics (HPIC-MS/MS), combined with KEGG and GO enrichment analyses, differentially expressed genes following administration of such metabolites can be identified to investigate how other metabolites in Gastrodia elata exert therapeutic effects by regulating gene expression (Song et al., 2024; Kim et al., 2022).
Regarding the research on Gastrodia elata, the specific parts of the plant also need to be considered. Ancient medical texts in China mainly focus on the utilization of the tuber, and current studies primarily investigate the extracts from the tuber. Research on the leaves, flowers, and stems of GE is relatively scarce (Martins and S, 2018; Hu et al., 2019). Our comparative analysis of three Gastrodia elata variants (GE Bl. f. elata, GE Bl. f. viridis, and GE Bl. f. glauca S. Chow) revealed significant differences in metabolite composition between stems and tubers (Table 1). The stems exhibited greater chemical diversity, containing 128 identified metabolites compared to 90 in tubers, with 80 metabolites shared between both parts (Wu et al., 2023). The quantitative analysis reveals significant variation in gastrodin content across different plant parts of Gastrodia elata Blume f. elata. Specifically, the fresh stem bark exhibits the highest concentration at 0.640%, followed by fruits (0.302%) and seeds (0.094%). Notably, both fresh stem barks and fruits meet the quality standard stipulated in the Chinese Pharmacopoeia (2020 edition), which mandates a minimum combined content of 0.25% for gastrodin and p-hydroxybenzyl alcohol. This compliance suggests these morphological parts possess adequate pharmacological potency for medicinal applications. The three-fold difference between stem bark (0.640%) and seed (0.094%) concentrations may reflect distinct biosynthetic activity or metabolite translocation patterns during plant development, warranting further phytochemical investigation (Commission ChP, 2020; Li, 2012). The comparative analysis of both phytochemical composition and pharmacological content reveals that non-tuber parts of GE (including stems, leaves, and flowers) possess considerable medicinal potential. Historically, these aerial portions have been underutilized in traditional Chinese medicine, as evidenced by the current Chinese Pharmacopoeia standards which exclusively regulate quality parameters for the tuberous rhizomes (Commission ChP, 2020). This phenomenon could be attributed to multiple historical and technological factors: (1) In ancient times, the absence of proper processing techniques for stem barks, (2) limited pharmacological understanding of stem bark metabolites, and (3) the empirically verified superior therapeutic efficacy of tubers compared to other plant parts through millennia of clinical practice. Modern analytical advancements—particularly the integration of ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) with high-performance liquid chromatography-ultraviolet detection (HPLC-UV) metabolomic platforms—enable comprehensive phytochemical profiling. These technologies not only elucidate compositional differences in bioactive metabolites among Gastrodia elata’s anatomical parts, but also establish metabolomic foundations for: (a) cultivar authentication, (b) quality standardization, and (c) pharmaceutical development of this medicinal species (Zeng et al., 2023).
In general, studies on gastrodia metabolites have found many different effects from traditional uses of Gastrodia elata, including improving immunity, anti-tumor, and delaying aging (Farooq et al., 2019; Liang et al., 2017). However, the research is relatively limited, and only gastrodin is widely accepted and used clinically (Kong et al., 2022). On the other hand, a critical gap is the overreliance on preclinical data: while antitumor and anti-osteoporosis effects show promise in in vitro (e.g., gastrodin in DBTRG-05MG cells) and in vivo models (e.g., WSS25 in ovariectomized mice), the absence of human clinical trials—particularly for postmenopausal osteoporosis—severely undermines their clinical relevance. Additionally, mechanistic insights into some active components are incomplete. Although β-sitosterol’s anti-inflammatory role via NF-κB pathway modulation is established, its precise binding sites (e.g., on p65) and molecular interactions remain undefined. Likewise, research on gastrodin polysaccharides and gut microbiota lacks definitive evidence connecting microbial structural shifts to immune improvement, resulting in unresolved mechanistic pathways. Therefore, other active metabolites of gastrodia have not been fully researched yet, leaving plenty of room for further exploration. In-depth research on other gastrodia metabolites can further explore their potential biological activities, functions, and applications.
Author contributions
XZ: Conceptualization, Investigation, Writing – original draft. MH: Conceptualization, Investigation, Writing – original draft. SY: Investigation, Writing – original draft. MW: Investigation, Writing – original draft. SZu: Investigation, Writing – original draft. SZh: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support was received from Southern Medical Branch of PLA General Hospital for the publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Salam, O. M., Youness, E. R., Mohammed, N. A., Morsy, S. M. Y., Omara, E. A., and Sleem, A. A. (2014). Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 17 (5), 588–598. doi:10.1089/jmf.2013.0065
Ahn, E. K., Jeon, H. J., Lim, E. J., Jung, H. J., and Park, E. H. (2007). Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. J. Ethnopharmacol. 110 (3), 476–482. doi:10.1016/j.jep.2006.10.006
Babu, S., and Jayaraman, S. (2020). An update on β-sitosterol: a potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 131, 110702. doi:10.1016/j.biopha.2020.110702
Bai, Y. X., Wu, H. L., Xie, W. L., Li, X., Han, J. J., Liu, J., et al. (2025). Efficacy and safety of gastrodin in preventing postoperative delirium following cardiac surgery: a randomized placebo controlled clinical trial. Crit. Care 29 (1), 108. doi:10.1186/s13054-025-05331-9
Baral, S., Pariyar, R., Yoon, C. S., Kim, D. C., Yun, J. M., Jang, S. O., et al. (2015). Effects of Gastrodiae rhizoma on proliferation and differentiation of human embryonic neural stem cells. Asian Pac J. Trop. Med. 8 (10), 792–797. doi:10.1016/j.apjtm.2015.09.004
Bing, W., Yan-Tao, S., Zhi-Dong, P., Ting-Guo, K., and Hui, Z. (2018). Pharmacokinetic and tissue distributions study of adenosine, 4-hydroxybenzyl alcohol and Parishin C from Gastrodia elata extract in rats. Pak J. Pharm. Sci. 31 (5(Supplementary)), 2053–2060.
