- 1Department of Clinical Pharmacology, Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Department of Oncology, Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
The integration of nanotechnology into oncology has profoundly reshaped cancer treatment, enabling drug delivery systems with remarkable precision, enhancing antitumor efficacy, and simultaneously addressing major challenges such as cardiotoxicity, one of the most prevalent and serious adverse effects of conventional chemotherapy. This review systematically examines the dual role of nanotechnology, highlighting its capacity to enhance the therapeutic effectiveness of anticancer treatments while concurrently mitigating cardiotoxic side effects. The discussion centers on a broad spectrum of nanocarrier platforms, such as liposome-based, polymeric nanocarriers, and inorganic nanocarriers organized according to their structural features and therapeutic benefits, thereby enabling a systematic comparison with conventional drug delivery strategies. By improving drug bioavailability, enabling controlled release, and achieving precise tumor-specific targeting, these nanocarrier systems enhance antitumor efficacy while concurrently reducing collateral damage to healthy tissues. Moreover, recent preclinical and clinical studies were summarized to demonstrate substantial advances in this interdisciplinary field, while also identifying persistent challenges that remain to be addressed. Finally, the review explores future directions, with particular emphasis on the integration of artificial intelligence to optimize nanocarrier design and the promise of personalized nanomedicine in transforming cancer care. Overall, this work provides a critical foundation for advancing next-generation, patient-tailored cancer therapies.
1 Introduction
Cancer continues to represent the leading cause of disease-related morbidity and mortality worldwide. By 2022, approximately 20 million new cancer cases were diagnosed globally, nearly seven million more than in 2020, underscoring the accelerating global cancer burden (Sung et al., 2021; Bray et al., 2024). Although substantial progress has been achieved, illustrated by a 15% increase in the 5-year survival rate of patients in China over the past decade and a half, the unintended consequences of treatment are becoming increasingly evident, raising serious concerns regarding the long-term survivorship of cancer patients (Siegel et al., 2024). Within these complications, cardiotoxicity has emerged as one of the most pressing challenges in oncology. Recent evidence suggests that over 40% of patients receiving chemotherapy experience cardiotoxic effects, making chemotherapy-induced cardiotoxicity (CIC) not a transient complication but a critical determinant of long-term quality of life (López-Sendón et al., 2020; Christidi and Brunham, 2021; Kong et al., 2022). Mechanistically, CIC arises from reactive oxygen species–induced mitochondrial injury, calcium dysregulation, and ferroptosis, manifesting clinically in a spectrum of conditions from arrhythmias to overt heart failure (Tai et al., 2023). This dual challenge, namely, sustaining durable tumor control while simultaneously protecting the cardiovascular system, underscores the urgent need for innovative therapeutic strategies that can preserve oncological efficacy while safeguarding cardiac health (Chen et al., 2022; Su et al., 2022; Cejas et al., 2024).
Nanotechnology provides a transformative strategy for drug delivery by overcoming many of the inherent limitations of conventional chemotherapy. Distinct from traditional nanomedicine that emphasizes tumor targeting alone, cancer nanocardiology advances a dual-functional paradigm that integrates tumor suppression with cardio-protection within a single nanoplatform (Lu et al., 2024). By encapsulating chemotherapeutic drugs, nanocarrier systems enhance solubility (Zeng et al., 2023), enhancing stability (Du et al., 2024), and increasing bioavailability (Itoo et al., 2024). By optimizing pharmacokinetic profiles, these systems enable tumor-specific delivery while minimizing off-target exposure (Lee et al., 2021; Wang et al., 2021; Zhu et al., 2023). Various nanocarriers, including liposomes (Su et al., 2022), polymeric nanocarriers (Feng et al., 2022), dendrimers (Dey et al., 2022), and inorganic nanomaterials (Pei et al., 2023) have demonstrated strong potential for precise drug delivery. Many of these systems can be engineered to achieve stimuli-responsive release triggered by pH, temperature, or enzymatic changes within the tumor microenvironment (Zhang J. et al., 2023). In addition, functionalization with targeting ligands or monoclonal antibodies further improves tumor specificity, markedly reducing the risk of cardiotoxicity (Su et al., 2022; Nevins et al., 2024).
Thus, the objective of this review is to offer a comprehensive synthesis of current applications of nanotechnology in cancer therapy, with particular emphasis on its capacity to improve therapeutic efficacy while simultaneously mitigating cardiotoxic side effects. It further examines recent advances in nanocarrier design and evaluates their translational potential across both preclinical and clinical settings. By synthesizing these innovations, the review seeks to elucidate the ways in which nanotechnology may reshape conventional cancer treatment paradigms and ultimately facilitate the development of safer and more effective therapeutic strategies.
2 Types and functions of nanocarriers
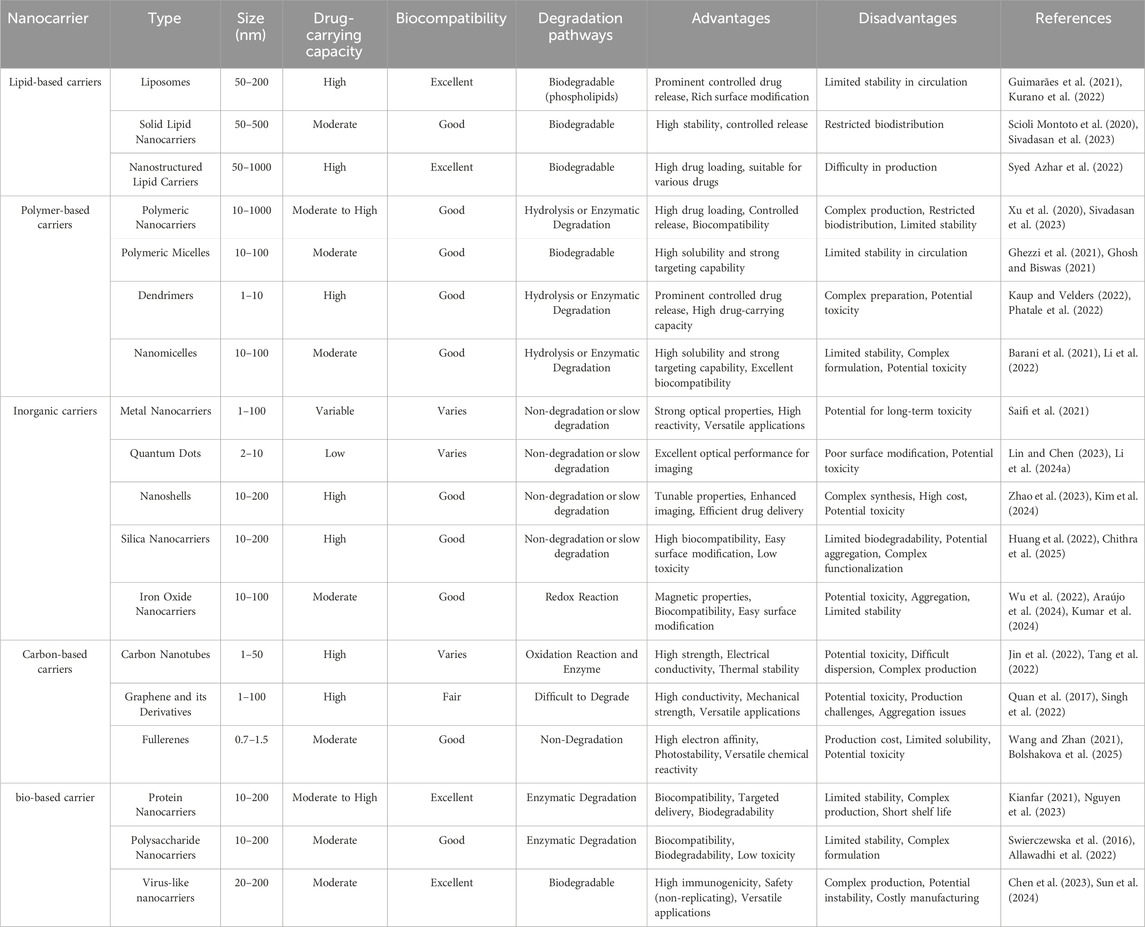
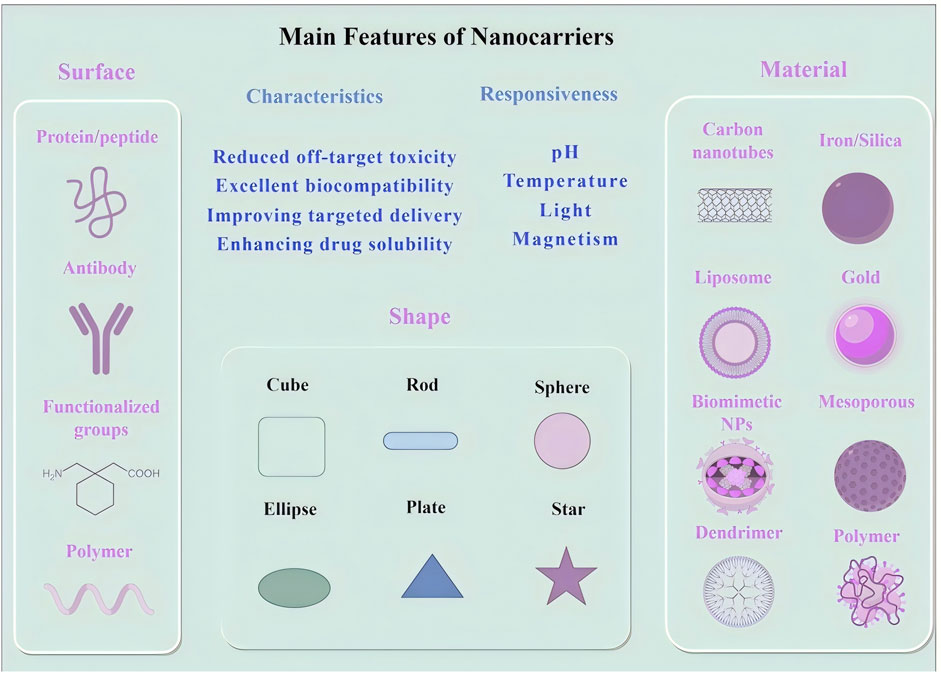
Nanotechnology has introduced innovative drug delivery strategies that are transforming cancer treatment by improving drug stability, solubility, and bioavailability. A diverse range of nanocarriers, including liposomes, polymeric nanocarriers, inorganic nanocarriers, and carbon-based materials, have been developed to enhance the precision and efficiency of oncological drug delivery (Garbayo et al., 2020; Vazhappilly et al., 2021) (Table 1 summarizes the characteristics of different nanocarrier types). Among these, liposomes constitute one of the earliest and most extensively utilized nanocarrier systems. Liposomes, composed of a phospholipid bilayer, can encapsulate both hydrophilic and hydrophobic agents. Their intrinsic ability to fuse with cellular membranes enables direct transport of therapeutic agents into tumor cells (Zhou et al., 2016; Vazhappilly et al., 2021). Polymeric nanocarriers, often synthesized from biodegradable polymers such as poly (lactic-co-glycolic acid) (PLGA), enable controlled drug release at tumor sites, thereby maintaining therapeutic concentrations while reducing systemic toxicity (Lin et al., 2023; Beach et al., 2024). Inorganic nanocarriers, such as gold-based or silica-based systems, exhibit unique physicochemical properties that enable their application in both therapeutic and diagnostic modalities (theranostics). For example, gold nanocarriers are particularly effective in photothermal therapy (Yang et al., 2020; Miao et al., 2022; Hirschbiegel et al., 2023). Carbon-based nanocarriers, such as carbon nanotubes and fullerenes, are structurally robust and capable of penetrating dense tissue matrices, thereby facilitating drug delivery into deep-seated tumors (Tang et al., 2021; Kaurav et al., 2023). Moreover, nanocarriers derived from natural biomaterials, such as protein-based nanocarriers and virus-like nanocarriers (VLPs), mimic viral architectures to promote cellular uptake. These carriers are biodegradable and display low immunogenicity, rendering them promising candidates for clinical translation (Zhang et al., 2017; Habibi et al., 2022; Tenchov et al., 2022) (Figure 1 illustrates the principal features of different nanocarrier types).
To further improve specificity, targeting ligands or monoclonal antibodies may be conjugated to the surface of nanocarriers, thereby enabling active targeting of tumor tissues while sparing healthy organs, especially the heart. This targeted approach is particularly valuable for stimulus-responsive drug release triggered by specific cues within the tumor microenvironment, such as alterations in pH or temperature, thereby maximizing therapeutic efficacy while minimizing off-target toxicity.
3 The role of nanotechnology in reducing cardiotoxicity in antitumor therapy
Nanotechnology enhances the efficacy of antitumor therapies by enabling targeted drug delivery, controlled release, and multimodal treatment strategies, thereby overcoming many limitations associated with conventional regimens.
3.1 Pathophysiology of cardiotoxicity induced by antitumor therapy
CIC encompasses a wide range of structural and functional cardiac complications-most notably heart failure (HF), arrhythmias, myocardial ischemia, and coronary artery disease (Carrasco et al., 2021; Li et al., 2021). These complications are frequently severe and potentially life-threatening, with HF representing the most critical clinical manifestation. Major contributors include anthracyclines (e.g., doxorubicin, DOX), targeted therapies (e.g., trastuzumab), and immune checkpoint inhibitors (Zhang et al., 2015). The pathophysiology is multifactorial, characterized by oxidative stress, mitochondrial dysfunction, and impaired cardiomyocyte signaling (Yang et al., 2023), as shown in Figure 2. DOX, for example, drives excessive ROS generation that results in DNA damage, lipid peroxidation, apoptosis or necrosis, and severe disruption of mitochondrial energy metabolism (Ding et al., 2023). Trastuzumab, in contrast, disrupts mitochondrial biogenesis and function through ErbB2 inhibition, thereby suppressing essential survival pathways and precipitating contractile dysfunction (Ye et al., 2023).
