- 1Department of Rehabilitation Medicine, School of Acupuncture-Moxibustion and Tuina and School of Health Preservation and Rehabilitation, Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Cardiology, Kunshan Hospital of Traditional Chinese Medicine, Kunshan, China
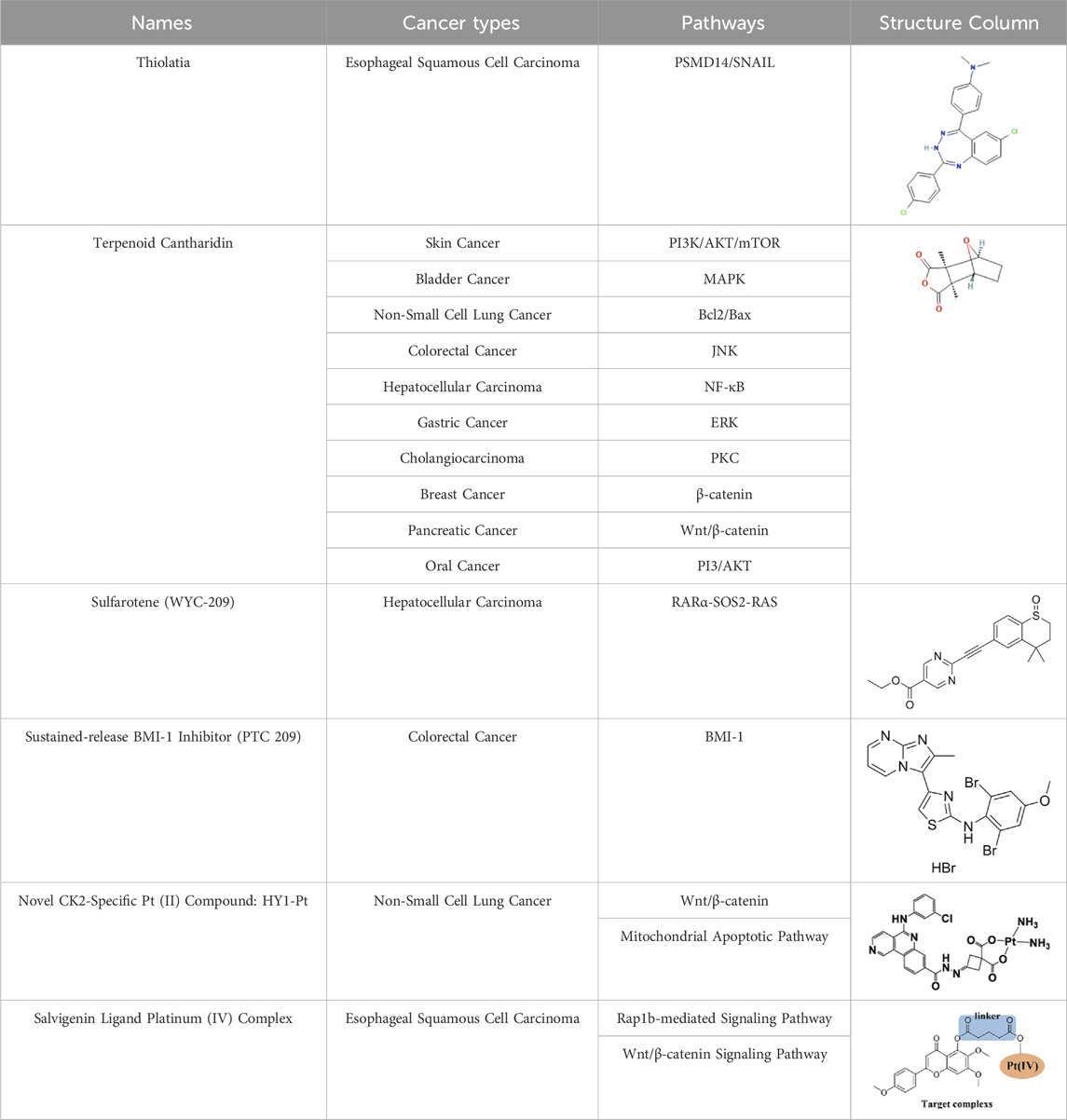
Cancer metastasis and stem cells (CSCs) drive resistance and most cancer deaths. Novel agents like Thiolatia (PSMD14 inhibitor) suppress metastasis and enhance chemotherapy efficacy. Sulfarotene targets tumor-repopulating cells in liver cancer with low toxicity. PTC 209 utilizes the high affinity of modified hyaluronic acid nanoparticles for colorectal cancer to reverse CSC stemness in colorectal cancer. Platinum hybrids (HY1-Pt, Salvigenin-Pt) overcome resistance through dual mechanisms. Natural compound Cantharidin inhibits metastasis but requires toxicity optimization. These strategies emphasize specificity, nanodelivery, and combination therapies to reduce toxicity and resistance, highlighting precision oncology potential. Clinical validation remains critical for translation.
1 Introduction
Cancer is a heterogeneous disease composed of multiple cells characterized by abnormal cell growth and proliferation (Roy and Saikia, 2016). About 90% of cancer patients die from tumor metastasis (Torre et al., 2016; Wang et al., 2018), and therapeutic options to stop tumor metastasis include inhibiting neoangiogenesis, blocking epithelial mesenchymal transition and targeting metastasis suppressors (Gerstberger et al., 2023). Some of the drugs that have been applied in the clinic such as Denosumab (RANKL monoclonal antibody) was approved in 2018 for the prevention of multiple myeloma and bone metastases, which reduces bone metastasis by inhibiting osteoclast activity (Clézardin et al., 2021); bevacizumab (VEGF monoclonal antibody) is used in the treatment of colon cancer and lung cancer by inhibiting tumor angiogenesis (Garcia et al., 2020), etc., but the current drugs used in the treatment of cancer metastasis have problems such as high toxicity. However, the current drugs for cancer metastasis have problems such as high toxicity, easy drug resistance, etc. Therefore the development of new anti-tumor metastasis drugs is imminent.
Cancer stem cells (CSCs) are a key subpopulation in tumors with properties such as self-renewal, differentiation, invasion, and drug resistance that drive tumorigenesis, metastasis, and recurrence (Batlle and Clevers, 2017). CSCs have become one of the main causes of therapeutic failure by mediating chemotherapy resistance through mechanisms such as transport proteins and gene mutations (Nassar and Blanpain, 2016). Tumor metastasis and drug resistance are both dependent on CSCs, and their unlimited proliferative capacity and resistance to standard therapies are major challenges in current tumor therapy (Walcher et al., 2020). Therefore, targeted reversal of CSCs stemness may become an important research direction for the treatment of tumor metastasis.
Currently, advanced stage II cancers are usually only detected after multiple metastases have occurred. Challenges associated with detecting dormant cancer cells or small metastases further complicate cancer treatment. In addition, drugs targeting cancer metastasis typically exhibit high cytotoxicity, inconsistent patient outcomes, and lead to the development of drug resistance (Bagchi et al., 2021; Wang M. et al., 2021). Therefore, there is an urgent need to develop novel small molecules, biologic drugs, and combination therapies that target key processes in cancer metastasis (Cha et al., 2020; Walcher et al., 2020). For example, Nethi and Li et al. integrated epidermal growth factor receptor (EGFR) targeted antibodies into mesenchymal stem cells (MSCs) and used them in combination with paclitaxel nanoparticles for delivery. This significantly inhibited the growth of in situ A549 tumors and effectively improved overall survival rates (Nethi et al., 2023). This paper summarizes recent research advances in drugs, mechanisms and potential therapeutic targets against tumor metastasis and stemness. The main topics include: novel inhibitors such as Thiolatia (PSMD14 inhibitor), PTC 209 (BMI-1 inhibitor); new and improved specific compounds such as HY1-Pt, Salvigenin platinum (IV) complex; multi-targeting inhibitor CTD, and low-toxicity inhibitor WYC-209, as show in Table 1.