Cai, Z., Huang, J., Luo, H., Lei, X., Yang, Z., Mai, Y., et al. (2013). Role of glucose transporters in the intestinal absorption of gastrodin, a highly water-soluble drug with good oral bioavailability. J. Drug Target 21 (6), 574–580. doi:10.3109/1061186X.2013.778263
Chen, P. J., and Sheen, L. Y. (2011). Gastrodiae Rhizoma (tiān má): a review of biological activity and antidepressant mechanisms. J. Tradit. Complement. Med. 1 (1), 31–40. doi:10.1016/s2225-4110(16)30054-2
Chen, P. J., Hsieh, C. L., Su, K. P., Hou, Y. C., Chiang, H. M., and Sheen, L. Y. (2009). Rhizomes of Gastrodia elata B(L) possess antidepressant-like effect via monoamine modulation in subchronic animal model. Am. J. Chin. Med. 37 (6), 1113–1124. doi:10.1142/S0192415X09007533
Chen, X., Xiao, F., Wang, Y., Fang, J., and Ding, K. (2012). Structure-activity relationship study of WSS25 derivatives with anti-angiogenesis effects. Glycoconj J. 29 (5-6), 389–398. doi:10.1007/s10719-012-9424-z
Chen, C., Qin, Y., Fang, J. p., Ni, X. y., Yao, J., Wang, H. y., et al. (2015). WSS25, a sulfated polysaccharide, inhibits RANKL-induced mouse osteoclast formation by blocking SMAD/ID1 signaling. Acta Pharmacol. Sin. 36 (9), 1053–1064. doi:10.1038/aps.2015.65
Chen, J., Tian, S., Shu, X., Du, H., Li, N., and Wang, J. (2016). Extraction, characterization and immunological activity of polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 17 (7), 1011. doi:10.3390/ijms17071011
Chen, J., Gu, Y. T., Xie, J. J., Wu, C. C., Xuan, J., Guo, W. J., et al. (2018). Gastrodin reduces IL-1β-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed. Pharmacother. 97, 642–651. doi:10.1016/j.biopha.2017.10.067
Chen, L., Wang, Y. C., Qin, L. Y., He, H. Y., Yu, X. L., Yang, M. Z., et al. (2019a). Dynamics of fungal communities during Gastrodia elata growth. BMC Microbiol. 19 (1), 158. doi:10.1186/s12866-019-1501-z
Chen, S. Y., Geng, C. A., Ma, Y. B., and Chen, J. J. (2019b). Melatonin receptors agonistic activities of phenols from Gastrodia elata. Nat. Prod. Bioprospect 9 (4), 297–302. doi:10.1007/s13659-019-0213-2
Chen, S. Y., Geng, C. A., Ma, Y. B., Huang, X. Y., Yang, X. T., Su, L. H., et al. (2019c). Polybenzyls from Gastrodia elata, their agonistic effects on melatonin receptors and structure-activity relationships. Bioorg Med. Chem. 27 (15), 3299–3306. doi:10.1016/j.bmc.2019.06.008
Cheng, L., Wang, H., Ma, K., Deng, Y., Li, M., and Ma, J. (2023). A novel alcohol steamed preparation from Gastrodia elata Blume: pharmacological assessment of a functional food. Front. Pharmacol. 14, 1092693. doi:10.3389/fphar.2023.1092693
China Medical Information Platform (2025). Available online at: https://www.dayi.org.cn/.
Commission ChP (2020). Pharmacopoeia of the People'S Republic of China. 2020. Beijing, China: China Medical Science Press, 59–60.
Dai, S., Zhang, W., Dou, Y., Liu, H., Chen, X., Shi, J., et al. (2021). Towards a better understanding of the relationships between the structure and antitumor activity of Gastrodia elata polysaccharides by asymmetrical flow field-flow fractionation. Food Res. Int. 149, 110673. doi:10.1016/j.foodres.2021.110673
de Feijter, M., Katimertzoglou, A., Tiemensma, J., Ikram, M. A., and Luik, A. I. (2022). Polysomnography-estimated sleep and the negative feedback loop of the hypothalamic-pituitary-adrenal (HPA) axis. Psychoneuroendocrinology 141, 105749. doi:10.1016/j.psyneuen.2022.105749
Dhanalakshmi, C., Manivasagam, T., Nataraj, J., Justin Thenmozhi, A., and Essa, M. M. (2015). Neurosupportive role of Vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evid. Based Complement. Altern. Med. 2015, 626028. doi:10.1155/2015/626028
Dong, Z., Bian, L., Wang, Y. L., and Sun, L. M. (2021). Gastrodin protects against high glucose-induced cardiomyocyte toxicity via GSK-3β-mediated nuclear translocation of Nrf2. Hum. Exp. Toxicol. 40 (9), 1584–1597. doi:10.1177/09603271211002885
Duan, X., Wang, W., Liu, X., Yan, H., Dai, R., and Lin, Q. (2015). Neuroprotective effect of ethyl acetate extract from gastrodia elata against transient focal cerebral ischemia in rats induced by middle cerebral artery occlusion. J. Tradit. Chin. Med. 35 (6), 671–678. doi:10.1016/s0254-6272(15)30158-8
Farooq, U., Pan, Y., Lin, Y., Wang, Y., Osada, H., Xiang, L., et al. (2019). Structure characterization and action mechanism of an antiaging NewCompound from Gastrodia elata blume. Oxid. Med. Cell Longev. 2019, 5459862. doi:10.1155/2019/5459862