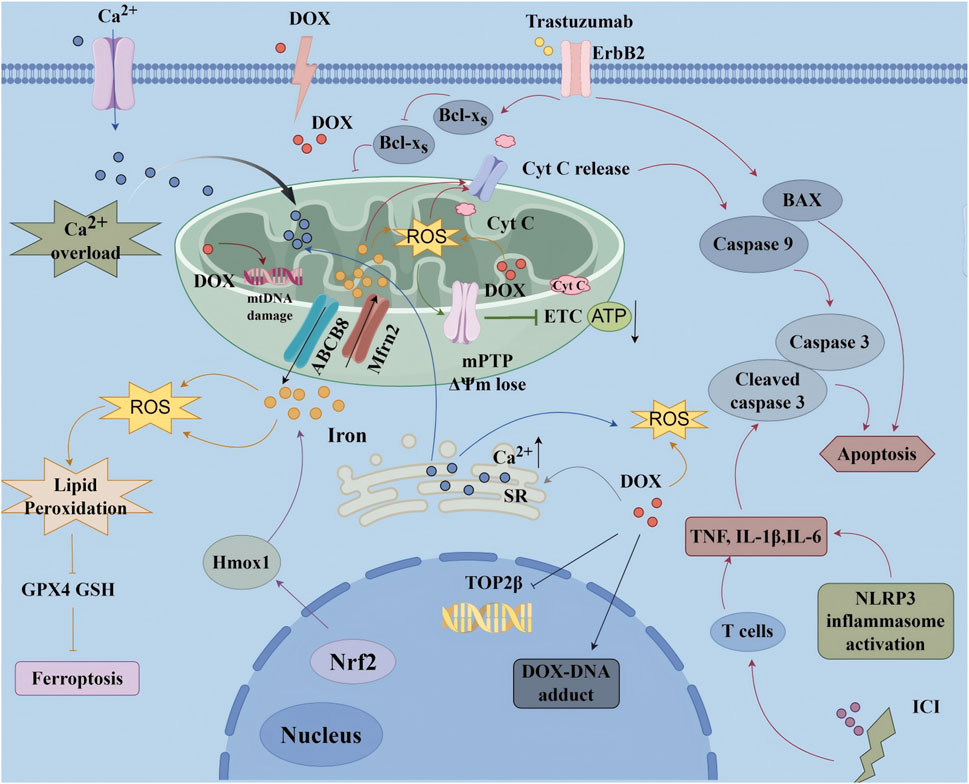
Figure 2. Molecular mechanism of chemotherapy-induced cardiotoxicity ATP, Adenosine Triphosphate; BAX, Bcl-2 Associated X Protein; Cyt C, Cytochrome C; DOX, Doxorubicin; ΔΨm, Mitochondrial Membrane Potential; ErbB2, Epidermal Growth Factor Receptor 2; ETC., Electron Transport Chain; GPX4, Glutathione Peroxidase 4; GSH, Glutathione; ICI, Immune Checkpoint Inhibitors; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; mPTP, Mitochondrial Permeability Transition Pore; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; ROS, Reactive Oxygen Species; TNF-α, Tumor Necrosis Factor-alpha.
Recent studies have highlighted ferroptosis as a central mechanism contributing to CIC. DOX, along with agents such as cisplatin and sorafenib, disrupts iron homeostasis, suppresses GPX4 and GSH, and activates ACSL4, collectively leading to iron overload, lipid peroxidation, and cardiomyocyte ferroptosis (Ding et al., 2023). Trastuzumab-induced activation of SLC7A11 appears to further sensitize cardiomyocytes to ferroptotic death (Zhang et al., 2023). Inflammation emerges as another critical factor: anticancer agents activate cardiac macrophages and recruit circulating monocytes, neutrophils, and T cells, which in turn release TNF-α, IL-1β, IL-6, chemokines, and reactive species, thereby exacerbating cardiomyocyte injury, fibrosis, and adverse remodeling. Notably, immune checkpoint inhibitors may provoke autoimmune-like myocarditis characterized by extensive T-cell infiltration (Wei et al., 2021; Zhao et al., 2025).
Another hallmark of CIC is the disruption of Ca2+ homeostasis. Anthracyclines and related agents impair SR Ca2+ reuptake through SERCA2a dysfunction and promote Ca2+ leakage via RyR2 channels, thereby inducing cytosolic Ca2+ overload. This disruption interferes with excitation-contraction coupling, facilitates arrhythmogenesis, and provokes ER stress with subsequent UPR activation, ultimately culminating in apoptosis (Ayza et al., 2020; Wei et al., 2021; Dridi et al., 2023; Li W. et al., 2024). Although cardioprotective strategies such as dexrazoxane, β-blockers, and ACEi/ARB have been developed, CIC persists as a formidable clinical challenge. Its multifaceted mechanisms limit optimal oncologic dosing and regimens, while also compromising long-term survivorship (Wei et al., 2021). These challenges underscore the urgent need for mechanism-driven innovations-such as rationally designed nanomaterials-that can selectively target ferroptosis and inflammation without undermining antitumor efficacy.
3.2 Applications of nanotechnology in reducing cardiotoxicity
Nanocarriers, including liposomes and polymeric or inorganic nanocarriers, significantly mitigate chemotherapeutic cardiotoxicity through selective drug delivery. These carriers reduce nonspecific drug accumulation in myocardial tissue by means of physical optimization and surface modification with targeting ligands (Zhao et al., 2020; Vazhappilly et al., 2021). This approach represents an innovative strategy for cardio-protection during chemotherapy (Garbayo et al., 2020).
3.2.1 Promoting targeted drug delivery
Nanocarriers employ both passive and active targeting mechanisms. Passive targeting is mediated by the enhanced permeability and retention (EPR) effect, which allows nanocarriers of 10–200 nm in size to preferentially accumulate in tumor tissues due to their leaky vasculature (Allawadhi et al., 2022; Singh et al., 2022; Chen et al., 2023; Sun et al., 2024), as shown in Figure 3. This selective distribution reduces systemic exposure, minimizes off-target toxicity, and enhances therapeutic efficacy. For instance, stimuli-responsive nanocarriers have been designed to release their drug payload in response to the acidic tumor microenvironment. These carriers remain stable under physiological pH (7.0) but release drugs efficiently at lower pH (5.0–6.5) (Liu et al., 2014). A dextran–DOX conjugate, for example, released only 11% of its payload at pH 7.4, compared to 96% at pH 4.0 (Behera and Padhi, 2022). This controlled release improves drug efficacy at tumor sites while protecting healthy tissues, including cardiomyocytes (Zhang et al., 2020; Yu et al., 2021).
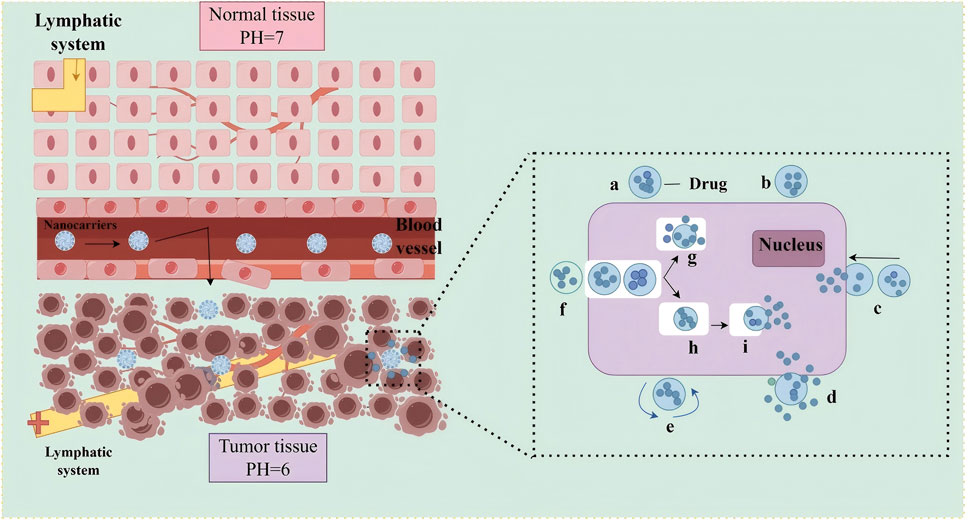
Figure 3. Schematic illustration of passive targeting drug delivery using nanocarriers. Left: Microvascular permeability comparison between normal and tumor tissues. In normal tissues (top), intact endothelial junctions and functional lymphatic drainage prevent nanonanocarrier extravasation. In tumor tissues (bottom), structurally abnormal vasculature with enlarged endothelial gaps (red arrows) and impaired lymphatic system (yellow cross) enable preferential nanonanocarrier accumulation via the EPR effect, facilitated by the acidic tumor microenvironment (pH 6.0 vs normal pH 7.0). Right: Intracellular delivery mechanism of nanocarriers, including: (a) Drug encapsulation in nanocarriers; (b) Surface receptor binding; (c) Endocytosis by tumor cells; (e) Endosomal escape; (g) Intracellular drug release; (h) Drug translocation to intracellular targets (e.g., nucleus). Abbreviation: EPR, Enhanced Permeability and Retention.
The EPR effect, however, can be inconsistent due to intertumoral heterogeneity (Fang et al., 2020; Irannejadrankouhi et al., 2025). Active targeting strategies have therefore been developed to improve reliability. These involve functionalizing nanocarriers with surface ligands such as antibodies or aptamers that recognize receptors overexpressed on tumor cells (Mi et al., 2020; Wang et al., 2023). This approach enhances specificity and facilitates intracellular drug delivery, as shown in Figure 4. Clinical evidence shows that liposomal DOX reduces the risk of cardiotoxicity by 54% compared to conventional DOX (OR = 0.46, p = 0.03) and is associated with a smaller decline in left ventricular ejection fraction (2.1% vs 5.6%, p = 0.0014) (Rayson et al., 2012; Xing et al., 2015). In HER2-positive breast cancer, trastuzumab-modified nanocarriers lowered the incidence of cardiac complications to 2.4% while enhancing therapeutic outcomes (Ngamcherdtrakul et al., 2015; Meng et al., 2018).
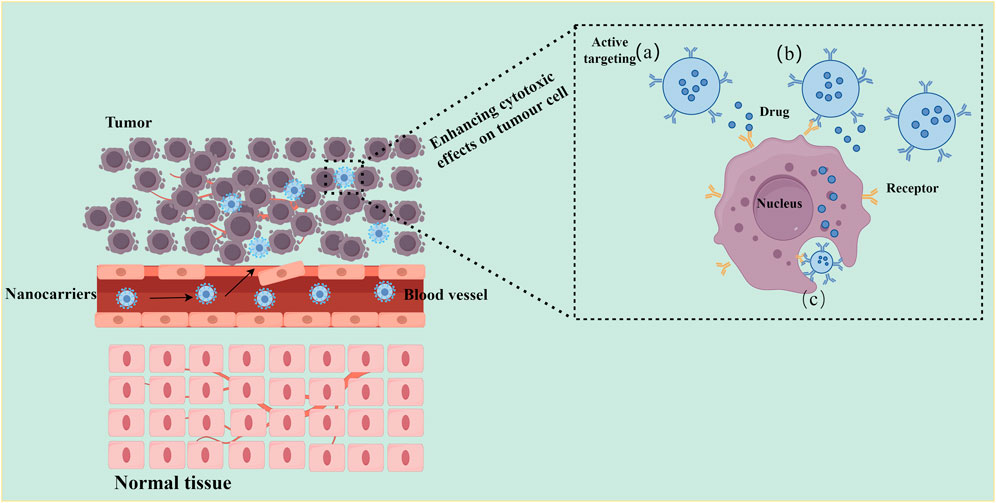
Figure 4. Schematic illustration of active targeting drug delivery using nanocarriers. Left: Schematic showing nanonanocarrier extravasation and tumor targeting. Nanocarriers modified with target ligands (blue stars) circulate through blood vessels, cross the abnormal tumor vasculature (dashed red arrows), and specifically bind to tumor cell surface receptors (orange Y-shaped structures), while being excluded from normal tissue (bottom) due to lack of target receptors. Right: Three active targeting delivery modes: (a) Proximity release: nanocarriers release drugs in the tumor microenvironment upon receptor binding; (b) Membrane depot: Ligand-receptor interaction anchors nanocarriers to the cell membrane for sustained drug release; (c) Receptor-mediated endocytosis: nanocarriers are internalized into tumor cells, delivering drugs directly to intracellular targets (e.g., nucleus). Key feature: Active targeting relies on specific ligand-receptor interactions (e.g., antibody-antigen, peptide-receptor), enabling selective drug accumulation in tumor tissues while minimizing uptake by normal cells.
By combining passive targeting through the EPR effect with active targeting via ligand modification, dual-targeting strategies significantly improve the therapeutic index of anticancer drugs (Izci et al., 2021). This dual approach also enables deeper penetration into the tumor microenvironment—a site where many conventional therapies fail due to inadequate drug diffusion. Figure 5 illustrates the process by which nanocarriers act as drug delivery vehicles within cancer cells.