2 Novel small molecules and drugs
2.1 Thiolatia: a PSMD14 inhibitor
Thiolatia (THL), a zinc chelator, is a disulfide-containing antibiotic and antiangiogenic compound. THL has been shown to inhibit adhesion to vitronectin and tumor-induced angiogenesis in vivo by decreasing paxillin in human umbilical vein endothelial cells (HUVECs) (Minamiguchi et al., 2001); and to block Hsp27 phosphorylation by inducing endothelial cell adhesion and wound/tumor-driven angiogenesis in vitro (Jia et al., 2010). Moreover, THL inhibits JAMM domain-containing proteases such as PSMD14 through catalytic Zn2+ ion complexes with the enzyme’s active center (Lauinger et al., 2017).
PSMD14 is highly expressed in a variety of cancers and acts as an oncogene to promote tumor development and progression (Zhu et al., 2018); it has been suggested that PSMD14 may be involved in esophageal squamous cell carcinoma (ESCC) tumorigenesis (Ma et al., 2016; Seo et al., 2019; Lv et al., 2020). As shown in Figure 1A that THL reverses the epidermal mesenchymal transition (EMT) process (Lamouille et al., 2014; Fischer et al., 2015; Zheng et al., 2015) (e.g., upregulation of E-Cadherin, inhibition of mesenchymal marker expression) by blocking PSMD14-mediated deubiquitination of SNAIL and decreasing the stability of SNAIL protein (Liu et al., 2017; Perez et al., 2017; Wu et al., 2017; Li et al., 2018). In addition, THL interferes with cytoskeletal reorganization and inhibits the formation of invagination cristae, which is essential for cell motility, weakening the motility and invasive ability of tumor cells. It also enhances the sensitivity of cancer cells to cisplatin, reducing side effects by reducing the dose of cisplatin, resulting in patient benefit (Jing et al., 2021).
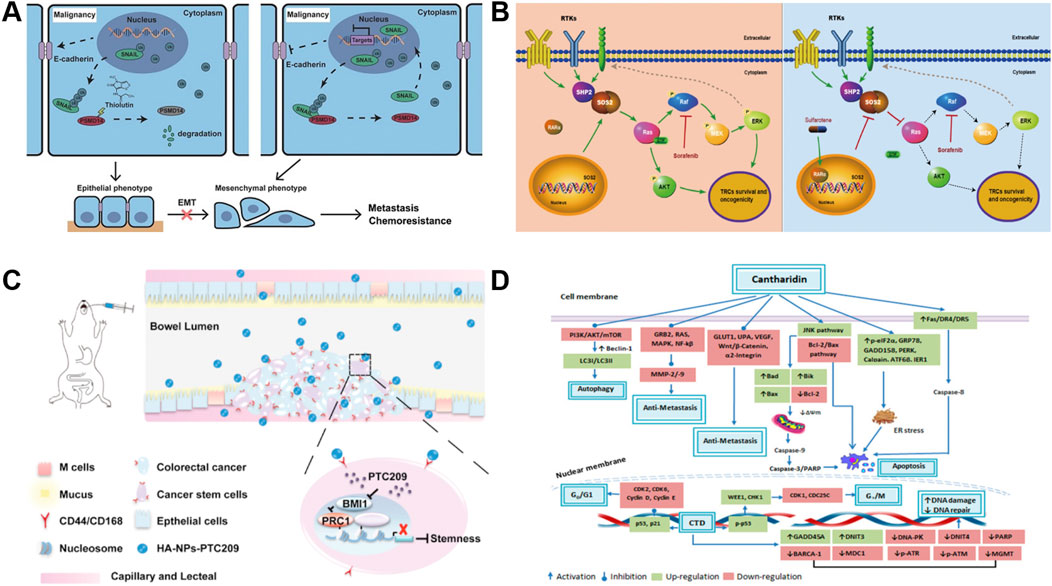
Figure 1. Mechanism of action of novel small molecules and drugs. (A) Schematic of the mechanism of THL for suppressing ESCC malignancy. Reproduced with permission from Jing et al. (2021). Image copyright belongs to Ivyspring International Publisher; no permission is required. (B) Schema depicting the mechanism by which sulfarotene targets the RAR SOS2-RAS signal axis to inhibit cancer cell growth and overcome drug resistance. Reproduced with permission from Qi et al. (2021), licensed under CC BY. (C) Proposed mechanism of HA-NPs-PTC209 action. After oral treatment, HA-NPs-PTC209 actively target CSCs in colorectal cancer (CRC) and are internalized. Consequently, the released PTC209 inhibits BMI-1 and downregulates the expression of stemness-re lated proteins to lower tumor stemness and recur rence. Reproduced with permission from Xu et al. (2019). Permission has been obtained from Elsevier. (D) Nticancer attributes of cantharidin and its molecular targets. Reproduced with permission from Naz et al. (2020), licensed under CC BY.
Traditional proteasome inhibitors (e.g., bortezomib, carfilzomib) have more serious toxic side effects and limited efficacy in solid tumors compared with specific target inhibitors such as THL (Hideshima et al., 2005; Li J. et al., 2017; Song et al., 2017). In contrast, THL, as a PSMD14-specific inhibitor, is more selective and may be a safer anti-metastatic drug by precisely regulating the EMT and chemo-sensitivity-related pathways, combining both anti-metastatic and chemo-sensitizing effects (Deshaies, 2014; Jing et al., 2021).
2.2 Sulfarotene (WYC-209)
Sulfarotene (WYC-209) is advantageous in that it possesses negligible toxicity and highly selective inhibition of the growth and tumor-inducing ability of tumor reconstructive cells (TRCs) (Jiang et al., 2020) in various types of cancers. It is believed that sulfarotene blocks the activation of pro-tumorigenic signals downstream of RAS by upregulating retinoic acid receptor α (RARα) in hepatocellular carcinoma (HCC) TRCs and inhibiting the expression of SOS2 (Liceras-Boillos et al., 2018), a key mediator of the RAS signaling pathway (Sheffels et al., 2018; Schwartz et al., 2019; Wołoszynowska-Fraser et al., 2020) (Figure 1B). This pathway not only drives the self-renewal and tumorigenicity of TRCs (Li et al., 2015), but is also closely associated with resistance to drugs such as sorafenib. In multiple preclinical models, sulfarotene demonstrated efficient and selective inhibition of HCC TRCs (Qi et al., 2021).
Traditional targeted drugs (e.g., sorafenib) are limited in their clinical application due to tumor cell stemness-induced resistance and their inherent toxicity (Wei et al., 2019; Wang et al., 2020; Xia et al., 2020). By precisely targeting the RARα-SOS2-RAS axis and directly interfering with the stemness maintenance and resistance mechanisms of TRCs, sulfarotene not only overcomes the limitations of existing drugs, but also demonstrates a potent inhibitory effect on metastatic foci. It provides a highly promising therapeutic strategy to improve the prognosis of HCC patients (Qi et al., 2021).
2.3 Sustained release BMI 1 inhibitor (PTC 209)
Many anticancer drugs belong to the class IV of the Biopharmaceutical Classification System (BCS), which comprises substances with both low solubility in aqueous fluids and low apparent permeability. The high recurrence and metastasis of colorectal cancer (CRC) (Todaro et al., 2010) are often attributed to the maintenance of stemness in cancer stem cells (CSCs) (Batlle and Clevers, 2017), whose self-renewal, drug-resistant, and invasive properties lead to therapeutic failures (Zhang Z. et al., 2016). BMI-1 (B-cell-specific Moloney Murine Leukemia Virus Integration Site 1), as a key regulator of the stemness of CSCs (Kreso et al., 2014), is overexpressed in CRC and correlates with tumor progression and poor prognosis (Siddique and Saleem, 2012). Inhibition of BMI-1 can reverse the stemness of CSCs and has been applied to the treatment of colon cancer, myeloma and acute myeloid leukemia (Mourgues et al., 2015). Based on this, the investigators developed PTC209, a specific inhibitor against BMI-1 (Bolomsky et al., 2016).