Feng, Q. (2022). Gastrodin attenuates lipopolysaccharide-induced inflammation and oxidative stress, and promotes the osteogenic differentiation of human periodontal ligament stem cells through enhancing sirtuin3 expression. Exp. Ther. Med. 23 (4), 296. doi:10.3892/etm.2022.11225
Feng, J., Yang, Q., Chen, M., Wang, Y., Luo, D., Hu, D., et al. (2025). Protective effects of 4-HBd on blood-brain barrier integrity in MCAO/R model rats based on brain pharmacokinetic characteristics. Front. Pharmacol. 16, 1528839. doi:10.3389/fphar.2025.1528839
Guan, J., Zhang, X., Feng, B., Zhao, D., Zhao, T., Chang, S., et al. (2017). Simultaneous determination of ferulic acid and gastrodin of Tianshu Capsule in rat plasma by ultra-fast liquid chromatography with tandem mass spectrometry and its application to a comparative pharmacokinetic study in normal and migraine rats. J. Sep. Sci. 40 (21), 4120–4127. doi:10.1002/jssc.201700665
Guo, Q. L., Wang, Y. N., Zhu, C. G., Chen, M. H., Jiang, Z. B., Chen, N. H., et al. (2015). 4-Hydroxybenzyl-substituted glutathione derivatives from Gastrodia elata. J. Asian Nat. Prod. Res. 17 (5), 439–454. doi:10.1080/10286020.2015.1040000
Ha, J. H., Shin, S. M., Lee, S. K., Kim, J. S., Shin, U. S., Huh, K., et al. (2001). In vitro effects of hydroxybenzaldehydes from Gastrodia elata and their analogues on GABAergic neurotransmission, and a structure-activity correlation. Planta Med. 67 (9), 877–880. doi:10.1055/s-2001-18844
Hardeland, R. (2021). Melatonin and Microglia. Int. J. Mol. Sci. 22 (15), 8296. doi:10.3390/ijms22158296
He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., et al. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson's disease. Food Funct. 12 (17), 7816–7824. doi:10.1039/d1fo00847a
Heese, K. (2020). Gastrodia elata blume (Tianma): hope for brain aging and Dementia. Evid. Based Complement. Altern. Med. 2020, 8870148. doi:10.1155/2020/8870148
Heo, J. C., Woo, S. U., Son, M., Park, J. Y., Choi, W. S., Chang, K. T., et al. (2007). Anti-tumor activity of Gastrodia elata Blume is closely associated with a GTP-Ras-dependent pathway. Oncol. Rep. 18 (4), 849–853. doi:10.3892/or.18.4.849
Hsieh, C. L., Tang, N. Y., Chiang, S. Y., Hsieh, C. T., and Lin, J. G. (1999). Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 65 (20), 2071–2082. doi:10.1016/s0024-3205(99)00473-7
Hsieh, C. L., Lin, J. J., Chiang, S. Y., Su, S. Y., Tang, N. Y., Lin, G. G., et al. (2007). Gastrodia elata modulated activator protein 1 via c-Jun N-terminal kinase signaling pathway in kainic acid-induced epilepsy in rats. J. Ethnopharmacol. 109 (2), 241–247. doi:10.1016/j.jep.2006.07.024
Hu, Y., Li, C., and Shen, W. (2014). Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer's disease. Neuropathology 34 (4), 370–377. doi:10.1111/neup.12115
Hu, H., Shen, X., Liao, B., Luo, L., Xu, J., and Chen, S. (2019). Herbgenomics: a stepping stone for research into herbal medicine. Sci. China Life Sci. 62 (7), 913–920. doi:10.1007/s11427-018-9472-y
Huang, T., Danaher, L. A., Brüschweiler, B. J., Kass, G. E. N., and Merten, C. (2019). Naturally occurring bisphenol F in plants used in traditional medicine. Arch. Toxicol. 93 (6), 1485–1490. doi:10.1007/s00204-019-02442-5
Huang, Y. J., Choong, L. X. C., Panyod, S., Lin, Y. E., Huang, H. S., Lu, K. H., et al. (2021). Gastrodia elata Blume water extract modulates neurotransmitters and alters the gut microbiota in a mild social defeat stress-induced depression mouse model. Phytother. Res. 35 (9), 5133–5142. doi:10.1002/ptr.7091
Hwang, S. M., Lee, Y. J., Kang, D. G., and Lee, H. S. (2009). Anti-inflammatory effect of Gastrodia elata rhizome in human umbilical vein endothelial cells. Am. J. Chin. Med. 37 (2), 395–406. doi:10.1142/S0192415X09006916
Jiang, T., Chu, J., Chen, H., Cheng, H., Su, J., Wang, X., et al. (2020). Gastrodin inhibits H(2)O(2)-Induced ferroptosis through its antioxidative effect in rat glioma cell line C6. Biol. Pharm. Bull. 43 (3), 480–487. doi:10.1248/bpb.b19-00824
Jiang, J., Shi, H., Jiang, S., Wang, A., Zou, X., Wang, Y., et al. (2023). Nutrition in Alzheimer's disease: a review of an underappreciated pathophysiological mechanism. Sci. China Life Sci. 66 (10), 2257–2279. doi:10.1007/s11427-022-2276-6
Khan, Z., Nath, N., Rauf, A., Emran, T. B., Mitra, S., Islam, F., et al. (2022). Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications. Chem. Biol. Interact. 365, 110117. doi:10.1016/j.cbi.2022.110117
Kim, K. J., Lee, O. H., Han, C. K., Kim, Y. C., and Hong, H. D. (2012). Acidic polysaccharide extracts from Gastrodia Rhizomes suppress the atherosclerosis risk index through inhibition of the serum cholesterol composition in Sprague Dawley rats fed a high-fat diet. Int. J. Mol. Sci. 13 (2), 1620–1631. doi:10.3390/ijms13021620