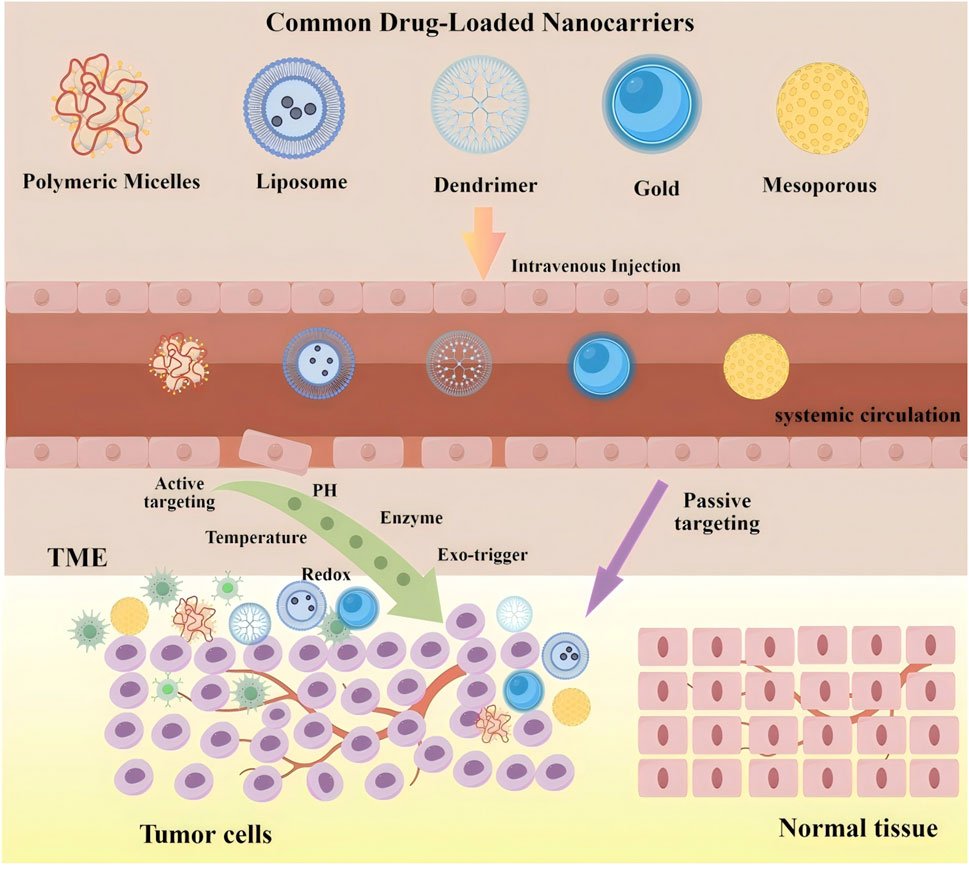
Figure 5. The process of nanocarriers as drug carriers acting on cancer cells. The upper shows structural diversity of common drug-loaded nanocarriers (left to right: polymeric micelles, liposomes, dendrimers, gold nanocarriers, mesoporous nanocarriers) as versatile drug encapsulation platforms. The lower depicts three-stage in vivo delivery: (1) Systemic circulation-intravenous injection enables bloodstream entry and systemic distribution; (2) tumor targeting via dual mechanisms: passive targeting (purple arrow, EPR effect-mediated extravasation across disorganized tumor vasculature) and active targeting [green arrow, triggered by TME cues (pH, enzymes, redox) or exogenous stimuli (temperature, light)]; and (3) Intratumoral action-drug release within the tumor microenvironment (TME) for selective cancer cell interaction while sparing normal tissues. Critical to this process, TME-established pathophysiological gradients (e.g., acidic pH, elevated enzyme levels) provide spatiotemporal control signals for stimuli-responsive nanocarriers. This multi-stage paradigm integrates dual targeting to overcome biological barriers, enhancing cancer therapeutic index.
3.2.2 Multifunctional nanocarriers for cardioprotection
Multifunctional nanocarriers integrate therapeutic and diagnostic functions by simultaneously enabling multi-drug co-delivery, controlled release, and synergistic effects. These platforms co-deliver chemotherapeutic agents, immunomodulators, and cardioprotectants such as coenzyme Q10, cardioprotective peptides, and natural bioactive compounds derived from Traditional Chinese Medicine (TCM) (e.g., resveratrol, quercetin, curcumin, berberine), which possess potent antioxidant and anti-inflammatory properties (Sun et al., 2025). In this way, they achieve both tumor suppression and organ protection (Majumder and Minko, 2021; Long et al., 2024). Mechanistically, multifunctional systems provide three major advantages. First, temporal release control coordinates the kinetics of drug and cardioprotectant delivery, preserving antitumor efficacy while reducing cardiotoxicity (Carvalho et al., 2021); Second, ROS-scavenging functions mediated by superoxide dismutase (SOD) mimetics protect against chemotherapy-induced oxidative myocardial injury (Quagliariello et al., 2020). Third, theranostic features allow real-time monitoring of treatment response, enabling personalized therapy adjustments (Shetty et al., 2019). Collectively, these advances highlight the potential of multifunctional nanocarriers as platforms for overcoming tumor drug resistance while simultaneously protecting cardiac function.
3.2.3 Distinct advantages of nano-cardio-oncology
Cardio-oncology nanocarriers establish a unique therapeutic paradigm that combines anticancer efficacy with cardio-protection, distinguishing them from conventional nanomedicine, which primarily focuses on tumor targeting and drug delivery efficiency. Poly (methacrylate citric acid)/DOX nanocarriers, for example, demonstrate 1.5-fold greater antitumor efficacy compared with free DOX in preclinical models, while simultaneously reducing systemic and cardiotoxicity (Yu et al., 2022). As shown in Figure 6, these nanocarriers must fulfill three critical requirements: they should achieve high tumor accumulation, enable effective cardio-protectant release in cardiac tissue, and prevent cross-interference between therapeutic components. Cascading-responsive nano-systems exemplify this concept by modulating drug release kinetics according to the distinct biological characteristics of tumor and cardiac microenvironments (Huang et al., 2025).
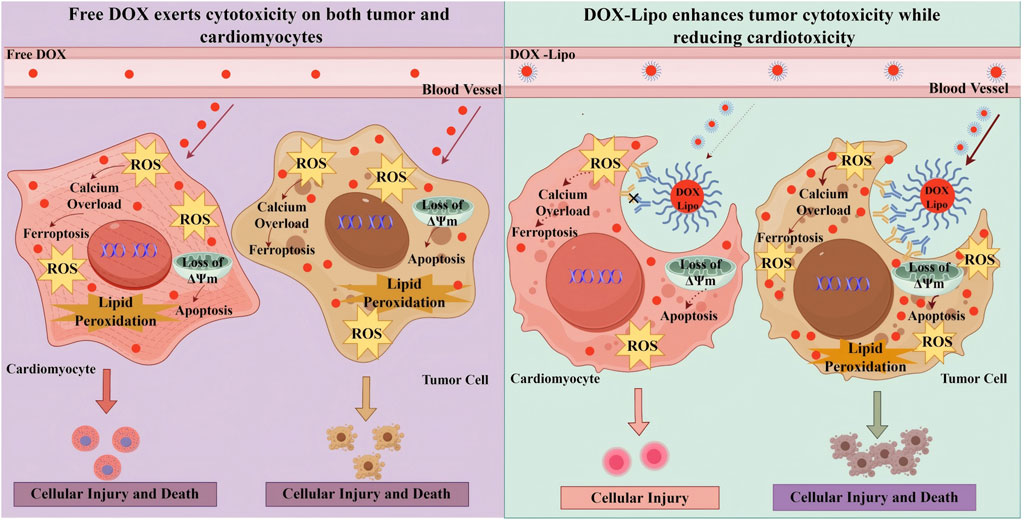
Figure 6. Pathological Mechanisms of Free DOX vs DOX-Lipo in Cardiomyocytes and tumor Cells. In DOX therapy, the use of free DOX often results in simultaneous damage to both cardiomyocytes and tumor cells due to its lack of selectivity, leading to significant cardiac toxicity and adverse effects. On the other hand, DOX-Lipo enhances the drug’s targeting ability, promoting tumor cell apoptosis and death while reducing cardiac toxicity. DOX,Doxorubicin; DOX-DNA adduct,Doxorubicin-DNA adduct; DOX-Lipo, Doxorubicin Liposome; ΔΨm, Mitochondrial Membrane Potential; TOP2β, Topoisomerase 2 beta.
Furthermore, cardio-oncology nanomedicine integrates advanced multidisciplinary approaches. Cardiovascular molecular imaging allows real-time monitoring of cardiac function; computational modeling predicts drug-induced cardiotoxicity; and organ-on-a-chip platforms simulate interactions between the heart and tumor tissues. Clinically, this discipline has opened transformative pathways, with several agents progressing through clinical trials. Notably, liposomal DOX formulations demonstrated more than 60% reduction in cardiac adverse events in Phase II trials (Moskowitz et al., 2021). Collectively, these innovations establish cardio-oncology nanomedicine as a distinct research ecosystem. By providing standardized frameworks and emphasizing the integration of therapy and protection, nanoplatform-based cardio-oncology is emerging as a new standard in comprehensive cancer care.
4 Preclinical and clinical research progress
4.1 Preclinical research
Lipid-based nanocarriers have emerged as effective drug delivery platforms due to their excellent biocompatibility and their ability to encapsulate both hydrophilic and hydrophobic compounds (Zhou et al., 2016). Liposomal formulations, such as liposomal DOX, are less cardiotoxic while maintaining strong antitumor efficacy. In preclinical studies, DOX-loaded liposomes administered to mice with triple-negative breast cancer reduced tumor growth by more than 50% while causing minimal cardiac injury, underscoring their therapeutic potential and the role of macrophage targeting (Lu et al., 2023). Similarly, polymeric micellar nanocarriers carrying paclitaxel reduced systemic toxicity, particularly in cardiac tissue, and improved survival in rat models of cancer-induced cardiotoxicity.
Polymeric nanocarriers further enhance therapeutic precision through controlled drug release and functionalization (Ahmed et al., 2021). For example, folate-targeted liposomes co-delivering paclitaxel and vinorelbine improved tumor suppression in non-small cell lung cancer (NSCLC) models while reducing systemic and cardiac damage compared with free drugs (Karpuz et al., 2021). PEG-b-PCL micelles delivering paclitaxel, cyclopamine, and gossypol demonstrated improved tumor control in ovarian cancer models with reduced cardiotoxicity (Cho et al., 2013). Nevertheless, long-term safety requires careful evaluation. Although polycaprolactone (PCL) is biodegradable, its hydrolysis product, ε-caprolactone, may gradually accumulate in cardiac tissues and induce oxidative stress over prolonged exposure, even though this effect was not evident in short-term studies (Inglut et al., 2020). These results indicate that polymeric platforms could expand therapeutic windows and minimize side effects, although long-term risks must be considered.
Inorganic nanocarriers, including gold nanocarriers and mesoporous silica nanocarriers, show considerable promise for imaging and drug delivery (Nam et al., 2013). Magnetic liposomes loaded with DOX significantly reduced breast tumor volume and caused less cardiotoxicity than conventional formulations (Maghsoudi et al., 2023). In thyroid cancer models, selenium nanocarriers combined with pH-responsive fingolimod enhanced drug release at tumor sites, reducing systemic side effects (Zou et al., 2021). In liver cancer, an UiO-66/Bi2S3 nanocomposite enabled controlled DOX release, suppressed tumor growth, and minimized systemic effects, including cardiac complications (Liu et al., 2022). However, preclinical studies also suggest that gold nanocarriers may accumulate in cardiac tissue over time, potentially inducing oxidative stress via Fenton chemistry reactions (Dulf et al., 2024).
Carbon-based nanomaterials, such as carbon nanotubes (CNTs) and graphene oxide, exhibit high drug-loading capacity and improved tissue penetration (Pei et al., 2023). For instance, RGD-conjugated PLGA nanocarriers increased the therapeutic index of cisplatin in lung cancer models by enhancing tumor regression while reducing systemic toxicity, including nephrotoxicity and cardiotoxicity (Yadav et al., 2023). Graphene oxide-based multilayer nanocarriers co-delivering DOX and methotrexate facilitated transdermal drug delivery, promoted tumor regression, and reduced systemic toxicity, including cardiotoxicity (Rajeev et al., 2023). Despite these advantages, carbon-based nanomaterials require careful assessment of long-term safety. While short-term cardiotoxic effects appear minimal, persistent concerns include aspect ratio-dependent toxicity, irreversible aggregation in physiological environments, and variability in large-scale production quality (Rezaei et al., 2025).
Bio-based nanocarriers derived from proteins, peptides, or polysaccharides offer superior biocompatibility and unique opportunities for functionalization (Torrini et al., 2024). For example, albumin-based nanocarriers carrying paclitaxel palmitate achieved high drug-loading efficiency and promoted significant tumor regression in mouse models, improving bioavailability while reducing systemic toxicity (Lan et al., 2023). Likewise, chitosan-coated silver nanocarriers loaded with 5-fluorouracil and nisin reduced tumor burden in skin cancer models and minimized systemic side effects (Rana et al., 2022). These findings highlight the potential of natural biomaterials for safer and more effective drug delivery.
The focus on targeting precision, functionalization, and biocompatibility provides a strong foundation for next-generation nanocarrier-based cancer therapies with improved safety profiles. Nonetheless, preclinical research has inherent limitations. Many studies rely on small sample sizes, which reduce statistical power and generalizability. Rodent models also differ physiologically from humans, limiting the accuracy with which they replicate human cardiotoxicity mechanisms and pharmacokinetics. Moreover, most studies are of short duration and cannot adequately assess long-term cardiac effects. These constraints emphasize the need for cautious interpretation of preclinical results and highlight the challenges of translating findings directly to clinical applications (L’Abbate et al., 2022). Table 2 summarizes key findings from animal studies employing different nanocarrier systems, providing an overview of their therapeutic potential and safety. Collectively, these investigations suggest that nanocarrier-based strategies could enhance anticancer efficacy while reducing cardiac and systemic toxicities.