To solve the problems of poor solubility, complex gastrointestinal environment and non-specific distribution faced by oral drug delivery (Mitragotri et al., 2014), the researchers developed a targeted delivery system based on poly (ethylene glycol)-poly (hydroxyglycolic acid) lactate (PEG-PLGA) nanoparticles (Wang et al., 2011; Mazzaferro et al., 2013; Lin et al., 2018). The nanoparticles were synthesized by a double emulsion method and modified with hyaluronic acid (HA) as a CD44/CD168-targeting ligand (HA-NPs-PTC209) (Huang et al., 2014; Choi et al., 2019; Leng et al., 2019), which significantly enhanced the targeting ability of the BMI-1 inhibitor (Zhang M. et al., 2016). The results of the in vivo antitumor experiments showed that HA-NPs-PTC209 significantly inhibited the growth and metastasis of CT26 orthogonal xenografts, which led to the in situ colon tumor accumulation in in situ colon tumors, thus reversing CSC stemness. The high in vitro stability of this targeted nano-example and the high permeability of the drug through the intestinal barrier offer the possibility of mitigating systemic adverse effects and improving therapeutic efficiency (Xu et al., 2019) (Figure 1C).
2.4 Novel CK2-specific platinum (II) compounds: HY1-Pt
The high mortality rate of non-small cell lung cancer (NSCLC) is closely related to chemoresistance and metastasis mediated by cancer stem cells (CSCs) (Martins-Neves et al., 2018). Conventional platinum-based drugs (e.g., cisplatin) have limited efficacy due to the DNA damage repair ability and drug resistance of CSCs (Nagasaka and Gadgeel, 2018; Wang et al., 2019). The CK2 inhibitor HY1 was found to have a strong inhibitory effect on CSCs in A549 cells (Schwind et al., 2017). Taking advantage of the inherent CK2 specificity and CSC inhibition of HY1, by conjugating HY1 with an active platinum (II) unit (Czarnomysy et al., 2018), the researchers developed a novel CK2-specific platinum (II) compound, HY1-Pt, which achieves the reversal of drug resistance and the inhibition of CSCs through the synergistic effect of targeting protein kinase CK2 with platinum-based drugs (Purwin et al., 2016). It was demonstrated that HY1-Pt specifically inhibited CK2 activity, blocked its mediated stemness-promoting signaling pathways such as Hedgehog/Gli1 and Wnt/β-catenin, and downregulated the expression of CSCs markers (e.g., Nanog, Oct-4) (Lu et al., 2013; Wang et al., 2017). Furthermore, HY1-Pt could enhance platinum drugs-induced DNA damage by interfering with the phosphorylation of DNA repair proteins by CK2 to inhibit the tumor cell repair ability. In vitro experiments demonstrated that HY1-Pt showed potent cytotoxicity (IC50 significantly lower than cisplatin) and selectively inhibited the formation of CSCs spheroids in A549/cDDP cells and in the A549/cDDP xenograft model, HY1-Pt significantly inhibited the tumor growth without triggering significant toxicity reactions.
Existing CK2 inhibitors (e.g., CX-4945) have limited clinical efficacy, while conventional platinum drugs are limited by drug resistance and toxicity. HY1-Pt’s synergistic effect through a “dual-targeting” strategy (CK2 inhibition + platinum DNA damage) is expected to improve the prospects for platinum-based therapies and to reverse the resistance to cisplatin (Wang et al., 2021b).
2.5 Salvigenin ligand platinum (IV) complexes
The high invasiveness and cisplatin resistance of esophageal squamous cell carcinoma (ESCC) are closely related to the maintenance of stemness in cancer stem cells (CSCs) (Najafi et al., 2019; Mao et al., 2021). The expression level of RAS-associated protein 1b (Rap1b), as a member of the RAS superfamily, has been confirmed to correlate positively with stemness of CSCs, and overexpression of Rap1b in ESCC positively regulates CSC proliferation, invasion, and stemness, making it a novel target for reversing ESCC drug resistance (Noguchi et al., 2015; Jia et al., 2017; Zhang et al., 2019). Guo et al. found that Rap1b was overexpressed in glioma stem cells (GSCs), and silencing Rap1b could effectively inhibit the growth and invasion of glioma cells (Guo et al., 2023). In addition, Rap1b has been shown to promote hematopoietic stem cell development by enhancing integrin-mediated cell adhesion (Rho et al., 2019).
Based on this, researchers have developed novel platinum (IV) complexes (e.g., complex-1) (Zhang et al., 2017) with the natural polyphenolic compound Salvigenin as a ligand to inhibit Rap1b and overcome cisplatin resistance through a dual mechanism. Complex-1, which consists of a platinum (IV) core conjugated with Salvigenin ligand, significantly downregulates cancer cell stemness by inhibiting Rap1b expression and blocking its mediated integrin signaling and Wnt/β-catenin/TCF pathway; and enhances the platinum accumulation in cisplatin-resistant cells (TE6/cDDP) and inhibits the DNA damage repair ability (Fang et al., 2019).
As the first platinum (IV) complex that potently inhibits Rap1b and effectively reverses cisplatin-induced drug resistance, complex-1 fills the gap that there is no effective solid molecule inhibitor for Rap1b, and provides a new way of thinking for the development of Rap1b inhibitors and overcoming cisplatin-induced drug resistance in cancer cells (Zhao et al., 2024).
2.6 Terpenoid cantharidin (CTD)
Cantharidin (CTD), a terpenoid isolated from blister beetles and used in traditional Chinese medicine for the treatment of a variety of diseases and cancers (Deng et al., 2013), has been shown to be an inhibitor of protein phosphatase 2A (PP2A) and heat shock transcription factor 1 (HSF-1), both of which are potential anticancer targets (Kim et al., 2013; Li W. et al., 2017). As shown in Figure 1D, CTD significantly inhibits the proliferation of a variety of solid tumors and leukemia cells, including liver cancer, pancreatic cancer, and colon cancer, by inhibiting PP1/PP2A activity, inducing apoptosis, interfering with cell cycle arrest and autophagy (Wu et al., 2014; Shen et al., 2015). By inhibiting PP1/PP2A activity, inducing apoptosis, interfering with protein synthesis, and triggering cell cycle arrest and autophagy, CTD significantly inhibits the proliferation of hepatocellular carcinoma, pancreatic carcinoma, colon carcinoma, and other solid tumors as well as leukemia cells (Huang et al., 2011; Hsia et al., 2015b; Ji et al., 2015; Su et al., 2015; Su et al., 2016; Liu et al., 2018). In addition, CTD can reduce the expression of DNA damage repair-related proteins, enhance the sensitivity of cancer cells to radiotherapy, and alleviate the sequelae of chemotherapy in combination therapy (Kuo et al., 2015; Xu et al., 2018).
Studies have demonstrated that CTD inhibits cancer cell invasion and migration by targeting and regulating metastasis-related signaling pathways in a variety of cancer cells. For example, in gastric cancer, CTD inhibited migration by down-regulating the CCAT1-mediated PI3K/AKT pathway (Song et al., 2020); in bladder cancer cells, it blocked cell adhesion and invasion by inhibiting the p38/JNK1/2 MAPK pathway and decreasing the enzyme activity and expression of MMP-2/9. In addition, CTD inhibited metastatic potential by inhibiting the PI3K/AKT/mTOR and NF-κB pathways and reducing UPA protein and matrix metalloproteinase activities in lung cancer models (Hsia et al., 2016).
CTD can effectively inhibit metastasis in different kinds of cancer cells; and the action of CTD involves key pathways such as MAPK, Bcl2/Bax, Wnt/β-catenin, ERK, etc., which inhibit tumor growth and metastasis through cross-regulation (Wang et al., 2015; Gu et al., 2017; Chun et al., 2018). Among them, PI3K/AKT/mTOR and MAPK pathways have been widely proved to be the core targets of CTD against tumor metastasis, and CTD, as a multi-targeted anticancer agent, has demonstrated its unique advantages in inhibiting tumor growth, metastasis, and synergistic radiotherapy. However, the toxicity of CTD itself remains to be solved (Hsia et al., 2015a; Naz et al., 2020).