Kim, M. E., Na, J. Y., Park, Y. D., and Lee, J. S. (2019). Anti-Neuroinflammatory effects of Vanillin through the regulation of inflammatory factors and NF-κB signaling in LPS-Stimulated microglia. Appl. Biochem. Biotechnol. 187 (3), 884–893. doi:10.1007/s12010-018-2857-5
Kim, S., Choi, J. G., Kim, S. W., Park, S. C., Kang, Y. R., Park, D. S., et al. (2022). Inhibition of α-synuclein aggregation by MT101-5 is neuroprotective in mouse models of Parkinson's disease. Biomed. Pharmacother. 154, 113637. doi:10.1016/j.biopha.2022.113637
Kim, S., Yang, M., Ku, B., Cha, E., Seo, W., Son, I., et al. (2023). Efficacy of mecasin for treatment of amyotrophic lateral sclerosis: a phase IIa multicenter randomized double-blinded placebo-controlled trial. J. Ethnopharmacol. 315, 116670. doi:10.1016/j.jep.2023.116670
Kong, F., Buse, D. C., Geng, J., Xu, J., Liu, H., and Ma, S. (2022). Efficacy and tolerability of oral gastrodin for medication overuse headache (EASTERN): study protocol for a multicenter randomized double-blind placebo-controlled trial. Front. Neurol. 13, 1095298. doi:10.3389/fneur.2022.1095298
Lai, M. C. (2022). Hydroxybenzyl alcohol antagonized the ROS-Dependent JNK/Jun/Caspase-3 pathway to produce neuroprotection in a cellular model of Parkinson's Disease. Nutrients 14 (23). doi:10.3390/nu14235002
Lai, C. J., Yuan, Y., Liu, D. H., Kang, C. Z., Zhang, Y., Zha, L., et al. (2017). Untargeted metabolite analysis-based UHPLC-Q-TOF-MS reveals significant enrichment of p-hydroxybenzyl dimers of citric acids in fresh beige-scape Gastrodia elata (Wutianma). J. Pharm. Biomed. Anal. 140, 287–294. doi:10.1016/j.jpba.2017.03.055
Lee, J. Y., Jang, Y. W., Kang, H. S., Moon, H., Sim, S. S., and Kim, C. J. (2006). Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch. Pharm. Res. 29 (10), 849–858. doi:10.1007/BF02973905
Lee, Y. K., Woo, M. H., Kim, C. H., Kim, Y., Lee, S. H., Jeong, B. S., et al. (2007). Two new benzofurans from Gastrodia elata and their DNA topoisomerases I and II inhibitory activities. Planta Med. 73 (12), 1287–1291. doi:10.1055/s-2007-981619
Lee, Y. J., Hwang, S. M., Kang, D. G., Kim, J. S., and Lee, H. S. (2009). Effect of Gastrodia elata on tumor necrosis factor-alpha-induced matrix metalloproteinase activity in endothelial cells. J. Nat. Med. 63 (4), 463–467. doi:10.1007/s11418-009-0352-6
Lee, O. H., Kim, K. I., Han, C. K., Kim, Y. C., and Hong, H. D. (2012). Effects of acidic polysaccharides from gastrodia rhizome on systolic blood pressure and serum lipid concentrations in spontaneously hypertensive rats fed a high-fat diet. Int. J. Mol. Sci. 13 (1), 698–709. doi:10.3390/ijms13010698
Li, J. H. m.Y. X. q.L. H. (2012). Contents of gastrodin from different growth periods and different tissues of Hong Gastrodia in Dejiang County of Guizhou Province. Guizhou Agric. Sci. 40 (05), 43–44.
Li, M., and Qian, S. (2016). Gastrodin protects neural progenitor cells against amyloid β (1-42)-Induced neurotoxicity and improves hippocampal neurogenesis in amyloid β (1-42)-Injected mice. J. Mol. Neurosci. 60 (1), 21–32. doi:10.1007/s12031-016-0758-z
Li, Q., Niu, C., Zhang, X., and Dong, M. (2018). Gastrodin and isorhynchophylline synergistically inhibit MPP(+)-Induced oxidative stress in SH-SY5Y cells by targeting ERK1/2 and GSK-3β pathways: involvement of Nrf2 nuclear translocation. ACS Chem. Neurosci. 9 (3), 482–493. doi:10.1021/acschemneuro.7b00247
Li, S., Bian, L., Fu, X., Ai, Q., Sui, Y., Zhang, A., et al. (2019). Gastrodin pretreatment alleviates rat brain injury caused by cerebral ischemic-reperfusion. Brain Res. 1712, 207–216. doi:10.1016/j.brainres.2019.02.006
Liang, W. Z., Jan, C. R., and Hsu, S. S. (2017). Cytotoxic effects of gastrodin extracted from the rhizome of Gastrodia elata Blume in glioblastoma cells, but not in normal astrocytes, via the induction of oxidative stress-associated apoptosis that involved cell cycle arrest and p53 activation. Food Chem. Toxicol. 107 (Pt A), 280–292. doi:10.1016/j.fct.2017.07.013
Lin, L. C., Chen, Y. F., Tsai, T. R., and Tsai, T. H. (2007). Analysis of brain distribution and biliary excretion of a nutrient supplement, gastrodin, in rat. Anal. Chim. Acta 590 (2), 173–179. doi:10.1016/j.aca.2007.03.035
Lin, Y. E., Lin, S. H., Chen, W. C., Ho, C. T., Lai, Y. S., Panyod, S., et al. (2016). Antidepressant-like effects of water extract of Gastrodia elata Blume in rats exposed to unpredictable chronic mild stress via modulation of monoamine regulatory pathways. J. Ethnopharmacol. 187, 57–65. doi:10.1016/j.jep.2016.04.032