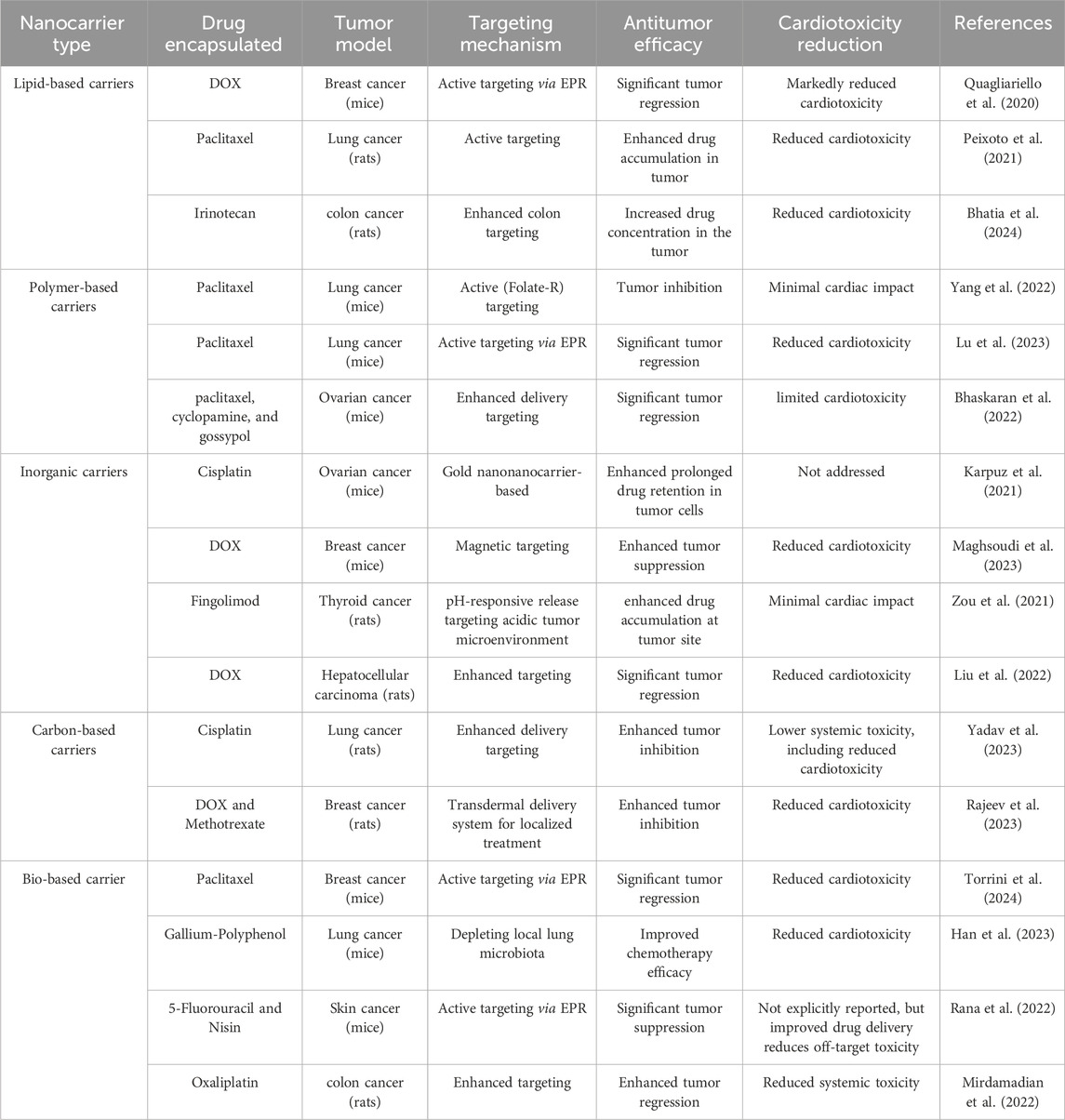
Table 2. Summarizes key findings from preclinical studies involving different nanocarrier systems in animal models.
4.2 Clinical trial progress
Lipid-based nanocarriers, particularly liposomes, have been extensively investigated due to their biocompatibility and capacity to encapsulate both hydrophilic and hydrophobic agents (Zhou et al., 2016). A meta-analysis demonstrated that pegylated liposomal doxorubicin (PLD) significantly reduced the risk of congestive heart failure compared with other anthracyclines (OR = 0.34, 95% CI: 0.24–0.47) (Rafiyath et al., 2012). Another study reported no significant difference in 3-year disease-free survival between PLD and epirubicin (94.9% vs 95.4%) in the neoadjuvant or adjuvant treatment of breast cancer, although the incidence of cardiotoxicity was markedly lower in the PLD group (Zhang et al., 2021). These findings underscore the clinical advantage of liposomal formulations in reducing cardiac risk without compromising therapeutic efficacy.
Polymeric nanocarriers, including those synthesized from PLGA and PEGylated materials, are particularly attractive due to their sustained drug release and stability in circulation, making them suitable for targeted cancer therapies (Maghsoudi et al., 2020). A Phase I/II clinical trial of CRLX101, a camptothecin-based nanocarrier, showed encouraging outcomes. In combination with bevacizumab, CRLX101 achieved an objective response rate of 21%, a disease control rate of 86%, and a median progression-free survival of 9.9 months in patients with advanced renal cell carcinoma (Keefe et al., 2016). These systems are often engineered for tumor accumulation, thereby reducing systemic toxicity and enhancing therapeutic efficacy (Li X. et al., 2024). Collectively, polymeric nanocarriers represent a promising approach for precise drug delivery, improving tumor targeting while minimizing damage to healthy organs.
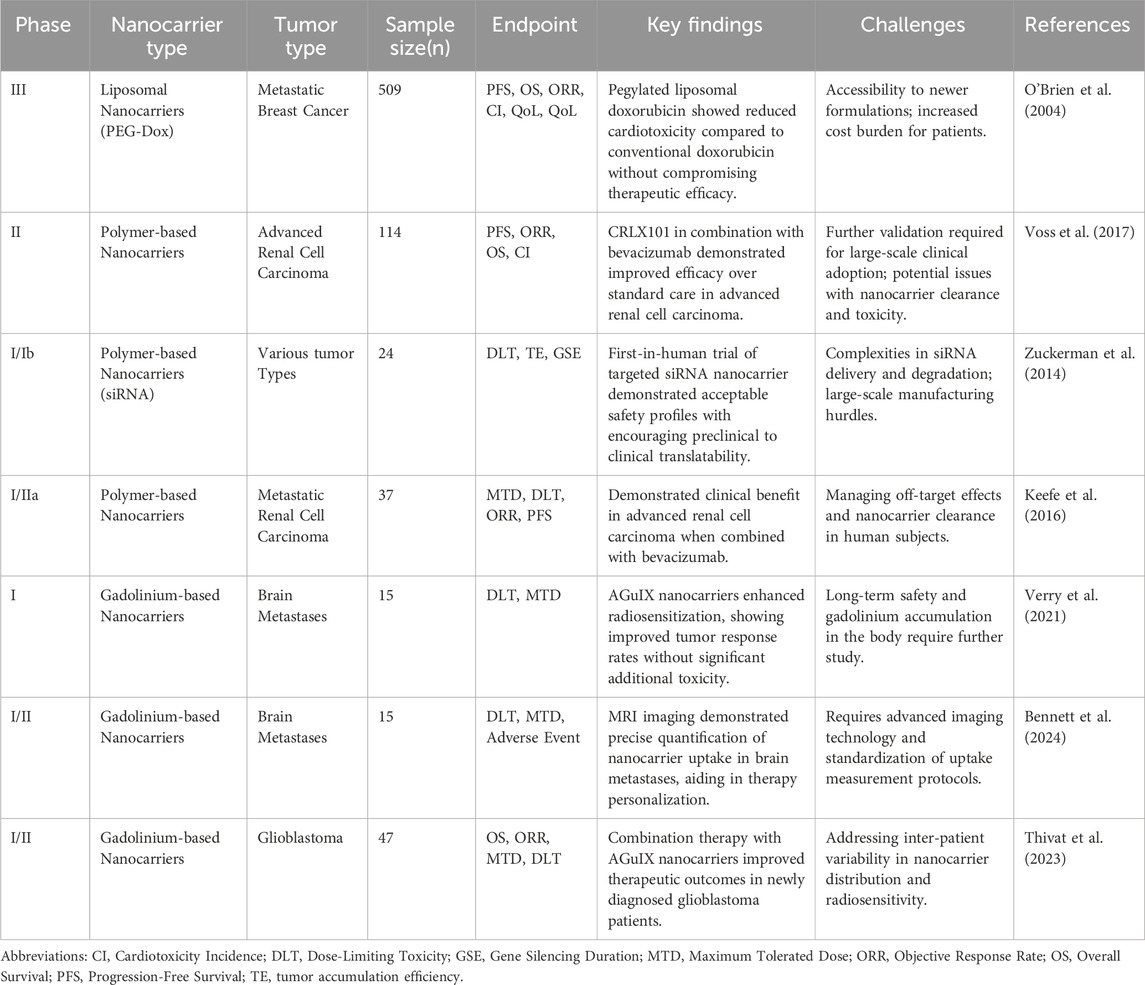
The growing body of clinical evidence highlights the potential of nanocarriers to improve cancer treatment outcomes while mitigating cardiotoxicity. Table 3 summarizes key clinical findings, providing an overview of the progress achieved thus far. Nevertheless, translating dual-purpose nanocarrier systems into clinical oncology remains challenging. Barriers include stringent regulatory requirements for therapies with both anticancer and cardioprotective functions, the complexity of evaluating long-term cardiotoxicity, and the technical difficulties of large-scale clinical-grade nanocarrier production (Makwana et al., 2021; Abdellatif et al., 2022; Santin et al., 2023; Sarfraz et al., 2023; Desai et al., 2025). Future research should focus on systematically assessing the long-term safety of nanotechnology platforms, particularly their potential immunological impacts (Moazzam et al., 2024). At the same time, standardized manufacturing protocols and advanced characterization methods are needed to optimize the precision of smart nanocarriers, thereby improving tumor specificity and minimizing off-target effects (Ali et al., 2021). To use nanotechnology to its fullest potential in cancer and heart defense, these kinds of improvements are needed.
Importantly, cardio-oncology nanomedicine distinguishes itself through its fundamental dual-targeting paradigm. By simultaneously enabling tumor-specific drug delivery and controlled release of cardioprotective agents, it addresses a long-standing challenge in oncology: enhancing anticancer efficacy while actively safeguarding cardiac function. This integrative approach elevates cardio-oncology nanomedicine as a distinct and emerging discipline within the broader field of precision oncology.
5 Innovation and future prospects
5.1 Development of nanotechnology integrated with artificial intelligence
The convergence of nanotechnology with artificial intelligence (AI) and machine learning (ML) is opening new frontiers for the design of next-generation nanocarriers in oncology (Tan et al., 2023). AI enables the analysis of large and complex biological datasets, facilitating the development of nanocarriers with enhanced specificity and reduced toxicity (Corti et al., 2023). For example, Chou et al. used an AI-assisted pharmacokinetic model to optimize nanocarrier size, surface chemistry, and dosing for targeted tumor delivery (Chou et al., 2023), while Zhang et al. applied machine learning to rapidly screen functional nanomedicines via drug-drug self-assembly (Zhang et al., 2025). Furthermore, real-time AI-driven monitoring systems can guide individualized dose adjustments according to patient responses, thereby improving therapeutic precision and outcomes (Bhinder et al., 2021; Pang et al., 2022). With continued advances, AI is expected to transform precision medicine by accelerating nanocarrier design and enabling more efficient, tumor-targeted interventions.
5.2 Personalized nanomedicine delivery
The rise of personalized medicine has intensified interest in patient-specific nanocarrier systems. Personalized nanomedicine leverages molecular and biological markers to optimize therapeutic efficacy (Passaro et al., 2024). By incorporating factors such as gene expression patterns, protein profiles, and metabolic signatures, nanocarriers can be tailored to improve drug delivery precision and clinical outcomes (Zhou et al., 2024). This approach is particularly valuable for addressing tumor heterogeneity and patient-to-patient variability in treatment response. For example, targeting receptors that are overexpressed in specific cancers, such as HER2 in breast cancer, enables direct delivery of chemotherapeutic agents to malignant cells while minimizing systemic toxicity (Krishnamurti and Silverman, 2014; Ratajczak et al., 2023). Ongoing progress in genomics and proteomics is accelerating the development of customized nanocarrier formulations aligned with each patient’s genetic and molecular landscape, positioning personalized nanomedicine as a central component of future cancer therapy.
5.3 Integration of multifunctional nanotechnology
A key future direction in cancer therapy lies in the integration of multifunctional nanotechnology with diverse therapeutic modalities. Multifunctional nanoplatforms can simultaneously combine chemotherapy with photothermal therapy, immunotherapy, or gene therapy, thereby enhancing therapeutic efficacy (Ashrafizadeh et al., 2023; Kang et al., 2023; Overchuk et al., 2023). For example, nanocarriers engineered to deliver both chemotherapeutics and immune checkpoint inhibitors can potentiate antitumor immune responses (Liang et al., 2024). The incorporation of photothermal agents into nanocarriers enables the concurrent release of drugs and localized hyperthermia, which increases tumor cell susceptibility to treatment (Dorjsuren et al., 2020). Moreover, nanocarriers are being developed as vehicles for gene therapy, enabling the correction of tumor-specific genetic alterations (Yu et al., 2021). Figure 7 illustrates multifunctional nanocarriers that integrate drug delivery, imaging, and cardio-protection within a single system, underscoring their potential to achieve multiple therapeutic objectives concurrently. Such multifunctional strategies represent a transformative shift in oncology, where a single nanoplatform can synergistically combine several treatment modalities, offering a comprehensive and highly effective approach to combating cancer.