3 Summary
Cancer metastasis and stemness maintenance of cancer stem cells (CSCs) are central causes of tumor treatment failure. About 90% of cancer patients die from metastasis, and the self-renewal, drug-resistant and invasive properties of CSCs drive tumor recurrence and spread (Torre et al., 2016; Wang et al., 2018). In recent years, significant progress has been made in the study of novel drugs and targets against CSCs and key pathways of metastasis, providing a new direction to break through the therapeutic bottleneck. We analyze and summarize the emergence of novel drugs and targets with potential translational ability against tumor metastasis and CSCs. Specific inhibitors serve as one of the hotspots for novel drug development by virtue of their low toxicity and high therapeutic efficiency.
New inhibitors such as Thiolatia (PSMD14 inhibitor) selectively inhibit the PSMD14 gene, which is highly expressed in many cancers, and its inhibition of key oncogenes ensures its efficacy in tumor metastasis; secondly, by virtue of its high specificity, it avoids the toxicity and side-effects of the traditional proteasome inhibitors; and the combination of the drug and cisplatin sensitization effect also gives it a wider scope of application. The combined sensitizing effect of the drug and cisplatin also gives it a wider scope of application (Deshaies, 2014; Jing et al., 2021). Sulfarotene, a sulfonamide drug, also has high selectivity for the target, inhibits RAS signaling pathway with high selectivity, blocks downstream oncogenic signals, and intervenes in the dry maintenance of TRCs; its low toxicity is also better than that of traditional drugs, and it is a drug with great therapeutic potential for cancer patients (Qi et al., 2021). The BMI 1 inhibitor (PTC 209) solves the problem of non-specific distribution of colorectal cancer drugs by using nanoparticles for targeted delivery, which enhances the permeability of the drug to the intestinal barrier. PTC 209 maintains the effect of the inhibitor while allowing the drug to accumulate in the in situ colon tumors, which effectively avoids the systemic adverse reactions of the patients and improves the therapeutic efficiency (Xu et al., 2019).
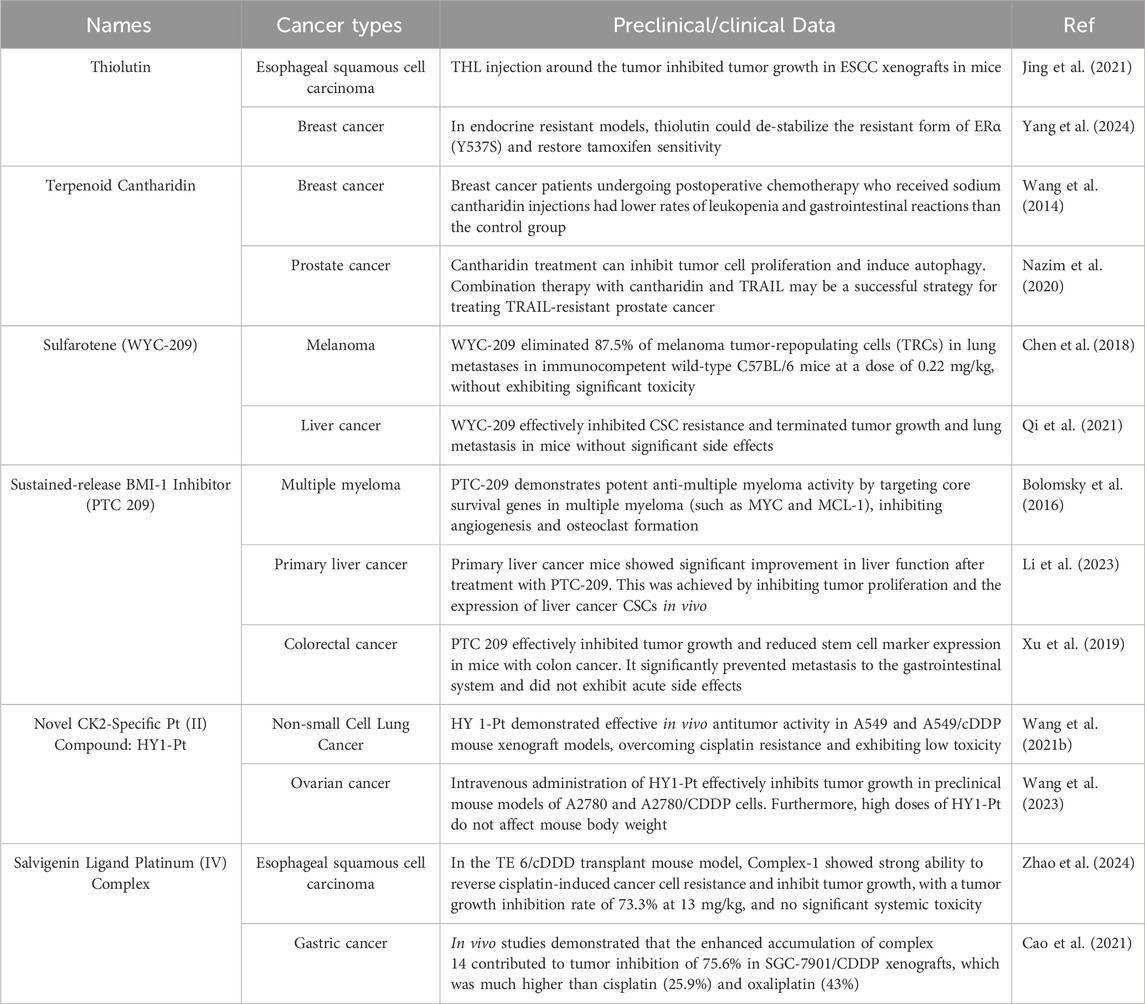
Platinum drugs such as cisplatin treat cancer by interfering with DNA and hindering the cell cycle. However, their non-specific therapeutic characteristics are characterized by problems such as drug resistance and toxic side effects (Nagasaka and Gadgeel, 2018; Wang et al., 2019). Current research has changed the therapeutic limitations of traditional platinum drugs by changing the chemical structure of platinum drugs and adding new ligands to form complexes. For example, HY1-Pt is a CK2 inhibitor HY1 conjugated to an active platinum (II) unit, which achieves resistance reversal and CSCs inhibition through the synergistic effect of targeting protein kinase CK2 and platinum drugs. This breaks through the limited clinical efficacy of existing CK2 inhibitors and significantly improves the resistance and toxicity of platinum drugs (Wang et al., 2021b). Some researchers have also used Salvigenin, a natural polyphenolic compound, as a ligand for novel platinum (IV) complexes, to potently inhibit Rap1b and effectively reverse cisplatin-induced drug resistance, filling the gap of no effective solid molecular inhibitor for Rap1b (Zhao et al., 2024). It provides a new idea to overcome cisplatin-induced drug resistance in cancer cells. The in-depth exploration of traditional drugs also continues, such as the terpenoid Cantharidin (CTD), a multi-targeted anticancer agent derived from traditional Chinese medicine, which plays an important role in inhibiting tumor proliferation and metastasis, enhancing sensitivity and mitigating side effects in combination with radiotherapy by inhibiting the activity of PP1/PP2A and modulating the pathways of PI3K/AKT/mTOR and MAPK. It plays an important role in inhibiting tumor proliferation and metastasis, enhancing sensitivity and alleviating side effects in combined radiotherapy (Hsia et al., 2015a; Naz et al., 2020). The toxicity problem may be solved in the future by nano-targeted delivery and structural modification optimization to enhance clinical safety. The above drugs have shown good therapeutic potential for tumor stemness in cell and animal experiments, and some of them have good performance in combining with sensitized classical anticancer drugs. Currently, some preclinical and clinical trials have demonstrated the efficacy of these drugs (Table 2). However, the potential toxicity, drug resistance, and clinical translation issues of these drugs still need to be solved.