Lin, Y. E., Lin, C. H., Ho, E. P., Ke, Y. C., Petridi, S., Elliott, C. J., et al. (2021). Glial Nrf2 signaling mediates the neuroprotection exerted by Gastrodia elata Blume in Lrrk2-G2019S Parkinson's disease. Elife 10, e73753. doi:10.7554/eLife.73753
Lindsay, J., Laurin, D., Verreault, R., Hébert, R., Helliwell, B., Hill, G. B., et al. (2002). Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 156 (5), 445–453. doi:10.1093/aje/kwf074
Liu, Z., Wang, W., Feng, N., Wang, L., Shi, J., and Wang, X. (2016a). Parishin C's prevention of Aβ 1-42-induced inhibition of long-term potentiation is related to NMDA receptors. Acta Pharm. Sin. B 6 (3), 189–197. doi:10.1016/j.apsb.2016.03.009
Liu, B., Li, F., Shi, J., Yang, D., Deng, Y., and Gong, Q. (2016b). Gastrodin ameliorates subacute phase cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis in rats. Mol. Med. Rep. 14 (5), 4144–4152. doi:10.3892/mmr.2016.5785
Liu, J., Chen, S., Cheng, J., Zhang, J., Wang, Y., and Liu, A. (2017). An optimized and sensitive pharmacokinetic quantitative method of investigating gastrodin, Parishin, and Parishin B, C and E in beagle dog plasma using LC-MS/MS after intragastric administration of tall Gastrodia Capsules. Molecules 22 (11), 1938. doi:10.3390/molecules22111938
Liu, Y., Gao, J., Peng, M., Meng, H., Ma, H., Cai, P., et al. (2018). A review on central nervous System effects of gastrodin. Front. Pharmacol. 9, 24. doi:10.3389/fphar.2018.00024
Liu, M., Zhao, L., Han, L., Li, H., Shi, Y., Cui, J., et al. (2020). Discovery and identification of proangiogenic chemical markers from Gastrodiae Rhizoma based on zebrafish model and metabolomics approach. Phytochem. Anal. 31 (6), 835–845. doi:10.1002/pca.2949
Liu, P., Zhao, Z., Zhang, H., Xiao, C., Wang, M., Yang, C., et al. (2025). A comprehensive pharmacology study reveals the molecular mechanisms underlying the antidepressant effects of Gastrodiae Rhizoma. Phytomedicine 142, 156761. doi:10.1016/j.phymed.2025.156761
Lu, C., Qu, S., Zhong, Z., Luo, H., Lei, S. S., Zhong, H. J., et al. (2022). The effects of bioactive components from the rhizome of gastrodia elata blume (Tianma) on the characteristics of Parkinson's disease. Front. Pharmacol. 13, 963327. doi:10.3389/fphar.2022.963327
Luo, Y., Chen, P., Yang, L., and Duan, X. (2022). Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Gastrodia elata Blume in the treatment of ischemic stroke. Exp. Ther. Med. 24 (6), 742. doi:10.3892/etm.2022.11678
Martins, J., and S, B. (2018). Phytochemistry and pharmacology of anti-depressant medicinal plants: a review. Biomed. Pharmacother. 104, 343–365. doi:10.1016/j.biopha.2018.05.044
Mi, Y., Wang, M., Liu, M., Cheng, H., and Li, S. (2020). Pharmacokinetic comparative study of GAS with different concentration of tetramethylpyrazine and ferulic acid on liver-yang hyperactivity migraine model by blood-brain microdialysis method. J. Pharm. Biomed. Anal. 191, 113643. doi:10.1016/j.jpba.2020.113643
Nepal, M. R., Jeong, K. S., Kim, G. H., Cha, D. H., Kang, M. J., Kim, J. S., et al. (2019). Role of intestinal microbiota in metabolism of gastrodin in vitro and in vivo. Metabolites 9 (4), 69. doi:10.3390/metabo9040069
Ng, C. F., Ko, C. H., Koon, C. M., Xian, J. W., Leung, P. C., Fung, K. P., et al. (2013). The aqueous extract of rhizome of Gastrodia elata protected drosophila and PC12 cells against beta-amyloid-induced neurotoxicity. Evid. Based Complement. Altern. Med. 2013, 516741. doi:10.1155/2013/516741
Ng, C. F., Ko, C. H., Koon, C. M., Chin, W. C., Kwong, H. C. S. T., Lo, A. W. I., et al. (2016). The aqueous extract of rhizome of Gastrodia elata Blume attenuates locomotor defect and inflammation after traumatic brain injury in rats. J. Ethnopharmacol. 185, 87–95. doi:10.1016/j.jep.2016.03.018
Pyo, M. K., Jin, J. L., Koo, Y. K., and Yun-Choi, H. S. (2004). Phenolic and furan type compounds isolated from Gastrodia elata and their anti-platelet effects. Arch. Pharm. Res. 27 (4), 381–385. doi:10.1007/BF02980077
Qiu, F., Liu, T. T., Qu, Z. W., Qiu, C. Y., Yang, Z., and Hu, W. P. (2014). Gastrodin inhibits the activity of acid-sensing ion channels in rat primary sensory neurons. Eur. J. Pharmacol. 731, 50–57. doi:10.1016/j.ejphar.2014.02.044
Russo, A. F., and Hay, D. L. (2023). CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol. Rev. 103 (2), 1565–1644. doi:10.1152/physrev.00059.2021