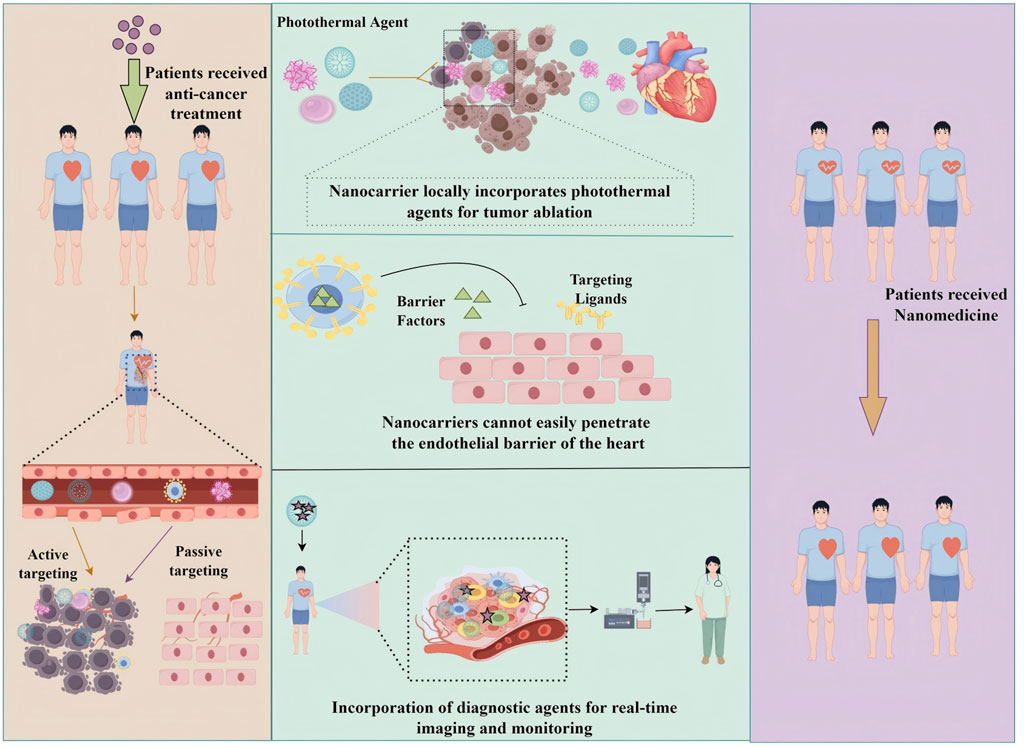
Figure 7. Schematic representation of multifunctional nanocarriers for simultaneous drug delivery, imaging, and cardiac protection.
6 Discussion and conclusion
Central to this review is the paradigm-shifting concept of cardio-oncology nanotechnology, which is defined by its dual commitment to antitumor efficacy and cardio-protection. This duality distinguishes it from conventional nanomedicine approaches that focus exclusively on tumor targeting (Lu et al., 2024). The analysis presented here highlights the transformative role of nanotechnology in cancer therapy, particularly in addressing CIC while maintaining robust antitumor activity. Nanocarriers such as liposomes, polymeric nanocarriers, and inorganic nanomaterials enhance the precision of drug delivery through both passive and active targeting mechanisms (Garbayo et al., 2020; Vazhappilly et al., 2021). More importantly, these platforms establish a novel therapeutic paradigm by integrating tumor suppression with active cardio-protection, a synergistic framework that defines the innovation of this emerging discipline (Yu et al., 2021). Recent advances in cardioprotective nanocarriers have reduced off-target effects and mitigated cardiac injury, while preclinical and clinical studies have demonstrated encouraging improvements in patient outcomes (Rafiyath et al., 2012; Keefe et al., 2016; Yang et al., 2022). Collectively, these findings establish cancer nanocardiology as a distinct research ecosystem characterized by standardized models for evaluating integrated therapeutic and protective efficacy. This dual-functional strategy underscores the capacity of nanotechnology to render cancer treatments both safer and more effective, while also pointing toward future developments in artificial intelligence-driven optimization and personalized medicine.
The findings of this review support prior evidence that nanocarrier-based drug delivery significantly reduces systemic damage compared to conventional formulations (Nooreen et al., 2022; Zhang L. et al., 2023; Alarcon et al., 2025). For example, liposomal DOX consistently reduces CIC by up to 54%, as reported in multiple studies and meta-analyses (Xing et al., 2015). However, this review extends current knowledge by emphasizing the incorporation of cardioprotective agents into nanocarriers, an underexplored yet promising strategy (Bruno et al., 2021; Kong et al., 2022). Additionally, the increasing use of pH-sensitive and multi-stimuli-responsive nanocarriers offers new opportunities to enhance therapeutic precision (Liu et al., 2014; Kong et al., 2023). By embedding cardioprotection into the broader framework of oncological nanomedicine, this review addresses critical gaps that remain in the field.
Despite these advances, several barriers limit the widespread clinical translation of nanocarrier systems. First, the variability of tumor microenvironments constrains the effectiveness of passive targeting strategies such as the EPR effect (Izci et al., 2021; Yang et al., 2021). Second, the long-term effects of nanocarriers, including their potential immunomodulatory properties and accumulation in tissues, remain insufficiently understood (Saifi et al., 2021; Zhao et al., 2022). Third, challenges in scaling up production and the high cost of manufacturing multifunctional nanocarriers pose significant practical obstacles (Pang et al., 2023). These limitations highlight the need for further optimization and rigorous evaluation of nanocarrier systems in experimental and clinical settings.
Thus, future innovation must refine the dual-functional architecture of nanocarriers, with AI serving as a key enabler for improving spatiotemporal precision in balancing tumor suppression and cardioprotection (Li et al., 2025). Machine learning approaches can facilitate predictive modeling of tumor characteristics, enabling the customization of nanocarrier properties such as size, charge, and surface chemistry (Chen, 2023). Furthermore, the development of recyclable or bio-derived nanocarriers may address concerns regarding the long-term health and environmental impacts of synthetic nanomaterials (Umapathi et al., 2022). Combining nanotechnology with gene therapy and immune-based strategies also presents considerable promise for expanding therapeutic capabilities (Kiaie et al., 2023; Birnboim-Perach and Benhar, 2024). Ultimately, large-scale, rigorously designed clinical trials remain essential for validating the safety, efficacy, and cost-effectiveness of nanocarriers, thereby enabling broader clinical adoption (Su et al., 2022; Saadh et al., 2024).
In summary, this review underscores the transformative potential of nanotechnology in cancer treatment, demonstrating its ability to enhance therapeutic efficacy while minimizing cardiotoxicity. Beyond oncology, the principles of dual-functional nanomedicine may serve as a model for other areas, including regenerative medicine and infectious disease management, underscoring the broad societal relevance of this field (Zhang P. et al., 2023; Abu Elella and Kolawole, 2024).
Author contributions
LM: Formal Analysis, Writing – original draft, Writing – review and editing. BZ: Data curation, Writing – review and editing. XL: Formal Analysis, Writing – review and editing. SG: Data curation, Writing – review and editing. SK: Data curation, Writing – review and editing. YaL: Data curation, Software, Writing – review and editing. RW: Data curation, Software, Writing – review and editing. ML: Data curation, Software, Writing – review and editing. XM: Data curation, Software, Writing – review and editing. YhL: Data curation, Software, Writing – review and editing. YLu: Validation, Writing – review and editing. LL: Validation, Writing – review and editing. CL: Formal Analysis, Validation, Visualization, Writing – original draft, Writing – review and editing. YH: Formal Analysis, Funding acquisition, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Major Project of Public Health in Tianjin (24ZXGZSY00190), the Haihe Laboratory of Modern Chinese Medicine Science and Technology Project (HYH20250102), the Key Research Project in Traditional Chinese Medicine of the Tianjin Health Commission (2024004), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (2021KJ160), and the Scientific Research Project of Integrated Traditional Chinese and Western Medicine of the Tianjin Health Commission (2023073).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdellatif, A. A. H., Younis, M. A., Alsharidah, M., Al Rugaie, O., and Tawfeek, H. M. (2022). Biomedical applications of quantum dots: overview, challenges, and clinical potential. Int. J. Nanomedicine 17, 1951–1970. doi:10.2147/IJN.S357980
Abu Elella, M. H., and Kolawole, O. M. (2024). Recent advances in modified chitosan-based drug delivery systems for transmucosal applications: a comprehensive review. Int. J. Biol. Macromol. 277, 134531. doi:10.1016/j.ijbiomac.2024.134531
Ahmed, A., Sarwar, S., Hu, Y., Munir, M. U., Nisar, M. F., Ikram, F., et al. (2021). Surface-modified polymeric nanoparticles for drug delivery to cancer cells. Expert Opin. Drug Deliv. 18, 1–24. doi:10.1080/17425247.2020.1822321
Alarcon, J. F., Karusan, N. R., Presciutti, C., Miras, J., Magana, J. R., Guerra-Rebollo, M., et al. (2025). Revolutionizing rheumatoid arthritis therapy: the potential of lipid nanocarriers. RSC Adv. 15, 27388–27402. doi:10.1039/d5ra04258e
Ali, E. S., Sharker, S. M., Islam, M. T., Khan, I. N., Shaw, S., Rahman, M. A., et al. (2021). Targeting cancer cells with nanotherapeutics and nanodiagnostics: current status and future perspectives. Semin. Cancer Biol. 69, 52–68. doi:10.1016/j.semcancer.2020.01.011
Allawadhi, P., Singh, V., Govindaraj, K., Khurana, I., Sarode, L. P., Navik, U., et al. (2022). Biomedical applications of polysaccharide nanoparticles for chronic inflammatory disorders: focus on rheumatoid arthritis, diabetes and organ fibrosis. Carbohydr. Polym. 281, 118923. doi:10.1016/j.carbpol.2021.118923
Araújo, E. V., Carneiro, S. V., Neto, D. M. A., Freire, T. M., Costa, V. M., Freire, R. M., et al. (2024). Advances in surface design and biomedical applications of magnetic nanoparticles. Adv. Colloid Interface Sci. 328, 103166. doi:10.1016/j.cis.2024.103166
Ashrafizadeh, M., Zarrabi, A., Bigham, A., Taheriazam, A., Saghari, Y., Mirzaei, S., et al. (2023). (Nano)platforms in breast cancer therapy: drug/gene delivery, advanced nanocarriers and immunotherapy. Med. Res. Rev. 43, 2115–2176. doi:10.1002/med.21971
Ayza, M. A., Zewdie, K. A., Tesfaye, B. A., Wondafrash, D. Z., and Berhe, A. H. (2020). The role of antioxidants in ameliorating cyclophosphamide-induced cardiotoxicity. Oxidative Med. Cell. Longev. 2020, 4965171. doi:10.1155/2020/4965171
Barani, M., Bilal, M., Sabir, F., Rahdar, A., and Kyzas, G. Z. (2021). Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci. 266, 118914. doi:10.1016/j.lfs.2020.118914
Beach, M. A., Nayanathara, U., Gao, Y., Zhang, C., Xiong, Y., Wang, Y., et al. (2024). Polymeric nanoparticles for drug delivery. Chem. Rev. 124, 5505–5616. doi:10.1021/acs.chemrev.3c00705
Behera, A., and Padhi, S. (2022). “pH-sensitive polymeric nanoparticles for cancer treatment,” in Polymeric nanoparticles for the treatment of solid tumors. Editors S. Padhi, A. Behera, and E. Lichtfouse (Cham: Springer International Publishing), 401–425. doi:10.1007/978-3-031-14848-4_15
Bennett, S., Verry, C., Kaza, E., Miao, X., Dufort, S., Boux, F., et al. (2024). Quantifying gadolinium-based nanoparticle uptake distributions in brain metastases via magnetic resonance imaging. Sci. Rep. 14, 11959. doi:10.1038/s41598-024-62389-1
Bhaskaran, N. A., Jitta, S. R., Salwa, , Cheruku, S., Kumar, N., and Kumar, L. (2022). Orally delivered solid lipid nanoparticles of irinotecan coupled with chitosan surface modification to treat colon cancer: preparation, in-vitro and in-vivo evaluations. Int. J. Biol. Macromol. 211, 301–315. doi:10.1016/j.ijbiomac.2022.05.060
Bhatia, R., Singh, A., Singh, S., and Rawal, R. K. (2024). Emerging trends in nano-carrier based gene delivery systems for targeted cancer therapy. J. Drug Deliv. Sci. Technol. 95, 105546. doi:10.1016/j.jddst.2024.105546
Bhinder, B., Gilvary, C., Madhukar, N. S., and Elemento, O. (2021). Artificial intelligence in cancer research and precision medicine. Cancer Discov. 11, 900–915. doi:10.1158/2159-8290.CD-21-0090
Birnboim-Perach, R., and Benhar, I. (2024). Using combination therapy to overcome diverse challenges of immune checkpoint inhibitors treatment. Int. J. Biol. Sci. 20, 3911–3922. doi:10.7150/ijbs.93697
Bolshakova, O., Zherebyatieva, O., and Sarantseva, S. V. (2025). Fullerenes in vivo. Toxicity and protective effects. Nanotoxicology 19, 233–258. doi:10.1080/17435390.2025.2471273
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi:10.3322/caac.21834