Currently, novel anticancer drugs targeting tumor metastasis and stem cells are characterized by high selectivity, optimized target delivery system, overcoming drug resistance and combination therapy, which provide diversified strategies to improve cancer prognosis. Further clinical validation and mechanism analysis will promote the arrival of the era of precision therapy.
Author contributions
SX: Formal Analysis, Validation, Writing – review and editing, Methodology, Writing – original draft, Investigation, Data curation, Conceptualization, Visualization. ZZ: Software, Formal Analysis, Validation, Methodology, Data curation, Writing – review and editing. YuZ: Conceptualization, Writing – review and editing, Data curation, Investigation, Formal Analysis, Software. CY: Visualization, Formal Analysis, Project administration, Writing – review and editing. WK: Methodology, Investigation, Writing – review and editing, Supervision. YC: Formal Analysis, Data curation, Writing – review and editing, Investigation. WS: Data curation, Investigation, Conceptualization, Writing – review and editing. FZ: Data curation, Validation, Writing – review and editing, Conceptualization. ZY: Validation, Conceptualization, Project administration, Writing – review and editing. RN: Writing – review and editing, Project administration, Data curation, Validation. CC: Visualization, Validation, Funding acquisition, Supervision, Resources, Writing – original draft, Writing – review and editing. YaZ: Writing – review and editing, Writing – original draft, Funding acquisition, Validation, Supervision, Visualization, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project supported by the Nanjing University of Traditional Chinese Medicine National Natural Science Foundation Youth Science Fund Supporting Project (No. 012062005001-26) and Nanjing 2024 Science and Technology Innovation Project for Overseas Students Optimal Funding Project (No. 013071025002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bagchi, S., Yuan, R., and Engleman, E. G. (2021). Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249. doi:10.1146/annurev-pathol-042020-042741
Batlle, E., and Clevers, H. (2017). Cancer stem cells revisited. Nat. Med. 23 (10), 1124–1134. doi:10.1038/nm.4409
Bolomsky, A., Schlangen, K., Schreiner, W., Zojer, N., and Ludwig, H. (2016). Targeting of BMI-1 with PTC-209 shows potent anti-myeloma activity and impairs the tumour microenvironment. J. Hematol. Oncol. 9, 17. doi:10.1186/s13045-016-0247-4
Cao, X., Li, R., Xiong, H., Su, J., Guo, C., An, T., et al. (2021). Novel Pt(IV) complexes to overcome multidrug resistance in gastric cancer by targeting P-glycoprotein. Eur. J. Med. Chem. 221, 113520. doi:10.1016/j.ejmech.2021.113520
Cha, J. H., Chan, L. C., Song, M. S., and Hung, M. C. (2020). New approaches on cancer immunotherapy. Cold Spring Harb. Perspect. Med. 10 (8), a036863. doi:10.1101/cshperspect.a036863
Chen, J., Cao, X., An, Q., Zhang, Y., Li, K., Yao, W., et al. (2018). Inhibition of cancer stem cell like cells by a synthetic retinoid. Nat. Commun. 9 (1), 1406. doi:10.1038/s41467-018-03877-7
Choi, K. Y., Han, H. S., Lee, E. S., Shin, J. M., Almquist, B. D., Lee, D. S., et al. (2019). Hyaluronic acid-based activatable nanomaterials for stimuli-responsive imaging and therapeutics: beyond CD44-Mediated drug delivery. Adv. Mater 31 (34), e1803549. doi:10.1002/adma.201803549
Chun, J., Park, M. K., Ko, H., Lee, K., and Kim, Y. S. (2018). Bioassay-guided isolation of cantharidin from blister beetles and its anticancer activity through inhibition of epidermal growth factor receptor-mediated STAT3 and akt pathways. J. Nat. Med. 72 (4), 937–945. doi:10.1007/s11418-018-1226-6
Clézardin, P., Coleman, R., Puppo, M., Ottewell, P., Bonnelye, E., Paycha, F., et al. (2021). Bone metastasis: mechanisms, therapies, and biomarkers. Physiol. Rev. 101 (3), 797–855. doi:10.1152/physrev.00012.2019
Czarnomysy, R., Surażyński, A., Muszynska, A., Gornowicz, A., Bielawska, A., and Bielawski, K. (2018). A novel series of pyrazole-platinum(II) complexes as potential anti-cancer agents that induce cell cycle arrest and apoptosis in breast cancer cells. J. Enzyme Inhib. Med. Chem. 33 (1), 1006–1023. doi:10.1080/14756366.2018.1471687
Deng, L. P., Dong, J., Cai, H., and Wang, W. (2013). Cantharidin as an antitumor agent: a retrospective review. Curr. Med. Chem. 20 (2), 159–166. doi:10.2174/092986713804806711
Deshaies, R. J. (2014). Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 12, 94. doi:10.1186/s12915-014-0094-0
Fang, L., Qin, X., Zhao, J., and Gou, S. (2019). Construction of dual stimuli-responsive Platinum(IV) hybrids with NQO1 targeting ability and overcoming Cisplatin resistance. Inorg. Chem. 58 (3), 2191–2200. doi:10.1021/acs.inorgchem.8b03386
Fischer, K. R., Durrans, A., Lee, S., Sheng, J., Li, F., Wong, S. T., et al. (2015). Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527 (7579), 472–476. doi:10.1038/nature15748
Garcia, J., Hurwitz, H. I., Sandler, A. B., Miles, D., Coleman, R. L., Deurloo, R., et al. (2020). Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017. doi:10.1016/j.ctrv.2020.102017
Gerstberger, S., Jiang, Q., and Ganesh, K. (2023). Metastasis. Cell 186 (8), 1564–1579. doi:10.1016/j.cell.2023.03.003
Gu, X. D., Xu, L. L., Zhao, H., Gu, J. Z., and Xie, X. H. (2017). Cantharidin suppressed breast cancer MDA-MB-231 cell growth and migration by inhibiting MAPK signaling pathway. Braz J. Med. Biol. Res. 50 (7), e5920. doi:10.1590/1414-431x20175920
Guo, S. J., Xiao, Y. B., Cherevan, A., Eder, D., Xu, L. L., Zeng, Q. H., et al. (2023). Catalytic multivariable metal-organic frameworks for lithium-sulfur batteries. Mater. Today 65, 37–46. doi:10.1016/j.mattod.2023.03.019
Hideshima, T., Bradner, J. E., Wong, J., Chauhan, D., Richardson, P., Schreiber, S. L., et al. (2005). Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci. U. S. A. 102 (24), 8567–8572. doi:10.1073/pnas.0503221102
Hsia, T. C., Lin, J. H., Hsu, S. C., Tang, N. Y., Lu, H. F., Wu, S. H., et al. (2015a). Cantharidin induces DNA damage and inhibits DNA repair-associated protein levels in NCI-H460 human lung cancer cells. Environ. Toxicol. 30 (10), 1135–1143. doi:10.1002/tox.21986
Hsia, T. C., Yu, C. C., Hsu, S. C., Tang, N. Y., Lu, H. F., Yu, C. S., et al. (2015b). cDNA microarray analysis of the effect of cantharidin on DNA damage, cell cycle and apoptosis-associated gene expression in NCI-H460 human lung cancer cells in vitro. Mol. Med. Rep. 12 (1), 1030–1042. doi:10.3892/mmr.2015.3538
Hsia, T. C., Yu, C. C., Hsiao, Y. T., Wu, S. H., Bau, D. T., Lu, H. F., et al. (2016). Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res. 36 (11), 5989–5997. doi:10.21873/anticanres.11187
Huang, W. W., Ko, S. W., Tsai, H. Y., Chung, J. G., Chiang, J. H., Chen, K. T., et al. (2011). Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. Int. J. Oncol. 38 (4), 1067–1073. doi:10.3892/ijo.2011.922
Huang, J., Zhang, H., Yu, Y., Chen, Y., Wang, D., Zhang, G., et al. (2014). Biodegradable self-assembled nanoparticles of poly (D,L-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel to breast cancer. Biomaterials 35 (1), 550–566. doi:10.1016/j.biomaterials.2013.09.089
Ji, B. C., Hsiao, Y. P., Tsai, C. H., Chang, S. J., Hsu, S. C., Liu, H. C., et al. (2015). Cantharidin impairs cell migration and invasion of A375.S2 human melanoma cells by suppressing MMP-2 and -9 through PI3K/NF-κB signaling pathways. Anticancer Res. 35 (2), 729–738.