Salau, V. F., Erukainure, O. L., Ibeji, C. U., Olasehinde, T. A., Koorbanally, N. A., and Islam, M. S. (2020). Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe(2+)-induced brain tissues damage. Metab. Brain Dis. 35 (5), 727–738. doi:10.1007/s11011-020-00545-y
Schloss, J., Ryan, K., and Steel, A. (2021). A randomised, double-blind, placebo-controlled clinical trial found that a novel herbal formula urox® (Bedtime buddy®) assisted children for the treatment of nocturnal enuresis. Phytomedicine 93, 153783. doi:10.1016/j.phymed.2021.153783
Shi, R., Zheng, C. B., Wang, H., Rao, Q., Du, T., Bai, C., et al. (2020). Gastrodin alleviates vascular dementia in a 2-VO-Vascular dementia rat model by altering amyloid and tau levels. Pharmacology 105 (7-8), 386–396. doi:10.1159/000504056
Shi, X., Yu, X., Yang, L., and Duan, X. (2023). Ethyl acetate extract of Gastrodia elata protects Caenorhabditis elegans from oxidative stress and amyloid β peptide toxicity. Exp. Ther. Med. 26 (2), 405. doi:10.3892/etm.2023.12104
Shu, G., Yang, T., Wang, C., Su, H., and Xiang, M. (2013). Gastrodin stimulates anticancer immune response and represses transplanted H22 hepatic ascitic tumor cell growth: involvement of NF-κB signaling activation in CD4+ T cells. Toxicol. Appl. Pharmacol. 269 (3), 270–279. doi:10.1016/j.taap.2013.02.019
Song, J. Z., Li, L. J., Ji, L., Shun, L., and Rui, Y. (2017). The pharmacokinetics of Tiangou antihypertensive capsule in rat in vivo. Biomed. Rep. 6 (1), 113–119. doi:10.3892/br.2016.810
Song, W., Zhao, B., Wu, Q., Gong, Y., Jia, Y., Zhang, Y., et al. (2024). Gastrodin alleviates diabetic peripheral neuropathy by regulating energy homeostasis via activating AMPK and inhibiting MMP9. Phytomedicine 135, 156033. doi:10.1016/j.phymed.2024.156033
Sulistio, Y. A., and Heese, K. (2016). The ubiquitin-proteasome System and molecular chaperone deregulation in Alzheimer's Disease. Mol. Neurobiol. 53 (2), 905–931. doi:10.1007/s12035-014-9063-4
Sun, X., Jia, B., Sun, J., Lin, J., Lu, B., Duan, J., et al. (2023). Gastrodia elata Blume: a review of its mechanisms and functions on cardiovascular systems. Fitoterapia 167, 105511. doi:10.1016/j.fitote.2023.105511
Tang, C., Wang, L., Liu, X., Cheng, M., Qu, Y., and Xiao, H. (2015). Comparative pharmacokinetics of gastrodin in rats after intragastric administration of free gastrodin, parishin and Gastrodia elata extract. J. Ethnopharmacol. 176, 49–54. doi:10.1016/j.jep.2015.10.007
Tang, C. L., Wang, L., Cheng, M., Liu, X., and Xiao, H. (2015). Determination of N6-(4-hydroxybenzyl)adenine riboside in rat plasma by ultra-performance liquid chromatography quadrupole time of flight mass spectrometry. Chin. J. Chromatogr. 33 (07), 699–703. doi:10.3724/sp.j.1123.2015.03017
Tang, D., Chen, K., Huang, L., and Li, J. (2017). Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 13 (3), 323–330. doi:10.1080/17425255.2017.1251903
Tian, J., Shi, J., Wei, M., Li, T., Ni, J., Zhang, X., et al. (2018). Efficacy and safety of Tianmabianchunzhigan in mild to moderate vascular dementia: protocol of a randomized controlled IIa trial. Med. Baltim. 97 (51), e13760. doi:10.1097/MD.0000000000013760
Tian, J., Qin, S., Han, J., Meng, J., and Liang, A. (2022). A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 289, 114984. doi:10.1016/j.jep.2022.114984
Wang, Q., Chen, G., and Zeng, S. (2008). Distribution and metabolism of gastrodin in rat brain. J. Pharm. Biomed. Anal. 46 (2), 399–404. doi:10.1016/j.jpba.2007.10.017
Wang, P. H., Zhao, L. X., Wan, J. Y., Zhang, L., Mao, X. N., Long, F. Y., et al. (2016a). Pharmacological characterization of a novel gastrodin derivative as a potential anti-migraine agent. Fitoterapia 109, 52–57. doi:10.1016/j.fitote.2015.12.007
Wang, X. S., Tian, Z., Zhang, N., Han, J., Guo, H. l., Zhao, M. g., et al. (2016b). Protective effects of gastrodin against autophagy-mediated astrocyte death. Phytother. Res. 30 (3), 386–396. doi:10.1002/ptr.5538
Wang, Z. W., Li, Y., Liu, D. H., Mu, Y., Dong, H. J., Zhou, H. L., et al. (2019). Four new phenolic constituents from the rhizomes of Gastrodia elata Blume. Nat. Prod. Res. 33 (8), 1140–1146. doi:10.1080/14786419.2018.1460836
Wu, Y. N., Wen, S. H., Zhang, W., Yu, S. S., Yang, K., Liu, D., et al. (2023). A comprehensive review of its traditional use, botany, phytochemistry, pharmacology, and pharmacokinetics. Evid. Based Complement. Altern. Med. 5606021. doi:10.1155/2023/560602