Bruno, K., Morales-Lara, A. C., Diflorio, D. N., Anastasiadis, Z., Landolfo, C., Ray, J. C., et al. (2021). Sex differences in cardio-oncology: utilizing a genetic variant as a therapeutic target doxorubicin-induced cardiomyopathy. Eur. Heart J. 42, ehab724.3263. doi:10.1093/eurheartj/ehab724.3263
Carrasco, R., Castillo, R. L., Gormaz, J. G., Carrillo, M., and Thavendiranathan, P. (2021). Role of oxidative stress in the mechanisms of anthracycline-induced cardiotoxicity: effects of preventive strategies. Oxidative Med. Cell. Longev. 2021, 8863789. doi:10.1155/2021/8863789
Carvalho, G. C., Sábio, R. M., and Chorilli, M. (2021). An overview of properties and analytical methods for lycopene in organic nanocarriers. Crit. Rev. Anal. Chem. 51, 674–686. doi:10.1080/10408347.2020.1763774
Cejas, R. B., Petrykey, K., Sapkota, Y., and Burridge, P. W. (2024). Anthracycline toxicity: light at the end of the tunnel? Annu. Rev. Pharmacol. Toxicol. 64, 115–134. doi:10.1146/annurev-pharmtox-022823-035521
Chen, Y. (2023). Nanotechnology for next-generation cancer immunotherapy: state of the art and future perspectives. J. Control. Release 356, 14–25. doi:10.1016/j.jconrel.2023.02.016
Chen, Y., Shi, S., and Dai, Y. (2022). Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 156, 113903. doi:10.1016/j.biopha.2022.113903
Chen, Y.-L., Bao, C.-J., Duan, J.-L., Xie, Y., and Lu, W.-L. (2023). Overcoming biological barriers by virus-like drug particles for drug delivery. Adv. Drug Deliv. Rev. 203, 115134. doi:10.1016/j.addr.2023.115134
Chithra, P., Bhatia, D., and Solanki, R. (2025). Advanced nanomicelles for targeted glioblastoma multiforme therapy. Biomater. Adv. 170, 214221. doi:10.1016/j.bioadv.2025.214221
Cho, H., Lai, T. C., and Kwon, G. S. (2013). Poly(ethylene glycol)-block-poly(ε-caprolactone) micelles for combination drug delivery: evaluation of paclitaxel, cyclopamine and gossypol in intraperitoneal xenograft models of ovarian cancer. J. Control Release 166, 1–9. doi:10.1016/j.jconrel.2012.12.005
Chou, W.-C., Chen, Q., Yuan, L., Cheng, Y.-H., He, C., Monteiro-Riviere, N. A., et al. (2023). An artificial intelligence-assisted physiologically-based pharmacokinetic model to predict nanoparticle delivery to tumors in mice. J. Control Release 361, 53–63. doi:10.1016/j.jconrel.2023.07.040
Christidi, E., and Brunham, L. R. (2021). Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 12, 339. doi:10.1038/s41419-021-03614-x
Corti, C., Cobanaj, M., Dee, E. C., Criscitiello, C., Tolaney, S. M., Celi, L. A., et al. (2023). Artificial intelligence in cancer research and precision medicine: applications, limitations and priorities to drive transformation in the delivery of equitable and unbiased care. Cancer Treat. Rev. 112, 102498. doi:10.1016/j.ctrv.2022.102498
Desai, N., Rana, D., Patel, M., Bajwa, N., Prasad, R., and Vora, L. K. (2025). Nanoparticle therapeutics in clinical perspective: classification, marketed products, and regulatory landscape. Small Weinh. (Bergstr. Ger.) 21, e2502315. doi:10.1002/smll.202502315
Dey, A. D., Bigham, A., Esmaeili, Y., Ashrafizadeh, M., Moghaddam, F. D., Tan, S. C., et al. (2022). Dendrimers as nanoscale vectors: unlocking the bars of cancer therapy. Semin. Cancer Biol. 86, 396–419. doi:10.1016/j.semcancer.2022.06.003
Ding, X., Zhang, Y., Pan, P., Long, C., Zhang, X., Zhuo, L., et al. (2023). Multiple mitochondria-targeted components screened from sini decoction improved cardiac energetics and mitochondrial dysfunction to attenuate doxorubicin-induced cardiomyopathy. Theranostics 13, 510–530. doi:10.7150/thno.80066
Dorjsuren, B., Chaurasiya, B., Ye, Z., Liu, Y., Li, W., Wang, C., et al. (2020). Cetuximab-coated thermo-sensitive liposomes loaded with magnetic nanoparticles and doxorubicin for targeted EGFR-expressing breast cancer combined therapy. Int. J. Nanomedicine 15, 8201–8215. doi:10.2147/IJN.S261671
Dridi, H., Liu, Y., Reiken, S., Liu, X., Argyrousi, E. K., Yuan, Q., et al. (2023). Heart failure-induced cognitive dysfunction is mediated by intracellular Ca2+ leak through ryanodine receptor type 2. Nat. Neurosci. 26, 1365–1378. doi:10.1038/s41593-023-01377-6
Du, Q., Gao, F., Cui, B., Wang, T., Chen, F., Zeng, Z., et al. (2024). Improving the stability, foliar utilization and biological activity of imidacloprid delivery systems: size effect of nanoparticles. Environ. Res. 257, 119386. doi:10.1016/j.envres.2024.119386
Dulf, P. L., Coadă, C. A., Florea, A., Moldovan, R., Baldea, I., Dulf, D. V., et al. (2024). Doxorubicin incorporation into gold nanoparticles: an in vivo study of its effects on cardiac tissue in rats. Nanomater. (Basel) 14, 1647. doi:10.3390/nano14201647
Fang, J., Islam, W., and Maeda, H. (2020). Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 157, 142–160. doi:10.1016/j.addr.2020.06.005
Feng, C., Li, Y., Ferdows, B. E., Patel, D. N., Ouyang, J., Tang, Z., et al. (2022). Emerging vaccine nanotechnology: from defense against infection to sniping cancer. Acta Pharm. Sin. B 12, 2206–2223. doi:10.1016/j.apsb.2021.12.021
Garbayo, E., Pascual-Gil, S., Rodríguez-Nogales, C., Saludas, L., Estella-Hermoso de Mendoza, A., and Blanco-Prieto, M. J. (2020). Nanomedicine and drug delivery systems in cancer and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12, e1637. doi:10.1002/wnan.1637
Ghezzi, M., Pescina, S., Padula, C., Santi, P., Del Favero, E., Cantù, L., et al. (2021). Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 332, 312–336. doi:10.1016/j.jconrel.2021.02.031
Ghosh, B., and Biswas, S. (2021). Polymeric micelles in cancer therapy: state of the art. J. Control. Release 332, 127–147. doi:10.1016/j.jconrel.2021.02.016
Guimarães, D., Cavaco-Paulo, A., and Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 601, 120571. doi:10.1016/j.ijpharm.2021.120571
Habibi, N., Mauser, A., Ko, Y., and Lahann, J. (2022). Protein nanoparticles: uniting the power of proteins with engineering design approaches. Adv. Sci. (Weinh) 9, e2104012. doi:10.1002/advs.202104012
Han, Z., Chen, Q., Zheng, D., Chen, K., Huang, Q., Zhuang, Z., et al. (2023). Inhalable capsular polysaccharide-camouflaged gallium-polyphenol nanoparticles enhance lung cancer chemotherapy by depleting local lung microbiota. Adv. Mater. 35, 2302551. doi:10.1002/adma.202302551
Hirschbiegel, C.-M., Zhang, X., Huang, R., Cicek, Y. A., Fedeli, S., and Rotello, V. M. (2023). Inorganic nanoparticles as scaffolds for bioorthogonal catalysts. Adv. Drug Deliv. Rev. 195, 114730. doi:10.1016/j.addr.2023.114730
Huang, Y., Li, P., Zhao, R., Zhao, L., Liu, J., Peng, S., et al. (2022). Silica nanoparticles: biomedical applications and toxicity. Biomed. Pharmacother. 151, 113053. doi:10.1016/j.biopha.2022.113053
Huang, J., Yang, J., Yang, Y., Lu, X., Xu, J., Lu, S., et al. (2025). Mitigating doxorubicin-induced cardiotoxicity and enhancing anti-tumor efficacy with a metformin-integrated self-assembled nanomedicine. Adv. Sci. Weinh. (Baden-Wurtt. Ger.) 12, e2415227. doi:10.1002/advs.202415227
Inglut, C. T., Sorrin, A. J., Kuruppu, T., Vig, S., Cicalo, J., Ahmad, H., et al. (2020). Immunological and toxicological considerations for the design of liposomes. Nanomater. (Basel Switz.) 10, 190. doi:10.3390/nano10020190
Irannejadrankouhi, S., Mivehchi, H., Eskandari-Yaghbastlo, A., Nejati, S. T., Emrahoglu, S., Nazarian, M., et al. (2025). Innovative nanoparticle strategies for treating oral cancers. Med. Oncol. N. (Lond. Engl.) 42, 182. doi:10.1007/s12032-025-02728-y
Itoo, A. M., Ghosh, B., and Biswas, S. (2024). Recent advancements in nanotechnology-mediated platinum-based cancer therapy. Coord. Chem. Rev. 508, 215796. doi:10.1016/j.ccr.2024.215796
Izci, M., Maksoudian, C., Manshian, B. B., and Soenen, S. J. (2021). The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem. Rev. 121, 1746–1803. doi:10.1021/acs.chemrev.0c00779
Jin, X., Wang, X., Liu, Y., Kim, M., Cao, M., Xie, H., et al. (2022). Nitrogen and sulfur Co-Doped hierarchically porous carbon nanotubes for fast potassium ion storage. Small 18, 2203545. doi:10.1002/smll.202203545
Kang, X., Zhang, Y., Song, J., Wang, L., Li, W., Qi, J., et al. (2023). A photo-triggered self-accelerated nanoplatform for multifunctional image-guided combination cancer immunotherapy. Nat. Commun. 14, 5216. doi:10.1038/s41467-023-40996-2
Karpuz, M., Silindir-Gunay, M., Ozer, A. Y., Ozturk, S. C., Yanik, H., Tuncel, M., et al. (2021). Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating Theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 156, 105576. doi:10.1016/j.ejps.2020.105576
Kaup, R., and Velders, A. H. (2022). Controlling trapping, release, and exchange dynamics of micellar core components. ACS Nano 16, 14611–14621. doi:10.1021/acsnano.2c05144
Kaurav, H., Verma, D., Bansal, A., Kapoor, D. N., and Sheth, S. (2023). Progress in drug delivery and diagnostic applications of carbon dots: a systematic review. Front. Chem. 11, 1227843. doi:10.3389/fchem.2023.1227843
Keefe, S. M., Hoffman-Censits, J., Cohen, R. B., Mamtani, R., Heitjan, D., Eliasof, S., et al. (2016). Efficacy of the nanoparticle–drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: results of an investigator-initiated phase I–IIa clinical trial. Ann. Oncol. 27, 1579–1585. doi:10.1093/annonc/mdw188
Kiaie, S. H., Salehi-Shadkami, H., Sanaei, M. J., Azizi, M., Shokrollahi Barough, M., Nasr, M. S., et al. (2023). Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy. J. Nanobiotechnol. 21, 339. doi:10.1186/s12951-023-02083-y
Kianfar, E. (2021). Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 19, 159. doi:10.1186/s12951-021-00896-3
Kim, M., Kubelick, K. P., Vanderlaan, D., Qin, D., Lee, J., Jhunjhunwala, A., et al. (2024). Coupling gold nanospheres into nanochain constructs for high-contrast, longitudinal photoacoustic imaging. Nano Lett. 24, 7202–7210. doi:10.1021/acs.nanolett.4c00992
Kong, C.-Y., Guo, Z., Song, P., Zhang, X., Yuan, Y.-P., Teng, T., et al. (2022). Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int. J. Biol. Sci. 18, 760–770. doi:10.7150/ijbs.65258
Kong, X., Qi, Y., Wang, X., Jiang, R., Wang, J., Fang, Y., et al. (2023). Nanoparticle drug delivery systems and their applications as targeted therapies for triple negative breast cancer. Prog. Mater Sci. 134, 101070. doi:10.1016/j.pmatsci.2023.101070
Krishnamurti, U., and Silverman, J. F. (2014). HER2 in breast cancer: a review and update. Adv. Anat. Pathol. 21, 100–107. doi:10.1097/PAP.0000000000000015
Kumar, P., Thakur, N., Kumar, K., Kumar, S., Dutt, A., Thakur, V. K., et al. (2024). Catalyzing innovation: exploring iron oxide nanoparticles - origins, advancements, and future application horizons. Coord. Chem. Rev. 507, 215750. doi:10.1016/j.ccr.2024.215750
Kurano, T., Kanazawa, T., Ooba, A., Masuyama, Y., Maruhana, N., Yamada, M., et al. (2022). Nose-to-brain/spinal cord delivery kinetics of liposomes with different surface properties. J. Control. Release 344, 225–234. doi:10.1016/j.jconrel.2022.03.017
Lan, H., Jamil, M., Ke, G., and Dong, N. (2023). The role of nanoparticles and nanomaterials in cancer diagnosis and treatment: a comprehensive review. Am. J. Cancer Res. 13, 5751–5784.