Jia, Y., Wu, S. L., Isenberg, J. S., Dai, S., Sipes, J. M., Field, L., et al. (2010). Thiolutin inhibits endothelial cell adhesion by perturbing Hsp27 interactions with components of the actin and intermediate filament cytoskeleton. Cell Stress Chaperones 15 (2), 165–181. doi:10.1007/s12192-009-0130-0
Jia, Z., Yang, Y., Dengyan, Z., Chunyang, Z., Donglei, L., Kai, W., et al. (2017). RAP1B, a DVL2 binding protein, activates wnt/Beta-Catenin signaling in esophageal squamous cell carcinoma. Gene 611, 15–20. doi:10.1016/j.gene.2017.01.021
Jiang, M. J., Chen, Y. Y., Dai, J. J., Gu, D. N., Mei, Z., Liu, F. R., et al. (2020). Dying tumor cell-derived exosomal miR-194-5p potentiates survival and repopulation of tumor repopulating cells upon radiotherapy in pancreatic cancer. Mol. Cancer 19 (1), 68. doi:10.1186/s12943-020-01178-6
Jing, C., Li, X., Zhou, M., Zhang, S., Lai, Q., Liu, D., et al. (2021). The PSMD14 inhibitor thiolutin as a novel therapeutic approach for esophageal squamous cell carcinoma through facilitating SNAIL degradation. Theranostics 11 (12), 5847–5862. doi:10.7150/thno.46109
Kim, J. A., Kim, Y., Kwon, B. M., and Han, D. C. (2013). The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 288 (40), 28713–28726. doi:10.1074/jbc.M113.488346
Kreso, A., van Galen, P., Pedley, N. M., Lima-Fernandes, E., Frelin, C., Davis, T., et al. (2014). Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 20 (1), 29–36. doi:10.1038/nm.3418
Kuo, J. H., Shih, T. Y., Lin, J. P., Lai, K. C., Lin, M. L., Yang, M. D., et al. (2015). Cantharidin induces DNA damage and inhibits DNA repair-associated protein expressions in TSGH8301 human bladder cancer cell. Anticancer Res. 35 (2), 795–804.
Lamouille, S., Xu, J., and Derynck, R. (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15 (3), 178–196. doi:10.1038/nrm3758
Lauinger, L., Li, J., Shostak, A., Cemel, I. A., Ha, N., Zhang, Y., et al. (2017). Thiolutin is a zinc chelator that inhibits the Rpn11 and other JAMM metalloproteases. Nat. Chem. Biol. 13 (7), 709–714. doi:10.1038/nchembio.2370
Leng, Y., Abdullah, A., Wendt, M. K., and Calve, S. (2019). Hyaluronic acid, CD44 and RHAMM regulate myoblast behavior during embryogenesis. Matrix Biol. 78-79, 236–254. doi:10.1016/j.matbio.2018.08.008
Li, Y., Luo, S., Ma, R., Liu, J., Xu, P., Zhang, H., et al. (2015). Upregulation of cytosolic phosphoenolpyruvate carboxykinase is a critical metabolic event in melanoma cells that repopulate tumors. Cancer Res. 75 (7), 1191–1196. doi:10.1158/0008-5472.Can-14-2615
Li, J., Yakushi, T., Parlati, F., Mackinnon, A. L., Perez, C., Ma, Y., et al. (2017a). Capzimin is a potent and specific inhibitor of proteasome isopeptidase Rpn11. Nat. Chem. Biol. 13 (5), 486–493. doi:10.1038/nchembio.2326
Li, W., Garcia, D., Cornell, R. F., Gailani, D., Laubach, J., Maglio, M. E., et al. (2017b). Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. 3 (7), 980–988. doi:10.1001/jamaoncol.2016.3350
Li, J., Zhang, Y., Da Silva Sil Dos Santos, B., Wang, F., Ma, Y., Perez, C., et al. (2018). Epidithiodiketopiperazines inhibit protein degradation by targeting proteasome deubiquitinase Rpn11. Cell Chem. Biol. 25 (11), 1350–1358. doi:10.1016/j.chembiol.2018.07.012
Li, W., Solenne, T., Wang, H., Li, B., Liu, Y., Wang, F., et al. (2023). Core-shell cisplatin/SiO(2) nanocapsules combined with PTC-209 overcome chemotherapy-acquired and intrinsic resistance in hepatocellular carcinoma. Acta Biomater. 170, 273–287. doi:10.1016/j.actbio.2023.08.021
Liceras-Boillos, P., Jimeno, D., García-Navas, R., Lorenzo-Martín, L. F., Menacho-Marquez, M., Segrelles, C., et al. (2018). Differential role of the RasGEFs Sos1 and Sos2 in mouse skin homeostasis and carcinogenesis. Mol. Cell Biol. 38 (16), e00049-18. doi:10.1128/mcb.00049-18
Lin, P. Y., Chiu, Y. L., Huang, J. H., Chuang, E. Y., Mi, F. L., Lin, K. J., et al. (2018). Oral nonviral gene delivery for chronic protein replacement therapy. Adv. Sci. (Weinh) 5 (8), 1701079. doi:10.1002/advs.201701079
Liu, T., Yu, J., Deng, M., Yin, Y., Zhang, H., Luo, K., et al. (2017). CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat. Commun. 8, 13923. doi:10.1038/ncomms13923
Liu, Y. P., Li, L., Xu, L., Dai, E. N., and Chen, W. D. (2018). Cantharidin suppresses cell growth and migration, and activates autophagy in human non-small cell lung cancer cells. Oncol. Lett. 15 (5), 6527–6532. doi:10.3892/ol.2018.8141
Lu, Y., Zhu, H., Shan, H., Lu, J., Chang, X., Li, X., et al. (2013). Knockdown of Oct4 and nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett. 340 (1), 113–123. doi:10.1016/j.canlet.2013.07.009
Lv, J., Zhang, S., Wu, H., Lu, J., Lu, Y., Wang, F., et al. (2020). Deubiquitinase PSMD14 enhances hepatocellular carcinoma growth and metastasis by stabilizing GRB2. Cancer Lett. 469, 22–34. doi:10.1016/j.canlet.2019.10.025
Ma, G., Jing, C., Li, L., Huang, F., Ding, F., Wang, B., et al. (2016). MicroRNA-92b represses invasion-metastasis Cascade of esophageal squamous cell carcinoma. Oncotarget 7 (15), 20209–20222. doi:10.18632/oncotarget.7747
Mao, C., Zeng, X., Zhang, C., Yang, Y., Xiao, X., Luan, S., et al. (2021). Mechanisms of pharmaceutical therapy and drug resistance in esophageal cancer. Front. Cell Dev. Biol. 9, 612451. doi:10.3389/fcell.2021.612451
Martins-Neves, S. R., Cleton-Jansen, A. M., and Gomes, C. M. F. (2018). Therapy-induced enrichment of cancer stem-like cells in solid human tumors: where do we stand? Pharmacol. Res. 137, 193–204. doi:10.1016/j.phrs.2018.10.011
Mazzaferro, S., Bouchemal, K., and Ponchel, G. (2013). Oral delivery of anticancer drugs III: formulation using drug delivery systems. Drug Discov. Today 18 (1-2), 99–104. doi:10.1016/j.drudis.2012.08.007
Minamiguchi, K., Kumagai, H., Masuda, T., Kawada, M., Ishizuka, M., and Takeuchi, T. (2001). Thiolutin, an inhibitor of HUVEC adhesion to vitronectin, reduces paxillin in HUVECs and suppresses tumor cell-induced angiogenesis. Int. J. Cancer 93 (3), 307–316. doi:10.1002/ijc.1321
Mitragotri, S., Burke, P. A., and Langer, R. (2014). Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 13 (9), 655–672. doi:10.1038/nrd4363