Xiao, M. M., Zhang, Y. Q., Wang, W. T., Han, W. J., Lin, Z., Xie, R. G., et al. (2016). Gastrodin protects against chronic inflammatory pain by inhibiting spinal synaptic potentiation. Sci. Rep. 6, 37251. doi:10.1038/srep37251
Xu, H., and Xu, H. E. (2009). Analysis of trace elements in Chinese therapeutic foods and herbs. Am. J. Chin. Med. 37 (4), 625–638. doi:10.1142/S0192415X09007119
Xu, H., Ruan, L. Y., Chen, C., Fan, J. T., Chen, J. F., Zhao, W. L., et al. (2020). Therapeutic assessment of fractions of Gastrodiae Rhizoma on chronic atrophic gastritis by (1)H NMR-based metabolomics. J. Ethnopharmacol. 254, 112403. doi:10.1016/j.jep.2019.112403
Yan, M. L. Y. Q. (2024). Study on transport of brain-protective components of Gastrodia elata across BBB. Chin. J. Hosp. Pharm. 44 (10), 1132–1138. doi:10.13286/j.1001-5213.2024.10.04
Yan, X., Liu, D. F., Zhang, X. Y., Liu, D., Xu, S. Y., Chen, G. X., et al. (2017). Vanillin protects dopaminergic neurons against inflammation-mediated cell death by inhibiting ERK1/2, P38 and the NF-κB signaling pathway. Int. J. Mol. Sci. 18 (2), 389. doi:10.3390/ijms18020389
Yang, F., Li, G., Lin, B., and Zhang, K. (2022). Gastrodin suppresses pyroptosis and exerts neuroprotective effect in traumatic brain injury model by inhibiting NLRP3 inflammasome signaling pathway. J. Integr. Neurosci. 21 (2), 72. doi:10.31083/j.jin2102072
Yu, H. (2022). Research progress on classification of chemical constituents from GE and their pharmacological effects. Chin. Traditional Herb. Drugs 53 (17), 5553–5564. doi:10.7501/j.issn.0253-2670.2022.17.033
Yu, Y., Tang, L., Cui, F., Jiao, F., Zhang, D., Ma, J., et al. (2021). Effect of Qizhitongluo capsule on lower limb rehabilitation after stroke: a randomized clinical trial. Pharmacol. Res. 165, 105464. doi:10.1016/j.phrs.2021.105464
Yun-Choi, H. S., Pyo, M. K., and Park, K. M. (1998). Isolation of 3-O-(4'-hydroxybenzyl)-beta-sitosterol and 4-[4′-(4″-hydroxybenzyloxy)benzyloxy]benzyl methyl ether from fresh tubers of Gastrodia elata. Arch. Pharm. Res. 21 (3), 357–360. doi:10.1007/BF02975302
Zeng, X., Li, J., Chen, T., Li, Y., and Guo, S. (2023). Global metabolic profile and multiple phytometabolites in the different varieties of Gastrodia elata Blume. Front. Plant Sci. 14, 1249456. doi:10.3389/fpls.2023.1249456
Zhan, H. D., Zhou, H. Y., Sui, Y. P., Du, X. L., Wang, W. H., Dai, L., et al. (2016). The rhizome of Gastrodia elata Blume - an ethnopharmacological review. J. Ethnopharmacol. 189, 361–385. doi:10.1016/j.jep.2016.06.057
Zhang, Q. Y. H., Yang, Y. m., and Yu, G. y. (2008). Effects of gastrodin injection on blood pressure and vasoactive substances in the treatment of elderly patients with refractory hypertension: a randomized controlled trial. J. Integr. Med. 6 (07), 695–699. doi:10.3736/jcim20080707
Zhang, Z., Ma, P., Xu, Y., Zhan, M., Zhang, Y., Yao, S., et al. (2011). Preventive effect of gastrodin on cognitive decline after cardiac surgery with cardiopulmonary bypass: a double-blind, randomized controlled study. J. Huazhong Univ. Sci. Technol. Med. Sci. 31 (1), 120–127. doi:10.1007/s11596-011-0162-4
Zhang, Y., Li, M., Kang, R. X., Shi, J. G., Liu, G. T., and Zhang, J. J. (2012). NHBA isolated from Gastrodia elata exerts sedative and hypnotic effects in sodium pentobarbital-treated mice. Pharmacol. Biochem. Behav. 102 (3), 450–457. doi:10.1016/j.pbb.2012.06.002
Zhang, Z. C., Su, G., Li, J., Wu, H., and Xie, X. D. (2013). Two new neuroprotective phenolic compounds from Gastrodia elata. J. Asian Nat. Prod. Res. 15 (6), 619–623. doi:10.1080/10286020.2013.791286
Zhang, J. S., Zhou, S. F., Wang, Q., Guo, J. N., Liang, H. M., Deng, J. B., et al. (2016). Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2α pathway in Alzheimer's disease. Neuroscience 325, 1–9. doi:10.1016/j.neuroscience.2016.03.024
Zhang, H., Yuan, B., Huang, H., Qu, S., Yang, S., and Zeng, Z. (2018). Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation. Braz J. Med. Biol. Res. 51 (10), e7439. doi:10.1590/1414-431X20187439
Zhang, D. Y., Cheng, Y. B., Guo, Q. H., Shan, X. L., Wei, F. F., Lu, F., et al. (2020). Treatment of masked hypertension with a Chinese herbal formula: a randomized, placebo-controlled trial. Circulation 142 (19), 1821–1830. doi:10.1161/CIRCULATIONAHA.120.046685
Zhao, Z., Guo, P., and Brand, E. (2012). The formation of daodi medicinal materials. J. Ethnopharmacol. 140 (3), 476–481. doi:10.1016/j.jep.2012.01.048
Zhao, W., Wang, J., Latta, M., Wang, C., Liu, Y., Ma, W., et al. (2022). Rhizoma gastrodiae water extract modulates the Gut Microbiota and pathological changes of P-Tau(Thr231) to protect against cognitive impairment in mice. Front. Pharmacol. 13, 903659. doi:10.3389/fphar.2022.903659