Lee, Y., Kamada, N., and Moon, J. J. (2021). Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv. Drug Deliv. Rev. 179, 114021. doi:10.1016/j.addr.2021.114021
Li, D., Yang, Y., Wang, S., He, X., Liu, M., Bai, B., et al. (2021). Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 46, 102089. doi:10.1016/j.redox.2021.102089
Li, L., Zeng, Y., Chen, M., and Liu, G. (2022). Application of nanomicelles in enhancing bioavailability and biological efficacy of bioactive nutrients. Polymers 14, 3278. doi:10.3390/polym14163278
Li, S., Fan, W., Chen, Q., and Zhang, X. (2024a). Facile light-driven synthesis of highly luminous sulfur quantum dots for fluorescence sensing and cell imaging. ACS Appl. Mater. Interfaces 16, 41281–41292. doi:10.1021/acsami.4c05739
Li, W., Cheng, X., Zhu, G., Hu, Y., Wang, Y., Niu, Y., et al. (2024b). A review of chemotherapeutic drugs-induced arrhythmia and potential intervention with traditional Chinese medicines. Front. Pharmacol. 15, 1340855. doi:10.3389/fphar.2024.1340855
Li, X., Qi, J., Wang, J., Hu, W., Zhou, W., Wang, Y., et al. (2024c). Nanoparticle technology for mRNA: delivery strategy, clinical application and developmental landscape. Theranostics 14, 738–760. doi:10.7150/thno.84291
Li, Z., Zhang, S., Xiao, Q., Shui, S., Dong, P., Jiang, Y., et al. (2025). Energy-confinement 3D flower-shaped cages for AI-driven decoding of metabolic fingerprints in cardiovascular disease diagnosis. ACS Nano 19, 6180–6194. doi:10.1021/acsnano.4c14656
Liang, S., Xiao, L., Chen, T., Roa, P., Cocco, E., Peng, Z., et al. (2024). Injectable nanocomposite hydrogels improve intraperitoneal Co-delivery of chemotherapeutics and immune checkpoint inhibitors for enhanced peritoneal metastasis therapy. ACS Nano 18, 18963–18979. doi:10.1021/acsnano.4c02312
Lin, X., and Chen, T. (2023). A review of in vivo toxicity of quantum dots in animal models. Int. J. Nanomed. 18, 8143–8168. doi:10.2147/IJN.S434842
Lin, J., Yang, H., Zhang, Y., Zou, F., He, H., Xie, W., et al. (2023). Ferrocene-based polymeric nanoparticles carrying doxorubicin for oncotherapeutic combination of chemotherapy and ferroptosis. Small 19, e2205024. doi:10.1002/smll.202205024
Liu, J., Huang, Y., Kumar, A., Tan, A., Jin, S., Mozhi, A., et al. (2014). pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 32, 693–710. doi:10.1016/j.biotechadv.2013.11.009
Liu, L., Zhuang, J., Tan, J., Liu, T., Fan, W., Zhang, Y., et al. (2022). Doxorubicin-loaded UiO-66/Bi2S3 nanocomposite-enhanced synergistic transarterial chemoembolization and photothermal therapy against hepatocellular carcinoma. ACS Appl. Mater. Interfaces 14, 7579–7591. doi:10.1021/acsami.1c19121
López-Sendón, J., Álvarez-Ortega, C., Auñon, P. Z., Soto, A. B., Lyon, A. B., Cardinale, D., et al. (2020). Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur. heart J. 41, 1720–1729. doi:10.1093/eurheartj/ehaa006
Long, Y., Jia, X., and Chu, L. (2024). Insight into the structure, function and the tumor suppression effect of gasdermin E. Biochem. Pharmacol. 226, 116348. doi:10.1016/j.bcp.2024.116348
Lu, J., Lou, Y., Zhang, Y., Zhong, R., Zhang, W., Zhang, X., et al. (2023). Paclitaxel has a reduced toxicity profile in healthy rats after polymeric micellar nanoparticle delivery. Int. J. Nanomedicine 18, 263–276. doi:10.2147/IJN.S372961
Lu, Y., Wang, Y.-D., Xu, T.-Q., Zhao, X.-H., Zhou, J., Jin, L.-H., et al. (2024). Pyridostigmine attenuates hypertension by inhibiting activation of the renin-angiotensin system in the hypothalamic paraventricular nucleus. Schmiedeb. Arch. Pharmacol. 397, 7995–8007. doi:10.1007/s00210-024-03156-x
L’Abbate, S., Chianca, M., Fabiani, I., Del Franco, A., Giannoni, A., Vergaro, G., et al. (2022). In vivo murine models of cardiotoxicity due to anticancer drugs: challenges and opportunities for clinical translation. J. Cardiovasc. Transl. Res. 15, 1143–1162. doi:10.1007/s12265-022-10231-2
Maghsoudi, S., Shahraki, B. T., Rabiee, N., Fatahi, Y., Dinarvand, R., Tavakolizadeh, M., et al. (2020). Burgeoning polymer nano blends for improved controlled drug release: a review. Int. J. Nanomed. 15, 4363. doi:10.2147/IJN.S252237
Maghsoudi, S., Hosseini, S. A., Soraya, H., Roosta, Y., and Mohammadzadeh, A. (2023). Development of doxorubicin-encapsulated magnetic liposome@PEG for treatment of breast cancer in BALB/c mice. Drug Deliv. Transl. Res. 13, 2589–2603. doi:10.1007/s13346-023-01339-2
Majumder, J., and Minko, T. (2021). Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 18, 205–227. doi:10.1080/17425247.2021.1828339
Makwana, V., Karanjia, J., Haselhorst, T., Anoopkumar-Dukie, S., and Rudrawar, S. (2021). Liposomal doxorubicin as targeted delivery platform: current trends in surface functionalization. Int. J. Pharm. 593, 120117. doi:10.1016/j.ijpharm.2020.120117
Meng, L.-X., Ren, Q., Meng, Q., Zheng, Y.-X., He, M.-L., Sun, S.-Y., et al. (2018). Trastuzumab modified silica nanoparticles loaded with doxorubicin for targeted and synergic therapy of breast cancer. Artif. Cells Nanomed. Biotechnol. 46, S556–S563. doi:10.1080/21691401.2018.1501380
Mi, P., Cabral, H., and Kataoka, K. (2020). Ligand-installed nanocarriers toward precision therapy. Adv. Mater. 32, 1902604. doi:10.1002/adma.201902604
Miao, Y., Yang, T., Yang, S., Yang, M., and Mao, C. (2022). Protein nanoparticles directed cancer imaging and therapy. Nano Converg. 9, 2. doi:10.1186/s40580-021-00293-4
Mirdamadian, S. Z., Varshosaz, J., Minaiyan, M., and Taheri, A. (2022). 3D printed tablets containing oxaliplatin loaded alginate nanoparticles for colon cancer targeted delivery. An in vitro/in vivo study. Int. J. Biol. Macromol. 205, 90–109. doi:10.1016/j.ijbiomac.2022.02.080
Moazzam, M., Zhang, M., Hussain, A., Yu, X., Huang, J., and Huang, Y. (2024). The landscape of nanoparticle-based siRNA delivery and therapeutic development. Mol. Ther. J. Am. Soc. Gene Ther. 32, 284–312. doi:10.1016/j.ymthe.2024.01.005
Moskowitz, A. J., Shah, G., Schöder, H., Ganesan, N., Drill, E., Hancock, H., et al. (2021). Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J. Clin. Oncol. 39, 3109–3117. doi:10.1200/JCO.21.01056
Nam, J., Won, N., Bang, J., Jin, H., Park, J., Jung, S., et al. (2013). Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 65, 622–648. doi:10.1016/j.addr.2012.08.015
Nevins, S., McLoughlin, C. D., Oliveros, A., Stein, J. B., Rashid, M. A., Hou, Y., et al. (2024). Nanotechnology approaches for prevention and treatment of chemotherapy-induced neurotoxicity, neuropathy, and cardiomyopathy in breast and ovarian cancer survivors. Small Weinh. (Bergstr. Ger.) 20, e2300744. doi:10.1002/smll.202300744
Ngamcherdtrakul, W., Morry, J., Gu, S., Castro, D. D. J., Goodyear, D. S. M., Sangvanich, T., et al. (2015). Cationic polymer modified mesoporous silica nanoparticles for targeted SiRNA delivery to HER2+ breast cancer. Adv. Funct. Mater. 25, 2646–2659. doi:10.1002/adfm.201404629
Nguyen, T. T. K., Pham, K.-Y., and Yook, S. (2023). Engineered therapeutic proteins for sustained-release drug delivery systems. Acta Biomater. 171, 131–154. doi:10.1016/j.actbio.2023.09.018
Nooreen, R., Nene, S., Jain, H., Prasannanjaneyulu, V., Chitlangya, P., Otavi, S., et al. (2022). Polymer nanotherapeutics: a versatile platform for effective rheumatoid arthritis therapy. J. Control. Release 348, 397–419. doi:10.1016/j.jconrel.2022.05.054
O’Brien, M. E. R., Wigler, N., Inbar, M., Rosso, R., Grischke, E., Santoro, A., et al. (2004). Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 15, 440–449. doi:10.1093/annonc/mdh097
Overchuk, M., Weersink, R. A., Wilson, B. C., and Zheng, G. (2023). Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano 17, 7979–8003. doi:10.1021/acsnano.3c00891
Pang, Y., Wang, H., and Li, H. (2022). Medical imaging biomarker discovery and integration towards AI-based personalized radiotherapy. Front. Oncol. 11, 764665. doi:10.3389/fonc.2021.764665
Pang, K., Shi, Z.-D., Wei, L.-Y., Dong, Y., Ma, Y.-Y., Wang, W., et al. (2023). Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade. Drug Resist. Updat. 66, 100907. doi:10.1016/j.drup.2022.100907
Passaro, A., Al Bakir, M., Hamilton, E. G., Diehn, M., André, F., Roy-Chowdhuri, S., et al. (2024). Cancer biomarkers: emerging trends and clinical implications for personalized treatment. Cell 187, 1617–1635. doi:10.1016/j.cell.2024.02.041
Pei, Z., Lei, H., and Cheng, L. (2023). Bioactive inorganic nanomaterials for cancer theranostics. Chem. Soc. Rev. 52, 2031–2081. doi:10.1039/d2cs00352j
Peixoto, D., Pereira, I., Pereira-Silva, M., Veiga, F., Hamblin, M. R., Lvov, Y., et al. (2021). Emerging role of nanoclays in cancer research, diagnosis, and therapy. Coord. Chem. Rev. 440, 213956. doi:10.1016/j.ccr.2021.213956
Phatale, V., Vaiphei, K. K., Jha, S., Patil, D., Agrawal, M., and Alexander, A. (2022). Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 351, 361–380. doi:10.1016/j.jconrel.2022.09.025
Quagliariello, V., Vecchione, R., De Capua, A., Lagreca, E., Iaffaioli, R. V., Botti, G., et al. (2020). Nano-encapsulation of coenzyme Q10 in secondary and tertiary nano-emulsions for enhanced cardioprotection and hepatoprotection in human cardiomyocytes and hepatocytes during exposure to anthracyclines and trastuzumab. Int. J. Nanomed. 15, 4859–4876. doi:10.2147/IJN.S245170
Quan, Q., Lin, X., Zhang, N., and Xu, Y.-J. (2017). Graphene and its derivatives as versatile templates for materials synthesis and functional applications. Nanoscale 9, 2398–2416. doi:10.1039/C6NR09439B
Rafiyath, S. M., Rasul, M., Lee, B., Wei, G., Lamba, G., and Liu, D. (2012). Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp. Hematol. Oncol. 1, 10. doi:10.1186/2162-3619-1-10
Rajeev, M. R., Manjusha, V., and Anirudhan, T. S. (2023). Transdermal delivery of doxorubicin and methotrexate from polyelectrolyte three layer nanoparticle of graphene oxide/polyethyleneimine/dextran sulphate for chemotherapy: in vitro and in vivo studies. Chem. Eng. J. 466, 143244. doi:10.1016/j.cej.2023.143244
Rana, K., Kumar Pandey, S., Chauhan, S., and Preet, S. (2022). Anticancer therapeutic potential of 5-fluorouracil and nisin co-loaded chitosan coated silver nanoparticles against murine skin cancer. Int. J. Pharm. 620, 121744. doi:10.1016/j.ijpharm.2022.121744
Ratajczak, K., Grel, H., Olejnik, P., Jakiela, S., and Stobiecka, M. (2023). Current progress, strategy, and prospects of PD-1/PDL-1 immune checkpoint biosensing platforms for cancer diagnostics, therapy monitoring, and drug screening. Biosens. Bioelectron. 240, 115644. doi:10.1016/j.bios.2023.115644
Rayson, D., Suter, T. M., Jackisch, C., van der Vegt, S., Bermejo, B., van den Bosch, J., et al. (2012). Cardiac safety of adjuvant pegylated liposomal doxorubicin with concurrent trastuzumab: a randomized phase II trial. Ann. Oncol. 23, 1780–1788. doi:10.1093/annonc/mdr519
Rezaei, Z., Wang, N., Yang, Y., Govindaraj, K., Velasco, J. J., Martinez Blanco, A. D., et al. (2025). Enhancing organoid technology with carbon-based nanomaterial biosensors: advancements, challenges, and future directions. Adv. Drug Deliv. Rev. 222, 115592. doi:10.1016/j.addr.2025.115592
Saadh, M. J., Mustafa, M. A., Kumar, A., Alamir, H. T. A., Kumar, A., Khudair, S. A., et al. (2024). Stealth nanocarriers in cancer therapy: a comprehensive review of design, functionality, and clinical applications. Aaps Pharmscitech 25, 140. doi:10.1208/s12249-024-02843-5
Saifi, M. A., Seal, S., and Godugu, C. (2021). Nanoceria, the versatile nanoparticles: promising biomedical applications. J. Control. Release 338, 164–189. doi:10.1016/j.jconrel.2021.08.033
Santin, Y., Formoso, K., Haidar, F., Fuentes, M. D. P. O., Bourgailh, F., Hifdi, N., et al. (2023). Inhalation of acidic nanoparticles prevents doxorubicin cardiotoxicity through improvement of lysosomal function. Theranostics 13, 5435–5451. doi:10.7150/thno.86310
Sarfraz, M., Arafat, M., Zaidi, S. H. H., Eltaib, L., Siddique, M. I., Kamal, M., et al. (2023). Resveratrol-laden nano-systems in the cancer environment: views and reviews. Cancers 15, 4499. doi:10.3390/cancers15184499
Scioli Montoto, S., Muraca, G., and Ruiz, M. E. (2020). Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 7, 587997. doi:10.3389/fmolb.2020.587997
Shetty, Y., Prabhu, P., and Prabhakar, B. (2019). Emerging vistas in theranostic medicine. Int. J. Pharm. 558, 29–42. doi:10.1016/j.ijpharm.2018.12.068