Mourgues, L., Imbert, V., Nebout, M., Colosetti, P., Neffati, Z., Lagadec, P., et al. (2015). The BMI1 polycomb protein represses cyclin G2-induced autophagy to support proliferation in chronic myeloid leukemia cells. Leukemia 29 (10), 1993–2002. doi:10.1038/leu.2015.112
Nagasaka, M., and Gadgeel, S. M. (2018). Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer Ther. 18 (1), 63–70. doi:10.1080/14737140.2018.1409624
Najafi, M., Farhood, B., and Mortezaee, K. (2019). Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell Physiol. 234 (6), 8381–8395. doi:10.1002/jcp.27740
Nassar, D., and Blanpain, C. (2016). Cancer stem cells: basic concepts and therapeutic implications. Annu. Rev. Pathol. 11, 47–76. doi:10.1146/annurev-pathol-012615-044438
Naz, F., Wu, Y. X., Zhang, N., Yang, Z., and Yu, C. Y. (2020). Anticancer attributes of Cantharidin: involved molecular mechanisms and pathways. Molecules 25 (14), 3279. doi:10.3390/molecules25143279
Nazim, U. M., Yin, H., and Park, S. Y. (2020). Downregulation of c-FLIP and upregulation of DR-5 by cantharidin sensitizes TRAIL-mediated apoptosis in prostate cancer cells via autophagy flux. Int. J. Mol. Med. 46 (1), 280–288. doi:10.3892/ijmm.2020.4566
Nethi, S. K., Li, X., Bhatnagar, S., and Prabha, S. (2023). Enhancing anticancer efficacy of chemotherapeutics using targeting ligand-functionalized synthetic antigen receptor-mesenchymal stem cells. Pharmaceutics 15 (6), 1742. doi:10.3390/pharmaceutics15061742
Noguchi, H., Ikegami, T., Nagadoi, A., Kamatari, Y. O., Park, S. Y., Tame, J. R., et al. (2015). The structure and conformational switching of Rap1B. Biochem. Biophys. Res. Commun. 462 (1), 46–51. doi:10.1016/j.bbrc.2015.04.103
Perez, C., Li, J., Parlati, F., Rouffet, M., Ma, Y., Mackinnon, A. L., et al. (2017). Discovery of an inhibitor of the proteasome subunit Rpn11. J. Med. Chem. 60 (4), 1343–1361. doi:10.1021/acs.jmedchem.6b01379
Purwin, M., Hernández-Toribio, J., Coderch, C., Panchuk, R., Skorokhyd, N., Filipiak, K., et al. (2016). Design and synthesis of novel dual-target agents for HDAC1 and CK2 inhibition. Rsc Adv. 6 (71), 66595–66608. doi:10.1039/c6ra09717k
Qi, F., Qin, W., Zhang, Y., Luo, Y., Niu, B., An, Q., et al. (2021). Sulfarotene, a synthetic retinoid, overcomes stemness and sorafenib resistance of hepatocellular carcinoma via suppressing SOS2-RAS pathway. J. Exp. Clin. Cancer Res. 40 (1), 280. doi:10.1186/s13046-021-02085-4
Rho, S. S., Kobayashi, I., Oguri-Nakamura, E., Ando, K., Fujiwara, M., Kamimura, N., et al. (2019). Rap1b promotes notch-signal-mediated hematopoietic stem cell development by enhancing integrin-mediated cell adhesion. Dev. Cell 49 (5), 681–696. doi:10.1016/j.devcel.2019.03.023
Roy, P. S., and Saikia, B. J. (2016). Cancer and cure: a critical analysis. Indian J. Cancer 53 (3), 441–442. doi:10.4103/0019-509x.200658
Schwartz, D. M., Farley, T. K., Richoz, N., Yao, C., Shih, H. Y., Petermann, F., et al. (2019). Retinoic acid receptor alpha represses a Th9 Transcriptional and Epigenomic program to reduce allergic pathology. Immunity 50 (1), 106–120. doi:10.1016/j.immuni.2018.12.014
Schwind, L., Schetting, S., and Montenarh, M. (2017). Inhibition of protein kinase CK2 prevents adipogenic differentiation of mesenchymal stem cells like C3H/10T1/2 cells. Pharm. (Basel) 10 (1), 22. doi:10.3390/ph10010022
Seo, D., Jung, S. M., Park, J. S., Lee, J., Ha, J., Kim, M., et al. (2019). The deubiquitinating enzyme PSMD14 facilitates tumor growth and chemoresistance through stabilizing the ALK2 receptor in the initiation of BMP6 signaling pathway. EBioMedicine 49, 55–71. doi:10.1016/j.ebiom.2019.10.039
Sheffels, E., Sealover, N. E., Wang, C., Kim, D. H., Vazirani, I. A., Lee, E., et al. (2018). Oncogenic RAS isoforms show a hierarchical requirement for the guanine nucleotide exchange factor SOS2 to mediate cell transformation. Sci. Signal 11 (546), eaar8371. doi:10.1126/scisignal.aar8371
Shen, M., Wu, M. Y., Chen, L. P., Zhi, Q., Gong, F. R., Chen, K., et al. (2015). Cantharidin represses invasion of pancreatic cancer cells through accelerated degradation of MMP2 mRNA. Sci. Rep. 5, 11836. doi:10.1038/srep11836
Siddique, H. R., and Saleem, M. (2012). Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells 30 (3), 372–378. doi:10.1002/stem.1035
Song, Y., Li, S., Ray, A., Das, D. S., Qi, J., Samur, M. K., et al. (2017). Blockade of deubiquitylating enzyme Rpn11 triggers apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Oncogene 36 (40), 5631–5638. doi:10.1038/onc.2017.172
Song, M., Wang, X., Luo, Y., Liu, Z., Tan, W., Ye, P., et al. (2020). Cantharidin suppresses gastric cancer cell migration/invasion by inhibiting the PI3K/Akt signaling pathway via CCAT1. Chem. Biol. Interact. 317, 108939. doi:10.1016/j.cbi.2020.108939
Su, C. C., Liu, S. H., Lee, K. I., Huang, K. T., Lu, T. H., Fang, K. M., et al. (2015). Cantharidin induces apoptosis through the Calcium/PKC-Regulated endoplasmic reticulum stress pathway in human bladder cancer cells. Am. J. Chin. Med. 43 (3), 581–600. doi:10.1142/s0192415x15500366
Su, C. C., Lee, K. I., Chen, M. K., Kuo, C. Y., Tang, C. H., and Liu, S. H. (2016). Cantharidin induced oral squamous cell carcinoma cell apoptosis via the JNK-regulated mitochondria and endoplasmic reticulum stress-related signaling pathways. PLoS One 11 (12), e0168095. doi:10.1371/journal.pone.0168095
Todaro, M., Francipane, M. G., Medema, J. P., and Stassi, G. (2010). Colon cancer stem cells: promise of targeted therapy. Gastroenterology 138 (6), 2151–2162. doi:10.1053/j.gastro.2009.12.063
Torre, L. A., Siegel, R. L., Ward, E. M., and Jemal, A. (2016). Global cancer incidence and mortality rates and Trends--An update. Cancer Epidemiol. Biomarkers Prev. 25 (1), 16–27. doi:10.1158/1055-9965.Epi-15-0578
Walcher, L., Kistenmacher, A. K., Suo, H., Kitte, R., Dluczek, S., Strauß, A., et al. (2020). Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front. Immunol. 11, 1280. doi:10.3389/fimmu.2020.01280
Wang, H., Zhao, Y., Wu, Y., Hu, Y. L., Nan, K., Nie, G., et al. (2011). Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 32 (32), 8281–8290. doi:10.1016/j.biomaterials.2011.07.032
Wang, L., Huang, X. E., and Cao, J. (2014). Clinical study on safety of cantharidin sodium and shenmai injection combined with chemotherapy in treating patients with breast cancer postoperatively. Asian Pac J. Cancer Prev. 15 (14), 5597–5600. doi:10.7314/apjcp.2014.15.14.5597