Zhou, C., He, M., Peng, C., Yu, J., Li, Z., Zhou, M., et al. (2020). Pharmacokinetic and lipidomic assessment of the in vivo effects of Parishin A-Isorhynchophylline in Rat migraine models. J. Anal. Methods Chem. 2020, 9101598. doi:10.1155/2020/9101598
Zhou, H. B., Lu, S. Z., Yu, Z. S., Zhang, J. L., and Mei, Z. N. (2024). Mechanisms for the biological activity of Gastrodia elata Blume and its constituents: a comprehensive review on sedative-hypnotic, and antidepressant properties. Phytomedicine 123, 155251. doi:10.1016/j.phymed.2023.155251
Zhu, M., Deng, W., Di, S., Qin, M., Liu, D., and Yi, B. (2018). Gastrodin protects cardiomyocytes from Anoxia/Reoxygenation injury by 14-3-3η. Oxid. Med. Cell Longev. 2018, 3685391. doi:10.1155/2018/3685391
Zhu, H., Liu, C., Hou, J., Long, H., Wang, B., Guo, D., et al. (2019). Gastrodia elata blume polysaccharides: a review of their acquisition, analysis, modification, and pharmacological activities. Molecules 24 (13), 2436. doi:10.3390/molecules24132436
Zhu, H., Wu, X., Huo, J., Hou, J., Long, H., Zhang, Z., et al. (2021). A five-dimensional data collection strategy for multicomponent discovery and characterization in Traditional Chinese Medicine: Gastrodia Rhizoma as a case study. J. Chromatogr. A 1653, 462405. doi:10.1016/j.chroma.2021.462405
Zuowei, T. t.Z. x.D. (2017). The research of efficacy and mechanism of gastrodin combined with betahistine mesylate in the treatment of posterior circulation ischemic dizziness. J. Chin. Med. Material 40 (11), 2706–2709. doi:10.13863/j.issn1001-4454.2017.11.047
Glossary
5-HT 5-Hydroxytryptamine
6-OHDA 6-Hydroxydopamine hydrobromide
A/R Anoxia/Reoxygenation
ACSL4 Acyl-CoA synthetase long-chain family member 4
AF4 Asymmetrical Flow Field-Flow Fractionation
APP Amyloid β precursor protein
BDNF Brain derived neurotrophic factor
BMP-2 Morphogenetic protein 2
CDK1/CDC2 Cyclin-dependent kinases 1/2
c-eEPSCs C-fiber evoked EPSCs
CGRP Calcitonin gene-related peptide
CIRI Cerebral ischemia-reperfusion injury
COX2 Cyclooxygenase-2
DA Dopamine
dRI Differential refractive index
EPSCs Excitatory postsynaptic currents
FPN1 Ferroportin 1
GABA γ-aminobutyric acid
GE Gastrodia elata
GSDMD Gasdermin D
HBA p-Hydroxybenzyl alcohol
HDL-C High cholesterol
HPA Hypothalamic-pituitary-adrenal
I/R Ischemia-reperfusion
ICAM-1 Intercellular cell adhesion molecule-1
IDO 1 Indoleamine 1
ISVs Intersegmental vessels
KA Kainic acid
LDL-C Lipoprotein cholesterol
LPS Lipopolysaccharide
LTP Long-term potentiation
MALS Multi-angle light scattering
MAO-A Monoamine oxidase
MCAO Middle cerebral artery occlusion
MCP-1 Macrophage chemotactic protein-1
MDA Malondialdehyde
MPO Myeloperoxidase
MT1/2 Melatonin 1/Melatonin 2
NFATc1 Nuclear factor of activated T cells cytoplasmic 1
NFTs Neurofibrillary tangles
NHBA N6-(4-hydroxybenzyl) adenine riboside
NLRP3 NOD-like receptor thermal protein domain associated protein 3
NMDAR N-methy-D-aspartate receptors
NSCs Neural stem cells
NTG Nitroglycerin
OCN Osteocalcin
OGD/R Oxygen-glucose deprivation reperfusion
ROS Reactive oxygen species
SHR Spontaneously hypertensive rats
TBI Traumatic brain injury
TC High cholesterol
TH Tyrosine hydroxylase
TPH 2 Tryptophan hydroxylase 2
UCMS Unpredictable chronic mild stress
Vanillin 4-hydroxy-3-methoxybenzaldehyde
VCAM-1 Vascular Cell Adhesion Molecule 1
WGEW Water-soluble polysaccharide extracted from Gastrodia elata
WSS25 A sulfated polysaccharide extracted from the rhizome of Gastrodia elata that binds to bone morphogenetic protein 2
Keywords: Gastrodia elata, biological activity, chemical composition, pharmacological effects, treatment ofdiseases, pharmacokinetics
Citation: Zhou X, Han M, Yan S, Wang M, Zu S and Zhong S (2025) From molecules to medicine: a systematic review of Gastrodia elata’s bioactive metabolites and therapeutic potential. Front. Pharmacol. 16:1641443. doi: 10.3389/fphar.2025.1641443
Received: 05 June 2025; Accepted: 20 October 2025;
Published: 07 November 2025.
Edited by:
Fabien Schultz, Bernhard Nocht Institute for Tropical Medicine (BNITM), GermanyReviewed by:
Adnan Amin, Yeungnam University, Republic of KoreaYuxiang Fei, China Pharmaceutical University, China
Copyright © 2025 Zhou, Han, Yan, Wang, Zu and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenghui Zhong, emhvbmdzaDE5ODhAMTI2LmNvbQ==
†These authors contributed equally to this work
 Xiantai Zhou1†
Xiantai Zhou1† Mengyuan Wang
Mengyuan Wang Shenghui Zhong
Shenghui Zhong