Siegel, R. L., Giaquinto, A. N., and Jemal, A. (2024). Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49. doi:10.3322/caac.21820
Singh, R., Alshaghdali, K., Saeed, A., Kausar, M. A., Aldakheel, F. M., Anwar, S., et al. (2022). Prospects of microbial-engineering for the production of graphene and its derivatives: application to design nanosystms for cancer theranostics. Semin. Cancer Biol. 86, 885–898. doi:10.1016/j.semcancer.2021.05.017
Sivadasan, D., Ramakrishnan, K., Mahendran, J., Ranganathan, H., Karuppaiah, A., and Rahman, H. (2023). Solid lipid nanoparticles: applications and prospects in cancer treatment. Int. J. Mol. Sci. 24, 6199. doi:10.3390/ijms24076199
Su, X., Zhang, X., Liu, W., Yang, X., An, N., Yang, F., et al. (2022). Advances in the application of nanotechnology in reducing cardiotoxicity induced by cancer chemotherapy. Semin. Cancer Biol. 86, 929–942. doi:10.1016/j.semcancer.2021.08.003
Sun, X., Lian, Y., Tian, T., and Cui, Z. (2024). Advancements in functional nanomaterials inspired by viral particles. Small 20, e2402980. doi:10.1002/smll.202402980
Sun, C., Chen, J., Bai, S., Liao, W., Chen, J., Guo, W., et al. (2025). Recent progress in nano-TCM active ingredient Co-delivery systems for inflammation-mediated diseases. Int. J. Nanomed. 20, 9573–9596. doi:10.2147/IJN.S526731
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Swierczewska, M., Han, H. S., Kim, K., Park, J. H., and Lee, S. (2016). Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 99, 70–84. doi:10.1016/j.addr.2015.11.015
Syed Azhar, S. N. A., Ashari, S. E., Zainuddin, N., and Hassan, M. (2022). Nanostructured lipid carriers-hydrogels system for drug delivery: nanohybrid technology perspective. Mol. (Basel Switz.) 27, 289. doi:10.3390/molecules27010289
Tai, P., Chen, X., Jia, G., Chen, G., Gong, L., Cheng, Y., et al. (2023). WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J. Transl. Med. 21, 823. doi:10.1186/s12967-023-04715-1
Tan, P., Chen, X., Zhang, H., Wei, Q., and Luo, K. (2023). Artificial intelligence aids in development of nanomedicines for cancer management. Semin. Cancer Biol. 89, 61–75. doi:10.1016/j.semcancer.2023.01.005
Tang, L., Xiao, Q., Mei, Y., He, S., Zhang, Z., Wang, R., et al. (2021). Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnology 19, 423. doi:10.1186/s12951-021-01174-y
Tang, L., Li, J., Pan, T., Yin, Y., Mei, Y., Xiao, Q., et al. (2022). Versatile carbon nanoplatforms for cancer treatment and diagnosis: strategies, applications and future perspectives. Theranostics 12, 2290–2321. doi:10.7150/thno.69628
Tenchov, R., Sasso, J. M., Wang, X., Liaw, W.-S., Chen, C.-A., and Zhou, Q. A. (2022). Exosomes─Nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano 16, 17802–17846. doi:10.1021/acsnano.2c08774
Thivat, E., Casile, M., Moreau, J., Molnar, I., Dufort, S., Seddik, K., et al. (2023). Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC Cancer 23, 344. doi:10.1186/s12885-023-10829-y
Torrini, F., Ferraro, G., Fratini, E., Palladino, P., Scarano, S., and Minunni, M. (2024). Toward nano-sized imprinted norepinephrine-derived biopolymer as artificial receptors for detecting IgG1 by surface plasmon resonance. Biosens. Bioelectron. 252, 116133. doi:10.1016/j.bios.2024.116133
Umapathi, A., Kumawat, M., and Daima, H. K. (2022). Engineered nanomaterials for biomedical applications and their toxicity: a review. Environ. Chem. Lett. 20, 445–468. doi:10.1007/s10311-021-01307-7
Vazhappilly, C. G., Amararathna, M., Cyril, A. C., Linger, R., Matar, R., Merheb, M., et al. (2021). Current methodologies to refine bioavailability, delivery, and therapeutic efficacy of plant flavonoids in cancer treatment. J. Nutr. Biochem. 94, 108623. doi:10.1016/j.jnutbio.2021.108623
Verry, C., Dufort, S., Villa, J., Gavard, M., Iriart, C., Grand, S., et al. (2021). Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 160, 159–165. doi:10.1016/j.radonc.2021.04.021
Voss, M. H., Hussain, A., Vogelzang, N., Lee, J. L., Keam, B., Rha, S. Y., et al. (2017). A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann. Oncol.28, 2754–2760. doi:10.1093/annonc/mdx493
Wang, J., and Zhan, X. (2021). Fused-ring electron acceptors for photovoltaics and beyond. Acc. Chem. Res. 54, 132–143. doi:10.1021/acs.accounts.0c00575
Wang, J., Tang, W., Yang, M., Yin, Y., Li, H., Hu, F., et al. (2021). Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials 273, 120784. doi:10.1016/j.biomaterials.2021.120784
Wang, L., Li, Z., Wang, Y., Gao, M., He, T., Zhan, Y., et al. (2023). Surface ligand-assisted synthesis and biomedical applications of metal-organic framework nanocomposites. Nanoscale 15, 10529–10557. doi:10.1039/d3nr01723k
Wei, S., Ma, W., Zhang, B., and Li, W. (2021). NLRP3 inflammasome: a promising therapeutic target for drug-induced toxicity. Front. Cell Dev. Biol. 9, 634607. doi:10.3389/fcell.2021.634607
Wu, L., Wen, W., Wang, X., Huang, D., Cao, J., Qi, X., et al. (2022). Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 19, 24. doi:10.1186/s12989-022-00465-y
Xing, M., Yan, F., Yu, S., and Shen, P. (2015). Efficacy and cardiotoxicity of liposomal doxorubicin-based chemotherapy in advanced breast cancer: a meta-analysis of ten randomized controlled trials. PLOS One 10, e0133569. doi:10.1371/journal.pone.0133569
Xu, Y., Zhai, X., Su, P., Liu, T., Zhou, L., Zhang, J., et al. (2020). Highly stable semiconducting polymer nanoparticles for multi-responsive chemo/photothermal combined cancer therapy. Theranostics 10, 5966–5978. doi:10.7150/thno.43090
Yadav, B., Chauhan, M., Shekhar, S., Kumar, A., Mehata, A. K., Nayak, A. K., et al. (2023). RGD-decorated PLGA nanoparticles improved effectiveness and safety of cisplatin for lung cancer therapy. Int. J. Pharm. 633, 122587. doi:10.1016/j.ijpharm.2023.122587
Yang, Z., Li, L., Jin, A. J., Huang, W., and Chen, X. (2020). Rational design of semiconducting polymer brushes as cancer theranostics. Mater Horiz. 7, 1474–1494. doi:10.1039/d0mh00012d
Yang, M., Li, J., Gu, P., and Fan, X. (2021). The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 6, 1973–1987. doi:10.1016/j.bioactmat.2020.12.010
Yang, L., Zhang, Y., Zhang, Y., Xu, Y., Li, Y., Xie, Z., et al. (2022). Live macrophage-delivered doxorubicin-loaded liposomes effectively treat triple-negative breast cancer. ACS Nano 16, 9799–9809. doi:10.1021/acsnano.2c03573
Yang, M., Abudureyimu, M., Wang, X., Zhou, Y., Zhang, Y., and Ren, J. (2023). PHB2 ameliorates Doxorubicin-induced cardiomyopathy through interaction with NDUFV2 and restoration of mitochondrial complex I function. Redox Biol. 65, 102812. doi:10.1016/j.redox.2023.102812
Ye, T., Yang, W., Gao, T., Yu, X., Chen, T., Yang, Y., et al. (2023). Trastuzumab-induced cardiomyopathy via ferroptosis-mediated mitochondrial dysfunction. Free Radic. Biol. Med. 206, 143–161. doi:10.1016/j.freeradbiomed.2023.06.019
Yu, C., Li, L., Hu, P., Yang, Y., Wei, W., Deng, X., et al. (2021). Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv. Sci. (Weinh) 8, 2100540. doi:10.1002/advs.202100540
Yu, B., Shen, Y., Zhang, X., Ding, L., Meng, Z., Wang, X., et al. (2022). Poly(methacrylate citric acid) as a dual functional carrier for tumor therapy. Pharmaceutics 14, 1765. doi:10.3390/pharmaceutics14091765
Zeng, S., Tang, Q., Xiao, M., Tong, X., Yang, T., Yin, D., et al. (2023). Cell membrane-coated nanomaterials for cancer therapy. Mater. Today Bio 20, 100633. doi:10.1016/j.mtbio.2023.100633
Zhang, Z., Yu, X., Wang, Z., Wu, P., and Huang, J. (2015). Anthracyclines potentiate anti-tumor immunity: a new opportunity for chemoimmunotherapy. Cancer Lett. 369, 331–335. doi:10.1016/j.canlet.2015.10.002
Zhang, T., Guo, W., Zhang, C., Yu, J., Xu, J., Li, S., et al. (2017). Transferrin-dressed virus-like ternary nanoparticles with aggregation-induced emission for targeted delivery and rapid cytosolic release of siRNA. ACS Appl. Mater Interfaces 9, 16006–16014. doi:10.1021/acsami.7b03402
Zhang, A., Meng, K., Liu, Y., Pan, Y., Qu, W., Chen, D., et al. (2020). Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv. Colloid Interface Sci. 284, 102261. doi:10.1016/j.cis.2020.102261
Zhang, J., Jiang, H., Zhang, J., Bao, G., Zhang, G., Wang, H., et al. (2021). Effectiveness and safety of pegylated liposomal doxorubicin versus epirubicin as neoadjuvant or adjuvant chemotherapy for breast cancer: a real-world study. BMC Cancer 21, 1301. doi:10.1186/s12885-021-09050-6
Zhang, J., Huang, L., Ge, G., and Hu, K. (2023a). Emerging epigenetic-based nanotechnology for cancer therapy: modulating the tumor microenvironment. Adv. Sci. Weinh. (Baden-wurtt. Ger.) 10, e2206169. doi:10.1002/advs.202206169
Zhang, L., Li, M., Wang, Y., Liu, Y., Zhang, F., Lin, Z., et al. (2023b). Hollow-polydopamine-nanocarrier-based near-infrared-light/pH-responsive drug delivery system for diffuse alveolar hemorrhage treatment. Front. Chem. 11, 1222107. doi:10.3389/fchem.2023.1222107
Zhang, P., Zhu, Y., Xiao, C., and Chen, X. (2023c). Activatable dual-functional molecular agents for imaging-guided cancer therapy. Adv. Drug Deliv. Rev. 195, 114725. doi:10.1016/j.addr.2023.114725
Zhang, C., Yuan, Y., Xia, Q., Wang, J., Xu, K., Gong, Z., et al. (2025). Machine learning-driven prediction, preparation, and evaluation of functional nanomedicines via drug-drug self-assembly. Adv. Sci. Weinh. (Baden-wurtt. Ger.) 12, e2415902. doi:10.1002/advs.202415902
Zhang, S., Liu, Q., Chang, M., Pan, Y., Yahaya, B. H., Liu, L., et al. (2023). Chemotherapy impairs ovarian function through excessive ROS-induced ferroptosis. Cell Death Dis. 14, 340. doi:10.1038/s41419-023-05859-0
Zhao, M., van Straten, D., Broekman, M. L. D., Preat, V., and Schiffelers, R. M. (2020). Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 10, 1355–1372. doi:10.7150/thno.38147
Zhao, Y., Liu, T., and Zhou, M. (2022). Immune-cell-derived exosomes for cancer therapy. Mol. Pharm. 19, 3042–3056. doi:10.1021/acs.molpharmaceut.2c00407
Zhao, L., Li, J., Song, Z., Wang, X., and Yu, S. (2023). Self-assembled growth of SnO2 nanoshells on copper nanowires for stable and transparent conductors. ACS Appl. Nano Mater. 6, 10658–10667. doi:10.1021/acsanm.3c01608
Zhao, X.-P., Duan, L., Zhao, Q.-R., Lv, X., Tian, N.-Y., Yang, S.-L., et al. (2025). NLRP3 inflammasome as a therapeutic target in doxorubicin-induced cardiotoxicity: role of phytochemicals. Front. Pharmacol. 16, 1567312. doi:10.3389/fphar.2025.1567312
Zhou, Z., Ji, Z., Wang, S., Haque, F., and Guo, P. (2016). Oriented single directional insertion of nanochannel of bacteriophage SPP1 DNA packaging motor into lipid bilayer via polar hydrophobicity. Biomaterials 105, 222–227. doi:10.1016/j.biomaterials.2016.08.002
Zhou, Y., Tao, L., Qiu, J., Xu, J., Yang, X., Zhang, Y., et al. (2024). Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 9, 132. doi:10.1038/s41392-024-01823-2
Zhu, M., Zhuang, J., Li, Z., Liu, Q., Zhao, R., Gao, Z., et al. (2023). Machine-learning-assisted single-vessel analysis of nanoparticle permeability in tumour vasculatures. Nat. Nanotechnol. 18, 657–666. doi:10.1038/s41565-023-01323-4
Zou, X., Jiang, Z., Li, L., and Huang, Z. (2021). Selenium nanoparticles coated with pH responsive silk fibroin complex for fingolimod release and enhanced targeting in thyroid cancer. Artif. Cells Nanomed Biotechnol. 49, 83–95. doi:10.1080/21691401.2021.1871620
Keywords: cardio-oncology, nanotechnology, chemotherapy, synergy, cancer therapy
Citation: Ma L, Zhang B, Liu X, Gao S, Kong S, Li Y, Wang R, Li M, Mao X, Li Y, Luo Y, Li L, Lv C and Huang Y (2025) Nanotechnology-driven synergy in cardio-oncology: enhancing tumor suppression and reducing cardiotoxicity. Front. Pharmacol. 16:1641618. doi: 10.3389/fphar.2025.1641618
Received: 05 June 2025; Accepted: 09 September 2025;
Published: 03 October 2025.
Edited by:
Qihua He, First Affiliated Hospital of Guangzhou Medical University, ChinaReviewed by:
Xinyu Wang, Philadelphia College of Osteopathic Medicine (PCOM), United StatesAmit Manhas, Stanford University, United States
Xun Guo, The First Branch of The First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2025 Ma, Zhang, Liu, Gao, Kong, Li, Wang, Li, Mao, Li, Luo, Li, Lv and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxiao Lv, bHZjaHVueGlhbzE5ODlAMTYzLmNvbQ==; Yuhong Huang, aHloMTAxQDEyNi5jb20=
†These authors have contributed equally to this work
 Luyao Ma1,2†
Luyao Ma1,2† Bowen Zhang
Bowen Zhang Yuhong Li
Yuhong Li Chunxiao Lv
Chunxiao Lv Yuhong Huang
Yuhong Huang