Wang, W. J., Wu, M. Y., Shen, M., Zhi, Q., Liu, Z. Y., Gong, F. R., et al. (2015). Cantharidin and norcantharidin impair stemness of pancreatic cancer cells by repressing the β-catenin pathway and strengthen the cytotoxicity of gemcitabine and erlotinib. Int. J. Oncol. 47 (5), 1912–1922. doi:10.3892/ijo.2015.3156
Wang, W., Yi, M., Chen, S., Li, J., Zhang, H., Xiong, W., et al. (2017). NOR1 suppresses cancer stem-like cells properties of tumor cells via the inhibition of the AKT-GSK-3β-Wnt/β-catenin-ALDH1A1 signal circuit. J. Cell Physiol. 232 (10), 2829–2840. doi:10.1002/jcp.25706
Wang, J. J., Lei, K. F., and Han, F. (2018). Tumor microenvironment: recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 22 (12), 3855–3864. doi:10.26355/eurrev_201806_15270
Wang, Z., Deng, Z., and Zhu, G. (2019). Emerging platinum(iv) prodrugs to combat cisplatin resistance: from isolated cancer cells to tumor microenvironment. Dalton Trans. 48 (8), 2536–2544. doi:10.1039/c8dt03923b
Wang, H., Xu, H., Ma, F., Zhan, M., Yang, X., Hua, S., et al. (2020). Zinc finger protein 703 induces EMT and sorafenib resistance in hepatocellular carcinoma by transactivating CLDN4 expression. Cell Death Dis. 11 (4), 225. doi:10.1038/s41419-020-2422-3
Wang, M., Herbst, R. S., and Boshoff, C. (2021a). Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 27 (8), 1345–1356. doi:10.1038/s41591-021-01450-2
Wang, Y., Wang, X., Xu, G., and Gou, S. (2021b). Novel CK2-Specific Pt(II) compound reverses cisplatin-induced resistance by inhibiting cancer cell stemness and suppressing DNA damage repair in non-small cell lung cancer treatments. J. Med. Chem. 64 (7), 4163–4178. doi:10.1021/acs.jmedchem.1c00079
Wang, X., Wang, Y., and Gou, S. (2023). A platinum(II) complex HY1-Pt overcomes cisplatin-induced resistance and attenuates metastasis of epithelial ovarian cancer by cancer cell stemness inhibition. Int. J. Biochem. Cell Biol. 157, 106395. doi:10.1016/j.biocel.2023.106395
Wei, Z., Jia, J., Heng, G., Xu, H., Shan, J., Wang, G., et al. (2019). Sirtuin-1/Mitochondrial ribosomal protein S5 axis enhances the metabolic flexibility of liver cancer stem cells. Hepatology 70 (4), 1197–1213. doi:10.1002/hep.30622
Wołoszynowska-Fraser, M. U., Kouchmeshky, A., and McCaffery, P. (2020). Vitamin A and retinoic acid in cognition and cognitive disease. Annu. Rev. Nutr. 40, 247–272. doi:10.1146/annurev-nutr-122319-034227
Wu, M. Y., Xie, X., Xu, Z. K., Xie, L., Chen, Z., Shou, L. M., et al. (2014). PP2A inhibitors suppress migration and growth of PANC-1 pancreatic cancer cells through inhibition on the Wnt/β-catenin pathway by phosphorylation and degradation of β-catenin. Oncol. Rep. 32 (2), 513–522. doi:10.3892/or.2014.3266
Wu, Y., Wang, Y., Lin, Y., Liu, Y., Wang, Y., Jia, J., et al. (2017). Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat. Commun. 8, 14228. doi:10.1038/ncomms14228
Xia, S., Pan, Y., Liang, Y., Xu, J., and Cai, X. (2020). The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine 51, 102610. doi:10.1016/j.ebiom.2019.102610
Xu, M. D., Liu, S. L., Zheng, B. B., Wu, J., Wu, M. Y., Zhang, Y., et al. (2018). The radiotherapy-sensitization effect of cantharidin: mechanisms involving cell cycle regulation, enhanced DNA damage, and inhibited DNA damage repair. Pancreatology 18 (7), 822–832. doi:10.1016/j.pan.2018.08.007
Xu, J., Zhang, Y., Xu, J., Wang, M., Liu, G., Wang, J., et al. (2019). Reversing tumor stemness via orally targeted nanoparticles achieves efficient Colon cancer treatment. Biomaterials 216, 119247. doi:10.1016/j.biomaterials.2019.119247
Yang, P., Yang, X., Wang, D., Yang, H., Li, Z., Zhang, C., et al. (2024). PSMD14 stabilizes estrogen signaling and facilitates breast cancer progression via deubiquitinating ERα. Oncogene 43 (4), 248–264. doi:10.1038/s41388-023-02905-1
Zhang, M., Xu, C., Wen, L., Han, M. K., Xiao, B., Zhou, J., et al. (2016a). A hyaluronidase-responsive nanoparticle-based drug delivery system for targeting Colon cancer cells. Cancer Res. 76 (24), 7208–7218. doi:10.1158/0008-5472.Can-16-1681
Zhang, Z., Bu, X., Chen, H., Wang, Q., and Sha, W. (2016b). Bmi-1 promotes the invasion and migration of Colon cancer stem cells through the downregulation of E-cadherin. Int. J. Mol. Med. 38 (4), 1199–1207. doi:10.3892/ijmm.2016.2730
Zhang, Z. H., Li, Y. J., Wan, J. X., Long, P. H., Guo, J., Chen, G. S., et al. (2017). Preparation of Pt(IV)-crosslinked polymer nanoparticles with an anti-detoxifying effect for enhanced anticancer therapy. Polym. Chem. 8 (15), 2410–2422. doi:10.1039/c6py02148d
Zhang, L., Cui, M., Song, L., Zhang, M., and Zhang, J. (2019). Function, significance, and regulation of Rap1b in malignancy. Crit. Rev. Eukaryot. Gene Expr. 29 (2), 151–160. doi:10.1615/CritRevEukaryotGeneExpr.2019025997
Zhao, J., Wu, K., Yang, Y., Liu, D., Zhang, C., and Li, X. (2024). Novel Pt(IV) complexes containing salvigenin ligand reverse cisplatin-induced resistance by inhibiting Rap1b-mediated cancer cell stemness in esophageal squamous cell carcinoma treatments. Bioorg Chem. 147, 107384. doi:10.1016/j.bioorg.2024.107384
Zheng, X., Carstens, J. L., Kim, J., Scheible, M., Kaye, J., Sugimoto, H., et al. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527 (7579), 525–530. doi:10.1038/nature16064
Keywords: cancer metastasis, cancer stem cells, targeted therapy, nanoparticle delivery, drug resistance
Citation: Xie S, Zhou Z, Zheng Y, Yu C, Kong W, Chen Y, Si W, Zhou F, Yang Z, Ni R, Chang C and Zhang Y (2025) Novel drug research and therapeutic strategies targeting tumor metastasis and cancer stem cells. Front. Pharmacol. 16:1643183. doi: 10.3389/fphar.2025.1643183
Received: 08 June 2025; Accepted: 05 September 2025;
Published: 16 September 2025.
Edited by:
Bo Wang, Zhengzhou University, ChinaReviewed by:
Xiaolei Li, University of Pennsylvania, United StatesHumphrey Omeoga, University at Albany, United States
Copyright © 2025 Xie, Zhou, Zheng, Yu, Kong, Chen, Si, Zhou, Yang, Ni, Chang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Chang, Y2MzOTQ1NjE0NThAaWNsb3VkLmNvbQ==; Yang Zhang, eWFuZ3poYW5nQG5qdWNtLmVkdS5jbg==
 Sicong Xie
Sicong Xie Zhiyi Zhou1
Zhiyi Zhou1 Yu Zheng
Yu Zheng Yang Zhang
Yang Zhang